User login
Repeat Admissions to Residential Substance Abuse Treatment Programs: A Descriptive Study
Maximizing Efficiency of Research Pharmacy Services: The VA New York Harbor Healthcare System Experience
Midfoot Arthritis
Bias in Research
MENOPAUSE
The author has received funding from Barr/Duramed, Bayer, and Warner Chilcott. He is a consultant for Barr/Duramed, Bayer, Warner Chilcott, Kenwood, Noven, and Johnson & Johnson. He holds stock with Sanofi Aventis and Procter & Gamble.
Does estrogen therapy carry more risk than benefit? The answer depends, new data suggest, on the age of the patient, route of administration, and type of progestin.
The past 12 months have yielded important new insights into the risks and benefits of menopausal hormone therapy (HT), including
- landmark reports from the Women’s Health Initiative (WHI) regarding HT and the risk of coronary artery disease
- data from France on the route of HT and risk of thrombosis and on progestin selection and the risk of breast cancer
- data from the Mayo Clinic regarding HT use and subsequent risk of dementia and parkinsonism.
User age determines effects of HT on coronary artery disease
Rossouw JE, Prentice PL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477.
Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602.
The WHI clinical trials were designed in 1991 and 1992 primarily to determine whether oral menopausal HT protects against coronary artery disease (CAD), as a large body of literature based on observational studies had suggested. Most of those observational studies had involved unopposed oral estrogen.1
When the estrogen–progestin arm of the WHI was halted in 2002, investigators noted that use of conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA) overall was associated with a 29% increase in the risk of CAD (hazard ratio [HR], 1.29; 95% confidence interval [CI], 1.02–1.63) and a more than 200% increase in the risk of venous thromboembolism (HR, 2.11; 95% CI, 1.49–2.87), compared with placebo. Subsequent reports explored this connection from different angles ( see the timeline).
In 2007, important—and, for some, startling—findings were published regarding HT and the risk of CAD, most notably:
- When estrogen users from both arms of the WHI trial were combined into one group, those who were less than 10 years since the onset of menopause had a HR for CAD of 0.76 (95% CI, 0.5–1.16), and oral HT was associated with six fewer cases of CAD for every 10,000 woman-years of use. Similar findings were reported for women 50 to 59 years old. Among older WHI participants and those more distant from menopause, HT was associated with an elevated risk of CAD.
- In the same cohort, mean coronary artery calcium scores overall were more favorable among women receiving estrogen than among those randomized to placebo (P=.02). Among women who took the study medication most consistently (at least 80% adherent), an even greater reduction in coronary artery calcification was noted with estrogen use, which was associated with a 61% reduction in the risk of having extensive coronary artery calcification (P=.004). The authors concluded: “… estrogen therapy may have cardioprotective effects in younger (menopausal) women.”
In contrast to earlier WHI reports, which failed to break out risks by user age, these recent publications are consistent with the earlier observational studies of HT and should reassure ObGyns that the patients most likely to experience menopausal symptoms (women in their 50s and early 60s) can use HT without increasing their risk of CAD.
Transdermal estrogen carries a lower risk of VTE than oral administration
Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: The ESTHER study. Circulation. 2007;115:840–845.
As I noted earlier in this article, the initial 2002 WHI report found that oral CEE plus MPA doubled the risk of venous thromboembolism (VTE). Although WHI clinical trials did not study transdermal estrogen, an important observational study comparing VTE risk between oral and transdermal estrogen therapy was conducted in France, where use of transdermal estrogen is more common than in the United States.
In a 2007 report from this large multicenter, case-control study (the Estrogen and Thromboembolism Risk study, or ESTHER), oral menopausal estrogen therapy was associated with a fourfold increase in the risk of VTE (including pulmonary embolism and deep venous thrombosis), compared with nonuse (P<.05), whereas use of transdermal estrogen was not associated with any increase in the risk of VTE.
Type of progestin also played a role
This report also assessed VTE by the type of progestin used by women taking combination estrogen–progestin HT. Micronized progesterone and MPA did not affect the risk of VTE, but norethindrone acetate as well as other progestins not used in the United States did appear to elevate VTE risk.
Transdermal estrogen is as effective as oral therapy
Like oral estrogen therapy, transdermal therapy effectively treats vasomotor symptoms, prevents loss of bone density, and treats genital atrophy.
Because transdermal menopausal estrogen therapy does not increase hepatic production of procoagulant factors, as does oral estrogen, it is biologically plausible that transdermal therapy is safer than oral therapy in terms of the risk of VTE.6
Combined with other evidence, the findings of this important French study suggest that ObGyns should consider transdermal therapy when helping menopausal women select a HT regimen.
Micronized progesterone might not raise the risk of breast cancer
Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103–111.
In contrast to estrogen-only therapy, long-term use of combination estrogen– progestin HT is associated with a modestly elevated risk of breast cancer.7-10
In France, micronized progesterone is the progestin most commonly used in HT. In 2008, results from a large French case-control study suggested that—in contrast to combination HT that contains MPA or norethindrone acetate—use of combination HT formulated with micronized progesterone was not associated with an elevated risk of breast cancer.
In women taking menopausal estrogen, the appropriate dosage of micronized progesterone to prevent endometrial hyperplasia is 100 mg nightly or 200 mg for 12 or more nights each month.
Avoid micronized progesterone in patients with peanut allergy
Because micronized progesterone contains peanut oil, patients with a history of peanut allergy should not use it.
Estrogen’s effects on cognition depend on, again, age at use
Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083.
Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209.
One intriguing possibility entertained in recent years is that HT prevents dementia, although data so far have been conflicting. A large, high-quality observational study performed in Utah and published in 2002 provided evidence that HT use by young menopausal women prevents cognitive decline later in life, particularly when HT is used over the long term.11
In contrast, the WHI Memory Study found that HT increases the risk of mild cognitive impairment and dementia.12 However, that study enrolled an older subgroup of WHI participants (65 to 79 years old at randomization).
Very young estrogen-deprived women stand to benefit from HT
Over the past year, Rocca and colleagues at the Mayo Clinic in Minnesota published two reports assessing the risk of neurologic disease among several thousand Midwestern women who had undergone oophorectomy (unilateral or bilateral) before reaching menopause. A history of oophorectomy, especially in women younger than 38 years, was associated with a significantly increased risk of cognitive impairment and dementia. However, when estrogen therapy was prescribed until at least 50 years of age following bilateral oophorectomy, no increased risk of cognitive impairment was found.
Using similar methods, the same research group at Mayo found that oophorectomy before menopause was associated with a significantly increased risk of parkinsonism (symptoms that did not meet the formal criteria for Parkinson’s disease) as well as an increased risk, which did not attain statistica significance, of Parkinson’s disease itself.
Taken in totality, the evidence suggests that when HT is initiated in young menopausal women, protection against dementia and other neurologic disease may result. These findings parallel the evidence on the risk of CAD during HT use presented at the beginning of this article.
1. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Michels KB, Manson JE. Postmenopausal hormone therapy: a reversal of fortune. Circulation. 2003;107:1830-1833.
3. Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
4. Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
5. Hsia J, Lander RD, Manson JE, et al. Women’s Health Initiative Investigators. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
6. Rexrode KM, Manson JE. Are some types of hormone therapy safer than others? Lessons from the Estrogen and Thromboembolism study [editorial]. Circulation. 2007;115:820-822.
7. Kaunitz AM. Update on menopause. OBG Management. 2006;18(5):45-54.
8. Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545-560.
9. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103-115.
10. Kaunitz AM. HT and breast cancer: Does the type of progestin matter? OBG Management. 2007;19(6):31-35.
11. Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache Country Study. JAMA. 2002;288:2123-2129.
12. Shumaker SA, Legault C, Kuller L, et al. Women’s Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
The author has received funding from Barr/Duramed, Bayer, and Warner Chilcott. He is a consultant for Barr/Duramed, Bayer, Warner Chilcott, Kenwood, Noven, and Johnson & Johnson. He holds stock with Sanofi Aventis and Procter & Gamble.
Does estrogen therapy carry more risk than benefit? The answer depends, new data suggest, on the age of the patient, route of administration, and type of progestin.
The past 12 months have yielded important new insights into the risks and benefits of menopausal hormone therapy (HT), including
- landmark reports from the Women’s Health Initiative (WHI) regarding HT and the risk of coronary artery disease
- data from France on the route of HT and risk of thrombosis and on progestin selection and the risk of breast cancer
- data from the Mayo Clinic regarding HT use and subsequent risk of dementia and parkinsonism.
User age determines effects of HT on coronary artery disease
Rossouw JE, Prentice PL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477.
Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602.
The WHI clinical trials were designed in 1991 and 1992 primarily to determine whether oral menopausal HT protects against coronary artery disease (CAD), as a large body of literature based on observational studies had suggested. Most of those observational studies had involved unopposed oral estrogen.1
When the estrogen–progestin arm of the WHI was halted in 2002, investigators noted that use of conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA) overall was associated with a 29% increase in the risk of CAD (hazard ratio [HR], 1.29; 95% confidence interval [CI], 1.02–1.63) and a more than 200% increase in the risk of venous thromboembolism (HR, 2.11; 95% CI, 1.49–2.87), compared with placebo. Subsequent reports explored this connection from different angles ( see the timeline).
In 2007, important—and, for some, startling—findings were published regarding HT and the risk of CAD, most notably:
- When estrogen users from both arms of the WHI trial were combined into one group, those who were less than 10 years since the onset of menopause had a HR for CAD of 0.76 (95% CI, 0.5–1.16), and oral HT was associated with six fewer cases of CAD for every 10,000 woman-years of use. Similar findings were reported for women 50 to 59 years old. Among older WHI participants and those more distant from menopause, HT was associated with an elevated risk of CAD.
- In the same cohort, mean coronary artery calcium scores overall were more favorable among women receiving estrogen than among those randomized to placebo (P=.02). Among women who took the study medication most consistently (at least 80% adherent), an even greater reduction in coronary artery calcification was noted with estrogen use, which was associated with a 61% reduction in the risk of having extensive coronary artery calcification (P=.004). The authors concluded: “… estrogen therapy may have cardioprotective effects in younger (menopausal) women.”
In contrast to earlier WHI reports, which failed to break out risks by user age, these recent publications are consistent with the earlier observational studies of HT and should reassure ObGyns that the patients most likely to experience menopausal symptoms (women in their 50s and early 60s) can use HT without increasing their risk of CAD.
Transdermal estrogen carries a lower risk of VTE than oral administration
Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: The ESTHER study. Circulation. 2007;115:840–845.
As I noted earlier in this article, the initial 2002 WHI report found that oral CEE plus MPA doubled the risk of venous thromboembolism (VTE). Although WHI clinical trials did not study transdermal estrogen, an important observational study comparing VTE risk between oral and transdermal estrogen therapy was conducted in France, where use of transdermal estrogen is more common than in the United States.
In a 2007 report from this large multicenter, case-control study (the Estrogen and Thromboembolism Risk study, or ESTHER), oral menopausal estrogen therapy was associated with a fourfold increase in the risk of VTE (including pulmonary embolism and deep venous thrombosis), compared with nonuse (P<.05), whereas use of transdermal estrogen was not associated with any increase in the risk of VTE.
Type of progestin also played a role
This report also assessed VTE by the type of progestin used by women taking combination estrogen–progestin HT. Micronized progesterone and MPA did not affect the risk of VTE, but norethindrone acetate as well as other progestins not used in the United States did appear to elevate VTE risk.
Transdermal estrogen is as effective as oral therapy
Like oral estrogen therapy, transdermal therapy effectively treats vasomotor symptoms, prevents loss of bone density, and treats genital atrophy.
Because transdermal menopausal estrogen therapy does not increase hepatic production of procoagulant factors, as does oral estrogen, it is biologically plausible that transdermal therapy is safer than oral therapy in terms of the risk of VTE.6
Combined with other evidence, the findings of this important French study suggest that ObGyns should consider transdermal therapy when helping menopausal women select a HT regimen.
Micronized progesterone might not raise the risk of breast cancer
Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103–111.
In contrast to estrogen-only therapy, long-term use of combination estrogen– progestin HT is associated with a modestly elevated risk of breast cancer.7-10
In France, micronized progesterone is the progestin most commonly used in HT. In 2008, results from a large French case-control study suggested that—in contrast to combination HT that contains MPA or norethindrone acetate—use of combination HT formulated with micronized progesterone was not associated with an elevated risk of breast cancer.
In women taking menopausal estrogen, the appropriate dosage of micronized progesterone to prevent endometrial hyperplasia is 100 mg nightly or 200 mg for 12 or more nights each month.
Avoid micronized progesterone in patients with peanut allergy
Because micronized progesterone contains peanut oil, patients with a history of peanut allergy should not use it.
Estrogen’s effects on cognition depend on, again, age at use
Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083.
Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209.
One intriguing possibility entertained in recent years is that HT prevents dementia, although data so far have been conflicting. A large, high-quality observational study performed in Utah and published in 2002 provided evidence that HT use by young menopausal women prevents cognitive decline later in life, particularly when HT is used over the long term.11
In contrast, the WHI Memory Study found that HT increases the risk of mild cognitive impairment and dementia.12 However, that study enrolled an older subgroup of WHI participants (65 to 79 years old at randomization).
Very young estrogen-deprived women stand to benefit from HT
Over the past year, Rocca and colleagues at the Mayo Clinic in Minnesota published two reports assessing the risk of neurologic disease among several thousand Midwestern women who had undergone oophorectomy (unilateral or bilateral) before reaching menopause. A history of oophorectomy, especially in women younger than 38 years, was associated with a significantly increased risk of cognitive impairment and dementia. However, when estrogen therapy was prescribed until at least 50 years of age following bilateral oophorectomy, no increased risk of cognitive impairment was found.
Using similar methods, the same research group at Mayo found that oophorectomy before menopause was associated with a significantly increased risk of parkinsonism (symptoms that did not meet the formal criteria for Parkinson’s disease) as well as an increased risk, which did not attain statistica significance, of Parkinson’s disease itself.
Taken in totality, the evidence suggests that when HT is initiated in young menopausal women, protection against dementia and other neurologic disease may result. These findings parallel the evidence on the risk of CAD during HT use presented at the beginning of this article.
The author has received funding from Barr/Duramed, Bayer, and Warner Chilcott. He is a consultant for Barr/Duramed, Bayer, Warner Chilcott, Kenwood, Noven, and Johnson & Johnson. He holds stock with Sanofi Aventis and Procter & Gamble.
Does estrogen therapy carry more risk than benefit? The answer depends, new data suggest, on the age of the patient, route of administration, and type of progestin.
The past 12 months have yielded important new insights into the risks and benefits of menopausal hormone therapy (HT), including
- landmark reports from the Women’s Health Initiative (WHI) regarding HT and the risk of coronary artery disease
- data from France on the route of HT and risk of thrombosis and on progestin selection and the risk of breast cancer
- data from the Mayo Clinic regarding HT use and subsequent risk of dementia and parkinsonism.
User age determines effects of HT on coronary artery disease
Rossouw JE, Prentice PL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477.
Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602.
The WHI clinical trials were designed in 1991 and 1992 primarily to determine whether oral menopausal HT protects against coronary artery disease (CAD), as a large body of literature based on observational studies had suggested. Most of those observational studies had involved unopposed oral estrogen.1
When the estrogen–progestin arm of the WHI was halted in 2002, investigators noted that use of conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA) overall was associated with a 29% increase in the risk of CAD (hazard ratio [HR], 1.29; 95% confidence interval [CI], 1.02–1.63) and a more than 200% increase in the risk of venous thromboembolism (HR, 2.11; 95% CI, 1.49–2.87), compared with placebo. Subsequent reports explored this connection from different angles ( see the timeline).
In 2007, important—and, for some, startling—findings were published regarding HT and the risk of CAD, most notably:
- When estrogen users from both arms of the WHI trial were combined into one group, those who were less than 10 years since the onset of menopause had a HR for CAD of 0.76 (95% CI, 0.5–1.16), and oral HT was associated with six fewer cases of CAD for every 10,000 woman-years of use. Similar findings were reported for women 50 to 59 years old. Among older WHI participants and those more distant from menopause, HT was associated with an elevated risk of CAD.
- In the same cohort, mean coronary artery calcium scores overall were more favorable among women receiving estrogen than among those randomized to placebo (P=.02). Among women who took the study medication most consistently (at least 80% adherent), an even greater reduction in coronary artery calcification was noted with estrogen use, which was associated with a 61% reduction in the risk of having extensive coronary artery calcification (P=.004). The authors concluded: “… estrogen therapy may have cardioprotective effects in younger (menopausal) women.”
In contrast to earlier WHI reports, which failed to break out risks by user age, these recent publications are consistent with the earlier observational studies of HT and should reassure ObGyns that the patients most likely to experience menopausal symptoms (women in their 50s and early 60s) can use HT without increasing their risk of CAD.
Transdermal estrogen carries a lower risk of VTE than oral administration
Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: The ESTHER study. Circulation. 2007;115:840–845.
As I noted earlier in this article, the initial 2002 WHI report found that oral CEE plus MPA doubled the risk of venous thromboembolism (VTE). Although WHI clinical trials did not study transdermal estrogen, an important observational study comparing VTE risk between oral and transdermal estrogen therapy was conducted in France, where use of transdermal estrogen is more common than in the United States.
In a 2007 report from this large multicenter, case-control study (the Estrogen and Thromboembolism Risk study, or ESTHER), oral menopausal estrogen therapy was associated with a fourfold increase in the risk of VTE (including pulmonary embolism and deep venous thrombosis), compared with nonuse (P<.05), whereas use of transdermal estrogen was not associated with any increase in the risk of VTE.
Type of progestin also played a role
This report also assessed VTE by the type of progestin used by women taking combination estrogen–progestin HT. Micronized progesterone and MPA did not affect the risk of VTE, but norethindrone acetate as well as other progestins not used in the United States did appear to elevate VTE risk.
Transdermal estrogen is as effective as oral therapy
Like oral estrogen therapy, transdermal therapy effectively treats vasomotor symptoms, prevents loss of bone density, and treats genital atrophy.
Because transdermal menopausal estrogen therapy does not increase hepatic production of procoagulant factors, as does oral estrogen, it is biologically plausible that transdermal therapy is safer than oral therapy in terms of the risk of VTE.6
Combined with other evidence, the findings of this important French study suggest that ObGyns should consider transdermal therapy when helping menopausal women select a HT regimen.
Micronized progesterone might not raise the risk of breast cancer
Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103–111.
In contrast to estrogen-only therapy, long-term use of combination estrogen– progestin HT is associated with a modestly elevated risk of breast cancer.7-10
In France, micronized progesterone is the progestin most commonly used in HT. In 2008, results from a large French case-control study suggested that—in contrast to combination HT that contains MPA or norethindrone acetate—use of combination HT formulated with micronized progesterone was not associated with an elevated risk of breast cancer.
In women taking menopausal estrogen, the appropriate dosage of micronized progesterone to prevent endometrial hyperplasia is 100 mg nightly or 200 mg for 12 or more nights each month.
Avoid micronized progesterone in patients with peanut allergy
Because micronized progesterone contains peanut oil, patients with a history of peanut allergy should not use it.
Estrogen’s effects on cognition depend on, again, age at use
Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083.
Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209.
One intriguing possibility entertained in recent years is that HT prevents dementia, although data so far have been conflicting. A large, high-quality observational study performed in Utah and published in 2002 provided evidence that HT use by young menopausal women prevents cognitive decline later in life, particularly when HT is used over the long term.11
In contrast, the WHI Memory Study found that HT increases the risk of mild cognitive impairment and dementia.12 However, that study enrolled an older subgroup of WHI participants (65 to 79 years old at randomization).
Very young estrogen-deprived women stand to benefit from HT
Over the past year, Rocca and colleagues at the Mayo Clinic in Minnesota published two reports assessing the risk of neurologic disease among several thousand Midwestern women who had undergone oophorectomy (unilateral or bilateral) before reaching menopause. A history of oophorectomy, especially in women younger than 38 years, was associated with a significantly increased risk of cognitive impairment and dementia. However, when estrogen therapy was prescribed until at least 50 years of age following bilateral oophorectomy, no increased risk of cognitive impairment was found.
Using similar methods, the same research group at Mayo found that oophorectomy before menopause was associated with a significantly increased risk of parkinsonism (symptoms that did not meet the formal criteria for Parkinson’s disease) as well as an increased risk, which did not attain statistica significance, of Parkinson’s disease itself.
Taken in totality, the evidence suggests that when HT is initiated in young menopausal women, protection against dementia and other neurologic disease may result. These findings parallel the evidence on the risk of CAD during HT use presented at the beginning of this article.
1. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Michels KB, Manson JE. Postmenopausal hormone therapy: a reversal of fortune. Circulation. 2003;107:1830-1833.
3. Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
4. Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
5. Hsia J, Lander RD, Manson JE, et al. Women’s Health Initiative Investigators. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
6. Rexrode KM, Manson JE. Are some types of hormone therapy safer than others? Lessons from the Estrogen and Thromboembolism study [editorial]. Circulation. 2007;115:820-822.
7. Kaunitz AM. Update on menopause. OBG Management. 2006;18(5):45-54.
8. Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545-560.
9. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103-115.
10. Kaunitz AM. HT and breast cancer: Does the type of progestin matter? OBG Management. 2007;19(6):31-35.
11. Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache Country Study. JAMA. 2002;288:2123-2129.
12. Shumaker SA, Legault C, Kuller L, et al. Women’s Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
1. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Michels KB, Manson JE. Postmenopausal hormone therapy: a reversal of fortune. Circulation. 2003;107:1830-1833.
3. Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
4. Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
5. Hsia J, Lander RD, Manson JE, et al. Women’s Health Initiative Investigators. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
6. Rexrode KM, Manson JE. Are some types of hormone therapy safer than others? Lessons from the Estrogen and Thromboembolism study [editorial]. Circulation. 2007;115:820-822.
7. Kaunitz AM. Update on menopause. OBG Management. 2006;18(5):45-54.
8. Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545-560.
9. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103-115.
10. Kaunitz AM. HT and breast cancer: Does the type of progestin matter? OBG Management. 2007;19(6):31-35.
11. Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache Country Study. JAMA. 2002;288:2123-2129.
12. Shumaker SA, Legault C, Kuller L, et al. Women’s Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
PART 1: Advising your patients Uterine fibroids: Childbearing, cancer, and hormone effects
The author reports no financial relationships relevant to this article.
CASE 1 Rapid growth=cancer?
Mrs. G., 47 years old, has had uterine fibroids the size of a 12-week pregnancy for about 6 years. At today’s examination, however, her uterus feels about the size of a 16-week pregnancy.
She is aware that her abdomen is bigger, and she complains of some abdominal pressure and urinary frequency. She reports no abnormal bleeding and no abdominal pain.
Mrs. G. is upset because another physician told her she might have cancer and needs a hysterectomy immediately. She tells you that she does not want a hysterectomy unless “it’s absolutely necessary.”
Ultrasonography at this visit reveals that two of the three fibroids noted on a previous sonogram have grown—one from 6 cm to 9 cm in diameter; the other from 5 cm to 8 cm.
What do you tell Mrs. G.?
Understanding myomas
Uterine fibroids, also called myomas, are benign, monoclonal tumors of the myometrium that contain collagen, fibronectin, and proteoglycan. The collagen fibrils are abnormally formed and in disarray; they look like the collagen found in keloids.1 Although the precise causes of fibroids are unknown, hormonal, genetic, and growth factors appear to be involved in their development and growth.2,3
About 40% of fibroids are chromosomally abnormal; the remaining 60% may have undetected mutations. More than 100 genes have been found to be up-regulated or down-regulated in fibroid cells. Many of these genes appear to regulate cell growth, differentiation, proliferation, and mitogenesis.
- A myoma is benign tumor of the myometrium
- In a premenopausal woman, rapid uterine growth almost never indicates the presence of uterine sarcoma
- In an older woman who experiences uterine growth, abdominal pain, and irregular vaginal bleeding, pelvic malignancy may be suspected; an increased level of LDH isoenzyme 3 with increased gadolinium uptake on MRI within 40 to 60 seconds suggests a diagnosis of leiomyosarcoma
- Most fibroids have no impact on fertility, but submucosal fibroids that distort the uterine cavity decrease fertility; removing them increases fertility
- Location, size, number, and extent of myoma penetration into the myometrium can be evaluated by pelvic MRI, with coronal, axial, and sagittal images without gadolinium contrast
- Given the risks associated with surgery and the lack of proof of efficacy, myomectomy to improve fertility should be undertaken with caution
- Most myomas do not grow during pregnancy. Unfavorable pregnancy outcomes are very rare in women with myomas.
- Oral contraceptives and postmenopausal hormone therapy almost never influence fibroid growth. Women with fibroids can usually use these therapies safely.
Differentiating benign myoma from uterine sarcoma
Myomas have chromosomal rearrangements similar to other benign lesions, whereas leiomyosarcomas are undifferentiated and have complex chromosomal rearrangements not seen in myomas.4 Genetic differences between myomas and leiomyosarcomas indicate they most likely have distinct origins, and that leiomyosarcomas do not result from malignant degeneration of myomas.2
In premenopausal women, rapid uterine growth almost never indicates uterine sarcoma: One study found only one sarcoma among 371 (0.26%) women operated on for rapid growth of a presumed myoma, and no sarcomas were found in the 198 women who had a 6-week-pregnancy-equivalent increase in uterine size over 1 year.5
Clinical indications. The clinical signs that would lead to suspicion of pelvic malignancy are:
- older age
- abdominal pain
- irregular vaginal bleeding.6
The average age of 2,098 women with uterine sarcoma reported in the SEER (Surveillance Epidemiology and End Results) cancer database from 1989 to 1999 was 63 years, whereas a review of the literature found a mean age of 36 years in women subjected to myomectomy who did not have sarcoma.3,7
Diagnostic tests. The distinction between benign myoma and leiomyosarcoma need not be based on clinical signs alone. Preoperative diagnosis of leiomyosarcoma may be possible, using laboratory values of total serum lactate dehydrogenase (LDH) and LDH isoenzyme 3 plus gadolinium-enhanced magnetic resonance imaging (MRI) scan (Gd-DTPA), with initial images taken 40 to 60 seconds after injection of gadolinium. A study of 87 women with fibroids, 10 women with leiomyosarcoma, and 130 women with degenerating fibroids reported 100% specificity, 100% positive predictive value, 100% negative predictive value, and 100% diagnostic accuracy for leiomyosarcoma with this combination diagnostic procedure.8
CASE 1 RESOLVED What advice for Mrs. G.?
You order a gadolinium-enhanced, dynamic MRI scan and have blood drawn for total LDH and LDH isoenzymes. Total LDH and isoenzyme 3 are normal. MRI shows no increased enhancement of the fibroids on images taken 40 to 60 seconds after injection of gadolinium. You advise the patient that there is no evidence of cancer (sarcoma) and no urgent need for hysterectomy. You also tell her that, because she is symptomatic, myomectomy is an option that will preserve her uterus.
CASE 2 Fibroids, and contemplating childbearing
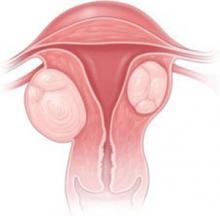
Mrs. H., a 35-year-old nulligravida, comes to the office for her first visit with you. She has no complaints, but your pelvic examination reveals a 10 week-size enlarged uterus. A sonogram shows a 6-cm subserosal fibroid and a 3-cm intramural fibroid near the endometrial cavity (see FIGURE). This patient was recently married and wants to become pregnant within the coming year.
When you tell Mrs. H. that she has fibroids, she grows concerned. She asks: “Will this affect my fertility, or a pregnancy?” and “Do I need surgery?”
You explain that the larger, subserosal fibroid is not a concern. The smaller, intramural fibroid could, however, have an impact on fertility and pregnancy if it distorts the uterine cavity.
To find out if that is the case, you order a saline infusion sonogram, which demonstrates, clearly, a normal cavity without distortion by the fibroid.
What is your advice to this woman?
Submucous fibroids that distort the uterine cavity decrease fertility; removal increases fertility. Otherwise, neither intramural nor subserosal fibroids appear to affect the fertility rate; removal has not been shown to increase fertility. Meta-analysis of 11 studies found that submucous myomas that distort the uterine cavity appear to decrease the pregnancy rate by 70% (relative risk [RR], 0.32; confidence interval [CI], 0.13–0.70).9
Assessing the uterine cavity. Evaluating a woman with fibroids for fertility requires reliable assessment of the uterine cavity. Hysteroscopy and saline infusion sonography have been shown to be far superior to transvaginal sonography or hysterosalpingography for detecting submucosal fibroids.10
The best modality for determining the extent of submucosal penetration of a myoma into the myometrium is MRI. It is also an excellent modality for evaluating the size, position, and number of multiple fibroids.11 The drawback to using MRI? It is more costly than other modalities.
Note: Classification of submucosal fibroids is based on the fraction of the mass within the cavity:
- Class 0 myomas are entirely intracavitary
- Class I myomas have 50% or more of the fibroid within the cavity
- Class II myomas have less than 50% within the cavity.12
Resection and fertility. Submucous fibroids can often be removed hysteroscopically. Systematic review of the evidence found that resection-restored fertility is equal to that of infertile controls undergoing in vitro fertilization who do not have fibroids (RR, 1.72; CI, 1.13–2.58).9 In that review, the presence of neither intramural nor subserosal fibroids decreased fertility (intramural: RR, 0.94, and CI, 0.73–1.20; subserosal: RR, 1.1, and CI, 0.06–1.72). Furthermore, removal of intramural and subserosal myomas by abdominal or laparoscopic myomectomy did not improve fertility. An updated unpublished meta-analysis, including studies published after 2001, came to the same conclusion (Pritts E, personal communication, 2008).
CASE 2: Subserosal, intramural myomas cause concern
In a woman contemplating childbearing, does a subserosal (left) or intramural (right) myoma present a problem because of potential to distort the uterine cavity?
Fibroids and pregnancy
The incidence of sonographically detected fibroids during pregnancy is low.13 Among 12,600 women at a prenatal clinic, routine second-trimester sonography identified myomas in 183 (mean age, 33 years)—an incidence of 1.5%.
Pregnancy has a variable and unpredictable effect on myoma growth, likely dependent on individual differences in genetics, circulating growth factors, and myoma-localized receptors. Most myomas do not, however, grow during pregnancy. A prospective study of pregnant women who had a single myoma found that 69% had no increase in volume throughout their pregnancy. In women who were noted to have an increase in the volume of their myoma, the greatest growth occurred before 10 weeks’ gestation. No relationship was found between initial myoma volume and myoma growth during the gestational period. 14
Do myomas complicate pregnancy?
Very rarely. Two studies reported on outcomes in large populations of pregnant women who were examined with routine second-trimester ultrasonography, with follow-up and delivery at the same institution.
In one of those studies, 12,600 pregnant women were evaluated, and the outcome in 167 women who were given a diagnosis of myoma was compared with the outcome in women who did not have a myoma.15 Despite similar clinical management between the two groups, no significant differences were seen in regard to the incidence of:
- preterm delivery
- premature rupture of membranes
- fetal growth restriction
- placenta previa
- placental abruption
- postpartum hemorrhage
- retained placenta.
Only cesarean section was more common among women with fibroids (23% vs 12%).
The second study reviewed 15,104 pregnancies and compared 401 women found to have myomas and the remaining women who did not.16 Although the presence of myoma did not increase the risk of premature rupture of membranes, operative vaginal delivery, chorioamnionitis, or endomyometritis, there was some increased risk of pre-term delivery (19.2% vs 12.7%), placenta previa (3.5% vs 1.8%), and post-partum hemorrhage (8.3% vs 2.9%). Cesarean section was, again, more common (49.1% vs 21.4%).
Do myomas injure the fetus?
Fetal injury as a consequence of fibroids has been reported very infrequently. A review of the literature from 1980 to 2005 revealed only four cases—one each of:
- fetal head anomalies with fetal growth restriction
- postural deformity
- limb reduction
- fetal head deformation with torticollis.17-19
CASE 2 RESOLVED Should Mrs. H. have a myomectomy?
Probably not. Abdominal and laparoscopic myomectomy involve substantial operative and anesthetic risks, including infection, postoperative adhesions, a very small risk of uterine rupture during pregnancy, and increased likelihood of cesarean section. Costs are also substantial, involving not only the expense of surgery, but also patient discomfort and time for recovery. Therefore, until it is proved that intramural myomas decrease fertility and myomectomy increases fertility, surgery should be undertaken with caution. As far as the effects of myoma on pregnancy are concerned, no data are available by which to compare pregnancy outcomes following myomectomy with pregnancy outcomes in women whose myomas are untreated. Randomized studies are needed to clarify these important issues.
CASES 3 & 4 The effects of oral contraceptives and hormone replacement therapy
Mrs. J. is a 32-year-old G0P0 woman who has a 5-cm fundal myoma. She is sexually active and wants to use an oral contraceptive (OC). She has heard from friends, however, that taking an OC makes fibroids grow, and she asks for your advice.
The same day, you see Mrs. K., a 54-year-old, recently menopausal woman. She complains of severe hot flashes and night sweats that disturb her sleep. She has had asymptomatic uterine fibroids for about 10 years and, although she would like to take menopausal hormone therapy, she is worried that the medication will make the fibroids larger.
How do you advise these two women?
OCs. OCs do not appear to influence the growth of fibroids. One study found a slightly increased risk of fibroids, another study found no increased risk, and a third found a decreased risk.18,19 These studies are retrospective, however, and may be marked by selection bias.
Postmenopausal hormone replacement therapy. Postmenopausal hormone therapy does not ordinarily cause fibroid growth. After 3 years, only three of 34 (8%) post-menopausal women who had fibroids and were treated with 0.625 mg of conjugated equine estrogen (CEE) and 5 mg of medroxyprogesterone acetate (MPA) a day had any increase in the size of fibroids.20 If any increase in the size of the uterus is noted, it is likely related to progestins.
A study found that 23% of women taking oral estrogen plus 2.5 mg of MPA a day for 1 year had a slight increase in the size of fibroids, whereas 50% of women taking 5 mg of MPA had an increase in size (mean increase in diameter, 3.2 cm).21 Transdermal estrogen plus oral MPA was shown, after 1 year, to cause, on average, a 0.5-cm increase in the diameter of fibroids; oral estrogen and MPA caused no increase in size.22
CASES RESOLVED Rx: Reassurance
Advise Mrs. J. that taking an OC is unlikely to make her fibroids grow larger.
Mrs. K., who is older, can seek relief from postmenopausal symptoms by taking hormone therapy without fear of her fibroids being stimulated to grow.
1. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900-906.
2. Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037-1054.
3. Parker W. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725-736.
4. Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97-108.
5. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
6. Boutselis JG, Ullery JC. Sarcoma of the uterus. Obstet Gynecol. 1962;20:23-35.
7. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204-208.
8. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12:354-361.
9. Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483-491.
10. Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76:350-357.
11. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:388-403.
12. Cohen LS, Valle RF. Role of vaginal sonography and hysterosonography in the endoscopic treatment of uterine myomas. Fertil Steril. 2000;73:197-204.
13. Cooper NP, Okolo S. Fibroids in pregnancy—common but poorly understood. Obstet Gynecol Surv. 2005;60:132-138.
14. Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med. 1992;11:511-515.
15. Vergani P, Ghidini A, Strobelt N, et al. Do uterine leiomyomas influence pregnancy outcome? Am J Perinatol. 1994;11:356-358.
16. Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376-382.
17. Joo JG, Inovay J, Silhavy M, Papp Z. Successful enucleation of a necrotizing fibroid causing oligohydramnios and fetal postural deformity in the 25th week of gestation. A case report. J Reprod Med. 2001;46:923-925.
18. Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:359-362.
19. Ratner H. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:1027.-
20. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas. 2002;43:35-39.
21. Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2002;102:199-201.
22. Sener AB, Seçkin NC, Ozmen S, Gökmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354-357.
The author reports no financial relationships relevant to this article.
CASE 1 Rapid growth=cancer?
Mrs. G., 47 years old, has had uterine fibroids the size of a 12-week pregnancy for about 6 years. At today’s examination, however, her uterus feels about the size of a 16-week pregnancy.
She is aware that her abdomen is bigger, and she complains of some abdominal pressure and urinary frequency. She reports no abnormal bleeding and no abdominal pain.
Mrs. G. is upset because another physician told her she might have cancer and needs a hysterectomy immediately. She tells you that she does not want a hysterectomy unless “it’s absolutely necessary.”
Ultrasonography at this visit reveals that two of the three fibroids noted on a previous sonogram have grown—one from 6 cm to 9 cm in diameter; the other from 5 cm to 8 cm.
What do you tell Mrs. G.?
Understanding myomas
Uterine fibroids, also called myomas, are benign, monoclonal tumors of the myometrium that contain collagen, fibronectin, and proteoglycan. The collagen fibrils are abnormally formed and in disarray; they look like the collagen found in keloids.1 Although the precise causes of fibroids are unknown, hormonal, genetic, and growth factors appear to be involved in their development and growth.2,3
About 40% of fibroids are chromosomally abnormal; the remaining 60% may have undetected mutations. More than 100 genes have been found to be up-regulated or down-regulated in fibroid cells. Many of these genes appear to regulate cell growth, differentiation, proliferation, and mitogenesis.
- A myoma is benign tumor of the myometrium
- In a premenopausal woman, rapid uterine growth almost never indicates the presence of uterine sarcoma
- In an older woman who experiences uterine growth, abdominal pain, and irregular vaginal bleeding, pelvic malignancy may be suspected; an increased level of LDH isoenzyme 3 with increased gadolinium uptake on MRI within 40 to 60 seconds suggests a diagnosis of leiomyosarcoma
- Most fibroids have no impact on fertility, but submucosal fibroids that distort the uterine cavity decrease fertility; removing them increases fertility
- Location, size, number, and extent of myoma penetration into the myometrium can be evaluated by pelvic MRI, with coronal, axial, and sagittal images without gadolinium contrast
- Given the risks associated with surgery and the lack of proof of efficacy, myomectomy to improve fertility should be undertaken with caution
- Most myomas do not grow during pregnancy. Unfavorable pregnancy outcomes are very rare in women with myomas.
- Oral contraceptives and postmenopausal hormone therapy almost never influence fibroid growth. Women with fibroids can usually use these therapies safely.
Differentiating benign myoma from uterine sarcoma
Myomas have chromosomal rearrangements similar to other benign lesions, whereas leiomyosarcomas are undifferentiated and have complex chromosomal rearrangements not seen in myomas.4 Genetic differences between myomas and leiomyosarcomas indicate they most likely have distinct origins, and that leiomyosarcomas do not result from malignant degeneration of myomas.2
In premenopausal women, rapid uterine growth almost never indicates uterine sarcoma: One study found only one sarcoma among 371 (0.26%) women operated on for rapid growth of a presumed myoma, and no sarcomas were found in the 198 women who had a 6-week-pregnancy-equivalent increase in uterine size over 1 year.5
Clinical indications. The clinical signs that would lead to suspicion of pelvic malignancy are:
- older age
- abdominal pain
- irregular vaginal bleeding.6
The average age of 2,098 women with uterine sarcoma reported in the SEER (Surveillance Epidemiology and End Results) cancer database from 1989 to 1999 was 63 years, whereas a review of the literature found a mean age of 36 years in women subjected to myomectomy who did not have sarcoma.3,7
Diagnostic tests. The distinction between benign myoma and leiomyosarcoma need not be based on clinical signs alone. Preoperative diagnosis of leiomyosarcoma may be possible, using laboratory values of total serum lactate dehydrogenase (LDH) and LDH isoenzyme 3 plus gadolinium-enhanced magnetic resonance imaging (MRI) scan (Gd-DTPA), with initial images taken 40 to 60 seconds after injection of gadolinium. A study of 87 women with fibroids, 10 women with leiomyosarcoma, and 130 women with degenerating fibroids reported 100% specificity, 100% positive predictive value, 100% negative predictive value, and 100% diagnostic accuracy for leiomyosarcoma with this combination diagnostic procedure.8
CASE 1 RESOLVED What advice for Mrs. G.?
You order a gadolinium-enhanced, dynamic MRI scan and have blood drawn for total LDH and LDH isoenzymes. Total LDH and isoenzyme 3 are normal. MRI shows no increased enhancement of the fibroids on images taken 40 to 60 seconds after injection of gadolinium. You advise the patient that there is no evidence of cancer (sarcoma) and no urgent need for hysterectomy. You also tell her that, because she is symptomatic, myomectomy is an option that will preserve her uterus.
CASE 2 Fibroids, and contemplating childbearing
Mrs. H., a 35-year-old nulligravida, comes to the office for her first visit with you. She has no complaints, but your pelvic examination reveals a 10 week-size enlarged uterus. A sonogram shows a 6-cm subserosal fibroid and a 3-cm intramural fibroid near the endometrial cavity (see FIGURE). This patient was recently married and wants to become pregnant within the coming year.
When you tell Mrs. H. that she has fibroids, she grows concerned. She asks: “Will this affect my fertility, or a pregnancy?” and “Do I need surgery?”
You explain that the larger, subserosal fibroid is not a concern. The smaller, intramural fibroid could, however, have an impact on fertility and pregnancy if it distorts the uterine cavity.
To find out if that is the case, you order a saline infusion sonogram, which demonstrates, clearly, a normal cavity without distortion by the fibroid.
What is your advice to this woman?
Submucous fibroids that distort the uterine cavity decrease fertility; removal increases fertility. Otherwise, neither intramural nor subserosal fibroids appear to affect the fertility rate; removal has not been shown to increase fertility. Meta-analysis of 11 studies found that submucous myomas that distort the uterine cavity appear to decrease the pregnancy rate by 70% (relative risk [RR], 0.32; confidence interval [CI], 0.13–0.70).9
Assessing the uterine cavity. Evaluating a woman with fibroids for fertility requires reliable assessment of the uterine cavity. Hysteroscopy and saline infusion sonography have been shown to be far superior to transvaginal sonography or hysterosalpingography for detecting submucosal fibroids.10
The best modality for determining the extent of submucosal penetration of a myoma into the myometrium is MRI. It is also an excellent modality for evaluating the size, position, and number of multiple fibroids.11 The drawback to using MRI? It is more costly than other modalities.
Note: Classification of submucosal fibroids is based on the fraction of the mass within the cavity:
- Class 0 myomas are entirely intracavitary
- Class I myomas have 50% or more of the fibroid within the cavity
- Class II myomas have less than 50% within the cavity.12
Resection and fertility. Submucous fibroids can often be removed hysteroscopically. Systematic review of the evidence found that resection-restored fertility is equal to that of infertile controls undergoing in vitro fertilization who do not have fibroids (RR, 1.72; CI, 1.13–2.58).9 In that review, the presence of neither intramural nor subserosal fibroids decreased fertility (intramural: RR, 0.94, and CI, 0.73–1.20; subserosal: RR, 1.1, and CI, 0.06–1.72). Furthermore, removal of intramural and subserosal myomas by abdominal or laparoscopic myomectomy did not improve fertility. An updated unpublished meta-analysis, including studies published after 2001, came to the same conclusion (Pritts E, personal communication, 2008).
CASE 2: Subserosal, intramural myomas cause concern
In a woman contemplating childbearing, does a subserosal (left) or intramural (right) myoma present a problem because of potential to distort the uterine cavity?
Fibroids and pregnancy
The incidence of sonographically detected fibroids during pregnancy is low.13 Among 12,600 women at a prenatal clinic, routine second-trimester sonography identified myomas in 183 (mean age, 33 years)—an incidence of 1.5%.
Pregnancy has a variable and unpredictable effect on myoma growth, likely dependent on individual differences in genetics, circulating growth factors, and myoma-localized receptors. Most myomas do not, however, grow during pregnancy. A prospective study of pregnant women who had a single myoma found that 69% had no increase in volume throughout their pregnancy. In women who were noted to have an increase in the volume of their myoma, the greatest growth occurred before 10 weeks’ gestation. No relationship was found between initial myoma volume and myoma growth during the gestational period. 14
Do myomas complicate pregnancy?
Very rarely. Two studies reported on outcomes in large populations of pregnant women who were examined with routine second-trimester ultrasonography, with follow-up and delivery at the same institution.
In one of those studies, 12,600 pregnant women were evaluated, and the outcome in 167 women who were given a diagnosis of myoma was compared with the outcome in women who did not have a myoma.15 Despite similar clinical management between the two groups, no significant differences were seen in regard to the incidence of:
- preterm delivery
- premature rupture of membranes
- fetal growth restriction
- placenta previa
- placental abruption
- postpartum hemorrhage
- retained placenta.
Only cesarean section was more common among women with fibroids (23% vs 12%).
The second study reviewed 15,104 pregnancies and compared 401 women found to have myomas and the remaining women who did not.16 Although the presence of myoma did not increase the risk of premature rupture of membranes, operative vaginal delivery, chorioamnionitis, or endomyometritis, there was some increased risk of pre-term delivery (19.2% vs 12.7%), placenta previa (3.5% vs 1.8%), and post-partum hemorrhage (8.3% vs 2.9%). Cesarean section was, again, more common (49.1% vs 21.4%).
Do myomas injure the fetus?
Fetal injury as a consequence of fibroids has been reported very infrequently. A review of the literature from 1980 to 2005 revealed only four cases—one each of:
- fetal head anomalies with fetal growth restriction
- postural deformity
- limb reduction
- fetal head deformation with torticollis.17-19
CASE 2 RESOLVED Should Mrs. H. have a myomectomy?
Probably not. Abdominal and laparoscopic myomectomy involve substantial operative and anesthetic risks, including infection, postoperative adhesions, a very small risk of uterine rupture during pregnancy, and increased likelihood of cesarean section. Costs are also substantial, involving not only the expense of surgery, but also patient discomfort and time for recovery. Therefore, until it is proved that intramural myomas decrease fertility and myomectomy increases fertility, surgery should be undertaken with caution. As far as the effects of myoma on pregnancy are concerned, no data are available by which to compare pregnancy outcomes following myomectomy with pregnancy outcomes in women whose myomas are untreated. Randomized studies are needed to clarify these important issues.
CASES 3 & 4 The effects of oral contraceptives and hormone replacement therapy
Mrs. J. is a 32-year-old G0P0 woman who has a 5-cm fundal myoma. She is sexually active and wants to use an oral contraceptive (OC). She has heard from friends, however, that taking an OC makes fibroids grow, and she asks for your advice.
The same day, you see Mrs. K., a 54-year-old, recently menopausal woman. She complains of severe hot flashes and night sweats that disturb her sleep. She has had asymptomatic uterine fibroids for about 10 years and, although she would like to take menopausal hormone therapy, she is worried that the medication will make the fibroids larger.
How do you advise these two women?
OCs. OCs do not appear to influence the growth of fibroids. One study found a slightly increased risk of fibroids, another study found no increased risk, and a third found a decreased risk.18,19 These studies are retrospective, however, and may be marked by selection bias.
Postmenopausal hormone replacement therapy. Postmenopausal hormone therapy does not ordinarily cause fibroid growth. After 3 years, only three of 34 (8%) post-menopausal women who had fibroids and were treated with 0.625 mg of conjugated equine estrogen (CEE) and 5 mg of medroxyprogesterone acetate (MPA) a day had any increase in the size of fibroids.20 If any increase in the size of the uterus is noted, it is likely related to progestins.
A study found that 23% of women taking oral estrogen plus 2.5 mg of MPA a day for 1 year had a slight increase in the size of fibroids, whereas 50% of women taking 5 mg of MPA had an increase in size (mean increase in diameter, 3.2 cm).21 Transdermal estrogen plus oral MPA was shown, after 1 year, to cause, on average, a 0.5-cm increase in the diameter of fibroids; oral estrogen and MPA caused no increase in size.22
CASES RESOLVED Rx: Reassurance
Advise Mrs. J. that taking an OC is unlikely to make her fibroids grow larger.
Mrs. K., who is older, can seek relief from postmenopausal symptoms by taking hormone therapy without fear of her fibroids being stimulated to grow.
The author reports no financial relationships relevant to this article.
CASE 1 Rapid growth=cancer?
Mrs. G., 47 years old, has had uterine fibroids the size of a 12-week pregnancy for about 6 years. At today’s examination, however, her uterus feels about the size of a 16-week pregnancy.
She is aware that her abdomen is bigger, and she complains of some abdominal pressure and urinary frequency. She reports no abnormal bleeding and no abdominal pain.
Mrs. G. is upset because another physician told her she might have cancer and needs a hysterectomy immediately. She tells you that she does not want a hysterectomy unless “it’s absolutely necessary.”
Ultrasonography at this visit reveals that two of the three fibroids noted on a previous sonogram have grown—one from 6 cm to 9 cm in diameter; the other from 5 cm to 8 cm.
What do you tell Mrs. G.?
Understanding myomas
Uterine fibroids, also called myomas, are benign, monoclonal tumors of the myometrium that contain collagen, fibronectin, and proteoglycan. The collagen fibrils are abnormally formed and in disarray; they look like the collagen found in keloids.1 Although the precise causes of fibroids are unknown, hormonal, genetic, and growth factors appear to be involved in their development and growth.2,3
About 40% of fibroids are chromosomally abnormal; the remaining 60% may have undetected mutations. More than 100 genes have been found to be up-regulated or down-regulated in fibroid cells. Many of these genes appear to regulate cell growth, differentiation, proliferation, and mitogenesis.
- A myoma is benign tumor of the myometrium
- In a premenopausal woman, rapid uterine growth almost never indicates the presence of uterine sarcoma
- In an older woman who experiences uterine growth, abdominal pain, and irregular vaginal bleeding, pelvic malignancy may be suspected; an increased level of LDH isoenzyme 3 with increased gadolinium uptake on MRI within 40 to 60 seconds suggests a diagnosis of leiomyosarcoma
- Most fibroids have no impact on fertility, but submucosal fibroids that distort the uterine cavity decrease fertility; removing them increases fertility
- Location, size, number, and extent of myoma penetration into the myometrium can be evaluated by pelvic MRI, with coronal, axial, and sagittal images without gadolinium contrast
- Given the risks associated with surgery and the lack of proof of efficacy, myomectomy to improve fertility should be undertaken with caution
- Most myomas do not grow during pregnancy. Unfavorable pregnancy outcomes are very rare in women with myomas.
- Oral contraceptives and postmenopausal hormone therapy almost never influence fibroid growth. Women with fibroids can usually use these therapies safely.
Differentiating benign myoma from uterine sarcoma
Myomas have chromosomal rearrangements similar to other benign lesions, whereas leiomyosarcomas are undifferentiated and have complex chromosomal rearrangements not seen in myomas.4 Genetic differences between myomas and leiomyosarcomas indicate they most likely have distinct origins, and that leiomyosarcomas do not result from malignant degeneration of myomas.2
In premenopausal women, rapid uterine growth almost never indicates uterine sarcoma: One study found only one sarcoma among 371 (0.26%) women operated on for rapid growth of a presumed myoma, and no sarcomas were found in the 198 women who had a 6-week-pregnancy-equivalent increase in uterine size over 1 year.5
Clinical indications. The clinical signs that would lead to suspicion of pelvic malignancy are:
- older age
- abdominal pain
- irregular vaginal bleeding.6
The average age of 2,098 women with uterine sarcoma reported in the SEER (Surveillance Epidemiology and End Results) cancer database from 1989 to 1999 was 63 years, whereas a review of the literature found a mean age of 36 years in women subjected to myomectomy who did not have sarcoma.3,7
Diagnostic tests. The distinction between benign myoma and leiomyosarcoma need not be based on clinical signs alone. Preoperative diagnosis of leiomyosarcoma may be possible, using laboratory values of total serum lactate dehydrogenase (LDH) and LDH isoenzyme 3 plus gadolinium-enhanced magnetic resonance imaging (MRI) scan (Gd-DTPA), with initial images taken 40 to 60 seconds after injection of gadolinium. A study of 87 women with fibroids, 10 women with leiomyosarcoma, and 130 women with degenerating fibroids reported 100% specificity, 100% positive predictive value, 100% negative predictive value, and 100% diagnostic accuracy for leiomyosarcoma with this combination diagnostic procedure.8
CASE 1 RESOLVED What advice for Mrs. G.?
You order a gadolinium-enhanced, dynamic MRI scan and have blood drawn for total LDH and LDH isoenzymes. Total LDH and isoenzyme 3 are normal. MRI shows no increased enhancement of the fibroids on images taken 40 to 60 seconds after injection of gadolinium. You advise the patient that there is no evidence of cancer (sarcoma) and no urgent need for hysterectomy. You also tell her that, because she is symptomatic, myomectomy is an option that will preserve her uterus.
CASE 2 Fibroids, and contemplating childbearing
Mrs. H., a 35-year-old nulligravida, comes to the office for her first visit with you. She has no complaints, but your pelvic examination reveals a 10 week-size enlarged uterus. A sonogram shows a 6-cm subserosal fibroid and a 3-cm intramural fibroid near the endometrial cavity (see FIGURE). This patient was recently married and wants to become pregnant within the coming year.
When you tell Mrs. H. that she has fibroids, she grows concerned. She asks: “Will this affect my fertility, or a pregnancy?” and “Do I need surgery?”
You explain that the larger, subserosal fibroid is not a concern. The smaller, intramural fibroid could, however, have an impact on fertility and pregnancy if it distorts the uterine cavity.
To find out if that is the case, you order a saline infusion sonogram, which demonstrates, clearly, a normal cavity without distortion by the fibroid.
What is your advice to this woman?
Submucous fibroids that distort the uterine cavity decrease fertility; removal increases fertility. Otherwise, neither intramural nor subserosal fibroids appear to affect the fertility rate; removal has not been shown to increase fertility. Meta-analysis of 11 studies found that submucous myomas that distort the uterine cavity appear to decrease the pregnancy rate by 70% (relative risk [RR], 0.32; confidence interval [CI], 0.13–0.70).9
Assessing the uterine cavity. Evaluating a woman with fibroids for fertility requires reliable assessment of the uterine cavity. Hysteroscopy and saline infusion sonography have been shown to be far superior to transvaginal sonography or hysterosalpingography for detecting submucosal fibroids.10
The best modality for determining the extent of submucosal penetration of a myoma into the myometrium is MRI. It is also an excellent modality for evaluating the size, position, and number of multiple fibroids.11 The drawback to using MRI? It is more costly than other modalities.
Note: Classification of submucosal fibroids is based on the fraction of the mass within the cavity:
- Class 0 myomas are entirely intracavitary
- Class I myomas have 50% or more of the fibroid within the cavity
- Class II myomas have less than 50% within the cavity.12
Resection and fertility. Submucous fibroids can often be removed hysteroscopically. Systematic review of the evidence found that resection-restored fertility is equal to that of infertile controls undergoing in vitro fertilization who do not have fibroids (RR, 1.72; CI, 1.13–2.58).9 In that review, the presence of neither intramural nor subserosal fibroids decreased fertility (intramural: RR, 0.94, and CI, 0.73–1.20; subserosal: RR, 1.1, and CI, 0.06–1.72). Furthermore, removal of intramural and subserosal myomas by abdominal or laparoscopic myomectomy did not improve fertility. An updated unpublished meta-analysis, including studies published after 2001, came to the same conclusion (Pritts E, personal communication, 2008).
CASE 2: Subserosal, intramural myomas cause concern
In a woman contemplating childbearing, does a subserosal (left) or intramural (right) myoma present a problem because of potential to distort the uterine cavity?
Fibroids and pregnancy
The incidence of sonographically detected fibroids during pregnancy is low.13 Among 12,600 women at a prenatal clinic, routine second-trimester sonography identified myomas in 183 (mean age, 33 years)—an incidence of 1.5%.
Pregnancy has a variable and unpredictable effect on myoma growth, likely dependent on individual differences in genetics, circulating growth factors, and myoma-localized receptors. Most myomas do not, however, grow during pregnancy. A prospective study of pregnant women who had a single myoma found that 69% had no increase in volume throughout their pregnancy. In women who were noted to have an increase in the volume of their myoma, the greatest growth occurred before 10 weeks’ gestation. No relationship was found between initial myoma volume and myoma growth during the gestational period. 14
Do myomas complicate pregnancy?
Very rarely. Two studies reported on outcomes in large populations of pregnant women who were examined with routine second-trimester ultrasonography, with follow-up and delivery at the same institution.
In one of those studies, 12,600 pregnant women were evaluated, and the outcome in 167 women who were given a diagnosis of myoma was compared with the outcome in women who did not have a myoma.15 Despite similar clinical management between the two groups, no significant differences were seen in regard to the incidence of:
- preterm delivery
- premature rupture of membranes
- fetal growth restriction
- placenta previa
- placental abruption
- postpartum hemorrhage
- retained placenta.
Only cesarean section was more common among women with fibroids (23% vs 12%).
The second study reviewed 15,104 pregnancies and compared 401 women found to have myomas and the remaining women who did not.16 Although the presence of myoma did not increase the risk of premature rupture of membranes, operative vaginal delivery, chorioamnionitis, or endomyometritis, there was some increased risk of pre-term delivery (19.2% vs 12.7%), placenta previa (3.5% vs 1.8%), and post-partum hemorrhage (8.3% vs 2.9%). Cesarean section was, again, more common (49.1% vs 21.4%).
Do myomas injure the fetus?
Fetal injury as a consequence of fibroids has been reported very infrequently. A review of the literature from 1980 to 2005 revealed only four cases—one each of:
- fetal head anomalies with fetal growth restriction
- postural deformity
- limb reduction
- fetal head deformation with torticollis.17-19
CASE 2 RESOLVED Should Mrs. H. have a myomectomy?
Probably not. Abdominal and laparoscopic myomectomy involve substantial operative and anesthetic risks, including infection, postoperative adhesions, a very small risk of uterine rupture during pregnancy, and increased likelihood of cesarean section. Costs are also substantial, involving not only the expense of surgery, but also patient discomfort and time for recovery. Therefore, until it is proved that intramural myomas decrease fertility and myomectomy increases fertility, surgery should be undertaken with caution. As far as the effects of myoma on pregnancy are concerned, no data are available by which to compare pregnancy outcomes following myomectomy with pregnancy outcomes in women whose myomas are untreated. Randomized studies are needed to clarify these important issues.
CASES 3 & 4 The effects of oral contraceptives and hormone replacement therapy
Mrs. J. is a 32-year-old G0P0 woman who has a 5-cm fundal myoma. She is sexually active and wants to use an oral contraceptive (OC). She has heard from friends, however, that taking an OC makes fibroids grow, and she asks for your advice.
The same day, you see Mrs. K., a 54-year-old, recently menopausal woman. She complains of severe hot flashes and night sweats that disturb her sleep. She has had asymptomatic uterine fibroids for about 10 years and, although she would like to take menopausal hormone therapy, she is worried that the medication will make the fibroids larger.
How do you advise these two women?
OCs. OCs do not appear to influence the growth of fibroids. One study found a slightly increased risk of fibroids, another study found no increased risk, and a third found a decreased risk.18,19 These studies are retrospective, however, and may be marked by selection bias.
Postmenopausal hormone replacement therapy. Postmenopausal hormone therapy does not ordinarily cause fibroid growth. After 3 years, only three of 34 (8%) post-menopausal women who had fibroids and were treated with 0.625 mg of conjugated equine estrogen (CEE) and 5 mg of medroxyprogesterone acetate (MPA) a day had any increase in the size of fibroids.20 If any increase in the size of the uterus is noted, it is likely related to progestins.
A study found that 23% of women taking oral estrogen plus 2.5 mg of MPA a day for 1 year had a slight increase in the size of fibroids, whereas 50% of women taking 5 mg of MPA had an increase in size (mean increase in diameter, 3.2 cm).21 Transdermal estrogen plus oral MPA was shown, after 1 year, to cause, on average, a 0.5-cm increase in the diameter of fibroids; oral estrogen and MPA caused no increase in size.22
CASES RESOLVED Rx: Reassurance
Advise Mrs. J. that taking an OC is unlikely to make her fibroids grow larger.
Mrs. K., who is older, can seek relief from postmenopausal symptoms by taking hormone therapy without fear of her fibroids being stimulated to grow.
1. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900-906.
2. Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037-1054.
3. Parker W. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725-736.
4. Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97-108.
5. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
6. Boutselis JG, Ullery JC. Sarcoma of the uterus. Obstet Gynecol. 1962;20:23-35.
7. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204-208.
8. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12:354-361.
9. Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483-491.
10. Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76:350-357.
11. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:388-403.
12. Cohen LS, Valle RF. Role of vaginal sonography and hysterosonography in the endoscopic treatment of uterine myomas. Fertil Steril. 2000;73:197-204.
13. Cooper NP, Okolo S. Fibroids in pregnancy—common but poorly understood. Obstet Gynecol Surv. 2005;60:132-138.
14. Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med. 1992;11:511-515.
15. Vergani P, Ghidini A, Strobelt N, et al. Do uterine leiomyomas influence pregnancy outcome? Am J Perinatol. 1994;11:356-358.
16. Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376-382.
17. Joo JG, Inovay J, Silhavy M, Papp Z. Successful enucleation of a necrotizing fibroid causing oligohydramnios and fetal postural deformity in the 25th week of gestation. A case report. J Reprod Med. 2001;46:923-925.
18. Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:359-362.
19. Ratner H. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:1027.-
20. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas. 2002;43:35-39.
21. Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2002;102:199-201.
22. Sener AB, Seçkin NC, Ozmen S, Gökmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354-357.
1. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900-906.
2. Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037-1054.
3. Parker W. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725-736.
4. Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97-108.
5. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
6. Boutselis JG, Ullery JC. Sarcoma of the uterus. Obstet Gynecol. 1962;20:23-35.
7. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204-208.
8. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12:354-361.
9. Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483-491.
10. Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76:350-357.
11. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:388-403.
12. Cohen LS, Valle RF. Role of vaginal sonography and hysterosonography in the endoscopic treatment of uterine myomas. Fertil Steril. 2000;73:197-204.
13. Cooper NP, Okolo S. Fibroids in pregnancy—common but poorly understood. Obstet Gynecol Surv. 2005;60:132-138.
14. Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med. 1992;11:511-515.
15. Vergani P, Ghidini A, Strobelt N, et al. Do uterine leiomyomas influence pregnancy outcome? Am J Perinatol. 1994;11:356-358.
16. Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376-382.
17. Joo JG, Inovay J, Silhavy M, Papp Z. Successful enucleation of a necrotizing fibroid causing oligohydramnios and fetal postural deformity in the 25th week of gestation. A case report. J Reprod Med. 2001;46:923-925.
18. Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:359-362.
19. Ratner H. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:1027.-
20. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas. 2002;43:35-39.
21. Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2002;102:199-201.
22. Sener AB, Seçkin NC, Ozmen S, Gökmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354-357.
IN THIS ARTICLE
“Doctor, I want a C-section.” How should you respond?
In general, when a patient inquires about elective primary C-section, it is best to consider the “6 C’s of elective cesarean” in a careful discussion with her. That approach entails consideration of the following:
- Clarification of her request
- Comorbidities in maternal health or surgical history
- number of Children planned overall
- clear Consent for the procedure
- Correct determination of gestational age at the time of planned delivery
- Confirmation of coverage by her insurance carrier.
One trend is clear: Maternal requests for primary cesarean delivery are on the rise in the United States, although we lack precise data on exactly how fast the rate is rising. Many experts estimate it to be 4% to 18%.1 In Brazil, the rate of elective C-section for women in private hospitals is thought to be as high as 80% to 90%.2
As more celebrities and other prominent figures undergo elective C-section, more American women are beginning to ask for the same “privilege.” In this article, I lay out an evidence-based and ethically sensitive approach to counseling patients who request C-section on an elective basis.
In 2004, the United States saw 4.1 million births, 18% of which—or nearly 750,000—involved primary C-section.13 However, it is difficult to discern how many of these primary C-sections were performed for nonobstetric, or elective, indications, because such data are not routinely collected.
Birth certificates are changing
Efforts to improve birth certificate data have begun. In 2003, the revised US Standard Certificate of Live Birth was adopted by seven states, allowing for a more detailed description of births. The new certificate provides for more robust information in several areas, including
- risk factors in the index pregnancy
- obstetric procedures performed
- characteristics of labor and delivery
- method of delivery
- normal conditions of the newborn
- congenital anomalies in the newborn.
It also specifies whether or not a trial of labor was attempted before cesarean delivery, but it is limited by the inclusion of breech presentation in the statistics.14
Data collection remains an inexact science
Even with the new birth certificate data, it remains difficult to accurately quantify the number of nonobstetrically indicated primary C-sections, although many experts have estimated the rate at 4% to 28%.1
The points raised in the list that begins this article are all discussed here.
The difficulty of calculating the rate of primary C-section
We are limited by terminology and data-collection practices, as well as a multitude of confounding obstetric factors. Practicing providers recognize the inherent difference between a planned C-section at term without the onset of labor and an unplanned C-section at term after the onset of labor—as well as every scenario in between.
Unplanned C-section can be performed to address fetal compromise or an unsuccessful attempt at vaginal delivery—each scenario replete with its own risks and potential complications. The urgency of C-section also confounds subsequent maternal and fetal complications. Underlying maternal factors such as obesity and medical and surgical history further complicate the scenario.
For these reasons, the discussion of elective C-section is best managed by limiting the parameters considered to the requested, scheduled, elective C-section at term without maternal or fetal indications. Most patients have this paradigm in mind when they make their request, even though physicians and midwives understand that this is the ideal and not generally the reality.
Medicolegal and ethical considerations
The ethical principles surrounding cesarean delivery upon maternal request balance on the tension between beneficence and patient autonomy. The former requires the promotion of the patient’s overall health and well-being, along with attention to the closely related dictum, primum non nocere, or “first do no harm.”
Patient autonomy requires respectful consideration of the patient and her world view when making a medical decision. The ethical principle of patient autonomy is usually understood as a right to decline medical intervention—not necessarily to demand dangerous or unproven intervention.1
This raises the question: Is a scheduled C-section in the absence of obstetric indications dangerous? Harmful? Imprudent? The medical community has accepted these inherent tensions in the field of aesthetic plastic surgery, but societies in obstetrics and gynecology continue to struggle with the ethical principles involved in maternal-choice cesarean.
FIGO: C-section for nonmedical reasons is not justified
The International Federation of Gynecology and Obstetrics (FIGO) Committee for the Ethical Aspects of Human Reproduction and Women’s Health bases its guidelines on the use of cesarean delivery for nonmedical reasons on the principles of beneficence and social justice. It concludes: “Cesarean section is a surgical intervention with potential hazards for both mother and child. It also uses more health-care resources than normal vaginal delivery…performing cesarean section for nonmedical reasons is ethically not justified.”3
ACOG: Individualize the decision consistent with ethical principles
The American College of Obstetricians and Gynecologists (ACOG), in a recent Committee Opinion, acknowledged the paucity of research data directly comparing cesarean delivery on maternal request with planned vaginal delivery. The document reviews the National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request (see below), which was convened in 2006, and notes the panel’s conclusion that the available body of evidence does not allow for a conclusive recommendation of one mode of delivery over another.4 The ACOG Committee Opinion states: “Any decision to perform a cesarean delivery on maternal request should be carefully individualized and consistent with ethical principles.”5
Different world views likely account for different conclusions
The difference in the FIGO and ACOG positions may arise from differences in cultural contexts between a general world health view and a highly patient-centered Western perspective. The former view bases the decision on universal good and the utilization of scarce health-care resources; the latter view recognizes the individual within an ethical context.
Both views acknowledge the limited data available to inform the decision. So what do the data say, and how can we help our patients understand it?
NIH State-of-the-Science Conference
In March of 2006, an independent panel of experts from a range of medical fields reviewed the scientific literature regarding cesarean delivery on maternal request at the NIH in Bethesda, Maryland. Although the panel found no Level I, or strong, evidence within the literature, it was able to characterize the risks and benefits of maternal-request C-section based on Level II (moderate), Level III (weak), and Level IV (absent) evidence.
Moderate evidence was scarce
From a maternal perspective, the panel found that “the frequency of postpartum hemorrhage associated with planned cesarean delivery is lower than that reported with the combination of planned vaginal delivery and unplanned cesarean delivery,”5 although hospital stay is longer than with vaginal delivery.
From a neonatal perspective, moderate evidence favors vaginal delivery because of a decreased incidence of respiratory morbidity, such as transient tachypnea of the newborn and respiratory distress syndrome. Respiratory morbidity is directly related to gestational age, and there is a risk of iatrogenic prematurity with scheduled C-section. The possibility of incorrect obstetric dating would seem to favor awaiting the spontaneous onset of labor at term and an attempt at vaginal delivery to reduce the risk of respiratory complications due to iatrogenic prematurity.
Weak evidence goes both ways
Weakly supported evidence favored both cesarean section and vaginal delivery for either the mother or fetus. Weak evidence favoring vaginal delivery for maternal interests included:
- decreased maternal infectious morbidity and anesthetic complications, compared with C-section
- greater ease establishing breastfeeding, due to logistical challenges surrounding mother–infant bonding after C-section
- greater freedom in planning family size because increasing numbers of repeat C-sections with subsequent pregnancies increase risk of uterine rupture, cesarean hysterectomy, and abnormal placentation.
- lower rate of postpartum stress urinary incontinence, compared with women undergoing vaginal delivery, in the short term
- lower risk of surgical morbidity and traumatic obstetric lacerations with elective C-section, compared with the injuries that can occur at the time of unscheduled C-section or vaginal delivery.
Weak evidence of neonatal benefit
From the neonatal perspective, the NIH Consensus Committee found weak evidence favoring C-section. A scheduled C-section protects the neonate from stillbirth arising from postdates intrauterine fetal demise, because, with elective cesarean, a pregnancy is not usually allowed to continue post-term.
The Committee also documented protection from intracranial hemorrhage, neonatal asphyxia, encephalopathy, birth injury, and neonatal infection with C-section, compared with vaginal delivery.5
The socioeconomic picture matters
From a socioeconomic standpoint, women who request C-section may have financial concerns such as the amount of time off from work that may be necessary for both themselves and their partners. The availability of family support may be relevant and improved if a specific time frame for delivery is anticipated.
In many cultures, “lucky days” exist, and women may have preferences or aspirations for their child to be born on one of them.
Last, although it may be more cost-effective for a patient to undergo vaginal delivery, we, as health-care providers, cannot predict who will be successful in that regard. A complicated labor that necessitates unscheduled, urgent, or emergent C-section costs more in health-care dollars than does a C-section without labor.
Canadian researchers in 2005 examined the hospital care costs over 18 years in 27,614 pregnancies associated with varying types of delivery and found that the cost of delivery was highest for a C-section performed after the onset of labor ($2,137). The lowest cost was for spontaneous vaginal delivery ($1,340), followed by C-section without labor ($1,532).6 Therefore, some could argue that the overall cost to the patient and system is lower with a scheduled cesarean delivery because it avoids the other possible comorbidities and utilization of resources.
TABLE
Risks and benefits of planned cesarean delivery
| BENEFIT | RISK | UNCLEAR EFFECTS |
|---|---|---|
| The mother | ||
| Protection against urinary incontinence Decreased surgical complications Decreased risk of postpartum hemorrhage Cultural factors Availability of social support Economic advantage | Increased length of stay Infection Anesthetic risk Subsequent placentation Difficulty breastfeeding Complication from future cesarean section Comorbidities related to obesity | Anorectal function Sexual function Pelvic organ prolapse Maternal mortality Postpartum pain Postpartum depression Thromboembolism |
| The child | ||
| Reduced mortality Decreased risk of intracranial hemorrhage Decreased risk of neonatal asphyxia Decreased risk of neonatal encephalopathy Decreased risk of brachial plexus injury | Iatrogenic prematurity Increased hospitalization Increased risk of respiratory complication | Breastfeeding Fetal laceration |
When a patient raises the subject
Your first responsibility is to clarify her request. Key to this discussion is the patient’s reason for requesting a scheduled C-section. Many women—especially primiparous women—have a fear of labor itself, not to mention concerns about their safety and the safety of their baby.7 Another major concern to many women is the risk of injury to their perineum and pelvic floor.1 These fears and concerns may motivate their request.
Educating patients about labor and discussing options for pain relief during labor can help soothe the patients’ fears. Clarifying long-term risks and benefits in regard to pelvic floor dysfunction also is important. Patients may have an unrealistic understanding of C-section and its potential complications. Often, education about the birth process and mode of delivery can alleviate a patient’s fears and change her hopes for delivery.
Explore any comorbidities
Because C-section is a major abdominal surgical procedure, maternal factors such as weight, age, surgical history, and medical comorbidities are relevant considerations when discussing the risks and benefits of cesarean in the absence of obstetric indications. Even in the absence of such comorbidities, certain risks of surgery should be clarified, including the risk of hemorrhage, infection, wound complication, thromboembolism, need for future surgery, and postoperative recovery.
The risks and benefits of vaginal delivery also should be discussed, including the factors that may lead up to an un-scheduled cesarean delivery despite the desire for a vaginal delivery.
How many children are planned?
Given the reluctance of health-care providers to manage attempted vaginal birth after C-section, women who opt for elective C-section for their first delivery may be committing themselves to C-section with subsequent pregnancies, too.8 Data suggest that an increasing number of C-sections place women at increasing risk of placenta accreta or previa, hysterectomy, blood transfusion, cystotomy, endometritis, prolonged operative time, and longer hospital stays. That said, overall maternal mortality from C-section remains low.9
Therefore, if a patient plans to have more than one or two children, she needs to understand the ramifications of repeat C-section at the time of her next delivery as well as in any additional pregnancies. Although a successful vaginal delivery cannot be guaranteed for any parturient, an attempt at vaginal delivery might be preferable for a woman hoping for a larger family.
Ensure clear consent
Chervenak and McCullough have provided an algorithm for offering C-section that balances the ethical concepts of autonomy and beneficence; that model is described above.10
If the patient requests C-section, but the clinician is uncomfortable performing one under the circumstances, referral is reasonable.
A patient’s thoughtful request can be considered out of respect for autonomy and supported by thorough counseling.
The decision to perform cesarean delivery is one of the most common clinical ethical challenges in obstetric practice today—“a challenge that will only increase with the growing influence of managed care,” observe Frank A. Chervenak, MD, and Laurence B. McCullough, PhD, who have written widely about ethical challenges in obstetrics and gynecology.10
In 1996, they proposed a model to help guide practitioners through the decision-making process of choosing cesarean delivery. According to that model, C-section is justified in four situations:
- when C-section is the only reasonable option based on clinical judgment, such as in a patient with a previous classical uterine incision. In this case, the clinician does not offer vaginal delivery but recommends only C-section based on beneficence
- when either C-section or vaginal delivery may be appropriate. This scenario warrants a clear discussion with the patient about the risks, benefits, and inherent controversy between delivery modes when all choices are equal in one’s best clinical judgment. An example might be the vertex/breech presentation of twins
- when vaginal delivery is preferable but C-section would also be indicated, such as in attempted vaginal birth after C-section
- when cesarean delivery is not generally supported over vaginal delivery, but the patient requests C-section and that request is based solely on autonomous principles. This is the case of cesarean delivery by maternal request, which necessitates clear counseling and education of the patient. Fear of pain is not a justifiable reason for cesarean delivery, because we can offer options for adequate pain management in labor.
Ensuring a correct gestational age
Once the decision to proceed with scheduled C-section is made, accurate determination of gestational age is crucial to avoid iatrogenic prematurity.
ACOG Educational Bulletin No. 230 (November 1996) lists a number of criteria by which to infer gestational age and, therefore, fetal lung maturity. The criteria include:
- documented fetal heart tone for 30 weeks by Doppler ultrasound
- 36 weeks having passed since reliable documentation of a positive urine or serum human chorionic gonadotropin pregnancy test
- crown–rump measurement by ultrasonography (US) at 6 to 11 weeks of gestation that supports the current gestational age of 39 weeks or more
- US measurement at 12 to 20 weeks’ gestation that supports the clinically determined estimated gestational age above 39 weeks.
Insurance concerns are vital to the decision
The Newborns’ and Mothers’ Health Protection Act (NMHPA) was passed in 1996. The law delineates a minimum requirement of coverage by insurers for hospital stays of 48 hours after vaginal delivery or 96 hours after C-section, thereby preventing health insurance plans from restricting hospital stays after delivery.11 The law was passed as a response to political concerns about “drive-thru deliveries.”
The NMHPA also allows for provider discretion regarding the length of stay required after childbirth, meaning that, if an attending-level provider deems discharge feasible in less than 48 or 96 hours, the insurer is not mandated to continue coverage beyond discharge.
The law, however, does not mandate coverage by health insurance plans for prenatal care, delivery, and postpartum care. Confounding the actions of health insurance companies are state laws governing the care of newborns and mothers, as these laws superceded the NMHPA. So, although most states have mandated benefit laws regarding a variety of services, as of 2002, only 18 states had laws mandating specific maternity services.12 Some states specifically mention elective C-sections as nonmandated services, meaning that a patient who elects a scheduled C-section at term without obstetric indications may be required to pay for her obstetric care.
1. Wax JR, Cartin A, Pinette MG, Blackstone J. Patient choice cesarean: an evidence-based review. Obstet Gynecol Surv. 2004;59:601-616.
2. Hopkins K. Are Brazilian women really choosing to deliver by cesarean? Soc Sci Med. 2000;51:725-740.
3. FIGO Committee for the Study of Ethical Aspects of Human Reproduction and Women’s Health. Ethical Issues in Obstetrics and Gynecology. November 2006. Available at www.figo.org/docs/Ethics%20Guidelines%20-%20English%20version%202006%20-2009.pdf. Accessed April 3, 2008.
4. ACOG Committee Opinion No 386: Cesarean delivery on maternal request. November 2007. Available at www.acog.org/publications/committee_opinions/co386.cfm. Accessed April 3, 2008.
5. National Institutes of Health state-of-the-science conference statement. Cesarean delivery on maternal request March 27-29, 2006. Obstet Gynecol. 2006;107:1386-1397.
6. Allen VM, O’Connell CM, Farrell SA, Baskett TF. Economic implications of method of delivery. Am J Obstet Gynecol. 2005;193:192-197.
7. McCourt C, Weaver J, Statham H, Beake S, Gamble J, Creedy DK. Elective cesarean section and decision making: a critical review of the literature. Birth. 2007;34:65-79.
8. Roberts RG, Deutchman M, King VJ, Fryer GE, Miyoshi TJ. Changing policies on vaginal birth after cesarean: impact on access. Birth. 2007;34:316-322.
9. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. National Institute of Child Health and Human Development Maternal-Fetal Medicine Unit Network. Obstet Gynecol. 2006;107:1226-1232.
10. Chervenak FA, McCullough LB. An ethically justified algorithm for offering, recommending, and performing cesarean delivery and its application in managed care practice. Obstet Gynecol. 1996;87:302-305.
11. Newborns’ and Mothers’ Health Protection Act of 1996, 29 U S.C.S. §1185.
12. Laugesen MJ, Paul RR, Luft HS, Aubry W, Ganiats TG. A comparative analysis of mandated benefit laws, 1949-2002. Health Serv Res. 2006;41(3 pt 2):1081-1103.
13. National Center for Health Statistics Technical Appendix. Vital statistics of the United States, 2004. Vol. I: Natality. US Department of Heath and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Hyattsville, Md. Available at www.cdc.gov/nchs/nvss.htm. Accessed April 3, 2008.
14. National Vital Statistics Reports. Vol. 55, No. 12, April 19, 2007. Available at www.cdc.gov/nchs/data/nvsr/nvsr55/nvsr55_12.pdf. Accessed April 3, 2008.
In general, when a patient inquires about elective primary C-section, it is best to consider the “6 C’s of elective cesarean” in a careful discussion with her. That approach entails consideration of the following:
- Clarification of her request
- Comorbidities in maternal health or surgical history
- number of Children planned overall
- clear Consent for the procedure
- Correct determination of gestational age at the time of planned delivery
- Confirmation of coverage by her insurance carrier.
One trend is clear: Maternal requests for primary cesarean delivery are on the rise in the United States, although we lack precise data on exactly how fast the rate is rising. Many experts estimate it to be 4% to 18%.1 In Brazil, the rate of elective C-section for women in private hospitals is thought to be as high as 80% to 90%.2
As more celebrities and other prominent figures undergo elective C-section, more American women are beginning to ask for the same “privilege.” In this article, I lay out an evidence-based and ethically sensitive approach to counseling patients who request C-section on an elective basis.
In 2004, the United States saw 4.1 million births, 18% of which—or nearly 750,000—involved primary C-section.13 However, it is difficult to discern how many of these primary C-sections were performed for nonobstetric, or elective, indications, because such data are not routinely collected.
Birth certificates are changing
Efforts to improve birth certificate data have begun. In 2003, the revised US Standard Certificate of Live Birth was adopted by seven states, allowing for a more detailed description of births. The new certificate provides for more robust information in several areas, including
- risk factors in the index pregnancy
- obstetric procedures performed
- characteristics of labor and delivery
- method of delivery
- normal conditions of the newborn
- congenital anomalies in the newborn.
It also specifies whether or not a trial of labor was attempted before cesarean delivery, but it is limited by the inclusion of breech presentation in the statistics.14
Data collection remains an inexact science
Even with the new birth certificate data, it remains difficult to accurately quantify the number of nonobstetrically indicated primary C-sections, although many experts have estimated the rate at 4% to 28%.1
The points raised in the list that begins this article are all discussed here.
The difficulty of calculating the rate of primary C-section
We are limited by terminology and data-collection practices, as well as a multitude of confounding obstetric factors. Practicing providers recognize the inherent difference between a planned C-section at term without the onset of labor and an unplanned C-section at term after the onset of labor—as well as every scenario in between.
Unplanned C-section can be performed to address fetal compromise or an unsuccessful attempt at vaginal delivery—each scenario replete with its own risks and potential complications. The urgency of C-section also confounds subsequent maternal and fetal complications. Underlying maternal factors such as obesity and medical and surgical history further complicate the scenario.
For these reasons, the discussion of elective C-section is best managed by limiting the parameters considered to the requested, scheduled, elective C-section at term without maternal or fetal indications. Most patients have this paradigm in mind when they make their request, even though physicians and midwives understand that this is the ideal and not generally the reality.
Medicolegal and ethical considerations
The ethical principles surrounding cesarean delivery upon maternal request balance on the tension between beneficence and patient autonomy. The former requires the promotion of the patient’s overall health and well-being, along with attention to the closely related dictum, primum non nocere, or “first do no harm.”
Patient autonomy requires respectful consideration of the patient and her world view when making a medical decision. The ethical principle of patient autonomy is usually understood as a right to decline medical intervention—not necessarily to demand dangerous or unproven intervention.1
This raises the question: Is a scheduled C-section in the absence of obstetric indications dangerous? Harmful? Imprudent? The medical community has accepted these inherent tensions in the field of aesthetic plastic surgery, but societies in obstetrics and gynecology continue to struggle with the ethical principles involved in maternal-choice cesarean.
FIGO: C-section for nonmedical reasons is not justified
The International Federation of Gynecology and Obstetrics (FIGO) Committee for the Ethical Aspects of Human Reproduction and Women’s Health bases its guidelines on the use of cesarean delivery for nonmedical reasons on the principles of beneficence and social justice. It concludes: “Cesarean section is a surgical intervention with potential hazards for both mother and child. It also uses more health-care resources than normal vaginal delivery…performing cesarean section for nonmedical reasons is ethically not justified.”3
ACOG: Individualize the decision consistent with ethical principles
The American College of Obstetricians and Gynecologists (ACOG), in a recent Committee Opinion, acknowledged the paucity of research data directly comparing cesarean delivery on maternal request with planned vaginal delivery. The document reviews the National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request (see below), which was convened in 2006, and notes the panel’s conclusion that the available body of evidence does not allow for a conclusive recommendation of one mode of delivery over another.4 The ACOG Committee Opinion states: “Any decision to perform a cesarean delivery on maternal request should be carefully individualized and consistent with ethical principles.”5
Different world views likely account for different conclusions
The difference in the FIGO and ACOG positions may arise from differences in cultural contexts between a general world health view and a highly patient-centered Western perspective. The former view bases the decision on universal good and the utilization of scarce health-care resources; the latter view recognizes the individual within an ethical context.
Both views acknowledge the limited data available to inform the decision. So what do the data say, and how can we help our patients understand it?
NIH State-of-the-Science Conference
In March of 2006, an independent panel of experts from a range of medical fields reviewed the scientific literature regarding cesarean delivery on maternal request at the NIH in Bethesda, Maryland. Although the panel found no Level I, or strong, evidence within the literature, it was able to characterize the risks and benefits of maternal-request C-section based on Level II (moderate), Level III (weak), and Level IV (absent) evidence.
Moderate evidence was scarce
From a maternal perspective, the panel found that “the frequency of postpartum hemorrhage associated with planned cesarean delivery is lower than that reported with the combination of planned vaginal delivery and unplanned cesarean delivery,”5 although hospital stay is longer than with vaginal delivery.
From a neonatal perspective, moderate evidence favors vaginal delivery because of a decreased incidence of respiratory morbidity, such as transient tachypnea of the newborn and respiratory distress syndrome. Respiratory morbidity is directly related to gestational age, and there is a risk of iatrogenic prematurity with scheduled C-section. The possibility of incorrect obstetric dating would seem to favor awaiting the spontaneous onset of labor at term and an attempt at vaginal delivery to reduce the risk of respiratory complications due to iatrogenic prematurity.
Weak evidence goes both ways
Weakly supported evidence favored both cesarean section and vaginal delivery for either the mother or fetus. Weak evidence favoring vaginal delivery for maternal interests included:
- decreased maternal infectious morbidity and anesthetic complications, compared with C-section
- greater ease establishing breastfeeding, due to logistical challenges surrounding mother–infant bonding after C-section
- greater freedom in planning family size because increasing numbers of repeat C-sections with subsequent pregnancies increase risk of uterine rupture, cesarean hysterectomy, and abnormal placentation.
- lower rate of postpartum stress urinary incontinence, compared with women undergoing vaginal delivery, in the short term
- lower risk of surgical morbidity and traumatic obstetric lacerations with elective C-section, compared with the injuries that can occur at the time of unscheduled C-section or vaginal delivery.
Weak evidence of neonatal benefit
From the neonatal perspective, the NIH Consensus Committee found weak evidence favoring C-section. A scheduled C-section protects the neonate from stillbirth arising from postdates intrauterine fetal demise, because, with elective cesarean, a pregnancy is not usually allowed to continue post-term.
The Committee also documented protection from intracranial hemorrhage, neonatal asphyxia, encephalopathy, birth injury, and neonatal infection with C-section, compared with vaginal delivery.5
The socioeconomic picture matters
From a socioeconomic standpoint, women who request C-section may have financial concerns such as the amount of time off from work that may be necessary for both themselves and their partners. The availability of family support may be relevant and improved if a specific time frame for delivery is anticipated.
In many cultures, “lucky days” exist, and women may have preferences or aspirations for their child to be born on one of them.
Last, although it may be more cost-effective for a patient to undergo vaginal delivery, we, as health-care providers, cannot predict who will be successful in that regard. A complicated labor that necessitates unscheduled, urgent, or emergent C-section costs more in health-care dollars than does a C-section without labor.
Canadian researchers in 2005 examined the hospital care costs over 18 years in 27,614 pregnancies associated with varying types of delivery and found that the cost of delivery was highest for a C-section performed after the onset of labor ($2,137). The lowest cost was for spontaneous vaginal delivery ($1,340), followed by C-section without labor ($1,532).6 Therefore, some could argue that the overall cost to the patient and system is lower with a scheduled cesarean delivery because it avoids the other possible comorbidities and utilization of resources.
TABLE
Risks and benefits of planned cesarean delivery
| BENEFIT | RISK | UNCLEAR EFFECTS |
|---|---|---|
| The mother | ||
| Protection against urinary incontinence Decreased surgical complications Decreased risk of postpartum hemorrhage Cultural factors Availability of social support Economic advantage | Increased length of stay Infection Anesthetic risk Subsequent placentation Difficulty breastfeeding Complication from future cesarean section Comorbidities related to obesity | Anorectal function Sexual function Pelvic organ prolapse Maternal mortality Postpartum pain Postpartum depression Thromboembolism |
| The child | ||
| Reduced mortality Decreased risk of intracranial hemorrhage Decreased risk of neonatal asphyxia Decreased risk of neonatal encephalopathy Decreased risk of brachial plexus injury | Iatrogenic prematurity Increased hospitalization Increased risk of respiratory complication | Breastfeeding Fetal laceration |
When a patient raises the subject
Your first responsibility is to clarify her request. Key to this discussion is the patient’s reason for requesting a scheduled C-section. Many women—especially primiparous women—have a fear of labor itself, not to mention concerns about their safety and the safety of their baby.7 Another major concern to many women is the risk of injury to their perineum and pelvic floor.1 These fears and concerns may motivate their request.
Educating patients about labor and discussing options for pain relief during labor can help soothe the patients’ fears. Clarifying long-term risks and benefits in regard to pelvic floor dysfunction also is important. Patients may have an unrealistic understanding of C-section and its potential complications. Often, education about the birth process and mode of delivery can alleviate a patient’s fears and change her hopes for delivery.
Explore any comorbidities
Because C-section is a major abdominal surgical procedure, maternal factors such as weight, age, surgical history, and medical comorbidities are relevant considerations when discussing the risks and benefits of cesarean in the absence of obstetric indications. Even in the absence of such comorbidities, certain risks of surgery should be clarified, including the risk of hemorrhage, infection, wound complication, thromboembolism, need for future surgery, and postoperative recovery.
The risks and benefits of vaginal delivery also should be discussed, including the factors that may lead up to an un-scheduled cesarean delivery despite the desire for a vaginal delivery.
How many children are planned?
Given the reluctance of health-care providers to manage attempted vaginal birth after C-section, women who opt for elective C-section for their first delivery may be committing themselves to C-section with subsequent pregnancies, too.8 Data suggest that an increasing number of C-sections place women at increasing risk of placenta accreta or previa, hysterectomy, blood transfusion, cystotomy, endometritis, prolonged operative time, and longer hospital stays. That said, overall maternal mortality from C-section remains low.9
Therefore, if a patient plans to have more than one or two children, she needs to understand the ramifications of repeat C-section at the time of her next delivery as well as in any additional pregnancies. Although a successful vaginal delivery cannot be guaranteed for any parturient, an attempt at vaginal delivery might be preferable for a woman hoping for a larger family.
Ensure clear consent
Chervenak and McCullough have provided an algorithm for offering C-section that balances the ethical concepts of autonomy and beneficence; that model is described above.10
If the patient requests C-section, but the clinician is uncomfortable performing one under the circumstances, referral is reasonable.
A patient’s thoughtful request can be considered out of respect for autonomy and supported by thorough counseling.
The decision to perform cesarean delivery is one of the most common clinical ethical challenges in obstetric practice today—“a challenge that will only increase with the growing influence of managed care,” observe Frank A. Chervenak, MD, and Laurence B. McCullough, PhD, who have written widely about ethical challenges in obstetrics and gynecology.10
In 1996, they proposed a model to help guide practitioners through the decision-making process of choosing cesarean delivery. According to that model, C-section is justified in four situations:
- when C-section is the only reasonable option based on clinical judgment, such as in a patient with a previous classical uterine incision. In this case, the clinician does not offer vaginal delivery but recommends only C-section based on beneficence
- when either C-section or vaginal delivery may be appropriate. This scenario warrants a clear discussion with the patient about the risks, benefits, and inherent controversy between delivery modes when all choices are equal in one’s best clinical judgment. An example might be the vertex/breech presentation of twins
- when vaginal delivery is preferable but C-section would also be indicated, such as in attempted vaginal birth after C-section
- when cesarean delivery is not generally supported over vaginal delivery, but the patient requests C-section and that request is based solely on autonomous principles. This is the case of cesarean delivery by maternal request, which necessitates clear counseling and education of the patient. Fear of pain is not a justifiable reason for cesarean delivery, because we can offer options for adequate pain management in labor.
Ensuring a correct gestational age
Once the decision to proceed with scheduled C-section is made, accurate determination of gestational age is crucial to avoid iatrogenic prematurity.
ACOG Educational Bulletin No. 230 (November 1996) lists a number of criteria by which to infer gestational age and, therefore, fetal lung maturity. The criteria include:
- documented fetal heart tone for 30 weeks by Doppler ultrasound
- 36 weeks having passed since reliable documentation of a positive urine or serum human chorionic gonadotropin pregnancy test
- crown–rump measurement by ultrasonography (US) at 6 to 11 weeks of gestation that supports the current gestational age of 39 weeks or more
- US measurement at 12 to 20 weeks’ gestation that supports the clinically determined estimated gestational age above 39 weeks.
Insurance concerns are vital to the decision
The Newborns’ and Mothers’ Health Protection Act (NMHPA) was passed in 1996. The law delineates a minimum requirement of coverage by insurers for hospital stays of 48 hours after vaginal delivery or 96 hours after C-section, thereby preventing health insurance plans from restricting hospital stays after delivery.11 The law was passed as a response to political concerns about “drive-thru deliveries.”
The NMHPA also allows for provider discretion regarding the length of stay required after childbirth, meaning that, if an attending-level provider deems discharge feasible in less than 48 or 96 hours, the insurer is not mandated to continue coverage beyond discharge.
The law, however, does not mandate coverage by health insurance plans for prenatal care, delivery, and postpartum care. Confounding the actions of health insurance companies are state laws governing the care of newborns and mothers, as these laws superceded the NMHPA. So, although most states have mandated benefit laws regarding a variety of services, as of 2002, only 18 states had laws mandating specific maternity services.12 Some states specifically mention elective C-sections as nonmandated services, meaning that a patient who elects a scheduled C-section at term without obstetric indications may be required to pay for her obstetric care.
In general, when a patient inquires about elective primary C-section, it is best to consider the “6 C’s of elective cesarean” in a careful discussion with her. That approach entails consideration of the following:
- Clarification of her request
- Comorbidities in maternal health or surgical history
- number of Children planned overall
- clear Consent for the procedure
- Correct determination of gestational age at the time of planned delivery
- Confirmation of coverage by her insurance carrier.
One trend is clear: Maternal requests for primary cesarean delivery are on the rise in the United States, although we lack precise data on exactly how fast the rate is rising. Many experts estimate it to be 4% to 18%.1 In Brazil, the rate of elective C-section for women in private hospitals is thought to be as high as 80% to 90%.2
As more celebrities and other prominent figures undergo elective C-section, more American women are beginning to ask for the same “privilege.” In this article, I lay out an evidence-based and ethically sensitive approach to counseling patients who request C-section on an elective basis.
In 2004, the United States saw 4.1 million births, 18% of which—or nearly 750,000—involved primary C-section.13 However, it is difficult to discern how many of these primary C-sections were performed for nonobstetric, or elective, indications, because such data are not routinely collected.
Birth certificates are changing
Efforts to improve birth certificate data have begun. In 2003, the revised US Standard Certificate of Live Birth was adopted by seven states, allowing for a more detailed description of births. The new certificate provides for more robust information in several areas, including
- risk factors in the index pregnancy
- obstetric procedures performed
- characteristics of labor and delivery
- method of delivery
- normal conditions of the newborn
- congenital anomalies in the newborn.
It also specifies whether or not a trial of labor was attempted before cesarean delivery, but it is limited by the inclusion of breech presentation in the statistics.14
Data collection remains an inexact science
Even with the new birth certificate data, it remains difficult to accurately quantify the number of nonobstetrically indicated primary C-sections, although many experts have estimated the rate at 4% to 28%.1
The points raised in the list that begins this article are all discussed here.
The difficulty of calculating the rate of primary C-section
We are limited by terminology and data-collection practices, as well as a multitude of confounding obstetric factors. Practicing providers recognize the inherent difference between a planned C-section at term without the onset of labor and an unplanned C-section at term after the onset of labor—as well as every scenario in between.
Unplanned C-section can be performed to address fetal compromise or an unsuccessful attempt at vaginal delivery—each scenario replete with its own risks and potential complications. The urgency of C-section also confounds subsequent maternal and fetal complications. Underlying maternal factors such as obesity and medical and surgical history further complicate the scenario.
For these reasons, the discussion of elective C-section is best managed by limiting the parameters considered to the requested, scheduled, elective C-section at term without maternal or fetal indications. Most patients have this paradigm in mind when they make their request, even though physicians and midwives understand that this is the ideal and not generally the reality.
Medicolegal and ethical considerations
The ethical principles surrounding cesarean delivery upon maternal request balance on the tension between beneficence and patient autonomy. The former requires the promotion of the patient’s overall health and well-being, along with attention to the closely related dictum, primum non nocere, or “first do no harm.”
Patient autonomy requires respectful consideration of the patient and her world view when making a medical decision. The ethical principle of patient autonomy is usually understood as a right to decline medical intervention—not necessarily to demand dangerous or unproven intervention.1
This raises the question: Is a scheduled C-section in the absence of obstetric indications dangerous? Harmful? Imprudent? The medical community has accepted these inherent tensions in the field of aesthetic plastic surgery, but societies in obstetrics and gynecology continue to struggle with the ethical principles involved in maternal-choice cesarean.
FIGO: C-section for nonmedical reasons is not justified
The International Federation of Gynecology and Obstetrics (FIGO) Committee for the Ethical Aspects of Human Reproduction and Women’s Health bases its guidelines on the use of cesarean delivery for nonmedical reasons on the principles of beneficence and social justice. It concludes: “Cesarean section is a surgical intervention with potential hazards for both mother and child. It also uses more health-care resources than normal vaginal delivery…performing cesarean section for nonmedical reasons is ethically not justified.”3
ACOG: Individualize the decision consistent with ethical principles
The American College of Obstetricians and Gynecologists (ACOG), in a recent Committee Opinion, acknowledged the paucity of research data directly comparing cesarean delivery on maternal request with planned vaginal delivery. The document reviews the National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request (see below), which was convened in 2006, and notes the panel’s conclusion that the available body of evidence does not allow for a conclusive recommendation of one mode of delivery over another.4 The ACOG Committee Opinion states: “Any decision to perform a cesarean delivery on maternal request should be carefully individualized and consistent with ethical principles.”5
Different world views likely account for different conclusions
The difference in the FIGO and ACOG positions may arise from differences in cultural contexts between a general world health view and a highly patient-centered Western perspective. The former view bases the decision on universal good and the utilization of scarce health-care resources; the latter view recognizes the individual within an ethical context.
Both views acknowledge the limited data available to inform the decision. So what do the data say, and how can we help our patients understand it?
NIH State-of-the-Science Conference
In March of 2006, an independent panel of experts from a range of medical fields reviewed the scientific literature regarding cesarean delivery on maternal request at the NIH in Bethesda, Maryland. Although the panel found no Level I, or strong, evidence within the literature, it was able to characterize the risks and benefits of maternal-request C-section based on Level II (moderate), Level III (weak), and Level IV (absent) evidence.
Moderate evidence was scarce
From a maternal perspective, the panel found that “the frequency of postpartum hemorrhage associated with planned cesarean delivery is lower than that reported with the combination of planned vaginal delivery and unplanned cesarean delivery,”5 although hospital stay is longer than with vaginal delivery.
From a neonatal perspective, moderate evidence favors vaginal delivery because of a decreased incidence of respiratory morbidity, such as transient tachypnea of the newborn and respiratory distress syndrome. Respiratory morbidity is directly related to gestational age, and there is a risk of iatrogenic prematurity with scheduled C-section. The possibility of incorrect obstetric dating would seem to favor awaiting the spontaneous onset of labor at term and an attempt at vaginal delivery to reduce the risk of respiratory complications due to iatrogenic prematurity.
Weak evidence goes both ways
Weakly supported evidence favored both cesarean section and vaginal delivery for either the mother or fetus. Weak evidence favoring vaginal delivery for maternal interests included:
- decreased maternal infectious morbidity and anesthetic complications, compared with C-section
- greater ease establishing breastfeeding, due to logistical challenges surrounding mother–infant bonding after C-section
- greater freedom in planning family size because increasing numbers of repeat C-sections with subsequent pregnancies increase risk of uterine rupture, cesarean hysterectomy, and abnormal placentation.
- lower rate of postpartum stress urinary incontinence, compared with women undergoing vaginal delivery, in the short term
- lower risk of surgical morbidity and traumatic obstetric lacerations with elective C-section, compared with the injuries that can occur at the time of unscheduled C-section or vaginal delivery.
Weak evidence of neonatal benefit
From the neonatal perspective, the NIH Consensus Committee found weak evidence favoring C-section. A scheduled C-section protects the neonate from stillbirth arising from postdates intrauterine fetal demise, because, with elective cesarean, a pregnancy is not usually allowed to continue post-term.
The Committee also documented protection from intracranial hemorrhage, neonatal asphyxia, encephalopathy, birth injury, and neonatal infection with C-section, compared with vaginal delivery.5
The socioeconomic picture matters
From a socioeconomic standpoint, women who request C-section may have financial concerns such as the amount of time off from work that may be necessary for both themselves and their partners. The availability of family support may be relevant and improved if a specific time frame for delivery is anticipated.
In many cultures, “lucky days” exist, and women may have preferences or aspirations for their child to be born on one of them.
Last, although it may be more cost-effective for a patient to undergo vaginal delivery, we, as health-care providers, cannot predict who will be successful in that regard. A complicated labor that necessitates unscheduled, urgent, or emergent C-section costs more in health-care dollars than does a C-section without labor.
Canadian researchers in 2005 examined the hospital care costs over 18 years in 27,614 pregnancies associated with varying types of delivery and found that the cost of delivery was highest for a C-section performed after the onset of labor ($2,137). The lowest cost was for spontaneous vaginal delivery ($1,340), followed by C-section without labor ($1,532).6 Therefore, some could argue that the overall cost to the patient and system is lower with a scheduled cesarean delivery because it avoids the other possible comorbidities and utilization of resources.
TABLE
Risks and benefits of planned cesarean delivery
| BENEFIT | RISK | UNCLEAR EFFECTS |
|---|---|---|
| The mother | ||
| Protection against urinary incontinence Decreased surgical complications Decreased risk of postpartum hemorrhage Cultural factors Availability of social support Economic advantage | Increased length of stay Infection Anesthetic risk Subsequent placentation Difficulty breastfeeding Complication from future cesarean section Comorbidities related to obesity | Anorectal function Sexual function Pelvic organ prolapse Maternal mortality Postpartum pain Postpartum depression Thromboembolism |
| The child | ||
| Reduced mortality Decreased risk of intracranial hemorrhage Decreased risk of neonatal asphyxia Decreased risk of neonatal encephalopathy Decreased risk of brachial plexus injury | Iatrogenic prematurity Increased hospitalization Increased risk of respiratory complication | Breastfeeding Fetal laceration |
When a patient raises the subject
Your first responsibility is to clarify her request. Key to this discussion is the patient’s reason for requesting a scheduled C-section. Many women—especially primiparous women—have a fear of labor itself, not to mention concerns about their safety and the safety of their baby.7 Another major concern to many women is the risk of injury to their perineum and pelvic floor.1 These fears and concerns may motivate their request.
Educating patients about labor and discussing options for pain relief during labor can help soothe the patients’ fears. Clarifying long-term risks and benefits in regard to pelvic floor dysfunction also is important. Patients may have an unrealistic understanding of C-section and its potential complications. Often, education about the birth process and mode of delivery can alleviate a patient’s fears and change her hopes for delivery.
Explore any comorbidities
Because C-section is a major abdominal surgical procedure, maternal factors such as weight, age, surgical history, and medical comorbidities are relevant considerations when discussing the risks and benefits of cesarean in the absence of obstetric indications. Even in the absence of such comorbidities, certain risks of surgery should be clarified, including the risk of hemorrhage, infection, wound complication, thromboembolism, need for future surgery, and postoperative recovery.
The risks and benefits of vaginal delivery also should be discussed, including the factors that may lead up to an un-scheduled cesarean delivery despite the desire for a vaginal delivery.
How many children are planned?
Given the reluctance of health-care providers to manage attempted vaginal birth after C-section, women who opt for elective C-section for their first delivery may be committing themselves to C-section with subsequent pregnancies, too.8 Data suggest that an increasing number of C-sections place women at increasing risk of placenta accreta or previa, hysterectomy, blood transfusion, cystotomy, endometritis, prolonged operative time, and longer hospital stays. That said, overall maternal mortality from C-section remains low.9
Therefore, if a patient plans to have more than one or two children, she needs to understand the ramifications of repeat C-section at the time of her next delivery as well as in any additional pregnancies. Although a successful vaginal delivery cannot be guaranteed for any parturient, an attempt at vaginal delivery might be preferable for a woman hoping for a larger family.
Ensure clear consent
Chervenak and McCullough have provided an algorithm for offering C-section that balances the ethical concepts of autonomy and beneficence; that model is described above.10
If the patient requests C-section, but the clinician is uncomfortable performing one under the circumstances, referral is reasonable.
A patient’s thoughtful request can be considered out of respect for autonomy and supported by thorough counseling.
The decision to perform cesarean delivery is one of the most common clinical ethical challenges in obstetric practice today—“a challenge that will only increase with the growing influence of managed care,” observe Frank A. Chervenak, MD, and Laurence B. McCullough, PhD, who have written widely about ethical challenges in obstetrics and gynecology.10
In 1996, they proposed a model to help guide practitioners through the decision-making process of choosing cesarean delivery. According to that model, C-section is justified in four situations:
- when C-section is the only reasonable option based on clinical judgment, such as in a patient with a previous classical uterine incision. In this case, the clinician does not offer vaginal delivery but recommends only C-section based on beneficence
- when either C-section or vaginal delivery may be appropriate. This scenario warrants a clear discussion with the patient about the risks, benefits, and inherent controversy between delivery modes when all choices are equal in one’s best clinical judgment. An example might be the vertex/breech presentation of twins
- when vaginal delivery is preferable but C-section would also be indicated, such as in attempted vaginal birth after C-section
- when cesarean delivery is not generally supported over vaginal delivery, but the patient requests C-section and that request is based solely on autonomous principles. This is the case of cesarean delivery by maternal request, which necessitates clear counseling and education of the patient. Fear of pain is not a justifiable reason for cesarean delivery, because we can offer options for adequate pain management in labor.
Ensuring a correct gestational age
Once the decision to proceed with scheduled C-section is made, accurate determination of gestational age is crucial to avoid iatrogenic prematurity.
ACOG Educational Bulletin No. 230 (November 1996) lists a number of criteria by which to infer gestational age and, therefore, fetal lung maturity. The criteria include:
- documented fetal heart tone for 30 weeks by Doppler ultrasound
- 36 weeks having passed since reliable documentation of a positive urine or serum human chorionic gonadotropin pregnancy test
- crown–rump measurement by ultrasonography (US) at 6 to 11 weeks of gestation that supports the current gestational age of 39 weeks or more
- US measurement at 12 to 20 weeks’ gestation that supports the clinically determined estimated gestational age above 39 weeks.
Insurance concerns are vital to the decision
The Newborns’ and Mothers’ Health Protection Act (NMHPA) was passed in 1996. The law delineates a minimum requirement of coverage by insurers for hospital stays of 48 hours after vaginal delivery or 96 hours after C-section, thereby preventing health insurance plans from restricting hospital stays after delivery.11 The law was passed as a response to political concerns about “drive-thru deliveries.”
The NMHPA also allows for provider discretion regarding the length of stay required after childbirth, meaning that, if an attending-level provider deems discharge feasible in less than 48 or 96 hours, the insurer is not mandated to continue coverage beyond discharge.
The law, however, does not mandate coverage by health insurance plans for prenatal care, delivery, and postpartum care. Confounding the actions of health insurance companies are state laws governing the care of newborns and mothers, as these laws superceded the NMHPA. So, although most states have mandated benefit laws regarding a variety of services, as of 2002, only 18 states had laws mandating specific maternity services.12 Some states specifically mention elective C-sections as nonmandated services, meaning that a patient who elects a scheduled C-section at term without obstetric indications may be required to pay for her obstetric care.
1. Wax JR, Cartin A, Pinette MG, Blackstone J. Patient choice cesarean: an evidence-based review. Obstet Gynecol Surv. 2004;59:601-616.
2. Hopkins K. Are Brazilian women really choosing to deliver by cesarean? Soc Sci Med. 2000;51:725-740.
3. FIGO Committee for the Study of Ethical Aspects of Human Reproduction and Women’s Health. Ethical Issues in Obstetrics and Gynecology. November 2006. Available at www.figo.org/docs/Ethics%20Guidelines%20-%20English%20version%202006%20-2009.pdf. Accessed April 3, 2008.
4. ACOG Committee Opinion No 386: Cesarean delivery on maternal request. November 2007. Available at www.acog.org/publications/committee_opinions/co386.cfm. Accessed April 3, 2008.
5. National Institutes of Health state-of-the-science conference statement. Cesarean delivery on maternal request March 27-29, 2006. Obstet Gynecol. 2006;107:1386-1397.
6. Allen VM, O’Connell CM, Farrell SA, Baskett TF. Economic implications of method of delivery. Am J Obstet Gynecol. 2005;193:192-197.
7. McCourt C, Weaver J, Statham H, Beake S, Gamble J, Creedy DK. Elective cesarean section and decision making: a critical review of the literature. Birth. 2007;34:65-79.
8. Roberts RG, Deutchman M, King VJ, Fryer GE, Miyoshi TJ. Changing policies on vaginal birth after cesarean: impact on access. Birth. 2007;34:316-322.
9. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. National Institute of Child Health and Human Development Maternal-Fetal Medicine Unit Network. Obstet Gynecol. 2006;107:1226-1232.
10. Chervenak FA, McCullough LB. An ethically justified algorithm for offering, recommending, and performing cesarean delivery and its application in managed care practice. Obstet Gynecol. 1996;87:302-305.
11. Newborns’ and Mothers’ Health Protection Act of 1996, 29 U S.C.S. §1185.
12. Laugesen MJ, Paul RR, Luft HS, Aubry W, Ganiats TG. A comparative analysis of mandated benefit laws, 1949-2002. Health Serv Res. 2006;41(3 pt 2):1081-1103.
13. National Center for Health Statistics Technical Appendix. Vital statistics of the United States, 2004. Vol. I: Natality. US Department of Heath and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Hyattsville, Md. Available at www.cdc.gov/nchs/nvss.htm. Accessed April 3, 2008.
14. National Vital Statistics Reports. Vol. 55, No. 12, April 19, 2007. Available at www.cdc.gov/nchs/data/nvsr/nvsr55/nvsr55_12.pdf. Accessed April 3, 2008.
1. Wax JR, Cartin A, Pinette MG, Blackstone J. Patient choice cesarean: an evidence-based review. Obstet Gynecol Surv. 2004;59:601-616.
2. Hopkins K. Are Brazilian women really choosing to deliver by cesarean? Soc Sci Med. 2000;51:725-740.
3. FIGO Committee for the Study of Ethical Aspects of Human Reproduction and Women’s Health. Ethical Issues in Obstetrics and Gynecology. November 2006. Available at www.figo.org/docs/Ethics%20Guidelines%20-%20English%20version%202006%20-2009.pdf. Accessed April 3, 2008.
4. ACOG Committee Opinion No 386: Cesarean delivery on maternal request. November 2007. Available at www.acog.org/publications/committee_opinions/co386.cfm. Accessed April 3, 2008.
5. National Institutes of Health state-of-the-science conference statement. Cesarean delivery on maternal request March 27-29, 2006. Obstet Gynecol. 2006;107:1386-1397.
6. Allen VM, O’Connell CM, Farrell SA, Baskett TF. Economic implications of method of delivery. Am J Obstet Gynecol. 2005;193:192-197.
7. McCourt C, Weaver J, Statham H, Beake S, Gamble J, Creedy DK. Elective cesarean section and decision making: a critical review of the literature. Birth. 2007;34:65-79.
8. Roberts RG, Deutchman M, King VJ, Fryer GE, Miyoshi TJ. Changing policies on vaginal birth after cesarean: impact on access. Birth. 2007;34:316-322.
9. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. National Institute of Child Health and Human Development Maternal-Fetal Medicine Unit Network. Obstet Gynecol. 2006;107:1226-1232.
10. Chervenak FA, McCullough LB. An ethically justified algorithm for offering, recommending, and performing cesarean delivery and its application in managed care practice. Obstet Gynecol. 1996;87:302-305.
11. Newborns’ and Mothers’ Health Protection Act of 1996, 29 U S.C.S. §1185.
12. Laugesen MJ, Paul RR, Luft HS, Aubry W, Ganiats TG. A comparative analysis of mandated benefit laws, 1949-2002. Health Serv Res. 2006;41(3 pt 2):1081-1103.
13. National Center for Health Statistics Technical Appendix. Vital statistics of the United States, 2004. Vol. I: Natality. US Department of Heath and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Hyattsville, Md. Available at www.cdc.gov/nchs/nvss.htm. Accessed April 3, 2008.
14. National Vital Statistics Reports. Vol. 55, No. 12, April 19, 2007. Available at www.cdc.gov/nchs/data/nvsr/nvsr55/nvsr55_12.pdf. Accessed April 3, 2008.