User login
Early Identification of Pancreatic Cancer
In the United States in the year 2012, it has been estimated that some 43,920 patients will receive a new diagnosis of pancreatic cancer, and about 37,390 will die of the disease.1 Although it represents less than 2% of new cancer diagnoses, pancreatic cancer is the fourth leading cause of cancer-related deaths in both women and men.2,3 Because the anatomic location of the pancreas makes the disease difficult to diagnose at an early stage, pancreatic cancer has a median survival of less than six months after diagnosis, and a 4.6% survival rate at 5 years.2 The current focus on pancreatic cancer is to improve survival through earlier diagnosis—before the tumor has reached an advanced stage.4
The incidence and mortality rates for pancreatic cancer have changed only minimally during the past 30 years. In recent decades, as reported in 2011 by the National Comprehensive Cancer Network (NCCN),5 the incidence of pancreatic cancer has increased steadily. Prevalence is greater in men than in women, and African-Americans have an increased incidence of pancreatic cancer, compared with whites. In the United States alone, the approximate medical costs associated with pancreatic cancer in 2006 amounted to nearly $1.9 billion.6
According to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database,7 derived from cases reported from 17 SEER regions between 1975 and 2009, the median patient age for a diagnosis of pancreatic cancer between 2004 and 2008 was 72 years. Prevalence of pancreatic cancer was greatest (28.6%) between ages 75 and 84, and age-adjusted incidence was reported at 12.0 per 100,000 men and women per year.7
The US median age for pancreatic cancer–associated mortality between 2004 and 2008, as reported by SEER, was 73.7 Patients in the 75-to-84 age-group accounted for 30.5% of pancreatic cancer–related deaths. In terms of all patient deaths reported in the US between 2004 and 2008, the overall pancreatic cancer–associated age-adjusted death rate was 10.8 per 100,000 men and women per year.7
In most cases, pancreatic cancer has a very unfavorable prognosis. It is estimated that 52% of all patients diagnosed with pancreatic cancer already have distant disease at the time of diagnosis; in 26%, cancer has already metastasized. According to data from the NCCN,5 survival rates for pancreatic cancer are 24% at one year after diagnosis, and 5% at five years. Treatment options eventually narrow down to palliative care.
Nevertheless, it is important for clinicians to be knowledgeable about pancreatic cancer, with an appreciation for the complexity of this disease. A clinical understanding of patients who are at high risk for pancreatic cancer may afford clinicians the opportunity to identify and treat pancreatic cancer earlier in the disease process.
THE DEVELOPMENT OF PANCREATIC CANCER
The progression from normal ductal epithelium to pancreatic ductal adenocarcinomas occurs through noninvasive precursor lesions (usually pancreatic intraepithelial neoplasias [PanINs]) that undergo clonally chosen genetic and epigenetic changes in the process.8-10Intraductal papillary mucinous neoplasms and mucinous cystic neoplasms also play a role in the development of pancreatic cancers.8,11,12
PanINs represent the most commonly identified neoplastic precursor to invasive ductal adenocarcinomas,8,13 which account for at least 90% of all pancreatic tumors and are considered one of the most lethal among all solid malignancies.13-15 PanINs measure less than 5.0 mm in diameter and cannot ordinarily be detected on pancreatic imaging.8 PanINs can store somatic genetic changes identified in invasive pancreatic cancers; these changes increase at the same rate that cytologic and architectural atypia develop in the precursor lesions.8,10 The genetic and epigenetic variations associated with pancreatic cancer may help explain the rapid progression of the tumor to an advanced stage.8
Pancreatic Cancer and Diabetes
The correlation between diabetes mellitus and pancreatic cancer has been acknowledged for many years; patients with type 1 or type 2 diabetes have an increased risk for pancreatic cancer—by 40% to 100% in patients with long-term diabetes.16 In nearly one-third of patients who develop “late-onset diabetes,” this condition may be an effect (and thus, a “harbinger”16) of pancreatic cancer. In the recently diagnosed diabetic patient, the risk for pancreatic cancer is increased by four- to sevenfold, and 1% to 2% of patients diagnosed with diabetes at age 50 or older will develop pancreatic cancer within three years.16,17
Pancreatic cancer should not be ruled out in the differential diagnosis in a patient who receives a new diagnosis of diabetes, despite an absence of risk factors for diabetes, after age 70.3
Other Risk Factors for Pancreatic Cancer
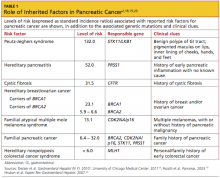
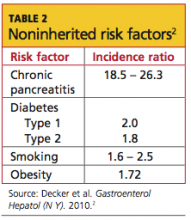
Cigarette smoking and family history (ie, at least one first-degree relative affected by the disease) are the predominant risk factors for pancreatic cancer. In addition to smoking, significant avoidable or reversible risk factors include alcohol abuse, obesity, a sedentary lifestyle, a diet high in fats and meats and low in vegetables and folate, and certain environmental exposures (eg, solvents used in dry cleaning, gasoline-related particles, nickel).2,4,8 Risk factors that are unavoidable or irreversible include advancing age, male gender, African-American ethnicity, a non–O blood group type, and a history of radiation treatment.4,8 Analyses of risk factors for pancreatic cancer appear in Table 12,18-20 (inherited risk factors) and Table 22 (noninherited risk factors).
It has been estimated that 20% to 25% of pancreatic tumors are attributable to cigarette smoking, and individuals who smoke carry more cancer-related genetic mutations than do nonsmokers.21,22 Additionally, patients with hereditary pancreatitis who smoke are at double the risk for pancreatic cancer and can develop the disease 20 years earlier than those with the associated genetic mutation who do not smoke.4 Among the germline mutations associated with cancer, BRCA2 mutations have been reported in 12% to 17% of patients with familial pancreatic cancer.23
As of 2010-2011, the US Preventive Services Task Force24 (USPSTF) recommended against routine screening (ie, abdominal palpation for organomegaly, serologic markers, or ultrasonography) for pancreatic cancer in asymptomatic adults; the USPSTF has not yet reviewed the effectiveness of screening patients with hereditary pancreatitis, even though they “may have a higher lifetime risk for developing pancreatic cancer.”24 Screening for pancreatic cancer is usually reserved for patients with a lifetime pancreatic cancer risk of at least 5%; it begins with a genetic analysis to detect mutations associated with pancreatic cancer.4,18 Imaging may follow, with the intent to detect precursor lesions or early pancreatic cancers.4
CLINICAL PRESENTATION
Clinically silent in its early stages, pancreatic cancer usually presents after the tumor has metastasized to distant organs or has invaded adjacent tissues. Typically, patients with pancreatic cancer have undergone abdominal CT for evaluation of other clinically indicated reasons before receiving this diagnosis.8
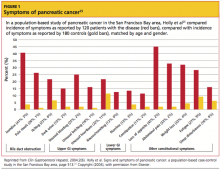
The presenting symptoms for pancreatic cancer include, but are not limited to, abdominal pain, midback pain, jaundice, loss of appetite and weight loss, floating stools, dyspepsia, nausea, and depression5,25 (see Figure 125 for a detailed analysis of self-reported symptoms in a study of patients with pancreatic cancer). Both deep and superficial venous thrombosis, which are not unusual on presentation, can be an indication of malignant disease.8,26 In patients with pancreatic cancer, incidence of thromboembolic disease, in its various manifestations, ranges from 17% to 57%.27,28
In a 2010 study, Raptis et al29 analyzed data from 355 patients with pancreatic cancer to evaluate whether a specific clinical presentation (abdominal pain, weight loss, jaundice) and delayed timing in referral, diagnosis, and treatment had any impact on the operability, resectability, and/or survival rate associated with pancreatic cancer. The researchers concluded that the time delay between referral and treatment had no significant impact on patient survival.
PHYSICAL EXAMINATION
Abdominal pain is the presenting complaint in about half of all adult patients who seek primary care.30,31 This presentation is contestable in both primary care and specialty practice, such as gastroenterology, because it is usually a benign symptom; it can also be an indication of serious acute pathology32 (see “Case Patient”33,34).
In the study by Raptis et al,29 abdominal pain was the symptom with which patients affected by pancreatic cancer most commonly presented to primary care. Thus, it is important for the clinician to perform a thorough abdominal exam—inspection, auscultation, percussion, light touch, and deep palpation—on any patient with such symptoms. Deep palpation is specifically sensitive, since it can sometimes help the provider feel the abdominal organs, especially if they are enlarged. Opioids may be given if needed while the assessment proceeds.30
Other key points to include in the physical exam of a patient presenting with abdominal pain are vital signs, observation for jaundice (which in one study was reported in 41% of patients with pancreatic cancer25), chest auscultation and percussion, examination of the rectal, pelvic, and genitourinary regions, and an evaluation of mental status.31-33
Abnormal findings can help guide clinical decision making and treatment planning. It should be emphasized, however, that tumors of the pancreas can be difficult to detect and diagnose because of the anatomic location of the pancreas and the disease’s insidious properties.4,14
Differential Diagnosis
It is critical to explore the many differential diagnoses for abdominal pain; the history and physical examination should guide the evaluation. Characterizing the pain according to location, chronology, severity, aggravating and alleviating factors, and associated symptoms can be most helpful. Pregnancy should be excluded in all women of childbearing age who present with abdominal pain.30,31
When pancreatic cancer is suspected, the differential diagnosis should include a malignant obstruction of the common bile duct, gastric cancer, cholangitis, cholelithiasis, choledolithiasis, cholecystitis, a choledochal cyst, duodenal or gastric ulcers, and acute or chronic pancreatitis.3 Additionally, conditions whose symptoms can mimic those of pancreatic cancer include abdominal aortic aneurysm, intestinal ischemia, and stricture of the bile duct. Symptoms associated with pancreatic ductal adenocarcinoma may also occur in patients with tumors of the bile duct, pancreatic lymphoma, gastric lymphoma, ampullary carcinoma, or hepatocellular carcinoma.3
LABORATORY WORKUP
Testing should include a complete metabolic panel, lipase, amylase, and a complete blood count with differential. These will reveal abnormalities in the total bilirubin and/or liver enzymes, and possible elevation of lipase and/or amylase, which could indicate acute, chronic, or hereditary pancreatitis—all of which are implicated in pancreatic cancer.2,35-37
No distinct tumor markers for pancreatic cancer have yet been identified. Though low in specificity for pancreatic cancer, the serum marker cancer antigen 19-9 (CA 19-9) is elevated in most patients when pancreatic cancer is diagnosed.38 Also, postsurgical levels of CA 19-9 are effective in evaluating patients’ response to neoadjuvant therapy and determining patient prognosis.39
DIAGNOSTIC IMAGING
Because it is noninvasive and relatively inexpensive, abdominal ultrasound (US) is often used first to investigate abdominal pain or jaundice; results suggestive of pancreatic cancer might include low echoic mass or dilatation of the pancreatic duct or of the common bile duct.40 However, conventional US is only 50% to 70% accurate for a diagnosis of pancreatic tumor.40,41
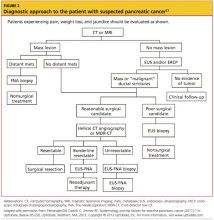
In many trials of imaging options for early detection and staging of pancreatic cancer, endoscopic US (EUS) has been deemed an effective modality for defining local T and N staging and for evaluating the potential for vascular involvement; CT has been pronounced effective for defining distant metastasis.23 For an algorithm detailing evaluation of the patient with a suspected pancreatic tumor, see Figure 2.23
All patients with suspected pancreatic cancer should be evaluated initially using CT or MRI.5Multidetector row CT (MDR-CT) is the most commonly used imaging modality for the detection and staging of pancreatic carcinoma.23 On contrast-enhanced CT, pancreatic adenocarcinoma commonly appears as a low-density area. EUS-guided fine-needle aspiration is an appropriate option for making a diagnosis with tissue samples.3,23,40 Triphasic pancreatic protocol CT provides about 90% accuracy for predicting resectability of a pancreatic tumor; EUS is comparably effective.3,5
MR cholangiopancreatography (MRCP) is a useful tool for visualizing the pancreas and the bile duct, while endoscopic retrograde cholangiopancreatography (ERCP) combines endoscopy and x-ray to visualize the pancreas and biliary tree.18,42 In one prospective, controlled study comparing these two modalities, MRCP and ERCP had 84% and 70% sensitivity, respectively, and 97% and 94% specificity, respectively, for diagnosis of pancreatic cancer (in cases actually diagnosed by histologic findings following surgical or fine-needle biopsy).42 Although ERCP makes it possible to obtain cytology and histology samples and to perform biliary stenting in patients with obstructive jaundice, it can miss tumors in the uncinate process, the accessory duct, and the tail of the pancreas.23,40
Tumor Staging for Pancreatic Cancer
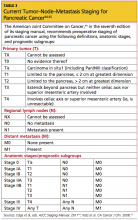
Pancreatic tumors can develop from various pancreatic cell lines; they are defined by appearance, hormonal function, and origin cell type.43 The staging system for pancreatic exocrine cancer continues to evolve.44,45 In the seventh edition of the American Joint Committee on Cancer’s AJCC Staging Manual (2011),44 the AJCC bases the preoperative clinical staging of pancreatic cancer on the results of high-quality cross-sectional imaging, through which local resectability and the absence or presence of distant disease are determined44,45 (see Table 3,44,45).
Clinical staging categories for the pancreatic tumor are:
- Resectable
- Borderline resectable
- Locally advanced, and
- Metastatic disease.8
The significance of tumor staging extending past resectable versus unresectable is currently unknown, as treatment has had little effect on survival. Cancers involving the pancreas are typically described according to the location of involvement within the organ: the head, the body, the tail, or the uncinate process.8
Confirming the presence of ductal adenocarcinoma by histology and cytology is essential; long-term survival may be increased in patients treated for other forms of pancreatic cancer (eg, ampullary or periampullary carcinomas; mucinous cystadenocarcinomas) or even benign lesions.15
TREATMENT AND MANAGEMENT
One reliable evidence-based clinical resource is the NCCN publication Clinical Guidelines in Oncology for Pancreatic Adenocarcinoma.5 A multidisciplinary approach to resectable or borderline resectable pancreatic cancer is the best management strategy and should involve a consulting gastroenterologist, a primary care provider, a surgeon, a medical oncologist, and a radiation oncologist, if one is needed.5,8,46 In patients with locally advanced or metastatic pancreatic cancer, a complete evaluation by a palliative care team is indicated.26
Surgery
Currently, the only known curative therapy for pancreatic cancer is surgical resection of the tumor and its surrounding tissue.47 The anatomic location and position of the tumor will guide the choice among surgical options:
- Classic pancreaticoduodenectomy (the Whipple procedure), recommended for tumors involving the head, neck, and uncinate process of the pancreas
- Pylorus-preserving pancreaticoduodenectomy (resection of the head, neck, uncinate process, and transection of the duodenum)
- Extended or radical pancreaticoduodenectomy (treatment, as for periampullary malignancies, that may involve resection of the head, neck, and uncinate process of the pancreas; the duodenum, gastric antrum and pylorus, common bile duct, and gallbladder; extensive dissection of retroperitoneal tissue and lymph nodes; possible vascular resection)
- Total pancreaticoduodenectomy, an option to treat a multicentric tumor or diffuse carcinoma of the entire gland; and
- Distal pancreatectomy, for adenocarcinomas in the body and tail of the pancreas.14,48-50
In patients with borderline resectable pancreatic cancer, the risk for positive surgical margins is high because tumors are likely to be involved with adjacent tissue (eg, nerve plexus, portal vein).46,51 This risk can be reduced, and prognosis improved, through neoadjuvant treatment regimens that combine gemcitabine-based chemotherapy and chemoradiotherapy.51
Palliative Chemotherapy
The stated purpose of palliative therapy, according to the NCCN,5 is to optimize quality of life by relieving cancer-related pain and other symptoms associated with biliary obstruction or gastric outlet obstruction. For patients with locally advanced, resectable or metastatic disease, NCCN recommendations (category 1 or 2A) for appropriate first-line therapy include:
- Gemcitabine, standard infusion (ie, 1,000 mg/m2 over 30 min, once per week for three weeks every 28 days), though not considered effective for certain pancreatic carcinomas8
- Gemcitabine plus cisplatin
- Gemcitabine plus erlotinib
- Gemcitabine plus capecitabine.5,52-55
In 2011, researchers in France reported results of a phase III trial comparing gemcitabine with the combination chemotherapy regimen FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin). According to Conroy et al,56 patients randomized to receive FOLFIRINOX experienced a survival advantage (median progression-free survival, 6.4 months vs 3.3 months) and delayed degradation of quality of life, but they were more likely to experience toxicity (eg, febrile neutropenia, thrombocytopenia) than patients in the gemcitabine group.5,56
NCCN-recommended second-line therapies include gemcitabine monotherapy (for patients who have not previously received this agent); capecitabine monotherapy; 5-fluorouracil combined with leucovorin and oxaliplatin; or oxaliplatin plus capecitabine.5,57,58
FOLLOW-UP
The primary care provider should initiate supportive care at the time the patient receives a diagnosis of pancreatic cancer, including information about prognosis and treatment options (particularly palliative care).8 The clinician should continue to follow the patient’s clinical course throughout treatment for advanced disease. Pain management should be a priority, particularly identifying and addressing its precise cause.26
As with other cancers, pancreatic cancer poses a considerable risk for recurrence after surgical resection and/or chemoradiation. Ongoing follow-up physical exams, routine laboratory studies (including screening for CA 19-9 every one to three months), and imaging studies should be ordered and results reviewed periodically (for example, CT at least every six months).23
Anticipatory Guidance for At-Risk Patients
Counseling patients at high risk for pancreatic cancer, targeting prevention and early detection, is key to reducing the incidence of this lethal disease. It is important to educate patients regarding the risk factors associated with pancreatic cancer—particularly smoking, the most reversible risk factor for pancreatic cancer.4 Patients who present with chronic pancreatitis and who consume excessive amounts of alcohol should be warned about the association between chronic pancreatitis and pancreatic cancer.4,8
Counseling should also focus on healthy eating, with a diet high in fruits and vegetables, routine physical activity, weight management, and an increased dietary intake of vitamin D (> 600 IU/d).24,59
Genetic counseling and testing options should be explored with at-risk patients and their families. These patients should be advised to seek immediate medical attention for abdominal pain, unexplained weight loss, and/or jaundice, and to return for further evaluation if symptoms persist despite initial treatment.59
The greater the clinician’s clinical understanding of the various pancreatic syndromes and their management, the better prepared he or she will be to evaluate each patient’s genetic risk and to provide high-quality clinical care.
CONCLUSION
Making a diagnosis of pancreatic cancer early enough to make effective treatment possible continues to challenge both researchers and clinicians. Current treatment modalities are not sufficiently effective, as evidenced by the high mortality rate associated with pancreatic cancer. Until clinical research provides increased insight into detecting this lethal disease and generating more effective therapies, it is up to the primary care clinician to routinely explore the family and social history of at-risk patients and to recognize the risk factors and symptomatology associated with pancreatic cancer. Early detection, followed by the most effective treatment options available, may lead to a more hopeful prognosis.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10-29.
2. Decker GA, Batheja MJ, Collins JM, et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y). 2010;6(4):246-254.
3. Dragovich T. Pancreatic cancer (2011). http://emedicine.medscape.com/article/280605 -overview#showall. Accessed February 17, 2012.
4. Stoita A, Penman ID, Williams DB. Review of screening for pancreatic cancer in high-risk individuals. World J Gastroenterol. 2011;17(19):
2365-2371.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma, Version 2.2012 (2011). www.nccn.org/profession als/physician_gls/pdf/pancreatic.pdf. Accessed March 2, 2012.
6. National Cancer Institute, US Department of Health and Human Services, NIH. Cancer Trends Progress Report, 2009/2010 Update. http://pro gressreport.cancer.gov/doc detail.asp?pid=1&did=2009&chid=95&coid=926&mid=. Accessed February 22, 2012.
7. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute. http://seer.cancer
.gov/csr/1975_2008/index.html. Accessed February 17, 2012.
8. Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791);607-620.
9. Koorstra JBM, Feldmann G, Habbe N, Maitra A. Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs). Langenbecks Arch Surg. 2008;393(4):561–570.
10. Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6(8):2969-2972.
11. Lubezky N, Ben-Haim M, Lahat G, et al. Intraductal papillary mucinous neoplasm of the pancreas: associated cancers, family history, genetic predisposition? Surgery. 2012;151(1):70-75.
12. Takaori K, Hruban RH, Maitra A, Tanigawa N. Current topics on precursors to pancreatic cancer. Adv Med Sci. 2006;51:23-30.
13. Hruban RH, Fukushima N. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod Pathol. 2007;20 suppl 1:S61-S70.
14. Dunphy EP. Pancreatic cancer: a review and update. Clin J Oncol Nurs. 2008:12(5):735-741.
15. Carpelan-Holström M, Nordling S, Pukkala E, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005; 54(3):385-387.
16. Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas. 2011;40(3):339-351.
17. Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005; 129(2):504-511.
18. University of Chicago Medical Center. Pancreatic cancer screening (2011). www.uchospitals
.edu/specialties/cancer/pancreatic/screening.html. Accessed February 22, 2012.
19. Pezzilli R, Morselli-Labate AM, Mantovani V, et al. Mutations of the CFTR gene in pancreatic disease. Pancreas. 2003;27(4):332-336.
20. Hruban RH, Klein AP, Eshleman JR, et al. Familial pancreatic cancer: from genes to improved patient care. Expert Rev Gastroenterol Hepatol. 2007;1(1):81-88.
21. Blackford A, Parmigiani G, Kensler TW, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009; 69(8):3681-3688.
22. Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium. Am J Epidemiol. 2009;170(4):403-413.
23. Fernández-Del Castillo C, Jiminez RE. Epidemiology and risk factors for exocrine pancreatic cancer (2011). www.uptodate.com/contents/epidemi ology-and-risk-factors-for-exocrine-pancreatic-cancer. Accessed February 17, 2012.
24. Agency for Healthcare Research and Quality, US Preventive Services Task Force. Guide to Clinical Preventive Services 2010-2011: Recommendations of the US Preventive Services Task Force. www
.ahrq.gov/clinic/pocketgd.htm. Accessed March 5, 2012.
25. Holly EA, Chaliha, I, Bracci PM, Gautam M. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol. 2004; 2(6):510-517.
26. Garcia AA, Egner JR. Pancreatic cancer and primary care providers. Cancer Pract. 1995;3(1):37-41.
27. Shah MM, Saif MW. Pancreatic cancer and thrombosis: highlights from the 2010 ASCO Annual Meeting, Chicago, IL, June 4-8, 2010. JOP. 2010; 11(4):331-333.
28. Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004; 5(11):655-663.
29. Raptis DA, Fessas C, Belasyse-Smith P, Kurzawinski TR. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surgeon. 2010;8(5):239-246.
30. Fishman MB, Aronson MD. History and physical examination in adults with abdominal pain (2011). www.uptodate.com/contents/history-and-physical-examination-in-adults-with-abdominal-pain. Accessed February 17, 2012.
31. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7):971-978.
32. Penner RM, Majumdar SR. Diagnostic approach to abdominal pain in adults (2011). www.uptodate.com/contents/diagnostic-approach-to-abdominal-pain-in-adults. Accessed February 22, 2012.
33. Töx U, Hackenberg R, Stelzer A, et al. Endosonographic diagnosis of solid pancreatic tumors: a retrospective analysis from a tertiary referral center. Z Gastroenterol. 2007;45(4):307-312.
34. Schattner A, Fenakel G, Malnick SD. Cholelithiasis and pancreatic cancer. A case-control study. J Clin Gastroenterol. 1997;25(4):602-604.
35. Jura N, Archer H, Bar-Saqi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15(1):72-77.
36. Thomas PC, Nash GF, Aldridge MC. Pancreatic acinar cell carcinoma presenting as acute pancreatitis. HPB (Oxford). 2003;5(2):111-113.
37. Fischer CP, Pope I, Garden OJ. Mucinous cystic tumour of the pancreas presenting with acute pancreatitis. HPB (Oxford). 2001;3(4):271-273.
38. National Cancer Institute, NIH. Pancreatic Cancer Treatment (PDQ®) (2011). www.cancer.gov/cancertopics/pdq/treatment/pancreatic/Health Professional. Accessed February 17, 2012.
39. Humphris JL, Chang DK, Johns AL, et al; NSW Pancreatic Cancer Network. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012 Jan 11 [Epub ahead of print].
40. Miura F, Takada T, Amano H, et al. Diagnosis of pancreatic cancer. HPB (Oxford). 2006;8(5):
337-342.
41. Rickes S, Unkrodt K, Neye H, et al. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol. 2002;37(11):1313-1320.
42. Adamek HE, Albert J, Breer H, et al. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356(9225):190-193.
43. Simianu VV, Zyromski NJ, Nakeeb A, Lillemoe KD. Pancreatic cancer: progress made. Acta Oncologica. 2010;49(4):407-417.
44. Edge SB, Byrd DR, Compton CC, et al, eds; American Joint Committee on Cancer. AJCC Staging Manual. 7th ed (2011). Staging of pancreatic tumors. www.cancerstaging.org/staging/posters/pancreas8.5x11.pdf. Accessed February 17, 2012.
45. Katz MH, Hwang R, Fleming JB, Evans DB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin. 2008;58(2):
111-125.
46. Takahashi S, Kinoshita T, Konishi M, et al. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. J Hepatobiliary Pancreat Sci. 2011;18(4):567-574.
47. Castellanos E, Berlin J, Cardin DB. Current treatment options for pancreatic carcinoma. Curr Oncol Rep. 2011;13(3):195-205.
48. Nguyen TC, Sohn TA, Cameron JL, et al. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7(1):1-9.
49. Kuvshinoff BW, Bryer MP. Treatment of resectable and locally advanced pancreatic cancer. Cancer Control. 2000;7(5):428-436.
50. Sauter PP, Coleman J. Pancreatic cancer: a continuum of care. Sem Oncol Nurs. 1999;15(1):36-47.
51. Springett GM, Hoffe SE. Borderline resectable pancreatic cancer: on the edge of survival. Cancer Control. 2008;15(4):295-307.
52. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25(15):1960-1966.
53. Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513-5518.
54. Colucci G, Labianca R, Di Costanzo F, et al; the GIP-1 study. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer. J Clin Oncol. 2010; 28(10):1645-1651.
55. Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006; 24(24):3946-3952.
56. Conroy T, Desseigne F, Ychou M, et al; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364(19):1817-1825.
57. Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine-refractory pancreatic cancer: final results of the CONKO 003 study. J Clin Oncol. 2008;26(15; May 20 suppl; abstr 4508, 2008 Annual Meeting, American Society of Clinical Oncology).
58. Xiong HQ, Varadhachary GR, Blais JC, et al. Phase ll trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008; 113(8):2046-2052.
59. Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counseling and surveillance of patients at risk for pancreatic cancer. Gut. 2007; 56(10):1460-1469.
In the United States in the year 2012, it has been estimated that some 43,920 patients will receive a new diagnosis of pancreatic cancer, and about 37,390 will die of the disease.1 Although it represents less than 2% of new cancer diagnoses, pancreatic cancer is the fourth leading cause of cancer-related deaths in both women and men.2,3 Because the anatomic location of the pancreas makes the disease difficult to diagnose at an early stage, pancreatic cancer has a median survival of less than six months after diagnosis, and a 4.6% survival rate at 5 years.2 The current focus on pancreatic cancer is to improve survival through earlier diagnosis—before the tumor has reached an advanced stage.4
The incidence and mortality rates for pancreatic cancer have changed only minimally during the past 30 years. In recent decades, as reported in 2011 by the National Comprehensive Cancer Network (NCCN),5 the incidence of pancreatic cancer has increased steadily. Prevalence is greater in men than in women, and African-Americans have an increased incidence of pancreatic cancer, compared with whites. In the United States alone, the approximate medical costs associated with pancreatic cancer in 2006 amounted to nearly $1.9 billion.6
According to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database,7 derived from cases reported from 17 SEER regions between 1975 and 2009, the median patient age for a diagnosis of pancreatic cancer between 2004 and 2008 was 72 years. Prevalence of pancreatic cancer was greatest (28.6%) between ages 75 and 84, and age-adjusted incidence was reported at 12.0 per 100,000 men and women per year.7
The US median age for pancreatic cancer–associated mortality between 2004 and 2008, as reported by SEER, was 73.7 Patients in the 75-to-84 age-group accounted for 30.5% of pancreatic cancer–related deaths. In terms of all patient deaths reported in the US between 2004 and 2008, the overall pancreatic cancer–associated age-adjusted death rate was 10.8 per 100,000 men and women per year.7
In most cases, pancreatic cancer has a very unfavorable prognosis. It is estimated that 52% of all patients diagnosed with pancreatic cancer already have distant disease at the time of diagnosis; in 26%, cancer has already metastasized. According to data from the NCCN,5 survival rates for pancreatic cancer are 24% at one year after diagnosis, and 5% at five years. Treatment options eventually narrow down to palliative care.
Nevertheless, it is important for clinicians to be knowledgeable about pancreatic cancer, with an appreciation for the complexity of this disease. A clinical understanding of patients who are at high risk for pancreatic cancer may afford clinicians the opportunity to identify and treat pancreatic cancer earlier in the disease process.
THE DEVELOPMENT OF PANCREATIC CANCER
The progression from normal ductal epithelium to pancreatic ductal adenocarcinomas occurs through noninvasive precursor lesions (usually pancreatic intraepithelial neoplasias [PanINs]) that undergo clonally chosen genetic and epigenetic changes in the process.8-10Intraductal papillary mucinous neoplasms and mucinous cystic neoplasms also play a role in the development of pancreatic cancers.8,11,12
PanINs represent the most commonly identified neoplastic precursor to invasive ductal adenocarcinomas,8,13 which account for at least 90% of all pancreatic tumors and are considered one of the most lethal among all solid malignancies.13-15 PanINs measure less than 5.0 mm in diameter and cannot ordinarily be detected on pancreatic imaging.8 PanINs can store somatic genetic changes identified in invasive pancreatic cancers; these changes increase at the same rate that cytologic and architectural atypia develop in the precursor lesions.8,10 The genetic and epigenetic variations associated with pancreatic cancer may help explain the rapid progression of the tumor to an advanced stage.8
Pancreatic Cancer and Diabetes
The correlation between diabetes mellitus and pancreatic cancer has been acknowledged for many years; patients with type 1 or type 2 diabetes have an increased risk for pancreatic cancer—by 40% to 100% in patients with long-term diabetes.16 In nearly one-third of patients who develop “late-onset diabetes,” this condition may be an effect (and thus, a “harbinger”16) of pancreatic cancer. In the recently diagnosed diabetic patient, the risk for pancreatic cancer is increased by four- to sevenfold, and 1% to 2% of patients diagnosed with diabetes at age 50 or older will develop pancreatic cancer within three years.16,17
Pancreatic cancer should not be ruled out in the differential diagnosis in a patient who receives a new diagnosis of diabetes, despite an absence of risk factors for diabetes, after age 70.3
Other Risk Factors for Pancreatic Cancer
Cigarette smoking and family history (ie, at least one first-degree relative affected by the disease) are the predominant risk factors for pancreatic cancer. In addition to smoking, significant avoidable or reversible risk factors include alcohol abuse, obesity, a sedentary lifestyle, a diet high in fats and meats and low in vegetables and folate, and certain environmental exposures (eg, solvents used in dry cleaning, gasoline-related particles, nickel).2,4,8 Risk factors that are unavoidable or irreversible include advancing age, male gender, African-American ethnicity, a non–O blood group type, and a history of radiation treatment.4,8 Analyses of risk factors for pancreatic cancer appear in Table 12,18-20 (inherited risk factors) and Table 22 (noninherited risk factors).
It has been estimated that 20% to 25% of pancreatic tumors are attributable to cigarette smoking, and individuals who smoke carry more cancer-related genetic mutations than do nonsmokers.21,22 Additionally, patients with hereditary pancreatitis who smoke are at double the risk for pancreatic cancer and can develop the disease 20 years earlier than those with the associated genetic mutation who do not smoke.4 Among the germline mutations associated with cancer, BRCA2 mutations have been reported in 12% to 17% of patients with familial pancreatic cancer.23
As of 2010-2011, the US Preventive Services Task Force24 (USPSTF) recommended against routine screening (ie, abdominal palpation for organomegaly, serologic markers, or ultrasonography) for pancreatic cancer in asymptomatic adults; the USPSTF has not yet reviewed the effectiveness of screening patients with hereditary pancreatitis, even though they “may have a higher lifetime risk for developing pancreatic cancer.”24 Screening for pancreatic cancer is usually reserved for patients with a lifetime pancreatic cancer risk of at least 5%; it begins with a genetic analysis to detect mutations associated with pancreatic cancer.4,18 Imaging may follow, with the intent to detect precursor lesions or early pancreatic cancers.4
CLINICAL PRESENTATION
Clinically silent in its early stages, pancreatic cancer usually presents after the tumor has metastasized to distant organs or has invaded adjacent tissues. Typically, patients with pancreatic cancer have undergone abdominal CT for evaluation of other clinically indicated reasons before receiving this diagnosis.8
The presenting symptoms for pancreatic cancer include, but are not limited to, abdominal pain, midback pain, jaundice, loss of appetite and weight loss, floating stools, dyspepsia, nausea, and depression5,25 (see Figure 125 for a detailed analysis of self-reported symptoms in a study of patients with pancreatic cancer). Both deep and superficial venous thrombosis, which are not unusual on presentation, can be an indication of malignant disease.8,26 In patients with pancreatic cancer, incidence of thromboembolic disease, in its various manifestations, ranges from 17% to 57%.27,28
In a 2010 study, Raptis et al29 analyzed data from 355 patients with pancreatic cancer to evaluate whether a specific clinical presentation (abdominal pain, weight loss, jaundice) and delayed timing in referral, diagnosis, and treatment had any impact on the operability, resectability, and/or survival rate associated with pancreatic cancer. The researchers concluded that the time delay between referral and treatment had no significant impact on patient survival.
PHYSICAL EXAMINATION
Abdominal pain is the presenting complaint in about half of all adult patients who seek primary care.30,31 This presentation is contestable in both primary care and specialty practice, such as gastroenterology, because it is usually a benign symptom; it can also be an indication of serious acute pathology32 (see “Case Patient”33,34).
In the study by Raptis et al,29 abdominal pain was the symptom with which patients affected by pancreatic cancer most commonly presented to primary care. Thus, it is important for the clinician to perform a thorough abdominal exam—inspection, auscultation, percussion, light touch, and deep palpation—on any patient with such symptoms. Deep palpation is specifically sensitive, since it can sometimes help the provider feel the abdominal organs, especially if they are enlarged. Opioids may be given if needed while the assessment proceeds.30
Other key points to include in the physical exam of a patient presenting with abdominal pain are vital signs, observation for jaundice (which in one study was reported in 41% of patients with pancreatic cancer25), chest auscultation and percussion, examination of the rectal, pelvic, and genitourinary regions, and an evaluation of mental status.31-33
Abnormal findings can help guide clinical decision making and treatment planning. It should be emphasized, however, that tumors of the pancreas can be difficult to detect and diagnose because of the anatomic location of the pancreas and the disease’s insidious properties.4,14
Differential Diagnosis
It is critical to explore the many differential diagnoses for abdominal pain; the history and physical examination should guide the evaluation. Characterizing the pain according to location, chronology, severity, aggravating and alleviating factors, and associated symptoms can be most helpful. Pregnancy should be excluded in all women of childbearing age who present with abdominal pain.30,31
When pancreatic cancer is suspected, the differential diagnosis should include a malignant obstruction of the common bile duct, gastric cancer, cholangitis, cholelithiasis, choledolithiasis, cholecystitis, a choledochal cyst, duodenal or gastric ulcers, and acute or chronic pancreatitis.3 Additionally, conditions whose symptoms can mimic those of pancreatic cancer include abdominal aortic aneurysm, intestinal ischemia, and stricture of the bile duct. Symptoms associated with pancreatic ductal adenocarcinoma may also occur in patients with tumors of the bile duct, pancreatic lymphoma, gastric lymphoma, ampullary carcinoma, or hepatocellular carcinoma.3
LABORATORY WORKUP
Testing should include a complete metabolic panel, lipase, amylase, and a complete blood count with differential. These will reveal abnormalities in the total bilirubin and/or liver enzymes, and possible elevation of lipase and/or amylase, which could indicate acute, chronic, or hereditary pancreatitis—all of which are implicated in pancreatic cancer.2,35-37
No distinct tumor markers for pancreatic cancer have yet been identified. Though low in specificity for pancreatic cancer, the serum marker cancer antigen 19-9 (CA 19-9) is elevated in most patients when pancreatic cancer is diagnosed.38 Also, postsurgical levels of CA 19-9 are effective in evaluating patients’ response to neoadjuvant therapy and determining patient prognosis.39
DIAGNOSTIC IMAGING
Because it is noninvasive and relatively inexpensive, abdominal ultrasound (US) is often used first to investigate abdominal pain or jaundice; results suggestive of pancreatic cancer might include low echoic mass or dilatation of the pancreatic duct or of the common bile duct.40 However, conventional US is only 50% to 70% accurate for a diagnosis of pancreatic tumor.40,41
In many trials of imaging options for early detection and staging of pancreatic cancer, endoscopic US (EUS) has been deemed an effective modality for defining local T and N staging and for evaluating the potential for vascular involvement; CT has been pronounced effective for defining distant metastasis.23 For an algorithm detailing evaluation of the patient with a suspected pancreatic tumor, see Figure 2.23
All patients with suspected pancreatic cancer should be evaluated initially using CT or MRI.5Multidetector row CT (MDR-CT) is the most commonly used imaging modality for the detection and staging of pancreatic carcinoma.23 On contrast-enhanced CT, pancreatic adenocarcinoma commonly appears as a low-density area. EUS-guided fine-needle aspiration is an appropriate option for making a diagnosis with tissue samples.3,23,40 Triphasic pancreatic protocol CT provides about 90% accuracy for predicting resectability of a pancreatic tumor; EUS is comparably effective.3,5
MR cholangiopancreatography (MRCP) is a useful tool for visualizing the pancreas and the bile duct, while endoscopic retrograde cholangiopancreatography (ERCP) combines endoscopy and x-ray to visualize the pancreas and biliary tree.18,42 In one prospective, controlled study comparing these two modalities, MRCP and ERCP had 84% and 70% sensitivity, respectively, and 97% and 94% specificity, respectively, for diagnosis of pancreatic cancer (in cases actually diagnosed by histologic findings following surgical or fine-needle biopsy).42 Although ERCP makes it possible to obtain cytology and histology samples and to perform biliary stenting in patients with obstructive jaundice, it can miss tumors in the uncinate process, the accessory duct, and the tail of the pancreas.23,40
Tumor Staging for Pancreatic Cancer
Pancreatic tumors can develop from various pancreatic cell lines; they are defined by appearance, hormonal function, and origin cell type.43 The staging system for pancreatic exocrine cancer continues to evolve.44,45 In the seventh edition of the American Joint Committee on Cancer’s AJCC Staging Manual (2011),44 the AJCC bases the preoperative clinical staging of pancreatic cancer on the results of high-quality cross-sectional imaging, through which local resectability and the absence or presence of distant disease are determined44,45 (see Table 3,44,45).
Clinical staging categories for the pancreatic tumor are:
- Resectable
- Borderline resectable
- Locally advanced, and
- Metastatic disease.8
The significance of tumor staging extending past resectable versus unresectable is currently unknown, as treatment has had little effect on survival. Cancers involving the pancreas are typically described according to the location of involvement within the organ: the head, the body, the tail, or the uncinate process.8
Confirming the presence of ductal adenocarcinoma by histology and cytology is essential; long-term survival may be increased in patients treated for other forms of pancreatic cancer (eg, ampullary or periampullary carcinomas; mucinous cystadenocarcinomas) or even benign lesions.15
TREATMENT AND MANAGEMENT
One reliable evidence-based clinical resource is the NCCN publication Clinical Guidelines in Oncology for Pancreatic Adenocarcinoma.5 A multidisciplinary approach to resectable or borderline resectable pancreatic cancer is the best management strategy and should involve a consulting gastroenterologist, a primary care provider, a surgeon, a medical oncologist, and a radiation oncologist, if one is needed.5,8,46 In patients with locally advanced or metastatic pancreatic cancer, a complete evaluation by a palliative care team is indicated.26
Surgery
Currently, the only known curative therapy for pancreatic cancer is surgical resection of the tumor and its surrounding tissue.47 The anatomic location and position of the tumor will guide the choice among surgical options:
- Classic pancreaticoduodenectomy (the Whipple procedure), recommended for tumors involving the head, neck, and uncinate process of the pancreas
- Pylorus-preserving pancreaticoduodenectomy (resection of the head, neck, uncinate process, and transection of the duodenum)
- Extended or radical pancreaticoduodenectomy (treatment, as for periampullary malignancies, that may involve resection of the head, neck, and uncinate process of the pancreas; the duodenum, gastric antrum and pylorus, common bile duct, and gallbladder; extensive dissection of retroperitoneal tissue and lymph nodes; possible vascular resection)
- Total pancreaticoduodenectomy, an option to treat a multicentric tumor or diffuse carcinoma of the entire gland; and
- Distal pancreatectomy, for adenocarcinomas in the body and tail of the pancreas.14,48-50
In patients with borderline resectable pancreatic cancer, the risk for positive surgical margins is high because tumors are likely to be involved with adjacent tissue (eg, nerve plexus, portal vein).46,51 This risk can be reduced, and prognosis improved, through neoadjuvant treatment regimens that combine gemcitabine-based chemotherapy and chemoradiotherapy.51
Palliative Chemotherapy
The stated purpose of palliative therapy, according to the NCCN,5 is to optimize quality of life by relieving cancer-related pain and other symptoms associated with biliary obstruction or gastric outlet obstruction. For patients with locally advanced, resectable or metastatic disease, NCCN recommendations (category 1 or 2A) for appropriate first-line therapy include:
- Gemcitabine, standard infusion (ie, 1,000 mg/m2 over 30 min, once per week for three weeks every 28 days), though not considered effective for certain pancreatic carcinomas8
- Gemcitabine plus cisplatin
- Gemcitabine plus erlotinib
- Gemcitabine plus capecitabine.5,52-55
In 2011, researchers in France reported results of a phase III trial comparing gemcitabine with the combination chemotherapy regimen FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin). According to Conroy et al,56 patients randomized to receive FOLFIRINOX experienced a survival advantage (median progression-free survival, 6.4 months vs 3.3 months) and delayed degradation of quality of life, but they were more likely to experience toxicity (eg, febrile neutropenia, thrombocytopenia) than patients in the gemcitabine group.5,56
NCCN-recommended second-line therapies include gemcitabine monotherapy (for patients who have not previously received this agent); capecitabine monotherapy; 5-fluorouracil combined with leucovorin and oxaliplatin; or oxaliplatin plus capecitabine.5,57,58
FOLLOW-UP
The primary care provider should initiate supportive care at the time the patient receives a diagnosis of pancreatic cancer, including information about prognosis and treatment options (particularly palliative care).8 The clinician should continue to follow the patient’s clinical course throughout treatment for advanced disease. Pain management should be a priority, particularly identifying and addressing its precise cause.26
As with other cancers, pancreatic cancer poses a considerable risk for recurrence after surgical resection and/or chemoradiation. Ongoing follow-up physical exams, routine laboratory studies (including screening for CA 19-9 every one to three months), and imaging studies should be ordered and results reviewed periodically (for example, CT at least every six months).23
Anticipatory Guidance for At-Risk Patients
Counseling patients at high risk for pancreatic cancer, targeting prevention and early detection, is key to reducing the incidence of this lethal disease. It is important to educate patients regarding the risk factors associated with pancreatic cancer—particularly smoking, the most reversible risk factor for pancreatic cancer.4 Patients who present with chronic pancreatitis and who consume excessive amounts of alcohol should be warned about the association between chronic pancreatitis and pancreatic cancer.4,8
Counseling should also focus on healthy eating, with a diet high in fruits and vegetables, routine physical activity, weight management, and an increased dietary intake of vitamin D (> 600 IU/d).24,59
Genetic counseling and testing options should be explored with at-risk patients and their families. These patients should be advised to seek immediate medical attention for abdominal pain, unexplained weight loss, and/or jaundice, and to return for further evaluation if symptoms persist despite initial treatment.59
The greater the clinician’s clinical understanding of the various pancreatic syndromes and their management, the better prepared he or she will be to evaluate each patient’s genetic risk and to provide high-quality clinical care.
CONCLUSION
Making a diagnosis of pancreatic cancer early enough to make effective treatment possible continues to challenge both researchers and clinicians. Current treatment modalities are not sufficiently effective, as evidenced by the high mortality rate associated with pancreatic cancer. Until clinical research provides increased insight into detecting this lethal disease and generating more effective therapies, it is up to the primary care clinician to routinely explore the family and social history of at-risk patients and to recognize the risk factors and symptomatology associated with pancreatic cancer. Early detection, followed by the most effective treatment options available, may lead to a more hopeful prognosis.
In the United States in the year 2012, it has been estimated that some 43,920 patients will receive a new diagnosis of pancreatic cancer, and about 37,390 will die of the disease.1 Although it represents less than 2% of new cancer diagnoses, pancreatic cancer is the fourth leading cause of cancer-related deaths in both women and men.2,3 Because the anatomic location of the pancreas makes the disease difficult to diagnose at an early stage, pancreatic cancer has a median survival of less than six months after diagnosis, and a 4.6% survival rate at 5 years.2 The current focus on pancreatic cancer is to improve survival through earlier diagnosis—before the tumor has reached an advanced stage.4
The incidence and mortality rates for pancreatic cancer have changed only minimally during the past 30 years. In recent decades, as reported in 2011 by the National Comprehensive Cancer Network (NCCN),5 the incidence of pancreatic cancer has increased steadily. Prevalence is greater in men than in women, and African-Americans have an increased incidence of pancreatic cancer, compared with whites. In the United States alone, the approximate medical costs associated with pancreatic cancer in 2006 amounted to nearly $1.9 billion.6
According to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database,7 derived from cases reported from 17 SEER regions between 1975 and 2009, the median patient age for a diagnosis of pancreatic cancer between 2004 and 2008 was 72 years. Prevalence of pancreatic cancer was greatest (28.6%) between ages 75 and 84, and age-adjusted incidence was reported at 12.0 per 100,000 men and women per year.7
The US median age for pancreatic cancer–associated mortality between 2004 and 2008, as reported by SEER, was 73.7 Patients in the 75-to-84 age-group accounted for 30.5% of pancreatic cancer–related deaths. In terms of all patient deaths reported in the US between 2004 and 2008, the overall pancreatic cancer–associated age-adjusted death rate was 10.8 per 100,000 men and women per year.7
In most cases, pancreatic cancer has a very unfavorable prognosis. It is estimated that 52% of all patients diagnosed with pancreatic cancer already have distant disease at the time of diagnosis; in 26%, cancer has already metastasized. According to data from the NCCN,5 survival rates for pancreatic cancer are 24% at one year after diagnosis, and 5% at five years. Treatment options eventually narrow down to palliative care.
Nevertheless, it is important for clinicians to be knowledgeable about pancreatic cancer, with an appreciation for the complexity of this disease. A clinical understanding of patients who are at high risk for pancreatic cancer may afford clinicians the opportunity to identify and treat pancreatic cancer earlier in the disease process.
THE DEVELOPMENT OF PANCREATIC CANCER
The progression from normal ductal epithelium to pancreatic ductal adenocarcinomas occurs through noninvasive precursor lesions (usually pancreatic intraepithelial neoplasias [PanINs]) that undergo clonally chosen genetic and epigenetic changes in the process.8-10Intraductal papillary mucinous neoplasms and mucinous cystic neoplasms also play a role in the development of pancreatic cancers.8,11,12
PanINs represent the most commonly identified neoplastic precursor to invasive ductal adenocarcinomas,8,13 which account for at least 90% of all pancreatic tumors and are considered one of the most lethal among all solid malignancies.13-15 PanINs measure less than 5.0 mm in diameter and cannot ordinarily be detected on pancreatic imaging.8 PanINs can store somatic genetic changes identified in invasive pancreatic cancers; these changes increase at the same rate that cytologic and architectural atypia develop in the precursor lesions.8,10 The genetic and epigenetic variations associated with pancreatic cancer may help explain the rapid progression of the tumor to an advanced stage.8
Pancreatic Cancer and Diabetes
The correlation between diabetes mellitus and pancreatic cancer has been acknowledged for many years; patients with type 1 or type 2 diabetes have an increased risk for pancreatic cancer—by 40% to 100% in patients with long-term diabetes.16 In nearly one-third of patients who develop “late-onset diabetes,” this condition may be an effect (and thus, a “harbinger”16) of pancreatic cancer. In the recently diagnosed diabetic patient, the risk for pancreatic cancer is increased by four- to sevenfold, and 1% to 2% of patients diagnosed with diabetes at age 50 or older will develop pancreatic cancer within three years.16,17
Pancreatic cancer should not be ruled out in the differential diagnosis in a patient who receives a new diagnosis of diabetes, despite an absence of risk factors for diabetes, after age 70.3
Other Risk Factors for Pancreatic Cancer
Cigarette smoking and family history (ie, at least one first-degree relative affected by the disease) are the predominant risk factors for pancreatic cancer. In addition to smoking, significant avoidable or reversible risk factors include alcohol abuse, obesity, a sedentary lifestyle, a diet high in fats and meats and low in vegetables and folate, and certain environmental exposures (eg, solvents used in dry cleaning, gasoline-related particles, nickel).2,4,8 Risk factors that are unavoidable or irreversible include advancing age, male gender, African-American ethnicity, a non–O blood group type, and a history of radiation treatment.4,8 Analyses of risk factors for pancreatic cancer appear in Table 12,18-20 (inherited risk factors) and Table 22 (noninherited risk factors).
It has been estimated that 20% to 25% of pancreatic tumors are attributable to cigarette smoking, and individuals who smoke carry more cancer-related genetic mutations than do nonsmokers.21,22 Additionally, patients with hereditary pancreatitis who smoke are at double the risk for pancreatic cancer and can develop the disease 20 years earlier than those with the associated genetic mutation who do not smoke.4 Among the germline mutations associated with cancer, BRCA2 mutations have been reported in 12% to 17% of patients with familial pancreatic cancer.23
As of 2010-2011, the US Preventive Services Task Force24 (USPSTF) recommended against routine screening (ie, abdominal palpation for organomegaly, serologic markers, or ultrasonography) for pancreatic cancer in asymptomatic adults; the USPSTF has not yet reviewed the effectiveness of screening patients with hereditary pancreatitis, even though they “may have a higher lifetime risk for developing pancreatic cancer.”24 Screening for pancreatic cancer is usually reserved for patients with a lifetime pancreatic cancer risk of at least 5%; it begins with a genetic analysis to detect mutations associated with pancreatic cancer.4,18 Imaging may follow, with the intent to detect precursor lesions or early pancreatic cancers.4
CLINICAL PRESENTATION
Clinically silent in its early stages, pancreatic cancer usually presents after the tumor has metastasized to distant organs or has invaded adjacent tissues. Typically, patients with pancreatic cancer have undergone abdominal CT for evaluation of other clinically indicated reasons before receiving this diagnosis.8
The presenting symptoms for pancreatic cancer include, but are not limited to, abdominal pain, midback pain, jaundice, loss of appetite and weight loss, floating stools, dyspepsia, nausea, and depression5,25 (see Figure 125 for a detailed analysis of self-reported symptoms in a study of patients with pancreatic cancer). Both deep and superficial venous thrombosis, which are not unusual on presentation, can be an indication of malignant disease.8,26 In patients with pancreatic cancer, incidence of thromboembolic disease, in its various manifestations, ranges from 17% to 57%.27,28
In a 2010 study, Raptis et al29 analyzed data from 355 patients with pancreatic cancer to evaluate whether a specific clinical presentation (abdominal pain, weight loss, jaundice) and delayed timing in referral, diagnosis, and treatment had any impact on the operability, resectability, and/or survival rate associated with pancreatic cancer. The researchers concluded that the time delay between referral and treatment had no significant impact on patient survival.
PHYSICAL EXAMINATION
Abdominal pain is the presenting complaint in about half of all adult patients who seek primary care.30,31 This presentation is contestable in both primary care and specialty practice, such as gastroenterology, because it is usually a benign symptom; it can also be an indication of serious acute pathology32 (see “Case Patient”33,34).
In the study by Raptis et al,29 abdominal pain was the symptom with which patients affected by pancreatic cancer most commonly presented to primary care. Thus, it is important for the clinician to perform a thorough abdominal exam—inspection, auscultation, percussion, light touch, and deep palpation—on any patient with such symptoms. Deep palpation is specifically sensitive, since it can sometimes help the provider feel the abdominal organs, especially if they are enlarged. Opioids may be given if needed while the assessment proceeds.30
Other key points to include in the physical exam of a patient presenting with abdominal pain are vital signs, observation for jaundice (which in one study was reported in 41% of patients with pancreatic cancer25), chest auscultation and percussion, examination of the rectal, pelvic, and genitourinary regions, and an evaluation of mental status.31-33
Abnormal findings can help guide clinical decision making and treatment planning. It should be emphasized, however, that tumors of the pancreas can be difficult to detect and diagnose because of the anatomic location of the pancreas and the disease’s insidious properties.4,14
Differential Diagnosis
It is critical to explore the many differential diagnoses for abdominal pain; the history and physical examination should guide the evaluation. Characterizing the pain according to location, chronology, severity, aggravating and alleviating factors, and associated symptoms can be most helpful. Pregnancy should be excluded in all women of childbearing age who present with abdominal pain.30,31
When pancreatic cancer is suspected, the differential diagnosis should include a malignant obstruction of the common bile duct, gastric cancer, cholangitis, cholelithiasis, choledolithiasis, cholecystitis, a choledochal cyst, duodenal or gastric ulcers, and acute or chronic pancreatitis.3 Additionally, conditions whose symptoms can mimic those of pancreatic cancer include abdominal aortic aneurysm, intestinal ischemia, and stricture of the bile duct. Symptoms associated with pancreatic ductal adenocarcinoma may also occur in patients with tumors of the bile duct, pancreatic lymphoma, gastric lymphoma, ampullary carcinoma, or hepatocellular carcinoma.3
LABORATORY WORKUP
Testing should include a complete metabolic panel, lipase, amylase, and a complete blood count with differential. These will reveal abnormalities in the total bilirubin and/or liver enzymes, and possible elevation of lipase and/or amylase, which could indicate acute, chronic, or hereditary pancreatitis—all of which are implicated in pancreatic cancer.2,35-37
No distinct tumor markers for pancreatic cancer have yet been identified. Though low in specificity for pancreatic cancer, the serum marker cancer antigen 19-9 (CA 19-9) is elevated in most patients when pancreatic cancer is diagnosed.38 Also, postsurgical levels of CA 19-9 are effective in evaluating patients’ response to neoadjuvant therapy and determining patient prognosis.39
DIAGNOSTIC IMAGING
Because it is noninvasive and relatively inexpensive, abdominal ultrasound (US) is often used first to investigate abdominal pain or jaundice; results suggestive of pancreatic cancer might include low echoic mass or dilatation of the pancreatic duct or of the common bile duct.40 However, conventional US is only 50% to 70% accurate for a diagnosis of pancreatic tumor.40,41
In many trials of imaging options for early detection and staging of pancreatic cancer, endoscopic US (EUS) has been deemed an effective modality for defining local T and N staging and for evaluating the potential for vascular involvement; CT has been pronounced effective for defining distant metastasis.23 For an algorithm detailing evaluation of the patient with a suspected pancreatic tumor, see Figure 2.23
All patients with suspected pancreatic cancer should be evaluated initially using CT or MRI.5Multidetector row CT (MDR-CT) is the most commonly used imaging modality for the detection and staging of pancreatic carcinoma.23 On contrast-enhanced CT, pancreatic adenocarcinoma commonly appears as a low-density area. EUS-guided fine-needle aspiration is an appropriate option for making a diagnosis with tissue samples.3,23,40 Triphasic pancreatic protocol CT provides about 90% accuracy for predicting resectability of a pancreatic tumor; EUS is comparably effective.3,5
MR cholangiopancreatography (MRCP) is a useful tool for visualizing the pancreas and the bile duct, while endoscopic retrograde cholangiopancreatography (ERCP) combines endoscopy and x-ray to visualize the pancreas and biliary tree.18,42 In one prospective, controlled study comparing these two modalities, MRCP and ERCP had 84% and 70% sensitivity, respectively, and 97% and 94% specificity, respectively, for diagnosis of pancreatic cancer (in cases actually diagnosed by histologic findings following surgical or fine-needle biopsy).42 Although ERCP makes it possible to obtain cytology and histology samples and to perform biliary stenting in patients with obstructive jaundice, it can miss tumors in the uncinate process, the accessory duct, and the tail of the pancreas.23,40
Tumor Staging for Pancreatic Cancer
Pancreatic tumors can develop from various pancreatic cell lines; they are defined by appearance, hormonal function, and origin cell type.43 The staging system for pancreatic exocrine cancer continues to evolve.44,45 In the seventh edition of the American Joint Committee on Cancer’s AJCC Staging Manual (2011),44 the AJCC bases the preoperative clinical staging of pancreatic cancer on the results of high-quality cross-sectional imaging, through which local resectability and the absence or presence of distant disease are determined44,45 (see Table 3,44,45).
Clinical staging categories for the pancreatic tumor are:
- Resectable
- Borderline resectable
- Locally advanced, and
- Metastatic disease.8
The significance of tumor staging extending past resectable versus unresectable is currently unknown, as treatment has had little effect on survival. Cancers involving the pancreas are typically described according to the location of involvement within the organ: the head, the body, the tail, or the uncinate process.8
Confirming the presence of ductal adenocarcinoma by histology and cytology is essential; long-term survival may be increased in patients treated for other forms of pancreatic cancer (eg, ampullary or periampullary carcinomas; mucinous cystadenocarcinomas) or even benign lesions.15
TREATMENT AND MANAGEMENT
One reliable evidence-based clinical resource is the NCCN publication Clinical Guidelines in Oncology for Pancreatic Adenocarcinoma.5 A multidisciplinary approach to resectable or borderline resectable pancreatic cancer is the best management strategy and should involve a consulting gastroenterologist, a primary care provider, a surgeon, a medical oncologist, and a radiation oncologist, if one is needed.5,8,46 In patients with locally advanced or metastatic pancreatic cancer, a complete evaluation by a palliative care team is indicated.26
Surgery
Currently, the only known curative therapy for pancreatic cancer is surgical resection of the tumor and its surrounding tissue.47 The anatomic location and position of the tumor will guide the choice among surgical options:
- Classic pancreaticoduodenectomy (the Whipple procedure), recommended for tumors involving the head, neck, and uncinate process of the pancreas
- Pylorus-preserving pancreaticoduodenectomy (resection of the head, neck, uncinate process, and transection of the duodenum)
- Extended or radical pancreaticoduodenectomy (treatment, as for periampullary malignancies, that may involve resection of the head, neck, and uncinate process of the pancreas; the duodenum, gastric antrum and pylorus, common bile duct, and gallbladder; extensive dissection of retroperitoneal tissue and lymph nodes; possible vascular resection)
- Total pancreaticoduodenectomy, an option to treat a multicentric tumor or diffuse carcinoma of the entire gland; and
- Distal pancreatectomy, for adenocarcinomas in the body and tail of the pancreas.14,48-50
In patients with borderline resectable pancreatic cancer, the risk for positive surgical margins is high because tumors are likely to be involved with adjacent tissue (eg, nerve plexus, portal vein).46,51 This risk can be reduced, and prognosis improved, through neoadjuvant treatment regimens that combine gemcitabine-based chemotherapy and chemoradiotherapy.51
Palliative Chemotherapy
The stated purpose of palliative therapy, according to the NCCN,5 is to optimize quality of life by relieving cancer-related pain and other symptoms associated with biliary obstruction or gastric outlet obstruction. For patients with locally advanced, resectable or metastatic disease, NCCN recommendations (category 1 or 2A) for appropriate first-line therapy include:
- Gemcitabine, standard infusion (ie, 1,000 mg/m2 over 30 min, once per week for three weeks every 28 days), though not considered effective for certain pancreatic carcinomas8
- Gemcitabine plus cisplatin
- Gemcitabine plus erlotinib
- Gemcitabine plus capecitabine.5,52-55
In 2011, researchers in France reported results of a phase III trial comparing gemcitabine with the combination chemotherapy regimen FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin). According to Conroy et al,56 patients randomized to receive FOLFIRINOX experienced a survival advantage (median progression-free survival, 6.4 months vs 3.3 months) and delayed degradation of quality of life, but they were more likely to experience toxicity (eg, febrile neutropenia, thrombocytopenia) than patients in the gemcitabine group.5,56
NCCN-recommended second-line therapies include gemcitabine monotherapy (for patients who have not previously received this agent); capecitabine monotherapy; 5-fluorouracil combined with leucovorin and oxaliplatin; or oxaliplatin plus capecitabine.5,57,58
FOLLOW-UP
The primary care provider should initiate supportive care at the time the patient receives a diagnosis of pancreatic cancer, including information about prognosis and treatment options (particularly palliative care).8 The clinician should continue to follow the patient’s clinical course throughout treatment for advanced disease. Pain management should be a priority, particularly identifying and addressing its precise cause.26
As with other cancers, pancreatic cancer poses a considerable risk for recurrence after surgical resection and/or chemoradiation. Ongoing follow-up physical exams, routine laboratory studies (including screening for CA 19-9 every one to three months), and imaging studies should be ordered and results reviewed periodically (for example, CT at least every six months).23
Anticipatory Guidance for At-Risk Patients
Counseling patients at high risk for pancreatic cancer, targeting prevention and early detection, is key to reducing the incidence of this lethal disease. It is important to educate patients regarding the risk factors associated with pancreatic cancer—particularly smoking, the most reversible risk factor for pancreatic cancer.4 Patients who present with chronic pancreatitis and who consume excessive amounts of alcohol should be warned about the association between chronic pancreatitis and pancreatic cancer.4,8
Counseling should also focus on healthy eating, with a diet high in fruits and vegetables, routine physical activity, weight management, and an increased dietary intake of vitamin D (> 600 IU/d).24,59
Genetic counseling and testing options should be explored with at-risk patients and their families. These patients should be advised to seek immediate medical attention for abdominal pain, unexplained weight loss, and/or jaundice, and to return for further evaluation if symptoms persist despite initial treatment.59
The greater the clinician’s clinical understanding of the various pancreatic syndromes and their management, the better prepared he or she will be to evaluate each patient’s genetic risk and to provide high-quality clinical care.
CONCLUSION
Making a diagnosis of pancreatic cancer early enough to make effective treatment possible continues to challenge both researchers and clinicians. Current treatment modalities are not sufficiently effective, as evidenced by the high mortality rate associated with pancreatic cancer. Until clinical research provides increased insight into detecting this lethal disease and generating more effective therapies, it is up to the primary care clinician to routinely explore the family and social history of at-risk patients and to recognize the risk factors and symptomatology associated with pancreatic cancer. Early detection, followed by the most effective treatment options available, may lead to a more hopeful prognosis.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10-29.
2. Decker GA, Batheja MJ, Collins JM, et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y). 2010;6(4):246-254.
3. Dragovich T. Pancreatic cancer (2011). http://emedicine.medscape.com/article/280605 -overview#showall. Accessed February 17, 2012.
4. Stoita A, Penman ID, Williams DB. Review of screening for pancreatic cancer in high-risk individuals. World J Gastroenterol. 2011;17(19):
2365-2371.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma, Version 2.2012 (2011). www.nccn.org/profession als/physician_gls/pdf/pancreatic.pdf. Accessed March 2, 2012.
6. National Cancer Institute, US Department of Health and Human Services, NIH. Cancer Trends Progress Report, 2009/2010 Update. http://pro gressreport.cancer.gov/doc detail.asp?pid=1&did=2009&chid=95&coid=926&mid=. Accessed February 22, 2012.
7. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute. http://seer.cancer
.gov/csr/1975_2008/index.html. Accessed February 17, 2012.
8. Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791);607-620.
9. Koorstra JBM, Feldmann G, Habbe N, Maitra A. Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs). Langenbecks Arch Surg. 2008;393(4):561–570.
10. Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6(8):2969-2972.
11. Lubezky N, Ben-Haim M, Lahat G, et al. Intraductal papillary mucinous neoplasm of the pancreas: associated cancers, family history, genetic predisposition? Surgery. 2012;151(1):70-75.
12. Takaori K, Hruban RH, Maitra A, Tanigawa N. Current topics on precursors to pancreatic cancer. Adv Med Sci. 2006;51:23-30.
13. Hruban RH, Fukushima N. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod Pathol. 2007;20 suppl 1:S61-S70.
14. Dunphy EP. Pancreatic cancer: a review and update. Clin J Oncol Nurs. 2008:12(5):735-741.
15. Carpelan-Holström M, Nordling S, Pukkala E, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005; 54(3):385-387.
16. Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas. 2011;40(3):339-351.
17. Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005; 129(2):504-511.
18. University of Chicago Medical Center. Pancreatic cancer screening (2011). www.uchospitals
.edu/specialties/cancer/pancreatic/screening.html. Accessed February 22, 2012.
19. Pezzilli R, Morselli-Labate AM, Mantovani V, et al. Mutations of the CFTR gene in pancreatic disease. Pancreas. 2003;27(4):332-336.
20. Hruban RH, Klein AP, Eshleman JR, et al. Familial pancreatic cancer: from genes to improved patient care. Expert Rev Gastroenterol Hepatol. 2007;1(1):81-88.
21. Blackford A, Parmigiani G, Kensler TW, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009; 69(8):3681-3688.
22. Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium. Am J Epidemiol. 2009;170(4):403-413.
23. Fernández-Del Castillo C, Jiminez RE. Epidemiology and risk factors for exocrine pancreatic cancer (2011). www.uptodate.com/contents/epidemi ology-and-risk-factors-for-exocrine-pancreatic-cancer. Accessed February 17, 2012.
24. Agency for Healthcare Research and Quality, US Preventive Services Task Force. Guide to Clinical Preventive Services 2010-2011: Recommendations of the US Preventive Services Task Force. www
.ahrq.gov/clinic/pocketgd.htm. Accessed March 5, 2012.
25. Holly EA, Chaliha, I, Bracci PM, Gautam M. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol. 2004; 2(6):510-517.
26. Garcia AA, Egner JR. Pancreatic cancer and primary care providers. Cancer Pract. 1995;3(1):37-41.
27. Shah MM, Saif MW. Pancreatic cancer and thrombosis: highlights from the 2010 ASCO Annual Meeting, Chicago, IL, June 4-8, 2010. JOP. 2010; 11(4):331-333.
28. Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004; 5(11):655-663.
29. Raptis DA, Fessas C, Belasyse-Smith P, Kurzawinski TR. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surgeon. 2010;8(5):239-246.
30. Fishman MB, Aronson MD. History and physical examination in adults with abdominal pain (2011). www.uptodate.com/contents/history-and-physical-examination-in-adults-with-abdominal-pain. Accessed February 17, 2012.
31. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7):971-978.
32. Penner RM, Majumdar SR. Diagnostic approach to abdominal pain in adults (2011). www.uptodate.com/contents/diagnostic-approach-to-abdominal-pain-in-adults. Accessed February 22, 2012.
33. Töx U, Hackenberg R, Stelzer A, et al. Endosonographic diagnosis of solid pancreatic tumors: a retrospective analysis from a tertiary referral center. Z Gastroenterol. 2007;45(4):307-312.
34. Schattner A, Fenakel G, Malnick SD. Cholelithiasis and pancreatic cancer. A case-control study. J Clin Gastroenterol. 1997;25(4):602-604.
35. Jura N, Archer H, Bar-Saqi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15(1):72-77.
36. Thomas PC, Nash GF, Aldridge MC. Pancreatic acinar cell carcinoma presenting as acute pancreatitis. HPB (Oxford). 2003;5(2):111-113.
37. Fischer CP, Pope I, Garden OJ. Mucinous cystic tumour of the pancreas presenting with acute pancreatitis. HPB (Oxford). 2001;3(4):271-273.
38. National Cancer Institute, NIH. Pancreatic Cancer Treatment (PDQ®) (2011). www.cancer.gov/cancertopics/pdq/treatment/pancreatic/Health Professional. Accessed February 17, 2012.
39. Humphris JL, Chang DK, Johns AL, et al; NSW Pancreatic Cancer Network. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012 Jan 11 [Epub ahead of print].
40. Miura F, Takada T, Amano H, et al. Diagnosis of pancreatic cancer. HPB (Oxford). 2006;8(5):
337-342.
41. Rickes S, Unkrodt K, Neye H, et al. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol. 2002;37(11):1313-1320.
42. Adamek HE, Albert J, Breer H, et al. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356(9225):190-193.
43. Simianu VV, Zyromski NJ, Nakeeb A, Lillemoe KD. Pancreatic cancer: progress made. Acta Oncologica. 2010;49(4):407-417.
44. Edge SB, Byrd DR, Compton CC, et al, eds; American Joint Committee on Cancer. AJCC Staging Manual. 7th ed (2011). Staging of pancreatic tumors. www.cancerstaging.org/staging/posters/pancreas8.5x11.pdf. Accessed February 17, 2012.
45. Katz MH, Hwang R, Fleming JB, Evans DB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin. 2008;58(2):
111-125.
46. Takahashi S, Kinoshita T, Konishi M, et al. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. J Hepatobiliary Pancreat Sci. 2011;18(4):567-574.
47. Castellanos E, Berlin J, Cardin DB. Current treatment options for pancreatic carcinoma. Curr Oncol Rep. 2011;13(3):195-205.
48. Nguyen TC, Sohn TA, Cameron JL, et al. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7(1):1-9.
49. Kuvshinoff BW, Bryer MP. Treatment of resectable and locally advanced pancreatic cancer. Cancer Control. 2000;7(5):428-436.
50. Sauter PP, Coleman J. Pancreatic cancer: a continuum of care. Sem Oncol Nurs. 1999;15(1):36-47.
51. Springett GM, Hoffe SE. Borderline resectable pancreatic cancer: on the edge of survival. Cancer Control. 2008;15(4):295-307.
52. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25(15):1960-1966.
53. Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513-5518.
54. Colucci G, Labianca R, Di Costanzo F, et al; the GIP-1 study. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer. J Clin Oncol. 2010; 28(10):1645-1651.
55. Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006; 24(24):3946-3952.
56. Conroy T, Desseigne F, Ychou M, et al; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364(19):1817-1825.
57. Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine-refractory pancreatic cancer: final results of the CONKO 003 study. J Clin Oncol. 2008;26(15; May 20 suppl; abstr 4508, 2008 Annual Meeting, American Society of Clinical Oncology).
58. Xiong HQ, Varadhachary GR, Blais JC, et al. Phase ll trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008; 113(8):2046-2052.
59. Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counseling and surveillance of patients at risk for pancreatic cancer. Gut. 2007; 56(10):1460-1469.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10-29.
2. Decker GA, Batheja MJ, Collins JM, et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y). 2010;6(4):246-254.
3. Dragovich T. Pancreatic cancer (2011). http://emedicine.medscape.com/article/280605 -overview#showall. Accessed February 17, 2012.
4. Stoita A, Penman ID, Williams DB. Review of screening for pancreatic cancer in high-risk individuals. World J Gastroenterol. 2011;17(19):
2365-2371.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma, Version 2.2012 (2011). www.nccn.org/profession als/physician_gls/pdf/pancreatic.pdf. Accessed March 2, 2012.
6. National Cancer Institute, US Department of Health and Human Services, NIH. Cancer Trends Progress Report, 2009/2010 Update. http://pro gressreport.cancer.gov/doc detail.asp?pid=1&did=2009&chid=95&coid=926&mid=. Accessed February 22, 2012.
7. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute. http://seer.cancer
.gov/csr/1975_2008/index.html. Accessed February 17, 2012.
8. Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791);607-620.
9. Koorstra JBM, Feldmann G, Habbe N, Maitra A. Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs). Langenbecks Arch Surg. 2008;393(4):561–570.
10. Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6(8):2969-2972.
11. Lubezky N, Ben-Haim M, Lahat G, et al. Intraductal papillary mucinous neoplasm of the pancreas: associated cancers, family history, genetic predisposition? Surgery. 2012;151(1):70-75.
12. Takaori K, Hruban RH, Maitra A, Tanigawa N. Current topics on precursors to pancreatic cancer. Adv Med Sci. 2006;51:23-30.
13. Hruban RH, Fukushima N. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod Pathol. 2007;20 suppl 1:S61-S70.
14. Dunphy EP. Pancreatic cancer: a review and update. Clin J Oncol Nurs. 2008:12(5):735-741.
15. Carpelan-Holström M, Nordling S, Pukkala E, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005; 54(3):385-387.
16. Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas. 2011;40(3):339-351.
17. Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005; 129(2):504-511.
18. University of Chicago Medical Center. Pancreatic cancer screening (2011). www.uchospitals
.edu/specialties/cancer/pancreatic/screening.html. Accessed February 22, 2012.
19. Pezzilli R, Morselli-Labate AM, Mantovani V, et al. Mutations of the CFTR gene in pancreatic disease. Pancreas. 2003;27(4):332-336.
20. Hruban RH, Klein AP, Eshleman JR, et al. Familial pancreatic cancer: from genes to improved patient care. Expert Rev Gastroenterol Hepatol. 2007;1(1):81-88.
21. Blackford A, Parmigiani G, Kensler TW, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009; 69(8):3681-3688.
22. Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium. Am J Epidemiol. 2009;170(4):403-413.
23. Fernández-Del Castillo C, Jiminez RE. Epidemiology and risk factors for exocrine pancreatic cancer (2011). www.uptodate.com/contents/epidemi ology-and-risk-factors-for-exocrine-pancreatic-cancer. Accessed February 17, 2012.
24. Agency for Healthcare Research and Quality, US Preventive Services Task Force. Guide to Clinical Preventive Services 2010-2011: Recommendations of the US Preventive Services Task Force. www
.ahrq.gov/clinic/pocketgd.htm. Accessed March 5, 2012.
25. Holly EA, Chaliha, I, Bracci PM, Gautam M. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol. 2004; 2(6):510-517.
26. Garcia AA, Egner JR. Pancreatic cancer and primary care providers. Cancer Pract. 1995;3(1):37-41.
27. Shah MM, Saif MW. Pancreatic cancer and thrombosis: highlights from the 2010 ASCO Annual Meeting, Chicago, IL, June 4-8, 2010. JOP. 2010; 11(4):331-333.
28. Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004; 5(11):655-663.
29. Raptis DA, Fessas C, Belasyse-Smith P, Kurzawinski TR. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surgeon. 2010;8(5):239-246.
30. Fishman MB, Aronson MD. History and physical examination in adults with abdominal pain (2011). www.uptodate.com/contents/history-and-physical-examination-in-adults-with-abdominal-pain. Accessed February 17, 2012.
31. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7):971-978.
32. Penner RM, Majumdar SR. Diagnostic approach to abdominal pain in adults (2011). www.uptodate.com/contents/diagnostic-approach-to-abdominal-pain-in-adults. Accessed February 22, 2012.
33. Töx U, Hackenberg R, Stelzer A, et al. Endosonographic diagnosis of solid pancreatic tumors: a retrospective analysis from a tertiary referral center. Z Gastroenterol. 2007;45(4):307-312.
34. Schattner A, Fenakel G, Malnick SD. Cholelithiasis and pancreatic cancer. A case-control study. J Clin Gastroenterol. 1997;25(4):602-604.
35. Jura N, Archer H, Bar-Saqi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15(1):72-77.
36. Thomas PC, Nash GF, Aldridge MC. Pancreatic acinar cell carcinoma presenting as acute pancreatitis. HPB (Oxford). 2003;5(2):111-113.
37. Fischer CP, Pope I, Garden OJ. Mucinous cystic tumour of the pancreas presenting with acute pancreatitis. HPB (Oxford). 2001;3(4):271-273.
38. National Cancer Institute, NIH. Pancreatic Cancer Treatment (PDQ®) (2011). www.cancer.gov/cancertopics/pdq/treatment/pancreatic/Health Professional. Accessed February 17, 2012.
39. Humphris JL, Chang DK, Johns AL, et al; NSW Pancreatic Cancer Network. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012 Jan 11 [Epub ahead of print].
40. Miura F, Takada T, Amano H, et al. Diagnosis of pancreatic cancer. HPB (Oxford). 2006;8(5):
337-342.
41. Rickes S, Unkrodt K, Neye H, et al. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol. 2002;37(11):1313-1320.
42. Adamek HE, Albert J, Breer H, et al. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356(9225):190-193.
43. Simianu VV, Zyromski NJ, Nakeeb A, Lillemoe KD. Pancreatic cancer: progress made. Acta Oncologica. 2010;49(4):407-417.
44. Edge SB, Byrd DR, Compton CC, et al, eds; American Joint Committee on Cancer. AJCC Staging Manual. 7th ed (2011). Staging of pancreatic tumors. www.cancerstaging.org/staging/posters/pancreas8.5x11.pdf. Accessed February 17, 2012.
45. Katz MH, Hwang R, Fleming JB, Evans DB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin. 2008;58(2):
111-125.
46. Takahashi S, Kinoshita T, Konishi M, et al. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. J Hepatobiliary Pancreat Sci. 2011;18(4):567-574.
47. Castellanos E, Berlin J, Cardin DB. Current treatment options for pancreatic carcinoma. Curr Oncol Rep. 2011;13(3):195-205.
48. Nguyen TC, Sohn TA, Cameron JL, et al. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7(1):1-9.
49. Kuvshinoff BW, Bryer MP. Treatment of resectable and locally advanced pancreatic cancer. Cancer Control. 2000;7(5):428-436.
50. Sauter PP, Coleman J. Pancreatic cancer: a continuum of care. Sem Oncol Nurs. 1999;15(1):36-47.
51. Springett GM, Hoffe SE. Borderline resectable pancreatic cancer: on the edge of survival. Cancer Control. 2008;15(4):295-307.
52. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25(15):1960-1966.
53. Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513-5518.
54. Colucci G, Labianca R, Di Costanzo F, et al; the GIP-1 study. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer. J Clin Oncol. 2010; 28(10):1645-1651.
55. Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006; 24(24):3946-3952.
56. Conroy T, Desseigne F, Ychou M, et al; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364(19):1817-1825.
57. Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine-refractory pancreatic cancer: final results of the CONKO 003 study. J Clin Oncol. 2008;26(15; May 20 suppl; abstr 4508, 2008 Annual Meeting, American Society of Clinical Oncology).
58. Xiong HQ, Varadhachary GR, Blais JC, et al. Phase ll trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008; 113(8):2046-2052.
59. Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counseling and surveillance of patients at risk for pancreatic cancer. Gut. 2007; 56(10):1460-1469.
Measuring the Benefits of Exercise for Cancer Patients
Medical Avatars—An Innovative Approach to Fostering Health Promotion and Lifestyle Change
A Shared Diabetes Clinic at a Veterans Affairs Medical Center
Anticholinergic Toxicity in a Patient With Sialorrhea
Grand Rounds: Man, 30, With Traumatic Finger Amputations
A 30-year-old man sustained traumatic amputations of three of his left fingers while at work. A heavy object fell when a supporting chain snapped; although he moved quickly, three of his left distal fingers were caught under the object. He was flown to a hospital for definitive hand care.
During the preadmission history and physical, it was noted that the patient had mild right knee pain in addition to his finger injuries. He had experienced no head injury and no loss of consciousness or other complaints. He did not remember injuring his leg, although he said it might have been struck by the falling object; all he could remember was the injury to his fingers.
On physical exam, the only abnormality other than the man’s traumatic finger amputations was mild right knee edema and a small bruised area medially. Initially, he complained of mild pain on palpation and moderate pain with passive range of motion, but range of motion was intact. His pain was worse at the proximal, medial tibial area, and he had mild lateral mid-calf tenderness though no bruising. Distally, his right lower extremity motor and sensory function were intact, and he had no open wounds or skin breakdown. He had 2+ dorsalis pedis pulse and 1+ posterior tibial pulse. The toes were pink and warm with brisk capillary refill. All compartments were soft and compressible.
Upon review of his plain radiographs (three views of the right knee), the patient was noted to have a severely comminuted medial tibial plateau fracture that extended to the midline in the region of the tibial spine, with mild depression of the fracture fragments measuring about 6 mm (see Figures 1a, 1b, and 1c). This would translate into a Schatzker IV classification type1 fracture (see Figure 22,3).
The man was admitted and underwent emergent surgery on his injured left fingers that night. Further diagnostic knee testing was performed, including CT and MRI (see Figures 3 and 4). Three days after admission, the patient underwent open reduction and internal fixation (plating) of the right medial, proximal tibia (see Figure 5). He has done very well since without issue.
DISCUSSION
Fractures of the tibial plateau occur along the articular, or joint, surface of the proximal tibia. The plateau consists of lateral and medial condylar surfaces. These concave structures function as an articulation point for the cartilaginous menisci and the femoral condyles.4 The medial plateau and condyle are stronger than those of the lateral side, and therefore are less commonly fractured. An elevated intercondylar eminence divides the lateral and medial plateaus, providing an attachment site for the cruciate ligaments.3
The Schatzker classification system1 is most commonly used to describe the types of tibial plateau fractures (as seen in Figure 22,3). Schatzker et al1 divided these injuries into six categories, according to the impact of increased energy exerted onto the bone; the rising classification numbers indicate an increase in complexity and severity and usually a worsening prognosis.
The type I fracture represents a split fracture of the lateral plateau. Typically, a fracture of this type has depression or displacement measuring less than 4 mm.
Type II tibial plateau fractures, the most common Schatzker injury, are lateral plateau fractures with depression noted at the split. Not always evident on plain radiographs, this depression can often be overlooked, and the injury mistaken for a type I fracture. The depression is measured vertically from the lower edge of the medial plateau to the lowest depression point of the lateral plateau.5
Type III fractures, the least common among the Schatzker injuries, are described as pure depression fractures of the lateral plateau. These fractures do not have an appreciable “split” along the plateau and are usually found in older patients with osteopenia.2
The Schatzker type IV injury is a medial fracture with displacement or depression to a portion of the plateau. The fracture may be split or comminuted and may originate in the intercondylar area.
Type V fractures, also known as “bicondylar fractures,” affect both the lateral and medial plateau. An inverted “Y” pattern is frequently seen, and there may be additional involvement of the intercondylar eminence. Type V fractures differ from type VI injuries in that there is no disturbance of the metaphyseal-diaphyseal connection. Thus, type VI fractures also include a transverse component that separates the condyles (metaphysis) of the bone from the shaft (diaphysis). Wide variation is seen among type VI fractures.5
Assessment and Diagnosis
Originally termed “fender fractures” due to their frequent association with automobile injuries, fractures of the tibial plateau account for 1% of all fractures and 8% of fractures in elderly patients.6 Tibial plateau fractures occur when varus or valgus force is combined with axial loading. The fracture itself occurs when the femoral condyle is driven into the lateral or medial plateau. Bicondylar injuries occur when rigorous axial force is sustained in a fully extended knee.
Injuries may also include those of the ligaments or menisci, resulting in joint instability. Patients may present with generalized knee pain or difficulty bearing weight after sustaining injuries, such as being struck in a motor vehicle accident, being tackled, or falling from some height.4
Evaluation of a patient with a suspected tibial plateau fracture begins with a detailed history and thorough physical examination. Details regarding the mechanism of injury help to predict the pattern of the fracture and may indicate whether a more focused neurovascular exam is warranted. Low-energy injuries (often seen with Schatzker types I to III) or twisting injuries yield low suspicion for neurovascular injury or compartment syndrome. However, high-energy injuries (seen often with Schatzker types IV through VI) have a greater likelihood of resulting in complicated injuries that must be urgently or emergently treated.5
The popliteal artery is bound posteriorly and distally to the tibial plateau, and the peroneal nerve is located laterally and positioned around the fibular head. It is essential to assess for the popliteal pulse, as well as lateral lower-extremity sensation and the patient’s ability to dorsiflex. Along with motor and neurovascular injuries, presentation with a painful, strikingly swollen knee and difficulty bearing weight may indicate a hemarthrosis. Soft tissue injuries over the knee resulting from direct trauma may require a saline arthrogram to rule out communication into the joint. Furthermore, a thorough ligamentous exam of the knee is helpful in determining the extent of the injuries.3
Compartment syndrome is a serious, emergent complication that can occur with tibial plateau fractures, especially those sustained during high-energy trauma.7 The health care provider must perform serial exams of the lower extremity to assess for classic signs of compartment syndrome. Are the compartments tense or noncompressible? Does the patient have pain with passive stretch or with range of motion of the lower extremity? Is there pallor or paresthesia to the affected limb? Is the pulse weak or absent? Presence of any of the aforementioned symptoms should prompt a high suspicion for compartment syndrome, and the patient must be sent to an emergency department for urgent evaluation.5
Treatment/Rehabilitation
For Schatzker types I through III, intervention focuses on the articular cartilage examination and repair. Type IV injuries often include corresponding damage to the popliteal artery and/or peroneal nerve, and types V and VI often have such overlying soft tissue damage that temporary placement of an external fixation device is required before definitive surgical intervention can be performed.8
However, it should be noted that conservative versus surgical treatment is often debated among surgeons for treatment of Schatzker fractures. The management of a tibial plateau fracture depends on the physical demands and health of the patient, the severity of the fracture, the stability of the joint, and the surgeon’s skill set and preferences.4 Operative intervention is generally indicated for fractures with depressions greater than 2 mm (although some surgeons allow up to 1 cm of depression), fractures with joint instability, or open fractures. Injuries with concern for vascular injury or compartment syndrome are also treated both operatively and emergently. Postoperatively, patients will remain non–weight-bearing for eight to 12 weeks after surgery, and in the interim, depending on the surgeon’s preference, may or may not engage in active or passive range of motion of the knee.
Advocates of open reduction and internal fixation (ORIF) argue that this method allows for the fracture reduction and anatomic alignment to be directly examined, but they also acknowledge that this approach compromises a great deal of soft tissue surrounding the proximal tibia.9,10
In order to reduce soft tissue damage, some surgeons favor external fixation. Initial use of this surgical technique results in minimal soft tissue swelling and allows early range of motion. While the external fixation device is in place, there is a risk for pin site infection, and proper site care must be provided.6,11
Generally, the treatment of tibial plateau fractures is considered successful when the fracture reduction is sustained, the patient’s functional capacity and axial loading are restored, and the articular surface is reconstructed. As a rule, nonoperative treatment is reserved for tibial plateau fractures that are minimally depressed or nondisplaced, or for patients with advanced osteoporosis. Under these circumstances, after a non–weight-bearing period of four to eight weeks, patients will begin to perform protected and partial weight bearing using a hinged knee brace.2 Early active range of motion, along with isometric exercises to strengthen the quadriceps, is recommended.
Whether surgical or conservative treatment is chosen, complications of tibial plateau fractures include knee stiffness, wound breakdown and infection, malunion or nonunion, vascular or neurologic injury, prominent or painful hardware, or avascular necrosis of fragmented bone pieces.4
CONCLUSION
The primary care practitioner must never overlook patients’ complaints of knee pain, especially after varus or valgus stress injuries or axial loading injuries to the knee. The patient may be able to ambulate; however, ordering a radiograph is an easy method for evaluation and for ruling out tibial plateau injuries. If there is any question regarding the presence of fracture with plain radiographs and/or the clinical exam warrants it, CT is an appropriate second diagnostic intervention.
Should a tibial plateau fracture present in a primary care or urgent care setting, thorough examination of neurovascular status and risk for compartment syndrome must be done urgently, followed by a referral to an orthopedic surgeon or emergency department.
REFERENCES
1. Schatzker J, McBroom R, Bruce D. The tibial plateau fracture: the Toronto experience, 1968–1975. Clin Orthop Relat Res. 1979;(138): 94-104.
2. Marsh JL. Tibial plateau fractures. In: Bucholz RW, Court-Brown CM, Heckman HD, Tornetta P. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:1780-1831.
3. Egol K, Koval KJ, Zuckerman JD. Tibial plateau. In: Egol K, Koval KJ, Zuckerman JD. Handbook of Fractures. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:455-463.
4. Fenton PP, Porter KK. Tibial plateau fractures: a review. Trauma. 2011;13(3):181-187.
5. Markhardt BK, Gross JM, Monu JU. Schatzker classification of tibial plateau fractures: use of CT and MR imaging improves assessment. Radiographics. 2009;29(2):585-597.
6. Lewis C. Does the mode of fixation of tibial plateau fractures, i.e. external fixation versus internal fixation, influence the time to union? A systematic review of the literature. Eur J Orthopaed Surg Traumatol. 2008;18(5):365-370.
7. Weinlein J, Schmidt A. Acute compartment syndrome in tibial plateau fractures—beware! J Knee Surg. 2010;31(1):9-16.
8. te Stroet MA, Holla M, Biert J, van Kampen A. The value of CT scan compared to plain radiographs for the classification and treatment plan in tibial plateau fractures. Emerg Radiol. 2011;18(4):279-283.
9. Musahl V, Tarkin I, Kobbe P, et al. New trends and techniques in open reduction and internal fixation of fractures of the tibial plateau. J Bone Joint Surg Br. 2009;91(4):426-433.
10. Toro-Arbelaez JB, Gardner MJ, Shindle MK, et al. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383.
11. Marsh JL, Smith ST, Do TT. External fixation and limited internal fixation for complex fractures of the tibial plateau. J Bone Joint Surg Am. 1995;77(5):661-673.
A 30-year-old man sustained traumatic amputations of three of his left fingers while at work. A heavy object fell when a supporting chain snapped; although he moved quickly, three of his left distal fingers were caught under the object. He was flown to a hospital for definitive hand care.
During the preadmission history and physical, it was noted that the patient had mild right knee pain in addition to his finger injuries. He had experienced no head injury and no loss of consciousness or other complaints. He did not remember injuring his leg, although he said it might have been struck by the falling object; all he could remember was the injury to his fingers.
On physical exam, the only abnormality other than the man’s traumatic finger amputations was mild right knee edema and a small bruised area medially. Initially, he complained of mild pain on palpation and moderate pain with passive range of motion, but range of motion was intact. His pain was worse at the proximal, medial tibial area, and he had mild lateral mid-calf tenderness though no bruising. Distally, his right lower extremity motor and sensory function were intact, and he had no open wounds or skin breakdown. He had 2+ dorsalis pedis pulse and 1+ posterior tibial pulse. The toes were pink and warm with brisk capillary refill. All compartments were soft and compressible.
Upon review of his plain radiographs (three views of the right knee), the patient was noted to have a severely comminuted medial tibial plateau fracture that extended to the midline in the region of the tibial spine, with mild depression of the fracture fragments measuring about 6 mm (see Figures 1a, 1b, and 1c). This would translate into a Schatzker IV classification type1 fracture (see Figure 22,3).
The man was admitted and underwent emergent surgery on his injured left fingers that night. Further diagnostic knee testing was performed, including CT and MRI (see Figures 3 and 4). Three days after admission, the patient underwent open reduction and internal fixation (plating) of the right medial, proximal tibia (see Figure 5). He has done very well since without issue.
DISCUSSION
Fractures of the tibial plateau occur along the articular, or joint, surface of the proximal tibia. The plateau consists of lateral and medial condylar surfaces. These concave structures function as an articulation point for the cartilaginous menisci and the femoral condyles.4 The medial plateau and condyle are stronger than those of the lateral side, and therefore are less commonly fractured. An elevated intercondylar eminence divides the lateral and medial plateaus, providing an attachment site for the cruciate ligaments.3
The Schatzker classification system1 is most commonly used to describe the types of tibial plateau fractures (as seen in Figure 22,3). Schatzker et al1 divided these injuries into six categories, according to the impact of increased energy exerted onto the bone; the rising classification numbers indicate an increase in complexity and severity and usually a worsening prognosis.
The type I fracture represents a split fracture of the lateral plateau. Typically, a fracture of this type has depression or displacement measuring less than 4 mm.
Type II tibial plateau fractures, the most common Schatzker injury, are lateral plateau fractures with depression noted at the split. Not always evident on plain radiographs, this depression can often be overlooked, and the injury mistaken for a type I fracture. The depression is measured vertically from the lower edge of the medial plateau to the lowest depression point of the lateral plateau.5
Type III fractures, the least common among the Schatzker injuries, are described as pure depression fractures of the lateral plateau. These fractures do not have an appreciable “split” along the plateau and are usually found in older patients with osteopenia.2
The Schatzker type IV injury is a medial fracture with displacement or depression to a portion of the plateau. The fracture may be split or comminuted and may originate in the intercondylar area.
Type V fractures, also known as “bicondylar fractures,” affect both the lateral and medial plateau. An inverted “Y” pattern is frequently seen, and there may be additional involvement of the intercondylar eminence. Type V fractures differ from type VI injuries in that there is no disturbance of the metaphyseal-diaphyseal connection. Thus, type VI fractures also include a transverse component that separates the condyles (metaphysis) of the bone from the shaft (diaphysis). Wide variation is seen among type VI fractures.5
Assessment and Diagnosis
Originally termed “fender fractures” due to their frequent association with automobile injuries, fractures of the tibial plateau account for 1% of all fractures and 8% of fractures in elderly patients.6 Tibial plateau fractures occur when varus or valgus force is combined with axial loading. The fracture itself occurs when the femoral condyle is driven into the lateral or medial plateau. Bicondylar injuries occur when rigorous axial force is sustained in a fully extended knee.
Injuries may also include those of the ligaments or menisci, resulting in joint instability. Patients may present with generalized knee pain or difficulty bearing weight after sustaining injuries, such as being struck in a motor vehicle accident, being tackled, or falling from some height.4
Evaluation of a patient with a suspected tibial plateau fracture begins with a detailed history and thorough physical examination. Details regarding the mechanism of injury help to predict the pattern of the fracture and may indicate whether a more focused neurovascular exam is warranted. Low-energy injuries (often seen with Schatzker types I to III) or twisting injuries yield low suspicion for neurovascular injury or compartment syndrome. However, high-energy injuries (seen often with Schatzker types IV through VI) have a greater likelihood of resulting in complicated injuries that must be urgently or emergently treated.5
The popliteal artery is bound posteriorly and distally to the tibial plateau, and the peroneal nerve is located laterally and positioned around the fibular head. It is essential to assess for the popliteal pulse, as well as lateral lower-extremity sensation and the patient’s ability to dorsiflex. Along with motor and neurovascular injuries, presentation with a painful, strikingly swollen knee and difficulty bearing weight may indicate a hemarthrosis. Soft tissue injuries over the knee resulting from direct trauma may require a saline arthrogram to rule out communication into the joint. Furthermore, a thorough ligamentous exam of the knee is helpful in determining the extent of the injuries.3
Compartment syndrome is a serious, emergent complication that can occur with tibial plateau fractures, especially those sustained during high-energy trauma.7 The health care provider must perform serial exams of the lower extremity to assess for classic signs of compartment syndrome. Are the compartments tense or noncompressible? Does the patient have pain with passive stretch or with range of motion of the lower extremity? Is there pallor or paresthesia to the affected limb? Is the pulse weak or absent? Presence of any of the aforementioned symptoms should prompt a high suspicion for compartment syndrome, and the patient must be sent to an emergency department for urgent evaluation.5
Treatment/Rehabilitation
For Schatzker types I through III, intervention focuses on the articular cartilage examination and repair. Type IV injuries often include corresponding damage to the popliteal artery and/or peroneal nerve, and types V and VI often have such overlying soft tissue damage that temporary placement of an external fixation device is required before definitive surgical intervention can be performed.8
However, it should be noted that conservative versus surgical treatment is often debated among surgeons for treatment of Schatzker fractures. The management of a tibial plateau fracture depends on the physical demands and health of the patient, the severity of the fracture, the stability of the joint, and the surgeon’s skill set and preferences.4 Operative intervention is generally indicated for fractures with depressions greater than 2 mm (although some surgeons allow up to 1 cm of depression), fractures with joint instability, or open fractures. Injuries with concern for vascular injury or compartment syndrome are also treated both operatively and emergently. Postoperatively, patients will remain non–weight-bearing for eight to 12 weeks after surgery, and in the interim, depending on the surgeon’s preference, may or may not engage in active or passive range of motion of the knee.
Advocates of open reduction and internal fixation (ORIF) argue that this method allows for the fracture reduction and anatomic alignment to be directly examined, but they also acknowledge that this approach compromises a great deal of soft tissue surrounding the proximal tibia.9,10
In order to reduce soft tissue damage, some surgeons favor external fixation. Initial use of this surgical technique results in minimal soft tissue swelling and allows early range of motion. While the external fixation device is in place, there is a risk for pin site infection, and proper site care must be provided.6,11
Generally, the treatment of tibial plateau fractures is considered successful when the fracture reduction is sustained, the patient’s functional capacity and axial loading are restored, and the articular surface is reconstructed. As a rule, nonoperative treatment is reserved for tibial plateau fractures that are minimally depressed or nondisplaced, or for patients with advanced osteoporosis. Under these circumstances, after a non–weight-bearing period of four to eight weeks, patients will begin to perform protected and partial weight bearing using a hinged knee brace.2 Early active range of motion, along with isometric exercises to strengthen the quadriceps, is recommended.
Whether surgical or conservative treatment is chosen, complications of tibial plateau fractures include knee stiffness, wound breakdown and infection, malunion or nonunion, vascular or neurologic injury, prominent or painful hardware, or avascular necrosis of fragmented bone pieces.4
CONCLUSION
The primary care practitioner must never overlook patients’ complaints of knee pain, especially after varus or valgus stress injuries or axial loading injuries to the knee. The patient may be able to ambulate; however, ordering a radiograph is an easy method for evaluation and for ruling out tibial plateau injuries. If there is any question regarding the presence of fracture with plain radiographs and/or the clinical exam warrants it, CT is an appropriate second diagnostic intervention.
Should a tibial plateau fracture present in a primary care or urgent care setting, thorough examination of neurovascular status and risk for compartment syndrome must be done urgently, followed by a referral to an orthopedic surgeon or emergency department.
REFERENCES
1. Schatzker J, McBroom R, Bruce D. The tibial plateau fracture: the Toronto experience, 1968–1975. Clin Orthop Relat Res. 1979;(138): 94-104.
2. Marsh JL. Tibial plateau fractures. In: Bucholz RW, Court-Brown CM, Heckman HD, Tornetta P. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:1780-1831.
3. Egol K, Koval KJ, Zuckerman JD. Tibial plateau. In: Egol K, Koval KJ, Zuckerman JD. Handbook of Fractures. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:455-463.
4. Fenton PP, Porter KK. Tibial plateau fractures: a review. Trauma. 2011;13(3):181-187.
5. Markhardt BK, Gross JM, Monu JU. Schatzker classification of tibial plateau fractures: use of CT and MR imaging improves assessment. Radiographics. 2009;29(2):585-597.
6. Lewis C. Does the mode of fixation of tibial plateau fractures, i.e. external fixation versus internal fixation, influence the time to union? A systematic review of the literature. Eur J Orthopaed Surg Traumatol. 2008;18(5):365-370.
7. Weinlein J, Schmidt A. Acute compartment syndrome in tibial plateau fractures—beware! J Knee Surg. 2010;31(1):9-16.
8. te Stroet MA, Holla M, Biert J, van Kampen A. The value of CT scan compared to plain radiographs for the classification and treatment plan in tibial plateau fractures. Emerg Radiol. 2011;18(4):279-283.
9. Musahl V, Tarkin I, Kobbe P, et al. New trends and techniques in open reduction and internal fixation of fractures of the tibial plateau. J Bone Joint Surg Br. 2009;91(4):426-433.
10. Toro-Arbelaez JB, Gardner MJ, Shindle MK, et al. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383.
11. Marsh JL, Smith ST, Do TT. External fixation and limited internal fixation for complex fractures of the tibial plateau. J Bone Joint Surg Am. 1995;77(5):661-673.
A 30-year-old man sustained traumatic amputations of three of his left fingers while at work. A heavy object fell when a supporting chain snapped; although he moved quickly, three of his left distal fingers were caught under the object. He was flown to a hospital for definitive hand care.
During the preadmission history and physical, it was noted that the patient had mild right knee pain in addition to his finger injuries. He had experienced no head injury and no loss of consciousness or other complaints. He did not remember injuring his leg, although he said it might have been struck by the falling object; all he could remember was the injury to his fingers.
On physical exam, the only abnormality other than the man’s traumatic finger amputations was mild right knee edema and a small bruised area medially. Initially, he complained of mild pain on palpation and moderate pain with passive range of motion, but range of motion was intact. His pain was worse at the proximal, medial tibial area, and he had mild lateral mid-calf tenderness though no bruising. Distally, his right lower extremity motor and sensory function were intact, and he had no open wounds or skin breakdown. He had 2+ dorsalis pedis pulse and 1+ posterior tibial pulse. The toes were pink and warm with brisk capillary refill. All compartments were soft and compressible.
Upon review of his plain radiographs (three views of the right knee), the patient was noted to have a severely comminuted medial tibial plateau fracture that extended to the midline in the region of the tibial spine, with mild depression of the fracture fragments measuring about 6 mm (see Figures 1a, 1b, and 1c). This would translate into a Schatzker IV classification type1 fracture (see Figure 22,3).
The man was admitted and underwent emergent surgery on his injured left fingers that night. Further diagnostic knee testing was performed, including CT and MRI (see Figures 3 and 4). Three days after admission, the patient underwent open reduction and internal fixation (plating) of the right medial, proximal tibia (see Figure 5). He has done very well since without issue.
DISCUSSION
Fractures of the tibial plateau occur along the articular, or joint, surface of the proximal tibia. The plateau consists of lateral and medial condylar surfaces. These concave structures function as an articulation point for the cartilaginous menisci and the femoral condyles.4 The medial plateau and condyle are stronger than those of the lateral side, and therefore are less commonly fractured. An elevated intercondylar eminence divides the lateral and medial plateaus, providing an attachment site for the cruciate ligaments.3
The Schatzker classification system1 is most commonly used to describe the types of tibial plateau fractures (as seen in Figure 22,3). Schatzker et al1 divided these injuries into six categories, according to the impact of increased energy exerted onto the bone; the rising classification numbers indicate an increase in complexity and severity and usually a worsening prognosis.
The type I fracture represents a split fracture of the lateral plateau. Typically, a fracture of this type has depression or displacement measuring less than 4 mm.
Type II tibial plateau fractures, the most common Schatzker injury, are lateral plateau fractures with depression noted at the split. Not always evident on plain radiographs, this depression can often be overlooked, and the injury mistaken for a type I fracture. The depression is measured vertically from the lower edge of the medial plateau to the lowest depression point of the lateral plateau.5
Type III fractures, the least common among the Schatzker injuries, are described as pure depression fractures of the lateral plateau. These fractures do not have an appreciable “split” along the plateau and are usually found in older patients with osteopenia.2
The Schatzker type IV injury is a medial fracture with displacement or depression to a portion of the plateau. The fracture may be split or comminuted and may originate in the intercondylar area.
Type V fractures, also known as “bicondylar fractures,” affect both the lateral and medial plateau. An inverted “Y” pattern is frequently seen, and there may be additional involvement of the intercondylar eminence. Type V fractures differ from type VI injuries in that there is no disturbance of the metaphyseal-diaphyseal connection. Thus, type VI fractures also include a transverse component that separates the condyles (metaphysis) of the bone from the shaft (diaphysis). Wide variation is seen among type VI fractures.5
Assessment and Diagnosis
Originally termed “fender fractures” due to their frequent association with automobile injuries, fractures of the tibial plateau account for 1% of all fractures and 8% of fractures in elderly patients.6 Tibial plateau fractures occur when varus or valgus force is combined with axial loading. The fracture itself occurs when the femoral condyle is driven into the lateral or medial plateau. Bicondylar injuries occur when rigorous axial force is sustained in a fully extended knee.
Injuries may also include those of the ligaments or menisci, resulting in joint instability. Patients may present with generalized knee pain or difficulty bearing weight after sustaining injuries, such as being struck in a motor vehicle accident, being tackled, or falling from some height.4
Evaluation of a patient with a suspected tibial plateau fracture begins with a detailed history and thorough physical examination. Details regarding the mechanism of injury help to predict the pattern of the fracture and may indicate whether a more focused neurovascular exam is warranted. Low-energy injuries (often seen with Schatzker types I to III) or twisting injuries yield low suspicion for neurovascular injury or compartment syndrome. However, high-energy injuries (seen often with Schatzker types IV through VI) have a greater likelihood of resulting in complicated injuries that must be urgently or emergently treated.5
The popliteal artery is bound posteriorly and distally to the tibial plateau, and the peroneal nerve is located laterally and positioned around the fibular head. It is essential to assess for the popliteal pulse, as well as lateral lower-extremity sensation and the patient’s ability to dorsiflex. Along with motor and neurovascular injuries, presentation with a painful, strikingly swollen knee and difficulty bearing weight may indicate a hemarthrosis. Soft tissue injuries over the knee resulting from direct trauma may require a saline arthrogram to rule out communication into the joint. Furthermore, a thorough ligamentous exam of the knee is helpful in determining the extent of the injuries.3
Compartment syndrome is a serious, emergent complication that can occur with tibial plateau fractures, especially those sustained during high-energy trauma.7 The health care provider must perform serial exams of the lower extremity to assess for classic signs of compartment syndrome. Are the compartments tense or noncompressible? Does the patient have pain with passive stretch or with range of motion of the lower extremity? Is there pallor or paresthesia to the affected limb? Is the pulse weak or absent? Presence of any of the aforementioned symptoms should prompt a high suspicion for compartment syndrome, and the patient must be sent to an emergency department for urgent evaluation.5
Treatment/Rehabilitation
For Schatzker types I through III, intervention focuses on the articular cartilage examination and repair. Type IV injuries often include corresponding damage to the popliteal artery and/or peroneal nerve, and types V and VI often have such overlying soft tissue damage that temporary placement of an external fixation device is required before definitive surgical intervention can be performed.8
However, it should be noted that conservative versus surgical treatment is often debated among surgeons for treatment of Schatzker fractures. The management of a tibial plateau fracture depends on the physical demands and health of the patient, the severity of the fracture, the stability of the joint, and the surgeon’s skill set and preferences.4 Operative intervention is generally indicated for fractures with depressions greater than 2 mm (although some surgeons allow up to 1 cm of depression), fractures with joint instability, or open fractures. Injuries with concern for vascular injury or compartment syndrome are also treated both operatively and emergently. Postoperatively, patients will remain non–weight-bearing for eight to 12 weeks after surgery, and in the interim, depending on the surgeon’s preference, may or may not engage in active or passive range of motion of the knee.
Advocates of open reduction and internal fixation (ORIF) argue that this method allows for the fracture reduction and anatomic alignment to be directly examined, but they also acknowledge that this approach compromises a great deal of soft tissue surrounding the proximal tibia.9,10
In order to reduce soft tissue damage, some surgeons favor external fixation. Initial use of this surgical technique results in minimal soft tissue swelling and allows early range of motion. While the external fixation device is in place, there is a risk for pin site infection, and proper site care must be provided.6,11
Generally, the treatment of tibial plateau fractures is considered successful when the fracture reduction is sustained, the patient’s functional capacity and axial loading are restored, and the articular surface is reconstructed. As a rule, nonoperative treatment is reserved for tibial plateau fractures that are minimally depressed or nondisplaced, or for patients with advanced osteoporosis. Under these circumstances, after a non–weight-bearing period of four to eight weeks, patients will begin to perform protected and partial weight bearing using a hinged knee brace.2 Early active range of motion, along with isometric exercises to strengthen the quadriceps, is recommended.
Whether surgical or conservative treatment is chosen, complications of tibial plateau fractures include knee stiffness, wound breakdown and infection, malunion or nonunion, vascular or neurologic injury, prominent or painful hardware, or avascular necrosis of fragmented bone pieces.4
CONCLUSION
The primary care practitioner must never overlook patients’ complaints of knee pain, especially after varus or valgus stress injuries or axial loading injuries to the knee. The patient may be able to ambulate; however, ordering a radiograph is an easy method for evaluation and for ruling out tibial plateau injuries. If there is any question regarding the presence of fracture with plain radiographs and/or the clinical exam warrants it, CT is an appropriate second diagnostic intervention.
Should a tibial plateau fracture present in a primary care or urgent care setting, thorough examination of neurovascular status and risk for compartment syndrome must be done urgently, followed by a referral to an orthopedic surgeon or emergency department.
REFERENCES
1. Schatzker J, McBroom R, Bruce D. The tibial plateau fracture: the Toronto experience, 1968–1975. Clin Orthop Relat Res. 1979;(138): 94-104.
2. Marsh JL. Tibial plateau fractures. In: Bucholz RW, Court-Brown CM, Heckman HD, Tornetta P. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:1780-1831.
3. Egol K, Koval KJ, Zuckerman JD. Tibial plateau. In: Egol K, Koval KJ, Zuckerman JD. Handbook of Fractures. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:455-463.
4. Fenton PP, Porter KK. Tibial plateau fractures: a review. Trauma. 2011;13(3):181-187.
5. Markhardt BK, Gross JM, Monu JU. Schatzker classification of tibial plateau fractures: use of CT and MR imaging improves assessment. Radiographics. 2009;29(2):585-597.
6. Lewis C. Does the mode of fixation of tibial plateau fractures, i.e. external fixation versus internal fixation, influence the time to union? A systematic review of the literature. Eur J Orthopaed Surg Traumatol. 2008;18(5):365-370.
7. Weinlein J, Schmidt A. Acute compartment syndrome in tibial plateau fractures—beware! J Knee Surg. 2010;31(1):9-16.
8. te Stroet MA, Holla M, Biert J, van Kampen A. The value of CT scan compared to plain radiographs for the classification and treatment plan in tibial plateau fractures. Emerg Radiol. 2011;18(4):279-283.
9. Musahl V, Tarkin I, Kobbe P, et al. New trends and techniques in open reduction and internal fixation of fractures of the tibial plateau. J Bone Joint Surg Br. 2009;91(4):426-433.
10. Toro-Arbelaez JB, Gardner MJ, Shindle MK, et al. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383.
11. Marsh JL, Smith ST, Do TT. External fixation and limited internal fixation for complex fractures of the tibial plateau. J Bone Joint Surg Am. 1995;77(5):661-673.
Cover
ABCs of CCBs
5 Electrical Injuries: Risk Stratification and Treatment
electrical injuries, severe electrical exposures, minor electrical exposures, High-voltage injuries, low-voltage injuries, pediatric electrical injuries, electrical injuries in pregnancy
electrical injuries, severe electrical exposures, minor electrical exposures, High-voltage injuries, low-voltage injuries, pediatric electrical injuries, electrical injuries in pregnancy
electrical injuries, severe electrical exposures, minor electrical exposures, High-voltage injuries, low-voltage injuries, pediatric electrical injuries, electrical injuries in pregnancy