User login
Vitamin D Deficiency
As increasing numbers of people work in windowless environments and as computer time, gaming consoles, and TV viewing keep more of them indoors during their leisure hours, many are losing access to their natural source of vitamin D: sunshine. In response to the justifiably publicized risk of skin cancers, people avoid sunlight or take great care to cover the skin with sunscreen—minimizing the risk of sun-related skin cancer, but greatly increasing the risk of vitamin D deficiency.
The importance of vitamin D was first recognized in the prevention of rickets and its role in absorption of calcium and phosphate in the diet.1 In recent decades, however, the growing understanding of vitamin D's influence on leukocytes, vascular smooth muscle cells, and other tissues2 has led to an increased awareness of this nutrient's contribution to numerous processes and functions.
Considering vitamin D's subtle but substantial impact on mental, cardiovascular, musculoskeletal, and autoimmune health (not to mention bone disorders and calcium deficiency), vitamin D deficiency is overlooked and undertreated with surprising frequency in the clinical setting, where clinicians are more likely to screen for and treat other disorders.
The Facts
Exposure of the skin to sunlight or ultraviolet (UV) light is the human body's natural way to synthesize vitamin D3.1,3 This nutrient can also be ingested in fish and fish liver oils; in the form of vitamin D2, which has been used since the 1930s in efforts to reduce rickets and other bone disorders by fortifying milk, cereals, and a variety of food products4,5; and in dietary supplements.
Unfortunately, the intake of vitamin D–fortified foods and/or supplements is often insufficient for the average person to maintain an adequate level of this essential substance.3 Fatty fish, including sardines, mackerel, tuna, and salmon,6 are among the few foods that represent a valuable source of vitamin D, but these are not commonly considered a staple in today's American diet. Additionally, it has been questioned whether the current recommended daily allowance guidelines for vitamin D intake are adequate for most of the population.7
Widespread Effects
The impact of vitamin D deficiency or insufficiency affects patients of both genders across the life span. Exclusive breastfeeding without adequate vitamin D supplementation can result in rickets in infants, children, and adolescents.3,4,8 Research indicates that even healthy-appearing adolescents may be deficient in this nutrient.9 Inadequate intake or supplementation of vitamin D during pregnancy has been shown to increase women's risk of preeclampsia, with potential impact on their infants' well-being.10
Adults with inadequate levels of vitamin D are at risk for periodontal disease and other dental concerns,11,12 hypertension and cardiovascular disease,2,13-16 musculoskeletal disorders, depression,17 and malignancies of the breast,18 colon,1,19,20 and prostate.13 Older persons with insufficient levels of this essential substance are at increased risk of falls and fractures,12,21 osteoporosis,21,22 hyperparathyroidism,23 impaired cognitive function, and depression.24
Vitamin D Synthesis
Vitamin D is synthesized in the skin by UV light between wavelengths of 290 and 315 nm,4,13 converting 7-dehydrocholesterol to previtamin D3, then by thermal isomerization to vitamin D3.1,3 Both vitamin D3 and vitamin D2 are incorporated into chylomicrons and absorbed by the lymph system, then put into systemic circulation by vitamin D–binding protein.4,13
Two additional steps—one that occurs in the liver, the other in the kidneys—are needed to complete the conversion from an inert form to usable vitamin D. In the liver, the molecule is hydroxylated by enzymes called the vitamin D-25-hydroxylases to form 25-hydroxyvitamin D. Then in the kidneys, the cytochrome P-450 enzyme 25-hydroxyvitamin D-1 alpha-hydroxylase continues the hydroxylation process, converting the molecule to vitamin D's biologically active form, 1,25-dihydroxyvitamin D.4,13,25 It is next bound to the vitamin D receptors and in an additional step is transcribed in RNA and replicated.
The known actions of vitamin D include increasing calcium and phosphorus absorption from the small bowel, enhancement of renal tubule resorption of phosphate, and maturation of osteoclasts to resorb calcium from the bones. Vitamin D also improves measurable bone mineral density.1
Who Is at Risk?
Many individuals may not recognize their risk for vitamin D deficiency or insufficiency. Clinicians must be aware of the conditions and factors that increase the risk. Many of these are identified in Table 1.3,5,6,8,10,24,26-31
Assessment
Clinicians in any number of specialties may encounter patients with vitamin D deficiency or insufficiency. Thus, it is important during the interview and review of systems to ask routinely about the patient's occupation, sun exposure, and use of sunscreen. Clinicians should also ask about dietary habits and dietary supplements, including multivitamins and supplemental vitamin D (eg, calcium with vitamin D).
The examining clinician should also key in on fatigue, bone pain, and muscle pain or weakness. While reviewing the patient's medical history and the current problem list, the clinician should maintain an awareness of disease processes that may mimic vitamin D insufficiency. These include fibromyalgia, chronic fatigue syndrome, myositis, hyperparathyroidism, and depression.13,17,23 Comorbidities that often coexist with vitamin D deficiency include hypertension and cardiovascular disease,16 obesity, type 1 diabetes mellitus,13 multiple sclerosis,5 secondary hyperparathyroidism,13 and prostate, breast, or colorectal cancer.1,2,9,13
Assessment of the patient's constitution, of course, includes vital signs and general appearance. As mentioned earlier, hypertension may coexist with vitamin D deficiency.2,13-16 Obesity, it is also important to note, has been associated with reduced vitamin D bioavailability.28 The type and coverage of the patient's clothing can provide an important clue to a potential lack of sunlight exposure and its impact on his or her vitamin D status.29 As for inspection of the integument, it should be noted that darker skin pigmentation is included among the risk factors for vitamin D insufficiency, as melanin in darker skin reduces vitamin D synthesis.9,31
Testing for Vitamin D
The most accurate means of meassuring the patient's vitamin D status is 25-hydroxyvitamin D, also known as serum 25(OH)D.4,25 With a relative half-life of two weeks,4 this marker reliably indicates the body's stores of vitamin D. Some laboratories report three aspects—total serum 25(OH)D, 25[OH]D3, and 25[OH]D2—while others report only total serum 25(OH)D. Interpretation of the latter is shown in Table 2.4,25
Additional research suggests that higher levels of serum 25(OH)D (ie, 36 to 48 ng/mL) may be desirable for the prevention of cancer.12
Treatment
Vitamin D insufficiency and deficiency are relatively easy and inexpensive to treat. With a target treatment goal of serum 25(OH)D greater than 30 ng/mL, the patient can be advised to increase his or her sunlight or UV exposure in moderate amounts, such as exposure of the hands and face to bright sunlight for 15 minutes daily. During winter or at northern latitudes with reduced sunlight, moderate exposure in a tanning bed (ie, one emitting 2% to 6% UVB radiation) can be helpful.6,32 For recommended supplementation to correct vitamin D deficiency or insufficiency, see Table 3.6,32
Oral supplementation for adults is an inexpensive, well-tolerated solution. A conscious effort to increase dietary intake of fortified dairy products and cereals or fatty fish may be adequate. OTC oral vitamin D3 supplements are available in 200, 400, and 1,000 IU for a few cents per dose. Prescription vitamin D2 ergocalciferol is also available.6
Infants who are exclusively breastfed or who consume less than 500 mL/d of vitamin D–fortified formula can be given a combination multivitamin containing 400 IU/mL for adequate supplementation3,6; Hollis and Wagner8 recommend that breastfeeding women have 4,000 IU/d of vitamin D intake to protect both themselves and their infants. Single-source or concentrated vitamin D is not recommended for infants.3 Gartner and Greer3 recommend a vitamin D intake of 200 IU/d from childhood through adolescence.
Research indicates that higher levels of vitamin D supplementation than previously recommended are needed for most people and are safe.7,12 Additionally, higher doses of vitamin D are not as toxic as were previously believed, as excess amounts are stored.33 Daily doses of no less than 1,000 IU (with or without sunlight exposure and/or dietary intake) may improve the serum 25(OH)D levels in the majority of the population.12 Results from one study suggest that a total of 3,600 to 4,200 IU/d from all sources is desirable and safe.33
Reevaluation
The serum 25(OH)D test should be repeated after six to eight weeks to ensure adequate vitamin D absorption, targeting a level of at least 30 ng/mL. If serum 25(OH)D falls persistently below that level, the clinician should consider vitamin D in an injectable form and reassess the patient for malabsorption or other interference issues.34
Conclusion
The health benefits of vitamin D are frequently overlooked in everyday practice. Screening and treatment are simple, cost-effective, and beneficial for patients' wellness.
1. Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-F28.
2. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063-1069.
3. Gartner LM, Greer FR; Section on Breastfeeding and Committee on Nutrition, American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111(4 pt 1):908-910.
4. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072.
5. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 suppl):1678S-1688S.
6. Office of Dietary Supplements, National Institutes of Health. Dietary supplement fact sheet: Vitamin D (2008). http://dietary-supplements.info.nih.gov/factsheets/vitamind.asp. Accessed June 26, 2008.
7. Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective (editorial). Am J Clin Nutr. 2007;85(3):649-650.
8. Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 suppl): 1752S-1758S.
9. Gordon CM, DePeter KC, Feldman HA, et al. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
10. Bodnar LM, Catov JM, Simhan HN, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517-3522.
11. Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80(1):108-113.
12. Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2008;624:55-71.
13. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
14. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503-511.
15. Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94(4):483-492.
16. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174-1180.
17. Jorde R, Waterloo K, Saleh F, et al. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels: the Tromsø study. J Neurol. 2006;253(4):464-470.
18. Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103(3-5):708-711.
19. Flanagan JN, Young MV, Persons KS, et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26(4A):2567-2572.
20. Spina CS, Ton L, Yao M, et al. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol. 2007;103(3-5):757-762.
21. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-351.
22. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634-639.
23. Harris SS, Soteriades E, Coolidge JA, et al. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125-4130.
24. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
25. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2008 Mar 8; [Epub ahead of print].
26. Elliott ME, Binkley NC, Carnes M, et al. Fracture risks for women in long-term care: high prevalence of calcaneal osteoporosis and hypovitaminosis D. Pharmacotherapy. 2003;23(6):702-710.
27. Johnson JM, Maher JW, DeMaria EJ, et al. The long-term effects of gastric bypass on vitamin D metabolism. Ann Surg. 2006;243(5):701-705.
28. Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
29. Mishal AA. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporos Int. 2001;12(11):931-935.
30. McDuffie JR, Calis KA, Booth SL, et al. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22(7):814-822.
31. Bell NH, Greene A, Epstein S, et al. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76(2):470-473.
32. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357(3):266-281.
33. Heaney RP. Vitamin D endocrine physiology. J Bone Miner Res. 2007;22 suppl 2:V25-V27.
34. Kumar R, Riggs BL. Vitamin D in the therapy of disorders of calcium and phosphorus metabolism. Mayo Clin Proc. 1981;56(5):327-333.
As increasing numbers of people work in windowless environments and as computer time, gaming consoles, and TV viewing keep more of them indoors during their leisure hours, many are losing access to their natural source of vitamin D: sunshine. In response to the justifiably publicized risk of skin cancers, people avoid sunlight or take great care to cover the skin with sunscreen—minimizing the risk of sun-related skin cancer, but greatly increasing the risk of vitamin D deficiency.
The importance of vitamin D was first recognized in the prevention of rickets and its role in absorption of calcium and phosphate in the diet.1 In recent decades, however, the growing understanding of vitamin D's influence on leukocytes, vascular smooth muscle cells, and other tissues2 has led to an increased awareness of this nutrient's contribution to numerous processes and functions.
Considering vitamin D's subtle but substantial impact on mental, cardiovascular, musculoskeletal, and autoimmune health (not to mention bone disorders and calcium deficiency), vitamin D deficiency is overlooked and undertreated with surprising frequency in the clinical setting, where clinicians are more likely to screen for and treat other disorders.
The Facts
Exposure of the skin to sunlight or ultraviolet (UV) light is the human body's natural way to synthesize vitamin D3.1,3 This nutrient can also be ingested in fish and fish liver oils; in the form of vitamin D2, which has been used since the 1930s in efforts to reduce rickets and other bone disorders by fortifying milk, cereals, and a variety of food products4,5; and in dietary supplements.
Unfortunately, the intake of vitamin D–fortified foods and/or supplements is often insufficient for the average person to maintain an adequate level of this essential substance.3 Fatty fish, including sardines, mackerel, tuna, and salmon,6 are among the few foods that represent a valuable source of vitamin D, but these are not commonly considered a staple in today's American diet. Additionally, it has been questioned whether the current recommended daily allowance guidelines for vitamin D intake are adequate for most of the population.7
Widespread Effects
The impact of vitamin D deficiency or insufficiency affects patients of both genders across the life span. Exclusive breastfeeding without adequate vitamin D supplementation can result in rickets in infants, children, and adolescents.3,4,8 Research indicates that even healthy-appearing adolescents may be deficient in this nutrient.9 Inadequate intake or supplementation of vitamin D during pregnancy has been shown to increase women's risk of preeclampsia, with potential impact on their infants' well-being.10
Adults with inadequate levels of vitamin D are at risk for periodontal disease and other dental concerns,11,12 hypertension and cardiovascular disease,2,13-16 musculoskeletal disorders, depression,17 and malignancies of the breast,18 colon,1,19,20 and prostate.13 Older persons with insufficient levels of this essential substance are at increased risk of falls and fractures,12,21 osteoporosis,21,22 hyperparathyroidism,23 impaired cognitive function, and depression.24
Vitamin D Synthesis
Vitamin D is synthesized in the skin by UV light between wavelengths of 290 and 315 nm,4,13 converting 7-dehydrocholesterol to previtamin D3, then by thermal isomerization to vitamin D3.1,3 Both vitamin D3 and vitamin D2 are incorporated into chylomicrons and absorbed by the lymph system, then put into systemic circulation by vitamin D–binding protein.4,13
Two additional steps—one that occurs in the liver, the other in the kidneys—are needed to complete the conversion from an inert form to usable vitamin D. In the liver, the molecule is hydroxylated by enzymes called the vitamin D-25-hydroxylases to form 25-hydroxyvitamin D. Then in the kidneys, the cytochrome P-450 enzyme 25-hydroxyvitamin D-1 alpha-hydroxylase continues the hydroxylation process, converting the molecule to vitamin D's biologically active form, 1,25-dihydroxyvitamin D.4,13,25 It is next bound to the vitamin D receptors and in an additional step is transcribed in RNA and replicated.
The known actions of vitamin D include increasing calcium and phosphorus absorption from the small bowel, enhancement of renal tubule resorption of phosphate, and maturation of osteoclasts to resorb calcium from the bones. Vitamin D also improves measurable bone mineral density.1
Who Is at Risk?
Many individuals may not recognize their risk for vitamin D deficiency or insufficiency. Clinicians must be aware of the conditions and factors that increase the risk. Many of these are identified in Table 1.3,5,6,8,10,24,26-31
Assessment
Clinicians in any number of specialties may encounter patients with vitamin D deficiency or insufficiency. Thus, it is important during the interview and review of systems to ask routinely about the patient's occupation, sun exposure, and use of sunscreen. Clinicians should also ask about dietary habits and dietary supplements, including multivitamins and supplemental vitamin D (eg, calcium with vitamin D).
The examining clinician should also key in on fatigue, bone pain, and muscle pain or weakness. While reviewing the patient's medical history and the current problem list, the clinician should maintain an awareness of disease processes that may mimic vitamin D insufficiency. These include fibromyalgia, chronic fatigue syndrome, myositis, hyperparathyroidism, and depression.13,17,23 Comorbidities that often coexist with vitamin D deficiency include hypertension and cardiovascular disease,16 obesity, type 1 diabetes mellitus,13 multiple sclerosis,5 secondary hyperparathyroidism,13 and prostate, breast, or colorectal cancer.1,2,9,13
Assessment of the patient's constitution, of course, includes vital signs and general appearance. As mentioned earlier, hypertension may coexist with vitamin D deficiency.2,13-16 Obesity, it is also important to note, has been associated with reduced vitamin D bioavailability.28 The type and coverage of the patient's clothing can provide an important clue to a potential lack of sunlight exposure and its impact on his or her vitamin D status.29 As for inspection of the integument, it should be noted that darker skin pigmentation is included among the risk factors for vitamin D insufficiency, as melanin in darker skin reduces vitamin D synthesis.9,31
Testing for Vitamin D
The most accurate means of meassuring the patient's vitamin D status is 25-hydroxyvitamin D, also known as serum 25(OH)D.4,25 With a relative half-life of two weeks,4 this marker reliably indicates the body's stores of vitamin D. Some laboratories report three aspects—total serum 25(OH)D, 25[OH]D3, and 25[OH]D2—while others report only total serum 25(OH)D. Interpretation of the latter is shown in Table 2.4,25
Additional research suggests that higher levels of serum 25(OH)D (ie, 36 to 48 ng/mL) may be desirable for the prevention of cancer.12
Treatment
Vitamin D insufficiency and deficiency are relatively easy and inexpensive to treat. With a target treatment goal of serum 25(OH)D greater than 30 ng/mL, the patient can be advised to increase his or her sunlight or UV exposure in moderate amounts, such as exposure of the hands and face to bright sunlight for 15 minutes daily. During winter or at northern latitudes with reduced sunlight, moderate exposure in a tanning bed (ie, one emitting 2% to 6% UVB radiation) can be helpful.6,32 For recommended supplementation to correct vitamin D deficiency or insufficiency, see Table 3.6,32
Oral supplementation for adults is an inexpensive, well-tolerated solution. A conscious effort to increase dietary intake of fortified dairy products and cereals or fatty fish may be adequate. OTC oral vitamin D3 supplements are available in 200, 400, and 1,000 IU for a few cents per dose. Prescription vitamin D2 ergocalciferol is also available.6
Infants who are exclusively breastfed or who consume less than 500 mL/d of vitamin D–fortified formula can be given a combination multivitamin containing 400 IU/mL for adequate supplementation3,6; Hollis and Wagner8 recommend that breastfeeding women have 4,000 IU/d of vitamin D intake to protect both themselves and their infants. Single-source or concentrated vitamin D is not recommended for infants.3 Gartner and Greer3 recommend a vitamin D intake of 200 IU/d from childhood through adolescence.
Research indicates that higher levels of vitamin D supplementation than previously recommended are needed for most people and are safe.7,12 Additionally, higher doses of vitamin D are not as toxic as were previously believed, as excess amounts are stored.33 Daily doses of no less than 1,000 IU (with or without sunlight exposure and/or dietary intake) may improve the serum 25(OH)D levels in the majority of the population.12 Results from one study suggest that a total of 3,600 to 4,200 IU/d from all sources is desirable and safe.33
Reevaluation
The serum 25(OH)D test should be repeated after six to eight weeks to ensure adequate vitamin D absorption, targeting a level of at least 30 ng/mL. If serum 25(OH)D falls persistently below that level, the clinician should consider vitamin D in an injectable form and reassess the patient for malabsorption or other interference issues.34
Conclusion
The health benefits of vitamin D are frequently overlooked in everyday practice. Screening and treatment are simple, cost-effective, and beneficial for patients' wellness.
As increasing numbers of people work in windowless environments and as computer time, gaming consoles, and TV viewing keep more of them indoors during their leisure hours, many are losing access to their natural source of vitamin D: sunshine. In response to the justifiably publicized risk of skin cancers, people avoid sunlight or take great care to cover the skin with sunscreen—minimizing the risk of sun-related skin cancer, but greatly increasing the risk of vitamin D deficiency.
The importance of vitamin D was first recognized in the prevention of rickets and its role in absorption of calcium and phosphate in the diet.1 In recent decades, however, the growing understanding of vitamin D's influence on leukocytes, vascular smooth muscle cells, and other tissues2 has led to an increased awareness of this nutrient's contribution to numerous processes and functions.
Considering vitamin D's subtle but substantial impact on mental, cardiovascular, musculoskeletal, and autoimmune health (not to mention bone disorders and calcium deficiency), vitamin D deficiency is overlooked and undertreated with surprising frequency in the clinical setting, where clinicians are more likely to screen for and treat other disorders.
The Facts
Exposure of the skin to sunlight or ultraviolet (UV) light is the human body's natural way to synthesize vitamin D3.1,3 This nutrient can also be ingested in fish and fish liver oils; in the form of vitamin D2, which has been used since the 1930s in efforts to reduce rickets and other bone disorders by fortifying milk, cereals, and a variety of food products4,5; and in dietary supplements.
Unfortunately, the intake of vitamin D–fortified foods and/or supplements is often insufficient for the average person to maintain an adequate level of this essential substance.3 Fatty fish, including sardines, mackerel, tuna, and salmon,6 are among the few foods that represent a valuable source of vitamin D, but these are not commonly considered a staple in today's American diet. Additionally, it has been questioned whether the current recommended daily allowance guidelines for vitamin D intake are adequate for most of the population.7
Widespread Effects
The impact of vitamin D deficiency or insufficiency affects patients of both genders across the life span. Exclusive breastfeeding without adequate vitamin D supplementation can result in rickets in infants, children, and adolescents.3,4,8 Research indicates that even healthy-appearing adolescents may be deficient in this nutrient.9 Inadequate intake or supplementation of vitamin D during pregnancy has been shown to increase women's risk of preeclampsia, with potential impact on their infants' well-being.10
Adults with inadequate levels of vitamin D are at risk for periodontal disease and other dental concerns,11,12 hypertension and cardiovascular disease,2,13-16 musculoskeletal disorders, depression,17 and malignancies of the breast,18 colon,1,19,20 and prostate.13 Older persons with insufficient levels of this essential substance are at increased risk of falls and fractures,12,21 osteoporosis,21,22 hyperparathyroidism,23 impaired cognitive function, and depression.24
Vitamin D Synthesis
Vitamin D is synthesized in the skin by UV light between wavelengths of 290 and 315 nm,4,13 converting 7-dehydrocholesterol to previtamin D3, then by thermal isomerization to vitamin D3.1,3 Both vitamin D3 and vitamin D2 are incorporated into chylomicrons and absorbed by the lymph system, then put into systemic circulation by vitamin D–binding protein.4,13
Two additional steps—one that occurs in the liver, the other in the kidneys—are needed to complete the conversion from an inert form to usable vitamin D. In the liver, the molecule is hydroxylated by enzymes called the vitamin D-25-hydroxylases to form 25-hydroxyvitamin D. Then in the kidneys, the cytochrome P-450 enzyme 25-hydroxyvitamin D-1 alpha-hydroxylase continues the hydroxylation process, converting the molecule to vitamin D's biologically active form, 1,25-dihydroxyvitamin D.4,13,25 It is next bound to the vitamin D receptors and in an additional step is transcribed in RNA and replicated.
The known actions of vitamin D include increasing calcium and phosphorus absorption from the small bowel, enhancement of renal tubule resorption of phosphate, and maturation of osteoclasts to resorb calcium from the bones. Vitamin D also improves measurable bone mineral density.1
Who Is at Risk?
Many individuals may not recognize their risk for vitamin D deficiency or insufficiency. Clinicians must be aware of the conditions and factors that increase the risk. Many of these are identified in Table 1.3,5,6,8,10,24,26-31
Assessment
Clinicians in any number of specialties may encounter patients with vitamin D deficiency or insufficiency. Thus, it is important during the interview and review of systems to ask routinely about the patient's occupation, sun exposure, and use of sunscreen. Clinicians should also ask about dietary habits and dietary supplements, including multivitamins and supplemental vitamin D (eg, calcium with vitamin D).
The examining clinician should also key in on fatigue, bone pain, and muscle pain or weakness. While reviewing the patient's medical history and the current problem list, the clinician should maintain an awareness of disease processes that may mimic vitamin D insufficiency. These include fibromyalgia, chronic fatigue syndrome, myositis, hyperparathyroidism, and depression.13,17,23 Comorbidities that often coexist with vitamin D deficiency include hypertension and cardiovascular disease,16 obesity, type 1 diabetes mellitus,13 multiple sclerosis,5 secondary hyperparathyroidism,13 and prostate, breast, or colorectal cancer.1,2,9,13
Assessment of the patient's constitution, of course, includes vital signs and general appearance. As mentioned earlier, hypertension may coexist with vitamin D deficiency.2,13-16 Obesity, it is also important to note, has been associated with reduced vitamin D bioavailability.28 The type and coverage of the patient's clothing can provide an important clue to a potential lack of sunlight exposure and its impact on his or her vitamin D status.29 As for inspection of the integument, it should be noted that darker skin pigmentation is included among the risk factors for vitamin D insufficiency, as melanin in darker skin reduces vitamin D synthesis.9,31
Testing for Vitamin D
The most accurate means of meassuring the patient's vitamin D status is 25-hydroxyvitamin D, also known as serum 25(OH)D.4,25 With a relative half-life of two weeks,4 this marker reliably indicates the body's stores of vitamin D. Some laboratories report three aspects—total serum 25(OH)D, 25[OH]D3, and 25[OH]D2—while others report only total serum 25(OH)D. Interpretation of the latter is shown in Table 2.4,25
Additional research suggests that higher levels of serum 25(OH)D (ie, 36 to 48 ng/mL) may be desirable for the prevention of cancer.12
Treatment
Vitamin D insufficiency and deficiency are relatively easy and inexpensive to treat. With a target treatment goal of serum 25(OH)D greater than 30 ng/mL, the patient can be advised to increase his or her sunlight or UV exposure in moderate amounts, such as exposure of the hands and face to bright sunlight for 15 minutes daily. During winter or at northern latitudes with reduced sunlight, moderate exposure in a tanning bed (ie, one emitting 2% to 6% UVB radiation) can be helpful.6,32 For recommended supplementation to correct vitamin D deficiency or insufficiency, see Table 3.6,32
Oral supplementation for adults is an inexpensive, well-tolerated solution. A conscious effort to increase dietary intake of fortified dairy products and cereals or fatty fish may be adequate. OTC oral vitamin D3 supplements are available in 200, 400, and 1,000 IU for a few cents per dose. Prescription vitamin D2 ergocalciferol is also available.6
Infants who are exclusively breastfed or who consume less than 500 mL/d of vitamin D–fortified formula can be given a combination multivitamin containing 400 IU/mL for adequate supplementation3,6; Hollis and Wagner8 recommend that breastfeeding women have 4,000 IU/d of vitamin D intake to protect both themselves and their infants. Single-source or concentrated vitamin D is not recommended for infants.3 Gartner and Greer3 recommend a vitamin D intake of 200 IU/d from childhood through adolescence.
Research indicates that higher levels of vitamin D supplementation than previously recommended are needed for most people and are safe.7,12 Additionally, higher doses of vitamin D are not as toxic as were previously believed, as excess amounts are stored.33 Daily doses of no less than 1,000 IU (with or without sunlight exposure and/or dietary intake) may improve the serum 25(OH)D levels in the majority of the population.12 Results from one study suggest that a total of 3,600 to 4,200 IU/d from all sources is desirable and safe.33
Reevaluation
The serum 25(OH)D test should be repeated after six to eight weeks to ensure adequate vitamin D absorption, targeting a level of at least 30 ng/mL. If serum 25(OH)D falls persistently below that level, the clinician should consider vitamin D in an injectable form and reassess the patient for malabsorption or other interference issues.34
Conclusion
The health benefits of vitamin D are frequently overlooked in everyday practice. Screening and treatment are simple, cost-effective, and beneficial for patients' wellness.
1. Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-F28.
2. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063-1069.
3. Gartner LM, Greer FR; Section on Breastfeeding and Committee on Nutrition, American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111(4 pt 1):908-910.
4. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072.
5. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 suppl):1678S-1688S.
6. Office of Dietary Supplements, National Institutes of Health. Dietary supplement fact sheet: Vitamin D (2008). http://dietary-supplements.info.nih.gov/factsheets/vitamind.asp. Accessed June 26, 2008.
7. Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective (editorial). Am J Clin Nutr. 2007;85(3):649-650.
8. Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 suppl): 1752S-1758S.
9. Gordon CM, DePeter KC, Feldman HA, et al. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
10. Bodnar LM, Catov JM, Simhan HN, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517-3522.
11. Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80(1):108-113.
12. Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2008;624:55-71.
13. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
14. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503-511.
15. Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94(4):483-492.
16. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174-1180.
17. Jorde R, Waterloo K, Saleh F, et al. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels: the Tromsø study. J Neurol. 2006;253(4):464-470.
18. Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103(3-5):708-711.
19. Flanagan JN, Young MV, Persons KS, et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26(4A):2567-2572.
20. Spina CS, Ton L, Yao M, et al. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol. 2007;103(3-5):757-762.
21. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-351.
22. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634-639.
23. Harris SS, Soteriades E, Coolidge JA, et al. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125-4130.
24. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
25. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2008 Mar 8; [Epub ahead of print].
26. Elliott ME, Binkley NC, Carnes M, et al. Fracture risks for women in long-term care: high prevalence of calcaneal osteoporosis and hypovitaminosis D. Pharmacotherapy. 2003;23(6):702-710.
27. Johnson JM, Maher JW, DeMaria EJ, et al. The long-term effects of gastric bypass on vitamin D metabolism. Ann Surg. 2006;243(5):701-705.
28. Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
29. Mishal AA. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporos Int. 2001;12(11):931-935.
30. McDuffie JR, Calis KA, Booth SL, et al. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22(7):814-822.
31. Bell NH, Greene A, Epstein S, et al. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76(2):470-473.
32. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357(3):266-281.
33. Heaney RP. Vitamin D endocrine physiology. J Bone Miner Res. 2007;22 suppl 2:V25-V27.
34. Kumar R, Riggs BL. Vitamin D in the therapy of disorders of calcium and phosphorus metabolism. Mayo Clin Proc. 1981;56(5):327-333.
1. Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-F28.
2. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063-1069.
3. Gartner LM, Greer FR; Section on Breastfeeding and Committee on Nutrition, American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111(4 pt 1):908-910.
4. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072.
5. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 suppl):1678S-1688S.
6. Office of Dietary Supplements, National Institutes of Health. Dietary supplement fact sheet: Vitamin D (2008). http://dietary-supplements.info.nih.gov/factsheets/vitamind.asp. Accessed June 26, 2008.
7. Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective (editorial). Am J Clin Nutr. 2007;85(3):649-650.
8. Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 suppl): 1752S-1758S.
9. Gordon CM, DePeter KC, Feldman HA, et al. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
10. Bodnar LM, Catov JM, Simhan HN, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517-3522.
11. Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80(1):108-113.
12. Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2008;624:55-71.
13. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
14. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503-511.
15. Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94(4):483-492.
16. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174-1180.
17. Jorde R, Waterloo K, Saleh F, et al. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels: the Tromsø study. J Neurol. 2006;253(4):464-470.
18. Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103(3-5):708-711.
19. Flanagan JN, Young MV, Persons KS, et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26(4A):2567-2572.
20. Spina CS, Ton L, Yao M, et al. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol. 2007;103(3-5):757-762.
21. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-351.
22. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634-639.
23. Harris SS, Soteriades E, Coolidge JA, et al. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125-4130.
24. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
25. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2008 Mar 8; [Epub ahead of print].
26. Elliott ME, Binkley NC, Carnes M, et al. Fracture risks for women in long-term care: high prevalence of calcaneal osteoporosis and hypovitaminosis D. Pharmacotherapy. 2003;23(6):702-710.
27. Johnson JM, Maher JW, DeMaria EJ, et al. The long-term effects of gastric bypass on vitamin D metabolism. Ann Surg. 2006;243(5):701-705.
28. Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
29. Mishal AA. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporos Int. 2001;12(11):931-935.
30. McDuffie JR, Calis KA, Booth SL, et al. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22(7):814-822.
31. Bell NH, Greene A, Epstein S, et al. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76(2):470-473.
32. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357(3):266-281.
33. Heaney RP. Vitamin D endocrine physiology. J Bone Miner Res. 2007;22 suppl 2:V25-V27.
34. Kumar R, Riggs BL. Vitamin D in the therapy of disorders of calcium and phosphorus metabolism. Mayo Clin Proc. 1981;56(5):327-333.
Hyphenated History: Erb-Duchenne Brachial Plexus Palsy
Type III Acromioclavicular Separation: Rationale for Anatomical Reconstruction
Consider retroperitoneal packing for postpartum hemorrhage
The authors report no financial relationships relevant to this article.
CASE: Postcesarean hemorrhage fails to respond to early maneuvers.
A 25-year-old G1P0 undergoes cesarean section at our hospital for fetal distress. She has no history of coagulopathy, and no intraoperative complications are noted during the procedure. Upon her arrival in the postanesthesia care unit, however, vaginal bleeding is observed. She is given 40 U of oxytocin in 1 L of lactated Ringer solution, two intramuscular doses of 0.2 mg of methylergonovine maleate, and 1,000 μg of misoprostol to treat the postpartum bleeding. Nevertheless, she loses almost 1 L of additional blood from her vagina and is returned to the operating room for exploration and resuscitation for hypotensive shock. What are the next steps?
Management of obstetrical hemorrhage often begins with conservative measures, circumstances permitting. It is common practice to give 20–40 U of oxytocin in 1 L of lactated Ringer solution after delivery of the placenta and to perform uterine massage as part of initial management of uterine atony, along with careful evaluation and repair of any laceration or hematoma. In addition, ultrasonography (US) can help detect any retained uterine products.
Medical management usually involves the use of various uterotonics, such as methylergonovine maleate, 15-methylprostaglandin F2α, dinoprostone, and misoprostol. If uterotonics fail, techniques of tamponade include uterine packing with gauze material or use of the Foley intrauterine catheter, Sengstaken–Blakemore tube, and Bakri balloon.1-3
Surgical management is often the last resort, and is limited by the clinician’s experience. Some surgical methods include uterine or hypogastric artery ligation, or both. Newer techniques include a variation of uterine compression sutures such as the B-Lynch suture or multiple-square suturing. The B-Lynch provides continuous compression of the uterus, thereby decreasing blood loss.4 Multiple-square suturing joins the anterior and posterior walls of the uterus, also compressing the uterus.
Hysterectomy should be a last resort, with the knowledge that bleeding may continue after the procedure, in which case pelvic packing becomes an alternative. Unfortunately, pelvic packing of the intraperitoneal cavity often has little effect on endometrial hemorrhage or retroperitoneal bleeding.2-5
Resuscitation calls for blood products. Our resuscitation regimen includes recent clinical recommendations from military medical units in Iraq and Afghanistan and from domestic trauma centers. These guidelines propose that 1 U of fresh frozen plasma be administered with every 1 or 2 U of packed red blood cells (RBCs) until the clinical situation stabilizes or coagulopathy is excluded. Because of massive blood loss in this case, however, fluid replacement continues throughout the procedure—totaling 6 U of packed RBCs, 6 U of fresh frozen plasma, and 5 U of cryoprecipitate with additional crystalloid.6-8
A decision is made to undertake surgical exploration. We open a Pfannenstiel incision and enter the peritoneal cavity, encountering scant dark red blood without gross intraperitoneal bleeding. The uterus is intact with apparent endometrial hemorrhage. Uterine vessels are not easily visualized because they are obscured by retroperitoneal blood and an engorged uterus. The uterus has increased in size severalfold during hemorrhage and occupies the entire pelvic cavity, making dissection difficult for emergent hysterectomy.
As the uterus is exteriorized, Péan clamps are placed on the cornua for retraction, and the round ligaments are transected and ligated bilaterally. Ecchymoses along the peritoneum suggest that retroperitoneal bleeding is occurring in addition to the endometrial blood loss.
What can be done about the retroperitoneal bleeding?
Although laparotomy and hysterectomy are last resorts in postpartum hemorrhage, the use of retroperitoneal packing during these procedures may hasten life-saving hemostasis. In pelvic trauma, a technique of retroperitoneal packing has significantly reduced mortality.9 The same technique of retroperitoneal packing is ideally suited for such devastating circumstances as life-threatening postpartum hemorrhage.
Retroperitoneal packing is a lesson gleaned from trauma surgery and has profound application in cases of severe postpartum hemorrhage.
CASE CONTINUED
Hemorrhage is stanched. Blood loss continues, and the patient remains in hypotensive shock. Vital signs are critical:
- systolic blood pressure, 40 mm Hg
- heart rate, 160 bpm
- minimal urine output
- hemoglobin level, 4 g/dL.
Hemorrhage is obvious from the appearance of the pelvis, and continuing blood loss suggests disseminated coagulopathy. Total abdominal hysterectomy cannot be safely or quickly performed.
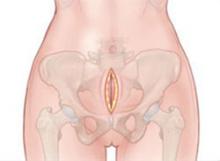
To quickly prevent further blood loss, we pack the retroperitoneum using a technique adapted from trauma surgery and first described by Smith and colleagues.9 We make a 5-cm incision into the space of Retzius just cephalad to the pubic symphysis (FIGURES 1 and 2). This incision is separate and inferior to the earlier laparotomy incision.
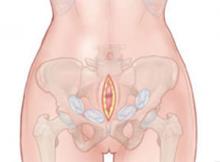
Four laparotomy sponges are packed along the retroperitoneal space to provide tamponade (FIGURE 3). Within seconds, the patient stabilizes, with systolic blood pressure rising to 90 mm Hg and the heart rate declining toward 100 bpm. Bleeding ceases immediately, and the hysterectomy is completed under stable conditions without further blood loss.
FIGURE 1 Packing begins with a 5-cm incision
Make a 5-cm incision just cephalad to the symphysis pubis, deep to the fascia, and into the space of Retzius.
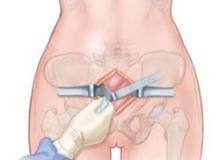
FIGURE 2 Preparing to place sponges
Use blunt dissection in the space of Retzius before placing packing material.
FIGURE 3 Insert packing into retroperitoneal space
Packing is usually sufficient when two or three laparotomy sponges are placed at each side of the retroperitoneal pelvis.
How retroperitoneal packing saves lives
Most of the pelvic packing that has been described in the literature has consistently involved intraperitoneal packing. However, packing of the peritoneal cavity is often insufficient tamponade for bleeding associated with retroperitoneal and endometrial bleeding. Direct compression in the retroperitoneal space stanches bleeding from the iliac vessels and branches. In trauma, this technique is used to provide quick relief of pelvic hemorrhage in any setting, including the emergency and operating rooms.9-11
Technique
Retroperitoneal packing consists of a few basic steps and can be easily reproduced and applied in life-threatening circumstances. First, a 5-cm incision is made just cephalad to the symphysis pubis deep to the fascia and into the space of Retzius (FIGURE 1). The buildup of blood often causes autodissection of this plane (FIGURE 4). It is often useful to keep this fascial incision separate from the laparotomy fascial incision to assist with tamponade. Next, blunt dissection is performed in the continuous space of Retzius and retroperitoneum to the level of the presacral space (FIGURE 2).
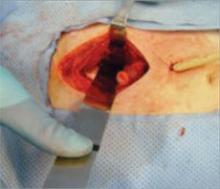
FIGURE 4 Expect autodissection
Blood loss frequently causes autodissection of the surgical plane. Tamponade is then achieved by placing laparotomy sponges into the retroperitoneal space (FIGURE 3). Packing with two or three laparotomy sponges at each side of the retroperitoneal pelvis is usually sufficient. In emergent situations, this entire procedure can be completed in an emergency room or postanesthesia care unit, with a drain left in place along with the packs (FIGURE 5). In trauma, this technique allows immediate stabilization of the patient until the underlying injury can be thoroughly addressed. Careful examination of the ureters and bladder should be completed to address any injury promptly.
FIGURE 5 A drain may be required
Packing can be left in place until bleeding is stanched, with a drain added for optimal recovery. The success of this technique is clear from the trauma literature, but the data have yet to be widely applied in nontraumatic applications.9 It is especially advantageous to have a space separate from the intraperitoneal cavity to provide tamponade because the uterus itself may obstruct visualization. It is possible that, in some cases, this technique may control bleeding without the need for postpartum hysterectomy.
CASE RESOLVED
When the retroperitoneal packs are removed after the hysterectomy, no further bleeding occurs. However, moderate hydronephrosis is apparent along the patient’s left ureter.
The wound is closed, and the patient is transferred to intensive care. She subsequently undergoes placement of a ureteral stent for the hydronephrosis and is discharged 5 days later. The stent is removed on an outpatient basis without further morbidity or the need for additional procedures.
Pelvic hemorrhage is a devastating complication in both trauma and postpartum situations. Postpartum hemorrhage complicates 6% of cesarean deliveries and leads to hysterectomy in 0.35 of every 1,000 deliveries. Maternal mortality approaches 13.6% in developing nations and 4% in industrialized nations.12
When does bleeding after delivery become “hemorrhage”?
Postpartum hemorrhage is often defined as more than 500 cc of blood loss after vaginal delivery or more than 1,000 cc during a cesarean delivery.13 Postpartum hemorrhage can be further classified into primary or secondary, depending on the timing of occurrence. Primary hemorrhage occurs within 24 hours of delivery; secondary hemorrhage occurs from 24 hours to 12 weeks after delivery.14
Causes of postpartum hemorrhage include uterine atony, retained placental products, genital laceration, inversion of the uterus, and coagulation disorders.
1. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
2. Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2003;(1):CD003249.-
3. Novello A, King JC. Health advisory: Prevention of maternal deaths through improved management of hemorrhage. Available at: www.acog.org/acog_districts/dist_notice.cfm?recno=1&bulletin=1517. Accessed May 14, 2008.
4. Allam MS, B-Lynch C. The B-Lynch and other uterine compression suture techniques. Int J Gynaecol Obstet. 2005;89:236-241.
5. Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstet Gynecol. 2000;96:129-131.
6. Burtelow M, Riley E, Druzin M, Fontaine M, Viele M, Goodnough LT. How we treat: management of life-threatening primary postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion. 2007;47:1564-1572.
7. Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307-310.
8. Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60(6 Suppl):S51-S58.
9. Smith WR, Moore EE, Osborn P, et al. Retroperitoneal packing as a resuscitation technique for hemodynamically unstable patients with pelvic fractures: report of two representative cases and a description of technique. J Trauma. 2005;59:1510-1514.
10. Hsu S, Rodgers B, Lele A, Yeh J. Use of packing in obstetric hemorrhage of uterine origin. J Reprod Med. 2003;48:69-71.
11. Maier RC. Control of postpartum hemorrhage with uterine packing. Obstet Gynecol. 1993;169:317-323.
12. Dildy GA, Scott JR, Saffer CS, Belfort MA. An effective pressure pack for severe pelvic hemorrhage. Obstet Gynecol. 2006;108:1222-1226.
13. Clark SL, Yeh SY, Phelan JP, Bruce S, Paul RH. Emergency hysterectomy for obstetric hemorrhage. Obstet Gynecol. 1984;64:376-380.
14. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetricians-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047.
The authors report no financial relationships relevant to this article.
CASE: Postcesarean hemorrhage fails to respond to early maneuvers.
A 25-year-old G1P0 undergoes cesarean section at our hospital for fetal distress. She has no history of coagulopathy, and no intraoperative complications are noted during the procedure. Upon her arrival in the postanesthesia care unit, however, vaginal bleeding is observed. She is given 40 U of oxytocin in 1 L of lactated Ringer solution, two intramuscular doses of 0.2 mg of methylergonovine maleate, and 1,000 μg of misoprostol to treat the postpartum bleeding. Nevertheless, she loses almost 1 L of additional blood from her vagina and is returned to the operating room for exploration and resuscitation for hypotensive shock. What are the next steps?
Management of obstetrical hemorrhage often begins with conservative measures, circumstances permitting. It is common practice to give 20–40 U of oxytocin in 1 L of lactated Ringer solution after delivery of the placenta and to perform uterine massage as part of initial management of uterine atony, along with careful evaluation and repair of any laceration or hematoma. In addition, ultrasonography (US) can help detect any retained uterine products.
Medical management usually involves the use of various uterotonics, such as methylergonovine maleate, 15-methylprostaglandin F2α, dinoprostone, and misoprostol. If uterotonics fail, techniques of tamponade include uterine packing with gauze material or use of the Foley intrauterine catheter, Sengstaken–Blakemore tube, and Bakri balloon.1-3
Surgical management is often the last resort, and is limited by the clinician’s experience. Some surgical methods include uterine or hypogastric artery ligation, or both. Newer techniques include a variation of uterine compression sutures such as the B-Lynch suture or multiple-square suturing. The B-Lynch provides continuous compression of the uterus, thereby decreasing blood loss.4 Multiple-square suturing joins the anterior and posterior walls of the uterus, also compressing the uterus.
Hysterectomy should be a last resort, with the knowledge that bleeding may continue after the procedure, in which case pelvic packing becomes an alternative. Unfortunately, pelvic packing of the intraperitoneal cavity often has little effect on endometrial hemorrhage or retroperitoneal bleeding.2-5
Resuscitation calls for blood products. Our resuscitation regimen includes recent clinical recommendations from military medical units in Iraq and Afghanistan and from domestic trauma centers. These guidelines propose that 1 U of fresh frozen plasma be administered with every 1 or 2 U of packed red blood cells (RBCs) until the clinical situation stabilizes or coagulopathy is excluded. Because of massive blood loss in this case, however, fluid replacement continues throughout the procedure—totaling 6 U of packed RBCs, 6 U of fresh frozen plasma, and 5 U of cryoprecipitate with additional crystalloid.6-8
A decision is made to undertake surgical exploration. We open a Pfannenstiel incision and enter the peritoneal cavity, encountering scant dark red blood without gross intraperitoneal bleeding. The uterus is intact with apparent endometrial hemorrhage. Uterine vessels are not easily visualized because they are obscured by retroperitoneal blood and an engorged uterus. The uterus has increased in size severalfold during hemorrhage and occupies the entire pelvic cavity, making dissection difficult for emergent hysterectomy.
As the uterus is exteriorized, Péan clamps are placed on the cornua for retraction, and the round ligaments are transected and ligated bilaterally. Ecchymoses along the peritoneum suggest that retroperitoneal bleeding is occurring in addition to the endometrial blood loss.
What can be done about the retroperitoneal bleeding?
Although laparotomy and hysterectomy are last resorts in postpartum hemorrhage, the use of retroperitoneal packing during these procedures may hasten life-saving hemostasis. In pelvic trauma, a technique of retroperitoneal packing has significantly reduced mortality.9 The same technique of retroperitoneal packing is ideally suited for such devastating circumstances as life-threatening postpartum hemorrhage.
Retroperitoneal packing is a lesson gleaned from trauma surgery and has profound application in cases of severe postpartum hemorrhage.
CASE CONTINUED
Hemorrhage is stanched. Blood loss continues, and the patient remains in hypotensive shock. Vital signs are critical:
- systolic blood pressure, 40 mm Hg
- heart rate, 160 bpm
- minimal urine output
- hemoglobin level, 4 g/dL.
Hemorrhage is obvious from the appearance of the pelvis, and continuing blood loss suggests disseminated coagulopathy. Total abdominal hysterectomy cannot be safely or quickly performed.
To quickly prevent further blood loss, we pack the retroperitoneum using a technique adapted from trauma surgery and first described by Smith and colleagues.9 We make a 5-cm incision into the space of Retzius just cephalad to the pubic symphysis (FIGURES 1 and 2). This incision is separate and inferior to the earlier laparotomy incision.
Four laparotomy sponges are packed along the retroperitoneal space to provide tamponade (FIGURE 3). Within seconds, the patient stabilizes, with systolic blood pressure rising to 90 mm Hg and the heart rate declining toward 100 bpm. Bleeding ceases immediately, and the hysterectomy is completed under stable conditions without further blood loss.
FIGURE 1 Packing begins with a 5-cm incision
Make a 5-cm incision just cephalad to the symphysis pubis, deep to the fascia, and into the space of Retzius.
FIGURE 2 Preparing to place sponges
Use blunt dissection in the space of Retzius before placing packing material.
FIGURE 3 Insert packing into retroperitoneal space
Packing is usually sufficient when two or three laparotomy sponges are placed at each side of the retroperitoneal pelvis.
How retroperitoneal packing saves lives
Most of the pelvic packing that has been described in the literature has consistently involved intraperitoneal packing. However, packing of the peritoneal cavity is often insufficient tamponade for bleeding associated with retroperitoneal and endometrial bleeding. Direct compression in the retroperitoneal space stanches bleeding from the iliac vessels and branches. In trauma, this technique is used to provide quick relief of pelvic hemorrhage in any setting, including the emergency and operating rooms.9-11
Technique
Retroperitoneal packing consists of a few basic steps and can be easily reproduced and applied in life-threatening circumstances. First, a 5-cm incision is made just cephalad to the symphysis pubis deep to the fascia and into the space of Retzius (FIGURE 1). The buildup of blood often causes autodissection of this plane (FIGURE 4). It is often useful to keep this fascial incision separate from the laparotomy fascial incision to assist with tamponade. Next, blunt dissection is performed in the continuous space of Retzius and retroperitoneum to the level of the presacral space (FIGURE 2).
FIGURE 4 Expect autodissection
Blood loss frequently causes autodissection of the surgical plane. Tamponade is then achieved by placing laparotomy sponges into the retroperitoneal space (FIGURE 3). Packing with two or three laparotomy sponges at each side of the retroperitoneal pelvis is usually sufficient. In emergent situations, this entire procedure can be completed in an emergency room or postanesthesia care unit, with a drain left in place along with the packs (FIGURE 5). In trauma, this technique allows immediate stabilization of the patient until the underlying injury can be thoroughly addressed. Careful examination of the ureters and bladder should be completed to address any injury promptly.
FIGURE 5 A drain may be required
Packing can be left in place until bleeding is stanched, with a drain added for optimal recovery. The success of this technique is clear from the trauma literature, but the data have yet to be widely applied in nontraumatic applications.9 It is especially advantageous to have a space separate from the intraperitoneal cavity to provide tamponade because the uterus itself may obstruct visualization. It is possible that, in some cases, this technique may control bleeding without the need for postpartum hysterectomy.
CASE RESOLVED
When the retroperitoneal packs are removed after the hysterectomy, no further bleeding occurs. However, moderate hydronephrosis is apparent along the patient’s left ureter.
The wound is closed, and the patient is transferred to intensive care. She subsequently undergoes placement of a ureteral stent for the hydronephrosis and is discharged 5 days later. The stent is removed on an outpatient basis without further morbidity or the need for additional procedures.
Pelvic hemorrhage is a devastating complication in both trauma and postpartum situations. Postpartum hemorrhage complicates 6% of cesarean deliveries and leads to hysterectomy in 0.35 of every 1,000 deliveries. Maternal mortality approaches 13.6% in developing nations and 4% in industrialized nations.12
When does bleeding after delivery become “hemorrhage”?
Postpartum hemorrhage is often defined as more than 500 cc of blood loss after vaginal delivery or more than 1,000 cc during a cesarean delivery.13 Postpartum hemorrhage can be further classified into primary or secondary, depending on the timing of occurrence. Primary hemorrhage occurs within 24 hours of delivery; secondary hemorrhage occurs from 24 hours to 12 weeks after delivery.14
Causes of postpartum hemorrhage include uterine atony, retained placental products, genital laceration, inversion of the uterus, and coagulation disorders.
The authors report no financial relationships relevant to this article.
CASE: Postcesarean hemorrhage fails to respond to early maneuvers.
A 25-year-old G1P0 undergoes cesarean section at our hospital for fetal distress. She has no history of coagulopathy, and no intraoperative complications are noted during the procedure. Upon her arrival in the postanesthesia care unit, however, vaginal bleeding is observed. She is given 40 U of oxytocin in 1 L of lactated Ringer solution, two intramuscular doses of 0.2 mg of methylergonovine maleate, and 1,000 μg of misoprostol to treat the postpartum bleeding. Nevertheless, she loses almost 1 L of additional blood from her vagina and is returned to the operating room for exploration and resuscitation for hypotensive shock. What are the next steps?
Management of obstetrical hemorrhage often begins with conservative measures, circumstances permitting. It is common practice to give 20–40 U of oxytocin in 1 L of lactated Ringer solution after delivery of the placenta and to perform uterine massage as part of initial management of uterine atony, along with careful evaluation and repair of any laceration or hematoma. In addition, ultrasonography (US) can help detect any retained uterine products.
Medical management usually involves the use of various uterotonics, such as methylergonovine maleate, 15-methylprostaglandin F2α, dinoprostone, and misoprostol. If uterotonics fail, techniques of tamponade include uterine packing with gauze material or use of the Foley intrauterine catheter, Sengstaken–Blakemore tube, and Bakri balloon.1-3
Surgical management is often the last resort, and is limited by the clinician’s experience. Some surgical methods include uterine or hypogastric artery ligation, or both. Newer techniques include a variation of uterine compression sutures such as the B-Lynch suture or multiple-square suturing. The B-Lynch provides continuous compression of the uterus, thereby decreasing blood loss.4 Multiple-square suturing joins the anterior and posterior walls of the uterus, also compressing the uterus.
Hysterectomy should be a last resort, with the knowledge that bleeding may continue after the procedure, in which case pelvic packing becomes an alternative. Unfortunately, pelvic packing of the intraperitoneal cavity often has little effect on endometrial hemorrhage or retroperitoneal bleeding.2-5
Resuscitation calls for blood products. Our resuscitation regimen includes recent clinical recommendations from military medical units in Iraq and Afghanistan and from domestic trauma centers. These guidelines propose that 1 U of fresh frozen plasma be administered with every 1 or 2 U of packed red blood cells (RBCs) until the clinical situation stabilizes or coagulopathy is excluded. Because of massive blood loss in this case, however, fluid replacement continues throughout the procedure—totaling 6 U of packed RBCs, 6 U of fresh frozen plasma, and 5 U of cryoprecipitate with additional crystalloid.6-8
A decision is made to undertake surgical exploration. We open a Pfannenstiel incision and enter the peritoneal cavity, encountering scant dark red blood without gross intraperitoneal bleeding. The uterus is intact with apparent endometrial hemorrhage. Uterine vessels are not easily visualized because they are obscured by retroperitoneal blood and an engorged uterus. The uterus has increased in size severalfold during hemorrhage and occupies the entire pelvic cavity, making dissection difficult for emergent hysterectomy.
As the uterus is exteriorized, Péan clamps are placed on the cornua for retraction, and the round ligaments are transected and ligated bilaterally. Ecchymoses along the peritoneum suggest that retroperitoneal bleeding is occurring in addition to the endometrial blood loss.
What can be done about the retroperitoneal bleeding?
Although laparotomy and hysterectomy are last resorts in postpartum hemorrhage, the use of retroperitoneal packing during these procedures may hasten life-saving hemostasis. In pelvic trauma, a technique of retroperitoneal packing has significantly reduced mortality.9 The same technique of retroperitoneal packing is ideally suited for such devastating circumstances as life-threatening postpartum hemorrhage.
Retroperitoneal packing is a lesson gleaned from trauma surgery and has profound application in cases of severe postpartum hemorrhage.
CASE CONTINUED
Hemorrhage is stanched. Blood loss continues, and the patient remains in hypotensive shock. Vital signs are critical:
- systolic blood pressure, 40 mm Hg
- heart rate, 160 bpm
- minimal urine output
- hemoglobin level, 4 g/dL.
Hemorrhage is obvious from the appearance of the pelvis, and continuing blood loss suggests disseminated coagulopathy. Total abdominal hysterectomy cannot be safely or quickly performed.
To quickly prevent further blood loss, we pack the retroperitoneum using a technique adapted from trauma surgery and first described by Smith and colleagues.9 We make a 5-cm incision into the space of Retzius just cephalad to the pubic symphysis (FIGURES 1 and 2). This incision is separate and inferior to the earlier laparotomy incision.
Four laparotomy sponges are packed along the retroperitoneal space to provide tamponade (FIGURE 3). Within seconds, the patient stabilizes, with systolic blood pressure rising to 90 mm Hg and the heart rate declining toward 100 bpm. Bleeding ceases immediately, and the hysterectomy is completed under stable conditions without further blood loss.
FIGURE 1 Packing begins with a 5-cm incision
Make a 5-cm incision just cephalad to the symphysis pubis, deep to the fascia, and into the space of Retzius.
FIGURE 2 Preparing to place sponges
Use blunt dissection in the space of Retzius before placing packing material.
FIGURE 3 Insert packing into retroperitoneal space
Packing is usually sufficient when two or three laparotomy sponges are placed at each side of the retroperitoneal pelvis.
How retroperitoneal packing saves lives
Most of the pelvic packing that has been described in the literature has consistently involved intraperitoneal packing. However, packing of the peritoneal cavity is often insufficient tamponade for bleeding associated with retroperitoneal and endometrial bleeding. Direct compression in the retroperitoneal space stanches bleeding from the iliac vessels and branches. In trauma, this technique is used to provide quick relief of pelvic hemorrhage in any setting, including the emergency and operating rooms.9-11
Technique
Retroperitoneal packing consists of a few basic steps and can be easily reproduced and applied in life-threatening circumstances. First, a 5-cm incision is made just cephalad to the symphysis pubis deep to the fascia and into the space of Retzius (FIGURE 1). The buildup of blood often causes autodissection of this plane (FIGURE 4). It is often useful to keep this fascial incision separate from the laparotomy fascial incision to assist with tamponade. Next, blunt dissection is performed in the continuous space of Retzius and retroperitoneum to the level of the presacral space (FIGURE 2).
FIGURE 4 Expect autodissection
Blood loss frequently causes autodissection of the surgical plane. Tamponade is then achieved by placing laparotomy sponges into the retroperitoneal space (FIGURE 3). Packing with two or three laparotomy sponges at each side of the retroperitoneal pelvis is usually sufficient. In emergent situations, this entire procedure can be completed in an emergency room or postanesthesia care unit, with a drain left in place along with the packs (FIGURE 5). In trauma, this technique allows immediate stabilization of the patient until the underlying injury can be thoroughly addressed. Careful examination of the ureters and bladder should be completed to address any injury promptly.
FIGURE 5 A drain may be required
Packing can be left in place until bleeding is stanched, with a drain added for optimal recovery. The success of this technique is clear from the trauma literature, but the data have yet to be widely applied in nontraumatic applications.9 It is especially advantageous to have a space separate from the intraperitoneal cavity to provide tamponade because the uterus itself may obstruct visualization. It is possible that, in some cases, this technique may control bleeding without the need for postpartum hysterectomy.
CASE RESOLVED
When the retroperitoneal packs are removed after the hysterectomy, no further bleeding occurs. However, moderate hydronephrosis is apparent along the patient’s left ureter.
The wound is closed, and the patient is transferred to intensive care. She subsequently undergoes placement of a ureteral stent for the hydronephrosis and is discharged 5 days later. The stent is removed on an outpatient basis without further morbidity or the need for additional procedures.
Pelvic hemorrhage is a devastating complication in both trauma and postpartum situations. Postpartum hemorrhage complicates 6% of cesarean deliveries and leads to hysterectomy in 0.35 of every 1,000 deliveries. Maternal mortality approaches 13.6% in developing nations and 4% in industrialized nations.12
When does bleeding after delivery become “hemorrhage”?
Postpartum hemorrhage is often defined as more than 500 cc of blood loss after vaginal delivery or more than 1,000 cc during a cesarean delivery.13 Postpartum hemorrhage can be further classified into primary or secondary, depending on the timing of occurrence. Primary hemorrhage occurs within 24 hours of delivery; secondary hemorrhage occurs from 24 hours to 12 weeks after delivery.14
Causes of postpartum hemorrhage include uterine atony, retained placental products, genital laceration, inversion of the uterus, and coagulation disorders.
1. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
2. Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2003;(1):CD003249.-
3. Novello A, King JC. Health advisory: Prevention of maternal deaths through improved management of hemorrhage. Available at: www.acog.org/acog_districts/dist_notice.cfm?recno=1&bulletin=1517. Accessed May 14, 2008.
4. Allam MS, B-Lynch C. The B-Lynch and other uterine compression suture techniques. Int J Gynaecol Obstet. 2005;89:236-241.
5. Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstet Gynecol. 2000;96:129-131.
6. Burtelow M, Riley E, Druzin M, Fontaine M, Viele M, Goodnough LT. How we treat: management of life-threatening primary postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion. 2007;47:1564-1572.
7. Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307-310.
8. Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60(6 Suppl):S51-S58.
9. Smith WR, Moore EE, Osborn P, et al. Retroperitoneal packing as a resuscitation technique for hemodynamically unstable patients with pelvic fractures: report of two representative cases and a description of technique. J Trauma. 2005;59:1510-1514.
10. Hsu S, Rodgers B, Lele A, Yeh J. Use of packing in obstetric hemorrhage of uterine origin. J Reprod Med. 2003;48:69-71.
11. Maier RC. Control of postpartum hemorrhage with uterine packing. Obstet Gynecol. 1993;169:317-323.
12. Dildy GA, Scott JR, Saffer CS, Belfort MA. An effective pressure pack for severe pelvic hemorrhage. Obstet Gynecol. 2006;108:1222-1226.
13. Clark SL, Yeh SY, Phelan JP, Bruce S, Paul RH. Emergency hysterectomy for obstetric hemorrhage. Obstet Gynecol. 1984;64:376-380.
14. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetricians-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047.
1. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
2. Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2003;(1):CD003249.-
3. Novello A, King JC. Health advisory: Prevention of maternal deaths through improved management of hemorrhage. Available at: www.acog.org/acog_districts/dist_notice.cfm?recno=1&bulletin=1517. Accessed May 14, 2008.
4. Allam MS, B-Lynch C. The B-Lynch and other uterine compression suture techniques. Int J Gynaecol Obstet. 2005;89:236-241.
5. Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstet Gynecol. 2000;96:129-131.
6. Burtelow M, Riley E, Druzin M, Fontaine M, Viele M, Goodnough LT. How we treat: management of life-threatening primary postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion. 2007;47:1564-1572.
7. Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307-310.
8. Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60(6 Suppl):S51-S58.
9. Smith WR, Moore EE, Osborn P, et al. Retroperitoneal packing as a resuscitation technique for hemodynamically unstable patients with pelvic fractures: report of two representative cases and a description of technique. J Trauma. 2005;59:1510-1514.
10. Hsu S, Rodgers B, Lele A, Yeh J. Use of packing in obstetric hemorrhage of uterine origin. J Reprod Med. 2003;48:69-71.
11. Maier RC. Control of postpartum hemorrhage with uterine packing. Obstet Gynecol. 1993;169:317-323.
12. Dildy GA, Scott JR, Saffer CS, Belfort MA. An effective pressure pack for severe pelvic hemorrhage. Obstet Gynecol. 2006;108:1222-1226.
13. Clark SL, Yeh SY, Phelan JP, Bruce S, Paul RH. Emergency hysterectomy for obstetric hemorrhage. Obstet Gynecol. 1984;64:376-380.
14. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetricians-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047.
Endometrial Cancer
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.
Use of Laser Doppler Imaging to Assess Microvascular Response to Thermal Stress
The Impact of Laboratory Automation on Performance Improvement
Methadone Maintenance Therapy for Opioid Addiction
Treating patients in pain has challenged health care providers for centuries. Long-term use of pain medication, particularly opioids, creates a potential for physical addiction. Addiction to opiates can cause irreparable damage in every aspect of life, including personal health, family, and finances.
For certain patients, undergoing pain management in their 20s or 30s for an acute injury, condition, or procedure can begin a cyclical pattern of abuse, physical addiction, cessation of use, and relapse. During this cycle, lack of access to legitimately prescribed opioids may lead to illegal activities, accidental overdose, or drug-related accidents that affect both the user and others.
More than 20% of Americans older than 12 report nonmedical use of various prescribed medications at some point during their lifetime.1 A large proportion of abuse involves opioid-based narcotics.
Benefits of Methadone
Methadone, a synthetic opiate whose analgesic properties were first discovered in the 1940s, was initially used to manage chronic pain. In the early 1960s, it was discovered that when taken daily at an appropriate maintenance dose, methadone benefited patients experiencing withdrawal from other opioids, including morphine and heroin.2,3 Research findings published by the NIH associate methadone maintenance treatment (MMT) with reductions in opioid drug use, crime, transmission of viral diseases, including HIV and hepatitis, and incidence of opioid-related death and overdose.4 This therapy is also credited for improved social productivity.5
Patients enrolled in MMT programs receive methadone for treatment of physical withdrawal symptoms (nausea, diarrhea, muscle aches, sweating, irritability, insomnia, "crawly" skin, anxiety) and cravings. Yet treatment with methadone is only one part of the recovery process.3 MMT also includes counseling, lifestyle modification, and other supportive services. It is important for patients to understand that constant personal reflection, ongoing counseling, and an awareness of the ramifications of continued use of other drugs all play a role in the success or failure of recovery from addiction. Methadone is not indicated for the treatment of addiction to drugs in other classes or to other substances.
Strict criteria are in place for persons to be admitted to MMT. These may include a history of at least six months' daily opioid use, positive urine screening for opioids, and the presence of active withdrawal symptoms. During the first 30 to 60 days, when daily attendance is required, the proper methadone maintenance dose is determined. Participants are monitored regularly with urine drug screening.
An integral part of MMT is to help patients reestablish a "normal" life: stability in employment, family status, finances, and personal goals. Treatment duration is highly individualized, with some patients requiring lifelong therapy to ensure continued success in recovery.3,5
How Methadone Works
Like the opioids, methadone acts on receptors in the brain that control pain and mood. Since methadone is metabolized in the liver through cytochrome P (CYP) enzymes (including CYP450, CYP3A4, CYP2C8, and CYP2D66), some care is warranted regarding use of other medications that may inhibit or induce substances in this enzyme class.7 (See table.7-10) Patients who take other medications that are influenced by CYP enzymes should be monitored for cross-reactions and may require medication adjustment. A thorough history of medication use and close monitoring of potential medication combinations are warranted.
Methadone possesses certain unique properties. Compared with most prescribed opioids (ie, hydrocodone, morphine, oxycodone), which have a half-life averaging less than three hours, the half-life for methadone exceeds 24 hours.9 This, coupled with a relatively slow onset of action, allows for once-daily dosing, making methadone a particularly effective tool in opioid addiction treatment. When taken properly, methadone is safe for the body and does not impede normal functioning. Additionally, methadone is cross-tolerant with other opioid medications,4 decreasing the likelihood of drug-seeking behavior.
Dosing is deemed adequate when the patient experiences relief from withdrawal and cravings without feeling "high" or oversedated. Because methadone is a full agonist, however, excessive dosing may produce euphoric effects.9
While methadone can in itself be physically addicting, research clearly shows that this agent helps normalize the function of body systems (particularly the immune, endocrine, and neurologic systems) that were previously impaired by opioid abuse.1
The most commonly reported adverse effects of methadone use are similar to those associated with the opioids: constipation, decreased libido, alterations in sexual functioning, amenorrhea, weight gain, and sweating. Methadone is not contraindicated for patients undergoing medical or dental procedures, but any dosing reduction should be coordinated through the MMT team. Abrupt reduction or cessation of methadone dosing may lead to drug craving and a reappearance of withdrawal symptoms.
Considerations Before MMT
For the patient who acknowledges an opioid addiction problem, the primary care provider's first step should be to attempt to wean the patient off the prescribed medication. Abruptly cutting the patient off will only generate panic and increase the likelihood of illegal behavior. If the patient is unable or unwilling to tolerate a weaning process, referral to a MMT center is indicated. To allow a reasonable time frame for the patient to enroll, providers are advised to prescribe a limited supply of the medication in question.
Methadone Maintenance Treatment
Upon admission to MMT, the patient undergoes an induction phase in which the proper methadone maintenance dose is determined by increasing the dosing every two to four days, with careful monitoring. Since methadone is taken by mouth as a pill, diskette, or liquid, the risk of exposure to needle-borne diseases is eliminated. Use of diskette or liquid formulations helps prevent diversion of medication, as does supervised consumption.11
Thus, patients are required to take the entire dose in the presence of a clinic employee (usually a dosing nurse). Starting doses are generally 30 to 40 mg per day.12 Maintenance doses are highly individualized, but most patients will require 60 to 120 mg per day to suppress opioid withdrawal symptoms and cravings.8 Some clinics arrange split dosing for patients who need to take lower doses more frequently.
Careful monitoring is an important feature of MMT. Because of methadone's long half-life, a too-rapid dosing increase or a drug reaction can trigger rapid elevation of methadone serum levels.9 Methadone-related mortality is most significant during the first few days and weeks of treatment, in part because of the use of opioids or other substances to cope with withdrawal symptoms that will subside once dosing is stabilized.
Most states require MMT clinics to register all patients in a statewide central database to prevent dosing at multiple sites.
Challenges for the Managing Clinician
One of the primary care provider's greatest concerns for patients who are receiving MMT is the treatment of pain. While NSAIDs or other nonnarcotic pain relievers may be appropriate for mild to moderate pain, severe pain will often require narcotic medication, possibly including opioids.13 Providers' general concern about oversedation in prescribing pain medication seems more significant when the patient has a known history of addiction. Many hesitate to prescribe adequate levels of medication for acute pain for fear of reinforcing a patient's addiction or supporting drug diversion.
Complicating this concern is a common mis-conception that methadone will provide adequate analgesia during an acute process.4,7 To the contrary, patients on MMT who experience acute pain still require effective pain management. Although methadone suppresses opioid withdrawal for longer than 24 hours, any analgesic relief generally lasts only four to six hours. Use of opioid-classified medications will "block" the euphoric effects of the drug, but with sufficient dosing, patients should begin to experience pain relief.13
For severe pain, more aggressive dosing or more frequent dosing with shorter-acting agents is indicated.4 Methadone alone will not provide adequate postsurgical pain relief. Certain contraindicated analgesics (mixed agonist/antagonist or partial agonists) will initiate immediate and extreme withdrawal symptoms (see table).
Another common misconception is that the use of opioid pain medication may lead to addiction relapse or drug-seeking behavior. There is no evidence to support either notion.13 It is generally thought that the stress associated with unrelieved pain may pose a greater threat for relapse.5
Drug-Seeking Behavior
Primary care practitioners may also be concerned about being manipulated by patients with a history of addiction and wary of drug-seeking behavior. A careful, objective, and subjective clinical assessment of pain will reduce the clinician's chance of being manipulated. In turn, providing reassurance and discussing pain management with the patient in a nonjudgmental manner will relieve any distrust of the primary care provider or fear of stigma the patient may harbor.13,14
Providers should coordinate care with the patient's MMT clinic and verify methadone dosing. For optimal pain management, ordering short-acting analgesics on continuous scheduled dosing is preferred over as-needed dosing.13
Careful documentation is essential for primary care practitioners who care for patients in MMT. Patients will need discharge information that clearly indicates dosing schedules of methadone, opioids, and other adjunct medications (eg, benzodiazepines) that may appear on urine drug screening panels. All new prescriptions should be listed on discharge forms. MMT program staff members generally work with patients before scheduled procedures or hospitalizations to avoid any disruption of treatment.
MMT During Pregnancy
Practitioners also need to be informed about methadone use during pregnancy—an area that has been studied fairly extensively. Compared with pregnant women who continue to use opioids or who attempt detoxification, those receiving MMT have a reduced risk of pregnancy-related complications (ie, miscarriage, fetal dis-tress, premature labor).1,5 When properly prescribed and monitored, MMT provides a favorable environment for the developing fetus. Dosing levels and frequency are adjusted as the pregnancy progresses.
Infants born to women who are methadone-dependent can be safely, successfully weaned with no adverse effects soon after delivery.13 Methadone levels are very low in breast milk; thus, breastfeeding is not necessarily contraindicated in women undergoing MMT.
Another Option
While this article focuses primarily on MMT, it is important to note that some patients will opt for treatment with buprenorphine. Approved in 2002 for patients with opioid addiction, this agent has a reduced potential for euphoria and abuse because of its partial agonist properties.12,15 Another advantage is that primary care providers certified in treatment can administer buprenorphine directly.
The downside of use of this agent is increased cost and lack of access to the counseling services that are such an important component of traditional MMT programs. Because research is limited in the treatment of acute pain for patients who take buprenorphine, more structure and greater caution are called for at times when opioid analgesics must be prescribed.12,13
Conclusion
Patients living with opioid addiction experience fear of discrimination, mistrust of health care providers, and often embarrassment and despair over their predicament. Practitioners who suspect opioid addiction should approach these patients with respect but be direct about their concerns and reassuring about the possibilities offered by treatment. An improved understanding of opioid addiction and the benefits of treatment with methadone should enhance management of addicted patients in the primary care setting.
1. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Results from the 2006 National Survey on Drug Use and Health: National Findings, 2007. www.oas.samhsa.gov/nsduh/2k6nsduh/2k6Results.htm. Accessed May 22, 2008.
2. O'Brien CP. Drug addiction and drug abuse. In: Hardman JG, Gilman AG, Limbird LE, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001:621-642.
3. Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303-1310.
4. Krambeer LL, von McKnelly W Jr, Gabrielli WF Jr, Penick EC. Methadone therapy for opioid dependence. Am Fam Physician. 2001;63(12):2404-2410.
5. CRC Health Corporation. A brief history of methadone. 2005. www.opiatesrx.com/methadone.php. Accessed May 22, 2008.
6. Wang JS, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab Dispos. 2003;31(6):742-747.
7. Leavitt SB. Methadone-drug interactions. 3rd ed. Addiction Treatment Forum. November 2005 revision/update. www.atforum.com/SiteRoot/pages/addiction_resources/Drug_Interactions.pdf. Accessed May 22, 2008.
8. Centers for Disease Control and Prevention. Methadone maintenance treatment. IDU HIV Prevention. February 2002. www.cdc.gov/IDU/facts/MethadoneFin.pdf. Accessed May 22, 2008.
9. Leavitt SB. Methadone dosing and safety in the treatment of opioid addiction. Addiction Treatment Forum. Special Report. 2003. http://atforum.com/SiteRoot/pages/addiction_resources/DosingandSafetyWP.pdf. Accessed May 22, 2008.
10. Flockhart DA. Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine (2007). http://medicine.iupui.edu/flockhart/table.htm. Accessed May 22, 2008.
11. Weinrich M, Stuart M. Provision of methadone treatment in primary care medical practices: review of the Scottish experience and implications for US policy. JAMA. 2000;283(10):1343-1348.
12. Curie CG, Clark HW; Substance Abuse and Mental Health Services Administration. Methadone-Associated Mortality: Report of a National Assessment. May 8-9, 2003. Rockville, MD: CSAT Publication No. 28-03. www.dpt.samhsa.gov/medications/methadonemortality2003/methadone_mortality.aspx. Accessed May 22, 2008.
13. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006; 144(2):127-134.
14. McMurphy S, Shea J, Switzer J, Turner BJ. Clinic-based treatment for opioid dependence: a qualitative inquiry. Am J Health Behav. 2006;30(5):544-554.
15. Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315(3):1320-1330.
In addition to working in private practice, Julia Lowe Behr is a faculty member at the Medical College of Georgia School of Nursing, Athens Campus.
Treating patients in pain has challenged health care providers for centuries. Long-term use of pain medication, particularly opioids, creates a potential for physical addiction. Addiction to opiates can cause irreparable damage in every aspect of life, including personal health, family, and finances.
For certain patients, undergoing pain management in their 20s or 30s for an acute injury, condition, or procedure can begin a cyclical pattern of abuse, physical addiction, cessation of use, and relapse. During this cycle, lack of access to legitimately prescribed opioids may lead to illegal activities, accidental overdose, or drug-related accidents that affect both the user and others.
More than 20% of Americans older than 12 report nonmedical use of various prescribed medications at some point during their lifetime.1 A large proportion of abuse involves opioid-based narcotics.
Benefits of Methadone
Methadone, a synthetic opiate whose analgesic properties were first discovered in the 1940s, was initially used to manage chronic pain. In the early 1960s, it was discovered that when taken daily at an appropriate maintenance dose, methadone benefited patients experiencing withdrawal from other opioids, including morphine and heroin.2,3 Research findings published by the NIH associate methadone maintenance treatment (MMT) with reductions in opioid drug use, crime, transmission of viral diseases, including HIV and hepatitis, and incidence of opioid-related death and overdose.4 This therapy is also credited for improved social productivity.5
Patients enrolled in MMT programs receive methadone for treatment of physical withdrawal symptoms (nausea, diarrhea, muscle aches, sweating, irritability, insomnia, "crawly" skin, anxiety) and cravings. Yet treatment with methadone is only one part of the recovery process.3 MMT also includes counseling, lifestyle modification, and other supportive services. It is important for patients to understand that constant personal reflection, ongoing counseling, and an awareness of the ramifications of continued use of other drugs all play a role in the success or failure of recovery from addiction. Methadone is not indicated for the treatment of addiction to drugs in other classes or to other substances.
Strict criteria are in place for persons to be admitted to MMT. These may include a history of at least six months' daily opioid use, positive urine screening for opioids, and the presence of active withdrawal symptoms. During the first 30 to 60 days, when daily attendance is required, the proper methadone maintenance dose is determined. Participants are monitored regularly with urine drug screening.
An integral part of MMT is to help patients reestablish a "normal" life: stability in employment, family status, finances, and personal goals. Treatment duration is highly individualized, with some patients requiring lifelong therapy to ensure continued success in recovery.3,5
How Methadone Works
Like the opioids, methadone acts on receptors in the brain that control pain and mood. Since methadone is metabolized in the liver through cytochrome P (CYP) enzymes (including CYP450, CYP3A4, CYP2C8, and CYP2D66), some care is warranted regarding use of other medications that may inhibit or induce substances in this enzyme class.7 (See table.7-10) Patients who take other medications that are influenced by CYP enzymes should be monitored for cross-reactions and may require medication adjustment. A thorough history of medication use and close monitoring of potential medication combinations are warranted.
Methadone possesses certain unique properties. Compared with most prescribed opioids (ie, hydrocodone, morphine, oxycodone), which have a half-life averaging less than three hours, the half-life for methadone exceeds 24 hours.9 This, coupled with a relatively slow onset of action, allows for once-daily dosing, making methadone a particularly effective tool in opioid addiction treatment. When taken properly, methadone is safe for the body and does not impede normal functioning. Additionally, methadone is cross-tolerant with other opioid medications,4 decreasing the likelihood of drug-seeking behavior.
Dosing is deemed adequate when the patient experiences relief from withdrawal and cravings without feeling "high" or oversedated. Because methadone is a full agonist, however, excessive dosing may produce euphoric effects.9
While methadone can in itself be physically addicting, research clearly shows that this agent helps normalize the function of body systems (particularly the immune, endocrine, and neurologic systems) that were previously impaired by opioid abuse.1
The most commonly reported adverse effects of methadone use are similar to those associated with the opioids: constipation, decreased libido, alterations in sexual functioning, amenorrhea, weight gain, and sweating. Methadone is not contraindicated for patients undergoing medical or dental procedures, but any dosing reduction should be coordinated through the MMT team. Abrupt reduction or cessation of methadone dosing may lead to drug craving and a reappearance of withdrawal symptoms.
Considerations Before MMT
For the patient who acknowledges an opioid addiction problem, the primary care provider's first step should be to attempt to wean the patient off the prescribed medication. Abruptly cutting the patient off will only generate panic and increase the likelihood of illegal behavior. If the patient is unable or unwilling to tolerate a weaning process, referral to a MMT center is indicated. To allow a reasonable time frame for the patient to enroll, providers are advised to prescribe a limited supply of the medication in question.
Methadone Maintenance Treatment
Upon admission to MMT, the patient undergoes an induction phase in which the proper methadone maintenance dose is determined by increasing the dosing every two to four days, with careful monitoring. Since methadone is taken by mouth as a pill, diskette, or liquid, the risk of exposure to needle-borne diseases is eliminated. Use of diskette or liquid formulations helps prevent diversion of medication, as does supervised consumption.11
Thus, patients are required to take the entire dose in the presence of a clinic employee (usually a dosing nurse). Starting doses are generally 30 to 40 mg per day.12 Maintenance doses are highly individualized, but most patients will require 60 to 120 mg per day to suppress opioid withdrawal symptoms and cravings.8 Some clinics arrange split dosing for patients who need to take lower doses more frequently.
Careful monitoring is an important feature of MMT. Because of methadone's long half-life, a too-rapid dosing increase or a drug reaction can trigger rapid elevation of methadone serum levels.9 Methadone-related mortality is most significant during the first few days and weeks of treatment, in part because of the use of opioids or other substances to cope with withdrawal symptoms that will subside once dosing is stabilized.
Most states require MMT clinics to register all patients in a statewide central database to prevent dosing at multiple sites.
Challenges for the Managing Clinician
One of the primary care provider's greatest concerns for patients who are receiving MMT is the treatment of pain. While NSAIDs or other nonnarcotic pain relievers may be appropriate for mild to moderate pain, severe pain will often require narcotic medication, possibly including opioids.13 Providers' general concern about oversedation in prescribing pain medication seems more significant when the patient has a known history of addiction. Many hesitate to prescribe adequate levels of medication for acute pain for fear of reinforcing a patient's addiction or supporting drug diversion.
Complicating this concern is a common mis-conception that methadone will provide adequate analgesia during an acute process.4,7 To the contrary, patients on MMT who experience acute pain still require effective pain management. Although methadone suppresses opioid withdrawal for longer than 24 hours, any analgesic relief generally lasts only four to six hours. Use of opioid-classified medications will "block" the euphoric effects of the drug, but with sufficient dosing, patients should begin to experience pain relief.13
For severe pain, more aggressive dosing or more frequent dosing with shorter-acting agents is indicated.4 Methadone alone will not provide adequate postsurgical pain relief. Certain contraindicated analgesics (mixed agonist/antagonist or partial agonists) will initiate immediate and extreme withdrawal symptoms (see table).
Another common misconception is that the use of opioid pain medication may lead to addiction relapse or drug-seeking behavior. There is no evidence to support either notion.13 It is generally thought that the stress associated with unrelieved pain may pose a greater threat for relapse.5
Drug-Seeking Behavior
Primary care practitioners may also be concerned about being manipulated by patients with a history of addiction and wary of drug-seeking behavior. A careful, objective, and subjective clinical assessment of pain will reduce the clinician's chance of being manipulated. In turn, providing reassurance and discussing pain management with the patient in a nonjudgmental manner will relieve any distrust of the primary care provider or fear of stigma the patient may harbor.13,14
Providers should coordinate care with the patient's MMT clinic and verify methadone dosing. For optimal pain management, ordering short-acting analgesics on continuous scheduled dosing is preferred over as-needed dosing.13
Careful documentation is essential for primary care practitioners who care for patients in MMT. Patients will need discharge information that clearly indicates dosing schedules of methadone, opioids, and other adjunct medications (eg, benzodiazepines) that may appear on urine drug screening panels. All new prescriptions should be listed on discharge forms. MMT program staff members generally work with patients before scheduled procedures or hospitalizations to avoid any disruption of treatment.
MMT During Pregnancy
Practitioners also need to be informed about methadone use during pregnancy—an area that has been studied fairly extensively. Compared with pregnant women who continue to use opioids or who attempt detoxification, those receiving MMT have a reduced risk of pregnancy-related complications (ie, miscarriage, fetal dis-tress, premature labor).1,5 When properly prescribed and monitored, MMT provides a favorable environment for the developing fetus. Dosing levels and frequency are adjusted as the pregnancy progresses.
Infants born to women who are methadone-dependent can be safely, successfully weaned with no adverse effects soon after delivery.13 Methadone levels are very low in breast milk; thus, breastfeeding is not necessarily contraindicated in women undergoing MMT.
Another Option
While this article focuses primarily on MMT, it is important to note that some patients will opt for treatment with buprenorphine. Approved in 2002 for patients with opioid addiction, this agent has a reduced potential for euphoria and abuse because of its partial agonist properties.12,15 Another advantage is that primary care providers certified in treatment can administer buprenorphine directly.
The downside of use of this agent is increased cost and lack of access to the counseling services that are such an important component of traditional MMT programs. Because research is limited in the treatment of acute pain for patients who take buprenorphine, more structure and greater caution are called for at times when opioid analgesics must be prescribed.12,13
Conclusion
Patients living with opioid addiction experience fear of discrimination, mistrust of health care providers, and often embarrassment and despair over their predicament. Practitioners who suspect opioid addiction should approach these patients with respect but be direct about their concerns and reassuring about the possibilities offered by treatment. An improved understanding of opioid addiction and the benefits of treatment with methadone should enhance management of addicted patients in the primary care setting.
Treating patients in pain has challenged health care providers for centuries. Long-term use of pain medication, particularly opioids, creates a potential for physical addiction. Addiction to opiates can cause irreparable damage in every aspect of life, including personal health, family, and finances.
For certain patients, undergoing pain management in their 20s or 30s for an acute injury, condition, or procedure can begin a cyclical pattern of abuse, physical addiction, cessation of use, and relapse. During this cycle, lack of access to legitimately prescribed opioids may lead to illegal activities, accidental overdose, or drug-related accidents that affect both the user and others.
More than 20% of Americans older than 12 report nonmedical use of various prescribed medications at some point during their lifetime.1 A large proportion of abuse involves opioid-based narcotics.
Benefits of Methadone
Methadone, a synthetic opiate whose analgesic properties were first discovered in the 1940s, was initially used to manage chronic pain. In the early 1960s, it was discovered that when taken daily at an appropriate maintenance dose, methadone benefited patients experiencing withdrawal from other opioids, including morphine and heroin.2,3 Research findings published by the NIH associate methadone maintenance treatment (MMT) with reductions in opioid drug use, crime, transmission of viral diseases, including HIV and hepatitis, and incidence of opioid-related death and overdose.4 This therapy is also credited for improved social productivity.5
Patients enrolled in MMT programs receive methadone for treatment of physical withdrawal symptoms (nausea, diarrhea, muscle aches, sweating, irritability, insomnia, "crawly" skin, anxiety) and cravings. Yet treatment with methadone is only one part of the recovery process.3 MMT also includes counseling, lifestyle modification, and other supportive services. It is important for patients to understand that constant personal reflection, ongoing counseling, and an awareness of the ramifications of continued use of other drugs all play a role in the success or failure of recovery from addiction. Methadone is not indicated for the treatment of addiction to drugs in other classes or to other substances.
Strict criteria are in place for persons to be admitted to MMT. These may include a history of at least six months' daily opioid use, positive urine screening for opioids, and the presence of active withdrawal symptoms. During the first 30 to 60 days, when daily attendance is required, the proper methadone maintenance dose is determined. Participants are monitored regularly with urine drug screening.
An integral part of MMT is to help patients reestablish a "normal" life: stability in employment, family status, finances, and personal goals. Treatment duration is highly individualized, with some patients requiring lifelong therapy to ensure continued success in recovery.3,5
How Methadone Works
Like the opioids, methadone acts on receptors in the brain that control pain and mood. Since methadone is metabolized in the liver through cytochrome P (CYP) enzymes (including CYP450, CYP3A4, CYP2C8, and CYP2D66), some care is warranted regarding use of other medications that may inhibit or induce substances in this enzyme class.7 (See table.7-10) Patients who take other medications that are influenced by CYP enzymes should be monitored for cross-reactions and may require medication adjustment. A thorough history of medication use and close monitoring of potential medication combinations are warranted.
Methadone possesses certain unique properties. Compared with most prescribed opioids (ie, hydrocodone, morphine, oxycodone), which have a half-life averaging less than three hours, the half-life for methadone exceeds 24 hours.9 This, coupled with a relatively slow onset of action, allows for once-daily dosing, making methadone a particularly effective tool in opioid addiction treatment. When taken properly, methadone is safe for the body and does not impede normal functioning. Additionally, methadone is cross-tolerant with other opioid medications,4 decreasing the likelihood of drug-seeking behavior.
Dosing is deemed adequate when the patient experiences relief from withdrawal and cravings without feeling "high" or oversedated. Because methadone is a full agonist, however, excessive dosing may produce euphoric effects.9
While methadone can in itself be physically addicting, research clearly shows that this agent helps normalize the function of body systems (particularly the immune, endocrine, and neurologic systems) that were previously impaired by opioid abuse.1
The most commonly reported adverse effects of methadone use are similar to those associated with the opioids: constipation, decreased libido, alterations in sexual functioning, amenorrhea, weight gain, and sweating. Methadone is not contraindicated for patients undergoing medical or dental procedures, but any dosing reduction should be coordinated through the MMT team. Abrupt reduction or cessation of methadone dosing may lead to drug craving and a reappearance of withdrawal symptoms.
Considerations Before MMT
For the patient who acknowledges an opioid addiction problem, the primary care provider's first step should be to attempt to wean the patient off the prescribed medication. Abruptly cutting the patient off will only generate panic and increase the likelihood of illegal behavior. If the patient is unable or unwilling to tolerate a weaning process, referral to a MMT center is indicated. To allow a reasonable time frame for the patient to enroll, providers are advised to prescribe a limited supply of the medication in question.
Methadone Maintenance Treatment
Upon admission to MMT, the patient undergoes an induction phase in which the proper methadone maintenance dose is determined by increasing the dosing every two to four days, with careful monitoring. Since methadone is taken by mouth as a pill, diskette, or liquid, the risk of exposure to needle-borne diseases is eliminated. Use of diskette or liquid formulations helps prevent diversion of medication, as does supervised consumption.11
Thus, patients are required to take the entire dose in the presence of a clinic employee (usually a dosing nurse). Starting doses are generally 30 to 40 mg per day.12 Maintenance doses are highly individualized, but most patients will require 60 to 120 mg per day to suppress opioid withdrawal symptoms and cravings.8 Some clinics arrange split dosing for patients who need to take lower doses more frequently.
Careful monitoring is an important feature of MMT. Because of methadone's long half-life, a too-rapid dosing increase or a drug reaction can trigger rapid elevation of methadone serum levels.9 Methadone-related mortality is most significant during the first few days and weeks of treatment, in part because of the use of opioids or other substances to cope with withdrawal symptoms that will subside once dosing is stabilized.
Most states require MMT clinics to register all patients in a statewide central database to prevent dosing at multiple sites.
Challenges for the Managing Clinician
One of the primary care provider's greatest concerns for patients who are receiving MMT is the treatment of pain. While NSAIDs or other nonnarcotic pain relievers may be appropriate for mild to moderate pain, severe pain will often require narcotic medication, possibly including opioids.13 Providers' general concern about oversedation in prescribing pain medication seems more significant when the patient has a known history of addiction. Many hesitate to prescribe adequate levels of medication for acute pain for fear of reinforcing a patient's addiction or supporting drug diversion.
Complicating this concern is a common mis-conception that methadone will provide adequate analgesia during an acute process.4,7 To the contrary, patients on MMT who experience acute pain still require effective pain management. Although methadone suppresses opioid withdrawal for longer than 24 hours, any analgesic relief generally lasts only four to six hours. Use of opioid-classified medications will "block" the euphoric effects of the drug, but with sufficient dosing, patients should begin to experience pain relief.13
For severe pain, more aggressive dosing or more frequent dosing with shorter-acting agents is indicated.4 Methadone alone will not provide adequate postsurgical pain relief. Certain contraindicated analgesics (mixed agonist/antagonist or partial agonists) will initiate immediate and extreme withdrawal symptoms (see table).
Another common misconception is that the use of opioid pain medication may lead to addiction relapse or drug-seeking behavior. There is no evidence to support either notion.13 It is generally thought that the stress associated with unrelieved pain may pose a greater threat for relapse.5
Drug-Seeking Behavior
Primary care practitioners may also be concerned about being manipulated by patients with a history of addiction and wary of drug-seeking behavior. A careful, objective, and subjective clinical assessment of pain will reduce the clinician's chance of being manipulated. In turn, providing reassurance and discussing pain management with the patient in a nonjudgmental manner will relieve any distrust of the primary care provider or fear of stigma the patient may harbor.13,14
Providers should coordinate care with the patient's MMT clinic and verify methadone dosing. For optimal pain management, ordering short-acting analgesics on continuous scheduled dosing is preferred over as-needed dosing.13
Careful documentation is essential for primary care practitioners who care for patients in MMT. Patients will need discharge information that clearly indicates dosing schedules of methadone, opioids, and other adjunct medications (eg, benzodiazepines) that may appear on urine drug screening panels. All new prescriptions should be listed on discharge forms. MMT program staff members generally work with patients before scheduled procedures or hospitalizations to avoid any disruption of treatment.
MMT During Pregnancy
Practitioners also need to be informed about methadone use during pregnancy—an area that has been studied fairly extensively. Compared with pregnant women who continue to use opioids or who attempt detoxification, those receiving MMT have a reduced risk of pregnancy-related complications (ie, miscarriage, fetal dis-tress, premature labor).1,5 When properly prescribed and monitored, MMT provides a favorable environment for the developing fetus. Dosing levels and frequency are adjusted as the pregnancy progresses.
Infants born to women who are methadone-dependent can be safely, successfully weaned with no adverse effects soon after delivery.13 Methadone levels are very low in breast milk; thus, breastfeeding is not necessarily contraindicated in women undergoing MMT.
Another Option
While this article focuses primarily on MMT, it is important to note that some patients will opt for treatment with buprenorphine. Approved in 2002 for patients with opioid addiction, this agent has a reduced potential for euphoria and abuse because of its partial agonist properties.12,15 Another advantage is that primary care providers certified in treatment can administer buprenorphine directly.
The downside of use of this agent is increased cost and lack of access to the counseling services that are such an important component of traditional MMT programs. Because research is limited in the treatment of acute pain for patients who take buprenorphine, more structure and greater caution are called for at times when opioid analgesics must be prescribed.12,13
Conclusion
Patients living with opioid addiction experience fear of discrimination, mistrust of health care providers, and often embarrassment and despair over their predicament. Practitioners who suspect opioid addiction should approach these patients with respect but be direct about their concerns and reassuring about the possibilities offered by treatment. An improved understanding of opioid addiction and the benefits of treatment with methadone should enhance management of addicted patients in the primary care setting.
1. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Results from the 2006 National Survey on Drug Use and Health: National Findings, 2007. www.oas.samhsa.gov/nsduh/2k6nsduh/2k6Results.htm. Accessed May 22, 2008.
2. O'Brien CP. Drug addiction and drug abuse. In: Hardman JG, Gilman AG, Limbird LE, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001:621-642.
3. Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303-1310.
4. Krambeer LL, von McKnelly W Jr, Gabrielli WF Jr, Penick EC. Methadone therapy for opioid dependence. Am Fam Physician. 2001;63(12):2404-2410.
5. CRC Health Corporation. A brief history of methadone. 2005. www.opiatesrx.com/methadone.php. Accessed May 22, 2008.
6. Wang JS, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab Dispos. 2003;31(6):742-747.
7. Leavitt SB. Methadone-drug interactions. 3rd ed. Addiction Treatment Forum. November 2005 revision/update. www.atforum.com/SiteRoot/pages/addiction_resources/Drug_Interactions.pdf. Accessed May 22, 2008.
8. Centers for Disease Control and Prevention. Methadone maintenance treatment. IDU HIV Prevention. February 2002. www.cdc.gov/IDU/facts/MethadoneFin.pdf. Accessed May 22, 2008.
9. Leavitt SB. Methadone dosing and safety in the treatment of opioid addiction. Addiction Treatment Forum. Special Report. 2003. http://atforum.com/SiteRoot/pages/addiction_resources/DosingandSafetyWP.pdf. Accessed May 22, 2008.
10. Flockhart DA. Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine (2007). http://medicine.iupui.edu/flockhart/table.htm. Accessed May 22, 2008.
11. Weinrich M, Stuart M. Provision of methadone treatment in primary care medical practices: review of the Scottish experience and implications for US policy. JAMA. 2000;283(10):1343-1348.
12. Curie CG, Clark HW; Substance Abuse and Mental Health Services Administration. Methadone-Associated Mortality: Report of a National Assessment. May 8-9, 2003. Rockville, MD: CSAT Publication No. 28-03. www.dpt.samhsa.gov/medications/methadonemortality2003/methadone_mortality.aspx. Accessed May 22, 2008.
13. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006; 144(2):127-134.
14. McMurphy S, Shea J, Switzer J, Turner BJ. Clinic-based treatment for opioid dependence: a qualitative inquiry. Am J Health Behav. 2006;30(5):544-554.
15. Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315(3):1320-1330.
In addition to working in private practice, Julia Lowe Behr is a faculty member at the Medical College of Georgia School of Nursing, Athens Campus.
1. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Results from the 2006 National Survey on Drug Use and Health: National Findings, 2007. www.oas.samhsa.gov/nsduh/2k6nsduh/2k6Results.htm. Accessed May 22, 2008.
2. O'Brien CP. Drug addiction and drug abuse. In: Hardman JG, Gilman AG, Limbird LE, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001:621-642.
3. Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303-1310.
4. Krambeer LL, von McKnelly W Jr, Gabrielli WF Jr, Penick EC. Methadone therapy for opioid dependence. Am Fam Physician. 2001;63(12):2404-2410.
5. CRC Health Corporation. A brief history of methadone. 2005. www.opiatesrx.com/methadone.php. Accessed May 22, 2008.
6. Wang JS, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab Dispos. 2003;31(6):742-747.
7. Leavitt SB. Methadone-drug interactions. 3rd ed. Addiction Treatment Forum. November 2005 revision/update. www.atforum.com/SiteRoot/pages/addiction_resources/Drug_Interactions.pdf. Accessed May 22, 2008.
8. Centers for Disease Control and Prevention. Methadone maintenance treatment. IDU HIV Prevention. February 2002. www.cdc.gov/IDU/facts/MethadoneFin.pdf. Accessed May 22, 2008.
9. Leavitt SB. Methadone dosing and safety in the treatment of opioid addiction. Addiction Treatment Forum. Special Report. 2003. http://atforum.com/SiteRoot/pages/addiction_resources/DosingandSafetyWP.pdf. Accessed May 22, 2008.
10. Flockhart DA. Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine (2007). http://medicine.iupui.edu/flockhart/table.htm. Accessed May 22, 2008.
11. Weinrich M, Stuart M. Provision of methadone treatment in primary care medical practices: review of the Scottish experience and implications for US policy. JAMA. 2000;283(10):1343-1348.
12. Curie CG, Clark HW; Substance Abuse and Mental Health Services Administration. Methadone-Associated Mortality: Report of a National Assessment. May 8-9, 2003. Rockville, MD: CSAT Publication No. 28-03. www.dpt.samhsa.gov/medications/methadonemortality2003/methadone_mortality.aspx. Accessed May 22, 2008.
13. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006; 144(2):127-134.
14. McMurphy S, Shea J, Switzer J, Turner BJ. Clinic-based treatment for opioid dependence: a qualitative inquiry. Am J Health Behav. 2006;30(5):544-554.
15. Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315(3):1320-1330.
In addition to working in private practice, Julia Lowe Behr is a faculty member at the Medical College of Georgia School of Nursing, Athens Campus.
Is hormonal contraception right for your perimenopausal patient?
CASE Perimenopausal complaints, and a request for contraception
At her annual visit, M.B., a healthy 48-year-old divorced woman, reports that her periods are increasingly erratic and that she has begun experiencing occasional hot flushes. Although her previous husband had a vasectomy, she has started to date and is concerned about contraception. A close friend became pregnant at the age of 46 and chose to have an abortion. M.B. hopes to avoid the same fate and asks specifically about birth control pills. Is this an appropriate option for her? What do you tell her?
Although only 11% of women 40 to 44 years old reported using oral contraceptives (OCs) in 2002 in the United States, that figure represents a 5% increase over 1995,1,2 and all indications are that the percentage is still rising.
In lean, nonsmoking, healthy perimenopausal women, OCs offer users not only effective contraception, but also benefits that include a reduction in heavy menstrual bleeding; regularization of the menstrual cycle; protection against ovarian, endometrial, and colorectal cancer; prevention of bone loss (with possible prevention of postmenopausal osteoporotic fractures); and some degree of relief from vasomotor symptoms. Although an increased risk of venous thromboembolism (VTE) is well documented in OC users, concerns also exist that use of the pill might increase the risk of myocardial infarction (MI), stroke, and breast cancer in older reproductive-age women.
To explore the range of hormonal contraceptive options and their risks and benefits in perimenopausal women in more depth, OBG Management recently caught up with Andrew M. Kaunitz, MD, an expert in both contraception and menopause and a member of the OBG Management Board of Editors. He describes and interprets the robust data in this field to answer our many questions—although he points out that perimenopausal women have been underrepresented in studies of OC use in particular and hormonal contraception in general.
Why hormonal contraception?
OBG Management: Why is effective contraception important in this age group? Aren’t perimenopausal women less fertile than younger women?
Kaunitz: Older women are less fecund, but irregular menstrual cycles make it difficult to predict when ovulation is occurring, making unplanned pregnancy a real possibility in sexually active women.
Pregnancy itself is fraught with risks in this age group. Pregnancy-related mortality among women 40 years or older in the United States is five times higher than among 25- to 29-year-olds. Older women are also more likely to have comorbidities such as hypertension and diabetes, further increasing the risks of pregnancy.3,4 In addition, perimenopausal women are more likely than any reproductive age group except adolescents to opt for induced abortion when they do become pregnant, with 304 abortions for every 1,000 live births in women 40 years or older in the United States.5
OBG Management: Why should a perimenopausal woman consider hormonal contraception?
Kaunitz: It is highly effective and offers a range of noncontraceptive benefits, and older women are more likely to use it properly, making contraceptive failure less likely than in younger patients.
Nor are combination OCs the only option for this age group. Progestin-only OCs, the levonorgestrel-releasing intrauterine system, the etonogestrel implant, and injectable depot medroxyprogesterone acetate (DMPA) are alternatives. Although the vaginal patch and ring have not been studied extensively, they may be appropriate in some instances. Until further data specific to these combination estrogen–progestin methods are available, let’s assume for our discussion that they carry the same risk–benefit profile as combination OCs.
Thromboembolism is the greatest risk
OBG Management: What is the greatest risk of OC use in perimenopausal women?
Kaunitz: That would be VTE. The risk rises sharply after 39 years of age among users of combination OCs, with approximately 100 cases for every 100,000 person-years, compared with 25 cases for every 100,000 person-years among adolescents.6 This already elevated risk almost doubles among obese women older than 39 years.7 In these women, progestin-only or intrauterine contraceptives are better options than combination OCs.8
Also, avoid prescribing combination OCs for women with a known thrombophilic defect. However, because screening for thrombophilia is not cost-effective, routinely evaluating candidates for combination contraception with testing for familial thrombophilic disorders is not recommended.
OBG Management: Does the dosage of estrogen determine the risk of VTE?
Kaunitz: That is the general assumption—that higher dosages of estrogen pose a greater risk—but we lack definitive evidence that OCs formulated with 20 μg of estrogen are any safer in this regard than those that contain 30 to 35 μg.7,9
There is some evidence that the progestin plays a role. OCs that contain desogestrel appear to carry almost twice the risk of VTE as those formulated with levonorgestrel or norgestimate.10
TABLE
How selected health conditions affect choice of contraceptive in women ≥35 years
| Condition | Recommendation* |
|---|---|
| Obesity | Avoid combination contraceptives (OCs, patch, and ring) |
| Smoking | |
| Diabetes | |
| Migraine | |
| Hypertension | |
| * Based on guidelines from the American College of Obstetricians and Gynecologists8 | |
| † Includes progestin-only OCs, progestin implants, depot medroxyprogesterone acetate, and copper and progestin-releasing intrauterine devices | |
Risk of MI, stroke may rise in some older women
OBG Management: Do perimenopausal women who take combination OCs face a heightened risk of MI or stroke?
Kaunitz: Yes, if they smoke or have hypertension. The reason: In women who use combination OCs, smoking and hypertension are synergistic risk factors for MI and stroke. That means perimenopausal women who smoke or have high blood pressure should avoid combination contraceptives.
Although it is limited, available evidence supports the safety of OCs in older women who do not smoke or have hypertension. One large case-control study from the United States found no increased risk of MI or stroke among this population when they used OCs containing less than 50 μg of ethinyl estradiol.11,12 However, this study included few women older than 35 years who used OCs and smoked or had hypertension.
A large, prospective study from Sweden that included 1,761 current OC users between 40 and 49 years of age found no increased risk of MI among former or current OC users.13 It also found that the initiation of OC use in women 30 years of age or older carried no higher risk of MI than did initiation at age 29 or younger.
Avoid OCs in older women who have diabetes
OBG Management: What about women 35 years of age or older who have diabetes? Is hormonal contraception appropriate for them?
Kaunitz: Both premenopausal and postmenopausal women who have diabetes have a higher risk of cardiovascular disease, so combination contraceptives are a bad idea when the woman has diabetes and is 35 years of age or older. OCs also should be avoided in women younger than 35 years who have diabetes, unless they are normotensive and free of nephropathy and other vascular disease. Intrauterine contraception and progestin-only formulations tend to be better options for diabetic women.
Avoid combination OCs in perimenopausal migraineurs
OBG Management: Isn’t there evidence that women who have migraine headaches have an elevated stroke risk? How does this affect their choice of contraceptive?
Kaunitz: One case-control study from a large US health maintenance organization found twice the risk of stroke among OC users who had migraines as among those who did not.12 However, this study did not distinguish between women who had migraines with aura and those who had migraines without aura.
Another study found an increased risk of stroke among OC users who had migraines with aura, but not among those who had migraines without aura.14
Accordingly, both the American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization recommend that older women who experience migraines use progestin-only or intrauterine contraception.8,15
Does estrogen use increase the risk of breast cancer?
OBG Management: It’s a common assumption that hormonal contraceptives that contain estrogen increase the risk of breast cancer. Is that assumption backed by data?
Kaunitz: Long-term use of combination estrogen–progestin menopausal hormone therapy modestly increases the risk of breast cancer. Accordingly, many clinicians and women assume that use of hormonal contraception must likewise increase risk. In fact, the evidence does not indicate that combination OCs or progestin-only contraceptives increase the risk of breast cancer. However, studies to date have involved a relatively small number of women older than 45 years.
For example, a large cohort study from the United Kingdom that involved more than 1 million person-years of follow-up found no association between use of OCs and breast cancer, even among long-term users.16 Most cases of OC use in this study involved OCs formulated with 50 μg or more of ethinyl estradiol. However, this study did not indicate the age at which women used OCs.
In the Women’s Contraceptive and Reproductive Experiences (CARE) study, current or previous users of OCs had no increased risk of invasive or in situ breast cancer, compared with never-users.17,18 This study did include a subgroup of women who had started using OCs after age 40. Nor did the CARE study find an association between progestin-only injectable DMPA or implantable contraceptives and breast cancer.19
Last, a population-based case-control study in the United States found no increased risk of death from breast cancer among previous users of OCs, compared with women who had never used them.20 This study included an analysis limited to women who had begun using OCs at 30 years of age or older.
OBG Management: What about women who have a family history of breast cancer? Do OCs and other hormonal contraceptives elevate their risk further?
Kaunitz: Women who have a family history of breast cancer are often cautioned that it would be unsafe for them to use hormonal contraception. However, use of hormonal contraception does not appear to impact the risk of breast cancer in women who have a family history of the disease.
A large prospective study from Canada involving women who had a family history of breast cancer and a mean age of 49 found no increased risk of breast cancer among former or current OC users.21 This study did not assess risk by BRCA mutation status.
A separate study found that the risk of breast cancer increased slightly among women who had a BRCA1 mutation, with an odds ratio of 1.20 (95% confidence interval, 1.02–1.40), but not among women who had a BRCA2 mutation.22 Another study found no significant increase in the risk of breast cancer among women who had either a BRCA1 or BRCA2 mutation.23
Benefits include improved bleeding patterns
OBG Management: Many perimenopausal women who have fibroids or adenomyosis experience menorrhagia or dysfunctional uterine bleeding (DUB) and opt for surgery such as endometrial ablation or hysterectomy. Can OCs or other hormonal contraceptives alleviate these patterns without the need for surgery?
Kaunitz: Yes. OCs can restore physiologic bleeding in older women who have DUB. One study involving women 15 to 50 years of age who had DUB found improved bleeding patterns in more than 80% of women randomized to OCs, compared with less than 50% of women randomized to placebo.24 In addition, women who have menorrhagia have reported a significant reduction of blood loss after using OCs.25
Another effective option for women who have menorrhagia is the levonorgestrel-releasing intrauterine system (LNG-IUS), even in women who have menorrhagia associated with fibroids and adenomyosis.26-28
Because long-term use of injectable forms of contraception tends to lead to amenorrhea, some physicians recommend DMPA as a treatment for menorrhagia. Data supporting this strategy are scant, however.29
OCs reduce the risk of three cancers
OBG Management: Oral contraceptives are known to reduce the risk of ovarian, endometrial, and colorectal cancer to varying degrees. Does this benefit hold up for older women, too?
Kaunitz: Yes. And because the incidence of ovarian cancer, in particular, increases with age, the protection afforded by combination OCs may be especially beneficial for women of older reproductive age.
OBG Management: Just how much protection against ovarian cancer does OC use afford?
Kaunitz: Among users of low-dose combination OCs, the risk of epithelial ovarian cancer declines by at least 50%, compared with women who have never used the pill—and, the longer the use, the greater the protection.16,30,31 Once OCs are discontinued, the protection diminishes over time, but some degree of reduced risk persists for three decades or longer.31
OBG Management: What about endometrial cancer?
Kaunitz: Not just OCs, but also DMPA, are associated with a significant reduction in the risk of endometrial cancer: 50% with use of OCs formulated with 30 μg or more of estrogen, and 80% with use of DMPA. In the case of OCs, the reduced risk is greater with longer use, and it persists after discontinuation for at least 20 years.25,32
OBG Management: Is the protection against colorectal cancer as great as the protection against these other cancers?
Kaunitz: No, it isn’t, but the protection is still significant. OC use reduces the risk of colorectal cancer by approximately 20%, but the protection against colorectal cancer does not appear to increase with duration of use.16,33 It also may be that more recent OC use (past 5 years) affords greater protection than use in the more distant past.16,33
OCs may reduce fracture risk postmenopausally
OBG Management: What effect do combination OCs and other forms of hormonal contraception have on the bone loss that accelerates around the time of menopause?
Kaunitz: One randomized trial found that OC use increases bone mineral density (BMD) in women of older reproductive age.34 And a population-based, case-control trial from Sweden found a 25% reduction in the risk of hip fracture among postmenopausal women who had a history of OC use. The reduction in risk was even greater when the women had used OCs in their 40s or for an extended duration.35
The Women’s Health Initiative found no reduction in the risk of fracture among previous users of OCs, but failed to stratify women by the age at which they used OCs.
OBG Management: Are any hormonal contraceptives associated with bone loss?
Kaunitz: Yes. Use of intramuscular DMPA (150 mg) or subcutaneous DMPA (104 mg) is linked to a loss of BMD. The good news is that BMD recovers after discontinuation of the drug, even in women who begin to use it after 40 years of age.29,36 However, we lack data on the risk of fracture among postmenopausal women with a history of DMPA use.
OCs may ease hot flushes and other menopausal symptoms
OBG Management: Is there any evidence that use of combination OCs by perimenopausal women relieves vasomotor symptoms?
Kaunitz: Yes, but the number of studies demonstrating this association so far has been limited. One small double-blind trial randomly assigned women to use of an OC containing 20 μg of estradiol or to placebo.37 Although the number and severity of symptoms diminished by about 50% in those taking the OC, the difference was not statistically significant.
A prospective observational study found that 90% of perimenopausal women experienced complete relief after taking an OC containing 30 μg of ethinyl estradiol, compared with only 40% of nonusers.38
OBG Management: What about other forms of hormonal contraception? Are any effective against vasomotor symptoms?
Kaunitz: One interesting option is to use menopausal doses of estrogen to treat vasomotor symptoms along with an LNG-IUS to prevent endometrial hyperplasia and provide contraception, if needed. This combination produced substantial improvement in a trial involving perimenopausal women who were experiencing vasomotor symptoms.39 Most of the women became amenorrheic, and there was no endometrial hyperplasia.
DMPA in contraceptive dosages also has relieved vasomotor symptoms in menopausal women, compared with placebo.40
OBG Management: What about women who experience vasomotor symptoms during the 7 placebo days of a 28-pill cycle? What options do they have?
Kaunitz: Some physicians either switch to a 24/4 OC formulation (Yaz or Lo-Estrin 24), an extended OC formulation with no placebo days (Seasonique), a continuous OC formulation (Lybrel), or simply prescribe pills from a traditional 21/7 pack in a continuous fashion so as to eliminate the hormone-free interval. However, this strategy has been studied to only a limited degree.
At what age should an OC be discontinued?
OBG Management: Perimenopausal women are, obviously, going to become menopausal at some point. How do you know when that transition occurs if they are taking OCs?
Kaunitz: It turns out that testing is not useful in this clinical setting. Some people have advocated measuring the follicle-stimulating hormone (FSH) level, but this strategy is unreliable. An elevated FSH level—thought to be indicative of menopause—has been found in older ovulatory women,41 and a depressed FSH level has been found in postmenopausal women for weeks after discontinuation of OCs.42
Rather than use this imperfect science to try and predict the point of menopause, I recommend discontinuing OCs once the woman has attained age 55, arbitrarily assuming that she is menopausal at this age. I use the same approach for women using other hormonal contraceptives.8,43
1. Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Advance data from vital and health statistics. No. 350. Hyattsville, MD: National Center for Health Statistics, December 10, 2004.
2. Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and health statistics. Series 23. No. 19. Hyattsville, MD: National Center for Health Statistics, May 1997:1–114. (DHHS publication no. (PHS) 97–1995.)
3. Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102:1015-1021.
4. Viegas OA, Leong WP, Ahmed S, Ratnam SS. Obstetrical outcome with increasing maternal age. J Biosoc Sci. 1994;26:261-267.
5. Strauss LT, Herndon J, Chang J, et al. Abortion surveillance—United States, 2001. MMWR Surveill Summ. 2004;53(SS–9):1-32.
6. Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Farmer RDT. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care. 2000;5:265-274.
7. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen–progestin oral contraceptives. Contraception. 2004;70:3-10.
8. ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 73: Use of hormonal contraceptive in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
9. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs. >20 microg estrogen oral contraceptives for contraception: systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
10. Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism with oral contraceptives containing levonorgestrel. Contraception. 2006;73:566-570.
11. Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98:1058-1063.
12. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
13. Margolis KL, Adami H-O, Luo J, Ye W, Weider-pass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertil Steril. 2007;88:310-316.
14. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2345.
15. Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: World Health Organization, 2004.
16. Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioners’ oral contraception study. BMJ. 2007;335:651.-
17. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
18. Gill JK, Press MF, Patel AV, Bernstein L. Oral contraceptive use and risk of breast carcinoma in situ (United States). Cancer Causes Control. 2006;17:1155-1162.
19. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69:353-360.
20. Wingo PA, Austin A, Marchbanks PA, et al. Oral contraceptives and the risk of death from breast cancer. Obstet Gynecol. 2007;110:793-800.
21. Silvera SA, Miller AB, Rohan TE. Oral contraceptive use and risk of breast cancer among women with a family history of breast cancer: a prospective cohort study. Cancer Causes Control. 2005;16:1059-1063.
22. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
23. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
24. Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynecol. 2000;96:913-920.
25. Kaunitz AM. Noncontraceptive health benefits of oral contraceptives. Rev Endocr Metab Disord. 2002;3:277-283.
26. Hurskainen R, Reperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
27. Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception. 2007;75 (6 Suppl):S130-S133.
28. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
29. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. In: Rose BD, ed. UpToDate. Wellesley, MA: UpToDate, 2008.
30. Petitti DB. Combination estrogen–progestin oral contraceptives. N Engl J Med. 2003;349:1443-1450.[Erratum, N Engl J Med. 2004;350:92.]
31. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303-314.
32. Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives: a practitioner’s guide to meta-analysis. Hum Reprod. 1997;12:1851-1863.
33. Fernandez E, LaVecchia C, Balducci A, Chatenoud L, Francheschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
34. Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54:176-180.
35. Michaëlsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: a case-control study. Lancet. 1999;353:1481-1484.
36. Rosenberg L, Zhang Y, Constant D, et al. Bone status after cessation of use of injectable progestin contraceptives. Contraception. 2007;76:425-431.
37. Casper RF, Dodin S, Reid RL. The effect of 20 μg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes, and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
38. Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: a three-year prospective study. Int J Fertil. 1985;30:15,18-28.
39. Hampton NRE, Rees MCP, Lowe DG, Rauramo I, Barlow D, Guillebaud J. Levonorgestrel intrauterine system (LNG-IUS) with conjugated equine estrogen: a successful regimen for HRT in perimenopausal women. Hum Reprod. 2005;20:2653-2669.
40. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
41. Gebbie AE, Glasier A, Sweeting V. Incidence of ovulation in perimenopausal women before and during hormone replacement therapy. Contraception. 1995;52:221-222.
42. Creinin MD. Laboratory criteria for menopause in women using oral contraceptives. Fertil Steril. 1996;66:101-104.
43. Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270.
CASE Perimenopausal complaints, and a request for contraception
At her annual visit, M.B., a healthy 48-year-old divorced woman, reports that her periods are increasingly erratic and that she has begun experiencing occasional hot flushes. Although her previous husband had a vasectomy, she has started to date and is concerned about contraception. A close friend became pregnant at the age of 46 and chose to have an abortion. M.B. hopes to avoid the same fate and asks specifically about birth control pills. Is this an appropriate option for her? What do you tell her?
Although only 11% of women 40 to 44 years old reported using oral contraceptives (OCs) in 2002 in the United States, that figure represents a 5% increase over 1995,1,2 and all indications are that the percentage is still rising.
In lean, nonsmoking, healthy perimenopausal women, OCs offer users not only effective contraception, but also benefits that include a reduction in heavy menstrual bleeding; regularization of the menstrual cycle; protection against ovarian, endometrial, and colorectal cancer; prevention of bone loss (with possible prevention of postmenopausal osteoporotic fractures); and some degree of relief from vasomotor symptoms. Although an increased risk of venous thromboembolism (VTE) is well documented in OC users, concerns also exist that use of the pill might increase the risk of myocardial infarction (MI), stroke, and breast cancer in older reproductive-age women.
To explore the range of hormonal contraceptive options and their risks and benefits in perimenopausal women in more depth, OBG Management recently caught up with Andrew M. Kaunitz, MD, an expert in both contraception and menopause and a member of the OBG Management Board of Editors. He describes and interprets the robust data in this field to answer our many questions—although he points out that perimenopausal women have been underrepresented in studies of OC use in particular and hormonal contraception in general.
Why hormonal contraception?
OBG Management: Why is effective contraception important in this age group? Aren’t perimenopausal women less fertile than younger women?
Kaunitz: Older women are less fecund, but irregular menstrual cycles make it difficult to predict when ovulation is occurring, making unplanned pregnancy a real possibility in sexually active women.
Pregnancy itself is fraught with risks in this age group. Pregnancy-related mortality among women 40 years or older in the United States is five times higher than among 25- to 29-year-olds. Older women are also more likely to have comorbidities such as hypertension and diabetes, further increasing the risks of pregnancy.3,4 In addition, perimenopausal women are more likely than any reproductive age group except adolescents to opt for induced abortion when they do become pregnant, with 304 abortions for every 1,000 live births in women 40 years or older in the United States.5
OBG Management: Why should a perimenopausal woman consider hormonal contraception?
Kaunitz: It is highly effective and offers a range of noncontraceptive benefits, and older women are more likely to use it properly, making contraceptive failure less likely than in younger patients.
Nor are combination OCs the only option for this age group. Progestin-only OCs, the levonorgestrel-releasing intrauterine system, the etonogestrel implant, and injectable depot medroxyprogesterone acetate (DMPA) are alternatives. Although the vaginal patch and ring have not been studied extensively, they may be appropriate in some instances. Until further data specific to these combination estrogen–progestin methods are available, let’s assume for our discussion that they carry the same risk–benefit profile as combination OCs.
Thromboembolism is the greatest risk
OBG Management: What is the greatest risk of OC use in perimenopausal women?
Kaunitz: That would be VTE. The risk rises sharply after 39 years of age among users of combination OCs, with approximately 100 cases for every 100,000 person-years, compared with 25 cases for every 100,000 person-years among adolescents.6 This already elevated risk almost doubles among obese women older than 39 years.7 In these women, progestin-only or intrauterine contraceptives are better options than combination OCs.8
Also, avoid prescribing combination OCs for women with a known thrombophilic defect. However, because screening for thrombophilia is not cost-effective, routinely evaluating candidates for combination contraception with testing for familial thrombophilic disorders is not recommended.
OBG Management: Does the dosage of estrogen determine the risk of VTE?
Kaunitz: That is the general assumption—that higher dosages of estrogen pose a greater risk—but we lack definitive evidence that OCs formulated with 20 μg of estrogen are any safer in this regard than those that contain 30 to 35 μg.7,9
There is some evidence that the progestin plays a role. OCs that contain desogestrel appear to carry almost twice the risk of VTE as those formulated with levonorgestrel or norgestimate.10
TABLE
How selected health conditions affect choice of contraceptive in women ≥35 years
| Condition | Recommendation* |
|---|---|
| Obesity | Avoid combination contraceptives (OCs, patch, and ring) |
| Smoking | |
| Diabetes | |
| Migraine | |
| Hypertension | |
| * Based on guidelines from the American College of Obstetricians and Gynecologists8 | |
| † Includes progestin-only OCs, progestin implants, depot medroxyprogesterone acetate, and copper and progestin-releasing intrauterine devices | |
Risk of MI, stroke may rise in some older women
OBG Management: Do perimenopausal women who take combination OCs face a heightened risk of MI or stroke?
Kaunitz: Yes, if they smoke or have hypertension. The reason: In women who use combination OCs, smoking and hypertension are synergistic risk factors for MI and stroke. That means perimenopausal women who smoke or have high blood pressure should avoid combination contraceptives.
Although it is limited, available evidence supports the safety of OCs in older women who do not smoke or have hypertension. One large case-control study from the United States found no increased risk of MI or stroke among this population when they used OCs containing less than 50 μg of ethinyl estradiol.11,12 However, this study included few women older than 35 years who used OCs and smoked or had hypertension.
A large, prospective study from Sweden that included 1,761 current OC users between 40 and 49 years of age found no increased risk of MI among former or current OC users.13 It also found that the initiation of OC use in women 30 years of age or older carried no higher risk of MI than did initiation at age 29 or younger.
Avoid OCs in older women who have diabetes
OBG Management: What about women 35 years of age or older who have diabetes? Is hormonal contraception appropriate for them?
Kaunitz: Both premenopausal and postmenopausal women who have diabetes have a higher risk of cardiovascular disease, so combination contraceptives are a bad idea when the woman has diabetes and is 35 years of age or older. OCs also should be avoided in women younger than 35 years who have diabetes, unless they are normotensive and free of nephropathy and other vascular disease. Intrauterine contraception and progestin-only formulations tend to be better options for diabetic women.
Avoid combination OCs in perimenopausal migraineurs
OBG Management: Isn’t there evidence that women who have migraine headaches have an elevated stroke risk? How does this affect their choice of contraceptive?
Kaunitz: One case-control study from a large US health maintenance organization found twice the risk of stroke among OC users who had migraines as among those who did not.12 However, this study did not distinguish between women who had migraines with aura and those who had migraines without aura.
Another study found an increased risk of stroke among OC users who had migraines with aura, but not among those who had migraines without aura.14
Accordingly, both the American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization recommend that older women who experience migraines use progestin-only or intrauterine contraception.8,15
Does estrogen use increase the risk of breast cancer?
OBG Management: It’s a common assumption that hormonal contraceptives that contain estrogen increase the risk of breast cancer. Is that assumption backed by data?
Kaunitz: Long-term use of combination estrogen–progestin menopausal hormone therapy modestly increases the risk of breast cancer. Accordingly, many clinicians and women assume that use of hormonal contraception must likewise increase risk. In fact, the evidence does not indicate that combination OCs or progestin-only contraceptives increase the risk of breast cancer. However, studies to date have involved a relatively small number of women older than 45 years.
For example, a large cohort study from the United Kingdom that involved more than 1 million person-years of follow-up found no association between use of OCs and breast cancer, even among long-term users.16 Most cases of OC use in this study involved OCs formulated with 50 μg or more of ethinyl estradiol. However, this study did not indicate the age at which women used OCs.
In the Women’s Contraceptive and Reproductive Experiences (CARE) study, current or previous users of OCs had no increased risk of invasive or in situ breast cancer, compared with never-users.17,18 This study did include a subgroup of women who had started using OCs after age 40. Nor did the CARE study find an association between progestin-only injectable DMPA or implantable contraceptives and breast cancer.19
Last, a population-based case-control study in the United States found no increased risk of death from breast cancer among previous users of OCs, compared with women who had never used them.20 This study included an analysis limited to women who had begun using OCs at 30 years of age or older.
OBG Management: What about women who have a family history of breast cancer? Do OCs and other hormonal contraceptives elevate their risk further?
Kaunitz: Women who have a family history of breast cancer are often cautioned that it would be unsafe for them to use hormonal contraception. However, use of hormonal contraception does not appear to impact the risk of breast cancer in women who have a family history of the disease.
A large prospective study from Canada involving women who had a family history of breast cancer and a mean age of 49 found no increased risk of breast cancer among former or current OC users.21 This study did not assess risk by BRCA mutation status.
A separate study found that the risk of breast cancer increased slightly among women who had a BRCA1 mutation, with an odds ratio of 1.20 (95% confidence interval, 1.02–1.40), but not among women who had a BRCA2 mutation.22 Another study found no significant increase in the risk of breast cancer among women who had either a BRCA1 or BRCA2 mutation.23
Benefits include improved bleeding patterns
OBG Management: Many perimenopausal women who have fibroids or adenomyosis experience menorrhagia or dysfunctional uterine bleeding (DUB) and opt for surgery such as endometrial ablation or hysterectomy. Can OCs or other hormonal contraceptives alleviate these patterns without the need for surgery?
Kaunitz: Yes. OCs can restore physiologic bleeding in older women who have DUB. One study involving women 15 to 50 years of age who had DUB found improved bleeding patterns in more than 80% of women randomized to OCs, compared with less than 50% of women randomized to placebo.24 In addition, women who have menorrhagia have reported a significant reduction of blood loss after using OCs.25
Another effective option for women who have menorrhagia is the levonorgestrel-releasing intrauterine system (LNG-IUS), even in women who have menorrhagia associated with fibroids and adenomyosis.26-28
Because long-term use of injectable forms of contraception tends to lead to amenorrhea, some physicians recommend DMPA as a treatment for menorrhagia. Data supporting this strategy are scant, however.29
OCs reduce the risk of three cancers
OBG Management: Oral contraceptives are known to reduce the risk of ovarian, endometrial, and colorectal cancer to varying degrees. Does this benefit hold up for older women, too?
Kaunitz: Yes. And because the incidence of ovarian cancer, in particular, increases with age, the protection afforded by combination OCs may be especially beneficial for women of older reproductive age.
OBG Management: Just how much protection against ovarian cancer does OC use afford?
Kaunitz: Among users of low-dose combination OCs, the risk of epithelial ovarian cancer declines by at least 50%, compared with women who have never used the pill—and, the longer the use, the greater the protection.16,30,31 Once OCs are discontinued, the protection diminishes over time, but some degree of reduced risk persists for three decades or longer.31
OBG Management: What about endometrial cancer?
Kaunitz: Not just OCs, but also DMPA, are associated with a significant reduction in the risk of endometrial cancer: 50% with use of OCs formulated with 30 μg or more of estrogen, and 80% with use of DMPA. In the case of OCs, the reduced risk is greater with longer use, and it persists after discontinuation for at least 20 years.25,32
OBG Management: Is the protection against colorectal cancer as great as the protection against these other cancers?
Kaunitz: No, it isn’t, but the protection is still significant. OC use reduces the risk of colorectal cancer by approximately 20%, but the protection against colorectal cancer does not appear to increase with duration of use.16,33 It also may be that more recent OC use (past 5 years) affords greater protection than use in the more distant past.16,33
OCs may reduce fracture risk postmenopausally
OBG Management: What effect do combination OCs and other forms of hormonal contraception have on the bone loss that accelerates around the time of menopause?
Kaunitz: One randomized trial found that OC use increases bone mineral density (BMD) in women of older reproductive age.34 And a population-based, case-control trial from Sweden found a 25% reduction in the risk of hip fracture among postmenopausal women who had a history of OC use. The reduction in risk was even greater when the women had used OCs in their 40s or for an extended duration.35
The Women’s Health Initiative found no reduction in the risk of fracture among previous users of OCs, but failed to stratify women by the age at which they used OCs.
OBG Management: Are any hormonal contraceptives associated with bone loss?
Kaunitz: Yes. Use of intramuscular DMPA (150 mg) or subcutaneous DMPA (104 mg) is linked to a loss of BMD. The good news is that BMD recovers after discontinuation of the drug, even in women who begin to use it after 40 years of age.29,36 However, we lack data on the risk of fracture among postmenopausal women with a history of DMPA use.
OCs may ease hot flushes and other menopausal symptoms
OBG Management: Is there any evidence that use of combination OCs by perimenopausal women relieves vasomotor symptoms?
Kaunitz: Yes, but the number of studies demonstrating this association so far has been limited. One small double-blind trial randomly assigned women to use of an OC containing 20 μg of estradiol or to placebo.37 Although the number and severity of symptoms diminished by about 50% in those taking the OC, the difference was not statistically significant.
A prospective observational study found that 90% of perimenopausal women experienced complete relief after taking an OC containing 30 μg of ethinyl estradiol, compared with only 40% of nonusers.38
OBG Management: What about other forms of hormonal contraception? Are any effective against vasomotor symptoms?
Kaunitz: One interesting option is to use menopausal doses of estrogen to treat vasomotor symptoms along with an LNG-IUS to prevent endometrial hyperplasia and provide contraception, if needed. This combination produced substantial improvement in a trial involving perimenopausal women who were experiencing vasomotor symptoms.39 Most of the women became amenorrheic, and there was no endometrial hyperplasia.
DMPA in contraceptive dosages also has relieved vasomotor symptoms in menopausal women, compared with placebo.40
OBG Management: What about women who experience vasomotor symptoms during the 7 placebo days of a 28-pill cycle? What options do they have?
Kaunitz: Some physicians either switch to a 24/4 OC formulation (Yaz or Lo-Estrin 24), an extended OC formulation with no placebo days (Seasonique), a continuous OC formulation (Lybrel), or simply prescribe pills from a traditional 21/7 pack in a continuous fashion so as to eliminate the hormone-free interval. However, this strategy has been studied to only a limited degree.
At what age should an OC be discontinued?
OBG Management: Perimenopausal women are, obviously, going to become menopausal at some point. How do you know when that transition occurs if they are taking OCs?
Kaunitz: It turns out that testing is not useful in this clinical setting. Some people have advocated measuring the follicle-stimulating hormone (FSH) level, but this strategy is unreliable. An elevated FSH level—thought to be indicative of menopause—has been found in older ovulatory women,41 and a depressed FSH level has been found in postmenopausal women for weeks after discontinuation of OCs.42
Rather than use this imperfect science to try and predict the point of menopause, I recommend discontinuing OCs once the woman has attained age 55, arbitrarily assuming that she is menopausal at this age. I use the same approach for women using other hormonal contraceptives.8,43
CASE Perimenopausal complaints, and a request for contraception
At her annual visit, M.B., a healthy 48-year-old divorced woman, reports that her periods are increasingly erratic and that she has begun experiencing occasional hot flushes. Although her previous husband had a vasectomy, she has started to date and is concerned about contraception. A close friend became pregnant at the age of 46 and chose to have an abortion. M.B. hopes to avoid the same fate and asks specifically about birth control pills. Is this an appropriate option for her? What do you tell her?
Although only 11% of women 40 to 44 years old reported using oral contraceptives (OCs) in 2002 in the United States, that figure represents a 5% increase over 1995,1,2 and all indications are that the percentage is still rising.
In lean, nonsmoking, healthy perimenopausal women, OCs offer users not only effective contraception, but also benefits that include a reduction in heavy menstrual bleeding; regularization of the menstrual cycle; protection against ovarian, endometrial, and colorectal cancer; prevention of bone loss (with possible prevention of postmenopausal osteoporotic fractures); and some degree of relief from vasomotor symptoms. Although an increased risk of venous thromboembolism (VTE) is well documented in OC users, concerns also exist that use of the pill might increase the risk of myocardial infarction (MI), stroke, and breast cancer in older reproductive-age women.
To explore the range of hormonal contraceptive options and their risks and benefits in perimenopausal women in more depth, OBG Management recently caught up with Andrew M. Kaunitz, MD, an expert in both contraception and menopause and a member of the OBG Management Board of Editors. He describes and interprets the robust data in this field to answer our many questions—although he points out that perimenopausal women have been underrepresented in studies of OC use in particular and hormonal contraception in general.
Why hormonal contraception?
OBG Management: Why is effective contraception important in this age group? Aren’t perimenopausal women less fertile than younger women?
Kaunitz: Older women are less fecund, but irregular menstrual cycles make it difficult to predict when ovulation is occurring, making unplanned pregnancy a real possibility in sexually active women.
Pregnancy itself is fraught with risks in this age group. Pregnancy-related mortality among women 40 years or older in the United States is five times higher than among 25- to 29-year-olds. Older women are also more likely to have comorbidities such as hypertension and diabetes, further increasing the risks of pregnancy.3,4 In addition, perimenopausal women are more likely than any reproductive age group except adolescents to opt for induced abortion when they do become pregnant, with 304 abortions for every 1,000 live births in women 40 years or older in the United States.5
OBG Management: Why should a perimenopausal woman consider hormonal contraception?
Kaunitz: It is highly effective and offers a range of noncontraceptive benefits, and older women are more likely to use it properly, making contraceptive failure less likely than in younger patients.
Nor are combination OCs the only option for this age group. Progestin-only OCs, the levonorgestrel-releasing intrauterine system, the etonogestrel implant, and injectable depot medroxyprogesterone acetate (DMPA) are alternatives. Although the vaginal patch and ring have not been studied extensively, they may be appropriate in some instances. Until further data specific to these combination estrogen–progestin methods are available, let’s assume for our discussion that they carry the same risk–benefit profile as combination OCs.
Thromboembolism is the greatest risk
OBG Management: What is the greatest risk of OC use in perimenopausal women?
Kaunitz: That would be VTE. The risk rises sharply after 39 years of age among users of combination OCs, with approximately 100 cases for every 100,000 person-years, compared with 25 cases for every 100,000 person-years among adolescents.6 This already elevated risk almost doubles among obese women older than 39 years.7 In these women, progestin-only or intrauterine contraceptives are better options than combination OCs.8
Also, avoid prescribing combination OCs for women with a known thrombophilic defect. However, because screening for thrombophilia is not cost-effective, routinely evaluating candidates for combination contraception with testing for familial thrombophilic disorders is not recommended.
OBG Management: Does the dosage of estrogen determine the risk of VTE?
Kaunitz: That is the general assumption—that higher dosages of estrogen pose a greater risk—but we lack definitive evidence that OCs formulated with 20 μg of estrogen are any safer in this regard than those that contain 30 to 35 μg.7,9
There is some evidence that the progestin plays a role. OCs that contain desogestrel appear to carry almost twice the risk of VTE as those formulated with levonorgestrel or norgestimate.10
TABLE
How selected health conditions affect choice of contraceptive in women ≥35 years
| Condition | Recommendation* |
|---|---|
| Obesity | Avoid combination contraceptives (OCs, patch, and ring) |
| Smoking | |
| Diabetes | |
| Migraine | |
| Hypertension | |
| * Based on guidelines from the American College of Obstetricians and Gynecologists8 | |
| † Includes progestin-only OCs, progestin implants, depot medroxyprogesterone acetate, and copper and progestin-releasing intrauterine devices | |
Risk of MI, stroke may rise in some older women
OBG Management: Do perimenopausal women who take combination OCs face a heightened risk of MI or stroke?
Kaunitz: Yes, if they smoke or have hypertension. The reason: In women who use combination OCs, smoking and hypertension are synergistic risk factors for MI and stroke. That means perimenopausal women who smoke or have high blood pressure should avoid combination contraceptives.
Although it is limited, available evidence supports the safety of OCs in older women who do not smoke or have hypertension. One large case-control study from the United States found no increased risk of MI or stroke among this population when they used OCs containing less than 50 μg of ethinyl estradiol.11,12 However, this study included few women older than 35 years who used OCs and smoked or had hypertension.
A large, prospective study from Sweden that included 1,761 current OC users between 40 and 49 years of age found no increased risk of MI among former or current OC users.13 It also found that the initiation of OC use in women 30 years of age or older carried no higher risk of MI than did initiation at age 29 or younger.
Avoid OCs in older women who have diabetes
OBG Management: What about women 35 years of age or older who have diabetes? Is hormonal contraception appropriate for them?
Kaunitz: Both premenopausal and postmenopausal women who have diabetes have a higher risk of cardiovascular disease, so combination contraceptives are a bad idea when the woman has diabetes and is 35 years of age or older. OCs also should be avoided in women younger than 35 years who have diabetes, unless they are normotensive and free of nephropathy and other vascular disease. Intrauterine contraception and progestin-only formulations tend to be better options for diabetic women.
Avoid combination OCs in perimenopausal migraineurs
OBG Management: Isn’t there evidence that women who have migraine headaches have an elevated stroke risk? How does this affect their choice of contraceptive?
Kaunitz: One case-control study from a large US health maintenance organization found twice the risk of stroke among OC users who had migraines as among those who did not.12 However, this study did not distinguish between women who had migraines with aura and those who had migraines without aura.
Another study found an increased risk of stroke among OC users who had migraines with aura, but not among those who had migraines without aura.14
Accordingly, both the American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization recommend that older women who experience migraines use progestin-only or intrauterine contraception.8,15
Does estrogen use increase the risk of breast cancer?
OBG Management: It’s a common assumption that hormonal contraceptives that contain estrogen increase the risk of breast cancer. Is that assumption backed by data?
Kaunitz: Long-term use of combination estrogen–progestin menopausal hormone therapy modestly increases the risk of breast cancer. Accordingly, many clinicians and women assume that use of hormonal contraception must likewise increase risk. In fact, the evidence does not indicate that combination OCs or progestin-only contraceptives increase the risk of breast cancer. However, studies to date have involved a relatively small number of women older than 45 years.
For example, a large cohort study from the United Kingdom that involved more than 1 million person-years of follow-up found no association between use of OCs and breast cancer, even among long-term users.16 Most cases of OC use in this study involved OCs formulated with 50 μg or more of ethinyl estradiol. However, this study did not indicate the age at which women used OCs.
In the Women’s Contraceptive and Reproductive Experiences (CARE) study, current or previous users of OCs had no increased risk of invasive or in situ breast cancer, compared with never-users.17,18 This study did include a subgroup of women who had started using OCs after age 40. Nor did the CARE study find an association between progestin-only injectable DMPA or implantable contraceptives and breast cancer.19
Last, a population-based case-control study in the United States found no increased risk of death from breast cancer among previous users of OCs, compared with women who had never used them.20 This study included an analysis limited to women who had begun using OCs at 30 years of age or older.
OBG Management: What about women who have a family history of breast cancer? Do OCs and other hormonal contraceptives elevate their risk further?
Kaunitz: Women who have a family history of breast cancer are often cautioned that it would be unsafe for them to use hormonal contraception. However, use of hormonal contraception does not appear to impact the risk of breast cancer in women who have a family history of the disease.
A large prospective study from Canada involving women who had a family history of breast cancer and a mean age of 49 found no increased risk of breast cancer among former or current OC users.21 This study did not assess risk by BRCA mutation status.
A separate study found that the risk of breast cancer increased slightly among women who had a BRCA1 mutation, with an odds ratio of 1.20 (95% confidence interval, 1.02–1.40), but not among women who had a BRCA2 mutation.22 Another study found no significant increase in the risk of breast cancer among women who had either a BRCA1 or BRCA2 mutation.23
Benefits include improved bleeding patterns
OBG Management: Many perimenopausal women who have fibroids or adenomyosis experience menorrhagia or dysfunctional uterine bleeding (DUB) and opt for surgery such as endometrial ablation or hysterectomy. Can OCs or other hormonal contraceptives alleviate these patterns without the need for surgery?
Kaunitz: Yes. OCs can restore physiologic bleeding in older women who have DUB. One study involving women 15 to 50 years of age who had DUB found improved bleeding patterns in more than 80% of women randomized to OCs, compared with less than 50% of women randomized to placebo.24 In addition, women who have menorrhagia have reported a significant reduction of blood loss after using OCs.25
Another effective option for women who have menorrhagia is the levonorgestrel-releasing intrauterine system (LNG-IUS), even in women who have menorrhagia associated with fibroids and adenomyosis.26-28
Because long-term use of injectable forms of contraception tends to lead to amenorrhea, some physicians recommend DMPA as a treatment for menorrhagia. Data supporting this strategy are scant, however.29
OCs reduce the risk of three cancers
OBG Management: Oral contraceptives are known to reduce the risk of ovarian, endometrial, and colorectal cancer to varying degrees. Does this benefit hold up for older women, too?
Kaunitz: Yes. And because the incidence of ovarian cancer, in particular, increases with age, the protection afforded by combination OCs may be especially beneficial for women of older reproductive age.
OBG Management: Just how much protection against ovarian cancer does OC use afford?
Kaunitz: Among users of low-dose combination OCs, the risk of epithelial ovarian cancer declines by at least 50%, compared with women who have never used the pill—and, the longer the use, the greater the protection.16,30,31 Once OCs are discontinued, the protection diminishes over time, but some degree of reduced risk persists for three decades or longer.31
OBG Management: What about endometrial cancer?
Kaunitz: Not just OCs, but also DMPA, are associated with a significant reduction in the risk of endometrial cancer: 50% with use of OCs formulated with 30 μg or more of estrogen, and 80% with use of DMPA. In the case of OCs, the reduced risk is greater with longer use, and it persists after discontinuation for at least 20 years.25,32
OBG Management: Is the protection against colorectal cancer as great as the protection against these other cancers?
Kaunitz: No, it isn’t, but the protection is still significant. OC use reduces the risk of colorectal cancer by approximately 20%, but the protection against colorectal cancer does not appear to increase with duration of use.16,33 It also may be that more recent OC use (past 5 years) affords greater protection than use in the more distant past.16,33
OCs may reduce fracture risk postmenopausally
OBG Management: What effect do combination OCs and other forms of hormonal contraception have on the bone loss that accelerates around the time of menopause?
Kaunitz: One randomized trial found that OC use increases bone mineral density (BMD) in women of older reproductive age.34 And a population-based, case-control trial from Sweden found a 25% reduction in the risk of hip fracture among postmenopausal women who had a history of OC use. The reduction in risk was even greater when the women had used OCs in their 40s or for an extended duration.35
The Women’s Health Initiative found no reduction in the risk of fracture among previous users of OCs, but failed to stratify women by the age at which they used OCs.
OBG Management: Are any hormonal contraceptives associated with bone loss?
Kaunitz: Yes. Use of intramuscular DMPA (150 mg) or subcutaneous DMPA (104 mg) is linked to a loss of BMD. The good news is that BMD recovers after discontinuation of the drug, even in women who begin to use it after 40 years of age.29,36 However, we lack data on the risk of fracture among postmenopausal women with a history of DMPA use.
OCs may ease hot flushes and other menopausal symptoms
OBG Management: Is there any evidence that use of combination OCs by perimenopausal women relieves vasomotor symptoms?
Kaunitz: Yes, but the number of studies demonstrating this association so far has been limited. One small double-blind trial randomly assigned women to use of an OC containing 20 μg of estradiol or to placebo.37 Although the number and severity of symptoms diminished by about 50% in those taking the OC, the difference was not statistically significant.
A prospective observational study found that 90% of perimenopausal women experienced complete relief after taking an OC containing 30 μg of ethinyl estradiol, compared with only 40% of nonusers.38
OBG Management: What about other forms of hormonal contraception? Are any effective against vasomotor symptoms?
Kaunitz: One interesting option is to use menopausal doses of estrogen to treat vasomotor symptoms along with an LNG-IUS to prevent endometrial hyperplasia and provide contraception, if needed. This combination produced substantial improvement in a trial involving perimenopausal women who were experiencing vasomotor symptoms.39 Most of the women became amenorrheic, and there was no endometrial hyperplasia.
DMPA in contraceptive dosages also has relieved vasomotor symptoms in menopausal women, compared with placebo.40
OBG Management: What about women who experience vasomotor symptoms during the 7 placebo days of a 28-pill cycle? What options do they have?
Kaunitz: Some physicians either switch to a 24/4 OC formulation (Yaz or Lo-Estrin 24), an extended OC formulation with no placebo days (Seasonique), a continuous OC formulation (Lybrel), or simply prescribe pills from a traditional 21/7 pack in a continuous fashion so as to eliminate the hormone-free interval. However, this strategy has been studied to only a limited degree.
At what age should an OC be discontinued?
OBG Management: Perimenopausal women are, obviously, going to become menopausal at some point. How do you know when that transition occurs if they are taking OCs?
Kaunitz: It turns out that testing is not useful in this clinical setting. Some people have advocated measuring the follicle-stimulating hormone (FSH) level, but this strategy is unreliable. An elevated FSH level—thought to be indicative of menopause—has been found in older ovulatory women,41 and a depressed FSH level has been found in postmenopausal women for weeks after discontinuation of OCs.42
Rather than use this imperfect science to try and predict the point of menopause, I recommend discontinuing OCs once the woman has attained age 55, arbitrarily assuming that she is menopausal at this age. I use the same approach for women using other hormonal contraceptives.8,43
1. Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Advance data from vital and health statistics. No. 350. Hyattsville, MD: National Center for Health Statistics, December 10, 2004.
2. Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and health statistics. Series 23. No. 19. Hyattsville, MD: National Center for Health Statistics, May 1997:1–114. (DHHS publication no. (PHS) 97–1995.)
3. Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102:1015-1021.
4. Viegas OA, Leong WP, Ahmed S, Ratnam SS. Obstetrical outcome with increasing maternal age. J Biosoc Sci. 1994;26:261-267.
5. Strauss LT, Herndon J, Chang J, et al. Abortion surveillance—United States, 2001. MMWR Surveill Summ. 2004;53(SS–9):1-32.
6. Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Farmer RDT. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care. 2000;5:265-274.
7. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen–progestin oral contraceptives. Contraception. 2004;70:3-10.
8. ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 73: Use of hormonal contraceptive in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
9. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs. >20 microg estrogen oral contraceptives for contraception: systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
10. Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism with oral contraceptives containing levonorgestrel. Contraception. 2006;73:566-570.
11. Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98:1058-1063.
12. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
13. Margolis KL, Adami H-O, Luo J, Ye W, Weider-pass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertil Steril. 2007;88:310-316.
14. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2345.
15. Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: World Health Organization, 2004.
16. Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioners’ oral contraception study. BMJ. 2007;335:651.-
17. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
18. Gill JK, Press MF, Patel AV, Bernstein L. Oral contraceptive use and risk of breast carcinoma in situ (United States). Cancer Causes Control. 2006;17:1155-1162.
19. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69:353-360.
20. Wingo PA, Austin A, Marchbanks PA, et al. Oral contraceptives and the risk of death from breast cancer. Obstet Gynecol. 2007;110:793-800.
21. Silvera SA, Miller AB, Rohan TE. Oral contraceptive use and risk of breast cancer among women with a family history of breast cancer: a prospective cohort study. Cancer Causes Control. 2005;16:1059-1063.
22. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
23. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
24. Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynecol. 2000;96:913-920.
25. Kaunitz AM. Noncontraceptive health benefits of oral contraceptives. Rev Endocr Metab Disord. 2002;3:277-283.
26. Hurskainen R, Reperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
27. Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception. 2007;75 (6 Suppl):S130-S133.
28. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
29. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. In: Rose BD, ed. UpToDate. Wellesley, MA: UpToDate, 2008.
30. Petitti DB. Combination estrogen–progestin oral contraceptives. N Engl J Med. 2003;349:1443-1450.[Erratum, N Engl J Med. 2004;350:92.]
31. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303-314.
32. Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives: a practitioner’s guide to meta-analysis. Hum Reprod. 1997;12:1851-1863.
33. Fernandez E, LaVecchia C, Balducci A, Chatenoud L, Francheschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
34. Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54:176-180.
35. Michaëlsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: a case-control study. Lancet. 1999;353:1481-1484.
36. Rosenberg L, Zhang Y, Constant D, et al. Bone status after cessation of use of injectable progestin contraceptives. Contraception. 2007;76:425-431.
37. Casper RF, Dodin S, Reid RL. The effect of 20 μg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes, and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
38. Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: a three-year prospective study. Int J Fertil. 1985;30:15,18-28.
39. Hampton NRE, Rees MCP, Lowe DG, Rauramo I, Barlow D, Guillebaud J. Levonorgestrel intrauterine system (LNG-IUS) with conjugated equine estrogen: a successful regimen for HRT in perimenopausal women. Hum Reprod. 2005;20:2653-2669.
40. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
41. Gebbie AE, Glasier A, Sweeting V. Incidence of ovulation in perimenopausal women before and during hormone replacement therapy. Contraception. 1995;52:221-222.
42. Creinin MD. Laboratory criteria for menopause in women using oral contraceptives. Fertil Steril. 1996;66:101-104.
43. Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270.
1. Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Advance data from vital and health statistics. No. 350. Hyattsville, MD: National Center for Health Statistics, December 10, 2004.
2. Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and health statistics. Series 23. No. 19. Hyattsville, MD: National Center for Health Statistics, May 1997:1–114. (DHHS publication no. (PHS) 97–1995.)
3. Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102:1015-1021.
4. Viegas OA, Leong WP, Ahmed S, Ratnam SS. Obstetrical outcome with increasing maternal age. J Biosoc Sci. 1994;26:261-267.
5. Strauss LT, Herndon J, Chang J, et al. Abortion surveillance—United States, 2001. MMWR Surveill Summ. 2004;53(SS–9):1-32.
6. Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Farmer RDT. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care. 2000;5:265-274.
7. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen–progestin oral contraceptives. Contraception. 2004;70:3-10.
8. ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 73: Use of hormonal contraceptive in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
9. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs. >20 microg estrogen oral contraceptives for contraception: systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
10. Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism with oral contraceptives containing levonorgestrel. Contraception. 2006;73:566-570.
11. Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98:1058-1063.
12. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
13. Margolis KL, Adami H-O, Luo J, Ye W, Weider-pass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertil Steril. 2007;88:310-316.
14. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2345.
15. Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: World Health Organization, 2004.
16. Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioners’ oral contraception study. BMJ. 2007;335:651.-
17. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
18. Gill JK, Press MF, Patel AV, Bernstein L. Oral contraceptive use and risk of breast carcinoma in situ (United States). Cancer Causes Control. 2006;17:1155-1162.
19. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69:353-360.
20. Wingo PA, Austin A, Marchbanks PA, et al. Oral contraceptives and the risk of death from breast cancer. Obstet Gynecol. 2007;110:793-800.
21. Silvera SA, Miller AB, Rohan TE. Oral contraceptive use and risk of breast cancer among women with a family history of breast cancer: a prospective cohort study. Cancer Causes Control. 2005;16:1059-1063.
22. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
23. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
24. Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynecol. 2000;96:913-920.
25. Kaunitz AM. Noncontraceptive health benefits of oral contraceptives. Rev Endocr Metab Disord. 2002;3:277-283.
26. Hurskainen R, Reperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
27. Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception. 2007;75 (6 Suppl):S130-S133.
28. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
29. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. In: Rose BD, ed. UpToDate. Wellesley, MA: UpToDate, 2008.
30. Petitti DB. Combination estrogen–progestin oral contraceptives. N Engl J Med. 2003;349:1443-1450.[Erratum, N Engl J Med. 2004;350:92.]
31. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303-314.
32. Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives: a practitioner’s guide to meta-analysis. Hum Reprod. 1997;12:1851-1863.
33. Fernandez E, LaVecchia C, Balducci A, Chatenoud L, Francheschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
34. Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54:176-180.
35. Michaëlsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: a case-control study. Lancet. 1999;353:1481-1484.
36. Rosenberg L, Zhang Y, Constant D, et al. Bone status after cessation of use of injectable progestin contraceptives. Contraception. 2007;76:425-431.
37. Casper RF, Dodin S, Reid RL. The effect of 20 μg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes, and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
38. Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: a three-year prospective study. Int J Fertil. 1985;30:15,18-28.
39. Hampton NRE, Rees MCP, Lowe DG, Rauramo I, Barlow D, Guillebaud J. Levonorgestrel intrauterine system (LNG-IUS) with conjugated equine estrogen: a successful regimen for HRT in perimenopausal women. Hum Reprod. 2005;20:2653-2669.
40. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
41. Gebbie AE, Glasier A, Sweeting V. Incidence of ovulation in perimenopausal women before and during hormone replacement therapy. Contraception. 1995;52:221-222.
42. Creinin MD. Laboratory criteria for menopause in women using oral contraceptives. Fertil Steril. 1996;66:101-104.
43. Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270.
UPDATE: INFECTIOUS DISEASE
- important new information regarding the effectiveness of hepatitis A vaccine for postexposure prophylaxis
- the need to confirm antimicrobial susceptibility of group B streptococcus (GBS) isolates in pregnant women who are allergic to penicillin
- a new guideline on administering antibiotic prophylaxis for cesarean delivery
- a valuable overview of diverticulitis, a disease that we will all see with increasing regularity as the US population ages
For most, the best hepatitis A postexposure prophylaxis is vaccination
Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685–1694.
The objective of this investigation, conducted in Almaty, Kazakhstan, was to compare the relative effectiveness of hepatitis A vaccine with that of immune globulin for prophylaxis after exposure to the hepatitis A virus. Participants were 2 to 40 years old and were household or day-care contacts of people who had hepatitis A. Five hundred sixty-eight susceptible patients received hepatitis A vaccine within 14 days of exposure; 522 susceptible patients received an age-appropriate dose of immune globulin. The primary endpoint was laboratoryconfirmed, symptomatic hepatitis A within 15 to 56 days of exposure.
FIGURE 1 Hepatitis A virus
Hepatitis A virus has single-stranded RNA, no envelope, and is approximately 27–30 nm in diameter.Symptomatic infection occurred in 25 of 568 (4.4%) vaccine recipients and 17 of 522 (3.3%) recipients of immune globulin. The relative risk of infection after the vaccine was 1.35 (95% confidence interval [CI], 0.70–2.67). No serious adverse effects occurred as the result of administration of either the vaccine or immune globulin. The authors concluded that both agents provide effective postexposure prophylaxis against hepatitis A infection.
Hepatitis A is caused by an RNA virus that is transmitted by fecal–oral contact. It is highly contagious and endemic in areas of the world where poverty, poor sanitation, and overcrowded living conditions prevail. Hepatitis A usually causes a symptomatic illness characterized by fever, malaise, anorexia, jaundice, acholic stools, darkened urine, and hepatic pain and tenderness. In poorly nourished or immunocompromised patients, severe morbidity and, rarely, mortality, may occur. Unlike other forms of hepatitis, hepatitis A does not cause a chronic carrier state, and perinatal transmission is extremely unlikely.
Best strategy? Prevention
At present, there is no specific antiviral therapy that is routinely used for treatment of hepatitis A. However, highly effective preventive measures are available. For preexposure prophylaxis, time permitting, the ideal agent is inactivated hepatitis A vaccine.1
The vaccine is usually given in two doses separated by 6 to 12 months and is highly immunogenic. In the United States, key candidates for vaccination are
- gay men
- residents and staff of chronic care facilities
- intravenous drug users
- individuals who live in areas where hepatitis A is endemic
- primate laboratory workers
- people 30 years of age and older who have chronic liver disease
- international travelers
- people who have received a liver transplant or are awaiting one.
The vaccine is safe for administration in pregnancy and is now recommended for children.
After exposure, vaccine trumps immune globulin in healthy patients
The standard agent for prophylaxis after exposure to the hepatitis A virus has been immune globulin 0.02 mL/kg, administered intramuscularly. Immune globulin is highly effective in this application and, in the present investigation, it was slightly more effective than the vaccine.
Despite the modest difference in effectiveness, however, hepatitis A vaccine offers several unique advantages for postexposure prophylaxis:
- It confers long-term immunity rather than just temporary protection.
- Because the volume of fluid injected is lower, the vaccine causes less pain upon administration.
- Immune globulin is now produced by a single manufacturer, and its supply has been limited. Its price also approaches that of the vaccine.
- Administration of immune globulin to a child may disrupt the normal childhood immunization schedule.
In older and immunocompromised patients, use immune globulin
For most patients, hepatitis A vaccine is the indicated method of postexposure immunoprophylaxis.
Because it is slightly more effective, however, immune globulin probably should remain the preferred agent for hepatitis A postexposure prophylaxis in older or immunocompromised patients who are more likely to develop severe illness if they be-come infected.
REFERENCES
We’re not following guidelines on GBS prophylaxis in penicillin allergy
Matheson KA, Lievense SP, Catanzaro B, Phipps MG. Intrapartum group B streptococci prophylaxis in patients reporting a penicillin allergy. Obstet Gynecol. 2008;111:356–364.
In this study, conducted at a single institution (Brown University), Matheson and colleagues sought to assess the adequacy of prophylaxis for GBS infection in women who had an allergy to penicillin. Specifically, the authors sought to determine how well practitioners at their institution adhered to the 2002 Centers for Disease Control and Prevention (CDC) guidelines, which specify that cefazolin should be used for prophylaxis in patients who are penicillin-allergic but not at high risk for anaphylaxis.1 For patients at high risk for anaphylaxis, clindamycin may be used for prophylaxis if the organism is known to be susceptible. If susceptibility has not been documented, vancomycin should be administered.1
Overall, 95% of GBS-positive, penicillin-allergic patients received prophylaxis (95% CI, 91–97). However, only 15% of these women received appropriate prophylaxis as defined by the CDC (95% CI, 11–12). Clindamycin was administered to 83% of patients, but susceptibility testing was performed in only 11%. At the time of this study, 26% of all GBS isolates at Brown were resistant to clindamycin; 37% were resistant to erythromycin.
The authors concluded that adherence to CDC guidelines was clearly less than optimal. Even at 1 year after adoption of the guidelines, only 20% of patients received appropriate prophylaxis.
Type of allergic reaction is key to selection of prophylactic agent
GBS is uniformly sensitive to penicillin and ampicillin. It also is 100% sensitive to cefazolin, the preferred drug for intrapartum prophylaxis in penicillin-allergic women who have a low risk of anaphylactic reaction to penicillin.
FIGURE 2 Group B streptococcus
A clear zone of hemolysis on blood agar is a key characteristic of group B streptococcus.However, it probably is better to avoid cephalosporins in patients who report a previous anaphylactic reaction to penicillin or ampicillin, even though the risk of cross-reactivity between penicillin and cephalosporin is low. In such patients, possible alternatives include erythromycin, clindamycin, and vancomycin.
Erythromycin is no longer recommended
At the University of Florida, we reported that 21% of GBS strains were resistant to erythromycin.2 At Brown University, Matheson and colleagues reported that 37% of GBS isolates were resistant to erythromycin. On the basis of similar reports, the CDC has concluded that erythromycin no longer should be used for GBS prophylaxis.
At our institution, we also have noted a disturbing trend of increased GBS resistance to clindamycin. In our recent report, 9% of GBS strains were resistant to this antibiotic. Similarly, Matheson and coworkers observed that 26% of GBS isolates in their center were resistant to clindamycin.
Neonatal GBS infection is now one of the leading causes for malpractice suits in obstetrics. Key issues presented in these suits include:
- failure to screen
- failure to use the correct culture medium for screening
- failure to obtain test results in a timely manner
- failure to use the correct drug for prophylaxis.
In GBS-positive patients, practitioners should inquire about penicillin allergy and document the exact type of reaction experienced by the patient, if it is accurately known. If the reported reaction to penicillin was not life-threatening, patients should receive cefazolin, 2 g IV initially, then 1 g every 8 hours until delivery. If the reaction to penicillin was immediate and life-threatening, the patient should receive clindamycin, 900 mg IV every 8 hours, if the organism is confirmed to be susceptible. Susceptibility testing should be documented in the medical record.
If such testing is unavailable, vancomycin is the drug of choice.
Susceptibility testing is vital in penicillin-allergic women
Because of observations such as these, the CDC now recommends that clindamycin be used for GBS prophylaxis only if antimicrobial susceptibility tests have confirmed that the organism is sensitive. If susceptibility testing cannot easily be performed, practitioners should use intravenous (IV) vancomycin, 1 g every 12 hours, for prophylaxis. Potential side effects of vancomycin include allergic reactions, gastrointestinal irritation, ototoxicity, and nephrotoxicity. The latter two effects are extremely unlikely in patients who receive only one or two IV doses of the drug.
20% to 30% of gravidas are colonized with GBS
GBS is one of the two major causes of pneumonia, meningitis, and sepsis in both pre-term and term newborns. Approximately 20% to 30% of women are colonized with the organism at some point during pregnancy. Universal screening for GBS at 35 to 37 weeks’ gestation, combined with intrapartum antibiotic prophylaxis, has been highly effective in reducing the frequency of invasive GBS infection.
REFERENCES
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1-24
2. Edwards RK, Clark P, Sistrom CL, Duff P. Intrapartum antibiotic prophylaxis 1: relative effects of recommended antibiotics on gram-negative pathogens. Obstet Gynecol. 2002;100:534-539
New data suggest that preincision prophylaxis is best for C-section
Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2007;196:455.e1–455.e5.
Is preoperative antibiotic prophylaxis superior to intraoperative prophylaxis in preventing postcesarean infection? Sullivan and colleagues set out to answer this question in a prospective, randomized, double-blinded, placebo-controlled study at the Medical University of South Carolina.
In the study group, 175 women undergoing cesarean delivery were randomized to receive 1 g of IV cefazolin 15 to 60 minutes before surgery, followed by a placebo infusion immediately after the umbilical cord was clamped. In the control group, 182 women received preoperative placebo, followed by 1 g of cefazolin immediately after cord clamping.
Two patients in the study group developed endomyometritis, compared with 10 in the control group (relative risk [RR], 0.2; 95% CI, 0.15–0.94). Five patients in the study group developed a wound infection, compared with 10 in the control group (RR, 0.52; 95% CI, 0.18–1.5, not significant). Overall, eight women in the study group and 21 women in the control group met the criteria for infectious morbidity (RR, 0.4; 95% CI, 0.18–0.87).
There were no differences between the groups in the frequency of neonatal sepsis, neonatal intensive care unit (NICU) admission, total length of hospital stay, metabolic acidosis, or sepsis evaluation. Infants in the study group had significantly fewer days in the NICU (P<.01).
How this study differs from earlier investigations
The classic studies of antibiotic prophylaxis were performed in an animal model by Burke.1 He demonstrated that prophylaxis had its greatest effect when the antibiotic was administered before the surgical incision. Essentially, no effect of prophylaxis was evident when antibiotic administration was delayed more than 4 hours beyond the start of surgery.
Early studies of antibiotic prophylaxis for cesarean delivery, conducted in the 1970s, administered antibiotics preoperatively and continued administration for several days after surgery. In 1979, Gordon and colleagues published an important investigation demonstrating that delay in administration of antibiotics until after the umbilical cord was clamped did not compromise the effectiveness of prophylaxis and significantly decreased the number of infants who required sepsis evaluations.2
This latter effect presumably occurred because infants were not exposed to antibiotics before delivery. Gordon’s investigation and subsequent reports also demonstrated that effective prophylaxis could be achieved with one to three doses of antibiotics.3
Why this new study may alter practice
Before Sullivan and colleagues published their findings, I believe that the best available evidence supported the use of a single dose of antibiotic, such as cefazolin, immediately after cord clamping. There are no convincing data that demonstrate an advantage for extended-spectrum agents (second- and third-generation cephalosporins, broad-spectrum penicillins, or carbapenems) over cefazolin.3
However, if the findings of Sullivan and coworkers are confirmed by other investigations in different patient populations, they definitely should lead to a change in the standard of care for prophylaxis. This investigation was exceptionally well designed and executed. The reduction in the frequency of endomyometritis and overall rate of infectious morbidity was impressive. This advantage was achieved without increasing the rate of neonatal sepsis evaluation.
REFERENCES
1. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168
2. Gordon HR, Phelps D. Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Surgery. 1979;53:151-156
3. Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost-effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157:794-798
Expect to see more women with diverticulitis as the population ages
Jacobs DO. Diverticulitis. N Engl J Med. 2007;357:2057–2066.
ObGyns continue to play a major role in providing primary care to women. With the steady aging of the American population, practitioners certainly can expect to care for more and more women who are 50 years of age or older, and diverticulitis is likely to turn up in an increasing number of these patients.
Diverticulitis is a relatively common condition in older patients and must consistently be considered in the differential diagnosis of women with acute abdominal pain—particularly left-sided pain.
It is present in approximately 10% of adults younger than 40 years of age and in 40 to 70% of people 80 years of age or older. It primarily affects the sigmoid and descending colon and is associated with diets that are low in fiber and high in refined carbohydrates.
The condition probably results from stasis or obstruction in the narrow neck of a diverticulum, which, in turn, leads to an overgrowth of bacteria. The principal micro-organisms isolated from affected patients are anaerobes, gram-negative aerobes, and some facultative gram-positive bacteria.
Range of severity can be wide
Presentation of diverticulitis may range in severity from mild to moderate lower abdominal pain associated with anorexia, nausea, and vomiting to abscess and fistula formation, colonic stricture, bowel obstruction, and peritonitis (“complicated diverticulitis”). Peritonitis may arise from rupture of a peridiverticular abscess or free rupture of an uninflamed diverticulum. Diverticulitis may be particularly severe in immunocompromised patients.
Classification of disease
The most accepted classification system for diverticulitis is the Hinchey system:
- in stage 1 disease, patients have small and confined pericolic or mesenteric abscesses
- in stage 2, the abscesses are larger but usually remain confined to the pelvis
- in stage 3, an abscess ruptures and causes purulent peritonitis
- stage 4 disease is distinguished by rupture of an uninflamed and unobstructed diverticulum (known as “free rupture”); this stage has the highest risk of adverse outcomes.
CT is best for diagnosis
The most useful diagnostic test for diverticulitis is a computed tomography (CT) scan. As a general rule, endoscopy should be avoided because of the risk of intestinal perforation.
Most patients can be treated as outpatients
Patients who have mild disease usually can be treated as outpatients. They should receive a 7- to 10-day course of oral antibiotics such as ciprofloxacin, 500 to 750 mg every 12 hours, plus metronidazole, 500 mg every 6 to 8 hours.
Alternate oral regimens include metronidazole, 500 mg every 6 to 8 hours, plus trimethoprim-sulfamethoxazole, double-strength, every 12 hours, or amoxicillin-clavulanate, 875 mg every 12 hours.
For seriously ill patients, hospitalization is warranted
Seriously ill patients—particularly those who are immunocompromised—should be hospitalized. If bowel obstruction is present, a nasogastric tube should be inserted. Appropriate IV antibiotic regimens for hospitalized patients include:
- metronidazole, 500 mg every 6 to 8 hours, plus ciprofloxacin, 400 mg every 12 hours
- metronidazole, 500 mg every 6 to 8 hours, plus ceftriaxone, 1 to 2 g every 24 hours
- ampicillin–sulbactam, 3 g every 6 hours
- piperacillin–tazobactam, 3.375 g every 6 hours
- ticarcillin–clavulanate, 3.1 g every 6 hours.
FIGURE 3 Consider diverticulitis in older women who complain of acute abdominal pain
Diverticulitis generally affects the sigmoid colon and descending colon and probably arises from stasis or obstruction in the narrow neck of a diverticulum, which leads to an overgrowth of bacteria and possible rupture.If an abscess is present and fails to respond promptly to medical therapy, drainage may be necessary. Some abscesses can be drained percutaneously under CT guidance. Large abscesses, in association with signs of generalized peritonitis, uncontrolled sepsis, or intestinal perforation, require surgical intervention, either via laparoscopy or open laparotomy. Preliminary data suggest that the laparoscopic approach may result in a shorter hospital stay, decreased postoperative pain, and an overall reduced rate of perioperative complication.
Diverticulitis may mimic appendicitis
The clinical presentation of diverticulitis is similar to that of appendicitis, except that the pain is usually on the left. Perforation, with resulting peritonitis, is an ever-present and potentially life-threatening complication. The dominant organisms are anaerobes and coliforms. The best diagnostic test is CT.
Patients in the early stages of disease can usually be treated as outpatients with antibiotics that are quite familiar to all ObGyns— i.e., metronidazole and a quinolone. More seriously ill patients should be hospitalized and treated with IV antibiotics and nasogastric suctioning.
Consultation with an interventional radiologist and general surgeon is recommended if operative intervention is necessary.
- important new information regarding the effectiveness of hepatitis A vaccine for postexposure prophylaxis
- the need to confirm antimicrobial susceptibility of group B streptococcus (GBS) isolates in pregnant women who are allergic to penicillin
- a new guideline on administering antibiotic prophylaxis for cesarean delivery
- a valuable overview of diverticulitis, a disease that we will all see with increasing regularity as the US population ages
For most, the best hepatitis A postexposure prophylaxis is vaccination
Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685–1694.
The objective of this investigation, conducted in Almaty, Kazakhstan, was to compare the relative effectiveness of hepatitis A vaccine with that of immune globulin for prophylaxis after exposure to the hepatitis A virus. Participants were 2 to 40 years old and were household or day-care contacts of people who had hepatitis A. Five hundred sixty-eight susceptible patients received hepatitis A vaccine within 14 days of exposure; 522 susceptible patients received an age-appropriate dose of immune globulin. The primary endpoint was laboratoryconfirmed, symptomatic hepatitis A within 15 to 56 days of exposure.
FIGURE 1 Hepatitis A virus
Hepatitis A virus has single-stranded RNA, no envelope, and is approximately 27–30 nm in diameter.Symptomatic infection occurred in 25 of 568 (4.4%) vaccine recipients and 17 of 522 (3.3%) recipients of immune globulin. The relative risk of infection after the vaccine was 1.35 (95% confidence interval [CI], 0.70–2.67). No serious adverse effects occurred as the result of administration of either the vaccine or immune globulin. The authors concluded that both agents provide effective postexposure prophylaxis against hepatitis A infection.
Hepatitis A is caused by an RNA virus that is transmitted by fecal–oral contact. It is highly contagious and endemic in areas of the world where poverty, poor sanitation, and overcrowded living conditions prevail. Hepatitis A usually causes a symptomatic illness characterized by fever, malaise, anorexia, jaundice, acholic stools, darkened urine, and hepatic pain and tenderness. In poorly nourished or immunocompromised patients, severe morbidity and, rarely, mortality, may occur. Unlike other forms of hepatitis, hepatitis A does not cause a chronic carrier state, and perinatal transmission is extremely unlikely.
Best strategy? Prevention
At present, there is no specific antiviral therapy that is routinely used for treatment of hepatitis A. However, highly effective preventive measures are available. For preexposure prophylaxis, time permitting, the ideal agent is inactivated hepatitis A vaccine.1
The vaccine is usually given in two doses separated by 6 to 12 months and is highly immunogenic. In the United States, key candidates for vaccination are
- gay men
- residents and staff of chronic care facilities
- intravenous drug users
- individuals who live in areas where hepatitis A is endemic
- primate laboratory workers
- people 30 years of age and older who have chronic liver disease
- international travelers
- people who have received a liver transplant or are awaiting one.
The vaccine is safe for administration in pregnancy and is now recommended for children.
After exposure, vaccine trumps immune globulin in healthy patients
The standard agent for prophylaxis after exposure to the hepatitis A virus has been immune globulin 0.02 mL/kg, administered intramuscularly. Immune globulin is highly effective in this application and, in the present investigation, it was slightly more effective than the vaccine.
Despite the modest difference in effectiveness, however, hepatitis A vaccine offers several unique advantages for postexposure prophylaxis:
- It confers long-term immunity rather than just temporary protection.
- Because the volume of fluid injected is lower, the vaccine causes less pain upon administration.
- Immune globulin is now produced by a single manufacturer, and its supply has been limited. Its price also approaches that of the vaccine.
- Administration of immune globulin to a child may disrupt the normal childhood immunization schedule.
In older and immunocompromised patients, use immune globulin
For most patients, hepatitis A vaccine is the indicated method of postexposure immunoprophylaxis.
Because it is slightly more effective, however, immune globulin probably should remain the preferred agent for hepatitis A postexposure prophylaxis in older or immunocompromised patients who are more likely to develop severe illness if they be-come infected.
REFERENCES
We’re not following guidelines on GBS prophylaxis in penicillin allergy
Matheson KA, Lievense SP, Catanzaro B, Phipps MG. Intrapartum group B streptococci prophylaxis in patients reporting a penicillin allergy. Obstet Gynecol. 2008;111:356–364.
In this study, conducted at a single institution (Brown University), Matheson and colleagues sought to assess the adequacy of prophylaxis for GBS infection in women who had an allergy to penicillin. Specifically, the authors sought to determine how well practitioners at their institution adhered to the 2002 Centers for Disease Control and Prevention (CDC) guidelines, which specify that cefazolin should be used for prophylaxis in patients who are penicillin-allergic but not at high risk for anaphylaxis.1 For patients at high risk for anaphylaxis, clindamycin may be used for prophylaxis if the organism is known to be susceptible. If susceptibility has not been documented, vancomycin should be administered.1
Overall, 95% of GBS-positive, penicillin-allergic patients received prophylaxis (95% CI, 91–97). However, only 15% of these women received appropriate prophylaxis as defined by the CDC (95% CI, 11–12). Clindamycin was administered to 83% of patients, but susceptibility testing was performed in only 11%. At the time of this study, 26% of all GBS isolates at Brown were resistant to clindamycin; 37% were resistant to erythromycin.
The authors concluded that adherence to CDC guidelines was clearly less than optimal. Even at 1 year after adoption of the guidelines, only 20% of patients received appropriate prophylaxis.
Type of allergic reaction is key to selection of prophylactic agent
GBS is uniformly sensitive to penicillin and ampicillin. It also is 100% sensitive to cefazolin, the preferred drug for intrapartum prophylaxis in penicillin-allergic women who have a low risk of anaphylactic reaction to penicillin.
FIGURE 2 Group B streptococcus
A clear zone of hemolysis on blood agar is a key characteristic of group B streptococcus.However, it probably is better to avoid cephalosporins in patients who report a previous anaphylactic reaction to penicillin or ampicillin, even though the risk of cross-reactivity between penicillin and cephalosporin is low. In such patients, possible alternatives include erythromycin, clindamycin, and vancomycin.
Erythromycin is no longer recommended
At the University of Florida, we reported that 21% of GBS strains were resistant to erythromycin.2 At Brown University, Matheson and colleagues reported that 37% of GBS isolates were resistant to erythromycin. On the basis of similar reports, the CDC has concluded that erythromycin no longer should be used for GBS prophylaxis.
At our institution, we also have noted a disturbing trend of increased GBS resistance to clindamycin. In our recent report, 9% of GBS strains were resistant to this antibiotic. Similarly, Matheson and coworkers observed that 26% of GBS isolates in their center were resistant to clindamycin.
Neonatal GBS infection is now one of the leading causes for malpractice suits in obstetrics. Key issues presented in these suits include:
- failure to screen
- failure to use the correct culture medium for screening
- failure to obtain test results in a timely manner
- failure to use the correct drug for prophylaxis.
In GBS-positive patients, practitioners should inquire about penicillin allergy and document the exact type of reaction experienced by the patient, if it is accurately known. If the reported reaction to penicillin was not life-threatening, patients should receive cefazolin, 2 g IV initially, then 1 g every 8 hours until delivery. If the reaction to penicillin was immediate and life-threatening, the patient should receive clindamycin, 900 mg IV every 8 hours, if the organism is confirmed to be susceptible. Susceptibility testing should be documented in the medical record.
If such testing is unavailable, vancomycin is the drug of choice.
Susceptibility testing is vital in penicillin-allergic women
Because of observations such as these, the CDC now recommends that clindamycin be used for GBS prophylaxis only if antimicrobial susceptibility tests have confirmed that the organism is sensitive. If susceptibility testing cannot easily be performed, practitioners should use intravenous (IV) vancomycin, 1 g every 12 hours, for prophylaxis. Potential side effects of vancomycin include allergic reactions, gastrointestinal irritation, ototoxicity, and nephrotoxicity. The latter two effects are extremely unlikely in patients who receive only one or two IV doses of the drug.
20% to 30% of gravidas are colonized with GBS
GBS is one of the two major causes of pneumonia, meningitis, and sepsis in both pre-term and term newborns. Approximately 20% to 30% of women are colonized with the organism at some point during pregnancy. Universal screening for GBS at 35 to 37 weeks’ gestation, combined with intrapartum antibiotic prophylaxis, has been highly effective in reducing the frequency of invasive GBS infection.
REFERENCES
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1-24
2. Edwards RK, Clark P, Sistrom CL, Duff P. Intrapartum antibiotic prophylaxis 1: relative effects of recommended antibiotics on gram-negative pathogens. Obstet Gynecol. 2002;100:534-539
New data suggest that preincision prophylaxis is best for C-section
Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2007;196:455.e1–455.e5.
Is preoperative antibiotic prophylaxis superior to intraoperative prophylaxis in preventing postcesarean infection? Sullivan and colleagues set out to answer this question in a prospective, randomized, double-blinded, placebo-controlled study at the Medical University of South Carolina.
In the study group, 175 women undergoing cesarean delivery were randomized to receive 1 g of IV cefazolin 15 to 60 minutes before surgery, followed by a placebo infusion immediately after the umbilical cord was clamped. In the control group, 182 women received preoperative placebo, followed by 1 g of cefazolin immediately after cord clamping.
Two patients in the study group developed endomyometritis, compared with 10 in the control group (relative risk [RR], 0.2; 95% CI, 0.15–0.94). Five patients in the study group developed a wound infection, compared with 10 in the control group (RR, 0.52; 95% CI, 0.18–1.5, not significant). Overall, eight women in the study group and 21 women in the control group met the criteria for infectious morbidity (RR, 0.4; 95% CI, 0.18–0.87).
There were no differences between the groups in the frequency of neonatal sepsis, neonatal intensive care unit (NICU) admission, total length of hospital stay, metabolic acidosis, or sepsis evaluation. Infants in the study group had significantly fewer days in the NICU (P<.01).
How this study differs from earlier investigations
The classic studies of antibiotic prophylaxis were performed in an animal model by Burke.1 He demonstrated that prophylaxis had its greatest effect when the antibiotic was administered before the surgical incision. Essentially, no effect of prophylaxis was evident when antibiotic administration was delayed more than 4 hours beyond the start of surgery.
Early studies of antibiotic prophylaxis for cesarean delivery, conducted in the 1970s, administered antibiotics preoperatively and continued administration for several days after surgery. In 1979, Gordon and colleagues published an important investigation demonstrating that delay in administration of antibiotics until after the umbilical cord was clamped did not compromise the effectiveness of prophylaxis and significantly decreased the number of infants who required sepsis evaluations.2
This latter effect presumably occurred because infants were not exposed to antibiotics before delivery. Gordon’s investigation and subsequent reports also demonstrated that effective prophylaxis could be achieved with one to three doses of antibiotics.3
Why this new study may alter practice
Before Sullivan and colleagues published their findings, I believe that the best available evidence supported the use of a single dose of antibiotic, such as cefazolin, immediately after cord clamping. There are no convincing data that demonstrate an advantage for extended-spectrum agents (second- and third-generation cephalosporins, broad-spectrum penicillins, or carbapenems) over cefazolin.3
However, if the findings of Sullivan and coworkers are confirmed by other investigations in different patient populations, they definitely should lead to a change in the standard of care for prophylaxis. This investigation was exceptionally well designed and executed. The reduction in the frequency of endomyometritis and overall rate of infectious morbidity was impressive. This advantage was achieved without increasing the rate of neonatal sepsis evaluation.
REFERENCES
1. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168
2. Gordon HR, Phelps D. Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Surgery. 1979;53:151-156
3. Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost-effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157:794-798
Expect to see more women with diverticulitis as the population ages
Jacobs DO. Diverticulitis. N Engl J Med. 2007;357:2057–2066.
ObGyns continue to play a major role in providing primary care to women. With the steady aging of the American population, practitioners certainly can expect to care for more and more women who are 50 years of age or older, and diverticulitis is likely to turn up in an increasing number of these patients.
Diverticulitis is a relatively common condition in older patients and must consistently be considered in the differential diagnosis of women with acute abdominal pain—particularly left-sided pain.
It is present in approximately 10% of adults younger than 40 years of age and in 40 to 70% of people 80 years of age or older. It primarily affects the sigmoid and descending colon and is associated with diets that are low in fiber and high in refined carbohydrates.
The condition probably results from stasis or obstruction in the narrow neck of a diverticulum, which, in turn, leads to an overgrowth of bacteria. The principal micro-organisms isolated from affected patients are anaerobes, gram-negative aerobes, and some facultative gram-positive bacteria.
Range of severity can be wide
Presentation of diverticulitis may range in severity from mild to moderate lower abdominal pain associated with anorexia, nausea, and vomiting to abscess and fistula formation, colonic stricture, bowel obstruction, and peritonitis (“complicated diverticulitis”). Peritonitis may arise from rupture of a peridiverticular abscess or free rupture of an uninflamed diverticulum. Diverticulitis may be particularly severe in immunocompromised patients.
Classification of disease
The most accepted classification system for diverticulitis is the Hinchey system:
- in stage 1 disease, patients have small and confined pericolic or mesenteric abscesses
- in stage 2, the abscesses are larger but usually remain confined to the pelvis
- in stage 3, an abscess ruptures and causes purulent peritonitis
- stage 4 disease is distinguished by rupture of an uninflamed and unobstructed diverticulum (known as “free rupture”); this stage has the highest risk of adverse outcomes.
CT is best for diagnosis
The most useful diagnostic test for diverticulitis is a computed tomography (CT) scan. As a general rule, endoscopy should be avoided because of the risk of intestinal perforation.
Most patients can be treated as outpatients
Patients who have mild disease usually can be treated as outpatients. They should receive a 7- to 10-day course of oral antibiotics such as ciprofloxacin, 500 to 750 mg every 12 hours, plus metronidazole, 500 mg every 6 to 8 hours.
Alternate oral regimens include metronidazole, 500 mg every 6 to 8 hours, plus trimethoprim-sulfamethoxazole, double-strength, every 12 hours, or amoxicillin-clavulanate, 875 mg every 12 hours.
For seriously ill patients, hospitalization is warranted
Seriously ill patients—particularly those who are immunocompromised—should be hospitalized. If bowel obstruction is present, a nasogastric tube should be inserted. Appropriate IV antibiotic regimens for hospitalized patients include:
- metronidazole, 500 mg every 6 to 8 hours, plus ciprofloxacin, 400 mg every 12 hours
- metronidazole, 500 mg every 6 to 8 hours, plus ceftriaxone, 1 to 2 g every 24 hours
- ampicillin–sulbactam, 3 g every 6 hours
- piperacillin–tazobactam, 3.375 g every 6 hours
- ticarcillin–clavulanate, 3.1 g every 6 hours.
FIGURE 3 Consider diverticulitis in older women who complain of acute abdominal pain
Diverticulitis generally affects the sigmoid colon and descending colon and probably arises from stasis or obstruction in the narrow neck of a diverticulum, which leads to an overgrowth of bacteria and possible rupture.If an abscess is present and fails to respond promptly to medical therapy, drainage may be necessary. Some abscesses can be drained percutaneously under CT guidance. Large abscesses, in association with signs of generalized peritonitis, uncontrolled sepsis, or intestinal perforation, require surgical intervention, either via laparoscopy or open laparotomy. Preliminary data suggest that the laparoscopic approach may result in a shorter hospital stay, decreased postoperative pain, and an overall reduced rate of perioperative complication.
Diverticulitis may mimic appendicitis
The clinical presentation of diverticulitis is similar to that of appendicitis, except that the pain is usually on the left. Perforation, with resulting peritonitis, is an ever-present and potentially life-threatening complication. The dominant organisms are anaerobes and coliforms. The best diagnostic test is CT.
Patients in the early stages of disease can usually be treated as outpatients with antibiotics that are quite familiar to all ObGyns— i.e., metronidazole and a quinolone. More seriously ill patients should be hospitalized and treated with IV antibiotics and nasogastric suctioning.
Consultation with an interventional radiologist and general surgeon is recommended if operative intervention is necessary.
- important new information regarding the effectiveness of hepatitis A vaccine for postexposure prophylaxis
- the need to confirm antimicrobial susceptibility of group B streptococcus (GBS) isolates in pregnant women who are allergic to penicillin
- a new guideline on administering antibiotic prophylaxis for cesarean delivery
- a valuable overview of diverticulitis, a disease that we will all see with increasing regularity as the US population ages
For most, the best hepatitis A postexposure prophylaxis is vaccination
Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685–1694.
The objective of this investigation, conducted in Almaty, Kazakhstan, was to compare the relative effectiveness of hepatitis A vaccine with that of immune globulin for prophylaxis after exposure to the hepatitis A virus. Participants were 2 to 40 years old and were household or day-care contacts of people who had hepatitis A. Five hundred sixty-eight susceptible patients received hepatitis A vaccine within 14 days of exposure; 522 susceptible patients received an age-appropriate dose of immune globulin. The primary endpoint was laboratoryconfirmed, symptomatic hepatitis A within 15 to 56 days of exposure.
FIGURE 1 Hepatitis A virus
Hepatitis A virus has single-stranded RNA, no envelope, and is approximately 27–30 nm in diameter.Symptomatic infection occurred in 25 of 568 (4.4%) vaccine recipients and 17 of 522 (3.3%) recipients of immune globulin. The relative risk of infection after the vaccine was 1.35 (95% confidence interval [CI], 0.70–2.67). No serious adverse effects occurred as the result of administration of either the vaccine or immune globulin. The authors concluded that both agents provide effective postexposure prophylaxis against hepatitis A infection.
Hepatitis A is caused by an RNA virus that is transmitted by fecal–oral contact. It is highly contagious and endemic in areas of the world where poverty, poor sanitation, and overcrowded living conditions prevail. Hepatitis A usually causes a symptomatic illness characterized by fever, malaise, anorexia, jaundice, acholic stools, darkened urine, and hepatic pain and tenderness. In poorly nourished or immunocompromised patients, severe morbidity and, rarely, mortality, may occur. Unlike other forms of hepatitis, hepatitis A does not cause a chronic carrier state, and perinatal transmission is extremely unlikely.
Best strategy? Prevention
At present, there is no specific antiviral therapy that is routinely used for treatment of hepatitis A. However, highly effective preventive measures are available. For preexposure prophylaxis, time permitting, the ideal agent is inactivated hepatitis A vaccine.1
The vaccine is usually given in two doses separated by 6 to 12 months and is highly immunogenic. In the United States, key candidates for vaccination are
- gay men
- residents and staff of chronic care facilities
- intravenous drug users
- individuals who live in areas where hepatitis A is endemic
- primate laboratory workers
- people 30 years of age and older who have chronic liver disease
- international travelers
- people who have received a liver transplant or are awaiting one.
The vaccine is safe for administration in pregnancy and is now recommended for children.
After exposure, vaccine trumps immune globulin in healthy patients
The standard agent for prophylaxis after exposure to the hepatitis A virus has been immune globulin 0.02 mL/kg, administered intramuscularly. Immune globulin is highly effective in this application and, in the present investigation, it was slightly more effective than the vaccine.
Despite the modest difference in effectiveness, however, hepatitis A vaccine offers several unique advantages for postexposure prophylaxis:
- It confers long-term immunity rather than just temporary protection.
- Because the volume of fluid injected is lower, the vaccine causes less pain upon administration.
- Immune globulin is now produced by a single manufacturer, and its supply has been limited. Its price also approaches that of the vaccine.
- Administration of immune globulin to a child may disrupt the normal childhood immunization schedule.
In older and immunocompromised patients, use immune globulin
For most patients, hepatitis A vaccine is the indicated method of postexposure immunoprophylaxis.
Because it is slightly more effective, however, immune globulin probably should remain the preferred agent for hepatitis A postexposure prophylaxis in older or immunocompromised patients who are more likely to develop severe illness if they be-come infected.
REFERENCES
We’re not following guidelines on GBS prophylaxis in penicillin allergy
Matheson KA, Lievense SP, Catanzaro B, Phipps MG. Intrapartum group B streptococci prophylaxis in patients reporting a penicillin allergy. Obstet Gynecol. 2008;111:356–364.
In this study, conducted at a single institution (Brown University), Matheson and colleagues sought to assess the adequacy of prophylaxis for GBS infection in women who had an allergy to penicillin. Specifically, the authors sought to determine how well practitioners at their institution adhered to the 2002 Centers for Disease Control and Prevention (CDC) guidelines, which specify that cefazolin should be used for prophylaxis in patients who are penicillin-allergic but not at high risk for anaphylaxis.1 For patients at high risk for anaphylaxis, clindamycin may be used for prophylaxis if the organism is known to be susceptible. If susceptibility has not been documented, vancomycin should be administered.1
Overall, 95% of GBS-positive, penicillin-allergic patients received prophylaxis (95% CI, 91–97). However, only 15% of these women received appropriate prophylaxis as defined by the CDC (95% CI, 11–12). Clindamycin was administered to 83% of patients, but susceptibility testing was performed in only 11%. At the time of this study, 26% of all GBS isolates at Brown were resistant to clindamycin; 37% were resistant to erythromycin.
The authors concluded that adherence to CDC guidelines was clearly less than optimal. Even at 1 year after adoption of the guidelines, only 20% of patients received appropriate prophylaxis.
Type of allergic reaction is key to selection of prophylactic agent
GBS is uniformly sensitive to penicillin and ampicillin. It also is 100% sensitive to cefazolin, the preferred drug for intrapartum prophylaxis in penicillin-allergic women who have a low risk of anaphylactic reaction to penicillin.
FIGURE 2 Group B streptococcus
A clear zone of hemolysis on blood agar is a key characteristic of group B streptococcus.However, it probably is better to avoid cephalosporins in patients who report a previous anaphylactic reaction to penicillin or ampicillin, even though the risk of cross-reactivity between penicillin and cephalosporin is low. In such patients, possible alternatives include erythromycin, clindamycin, and vancomycin.
Erythromycin is no longer recommended
At the University of Florida, we reported that 21% of GBS strains were resistant to erythromycin.2 At Brown University, Matheson and colleagues reported that 37% of GBS isolates were resistant to erythromycin. On the basis of similar reports, the CDC has concluded that erythromycin no longer should be used for GBS prophylaxis.
At our institution, we also have noted a disturbing trend of increased GBS resistance to clindamycin. In our recent report, 9% of GBS strains were resistant to this antibiotic. Similarly, Matheson and coworkers observed that 26% of GBS isolates in their center were resistant to clindamycin.
Neonatal GBS infection is now one of the leading causes for malpractice suits in obstetrics. Key issues presented in these suits include:
- failure to screen
- failure to use the correct culture medium for screening
- failure to obtain test results in a timely manner
- failure to use the correct drug for prophylaxis.
In GBS-positive patients, practitioners should inquire about penicillin allergy and document the exact type of reaction experienced by the patient, if it is accurately known. If the reported reaction to penicillin was not life-threatening, patients should receive cefazolin, 2 g IV initially, then 1 g every 8 hours until delivery. If the reaction to penicillin was immediate and life-threatening, the patient should receive clindamycin, 900 mg IV every 8 hours, if the organism is confirmed to be susceptible. Susceptibility testing should be documented in the medical record.
If such testing is unavailable, vancomycin is the drug of choice.
Susceptibility testing is vital in penicillin-allergic women
Because of observations such as these, the CDC now recommends that clindamycin be used for GBS prophylaxis only if antimicrobial susceptibility tests have confirmed that the organism is sensitive. If susceptibility testing cannot easily be performed, practitioners should use intravenous (IV) vancomycin, 1 g every 12 hours, for prophylaxis. Potential side effects of vancomycin include allergic reactions, gastrointestinal irritation, ototoxicity, and nephrotoxicity. The latter two effects are extremely unlikely in patients who receive only one or two IV doses of the drug.
20% to 30% of gravidas are colonized with GBS
GBS is one of the two major causes of pneumonia, meningitis, and sepsis in both pre-term and term newborns. Approximately 20% to 30% of women are colonized with the organism at some point during pregnancy. Universal screening for GBS at 35 to 37 weeks’ gestation, combined with intrapartum antibiotic prophylaxis, has been highly effective in reducing the frequency of invasive GBS infection.
REFERENCES
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1-24
2. Edwards RK, Clark P, Sistrom CL, Duff P. Intrapartum antibiotic prophylaxis 1: relative effects of recommended antibiotics on gram-negative pathogens. Obstet Gynecol. 2002;100:534-539
New data suggest that preincision prophylaxis is best for C-section
Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2007;196:455.e1–455.e5.
Is preoperative antibiotic prophylaxis superior to intraoperative prophylaxis in preventing postcesarean infection? Sullivan and colleagues set out to answer this question in a prospective, randomized, double-blinded, placebo-controlled study at the Medical University of South Carolina.
In the study group, 175 women undergoing cesarean delivery were randomized to receive 1 g of IV cefazolin 15 to 60 minutes before surgery, followed by a placebo infusion immediately after the umbilical cord was clamped. In the control group, 182 women received preoperative placebo, followed by 1 g of cefazolin immediately after cord clamping.
Two patients in the study group developed endomyometritis, compared with 10 in the control group (relative risk [RR], 0.2; 95% CI, 0.15–0.94). Five patients in the study group developed a wound infection, compared with 10 in the control group (RR, 0.52; 95% CI, 0.18–1.5, not significant). Overall, eight women in the study group and 21 women in the control group met the criteria for infectious morbidity (RR, 0.4; 95% CI, 0.18–0.87).
There were no differences between the groups in the frequency of neonatal sepsis, neonatal intensive care unit (NICU) admission, total length of hospital stay, metabolic acidosis, or sepsis evaluation. Infants in the study group had significantly fewer days in the NICU (P<.01).
How this study differs from earlier investigations
The classic studies of antibiotic prophylaxis were performed in an animal model by Burke.1 He demonstrated that prophylaxis had its greatest effect when the antibiotic was administered before the surgical incision. Essentially, no effect of prophylaxis was evident when antibiotic administration was delayed more than 4 hours beyond the start of surgery.
Early studies of antibiotic prophylaxis for cesarean delivery, conducted in the 1970s, administered antibiotics preoperatively and continued administration for several days after surgery. In 1979, Gordon and colleagues published an important investigation demonstrating that delay in administration of antibiotics until after the umbilical cord was clamped did not compromise the effectiveness of prophylaxis and significantly decreased the number of infants who required sepsis evaluations.2
This latter effect presumably occurred because infants were not exposed to antibiotics before delivery. Gordon’s investigation and subsequent reports also demonstrated that effective prophylaxis could be achieved with one to three doses of antibiotics.3
Why this new study may alter practice
Before Sullivan and colleagues published their findings, I believe that the best available evidence supported the use of a single dose of antibiotic, such as cefazolin, immediately after cord clamping. There are no convincing data that demonstrate an advantage for extended-spectrum agents (second- and third-generation cephalosporins, broad-spectrum penicillins, or carbapenems) over cefazolin.3
However, if the findings of Sullivan and coworkers are confirmed by other investigations in different patient populations, they definitely should lead to a change in the standard of care for prophylaxis. This investigation was exceptionally well designed and executed. The reduction in the frequency of endomyometritis and overall rate of infectious morbidity was impressive. This advantage was achieved without increasing the rate of neonatal sepsis evaluation.
REFERENCES
1. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168
2. Gordon HR, Phelps D. Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Surgery. 1979;53:151-156
3. Duff P. Prophylactic antibiotics for cesarean delivery: a simple cost-effective strategy for prevention of postoperative morbidity. Am J Obstet Gynecol. 1987;157:794-798
Expect to see more women with diverticulitis as the population ages
Jacobs DO. Diverticulitis. N Engl J Med. 2007;357:2057–2066.
ObGyns continue to play a major role in providing primary care to women. With the steady aging of the American population, practitioners certainly can expect to care for more and more women who are 50 years of age or older, and diverticulitis is likely to turn up in an increasing number of these patients.
Diverticulitis is a relatively common condition in older patients and must consistently be considered in the differential diagnosis of women with acute abdominal pain—particularly left-sided pain.
It is present in approximately 10% of adults younger than 40 years of age and in 40 to 70% of people 80 years of age or older. It primarily affects the sigmoid and descending colon and is associated with diets that are low in fiber and high in refined carbohydrates.
The condition probably results from stasis or obstruction in the narrow neck of a diverticulum, which, in turn, leads to an overgrowth of bacteria. The principal micro-organisms isolated from affected patients are anaerobes, gram-negative aerobes, and some facultative gram-positive bacteria.
Range of severity can be wide
Presentation of diverticulitis may range in severity from mild to moderate lower abdominal pain associated with anorexia, nausea, and vomiting to abscess and fistula formation, colonic stricture, bowel obstruction, and peritonitis (“complicated diverticulitis”). Peritonitis may arise from rupture of a peridiverticular abscess or free rupture of an uninflamed diverticulum. Diverticulitis may be particularly severe in immunocompromised patients.
Classification of disease
The most accepted classification system for diverticulitis is the Hinchey system:
- in stage 1 disease, patients have small and confined pericolic or mesenteric abscesses
- in stage 2, the abscesses are larger but usually remain confined to the pelvis
- in stage 3, an abscess ruptures and causes purulent peritonitis
- stage 4 disease is distinguished by rupture of an uninflamed and unobstructed diverticulum (known as “free rupture”); this stage has the highest risk of adverse outcomes.
CT is best for diagnosis
The most useful diagnostic test for diverticulitis is a computed tomography (CT) scan. As a general rule, endoscopy should be avoided because of the risk of intestinal perforation.
Most patients can be treated as outpatients
Patients who have mild disease usually can be treated as outpatients. They should receive a 7- to 10-day course of oral antibiotics such as ciprofloxacin, 500 to 750 mg every 12 hours, plus metronidazole, 500 mg every 6 to 8 hours.
Alternate oral regimens include metronidazole, 500 mg every 6 to 8 hours, plus trimethoprim-sulfamethoxazole, double-strength, every 12 hours, or amoxicillin-clavulanate, 875 mg every 12 hours.
For seriously ill patients, hospitalization is warranted
Seriously ill patients—particularly those who are immunocompromised—should be hospitalized. If bowel obstruction is present, a nasogastric tube should be inserted. Appropriate IV antibiotic regimens for hospitalized patients include:
- metronidazole, 500 mg every 6 to 8 hours, plus ciprofloxacin, 400 mg every 12 hours
- metronidazole, 500 mg every 6 to 8 hours, plus ceftriaxone, 1 to 2 g every 24 hours
- ampicillin–sulbactam, 3 g every 6 hours
- piperacillin–tazobactam, 3.375 g every 6 hours
- ticarcillin–clavulanate, 3.1 g every 6 hours.
FIGURE 3 Consider diverticulitis in older women who complain of acute abdominal pain
Diverticulitis generally affects the sigmoid colon and descending colon and probably arises from stasis or obstruction in the narrow neck of a diverticulum, which leads to an overgrowth of bacteria and possible rupture.If an abscess is present and fails to respond promptly to medical therapy, drainage may be necessary. Some abscesses can be drained percutaneously under CT guidance. Large abscesses, in association with signs of generalized peritonitis, uncontrolled sepsis, or intestinal perforation, require surgical intervention, either via laparoscopy or open laparotomy. Preliminary data suggest that the laparoscopic approach may result in a shorter hospital stay, decreased postoperative pain, and an overall reduced rate of perioperative complication.
Diverticulitis may mimic appendicitis
The clinical presentation of diverticulitis is similar to that of appendicitis, except that the pain is usually on the left. Perforation, with resulting peritonitis, is an ever-present and potentially life-threatening complication. The dominant organisms are anaerobes and coliforms. The best diagnostic test is CT.
Patients in the early stages of disease can usually be treated as outpatients with antibiotics that are quite familiar to all ObGyns— i.e., metronidazole and a quinolone. More seriously ill patients should be hospitalized and treated with IV antibiotics and nasogastric suctioning.
Consultation with an interventional radiologist and general surgeon is recommended if operative intervention is necessary.