User login
The Effect of Colestipol on Glycemic Control in Patients With Type 2 Diabetes
Optimizing Office Blood Pressure Measurement at a VAMC
High-Speed Hyponatremia: Exercise-Associated Hyponatremia in a Young Male Soldier
Grand Rounds: Man, 62, With New-Onset Atrial Fibrillation
A 62-year-old black nursing home resident was transported to the hospital emergency department with fever of 102°F, new-onset atrial fibrillation (A-fib), and dementia. His medical history was significant for hypertension and multiple strokes.
His inpatient work-up for A-fib and dementia revealed a thyroid-stimulating hormone (TSH) level below 0.005 µIU/mL (normal range, 0.3 to 3.0 µIU/mL). Results of thyroid function testing (TFT) revealed a triiodothyronine (T3) level within normal range but a free thyroxine (T4) level of 2.9 ng/dL (normal range, 0.7 to 1.5 ng/dL) and a total T4 of 17.8 µg/dL (normal, 4.5 to 12.0 µg/dL). The abnormal TSH and T4 levels were considered suggestive of a thyrotoxic state, warranting an endocrinology consult. Cardiology was consulted regarding new-onset A-fib.
During history taking, the patient denied any shortness of breath, cough, palpitations, heat intolerance, anxiety, tremors, insomnia, dysphagia, diarrhea, dysuria, weight loss, or recent ingestion of iodine-containing medications or supplements.
On examination, the patient was febrile, with a blood pressure of 106/71 mm Hg; pulse, 74 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 98% to 99% on room air. ECG showed a normal sinus rhythm and a ventricular rate of 64 beats/min.
The patient's weight was 58.9 kg, and his height, 63" (BMI, 22.8). The patient had no skin changes, and his mucous membranes were slightly moist. The patient's head was atraumatic and normocephalic. His extraocular movements were intact, and his pupils were equal, round, and reactive to light, with nonicteric sclera. There was no proptosis or ophthalmoplegia. The patient's neck was supple, with no jugular venous distension, tracheal deviation, or thyromegaly.
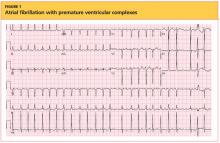
The cardiovascular exam revealed an irregular heartbeat, and repeat ECG showed A-fib with a ventricular rate of 151 beats/min (see Figure 1). The patient's chest was clear, with no wheezing or rhonchi. The abdomen was soft and slightly obese, and bowel sounds were present. The neurologic examination revealed no hyperreflexia. The patient's mental status was altered at times and he was alert, awake, and oriented to others. His speech was slightly slow, and some left-sided weakness was noted.
As recommended during the endocrinology consult, the patient underwent an I-123 sodium iodide thyroid scan, which showed faint uptake at the base of the neck, slightly to the left of midline; and a 24-hour radioactive iodide uptake (RAIU), which measured 2.8% (normal range, 8% to 35%).
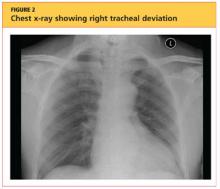
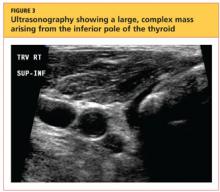
The patient's chest X-ray showed a right tracheal deviation not previously noted on physical examination (see Figure 2); the possible cause of a thyroid mass was considered. Subsequent ultrasonography of the thyroid revealed generally normal dimensions and parenchymal echogenicity. However, a large complex mass was detected, arising from the inferior pole of the thyroid and displacing the trachea toward the right (see Figure 3). According to the radiologist's notes, the mass contained both solid and cystic elements, scattered calcifications, and foci of flow on color Doppler. It measured about 6 cm in the largest (transverse) dimension. A 2.0-mm nodule was noted in the isthmus, slightly to the right of midline, consistent with multinodular goiter.
Following the cardiology consult, a diltiazem drip was initiated, but the patient was later optimized on flecainide for rhythm control and metoprolol for rate control. He was also initially anticoagulated using a heparin drip and bridged to warfarin, with target international normalized ratio (INR) between 2.0 and 3.0. Echocardiography revealed normal systolic function with ejection fraction of 55%, left ventricular hypertrophy, pulmonary artery systolic pressure of 35 mm Hg, and no pericardial effusions or valvular disease.
Regarding the patient's unexplained fever, results of chest imaging were negative for signs of pneumonia or atelectasis, which might have suggested a pulmonary cause. Urinalysis results were normal. Complete blood count showed no leukocytosis. The patient's fever subsided within 48 hours.
The differential diagnosis included Graves' disease, toxic multinodular goiter, Jod-Basedow syndrome, and subacute thyroiditis.
Graves' disease, an autoimmune disease with an unknown trigger, is the most common cause of hyperthyroidism. In affected patients, the thyroid gland overproduces thyroid hormones, leading to thyrotoxicosis. Thyrotoxicosis can result in multiple clinical signs and symptoms, including Graves' ophthalmopathy, pretibial myxedema, and goiter; TFT results typically include elevated T3 and T4 and low TSH.1-5 In the case patient (who had no history of thyroid disease, nor clinical signs or symptoms of Graves' disease), low uptake of iodine on thyroid scan precluded this diagnosis.
Toxic multinodular goiter, the second most common cause of hyperthyroidism, can be responsible for A-fib, tachycardia, and congestive heart failure.6,7 Iodine deficiency causes enlargement of the thyroid gland, where numerous nodules can develop, as seen in the case patient. These nodules can function independently, sometimes producing excess thyroid hormone; this leads to hyperplasia of the thyroid gland, resulting in a nontoxic multinodular goiter. From this goiter, a toxic multinodular goiter can emerge insidiously. However, in this condition, RAIU typically exceeds 30%; in the case patient, low 24-hour RAIU (2.8%) and the absence of functioning nodules on scanning made it possible to rule out this diagnosis.
Jod-Basedow syndrome refers to hyperthyroidism that develops as a result of administration of iodide, either as a dietary supplement or as IV contrast medium, or as an adverse effect of the antiarrhythmic drug amiodarone. This phenomenon is usually seen in a patient with endemic goiter.8-11 The relatively limited nature of the case patient's goiter and absence of a precipitating exposure to iodine made this diagnosis highly unlikely.
Subacute thyroiditis is a condition to which the patient's abnormal TFT results could reasonably be attributed. The patient had a substernal multinodular goiter that could not be palpated on physical examination, but it was visualized in the extended lower neck during thyroid scintigraphy.3 RAIU was minimal—a typical finding in this disorder,6 as TSH is suppressed by leakage of the excessive amounts of thyroid hormone. A tentative diagnosis of subacute thyroiditis was made.
As subacute thyroiditis is a self-limiting disorder, the patient was not started on any medications for hyperthyroidism but was advised to follow up with his primary care provider or an endocrinologist for repeat TFT and for fine-needle aspiration biopsy of the large thyroid nodule (a complex mass, containing cystic elements and calcifications, with a potential for malignancy) to rule out thyroid cancer.
Repeat ECG before discharge showed normal sinus rhythm with a ventricular rate of 74 beats/min. The patient was alert, awake, and oriented at discharge. He was continued on flecainide, metoprolol, and warfarin and advised to follow up with his primary care provider regarding his target INR.
DISCUSSION
The incidence of subacute thyroiditis, according to findings reported in 2003 from the Rochester Epidemiology Project in Olmsted County, Minnesota,12 is 12.1 cases per 100,000/year, with a higher incidence in women than men. It is most common in young adults and decreases with advancing age. Coxsackie virus, adenovirus, mumps, echovirus, influenza, and Epstein-Barr virus have been implicated in the disorder.12,13
Subacute thyroiditis is associated with a triphasic clinical course of hyperthyroidism, then hypothyroidism, then a return to normal thyroid function—as was seen in the case patient. Onset of subacute thyroiditis has been associated with recent viral infection, which may serve as a precipitant. The cause of this patient's high fever was never identified; thus, the etiology may have been viral.
The initial high thyroid hormone levels result from inflammation of thyroid tissue and release of preformed thyroid hormone into the circulation.6 At this point, TSH is suppressed and patients have very low RAIU, as was true in the case patient.
The condition is self-limiting and does not require treatment in the majority of patients, as TFT results return to normal levels within about two months.6 Patients can appear extremely ill due to thyrotoxicosis from subacute thyroiditis, but this usually lasts no longer than six to eight weeks.3 Subacute thyroiditis can be associated with atrial arrhythmia or heart failure.14,15
PATIENT OUTCOME
New-onset A-fib was attributed to the patient's thyrotoxicosis, which in turn was caused by subacute thyroiditis. He had a multinodular goiter, although he had not received any iodine supplements or IV contrast. As in most cases of subacute thyroiditis, no precipitating event was identified. However, given this patient's residence in a nursing facility and presentation with a high fever with no identifiable cause, a viral etiology for his subacute thyroiditis is possible.6
The patient's dementia may have been secondary to acute thyrotoxicosis, as his mental state improved during the hospital stay. His vitamin B12, folate, and A1C levels were within normal range. CT of the head showed multiple chronic infarcts and cerebral atrophy, and MRI of the brain indicated microvascular ischemic disease.
The patient was readmitted one month later for an episode of near-syncope (which, it was concluded, was a vasovagal episode). At that time, his TSH was found normal at 1.350 µIU/mL. Flecainide and metoprolol were discontinued; he was started on diltiazem for continued rate and rhythm control (as recommended by cardiology) and continued on warfarin.
CONCLUSION
In this case, subacute thyroiditis was most likely caused by a viral infection that led to destruction of the normal thyroid follicles and release of their preformed thyroid hormone into the circulation; this in turn led to sudden-onset A-fib. The diagnosis of subacute thyroiditis was suggested based on the abnormalities seen in this patient's TFT results, coupled with the suppressed RAIU—a typical finding in this disease.
Because subacute thyroiditis is a self-limiting condition, there is no role for antithyroid medication. Instead, treatment should be focused on relieving the patient's symptoms, such as ß-blockade or calcium channel blockers for tachycardia and corticosteroids or NSAIDs for neck pain.
REFERENCES
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-1248.
2. Delgado Hurtado JJ, Pineda M. Images in medicine: Graves' disease. N Engl J Med. 2011; 364(20):1955.
3. Al-Sharif AA, Abujbara MA, Chiacchio S, et al. Contribution of radioiodine uptake measurement and thyroid scintigraphy to the differential diagnosis of thyrotoxicosis. Hell J Nucl Med. 2010;13(2):132-137.
4. Buccelletti F, Carroccia A, Marsiliani D, et al. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med. 2011;29(9):1158-1162.
5. Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364(6):542-550.
6. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17(3):456-520.
7. Erickson D, Gharib H, Li H, van Heerden JA. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8(4):277-282.
8. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118(7):706-714.
9. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010;95(6):2529-2535.
10. El-Shirbiny AM, Stavrou SS, Dnistrian A, et al. Jod-Basedow syndrome following oral iodine and radioiodinated-antibody administration. J Nucl Med. 1997;38(11):1816-1817.
11. Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83-100.
12. Fatourechi V, Aniszewski JP, Fatourechi GZ, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100-2105.
13. Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
14. Volpé R. The management of subacute (DeQuervain's) thyroiditis. Thyroid. 1993;3(3):253-255.
15. Lee SL. Subacute thyroiditis (2009). http://emedicine.medscape.com/article/125648-overview. Accessed April 17, 2012.
A 62-year-old black nursing home resident was transported to the hospital emergency department with fever of 102°F, new-onset atrial fibrillation (A-fib), and dementia. His medical history was significant for hypertension and multiple strokes.
His inpatient work-up for A-fib and dementia revealed a thyroid-stimulating hormone (TSH) level below 0.005 µIU/mL (normal range, 0.3 to 3.0 µIU/mL). Results of thyroid function testing (TFT) revealed a triiodothyronine (T3) level within normal range but a free thyroxine (T4) level of 2.9 ng/dL (normal range, 0.7 to 1.5 ng/dL) and a total T4 of 17.8 µg/dL (normal, 4.5 to 12.0 µg/dL). The abnormal TSH and T4 levels were considered suggestive of a thyrotoxic state, warranting an endocrinology consult. Cardiology was consulted regarding new-onset A-fib.
During history taking, the patient denied any shortness of breath, cough, palpitations, heat intolerance, anxiety, tremors, insomnia, dysphagia, diarrhea, dysuria, weight loss, or recent ingestion of iodine-containing medications or supplements.
On examination, the patient was febrile, with a blood pressure of 106/71 mm Hg; pulse, 74 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 98% to 99% on room air. ECG showed a normal sinus rhythm and a ventricular rate of 64 beats/min.
The patient's weight was 58.9 kg, and his height, 63" (BMI, 22.8). The patient had no skin changes, and his mucous membranes were slightly moist. The patient's head was atraumatic and normocephalic. His extraocular movements were intact, and his pupils were equal, round, and reactive to light, with nonicteric sclera. There was no proptosis or ophthalmoplegia. The patient's neck was supple, with no jugular venous distension, tracheal deviation, or thyromegaly.
The cardiovascular exam revealed an irregular heartbeat, and repeat ECG showed A-fib with a ventricular rate of 151 beats/min (see Figure 1). The patient's chest was clear, with no wheezing or rhonchi. The abdomen was soft and slightly obese, and bowel sounds were present. The neurologic examination revealed no hyperreflexia. The patient's mental status was altered at times and he was alert, awake, and oriented to others. His speech was slightly slow, and some left-sided weakness was noted.
As recommended during the endocrinology consult, the patient underwent an I-123 sodium iodide thyroid scan, which showed faint uptake at the base of the neck, slightly to the left of midline; and a 24-hour radioactive iodide uptake (RAIU), which measured 2.8% (normal range, 8% to 35%).
The patient's chest X-ray showed a right tracheal deviation not previously noted on physical examination (see Figure 2); the possible cause of a thyroid mass was considered. Subsequent ultrasonography of the thyroid revealed generally normal dimensions and parenchymal echogenicity. However, a large complex mass was detected, arising from the inferior pole of the thyroid and displacing the trachea toward the right (see Figure 3). According to the radiologist's notes, the mass contained both solid and cystic elements, scattered calcifications, and foci of flow on color Doppler. It measured about 6 cm in the largest (transverse) dimension. A 2.0-mm nodule was noted in the isthmus, slightly to the right of midline, consistent with multinodular goiter.
Following the cardiology consult, a diltiazem drip was initiated, but the patient was later optimized on flecainide for rhythm control and metoprolol for rate control. He was also initially anticoagulated using a heparin drip and bridged to warfarin, with target international normalized ratio (INR) between 2.0 and 3.0. Echocardiography revealed normal systolic function with ejection fraction of 55%, left ventricular hypertrophy, pulmonary artery systolic pressure of 35 mm Hg, and no pericardial effusions or valvular disease.
Regarding the patient's unexplained fever, results of chest imaging were negative for signs of pneumonia or atelectasis, which might have suggested a pulmonary cause. Urinalysis results were normal. Complete blood count showed no leukocytosis. The patient's fever subsided within 48 hours.
The differential diagnosis included Graves' disease, toxic multinodular goiter, Jod-Basedow syndrome, and subacute thyroiditis.
Graves' disease, an autoimmune disease with an unknown trigger, is the most common cause of hyperthyroidism. In affected patients, the thyroid gland overproduces thyroid hormones, leading to thyrotoxicosis. Thyrotoxicosis can result in multiple clinical signs and symptoms, including Graves' ophthalmopathy, pretibial myxedema, and goiter; TFT results typically include elevated T3 and T4 and low TSH.1-5 In the case patient (who had no history of thyroid disease, nor clinical signs or symptoms of Graves' disease), low uptake of iodine on thyroid scan precluded this diagnosis.
Toxic multinodular goiter, the second most common cause of hyperthyroidism, can be responsible for A-fib, tachycardia, and congestive heart failure.6,7 Iodine deficiency causes enlargement of the thyroid gland, where numerous nodules can develop, as seen in the case patient. These nodules can function independently, sometimes producing excess thyroid hormone; this leads to hyperplasia of the thyroid gland, resulting in a nontoxic multinodular goiter. From this goiter, a toxic multinodular goiter can emerge insidiously. However, in this condition, RAIU typically exceeds 30%; in the case patient, low 24-hour RAIU (2.8%) and the absence of functioning nodules on scanning made it possible to rule out this diagnosis.
Jod-Basedow syndrome refers to hyperthyroidism that develops as a result of administration of iodide, either as a dietary supplement or as IV contrast medium, or as an adverse effect of the antiarrhythmic drug amiodarone. This phenomenon is usually seen in a patient with endemic goiter.8-11 The relatively limited nature of the case patient's goiter and absence of a precipitating exposure to iodine made this diagnosis highly unlikely.
Subacute thyroiditis is a condition to which the patient's abnormal TFT results could reasonably be attributed. The patient had a substernal multinodular goiter that could not be palpated on physical examination, but it was visualized in the extended lower neck during thyroid scintigraphy.3 RAIU was minimal—a typical finding in this disorder,6 as TSH is suppressed by leakage of the excessive amounts of thyroid hormone. A tentative diagnosis of subacute thyroiditis was made.
As subacute thyroiditis is a self-limiting disorder, the patient was not started on any medications for hyperthyroidism but was advised to follow up with his primary care provider or an endocrinologist for repeat TFT and for fine-needle aspiration biopsy of the large thyroid nodule (a complex mass, containing cystic elements and calcifications, with a potential for malignancy) to rule out thyroid cancer.
Repeat ECG before discharge showed normal sinus rhythm with a ventricular rate of 74 beats/min. The patient was alert, awake, and oriented at discharge. He was continued on flecainide, metoprolol, and warfarin and advised to follow up with his primary care provider regarding his target INR.
DISCUSSION
The incidence of subacute thyroiditis, according to findings reported in 2003 from the Rochester Epidemiology Project in Olmsted County, Minnesota,12 is 12.1 cases per 100,000/year, with a higher incidence in women than men. It is most common in young adults and decreases with advancing age. Coxsackie virus, adenovirus, mumps, echovirus, influenza, and Epstein-Barr virus have been implicated in the disorder.12,13
Subacute thyroiditis is associated with a triphasic clinical course of hyperthyroidism, then hypothyroidism, then a return to normal thyroid function—as was seen in the case patient. Onset of subacute thyroiditis has been associated with recent viral infection, which may serve as a precipitant. The cause of this patient's high fever was never identified; thus, the etiology may have been viral.
The initial high thyroid hormone levels result from inflammation of thyroid tissue and release of preformed thyroid hormone into the circulation.6 At this point, TSH is suppressed and patients have very low RAIU, as was true in the case patient.
The condition is self-limiting and does not require treatment in the majority of patients, as TFT results return to normal levels within about two months.6 Patients can appear extremely ill due to thyrotoxicosis from subacute thyroiditis, but this usually lasts no longer than six to eight weeks.3 Subacute thyroiditis can be associated with atrial arrhythmia or heart failure.14,15
PATIENT OUTCOME
New-onset A-fib was attributed to the patient's thyrotoxicosis, which in turn was caused by subacute thyroiditis. He had a multinodular goiter, although he had not received any iodine supplements or IV contrast. As in most cases of subacute thyroiditis, no precipitating event was identified. However, given this patient's residence in a nursing facility and presentation with a high fever with no identifiable cause, a viral etiology for his subacute thyroiditis is possible.6
The patient's dementia may have been secondary to acute thyrotoxicosis, as his mental state improved during the hospital stay. His vitamin B12, folate, and A1C levels were within normal range. CT of the head showed multiple chronic infarcts and cerebral atrophy, and MRI of the brain indicated microvascular ischemic disease.
The patient was readmitted one month later for an episode of near-syncope (which, it was concluded, was a vasovagal episode). At that time, his TSH was found normal at 1.350 µIU/mL. Flecainide and metoprolol were discontinued; he was started on diltiazem for continued rate and rhythm control (as recommended by cardiology) and continued on warfarin.
CONCLUSION
In this case, subacute thyroiditis was most likely caused by a viral infection that led to destruction of the normal thyroid follicles and release of their preformed thyroid hormone into the circulation; this in turn led to sudden-onset A-fib. The diagnosis of subacute thyroiditis was suggested based on the abnormalities seen in this patient's TFT results, coupled with the suppressed RAIU—a typical finding in this disease.
Because subacute thyroiditis is a self-limiting condition, there is no role for antithyroid medication. Instead, treatment should be focused on relieving the patient's symptoms, such as ß-blockade or calcium channel blockers for tachycardia and corticosteroids or NSAIDs for neck pain.
REFERENCES
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-1248.
2. Delgado Hurtado JJ, Pineda M. Images in medicine: Graves' disease. N Engl J Med. 2011; 364(20):1955.
3. Al-Sharif AA, Abujbara MA, Chiacchio S, et al. Contribution of radioiodine uptake measurement and thyroid scintigraphy to the differential diagnosis of thyrotoxicosis. Hell J Nucl Med. 2010;13(2):132-137.
4. Buccelletti F, Carroccia A, Marsiliani D, et al. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med. 2011;29(9):1158-1162.
5. Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364(6):542-550.
6. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17(3):456-520.
7. Erickson D, Gharib H, Li H, van Heerden JA. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8(4):277-282.
8. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118(7):706-714.
9. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010;95(6):2529-2535.
10. El-Shirbiny AM, Stavrou SS, Dnistrian A, et al. Jod-Basedow syndrome following oral iodine and radioiodinated-antibody administration. J Nucl Med. 1997;38(11):1816-1817.
11. Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83-100.
12. Fatourechi V, Aniszewski JP, Fatourechi GZ, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100-2105.
13. Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
14. Volpé R. The management of subacute (DeQuervain's) thyroiditis. Thyroid. 1993;3(3):253-255.
15. Lee SL. Subacute thyroiditis (2009). http://emedicine.medscape.com/article/125648-overview. Accessed April 17, 2012.
A 62-year-old black nursing home resident was transported to the hospital emergency department with fever of 102°F, new-onset atrial fibrillation (A-fib), and dementia. His medical history was significant for hypertension and multiple strokes.
His inpatient work-up for A-fib and dementia revealed a thyroid-stimulating hormone (TSH) level below 0.005 µIU/mL (normal range, 0.3 to 3.0 µIU/mL). Results of thyroid function testing (TFT) revealed a triiodothyronine (T3) level within normal range but a free thyroxine (T4) level of 2.9 ng/dL (normal range, 0.7 to 1.5 ng/dL) and a total T4 of 17.8 µg/dL (normal, 4.5 to 12.0 µg/dL). The abnormal TSH and T4 levels were considered suggestive of a thyrotoxic state, warranting an endocrinology consult. Cardiology was consulted regarding new-onset A-fib.
During history taking, the patient denied any shortness of breath, cough, palpitations, heat intolerance, anxiety, tremors, insomnia, dysphagia, diarrhea, dysuria, weight loss, or recent ingestion of iodine-containing medications or supplements.
On examination, the patient was febrile, with a blood pressure of 106/71 mm Hg; pulse, 74 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 98% to 99% on room air. ECG showed a normal sinus rhythm and a ventricular rate of 64 beats/min.
The patient's weight was 58.9 kg, and his height, 63" (BMI, 22.8). The patient had no skin changes, and his mucous membranes were slightly moist. The patient's head was atraumatic and normocephalic. His extraocular movements were intact, and his pupils were equal, round, and reactive to light, with nonicteric sclera. There was no proptosis or ophthalmoplegia. The patient's neck was supple, with no jugular venous distension, tracheal deviation, or thyromegaly.
The cardiovascular exam revealed an irregular heartbeat, and repeat ECG showed A-fib with a ventricular rate of 151 beats/min (see Figure 1). The patient's chest was clear, with no wheezing or rhonchi. The abdomen was soft and slightly obese, and bowel sounds were present. The neurologic examination revealed no hyperreflexia. The patient's mental status was altered at times and he was alert, awake, and oriented to others. His speech was slightly slow, and some left-sided weakness was noted.
As recommended during the endocrinology consult, the patient underwent an I-123 sodium iodide thyroid scan, which showed faint uptake at the base of the neck, slightly to the left of midline; and a 24-hour radioactive iodide uptake (RAIU), which measured 2.8% (normal range, 8% to 35%).
The patient's chest X-ray showed a right tracheal deviation not previously noted on physical examination (see Figure 2); the possible cause of a thyroid mass was considered. Subsequent ultrasonography of the thyroid revealed generally normal dimensions and parenchymal echogenicity. However, a large complex mass was detected, arising from the inferior pole of the thyroid and displacing the trachea toward the right (see Figure 3). According to the radiologist's notes, the mass contained both solid and cystic elements, scattered calcifications, and foci of flow on color Doppler. It measured about 6 cm in the largest (transverse) dimension. A 2.0-mm nodule was noted in the isthmus, slightly to the right of midline, consistent with multinodular goiter.
Following the cardiology consult, a diltiazem drip was initiated, but the patient was later optimized on flecainide for rhythm control and metoprolol for rate control. He was also initially anticoagulated using a heparin drip and bridged to warfarin, with target international normalized ratio (INR) between 2.0 and 3.0. Echocardiography revealed normal systolic function with ejection fraction of 55%, left ventricular hypertrophy, pulmonary artery systolic pressure of 35 mm Hg, and no pericardial effusions or valvular disease.
Regarding the patient's unexplained fever, results of chest imaging were negative for signs of pneumonia or atelectasis, which might have suggested a pulmonary cause. Urinalysis results were normal. Complete blood count showed no leukocytosis. The patient's fever subsided within 48 hours.
The differential diagnosis included Graves' disease, toxic multinodular goiter, Jod-Basedow syndrome, and subacute thyroiditis.
Graves' disease, an autoimmune disease with an unknown trigger, is the most common cause of hyperthyroidism. In affected patients, the thyroid gland overproduces thyroid hormones, leading to thyrotoxicosis. Thyrotoxicosis can result in multiple clinical signs and symptoms, including Graves' ophthalmopathy, pretibial myxedema, and goiter; TFT results typically include elevated T3 and T4 and low TSH.1-5 In the case patient (who had no history of thyroid disease, nor clinical signs or symptoms of Graves' disease), low uptake of iodine on thyroid scan precluded this diagnosis.
Toxic multinodular goiter, the second most common cause of hyperthyroidism, can be responsible for A-fib, tachycardia, and congestive heart failure.6,7 Iodine deficiency causes enlargement of the thyroid gland, where numerous nodules can develop, as seen in the case patient. These nodules can function independently, sometimes producing excess thyroid hormone; this leads to hyperplasia of the thyroid gland, resulting in a nontoxic multinodular goiter. From this goiter, a toxic multinodular goiter can emerge insidiously. However, in this condition, RAIU typically exceeds 30%; in the case patient, low 24-hour RAIU (2.8%) and the absence of functioning nodules on scanning made it possible to rule out this diagnosis.
Jod-Basedow syndrome refers to hyperthyroidism that develops as a result of administration of iodide, either as a dietary supplement or as IV contrast medium, or as an adverse effect of the antiarrhythmic drug amiodarone. This phenomenon is usually seen in a patient with endemic goiter.8-11 The relatively limited nature of the case patient's goiter and absence of a precipitating exposure to iodine made this diagnosis highly unlikely.
Subacute thyroiditis is a condition to which the patient's abnormal TFT results could reasonably be attributed. The patient had a substernal multinodular goiter that could not be palpated on physical examination, but it was visualized in the extended lower neck during thyroid scintigraphy.3 RAIU was minimal—a typical finding in this disorder,6 as TSH is suppressed by leakage of the excessive amounts of thyroid hormone. A tentative diagnosis of subacute thyroiditis was made.
As subacute thyroiditis is a self-limiting disorder, the patient was not started on any medications for hyperthyroidism but was advised to follow up with his primary care provider or an endocrinologist for repeat TFT and for fine-needle aspiration biopsy of the large thyroid nodule (a complex mass, containing cystic elements and calcifications, with a potential for malignancy) to rule out thyroid cancer.
Repeat ECG before discharge showed normal sinus rhythm with a ventricular rate of 74 beats/min. The patient was alert, awake, and oriented at discharge. He was continued on flecainide, metoprolol, and warfarin and advised to follow up with his primary care provider regarding his target INR.
DISCUSSION
The incidence of subacute thyroiditis, according to findings reported in 2003 from the Rochester Epidemiology Project in Olmsted County, Minnesota,12 is 12.1 cases per 100,000/year, with a higher incidence in women than men. It is most common in young adults and decreases with advancing age. Coxsackie virus, adenovirus, mumps, echovirus, influenza, and Epstein-Barr virus have been implicated in the disorder.12,13
Subacute thyroiditis is associated with a triphasic clinical course of hyperthyroidism, then hypothyroidism, then a return to normal thyroid function—as was seen in the case patient. Onset of subacute thyroiditis has been associated with recent viral infection, which may serve as a precipitant. The cause of this patient's high fever was never identified; thus, the etiology may have been viral.
The initial high thyroid hormone levels result from inflammation of thyroid tissue and release of preformed thyroid hormone into the circulation.6 At this point, TSH is suppressed and patients have very low RAIU, as was true in the case patient.
The condition is self-limiting and does not require treatment in the majority of patients, as TFT results return to normal levels within about two months.6 Patients can appear extremely ill due to thyrotoxicosis from subacute thyroiditis, but this usually lasts no longer than six to eight weeks.3 Subacute thyroiditis can be associated with atrial arrhythmia or heart failure.14,15
PATIENT OUTCOME
New-onset A-fib was attributed to the patient's thyrotoxicosis, which in turn was caused by subacute thyroiditis. He had a multinodular goiter, although he had not received any iodine supplements or IV contrast. As in most cases of subacute thyroiditis, no precipitating event was identified. However, given this patient's residence in a nursing facility and presentation with a high fever with no identifiable cause, a viral etiology for his subacute thyroiditis is possible.6
The patient's dementia may have been secondary to acute thyrotoxicosis, as his mental state improved during the hospital stay. His vitamin B12, folate, and A1C levels were within normal range. CT of the head showed multiple chronic infarcts and cerebral atrophy, and MRI of the brain indicated microvascular ischemic disease.
The patient was readmitted one month later for an episode of near-syncope (which, it was concluded, was a vasovagal episode). At that time, his TSH was found normal at 1.350 µIU/mL. Flecainide and metoprolol were discontinued; he was started on diltiazem for continued rate and rhythm control (as recommended by cardiology) and continued on warfarin.
CONCLUSION
In this case, subacute thyroiditis was most likely caused by a viral infection that led to destruction of the normal thyroid follicles and release of their preformed thyroid hormone into the circulation; this in turn led to sudden-onset A-fib. The diagnosis of subacute thyroiditis was suggested based on the abnormalities seen in this patient's TFT results, coupled with the suppressed RAIU—a typical finding in this disease.
Because subacute thyroiditis is a self-limiting condition, there is no role for antithyroid medication. Instead, treatment should be focused on relieving the patient's symptoms, such as ß-blockade or calcium channel blockers for tachycardia and corticosteroids or NSAIDs for neck pain.
REFERENCES
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-1248.
2. Delgado Hurtado JJ, Pineda M. Images in medicine: Graves' disease. N Engl J Med. 2011; 364(20):1955.
3. Al-Sharif AA, Abujbara MA, Chiacchio S, et al. Contribution of radioiodine uptake measurement and thyroid scintigraphy to the differential diagnosis of thyrotoxicosis. Hell J Nucl Med. 2010;13(2):132-137.
4. Buccelletti F, Carroccia A, Marsiliani D, et al. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med. 2011;29(9):1158-1162.
5. Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364(6):542-550.
6. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17(3):456-520.
7. Erickson D, Gharib H, Li H, van Heerden JA. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8(4):277-282.
8. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118(7):706-714.
9. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010;95(6):2529-2535.
10. El-Shirbiny AM, Stavrou SS, Dnistrian A, et al. Jod-Basedow syndrome following oral iodine and radioiodinated-antibody administration. J Nucl Med. 1997;38(11):1816-1817.
11. Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83-100.
12. Fatourechi V, Aniszewski JP, Fatourechi GZ, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100-2105.
13. Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
14. Volpé R. The management of subacute (DeQuervain's) thyroiditis. Thyroid. 1993;3(3):253-255.
15. Lee SL. Subacute thyroiditis (2009). http://emedicine.medscape.com/article/125648-overview. Accessed April 17, 2012.
Pediatric Abdominal Trauma: Making a Difficult Diagnosis
Cover
The Treatment of Cartilage Defects in the Knee Joint: Microfracture, Mosaicplasty, and Autologous Chondrocyte Implantation
Bone Graft Extenders and Substitutes in the Thoracolumbar Spine
Which sling for which SUI patient?
- Placement of TVT-O transobturator tape
- Monarc transobturator procedure
- TVT Exact retropubic sling operation
These videos were selected by Mark D. Walters, MD, and presented courtesy of the International Academy of Pelvic Surgery (IAPS).
Only 15 years ago, when surgery was recommended for patients who had bothersome stress urinary incontinence (SUI), they were offered operations such as suburethral (Kelly) plication, needle urethropexy, open or laparoscopic Burch procedure, and pubovaginal fascial sling procedure. Today, virtually all of these operations have been replaced in general practice by retropubic or transobturator (TOT) midurethral synthetic slings.
Although Burch colposuspension and the pubovaginal fascial sling procedure are effective for both primary and recurrent SUI, they are more invasive than midurethral slings, cause more voiding dysfunction, and have significantly longer recovery times, making them less attractive for most primary and recurrent cases of SUI.
The evolution of SUI surgeries has shifted so far toward midurethral slings that Burch colposuspension and the pubovaginal sling procedure are rarely performed or taught in obstetrics and gynecology or urology residency programs; these procedures are now mostly done in fellowship programs by specialists in female pelvic medicine and reconstructive surgery.
In this article, we describe how an ObGyn generalist can approach the surgical treatment of women who have either primary or recurrent SUI. Using evidence-based principles, when available, we also discuss how different clinical characteristics—as well as the characteristics of the available slings—affect the suitability of the sling for individual patients.
One caveat: This article assumes that the surgeon knows how to, and is able to, perform retropubic and TOT sling procedures equally well. However, when this is not the case, the surgeon should perform the sling procedure that she or he does best, assuming that it is appropriate for that particular patient.
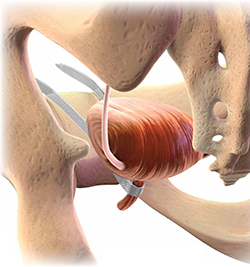
Almost all surgical procedures for stress urinary incontinence performed today involve placement of a retropubic or transobturator midurethral synthetic sling.
Illustration: Craig Zuckerman for OBG Management
CASE: SUI and Stage II anterior vaginal prolapse
A healthy 45-year-old G2P2 woman complains of a 5-year history of worsening SUI symptoms, mostly occurring during activities such as coughing, laughing, and running. The incontinence has become so severe that she requires several pads daily. She is able to void without difficulty or pain, and her bowel movements are normal. She has regular menses, has had a tubal ligation, and is sexually active.
She reports that she has been performing daily Kegel pelvic muscle exercises, without improvement.
On physical examination, she is found to have Stage II anterior vaginal prolapse and urethral hypermobility, with normal uterine and posterior vaginal support. The uterus and ovaries are of normal size.
A full bladder stress test in the office reveals immediate loss of urine from the urethra upon coughing in a semi-sitting position. She voids 325 mL after the examination and has a post-void residual urine volume, as measured by ultrasonography (US), of 25 mL. Urinalysis is negative.
When discussing her goals, the patient expresses a desire for a cure of her urinary incontinence, if possible.
What further testing and treatment options do you offer to her?
If you and the patient agree that surgery is warranted, which procedure do you recommend?
Recommended assessment of women who report SUI
Women who have bothersome urine loss during activities such as exercise, coughing, or laughing should undergo a history, physical examination, and urinalysis. During the pelvic examination, it is important to assess pelvic organ support defects, especially those involving the anterior vagina and urethra. Also note levator ani muscle contraction and strength. In addition, you can use this time to discuss whether the patient is doing, or has done, pelvic muscle (Kegel) exercises; teach the exercises, if necessary; and encourage her to do them in the future.
If the patient has no urinary infection, has performed Kegel exercises without further benefit, and wishes to consider surgical treatment, basic assessment of lower urinary function is indicated. Basic office urodynamic testing includes:
- a measured void
- measurement of post-void residual volume (by catheter or US)
- assessment of bladder sensation and capacity
- provocation for overactive bladder
- a full-bladder cough stress test (a positive test is direct observation of urethral loss of urine upon coughing).
Patients who have a complex history or mixed symptoms, previous failed surgery, or other characteristics that suggest a diagnosis other than simple SUI should undergo formal electronic urodynamic testing.1
Patient selection criteria
Primary sling surgery is an option for patients who have:
- no urinary infection
- normal voiding and bladder-filling function
- urethral hypermobility on examination
- SUI on a cough-stress test
- failure to improve sufficiently with pelvic muscle exercises.
Suburethral slings were initially developed as a treatment for recurrent, urodynamically confirmed SUI, particularly SUI caused by intrinsic sphincter deficiency (ISD). Pubovaginal slings, usually consisting of autologous fascia, were placed at the bladder neck to both support and slightly compress the proximal urethra. Compared with synthetic slings, fascial slings are effective but take longer to place and have a higher rate of surgical morbidity and more postoperative voiding dysfunction. They are now mostly indicated for complex recurrent SUI, usually managed by specialists in female pelvic medicine and reconstructive surgery.
Current slings are lightweight polypropylene mesh
Most slings today are tension-free midurethral slings consisting of synthetic, large-pore polypropylene mesh; they are sold in kits available from several different companies. Sling procedures can also be performed using hand-cut polypropylene mesh and a reusable needle passer.
These slings are placed at the midurethra and work by mechanical kinking or folding of the urethra over the sling, with an increase in intra-abdominal pressure. Ideally, the midurethral sling will not compress the urethra at rest and have no effect on the normal voiding mechanism.
Three main techniques are used to place synthetic midurethral slings:
- the retropubic approach
- the TOT approach
- variations of single-incision “mini-sling” procedures.
Early studies of mini-slings showed few complications but lower effectiveness, compared with retropubic and TOT midurethral slings, according to short-term follow-up data.2-4 A mini-sling might be an option for some patients in whom surgical complications must be kept to a minimum; otherwise, they will not be discussed further.
Retropubic midurethral slings
The tension-free vaginal tape (TVT) procedure described by Petros and Ulmsten was the first synthetic midurethral sling.5 This ambulatory procedure aims to restore the pubourethral ligament and suburethral vaginal hammock by using specially designed needles attached to synthetic sling material.
The synthetic sling consists of polypropylene, approximately 1 cm wide and 40 cm long. The sling material is attached to two stainless steel needles that are passed from a vaginal incision made at the level of the midurethra, through the retropubic space, and exiting at a previously created mark or stab incision in the suprapubic area (FIGURE 1).
Variations of the retropubic midurethral sling have been developed, with sling passers going from the vagina upward (“bottom to top”) and also from the suprapubic area downward (“top to bottom”). A recent Cochrane review reported that the bottom-to-top variation is slightly more effective.6
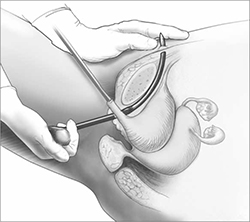
FIGURE 1 Retropubic sling
Placement of the tension-free vaginal tape trocar into the retropubic space.
Illustration: Craig Zuckerman for OBG Management
Transobturator midurethral slings
The TOT sling has become one of the most popular and effective surgical treatments for female SUI worldwide (Video 1 and Video 2). It is a relatively rapid and low-risk surgery that is comparable to other surgical options in effectiveness while avoiding an abdominal incision and the passage of a needle or trocar through the space of Retzius.
The TOT sling lies flatter under the urethra and carries a lower risk of urethral obstruction, urinary retention, and subsequent need for sling release, compared with retropubic slings.7-9 Compared with the retropubic TVT, the TOT sling produces similar rates of cure, with fewer bladder perforations and less postoperative irritative voiding symptoms.6,10-12 It nearly eliminates the rare but catastrophic risk of bowel or major vessel perforation. The trade-off is that patients experience more complications referable to the groin (pain and leg weakness or numbness) with the TOT approach.9,13
All TOT slings on the market consist of a large-pore, lightweight, polypropylene mesh strip, usually covered with a plastic sheath. Various devices are used to place the sling, but most of them involve a helical trocar that curves around the ischiopubic ramus, passing through the inner thigh and obturator membrane to a space created in the ipsilateral peri-urethral tissues.
TOT slings can be placed outside-to-inside or inside-to-outside (FIGURE 2), and the indications, effectiveness, and frequency of complications seem to be similar between these two approaches.12 One study found a higher frequency of new sexual dysfunction (tender, palpable sling; penile pain in male partner) in women after the “outside-in” approach,14 but this clinical issue has not been observed in all studies.15,16
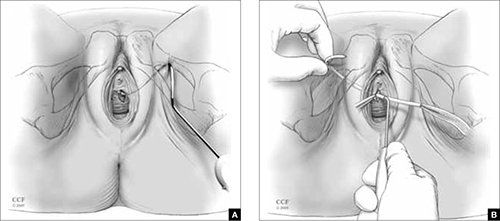
FIGURE 2 TOT sling variations
Placement of the transobturator (TOT) sling helical trocar using the (A) “outside-in” variation and (B) “inside-out” variation.
Illustration: Craig Zuckerman for OBG Management
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography
© 2005-2012. All Rights Reserved.
Success rates are similar for retropubic and TOT slings
Despite differences in technique and brand of mesh used, treatment success rates for uncomplicated primary SUI are similar for the retropubic (Video 3) and TOT tension-free slings.6-8,10-12,17 The percentage of patients treated successfully depends on the definition used, ranging from a high of 96% to a low of 60%. When the definition of success is restricted to stress incontinence symptoms, especially over a short period of time, the reported effectiveness is high.
In contrast, when the definition of success includes incontinence of any type, the reported effectiveness is lower. For example, in the study that reported 60% effectiveness, success was defined as no incontinence symptoms of any type, a negative cough stress test, and no retreatment for stress incontinence or postoperative urinary retention.11
Retropubic slings, especially TVT, may be somewhat more effective for ISD,18-20 although this conclusion must be tempered by the small number of studies addressing the issue and differences in the diagnosis of ISD.21
Some studies have reported good success in treating mixed urinary incontinence with the retropubic and TOT slings,2,8 although other studies have reported that the initial benefit for urgency or urge incontinence is not sustained over time, compared with the benefit for stress incontinence.22 It is important to counsel patients before surgery that improvement in stress incontinence symptoms and general satisfaction is highly likely, but perfect bladder function is not.
Serious complications are uncommon
Complications are common after both retropubic and TOT slings, although serious complications are uncommon. Cystitis and temporary voiding difficulties are the most common problems after a sling procedure. If the patient is unable to void on the day of surgery, it is reasonable to discharge her with a Foley catheter in place for a few days or teach her to perform intermittent self-catheterization at home. In most cases, normal voiding will resume within a few days. Cystitis is at least partially related to the surgery itself and the duration of postoperative catheterization.
The frequency of some complications differs between the retropubic and TOT approaches to midurethral slings. For example, some literature suggests that irritative voiding symptoms such as urgency or voiding difficulty are somewhat less common after TOT slings, compared with retropubic slings. However, symptoms referable to the groin (pain and leg weakness or numbness) occur more commonly with the TOT approach.6
After placement of a TOT sling, 10% to 15% of women experience temporary inner thigh or groin pain or leg weakness and are usually managed conservatively with nonsteroidal anti-inflammatory drugs and physical therapy. Long-term or severe complications related to TOT sling passage are rare.
Major intraoperative complications are rare
The rate of these complications does not differ between retropubic and TOT approaches. Minor intraoperative complications—primarily, bladder perforation—occur more commonly with the retropubic approach.6
Bladder perforation with the TVT occurs in 4% to 7% of patients. However, the clinical significance of bladder perforation is minimal as long as the surgeon performs careful cystoscopy, recognizes bladder perforation, and repositions the trocar and mesh outside the bladder lumen. Bladder perforation caused by the trocar usually does not require specific treatment (except repositioning of the trocar outside the bladder lumen) and rarely results in later problems.
Mesh exposures occur with similar frequency for the different sling types as long as large-pore lightweight polypropylene is used. Dehiscence of the suburethral incision (mesh exposure) is uncommon with midurethral slings, occurring in 1% to 2% of patients. Dehiscence can be managed with estrogen cream or trimming of the exposed portion of the sling in the office. If symptoms or signs persist, removal of the exposed segment or the entire central portion of the sling, with closure of the vaginal epithelium, is indicated to allow for healing and resolution of symptoms. However, removal may lead to recurrence of the original SUI symptoms.
Retropubic hematomas occur in 1% to 2% of patients after placement of a retropubic sling, but major vascular injuries are rare—occurring in, perhaps, 3 in every 1,000 cases.
Bowel perforations are very rare but serious complications. A retropubic sling should be placed with caution or avoided in women who have a history of peritonitis, bowel surgery, ruptured appendix, or known extensive pelvic adhesions.
Major vascular injuries are also rare with TOT slings, occurring in approximately 1 to 2 cases in every 1,000.
Bladder injury occurs much less frequently after placement of a TOT sling, compared with the retropubic approach, although one study reported bladder injury in 2% of TOT cases.17 Although bladder injury is uncommon with the TOT approach, the morbidity associated with delayed detection of bladder injury is much higher than the morbidity associated with intraoperative detection and management. Therefore, we believe that cystoscopy should be performed in all TOT and retropubic sling procedures to either exclude bladder damage or detect and appropriately manage it.
For reassurance that intraoperative and postoperative blood loss is not excessive, it is reasonable to check one hemoglobin level before discharge, if desired.
How to individualize the choice of sling
Patients who have primary SUI: Retropubic or TOT sling. Objective and subjective success rates are similar, regardless of approach, and serious complications are infrequent. The retropubic approach has longer-term evidence of sustained benefit, compared with the newer TOT approach. We tend to treat younger patients with TVT and older patients with TOT. Surgeon experience and informed patient preferences may dictate the choice of sling (TABLE).
What we recommend surgically for our patients who have SUI—and why
| Clinical problem and patient characteristics | Surgery | Rationale |
|---|---|---|
| Primary SUI with urethral hypermobility—young patient | TVT | TVT has similar effectiveness and more long-term data than TOT; TVT may result in less sexual pain than TOT |
| Primary SUI with urethral hypermobility—older patient; leak point pressure >60 cmH20 | TOT | Similar effectiveness, fewer complications with TOT |
| Recurrent SUI with urethral hypermobility—any age; leak point pressure >60 cmH20 | TVT | Limited data suggest effectiveness of TVT after TOT failure |
| Recurrent SUI with urethral hypermobility—leak point pressure <60 cmH20 (ISD) | TVT or pubovaginal fascial sling | Some but not all data indicate that TVT is more effective for ISD; fascial slings in expert hands are effective, based on cohort studies |
| Recurrent SUI with nonmobile bladder neck; any leak point pressure | Urethral bulking | All sling procedures have lowered effectiveness when the bladder neck is immobile |
| SUI mixed with dominant urgency or voiding dysfunction | TOT | TOT improves or does not exacerbate mixed urinary symptoms to the extent that TVT may |
| SUI with prolapse and planned vaginal prolapse repair | TVT or TOT | Limited data support similar effectiveness for either approach |
| “Occult” SUI with prolapse reduced and planned vaginal prolapse repair | TOT or “wait and see” | TOT has a lower chance of creating new irritative voiding symptoms; “wait-and-see” approach allows treatment of SUI if it develops after prolapse repair |
| Recurrent SUI with previous synthetic sling mesh complication (or patients who desire treatment without mesh) | Pubovaginal fascial sling or Burch colposuspension | These nonmesh options are effective for recurrent SUI, but have higher surgical morbidity |
| ISD = intrinsic sphincter deficiency; SUI = stress urinary incontinence; TVT = tension-free vaginal tape or similar retropubic midurethral sling; TOT = transobturator sling placed either by outside-in or inside-out variations | ||
Patients who have recurrent SUI: Retropubic sling. Comparative data are limited regarding the retropubic and TOT approaches for recurrent SUI that does not involve ISD. One case series reported good results with the use of retropubic TVT for recurrent SUI after an initial TOT approach.23
Patients who have ISD: Retropubic sling (synthetic midurethral sling or fascial sling placed at the bladder neck). A few studies suggest that patients with ISD have better outcomes with the retropubic approach.19,20 However, with differing definitions of ISD and relatively few patients with ISD included in these trials, it is not possible to conclude definitively that the retropubic approach is more effective than the TOT approach for patients who have SUI and ISD. However, the retropubic approach has longer-term data to support its effectiveness; therefore, with some but not all evidence suggesting its superiority for ISD, it is reasonable to choose the retropubic midurethral approach.
A pubovaginal fascial sling placed at the proximal urethra is also an effective option, based on numerous cohort studies.12
Patients who have recurrent SUI or ISD, or both, with a non-mobile bladder neck: Urethral bulking. Although data are scant, urethral injection therapy is beneficial for SUI in the short-term, but long-term studies are lacking. Bulking agents include silicone particles, calcium hydroxylapatite, and carbon spheres; studies have not shown one to be more or less efficacious than the others.24
It is reasonable to use urethral bulking first in these patients as the morbidity is very low and some patients become continent. A retropubic sling can be performed if urethral bulking fails to adequately improve symptoms, although the effectiveness is lower in this population than in women with SUI and urethral hypermobility.
Patients who have mixed stress and urge incontinence or voiding dysfunction: TOT sling. Limited data suggest that the TOT approach improves symptoms of mixed incontinence—or, at least, exacerbates them to a lesser degree than the retropubic approach. Rarely is a sling release needed to treat obstructive urinary symptoms after the TOT approach.
Patients who have prolapse and SUI: Retropubic or TOT sling. When a sling procedure is performed at the same time as reconstructive surgery for prolapse, it has similar effectiveness, regardless of whether the retropubic or TOT approach is selected.8,11 A sling placed during prolapse surgery (placed through a separate midurethral incision) appears to be as effective as a sling placed as a sole procedure.
Patients who have prolapse and occult SUI: TOT sling. If you recommend a sling to prevent SUI after prolapse surgery by any route, pick the sling with acceptable efficacy and the lowest rates of complications and voiding dysfunction. Patients are especially intolerant of complications from a sling to prevent SUI. Given the ease of placement and low morbidity of a later outpatient sling procedure, it is also reasonable to offer patients the “wait-and-see” alternative to see if SUI develops after prolapse surgery and only then proceeding with sling surgery. In this way, overtreatment is avoided, and any complications that occur after sling surgery for SUI treatment may be better tolerated by the patient. The preferences of an informed patient may guide decisions in this setting.
Patients who have recurrent SUI with mesh complication: Pubovaginal fascial sling or Burch colposuspension. These non-mesh options are effective for recurrent SUI and can be performed at the same time as mesh removal. They carry higher surgical morbidity, longer operative time, and greater postoperative voiding dysfunction.
An informed patient can help guide the approach
The retropubic and TOT approaches to tension-free midurethral slings are similar in effectiveness. Most women experience significant improvement of SUI symptoms after sling placement, although many women continue to have some urinary symptoms.
Depending on their training, experience, and personal results—as well as the preferences of an informed patient—surgeons may recommend one approach over the other. In addition, certain clinical situations may favor one sling over another. Studies with longer-term follow-up in different patient subgroups are needed to adequately counsel women about the durability of results.
CASE: Resolved
After discussing the options with your patient, she opts to undergo anterior prolapse repair with concurrent placement of a TOT sling. The surgery is completed without complication. She is discharged later that day without a catheter after demonstrating normal voiding with low residual urine volume. Postoperatively, she reports mild pain referred to the groin. You instruct her to take nonsteroidal anti-inflammatory drugs for pain relief. On her postoperative visit, she reports that the pain is gone and the SUI has almost completely resolved.
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #63: Urinary incontinence in women. Obstet Gynecol. 2005;105(6):1533-1545.
2. Abdel-Fattah M, Ford JA, Lim CP, Madhuvrata P. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: a meta-analysis of effectiveness and complications. Eur Urol. 2011;60(3):468-480.
3. Hinoul P, Vervest HA, den Boon J, et al. A randomized, controlled trial comparing an innovative single-incision sling with an established transobturator sling to treat female stress urinary incontinence. J Urol. 2011;185(4):1356-1362.
4. Barber MD, Weidner AC, Sokol AI, et al. Foundation for Female Health Awareness Research Network. Single-incision mini-sling compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2012;119(2 pt 1):328-337.
5. Petros P, Ulmsten U. Intravaginal sling plasty (IVS): An ambulatory surgical procedure for treatment of female urinary stress incontinence. Scand J Urol Nephrol. 1995;29(1):75-82.
6. Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2011;30(3):284-291.
7. Meschia M, Bertozzi R, Pifarotti P, et al. Perioperative morbidity and early results of a randomised trial comparing TVT and TVT-O. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1257-1261.
8. Richter HE, Albo ME, Zyczynski HM, et al. Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066-2076.
9. Brubaker L, Norton PA, Albo ME, et al. Urinary Incontinence Treatment Network. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011;205(5):498.e1-6.
10. Sung VW, Schleinitz MD, Rardin CR, Ward RM, Myers DL. Comparison of retropubic vs transobturator approach to midurethral slings: a systematic review and meta-analysis. Am J Obstet Gynecol. 2007;197(1):3-11.
11. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2008;111(3):611-621.
12. Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58(2):218-238.
13. Ross S, Robert M, Swaby C, et al. Transobturator tape compared with tension-free vaginal tape for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2009;114(6):1287-1294.
14. Scheiner DA, Betschart C, Wiederkehr S, Seifert B, Fink D, Perucchini D. Twelve months effect on voiding function of retropubic compared with outside-in and inside-out transobturator midurethral slings. Int Urogynecol J. 2012;23(2):197-206.
15. De Souza A, Dwyer PL, Rosamilia A, et al. Sexual function following retropubic TVT and transobturator Monarc sling in women with intrinsic sphincter deficiency: a multicenter prospective study. Int Urogynecol J. 2012;23(2):153-158.
16. Sentilhes L, Berthier A, Loisel C, Descamps P, Marpeau L, Grise P. Female sexual function following surgery for stress urinary incontinence: tension-free vaginal versus transobturator tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):393-399.
17. Deffieux X, Daher N, Mansoor A, Debodinance P, Muhlstein J, Fernandez H. Transobturator TVT-O versus retropubic TVT: results of a multicenter randomized controlled trial at 24 months follow-up. Int Urogynecol J. 2010;21(11):1337-1345.
18. Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112(6):1253-1261.
19. Rechberger T, Futyma K, Jankiewicz K, Adamiak A, Skorupski P. The clinical effectiveness of retropubic (IVS-02) and transobturator (IVS-04) midurethral slings: randomized trial. Eur Urol. 2009;56(1):24-30.
20. Schierlitz L, Dwyer PL, Rosamilia AN, et al. Three-year follow-up of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency. Obstet Gynecol. 2012;119(2 pt 1):321-327.
21. Nager C, Siris L, Litman HJ, et al. Urinary Incontinence Treatment Network. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186(2):597-603.
22. Jain P, Jirschele K, Botros SM, Latthe PM. Effectiveness of midurethral slings in mixed urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2011;22(8):923-932.
23. Sabadell J, Poza JL, Esgueva A, Morales JC, Sanchez-Iglesias JL, Xercavins J. Usefulness of retropubic tape for recurrent stress incontinence after transobturator tape failure. Int Urogynecol J. 2011;22(12):1543-1547.
24. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012; Feb 15;2:CD003881.-
- Placement of TVT-O transobturator tape
- Monarc transobturator procedure
- TVT Exact retropubic sling operation
These videos were selected by Mark D. Walters, MD, and presented courtesy of the International Academy of Pelvic Surgery (IAPS).
Only 15 years ago, when surgery was recommended for patients who had bothersome stress urinary incontinence (SUI), they were offered operations such as suburethral (Kelly) plication, needle urethropexy, open or laparoscopic Burch procedure, and pubovaginal fascial sling procedure. Today, virtually all of these operations have been replaced in general practice by retropubic or transobturator (TOT) midurethral synthetic slings.
Although Burch colposuspension and the pubovaginal fascial sling procedure are effective for both primary and recurrent SUI, they are more invasive than midurethral slings, cause more voiding dysfunction, and have significantly longer recovery times, making them less attractive for most primary and recurrent cases of SUI.
The evolution of SUI surgeries has shifted so far toward midurethral slings that Burch colposuspension and the pubovaginal sling procedure are rarely performed or taught in obstetrics and gynecology or urology residency programs; these procedures are now mostly done in fellowship programs by specialists in female pelvic medicine and reconstructive surgery.
In this article, we describe how an ObGyn generalist can approach the surgical treatment of women who have either primary or recurrent SUI. Using evidence-based principles, when available, we also discuss how different clinical characteristics—as well as the characteristics of the available slings—affect the suitability of the sling for individual patients.
One caveat: This article assumes that the surgeon knows how to, and is able to, perform retropubic and TOT sling procedures equally well. However, when this is not the case, the surgeon should perform the sling procedure that she or he does best, assuming that it is appropriate for that particular patient.

Almost all surgical procedures for stress urinary incontinence performed today involve placement of a retropubic or transobturator midurethral synthetic sling.
Illustration: Craig Zuckerman for OBG Management
CASE: SUI and Stage II anterior vaginal prolapse
A healthy 45-year-old G2P2 woman complains of a 5-year history of worsening SUI symptoms, mostly occurring during activities such as coughing, laughing, and running. The incontinence has become so severe that she requires several pads daily. She is able to void without difficulty or pain, and her bowel movements are normal. She has regular menses, has had a tubal ligation, and is sexually active.
She reports that she has been performing daily Kegel pelvic muscle exercises, without improvement.
On physical examination, she is found to have Stage II anterior vaginal prolapse and urethral hypermobility, with normal uterine and posterior vaginal support. The uterus and ovaries are of normal size.
A full bladder stress test in the office reveals immediate loss of urine from the urethra upon coughing in a semi-sitting position. She voids 325 mL after the examination and has a post-void residual urine volume, as measured by ultrasonography (US), of 25 mL. Urinalysis is negative.
When discussing her goals, the patient expresses a desire for a cure of her urinary incontinence, if possible.
What further testing and treatment options do you offer to her?
If you and the patient agree that surgery is warranted, which procedure do you recommend?
Recommended assessment of women who report SUI
Women who have bothersome urine loss during activities such as exercise, coughing, or laughing should undergo a history, physical examination, and urinalysis. During the pelvic examination, it is important to assess pelvic organ support defects, especially those involving the anterior vagina and urethra. Also note levator ani muscle contraction and strength. In addition, you can use this time to discuss whether the patient is doing, or has done, pelvic muscle (Kegel) exercises; teach the exercises, if necessary; and encourage her to do them in the future.
If the patient has no urinary infection, has performed Kegel exercises without further benefit, and wishes to consider surgical treatment, basic assessment of lower urinary function is indicated. Basic office urodynamic testing includes:
- a measured void
- measurement of post-void residual volume (by catheter or US)
- assessment of bladder sensation and capacity
- provocation for overactive bladder
- a full-bladder cough stress test (a positive test is direct observation of urethral loss of urine upon coughing).
Patients who have a complex history or mixed symptoms, previous failed surgery, or other characteristics that suggest a diagnosis other than simple SUI should undergo formal electronic urodynamic testing.1
Patient selection criteria
Primary sling surgery is an option for patients who have:
- no urinary infection
- normal voiding and bladder-filling function
- urethral hypermobility on examination
- SUI on a cough-stress test
- failure to improve sufficiently with pelvic muscle exercises.
Suburethral slings were initially developed as a treatment for recurrent, urodynamically confirmed SUI, particularly SUI caused by intrinsic sphincter deficiency (ISD). Pubovaginal slings, usually consisting of autologous fascia, were placed at the bladder neck to both support and slightly compress the proximal urethra. Compared with synthetic slings, fascial slings are effective but take longer to place and have a higher rate of surgical morbidity and more postoperative voiding dysfunction. They are now mostly indicated for complex recurrent SUI, usually managed by specialists in female pelvic medicine and reconstructive surgery.
Current slings are lightweight polypropylene mesh
Most slings today are tension-free midurethral slings consisting of synthetic, large-pore polypropylene mesh; they are sold in kits available from several different companies. Sling procedures can also be performed using hand-cut polypropylene mesh and a reusable needle passer.
These slings are placed at the midurethra and work by mechanical kinking or folding of the urethra over the sling, with an increase in intra-abdominal pressure. Ideally, the midurethral sling will not compress the urethra at rest and have no effect on the normal voiding mechanism.
Three main techniques are used to place synthetic midurethral slings:
- the retropubic approach
- the TOT approach
- variations of single-incision “mini-sling” procedures.
Early studies of mini-slings showed few complications but lower effectiveness, compared with retropubic and TOT midurethral slings, according to short-term follow-up data.2-4 A mini-sling might be an option for some patients in whom surgical complications must be kept to a minimum; otherwise, they will not be discussed further.
Retropubic midurethral slings
The tension-free vaginal tape (TVT) procedure described by Petros and Ulmsten was the first synthetic midurethral sling.5 This ambulatory procedure aims to restore the pubourethral ligament and suburethral vaginal hammock by using specially designed needles attached to synthetic sling material.
The synthetic sling consists of polypropylene, approximately 1 cm wide and 40 cm long. The sling material is attached to two stainless steel needles that are passed from a vaginal incision made at the level of the midurethra, through the retropubic space, and exiting at a previously created mark or stab incision in the suprapubic area (FIGURE 1).
Variations of the retropubic midurethral sling have been developed, with sling passers going from the vagina upward (“bottom to top”) and also from the suprapubic area downward (“top to bottom”). A recent Cochrane review reported that the bottom-to-top variation is slightly more effective.6

FIGURE 1 Retropubic sling
Placement of the tension-free vaginal tape trocar into the retropubic space.
Illustration: Craig Zuckerman for OBG Management
Transobturator midurethral slings
The TOT sling has become one of the most popular and effective surgical treatments for female SUI worldwide (Video 1 and Video 2). It is a relatively rapid and low-risk surgery that is comparable to other surgical options in effectiveness while avoiding an abdominal incision and the passage of a needle or trocar through the space of Retzius.
The TOT sling lies flatter under the urethra and carries a lower risk of urethral obstruction, urinary retention, and subsequent need for sling release, compared with retropubic slings.7-9 Compared with the retropubic TVT, the TOT sling produces similar rates of cure, with fewer bladder perforations and less postoperative irritative voiding symptoms.6,10-12 It nearly eliminates the rare but catastrophic risk of bowel or major vessel perforation. The trade-off is that patients experience more complications referable to the groin (pain and leg weakness or numbness) with the TOT approach.9,13
All TOT slings on the market consist of a large-pore, lightweight, polypropylene mesh strip, usually covered with a plastic sheath. Various devices are used to place the sling, but most of them involve a helical trocar that curves around the ischiopubic ramus, passing through the inner thigh and obturator membrane to a space created in the ipsilateral peri-urethral tissues.
TOT slings can be placed outside-to-inside or inside-to-outside (FIGURE 2), and the indications, effectiveness, and frequency of complications seem to be similar between these two approaches.12 One study found a higher frequency of new sexual dysfunction (tender, palpable sling; penile pain in male partner) in women after the “outside-in” approach,14 but this clinical issue has not been observed in all studies.15,16

FIGURE 2 TOT sling variations
Placement of the transobturator (TOT) sling helical trocar using the (A) “outside-in” variation and (B) “inside-out” variation.
Illustration: Craig Zuckerman for OBG Management
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography
© 2005-2012. All Rights Reserved.
Success rates are similar for retropubic and TOT slings
Despite differences in technique and brand of mesh used, treatment success rates for uncomplicated primary SUI are similar for the retropubic (Video 3) and TOT tension-free slings.6-8,10-12,17 The percentage of patients treated successfully depends on the definition used, ranging from a high of 96% to a low of 60%. When the definition of success is restricted to stress incontinence symptoms, especially over a short period of time, the reported effectiveness is high.
In contrast, when the definition of success includes incontinence of any type, the reported effectiveness is lower. For example, in the study that reported 60% effectiveness, success was defined as no incontinence symptoms of any type, a negative cough stress test, and no retreatment for stress incontinence or postoperative urinary retention.11
Retropubic slings, especially TVT, may be somewhat more effective for ISD,18-20 although this conclusion must be tempered by the small number of studies addressing the issue and differences in the diagnosis of ISD.21
Some studies have reported good success in treating mixed urinary incontinence with the retropubic and TOT slings,2,8 although other studies have reported that the initial benefit for urgency or urge incontinence is not sustained over time, compared with the benefit for stress incontinence.22 It is important to counsel patients before surgery that improvement in stress incontinence symptoms and general satisfaction is highly likely, but perfect bladder function is not.
Serious complications are uncommon
Complications are common after both retropubic and TOT slings, although serious complications are uncommon. Cystitis and temporary voiding difficulties are the most common problems after a sling procedure. If the patient is unable to void on the day of surgery, it is reasonable to discharge her with a Foley catheter in place for a few days or teach her to perform intermittent self-catheterization at home. In most cases, normal voiding will resume within a few days. Cystitis is at least partially related to the surgery itself and the duration of postoperative catheterization.
The frequency of some complications differs between the retropubic and TOT approaches to midurethral slings. For example, some literature suggests that irritative voiding symptoms such as urgency or voiding difficulty are somewhat less common after TOT slings, compared with retropubic slings. However, symptoms referable to the groin (pain and leg weakness or numbness) occur more commonly with the TOT approach.6
After placement of a TOT sling, 10% to 15% of women experience temporary inner thigh or groin pain or leg weakness and are usually managed conservatively with nonsteroidal anti-inflammatory drugs and physical therapy. Long-term or severe complications related to TOT sling passage are rare.
Major intraoperative complications are rare
The rate of these complications does not differ between retropubic and TOT approaches. Minor intraoperative complications—primarily, bladder perforation—occur more commonly with the retropubic approach.6
Bladder perforation with the TVT occurs in 4% to 7% of patients. However, the clinical significance of bladder perforation is minimal as long as the surgeon performs careful cystoscopy, recognizes bladder perforation, and repositions the trocar and mesh outside the bladder lumen. Bladder perforation caused by the trocar usually does not require specific treatment (except repositioning of the trocar outside the bladder lumen) and rarely results in later problems.
Mesh exposures occur with similar frequency for the different sling types as long as large-pore lightweight polypropylene is used. Dehiscence of the suburethral incision (mesh exposure) is uncommon with midurethral slings, occurring in 1% to 2% of patients. Dehiscence can be managed with estrogen cream or trimming of the exposed portion of the sling in the office. If symptoms or signs persist, removal of the exposed segment or the entire central portion of the sling, with closure of the vaginal epithelium, is indicated to allow for healing and resolution of symptoms. However, removal may lead to recurrence of the original SUI symptoms.
Retropubic hematomas occur in 1% to 2% of patients after placement of a retropubic sling, but major vascular injuries are rare—occurring in, perhaps, 3 in every 1,000 cases.
Bowel perforations are very rare but serious complications. A retropubic sling should be placed with caution or avoided in women who have a history of peritonitis, bowel surgery, ruptured appendix, or known extensive pelvic adhesions.
Major vascular injuries are also rare with TOT slings, occurring in approximately 1 to 2 cases in every 1,000.
Bladder injury occurs much less frequently after placement of a TOT sling, compared with the retropubic approach, although one study reported bladder injury in 2% of TOT cases.17 Although bladder injury is uncommon with the TOT approach, the morbidity associated with delayed detection of bladder injury is much higher than the morbidity associated with intraoperative detection and management. Therefore, we believe that cystoscopy should be performed in all TOT and retropubic sling procedures to either exclude bladder damage or detect and appropriately manage it.
For reassurance that intraoperative and postoperative blood loss is not excessive, it is reasonable to check one hemoglobin level before discharge, if desired.
How to individualize the choice of sling
Patients who have primary SUI: Retropubic or TOT sling. Objective and subjective success rates are similar, regardless of approach, and serious complications are infrequent. The retropubic approach has longer-term evidence of sustained benefit, compared with the newer TOT approach. We tend to treat younger patients with TVT and older patients with TOT. Surgeon experience and informed patient preferences may dictate the choice of sling (TABLE).
What we recommend surgically for our patients who have SUI—and why
| Clinical problem and patient characteristics | Surgery | Rationale |
|---|---|---|
| Primary SUI with urethral hypermobility—young patient | TVT | TVT has similar effectiveness and more long-term data than TOT; TVT may result in less sexual pain than TOT |
| Primary SUI with urethral hypermobility—older patient; leak point pressure >60 cmH20 | TOT | Similar effectiveness, fewer complications with TOT |
| Recurrent SUI with urethral hypermobility—any age; leak point pressure >60 cmH20 | TVT | Limited data suggest effectiveness of TVT after TOT failure |
| Recurrent SUI with urethral hypermobility—leak point pressure <60 cmH20 (ISD) | TVT or pubovaginal fascial sling | Some but not all data indicate that TVT is more effective for ISD; fascial slings in expert hands are effective, based on cohort studies |
| Recurrent SUI with nonmobile bladder neck; any leak point pressure | Urethral bulking | All sling procedures have lowered effectiveness when the bladder neck is immobile |
| SUI mixed with dominant urgency or voiding dysfunction | TOT | TOT improves or does not exacerbate mixed urinary symptoms to the extent that TVT may |
| SUI with prolapse and planned vaginal prolapse repair | TVT or TOT | Limited data support similar effectiveness for either approach |
| “Occult” SUI with prolapse reduced and planned vaginal prolapse repair | TOT or “wait and see” | TOT has a lower chance of creating new irritative voiding symptoms; “wait-and-see” approach allows treatment of SUI if it develops after prolapse repair |
| Recurrent SUI with previous synthetic sling mesh complication (or patients who desire treatment without mesh) | Pubovaginal fascial sling or Burch colposuspension | These nonmesh options are effective for recurrent SUI, but have higher surgical morbidity |
| ISD = intrinsic sphincter deficiency; SUI = stress urinary incontinence; TVT = tension-free vaginal tape or similar retropubic midurethral sling; TOT = transobturator sling placed either by outside-in or inside-out variations | ||
Patients who have recurrent SUI: Retropubic sling. Comparative data are limited regarding the retropubic and TOT approaches for recurrent SUI that does not involve ISD. One case series reported good results with the use of retropubic TVT for recurrent SUI after an initial TOT approach.23
Patients who have ISD: Retropubic sling (synthetic midurethral sling or fascial sling placed at the bladder neck). A few studies suggest that patients with ISD have better outcomes with the retropubic approach.19,20 However, with differing definitions of ISD and relatively few patients with ISD included in these trials, it is not possible to conclude definitively that the retropubic approach is more effective than the TOT approach for patients who have SUI and ISD. However, the retropubic approach has longer-term data to support its effectiveness; therefore, with some but not all evidence suggesting its superiority for ISD, it is reasonable to choose the retropubic midurethral approach.
A pubovaginal fascial sling placed at the proximal urethra is also an effective option, based on numerous cohort studies.12
Patients who have recurrent SUI or ISD, or both, with a non-mobile bladder neck: Urethral bulking. Although data are scant, urethral injection therapy is beneficial for SUI in the short-term, but long-term studies are lacking. Bulking agents include silicone particles, calcium hydroxylapatite, and carbon spheres; studies have not shown one to be more or less efficacious than the others.24
It is reasonable to use urethral bulking first in these patients as the morbidity is very low and some patients become continent. A retropubic sling can be performed if urethral bulking fails to adequately improve symptoms, although the effectiveness is lower in this population than in women with SUI and urethral hypermobility.
Patients who have mixed stress and urge incontinence or voiding dysfunction: TOT sling. Limited data suggest that the TOT approach improves symptoms of mixed incontinence—or, at least, exacerbates them to a lesser degree than the retropubic approach. Rarely is a sling release needed to treat obstructive urinary symptoms after the TOT approach.
Patients who have prolapse and SUI: Retropubic or TOT sling. When a sling procedure is performed at the same time as reconstructive surgery for prolapse, it has similar effectiveness, regardless of whether the retropubic or TOT approach is selected.8,11 A sling placed during prolapse surgery (placed through a separate midurethral incision) appears to be as effective as a sling placed as a sole procedure.
Patients who have prolapse and occult SUI: TOT sling. If you recommend a sling to prevent SUI after prolapse surgery by any route, pick the sling with acceptable efficacy and the lowest rates of complications and voiding dysfunction. Patients are especially intolerant of complications from a sling to prevent SUI. Given the ease of placement and low morbidity of a later outpatient sling procedure, it is also reasonable to offer patients the “wait-and-see” alternative to see if SUI develops after prolapse surgery and only then proceeding with sling surgery. In this way, overtreatment is avoided, and any complications that occur after sling surgery for SUI treatment may be better tolerated by the patient. The preferences of an informed patient may guide decisions in this setting.
Patients who have recurrent SUI with mesh complication: Pubovaginal fascial sling or Burch colposuspension. These non-mesh options are effective for recurrent SUI and can be performed at the same time as mesh removal. They carry higher surgical morbidity, longer operative time, and greater postoperative voiding dysfunction.
An informed patient can help guide the approach
The retropubic and TOT approaches to tension-free midurethral slings are similar in effectiveness. Most women experience significant improvement of SUI symptoms after sling placement, although many women continue to have some urinary symptoms.
Depending on their training, experience, and personal results—as well as the preferences of an informed patient—surgeons may recommend one approach over the other. In addition, certain clinical situations may favor one sling over another. Studies with longer-term follow-up in different patient subgroups are needed to adequately counsel women about the durability of results.
CASE: Resolved
After discussing the options with your patient, she opts to undergo anterior prolapse repair with concurrent placement of a TOT sling. The surgery is completed without complication. She is discharged later that day without a catheter after demonstrating normal voiding with low residual urine volume. Postoperatively, she reports mild pain referred to the groin. You instruct her to take nonsteroidal anti-inflammatory drugs for pain relief. On her postoperative visit, she reports that the pain is gone and the SUI has almost completely resolved.
We want to hear from you! Tell us what you think.
- Placement of TVT-O transobturator tape
- Monarc transobturator procedure
- TVT Exact retropubic sling operation
These videos were selected by Mark D. Walters, MD, and presented courtesy of the International Academy of Pelvic Surgery (IAPS).
Only 15 years ago, when surgery was recommended for patients who had bothersome stress urinary incontinence (SUI), they were offered operations such as suburethral (Kelly) plication, needle urethropexy, open or laparoscopic Burch procedure, and pubovaginal fascial sling procedure. Today, virtually all of these operations have been replaced in general practice by retropubic or transobturator (TOT) midurethral synthetic slings.
Although Burch colposuspension and the pubovaginal fascial sling procedure are effective for both primary and recurrent SUI, they are more invasive than midurethral slings, cause more voiding dysfunction, and have significantly longer recovery times, making them less attractive for most primary and recurrent cases of SUI.
The evolution of SUI surgeries has shifted so far toward midurethral slings that Burch colposuspension and the pubovaginal sling procedure are rarely performed or taught in obstetrics and gynecology or urology residency programs; these procedures are now mostly done in fellowship programs by specialists in female pelvic medicine and reconstructive surgery.
In this article, we describe how an ObGyn generalist can approach the surgical treatment of women who have either primary or recurrent SUI. Using evidence-based principles, when available, we also discuss how different clinical characteristics—as well as the characteristics of the available slings—affect the suitability of the sling for individual patients.
One caveat: This article assumes that the surgeon knows how to, and is able to, perform retropubic and TOT sling procedures equally well. However, when this is not the case, the surgeon should perform the sling procedure that she or he does best, assuming that it is appropriate for that particular patient.

Almost all surgical procedures for stress urinary incontinence performed today involve placement of a retropubic or transobturator midurethral synthetic sling.
Illustration: Craig Zuckerman for OBG Management
CASE: SUI and Stage II anterior vaginal prolapse
A healthy 45-year-old G2P2 woman complains of a 5-year history of worsening SUI symptoms, mostly occurring during activities such as coughing, laughing, and running. The incontinence has become so severe that she requires several pads daily. She is able to void without difficulty or pain, and her bowel movements are normal. She has regular menses, has had a tubal ligation, and is sexually active.
She reports that she has been performing daily Kegel pelvic muscle exercises, without improvement.
On physical examination, she is found to have Stage II anterior vaginal prolapse and urethral hypermobility, with normal uterine and posterior vaginal support. The uterus and ovaries are of normal size.
A full bladder stress test in the office reveals immediate loss of urine from the urethra upon coughing in a semi-sitting position. She voids 325 mL after the examination and has a post-void residual urine volume, as measured by ultrasonography (US), of 25 mL. Urinalysis is negative.
When discussing her goals, the patient expresses a desire for a cure of her urinary incontinence, if possible.
What further testing and treatment options do you offer to her?
If you and the patient agree that surgery is warranted, which procedure do you recommend?
Recommended assessment of women who report SUI
Women who have bothersome urine loss during activities such as exercise, coughing, or laughing should undergo a history, physical examination, and urinalysis. During the pelvic examination, it is important to assess pelvic organ support defects, especially those involving the anterior vagina and urethra. Also note levator ani muscle contraction and strength. In addition, you can use this time to discuss whether the patient is doing, or has done, pelvic muscle (Kegel) exercises; teach the exercises, if necessary; and encourage her to do them in the future.
If the patient has no urinary infection, has performed Kegel exercises without further benefit, and wishes to consider surgical treatment, basic assessment of lower urinary function is indicated. Basic office urodynamic testing includes:
- a measured void
- measurement of post-void residual volume (by catheter or US)
- assessment of bladder sensation and capacity
- provocation for overactive bladder
- a full-bladder cough stress test (a positive test is direct observation of urethral loss of urine upon coughing).
Patients who have a complex history or mixed symptoms, previous failed surgery, or other characteristics that suggest a diagnosis other than simple SUI should undergo formal electronic urodynamic testing.1
Patient selection criteria
Primary sling surgery is an option for patients who have:
- no urinary infection
- normal voiding and bladder-filling function
- urethral hypermobility on examination
- SUI on a cough-stress test
- failure to improve sufficiently with pelvic muscle exercises.
Suburethral slings were initially developed as a treatment for recurrent, urodynamically confirmed SUI, particularly SUI caused by intrinsic sphincter deficiency (ISD). Pubovaginal slings, usually consisting of autologous fascia, were placed at the bladder neck to both support and slightly compress the proximal urethra. Compared with synthetic slings, fascial slings are effective but take longer to place and have a higher rate of surgical morbidity and more postoperative voiding dysfunction. They are now mostly indicated for complex recurrent SUI, usually managed by specialists in female pelvic medicine and reconstructive surgery.
Current slings are lightweight polypropylene mesh
Most slings today are tension-free midurethral slings consisting of synthetic, large-pore polypropylene mesh; they are sold in kits available from several different companies. Sling procedures can also be performed using hand-cut polypropylene mesh and a reusable needle passer.
These slings are placed at the midurethra and work by mechanical kinking or folding of the urethra over the sling, with an increase in intra-abdominal pressure. Ideally, the midurethral sling will not compress the urethra at rest and have no effect on the normal voiding mechanism.
Three main techniques are used to place synthetic midurethral slings:
- the retropubic approach
- the TOT approach
- variations of single-incision “mini-sling” procedures.
Early studies of mini-slings showed few complications but lower effectiveness, compared with retropubic and TOT midurethral slings, according to short-term follow-up data.2-4 A mini-sling might be an option for some patients in whom surgical complications must be kept to a minimum; otherwise, they will not be discussed further.
Retropubic midurethral slings
The tension-free vaginal tape (TVT) procedure described by Petros and Ulmsten was the first synthetic midurethral sling.5 This ambulatory procedure aims to restore the pubourethral ligament and suburethral vaginal hammock by using specially designed needles attached to synthetic sling material.
The synthetic sling consists of polypropylene, approximately 1 cm wide and 40 cm long. The sling material is attached to two stainless steel needles that are passed from a vaginal incision made at the level of the midurethra, through the retropubic space, and exiting at a previously created mark or stab incision in the suprapubic area (FIGURE 1).
Variations of the retropubic midurethral sling have been developed, with sling passers going from the vagina upward (“bottom to top”) and also from the suprapubic area downward (“top to bottom”). A recent Cochrane review reported that the bottom-to-top variation is slightly more effective.6

FIGURE 1 Retropubic sling
Placement of the tension-free vaginal tape trocar into the retropubic space.
Illustration: Craig Zuckerman for OBG Management
Transobturator midurethral slings
The TOT sling has become one of the most popular and effective surgical treatments for female SUI worldwide (Video 1 and Video 2). It is a relatively rapid and low-risk surgery that is comparable to other surgical options in effectiveness while avoiding an abdominal incision and the passage of a needle or trocar through the space of Retzius.
The TOT sling lies flatter under the urethra and carries a lower risk of urethral obstruction, urinary retention, and subsequent need for sling release, compared with retropubic slings.7-9 Compared with the retropubic TVT, the TOT sling produces similar rates of cure, with fewer bladder perforations and less postoperative irritative voiding symptoms.6,10-12 It nearly eliminates the rare but catastrophic risk of bowel or major vessel perforation. The trade-off is that patients experience more complications referable to the groin (pain and leg weakness or numbness) with the TOT approach.9,13
All TOT slings on the market consist of a large-pore, lightweight, polypropylene mesh strip, usually covered with a plastic sheath. Various devices are used to place the sling, but most of them involve a helical trocar that curves around the ischiopubic ramus, passing through the inner thigh and obturator membrane to a space created in the ipsilateral peri-urethral tissues.
TOT slings can be placed outside-to-inside or inside-to-outside (FIGURE 2), and the indications, effectiveness, and frequency of complications seem to be similar between these two approaches.12 One study found a higher frequency of new sexual dysfunction (tender, palpable sling; penile pain in male partner) in women after the “outside-in” approach,14 but this clinical issue has not been observed in all studies.15,16

FIGURE 2 TOT sling variations
Placement of the transobturator (TOT) sling helical trocar using the (A) “outside-in” variation and (B) “inside-out” variation.
Illustration: Craig Zuckerman for OBG Management
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography
© 2005-2012. All Rights Reserved.
Success rates are similar for retropubic and TOT slings
Despite differences in technique and brand of mesh used, treatment success rates for uncomplicated primary SUI are similar for the retropubic (Video 3) and TOT tension-free slings.6-8,10-12,17 The percentage of patients treated successfully depends on the definition used, ranging from a high of 96% to a low of 60%. When the definition of success is restricted to stress incontinence symptoms, especially over a short period of time, the reported effectiveness is high.
In contrast, when the definition of success includes incontinence of any type, the reported effectiveness is lower. For example, in the study that reported 60% effectiveness, success was defined as no incontinence symptoms of any type, a negative cough stress test, and no retreatment for stress incontinence or postoperative urinary retention.11
Retropubic slings, especially TVT, may be somewhat more effective for ISD,18-20 although this conclusion must be tempered by the small number of studies addressing the issue and differences in the diagnosis of ISD.21
Some studies have reported good success in treating mixed urinary incontinence with the retropubic and TOT slings,2,8 although other studies have reported that the initial benefit for urgency or urge incontinence is not sustained over time, compared with the benefit for stress incontinence.22 It is important to counsel patients before surgery that improvement in stress incontinence symptoms and general satisfaction is highly likely, but perfect bladder function is not.
Serious complications are uncommon
Complications are common after both retropubic and TOT slings, although serious complications are uncommon. Cystitis and temporary voiding difficulties are the most common problems after a sling procedure. If the patient is unable to void on the day of surgery, it is reasonable to discharge her with a Foley catheter in place for a few days or teach her to perform intermittent self-catheterization at home. In most cases, normal voiding will resume within a few days. Cystitis is at least partially related to the surgery itself and the duration of postoperative catheterization.
The frequency of some complications differs between the retropubic and TOT approaches to midurethral slings. For example, some literature suggests that irritative voiding symptoms such as urgency or voiding difficulty are somewhat less common after TOT slings, compared with retropubic slings. However, symptoms referable to the groin (pain and leg weakness or numbness) occur more commonly with the TOT approach.6
After placement of a TOT sling, 10% to 15% of women experience temporary inner thigh or groin pain or leg weakness and are usually managed conservatively with nonsteroidal anti-inflammatory drugs and physical therapy. Long-term or severe complications related to TOT sling passage are rare.
Major intraoperative complications are rare
The rate of these complications does not differ between retropubic and TOT approaches. Minor intraoperative complications—primarily, bladder perforation—occur more commonly with the retropubic approach.6
Bladder perforation with the TVT occurs in 4% to 7% of patients. However, the clinical significance of bladder perforation is minimal as long as the surgeon performs careful cystoscopy, recognizes bladder perforation, and repositions the trocar and mesh outside the bladder lumen. Bladder perforation caused by the trocar usually does not require specific treatment (except repositioning of the trocar outside the bladder lumen) and rarely results in later problems.
Mesh exposures occur with similar frequency for the different sling types as long as large-pore lightweight polypropylene is used. Dehiscence of the suburethral incision (mesh exposure) is uncommon with midurethral slings, occurring in 1% to 2% of patients. Dehiscence can be managed with estrogen cream or trimming of the exposed portion of the sling in the office. If symptoms or signs persist, removal of the exposed segment or the entire central portion of the sling, with closure of the vaginal epithelium, is indicated to allow for healing and resolution of symptoms. However, removal may lead to recurrence of the original SUI symptoms.
Retropubic hematomas occur in 1% to 2% of patients after placement of a retropubic sling, but major vascular injuries are rare—occurring in, perhaps, 3 in every 1,000 cases.
Bowel perforations are very rare but serious complications. A retropubic sling should be placed with caution or avoided in women who have a history of peritonitis, bowel surgery, ruptured appendix, or known extensive pelvic adhesions.
Major vascular injuries are also rare with TOT slings, occurring in approximately 1 to 2 cases in every 1,000.
Bladder injury occurs much less frequently after placement of a TOT sling, compared with the retropubic approach, although one study reported bladder injury in 2% of TOT cases.17 Although bladder injury is uncommon with the TOT approach, the morbidity associated with delayed detection of bladder injury is much higher than the morbidity associated with intraoperative detection and management. Therefore, we believe that cystoscopy should be performed in all TOT and retropubic sling procedures to either exclude bladder damage or detect and appropriately manage it.
For reassurance that intraoperative and postoperative blood loss is not excessive, it is reasonable to check one hemoglobin level before discharge, if desired.
How to individualize the choice of sling
Patients who have primary SUI: Retropubic or TOT sling. Objective and subjective success rates are similar, regardless of approach, and serious complications are infrequent. The retropubic approach has longer-term evidence of sustained benefit, compared with the newer TOT approach. We tend to treat younger patients with TVT and older patients with TOT. Surgeon experience and informed patient preferences may dictate the choice of sling (TABLE).
What we recommend surgically for our patients who have SUI—and why
| Clinical problem and patient characteristics | Surgery | Rationale |
|---|---|---|
| Primary SUI with urethral hypermobility—young patient | TVT | TVT has similar effectiveness and more long-term data than TOT; TVT may result in less sexual pain than TOT |
| Primary SUI with urethral hypermobility—older patient; leak point pressure >60 cmH20 | TOT | Similar effectiveness, fewer complications with TOT |
| Recurrent SUI with urethral hypermobility—any age; leak point pressure >60 cmH20 | TVT | Limited data suggest effectiveness of TVT after TOT failure |
| Recurrent SUI with urethral hypermobility—leak point pressure <60 cmH20 (ISD) | TVT or pubovaginal fascial sling | Some but not all data indicate that TVT is more effective for ISD; fascial slings in expert hands are effective, based on cohort studies |
| Recurrent SUI with nonmobile bladder neck; any leak point pressure | Urethral bulking | All sling procedures have lowered effectiveness when the bladder neck is immobile |
| SUI mixed with dominant urgency or voiding dysfunction | TOT | TOT improves or does not exacerbate mixed urinary symptoms to the extent that TVT may |
| SUI with prolapse and planned vaginal prolapse repair | TVT or TOT | Limited data support similar effectiveness for either approach |
| “Occult” SUI with prolapse reduced and planned vaginal prolapse repair | TOT or “wait and see” | TOT has a lower chance of creating new irritative voiding symptoms; “wait-and-see” approach allows treatment of SUI if it develops after prolapse repair |
| Recurrent SUI with previous synthetic sling mesh complication (or patients who desire treatment without mesh) | Pubovaginal fascial sling or Burch colposuspension | These nonmesh options are effective for recurrent SUI, but have higher surgical morbidity |
| ISD = intrinsic sphincter deficiency; SUI = stress urinary incontinence; TVT = tension-free vaginal tape or similar retropubic midurethral sling; TOT = transobturator sling placed either by outside-in or inside-out variations | ||
Patients who have recurrent SUI: Retropubic sling. Comparative data are limited regarding the retropubic and TOT approaches for recurrent SUI that does not involve ISD. One case series reported good results with the use of retropubic TVT for recurrent SUI after an initial TOT approach.23
Patients who have ISD: Retropubic sling (synthetic midurethral sling or fascial sling placed at the bladder neck). A few studies suggest that patients with ISD have better outcomes with the retropubic approach.19,20 However, with differing definitions of ISD and relatively few patients with ISD included in these trials, it is not possible to conclude definitively that the retropubic approach is more effective than the TOT approach for patients who have SUI and ISD. However, the retropubic approach has longer-term data to support its effectiveness; therefore, with some but not all evidence suggesting its superiority for ISD, it is reasonable to choose the retropubic midurethral approach.
A pubovaginal fascial sling placed at the proximal urethra is also an effective option, based on numerous cohort studies.12
Patients who have recurrent SUI or ISD, or both, with a non-mobile bladder neck: Urethral bulking. Although data are scant, urethral injection therapy is beneficial for SUI in the short-term, but long-term studies are lacking. Bulking agents include silicone particles, calcium hydroxylapatite, and carbon spheres; studies have not shown one to be more or less efficacious than the others.24
It is reasonable to use urethral bulking first in these patients as the morbidity is very low and some patients become continent. A retropubic sling can be performed if urethral bulking fails to adequately improve symptoms, although the effectiveness is lower in this population than in women with SUI and urethral hypermobility.
Patients who have mixed stress and urge incontinence or voiding dysfunction: TOT sling. Limited data suggest that the TOT approach improves symptoms of mixed incontinence—or, at least, exacerbates them to a lesser degree than the retropubic approach. Rarely is a sling release needed to treat obstructive urinary symptoms after the TOT approach.
Patients who have prolapse and SUI: Retropubic or TOT sling. When a sling procedure is performed at the same time as reconstructive surgery for prolapse, it has similar effectiveness, regardless of whether the retropubic or TOT approach is selected.8,11 A sling placed during prolapse surgery (placed through a separate midurethral incision) appears to be as effective as a sling placed as a sole procedure.
Patients who have prolapse and occult SUI: TOT sling. If you recommend a sling to prevent SUI after prolapse surgery by any route, pick the sling with acceptable efficacy and the lowest rates of complications and voiding dysfunction. Patients are especially intolerant of complications from a sling to prevent SUI. Given the ease of placement and low morbidity of a later outpatient sling procedure, it is also reasonable to offer patients the “wait-and-see” alternative to see if SUI develops after prolapse surgery and only then proceeding with sling surgery. In this way, overtreatment is avoided, and any complications that occur after sling surgery for SUI treatment may be better tolerated by the patient. The preferences of an informed patient may guide decisions in this setting.
Patients who have recurrent SUI with mesh complication: Pubovaginal fascial sling or Burch colposuspension. These non-mesh options are effective for recurrent SUI and can be performed at the same time as mesh removal. They carry higher surgical morbidity, longer operative time, and greater postoperative voiding dysfunction.
An informed patient can help guide the approach
The retropubic and TOT approaches to tension-free midurethral slings are similar in effectiveness. Most women experience significant improvement of SUI symptoms after sling placement, although many women continue to have some urinary symptoms.
Depending on their training, experience, and personal results—as well as the preferences of an informed patient—surgeons may recommend one approach over the other. In addition, certain clinical situations may favor one sling over another. Studies with longer-term follow-up in different patient subgroups are needed to adequately counsel women about the durability of results.
CASE: Resolved
After discussing the options with your patient, she opts to undergo anterior prolapse repair with concurrent placement of a TOT sling. The surgery is completed without complication. She is discharged later that day without a catheter after demonstrating normal voiding with low residual urine volume. Postoperatively, she reports mild pain referred to the groin. You instruct her to take nonsteroidal anti-inflammatory drugs for pain relief. On her postoperative visit, she reports that the pain is gone and the SUI has almost completely resolved.
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #63: Urinary incontinence in women. Obstet Gynecol. 2005;105(6):1533-1545.
2. Abdel-Fattah M, Ford JA, Lim CP, Madhuvrata P. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: a meta-analysis of effectiveness and complications. Eur Urol. 2011;60(3):468-480.
3. Hinoul P, Vervest HA, den Boon J, et al. A randomized, controlled trial comparing an innovative single-incision sling with an established transobturator sling to treat female stress urinary incontinence. J Urol. 2011;185(4):1356-1362.
4. Barber MD, Weidner AC, Sokol AI, et al. Foundation for Female Health Awareness Research Network. Single-incision mini-sling compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2012;119(2 pt 1):328-337.
5. Petros P, Ulmsten U. Intravaginal sling plasty (IVS): An ambulatory surgical procedure for treatment of female urinary stress incontinence. Scand J Urol Nephrol. 1995;29(1):75-82.
6. Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2011;30(3):284-291.
7. Meschia M, Bertozzi R, Pifarotti P, et al. Perioperative morbidity and early results of a randomised trial comparing TVT and TVT-O. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1257-1261.
8. Richter HE, Albo ME, Zyczynski HM, et al. Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066-2076.
9. Brubaker L, Norton PA, Albo ME, et al. Urinary Incontinence Treatment Network. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011;205(5):498.e1-6.
10. Sung VW, Schleinitz MD, Rardin CR, Ward RM, Myers DL. Comparison of retropubic vs transobturator approach to midurethral slings: a systematic review and meta-analysis. Am J Obstet Gynecol. 2007;197(1):3-11.
11. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2008;111(3):611-621.
12. Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58(2):218-238.
13. Ross S, Robert M, Swaby C, et al. Transobturator tape compared with tension-free vaginal tape for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2009;114(6):1287-1294.
14. Scheiner DA, Betschart C, Wiederkehr S, Seifert B, Fink D, Perucchini D. Twelve months effect on voiding function of retropubic compared with outside-in and inside-out transobturator midurethral slings. Int Urogynecol J. 2012;23(2):197-206.
15. De Souza A, Dwyer PL, Rosamilia A, et al. Sexual function following retropubic TVT and transobturator Monarc sling in women with intrinsic sphincter deficiency: a multicenter prospective study. Int Urogynecol J. 2012;23(2):153-158.
16. Sentilhes L, Berthier A, Loisel C, Descamps P, Marpeau L, Grise P. Female sexual function following surgery for stress urinary incontinence: tension-free vaginal versus transobturator tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):393-399.
17. Deffieux X, Daher N, Mansoor A, Debodinance P, Muhlstein J, Fernandez H. Transobturator TVT-O versus retropubic TVT: results of a multicenter randomized controlled trial at 24 months follow-up. Int Urogynecol J. 2010;21(11):1337-1345.
18. Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112(6):1253-1261.
19. Rechberger T, Futyma K, Jankiewicz K, Adamiak A, Skorupski P. The clinical effectiveness of retropubic (IVS-02) and transobturator (IVS-04) midurethral slings: randomized trial. Eur Urol. 2009;56(1):24-30.
20. Schierlitz L, Dwyer PL, Rosamilia AN, et al. Three-year follow-up of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency. Obstet Gynecol. 2012;119(2 pt 1):321-327.
21. Nager C, Siris L, Litman HJ, et al. Urinary Incontinence Treatment Network. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186(2):597-603.
22. Jain P, Jirschele K, Botros SM, Latthe PM. Effectiveness of midurethral slings in mixed urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2011;22(8):923-932.
23. Sabadell J, Poza JL, Esgueva A, Morales JC, Sanchez-Iglesias JL, Xercavins J. Usefulness of retropubic tape for recurrent stress incontinence after transobturator tape failure. Int Urogynecol J. 2011;22(12):1543-1547.
24. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012; Feb 15;2:CD003881.-
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #63: Urinary incontinence in women. Obstet Gynecol. 2005;105(6):1533-1545.
2. Abdel-Fattah M, Ford JA, Lim CP, Madhuvrata P. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: a meta-analysis of effectiveness and complications. Eur Urol. 2011;60(3):468-480.
3. Hinoul P, Vervest HA, den Boon J, et al. A randomized, controlled trial comparing an innovative single-incision sling with an established transobturator sling to treat female stress urinary incontinence. J Urol. 2011;185(4):1356-1362.
4. Barber MD, Weidner AC, Sokol AI, et al. Foundation for Female Health Awareness Research Network. Single-incision mini-sling compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2012;119(2 pt 1):328-337.
5. Petros P, Ulmsten U. Intravaginal sling plasty (IVS): An ambulatory surgical procedure for treatment of female urinary stress incontinence. Scand J Urol Nephrol. 1995;29(1):75-82.
6. Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2011;30(3):284-291.
7. Meschia M, Bertozzi R, Pifarotti P, et al. Perioperative morbidity and early results of a randomised trial comparing TVT and TVT-O. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1257-1261.
8. Richter HE, Albo ME, Zyczynski HM, et al. Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066-2076.
9. Brubaker L, Norton PA, Albo ME, et al. Urinary Incontinence Treatment Network. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011;205(5):498.e1-6.
10. Sung VW, Schleinitz MD, Rardin CR, Ward RM, Myers DL. Comparison of retropubic vs transobturator approach to midurethral slings: a systematic review and meta-analysis. Am J Obstet Gynecol. 2007;197(1):3-11.
11. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2008;111(3):611-621.
12. Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58(2):218-238.
13. Ross S, Robert M, Swaby C, et al. Transobturator tape compared with tension-free vaginal tape for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2009;114(6):1287-1294.
14. Scheiner DA, Betschart C, Wiederkehr S, Seifert B, Fink D, Perucchini D. Twelve months effect on voiding function of retropubic compared with outside-in and inside-out transobturator midurethral slings. Int Urogynecol J. 2012;23(2):197-206.
15. De Souza A, Dwyer PL, Rosamilia A, et al. Sexual function following retropubic TVT and transobturator Monarc sling in women with intrinsic sphincter deficiency: a multicenter prospective study. Int Urogynecol J. 2012;23(2):153-158.
16. Sentilhes L, Berthier A, Loisel C, Descamps P, Marpeau L, Grise P. Female sexual function following surgery for stress urinary incontinence: tension-free vaginal versus transobturator tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):393-399.
17. Deffieux X, Daher N, Mansoor A, Debodinance P, Muhlstein J, Fernandez H. Transobturator TVT-O versus retropubic TVT: results of a multicenter randomized controlled trial at 24 months follow-up. Int Urogynecol J. 2010;21(11):1337-1345.
18. Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112(6):1253-1261.
19. Rechberger T, Futyma K, Jankiewicz K, Adamiak A, Skorupski P. The clinical effectiveness of retropubic (IVS-02) and transobturator (IVS-04) midurethral slings: randomized trial. Eur Urol. 2009;56(1):24-30.
20. Schierlitz L, Dwyer PL, Rosamilia AN, et al. Three-year follow-up of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency. Obstet Gynecol. 2012;119(2 pt 1):321-327.
21. Nager C, Siris L, Litman HJ, et al. Urinary Incontinence Treatment Network. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186(2):597-603.
22. Jain P, Jirschele K, Botros SM, Latthe PM. Effectiveness of midurethral slings in mixed urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2011;22(8):923-932.
23. Sabadell J, Poza JL, Esgueva A, Morales JC, Sanchez-Iglesias JL, Xercavins J. Usefulness of retropubic tape for recurrent stress incontinence after transobturator tape failure. Int Urogynecol J. 2011;22(12):1543-1547.
24. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012; Feb 15;2:CD003881.-
UPDATE: MENOPAUSE
Important developments in the care of menopausal women in the past 12 months include:
- new evidence about the duration, and nonhormonal management, of hot flushes
- new data on the risk of venous thromboembolism when oral and transdermal hormone therapy (HT) are compared
- trends in thinking regarding ovarian conservation at the time of hysterectomy, as well as a new report on the impact of hysterectomy on subsequent ovarian function
- a new Position Statement on HT from the North American Menopause Society.
Hot flushes can last 10 years or longer
Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104.
Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch Intern Med. 2011;171(15):1363–1369.
Hot flushes are more persistent than has been recognized
Previous reports have suggested that hot flushes, the most prevalent menopausal symptom, persist from 6 months to longer than 5 years. Freeman and colleagues carried out a prospective, population-based study in the Northeastern United States that enrolled more than 250 women (age range at enrollment, 35 to 47 years) who did not use HT. Subjects in this cohort were followed for 13 years as they progressed through menopause.
Surprisingly, the researchers found that the median duration of moderate-to-severe hot flushes was 10.2 years. Hot flushes persisted longer in black women than in white women (P = .02) and longer in non-obese women than in obese women (P = .003). Duration of symptoms was similar in smokers and nonsmokers.
Once again, soy fails to relieve menopausal symptoms
A number of clinical trials performed since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI) have failed to demonstrate that soy is efficacious for treating menopausal symptoms. Nevertheless, many women remain intrigued by the potential for obtaining symptom relief with over-the-counter supplements.
Investigators in Florida randomized women who had been menopausal for at least 5 years to receive daily soy isoflavones (equivalent to about twice the amount ingested in a typical Asian diet) or placebo for 2 years. Outcomes assessed at baseline and again at 12 and at 24 months included spine and hip bone-mineral density (BMD), menopausal symptoms, and vaginal epithelial maturation. Almost 250 women (mean age, 52 years) were randomized.
At 2 years, researchers found that:
- BMD had declined at all sites by about 2% in both groups
- approximately one half of subjects in the soy group and approximately one third who were randomized to the placebo group reported experiencing hot flushes (P = .02)
- vaginal epithelial maturation did not change appreciably from baseline in either group
- constipation was reported by 31% of women in the soy group and 21% in the placebo group—a difference that only marginally achieved statistical significance.
Hormone therapy remains far and away the most effective treatment for vasomotor symptoms. The long-term prospective study of Freeman and colleagues clarifies that bothersome symptoms may persist for many years—an important (though not upbeat) counseling point for symptomatic women.
Highly effective nonhormonal treatment of vasomotor symptoms would represent a major advance for our menopausal patients. Regrettably, neither soy nor black cohosh1 offers relief greater than placebo.
Gabapentin and some serotonin reuptake inhibitor and serotonin–norepinephrine reuptake inhibitor antidepressants do offer a modestly more effective off-label treatment of hot flushes than does placebo,2 but their efficacy does not approach that of HT. In my practice, I find that many patients who suffer bothersome hot flushes are reluctant to try off-label use of antidepressants.
Hormone therapy and risk of venous thromboembolism
Laliberté F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059.
Olié V, Plu-Bureau G, Conard J, Horellou MH, Canonico M, Scarabin PY. Hormone therapy and recurrence of venous thromboembolism among postmenopausal women. Menopause. 2011;18(5):488–493.
Transdermal HT appears to be safer than oral therapy
Yet another observational study adds evidence that venous thromboembolism (VTE) is less of a risk in women using transdermal estrogen therapy than it is in women taking oral therapy.
To compare oral and transdermal estrogen formulations in regard to the risk of VTE that they pose, Laliberte and colleagues conducted a retrospective cohort study of US and Canadian women, using health insurance claims data from women who were starting transdermal or oral estrogen. In all, 27,018 users of transdermal estrogen were matched with an equal number of oral users.
VTE was diagnosed in 115 women using transdermal estradiol and 164 women using oral estrogen. Compared with the rate in women initiating oral estrogen, women using transdermal estradiol had a significantly lower incidence of VTE than oral estrogen users (adjusted incidence rate ratio, 0.67).
Is HT safe for women who have a history of VTE?
The US Food and Drug Administration has designated a personal history of VTE as a contraindication to all estrogen and estrogen-progestin HT formulations in the package labeling for these products. Because accumulating evidence is reassuring in regard to the risk of VTE with transdermal HT, however, it seems reasonable to consider using HT in selected women who have a history of VTE.
In a retrospective cohort study, French investigators assessed the impact of oral and transdermal estrogen on the risk of recurrent VTE in 1,023 postmenopausal women who had an earlier diagnosis of VTE. During follow-up, most of the subjects did not use HT, although 103 used transdermal estrogen and 10 used oral estrogen.
Seventy-seven women experienced recurrent VTE during a mean of 79 months after discontinuing anticoagulation. Compared with non-use of estrogen therapy, use of transdermal estrogen was not significantly associated with recurrent VTE (hazard ratio [HR], 1.0); oral estrogen, however, was associated with a substantial and significantly increased risk of recurrent VTE (HR, 6.4).
In the 2011 OBG Management Update on Menopause, I examined two large observational studies3,4—one from France, the other from Great Britain—that provided convincing evidence that transdermal HT does not, in contrast with oral HT, raise the risk of VTE. These new reports, from North America and France, provide further support for the hypothesis that transdermal HT is safer from the perspective of VTE risk. Although a randomized trial that compares the risk of VTE in women using oral estrogen with the risk in women using transdermal estrogen might put this matter to rest, I don’t anticipate that a trial to address this outcome, with adequate statistical power, will be performed any time soon.
In my practice, most of the estrogen that I prescribe for menopausal women is transdermal. Using transdermal estrogen may be particularly important in patients who are at increased risk of VTE at baseline, including obese women.
The small numbers of thrombotic events in the cohort of women who had a history of VTE limits confidence in the findings of this French report. Nevertheless, this study provides a small measure of reassurance regarding use of transdermal estrogen after VTE.
Only rarely have I prescribed HT to women who have a history of VTE. These exceptional patients have been highly symptomatic and extensively counseled about the risk of recurrent thrombosis as well as the off-label status of hormone use, given their medical history. Certainly, if you consider prescribing HT to such women, the transdermal route (preferably at a dosage of 0.05 mg, or lower) would be more prudent that oral HT.
Hysterectomy may accelerate the onset of menopause
Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118(6):1271–1279.
Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol. 2011;118(6):1280–1286.
Does hysterectomy hasten ovarian failure?
In a prospective cohort study from North Carolina, Moorman and colleagues followed 1) 406 women who did not have malignancy who underwent hysterectomy, with conservation of at least one ovary and 2) 465 women who had an intact uterus (overall age range, 30 to 47 years). Within 5 years of follow-up, ovarian failure had occurred in 60 women who had undergone a hysterectomy and in 46 women who had an intact uterus (adjusted HR, 1.9).
Ovarian failure occurred almost 2 years earlier in women who had undergone a hysterectomy than it did in those whose uterus was intact. The likelihood of ovarian failure was higher in the setting of unilateral oophorectomy than when both ovaries had been conserved.
Hysterectomy for benign disease: Are we performing fewer oophorectomies?
Investigators in New York State followed trends in concomitant bilateral oophorectomy among women undergoing hysterectomy for benign disease, from 2000 to 2006. Overall, the rate of concomitant oophorectomy declined by 8% during this period; among women younger than 55 years, the rate of oophorectomy declined by more than 10%. The rate of concomitant bilateral oophorectomy was higher among women who had a family history of breast or ovarian cancer and among those who had a personal history of breast cancer, ovarian cysts, or endometriosis.
Early menopause puts our patients at elevated risk of osteoporosis, cardiovascular disease, neurodegenerative disease (possibly), and sexual dysfunction. We have long suspected that hysterectomy may accelerate the onset of menopause, and the North Carolina cohort study provides strong support for this hypothesis.
The New York State report reveals that ObGyns are more often practicing ovarian conservation in women (particularly younger women) undergoing hysterectomy for benign indications.
In 2008, ACOG revised its guidance on this matter—stating that “strong consideration should be given to retaining normal ovaries in premenopausal women who are not at increased genetic risk of ovarian cancer.”5 Evidence that we are increasingly following this prudent guideline is welcome news.
Breaking news: NAMS updates guidance on hormone therapy
Position Statement emphasizes differences in the benefit-risk profile of estrogen–only HT and estrogen-progestin HT
Periodically, NAMS assembles a multidisciplinary panel of clinicians and researchers to evaluate new evidence about HT and reach consensus on guidance about using hormones, and then publishes a Position Statement on the subject. In March, NAMS published its updated (2012) position on HT.
Two recent, and important, analyses of data from the Women’s Health Initiative (WHI)6,7 made an impact on the current revision to an earlier (2010) Position Statement; I had summarized those studies in the 2011 OBG Management Update on Menopause. One focused on breast cancer characteristics and mortality associated with use of combination estrogen-progestin HT; the other, outcomes after use of estrogen-only HT. Recap. Initial findings in the estrogen– progestin arm of the WHI, published in 2002,8 found that, after participants had used study medications (HT or placebo) for a mean of 5.2 years, their risk of invasive breast cancer was increased (HR, 1.26). This modestly elevated risk was only marginally significant (95% confidence limit, 1.00–1.59).
In 2010, investigators reported on breast cancer characteristics and mortality in WHI participants at a mean follow-up of 11 years. They found that combination HT users had breast cancer histology similar to that of subjects assigned to placebo, but that the tumors were more likely to be node-positive in combination HT users (23.7%, compared with 16.2% among placebo users). In addition, breast cancer mortality was slightly higher among users of HT (2.6 deaths, compared with 1.3 deaths, for every 10,000 woman-years of use) (HR, 1.96; 95% confidence interval [CI], 1.00–4.04); again, this elevated risk reached only marginal statistical significance.
Then, in 2011, WHI investigators reported their findings from the estrogen-alone arm of the study, in which post-menopausal, hysterectomized women were randomized to oral estrogen or placebo and took study medications for a mean of 6.8 years. (Recall that initial findings from the estrogen-only arm of WHI, published in 2004, found that the risk of invasive cancer was lower in women randomized to estrogen [HR, 0.77]—a reduction in risk that approached, but did not achieve, statistical significance [95% CI, 0.59–1.01].9) In the 2011 report, the lower risk of breast cancer in the estrogen group persisted; with almost 11 years mean follow-up, this prevention was found to be robust and statistically significant (HR, 0.77; 95% CI, 0.62–0.95).
The sobering increased risk of advanced-stage tumors and the marginally higher likelihood of fatal breast cancer associated with use of estrogen–progestin HT stands in stark contrast with the significant reduction in breast cancer associated with estrogen- only HT.
Estrogen. Most of my patients who are taking systemic menopausal hormone therapy (HT) use transdermal estrogen, with 0.05 mg the most common starting dose. Given the elevated baseline risk of thrombosis among obese women, I particularly encourage them to use transdermal estrogen when starting systemic HT.
When I prescribe oral estrogen, the formulation I use most often is generic micronized estradiol; the most common starting dosage is a 1 mg tablet.
Progestin. To protect the endometrium in menopausal women whose uterus is intact and who are opting for systemic HT, I often use micronized progesterone, 100 mg nightly (provided no peanut allergy is present). My rationale? Progesterone is less likely than other progestational agents to cause unpleasant mood changes, and may offer a safety advantage vis a vis breast cancer.
When cost is a concern, generic medroxyprogesterone acetate tablets are well studied and inexpensive (2.5 mg tablets are appropriate when using the dosages of transdermal or oral estradiol given above).
When treating vasomotor symptoms/irregular bleeding in perimenopausal women, symptomatic relief may be more likely if HT formulations with sufficient progestin to consistently suppress ovulation are employed. Therefore, in such patients, I often use approaches such as femHRT 1/5 (also available as a generic) and Activella (also available as a generic).
Last, my experience is favorable using a combination of transdermal estrogen and the progestin-releasing IUD in symptomatic perimenopausal women.
Note: Using any sex steroids to manage perimenopausal symptoms constitutes an off-label use.
Accordingly, NAMS has modified its guidance. To step back for a moment, in the abstract of its 2010 Position Statement, NAMS had concluded that:
- Recent data support the initiation of HT around the time of menopause to treat menopause-related symptoms; to treat or reduce the risk of certain disorders, such as osteoporosis or fractures in select postmenopausal women; or both. The benefit-risk ratio for menopausal HT is favorable for women who initiate HT close to menopause but decreases in older women and with time since menopause in previously untreated women.
Contrast that with the conclusion in the abstract of the Society’s 2012 Position Statement:
- Recent data support the initiation of HT around the time of menopause to treat menopause-related symptoms and to prevent osteoporosis in women at high risk of fracture. The more favorable benefit-risk ratio for ET allows more flexibility in extending duration of use compared to EPT where the earlier appearance of increased breast cancer risk precludes a recommendation for use beyond 3 to 5 years.
When counseling menopausal women who are considering starting or continuing HT, I point out that HT represents the most effective treatment for bothersome menopausal symptoms and is highly effective for preventing osteoporotic fractures and genital atrophy.
Almost all of my patients who are considering starting systemic HT are in their late 40s or in their 50s—within a decade of the onset of menopause. If these women have had a hysterectomy, I counsel them that estrogen-only HT is likely to reduce their risk of coronary artery disease (CAD). On the other hand, if these women have an intact uterus, I counsel them that combination estrogen–progestin HT does not increase their risk of CAD—and might prevent it.
I also point out that starting HT and continuing it over the long term may reduce their risk of dementia later in life.
I do prescribe oral and transdermal estrogen, but I more often prescribe transdermal formulations because of their apparent safety in regard to the risk of venous thromboembolism. This preference for transdermal estrogen applies, in particular, to overweight women because their baseline risk of VTE is elevated.
Regarding breast cancer, I point out to estrogen-only HT candidates that HT prevents breast cancer. I counsel women whose uterus is intact that women who use combination HT for longer than 3 to 5 years experience a modest increase in their risk of having a diagnosis of breast cancer—similar to the elevation associated with moderate alcohol consumption. I also point out that the risk of dying from breast cancer might be increased with long-term combination HT use.
In women for whom the only indication for HT is prevention of genital atrophy, I prefer to prescribe vaginal formulations of estrogen.
Some of my patients—particularly those who do not have a uterus—who are extensively counseled, choose to continue HT indefinitely. Such very-long-term users often focus on either 1) their greater sense of well-being with HT or 2) the benefit of the prevention of osteoporosis in the face of their desire to avoid long-term bisphosphonate therapy.
Last, over the course of patients’ years of taking HT, I encourage them to try lower dosages, until they either discontinue HT or remain on a very low dosage.
We want to hear from you! Tell us what you think.
1. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: A randomized controlled trial. Menopause. 2009;16(6):1156-1166.
2. Pachman DR, Jones JM, Loprinzi CL. Management of menopause-associated vasomotor symptoms: Current treatment options challenges and future directions. Int J Womens Health. 2010;2:123-135.
3. Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340-345.
4. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519.
5. ACOG Practice Bulletin No 89. Elective and risk-reducing salpingo-oophorectomy. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;111(1):231-241.
6. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684-1692.
7. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305-1314.
8. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288(3):321-333.
9. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701-1712.
Important developments in the care of menopausal women in the past 12 months include:
- new evidence about the duration, and nonhormonal management, of hot flushes
- new data on the risk of venous thromboembolism when oral and transdermal hormone therapy (HT) are compared
- trends in thinking regarding ovarian conservation at the time of hysterectomy, as well as a new report on the impact of hysterectomy on subsequent ovarian function
- a new Position Statement on HT from the North American Menopause Society.
Hot flushes can last 10 years or longer
Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104.
Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch Intern Med. 2011;171(15):1363–1369.
Hot flushes are more persistent than has been recognized
Previous reports have suggested that hot flushes, the most prevalent menopausal symptom, persist from 6 months to longer than 5 years. Freeman and colleagues carried out a prospective, population-based study in the Northeastern United States that enrolled more than 250 women (age range at enrollment, 35 to 47 years) who did not use HT. Subjects in this cohort were followed for 13 years as they progressed through menopause.
Surprisingly, the researchers found that the median duration of moderate-to-severe hot flushes was 10.2 years. Hot flushes persisted longer in black women than in white women (P = .02) and longer in non-obese women than in obese women (P = .003). Duration of symptoms was similar in smokers and nonsmokers.
Once again, soy fails to relieve menopausal symptoms
A number of clinical trials performed since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI) have failed to demonstrate that soy is efficacious for treating menopausal symptoms. Nevertheless, many women remain intrigued by the potential for obtaining symptom relief with over-the-counter supplements.
Investigators in Florida randomized women who had been menopausal for at least 5 years to receive daily soy isoflavones (equivalent to about twice the amount ingested in a typical Asian diet) or placebo for 2 years. Outcomes assessed at baseline and again at 12 and at 24 months included spine and hip bone-mineral density (BMD), menopausal symptoms, and vaginal epithelial maturation. Almost 250 women (mean age, 52 years) were randomized.
At 2 years, researchers found that:
- BMD had declined at all sites by about 2% in both groups
- approximately one half of subjects in the soy group and approximately one third who were randomized to the placebo group reported experiencing hot flushes (P = .02)
- vaginal epithelial maturation did not change appreciably from baseline in either group
- constipation was reported by 31% of women in the soy group and 21% in the placebo group—a difference that only marginally achieved statistical significance.
Hormone therapy remains far and away the most effective treatment for vasomotor symptoms. The long-term prospective study of Freeman and colleagues clarifies that bothersome symptoms may persist for many years—an important (though not upbeat) counseling point for symptomatic women.
Highly effective nonhormonal treatment of vasomotor symptoms would represent a major advance for our menopausal patients. Regrettably, neither soy nor black cohosh1 offers relief greater than placebo.
Gabapentin and some serotonin reuptake inhibitor and serotonin–norepinephrine reuptake inhibitor antidepressants do offer a modestly more effective off-label treatment of hot flushes than does placebo,2 but their efficacy does not approach that of HT. In my practice, I find that many patients who suffer bothersome hot flushes are reluctant to try off-label use of antidepressants.
Hormone therapy and risk of venous thromboembolism
Laliberté F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059.
Olié V, Plu-Bureau G, Conard J, Horellou MH, Canonico M, Scarabin PY. Hormone therapy and recurrence of venous thromboembolism among postmenopausal women. Menopause. 2011;18(5):488–493.
Transdermal HT appears to be safer than oral therapy
Yet another observational study adds evidence that venous thromboembolism (VTE) is less of a risk in women using transdermal estrogen therapy than it is in women taking oral therapy.
To compare oral and transdermal estrogen formulations in regard to the risk of VTE that they pose, Laliberte and colleagues conducted a retrospective cohort study of US and Canadian women, using health insurance claims data from women who were starting transdermal or oral estrogen. In all, 27,018 users of transdermal estrogen were matched with an equal number of oral users.
VTE was diagnosed in 115 women using transdermal estradiol and 164 women using oral estrogen. Compared with the rate in women initiating oral estrogen, women using transdermal estradiol had a significantly lower incidence of VTE than oral estrogen users (adjusted incidence rate ratio, 0.67).
Is HT safe for women who have a history of VTE?
The US Food and Drug Administration has designated a personal history of VTE as a contraindication to all estrogen and estrogen-progestin HT formulations in the package labeling for these products. Because accumulating evidence is reassuring in regard to the risk of VTE with transdermal HT, however, it seems reasonable to consider using HT in selected women who have a history of VTE.
In a retrospective cohort study, French investigators assessed the impact of oral and transdermal estrogen on the risk of recurrent VTE in 1,023 postmenopausal women who had an earlier diagnosis of VTE. During follow-up, most of the subjects did not use HT, although 103 used transdermal estrogen and 10 used oral estrogen.
Seventy-seven women experienced recurrent VTE during a mean of 79 months after discontinuing anticoagulation. Compared with non-use of estrogen therapy, use of transdermal estrogen was not significantly associated with recurrent VTE (hazard ratio [HR], 1.0); oral estrogen, however, was associated with a substantial and significantly increased risk of recurrent VTE (HR, 6.4).
In the 2011 OBG Management Update on Menopause, I examined two large observational studies3,4—one from France, the other from Great Britain—that provided convincing evidence that transdermal HT does not, in contrast with oral HT, raise the risk of VTE. These new reports, from North America and France, provide further support for the hypothesis that transdermal HT is safer from the perspective of VTE risk. Although a randomized trial that compares the risk of VTE in women using oral estrogen with the risk in women using transdermal estrogen might put this matter to rest, I don’t anticipate that a trial to address this outcome, with adequate statistical power, will be performed any time soon.
In my practice, most of the estrogen that I prescribe for menopausal women is transdermal. Using transdermal estrogen may be particularly important in patients who are at increased risk of VTE at baseline, including obese women.
The small numbers of thrombotic events in the cohort of women who had a history of VTE limits confidence in the findings of this French report. Nevertheless, this study provides a small measure of reassurance regarding use of transdermal estrogen after VTE.
Only rarely have I prescribed HT to women who have a history of VTE. These exceptional patients have been highly symptomatic and extensively counseled about the risk of recurrent thrombosis as well as the off-label status of hormone use, given their medical history. Certainly, if you consider prescribing HT to such women, the transdermal route (preferably at a dosage of 0.05 mg, or lower) would be more prudent that oral HT.
Hysterectomy may accelerate the onset of menopause
Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118(6):1271–1279.
Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol. 2011;118(6):1280–1286.
Does hysterectomy hasten ovarian failure?
In a prospective cohort study from North Carolina, Moorman and colleagues followed 1) 406 women who did not have malignancy who underwent hysterectomy, with conservation of at least one ovary and 2) 465 women who had an intact uterus (overall age range, 30 to 47 years). Within 5 years of follow-up, ovarian failure had occurred in 60 women who had undergone a hysterectomy and in 46 women who had an intact uterus (adjusted HR, 1.9).
Ovarian failure occurred almost 2 years earlier in women who had undergone a hysterectomy than it did in those whose uterus was intact. The likelihood of ovarian failure was higher in the setting of unilateral oophorectomy than when both ovaries had been conserved.
Hysterectomy for benign disease: Are we performing fewer oophorectomies?
Investigators in New York State followed trends in concomitant bilateral oophorectomy among women undergoing hysterectomy for benign disease, from 2000 to 2006. Overall, the rate of concomitant oophorectomy declined by 8% during this period; among women younger than 55 years, the rate of oophorectomy declined by more than 10%. The rate of concomitant bilateral oophorectomy was higher among women who had a family history of breast or ovarian cancer and among those who had a personal history of breast cancer, ovarian cysts, or endometriosis.
Early menopause puts our patients at elevated risk of osteoporosis, cardiovascular disease, neurodegenerative disease (possibly), and sexual dysfunction. We have long suspected that hysterectomy may accelerate the onset of menopause, and the North Carolina cohort study provides strong support for this hypothesis.
The New York State report reveals that ObGyns are more often practicing ovarian conservation in women (particularly younger women) undergoing hysterectomy for benign indications.
In 2008, ACOG revised its guidance on this matter—stating that “strong consideration should be given to retaining normal ovaries in premenopausal women who are not at increased genetic risk of ovarian cancer.”5 Evidence that we are increasingly following this prudent guideline is welcome news.
Breaking news: NAMS updates guidance on hormone therapy
Position Statement emphasizes differences in the benefit-risk profile of estrogen–only HT and estrogen-progestin HT
Periodically, NAMS assembles a multidisciplinary panel of clinicians and researchers to evaluate new evidence about HT and reach consensus on guidance about using hormones, and then publishes a Position Statement on the subject. In March, NAMS published its updated (2012) position on HT.
Two recent, and important, analyses of data from the Women’s Health Initiative (WHI)6,7 made an impact on the current revision to an earlier (2010) Position Statement; I had summarized those studies in the 2011 OBG Management Update on Menopause. One focused on breast cancer characteristics and mortality associated with use of combination estrogen-progestin HT; the other, outcomes after use of estrogen-only HT. Recap. Initial findings in the estrogen– progestin arm of the WHI, published in 2002,8 found that, after participants had used study medications (HT or placebo) for a mean of 5.2 years, their risk of invasive breast cancer was increased (HR, 1.26). This modestly elevated risk was only marginally significant (95% confidence limit, 1.00–1.59).
In 2010, investigators reported on breast cancer characteristics and mortality in WHI participants at a mean follow-up of 11 years. They found that combination HT users had breast cancer histology similar to that of subjects assigned to placebo, but that the tumors were more likely to be node-positive in combination HT users (23.7%, compared with 16.2% among placebo users). In addition, breast cancer mortality was slightly higher among users of HT (2.6 deaths, compared with 1.3 deaths, for every 10,000 woman-years of use) (HR, 1.96; 95% confidence interval [CI], 1.00–4.04); again, this elevated risk reached only marginal statistical significance.
Then, in 2011, WHI investigators reported their findings from the estrogen-alone arm of the study, in which post-menopausal, hysterectomized women were randomized to oral estrogen or placebo and took study medications for a mean of 6.8 years. (Recall that initial findings from the estrogen-only arm of WHI, published in 2004, found that the risk of invasive cancer was lower in women randomized to estrogen [HR, 0.77]—a reduction in risk that approached, but did not achieve, statistical significance [95% CI, 0.59–1.01].9) In the 2011 report, the lower risk of breast cancer in the estrogen group persisted; with almost 11 years mean follow-up, this prevention was found to be robust and statistically significant (HR, 0.77; 95% CI, 0.62–0.95).
The sobering increased risk of advanced-stage tumors and the marginally higher likelihood of fatal breast cancer associated with use of estrogen–progestin HT stands in stark contrast with the significant reduction in breast cancer associated with estrogen- only HT.
Estrogen. Most of my patients who are taking systemic menopausal hormone therapy (HT) use transdermal estrogen, with 0.05 mg the most common starting dose. Given the elevated baseline risk of thrombosis among obese women, I particularly encourage them to use transdermal estrogen when starting systemic HT.
When I prescribe oral estrogen, the formulation I use most often is generic micronized estradiol; the most common starting dosage is a 1 mg tablet.
Progestin. To protect the endometrium in menopausal women whose uterus is intact and who are opting for systemic HT, I often use micronized progesterone, 100 mg nightly (provided no peanut allergy is present). My rationale? Progesterone is less likely than other progestational agents to cause unpleasant mood changes, and may offer a safety advantage vis a vis breast cancer.
When cost is a concern, generic medroxyprogesterone acetate tablets are well studied and inexpensive (2.5 mg tablets are appropriate when using the dosages of transdermal or oral estradiol given above).
When treating vasomotor symptoms/irregular bleeding in perimenopausal women, symptomatic relief may be more likely if HT formulations with sufficient progestin to consistently suppress ovulation are employed. Therefore, in such patients, I often use approaches such as femHRT 1/5 (also available as a generic) and Activella (also available as a generic).
Last, my experience is favorable using a combination of transdermal estrogen and the progestin-releasing IUD in symptomatic perimenopausal women.
Note: Using any sex steroids to manage perimenopausal symptoms constitutes an off-label use.
Accordingly, NAMS has modified its guidance. To step back for a moment, in the abstract of its 2010 Position Statement, NAMS had concluded that:
- Recent data support the initiation of HT around the time of menopause to treat menopause-related symptoms; to treat or reduce the risk of certain disorders, such as osteoporosis or fractures in select postmenopausal women; or both. The benefit-risk ratio for menopausal HT is favorable for women who initiate HT close to menopause but decreases in older women and with time since menopause in previously untreated women.
Contrast that with the conclusion in the abstract of the Society’s 2012 Position Statement:
- Recent data support the initiation of HT around the time of menopause to treat menopause-related symptoms and to prevent osteoporosis in women at high risk of fracture. The more favorable benefit-risk ratio for ET allows more flexibility in extending duration of use compared to EPT where the earlier appearance of increased breast cancer risk precludes a recommendation for use beyond 3 to 5 years.
When counseling menopausal women who are considering starting or continuing HT, I point out that HT represents the most effective treatment for bothersome menopausal symptoms and is highly effective for preventing osteoporotic fractures and genital atrophy.
Almost all of my patients who are considering starting systemic HT are in their late 40s or in their 50s—within a decade of the onset of menopause. If these women have had a hysterectomy, I counsel them that estrogen-only HT is likely to reduce their risk of coronary artery disease (CAD). On the other hand, if these women have an intact uterus, I counsel them that combination estrogen–progestin HT does not increase their risk of CAD—and might prevent it.
I also point out that starting HT and continuing it over the long term may reduce their risk of dementia later in life.
I do prescribe oral and transdermal estrogen, but I more often prescribe transdermal formulations because of their apparent safety in regard to the risk of venous thromboembolism. This preference for transdermal estrogen applies, in particular, to overweight women because their baseline risk of VTE is elevated.
Regarding breast cancer, I point out to estrogen-only HT candidates that HT prevents breast cancer. I counsel women whose uterus is intact that women who use combination HT for longer than 3 to 5 years experience a modest increase in their risk of having a diagnosis of breast cancer—similar to the elevation associated with moderate alcohol consumption. I also point out that the risk of dying from breast cancer might be increased with long-term combination HT use.
In women for whom the only indication for HT is prevention of genital atrophy, I prefer to prescribe vaginal formulations of estrogen.
Some of my patients—particularly those who do not have a uterus—who are extensively counseled, choose to continue HT indefinitely. Such very-long-term users often focus on either 1) their greater sense of well-being with HT or 2) the benefit of the prevention of osteoporosis in the face of their desire to avoid long-term bisphosphonate therapy.
Last, over the course of patients’ years of taking HT, I encourage them to try lower dosages, until they either discontinue HT or remain on a very low dosage.
We want to hear from you! Tell us what you think.
Important developments in the care of menopausal women in the past 12 months include:
- new evidence about the duration, and nonhormonal management, of hot flushes
- new data on the risk of venous thromboembolism when oral and transdermal hormone therapy (HT) are compared
- trends in thinking regarding ovarian conservation at the time of hysterectomy, as well as a new report on the impact of hysterectomy on subsequent ovarian function
- a new Position Statement on HT from the North American Menopause Society.
Hot flushes can last 10 years or longer
Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104.
Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch Intern Med. 2011;171(15):1363–1369.
Hot flushes are more persistent than has been recognized
Previous reports have suggested that hot flushes, the most prevalent menopausal symptom, persist from 6 months to longer than 5 years. Freeman and colleagues carried out a prospective, population-based study in the Northeastern United States that enrolled more than 250 women (age range at enrollment, 35 to 47 years) who did not use HT. Subjects in this cohort were followed for 13 years as they progressed through menopause.
Surprisingly, the researchers found that the median duration of moderate-to-severe hot flushes was 10.2 years. Hot flushes persisted longer in black women than in white women (P = .02) and longer in non-obese women than in obese women (P = .003). Duration of symptoms was similar in smokers and nonsmokers.
Once again, soy fails to relieve menopausal symptoms
A number of clinical trials performed since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI) have failed to demonstrate that soy is efficacious for treating menopausal symptoms. Nevertheless, many women remain intrigued by the potential for obtaining symptom relief with over-the-counter supplements.
Investigators in Florida randomized women who had been menopausal for at least 5 years to receive daily soy isoflavones (equivalent to about twice the amount ingested in a typical Asian diet) or placebo for 2 years. Outcomes assessed at baseline and again at 12 and at 24 months included spine and hip bone-mineral density (BMD), menopausal symptoms, and vaginal epithelial maturation. Almost 250 women (mean age, 52 years) were randomized.
At 2 years, researchers found that:
- BMD had declined at all sites by about 2% in both groups
- approximately one half of subjects in the soy group and approximately one third who were randomized to the placebo group reported experiencing hot flushes (P = .02)
- vaginal epithelial maturation did not change appreciably from baseline in either group
- constipation was reported by 31% of women in the soy group and 21% in the placebo group—a difference that only marginally achieved statistical significance.
Hormone therapy remains far and away the most effective treatment for vasomotor symptoms. The long-term prospective study of Freeman and colleagues clarifies that bothersome symptoms may persist for many years—an important (though not upbeat) counseling point for symptomatic women.
Highly effective nonhormonal treatment of vasomotor symptoms would represent a major advance for our menopausal patients. Regrettably, neither soy nor black cohosh1 offers relief greater than placebo.
Gabapentin and some serotonin reuptake inhibitor and serotonin–norepinephrine reuptake inhibitor antidepressants do offer a modestly more effective off-label treatment of hot flushes than does placebo,2 but their efficacy does not approach that of HT. In my practice, I find that many patients who suffer bothersome hot flushes are reluctant to try off-label use of antidepressants.
Hormone therapy and risk of venous thromboembolism
Laliberté F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059.
Olié V, Plu-Bureau G, Conard J, Horellou MH, Canonico M, Scarabin PY. Hormone therapy and recurrence of venous thromboembolism among postmenopausal women. Menopause. 2011;18(5):488–493.
Transdermal HT appears to be safer than oral therapy
Yet another observational study adds evidence that venous thromboembolism (VTE) is less of a risk in women using transdermal estrogen therapy than it is in women taking oral therapy.
To compare oral and transdermal estrogen formulations in regard to the risk of VTE that they pose, Laliberte and colleagues conducted a retrospective cohort study of US and Canadian women, using health insurance claims data from women who were starting transdermal or oral estrogen. In all, 27,018 users of transdermal estrogen were matched with an equal number of oral users.
VTE was diagnosed in 115 women using transdermal estradiol and 164 women using oral estrogen. Compared with the rate in women initiating oral estrogen, women using transdermal estradiol had a significantly lower incidence of VTE than oral estrogen users (adjusted incidence rate ratio, 0.67).
Is HT safe for women who have a history of VTE?
The US Food and Drug Administration has designated a personal history of VTE as a contraindication to all estrogen and estrogen-progestin HT formulations in the package labeling for these products. Because accumulating evidence is reassuring in regard to the risk of VTE with transdermal HT, however, it seems reasonable to consider using HT in selected women who have a history of VTE.
In a retrospective cohort study, French investigators assessed the impact of oral and transdermal estrogen on the risk of recurrent VTE in 1,023 postmenopausal women who had an earlier diagnosis of VTE. During follow-up, most of the subjects did not use HT, although 103 used transdermal estrogen and 10 used oral estrogen.
Seventy-seven women experienced recurrent VTE during a mean of 79 months after discontinuing anticoagulation. Compared with non-use of estrogen therapy, use of transdermal estrogen was not significantly associated with recurrent VTE (hazard ratio [HR], 1.0); oral estrogen, however, was associated with a substantial and significantly increased risk of recurrent VTE (HR, 6.4).
In the 2011 OBG Management Update on Menopause, I examined two large observational studies3,4—one from France, the other from Great Britain—that provided convincing evidence that transdermal HT does not, in contrast with oral HT, raise the risk of VTE. These new reports, from North America and France, provide further support for the hypothesis that transdermal HT is safer from the perspective of VTE risk. Although a randomized trial that compares the risk of VTE in women using oral estrogen with the risk in women using transdermal estrogen might put this matter to rest, I don’t anticipate that a trial to address this outcome, with adequate statistical power, will be performed any time soon.
In my practice, most of the estrogen that I prescribe for menopausal women is transdermal. Using transdermal estrogen may be particularly important in patients who are at increased risk of VTE at baseline, including obese women.
The small numbers of thrombotic events in the cohort of women who had a history of VTE limits confidence in the findings of this French report. Nevertheless, this study provides a small measure of reassurance regarding use of transdermal estrogen after VTE.
Only rarely have I prescribed HT to women who have a history of VTE. These exceptional patients have been highly symptomatic and extensively counseled about the risk of recurrent thrombosis as well as the off-label status of hormone use, given their medical history. Certainly, if you consider prescribing HT to such women, the transdermal route (preferably at a dosage of 0.05 mg, or lower) would be more prudent that oral HT.
Hysterectomy may accelerate the onset of menopause
Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118(6):1271–1279.
Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol. 2011;118(6):1280–1286.
Does hysterectomy hasten ovarian failure?
In a prospective cohort study from North Carolina, Moorman and colleagues followed 1) 406 women who did not have malignancy who underwent hysterectomy, with conservation of at least one ovary and 2) 465 women who had an intact uterus (overall age range, 30 to 47 years). Within 5 years of follow-up, ovarian failure had occurred in 60 women who had undergone a hysterectomy and in 46 women who had an intact uterus (adjusted HR, 1.9).
Ovarian failure occurred almost 2 years earlier in women who had undergone a hysterectomy than it did in those whose uterus was intact. The likelihood of ovarian failure was higher in the setting of unilateral oophorectomy than when both ovaries had been conserved.
Hysterectomy for benign disease: Are we performing fewer oophorectomies?
Investigators in New York State followed trends in concomitant bilateral oophorectomy among women undergoing hysterectomy for benign disease, from 2000 to 2006. Overall, the rate of concomitant oophorectomy declined by 8% during this period; among women younger than 55 years, the rate of oophorectomy declined by more than 10%. The rate of concomitant bilateral oophorectomy was higher among women who had a family history of breast or ovarian cancer and among those who had a personal history of breast cancer, ovarian cysts, or endometriosis.
Early menopause puts our patients at elevated risk of osteoporosis, cardiovascular disease, neurodegenerative disease (possibly), and sexual dysfunction. We have long suspected that hysterectomy may accelerate the onset of menopause, and the North Carolina cohort study provides strong support for this hypothesis.
The New York State report reveals that ObGyns are more often practicing ovarian conservation in women (particularly younger women) undergoing hysterectomy for benign indications.
In 2008, ACOG revised its guidance on this matter—stating that “strong consideration should be given to retaining normal ovaries in premenopausal women who are not at increased genetic risk of ovarian cancer.”5 Evidence that we are increasingly following this prudent guideline is welcome news.
Breaking news: NAMS updates guidance on hormone therapy
Position Statement emphasizes differences in the benefit-risk profile of estrogen–only HT and estrogen-progestin HT
Periodically, NAMS assembles a multidisciplinary panel of clinicians and researchers to evaluate new evidence about HT and reach consensus on guidance about using hormones, and then publishes a Position Statement on the subject. In March, NAMS published its updated (2012) position on HT.
Two recent, and important, analyses of data from the Women’s Health Initiative (WHI)6,7 made an impact on the current revision to an earlier (2010) Position Statement; I had summarized those studies in the 2011 OBG Management Update on Menopause. One focused on breast cancer characteristics and mortality associated with use of combination estrogen-progestin HT; the other, outcomes after use of estrogen-only HT. Recap. Initial findings in the estrogen– progestin arm of the WHI, published in 2002,8 found that, after participants had used study medications (HT or placebo) for a mean of 5.2 years, their risk of invasive breast cancer was increased (HR, 1.26). This modestly elevated risk was only marginally significant (95% confidence limit, 1.00–1.59).
In 2010, investigators reported on breast cancer characteristics and mortality in WHI participants at a mean follow-up of 11 years. They found that combination HT users had breast cancer histology similar to that of subjects assigned to placebo, but that the tumors were more likely to be node-positive in combination HT users (23.7%, compared with 16.2% among placebo users). In addition, breast cancer mortality was slightly higher among users of HT (2.6 deaths, compared with 1.3 deaths, for every 10,000 woman-years of use) (HR, 1.96; 95% confidence interval [CI], 1.00–4.04); again, this elevated risk reached only marginal statistical significance.
Then, in 2011, WHI investigators reported their findings from the estrogen-alone arm of the study, in which post-menopausal, hysterectomized women were randomized to oral estrogen or placebo and took study medications for a mean of 6.8 years. (Recall that initial findings from the estrogen-only arm of WHI, published in 2004, found that the risk of invasive cancer was lower in women randomized to estrogen [HR, 0.77]—a reduction in risk that approached, but did not achieve, statistical significance [95% CI, 0.59–1.01].9) In the 2011 report, the lower risk of breast cancer in the estrogen group persisted; with almost 11 years mean follow-up, this prevention was found to be robust and statistically significant (HR, 0.77; 95% CI, 0.62–0.95).
The sobering increased risk of advanced-stage tumors and the marginally higher likelihood of fatal breast cancer associated with use of estrogen–progestin HT stands in stark contrast with the significant reduction in breast cancer associated with estrogen- only HT.
Estrogen. Most of my patients who are taking systemic menopausal hormone therapy (HT) use transdermal estrogen, with 0.05 mg the most common starting dose. Given the elevated baseline risk of thrombosis among obese women, I particularly encourage them to use transdermal estrogen when starting systemic HT.
When I prescribe oral estrogen, the formulation I use most often is generic micronized estradiol; the most common starting dosage is a 1 mg tablet.
Progestin. To protect the endometrium in menopausal women whose uterus is intact and who are opting for systemic HT, I often use micronized progesterone, 100 mg nightly (provided no peanut allergy is present). My rationale? Progesterone is less likely than other progestational agents to cause unpleasant mood changes, and may offer a safety advantage vis a vis breast cancer.
When cost is a concern, generic medroxyprogesterone acetate tablets are well studied and inexpensive (2.5 mg tablets are appropriate when using the dosages of transdermal or oral estradiol given above).
When treating vasomotor symptoms/irregular bleeding in perimenopausal women, symptomatic relief may be more likely if HT formulations with sufficient progestin to consistently suppress ovulation are employed. Therefore, in such patients, I often use approaches such as femHRT 1/5 (also available as a generic) and Activella (also available as a generic).
Last, my experience is favorable using a combination of transdermal estrogen and the progestin-releasing IUD in symptomatic perimenopausal women.
Note: Using any sex steroids to manage perimenopausal symptoms constitutes an off-label use.
Accordingly, NAMS has modified its guidance. To step back for a moment, in the abstract of its 2010 Position Statement, NAMS had concluded that:
- Recent data support the initiation of HT around the time of menopause to treat menopause-related symptoms; to treat or reduce the risk of certain disorders, such as osteoporosis or fractures in select postmenopausal women; or both. The benefit-risk ratio for menopausal HT is favorable for women who initiate HT close to menopause but decreases in older women and with time since menopause in previously untreated women.
Contrast that with the conclusion in the abstract of the Society’s 2012 Position Statement:
- Recent data support the initiation of HT around the time of menopause to treat menopause-related symptoms and to prevent osteoporosis in women at high risk of fracture. The more favorable benefit-risk ratio for ET allows more flexibility in extending duration of use compared to EPT where the earlier appearance of increased breast cancer risk precludes a recommendation for use beyond 3 to 5 years.
When counseling menopausal women who are considering starting or continuing HT, I point out that HT represents the most effective treatment for bothersome menopausal symptoms and is highly effective for preventing osteoporotic fractures and genital atrophy.
Almost all of my patients who are considering starting systemic HT are in their late 40s or in their 50s—within a decade of the onset of menopause. If these women have had a hysterectomy, I counsel them that estrogen-only HT is likely to reduce their risk of coronary artery disease (CAD). On the other hand, if these women have an intact uterus, I counsel them that combination estrogen–progestin HT does not increase their risk of CAD—and might prevent it.
I also point out that starting HT and continuing it over the long term may reduce their risk of dementia later in life.
I do prescribe oral and transdermal estrogen, but I more often prescribe transdermal formulations because of their apparent safety in regard to the risk of venous thromboembolism. This preference for transdermal estrogen applies, in particular, to overweight women because their baseline risk of VTE is elevated.
Regarding breast cancer, I point out to estrogen-only HT candidates that HT prevents breast cancer. I counsel women whose uterus is intact that women who use combination HT for longer than 3 to 5 years experience a modest increase in their risk of having a diagnosis of breast cancer—similar to the elevation associated with moderate alcohol consumption. I also point out that the risk of dying from breast cancer might be increased with long-term combination HT use.
In women for whom the only indication for HT is prevention of genital atrophy, I prefer to prescribe vaginal formulations of estrogen.
Some of my patients—particularly those who do not have a uterus—who are extensively counseled, choose to continue HT indefinitely. Such very-long-term users often focus on either 1) their greater sense of well-being with HT or 2) the benefit of the prevention of osteoporosis in the face of their desire to avoid long-term bisphosphonate therapy.
Last, over the course of patients’ years of taking HT, I encourage them to try lower dosages, until they either discontinue HT or remain on a very low dosage.
We want to hear from you! Tell us what you think.
1. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: A randomized controlled trial. Menopause. 2009;16(6):1156-1166.
2. Pachman DR, Jones JM, Loprinzi CL. Management of menopause-associated vasomotor symptoms: Current treatment options challenges and future directions. Int J Womens Health. 2010;2:123-135.
3. Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340-345.
4. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519.
5. ACOG Practice Bulletin No 89. Elective and risk-reducing salpingo-oophorectomy. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;111(1):231-241.
6. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684-1692.
7. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305-1314.
8. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288(3):321-333.
9. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701-1712.
1. Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: A randomized controlled trial. Menopause. 2009;16(6):1156-1166.
2. Pachman DR, Jones JM, Loprinzi CL. Management of menopause-associated vasomotor symptoms: Current treatment options challenges and future directions. Int J Womens Health. 2010;2:123-135.
3. Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340-345.
4. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519.
5. ACOG Practice Bulletin No 89. Elective and risk-reducing salpingo-oophorectomy. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;111(1):231-241.
6. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684-1692.
7. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305-1314.
8. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288(3):321-333.
9. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701-1712.