User login
Metastatic Crohn Disease: A Review of Dermatologic Manifestations and Treatment
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
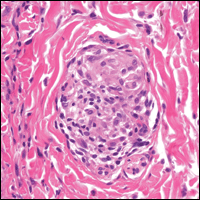
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34
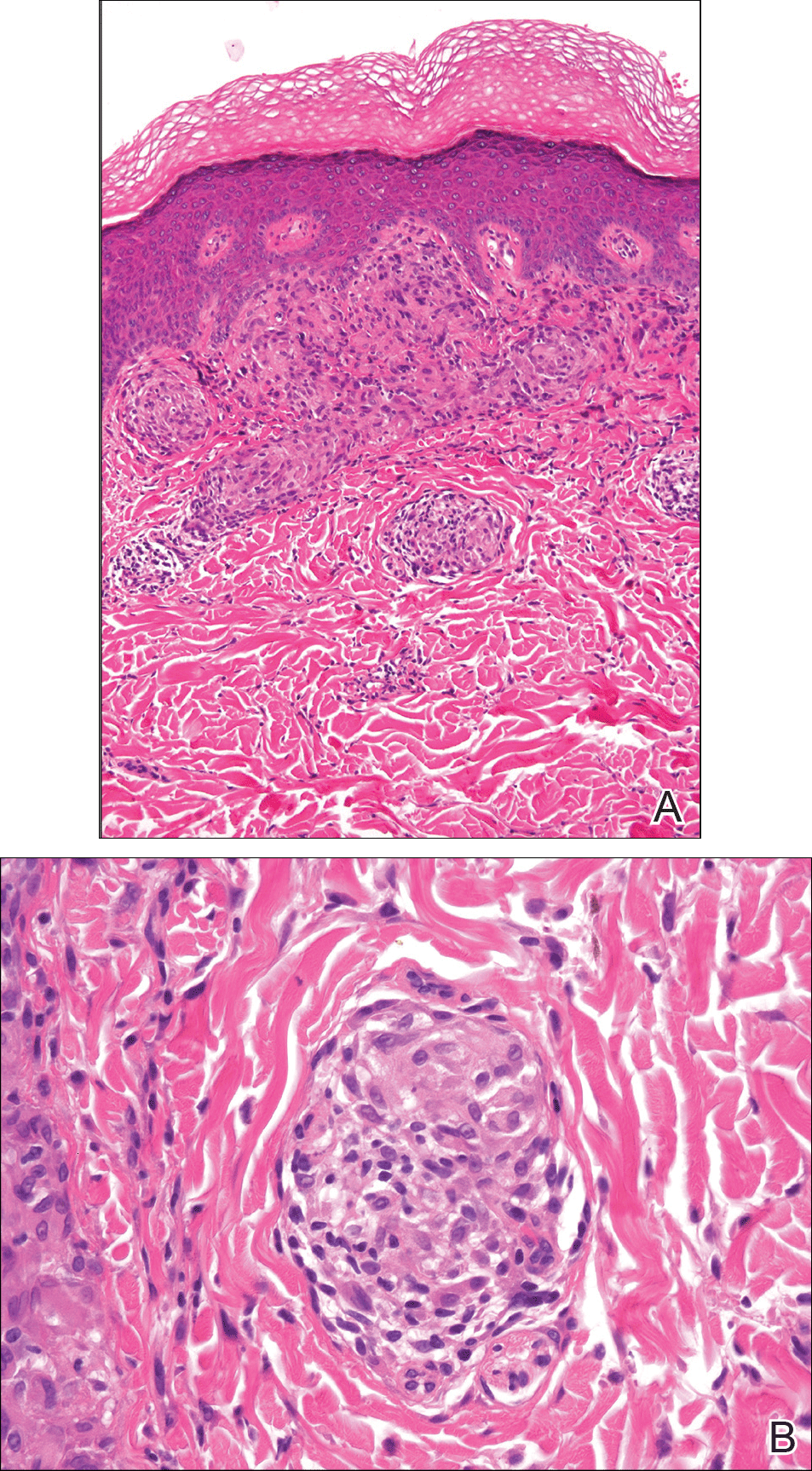
On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34

On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34

On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
Practice Points
- Almost half of patients with Crohn disease develop a dermatologic manifestation of the disease.
- The etiology of metastatic Crohn disease is unknown and diagnosis requires a high index of suspicion with exclusion of other processes.
2017 Update on menopause
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
Is Diabetes Distress on Your Radar Screen?

Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of persons with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder; rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.13-15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings, this review is intended to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.19
DIABETES DISTRESS: WHAT IT IS, WHAT IT'S NOT
For patients with type 2 diabetes, diabetes distress centers around four main issues
- Frustration with the demands of self-care
- Apprehension about the future and the possibility of developing serious complications
- Concern about both the quality and the cost of required medical care
- Perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least five of the nine symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least two weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes, and the co-occurrence of a psychiatric disorder and diabetes distress is high.24,25Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that persons diagnosed with diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
SCREENING FOR DIABETES DISTRESS
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (see Table).26-30 Some of the measures are easily accessible online; others require a subscription to MEDLINE.
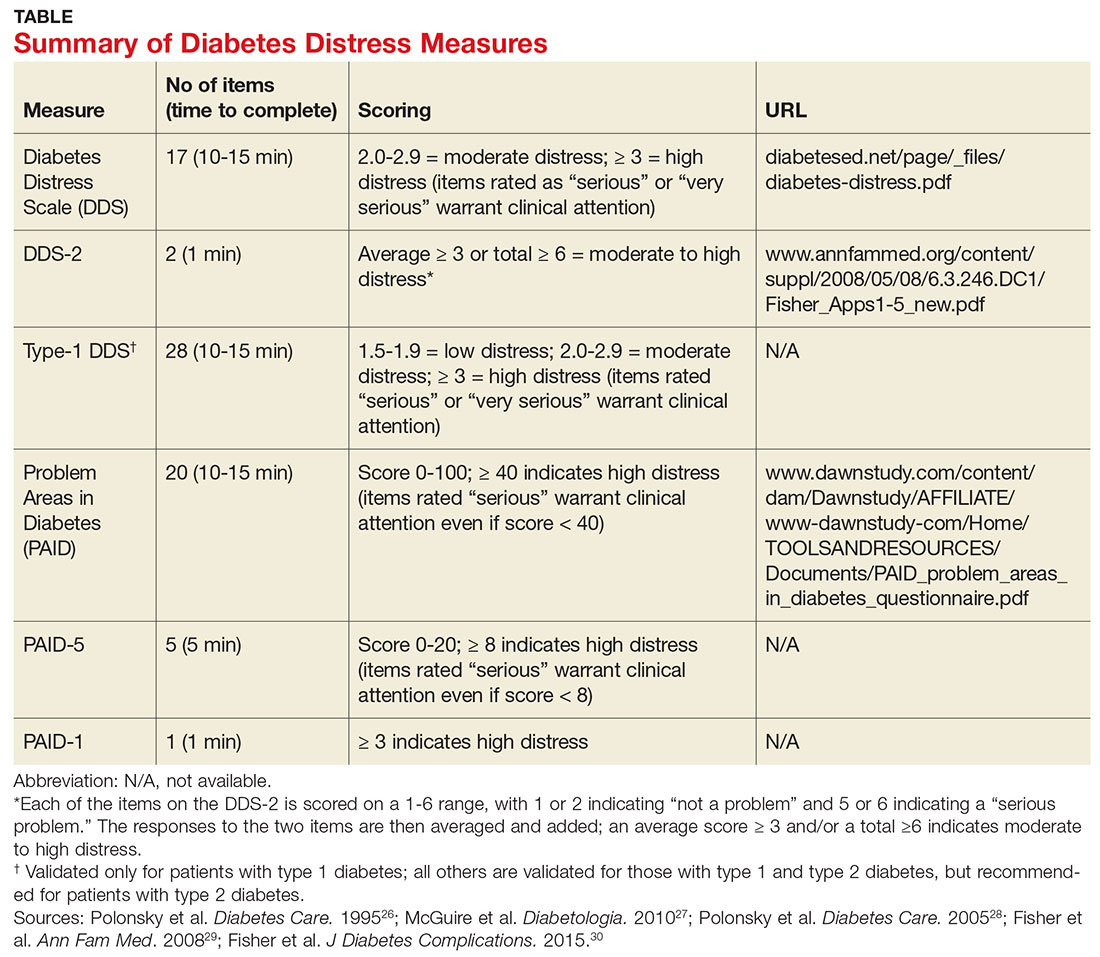
Problem Areas in Diabetes (PAID). There are three versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a five-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS). This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with two items and a 28-item scale specifically for patients with type 1 diabetes.29,30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the seven sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but also that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
HOW TO RESPOND TO DIABETES DISTRESS
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only two studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT).31 The trial had three arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress–specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), self-management behaviors, and A1C.
Participants in all three arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in A1C were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other two groups.31
The DIAMOS trial recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a two-arm RCT.32 One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, A1C, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, A1C, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function, the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors.36-38 Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care providers without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care providers to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Clinicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussions. (See “Directing Help Where It’s Most Needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46

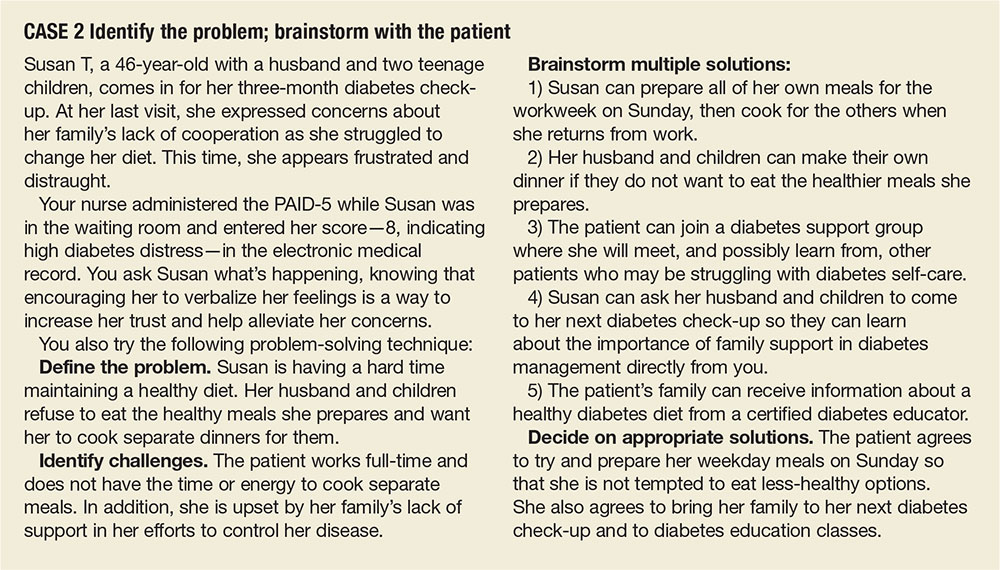
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educ. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psychol. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. N Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectr. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl):S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:1822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33:1080-1106.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatry. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychol. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diab Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Educ Couns. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectr. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed May 16, 2017.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.

Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of persons with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder; rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.13-15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings, this review is intended to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.19
DIABETES DISTRESS: WHAT IT IS, WHAT IT'S NOT
For patients with type 2 diabetes, diabetes distress centers around four main issues
- Frustration with the demands of self-care
- Apprehension about the future and the possibility of developing serious complications
- Concern about both the quality and the cost of required medical care
- Perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least five of the nine symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least two weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes, and the co-occurrence of a psychiatric disorder and diabetes distress is high.24,25Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that persons diagnosed with diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
SCREENING FOR DIABETES DISTRESS
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (see Table).26-30 Some of the measures are easily accessible online; others require a subscription to MEDLINE.

Problem Areas in Diabetes (PAID). There are three versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a five-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS). This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with two items and a 28-item scale specifically for patients with type 1 diabetes.29,30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the seven sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but also that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
HOW TO RESPOND TO DIABETES DISTRESS
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only two studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT).31 The trial had three arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress–specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), self-management behaviors, and A1C.
Participants in all three arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in A1C were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other two groups.31
The DIAMOS trial recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a two-arm RCT.32 One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, A1C, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, A1C, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function, the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors.36-38 Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care providers without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care providers to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Clinicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussions. (See “Directing Help Where It’s Most Needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46



Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of persons with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder; rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.13-15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings, this review is intended to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.19
DIABETES DISTRESS: WHAT IT IS, WHAT IT'S NOT
For patients with type 2 diabetes, diabetes distress centers around four main issues
- Frustration with the demands of self-care
- Apprehension about the future and the possibility of developing serious complications
- Concern about both the quality and the cost of required medical care
- Perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least five of the nine symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least two weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes, and the co-occurrence of a psychiatric disorder and diabetes distress is high.24,25Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that persons diagnosed with diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
SCREENING FOR DIABETES DISTRESS
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (see Table).26-30 Some of the measures are easily accessible online; others require a subscription to MEDLINE.

Problem Areas in Diabetes (PAID). There are three versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a five-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS). This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with two items and a 28-item scale specifically for patients with type 1 diabetes.29,30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the seven sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but also that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
HOW TO RESPOND TO DIABETES DISTRESS
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only two studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT).31 The trial had three arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress–specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), self-management behaviors, and A1C.
Participants in all three arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in A1C were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other two groups.31
The DIAMOS trial recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a two-arm RCT.32 One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, A1C, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, A1C, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function, the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors.36-38 Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care providers without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care providers to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Clinicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussions. (See “Directing Help Where It’s Most Needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46


1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educ. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psychol. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. N Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectr. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl):S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:1822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33:1080-1106.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatry. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychol. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diab Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Educ Couns. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectr. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed May 16, 2017.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educ. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psychol. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. N Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectr. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl):S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:1822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33:1080-1106.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatry. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychol. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diab Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Educ Couns. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectr. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed May 16, 2017.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
Common Variable Immunodeficiency: A Clinical Overview
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23
The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23

The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23

The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
First EDition: Novel Blood Collection System May Reduce Contamination Rates, more
BY JEFF BAUER
Use of a blood collection system that diverts and sequesters the initial 1.5 to 2 mL of blood was associated with a significant decrease in blood culture contamination compared to standard practice, according to an open-label trial conducted at a single ED. The results were published online in the journal Clinical Infectious Diseases.
An estimated 0.6% to 6% of blood cultures are contaminated. Some blood cultures may become contaminated by skin fragments colonized with bacteria that are dislodged during venipuncture. Such false-positive results lead to increased costs and harm associated with unnecessary additional testing and treatment.
Researchers at the University of Nebraska Medical Center evaluated a novel sterile blood collection system, the SteriPath initial specimen diversion device (ISDD), to determine if it could reduce contamination rates by diverting and excluding the initial portion of collected blood. Investigators evaluated 1,808 blood cultures from 904 adult ED patients at an urban 689-bed university hospital. The patients’ mean age was 59 years, and 55% were male. For each patient, the first 20-mL blood sample was obtained using a standard procedure in which blood was drawn into a syringe and then injected into blood culture vials. A second 20-mL sample was obtained using the ISDD; the initial 1.5 to 2 mL of blood was diverted into a holding chamber, and the rest of the sample was directed into the blood culture vials. A culture was determined to be contaminated if one or more of several skin-residing organisms, including coagulase-negative staphylococci, Propionibacterium species, Micrococcus species, viridans group streptococci, Corynebacterium species, or Bacillus species, was recovered from only one of the paired cultures.
Compared to standard practice, use of the ISDD was associated with a significant reduction in blood culture contamination. Overall, two of the 904 samples (0.22%) collected with the ISDD were contaminated, compared to 16 of the 904 samples (1.78%) collected via standard practice (P = .001). Sensitivity was not affected by use of the ISDD; true septicemia was observed in 65 of 904 samples (7.2%) collected via ISDD and 69 of 904 samples (7.6%) collected via standard procedure (P = .41).
Rupp ME, Cavalieri RJ, Marolf C, Lyden E. Reduction in blood culture contamination through use of initial specimen diversion device. Clin Infect Dis. 2017 Apr 3. [Epub ahead of print]. doi:10.1093/cid/cix304.
FDA: Fluoroquinolone Use Not Linked to Retinal Detachment, Aortic Problems
LUCAS FRANKI
FRONTLINE MEDICAL NEWS
The Food and Drug Administration (FDA) has found no evidence of a link between fluoroquinolone antibiotic use and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling adverse effects of oral and injectable fluoroquinolones.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
In a Safety Communication published May 12, 2016, the FDA noted that serious adverse effects were possible from fluoroquinolone usage and that fluoroquinolones should be prescribed only when no other treatment options are possible. Serious adverse effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a pins-and-needles feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
After reviewing patient cases and study findings, the FDA said the evidence did not support an association between fluoroquinolone use and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. May 10, 2017. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Accessed May 25, 2017.
Intravenous tPA Increases Risk of Mortality in Children With Acute Ischemic Stroke
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients ages 0 to 17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in five patients, and both tPA and IAT in 24 patients. The overall mortality rate was 7.8%, but in those who received tPA, it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, of Rush University Medical Center, Chicago, reported at the annual meeting of the American Academy of Neurology. No deaths occurred in those who underwent only IAT, said Dr Ess.
Other outcomes were also worse for those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs 47.5%), and intracerebral hemorrhage was more common in treated vs untreated patients (10.1% vs 3.8%). Costs for treated patients averaged $200,346 vs $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session. “We need prospective clinical trials in children,” he said.
HCV Seroconversion Rate 0.1% After Occupational Exposure
BIANCA NOGRADY
FRONTLINE MEDICAL NEWS
An analysis of 13 years of accidental occupational exposures to hepatitis C virus (HCV)-contaminated fluids or instruments has revealed a seroconversion rate of just 0.1%, significantly lower than that previously reported in the literature. This finding is from a longitudinal analysis of data from a prospectively maintained database of 1,361 occupational injuries involving HCV-positive source that occurred between 2002 and 2015 conducted by Francesco M. Egro, MD, and his colleagues from the University of Pittsburgh Medical Center. Results were published online in the American Journal of Infection Control.
The two incidents of seroconversion occurred in patients who were exposed to blood from an HCV-positive patient via percutaneous injuries to the thumb from a hollow-bore needle, representing an overall seroconversion rate of 0.1%. In both cases, the source patients whose blood was involved were not coinfected with hepatitis B virus or human immunodeficiency virus.
Researchers also conducted a review of literature on needlestick injuries and occupational exposure to HCV-infected blood and fluids; from this review, they calculated an overall seroconversion rate average of 0.7%, with an average rate of 0.8% for percutaneous exposures. The review did not include mucomembranous exposure, as there were not enough data.
In this study, 65% of exposures were caused by percutaneous injuries and 34% were caused by mucocutaneous injuries; the cause of the remaining 1% was uncertain.
The hand was the most common site of injury (63%), followed by the face and neck (28%), and the arm, foot, leg, or trunk (4%). There was no record of the anatomical location of the injury in 5% of cases.
In nearly three-quarters of cases, blood was the source of exposure, while blood-containing saliva accounted for 3% of cases. The remaining 24% of c
“The risk of transmission after exposure to HCV-positive patients’ fluids or tissues other than blood is expected to be low, but has not been formally quantified,” the authors wrote. “Although there have been reports of HCV seroconversion after human bites and after punching a HCV-positive individual in the teeth, percutaneous exposures to the blood of a HCV-positive source remain the most common cause of occupational HCV transmission.”
While the rate of seroconversion was low, the authors encouraged prompt reporting, testing, and follow-up of exposed individuals.
Egro FM, Nwaiwu CA, Smith S, Harper JD, Spiess AM. Seroconversion rates among health care workers exposed to hepatitis C virus-contaminated body fluids: The University of Pittsburgh 13-year experience. Am J Infect Control. 2017 Apr 24. [Epub ahead of print]. doi:10.1016/j.ajic.2017.03.011.
BY JEFF BAUER
Use of a blood collection system that diverts and sequesters the initial 1.5 to 2 mL of blood was associated with a significant decrease in blood culture contamination compared to standard practice, according to an open-label trial conducted at a single ED. The results were published online in the journal Clinical Infectious Diseases.
An estimated 0.6% to 6% of blood cultures are contaminated. Some blood cultures may become contaminated by skin fragments colonized with bacteria that are dislodged during venipuncture. Such false-positive results lead to increased costs and harm associated with unnecessary additional testing and treatment.
Researchers at the University of Nebraska Medical Center evaluated a novel sterile blood collection system, the SteriPath initial specimen diversion device (ISDD), to determine if it could reduce contamination rates by diverting and excluding the initial portion of collected blood. Investigators evaluated 1,808 blood cultures from 904 adult ED patients at an urban 689-bed university hospital. The patients’ mean age was 59 years, and 55% were male. For each patient, the first 20-mL blood sample was obtained using a standard procedure in which blood was drawn into a syringe and then injected into blood culture vials. A second 20-mL sample was obtained using the ISDD; the initial 1.5 to 2 mL of blood was diverted into a holding chamber, and the rest of the sample was directed into the blood culture vials. A culture was determined to be contaminated if one or more of several skin-residing organisms, including coagulase-negative staphylococci, Propionibacterium species, Micrococcus species, viridans group streptococci, Corynebacterium species, or Bacillus species, was recovered from only one of the paired cultures.
Compared to standard practice, use of the ISDD was associated with a significant reduction in blood culture contamination. Overall, two of the 904 samples (0.22%) collected with the ISDD were contaminated, compared to 16 of the 904 samples (1.78%) collected via standard practice (P = .001). Sensitivity was not affected by use of the ISDD; true septicemia was observed in 65 of 904 samples (7.2%) collected via ISDD and 69 of 904 samples (7.6%) collected via standard procedure (P = .41).
Rupp ME, Cavalieri RJ, Marolf C, Lyden E. Reduction in blood culture contamination through use of initial specimen diversion device. Clin Infect Dis. 2017 Apr 3. [Epub ahead of print]. doi:10.1093/cid/cix304.
FDA: Fluoroquinolone Use Not Linked to Retinal Detachment, Aortic Problems
LUCAS FRANKI
FRONTLINE MEDICAL NEWS
The Food and Drug Administration (FDA) has found no evidence of a link between fluoroquinolone antibiotic use and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling adverse effects of oral and injectable fluoroquinolones.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
In a Safety Communication published May 12, 2016, the FDA noted that serious adverse effects were possible from fluoroquinolone usage and that fluoroquinolones should be prescribed only when no other treatment options are possible. Serious adverse effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a pins-and-needles feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
After reviewing patient cases and study findings, the FDA said the evidence did not support an association between fluoroquinolone use and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. May 10, 2017. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Accessed May 25, 2017.
Intravenous tPA Increases Risk of Mortality in Children With Acute Ischemic Stroke
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients ages 0 to 17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in five patients, and both tPA and IAT in 24 patients. The overall mortality rate was 7.8%, but in those who received tPA, it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, of Rush University Medical Center, Chicago, reported at the annual meeting of the American Academy of Neurology. No deaths occurred in those who underwent only IAT, said Dr Ess.
Other outcomes were also worse for those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs 47.5%), and intracerebral hemorrhage was more common in treated vs untreated patients (10.1% vs 3.8%). Costs for treated patients averaged $200,346 vs $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session. “We need prospective clinical trials in children,” he said.
HCV Seroconversion Rate 0.1% After Occupational Exposure
BIANCA NOGRADY
FRONTLINE MEDICAL NEWS
An analysis of 13 years of accidental occupational exposures to hepatitis C virus (HCV)-contaminated fluids or instruments has revealed a seroconversion rate of just 0.1%, significantly lower than that previously reported in the literature. This finding is from a longitudinal analysis of data from a prospectively maintained database of 1,361 occupational injuries involving HCV-positive source that occurred between 2002 and 2015 conducted by Francesco M. Egro, MD, and his colleagues from the University of Pittsburgh Medical Center. Results were published online in the American Journal of Infection Control.
The two incidents of seroconversion occurred in patients who were exposed to blood from an HCV-positive patient via percutaneous injuries to the thumb from a hollow-bore needle, representing an overall seroconversion rate of 0.1%. In both cases, the source patients whose blood was involved were not coinfected with hepatitis B virus or human immunodeficiency virus.
Researchers also conducted a review of literature on needlestick injuries and occupational exposure to HCV-infected blood and fluids; from this review, they calculated an overall seroconversion rate average of 0.7%, with an average rate of 0.8% for percutaneous exposures. The review did not include mucomembranous exposure, as there were not enough data.
In this study, 65% of exposures were caused by percutaneous injuries and 34% were caused by mucocutaneous injuries; the cause of the remaining 1% was uncertain.
The hand was the most common site of injury (63%), followed by the face and neck (28%), and the arm, foot, leg, or trunk (4%). There was no record of the anatomical location of the injury in 5% of cases.
In nearly three-quarters of cases, blood was the source of exposure, while blood-containing saliva accounted for 3% of cases. The remaining 24% of c
“The risk of transmission after exposure to HCV-positive patients’ fluids or tissues other than blood is expected to be low, but has not been formally quantified,” the authors wrote. “Although there have been reports of HCV seroconversion after human bites and after punching a HCV-positive individual in the teeth, percutaneous exposures to the blood of a HCV-positive source remain the most common cause of occupational HCV transmission.”
While the rate of seroconversion was low, the authors encouraged prompt reporting, testing, and follow-up of exposed individuals.
Egro FM, Nwaiwu CA, Smith S, Harper JD, Spiess AM. Seroconversion rates among health care workers exposed to hepatitis C virus-contaminated body fluids: The University of Pittsburgh 13-year experience. Am J Infect Control. 2017 Apr 24. [Epub ahead of print]. doi:10.1016/j.ajic.2017.03.011.
BY JEFF BAUER
Use of a blood collection system that diverts and sequesters the initial 1.5 to 2 mL of blood was associated with a significant decrease in blood culture contamination compared to standard practice, according to an open-label trial conducted at a single ED. The results were published online in the journal Clinical Infectious Diseases.
An estimated 0.6% to 6% of blood cultures are contaminated. Some blood cultures may become contaminated by skin fragments colonized with bacteria that are dislodged during venipuncture. Such false-positive results lead to increased costs and harm associated with unnecessary additional testing and treatment.
Researchers at the University of Nebraska Medical Center evaluated a novel sterile blood collection system, the SteriPath initial specimen diversion device (ISDD), to determine if it could reduce contamination rates by diverting and excluding the initial portion of collected blood. Investigators evaluated 1,808 blood cultures from 904 adult ED patients at an urban 689-bed university hospital. The patients’ mean age was 59 years, and 55% were male. For each patient, the first 20-mL blood sample was obtained using a standard procedure in which blood was drawn into a syringe and then injected into blood culture vials. A second 20-mL sample was obtained using the ISDD; the initial 1.5 to 2 mL of blood was diverted into a holding chamber, and the rest of the sample was directed into the blood culture vials. A culture was determined to be contaminated if one or more of several skin-residing organisms, including coagulase-negative staphylococci, Propionibacterium species, Micrococcus species, viridans group streptococci, Corynebacterium species, or Bacillus species, was recovered from only one of the paired cultures.
Compared to standard practice, use of the ISDD was associated with a significant reduction in blood culture contamination. Overall, two of the 904 samples (0.22%) collected with the ISDD were contaminated, compared to 16 of the 904 samples (1.78%) collected via standard practice (P = .001). Sensitivity was not affected by use of the ISDD; true septicemia was observed in 65 of 904 samples (7.2%) collected via ISDD and 69 of 904 samples (7.6%) collected via standard procedure (P = .41).
Rupp ME, Cavalieri RJ, Marolf C, Lyden E. Reduction in blood culture contamination through use of initial specimen diversion device. Clin Infect Dis. 2017 Apr 3. [Epub ahead of print]. doi:10.1093/cid/cix304.
FDA: Fluoroquinolone Use Not Linked to Retinal Detachment, Aortic Problems
LUCAS FRANKI
FRONTLINE MEDICAL NEWS
The Food and Drug Administration (FDA) has found no evidence of a link between fluoroquinolone antibiotic use and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling adverse effects of oral and injectable fluoroquinolones.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
In a Safety Communication published May 12, 2016, the FDA noted that serious adverse effects were possible from fluoroquinolone usage and that fluoroquinolones should be prescribed only when no other treatment options are possible. Serious adverse effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a pins-and-needles feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
After reviewing patient cases and study findings, the FDA said the evidence did not support an association between fluoroquinolone use and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. May 10, 2017. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Accessed May 25, 2017.
Intravenous tPA Increases Risk of Mortality in Children With Acute Ischemic Stroke
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients ages 0 to 17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in five patients, and both tPA and IAT in 24 patients. The overall mortality rate was 7.8%, but in those who received tPA, it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, of Rush University Medical Center, Chicago, reported at the annual meeting of the American Academy of Neurology. No deaths occurred in those who underwent only IAT, said Dr Ess.
Other outcomes were also worse for those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs 47.5%), and intracerebral hemorrhage was more common in treated vs untreated patients (10.1% vs 3.8%). Costs for treated patients averaged $200,346 vs $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session. “We need prospective clinical trials in children,” he said.
HCV Seroconversion Rate 0.1% After Occupational Exposure
BIANCA NOGRADY
FRONTLINE MEDICAL NEWS
An analysis of 13 years of accidental occupational exposures to hepatitis C virus (HCV)-contaminated fluids or instruments has revealed a seroconversion rate of just 0.1%, significantly lower than that previously reported in the literature. This finding is from a longitudinal analysis of data from a prospectively maintained database of 1,361 occupational injuries involving HCV-positive source that occurred between 2002 and 2015 conducted by Francesco M. Egro, MD, and his colleagues from the University of Pittsburgh Medical Center. Results were published online in the American Journal of Infection Control.
The two incidents of seroconversion occurred in patients who were exposed to blood from an HCV-positive patient via percutaneous injuries to the thumb from a hollow-bore needle, representing an overall seroconversion rate of 0.1%. In both cases, the source patients whose blood was involved were not coinfected with hepatitis B virus or human immunodeficiency virus.
Researchers also conducted a review of literature on needlestick injuries and occupational exposure to HCV-infected blood and fluids; from this review, they calculated an overall seroconversion rate average of 0.7%, with an average rate of 0.8% for percutaneous exposures. The review did not include mucomembranous exposure, as there were not enough data.
In this study, 65% of exposures were caused by percutaneous injuries and 34% were caused by mucocutaneous injuries; the cause of the remaining 1% was uncertain.
The hand was the most common site of injury (63%), followed by the face and neck (28%), and the arm, foot, leg, or trunk (4%). There was no record of the anatomical location of the injury in 5% of cases.
In nearly three-quarters of cases, blood was the source of exposure, while blood-containing saliva accounted for 3% of cases. The remaining 24% of c
“The risk of transmission after exposure to HCV-positive patients’ fluids or tissues other than blood is expected to be low, but has not been formally quantified,” the authors wrote. “Although there have been reports of HCV seroconversion after human bites and after punching a HCV-positive individual in the teeth, percutaneous exposures to the blood of a HCV-positive source remain the most common cause of occupational HCV transmission.”
While the rate of seroconversion was low, the authors encouraged prompt reporting, testing, and follow-up of exposed individuals.
Egro FM, Nwaiwu CA, Smith S, Harper JD, Spiess AM. Seroconversion rates among health care workers exposed to hepatitis C virus-contaminated body fluids: The University of Pittsburgh 13-year experience. Am J Infect Control. 2017 Apr 24. [Epub ahead of print]. doi:10.1016/j.ajic.2017.03.011.
Caring for the transgender patient: The role of the gynecologist
CASE: Transgender man consults gynecologist for fertility options
A 36-year-old transgender man considering the possibility of having his own biological children presents to the gynecology office to discuss hysterectomy as gender dysphoria treatment as well as his fertility preservation options. He has never had a gynecologic examination. Since age 24, he has been on testosterone therapy. Although his menses initially ceased, each month over the past 2 years he has had breakthrough spotting lasting 2 to 4 days, sometimes accompanied by pelvic pain and cramping. These symptoms have caused him distress and anxiety, which have led to his missing work 1 to 3 days each month. On presentation, he appears anxious and makes little eye contact. His girlfriend of 6 years has come in with him and is very supportive.
Over the past decade, transgender health care has moved to the forefront of the medical conversation. At many prominent medical centers across the United States, clinicians are forming multidisciplinary teams to help improve the health care of this patient population. Outcomes are being studied, and the literature is becoming more robust.
People tend to think of transgender women—male-assigned persons who self-identify as female—as the typical prototype for transgender people, but this focus is skewed in both society and the medical community. Transgender men—female-assigned persons who self-identify as male—remain underrepresented, mostly because they want to stay “under the radar,” especially with respect to medical care and, more specifically, routine gynecologic care.
Although the transgender woman has unique health needs and may present to a gynecologist for care after gender-affirmationsurgery, the transgender man’s many health care needs and their subtleties can be addressed only by a gynecologist. In this article, I review these intricacies of care to help increase clinician comfort in treating these patients.
Clinicians can take steps to:
- ensure all patients have the correct identifiers in their medical records
- provide staff with the proper education and diversity training
- instruct staff in proper use of pronouns
- set up unisex or gender-nonbinary restrooms with appropriate signage
- make the decor gender nonspecific.
Beth Cronin, MD, a practicing general gynecologist in Providence, Rhode Island, says that you also should consider a general sign, placed in a highly visible area, that represents your nondiscrimination policy. The AMA offers this wording: "This office appreciates the diversity of human beings and does not discriminate based on race, age, religion, ability, marital status, sexual orientation, sex or gender identity." She also recommends having education and marketing materials with affirmative imagery and content and providing educational brochures on transgender health topics.
Why transgender patients may delay seeking health care
Transgender patients remain underserved because of the health care barriers they encounter. Factors contributing to poor access include lack of health insurance, inability to pay for services, clinician insensitivity and hostility, and fear of exposure of transgender status during health care encounters.1 In a recent large survey study, 30% of transgender respondents indicated that they delayed or did not seek medical care as a result of discrimination, and those who had needed to teach their clinicians about transgenderism were 4 times more likely to postpone or not seek care.2
In a 2015 survey of ObGyns’ current knowledge and practice regarding LGBT (lesbian, gay, bisexual, transgender) care, only one-third of respondents indicated they were comfortable caring for transgender patients.3 In addition, only one-third indicated being knowledgeable about the steps transgender patients must take to transition to their self-identified gender, and less than half were familiar with the recommendations for the routine health maintenance and screening of these patients.
Much of this discomfort derives from the lack of incorporation of LGBT-specific topics in medical curricula. In 2011, Obedin-Maliver and colleagues found that, at 176 US and Canadian allopathic and osteopathic medical schools, the median time dedicated to LGBT health care needs and related topics was unsatisfactory.4 This deficiency is slowly being reduced with changes in the curricula of many health care specialties. In ObGyn residency programs, for example, transgender-specific questions have been added to annual in-service examinations. The hope is that, as education initiatives improve, clinicians will become more comfortable caring for gender-minority patients, who with improved access to care will no longer need to seek subspecialists in transgender services.
Read about the need for gyn exams, managing benign disorders, and cervical cancer screening
Considerations for the gynecologic visit and examination
Transgender men visit the gynecology office for many reasons, including routine gynecologic care and health maintenance, care for acute and chronic gynecologic conditions (abnormal bleeding, pelvic pain, vaginitis), evaluation and management of pelvic floor disorders, consultation on hysterectomy for gender transition, and fertility counseling.
However, transgender men who reach their third, fourth, or fifth decade without having had a pelvic examination cite many reasons for avoiding the gynecology office. Most commonly, gynecologic visits and genital examination can severely exacerbate these patients’ gender dysphoria. In addition, many patients who do not engage in penetrative vaginal sex think their health risks are so low that they can forgo or delay pelvic exams. Patients who have stopped menstruating while on testosterone therapy may think there is no need for routine gynecologic care. Other reasons for avoiding pelvic exams are pain and traumatic sexual memories.5
Related Article:
Four pillars of a successful practice: 4. Motivate your staff
Transgender men need to receive the regular guideline-recommended pelvic exams and screenings used for cisgender women. (Cisgender refers to a person whose sense of gender identity corresponds with their birth sex.) We need to educate patients in this regard and to discuss several issues before performing an examination. First, take a thorough history and avoid making assumptions about sexual orientation and sex practices. Some patients have penetrative vaginal intercourse with either men or women. For some patients, the exam may cause dysphoria symptoms, and we need to validate patients’ fears. Discussing these issues ahead of time helps patients get used to the idea of undergoing an exam and assures them that the clinician is experienced in performing these exams for transgender men. In my practice, we explain the exam’s purpose (screening or diagnosis) and importance. We also counsel patients that they may experience some normal, and temporary, spotting after the exam. For those who experience severe dysphoria with vaginal bleeding of any kind, we acknowledge that postexam spotting may cause some anxiety. Patients with severe anxiety before the exam may be premedicated with an anxiolytic agent as long as someone can transport them to and from the office.
The bimanual exam should be performed with care and efficiency and with the patient given as much control as possible. In most cases, we ask patients to undress only from the waist down, and their genitals stay covered. Patients uncomfortable in stirrups are asked to show us the position that suits them best, and we try to accommodate them. Although speed is a goal, remember that many patients are nulliparous, have had limited or no vaginal penetration, or are on testosterone and have significant vaginal dryness. Use the smallest speculum possible, a pediatric or long and narrow adult speculum, and apply lubricant copiously. Pre-exam application of topical lidocaine jelly to the introitus can help reduce pain. To help a patient relax the pelvic floor muscles and habituate to the presence of a foreign object in the vagina, start the exam by inserting a single digit. In addition, ask the patient about speculum placement inside the vagina: Does he want to place the speculum himself or guide the clinician’s hand? Open the speculum only as much as needed to adequately visualize the cervix and then remove it with care.
Managing benign gynecologic disorders
The same algorithms are used to evaluate abnormal bleeding in all patients, but the differential diagnosis expands for those on testosterone therapy. Testosterone may no longer be suppressing their cycles, and abnormal bleeding could simply be the return of menses, which would present as regular cyclic bleeding. Increasing the testosterone dosing or changing the testosterone formulation may help, and the gynecologist should discuss these options with the patient’s prescribing clinician. In addition, progesterone in any form (for example, medroxyprogesterone acetate 5 to 30 mg daily) can be added to testosterone regimens to help suppress menses. The levonorgestrel-releasing intrauterine device (LNG-IUD) can be very effective, but placement can induce anxiety, and some patients decline this treatment option.
In patients with intermenstrual spotting, assess the vagina for atrophy. Both over-the-counter vaginal moisturizers and DHEA (dehydroepiandrosterone) suppositories (1% compounded) can help treat atrophy, but not all patients are comfortable using them. Most patients decline vaginal estrogen products for symptomatic vaginal atrophy even though the systemic effects are minimal.
The historic literature suggests that female-to-male patients’ long-term exposure to androgens leads to atrophic changes in the endometrium and myometrium, and clinical studies of menopausal women who take exogenous androgens have confirmed this effect.6 However, new data point to a different histologic scenario. A recent study found a possible association between long-term testosterone use in transgender men of reproductive age and a low proliferative active endometrium, as well as hypertrophic changes in the myometrium.7 The causes may be peripheral aromatization of androgens and expression and up-regulation of androgen receptors within the endometrial stroma and myometrial cells.8 Given these emerging data and anecdotal cases reported by clinicians who perform hysterectomies for transgender men, imaging and tissue sampling should be used to evaluate abnormal uterine bleeding, particularly in patients previously amenorrheic on testosterone. Be aware that transvaginal ultrasound or endometrial biopsy are challenging procedures for these patients. Counsel patients to ensure that they adhere to follow-up.
Related Article:
2017 Update on cervical disease
The ongoing need for cervical cancer screening
The concept of “original gender surveillance” was presented in a 2-case series of transgender men with uterine and cervical cancer that might have been detected earlier with better screening and routine care.9 There is no evidence, however, that long-term high-dose androgen therapy causes endometrial or cervical cancer,10 and the data on endometrial cancer in patients on cross-sex hormone therapy are limited such that a causal relationship between testosterone and these malignancies cannot be established.9,11–14
The rate of unsatisfactory Pap smears is higher in transgender men than in cisgender women. The difference was anecdotally noted by clinicians who routinely cared for transgender patients over time and was confirmed with a retrospective chart review.15
Peitzmeier and colleagues reviewed the records of 233 transgender men and 3,625 cisgender women with Pap tests performed at an urban community health center over 6 years.15 The transgender cohort, with its prevalence rate of 10%, was 10 times more likely to have an unsatisfactory or inadequate Pap smear. Moreover, the transgender patients were more likely to have longer latency to follow-up for a repeat Pap test. In addition, testosterone therapy was more likely associated with inadequate Pap smears, and time on testosterone therapy was associated with higher odds of Pap smear inadequacy. Besides the exogenous hormone therapy, clinician comfort level and experience may have contributed to the high prevalence of inadequate Pap smears.
As mentioned earlier, it is important to become comfortable performing pelvic exams for transgender men and to prepare patients for the possibility that a Pap smear might be inadequate, making a follow-up visit and repeat Pap test necessary.16
Read about hysterectomy, oophorectomy, and vaginectomy choices
Consultation for hysterectomy: Perioperative considerations
Transgender men may undergo hysterectomy, oophorectomy, and/or vaginectomy. The TABLE summarizes the indications and perioperative considerations for each procedure.

Some transgender men undergo hysterectomy for benign gynecologic disease. Counseling and perioperative planning are the same for these patients as for cisgender women, although some of the considerations discussed here remain important.
Other patients undergo hysterectomy as part of transitioning to their self-affirmed gender. The World Professional Association for Transgender Health (WPATH) Standards of Care should be used to guide counseling and treatment.17 These guidelines were designed as a framework for performing hysterectomy and other gender-affirming procedures. According to the WPATH standards, the criteria for hysterectomy and oophorectomy are:
- 2 referral letters from qualified mental health professionals
- well-documented persistent gender dysphoria
- capacity to make fully informed decisions and to consent to treatment
- age of majority in given country
- good control of any concurrent medical or mental health concerns, and
- hormone therapy for 12 continuous months, as appropriate to gender goals, unless the patient has a medical contraindication or is otherwise unable or unwilling to take hormones.
As the guidelines emphasize, these criteria do not apply to patients undergoing either procedure for medical indications other than gender dysphoria.
Hysterectomy approach. Most surgeons perform gender-affirming hysterectomies laparoscopically. Many clinicians hesitate to perform these hysterectomies vaginally, as the patients are often nulliparous. In general, the best operative route is the one the surgeon feels most comfortable performing safely and efficiently. For a nulliparous patient with minimal pelvic organ descensus and a narrow pelvis, the laparoscopic approach is reasonable. A recent study in a small cohort of transgender men found that vaginal hysterectomy was successful in only 1 in 4 patients.18 Nevertheless, the American College of Obstetricians and Gynecologists (ACOG) recommends vaginal hysterectomy, when appropriate, for limiting complications and morbidity while maximizing cost-effectiveness.19 Although data are limited, vaginal hysterectomy seems feasible and should be considered in a subset of patients who pre‑sent for gender-affirming hysterectomy.
Related Article:
Total laparoscopic versus laparoscopic supracervical hysterectomy
The oophorectomy debate
Oophorectomy concurrent with hysterectomy remains a topic of debate among gynecologists who perform hysterectomy for gender transition. Some clinicians think gonadectomy poses a significant risk for bone health compromise at an early age. The long-term effects of testosterone on bone have not been well studied. Although bone metabolism is thought to increase over the short term, there are no major changes in bone density over the long term. In fact, in the setting of long-term testosterone therapy, cortical bone was found to be larger in transgender men than in cisgender women.20 The issue is for patients who stop taking exogenous testosterone after oophorectomy. This subset of patients has not been well studied but clearly needs bone health surveillance and supplementation.
Another concern about oophorectomy is its effect on fertility. Because it is important to discuss fertility-preserving options, during consultation for a hysterectomy I spend a large portion of time addressing fertility goals. Patients who want to become a parent but do not want to carry a child (they want a current or future partner or surrogate to carry) are candidates for hysterectomy; those who do not want a genetic child are candidates for oophorectomy; and those who do not want to preserve their fertility (or have already ended it) and who meet the WPATH criteria for surgery are candidates for oophorectomy concurrent with hysterectomy. The discussion can be particularly challenging with young transgender men, since their ability to project their family planning goals may be compromised by their gender dysphoria. Clinicians can counsel patients about another option: isolated hysterectomy with subsequent staged oophorectomy.
Similar to cisgender women with polycystic ovary syndrome, transgender men on exogenous testosterone therapy are at risk for ovarian cysts,7 which can cause pain and should be evaluated and managed. As mentioned, these patients may find it difficult to visit a gynecologist and tolerate a vaginal examination, and many fear presenting to an emergency room, as they will need to disclose their transgender status and risk being discriminated against or, worse, not being triaged or cared for properly. Patients should be thoroughly counseled about the risks and benefits of having oophorectomy performed concurrently with hysterectomy.
Related Article:
Vaginal hysterectomy with basic instrumentation
The question of vaginectomy
Patients and clinicians often ask about concurrent vaginectomy procedures. In some cases, patients with severe gender dysphoria and absence of penetrative vaginal activity request excision or obliteration of the vagina. There is no standard of care, however. Vaginectomy can be done transvaginally or abdominally: open, laparoscopically, or robotically. It therefore should be performed by surgeons experienced in the procedure. Patients should be advised that a portion of the vaginal epithelium is sometimes used for certain phalloplasty procedures and that, if they are considering genital reconstruction in the future, it may be beneficial to preserve the vagina until that time.
There are no guidelines on stopping or continuing testosterone therapy perioperatively. Some clinicians are concerned about possible venous thromboembolic events related to perioperative use of testosterone, but there are no data supporting increased risk. The risk of postoperative vaginal cuff bleeding in patients on and off testosterone has not been well studied. Since patients who stop taking testosterone may develop severe mood swings and malaise, they should be counseled on recognizing and managing such changes. There are also no data on the risk of vaginal cuff dehiscence in this patient population. Testosterone usually causes the vagina to become very atrophic, so proper closure should be ensured to avoid cuff evisceration. In my practice, the vaginal cuff is closed in 2 layers using at least 1 layer of delayed absorbable suture.
Read about addressing fertility, contraception, OB care, and your role
Addressing fertility, contraception, and obstetric care
Most transgender men are able to conceive a child.21 Data in this area, however, are sparse. Most of the literature on reproductive health in this patient population is focused on human immunodeficiency virus (HIV) and other sexually transmitted infections.22 Nevertheless, patient-physician dialogue on fertility and reproductive health has increased since more patients started seeking surgical transition services (likely a result of improved coverage for these surgeries). In addition, we are learning more about patients’ ability and desire to conceive after long-term use of cross-sex hormone therapy. The importance of this dialogue is becoming apparent. One survey study found that more than half of the transgender men who had undergone affirmation surgery wanted to become parents.23
Before initiating cross-sex hormone therapy or before undergoing hysterectomy and/or oophorectomy, patients must be counseled about their fertility options. Testosterone may affect fertility and fecundity, but there are case reports of successful pregnancy after discontinuation of testosterone.21 Reproductive endocrinology and fertility specialists have begun to recognize the importance of fertility preservation in this patient population and to apply the principles of oncofertility care beyond patients with cancer. In a 2015 opinion paper on access to fertility services by transgender persons, the Ethics Committee of the American Society for Reproductive Medicine focused on this population’s unique fertility needs.24 Currently, oocyte and embryo cryopreservation are options for transgender men planning to start cross-sex hormones or undergo surgery.25 Other methods being investigated may become options in the future.25
There are even fewer data on transgender men’s contraceptive needs. Many clinicians mistakenly think these patients are at low risk for pregnancy. Some patients have male partners and engage in penetrative penile-vaginal intercourse; others are not on testosterone therapy; and still others, despite taking testosterone, are not always amenorrheic and may be ovulating. In a small cross-sectional study, Light and colleagues found that 12% of transgender men who were surveyed after conceiving had been amenorrheic on testosterone therapy, and 24% of these pregnancies were not planned.21
In a study by Cipres and colleagues, half of the 26 transgender men were considered at risk for pregnancy: These patients still had a uterus, not all were on testosterone, not all on testosterone were amenorrheic, they were having vaginal intercourse with cisgender men, and none were using condoms or other contraception.26 The authors noted several potential underlying reasons for poor counseling on contraceptive needs: patients feel stigmatized, clinicians assume these patients are not candidates for “female” hormone therapy, patients fear these modalities may feminize them and compromise their affirmed identities, patients poorly understand how testosterone works and have mistaken ideas about its contraceptive properties, and clinician discomfort with broaching fertility and reproductive health discussions.
Data are also limited on pregnancy in transgender men. We do know that clinicians are not well equipped to help patients during the peripartum period and better resources are needed.21 Gender dysphoria can worsen during and immediately after pregnancy, and patients may be at significant risk for postpartum depression. More research is needed.
Related Article:
Care of the transgender patient: What is the gynecologist's role?
Gynecologists play key role in transgender care
Transgender men’s unique health care needs can be addressed only by gynecologists.It is important to become comfortable with and educated about these needs and their subtleties. This starts with understanding transgender patients’ gender dysphoria associated with the gynecologic visit and examination. Learning more about these patients and their needs will improve health care delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Xavier JM, Simmons R. The Washington Transgender Needs Assessment Survey, 2000. http://www.glaa.org/archive/2000/tgneedsassessment1112.shtml. Accessed January 2, 2017.
- Jaffee KD, Shires DA, Stroumsa D. Discrimination and delayed health care among transgender women and men: implications for improving medical education and health care delivery. Med Care. 2016;54(11):1010–1016.
- Unger CA. Care of the transgender patient: a survey of gynecologists’ current knowledge and practice. J Womens Health. 2015;24(2):114–118.
- Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–977.
- Feldman J. Medical and surgical management of the transgender patient: what the primary care clinician needs to know. In: Makadon H, Mayer K, Potter J, Goldhammer H, eds. Fenway Guide to Lesbian, Gay, Bisexual, and Transgender Health. Philadelphia, PA: American College of Physicians; 2008:365–392.
- Hickok LR, Toomey C, Speroff L. A comparison of esterified estrogens with and without methyltestosterone: effects on endometrial histology and serum lipoproteins in postmenopausal women. Obstet Gynecol. 1993;82(6):919–924.
- Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691.
- Mertens HJ, Heineman MJ, Koudstaal J, Theunissen P, Evers JL. Androgen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):11–13.
- Urban RR, Teng NN, Kapp DS. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204(5):e9–e12.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159(3):197–202.
- Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–497.
- Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76(3):413–415.
- Dizon DS, Tejada-Berges T, Keolliker S, Steinhoff M, Grania CO. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Oncol Invest. 2006;62(4):226–228.
- Schenck TL, Holzbach T, Zantl N, et al. Vaginal carcinoma in a female-to-male transsexual. J Sex Med. 2010;7(8):2899–2902.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med. 2014;29(5):778–784.
- Potter J, Peitzmeier SM, Bernstein I, et al. Cervical cancer screening for patients on the female-to-male spectrum: a narrative review and guide for clinicians. J Gen Intern Med. 2015;30(12):1857–1864.
- Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Version 7. https://s3.amazonaws.com/amo_hub_content/Association140/files/Standards_of_Care_V7_2011_WPATH(2)(1).pdf. Published 2011. Accessed January 21, 2017.
- Obedin-Maliver J, Light A, de Haan G, Jackson RA. Feasibility of vaginal hysterectomy for female-to-male transgender men. Obstet Gynecol. 2017;129(3):457–463.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–466.
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127.
- Stephens SC, Bernstein KT, Philip SS. Male to female and female to male transgender persons have different sexual risk behaviors yet similar rates of STDs and HIV. AIDS Behav. 2011;15(3):683–686.
- Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487.
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104(5):1111–1115.
- Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol. 2014;30(12):868–871.
- Cipres D, Seidman D, Cloniger C 3rd, Nova C, O’Shea A, Obedin-Maliver J. Contraceptive use and pregnancy intentions among transgender men presenting to a clinic for sex workers and their families in San Francisco. Contraception. 2016;95(2):186–189.
CASE: Transgender man consults gynecologist for fertility options
A 36-year-old transgender man considering the possibility of having his own biological children presents to the gynecology office to discuss hysterectomy as gender dysphoria treatment as well as his fertility preservation options. He has never had a gynecologic examination. Since age 24, he has been on testosterone therapy. Although his menses initially ceased, each month over the past 2 years he has had breakthrough spotting lasting 2 to 4 days, sometimes accompanied by pelvic pain and cramping. These symptoms have caused him distress and anxiety, which have led to his missing work 1 to 3 days each month. On presentation, he appears anxious and makes little eye contact. His girlfriend of 6 years has come in with him and is very supportive.
Over the past decade, transgender health care has moved to the forefront of the medical conversation. At many prominent medical centers across the United States, clinicians are forming multidisciplinary teams to help improve the health care of this patient population. Outcomes are being studied, and the literature is becoming more robust.
People tend to think of transgender women—male-assigned persons who self-identify as female—as the typical prototype for transgender people, but this focus is skewed in both society and the medical community. Transgender men—female-assigned persons who self-identify as male—remain underrepresented, mostly because they want to stay “under the radar,” especially with respect to medical care and, more specifically, routine gynecologic care.
Although the transgender woman has unique health needs and may present to a gynecologist for care after gender-affirmationsurgery, the transgender man’s many health care needs and their subtleties can be addressed only by a gynecologist. In this article, I review these intricacies of care to help increase clinician comfort in treating these patients.
Clinicians can take steps to:
- ensure all patients have the correct identifiers in their medical records
- provide staff with the proper education and diversity training
- instruct staff in proper use of pronouns
- set up unisex or gender-nonbinary restrooms with appropriate signage
- make the decor gender nonspecific.
Beth Cronin, MD, a practicing general gynecologist in Providence, Rhode Island, says that you also should consider a general sign, placed in a highly visible area, that represents your nondiscrimination policy. The AMA offers this wording: "This office appreciates the diversity of human beings and does not discriminate based on race, age, religion, ability, marital status, sexual orientation, sex or gender identity." She also recommends having education and marketing materials with affirmative imagery and content and providing educational brochures on transgender health topics.
Why transgender patients may delay seeking health care
Transgender patients remain underserved because of the health care barriers they encounter. Factors contributing to poor access include lack of health insurance, inability to pay for services, clinician insensitivity and hostility, and fear of exposure of transgender status during health care encounters.1 In a recent large survey study, 30% of transgender respondents indicated that they delayed or did not seek medical care as a result of discrimination, and those who had needed to teach their clinicians about transgenderism were 4 times more likely to postpone or not seek care.2
In a 2015 survey of ObGyns’ current knowledge and practice regarding LGBT (lesbian, gay, bisexual, transgender) care, only one-third of respondents indicated they were comfortable caring for transgender patients.3 In addition, only one-third indicated being knowledgeable about the steps transgender patients must take to transition to their self-identified gender, and less than half were familiar with the recommendations for the routine health maintenance and screening of these patients.
Much of this discomfort derives from the lack of incorporation of LGBT-specific topics in medical curricula. In 2011, Obedin-Maliver and colleagues found that, at 176 US and Canadian allopathic and osteopathic medical schools, the median time dedicated to LGBT health care needs and related topics was unsatisfactory.4 This deficiency is slowly being reduced with changes in the curricula of many health care specialties. In ObGyn residency programs, for example, transgender-specific questions have been added to annual in-service examinations. The hope is that, as education initiatives improve, clinicians will become more comfortable caring for gender-minority patients, who with improved access to care will no longer need to seek subspecialists in transgender services.
Read about the need for gyn exams, managing benign disorders, and cervical cancer screening
Considerations for the gynecologic visit and examination
Transgender men visit the gynecology office for many reasons, including routine gynecologic care and health maintenance, care for acute and chronic gynecologic conditions (abnormal bleeding, pelvic pain, vaginitis), evaluation and management of pelvic floor disorders, consultation on hysterectomy for gender transition, and fertility counseling.
However, transgender men who reach their third, fourth, or fifth decade without having had a pelvic examination cite many reasons for avoiding the gynecology office. Most commonly, gynecologic visits and genital examination can severely exacerbate these patients’ gender dysphoria. In addition, many patients who do not engage in penetrative vaginal sex think their health risks are so low that they can forgo or delay pelvic exams. Patients who have stopped menstruating while on testosterone therapy may think there is no need for routine gynecologic care. Other reasons for avoiding pelvic exams are pain and traumatic sexual memories.5
Related Article:
Four pillars of a successful practice: 4. Motivate your staff
Transgender men need to receive the regular guideline-recommended pelvic exams and screenings used for cisgender women. (Cisgender refers to a person whose sense of gender identity corresponds with their birth sex.) We need to educate patients in this regard and to discuss several issues before performing an examination. First, take a thorough history and avoid making assumptions about sexual orientation and sex practices. Some patients have penetrative vaginal intercourse with either men or women. For some patients, the exam may cause dysphoria symptoms, and we need to validate patients’ fears. Discussing these issues ahead of time helps patients get used to the idea of undergoing an exam and assures them that the clinician is experienced in performing these exams for transgender men. In my practice, we explain the exam’s purpose (screening or diagnosis) and importance. We also counsel patients that they may experience some normal, and temporary, spotting after the exam. For those who experience severe dysphoria with vaginal bleeding of any kind, we acknowledge that postexam spotting may cause some anxiety. Patients with severe anxiety before the exam may be premedicated with an anxiolytic agent as long as someone can transport them to and from the office.
The bimanual exam should be performed with care and efficiency and with the patient given as much control as possible. In most cases, we ask patients to undress only from the waist down, and their genitals stay covered. Patients uncomfortable in stirrups are asked to show us the position that suits them best, and we try to accommodate them. Although speed is a goal, remember that many patients are nulliparous, have had limited or no vaginal penetration, or are on testosterone and have significant vaginal dryness. Use the smallest speculum possible, a pediatric or long and narrow adult speculum, and apply lubricant copiously. Pre-exam application of topical lidocaine jelly to the introitus can help reduce pain. To help a patient relax the pelvic floor muscles and habituate to the presence of a foreign object in the vagina, start the exam by inserting a single digit. In addition, ask the patient about speculum placement inside the vagina: Does he want to place the speculum himself or guide the clinician’s hand? Open the speculum only as much as needed to adequately visualize the cervix and then remove it with care.
Managing benign gynecologic disorders
The same algorithms are used to evaluate abnormal bleeding in all patients, but the differential diagnosis expands for those on testosterone therapy. Testosterone may no longer be suppressing their cycles, and abnormal bleeding could simply be the return of menses, which would present as regular cyclic bleeding. Increasing the testosterone dosing or changing the testosterone formulation may help, and the gynecologist should discuss these options with the patient’s prescribing clinician. In addition, progesterone in any form (for example, medroxyprogesterone acetate 5 to 30 mg daily) can be added to testosterone regimens to help suppress menses. The levonorgestrel-releasing intrauterine device (LNG-IUD) can be very effective, but placement can induce anxiety, and some patients decline this treatment option.
In patients with intermenstrual spotting, assess the vagina for atrophy. Both over-the-counter vaginal moisturizers and DHEA (dehydroepiandrosterone) suppositories (1% compounded) can help treat atrophy, but not all patients are comfortable using them. Most patients decline vaginal estrogen products for symptomatic vaginal atrophy even though the systemic effects are minimal.
The historic literature suggests that female-to-male patients’ long-term exposure to androgens leads to atrophic changes in the endometrium and myometrium, and clinical studies of menopausal women who take exogenous androgens have confirmed this effect.6 However, new data point to a different histologic scenario. A recent study found a possible association between long-term testosterone use in transgender men of reproductive age and a low proliferative active endometrium, as well as hypertrophic changes in the myometrium.7 The causes may be peripheral aromatization of androgens and expression and up-regulation of androgen receptors within the endometrial stroma and myometrial cells.8 Given these emerging data and anecdotal cases reported by clinicians who perform hysterectomies for transgender men, imaging and tissue sampling should be used to evaluate abnormal uterine bleeding, particularly in patients previously amenorrheic on testosterone. Be aware that transvaginal ultrasound or endometrial biopsy are challenging procedures for these patients. Counsel patients to ensure that they adhere to follow-up.
Related Article:
2017 Update on cervical disease
The ongoing need for cervical cancer screening
The concept of “original gender surveillance” was presented in a 2-case series of transgender men with uterine and cervical cancer that might have been detected earlier with better screening and routine care.9 There is no evidence, however, that long-term high-dose androgen therapy causes endometrial or cervical cancer,10 and the data on endometrial cancer in patients on cross-sex hormone therapy are limited such that a causal relationship between testosterone and these malignancies cannot be established.9,11–14
The rate of unsatisfactory Pap smears is higher in transgender men than in cisgender women. The difference was anecdotally noted by clinicians who routinely cared for transgender patients over time and was confirmed with a retrospective chart review.15
Peitzmeier and colleagues reviewed the records of 233 transgender men and 3,625 cisgender women with Pap tests performed at an urban community health center over 6 years.15 The transgender cohort, with its prevalence rate of 10%, was 10 times more likely to have an unsatisfactory or inadequate Pap smear. Moreover, the transgender patients were more likely to have longer latency to follow-up for a repeat Pap test. In addition, testosterone therapy was more likely associated with inadequate Pap smears, and time on testosterone therapy was associated with higher odds of Pap smear inadequacy. Besides the exogenous hormone therapy, clinician comfort level and experience may have contributed to the high prevalence of inadequate Pap smears.
As mentioned earlier, it is important to become comfortable performing pelvic exams for transgender men and to prepare patients for the possibility that a Pap smear might be inadequate, making a follow-up visit and repeat Pap test necessary.16
Read about hysterectomy, oophorectomy, and vaginectomy choices
Consultation for hysterectomy: Perioperative considerations
Transgender men may undergo hysterectomy, oophorectomy, and/or vaginectomy. The TABLE summarizes the indications and perioperative considerations for each procedure.

Some transgender men undergo hysterectomy for benign gynecologic disease. Counseling and perioperative planning are the same for these patients as for cisgender women, although some of the considerations discussed here remain important.
Other patients undergo hysterectomy as part of transitioning to their self-affirmed gender. The World Professional Association for Transgender Health (WPATH) Standards of Care should be used to guide counseling and treatment.17 These guidelines were designed as a framework for performing hysterectomy and other gender-affirming procedures. According to the WPATH standards, the criteria for hysterectomy and oophorectomy are:
- 2 referral letters from qualified mental health professionals
- well-documented persistent gender dysphoria
- capacity to make fully informed decisions and to consent to treatment
- age of majority in given country
- good control of any concurrent medical or mental health concerns, and
- hormone therapy for 12 continuous months, as appropriate to gender goals, unless the patient has a medical contraindication or is otherwise unable or unwilling to take hormones.
As the guidelines emphasize, these criteria do not apply to patients undergoing either procedure for medical indications other than gender dysphoria.
Hysterectomy approach. Most surgeons perform gender-affirming hysterectomies laparoscopically. Many clinicians hesitate to perform these hysterectomies vaginally, as the patients are often nulliparous. In general, the best operative route is the one the surgeon feels most comfortable performing safely and efficiently. For a nulliparous patient with minimal pelvic organ descensus and a narrow pelvis, the laparoscopic approach is reasonable. A recent study in a small cohort of transgender men found that vaginal hysterectomy was successful in only 1 in 4 patients.18 Nevertheless, the American College of Obstetricians and Gynecologists (ACOG) recommends vaginal hysterectomy, when appropriate, for limiting complications and morbidity while maximizing cost-effectiveness.19 Although data are limited, vaginal hysterectomy seems feasible and should be considered in a subset of patients who pre‑sent for gender-affirming hysterectomy.
Related Article:
Total laparoscopic versus laparoscopic supracervical hysterectomy
The oophorectomy debate
Oophorectomy concurrent with hysterectomy remains a topic of debate among gynecologists who perform hysterectomy for gender transition. Some clinicians think gonadectomy poses a significant risk for bone health compromise at an early age. The long-term effects of testosterone on bone have not been well studied. Although bone metabolism is thought to increase over the short term, there are no major changes in bone density over the long term. In fact, in the setting of long-term testosterone therapy, cortical bone was found to be larger in transgender men than in cisgender women.20 The issue is for patients who stop taking exogenous testosterone after oophorectomy. This subset of patients has not been well studied but clearly needs bone health surveillance and supplementation.
Another concern about oophorectomy is its effect on fertility. Because it is important to discuss fertility-preserving options, during consultation for a hysterectomy I spend a large portion of time addressing fertility goals. Patients who want to become a parent but do not want to carry a child (they want a current or future partner or surrogate to carry) are candidates for hysterectomy; those who do not want a genetic child are candidates for oophorectomy; and those who do not want to preserve their fertility (or have already ended it) and who meet the WPATH criteria for surgery are candidates for oophorectomy concurrent with hysterectomy. The discussion can be particularly challenging with young transgender men, since their ability to project their family planning goals may be compromised by their gender dysphoria. Clinicians can counsel patients about another option: isolated hysterectomy with subsequent staged oophorectomy.
Similar to cisgender women with polycystic ovary syndrome, transgender men on exogenous testosterone therapy are at risk for ovarian cysts,7 which can cause pain and should be evaluated and managed. As mentioned, these patients may find it difficult to visit a gynecologist and tolerate a vaginal examination, and many fear presenting to an emergency room, as they will need to disclose their transgender status and risk being discriminated against or, worse, not being triaged or cared for properly. Patients should be thoroughly counseled about the risks and benefits of having oophorectomy performed concurrently with hysterectomy.
Related Article:
Vaginal hysterectomy with basic instrumentation
The question of vaginectomy
Patients and clinicians often ask about concurrent vaginectomy procedures. In some cases, patients with severe gender dysphoria and absence of penetrative vaginal activity request excision or obliteration of the vagina. There is no standard of care, however. Vaginectomy can be done transvaginally or abdominally: open, laparoscopically, or robotically. It therefore should be performed by surgeons experienced in the procedure. Patients should be advised that a portion of the vaginal epithelium is sometimes used for certain phalloplasty procedures and that, if they are considering genital reconstruction in the future, it may be beneficial to preserve the vagina until that time.
There are no guidelines on stopping or continuing testosterone therapy perioperatively. Some clinicians are concerned about possible venous thromboembolic events related to perioperative use of testosterone, but there are no data supporting increased risk. The risk of postoperative vaginal cuff bleeding in patients on and off testosterone has not been well studied. Since patients who stop taking testosterone may develop severe mood swings and malaise, they should be counseled on recognizing and managing such changes. There are also no data on the risk of vaginal cuff dehiscence in this patient population. Testosterone usually causes the vagina to become very atrophic, so proper closure should be ensured to avoid cuff evisceration. In my practice, the vaginal cuff is closed in 2 layers using at least 1 layer of delayed absorbable suture.
Read about addressing fertility, contraception, OB care, and your role
Addressing fertility, contraception, and obstetric care
Most transgender men are able to conceive a child.21 Data in this area, however, are sparse. Most of the literature on reproductive health in this patient population is focused on human immunodeficiency virus (HIV) and other sexually transmitted infections.22 Nevertheless, patient-physician dialogue on fertility and reproductive health has increased since more patients started seeking surgical transition services (likely a result of improved coverage for these surgeries). In addition, we are learning more about patients’ ability and desire to conceive after long-term use of cross-sex hormone therapy. The importance of this dialogue is becoming apparent. One survey study found that more than half of the transgender men who had undergone affirmation surgery wanted to become parents.23
Before initiating cross-sex hormone therapy or before undergoing hysterectomy and/or oophorectomy, patients must be counseled about their fertility options. Testosterone may affect fertility and fecundity, but there are case reports of successful pregnancy after discontinuation of testosterone.21 Reproductive endocrinology and fertility specialists have begun to recognize the importance of fertility preservation in this patient population and to apply the principles of oncofertility care beyond patients with cancer. In a 2015 opinion paper on access to fertility services by transgender persons, the Ethics Committee of the American Society for Reproductive Medicine focused on this population’s unique fertility needs.24 Currently, oocyte and embryo cryopreservation are options for transgender men planning to start cross-sex hormones or undergo surgery.25 Other methods being investigated may become options in the future.25
There are even fewer data on transgender men’s contraceptive needs. Many clinicians mistakenly think these patients are at low risk for pregnancy. Some patients have male partners and engage in penetrative penile-vaginal intercourse; others are not on testosterone therapy; and still others, despite taking testosterone, are not always amenorrheic and may be ovulating. In a small cross-sectional study, Light and colleagues found that 12% of transgender men who were surveyed after conceiving had been amenorrheic on testosterone therapy, and 24% of these pregnancies were not planned.21
In a study by Cipres and colleagues, half of the 26 transgender men were considered at risk for pregnancy: These patients still had a uterus, not all were on testosterone, not all on testosterone were amenorrheic, they were having vaginal intercourse with cisgender men, and none were using condoms or other contraception.26 The authors noted several potential underlying reasons for poor counseling on contraceptive needs: patients feel stigmatized, clinicians assume these patients are not candidates for “female” hormone therapy, patients fear these modalities may feminize them and compromise their affirmed identities, patients poorly understand how testosterone works and have mistaken ideas about its contraceptive properties, and clinician discomfort with broaching fertility and reproductive health discussions.
Data are also limited on pregnancy in transgender men. We do know that clinicians are not well equipped to help patients during the peripartum period and better resources are needed.21 Gender dysphoria can worsen during and immediately after pregnancy, and patients may be at significant risk for postpartum depression. More research is needed.
Related Article:
Care of the transgender patient: What is the gynecologist's role?
Gynecologists play key role in transgender care
Transgender men’s unique health care needs can be addressed only by gynecologists.It is important to become comfortable with and educated about these needs and their subtleties. This starts with understanding transgender patients’ gender dysphoria associated with the gynecologic visit and examination. Learning more about these patients and their needs will improve health care delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Transgender man consults gynecologist for fertility options
A 36-year-old transgender man considering the possibility of having his own biological children presents to the gynecology office to discuss hysterectomy as gender dysphoria treatment as well as his fertility preservation options. He has never had a gynecologic examination. Since age 24, he has been on testosterone therapy. Although his menses initially ceased, each month over the past 2 years he has had breakthrough spotting lasting 2 to 4 days, sometimes accompanied by pelvic pain and cramping. These symptoms have caused him distress and anxiety, which have led to his missing work 1 to 3 days each month. On presentation, he appears anxious and makes little eye contact. His girlfriend of 6 years has come in with him and is very supportive.
Over the past decade, transgender health care has moved to the forefront of the medical conversation. At many prominent medical centers across the United States, clinicians are forming multidisciplinary teams to help improve the health care of this patient population. Outcomes are being studied, and the literature is becoming more robust.
People tend to think of transgender women—male-assigned persons who self-identify as female—as the typical prototype for transgender people, but this focus is skewed in both society and the medical community. Transgender men—female-assigned persons who self-identify as male—remain underrepresented, mostly because they want to stay “under the radar,” especially with respect to medical care and, more specifically, routine gynecologic care.
Although the transgender woman has unique health needs and may present to a gynecologist for care after gender-affirmationsurgery, the transgender man’s many health care needs and their subtleties can be addressed only by a gynecologist. In this article, I review these intricacies of care to help increase clinician comfort in treating these patients.
Clinicians can take steps to:
- ensure all patients have the correct identifiers in their medical records
- provide staff with the proper education and diversity training
- instruct staff in proper use of pronouns
- set up unisex or gender-nonbinary restrooms with appropriate signage
- make the decor gender nonspecific.
Beth Cronin, MD, a practicing general gynecologist in Providence, Rhode Island, says that you also should consider a general sign, placed in a highly visible area, that represents your nondiscrimination policy. The AMA offers this wording: "This office appreciates the diversity of human beings and does not discriminate based on race, age, religion, ability, marital status, sexual orientation, sex or gender identity." She also recommends having education and marketing materials with affirmative imagery and content and providing educational brochures on transgender health topics.
Why transgender patients may delay seeking health care
Transgender patients remain underserved because of the health care barriers they encounter. Factors contributing to poor access include lack of health insurance, inability to pay for services, clinician insensitivity and hostility, and fear of exposure of transgender status during health care encounters.1 In a recent large survey study, 30% of transgender respondents indicated that they delayed or did not seek medical care as a result of discrimination, and those who had needed to teach their clinicians about transgenderism were 4 times more likely to postpone or not seek care.2
In a 2015 survey of ObGyns’ current knowledge and practice regarding LGBT (lesbian, gay, bisexual, transgender) care, only one-third of respondents indicated they were comfortable caring for transgender patients.3 In addition, only one-third indicated being knowledgeable about the steps transgender patients must take to transition to their self-identified gender, and less than half were familiar with the recommendations for the routine health maintenance and screening of these patients.
Much of this discomfort derives from the lack of incorporation of LGBT-specific topics in medical curricula. In 2011, Obedin-Maliver and colleagues found that, at 176 US and Canadian allopathic and osteopathic medical schools, the median time dedicated to LGBT health care needs and related topics was unsatisfactory.4 This deficiency is slowly being reduced with changes in the curricula of many health care specialties. In ObGyn residency programs, for example, transgender-specific questions have been added to annual in-service examinations. The hope is that, as education initiatives improve, clinicians will become more comfortable caring for gender-minority patients, who with improved access to care will no longer need to seek subspecialists in transgender services.
Read about the need for gyn exams, managing benign disorders, and cervical cancer screening
Considerations for the gynecologic visit and examination
Transgender men visit the gynecology office for many reasons, including routine gynecologic care and health maintenance, care for acute and chronic gynecologic conditions (abnormal bleeding, pelvic pain, vaginitis), evaluation and management of pelvic floor disorders, consultation on hysterectomy for gender transition, and fertility counseling.
However, transgender men who reach their third, fourth, or fifth decade without having had a pelvic examination cite many reasons for avoiding the gynecology office. Most commonly, gynecologic visits and genital examination can severely exacerbate these patients’ gender dysphoria. In addition, many patients who do not engage in penetrative vaginal sex think their health risks are so low that they can forgo or delay pelvic exams. Patients who have stopped menstruating while on testosterone therapy may think there is no need for routine gynecologic care. Other reasons for avoiding pelvic exams are pain and traumatic sexual memories.5
Related Article:
Four pillars of a successful practice: 4. Motivate your staff
Transgender men need to receive the regular guideline-recommended pelvic exams and screenings used for cisgender women. (Cisgender refers to a person whose sense of gender identity corresponds with their birth sex.) We need to educate patients in this regard and to discuss several issues before performing an examination. First, take a thorough history and avoid making assumptions about sexual orientation and sex practices. Some patients have penetrative vaginal intercourse with either men or women. For some patients, the exam may cause dysphoria symptoms, and we need to validate patients’ fears. Discussing these issues ahead of time helps patients get used to the idea of undergoing an exam and assures them that the clinician is experienced in performing these exams for transgender men. In my practice, we explain the exam’s purpose (screening or diagnosis) and importance. We also counsel patients that they may experience some normal, and temporary, spotting after the exam. For those who experience severe dysphoria with vaginal bleeding of any kind, we acknowledge that postexam spotting may cause some anxiety. Patients with severe anxiety before the exam may be premedicated with an anxiolytic agent as long as someone can transport them to and from the office.
The bimanual exam should be performed with care and efficiency and with the patient given as much control as possible. In most cases, we ask patients to undress only from the waist down, and their genitals stay covered. Patients uncomfortable in stirrups are asked to show us the position that suits them best, and we try to accommodate them. Although speed is a goal, remember that many patients are nulliparous, have had limited or no vaginal penetration, or are on testosterone and have significant vaginal dryness. Use the smallest speculum possible, a pediatric or long and narrow adult speculum, and apply lubricant copiously. Pre-exam application of topical lidocaine jelly to the introitus can help reduce pain. To help a patient relax the pelvic floor muscles and habituate to the presence of a foreign object in the vagina, start the exam by inserting a single digit. In addition, ask the patient about speculum placement inside the vagina: Does he want to place the speculum himself or guide the clinician’s hand? Open the speculum only as much as needed to adequately visualize the cervix and then remove it with care.
Managing benign gynecologic disorders
The same algorithms are used to evaluate abnormal bleeding in all patients, but the differential diagnosis expands for those on testosterone therapy. Testosterone may no longer be suppressing their cycles, and abnormal bleeding could simply be the return of menses, which would present as regular cyclic bleeding. Increasing the testosterone dosing or changing the testosterone formulation may help, and the gynecologist should discuss these options with the patient’s prescribing clinician. In addition, progesterone in any form (for example, medroxyprogesterone acetate 5 to 30 mg daily) can be added to testosterone regimens to help suppress menses. The levonorgestrel-releasing intrauterine device (LNG-IUD) can be very effective, but placement can induce anxiety, and some patients decline this treatment option.
In patients with intermenstrual spotting, assess the vagina for atrophy. Both over-the-counter vaginal moisturizers and DHEA (dehydroepiandrosterone) suppositories (1% compounded) can help treat atrophy, but not all patients are comfortable using them. Most patients decline vaginal estrogen products for symptomatic vaginal atrophy even though the systemic effects are minimal.
The historic literature suggests that female-to-male patients’ long-term exposure to androgens leads to atrophic changes in the endometrium and myometrium, and clinical studies of menopausal women who take exogenous androgens have confirmed this effect.6 However, new data point to a different histologic scenario. A recent study found a possible association between long-term testosterone use in transgender men of reproductive age and a low proliferative active endometrium, as well as hypertrophic changes in the myometrium.7 The causes may be peripheral aromatization of androgens and expression and up-regulation of androgen receptors within the endometrial stroma and myometrial cells.8 Given these emerging data and anecdotal cases reported by clinicians who perform hysterectomies for transgender men, imaging and tissue sampling should be used to evaluate abnormal uterine bleeding, particularly in patients previously amenorrheic on testosterone. Be aware that transvaginal ultrasound or endometrial biopsy are challenging procedures for these patients. Counsel patients to ensure that they adhere to follow-up.
Related Article:
2017 Update on cervical disease
The ongoing need for cervical cancer screening
The concept of “original gender surveillance” was presented in a 2-case series of transgender men with uterine and cervical cancer that might have been detected earlier with better screening and routine care.9 There is no evidence, however, that long-term high-dose androgen therapy causes endometrial or cervical cancer,10 and the data on endometrial cancer in patients on cross-sex hormone therapy are limited such that a causal relationship between testosterone and these malignancies cannot be established.9,11–14
The rate of unsatisfactory Pap smears is higher in transgender men than in cisgender women. The difference was anecdotally noted by clinicians who routinely cared for transgender patients over time and was confirmed with a retrospective chart review.15
Peitzmeier and colleagues reviewed the records of 233 transgender men and 3,625 cisgender women with Pap tests performed at an urban community health center over 6 years.15 The transgender cohort, with its prevalence rate of 10%, was 10 times more likely to have an unsatisfactory or inadequate Pap smear. Moreover, the transgender patients were more likely to have longer latency to follow-up for a repeat Pap test. In addition, testosterone therapy was more likely associated with inadequate Pap smears, and time on testosterone therapy was associated with higher odds of Pap smear inadequacy. Besides the exogenous hormone therapy, clinician comfort level and experience may have contributed to the high prevalence of inadequate Pap smears.
As mentioned earlier, it is important to become comfortable performing pelvic exams for transgender men and to prepare patients for the possibility that a Pap smear might be inadequate, making a follow-up visit and repeat Pap test necessary.16
Read about hysterectomy, oophorectomy, and vaginectomy choices
Consultation for hysterectomy: Perioperative considerations
Transgender men may undergo hysterectomy, oophorectomy, and/or vaginectomy. The TABLE summarizes the indications and perioperative considerations for each procedure.

Some transgender men undergo hysterectomy for benign gynecologic disease. Counseling and perioperative planning are the same for these patients as for cisgender women, although some of the considerations discussed here remain important.
Other patients undergo hysterectomy as part of transitioning to their self-affirmed gender. The World Professional Association for Transgender Health (WPATH) Standards of Care should be used to guide counseling and treatment.17 These guidelines were designed as a framework for performing hysterectomy and other gender-affirming procedures. According to the WPATH standards, the criteria for hysterectomy and oophorectomy are:
- 2 referral letters from qualified mental health professionals
- well-documented persistent gender dysphoria
- capacity to make fully informed decisions and to consent to treatment
- age of majority in given country
- good control of any concurrent medical or mental health concerns, and
- hormone therapy for 12 continuous months, as appropriate to gender goals, unless the patient has a medical contraindication or is otherwise unable or unwilling to take hormones.
As the guidelines emphasize, these criteria do not apply to patients undergoing either procedure for medical indications other than gender dysphoria.
Hysterectomy approach. Most surgeons perform gender-affirming hysterectomies laparoscopically. Many clinicians hesitate to perform these hysterectomies vaginally, as the patients are often nulliparous. In general, the best operative route is the one the surgeon feels most comfortable performing safely and efficiently. For a nulliparous patient with minimal pelvic organ descensus and a narrow pelvis, the laparoscopic approach is reasonable. A recent study in a small cohort of transgender men found that vaginal hysterectomy was successful in only 1 in 4 patients.18 Nevertheless, the American College of Obstetricians and Gynecologists (ACOG) recommends vaginal hysterectomy, when appropriate, for limiting complications and morbidity while maximizing cost-effectiveness.19 Although data are limited, vaginal hysterectomy seems feasible and should be considered in a subset of patients who pre‑sent for gender-affirming hysterectomy.
Related Article:
Total laparoscopic versus laparoscopic supracervical hysterectomy
The oophorectomy debate
Oophorectomy concurrent with hysterectomy remains a topic of debate among gynecologists who perform hysterectomy for gender transition. Some clinicians think gonadectomy poses a significant risk for bone health compromise at an early age. The long-term effects of testosterone on bone have not been well studied. Although bone metabolism is thought to increase over the short term, there are no major changes in bone density over the long term. In fact, in the setting of long-term testosterone therapy, cortical bone was found to be larger in transgender men than in cisgender women.20 The issue is for patients who stop taking exogenous testosterone after oophorectomy. This subset of patients has not been well studied but clearly needs bone health surveillance and supplementation.
Another concern about oophorectomy is its effect on fertility. Because it is important to discuss fertility-preserving options, during consultation for a hysterectomy I spend a large portion of time addressing fertility goals. Patients who want to become a parent but do not want to carry a child (they want a current or future partner or surrogate to carry) are candidates for hysterectomy; those who do not want a genetic child are candidates for oophorectomy; and those who do not want to preserve their fertility (or have already ended it) and who meet the WPATH criteria for surgery are candidates for oophorectomy concurrent with hysterectomy. The discussion can be particularly challenging with young transgender men, since their ability to project their family planning goals may be compromised by their gender dysphoria. Clinicians can counsel patients about another option: isolated hysterectomy with subsequent staged oophorectomy.
Similar to cisgender women with polycystic ovary syndrome, transgender men on exogenous testosterone therapy are at risk for ovarian cysts,7 which can cause pain and should be evaluated and managed. As mentioned, these patients may find it difficult to visit a gynecologist and tolerate a vaginal examination, and many fear presenting to an emergency room, as they will need to disclose their transgender status and risk being discriminated against or, worse, not being triaged or cared for properly. Patients should be thoroughly counseled about the risks and benefits of having oophorectomy performed concurrently with hysterectomy.
Related Article:
Vaginal hysterectomy with basic instrumentation
The question of vaginectomy
Patients and clinicians often ask about concurrent vaginectomy procedures. In some cases, patients with severe gender dysphoria and absence of penetrative vaginal activity request excision or obliteration of the vagina. There is no standard of care, however. Vaginectomy can be done transvaginally or abdominally: open, laparoscopically, or robotically. It therefore should be performed by surgeons experienced in the procedure. Patients should be advised that a portion of the vaginal epithelium is sometimes used for certain phalloplasty procedures and that, if they are considering genital reconstruction in the future, it may be beneficial to preserve the vagina until that time.
There are no guidelines on stopping or continuing testosterone therapy perioperatively. Some clinicians are concerned about possible venous thromboembolic events related to perioperative use of testosterone, but there are no data supporting increased risk. The risk of postoperative vaginal cuff bleeding in patients on and off testosterone has not been well studied. Since patients who stop taking testosterone may develop severe mood swings and malaise, they should be counseled on recognizing and managing such changes. There are also no data on the risk of vaginal cuff dehiscence in this patient population. Testosterone usually causes the vagina to become very atrophic, so proper closure should be ensured to avoid cuff evisceration. In my practice, the vaginal cuff is closed in 2 layers using at least 1 layer of delayed absorbable suture.
Read about addressing fertility, contraception, OB care, and your role
Addressing fertility, contraception, and obstetric care
Most transgender men are able to conceive a child.21 Data in this area, however, are sparse. Most of the literature on reproductive health in this patient population is focused on human immunodeficiency virus (HIV) and other sexually transmitted infections.22 Nevertheless, patient-physician dialogue on fertility and reproductive health has increased since more patients started seeking surgical transition services (likely a result of improved coverage for these surgeries). In addition, we are learning more about patients’ ability and desire to conceive after long-term use of cross-sex hormone therapy. The importance of this dialogue is becoming apparent. One survey study found that more than half of the transgender men who had undergone affirmation surgery wanted to become parents.23
Before initiating cross-sex hormone therapy or before undergoing hysterectomy and/or oophorectomy, patients must be counseled about their fertility options. Testosterone may affect fertility and fecundity, but there are case reports of successful pregnancy after discontinuation of testosterone.21 Reproductive endocrinology and fertility specialists have begun to recognize the importance of fertility preservation in this patient population and to apply the principles of oncofertility care beyond patients with cancer. In a 2015 opinion paper on access to fertility services by transgender persons, the Ethics Committee of the American Society for Reproductive Medicine focused on this population’s unique fertility needs.24 Currently, oocyte and embryo cryopreservation are options for transgender men planning to start cross-sex hormones or undergo surgery.25 Other methods being investigated may become options in the future.25
There are even fewer data on transgender men’s contraceptive needs. Many clinicians mistakenly think these patients are at low risk for pregnancy. Some patients have male partners and engage in penetrative penile-vaginal intercourse; others are not on testosterone therapy; and still others, despite taking testosterone, are not always amenorrheic and may be ovulating. In a small cross-sectional study, Light and colleagues found that 12% of transgender men who were surveyed after conceiving had been amenorrheic on testosterone therapy, and 24% of these pregnancies were not planned.21
In a study by Cipres and colleagues, half of the 26 transgender men were considered at risk for pregnancy: These patients still had a uterus, not all were on testosterone, not all on testosterone were amenorrheic, they were having vaginal intercourse with cisgender men, and none were using condoms or other contraception.26 The authors noted several potential underlying reasons for poor counseling on contraceptive needs: patients feel stigmatized, clinicians assume these patients are not candidates for “female” hormone therapy, patients fear these modalities may feminize them and compromise their affirmed identities, patients poorly understand how testosterone works and have mistaken ideas about its contraceptive properties, and clinician discomfort with broaching fertility and reproductive health discussions.
Data are also limited on pregnancy in transgender men. We do know that clinicians are not well equipped to help patients during the peripartum period and better resources are needed.21 Gender dysphoria can worsen during and immediately after pregnancy, and patients may be at significant risk for postpartum depression. More research is needed.
Related Article:
Care of the transgender patient: What is the gynecologist's role?
Gynecologists play key role in transgender care
Transgender men’s unique health care needs can be addressed only by gynecologists.It is important to become comfortable with and educated about these needs and their subtleties. This starts with understanding transgender patients’ gender dysphoria associated with the gynecologic visit and examination. Learning more about these patients and their needs will improve health care delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Xavier JM, Simmons R. The Washington Transgender Needs Assessment Survey, 2000. http://www.glaa.org/archive/2000/tgneedsassessment1112.shtml. Accessed January 2, 2017.
- Jaffee KD, Shires DA, Stroumsa D. Discrimination and delayed health care among transgender women and men: implications for improving medical education and health care delivery. Med Care. 2016;54(11):1010–1016.
- Unger CA. Care of the transgender patient: a survey of gynecologists’ current knowledge and practice. J Womens Health. 2015;24(2):114–118.
- Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–977.
- Feldman J. Medical and surgical management of the transgender patient: what the primary care clinician needs to know. In: Makadon H, Mayer K, Potter J, Goldhammer H, eds. Fenway Guide to Lesbian, Gay, Bisexual, and Transgender Health. Philadelphia, PA: American College of Physicians; 2008:365–392.
- Hickok LR, Toomey C, Speroff L. A comparison of esterified estrogens with and without methyltestosterone: effects on endometrial histology and serum lipoproteins in postmenopausal women. Obstet Gynecol. 1993;82(6):919–924.
- Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691.
- Mertens HJ, Heineman MJ, Koudstaal J, Theunissen P, Evers JL. Androgen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):11–13.
- Urban RR, Teng NN, Kapp DS. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204(5):e9–e12.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159(3):197–202.
- Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–497.
- Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76(3):413–415.
- Dizon DS, Tejada-Berges T, Keolliker S, Steinhoff M, Grania CO. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Oncol Invest. 2006;62(4):226–228.
- Schenck TL, Holzbach T, Zantl N, et al. Vaginal carcinoma in a female-to-male transsexual. J Sex Med. 2010;7(8):2899–2902.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med. 2014;29(5):778–784.
- Potter J, Peitzmeier SM, Bernstein I, et al. Cervical cancer screening for patients on the female-to-male spectrum: a narrative review and guide for clinicians. J Gen Intern Med. 2015;30(12):1857–1864.
- Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Version 7. https://s3.amazonaws.com/amo_hub_content/Association140/files/Standards_of_Care_V7_2011_WPATH(2)(1).pdf. Published 2011. Accessed January 21, 2017.
- Obedin-Maliver J, Light A, de Haan G, Jackson RA. Feasibility of vaginal hysterectomy for female-to-male transgender men. Obstet Gynecol. 2017;129(3):457–463.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–466.
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127.
- Stephens SC, Bernstein KT, Philip SS. Male to female and female to male transgender persons have different sexual risk behaviors yet similar rates of STDs and HIV. AIDS Behav. 2011;15(3):683–686.
- Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487.
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104(5):1111–1115.
- Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol. 2014;30(12):868–871.
- Cipres D, Seidman D, Cloniger C 3rd, Nova C, O’Shea A, Obedin-Maliver J. Contraceptive use and pregnancy intentions among transgender men presenting to a clinic for sex workers and their families in San Francisco. Contraception. 2016;95(2):186–189.
- Xavier JM, Simmons R. The Washington Transgender Needs Assessment Survey, 2000. http://www.glaa.org/archive/2000/tgneedsassessment1112.shtml. Accessed January 2, 2017.
- Jaffee KD, Shires DA, Stroumsa D. Discrimination and delayed health care among transgender women and men: implications for improving medical education and health care delivery. Med Care. 2016;54(11):1010–1016.
- Unger CA. Care of the transgender patient: a survey of gynecologists’ current knowledge and practice. J Womens Health. 2015;24(2):114–118.
- Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–977.
- Feldman J. Medical and surgical management of the transgender patient: what the primary care clinician needs to know. In: Makadon H, Mayer K, Potter J, Goldhammer H, eds. Fenway Guide to Lesbian, Gay, Bisexual, and Transgender Health. Philadelphia, PA: American College of Physicians; 2008:365–392.
- Hickok LR, Toomey C, Speroff L. A comparison of esterified estrogens with and without methyltestosterone: effects on endometrial histology and serum lipoproteins in postmenopausal women. Obstet Gynecol. 1993;82(6):919–924.
- Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691.
- Mertens HJ, Heineman MJ, Koudstaal J, Theunissen P, Evers JL. Androgen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):11–13.
- Urban RR, Teng NN, Kapp DS. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204(5):e9–e12.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159(3):197–202.
- Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–497.
- Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76(3):413–415.
- Dizon DS, Tejada-Berges T, Keolliker S, Steinhoff M, Grania CO. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Oncol Invest. 2006;62(4):226–228.
- Schenck TL, Holzbach T, Zantl N, et al. Vaginal carcinoma in a female-to-male transsexual. J Sex Med. 2010;7(8):2899–2902.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med. 2014;29(5):778–784.
- Potter J, Peitzmeier SM, Bernstein I, et al. Cervical cancer screening for patients on the female-to-male spectrum: a narrative review and guide for clinicians. J Gen Intern Med. 2015;30(12):1857–1864.
- Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Version 7. https://s3.amazonaws.com/amo_hub_content/Association140/files/Standards_of_Care_V7_2011_WPATH(2)(1).pdf. Published 2011. Accessed January 21, 2017.
- Obedin-Maliver J, Light A, de Haan G, Jackson RA. Feasibility of vaginal hysterectomy for female-to-male transgender men. Obstet Gynecol. 2017;129(3):457–463.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–466.
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127.
- Stephens SC, Bernstein KT, Philip SS. Male to female and female to male transgender persons have different sexual risk behaviors yet similar rates of STDs and HIV. AIDS Behav. 2011;15(3):683–686.
- Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487.
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104(5):1111–1115.
- Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol. 2014;30(12):868–871.
- Cipres D, Seidman D, Cloniger C 3rd, Nova C, O’Shea A, Obedin-Maliver J. Contraceptive use and pregnancy intentions among transgender men presenting to a clinic for sex workers and their families in San Francisco. Contraception. 2016;95(2):186–189.
5 Points on Pyogenic Flexor Tenosynovitis of the Hand
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.
Clinical Assessment and Management of Cancer-Related Fatigue
From the University of Texas MD Anderson Cancer Center, Houston, TX.
Abstract
- Objective: To review the evidence on interventions for managing cancer-related fatigue (CRF) and provide evidence-based guidance on approaches to its management.
- Methods: Nonsystematic review of the literature.
- Results: Several theories have been proposed to explain the biology of CRF, but there is no single clear mechanism that can be targeted for therapy. The approach to patients begins with screening for fatigue and assessing its intensity, followed by a thorough history and examination to determine whether any reversible medical conditions are contributing to fatigue. Management of underlying medical comorbidities may help some patients. For patients whose fatigue persists, pharmacologic and nonpharmacologic treatment options are available. Pharmacologic options include psychostimulants, such as methylphenidate and modafinil, and corticosteroids. Nonpharmacologic approaches include exercise, cognitive behavior therapy, yoga, acupuncture, and tai chi.
- Conclusion: We recommend an individualized approach, often with a combination of the available options. Patients need to be evaluated periodically to assess their fatigue, and since cancer-related fatigue affects survivors, long-term follow-up is needed.
Key words: fatigue; cancer; pro-inflammatory cytokines; nonpharmacologic; psychostimulants.
Fatigue is a common distressing effect of cancer [1].It impairs the quality of life of patients undergoing active cancer treatment and of post-treatment survivors. The National Comprehensive Cancer Network (NCCN) defines cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning [2].” Differences between CRF and fatigue reported by individuals without cancer are that CRF is more severe and is not relieved by rest. The prevalence of CRF in cancer patients and survivors is highly variable, ranging between 25% and 99% [2,3]. This variability may be secondary to methods used for screening fatigue and characteristics of the patient groups. In this article, we discuss recognition of CRF and approaches to its management.
Pathophysiology
The specific pathophysiologic mechanism underlying CRF is unknown, making targeted treatment a challenge. The multidimensional and subjective nature of CRF has limited the development of research methodologies to explain this condition. However, research has been done in both human and animal models, and several theories have been proposed to explain the pathophysiology of CRF. While pro-inflammatory cytokines remain the central factor playing a significant role at multiple levels in CRF, there may be a complex interplay of more than 1 mechanism contributing to fatigue in an individual patient.
Central Nervous System Disturbances
The basal ganglia are known to influence motivation. Lack of motivation and drive may cause failure to complete physical and mental tasks, even with preserved cognitive ability and motor function. In a study of melanoma patients receiving interferon, increased activity of the basal ganglia and the cerebellum resulted in higher fatigue scores [4]. Higher levels of cytokines may alter blood flow to the cerebellum and lead to the perception of fatigue. In a study of 12 patients and matched controls, when patients were asked to perform sustained elbow flexion until they perceived exhaustion, CRF patients perceived physical exhaustion sooner than controls. In CRF patients in this study, muscle fatigue measured by electromyogram was less than that in healthy individuals at the time of exhaustion, suggesting the role of the central nervous system in CRF [5]. However, there is not enough evidence at this time to support central nervous system disturbance as the main contributing factor to fatigue in cancer patients.
Circadian Rhythm Dysregulation
Circadian rhythm is regulated by the suprachiasmatic nucleus in the hypothalamus through cortisol and melatonin. Sleep disturbances occur with disruption of the circadian rhythm. Tumor-related peptides such as epidermal growth factor or alterations in serotonin and cortisol can influence the suprachiasmatic nucleus and the complex signaling pathways [2]. Positive feedback loops that are activated by cortisol under the influence of cytokines may lead to continuous cytokine production and altered circadian rhythm. Bower et al showed that changes in the cortisol curve influence fatigue in breast cancer survivors [6]. These patients had a late evening peak in cortisol levels, compared with an early morning peak in individuals without cancer.
Inhibition of Hypothalamic–Pituitary–Adrenal Axis
The hypothalamic–pituitary–adrenal (HPA) axis regulates the release of the stress hormone cortisol. One of several hypotheses advanced to explain the effect of serotonin and the HPA axis on CRF suggests that lower serotonin levels cause decreased activation of 5-hydroxytrytophan 1-a (5-HT1-a) receptors in the hypothalamus, leading to decreased activity of the HPA axis [6]. The inhibition of the HPA axis may occur with higher levels of serotonin as well [7]. The 5-HT1-a receptors are also triggered by cytokines. However, the correction of serotonin levels by antidepressants was not shown to improve fatigue [8]. Inhibition of the HPA axis can also lead to lower testosterone, progesterone, or estrogen levels, which may indirectly contribute to fatigue [2].
Skeletal Muscle Effect
Chemotherapy- and tumor-related cachexia have a direct effect on the metabolism of skeletal muscles. This effect may lead to impaired adenosine triphosphate (ATP) generation during muscle contraction [9]. ATP infusion improved muscle strength in one trial, but this was not confirmed in another trial [10,11]. Muscle contraction studies showed no differences in the contractile properties of muscles in fatigued patients who failed earlier in motor tasks and healthy controls [12]. This finding suggests that there could be a failure of skeletal muscle activation by the central nervous system or inhibition of skeletal muscle activity. Cytokines and other neurotransmitters activate vagal efferent nerve fibers, which may lead to reflex inhibition in skeletal muscles [13,14].
Pro-inflammatory Cytokines
Tumors or treatment of them may cause tissue injury, which triggers immune cells to release cytokines, signaling the brain to manifest the symptom fatigue. Inflammatory pathways are influenced by psychological, behavioral, and biological factors, which play a role as risk factors in CRF. Interleukin 6 (IL-6), interleukin-1 receptor antagonist, interleukin-1, and tumor necrosis factor (TNF) have been shown to be elevated in fatigued patients being treated for leukemia and non-Hodgkin lymphoma [15]. IL-6 was also associated with increased fatigue in breast cancer survivors [16]. Similar findings were reported in patients undergoing stem cell transplantation and high-dose chemotherapy [17]. Elevated levels of IL-6 and C-reactive protein were also linked to fatigue in terminally ill cancer patients [18,19]. Furthermore, TNF-α signaling was associated with post-chemotherapy fatigue in breast cancer patients [20]. Leukocytes in breast cancer survivors with fatigue also have increased gene expression of pro-inflammatory cytokines, emphasizing the role of cytokines and inflammation in the pathogenesis of CRF [21].
Other Hypotheses
Several other hypotheses for CRF pathogenesis have been proposed. Activation of latent viruses such as Epstein-Barr virus, lack of social support [22], genetic alterations in immune pathway [23], epigenetic changes [24], accumulation of neurotoxic metabolites and depletion of serotonin by indoleamine 2,3-dioxygenase pathway activation [25], elevated vascular endothelial growth factor levels [26], and hypoxia-related organ dysfunction due to anemia or hemoglobin dysfunction [13] all have been postulated to cause CRF.
Approach to Evaluation and Treatment
Screening
Because patients and health care professionals may be unaware of the treatment options available for CRF, patients may not report fatigue levels to their clinicians, and clinicians may not understand the impact of fatigue on their patients’ quality of life. This leads to underrecognition of the problem. The NCCN recommends screening every cancer patient and post-treatment survivor for fatigue [2]. Patients should be screened at their first visit and then at periodic intervals during and after cancer treatment.
Many scales are available to screen patients for CRF in clinical practice and clinical trials [27]. A single item that asks patients to rate their fatigue on a scale from 0 to 10—in which 0 indicates no fatigue, 1 to 3 indicates mild fatigue, 4 to 6 indicates moderate fatigue, 7 to 9 indicates severe fatigue, and 10 indicates the worst fatigue imaginable—is commonly used to screen for CRF [2]. This scale was adapted from the MD Anderson Symptom Inventory scale and is based on a large nationwide study of cancer patients and survivors [28]. The statistically derived cutoff points in this study are consistent with other scales such as the Brief Fatigue Inventory (BFI) and support the cutoff points (4–6 for moderate and ≥ 7 for severe fatigue) used in various fatigue management guidelines. Furthermore, studies of fatigue in cancer patients have revealed a marked decrease in physical function at levels of 7 or higher, suggesting 7 as an optimal cutoff to identify severe fatigue [29,30]. The Visual Analog Scale is another simple-to-use tool that helps in understanding variations in fatigue throughout the course of the day [31]. The 9-item BFI is often used in clinical trials [29]. It measures the severity of fatigue over the previous 24 hours and has been validated in non-English speaking patients [32].
CRF affects not only the somatic domain, but also the cognitive, behavioral, and affective domains; therefore, multidimensional scales have been developed for screening. One such tool is the Multidimensional Fatigue Inventory, which measures general, physical, mental, and emotional fatigue domains as well as activity and compares them with those of individuals without cancer [33,34]. The Functional Assessment of Cancer Therapy for Fatigue (FACT-F) is a 13-item questionnaire that has been used to measure CRF in clinical trials as well as in patients receiving various treatments [35].
Although many scales are available, the validity of self-reporting on simple fatigue-rating scales is equal to or better than most complex, lengthy scales [36]. Therefore, unidimensional tools such as the numeric rating scale of 0–10 are commonly used in clinical practice. Mild fatigue (0–3) requires periodic re-evaluation, and moderate and severe fatigue need further evaluation and management [37].
Primary Evaluation
This phase involves a focused history and physical examination and assessment of concurrent symptoms and contributing factors.
History and Physical Examination
A detailed history of the patient’s malignancy and type of previous and current treatment may help reveal the cause of fatigue. New-onset fatigue or increase in fatigue may be related to the progression of disease in patients with active malignancy or recurrence of cancer in survivors. These patients may require appropriate testing to assess the underlying disease pattern. A detailed review of systems may help identify some of the contributing factors, which are discussed below. A detailed history regarding medications, including over-the-counter drugs, complementary agents, and past and prior cancer therapies, is helpful as medications can contribute to fatigue. For example, opioids may cause drowsiness and fatigue, which could be improved by dose adjustments. A focused history of fatigue should be obtained in all patients with moderate to severe CRF, which includes the onset, pattern, duration, associated or alleviating factors, and interference with functioning, including activities of daily living [37]. Physical examination should focus on identifying signs of organ dysfunction and features of substance or alcohol abuse which may cause poor sleep and fatigue.
Assessment of Contributing Factors
The management of fatigue should be multifactorial, with a comprehensive assessment and treatment plan to address all modifiable fatigue etiologies. The Table lists potential contributing factors to fatigue that should be considered when evaluating patients for CRF; several common conditions are discussed below.
Anemia. Anemia has been correlated with fatigue and quality of life. In a study of 4382 cancer patients receiving chemotherapy, quality-of-life measures using FACT-Anemia scores improved with increased hemoglobin levels [38]. Cancer patients may have anemia due to marrow-suppressing effects of chemotherapy and may also have iron deficiency anemia due to blood loss or autoimmune hemolytic anemia. Therefore, a detailed work-up is required to identify the etiology of anemia. Patients with CRF whose anemia is related to chemotherapy or anemia of chronic disease may benefit from red blood cell transfusion or erythropoiesis-stimulating agents (ESAs). ESAs have been studied extensively; however, their use is controversial because of concerns about thromboembolic side effects leading to adverse outcomes [39]. Also, ESA therapy is not recommended in patients with hematologic malignancies. ESA use should be restricted to patients with chemotherapy-related anemia with hemoglobin below 10 mg/dL and should be discontinued in 6 to 8 weeks if patients do not respond [40]. Other patients may benefit from blood transfusions, which were shown to help in patients with hemoglobin levels between 7.5 and 8.5 g/dL [41].
Sleep disturbance. Poor sleep is common in fatigued cancer survivors [42]. Pro-inflammatory cytokines can disrupt the sleep–wake cycle, causing changes in the HPA axis and neuroendocrine system, which in turn may lead to increasing fatigue. Heckler et al showed that improvement in nighttime sleep leads to improvement of fatigue [43]. Cognitive behavioral therapy and sleep hygiene are important in caring for patients with CRF and poor sleep [44]. Taking a warm bath and/or drinking a glass of milk before bedtime, avoiding caffeinated drinks, and avoiding frequent napping in the day might help. Some patients may require medications such as benzodiazepines or non-benzodiazepine hypnotics (eg, zolpidem) to promote sleep [45]. Melatonin agonists are approved for insomnia in the United states, but not in Europe [46].
Malnutrition. Patients with advanced-stage cancer and with cancers affecting the gastrointestinal tract frequently develop mechanical bowel obstructions, especially at the end of their life, which cause malnutrition. Chemotherapy-related nausea and vomiting may also cause poor oral intake and malnutrition, causing fatigue from muscle weakness. Dehydration and electrolyte imbalances frequently occur as a result of poor oral intake, which might worsen fatigue. In our experience, modifying dietary intake with appropriate caloric exchanges with the help of a nutrition expert has lessened fatigue in some patients. However, terminally ill patients are advised to eat based on their comfort.
Medical comorbidities. Congestive heart failure from anthracycline chemotherapy, hypothyroidism after radiation therapy for head and neck cancers, renal failure, or hepatic failure from chemotherapy may indirectly lead to fatigue. Chemotherapy, opioids, and steroids may cause hypogonadism, which can contribute to fatigue in men [47].
Assessment of Concurrent Symptoms
Depression is common in cancer patients and coexists with pain, insomnia, fatigue, and anxiety as a symptom cluster [48]. A symptom cluster is defined as 2 or more concurrent and interrelated symptoms occurring together; treating of one of these symptoms without addressing other symptoms is not effective [49]. Therefore, screening for and management of depression, anxiety, and insomnia play an important role in the management of CRF.
Physical symptoms due to the tumor or to therapy—such as pain, dyspnea, nausea, and decreased physical activity—may also contribute to fatigue both directly and indirectly. Patients with lung cancer may have hypoxemia, which can contribute to dyspnea with activity and a perception of fatigue. Optimal management of pain and other physical symptoms in patients with cancer may significantly alleviate fatigue [50].
Management
Management of CRF is individualized based on the patient’s clinical status: active cancer treatment, survivor, or end of life. Education and counselling of patients and their caregivers play an important role in CRF. NCCN guidelines recommend focusing on pain control, distress management, energy conservation, physical activity, nutrition, and sleep hygiene.
Nonpharmacologic Interventions
Energy conservation. Energy conservation strategies, in which patients are advised to set priorities and realistic expectations, are highly recommended. Some energy-conserving strategies are to pace oneself, delegate and schedule activities at times of peak energy, postpone nonessential activities, attend to 1 activity at a time, structure daily routines, and maintain a diary to identify their peak energy period and structure activities around that time [51,52]. When patients approach the end of life, increasing fatigue may limit their activity level, and they may depend on caregivers for assistance with activities of daily living, monitoring treatment-related adverse effects, and taking medications, especially elderly patients [53].
Cognitive behavioral therapy. Cognitive behavioral therapy (CBT) has been shown to improve CRF during active treatment, and the benefits persist for a minimum of 2 years after therapy [54]. CBT interventions that optimize sleep quality may improve fatigue [55]. More studies are needed to understand whether referral to a psychologist for formal CBT is required. Randomized clinical trials (RCTs) showed patient fatigue education, learned self-care, coping techniques, and balancing rest and activity benefit patients with CRF [56].
Exercise. Physical activity is highly encouraged in patients with CRF. Exercise increases muscle protein synthesis, improves cytokine response, and decreases the rate of sarcopenia in healthy populations [57]. Studies have shown that exercise helps CRF at all phases of the cancer journey, including radiation therapy, chemotherapy, and survivorship [58]. Some patients may feel less motivated to exercise and may not believe that exercise is possible or could potentially help them. Counselling is needed for such patients.
Older cancer survivors have a decline in their functional capacity and reduced muscle mass. Exercise can improve cardiorespiratory fitness, muscle strength, and body composition [57]. Exercise not only helps at the cellular level but also has psychosocial benefits from improved self-esteem. Therefore, exercise may be recommended not only for younger patients, but also in the older population, who may have comorbidities and less motivation than younger patients.
In a meta-analysis of 56 randomized controlled trials involving 4068 participants, aerobic exercise was found to have beneficial effects on CRF for patients during and after chemotherapy, specifically for patients with solid tumors [59]. In another meta-analysis of breast and prostate cancer survivors, a combination of aerobic exercise with resistance training (3–6 metabolic equivalents, 60%–80% range of motion) was shown to reduce CRF more than aerobic exercise alone [60]. This effect was also shown in an RCT of 160 patients with stage 0 to III breast cancer undergoing radiation therapy [61]. The control group in this study had a group-based non-exercise intervention/relaxation; therefore, the study showed that the effect of resistance training extends beyond the psychosocial benefits of group-based interventions. The intervention comprised 8 progressive machine-based resistance exercises (3 sets, 8–12 repetitions at 60%–80% of 1 repetition maximum) for 60 minutes twice weekly for 12 weeks. However, fatigue assessment questionnaire scores showed benefits in the physical fatigue but not the affective and cognitive components.
The American Society of Clinical Oncology’s guidelines for cancer survivors with fatigue recommends 150 minutes of moderate aerobic exercise (eg, fast walking, cycling, or swimming) per week, with 2 or 3 sessions of strength training per week [62]. An individualized approach to exercise is recommended, as patients’ ability to perform certain types of exercises may be limited by thrombocytopenia, neutropenia, or lytic bone metastasis. Routine use of pre-exercise cardiovascular testing is not recommended but may be considered in high-risk populations, especially patients with risk factors for coronary heart disease and diabetes [63]. Patients withcomorbidities, substantial deconditioning, functional and anatomic defects, or recent major surgery may benefit from referral to physical therapy [37]. Patients near end of life may also benefit from an exercise program, as demonstrated in several studies that showed benefit in CRF and quality of life [64,65]. We recommend that physicians use their best clinical judgement in suggesting the type and intensity of exercise program, as it may not be feasible in some patients.
Mind-body interventions. Mindfulness-based stress reduction (MBSR) has shown promise in breast cancer survivors, who reported immediate improvements in fatigue severity that continued up to 6 weeks after cessation of the training [66]. Prior studies had similar findings, suggesting that MBSR modestly decreases fatigue and sleep disturbances and has a greater effect on the degree to which symptoms interfere with many facets of life [67].
Yoga. A study of a yoga intervention showed a benefit in older cancer survivors [68]. In breast cancer patients undergoing chemotherapy, yoga was shown to benefit not only physical fatigue, but also cognitive fatigue [69]. DVD-based yoga had benefits similar to strengthening exercises in a study of 34 early-stage breast cancer survivors with CRF [70]. More studies are needed in men and patients and survivors of other cancers, as most studies of yoga were conducted in women with breast cancer.
Tai chi/qigong. Like yoga, tai chi and qigong are practices of meditative movement. These practices use postures or movements with a focus on breath and a meditative state to bring about deep states of relaxation. Qigong is a series of simple, repeated practices including body posture/movement, breath practice, and meditation performed in synchrony. Tai chi easy (TCE) is a simplified set of common, repetitive tai chi movements. In a trial, qigong/TCE was compared with sham qigong, which had physical movements but no breathing or meditative practice. Breast cancer survivors in the qigong/TCE group had improved fatigue scores, and the effect persisted for 3 months [71]. Additional research is needed in this area.
Acupuncture. An RCT in breast cancer patients with CRF showed an improvement in the mean general fatigue score (per the Multidimensional Fatigue Inventory) in patients who received acupuncture versus those who did not (−3.11 [95% confidence interval −3.97 to −2.25]; P < 0.001) at 6 weeks. Improvements were seen in both the mental and physical aspects of fatigue [72]. However, Deng et al noted that true acupuncture was no more effective than sham acupuncture for reducing post-chemotherapy chronic fatigue [73]. Presently, there is not sufficient evidence to evaluate the benefits of acupuncture in CRF.
Other modalities. Massage therapy, music therapy, hypnosis, therapeutic touch, biofield therapies, relaxation, and reiki are other therapies for which few studies have been done, with mixed results, and additional research is needed [74]. Currently, there are not sufficient data to recommend any of these modalities.
Pharmacologic Interventions
Psychostimulants. Methylphenidate and modafinil are psychostimulants or wakefulness-promoting agents. Pilot studies showed benefit from methylphenidate and modafinil in CRF [75–77], but RCTs have yielded mixed results. Therefore, in patients with severe fatigue during cancer therapy, the initial management strategy involves evaluation and treatment of medical conditions such as anemia and a trial of non-pharmacological strategies as discussed above. If symptoms persist, then a therapeutic trial of a psychostimulant may be considered per NCCN guidelines for patients undergoing active cancer treatment [37].
Methylphenidate directly stimulates adrenergic receptors and indirectly releases dopamine and norepinephrine from presynaptic terminals, which may explain why the drug benefits patients receiving opioid-induced sedation. It is a commonly studied psychostimulant, though its mechanism of action in CRF is unclear. RCTs of methylphenidate have resulted in a wide range of findings due to the heterogeneity of study populations and variations in the dosage of methylphenidate. A meta-analysis of 7 studies indicates that methylphenidate benefitted the subgroup of patients with CRF [78]. Likewise, in an analysis of 5 RCTs, Minton et al showed a benefit of psychostimulants in fatigue compared with placebo [79]. However, another study of methylphenidate in patients with CRF showed a benefit only in patients with severe fatigue or advanced disease [80]. Methylphenidate was found to benefit cancer patients receiving opioid-induced sedation, as methylphenidate promotes wakefulness, though fatigue was not studied specifically [81]. In a trial with 30 hospice patients in which the methylphenidate dose was titrated based on response and adverse effects, Kerr at al found that the drug improved fatigue in a dose-dependent manner [82]. However, a study in patients with CRF at the University of Texas MD Anderson Cancer Center found no significant difference in BFI scores between patients receiving methylphenidate and those receiving placebo at the end of 2 weeks of treatment [83]. Also, other RCTs in patients undergoing adjuvant chemotherapy for breast cancer [84] and patients receiving radiation therapy for brain tumors [85] failed to demonstrate the efficacy of methylphenidate in CRF. It should be used cautiously after ruling out other causes of fatigue. The drug is overall well tolerated and side effects include headache and nausea.
Modafinil is a non-amphetamine psychostimulant that has been approved for the treatment of narcolepsy. In a trial studying the effect of modafinil on patients receiving docetaxel-based chemotherapy for metastatic breast or prostate cancer, there was a modest but not statistically significant improvement in fatigue scores on the MD Anderson Symptom Inventory compared with placebo. Nausea and vomiting were higher in the modafinil arm than in the placebo arm [86]. Similarly, modafinil was not superior to placebo for CRF in 208 patients with non-squamous cell lung cancer not undergoing chemotherapy or radiation [87]. A placebo effect was also noted in patients with multiple myeloma [88] and patients with primary brain tumors [89]. In a phase 3, multicenter, randomized, placebo-controlled, double-blind clinical trial of modafinil for CRF in 867 patients undergoing chemotherapy, there was a reduction in fatigue only for patients with severe baseline fatigue, with no significant effect on mild to moderate fatigue [90]. In another recent study, modafinil was shown to reduce depressive symptoms only in patients with severe fatigue (BFI item 3 score ≥ 7) [91]. This finding is consistent with previous studies showing benefit in patients with high baseline fatigue, but additional RCTs are needed to provide clarity. NCCN guidelines do not recommend the use of modafinil to treat CRF [37].
Other pharmacologic interventions. Corticosteroids are often used for symptom control in cancer patients. These drugs have anti-inflammatory effects through their modulation of pro-inflammatory cytokines [92]. In a RCT evaluating the efficacy of corticosteroids, patients receiving dexamethasone (4 mg twice daily) experienced significant improvement in their FACT-F scores compared with patients receiving placebo [93]. A similar benefit in fatigue was demonstrated in another study of methylprednisolone (32 mg daily) versus placebo [94]. Despite the benefits of steroids, their adverse effects, such as mood swings, gastritis, hyperglycemia, and immune suppression, limit their long-term use. Therefore, the use of steroids should be restricted to terminally ill fatigued patients with other symptoms such as anorexia, brain metastasis, or pain related to bone metastasis [37].
Testosterone replacement has been shown to diminish fatigue in non-cancer patients. Many men with advanced cancer have hypogonadism leading to low serum testosterone, which may cause fatigue. In a small trial in which cancer patients with hypogonadism received intramuscular testosterone every 14 days or placebo, the group receiving testosterone showed improvement in FACT-F scores, but there was no significant difference in FACT-F scores between the 2 groups [95].
Antidepressants have failed to demonstrate benefit in CRF without depression [8]. However, if a patient has both fatigue and depression, antidepressants may help [96]. A selective serotonin receptor inhibitor is recommended as a first-line antidepressant [97]. Patients with cancer are often receiving multiple medications, and medication interactions should be considered to prevent adverse events such as serotonin syndrome.
Complementary and Alternative Supplements
Studies using vitamin supplementation have been inconclusive in patients with CRF [74]. The use of other dietary supplements has yielded mixed results, and coenzyme Q has shown no benefit for patients with CRF [98].
The benefit of ginseng was studied in a RCT involving 364 patients with CRF. There was an improvement in Multidimensional Fatigue Symptom Inventory-short form (MFSI-SF) scores at 8 weeks in patients receiving 2000 mg of Wisconsin ginseng compared with patients receiving placebo [99]. Patients on active treatment had greater improvement as compared to the post-treatment group in this trial. In another study of high-dose panax ginseng (ginseng root) at 800 mg daily for 29 days, patients had improvement of CRF as well as overall quality of life, appetite, and sleep at night. It was also well tolerated with few adverse effects [100]. Interaction with warfarin, calcium channel blockers, antiplatelet agents, thrombolytic agents, imatinib, and other agents may occur; therefore, ginseng must be used with careful monitoring in selected patients. There is not enough evidence at this time to support the routine use of ginseng in CRF.
The seed extract of the guarana plant (Paullinia cupana) traditionally has been used as a stimulant. An improvement in fatigue scores was seen with the use of oral guarana (100 mg daily) at the end of 21 days in breast cancer patients receiving chemotherapy [101]. Further studies are needed for these results to be generalized and to understand the adverse effects and interaction profile of guarana.
Re-evaluation
Patients who have completed cancer treatment must be monitored for fatigue over the long term, as fatigue may exist beyond the period of active treatment. Many studies have shown fatigue in breast cancer survivors, and fatigue has been demonstrated in survivors of colorectal, lung, and prostate cancers as well as myeloproliferative neoplasms [28]. Therefore, it is important to screen patients for fatigue during follow-up visits. There are currently no studies evaluating the long-term treatment of fatigue. In our experience, the timing of follow-up depends on the level of fatigue and interventions prescribed. Once fatigue is stabilized to a level with which the patient is able to cope, the time interval for follow up may be lengthened. Annual visits may suffice in patients with mild fatigue. Follow-up of patients with moderate to severe fatigue depends on the level of fatigue, the ability to cope, choice of treatment, and presence of contributing factors.
Conclusion
CRF is a complex condition that places a significant burden on patients and caregivers, resulting in emotional distress, poor functioning, and suffering. Fatigue can occur before, during, and long after cancer treatment. The approach to CRF begins with screening for and educating patients and their caregivers about the symptoms. Many screening scales are available that may be used to follow patients’ progress over time. The evaluation and management of contributing conditions may help improve fatigue. If the fatigue persists, an individualized approach with a combination of nonpharmacologic and pharmacologic approaches should be considered. More research is needed to understand brain signaling pathways, cytokine changes, and genomic changes in cancer patients with fatigue. Though many hypotheses have been proposed, to date there is no biological marker to assess this condition. Biomarker research needs to be advanced to help to identify patients at risk for fatigue. As cytokines have a major role in CRF, targeted therapy to block cytokine pathways may also be explored in the future.
Acknowledgment: Bryan Tutt provided editorial assistance.
Corresponding author: Carmelita P. Escalante, MD, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Houston, TX 77030, [email protected].
Financial disclosures: None.
1. Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016;122:477–85.
2. Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013;18:1135–43.
3. Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients -- an EAPC approach. Palliat Med 2008;22:13–32.
4. Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 2007;32:2384–92.
5. Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One 2013;8:e83636.
6. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 2005;67:277–80.
7. Barsevick A, Frost M, Zwinderman A, et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 2010;19:1419–27.
8. Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol 2003;21:4635–41.
9. Fontes-Oliveira CC, Busquets S, Toledo M, et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta 2013;1830:2770–8.
10. Agteresch HJ, Dagnelie PC, van der Gaast A, et al. Randomized clinical trial of adenosine 5’-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2000;92:321–8.
11. Beijer S, Hupperets PS, van den Borne BE, et al. Randomized clinical trial on the effects of adenosine 5’-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage 2010;40:520–30.
12. Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44:351–61.
13. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist 2007;12 Suppl 1:22–34.
14. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887–99.
15. Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:1319–28.
16. Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006;12:2759–66.
17. Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer 2008;113:2102–9.
18. Inagaki M, Isono M, Okuyama T, et al. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage 2008;35:153–61.
19. Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5.
20. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29:3517–22.
21. Whistler T, Taylor R, Craddock RC, et al. Gene expression correlates of unexplained fatigue. Pharmacogenomics 2006;7:395–405.
22. Fagundes CP, Bennett JM, Alfano CM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychol 2012;31:11–19.
23. Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J 2009;9:333–40.
24. Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun 2014;38:227–36.
25. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015;121:2129–36.
26. Mills PJ, Parker B, Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 2005;69:85–96.
27. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 Suppl 1:11–21.
28. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014;120:425–32.
29. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186–96.
30. Mendoza ME, Capafons A, Gralow JR, et al. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psychooncology 2016 Jul 28.
31. Glaus A. Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer 1993;1:305–15.
32. Seyidova-Khoshknabi D, Davis MP, Walsh D. A systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care 2011;28:119–29.
33. Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol 2002;13:965–73.
34. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004;27:14–23.
35. Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 2003;26:604–14.
36. Peterspm DR. Scope and generality of verbally defined personality factors. Psychol Rev 1965;72:48–59.
37. Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw 2010;8:904–31.
38. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002;95:888–95.
39. Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
40. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010;116:4045–59.
41. Preston NJ, Hurlow A, Brine J, Bennett MI. Blood transfusions for anaemia in patients with advanced cancer. Cochrane Database Syst Rev 2012(2):CD009007.
42. Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care 2012;2:231–8.
43. Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer 2016;24:2059–66.
44. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791–800.
45. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12.
46. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med 2014;15:385–92.
47. Strasser F, Palmer JL, Schover LR, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 2006;107:2949–57.
48. Agasi-Idenburg SC, Thong MS, Punt CJ, et al. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer 2017;25:625–32.
49. Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs 2007;23:99–105.
50. de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol 2013;31:716–23.
51. Barsevick AM, Whitmer K, Sweeney C, Nail LM. A pilot study examining energy conservation for cancer treatment-related fatigue. Cancer Nurs 2002;25:333–41.
52. Barsevick AM, Dudley W, Beck S, et a;. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004;100:1302–10.
53. Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31:424–30.
54. Abrahams HJ, Gielissen MF, Goedendorp MM, et al. A randomized controlled trial of web-based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE-study): study protocol. BMC Cancer 2015;15:765.
55. Quesnel C, Savard J, Simard S, et al. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003;71:189–200.
56. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009(1):CD006953.
57. Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82.
58. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;(9):CD005001.
59. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;(11):CD006145.
60. Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33.
61. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014;25:2237–43.
62. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50.
63. Kenjale AA, Hornsby WE, Crowgey T, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist 2014;19:999–1005.
64. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage 2006;31:421–30.
65. Porock D, Kristjanson LJ, Tinnelly K, et al. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000;16:30–6.
66. Lengacher CA, Kip KE, Reich RR, et al. A cost-effective mindfulness stress reduction program: a randomized control trial for breast cancer survivors. Nursing Econ 2015;33:210–8, 32.
67. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med 2012;35:86–94.
68. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol 2015;6:8–14.
69. Wang G, Wang S, Jiang P, Zeng C. [Effect of Yoga on cancer related fatigue in breast cancer patients with chemotherapy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1077–82.
70. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer 2016;24:4005–15.
71. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med 2015;49:165–76.
72. Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470–6.
73. Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer 2013;21:1735–41.
74. Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90.
75. Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum 2002;29:E85–90.
76. Spathis A, Dhillan R, Booden D, et al. Modafinil for the treatment of fatigue in lung cancer: a pilot study. Palliat Med 2009;23:325–31.
77. Blackhall L, Petroni G, Shu J, et al. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med 2009;12:433–9.
78. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl) 2015;25:970–9.
79. Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010(7):CD006704.
80. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010;28:3673–9.
81. Bruera E, Driver L, Barnes EA, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol 2003;21:4439–43.
82. Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012;43:68–77.
83. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J 2014;20:8–14.
84. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 2008;16:577–83.
85. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007;69:1496–501.
86. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer 2014;22:1233–42.
87. Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol 2014;32:1882–8.
88. Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer 2015;23:1503–12.
89. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol 2013;15:1420–8.
90. Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010;116:3513–20.
91. Conley CC, Kamen CS, Heckler CE, et al. Modafinil moderates the relationship between cancer-related fatigue and depression in 541 patients receiving chemotherapy. J Clin Psychopharmacol 2016;36:82–5.
92. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 Suppl 2:81–90.
93. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol 2013;31:3076–82.
94. Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751–4.
95. Pulivarthi K, Dev R, Garcia J, et al. Testosterone replacement for fatigue in male hypogonadic patients with advanced cancer: A preliminary double-blind placebo-controlled trial. J Clin Oncol 2012;30 (suppl). Abstract e19643.
96. Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012;13:1184–90.
97. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in Cancer. Curr Psychiatry Rep 2014;17:529.
98. Lesser GJ. Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol 2013;11:31–42.
99. Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 2013;105:1230–8.
100. Yennurajalingam S, Reddy A, Tannir NM, et al. High-dose Asian ginseng (panax ginseng) for cancer-related fatigue: a preliminary report. Integr Cancer Ther 2015;14:419–27.
101. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233–46.
From the University of Texas MD Anderson Cancer Center, Houston, TX.
Abstract
- Objective: To review the evidence on interventions for managing cancer-related fatigue (CRF) and provide evidence-based guidance on approaches to its management.
- Methods: Nonsystematic review of the literature.
- Results: Several theories have been proposed to explain the biology of CRF, but there is no single clear mechanism that can be targeted for therapy. The approach to patients begins with screening for fatigue and assessing its intensity, followed by a thorough history and examination to determine whether any reversible medical conditions are contributing to fatigue. Management of underlying medical comorbidities may help some patients. For patients whose fatigue persists, pharmacologic and nonpharmacologic treatment options are available. Pharmacologic options include psychostimulants, such as methylphenidate and modafinil, and corticosteroids. Nonpharmacologic approaches include exercise, cognitive behavior therapy, yoga, acupuncture, and tai chi.
- Conclusion: We recommend an individualized approach, often with a combination of the available options. Patients need to be evaluated periodically to assess their fatigue, and since cancer-related fatigue affects survivors, long-term follow-up is needed.
Key words: fatigue; cancer; pro-inflammatory cytokines; nonpharmacologic; psychostimulants.
Fatigue is a common distressing effect of cancer [1].It impairs the quality of life of patients undergoing active cancer treatment and of post-treatment survivors. The National Comprehensive Cancer Network (NCCN) defines cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning [2].” Differences between CRF and fatigue reported by individuals without cancer are that CRF is more severe and is not relieved by rest. The prevalence of CRF in cancer patients and survivors is highly variable, ranging between 25% and 99% [2,3]. This variability may be secondary to methods used for screening fatigue and characteristics of the patient groups. In this article, we discuss recognition of CRF and approaches to its management.
Pathophysiology
The specific pathophysiologic mechanism underlying CRF is unknown, making targeted treatment a challenge. The multidimensional and subjective nature of CRF has limited the development of research methodologies to explain this condition. However, research has been done in both human and animal models, and several theories have been proposed to explain the pathophysiology of CRF. While pro-inflammatory cytokines remain the central factor playing a significant role at multiple levels in CRF, there may be a complex interplay of more than 1 mechanism contributing to fatigue in an individual patient.
Central Nervous System Disturbances
The basal ganglia are known to influence motivation. Lack of motivation and drive may cause failure to complete physical and mental tasks, even with preserved cognitive ability and motor function. In a study of melanoma patients receiving interferon, increased activity of the basal ganglia and the cerebellum resulted in higher fatigue scores [4]. Higher levels of cytokines may alter blood flow to the cerebellum and lead to the perception of fatigue. In a study of 12 patients and matched controls, when patients were asked to perform sustained elbow flexion until they perceived exhaustion, CRF patients perceived physical exhaustion sooner than controls. In CRF patients in this study, muscle fatigue measured by electromyogram was less than that in healthy individuals at the time of exhaustion, suggesting the role of the central nervous system in CRF [5]. However, there is not enough evidence at this time to support central nervous system disturbance as the main contributing factor to fatigue in cancer patients.
Circadian Rhythm Dysregulation
Circadian rhythm is regulated by the suprachiasmatic nucleus in the hypothalamus through cortisol and melatonin. Sleep disturbances occur with disruption of the circadian rhythm. Tumor-related peptides such as epidermal growth factor or alterations in serotonin and cortisol can influence the suprachiasmatic nucleus and the complex signaling pathways [2]. Positive feedback loops that are activated by cortisol under the influence of cytokines may lead to continuous cytokine production and altered circadian rhythm. Bower et al showed that changes in the cortisol curve influence fatigue in breast cancer survivors [6]. These patients had a late evening peak in cortisol levels, compared with an early morning peak in individuals without cancer.
Inhibition of Hypothalamic–Pituitary–Adrenal Axis
The hypothalamic–pituitary–adrenal (HPA) axis regulates the release of the stress hormone cortisol. One of several hypotheses advanced to explain the effect of serotonin and the HPA axis on CRF suggests that lower serotonin levels cause decreased activation of 5-hydroxytrytophan 1-a (5-HT1-a) receptors in the hypothalamus, leading to decreased activity of the HPA axis [6]. The inhibition of the HPA axis may occur with higher levels of serotonin as well [7]. The 5-HT1-a receptors are also triggered by cytokines. However, the correction of serotonin levels by antidepressants was not shown to improve fatigue [8]. Inhibition of the HPA axis can also lead to lower testosterone, progesterone, or estrogen levels, which may indirectly contribute to fatigue [2].
Skeletal Muscle Effect
Chemotherapy- and tumor-related cachexia have a direct effect on the metabolism of skeletal muscles. This effect may lead to impaired adenosine triphosphate (ATP) generation during muscle contraction [9]. ATP infusion improved muscle strength in one trial, but this was not confirmed in another trial [10,11]. Muscle contraction studies showed no differences in the contractile properties of muscles in fatigued patients who failed earlier in motor tasks and healthy controls [12]. This finding suggests that there could be a failure of skeletal muscle activation by the central nervous system or inhibition of skeletal muscle activity. Cytokines and other neurotransmitters activate vagal efferent nerve fibers, which may lead to reflex inhibition in skeletal muscles [13,14].
Pro-inflammatory Cytokines
Tumors or treatment of them may cause tissue injury, which triggers immune cells to release cytokines, signaling the brain to manifest the symptom fatigue. Inflammatory pathways are influenced by psychological, behavioral, and biological factors, which play a role as risk factors in CRF. Interleukin 6 (IL-6), interleukin-1 receptor antagonist, interleukin-1, and tumor necrosis factor (TNF) have been shown to be elevated in fatigued patients being treated for leukemia and non-Hodgkin lymphoma [15]. IL-6 was also associated with increased fatigue in breast cancer survivors [16]. Similar findings were reported in patients undergoing stem cell transplantation and high-dose chemotherapy [17]. Elevated levels of IL-6 and C-reactive protein were also linked to fatigue in terminally ill cancer patients [18,19]. Furthermore, TNF-α signaling was associated with post-chemotherapy fatigue in breast cancer patients [20]. Leukocytes in breast cancer survivors with fatigue also have increased gene expression of pro-inflammatory cytokines, emphasizing the role of cytokines and inflammation in the pathogenesis of CRF [21].
Other Hypotheses
Several other hypotheses for CRF pathogenesis have been proposed. Activation of latent viruses such as Epstein-Barr virus, lack of social support [22], genetic alterations in immune pathway [23], epigenetic changes [24], accumulation of neurotoxic metabolites and depletion of serotonin by indoleamine 2,3-dioxygenase pathway activation [25], elevated vascular endothelial growth factor levels [26], and hypoxia-related organ dysfunction due to anemia or hemoglobin dysfunction [13] all have been postulated to cause CRF.
Approach to Evaluation and Treatment
Screening
Because patients and health care professionals may be unaware of the treatment options available for CRF, patients may not report fatigue levels to their clinicians, and clinicians may not understand the impact of fatigue on their patients’ quality of life. This leads to underrecognition of the problem. The NCCN recommends screening every cancer patient and post-treatment survivor for fatigue [2]. Patients should be screened at their first visit and then at periodic intervals during and after cancer treatment.
Many scales are available to screen patients for CRF in clinical practice and clinical trials [27]. A single item that asks patients to rate their fatigue on a scale from 0 to 10—in which 0 indicates no fatigue, 1 to 3 indicates mild fatigue, 4 to 6 indicates moderate fatigue, 7 to 9 indicates severe fatigue, and 10 indicates the worst fatigue imaginable—is commonly used to screen for CRF [2]. This scale was adapted from the MD Anderson Symptom Inventory scale and is based on a large nationwide study of cancer patients and survivors [28]. The statistically derived cutoff points in this study are consistent with other scales such as the Brief Fatigue Inventory (BFI) and support the cutoff points (4–6 for moderate and ≥ 7 for severe fatigue) used in various fatigue management guidelines. Furthermore, studies of fatigue in cancer patients have revealed a marked decrease in physical function at levels of 7 or higher, suggesting 7 as an optimal cutoff to identify severe fatigue [29,30]. The Visual Analog Scale is another simple-to-use tool that helps in understanding variations in fatigue throughout the course of the day [31]. The 9-item BFI is often used in clinical trials [29]. It measures the severity of fatigue over the previous 24 hours and has been validated in non-English speaking patients [32].
CRF affects not only the somatic domain, but also the cognitive, behavioral, and affective domains; therefore, multidimensional scales have been developed for screening. One such tool is the Multidimensional Fatigue Inventory, which measures general, physical, mental, and emotional fatigue domains as well as activity and compares them with those of individuals without cancer [33,34]. The Functional Assessment of Cancer Therapy for Fatigue (FACT-F) is a 13-item questionnaire that has been used to measure CRF in clinical trials as well as in patients receiving various treatments [35].
Although many scales are available, the validity of self-reporting on simple fatigue-rating scales is equal to or better than most complex, lengthy scales [36]. Therefore, unidimensional tools such as the numeric rating scale of 0–10 are commonly used in clinical practice. Mild fatigue (0–3) requires periodic re-evaluation, and moderate and severe fatigue need further evaluation and management [37].
Primary Evaluation
This phase involves a focused history and physical examination and assessment of concurrent symptoms and contributing factors.
History and Physical Examination
A detailed history of the patient’s malignancy and type of previous and current treatment may help reveal the cause of fatigue. New-onset fatigue or increase in fatigue may be related to the progression of disease in patients with active malignancy or recurrence of cancer in survivors. These patients may require appropriate testing to assess the underlying disease pattern. A detailed review of systems may help identify some of the contributing factors, which are discussed below. A detailed history regarding medications, including over-the-counter drugs, complementary agents, and past and prior cancer therapies, is helpful as medications can contribute to fatigue. For example, opioids may cause drowsiness and fatigue, which could be improved by dose adjustments. A focused history of fatigue should be obtained in all patients with moderate to severe CRF, which includes the onset, pattern, duration, associated or alleviating factors, and interference with functioning, including activities of daily living [37]. Physical examination should focus on identifying signs of organ dysfunction and features of substance or alcohol abuse which may cause poor sleep and fatigue.
Assessment of Contributing Factors
The management of fatigue should be multifactorial, with a comprehensive assessment and treatment plan to address all modifiable fatigue etiologies. The Table lists potential contributing factors to fatigue that should be considered when evaluating patients for CRF; several common conditions are discussed below.
Anemia. Anemia has been correlated with fatigue and quality of life. In a study of 4382 cancer patients receiving chemotherapy, quality-of-life measures using FACT-Anemia scores improved with increased hemoglobin levels [38]. Cancer patients may have anemia due to marrow-suppressing effects of chemotherapy and may also have iron deficiency anemia due to blood loss or autoimmune hemolytic anemia. Therefore, a detailed work-up is required to identify the etiology of anemia. Patients with CRF whose anemia is related to chemotherapy or anemia of chronic disease may benefit from red blood cell transfusion or erythropoiesis-stimulating agents (ESAs). ESAs have been studied extensively; however, their use is controversial because of concerns about thromboembolic side effects leading to adverse outcomes [39]. Also, ESA therapy is not recommended in patients with hematologic malignancies. ESA use should be restricted to patients with chemotherapy-related anemia with hemoglobin below 10 mg/dL and should be discontinued in 6 to 8 weeks if patients do not respond [40]. Other patients may benefit from blood transfusions, which were shown to help in patients with hemoglobin levels between 7.5 and 8.5 g/dL [41].
Sleep disturbance. Poor sleep is common in fatigued cancer survivors [42]. Pro-inflammatory cytokines can disrupt the sleep–wake cycle, causing changes in the HPA axis and neuroendocrine system, which in turn may lead to increasing fatigue. Heckler et al showed that improvement in nighttime sleep leads to improvement of fatigue [43]. Cognitive behavioral therapy and sleep hygiene are important in caring for patients with CRF and poor sleep [44]. Taking a warm bath and/or drinking a glass of milk before bedtime, avoiding caffeinated drinks, and avoiding frequent napping in the day might help. Some patients may require medications such as benzodiazepines or non-benzodiazepine hypnotics (eg, zolpidem) to promote sleep [45]. Melatonin agonists are approved for insomnia in the United states, but not in Europe [46].
Malnutrition. Patients with advanced-stage cancer and with cancers affecting the gastrointestinal tract frequently develop mechanical bowel obstructions, especially at the end of their life, which cause malnutrition. Chemotherapy-related nausea and vomiting may also cause poor oral intake and malnutrition, causing fatigue from muscle weakness. Dehydration and electrolyte imbalances frequently occur as a result of poor oral intake, which might worsen fatigue. In our experience, modifying dietary intake with appropriate caloric exchanges with the help of a nutrition expert has lessened fatigue in some patients. However, terminally ill patients are advised to eat based on their comfort.
Medical comorbidities. Congestive heart failure from anthracycline chemotherapy, hypothyroidism after radiation therapy for head and neck cancers, renal failure, or hepatic failure from chemotherapy may indirectly lead to fatigue. Chemotherapy, opioids, and steroids may cause hypogonadism, which can contribute to fatigue in men [47].
Assessment of Concurrent Symptoms
Depression is common in cancer patients and coexists with pain, insomnia, fatigue, and anxiety as a symptom cluster [48]. A symptom cluster is defined as 2 or more concurrent and interrelated symptoms occurring together; treating of one of these symptoms without addressing other symptoms is not effective [49]. Therefore, screening for and management of depression, anxiety, and insomnia play an important role in the management of CRF.
Physical symptoms due to the tumor or to therapy—such as pain, dyspnea, nausea, and decreased physical activity—may also contribute to fatigue both directly and indirectly. Patients with lung cancer may have hypoxemia, which can contribute to dyspnea with activity and a perception of fatigue. Optimal management of pain and other physical symptoms in patients with cancer may significantly alleviate fatigue [50].
Management
Management of CRF is individualized based on the patient’s clinical status: active cancer treatment, survivor, or end of life. Education and counselling of patients and their caregivers play an important role in CRF. NCCN guidelines recommend focusing on pain control, distress management, energy conservation, physical activity, nutrition, and sleep hygiene.
Nonpharmacologic Interventions
Energy conservation. Energy conservation strategies, in which patients are advised to set priorities and realistic expectations, are highly recommended. Some energy-conserving strategies are to pace oneself, delegate and schedule activities at times of peak energy, postpone nonessential activities, attend to 1 activity at a time, structure daily routines, and maintain a diary to identify their peak energy period and structure activities around that time [51,52]. When patients approach the end of life, increasing fatigue may limit their activity level, and they may depend on caregivers for assistance with activities of daily living, monitoring treatment-related adverse effects, and taking medications, especially elderly patients [53].
Cognitive behavioral therapy. Cognitive behavioral therapy (CBT) has been shown to improve CRF during active treatment, and the benefits persist for a minimum of 2 years after therapy [54]. CBT interventions that optimize sleep quality may improve fatigue [55]. More studies are needed to understand whether referral to a psychologist for formal CBT is required. Randomized clinical trials (RCTs) showed patient fatigue education, learned self-care, coping techniques, and balancing rest and activity benefit patients with CRF [56].
Exercise. Physical activity is highly encouraged in patients with CRF. Exercise increases muscle protein synthesis, improves cytokine response, and decreases the rate of sarcopenia in healthy populations [57]. Studies have shown that exercise helps CRF at all phases of the cancer journey, including radiation therapy, chemotherapy, and survivorship [58]. Some patients may feel less motivated to exercise and may not believe that exercise is possible or could potentially help them. Counselling is needed for such patients.
Older cancer survivors have a decline in their functional capacity and reduced muscle mass. Exercise can improve cardiorespiratory fitness, muscle strength, and body composition [57]. Exercise not only helps at the cellular level but also has psychosocial benefits from improved self-esteem. Therefore, exercise may be recommended not only for younger patients, but also in the older population, who may have comorbidities and less motivation than younger patients.
In a meta-analysis of 56 randomized controlled trials involving 4068 participants, aerobic exercise was found to have beneficial effects on CRF for patients during and after chemotherapy, specifically for patients with solid tumors [59]. In another meta-analysis of breast and prostate cancer survivors, a combination of aerobic exercise with resistance training (3–6 metabolic equivalents, 60%–80% range of motion) was shown to reduce CRF more than aerobic exercise alone [60]. This effect was also shown in an RCT of 160 patients with stage 0 to III breast cancer undergoing radiation therapy [61]. The control group in this study had a group-based non-exercise intervention/relaxation; therefore, the study showed that the effect of resistance training extends beyond the psychosocial benefits of group-based interventions. The intervention comprised 8 progressive machine-based resistance exercises (3 sets, 8–12 repetitions at 60%–80% of 1 repetition maximum) for 60 minutes twice weekly for 12 weeks. However, fatigue assessment questionnaire scores showed benefits in the physical fatigue but not the affective and cognitive components.
The American Society of Clinical Oncology’s guidelines for cancer survivors with fatigue recommends 150 minutes of moderate aerobic exercise (eg, fast walking, cycling, or swimming) per week, with 2 or 3 sessions of strength training per week [62]. An individualized approach to exercise is recommended, as patients’ ability to perform certain types of exercises may be limited by thrombocytopenia, neutropenia, or lytic bone metastasis. Routine use of pre-exercise cardiovascular testing is not recommended but may be considered in high-risk populations, especially patients with risk factors for coronary heart disease and diabetes [63]. Patients withcomorbidities, substantial deconditioning, functional and anatomic defects, or recent major surgery may benefit from referral to physical therapy [37]. Patients near end of life may also benefit from an exercise program, as demonstrated in several studies that showed benefit in CRF and quality of life [64,65]. We recommend that physicians use their best clinical judgement in suggesting the type and intensity of exercise program, as it may not be feasible in some patients.
Mind-body interventions. Mindfulness-based stress reduction (MBSR) has shown promise in breast cancer survivors, who reported immediate improvements in fatigue severity that continued up to 6 weeks after cessation of the training [66]. Prior studies had similar findings, suggesting that MBSR modestly decreases fatigue and sleep disturbances and has a greater effect on the degree to which symptoms interfere with many facets of life [67].
Yoga. A study of a yoga intervention showed a benefit in older cancer survivors [68]. In breast cancer patients undergoing chemotherapy, yoga was shown to benefit not only physical fatigue, but also cognitive fatigue [69]. DVD-based yoga had benefits similar to strengthening exercises in a study of 34 early-stage breast cancer survivors with CRF [70]. More studies are needed in men and patients and survivors of other cancers, as most studies of yoga were conducted in women with breast cancer.
Tai chi/qigong. Like yoga, tai chi and qigong are practices of meditative movement. These practices use postures or movements with a focus on breath and a meditative state to bring about deep states of relaxation. Qigong is a series of simple, repeated practices including body posture/movement, breath practice, and meditation performed in synchrony. Tai chi easy (TCE) is a simplified set of common, repetitive tai chi movements. In a trial, qigong/TCE was compared with sham qigong, which had physical movements but no breathing or meditative practice. Breast cancer survivors in the qigong/TCE group had improved fatigue scores, and the effect persisted for 3 months [71]. Additional research is needed in this area.
Acupuncture. An RCT in breast cancer patients with CRF showed an improvement in the mean general fatigue score (per the Multidimensional Fatigue Inventory) in patients who received acupuncture versus those who did not (−3.11 [95% confidence interval −3.97 to −2.25]; P < 0.001) at 6 weeks. Improvements were seen in both the mental and physical aspects of fatigue [72]. However, Deng et al noted that true acupuncture was no more effective than sham acupuncture for reducing post-chemotherapy chronic fatigue [73]. Presently, there is not sufficient evidence to evaluate the benefits of acupuncture in CRF.
Other modalities. Massage therapy, music therapy, hypnosis, therapeutic touch, biofield therapies, relaxation, and reiki are other therapies for which few studies have been done, with mixed results, and additional research is needed [74]. Currently, there are not sufficient data to recommend any of these modalities.
Pharmacologic Interventions
Psychostimulants. Methylphenidate and modafinil are psychostimulants or wakefulness-promoting agents. Pilot studies showed benefit from methylphenidate and modafinil in CRF [75–77], but RCTs have yielded mixed results. Therefore, in patients with severe fatigue during cancer therapy, the initial management strategy involves evaluation and treatment of medical conditions such as anemia and a trial of non-pharmacological strategies as discussed above. If symptoms persist, then a therapeutic trial of a psychostimulant may be considered per NCCN guidelines for patients undergoing active cancer treatment [37].
Methylphenidate directly stimulates adrenergic receptors and indirectly releases dopamine and norepinephrine from presynaptic terminals, which may explain why the drug benefits patients receiving opioid-induced sedation. It is a commonly studied psychostimulant, though its mechanism of action in CRF is unclear. RCTs of methylphenidate have resulted in a wide range of findings due to the heterogeneity of study populations and variations in the dosage of methylphenidate. A meta-analysis of 7 studies indicates that methylphenidate benefitted the subgroup of patients with CRF [78]. Likewise, in an analysis of 5 RCTs, Minton et al showed a benefit of psychostimulants in fatigue compared with placebo [79]. However, another study of methylphenidate in patients with CRF showed a benefit only in patients with severe fatigue or advanced disease [80]. Methylphenidate was found to benefit cancer patients receiving opioid-induced sedation, as methylphenidate promotes wakefulness, though fatigue was not studied specifically [81]. In a trial with 30 hospice patients in which the methylphenidate dose was titrated based on response and adverse effects, Kerr at al found that the drug improved fatigue in a dose-dependent manner [82]. However, a study in patients with CRF at the University of Texas MD Anderson Cancer Center found no significant difference in BFI scores between patients receiving methylphenidate and those receiving placebo at the end of 2 weeks of treatment [83]. Also, other RCTs in patients undergoing adjuvant chemotherapy for breast cancer [84] and patients receiving radiation therapy for brain tumors [85] failed to demonstrate the efficacy of methylphenidate in CRF. It should be used cautiously after ruling out other causes of fatigue. The drug is overall well tolerated and side effects include headache and nausea.
Modafinil is a non-amphetamine psychostimulant that has been approved for the treatment of narcolepsy. In a trial studying the effect of modafinil on patients receiving docetaxel-based chemotherapy for metastatic breast or prostate cancer, there was a modest but not statistically significant improvement in fatigue scores on the MD Anderson Symptom Inventory compared with placebo. Nausea and vomiting were higher in the modafinil arm than in the placebo arm [86]. Similarly, modafinil was not superior to placebo for CRF in 208 patients with non-squamous cell lung cancer not undergoing chemotherapy or radiation [87]. A placebo effect was also noted in patients with multiple myeloma [88] and patients with primary brain tumors [89]. In a phase 3, multicenter, randomized, placebo-controlled, double-blind clinical trial of modafinil for CRF in 867 patients undergoing chemotherapy, there was a reduction in fatigue only for patients with severe baseline fatigue, with no significant effect on mild to moderate fatigue [90]. In another recent study, modafinil was shown to reduce depressive symptoms only in patients with severe fatigue (BFI item 3 score ≥ 7) [91]. This finding is consistent with previous studies showing benefit in patients with high baseline fatigue, but additional RCTs are needed to provide clarity. NCCN guidelines do not recommend the use of modafinil to treat CRF [37].
Other pharmacologic interventions. Corticosteroids are often used for symptom control in cancer patients. These drugs have anti-inflammatory effects through their modulation of pro-inflammatory cytokines [92]. In a RCT evaluating the efficacy of corticosteroids, patients receiving dexamethasone (4 mg twice daily) experienced significant improvement in their FACT-F scores compared with patients receiving placebo [93]. A similar benefit in fatigue was demonstrated in another study of methylprednisolone (32 mg daily) versus placebo [94]. Despite the benefits of steroids, their adverse effects, such as mood swings, gastritis, hyperglycemia, and immune suppression, limit their long-term use. Therefore, the use of steroids should be restricted to terminally ill fatigued patients with other symptoms such as anorexia, brain metastasis, or pain related to bone metastasis [37].
Testosterone replacement has been shown to diminish fatigue in non-cancer patients. Many men with advanced cancer have hypogonadism leading to low serum testosterone, which may cause fatigue. In a small trial in which cancer patients with hypogonadism received intramuscular testosterone every 14 days or placebo, the group receiving testosterone showed improvement in FACT-F scores, but there was no significant difference in FACT-F scores between the 2 groups [95].
Antidepressants have failed to demonstrate benefit in CRF without depression [8]. However, if a patient has both fatigue and depression, antidepressants may help [96]. A selective serotonin receptor inhibitor is recommended as a first-line antidepressant [97]. Patients with cancer are often receiving multiple medications, and medication interactions should be considered to prevent adverse events such as serotonin syndrome.
Complementary and Alternative Supplements
Studies using vitamin supplementation have been inconclusive in patients with CRF [74]. The use of other dietary supplements has yielded mixed results, and coenzyme Q has shown no benefit for patients with CRF [98].
The benefit of ginseng was studied in a RCT involving 364 patients with CRF. There was an improvement in Multidimensional Fatigue Symptom Inventory-short form (MFSI-SF) scores at 8 weeks in patients receiving 2000 mg of Wisconsin ginseng compared with patients receiving placebo [99]. Patients on active treatment had greater improvement as compared to the post-treatment group in this trial. In another study of high-dose panax ginseng (ginseng root) at 800 mg daily for 29 days, patients had improvement of CRF as well as overall quality of life, appetite, and sleep at night. It was also well tolerated with few adverse effects [100]. Interaction with warfarin, calcium channel blockers, antiplatelet agents, thrombolytic agents, imatinib, and other agents may occur; therefore, ginseng must be used with careful monitoring in selected patients. There is not enough evidence at this time to support the routine use of ginseng in CRF.
The seed extract of the guarana plant (Paullinia cupana) traditionally has been used as a stimulant. An improvement in fatigue scores was seen with the use of oral guarana (100 mg daily) at the end of 21 days in breast cancer patients receiving chemotherapy [101]. Further studies are needed for these results to be generalized and to understand the adverse effects and interaction profile of guarana.
Re-evaluation
Patients who have completed cancer treatment must be monitored for fatigue over the long term, as fatigue may exist beyond the period of active treatment. Many studies have shown fatigue in breast cancer survivors, and fatigue has been demonstrated in survivors of colorectal, lung, and prostate cancers as well as myeloproliferative neoplasms [28]. Therefore, it is important to screen patients for fatigue during follow-up visits. There are currently no studies evaluating the long-term treatment of fatigue. In our experience, the timing of follow-up depends on the level of fatigue and interventions prescribed. Once fatigue is stabilized to a level with which the patient is able to cope, the time interval for follow up may be lengthened. Annual visits may suffice in patients with mild fatigue. Follow-up of patients with moderate to severe fatigue depends on the level of fatigue, the ability to cope, choice of treatment, and presence of contributing factors.
Conclusion
CRF is a complex condition that places a significant burden on patients and caregivers, resulting in emotional distress, poor functioning, and suffering. Fatigue can occur before, during, and long after cancer treatment. The approach to CRF begins with screening for and educating patients and their caregivers about the symptoms. Many screening scales are available that may be used to follow patients’ progress over time. The evaluation and management of contributing conditions may help improve fatigue. If the fatigue persists, an individualized approach with a combination of nonpharmacologic and pharmacologic approaches should be considered. More research is needed to understand brain signaling pathways, cytokine changes, and genomic changes in cancer patients with fatigue. Though many hypotheses have been proposed, to date there is no biological marker to assess this condition. Biomarker research needs to be advanced to help to identify patients at risk for fatigue. As cytokines have a major role in CRF, targeted therapy to block cytokine pathways may also be explored in the future.
Acknowledgment: Bryan Tutt provided editorial assistance.
Corresponding author: Carmelita P. Escalante, MD, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Houston, TX 77030, [email protected].
Financial disclosures: None.
From the University of Texas MD Anderson Cancer Center, Houston, TX.
Abstract
- Objective: To review the evidence on interventions for managing cancer-related fatigue (CRF) and provide evidence-based guidance on approaches to its management.
- Methods: Nonsystematic review of the literature.
- Results: Several theories have been proposed to explain the biology of CRF, but there is no single clear mechanism that can be targeted for therapy. The approach to patients begins with screening for fatigue and assessing its intensity, followed by a thorough history and examination to determine whether any reversible medical conditions are contributing to fatigue. Management of underlying medical comorbidities may help some patients. For patients whose fatigue persists, pharmacologic and nonpharmacologic treatment options are available. Pharmacologic options include psychostimulants, such as methylphenidate and modafinil, and corticosteroids. Nonpharmacologic approaches include exercise, cognitive behavior therapy, yoga, acupuncture, and tai chi.
- Conclusion: We recommend an individualized approach, often with a combination of the available options. Patients need to be evaluated periodically to assess their fatigue, and since cancer-related fatigue affects survivors, long-term follow-up is needed.
Key words: fatigue; cancer; pro-inflammatory cytokines; nonpharmacologic; psychostimulants.
Fatigue is a common distressing effect of cancer [1].It impairs the quality of life of patients undergoing active cancer treatment and of post-treatment survivors. The National Comprehensive Cancer Network (NCCN) defines cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning [2].” Differences between CRF and fatigue reported by individuals without cancer are that CRF is more severe and is not relieved by rest. The prevalence of CRF in cancer patients and survivors is highly variable, ranging between 25% and 99% [2,3]. This variability may be secondary to methods used for screening fatigue and characteristics of the patient groups. In this article, we discuss recognition of CRF and approaches to its management.
Pathophysiology
The specific pathophysiologic mechanism underlying CRF is unknown, making targeted treatment a challenge. The multidimensional and subjective nature of CRF has limited the development of research methodologies to explain this condition. However, research has been done in both human and animal models, and several theories have been proposed to explain the pathophysiology of CRF. While pro-inflammatory cytokines remain the central factor playing a significant role at multiple levels in CRF, there may be a complex interplay of more than 1 mechanism contributing to fatigue in an individual patient.
Central Nervous System Disturbances
The basal ganglia are known to influence motivation. Lack of motivation and drive may cause failure to complete physical and mental tasks, even with preserved cognitive ability and motor function. In a study of melanoma patients receiving interferon, increased activity of the basal ganglia and the cerebellum resulted in higher fatigue scores [4]. Higher levels of cytokines may alter blood flow to the cerebellum and lead to the perception of fatigue. In a study of 12 patients and matched controls, when patients were asked to perform sustained elbow flexion until they perceived exhaustion, CRF patients perceived physical exhaustion sooner than controls. In CRF patients in this study, muscle fatigue measured by electromyogram was less than that in healthy individuals at the time of exhaustion, suggesting the role of the central nervous system in CRF [5]. However, there is not enough evidence at this time to support central nervous system disturbance as the main contributing factor to fatigue in cancer patients.
Circadian Rhythm Dysregulation
Circadian rhythm is regulated by the suprachiasmatic nucleus in the hypothalamus through cortisol and melatonin. Sleep disturbances occur with disruption of the circadian rhythm. Tumor-related peptides such as epidermal growth factor or alterations in serotonin and cortisol can influence the suprachiasmatic nucleus and the complex signaling pathways [2]. Positive feedback loops that are activated by cortisol under the influence of cytokines may lead to continuous cytokine production and altered circadian rhythm. Bower et al showed that changes in the cortisol curve influence fatigue in breast cancer survivors [6]. These patients had a late evening peak in cortisol levels, compared with an early morning peak in individuals without cancer.
Inhibition of Hypothalamic–Pituitary–Adrenal Axis
The hypothalamic–pituitary–adrenal (HPA) axis regulates the release of the stress hormone cortisol. One of several hypotheses advanced to explain the effect of serotonin and the HPA axis on CRF suggests that lower serotonin levels cause decreased activation of 5-hydroxytrytophan 1-a (5-HT1-a) receptors in the hypothalamus, leading to decreased activity of the HPA axis [6]. The inhibition of the HPA axis may occur with higher levels of serotonin as well [7]. The 5-HT1-a receptors are also triggered by cytokines. However, the correction of serotonin levels by antidepressants was not shown to improve fatigue [8]. Inhibition of the HPA axis can also lead to lower testosterone, progesterone, or estrogen levels, which may indirectly contribute to fatigue [2].
Skeletal Muscle Effect
Chemotherapy- and tumor-related cachexia have a direct effect on the metabolism of skeletal muscles. This effect may lead to impaired adenosine triphosphate (ATP) generation during muscle contraction [9]. ATP infusion improved muscle strength in one trial, but this was not confirmed in another trial [10,11]. Muscle contraction studies showed no differences in the contractile properties of muscles in fatigued patients who failed earlier in motor tasks and healthy controls [12]. This finding suggests that there could be a failure of skeletal muscle activation by the central nervous system or inhibition of skeletal muscle activity. Cytokines and other neurotransmitters activate vagal efferent nerve fibers, which may lead to reflex inhibition in skeletal muscles [13,14].
Pro-inflammatory Cytokines
Tumors or treatment of them may cause tissue injury, which triggers immune cells to release cytokines, signaling the brain to manifest the symptom fatigue. Inflammatory pathways are influenced by psychological, behavioral, and biological factors, which play a role as risk factors in CRF. Interleukin 6 (IL-6), interleukin-1 receptor antagonist, interleukin-1, and tumor necrosis factor (TNF) have been shown to be elevated in fatigued patients being treated for leukemia and non-Hodgkin lymphoma [15]. IL-6 was also associated with increased fatigue in breast cancer survivors [16]. Similar findings were reported in patients undergoing stem cell transplantation and high-dose chemotherapy [17]. Elevated levels of IL-6 and C-reactive protein were also linked to fatigue in terminally ill cancer patients [18,19]. Furthermore, TNF-α signaling was associated with post-chemotherapy fatigue in breast cancer patients [20]. Leukocytes in breast cancer survivors with fatigue also have increased gene expression of pro-inflammatory cytokines, emphasizing the role of cytokines and inflammation in the pathogenesis of CRF [21].
Other Hypotheses
Several other hypotheses for CRF pathogenesis have been proposed. Activation of latent viruses such as Epstein-Barr virus, lack of social support [22], genetic alterations in immune pathway [23], epigenetic changes [24], accumulation of neurotoxic metabolites and depletion of serotonin by indoleamine 2,3-dioxygenase pathway activation [25], elevated vascular endothelial growth factor levels [26], and hypoxia-related organ dysfunction due to anemia or hemoglobin dysfunction [13] all have been postulated to cause CRF.
Approach to Evaluation and Treatment
Screening
Because patients and health care professionals may be unaware of the treatment options available for CRF, patients may not report fatigue levels to their clinicians, and clinicians may not understand the impact of fatigue on their patients’ quality of life. This leads to underrecognition of the problem. The NCCN recommends screening every cancer patient and post-treatment survivor for fatigue [2]. Patients should be screened at their first visit and then at periodic intervals during and after cancer treatment.
Many scales are available to screen patients for CRF in clinical practice and clinical trials [27]. A single item that asks patients to rate their fatigue on a scale from 0 to 10—in which 0 indicates no fatigue, 1 to 3 indicates mild fatigue, 4 to 6 indicates moderate fatigue, 7 to 9 indicates severe fatigue, and 10 indicates the worst fatigue imaginable—is commonly used to screen for CRF [2]. This scale was adapted from the MD Anderson Symptom Inventory scale and is based on a large nationwide study of cancer patients and survivors [28]. The statistically derived cutoff points in this study are consistent with other scales such as the Brief Fatigue Inventory (BFI) and support the cutoff points (4–6 for moderate and ≥ 7 for severe fatigue) used in various fatigue management guidelines. Furthermore, studies of fatigue in cancer patients have revealed a marked decrease in physical function at levels of 7 or higher, suggesting 7 as an optimal cutoff to identify severe fatigue [29,30]. The Visual Analog Scale is another simple-to-use tool that helps in understanding variations in fatigue throughout the course of the day [31]. The 9-item BFI is often used in clinical trials [29]. It measures the severity of fatigue over the previous 24 hours and has been validated in non-English speaking patients [32].
CRF affects not only the somatic domain, but also the cognitive, behavioral, and affective domains; therefore, multidimensional scales have been developed for screening. One such tool is the Multidimensional Fatigue Inventory, which measures general, physical, mental, and emotional fatigue domains as well as activity and compares them with those of individuals without cancer [33,34]. The Functional Assessment of Cancer Therapy for Fatigue (FACT-F) is a 13-item questionnaire that has been used to measure CRF in clinical trials as well as in patients receiving various treatments [35].
Although many scales are available, the validity of self-reporting on simple fatigue-rating scales is equal to or better than most complex, lengthy scales [36]. Therefore, unidimensional tools such as the numeric rating scale of 0–10 are commonly used in clinical practice. Mild fatigue (0–3) requires periodic re-evaluation, and moderate and severe fatigue need further evaluation and management [37].
Primary Evaluation
This phase involves a focused history and physical examination and assessment of concurrent symptoms and contributing factors.
History and Physical Examination
A detailed history of the patient’s malignancy and type of previous and current treatment may help reveal the cause of fatigue. New-onset fatigue or increase in fatigue may be related to the progression of disease in patients with active malignancy or recurrence of cancer in survivors. These patients may require appropriate testing to assess the underlying disease pattern. A detailed review of systems may help identify some of the contributing factors, which are discussed below. A detailed history regarding medications, including over-the-counter drugs, complementary agents, and past and prior cancer therapies, is helpful as medications can contribute to fatigue. For example, opioids may cause drowsiness and fatigue, which could be improved by dose adjustments. A focused history of fatigue should be obtained in all patients with moderate to severe CRF, which includes the onset, pattern, duration, associated or alleviating factors, and interference with functioning, including activities of daily living [37]. Physical examination should focus on identifying signs of organ dysfunction and features of substance or alcohol abuse which may cause poor sleep and fatigue.
Assessment of Contributing Factors
The management of fatigue should be multifactorial, with a comprehensive assessment and treatment plan to address all modifiable fatigue etiologies. The Table lists potential contributing factors to fatigue that should be considered when evaluating patients for CRF; several common conditions are discussed below.
Anemia. Anemia has been correlated with fatigue and quality of life. In a study of 4382 cancer patients receiving chemotherapy, quality-of-life measures using FACT-Anemia scores improved with increased hemoglobin levels [38]. Cancer patients may have anemia due to marrow-suppressing effects of chemotherapy and may also have iron deficiency anemia due to blood loss or autoimmune hemolytic anemia. Therefore, a detailed work-up is required to identify the etiology of anemia. Patients with CRF whose anemia is related to chemotherapy or anemia of chronic disease may benefit from red blood cell transfusion or erythropoiesis-stimulating agents (ESAs). ESAs have been studied extensively; however, their use is controversial because of concerns about thromboembolic side effects leading to adverse outcomes [39]. Also, ESA therapy is not recommended in patients with hematologic malignancies. ESA use should be restricted to patients with chemotherapy-related anemia with hemoglobin below 10 mg/dL and should be discontinued in 6 to 8 weeks if patients do not respond [40]. Other patients may benefit from blood transfusions, which were shown to help in patients with hemoglobin levels between 7.5 and 8.5 g/dL [41].
Sleep disturbance. Poor sleep is common in fatigued cancer survivors [42]. Pro-inflammatory cytokines can disrupt the sleep–wake cycle, causing changes in the HPA axis and neuroendocrine system, which in turn may lead to increasing fatigue. Heckler et al showed that improvement in nighttime sleep leads to improvement of fatigue [43]. Cognitive behavioral therapy and sleep hygiene are important in caring for patients with CRF and poor sleep [44]. Taking a warm bath and/or drinking a glass of milk before bedtime, avoiding caffeinated drinks, and avoiding frequent napping in the day might help. Some patients may require medications such as benzodiazepines or non-benzodiazepine hypnotics (eg, zolpidem) to promote sleep [45]. Melatonin agonists are approved for insomnia in the United states, but not in Europe [46].
Malnutrition. Patients with advanced-stage cancer and with cancers affecting the gastrointestinal tract frequently develop mechanical bowel obstructions, especially at the end of their life, which cause malnutrition. Chemotherapy-related nausea and vomiting may also cause poor oral intake and malnutrition, causing fatigue from muscle weakness. Dehydration and electrolyte imbalances frequently occur as a result of poor oral intake, which might worsen fatigue. In our experience, modifying dietary intake with appropriate caloric exchanges with the help of a nutrition expert has lessened fatigue in some patients. However, terminally ill patients are advised to eat based on their comfort.
Medical comorbidities. Congestive heart failure from anthracycline chemotherapy, hypothyroidism after radiation therapy for head and neck cancers, renal failure, or hepatic failure from chemotherapy may indirectly lead to fatigue. Chemotherapy, opioids, and steroids may cause hypogonadism, which can contribute to fatigue in men [47].
Assessment of Concurrent Symptoms
Depression is common in cancer patients and coexists with pain, insomnia, fatigue, and anxiety as a symptom cluster [48]. A symptom cluster is defined as 2 or more concurrent and interrelated symptoms occurring together; treating of one of these symptoms without addressing other symptoms is not effective [49]. Therefore, screening for and management of depression, anxiety, and insomnia play an important role in the management of CRF.
Physical symptoms due to the tumor or to therapy—such as pain, dyspnea, nausea, and decreased physical activity—may also contribute to fatigue both directly and indirectly. Patients with lung cancer may have hypoxemia, which can contribute to dyspnea with activity and a perception of fatigue. Optimal management of pain and other physical symptoms in patients with cancer may significantly alleviate fatigue [50].
Management
Management of CRF is individualized based on the patient’s clinical status: active cancer treatment, survivor, or end of life. Education and counselling of patients and their caregivers play an important role in CRF. NCCN guidelines recommend focusing on pain control, distress management, energy conservation, physical activity, nutrition, and sleep hygiene.
Nonpharmacologic Interventions
Energy conservation. Energy conservation strategies, in which patients are advised to set priorities and realistic expectations, are highly recommended. Some energy-conserving strategies are to pace oneself, delegate and schedule activities at times of peak energy, postpone nonessential activities, attend to 1 activity at a time, structure daily routines, and maintain a diary to identify their peak energy period and structure activities around that time [51,52]. When patients approach the end of life, increasing fatigue may limit their activity level, and they may depend on caregivers for assistance with activities of daily living, monitoring treatment-related adverse effects, and taking medications, especially elderly patients [53].
Cognitive behavioral therapy. Cognitive behavioral therapy (CBT) has been shown to improve CRF during active treatment, and the benefits persist for a minimum of 2 years after therapy [54]. CBT interventions that optimize sleep quality may improve fatigue [55]. More studies are needed to understand whether referral to a psychologist for formal CBT is required. Randomized clinical trials (RCTs) showed patient fatigue education, learned self-care, coping techniques, and balancing rest and activity benefit patients with CRF [56].
Exercise. Physical activity is highly encouraged in patients with CRF. Exercise increases muscle protein synthesis, improves cytokine response, and decreases the rate of sarcopenia in healthy populations [57]. Studies have shown that exercise helps CRF at all phases of the cancer journey, including radiation therapy, chemotherapy, and survivorship [58]. Some patients may feel less motivated to exercise and may not believe that exercise is possible or could potentially help them. Counselling is needed for such patients.
Older cancer survivors have a decline in their functional capacity and reduced muscle mass. Exercise can improve cardiorespiratory fitness, muscle strength, and body composition [57]. Exercise not only helps at the cellular level but also has psychosocial benefits from improved self-esteem. Therefore, exercise may be recommended not only for younger patients, but also in the older population, who may have comorbidities and less motivation than younger patients.
In a meta-analysis of 56 randomized controlled trials involving 4068 participants, aerobic exercise was found to have beneficial effects on CRF for patients during and after chemotherapy, specifically for patients with solid tumors [59]. In another meta-analysis of breast and prostate cancer survivors, a combination of aerobic exercise with resistance training (3–6 metabolic equivalents, 60%–80% range of motion) was shown to reduce CRF more than aerobic exercise alone [60]. This effect was also shown in an RCT of 160 patients with stage 0 to III breast cancer undergoing radiation therapy [61]. The control group in this study had a group-based non-exercise intervention/relaxation; therefore, the study showed that the effect of resistance training extends beyond the psychosocial benefits of group-based interventions. The intervention comprised 8 progressive machine-based resistance exercises (3 sets, 8–12 repetitions at 60%–80% of 1 repetition maximum) for 60 minutes twice weekly for 12 weeks. However, fatigue assessment questionnaire scores showed benefits in the physical fatigue but not the affective and cognitive components.
The American Society of Clinical Oncology’s guidelines for cancer survivors with fatigue recommends 150 minutes of moderate aerobic exercise (eg, fast walking, cycling, or swimming) per week, with 2 or 3 sessions of strength training per week [62]. An individualized approach to exercise is recommended, as patients’ ability to perform certain types of exercises may be limited by thrombocytopenia, neutropenia, or lytic bone metastasis. Routine use of pre-exercise cardiovascular testing is not recommended but may be considered in high-risk populations, especially patients with risk factors for coronary heart disease and diabetes [63]. Patients withcomorbidities, substantial deconditioning, functional and anatomic defects, or recent major surgery may benefit from referral to physical therapy [37]. Patients near end of life may also benefit from an exercise program, as demonstrated in several studies that showed benefit in CRF and quality of life [64,65]. We recommend that physicians use their best clinical judgement in suggesting the type and intensity of exercise program, as it may not be feasible in some patients.
Mind-body interventions. Mindfulness-based stress reduction (MBSR) has shown promise in breast cancer survivors, who reported immediate improvements in fatigue severity that continued up to 6 weeks after cessation of the training [66]. Prior studies had similar findings, suggesting that MBSR modestly decreases fatigue and sleep disturbances and has a greater effect on the degree to which symptoms interfere with many facets of life [67].
Yoga. A study of a yoga intervention showed a benefit in older cancer survivors [68]. In breast cancer patients undergoing chemotherapy, yoga was shown to benefit not only physical fatigue, but also cognitive fatigue [69]. DVD-based yoga had benefits similar to strengthening exercises in a study of 34 early-stage breast cancer survivors with CRF [70]. More studies are needed in men and patients and survivors of other cancers, as most studies of yoga were conducted in women with breast cancer.
Tai chi/qigong. Like yoga, tai chi and qigong are practices of meditative movement. These practices use postures or movements with a focus on breath and a meditative state to bring about deep states of relaxation. Qigong is a series of simple, repeated practices including body posture/movement, breath practice, and meditation performed in synchrony. Tai chi easy (TCE) is a simplified set of common, repetitive tai chi movements. In a trial, qigong/TCE was compared with sham qigong, which had physical movements but no breathing or meditative practice. Breast cancer survivors in the qigong/TCE group had improved fatigue scores, and the effect persisted for 3 months [71]. Additional research is needed in this area.
Acupuncture. An RCT in breast cancer patients with CRF showed an improvement in the mean general fatigue score (per the Multidimensional Fatigue Inventory) in patients who received acupuncture versus those who did not (−3.11 [95% confidence interval −3.97 to −2.25]; P < 0.001) at 6 weeks. Improvements were seen in both the mental and physical aspects of fatigue [72]. However, Deng et al noted that true acupuncture was no more effective than sham acupuncture for reducing post-chemotherapy chronic fatigue [73]. Presently, there is not sufficient evidence to evaluate the benefits of acupuncture in CRF.
Other modalities. Massage therapy, music therapy, hypnosis, therapeutic touch, biofield therapies, relaxation, and reiki are other therapies for which few studies have been done, with mixed results, and additional research is needed [74]. Currently, there are not sufficient data to recommend any of these modalities.
Pharmacologic Interventions
Psychostimulants. Methylphenidate and modafinil are psychostimulants or wakefulness-promoting agents. Pilot studies showed benefit from methylphenidate and modafinil in CRF [75–77], but RCTs have yielded mixed results. Therefore, in patients with severe fatigue during cancer therapy, the initial management strategy involves evaluation and treatment of medical conditions such as anemia and a trial of non-pharmacological strategies as discussed above. If symptoms persist, then a therapeutic trial of a psychostimulant may be considered per NCCN guidelines for patients undergoing active cancer treatment [37].
Methylphenidate directly stimulates adrenergic receptors and indirectly releases dopamine and norepinephrine from presynaptic terminals, which may explain why the drug benefits patients receiving opioid-induced sedation. It is a commonly studied psychostimulant, though its mechanism of action in CRF is unclear. RCTs of methylphenidate have resulted in a wide range of findings due to the heterogeneity of study populations and variations in the dosage of methylphenidate. A meta-analysis of 7 studies indicates that methylphenidate benefitted the subgroup of patients with CRF [78]. Likewise, in an analysis of 5 RCTs, Minton et al showed a benefit of psychostimulants in fatigue compared with placebo [79]. However, another study of methylphenidate in patients with CRF showed a benefit only in patients with severe fatigue or advanced disease [80]. Methylphenidate was found to benefit cancer patients receiving opioid-induced sedation, as methylphenidate promotes wakefulness, though fatigue was not studied specifically [81]. In a trial with 30 hospice patients in which the methylphenidate dose was titrated based on response and adverse effects, Kerr at al found that the drug improved fatigue in a dose-dependent manner [82]. However, a study in patients with CRF at the University of Texas MD Anderson Cancer Center found no significant difference in BFI scores between patients receiving methylphenidate and those receiving placebo at the end of 2 weeks of treatment [83]. Also, other RCTs in patients undergoing adjuvant chemotherapy for breast cancer [84] and patients receiving radiation therapy for brain tumors [85] failed to demonstrate the efficacy of methylphenidate in CRF. It should be used cautiously after ruling out other causes of fatigue. The drug is overall well tolerated and side effects include headache and nausea.
Modafinil is a non-amphetamine psychostimulant that has been approved for the treatment of narcolepsy. In a trial studying the effect of modafinil on patients receiving docetaxel-based chemotherapy for metastatic breast or prostate cancer, there was a modest but not statistically significant improvement in fatigue scores on the MD Anderson Symptom Inventory compared with placebo. Nausea and vomiting were higher in the modafinil arm than in the placebo arm [86]. Similarly, modafinil was not superior to placebo for CRF in 208 patients with non-squamous cell lung cancer not undergoing chemotherapy or radiation [87]. A placebo effect was also noted in patients with multiple myeloma [88] and patients with primary brain tumors [89]. In a phase 3, multicenter, randomized, placebo-controlled, double-blind clinical trial of modafinil for CRF in 867 patients undergoing chemotherapy, there was a reduction in fatigue only for patients with severe baseline fatigue, with no significant effect on mild to moderate fatigue [90]. In another recent study, modafinil was shown to reduce depressive symptoms only in patients with severe fatigue (BFI item 3 score ≥ 7) [91]. This finding is consistent with previous studies showing benefit in patients with high baseline fatigue, but additional RCTs are needed to provide clarity. NCCN guidelines do not recommend the use of modafinil to treat CRF [37].
Other pharmacologic interventions. Corticosteroids are often used for symptom control in cancer patients. These drugs have anti-inflammatory effects through their modulation of pro-inflammatory cytokines [92]. In a RCT evaluating the efficacy of corticosteroids, patients receiving dexamethasone (4 mg twice daily) experienced significant improvement in their FACT-F scores compared with patients receiving placebo [93]. A similar benefit in fatigue was demonstrated in another study of methylprednisolone (32 mg daily) versus placebo [94]. Despite the benefits of steroids, their adverse effects, such as mood swings, gastritis, hyperglycemia, and immune suppression, limit their long-term use. Therefore, the use of steroids should be restricted to terminally ill fatigued patients with other symptoms such as anorexia, brain metastasis, or pain related to bone metastasis [37].
Testosterone replacement has been shown to diminish fatigue in non-cancer patients. Many men with advanced cancer have hypogonadism leading to low serum testosterone, which may cause fatigue. In a small trial in which cancer patients with hypogonadism received intramuscular testosterone every 14 days or placebo, the group receiving testosterone showed improvement in FACT-F scores, but there was no significant difference in FACT-F scores between the 2 groups [95].
Antidepressants have failed to demonstrate benefit in CRF without depression [8]. However, if a patient has both fatigue and depression, antidepressants may help [96]. A selective serotonin receptor inhibitor is recommended as a first-line antidepressant [97]. Patients with cancer are often receiving multiple medications, and medication interactions should be considered to prevent adverse events such as serotonin syndrome.
Complementary and Alternative Supplements
Studies using vitamin supplementation have been inconclusive in patients with CRF [74]. The use of other dietary supplements has yielded mixed results, and coenzyme Q has shown no benefit for patients with CRF [98].
The benefit of ginseng was studied in a RCT involving 364 patients with CRF. There was an improvement in Multidimensional Fatigue Symptom Inventory-short form (MFSI-SF) scores at 8 weeks in patients receiving 2000 mg of Wisconsin ginseng compared with patients receiving placebo [99]. Patients on active treatment had greater improvement as compared to the post-treatment group in this trial. In another study of high-dose panax ginseng (ginseng root) at 800 mg daily for 29 days, patients had improvement of CRF as well as overall quality of life, appetite, and sleep at night. It was also well tolerated with few adverse effects [100]. Interaction with warfarin, calcium channel blockers, antiplatelet agents, thrombolytic agents, imatinib, and other agents may occur; therefore, ginseng must be used with careful monitoring in selected patients. There is not enough evidence at this time to support the routine use of ginseng in CRF.
The seed extract of the guarana plant (Paullinia cupana) traditionally has been used as a stimulant. An improvement in fatigue scores was seen with the use of oral guarana (100 mg daily) at the end of 21 days in breast cancer patients receiving chemotherapy [101]. Further studies are needed for these results to be generalized and to understand the adverse effects and interaction profile of guarana.
Re-evaluation
Patients who have completed cancer treatment must be monitored for fatigue over the long term, as fatigue may exist beyond the period of active treatment. Many studies have shown fatigue in breast cancer survivors, and fatigue has been demonstrated in survivors of colorectal, lung, and prostate cancers as well as myeloproliferative neoplasms [28]. Therefore, it is important to screen patients for fatigue during follow-up visits. There are currently no studies evaluating the long-term treatment of fatigue. In our experience, the timing of follow-up depends on the level of fatigue and interventions prescribed. Once fatigue is stabilized to a level with which the patient is able to cope, the time interval for follow up may be lengthened. Annual visits may suffice in patients with mild fatigue. Follow-up of patients with moderate to severe fatigue depends on the level of fatigue, the ability to cope, choice of treatment, and presence of contributing factors.
Conclusion
CRF is a complex condition that places a significant burden on patients and caregivers, resulting in emotional distress, poor functioning, and suffering. Fatigue can occur before, during, and long after cancer treatment. The approach to CRF begins with screening for and educating patients and their caregivers about the symptoms. Many screening scales are available that may be used to follow patients’ progress over time. The evaluation and management of contributing conditions may help improve fatigue. If the fatigue persists, an individualized approach with a combination of nonpharmacologic and pharmacologic approaches should be considered. More research is needed to understand brain signaling pathways, cytokine changes, and genomic changes in cancer patients with fatigue. Though many hypotheses have been proposed, to date there is no biological marker to assess this condition. Biomarker research needs to be advanced to help to identify patients at risk for fatigue. As cytokines have a major role in CRF, targeted therapy to block cytokine pathways may also be explored in the future.
Acknowledgment: Bryan Tutt provided editorial assistance.
Corresponding author: Carmelita P. Escalante, MD, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Houston, TX 77030, [email protected].
Financial disclosures: None.
1. Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016;122:477–85.
2. Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013;18:1135–43.
3. Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients -- an EAPC approach. Palliat Med 2008;22:13–32.
4. Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 2007;32:2384–92.
5. Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One 2013;8:e83636.
6. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 2005;67:277–80.
7. Barsevick A, Frost M, Zwinderman A, et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 2010;19:1419–27.
8. Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol 2003;21:4635–41.
9. Fontes-Oliveira CC, Busquets S, Toledo M, et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta 2013;1830:2770–8.
10. Agteresch HJ, Dagnelie PC, van der Gaast A, et al. Randomized clinical trial of adenosine 5’-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2000;92:321–8.
11. Beijer S, Hupperets PS, van den Borne BE, et al. Randomized clinical trial on the effects of adenosine 5’-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage 2010;40:520–30.
12. Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44:351–61.
13. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist 2007;12 Suppl 1:22–34.
14. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887–99.
15. Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:1319–28.
16. Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006;12:2759–66.
17. Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer 2008;113:2102–9.
18. Inagaki M, Isono M, Okuyama T, et al. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage 2008;35:153–61.
19. Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5.
20. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29:3517–22.
21. Whistler T, Taylor R, Craddock RC, et al. Gene expression correlates of unexplained fatigue. Pharmacogenomics 2006;7:395–405.
22. Fagundes CP, Bennett JM, Alfano CM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychol 2012;31:11–19.
23. Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J 2009;9:333–40.
24. Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun 2014;38:227–36.
25. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015;121:2129–36.
26. Mills PJ, Parker B, Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 2005;69:85–96.
27. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 Suppl 1:11–21.
28. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014;120:425–32.
29. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186–96.
30. Mendoza ME, Capafons A, Gralow JR, et al. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psychooncology 2016 Jul 28.
31. Glaus A. Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer 1993;1:305–15.
32. Seyidova-Khoshknabi D, Davis MP, Walsh D. A systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care 2011;28:119–29.
33. Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol 2002;13:965–73.
34. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004;27:14–23.
35. Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 2003;26:604–14.
36. Peterspm DR. Scope and generality of verbally defined personality factors. Psychol Rev 1965;72:48–59.
37. Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw 2010;8:904–31.
38. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002;95:888–95.
39. Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
40. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010;116:4045–59.
41. Preston NJ, Hurlow A, Brine J, Bennett MI. Blood transfusions for anaemia in patients with advanced cancer. Cochrane Database Syst Rev 2012(2):CD009007.
42. Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care 2012;2:231–8.
43. Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer 2016;24:2059–66.
44. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791–800.
45. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12.
46. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med 2014;15:385–92.
47. Strasser F, Palmer JL, Schover LR, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 2006;107:2949–57.
48. Agasi-Idenburg SC, Thong MS, Punt CJ, et al. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer 2017;25:625–32.
49. Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs 2007;23:99–105.
50. de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol 2013;31:716–23.
51. Barsevick AM, Whitmer K, Sweeney C, Nail LM. A pilot study examining energy conservation for cancer treatment-related fatigue. Cancer Nurs 2002;25:333–41.
52. Barsevick AM, Dudley W, Beck S, et a;. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004;100:1302–10.
53. Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31:424–30.
54. Abrahams HJ, Gielissen MF, Goedendorp MM, et al. A randomized controlled trial of web-based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE-study): study protocol. BMC Cancer 2015;15:765.
55. Quesnel C, Savard J, Simard S, et al. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003;71:189–200.
56. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009(1):CD006953.
57. Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82.
58. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;(9):CD005001.
59. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;(11):CD006145.
60. Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33.
61. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014;25:2237–43.
62. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50.
63. Kenjale AA, Hornsby WE, Crowgey T, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist 2014;19:999–1005.
64. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage 2006;31:421–30.
65. Porock D, Kristjanson LJ, Tinnelly K, et al. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000;16:30–6.
66. Lengacher CA, Kip KE, Reich RR, et al. A cost-effective mindfulness stress reduction program: a randomized control trial for breast cancer survivors. Nursing Econ 2015;33:210–8, 32.
67. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med 2012;35:86–94.
68. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol 2015;6:8–14.
69. Wang G, Wang S, Jiang P, Zeng C. [Effect of Yoga on cancer related fatigue in breast cancer patients with chemotherapy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1077–82.
70. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer 2016;24:4005–15.
71. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med 2015;49:165–76.
72. Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470–6.
73. Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer 2013;21:1735–41.
74. Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90.
75. Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum 2002;29:E85–90.
76. Spathis A, Dhillan R, Booden D, et al. Modafinil for the treatment of fatigue in lung cancer: a pilot study. Palliat Med 2009;23:325–31.
77. Blackhall L, Petroni G, Shu J, et al. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med 2009;12:433–9.
78. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl) 2015;25:970–9.
79. Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010(7):CD006704.
80. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010;28:3673–9.
81. Bruera E, Driver L, Barnes EA, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol 2003;21:4439–43.
82. Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012;43:68–77.
83. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J 2014;20:8–14.
84. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 2008;16:577–83.
85. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007;69:1496–501.
86. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer 2014;22:1233–42.
87. Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol 2014;32:1882–8.
88. Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer 2015;23:1503–12.
89. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol 2013;15:1420–8.
90. Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010;116:3513–20.
91. Conley CC, Kamen CS, Heckler CE, et al. Modafinil moderates the relationship between cancer-related fatigue and depression in 541 patients receiving chemotherapy. J Clin Psychopharmacol 2016;36:82–5.
92. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 Suppl 2:81–90.
93. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol 2013;31:3076–82.
94. Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751–4.
95. Pulivarthi K, Dev R, Garcia J, et al. Testosterone replacement for fatigue in male hypogonadic patients with advanced cancer: A preliminary double-blind placebo-controlled trial. J Clin Oncol 2012;30 (suppl). Abstract e19643.
96. Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012;13:1184–90.
97. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in Cancer. Curr Psychiatry Rep 2014;17:529.
98. Lesser GJ. Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol 2013;11:31–42.
99. Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 2013;105:1230–8.
100. Yennurajalingam S, Reddy A, Tannir NM, et al. High-dose Asian ginseng (panax ginseng) for cancer-related fatigue: a preliminary report. Integr Cancer Ther 2015;14:419–27.
101. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233–46.
1. Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016;122:477–85.
2. Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013;18:1135–43.
3. Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients -- an EAPC approach. Palliat Med 2008;22:13–32.
4. Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 2007;32:2384–92.
5. Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One 2013;8:e83636.
6. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 2005;67:277–80.
7. Barsevick A, Frost M, Zwinderman A, et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 2010;19:1419–27.
8. Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol 2003;21:4635–41.
9. Fontes-Oliveira CC, Busquets S, Toledo M, et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta 2013;1830:2770–8.
10. Agteresch HJ, Dagnelie PC, van der Gaast A, et al. Randomized clinical trial of adenosine 5’-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2000;92:321–8.
11. Beijer S, Hupperets PS, van den Borne BE, et al. Randomized clinical trial on the effects of adenosine 5’-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage 2010;40:520–30.
12. Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44:351–61.
13. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist 2007;12 Suppl 1:22–34.
14. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887–99.
15. Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:1319–28.
16. Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006;12:2759–66.
17. Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer 2008;113:2102–9.
18. Inagaki M, Isono M, Okuyama T, et al. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage 2008;35:153–61.
19. Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5.
20. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29:3517–22.
21. Whistler T, Taylor R, Craddock RC, et al. Gene expression correlates of unexplained fatigue. Pharmacogenomics 2006;7:395–405.
22. Fagundes CP, Bennett JM, Alfano CM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychol 2012;31:11–19.
23. Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J 2009;9:333–40.
24. Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun 2014;38:227–36.
25. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015;121:2129–36.
26. Mills PJ, Parker B, Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 2005;69:85–96.
27. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 Suppl 1:11–21.
28. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014;120:425–32.
29. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186–96.
30. Mendoza ME, Capafons A, Gralow JR, et al. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psychooncology 2016 Jul 28.
31. Glaus A. Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer 1993;1:305–15.
32. Seyidova-Khoshknabi D, Davis MP, Walsh D. A systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care 2011;28:119–29.
33. Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol 2002;13:965–73.
34. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004;27:14–23.
35. Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 2003;26:604–14.
36. Peterspm DR. Scope and generality of verbally defined personality factors. Psychol Rev 1965;72:48–59.
37. Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw 2010;8:904–31.
38. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002;95:888–95.
39. Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
40. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010;116:4045–59.
41. Preston NJ, Hurlow A, Brine J, Bennett MI. Blood transfusions for anaemia in patients with advanced cancer. Cochrane Database Syst Rev 2012(2):CD009007.
42. Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care 2012;2:231–8.
43. Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer 2016;24:2059–66.
44. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791–800.
45. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12.
46. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med 2014;15:385–92.
47. Strasser F, Palmer JL, Schover LR, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 2006;107:2949–57.
48. Agasi-Idenburg SC, Thong MS, Punt CJ, et al. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer 2017;25:625–32.
49. Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs 2007;23:99–105.
50. de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol 2013;31:716–23.
51. Barsevick AM, Whitmer K, Sweeney C, Nail LM. A pilot study examining energy conservation for cancer treatment-related fatigue. Cancer Nurs 2002;25:333–41.
52. Barsevick AM, Dudley W, Beck S, et a;. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004;100:1302–10.
53. Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31:424–30.
54. Abrahams HJ, Gielissen MF, Goedendorp MM, et al. A randomized controlled trial of web-based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE-study): study protocol. BMC Cancer 2015;15:765.
55. Quesnel C, Savard J, Simard S, et al. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003;71:189–200.
56. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009(1):CD006953.
57. Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82.
58. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;(9):CD005001.
59. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;(11):CD006145.
60. Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33.
61. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014;25:2237–43.
62. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50.
63. Kenjale AA, Hornsby WE, Crowgey T, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist 2014;19:999–1005.
64. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage 2006;31:421–30.
65. Porock D, Kristjanson LJ, Tinnelly K, et al. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000;16:30–6.
66. Lengacher CA, Kip KE, Reich RR, et al. A cost-effective mindfulness stress reduction program: a randomized control trial for breast cancer survivors. Nursing Econ 2015;33:210–8, 32.
67. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med 2012;35:86–94.
68. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol 2015;6:8–14.
69. Wang G, Wang S, Jiang P, Zeng C. [Effect of Yoga on cancer related fatigue in breast cancer patients with chemotherapy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1077–82.
70. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer 2016;24:4005–15.
71. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med 2015;49:165–76.
72. Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470–6.
73. Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer 2013;21:1735–41.
74. Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90.
75. Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum 2002;29:E85–90.
76. Spathis A, Dhillan R, Booden D, et al. Modafinil for the treatment of fatigue in lung cancer: a pilot study. Palliat Med 2009;23:325–31.
77. Blackhall L, Petroni G, Shu J, et al. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med 2009;12:433–9.
78. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl) 2015;25:970–9.
79. Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010(7):CD006704.
80. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010;28:3673–9.
81. Bruera E, Driver L, Barnes EA, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol 2003;21:4439–43.
82. Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012;43:68–77.
83. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J 2014;20:8–14.
84. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 2008;16:577–83.
85. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007;69:1496–501.
86. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer 2014;22:1233–42.
87. Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol 2014;32:1882–8.
88. Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer 2015;23:1503–12.
89. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol 2013;15:1420–8.
90. Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010;116:3513–20.
91. Conley CC, Kamen CS, Heckler CE, et al. Modafinil moderates the relationship between cancer-related fatigue and depression in 541 patients receiving chemotherapy. J Clin Psychopharmacol 2016;36:82–5.
92. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 Suppl 2:81–90.
93. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol 2013;31:3076–82.
94. Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751–4.
95. Pulivarthi K, Dev R, Garcia J, et al. Testosterone replacement for fatigue in male hypogonadic patients with advanced cancer: A preliminary double-blind placebo-controlled trial. J Clin Oncol 2012;30 (suppl). Abstract e19643.
96. Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012;13:1184–90.
97. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in Cancer. Curr Psychiatry Rep 2014;17:529.
98. Lesser GJ. Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol 2013;11:31–42.
99. Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 2013;105:1230–8.
100. Yennurajalingam S, Reddy A, Tannir NM, et al. High-dose Asian ginseng (panax ginseng) for cancer-related fatigue: a preliminary report. Integr Cancer Ther 2015;14:419–27.
101. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233–46.