User login
What’s the gist of a new FDA label for the LNG-IUS?
Since the levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena, Bayer Health Care Pharmaceuticals) ( FIGURE 1 ) was introduced in the United States in 2001, more than 2 million devices have been used by women here. This use has contributed to a cumulative experience of 14 million women (36 million woman-years of experience) in 120 countries over the last 18 years.
Recent Food and Drug Administration (FDA)-approved labeling changes for the LNG-IUS1 expand the pool of women who are candidates for this convenient, reversible method of contraception. This article summarizes those labeling changes and answers questions that are often asked by clinicians who, more and more, insert the LNG-IUS for their patients.
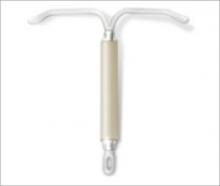
FIGURE 1 LNG-IUS, arms open
The LNG-IUS device is readily visible on radiographs. Visualization by ultrasonography is more challenging.
SOURCE: BAYER HEALTH CARE PHARMACEUTICALS. USED WITH PERMISSION.
What changes have been made to labeling?
Patient profile. The so-called recommended patient profile has been streamlined. The label now indicates only that the LNG-IUS is recommended for women who have given birth to at least one child. A more detailed description of women who were included in the phase-II US clinical trial is included in that section of the labeling. Clinicians should recognize that neither nulliparity nor nulligravity is listed in labeling as a contraindication to the LNG-IUS.
Depth of cavity. Another important change in the new labeling is that the LNG-IUS can be used in women whose uterine cavity sounds to a depth of 6 to 10 cm (no longer only 6 to 9 cm). This will permit more multiparous women, who may have a larger cavity, to use the LNG-IUS.
Pregnancy risk and consequences. The risk of pregnancy is rare when the LNG-IUS is used; pregnancy with the device in situ does not appear to be associated with an increased risk of birth defects. As of September 2006, there had been only 390 live births among an estimated cumulative 9.9 million LNG-IUS users worldwide. Of those births, congenital anomalies have been infrequent; no clear trend was seen toward an increased risk of any specific anomaly after exposure to the LNG-IUS.
Adverse events. As worldwide experience with the LNG-IUS has expanded since the last labeling, the number of reported cases of relatively rare adverse events has also been updated:
- Only nine cases of infection with group A Streptococcus have been reported in 9.9 million users, constituting a risk of approximately one infection for every 1 million users
- Based on postmarketing experience, new wording has been added about the possibility of 1) device breakage (before insertion) and 2) angioedema—a rare allergic reaction that is not specific to levonorgestrel (or the IUS)
- Wording has been added to the labeling that provides reassurance, based on observational studies, that there is no evidence of an increase in the risk of breast cancer risk with use of the LNG-IUS
- Similarly, the new labeling declares that, in general, no adverse eff ects have been found with use of the LNG-IUS on breast-feeding performance in regard to the health, growth, or development of an infant—even though isolated cases of a decrease in milk production have been reported.
Contraindications. The roster of contraindications ( TABLE ) has been significantly modified to reflect scientific evidence. Removed from that list are 1) a history of ectopic pregnancy and 2) risk factors for ectopic pregnancy.
TABLE A new label lists 12 contraindications to the LNG-IUS
| The LNG-IUS is contraindicated in the presence of one or more of the following: |
| Pregnancy or suspicion of pregnancy |
| Congenital or acquired uterine anomaly, including fibroids if they distort the uterine cavity |
| Acute pelvic inflammatory disease or a history of pelvic inflammatory disease, unless there has been a subsequent intrauterine pregnancy |
| Postpartum endometritis or infected abortion in the past 3 months |
| Known or suspected uterine or cervical neoplasia or unresolved, abnormal Pap smear |
| Genital bleeding of unknown cause |
| Untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infection, until infection is controlled |
| Acute liver disease or liver tumor (benign or malignant |
| Conditions associated with an increased susceptibility to pelvic infection |
| Previously inserted intrauterine device that has not been removed |
| Hypersensitivity to any device component |
| Known or suspected carcinoma of the breast |
When should I place the LNG-IUS?
Labeling recommends that, in a cycling woman, the LNG-IUS be placed sometime during the first 7 days of menses. A woman who has it placed at any other time in the cycle needs to be screened for pregnancy and needs to use back-up contraception for 7 days after the LNG-IUS is placed.
How should I place the device?
Complete instructions on insertion are provided in the package insert for the LNG-IUS. A few points about placement highlighted in the new labeling should be noted:
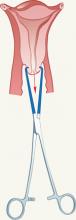
- The need to use a tenaculum to stabilize the cervix and to straighten the uterine axis has been reinforced in the new labeling ( FIGURE 2 )
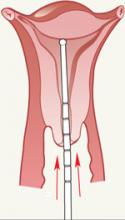
- Careful uterine sounding ( FIGURE 3 ) is needed to evaluate the uterine cavity to rule out any significant distortion of the cavity and to ensure appropriate uterine size before the LNG-IUS package is opened
- Uterine perforation is rare with the LNG-IUS, but to achieve that low risk you must wait at least 10 seconds for the arms of the device to open within the uterine cavity before it is advanced to the fundus
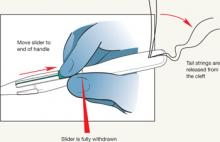
- The procedure-related expulsion rate can be reduced if the clinician moves the slider to the end of the handle (Position 3) carefully and waits for the tail strings to be released from the cleft ( FIGURE 4 ) before trying to withdraw the insertion device.
FIGURE 2 Tenaculum use is key
FIGURE 3 Sound the uterus
FIGURE 4 Release tail strings before withdrawing insertion device
What should I do if the LNG-IUS isn’t at the fundus?
Studies have shown there can be significant migration of the LNG-IUS within the uterine cavity. Fundal placement insures that the tail strings will be long enough to remove the device regardless of where it settles. A device that settles within the lower uterine segment is still effective. Removal of the device is necessary only if 1) a portion of it protrudes from the cervix or 2) the woman has excessive cramping with a low-lying device.
How can I visualize an LNG-IUS?
The LNG-IUS is visible on a radiograph. It is more challenging to image the device by ultrasonography; visualization of the shadowing beneath the device can help localize the unit. It is important to know that the tail strings are more echogenic than the device. Failure to recognize this allows the false impression that the IUD is lower than it actually is.
A woman can safely have a magnetic resonance imaging study without disrupting the position of the LNG-IUS device. It will not set off alarms in security systems—such as those used at an airport
I place only a few LNG-IUS devices each year. What should I do to remain competent and confident at performing this procedure?
Many teaching aids are available to refresh your skills. Options include instructional CD-ROMs and manuals and hands-on practice with a representative of the manufacturer of the device. As noted, the LNG-IUS package insert offers step-by-step instructions on the procedure for placing the device.
What about concerns over cost that some patients express to me?
Women can charge the cost of the device to a credit card if their health insurance does not cover it, or its insertion. Payment plans are also available from the manufacturer.
If you attempt to assist your patients by finding less expensive LNG-IUS units (that is, from a source other than the manufacturer), be aware that the device must be stored under controlled conditions in transit, similar to the way other delicate devices are (e.g., NuvaRing, Implanon). LNG-IUS devices that are shipped under less-than-optimal controlled conditions may not maintain their stability or provide the appropriate rate of release of the contraceptive hormone.
1. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ: Bayer Health Care Pharmaceuticals; 2008.
Since the levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena, Bayer Health Care Pharmaceuticals) ( FIGURE 1 ) was introduced in the United States in 2001, more than 2 million devices have been used by women here. This use has contributed to a cumulative experience of 14 million women (36 million woman-years of experience) in 120 countries over the last 18 years.
Recent Food and Drug Administration (FDA)-approved labeling changes for the LNG-IUS1 expand the pool of women who are candidates for this convenient, reversible method of contraception. This article summarizes those labeling changes and answers questions that are often asked by clinicians who, more and more, insert the LNG-IUS for their patients.
FIGURE 1 LNG-IUS, arms open
The LNG-IUS device is readily visible on radiographs. Visualization by ultrasonography is more challenging.
SOURCE: BAYER HEALTH CARE PHARMACEUTICALS. USED WITH PERMISSION.
What changes have been made to labeling?
Patient profile. The so-called recommended patient profile has been streamlined. The label now indicates only that the LNG-IUS is recommended for women who have given birth to at least one child. A more detailed description of women who were included in the phase-II US clinical trial is included in that section of the labeling. Clinicians should recognize that neither nulliparity nor nulligravity is listed in labeling as a contraindication to the LNG-IUS.
Depth of cavity. Another important change in the new labeling is that the LNG-IUS can be used in women whose uterine cavity sounds to a depth of 6 to 10 cm (no longer only 6 to 9 cm). This will permit more multiparous women, who may have a larger cavity, to use the LNG-IUS.
Pregnancy risk and consequences. The risk of pregnancy is rare when the LNG-IUS is used; pregnancy with the device in situ does not appear to be associated with an increased risk of birth defects. As of September 2006, there had been only 390 live births among an estimated cumulative 9.9 million LNG-IUS users worldwide. Of those births, congenital anomalies have been infrequent; no clear trend was seen toward an increased risk of any specific anomaly after exposure to the LNG-IUS.
Adverse events. As worldwide experience with the LNG-IUS has expanded since the last labeling, the number of reported cases of relatively rare adverse events has also been updated:
- Only nine cases of infection with group A Streptococcus have been reported in 9.9 million users, constituting a risk of approximately one infection for every 1 million users
- Based on postmarketing experience, new wording has been added about the possibility of 1) device breakage (before insertion) and 2) angioedema—a rare allergic reaction that is not specific to levonorgestrel (or the IUS)
- Wording has been added to the labeling that provides reassurance, based on observational studies, that there is no evidence of an increase in the risk of breast cancer risk with use of the LNG-IUS
- Similarly, the new labeling declares that, in general, no adverse eff ects have been found with use of the LNG-IUS on breast-feeding performance in regard to the health, growth, or development of an infant—even though isolated cases of a decrease in milk production have been reported.
Contraindications. The roster of contraindications ( TABLE ) has been significantly modified to reflect scientific evidence. Removed from that list are 1) a history of ectopic pregnancy and 2) risk factors for ectopic pregnancy.
TABLE A new label lists 12 contraindications to the LNG-IUS
| The LNG-IUS is contraindicated in the presence of one or more of the following: |
| Pregnancy or suspicion of pregnancy |
| Congenital or acquired uterine anomaly, including fibroids if they distort the uterine cavity |
| Acute pelvic inflammatory disease or a history of pelvic inflammatory disease, unless there has been a subsequent intrauterine pregnancy |
| Postpartum endometritis or infected abortion in the past 3 months |
| Known or suspected uterine or cervical neoplasia or unresolved, abnormal Pap smear |
| Genital bleeding of unknown cause |
| Untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infection, until infection is controlled |
| Acute liver disease or liver tumor (benign or malignant |
| Conditions associated with an increased susceptibility to pelvic infection |
| Previously inserted intrauterine device that has not been removed |
| Hypersensitivity to any device component |
| Known or suspected carcinoma of the breast |
When should I place the LNG-IUS?
Labeling recommends that, in a cycling woman, the LNG-IUS be placed sometime during the first 7 days of menses. A woman who has it placed at any other time in the cycle needs to be screened for pregnancy and needs to use back-up contraception for 7 days after the LNG-IUS is placed.
How should I place the device?
Complete instructions on insertion are provided in the package insert for the LNG-IUS. A few points about placement highlighted in the new labeling should be noted:
- The need to use a tenaculum to stabilize the cervix and to straighten the uterine axis has been reinforced in the new labeling ( FIGURE 2 )
- Careful uterine sounding ( FIGURE 3 ) is needed to evaluate the uterine cavity to rule out any significant distortion of the cavity and to ensure appropriate uterine size before the LNG-IUS package is opened
- Uterine perforation is rare with the LNG-IUS, but to achieve that low risk you must wait at least 10 seconds for the arms of the device to open within the uterine cavity before it is advanced to the fundus
- The procedure-related expulsion rate can be reduced if the clinician moves the slider to the end of the handle (Position 3) carefully and waits for the tail strings to be released from the cleft ( FIGURE 4 ) before trying to withdraw the insertion device.
FIGURE 2 Tenaculum use is key
FIGURE 3 Sound the uterus
FIGURE 4 Release tail strings before withdrawing insertion device
What should I do if the LNG-IUS isn’t at the fundus?
Studies have shown there can be significant migration of the LNG-IUS within the uterine cavity. Fundal placement insures that the tail strings will be long enough to remove the device regardless of where it settles. A device that settles within the lower uterine segment is still effective. Removal of the device is necessary only if 1) a portion of it protrudes from the cervix or 2) the woman has excessive cramping with a low-lying device.
How can I visualize an LNG-IUS?
The LNG-IUS is visible on a radiograph. It is more challenging to image the device by ultrasonography; visualization of the shadowing beneath the device can help localize the unit. It is important to know that the tail strings are more echogenic than the device. Failure to recognize this allows the false impression that the IUD is lower than it actually is.
A woman can safely have a magnetic resonance imaging study without disrupting the position of the LNG-IUS device. It will not set off alarms in security systems—such as those used at an airport
I place only a few LNG-IUS devices each year. What should I do to remain competent and confident at performing this procedure?
Many teaching aids are available to refresh your skills. Options include instructional CD-ROMs and manuals and hands-on practice with a representative of the manufacturer of the device. As noted, the LNG-IUS package insert offers step-by-step instructions on the procedure for placing the device.
What about concerns over cost that some patients express to me?
Women can charge the cost of the device to a credit card if their health insurance does not cover it, or its insertion. Payment plans are also available from the manufacturer.
If you attempt to assist your patients by finding less expensive LNG-IUS units (that is, from a source other than the manufacturer), be aware that the device must be stored under controlled conditions in transit, similar to the way other delicate devices are (e.g., NuvaRing, Implanon). LNG-IUS devices that are shipped under less-than-optimal controlled conditions may not maintain their stability or provide the appropriate rate of release of the contraceptive hormone.
Since the levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena, Bayer Health Care Pharmaceuticals) ( FIGURE 1 ) was introduced in the United States in 2001, more than 2 million devices have been used by women here. This use has contributed to a cumulative experience of 14 million women (36 million woman-years of experience) in 120 countries over the last 18 years.
Recent Food and Drug Administration (FDA)-approved labeling changes for the LNG-IUS1 expand the pool of women who are candidates for this convenient, reversible method of contraception. This article summarizes those labeling changes and answers questions that are often asked by clinicians who, more and more, insert the LNG-IUS for their patients.
FIGURE 1 LNG-IUS, arms open
The LNG-IUS device is readily visible on radiographs. Visualization by ultrasonography is more challenging.
SOURCE: BAYER HEALTH CARE PHARMACEUTICALS. USED WITH PERMISSION.
What changes have been made to labeling?
Patient profile. The so-called recommended patient profile has been streamlined. The label now indicates only that the LNG-IUS is recommended for women who have given birth to at least one child. A more detailed description of women who were included in the phase-II US clinical trial is included in that section of the labeling. Clinicians should recognize that neither nulliparity nor nulligravity is listed in labeling as a contraindication to the LNG-IUS.
Depth of cavity. Another important change in the new labeling is that the LNG-IUS can be used in women whose uterine cavity sounds to a depth of 6 to 10 cm (no longer only 6 to 9 cm). This will permit more multiparous women, who may have a larger cavity, to use the LNG-IUS.
Pregnancy risk and consequences. The risk of pregnancy is rare when the LNG-IUS is used; pregnancy with the device in situ does not appear to be associated with an increased risk of birth defects. As of September 2006, there had been only 390 live births among an estimated cumulative 9.9 million LNG-IUS users worldwide. Of those births, congenital anomalies have been infrequent; no clear trend was seen toward an increased risk of any specific anomaly after exposure to the LNG-IUS.
Adverse events. As worldwide experience with the LNG-IUS has expanded since the last labeling, the number of reported cases of relatively rare adverse events has also been updated:
- Only nine cases of infection with group A Streptococcus have been reported in 9.9 million users, constituting a risk of approximately one infection for every 1 million users
- Based on postmarketing experience, new wording has been added about the possibility of 1) device breakage (before insertion) and 2) angioedema—a rare allergic reaction that is not specific to levonorgestrel (or the IUS)
- Wording has been added to the labeling that provides reassurance, based on observational studies, that there is no evidence of an increase in the risk of breast cancer risk with use of the LNG-IUS
- Similarly, the new labeling declares that, in general, no adverse eff ects have been found with use of the LNG-IUS on breast-feeding performance in regard to the health, growth, or development of an infant—even though isolated cases of a decrease in milk production have been reported.
Contraindications. The roster of contraindications ( TABLE ) has been significantly modified to reflect scientific evidence. Removed from that list are 1) a history of ectopic pregnancy and 2) risk factors for ectopic pregnancy.
TABLE A new label lists 12 contraindications to the LNG-IUS
| The LNG-IUS is contraindicated in the presence of one or more of the following: |
| Pregnancy or suspicion of pregnancy |
| Congenital or acquired uterine anomaly, including fibroids if they distort the uterine cavity |
| Acute pelvic inflammatory disease or a history of pelvic inflammatory disease, unless there has been a subsequent intrauterine pregnancy |
| Postpartum endometritis or infected abortion in the past 3 months |
| Known or suspected uterine or cervical neoplasia or unresolved, abnormal Pap smear |
| Genital bleeding of unknown cause |
| Untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infection, until infection is controlled |
| Acute liver disease or liver tumor (benign or malignant |
| Conditions associated with an increased susceptibility to pelvic infection |
| Previously inserted intrauterine device that has not been removed |
| Hypersensitivity to any device component |
| Known or suspected carcinoma of the breast |
When should I place the LNG-IUS?
Labeling recommends that, in a cycling woman, the LNG-IUS be placed sometime during the first 7 days of menses. A woman who has it placed at any other time in the cycle needs to be screened for pregnancy and needs to use back-up contraception for 7 days after the LNG-IUS is placed.
How should I place the device?
Complete instructions on insertion are provided in the package insert for the LNG-IUS. A few points about placement highlighted in the new labeling should be noted:
- The need to use a tenaculum to stabilize the cervix and to straighten the uterine axis has been reinforced in the new labeling ( FIGURE 2 )
- Careful uterine sounding ( FIGURE 3 ) is needed to evaluate the uterine cavity to rule out any significant distortion of the cavity and to ensure appropriate uterine size before the LNG-IUS package is opened
- Uterine perforation is rare with the LNG-IUS, but to achieve that low risk you must wait at least 10 seconds for the arms of the device to open within the uterine cavity before it is advanced to the fundus
- The procedure-related expulsion rate can be reduced if the clinician moves the slider to the end of the handle (Position 3) carefully and waits for the tail strings to be released from the cleft ( FIGURE 4 ) before trying to withdraw the insertion device.
FIGURE 2 Tenaculum use is key
FIGURE 3 Sound the uterus
FIGURE 4 Release tail strings before withdrawing insertion device
What should I do if the LNG-IUS isn’t at the fundus?
Studies have shown there can be significant migration of the LNG-IUS within the uterine cavity. Fundal placement insures that the tail strings will be long enough to remove the device regardless of where it settles. A device that settles within the lower uterine segment is still effective. Removal of the device is necessary only if 1) a portion of it protrudes from the cervix or 2) the woman has excessive cramping with a low-lying device.
How can I visualize an LNG-IUS?
The LNG-IUS is visible on a radiograph. It is more challenging to image the device by ultrasonography; visualization of the shadowing beneath the device can help localize the unit. It is important to know that the tail strings are more echogenic than the device. Failure to recognize this allows the false impression that the IUD is lower than it actually is.
A woman can safely have a magnetic resonance imaging study without disrupting the position of the LNG-IUS device. It will not set off alarms in security systems—such as those used at an airport
I place only a few LNG-IUS devices each year. What should I do to remain competent and confident at performing this procedure?
Many teaching aids are available to refresh your skills. Options include instructional CD-ROMs and manuals and hands-on practice with a representative of the manufacturer of the device. As noted, the LNG-IUS package insert offers step-by-step instructions on the procedure for placing the device.
What about concerns over cost that some patients express to me?
Women can charge the cost of the device to a credit card if their health insurance does not cover it, or its insertion. Payment plans are also available from the manufacturer.
If you attempt to assist your patients by finding less expensive LNG-IUS units (that is, from a source other than the manufacturer), be aware that the device must be stored under controlled conditions in transit, similar to the way other delicate devices are (e.g., NuvaRing, Implanon). LNG-IUS devices that are shipped under less-than-optimal controlled conditions may not maintain their stability or provide the appropriate rate of release of the contraceptive hormone.
1. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ: Bayer Health Care Pharmaceuticals; 2008.
1. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ: Bayer Health Care Pharmaceuticals; 2008.
FERTILITY
The diagnosis and treatment of fertility are evolving rapidly as a result of clinical studies, scientific research, and changing socioeconomic and ethical perspectives. These developments benefit health-care consumers, but they also pose new challenges to general ObGyns and other practitioners committed to the best possible care for their patients.
In this Update, I focus on a number of these areas of change:
- care of women who have polycystic ovary syndrome (PCOS)
- the impact of myomas on fertility
- treatment of infertility in women who have endometriosis
- when tubal reconstruction is appropriate
- the impact of a woman’s age on fertility
- patient-friendly strategies to enhance fertility
- cross-border reproductive travel.
Use clomiphene citrate to stimulate ovulation in women who have PCOS
Practice Committee of the American Society for Reproductive Medicine. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90(5 Suppl):S69–S73.
A new Committee Opinion from the American Society for Reproductive Medicine (ASRM) Practice Committee tackles the challenge of treating women with PCOS for infertility.
PCOS is associated with an increased risk of insulin resistance and diabetes mellitus. The first line of treatment for all women who have PCOS, especially those with an elevated body mass index, is lifestyle modification through diet and exercise, with the goal of losing weight.
Clomiphene is first-line therapy when ovulation is the aim
Metformin and other insulin-sensitizing agents may enhance ovulation and increase the response to clomiphene citrate in women who have PCOS and insulin resistance, but their use solely to enhance ovulation is unwarranted, and they do not reduce the rate of miscarriage. Clomiphene citrate should be the first-line treatment because it is much more effective. Long-term use of metformin to prevent disease is not advised.
Screen for insulin resistance at the time of diagnosis
Women who have PCOS should be given a 2-hour oral glucose tolerance test and have their lipid profile measured at the time of diagnosis and then at an interval of every 2 years. Insulin-sensitizing agents should be used for long-term health issues only after impaired glucose tolerance has been measured, if diet and exercise alone prove to be ineffective.
My strategy for stimulating ovulation in this population involves the following:
- Perform vaginal ultrasonography (US) on cycle day 3 for an antral follicle count and to rule out ovarian cysts >1 cm.
- Give clomiphene citrate, 50 mg, on cycle days 3 through 7 (or 5 through 9).
- Repeat vaginal US on cycle day 11 (or 13) to evaluate ovarian response. The optimal response is 1 to 2, and not more than 3, follicles ≥15 mm in size.
- Recommend timed intercourse, starting on cycle day 10 and then every 2±0.5 days until 1 to 2 days after ovulation.
- Measure urinary luteinizing hormone (uLH) daily, to detect uLH surge, starting on cycle day 11. A positive surge indicates that ovulation is likely within the next 12–48 hours. Absence of a surge indicates the likely absence of ovulation, which can be treated by giving 10,000 IU of human chorionic gonadotropin (hCG) subcutaneously or intramuscularly when the largest follicle is 18 to 25 mm in size.—G. DAVID ADAMSON, MD
When choosing a treatment for myoma, consider impacts on fertility
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproduction Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(5 Suppl): S125–S130.
A recent educational bulletin from the ASRM Practice Committee examined the relationship between myomas and reproductive function and reviewed management of this pathology.
The effects of myomas on reproductive outcome are ill-defined, but fibroids that distort the uterine cavity, as well as larger intramural myomas, may have adverse effects on fertility.
Select interventions carefully
Among women who have infertility and those who have recurrent pregnancy loss, myomectomy should be considered only after thorough evaluation. The reason? Postoperative adhesions as a result of abdominal myomectomy are common and may reduce subsequent fertility.
As for uterine artery embolization, myolysis, and MRI-guided ultrasonic treatment, these are not recommended for women who have myomas and who are seeking to maintain or improve fertility. The safety and efficacy of these procedures in this population have not been established.
Is a GnRH agonist useful?
Treatment of myomas with a gonadotropin-releasing hormone (GnRH) agonist does not improve fertility but may be helpful before surgery in anemic women and in those who might be able to undergo a less invasive procedure if the myoma volume were moderately smaller.
Sequence of infertility treatments is critical in endometriosis patients
Adamson G. Management of endometriosis and infertility following surgery. In: Sutton C, Jones K, Adamson GD, eds. Modern Management of Endometriosis. London: Taylor & Francis; 2006:273–287.
New data make it easier to treat infertility in women thought to have endometriosis, although further randomized trials are needed. If other fertility variables are normal, and minimal to mild endometriosis is suspected but not confirmed, clomiphene citrate, 100 mg on cycle days 3 through 7, followed by intrauterine insemination (IUI) for 3 to 6 cycles, is a reasonable initial treatment, with the higher number of cycles being reserved for younger patients and those who have a better prognosis.
When is surgery helpful?
Diagnostic or operative laparoscopy, or both, is often indicated when one or more of the following are present:
- The patient experiences pain
- She fails to conceive after clomiphene citrate is administered and IUI is attempted for 3 to 6 cycles
- She has other factors associated with infertility.
Generally, if pregnancy does not occur within 9 to 15 months after surgery, repeat surgery is of limited benefit for infertility, but may have some benefit for pain. In women who do not conceive after surgery, ovarian suppression for 2 months is of possible benefit before assisted reproductive technology (ART) and should be considered in patients who are also suffering from pain. Pre-ART surgery for large endometriomas is frequently indicated, and excision of the cyst capsule produces results superior to those of drainage, coagulation, or both.
Postoperative management
After complete destruction of endometriosis in women who have infertility, ovarian suppression is not indicated. Rather, the patient should usually attempt to conceive for 9 to 15 months, with an outside range of 3 to 24 months for much older women who have an unfavorable prognosis, and for much younger women who have a good prognosis, respectively. If pregnancy does not occur, clomiphene citrate and IUI for 3 to 6 months are then indicated.
If this last strategy is unsuccessful, the options include:
- gonadotropins and IUI for 3 months to a maximum of 6 months in the young patient who has a good prognosis
- repeat laparoscopy (although this option is rare), possibly in conjunction with gamete intrafallopian transfer (GIFT), or, alternatively, in vitro fertilization (IVF). If the patient had a technically inadequate operation the first time, it sometimes is appropriate to repeat the surgery or go directly to IVF.
Consider tubal reconstruction in carefully selected patients
Practice Committee of the American Society for Reproductive Medicine. The role of tubal reconstructive surgery in the era of assisted reproductive technologies. Fertil Steril. 2008;90(5 Suppl):S250–S253.
In the era of ART, tubal reconstruction has fewer indications but is still appropriate and effective in properly selected individuals.
Determine the extent of tubal disease before reconstructive surgery
Hysterosalpingography is a useful initial test for the evaluation of tubal patency, but laparoscopy often is necessary to identify the nature and extent of pelvic disease. Selective salpingography or hysteroscopic tubal recanalization can help confirm the diagnosis of true proximal tubal occlusion.
Advise the patient of risks of surgery
Generally, the risk of ectopic pregnancy after tubal reconstruction is comparable to the risk of ectopic pregnancy associated with IVF, but the extent of tubal disease and pelvic pathology are important variables in predicting intrauterine and ectopic pregnancy rates.
The pregnancy rate after reversal of tubal sterilization depends on 1) the type of sterilization procedure that was performed, 2) site of anastomosis, and 3) postoperative tubal length, as well as 4) sperm quality and 5) the age of the female patient.
Maternal age, number of children desired, coexisting infertility variables, risk of ectopic and multiple pregnancy, and treatment cost are important considerations when counseling patients about the relative advantages and disadvantages of tubal surgery and IVF.
IVF is the best treatment for older women of reproductive age who have significant tubal pathology, and for women who have both proximal and distal occlusion.
Age, and duration of infertility, are key determinants of treatment
Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists, and Practice Committee of the American Society for Reproductive Medicine. Age-related fertility decline: a Committee Opinion. Fertil Steril. 2008;90(5 Suppl):S154–S155.
Women older than 35 years should receive expedited evaluation and treatment for infertility if they have not conceived after 6 months, or earlier if clinically indicated. That’s one of the conclusions from a recent ACOG–ASRM joint Committee Opinion on age-related fertility decline.
Age remains a major variable influencing a woman’s fertility and risk of pregnancy loss, and is increasingly important because of the social trend toward deferred child-bearing. The fertility rate peaks in a woman’s mid-20s and decreases by approximately 25% by age 35 and 50% by age 40, with a concomitant (and significant) increase in rates of aneuploidy and miscarriage.
The duration of infertility also is key. Of any given 100 women attempting to conceive:
- 78 will succeed within 1 year
- 88 will conceive within 2 years
- only an additional two or three women will conceive in the third year
- one more will conceive in each of the fourth and fifth years
- only three more will conceive over the rest of their reproductive life.
Recurrent pregnancy loss and infertility are separate entities
By definition, recurrent pregnancy loss entails the loss of two or more pregnancies. When the cause is unknown, each loss merits careful review to determine whether specific evaluation may be appropriate. After three losses, thorough evaluation is warranted.1,2
To distinguish infertility from recurrent pregnancy loss, define clinical pregnancy as one documented by US or histopathology.
New technologies remain unproven
Although ovarian tissue and oocyte cryopreservation offer the promise of female fertility preservation, these technologies remain investigational to date.
The greatest benefit to patients who wish to preserve their fertility is appropriate counseling about their reproductive health.3, 4
Fertility can be enhanced with a few patient-friendly strategies
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(5 Suppl):S1–S6.
Another Committee Opinion from ASRM, in collaboration with the Society for Reproductive Endocrinology and Infertility, offers simple but effective steps for patients to take to optimize fertility. ObGyns should recommend these strategies to any woman planning to conceive in the near future.
Frequent intercourse is best
Intercourse every day or every other day yields the highest pregnancy rate, but intercourse two to three times a week is nearly equivalent. There is a “fertile window” that spans the 6-day interval ending on the day of ovulation, and it correlates with the volume and character of cervical mucus.
Among women who have regular menstrual cycles, frequent intercourse that begins soon after the cessation of menses can help maximize fecundity.
Devices that determine or predict the time of ovulation may be useful for couples who have infrequent intercourse.
Neither specific coital timing, nor position during coitus, nor rest in a supine position after intercourse has a significant impact on fertility.
Caffeine, alcohol OK—in moderation
Moderate caffeine or alcohol consumption (1 or 2 drinks daily) has no demonstrable adverse effect on fertility. Smoking, a higher level of alcohol consumption (≥2 drinks daily), use of recreational drugs, and most commercially available vaginal lubricants should be discouraged among patients who are trying to conceive.
Fertility rates are lower in women who are very thin or obese, but there is little evidence that dietary variations improve fertility or affect the gender of the infant.
Elevated blood mercury levels from heavy seafood consumption have been associated with infertility.
Saunas do not reduce fertility in women. In normal men, attempts to protect the testicles from excessive heat are unjustified.
Avoid solvents and pesticides
- Fecundity may be diminished in women who are exposed to certain toxins and solvents, such as those used in the dry-cleaning and printing industries.
- Men who are exposed to heavy metals may be more likely to have abnormal semen parameters.
- Pesticide exposure may be a problem for both male and female agricultural workers.
- Despite limited data on exposure to lead and use of industrial microwaves, they are probably best avoided or minimized.
- Prescription drug use should be carefully controlled and managed on an individual basis.
Recommend 400 µg of folic acid daily
Any woman hoping to conceive should be advised to initiate this regimen to reduce the risk of neural tube defects.
The Centers for Disease Control and Prevention (CDC) held its first Public Health Symposium on Infertility in September 2008. Consensus is growing that infertility is a common disease or disability that has serious consequences for the well-being of families—making it a public health concern.
Because only approximately 50% of patients who have infertility ever seek treatment, it is hoped that new programs will improve access to fertility treatment for many more women.
For more information on the CDC’s initiatives in reproductive health, visit: http://www.cdc.gov/reproductivehealth/
WHO focuses on international inequities
The World Health Organization (WHO) held a meeting in Geneva in December 2008 to modify its glossary of ART definitions and develop new terminology to allow the collection of better data on the use of IVF internationally.5, 6
The prevalence of infertility is about the same in all countries of the world, affecting, on average, about 9% of people of reproductive age. However, there is a greater degree of secondary infertility—mostly as a result of infectious disease and obstetric complications—in low-resource (developing) countries.
Infertility is a major burden with serious medical and psychological consequences in American society, but its impact on women in other cultures is often more profound, with loss of personal status, divorce, and social ostracism adding to the burden.
More and more women seek care in countries other than their own
“Medical tourism” is an interesting phenomenon that has received widespread media attention. When it applies to infertility, a more appropriate term may be “cross-border reproductive care.”
This is an international phenomenon that is, so far, poorly documented. Common reasons to travel for medical care include cost and availability of specialized services. Women grappling with infertility may also seek to bypass regulations or ethical issues that limit availability of treatment in their home country. Among the issues that prompt travel are:
- gamete and embryo donation
- payment of donors and surrogates
- nontraditional relationships.
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5 Suppl):S60.-
2. Adamson GD. Update in fertility. OBG Management. 2007;19(2):37-38, 41-44,-76.
3. Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90(5 Suppl):S241-S246.
4. Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2008;90(5 Suppl):S134-S135.
5. Zegers-Hochschild F, Nygren K-G, Adamson GD, et al. International Committee Monitoring Assisted Reproductive Technologies. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertil Steril. 2006;86:16-19.
6. International Committee for Monitoring Assisted Reproductive Technology, Adamson GD, de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F. World collaborative report on in vitro fertilization for year 2000. Fertil Steril. 2006;85:1586-1622.
The diagnosis and treatment of fertility are evolving rapidly as a result of clinical studies, scientific research, and changing socioeconomic and ethical perspectives. These developments benefit health-care consumers, but they also pose new challenges to general ObGyns and other practitioners committed to the best possible care for their patients.
In this Update, I focus on a number of these areas of change:
- care of women who have polycystic ovary syndrome (PCOS)
- the impact of myomas on fertility
- treatment of infertility in women who have endometriosis
- when tubal reconstruction is appropriate
- the impact of a woman’s age on fertility
- patient-friendly strategies to enhance fertility
- cross-border reproductive travel.
Use clomiphene citrate to stimulate ovulation in women who have PCOS
Practice Committee of the American Society for Reproductive Medicine. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90(5 Suppl):S69–S73.
A new Committee Opinion from the American Society for Reproductive Medicine (ASRM) Practice Committee tackles the challenge of treating women with PCOS for infertility.
PCOS is associated with an increased risk of insulin resistance and diabetes mellitus. The first line of treatment for all women who have PCOS, especially those with an elevated body mass index, is lifestyle modification through diet and exercise, with the goal of losing weight.
Clomiphene is first-line therapy when ovulation is the aim
Metformin and other insulin-sensitizing agents may enhance ovulation and increase the response to clomiphene citrate in women who have PCOS and insulin resistance, but their use solely to enhance ovulation is unwarranted, and they do not reduce the rate of miscarriage. Clomiphene citrate should be the first-line treatment because it is much more effective. Long-term use of metformin to prevent disease is not advised.
Screen for insulin resistance at the time of diagnosis
Women who have PCOS should be given a 2-hour oral glucose tolerance test and have their lipid profile measured at the time of diagnosis and then at an interval of every 2 years. Insulin-sensitizing agents should be used for long-term health issues only after impaired glucose tolerance has been measured, if diet and exercise alone prove to be ineffective.
My strategy for stimulating ovulation in this population involves the following:
- Perform vaginal ultrasonography (US) on cycle day 3 for an antral follicle count and to rule out ovarian cysts >1 cm.
- Give clomiphene citrate, 50 mg, on cycle days 3 through 7 (or 5 through 9).
- Repeat vaginal US on cycle day 11 (or 13) to evaluate ovarian response. The optimal response is 1 to 2, and not more than 3, follicles ≥15 mm in size.
- Recommend timed intercourse, starting on cycle day 10 and then every 2±0.5 days until 1 to 2 days after ovulation.
- Measure urinary luteinizing hormone (uLH) daily, to detect uLH surge, starting on cycle day 11. A positive surge indicates that ovulation is likely within the next 12–48 hours. Absence of a surge indicates the likely absence of ovulation, which can be treated by giving 10,000 IU of human chorionic gonadotropin (hCG) subcutaneously or intramuscularly when the largest follicle is 18 to 25 mm in size.—G. DAVID ADAMSON, MD
When choosing a treatment for myoma, consider impacts on fertility
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproduction Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(5 Suppl): S125–S130.
A recent educational bulletin from the ASRM Practice Committee examined the relationship between myomas and reproductive function and reviewed management of this pathology.
The effects of myomas on reproductive outcome are ill-defined, but fibroids that distort the uterine cavity, as well as larger intramural myomas, may have adverse effects on fertility.
Select interventions carefully
Among women who have infertility and those who have recurrent pregnancy loss, myomectomy should be considered only after thorough evaluation. The reason? Postoperative adhesions as a result of abdominal myomectomy are common and may reduce subsequent fertility.
As for uterine artery embolization, myolysis, and MRI-guided ultrasonic treatment, these are not recommended for women who have myomas and who are seeking to maintain or improve fertility. The safety and efficacy of these procedures in this population have not been established.
Is a GnRH agonist useful?
Treatment of myomas with a gonadotropin-releasing hormone (GnRH) agonist does not improve fertility but may be helpful before surgery in anemic women and in those who might be able to undergo a less invasive procedure if the myoma volume were moderately smaller.
Sequence of infertility treatments is critical in endometriosis patients
Adamson G. Management of endometriosis and infertility following surgery. In: Sutton C, Jones K, Adamson GD, eds. Modern Management of Endometriosis. London: Taylor & Francis; 2006:273–287.
New data make it easier to treat infertility in women thought to have endometriosis, although further randomized trials are needed. If other fertility variables are normal, and minimal to mild endometriosis is suspected but not confirmed, clomiphene citrate, 100 mg on cycle days 3 through 7, followed by intrauterine insemination (IUI) for 3 to 6 cycles, is a reasonable initial treatment, with the higher number of cycles being reserved for younger patients and those who have a better prognosis.
When is surgery helpful?
Diagnostic or operative laparoscopy, or both, is often indicated when one or more of the following are present:
- The patient experiences pain
- She fails to conceive after clomiphene citrate is administered and IUI is attempted for 3 to 6 cycles
- She has other factors associated with infertility.
Generally, if pregnancy does not occur within 9 to 15 months after surgery, repeat surgery is of limited benefit for infertility, but may have some benefit for pain. In women who do not conceive after surgery, ovarian suppression for 2 months is of possible benefit before assisted reproductive technology (ART) and should be considered in patients who are also suffering from pain. Pre-ART surgery for large endometriomas is frequently indicated, and excision of the cyst capsule produces results superior to those of drainage, coagulation, or both.
Postoperative management
After complete destruction of endometriosis in women who have infertility, ovarian suppression is not indicated. Rather, the patient should usually attempt to conceive for 9 to 15 months, with an outside range of 3 to 24 months for much older women who have an unfavorable prognosis, and for much younger women who have a good prognosis, respectively. If pregnancy does not occur, clomiphene citrate and IUI for 3 to 6 months are then indicated.
If this last strategy is unsuccessful, the options include:
- gonadotropins and IUI for 3 months to a maximum of 6 months in the young patient who has a good prognosis
- repeat laparoscopy (although this option is rare), possibly in conjunction with gamete intrafallopian transfer (GIFT), or, alternatively, in vitro fertilization (IVF). If the patient had a technically inadequate operation the first time, it sometimes is appropriate to repeat the surgery or go directly to IVF.
Consider tubal reconstruction in carefully selected patients
Practice Committee of the American Society for Reproductive Medicine. The role of tubal reconstructive surgery in the era of assisted reproductive technologies. Fertil Steril. 2008;90(5 Suppl):S250–S253.
In the era of ART, tubal reconstruction has fewer indications but is still appropriate and effective in properly selected individuals.
Determine the extent of tubal disease before reconstructive surgery
Hysterosalpingography is a useful initial test for the evaluation of tubal patency, but laparoscopy often is necessary to identify the nature and extent of pelvic disease. Selective salpingography or hysteroscopic tubal recanalization can help confirm the diagnosis of true proximal tubal occlusion.
Advise the patient of risks of surgery
Generally, the risk of ectopic pregnancy after tubal reconstruction is comparable to the risk of ectopic pregnancy associated with IVF, but the extent of tubal disease and pelvic pathology are important variables in predicting intrauterine and ectopic pregnancy rates.
The pregnancy rate after reversal of tubal sterilization depends on 1) the type of sterilization procedure that was performed, 2) site of anastomosis, and 3) postoperative tubal length, as well as 4) sperm quality and 5) the age of the female patient.
Maternal age, number of children desired, coexisting infertility variables, risk of ectopic and multiple pregnancy, and treatment cost are important considerations when counseling patients about the relative advantages and disadvantages of tubal surgery and IVF.
IVF is the best treatment for older women of reproductive age who have significant tubal pathology, and for women who have both proximal and distal occlusion.
Age, and duration of infertility, are key determinants of treatment
Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists, and Practice Committee of the American Society for Reproductive Medicine. Age-related fertility decline: a Committee Opinion. Fertil Steril. 2008;90(5 Suppl):S154–S155.
Women older than 35 years should receive expedited evaluation and treatment for infertility if they have not conceived after 6 months, or earlier if clinically indicated. That’s one of the conclusions from a recent ACOG–ASRM joint Committee Opinion on age-related fertility decline.
Age remains a major variable influencing a woman’s fertility and risk of pregnancy loss, and is increasingly important because of the social trend toward deferred child-bearing. The fertility rate peaks in a woman’s mid-20s and decreases by approximately 25% by age 35 and 50% by age 40, with a concomitant (and significant) increase in rates of aneuploidy and miscarriage.
The duration of infertility also is key. Of any given 100 women attempting to conceive:
- 78 will succeed within 1 year
- 88 will conceive within 2 years
- only an additional two or three women will conceive in the third year
- one more will conceive in each of the fourth and fifth years
- only three more will conceive over the rest of their reproductive life.
Recurrent pregnancy loss and infertility are separate entities
By definition, recurrent pregnancy loss entails the loss of two or more pregnancies. When the cause is unknown, each loss merits careful review to determine whether specific evaluation may be appropriate. After three losses, thorough evaluation is warranted.1,2
To distinguish infertility from recurrent pregnancy loss, define clinical pregnancy as one documented by US or histopathology.
New technologies remain unproven
Although ovarian tissue and oocyte cryopreservation offer the promise of female fertility preservation, these technologies remain investigational to date.
The greatest benefit to patients who wish to preserve their fertility is appropriate counseling about their reproductive health.3, 4
Fertility can be enhanced with a few patient-friendly strategies
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(5 Suppl):S1–S6.
Another Committee Opinion from ASRM, in collaboration with the Society for Reproductive Endocrinology and Infertility, offers simple but effective steps for patients to take to optimize fertility. ObGyns should recommend these strategies to any woman planning to conceive in the near future.
Frequent intercourse is best
Intercourse every day or every other day yields the highest pregnancy rate, but intercourse two to three times a week is nearly equivalent. There is a “fertile window” that spans the 6-day interval ending on the day of ovulation, and it correlates with the volume and character of cervical mucus.
Among women who have regular menstrual cycles, frequent intercourse that begins soon after the cessation of menses can help maximize fecundity.
Devices that determine or predict the time of ovulation may be useful for couples who have infrequent intercourse.
Neither specific coital timing, nor position during coitus, nor rest in a supine position after intercourse has a significant impact on fertility.
Caffeine, alcohol OK—in moderation
Moderate caffeine or alcohol consumption (1 or 2 drinks daily) has no demonstrable adverse effect on fertility. Smoking, a higher level of alcohol consumption (≥2 drinks daily), use of recreational drugs, and most commercially available vaginal lubricants should be discouraged among patients who are trying to conceive.
Fertility rates are lower in women who are very thin or obese, but there is little evidence that dietary variations improve fertility or affect the gender of the infant.
Elevated blood mercury levels from heavy seafood consumption have been associated with infertility.
Saunas do not reduce fertility in women. In normal men, attempts to protect the testicles from excessive heat are unjustified.
Avoid solvents and pesticides
- Fecundity may be diminished in women who are exposed to certain toxins and solvents, such as those used in the dry-cleaning and printing industries.
- Men who are exposed to heavy metals may be more likely to have abnormal semen parameters.
- Pesticide exposure may be a problem for both male and female agricultural workers.
- Despite limited data on exposure to lead and use of industrial microwaves, they are probably best avoided or minimized.
- Prescription drug use should be carefully controlled and managed on an individual basis.
Recommend 400 µg of folic acid daily
Any woman hoping to conceive should be advised to initiate this regimen to reduce the risk of neural tube defects.
The Centers for Disease Control and Prevention (CDC) held its first Public Health Symposium on Infertility in September 2008. Consensus is growing that infertility is a common disease or disability that has serious consequences for the well-being of families—making it a public health concern.
Because only approximately 50% of patients who have infertility ever seek treatment, it is hoped that new programs will improve access to fertility treatment for many more women.
For more information on the CDC’s initiatives in reproductive health, visit: http://www.cdc.gov/reproductivehealth/
WHO focuses on international inequities
The World Health Organization (WHO) held a meeting in Geneva in December 2008 to modify its glossary of ART definitions and develop new terminology to allow the collection of better data on the use of IVF internationally.5, 6
The prevalence of infertility is about the same in all countries of the world, affecting, on average, about 9% of people of reproductive age. However, there is a greater degree of secondary infertility—mostly as a result of infectious disease and obstetric complications—in low-resource (developing) countries.
Infertility is a major burden with serious medical and psychological consequences in American society, but its impact on women in other cultures is often more profound, with loss of personal status, divorce, and social ostracism adding to the burden.
More and more women seek care in countries other than their own
“Medical tourism” is an interesting phenomenon that has received widespread media attention. When it applies to infertility, a more appropriate term may be “cross-border reproductive care.”
This is an international phenomenon that is, so far, poorly documented. Common reasons to travel for medical care include cost and availability of specialized services. Women grappling with infertility may also seek to bypass regulations or ethical issues that limit availability of treatment in their home country. Among the issues that prompt travel are:
- gamete and embryo donation
- payment of donors and surrogates
- nontraditional relationships.
The diagnosis and treatment of fertility are evolving rapidly as a result of clinical studies, scientific research, and changing socioeconomic and ethical perspectives. These developments benefit health-care consumers, but they also pose new challenges to general ObGyns and other practitioners committed to the best possible care for their patients.
In this Update, I focus on a number of these areas of change:
- care of women who have polycystic ovary syndrome (PCOS)
- the impact of myomas on fertility
- treatment of infertility in women who have endometriosis
- when tubal reconstruction is appropriate
- the impact of a woman’s age on fertility
- patient-friendly strategies to enhance fertility
- cross-border reproductive travel.
Use clomiphene citrate to stimulate ovulation in women who have PCOS
Practice Committee of the American Society for Reproductive Medicine. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90(5 Suppl):S69–S73.
A new Committee Opinion from the American Society for Reproductive Medicine (ASRM) Practice Committee tackles the challenge of treating women with PCOS for infertility.
PCOS is associated with an increased risk of insulin resistance and diabetes mellitus. The first line of treatment for all women who have PCOS, especially those with an elevated body mass index, is lifestyle modification through diet and exercise, with the goal of losing weight.
Clomiphene is first-line therapy when ovulation is the aim
Metformin and other insulin-sensitizing agents may enhance ovulation and increase the response to clomiphene citrate in women who have PCOS and insulin resistance, but their use solely to enhance ovulation is unwarranted, and they do not reduce the rate of miscarriage. Clomiphene citrate should be the first-line treatment because it is much more effective. Long-term use of metformin to prevent disease is not advised.
Screen for insulin resistance at the time of diagnosis
Women who have PCOS should be given a 2-hour oral glucose tolerance test and have their lipid profile measured at the time of diagnosis and then at an interval of every 2 years. Insulin-sensitizing agents should be used for long-term health issues only after impaired glucose tolerance has been measured, if diet and exercise alone prove to be ineffective.
My strategy for stimulating ovulation in this population involves the following:
- Perform vaginal ultrasonography (US) on cycle day 3 for an antral follicle count and to rule out ovarian cysts >1 cm.
- Give clomiphene citrate, 50 mg, on cycle days 3 through 7 (or 5 through 9).
- Repeat vaginal US on cycle day 11 (or 13) to evaluate ovarian response. The optimal response is 1 to 2, and not more than 3, follicles ≥15 mm in size.
- Recommend timed intercourse, starting on cycle day 10 and then every 2±0.5 days until 1 to 2 days after ovulation.
- Measure urinary luteinizing hormone (uLH) daily, to detect uLH surge, starting on cycle day 11. A positive surge indicates that ovulation is likely within the next 12–48 hours. Absence of a surge indicates the likely absence of ovulation, which can be treated by giving 10,000 IU of human chorionic gonadotropin (hCG) subcutaneously or intramuscularly when the largest follicle is 18 to 25 mm in size.—G. DAVID ADAMSON, MD
When choosing a treatment for myoma, consider impacts on fertility
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproduction Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(5 Suppl): S125–S130.
A recent educational bulletin from the ASRM Practice Committee examined the relationship between myomas and reproductive function and reviewed management of this pathology.
The effects of myomas on reproductive outcome are ill-defined, but fibroids that distort the uterine cavity, as well as larger intramural myomas, may have adverse effects on fertility.
Select interventions carefully
Among women who have infertility and those who have recurrent pregnancy loss, myomectomy should be considered only after thorough evaluation. The reason? Postoperative adhesions as a result of abdominal myomectomy are common and may reduce subsequent fertility.
As for uterine artery embolization, myolysis, and MRI-guided ultrasonic treatment, these are not recommended for women who have myomas and who are seeking to maintain or improve fertility. The safety and efficacy of these procedures in this population have not been established.
Is a GnRH agonist useful?
Treatment of myomas with a gonadotropin-releasing hormone (GnRH) agonist does not improve fertility but may be helpful before surgery in anemic women and in those who might be able to undergo a less invasive procedure if the myoma volume were moderately smaller.
Sequence of infertility treatments is critical in endometriosis patients
Adamson G. Management of endometriosis and infertility following surgery. In: Sutton C, Jones K, Adamson GD, eds. Modern Management of Endometriosis. London: Taylor & Francis; 2006:273–287.
New data make it easier to treat infertility in women thought to have endometriosis, although further randomized trials are needed. If other fertility variables are normal, and minimal to mild endometriosis is suspected but not confirmed, clomiphene citrate, 100 mg on cycle days 3 through 7, followed by intrauterine insemination (IUI) for 3 to 6 cycles, is a reasonable initial treatment, with the higher number of cycles being reserved for younger patients and those who have a better prognosis.
When is surgery helpful?
Diagnostic or operative laparoscopy, or both, is often indicated when one or more of the following are present:
- The patient experiences pain
- She fails to conceive after clomiphene citrate is administered and IUI is attempted for 3 to 6 cycles
- She has other factors associated with infertility.
Generally, if pregnancy does not occur within 9 to 15 months after surgery, repeat surgery is of limited benefit for infertility, but may have some benefit for pain. In women who do not conceive after surgery, ovarian suppression for 2 months is of possible benefit before assisted reproductive technology (ART) and should be considered in patients who are also suffering from pain. Pre-ART surgery for large endometriomas is frequently indicated, and excision of the cyst capsule produces results superior to those of drainage, coagulation, or both.
Postoperative management
After complete destruction of endometriosis in women who have infertility, ovarian suppression is not indicated. Rather, the patient should usually attempt to conceive for 9 to 15 months, with an outside range of 3 to 24 months for much older women who have an unfavorable prognosis, and for much younger women who have a good prognosis, respectively. If pregnancy does not occur, clomiphene citrate and IUI for 3 to 6 months are then indicated.
If this last strategy is unsuccessful, the options include:
- gonadotropins and IUI for 3 months to a maximum of 6 months in the young patient who has a good prognosis
- repeat laparoscopy (although this option is rare), possibly in conjunction with gamete intrafallopian transfer (GIFT), or, alternatively, in vitro fertilization (IVF). If the patient had a technically inadequate operation the first time, it sometimes is appropriate to repeat the surgery or go directly to IVF.
Consider tubal reconstruction in carefully selected patients
Practice Committee of the American Society for Reproductive Medicine. The role of tubal reconstructive surgery in the era of assisted reproductive technologies. Fertil Steril. 2008;90(5 Suppl):S250–S253.
In the era of ART, tubal reconstruction has fewer indications but is still appropriate and effective in properly selected individuals.
Determine the extent of tubal disease before reconstructive surgery
Hysterosalpingography is a useful initial test for the evaluation of tubal patency, but laparoscopy often is necessary to identify the nature and extent of pelvic disease. Selective salpingography or hysteroscopic tubal recanalization can help confirm the diagnosis of true proximal tubal occlusion.
Advise the patient of risks of surgery
Generally, the risk of ectopic pregnancy after tubal reconstruction is comparable to the risk of ectopic pregnancy associated with IVF, but the extent of tubal disease and pelvic pathology are important variables in predicting intrauterine and ectopic pregnancy rates.
The pregnancy rate after reversal of tubal sterilization depends on 1) the type of sterilization procedure that was performed, 2) site of anastomosis, and 3) postoperative tubal length, as well as 4) sperm quality and 5) the age of the female patient.
Maternal age, number of children desired, coexisting infertility variables, risk of ectopic and multiple pregnancy, and treatment cost are important considerations when counseling patients about the relative advantages and disadvantages of tubal surgery and IVF.
IVF is the best treatment for older women of reproductive age who have significant tubal pathology, and for women who have both proximal and distal occlusion.
Age, and duration of infertility, are key determinants of treatment
Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists, and Practice Committee of the American Society for Reproductive Medicine. Age-related fertility decline: a Committee Opinion. Fertil Steril. 2008;90(5 Suppl):S154–S155.
Women older than 35 years should receive expedited evaluation and treatment for infertility if they have not conceived after 6 months, or earlier if clinically indicated. That’s one of the conclusions from a recent ACOG–ASRM joint Committee Opinion on age-related fertility decline.
Age remains a major variable influencing a woman’s fertility and risk of pregnancy loss, and is increasingly important because of the social trend toward deferred child-bearing. The fertility rate peaks in a woman’s mid-20s and decreases by approximately 25% by age 35 and 50% by age 40, with a concomitant (and significant) increase in rates of aneuploidy and miscarriage.
The duration of infertility also is key. Of any given 100 women attempting to conceive:
- 78 will succeed within 1 year
- 88 will conceive within 2 years
- only an additional two or three women will conceive in the third year
- one more will conceive in each of the fourth and fifth years
- only three more will conceive over the rest of their reproductive life.
Recurrent pregnancy loss and infertility are separate entities
By definition, recurrent pregnancy loss entails the loss of two or more pregnancies. When the cause is unknown, each loss merits careful review to determine whether specific evaluation may be appropriate. After three losses, thorough evaluation is warranted.1,2
To distinguish infertility from recurrent pregnancy loss, define clinical pregnancy as one documented by US or histopathology.
New technologies remain unproven
Although ovarian tissue and oocyte cryopreservation offer the promise of female fertility preservation, these technologies remain investigational to date.
The greatest benefit to patients who wish to preserve their fertility is appropriate counseling about their reproductive health.3, 4
Fertility can be enhanced with a few patient-friendly strategies
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(5 Suppl):S1–S6.
Another Committee Opinion from ASRM, in collaboration with the Society for Reproductive Endocrinology and Infertility, offers simple but effective steps for patients to take to optimize fertility. ObGyns should recommend these strategies to any woman planning to conceive in the near future.
Frequent intercourse is best
Intercourse every day or every other day yields the highest pregnancy rate, but intercourse two to three times a week is nearly equivalent. There is a “fertile window” that spans the 6-day interval ending on the day of ovulation, and it correlates with the volume and character of cervical mucus.
Among women who have regular menstrual cycles, frequent intercourse that begins soon after the cessation of menses can help maximize fecundity.
Devices that determine or predict the time of ovulation may be useful for couples who have infrequent intercourse.
Neither specific coital timing, nor position during coitus, nor rest in a supine position after intercourse has a significant impact on fertility.
Caffeine, alcohol OK—in moderation
Moderate caffeine or alcohol consumption (1 or 2 drinks daily) has no demonstrable adverse effect on fertility. Smoking, a higher level of alcohol consumption (≥2 drinks daily), use of recreational drugs, and most commercially available vaginal lubricants should be discouraged among patients who are trying to conceive.
Fertility rates are lower in women who are very thin or obese, but there is little evidence that dietary variations improve fertility or affect the gender of the infant.
Elevated blood mercury levels from heavy seafood consumption have been associated with infertility.
Saunas do not reduce fertility in women. In normal men, attempts to protect the testicles from excessive heat are unjustified.
Avoid solvents and pesticides
- Fecundity may be diminished in women who are exposed to certain toxins and solvents, such as those used in the dry-cleaning and printing industries.
- Men who are exposed to heavy metals may be more likely to have abnormal semen parameters.
- Pesticide exposure may be a problem for both male and female agricultural workers.
- Despite limited data on exposure to lead and use of industrial microwaves, they are probably best avoided or minimized.
- Prescription drug use should be carefully controlled and managed on an individual basis.
Recommend 400 µg of folic acid daily
Any woman hoping to conceive should be advised to initiate this regimen to reduce the risk of neural tube defects.
The Centers for Disease Control and Prevention (CDC) held its first Public Health Symposium on Infertility in September 2008. Consensus is growing that infertility is a common disease or disability that has serious consequences for the well-being of families—making it a public health concern.
Because only approximately 50% of patients who have infertility ever seek treatment, it is hoped that new programs will improve access to fertility treatment for many more women.
For more information on the CDC’s initiatives in reproductive health, visit: http://www.cdc.gov/reproductivehealth/
WHO focuses on international inequities
The World Health Organization (WHO) held a meeting in Geneva in December 2008 to modify its glossary of ART definitions and develop new terminology to allow the collection of better data on the use of IVF internationally.5, 6
The prevalence of infertility is about the same in all countries of the world, affecting, on average, about 9% of people of reproductive age. However, there is a greater degree of secondary infertility—mostly as a result of infectious disease and obstetric complications—in low-resource (developing) countries.
Infertility is a major burden with serious medical and psychological consequences in American society, but its impact on women in other cultures is often more profound, with loss of personal status, divorce, and social ostracism adding to the burden.
More and more women seek care in countries other than their own
“Medical tourism” is an interesting phenomenon that has received widespread media attention. When it applies to infertility, a more appropriate term may be “cross-border reproductive care.”
This is an international phenomenon that is, so far, poorly documented. Common reasons to travel for medical care include cost and availability of specialized services. Women grappling with infertility may also seek to bypass regulations or ethical issues that limit availability of treatment in their home country. Among the issues that prompt travel are:
- gamete and embryo donation
- payment of donors and surrogates
- nontraditional relationships.
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5 Suppl):S60.-
2. Adamson GD. Update in fertility. OBG Management. 2007;19(2):37-38, 41-44,-76.
3. Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90(5 Suppl):S241-S246.
4. Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2008;90(5 Suppl):S134-S135.
5. Zegers-Hochschild F, Nygren K-G, Adamson GD, et al. International Committee Monitoring Assisted Reproductive Technologies. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertil Steril. 2006;86:16-19.
6. International Committee for Monitoring Assisted Reproductive Technology, Adamson GD, de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F. World collaborative report on in vitro fertilization for year 2000. Fertil Steril. 2006;85:1586-1622.
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5 Suppl):S60.-
2. Adamson GD. Update in fertility. OBG Management. 2007;19(2):37-38, 41-44,-76.
3. Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90(5 Suppl):S241-S246.
4. Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2008;90(5 Suppl):S134-S135.
5. Zegers-Hochschild F, Nygren K-G, Adamson GD, et al. International Committee Monitoring Assisted Reproductive Technologies. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertil Steril. 2006;86:16-19.
6. International Committee for Monitoring Assisted Reproductive Technology, Adamson GD, de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F. World collaborative report on in vitro fertilization for year 2000. Fertil Steril. 2006;85:1586-1622.
Part 2 of 2: Delivery and postpartum concerns in the obese gravida
The authors report no financial relationships relevant to this article.
Hear Dr Phillips discuss the key points of this series
With the ranks of the obese expanding, and the severity of obesity increasing as well, obstetricians see a greater percentage of obese and morbidly obese gravidas in their practice. At the end of pregnancy, when it is time to deliver the infant, a number of issues must be taken into account to ensure the safety and health of both mother and child. Part 2 of this two-part article discusses these issues, from intrapartum and intraoperative considerations to care during the postpartum period.
Intrapartum concerns
Induction of labor
Obese women are more likely to require induction of labor; they should be counseled accordingly. The rate of labor induction is as high as 35% in women who have a body mass index (BMI) above 35.1 Many of these inductions are indicated for maternal disease such as diabetes or preeclampsia. However, data suggest that obesity alone increases the induction rate, possibly through increased levels of leptin (an adipokine involved in obesity, appetite, and satiety), which may inhibit uterine contractions.2
Uterine and fetal monitoring
Monitoring of uterine contractions and fetal heart rate can be challenging in obese women. Early use of an intra uterine pressure catheter and fetal scalp electrode can help.
Shoulder dystocia
Obesity is a risk factor for macrosomia, which increases the likelihood of shoulder dystocia. In addition, at least one study supports maternal obesity as an independent risk factor for shoulder dystocia. Women who had a BMI above 30 were 2.7 times more likely to have a pregnancy complicated by shoulder dystocia, after adjustment for macrosomia, diabetes, parity, and induction of labor.3
Because of this risk, appropriate staff should be readily available for assistance, and operative vaginal delivery should be performed with caution, if at all.
Vaginal birth after cesarean delivery
Obese women who have a history of cesarean delivery need to be counseled about the higher risk of failed vaginal birth after cesarean (VBAC) and the likely need for repeat cesarean delivery. They also need to be told about the elevated risk of infection in the event that VBAC fails.
In women of normal weight, the likelihood of successful VBAC exceeds 80%, compared with a success rate of 68% for women who have a BMI above 29, and 60% for women who have a BMI above 40.4,5
Appropriate counseling and patient selection are critical components of the decision to proceed with a trial of labor in obese women who have a history of cesarean delivery. Twenty-four-hour anesthesia and NICU coverage are also necessary in case emergent cesarean delivery becomes necessary.
Cesarean delivery
Morbidly obese women (BMI >35) have approximately a 50% greater chance of requiring cesarean delivery than do women of normal weight.6 The need for cesarean delivery rises with increasing BMI, probably because of a greater degree of maternal pelvic soft tissue, which increases the risk of both dystocia and cephalopelvic disproportion.
Anesthesia
It may be advisable to place an early epidural in laboring obese patients because they are more likely to require multiple placement attempts and cesarean delivery.
Intraoperative considerations
Ensure availability of the proper equipment
Table extenders, appropriate retractors, and long instruments should be readily available before surgery. For example, obstetricians often retract the panniculus to allow for a Pfannenstiel skin incision ( FIGURE 1 ), which works well to increase exposure. Moreover, the depth of the abdominal wall is much thinner beneath the panniculus.
However, be aware that, even in a supine patient with a leftward tilt, the weight of the panniculus can decrease vena caval return and cause uteroplacental insufficiency and compromised maternal respiration. Cesarean delivery should be performed expediently for these reasons.
A self-retaining elastic abdominal retractor may improve exposure at the same time that it minimizes the amount of retraction that must be exerted by the surgical assistant ( FIGURE 2 ).
FIGURE 1 Retract the panniculus
Retract the panniculus toward the patient’s head and use straps to secure it to facilitate a Pfannenstiel incision.
FIGURE 2 Elastic abdominal retractor improves visibility and access
Insert the inner ring of the adjustable elastic retractor into the abdominal cavity toward the patient’s head until it springs open against the parietal peritoneum. The outer ring is then rolled onto the plastic sleeve until it is snug against the skin.
Prophylaxis is warranted
Preoperative prophylactic antibiotics (weight-based) and prophylaxis for venous thromboembolism using sequential compression devices are recommended prior to surgery.
Data do not indicate whether vertical or transverse skin incisions for cesarean delivery are associated with higher infectious morbidity. In general, a Pfannenstiel incision is associated with less postoperative discomfort, allowing earlier mobilization and better respiratory function. A vertical incision allows optimal exposure, especially in the case of a macrosomic infant, and may thereby limit operating time and blood loss.7,8
A vertical supraumbilical incision is another option. If the lower uterine segment is not accessible, a fundal uterine incision can be made ( FIGURE 3 ). The advantage of this method is increased exposure as well as access to the incision for wound care. However, because this method may necessitate a classical uterine incision, counseling must be given regarding future pregnancy.
FIGURE 3 Pair incisions strategically to ease cesarean delivery
In an obese patient, a supraumbilical skin incision paired with a classical uterine incision may simplify the surgery.
Other intraoperative considerations
If the surgery is prolonged, consider administering another dose of antibiotics.
If the subcutaneous tissue is thicker than 2 cm, it should be reapproximated to minimize the risk of hematoma and seroma formation and wound infection.8,9
A subcutaneous drain should also be considered.
Be aware of special risks
One retrospective study showed an increased risk of postpartum hemorrhage in women who had a BMI above 25, compared with women of normal weight. This risk increased as BMI increased. Knowledge of this complication and early use of uterotonic agents can minimize the morbidity associated with postpartum hemorrhage.
Postpartum infection is more likely in obese women, with three times the risk of endometritis of women of normal weight, and increased risks of wound infection, urinary tract infection, septic pelvic thrombophlebitis, and pneumonia.10 Early recognition of infection and administration of antibiotics can minimize morbidity.
Venous thromboembolism is more likely throughout pregnancy in obese women. Risk is heightened, however, during the postpartum period, when it is four times as high as the risk in women of normal weight.11 Early ambulation and use of venous compression devices are encouraged. Consider heparin in the immediate postpartum period, when mobility is compromised for a longer period, particularly for women undergoing cesarean delivery who have comorbidities.
Suggest breastfeeding, weight loss
Women who breastfeed longer than 1 month have a 22% reduction in the risk of developing metabolic syndrome later in life, compared with women who do not. It also appears that a longer duration of breastfeeding reduces the risk of diabetes.12
Weight loss should be strongly encouraged. Women who retained their pregnancy weight at 6 months were 18 lb heavier after 10 years than women who lost their pregnancy weight.
The risk of metabolic syndrome increases with parity, probably because of the tendency of many obese women to retain their pregnancy weight.12
1. Sukalich S, Mingione MJ, Glantz JC. Obstetric outcomes in overweight and obese adolescents. Am J Obstet Gynecol. 2006;195:851-855.
2. Vickers M. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes. 2007;14:17-22.
3. Mazouni C, Porcu G, Cohen-Solal E, et al. Maternal and anthropomorphic risk factors for shoulder dystocia. Acta Obstet Gynecol Scand. 2006;85:567-570.
4. Juhasz G, Gyamfi C, Gyamfi P, Tocce K, Stone JL. Effect of body mass index and excessive weight gain on success of vaginal birth after cesarean delivery. Obstet Gynecol. 2005;106:741-746.
5. Hibbard JU, Gilbert S, Landon MB, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Trial of labor or repeat cesarean delivery in women with morbid obesity and previous cesarean delivery. Obstet Gynecol. 2006;108:125-133.
6. Weiss JL, Malone FD, Emig D, et al:. FASTER Research Consortium. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190:1091-1097.
7. Wall PD, Deucy EE, Glantz JC, Pressman EK. Vertical skin incisions and wound complications in the obese parturient. Obstet Gynecol. 2003;102(5 Pt 1):952-956.
8. Alexander CI, Liston WA. Operating on the obese woman—a review. BJOG. 2006;113:1167-1172.
9. Ramsey PS, White AM, Guinn DA, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105(5 Pt 1):967-973.
10. Myles TD, Gooch J, Santolaya J. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet Gynecol. 2002;100(5 Pt 1):959-964.
11. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311-1315.
12. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3:696-704.
The authors report no financial relationships relevant to this article.
Hear Dr Phillips discuss the key points of this series
With the ranks of the obese expanding, and the severity of obesity increasing as well, obstetricians see a greater percentage of obese and morbidly obese gravidas in their practice. At the end of pregnancy, when it is time to deliver the infant, a number of issues must be taken into account to ensure the safety and health of both mother and child. Part 2 of this two-part article discusses these issues, from intrapartum and intraoperative considerations to care during the postpartum period.
Intrapartum concerns
Induction of labor
Obese women are more likely to require induction of labor; they should be counseled accordingly. The rate of labor induction is as high as 35% in women who have a body mass index (BMI) above 35.1 Many of these inductions are indicated for maternal disease such as diabetes or preeclampsia. However, data suggest that obesity alone increases the induction rate, possibly through increased levels of leptin (an adipokine involved in obesity, appetite, and satiety), which may inhibit uterine contractions.2
Uterine and fetal monitoring
Monitoring of uterine contractions and fetal heart rate can be challenging in obese women. Early use of an intra uterine pressure catheter and fetal scalp electrode can help.
Shoulder dystocia
Obesity is a risk factor for macrosomia, which increases the likelihood of shoulder dystocia. In addition, at least one study supports maternal obesity as an independent risk factor for shoulder dystocia. Women who had a BMI above 30 were 2.7 times more likely to have a pregnancy complicated by shoulder dystocia, after adjustment for macrosomia, diabetes, parity, and induction of labor.3
Because of this risk, appropriate staff should be readily available for assistance, and operative vaginal delivery should be performed with caution, if at all.
Vaginal birth after cesarean delivery
Obese women who have a history of cesarean delivery need to be counseled about the higher risk of failed vaginal birth after cesarean (VBAC) and the likely need for repeat cesarean delivery. They also need to be told about the elevated risk of infection in the event that VBAC fails.
In women of normal weight, the likelihood of successful VBAC exceeds 80%, compared with a success rate of 68% for women who have a BMI above 29, and 60% for women who have a BMI above 40.4,5
Appropriate counseling and patient selection are critical components of the decision to proceed with a trial of labor in obese women who have a history of cesarean delivery. Twenty-four-hour anesthesia and NICU coverage are also necessary in case emergent cesarean delivery becomes necessary.
Cesarean delivery
Morbidly obese women (BMI >35) have approximately a 50% greater chance of requiring cesarean delivery than do women of normal weight.6 The need for cesarean delivery rises with increasing BMI, probably because of a greater degree of maternal pelvic soft tissue, which increases the risk of both dystocia and cephalopelvic disproportion.
Anesthesia
It may be advisable to place an early epidural in laboring obese patients because they are more likely to require multiple placement attempts and cesarean delivery.
Intraoperative considerations
Ensure availability of the proper equipment
Table extenders, appropriate retractors, and long instruments should be readily available before surgery. For example, obstetricians often retract the panniculus to allow for a Pfannenstiel skin incision ( FIGURE 1 ), which works well to increase exposure. Moreover, the depth of the abdominal wall is much thinner beneath the panniculus.
However, be aware that, even in a supine patient with a leftward tilt, the weight of the panniculus can decrease vena caval return and cause uteroplacental insufficiency and compromised maternal respiration. Cesarean delivery should be performed expediently for these reasons.
A self-retaining elastic abdominal retractor may improve exposure at the same time that it minimizes the amount of retraction that must be exerted by the surgical assistant ( FIGURE 2 ).
FIGURE 1 Retract the panniculus
Retract the panniculus toward the patient’s head and use straps to secure it to facilitate a Pfannenstiel incision.
FIGURE 2 Elastic abdominal retractor improves visibility and access
Insert the inner ring of the adjustable elastic retractor into the abdominal cavity toward the patient’s head until it springs open against the parietal peritoneum. The outer ring is then rolled onto the plastic sleeve until it is snug against the skin.
Prophylaxis is warranted
Preoperative prophylactic antibiotics (weight-based) and prophylaxis for venous thromboembolism using sequential compression devices are recommended prior to surgery.
Data do not indicate whether vertical or transverse skin incisions for cesarean delivery are associated with higher infectious morbidity. In general, a Pfannenstiel incision is associated with less postoperative discomfort, allowing earlier mobilization and better respiratory function. A vertical incision allows optimal exposure, especially in the case of a macrosomic infant, and may thereby limit operating time and blood loss.7,8
A vertical supraumbilical incision is another option. If the lower uterine segment is not accessible, a fundal uterine incision can be made ( FIGURE 3 ). The advantage of this method is increased exposure as well as access to the incision for wound care. However, because this method may necessitate a classical uterine incision, counseling must be given regarding future pregnancy.
FIGURE 3 Pair incisions strategically to ease cesarean delivery
In an obese patient, a supraumbilical skin incision paired with a classical uterine incision may simplify the surgery.
Other intraoperative considerations
If the surgery is prolonged, consider administering another dose of antibiotics.
If the subcutaneous tissue is thicker than 2 cm, it should be reapproximated to minimize the risk of hematoma and seroma formation and wound infection.8,9
A subcutaneous drain should also be considered.
Be aware of special risks
One retrospective study showed an increased risk of postpartum hemorrhage in women who had a BMI above 25, compared with women of normal weight. This risk increased as BMI increased. Knowledge of this complication and early use of uterotonic agents can minimize the morbidity associated with postpartum hemorrhage.
Postpartum infection is more likely in obese women, with three times the risk of endometritis of women of normal weight, and increased risks of wound infection, urinary tract infection, septic pelvic thrombophlebitis, and pneumonia.10 Early recognition of infection and administration of antibiotics can minimize morbidity.
Venous thromboembolism is more likely throughout pregnancy in obese women. Risk is heightened, however, during the postpartum period, when it is four times as high as the risk in women of normal weight.11 Early ambulation and use of venous compression devices are encouraged. Consider heparin in the immediate postpartum period, when mobility is compromised for a longer period, particularly for women undergoing cesarean delivery who have comorbidities.
Suggest breastfeeding, weight loss
Women who breastfeed longer than 1 month have a 22% reduction in the risk of developing metabolic syndrome later in life, compared with women who do not. It also appears that a longer duration of breastfeeding reduces the risk of diabetes.12
Weight loss should be strongly encouraged. Women who retained their pregnancy weight at 6 months were 18 lb heavier after 10 years than women who lost their pregnancy weight.
The risk of metabolic syndrome increases with parity, probably because of the tendency of many obese women to retain their pregnancy weight.12
The authors report no financial relationships relevant to this article.
Hear Dr Phillips discuss the key points of this series
With the ranks of the obese expanding, and the severity of obesity increasing as well, obstetricians see a greater percentage of obese and morbidly obese gravidas in their practice. At the end of pregnancy, when it is time to deliver the infant, a number of issues must be taken into account to ensure the safety and health of both mother and child. Part 2 of this two-part article discusses these issues, from intrapartum and intraoperative considerations to care during the postpartum period.
Intrapartum concerns
Induction of labor
Obese women are more likely to require induction of labor; they should be counseled accordingly. The rate of labor induction is as high as 35% in women who have a body mass index (BMI) above 35.1 Many of these inductions are indicated for maternal disease such as diabetes or preeclampsia. However, data suggest that obesity alone increases the induction rate, possibly through increased levels of leptin (an adipokine involved in obesity, appetite, and satiety), which may inhibit uterine contractions.2
Uterine and fetal monitoring
Monitoring of uterine contractions and fetal heart rate can be challenging in obese women. Early use of an intra uterine pressure catheter and fetal scalp electrode can help.
Shoulder dystocia
Obesity is a risk factor for macrosomia, which increases the likelihood of shoulder dystocia. In addition, at least one study supports maternal obesity as an independent risk factor for shoulder dystocia. Women who had a BMI above 30 were 2.7 times more likely to have a pregnancy complicated by shoulder dystocia, after adjustment for macrosomia, diabetes, parity, and induction of labor.3
Because of this risk, appropriate staff should be readily available for assistance, and operative vaginal delivery should be performed with caution, if at all.
Vaginal birth after cesarean delivery
Obese women who have a history of cesarean delivery need to be counseled about the higher risk of failed vaginal birth after cesarean (VBAC) and the likely need for repeat cesarean delivery. They also need to be told about the elevated risk of infection in the event that VBAC fails.
In women of normal weight, the likelihood of successful VBAC exceeds 80%, compared with a success rate of 68% for women who have a BMI above 29, and 60% for women who have a BMI above 40.4,5
Appropriate counseling and patient selection are critical components of the decision to proceed with a trial of labor in obese women who have a history of cesarean delivery. Twenty-four-hour anesthesia and NICU coverage are also necessary in case emergent cesarean delivery becomes necessary.
Cesarean delivery
Morbidly obese women (BMI >35) have approximately a 50% greater chance of requiring cesarean delivery than do women of normal weight.6 The need for cesarean delivery rises with increasing BMI, probably because of a greater degree of maternal pelvic soft tissue, which increases the risk of both dystocia and cephalopelvic disproportion.
Anesthesia
It may be advisable to place an early epidural in laboring obese patients because they are more likely to require multiple placement attempts and cesarean delivery.
Intraoperative considerations
Ensure availability of the proper equipment
Table extenders, appropriate retractors, and long instruments should be readily available before surgery. For example, obstetricians often retract the panniculus to allow for a Pfannenstiel skin incision ( FIGURE 1 ), which works well to increase exposure. Moreover, the depth of the abdominal wall is much thinner beneath the panniculus.
However, be aware that, even in a supine patient with a leftward tilt, the weight of the panniculus can decrease vena caval return and cause uteroplacental insufficiency and compromised maternal respiration. Cesarean delivery should be performed expediently for these reasons.
A self-retaining elastic abdominal retractor may improve exposure at the same time that it minimizes the amount of retraction that must be exerted by the surgical assistant ( FIGURE 2 ).
FIGURE 1 Retract the panniculus
Retract the panniculus toward the patient’s head and use straps to secure it to facilitate a Pfannenstiel incision.
FIGURE 2 Elastic abdominal retractor improves visibility and access
Insert the inner ring of the adjustable elastic retractor into the abdominal cavity toward the patient’s head until it springs open against the parietal peritoneum. The outer ring is then rolled onto the plastic sleeve until it is snug against the skin.
Prophylaxis is warranted
Preoperative prophylactic antibiotics (weight-based) and prophylaxis for venous thromboembolism using sequential compression devices are recommended prior to surgery.
Data do not indicate whether vertical or transverse skin incisions for cesarean delivery are associated with higher infectious morbidity. In general, a Pfannenstiel incision is associated with less postoperative discomfort, allowing earlier mobilization and better respiratory function. A vertical incision allows optimal exposure, especially in the case of a macrosomic infant, and may thereby limit operating time and blood loss.7,8
A vertical supraumbilical incision is another option. If the lower uterine segment is not accessible, a fundal uterine incision can be made ( FIGURE 3 ). The advantage of this method is increased exposure as well as access to the incision for wound care. However, because this method may necessitate a classical uterine incision, counseling must be given regarding future pregnancy.
FIGURE 3 Pair incisions strategically to ease cesarean delivery
In an obese patient, a supraumbilical skin incision paired with a classical uterine incision may simplify the surgery.
Other intraoperative considerations
If the surgery is prolonged, consider administering another dose of antibiotics.
If the subcutaneous tissue is thicker than 2 cm, it should be reapproximated to minimize the risk of hematoma and seroma formation and wound infection.8,9
A subcutaneous drain should also be considered.
Be aware of special risks
One retrospective study showed an increased risk of postpartum hemorrhage in women who had a BMI above 25, compared with women of normal weight. This risk increased as BMI increased. Knowledge of this complication and early use of uterotonic agents can minimize the morbidity associated with postpartum hemorrhage.
Postpartum infection is more likely in obese women, with three times the risk of endometritis of women of normal weight, and increased risks of wound infection, urinary tract infection, septic pelvic thrombophlebitis, and pneumonia.10 Early recognition of infection and administration of antibiotics can minimize morbidity.
Venous thromboembolism is more likely throughout pregnancy in obese women. Risk is heightened, however, during the postpartum period, when it is four times as high as the risk in women of normal weight.11 Early ambulation and use of venous compression devices are encouraged. Consider heparin in the immediate postpartum period, when mobility is compromised for a longer period, particularly for women undergoing cesarean delivery who have comorbidities.
Suggest breastfeeding, weight loss
Women who breastfeed longer than 1 month have a 22% reduction in the risk of developing metabolic syndrome later in life, compared with women who do not. It also appears that a longer duration of breastfeeding reduces the risk of diabetes.12
Weight loss should be strongly encouraged. Women who retained their pregnancy weight at 6 months were 18 lb heavier after 10 years than women who lost their pregnancy weight.
The risk of metabolic syndrome increases with parity, probably because of the tendency of many obese women to retain their pregnancy weight.12
1. Sukalich S, Mingione MJ, Glantz JC. Obstetric outcomes in overweight and obese adolescents. Am J Obstet Gynecol. 2006;195:851-855.
2. Vickers M. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes. 2007;14:17-22.
3. Mazouni C, Porcu G, Cohen-Solal E, et al. Maternal and anthropomorphic risk factors for shoulder dystocia. Acta Obstet Gynecol Scand. 2006;85:567-570.
4. Juhasz G, Gyamfi C, Gyamfi P, Tocce K, Stone JL. Effect of body mass index and excessive weight gain on success of vaginal birth after cesarean delivery. Obstet Gynecol. 2005;106:741-746.
5. Hibbard JU, Gilbert S, Landon MB, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Trial of labor or repeat cesarean delivery in women with morbid obesity and previous cesarean delivery. Obstet Gynecol. 2006;108:125-133.
6. Weiss JL, Malone FD, Emig D, et al:. FASTER Research Consortium. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190:1091-1097.
7. Wall PD, Deucy EE, Glantz JC, Pressman EK. Vertical skin incisions and wound complications in the obese parturient. Obstet Gynecol. 2003;102(5 Pt 1):952-956.
8. Alexander CI, Liston WA. Operating on the obese woman—a review. BJOG. 2006;113:1167-1172.
9. Ramsey PS, White AM, Guinn DA, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105(5 Pt 1):967-973.
10. Myles TD, Gooch J, Santolaya J. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet Gynecol. 2002;100(5 Pt 1):959-964.
11. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311-1315.
12. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3:696-704.
1. Sukalich S, Mingione MJ, Glantz JC. Obstetric outcomes in overweight and obese adolescents. Am J Obstet Gynecol. 2006;195:851-855.
2. Vickers M. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes. 2007;14:17-22.
3. Mazouni C, Porcu G, Cohen-Solal E, et al. Maternal and anthropomorphic risk factors for shoulder dystocia. Acta Obstet Gynecol Scand. 2006;85:567-570.
4. Juhasz G, Gyamfi C, Gyamfi P, Tocce K, Stone JL. Effect of body mass index and excessive weight gain on success of vaginal birth after cesarean delivery. Obstet Gynecol. 2005;106:741-746.
5. Hibbard JU, Gilbert S, Landon MB, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Trial of labor or repeat cesarean delivery in women with morbid obesity and previous cesarean delivery. Obstet Gynecol. 2006;108:125-133.
6. Weiss JL, Malone FD, Emig D, et al:. FASTER Research Consortium. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190:1091-1097.
7. Wall PD, Deucy EE, Glantz JC, Pressman EK. Vertical skin incisions and wound complications in the obese parturient. Obstet Gynecol. 2003;102(5 Pt 1):952-956.
8. Alexander CI, Liston WA. Operating on the obese woman—a review. BJOG. 2006;113:1167-1172.
9. Ramsey PS, White AM, Guinn DA, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105(5 Pt 1):967-973.
10. Myles TD, Gooch J, Santolaya J. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet Gynecol. 2002;100(5 Pt 1):959-964.
11. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311-1315.
12. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3:696-704.
PART 1 OF 2: For the obese gravida, try strong counseling and close follow-up
Hear Dr Phillips discuss the key points of this series
CASE: Obesity + coexisting conditions = complicated pregnancy and delivery
A 30-year-old gravida 8 para 5026 is referred from the clinic for evaluation of elevated blood pressure at 36 4/7 weeks’ gestation. She is morbidly obese, with a weight of 440 lb and a body mass index (BMI) of 67. She also has a history of chronic hypertension and was recently given a diagnosis of gestational diabetes, which has been controlled through diet.
Her reproductive history includes four full-term vaginal deliveries followed by cesarean delivery for malpresentation of twins. Her blood pressure is 180/100 mm Hg, and she has new-onset proteinuria (3+) and a headache. The diagnosis? Preeclampsia superimposed on chronic hypertension.
Induction of labor is initiated using a Foley bulb and oxytocin, and magnesium sulfate is given to prevent seizures. Over the next 48 hours there is minimal cervical change, and the patient develops chorioamnionitis, for which she is given intravenous antibiotics. A repeat cesarean delivery is performed via a Pfannenstiel skin incision. The surgery is uneventful, and the infant is healthy.
Are further complications likely?
Yes—additional complications are considerably more likely in this scenario than in one involving a patient of normal weight, especially given the patient’s chronic hypertension and gestational diabetes. Obesity can affect all aspects of pregnancy, from conception through the postpartum period, with the potential for significant adverse maternal and fetal outcomes, including maternal mortality.
As the number of obese women of reproductive age increases, obstetricians face new challenges in the management of complications during pregnancy, labor, delivery, and beyond. In Part 1 of this two-part article, we offer advice on how to counsel the obese patient about the very real risks she faces in pregnancy, and detail trimester-specific recommendations. In Part 2, which follows on page 51, we offer practical management strategies during intrapartum, intraoperative, and postpartum periods.
CASE CONTINUED
The patient becomes febrile and hypoxic on postoperative day 1. When a computed tomography scan fails to rule out pulmonary embolism, she is started on heparin.
On postoperative day 7, omentum is detected at the incision, and the patient is taken to the operating room, where fascial dehiscence is identified and necrotic tissue is debrided. Two days later, a wound vac and inferior vena cava filter are placed.
The patient is discharged to a rehabilitation center on postoperative day 22.
Management starts before conception
The most important strategy to prevent complications associated with obesity and pregnancy is prepregnancy weight loss. Ideally, all obese patients should have a prepregnancy consultation that includes the recommendation to lose weight before conception. At this consultation, the ObGyn should determine the patient’s BMI and risk category and advise her of the relevant maternal and fetal risks (page 51, we take up intrapartum and postpartum concerns.
1. Gray AD, Power ML, Zinberg S, Schulkin J. Assessment and management of obesity. Obstet Gynecol Surv. 2006;61:742-748.
2. Centers for Disease Control and Prevention. Physical activity for everyone. Atlanta: CDC. Available at www.cdc.gov/nccdphp/dnpa/physical/everyone/recommendations/index.htm. Accessed December 30, 2008.
3. Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19:1644-1646.
4. Institute of Medicine, Committee on Nutritional Status During Pregnancy. Nutrition during pregnancy. Washington, DC: National Academy Press; 1990.
5. Doherty DA, Magann EF, Francis J, Morrison JC, Newnham JP. Pre-pregnancy body mass index and pregnancy outcomes. Int J Gynaecol Obstet. 2006;95:242-247.
6. Waller DK, Shaw GM, Rasmussen SA, et al. National Birth Defects Prevention Study. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161:745-750.
7. O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
8. Heiskanen N, Raatikainen K, Heinonen S. Fetal macrosomia—a continuing obstetric challenge. Biol Neonate. 2006;90:98-103.
9. Endler GC, Mariona FG, Sokol RJ, Stevenson LB. Anesthesia-related maternal mortality in Michigan, 1972 to 1984. Am J Obstet Gynecol. 1988;159:187-193.
10. Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61:36-48.
11. Salihu HM, Dunlop AL, Hedayatzadeh M, Alio AP, Kirby RS, Alexander GR. Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol. 2007;110:552-557.
12. World Health Organization. Obesity and overweight. Fact sheet No. 311. Geneva, Switzerland: WHO; 2006. Available at: www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 30, 2008.
13. Centers for Disease Control and Prevention. Overweight and obesity. Atlanta: CDC. Available at: www.cdc.gov/nccdphp/dnpa/obesity/index.htm. Accessed December 30, 2008.
14. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3:696-704.
15. Dixon JB, Dixon ME, O’Brien PE. Birth outcomes in obese women after laparoscopic adjustable gastric banding. Obstet Gynecol. 2005;106(5 Pt 1):966-972.
16. American College of Obstetricians and Gynecologists. Committee Opinion No. 315. Obesity in pregnancy. Washington, DC: ACOG; 2005.
Hear Dr Phillips discuss the key points of this series
CASE: Obesity + coexisting conditions = complicated pregnancy and delivery
A 30-year-old gravida 8 para 5026 is referred from the clinic for evaluation of elevated blood pressure at 36 4/7 weeks’ gestation. She is morbidly obese, with a weight of 440 lb and a body mass index (BMI) of 67. She also has a history of chronic hypertension and was recently given a diagnosis of gestational diabetes, which has been controlled through diet.
Her reproductive history includes four full-term vaginal deliveries followed by cesarean delivery for malpresentation of twins. Her blood pressure is 180/100 mm Hg, and she has new-onset proteinuria (3+) and a headache. The diagnosis? Preeclampsia superimposed on chronic hypertension.
Induction of labor is initiated using a Foley bulb and oxytocin, and magnesium sulfate is given to prevent seizures. Over the next 48 hours there is minimal cervical change, and the patient develops chorioamnionitis, for which she is given intravenous antibiotics. A repeat cesarean delivery is performed via a Pfannenstiel skin incision. The surgery is uneventful, and the infant is healthy.
Are further complications likely?
Yes—additional complications are considerably more likely in this scenario than in one involving a patient of normal weight, especially given the patient’s chronic hypertension and gestational diabetes. Obesity can affect all aspects of pregnancy, from conception through the postpartum period, with the potential for significant adverse maternal and fetal outcomes, including maternal mortality.
As the number of obese women of reproductive age increases, obstetricians face new challenges in the management of complications during pregnancy, labor, delivery, and beyond. In Part 1 of this two-part article, we offer advice on how to counsel the obese patient about the very real risks she faces in pregnancy, and detail trimester-specific recommendations. In Part 2, which follows on page 51, we offer practical management strategies during intrapartum, intraoperative, and postpartum periods.
CASE CONTINUED
The patient becomes febrile and hypoxic on postoperative day 1. When a computed tomography scan fails to rule out pulmonary embolism, she is started on heparin.
On postoperative day 7, omentum is detected at the incision, and the patient is taken to the operating room, where fascial dehiscence is identified and necrotic tissue is debrided. Two days later, a wound vac and inferior vena cava filter are placed.
The patient is discharged to a rehabilitation center on postoperative day 22.
Management starts before conception
The most important strategy to prevent complications associated with obesity and pregnancy is prepregnancy weight loss. Ideally, all obese patients should have a prepregnancy consultation that includes the recommendation to lose weight before conception. At this consultation, the ObGyn should determine the patient’s BMI and risk category and advise her of the relevant maternal and fetal risks (page 51, we take up intrapartum and postpartum concerns.
Hear Dr Phillips discuss the key points of this series
CASE: Obesity + coexisting conditions = complicated pregnancy and delivery
A 30-year-old gravida 8 para 5026 is referred from the clinic for evaluation of elevated blood pressure at 36 4/7 weeks’ gestation. She is morbidly obese, with a weight of 440 lb and a body mass index (BMI) of 67. She also has a history of chronic hypertension and was recently given a diagnosis of gestational diabetes, which has been controlled through diet.
Her reproductive history includes four full-term vaginal deliveries followed by cesarean delivery for malpresentation of twins. Her blood pressure is 180/100 mm Hg, and she has new-onset proteinuria (3+) and a headache. The diagnosis? Preeclampsia superimposed on chronic hypertension.
Induction of labor is initiated using a Foley bulb and oxytocin, and magnesium sulfate is given to prevent seizures. Over the next 48 hours there is minimal cervical change, and the patient develops chorioamnionitis, for which she is given intravenous antibiotics. A repeat cesarean delivery is performed via a Pfannenstiel skin incision. The surgery is uneventful, and the infant is healthy.
Are further complications likely?
Yes—additional complications are considerably more likely in this scenario than in one involving a patient of normal weight, especially given the patient’s chronic hypertension and gestational diabetes. Obesity can affect all aspects of pregnancy, from conception through the postpartum period, with the potential for significant adverse maternal and fetal outcomes, including maternal mortality.
As the number of obese women of reproductive age increases, obstetricians face new challenges in the management of complications during pregnancy, labor, delivery, and beyond. In Part 1 of this two-part article, we offer advice on how to counsel the obese patient about the very real risks she faces in pregnancy, and detail trimester-specific recommendations. In Part 2, which follows on page 51, we offer practical management strategies during intrapartum, intraoperative, and postpartum periods.
CASE CONTINUED
The patient becomes febrile and hypoxic on postoperative day 1. When a computed tomography scan fails to rule out pulmonary embolism, she is started on heparin.
On postoperative day 7, omentum is detected at the incision, and the patient is taken to the operating room, where fascial dehiscence is identified and necrotic tissue is debrided. Two days later, a wound vac and inferior vena cava filter are placed.
The patient is discharged to a rehabilitation center on postoperative day 22.
Management starts before conception
The most important strategy to prevent complications associated with obesity and pregnancy is prepregnancy weight loss. Ideally, all obese patients should have a prepregnancy consultation that includes the recommendation to lose weight before conception. At this consultation, the ObGyn should determine the patient’s BMI and risk category and advise her of the relevant maternal and fetal risks (page 51, we take up intrapartum and postpartum concerns.
1. Gray AD, Power ML, Zinberg S, Schulkin J. Assessment and management of obesity. Obstet Gynecol Surv. 2006;61:742-748.
2. Centers for Disease Control and Prevention. Physical activity for everyone. Atlanta: CDC. Available at www.cdc.gov/nccdphp/dnpa/physical/everyone/recommendations/index.htm. Accessed December 30, 2008.
3. Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19:1644-1646.
4. Institute of Medicine, Committee on Nutritional Status During Pregnancy. Nutrition during pregnancy. Washington, DC: National Academy Press; 1990.
5. Doherty DA, Magann EF, Francis J, Morrison JC, Newnham JP. Pre-pregnancy body mass index and pregnancy outcomes. Int J Gynaecol Obstet. 2006;95:242-247.
6. Waller DK, Shaw GM, Rasmussen SA, et al. National Birth Defects Prevention Study. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161:745-750.
7. O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
8. Heiskanen N, Raatikainen K, Heinonen S. Fetal macrosomia—a continuing obstetric challenge. Biol Neonate. 2006;90:98-103.
9. Endler GC, Mariona FG, Sokol RJ, Stevenson LB. Anesthesia-related maternal mortality in Michigan, 1972 to 1984. Am J Obstet Gynecol. 1988;159:187-193.
10. Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61:36-48.
11. Salihu HM, Dunlop AL, Hedayatzadeh M, Alio AP, Kirby RS, Alexander GR. Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol. 2007;110:552-557.
12. World Health Organization. Obesity and overweight. Fact sheet No. 311. Geneva, Switzerland: WHO; 2006. Available at: www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 30, 2008.
13. Centers for Disease Control and Prevention. Overweight and obesity. Atlanta: CDC. Available at: www.cdc.gov/nccdphp/dnpa/obesity/index.htm. Accessed December 30, 2008.
14. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3:696-704.
15. Dixon JB, Dixon ME, O’Brien PE. Birth outcomes in obese women after laparoscopic adjustable gastric banding. Obstet Gynecol. 2005;106(5 Pt 1):966-972.
16. American College of Obstetricians and Gynecologists. Committee Opinion No. 315. Obesity in pregnancy. Washington, DC: ACOG; 2005.
1. Gray AD, Power ML, Zinberg S, Schulkin J. Assessment and management of obesity. Obstet Gynecol Surv. 2006;61:742-748.
2. Centers for Disease Control and Prevention. Physical activity for everyone. Atlanta: CDC. Available at www.cdc.gov/nccdphp/dnpa/physical/everyone/recommendations/index.htm. Accessed December 30, 2008.
3. Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19:1644-1646.
4. Institute of Medicine, Committee on Nutritional Status During Pregnancy. Nutrition during pregnancy. Washington, DC: National Academy Press; 1990.
5. Doherty DA, Magann EF, Francis J, Morrison JC, Newnham JP. Pre-pregnancy body mass index and pregnancy outcomes. Int J Gynaecol Obstet. 2006;95:242-247.
6. Waller DK, Shaw GM, Rasmussen SA, et al. National Birth Defects Prevention Study. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161:745-750.
7. O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
8. Heiskanen N, Raatikainen K, Heinonen S. Fetal macrosomia—a continuing obstetric challenge. Biol Neonate. 2006;90:98-103.
9. Endler GC, Mariona FG, Sokol RJ, Stevenson LB. Anesthesia-related maternal mortality in Michigan, 1972 to 1984. Am J Obstet Gynecol. 1988;159:187-193.
10. Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61:36-48.
11. Salihu HM, Dunlop AL, Hedayatzadeh M, Alio AP, Kirby RS, Alexander GR. Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol. 2007;110:552-557.
12. World Health Organization. Obesity and overweight. Fact sheet No. 311. Geneva, Switzerland: WHO; 2006. Available at: www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 30, 2008.
13. Centers for Disease Control and Prevention. Overweight and obesity. Atlanta: CDC. Available at: www.cdc.gov/nccdphp/dnpa/obesity/index.htm. Accessed December 30, 2008.
14. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3:696-704.
15. Dixon JB, Dixon ME, O’Brien PE. Birth outcomes in obese women after laparoscopic adjustable gastric banding. Obstet Gynecol. 2005;106(5 Pt 1):966-972.
16. American College of Obstetricians and Gynecologists. Committee Opinion No. 315. Obesity in pregnancy. Washington, DC: ACOG; 2005.