User login
Prostate Cancer
Prostate cancer is the sixth most common cancer in the world and the second most common cancer among American men, surpassed only by nonmelanoma skin cancer. Prostate cancer led to approximately 28,660 deaths in 2008 (the second leading cause of cancer deaths that year), and 192,000 new cases of prostate cancer were diagnosed in 2009.1-7
The incidence of prostate cancer has been rising about 1% annually since 1995.4 Explanations for the increasing incidence, though not certain, are believed to be both genetic and environmental. In the US, prostate cancer–associated morbidity and mortality rates are highest in African-American men.8 Men of African and Caribbean descent are at three times the risk for prostate cancer, compared with white men.9
EPIDEMIOLOGY AND RISK FACTORS
Prostate-specific antigen (PSA) testing has nearly doubled the chance that a man will be diagnosed with prostate cancer in his lifetime. Prior to widespread use of PSA testing, a white man had a one-in-eleven chance of being diagnosed with prostate cancer in his lifetime; currently, that man’s chances are one in six.6
The incidence of prostate cancer increases as men age, with those in their 40s accounting for less than 1% of prostate cancer cases, compared with men older than 65, who account for more than 75% of cases.10 Besides age, other risk factors include a positive family history of prostate cancer in the father or brother(s) and African-American ethnicity.2,3
No clear link has been demonstrated between diet and prostate cancer. Tobacco use is not currently considered a risk factor for prostate cancer, but pooled data from 24 cohort studies enrolling more than 26,000 participants with prostate cancer revealed a 9% to 30% increase in both incident and fatal prostate cancer among men who smoked.3
ANATOMY AND FUNCTION
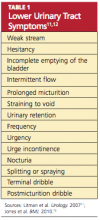
The prostate gland sits under the bladder and surrounds the urethra; in a young man, it is approximately the size of a walnut. As a man ages, the prostate begins to enlarge. Once a man reaches his 50s, he may begin to experience lower urinary tract symptoms11,12 (see Table 111,12).
The prostate produces fluid containing prostate-specific antigen, a type of protein that helps liquefy the semen and facilitate sperm motility. PSA is a component of the seminal fluid that is necessary for ejaculation. The prostate and the seminal vessels contract during ejaculation, expelling fluid through the prostate’s ejaculatory ducts and out along the urethra.10 PSA levels in the serum are normally very low. Prostatic disease, inflammation, or trauma can lead to increased levels of PSA in the serum. Elevated serum PSA has become an important marker of many prostate diseases, including benign prostatic hyperplasia, prostatitis, and prostate cancer13—the focus of this article.
The prostate gland is comprised of three zones: central, transitional, and peripheral. The peripheral zone, located at the back of the prostate, is the most susceptible to cancer. Prostate cancer is typically adenocarcinoma.14
CLINICAL PRESENTATION
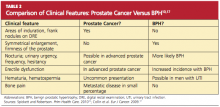
As a result of widespread screening of serum PSA, prostate cancer is often diagnosed before symptoms develop or a palpable nodule appears.5,15,16 Prostate cancer not identified through PSA screening is typically detected either by digital rectal examination (DRE) or in an investigation of genitourinary symptoms. For men who are symptomatic, the primary care provider can order a urine test to rule out infection (which can raise the PSA level), order PSA screening, and perform a DRE, which can also help to rule out benign prostatic hypertrophy (BPH)17 (see Table 210,17).
PSA TESTING
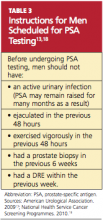
Primary care providers can make an important contribution to men’s health by educating those older than 50 regarding the pros and cons of PSA testing. It has been found that 47% of men between ages 50 and 70 have no knowledge of the PSA test. Men in the upper socioeconomic groups are more likely to be aware of the test.10 Patients scheduled to undergo PSA testing should be advised in advance to avoid certain activities and circumstances (see Table 3,13,18).
Screening serum PSA can reduce the number of prostate cancer–associated deaths by 31%, but this benefit must be weighed against a degree of overdiagnosis and overtreatment.2,5,6,16,19 Although the incidence of prostate cancer is 16%, only 2% of affected men will succumb to the disease.16 Up to 30% of prostate cancers detected by PSA may have otherwise remained clinically silent throughout the patient’s life.19
Additionally, PSA screening rates remain high in men between ages 75 and 80, who may be at risk for screening that is unnecessary due to an increase in competing causes of death.19 In the Baltimore Longitudinal Study of Aging,19,20 a longitudinal cohort study in which 849 men were enrolled, researchers measured the proportion of men, by PSA and age, who died of prostate cancer or in whom aggressive prostate cancer developed. It was found that no participants between ages 75 and 80 with a PSA below 3.0 ng/mL died of prostate cancer, but men of all ages with a PSA greater than 3.0 ng/mL had a continually increasing probability of death from prostate cancer. These findings suggest that it may be safe to discontinue PSA testing in men older than 75 whose PSA level is lower than 3.0 ng/mL.19
In the absence of a family history of prostate cancer, it is recommended that men undergo annual DRE and PSA testing beginning at age 50.21,22 The American Urological Association13 recommends annual PSA testing beginning at age 40 for African-American men and other men with a positive family history of prostate cancer. The American Cancer Society23 recommends that these tests be offered annually to men who are 50 and older and who have at least a 10-year life expectancy.
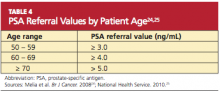
The serum PSA level alone does not automatically trigger a referral for a prostate biopsy (see Table 424,25). Other factors that should also be considered are family history, nonsymmetrical enlargement of the prostate, DRE findings of firmness, induration, or frank nodules, age, ethnicity, comorbidities, a history of previous negative prostate biopsy, and previous PSA levels. The patient should be involved in any decision regarding referral to another health care provider and the decision to perform biopsy.18
PSA levels are also used to monitor response to cancer treatments and to detect disease recurrence after treatment.2
Serum PSA Elevation
PSA is a prostate-specific marker, and elevations can be caused by prostate cancer or by benign conditions, such as BPH or infection. Malignant prostate tissue generates more PSA than does normal or hyperplastic tissue. The likelihood of finding cancer on a prostate biopsy increases as PSA values rise. Two PSA tests performed seven weeks apart allow for more accurate risk prediction and may assist in decision-making regarding referral and/or biopsy.18
Prostate biopsy is recommended for men with a total serum PSA of 4.0 ng/mL or greater, regardless of DRE findings. Biopsy at this time increases the likelihood of diagnosing disease while it is still organ-confined.26 In men with serum PSA of 10 ng/mL or more, the chance of finding prostate cancer exceeds 50%, and many such men will have disease that has already extended beyond the prostate.27
Managing men with PSA measurements below 4.0 ng/mL is more difficult. The majority of men in this category will have negative biopsies. However, a significant number of men with prostate cancer present with serum PSA concentrations below 4.0 ng/mL.28 Because cancer detected in these men is likely to be organ-confined, the National Comprehensive Cancer Network altered its guidelines, recommending that prostate biopsy be considered in men with a serum PSA between 2.6 and 4.0 ng/mL; or in those with a rate of rise in the serum PSA (PSA velocity) of at least 0.35 ng/mL/year in men with a serum PSA of 2.5 ng/mL or less.26
DIAGNOSIS
The diagnosis of prostate cancer is accomplished through a histologic examination of biopsied prostate tumor. The biopsy is performed using transrectal ultrasound. A prostate biopsy may be indicated based upon clinical symptoms, an abnormal DRE, or an elevated serum PSA.29
Transrectal ultrasonography with prostate biopsy is indicated in men with DRE findings that are suspicious for cancer (ie, induration, asymmetry, or palpable nodularity of the prostate gland), even if the serum PSA is not elevated; such findings require that prostate cancer be ruled out. This is particularly important if the patient is older than 45 or has other risk factors for the disease.17,30
Tumors located in the posterior and lateral aspects of the prostate gland are most often detected by DRE. About 25% to 35% of prostate tumors develop in other areas of the prostate or are too small to be detected by DRE.31
A serum PSA level should be ordered before biopsy in men with an abnormal DRE for diagnostic and prognostic purposes. Serum PSA concentrations rise slightly during the first several hours after a rectal examination in some men; if possible, the PSA should not be drawn within one week of the DRE but should be obtained prior to the biopsy.18,32
Staging
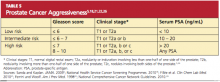
Prostate cancer is divided into low, intermediate, and high risk, based on the serum PSA, the Gleason score, and the clinical stage (see Table 5,5,18,21,22,26 ). Imaging studies, including CT of the abdomen and pelvis, radionuclide bone scans, and endorectal coil MRI, are used selectively to assess for extraprostatic extension, regional adenopathy, or distant metastases.33
PROSTATE BIOPSY
Transrectal biopsy of the prostate is an office procedure. Local and topical anesthetics can be used to minimize associated discomfort,34 although local anesthetics may not completely eliminate the discomfort of the introduction of the probe.35 A combination of local infiltration and topical gel application seems to provide the best pain relief.36 Use of intraprostatic administration of local anesthetic and of NSAID-containing rectal suppositories is currently being explored.37
Many men who undergo prostate biopsy are middle-aged or elderly and may be receiving antiplatelet therapy (most commonly with low-dose aspirin).38 To decrease the risk for bleeding complications, patients should be advised to discontinue antiplatelet therapy seven to 10 days before biopsy, unless even temporary withdrawal places them at increased risk for cardiovascular complications. Continuing antiplatelet drugs does not appear to increase the incidence of severe hemorrhagic complications and is an acceptable option for patients at high risk for cardiovascular incidents.38
Endorectal coil MRI, in which a coil is inserted into the rectum near the prostate to pick up the MRI signal, has been used to guide biopsies in men with persistently elevated PSA and negative results following transrectal ultrasound–guided biopsies. Complications of transrectal ultrasound–guided biopsies include hematospermia, hematuria (for as long as three days), fever, and rectal bleeding. A small percentage of men develop urinary retention or require hospitalization—usually for urosepsis.39,40 Antibiotic prophylaxis with ciprofloxacin to minimize the risk for infection is considered standard care.41
Patients may be concerned by the possibility that cancer cells will spread during the transrectal biopsy. While there have been isolated reports of tumors recurring in the needle biopsy tract, the incidence is low and this risk should not prevent an indicated biopsy.42
TREATMENT
For patients with disease confined to the prostate gland, treatment options include radical prostatectomy (RP), radiation therapy (RT, external beam and/or brachytherapy), and active surveillance. When disease extends through the prostatic capsule into the seminal vesicles or into regional lymph nodes, definitive local therapy may be combined with adjuvant RT and/or androgen deprivation therapy.43
When choosing treatment for an individual patient, the clinician should consider several factors, including the extent of disease, the patient’s age, and the presence or absence of significant comorbidity. In untreated patients, prostate cancer–related mortality occurs 10 to 20 years after diagnosis.5,44,45
The patient’s chance of being cured following definitive therapy is high if the tumors are confined to the prostate gland.46 The chances of cure decrease when the tumor has spread beyond the prostate capsule, invaded the seminal vesicles, or metastasized to regional lymph nodes.
The overall survival of men with early-stage prostate cancer is prolonged. Ten-year survival rates after RP or RT are high (averaging between 60% and 90%).5,47 Because of the risk for late relapse and mortality, biochemical relapse (as detected by a rise in serum PSA) is monitored. The patient’s likelihood of remaining disease-free, as evidenced by an undetectable serum PSA level (“biochemical progression–free survival”5), is inversely related to the presence of extraprostatic extension, seminal vesicle extension, evidence of tumor spread to the lymph nodes, or evidence of positive margins at surgery.46 Men in the Scandinavian Prostate Cancer Group-4 Randomized Trial (SPCG-4)46 who underwent RP were evaluated 12 years after surgery; those with extracapsular tumor growth had 14 times the risk for prostate cancer death than those without it.
Increasing numbers of selected low-risk patients are being placed on active surveillance for the management of prostate cancer—a protocol that includes repeat prostatic biopsies and routine follow-up visits.48 The PSA should be reassessed two to three times annually, with annual biopsies to determine whether the cancer has become more aggressive.5,16 Patients with cancer that is becoming more aggressive should convert to primary treatment.5
Difficulties with this option include psychological distress, poor compliance with scheduled appointments and repeat biopsies, and the risk for missing the therapeutic window in which the cancer can be cured.5,16 No results are yet available from randomized clinical trials comparing active surveillance with immediate definitive treatment; two large trials are under way.49,50
Radical Prostatectomy
RP is the definitive treatment for localized prostatic cancer.5,16 The potential for cure in men who undergo RP is highest when the cancer is confined to the prostate gland (clinical stage, T1-T2). RP is also an appropriate option for some men with locally advanced prostate cancer. Additionally, RP is used as a potentially curative salvage procedure to treat carefully selected men with a local recurrence after RT for localized prostate cancer.
The SPCG-4,46 which included predominately men whose cancer was not detected by PSA, was the first randomized trial to show that RP decreased the risk for prostate cancer mortality as well as the risk for metastases.2,46 Results were analyzed at 8.2 years’ and 10.8 years’ follow-up. Almost all men in the RP group who died of prostate cancer initially had tumor growth outside the prostate capsule. No men who underwent RP and had specimen Gleason scores of 2 to 6 died of prostate cancer. Rates of local recurrence and/or progression were lower in the RP group than in the “watchful waiting” group (whose members used hormonal and other palliative treatments).46 Subgroup analysis by age showed that men younger than 65 received the greatest benefit from prostatectomy.46,51
Surgical options include open retropubic RP, laparoscopic RP, and perineal RP. Da Vinci (robotic) RP is the method most commonly used in the US to achieve surgical removal of the prostate.16 The procedure is associated with reduced blood loss, compared with other methods, and the 10x magnified vision allows for nerve-sparing techniques, improving postoperative sexual function.16,52
Radiation Therapy
The goal of RT for men with localized prostate cancer is to deliver a therapeutic dose of radiation to the tumor while minimizing radiation to adjacent healthy tissues. Because of the prostate’s close anatomic proximity to the bowel, rectum, and bladder, the most common complications of RT are due to damage from radiation to surrounding structures in the gastrointestinal and genitourinary tracts.
Several forms of RT are available, including external beam radiation and brachytherapy. Radiation combined with hormonal therapy in high-risk patients has significantly improved outcomes, compared with men undergoing radiation alone.53Conformal 3D technology allows radiation oncologists to pinpoint the prostate and follow minute motion of the gland. This technology has helped to decrease the adverse effects of periprostatic radiation exposure.5,16Intensity-modulated radiation therapy offers increased accuracy in radiation dosage, improving cancer cure rates. External beam RT is used as adjuvant therapy in men with advanced disease and as salvage therapy for selected men who experience a rising serum PSA after RP.7,54
Brachytherapy involves the placement of radioactive seeds into the prostate under ultrasound guidance. It is an option for patients with low-risk prostate cancer and is performed as an outpatient procedure.5
Treatment for Disseminated Disease
Among men with disseminated disease, bone metastases are most common. Androgen deprivation therapy (ADT) is used to control the disease while maintaining the patient’s quality of life.2 Once prostate cancer no longer responds to this treatment, it is referred to as hormone-refractory prostate cancer.
Chemotherapy with docetaxel has been shown to extend survival in patients with hormone-refractory prostate cancer.2 Cabazitaxel is a new chemotherapy agent recently approved for use in combination with prednisone for the treatment of patients with metastatic hormone-refractory prostate cancer previously treated with a docetaxel-containing regimen.55,56
Currently, a large phase 3 clinical trial, led by the Cancer and Leukemia Group B,57 is under way to examine the addition of bevacizumab to docetaxel and prednisone for possible improvement in overall prostate cancer survival.2
Adverse Effects of Treatment
As successful as treatments for prostate cancer can be, they are not without significant adverse effects. Their severity varies by treatment plan and by patient, but they usually include physical and psychological effects. Men with prostate cancer are often less satisfied with the management of their disease than are other cancer patients.58 Adverse effects that they find particularly troublesome include urinary incontinence, erectile dysfunction, and effects associated with ADT (see Table 659,60).
Urinary incontinence is a resulting feature of both surgery and RT. After RP, men may have varying degrees of incontinence, ranging from stress incontinence to complete loss of urinary control. Incontinence usually improves within six to 12 months following surgery, with 2% to 22% of men experiencing long-term incontinence.5
Toxic effects of RT can occur immediately or after many years.5 Radiotherapy can result in cystitis, which usually develops a few weeks into treatment and improves after treatment is completed. Approximately one-half of patients who undergo RT experience urinary frequency, dysuria, or urgency due to cystitis, urethritis, or both. Long-term effects of radiation include persistent cystitis or urethral strictures.
Brachytherapy is associated with urinary symptoms that may take several weeks to develop. The side effects may be long lasting and can lead to obstruction of the urethra and urinary retention. Patients should be counseled to avoid bladder irritants, such as tea, coffee, alcohol, and carbonated beverages.
Reports of acute radiation proctitis vary from 2% to 40% of treated men.5,58 If the pelvic lymph nodes are within the treatment field, radiation enteritis may also be observed. Symptoms can include abdominal cramping, tenesmus, urgency, and frequent defecation. These can usually be controlled with antidiarrheal agents or topical anti-inflammatory preparations. Acute symptoms usually subside within three to eight weeks of RT completion. Long-term intestinal side effects persist in up to 10% of patients; these include diarrhea, tenesmus, rectal urgency, or hematochezia. Rectal or anal strictures, ulcers, and perforations are rare.5
Erectile dysfunction is the most common long-term adverse effect of RP, with reports of affected men (who have been advised to abstain from sexual intercourse for six to eight weeks following RP) ranging from 20% to 90%.5,52,61 Maximal recovery usually takes one to two years. Laparoscopic nerve-sparing surgeries have been developed to reduce the risk for erectile dysfunction.5,16,52
Thirty percent to 45% of men who were sexually potent before RT became impotent afterward, with frequency increasing over time.5,58 Factors that contribute to posttreatment impotence in this population include increasing age, intercurrent disease (eg, hypertension, cardiovascular disease, diabetes), and use of ADT.
Research among prostate cancer survivors and their spouses reveals that erectile dysfunction is the most important quality-of-life issue for the 50-to-64 age-group.62 Some men will recover a presurgical level of function, while others may require lengthy treatments, including surgical interventions, that may yield little or no success. Erectile dysfunction can be treated with PDE-5 inhibitors, penile injection therapy, vacuum erection devices, and penile prosthesis implantation.61
Choosing Treatment
Many men will turn to their primary care providers for objective information regarding their treatment options. Long-term morbidity profiles of RP, RT, and brachytherapy do not clearly show one treatment to be superior to the others.5 Each patient’s risks must be assessed, and evidence-based information must be provided for patients to make an informed decision regarding treatment options.
A highly selected group of low-risk patients have the option of management by active surveillance.16,48 Patients with intermediate- or high-risk cancers who have a life expectancy exceeding 10 years should be encouraged to proceed with RP or RT.5 Class I evidence has shown a survival benefit with RP alone.46 RT has shown survival benefit only when used in combination with ADT.53 At this time, there is no direct evidence as to whether brachytherapy reduces prostate cancer mortality because randomized clinical trials to address this question have not been completed.5
Up to one-half of patients who undergo RP and one-third of those undergoing RT develop erectile dysfunction.5,52,58,61,63 Ten percent of RP patients develop long-term urinary incontinence,61 and a similar proportion of RT patients develop long-term proctitis.58 For some patients with low-risk cancers, baseline function and quality-of-life consequences may be enough to sway their decision regarding which treatment option to choose.5,58
EMERGING THERAPIES
In order to reduce the adverse effects of radical therapy, new focal therapies are being developed. Cryotherapy freezes specific areas of the prostate with cooling probes,64 and high-intensity focused ultrasound uses hyperthermia to cause instantaneous irreversible coagulative necrosis of the targeted tissue65; these are two of the most widely accepted focal therapies. Both options offer the advantages of diminished side effects of incontinence and erectile dysfunction and can be completed in a single outpatient treatment session.16,64,65 These procedures require considerable technical skill and are not currently offered in all communities.
Primary prevention may be a possible future direction for the management of prostate cancer. One large trial demonstrated the use of 5- reductase inhibitors to prevent prostate cancer in at-risk men66; further trials are ongoing. 5- Reductase inhibitors (eg, finasteride, dutasteride) prevent the conversion of testosterone to dihydrotestosterone (DHT), which is a more potent agonist for prostate growth.16 Finasteride has been proven safe and effective in reducing the risk for prostate cancer, regardless of risk stratum, and may reduce the risk for high-grade cancers.6,66,67
Attempts to treat prostate cancer with immunotherapy have begun to yield encouraging results. Treatment of metastatic castration-recurrent prostate cancer with sipuleucel-T cancer, a vaccine for the treatment of prostate cancer, showed a four-month survival benefit.2,68 Sipuleucel-T is made from dendritic cells in the patient’s immune system; currently, the vaccine is being produced in small quantities due to limitations in insurance coverage.2
Statin use may reduce the risk for prostate cancer recurrence among men who have undergone RP or RT for localized or locally advanced prostate cancer.69,70 Researchers analyzed database records of 1,319 prostate cancer patients who had undergone RP. For each patient, use or nonuse of statins at the time of surgery was determined, as was PSA progression following surgery. Statin use was associated with a 30% lower risk for PSA recurrence, with statin users taking the highest doses experiencing the most benefit. Additional studies are needed to confirm these results.69,70
DIETARY SUPPLEMENTATION
It is largely unknown whether prostate cancer can be prevented or modified by diet and lifestyle. Global differences in mortality rates and disease patterns associated with immigrant populations suggest that nutrition may play a role in the development of prostate cancer, but data are lacking.
A population-based cohort study of 525 men diagnosed with localized or advanced-stage prostate cancer examined the association of dietary intake of folate, riboflavin, vitamin B6, vitamin B12, and methionine with prostate cancer survival.71 Use of vitamin B6 was found to improve survival of men with localized disease but not with advanced-stage cancer. Dietary intake of folate, riboflavin, vitamin B6, and methionine was not associated with increased prostate cancer survival.71
Investigators for the randomized, placebo-controlled Selenium and Vitamin E Cancer Prevention Trial (SELECT),1 which included 35,533 men from 427 participating sites throughout North America, concluded that selenium or vitamin E, alone or in combination, did not prevent prostate cancer in this population of relatively healthy men.1,16,72
CONCLUSION
Screening for prostate cancer has almost doubled the chance that a man will be diagnosed with prostate cancer in his lifetime, and about 85% of men diagnosed with prostate cancer will undergo active treatment.19 Overtreatment of nonaggressive tumors may result in adverse effects detrimental to the patient’s quality of life, whereas early detection of aggressive tumors may lead to curative therapies being performed while the cancer is still confined to the prostate.1,2,16,19 Differences between outcomes of localized versus advanced disease are remarkable, with associated five-year survival rates of 100% versus 31.7%, respectively.6
New strategies to differentiate between aggressive and nonaggressive tumors would have substantial public heath benefits. Tools for clinical and patient use should be developed to support informed decisions regarding prevention, screening, and biopsy, and to tailor treatments to tumor biology.6 Patients at high risk for prostate cancer should be identified in order to take preventive measures that will improve survival in this group.
REFERENCES
1. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39-51.
2. Kohli M, Tindall DJ. New developments in the medical management of prostate cancer. Mayo Clin Proc. 2010;85(1):77-86.
3. Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100(4):693-701.
4. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-229.
5. Sanda MG, Kaplan ID. A 64-year-old man with low-risk prostate cancer: review of prostate cancer treatment. JAMA. 2009;301(20):2141-2151.
6. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685-1692.
7. Talcott JA, Rossi C, Shipley WU, et al. Patient-reported long-term outcomes after conventional and high-dose combination proton and photon radiation for early prostate cancer. JAMA. 2010;303(11):1046-1053.
8. Odedina FT, Akinremi TO, Chinegwundoh F, et al. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009; 4(suppl 1):S2.
9. Ben-Shlomo Y, Evans S, Ibrahim F, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53(1):99-105.
10. Spickett I, Robertson J. Prostate cancer: the ongoing challenge. Prim Health Care. 2010;20(2): 16-21.
11. Litman HJ, Steers WD, Wei JT, et al. Relationship of lifestyle and clinical factors to lower urinary tract symptoms: results from Boston Area Community Health survey. Urology. 2007;70(5): 916-921.
12. Jones C, Jill J, Chapple C; Guideline Development Group. Management of lower urinary tract symptoms in men: summary of NICE guidance. BMJ. 2010 May 19;340:c2354. doi: 10.1136/bmj.c2354.
13. American Urological Association. Prostate Specific Antigen Best Practice Update: 2009 Update. www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf. Accessed December 21, 2010.
14. Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371-2490.
15. Cooperberg MR, Moul JS, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23(32):8146-8151.
16. Krane LS, Patel MN, Hemal AK. Advances and future directions in management of prostate cancer. Indian J Surg. 2009;71(6):337-341.
17. Collin SM, Metcalfe C, Donovan JL, et al. Associations of sexual dysfunction symptoms with PSA-detected localised and prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. Eur J Cancer. 2009; 45(18):3254-3261.
18. National Health Service Cancer Screening Programmes. Information for primary care; PSA testing in asymptomatic men (2010). www.cancer screening.nhs.uk/prostate/pcrmp-guide-2.html. Accessed December 21, 2010.
19. Schaeffer EM, Carter HB, Kettermann A, et al. Prostate specific antigen testing among the elderly—when to stop? J Urol. 2009;181(4):1606-1614.
20. National Institute on Aging. BLSA: Baltimore Longitudinal Study of Aging; NCT00233272. http://clinicaltrials.gov/ct2/show/NCT00233272. Accessed December 21, 2010.
21. Fillée C, Tombal B, Philippe M. Prostate cancer screening: clinical impact of WHO calibration of Beckman Coulter Access prostate-specific antigen assays. Clin Chem Lab Med. 2010;48(2):285-288.
22. Ferrini R, Woolf SH. Screening for prostate cancer in American men: American College of Preventive Medicine Practice Policy Statement. Am J Prev Med. 1998;15(1):81-84.
23. Wolf AMD, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98.
24. Melia J, Coulson P, Coleman D, Moss S. Urological referral of asymptomatic men in general practice in England. Br J Cancer. 2008;98(7):1176-1181.
25. NHS Cancer Screening Programmes. PCRMP Guide No 2. Information for primary care: PSA testing in asymptomatic men (evidence document, Jan 2010). www.cancerscreening.nhs.uk/prostate/pcrmp-guide-2.html. Accessed December 21, 2010.
26. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™: Prostate cancer. V.3.2010. www.nccn.org/profes sionals/physician_gls/PDF/prostate.pdf. Accessed December 21, 2010.
27. Terakawa T, Miyake, H, Kanomata N, et al. Inverse association between histologic inflammation in needle biopsy specimens and prostate cancer in men with serum PSA of 10-50 ng/mL. Urology. 2008;72(6):1194-1197.
28. Makarov DV, Humphreys EB, Mangold LA, et al. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 ng/mL. J Urol. 2006;176 (2):554-558.
29. Taneja SS. Optimizing prostate biopsy strategies for the diagnosis of prostate cancer. Rev Urol. 2003;5(3):149-155.
30. Delongchamps NB, Singh A, Hass GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. 2006;13(3):158-168.
31. Baumgart LA, Gerling GJ, Bass EJ. Characterizing the range of simulated prostate abnormalities palpable by digital rectal examination. Cancer Epidemiol. 2010;34(1):79-84.
32. Crawford ED, Schutz MJ, Clejan S, et al. The effect of digital rectal examination on prostate-specific antigen levels. JAMA. 1992;267(16):2227-2228.
33. Stephens NJ, Bharwani N, Heenan SD. Prostate cancer staging. Imaging. 2008;20:112-121.
34. Richman JM, Carter HB, Hanna MN, et al. Efficacy of periprostatic local anesthetic for prostate biopsy analgesia: a meta-analysis. Urology. 2006;67(6):1224-1228.
35. Luscombe CJ, Cooke PW. Pain during prostate biopsy. Lancet. 2004;363(9424):1840-1841.
36. Yun TJ, Lee HJ, Kim SH, et al. Does the intrarectal instillation of lidocaine gel before periprostatic neurovascular bundle block during transrectal ultrasound-guided prostate biopsies improve analgesic efficacy? A prospective, randomized trial. J Urol. 2007;178(1):103-106.
37. Lee HY, Lee HJ, Byun SS, et al. Effect of intraprostatic local anesthesia during transrectal ultrasound guided prostate biopsy: comparison of 3 methods in a randomized, double-blind, placebo controlled trial. J Urol. 2007;178(2):469-472.
38. Giannarini G, Mogorovich, A, Valent F, et al. Continuing or discontinuing low-dose aspirin before transrectal prostate biopsy: results of a prospective randomized trial. Urology. 2007;70(3): 501-505.
39. Park DS, Oh JJ, Lee JH, et al. Simple use of the suppository type povidone-iodine can prevent infectious complications in transrectal ultrasound-guided prostate biopsy. Adv Urol. 2009:750598. Epub 2009 Apr 23.
40. Rietbergen JB, Kruger AE, Kranse R, Schröder FH. Complications of transrectal ultrasound–guided systemic sextant biopsies of the prostate: evaluation of complication rates and risks factors within a population-based screening program. Urology. 1997;49(6):875-880.
41. Weber B, Saliken J, Jadavji T, et al. A near-fatal case of sepsis with an antibiotic-resistant organism complicating a routine transrectal prostate biopsy in a health care worker. Can Urol Assoc J. 2008;2 (5):543-545.
42. Vaghefi H, Magi-Galluzzi C, Klein EA. Local recurrence of prostate cancer in rectal submucosa after transrectal needle biopsy and radical prostatectomy. Urology. 2005;66(4):881.
43. Widmark A, Klepp O, Solberg A, et al; Swedish Association for Urological Oncology 3. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009; 373(9660):301-308.
44. Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280(11):975-980.
45. Johansson JE, Andrén O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713-2719.
46. Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
47. Inman BA, Davies JD, Rangel LJ, et al. Long-term outcomes of radical prostatectomy with multimodal adjuvant therapy in men with a preoperative serum prostate-specific antigen level > or =50 ng/mL. Cancer. 2008;113(7):1544-1551.
48. Klotz L. Active surveillance for prostate cancer: patient selection and management. Curr Oncol. 2010;17 suppl 2:S111-S117.
49. Oxford Radcliffe Hospital, National Cancer Institute. Active surveillance, radical prostatectomy, or radiation therapy in treating patients with localized prostate cancer; NCT00632983. http://clinicaltrials.gov/ct2/show/NCT00632983?term=prostate%2C+surveillance&rank=7. Accessed December 21, 2010.
50. NCIC Clinical Trials Group, National Cancer Institute, Cancer and Leukemia Group B, Eastern Cooperative Oncology Group, Southwest Oncology Group. Observation or radical treatment in patients with prostate cancer; NCT00499174. http://clinicaltrials.gov/ct2/show/NCT00499174? term=prostate%2C+surveillance&rank=12. Accessed December 21, 2010.
51. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11): 1202-1209.
52. Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162(2):433-438.
53. D’Amico AV, Manola J, Loffredo M, et al. 6-Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821-827.
54. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008; 299(23):2760-2769.
55. de Bono JS, Oudard S, Ozguroglu M, et al; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
56. US Food and Drug Administration (FDA news release, June 17, 2010). FDA approves new treatment for advanced prostate cancer. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm216143.htm Accessed December 21, 2010.
57. Cancer and Leukemia Group B, National Cancer Institute, Eastern Cooperative Oncology Group. Docetaxel and prednisone with or without bevacizumab in treating patients with prostate cancer that did not respond to hormone therapy; NCT00110214. http://clinicaltrials.gov/ct2/show/NCT00110214. Accessed December 21, 2010.
58. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12): 1250-1261.
59. Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115(11): 2388-2399.
60. Nanda A, Chen MH, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease–induced congestive heart failure or myocardial infarction. JAMA. 2009;302(8):866-873.
61. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000; 283(3):354-360.
62. Harden J, Northouse L, Cimprich B, et al. The influence of developmental life stage in quality of life in survivors of prostate cancer and their partners. J Cancer Surviv. 2008;2(2):84-94.
63. Potosky A, Legler J, Albertsen P, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92 (19):1582-1592.
64. Tsivian M, Polascik TJ. Focal cryotherapy for prostate cancer. Curr Urol Rep. 2010;11(3):
147-151.
65. Lee HM, Hong JH, Choi HY. High-intensity focused ultrasound therapy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(4):439-443.
66. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349 (3):215-224.
67. Kramer BS, Hagerty KL, Justman S, et al. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Clin Oncol. 2009;27 (9):1502-1516.
68. Schellhammer PF, Higano C, Berger ER, et al; IMPACT Study Investigators. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen-independent prostatic adenocarcinoma (AIPC). Presented at: American Urological Association 104th Annual Scientific Meeting; April 28, 2009; Chicago, IL. Abstract 9.
69. Hamilton RJ, Banez LL, Aronson WJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010; 116(14):3389-3398.
70. Gutt R, Tonlaar N, Kunnavakkam R, et al. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28(16):2653-2659.
71. Kasperzyk JL, Fall K, Mucci LA, et al. One-carbon metabolism-related nutrients and prostate cancer survival. Am J Clin Nutr. 2009;90(3): 561-569.
72. Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1): 52-62.
Prostate cancer is the sixth most common cancer in the world and the second most common cancer among American men, surpassed only by nonmelanoma skin cancer. Prostate cancer led to approximately 28,660 deaths in 2008 (the second leading cause of cancer deaths that year), and 192,000 new cases of prostate cancer were diagnosed in 2009.1-7
The incidence of prostate cancer has been rising about 1% annually since 1995.4 Explanations for the increasing incidence, though not certain, are believed to be both genetic and environmental. In the US, prostate cancer–associated morbidity and mortality rates are highest in African-American men.8 Men of African and Caribbean descent are at three times the risk for prostate cancer, compared with white men.9
EPIDEMIOLOGY AND RISK FACTORS
Prostate-specific antigen (PSA) testing has nearly doubled the chance that a man will be diagnosed with prostate cancer in his lifetime. Prior to widespread use of PSA testing, a white man had a one-in-eleven chance of being diagnosed with prostate cancer in his lifetime; currently, that man’s chances are one in six.6
The incidence of prostate cancer increases as men age, with those in their 40s accounting for less than 1% of prostate cancer cases, compared with men older than 65, who account for more than 75% of cases.10 Besides age, other risk factors include a positive family history of prostate cancer in the father or brother(s) and African-American ethnicity.2,3
No clear link has been demonstrated between diet and prostate cancer. Tobacco use is not currently considered a risk factor for prostate cancer, but pooled data from 24 cohort studies enrolling more than 26,000 participants with prostate cancer revealed a 9% to 30% increase in both incident and fatal prostate cancer among men who smoked.3
ANATOMY AND FUNCTION
The prostate gland sits under the bladder and surrounds the urethra; in a young man, it is approximately the size of a walnut. As a man ages, the prostate begins to enlarge. Once a man reaches his 50s, he may begin to experience lower urinary tract symptoms11,12 (see Table 111,12).
The prostate produces fluid containing prostate-specific antigen, a type of protein that helps liquefy the semen and facilitate sperm motility. PSA is a component of the seminal fluid that is necessary for ejaculation. The prostate and the seminal vessels contract during ejaculation, expelling fluid through the prostate’s ejaculatory ducts and out along the urethra.10 PSA levels in the serum are normally very low. Prostatic disease, inflammation, or trauma can lead to increased levels of PSA in the serum. Elevated serum PSA has become an important marker of many prostate diseases, including benign prostatic hyperplasia, prostatitis, and prostate cancer13—the focus of this article.
The prostate gland is comprised of three zones: central, transitional, and peripheral. The peripheral zone, located at the back of the prostate, is the most susceptible to cancer. Prostate cancer is typically adenocarcinoma.14
CLINICAL PRESENTATION
As a result of widespread screening of serum PSA, prostate cancer is often diagnosed before symptoms develop or a palpable nodule appears.5,15,16 Prostate cancer not identified through PSA screening is typically detected either by digital rectal examination (DRE) or in an investigation of genitourinary symptoms. For men who are symptomatic, the primary care provider can order a urine test to rule out infection (which can raise the PSA level), order PSA screening, and perform a DRE, which can also help to rule out benign prostatic hypertrophy (BPH)17 (see Table 210,17).
PSA TESTING
Primary care providers can make an important contribution to men’s health by educating those older than 50 regarding the pros and cons of PSA testing. It has been found that 47% of men between ages 50 and 70 have no knowledge of the PSA test. Men in the upper socioeconomic groups are more likely to be aware of the test.10 Patients scheduled to undergo PSA testing should be advised in advance to avoid certain activities and circumstances (see Table 3,13,18).
Screening serum PSA can reduce the number of prostate cancer–associated deaths by 31%, but this benefit must be weighed against a degree of overdiagnosis and overtreatment.2,5,6,16,19 Although the incidence of prostate cancer is 16%, only 2% of affected men will succumb to the disease.16 Up to 30% of prostate cancers detected by PSA may have otherwise remained clinically silent throughout the patient’s life.19
Additionally, PSA screening rates remain high in men between ages 75 and 80, who may be at risk for screening that is unnecessary due to an increase in competing causes of death.19 In the Baltimore Longitudinal Study of Aging,19,20 a longitudinal cohort study in which 849 men were enrolled, researchers measured the proportion of men, by PSA and age, who died of prostate cancer or in whom aggressive prostate cancer developed. It was found that no participants between ages 75 and 80 with a PSA below 3.0 ng/mL died of prostate cancer, but men of all ages with a PSA greater than 3.0 ng/mL had a continually increasing probability of death from prostate cancer. These findings suggest that it may be safe to discontinue PSA testing in men older than 75 whose PSA level is lower than 3.0 ng/mL.19
In the absence of a family history of prostate cancer, it is recommended that men undergo annual DRE and PSA testing beginning at age 50.21,22 The American Urological Association13 recommends annual PSA testing beginning at age 40 for African-American men and other men with a positive family history of prostate cancer. The American Cancer Society23 recommends that these tests be offered annually to men who are 50 and older and who have at least a 10-year life expectancy.
The serum PSA level alone does not automatically trigger a referral for a prostate biopsy (see Table 424,25). Other factors that should also be considered are family history, nonsymmetrical enlargement of the prostate, DRE findings of firmness, induration, or frank nodules, age, ethnicity, comorbidities, a history of previous negative prostate biopsy, and previous PSA levels. The patient should be involved in any decision regarding referral to another health care provider and the decision to perform biopsy.18
PSA levels are also used to monitor response to cancer treatments and to detect disease recurrence after treatment.2
Serum PSA Elevation
PSA is a prostate-specific marker, and elevations can be caused by prostate cancer or by benign conditions, such as BPH or infection. Malignant prostate tissue generates more PSA than does normal or hyperplastic tissue. The likelihood of finding cancer on a prostate biopsy increases as PSA values rise. Two PSA tests performed seven weeks apart allow for more accurate risk prediction and may assist in decision-making regarding referral and/or biopsy.18
Prostate biopsy is recommended for men with a total serum PSA of 4.0 ng/mL or greater, regardless of DRE findings. Biopsy at this time increases the likelihood of diagnosing disease while it is still organ-confined.26 In men with serum PSA of 10 ng/mL or more, the chance of finding prostate cancer exceeds 50%, and many such men will have disease that has already extended beyond the prostate.27
Managing men with PSA measurements below 4.0 ng/mL is more difficult. The majority of men in this category will have negative biopsies. However, a significant number of men with prostate cancer present with serum PSA concentrations below 4.0 ng/mL.28 Because cancer detected in these men is likely to be organ-confined, the National Comprehensive Cancer Network altered its guidelines, recommending that prostate biopsy be considered in men with a serum PSA between 2.6 and 4.0 ng/mL; or in those with a rate of rise in the serum PSA (PSA velocity) of at least 0.35 ng/mL/year in men with a serum PSA of 2.5 ng/mL or less.26
DIAGNOSIS
The diagnosis of prostate cancer is accomplished through a histologic examination of biopsied prostate tumor. The biopsy is performed using transrectal ultrasound. A prostate biopsy may be indicated based upon clinical symptoms, an abnormal DRE, or an elevated serum PSA.29
Transrectal ultrasonography with prostate biopsy is indicated in men with DRE findings that are suspicious for cancer (ie, induration, asymmetry, or palpable nodularity of the prostate gland), even if the serum PSA is not elevated; such findings require that prostate cancer be ruled out. This is particularly important if the patient is older than 45 or has other risk factors for the disease.17,30
Tumors located in the posterior and lateral aspects of the prostate gland are most often detected by DRE. About 25% to 35% of prostate tumors develop in other areas of the prostate or are too small to be detected by DRE.31
A serum PSA level should be ordered before biopsy in men with an abnormal DRE for diagnostic and prognostic purposes. Serum PSA concentrations rise slightly during the first several hours after a rectal examination in some men; if possible, the PSA should not be drawn within one week of the DRE but should be obtained prior to the biopsy.18,32
Staging
Prostate cancer is divided into low, intermediate, and high risk, based on the serum PSA, the Gleason score, and the clinical stage (see Table 5,5,18,21,22,26 ). Imaging studies, including CT of the abdomen and pelvis, radionuclide bone scans, and endorectal coil MRI, are used selectively to assess for extraprostatic extension, regional adenopathy, or distant metastases.33
PROSTATE BIOPSY
Transrectal biopsy of the prostate is an office procedure. Local and topical anesthetics can be used to minimize associated discomfort,34 although local anesthetics may not completely eliminate the discomfort of the introduction of the probe.35 A combination of local infiltration and topical gel application seems to provide the best pain relief.36 Use of intraprostatic administration of local anesthetic and of NSAID-containing rectal suppositories is currently being explored.37
Many men who undergo prostate biopsy are middle-aged or elderly and may be receiving antiplatelet therapy (most commonly with low-dose aspirin).38 To decrease the risk for bleeding complications, patients should be advised to discontinue antiplatelet therapy seven to 10 days before biopsy, unless even temporary withdrawal places them at increased risk for cardiovascular complications. Continuing antiplatelet drugs does not appear to increase the incidence of severe hemorrhagic complications and is an acceptable option for patients at high risk for cardiovascular incidents.38
Endorectal coil MRI, in which a coil is inserted into the rectum near the prostate to pick up the MRI signal, has been used to guide biopsies in men with persistently elevated PSA and negative results following transrectal ultrasound–guided biopsies. Complications of transrectal ultrasound–guided biopsies include hematospermia, hematuria (for as long as three days), fever, and rectal bleeding. A small percentage of men develop urinary retention or require hospitalization—usually for urosepsis.39,40 Antibiotic prophylaxis with ciprofloxacin to minimize the risk for infection is considered standard care.41
Patients may be concerned by the possibility that cancer cells will spread during the transrectal biopsy. While there have been isolated reports of tumors recurring in the needle biopsy tract, the incidence is low and this risk should not prevent an indicated biopsy.42
TREATMENT
For patients with disease confined to the prostate gland, treatment options include radical prostatectomy (RP), radiation therapy (RT, external beam and/or brachytherapy), and active surveillance. When disease extends through the prostatic capsule into the seminal vesicles or into regional lymph nodes, definitive local therapy may be combined with adjuvant RT and/or androgen deprivation therapy.43
When choosing treatment for an individual patient, the clinician should consider several factors, including the extent of disease, the patient’s age, and the presence or absence of significant comorbidity. In untreated patients, prostate cancer–related mortality occurs 10 to 20 years after diagnosis.5,44,45
The patient’s chance of being cured following definitive therapy is high if the tumors are confined to the prostate gland.46 The chances of cure decrease when the tumor has spread beyond the prostate capsule, invaded the seminal vesicles, or metastasized to regional lymph nodes.
The overall survival of men with early-stage prostate cancer is prolonged. Ten-year survival rates after RP or RT are high (averaging between 60% and 90%).5,47 Because of the risk for late relapse and mortality, biochemical relapse (as detected by a rise in serum PSA) is monitored. The patient’s likelihood of remaining disease-free, as evidenced by an undetectable serum PSA level (“biochemical progression–free survival”5), is inversely related to the presence of extraprostatic extension, seminal vesicle extension, evidence of tumor spread to the lymph nodes, or evidence of positive margins at surgery.46 Men in the Scandinavian Prostate Cancer Group-4 Randomized Trial (SPCG-4)46 who underwent RP were evaluated 12 years after surgery; those with extracapsular tumor growth had 14 times the risk for prostate cancer death than those without it.
Increasing numbers of selected low-risk patients are being placed on active surveillance for the management of prostate cancer—a protocol that includes repeat prostatic biopsies and routine follow-up visits.48 The PSA should be reassessed two to three times annually, with annual biopsies to determine whether the cancer has become more aggressive.5,16 Patients with cancer that is becoming more aggressive should convert to primary treatment.5
Difficulties with this option include psychological distress, poor compliance with scheduled appointments and repeat biopsies, and the risk for missing the therapeutic window in which the cancer can be cured.5,16 No results are yet available from randomized clinical trials comparing active surveillance with immediate definitive treatment; two large trials are under way.49,50
Radical Prostatectomy
RP is the definitive treatment for localized prostatic cancer.5,16 The potential for cure in men who undergo RP is highest when the cancer is confined to the prostate gland (clinical stage, T1-T2). RP is also an appropriate option for some men with locally advanced prostate cancer. Additionally, RP is used as a potentially curative salvage procedure to treat carefully selected men with a local recurrence after RT for localized prostate cancer.
The SPCG-4,46 which included predominately men whose cancer was not detected by PSA, was the first randomized trial to show that RP decreased the risk for prostate cancer mortality as well as the risk for metastases.2,46 Results were analyzed at 8.2 years’ and 10.8 years’ follow-up. Almost all men in the RP group who died of prostate cancer initially had tumor growth outside the prostate capsule. No men who underwent RP and had specimen Gleason scores of 2 to 6 died of prostate cancer. Rates of local recurrence and/or progression were lower in the RP group than in the “watchful waiting” group (whose members used hormonal and other palliative treatments).46 Subgroup analysis by age showed that men younger than 65 received the greatest benefit from prostatectomy.46,51
Surgical options include open retropubic RP, laparoscopic RP, and perineal RP. Da Vinci (robotic) RP is the method most commonly used in the US to achieve surgical removal of the prostate.16 The procedure is associated with reduced blood loss, compared with other methods, and the 10x magnified vision allows for nerve-sparing techniques, improving postoperative sexual function.16,52
Radiation Therapy
The goal of RT for men with localized prostate cancer is to deliver a therapeutic dose of radiation to the tumor while minimizing radiation to adjacent healthy tissues. Because of the prostate’s close anatomic proximity to the bowel, rectum, and bladder, the most common complications of RT are due to damage from radiation to surrounding structures in the gastrointestinal and genitourinary tracts.
Several forms of RT are available, including external beam radiation and brachytherapy. Radiation combined with hormonal therapy in high-risk patients has significantly improved outcomes, compared with men undergoing radiation alone.53Conformal 3D technology allows radiation oncologists to pinpoint the prostate and follow minute motion of the gland. This technology has helped to decrease the adverse effects of periprostatic radiation exposure.5,16Intensity-modulated radiation therapy offers increased accuracy in radiation dosage, improving cancer cure rates. External beam RT is used as adjuvant therapy in men with advanced disease and as salvage therapy for selected men who experience a rising serum PSA after RP.7,54
Brachytherapy involves the placement of radioactive seeds into the prostate under ultrasound guidance. It is an option for patients with low-risk prostate cancer and is performed as an outpatient procedure.5
Treatment for Disseminated Disease
Among men with disseminated disease, bone metastases are most common. Androgen deprivation therapy (ADT) is used to control the disease while maintaining the patient’s quality of life.2 Once prostate cancer no longer responds to this treatment, it is referred to as hormone-refractory prostate cancer.
Chemotherapy with docetaxel has been shown to extend survival in patients with hormone-refractory prostate cancer.2 Cabazitaxel is a new chemotherapy agent recently approved for use in combination with prednisone for the treatment of patients with metastatic hormone-refractory prostate cancer previously treated with a docetaxel-containing regimen.55,56
Currently, a large phase 3 clinical trial, led by the Cancer and Leukemia Group B,57 is under way to examine the addition of bevacizumab to docetaxel and prednisone for possible improvement in overall prostate cancer survival.2
Adverse Effects of Treatment
As successful as treatments for prostate cancer can be, they are not without significant adverse effects. Their severity varies by treatment plan and by patient, but they usually include physical and psychological effects. Men with prostate cancer are often less satisfied with the management of their disease than are other cancer patients.58 Adverse effects that they find particularly troublesome include urinary incontinence, erectile dysfunction, and effects associated with ADT (see Table 659,60).
Urinary incontinence is a resulting feature of both surgery and RT. After RP, men may have varying degrees of incontinence, ranging from stress incontinence to complete loss of urinary control. Incontinence usually improves within six to 12 months following surgery, with 2% to 22% of men experiencing long-term incontinence.5
Toxic effects of RT can occur immediately or after many years.5 Radiotherapy can result in cystitis, which usually develops a few weeks into treatment and improves after treatment is completed. Approximately one-half of patients who undergo RT experience urinary frequency, dysuria, or urgency due to cystitis, urethritis, or both. Long-term effects of radiation include persistent cystitis or urethral strictures.
Brachytherapy is associated with urinary symptoms that may take several weeks to develop. The side effects may be long lasting and can lead to obstruction of the urethra and urinary retention. Patients should be counseled to avoid bladder irritants, such as tea, coffee, alcohol, and carbonated beverages.
Reports of acute radiation proctitis vary from 2% to 40% of treated men.5,58 If the pelvic lymph nodes are within the treatment field, radiation enteritis may also be observed. Symptoms can include abdominal cramping, tenesmus, urgency, and frequent defecation. These can usually be controlled with antidiarrheal agents or topical anti-inflammatory preparations. Acute symptoms usually subside within three to eight weeks of RT completion. Long-term intestinal side effects persist in up to 10% of patients; these include diarrhea, tenesmus, rectal urgency, or hematochezia. Rectal or anal strictures, ulcers, and perforations are rare.5
Erectile dysfunction is the most common long-term adverse effect of RP, with reports of affected men (who have been advised to abstain from sexual intercourse for six to eight weeks following RP) ranging from 20% to 90%.5,52,61 Maximal recovery usually takes one to two years. Laparoscopic nerve-sparing surgeries have been developed to reduce the risk for erectile dysfunction.5,16,52
Thirty percent to 45% of men who were sexually potent before RT became impotent afterward, with frequency increasing over time.5,58 Factors that contribute to posttreatment impotence in this population include increasing age, intercurrent disease (eg, hypertension, cardiovascular disease, diabetes), and use of ADT.
Research among prostate cancer survivors and their spouses reveals that erectile dysfunction is the most important quality-of-life issue for the 50-to-64 age-group.62 Some men will recover a presurgical level of function, while others may require lengthy treatments, including surgical interventions, that may yield little or no success. Erectile dysfunction can be treated with PDE-5 inhibitors, penile injection therapy, vacuum erection devices, and penile prosthesis implantation.61
Choosing Treatment
Many men will turn to their primary care providers for objective information regarding their treatment options. Long-term morbidity profiles of RP, RT, and brachytherapy do not clearly show one treatment to be superior to the others.5 Each patient’s risks must be assessed, and evidence-based information must be provided for patients to make an informed decision regarding treatment options.
A highly selected group of low-risk patients have the option of management by active surveillance.16,48 Patients with intermediate- or high-risk cancers who have a life expectancy exceeding 10 years should be encouraged to proceed with RP or RT.5 Class I evidence has shown a survival benefit with RP alone.46 RT has shown survival benefit only when used in combination with ADT.53 At this time, there is no direct evidence as to whether brachytherapy reduces prostate cancer mortality because randomized clinical trials to address this question have not been completed.5
Up to one-half of patients who undergo RP and one-third of those undergoing RT develop erectile dysfunction.5,52,58,61,63 Ten percent of RP patients develop long-term urinary incontinence,61 and a similar proportion of RT patients develop long-term proctitis.58 For some patients with low-risk cancers, baseline function and quality-of-life consequences may be enough to sway their decision regarding which treatment option to choose.5,58
EMERGING THERAPIES
In order to reduce the adverse effects of radical therapy, new focal therapies are being developed. Cryotherapy freezes specific areas of the prostate with cooling probes,64 and high-intensity focused ultrasound uses hyperthermia to cause instantaneous irreversible coagulative necrosis of the targeted tissue65; these are two of the most widely accepted focal therapies. Both options offer the advantages of diminished side effects of incontinence and erectile dysfunction and can be completed in a single outpatient treatment session.16,64,65 These procedures require considerable technical skill and are not currently offered in all communities.
Primary prevention may be a possible future direction for the management of prostate cancer. One large trial demonstrated the use of 5- reductase inhibitors to prevent prostate cancer in at-risk men66; further trials are ongoing. 5- Reductase inhibitors (eg, finasteride, dutasteride) prevent the conversion of testosterone to dihydrotestosterone (DHT), which is a more potent agonist for prostate growth.16 Finasteride has been proven safe and effective in reducing the risk for prostate cancer, regardless of risk stratum, and may reduce the risk for high-grade cancers.6,66,67
Attempts to treat prostate cancer with immunotherapy have begun to yield encouraging results. Treatment of metastatic castration-recurrent prostate cancer with sipuleucel-T cancer, a vaccine for the treatment of prostate cancer, showed a four-month survival benefit.2,68 Sipuleucel-T is made from dendritic cells in the patient’s immune system; currently, the vaccine is being produced in small quantities due to limitations in insurance coverage.2
Statin use may reduce the risk for prostate cancer recurrence among men who have undergone RP or RT for localized or locally advanced prostate cancer.69,70 Researchers analyzed database records of 1,319 prostate cancer patients who had undergone RP. For each patient, use or nonuse of statins at the time of surgery was determined, as was PSA progression following surgery. Statin use was associated with a 30% lower risk for PSA recurrence, with statin users taking the highest doses experiencing the most benefit. Additional studies are needed to confirm these results.69,70
DIETARY SUPPLEMENTATION
It is largely unknown whether prostate cancer can be prevented or modified by diet and lifestyle. Global differences in mortality rates and disease patterns associated with immigrant populations suggest that nutrition may play a role in the development of prostate cancer, but data are lacking.
A population-based cohort study of 525 men diagnosed with localized or advanced-stage prostate cancer examined the association of dietary intake of folate, riboflavin, vitamin B6, vitamin B12, and methionine with prostate cancer survival.71 Use of vitamin B6 was found to improve survival of men with localized disease but not with advanced-stage cancer. Dietary intake of folate, riboflavin, vitamin B6, and methionine was not associated with increased prostate cancer survival.71
Investigators for the randomized, placebo-controlled Selenium and Vitamin E Cancer Prevention Trial (SELECT),1 which included 35,533 men from 427 participating sites throughout North America, concluded that selenium or vitamin E, alone or in combination, did not prevent prostate cancer in this population of relatively healthy men.1,16,72
CONCLUSION
Screening for prostate cancer has almost doubled the chance that a man will be diagnosed with prostate cancer in his lifetime, and about 85% of men diagnosed with prostate cancer will undergo active treatment.19 Overtreatment of nonaggressive tumors may result in adverse effects detrimental to the patient’s quality of life, whereas early detection of aggressive tumors may lead to curative therapies being performed while the cancer is still confined to the prostate.1,2,16,19 Differences between outcomes of localized versus advanced disease are remarkable, with associated five-year survival rates of 100% versus 31.7%, respectively.6
New strategies to differentiate between aggressive and nonaggressive tumors would have substantial public heath benefits. Tools for clinical and patient use should be developed to support informed decisions regarding prevention, screening, and biopsy, and to tailor treatments to tumor biology.6 Patients at high risk for prostate cancer should be identified in order to take preventive measures that will improve survival in this group.
REFERENCES
1. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39-51.
2. Kohli M, Tindall DJ. New developments in the medical management of prostate cancer. Mayo Clin Proc. 2010;85(1):77-86.
3. Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100(4):693-701.
4. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-229.
5. Sanda MG, Kaplan ID. A 64-year-old man with low-risk prostate cancer: review of prostate cancer treatment. JAMA. 2009;301(20):2141-2151.
6. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685-1692.
7. Talcott JA, Rossi C, Shipley WU, et al. Patient-reported long-term outcomes after conventional and high-dose combination proton and photon radiation for early prostate cancer. JAMA. 2010;303(11):1046-1053.
8. Odedina FT, Akinremi TO, Chinegwundoh F, et al. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009; 4(suppl 1):S2.
9. Ben-Shlomo Y, Evans S, Ibrahim F, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53(1):99-105.
10. Spickett I, Robertson J. Prostate cancer: the ongoing challenge. Prim Health Care. 2010;20(2): 16-21.
11. Litman HJ, Steers WD, Wei JT, et al. Relationship of lifestyle and clinical factors to lower urinary tract symptoms: results from Boston Area Community Health survey. Urology. 2007;70(5): 916-921.
12. Jones C, Jill J, Chapple C; Guideline Development Group. Management of lower urinary tract symptoms in men: summary of NICE guidance. BMJ. 2010 May 19;340:c2354. doi: 10.1136/bmj.c2354.
13. American Urological Association. Prostate Specific Antigen Best Practice Update: 2009 Update. www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf. Accessed December 21, 2010.
14. Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371-2490.
15. Cooperberg MR, Moul JS, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23(32):8146-8151.
16. Krane LS, Patel MN, Hemal AK. Advances and future directions in management of prostate cancer. Indian J Surg. 2009;71(6):337-341.
17. Collin SM, Metcalfe C, Donovan JL, et al. Associations of sexual dysfunction symptoms with PSA-detected localised and prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. Eur J Cancer. 2009; 45(18):3254-3261.
18. National Health Service Cancer Screening Programmes. Information for primary care; PSA testing in asymptomatic men (2010). www.cancer screening.nhs.uk/prostate/pcrmp-guide-2.html. Accessed December 21, 2010.
19. Schaeffer EM, Carter HB, Kettermann A, et al. Prostate specific antigen testing among the elderly—when to stop? J Urol. 2009;181(4):1606-1614.
20. National Institute on Aging. BLSA: Baltimore Longitudinal Study of Aging; NCT00233272. http://clinicaltrials.gov/ct2/show/NCT00233272. Accessed December 21, 2010.
21. Fillée C, Tombal B, Philippe M. Prostate cancer screening: clinical impact of WHO calibration of Beckman Coulter Access prostate-specific antigen assays. Clin Chem Lab Med. 2010;48(2):285-288.
22. Ferrini R, Woolf SH. Screening for prostate cancer in American men: American College of Preventive Medicine Practice Policy Statement. Am J Prev Med. 1998;15(1):81-84.
23. Wolf AMD, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98.
24. Melia J, Coulson P, Coleman D, Moss S. Urological referral of asymptomatic men in general practice in England. Br J Cancer. 2008;98(7):1176-1181.
25. NHS Cancer Screening Programmes. PCRMP Guide No 2. Information for primary care: PSA testing in asymptomatic men (evidence document, Jan 2010). www.cancerscreening.nhs.uk/prostate/pcrmp-guide-2.html. Accessed December 21, 2010.
26. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™: Prostate cancer. V.3.2010. www.nccn.org/profes sionals/physician_gls/PDF/prostate.pdf. Accessed December 21, 2010.
27. Terakawa T, Miyake, H, Kanomata N, et al. Inverse association between histologic inflammation in needle biopsy specimens and prostate cancer in men with serum PSA of 10-50 ng/mL. Urology. 2008;72(6):1194-1197.
28. Makarov DV, Humphreys EB, Mangold LA, et al. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 ng/mL. J Urol. 2006;176 (2):554-558.
29. Taneja SS. Optimizing prostate biopsy strategies for the diagnosis of prostate cancer. Rev Urol. 2003;5(3):149-155.
30. Delongchamps NB, Singh A, Hass GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. 2006;13(3):158-168.
31. Baumgart LA, Gerling GJ, Bass EJ. Characterizing the range of simulated prostate abnormalities palpable by digital rectal examination. Cancer Epidemiol. 2010;34(1):79-84.
32. Crawford ED, Schutz MJ, Clejan S, et al. The effect of digital rectal examination on prostate-specific antigen levels. JAMA. 1992;267(16):2227-2228.
33. Stephens NJ, Bharwani N, Heenan SD. Prostate cancer staging. Imaging. 2008;20:112-121.
34. Richman JM, Carter HB, Hanna MN, et al. Efficacy of periprostatic local anesthetic for prostate biopsy analgesia: a meta-analysis. Urology. 2006;67(6):1224-1228.
35. Luscombe CJ, Cooke PW. Pain during prostate biopsy. Lancet. 2004;363(9424):1840-1841.
36. Yun TJ, Lee HJ, Kim SH, et al. Does the intrarectal instillation of lidocaine gel before periprostatic neurovascular bundle block during transrectal ultrasound-guided prostate biopsies improve analgesic efficacy? A prospective, randomized trial. J Urol. 2007;178(1):103-106.
37. Lee HY, Lee HJ, Byun SS, et al. Effect of intraprostatic local anesthesia during transrectal ultrasound guided prostate biopsy: comparison of 3 methods in a randomized, double-blind, placebo controlled trial. J Urol. 2007;178(2):469-472.
38. Giannarini G, Mogorovich, A, Valent F, et al. Continuing or discontinuing low-dose aspirin before transrectal prostate biopsy: results of a prospective randomized trial. Urology. 2007;70(3): 501-505.
39. Park DS, Oh JJ, Lee JH, et al. Simple use of the suppository type povidone-iodine can prevent infectious complications in transrectal ultrasound-guided prostate biopsy. Adv Urol. 2009:750598. Epub 2009 Apr 23.
40. Rietbergen JB, Kruger AE, Kranse R, Schröder FH. Complications of transrectal ultrasound–guided systemic sextant biopsies of the prostate: evaluation of complication rates and risks factors within a population-based screening program. Urology. 1997;49(6):875-880.
41. Weber B, Saliken J, Jadavji T, et al. A near-fatal case of sepsis with an antibiotic-resistant organism complicating a routine transrectal prostate biopsy in a health care worker. Can Urol Assoc J. 2008;2 (5):543-545.
42. Vaghefi H, Magi-Galluzzi C, Klein EA. Local recurrence of prostate cancer in rectal submucosa after transrectal needle biopsy and radical prostatectomy. Urology. 2005;66(4):881.
43. Widmark A, Klepp O, Solberg A, et al; Swedish Association for Urological Oncology 3. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009; 373(9660):301-308.
44. Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280(11):975-980.
45. Johansson JE, Andrén O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713-2719.
46. Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
47. Inman BA, Davies JD, Rangel LJ, et al. Long-term outcomes of radical prostatectomy with multimodal adjuvant therapy in men with a preoperative serum prostate-specific antigen level > or =50 ng/mL. Cancer. 2008;113(7):1544-1551.
48. Klotz L. Active surveillance for prostate cancer: patient selection and management. Curr Oncol. 2010;17 suppl 2:S111-S117.
49. Oxford Radcliffe Hospital, National Cancer Institute. Active surveillance, radical prostatectomy, or radiation therapy in treating patients with localized prostate cancer; NCT00632983. http://clinicaltrials.gov/ct2/show/NCT00632983?term=prostate%2C+surveillance&rank=7. Accessed December 21, 2010.
50. NCIC Clinical Trials Group, National Cancer Institute, Cancer and Leukemia Group B, Eastern Cooperative Oncology Group, Southwest Oncology Group. Observation or radical treatment in patients with prostate cancer; NCT00499174. http://clinicaltrials.gov/ct2/show/NCT00499174? term=prostate%2C+surveillance&rank=12. Accessed December 21, 2010.
51. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11): 1202-1209.
52. Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162(2):433-438.
53. D’Amico AV, Manola J, Loffredo M, et al. 6-Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821-827.
54. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008; 299(23):2760-2769.
55. de Bono JS, Oudard S, Ozguroglu M, et al; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
56. US Food and Drug Administration (FDA news release, June 17, 2010). FDA approves new treatment for advanced prostate cancer. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm216143.htm Accessed December 21, 2010.
57. Cancer and Leukemia Group B, National Cancer Institute, Eastern Cooperative Oncology Group. Docetaxel and prednisone with or without bevacizumab in treating patients with prostate cancer that did not respond to hormone therapy; NCT00110214. http://clinicaltrials.gov/ct2/show/NCT00110214. Accessed December 21, 2010.
58. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12): 1250-1261.
59. Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115(11): 2388-2399.
60. Nanda A, Chen MH, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease–induced congestive heart failure or myocardial infarction. JAMA. 2009;302(8):866-873.
61. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000; 283(3):354-360.
62. Harden J, Northouse L, Cimprich B, et al. The influence of developmental life stage in quality of life in survivors of prostate cancer and their partners. J Cancer Surviv. 2008;2(2):84-94.
63. Potosky A, Legler J, Albertsen P, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92 (19):1582-1592.
64. Tsivian M, Polascik TJ. Focal cryotherapy for prostate cancer. Curr Urol Rep. 2010;11(3):
147-151.
65. Lee HM, Hong JH, Choi HY. High-intensity focused ultrasound therapy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(4):439-443.
66. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349 (3):215-224.
67. Kramer BS, Hagerty KL, Justman S, et al. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Clin Oncol. 2009;27 (9):1502-1516.
68. Schellhammer PF, Higano C, Berger ER, et al; IMPACT Study Investigators. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen-independent prostatic adenocarcinoma (AIPC). Presented at: American Urological Association 104th Annual Scientific Meeting; April 28, 2009; Chicago, IL. Abstract 9.
69. Hamilton RJ, Banez LL, Aronson WJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010; 116(14):3389-3398.
70. Gutt R, Tonlaar N, Kunnavakkam R, et al. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28(16):2653-2659.
71. Kasperzyk JL, Fall K, Mucci LA, et al. One-carbon metabolism-related nutrients and prostate cancer survival. Am J Clin Nutr. 2009;90(3): 561-569.
72. Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1): 52-62.
Prostate cancer is the sixth most common cancer in the world and the second most common cancer among American men, surpassed only by nonmelanoma skin cancer. Prostate cancer led to approximately 28,660 deaths in 2008 (the second leading cause of cancer deaths that year), and 192,000 new cases of prostate cancer were diagnosed in 2009.1-7
The incidence of prostate cancer has been rising about 1% annually since 1995.4 Explanations for the increasing incidence, though not certain, are believed to be both genetic and environmental. In the US, prostate cancer–associated morbidity and mortality rates are highest in African-American men.8 Men of African and Caribbean descent are at three times the risk for prostate cancer, compared with white men.9
EPIDEMIOLOGY AND RISK FACTORS
Prostate-specific antigen (PSA) testing has nearly doubled the chance that a man will be diagnosed with prostate cancer in his lifetime. Prior to widespread use of PSA testing, a white man had a one-in-eleven chance of being diagnosed with prostate cancer in his lifetime; currently, that man’s chances are one in six.6
The incidence of prostate cancer increases as men age, with those in their 40s accounting for less than 1% of prostate cancer cases, compared with men older than 65, who account for more than 75% of cases.10 Besides age, other risk factors include a positive family history of prostate cancer in the father or brother(s) and African-American ethnicity.2,3
No clear link has been demonstrated between diet and prostate cancer. Tobacco use is not currently considered a risk factor for prostate cancer, but pooled data from 24 cohort studies enrolling more than 26,000 participants with prostate cancer revealed a 9% to 30% increase in both incident and fatal prostate cancer among men who smoked.3
ANATOMY AND FUNCTION
The prostate gland sits under the bladder and surrounds the urethra; in a young man, it is approximately the size of a walnut. As a man ages, the prostate begins to enlarge. Once a man reaches his 50s, he may begin to experience lower urinary tract symptoms11,12 (see Table 111,12).
The prostate produces fluid containing prostate-specific antigen, a type of protein that helps liquefy the semen and facilitate sperm motility. PSA is a component of the seminal fluid that is necessary for ejaculation. The prostate and the seminal vessels contract during ejaculation, expelling fluid through the prostate’s ejaculatory ducts and out along the urethra.10 PSA levels in the serum are normally very low. Prostatic disease, inflammation, or trauma can lead to increased levels of PSA in the serum. Elevated serum PSA has become an important marker of many prostate diseases, including benign prostatic hyperplasia, prostatitis, and prostate cancer13—the focus of this article.
The prostate gland is comprised of three zones: central, transitional, and peripheral. The peripheral zone, located at the back of the prostate, is the most susceptible to cancer. Prostate cancer is typically adenocarcinoma.14
CLINICAL PRESENTATION
As a result of widespread screening of serum PSA, prostate cancer is often diagnosed before symptoms develop or a palpable nodule appears.5,15,16 Prostate cancer not identified through PSA screening is typically detected either by digital rectal examination (DRE) or in an investigation of genitourinary symptoms. For men who are symptomatic, the primary care provider can order a urine test to rule out infection (which can raise the PSA level), order PSA screening, and perform a DRE, which can also help to rule out benign prostatic hypertrophy (BPH)17 (see Table 210,17).
PSA TESTING
Primary care providers can make an important contribution to men’s health by educating those older than 50 regarding the pros and cons of PSA testing. It has been found that 47% of men between ages 50 and 70 have no knowledge of the PSA test. Men in the upper socioeconomic groups are more likely to be aware of the test.10 Patients scheduled to undergo PSA testing should be advised in advance to avoid certain activities and circumstances (see Table 3,13,18).
Screening serum PSA can reduce the number of prostate cancer–associated deaths by 31%, but this benefit must be weighed against a degree of overdiagnosis and overtreatment.2,5,6,16,19 Although the incidence of prostate cancer is 16%, only 2% of affected men will succumb to the disease.16 Up to 30% of prostate cancers detected by PSA may have otherwise remained clinically silent throughout the patient’s life.19
Additionally, PSA screening rates remain high in men between ages 75 and 80, who may be at risk for screening that is unnecessary due to an increase in competing causes of death.19 In the Baltimore Longitudinal Study of Aging,19,20 a longitudinal cohort study in which 849 men were enrolled, researchers measured the proportion of men, by PSA and age, who died of prostate cancer or in whom aggressive prostate cancer developed. It was found that no participants between ages 75 and 80 with a PSA below 3.0 ng/mL died of prostate cancer, but men of all ages with a PSA greater than 3.0 ng/mL had a continually increasing probability of death from prostate cancer. These findings suggest that it may be safe to discontinue PSA testing in men older than 75 whose PSA level is lower than 3.0 ng/mL.19
In the absence of a family history of prostate cancer, it is recommended that men undergo annual DRE and PSA testing beginning at age 50.21,22 The American Urological Association13 recommends annual PSA testing beginning at age 40 for African-American men and other men with a positive family history of prostate cancer. The American Cancer Society23 recommends that these tests be offered annually to men who are 50 and older and who have at least a 10-year life expectancy.
The serum PSA level alone does not automatically trigger a referral for a prostate biopsy (see Table 424,25). Other factors that should also be considered are family history, nonsymmetrical enlargement of the prostate, DRE findings of firmness, induration, or frank nodules, age, ethnicity, comorbidities, a history of previous negative prostate biopsy, and previous PSA levels. The patient should be involved in any decision regarding referral to another health care provider and the decision to perform biopsy.18
PSA levels are also used to monitor response to cancer treatments and to detect disease recurrence after treatment.2
Serum PSA Elevation
PSA is a prostate-specific marker, and elevations can be caused by prostate cancer or by benign conditions, such as BPH or infection. Malignant prostate tissue generates more PSA than does normal or hyperplastic tissue. The likelihood of finding cancer on a prostate biopsy increases as PSA values rise. Two PSA tests performed seven weeks apart allow for more accurate risk prediction and may assist in decision-making regarding referral and/or biopsy.18
Prostate biopsy is recommended for men with a total serum PSA of 4.0 ng/mL or greater, regardless of DRE findings. Biopsy at this time increases the likelihood of diagnosing disease while it is still organ-confined.26 In men with serum PSA of 10 ng/mL or more, the chance of finding prostate cancer exceeds 50%, and many such men will have disease that has already extended beyond the prostate.27
Managing men with PSA measurements below 4.0 ng/mL is more difficult. The majority of men in this category will have negative biopsies. However, a significant number of men with prostate cancer present with serum PSA concentrations below 4.0 ng/mL.28 Because cancer detected in these men is likely to be organ-confined, the National Comprehensive Cancer Network altered its guidelines, recommending that prostate biopsy be considered in men with a serum PSA between 2.6 and 4.0 ng/mL; or in those with a rate of rise in the serum PSA (PSA velocity) of at least 0.35 ng/mL/year in men with a serum PSA of 2.5 ng/mL or less.26
DIAGNOSIS
The diagnosis of prostate cancer is accomplished through a histologic examination of biopsied prostate tumor. The biopsy is performed using transrectal ultrasound. A prostate biopsy may be indicated based upon clinical symptoms, an abnormal DRE, or an elevated serum PSA.29
Transrectal ultrasonography with prostate biopsy is indicated in men with DRE findings that are suspicious for cancer (ie, induration, asymmetry, or palpable nodularity of the prostate gland), even if the serum PSA is not elevated; such findings require that prostate cancer be ruled out. This is particularly important if the patient is older than 45 or has other risk factors for the disease.17,30
Tumors located in the posterior and lateral aspects of the prostate gland are most often detected by DRE. About 25% to 35% of prostate tumors develop in other areas of the prostate or are too small to be detected by DRE.31
A serum PSA level should be ordered before biopsy in men with an abnormal DRE for diagnostic and prognostic purposes. Serum PSA concentrations rise slightly during the first several hours after a rectal examination in some men; if possible, the PSA should not be drawn within one week of the DRE but should be obtained prior to the biopsy.18,32
Staging
Prostate cancer is divided into low, intermediate, and high risk, based on the serum PSA, the Gleason score, and the clinical stage (see Table 5,5,18,21,22,26 ). Imaging studies, including CT of the abdomen and pelvis, radionuclide bone scans, and endorectal coil MRI, are used selectively to assess for extraprostatic extension, regional adenopathy, or distant metastases.33
PROSTATE BIOPSY
Transrectal biopsy of the prostate is an office procedure. Local and topical anesthetics can be used to minimize associated discomfort,34 although local anesthetics may not completely eliminate the discomfort of the introduction of the probe.35 A combination of local infiltration and topical gel application seems to provide the best pain relief.36 Use of intraprostatic administration of local anesthetic and of NSAID-containing rectal suppositories is currently being explored.37
Many men who undergo prostate biopsy are middle-aged or elderly and may be receiving antiplatelet therapy (most commonly with low-dose aspirin).38 To decrease the risk for bleeding complications, patients should be advised to discontinue antiplatelet therapy seven to 10 days before biopsy, unless even temporary withdrawal places them at increased risk for cardiovascular complications. Continuing antiplatelet drugs does not appear to increase the incidence of severe hemorrhagic complications and is an acceptable option for patients at high risk for cardiovascular incidents.38
Endorectal coil MRI, in which a coil is inserted into the rectum near the prostate to pick up the MRI signal, has been used to guide biopsies in men with persistently elevated PSA and negative results following transrectal ultrasound–guided biopsies. Complications of transrectal ultrasound–guided biopsies include hematospermia, hematuria (for as long as three days), fever, and rectal bleeding. A small percentage of men develop urinary retention or require hospitalization—usually for urosepsis.39,40 Antibiotic prophylaxis with ciprofloxacin to minimize the risk for infection is considered standard care.41
Patients may be concerned by the possibility that cancer cells will spread during the transrectal biopsy. While there have been isolated reports of tumors recurring in the needle biopsy tract, the incidence is low and this risk should not prevent an indicated biopsy.42
TREATMENT
For patients with disease confined to the prostate gland, treatment options include radical prostatectomy (RP), radiation therapy (RT, external beam and/or brachytherapy), and active surveillance. When disease extends through the prostatic capsule into the seminal vesicles or into regional lymph nodes, definitive local therapy may be combined with adjuvant RT and/or androgen deprivation therapy.43
When choosing treatment for an individual patient, the clinician should consider several factors, including the extent of disease, the patient’s age, and the presence or absence of significant comorbidity. In untreated patients, prostate cancer–related mortality occurs 10 to 20 years after diagnosis.5,44,45
The patient’s chance of being cured following definitive therapy is high if the tumors are confined to the prostate gland.46 The chances of cure decrease when the tumor has spread beyond the prostate capsule, invaded the seminal vesicles, or metastasized to regional lymph nodes.
The overall survival of men with early-stage prostate cancer is prolonged. Ten-year survival rates after RP or RT are high (averaging between 60% and 90%).5,47 Because of the risk for late relapse and mortality, biochemical relapse (as detected by a rise in serum PSA) is monitored. The patient’s likelihood of remaining disease-free, as evidenced by an undetectable serum PSA level (“biochemical progression–free survival”5), is inversely related to the presence of extraprostatic extension, seminal vesicle extension, evidence of tumor spread to the lymph nodes, or evidence of positive margins at surgery.46 Men in the Scandinavian Prostate Cancer Group-4 Randomized Trial (SPCG-4)46 who underwent RP were evaluated 12 years after surgery; those with extracapsular tumor growth had 14 times the risk for prostate cancer death than those without it.
Increasing numbers of selected low-risk patients are being placed on active surveillance for the management of prostate cancer—a protocol that includes repeat prostatic biopsies and routine follow-up visits.48 The PSA should be reassessed two to three times annually, with annual biopsies to determine whether the cancer has become more aggressive.5,16 Patients with cancer that is becoming more aggressive should convert to primary treatment.5
Difficulties with this option include psychological distress, poor compliance with scheduled appointments and repeat biopsies, and the risk for missing the therapeutic window in which the cancer can be cured.5,16 No results are yet available from randomized clinical trials comparing active surveillance with immediate definitive treatment; two large trials are under way.49,50
Radical Prostatectomy
RP is the definitive treatment for localized prostatic cancer.5,16 The potential for cure in men who undergo RP is highest when the cancer is confined to the prostate gland (clinical stage, T1-T2). RP is also an appropriate option for some men with locally advanced prostate cancer. Additionally, RP is used as a potentially curative salvage procedure to treat carefully selected men with a local recurrence after RT for localized prostate cancer.
The SPCG-4,46 which included predominately men whose cancer was not detected by PSA, was the first randomized trial to show that RP decreased the risk for prostate cancer mortality as well as the risk for metastases.2,46 Results were analyzed at 8.2 years’ and 10.8 years’ follow-up. Almost all men in the RP group who died of prostate cancer initially had tumor growth outside the prostate capsule. No men who underwent RP and had specimen Gleason scores of 2 to 6 died of prostate cancer. Rates of local recurrence and/or progression were lower in the RP group than in the “watchful waiting” group (whose members used hormonal and other palliative treatments).46 Subgroup analysis by age showed that men younger than 65 received the greatest benefit from prostatectomy.46,51
Surgical options include open retropubic RP, laparoscopic RP, and perineal RP. Da Vinci (robotic) RP is the method most commonly used in the US to achieve surgical removal of the prostate.16 The procedure is associated with reduced blood loss, compared with other methods, and the 10x magnified vision allows for nerve-sparing techniques, improving postoperative sexual function.16,52
Radiation Therapy
The goal of RT for men with localized prostate cancer is to deliver a therapeutic dose of radiation to the tumor while minimizing radiation to adjacent healthy tissues. Because of the prostate’s close anatomic proximity to the bowel, rectum, and bladder, the most common complications of RT are due to damage from radiation to surrounding structures in the gastrointestinal and genitourinary tracts.
Several forms of RT are available, including external beam radiation and brachytherapy. Radiation combined with hormonal therapy in high-risk patients has significantly improved outcomes, compared with men undergoing radiation alone.53Conformal 3D technology allows radiation oncologists to pinpoint the prostate and follow minute motion of the gland. This technology has helped to decrease the adverse effects of periprostatic radiation exposure.5,16Intensity-modulated radiation therapy offers increased accuracy in radiation dosage, improving cancer cure rates. External beam RT is used as adjuvant therapy in men with advanced disease and as salvage therapy for selected men who experience a rising serum PSA after RP.7,54
Brachytherapy involves the placement of radioactive seeds into the prostate under ultrasound guidance. It is an option for patients with low-risk prostate cancer and is performed as an outpatient procedure.5
Treatment for Disseminated Disease
Among men with disseminated disease, bone metastases are most common. Androgen deprivation therapy (ADT) is used to control the disease while maintaining the patient’s quality of life.2 Once prostate cancer no longer responds to this treatment, it is referred to as hormone-refractory prostate cancer.
Chemotherapy with docetaxel has been shown to extend survival in patients with hormone-refractory prostate cancer.2 Cabazitaxel is a new chemotherapy agent recently approved for use in combination with prednisone for the treatment of patients with metastatic hormone-refractory prostate cancer previously treated with a docetaxel-containing regimen.55,56
Currently, a large phase 3 clinical trial, led by the Cancer and Leukemia Group B,57 is under way to examine the addition of bevacizumab to docetaxel and prednisone for possible improvement in overall prostate cancer survival.2
Adverse Effects of Treatment
As successful as treatments for prostate cancer can be, they are not without significant adverse effects. Their severity varies by treatment plan and by patient, but they usually include physical and psychological effects. Men with prostate cancer are often less satisfied with the management of their disease than are other cancer patients.58 Adverse effects that they find particularly troublesome include urinary incontinence, erectile dysfunction, and effects associated with ADT (see Table 659,60).
Urinary incontinence is a resulting feature of both surgery and RT. After RP, men may have varying degrees of incontinence, ranging from stress incontinence to complete loss of urinary control. Incontinence usually improves within six to 12 months following surgery, with 2% to 22% of men experiencing long-term incontinence.5
Toxic effects of RT can occur immediately or after many years.5 Radiotherapy can result in cystitis, which usually develops a few weeks into treatment and improves after treatment is completed. Approximately one-half of patients who undergo RT experience urinary frequency, dysuria, or urgency due to cystitis, urethritis, or both. Long-term effects of radiation include persistent cystitis or urethral strictures.
Brachytherapy is associated with urinary symptoms that may take several weeks to develop. The side effects may be long lasting and can lead to obstruction of the urethra and urinary retention. Patients should be counseled to avoid bladder irritants, such as tea, coffee, alcohol, and carbonated beverages.
Reports of acute radiation proctitis vary from 2% to 40% of treated men.5,58 If the pelvic lymph nodes are within the treatment field, radiation enteritis may also be observed. Symptoms can include abdominal cramping, tenesmus, urgency, and frequent defecation. These can usually be controlled with antidiarrheal agents or topical anti-inflammatory preparations. Acute symptoms usually subside within three to eight weeks of RT completion. Long-term intestinal side effects persist in up to 10% of patients; these include diarrhea, tenesmus, rectal urgency, or hematochezia. Rectal or anal strictures, ulcers, and perforations are rare.5
Erectile dysfunction is the most common long-term adverse effect of RP, with reports of affected men (who have been advised to abstain from sexual intercourse for six to eight weeks following RP) ranging from 20% to 90%.5,52,61 Maximal recovery usually takes one to two years. Laparoscopic nerve-sparing surgeries have been developed to reduce the risk for erectile dysfunction.5,16,52
Thirty percent to 45% of men who were sexually potent before RT became impotent afterward, with frequency increasing over time.5,58 Factors that contribute to posttreatment impotence in this population include increasing age, intercurrent disease (eg, hypertension, cardiovascular disease, diabetes), and use of ADT.
Research among prostate cancer survivors and their spouses reveals that erectile dysfunction is the most important quality-of-life issue for the 50-to-64 age-group.62 Some men will recover a presurgical level of function, while others may require lengthy treatments, including surgical interventions, that may yield little or no success. Erectile dysfunction can be treated with PDE-5 inhibitors, penile injection therapy, vacuum erection devices, and penile prosthesis implantation.61
Choosing Treatment
Many men will turn to their primary care providers for objective information regarding their treatment options. Long-term morbidity profiles of RP, RT, and brachytherapy do not clearly show one treatment to be superior to the others.5 Each patient’s risks must be assessed, and evidence-based information must be provided for patients to make an informed decision regarding treatment options.
A highly selected group of low-risk patients have the option of management by active surveillance.16,48 Patients with intermediate- or high-risk cancers who have a life expectancy exceeding 10 years should be encouraged to proceed with RP or RT.5 Class I evidence has shown a survival benefit with RP alone.46 RT has shown survival benefit only when used in combination with ADT.53 At this time, there is no direct evidence as to whether brachytherapy reduces prostate cancer mortality because randomized clinical trials to address this question have not been completed.5
Up to one-half of patients who undergo RP and one-third of those undergoing RT develop erectile dysfunction.5,52,58,61,63 Ten percent of RP patients develop long-term urinary incontinence,61 and a similar proportion of RT patients develop long-term proctitis.58 For some patients with low-risk cancers, baseline function and quality-of-life consequences may be enough to sway their decision regarding which treatment option to choose.5,58
EMERGING THERAPIES
In order to reduce the adverse effects of radical therapy, new focal therapies are being developed. Cryotherapy freezes specific areas of the prostate with cooling probes,64 and high-intensity focused ultrasound uses hyperthermia to cause instantaneous irreversible coagulative necrosis of the targeted tissue65; these are two of the most widely accepted focal therapies. Both options offer the advantages of diminished side effects of incontinence and erectile dysfunction and can be completed in a single outpatient treatment session.16,64,65 These procedures require considerable technical skill and are not currently offered in all communities.
Primary prevention may be a possible future direction for the management of prostate cancer. One large trial demonstrated the use of 5- reductase inhibitors to prevent prostate cancer in at-risk men66; further trials are ongoing. 5- Reductase inhibitors (eg, finasteride, dutasteride) prevent the conversion of testosterone to dihydrotestosterone (DHT), which is a more potent agonist for prostate growth.16 Finasteride has been proven safe and effective in reducing the risk for prostate cancer, regardless of risk stratum, and may reduce the risk for high-grade cancers.6,66,67
Attempts to treat prostate cancer with immunotherapy have begun to yield encouraging results. Treatment of metastatic castration-recurrent prostate cancer with sipuleucel-T cancer, a vaccine for the treatment of prostate cancer, showed a four-month survival benefit.2,68 Sipuleucel-T is made from dendritic cells in the patient’s immune system; currently, the vaccine is being produced in small quantities due to limitations in insurance coverage.2
Statin use may reduce the risk for prostate cancer recurrence among men who have undergone RP or RT for localized or locally advanced prostate cancer.69,70 Researchers analyzed database records of 1,319 prostate cancer patients who had undergone RP. For each patient, use or nonuse of statins at the time of surgery was determined, as was PSA progression following surgery. Statin use was associated with a 30% lower risk for PSA recurrence, with statin users taking the highest doses experiencing the most benefit. Additional studies are needed to confirm these results.69,70
DIETARY SUPPLEMENTATION
It is largely unknown whether prostate cancer can be prevented or modified by diet and lifestyle. Global differences in mortality rates and disease patterns associated with immigrant populations suggest that nutrition may play a role in the development of prostate cancer, but data are lacking.
A population-based cohort study of 525 men diagnosed with localized or advanced-stage prostate cancer examined the association of dietary intake of folate, riboflavin, vitamin B6, vitamin B12, and methionine with prostate cancer survival.71 Use of vitamin B6 was found to improve survival of men with localized disease but not with advanced-stage cancer. Dietary intake of folate, riboflavin, vitamin B6, and methionine was not associated with increased prostate cancer survival.71
Investigators for the randomized, placebo-controlled Selenium and Vitamin E Cancer Prevention Trial (SELECT),1 which included 35,533 men from 427 participating sites throughout North America, concluded that selenium or vitamin E, alone or in combination, did not prevent prostate cancer in this population of relatively healthy men.1,16,72
CONCLUSION
Screening for prostate cancer has almost doubled the chance that a man will be diagnosed with prostate cancer in his lifetime, and about 85% of men diagnosed with prostate cancer will undergo active treatment.19 Overtreatment of nonaggressive tumors may result in adverse effects detrimental to the patient’s quality of life, whereas early detection of aggressive tumors may lead to curative therapies being performed while the cancer is still confined to the prostate.1,2,16,19 Differences between outcomes of localized versus advanced disease are remarkable, with associated five-year survival rates of 100% versus 31.7%, respectively.6
New strategies to differentiate between aggressive and nonaggressive tumors would have substantial public heath benefits. Tools for clinical and patient use should be developed to support informed decisions regarding prevention, screening, and biopsy, and to tailor treatments to tumor biology.6 Patients at high risk for prostate cancer should be identified in order to take preventive measures that will improve survival in this group.
REFERENCES
1. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39-51.
2. Kohli M, Tindall DJ. New developments in the medical management of prostate cancer. Mayo Clin Proc. 2010;85(1):77-86.
3. Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100(4):693-701.
4. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-229.
5. Sanda MG, Kaplan ID. A 64-year-old man with low-risk prostate cancer: review of prostate cancer treatment. JAMA. 2009;301(20):2141-2151.
6. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685-1692.
7. Talcott JA, Rossi C, Shipley WU, et al. Patient-reported long-term outcomes after conventional and high-dose combination proton and photon radiation for early prostate cancer. JAMA. 2010;303(11):1046-1053.
8. Odedina FT, Akinremi TO, Chinegwundoh F, et al. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009; 4(suppl 1):S2.
9. Ben-Shlomo Y, Evans S, Ibrahim F, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53(1):99-105.
10. Spickett I, Robertson J. Prostate cancer: the ongoing challenge. Prim Health Care. 2010;20(2): 16-21.
11. Litman HJ, Steers WD, Wei JT, et al. Relationship of lifestyle and clinical factors to lower urinary tract symptoms: results from Boston Area Community Health survey. Urology. 2007;70(5): 916-921.
12. Jones C, Jill J, Chapple C; Guideline Development Group. Management of lower urinary tract symptoms in men: summary of NICE guidance. BMJ. 2010 May 19;340:c2354. doi: 10.1136/bmj.c2354.
13. American Urological Association. Prostate Specific Antigen Best Practice Update: 2009 Update. www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf. Accessed December 21, 2010.
14. Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371-2490.
15. Cooperberg MR, Moul JS, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23(32):8146-8151.
16. Krane LS, Patel MN, Hemal AK. Advances and future directions in management of prostate cancer. Indian J Surg. 2009;71(6):337-341.
17. Collin SM, Metcalfe C, Donovan JL, et al. Associations of sexual dysfunction symptoms with PSA-detected localised and prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. Eur J Cancer. 2009; 45(18):3254-3261.
18. National Health Service Cancer Screening Programmes. Information for primary care; PSA testing in asymptomatic men (2010). www.cancer screening.nhs.uk/prostate/pcrmp-guide-2.html. Accessed December 21, 2010.
19. Schaeffer EM, Carter HB, Kettermann A, et al. Prostate specific antigen testing among the elderly—when to stop? J Urol. 2009;181(4):1606-1614.
20. National Institute on Aging. BLSA: Baltimore Longitudinal Study of Aging; NCT00233272. http://clinicaltrials.gov/ct2/show/NCT00233272. Accessed December 21, 2010.
21. Fillée C, Tombal B, Philippe M. Prostate cancer screening: clinical impact of WHO calibration of Beckman Coulter Access prostate-specific antigen assays. Clin Chem Lab Med. 2010;48(2):285-288.
22. Ferrini R, Woolf SH. Screening for prostate cancer in American men: American College of Preventive Medicine Practice Policy Statement. Am J Prev Med. 1998;15(1):81-84.
23. Wolf AMD, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98.
24. Melia J, Coulson P, Coleman D, Moss S. Urological referral of asymptomatic men in general practice in England. Br J Cancer. 2008;98(7):1176-1181.
25. NHS Cancer Screening Programmes. PCRMP Guide No 2. Information for primary care: PSA testing in asymptomatic men (evidence document, Jan 2010). www.cancerscreening.nhs.uk/prostate/pcrmp-guide-2.html. Accessed December 21, 2010.
26. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™: Prostate cancer. V.3.2010. www.nccn.org/profes sionals/physician_gls/PDF/prostate.pdf. Accessed December 21, 2010.
27. Terakawa T, Miyake, H, Kanomata N, et al. Inverse association between histologic inflammation in needle biopsy specimens and prostate cancer in men with serum PSA of 10-50 ng/mL. Urology. 2008;72(6):1194-1197.
28. Makarov DV, Humphreys EB, Mangold LA, et al. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 ng/mL. J Urol. 2006;176 (2):554-558.
29. Taneja SS. Optimizing prostate biopsy strategies for the diagnosis of prostate cancer. Rev Urol. 2003;5(3):149-155.
30. Delongchamps NB, Singh A, Hass GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. 2006;13(3):158-168.
31. Baumgart LA, Gerling GJ, Bass EJ. Characterizing the range of simulated prostate abnormalities palpable by digital rectal examination. Cancer Epidemiol. 2010;34(1):79-84.
32. Crawford ED, Schutz MJ, Clejan S, et al. The effect of digital rectal examination on prostate-specific antigen levels. JAMA. 1992;267(16):2227-2228.
33. Stephens NJ, Bharwani N, Heenan SD. Prostate cancer staging. Imaging. 2008;20:112-121.
34. Richman JM, Carter HB, Hanna MN, et al. Efficacy of periprostatic local anesthetic for prostate biopsy analgesia: a meta-analysis. Urology. 2006;67(6):1224-1228.
35. Luscombe CJ, Cooke PW. Pain during prostate biopsy. Lancet. 2004;363(9424):1840-1841.
36. Yun TJ, Lee HJ, Kim SH, et al. Does the intrarectal instillation of lidocaine gel before periprostatic neurovascular bundle block during transrectal ultrasound-guided prostate biopsies improve analgesic efficacy? A prospective, randomized trial. J Urol. 2007;178(1):103-106.
37. Lee HY, Lee HJ, Byun SS, et al. Effect of intraprostatic local anesthesia during transrectal ultrasound guided prostate biopsy: comparison of 3 methods in a randomized, double-blind, placebo controlled trial. J Urol. 2007;178(2):469-472.
38. Giannarini G, Mogorovich, A, Valent F, et al. Continuing or discontinuing low-dose aspirin before transrectal prostate biopsy: results of a prospective randomized trial. Urology. 2007;70(3): 501-505.
39. Park DS, Oh JJ, Lee JH, et al. Simple use of the suppository type povidone-iodine can prevent infectious complications in transrectal ultrasound-guided prostate biopsy. Adv Urol. 2009:750598. Epub 2009 Apr 23.
40. Rietbergen JB, Kruger AE, Kranse R, Schröder FH. Complications of transrectal ultrasound–guided systemic sextant biopsies of the prostate: evaluation of complication rates and risks factors within a population-based screening program. Urology. 1997;49(6):875-880.
41. Weber B, Saliken J, Jadavji T, et al. A near-fatal case of sepsis with an antibiotic-resistant organism complicating a routine transrectal prostate biopsy in a health care worker. Can Urol Assoc J. 2008;2 (5):543-545.
42. Vaghefi H, Magi-Galluzzi C, Klein EA. Local recurrence of prostate cancer in rectal submucosa after transrectal needle biopsy and radical prostatectomy. Urology. 2005;66(4):881.
43. Widmark A, Klepp O, Solberg A, et al; Swedish Association for Urological Oncology 3. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009; 373(9660):301-308.
44. Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280(11):975-980.
45. Johansson JE, Andrén O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713-2719.
46. Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
47. Inman BA, Davies JD, Rangel LJ, et al. Long-term outcomes of radical prostatectomy with multimodal adjuvant therapy in men with a preoperative serum prostate-specific antigen level > or =50 ng/mL. Cancer. 2008;113(7):1544-1551.
48. Klotz L. Active surveillance for prostate cancer: patient selection and management. Curr Oncol. 2010;17 suppl 2:S111-S117.
49. Oxford Radcliffe Hospital, National Cancer Institute. Active surveillance, radical prostatectomy, or radiation therapy in treating patients with localized prostate cancer; NCT00632983. http://clinicaltrials.gov/ct2/show/NCT00632983?term=prostate%2C+surveillance&rank=7. Accessed December 21, 2010.
50. NCIC Clinical Trials Group, National Cancer Institute, Cancer and Leukemia Group B, Eastern Cooperative Oncology Group, Southwest Oncology Group. Observation or radical treatment in patients with prostate cancer; NCT00499174. http://clinicaltrials.gov/ct2/show/NCT00499174? term=prostate%2C+surveillance&rank=12. Accessed December 21, 2010.
51. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11): 1202-1209.
52. Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162(2):433-438.
53. D’Amico AV, Manola J, Loffredo M, et al. 6-Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821-827.
54. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008; 299(23):2760-2769.
55. de Bono JS, Oudard S, Ozguroglu M, et al; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
56. US Food and Drug Administration (FDA news release, June 17, 2010). FDA approves new treatment for advanced prostate cancer. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm216143.htm Accessed December 21, 2010.
57. Cancer and Leukemia Group B, National Cancer Institute, Eastern Cooperative Oncology Group. Docetaxel and prednisone with or without bevacizumab in treating patients with prostate cancer that did not respond to hormone therapy; NCT00110214. http://clinicaltrials.gov/ct2/show/NCT00110214. Accessed December 21, 2010.
58. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12): 1250-1261.
59. Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115(11): 2388-2399.
60. Nanda A, Chen MH, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease–induced congestive heart failure or myocardial infarction. JAMA. 2009;302(8):866-873.
61. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000; 283(3):354-360.
62. Harden J, Northouse L, Cimprich B, et al. The influence of developmental life stage in quality of life in survivors of prostate cancer and their partners. J Cancer Surviv. 2008;2(2):84-94.
63. Potosky A, Legler J, Albertsen P, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92 (19):1582-1592.
64. Tsivian M, Polascik TJ. Focal cryotherapy for prostate cancer. Curr Urol Rep. 2010;11(3):
147-151.
65. Lee HM, Hong JH, Choi HY. High-intensity focused ultrasound therapy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(4):439-443.
66. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349 (3):215-224.
67. Kramer BS, Hagerty KL, Justman S, et al. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Clin Oncol. 2009;27 (9):1502-1516.
68. Schellhammer PF, Higano C, Berger ER, et al; IMPACT Study Investigators. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen-independent prostatic adenocarcinoma (AIPC). Presented at: American Urological Association 104th Annual Scientific Meeting; April 28, 2009; Chicago, IL. Abstract 9.
69. Hamilton RJ, Banez LL, Aronson WJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010; 116(14):3389-3398.
70. Gutt R, Tonlaar N, Kunnavakkam R, et al. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28(16):2653-2659.
71. Kasperzyk JL, Fall K, Mucci LA, et al. One-carbon metabolism-related nutrients and prostate cancer survival. Am J Clin Nutr. 2009;90(3): 561-569.
72. Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1): 52-62.
Effect of High Left Ventricular Ejection Fraction in Older Women
Argyria Associated With Use of Systemic Colloidal Silver
Assessing Health-Related Quality of Life in Veterans With Nonmuscle-Invasive Bladder Cancer
Chronic Obstructive Pulmonary Disease: Focus on Early Diagnosis
Grand Rounds: Girl, 6, With Facial Weakness
A 6-year-old girl was brought to a pediatric emergency department (ED) in Atlanta by her mother. The mother stated that during the previous hour, she had noticed that her daughter’s face seemed weaker on the right side.
The night before, the child had said, “I can’t blink my eye”; when her mother asked her to demonstrate, the child seemed to be able to blink both eyes appropriately, and she had no further complaints. The next morning, the child complained of the light being too bright and asked to wear her mother’s sunglasses. In the course of the day, she continued to complain of eye discomfort, which she described as “stinging” and “sore.” The mother could see nothing abnormal, but by late afternoon noticed that her daughter’s smile and facial movements were asymmetrical. She immediately took her to the pediatric ED.
The child had no significant medical history and no surgical history. Her vaccination schedule was current, and she denied any recent illnesses. The mother could recall no exposures to infections or tick bites, no rashes, and no trauma to the face or head. The mother and child were visiting Atlanta from northeastern Florida.
The review of systems was negative for headache, fever, chills, rash, earache, sore throat, cough, rhinorrhea, vision changes, weight loss, or change in appetite or disposition. The child was afebrile, and the other vital signs were within normal limits.
Physical examination revealed an alert child who was calm and conversant. Her height was 45” and weight, 43 lb. Otoscopic exam showed normal ears and tympanic membranes with no sign of otitis media or ear pathology. No throat redness, tonsillar enlargement, or lymphadenopathies were noted. Breath sounds were clear, and heart rhythm and rate were regular without murmur.
The patient’s left eye appeared normal, and the right eye was mildly erythematic without drainage or swelling; since corneal abrasion was not suspected, a slit lamp examination was not performed. Upon neurologic examination, right eye ptosis with incomplete lid closure, asymmetrical mouth movement with smile, and a diminished nasal labial fold crease were noted on the right side. When the child was asked to raise her eyebrows and wrinkle her forehead, asymmetrical forehead creases were apparent. All other cranial nerve functions were intact, and motor and sensory responses, including gait and reflexes, were assessed as normal. Unilateral dysfunction of right-sided cranial nerve VII (CN VII), including forehead involvement, was confirmed, consistent with a grade of III to IV on the House-Brackmann (maximum, VI)1,2 facial nerve grading scale.
Based on the rapid onset of unilateral facial nerve paresis (FNP) and an otherwise normal exam, the patient was diagnosed with Bell’s palsy. No further testing was done, and the child was given a dose of oral prednisolone 40 mg in the ED, with a prescription for four more days of oral prednisolone at 15 mg bid. The need for eye protection and lubrication was emphasized to the mother, who was given lubricating eye drops to administer. The mother was also instructed to follow up with the child’s primary care practitioner upon their return to Florida.
The child was seen by her pediatrician three days later. Her facial paresis had not worsened in the interim, and the pediatrician declined to extend the course of corticosteroids or to add an antiviral medication. At the mother’s request, the child was referred to a pediatric otolaryngologist, who saw her the following day and adjusted the treatment plan. The child was prescribed prednisolone elixir 20 mg bid for one week, followed by a tapering dose for the second week. In addition, she was prescribed oral acyclovir 400 mg qid for 10 days. Her mother was instructed to return with the child in one week for audiometry testing.
Discussion
Idiopathic FNP, commonly referred to as Bell’s palsy, is defined as an acute unilateral paresis of the facial nerve without detectable underlying cause.3,4 It most commonly occurs among persons ages 15 to 45, with a prevalence rate of 15 to 30 cases per 100,000 persons. The peak incidence of Bell’s palsy is in the fourth decade of life. Diabetic patients and pregnant women are disproportionately affected by idiopathic FNP.2,5 About 8% to 10% of patients will experience a recurrence of Bell’s palsy within 10 years.2,6
Pediatric FNP can be congenital or acquired. Congenital FNP is most often associated with birth trauma and occurs at a rate of 2.1 cases per 1,000 births. Rare genetic syndromes can also manifest with FNP and will most often present with other syndromic anomalies noted at birth.7
Acquired FNP is two to four times less common in children than adults, with an estimated prevalence of 2.7 per 100,000 patients younger than 10. Children account for only a small proportion of subjects in published studies that address diagnosis and management of FNP.3 While the presentation of FNP is much the same in adults and children, some notable differences in etiology exist.2,3,7-9 Infectious, traumatic, or neoplastic causes of FNP are more common among children than adults and must be distinguished from idiopathic FNP.7,9-11
Decisions regarding diagnostic testing, pharmacologic treatment, and referral must be guided by the history and physical exam, neurologic exam, and clinical judgment. Being able to identify or exclude alarming causes of FNP, such as neoplasm, will aid the primary care practitioner in treatment and referral practices for this condition.
Pathophysiology
CN VII, the facial nerve, has a broad scope of function that incorporates both sensory and motor pathways. The brachial nerve portion of CN VII controls the muscles of voluntary facial expression. CN VII also autonomically innervates the lacrimal gland and submandibular gland and governs sensation from part of the ear as well as taste from the anterior two-thirds of the tongue.4
The precise pathophysiology involved in FNP remains an area of continuing debate, but infectious, vascular, immunologic, and genetic causes have been hypothesized.7,12 Inflammation and subsequent nerve damage along CN VII caused by an infectious process is thought to be the most likely explanation for the pathogenesis of acquired FNP in both adults and children.5,13
Herpes simplex virus 1 (HSV-1) has been suggested as the virus most commonly linked to FNP in both adults and children, but it is unlikely to be the sole cause.5,6,9 Data from a three-year prospective study of FNP cases in children support a relationship between pediatric FNP and HSV-1 infection.14 Other infectious causes implicated in pediatric FNP are Lyme disease, Epstein-Barr, varicella zoster virus, rubella, coxsackie virus, adenovirus, and otitis media.4,7,9
Presentation, History, and Physical Exam
Most children with idiopathic FNP will present with sudden-onset facial asymmetry and may have decreased tearing, loss of the conjunctival reflex (leading to difficulty closing the eye), an inability to hold the lips tightly together, and difficulty keeping food in the mouth. Complaints of otalgia, speech disturbances, hyperacusis, and altered sense of taste are common.2,7 Recent occurrence of an upper respiratory infection is often reported in the history of a pediatric patient with FNP.3,7,15,16
Idiopathic FNP is essentially a diagnosis of exclusion.3,5 A meticulous history must be conducted, including any recent illnesses, trauma to the face or head, vaccines, rashes, and travel. Assessment of the head, eyes, ears, nose, and throat, and a careful neurologic history must be conducted to identify nonidiopathic causes of FNP (see Table 15-7,9). Facial weakness can progress from mild palsy to complete paralysis over one to two weeks5; therefore, a careful history of the progression of facial weakness should be ascertained and documented.5,17
A full neurologic exam is essential. Cranial nerves I through XII should be evaluated; any malfunction of a cranial nerve other than CN VII could be indicative of a tumor or process other than idiopathic FNP. Assessment of facial nerve function is imperative, as this factor is the most important for predicting recovery; it can also aid in formulating a prognosis and directing treatment.5,9,17
The House-Brackmann facial nerve grading system1,2 is considered the gold standard for grading severity of facial paresis9 (see Table 21,2 ). A clear distinction between paresis (partial or incomplete palsy) and paralysis (complete palsy) must be made. Pediatric patients with an incomplete palsy have an improved chance of full recovery.17,18
Any abnormalities in the peripheral neurologic exam should prompt further testing. FNP not involving the forehead musculature, gradual progression of paresis, and weakness in any extremity could be indicative of a central lesion. FNP has been the presenting symptom in various neoplastic processes, including leukemia, cholesteatoma, and astrocytoma.3,7,9
Otitis media is a frequent cause of FNP among children.9-11 Thus, a thorough examination of the ear canal, tympanic membrane, and hearing should be performed. The throat and oropharynx should be inspected, and the parotid gland palpated. Any swelling or abnormalities warrant further investigation.
Lyme disease presenting with FNP is more common in children than adults. This may be related to the increased likelihood for children to be bitten by ticks in the head and neck areas. Frequently, FNP associated with Lyme disease is bilateral—as often as 25% of the time.19 Headache, onset of symptoms during peak Lyme season, or bilateral FNP should raise the clinician’s suspicion for Lyme disease.7,9,19
An accurate assessment of blood pressure is essential, as severe hypertension may be implicated in FNP in children.3,5,7 One literature review reported that hypertension was the origin of FNP in 3% to 17% of affected children.20 Vascular hemorrhage induced by hypertension is thought to cause nerve compression and subsequent FNP.7
A bilateral eye exam is also important. Irritation is likely, and the patient with any suspected corneal abrasion or damage should be referred to an ophthalmologist.6,18
Laboratory Testing and Imaging
Diagnostic testing that facilitates the exclusion of known causes of FNP should be considered, as there is no specific laboratory test to confirm the diagnosis. A complete blood count, Lyme titers, cerebrospinal fluid analysis, CT, and/or MRI may be warranted, based on the clinical presentation.7-9 In children in whom Lyme disease is suspected (ie, those living in tick-endemic areas or with recent tick bites), serologic testing should be performed. Lumbar puncture and an evaluation of cerebrospinal fluid may be necessary in cases in which meningitis cannot be excluded.7,9
Specialized diagnostic tests are not routinely recommended for patients with paresis that is improving. Audiometry and evaluation of the stapedial reflex may help guide treatment decisions for patients whose condition is not improving. In children, the presence or return of the stapedial reflex within three weeks of disease onset is predictive of complete recovery.5 In patients who experience complete paralysis or unimproved paresis, results of electrodiagnostic testing (in particular, evoked facial nerve electroneuronography) can help forecast recovery of facial nerve function.5,17
Treatment and Management
Treatment for FNP in adults is controversial, and even more so for the pediatric patient. Treatment decisions consist of eye care, corticosteroids, antiviral medications, and appropriate referrals.
Eye care. Eye lubrication and protection should be implemented immediately. Protecting the cornea is paramount; thorough lubrication of the eye is the mainstay of treatment.18 Artificial tears should be used frequently during the day, and an ointment should be applied to the eye at night. Use of eye patches is controversial, as they may actually cause corneal injury.7,9 Taping the eye shut at night may prevent trauma during sleep, but this option must be considered carefully.9,18
Corticosteroids. Early initiation of corticosteroids should be considered for all patients with FNP, including children.2,7,9,17 Studies are inconclusive as to whether steroid therapy is beneficial in children with idiopathic FNP. However, two 2010 reviews of pediatric FNP recommend early initiation of steroids for children with acute-onset FNP, particularly when facial paresis is evaluated at a House-Brackmann grade V or VI.7,9 The American Academy of Family Physicians (AAFP) recommends a tapering course of prednisone for all patients, begun as soon as possible.6 The prednisone dosage for pediatric patients is usually 1.0 mg/kg/d, split into two doses, for six days, followed by a tapering dose for four days.5
Antivirals and antibiotic therapy. When an infectious cause of FNP is known, appropriate antibiotic or antiviral therapy should begin. If the patient lives in or has traveled to an area endemic for Lyme disease, empiric treatment may be appropriate. When Ramsay Hunt syndrome is diagnosed or herpetic lesions are visible, antiviral treatment should be initiated.7
Antiviral therapy for idiopathic FNP is the most controversial of the treatment decisions. In 2001, the American Academy of Neurology concluded that no clear benefit from acyclovir could be ascertained, although it might be effective.13 This was affirmed in a recently updated Cochrane review of antiviral therapy for idiopathic FNP.12 Antiviral therapy alone showed no benefit, compared with placebo; however, combined antiviral and corticosteroid therapy was more effective than placebo alone in recovery outcomes. Antivirals may benefit pediatric patients and should be considered early when the cause of FNP is viral or idiopathic.7,9
Referrals. Initial presentation and course of paresis should guide referral patterns for the pediatric patient presenting with FNP. The American Academy of Pediatrics (AAP) recommends referral to an otolaryngologist for any infant or child with FNP.21 The AAFP recommends referral to a specialist for any patient who does not show improvement within two weeks.6
In patients with complete paralysis, early surgical intervention may be considered, and referral should be made promptly for electrodiagnostic testing and surgical consult. In cases in which otitis media causes FNP, myringotomy and tube insertion are indicated, and appropriate referral should be made.7,9
Outcomes
|The prognosis in children with FNP is good, and most will recover completely.2,9-11,22 Idiopathic and infectious etiologies of FNP seem to have the greatest likelihood for complete recovery.10,11,16,17 Recovery appears to be affected by etiology, degree of paresis, and treatment. How these factors coalesce is not fully understood, and up to 20% of children may have mild to moderate residual facial nerve dysfunction.10,11,19,22
The Case Patient
The child’s facial nerve function gradually returned over a three-week period, with no residual deficit (see Figures 1a, 1b, and 1c). Results of the audiometry screening on day 10 were normal, showing a positive stapedial reflex. An MRI, performed four months after the initial paralysis to rule out any tumors, yielded normal results.
This case highlights the differing management of pediatric Bell’s palsy among emergency, pediatric, and specialized providers. This child was managed more aggressively under the care of an otolaryngologist with a two-week course of steroids, antiviral medication for 10 days, and a follow-up MRI to rule out any evidence of a tumor. The need for further research to guide practice in the pediatric patient with Bell’s palsy is apparent.
Conclusion
FNP in the pediatric population is rare and more likely to have an identifiable cause than among adults. Careful examination should reveal differential diagnoses that warrant treatment and referrals. The main causes of FNP that should not be missed are otitis media, hypertension, varicella zoster virus (Ramsay Hunt syndrome), neoplastic processes, and Lyme disease.
Practitioners should have a high index of suspicion for nonidiopathic causes of FNP when a child has a neurologic exam that includes facial paresis of gradual onset, abnormal function of other cranial nerves, lack of forehead muscle weakness, or peripheral abnormalities. In addition to the history and exam, blood work and radiologic imaging can aid the practitioner in ruling in or out nonidiopathic causes of FNP.
Grading of facial palsy severity using the House-Brackmann scale helps guide prognosis and referral choices. Referral to a specialist in otolaryngology is appropriate and recommended by the AAP. Referral should be made to an ophthalmologist if any suspicion of corneal abrasion exists.
Treatment in children should consist of eye care and steroids. Antiviral therapy should be considered on an individualized basis and when evidence of HSV or varicella exists. Parents should be advised about the importance of eye care in a child with FNP (see Table 35-7,9,17,18,22).
The emotional stress associated with FNP can be significant for both children and adults; fear of lifelong facial deformity can be psychologically debilitating. Yet a favorable prognosis for recovery of facial nerve function can be relayed to anxious parents.
1. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2): 146-147.
2. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265(7):743-752.
3. Lunan R, Nagarajan L. Bell’s palsy: a guideline proposal following a review of practice. J Paediatr Child Health. 2008;44(4):219-220.
4. Blosser CG, Reider-Demer M. Neurologic disorders. In: Burns CE, Dunn AM, Brady MA, et al, eds. Pediatric Primary Care. 4th ed. St. Louis: Saunders Elsevier; 2008:634-672.
5. Singhi P, Jain V. Bell’s palsy in children. Semin Pediatr Neurol. 2003;10(4):289-297.
6. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
7. Lorch M, Teach SJ. Facial nerve palsy: Etiology and approach to diagnosis and treatment. Pediatr Emerg Care. 2010;26(10):763-769.
8. El-Hawrani AS, Eng CY, Ahmed SK, et al. General practitioners’ referral pattern for children with acute facial paralysis. J Laryngol Otol. 2005;119(7):540-542.
9. Shargorodsky J, Lin HW, Gopen Q. Facial nerve palsy in the pediatric population. Clin Pediatr (Phila). 2010;49(5):411-417.
10. Wang CH, Chang YC, Shih HM, et al. Facial palsy in children: emergency department management and outcome. Pediatr Emerg Care. 2010;26(2):121-125.
11. Evans AK, Licameli G, Brietzke S, et al. Pediatric facial nerve paralysis: patients, management and outcomes. Int J Pediatr Otorhinolaryngol. 2005;69(11):1521-1528.
12. Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2009;(4):CD001869.
13. Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(7):830-836.
14. Khine H, Mayers M, Avner JR, et al. Association between herpes simplex virus-1 infection and idiopathic unilateral facial paralysis in children and adolescents. Pediatr Infect Dis J. 2008;27(5):468-469.
15. Tsai HS, Chang LY, Lu CY, et al. Epidemiology and treatment of Bell’s palsy in children in northern Taiwan. J Microbiol Immunol Infect. 2009;42(4):351-356.
16. Cha CI, Hong CK, Park MS, Yeo SG. Comparison of facial nerve paralysis in adults and children. Yonsei Med J. 2008;49(5):725-734.
17. Linder TE, Abdelkafy W, Cavero-Vanek S. The management of peripheral facial nerve palsy: “paresis” versus “paralysis” and sources of ambiguity in study designs. Otol Neurotol. 2010;31(2):319-327.
18. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: a review. Surv Ophthalmol. 2007;52(2):121-144.
19. Skogman BH, Croner S, Odkvist L. Acute facial palsy in children: a 2-year follow-up with focus on Lyme neuroborreliosis. Int J Pediatr Otorhinolaryngol. 2003;67(6):597-602.
20. Siegler RL, Brewer ED, Corneli HM, Thompson JA. Hypertension first seen as facial paralysis: case reports and review of the literature. Pediatrics. 1991;87(3):387-389.
21. Surgical Advisory Panel, American Academy of Pediatrics. Guidelines for referral to pediatric surgical specialists. Pediatrics. 2002;110(1 pt 1):187-191.
22. Chen WX, Wong V. Prognosis of Bell’s palsy in children: analysis of 29 cases. Brain Dev. 2005; 27(7):504-508.
A 6-year-old girl was brought to a pediatric emergency department (ED) in Atlanta by her mother. The mother stated that during the previous hour, she had noticed that her daughter’s face seemed weaker on the right side.
The night before, the child had said, “I can’t blink my eye”; when her mother asked her to demonstrate, the child seemed to be able to blink both eyes appropriately, and she had no further complaints. The next morning, the child complained of the light being too bright and asked to wear her mother’s sunglasses. In the course of the day, she continued to complain of eye discomfort, which she described as “stinging” and “sore.” The mother could see nothing abnormal, but by late afternoon noticed that her daughter’s smile and facial movements were asymmetrical. She immediately took her to the pediatric ED.
The child had no significant medical history and no surgical history. Her vaccination schedule was current, and she denied any recent illnesses. The mother could recall no exposures to infections or tick bites, no rashes, and no trauma to the face or head. The mother and child were visiting Atlanta from northeastern Florida.
The review of systems was negative for headache, fever, chills, rash, earache, sore throat, cough, rhinorrhea, vision changes, weight loss, or change in appetite or disposition. The child was afebrile, and the other vital signs were within normal limits.
Physical examination revealed an alert child who was calm and conversant. Her height was 45” and weight, 43 lb. Otoscopic exam showed normal ears and tympanic membranes with no sign of otitis media or ear pathology. No throat redness, tonsillar enlargement, or lymphadenopathies were noted. Breath sounds were clear, and heart rhythm and rate were regular without murmur.
The patient’s left eye appeared normal, and the right eye was mildly erythematic without drainage or swelling; since corneal abrasion was not suspected, a slit lamp examination was not performed. Upon neurologic examination, right eye ptosis with incomplete lid closure, asymmetrical mouth movement with smile, and a diminished nasal labial fold crease were noted on the right side. When the child was asked to raise her eyebrows and wrinkle her forehead, asymmetrical forehead creases were apparent. All other cranial nerve functions were intact, and motor and sensory responses, including gait and reflexes, were assessed as normal. Unilateral dysfunction of right-sided cranial nerve VII (CN VII), including forehead involvement, was confirmed, consistent with a grade of III to IV on the House-Brackmann (maximum, VI)1,2 facial nerve grading scale.
Based on the rapid onset of unilateral facial nerve paresis (FNP) and an otherwise normal exam, the patient was diagnosed with Bell’s palsy. No further testing was done, and the child was given a dose of oral prednisolone 40 mg in the ED, with a prescription for four more days of oral prednisolone at 15 mg bid. The need for eye protection and lubrication was emphasized to the mother, who was given lubricating eye drops to administer. The mother was also instructed to follow up with the child’s primary care practitioner upon their return to Florida.
The child was seen by her pediatrician three days later. Her facial paresis had not worsened in the interim, and the pediatrician declined to extend the course of corticosteroids or to add an antiviral medication. At the mother’s request, the child was referred to a pediatric otolaryngologist, who saw her the following day and adjusted the treatment plan. The child was prescribed prednisolone elixir 20 mg bid for one week, followed by a tapering dose for the second week. In addition, she was prescribed oral acyclovir 400 mg qid for 10 days. Her mother was instructed to return with the child in one week for audiometry testing.
Discussion
Idiopathic FNP, commonly referred to as Bell’s palsy, is defined as an acute unilateral paresis of the facial nerve without detectable underlying cause.3,4 It most commonly occurs among persons ages 15 to 45, with a prevalence rate of 15 to 30 cases per 100,000 persons. The peak incidence of Bell’s palsy is in the fourth decade of life. Diabetic patients and pregnant women are disproportionately affected by idiopathic FNP.2,5 About 8% to 10% of patients will experience a recurrence of Bell’s palsy within 10 years.2,6
Pediatric FNP can be congenital or acquired. Congenital FNP is most often associated with birth trauma and occurs at a rate of 2.1 cases per 1,000 births. Rare genetic syndromes can also manifest with FNP and will most often present with other syndromic anomalies noted at birth.7
Acquired FNP is two to four times less common in children than adults, with an estimated prevalence of 2.7 per 100,000 patients younger than 10. Children account for only a small proportion of subjects in published studies that address diagnosis and management of FNP.3 While the presentation of FNP is much the same in adults and children, some notable differences in etiology exist.2,3,7-9 Infectious, traumatic, or neoplastic causes of FNP are more common among children than adults and must be distinguished from idiopathic FNP.7,9-11
Decisions regarding diagnostic testing, pharmacologic treatment, and referral must be guided by the history and physical exam, neurologic exam, and clinical judgment. Being able to identify or exclude alarming causes of FNP, such as neoplasm, will aid the primary care practitioner in treatment and referral practices for this condition.
Pathophysiology
CN VII, the facial nerve, has a broad scope of function that incorporates both sensory and motor pathways. The brachial nerve portion of CN VII controls the muscles of voluntary facial expression. CN VII also autonomically innervates the lacrimal gland and submandibular gland and governs sensation from part of the ear as well as taste from the anterior two-thirds of the tongue.4
The precise pathophysiology involved in FNP remains an area of continuing debate, but infectious, vascular, immunologic, and genetic causes have been hypothesized.7,12 Inflammation and subsequent nerve damage along CN VII caused by an infectious process is thought to be the most likely explanation for the pathogenesis of acquired FNP in both adults and children.5,13
Herpes simplex virus 1 (HSV-1) has been suggested as the virus most commonly linked to FNP in both adults and children, but it is unlikely to be the sole cause.5,6,9 Data from a three-year prospective study of FNP cases in children support a relationship between pediatric FNP and HSV-1 infection.14 Other infectious causes implicated in pediatric FNP are Lyme disease, Epstein-Barr, varicella zoster virus, rubella, coxsackie virus, adenovirus, and otitis media.4,7,9
Presentation, History, and Physical Exam
Most children with idiopathic FNP will present with sudden-onset facial asymmetry and may have decreased tearing, loss of the conjunctival reflex (leading to difficulty closing the eye), an inability to hold the lips tightly together, and difficulty keeping food in the mouth. Complaints of otalgia, speech disturbances, hyperacusis, and altered sense of taste are common.2,7 Recent occurrence of an upper respiratory infection is often reported in the history of a pediatric patient with FNP.3,7,15,16
Idiopathic FNP is essentially a diagnosis of exclusion.3,5 A meticulous history must be conducted, including any recent illnesses, trauma to the face or head, vaccines, rashes, and travel. Assessment of the head, eyes, ears, nose, and throat, and a careful neurologic history must be conducted to identify nonidiopathic causes of FNP (see Table 15-7,9). Facial weakness can progress from mild palsy to complete paralysis over one to two weeks5; therefore, a careful history of the progression of facial weakness should be ascertained and documented.5,17
A full neurologic exam is essential. Cranial nerves I through XII should be evaluated; any malfunction of a cranial nerve other than CN VII could be indicative of a tumor or process other than idiopathic FNP. Assessment of facial nerve function is imperative, as this factor is the most important for predicting recovery; it can also aid in formulating a prognosis and directing treatment.5,9,17
The House-Brackmann facial nerve grading system1,2 is considered the gold standard for grading severity of facial paresis9 (see Table 21,2 ). A clear distinction between paresis (partial or incomplete palsy) and paralysis (complete palsy) must be made. Pediatric patients with an incomplete palsy have an improved chance of full recovery.17,18
Any abnormalities in the peripheral neurologic exam should prompt further testing. FNP not involving the forehead musculature, gradual progression of paresis, and weakness in any extremity could be indicative of a central lesion. FNP has been the presenting symptom in various neoplastic processes, including leukemia, cholesteatoma, and astrocytoma.3,7,9
Otitis media is a frequent cause of FNP among children.9-11 Thus, a thorough examination of the ear canal, tympanic membrane, and hearing should be performed. The throat and oropharynx should be inspected, and the parotid gland palpated. Any swelling or abnormalities warrant further investigation.
Lyme disease presenting with FNP is more common in children than adults. This may be related to the increased likelihood for children to be bitten by ticks in the head and neck areas. Frequently, FNP associated with Lyme disease is bilateral—as often as 25% of the time.19 Headache, onset of symptoms during peak Lyme season, or bilateral FNP should raise the clinician’s suspicion for Lyme disease.7,9,19
An accurate assessment of blood pressure is essential, as severe hypertension may be implicated in FNP in children.3,5,7 One literature review reported that hypertension was the origin of FNP in 3% to 17% of affected children.20 Vascular hemorrhage induced by hypertension is thought to cause nerve compression and subsequent FNP.7
A bilateral eye exam is also important. Irritation is likely, and the patient with any suspected corneal abrasion or damage should be referred to an ophthalmologist.6,18
Laboratory Testing and Imaging
Diagnostic testing that facilitates the exclusion of known causes of FNP should be considered, as there is no specific laboratory test to confirm the diagnosis. A complete blood count, Lyme titers, cerebrospinal fluid analysis, CT, and/or MRI may be warranted, based on the clinical presentation.7-9 In children in whom Lyme disease is suspected (ie, those living in tick-endemic areas or with recent tick bites), serologic testing should be performed. Lumbar puncture and an evaluation of cerebrospinal fluid may be necessary in cases in which meningitis cannot be excluded.7,9
Specialized diagnostic tests are not routinely recommended for patients with paresis that is improving. Audiometry and evaluation of the stapedial reflex may help guide treatment decisions for patients whose condition is not improving. In children, the presence or return of the stapedial reflex within three weeks of disease onset is predictive of complete recovery.5 In patients who experience complete paralysis or unimproved paresis, results of electrodiagnostic testing (in particular, evoked facial nerve electroneuronography) can help forecast recovery of facial nerve function.5,17
Treatment and Management
Treatment for FNP in adults is controversial, and even more so for the pediatric patient. Treatment decisions consist of eye care, corticosteroids, antiviral medications, and appropriate referrals.
Eye care. Eye lubrication and protection should be implemented immediately. Protecting the cornea is paramount; thorough lubrication of the eye is the mainstay of treatment.18 Artificial tears should be used frequently during the day, and an ointment should be applied to the eye at night. Use of eye patches is controversial, as they may actually cause corneal injury.7,9 Taping the eye shut at night may prevent trauma during sleep, but this option must be considered carefully.9,18
Corticosteroids. Early initiation of corticosteroids should be considered for all patients with FNP, including children.2,7,9,17 Studies are inconclusive as to whether steroid therapy is beneficial in children with idiopathic FNP. However, two 2010 reviews of pediatric FNP recommend early initiation of steroids for children with acute-onset FNP, particularly when facial paresis is evaluated at a House-Brackmann grade V or VI.7,9 The American Academy of Family Physicians (AAFP) recommends a tapering course of prednisone for all patients, begun as soon as possible.6 The prednisone dosage for pediatric patients is usually 1.0 mg/kg/d, split into two doses, for six days, followed by a tapering dose for four days.5
Antivirals and antibiotic therapy. When an infectious cause of FNP is known, appropriate antibiotic or antiviral therapy should begin. If the patient lives in or has traveled to an area endemic for Lyme disease, empiric treatment may be appropriate. When Ramsay Hunt syndrome is diagnosed or herpetic lesions are visible, antiviral treatment should be initiated.7
Antiviral therapy for idiopathic FNP is the most controversial of the treatment decisions. In 2001, the American Academy of Neurology concluded that no clear benefit from acyclovir could be ascertained, although it might be effective.13 This was affirmed in a recently updated Cochrane review of antiviral therapy for idiopathic FNP.12 Antiviral therapy alone showed no benefit, compared with placebo; however, combined antiviral and corticosteroid therapy was more effective than placebo alone in recovery outcomes. Antivirals may benefit pediatric patients and should be considered early when the cause of FNP is viral or idiopathic.7,9
Referrals. Initial presentation and course of paresis should guide referral patterns for the pediatric patient presenting with FNP. The American Academy of Pediatrics (AAP) recommends referral to an otolaryngologist for any infant or child with FNP.21 The AAFP recommends referral to a specialist for any patient who does not show improvement within two weeks.6
In patients with complete paralysis, early surgical intervention may be considered, and referral should be made promptly for electrodiagnostic testing and surgical consult. In cases in which otitis media causes FNP, myringotomy and tube insertion are indicated, and appropriate referral should be made.7,9
Outcomes
|The prognosis in children with FNP is good, and most will recover completely.2,9-11,22 Idiopathic and infectious etiologies of FNP seem to have the greatest likelihood for complete recovery.10,11,16,17 Recovery appears to be affected by etiology, degree of paresis, and treatment. How these factors coalesce is not fully understood, and up to 20% of children may have mild to moderate residual facial nerve dysfunction.10,11,19,22
The Case Patient
The child’s facial nerve function gradually returned over a three-week period, with no residual deficit (see Figures 1a, 1b, and 1c). Results of the audiometry screening on day 10 were normal, showing a positive stapedial reflex. An MRI, performed four months after the initial paralysis to rule out any tumors, yielded normal results.
This case highlights the differing management of pediatric Bell’s palsy among emergency, pediatric, and specialized providers. This child was managed more aggressively under the care of an otolaryngologist with a two-week course of steroids, antiviral medication for 10 days, and a follow-up MRI to rule out any evidence of a tumor. The need for further research to guide practice in the pediatric patient with Bell’s palsy is apparent.
Conclusion
FNP in the pediatric population is rare and more likely to have an identifiable cause than among adults. Careful examination should reveal differential diagnoses that warrant treatment and referrals. The main causes of FNP that should not be missed are otitis media, hypertension, varicella zoster virus (Ramsay Hunt syndrome), neoplastic processes, and Lyme disease.
Practitioners should have a high index of suspicion for nonidiopathic causes of FNP when a child has a neurologic exam that includes facial paresis of gradual onset, abnormal function of other cranial nerves, lack of forehead muscle weakness, or peripheral abnormalities. In addition to the history and exam, blood work and radiologic imaging can aid the practitioner in ruling in or out nonidiopathic causes of FNP.
Grading of facial palsy severity using the House-Brackmann scale helps guide prognosis and referral choices. Referral to a specialist in otolaryngology is appropriate and recommended by the AAP. Referral should be made to an ophthalmologist if any suspicion of corneal abrasion exists.
Treatment in children should consist of eye care and steroids. Antiviral therapy should be considered on an individualized basis and when evidence of HSV or varicella exists. Parents should be advised about the importance of eye care in a child with FNP (see Table 35-7,9,17,18,22).
The emotional stress associated with FNP can be significant for both children and adults; fear of lifelong facial deformity can be psychologically debilitating. Yet a favorable prognosis for recovery of facial nerve function can be relayed to anxious parents.
A 6-year-old girl was brought to a pediatric emergency department (ED) in Atlanta by her mother. The mother stated that during the previous hour, she had noticed that her daughter’s face seemed weaker on the right side.
The night before, the child had said, “I can’t blink my eye”; when her mother asked her to demonstrate, the child seemed to be able to blink both eyes appropriately, and she had no further complaints. The next morning, the child complained of the light being too bright and asked to wear her mother’s sunglasses. In the course of the day, she continued to complain of eye discomfort, which she described as “stinging” and “sore.” The mother could see nothing abnormal, but by late afternoon noticed that her daughter’s smile and facial movements were asymmetrical. She immediately took her to the pediatric ED.
The child had no significant medical history and no surgical history. Her vaccination schedule was current, and she denied any recent illnesses. The mother could recall no exposures to infections or tick bites, no rashes, and no trauma to the face or head. The mother and child were visiting Atlanta from northeastern Florida.
The review of systems was negative for headache, fever, chills, rash, earache, sore throat, cough, rhinorrhea, vision changes, weight loss, or change in appetite or disposition. The child was afebrile, and the other vital signs were within normal limits.
Physical examination revealed an alert child who was calm and conversant. Her height was 45” and weight, 43 lb. Otoscopic exam showed normal ears and tympanic membranes with no sign of otitis media or ear pathology. No throat redness, tonsillar enlargement, or lymphadenopathies were noted. Breath sounds were clear, and heart rhythm and rate were regular without murmur.
The patient’s left eye appeared normal, and the right eye was mildly erythematic without drainage or swelling; since corneal abrasion was not suspected, a slit lamp examination was not performed. Upon neurologic examination, right eye ptosis with incomplete lid closure, asymmetrical mouth movement with smile, and a diminished nasal labial fold crease were noted on the right side. When the child was asked to raise her eyebrows and wrinkle her forehead, asymmetrical forehead creases were apparent. All other cranial nerve functions were intact, and motor and sensory responses, including gait and reflexes, were assessed as normal. Unilateral dysfunction of right-sided cranial nerve VII (CN VII), including forehead involvement, was confirmed, consistent with a grade of III to IV on the House-Brackmann (maximum, VI)1,2 facial nerve grading scale.
Based on the rapid onset of unilateral facial nerve paresis (FNP) and an otherwise normal exam, the patient was diagnosed with Bell’s palsy. No further testing was done, and the child was given a dose of oral prednisolone 40 mg in the ED, with a prescription for four more days of oral prednisolone at 15 mg bid. The need for eye protection and lubrication was emphasized to the mother, who was given lubricating eye drops to administer. The mother was also instructed to follow up with the child’s primary care practitioner upon their return to Florida.
The child was seen by her pediatrician three days later. Her facial paresis had not worsened in the interim, and the pediatrician declined to extend the course of corticosteroids or to add an antiviral medication. At the mother’s request, the child was referred to a pediatric otolaryngologist, who saw her the following day and adjusted the treatment plan. The child was prescribed prednisolone elixir 20 mg bid for one week, followed by a tapering dose for the second week. In addition, she was prescribed oral acyclovir 400 mg qid for 10 days. Her mother was instructed to return with the child in one week for audiometry testing.
Discussion
Idiopathic FNP, commonly referred to as Bell’s palsy, is defined as an acute unilateral paresis of the facial nerve without detectable underlying cause.3,4 It most commonly occurs among persons ages 15 to 45, with a prevalence rate of 15 to 30 cases per 100,000 persons. The peak incidence of Bell’s palsy is in the fourth decade of life. Diabetic patients and pregnant women are disproportionately affected by idiopathic FNP.2,5 About 8% to 10% of patients will experience a recurrence of Bell’s palsy within 10 years.2,6
Pediatric FNP can be congenital or acquired. Congenital FNP is most often associated with birth trauma and occurs at a rate of 2.1 cases per 1,000 births. Rare genetic syndromes can also manifest with FNP and will most often present with other syndromic anomalies noted at birth.7
Acquired FNP is two to four times less common in children than adults, with an estimated prevalence of 2.7 per 100,000 patients younger than 10. Children account for only a small proportion of subjects in published studies that address diagnosis and management of FNP.3 While the presentation of FNP is much the same in adults and children, some notable differences in etiology exist.2,3,7-9 Infectious, traumatic, or neoplastic causes of FNP are more common among children than adults and must be distinguished from idiopathic FNP.7,9-11
Decisions regarding diagnostic testing, pharmacologic treatment, and referral must be guided by the history and physical exam, neurologic exam, and clinical judgment. Being able to identify or exclude alarming causes of FNP, such as neoplasm, will aid the primary care practitioner in treatment and referral practices for this condition.
Pathophysiology
CN VII, the facial nerve, has a broad scope of function that incorporates both sensory and motor pathways. The brachial nerve portion of CN VII controls the muscles of voluntary facial expression. CN VII also autonomically innervates the lacrimal gland and submandibular gland and governs sensation from part of the ear as well as taste from the anterior two-thirds of the tongue.4
The precise pathophysiology involved in FNP remains an area of continuing debate, but infectious, vascular, immunologic, and genetic causes have been hypothesized.7,12 Inflammation and subsequent nerve damage along CN VII caused by an infectious process is thought to be the most likely explanation for the pathogenesis of acquired FNP in both adults and children.5,13
Herpes simplex virus 1 (HSV-1) has been suggested as the virus most commonly linked to FNP in both adults and children, but it is unlikely to be the sole cause.5,6,9 Data from a three-year prospective study of FNP cases in children support a relationship between pediatric FNP and HSV-1 infection.14 Other infectious causes implicated in pediatric FNP are Lyme disease, Epstein-Barr, varicella zoster virus, rubella, coxsackie virus, adenovirus, and otitis media.4,7,9
Presentation, History, and Physical Exam
Most children with idiopathic FNP will present with sudden-onset facial asymmetry and may have decreased tearing, loss of the conjunctival reflex (leading to difficulty closing the eye), an inability to hold the lips tightly together, and difficulty keeping food in the mouth. Complaints of otalgia, speech disturbances, hyperacusis, and altered sense of taste are common.2,7 Recent occurrence of an upper respiratory infection is often reported in the history of a pediatric patient with FNP.3,7,15,16
Idiopathic FNP is essentially a diagnosis of exclusion.3,5 A meticulous history must be conducted, including any recent illnesses, trauma to the face or head, vaccines, rashes, and travel. Assessment of the head, eyes, ears, nose, and throat, and a careful neurologic history must be conducted to identify nonidiopathic causes of FNP (see Table 15-7,9). Facial weakness can progress from mild palsy to complete paralysis over one to two weeks5; therefore, a careful history of the progression of facial weakness should be ascertained and documented.5,17
A full neurologic exam is essential. Cranial nerves I through XII should be evaluated; any malfunction of a cranial nerve other than CN VII could be indicative of a tumor or process other than idiopathic FNP. Assessment of facial nerve function is imperative, as this factor is the most important for predicting recovery; it can also aid in formulating a prognosis and directing treatment.5,9,17
The House-Brackmann facial nerve grading system1,2 is considered the gold standard for grading severity of facial paresis9 (see Table 21,2 ). A clear distinction between paresis (partial or incomplete palsy) and paralysis (complete palsy) must be made. Pediatric patients with an incomplete palsy have an improved chance of full recovery.17,18
Any abnormalities in the peripheral neurologic exam should prompt further testing. FNP not involving the forehead musculature, gradual progression of paresis, and weakness in any extremity could be indicative of a central lesion. FNP has been the presenting symptom in various neoplastic processes, including leukemia, cholesteatoma, and astrocytoma.3,7,9
Otitis media is a frequent cause of FNP among children.9-11 Thus, a thorough examination of the ear canal, tympanic membrane, and hearing should be performed. The throat and oropharynx should be inspected, and the parotid gland palpated. Any swelling or abnormalities warrant further investigation.
Lyme disease presenting with FNP is more common in children than adults. This may be related to the increased likelihood for children to be bitten by ticks in the head and neck areas. Frequently, FNP associated with Lyme disease is bilateral—as often as 25% of the time.19 Headache, onset of symptoms during peak Lyme season, or bilateral FNP should raise the clinician’s suspicion for Lyme disease.7,9,19
An accurate assessment of blood pressure is essential, as severe hypertension may be implicated in FNP in children.3,5,7 One literature review reported that hypertension was the origin of FNP in 3% to 17% of affected children.20 Vascular hemorrhage induced by hypertension is thought to cause nerve compression and subsequent FNP.7
A bilateral eye exam is also important. Irritation is likely, and the patient with any suspected corneal abrasion or damage should be referred to an ophthalmologist.6,18
Laboratory Testing and Imaging
Diagnostic testing that facilitates the exclusion of known causes of FNP should be considered, as there is no specific laboratory test to confirm the diagnosis. A complete blood count, Lyme titers, cerebrospinal fluid analysis, CT, and/or MRI may be warranted, based on the clinical presentation.7-9 In children in whom Lyme disease is suspected (ie, those living in tick-endemic areas or with recent tick bites), serologic testing should be performed. Lumbar puncture and an evaluation of cerebrospinal fluid may be necessary in cases in which meningitis cannot be excluded.7,9
Specialized diagnostic tests are not routinely recommended for patients with paresis that is improving. Audiometry and evaluation of the stapedial reflex may help guide treatment decisions for patients whose condition is not improving. In children, the presence or return of the stapedial reflex within three weeks of disease onset is predictive of complete recovery.5 In patients who experience complete paralysis or unimproved paresis, results of electrodiagnostic testing (in particular, evoked facial nerve electroneuronography) can help forecast recovery of facial nerve function.5,17
Treatment and Management
Treatment for FNP in adults is controversial, and even more so for the pediatric patient. Treatment decisions consist of eye care, corticosteroids, antiviral medications, and appropriate referrals.
Eye care. Eye lubrication and protection should be implemented immediately. Protecting the cornea is paramount; thorough lubrication of the eye is the mainstay of treatment.18 Artificial tears should be used frequently during the day, and an ointment should be applied to the eye at night. Use of eye patches is controversial, as they may actually cause corneal injury.7,9 Taping the eye shut at night may prevent trauma during sleep, but this option must be considered carefully.9,18
Corticosteroids. Early initiation of corticosteroids should be considered for all patients with FNP, including children.2,7,9,17 Studies are inconclusive as to whether steroid therapy is beneficial in children with idiopathic FNP. However, two 2010 reviews of pediatric FNP recommend early initiation of steroids for children with acute-onset FNP, particularly when facial paresis is evaluated at a House-Brackmann grade V or VI.7,9 The American Academy of Family Physicians (AAFP) recommends a tapering course of prednisone for all patients, begun as soon as possible.6 The prednisone dosage for pediatric patients is usually 1.0 mg/kg/d, split into two doses, for six days, followed by a tapering dose for four days.5
Antivirals and antibiotic therapy. When an infectious cause of FNP is known, appropriate antibiotic or antiviral therapy should begin. If the patient lives in or has traveled to an area endemic for Lyme disease, empiric treatment may be appropriate. When Ramsay Hunt syndrome is diagnosed or herpetic lesions are visible, antiviral treatment should be initiated.7
Antiviral therapy for idiopathic FNP is the most controversial of the treatment decisions. In 2001, the American Academy of Neurology concluded that no clear benefit from acyclovir could be ascertained, although it might be effective.13 This was affirmed in a recently updated Cochrane review of antiviral therapy for idiopathic FNP.12 Antiviral therapy alone showed no benefit, compared with placebo; however, combined antiviral and corticosteroid therapy was more effective than placebo alone in recovery outcomes. Antivirals may benefit pediatric patients and should be considered early when the cause of FNP is viral or idiopathic.7,9
Referrals. Initial presentation and course of paresis should guide referral patterns for the pediatric patient presenting with FNP. The American Academy of Pediatrics (AAP) recommends referral to an otolaryngologist for any infant or child with FNP.21 The AAFP recommends referral to a specialist for any patient who does not show improvement within two weeks.6
In patients with complete paralysis, early surgical intervention may be considered, and referral should be made promptly for electrodiagnostic testing and surgical consult. In cases in which otitis media causes FNP, myringotomy and tube insertion are indicated, and appropriate referral should be made.7,9
Outcomes
|The prognosis in children with FNP is good, and most will recover completely.2,9-11,22 Idiopathic and infectious etiologies of FNP seem to have the greatest likelihood for complete recovery.10,11,16,17 Recovery appears to be affected by etiology, degree of paresis, and treatment. How these factors coalesce is not fully understood, and up to 20% of children may have mild to moderate residual facial nerve dysfunction.10,11,19,22
The Case Patient
The child’s facial nerve function gradually returned over a three-week period, with no residual deficit (see Figures 1a, 1b, and 1c). Results of the audiometry screening on day 10 were normal, showing a positive stapedial reflex. An MRI, performed four months after the initial paralysis to rule out any tumors, yielded normal results.
This case highlights the differing management of pediatric Bell’s palsy among emergency, pediatric, and specialized providers. This child was managed more aggressively under the care of an otolaryngologist with a two-week course of steroids, antiviral medication for 10 days, and a follow-up MRI to rule out any evidence of a tumor. The need for further research to guide practice in the pediatric patient with Bell’s palsy is apparent.
Conclusion
FNP in the pediatric population is rare and more likely to have an identifiable cause than among adults. Careful examination should reveal differential diagnoses that warrant treatment and referrals. The main causes of FNP that should not be missed are otitis media, hypertension, varicella zoster virus (Ramsay Hunt syndrome), neoplastic processes, and Lyme disease.
Practitioners should have a high index of suspicion for nonidiopathic causes of FNP when a child has a neurologic exam that includes facial paresis of gradual onset, abnormal function of other cranial nerves, lack of forehead muscle weakness, or peripheral abnormalities. In addition to the history and exam, blood work and radiologic imaging can aid the practitioner in ruling in or out nonidiopathic causes of FNP.
Grading of facial palsy severity using the House-Brackmann scale helps guide prognosis and referral choices. Referral to a specialist in otolaryngology is appropriate and recommended by the AAP. Referral should be made to an ophthalmologist if any suspicion of corneal abrasion exists.
Treatment in children should consist of eye care and steroids. Antiviral therapy should be considered on an individualized basis and when evidence of HSV or varicella exists. Parents should be advised about the importance of eye care in a child with FNP (see Table 35-7,9,17,18,22).
The emotional stress associated with FNP can be significant for both children and adults; fear of lifelong facial deformity can be psychologically debilitating. Yet a favorable prognosis for recovery of facial nerve function can be relayed to anxious parents.
1. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2): 146-147.
2. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265(7):743-752.
3. Lunan R, Nagarajan L. Bell’s palsy: a guideline proposal following a review of practice. J Paediatr Child Health. 2008;44(4):219-220.
4. Blosser CG, Reider-Demer M. Neurologic disorders. In: Burns CE, Dunn AM, Brady MA, et al, eds. Pediatric Primary Care. 4th ed. St. Louis: Saunders Elsevier; 2008:634-672.
5. Singhi P, Jain V. Bell’s palsy in children. Semin Pediatr Neurol. 2003;10(4):289-297.
6. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
7. Lorch M, Teach SJ. Facial nerve palsy: Etiology and approach to diagnosis and treatment. Pediatr Emerg Care. 2010;26(10):763-769.
8. El-Hawrani AS, Eng CY, Ahmed SK, et al. General practitioners’ referral pattern for children with acute facial paralysis. J Laryngol Otol. 2005;119(7):540-542.
9. Shargorodsky J, Lin HW, Gopen Q. Facial nerve palsy in the pediatric population. Clin Pediatr (Phila). 2010;49(5):411-417.
10. Wang CH, Chang YC, Shih HM, et al. Facial palsy in children: emergency department management and outcome. Pediatr Emerg Care. 2010;26(2):121-125.
11. Evans AK, Licameli G, Brietzke S, et al. Pediatric facial nerve paralysis: patients, management and outcomes. Int J Pediatr Otorhinolaryngol. 2005;69(11):1521-1528.
12. Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2009;(4):CD001869.
13. Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(7):830-836.
14. Khine H, Mayers M, Avner JR, et al. Association between herpes simplex virus-1 infection and idiopathic unilateral facial paralysis in children and adolescents. Pediatr Infect Dis J. 2008;27(5):468-469.
15. Tsai HS, Chang LY, Lu CY, et al. Epidemiology and treatment of Bell’s palsy in children in northern Taiwan. J Microbiol Immunol Infect. 2009;42(4):351-356.
16. Cha CI, Hong CK, Park MS, Yeo SG. Comparison of facial nerve paralysis in adults and children. Yonsei Med J. 2008;49(5):725-734.
17. Linder TE, Abdelkafy W, Cavero-Vanek S. The management of peripheral facial nerve palsy: “paresis” versus “paralysis” and sources of ambiguity in study designs. Otol Neurotol. 2010;31(2):319-327.
18. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: a review. Surv Ophthalmol. 2007;52(2):121-144.
19. Skogman BH, Croner S, Odkvist L. Acute facial palsy in children: a 2-year follow-up with focus on Lyme neuroborreliosis. Int J Pediatr Otorhinolaryngol. 2003;67(6):597-602.
20. Siegler RL, Brewer ED, Corneli HM, Thompson JA. Hypertension first seen as facial paralysis: case reports and review of the literature. Pediatrics. 1991;87(3):387-389.
21. Surgical Advisory Panel, American Academy of Pediatrics. Guidelines for referral to pediatric surgical specialists. Pediatrics. 2002;110(1 pt 1):187-191.
22. Chen WX, Wong V. Prognosis of Bell’s palsy in children: analysis of 29 cases. Brain Dev. 2005; 27(7):504-508.
1. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2): 146-147.
2. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265(7):743-752.
3. Lunan R, Nagarajan L. Bell’s palsy: a guideline proposal following a review of practice. J Paediatr Child Health. 2008;44(4):219-220.
4. Blosser CG, Reider-Demer M. Neurologic disorders. In: Burns CE, Dunn AM, Brady MA, et al, eds. Pediatric Primary Care. 4th ed. St. Louis: Saunders Elsevier; 2008:634-672.
5. Singhi P, Jain V. Bell’s palsy in children. Semin Pediatr Neurol. 2003;10(4):289-297.
6. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
7. Lorch M, Teach SJ. Facial nerve palsy: Etiology and approach to diagnosis and treatment. Pediatr Emerg Care. 2010;26(10):763-769.
8. El-Hawrani AS, Eng CY, Ahmed SK, et al. General practitioners’ referral pattern for children with acute facial paralysis. J Laryngol Otol. 2005;119(7):540-542.
9. Shargorodsky J, Lin HW, Gopen Q. Facial nerve palsy in the pediatric population. Clin Pediatr (Phila). 2010;49(5):411-417.
10. Wang CH, Chang YC, Shih HM, et al. Facial palsy in children: emergency department management and outcome. Pediatr Emerg Care. 2010;26(2):121-125.
11. Evans AK, Licameli G, Brietzke S, et al. Pediatric facial nerve paralysis: patients, management and outcomes. Int J Pediatr Otorhinolaryngol. 2005;69(11):1521-1528.
12. Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2009;(4):CD001869.
13. Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(7):830-836.
14. Khine H, Mayers M, Avner JR, et al. Association between herpes simplex virus-1 infection and idiopathic unilateral facial paralysis in children and adolescents. Pediatr Infect Dis J. 2008;27(5):468-469.
15. Tsai HS, Chang LY, Lu CY, et al. Epidemiology and treatment of Bell’s palsy in children in northern Taiwan. J Microbiol Immunol Infect. 2009;42(4):351-356.
16. Cha CI, Hong CK, Park MS, Yeo SG. Comparison of facial nerve paralysis in adults and children. Yonsei Med J. 2008;49(5):725-734.
17. Linder TE, Abdelkafy W, Cavero-Vanek S. The management of peripheral facial nerve palsy: “paresis” versus “paralysis” and sources of ambiguity in study designs. Otol Neurotol. 2010;31(2):319-327.
18. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: a review. Surv Ophthalmol. 2007;52(2):121-144.
19. Skogman BH, Croner S, Odkvist L. Acute facial palsy in children: a 2-year follow-up with focus on Lyme neuroborreliosis. Int J Pediatr Otorhinolaryngol. 2003;67(6):597-602.
20. Siegler RL, Brewer ED, Corneli HM, Thompson JA. Hypertension first seen as facial paralysis: case reports and review of the literature. Pediatrics. 1991;87(3):387-389.
21. Surgical Advisory Panel, American Academy of Pediatrics. Guidelines for referral to pediatric surgical specialists. Pediatrics. 2002;110(1 pt 1):187-191.
22. Chen WX, Wong V. Prognosis of Bell’s palsy in children: analysis of 29 cases. Brain Dev. 2005; 27(7):504-508.
Inhalation of Baby Powder
Emergency Management of Cold-Induced Injuries
Treatment Options for Symptomatic Degenerative Joint Disease Secondary to Legg-Calvé-Perthes Disease
UPDATE ON OBSTETRICS
- How to manage a short cervix to lower the risk of preterm delivery
Joseph R. Wax, MD (May 2010) - When is VBAC appropriate?
Aviva Lee-Parritz, MD (July 2010) - What can be safer than having a baby in the USA? (Commentary)
Louis L. Weinstein, MD (May 2010)
From an evolutionary standpoint, not much has changed in pregnancy and childbirth. From a clinical perspective, however, flux is a constant. Three issues, in particular, have seen notable development over the past year:
- optimal timing of elective delivery
- screening for thrombophilias in women who experience recurrent pregnancy loss, fetal growth restriction, preeclampsia, or placental abruption
- use of magnesium sulfate for fetal neuroprotection.
Of course, in the specialties of obstetrics and perinatal medicine, research continues in a variety of other subject areas, as well. Simulation training, diagnosis and management of gestational diabetes, and rescue steroid treatment are three examples. Other issues being explored include the use of progesterone to prevent prematurity, the use of ultrasonography to measure cervical length, and the safety of vaginal birth after cesarean delivery. The three areas highlighted here are not the only ones “ready for prime time,” but they are areas of considerable interest and debate.
We have a tradition in obstetrics of not embracing change too quickly. We learned this lesson through our experience with diethylstilbestrol (DES) and thalidomide, and we must continue to use caution whenever new technologies or management approaches are proposed.
30 weeks is the rule, provided delivery is truly elective
Tita ATN, Landon MB, Spong CY, et al. Timing of elective cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
When it comes to elective delivery, no one would argue against the wisdom of continuing pregnancy until at least 39 weeks’ gestation in the absence of complications. But what data form the basis of this wisdom, and when might it be prudent to consider earlier delivery?
In a widely publicized study, Tita and colleagues concluded that elective repeat cesarean delivery before 39 weeks of gestation (i.e., 37 through 38-6/7 weeks) is associated with a higher rate of neonatal respiratory distress and other adverse neonatal outcomes than is delivery at 39 to 40 weeks. Note, however, that the primary outcome of this study was a composite. Therefore, the findings should be interpreted with some caution.
In their report, Tita and coworkers acknowledged that the transient and predominantly minor complications associated with delivery before 39 weeks must be weighed against the risk of fetal death inherent in delaying delivery through 38 full weeks—and an accompanying editorial made the same point.1 Stillbirth occurs at a rate of 1 case for every 1,000 births in the 37- to 39-week gestational age range—a rate that may be higher than the risks associated with delivery. Even so, the risk of stillbirth at 37 to 39 weeks is very small, and that risk is unlikely to be lowered through routine antenatal fetal testing. We should also remember that the risks of neonatal respiratory distress, transient tachypnea, admission to the neonatal intensive care unit (NICU), and even cerebral palsy2 may be increased with delivery at 37 to 38 weeks, or at 42 weeks or later, compared with delivery at 40 weeks.
All truly elective deliveries should occur at or after 39 weeks of gestation. However, when indicated, earlier delivery is acceptable—even essential—if we are to minimize maternal and neonatal morbidity and mortality in high-risk circumstances, such as hypertensive disorders of pregnancy, placenta previa, fetal growth restriction, and other conditions.
Investigations are under way to determine whether there is a role for routine betamethasone administration (regardless of indication or gestational age) in the absence of labor before 39 weeks. Until those data come in, we should continue to follow current practice guidelines for antenatal maternal administration of betamethasone—namely, a single course given between 24 and 34 weeks in women who have an elevated risk of preterm delivery.
Population-based screening for thrombophilias is not recommended
Silver RM, Zhao Y, Spong CY, et al, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (NICHD MFMU) Network. Prothrombin gene G20210A mutation and obstetric complications. Obstet Gynecol. 2010;115(1):14–20.
Said JM, Higgins JR, Moses EK, et al. Inherited thrombophilia polymorphisms and pregnancy outcomes in nulliparous women. Obstet Gynecol. 2010;115(1):5–13.
Since the mid-1990s, screening for thrombophilias has been recommended in the evaluation of a variety of adverse reproductive outcomes, including, but not limited to:
- recurrent pregnancy loss
- unexplained stillbirth
- placental abruption
- preeclampsia
- fetal growth restriction.
When a thrombophilia is detected in these settings, the practitioner faces a dilemma—namely, what to do when the mother is otherwise healthy and asymptomatic. All too often the finding of a thrombophilia leads to the initiation of some anticoagulation regimen, ranging from low-dose aspirin all the way to therapeutic anticoagulation with heparin.
Over the past year, several studies and expert opinions have been published that recast the role of thrombophilia screening in obstetric practice.
Writing for the Maternal-Fetal Medicine Units (MFMU) Network, Silver and colleagues concluded that the prothrombin (PT) gene mutation G20210A was not associated with pregnancy loss, preeclampsia, fetal growth restriction, or placental abruption in a low-risk, prospective cohort.
Said and coworkers reached a similar conclusion. In a blinded, prospective cohort study, they screened for the following mutations in 2,034 healthy nulliparous women before 22 weeks’ gestation:
- factor V Leiden mutation
- PT gene G20210A mutation
- methylenetetrahydrofolate reductase enzyme (MTHFR) C677T
- MTHFR A1298C
- thrombomodulin polymorphism.
The majority of asymptomatic women who carried an inherited thrombophilia polymorphism had a successful pregnancy outcome. In fact, homozygosity of the MTHFR A1298C mutation was found to be protective.
Population-based screening for thrombophilias is not recommended. In fact, some authors have advised against screening for thrombophilias even in the setting of a thrombotic event, suggesting it has limited utility.3
The main reason to screen for a thrombophilia at this time is to explore idiopathic thrombosis or a strong family history of the same. There is no need to screen for thrombophilias when the patient has a history of pregnancy loss, placental abruption, preeclampsia, and fetal growth restriction.
If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol
ACOG Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115(3):669–671.
Magnesium sulfate has a long and glorious history in obstetrics, having been used to prevent eclampsia, to treat preterm uterine contractions, and now, potentially, to reduce the incidence of central nervous system damage among prematurely delivered infants.
Cerebral palsy (CP) is most commonly associated with prematurity and intrauterine fetal infection. Only in the past two decades has there been a shift away from assigning the cause of most cases of CP to intrapartum events. Nevertheless, CP remains an important concern among patients and providers, and research continues to find ways to prevent CP or minimize its effects.
Numerous large trials have explored the use of magnesium sulfate to prevent CP in preterm infants. Although their findings have not been as definitive as researchers had hoped, some data do suggest that magnesium sulfate has a protective effect.
The American Congress of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) urge caution in regard to the use of magnesium sulfate for fetal neuroprotection. The SMFM points out that the reported benefits of magnesium sulfate in this setting have been derived largely from secondary analyses. The SMFM recommends that, if magnesium is used at all, it should be administered according to one of the published protocols (three are cited in the ACOG opinion).
The SMFM goes on to warn against choosing magnesium sulfate as a tocolytic solely because of its possible neuroprotective effects.
In a Committee Opinion published last year, ACOG was a bit more definite. “The Committee on Obstetric Practice and the Society for Maternal-Fetal Medicine recognize that none of the individual studies found a benefit with regard to their primary outcome,” the opinion states. “However, the available evidence suggests that magnesium sulfate given before anticipated early preterm birth reduces the risk of cerebral palsy in surviving infants. Physicians electing to use magnesium sulfate for fetal neuroprotection should develop specific guidelines regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in concordance with one of the larger trials.”
Recent editorials have cautioned against using magnesium sulfate for fetal neuroprotection until more data become available,4 or have left it up to the individual practitioners (or institution) to decide whether it is advisable.5
The use of magnesium sulfate for fetal neuroprotection when preterm delivery seems likely requires additional research. For now, this practice should be governed strictly by protocol. And it should not be viewed as “standard of care” by our legal colleagues.
When administering magnesium sulfate, avoid giving a cumulative total in excess of 50 g, as this amount may increase the risk of pediatric death.6
We want to hear from you! Tell us what you think.
1. Greene MF. Making small risks even smaller. N Engl J Med. 2009;360(2):183-184.
2. Moster D, Wilcox AJ, Vollset SE, Markestad T, Lie RT. Cerebral palsy among term and postterm births. JAMA. 2010;304(9):976-982.
3. Scifres CM, Macones GA. The utility of thrombophilia testing in pregnant women with thrombosis; fact or fiction? Am J Obstet Gynecol. 2008;199(4):344.e1–7.-
4. Stanley FJ, Crowther C. Antenatal magnesium sulfate for neuroprotection before preterm birth? N Engl J Med. 2008;359(9):962-964.
5. Macones GA. MgSO4 for CP prevention: too good to be true? Am J Obstet Gynecol. 2009;200(6):589.-
6. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet. 1997;350(9090):1517-1518.
- How to manage a short cervix to lower the risk of preterm delivery
Joseph R. Wax, MD (May 2010) - When is VBAC appropriate?
Aviva Lee-Parritz, MD (July 2010) - What can be safer than having a baby in the USA? (Commentary)
Louis L. Weinstein, MD (May 2010)
From an evolutionary standpoint, not much has changed in pregnancy and childbirth. From a clinical perspective, however, flux is a constant. Three issues, in particular, have seen notable development over the past year:
- optimal timing of elective delivery
- screening for thrombophilias in women who experience recurrent pregnancy loss, fetal growth restriction, preeclampsia, or placental abruption
- use of magnesium sulfate for fetal neuroprotection.
Of course, in the specialties of obstetrics and perinatal medicine, research continues in a variety of other subject areas, as well. Simulation training, diagnosis and management of gestational diabetes, and rescue steroid treatment are three examples. Other issues being explored include the use of progesterone to prevent prematurity, the use of ultrasonography to measure cervical length, and the safety of vaginal birth after cesarean delivery. The three areas highlighted here are not the only ones “ready for prime time,” but they are areas of considerable interest and debate.
We have a tradition in obstetrics of not embracing change too quickly. We learned this lesson through our experience with diethylstilbestrol (DES) and thalidomide, and we must continue to use caution whenever new technologies or management approaches are proposed.
30 weeks is the rule, provided delivery is truly elective
Tita ATN, Landon MB, Spong CY, et al. Timing of elective cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
When it comes to elective delivery, no one would argue against the wisdom of continuing pregnancy until at least 39 weeks’ gestation in the absence of complications. But what data form the basis of this wisdom, and when might it be prudent to consider earlier delivery?
In a widely publicized study, Tita and colleagues concluded that elective repeat cesarean delivery before 39 weeks of gestation (i.e., 37 through 38-6/7 weeks) is associated with a higher rate of neonatal respiratory distress and other adverse neonatal outcomes than is delivery at 39 to 40 weeks. Note, however, that the primary outcome of this study was a composite. Therefore, the findings should be interpreted with some caution.
In their report, Tita and coworkers acknowledged that the transient and predominantly minor complications associated with delivery before 39 weeks must be weighed against the risk of fetal death inherent in delaying delivery through 38 full weeks—and an accompanying editorial made the same point.1 Stillbirth occurs at a rate of 1 case for every 1,000 births in the 37- to 39-week gestational age range—a rate that may be higher than the risks associated with delivery. Even so, the risk of stillbirth at 37 to 39 weeks is very small, and that risk is unlikely to be lowered through routine antenatal fetal testing. We should also remember that the risks of neonatal respiratory distress, transient tachypnea, admission to the neonatal intensive care unit (NICU), and even cerebral palsy2 may be increased with delivery at 37 to 38 weeks, or at 42 weeks or later, compared with delivery at 40 weeks.
All truly elective deliveries should occur at or after 39 weeks of gestation. However, when indicated, earlier delivery is acceptable—even essential—if we are to minimize maternal and neonatal morbidity and mortality in high-risk circumstances, such as hypertensive disorders of pregnancy, placenta previa, fetal growth restriction, and other conditions.
Investigations are under way to determine whether there is a role for routine betamethasone administration (regardless of indication or gestational age) in the absence of labor before 39 weeks. Until those data come in, we should continue to follow current practice guidelines for antenatal maternal administration of betamethasone—namely, a single course given between 24 and 34 weeks in women who have an elevated risk of preterm delivery.
Population-based screening for thrombophilias is not recommended
Silver RM, Zhao Y, Spong CY, et al, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (NICHD MFMU) Network. Prothrombin gene G20210A mutation and obstetric complications. Obstet Gynecol. 2010;115(1):14–20.
Said JM, Higgins JR, Moses EK, et al. Inherited thrombophilia polymorphisms and pregnancy outcomes in nulliparous women. Obstet Gynecol. 2010;115(1):5–13.
Since the mid-1990s, screening for thrombophilias has been recommended in the evaluation of a variety of adverse reproductive outcomes, including, but not limited to:
- recurrent pregnancy loss
- unexplained stillbirth
- placental abruption
- preeclampsia
- fetal growth restriction.
When a thrombophilia is detected in these settings, the practitioner faces a dilemma—namely, what to do when the mother is otherwise healthy and asymptomatic. All too often the finding of a thrombophilia leads to the initiation of some anticoagulation regimen, ranging from low-dose aspirin all the way to therapeutic anticoagulation with heparin.
Over the past year, several studies and expert opinions have been published that recast the role of thrombophilia screening in obstetric practice.
Writing for the Maternal-Fetal Medicine Units (MFMU) Network, Silver and colleagues concluded that the prothrombin (PT) gene mutation G20210A was not associated with pregnancy loss, preeclampsia, fetal growth restriction, or placental abruption in a low-risk, prospective cohort.
Said and coworkers reached a similar conclusion. In a blinded, prospective cohort study, they screened for the following mutations in 2,034 healthy nulliparous women before 22 weeks’ gestation:
- factor V Leiden mutation
- PT gene G20210A mutation
- methylenetetrahydrofolate reductase enzyme (MTHFR) C677T
- MTHFR A1298C
- thrombomodulin polymorphism.
The majority of asymptomatic women who carried an inherited thrombophilia polymorphism had a successful pregnancy outcome. In fact, homozygosity of the MTHFR A1298C mutation was found to be protective.
Population-based screening for thrombophilias is not recommended. In fact, some authors have advised against screening for thrombophilias even in the setting of a thrombotic event, suggesting it has limited utility.3
The main reason to screen for a thrombophilia at this time is to explore idiopathic thrombosis or a strong family history of the same. There is no need to screen for thrombophilias when the patient has a history of pregnancy loss, placental abruption, preeclampsia, and fetal growth restriction.
If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol
ACOG Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115(3):669–671.
Magnesium sulfate has a long and glorious history in obstetrics, having been used to prevent eclampsia, to treat preterm uterine contractions, and now, potentially, to reduce the incidence of central nervous system damage among prematurely delivered infants.
Cerebral palsy (CP) is most commonly associated with prematurity and intrauterine fetal infection. Only in the past two decades has there been a shift away from assigning the cause of most cases of CP to intrapartum events. Nevertheless, CP remains an important concern among patients and providers, and research continues to find ways to prevent CP or minimize its effects.
Numerous large trials have explored the use of magnesium sulfate to prevent CP in preterm infants. Although their findings have not been as definitive as researchers had hoped, some data do suggest that magnesium sulfate has a protective effect.
The American Congress of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) urge caution in regard to the use of magnesium sulfate for fetal neuroprotection. The SMFM points out that the reported benefits of magnesium sulfate in this setting have been derived largely from secondary analyses. The SMFM recommends that, if magnesium is used at all, it should be administered according to one of the published protocols (three are cited in the ACOG opinion).
The SMFM goes on to warn against choosing magnesium sulfate as a tocolytic solely because of its possible neuroprotective effects.
In a Committee Opinion published last year, ACOG was a bit more definite. “The Committee on Obstetric Practice and the Society for Maternal-Fetal Medicine recognize that none of the individual studies found a benefit with regard to their primary outcome,” the opinion states. “However, the available evidence suggests that magnesium sulfate given before anticipated early preterm birth reduces the risk of cerebral palsy in surviving infants. Physicians electing to use magnesium sulfate for fetal neuroprotection should develop specific guidelines regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in concordance with one of the larger trials.”
Recent editorials have cautioned against using magnesium sulfate for fetal neuroprotection until more data become available,4 or have left it up to the individual practitioners (or institution) to decide whether it is advisable.5
The use of magnesium sulfate for fetal neuroprotection when preterm delivery seems likely requires additional research. For now, this practice should be governed strictly by protocol. And it should not be viewed as “standard of care” by our legal colleagues.
When administering magnesium sulfate, avoid giving a cumulative total in excess of 50 g, as this amount may increase the risk of pediatric death.6
We want to hear from you! Tell us what you think.
- How to manage a short cervix to lower the risk of preterm delivery
Joseph R. Wax, MD (May 2010) - When is VBAC appropriate?
Aviva Lee-Parritz, MD (July 2010) - What can be safer than having a baby in the USA? (Commentary)
Louis L. Weinstein, MD (May 2010)
From an evolutionary standpoint, not much has changed in pregnancy and childbirth. From a clinical perspective, however, flux is a constant. Three issues, in particular, have seen notable development over the past year:
- optimal timing of elective delivery
- screening for thrombophilias in women who experience recurrent pregnancy loss, fetal growth restriction, preeclampsia, or placental abruption
- use of magnesium sulfate for fetal neuroprotection.
Of course, in the specialties of obstetrics and perinatal medicine, research continues in a variety of other subject areas, as well. Simulation training, diagnosis and management of gestational diabetes, and rescue steroid treatment are three examples. Other issues being explored include the use of progesterone to prevent prematurity, the use of ultrasonography to measure cervical length, and the safety of vaginal birth after cesarean delivery. The three areas highlighted here are not the only ones “ready for prime time,” but they are areas of considerable interest and debate.
We have a tradition in obstetrics of not embracing change too quickly. We learned this lesson through our experience with diethylstilbestrol (DES) and thalidomide, and we must continue to use caution whenever new technologies or management approaches are proposed.
30 weeks is the rule, provided delivery is truly elective
Tita ATN, Landon MB, Spong CY, et al. Timing of elective cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
When it comes to elective delivery, no one would argue against the wisdom of continuing pregnancy until at least 39 weeks’ gestation in the absence of complications. But what data form the basis of this wisdom, and when might it be prudent to consider earlier delivery?
In a widely publicized study, Tita and colleagues concluded that elective repeat cesarean delivery before 39 weeks of gestation (i.e., 37 through 38-6/7 weeks) is associated with a higher rate of neonatal respiratory distress and other adverse neonatal outcomes than is delivery at 39 to 40 weeks. Note, however, that the primary outcome of this study was a composite. Therefore, the findings should be interpreted with some caution.
In their report, Tita and coworkers acknowledged that the transient and predominantly minor complications associated with delivery before 39 weeks must be weighed against the risk of fetal death inherent in delaying delivery through 38 full weeks—and an accompanying editorial made the same point.1 Stillbirth occurs at a rate of 1 case for every 1,000 births in the 37- to 39-week gestational age range—a rate that may be higher than the risks associated with delivery. Even so, the risk of stillbirth at 37 to 39 weeks is very small, and that risk is unlikely to be lowered through routine antenatal fetal testing. We should also remember that the risks of neonatal respiratory distress, transient tachypnea, admission to the neonatal intensive care unit (NICU), and even cerebral palsy2 may be increased with delivery at 37 to 38 weeks, or at 42 weeks or later, compared with delivery at 40 weeks.
All truly elective deliveries should occur at or after 39 weeks of gestation. However, when indicated, earlier delivery is acceptable—even essential—if we are to minimize maternal and neonatal morbidity and mortality in high-risk circumstances, such as hypertensive disorders of pregnancy, placenta previa, fetal growth restriction, and other conditions.
Investigations are under way to determine whether there is a role for routine betamethasone administration (regardless of indication or gestational age) in the absence of labor before 39 weeks. Until those data come in, we should continue to follow current practice guidelines for antenatal maternal administration of betamethasone—namely, a single course given between 24 and 34 weeks in women who have an elevated risk of preterm delivery.
Population-based screening for thrombophilias is not recommended
Silver RM, Zhao Y, Spong CY, et al, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (NICHD MFMU) Network. Prothrombin gene G20210A mutation and obstetric complications. Obstet Gynecol. 2010;115(1):14–20.
Said JM, Higgins JR, Moses EK, et al. Inherited thrombophilia polymorphisms and pregnancy outcomes in nulliparous women. Obstet Gynecol. 2010;115(1):5–13.
Since the mid-1990s, screening for thrombophilias has been recommended in the evaluation of a variety of adverse reproductive outcomes, including, but not limited to:
- recurrent pregnancy loss
- unexplained stillbirth
- placental abruption
- preeclampsia
- fetal growth restriction.
When a thrombophilia is detected in these settings, the practitioner faces a dilemma—namely, what to do when the mother is otherwise healthy and asymptomatic. All too often the finding of a thrombophilia leads to the initiation of some anticoagulation regimen, ranging from low-dose aspirin all the way to therapeutic anticoagulation with heparin.
Over the past year, several studies and expert opinions have been published that recast the role of thrombophilia screening in obstetric practice.
Writing for the Maternal-Fetal Medicine Units (MFMU) Network, Silver and colleagues concluded that the prothrombin (PT) gene mutation G20210A was not associated with pregnancy loss, preeclampsia, fetal growth restriction, or placental abruption in a low-risk, prospective cohort.
Said and coworkers reached a similar conclusion. In a blinded, prospective cohort study, they screened for the following mutations in 2,034 healthy nulliparous women before 22 weeks’ gestation:
- factor V Leiden mutation
- PT gene G20210A mutation
- methylenetetrahydrofolate reductase enzyme (MTHFR) C677T
- MTHFR A1298C
- thrombomodulin polymorphism.
The majority of asymptomatic women who carried an inherited thrombophilia polymorphism had a successful pregnancy outcome. In fact, homozygosity of the MTHFR A1298C mutation was found to be protective.
Population-based screening for thrombophilias is not recommended. In fact, some authors have advised against screening for thrombophilias even in the setting of a thrombotic event, suggesting it has limited utility.3
The main reason to screen for a thrombophilia at this time is to explore idiopathic thrombosis or a strong family history of the same. There is no need to screen for thrombophilias when the patient has a history of pregnancy loss, placental abruption, preeclampsia, and fetal growth restriction.
If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol
ACOG Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115(3):669–671.
Magnesium sulfate has a long and glorious history in obstetrics, having been used to prevent eclampsia, to treat preterm uterine contractions, and now, potentially, to reduce the incidence of central nervous system damage among prematurely delivered infants.
Cerebral palsy (CP) is most commonly associated with prematurity and intrauterine fetal infection. Only in the past two decades has there been a shift away from assigning the cause of most cases of CP to intrapartum events. Nevertheless, CP remains an important concern among patients and providers, and research continues to find ways to prevent CP or minimize its effects.
Numerous large trials have explored the use of magnesium sulfate to prevent CP in preterm infants. Although their findings have not been as definitive as researchers had hoped, some data do suggest that magnesium sulfate has a protective effect.
The American Congress of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) urge caution in regard to the use of magnesium sulfate for fetal neuroprotection. The SMFM points out that the reported benefits of magnesium sulfate in this setting have been derived largely from secondary analyses. The SMFM recommends that, if magnesium is used at all, it should be administered according to one of the published protocols (three are cited in the ACOG opinion).
The SMFM goes on to warn against choosing magnesium sulfate as a tocolytic solely because of its possible neuroprotective effects.
In a Committee Opinion published last year, ACOG was a bit more definite. “The Committee on Obstetric Practice and the Society for Maternal-Fetal Medicine recognize that none of the individual studies found a benefit with regard to their primary outcome,” the opinion states. “However, the available evidence suggests that magnesium sulfate given before anticipated early preterm birth reduces the risk of cerebral palsy in surviving infants. Physicians electing to use magnesium sulfate for fetal neuroprotection should develop specific guidelines regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in concordance with one of the larger trials.”
Recent editorials have cautioned against using magnesium sulfate for fetal neuroprotection until more data become available,4 or have left it up to the individual practitioners (or institution) to decide whether it is advisable.5
The use of magnesium sulfate for fetal neuroprotection when preterm delivery seems likely requires additional research. For now, this practice should be governed strictly by protocol. And it should not be viewed as “standard of care” by our legal colleagues.
When administering magnesium sulfate, avoid giving a cumulative total in excess of 50 g, as this amount may increase the risk of pediatric death.6
We want to hear from you! Tell us what you think.
1. Greene MF. Making small risks even smaller. N Engl J Med. 2009;360(2):183-184.
2. Moster D, Wilcox AJ, Vollset SE, Markestad T, Lie RT. Cerebral palsy among term and postterm births. JAMA. 2010;304(9):976-982.
3. Scifres CM, Macones GA. The utility of thrombophilia testing in pregnant women with thrombosis; fact or fiction? Am J Obstet Gynecol. 2008;199(4):344.e1–7.-
4. Stanley FJ, Crowther C. Antenatal magnesium sulfate for neuroprotection before preterm birth? N Engl J Med. 2008;359(9):962-964.
5. Macones GA. MgSO4 for CP prevention: too good to be true? Am J Obstet Gynecol. 2009;200(6):589.-
6. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet. 1997;350(9090):1517-1518.
1. Greene MF. Making small risks even smaller. N Engl J Med. 2009;360(2):183-184.
2. Moster D, Wilcox AJ, Vollset SE, Markestad T, Lie RT. Cerebral palsy among term and postterm births. JAMA. 2010;304(9):976-982.
3. Scifres CM, Macones GA. The utility of thrombophilia testing in pregnant women with thrombosis; fact or fiction? Am J Obstet Gynecol. 2008;199(4):344.e1–7.-
4. Stanley FJ, Crowther C. Antenatal magnesium sulfate for neuroprotection before preterm birth? N Engl J Med. 2008;359(9):962-964.
5. Macones GA. MgSO4 for CP prevention: too good to be true? Am J Obstet Gynecol. 2009;200(6):589.-
6. Mittendorf R, Covert R, Boman J, et al. Is tocolytic magnesium sulfate associated with increased total paediatric mortality? Lancet. 1997;350(9090):1517-1518.