User login
Inappropriate Use of Proton Pump Inhibitors for Stress Ulcer Prophylaxis
Treating CA-MRSA Infections: A Review of Antibiotic Therapy Selection and Patient Treatment Outcomes at a VA Institution
Synthetic Cannabinoids: The Newest, Almost Illicit Drug of Abuse
Acute Disorders of the Joints and Bursae: Radiographic Clues to Diagnosis
Acute Disorders of the Joints and Bursae: Radiographic Clues to Diagnosis
Partial Tears of the Anterior Cruciate Ligament: Diagnosis and Treatment
Challenges and Solutions for Total Hip Arthroplasty in Treatment of Patients With Symptomatic Sequelae of Developmental Dysplasia of the Hip
2011 Update on fertility
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.
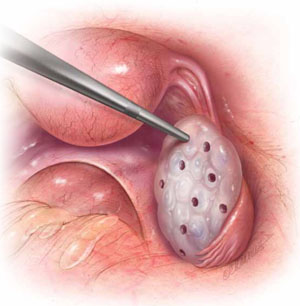
FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.
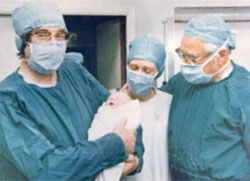
Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | |||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.

FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.

Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | * | ||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.

FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.

Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | * | ||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
What we’ve learned from 2 decades’ experience with the LNG-IUS
With the total fertility rate in the United States at just over two children for every woman, one thing seems obvious: The “average” woman needs several decades of effective contraception during her fertile life span.1 The situation is even more compelling in Europe, where several countries are experiencing a decline in population. Clearly, women are choosing to have smaller families, or none at all, or are postponing childbearing longer than ever before.
In the past, many women opted for sterilization once childbearing was completed. Today, however, the sterilization rate is declining, in part because of the emerging use of long-acting, reversible contraception.2 The levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena) is one of these long-acting contraceptives. It also offers benefits besides contraception: It reduces the severity of heavy menstrual bleeding, requires no daily or monthly attention, and, when priced over its full 5-year lifetime, is economical. Because of its effect on heavy menstrual bleeding, for which it was FDA-approved in 2009, the LNG-IUS also is emerging as an alternative to endometrial ablation and hysterectomy.3-5
To elucidate the benefits and risks of the LNG-IUS and explore its ultra-long-term use, we contacted Oskari Heikinheimo, MD, PhD, an expert on the subject. Dr. Heikinheimo is adjunct professor of obstetrics and gynecology at Helsinki University Central Hospital in Helsinki, Finland, and an integral figure in early use of the LNG-IUS. In this interview, he discusses the LNG-IUS overall and offers insight from Scandinavia, which has a long history of LNG-IUS use.
Does the LNG-IUS provide effective contraception?
OBG Management: Let’s begin by focusing on the primary indication for the LNG-IUS— as a contraceptive. The device was approved as a contraceptive in the United States in 2001. That means we have a decade of experience. What have we learned?
Dr. Heikinheimo: We have considerably more than 10 years of experience with the LNG-IUS, as it was first approved in Finland in 1990 and in Sweden in 1992. We know that the system is safe and highly effective, with a 5-year cumulative pregnancy rate of 0.1%–0.5%.
OBG Management: Where does that statistic originate?
Dr. Heikinheimo: The safety and efficacy of the LNG-IUS were first established in a Finnish as well as a large European multicenter trial of more than 2,000 women. The women were 18 to 38 years old at the time of enrollment, of proven fertility, and used the system for as long as 5 years, providing 110,000 woman-months of exposure.6,7 These results were confirmed in several later studies, most recently in a large post-marketing study of more than 17,000 women and 58,000 woman–years.8
OBG Management: Were you involved in development of the LNG-IUS?
Dr. Heikinheimo: No, development of the LNG-IUS began in the 1970s—at that time I was attending elementary school. However, I do have the privilege of knowing the masterminds behind the LNG-IUS, most importantly Professor Tapani Luukkainen.
OBG Management: What amount of levonorgestrel is released by the LNG-IUS?
Dr. Heikinheimo: The progestin is released at a rate of approximately 20 μg daily when the LNG-IUS is first inserted, although that rate gradually declines, decreasing by approximately 50% after 5 years of use, when the system should be replaced.9
A stable serum concentration of levonorgestrel of 150 to 200 pg/mL is found within a few weeks of insertion. After 12, 24, and 60 months, that level is 180±66 pg/mL, 192±140 pg/mL, and 159±59 pg/mL, respectively.10
OBG Management: What is the mechanism of action of the LNG-IUS as a contraceptive?
Dr. Heikinheimo: It isn’t completely clear. It is thought that the system thickens the cervical mucus, preventing passage of sperm into the uterus. It may also inhibit sperm capacitation or survival, or alter the endometrium, or all of these things. A recent study from Los Angeles showed convincingly that sperm penetration through samples of mid-cycle cervical mucus, collected from women using the LNG-IUS, is zero.11 Thickening of cervical mucus plays an important role in the contraceptive efficacy of the LNG-IUS. The main mechanism of action is prevention of fertilization.
OBG Management: Can a woman who has used the LNG-IUS readily conceive once it is removed?
Dr. Heikinheimo: Yes. Approximately 80% of women who wish to become pregnant do so within 12 months after the device is removed.9 That figure is similar in women who have not used the LNG-IUS.
What is the effect on bleeding patterns?
OBG Management: In the United States, in 2009, the LNG-IUS was approved for a second indication—to reduce heavy menstrual bleeding. What do we know about its efficacy in that regard?
Dr. Heikinheimo: A marked reduction in uterine bleeding is a hallmark of LNG-IUS use. The typical bleeding pattern during its use is oligomenorrhea or amenorrhea, with minor occasional bleeding.
OBG Management: What data do we have on the effect of the LNG-IUS on heavy menstrual bleeding?
Dr. Heikinheimo: This effect was explored in a randomized, open-label, active-control, parallel-group trial of 79 women who used the LNG-IUS and 81 women who were given medroxyprogesterone acetate (MPA) over six menstrual cycles.4 When the trial began, all of these women experienced menstrual blood loss of 80 mL or more. By trial’s end, the LNG-IUS had caused a significantly greater reduction in menstrual blood loss than MPA had, and more women using the LNG-IUS had successful treatment. Success was defined as menstrual blood loss below 80 mL and a reduction in menstrual blood loss of 50% or more from baseline.
Women who had organic or systemic conditions that may cause heavy uterine bleeding were excluded, except for women who had small fibroids that did not exceed 5 mL in volume.
OBG Management: What is the mechanism of action for the LNG-IUS in the reduction of heavy menstrual bleeding?
Dr. Heikinheimo: The high local concentration of levonorgestrel causes marked suppression of the endometrium. This suppression is associated with several biochemical events, such as reduced expression of steroid receptors, altered expression of steroid-metabolizing enzymes, and inhibition of insulin-like growth factor activity.12 These alterations render the endometrium insensitive to growth-promoting entities, such as estradiol. The result is thin endometrium and uterine bleeding that is either minor or nonexistent.
How does the LNG-IUS compare with endometrial ablation
and hysterectomy?
OBG Management: How does the LNG-IUS compare with endometrial ablation and hysterectomy in the treatment of heavy menstrual bleeding?
Dr. Heikinheimo: The LNG-IUS is increasingly used in the treatment of heavy menstrual bleeding. In the Finnish VUOKKO study, women who were referred to a gynecologic outpatient clinic because of heavy menstrual bleeding were randomized to hysterectomy or to treatment with the LNG-IUS. At 5 years, approximately half of the women randomized to the LNG-IUS were still using the device.5 Also, quality of life and psychological well-being were similar between the groups. Although 40% of the women randomized to the LNG-IUS eventually underwent hysterectomy, the cost of the treatment was significantly lower in the LNG-IUS group than in the hysterectomy group ($2,820 versus $4,660).5
Endometrial resection is less commonly used to treat heavy menstrual bleeding in Scandinavia. However, in research studies, the efficacy of the LNG-IUS has been comparable to that of endometrial resection.3
Is the LNG-IUS completely benign?
OBG Management: What adverse reactions are associated with the LNG-IUS?
Dr. Heikinheimo: The main effect is an altered bleeding pattern. The device can cause spotting and irregular bleeding, oligomenorrhea, amenorrhea, or even heavy bleeding. Most of these changes occur during the first 3 to 6 months after insertion. Altered bleeding is seen in approximately 30% of women using the LNG-IUS. Proper patient information, provided before and at insertion, is the key element in guiding these women through these initial inconveniences.13 After the first months, the number of bleeding and spotting days commonly decreases, although bleeding may remain irregular. Amenorrhea develops in about 20% of users by the end of the first year of use.9
In most women who experience heavy menstrual bleeding, the number of bleeding and spotting days may increase during the first months of therapy but declines with continued use, as does the volume of blood loss each month.
A potential concern with irregular bleeding is that it may mask the signs and symptoms of endometrial polyps or malignancy. For this reason, abnormal uterine bleeding should be evaluated before insertion of the LNG-IUS. Similarly, any woman who develops unexplained bleeding during prolonged use of the device should also be evaluated.
Does the LNG-IUS raise the risk of breast cancer?
OBG Management: Because the LNG-IUS is hormonal contraception, some women may worry about their risk of breast cancer when using it. What do we know about that risk?
Dr. Heikinheimo: A large post-marketing study in Finland revealed that the risk of breast cancer among users of the LNG-IUS is similar to that among the general population.14 The results are clear: When used for contraception, the LNG-IUS is not associated with an increased risk of breast cancer.
Is patient satisfaction high?
OBG Management: Here’s a critical question—are women happy with the LNG-IUS?
Dr. Heikinheimo: They certainly appear to be. Continuation rates in large post-marketing trials have been high, in the range of 65% at 5 years.13 I often tell my patients and students that, as a gynecologist, I see lots of happy women; many of them are using the LNG-IUS. That means that many women are likely to use more than one LNG-IUS during their fertile years.
OBG Management: You were coauthor of a study on consecutive use of the system, were you not? What did you find?
Dr. Heikinheimo: We enrolled 204 women 23 to 45 years old who had used an LNG-IUS for 4 years and 3 to 9 months and who opted to have a second system inserted at the time the first one was removed. Overall, we found the LNG-IUS to be well tolerated and highly acceptable among the women. In addition, the pattern of reduced menstrual bleeding that had developed during use of the first LNG-IUS continued after it was replaced; in some cases, it was even further reduced.15
Removal of the previous system and insertion of a new one at the same visit ensures that the initial irregular spotting period, which is typical of the first months after LNG-IUS insertion, does not recur in consecutive use. The rate of overall satisfaction with the system, assessed at the end of the first year after insertion of the second LNG-IUS, was high—93%. The women who were amenorrheic were most satisfied (100%).
OBG Management: As you noted earlier, the LNG-IUS has been widely used in Finland and Sweden for 20 years now. What else have we learned about the benefits and risks of the system from that long experience?
Dr. Heikinheimo: More and more women are asking for a bleeding-free contraceptive method! Also, the widespread use of the LNG-IUS has had an important impact on the entire specialty of obstetrics and gynecology. Because women are happy with the high contraceptive efficacy and reduced uterine bleeding, there has been a marked reduction in female sterilization. Similarly, the number of hysterectomies performed for benign causes has decreased by 40% over the past 10 years. These figures also translate into effective use of the surgical ward.
OBG Management: What other features of the LNG-IUS are worth mentioning here?
Dr. Heikinheimo: Besides the conventional users of intrauterine contraception—married parous women—nulliparous women are increasingly using the LNG-IUS.16 Young, highly fertile women need effective contraception that does not need to be remembered on a daily basis.
There is also an increasing number of publications describing the use of the LNG-IUS in women with various pre-existing conditions, such as insulin-dependent diabetes, HIV infection, and inherited bleeding disorders, as well as in institutionalized women. It is reassuring to see that the benefits of the LNG-IUS—safety, high contraceptive efficacy, and markedly reduced uterine bleeding—are also apparent in these women. I’m convinced that there are still several additional subgroups of women who will benefit from use of the LNG-IUS.
We want to hear from you! Tell us what you think.
1. United Nations Department of Economic and Social Affairs, Population Division. United Nations World Population Prospects: 2006 revision. New York: United Nations; 2007.
2. Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
3. Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113(5):1104-1116.
4. Kaunitz AM, Bissonnette F, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116(3):625-632.
5. Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291(12):1456-1463.
6. Luukkainen T, Allonen H, Haukkamaa M, Lähteenmäki P, Nilsson CG, Toivonen J. Five years’ experience with levonorgestrel releasing IUDs. Contraception. 1986;33(2):139-148.
7. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49(1):56-72.
8. Backman T, Rauramo I, Huhtala S, Koskenvuo M. Pregnancy during the use of levonorgstrel intrauterine system. Am J Obst Gynecol. 2004;190(1):50-54.
9. Package insert: Mirena 8th ed. Bayer Schering Pharma AG; 2009.
10. Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf). 1982;17(6):529-536.
11. Lewis R, Taylor D, Natavio M, Melamed A, Felix J, Mishell D. Effects of the levonorgestrel-releasing intrauterine system on cervical mucus quantity and sperm penetration. Contraception. 2010;82(6):491-496.
12. Guttinger A, Critchley H. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75(supple 6):S93-S98.
13. Backman T, Huhtala S, Luoto R, Tuominen J, Rauramo I, Koskenvuo M. Advance information improves user satisfaction with the levonorgestrel intrauterine system. Obstet Gynecol. 2002;99(4):608-613.
14. Backman T, Rauramo I, Jaakkola K, Inki P, Vaahtera K, Launonen A, Koskenvuo M. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106(4):813-817.
15. Gemzell-Danielsson K, Inki P, Boubli L, O’Flynn M, Kunz M, Keikinheimo O. Bleeding pattern and safety of consecutive use of the LNG-IUS—a multicenter prospective study. Hum Reprod. 2010;25(2):354-359.
16. Suhonen S, Haukkamaa M, Jakobsson T, et al. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69(5):407-412.
With the total fertility rate in the United States at just over two children for every woman, one thing seems obvious: The “average” woman needs several decades of effective contraception during her fertile life span.1 The situation is even more compelling in Europe, where several countries are experiencing a decline in population. Clearly, women are choosing to have smaller families, or none at all, or are postponing childbearing longer than ever before.
In the past, many women opted for sterilization once childbearing was completed. Today, however, the sterilization rate is declining, in part because of the emerging use of long-acting, reversible contraception.2 The levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena) is one of these long-acting contraceptives. It also offers benefits besides contraception: It reduces the severity of heavy menstrual bleeding, requires no daily or monthly attention, and, when priced over its full 5-year lifetime, is economical. Because of its effect on heavy menstrual bleeding, for which it was FDA-approved in 2009, the LNG-IUS also is emerging as an alternative to endometrial ablation and hysterectomy.3-5
To elucidate the benefits and risks of the LNG-IUS and explore its ultra-long-term use, we contacted Oskari Heikinheimo, MD, PhD, an expert on the subject. Dr. Heikinheimo is adjunct professor of obstetrics and gynecology at Helsinki University Central Hospital in Helsinki, Finland, and an integral figure in early use of the LNG-IUS. In this interview, he discusses the LNG-IUS overall and offers insight from Scandinavia, which has a long history of LNG-IUS use.
Does the LNG-IUS provide effective contraception?
OBG Management: Let’s begin by focusing on the primary indication for the LNG-IUS— as a contraceptive. The device was approved as a contraceptive in the United States in 2001. That means we have a decade of experience. What have we learned?
Dr. Heikinheimo: We have considerably more than 10 years of experience with the LNG-IUS, as it was first approved in Finland in 1990 and in Sweden in 1992. We know that the system is safe and highly effective, with a 5-year cumulative pregnancy rate of 0.1%–0.5%.
OBG Management: Where does that statistic originate?
Dr. Heikinheimo: The safety and efficacy of the LNG-IUS were first established in a Finnish as well as a large European multicenter trial of more than 2,000 women. The women were 18 to 38 years old at the time of enrollment, of proven fertility, and used the system for as long as 5 years, providing 110,000 woman-months of exposure.6,7 These results were confirmed in several later studies, most recently in a large post-marketing study of more than 17,000 women and 58,000 woman–years.8
OBG Management: Were you involved in development of the LNG-IUS?
Dr. Heikinheimo: No, development of the LNG-IUS began in the 1970s—at that time I was attending elementary school. However, I do have the privilege of knowing the masterminds behind the LNG-IUS, most importantly Professor Tapani Luukkainen.
OBG Management: What amount of levonorgestrel is released by the LNG-IUS?
Dr. Heikinheimo: The progestin is released at a rate of approximately 20 μg daily when the LNG-IUS is first inserted, although that rate gradually declines, decreasing by approximately 50% after 5 years of use, when the system should be replaced.9
A stable serum concentration of levonorgestrel of 150 to 200 pg/mL is found within a few weeks of insertion. After 12, 24, and 60 months, that level is 180±66 pg/mL, 192±140 pg/mL, and 159±59 pg/mL, respectively.10
OBG Management: What is the mechanism of action of the LNG-IUS as a contraceptive?
Dr. Heikinheimo: It isn’t completely clear. It is thought that the system thickens the cervical mucus, preventing passage of sperm into the uterus. It may also inhibit sperm capacitation or survival, or alter the endometrium, or all of these things. A recent study from Los Angeles showed convincingly that sperm penetration through samples of mid-cycle cervical mucus, collected from women using the LNG-IUS, is zero.11 Thickening of cervical mucus plays an important role in the contraceptive efficacy of the LNG-IUS. The main mechanism of action is prevention of fertilization.
OBG Management: Can a woman who has used the LNG-IUS readily conceive once it is removed?
Dr. Heikinheimo: Yes. Approximately 80% of women who wish to become pregnant do so within 12 months after the device is removed.9 That figure is similar in women who have not used the LNG-IUS.
What is the effect on bleeding patterns?
OBG Management: In the United States, in 2009, the LNG-IUS was approved for a second indication—to reduce heavy menstrual bleeding. What do we know about its efficacy in that regard?
Dr. Heikinheimo: A marked reduction in uterine bleeding is a hallmark of LNG-IUS use. The typical bleeding pattern during its use is oligomenorrhea or amenorrhea, with minor occasional bleeding.
OBG Management: What data do we have on the effect of the LNG-IUS on heavy menstrual bleeding?
Dr. Heikinheimo: This effect was explored in a randomized, open-label, active-control, parallel-group trial of 79 women who used the LNG-IUS and 81 women who were given medroxyprogesterone acetate (MPA) over six menstrual cycles.4 When the trial began, all of these women experienced menstrual blood loss of 80 mL or more. By trial’s end, the LNG-IUS had caused a significantly greater reduction in menstrual blood loss than MPA had, and more women using the LNG-IUS had successful treatment. Success was defined as menstrual blood loss below 80 mL and a reduction in menstrual blood loss of 50% or more from baseline.
Women who had organic or systemic conditions that may cause heavy uterine bleeding were excluded, except for women who had small fibroids that did not exceed 5 mL in volume.
OBG Management: What is the mechanism of action for the LNG-IUS in the reduction of heavy menstrual bleeding?
Dr. Heikinheimo: The high local concentration of levonorgestrel causes marked suppression of the endometrium. This suppression is associated with several biochemical events, such as reduced expression of steroid receptors, altered expression of steroid-metabolizing enzymes, and inhibition of insulin-like growth factor activity.12 These alterations render the endometrium insensitive to growth-promoting entities, such as estradiol. The result is thin endometrium and uterine bleeding that is either minor or nonexistent.
How does the LNG-IUS compare with endometrial ablation
and hysterectomy?
OBG Management: How does the LNG-IUS compare with endometrial ablation and hysterectomy in the treatment of heavy menstrual bleeding?
Dr. Heikinheimo: The LNG-IUS is increasingly used in the treatment of heavy menstrual bleeding. In the Finnish VUOKKO study, women who were referred to a gynecologic outpatient clinic because of heavy menstrual bleeding were randomized to hysterectomy or to treatment with the LNG-IUS. At 5 years, approximately half of the women randomized to the LNG-IUS were still using the device.5 Also, quality of life and psychological well-being were similar between the groups. Although 40% of the women randomized to the LNG-IUS eventually underwent hysterectomy, the cost of the treatment was significantly lower in the LNG-IUS group than in the hysterectomy group ($2,820 versus $4,660).5
Endometrial resection is less commonly used to treat heavy menstrual bleeding in Scandinavia. However, in research studies, the efficacy of the LNG-IUS has been comparable to that of endometrial resection.3
Is the LNG-IUS completely benign?
OBG Management: What adverse reactions are associated with the LNG-IUS?
Dr. Heikinheimo: The main effect is an altered bleeding pattern. The device can cause spotting and irregular bleeding, oligomenorrhea, amenorrhea, or even heavy bleeding. Most of these changes occur during the first 3 to 6 months after insertion. Altered bleeding is seen in approximately 30% of women using the LNG-IUS. Proper patient information, provided before and at insertion, is the key element in guiding these women through these initial inconveniences.13 After the first months, the number of bleeding and spotting days commonly decreases, although bleeding may remain irregular. Amenorrhea develops in about 20% of users by the end of the first year of use.9
In most women who experience heavy menstrual bleeding, the number of bleeding and spotting days may increase during the first months of therapy but declines with continued use, as does the volume of blood loss each month.
A potential concern with irregular bleeding is that it may mask the signs and symptoms of endometrial polyps or malignancy. For this reason, abnormal uterine bleeding should be evaluated before insertion of the LNG-IUS. Similarly, any woman who develops unexplained bleeding during prolonged use of the device should also be evaluated.
Does the LNG-IUS raise the risk of breast cancer?
OBG Management: Because the LNG-IUS is hormonal contraception, some women may worry about their risk of breast cancer when using it. What do we know about that risk?
Dr. Heikinheimo: A large post-marketing study in Finland revealed that the risk of breast cancer among users of the LNG-IUS is similar to that among the general population.14 The results are clear: When used for contraception, the LNG-IUS is not associated with an increased risk of breast cancer.
Is patient satisfaction high?
OBG Management: Here’s a critical question—are women happy with the LNG-IUS?
Dr. Heikinheimo: They certainly appear to be. Continuation rates in large post-marketing trials have been high, in the range of 65% at 5 years.13 I often tell my patients and students that, as a gynecologist, I see lots of happy women; many of them are using the LNG-IUS. That means that many women are likely to use more than one LNG-IUS during their fertile years.
OBG Management: You were coauthor of a study on consecutive use of the system, were you not? What did you find?
Dr. Heikinheimo: We enrolled 204 women 23 to 45 years old who had used an LNG-IUS for 4 years and 3 to 9 months and who opted to have a second system inserted at the time the first one was removed. Overall, we found the LNG-IUS to be well tolerated and highly acceptable among the women. In addition, the pattern of reduced menstrual bleeding that had developed during use of the first LNG-IUS continued after it was replaced; in some cases, it was even further reduced.15
Removal of the previous system and insertion of a new one at the same visit ensures that the initial irregular spotting period, which is typical of the first months after LNG-IUS insertion, does not recur in consecutive use. The rate of overall satisfaction with the system, assessed at the end of the first year after insertion of the second LNG-IUS, was high—93%. The women who were amenorrheic were most satisfied (100%).
OBG Management: As you noted earlier, the LNG-IUS has been widely used in Finland and Sweden for 20 years now. What else have we learned about the benefits and risks of the system from that long experience?
Dr. Heikinheimo: More and more women are asking for a bleeding-free contraceptive method! Also, the widespread use of the LNG-IUS has had an important impact on the entire specialty of obstetrics and gynecology. Because women are happy with the high contraceptive efficacy and reduced uterine bleeding, there has been a marked reduction in female sterilization. Similarly, the number of hysterectomies performed for benign causes has decreased by 40% over the past 10 years. These figures also translate into effective use of the surgical ward.
OBG Management: What other features of the LNG-IUS are worth mentioning here?
Dr. Heikinheimo: Besides the conventional users of intrauterine contraception—married parous women—nulliparous women are increasingly using the LNG-IUS.16 Young, highly fertile women need effective contraception that does not need to be remembered on a daily basis.
There is also an increasing number of publications describing the use of the LNG-IUS in women with various pre-existing conditions, such as insulin-dependent diabetes, HIV infection, and inherited bleeding disorders, as well as in institutionalized women. It is reassuring to see that the benefits of the LNG-IUS—safety, high contraceptive efficacy, and markedly reduced uterine bleeding—are also apparent in these women. I’m convinced that there are still several additional subgroups of women who will benefit from use of the LNG-IUS.
We want to hear from you! Tell us what you think.
With the total fertility rate in the United States at just over two children for every woman, one thing seems obvious: The “average” woman needs several decades of effective contraception during her fertile life span.1 The situation is even more compelling in Europe, where several countries are experiencing a decline in population. Clearly, women are choosing to have smaller families, or none at all, or are postponing childbearing longer than ever before.
In the past, many women opted for sterilization once childbearing was completed. Today, however, the sterilization rate is declining, in part because of the emerging use of long-acting, reversible contraception.2 The levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena) is one of these long-acting contraceptives. It also offers benefits besides contraception: It reduces the severity of heavy menstrual bleeding, requires no daily or monthly attention, and, when priced over its full 5-year lifetime, is economical. Because of its effect on heavy menstrual bleeding, for which it was FDA-approved in 2009, the LNG-IUS also is emerging as an alternative to endometrial ablation and hysterectomy.3-5
To elucidate the benefits and risks of the LNG-IUS and explore its ultra-long-term use, we contacted Oskari Heikinheimo, MD, PhD, an expert on the subject. Dr. Heikinheimo is adjunct professor of obstetrics and gynecology at Helsinki University Central Hospital in Helsinki, Finland, and an integral figure in early use of the LNG-IUS. In this interview, he discusses the LNG-IUS overall and offers insight from Scandinavia, which has a long history of LNG-IUS use.
Does the LNG-IUS provide effective contraception?
OBG Management: Let’s begin by focusing on the primary indication for the LNG-IUS— as a contraceptive. The device was approved as a contraceptive in the United States in 2001. That means we have a decade of experience. What have we learned?
Dr. Heikinheimo: We have considerably more than 10 years of experience with the LNG-IUS, as it was first approved in Finland in 1990 and in Sweden in 1992. We know that the system is safe and highly effective, with a 5-year cumulative pregnancy rate of 0.1%–0.5%.
OBG Management: Where does that statistic originate?
Dr. Heikinheimo: The safety and efficacy of the LNG-IUS were first established in a Finnish as well as a large European multicenter trial of more than 2,000 women. The women were 18 to 38 years old at the time of enrollment, of proven fertility, and used the system for as long as 5 years, providing 110,000 woman-months of exposure.6,7 These results were confirmed in several later studies, most recently in a large post-marketing study of more than 17,000 women and 58,000 woman–years.8
OBG Management: Were you involved in development of the LNG-IUS?
Dr. Heikinheimo: No, development of the LNG-IUS began in the 1970s—at that time I was attending elementary school. However, I do have the privilege of knowing the masterminds behind the LNG-IUS, most importantly Professor Tapani Luukkainen.
OBG Management: What amount of levonorgestrel is released by the LNG-IUS?
Dr. Heikinheimo: The progestin is released at a rate of approximately 20 μg daily when the LNG-IUS is first inserted, although that rate gradually declines, decreasing by approximately 50% after 5 years of use, when the system should be replaced.9
A stable serum concentration of levonorgestrel of 150 to 200 pg/mL is found within a few weeks of insertion. After 12, 24, and 60 months, that level is 180±66 pg/mL, 192±140 pg/mL, and 159±59 pg/mL, respectively.10
OBG Management: What is the mechanism of action of the LNG-IUS as a contraceptive?
Dr. Heikinheimo: It isn’t completely clear. It is thought that the system thickens the cervical mucus, preventing passage of sperm into the uterus. It may also inhibit sperm capacitation or survival, or alter the endometrium, or all of these things. A recent study from Los Angeles showed convincingly that sperm penetration through samples of mid-cycle cervical mucus, collected from women using the LNG-IUS, is zero.11 Thickening of cervical mucus plays an important role in the contraceptive efficacy of the LNG-IUS. The main mechanism of action is prevention of fertilization.
OBG Management: Can a woman who has used the LNG-IUS readily conceive once it is removed?
Dr. Heikinheimo: Yes. Approximately 80% of women who wish to become pregnant do so within 12 months after the device is removed.9 That figure is similar in women who have not used the LNG-IUS.
What is the effect on bleeding patterns?
OBG Management: In the United States, in 2009, the LNG-IUS was approved for a second indication—to reduce heavy menstrual bleeding. What do we know about its efficacy in that regard?
Dr. Heikinheimo: A marked reduction in uterine bleeding is a hallmark of LNG-IUS use. The typical bleeding pattern during its use is oligomenorrhea or amenorrhea, with minor occasional bleeding.
OBG Management: What data do we have on the effect of the LNG-IUS on heavy menstrual bleeding?
Dr. Heikinheimo: This effect was explored in a randomized, open-label, active-control, parallel-group trial of 79 women who used the LNG-IUS and 81 women who were given medroxyprogesterone acetate (MPA) over six menstrual cycles.4 When the trial began, all of these women experienced menstrual blood loss of 80 mL or more. By trial’s end, the LNG-IUS had caused a significantly greater reduction in menstrual blood loss than MPA had, and more women using the LNG-IUS had successful treatment. Success was defined as menstrual blood loss below 80 mL and a reduction in menstrual blood loss of 50% or more from baseline.
Women who had organic or systemic conditions that may cause heavy uterine bleeding were excluded, except for women who had small fibroids that did not exceed 5 mL in volume.
OBG Management: What is the mechanism of action for the LNG-IUS in the reduction of heavy menstrual bleeding?
Dr. Heikinheimo: The high local concentration of levonorgestrel causes marked suppression of the endometrium. This suppression is associated with several biochemical events, such as reduced expression of steroid receptors, altered expression of steroid-metabolizing enzymes, and inhibition of insulin-like growth factor activity.12 These alterations render the endometrium insensitive to growth-promoting entities, such as estradiol. The result is thin endometrium and uterine bleeding that is either minor or nonexistent.
How does the LNG-IUS compare with endometrial ablation
and hysterectomy?
OBG Management: How does the LNG-IUS compare with endometrial ablation and hysterectomy in the treatment of heavy menstrual bleeding?
Dr. Heikinheimo: The LNG-IUS is increasingly used in the treatment of heavy menstrual bleeding. In the Finnish VUOKKO study, women who were referred to a gynecologic outpatient clinic because of heavy menstrual bleeding were randomized to hysterectomy or to treatment with the LNG-IUS. At 5 years, approximately half of the women randomized to the LNG-IUS were still using the device.5 Also, quality of life and psychological well-being were similar between the groups. Although 40% of the women randomized to the LNG-IUS eventually underwent hysterectomy, the cost of the treatment was significantly lower in the LNG-IUS group than in the hysterectomy group ($2,820 versus $4,660).5
Endometrial resection is less commonly used to treat heavy menstrual bleeding in Scandinavia. However, in research studies, the efficacy of the LNG-IUS has been comparable to that of endometrial resection.3
Is the LNG-IUS completely benign?
OBG Management: What adverse reactions are associated with the LNG-IUS?
Dr. Heikinheimo: The main effect is an altered bleeding pattern. The device can cause spotting and irregular bleeding, oligomenorrhea, amenorrhea, or even heavy bleeding. Most of these changes occur during the first 3 to 6 months after insertion. Altered bleeding is seen in approximately 30% of women using the LNG-IUS. Proper patient information, provided before and at insertion, is the key element in guiding these women through these initial inconveniences.13 After the first months, the number of bleeding and spotting days commonly decreases, although bleeding may remain irregular. Amenorrhea develops in about 20% of users by the end of the first year of use.9
In most women who experience heavy menstrual bleeding, the number of bleeding and spotting days may increase during the first months of therapy but declines with continued use, as does the volume of blood loss each month.
A potential concern with irregular bleeding is that it may mask the signs and symptoms of endometrial polyps or malignancy. For this reason, abnormal uterine bleeding should be evaluated before insertion of the LNG-IUS. Similarly, any woman who develops unexplained bleeding during prolonged use of the device should also be evaluated.
Does the LNG-IUS raise the risk of breast cancer?
OBG Management: Because the LNG-IUS is hormonal contraception, some women may worry about their risk of breast cancer when using it. What do we know about that risk?
Dr. Heikinheimo: A large post-marketing study in Finland revealed that the risk of breast cancer among users of the LNG-IUS is similar to that among the general population.14 The results are clear: When used for contraception, the LNG-IUS is not associated with an increased risk of breast cancer.
Is patient satisfaction high?
OBG Management: Here’s a critical question—are women happy with the LNG-IUS?
Dr. Heikinheimo: They certainly appear to be. Continuation rates in large post-marketing trials have been high, in the range of 65% at 5 years.13 I often tell my patients and students that, as a gynecologist, I see lots of happy women; many of them are using the LNG-IUS. That means that many women are likely to use more than one LNG-IUS during their fertile years.
OBG Management: You were coauthor of a study on consecutive use of the system, were you not? What did you find?
Dr. Heikinheimo: We enrolled 204 women 23 to 45 years old who had used an LNG-IUS for 4 years and 3 to 9 months and who opted to have a second system inserted at the time the first one was removed. Overall, we found the LNG-IUS to be well tolerated and highly acceptable among the women. In addition, the pattern of reduced menstrual bleeding that had developed during use of the first LNG-IUS continued after it was replaced; in some cases, it was even further reduced.15
Removal of the previous system and insertion of a new one at the same visit ensures that the initial irregular spotting period, which is typical of the first months after LNG-IUS insertion, does not recur in consecutive use. The rate of overall satisfaction with the system, assessed at the end of the first year after insertion of the second LNG-IUS, was high—93%. The women who were amenorrheic were most satisfied (100%).
OBG Management: As you noted earlier, the LNG-IUS has been widely used in Finland and Sweden for 20 years now. What else have we learned about the benefits and risks of the system from that long experience?
Dr. Heikinheimo: More and more women are asking for a bleeding-free contraceptive method! Also, the widespread use of the LNG-IUS has had an important impact on the entire specialty of obstetrics and gynecology. Because women are happy with the high contraceptive efficacy and reduced uterine bleeding, there has been a marked reduction in female sterilization. Similarly, the number of hysterectomies performed for benign causes has decreased by 40% over the past 10 years. These figures also translate into effective use of the surgical ward.
OBG Management: What other features of the LNG-IUS are worth mentioning here?
Dr. Heikinheimo: Besides the conventional users of intrauterine contraception—married parous women—nulliparous women are increasingly using the LNG-IUS.16 Young, highly fertile women need effective contraception that does not need to be remembered on a daily basis.
There is also an increasing number of publications describing the use of the LNG-IUS in women with various pre-existing conditions, such as insulin-dependent diabetes, HIV infection, and inherited bleeding disorders, as well as in institutionalized women. It is reassuring to see that the benefits of the LNG-IUS—safety, high contraceptive efficacy, and markedly reduced uterine bleeding—are also apparent in these women. I’m convinced that there are still several additional subgroups of women who will benefit from use of the LNG-IUS.
We want to hear from you! Tell us what you think.
1. United Nations Department of Economic and Social Affairs, Population Division. United Nations World Population Prospects: 2006 revision. New York: United Nations; 2007.
2. Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
3. Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113(5):1104-1116.
4. Kaunitz AM, Bissonnette F, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116(3):625-632.
5. Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291(12):1456-1463.
6. Luukkainen T, Allonen H, Haukkamaa M, Lähteenmäki P, Nilsson CG, Toivonen J. Five years’ experience with levonorgestrel releasing IUDs. Contraception. 1986;33(2):139-148.
7. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49(1):56-72.
8. Backman T, Rauramo I, Huhtala S, Koskenvuo M. Pregnancy during the use of levonorgstrel intrauterine system. Am J Obst Gynecol. 2004;190(1):50-54.
9. Package insert: Mirena 8th ed. Bayer Schering Pharma AG; 2009.
10. Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf). 1982;17(6):529-536.
11. Lewis R, Taylor D, Natavio M, Melamed A, Felix J, Mishell D. Effects of the levonorgestrel-releasing intrauterine system on cervical mucus quantity and sperm penetration. Contraception. 2010;82(6):491-496.
12. Guttinger A, Critchley H. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75(supple 6):S93-S98.
13. Backman T, Huhtala S, Luoto R, Tuominen J, Rauramo I, Koskenvuo M. Advance information improves user satisfaction with the levonorgestrel intrauterine system. Obstet Gynecol. 2002;99(4):608-613.
14. Backman T, Rauramo I, Jaakkola K, Inki P, Vaahtera K, Launonen A, Koskenvuo M. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106(4):813-817.
15. Gemzell-Danielsson K, Inki P, Boubli L, O’Flynn M, Kunz M, Keikinheimo O. Bleeding pattern and safety of consecutive use of the LNG-IUS—a multicenter prospective study. Hum Reprod. 2010;25(2):354-359.
16. Suhonen S, Haukkamaa M, Jakobsson T, et al. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69(5):407-412.
1. United Nations Department of Economic and Social Affairs, Population Division. United Nations World Population Prospects: 2006 revision. New York: United Nations; 2007.
2. Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
3. Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113(5):1104-1116.
4. Kaunitz AM, Bissonnette F, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116(3):625-632.
5. Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291(12):1456-1463.
6. Luukkainen T, Allonen H, Haukkamaa M, Lähteenmäki P, Nilsson CG, Toivonen J. Five years’ experience with levonorgestrel releasing IUDs. Contraception. 1986;33(2):139-148.
7. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49(1):56-72.
8. Backman T, Rauramo I, Huhtala S, Koskenvuo M. Pregnancy during the use of levonorgstrel intrauterine system. Am J Obst Gynecol. 2004;190(1):50-54.
9. Package insert: Mirena 8th ed. Bayer Schering Pharma AG; 2009.
10. Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf). 1982;17(6):529-536.
11. Lewis R, Taylor D, Natavio M, Melamed A, Felix J, Mishell D. Effects of the levonorgestrel-releasing intrauterine system on cervical mucus quantity and sperm penetration. Contraception. 2010;82(6):491-496.
12. Guttinger A, Critchley H. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75(supple 6):S93-S98.
13. Backman T, Huhtala S, Luoto R, Tuominen J, Rauramo I, Koskenvuo M. Advance information improves user satisfaction with the levonorgestrel intrauterine system. Obstet Gynecol. 2002;99(4):608-613.
14. Backman T, Rauramo I, Jaakkola K, Inki P, Vaahtera K, Launonen A, Koskenvuo M. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106(4):813-817.
15. Gemzell-Danielsson K, Inki P, Boubli L, O’Flynn M, Kunz M, Keikinheimo O. Bleeding pattern and safety of consecutive use of the LNG-IUS—a multicenter prospective study. Hum Reprod. 2010;25(2):354-359.
16. Suhonen S, Haukkamaa M, Jakobsson T, et al. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69(5):407-412.
How to choose a contraceptive for a patient who has headaches
- The gynecologist’s role in managing menstrual migraine
Anne H. Calhoun, MD (April 2010)
Headaches are highly prevalent in women during their reproductive years. Most are a painful nuisance and do not present a risk of serious morbidity. Some, however, can be dangerous, and the addition of an estrogen-containing contraceptive can increase that risk.
Combination estrogen-progestin contraceptives are effective, popular, and easy to use—but are they safe for women who have headaches? This is a critical question. Some women who have preexisting headaches experience relief with hormonal contraception; others report stable or worsening symptoms; still others do not develop headaches until they begin hormonal contraception.
The differentiation between nuisance and true medical risk in this population depends on an accurate diagnosis of headache subtype. Taking a few moments to confirm whether a patient with headache has a true risk if she chooses hormonal contraception will prevent unnecessary restriction of a method and promote contraceptive success.
In this article, we present three cases that facilitate discussion of the safety, adverse effects, and benefits of various contraceptive strategies in women who have headaches.
Many women who report migraines don’t have them
Most women who report headaches to their gynecologist have not received a clinical diagnosis of headache subtype. They may say that they have “migraines” because that is the term used most commonly in the United States to indicate a severe level of distress with a headache. In actuality, although migraine is common in women, tension-type headaches are more prevalent.
The evaluation of a patient with headaches who is seeking contraception should begin with a simple diagnostic algorithm for headache type. Accurate diagnosis can be made using the International Headache Society (IHS) comprehensive guide for headache subtypes, last updated in 2004.1 TABLE 1, presents a simple classification of chronic headache syndromes, which account for more than 90% of headaches.
TABLE 1
Diagnostic criteria for headache subtypes
| Tension-type | |
| Infrequent episodic | |
| A. | At least 10 episodes <1 day per month on average (<12 days per year) and fulfilling criteria B–D below |
| Frequent episodic | |
| A. | At least 10 episodes occurring ≥1 but <15 days per month for at least 3 months (≥12 and <180 days per year) and fulfilling criteria B–D |
| B. | Headache lasting for 30 minutes to 7 days |
| C. | Headache has at least two of the following characteristics: |
| |
| D. | Both of the following: |
| |
| E. | Not attributable to another disorder |
| Cluster | |
| A. | At least 5 attacks fulfilling criteria B–D |
| B. | Severe or very severe unilateral orbital, supraorbital and/or temporal pain lasting 15–180 minutes if untreated |
| C. | Headache is accompanied by at least one of the following: |
| |
| D. | Attacks have a frequency from one every other day to 8 per day |
| E. | Not attributable to another disorder |
| Migraine without aura | |
| At least 5 attacks fulfilling criteria B–D | |
| A. | Headache attacks lasting 4–72 hours (untreated or successfully treated) |
| B. | Headache has at least two of the following characteristics: |
| |
| C. | During headache at least one of the following: |
| |
| D. | Not attributable to another disorder |
| Typical migraine with aura headache | |
| A. | At least 2 attacks fulfilling criteria B–D |
| B. | Aura consisting of at least one of the following, but no motor weakness: |
| |
| C. | At least two of the following: |
| |
| D. | Headache fulfilling criteria B–D for migraine without aura begins during the aura or follows aura within 60 minutes |
| E. | Not attributable to another disorder |
| Pure menstrual migraine without aura | |
| A. | Attacks, in a menstruating woman, fulfilling criteria for migraine without aura |
| B. | Attacks occur exclusively on Day 1 ±2 days (i.e., Days +2 to –3) of menstruation in at least two out of three menstrual cycles and at no other times of the cycle |
| Estrogen-withdrawal headache | |
| A. | Headache or migraine fulfilling criteria C and D |
| B. | Daily use of exogenous estrogen for >3 weeks, which is interrupted |
| C. | Headache or migraine develops within 5 days after last use of estrogen |
| D. | Headache or migraine resolves within 3 days |
| Exogenous hormone-induced headache | |
| A. | Headache or migraine fulfilling criteria C and D |
| B. | Regular use of exogenous hormones |
| C. | Headache or migraine develops or markedly worsens within 3 months of commencing exogenous hormones |
| D. | Headache or migraine resolves or reverts to its previous pattern within 3 months after total discontinuation of exogenous hormones |
| Source: International Headache Society1 | |
CASE 1: Patient reports a history of migraine
A 21-year-old nulliparous woman has severe dysmenorrhea that has been unresponsive to treatment with nonsteroidal anti-inflammatory drugs (NSAIDs). She also desires contraception. Her primary care provider has recommended combination oral contraceptives (OCs) as a solution to both problems. However, the patient has heard from friends that she should not use OCs because of her history of migraine headache, and she has come to see you for a second opinion.
She describes her headaches as bilateral and reports a “tightening” sensation. The headaches are associated with photophobia and are not aggravated by routine physical activity. They respond to NSAIDs.
She also reports that her mother and sister have been on a prescription medication for migraines for many years.
Is an OC appropriate for this patient?
This young woman’s history is consistent with tension-type headache, not migraine. Tension headache is the most common subtype, with prevalence as high as 59% in women of reproductive age.2 It is generally characterized by mild or moderate pain that is bilateral, pressing, or tightening in quality. The pain does not worsen with routine physical activity. There is no nausea, but photophobia or phonophobia may be present.1
A systematic review of the risk of stroke associated with combination OC use and headaches did not find any studies examining the association between nonmigraine headache and the risk of stroke among combination OC users.3 In contrast to some migraines, however, tension-type headache has not been associated with an increased risk of stroke in the general population. Nor is there evidence that hormonal fluctuations play a role in the pathogenesis or clinical course of tension headache.
In summary, there are no contraindications to combination hormonal contraceptives—including estrogen-progestin OCs, the contraceptive patch, and the contraceptive ring—in women who have tension headache.4
Explore any family history of migraine
The patient in Case 1 appears to have a family history of migraine. Some evidence suggests that such a family history increases the risk of new-onset migraine with use of a combination OC.5 Because the background prevalence of migraine is so high in the population of women likely to use a combination OC, it can be difficult to determine whether worsening headache or development of migraine with OC use is causal or coincidental.
Were this patient to express concern over even a theoretical risk of triggering migraine headache, then a combination OC would probably not be appropriate. In the absence of such concern, however, there is no reason to withhold hormonal contraception. Progestin-only options exist that will provide her with excellent contraceptive efficacy and help relieve her dysmenorrhea:
- the etonogestrel subdermal implant (Implanon)
- depot medroxyprogesterone acetate (DMPA) injection (Depo-Provera)
- the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena).
Although some women do develop headaches while using progestin-only contraceptives, there is no evidence that such use can trigger a new migraine syndrome in a woman with a family history of such. Again, however, the data are limited.
Progestin methods are safe
The use of progestin-only methods has been promoted in headache sufferers, especially those who have a specific diagnosis of migraine, because progestins do not add to the elevated risk of stroke that accompanies migraine with aura.
Because headache is common in women of reproductive age, it is not surprising that it is listed as a common adverse event for all contraceptives, including progestin-only methods. Evidence that progestin-only methods cause or worsen headaches is slim, however. Preliminary studies indicate that mid-luteal elevations of progesterone or its metabolites could prevent migraine, compared with other times in the cycle.6 Older studies report that a daily oral progestin could prevent migraine in premenopausal women, possibly secondary to induction of anovulation. At the same time, there are clinical reports that DMPA may trigger headache as a side effect in susceptible women.
Generally, then, although progestin-only methods are likely to be safe in all patients with headache, and ovulation suppression may improve the headaches, some patients may experience worsening symptoms.
CASE 2: OC user reports migraine with aura
A 26-year-old mother of one comes to your office for her annual exam. She has used combination OCs for 2 years. She also has a history of severe headaches, which occur four or five times a year. She says the headaches are unilateral, pulsating, and associated with photophobia. The symptoms worsen when she is active and are preceded by a flashing zigzag line that migrates from the center of her visual field to the lateral periphery. The headaches are not associated with her menstrual cycle and have not changed in character or frequency since she began using an OC. She does not smoke.
Should she continue taking an OC?
This patient’s history is consistent with migraine headache with aura. Migraine is a common, disabling primary headache disorder, with an estimated 1-year prevalence in adult women of 15% to 18% and a lifetime prevalence of about 30%.2 Approximately 10% to 20% of people who have migraine experience auras.7
Research on the association between combination OCs, migraine, and stroke has been limited by the rarity of the outcome in the population of concern. Most data come from case-controlled studies and are fettered by a lack of standardized criteria for the diagnosis of migraine (few studies use criteria from the IHS); by recall bias (such as self-reported OC use); and by survivorship bias. Many studies fail to differentiate by the presence of aura, which indicates a different effect on cerebral blood flow patterns than does migraine without aura.
Although most studies attempt to control for the confounding effect of smoking, some do not, and in others the prevalence of smoking is so high it can be difficult to remove from the equation. Some of the studies examining the association between migraine and stroke do not differentiate by gender.
Taking all these variables into account, migraine in women independently appears to carry a twofold to threefold increased risk of ischemic stroke, compared with the risk in similarly aged women who do not have migraine.8 Among women who have a history of migraine, those who use combination OCs are two times to four times more likely to experience ischemic stroke than nonusers are. Among women with the highest risk (combination OC users with migraine), the odds ratio for ischemic stroke ranges from 6 to almost 14, compared with women with the lowest risk (nonusers without migraine).3
To put all this in perspective, the absolute risk of stroke for a 26-year-old nonsmoker like our patient is 6 cases in every 100,000 woman-years.9 Multiplying this risk by a factor of 3 to account for her migraines, and by 3 again to account for her OC use, we can roughly estimate her absolute risk of stroke as about 54 cases for every 100,000 woman-years. Although this absolute risk is extremely low, the outcome can be catastrophic. It behooves us to proceed with caution.
A common misperception among health-care providers is that nausea, vomiting, photophobia, and phonophobia represent migraine aura, when in fact these symptoms are part of the associated diagnostic symptoms of all migraines.
About 20% of people who have migraine experience an aura. The aura begins before the headache and typically lasts 5 to 20 minutes—rarely does it last more than 60 minutes. The headache occurs soon after the aura stops.
The aura may include zigzag lines of light, flashing lights or bright spots, blurred or darkened spots, or focal neurologic symptoms such as numbness or tingling in the fingers of one hand, lips, tongue, or lower face. Auras may involve other senses, such as smell, and can occasionally cause temporary focal weakness or changes in speech.
Presence of aura likely confers greater risk
Many studies of migraine do not explore contraceptive use. They report a higher risk of stroke when aura is present.8,10-15 One of the few prospective studies found no increased risk of stroke in migraineurs who did not experience aura.12
No studies examining a link between combination OCs and ischemic stroke in migraineurs have been large enough to stratify the risk of stroke by the presence or absence of aura. The assumption has been that this risk is amplified by use of combination OCs. Consequently, migraine with aura is designated as an absolute contraindication to the use of combination OCs by the World Health Organization (WHO), ACOG, and the IHS.4,16Although no studies have included women using the contraceptive patch or ring, it is assumed that these methods carry a risk of ischemic stroke similar to that of combination OCs.17 The IHS recommendations on combination hormonal contraception in women with migraine are given in TABLE 2.18
TABLE 2
When combination OCs are appropriate for a woman who has migraine
|
| Source: International Headache Society18 |
Although the patient in Case 2 has been experiencing migraine with aura for some time without a change in headache pattern, combination hormonal contraception is contraindicated. Her history of migraine with aura may have been missed during previous evaluation if she did not consider it to be a “medical problem.” All candidates for combination OCs should be directly questioned about any history of migraine, thrombosis, cigarette smoking, and hypertension.
The patient described in Case 2 should be encouraged to switch to a progestin-only method of contraception or a highly effective nonhormonal method, such as a copper-containing IUD (Paragard). Because she had no change in her headache pattern on ovulation-suppression therapy (OCs), she likely will have no change with continued ovulation suppression (DMPA, subdermal implant) or without it (LNG-IUS, copper lUD).
Be sure to time the switch in methods so that contraception is maintained throughout the transition. This patient would also benefit from referral to a headache specialist for evaluation and treatment.
CASE 3 Perimenstrual headache in a smoker
A 32-year-old mother of two visits your office for contraceptive counseling. She reports that she smokes two to five cigarettes a day and has a history of migraine without aura. Her headaches occur exclusively during the two days just before her period, and they resolve within the first few days of bleeding. NSAIDs provide limited relief. Is hormonal contraception appropriate?
When a woman with perimenstrual headache is choosing a contraceptive method, we need to ensure that she is not exposed to undue risks and focus on improving her quality of life, if possible.
TABLE 1, reviews the diagnostic criteria for pure menstrual migraine, in which symptoms occur only in the perimenstrual period, as in this patient. Most practitioners use a broader definition of menstrual-related migraine, in which women may have other migraine triggers but still experience predictable headaches with menses. More than 50% of women who have migraine report an association between their headaches and menstruation, but only about 10% of women report migraine exclusively with menstruation.7
Hormonal manipulation is not first-line management for menstrual migraine.19 However, for the patient seeking contraception, it is appropriate to consider the effect of a given method on her headaches because that effect is sure to influence her satisfaction, quality of life, and compliance.
Menstrual migraine is rarely associated with aura, even in women who have migraine with aura at other times in the cycle.7However, the headaches experienced at the time of menstruation tend to be more severe and longer-lasting and less responsive to medication than are migraines experienced at other times of the month.20
Eliminate the placebo week
Research into the pathogenesis of menstrual migraine has focused on the withdrawal of estrogen in the luteal phase of the menstrual cycle. Ovulation is not a requisite for menstrual migraine attacks, which frequently occur during the hormone-free interval in combination hormonal contraceptive regimens. Several lines of evidence suggest that stabilizing estrogen fluctuations can prevent migraine (FIGURE).7
Hormonal fluctuations can trigger migraine
Fluctuations in estrogen during the menstrual cycle may trigger dilation and constriction of blood vessels in the brain, causing migraine.If a patient who has pure menstrual migraine desires to use an estrogen-containing contraceptive, she is likely to be as safe in that choice as a patient without migraine. However, she is not likely to experience improvement in her migraines if she uses a traditional 21/7 active-to-placebo pill regimen. It is necessary to maintain an adequate estrogen level during the placebo week, either through estrogen supplementation or extended-cycle dosing, to prevent the estrogen-withdrawal trigger for headaches.
A few OC regimens reduce the estrogen-free interval to 4 days or provide estrogen supplementation during the placebo week; these regimens may benefit women who have menstrual migraine, although data are scant. If most, or all, of the patient’s migraines are suppressed on combined hormonal contraception, continuous use with rare (e.g., yearly) withdrawals may be indicated.
Alternatives to estrogen-containing contraceptives
If the patient does not desire a combination OC or has other contraindications to estrogen, she may benefit from a progestin-only contraceptive that suppresses ovulation, such as DMPA or the etonogestrel implant. Even with ovulation suppression, however, estrogen fluctuations may occur, and the evidence supporting the use of these methods in the concurrent management of contraceptive needs and menstrual migraine is less clear. Although they are safe in women who have migraine, progestin-only pills and the LNG-IUS do not suppress ovulation reliably enough to be considered useful in managing menstrual migraine.
Take a careful history
Choosing a contraceptive for a woman who has headaches begins the same way as with any other patient: with careful assessment of her short-term and long-term family-planning goals, along with a history and physical to elicit any “red flags,” cultural preferences, and tolerance for specific side effects.
Once you become aware of a history of headache, careful history-taking can usually differentiate between the major headache subtypes and help you avoid limiting contraceptive options unnecessarily.
Women who start any contraceptive may report improvement, worsening, or new onset of headache symptoms. This change usually occurs within the first 3 months, and headaches associated with hormone use tend to improve with continued use.21
In women who have preexisting migraine, you should undertake prompt evaluation and consider a change of contraceptive method in response to 1) any increase in severity or frequency or 2) the onset of associated neurologic symptoms.
We want to hear from you! Tell us what you think.
1. International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(suppl 1):9-160.
2. Rasmussen BK. Epidemiology of headache. Cephalalgia. 2001;21(7):774-777.
3. Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006;73(2):189-194.
4. Harris M, Kaneshiro B. An evidence-based approach to hormonal contraception and headaches. Contraception. 2009;80(5):417-421.
5. Loder EW, Buse DC, Golub JR. Headache and combination estrogen-progestin oral contraceptives: integrating evidence, guidelines, and clinical practice. Headache. 2005;45(3):224.
6. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis— part 2. Headache. 2006;46(3):365-386.
7. Macgregor EA. Menstrual migraine: a clinical review. J Fam Plann Reprod Health Care. 2007;33(1):36-47.
8. Etminan M, Takkouche B, Isorna FC, et al. Risk of ischaemic stroke in people with migraine: systematic review and metaanalysis of observational studies. BMJ. 2005;330(7482):63.-
9. Petitti DB, Sidney S, Quesenberry CP, Jr. Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28(2):280-283.
10. Carolei A, Marini C, De Matteis G. History of migraine and risk of cerebral ischaemia in young adults. Lancet. 1996;347(9014):1503-1506.
11. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. BMJ. 1999;318(7175):13.
12. Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64(6):1020-1026.
13. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38(9):2438-2445.
14. Tzourio C, Kittner SJ, Bousser MG, Alperovitch A. Migraine and stroke in young women. Cephalalgia. 2000;20(3):190-199.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310(6983):830-833.
16. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107(6):1453-1472.
17. Department of Reproductive Health. Medical Eligibility Criteria for Contraceptive Use. 4th ed; 2009. Geneva, Switzerland: World Health Organization; 2010.
18. Bousser MG, Conard J, Kittner S, et al. Recommendations on the risk of ischaemic stroke associated with use of combined oral contraceptives and hormone replacement therapy in women with migraine. The International Headache Society Task Force on Combined Oral Contraceptives & Hormone Replacement Therapy. Cephalalgia. 2000;20(3):155-156.
19. Calhoun AH. The gynecologist’s role in managing menstrual migraine. OBG Manage. 2010;22(4):30-43.
20. Martin VT, Wernke S, Mandell K, et al. Defining the relationship between ovarian hormones and migraine headache. Headache. 2005;45(9):1190-1201.
21. Allais G, Gabellari IC, De Lorenzo C, C, Mana O, Benedetto C. Oral contraceptives in migraine. Expert Rev Neurother. 2009;9(3):381-393.
- The gynecologist’s role in managing menstrual migraine
Anne H. Calhoun, MD (April 2010)
Headaches are highly prevalent in women during their reproductive years. Most are a painful nuisance and do not present a risk of serious morbidity. Some, however, can be dangerous, and the addition of an estrogen-containing contraceptive can increase that risk.
Combination estrogen-progestin contraceptives are effective, popular, and easy to use—but are they safe for women who have headaches? This is a critical question. Some women who have preexisting headaches experience relief with hormonal contraception; others report stable or worsening symptoms; still others do not develop headaches until they begin hormonal contraception.
The differentiation between nuisance and true medical risk in this population depends on an accurate diagnosis of headache subtype. Taking a few moments to confirm whether a patient with headache has a true risk if she chooses hormonal contraception will prevent unnecessary restriction of a method and promote contraceptive success.
In this article, we present three cases that facilitate discussion of the safety, adverse effects, and benefits of various contraceptive strategies in women who have headaches.
Many women who report migraines don’t have them
Most women who report headaches to their gynecologist have not received a clinical diagnosis of headache subtype. They may say that they have “migraines” because that is the term used most commonly in the United States to indicate a severe level of distress with a headache. In actuality, although migraine is common in women, tension-type headaches are more prevalent.
The evaluation of a patient with headaches who is seeking contraception should begin with a simple diagnostic algorithm for headache type. Accurate diagnosis can be made using the International Headache Society (IHS) comprehensive guide for headache subtypes, last updated in 2004.1 TABLE 1, presents a simple classification of chronic headache syndromes, which account for more than 90% of headaches.
TABLE 1
Diagnostic criteria for headache subtypes
| Tension-type | |
| Infrequent episodic | |
| A. | At least 10 episodes <1 day per month on average (<12 days per year) and fulfilling criteria B–D below |
| Frequent episodic | |
| A. | At least 10 episodes occurring ≥1 but <15 days per month for at least 3 months (≥12 and <180 days per year) and fulfilling criteria B–D |
| B. | Headache lasting for 30 minutes to 7 days |
| C. | Headache has at least two of the following characteristics: |
| |
| D. | Both of the following: |
| |
| E. | Not attributable to another disorder |
| Cluster | |
| A. | At least 5 attacks fulfilling criteria B–D |
| B. | Severe or very severe unilateral orbital, supraorbital and/or temporal pain lasting 15–180 minutes if untreated |
| C. | Headache is accompanied by at least one of the following: |
| |
| D. | Attacks have a frequency from one every other day to 8 per day |
| E. | Not attributable to another disorder |
| Migraine without aura | |
| At least 5 attacks fulfilling criteria B–D | |
| A. | Headache attacks lasting 4–72 hours (untreated or successfully treated) |
| B. | Headache has at least two of the following characteristics: |
| |
| C. | During headache at least one of the following: |
| |
| D. | Not attributable to another disorder |
| Typical migraine with aura headache | |
| A. | At least 2 attacks fulfilling criteria B–D |
| B. | Aura consisting of at least one of the following, but no motor weakness: |
| |
| C. | At least two of the following: |
| |
| D. | Headache fulfilling criteria B–D for migraine without aura begins during the aura or follows aura within 60 minutes |
| E. | Not attributable to another disorder |
| Pure menstrual migraine without aura | |
| A. | Attacks, in a menstruating woman, fulfilling criteria for migraine without aura |
| B. | Attacks occur exclusively on Day 1 ±2 days (i.e., Days +2 to –3) of menstruation in at least two out of three menstrual cycles and at no other times of the cycle |
| Estrogen-withdrawal headache | |
| A. | Headache or migraine fulfilling criteria C and D |
| B. | Daily use of exogenous estrogen for >3 weeks, which is interrupted |
| C. | Headache or migraine develops within 5 days after last use of estrogen |
| D. | Headache or migraine resolves within 3 days |
| Exogenous hormone-induced headache | |
| A. | Headache or migraine fulfilling criteria C and D |
| B. | Regular use of exogenous hormones |
| C. | Headache or migraine develops or markedly worsens within 3 months of commencing exogenous hormones |
| D. | Headache or migraine resolves or reverts to its previous pattern within 3 months after total discontinuation of exogenous hormones |
| Source: International Headache Society1 | |
CASE 1: Patient reports a history of migraine
A 21-year-old nulliparous woman has severe dysmenorrhea that has been unresponsive to treatment with nonsteroidal anti-inflammatory drugs (NSAIDs). She also desires contraception. Her primary care provider has recommended combination oral contraceptives (OCs) as a solution to both problems. However, the patient has heard from friends that she should not use OCs because of her history of migraine headache, and she has come to see you for a second opinion.
She describes her headaches as bilateral and reports a “tightening” sensation. The headaches are associated with photophobia and are not aggravated by routine physical activity. They respond to NSAIDs.
She also reports that her mother and sister have been on a prescription medication for migraines for many years.
Is an OC appropriate for this patient?
This young woman’s history is consistent with tension-type headache, not migraine. Tension headache is the most common subtype, with prevalence as high as 59% in women of reproductive age.2 It is generally characterized by mild or moderate pain that is bilateral, pressing, or tightening in quality. The pain does not worsen with routine physical activity. There is no nausea, but photophobia or phonophobia may be present.1
A systematic review of the risk of stroke associated with combination OC use and headaches did not find any studies examining the association between nonmigraine headache and the risk of stroke among combination OC users.3 In contrast to some migraines, however, tension-type headache has not been associated with an increased risk of stroke in the general population. Nor is there evidence that hormonal fluctuations play a role in the pathogenesis or clinical course of tension headache.
In summary, there are no contraindications to combination hormonal contraceptives—including estrogen-progestin OCs, the contraceptive patch, and the contraceptive ring—in women who have tension headache.4
Explore any family history of migraine
The patient in Case 1 appears to have a family history of migraine. Some evidence suggests that such a family history increases the risk of new-onset migraine with use of a combination OC.5 Because the background prevalence of migraine is so high in the population of women likely to use a combination OC, it can be difficult to determine whether worsening headache or development of migraine with OC use is causal or coincidental.
Were this patient to express concern over even a theoretical risk of triggering migraine headache, then a combination OC would probably not be appropriate. In the absence of such concern, however, there is no reason to withhold hormonal contraception. Progestin-only options exist that will provide her with excellent contraceptive efficacy and help relieve her dysmenorrhea:
- the etonogestrel subdermal implant (Implanon)
- depot medroxyprogesterone acetate (DMPA) injection (Depo-Provera)
- the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena).
Although some women do develop headaches while using progestin-only contraceptives, there is no evidence that such use can trigger a new migraine syndrome in a woman with a family history of such. Again, however, the data are limited.
Progestin methods are safe
The use of progestin-only methods has been promoted in headache sufferers, especially those who have a specific diagnosis of migraine, because progestins do not add to the elevated risk of stroke that accompanies migraine with aura.
Because headache is common in women of reproductive age, it is not surprising that it is listed as a common adverse event for all contraceptives, including progestin-only methods. Evidence that progestin-only methods cause or worsen headaches is slim, however. Preliminary studies indicate that mid-luteal elevations of progesterone or its metabolites could prevent migraine, compared with other times in the cycle.6 Older studies report that a daily oral progestin could prevent migraine in premenopausal women, possibly secondary to induction of anovulation. At the same time, there are clinical reports that DMPA may trigger headache as a side effect in susceptible women.
Generally, then, although progestin-only methods are likely to be safe in all patients with headache, and ovulation suppression may improve the headaches, some patients may experience worsening symptoms.
CASE 2: OC user reports migraine with aura
A 26-year-old mother of one comes to your office for her annual exam. She has used combination OCs for 2 years. She also has a history of severe headaches, which occur four or five times a year. She says the headaches are unilateral, pulsating, and associated with photophobia. The symptoms worsen when she is active and are preceded by a flashing zigzag line that migrates from the center of her visual field to the lateral periphery. The headaches are not associated with her menstrual cycle and have not changed in character or frequency since she began using an OC. She does not smoke.
Should she continue taking an OC?
This patient’s history is consistent with migraine headache with aura. Migraine is a common, disabling primary headache disorder, with an estimated 1-year prevalence in adult women of 15% to 18% and a lifetime prevalence of about 30%.2 Approximately 10% to 20% of people who have migraine experience auras.7
Research on the association between combination OCs, migraine, and stroke has been limited by the rarity of the outcome in the population of concern. Most data come from case-controlled studies and are fettered by a lack of standardized criteria for the diagnosis of migraine (few studies use criteria from the IHS); by recall bias (such as self-reported OC use); and by survivorship bias. Many studies fail to differentiate by the presence of aura, which indicates a different effect on cerebral blood flow patterns than does migraine without aura.
Although most studies attempt to control for the confounding effect of smoking, some do not, and in others the prevalence of smoking is so high it can be difficult to remove from the equation. Some of the studies examining the association between migraine and stroke do not differentiate by gender.
Taking all these variables into account, migraine in women independently appears to carry a twofold to threefold increased risk of ischemic stroke, compared with the risk in similarly aged women who do not have migraine.8 Among women who have a history of migraine, those who use combination OCs are two times to four times more likely to experience ischemic stroke than nonusers are. Among women with the highest risk (combination OC users with migraine), the odds ratio for ischemic stroke ranges from 6 to almost 14, compared with women with the lowest risk (nonusers without migraine).3
To put all this in perspective, the absolute risk of stroke for a 26-year-old nonsmoker like our patient is 6 cases in every 100,000 woman-years.9 Multiplying this risk by a factor of 3 to account for her migraines, and by 3 again to account for her OC use, we can roughly estimate her absolute risk of stroke as about 54 cases for every 100,000 woman-years. Although this absolute risk is extremely low, the outcome can be catastrophic. It behooves us to proceed with caution.
A common misperception among health-care providers is that nausea, vomiting, photophobia, and phonophobia represent migraine aura, when in fact these symptoms are part of the associated diagnostic symptoms of all migraines.
About 20% of people who have migraine experience an aura. The aura begins before the headache and typically lasts 5 to 20 minutes—rarely does it last more than 60 minutes. The headache occurs soon after the aura stops.
The aura may include zigzag lines of light, flashing lights or bright spots, blurred or darkened spots, or focal neurologic symptoms such as numbness or tingling in the fingers of one hand, lips, tongue, or lower face. Auras may involve other senses, such as smell, and can occasionally cause temporary focal weakness or changes in speech.
Presence of aura likely confers greater risk
Many studies of migraine do not explore contraceptive use. They report a higher risk of stroke when aura is present.8,10-15 One of the few prospective studies found no increased risk of stroke in migraineurs who did not experience aura.12
No studies examining a link between combination OCs and ischemic stroke in migraineurs have been large enough to stratify the risk of stroke by the presence or absence of aura. The assumption has been that this risk is amplified by use of combination OCs. Consequently, migraine with aura is designated as an absolute contraindication to the use of combination OCs by the World Health Organization (WHO), ACOG, and the IHS.4,16Although no studies have included women using the contraceptive patch or ring, it is assumed that these methods carry a risk of ischemic stroke similar to that of combination OCs.17 The IHS recommendations on combination hormonal contraception in women with migraine are given in TABLE 2.18
TABLE 2
When combination OCs are appropriate for a woman who has migraine
|
| Source: International Headache Society18 |
Although the patient in Case 2 has been experiencing migraine with aura for some time without a change in headache pattern, combination hormonal contraception is contraindicated. Her history of migraine with aura may have been missed during previous evaluation if she did not consider it to be a “medical problem.” All candidates for combination OCs should be directly questioned about any history of migraine, thrombosis, cigarette smoking, and hypertension.
The patient described in Case 2 should be encouraged to switch to a progestin-only method of contraception or a highly effective nonhormonal method, such as a copper-containing IUD (Paragard). Because she had no change in her headache pattern on ovulation-suppression therapy (OCs), she likely will have no change with continued ovulation suppression (DMPA, subdermal implant) or without it (LNG-IUS, copper lUD).
Be sure to time the switch in methods so that contraception is maintained throughout the transition. This patient would also benefit from referral to a headache specialist for evaluation and treatment.
CASE 3 Perimenstrual headache in a smoker
A 32-year-old mother of two visits your office for contraceptive counseling. She reports that she smokes two to five cigarettes a day and has a history of migraine without aura. Her headaches occur exclusively during the two days just before her period, and they resolve within the first few days of bleeding. NSAIDs provide limited relief. Is hormonal contraception appropriate?
When a woman with perimenstrual headache is choosing a contraceptive method, we need to ensure that she is not exposed to undue risks and focus on improving her quality of life, if possible.
TABLE 1, reviews the diagnostic criteria for pure menstrual migraine, in which symptoms occur only in the perimenstrual period, as in this patient. Most practitioners use a broader definition of menstrual-related migraine, in which women may have other migraine triggers but still experience predictable headaches with menses. More than 50% of women who have migraine report an association between their headaches and menstruation, but only about 10% of women report migraine exclusively with menstruation.7
Hormonal manipulation is not first-line management for menstrual migraine.19 However, for the patient seeking contraception, it is appropriate to consider the effect of a given method on her headaches because that effect is sure to influence her satisfaction, quality of life, and compliance.
Menstrual migraine is rarely associated with aura, even in women who have migraine with aura at other times in the cycle.7However, the headaches experienced at the time of menstruation tend to be more severe and longer-lasting and less responsive to medication than are migraines experienced at other times of the month.20
Eliminate the placebo week
Research into the pathogenesis of menstrual migraine has focused on the withdrawal of estrogen in the luteal phase of the menstrual cycle. Ovulation is not a requisite for menstrual migraine attacks, which frequently occur during the hormone-free interval in combination hormonal contraceptive regimens. Several lines of evidence suggest that stabilizing estrogen fluctuations can prevent migraine (FIGURE).7

Hormonal fluctuations can trigger migraine
Fluctuations in estrogen during the menstrual cycle may trigger dilation and constriction of blood vessels in the brain, causing migraine.If a patient who has pure menstrual migraine desires to use an estrogen-containing contraceptive, she is likely to be as safe in that choice as a patient without migraine. However, she is not likely to experience improvement in her migraines if she uses a traditional 21/7 active-to-placebo pill regimen. It is necessary to maintain an adequate estrogen level during the placebo week, either through estrogen supplementation or extended-cycle dosing, to prevent the estrogen-withdrawal trigger for headaches.
A few OC regimens reduce the estrogen-free interval to 4 days or provide estrogen supplementation during the placebo week; these regimens may benefit women who have menstrual migraine, although data are scant. If most, or all, of the patient’s migraines are suppressed on combined hormonal contraception, continuous use with rare (e.g., yearly) withdrawals may be indicated.
Alternatives to estrogen-containing contraceptives
If the patient does not desire a combination OC or has other contraindications to estrogen, she may benefit from a progestin-only contraceptive that suppresses ovulation, such as DMPA or the etonogestrel implant. Even with ovulation suppression, however, estrogen fluctuations may occur, and the evidence supporting the use of these methods in the concurrent management of contraceptive needs and menstrual migraine is less clear. Although they are safe in women who have migraine, progestin-only pills and the LNG-IUS do not suppress ovulation reliably enough to be considered useful in managing menstrual migraine.
Take a careful history
Choosing a contraceptive for a woman who has headaches begins the same way as with any other patient: with careful assessment of her short-term and long-term family-planning goals, along with a history and physical to elicit any “red flags,” cultural preferences, and tolerance for specific side effects.
Once you become aware of a history of headache, careful history-taking can usually differentiate between the major headache subtypes and help you avoid limiting contraceptive options unnecessarily.
Women who start any contraceptive may report improvement, worsening, or new onset of headache symptoms. This change usually occurs within the first 3 months, and headaches associated with hormone use tend to improve with continued use.21
In women who have preexisting migraine, you should undertake prompt evaluation and consider a change of contraceptive method in response to 1) any increase in severity or frequency or 2) the onset of associated neurologic symptoms.
We want to hear from you! Tell us what you think.
- The gynecologist’s role in managing menstrual migraine
Anne H. Calhoun, MD (April 2010)
Headaches are highly prevalent in women during their reproductive years. Most are a painful nuisance and do not present a risk of serious morbidity. Some, however, can be dangerous, and the addition of an estrogen-containing contraceptive can increase that risk.
Combination estrogen-progestin contraceptives are effective, popular, and easy to use—but are they safe for women who have headaches? This is a critical question. Some women who have preexisting headaches experience relief with hormonal contraception; others report stable or worsening symptoms; still others do not develop headaches until they begin hormonal contraception.
The differentiation between nuisance and true medical risk in this population depends on an accurate diagnosis of headache subtype. Taking a few moments to confirm whether a patient with headache has a true risk if she chooses hormonal contraception will prevent unnecessary restriction of a method and promote contraceptive success.
In this article, we present three cases that facilitate discussion of the safety, adverse effects, and benefits of various contraceptive strategies in women who have headaches.
Many women who report migraines don’t have them
Most women who report headaches to their gynecologist have not received a clinical diagnosis of headache subtype. They may say that they have “migraines” because that is the term used most commonly in the United States to indicate a severe level of distress with a headache. In actuality, although migraine is common in women, tension-type headaches are more prevalent.
The evaluation of a patient with headaches who is seeking contraception should begin with a simple diagnostic algorithm for headache type. Accurate diagnosis can be made using the International Headache Society (IHS) comprehensive guide for headache subtypes, last updated in 2004.1 TABLE 1, presents a simple classification of chronic headache syndromes, which account for more than 90% of headaches.
TABLE 1
Diagnostic criteria for headache subtypes
| Tension-type | |
| Infrequent episodic | |
| A. | At least 10 episodes <1 day per month on average (<12 days per year) and fulfilling criteria B–D below |
| Frequent episodic | |
| A. | At least 10 episodes occurring ≥1 but <15 days per month for at least 3 months (≥12 and <180 days per year) and fulfilling criteria B–D |
| B. | Headache lasting for 30 minutes to 7 days |
| C. | Headache has at least two of the following characteristics: |
| |
| D. | Both of the following: |
| |
| E. | Not attributable to another disorder |
| Cluster | |
| A. | At least 5 attacks fulfilling criteria B–D |
| B. | Severe or very severe unilateral orbital, supraorbital and/or temporal pain lasting 15–180 minutes if untreated |
| C. | Headache is accompanied by at least one of the following: |
| |
| D. | Attacks have a frequency from one every other day to 8 per day |
| E. | Not attributable to another disorder |
| Migraine without aura | |
| At least 5 attacks fulfilling criteria B–D | |
| A. | Headache attacks lasting 4–72 hours (untreated or successfully treated) |
| B. | Headache has at least two of the following characteristics: |
| |
| C. | During headache at least one of the following: |
| |
| D. | Not attributable to another disorder |
| Typical migraine with aura headache | |
| A. | At least 2 attacks fulfilling criteria B–D |
| B. | Aura consisting of at least one of the following, but no motor weakness: |
| |
| C. | At least two of the following: |
| |
| D. | Headache fulfilling criteria B–D for migraine without aura begins during the aura or follows aura within 60 minutes |
| E. | Not attributable to another disorder |
| Pure menstrual migraine without aura | |
| A. | Attacks, in a menstruating woman, fulfilling criteria for migraine without aura |
| B. | Attacks occur exclusively on Day 1 ±2 days (i.e., Days +2 to –3) of menstruation in at least two out of three menstrual cycles and at no other times of the cycle |
| Estrogen-withdrawal headache | |
| A. | Headache or migraine fulfilling criteria C and D |
| B. | Daily use of exogenous estrogen for >3 weeks, which is interrupted |
| C. | Headache or migraine develops within 5 days after last use of estrogen |
| D. | Headache or migraine resolves within 3 days |
| Exogenous hormone-induced headache | |
| A. | Headache or migraine fulfilling criteria C and D |
| B. | Regular use of exogenous hormones |
| C. | Headache or migraine develops or markedly worsens within 3 months of commencing exogenous hormones |
| D. | Headache or migraine resolves or reverts to its previous pattern within 3 months after total discontinuation of exogenous hormones |
| Source: International Headache Society1 | |
CASE 1: Patient reports a history of migraine
A 21-year-old nulliparous woman has severe dysmenorrhea that has been unresponsive to treatment with nonsteroidal anti-inflammatory drugs (NSAIDs). She also desires contraception. Her primary care provider has recommended combination oral contraceptives (OCs) as a solution to both problems. However, the patient has heard from friends that she should not use OCs because of her history of migraine headache, and she has come to see you for a second opinion.
She describes her headaches as bilateral and reports a “tightening” sensation. The headaches are associated with photophobia and are not aggravated by routine physical activity. They respond to NSAIDs.
She also reports that her mother and sister have been on a prescription medication for migraines for many years.
Is an OC appropriate for this patient?
This young woman’s history is consistent with tension-type headache, not migraine. Tension headache is the most common subtype, with prevalence as high as 59% in women of reproductive age.2 It is generally characterized by mild or moderate pain that is bilateral, pressing, or tightening in quality. The pain does not worsen with routine physical activity. There is no nausea, but photophobia or phonophobia may be present.1
A systematic review of the risk of stroke associated with combination OC use and headaches did not find any studies examining the association between nonmigraine headache and the risk of stroke among combination OC users.3 In contrast to some migraines, however, tension-type headache has not been associated with an increased risk of stroke in the general population. Nor is there evidence that hormonal fluctuations play a role in the pathogenesis or clinical course of tension headache.
In summary, there are no contraindications to combination hormonal contraceptives—including estrogen-progestin OCs, the contraceptive patch, and the contraceptive ring—in women who have tension headache.4
Explore any family history of migraine
The patient in Case 1 appears to have a family history of migraine. Some evidence suggests that such a family history increases the risk of new-onset migraine with use of a combination OC.5 Because the background prevalence of migraine is so high in the population of women likely to use a combination OC, it can be difficult to determine whether worsening headache or development of migraine with OC use is causal or coincidental.
Were this patient to express concern over even a theoretical risk of triggering migraine headache, then a combination OC would probably not be appropriate. In the absence of such concern, however, there is no reason to withhold hormonal contraception. Progestin-only options exist that will provide her with excellent contraceptive efficacy and help relieve her dysmenorrhea:
- the etonogestrel subdermal implant (Implanon)
- depot medroxyprogesterone acetate (DMPA) injection (Depo-Provera)
- the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena).
Although some women do develop headaches while using progestin-only contraceptives, there is no evidence that such use can trigger a new migraine syndrome in a woman with a family history of such. Again, however, the data are limited.
Progestin methods are safe
The use of progestin-only methods has been promoted in headache sufferers, especially those who have a specific diagnosis of migraine, because progestins do not add to the elevated risk of stroke that accompanies migraine with aura.
Because headache is common in women of reproductive age, it is not surprising that it is listed as a common adverse event for all contraceptives, including progestin-only methods. Evidence that progestin-only methods cause or worsen headaches is slim, however. Preliminary studies indicate that mid-luteal elevations of progesterone or its metabolites could prevent migraine, compared with other times in the cycle.6 Older studies report that a daily oral progestin could prevent migraine in premenopausal women, possibly secondary to induction of anovulation. At the same time, there are clinical reports that DMPA may trigger headache as a side effect in susceptible women.
Generally, then, although progestin-only methods are likely to be safe in all patients with headache, and ovulation suppression may improve the headaches, some patients may experience worsening symptoms.
CASE 2: OC user reports migraine with aura
A 26-year-old mother of one comes to your office for her annual exam. She has used combination OCs for 2 years. She also has a history of severe headaches, which occur four or five times a year. She says the headaches are unilateral, pulsating, and associated with photophobia. The symptoms worsen when she is active and are preceded by a flashing zigzag line that migrates from the center of her visual field to the lateral periphery. The headaches are not associated with her menstrual cycle and have not changed in character or frequency since she began using an OC. She does not smoke.
Should she continue taking an OC?
This patient’s history is consistent with migraine headache with aura. Migraine is a common, disabling primary headache disorder, with an estimated 1-year prevalence in adult women of 15% to 18% and a lifetime prevalence of about 30%.2 Approximately 10% to 20% of people who have migraine experience auras.7
Research on the association between combination OCs, migraine, and stroke has been limited by the rarity of the outcome in the population of concern. Most data come from case-controlled studies and are fettered by a lack of standardized criteria for the diagnosis of migraine (few studies use criteria from the IHS); by recall bias (such as self-reported OC use); and by survivorship bias. Many studies fail to differentiate by the presence of aura, which indicates a different effect on cerebral blood flow patterns than does migraine without aura.
Although most studies attempt to control for the confounding effect of smoking, some do not, and in others the prevalence of smoking is so high it can be difficult to remove from the equation. Some of the studies examining the association between migraine and stroke do not differentiate by gender.
Taking all these variables into account, migraine in women independently appears to carry a twofold to threefold increased risk of ischemic stroke, compared with the risk in similarly aged women who do not have migraine.8 Among women who have a history of migraine, those who use combination OCs are two times to four times more likely to experience ischemic stroke than nonusers are. Among women with the highest risk (combination OC users with migraine), the odds ratio for ischemic stroke ranges from 6 to almost 14, compared with women with the lowest risk (nonusers without migraine).3
To put all this in perspective, the absolute risk of stroke for a 26-year-old nonsmoker like our patient is 6 cases in every 100,000 woman-years.9 Multiplying this risk by a factor of 3 to account for her migraines, and by 3 again to account for her OC use, we can roughly estimate her absolute risk of stroke as about 54 cases for every 100,000 woman-years. Although this absolute risk is extremely low, the outcome can be catastrophic. It behooves us to proceed with caution.
A common misperception among health-care providers is that nausea, vomiting, photophobia, and phonophobia represent migraine aura, when in fact these symptoms are part of the associated diagnostic symptoms of all migraines.
About 20% of people who have migraine experience an aura. The aura begins before the headache and typically lasts 5 to 20 minutes—rarely does it last more than 60 minutes. The headache occurs soon after the aura stops.
The aura may include zigzag lines of light, flashing lights or bright spots, blurred or darkened spots, or focal neurologic symptoms such as numbness or tingling in the fingers of one hand, lips, tongue, or lower face. Auras may involve other senses, such as smell, and can occasionally cause temporary focal weakness or changes in speech.
Presence of aura likely confers greater risk
Many studies of migraine do not explore contraceptive use. They report a higher risk of stroke when aura is present.8,10-15 One of the few prospective studies found no increased risk of stroke in migraineurs who did not experience aura.12
No studies examining a link between combination OCs and ischemic stroke in migraineurs have been large enough to stratify the risk of stroke by the presence or absence of aura. The assumption has been that this risk is amplified by use of combination OCs. Consequently, migraine with aura is designated as an absolute contraindication to the use of combination OCs by the World Health Organization (WHO), ACOG, and the IHS.4,16Although no studies have included women using the contraceptive patch or ring, it is assumed that these methods carry a risk of ischemic stroke similar to that of combination OCs.17 The IHS recommendations on combination hormonal contraception in women with migraine are given in TABLE 2.18
TABLE 2
When combination OCs are appropriate for a woman who has migraine
|
| Source: International Headache Society18 |
Although the patient in Case 2 has been experiencing migraine with aura for some time without a change in headache pattern, combination hormonal contraception is contraindicated. Her history of migraine with aura may have been missed during previous evaluation if she did not consider it to be a “medical problem.” All candidates for combination OCs should be directly questioned about any history of migraine, thrombosis, cigarette smoking, and hypertension.
The patient described in Case 2 should be encouraged to switch to a progestin-only method of contraception or a highly effective nonhormonal method, such as a copper-containing IUD (Paragard). Because she had no change in her headache pattern on ovulation-suppression therapy (OCs), she likely will have no change with continued ovulation suppression (DMPA, subdermal implant) or without it (LNG-IUS, copper lUD).
Be sure to time the switch in methods so that contraception is maintained throughout the transition. This patient would also benefit from referral to a headache specialist for evaluation and treatment.
CASE 3 Perimenstrual headache in a smoker
A 32-year-old mother of two visits your office for contraceptive counseling. She reports that she smokes two to five cigarettes a day and has a history of migraine without aura. Her headaches occur exclusively during the two days just before her period, and they resolve within the first few days of bleeding. NSAIDs provide limited relief. Is hormonal contraception appropriate?
When a woman with perimenstrual headache is choosing a contraceptive method, we need to ensure that she is not exposed to undue risks and focus on improving her quality of life, if possible.
TABLE 1, reviews the diagnostic criteria for pure menstrual migraine, in which symptoms occur only in the perimenstrual period, as in this patient. Most practitioners use a broader definition of menstrual-related migraine, in which women may have other migraine triggers but still experience predictable headaches with menses. More than 50% of women who have migraine report an association between their headaches and menstruation, but only about 10% of women report migraine exclusively with menstruation.7
Hormonal manipulation is not first-line management for menstrual migraine.19 However, for the patient seeking contraception, it is appropriate to consider the effect of a given method on her headaches because that effect is sure to influence her satisfaction, quality of life, and compliance.
Menstrual migraine is rarely associated with aura, even in women who have migraine with aura at other times in the cycle.7However, the headaches experienced at the time of menstruation tend to be more severe and longer-lasting and less responsive to medication than are migraines experienced at other times of the month.20
Eliminate the placebo week
Research into the pathogenesis of menstrual migraine has focused on the withdrawal of estrogen in the luteal phase of the menstrual cycle. Ovulation is not a requisite for menstrual migraine attacks, which frequently occur during the hormone-free interval in combination hormonal contraceptive regimens. Several lines of evidence suggest that stabilizing estrogen fluctuations can prevent migraine (FIGURE).7

Hormonal fluctuations can trigger migraine
Fluctuations in estrogen during the menstrual cycle may trigger dilation and constriction of blood vessels in the brain, causing migraine.If a patient who has pure menstrual migraine desires to use an estrogen-containing contraceptive, she is likely to be as safe in that choice as a patient without migraine. However, she is not likely to experience improvement in her migraines if she uses a traditional 21/7 active-to-placebo pill regimen. It is necessary to maintain an adequate estrogen level during the placebo week, either through estrogen supplementation or extended-cycle dosing, to prevent the estrogen-withdrawal trigger for headaches.
A few OC regimens reduce the estrogen-free interval to 4 days or provide estrogen supplementation during the placebo week; these regimens may benefit women who have menstrual migraine, although data are scant. If most, or all, of the patient’s migraines are suppressed on combined hormonal contraception, continuous use with rare (e.g., yearly) withdrawals may be indicated.
Alternatives to estrogen-containing contraceptives
If the patient does not desire a combination OC or has other contraindications to estrogen, she may benefit from a progestin-only contraceptive that suppresses ovulation, such as DMPA or the etonogestrel implant. Even with ovulation suppression, however, estrogen fluctuations may occur, and the evidence supporting the use of these methods in the concurrent management of contraceptive needs and menstrual migraine is less clear. Although they are safe in women who have migraine, progestin-only pills and the LNG-IUS do not suppress ovulation reliably enough to be considered useful in managing menstrual migraine.
Take a careful history
Choosing a contraceptive for a woman who has headaches begins the same way as with any other patient: with careful assessment of her short-term and long-term family-planning goals, along with a history and physical to elicit any “red flags,” cultural preferences, and tolerance for specific side effects.
Once you become aware of a history of headache, careful history-taking can usually differentiate between the major headache subtypes and help you avoid limiting contraceptive options unnecessarily.
Women who start any contraceptive may report improvement, worsening, or new onset of headache symptoms. This change usually occurs within the first 3 months, and headaches associated with hormone use tend to improve with continued use.21
In women who have preexisting migraine, you should undertake prompt evaluation and consider a change of contraceptive method in response to 1) any increase in severity or frequency or 2) the onset of associated neurologic symptoms.
We want to hear from you! Tell us what you think.
1. International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(suppl 1):9-160.
2. Rasmussen BK. Epidemiology of headache. Cephalalgia. 2001;21(7):774-777.
3. Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006;73(2):189-194.
4. Harris M, Kaneshiro B. An evidence-based approach to hormonal contraception and headaches. Contraception. 2009;80(5):417-421.
5. Loder EW, Buse DC, Golub JR. Headache and combination estrogen-progestin oral contraceptives: integrating evidence, guidelines, and clinical practice. Headache. 2005;45(3):224.
6. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis— part 2. Headache. 2006;46(3):365-386.
7. Macgregor EA. Menstrual migraine: a clinical review. J Fam Plann Reprod Health Care. 2007;33(1):36-47.
8. Etminan M, Takkouche B, Isorna FC, et al. Risk of ischaemic stroke in people with migraine: systematic review and metaanalysis of observational studies. BMJ. 2005;330(7482):63.-
9. Petitti DB, Sidney S, Quesenberry CP, Jr. Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28(2):280-283.
10. Carolei A, Marini C, De Matteis G. History of migraine and risk of cerebral ischaemia in young adults. Lancet. 1996;347(9014):1503-1506.
11. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. BMJ. 1999;318(7175):13.
12. Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64(6):1020-1026.
13. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38(9):2438-2445.
14. Tzourio C, Kittner SJ, Bousser MG, Alperovitch A. Migraine and stroke in young women. Cephalalgia. 2000;20(3):190-199.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310(6983):830-833.
16. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107(6):1453-1472.
17. Department of Reproductive Health. Medical Eligibility Criteria for Contraceptive Use. 4th ed; 2009. Geneva, Switzerland: World Health Organization; 2010.
18. Bousser MG, Conard J, Kittner S, et al. Recommendations on the risk of ischaemic stroke associated with use of combined oral contraceptives and hormone replacement therapy in women with migraine. The International Headache Society Task Force on Combined Oral Contraceptives & Hormone Replacement Therapy. Cephalalgia. 2000;20(3):155-156.
19. Calhoun AH. The gynecologist’s role in managing menstrual migraine. OBG Manage. 2010;22(4):30-43.
20. Martin VT, Wernke S, Mandell K, et al. Defining the relationship between ovarian hormones and migraine headache. Headache. 2005;45(9):1190-1201.
21. Allais G, Gabellari IC, De Lorenzo C, C, Mana O, Benedetto C. Oral contraceptives in migraine. Expert Rev Neurother. 2009;9(3):381-393.
1. International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(suppl 1):9-160.
2. Rasmussen BK. Epidemiology of headache. Cephalalgia. 2001;21(7):774-777.
3. Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006;73(2):189-194.
4. Harris M, Kaneshiro B. An evidence-based approach to hormonal contraception and headaches. Contraception. 2009;80(5):417-421.
5. Loder EW, Buse DC, Golub JR. Headache and combination estrogen-progestin oral contraceptives: integrating evidence, guidelines, and clinical practice. Headache. 2005;45(3):224.
6. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis— part 2. Headache. 2006;46(3):365-386.
7. Macgregor EA. Menstrual migraine: a clinical review. J Fam Plann Reprod Health Care. 2007;33(1):36-47.
8. Etminan M, Takkouche B, Isorna FC, et al. Risk of ischaemic stroke in people with migraine: systematic review and metaanalysis of observational studies. BMJ. 2005;330(7482):63.-
9. Petitti DB, Sidney S, Quesenberry CP, Jr. Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28(2):280-283.
10. Carolei A, Marini C, De Matteis G. History of migraine and risk of cerebral ischaemia in young adults. Lancet. 1996;347(9014):1503-1506.
11. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. BMJ. 1999;318(7175):13.
12. Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64(6):1020-1026.
13. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38(9):2438-2445.
14. Tzourio C, Kittner SJ, Bousser MG, Alperovitch A. Migraine and stroke in young women. Cephalalgia. 2000;20(3):190-199.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310(6983):830-833.
16. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107(6):1453-1472.
17. Department of Reproductive Health. Medical Eligibility Criteria for Contraceptive Use. 4th ed; 2009. Geneva, Switzerland: World Health Organization; 2010.
18. Bousser MG, Conard J, Kittner S, et al. Recommendations on the risk of ischaemic stroke associated with use of combined oral contraceptives and hormone replacement therapy in women with migraine. The International Headache Society Task Force on Combined Oral Contraceptives & Hormone Replacement Therapy. Cephalalgia. 2000;20(3):155-156.
19. Calhoun AH. The gynecologist’s role in managing menstrual migraine. OBG Manage. 2010;22(4):30-43.
20. Martin VT, Wernke S, Mandell K, et al. Defining the relationship between ovarian hormones and migraine headache. Headache. 2005;45(9):1190-1201.
21. Allais G, Gabellari IC, De Lorenzo C, C, Mana O, Benedetto C. Oral contraceptives in migraine. Expert Rev Neurother. 2009;9(3):381-393.

