User login
Taking the Mystery Out of the Match: Histocompatibility Testing and Kidney Transplantation
Effect of Statins on Total Testosterone Levels in Male Veterans
Restless Legs Syndrome Complicating Iron Deficiency
Diagnosing Infantile Hypertrophic Pyloric Stenosis
Infantile hypertrophic pyloric stenosis (IHPS) is a common yet treatable condition in young infants, characterized by forceful vomiting after feeding as a result of hypertrophy of the pyloric muscle. Without proper diagnosis and surgical intervention, IHPS can eventually lead to dehydration, weight loss, and electrolyte disturbances, including the classic finding of hypochloremic alkalosis.1,2
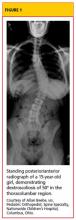
IHPS occurs in two to four of every 1,000 births and is most common among white male infants.3,4 It develops between the first three to five weeks of life but rarely after age 12 weeks.1,2,5 IHPS is characterized by hypertrophy of the pyloric muscle (see figure), which eventually leads to gastric outlet obstruction.6,7
Because IHPS presents with symptoms that resemble those associated with other gastrointestinal disturbances, such as gastroesophageal reflux disease (GERD), it can be misdiagnosed in its early stages.1 Once it is identified, however, surgical correction by pyloromyotomy is considered curative, with a very low mortality rate (ie, 0.1%) and incidence recurrence in only 1% of patients. Typically, treated infants recover quickly and can begin feeding within hours after surgery.3,8
Although the etiology of IHPS remains unknown, providers can quickly recognize the signs and symptoms of IHPS in order for surgical treatment to be scheduled in a timely manner.
PATIENT PRESENTATION
Typically, an infant with IHPS will have a period of normal feeding for the first two to three weeks of life, followed by onset of nonbilious vomiting soon after feeding. Vomiting may become increasingly frequent and forceful—possibly described as “projectile.” Despite stomach distention, affected infants seem to have an insatiable appetite and may cry inconsolably. Depending on the duration of symptoms, patients may suffer significant weight loss, even falling below birth weight. In severe cases, a scaphoid abdomen and protruding ribcage may be present.1,2
Atypical Presentations
Premature infants with IHPS and those with certain medical or surgical conditions may present atypically. Vomiting may be less forceful, and the classic finding of visible gastric peristalsis may or may not be present.9 Researchers have documented cases in which hospitalized premature infants had nonprojectile vomiting, weight loss, and lethargy that were erroneously attributed to sepsis. IHPS should have been considered over sepsis when the infants’ clinical condition improved rapidly with rehydration, and when metabolic alkalosis (rather than acidosis) was identified.1,10,11
Projectile vomiting may not occur in infants with congenital anomalies that affect swallowing, such as cleft lip/palate or central nervous system disturbances. In infants who have recently undergone gastrointestinal (GI) surgery, IHPS may be misdiagnosed as adhesions or obstruction at an anastomotic site.1,10
RISK FACTORS
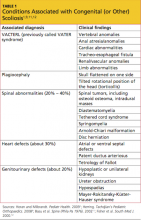
Despite the fact that IHPS is a relatively common condition, the etiology remains unknown. Research findings indicate that IHPS is not present at birth. Several hypotheses exist about potential risk factors, including genetics, use of macrolide antibiotics, and mechanical or environmental factors.6 IHPS has been associated with certain genetic conditions, including Cornelia de Lange syndrome and Smith-Lemli-Opitz syndrome, as well as chromosomal abnormalities, such as the translocation of chromosomes 8 and 17, and partial trisomy of chromosome 9.6,12
According to Chung,13 additional research has implicated the genetic loci IHPS1, also known as nitric oxide synthase 1 (NOS1), which encodes the gene for neuronal nitric oxide synthase (nNOS). This is the key enzyme for production of nitric oxide, which mediates relaxation of the pyloric smooth muscle.13
In a study by Mahon et al,12 an increased risk for IHPS was confirmed in young infants for whom systemic erythromycin had been prescribed as prophylactic treatment for pertussis. This may result from the agent’s motilin-like effects on antral smooth-muscle function.13
Mechanical defects, such as abnormal innervation of the pyloric muscle and neonatal hypergastrinemia and hyperacidity, have also been implicated.6 Infant sleeping position has been investigated as a possible environmental factor in the development of IHPS, correlating the decline in sudden infant death syndrome (attributed to decreased prone sleep position) with a decline in IHPS in recent years. However, no conclusive evidence has been shown to support this hypothesis.13
DIFFERENTIAL DIAGNOSIS
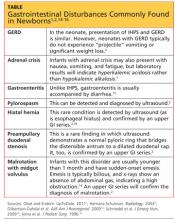
Typically, infants with nonbilious vomiting have either IHPS or GERD. Two factors to consider when evaluating the infant are its age and whether emesis is bilious or nonbilious. IHPS rarely causes bilious vomiting and most frequently occurs in infants between ages 3 and 6 weeks. Other GI disturbances that cause nonbilious emesis include adrenal crisis, gastroenteritis, pylorospasm, hiatal hernia, and preampullary duodenal stenosis.2 Malrotation or midgut volvulus may also be considered in the differential14 (see table1,2,14-16).
DIAGNOSIS
The diagnosis of IHPS is often made based on the history and physical exam. Imaging studies and labs can confirm the diagnosis, and surgery is usually deferred until dehydration has been addressed and any electrolyte disturbances corrected.1,17
Physical Examination
The infant may appear underweight and dehydrated, with visible peristaltic waves across the upper abdomen prior to emesis. Severe illness may be indicated in an underweight infant with the classic scaphoid abdomen.18
Palpation of an olive-shaped mass in the upper left quadrant of the epigastric region is pathognomonic for IHPS; the mass may be found more easily after emesis has occurred.19 To facilitate palpation, the hips can first be flexed to relax the abdominal wall. Next, the examiner should palpate gently for the “olive” in the space midway between the umbilicus and the xiphoid, between the two rectus muscles.3 All other findings in the physical exam should be within normal limits.
Imaging and Laboratory
Studies
An experienced provider can make a diagnosis of IHPS based on clinical examination alone in 60% to 80% of cases.20 However, most surgeons require the diagnosis to be confirmed with one of the following imaging studies before surgery.19
The diagnosis of IHPS can be confirmed by an upper GI series, but this is not commonly ordered as the primary diagnostic study. Ultrasound has become the modality of choice9; it can be used to quantify the size of an elongated, thickened pyloric muscle. The hypertrophied pylorus ranges in length from 14 to 16 mm, with thickness measuring more than 3.0 to 3.5 mm.1,19,21
An upper GI contrast study is rarely used for diagnosis of IHPS, but when this test is ordered to detect other gastrointestinal disease processes (eg, malrotation), IHPS may be identified incidentally.19 During an upper GI study, contrast material is propelled through the pyloric mucosa, and the string sign or the double-track sign may be visualized, revealing the mucosal filling defect.2
Abdominal x-ray may reveal a dilated stomach or a blockage, with a possible finding of gas in the gastric bubble but extending no further into the intestine.19
A study by Hernanz-Schulman2 was conducted to quantify the sensitivities and specificities associated with different diagnostic tools and the clinician’s experience and proficiency in using them. According to this study, palpation by a surgeon has a sensitivity of 31% to 99% and a specificity of 85% to 99% for detection of the pathognomonic olive-shaped mass. Ultrasound performed by an experienced technician has 97% to 100% sensitivity and 99% to 100% specificity for detecting IHPS. An upper GI series has 90% to 100% sensitivity and 99.5% specificity.
Venous blood gas and electrolyte levels are both helpful in making a diagnosis of IHPS.19,22 In a study by Oakley and Barnett,22 the following lab values were found useful in confirming the presence of pyloric stenosis: pH > 7.45; chloride 3 mEq/L. Sodium and potassium deficits may also be present.2,22
Before treatment is considered, the degree of dehydration must be determined by clinical examination and urinary output, as well as serum bicarbonate and chloride levels.3 Other laboratory tests to be ordered include urinalysis and a complete blood count (including platelets).
TREATMENT/MANAGEMENT
Initial management of vomiting in children should begin with fluid replacement if the patient appears dehydrated or if lab findings suggest an electrolyte imbalance. If surgery is later deemed necessary, this will reduce the risk for postoperative apnea.1,23
If IHPS is suspected, a pediatric surgery consult should be obtained. Before anesthesia is considered, serum bicarbonate should be measured with results no higher than 28 mEq/L, and the serum chloride level should be at least 100 mEq/L.3 Imaging, such as ultrasound or the upper GI series, may be ordered to confirm the diagnosis.
Surgical correction by a pyloromyotomy is curative. Presurgical gastric decompression via nasogastric tube placement will reduce the risk for postoperative vomiting and gastritis.20
Surgery
Although a variety of nonsurgical interventions, including oral and IV atropine and balloon dilation, have been described in the literature,3,24-26 the preferred treatment for IHPS is surgical intervention. Surgical correction has been so consistently successful (that is, provided the procedure is performed by a pediatric surgeon or other surgeon with appropriate experience3) that the treatment of choice for IHPS is the Ramstedt pyloromyotomy, which was first performed in 1912.6,27,28
Although the approach may differ based on the individual surgeon’s preference, the pyloric muscle is pulled through an incision in the abdominal wall. A longitudinal incision is made through the muscle with blunt dissection to the submucosa on the anterior surface of the pylorus. The pylorus is then returned to the abdominal cavity, and the abdominal incision is sutured.3,20
The laparoscopic approach, first described in the literature in the mid-1990s,29 is gaining popularity among surgeons, although recent studies have demonstrated that open and laparoscopic procedures are comparably safe and effective for the management of IHPS.30-33 Results from a recent study by Jia et al30 indicate that the laparoscopic approach results in reduced postoperative emesis, shorter length of hospital stay, and shorter recovery times; Hall et al31 emphasize the advantages of laparoscopic pyloromyotomy and recommend it over open surgery in facilities with experienced surgeons.
In follow-up ultrasound studies, hypertrophy of the pyloric muscle was reportedly resolved between two and 12 weeks after pyloromyotomy.34
Postoperative Care
The gastric tube can be removed and oral fluids reintroduced slowly at the surgeon’s discretion, between 8 and 12 hours after surgery. Postoperative vomiting is common (occurring in up to 80% of patients) but should resolve within 24 hours. Mild emesis should not delay the refeeding schedule.3
Patients should be evaluated by the surgeon one to two weeks following surgery unless the infant shows signs of infection (ie, fever, erythema, edema, bleeding, purulent drainage, excess pain, decreased fluid or nutritional intake). Follow-up imaging and laboratory studies are not indicated in an otherwise healthy infant.20
CONCLUSION
Early detection of IHPS, a condition characterized by hypertrophy of the pyloric muscle that results in gastric obstruction and projectile vomiting, can prevent complications of dehydration, malnutrition, and electrolyte disturbances. Surgery by open or laparoscopic pyloromyotomy is curative. Knowing the key physical exam findings, laboratory values, and typical patient history in IHPS enhances the clinician’s ability to make a timely diagnosis.
REFERENCES
1. Olivé AP, Endom EE. Infantile hypertrophic pyloric stenosis (2011). www.uptodate.com/contents/infan tile-hypertrophic-pyloric-stenosis. Accessed August 20, 2012.
2. Hernanz-Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227(2):319-331.
3. Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg. 2007;16(1):27-33.
4. To T, Wajja A, Wales PW, Langer JC. Population demographic indicators associated with incidence of pyloric stenosis. Arch Pediatr Adolesc Med. 2005;159(6):520-525.
5. MacMahon B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006; 17(2):195-201.
6. Panteli C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009;25 (12):1043-1052.
7. Stone CK, Humphries RL, eds. Current Medical Diagnosis and Treatment: Emergency Medicine. 7th ed. Chapter 50. Pediatric emergencies (2008). http://accessmedicine.com/resourceTOC.aspx?resource ID=718. Accessed August 20, 2012.
8. Hulka F, Harrison MW, Campbell TJ, Campbell JR. Complications of pyloromyotomy for infantile hypertrophic pyloric stenosis. Am J Surg. 1997;173 (5):450-452.
9. Shaoul R, Enav B, Steiner Z, et al. Clinical presentation of pyloric stenosis: the change is in our hands. Isr Med Assoc J. 2004;6(3):134-137.
10. Weinstein MM, Seibert JJ, Ehrenberg A. Six atypical presentations of congenital hypertrophic pyloric stenosis. Clin Pediatr. 1979;18(2):120-122.
11. Eyal O, Asia A, Yorgenson U, et al. Atypical infantile hypertrophic pyloric stenosis [in Hebrew]. Harefuah. 1999;136(2):113-114, 175.
12. Mahon BE, Rosenman MB, Kleiman MB. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139(3):380-384.
13. Chung E. Infantile hypertrophic pyloric stenosis: genes and environment. Arch Dis Child. 2008;93 (12):1003-1004.
14. Gilbertson-Dahdal DL, Dutta S, Varich LJ, Barth RA. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. AJR Am J Roentgenol. 2009;192(5):1269-1271.
15. Schmedel W, Ashe L, Kuznicki K. A 24-day-old child with projectile vomiting. J Emerg Nurs. 2009; 35(2):163-164.
16. Iijima T, Okamatsu T, Matsumura M, Yatsuzuka M. Hypertrophic pyloric stenosis associated with hiatal hernia. J Pediatr Surg. 1996;31(2):277-279.
17. Nakayama DK, Taylor LA. Hypertrophic pyloric stenosis in infancy: an analysis of treatment outcome. N C Med J. 1998;59(5):310-313.
18. Irish MS, Pearl RH, Caty M, Glick PL. The approach to common abdominal diagnoses in infants and children. Pediatr Clin North Am. 1998; 45(4):729-772.
19. Askew N. An overview of infantile hypertrophic pyloric stenosis: literature review. Paediatric Nurs. 2010;22(8):27-30.
20. Rudolph CD. Infantile hypertrophic pyloric stenosis. In: Rudolph CD, Rudolph AM, eds. Rudolph’s Pediatrics. 21st ed. New York, NY: McGraw-Hill; 2002:1402-1403.
21. Hallam D, Hansen B, Bødker B, et al. Pyloric size in normal infants and in infants suspected of having hypertrophic pyloric stenosis. Acta Radiol. 2005;36(3):261.
22. Oakley EA, Barnett PL. Is acid base determination an accurate predictor of pyloric stenosis? J Paediatr Child Health. 2000;36(6):587-589.
23. Steven IM, Allen TH, Sweeney DB. Congenital hypertrophic pyloric stenosis: the anaesthetist’s view. Anaesth Intensive Care. 1973;1(6):544-546.
24. Singh UK, Kumar R, Prasad R. Oral atropine sulfate for infantile hypertrophic pyloric stenosis. Indian Pediatr. 2005;42(5):473-476.
25. Nagita A, Yamaguchi J, Amemoto K, et al. Management and ultrasonographic appearance of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate. J Pediatr Gastroenterol Nutr. 1996;23(2):172-177.
26. Ogawa Y, Higashimoto U, Nishijima E, et al. Successful endoscopic balloon dilatation for hypertrophic pyloric stenosis. J Pediatr Surg. 1996;31(12):1712-1714.
27. Langer JC, To T. Does pediatric surgical specialty training affect outcome after Ramstedt pyloromyotomy? A population-based study. Pediatrics. 2004;113(5):1342-1347.
28. Pollock WF, Norris WJ. Dr. Conrad Ramstedt and pyloromyotomy. Surgery. 1957;42(5):966-970.
29. Najmaldin A, Tan HL. Early experience with laparoscopic pyloromyotomy for infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1995;30(1):37-38.
30. Jia WQ, Tian JH, Yang KH, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a meta-analysis of randomized controlled trials. Eur J Pediatr Surg. 2011;21(2):77-81.
31. Hall NJ, Pacilli M, Eaton S, et al. Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet. 2009;373(9661):390-398.
32. Adibe OO, Nichol PF, Flake AAW, Mattei P. Comparison of outcomes after laparoscopic and open pyloromyotomy at a high-volume pediatric teaching hospital. J Pediatr Surg. 2006;41(10):1676-1678.
33. St. Peter SD, Holcomb GW, Calkins CM, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg. 2006;244(3):363-370.
34. Okorie NM, Dickson JA, Carver RA, Steiner GM. What happens to the pylorus after pyloromyotomy? Arch Dis Child. 1988;63(11):1339-1341.
Infantile hypertrophic pyloric stenosis (IHPS) is a common yet treatable condition in young infants, characterized by forceful vomiting after feeding as a result of hypertrophy of the pyloric muscle. Without proper diagnosis and surgical intervention, IHPS can eventually lead to dehydration, weight loss, and electrolyte disturbances, including the classic finding of hypochloremic alkalosis.1,2
IHPS occurs in two to four of every 1,000 births and is most common among white male infants.3,4 It develops between the first three to five weeks of life but rarely after age 12 weeks.1,2,5 IHPS is characterized by hypertrophy of the pyloric muscle (see figure), which eventually leads to gastric outlet obstruction.6,7
Because IHPS presents with symptoms that resemble those associated with other gastrointestinal disturbances, such as gastroesophageal reflux disease (GERD), it can be misdiagnosed in its early stages.1 Once it is identified, however, surgical correction by pyloromyotomy is considered curative, with a very low mortality rate (ie, 0.1%) and incidence recurrence in only 1% of patients. Typically, treated infants recover quickly and can begin feeding within hours after surgery.3,8
Although the etiology of IHPS remains unknown, providers can quickly recognize the signs and symptoms of IHPS in order for surgical treatment to be scheduled in a timely manner.
PATIENT PRESENTATION
Typically, an infant with IHPS will have a period of normal feeding for the first two to three weeks of life, followed by onset of nonbilious vomiting soon after feeding. Vomiting may become increasingly frequent and forceful—possibly described as “projectile.” Despite stomach distention, affected infants seem to have an insatiable appetite and may cry inconsolably. Depending on the duration of symptoms, patients may suffer significant weight loss, even falling below birth weight. In severe cases, a scaphoid abdomen and protruding ribcage may be present.1,2
Atypical Presentations
Premature infants with IHPS and those with certain medical or surgical conditions may present atypically. Vomiting may be less forceful, and the classic finding of visible gastric peristalsis may or may not be present.9 Researchers have documented cases in which hospitalized premature infants had nonprojectile vomiting, weight loss, and lethargy that were erroneously attributed to sepsis. IHPS should have been considered over sepsis when the infants’ clinical condition improved rapidly with rehydration, and when metabolic alkalosis (rather than acidosis) was identified.1,10,11
Projectile vomiting may not occur in infants with congenital anomalies that affect swallowing, such as cleft lip/palate or central nervous system disturbances. In infants who have recently undergone gastrointestinal (GI) surgery, IHPS may be misdiagnosed as adhesions or obstruction at an anastomotic site.1,10
RISK FACTORS
Despite the fact that IHPS is a relatively common condition, the etiology remains unknown. Research findings indicate that IHPS is not present at birth. Several hypotheses exist about potential risk factors, including genetics, use of macrolide antibiotics, and mechanical or environmental factors.6 IHPS has been associated with certain genetic conditions, including Cornelia de Lange syndrome and Smith-Lemli-Opitz syndrome, as well as chromosomal abnormalities, such as the translocation of chromosomes 8 and 17, and partial trisomy of chromosome 9.6,12
According to Chung,13 additional research has implicated the genetic loci IHPS1, also known as nitric oxide synthase 1 (NOS1), which encodes the gene for neuronal nitric oxide synthase (nNOS). This is the key enzyme for production of nitric oxide, which mediates relaxation of the pyloric smooth muscle.13
In a study by Mahon et al,12 an increased risk for IHPS was confirmed in young infants for whom systemic erythromycin had been prescribed as prophylactic treatment for pertussis. This may result from the agent’s motilin-like effects on antral smooth-muscle function.13
Mechanical defects, such as abnormal innervation of the pyloric muscle and neonatal hypergastrinemia and hyperacidity, have also been implicated.6 Infant sleeping position has been investigated as a possible environmental factor in the development of IHPS, correlating the decline in sudden infant death syndrome (attributed to decreased prone sleep position) with a decline in IHPS in recent years. However, no conclusive evidence has been shown to support this hypothesis.13
DIFFERENTIAL DIAGNOSIS
Typically, infants with nonbilious vomiting have either IHPS or GERD. Two factors to consider when evaluating the infant are its age and whether emesis is bilious or nonbilious. IHPS rarely causes bilious vomiting and most frequently occurs in infants between ages 3 and 6 weeks. Other GI disturbances that cause nonbilious emesis include adrenal crisis, gastroenteritis, pylorospasm, hiatal hernia, and preampullary duodenal stenosis.2 Malrotation or midgut volvulus may also be considered in the differential14 (see table1,2,14-16).
DIAGNOSIS
The diagnosis of IHPS is often made based on the history and physical exam. Imaging studies and labs can confirm the diagnosis, and surgery is usually deferred until dehydration has been addressed and any electrolyte disturbances corrected.1,17
Physical Examination
The infant may appear underweight and dehydrated, with visible peristaltic waves across the upper abdomen prior to emesis. Severe illness may be indicated in an underweight infant with the classic scaphoid abdomen.18
Palpation of an olive-shaped mass in the upper left quadrant of the epigastric region is pathognomonic for IHPS; the mass may be found more easily after emesis has occurred.19 To facilitate palpation, the hips can first be flexed to relax the abdominal wall. Next, the examiner should palpate gently for the “olive” in the space midway between the umbilicus and the xiphoid, between the two rectus muscles.3 All other findings in the physical exam should be within normal limits.
Imaging and Laboratory
Studies
An experienced provider can make a diagnosis of IHPS based on clinical examination alone in 60% to 80% of cases.20 However, most surgeons require the diagnosis to be confirmed with one of the following imaging studies before surgery.19
The diagnosis of IHPS can be confirmed by an upper GI series, but this is not commonly ordered as the primary diagnostic study. Ultrasound has become the modality of choice9; it can be used to quantify the size of an elongated, thickened pyloric muscle. The hypertrophied pylorus ranges in length from 14 to 16 mm, with thickness measuring more than 3.0 to 3.5 mm.1,19,21
An upper GI contrast study is rarely used for diagnosis of IHPS, but when this test is ordered to detect other gastrointestinal disease processes (eg, malrotation), IHPS may be identified incidentally.19 During an upper GI study, contrast material is propelled through the pyloric mucosa, and the string sign or the double-track sign may be visualized, revealing the mucosal filling defect.2
Abdominal x-ray may reveal a dilated stomach or a blockage, with a possible finding of gas in the gastric bubble but extending no further into the intestine.19
A study by Hernanz-Schulman2 was conducted to quantify the sensitivities and specificities associated with different diagnostic tools and the clinician’s experience and proficiency in using them. According to this study, palpation by a surgeon has a sensitivity of 31% to 99% and a specificity of 85% to 99% for detection of the pathognomonic olive-shaped mass. Ultrasound performed by an experienced technician has 97% to 100% sensitivity and 99% to 100% specificity for detecting IHPS. An upper GI series has 90% to 100% sensitivity and 99.5% specificity.
Venous blood gas and electrolyte levels are both helpful in making a diagnosis of IHPS.19,22 In a study by Oakley and Barnett,22 the following lab values were found useful in confirming the presence of pyloric stenosis: pH > 7.45; chloride 3 mEq/L. Sodium and potassium deficits may also be present.2,22
Before treatment is considered, the degree of dehydration must be determined by clinical examination and urinary output, as well as serum bicarbonate and chloride levels.3 Other laboratory tests to be ordered include urinalysis and a complete blood count (including platelets).
TREATMENT/MANAGEMENT
Initial management of vomiting in children should begin with fluid replacement if the patient appears dehydrated or if lab findings suggest an electrolyte imbalance. If surgery is later deemed necessary, this will reduce the risk for postoperative apnea.1,23
If IHPS is suspected, a pediatric surgery consult should be obtained. Before anesthesia is considered, serum bicarbonate should be measured with results no higher than 28 mEq/L, and the serum chloride level should be at least 100 mEq/L.3 Imaging, such as ultrasound or the upper GI series, may be ordered to confirm the diagnosis.
Surgical correction by a pyloromyotomy is curative. Presurgical gastric decompression via nasogastric tube placement will reduce the risk for postoperative vomiting and gastritis.20
Surgery
Although a variety of nonsurgical interventions, including oral and IV atropine and balloon dilation, have been described in the literature,3,24-26 the preferred treatment for IHPS is surgical intervention. Surgical correction has been so consistently successful (that is, provided the procedure is performed by a pediatric surgeon or other surgeon with appropriate experience3) that the treatment of choice for IHPS is the Ramstedt pyloromyotomy, which was first performed in 1912.6,27,28
Although the approach may differ based on the individual surgeon’s preference, the pyloric muscle is pulled through an incision in the abdominal wall. A longitudinal incision is made through the muscle with blunt dissection to the submucosa on the anterior surface of the pylorus. The pylorus is then returned to the abdominal cavity, and the abdominal incision is sutured.3,20
The laparoscopic approach, first described in the literature in the mid-1990s,29 is gaining popularity among surgeons, although recent studies have demonstrated that open and laparoscopic procedures are comparably safe and effective for the management of IHPS.30-33 Results from a recent study by Jia et al30 indicate that the laparoscopic approach results in reduced postoperative emesis, shorter length of hospital stay, and shorter recovery times; Hall et al31 emphasize the advantages of laparoscopic pyloromyotomy and recommend it over open surgery in facilities with experienced surgeons.
In follow-up ultrasound studies, hypertrophy of the pyloric muscle was reportedly resolved between two and 12 weeks after pyloromyotomy.34
Postoperative Care
The gastric tube can be removed and oral fluids reintroduced slowly at the surgeon’s discretion, between 8 and 12 hours after surgery. Postoperative vomiting is common (occurring in up to 80% of patients) but should resolve within 24 hours. Mild emesis should not delay the refeeding schedule.3
Patients should be evaluated by the surgeon one to two weeks following surgery unless the infant shows signs of infection (ie, fever, erythema, edema, bleeding, purulent drainage, excess pain, decreased fluid or nutritional intake). Follow-up imaging and laboratory studies are not indicated in an otherwise healthy infant.20
CONCLUSION
Early detection of IHPS, a condition characterized by hypertrophy of the pyloric muscle that results in gastric obstruction and projectile vomiting, can prevent complications of dehydration, malnutrition, and electrolyte disturbances. Surgery by open or laparoscopic pyloromyotomy is curative. Knowing the key physical exam findings, laboratory values, and typical patient history in IHPS enhances the clinician’s ability to make a timely diagnosis.
REFERENCES
1. Olivé AP, Endom EE. Infantile hypertrophic pyloric stenosis (2011). www.uptodate.com/contents/infan tile-hypertrophic-pyloric-stenosis. Accessed August 20, 2012.
2. Hernanz-Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227(2):319-331.
3. Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg. 2007;16(1):27-33.
4. To T, Wajja A, Wales PW, Langer JC. Population demographic indicators associated with incidence of pyloric stenosis. Arch Pediatr Adolesc Med. 2005;159(6):520-525.
5. MacMahon B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006; 17(2):195-201.
6. Panteli C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009;25 (12):1043-1052.
7. Stone CK, Humphries RL, eds. Current Medical Diagnosis and Treatment: Emergency Medicine. 7th ed. Chapter 50. Pediatric emergencies (2008). http://accessmedicine.com/resourceTOC.aspx?resource ID=718. Accessed August 20, 2012.
8. Hulka F, Harrison MW, Campbell TJ, Campbell JR. Complications of pyloromyotomy for infantile hypertrophic pyloric stenosis. Am J Surg. 1997;173 (5):450-452.
9. Shaoul R, Enav B, Steiner Z, et al. Clinical presentation of pyloric stenosis: the change is in our hands. Isr Med Assoc J. 2004;6(3):134-137.
10. Weinstein MM, Seibert JJ, Ehrenberg A. Six atypical presentations of congenital hypertrophic pyloric stenosis. Clin Pediatr. 1979;18(2):120-122.
11. Eyal O, Asia A, Yorgenson U, et al. Atypical infantile hypertrophic pyloric stenosis [in Hebrew]. Harefuah. 1999;136(2):113-114, 175.
12. Mahon BE, Rosenman MB, Kleiman MB. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139(3):380-384.
13. Chung E. Infantile hypertrophic pyloric stenosis: genes and environment. Arch Dis Child. 2008;93 (12):1003-1004.
14. Gilbertson-Dahdal DL, Dutta S, Varich LJ, Barth RA. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. AJR Am J Roentgenol. 2009;192(5):1269-1271.
15. Schmedel W, Ashe L, Kuznicki K. A 24-day-old child with projectile vomiting. J Emerg Nurs. 2009; 35(2):163-164.
16. Iijima T, Okamatsu T, Matsumura M, Yatsuzuka M. Hypertrophic pyloric stenosis associated with hiatal hernia. J Pediatr Surg. 1996;31(2):277-279.
17. Nakayama DK, Taylor LA. Hypertrophic pyloric stenosis in infancy: an analysis of treatment outcome. N C Med J. 1998;59(5):310-313.
18. Irish MS, Pearl RH, Caty M, Glick PL. The approach to common abdominal diagnoses in infants and children. Pediatr Clin North Am. 1998; 45(4):729-772.
19. Askew N. An overview of infantile hypertrophic pyloric stenosis: literature review. Paediatric Nurs. 2010;22(8):27-30.
20. Rudolph CD. Infantile hypertrophic pyloric stenosis. In: Rudolph CD, Rudolph AM, eds. Rudolph’s Pediatrics. 21st ed. New York, NY: McGraw-Hill; 2002:1402-1403.
21. Hallam D, Hansen B, Bødker B, et al. Pyloric size in normal infants and in infants suspected of having hypertrophic pyloric stenosis. Acta Radiol. 2005;36(3):261.
22. Oakley EA, Barnett PL. Is acid base determination an accurate predictor of pyloric stenosis? J Paediatr Child Health. 2000;36(6):587-589.
23. Steven IM, Allen TH, Sweeney DB. Congenital hypertrophic pyloric stenosis: the anaesthetist’s view. Anaesth Intensive Care. 1973;1(6):544-546.
24. Singh UK, Kumar R, Prasad R. Oral atropine sulfate for infantile hypertrophic pyloric stenosis. Indian Pediatr. 2005;42(5):473-476.
25. Nagita A, Yamaguchi J, Amemoto K, et al. Management and ultrasonographic appearance of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate. J Pediatr Gastroenterol Nutr. 1996;23(2):172-177.
26. Ogawa Y, Higashimoto U, Nishijima E, et al. Successful endoscopic balloon dilatation for hypertrophic pyloric stenosis. J Pediatr Surg. 1996;31(12):1712-1714.
27. Langer JC, To T. Does pediatric surgical specialty training affect outcome after Ramstedt pyloromyotomy? A population-based study. Pediatrics. 2004;113(5):1342-1347.
28. Pollock WF, Norris WJ. Dr. Conrad Ramstedt and pyloromyotomy. Surgery. 1957;42(5):966-970.
29. Najmaldin A, Tan HL. Early experience with laparoscopic pyloromyotomy for infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1995;30(1):37-38.
30. Jia WQ, Tian JH, Yang KH, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a meta-analysis of randomized controlled trials. Eur J Pediatr Surg. 2011;21(2):77-81.
31. Hall NJ, Pacilli M, Eaton S, et al. Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet. 2009;373(9661):390-398.
32. Adibe OO, Nichol PF, Flake AAW, Mattei P. Comparison of outcomes after laparoscopic and open pyloromyotomy at a high-volume pediatric teaching hospital. J Pediatr Surg. 2006;41(10):1676-1678.
33. St. Peter SD, Holcomb GW, Calkins CM, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg. 2006;244(3):363-370.
34. Okorie NM, Dickson JA, Carver RA, Steiner GM. What happens to the pylorus after pyloromyotomy? Arch Dis Child. 1988;63(11):1339-1341.
Infantile hypertrophic pyloric stenosis (IHPS) is a common yet treatable condition in young infants, characterized by forceful vomiting after feeding as a result of hypertrophy of the pyloric muscle. Without proper diagnosis and surgical intervention, IHPS can eventually lead to dehydration, weight loss, and electrolyte disturbances, including the classic finding of hypochloremic alkalosis.1,2
IHPS occurs in two to four of every 1,000 births and is most common among white male infants.3,4 It develops between the first three to five weeks of life but rarely after age 12 weeks.1,2,5 IHPS is characterized by hypertrophy of the pyloric muscle (see figure), which eventually leads to gastric outlet obstruction.6,7
Because IHPS presents with symptoms that resemble those associated with other gastrointestinal disturbances, such as gastroesophageal reflux disease (GERD), it can be misdiagnosed in its early stages.1 Once it is identified, however, surgical correction by pyloromyotomy is considered curative, with a very low mortality rate (ie, 0.1%) and incidence recurrence in only 1% of patients. Typically, treated infants recover quickly and can begin feeding within hours after surgery.3,8
Although the etiology of IHPS remains unknown, providers can quickly recognize the signs and symptoms of IHPS in order for surgical treatment to be scheduled in a timely manner.
PATIENT PRESENTATION
Typically, an infant with IHPS will have a period of normal feeding for the first two to three weeks of life, followed by onset of nonbilious vomiting soon after feeding. Vomiting may become increasingly frequent and forceful—possibly described as “projectile.” Despite stomach distention, affected infants seem to have an insatiable appetite and may cry inconsolably. Depending on the duration of symptoms, patients may suffer significant weight loss, even falling below birth weight. In severe cases, a scaphoid abdomen and protruding ribcage may be present.1,2
Atypical Presentations
Premature infants with IHPS and those with certain medical or surgical conditions may present atypically. Vomiting may be less forceful, and the classic finding of visible gastric peristalsis may or may not be present.9 Researchers have documented cases in which hospitalized premature infants had nonprojectile vomiting, weight loss, and lethargy that were erroneously attributed to sepsis. IHPS should have been considered over sepsis when the infants’ clinical condition improved rapidly with rehydration, and when metabolic alkalosis (rather than acidosis) was identified.1,10,11
Projectile vomiting may not occur in infants with congenital anomalies that affect swallowing, such as cleft lip/palate or central nervous system disturbances. In infants who have recently undergone gastrointestinal (GI) surgery, IHPS may be misdiagnosed as adhesions or obstruction at an anastomotic site.1,10
RISK FACTORS
Despite the fact that IHPS is a relatively common condition, the etiology remains unknown. Research findings indicate that IHPS is not present at birth. Several hypotheses exist about potential risk factors, including genetics, use of macrolide antibiotics, and mechanical or environmental factors.6 IHPS has been associated with certain genetic conditions, including Cornelia de Lange syndrome and Smith-Lemli-Opitz syndrome, as well as chromosomal abnormalities, such as the translocation of chromosomes 8 and 17, and partial trisomy of chromosome 9.6,12
According to Chung,13 additional research has implicated the genetic loci IHPS1, also known as nitric oxide synthase 1 (NOS1), which encodes the gene for neuronal nitric oxide synthase (nNOS). This is the key enzyme for production of nitric oxide, which mediates relaxation of the pyloric smooth muscle.13
In a study by Mahon et al,12 an increased risk for IHPS was confirmed in young infants for whom systemic erythromycin had been prescribed as prophylactic treatment for pertussis. This may result from the agent’s motilin-like effects on antral smooth-muscle function.13
Mechanical defects, such as abnormal innervation of the pyloric muscle and neonatal hypergastrinemia and hyperacidity, have also been implicated.6 Infant sleeping position has been investigated as a possible environmental factor in the development of IHPS, correlating the decline in sudden infant death syndrome (attributed to decreased prone sleep position) with a decline in IHPS in recent years. However, no conclusive evidence has been shown to support this hypothesis.13
DIFFERENTIAL DIAGNOSIS
Typically, infants with nonbilious vomiting have either IHPS or GERD. Two factors to consider when evaluating the infant are its age and whether emesis is bilious or nonbilious. IHPS rarely causes bilious vomiting and most frequently occurs in infants between ages 3 and 6 weeks. Other GI disturbances that cause nonbilious emesis include adrenal crisis, gastroenteritis, pylorospasm, hiatal hernia, and preampullary duodenal stenosis.2 Malrotation or midgut volvulus may also be considered in the differential14 (see table1,2,14-16).
DIAGNOSIS
The diagnosis of IHPS is often made based on the history and physical exam. Imaging studies and labs can confirm the diagnosis, and surgery is usually deferred until dehydration has been addressed and any electrolyte disturbances corrected.1,17
Physical Examination
The infant may appear underweight and dehydrated, with visible peristaltic waves across the upper abdomen prior to emesis. Severe illness may be indicated in an underweight infant with the classic scaphoid abdomen.18
Palpation of an olive-shaped mass in the upper left quadrant of the epigastric region is pathognomonic for IHPS; the mass may be found more easily after emesis has occurred.19 To facilitate palpation, the hips can first be flexed to relax the abdominal wall. Next, the examiner should palpate gently for the “olive” in the space midway between the umbilicus and the xiphoid, between the two rectus muscles.3 All other findings in the physical exam should be within normal limits.
Imaging and Laboratory
Studies
An experienced provider can make a diagnosis of IHPS based on clinical examination alone in 60% to 80% of cases.20 However, most surgeons require the diagnosis to be confirmed with one of the following imaging studies before surgery.19
The diagnosis of IHPS can be confirmed by an upper GI series, but this is not commonly ordered as the primary diagnostic study. Ultrasound has become the modality of choice9; it can be used to quantify the size of an elongated, thickened pyloric muscle. The hypertrophied pylorus ranges in length from 14 to 16 mm, with thickness measuring more than 3.0 to 3.5 mm.1,19,21
An upper GI contrast study is rarely used for diagnosis of IHPS, but when this test is ordered to detect other gastrointestinal disease processes (eg, malrotation), IHPS may be identified incidentally.19 During an upper GI study, contrast material is propelled through the pyloric mucosa, and the string sign or the double-track sign may be visualized, revealing the mucosal filling defect.2
Abdominal x-ray may reveal a dilated stomach or a blockage, with a possible finding of gas in the gastric bubble but extending no further into the intestine.19
A study by Hernanz-Schulman2 was conducted to quantify the sensitivities and specificities associated with different diagnostic tools and the clinician’s experience and proficiency in using them. According to this study, palpation by a surgeon has a sensitivity of 31% to 99% and a specificity of 85% to 99% for detection of the pathognomonic olive-shaped mass. Ultrasound performed by an experienced technician has 97% to 100% sensitivity and 99% to 100% specificity for detecting IHPS. An upper GI series has 90% to 100% sensitivity and 99.5% specificity.
Venous blood gas and electrolyte levels are both helpful in making a diagnosis of IHPS.19,22 In a study by Oakley and Barnett,22 the following lab values were found useful in confirming the presence of pyloric stenosis: pH > 7.45; chloride 3 mEq/L. Sodium and potassium deficits may also be present.2,22
Before treatment is considered, the degree of dehydration must be determined by clinical examination and urinary output, as well as serum bicarbonate and chloride levels.3 Other laboratory tests to be ordered include urinalysis and a complete blood count (including platelets).
TREATMENT/MANAGEMENT
Initial management of vomiting in children should begin with fluid replacement if the patient appears dehydrated or if lab findings suggest an electrolyte imbalance. If surgery is later deemed necessary, this will reduce the risk for postoperative apnea.1,23
If IHPS is suspected, a pediatric surgery consult should be obtained. Before anesthesia is considered, serum bicarbonate should be measured with results no higher than 28 mEq/L, and the serum chloride level should be at least 100 mEq/L.3 Imaging, such as ultrasound or the upper GI series, may be ordered to confirm the diagnosis.
Surgical correction by a pyloromyotomy is curative. Presurgical gastric decompression via nasogastric tube placement will reduce the risk for postoperative vomiting and gastritis.20
Surgery
Although a variety of nonsurgical interventions, including oral and IV atropine and balloon dilation, have been described in the literature,3,24-26 the preferred treatment for IHPS is surgical intervention. Surgical correction has been so consistently successful (that is, provided the procedure is performed by a pediatric surgeon or other surgeon with appropriate experience3) that the treatment of choice for IHPS is the Ramstedt pyloromyotomy, which was first performed in 1912.6,27,28
Although the approach may differ based on the individual surgeon’s preference, the pyloric muscle is pulled through an incision in the abdominal wall. A longitudinal incision is made through the muscle with blunt dissection to the submucosa on the anterior surface of the pylorus. The pylorus is then returned to the abdominal cavity, and the abdominal incision is sutured.3,20
The laparoscopic approach, first described in the literature in the mid-1990s,29 is gaining popularity among surgeons, although recent studies have demonstrated that open and laparoscopic procedures are comparably safe and effective for the management of IHPS.30-33 Results from a recent study by Jia et al30 indicate that the laparoscopic approach results in reduced postoperative emesis, shorter length of hospital stay, and shorter recovery times; Hall et al31 emphasize the advantages of laparoscopic pyloromyotomy and recommend it over open surgery in facilities with experienced surgeons.
In follow-up ultrasound studies, hypertrophy of the pyloric muscle was reportedly resolved between two and 12 weeks after pyloromyotomy.34
Postoperative Care
The gastric tube can be removed and oral fluids reintroduced slowly at the surgeon’s discretion, between 8 and 12 hours after surgery. Postoperative vomiting is common (occurring in up to 80% of patients) but should resolve within 24 hours. Mild emesis should not delay the refeeding schedule.3
Patients should be evaluated by the surgeon one to two weeks following surgery unless the infant shows signs of infection (ie, fever, erythema, edema, bleeding, purulent drainage, excess pain, decreased fluid or nutritional intake). Follow-up imaging and laboratory studies are not indicated in an otherwise healthy infant.20
CONCLUSION
Early detection of IHPS, a condition characterized by hypertrophy of the pyloric muscle that results in gastric obstruction and projectile vomiting, can prevent complications of dehydration, malnutrition, and electrolyte disturbances. Surgery by open or laparoscopic pyloromyotomy is curative. Knowing the key physical exam findings, laboratory values, and typical patient history in IHPS enhances the clinician’s ability to make a timely diagnosis.
REFERENCES
1. Olivé AP, Endom EE. Infantile hypertrophic pyloric stenosis (2011). www.uptodate.com/contents/infan tile-hypertrophic-pyloric-stenosis. Accessed August 20, 2012.
2. Hernanz-Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227(2):319-331.
3. Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg. 2007;16(1):27-33.
4. To T, Wajja A, Wales PW, Langer JC. Population demographic indicators associated with incidence of pyloric stenosis. Arch Pediatr Adolesc Med. 2005;159(6):520-525.
5. MacMahon B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006; 17(2):195-201.
6. Panteli C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009;25 (12):1043-1052.
7. Stone CK, Humphries RL, eds. Current Medical Diagnosis and Treatment: Emergency Medicine. 7th ed. Chapter 50. Pediatric emergencies (2008). http://accessmedicine.com/resourceTOC.aspx?resource ID=718. Accessed August 20, 2012.
8. Hulka F, Harrison MW, Campbell TJ, Campbell JR. Complications of pyloromyotomy for infantile hypertrophic pyloric stenosis. Am J Surg. 1997;173 (5):450-452.
9. Shaoul R, Enav B, Steiner Z, et al. Clinical presentation of pyloric stenosis: the change is in our hands. Isr Med Assoc J. 2004;6(3):134-137.
10. Weinstein MM, Seibert JJ, Ehrenberg A. Six atypical presentations of congenital hypertrophic pyloric stenosis. Clin Pediatr. 1979;18(2):120-122.
11. Eyal O, Asia A, Yorgenson U, et al. Atypical infantile hypertrophic pyloric stenosis [in Hebrew]. Harefuah. 1999;136(2):113-114, 175.
12. Mahon BE, Rosenman MB, Kleiman MB. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139(3):380-384.
13. Chung E. Infantile hypertrophic pyloric stenosis: genes and environment. Arch Dis Child. 2008;93 (12):1003-1004.
14. Gilbertson-Dahdal DL, Dutta S, Varich LJ, Barth RA. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. AJR Am J Roentgenol. 2009;192(5):1269-1271.
15. Schmedel W, Ashe L, Kuznicki K. A 24-day-old child with projectile vomiting. J Emerg Nurs. 2009; 35(2):163-164.
16. Iijima T, Okamatsu T, Matsumura M, Yatsuzuka M. Hypertrophic pyloric stenosis associated with hiatal hernia. J Pediatr Surg. 1996;31(2):277-279.
17. Nakayama DK, Taylor LA. Hypertrophic pyloric stenosis in infancy: an analysis of treatment outcome. N C Med J. 1998;59(5):310-313.
18. Irish MS, Pearl RH, Caty M, Glick PL. The approach to common abdominal diagnoses in infants and children. Pediatr Clin North Am. 1998; 45(4):729-772.
19. Askew N. An overview of infantile hypertrophic pyloric stenosis: literature review. Paediatric Nurs. 2010;22(8):27-30.
20. Rudolph CD. Infantile hypertrophic pyloric stenosis. In: Rudolph CD, Rudolph AM, eds. Rudolph’s Pediatrics. 21st ed. New York, NY: McGraw-Hill; 2002:1402-1403.
21. Hallam D, Hansen B, Bødker B, et al. Pyloric size in normal infants and in infants suspected of having hypertrophic pyloric stenosis. Acta Radiol. 2005;36(3):261.
22. Oakley EA, Barnett PL. Is acid base determination an accurate predictor of pyloric stenosis? J Paediatr Child Health. 2000;36(6):587-589.
23. Steven IM, Allen TH, Sweeney DB. Congenital hypertrophic pyloric stenosis: the anaesthetist’s view. Anaesth Intensive Care. 1973;1(6):544-546.
24. Singh UK, Kumar R, Prasad R. Oral atropine sulfate for infantile hypertrophic pyloric stenosis. Indian Pediatr. 2005;42(5):473-476.
25. Nagita A, Yamaguchi J, Amemoto K, et al. Management and ultrasonographic appearance of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate. J Pediatr Gastroenterol Nutr. 1996;23(2):172-177.
26. Ogawa Y, Higashimoto U, Nishijima E, et al. Successful endoscopic balloon dilatation for hypertrophic pyloric stenosis. J Pediatr Surg. 1996;31(12):1712-1714.
27. Langer JC, To T. Does pediatric surgical specialty training affect outcome after Ramstedt pyloromyotomy? A population-based study. Pediatrics. 2004;113(5):1342-1347.
28. Pollock WF, Norris WJ. Dr. Conrad Ramstedt and pyloromyotomy. Surgery. 1957;42(5):966-970.
29. Najmaldin A, Tan HL. Early experience with laparoscopic pyloromyotomy for infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1995;30(1):37-38.
30. Jia WQ, Tian JH, Yang KH, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a meta-analysis of randomized controlled trials. Eur J Pediatr Surg. 2011;21(2):77-81.
31. Hall NJ, Pacilli M, Eaton S, et al. Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet. 2009;373(9661):390-398.
32. Adibe OO, Nichol PF, Flake AAW, Mattei P. Comparison of outcomes after laparoscopic and open pyloromyotomy at a high-volume pediatric teaching hospital. J Pediatr Surg. 2006;41(10):1676-1678.
33. St. Peter SD, Holcomb GW, Calkins CM, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg. 2006;244(3):363-370.
34. Okorie NM, Dickson JA, Carver RA, Steiner GM. What happens to the pylorus after pyloromyotomy? Arch Dis Child. 1988;63(11):1339-1341.
cover
Status Asthmaticus and Lactic Acidosis:
Is There a Toxicologic Link?
Polycystic ovary syndrome: Where we stand with diagnosis and treatment and where we’re going
Part 2. How are obesity and insulin resistance involved?
Polycystic ovary syndrome, or PCOS, is a condition characterized by hyperandrogenism and chronic anovulation—the most common endocrinopathy in women of reproductive age, affecting at least 1 in every 15. Associated metabolic and health complications are significant and serious, and include obesity, insulin resistance, dyslipidemia, pancreatic ß-cell dysfunction, type-2 diabetes, cardiovascular disease, endometrial cancer, sleep apnea, inflammation, and infertility. To the frustration of the medical community and patients, the exact cause (or causes) of PCOS remains largely unknown; making the diagnosis means, essentially, excluding disorders that mimic PCOS—including congenital adrenal hyperplasia, hyperprolactinemia, and thyroid disease. PCOS is an enigma, in that it is a heterogeneous disorder, with the severity of clinical hyperandrogenism (hirsutism, acne, alopecia), obesity, and menstrual disturbance being considerably variable.
Furthermore, as many as 40% of women who have PCOS do not express classic signs of hyperandrogenism, making the diagnosis exceedingly challenging, particularly in the case of a patient of the lean (i.e., physical appearance) phenotype.
The picture is further confused. The appearance of polycystic-appearing ovaries (multiple tiny cysts) on ultrasonography (US) is noted in as many as 20% of women who have polycystic ovaries without evidence of androgen excess. The significance of this as an isolated finding on imaging in an otherwise normal woman is unclear. Some experts have described the presence of this finding as, again, signaling a cryptic or unexpressed form of PCOS or a prelude to the manifestation of signs of PCOS later.
The four parts of this article that will be posted here on the OBG Management Web site over coming months address questions that are often asked by clinicians about this challenging clinical entity. [Editor’s note: Those four installments will, as they are published, be collected on a single Web page for ease of access.]
![]()
In its original description in the medical literature in the 1800s, PCOS was called cystic oophoritis.1,2 However, it wasn’t until the early 1930s that Stein and Leventhal first diagnosed what was initially coined Stein-Leventhal syndrome, reporting their findings in 1935.3 Later, the condition was referred to as polycystic ovarian disease.
In 1945, Stein published a follow-up report in which he added excessive male-pattern hair growth and obesity to the list of described symptoms. Although other associated symptoms have been noted in women who have the syndrome, the four principal ones established by Stein and Leventhal between 1935 and 1945 are irregular menstruation, infertility, obesity, and hirsutism.
Evolution as a disorder. PCOS was, initially, thought to be an anatomic disorder that specifically involved the ovaries and their thickened capsules. By the 1960s, with the advent of the radioimmunoassay, researchers could measure hormone levels in women who had the disorder. Studies confirmed that PCOS was associated with 1) increased androgen production from the ovaries and 2) abnormal gonadotropin secretion. Specifically, luteinizing hormone (LH) stimulated excess ovarian androgen production. From a historical perspective, then, the view of PCOS changed from anatomic disorder to, primarily, an endocrine disorder.
By the 1980s, clinical observations suggested a strong relationship between hyperinsulinemia and hyperandrogenism. The constellation of hyperandrogenism (HA), insulin resistance (IR), and acanthosis nigricans (AN) was then called HAIR-AN syndrome. Dunaif described how insulin, acting through ovarian insulin growth-factor receptors as mediators in ovarian dysfunction, led to hyperandrogenism.4 Two mechanisms appeared to account for HAIR-AN syndrome:
- hyperinsulinemia induced by insulin resistance causes hyperandrogenism
- hyperandrogenism causes insulin resistance and hyperinsulinemia (acanthosis nigricans is considered to be an epiphenomenon caused by hyperinsulinemia).
In the late 1980s, Reaven theorized that central obesity (male-type, or apple-shaped, obesity), diabetes, and hypertension have a common cause in insulin resistance (IR) and impaired glucose tolerance (IGT).5 This constellation of symptoms, at first called syndrome X, is known today as metabolic syndrome and is an object of extensive scientific inquiry—especially because the combination of findings strongly predisposes an affected person to cardiovascular disease.
By 2000, PCOS was viewed more as a metabolic disorder, with an array of cardiac and metabolic risk factors (insulin resistance and glucose intolerance; hypertension; dyslipidemia; and dysfibrinolysis) that have been linked to a number of health disorders, including type-2 diabetes and atherosclerotic cardiovascular and cerebrovascular disease. Today, diagnostic criteria for metabolic syndrome are at least three of these five findings:
- waist circumference, more than 35 inches
- triglycerides level, at least 50 mg/dL
- high-density lipoprotein-C level, greater than 50 mg/dL
- systolic BP, 130 mm Hg or higher, and diastolic BP, 85 mm Hg or higher
- fasting glucose level (after an oral glucose tolerance test) between 110 and 126 mg/dL or a 2-hour postprandial glucose level between 140 and 199 mg/dL, or both.
![]()
Data are scarce, making the prevalence of PCOS difficult to estimate—in part, because PCOS is a heterogeneous condition that can manifest with variable clinical evidence:
- hyperandrogenism—as either hirsutism, acne, or alopecia, or a combination of these signs
- menstrual or ovulatory dysfunction, or both
- overweight or obesity
- infertility
- insulin resistance and other metabolic abnormalities
- polycystic ovaries
Now that accepted diagnostic criteria for PCOS are in place, the prevalence rate of the syndrome will be easier to establish. In the discussion that follows, we attempt to establish estimates of prevalence based on histopathology, signs of clinical hyperandrogenism, and the US appearance of polycystic ovaries.
When PCOS is defined histopathologically (i.e., by the presence of polycystic ovaries at the time of oophorectomy or wedge resection), 1.4% to 3.5% of unselected women6 and 0.6% to 4.3% of infertile women7 have this syndrome.
When clinical criteria are used, prevalence varies with the clinical complaint. Hirsutism is usually a mark of increased ovarian or adrenal androgen production. Studies—including one in which more than 1,000 women were evaluated using the 1990 National Institutes of Health (NIH) criteria (see the next section)—suggest that, in fact, more than 75% of hirsute women have PCOS.8,9
In the absence of frank hirsutism, when only unwanted facial hair is present, approximately 50% of these women meet the definition for having PCOS.10
Among women whose only complaint is acne, prevalence has been reported in as many as one-third (range, 19% to 37%, although diagnostic criteria for PCOS were not well defined in these three studies).11-13
Last, in women who had any manifestation of clinical hyperandrogenism by the 2003 Rotterdam criteria (hirsutism, acne, or alopecia, or a combination; again, see the next section), PCOS was diagnosed in 72%.14
When PCOS is defined by a finding of polycystic ovaries on US, prevalence varies by study settings. Polycystic ovaries are seen in 92% of women who have idiopathic hirsutism15; in 87% of women who have oligomenorrhea15; in 21% to 23% of randomly selected women14,16; and in 23% of women who described themselves as “normal” and reported having a “regular” menstrual cycle.17 However, up to 25% of women with polycystic appearing ovaries may be entirely asymptomatic.18
In contrast, not all women who have an excess of androgens have polycystic-appearing ovaries19-21; this situation has been observed in 20% to 30% of young, healthy women.9
When biochemical parameters are used as diagnostic criteria, the prevalence of PCOS varies from 2.5% to 7.5%.22 In an unselected, minimally-biased population of women, overall prevalence of PCOS appears to be approximately 4.6%, although it could be as low as 3.5% and as high as 11.2%.23
All these observations, findings, and criteria considered, it is generally accepted that PCOS is one of the most common reproductive endocrine disorders of women.
What are the diagnostic criteria for PCOS?
![]()
Since the original description in 1945 of the diagnostic criteria of PCOS—irregular menstruation, infertility, obesity, hirsutism—it’s become clear that this disorder is a heterogeneous condition. Some patients display classic symptoms; many have a mild variant.
NIH seeks clarity. To further understand and study PCOS, it was essential to standardize the definition to facilitate collaborative clinical trials. In 1990, an NIH-sponsored consensus workshop attempted to standardize the criteria for making a diagnosis of PCOS.24 This included a combination of:
- chronic anovulation
- clinical (hirsutism) or biochemical (or both) signs of hyperandrogenism
- exclusion of other causes (including thyroid dysfunction, hyperprolactinemia, and adult-onset congenital adrenal hyperplasia).
A diagnosis of PCOS did not, however, require that the ovaries have polycystic characteristics on US imaging. In contrast, the European definition of PCOS was a syndrome that included polycystic ovaries on US in conjunction with clinical or biochemical hyperandrogenism; oligomenorrhea or amenorrhea; and obesity.
International consensus sought. To foster agreement across borders, a joint workshop of the European Society of Human Reproduction and Embryology and the American Society for Reproductive Endocrinology workshop was held in Rotterdam in 2003,25,26 resulting in an updated definition of PCOS. Ovarian morphology of multifollicular-appearing ovaries on US was recognized as an important component of the diagnosis; women who had clinical or biochemical hyperandrogenism in the face of a normal menstrual cycle could, therefore, have PCOS.
Workshop participants also agreed that a PCOS diagnosis required two of three criteria:
- oligo-ovulation or anovulation
- clinical or biochemical signs (or both) of hyperandrogenism
- polycystic ovaries on ultrasonography.
In addition, participants agreed that the exclusion of other causes of these findings—such as congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome, thyroid dysfunction, and an elevated prolactin level—was still critical to the diagnosis. (Note: We’ll discuss details of the diagnostic work-up for PCOS in a subsequent part of this article.)
The 2003 consensus meeting further described, in detail, US criteria by which to make a diagnosis of PCOS:
- at least 12 follicles in each ovary that are each 2 to 9 mm in diameter or
- ovarian volume greater than 10 mL.
These criteria do not apply to patients who are being treated with an oral contraceptive because their ovarian volume often appears smaller. In addition, having one ovary only that fits this definition was, and remains, sufficient to meet the US definition of PCOS. A so-called asymptomatic polycystic ovary—that is, positive US imaging in a woman who has regular cycles and a normal endocrine profile—should not be considered PCOS.
In the next installment
The authors begin by taking on two common areas of questioning in the care of women who have PCOS:
- “How is obesity defined and how is associated insulin resistance explained in the pathology of PCOS?
- “What is the prevalence of, and best way to screen for, insulin resistance?”
We want to hear from you! Tell us what you think.
Part 2. How are obesity and insulin resistance involved?
Polycystic ovary syndrome, or PCOS, is a condition characterized by hyperandrogenism and chronic anovulation—the most common endocrinopathy in women of reproductive age, affecting at least 1 in every 15. Associated metabolic and health complications are significant and serious, and include obesity, insulin resistance, dyslipidemia, pancreatic ß-cell dysfunction, type-2 diabetes, cardiovascular disease, endometrial cancer, sleep apnea, inflammation, and infertility. To the frustration of the medical community and patients, the exact cause (or causes) of PCOS remains largely unknown; making the diagnosis means, essentially, excluding disorders that mimic PCOS—including congenital adrenal hyperplasia, hyperprolactinemia, and thyroid disease. PCOS is an enigma, in that it is a heterogeneous disorder, with the severity of clinical hyperandrogenism (hirsutism, acne, alopecia), obesity, and menstrual disturbance being considerably variable.
Furthermore, as many as 40% of women who have PCOS do not express classic signs of hyperandrogenism, making the diagnosis exceedingly challenging, particularly in the case of a patient of the lean (i.e., physical appearance) phenotype.
The picture is further confused. The appearance of polycystic-appearing ovaries (multiple tiny cysts) on ultrasonography (US) is noted in as many as 20% of women who have polycystic ovaries without evidence of androgen excess. The significance of this as an isolated finding on imaging in an otherwise normal woman is unclear. Some experts have described the presence of this finding as, again, signaling a cryptic or unexpressed form of PCOS or a prelude to the manifestation of signs of PCOS later.
The four parts of this article that will be posted here on the OBG Management Web site over coming months address questions that are often asked by clinicians about this challenging clinical entity. [Editor’s note: Those four installments will, as they are published, be collected on a single Web page for ease of access.]
![]()
In its original description in the medical literature in the 1800s, PCOS was called cystic oophoritis.1,2 However, it wasn’t until the early 1930s that Stein and Leventhal first diagnosed what was initially coined Stein-Leventhal syndrome, reporting their findings in 1935.3 Later, the condition was referred to as polycystic ovarian disease.
In 1945, Stein published a follow-up report in which he added excessive male-pattern hair growth and obesity to the list of described symptoms. Although other associated symptoms have been noted in women who have the syndrome, the four principal ones established by Stein and Leventhal between 1935 and 1945 are irregular menstruation, infertility, obesity, and hirsutism.
Evolution as a disorder. PCOS was, initially, thought to be an anatomic disorder that specifically involved the ovaries and their thickened capsules. By the 1960s, with the advent of the radioimmunoassay, researchers could measure hormone levels in women who had the disorder. Studies confirmed that PCOS was associated with 1) increased androgen production from the ovaries and 2) abnormal gonadotropin secretion. Specifically, luteinizing hormone (LH) stimulated excess ovarian androgen production. From a historical perspective, then, the view of PCOS changed from anatomic disorder to, primarily, an endocrine disorder.
By the 1980s, clinical observations suggested a strong relationship between hyperinsulinemia and hyperandrogenism. The constellation of hyperandrogenism (HA), insulin resistance (IR), and acanthosis nigricans (AN) was then called HAIR-AN syndrome. Dunaif described how insulin, acting through ovarian insulin growth-factor receptors as mediators in ovarian dysfunction, led to hyperandrogenism.4 Two mechanisms appeared to account for HAIR-AN syndrome:
- hyperinsulinemia induced by insulin resistance causes hyperandrogenism
- hyperandrogenism causes insulin resistance and hyperinsulinemia (acanthosis nigricans is considered to be an epiphenomenon caused by hyperinsulinemia).
In the late 1980s, Reaven theorized that central obesity (male-type, or apple-shaped, obesity), diabetes, and hypertension have a common cause in insulin resistance (IR) and impaired glucose tolerance (IGT).5 This constellation of symptoms, at first called syndrome X, is known today as metabolic syndrome and is an object of extensive scientific inquiry—especially because the combination of findings strongly predisposes an affected person to cardiovascular disease.
By 2000, PCOS was viewed more as a metabolic disorder, with an array of cardiac and metabolic risk factors (insulin resistance and glucose intolerance; hypertension; dyslipidemia; and dysfibrinolysis) that have been linked to a number of health disorders, including type-2 diabetes and atherosclerotic cardiovascular and cerebrovascular disease. Today, diagnostic criteria for metabolic syndrome are at least three of these five findings:
- waist circumference, more than 35 inches
- triglycerides level, at least 50 mg/dL
- high-density lipoprotein-C level, greater than 50 mg/dL
- systolic BP, 130 mm Hg or higher, and diastolic BP, 85 mm Hg or higher
- fasting glucose level (after an oral glucose tolerance test) between 110 and 126 mg/dL or a 2-hour postprandial glucose level between 140 and 199 mg/dL, or both.
![]()
Data are scarce, making the prevalence of PCOS difficult to estimate—in part, because PCOS is a heterogeneous condition that can manifest with variable clinical evidence:
- hyperandrogenism—as either hirsutism, acne, or alopecia, or a combination of these signs
- menstrual or ovulatory dysfunction, or both
- overweight or obesity
- infertility
- insulin resistance and other metabolic abnormalities
- polycystic ovaries
Now that accepted diagnostic criteria for PCOS are in place, the prevalence rate of the syndrome will be easier to establish. In the discussion that follows, we attempt to establish estimates of prevalence based on histopathology, signs of clinical hyperandrogenism, and the US appearance of polycystic ovaries.
When PCOS is defined histopathologically (i.e., by the presence of polycystic ovaries at the time of oophorectomy or wedge resection), 1.4% to 3.5% of unselected women6 and 0.6% to 4.3% of infertile women7 have this syndrome.
When clinical criteria are used, prevalence varies with the clinical complaint. Hirsutism is usually a mark of increased ovarian or adrenal androgen production. Studies—including one in which more than 1,000 women were evaluated using the 1990 National Institutes of Health (NIH) criteria (see the next section)—suggest that, in fact, more than 75% of hirsute women have PCOS.8,9
In the absence of frank hirsutism, when only unwanted facial hair is present, approximately 50% of these women meet the definition for having PCOS.10
Among women whose only complaint is acne, prevalence has been reported in as many as one-third (range, 19% to 37%, although diagnostic criteria for PCOS were not well defined in these three studies).11-13
Last, in women who had any manifestation of clinical hyperandrogenism by the 2003 Rotterdam criteria (hirsutism, acne, or alopecia, or a combination; again, see the next section), PCOS was diagnosed in 72%.14
When PCOS is defined by a finding of polycystic ovaries on US, prevalence varies by study settings. Polycystic ovaries are seen in 92% of women who have idiopathic hirsutism15; in 87% of women who have oligomenorrhea15; in 21% to 23% of randomly selected women14,16; and in 23% of women who described themselves as “normal” and reported having a “regular” menstrual cycle.17 However, up to 25% of women with polycystic appearing ovaries may be entirely asymptomatic.18
In contrast, not all women who have an excess of androgens have polycystic-appearing ovaries19-21; this situation has been observed in 20% to 30% of young, healthy women.9
When biochemical parameters are used as diagnostic criteria, the prevalence of PCOS varies from 2.5% to 7.5%.22 In an unselected, minimally-biased population of women, overall prevalence of PCOS appears to be approximately 4.6%, although it could be as low as 3.5% and as high as 11.2%.23
All these observations, findings, and criteria considered, it is generally accepted that PCOS is one of the most common reproductive endocrine disorders of women.
What are the diagnostic criteria for PCOS?
![]()
Since the original description in 1945 of the diagnostic criteria of PCOS—irregular menstruation, infertility, obesity, hirsutism—it’s become clear that this disorder is a heterogeneous condition. Some patients display classic symptoms; many have a mild variant.
NIH seeks clarity. To further understand and study PCOS, it was essential to standardize the definition to facilitate collaborative clinical trials. In 1990, an NIH-sponsored consensus workshop attempted to standardize the criteria for making a diagnosis of PCOS.24 This included a combination of:
- chronic anovulation
- clinical (hirsutism) or biochemical (or both) signs of hyperandrogenism
- exclusion of other causes (including thyroid dysfunction, hyperprolactinemia, and adult-onset congenital adrenal hyperplasia).
A diagnosis of PCOS did not, however, require that the ovaries have polycystic characteristics on US imaging. In contrast, the European definition of PCOS was a syndrome that included polycystic ovaries on US in conjunction with clinical or biochemical hyperandrogenism; oligomenorrhea or amenorrhea; and obesity.
International consensus sought. To foster agreement across borders, a joint workshop of the European Society of Human Reproduction and Embryology and the American Society for Reproductive Endocrinology workshop was held in Rotterdam in 2003,25,26 resulting in an updated definition of PCOS. Ovarian morphology of multifollicular-appearing ovaries on US was recognized as an important component of the diagnosis; women who had clinical or biochemical hyperandrogenism in the face of a normal menstrual cycle could, therefore, have PCOS.
Workshop participants also agreed that a PCOS diagnosis required two of three criteria:
- oligo-ovulation or anovulation
- clinical or biochemical signs (or both) of hyperandrogenism
- polycystic ovaries on ultrasonography.
In addition, participants agreed that the exclusion of other causes of these findings—such as congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome, thyroid dysfunction, and an elevated prolactin level—was still critical to the diagnosis. (Note: We’ll discuss details of the diagnostic work-up for PCOS in a subsequent part of this article.)
The 2003 consensus meeting further described, in detail, US criteria by which to make a diagnosis of PCOS:
- at least 12 follicles in each ovary that are each 2 to 9 mm in diameter or
- ovarian volume greater than 10 mL.
These criteria do not apply to patients who are being treated with an oral contraceptive because their ovarian volume often appears smaller. In addition, having one ovary only that fits this definition was, and remains, sufficient to meet the US definition of PCOS. A so-called asymptomatic polycystic ovary—that is, positive US imaging in a woman who has regular cycles and a normal endocrine profile—should not be considered PCOS.
In the next installment
The authors begin by taking on two common areas of questioning in the care of women who have PCOS:
- “How is obesity defined and how is associated insulin resistance explained in the pathology of PCOS?
- “What is the prevalence of, and best way to screen for, insulin resistance?”
We want to hear from you! Tell us what you think.
Part 2. How are obesity and insulin resistance involved?
Polycystic ovary syndrome, or PCOS, is a condition characterized by hyperandrogenism and chronic anovulation—the most common endocrinopathy in women of reproductive age, affecting at least 1 in every 15. Associated metabolic and health complications are significant and serious, and include obesity, insulin resistance, dyslipidemia, pancreatic ß-cell dysfunction, type-2 diabetes, cardiovascular disease, endometrial cancer, sleep apnea, inflammation, and infertility. To the frustration of the medical community and patients, the exact cause (or causes) of PCOS remains largely unknown; making the diagnosis means, essentially, excluding disorders that mimic PCOS—including congenital adrenal hyperplasia, hyperprolactinemia, and thyroid disease. PCOS is an enigma, in that it is a heterogeneous disorder, with the severity of clinical hyperandrogenism (hirsutism, acne, alopecia), obesity, and menstrual disturbance being considerably variable.
Furthermore, as many as 40% of women who have PCOS do not express classic signs of hyperandrogenism, making the diagnosis exceedingly challenging, particularly in the case of a patient of the lean (i.e., physical appearance) phenotype.
The picture is further confused. The appearance of polycystic-appearing ovaries (multiple tiny cysts) on ultrasonography (US) is noted in as many as 20% of women who have polycystic ovaries without evidence of androgen excess. The significance of this as an isolated finding on imaging in an otherwise normal woman is unclear. Some experts have described the presence of this finding as, again, signaling a cryptic or unexpressed form of PCOS or a prelude to the manifestation of signs of PCOS later.
The four parts of this article that will be posted here on the OBG Management Web site over coming months address questions that are often asked by clinicians about this challenging clinical entity. [Editor’s note: Those four installments will, as they are published, be collected on a single Web page for ease of access.]
![]()
In its original description in the medical literature in the 1800s, PCOS was called cystic oophoritis.1,2 However, it wasn’t until the early 1930s that Stein and Leventhal first diagnosed what was initially coined Stein-Leventhal syndrome, reporting their findings in 1935.3 Later, the condition was referred to as polycystic ovarian disease.
In 1945, Stein published a follow-up report in which he added excessive male-pattern hair growth and obesity to the list of described symptoms. Although other associated symptoms have been noted in women who have the syndrome, the four principal ones established by Stein and Leventhal between 1935 and 1945 are irregular menstruation, infertility, obesity, and hirsutism.
Evolution as a disorder. PCOS was, initially, thought to be an anatomic disorder that specifically involved the ovaries and their thickened capsules. By the 1960s, with the advent of the radioimmunoassay, researchers could measure hormone levels in women who had the disorder. Studies confirmed that PCOS was associated with 1) increased androgen production from the ovaries and 2) abnormal gonadotropin secretion. Specifically, luteinizing hormone (LH) stimulated excess ovarian androgen production. From a historical perspective, then, the view of PCOS changed from anatomic disorder to, primarily, an endocrine disorder.
By the 1980s, clinical observations suggested a strong relationship between hyperinsulinemia and hyperandrogenism. The constellation of hyperandrogenism (HA), insulin resistance (IR), and acanthosis nigricans (AN) was then called HAIR-AN syndrome. Dunaif described how insulin, acting through ovarian insulin growth-factor receptors as mediators in ovarian dysfunction, led to hyperandrogenism.4 Two mechanisms appeared to account for HAIR-AN syndrome:
- hyperinsulinemia induced by insulin resistance causes hyperandrogenism
- hyperandrogenism causes insulin resistance and hyperinsulinemia (acanthosis nigricans is considered to be an epiphenomenon caused by hyperinsulinemia).
In the late 1980s, Reaven theorized that central obesity (male-type, or apple-shaped, obesity), diabetes, and hypertension have a common cause in insulin resistance (IR) and impaired glucose tolerance (IGT).5 This constellation of symptoms, at first called syndrome X, is known today as metabolic syndrome and is an object of extensive scientific inquiry—especially because the combination of findings strongly predisposes an affected person to cardiovascular disease.
By 2000, PCOS was viewed more as a metabolic disorder, with an array of cardiac and metabolic risk factors (insulin resistance and glucose intolerance; hypertension; dyslipidemia; and dysfibrinolysis) that have been linked to a number of health disorders, including type-2 diabetes and atherosclerotic cardiovascular and cerebrovascular disease. Today, diagnostic criteria for metabolic syndrome are at least three of these five findings:
- waist circumference, more than 35 inches
- triglycerides level, at least 50 mg/dL
- high-density lipoprotein-C level, greater than 50 mg/dL
- systolic BP, 130 mm Hg or higher, and diastolic BP, 85 mm Hg or higher
- fasting glucose level (after an oral glucose tolerance test) between 110 and 126 mg/dL or a 2-hour postprandial glucose level between 140 and 199 mg/dL, or both.
![]()
Data are scarce, making the prevalence of PCOS difficult to estimate—in part, because PCOS is a heterogeneous condition that can manifest with variable clinical evidence:
- hyperandrogenism—as either hirsutism, acne, or alopecia, or a combination of these signs
- menstrual or ovulatory dysfunction, or both
- overweight or obesity
- infertility
- insulin resistance and other metabolic abnormalities
- polycystic ovaries
Now that accepted diagnostic criteria for PCOS are in place, the prevalence rate of the syndrome will be easier to establish. In the discussion that follows, we attempt to establish estimates of prevalence based on histopathology, signs of clinical hyperandrogenism, and the US appearance of polycystic ovaries.
When PCOS is defined histopathologically (i.e., by the presence of polycystic ovaries at the time of oophorectomy or wedge resection), 1.4% to 3.5% of unselected women6 and 0.6% to 4.3% of infertile women7 have this syndrome.
When clinical criteria are used, prevalence varies with the clinical complaint. Hirsutism is usually a mark of increased ovarian or adrenal androgen production. Studies—including one in which more than 1,000 women were evaluated using the 1990 National Institutes of Health (NIH) criteria (see the next section)—suggest that, in fact, more than 75% of hirsute women have PCOS.8,9
In the absence of frank hirsutism, when only unwanted facial hair is present, approximately 50% of these women meet the definition for having PCOS.10
Among women whose only complaint is acne, prevalence has been reported in as many as one-third (range, 19% to 37%, although diagnostic criteria for PCOS were not well defined in these three studies).11-13
Last, in women who had any manifestation of clinical hyperandrogenism by the 2003 Rotterdam criteria (hirsutism, acne, or alopecia, or a combination; again, see the next section), PCOS was diagnosed in 72%.14
When PCOS is defined by a finding of polycystic ovaries on US, prevalence varies by study settings. Polycystic ovaries are seen in 92% of women who have idiopathic hirsutism15; in 87% of women who have oligomenorrhea15; in 21% to 23% of randomly selected women14,16; and in 23% of women who described themselves as “normal” and reported having a “regular” menstrual cycle.17 However, up to 25% of women with polycystic appearing ovaries may be entirely asymptomatic.18
In contrast, not all women who have an excess of androgens have polycystic-appearing ovaries19-21; this situation has been observed in 20% to 30% of young, healthy women.9
When biochemical parameters are used as diagnostic criteria, the prevalence of PCOS varies from 2.5% to 7.5%.22 In an unselected, minimally-biased population of women, overall prevalence of PCOS appears to be approximately 4.6%, although it could be as low as 3.5% and as high as 11.2%.23
All these observations, findings, and criteria considered, it is generally accepted that PCOS is one of the most common reproductive endocrine disorders of women.
What are the diagnostic criteria for PCOS?
![]()
Since the original description in 1945 of the diagnostic criteria of PCOS—irregular menstruation, infertility, obesity, hirsutism—it’s become clear that this disorder is a heterogeneous condition. Some patients display classic symptoms; many have a mild variant.
NIH seeks clarity. To further understand and study PCOS, it was essential to standardize the definition to facilitate collaborative clinical trials. In 1990, an NIH-sponsored consensus workshop attempted to standardize the criteria for making a diagnosis of PCOS.24 This included a combination of:
- chronic anovulation
- clinical (hirsutism) or biochemical (or both) signs of hyperandrogenism
- exclusion of other causes (including thyroid dysfunction, hyperprolactinemia, and adult-onset congenital adrenal hyperplasia).
A diagnosis of PCOS did not, however, require that the ovaries have polycystic characteristics on US imaging. In contrast, the European definition of PCOS was a syndrome that included polycystic ovaries on US in conjunction with clinical or biochemical hyperandrogenism; oligomenorrhea or amenorrhea; and obesity.
International consensus sought. To foster agreement across borders, a joint workshop of the European Society of Human Reproduction and Embryology and the American Society for Reproductive Endocrinology workshop was held in Rotterdam in 2003,25,26 resulting in an updated definition of PCOS. Ovarian morphology of multifollicular-appearing ovaries on US was recognized as an important component of the diagnosis; women who had clinical or biochemical hyperandrogenism in the face of a normal menstrual cycle could, therefore, have PCOS.
Workshop participants also agreed that a PCOS diagnosis required two of three criteria:
- oligo-ovulation or anovulation
- clinical or biochemical signs (or both) of hyperandrogenism
- polycystic ovaries on ultrasonography.
In addition, participants agreed that the exclusion of other causes of these findings—such as congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome, thyroid dysfunction, and an elevated prolactin level—was still critical to the diagnosis. (Note: We’ll discuss details of the diagnostic work-up for PCOS in a subsequent part of this article.)
The 2003 consensus meeting further described, in detail, US criteria by which to make a diagnosis of PCOS:
- at least 12 follicles in each ovary that are each 2 to 9 mm in diameter or
- ovarian volume greater than 10 mL.
These criteria do not apply to patients who are being treated with an oral contraceptive because their ovarian volume often appears smaller. In addition, having one ovary only that fits this definition was, and remains, sufficient to meet the US definition of PCOS. A so-called asymptomatic polycystic ovary—that is, positive US imaging in a woman who has regular cycles and a normal endocrine profile—should not be considered PCOS.
In the next installment
The authors begin by taking on two common areas of questioning in the care of women who have PCOS:
- “How is obesity defined and how is associated insulin resistance explained in the pathology of PCOS?
- “What is the prevalence of, and best way to screen for, insulin resistance?”
We want to hear from you! Tell us what you think.
UPDATE: SEXUAL DYSFUNCTION
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD; Sheryl Kingsberg, PhD (January 2012)
Update on sexual dysfunction
Barbara S. Levy, MD (September 2011)
Sexual dysfunction is common among women, with an overall incidence of about 40%—even higher in some populations.1,2 All the more surprising, then, that only a minority of women raise the topic with their physician. In one well-regarded study, for example, only 22% of older women reported having discussed sex with their physician after the age of 50.3
One reason for the lack of communication may be a sense of discomfort around sexuality, among physicians as well as patients. Other reasons may include limited time on the part of physicians, and a lack of clarity about how to evaluate sexual function in women.
How, then, to assess a woman’s sexual function? In this Update, I address this question, and offer numerous others you can discuss with your patients without adding a significant time burden to your day. “A sidebar on page 27 focuses on a few strategies for tackling sexual function in an efficient and timely manner.”
In an ObGyn practice already pressed for time, adding a new domain of concern to the mix can be a challenge. (This is assuming you do not already ask patients routinely about sexual function.) It may not be as challenging as you think, however. One way to start is to add a few basic questions about sexual function to the intake form. This approach serves two purposes:
- It validates sexual function as an important part of health
- It allows the patient to identify any problems without having to raise the subject herself.
The second purpose is especially important because many patients are reluctant to broach the topic of sex.
After reviewing the intake form, you can take a more detailed history, addressing the concerns gently and matter of factly, to determine the scope of the problem, its duration, and any steps the patient has already taken to remedy it. The physical exam then can be more appropriately focused.
Straightforward areas of dysfunction, such as perimenopausal vaginal dryness, usually can be addressed in the same visit. More extensive problems may merit a separate office visit.
Linear model of female sexual function is not clinically useful
The classical linear model that proposes that women progress from sexual excitement to plateau, orgasm, and resolution is not that helpful when we are trying to determine the cause of our patient’s sexual problem and choose a course of treatment. More revealing is the biopsychosocial model, which considers physical, psychological, relational, and situational variables in exploring sexual function. If a physician focuses the history on these four aspects of sexual function, she generally can discover the source of any problem.
According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), female sexual dysfunction typically falls into one or more categories:
- desire disorders – hypoactive sexual desire disorder (HSDD) and sexual aversion disorder
- problems with arousal – female sexual arousal disorder
- pain disorders – dyspareunia and vaginismus
- orgasmic disorders – female orgasmic disorder.4
If we superimpose this framework over the biopsychosocial model, diagnosis and management can be elucidated further. For example, we might see a 57-year-old patient who complains during her annual exam about decreased libido. We ask about menopausal symptoms and general health and screen for depression and intimate partner violence. On questioning, we discover that her partner was diagnosed with prostate cancer 2 years earlier and is able to achieve an erection on occasion with the use of medication, but there is fear, for both of them, that the erection will not last. This development has changed their sexual behaviors in ways that are not satisfying for either of them.
Clearly, addressing this fairly common scenario as “female sexual dysfunction” will overlook the main issues. This couple needs help communicating their love and support for each other and some direction to help them learn new ways to express themselves sexually that can be satisfying for both. It is important to remain sensitive to the patient’s (and partner’s) social and medical history, age, and physiological status.
Keep in mind, however, that it is common to see overlapping disorders in a patient with sexual dysfunction. For example, a lack of desire can lead to diminished arousal and anorgasmia. For this reason, when one disorder is diagnosed, ask the patient to describe any other problems she may have. Also inquire about the chronology of multiple sexual disorders in order to determine which problem came first.
One last caveat: When you are evaluating a patient’s sexual function, don’t assume that she has a male partner—or any partner at all.
Don’t overlook psychosocial variables when assessing desire disorders
Prevalence of low sexual desire ranges from 26.7% among premenopausal women to 52.4% among naturally menopausal women—no small problem.5
The Female Sexual Function Index (FSFI), a multidimensional self-reporting tool used widely in research, describes sexual desire as “a feeling that includes wanting to have a sexual experience, feeling receptive to a partner’s sexual initiation, and thinking or fantasizing about having sex.”6 The FSFI attempts to discern whether a desire disorder is present by asking about the patient’s feelings over the preceding 4 weeks.
Among the questions it poses are:
- How often have you felt sexual desire?
- How would you rate your level of sexual desire or interest?
These questions may be useful as a starting point when a patient complains of low desire.
Low desire may have multiple causes
Desire disorders may be associated with depression, but they also may arise from experiences and attitudes that occurred during childhood. For example, women who had a strict religious upbringing or were exposed to negative parental attitudes toward sex may suffer lifelong psychological effects. Other deep-seated sources of impaired desire include childhood physical, sexual, and emotional abuse.
These influences may not be readily apparent if the woman is in a new relationship, when powerful hormonal determinants of attraction—driven by phenylethylamine—hold sway. Once the relationship matures, however, and the “lust” begins to recede and a more comfortable, stable relationship emerges—these psychological barriers to physical enjoyment may come to the fore.
Referral is indicated for SAD
Sexual aversion disorder (SAD) is characterized by “persistent or recurrent extreme aversion to and avoidance of all (or almost all) genital sexual contact with a sexual partner,” according to DSM-IV.2 Unlike HSDD, which reflects a lack of interest in sex, SAD may involve physiologic aversion responses such as nausea, revulsion, and shortness of breath.3
SAD is a psychiatric illness that requires management by a qualified mental health professional, preferably a sex therapist. (For information on how to find a qualified therapist, consult the American Association of Sexuality Educators, Counselors, and Therapists at www.aasect.org.)
No FDA-approved drug for HSDD
HSDD is characterized by “a deficiency or absence of sexual fantasies and desire for sexual activity,” according to DSM-IV.4 It may be treated by an experienced psychotherapist without additional training in sexual therapy.
Regrettably, attempts to treat HSDD with pharmacologic agents have been modestly successful at best, and we lack FDA-approved medications. Some trials demonstrated a slight improvement in desire among surgically menopausal women on estrogen therapy when a supraphysiologic dose of testosterone was given. Off-label use of a compounded testosterone or a lower dose of a male testosterone product may be useful.
Avoid laboratory assays for testosterone—serum or salivary—in the diagnosis of HSDD. However, if a commercial testosterone product is given, testing may be useful to prevent excessive levels of testosterone and associated (and sometimes irreversible) physiologic changes, such as male-pattern hair loss and deepening of the voice.
Failure to lubricate is the hallmark of female sexual arousal disorder
The DSM-IV defines female sexual arousal disorder as persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate physiologic response (lubrication, swelling) of sexual excitement.4 It is analogous to erectile dysfunction in men. In women, however, it may be difficult to distinguish this condition from primary desire disorder, particularly in cases in which a pattern of poor arousal, dryness, and dyspareunia has developed. The hallmark of female sexual arousal disorder is a failure to lubricate.
A few simple questions
Among the questions you might ask the patient:
- Do you feel interested in sexual activity?
- Do you have a problem lubricating well?
- Do you use a lubricant for sexual activity? If so, does it work to make sexual intercourse comfortable?
Other variables to consider
Arousal occurs secondary to genital vasodilation and tissue engorgement. It may be disturbed by any physiologic condition that reduces blood flow, such as smoking, hypertension, diabetes, and hypoestrogenism.
Decreased sensation sometimes may contribute to arousal disorder. For example, when the vagina and external genitalia experience decreased sensation, the cause may be physiologic, neurologic, or supratentorial.
Unlike men, women experience very little direct feedback regarding arousal. A disconnect between the sensory afferent input and higher-level awareness is not unusual. A thorough physical and neurologic exam may be necessary to assess the sensory nerves, integrity of the skin (signs of any inflammation), and blood flow to the genitalia.
Referral to a therapist also may be necessary so that other barriers to intimacy and sexuality can be determined.
As menopause approaches, so do a number of interrelated issues that may affect a woman’s sexual function. Here are five of them:
Body image may deteriorate, leading to feelings of unattractiveness that can lower interest in sexual activity.
Testosterone levels decline throughout a woman’s reproductive life, as does estrogen—but the decline in testosterone is much more gradual without any abrupt reduction at menopause. In addition, some common medications, such as oral contraceptives and oral estrogen formulations, increase sex hormone binding globulin, which can reduce dramatically the free testosterone level. Some women are especially sensitive to the effects of these drugs and may report a rapid loss of sexual desire when these agents are given. To sidestep the problem, give transdermal or transvaginal estrogen, when possible.
Comorbidities take a toll on sexual function in many cases. Arthritis, diabetes, cardiovascular disease, and other impairments may hamper sexual satisfaction. In addition, fatigue, insomnia, and depression are common with age.
Partner issues also play a role in diagnosis and treatment of female sexual dysfunction. For example, poor health in the partner can cause or exacerbate sexual problems.
Pain during sex can trigger desire and arousal disorders
Pain during sexual activity can lead to disorders of desire as well as arousal. When a patient reports pain during sex, pay careful attention to her medical history and perform a detailed physical examination. Patience is vital. Successful treatment of pain disorders requires commitment from the patient and her partner as well as the medical team.
Consider asking the following questions:
- Over the past 4 weeks, how often have you experienced discomfort or pain during vaginal penetration?
- How often have you experienced discomfort or pain following vaginal penetration?
- How would you rate your degree of discomfort or pain during or following vaginal penetration?6
Consider pain as a cause when any patient reports low libido, as pain is a potent suppressor of desire. A meticulous clinical history is required to determine the cause. For example, it is important to uncover whether the pain is of recent onset or of long duration, or whether it is related to childbirth, lactation, or menopausal changes.
Pain upon penetration could be caused by chemical, infectious, or atrophic vulvovaginitis. Dryness and pain upon penetration are often caused by:
- contact dermatitis
- irritation from soaps or scrubbing
- daily use of panty liners
- use of so-called feminine hygiene products
- regular use of swimming pools or hot tubs that contain chemicals.
Another cause of pain to consider is vulvar dystrophy. When lichen sclerosis or hypertrophic dystrophy goes untreated, the result may be fibrosis, lack of elasticity, painful fissures, and loss of normal architecture. These changes usually occur in postmenopausal women, so it is important that treatment address both the fibrosis and the hypoestrogenic atrophy.
If treated early, vulvar vestibulitis may not require surgery
Vulvar vestibulitis is poorly understood. It tends to occur most often in premenopausal women, frequently as a result of vulvar infection or during the postpartum period.
Vulvar vestibulitis involves point tenderness—sometimes experienced as a burning, searing sensation—around the introitus, specifically, the vestibular glands. When this condition is suspected, examine the vulva and vestibule with a moistened cotton swab to assess whether the classic distribution of pain is present. The necks of the vestibular glands may appear inflamed and erythematous.
If the condition is treated early enough with topical steroids and, in some cases, hydroxyzine, surgery may be avoided, provided the patient also avoids topical irritants. In many women, however, vestibulectomy is required to eliminate symptoms.
Vulvodynia may be associated with other pain syndromes
This disorder is a more generalized pain syndrome that involves the entire vulvar region. Like vulvar vestibulitis, it can cause painful penetration. It is also associated with other pain syndromes, including interstitial cystitis and endometriosis. Sensitization to pain at a central level may lead to hyperesthesia and allodynia. Also consider pudendal neuropathy, especially if the patient is a regular bicycle rider.
Treatment usually consists of off-label use of neuromodulators, such as gabapentin, tricyclic antidepressants, or duloxetine. The use of topical local anesthetic creams or gels may also permit pain-free sexual activity.
Vaginismus may indicate a history of sexual abuse
When the perineal muscles surrounding the outer third of the vagina contract involuntarily upon contact with a penis, speculum, or other item, vaginismus may be present. This disorder can be primary or secondary. In primary vaginismus, the patient may be unable to tolerate any vaginal penetration at all, not even a single digit or tampon. When this is the case, the patient may have a history of childhood sexual abuse. Explore her history, including any medical examinations that may have been painful or generated fear and anxiety. Also be aware that women with sexual aversion disorder may present with primary vaginismus.
Secondary vaginismus can occur even after years of satisfying sexual activity when a woman undergoes pelvic reconstructive surgery or develops vulvar dystrophy or vulvovaginal atrophy. Pain or the fear of pain can trigger a powerful reflex spasm of the levator ani musculature. Also keep in mind that secondary vaginismus may not be reproducible during the pelvic examination.
Treatment of both primary and secondary vaginismus includes physical therapy of the pelvic floor using biofeedback. The patient’s partner also needs to attend at least one session to learn techniques to prevent levator spasm and disable the reflex.
In the early phase of treatment, it may be helpful for the couple to agree to participate in a pact to avoid penetration during sexual activity. This approach may help reawaken sexual desire and arousal by eliminating the fear of pain.
The nature of the patient’s relationship with her partner is a powerful determinant of outcome. For example, intimacy and good communication are more likely to resolve the problem, whereas a difficult relationship may inhibit success. Depending on the scenario, counseling and psychological assessment may be necessary in the treatment of vaginismus, especially when a patient has a history of abuse.
Deep dyspareunia may be linked to pelvic pathology
This pain disorder may be associated with poor arousal or with fixation of the pelvic organs as a result of endometriosis, adhesions, or posthysterectomy scarring.
In a “normal” scenario, when arousal is unimpeded, the vagina lengthens by about 30%, and the uterus and cervix lift out of the cul-de-sac. This helps explain why not all women who have retroverted uteri or an obliterated cul-de-sac experience deep dyspareunia.
The patient’s history is a critical component of diagnosis. Ask her about foreplay, lubrication, and arousal prior to penetration. During the physical examination, be vigilant for point tenderness along the vaginal cuff and painful nodularity along the uterosacral ligaments.
To successfully treat deep dyspareunia, you must address any pelvic pathology as well as arousal problems. If penetration occurs prior to adequate arousal, the vagina remains shorter and the uterus has not yet engorged and lifted out of the cul-de-sac, resulting in “bump” dyspareunia. Surgery can elevate and alter any uterine retroversion that is present, but it is very rarely needed when adequate arousal can be achieved.
Pain with orgasm may arise from uterine pathology
At the time of orgasm, the levator ani musculature and myometrium contract strongly. When adenomyosis or degenerating uterine fibroids are present, pain may occur during or after orgasm. Women who have pelvic floor tension myalgia also may experience pelvic pain and aching after orgasm.
To tease out the cause of orgasm-related pain, perform a careful physical examination. To distinguish uterine pain from pain at the pelvic floor, perform a single-digit examination of each pelvic floor muscle before touching the cervix and uterus. Compression applied to a tender uterus often triggers a muscle spasm at the pelvic floor, so it is important to evaluate the pelvic floor muscles for tone and discomfort before performing a bimanual exam.
Treatment of uterine pathology usually entails medical or surgical intervention, whereas pelvic floor tension myalgia is treated with physical therapy and biofeedback.
Female orgasmic disorder may indicate a need for basic education
When a woman reports a persistent delay in or absence of orgasm after an otherwise satisfying episode of sexual activity and excitement, female orgasmic disorder is the likely cause. It may be primary or secondary.
Primary anorgasmia is often related to sexual inexperience and ignorance. Management may require education, use of a vibrator, and permission to engage in self-exploration—or it may necessitate evaluation and management by a trained sexual therapist. Resources for the patient, including educational materials and video, can be found at www.bettersex.com.
Secondary anorgasmia occurs in women who have a history of regular orgasm; the cause is generally drug-related. Among the usual culprits are selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants. Anorgasmia can be difficult to treat in these cases because discontinuation of the antidepressant can worsen depression—and depression is often associated with disorders of desire. One option is switching the class of the antidepressant to one less likely to disrupt orgasm, such as buproprion or trazodone. Off-label use of low-dose sildenafil may reverse the effect of SSRIs on orgasmic function, according to recent evidence.8
We want to hear from you! Tell us what you think.
1. Trompeter SE, Bettencourt R, Barrett-Connor E. Sexual activity and satisfaction in healthy community-dwelling older women. Am J Med. 2012;125(1):37-43.e1.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544.
3. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text revision. Washington, DC: APA; 2000.
5. West SL, D’Aloisio AA, Agans RP, Kalsbeek WB, Borisov NN, Thorp JM. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168(13):1441-1449.
6. Female Sexual Function Index. http://www.fsfi-questionnaire.com/FSFI%20questionnaire2000.pdf. Published 2000. Accessed August 14 2012.
7. Janata JW, Kingsberg SA. Sexual aversion disorder. In: Balon R Segraves RT, eds. Handbook of Sexual Dysfunction. Boca Raton, FL: Taylor and Francis; 2005.
8. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-assocated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300(4):395-404.
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD; Sheryl Kingsberg, PhD (January 2012)
Update on sexual dysfunction
Barbara S. Levy, MD (September 2011)
Sexual dysfunction is common among women, with an overall incidence of about 40%—even higher in some populations.1,2 All the more surprising, then, that only a minority of women raise the topic with their physician. In one well-regarded study, for example, only 22% of older women reported having discussed sex with their physician after the age of 50.3
One reason for the lack of communication may be a sense of discomfort around sexuality, among physicians as well as patients. Other reasons may include limited time on the part of physicians, and a lack of clarity about how to evaluate sexual function in women.
How, then, to assess a woman’s sexual function? In this Update, I address this question, and offer numerous others you can discuss with your patients without adding a significant time burden to your day. “A sidebar on page 27 focuses on a few strategies for tackling sexual function in an efficient and timely manner.”
In an ObGyn practice already pressed for time, adding a new domain of concern to the mix can be a challenge. (This is assuming you do not already ask patients routinely about sexual function.) It may not be as challenging as you think, however. One way to start is to add a few basic questions about sexual function to the intake form. This approach serves two purposes:
- It validates sexual function as an important part of health
- It allows the patient to identify any problems without having to raise the subject herself.
The second purpose is especially important because many patients are reluctant to broach the topic of sex.
After reviewing the intake form, you can take a more detailed history, addressing the concerns gently and matter of factly, to determine the scope of the problem, its duration, and any steps the patient has already taken to remedy it. The physical exam then can be more appropriately focused.
Straightforward areas of dysfunction, such as perimenopausal vaginal dryness, usually can be addressed in the same visit. More extensive problems may merit a separate office visit.
Linear model of female sexual function is not clinically useful
The classical linear model that proposes that women progress from sexual excitement to plateau, orgasm, and resolution is not that helpful when we are trying to determine the cause of our patient’s sexual problem and choose a course of treatment. More revealing is the biopsychosocial model, which considers physical, psychological, relational, and situational variables in exploring sexual function. If a physician focuses the history on these four aspects of sexual function, she generally can discover the source of any problem.
According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), female sexual dysfunction typically falls into one or more categories:
- desire disorders – hypoactive sexual desire disorder (HSDD) and sexual aversion disorder
- problems with arousal – female sexual arousal disorder
- pain disorders – dyspareunia and vaginismus
- orgasmic disorders – female orgasmic disorder.4
If we superimpose this framework over the biopsychosocial model, diagnosis and management can be elucidated further. For example, we might see a 57-year-old patient who complains during her annual exam about decreased libido. We ask about menopausal symptoms and general health and screen for depression and intimate partner violence. On questioning, we discover that her partner was diagnosed with prostate cancer 2 years earlier and is able to achieve an erection on occasion with the use of medication, but there is fear, for both of them, that the erection will not last. This development has changed their sexual behaviors in ways that are not satisfying for either of them.
Clearly, addressing this fairly common scenario as “female sexual dysfunction” will overlook the main issues. This couple needs help communicating their love and support for each other and some direction to help them learn new ways to express themselves sexually that can be satisfying for both. It is important to remain sensitive to the patient’s (and partner’s) social and medical history, age, and physiological status.
Keep in mind, however, that it is common to see overlapping disorders in a patient with sexual dysfunction. For example, a lack of desire can lead to diminished arousal and anorgasmia. For this reason, when one disorder is diagnosed, ask the patient to describe any other problems she may have. Also inquire about the chronology of multiple sexual disorders in order to determine which problem came first.
One last caveat: When you are evaluating a patient’s sexual function, don’t assume that she has a male partner—or any partner at all.
Don’t overlook psychosocial variables when assessing desire disorders
Prevalence of low sexual desire ranges from 26.7% among premenopausal women to 52.4% among naturally menopausal women—no small problem.5
The Female Sexual Function Index (FSFI), a multidimensional self-reporting tool used widely in research, describes sexual desire as “a feeling that includes wanting to have a sexual experience, feeling receptive to a partner’s sexual initiation, and thinking or fantasizing about having sex.”6 The FSFI attempts to discern whether a desire disorder is present by asking about the patient’s feelings over the preceding 4 weeks.
Among the questions it poses are:
- How often have you felt sexual desire?
- How would you rate your level of sexual desire or interest?
These questions may be useful as a starting point when a patient complains of low desire.
Low desire may have multiple causes
Desire disorders may be associated with depression, but they also may arise from experiences and attitudes that occurred during childhood. For example, women who had a strict religious upbringing or were exposed to negative parental attitudes toward sex may suffer lifelong psychological effects. Other deep-seated sources of impaired desire include childhood physical, sexual, and emotional abuse.
These influences may not be readily apparent if the woman is in a new relationship, when powerful hormonal determinants of attraction—driven by phenylethylamine—hold sway. Once the relationship matures, however, and the “lust” begins to recede and a more comfortable, stable relationship emerges—these psychological barriers to physical enjoyment may come to the fore.
Referral is indicated for SAD
Sexual aversion disorder (SAD) is characterized by “persistent or recurrent extreme aversion to and avoidance of all (or almost all) genital sexual contact with a sexual partner,” according to DSM-IV.2 Unlike HSDD, which reflects a lack of interest in sex, SAD may involve physiologic aversion responses such as nausea, revulsion, and shortness of breath.3
SAD is a psychiatric illness that requires management by a qualified mental health professional, preferably a sex therapist. (For information on how to find a qualified therapist, consult the American Association of Sexuality Educators, Counselors, and Therapists at www.aasect.org.)
No FDA-approved drug for HSDD
HSDD is characterized by “a deficiency or absence of sexual fantasies and desire for sexual activity,” according to DSM-IV.4 It may be treated by an experienced psychotherapist without additional training in sexual therapy.
Regrettably, attempts to treat HSDD with pharmacologic agents have been modestly successful at best, and we lack FDA-approved medications. Some trials demonstrated a slight improvement in desire among surgically menopausal women on estrogen therapy when a supraphysiologic dose of testosterone was given. Off-label use of a compounded testosterone or a lower dose of a male testosterone product may be useful.
Avoid laboratory assays for testosterone—serum or salivary—in the diagnosis of HSDD. However, if a commercial testosterone product is given, testing may be useful to prevent excessive levels of testosterone and associated (and sometimes irreversible) physiologic changes, such as male-pattern hair loss and deepening of the voice.
Failure to lubricate is the hallmark of female sexual arousal disorder
The DSM-IV defines female sexual arousal disorder as persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate physiologic response (lubrication, swelling) of sexual excitement.4 It is analogous to erectile dysfunction in men. In women, however, it may be difficult to distinguish this condition from primary desire disorder, particularly in cases in which a pattern of poor arousal, dryness, and dyspareunia has developed. The hallmark of female sexual arousal disorder is a failure to lubricate.
A few simple questions
Among the questions you might ask the patient:
- Do you feel interested in sexual activity?
- Do you have a problem lubricating well?
- Do you use a lubricant for sexual activity? If so, does it work to make sexual intercourse comfortable?
Other variables to consider
Arousal occurs secondary to genital vasodilation and tissue engorgement. It may be disturbed by any physiologic condition that reduces blood flow, such as smoking, hypertension, diabetes, and hypoestrogenism.
Decreased sensation sometimes may contribute to arousal disorder. For example, when the vagina and external genitalia experience decreased sensation, the cause may be physiologic, neurologic, or supratentorial.
Unlike men, women experience very little direct feedback regarding arousal. A disconnect between the sensory afferent input and higher-level awareness is not unusual. A thorough physical and neurologic exam may be necessary to assess the sensory nerves, integrity of the skin (signs of any inflammation), and blood flow to the genitalia.
Referral to a therapist also may be necessary so that other barriers to intimacy and sexuality can be determined.
As menopause approaches, so do a number of interrelated issues that may affect a woman’s sexual function. Here are five of them:
Body image may deteriorate, leading to feelings of unattractiveness that can lower interest in sexual activity.
Testosterone levels decline throughout a woman’s reproductive life, as does estrogen—but the decline in testosterone is much more gradual without any abrupt reduction at menopause. In addition, some common medications, such as oral contraceptives and oral estrogen formulations, increase sex hormone binding globulin, which can reduce dramatically the free testosterone level. Some women are especially sensitive to the effects of these drugs and may report a rapid loss of sexual desire when these agents are given. To sidestep the problem, give transdermal or transvaginal estrogen, when possible.
Comorbidities take a toll on sexual function in many cases. Arthritis, diabetes, cardiovascular disease, and other impairments may hamper sexual satisfaction. In addition, fatigue, insomnia, and depression are common with age.
Partner issues also play a role in diagnosis and treatment of female sexual dysfunction. For example, poor health in the partner can cause or exacerbate sexual problems.
Pain during sex can trigger desire and arousal disorders
Pain during sexual activity can lead to disorders of desire as well as arousal. When a patient reports pain during sex, pay careful attention to her medical history and perform a detailed physical examination. Patience is vital. Successful treatment of pain disorders requires commitment from the patient and her partner as well as the medical team.
Consider asking the following questions:
- Over the past 4 weeks, how often have you experienced discomfort or pain during vaginal penetration?
- How often have you experienced discomfort or pain following vaginal penetration?
- How would you rate your degree of discomfort or pain during or following vaginal penetration?6
Consider pain as a cause when any patient reports low libido, as pain is a potent suppressor of desire. A meticulous clinical history is required to determine the cause. For example, it is important to uncover whether the pain is of recent onset or of long duration, or whether it is related to childbirth, lactation, or menopausal changes.
Pain upon penetration could be caused by chemical, infectious, or atrophic vulvovaginitis. Dryness and pain upon penetration are often caused by:
- contact dermatitis
- irritation from soaps or scrubbing
- daily use of panty liners
- use of so-called feminine hygiene products
- regular use of swimming pools or hot tubs that contain chemicals.
Another cause of pain to consider is vulvar dystrophy. When lichen sclerosis or hypertrophic dystrophy goes untreated, the result may be fibrosis, lack of elasticity, painful fissures, and loss of normal architecture. These changes usually occur in postmenopausal women, so it is important that treatment address both the fibrosis and the hypoestrogenic atrophy.
If treated early, vulvar vestibulitis may not require surgery
Vulvar vestibulitis is poorly understood. It tends to occur most often in premenopausal women, frequently as a result of vulvar infection or during the postpartum period.
Vulvar vestibulitis involves point tenderness—sometimes experienced as a burning, searing sensation—around the introitus, specifically, the vestibular glands. When this condition is suspected, examine the vulva and vestibule with a moistened cotton swab to assess whether the classic distribution of pain is present. The necks of the vestibular glands may appear inflamed and erythematous.
If the condition is treated early enough with topical steroids and, in some cases, hydroxyzine, surgery may be avoided, provided the patient also avoids topical irritants. In many women, however, vestibulectomy is required to eliminate symptoms.
Vulvodynia may be associated with other pain syndromes
This disorder is a more generalized pain syndrome that involves the entire vulvar region. Like vulvar vestibulitis, it can cause painful penetration. It is also associated with other pain syndromes, including interstitial cystitis and endometriosis. Sensitization to pain at a central level may lead to hyperesthesia and allodynia. Also consider pudendal neuropathy, especially if the patient is a regular bicycle rider.
Treatment usually consists of off-label use of neuromodulators, such as gabapentin, tricyclic antidepressants, or duloxetine. The use of topical local anesthetic creams or gels may also permit pain-free sexual activity.
Vaginismus may indicate a history of sexual abuse
When the perineal muscles surrounding the outer third of the vagina contract involuntarily upon contact with a penis, speculum, or other item, vaginismus may be present. This disorder can be primary or secondary. In primary vaginismus, the patient may be unable to tolerate any vaginal penetration at all, not even a single digit or tampon. When this is the case, the patient may have a history of childhood sexual abuse. Explore her history, including any medical examinations that may have been painful or generated fear and anxiety. Also be aware that women with sexual aversion disorder may present with primary vaginismus.
Secondary vaginismus can occur even after years of satisfying sexual activity when a woman undergoes pelvic reconstructive surgery or develops vulvar dystrophy or vulvovaginal atrophy. Pain or the fear of pain can trigger a powerful reflex spasm of the levator ani musculature. Also keep in mind that secondary vaginismus may not be reproducible during the pelvic examination.
Treatment of both primary and secondary vaginismus includes physical therapy of the pelvic floor using biofeedback. The patient’s partner also needs to attend at least one session to learn techniques to prevent levator spasm and disable the reflex.
In the early phase of treatment, it may be helpful for the couple to agree to participate in a pact to avoid penetration during sexual activity. This approach may help reawaken sexual desire and arousal by eliminating the fear of pain.
The nature of the patient’s relationship with her partner is a powerful determinant of outcome. For example, intimacy and good communication are more likely to resolve the problem, whereas a difficult relationship may inhibit success. Depending on the scenario, counseling and psychological assessment may be necessary in the treatment of vaginismus, especially when a patient has a history of abuse.
Deep dyspareunia may be linked to pelvic pathology
This pain disorder may be associated with poor arousal or with fixation of the pelvic organs as a result of endometriosis, adhesions, or posthysterectomy scarring.
In a “normal” scenario, when arousal is unimpeded, the vagina lengthens by about 30%, and the uterus and cervix lift out of the cul-de-sac. This helps explain why not all women who have retroverted uteri or an obliterated cul-de-sac experience deep dyspareunia.
The patient’s history is a critical component of diagnosis. Ask her about foreplay, lubrication, and arousal prior to penetration. During the physical examination, be vigilant for point tenderness along the vaginal cuff and painful nodularity along the uterosacral ligaments.
To successfully treat deep dyspareunia, you must address any pelvic pathology as well as arousal problems. If penetration occurs prior to adequate arousal, the vagina remains shorter and the uterus has not yet engorged and lifted out of the cul-de-sac, resulting in “bump” dyspareunia. Surgery can elevate and alter any uterine retroversion that is present, but it is very rarely needed when adequate arousal can be achieved.
Pain with orgasm may arise from uterine pathology
At the time of orgasm, the levator ani musculature and myometrium contract strongly. When adenomyosis or degenerating uterine fibroids are present, pain may occur during or after orgasm. Women who have pelvic floor tension myalgia also may experience pelvic pain and aching after orgasm.
To tease out the cause of orgasm-related pain, perform a careful physical examination. To distinguish uterine pain from pain at the pelvic floor, perform a single-digit examination of each pelvic floor muscle before touching the cervix and uterus. Compression applied to a tender uterus often triggers a muscle spasm at the pelvic floor, so it is important to evaluate the pelvic floor muscles for tone and discomfort before performing a bimanual exam.
Treatment of uterine pathology usually entails medical or surgical intervention, whereas pelvic floor tension myalgia is treated with physical therapy and biofeedback.
Female orgasmic disorder may indicate a need for basic education
When a woman reports a persistent delay in or absence of orgasm after an otherwise satisfying episode of sexual activity and excitement, female orgasmic disorder is the likely cause. It may be primary or secondary.
Primary anorgasmia is often related to sexual inexperience and ignorance. Management may require education, use of a vibrator, and permission to engage in self-exploration—or it may necessitate evaluation and management by a trained sexual therapist. Resources for the patient, including educational materials and video, can be found at www.bettersex.com.
Secondary anorgasmia occurs in women who have a history of regular orgasm; the cause is generally drug-related. Among the usual culprits are selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants. Anorgasmia can be difficult to treat in these cases because discontinuation of the antidepressant can worsen depression—and depression is often associated with disorders of desire. One option is switching the class of the antidepressant to one less likely to disrupt orgasm, such as buproprion or trazodone. Off-label use of low-dose sildenafil may reverse the effect of SSRIs on orgasmic function, according to recent evidence.8
We want to hear from you! Tell us what you think.
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD; Sheryl Kingsberg, PhD (January 2012)
Update on sexual dysfunction
Barbara S. Levy, MD (September 2011)
Sexual dysfunction is common among women, with an overall incidence of about 40%—even higher in some populations.1,2 All the more surprising, then, that only a minority of women raise the topic with their physician. In one well-regarded study, for example, only 22% of older women reported having discussed sex with their physician after the age of 50.3
One reason for the lack of communication may be a sense of discomfort around sexuality, among physicians as well as patients. Other reasons may include limited time on the part of physicians, and a lack of clarity about how to evaluate sexual function in women.
How, then, to assess a woman’s sexual function? In this Update, I address this question, and offer numerous others you can discuss with your patients without adding a significant time burden to your day. “A sidebar on page 27 focuses on a few strategies for tackling sexual function in an efficient and timely manner.”
In an ObGyn practice already pressed for time, adding a new domain of concern to the mix can be a challenge. (This is assuming you do not already ask patients routinely about sexual function.) It may not be as challenging as you think, however. One way to start is to add a few basic questions about sexual function to the intake form. This approach serves two purposes:
- It validates sexual function as an important part of health
- It allows the patient to identify any problems without having to raise the subject herself.
The second purpose is especially important because many patients are reluctant to broach the topic of sex.
After reviewing the intake form, you can take a more detailed history, addressing the concerns gently and matter of factly, to determine the scope of the problem, its duration, and any steps the patient has already taken to remedy it. The physical exam then can be more appropriately focused.
Straightforward areas of dysfunction, such as perimenopausal vaginal dryness, usually can be addressed in the same visit. More extensive problems may merit a separate office visit.
Linear model of female sexual function is not clinically useful
The classical linear model that proposes that women progress from sexual excitement to plateau, orgasm, and resolution is not that helpful when we are trying to determine the cause of our patient’s sexual problem and choose a course of treatment. More revealing is the biopsychosocial model, which considers physical, psychological, relational, and situational variables in exploring sexual function. If a physician focuses the history on these four aspects of sexual function, she generally can discover the source of any problem.
According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), female sexual dysfunction typically falls into one or more categories:
- desire disorders – hypoactive sexual desire disorder (HSDD) and sexual aversion disorder
- problems with arousal – female sexual arousal disorder
- pain disorders – dyspareunia and vaginismus
- orgasmic disorders – female orgasmic disorder.4
If we superimpose this framework over the biopsychosocial model, diagnosis and management can be elucidated further. For example, we might see a 57-year-old patient who complains during her annual exam about decreased libido. We ask about menopausal symptoms and general health and screen for depression and intimate partner violence. On questioning, we discover that her partner was diagnosed with prostate cancer 2 years earlier and is able to achieve an erection on occasion with the use of medication, but there is fear, for both of them, that the erection will not last. This development has changed their sexual behaviors in ways that are not satisfying for either of them.
Clearly, addressing this fairly common scenario as “female sexual dysfunction” will overlook the main issues. This couple needs help communicating their love and support for each other and some direction to help them learn new ways to express themselves sexually that can be satisfying for both. It is important to remain sensitive to the patient’s (and partner’s) social and medical history, age, and physiological status.
Keep in mind, however, that it is common to see overlapping disorders in a patient with sexual dysfunction. For example, a lack of desire can lead to diminished arousal and anorgasmia. For this reason, when one disorder is diagnosed, ask the patient to describe any other problems she may have. Also inquire about the chronology of multiple sexual disorders in order to determine which problem came first.
One last caveat: When you are evaluating a patient’s sexual function, don’t assume that she has a male partner—or any partner at all.
Don’t overlook psychosocial variables when assessing desire disorders
Prevalence of low sexual desire ranges from 26.7% among premenopausal women to 52.4% among naturally menopausal women—no small problem.5
The Female Sexual Function Index (FSFI), a multidimensional self-reporting tool used widely in research, describes sexual desire as “a feeling that includes wanting to have a sexual experience, feeling receptive to a partner’s sexual initiation, and thinking or fantasizing about having sex.”6 The FSFI attempts to discern whether a desire disorder is present by asking about the patient’s feelings over the preceding 4 weeks.
Among the questions it poses are:
- How often have you felt sexual desire?
- How would you rate your level of sexual desire or interest?
These questions may be useful as a starting point when a patient complains of low desire.
Low desire may have multiple causes
Desire disorders may be associated with depression, but they also may arise from experiences and attitudes that occurred during childhood. For example, women who had a strict religious upbringing or were exposed to negative parental attitudes toward sex may suffer lifelong psychological effects. Other deep-seated sources of impaired desire include childhood physical, sexual, and emotional abuse.
These influences may not be readily apparent if the woman is in a new relationship, when powerful hormonal determinants of attraction—driven by phenylethylamine—hold sway. Once the relationship matures, however, and the “lust” begins to recede and a more comfortable, stable relationship emerges—these psychological barriers to physical enjoyment may come to the fore.
Referral is indicated for SAD
Sexual aversion disorder (SAD) is characterized by “persistent or recurrent extreme aversion to and avoidance of all (or almost all) genital sexual contact with a sexual partner,” according to DSM-IV.2 Unlike HSDD, which reflects a lack of interest in sex, SAD may involve physiologic aversion responses such as nausea, revulsion, and shortness of breath.3
SAD is a psychiatric illness that requires management by a qualified mental health professional, preferably a sex therapist. (For information on how to find a qualified therapist, consult the American Association of Sexuality Educators, Counselors, and Therapists at www.aasect.org.)
No FDA-approved drug for HSDD
HSDD is characterized by “a deficiency or absence of sexual fantasies and desire for sexual activity,” according to DSM-IV.4 It may be treated by an experienced psychotherapist without additional training in sexual therapy.
Regrettably, attempts to treat HSDD with pharmacologic agents have been modestly successful at best, and we lack FDA-approved medications. Some trials demonstrated a slight improvement in desire among surgically menopausal women on estrogen therapy when a supraphysiologic dose of testosterone was given. Off-label use of a compounded testosterone or a lower dose of a male testosterone product may be useful.
Avoid laboratory assays for testosterone—serum or salivary—in the diagnosis of HSDD. However, if a commercial testosterone product is given, testing may be useful to prevent excessive levels of testosterone and associated (and sometimes irreversible) physiologic changes, such as male-pattern hair loss and deepening of the voice.
Failure to lubricate is the hallmark of female sexual arousal disorder
The DSM-IV defines female sexual arousal disorder as persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate physiologic response (lubrication, swelling) of sexual excitement.4 It is analogous to erectile dysfunction in men. In women, however, it may be difficult to distinguish this condition from primary desire disorder, particularly in cases in which a pattern of poor arousal, dryness, and dyspareunia has developed. The hallmark of female sexual arousal disorder is a failure to lubricate.
A few simple questions
Among the questions you might ask the patient:
- Do you feel interested in sexual activity?
- Do you have a problem lubricating well?
- Do you use a lubricant for sexual activity? If so, does it work to make sexual intercourse comfortable?
Other variables to consider
Arousal occurs secondary to genital vasodilation and tissue engorgement. It may be disturbed by any physiologic condition that reduces blood flow, such as smoking, hypertension, diabetes, and hypoestrogenism.
Decreased sensation sometimes may contribute to arousal disorder. For example, when the vagina and external genitalia experience decreased sensation, the cause may be physiologic, neurologic, or supratentorial.
Unlike men, women experience very little direct feedback regarding arousal. A disconnect between the sensory afferent input and higher-level awareness is not unusual. A thorough physical and neurologic exam may be necessary to assess the sensory nerves, integrity of the skin (signs of any inflammation), and blood flow to the genitalia.
Referral to a therapist also may be necessary so that other barriers to intimacy and sexuality can be determined.
As menopause approaches, so do a number of interrelated issues that may affect a woman’s sexual function. Here are five of them:
Body image may deteriorate, leading to feelings of unattractiveness that can lower interest in sexual activity.
Testosterone levels decline throughout a woman’s reproductive life, as does estrogen—but the decline in testosterone is much more gradual without any abrupt reduction at menopause. In addition, some common medications, such as oral contraceptives and oral estrogen formulations, increase sex hormone binding globulin, which can reduce dramatically the free testosterone level. Some women are especially sensitive to the effects of these drugs and may report a rapid loss of sexual desire when these agents are given. To sidestep the problem, give transdermal or transvaginal estrogen, when possible.
Comorbidities take a toll on sexual function in many cases. Arthritis, diabetes, cardiovascular disease, and other impairments may hamper sexual satisfaction. In addition, fatigue, insomnia, and depression are common with age.
Partner issues also play a role in diagnosis and treatment of female sexual dysfunction. For example, poor health in the partner can cause or exacerbate sexual problems.
Pain during sex can trigger desire and arousal disorders
Pain during sexual activity can lead to disorders of desire as well as arousal. When a patient reports pain during sex, pay careful attention to her medical history and perform a detailed physical examination. Patience is vital. Successful treatment of pain disorders requires commitment from the patient and her partner as well as the medical team.
Consider asking the following questions:
- Over the past 4 weeks, how often have you experienced discomfort or pain during vaginal penetration?
- How often have you experienced discomfort or pain following vaginal penetration?
- How would you rate your degree of discomfort or pain during or following vaginal penetration?6
Consider pain as a cause when any patient reports low libido, as pain is a potent suppressor of desire. A meticulous clinical history is required to determine the cause. For example, it is important to uncover whether the pain is of recent onset or of long duration, or whether it is related to childbirth, lactation, or menopausal changes.
Pain upon penetration could be caused by chemical, infectious, or atrophic vulvovaginitis. Dryness and pain upon penetration are often caused by:
- contact dermatitis
- irritation from soaps or scrubbing
- daily use of panty liners
- use of so-called feminine hygiene products
- regular use of swimming pools or hot tubs that contain chemicals.
Another cause of pain to consider is vulvar dystrophy. When lichen sclerosis or hypertrophic dystrophy goes untreated, the result may be fibrosis, lack of elasticity, painful fissures, and loss of normal architecture. These changes usually occur in postmenopausal women, so it is important that treatment address both the fibrosis and the hypoestrogenic atrophy.
If treated early, vulvar vestibulitis may not require surgery
Vulvar vestibulitis is poorly understood. It tends to occur most often in premenopausal women, frequently as a result of vulvar infection or during the postpartum period.
Vulvar vestibulitis involves point tenderness—sometimes experienced as a burning, searing sensation—around the introitus, specifically, the vestibular glands. When this condition is suspected, examine the vulva and vestibule with a moistened cotton swab to assess whether the classic distribution of pain is present. The necks of the vestibular glands may appear inflamed and erythematous.
If the condition is treated early enough with topical steroids and, in some cases, hydroxyzine, surgery may be avoided, provided the patient also avoids topical irritants. In many women, however, vestibulectomy is required to eliminate symptoms.
Vulvodynia may be associated with other pain syndromes
This disorder is a more generalized pain syndrome that involves the entire vulvar region. Like vulvar vestibulitis, it can cause painful penetration. It is also associated with other pain syndromes, including interstitial cystitis and endometriosis. Sensitization to pain at a central level may lead to hyperesthesia and allodynia. Also consider pudendal neuropathy, especially if the patient is a regular bicycle rider.
Treatment usually consists of off-label use of neuromodulators, such as gabapentin, tricyclic antidepressants, or duloxetine. The use of topical local anesthetic creams or gels may also permit pain-free sexual activity.
Vaginismus may indicate a history of sexual abuse
When the perineal muscles surrounding the outer third of the vagina contract involuntarily upon contact with a penis, speculum, or other item, vaginismus may be present. This disorder can be primary or secondary. In primary vaginismus, the patient may be unable to tolerate any vaginal penetration at all, not even a single digit or tampon. When this is the case, the patient may have a history of childhood sexual abuse. Explore her history, including any medical examinations that may have been painful or generated fear and anxiety. Also be aware that women with sexual aversion disorder may present with primary vaginismus.
Secondary vaginismus can occur even after years of satisfying sexual activity when a woman undergoes pelvic reconstructive surgery or develops vulvar dystrophy or vulvovaginal atrophy. Pain or the fear of pain can trigger a powerful reflex spasm of the levator ani musculature. Also keep in mind that secondary vaginismus may not be reproducible during the pelvic examination.
Treatment of both primary and secondary vaginismus includes physical therapy of the pelvic floor using biofeedback. The patient’s partner also needs to attend at least one session to learn techniques to prevent levator spasm and disable the reflex.
In the early phase of treatment, it may be helpful for the couple to agree to participate in a pact to avoid penetration during sexual activity. This approach may help reawaken sexual desire and arousal by eliminating the fear of pain.
The nature of the patient’s relationship with her partner is a powerful determinant of outcome. For example, intimacy and good communication are more likely to resolve the problem, whereas a difficult relationship may inhibit success. Depending on the scenario, counseling and psychological assessment may be necessary in the treatment of vaginismus, especially when a patient has a history of abuse.
Deep dyspareunia may be linked to pelvic pathology
This pain disorder may be associated with poor arousal or with fixation of the pelvic organs as a result of endometriosis, adhesions, or posthysterectomy scarring.
In a “normal” scenario, when arousal is unimpeded, the vagina lengthens by about 30%, and the uterus and cervix lift out of the cul-de-sac. This helps explain why not all women who have retroverted uteri or an obliterated cul-de-sac experience deep dyspareunia.
The patient’s history is a critical component of diagnosis. Ask her about foreplay, lubrication, and arousal prior to penetration. During the physical examination, be vigilant for point tenderness along the vaginal cuff and painful nodularity along the uterosacral ligaments.
To successfully treat deep dyspareunia, you must address any pelvic pathology as well as arousal problems. If penetration occurs prior to adequate arousal, the vagina remains shorter and the uterus has not yet engorged and lifted out of the cul-de-sac, resulting in “bump” dyspareunia. Surgery can elevate and alter any uterine retroversion that is present, but it is very rarely needed when adequate arousal can be achieved.
Pain with orgasm may arise from uterine pathology
At the time of orgasm, the levator ani musculature and myometrium contract strongly. When adenomyosis or degenerating uterine fibroids are present, pain may occur during or after orgasm. Women who have pelvic floor tension myalgia also may experience pelvic pain and aching after orgasm.
To tease out the cause of orgasm-related pain, perform a careful physical examination. To distinguish uterine pain from pain at the pelvic floor, perform a single-digit examination of each pelvic floor muscle before touching the cervix and uterus. Compression applied to a tender uterus often triggers a muscle spasm at the pelvic floor, so it is important to evaluate the pelvic floor muscles for tone and discomfort before performing a bimanual exam.
Treatment of uterine pathology usually entails medical or surgical intervention, whereas pelvic floor tension myalgia is treated with physical therapy and biofeedback.
Female orgasmic disorder may indicate a need for basic education
When a woman reports a persistent delay in or absence of orgasm after an otherwise satisfying episode of sexual activity and excitement, female orgasmic disorder is the likely cause. It may be primary or secondary.
Primary anorgasmia is often related to sexual inexperience and ignorance. Management may require education, use of a vibrator, and permission to engage in self-exploration—or it may necessitate evaluation and management by a trained sexual therapist. Resources for the patient, including educational materials and video, can be found at www.bettersex.com.
Secondary anorgasmia occurs in women who have a history of regular orgasm; the cause is generally drug-related. Among the usual culprits are selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants. Anorgasmia can be difficult to treat in these cases because discontinuation of the antidepressant can worsen depression—and depression is often associated with disorders of desire. One option is switching the class of the antidepressant to one less likely to disrupt orgasm, such as buproprion or trazodone. Off-label use of low-dose sildenafil may reverse the effect of SSRIs on orgasmic function, according to recent evidence.8
We want to hear from you! Tell us what you think.
1. Trompeter SE, Bettencourt R, Barrett-Connor E. Sexual activity and satisfaction in healthy community-dwelling older women. Am J Med. 2012;125(1):37-43.e1.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544.
3. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text revision. Washington, DC: APA; 2000.
5. West SL, D’Aloisio AA, Agans RP, Kalsbeek WB, Borisov NN, Thorp JM. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168(13):1441-1449.
6. Female Sexual Function Index. http://www.fsfi-questionnaire.com/FSFI%20questionnaire2000.pdf. Published 2000. Accessed August 14 2012.
7. Janata JW, Kingsberg SA. Sexual aversion disorder. In: Balon R Segraves RT, eds. Handbook of Sexual Dysfunction. Boca Raton, FL: Taylor and Francis; 2005.
8. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-assocated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300(4):395-404.
1. Trompeter SE, Bettencourt R, Barrett-Connor E. Sexual activity and satisfaction in healthy community-dwelling older women. Am J Med. 2012;125(1):37-43.e1.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544.
3. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text revision. Washington, DC: APA; 2000.
5. West SL, D’Aloisio AA, Agans RP, Kalsbeek WB, Borisov NN, Thorp JM. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168(13):1441-1449.
6. Female Sexual Function Index. http://www.fsfi-questionnaire.com/FSFI%20questionnaire2000.pdf. Published 2000. Accessed August 14 2012.
7. Janata JW, Kingsberg SA. Sexual aversion disorder. In: Balon R Segraves RT, eds. Handbook of Sexual Dysfunction. Boca Raton, FL: Taylor and Francis; 2005.
8. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-assocated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300(4):395-404.
Scoliosis Early Identification of Affected Patients
A majority of practitioners will at some time encounter scoliosis, the most common spinal deformity seen in pediatric practice.1 This musculoskeletal condition can be found incidentally during routine exams, in the urgent care setting, in the emergency department (ED), or on chest x-rays or other radiographs. In primary care, scoliosis may be overlooked, particularly when the etiology is idiopathic or when well-child visits or routine back exams have been missed.1
The focus of this review will be on early identification of affected patients (most commonly those with adolescent idiopathic scoliosis), with emphasis on evaluation through an excellent physical examination and ordering of appropriate radiographs. Watchful waiting and monitoring are the norm; however, implementing carefully selected treatment options, such as bracing, or making an appropriate referral may make it possible to slow or stop curve progression.1-4
SCOLIOSIS: THE DEFINITION
Scoliosis is a coronal curvature (lateral deviation of the spine) of more than 10°, usually with a rotational component.1,2 A healthy spine is straight in the frontal plane, but curved in the sagittal plane. Normal circumstances include thoracic kyphosis (outward curve of the upper back) and lumbar lordosis (inward curve of the lower back).5 The scoliotic spine deviates away from the midline, and maximal rotation is at the apex of the curve. The apex or point of the curve defines its center, that is, the most laterally deviated vertebra or disc. The first curve to develop is the primary curve; however, when a patient presents with two or three curves, it is difficult to determine which curve is primary.
The secondary curve is a compensatory curve, which develops as a means to balance the head and trunk over the pelvis. A similar compensation may occur in the sagittal plane, exacerbating the normal kyphosis or lordosis or possibly causing rib or scapular prominences.6 Cervical curves can also develop but are more commonly associated with conditions other than idiopathic scoliosis.3 Cervical scoliosis will not be reviewed here.
THE EPIDEMIOLOGY OF SCOLIOSIS TYPES
Eighty percent of all scoliosis patients are diagnosed with idiopathic scoliosis (which may be present in 2% to 3% of the population).1,5 The remaining 20% are diagnosed with congenital, neuromuscular, or syndrome-related spinal deformities.1 The overall prevalence of idiopathic scoliosis (with curves greater than 10°) is 1 to 3 per 100, with a comparable proportion of girls and boys. The prevalence of those with curves greater than 30° is 1 to 3 per 1,000, with a boy-girl ratio of 1:8.7 In the child who is skeletally mature, scoliosis curves measuring less than 30° usually do not progress. However, most curves greater than 50° tend to progress at about 1° per year.7
Idiopathic scoliosis is broken down into age ranges.8Infantile scoliosis is diagnosed from age 0 to 3 years and comprises 0.5% of cases; juvenile scoliosis, with an age range of 4 to 9 years, represents 10.5% of cases; and adolescent scoliosis, diagnosed any time after age 10, accounts for 89% of cases.7 The four main types of scoliosis will be discussed.
Congenital
Congenital scoliosis is defined as a malformed or segmented portion of the spine, a failure of formation of a portion of the spine, or a combination of the two.9 This results in block vertebrae or hemivertebrae, or possibly the fusing of two vertebrae with a bony bridge. Because the associated curves are often rigid, they can be resistant to correction. Worldwide, prevalence of congenital vertebral anomalies is 0.5 to 1.0 per 1,000 live births.10
Congenital scoliosis can be associated with many other congenital abnormalities, as embryonic development of the spine occurs at the same time as that of the heart, kidneys, bowel, and bladder.1 Congenital scoliosis can occur with any of these defects or in the setting of a syndrome, such as VACTERL1,9-11 (ie, vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal defects, and limb abnormalities1; see Table 11,9,11,12). If even one such defect or abnormality is found, associated comorbid conditions should be actively sought.1
In many instances, these abnormalities are noted when children are young; in children younger than 5 years, MRI is warranted, and these patients almost always need sedation during imaging. In the young child who also exhibits urologic issues, an MRI work-up for genitourinary defects can be performed at the same time.1
Patients with abnormal cardiac findings (eg, heart murmur) should be referred for a cardiology work-up.11,13 Chest wall deformities may also be present in children with congenital scoliosis. Multiple congenital rib fusions can cause chest wall constriction, possibly inhibiting growth and lung development. Untreated, this condition can ultimately shorten a child’s lifespan.14
Neuromuscular
Spinal curvature in a patient with a neuromuscular disease is referred to as neuromuscular scoliosis (see Table 2).1,4,5 Most diagnoses within this category are genetic in nature, although some can result from a traumatic brain injury and/or anoxia (eg, cerebral palsy). Given that this disease state is detected early in most patients’ lives, they are generally followed by an orthopedist (or more specifically, by a pediatric orthopedist) once necessary testing, such as radiography or MRI, has been completed.1,15
Syndrome-related
Patients may have certain genetic conditions or syndromes that put them at high risk for scoliosis.
The two conditions most commonly associated with scoliosis are Marfan syndrome (60%) and neurofibromatosis (10% to 15%).1 Many patients in both groups will require MRI for evaluation; given that they are at high risk for lumbar spondylolisthesis (slippage between two lumbar vertebrae), care should be taken to evaluate for this possible secondary finding.16,17 It should be noted that patients with Marfan syndrome are at high risk for cardiac abnormalities, and those with neurofibromatosis, for dural ectasia and/ or Chiari malformations (which would be detected by MRI).16,18
Patients who have undergone chest wall surgery, such as thoracotomy for congenital heart defect repair, should be evaluated for scoliosis. The incidence of scoliosis is elevated in patients with heart defects, and the association is even greater in those who have had chest wall surgery, especially before age 18 months.19
IDIOPATHIC SCOLIOSIS
Because, by definition, no cause is known, idiopathic scoliosis is considered a diagnosis of exclusion. Affected patients have a normal neurologic exam and usually have no presenting symptoms or evidence of spinal abnormalities.1 Since 1954, idiopathic scoliosis has been divided into three age-groups, as these periods correspond to phases of increased growth velocity that occur during childhood and adolescence.8,20
Infant
A diagnosis of early-onset scoliosis is made when a child presents with scoliosis before age 5; any affected child between 0 and 3 years of age may be included in the infant scoliosis classification.2 All congenital, neuromuscular, and syndrome-related causes will have been excluded. It is felt among many experts that there is always a genetic or familial component to scoliosis. Therefore, when a child's family history is positive for scoliosis, the child should be aggressively screened, and the family should be educated to observe for signs of scoliosis and seek practitioner involvement as soon as any are evident.2
A thorough examination should be completed to rule out organ involvement in the presence of infant scoliosis.1 It is recommended that any infant with scoliotic curves greater than 20° undergo MRI of the head and spine to rule out central nervous system lesions or structural abnormalities. The risk for respiratory failure-related morbidity and mortality is increased in children with untreated early-onset scoliosis who experience curve progression. Rare progressive curves often can be addressed surgically, preserving spine and trunk growth while also achieving curve correction.2
In the majority of cases of infant scoliosis (75% to 90%), curves are left-sided (levoscoliosis)—in contrast to the right-sided curves (dextroscoliosis) more commonly noted in late-onset (juvenile or adolescent) scoliosis3,21 (see Figure 1). Infant scoliosis is slightly more common in boys (60%) than in girls.21
Neither curve lateralization nor gender makes a difference in treatment.2 Approximately 90% of early-onset curves resolve spontaneously, especially in those who are diagnosed before age 1 year. Progression occurring at a rate of 2° to 3° per year is considered gradual, whereas malignant progression often worsens by 10° or more per year, with the condition possibly becoming severe and disabling.2,21
Any infant given a diagnosis of scoliosis by a practitioner other than a pediatric orthopedist should be referred as soon as possible. On referral, copies of previously obtained radiographs and/or MRI should be presented or sent to the orthopedic practitioner, along with the history and physical exam findings, to avoid the need for repeated imaging.
Juvenile
Juvenile idiopathic scoliosis is diagnosed in patients between ages 4 and 9. Presentation in younger members of this age-group tends to resemble that in infantile idiopathic scoliosis, and children in the middle to upper age range tend to present as adolescent patients do.3 Most juvenile patients' curves will resolve without treatment (especially younger children's).
It is recommended that children with juvenile idiopathic scoliosis undergo MRI to rule out brain or spinal abnormalities. In up to 20% of children younger than 10 with curves exceeding 20°, findings on MRI are abnormal.3 Observation with close monitoring of curve progression is key in this population, especially if rapid progression is evident. In children with juvenile idiopathic scoliosis and rapid-onset curve progression, severe trunk deformity or pulmonary and/or cardiac compromise can develop. Curves that reach 30° almost always continue to worsen unless treatment is given.3,7 Timely referral to a pediatric spine orthopedist is warranted for children in this age-group.
Adolescent
It is during adolescence (ages 10 to 18) that idiopathic scoliosis is most common. Approximately 2% to 3% of children ages 10 to 15 have scoliosis with a curve of at least 10°. Among these patients, however, just 5% have scoliosis of clinical significance to warrant treatment (ie, curve progression > 30°).5,22
Each child should be examined initially for nonstructural scoliosis. This includes postural scoliosis, which resolves when a child is recumbent; compensatory scoliosis, which can be the result of a leg-length discrepancy (with no fixed vertebral rotation); sciatic scoliosis, an abnormality that results from avoiding the pain of an irritated sciatic nerve; inflammatory scoliosis, which could result from an infectious process, such as acute appendicitis; and hysterical scoliosis, a very rare form with an underlying psychological factor.4
Structural scoliosis comprises the remaining types of scoliosis, including the idiopathic variety. Patients with adolescent idiopathic scoliosis (AIS) are older than 10 and usually undergoing a rapid growth phase. Thus, some adolescent patients' curves can progress rapidly.4
Adolescents with idiopathic scoliosis rarely complain of pain or neurologic symptoms. Generally, their spinal curves do not cause organ pressure or shortness of breath. Many patients with AIS are involved in extracurricular activities that can trigger low back pain, yet activity-related low back pain is not an uncommon complaint among teens in general. Therefore, it is felt that when a patient with AIS has intermittent low back pain, it is most likely not related to their curve(s). Nevertheless, if a patient with AIS develops continued or unexplained low back pain and/or new neurologic symptoms, MRI is warranted.3
HISTORY, PHYSICAL EXAMINATION, AND DIAGNOSIS
During the exam, elicit any family history of scoliosis, especially in the setting of known diseases, such as Marfan syndrome, neurofibromatosis, muscular dystrophy, or other related diseases. Approximately 30% of AIS patients have a family history of scoliosis, so although the cause may not be clear, some genetic component is possible.3 The examination should stem from the patient's current stage of growth and development (see Table 31,3,4,7,23). The diagnosis of scoliosis is typically made during the examination and confirmed by radiography.
The newborn patient should be kept warm but be wearing only a diaper during the exam. A child or adolescent should be undressed and wearing a patient gown (unless at a school screening).
A general observation should be done initially, including the child's overall shape. The examiner should look for marfanoid features, such as excessive height, long arms, long trunk, and joint laxity.7 In the examination of the feet, an extremely high arch or cavus foot may be indicative of tethered spinal cord, spinal syrinx, or Charcot-Marie-Tooth disease, especially if the cavus is unilateral.1,7,23 MRI would be warranted in these instances.1
The examining clinician should note the child's Tanner stage. Height and weight measurements should be taken, and for a girl, age at menarche should be recorded.4
Next, the skin should be carefully inspected. In the infant, the examiner should look for hairy patches at the base of the spine, cutaneous sinuses, sacral dimpling, or other skin lesions that may suggest spinal dysraphism (ie, failure of the posterior elements to fuse around the spinal cord).4 Examination for café au lait spots and/or freckling in the axilla or groin area should be included, as this could signify neurofibromatosis.1,4,7
Examine the back in a standing child. Note whether the head appears to be centered over the sacrum. Evaluate for shoulder height or truncal asymmetry, scapular or posterior rib prominence, and excessive thoracic kyphosis and/or lumbar lordosis. Examine the pelvic bones, looking for obliquity (ie, one side higher than the other). Look for leg length discrepancy, both while the patient is standing still and during a gait review.1
During an anterior exam, look for shoulder or truncal asymmetry, leg length discrepancy, and anterior rib prominence. If shoulder height asymmetry is apparent, measure from each acromioclavicular joint to the floor and note the difference in centimeters. In the case of pelvic obliquity, measure from each anterior (or posterior) superior iliac spine to the floor and note the difference in centimeters. If the head does not appear to align over the sacrum, lower a plum line from the spinous process of C7 down to the gluteal cleft level. Measure by centimeters the distance from the cleft to the line, noting the side to which the line sways.
View the patient from the side. The sagittal curve can be assessed from the occiput to the sacrum; observe for an increased lordotic or kyphotic curve or abnormal positioning related to abnormal curves.4
The Adams forward bend test should be conducted.24,25 The child who is able to stand faces away from the examiner, then bends forward at the waist with the knees straight and the arms hanging down passively (see Figure 2). The practitioner looks at the spine from behind and notes any rib, thoracic, scapular, or lumbar prominence that could indicate a rotational spine deformity.1,4,7 In some instances, the use of a scoliometer can be helpful to assign a number to the amount of rotation to the spine during the Adams forward bend test. However, primary care offices are not often equipped with this device.26
Motor and Neurologic Examinations
The motor exam includes testing motor strength in all four extremities and noting any asymmetry. The examiner should have the child actively bend in every direction, making note of any deformity, stiffness, or asymmetry. The patient's spine should be palpated, with any tenderness noted, and any available radiographs reviewed.1,4,7
The neurologic exam includes testing cranial nerves, sensation, and reflexes, including the patellar, Achilles, and umbilical reflexes, as well as Babinski's sign. Many patients are areflexic in one or more areas. For instance, a finding of no umbilical (abdominal wink) reflex on either side is not suspicious in itself, but it should alert the practitioner to evaluate carefully for asymmetrical reflexes in one or more areas, especially if they are ipsilateral. Any positive neurologic findings, especially in the setting of scoliosis, warrant an MRI.1,7
Scoliosis Screening in the School Setting
One controversial subject related to scoliosis is school screenings. These screenings were developed in the 1980s for early identification of children with scoliosis curves so that nonsurgical treatment could be initiated promptly, or that surgery, when deemed necessary, could be performed in a timely fashion for optimal results.27
Since then, the effectiveness of screening programs has come under debate; in 2004, the US Preventive Services Task Force28 issued a recommendation (rating, "D Recommendation") against routine screening for idiopathic scoliosis in asymptomatic adolescents, stating that its effectiveness had not been shown in clinical trials. In response, a task force was convened by the American Academy of Orthopaedic Surgeons, the Scoliosis Research Society, the Pediatric Orthopaedic Society of North America, and the American Academy of Pediatrics, with findings published in a 2008 information statement by Richards and Vitale.25 While acknowledging the limitations of support for screening in the literature, the task force concluded:
- Early treatment of the deformities associated with idiopathic scoliosis can result in substantial benefits
- Screening (whether in the school environment or other settings) offers an opportunity for early diagnosis and appropriate referral of otherwise undetected deformities, especially in under-served populations
- In many cases, brace treatment may preclude the need for surgery in children with significant scoliosis
- Identifying deformities that require surgery as early as possible through screening increases the likelihood of good treatment outcomes
- Girls, who reach adolescence two years earlier than boys, should be screened at ages 10 and 12; boys, at age 13 or 14
- School personnel who conduct screening should receive training to detect spinal deformities
- While no screening test is entirely reliable, the forward bend test is considered the most specific test for detection of scoliosis29
- The participating organizations remain committed to preventing inappropriate use of spinal x-rays.25
Despite the ongoing debate, more than half of the US states have screening programs in place.24,27
RADIOGRAPHS/LABORATORY TESTING
Physical examination and a thorough history are most important in evaluation for scoliosis in the primary care setting. When radiographs (x-rays) are considered necessary, posterior-anterior and lateral spine series should be ordered. These are full-length (3-ft)
x-rays of the patient standing with the knees straight (when the child is able to do so).1,4,7 The x-rays are taken in the posterior-anterior direction to minimize breast and organ radiation exposure and to produce an x-ray similar to that of viewing the patient from behind.
If scoliosis is observed on radiography, note the apex of the curve, the location, the direction in which it curves, and any rotational component. Examine for pelvic obliquity, shoulder asymmetry, spondylosis, or spondylolisthesis.7
The Cobb angle is the measurement used to quantify the degree of scoliosis.4,5,7 Using the posterior-anterior radiograph, a line is drawn along the superior endplate of the most curved cephalad vertebra and the inferior endplate of the caudal vertebra. When perpendicular lines are drawn, the intersecting lines form the Cobb angle (see Figure 3, in which "X" indicates the Cobb angle, measured in degrees). An increasing angle reflects the increasing severity of scoliosis.
Other radiographs that may be ordered include supine lateral (bending) x-rays or supine traction radiographs to evaluate curve flexibility before surgery30; recently, hanging total spine x-rays have been investigated to evaluate spine flexibility before brace treatment.31 Additionally, hand/wrist x-rays may be ordered to determine the patient's remaining growth potential. These are most often taken in an orthopedist's office.3
If a diagnosis of scoliosis is made, Risser staging should be completed. Risser sign is a growth marker noted in adolescents by viewing iliac apophyseal ossification on radiograph. The progress of bone fusion over the iliac apophysis reflects remaining skeletal growth and is graded from 0 to 5, with 0 representing no ossification of the iliac apophysis and 5, complete ossification. The lower the grade noted at curve detection, the greater the risk for curve progression.3,4
Other tests may be warranted. If a patient has persistent back or side pain, especially pain that wakens the patient at night, and/ or pain not relieved by aspirin or NSAIDs, then a bone scan should be ordered to rule out discitis, rib or spine osteoid osteoma, spinal or intradural tumors, osteomyelitis, or other abnormalities.7 An MRI is warranted in children younger than 10; those who have left-sided curves, headaches, or neck pain; patients with quickly progressing curves; and those with any abnormality on neurologic exam. This test is generally not necessary in those with AIS if no family history is present and physical exam findings are unremarkable.1,2,5,7 Laboratory tests are generally not ordered for patients with idiopathic scoliosis unless inflammatory or infectious processes are suspected; standard laboratory tests would then follow. The ScoliScoreTM (Axial Biotech, Salt Lake City), a newer genetic screening test, can be used along with clinical and radiographic testing in patients with AIS. It is felt that specific genes are associated with AIS, and this test may help detect them and determine any associated risk for curve progression.1,3
There are several genetic tests for conditions associated with congenital neuromuscular or syndrome-related scoliosis.12,32 These may be ordered by the primary care provider, an orthopedist, or the geneticist.
REFERRAL
According to some researchers, any child with scoliosis greater than 10° should be referred to an orthopedist, preferably a pediatric spine orthopedist, especially when the patient is younger than 10. Referral is also recommended when the curve is left thoracic, or if any abnormal physical findings or a positive family history is present.4,5,7 There is also support for a strategy in which the practitioner closely observes the patient older than 10 with scoliosis but no other abnormalities, then refers the child once the curve reaches 20° to 25°.7 Referral within three months is most likely indicated for those with typical scoliosis and within one month for atypical scoliosis.7
Each practitioner will develop his or her own comfort level in the management of idiopathic scoliosis. Once a referral has been made, the practitioner should follow up to be sure the patient has seen the specialist in a timely fashion, with no appointments missed. Curve progression can develop rapidly, possibly making treatment more difficult once it begins.
TREATMENT
Three main types of treatment are provided for scoliosis: observation, bracing, and surgery.3,5,7 Observation is generally considered sufficient for immature patients whose curves measure less than 25°; usually no treatment will be needed for mature patients with curves less than 25°. Depending on risk stratification, children will initially need serial radiographs every four to six months.2
Most children will be seen by an orthopedist at this point.2,3,22 Oftentimes orthopedists with adult patients will order radiographs every three to five years to be sure the scoliosis has not progressed.3 Treatment of children with neuromuscular or syndrome-related scoliosis is lengthy and will not be addressed in this review.
Orthotic management (bracing or casting) is usually started once the immature patient's curve is between 25° and 45°. Brace treatment is not used to alter or correct scoliosis, but rather to halt its progression until the patient is skeletally mature (more common), a spinal fusion occurs (less common), or both.5 A 20% to 24% risk for eventual surgery has been reported in patients treated with braces.25,33 In infants or young children with congenital (or other) scoliosis, several temporary management options can be used to prevent curve progression while allowing the child's thorax to grow and expand.2
Several surgical interventions are appropriate for scoliosis. For infants or young children, one surgical intervention is the vertical expandable prosthetic titanium rib (VEPTRTM [Synthes, Inc, West Chester, Pennsylvania]). This is a longitudinal rib distraction device that can attach from rib to rib, rib to spine, or rib to pelvis. The device is indicated in the presence of a constrictive chest wall syndrome, which can decrease lung volumes and cause severe deformity if untreated. The device is lengthened every 4 to 6 months, with the goal to delay a final fusion until skeletal maturity.2
The growing rod, a comparable temporary device, is inserted spine to spine and lengthened similarly at various intervals. Growing rods are usually used in older children with scoliosis who await maturity before undergoing a definitive spinal fusion.2
A newer surgical procedure being used at Morgan Stanley Children's Hospital of NewYork-Presbyterian is spinal stapling.34 This involves minimally invasive surgery to implant inch-long staples across the spinal growth plates, which can stop scoliosis from worsening, or even correct the curve.
Spinal fusion with instrumentation, though used in less than 5% of patients with AIS, is considered the definitive surgical treatment for scoliosis.6 Surgical correction is almost always deemed necessary in immature patients with curves greater than 45° and mature patients with curves greater than 50°.3,7,22 The fusion may involve posterior (and sometimes anterior) discectomy and/or bone grafting between the involved vertebrae to help the bones fuse together so that curves cannot progress. The instrumentation (rods, hooks, wires, and/or screws) is used simply to maintain the spine in the best possible position until the actual fusion occurs; this can take months to a year.2,5,7
Risks and benefits of spinal fusion must be thoroughly reviewed with each parent and patient. The reported neurologic injury and mortality rates are less than 1% and less than 0.02%, respectively, in patients with idiopathic scoliosis who undergo the surgery.7 There is an increased incidence of such concerns in patients with other types of scoliosis, given their comorbid issues.35
Alternative treatments such as physical therapy, yoga, massage, acupuncture, and chiropractic medicine do not put patients at risk, and some have found them beneficial.3
EDUCATION
Both patient and parent must be acquainted with the goal of scoliosis diagnosis and treatment: to prevent curve progression, altered body image, and, ultimately, morbidity. Screening, history, examination, and radiographs are important first steps, with the information gained determining when referral to a pediatric spine orthopedist/specialist (if possible) should be made. Once curves reach 25° to 30°, bracing can successfully prevent curve progression; it will not correct the scoliosis.7 Teens who are resistant or reluctant to wear their brace must be made to understand the consequences of not following the treatment regimen, and the clinician's encouragement is essential.
Patients should be encouraged to stay active, with special emphasis on flexibility. Generally, there is no contraindication regarding activities or sports, unless the patient has undergone a surgical procedure; in this case, the practitioner will determine the patient's optimal level of participation in physical activities.
Surgical treatment with fusion and instrumentation is the only proven scoliosis treatment to correct curves (fully or partially) and prevent progression.3,5,7 Curves that reach 50° without treatment in the fully mature individual may continue to worsen into adulthood.
For patients with scoliosis having a possible familial and/or genetic component,2,3,7 there are several Web sites for patients and parents (see Table 4). Some of these address alternative therapy options.
CONCLUSION
Scoliosis is common, but the sequelae of idiopathic scoliosis can be minimized if the condition is diagnosed early and measures to prevent curve progression are initiated in a timely fashion. The key to diagnosing associated comorbid disorders and other related issues is to conduct a thorough patient and family history and complete physical examination. Timely radiographs are warranted in any patient with a positive finding. Ordering appropriate radiographs accurately is essential to facilitate patient care while reducing radiation exposure. Each practitioner must determine his or her level of comfort in managing patients with idiopathic scoliosis or immediately referring the patient once the diagnosis is made. Early referral is best in the primary care practice.
1. Horan MP, Milbrandt TA. Scoliosis in pediatric patients: comorbid disorders and screening. Pediatr Health. 2009;3(5):451-456.
2. Debnath UK. Current concepts in the management of early-onset idiopathic scoliosis. Pediatr Health. 2010;4(3):343-354.
3. Scoliosis Research Society. Idiopathic scoliosis. www.srs.org/patient_and_family/scoliosis/idiopathic. Accessed July 19, 2012.
4. Taft E, Francis R. Evaluation and management of scoliosis. J Pediatr Health Care. 2003;17(1):42- 44.
5. Speigel DA, Sarwark JF. Pediatric orthopaedics. In: Sarwark JF, ed. Essentials of Musculoskeletal Care. 4th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010:976-1201.
6. Horn PL, Beebe AC. Scoliosis: incidence and current medical and surgical interventions. Heartbeat. 2008;19(3):6-7,17.
7. Janicki JA. Scoliosis. In: Miller MD, Hart JA, MacKnight JM. Essential Orthopaedics. Marietta, GA: Saunders/Elsevier; 2010:881-886.
8. James JIP. Idiopathic scoliosis; the prognosis, diagnosis, and operative indications related to curve patterns and the age at onset. J Bone Joint Surg Br. 1954;36-B(1):36-49.
9. Scoliosis. In: Herring JA. Tachdjian's Pediatric Orthopaedics. Vol I. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2008:265-411.
10. Debnath UK, Goel V, Harshavarfhana N, Webb JK. Congenital scoliosis - Quo vadis? Indian J Orthop. 2010;44(2):137-147.
11. Basu PS, Elsebaie H, Noordeen MH. Congenital spine deformity: a comprehensive assessment at presentation. Spine (Phila Pa 1976). 2002;27(20):2255-2259.
12. Fisher K, Esham RH, Thorneycroft I. Scoliosis associated with typical Mayer-Rokitansky-Küster-Hauser syndrome. South Med J. 2000;93(2):243-246.
13. Hedequist D, Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop. 2007;27(1):106-116.
14. Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis: a study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976). 1992;17(9):1091-1096.
15. Ferguson RL. Medical and congenital comorbidities associated with spinal deformities in the immature spine. J Bone Joint Surg Am. 2007;89(suppl 1):34-41.
16. Demetracopoulos CA, Sponseller PD. Spinal deformities in Marfan syndrome. Orthop Clin North Am. 2007;38(4):563-572.
17. Crawford AH, Herrera-Soto J. Scoliosis associated with neurofibromatosis. Orthop Clin North Am. 2007;38(4):553-562.
18. Disorders of the spinal cord. In: Herring JA. Tachdjian's Pediatric Orthopaedics. Vol II. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2008:1405-1482.
19. Ruiz-Iban MA, Burgos J, Aguado HJ, et al. Scoliosis after median sternotomy in children with congenital heart disease. Spine (Phila Pa 1976). 2005;30(8):E214-E218.
20. Dimeglio A. Growth of the spine before age 5 years. J Pediatr Orthop B. 1993;1:102-107.
21. Lloyd-Roberts GC, Pilcher MF. Structural idiopathic scoliosis in infancy: a study of natural history of 100 patients. J Bone Joint Surg Br. 1965;47:520-523.
22. Gutknecht S, Lonstein J, Novacheck T. Adolescent idiopathic scoliosis. Pediatric Perspective. 2009;18(4):1-4.
23. Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg. 2007;43(3):236-248.
24. Bunge EM, Juttmann RE, van Biezen FC, et al; Netherlands Evaluation Study on Screening for Scoliosis (NESCIO) Group. Estimating the effectiveness of screening for scoliosis: a case-control study. Pediatrics. 2008;121(1):9-14.
25. Richards BS, Vitale MG. Screening for idiopathic scoliosis in adolescents: an information statement. J Bone Joint Surg. 2008;90(1):195-198.
26. Côté P, Kreitz BG, Cassidy JD, et al. A study of the diagnostic accuracy and reliability of the scoliometer and Adam's forward bend test. Spine. 1998;23(7):796-802.
27. Richards BS, Beaty JH, Thompson GH, Willis RB. Estimating the effectiveness of screening for scoliosis. Pediatrics. 2008:121(6):1297-1298.
28. US Preventive Services Task Force. Screening for idiopathic scoliosis in adolescents: recommendation statement (2004). www.uspreventiveservicestaskforce.org/3rduspstf/scoliosis/scoliors.htm. Accessed July 19, 2012.
29. Karachalios T, Sofianos J, Roidis N, et al. Ten-year follow-up evaluation of a school screening program for scoliosis. Is the forward-bending test an accurate diagnostic criterion for the screening of scoliosis? Spine (Phila Pa 1976). 1999;24(22):2318-2324.
30. Hamzaoglu A, Talu U, Tezer M, et al. Assessment of curve flexibility in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2005;30(14):1627-1642.
31. Kuroki H, Inomata N, Hamanaka H, et al. Significance of hanging total spine x-ray to estimate the indicative correction angle by brace wearing in idiopathic scoliosis patients. Scoliosis. 2012;7:8.
32. Al-Otibi M, Rutka JT. Neurosurgical implications of neurofibromatosis type I in children. Neurosurg Focus. 2006;20(1):E2.
33. Dolan LA, Weinstein SL. Surgical rates after observation and bracing for adolescent idiopathic scoliosis: an evidence-based review. Spine (Phila Pa 1976). 2007;32(19 suppl):S91-S100.
34. American Academy of Pediatrics. New stapling treatment may help reverse scoliosis: report from the Morgan Stanley Children's Hospital Department of Pediatric Orthopaedics. Section on Orthopaedics Newsletter. Spring 2009. www2.aap.org/sections/ortho/spring2009news.pdf. Accessed July 19, 2012.
35. Reames DL, Smith JS, Fu KG, et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis. Spine. 2011;36(18):1484-1491.
A majority of practitioners will at some time encounter scoliosis, the most common spinal deformity seen in pediatric practice.1 This musculoskeletal condition can be found incidentally during routine exams, in the urgent care setting, in the emergency department (ED), or on chest x-rays or other radiographs. In primary care, scoliosis may be overlooked, particularly when the etiology is idiopathic or when well-child visits or routine back exams have been missed.1
The focus of this review will be on early identification of affected patients (most commonly those with adolescent idiopathic scoliosis), with emphasis on evaluation through an excellent physical examination and ordering of appropriate radiographs. Watchful waiting and monitoring are the norm; however, implementing carefully selected treatment options, such as bracing, or making an appropriate referral may make it possible to slow or stop curve progression.1-4
SCOLIOSIS: THE DEFINITION
Scoliosis is a coronal curvature (lateral deviation of the spine) of more than 10°, usually with a rotational component.1,2 A healthy spine is straight in the frontal plane, but curved in the sagittal plane. Normal circumstances include thoracic kyphosis (outward curve of the upper back) and lumbar lordosis (inward curve of the lower back).5 The scoliotic spine deviates away from the midline, and maximal rotation is at the apex of the curve. The apex or point of the curve defines its center, that is, the most laterally deviated vertebra or disc. The first curve to develop is the primary curve; however, when a patient presents with two or three curves, it is difficult to determine which curve is primary.
The secondary curve is a compensatory curve, which develops as a means to balance the head and trunk over the pelvis. A similar compensation may occur in the sagittal plane, exacerbating the normal kyphosis or lordosis or possibly causing rib or scapular prominences.6 Cervical curves can also develop but are more commonly associated with conditions other than idiopathic scoliosis.3 Cervical scoliosis will not be reviewed here.
THE EPIDEMIOLOGY OF SCOLIOSIS TYPES
Eighty percent of all scoliosis patients are diagnosed with idiopathic scoliosis (which may be present in 2% to 3% of the population).1,5 The remaining 20% are diagnosed with congenital, neuromuscular, or syndrome-related spinal deformities.1 The overall prevalence of idiopathic scoliosis (with curves greater than 10°) is 1 to 3 per 100, with a comparable proportion of girls and boys. The prevalence of those with curves greater than 30° is 1 to 3 per 1,000, with a boy-girl ratio of 1:8.7 In the child who is skeletally mature, scoliosis curves measuring less than 30° usually do not progress. However, most curves greater than 50° tend to progress at about 1° per year.7
Idiopathic scoliosis is broken down into age ranges.8Infantile scoliosis is diagnosed from age 0 to 3 years and comprises 0.5% of cases; juvenile scoliosis, with an age range of 4 to 9 years, represents 10.5% of cases; and adolescent scoliosis, diagnosed any time after age 10, accounts for 89% of cases.7 The four main types of scoliosis will be discussed.
Congenital
Congenital scoliosis is defined as a malformed or segmented portion of the spine, a failure of formation of a portion of the spine, or a combination of the two.9 This results in block vertebrae or hemivertebrae, or possibly the fusing of two vertebrae with a bony bridge. Because the associated curves are often rigid, they can be resistant to correction. Worldwide, prevalence of congenital vertebral anomalies is 0.5 to 1.0 per 1,000 live births.10
Congenital scoliosis can be associated with many other congenital abnormalities, as embryonic development of the spine occurs at the same time as that of the heart, kidneys, bowel, and bladder.1 Congenital scoliosis can occur with any of these defects or in the setting of a syndrome, such as VACTERL1,9-11 (ie, vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal defects, and limb abnormalities1; see Table 11,9,11,12). If even one such defect or abnormality is found, associated comorbid conditions should be actively sought.1
In many instances, these abnormalities are noted when children are young; in children younger than 5 years, MRI is warranted, and these patients almost always need sedation during imaging. In the young child who also exhibits urologic issues, an MRI work-up for genitourinary defects can be performed at the same time.1
Patients with abnormal cardiac findings (eg, heart murmur) should be referred for a cardiology work-up.11,13 Chest wall deformities may also be present in children with congenital scoliosis. Multiple congenital rib fusions can cause chest wall constriction, possibly inhibiting growth and lung development. Untreated, this condition can ultimately shorten a child’s lifespan.14
Neuromuscular
Spinal curvature in a patient with a neuromuscular disease is referred to as neuromuscular scoliosis (see Table 2).1,4,5 Most diagnoses within this category are genetic in nature, although some can result from a traumatic brain injury and/or anoxia (eg, cerebral palsy). Given that this disease state is detected early in most patients’ lives, they are generally followed by an orthopedist (or more specifically, by a pediatric orthopedist) once necessary testing, such as radiography or MRI, has been completed.1,15
Syndrome-related
Patients may have certain genetic conditions or syndromes that put them at high risk for scoliosis.
The two conditions most commonly associated with scoliosis are Marfan syndrome (60%) and neurofibromatosis (10% to 15%).1 Many patients in both groups will require MRI for evaluation; given that they are at high risk for lumbar spondylolisthesis (slippage between two lumbar vertebrae), care should be taken to evaluate for this possible secondary finding.16,17 It should be noted that patients with Marfan syndrome are at high risk for cardiac abnormalities, and those with neurofibromatosis, for dural ectasia and/ or Chiari malformations (which would be detected by MRI).16,18
Patients who have undergone chest wall surgery, such as thoracotomy for congenital heart defect repair, should be evaluated for scoliosis. The incidence of scoliosis is elevated in patients with heart defects, and the association is even greater in those who have had chest wall surgery, especially before age 18 months.19
IDIOPATHIC SCOLIOSIS
Because, by definition, no cause is known, idiopathic scoliosis is considered a diagnosis of exclusion. Affected patients have a normal neurologic exam and usually have no presenting symptoms or evidence of spinal abnormalities.1 Since 1954, idiopathic scoliosis has been divided into three age-groups, as these periods correspond to phases of increased growth velocity that occur during childhood and adolescence.8,20
Infant
A diagnosis of early-onset scoliosis is made when a child presents with scoliosis before age 5; any affected child between 0 and 3 years of age may be included in the infant scoliosis classification.2 All congenital, neuromuscular, and syndrome-related causes will have been excluded. It is felt among many experts that there is always a genetic or familial component to scoliosis. Therefore, when a child's family history is positive for scoliosis, the child should be aggressively screened, and the family should be educated to observe for signs of scoliosis and seek practitioner involvement as soon as any are evident.2
A thorough examination should be completed to rule out organ involvement in the presence of infant scoliosis.1 It is recommended that any infant with scoliotic curves greater than 20° undergo MRI of the head and spine to rule out central nervous system lesions or structural abnormalities. The risk for respiratory failure-related morbidity and mortality is increased in children with untreated early-onset scoliosis who experience curve progression. Rare progressive curves often can be addressed surgically, preserving spine and trunk growth while also achieving curve correction.2
In the majority of cases of infant scoliosis (75% to 90%), curves are left-sided (levoscoliosis)—in contrast to the right-sided curves (dextroscoliosis) more commonly noted in late-onset (juvenile or adolescent) scoliosis3,21 (see Figure 1). Infant scoliosis is slightly more common in boys (60%) than in girls.21
Neither curve lateralization nor gender makes a difference in treatment.2 Approximately 90% of early-onset curves resolve spontaneously, especially in those who are diagnosed before age 1 year. Progression occurring at a rate of 2° to 3° per year is considered gradual, whereas malignant progression often worsens by 10° or more per year, with the condition possibly becoming severe and disabling.2,21
Any infant given a diagnosis of scoliosis by a practitioner other than a pediatric orthopedist should be referred as soon as possible. On referral, copies of previously obtained radiographs and/or MRI should be presented or sent to the orthopedic practitioner, along with the history and physical exam findings, to avoid the need for repeated imaging.
Juvenile
Juvenile idiopathic scoliosis is diagnosed in patients between ages 4 and 9. Presentation in younger members of this age-group tends to resemble that in infantile idiopathic scoliosis, and children in the middle to upper age range tend to present as adolescent patients do.3 Most juvenile patients' curves will resolve without treatment (especially younger children's).
It is recommended that children with juvenile idiopathic scoliosis undergo MRI to rule out brain or spinal abnormalities. In up to 20% of children younger than 10 with curves exceeding 20°, findings on MRI are abnormal.3 Observation with close monitoring of curve progression is key in this population, especially if rapid progression is evident. In children with juvenile idiopathic scoliosis and rapid-onset curve progression, severe trunk deformity or pulmonary and/or cardiac compromise can develop. Curves that reach 30° almost always continue to worsen unless treatment is given.3,7 Timely referral to a pediatric spine orthopedist is warranted for children in this age-group.
Adolescent
It is during adolescence (ages 10 to 18) that idiopathic scoliosis is most common. Approximately 2% to 3% of children ages 10 to 15 have scoliosis with a curve of at least 10°. Among these patients, however, just 5% have scoliosis of clinical significance to warrant treatment (ie, curve progression > 30°).5,22
Each child should be examined initially for nonstructural scoliosis. This includes postural scoliosis, which resolves when a child is recumbent; compensatory scoliosis, which can be the result of a leg-length discrepancy (with no fixed vertebral rotation); sciatic scoliosis, an abnormality that results from avoiding the pain of an irritated sciatic nerve; inflammatory scoliosis, which could result from an infectious process, such as acute appendicitis; and hysterical scoliosis, a very rare form with an underlying psychological factor.4
Structural scoliosis comprises the remaining types of scoliosis, including the idiopathic variety. Patients with adolescent idiopathic scoliosis (AIS) are older than 10 and usually undergoing a rapid growth phase. Thus, some adolescent patients' curves can progress rapidly.4
Adolescents with idiopathic scoliosis rarely complain of pain or neurologic symptoms. Generally, their spinal curves do not cause organ pressure or shortness of breath. Many patients with AIS are involved in extracurricular activities that can trigger low back pain, yet activity-related low back pain is not an uncommon complaint among teens in general. Therefore, it is felt that when a patient with AIS has intermittent low back pain, it is most likely not related to their curve(s). Nevertheless, if a patient with AIS develops continued or unexplained low back pain and/or new neurologic symptoms, MRI is warranted.3
HISTORY, PHYSICAL EXAMINATION, AND DIAGNOSIS
During the exam, elicit any family history of scoliosis, especially in the setting of known diseases, such as Marfan syndrome, neurofibromatosis, muscular dystrophy, or other related diseases. Approximately 30% of AIS patients have a family history of scoliosis, so although the cause may not be clear, some genetic component is possible.3 The examination should stem from the patient's current stage of growth and development (see Table 31,3,4,7,23). The diagnosis of scoliosis is typically made during the examination and confirmed by radiography.
The newborn patient should be kept warm but be wearing only a diaper during the exam. A child or adolescent should be undressed and wearing a patient gown (unless at a school screening).
A general observation should be done initially, including the child's overall shape. The examiner should look for marfanoid features, such as excessive height, long arms, long trunk, and joint laxity.7 In the examination of the feet, an extremely high arch or cavus foot may be indicative of tethered spinal cord, spinal syrinx, or Charcot-Marie-Tooth disease, especially if the cavus is unilateral.1,7,23 MRI would be warranted in these instances.1
The examining clinician should note the child's Tanner stage. Height and weight measurements should be taken, and for a girl, age at menarche should be recorded.4
Next, the skin should be carefully inspected. In the infant, the examiner should look for hairy patches at the base of the spine, cutaneous sinuses, sacral dimpling, or other skin lesions that may suggest spinal dysraphism (ie, failure of the posterior elements to fuse around the spinal cord).4 Examination for café au lait spots and/or freckling in the axilla or groin area should be included, as this could signify neurofibromatosis.1,4,7
Examine the back in a standing child. Note whether the head appears to be centered over the sacrum. Evaluate for shoulder height or truncal asymmetry, scapular or posterior rib prominence, and excessive thoracic kyphosis and/or lumbar lordosis. Examine the pelvic bones, looking for obliquity (ie, one side higher than the other). Look for leg length discrepancy, both while the patient is standing still and during a gait review.1
During an anterior exam, look for shoulder or truncal asymmetry, leg length discrepancy, and anterior rib prominence. If shoulder height asymmetry is apparent, measure from each acromioclavicular joint to the floor and note the difference in centimeters. In the case of pelvic obliquity, measure from each anterior (or posterior) superior iliac spine to the floor and note the difference in centimeters. If the head does not appear to align over the sacrum, lower a plum line from the spinous process of C7 down to the gluteal cleft level. Measure by centimeters the distance from the cleft to the line, noting the side to which the line sways.
View the patient from the side. The sagittal curve can be assessed from the occiput to the sacrum; observe for an increased lordotic or kyphotic curve or abnormal positioning related to abnormal curves.4
The Adams forward bend test should be conducted.24,25 The child who is able to stand faces away from the examiner, then bends forward at the waist with the knees straight and the arms hanging down passively (see Figure 2). The practitioner looks at the spine from behind and notes any rib, thoracic, scapular, or lumbar prominence that could indicate a rotational spine deformity.1,4,7 In some instances, the use of a scoliometer can be helpful to assign a number to the amount of rotation to the spine during the Adams forward bend test. However, primary care offices are not often equipped with this device.26
Motor and Neurologic Examinations
The motor exam includes testing motor strength in all four extremities and noting any asymmetry. The examiner should have the child actively bend in every direction, making note of any deformity, stiffness, or asymmetry. The patient's spine should be palpated, with any tenderness noted, and any available radiographs reviewed.1,4,7
The neurologic exam includes testing cranial nerves, sensation, and reflexes, including the patellar, Achilles, and umbilical reflexes, as well as Babinski's sign. Many patients are areflexic in one or more areas. For instance, a finding of no umbilical (abdominal wink) reflex on either side is not suspicious in itself, but it should alert the practitioner to evaluate carefully for asymmetrical reflexes in one or more areas, especially if they are ipsilateral. Any positive neurologic findings, especially in the setting of scoliosis, warrant an MRI.1,7
Scoliosis Screening in the School Setting
One controversial subject related to scoliosis is school screenings. These screenings were developed in the 1980s for early identification of children with scoliosis curves so that nonsurgical treatment could be initiated promptly, or that surgery, when deemed necessary, could be performed in a timely fashion for optimal results.27
Since then, the effectiveness of screening programs has come under debate; in 2004, the US Preventive Services Task Force28 issued a recommendation (rating, "D Recommendation") against routine screening for idiopathic scoliosis in asymptomatic adolescents, stating that its effectiveness had not been shown in clinical trials. In response, a task force was convened by the American Academy of Orthopaedic Surgeons, the Scoliosis Research Society, the Pediatric Orthopaedic Society of North America, and the American Academy of Pediatrics, with findings published in a 2008 information statement by Richards and Vitale.25 While acknowledging the limitations of support for screening in the literature, the task force concluded:
- Early treatment of the deformities associated with idiopathic scoliosis can result in substantial benefits
- Screening (whether in the school environment or other settings) offers an opportunity for early diagnosis and appropriate referral of otherwise undetected deformities, especially in under-served populations
- In many cases, brace treatment may preclude the need for surgery in children with significant scoliosis
- Identifying deformities that require surgery as early as possible through screening increases the likelihood of good treatment outcomes
- Girls, who reach adolescence two years earlier than boys, should be screened at ages 10 and 12; boys, at age 13 or 14
- School personnel who conduct screening should receive training to detect spinal deformities
- While no screening test is entirely reliable, the forward bend test is considered the most specific test for detection of scoliosis29
- The participating organizations remain committed to preventing inappropriate use of spinal x-rays.25
Despite the ongoing debate, more than half of the US states have screening programs in place.24,27
RADIOGRAPHS/LABORATORY TESTING
Physical examination and a thorough history are most important in evaluation for scoliosis in the primary care setting. When radiographs (x-rays) are considered necessary, posterior-anterior and lateral spine series should be ordered. These are full-length (3-ft)
x-rays of the patient standing with the knees straight (when the child is able to do so).1,4,7 The x-rays are taken in the posterior-anterior direction to minimize breast and organ radiation exposure and to produce an x-ray similar to that of viewing the patient from behind.
If scoliosis is observed on radiography, note the apex of the curve, the location, the direction in which it curves, and any rotational component. Examine for pelvic obliquity, shoulder asymmetry, spondylosis, or spondylolisthesis.7
The Cobb angle is the measurement used to quantify the degree of scoliosis.4,5,7 Using the posterior-anterior radiograph, a line is drawn along the superior endplate of the most curved cephalad vertebra and the inferior endplate of the caudal vertebra. When perpendicular lines are drawn, the intersecting lines form the Cobb angle (see Figure 3, in which "X" indicates the Cobb angle, measured in degrees). An increasing angle reflects the increasing severity of scoliosis.
Other radiographs that may be ordered include supine lateral (bending) x-rays or supine traction radiographs to evaluate curve flexibility before surgery30; recently, hanging total spine x-rays have been investigated to evaluate spine flexibility before brace treatment.31 Additionally, hand/wrist x-rays may be ordered to determine the patient's remaining growth potential. These are most often taken in an orthopedist's office.3
If a diagnosis of scoliosis is made, Risser staging should be completed. Risser sign is a growth marker noted in adolescents by viewing iliac apophyseal ossification on radiograph. The progress of bone fusion over the iliac apophysis reflects remaining skeletal growth and is graded from 0 to 5, with 0 representing no ossification of the iliac apophysis and 5, complete ossification. The lower the grade noted at curve detection, the greater the risk for curve progression.3,4
Other tests may be warranted. If a patient has persistent back or side pain, especially pain that wakens the patient at night, and/ or pain not relieved by aspirin or NSAIDs, then a bone scan should be ordered to rule out discitis, rib or spine osteoid osteoma, spinal or intradural tumors, osteomyelitis, or other abnormalities.7 An MRI is warranted in children younger than 10; those who have left-sided curves, headaches, or neck pain; patients with quickly progressing curves; and those with any abnormality on neurologic exam. This test is generally not necessary in those with AIS if no family history is present and physical exam findings are unremarkable.1,2,5,7 Laboratory tests are generally not ordered for patients with idiopathic scoliosis unless inflammatory or infectious processes are suspected; standard laboratory tests would then follow. The ScoliScoreTM (Axial Biotech, Salt Lake City), a newer genetic screening test, can be used along with clinical and radiographic testing in patients with AIS. It is felt that specific genes are associated with AIS, and this test may help detect them and determine any associated risk for curve progression.1,3
There are several genetic tests for conditions associated with congenital neuromuscular or syndrome-related scoliosis.12,32 These may be ordered by the primary care provider, an orthopedist, or the geneticist.
REFERRAL
According to some researchers, any child with scoliosis greater than 10° should be referred to an orthopedist, preferably a pediatric spine orthopedist, especially when the patient is younger than 10. Referral is also recommended when the curve is left thoracic, or if any abnormal physical findings or a positive family history is present.4,5,7 There is also support for a strategy in which the practitioner closely observes the patient older than 10 with scoliosis but no other abnormalities, then refers the child once the curve reaches 20° to 25°.7 Referral within three months is most likely indicated for those with typical scoliosis and within one month for atypical scoliosis.7
Each practitioner will develop his or her own comfort level in the management of idiopathic scoliosis. Once a referral has been made, the practitioner should follow up to be sure the patient has seen the specialist in a timely fashion, with no appointments missed. Curve progression can develop rapidly, possibly making treatment more difficult once it begins.
TREATMENT
Three main types of treatment are provided for scoliosis: observation, bracing, and surgery.3,5,7 Observation is generally considered sufficient for immature patients whose curves measure less than 25°; usually no treatment will be needed for mature patients with curves less than 25°. Depending on risk stratification, children will initially need serial radiographs every four to six months.2
Most children will be seen by an orthopedist at this point.2,3,22 Oftentimes orthopedists with adult patients will order radiographs every three to five years to be sure the scoliosis has not progressed.3 Treatment of children with neuromuscular or syndrome-related scoliosis is lengthy and will not be addressed in this review.
Orthotic management (bracing or casting) is usually started once the immature patient's curve is between 25° and 45°. Brace treatment is not used to alter or correct scoliosis, but rather to halt its progression until the patient is skeletally mature (more common), a spinal fusion occurs (less common), or both.5 A 20% to 24% risk for eventual surgery has been reported in patients treated with braces.25,33 In infants or young children with congenital (or other) scoliosis, several temporary management options can be used to prevent curve progression while allowing the child's thorax to grow and expand.2
Several surgical interventions are appropriate for scoliosis. For infants or young children, one surgical intervention is the vertical expandable prosthetic titanium rib (VEPTRTM [Synthes, Inc, West Chester, Pennsylvania]). This is a longitudinal rib distraction device that can attach from rib to rib, rib to spine, or rib to pelvis. The device is indicated in the presence of a constrictive chest wall syndrome, which can decrease lung volumes and cause severe deformity if untreated. The device is lengthened every 4 to 6 months, with the goal to delay a final fusion until skeletal maturity.2
The growing rod, a comparable temporary device, is inserted spine to spine and lengthened similarly at various intervals. Growing rods are usually used in older children with scoliosis who await maturity before undergoing a definitive spinal fusion.2
A newer surgical procedure being used at Morgan Stanley Children's Hospital of NewYork-Presbyterian is spinal stapling.34 This involves minimally invasive surgery to implant inch-long staples across the spinal growth plates, which can stop scoliosis from worsening, or even correct the curve.
Spinal fusion with instrumentation, though used in less than 5% of patients with AIS, is considered the definitive surgical treatment for scoliosis.6 Surgical correction is almost always deemed necessary in immature patients with curves greater than 45° and mature patients with curves greater than 50°.3,7,22 The fusion may involve posterior (and sometimes anterior) discectomy and/or bone grafting between the involved vertebrae to help the bones fuse together so that curves cannot progress. The instrumentation (rods, hooks, wires, and/or screws) is used simply to maintain the spine in the best possible position until the actual fusion occurs; this can take months to a year.2,5,7
Risks and benefits of spinal fusion must be thoroughly reviewed with each parent and patient. The reported neurologic injury and mortality rates are less than 1% and less than 0.02%, respectively, in patients with idiopathic scoliosis who undergo the surgery.7 There is an increased incidence of such concerns in patients with other types of scoliosis, given their comorbid issues.35
Alternative treatments such as physical therapy, yoga, massage, acupuncture, and chiropractic medicine do not put patients at risk, and some have found them beneficial.3
EDUCATION
Both patient and parent must be acquainted with the goal of scoliosis diagnosis and treatment: to prevent curve progression, altered body image, and, ultimately, morbidity. Screening, history, examination, and radiographs are important first steps, with the information gained determining when referral to a pediatric spine orthopedist/specialist (if possible) should be made. Once curves reach 25° to 30°, bracing can successfully prevent curve progression; it will not correct the scoliosis.7 Teens who are resistant or reluctant to wear their brace must be made to understand the consequences of not following the treatment regimen, and the clinician's encouragement is essential.
Patients should be encouraged to stay active, with special emphasis on flexibility. Generally, there is no contraindication regarding activities or sports, unless the patient has undergone a surgical procedure; in this case, the practitioner will determine the patient's optimal level of participation in physical activities.
Surgical treatment with fusion and instrumentation is the only proven scoliosis treatment to correct curves (fully or partially) and prevent progression.3,5,7 Curves that reach 50° without treatment in the fully mature individual may continue to worsen into adulthood.
For patients with scoliosis having a possible familial and/or genetic component,2,3,7 there are several Web sites for patients and parents (see Table 4). Some of these address alternative therapy options.
CONCLUSION
Scoliosis is common, but the sequelae of idiopathic scoliosis can be minimized if the condition is diagnosed early and measures to prevent curve progression are initiated in a timely fashion. The key to diagnosing associated comorbid disorders and other related issues is to conduct a thorough patient and family history and complete physical examination. Timely radiographs are warranted in any patient with a positive finding. Ordering appropriate radiographs accurately is essential to facilitate patient care while reducing radiation exposure. Each practitioner must determine his or her level of comfort in managing patients with idiopathic scoliosis or immediately referring the patient once the diagnosis is made. Early referral is best in the primary care practice.
A majority of practitioners will at some time encounter scoliosis, the most common spinal deformity seen in pediatric practice.1 This musculoskeletal condition can be found incidentally during routine exams, in the urgent care setting, in the emergency department (ED), or on chest x-rays or other radiographs. In primary care, scoliosis may be overlooked, particularly when the etiology is idiopathic or when well-child visits or routine back exams have been missed.1
The focus of this review will be on early identification of affected patients (most commonly those with adolescent idiopathic scoliosis), with emphasis on evaluation through an excellent physical examination and ordering of appropriate radiographs. Watchful waiting and monitoring are the norm; however, implementing carefully selected treatment options, such as bracing, or making an appropriate referral may make it possible to slow or stop curve progression.1-4
SCOLIOSIS: THE DEFINITION
Scoliosis is a coronal curvature (lateral deviation of the spine) of more than 10°, usually with a rotational component.1,2 A healthy spine is straight in the frontal plane, but curved in the sagittal plane. Normal circumstances include thoracic kyphosis (outward curve of the upper back) and lumbar lordosis (inward curve of the lower back).5 The scoliotic spine deviates away from the midline, and maximal rotation is at the apex of the curve. The apex or point of the curve defines its center, that is, the most laterally deviated vertebra or disc. The first curve to develop is the primary curve; however, when a patient presents with two or three curves, it is difficult to determine which curve is primary.
The secondary curve is a compensatory curve, which develops as a means to balance the head and trunk over the pelvis. A similar compensation may occur in the sagittal plane, exacerbating the normal kyphosis or lordosis or possibly causing rib or scapular prominences.6 Cervical curves can also develop but are more commonly associated with conditions other than idiopathic scoliosis.3 Cervical scoliosis will not be reviewed here.
THE EPIDEMIOLOGY OF SCOLIOSIS TYPES
Eighty percent of all scoliosis patients are diagnosed with idiopathic scoliosis (which may be present in 2% to 3% of the population).1,5 The remaining 20% are diagnosed with congenital, neuromuscular, or syndrome-related spinal deformities.1 The overall prevalence of idiopathic scoliosis (with curves greater than 10°) is 1 to 3 per 100, with a comparable proportion of girls and boys. The prevalence of those with curves greater than 30° is 1 to 3 per 1,000, with a boy-girl ratio of 1:8.7 In the child who is skeletally mature, scoliosis curves measuring less than 30° usually do not progress. However, most curves greater than 50° tend to progress at about 1° per year.7
Idiopathic scoliosis is broken down into age ranges.8Infantile scoliosis is diagnosed from age 0 to 3 years and comprises 0.5% of cases; juvenile scoliosis, with an age range of 4 to 9 years, represents 10.5% of cases; and adolescent scoliosis, diagnosed any time after age 10, accounts for 89% of cases.7 The four main types of scoliosis will be discussed.
Congenital
Congenital scoliosis is defined as a malformed or segmented portion of the spine, a failure of formation of a portion of the spine, or a combination of the two.9 This results in block vertebrae or hemivertebrae, or possibly the fusing of two vertebrae with a bony bridge. Because the associated curves are often rigid, they can be resistant to correction. Worldwide, prevalence of congenital vertebral anomalies is 0.5 to 1.0 per 1,000 live births.10
Congenital scoliosis can be associated with many other congenital abnormalities, as embryonic development of the spine occurs at the same time as that of the heart, kidneys, bowel, and bladder.1 Congenital scoliosis can occur with any of these defects or in the setting of a syndrome, such as VACTERL1,9-11 (ie, vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal defects, and limb abnormalities1; see Table 11,9,11,12). If even one such defect or abnormality is found, associated comorbid conditions should be actively sought.1
In many instances, these abnormalities are noted when children are young; in children younger than 5 years, MRI is warranted, and these patients almost always need sedation during imaging. In the young child who also exhibits urologic issues, an MRI work-up for genitourinary defects can be performed at the same time.1
Patients with abnormal cardiac findings (eg, heart murmur) should be referred for a cardiology work-up.11,13 Chest wall deformities may also be present in children with congenital scoliosis. Multiple congenital rib fusions can cause chest wall constriction, possibly inhibiting growth and lung development. Untreated, this condition can ultimately shorten a child’s lifespan.14
Neuromuscular
Spinal curvature in a patient with a neuromuscular disease is referred to as neuromuscular scoliosis (see Table 2).1,4,5 Most diagnoses within this category are genetic in nature, although some can result from a traumatic brain injury and/or anoxia (eg, cerebral palsy). Given that this disease state is detected early in most patients’ lives, they are generally followed by an orthopedist (or more specifically, by a pediatric orthopedist) once necessary testing, such as radiography or MRI, has been completed.1,15
Syndrome-related
Patients may have certain genetic conditions or syndromes that put them at high risk for scoliosis.
The two conditions most commonly associated with scoliosis are Marfan syndrome (60%) and neurofibromatosis (10% to 15%).1 Many patients in both groups will require MRI for evaluation; given that they are at high risk for lumbar spondylolisthesis (slippage between two lumbar vertebrae), care should be taken to evaluate for this possible secondary finding.16,17 It should be noted that patients with Marfan syndrome are at high risk for cardiac abnormalities, and those with neurofibromatosis, for dural ectasia and/ or Chiari malformations (which would be detected by MRI).16,18
Patients who have undergone chest wall surgery, such as thoracotomy for congenital heart defect repair, should be evaluated for scoliosis. The incidence of scoliosis is elevated in patients with heart defects, and the association is even greater in those who have had chest wall surgery, especially before age 18 months.19
IDIOPATHIC SCOLIOSIS
Because, by definition, no cause is known, idiopathic scoliosis is considered a diagnosis of exclusion. Affected patients have a normal neurologic exam and usually have no presenting symptoms or evidence of spinal abnormalities.1 Since 1954, idiopathic scoliosis has been divided into three age-groups, as these periods correspond to phases of increased growth velocity that occur during childhood and adolescence.8,20
Infant
A diagnosis of early-onset scoliosis is made when a child presents with scoliosis before age 5; any affected child between 0 and 3 years of age may be included in the infant scoliosis classification.2 All congenital, neuromuscular, and syndrome-related causes will have been excluded. It is felt among many experts that there is always a genetic or familial component to scoliosis. Therefore, when a child's family history is positive for scoliosis, the child should be aggressively screened, and the family should be educated to observe for signs of scoliosis and seek practitioner involvement as soon as any are evident.2
A thorough examination should be completed to rule out organ involvement in the presence of infant scoliosis.1 It is recommended that any infant with scoliotic curves greater than 20° undergo MRI of the head and spine to rule out central nervous system lesions or structural abnormalities. The risk for respiratory failure-related morbidity and mortality is increased in children with untreated early-onset scoliosis who experience curve progression. Rare progressive curves often can be addressed surgically, preserving spine and trunk growth while also achieving curve correction.2
In the majority of cases of infant scoliosis (75% to 90%), curves are left-sided (levoscoliosis)—in contrast to the right-sided curves (dextroscoliosis) more commonly noted in late-onset (juvenile or adolescent) scoliosis3,21 (see Figure 1). Infant scoliosis is slightly more common in boys (60%) than in girls.21
Neither curve lateralization nor gender makes a difference in treatment.2 Approximately 90% of early-onset curves resolve spontaneously, especially in those who are diagnosed before age 1 year. Progression occurring at a rate of 2° to 3° per year is considered gradual, whereas malignant progression often worsens by 10° or more per year, with the condition possibly becoming severe and disabling.2,21
Any infant given a diagnosis of scoliosis by a practitioner other than a pediatric orthopedist should be referred as soon as possible. On referral, copies of previously obtained radiographs and/or MRI should be presented or sent to the orthopedic practitioner, along with the history and physical exam findings, to avoid the need for repeated imaging.
Juvenile
Juvenile idiopathic scoliosis is diagnosed in patients between ages 4 and 9. Presentation in younger members of this age-group tends to resemble that in infantile idiopathic scoliosis, and children in the middle to upper age range tend to present as adolescent patients do.3 Most juvenile patients' curves will resolve without treatment (especially younger children's).
It is recommended that children with juvenile idiopathic scoliosis undergo MRI to rule out brain or spinal abnormalities. In up to 20% of children younger than 10 with curves exceeding 20°, findings on MRI are abnormal.3 Observation with close monitoring of curve progression is key in this population, especially if rapid progression is evident. In children with juvenile idiopathic scoliosis and rapid-onset curve progression, severe trunk deformity or pulmonary and/or cardiac compromise can develop. Curves that reach 30° almost always continue to worsen unless treatment is given.3,7 Timely referral to a pediatric spine orthopedist is warranted for children in this age-group.
Adolescent
It is during adolescence (ages 10 to 18) that idiopathic scoliosis is most common. Approximately 2% to 3% of children ages 10 to 15 have scoliosis with a curve of at least 10°. Among these patients, however, just 5% have scoliosis of clinical significance to warrant treatment (ie, curve progression > 30°).5,22
Each child should be examined initially for nonstructural scoliosis. This includes postural scoliosis, which resolves when a child is recumbent; compensatory scoliosis, which can be the result of a leg-length discrepancy (with no fixed vertebral rotation); sciatic scoliosis, an abnormality that results from avoiding the pain of an irritated sciatic nerve; inflammatory scoliosis, which could result from an infectious process, such as acute appendicitis; and hysterical scoliosis, a very rare form with an underlying psychological factor.4
Structural scoliosis comprises the remaining types of scoliosis, including the idiopathic variety. Patients with adolescent idiopathic scoliosis (AIS) are older than 10 and usually undergoing a rapid growth phase. Thus, some adolescent patients' curves can progress rapidly.4
Adolescents with idiopathic scoliosis rarely complain of pain or neurologic symptoms. Generally, their spinal curves do not cause organ pressure or shortness of breath. Many patients with AIS are involved in extracurricular activities that can trigger low back pain, yet activity-related low back pain is not an uncommon complaint among teens in general. Therefore, it is felt that when a patient with AIS has intermittent low back pain, it is most likely not related to their curve(s). Nevertheless, if a patient with AIS develops continued or unexplained low back pain and/or new neurologic symptoms, MRI is warranted.3
HISTORY, PHYSICAL EXAMINATION, AND DIAGNOSIS
During the exam, elicit any family history of scoliosis, especially in the setting of known diseases, such as Marfan syndrome, neurofibromatosis, muscular dystrophy, or other related diseases. Approximately 30% of AIS patients have a family history of scoliosis, so although the cause may not be clear, some genetic component is possible.3 The examination should stem from the patient's current stage of growth and development (see Table 31,3,4,7,23). The diagnosis of scoliosis is typically made during the examination and confirmed by radiography.
The newborn patient should be kept warm but be wearing only a diaper during the exam. A child or adolescent should be undressed and wearing a patient gown (unless at a school screening).
A general observation should be done initially, including the child's overall shape. The examiner should look for marfanoid features, such as excessive height, long arms, long trunk, and joint laxity.7 In the examination of the feet, an extremely high arch or cavus foot may be indicative of tethered spinal cord, spinal syrinx, or Charcot-Marie-Tooth disease, especially if the cavus is unilateral.1,7,23 MRI would be warranted in these instances.1
The examining clinician should note the child's Tanner stage. Height and weight measurements should be taken, and for a girl, age at menarche should be recorded.4
Next, the skin should be carefully inspected. In the infant, the examiner should look for hairy patches at the base of the spine, cutaneous sinuses, sacral dimpling, or other skin lesions that may suggest spinal dysraphism (ie, failure of the posterior elements to fuse around the spinal cord).4 Examination for café au lait spots and/or freckling in the axilla or groin area should be included, as this could signify neurofibromatosis.1,4,7
Examine the back in a standing child. Note whether the head appears to be centered over the sacrum. Evaluate for shoulder height or truncal asymmetry, scapular or posterior rib prominence, and excessive thoracic kyphosis and/or lumbar lordosis. Examine the pelvic bones, looking for obliquity (ie, one side higher than the other). Look for leg length discrepancy, both while the patient is standing still and during a gait review.1
During an anterior exam, look for shoulder or truncal asymmetry, leg length discrepancy, and anterior rib prominence. If shoulder height asymmetry is apparent, measure from each acromioclavicular joint to the floor and note the difference in centimeters. In the case of pelvic obliquity, measure from each anterior (or posterior) superior iliac spine to the floor and note the difference in centimeters. If the head does not appear to align over the sacrum, lower a plum line from the spinous process of C7 down to the gluteal cleft level. Measure by centimeters the distance from the cleft to the line, noting the side to which the line sways.
View the patient from the side. The sagittal curve can be assessed from the occiput to the sacrum; observe for an increased lordotic or kyphotic curve or abnormal positioning related to abnormal curves.4
The Adams forward bend test should be conducted.24,25 The child who is able to stand faces away from the examiner, then bends forward at the waist with the knees straight and the arms hanging down passively (see Figure 2). The practitioner looks at the spine from behind and notes any rib, thoracic, scapular, or lumbar prominence that could indicate a rotational spine deformity.1,4,7 In some instances, the use of a scoliometer can be helpful to assign a number to the amount of rotation to the spine during the Adams forward bend test. However, primary care offices are not often equipped with this device.26
Motor and Neurologic Examinations
The motor exam includes testing motor strength in all four extremities and noting any asymmetry. The examiner should have the child actively bend in every direction, making note of any deformity, stiffness, or asymmetry. The patient's spine should be palpated, with any tenderness noted, and any available radiographs reviewed.1,4,7
The neurologic exam includes testing cranial nerves, sensation, and reflexes, including the patellar, Achilles, and umbilical reflexes, as well as Babinski's sign. Many patients are areflexic in one or more areas. For instance, a finding of no umbilical (abdominal wink) reflex on either side is not suspicious in itself, but it should alert the practitioner to evaluate carefully for asymmetrical reflexes in one or more areas, especially if they are ipsilateral. Any positive neurologic findings, especially in the setting of scoliosis, warrant an MRI.1,7
Scoliosis Screening in the School Setting
One controversial subject related to scoliosis is school screenings. These screenings were developed in the 1980s for early identification of children with scoliosis curves so that nonsurgical treatment could be initiated promptly, or that surgery, when deemed necessary, could be performed in a timely fashion for optimal results.27
Since then, the effectiveness of screening programs has come under debate; in 2004, the US Preventive Services Task Force28 issued a recommendation (rating, "D Recommendation") against routine screening for idiopathic scoliosis in asymptomatic adolescents, stating that its effectiveness had not been shown in clinical trials. In response, a task force was convened by the American Academy of Orthopaedic Surgeons, the Scoliosis Research Society, the Pediatric Orthopaedic Society of North America, and the American Academy of Pediatrics, with findings published in a 2008 information statement by Richards and Vitale.25 While acknowledging the limitations of support for screening in the literature, the task force concluded:
- Early treatment of the deformities associated with idiopathic scoliosis can result in substantial benefits
- Screening (whether in the school environment or other settings) offers an opportunity for early diagnosis and appropriate referral of otherwise undetected deformities, especially in under-served populations
- In many cases, brace treatment may preclude the need for surgery in children with significant scoliosis
- Identifying deformities that require surgery as early as possible through screening increases the likelihood of good treatment outcomes
- Girls, who reach adolescence two years earlier than boys, should be screened at ages 10 and 12; boys, at age 13 or 14
- School personnel who conduct screening should receive training to detect spinal deformities
- While no screening test is entirely reliable, the forward bend test is considered the most specific test for detection of scoliosis29
- The participating organizations remain committed to preventing inappropriate use of spinal x-rays.25
Despite the ongoing debate, more than half of the US states have screening programs in place.24,27
RADIOGRAPHS/LABORATORY TESTING
Physical examination and a thorough history are most important in evaluation for scoliosis in the primary care setting. When radiographs (x-rays) are considered necessary, posterior-anterior and lateral spine series should be ordered. These are full-length (3-ft)
x-rays of the patient standing with the knees straight (when the child is able to do so).1,4,7 The x-rays are taken in the posterior-anterior direction to minimize breast and organ radiation exposure and to produce an x-ray similar to that of viewing the patient from behind.
If scoliosis is observed on radiography, note the apex of the curve, the location, the direction in which it curves, and any rotational component. Examine for pelvic obliquity, shoulder asymmetry, spondylosis, or spondylolisthesis.7
The Cobb angle is the measurement used to quantify the degree of scoliosis.4,5,7 Using the posterior-anterior radiograph, a line is drawn along the superior endplate of the most curved cephalad vertebra and the inferior endplate of the caudal vertebra. When perpendicular lines are drawn, the intersecting lines form the Cobb angle (see Figure 3, in which "X" indicates the Cobb angle, measured in degrees). An increasing angle reflects the increasing severity of scoliosis.
Other radiographs that may be ordered include supine lateral (bending) x-rays or supine traction radiographs to evaluate curve flexibility before surgery30; recently, hanging total spine x-rays have been investigated to evaluate spine flexibility before brace treatment.31 Additionally, hand/wrist x-rays may be ordered to determine the patient's remaining growth potential. These are most often taken in an orthopedist's office.3
If a diagnosis of scoliosis is made, Risser staging should be completed. Risser sign is a growth marker noted in adolescents by viewing iliac apophyseal ossification on radiograph. The progress of bone fusion over the iliac apophysis reflects remaining skeletal growth and is graded from 0 to 5, with 0 representing no ossification of the iliac apophysis and 5, complete ossification. The lower the grade noted at curve detection, the greater the risk for curve progression.3,4
Other tests may be warranted. If a patient has persistent back or side pain, especially pain that wakens the patient at night, and/ or pain not relieved by aspirin or NSAIDs, then a bone scan should be ordered to rule out discitis, rib or spine osteoid osteoma, spinal or intradural tumors, osteomyelitis, or other abnormalities.7 An MRI is warranted in children younger than 10; those who have left-sided curves, headaches, or neck pain; patients with quickly progressing curves; and those with any abnormality on neurologic exam. This test is generally not necessary in those with AIS if no family history is present and physical exam findings are unremarkable.1,2,5,7 Laboratory tests are generally not ordered for patients with idiopathic scoliosis unless inflammatory or infectious processes are suspected; standard laboratory tests would then follow. The ScoliScoreTM (Axial Biotech, Salt Lake City), a newer genetic screening test, can be used along with clinical and radiographic testing in patients with AIS. It is felt that specific genes are associated with AIS, and this test may help detect them and determine any associated risk for curve progression.1,3
There are several genetic tests for conditions associated with congenital neuromuscular or syndrome-related scoliosis.12,32 These may be ordered by the primary care provider, an orthopedist, or the geneticist.
REFERRAL
According to some researchers, any child with scoliosis greater than 10° should be referred to an orthopedist, preferably a pediatric spine orthopedist, especially when the patient is younger than 10. Referral is also recommended when the curve is left thoracic, or if any abnormal physical findings or a positive family history is present.4,5,7 There is also support for a strategy in which the practitioner closely observes the patient older than 10 with scoliosis but no other abnormalities, then refers the child once the curve reaches 20° to 25°.7 Referral within three months is most likely indicated for those with typical scoliosis and within one month for atypical scoliosis.7
Each practitioner will develop his or her own comfort level in the management of idiopathic scoliosis. Once a referral has been made, the practitioner should follow up to be sure the patient has seen the specialist in a timely fashion, with no appointments missed. Curve progression can develop rapidly, possibly making treatment more difficult once it begins.
TREATMENT
Three main types of treatment are provided for scoliosis: observation, bracing, and surgery.3,5,7 Observation is generally considered sufficient for immature patients whose curves measure less than 25°; usually no treatment will be needed for mature patients with curves less than 25°. Depending on risk stratification, children will initially need serial radiographs every four to six months.2
Most children will be seen by an orthopedist at this point.2,3,22 Oftentimes orthopedists with adult patients will order radiographs every three to five years to be sure the scoliosis has not progressed.3 Treatment of children with neuromuscular or syndrome-related scoliosis is lengthy and will not be addressed in this review.
Orthotic management (bracing or casting) is usually started once the immature patient's curve is between 25° and 45°. Brace treatment is not used to alter or correct scoliosis, but rather to halt its progression until the patient is skeletally mature (more common), a spinal fusion occurs (less common), or both.5 A 20% to 24% risk for eventual surgery has been reported in patients treated with braces.25,33 In infants or young children with congenital (or other) scoliosis, several temporary management options can be used to prevent curve progression while allowing the child's thorax to grow and expand.2
Several surgical interventions are appropriate for scoliosis. For infants or young children, one surgical intervention is the vertical expandable prosthetic titanium rib (VEPTRTM [Synthes, Inc, West Chester, Pennsylvania]). This is a longitudinal rib distraction device that can attach from rib to rib, rib to spine, or rib to pelvis. The device is indicated in the presence of a constrictive chest wall syndrome, which can decrease lung volumes and cause severe deformity if untreated. The device is lengthened every 4 to 6 months, with the goal to delay a final fusion until skeletal maturity.2
The growing rod, a comparable temporary device, is inserted spine to spine and lengthened similarly at various intervals. Growing rods are usually used in older children with scoliosis who await maturity before undergoing a definitive spinal fusion.2
A newer surgical procedure being used at Morgan Stanley Children's Hospital of NewYork-Presbyterian is spinal stapling.34 This involves minimally invasive surgery to implant inch-long staples across the spinal growth plates, which can stop scoliosis from worsening, or even correct the curve.
Spinal fusion with instrumentation, though used in less than 5% of patients with AIS, is considered the definitive surgical treatment for scoliosis.6 Surgical correction is almost always deemed necessary in immature patients with curves greater than 45° and mature patients with curves greater than 50°.3,7,22 The fusion may involve posterior (and sometimes anterior) discectomy and/or bone grafting between the involved vertebrae to help the bones fuse together so that curves cannot progress. The instrumentation (rods, hooks, wires, and/or screws) is used simply to maintain the spine in the best possible position until the actual fusion occurs; this can take months to a year.2,5,7
Risks and benefits of spinal fusion must be thoroughly reviewed with each parent and patient. The reported neurologic injury and mortality rates are less than 1% and less than 0.02%, respectively, in patients with idiopathic scoliosis who undergo the surgery.7 There is an increased incidence of such concerns in patients with other types of scoliosis, given their comorbid issues.35
Alternative treatments such as physical therapy, yoga, massage, acupuncture, and chiropractic medicine do not put patients at risk, and some have found them beneficial.3
EDUCATION
Both patient and parent must be acquainted with the goal of scoliosis diagnosis and treatment: to prevent curve progression, altered body image, and, ultimately, morbidity. Screening, history, examination, and radiographs are important first steps, with the information gained determining when referral to a pediatric spine orthopedist/specialist (if possible) should be made. Once curves reach 25° to 30°, bracing can successfully prevent curve progression; it will not correct the scoliosis.7 Teens who are resistant or reluctant to wear their brace must be made to understand the consequences of not following the treatment regimen, and the clinician's encouragement is essential.
Patients should be encouraged to stay active, with special emphasis on flexibility. Generally, there is no contraindication regarding activities or sports, unless the patient has undergone a surgical procedure; in this case, the practitioner will determine the patient's optimal level of participation in physical activities.
Surgical treatment with fusion and instrumentation is the only proven scoliosis treatment to correct curves (fully or partially) and prevent progression.3,5,7 Curves that reach 50° without treatment in the fully mature individual may continue to worsen into adulthood.
For patients with scoliosis having a possible familial and/or genetic component,2,3,7 there are several Web sites for patients and parents (see Table 4). Some of these address alternative therapy options.
CONCLUSION
Scoliosis is common, but the sequelae of idiopathic scoliosis can be minimized if the condition is diagnosed early and measures to prevent curve progression are initiated in a timely fashion. The key to diagnosing associated comorbid disorders and other related issues is to conduct a thorough patient and family history and complete physical examination. Timely radiographs are warranted in any patient with a positive finding. Ordering appropriate radiographs accurately is essential to facilitate patient care while reducing radiation exposure. Each practitioner must determine his or her level of comfort in managing patients with idiopathic scoliosis or immediately referring the patient once the diagnosis is made. Early referral is best in the primary care practice.
1. Horan MP, Milbrandt TA. Scoliosis in pediatric patients: comorbid disorders and screening. Pediatr Health. 2009;3(5):451-456.
2. Debnath UK. Current concepts in the management of early-onset idiopathic scoliosis. Pediatr Health. 2010;4(3):343-354.
3. Scoliosis Research Society. Idiopathic scoliosis. www.srs.org/patient_and_family/scoliosis/idiopathic. Accessed July 19, 2012.
4. Taft E, Francis R. Evaluation and management of scoliosis. J Pediatr Health Care. 2003;17(1):42- 44.
5. Speigel DA, Sarwark JF. Pediatric orthopaedics. In: Sarwark JF, ed. Essentials of Musculoskeletal Care. 4th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010:976-1201.
6. Horn PL, Beebe AC. Scoliosis: incidence and current medical and surgical interventions. Heartbeat. 2008;19(3):6-7,17.
7. Janicki JA. Scoliosis. In: Miller MD, Hart JA, MacKnight JM. Essential Orthopaedics. Marietta, GA: Saunders/Elsevier; 2010:881-886.
8. James JIP. Idiopathic scoliosis; the prognosis, diagnosis, and operative indications related to curve patterns and the age at onset. J Bone Joint Surg Br. 1954;36-B(1):36-49.
9. Scoliosis. In: Herring JA. Tachdjian's Pediatric Orthopaedics. Vol I. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2008:265-411.
10. Debnath UK, Goel V, Harshavarfhana N, Webb JK. Congenital scoliosis - Quo vadis? Indian J Orthop. 2010;44(2):137-147.
11. Basu PS, Elsebaie H, Noordeen MH. Congenital spine deformity: a comprehensive assessment at presentation. Spine (Phila Pa 1976). 2002;27(20):2255-2259.
12. Fisher K, Esham RH, Thorneycroft I. Scoliosis associated with typical Mayer-Rokitansky-Küster-Hauser syndrome. South Med J. 2000;93(2):243-246.
13. Hedequist D, Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop. 2007;27(1):106-116.
14. Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis: a study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976). 1992;17(9):1091-1096.
15. Ferguson RL. Medical and congenital comorbidities associated with spinal deformities in the immature spine. J Bone Joint Surg Am. 2007;89(suppl 1):34-41.
16. Demetracopoulos CA, Sponseller PD. Spinal deformities in Marfan syndrome. Orthop Clin North Am. 2007;38(4):563-572.
17. Crawford AH, Herrera-Soto J. Scoliosis associated with neurofibromatosis. Orthop Clin North Am. 2007;38(4):553-562.
18. Disorders of the spinal cord. In: Herring JA. Tachdjian's Pediatric Orthopaedics. Vol II. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2008:1405-1482.
19. Ruiz-Iban MA, Burgos J, Aguado HJ, et al. Scoliosis after median sternotomy in children with congenital heart disease. Spine (Phila Pa 1976). 2005;30(8):E214-E218.
20. Dimeglio A. Growth of the spine before age 5 years. J Pediatr Orthop B. 1993;1:102-107.
21. Lloyd-Roberts GC, Pilcher MF. Structural idiopathic scoliosis in infancy: a study of natural history of 100 patients. J Bone Joint Surg Br. 1965;47:520-523.
22. Gutknecht S, Lonstein J, Novacheck T. Adolescent idiopathic scoliosis. Pediatric Perspective. 2009;18(4):1-4.
23. Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg. 2007;43(3):236-248.
24. Bunge EM, Juttmann RE, van Biezen FC, et al; Netherlands Evaluation Study on Screening for Scoliosis (NESCIO) Group. Estimating the effectiveness of screening for scoliosis: a case-control study. Pediatrics. 2008;121(1):9-14.
25. Richards BS, Vitale MG. Screening for idiopathic scoliosis in adolescents: an information statement. J Bone Joint Surg. 2008;90(1):195-198.
26. Côté P, Kreitz BG, Cassidy JD, et al. A study of the diagnostic accuracy and reliability of the scoliometer and Adam's forward bend test. Spine. 1998;23(7):796-802.
27. Richards BS, Beaty JH, Thompson GH, Willis RB. Estimating the effectiveness of screening for scoliosis. Pediatrics. 2008:121(6):1297-1298.
28. US Preventive Services Task Force. Screening for idiopathic scoliosis in adolescents: recommendation statement (2004). www.uspreventiveservicestaskforce.org/3rduspstf/scoliosis/scoliors.htm. Accessed July 19, 2012.
29. Karachalios T, Sofianos J, Roidis N, et al. Ten-year follow-up evaluation of a school screening program for scoliosis. Is the forward-bending test an accurate diagnostic criterion for the screening of scoliosis? Spine (Phila Pa 1976). 1999;24(22):2318-2324.
30. Hamzaoglu A, Talu U, Tezer M, et al. Assessment of curve flexibility in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2005;30(14):1627-1642.
31. Kuroki H, Inomata N, Hamanaka H, et al. Significance of hanging total spine x-ray to estimate the indicative correction angle by brace wearing in idiopathic scoliosis patients. Scoliosis. 2012;7:8.
32. Al-Otibi M, Rutka JT. Neurosurgical implications of neurofibromatosis type I in children. Neurosurg Focus. 2006;20(1):E2.
33. Dolan LA, Weinstein SL. Surgical rates after observation and bracing for adolescent idiopathic scoliosis: an evidence-based review. Spine (Phila Pa 1976). 2007;32(19 suppl):S91-S100.
34. American Academy of Pediatrics. New stapling treatment may help reverse scoliosis: report from the Morgan Stanley Children's Hospital Department of Pediatric Orthopaedics. Section on Orthopaedics Newsletter. Spring 2009. www2.aap.org/sections/ortho/spring2009news.pdf. Accessed July 19, 2012.
35. Reames DL, Smith JS, Fu KG, et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis. Spine. 2011;36(18):1484-1491.
1. Horan MP, Milbrandt TA. Scoliosis in pediatric patients: comorbid disorders and screening. Pediatr Health. 2009;3(5):451-456.
2. Debnath UK. Current concepts in the management of early-onset idiopathic scoliosis. Pediatr Health. 2010;4(3):343-354.
3. Scoliosis Research Society. Idiopathic scoliosis. www.srs.org/patient_and_family/scoliosis/idiopathic. Accessed July 19, 2012.
4. Taft E, Francis R. Evaluation and management of scoliosis. J Pediatr Health Care. 2003;17(1):42- 44.
5. Speigel DA, Sarwark JF. Pediatric orthopaedics. In: Sarwark JF, ed. Essentials of Musculoskeletal Care. 4th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010:976-1201.
6. Horn PL, Beebe AC. Scoliosis: incidence and current medical and surgical interventions. Heartbeat. 2008;19(3):6-7,17.
7. Janicki JA. Scoliosis. In: Miller MD, Hart JA, MacKnight JM. Essential Orthopaedics. Marietta, GA: Saunders/Elsevier; 2010:881-886.
8. James JIP. Idiopathic scoliosis; the prognosis, diagnosis, and operative indications related to curve patterns and the age at onset. J Bone Joint Surg Br. 1954;36-B(1):36-49.
9. Scoliosis. In: Herring JA. Tachdjian's Pediatric Orthopaedics. Vol I. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2008:265-411.
10. Debnath UK, Goel V, Harshavarfhana N, Webb JK. Congenital scoliosis - Quo vadis? Indian J Orthop. 2010;44(2):137-147.
11. Basu PS, Elsebaie H, Noordeen MH. Congenital spine deformity: a comprehensive assessment at presentation. Spine (Phila Pa 1976). 2002;27(20):2255-2259.
12. Fisher K, Esham RH, Thorneycroft I. Scoliosis associated with typical Mayer-Rokitansky-Küster-Hauser syndrome. South Med J. 2000;93(2):243-246.
13. Hedequist D, Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop. 2007;27(1):106-116.
14. Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis: a study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976). 1992;17(9):1091-1096.
15. Ferguson RL. Medical and congenital comorbidities associated with spinal deformities in the immature spine. J Bone Joint Surg Am. 2007;89(suppl 1):34-41.
16. Demetracopoulos CA, Sponseller PD. Spinal deformities in Marfan syndrome. Orthop Clin North Am. 2007;38(4):563-572.
17. Crawford AH, Herrera-Soto J. Scoliosis associated with neurofibromatosis. Orthop Clin North Am. 2007;38(4):553-562.
18. Disorders of the spinal cord. In: Herring JA. Tachdjian's Pediatric Orthopaedics. Vol II. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2008:1405-1482.
19. Ruiz-Iban MA, Burgos J, Aguado HJ, et al. Scoliosis after median sternotomy in children with congenital heart disease. Spine (Phila Pa 1976). 2005;30(8):E214-E218.
20. Dimeglio A. Growth of the spine before age 5 years. J Pediatr Orthop B. 1993;1:102-107.
21. Lloyd-Roberts GC, Pilcher MF. Structural idiopathic scoliosis in infancy: a study of natural history of 100 patients. J Bone Joint Surg Br. 1965;47:520-523.
22. Gutknecht S, Lonstein J, Novacheck T. Adolescent idiopathic scoliosis. Pediatric Perspective. 2009;18(4):1-4.
23. Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg. 2007;43(3):236-248.
24. Bunge EM, Juttmann RE, van Biezen FC, et al; Netherlands Evaluation Study on Screening for Scoliosis (NESCIO) Group. Estimating the effectiveness of screening for scoliosis: a case-control study. Pediatrics. 2008;121(1):9-14.
25. Richards BS, Vitale MG. Screening for idiopathic scoliosis in adolescents: an information statement. J Bone Joint Surg. 2008;90(1):195-198.
26. Côté P, Kreitz BG, Cassidy JD, et al. A study of the diagnostic accuracy and reliability of the scoliometer and Adam's forward bend test. Spine. 1998;23(7):796-802.
27. Richards BS, Beaty JH, Thompson GH, Willis RB. Estimating the effectiveness of screening for scoliosis. Pediatrics. 2008:121(6):1297-1298.
28. US Preventive Services Task Force. Screening for idiopathic scoliosis in adolescents: recommendation statement (2004). www.uspreventiveservicestaskforce.org/3rduspstf/scoliosis/scoliors.htm. Accessed July 19, 2012.
29. Karachalios T, Sofianos J, Roidis N, et al. Ten-year follow-up evaluation of a school screening program for scoliosis. Is the forward-bending test an accurate diagnostic criterion for the screening of scoliosis? Spine (Phila Pa 1976). 1999;24(22):2318-2324.
30. Hamzaoglu A, Talu U, Tezer M, et al. Assessment of curve flexibility in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2005;30(14):1627-1642.
31. Kuroki H, Inomata N, Hamanaka H, et al. Significance of hanging total spine x-ray to estimate the indicative correction angle by brace wearing in idiopathic scoliosis patients. Scoliosis. 2012;7:8.
32. Al-Otibi M, Rutka JT. Neurosurgical implications of neurofibromatosis type I in children. Neurosurg Focus. 2006;20(1):E2.
33. Dolan LA, Weinstein SL. Surgical rates after observation and bracing for adolescent idiopathic scoliosis: an evidence-based review. Spine (Phila Pa 1976). 2007;32(19 suppl):S91-S100.
34. American Academy of Pediatrics. New stapling treatment may help reverse scoliosis: report from the Morgan Stanley Children's Hospital Department of Pediatric Orthopaedics. Section on Orthopaedics Newsletter. Spring 2009. www2.aap.org/sections/ortho/spring2009news.pdf. Accessed July 19, 2012.
35. Reames DL, Smith JS, Fu KG, et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis. Spine. 2011;36(18):1484-1491.