User login
Shoulder dystocia: Clarifying the care of an old problem
The author reports no financial relationships relevant to this article.
Brachial plexus injury is a dreaded sequela of shoulder dystocia, one that lies at the root of many medical liability disputes. Although brachial plexus injury cannot be prevented, most of the commonly used maneuvers for freeing a stuck shoulder are designed to maximize fetal safety and minimize injury.
What is the standard of care when dystocia occurs? Several respected sources have offered conflicting recommendations, particularly in regard to maternal pushing once dystocia is diagnosed. My aim in this article is to clarify the issue.
Endogenous force versus exogenous force
The introduction to the American College of Obstetricians and Gynecologists’ practice bulletin on shoulder dystocia, published in November 2002, provides a useful summary of much of our current knowledge:
Shoulder dystocia is most often an unpredictable and unpreventable obstetric emergency. Failure of the shoulders to deliver spontaneously places both the pregnant woman and fetus at risk for injury. Several maneuvers to release impacted shoulders have been developed, but the urgency of this event makes prospective studies impractical for comparing their effectiveness.1
Because prospective studies are unlikely ever to be performed, some investigators have turned to mathematical modeling to learn more about the forces exerted on the fetal neck overlying the roots of the brachial plexus when a shoulder is impacted against the symphysis pubis.
Gonik and colleagues2 performed elegant modeling of the pressure between the base of the fetal neck and symphysis pubis during dystocia. They utilized data on birth forces gathered by CaldeyroBarcia and Poseiro3 in their classic work on intrauterine pressure. Gonik and colleagues also used data from Allen and associates,4 who measured the force of clinician-applied traction after delivery of the head by having clinicians wear sensory gloves that recorded the force of traction applied.
Gonik and associates2 concluded that the pressure resulting from endogenous forces is four to nine times greater than the pressure generated by a clinician. “Neonatal brachial plexus injury is not a priori explained by iatrogenically induced excessive traction,” they wrote. “Spontaneous endogenous forces may contribute substantially to this type of neonatal trauma.”
How an understanding of endogenous forces alters management
Although fewer than 10% of cases of shoulder dystocia result in permanent brachial plexus injury,1 such injuries are a major source of malpractice litigation in obstetrics, as I noted at the beginning of this article. In most such cases, injury is blamed on excessive traction by the physician (FIGURE). Newer data, such as the study by Gonik and colleagues,2 may implicate expulsive force (ie, maternal pushing) as another, perhaps greater, cause.
As long ago as 1988, Acker and colleagues5 reported on their experience with Erb’s palsy, which was associated with rapid delivery and unusually forceful expulsive effort in one third of cases. Their findings suggest that, when shoulder dystocia occurs and additional maneuvers are necessary to deliver the impacted anterior shoulder, the contribution of potentially harmful endogenous forces should be kept in mind. Counterintuitive strategies, including having the mother stop pushing until the anterior shoulder is freed, may help limit injury.
FIGURE Maternal pushing may contribute to injury
When shoulder dystocia occurs, the progress of labor is interrupted and brachial plexus injury can result, a common cause of litigation. Until now, plaintiff’s attorneys have tended to blame these injuries on the obstetrician and “excess” traction, but it now appears that maternal pushing contributes as well—possibly, to a greater degree than any effort by the physician.
How confusion crept into the literature
The 21st (current) edition of Williams Obstetrics,6 in the section on management of shoulder dystocia, states that “an initial gentle attempt at traction assisted by maternal expulsive efforts is recommended.” There is no reference for this statement. However, a look at the 19th edition of the textbook7 reveals identical wording, and the reference cited is ACOG Technical Bulletin No. 152 (August 1991), entitled Operative Vaginal Delivery. A subsequent version of the same bulletin (no. 196 from August 1994) contains identical wording. By the time that version was replaced by ACOG Practice Bulletin No. 17 (June 2000), however, it no longer contained any information on shoulder dystocia. Instead, ACOG published Practice Pattern No. 7 (October 1997), entitled Shoulder Dystocia. This document did not recommend maternal expulsive force after a diagnosis of shoulder dystocia—in fact, maternal force was not even mentioned. Nor is it mentioned in the current practice bulletin (no. 40 from November 2002), which replaced the previous version of Shoulder Dystocia.
Confusion doesn’t end there
In its section on shoulder dystocia, ACOG’s publication Precis (1998) states:
Management of shoulder dystocia involves both anticipation of and preparation for problems. The key to preventing fetal injury is avoidance of excess traction on the fetal head. When shoulder dystocia is diagnosed, a deliberate and planned sequence of events should be initiated. Pushing should be halted and obstructive causes should be considered. Aggressive fundal pressure or continued pushing will only further impact the anterior shoulder.
We are left with the paradox that the current edition of Williams Obstetrics, in its discussion of shoulder dystocia, carries a statement recommending maternal pushing based on a 1994 ACOG document—a statement that subsequent ACOG documents no longer contain. In fact, one of those documents—Precis—tells us that pushing should be halted, an instruction supported by the mathematical modeling of Gonik and colleagues.2 And a popular online text (UpToDate.com) advises: “The mother should be told not to push during attempts to reposition the fetus.”8 Once the fetus is successfully repositioned, maternal pushing or traction, or both, can be reinstated.
Putting it all into clinical perspective
The current ACOG practice bulletin on shoulder dystocia (no. 40 from November 2002) observes that “retraction of the delivered fetal head against the maternal perineum (turtle sign) may be present and may assist in the diagnosis of shoulder dystocia.” When present, the turtle sign strongly suggests that the anterior shoulder is already impacted against the symphysis pubis. Maternal expulsive forces may have already put enough pressure on the nerve roots of the brachial plexus to cause damage. Any degree of traction or continued maternal pushing is likely to compound an already potentially serious problem.
In such cases, it is prudent to resort to known maneuvers, avoid encouraging continued maternal pushing, and simply support and guide the head without supplying any real traction.
When the turtle sign is absent, shoulder dystocia can be diagnosed only after the head is delivered, when the usual methods (ie, downward traction and continued maternal pushing) fail to advance delivery. Diagnosis in these cases requires recognition on the part of the delivering physician that shoulder dystocia is present. At that point, continued expulsive force and any real degree of traction no longer are appropriate.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 40: Shoulder dystocia. Washington, DC: American College of Obstetricians and Gynecologists; 2002.
2. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182:689-691.
3. Caldeyro-Barcia R, Poseiro JJ. Physiology of the uterine contraction. Clin Obstet Gynecol. 1960;3:386-392.
4. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77:352-355.
5. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71:389-392.
6. Dystocia: abnormal presentation position and development of the fetus: shoulder dystocia. In: Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD (eds). Williams Obstetrics. 21st ed. New York: McGraw-Hill; 2001:459-464.
7. Dystocia due to abnormalities in presentation position or development of the fetus: shoulder dystocia. In: Cunningham FG, MacDonald PC, Gant NF (eds). Williams Obstetrics. 19th ed. Norwalk, Conn: Appleton & Lange; 1993:509-514.
8. Rodis JF. Management of fetal macrosomia and shoulder dystocia. UpToDate [serial online]. Waltham, MA; November 7, 2007.
The author reports no financial relationships relevant to this article.
Brachial plexus injury is a dreaded sequela of shoulder dystocia, one that lies at the root of many medical liability disputes. Although brachial plexus injury cannot be prevented, most of the commonly used maneuvers for freeing a stuck shoulder are designed to maximize fetal safety and minimize injury.
What is the standard of care when dystocia occurs? Several respected sources have offered conflicting recommendations, particularly in regard to maternal pushing once dystocia is diagnosed. My aim in this article is to clarify the issue.
Endogenous force versus exogenous force
The introduction to the American College of Obstetricians and Gynecologists’ practice bulletin on shoulder dystocia, published in November 2002, provides a useful summary of much of our current knowledge:
Shoulder dystocia is most often an unpredictable and unpreventable obstetric emergency. Failure of the shoulders to deliver spontaneously places both the pregnant woman and fetus at risk for injury. Several maneuvers to release impacted shoulders have been developed, but the urgency of this event makes prospective studies impractical for comparing their effectiveness.1
Because prospective studies are unlikely ever to be performed, some investigators have turned to mathematical modeling to learn more about the forces exerted on the fetal neck overlying the roots of the brachial plexus when a shoulder is impacted against the symphysis pubis.
Gonik and colleagues2 performed elegant modeling of the pressure between the base of the fetal neck and symphysis pubis during dystocia. They utilized data on birth forces gathered by CaldeyroBarcia and Poseiro3 in their classic work on intrauterine pressure. Gonik and colleagues also used data from Allen and associates,4 who measured the force of clinician-applied traction after delivery of the head by having clinicians wear sensory gloves that recorded the force of traction applied.
Gonik and associates2 concluded that the pressure resulting from endogenous forces is four to nine times greater than the pressure generated by a clinician. “Neonatal brachial plexus injury is not a priori explained by iatrogenically induced excessive traction,” they wrote. “Spontaneous endogenous forces may contribute substantially to this type of neonatal trauma.”
How an understanding of endogenous forces alters management
Although fewer than 10% of cases of shoulder dystocia result in permanent brachial plexus injury,1 such injuries are a major source of malpractice litigation in obstetrics, as I noted at the beginning of this article. In most such cases, injury is blamed on excessive traction by the physician (FIGURE). Newer data, such as the study by Gonik and colleagues,2 may implicate expulsive force (ie, maternal pushing) as another, perhaps greater, cause.
As long ago as 1988, Acker and colleagues5 reported on their experience with Erb’s palsy, which was associated with rapid delivery and unusually forceful expulsive effort in one third of cases. Their findings suggest that, when shoulder dystocia occurs and additional maneuvers are necessary to deliver the impacted anterior shoulder, the contribution of potentially harmful endogenous forces should be kept in mind. Counterintuitive strategies, including having the mother stop pushing until the anterior shoulder is freed, may help limit injury.
FIGURE Maternal pushing may contribute to injury
When shoulder dystocia occurs, the progress of labor is interrupted and brachial plexus injury can result, a common cause of litigation. Until now, plaintiff’s attorneys have tended to blame these injuries on the obstetrician and “excess” traction, but it now appears that maternal pushing contributes as well—possibly, to a greater degree than any effort by the physician.
How confusion crept into the literature
The 21st (current) edition of Williams Obstetrics,6 in the section on management of shoulder dystocia, states that “an initial gentle attempt at traction assisted by maternal expulsive efforts is recommended.” There is no reference for this statement. However, a look at the 19th edition of the textbook7 reveals identical wording, and the reference cited is ACOG Technical Bulletin No. 152 (August 1991), entitled Operative Vaginal Delivery. A subsequent version of the same bulletin (no. 196 from August 1994) contains identical wording. By the time that version was replaced by ACOG Practice Bulletin No. 17 (June 2000), however, it no longer contained any information on shoulder dystocia. Instead, ACOG published Practice Pattern No. 7 (October 1997), entitled Shoulder Dystocia. This document did not recommend maternal expulsive force after a diagnosis of shoulder dystocia—in fact, maternal force was not even mentioned. Nor is it mentioned in the current practice bulletin (no. 40 from November 2002), which replaced the previous version of Shoulder Dystocia.
Confusion doesn’t end there
In its section on shoulder dystocia, ACOG’s publication Precis (1998) states:
Management of shoulder dystocia involves both anticipation of and preparation for problems. The key to preventing fetal injury is avoidance of excess traction on the fetal head. When shoulder dystocia is diagnosed, a deliberate and planned sequence of events should be initiated. Pushing should be halted and obstructive causes should be considered. Aggressive fundal pressure or continued pushing will only further impact the anterior shoulder.
We are left with the paradox that the current edition of Williams Obstetrics, in its discussion of shoulder dystocia, carries a statement recommending maternal pushing based on a 1994 ACOG document—a statement that subsequent ACOG documents no longer contain. In fact, one of those documents—Precis—tells us that pushing should be halted, an instruction supported by the mathematical modeling of Gonik and colleagues.2 And a popular online text (UpToDate.com) advises: “The mother should be told not to push during attempts to reposition the fetus.”8 Once the fetus is successfully repositioned, maternal pushing or traction, or both, can be reinstated.
Putting it all into clinical perspective
The current ACOG practice bulletin on shoulder dystocia (no. 40 from November 2002) observes that “retraction of the delivered fetal head against the maternal perineum (turtle sign) may be present and may assist in the diagnosis of shoulder dystocia.” When present, the turtle sign strongly suggests that the anterior shoulder is already impacted against the symphysis pubis. Maternal expulsive forces may have already put enough pressure on the nerve roots of the brachial plexus to cause damage. Any degree of traction or continued maternal pushing is likely to compound an already potentially serious problem.
In such cases, it is prudent to resort to known maneuvers, avoid encouraging continued maternal pushing, and simply support and guide the head without supplying any real traction.
When the turtle sign is absent, shoulder dystocia can be diagnosed only after the head is delivered, when the usual methods (ie, downward traction and continued maternal pushing) fail to advance delivery. Diagnosis in these cases requires recognition on the part of the delivering physician that shoulder dystocia is present. At that point, continued expulsive force and any real degree of traction no longer are appropriate.
The author reports no financial relationships relevant to this article.
Brachial plexus injury is a dreaded sequela of shoulder dystocia, one that lies at the root of many medical liability disputes. Although brachial plexus injury cannot be prevented, most of the commonly used maneuvers for freeing a stuck shoulder are designed to maximize fetal safety and minimize injury.
What is the standard of care when dystocia occurs? Several respected sources have offered conflicting recommendations, particularly in regard to maternal pushing once dystocia is diagnosed. My aim in this article is to clarify the issue.
Endogenous force versus exogenous force
The introduction to the American College of Obstetricians and Gynecologists’ practice bulletin on shoulder dystocia, published in November 2002, provides a useful summary of much of our current knowledge:
Shoulder dystocia is most often an unpredictable and unpreventable obstetric emergency. Failure of the shoulders to deliver spontaneously places both the pregnant woman and fetus at risk for injury. Several maneuvers to release impacted shoulders have been developed, but the urgency of this event makes prospective studies impractical for comparing their effectiveness.1
Because prospective studies are unlikely ever to be performed, some investigators have turned to mathematical modeling to learn more about the forces exerted on the fetal neck overlying the roots of the brachial plexus when a shoulder is impacted against the symphysis pubis.
Gonik and colleagues2 performed elegant modeling of the pressure between the base of the fetal neck and symphysis pubis during dystocia. They utilized data on birth forces gathered by CaldeyroBarcia and Poseiro3 in their classic work on intrauterine pressure. Gonik and colleagues also used data from Allen and associates,4 who measured the force of clinician-applied traction after delivery of the head by having clinicians wear sensory gloves that recorded the force of traction applied.
Gonik and associates2 concluded that the pressure resulting from endogenous forces is four to nine times greater than the pressure generated by a clinician. “Neonatal brachial plexus injury is not a priori explained by iatrogenically induced excessive traction,” they wrote. “Spontaneous endogenous forces may contribute substantially to this type of neonatal trauma.”
How an understanding of endogenous forces alters management
Although fewer than 10% of cases of shoulder dystocia result in permanent brachial plexus injury,1 such injuries are a major source of malpractice litigation in obstetrics, as I noted at the beginning of this article. In most such cases, injury is blamed on excessive traction by the physician (FIGURE). Newer data, such as the study by Gonik and colleagues,2 may implicate expulsive force (ie, maternal pushing) as another, perhaps greater, cause.
As long ago as 1988, Acker and colleagues5 reported on their experience with Erb’s palsy, which was associated with rapid delivery and unusually forceful expulsive effort in one third of cases. Their findings suggest that, when shoulder dystocia occurs and additional maneuvers are necessary to deliver the impacted anterior shoulder, the contribution of potentially harmful endogenous forces should be kept in mind. Counterintuitive strategies, including having the mother stop pushing until the anterior shoulder is freed, may help limit injury.
FIGURE Maternal pushing may contribute to injury
When shoulder dystocia occurs, the progress of labor is interrupted and brachial plexus injury can result, a common cause of litigation. Until now, plaintiff’s attorneys have tended to blame these injuries on the obstetrician and “excess” traction, but it now appears that maternal pushing contributes as well—possibly, to a greater degree than any effort by the physician.
How confusion crept into the literature
The 21st (current) edition of Williams Obstetrics,6 in the section on management of shoulder dystocia, states that “an initial gentle attempt at traction assisted by maternal expulsive efforts is recommended.” There is no reference for this statement. However, a look at the 19th edition of the textbook7 reveals identical wording, and the reference cited is ACOG Technical Bulletin No. 152 (August 1991), entitled Operative Vaginal Delivery. A subsequent version of the same bulletin (no. 196 from August 1994) contains identical wording. By the time that version was replaced by ACOG Practice Bulletin No. 17 (June 2000), however, it no longer contained any information on shoulder dystocia. Instead, ACOG published Practice Pattern No. 7 (October 1997), entitled Shoulder Dystocia. This document did not recommend maternal expulsive force after a diagnosis of shoulder dystocia—in fact, maternal force was not even mentioned. Nor is it mentioned in the current practice bulletin (no. 40 from November 2002), which replaced the previous version of Shoulder Dystocia.
Confusion doesn’t end there
In its section on shoulder dystocia, ACOG’s publication Precis (1998) states:
Management of shoulder dystocia involves both anticipation of and preparation for problems. The key to preventing fetal injury is avoidance of excess traction on the fetal head. When shoulder dystocia is diagnosed, a deliberate and planned sequence of events should be initiated. Pushing should be halted and obstructive causes should be considered. Aggressive fundal pressure or continued pushing will only further impact the anterior shoulder.
We are left with the paradox that the current edition of Williams Obstetrics, in its discussion of shoulder dystocia, carries a statement recommending maternal pushing based on a 1994 ACOG document—a statement that subsequent ACOG documents no longer contain. In fact, one of those documents—Precis—tells us that pushing should be halted, an instruction supported by the mathematical modeling of Gonik and colleagues.2 And a popular online text (UpToDate.com) advises: “The mother should be told not to push during attempts to reposition the fetus.”8 Once the fetus is successfully repositioned, maternal pushing or traction, or both, can be reinstated.
Putting it all into clinical perspective
The current ACOG practice bulletin on shoulder dystocia (no. 40 from November 2002) observes that “retraction of the delivered fetal head against the maternal perineum (turtle sign) may be present and may assist in the diagnosis of shoulder dystocia.” When present, the turtle sign strongly suggests that the anterior shoulder is already impacted against the symphysis pubis. Maternal expulsive forces may have already put enough pressure on the nerve roots of the brachial plexus to cause damage. Any degree of traction or continued maternal pushing is likely to compound an already potentially serious problem.
In such cases, it is prudent to resort to known maneuvers, avoid encouraging continued maternal pushing, and simply support and guide the head without supplying any real traction.
When the turtle sign is absent, shoulder dystocia can be diagnosed only after the head is delivered, when the usual methods (ie, downward traction and continued maternal pushing) fail to advance delivery. Diagnosis in these cases requires recognition on the part of the delivering physician that shoulder dystocia is present. At that point, continued expulsive force and any real degree of traction no longer are appropriate.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 40: Shoulder dystocia. Washington, DC: American College of Obstetricians and Gynecologists; 2002.
2. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182:689-691.
3. Caldeyro-Barcia R, Poseiro JJ. Physiology of the uterine contraction. Clin Obstet Gynecol. 1960;3:386-392.
4. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77:352-355.
5. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71:389-392.
6. Dystocia: abnormal presentation position and development of the fetus: shoulder dystocia. In: Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD (eds). Williams Obstetrics. 21st ed. New York: McGraw-Hill; 2001:459-464.
7. Dystocia due to abnormalities in presentation position or development of the fetus: shoulder dystocia. In: Cunningham FG, MacDonald PC, Gant NF (eds). Williams Obstetrics. 19th ed. Norwalk, Conn: Appleton & Lange; 1993:509-514.
8. Rodis JF. Management of fetal macrosomia and shoulder dystocia. UpToDate [serial online]. Waltham, MA; November 7, 2007.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 40: Shoulder dystocia. Washington, DC: American College of Obstetricians and Gynecologists; 2002.
2. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182:689-691.
3. Caldeyro-Barcia R, Poseiro JJ. Physiology of the uterine contraction. Clin Obstet Gynecol. 1960;3:386-392.
4. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77:352-355.
5. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71:389-392.
6. Dystocia: abnormal presentation position and development of the fetus: shoulder dystocia. In: Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD (eds). Williams Obstetrics. 21st ed. New York: McGraw-Hill; 2001:459-464.
7. Dystocia due to abnormalities in presentation position or development of the fetus: shoulder dystocia. In: Cunningham FG, MacDonald PC, Gant NF (eds). Williams Obstetrics. 19th ed. Norwalk, Conn: Appleton & Lange; 1993:509-514.
8. Rodis JF. Management of fetal macrosomia and shoulder dystocia. UpToDate [serial online]. Waltham, MA; November 7, 2007.
PELVIC SURGERY
The authors report no financial relationships relevant to this article.
The use of transvaginal mesh—with or without trocar placement—is surrounded by controversy. A number of minimally invasive vaginal mesh kits are commercially available for the repair of pelvic organ prolapse, and new kits are entering the market rapidly. The challenge is determining whether these new techniques are as effective and safe as traditional prolapse repairs.
Although the use of permanent mesh to repair prolapse has been explored in retrospective and prospective studies, no rigorous controlled trials have compared these new procedures with abdominal sacrocolpopexy or uterosacral ligament suspension, for example. The current body of literature does suggest a high rate of recurrent prolapse after traditional anterior or posterior colporrhaphy, and the use of allograft material has not been shown to improve outcomes. Surgeons are now turning their attention to permanent polypropylene mesh as a possible alternative. In addition, repair of the vaginal apex at the time of anterior and posterior vaginal wall repair is being explored as a way to increase durability of the repair. The new trocar-delivered mesh kits address this issue by suspending the vaginal vault while providing support to the vaginal walls.
This article highlights three recent studies that focus on a new trocar-delivered, protected, low-weight polypropylene mesh (Ugytex, distributed by Bard as Pelvitex) and three trocar-delivered mesh kits (Prolift, Apogee, and Perigee).
One-year outcomes encouraging for low-weight polypropylene mesh
De Tayrac R, Devoldere G, Renaudie J, Villard P, Guilbaud O, Eglin G. Prolapse repair by vaginal route using a new protected low-weight polypropylene mesh: 1-year functional and anatomical outcome in a prospective multicentre study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:251–256.
This study evaluated functional and anatomic outcomes after placement for prolapse repair of low-weight polypropylene mesh protected by absorbable hydrophilic film. The film, a combination of atelocollagen, polyethylene glycol, and glycerol, is designed to protect pelvic organs from acute inflammation during healing. In a separate investigation of unprotected, heavyweight polypropylene mesh in prolapse repair, the anatomic success rate ranged from 75% to 100%, but the rate of mesh erosion (13%) and dyspareunia (69%) seemed unacceptably high.1
Rigorous preoperative assessment
In this trial, 230 women with symptomatic vaginal wall prolapse were recruited at 13 centers in a consecutive fashion. At enrollment, all patients were measured using the pelvic organ prolapse quantitative staging system (POP-Q). They also completed the validated Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. The presence and severity of dyspareunia were also recorded, as well as the Urinary Dysfunction Measurement Scale. All participants had prolapse equal to or exceeding stage II.
Surgeons used trocars to percutaneously place a low-weight (38 g/m2) and highly porous polypropylene monofilament mesh (Ugytex/Pelvitex) for vaginal repair and performed any concomitant procedures. Perioperative and postoperative complications were recorded. Patients were evaluated at 6 weeks, 6 months, and 1 year. The first 143 patients with at least 10 months of follow-up were analyzed, with a mean follow-up of 13±2 months (range: 10–19). Anatomic cure was defined as no prolapse greater than or equal to stage II.
Patient satisfaction was high
The anatomic cure rate was 92.3%, with a 6.8% recurrence of anterior vaginal wall prolapse and 2.6% recurrence of posterior vaginal wall prolapse. Only one patient with recurrence was symptomatic.
Six of 143 patients (4.2%) sustained an intraoperative complication: three bladder injuries, one rectal injury, one uterine artery hemorrhage (during hysterectomy), and one vaginal sulcus perforation (during transobturator tape placement). The most significant postoperative complication related to the vaginal mesh kit was vaginal hematoma; one of the two cases required reoperation and partial removal of the mesh.
Nine patients developed mesh erosion in the first 3 months, for an erosion rate of 6.3%. Six required partial excision of the mesh. Overall, symptoms and quality of life improved significantly, with an overall satisfaction rate at follow-up of 96.5%. No significant difference was noted between pre- and postoperative rates of dyspareunia.
Further evaluation is warranted
The authors are already conducting a randomized trial to compare anterior vaginal wall repair using this low-weight polypropylene mesh with traditional anterior colporrhaphy to confirm and explore these results.
Note: Bard now offers a kit called Avaulta Plus that uses the same mesh material with a trocar delivery system, previously lacking (although investigators used trocars in this study).
Perioperative complications were uncommon with Prolift system
Altman D, Falconer C. Perioperative morbidity using transvaginal mesh in pelvic organ prolapse repair. Obstet Gynecol. 2007;109:303–308.
This study explored the frequency and characteristics of perioperative complications associated with the use of Prolift, a transvaginal mesh system for the repair of pelvic organ prolapse (FIGURE). Twenty-five centers participated by registering a standardized safety protocol form for 248 women who underwent surgery using the system over a 6-month period. The form included information about perioperative complications, adverse intraoperative events, and the associated hospital stay, as well as obstetric and gynecologic medical history and previous pelvic surgery.
Pelvic organ perforation (lower urinary tract or anorectal injury) and blood loss greater than 1,000 mL were recorded as major complications, and any other adverse events related to the hospital stay were documented as minor complications. Most of the cohort had already undergone prolapse repair, and prolapse had recurred in the same vaginal compartment.
One author was an educational adviser for Gynecare Sweden AB, and the other an adviser for Johnson & Johnson. Although the study was funded entirely by university-administered research funds, pretrial scientific meetings were paid for by Gynecare Sweden AB.
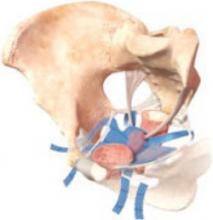
FIGURE: Mesh support of pelvic organs
Prolift mesh in final position, with extension arms passed through the sacrospinous ligaments and the obturator foramen bilaterally.
4.4% rate of serious complications
Serious complications occurred in 4.4% (11 of 248) of cases (95% confidence interval [CI], 2.5–7.8). The predominant complication was visceral injury, which included bladder, urethral, and rectal perforation. One patient had blood loss exceeding 1,000 mL.
Minor complications occurred in 44 patients (18%). The most common minor complication was urinary tract infection. Adverse events included urinary retention requiring catheterization, anemia, transfusion, fever, groin and buttock pain, and vaginal hematoma, among others.
Concurrent pelvic floor surgery increased the risk for minor complications (odds ratio, 2.8; 95% CI, 1.1–6.9). Concurrent procedures included vaginal hysterectomy, sling procedure with tension-free vaginal tape or transobturator tension-free tape, sacrospinal fixation, repair of vaginal enterocele, and bilateral salpingo-oophorectomy. This risk analysis identified no other predictors of outcome.
Posterior/apical repair
- Adequately infiltrate the vaginal epithelium with diluted epinephrine solution, especially toward the lateral apices, to facilitate hemostasis and dissection
- Be thorough in lateral dissection toward the ischial spine and stay in the proper surgical plane to create a thick vaginal epithelial flap
- Palpate the ischial spine, with the preoperatively packed rectum retracted medially
- During passage of the trocar, place an index finger along the vaginal dissection to palpate the trocar in the ischiorectal fossa and deep to the levator ani muscles until the tip is palpated at the level of the ischial spine
- Pass the trocar through the arcus tendineus/levator fascia at the level of the ischial spine, as shown below:
- Do not apply excess tension to the straps of the graft material
- Do not trim the vaginal epithelium
Anterior wall (obturator foramen trocar passage)
- Same key points as posterior wall technique, but in anterior repair, there are two passes through the obturator foramen
- The first trocar is inserted into the inferior obturator foramen, rotated, and guided with the surgeon’s finger inserted into and held in the vaginal dissection, as shown below:
- The superior passage exits close to the bladder neck, and the inferior passage approximates the ischial spine. Penetrate along the arcus tendineus approximately 1 cm from the ischial spine
Caution! Keep summary points in context
These key points are not intended as formal medical training, but as general information only. Continued research into these techniques is needed to assess long-term outcomes.
Short-term outcomes data only
Because this study focused on immediate complications, no long-term data on such complications as persistent pain, mesh erosion, or infection were collected.
All surgeons underwent hands-on training with the transvaginal repair system before patients were enrolled in the study. Nevertheless, the authors observe that many repair procedures were performed at the beginning of the physicians’ learning curve, with a higher number of complications than would be expected from more experienced surgeons.
The data may also have been affected by selection bias (ie, toward more complicated cases), given that most patients had already undergone prolapse repair.
Two systems yield excellent short-term results in women with recurrent prolapse
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R. Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1059–1064.
This retrospective study involved women who had already undergone one or more prolapse repairs. These patients then underwent reoperation with mesh-reinforced repair. The authors hypothesized that recurrent prolapse represents poor tissue quality, necessitating the use of mesh in subsequent repairs. Both pre- and postoperative symptoms and functional changes were analyzed, with a special focus on mesh erosion and sexual function.
Details of the study
Of 145 women who underwent repair with the Apogee (apical posterior) or Perigee (anterior wall) system during a 1-year period, 120 were included in the analysis. The other 25 patients were excluded because they did not return for follow-up, were missing urodynamic data, or had inaccurate POP-Q staging. All patients had recurrent stage III posterior or anterior vaginal wall prolapse. Forty percent of patients had an apical posterior repair, and 60% had anterior wall repair. None had both procedures performed simultaneously.
All patients had undergone hysterectomy and received vaginal estrogen before and after surgery. Urinary incontinence was treated in a two-step fashion; that is, it was not addressed until 3 months after repair of the prolapse. Routine follow-up occurred at 1 month and 1 year after surgery.
One-year cure rate was 93%
No perioperative or intraoperative complications occurred, and mean operative time was 35±4.5 minutes. Mesh erosion occurred in four patients (3%) and involved anterior mesh placement only. No mesh infections were noted.
At 1 year, 93% of women were anatomically cured of prolapse (ie, they had less than stage II prolapse). Prolapse recurred in eight women; all cases involved the anterior compartment.
No dyspareunia was associated with the repair. In fact, prolapse-associated dyspareunia resolved in all 15 women who reported this symptom before surgery. In addition, questionnaires about quality of life and satisfaction revealed significant improvement after mesh placement (P=.023).
The authors attribute the positive results to the fact that both surgeons involved in the study used the technique on 15 patients before operating on study participants, minimizing the effect of the learning curve. The authors were also careful about patient selection.
Results merit cautious optimism
The authors propose that the low erosion rate and lack of new-onset dyspareunia after surgery may be misleading because long-term results have not yet been obtained. We also speculate that precise dissection in the proper surgical plane likely minimized early erosions.
Reference
1. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. Br J Obstet Gynaecol. 2004;111:1-5.
The authors report no financial relationships relevant to this article.
The use of transvaginal mesh—with or without trocar placement—is surrounded by controversy. A number of minimally invasive vaginal mesh kits are commercially available for the repair of pelvic organ prolapse, and new kits are entering the market rapidly. The challenge is determining whether these new techniques are as effective and safe as traditional prolapse repairs.
Although the use of permanent mesh to repair prolapse has been explored in retrospective and prospective studies, no rigorous controlled trials have compared these new procedures with abdominal sacrocolpopexy or uterosacral ligament suspension, for example. The current body of literature does suggest a high rate of recurrent prolapse after traditional anterior or posterior colporrhaphy, and the use of allograft material has not been shown to improve outcomes. Surgeons are now turning their attention to permanent polypropylene mesh as a possible alternative. In addition, repair of the vaginal apex at the time of anterior and posterior vaginal wall repair is being explored as a way to increase durability of the repair. The new trocar-delivered mesh kits address this issue by suspending the vaginal vault while providing support to the vaginal walls.
This article highlights three recent studies that focus on a new trocar-delivered, protected, low-weight polypropylene mesh (Ugytex, distributed by Bard as Pelvitex) and three trocar-delivered mesh kits (Prolift, Apogee, and Perigee).
One-year outcomes encouraging for low-weight polypropylene mesh
De Tayrac R, Devoldere G, Renaudie J, Villard P, Guilbaud O, Eglin G. Prolapse repair by vaginal route using a new protected low-weight polypropylene mesh: 1-year functional and anatomical outcome in a prospective multicentre study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:251–256.
This study evaluated functional and anatomic outcomes after placement for prolapse repair of low-weight polypropylene mesh protected by absorbable hydrophilic film. The film, a combination of atelocollagen, polyethylene glycol, and glycerol, is designed to protect pelvic organs from acute inflammation during healing. In a separate investigation of unprotected, heavyweight polypropylene mesh in prolapse repair, the anatomic success rate ranged from 75% to 100%, but the rate of mesh erosion (13%) and dyspareunia (69%) seemed unacceptably high.1
Rigorous preoperative assessment
In this trial, 230 women with symptomatic vaginal wall prolapse were recruited at 13 centers in a consecutive fashion. At enrollment, all patients were measured using the pelvic organ prolapse quantitative staging system (POP-Q). They also completed the validated Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. The presence and severity of dyspareunia were also recorded, as well as the Urinary Dysfunction Measurement Scale. All participants had prolapse equal to or exceeding stage II.
Surgeons used trocars to percutaneously place a low-weight (38 g/m2) and highly porous polypropylene monofilament mesh (Ugytex/Pelvitex) for vaginal repair and performed any concomitant procedures. Perioperative and postoperative complications were recorded. Patients were evaluated at 6 weeks, 6 months, and 1 year. The first 143 patients with at least 10 months of follow-up were analyzed, with a mean follow-up of 13±2 months (range: 10–19). Anatomic cure was defined as no prolapse greater than or equal to stage II.
Patient satisfaction was high
The anatomic cure rate was 92.3%, with a 6.8% recurrence of anterior vaginal wall prolapse and 2.6% recurrence of posterior vaginal wall prolapse. Only one patient with recurrence was symptomatic.
Six of 143 patients (4.2%) sustained an intraoperative complication: three bladder injuries, one rectal injury, one uterine artery hemorrhage (during hysterectomy), and one vaginal sulcus perforation (during transobturator tape placement). The most significant postoperative complication related to the vaginal mesh kit was vaginal hematoma; one of the two cases required reoperation and partial removal of the mesh.
Nine patients developed mesh erosion in the first 3 months, for an erosion rate of 6.3%. Six required partial excision of the mesh. Overall, symptoms and quality of life improved significantly, with an overall satisfaction rate at follow-up of 96.5%. No significant difference was noted between pre- and postoperative rates of dyspareunia.
Further evaluation is warranted
The authors are already conducting a randomized trial to compare anterior vaginal wall repair using this low-weight polypropylene mesh with traditional anterior colporrhaphy to confirm and explore these results.
Note: Bard now offers a kit called Avaulta Plus that uses the same mesh material with a trocar delivery system, previously lacking (although investigators used trocars in this study).
Perioperative complications were uncommon with Prolift system
Altman D, Falconer C. Perioperative morbidity using transvaginal mesh in pelvic organ prolapse repair. Obstet Gynecol. 2007;109:303–308.
This study explored the frequency and characteristics of perioperative complications associated with the use of Prolift, a transvaginal mesh system for the repair of pelvic organ prolapse (FIGURE). Twenty-five centers participated by registering a standardized safety protocol form for 248 women who underwent surgery using the system over a 6-month period. The form included information about perioperative complications, adverse intraoperative events, and the associated hospital stay, as well as obstetric and gynecologic medical history and previous pelvic surgery.
Pelvic organ perforation (lower urinary tract or anorectal injury) and blood loss greater than 1,000 mL were recorded as major complications, and any other adverse events related to the hospital stay were documented as minor complications. Most of the cohort had already undergone prolapse repair, and prolapse had recurred in the same vaginal compartment.
One author was an educational adviser for Gynecare Sweden AB, and the other an adviser for Johnson & Johnson. Although the study was funded entirely by university-administered research funds, pretrial scientific meetings were paid for by Gynecare Sweden AB.
FIGURE: Mesh support of pelvic organs
Prolift mesh in final position, with extension arms passed through the sacrospinous ligaments and the obturator foramen bilaterally.
4.4% rate of serious complications
Serious complications occurred in 4.4% (11 of 248) of cases (95% confidence interval [CI], 2.5–7.8). The predominant complication was visceral injury, which included bladder, urethral, and rectal perforation. One patient had blood loss exceeding 1,000 mL.
Minor complications occurred in 44 patients (18%). The most common minor complication was urinary tract infection. Adverse events included urinary retention requiring catheterization, anemia, transfusion, fever, groin and buttock pain, and vaginal hematoma, among others.
Concurrent pelvic floor surgery increased the risk for minor complications (odds ratio, 2.8; 95% CI, 1.1–6.9). Concurrent procedures included vaginal hysterectomy, sling procedure with tension-free vaginal tape or transobturator tension-free tape, sacrospinal fixation, repair of vaginal enterocele, and bilateral salpingo-oophorectomy. This risk analysis identified no other predictors of outcome.
Posterior/apical repair
- Adequately infiltrate the vaginal epithelium with diluted epinephrine solution, especially toward the lateral apices, to facilitate hemostasis and dissection
- Be thorough in lateral dissection toward the ischial spine and stay in the proper surgical plane to create a thick vaginal epithelial flap
- Palpate the ischial spine, with the preoperatively packed rectum retracted medially
- During passage of the trocar, place an index finger along the vaginal dissection to palpate the trocar in the ischiorectal fossa and deep to the levator ani muscles until the tip is palpated at the level of the ischial spine
- Pass the trocar through the arcus tendineus/levator fascia at the level of the ischial spine, as shown below:
- Do not apply excess tension to the straps of the graft material
- Do not trim the vaginal epithelium
Anterior wall (obturator foramen trocar passage)
- Same key points as posterior wall technique, but in anterior repair, there are two passes through the obturator foramen
- The first trocar is inserted into the inferior obturator foramen, rotated, and guided with the surgeon’s finger inserted into and held in the vaginal dissection, as shown below:
- The superior passage exits close to the bladder neck, and the inferior passage approximates the ischial spine. Penetrate along the arcus tendineus approximately 1 cm from the ischial spine
Caution! Keep summary points in context
These key points are not intended as formal medical training, but as general information only. Continued research into these techniques is needed to assess long-term outcomes.
Short-term outcomes data only
Because this study focused on immediate complications, no long-term data on such complications as persistent pain, mesh erosion, or infection were collected.
All surgeons underwent hands-on training with the transvaginal repair system before patients were enrolled in the study. Nevertheless, the authors observe that many repair procedures were performed at the beginning of the physicians’ learning curve, with a higher number of complications than would be expected from more experienced surgeons.
The data may also have been affected by selection bias (ie, toward more complicated cases), given that most patients had already undergone prolapse repair.
Two systems yield excellent short-term results in women with recurrent prolapse
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R. Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1059–1064.
This retrospective study involved women who had already undergone one or more prolapse repairs. These patients then underwent reoperation with mesh-reinforced repair. The authors hypothesized that recurrent prolapse represents poor tissue quality, necessitating the use of mesh in subsequent repairs. Both pre- and postoperative symptoms and functional changes were analyzed, with a special focus on mesh erosion and sexual function.
Details of the study
Of 145 women who underwent repair with the Apogee (apical posterior) or Perigee (anterior wall) system during a 1-year period, 120 were included in the analysis. The other 25 patients were excluded because they did not return for follow-up, were missing urodynamic data, or had inaccurate POP-Q staging. All patients had recurrent stage III posterior or anterior vaginal wall prolapse. Forty percent of patients had an apical posterior repair, and 60% had anterior wall repair. None had both procedures performed simultaneously.
All patients had undergone hysterectomy and received vaginal estrogen before and after surgery. Urinary incontinence was treated in a two-step fashion; that is, it was not addressed until 3 months after repair of the prolapse. Routine follow-up occurred at 1 month and 1 year after surgery.
One-year cure rate was 93%
No perioperative or intraoperative complications occurred, and mean operative time was 35±4.5 minutes. Mesh erosion occurred in four patients (3%) and involved anterior mesh placement only. No mesh infections were noted.
At 1 year, 93% of women were anatomically cured of prolapse (ie, they had less than stage II prolapse). Prolapse recurred in eight women; all cases involved the anterior compartment.
No dyspareunia was associated with the repair. In fact, prolapse-associated dyspareunia resolved in all 15 women who reported this symptom before surgery. In addition, questionnaires about quality of life and satisfaction revealed significant improvement after mesh placement (P=.023).
The authors attribute the positive results to the fact that both surgeons involved in the study used the technique on 15 patients before operating on study participants, minimizing the effect of the learning curve. The authors were also careful about patient selection.
Results merit cautious optimism
The authors propose that the low erosion rate and lack of new-onset dyspareunia after surgery may be misleading because long-term results have not yet been obtained. We also speculate that precise dissection in the proper surgical plane likely minimized early erosions.
The authors report no financial relationships relevant to this article.
The use of transvaginal mesh—with or without trocar placement—is surrounded by controversy. A number of minimally invasive vaginal mesh kits are commercially available for the repair of pelvic organ prolapse, and new kits are entering the market rapidly. The challenge is determining whether these new techniques are as effective and safe as traditional prolapse repairs.
Although the use of permanent mesh to repair prolapse has been explored in retrospective and prospective studies, no rigorous controlled trials have compared these new procedures with abdominal sacrocolpopexy or uterosacral ligament suspension, for example. The current body of literature does suggest a high rate of recurrent prolapse after traditional anterior or posterior colporrhaphy, and the use of allograft material has not been shown to improve outcomes. Surgeons are now turning their attention to permanent polypropylene mesh as a possible alternative. In addition, repair of the vaginal apex at the time of anterior and posterior vaginal wall repair is being explored as a way to increase durability of the repair. The new trocar-delivered mesh kits address this issue by suspending the vaginal vault while providing support to the vaginal walls.
This article highlights three recent studies that focus on a new trocar-delivered, protected, low-weight polypropylene mesh (Ugytex, distributed by Bard as Pelvitex) and three trocar-delivered mesh kits (Prolift, Apogee, and Perigee).
One-year outcomes encouraging for low-weight polypropylene mesh
De Tayrac R, Devoldere G, Renaudie J, Villard P, Guilbaud O, Eglin G. Prolapse repair by vaginal route using a new protected low-weight polypropylene mesh: 1-year functional and anatomical outcome in a prospective multicentre study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:251–256.
This study evaluated functional and anatomic outcomes after placement for prolapse repair of low-weight polypropylene mesh protected by absorbable hydrophilic film. The film, a combination of atelocollagen, polyethylene glycol, and glycerol, is designed to protect pelvic organs from acute inflammation during healing. In a separate investigation of unprotected, heavyweight polypropylene mesh in prolapse repair, the anatomic success rate ranged from 75% to 100%, but the rate of mesh erosion (13%) and dyspareunia (69%) seemed unacceptably high.1
Rigorous preoperative assessment
In this trial, 230 women with symptomatic vaginal wall prolapse were recruited at 13 centers in a consecutive fashion. At enrollment, all patients were measured using the pelvic organ prolapse quantitative staging system (POP-Q). They also completed the validated Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. The presence and severity of dyspareunia were also recorded, as well as the Urinary Dysfunction Measurement Scale. All participants had prolapse equal to or exceeding stage II.
Surgeons used trocars to percutaneously place a low-weight (38 g/m2) and highly porous polypropylene monofilament mesh (Ugytex/Pelvitex) for vaginal repair and performed any concomitant procedures. Perioperative and postoperative complications were recorded. Patients were evaluated at 6 weeks, 6 months, and 1 year. The first 143 patients with at least 10 months of follow-up were analyzed, with a mean follow-up of 13±2 months (range: 10–19). Anatomic cure was defined as no prolapse greater than or equal to stage II.
Patient satisfaction was high
The anatomic cure rate was 92.3%, with a 6.8% recurrence of anterior vaginal wall prolapse and 2.6% recurrence of posterior vaginal wall prolapse. Only one patient with recurrence was symptomatic.
Six of 143 patients (4.2%) sustained an intraoperative complication: three bladder injuries, one rectal injury, one uterine artery hemorrhage (during hysterectomy), and one vaginal sulcus perforation (during transobturator tape placement). The most significant postoperative complication related to the vaginal mesh kit was vaginal hematoma; one of the two cases required reoperation and partial removal of the mesh.
Nine patients developed mesh erosion in the first 3 months, for an erosion rate of 6.3%. Six required partial excision of the mesh. Overall, symptoms and quality of life improved significantly, with an overall satisfaction rate at follow-up of 96.5%. No significant difference was noted between pre- and postoperative rates of dyspareunia.
Further evaluation is warranted
The authors are already conducting a randomized trial to compare anterior vaginal wall repair using this low-weight polypropylene mesh with traditional anterior colporrhaphy to confirm and explore these results.
Note: Bard now offers a kit called Avaulta Plus that uses the same mesh material with a trocar delivery system, previously lacking (although investigators used trocars in this study).
Perioperative complications were uncommon with Prolift system
Altman D, Falconer C. Perioperative morbidity using transvaginal mesh in pelvic organ prolapse repair. Obstet Gynecol. 2007;109:303–308.
This study explored the frequency and characteristics of perioperative complications associated with the use of Prolift, a transvaginal mesh system for the repair of pelvic organ prolapse (FIGURE). Twenty-five centers participated by registering a standardized safety protocol form for 248 women who underwent surgery using the system over a 6-month period. The form included information about perioperative complications, adverse intraoperative events, and the associated hospital stay, as well as obstetric and gynecologic medical history and previous pelvic surgery.
Pelvic organ perforation (lower urinary tract or anorectal injury) and blood loss greater than 1,000 mL were recorded as major complications, and any other adverse events related to the hospital stay were documented as minor complications. Most of the cohort had already undergone prolapse repair, and prolapse had recurred in the same vaginal compartment.
One author was an educational adviser for Gynecare Sweden AB, and the other an adviser for Johnson & Johnson. Although the study was funded entirely by university-administered research funds, pretrial scientific meetings were paid for by Gynecare Sweden AB.
FIGURE: Mesh support of pelvic organs
Prolift mesh in final position, with extension arms passed through the sacrospinous ligaments and the obturator foramen bilaterally.
4.4% rate of serious complications
Serious complications occurred in 4.4% (11 of 248) of cases (95% confidence interval [CI], 2.5–7.8). The predominant complication was visceral injury, which included bladder, urethral, and rectal perforation. One patient had blood loss exceeding 1,000 mL.
Minor complications occurred in 44 patients (18%). The most common minor complication was urinary tract infection. Adverse events included urinary retention requiring catheterization, anemia, transfusion, fever, groin and buttock pain, and vaginal hematoma, among others.
Concurrent pelvic floor surgery increased the risk for minor complications (odds ratio, 2.8; 95% CI, 1.1–6.9). Concurrent procedures included vaginal hysterectomy, sling procedure with tension-free vaginal tape or transobturator tension-free tape, sacrospinal fixation, repair of vaginal enterocele, and bilateral salpingo-oophorectomy. This risk analysis identified no other predictors of outcome.
Posterior/apical repair
- Adequately infiltrate the vaginal epithelium with diluted epinephrine solution, especially toward the lateral apices, to facilitate hemostasis and dissection
- Be thorough in lateral dissection toward the ischial spine and stay in the proper surgical plane to create a thick vaginal epithelial flap
- Palpate the ischial spine, with the preoperatively packed rectum retracted medially
- During passage of the trocar, place an index finger along the vaginal dissection to palpate the trocar in the ischiorectal fossa and deep to the levator ani muscles until the tip is palpated at the level of the ischial spine
- Pass the trocar through the arcus tendineus/levator fascia at the level of the ischial spine, as shown below:
- Do not apply excess tension to the straps of the graft material
- Do not trim the vaginal epithelium
Anterior wall (obturator foramen trocar passage)
- Same key points as posterior wall technique, but in anterior repair, there are two passes through the obturator foramen
- The first trocar is inserted into the inferior obturator foramen, rotated, and guided with the surgeon’s finger inserted into and held in the vaginal dissection, as shown below:
- The superior passage exits close to the bladder neck, and the inferior passage approximates the ischial spine. Penetrate along the arcus tendineus approximately 1 cm from the ischial spine
Caution! Keep summary points in context
These key points are not intended as formal medical training, but as general information only. Continued research into these techniques is needed to assess long-term outcomes.
Short-term outcomes data only
Because this study focused on immediate complications, no long-term data on such complications as persistent pain, mesh erosion, or infection were collected.
All surgeons underwent hands-on training with the transvaginal repair system before patients were enrolled in the study. Nevertheless, the authors observe that many repair procedures were performed at the beginning of the physicians’ learning curve, with a higher number of complications than would be expected from more experienced surgeons.
The data may also have been affected by selection bias (ie, toward more complicated cases), given that most patients had already undergone prolapse repair.
Two systems yield excellent short-term results in women with recurrent prolapse
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R. Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1059–1064.
This retrospective study involved women who had already undergone one or more prolapse repairs. These patients then underwent reoperation with mesh-reinforced repair. The authors hypothesized that recurrent prolapse represents poor tissue quality, necessitating the use of mesh in subsequent repairs. Both pre- and postoperative symptoms and functional changes were analyzed, with a special focus on mesh erosion and sexual function.
Details of the study
Of 145 women who underwent repair with the Apogee (apical posterior) or Perigee (anterior wall) system during a 1-year period, 120 were included in the analysis. The other 25 patients were excluded because they did not return for follow-up, were missing urodynamic data, or had inaccurate POP-Q staging. All patients had recurrent stage III posterior or anterior vaginal wall prolapse. Forty percent of patients had an apical posterior repair, and 60% had anterior wall repair. None had both procedures performed simultaneously.
All patients had undergone hysterectomy and received vaginal estrogen before and after surgery. Urinary incontinence was treated in a two-step fashion; that is, it was not addressed until 3 months after repair of the prolapse. Routine follow-up occurred at 1 month and 1 year after surgery.
One-year cure rate was 93%
No perioperative or intraoperative complications occurred, and mean operative time was 35±4.5 minutes. Mesh erosion occurred in four patients (3%) and involved anterior mesh placement only. No mesh infections were noted.
At 1 year, 93% of women were anatomically cured of prolapse (ie, they had less than stage II prolapse). Prolapse recurred in eight women; all cases involved the anterior compartment.
No dyspareunia was associated with the repair. In fact, prolapse-associated dyspareunia resolved in all 15 women who reported this symptom before surgery. In addition, questionnaires about quality of life and satisfaction revealed significant improvement after mesh placement (P=.023).
The authors attribute the positive results to the fact that both surgeons involved in the study used the technique on 15 patients before operating on study participants, minimizing the effect of the learning curve. The authors were also careful about patient selection.
Results merit cautious optimism
The authors propose that the low erosion rate and lack of new-onset dyspareunia after surgery may be misleading because long-term results have not yet been obtained. We also speculate that precise dissection in the proper surgical plane likely minimized early erosions.
Reference
1. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. Br J Obstet Gynaecol. 2004;111:1-5.
Reference
1. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. Br J Obstet Gynaecol. 2004;111:1-5.
A Hepatitis C Support Group
Gram-negative Toe Web Infection Complicated by Myiasis
5 Points on Ankle Fractures: It Is Not Just a "Simple" Ankle Fracture
TECHNOLOGY
Over its history, surgery has been defined by the tools available to practitioners. In our era, opportunities to offer patients minimally invasive surgery have expanded dramatically as methods of establishing visualization, achieving hemostasis, and performing tissue dissection have improved. (I remember trying to treat ectopic pregnancy laparoscopically in the early 1980s without benefit of a camera or suction irrigator!)
For surgeons of my generation, the ability to access the abdominal cavity minimally invasively and to clearly visualize the contents was a significant step forward. Hysteroscopic myomectomy was another tremendous incremental improvement for patients with submucous myomas. But there is much more in store for the coming years.
Where are we headed in the next wave of gynecologic surgery? Will patients require an incision at all? Is there room to advance beyond laparoscopy and hysteroscopy? What innovations will industry offer us in the 21st century?
In this article, I describe something that is fairly familiar to most of us by now, but which is not yet practical for routine gynecologic procedures—robotically assisted endoscopic surgery. I then move on to a phenomenon that, in many respects, is still being imagined—natural orifice transluminal endoscopic surgery, or NOTES.
Robotic systems are best suited for complex surgery
Laparoscopic surgery is limited by the two-dimensional view and need for hand control of long, rigid instruments through ancillary trocar sites. Although these impediments can be overcome with practice and experience, the inability to see in three dimensions and the compromised range of motion hamper optimal management of some surgical procedures.
A number of technological advances may significantly improve our ability to perform suture-intensive or anatomically challenging operations. Several companies are developing camera systems that will permit a three-dimensional view without the need for multiple visual ports. The technology is borrowed from the world of insects, which “see” through multiple lenses within the same eye. The application of such visual processing to optical systems for endoscopic surgery will be a huge advance for laparoscopy—one that is still being perfected by industry. In 2007, the da Vinci robot system (Intuitive Surgical) offers the best opportunity to achieve both three-dimensional visualization and an ability to “feel” tissue and manipulate instruments with markedly increased range of motion.
Cost is the limiting factor
Although the da Vinci system has revolutionized the practice of urology, enabling radical nerve-sparing prostatectomy, its utility in gynecology is still being investigated. Several centers use the robot for a significant percentage of their laparoscopic gynecologic surgery, but the setup time, learning curve, and intraoperative time required make the da Vinci system an impractical tool for many routine procedures. Its true advantage lies in suture-intensive procedures and in surgeries that require meticulous dissection close to major structures. In gynecology, the laparoscopic procedures most likely to benefit from the three-dimensional view and articulating instruments are sacral colpopexy, myomectomy (FIGURE), radical hysterectomy, and lymph node dissection.
Although it is interesting and enjoyable to use robotic technology for routine laparoscopic procedures, I believe the cost is prohibitive—several million dollars for each robot. If the financial barriers are removed, however, this system will be a welcome addition to the toolset for gynecologic laparoscopic surgery. Until then, we need to make intelligent use of this powerful tool.
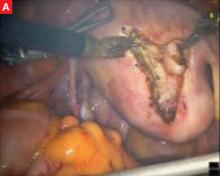
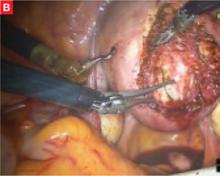
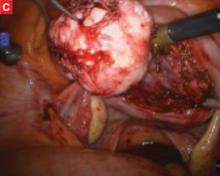
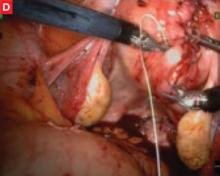
FIGURE Robotic myomectomy
A: Using the da Vinci robot, the surgeon incises the myometrium down to the fibroid.
B: After grasping the fibroid, the surgeon dissects it away from the surrounding myometrium.
C: As the fibroid is freed, another small tumor becomes apparent at the bottom right, and is also removed.
D: The myometrium is sutured in layers after removal of the fibroids. Photos courtesy of Paul Indman, MD.Just as many of us were able to perform laparoscopic surgery without a three-chip camera and high-tech energy system for hemostasis until the cost of those technologies could be recouped in reduced operating room time and fewer conversions to laparotomy, so will today’s surgeons have to continue performing laparoscopic adnexal surgery, routine hysterectomy, and treatment of ectopic pregnancy the “old-fashioned” way. For complex procedures, however, the da Vinci system is proving to be a major advance in endoscopic surgery.
Look for other, perhaps less expensive, technologies coming down the road that will, at the very least, permit three-dimensional visualization without the need for robotics. In addition, as I discuss in the next section, miniaturization of robotics is on the horizon. Only our imagination limits our thinking about how robotic technology may be used in the not-too-distant future.
ROBOTICS IN GYNECOLOGY
Selected studies
- Bocca S, Stadtmauer L, Oehninger S. Uncomplicated fullterm pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online. 2007;14:246–249.
- Elliott DS, Chow GK, Gettman M. Current status of robotics in female urology and gynecology. World J Urol. 2006;24:188–192.
- Fiorentino RP, Zepeda MA, Goldstein BH, John CR, Rettenmaier MA. Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol. 2006;13:60–63.
- Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28:77–82.
NOTES takes “minimally invasive” to a new level
Imagine performing surgery for ectopic pregnancy or endometriosis in your office, without anesthesia. Think this is impossible? Think again!
A newer, perhaps better, and definitely less invasive version of endoscopic surgery is on the horizon—natural orifice transluminal endoscopic surgery, or NOTES. In May, a surgeon in Portland, Oregon, performed a cholecystectomy by dropping an endoscope through the patient’s mouth into the stomach, drilling an opening in the gastric wall, and placing small instruments through that opening to perform the surgery. The specimen was then pulled through the small opening in the stomach and retrieved through the patient’s mouth! The stomach was closed with an endoscopic stapling device.
NOTES appears to be the next true advance in minimally invasive surgery. This should come as no surprise to gynecologists. We are the champions of transcervical and transvaginal surgery. General surgeons and gastroenterologists are recognizing what we have long known—that operating through these natural orifices is less uncomfortable for the patient and provides faster, less complicated recovery. They are also recognizing the challenges involved in such an approach.
A new generation of instruments is in the works
Clearly, operating through the vagina, cervix, or stomach necessitates excellent visualization and instruments flexible enough to navigate through tiny openings but strong enough to transect and retrieve tissue. Many of our industry partners are working diligently to create and perfect new instrumentation for NOTES procedures, and research is under way at many centers in this country and overseas into transgastric and transrectal procedures.
Consider what we might be able to achieve with this technology! By eliminating the need for transabdominal access, we can vastly reduce the risk of intestinal and major vessel injury and eliminate the risk of hernia. We can also markedly reduce the discomfort associated with abdominal incisions.
How might this technology be applied in gynecology? I anticipate that ovarian pathology, endometriosis, and ectopic pregnancy will be managed transvaginally or via a small opening in the uterus. Transvaginal hydrolaparoscopy—in which warm saline is used as the distention medium instead of carbon dioxide, and access to the pelvis is achieved through a small culpotomy—has been around for many years but is limited by the rigid instrumentation and restricted visualization now available. With flexible instruments that can “see” around corners yet provide a wide visual field, microrobots that can be placed through a tiny opening and then deployed to accomplish the surgical task, and systems to achieve hemostasis, NOTES may be the next revolution in gynecologic surgery.
Still in a very early stage of development, natural orifice transluminal endoscopic surgery (NOTES) has generated considerable enthusiasm among physicians leading research and development efforts. Hoping to steer these efforts in a responsible direction—and avoid the problems encountered during the early days of laparoscopic surgery, when many inexperienced practitioners began adopting the technique prematurely—a working group from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons was formed in 2005, calling itself the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). So far, this group has convened two international conferences and penned two white papers, noting that “the overwhelming sense [at the first international conference]…was that NOTES will develop into a mainstream clinical capability in the near future.”1
Some of the needs NOSCAR has identified are:
- determining the optimal technique and site to achieve access to the peritoneal cavity
- developing a gastric closure method that is 100% reliable
- reducing the risk of intraperitoneal contamination and infection, given the transgastric route that has dominated NOTES so far
- developing the ability to suture
- maintaining spatial orientation during surgery, as well as a multitasking platform that would allow manipulation of tissue, clear visualization, and safe access
- preventing intraperitoneal complications such as bleeding and bowel perforation
- exploring the physiology of pneumoperitoneum in the setting of NOTES
- establishing guidelines for training physicians and reporting both positive and negative outcomes.
In the meantime, NOSCAR recommends that all NOTES procedures in humans be approved by the Institutional Review Board and reported to a registry.
So far, the technology has been used to perform appendectomy and cholecystectomy in humans. Research grants totaling $1.5 million have been pledged by industry.
Reference
1. NOSCAR Working Group. NOTES: gathering momentum. White Paper. May 2006. Available at: http://www.noscar.org/documents/NOTES_White_Paper_May06.pdf. Accessed July 3, 2007.
NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY (NOTES)
Selected studies
To date, 19 abstracts on PubMed discuss the impressive opportunities NOTES will provide. Here is a sample:
- de la Fuente SG, Demaria EJ, Reynolds JD, Portenier DD, Pryor AD. New developments in surgery: natural orifi ce transluminal endoscopic surgery (NOTES). Arch Surg. 2007;142:295–297.
- Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–318.
- Malik A, Mellinger JD, Hazey JW, Dunkin BJ, MacFadyen BV. Endoluminal and transluminal surgery: current status and future possibilities. Surg Endosc. 2006;20:1179–1192.
- McGee MF, Rosen MJ, Marks J, et al. A primer on natural orifi ce transluminal endoscopic surgery: building a new paradigm. Surg Innov. 2006;13:86–93.
- Wilhelm D, Meining A, von Delius S, et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39:401–406.
Over its history, surgery has been defined by the tools available to practitioners. In our era, opportunities to offer patients minimally invasive surgery have expanded dramatically as methods of establishing visualization, achieving hemostasis, and performing tissue dissection have improved. (I remember trying to treat ectopic pregnancy laparoscopically in the early 1980s without benefit of a camera or suction irrigator!)
For surgeons of my generation, the ability to access the abdominal cavity minimally invasively and to clearly visualize the contents was a significant step forward. Hysteroscopic myomectomy was another tremendous incremental improvement for patients with submucous myomas. But there is much more in store for the coming years.
Where are we headed in the next wave of gynecologic surgery? Will patients require an incision at all? Is there room to advance beyond laparoscopy and hysteroscopy? What innovations will industry offer us in the 21st century?
In this article, I describe something that is fairly familiar to most of us by now, but which is not yet practical for routine gynecologic procedures—robotically assisted endoscopic surgery. I then move on to a phenomenon that, in many respects, is still being imagined—natural orifice transluminal endoscopic surgery, or NOTES.
Robotic systems are best suited for complex surgery
Laparoscopic surgery is limited by the two-dimensional view and need for hand control of long, rigid instruments through ancillary trocar sites. Although these impediments can be overcome with practice and experience, the inability to see in three dimensions and the compromised range of motion hamper optimal management of some surgical procedures.
A number of technological advances may significantly improve our ability to perform suture-intensive or anatomically challenging operations. Several companies are developing camera systems that will permit a three-dimensional view without the need for multiple visual ports. The technology is borrowed from the world of insects, which “see” through multiple lenses within the same eye. The application of such visual processing to optical systems for endoscopic surgery will be a huge advance for laparoscopy—one that is still being perfected by industry. In 2007, the da Vinci robot system (Intuitive Surgical) offers the best opportunity to achieve both three-dimensional visualization and an ability to “feel” tissue and manipulate instruments with markedly increased range of motion.
Cost is the limiting factor
Although the da Vinci system has revolutionized the practice of urology, enabling radical nerve-sparing prostatectomy, its utility in gynecology is still being investigated. Several centers use the robot for a significant percentage of their laparoscopic gynecologic surgery, but the setup time, learning curve, and intraoperative time required make the da Vinci system an impractical tool for many routine procedures. Its true advantage lies in suture-intensive procedures and in surgeries that require meticulous dissection close to major structures. In gynecology, the laparoscopic procedures most likely to benefit from the three-dimensional view and articulating instruments are sacral colpopexy, myomectomy (FIGURE), radical hysterectomy, and lymph node dissection.
Although it is interesting and enjoyable to use robotic technology for routine laparoscopic procedures, I believe the cost is prohibitive—several million dollars for each robot. If the financial barriers are removed, however, this system will be a welcome addition to the toolset for gynecologic laparoscopic surgery. Until then, we need to make intelligent use of this powerful tool.
FIGURE Robotic myomectomy
A: Using the da Vinci robot, the surgeon incises the myometrium down to the fibroid.
B: After grasping the fibroid, the surgeon dissects it away from the surrounding myometrium.
C: As the fibroid is freed, another small tumor becomes apparent at the bottom right, and is also removed.
D: The myometrium is sutured in layers after removal of the fibroids. Photos courtesy of Paul Indman, MD.Just as many of us were able to perform laparoscopic surgery without a three-chip camera and high-tech energy system for hemostasis until the cost of those technologies could be recouped in reduced operating room time and fewer conversions to laparotomy, so will today’s surgeons have to continue performing laparoscopic adnexal surgery, routine hysterectomy, and treatment of ectopic pregnancy the “old-fashioned” way. For complex procedures, however, the da Vinci system is proving to be a major advance in endoscopic surgery.
Look for other, perhaps less expensive, technologies coming down the road that will, at the very least, permit three-dimensional visualization without the need for robotics. In addition, as I discuss in the next section, miniaturization of robotics is on the horizon. Only our imagination limits our thinking about how robotic technology may be used in the not-too-distant future.
ROBOTICS IN GYNECOLOGY
Selected studies
- Bocca S, Stadtmauer L, Oehninger S. Uncomplicated fullterm pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online. 2007;14:246–249.
- Elliott DS, Chow GK, Gettman M. Current status of robotics in female urology and gynecology. World J Urol. 2006;24:188–192.
- Fiorentino RP, Zepeda MA, Goldstein BH, John CR, Rettenmaier MA. Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol. 2006;13:60–63.
- Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28:77–82.
NOTES takes “minimally invasive” to a new level
Imagine performing surgery for ectopic pregnancy or endometriosis in your office, without anesthesia. Think this is impossible? Think again!
A newer, perhaps better, and definitely less invasive version of endoscopic surgery is on the horizon—natural orifice transluminal endoscopic surgery, or NOTES. In May, a surgeon in Portland, Oregon, performed a cholecystectomy by dropping an endoscope through the patient’s mouth into the stomach, drilling an opening in the gastric wall, and placing small instruments through that opening to perform the surgery. The specimen was then pulled through the small opening in the stomach and retrieved through the patient’s mouth! The stomach was closed with an endoscopic stapling device.
NOTES appears to be the next true advance in minimally invasive surgery. This should come as no surprise to gynecologists. We are the champions of transcervical and transvaginal surgery. General surgeons and gastroenterologists are recognizing what we have long known—that operating through these natural orifices is less uncomfortable for the patient and provides faster, less complicated recovery. They are also recognizing the challenges involved in such an approach.
A new generation of instruments is in the works
Clearly, operating through the vagina, cervix, or stomach necessitates excellent visualization and instruments flexible enough to navigate through tiny openings but strong enough to transect and retrieve tissue. Many of our industry partners are working diligently to create and perfect new instrumentation for NOTES procedures, and research is under way at many centers in this country and overseas into transgastric and transrectal procedures.
Consider what we might be able to achieve with this technology! By eliminating the need for transabdominal access, we can vastly reduce the risk of intestinal and major vessel injury and eliminate the risk of hernia. We can also markedly reduce the discomfort associated with abdominal incisions.
How might this technology be applied in gynecology? I anticipate that ovarian pathology, endometriosis, and ectopic pregnancy will be managed transvaginally or via a small opening in the uterus. Transvaginal hydrolaparoscopy—in which warm saline is used as the distention medium instead of carbon dioxide, and access to the pelvis is achieved through a small culpotomy—has been around for many years but is limited by the rigid instrumentation and restricted visualization now available. With flexible instruments that can “see” around corners yet provide a wide visual field, microrobots that can be placed through a tiny opening and then deployed to accomplish the surgical task, and systems to achieve hemostasis, NOTES may be the next revolution in gynecologic surgery.
Still in a very early stage of development, natural orifice transluminal endoscopic surgery (NOTES) has generated considerable enthusiasm among physicians leading research and development efforts. Hoping to steer these efforts in a responsible direction—and avoid the problems encountered during the early days of laparoscopic surgery, when many inexperienced practitioners began adopting the technique prematurely—a working group from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons was formed in 2005, calling itself the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). So far, this group has convened two international conferences and penned two white papers, noting that “the overwhelming sense [at the first international conference]…was that NOTES will develop into a mainstream clinical capability in the near future.”1
Some of the needs NOSCAR has identified are:
- determining the optimal technique and site to achieve access to the peritoneal cavity
- developing a gastric closure method that is 100% reliable
- reducing the risk of intraperitoneal contamination and infection, given the transgastric route that has dominated NOTES so far
- developing the ability to suture
- maintaining spatial orientation during surgery, as well as a multitasking platform that would allow manipulation of tissue, clear visualization, and safe access
- preventing intraperitoneal complications such as bleeding and bowel perforation
- exploring the physiology of pneumoperitoneum in the setting of NOTES
- establishing guidelines for training physicians and reporting both positive and negative outcomes.
In the meantime, NOSCAR recommends that all NOTES procedures in humans be approved by the Institutional Review Board and reported to a registry.
So far, the technology has been used to perform appendectomy and cholecystectomy in humans. Research grants totaling $1.5 million have been pledged by industry.
Reference
1. NOSCAR Working Group. NOTES: gathering momentum. White Paper. May 2006. Available at: http://www.noscar.org/documents/NOTES_White_Paper_May06.pdf. Accessed July 3, 2007.
NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY (NOTES)
Selected studies
To date, 19 abstracts on PubMed discuss the impressive opportunities NOTES will provide. Here is a sample:
- de la Fuente SG, Demaria EJ, Reynolds JD, Portenier DD, Pryor AD. New developments in surgery: natural orifi ce transluminal endoscopic surgery (NOTES). Arch Surg. 2007;142:295–297.
- Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–318.
- Malik A, Mellinger JD, Hazey JW, Dunkin BJ, MacFadyen BV. Endoluminal and transluminal surgery: current status and future possibilities. Surg Endosc. 2006;20:1179–1192.
- McGee MF, Rosen MJ, Marks J, et al. A primer on natural orifi ce transluminal endoscopic surgery: building a new paradigm. Surg Innov. 2006;13:86–93.
- Wilhelm D, Meining A, von Delius S, et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39:401–406.
Over its history, surgery has been defined by the tools available to practitioners. In our era, opportunities to offer patients minimally invasive surgery have expanded dramatically as methods of establishing visualization, achieving hemostasis, and performing tissue dissection have improved. (I remember trying to treat ectopic pregnancy laparoscopically in the early 1980s without benefit of a camera or suction irrigator!)
For surgeons of my generation, the ability to access the abdominal cavity minimally invasively and to clearly visualize the contents was a significant step forward. Hysteroscopic myomectomy was another tremendous incremental improvement for patients with submucous myomas. But there is much more in store for the coming years.
Where are we headed in the next wave of gynecologic surgery? Will patients require an incision at all? Is there room to advance beyond laparoscopy and hysteroscopy? What innovations will industry offer us in the 21st century?
In this article, I describe something that is fairly familiar to most of us by now, but which is not yet practical for routine gynecologic procedures—robotically assisted endoscopic surgery. I then move on to a phenomenon that, in many respects, is still being imagined—natural orifice transluminal endoscopic surgery, or NOTES.
Robotic systems are best suited for complex surgery
Laparoscopic surgery is limited by the two-dimensional view and need for hand control of long, rigid instruments through ancillary trocar sites. Although these impediments can be overcome with practice and experience, the inability to see in three dimensions and the compromised range of motion hamper optimal management of some surgical procedures.
A number of technological advances may significantly improve our ability to perform suture-intensive or anatomically challenging operations. Several companies are developing camera systems that will permit a three-dimensional view without the need for multiple visual ports. The technology is borrowed from the world of insects, which “see” through multiple lenses within the same eye. The application of such visual processing to optical systems for endoscopic surgery will be a huge advance for laparoscopy—one that is still being perfected by industry. In 2007, the da Vinci robot system (Intuitive Surgical) offers the best opportunity to achieve both three-dimensional visualization and an ability to “feel” tissue and manipulate instruments with markedly increased range of motion.
Cost is the limiting factor
Although the da Vinci system has revolutionized the practice of urology, enabling radical nerve-sparing prostatectomy, its utility in gynecology is still being investigated. Several centers use the robot for a significant percentage of their laparoscopic gynecologic surgery, but the setup time, learning curve, and intraoperative time required make the da Vinci system an impractical tool for many routine procedures. Its true advantage lies in suture-intensive procedures and in surgeries that require meticulous dissection close to major structures. In gynecology, the laparoscopic procedures most likely to benefit from the three-dimensional view and articulating instruments are sacral colpopexy, myomectomy (FIGURE), radical hysterectomy, and lymph node dissection.
Although it is interesting and enjoyable to use robotic technology for routine laparoscopic procedures, I believe the cost is prohibitive—several million dollars for each robot. If the financial barriers are removed, however, this system will be a welcome addition to the toolset for gynecologic laparoscopic surgery. Until then, we need to make intelligent use of this powerful tool.
FIGURE Robotic myomectomy
A: Using the da Vinci robot, the surgeon incises the myometrium down to the fibroid.
B: After grasping the fibroid, the surgeon dissects it away from the surrounding myometrium.
C: As the fibroid is freed, another small tumor becomes apparent at the bottom right, and is also removed.
D: The myometrium is sutured in layers after removal of the fibroids. Photos courtesy of Paul Indman, MD.Just as many of us were able to perform laparoscopic surgery without a three-chip camera and high-tech energy system for hemostasis until the cost of those technologies could be recouped in reduced operating room time and fewer conversions to laparotomy, so will today’s surgeons have to continue performing laparoscopic adnexal surgery, routine hysterectomy, and treatment of ectopic pregnancy the “old-fashioned” way. For complex procedures, however, the da Vinci system is proving to be a major advance in endoscopic surgery.
Look for other, perhaps less expensive, technologies coming down the road that will, at the very least, permit three-dimensional visualization without the need for robotics. In addition, as I discuss in the next section, miniaturization of robotics is on the horizon. Only our imagination limits our thinking about how robotic technology may be used in the not-too-distant future.
ROBOTICS IN GYNECOLOGY
Selected studies
- Bocca S, Stadtmauer L, Oehninger S. Uncomplicated fullterm pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online. 2007;14:246–249.
- Elliott DS, Chow GK, Gettman M. Current status of robotics in female urology and gynecology. World J Urol. 2006;24:188–192.
- Fiorentino RP, Zepeda MA, Goldstein BH, John CR, Rettenmaier MA. Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol. 2006;13:60–63.
- Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28:77–82.
NOTES takes “minimally invasive” to a new level
Imagine performing surgery for ectopic pregnancy or endometriosis in your office, without anesthesia. Think this is impossible? Think again!
A newer, perhaps better, and definitely less invasive version of endoscopic surgery is on the horizon—natural orifice transluminal endoscopic surgery, or NOTES. In May, a surgeon in Portland, Oregon, performed a cholecystectomy by dropping an endoscope through the patient’s mouth into the stomach, drilling an opening in the gastric wall, and placing small instruments through that opening to perform the surgery. The specimen was then pulled through the small opening in the stomach and retrieved through the patient’s mouth! The stomach was closed with an endoscopic stapling device.
NOTES appears to be the next true advance in minimally invasive surgery. This should come as no surprise to gynecologists. We are the champions of transcervical and transvaginal surgery. General surgeons and gastroenterologists are recognizing what we have long known—that operating through these natural orifices is less uncomfortable for the patient and provides faster, less complicated recovery. They are also recognizing the challenges involved in such an approach.
A new generation of instruments is in the works
Clearly, operating through the vagina, cervix, or stomach necessitates excellent visualization and instruments flexible enough to navigate through tiny openings but strong enough to transect and retrieve tissue. Many of our industry partners are working diligently to create and perfect new instrumentation for NOTES procedures, and research is under way at many centers in this country and overseas into transgastric and transrectal procedures.
Consider what we might be able to achieve with this technology! By eliminating the need for transabdominal access, we can vastly reduce the risk of intestinal and major vessel injury and eliminate the risk of hernia. We can also markedly reduce the discomfort associated with abdominal incisions.
How might this technology be applied in gynecology? I anticipate that ovarian pathology, endometriosis, and ectopic pregnancy will be managed transvaginally or via a small opening in the uterus. Transvaginal hydrolaparoscopy—in which warm saline is used as the distention medium instead of carbon dioxide, and access to the pelvis is achieved through a small culpotomy—has been around for many years but is limited by the rigid instrumentation and restricted visualization now available. With flexible instruments that can “see” around corners yet provide a wide visual field, microrobots that can be placed through a tiny opening and then deployed to accomplish the surgical task, and systems to achieve hemostasis, NOTES may be the next revolution in gynecologic surgery.
Still in a very early stage of development, natural orifice transluminal endoscopic surgery (NOTES) has generated considerable enthusiasm among physicians leading research and development efforts. Hoping to steer these efforts in a responsible direction—and avoid the problems encountered during the early days of laparoscopic surgery, when many inexperienced practitioners began adopting the technique prematurely—a working group from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons was formed in 2005, calling itself the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). So far, this group has convened two international conferences and penned two white papers, noting that “the overwhelming sense [at the first international conference]…was that NOTES will develop into a mainstream clinical capability in the near future.”1
Some of the needs NOSCAR has identified are:
- determining the optimal technique and site to achieve access to the peritoneal cavity
- developing a gastric closure method that is 100% reliable
- reducing the risk of intraperitoneal contamination and infection, given the transgastric route that has dominated NOTES so far
- developing the ability to suture
- maintaining spatial orientation during surgery, as well as a multitasking platform that would allow manipulation of tissue, clear visualization, and safe access
- preventing intraperitoneal complications such as bleeding and bowel perforation
- exploring the physiology of pneumoperitoneum in the setting of NOTES
- establishing guidelines for training physicians and reporting both positive and negative outcomes.
In the meantime, NOSCAR recommends that all NOTES procedures in humans be approved by the Institutional Review Board and reported to a registry.
So far, the technology has been used to perform appendectomy and cholecystectomy in humans. Research grants totaling $1.5 million have been pledged by industry.
Reference
1. NOSCAR Working Group. NOTES: gathering momentum. White Paper. May 2006. Available at: http://www.noscar.org/documents/NOTES_White_Paper_May06.pdf. Accessed July 3, 2007.
NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY (NOTES)
Selected studies
To date, 19 abstracts on PubMed discuss the impressive opportunities NOTES will provide. Here is a sample:
- de la Fuente SG, Demaria EJ, Reynolds JD, Portenier DD, Pryor AD. New developments in surgery: natural orifi ce transluminal endoscopic surgery (NOTES). Arch Surg. 2007;142:295–297.
- Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–318.
- Malik A, Mellinger JD, Hazey JW, Dunkin BJ, MacFadyen BV. Endoluminal and transluminal surgery: current status and future possibilities. Surg Endosc. 2006;20:1179–1192.
- McGee MF, Rosen MJ, Marks J, et al. A primer on natural orifi ce transluminal endoscopic surgery: building a new paradigm. Surg Innov. 2006;13:86–93.
- Wilhelm D, Meining A, von Delius S, et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39:401–406.
Chronic genital skin disorders: 6 challenging conditions
Six common dermatologic disorders of the vulva and vagina can present considerable challenges:
- Allergic contact dermatitis: 100% of the population may be at risk for this disorder, and the vulvar skin is especially vulnerable.
- Irritant contact dermatitis: Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to the acquisition of sexually transmitted disease.
- Lichen sclerosus: Women with this condition are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
- Lichen planus: Antibiotic therapy is ineffective against this disorder; symptoms reappear as soon as antibiotics are stopped.
- Yeast infection: Yeast organisms release proteins that activate a local allergic response and perpetuate an environment that supports infection.
- Human papillomavirus (HPV): The small HPV particle easily gains entry to minimally traumatized vulvar skin.
These common conditions sometimes compromise the skin barrier and prevent an adequate immune response to invading microbes. Some degree of skin immune dysfunction is generally associated with each genital dermatologic disorder. Identifying and treating the underlying dermatologic disorder, then, often corrects the associated immune dysfunction and may restore the skin barrier and prevent further microbial invasion.
Allergic contact dermatitis
Allergic dermatitis, or atopic dermatitis (formerly called eczema), is a highly prevalent skin disorder (FIGURE 1). Depending on the environment and genetic factors, as many as 40% of adults have a history of atopic dermatitis, and essentially 100% of the population may be at risk. Women have a higher rate of atopic dermatitis than men do.
Recognized vulvar allergens or triggers include dry climate, elastic, latex, fragrances in soaps or body lotions, and residues of detergent and fabric softener in clothing. In most biopsy-proven cases of vulvar eczema, the patient is unable to identify specific allergens. Such a patient often has a history of asthma, allergic rhinitis, sinusitis, or atopic dermatitis on other parts of the body.
Patch testing is no help in identifying specific allergens in the pelvic area. Testing the tougher skin of the back may not disclose all vulvar sensitivities.
But biopsy is useful. Women with vulvar allergic contact dermatitis often complain of persistent itching. A history of allergy elsewhere on the body is diagnostically helpful, but a biopsy submitted to a dermatopathologist confirms the diagnosis.
Not all women with vulvar allergic dermatitis have atopy at other body sites. Hyperkeratosis and spongiosis in the pathology specimen are characteristic. A small (3-or 4-mm) biopsy at the most symptomatic site is appropriate in any woman with chronic vulvar pruritis.
Local immune dysfunction is involved. Allergic vulvar dermatitis is characterized by a locally dysfunctional cell-mediated immune response. Langerhans cells are involved in this allergic reaction, directed away from their normal protective role.1 (Read about the role of Langerhans cells in “Three barriers to microbial infection: The skin’s built-in defense system,”) Viruses, bacteria, and yeast that gain entry into the skin have greater freedom to proliferate and persist, and skin-cancer surveillance by Langerhans cells is also compromised, with an increased risk for squamous carcinoma. This may account for a large portion of the 50% of vulvar carcinomas that cannot be attributed to HPV infection. Langerhans-cell dysfunction also contributes to the progression of HPV-associated carcinoma.
Allergic dermatitis inhibits the production of human cathelicidin and the ß-defensins, natural skin microbicides. As a result, vulvar skin affected by allergic dermatitis has a higher yeast and bacterial colonization rate.
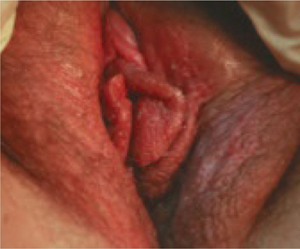
FIGURE 1 Allergic contact dermatitis
This case of severe allergic contact dermatitis has been aggravated by chronic scratching, especially of the left labia. Cases typically are much more subtle.
Look for telltale flaking skin
Allergic dermatitis involves flaking of the skin, probably due to allergic stimulation of epithelial cell proliferation. Flakes of skin are exfoliated before the desmosomes that hold individual skin cells together deteriorate. The flaking compromises the stratum corneum barrier, and likely facilitates skin invasion of yeast and bacteria that have colonized the surface.
Because the background rate of dermatitis in the general population is relatively high, skin flakes often appear in the saline wet prep and are referred to as “reactive, reparative” changes in the Papanicolaou smear.2
Other diagnostic clues. Chronic vulvar pruritus with a history of asthma, hay fever, sinusitis, atopic dermatitis, or dry skin is vulvar dermatitis until it is proved otherwise. Recurrent yeast infection is often reported as well.
In many cases, the dermatitis may exhibit no clinical signs beyond flakes of skin in the saline wet prep.
Start with a topical steroid
A trial of topical steroid ointment is appropriate, using a low-to medium-strength ointment such as 0.1% hydrocortisone butyrate, which may also lower the risk of yeast infection.
Several weeks of treatment may be necessary. It may take 4 to 6 weeks for a full layer of skin to be replaced. Subdermal atrophy, skin neovascularization, and other risks of topical steroids are of less concern during extended use of low-potency steroids, and may be more acceptable on an unexposed part of the body such as the vulva.
To test for therapeutic success, look for a reduction in pruritus and a lower incidence of yeast infection. Failure of steroid ointment and oral yeast suppression may justify vulvar biopsy, which should be submitted to a dermatopathologist.
Occasionally, a high-potency topical steroid such as clobetasol 0.05% ointment may be necessary (applied twice daily and rubbed in), but adrenal suppression may develop if therapy exceeds 3 to 4 weeks. The agent should be tapered rather than stopped abruptly.
Irritant contact dermatitis
This condition is characterized by a burning sensation. Common vulvar irritants include oxylate (in urine), propylene glycol (in medicated creams and lotions), and abrasive toilet paper. The list of potential irritants is long, and each irritant may have a different mechanism of action. A burning reaction after application of a topical cream suggests significant compromise of the skin barrier that would otherwise have prevented entry of the irritant. Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to acquisition of sexually transmitted disease.
Begin by identifying the culprits
The first step of treatment is recognizing and eliminating potential irritants such as bath soap, urine, topical creams that contain propylene glycol, and soap residue in clothing. Have the patient use a squirt bottle to rinse the genital area after urination to eliminate irritants such as oxylate. Also suggest that she rinse undergarments twice and use liquid rather than powder detergent. Cotton undergarments are more skin-friendly than synthetics.
Twice-daily or more frequent application of a skin moisturizer such as vegetable shortening, MimyX cream, or mineral oil/petrolatum cream (Eletone) helps to heal the skin, and continued use may prevent recurrence of symptoms.
When a patient complains of persistent vulvar pruritus and exhibits a figure-of-eight vulvar rash, suspect lichen sclerosus (FIGURE 2). The cause of this condition is unclear. Often, allergic contact dermatitis is superimposed on it. In older gynecologic terminology, this was referred to as mixed vulvar dystrophy.
Women with lichen sclerosus are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
Vulvar biopsy occasionally discloses unexpected early lichen sclerosus.
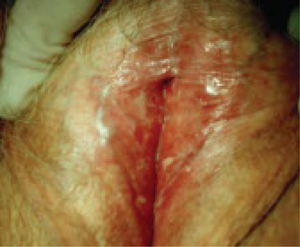
FIGURE 2 Lichen sclerosus
Note the labial agglutination (labia minora) and diffuse white epithelium, which are characteristic findings. If the areas of thickened epithelium do not resolve with topical steroid ointment, biopsy is appropriate.
Topical steroids are key to therapy
The degree of involvement that is grossly apparent determines the strength of the steroid ointment. If tissue is thickened, with areas of deep white change, a high-potency ointment such as clobetasol 0.05% may be necessary, applied twice daily for as long as several weeks. Milder cases may respond to a medium-strength topical steroid, such as fluticasone propionate 0.005% ointment.
If a higher-strength steroid is selected, it is appropriate to switch to a milder steroid as soon as symptoms resolve, with the goal of maintaining control with 1% hydrocortisone ointment or even continuous use of one of the skin moisturizers recommended for irritant dermatitis. Biopsy any thickened white patch, ulcerated area, or nonhealing skin fissure to check for squamous cancer.
Lichen planus
Erosive lichen planus (desquamative inflammatory vaginitis) of the vulva and vagina is an autoimmune skin disorder that causes superficial ulceration of the vaginal mucosa (FIGURE 3). An increase in vaginal discharge represents a shift in microflora away from lactobacillus dominance and an increase in the number of white blood cells and parabasal epithelial cells, with markedly heightened skin turnover. Local cellulitis does not develop, despite an overgrowth of various enteric organisms.
FIGURE 3 Lichen planus
This severe case resolved with a 3-month course of daily azathioprine (150 mg) but recurred after therapy ended.
A dermatologist may be required
Lichen planus is characterized by purulent discharge that contains bacteria, white blood cells, and parabasal cells. Unusual enteric microbes are often detected by routine culture, but antibiotic therapy is not helpful. Potent systemic anti-inflammatory therapy is often necessary rather than antimicrobial therapy. Daily azathioprine (Imuran) in doses ranging from 25 mg to 150 mg orally have been used, depending on the degree of vulvovaginal involvement. Tacrolimus ointment 0.1% applied twice daily may help in milder cases, but this agent typically causes an irritant reaction (burning) until the disorder partially resolves.
It may be helpful to seek the assistance of an experienced dermatologist if a biopsy demonstrates this disorder.
Vulvovaginal yeast infection is often found in conjunction with chronic vulvar eczema.3 Infection is promoted by:
- deficient skin microbicides
- skin-surface disruption with flaking
- ineffective Langerhans-cell response to invading yeast.
Yeast organisms release proteins that further activate a local allergic response to perpetuate an environment that supports infection (FIGURE 4). This represents a breakdown in the skin’s natural defenses.
FIGURE 4 Yeast infection
Vulvar yeast rash in the normally occluded area of the vulva with large satellite lesions (erythematous patches around the margin of the vulvar rash). Satellite lesions are typically much smaller.
Oral therapy may be preferred
Topical anti-yeast creams often contain propylene glycol, an irritant to fragile skin, so oral therapy may be more appropriate. For oral therapy, 200 mg of fluconazole every 3 days for three doses is a useful starting point. For severe cases, this can be followed by a weekly 200-mg oral dose for 2 to 3 months to maintain yeast suppression while the underlying skin disorder begins to resolve. An extended course of oral fluconazole may not be appropriate during pregnancy or anticoagulation or while the patient is taking a statin drug to lower cholesterol. If oral therapy is not appropriate, 1% clotrimazole 7-day vaginal cream is the only topical agent in the United States that does not contain propylene glycol.
Beauty may be only skin deep, but that layer of epidermis is a pretty busy place. Among its activities is the production of hundreds of substances that regulate susceptibility to infection. More than 50 of these chemicals fall into the class of skin microbicides.8
We began to learn about these microbicides a decade ago, when researchers asked why eczema usually is secondarily infected with pathogenic staphylococcus, streptococcus, and yeast, and psoriatic skin isn’t. The answer: Both healthy and psoriatic skin produce natural microbicides, but allergic dermatitis (eczema) prevents their release on the skin surface.9
1. Proteins
Some proteins fight microbes better than pharmaceutical agents do. The most important antimicrobial proteins in the skin are defensins and cathelicidins, which are found in all epithelial structures, including the vulva and vagina.10 In the defensin category, human ß-defensins 2 and 3 are the most important proteins and are present in the surface epithelium. An inflammatory response triggers their release to inhibit microbes on the skin surface. The mean inhibitory concentration of human ß-defensin 3 against the relatively resistant yeast, Saccharomyces cerevisiae, is about 14 μg/mL.11 This inhibitory action is superior to many azole anti-yeast agents. Human cathelicidin is an equally effective skin microbicide, with antiviral, antifungal, and antibacterial activity. In normal function, these natural antimicrobial substances prevent colonization of pathogenic organisms in healthy skin.
2. Stratum corneum
The stratum corneum comprises the outer few microns of the epithelium. When it remains intact, the stratum corneum is an effective barrier to microbial invasion. Intact skin prevents substances with a molecular weight greater than 500 daltons from passing into the skin.
This barrier may be compromised by microtrauma or dermatologic disorders such as irritant or allergic dermatitis. Minimal microtrauma is all that is necessary to allow small microbes such as viruses to pass through the stratum corneum. Larger organisms (spirochetes, yeast) may require a greater degree of compromise, such as flaking skin. Environmental and dermatologic factors often compromise this natural barrier.
The vaginal epithelium is not keratinized and lacks an effective stratum corneum. Instead, vaginal tissue produces mucus, which floats on a thin transudate of intercellular fluid. Potential pathogens are captured in the mucus and drain out of the vagina. The vaginal epithelium produces several milliliters of mucus daily that is constantly draining out of the vaginal lumen.
3. Langerhans cells
The skin has a final layer of defense within its structure. Microbes that pass through the stratum corneum and enter the skin are attacked by defensive cells that reside there, known as antigen-presenting cells. Langerhans cells are the chief antigen-presenting cells in the skin. They originate in the bone marrow, but rest in the skin, awaiting microbial invasion.
Antigen-presenting cells kill intraepithelial microbes as they are detected, and then process the microbial antigens, enabling a cell-mediated immune response against the microbes. Langerhans cells also destroy individual cancer cells that appear randomly in the epithelium.
The genital skin and the skin around the mouth and eyes carry the highest concentration of Langerhans cells.12 Under normal conditions, Langerhans cells constitute as much as 8% of the cells in vulvar skin. In the genital area, the cervical transformation zone has the highest count of Langerhans cells13—possibly compensation for a highly vulnerable epithelial barrier, owing to the immature squamous epithelium at this site.
Maturation of a Langerhans cell
Langerhans cells, the main antigen-presenting cells in skin, defend it from microbes that breach the stratum corneum. Although Langerhans cells originate in bone marrow, mature cells reside in the epidermis.
With its high concentration of Langerhans cells, the cervical transformation zone may be the primary port of entry of HIV.14 Langerhans cells have a surface CD4 receptor to which HIV attaches. The Langerhans cells are unable to kill the HIV after phagocytosis. HIV-infected Langerhans cells then lead to systemic spread of the virus.
Overall, the antimicrobial function of Langerhans cells is imperfect. When a pathogen is located within a cell, some microbes, such as Chlamydia trachomatis, herpesvirus, and HPV, may evade detection. In addition, some dermatologic conditions are associated with significant dysfunction of Langerhans cells.
HPV infection
The small HPV particle easily gains entry to minimally traumatized vulvar skin—with a high transmission rate with even a single exposure—and the immature epithelium of the cervical transformation zone makes that site a naturally compromised barrier to infection.
Under normal conditions, a cell-mediated immune response eliminates the HPV virus within 12 months, with lasting protection from reinfection. If cell-mediated immunity is compromised, the virus cannot be eliminated. The result is genital warts, variable degrees of dysplasia, or cancer, depending on the degree of immune compromise (FIGURE 5).
Risk factors for HPV-associated warts and dysplasia include allergy, immunosuppressant drugs to prevent rejection of a transplanted organ, and smoking. Smoking cessation is particularly important because control of the virus is dependent on Langerhans-cell function.
FIGURE 5 Genital warts
Debulking of warts with cryocautery or electrocautery may be appropriate, followed by imiquimod cream. It is prudent to biopsy persistent warts to exclude carcinoma, especially in an immunocompromised patient.
Begin by debulking warts
Electro- or cryocautery of large warts is an appropriate first step. Follow debulking with thrice-weekly application of one packet of imiquimod cream, to be washed off in the morning, for 4 to 6 weeks. This therapy may not eliminate genital warts in women who are taking immunosuppressant drugs to prevent organ-transplant rejection. Unfortunately, control of genital warts with monthly cautery of new warts may be the only useful option in these patients. Immunocompromised patients are at high risk for squamous carcinoma, and biopsy of persistent warts may be wise.
Vulnerabilities of vulvovaginal skin
The skin of the vulva and vagina is far from invincible. Some factors that affect it adversely are aging,4 tobacco use,5 estrogen deficiency,6 immunosuppressant drugs, and human immunodeficiency virus (HIV) infection.7 Look for these risk factors in women with persistent genital infection, so that the management plan can include treatment of the underlying dermatologic or immune disorder, as well as any microbes that are identified.
Immunosuppressed patients
Rejection of a transplanted organ is a function of cell-mediated immunity, so it is not surprising that drugs that suppress transplant rejection also inhibit vulvovaginal cell-mediated immunity. This increases the risk that HPV-associated disease will progress. Imiquimod cream promotes cell-mediated immunity by activating the release of interferon in the vulvar skin, and may compensate for depressed immune-cell function in the nontransplant population, but it is less effective in the transplant recipient. Sadly, there is no long-term solution to the effects of immunosuppressant therapy in this population; special surveillance for vulvar cancer and cervical dysplasia is necessary. Smoking cessation is also essential, especially for women with HPV-associated disease.
When HIV infection progresses to AIDS, Langerhans cells that carry HIV are depleted from the skin and substantially decrease in number, completely compromising cell-mediated immunity. This explains why AIDS patients often have severe genital herpes infections, severe chronic yeast vulvovaginitis, extensive molluscum disease, and unusual skin cancers. Antiretroviral therapy may restore some Langerhans-cell function.
Consider screening for HIV/AIDS when a woman has severe recurrent genital viral or yeast infection.
Aging and estrogen deficiency
Cell-mediated immune function declines with age. A higher risk of skin cancer, herpes (and its recurrence), and irritant and allergic vulvar dermatitis are the results. Increased surveillance for skin cancer, and varicella vaccination to lower the risk for herpes zoster, may be important.
Topical estrogen may be indicated if saline wet-prep evaluation reveals parabasal cells in vaginal secretions of a symptomatic postmenopausal woman. The estrogen may gradually alleviate burning and restrict potentially pathogenic bacterial flora. Be aware, however, that commercially available estrogen creams often contain propylene glycol, a recognized irritant of fragile skin. One solution: Have a compounding pharmacist formulate an equivalent cream (estradiol, 0.1 mg/g) for twice-daily topical application, using a base of petrolatum or solid vegetable oil.
1. Cruz PD. The epidermis: an outpost of the immune system. In: Freinkel RK, Woodley D, eds. The Biology of the Skin. New York: Parthenon Publishing Group; 2000:256-260.
2. Bonfiglio TA, Erozan YS. Gynecologic Cytopathology. Philadelphia: Lippincott-Raven; 1997:42-45.
3. Fidel PL, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335-348.
4. Gilchrest B, Murphy G, Soter N. Effect of chronological aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982;79:85-88.
5. Ouyang Y, Virasch N, Hao P, et al. Suppression of human IL-1beta, IL-2, IFN-gamma, and TNF-alpha by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280-287.
6. Mao A, Paharkova-Vatchkova V, Hardy J, et al. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146-5151.
7. Memar OM, Geraminejad P, Arany I, Tyring SK. Cutaneous resistance to viral infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York: Marcel Dekker; 2002:25-28.
8. Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9-13.
9. Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262-3269.
10. Harder J, Bartels J, Christophers E, Schroder JN. Isolation and characterization of human beta defensin-3, a novel inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713.
11. Garcia JR, Jaumann F, Schultz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 2001;306:257-264.
12. Udey MC. Cadherins and Langerhans cell immunobiology. Clin Exp Immunol. 1997;107(suppl. 1):6-8.
13. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253-1263.
14. Tschachler E, Groh V Popovic, et al. Epidermal Langerhans cells—a target for HTLV III/LAV infection. J Invest Dermatol. 1987;88:233-237.
Six common dermatologic disorders of the vulva and vagina can present considerable challenges:
- Allergic contact dermatitis: 100% of the population may be at risk for this disorder, and the vulvar skin is especially vulnerable.
- Irritant contact dermatitis: Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to the acquisition of sexually transmitted disease.
- Lichen sclerosus: Women with this condition are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
- Lichen planus: Antibiotic therapy is ineffective against this disorder; symptoms reappear as soon as antibiotics are stopped.
- Yeast infection: Yeast organisms release proteins that activate a local allergic response and perpetuate an environment that supports infection.
- Human papillomavirus (HPV): The small HPV particle easily gains entry to minimally traumatized vulvar skin.
These common conditions sometimes compromise the skin barrier and prevent an adequate immune response to invading microbes. Some degree of skin immune dysfunction is generally associated with each genital dermatologic disorder. Identifying and treating the underlying dermatologic disorder, then, often corrects the associated immune dysfunction and may restore the skin barrier and prevent further microbial invasion.
Allergic contact dermatitis
Allergic dermatitis, or atopic dermatitis (formerly called eczema), is a highly prevalent skin disorder (FIGURE 1). Depending on the environment and genetic factors, as many as 40% of adults have a history of atopic dermatitis, and essentially 100% of the population may be at risk. Women have a higher rate of atopic dermatitis than men do.
Recognized vulvar allergens or triggers include dry climate, elastic, latex, fragrances in soaps or body lotions, and residues of detergent and fabric softener in clothing. In most biopsy-proven cases of vulvar eczema, the patient is unable to identify specific allergens. Such a patient often has a history of asthma, allergic rhinitis, sinusitis, or atopic dermatitis on other parts of the body.
Patch testing is no help in identifying specific allergens in the pelvic area. Testing the tougher skin of the back may not disclose all vulvar sensitivities.
But biopsy is useful. Women with vulvar allergic contact dermatitis often complain of persistent itching. A history of allergy elsewhere on the body is diagnostically helpful, but a biopsy submitted to a dermatopathologist confirms the diagnosis.
Not all women with vulvar allergic dermatitis have atopy at other body sites. Hyperkeratosis and spongiosis in the pathology specimen are characteristic. A small (3-or 4-mm) biopsy at the most symptomatic site is appropriate in any woman with chronic vulvar pruritis.
Local immune dysfunction is involved. Allergic vulvar dermatitis is characterized by a locally dysfunctional cell-mediated immune response. Langerhans cells are involved in this allergic reaction, directed away from their normal protective role.1 (Read about the role of Langerhans cells in “Three barriers to microbial infection: The skin’s built-in defense system,”) Viruses, bacteria, and yeast that gain entry into the skin have greater freedom to proliferate and persist, and skin-cancer surveillance by Langerhans cells is also compromised, with an increased risk for squamous carcinoma. This may account for a large portion of the 50% of vulvar carcinomas that cannot be attributed to HPV infection. Langerhans-cell dysfunction also contributes to the progression of HPV-associated carcinoma.
Allergic dermatitis inhibits the production of human cathelicidin and the ß-defensins, natural skin microbicides. As a result, vulvar skin affected by allergic dermatitis has a higher yeast and bacterial colonization rate.

FIGURE 1 Allergic contact dermatitis
This case of severe allergic contact dermatitis has been aggravated by chronic scratching, especially of the left labia. Cases typically are much more subtle.
Look for telltale flaking skin
Allergic dermatitis involves flaking of the skin, probably due to allergic stimulation of epithelial cell proliferation. Flakes of skin are exfoliated before the desmosomes that hold individual skin cells together deteriorate. The flaking compromises the stratum corneum barrier, and likely facilitates skin invasion of yeast and bacteria that have colonized the surface.
Because the background rate of dermatitis in the general population is relatively high, skin flakes often appear in the saline wet prep and are referred to as “reactive, reparative” changes in the Papanicolaou smear.2
Other diagnostic clues. Chronic vulvar pruritus with a history of asthma, hay fever, sinusitis, atopic dermatitis, or dry skin is vulvar dermatitis until it is proved otherwise. Recurrent yeast infection is often reported as well.
In many cases, the dermatitis may exhibit no clinical signs beyond flakes of skin in the saline wet prep.
Start with a topical steroid
A trial of topical steroid ointment is appropriate, using a low-to medium-strength ointment such as 0.1% hydrocortisone butyrate, which may also lower the risk of yeast infection.
Several weeks of treatment may be necessary. It may take 4 to 6 weeks for a full layer of skin to be replaced. Subdermal atrophy, skin neovascularization, and other risks of topical steroids are of less concern during extended use of low-potency steroids, and may be more acceptable on an unexposed part of the body such as the vulva.
To test for therapeutic success, look for a reduction in pruritus and a lower incidence of yeast infection. Failure of steroid ointment and oral yeast suppression may justify vulvar biopsy, which should be submitted to a dermatopathologist.
Occasionally, a high-potency topical steroid such as clobetasol 0.05% ointment may be necessary (applied twice daily and rubbed in), but adrenal suppression may develop if therapy exceeds 3 to 4 weeks. The agent should be tapered rather than stopped abruptly.
Irritant contact dermatitis
This condition is characterized by a burning sensation. Common vulvar irritants include oxylate (in urine), propylene glycol (in medicated creams and lotions), and abrasive toilet paper. The list of potential irritants is long, and each irritant may have a different mechanism of action. A burning reaction after application of a topical cream suggests significant compromise of the skin barrier that would otherwise have prevented entry of the irritant. Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to acquisition of sexually transmitted disease.
Begin by identifying the culprits
The first step of treatment is recognizing and eliminating potential irritants such as bath soap, urine, topical creams that contain propylene glycol, and soap residue in clothing. Have the patient use a squirt bottle to rinse the genital area after urination to eliminate irritants such as oxylate. Also suggest that she rinse undergarments twice and use liquid rather than powder detergent. Cotton undergarments are more skin-friendly than synthetics.
Twice-daily or more frequent application of a skin moisturizer such as vegetable shortening, MimyX cream, or mineral oil/petrolatum cream (Eletone) helps to heal the skin, and continued use may prevent recurrence of symptoms.
When a patient complains of persistent vulvar pruritus and exhibits a figure-of-eight vulvar rash, suspect lichen sclerosus (FIGURE 2). The cause of this condition is unclear. Often, allergic contact dermatitis is superimposed on it. In older gynecologic terminology, this was referred to as mixed vulvar dystrophy.
Women with lichen sclerosus are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
Vulvar biopsy occasionally discloses unexpected early lichen sclerosus.

FIGURE 2 Lichen sclerosus
Note the labial agglutination (labia minora) and diffuse white epithelium, which are characteristic findings. If the areas of thickened epithelium do not resolve with topical steroid ointment, biopsy is appropriate.
Topical steroids are key to therapy
The degree of involvement that is grossly apparent determines the strength of the steroid ointment. If tissue is thickened, with areas of deep white change, a high-potency ointment such as clobetasol 0.05% may be necessary, applied twice daily for as long as several weeks. Milder cases may respond to a medium-strength topical steroid, such as fluticasone propionate 0.005% ointment.
If a higher-strength steroid is selected, it is appropriate to switch to a milder steroid as soon as symptoms resolve, with the goal of maintaining control with 1% hydrocortisone ointment or even continuous use of one of the skin moisturizers recommended for irritant dermatitis. Biopsy any thickened white patch, ulcerated area, or nonhealing skin fissure to check for squamous cancer.
Lichen planus
Erosive lichen planus (desquamative inflammatory vaginitis) of the vulva and vagina is an autoimmune skin disorder that causes superficial ulceration of the vaginal mucosa (FIGURE 3). An increase in vaginal discharge represents a shift in microflora away from lactobacillus dominance and an increase in the number of white blood cells and parabasal epithelial cells, with markedly heightened skin turnover. Local cellulitis does not develop, despite an overgrowth of various enteric organisms.

FIGURE 3 Lichen planus
This severe case resolved with a 3-month course of daily azathioprine (150 mg) but recurred after therapy ended.
A dermatologist may be required
Lichen planus is characterized by purulent discharge that contains bacteria, white blood cells, and parabasal cells. Unusual enteric microbes are often detected by routine culture, but antibiotic therapy is not helpful. Potent systemic anti-inflammatory therapy is often necessary rather than antimicrobial therapy. Daily azathioprine (Imuran) in doses ranging from 25 mg to 150 mg orally have been used, depending on the degree of vulvovaginal involvement. Tacrolimus ointment 0.1% applied twice daily may help in milder cases, but this agent typically causes an irritant reaction (burning) until the disorder partially resolves.
It may be helpful to seek the assistance of an experienced dermatologist if a biopsy demonstrates this disorder.
Vulvovaginal yeast infection is often found in conjunction with chronic vulvar eczema.3 Infection is promoted by:
- deficient skin microbicides
- skin-surface disruption with flaking
- ineffective Langerhans-cell response to invading yeast.
Yeast organisms release proteins that further activate a local allergic response to perpetuate an environment that supports infection (FIGURE 4). This represents a breakdown in the skin’s natural defenses.

FIGURE 4 Yeast infection
Vulvar yeast rash in the normally occluded area of the vulva with large satellite lesions (erythematous patches around the margin of the vulvar rash). Satellite lesions are typically much smaller.
Oral therapy may be preferred
Topical anti-yeast creams often contain propylene glycol, an irritant to fragile skin, so oral therapy may be more appropriate. For oral therapy, 200 mg of fluconazole every 3 days for three doses is a useful starting point. For severe cases, this can be followed by a weekly 200-mg oral dose for 2 to 3 months to maintain yeast suppression while the underlying skin disorder begins to resolve. An extended course of oral fluconazole may not be appropriate during pregnancy or anticoagulation or while the patient is taking a statin drug to lower cholesterol. If oral therapy is not appropriate, 1% clotrimazole 7-day vaginal cream is the only topical agent in the United States that does not contain propylene glycol.
Beauty may be only skin deep, but that layer of epidermis is a pretty busy place. Among its activities is the production of hundreds of substances that regulate susceptibility to infection. More than 50 of these chemicals fall into the class of skin microbicides.8
We began to learn about these microbicides a decade ago, when researchers asked why eczema usually is secondarily infected with pathogenic staphylococcus, streptococcus, and yeast, and psoriatic skin isn’t. The answer: Both healthy and psoriatic skin produce natural microbicides, but allergic dermatitis (eczema) prevents their release on the skin surface.9
1. Proteins
Some proteins fight microbes better than pharmaceutical agents do. The most important antimicrobial proteins in the skin are defensins and cathelicidins, which are found in all epithelial structures, including the vulva and vagina.10 In the defensin category, human ß-defensins 2 and 3 are the most important proteins and are present in the surface epithelium. An inflammatory response triggers their release to inhibit microbes on the skin surface. The mean inhibitory concentration of human ß-defensin 3 against the relatively resistant yeast, Saccharomyces cerevisiae, is about 14 μg/mL.11 This inhibitory action is superior to many azole anti-yeast agents. Human cathelicidin is an equally effective skin microbicide, with antiviral, antifungal, and antibacterial activity. In normal function, these natural antimicrobial substances prevent colonization of pathogenic organisms in healthy skin.
2. Stratum corneum
The stratum corneum comprises the outer few microns of the epithelium. When it remains intact, the stratum corneum is an effective barrier to microbial invasion. Intact skin prevents substances with a molecular weight greater than 500 daltons from passing into the skin.
This barrier may be compromised by microtrauma or dermatologic disorders such as irritant or allergic dermatitis. Minimal microtrauma is all that is necessary to allow small microbes such as viruses to pass through the stratum corneum. Larger organisms (spirochetes, yeast) may require a greater degree of compromise, such as flaking skin. Environmental and dermatologic factors often compromise this natural barrier.
The vaginal epithelium is not keratinized and lacks an effective stratum corneum. Instead, vaginal tissue produces mucus, which floats on a thin transudate of intercellular fluid. Potential pathogens are captured in the mucus and drain out of the vagina. The vaginal epithelium produces several milliliters of mucus daily that is constantly draining out of the vaginal lumen.
3. Langerhans cells
The skin has a final layer of defense within its structure. Microbes that pass through the stratum corneum and enter the skin are attacked by defensive cells that reside there, known as antigen-presenting cells. Langerhans cells are the chief antigen-presenting cells in the skin. They originate in the bone marrow, but rest in the skin, awaiting microbial invasion.
Antigen-presenting cells kill intraepithelial microbes as they are detected, and then process the microbial antigens, enabling a cell-mediated immune response against the microbes. Langerhans cells also destroy individual cancer cells that appear randomly in the epithelium.
The genital skin and the skin around the mouth and eyes carry the highest concentration of Langerhans cells.12 Under normal conditions, Langerhans cells constitute as much as 8% of the cells in vulvar skin. In the genital area, the cervical transformation zone has the highest count of Langerhans cells13—possibly compensation for a highly vulnerable epithelial barrier, owing to the immature squamous epithelium at this site.

Maturation of a Langerhans cell
Langerhans cells, the main antigen-presenting cells in skin, defend it from microbes that breach the stratum corneum. Although Langerhans cells originate in bone marrow, mature cells reside in the epidermis.
With its high concentration of Langerhans cells, the cervical transformation zone may be the primary port of entry of HIV.14 Langerhans cells have a surface CD4 receptor to which HIV attaches. The Langerhans cells are unable to kill the HIV after phagocytosis. HIV-infected Langerhans cells then lead to systemic spread of the virus.
Overall, the antimicrobial function of Langerhans cells is imperfect. When a pathogen is located within a cell, some microbes, such as Chlamydia trachomatis, herpesvirus, and HPV, may evade detection. In addition, some dermatologic conditions are associated with significant dysfunction of Langerhans cells.
HPV infection
The small HPV particle easily gains entry to minimally traumatized vulvar skin—with a high transmission rate with even a single exposure—and the immature epithelium of the cervical transformation zone makes that site a naturally compromised barrier to infection.
Under normal conditions, a cell-mediated immune response eliminates the HPV virus within 12 months, with lasting protection from reinfection. If cell-mediated immunity is compromised, the virus cannot be eliminated. The result is genital warts, variable degrees of dysplasia, or cancer, depending on the degree of immune compromise (FIGURE 5).
Risk factors for HPV-associated warts and dysplasia include allergy, immunosuppressant drugs to prevent rejection of a transplanted organ, and smoking. Smoking cessation is particularly important because control of the virus is dependent on Langerhans-cell function.

FIGURE 5 Genital warts
Debulking of warts with cryocautery or electrocautery may be appropriate, followed by imiquimod cream. It is prudent to biopsy persistent warts to exclude carcinoma, especially in an immunocompromised patient.
Begin by debulking warts
Electro- or cryocautery of large warts is an appropriate first step. Follow debulking with thrice-weekly application of one packet of imiquimod cream, to be washed off in the morning, for 4 to 6 weeks. This therapy may not eliminate genital warts in women who are taking immunosuppressant drugs to prevent organ-transplant rejection. Unfortunately, control of genital warts with monthly cautery of new warts may be the only useful option in these patients. Immunocompromised patients are at high risk for squamous carcinoma, and biopsy of persistent warts may be wise.
Vulnerabilities of vulvovaginal skin
The skin of the vulva and vagina is far from invincible. Some factors that affect it adversely are aging,4 tobacco use,5 estrogen deficiency,6 immunosuppressant drugs, and human immunodeficiency virus (HIV) infection.7 Look for these risk factors in women with persistent genital infection, so that the management plan can include treatment of the underlying dermatologic or immune disorder, as well as any microbes that are identified.
Immunosuppressed patients
Rejection of a transplanted organ is a function of cell-mediated immunity, so it is not surprising that drugs that suppress transplant rejection also inhibit vulvovaginal cell-mediated immunity. This increases the risk that HPV-associated disease will progress. Imiquimod cream promotes cell-mediated immunity by activating the release of interferon in the vulvar skin, and may compensate for depressed immune-cell function in the nontransplant population, but it is less effective in the transplant recipient. Sadly, there is no long-term solution to the effects of immunosuppressant therapy in this population; special surveillance for vulvar cancer and cervical dysplasia is necessary. Smoking cessation is also essential, especially for women with HPV-associated disease.
When HIV infection progresses to AIDS, Langerhans cells that carry HIV are depleted from the skin and substantially decrease in number, completely compromising cell-mediated immunity. This explains why AIDS patients often have severe genital herpes infections, severe chronic yeast vulvovaginitis, extensive molluscum disease, and unusual skin cancers. Antiretroviral therapy may restore some Langerhans-cell function.
Consider screening for HIV/AIDS when a woman has severe recurrent genital viral or yeast infection.
Aging and estrogen deficiency
Cell-mediated immune function declines with age. A higher risk of skin cancer, herpes (and its recurrence), and irritant and allergic vulvar dermatitis are the results. Increased surveillance for skin cancer, and varicella vaccination to lower the risk for herpes zoster, may be important.
Topical estrogen may be indicated if saline wet-prep evaluation reveals parabasal cells in vaginal secretions of a symptomatic postmenopausal woman. The estrogen may gradually alleviate burning and restrict potentially pathogenic bacterial flora. Be aware, however, that commercially available estrogen creams often contain propylene glycol, a recognized irritant of fragile skin. One solution: Have a compounding pharmacist formulate an equivalent cream (estradiol, 0.1 mg/g) for twice-daily topical application, using a base of petrolatum or solid vegetable oil.
Six common dermatologic disorders of the vulva and vagina can present considerable challenges:
- Allergic contact dermatitis: 100% of the population may be at risk for this disorder, and the vulvar skin is especially vulnerable.
- Irritant contact dermatitis: Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to the acquisition of sexually transmitted disease.
- Lichen sclerosus: Women with this condition are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
- Lichen planus: Antibiotic therapy is ineffective against this disorder; symptoms reappear as soon as antibiotics are stopped.
- Yeast infection: Yeast organisms release proteins that activate a local allergic response and perpetuate an environment that supports infection.
- Human papillomavirus (HPV): The small HPV particle easily gains entry to minimally traumatized vulvar skin.
These common conditions sometimes compromise the skin barrier and prevent an adequate immune response to invading microbes. Some degree of skin immune dysfunction is generally associated with each genital dermatologic disorder. Identifying and treating the underlying dermatologic disorder, then, often corrects the associated immune dysfunction and may restore the skin barrier and prevent further microbial invasion.
Allergic contact dermatitis
Allergic dermatitis, or atopic dermatitis (formerly called eczema), is a highly prevalent skin disorder (FIGURE 1). Depending on the environment and genetic factors, as many as 40% of adults have a history of atopic dermatitis, and essentially 100% of the population may be at risk. Women have a higher rate of atopic dermatitis than men do.
Recognized vulvar allergens or triggers include dry climate, elastic, latex, fragrances in soaps or body lotions, and residues of detergent and fabric softener in clothing. In most biopsy-proven cases of vulvar eczema, the patient is unable to identify specific allergens. Such a patient often has a history of asthma, allergic rhinitis, sinusitis, or atopic dermatitis on other parts of the body.
Patch testing is no help in identifying specific allergens in the pelvic area. Testing the tougher skin of the back may not disclose all vulvar sensitivities.
But biopsy is useful. Women with vulvar allergic contact dermatitis often complain of persistent itching. A history of allergy elsewhere on the body is diagnostically helpful, but a biopsy submitted to a dermatopathologist confirms the diagnosis.
Not all women with vulvar allergic dermatitis have atopy at other body sites. Hyperkeratosis and spongiosis in the pathology specimen are characteristic. A small (3-or 4-mm) biopsy at the most symptomatic site is appropriate in any woman with chronic vulvar pruritis.
Local immune dysfunction is involved. Allergic vulvar dermatitis is characterized by a locally dysfunctional cell-mediated immune response. Langerhans cells are involved in this allergic reaction, directed away from their normal protective role.1 (Read about the role of Langerhans cells in “Three barriers to microbial infection: The skin’s built-in defense system,”) Viruses, bacteria, and yeast that gain entry into the skin have greater freedom to proliferate and persist, and skin-cancer surveillance by Langerhans cells is also compromised, with an increased risk for squamous carcinoma. This may account for a large portion of the 50% of vulvar carcinomas that cannot be attributed to HPV infection. Langerhans-cell dysfunction also contributes to the progression of HPV-associated carcinoma.
Allergic dermatitis inhibits the production of human cathelicidin and the ß-defensins, natural skin microbicides. As a result, vulvar skin affected by allergic dermatitis has a higher yeast and bacterial colonization rate.

FIGURE 1 Allergic contact dermatitis
This case of severe allergic contact dermatitis has been aggravated by chronic scratching, especially of the left labia. Cases typically are much more subtle.
Look for telltale flaking skin
Allergic dermatitis involves flaking of the skin, probably due to allergic stimulation of epithelial cell proliferation. Flakes of skin are exfoliated before the desmosomes that hold individual skin cells together deteriorate. The flaking compromises the stratum corneum barrier, and likely facilitates skin invasion of yeast and bacteria that have colonized the surface.
Because the background rate of dermatitis in the general population is relatively high, skin flakes often appear in the saline wet prep and are referred to as “reactive, reparative” changes in the Papanicolaou smear.2
Other diagnostic clues. Chronic vulvar pruritus with a history of asthma, hay fever, sinusitis, atopic dermatitis, or dry skin is vulvar dermatitis until it is proved otherwise. Recurrent yeast infection is often reported as well.
In many cases, the dermatitis may exhibit no clinical signs beyond flakes of skin in the saline wet prep.
Start with a topical steroid
A trial of topical steroid ointment is appropriate, using a low-to medium-strength ointment such as 0.1% hydrocortisone butyrate, which may also lower the risk of yeast infection.
Several weeks of treatment may be necessary. It may take 4 to 6 weeks for a full layer of skin to be replaced. Subdermal atrophy, skin neovascularization, and other risks of topical steroids are of less concern during extended use of low-potency steroids, and may be more acceptable on an unexposed part of the body such as the vulva.
To test for therapeutic success, look for a reduction in pruritus and a lower incidence of yeast infection. Failure of steroid ointment and oral yeast suppression may justify vulvar biopsy, which should be submitted to a dermatopathologist.
Occasionally, a high-potency topical steroid such as clobetasol 0.05% ointment may be necessary (applied twice daily and rubbed in), but adrenal suppression may develop if therapy exceeds 3 to 4 weeks. The agent should be tapered rather than stopped abruptly.
Irritant contact dermatitis
This condition is characterized by a burning sensation. Common vulvar irritants include oxylate (in urine), propylene glycol (in medicated creams and lotions), and abrasive toilet paper. The list of potential irritants is long, and each irritant may have a different mechanism of action. A burning reaction after application of a topical cream suggests significant compromise of the skin barrier that would otherwise have prevented entry of the irritant. Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to acquisition of sexually transmitted disease.
Begin by identifying the culprits
The first step of treatment is recognizing and eliminating potential irritants such as bath soap, urine, topical creams that contain propylene glycol, and soap residue in clothing. Have the patient use a squirt bottle to rinse the genital area after urination to eliminate irritants such as oxylate. Also suggest that she rinse undergarments twice and use liquid rather than powder detergent. Cotton undergarments are more skin-friendly than synthetics.
Twice-daily or more frequent application of a skin moisturizer such as vegetable shortening, MimyX cream, or mineral oil/petrolatum cream (Eletone) helps to heal the skin, and continued use may prevent recurrence of symptoms.
When a patient complains of persistent vulvar pruritus and exhibits a figure-of-eight vulvar rash, suspect lichen sclerosus (FIGURE 2). The cause of this condition is unclear. Often, allergic contact dermatitis is superimposed on it. In older gynecologic terminology, this was referred to as mixed vulvar dystrophy.
Women with lichen sclerosus are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
Vulvar biopsy occasionally discloses unexpected early lichen sclerosus.

FIGURE 2 Lichen sclerosus
Note the labial agglutination (labia minora) and diffuse white epithelium, which are characteristic findings. If the areas of thickened epithelium do not resolve with topical steroid ointment, biopsy is appropriate.
Topical steroids are key to therapy
The degree of involvement that is grossly apparent determines the strength of the steroid ointment. If tissue is thickened, with areas of deep white change, a high-potency ointment such as clobetasol 0.05% may be necessary, applied twice daily for as long as several weeks. Milder cases may respond to a medium-strength topical steroid, such as fluticasone propionate 0.005% ointment.
If a higher-strength steroid is selected, it is appropriate to switch to a milder steroid as soon as symptoms resolve, with the goal of maintaining control with 1% hydrocortisone ointment or even continuous use of one of the skin moisturizers recommended for irritant dermatitis. Biopsy any thickened white patch, ulcerated area, or nonhealing skin fissure to check for squamous cancer.
Lichen planus
Erosive lichen planus (desquamative inflammatory vaginitis) of the vulva and vagina is an autoimmune skin disorder that causes superficial ulceration of the vaginal mucosa (FIGURE 3). An increase in vaginal discharge represents a shift in microflora away from lactobacillus dominance and an increase in the number of white blood cells and parabasal epithelial cells, with markedly heightened skin turnover. Local cellulitis does not develop, despite an overgrowth of various enteric organisms.

FIGURE 3 Lichen planus
This severe case resolved with a 3-month course of daily azathioprine (150 mg) but recurred after therapy ended.
A dermatologist may be required
Lichen planus is characterized by purulent discharge that contains bacteria, white blood cells, and parabasal cells. Unusual enteric microbes are often detected by routine culture, but antibiotic therapy is not helpful. Potent systemic anti-inflammatory therapy is often necessary rather than antimicrobial therapy. Daily azathioprine (Imuran) in doses ranging from 25 mg to 150 mg orally have been used, depending on the degree of vulvovaginal involvement. Tacrolimus ointment 0.1% applied twice daily may help in milder cases, but this agent typically causes an irritant reaction (burning) until the disorder partially resolves.
It may be helpful to seek the assistance of an experienced dermatologist if a biopsy demonstrates this disorder.
Vulvovaginal yeast infection is often found in conjunction with chronic vulvar eczema.3 Infection is promoted by:
- deficient skin microbicides
- skin-surface disruption with flaking
- ineffective Langerhans-cell response to invading yeast.
Yeast organisms release proteins that further activate a local allergic response to perpetuate an environment that supports infection (FIGURE 4). This represents a breakdown in the skin’s natural defenses.

FIGURE 4 Yeast infection
Vulvar yeast rash in the normally occluded area of the vulva with large satellite lesions (erythematous patches around the margin of the vulvar rash). Satellite lesions are typically much smaller.
Oral therapy may be preferred
Topical anti-yeast creams often contain propylene glycol, an irritant to fragile skin, so oral therapy may be more appropriate. For oral therapy, 200 mg of fluconazole every 3 days for three doses is a useful starting point. For severe cases, this can be followed by a weekly 200-mg oral dose for 2 to 3 months to maintain yeast suppression while the underlying skin disorder begins to resolve. An extended course of oral fluconazole may not be appropriate during pregnancy or anticoagulation or while the patient is taking a statin drug to lower cholesterol. If oral therapy is not appropriate, 1% clotrimazole 7-day vaginal cream is the only topical agent in the United States that does not contain propylene glycol.
Beauty may be only skin deep, but that layer of epidermis is a pretty busy place. Among its activities is the production of hundreds of substances that regulate susceptibility to infection. More than 50 of these chemicals fall into the class of skin microbicides.8
We began to learn about these microbicides a decade ago, when researchers asked why eczema usually is secondarily infected with pathogenic staphylococcus, streptococcus, and yeast, and psoriatic skin isn’t. The answer: Both healthy and psoriatic skin produce natural microbicides, but allergic dermatitis (eczema) prevents their release on the skin surface.9
1. Proteins
Some proteins fight microbes better than pharmaceutical agents do. The most important antimicrobial proteins in the skin are defensins and cathelicidins, which are found in all epithelial structures, including the vulva and vagina.10 In the defensin category, human ß-defensins 2 and 3 are the most important proteins and are present in the surface epithelium. An inflammatory response triggers their release to inhibit microbes on the skin surface. The mean inhibitory concentration of human ß-defensin 3 against the relatively resistant yeast, Saccharomyces cerevisiae, is about 14 μg/mL.11 This inhibitory action is superior to many azole anti-yeast agents. Human cathelicidin is an equally effective skin microbicide, with antiviral, antifungal, and antibacterial activity. In normal function, these natural antimicrobial substances prevent colonization of pathogenic organisms in healthy skin.
2. Stratum corneum
The stratum corneum comprises the outer few microns of the epithelium. When it remains intact, the stratum corneum is an effective barrier to microbial invasion. Intact skin prevents substances with a molecular weight greater than 500 daltons from passing into the skin.
This barrier may be compromised by microtrauma or dermatologic disorders such as irritant or allergic dermatitis. Minimal microtrauma is all that is necessary to allow small microbes such as viruses to pass through the stratum corneum. Larger organisms (spirochetes, yeast) may require a greater degree of compromise, such as flaking skin. Environmental and dermatologic factors often compromise this natural barrier.
The vaginal epithelium is not keratinized and lacks an effective stratum corneum. Instead, vaginal tissue produces mucus, which floats on a thin transudate of intercellular fluid. Potential pathogens are captured in the mucus and drain out of the vagina. The vaginal epithelium produces several milliliters of mucus daily that is constantly draining out of the vaginal lumen.
3. Langerhans cells
The skin has a final layer of defense within its structure. Microbes that pass through the stratum corneum and enter the skin are attacked by defensive cells that reside there, known as antigen-presenting cells. Langerhans cells are the chief antigen-presenting cells in the skin. They originate in the bone marrow, but rest in the skin, awaiting microbial invasion.
Antigen-presenting cells kill intraepithelial microbes as they are detected, and then process the microbial antigens, enabling a cell-mediated immune response against the microbes. Langerhans cells also destroy individual cancer cells that appear randomly in the epithelium.
The genital skin and the skin around the mouth and eyes carry the highest concentration of Langerhans cells.12 Under normal conditions, Langerhans cells constitute as much as 8% of the cells in vulvar skin. In the genital area, the cervical transformation zone has the highest count of Langerhans cells13—possibly compensation for a highly vulnerable epithelial barrier, owing to the immature squamous epithelium at this site.

Maturation of a Langerhans cell
Langerhans cells, the main antigen-presenting cells in skin, defend it from microbes that breach the stratum corneum. Although Langerhans cells originate in bone marrow, mature cells reside in the epidermis.
With its high concentration of Langerhans cells, the cervical transformation zone may be the primary port of entry of HIV.14 Langerhans cells have a surface CD4 receptor to which HIV attaches. The Langerhans cells are unable to kill the HIV after phagocytosis. HIV-infected Langerhans cells then lead to systemic spread of the virus.
Overall, the antimicrobial function of Langerhans cells is imperfect. When a pathogen is located within a cell, some microbes, such as Chlamydia trachomatis, herpesvirus, and HPV, may evade detection. In addition, some dermatologic conditions are associated with significant dysfunction of Langerhans cells.
HPV infection
The small HPV particle easily gains entry to minimally traumatized vulvar skin—with a high transmission rate with even a single exposure—and the immature epithelium of the cervical transformation zone makes that site a naturally compromised barrier to infection.
Under normal conditions, a cell-mediated immune response eliminates the HPV virus within 12 months, with lasting protection from reinfection. If cell-mediated immunity is compromised, the virus cannot be eliminated. The result is genital warts, variable degrees of dysplasia, or cancer, depending on the degree of immune compromise (FIGURE 5).
Risk factors for HPV-associated warts and dysplasia include allergy, immunosuppressant drugs to prevent rejection of a transplanted organ, and smoking. Smoking cessation is particularly important because control of the virus is dependent on Langerhans-cell function.

FIGURE 5 Genital warts
Debulking of warts with cryocautery or electrocautery may be appropriate, followed by imiquimod cream. It is prudent to biopsy persistent warts to exclude carcinoma, especially in an immunocompromised patient.
Begin by debulking warts
Electro- or cryocautery of large warts is an appropriate first step. Follow debulking with thrice-weekly application of one packet of imiquimod cream, to be washed off in the morning, for 4 to 6 weeks. This therapy may not eliminate genital warts in women who are taking immunosuppressant drugs to prevent organ-transplant rejection. Unfortunately, control of genital warts with monthly cautery of new warts may be the only useful option in these patients. Immunocompromised patients are at high risk for squamous carcinoma, and biopsy of persistent warts may be wise.
Vulnerabilities of vulvovaginal skin
The skin of the vulva and vagina is far from invincible. Some factors that affect it adversely are aging,4 tobacco use,5 estrogen deficiency,6 immunosuppressant drugs, and human immunodeficiency virus (HIV) infection.7 Look for these risk factors in women with persistent genital infection, so that the management plan can include treatment of the underlying dermatologic or immune disorder, as well as any microbes that are identified.
Immunosuppressed patients
Rejection of a transplanted organ is a function of cell-mediated immunity, so it is not surprising that drugs that suppress transplant rejection also inhibit vulvovaginal cell-mediated immunity. This increases the risk that HPV-associated disease will progress. Imiquimod cream promotes cell-mediated immunity by activating the release of interferon in the vulvar skin, and may compensate for depressed immune-cell function in the nontransplant population, but it is less effective in the transplant recipient. Sadly, there is no long-term solution to the effects of immunosuppressant therapy in this population; special surveillance for vulvar cancer and cervical dysplasia is necessary. Smoking cessation is also essential, especially for women with HPV-associated disease.
When HIV infection progresses to AIDS, Langerhans cells that carry HIV are depleted from the skin and substantially decrease in number, completely compromising cell-mediated immunity. This explains why AIDS patients often have severe genital herpes infections, severe chronic yeast vulvovaginitis, extensive molluscum disease, and unusual skin cancers. Antiretroviral therapy may restore some Langerhans-cell function.
Consider screening for HIV/AIDS when a woman has severe recurrent genital viral or yeast infection.
Aging and estrogen deficiency
Cell-mediated immune function declines with age. A higher risk of skin cancer, herpes (and its recurrence), and irritant and allergic vulvar dermatitis are the results. Increased surveillance for skin cancer, and varicella vaccination to lower the risk for herpes zoster, may be important.
Topical estrogen may be indicated if saline wet-prep evaluation reveals parabasal cells in vaginal secretions of a symptomatic postmenopausal woman. The estrogen may gradually alleviate burning and restrict potentially pathogenic bacterial flora. Be aware, however, that commercially available estrogen creams often contain propylene glycol, a recognized irritant of fragile skin. One solution: Have a compounding pharmacist formulate an equivalent cream (estradiol, 0.1 mg/g) for twice-daily topical application, using a base of petrolatum or solid vegetable oil.
1. Cruz PD. The epidermis: an outpost of the immune system. In: Freinkel RK, Woodley D, eds. The Biology of the Skin. New York: Parthenon Publishing Group; 2000:256-260.
2. Bonfiglio TA, Erozan YS. Gynecologic Cytopathology. Philadelphia: Lippincott-Raven; 1997:42-45.
3. Fidel PL, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335-348.
4. Gilchrest B, Murphy G, Soter N. Effect of chronological aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982;79:85-88.
5. Ouyang Y, Virasch N, Hao P, et al. Suppression of human IL-1beta, IL-2, IFN-gamma, and TNF-alpha by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280-287.
6. Mao A, Paharkova-Vatchkova V, Hardy J, et al. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146-5151.
7. Memar OM, Geraminejad P, Arany I, Tyring SK. Cutaneous resistance to viral infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York: Marcel Dekker; 2002:25-28.
8. Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9-13.
9. Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262-3269.
10. Harder J, Bartels J, Christophers E, Schroder JN. Isolation and characterization of human beta defensin-3, a novel inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713.
11. Garcia JR, Jaumann F, Schultz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 2001;306:257-264.
12. Udey MC. Cadherins and Langerhans cell immunobiology. Clin Exp Immunol. 1997;107(suppl. 1):6-8.
13. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253-1263.
14. Tschachler E, Groh V Popovic, et al. Epidermal Langerhans cells—a target for HTLV III/LAV infection. J Invest Dermatol. 1987;88:233-237.
1. Cruz PD. The epidermis: an outpost of the immune system. In: Freinkel RK, Woodley D, eds. The Biology of the Skin. New York: Parthenon Publishing Group; 2000:256-260.
2. Bonfiglio TA, Erozan YS. Gynecologic Cytopathology. Philadelphia: Lippincott-Raven; 1997:42-45.
3. Fidel PL, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335-348.
4. Gilchrest B, Murphy G, Soter N. Effect of chronological aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982;79:85-88.
5. Ouyang Y, Virasch N, Hao P, et al. Suppression of human IL-1beta, IL-2, IFN-gamma, and TNF-alpha by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280-287.
6. Mao A, Paharkova-Vatchkova V, Hardy J, et al. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146-5151.
7. Memar OM, Geraminejad P, Arany I, Tyring SK. Cutaneous resistance to viral infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York: Marcel Dekker; 2002:25-28.
8. Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9-13.
9. Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262-3269.
10. Harder J, Bartels J, Christophers E, Schroder JN. Isolation and characterization of human beta defensin-3, a novel inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713.
11. Garcia JR, Jaumann F, Schultz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 2001;306:257-264.
12. Udey MC. Cadherins and Langerhans cell immunobiology. Clin Exp Immunol. 1997;107(suppl. 1):6-8.
13. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253-1263.
14. Tschachler E, Groh V Popovic, et al. Epidermal Langerhans cells—a target for HTLV III/LAV infection. J Invest Dermatol. 1987;88:233-237.