User login
Primary Total Knee Arthroplasty for Distal Femur Fractures: A Systematic Review of Indications, Implants, Techniques, and Results
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
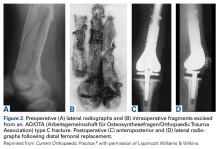
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
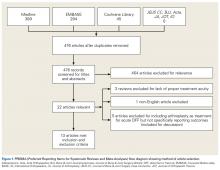
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
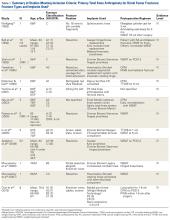
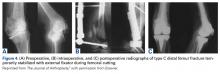
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
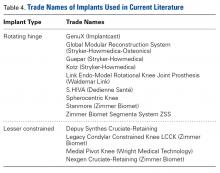
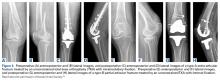
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
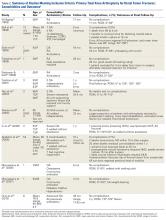
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
Endometriosis: From Identification to Management
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
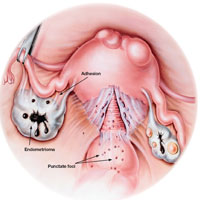
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
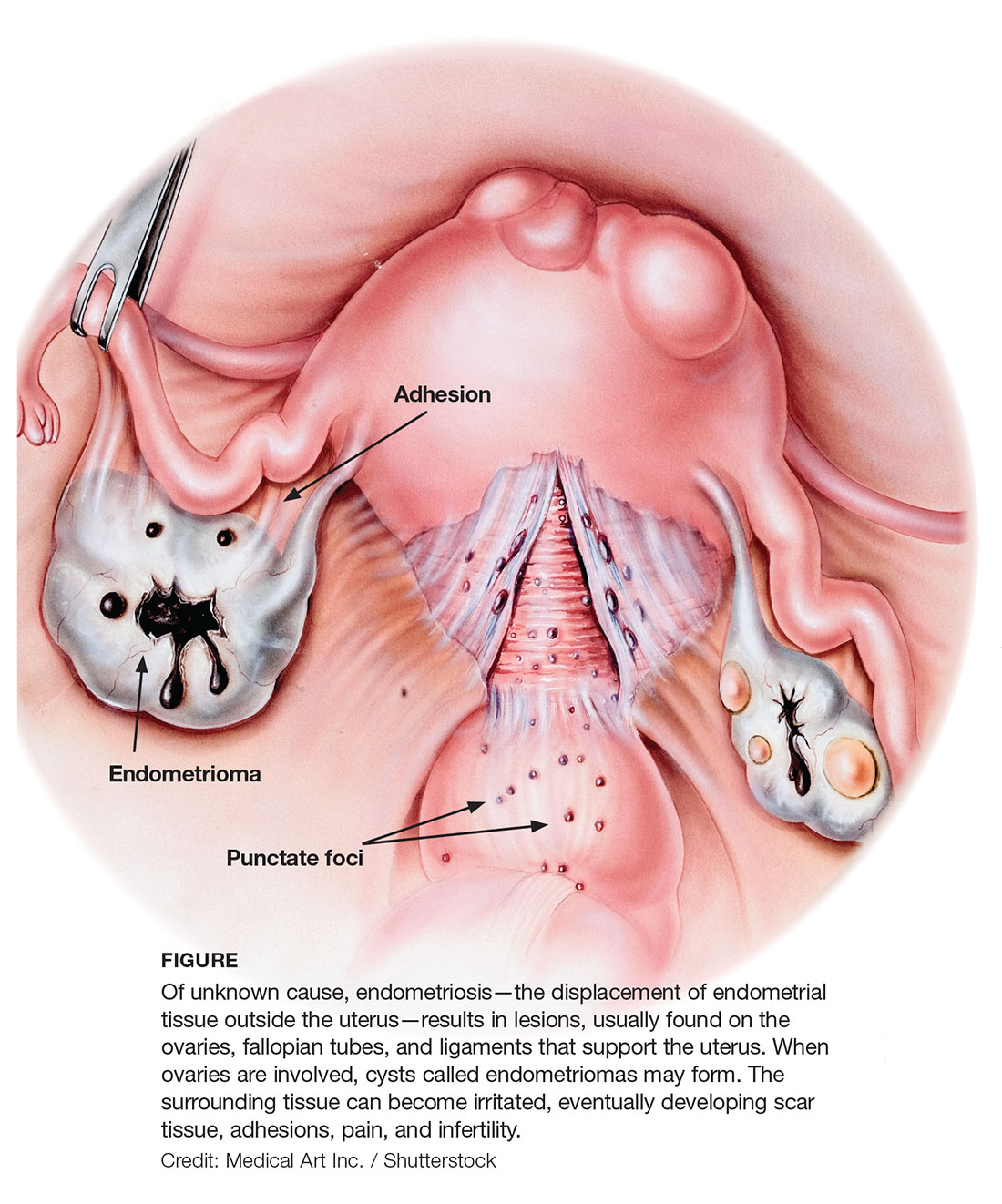
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
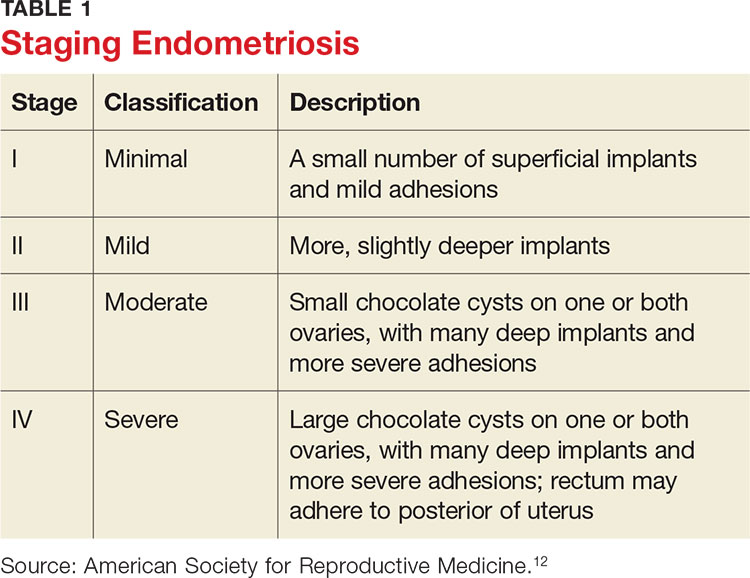
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
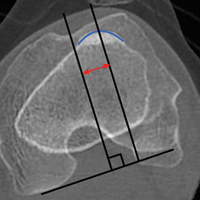
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
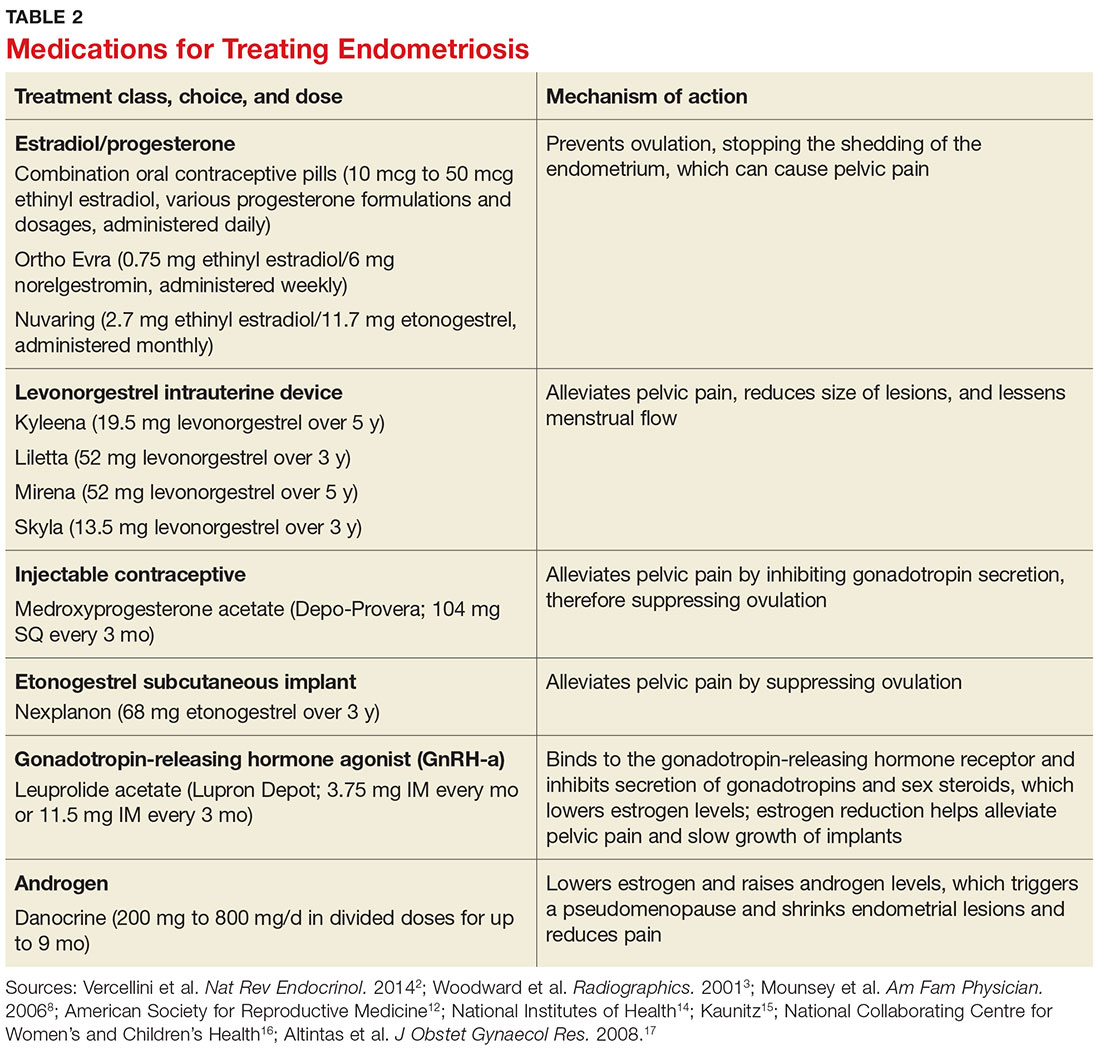
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3

Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12

DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.

Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3

Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12

DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.

Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
2017 Update on cervical disease
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
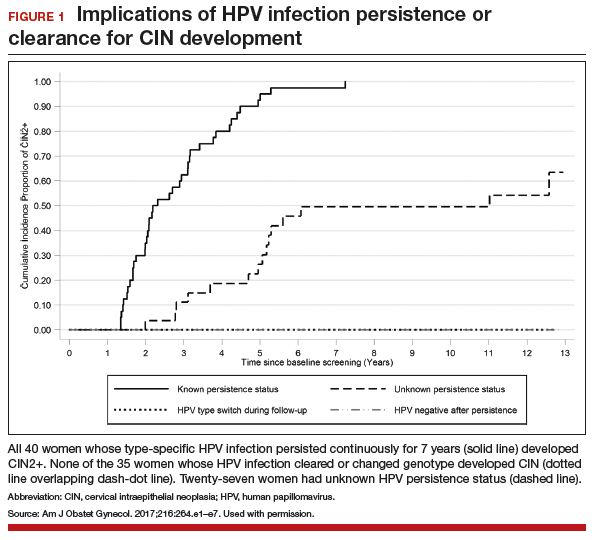
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
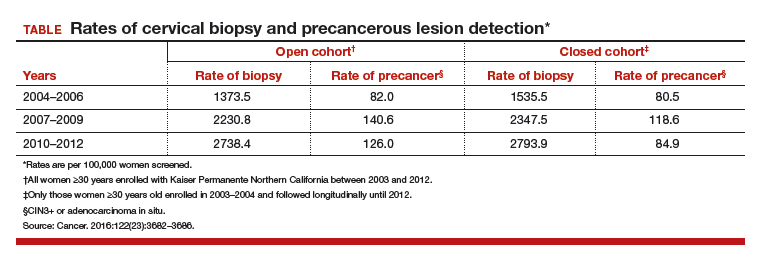
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.

Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).

In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.

Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).

In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
Recognizing and Managing Elder Abuse in the Emergency Department
Case
An 85-year-old right-handed woman who recently had been diagnosed with mild cognitive impairment arrived at the ED via emergency medical services (EMS) for evaluation of a reported fall. She was accompanied by her daughter, who resided with the patient and was her primary caregiver. The patient stated that she had tripped on a wet rug in the bathroom of her home, striking her head and face on the edge of the sink without losing consciousness. Her daughter reported that she was not assisting her mother when the fall occurred, but had witnessed the fall from the hallway and called EMS. At the patient’s home, EMS found the patient to be alert, oriented, and ambulatory with normal vital signs that remained stable throughout prehospital transport.
The remainder of the patient’s history was provided almost entirely by her daughter, who constantly interrupted her mother whenever she attempted to directly answer a question or provide information. On physical examination, the patient had bilateral tenderness, edema, and periorbital ecchymoses, and a left eye that was nearly swollen shut. Extraocular movements were normal, visual acuity was intact, and sclerae were noninjected. The patient had tenderness over both maxillary sinuses, and edema and ecchymosis of her left cheek. There was also tenderness, ecchymoses, and edema on the lateral aspects of both forearms, and decreased range of motion of her right lower arm and wrist. With the exception of the patient not knowing the date during the orientation part of the thorough neurological examination, the remainder of the physical examination was unremarkable.
Radiological evaluation found no evidence of traumatic brain injury, but did reveal an acute fracture of the left zygomatic arch, an acute displaced nasal bone fracture, an age-indeterminate fracture of the right zygomatic arch, and an acute right ulnar fracture. Considering all of these findings, particularly the pattern of acute injuries, the emergency physician (EP) considered elder abuse as the possible etiology of the patient’s acute and chronic injuries.
Although the patient had initially agreed with her daughter’s description of the events—including her claim that she had fallen—when the EP questioned the patient alone, she related a history of frequent verbal and less frequent physical abuse by her daughter. The patient further noted that immediately before sustaining the injuries that brought her to the ED, her daughter had been insisting that she sign documents to give her control of her banking and finances. After refusing to sign the papers, the patient said that she and her daughter got into an argument, which she noted “they tended to do frequently.” The patient admitted that during this argument, her daughter struck her in the face repeatedly with the cane that the daughter had grabbed with her right hand.
The EP admitted the patient to the hospital for management of her orthopedic injuries and related pain, and to formulate a safe discharge plan. During admission, additional diagnostic testing revealed multiple old rib fractures, anemia, and a low-serum albumin, which suggested poor nutritional status.
Epidemiology
The term elder abuse refers to harm or the risk of harm to an older adult from either action or negligence committed by someone in a relationship of trust, or when a victim has been targeted because of age or disability. Elder abuse encompasses physical, sexual, or psychological abuse, neglect, and financial exploitation.1-5 Identified victims of elder abuse typically suffer from multiple forms of abuse.1-5
At present, elder abuse annually affects 5% to 10% of community-dwelling older adults,1-6 and nursing-home residents are at increased risk of abuse.7-10 Poor medical outcomes, including depression and dementia,11 and much higher mortality6,12,13 have been linked to victims of elder abuse.
Etiology
When treating older adults, it is critically important for EPs and the ED staff to consider and identify elder abuse in the differential diagnosis.14,15 Presently, only an estimated 1 in 24 cases of abuse is recognized and reported to the authorities,2 and much of the subsequent morbidity and mortality of elder abuse results from poor detection. A visit to the ED for an acute injury or illness may be the only time socially isolated older adults leave their homes.15-17 Additionally, the ED setting is uniquely suited to identify mistreatment, as a patient typically may be evaluated for several hours by providers from multiple disciplines who are able to observe, interact with, and examine the patient.15 The ED already exercises a similar essential role in the identification and initial intervention for both child abuse18,19 and intimate partner violence among younger adults.20,21
Recognition
Unfortunately, at present, ED providers rarely recognize and report elder abuse.22-24 Though the reasons for this are not entirely understood, inadequate training, lack of time and space to conduct complete evaluations, reluctance to become involved with the legal system, and challenges to distinguishing intentional from unintentional injuries may be contributing factors.24,25 A focus on improving EP and ED staff approaches to elder abuse is relevant and timely given the growing elderly population.
Risk Factors
When evaluating elderly patients, providers should consider research suggesting that some older adults may be at particularly high risk for abuse.4,26-29 Notably, individuals who have cognitive impairment are more likely to be victims of abuse.30-32 Health-related demographic characteristics such as poor physical and mental health, substance abuse, low income/socioeconomic status, and social isolation all may increase the potential for mistreatment.
Family History
Similar to situations resulting in intimate partner violence, a family history of abuse and exposure to traumatic events may increase risk, and those responsible for elder abuse often turn out to be spouses, romantic partners, or an adult child living with the elderly parent—though paid caregivers also can be abusive.
Suspicion of abuse should be increased when individuals in caregiving roles have a history or show signs of mental illness, substance abuse, financial dependence on the victim, or caregiver stress. Considering that a caregiver may be overwhelmed is particularly relevant when an elderly patient exhibits behavioral issues.
Medical History
Obtaining a clear and thorough medical history from the patient and caregiver, both together and alone, is paramount to assessing the potential for abuse. Many indicators from the history may suggest the possibility of mistreatment (Table 1)33-37 and although challenging in a busy ED, a comprehensive head-to-toe examination is crucial to adequately assess abuse. Suspicious physical findings and injury patterns of physical abuse, sexual abuse, and neglect are listed in Table 2.33-37 Ongoing research is aimed at improving ED providers’ ability to differentiate accidental injuries, such as fall injuries, from injuries caused by physical elder abuse.
Injury Patterns
Preliminary studies have indicated that physical abuse injuries most commonly occur on the head, neck, and upper extremities.38,39 A study comparing abuse victims to accidental injury sufferers found that abuse victims often had large bruises (>5 cm) on the face, lateral right arm, or posterior torso.40 Preliminary results from a study in progress suggest that injuries to the left periorbital area, neck, and ulnar forearm may be much more common in abuse than in accident.
Imaging Studies
Emergency radiologists are contributing additional concerning findings indicative of elder abuse,38,41,42 such as the concomitant presence of old and new fractures, high-energy fractures inconsistent with the purported mechanism, and distal ulnar diaphyseal fractures.41,42 The ultimate goal is to identify pathognomonic injury patterns similar to those found in child abuse cases, to assist ED providers.
Laboratory Studies
Although there are no laboratory tests to definitively identify abuse or neglect, specific findings that may indicate abuse include anemia, dehydration, malnutrition, hypothermia/hyperthermia, and rhabdomyolysis.43 In addition, inappropriately high- or low-medication levels and the presence of illicit drugs, which are not often checked in elderly patients in the ED, may be a sign of abuse.43
Laboratory studies that reveal undetectable levels of a patient’s prescription medications may indicate a caregiver’s intentional or neglectful withholding of such medications—especially diversion of opioid medications prescribed for painful conditions.43 Likewise, elevated levels of prescribed drugs may point to intentional or unintentional overdose, whereas the presence of nonprescribed drugs or toxins may suggest poisoning.43
Screening Tools
To improve identification of elder abuse in the ED, universal or targeted screening tools are under consideration. Though several screening tools for elder abuse are already available, none have been validated in the ED.15,44,45 Research sponsored by the National Institute of Justice to identify an ED-specific screening tool is ongoing.15
Elder Abuse Suspicion Index
The Elder Abuse Suspicion Index (EASI) is a short screening tool that has been validated for cognitively intact patients being treated in family practice and ambulatory care settings, and may be used in EDs.44 The tool comprises six questions: five for patient response, and a sixth question for clinician response. This tool is available at http://www.nicenet.ca/tools-easi-elder-abuse-suspicion-index.46
Interventional Measures
When elder mistreatment is suspected or confirmed, health care providers must first address any acute medical, traumatic, or psychological issues. Bleeding, orthopedic injuries, metabolic abnormalities, infections, and agitation must be treated and/or stabilized, while neglected or inappropriately managed chronic medical conditions may require treatment.
Hospitalization should be considered for an older adult who needs extended treatment or observation and, in cases of immediate or continued danger of abuse, separation from contact with the suspected abuser. These measures present several challenges, particularly if the suspected abuser is the patient’s health care proxy, in which case early involvement of the hospital’s legal department, social services, and administration may be necessary—especially in navigating the guardianship process.
Engaging security also may be necessary if the patient requires one-to-one patient watch or when the perpetrator must be removed from the ED. Social workers, patient services representatives, and law enforcement officials should be informed when such intervention is necessary.
In instances when a patient is not at risk of immediate harm, interventions can be more individualized. Coordination with primary care physicians (PCPs) must also be facilitated prior to discharge, to ensure consistent longitudinal follow-up care, and social workers should provide any needed out-of-hospital resources to the patient—and caregiver—such as Meals-on-Wheels, medical transportation services, adult day care/senior center participation, and substance abuse treatment.
Patient Decision-Making Capacity
When a patient experiencing abuse declines interventions or services, the EP must evaluate the patient’s decision-making capacity. In unclear cases, a psychiatric evaluation can help to assess decision-making capacity. If the victim is deemed to have capacity with regard to care and/or discharge, the patient’s choice of returning to an unsafe environment must be respected, as is true in instances of intimate partner violence among younger adults—but not in child abuse cases. In such situations, the EP should nevertheless discuss safety planning, offer psychoeducation about violence and abuse, suggest appropriate community referrals, and encourage abused patients to return or call a contact person whenever they desire or feel the need to talk further. For a victim who is deemed not to possess capacity, providers should proceed with treatment considered to be in the best interest of the patient.
Reporting Abuse
Emergency department providers should notify the appropriate authorities when elder abuse is suspected or identified. A report may be made to the local Adult Protective Services (APS), but this agency operates much differently than Child Protective Services. Case workers with APS will not open a case while a patient is in the ED or hospital, as it is deemed a safe environment and any investigation they undertake will only commence upon discharge. Because of this, contacting the local police department prior to discharge should be considered.
Mandatory elder abuse-reporting laws vary from state to state. Health care providers should therefore contact their respective state or city department of health to obtain local legislation.
Multidisciplinary Approach
Ideally, a multidisciplinary, ED-based intervention team modeled on child abuse teams18,19 would help to optimize treatment and ensure the safety and treatment of vulnerable older adults. These teams could conduct thorough medical, forensic, and social work assessments, allowing ED providers to attend to other patients. The team could also assist in arranging for appropriate and safe dispositions. An innovative Vulnerable Elder Protection Team was recently launched at New York-Presbyterian Weill Cornell Medical Center to provide these services, and its impact is currently being evaluated.
Case Conclusion
The EP who treated the patient realized that blows from a blunt object held by a right-handed person would tend to land on the left side of the victim’s face and upper torso, and that a right-handed victim who successfully blocked the blows intended for her face would instead sustain an isolated right ulna or radius midshaft fracture. These findings, together with the concomitant presence of both old and new fractures, led the EP to question the patient alone and, after obtaining a different history of the events that led to the injuries, admit her for further evaluation, treatment, and interventions to prevent continuing abuse.
Summary
Elder abuse has the potential to affect an increasing number of older adults in this growing population, and an ED visit may offer the only opportunity to identify victims and provide intervention, in turn reducing morbidity and mortality. The results of ongoing research will improve the ability of EPs and ED staff to accurately assess the presence or risk of elder abuse and respond more effectively. It is essential that EPs always consider elder abuse and neglect as a possible etiology when evaluating injuries in this population. Moreover, when identified, addressing elder mistreatment may dramatically improve quality of life or save the lives of these vulnerable patients.
1. Elder Mistreatment: Abuse, Neglect, and Exploitation in an Aging America. Bonnie RJ, Wallace RB, eds. Washington, DC: National Academies Press; 2003:1-552. https://www.nap.edu/read/10406/chapter/1. Accessed April 4, 2017.
2. Lifespan of Greater Rochester, Inc; Weill Cornell Medical Center of Cornell University; New York City Department for the Aging. Under the radar: New York state elder abuse prevalence study: self-reported prevalence and documented case surveys 2011.http://ocfs.ny.gov/main/reports/Under%20the%20Radar%2005%2012%2011%20final%20report.pdf. Published May 2011. Accessed April 4, 2017.
3. Connolly MT, Brandl B, Breckman R. The Elder Justice Roadmap: A Stakeholder Initiative to Respond to an Emerging Health, Justice, Financial, and Social Crisis. https://www.justice.gov/elderjustice/file/829266/download. National Center for Elder Abuse. Published January 2014. Accessed April 4, 2017.
4. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297. doi:10.2105/AJPH.2009.163089.
5. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272. doi:10.1016/S0140-6736(04)17144-4.
6. Lachs MS, Pillemer KA. Elder abuse. N Engl J Med. 2015;373(20):1947-1956. doi:10.1056/NEJMra1404688.
7. Ortmann C, Fechner G, Bajanowski T, Brinkmann B. Fatal neglect of the elderly. Int J Legal Med. 2001;114(3):191-193.
8. Schiamberg LB, Oehmke J, Zhang Z, et al. Physical abuse of older adults in nursing homes: a random sample survey of adults with an elderly family member in a nursing home. J Elder Abuse Negl. 2012;24(1):65-83. doi:10.1080/08946566.2011.608056.
9. Rosen T, Pillemer K, Lachs M. Resident-to-resident aggression in long-term care facilities: an understudied problem. Aggress Violent Behav. 2008;13(2):77-87. doi:10.1016/j.avb.2007.12.001.
10. Shinoda-Tagawa T, Leonard R, Pontikas J, McDonough JE, Allen D, Dreyer PI. Resident-to-resident violent incidents in nursing homes. JAMA. 2004;291(5):591-598. doi:10.1001/jama.291.5.591.
11. Dyer CB, Pavlik VN, Murphy KP, Hyman DJ. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
12. Lachs MS, Williams CS, O’Brien S, Pillemer KA, Charlson ME. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
13. Dong XQ, Simon MA, Beck TT, et al. Elder abuse and mortality: the role of psychological and social wellbeing. Gerontology. 2011;57(6):549-658. doi:10.1159/000321881.
14. Stevens TB, Richmond NL, Pereira GF, Shenvi CL, Platts-Mills TF. Prevalence of nonmedical problems among older adults presenting to the emergency department. Acad Emerg Med. 2014;21(6):651-658. doi:10.1111/acem.12395.
15. Rosen T, Hargarten S, Flomenbaum NE, Platts-Mills TF. Identifying elder abuse in the emergency department: toward a multidisciplinary team-based approach. Ann Emerg Med. 2016;68(3):378-382. doi:10.1016/j.annemergmed.2016.01.037.
16. Bond MC, Butler KH. Elder abuse and neglect: definitions, epidemiology, and approaches to emergency department screening. Clin Geriatr Med. 2013;29(1):257-273. doi:10.1016/j.cger.2012.09.004.
17. Heyborne RD. Elder abuse: keeping the unthinkable in the differential. Acad Emerg Med. 2007;14(6):566-567. doi:10.1197/j.aem.2007.01.015.
18. Kistin CJ, Tien I, Bauchner H, Parker V, Leventhal JM. Factors that influence the effectiveness of child protection teams. Pediatrics. 2010;126(1):94-100. doi:10.1542/peds.2009-3446.
19. Hochstadt NJ, Harwicke NJ. How effective is the multidisciplinary approach? A follow-up study. Child Abuse Negl. 1985;9(3):365-372.
20. Choo EK, Gottlieb AS, DeLuca M, Tape C, Colwell L, Zlotnick C. Systematic review of ED-based intimate partner violence intervention research. West J Emerg Med. 2015;16(7):1037-1042. doi:10.5811/westjem.2015.10.27586.
21. Rhodes KV, Rodgers M, Sommers M, et al. Brief motivational intervention for intimate partner violence and heavy drinking in the emergency department: a randomized clinical trial. JAMA. 2015;314(5):466-477. doi:10.1001/jama.2015.8369.
22. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 survey of state adult protective services: abuse of adults 60 years of age and older. http://www.napsa-now.org/wp-content/uploads/2012/09/2-14-06-FINAL-60+REPORT.pdf. Published February 2006. Accessed March 10, 2017.
23. Blakely BE, Dolon R. Another look at the helpfulness of occupational groups in the discovery of elder abuse and neglect. J Elder Abuse Negl. 2003;13:1-23.
24. Evans CS, Hunold KM, Rosen T, Platts-Mills TF. Diagnosis of elder abuse in U.S. emergency departments. J Am Geriatr Soc. 2017;65(1):91-97. doi:10.1111/jgs.14480.
25. Jones JS, Veenstra TR, Seamon JP, Krohmer J. Elder mistreatment: national survey of emergency physicians. Ann Emerg Med. 1997;30(4):473-479.
26. Amstadter AB, Zajac K, Strachan M, Hernandez MA, Kilpatrick DG, Acierno R. Prevalence and correlates of elder mistreatment in South Carolina: the South Carolina elder mistreatment study. J Interpers Violence. 2011;26(15):2947-2972. doi:10.1177/0886260510390959.
27. Friedman LS, Avila S, Tanouye K, Joseph K. A case-control study of severe physical abuse of older adults. J Am Geriatr Soc. 2011;59(3):417-422. doi:10.1111/j.1532-5415.2010.03313.x.
28. Pillemer K, Burnes D, Riffin C, Lachs MS. Elder Abuse: global situation, risk factors, and prevention strategies. Gerontologist. 2016;56 Suppl 2:S194-S205. doi:10.1093/geront/gnw004.
29. Laumann EO, Leitsch SA, Waite LJ. Elder mistreatment in the United States: prevalence estimates from a nationally representative study. J Gerontol B Psychol Sci Soc Sci. 2008;63(4):S248-S254.
30. Cooney C, Howard R, Lawlor B. Abuse of vulnerable people with dementia by their carers: can we identify those most at risk? Int J Geriatr Psychiatry. 2006;21(6):564-571. doi:10.1002/gps.1525.
31. Lachs MS, Williams C, O’Brien S, Hurst L, Horwitz R. Risk factors for reported elder abuse and neglect: a nine-year observational cohort study. Gerontologist. 1997;37(4):469-474.
32. Wiglesworth A, Mosqueda L, Mulnard R, Liao S, Gibbs L, Fitzgerald W. Screening for abuse and neglect of people with dementia. J Am Geriatr Soc. 2010;58(3):493-500. doi:10.1111/j.1532-5415.2010.02737.x.
33. Collins KA. Elder maltreatment: a review. Arch Pathol Lab Med. 2006;130(9):1290-1296. doi:10.1043/1543-2165(2006)130[1290:EMAR]2.0.CO;2.
34. Gibbs LM. Understanding the medical markers of elder abuse and neglect: physical examination findings. Clin Geriatr Med. 2014 Nov;30(4):687-712. doi:10.1016/j.cger.2014.08.002.
35. Palmer M, Brodell RT, Mostow EN. Elder abuse: dermatologic clues and critical solutions. J Am Acad Dermatol. 2013;68(2):e37-e42. doi:10.1016/j.jaad.2011.03.016.
36. Speck PM, Hartig MT, Likes W, et al. Case series of sexual assault in older persons. Clin Geriatr Med. 2014;30(4):779-806. doi:10.1016/j.cger.2014.08.007.
37. Chang AL, Wong JW, Endo JO, Norman RA. Geriatric dermatology: part II. Risk factors and cutaneous signs of elder mistreatment for the dermatologist. J Am Acad Dermatol. 2013;68(4):533.e1-.e10. doi:10.1016/j.jaad.2013.01.001.
38. Murphy K, Waa S, Jaffer H, Sauter A, Chan A. A literature review of findings in physical elder abuse. Can Assoc Radiol J. 2013;64(1):10-14. doi:10.1016/j.carj.2012.12.001.
39. Rosen T, Bloemen EM, LoFaso VM, Clark S, Flomenbaum NE, Lachs MS. Emergency department presentations for injuries in older adults independently known to be victims of elder abuse. J Emerg Med. 2016;50(3):518-526. doi:10.1016/j.jemermed.2015.10.037.
40. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196. doi:10.1111/j.1532-5415.2009.02330.x.
41. Rosen T, Bloemen EM, Harpe J, et al. Radiologists’ training, experience, and attitudes about elder abuse detection. AJR Am J Roentgenol. 2016;207:1210-1214.
42. Wong NZ, Rosen T, Sanchez AM, et al. Imaging findings in elder abuse: a role for radiologists in detection. Can Assoc Radiol J. 2017;68(1):16-20. doi:10.1016/j.carj.2016.06.001.
43. LoFaso VM, Rosen T. Medical and laboratory indicators of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):713-28. doi:10.1016/j.cger.2014.08.003.
44. Fulmer T, Guadagno L, Bitondo Dyer C, Connolly MT. Progress in elder abuse screening and assessment instruments. J Am Geriatr Soc. 2004;52(2):297-304.
45. Yaffe MJ, Wolfson C, Lithwick M, Weiss D. Development and validation of a tool to improve physician identification of elder abuse: the Elder Abuse Suspicion Index (EASI). J Elder Abuse Negl. 2008;20(3):276-300. doi:10.1080/08946560801973168.
46. National Initiative for the Care of the Elderly. EASI: Elder Abuse Suspicion Index. http://www.nicenet.ca/tools-easi-elder-abuse-suspicion-index. Accessed April 5, 2017.
Case
An 85-year-old right-handed woman who recently had been diagnosed with mild cognitive impairment arrived at the ED via emergency medical services (EMS) for evaluation of a reported fall. She was accompanied by her daughter, who resided with the patient and was her primary caregiver. The patient stated that she had tripped on a wet rug in the bathroom of her home, striking her head and face on the edge of the sink without losing consciousness. Her daughter reported that she was not assisting her mother when the fall occurred, but had witnessed the fall from the hallway and called EMS. At the patient’s home, EMS found the patient to be alert, oriented, and ambulatory with normal vital signs that remained stable throughout prehospital transport.
The remainder of the patient’s history was provided almost entirely by her daughter, who constantly interrupted her mother whenever she attempted to directly answer a question or provide information. On physical examination, the patient had bilateral tenderness, edema, and periorbital ecchymoses, and a left eye that was nearly swollen shut. Extraocular movements were normal, visual acuity was intact, and sclerae were noninjected. The patient had tenderness over both maxillary sinuses, and edema and ecchymosis of her left cheek. There was also tenderness, ecchymoses, and edema on the lateral aspects of both forearms, and decreased range of motion of her right lower arm and wrist. With the exception of the patient not knowing the date during the orientation part of the thorough neurological examination, the remainder of the physical examination was unremarkable.
Radiological evaluation found no evidence of traumatic brain injury, but did reveal an acute fracture of the left zygomatic arch, an acute displaced nasal bone fracture, an age-indeterminate fracture of the right zygomatic arch, and an acute right ulnar fracture. Considering all of these findings, particularly the pattern of acute injuries, the emergency physician (EP) considered elder abuse as the possible etiology of the patient’s acute and chronic injuries.
Although the patient had initially agreed with her daughter’s description of the events—including her claim that she had fallen—when the EP questioned the patient alone, she related a history of frequent verbal and less frequent physical abuse by her daughter. The patient further noted that immediately before sustaining the injuries that brought her to the ED, her daughter had been insisting that she sign documents to give her control of her banking and finances. After refusing to sign the papers, the patient said that she and her daughter got into an argument, which she noted “they tended to do frequently.” The patient admitted that during this argument, her daughter struck her in the face repeatedly with the cane that the daughter had grabbed with her right hand.
The EP admitted the patient to the hospital for management of her orthopedic injuries and related pain, and to formulate a safe discharge plan. During admission, additional diagnostic testing revealed multiple old rib fractures, anemia, and a low-serum albumin, which suggested poor nutritional status.
Epidemiology
The term elder abuse refers to harm or the risk of harm to an older adult from either action or negligence committed by someone in a relationship of trust, or when a victim has been targeted because of age or disability. Elder abuse encompasses physical, sexual, or psychological abuse, neglect, and financial exploitation.1-5 Identified victims of elder abuse typically suffer from multiple forms of abuse.1-5
At present, elder abuse annually affects 5% to 10% of community-dwelling older adults,1-6 and nursing-home residents are at increased risk of abuse.7-10 Poor medical outcomes, including depression and dementia,11 and much higher mortality6,12,13 have been linked to victims of elder abuse.
Etiology
When treating older adults, it is critically important for EPs and the ED staff to consider and identify elder abuse in the differential diagnosis.14,15 Presently, only an estimated 1 in 24 cases of abuse is recognized and reported to the authorities,2 and much of the subsequent morbidity and mortality of elder abuse results from poor detection. A visit to the ED for an acute injury or illness may be the only time socially isolated older adults leave their homes.15-17 Additionally, the ED setting is uniquely suited to identify mistreatment, as a patient typically may be evaluated for several hours by providers from multiple disciplines who are able to observe, interact with, and examine the patient.15 The ED already exercises a similar essential role in the identification and initial intervention for both child abuse18,19 and intimate partner violence among younger adults.20,21
Recognition
Unfortunately, at present, ED providers rarely recognize and report elder abuse.22-24 Though the reasons for this are not entirely understood, inadequate training, lack of time and space to conduct complete evaluations, reluctance to become involved with the legal system, and challenges to distinguishing intentional from unintentional injuries may be contributing factors.24,25 A focus on improving EP and ED staff approaches to elder abuse is relevant and timely given the growing elderly population.
Risk Factors
When evaluating elderly patients, providers should consider research suggesting that some older adults may be at particularly high risk for abuse.4,26-29 Notably, individuals who have cognitive impairment are more likely to be victims of abuse.30-32 Health-related demographic characteristics such as poor physical and mental health, substance abuse, low income/socioeconomic status, and social isolation all may increase the potential for mistreatment.
Family History
Similar to situations resulting in intimate partner violence, a family history of abuse and exposure to traumatic events may increase risk, and those responsible for elder abuse often turn out to be spouses, romantic partners, or an adult child living with the elderly parent—though paid caregivers also can be abusive.
Suspicion of abuse should be increased when individuals in caregiving roles have a history or show signs of mental illness, substance abuse, financial dependence on the victim, or caregiver stress. Considering that a caregiver may be overwhelmed is particularly relevant when an elderly patient exhibits behavioral issues.
Medical History
Obtaining a clear and thorough medical history from the patient and caregiver, both together and alone, is paramount to assessing the potential for abuse. Many indicators from the history may suggest the possibility of mistreatment (Table 1)33-37 and although challenging in a busy ED, a comprehensive head-to-toe examination is crucial to adequately assess abuse. Suspicious physical findings and injury patterns of physical abuse, sexual abuse, and neglect are listed in Table 2.33-37 Ongoing research is aimed at improving ED providers’ ability to differentiate accidental injuries, such as fall injuries, from injuries caused by physical elder abuse.
Injury Patterns
Preliminary studies have indicated that physical abuse injuries most commonly occur on the head, neck, and upper extremities.38,39 A study comparing abuse victims to accidental injury sufferers found that abuse victims often had large bruises (>5 cm) on the face, lateral right arm, or posterior torso.40 Preliminary results from a study in progress suggest that injuries to the left periorbital area, neck, and ulnar forearm may be much more common in abuse than in accident.
Imaging Studies
Emergency radiologists are contributing additional concerning findings indicative of elder abuse,38,41,42 such as the concomitant presence of old and new fractures, high-energy fractures inconsistent with the purported mechanism, and distal ulnar diaphyseal fractures.41,42 The ultimate goal is to identify pathognomonic injury patterns similar to those found in child abuse cases, to assist ED providers.
Laboratory Studies
Although there are no laboratory tests to definitively identify abuse or neglect, specific findings that may indicate abuse include anemia, dehydration, malnutrition, hypothermia/hyperthermia, and rhabdomyolysis.43 In addition, inappropriately high- or low-medication levels and the presence of illicit drugs, which are not often checked in elderly patients in the ED, may be a sign of abuse.43
Laboratory studies that reveal undetectable levels of a patient’s prescription medications may indicate a caregiver’s intentional or neglectful withholding of such medications—especially diversion of opioid medications prescribed for painful conditions.43 Likewise, elevated levels of prescribed drugs may point to intentional or unintentional overdose, whereas the presence of nonprescribed drugs or toxins may suggest poisoning.43
Screening Tools
To improve identification of elder abuse in the ED, universal or targeted screening tools are under consideration. Though several screening tools for elder abuse are already available, none have been validated in the ED.15,44,45 Research sponsored by the National Institute of Justice to identify an ED-specific screening tool is ongoing.15
Elder Abuse Suspicion Index
The Elder Abuse Suspicion Index (EASI) is a short screening tool that has been validated for cognitively intact patients being treated in family practice and ambulatory care settings, and may be used in EDs.44 The tool comprises six questions: five for patient response, and a sixth question for clinician response. This tool is available at http://www.nicenet.ca/tools-easi-elder-abuse-suspicion-index.46
Interventional Measures
When elder mistreatment is suspected or confirmed, health care providers must first address any acute medical, traumatic, or psychological issues. Bleeding, orthopedic injuries, metabolic abnormalities, infections, and agitation must be treated and/or stabilized, while neglected or inappropriately managed chronic medical conditions may require treatment.
Hospitalization should be considered for an older adult who needs extended treatment or observation and, in cases of immediate or continued danger of abuse, separation from contact with the suspected abuser. These measures present several challenges, particularly if the suspected abuser is the patient’s health care proxy, in which case early involvement of the hospital’s legal department, social services, and administration may be necessary—especially in navigating the guardianship process.
Engaging security also may be necessary if the patient requires one-to-one patient watch or when the perpetrator must be removed from the ED. Social workers, patient services representatives, and law enforcement officials should be informed when such intervention is necessary.
In instances when a patient is not at risk of immediate harm, interventions can be more individualized. Coordination with primary care physicians (PCPs) must also be facilitated prior to discharge, to ensure consistent longitudinal follow-up care, and social workers should provide any needed out-of-hospital resources to the patient—and caregiver—such as Meals-on-Wheels, medical transportation services, adult day care/senior center participation, and substance abuse treatment.
Patient Decision-Making Capacity
When a patient experiencing abuse declines interventions or services, the EP must evaluate the patient’s decision-making capacity. In unclear cases, a psychiatric evaluation can help to assess decision-making capacity. If the victim is deemed to have capacity with regard to care and/or discharge, the patient’s choice of returning to an unsafe environment must be respected, as is true in instances of intimate partner violence among younger adults—but not in child abuse cases. In such situations, the EP should nevertheless discuss safety planning, offer psychoeducation about violence and abuse, suggest appropriate community referrals, and encourage abused patients to return or call a contact person whenever they desire or feel the need to talk further. For a victim who is deemed not to possess capacity, providers should proceed with treatment considered to be in the best interest of the patient.
Reporting Abuse
Emergency department providers should notify the appropriate authorities when elder abuse is suspected or identified. A report may be made to the local Adult Protective Services (APS), but this agency operates much differently than Child Protective Services. Case workers with APS will not open a case while a patient is in the ED or hospital, as it is deemed a safe environment and any investigation they undertake will only commence upon discharge. Because of this, contacting the local police department prior to discharge should be considered.
Mandatory elder abuse-reporting laws vary from state to state. Health care providers should therefore contact their respective state or city department of health to obtain local legislation.
Multidisciplinary Approach
Ideally, a multidisciplinary, ED-based intervention team modeled on child abuse teams18,19 would help to optimize treatment and ensure the safety and treatment of vulnerable older adults. These teams could conduct thorough medical, forensic, and social work assessments, allowing ED providers to attend to other patients. The team could also assist in arranging for appropriate and safe dispositions. An innovative Vulnerable Elder Protection Team was recently launched at New York-Presbyterian Weill Cornell Medical Center to provide these services, and its impact is currently being evaluated.
Case Conclusion
The EP who treated the patient realized that blows from a blunt object held by a right-handed person would tend to land on the left side of the victim’s face and upper torso, and that a right-handed victim who successfully blocked the blows intended for her face would instead sustain an isolated right ulna or radius midshaft fracture. These findings, together with the concomitant presence of both old and new fractures, led the EP to question the patient alone and, after obtaining a different history of the events that led to the injuries, admit her for further evaluation, treatment, and interventions to prevent continuing abuse.
Summary
Elder abuse has the potential to affect an increasing number of older adults in this growing population, and an ED visit may offer the only opportunity to identify victims and provide intervention, in turn reducing morbidity and mortality. The results of ongoing research will improve the ability of EPs and ED staff to accurately assess the presence or risk of elder abuse and respond more effectively. It is essential that EPs always consider elder abuse and neglect as a possible etiology when evaluating injuries in this population. Moreover, when identified, addressing elder mistreatment may dramatically improve quality of life or save the lives of these vulnerable patients.
Case
An 85-year-old right-handed woman who recently had been diagnosed with mild cognitive impairment arrived at the ED via emergency medical services (EMS) for evaluation of a reported fall. She was accompanied by her daughter, who resided with the patient and was her primary caregiver. The patient stated that she had tripped on a wet rug in the bathroom of her home, striking her head and face on the edge of the sink without losing consciousness. Her daughter reported that she was not assisting her mother when the fall occurred, but had witnessed the fall from the hallway and called EMS. At the patient’s home, EMS found the patient to be alert, oriented, and ambulatory with normal vital signs that remained stable throughout prehospital transport.
The remainder of the patient’s history was provided almost entirely by her daughter, who constantly interrupted her mother whenever she attempted to directly answer a question or provide information. On physical examination, the patient had bilateral tenderness, edema, and periorbital ecchymoses, and a left eye that was nearly swollen shut. Extraocular movements were normal, visual acuity was intact, and sclerae were noninjected. The patient had tenderness over both maxillary sinuses, and edema and ecchymosis of her left cheek. There was also tenderness, ecchymoses, and edema on the lateral aspects of both forearms, and decreased range of motion of her right lower arm and wrist. With the exception of the patient not knowing the date during the orientation part of the thorough neurological examination, the remainder of the physical examination was unremarkable.
Radiological evaluation found no evidence of traumatic brain injury, but did reveal an acute fracture of the left zygomatic arch, an acute displaced nasal bone fracture, an age-indeterminate fracture of the right zygomatic arch, and an acute right ulnar fracture. Considering all of these findings, particularly the pattern of acute injuries, the emergency physician (EP) considered elder abuse as the possible etiology of the patient’s acute and chronic injuries.
Although the patient had initially agreed with her daughter’s description of the events—including her claim that she had fallen—when the EP questioned the patient alone, she related a history of frequent verbal and less frequent physical abuse by her daughter. The patient further noted that immediately before sustaining the injuries that brought her to the ED, her daughter had been insisting that she sign documents to give her control of her banking and finances. After refusing to sign the papers, the patient said that she and her daughter got into an argument, which she noted “they tended to do frequently.” The patient admitted that during this argument, her daughter struck her in the face repeatedly with the cane that the daughter had grabbed with her right hand.
The EP admitted the patient to the hospital for management of her orthopedic injuries and related pain, and to formulate a safe discharge plan. During admission, additional diagnostic testing revealed multiple old rib fractures, anemia, and a low-serum albumin, which suggested poor nutritional status.
Epidemiology
The term elder abuse refers to harm or the risk of harm to an older adult from either action or negligence committed by someone in a relationship of trust, or when a victim has been targeted because of age or disability. Elder abuse encompasses physical, sexual, or psychological abuse, neglect, and financial exploitation.1-5 Identified victims of elder abuse typically suffer from multiple forms of abuse.1-5
At present, elder abuse annually affects 5% to 10% of community-dwelling older adults,1-6 and nursing-home residents are at increased risk of abuse.7-10 Poor medical outcomes, including depression and dementia,11 and much higher mortality6,12,13 have been linked to victims of elder abuse.
Etiology
When treating older adults, it is critically important for EPs and the ED staff to consider and identify elder abuse in the differential diagnosis.14,15 Presently, only an estimated 1 in 24 cases of abuse is recognized and reported to the authorities,2 and much of the subsequent morbidity and mortality of elder abuse results from poor detection. A visit to the ED for an acute injury or illness may be the only time socially isolated older adults leave their homes.15-17 Additionally, the ED setting is uniquely suited to identify mistreatment, as a patient typically may be evaluated for several hours by providers from multiple disciplines who are able to observe, interact with, and examine the patient.15 The ED already exercises a similar essential role in the identification and initial intervention for both child abuse18,19 and intimate partner violence among younger adults.20,21
Recognition
Unfortunately, at present, ED providers rarely recognize and report elder abuse.22-24 Though the reasons for this are not entirely understood, inadequate training, lack of time and space to conduct complete evaluations, reluctance to become involved with the legal system, and challenges to distinguishing intentional from unintentional injuries may be contributing factors.24,25 A focus on improving EP and ED staff approaches to elder abuse is relevant and timely given the growing elderly population.
Risk Factors
When evaluating elderly patients, providers should consider research suggesting that some older adults may be at particularly high risk for abuse.4,26-29 Notably, individuals who have cognitive impairment are more likely to be victims of abuse.30-32 Health-related demographic characteristics such as poor physical and mental health, substance abuse, low income/socioeconomic status, and social isolation all may increase the potential for mistreatment.
Family History
Similar to situations resulting in intimate partner violence, a family history of abuse and exposure to traumatic events may increase risk, and those responsible for elder abuse often turn out to be spouses, romantic partners, or an adult child living with the elderly parent—though paid caregivers also can be abusive.
Suspicion of abuse should be increased when individuals in caregiving roles have a history or show signs of mental illness, substance abuse, financial dependence on the victim, or caregiver stress. Considering that a caregiver may be overwhelmed is particularly relevant when an elderly patient exhibits behavioral issues.
Medical History
Obtaining a clear and thorough medical history from the patient and caregiver, both together and alone, is paramount to assessing the potential for abuse. Many indicators from the history may suggest the possibility of mistreatment (Table 1)33-37 and although challenging in a busy ED, a comprehensive head-to-toe examination is crucial to adequately assess abuse. Suspicious physical findings and injury patterns of physical abuse, sexual abuse, and neglect are listed in Table 2.33-37 Ongoing research is aimed at improving ED providers’ ability to differentiate accidental injuries, such as fall injuries, from injuries caused by physical elder abuse.
Injury Patterns
Preliminary studies have indicated that physical abuse injuries most commonly occur on the head, neck, and upper extremities.38,39 A study comparing abuse victims to accidental injury sufferers found that abuse victims often had large bruises (>5 cm) on the face, lateral right arm, or posterior torso.40 Preliminary results from a study in progress suggest that injuries to the left periorbital area, neck, and ulnar forearm may be much more common in abuse than in accident.
Imaging Studies
Emergency radiologists are contributing additional concerning findings indicative of elder abuse,38,41,42 such as the concomitant presence of old and new fractures, high-energy fractures inconsistent with the purported mechanism, and distal ulnar diaphyseal fractures.41,42 The ultimate goal is to identify pathognomonic injury patterns similar to those found in child abuse cases, to assist ED providers.
Laboratory Studies
Although there are no laboratory tests to definitively identify abuse or neglect, specific findings that may indicate abuse include anemia, dehydration, malnutrition, hypothermia/hyperthermia, and rhabdomyolysis.43 In addition, inappropriately high- or low-medication levels and the presence of illicit drugs, which are not often checked in elderly patients in the ED, may be a sign of abuse.43
Laboratory studies that reveal undetectable levels of a patient’s prescription medications may indicate a caregiver’s intentional or neglectful withholding of such medications—especially diversion of opioid medications prescribed for painful conditions.43 Likewise, elevated levels of prescribed drugs may point to intentional or unintentional overdose, whereas the presence of nonprescribed drugs or toxins may suggest poisoning.43
Screening Tools
To improve identification of elder abuse in the ED, universal or targeted screening tools are under consideration. Though several screening tools for elder abuse are already available, none have been validated in the ED.15,44,45 Research sponsored by the National Institute of Justice to identify an ED-specific screening tool is ongoing.15
Elder Abuse Suspicion Index
The Elder Abuse Suspicion Index (EASI) is a short screening tool that has been validated for cognitively intact patients being treated in family practice and ambulatory care settings, and may be used in EDs.44 The tool comprises six questions: five for patient response, and a sixth question for clinician response. This tool is available at http://www.nicenet.ca/tools-easi-elder-abuse-suspicion-index.46
Interventional Measures
When elder mistreatment is suspected or confirmed, health care providers must first address any acute medical, traumatic, or psychological issues. Bleeding, orthopedic injuries, metabolic abnormalities, infections, and agitation must be treated and/or stabilized, while neglected or inappropriately managed chronic medical conditions may require treatment.
Hospitalization should be considered for an older adult who needs extended treatment or observation and, in cases of immediate or continued danger of abuse, separation from contact with the suspected abuser. These measures present several challenges, particularly if the suspected abuser is the patient’s health care proxy, in which case early involvement of the hospital’s legal department, social services, and administration may be necessary—especially in navigating the guardianship process.
Engaging security also may be necessary if the patient requires one-to-one patient watch or when the perpetrator must be removed from the ED. Social workers, patient services representatives, and law enforcement officials should be informed when such intervention is necessary.
In instances when a patient is not at risk of immediate harm, interventions can be more individualized. Coordination with primary care physicians (PCPs) must also be facilitated prior to discharge, to ensure consistent longitudinal follow-up care, and social workers should provide any needed out-of-hospital resources to the patient—and caregiver—such as Meals-on-Wheels, medical transportation services, adult day care/senior center participation, and substance abuse treatment.
Patient Decision-Making Capacity
When a patient experiencing abuse declines interventions or services, the EP must evaluate the patient’s decision-making capacity. In unclear cases, a psychiatric evaluation can help to assess decision-making capacity. If the victim is deemed to have capacity with regard to care and/or discharge, the patient’s choice of returning to an unsafe environment must be respected, as is true in instances of intimate partner violence among younger adults—but not in child abuse cases. In such situations, the EP should nevertheless discuss safety planning, offer psychoeducation about violence and abuse, suggest appropriate community referrals, and encourage abused patients to return or call a contact person whenever they desire or feel the need to talk further. For a victim who is deemed not to possess capacity, providers should proceed with treatment considered to be in the best interest of the patient.
Reporting Abuse
Emergency department providers should notify the appropriate authorities when elder abuse is suspected or identified. A report may be made to the local Adult Protective Services (APS), but this agency operates much differently than Child Protective Services. Case workers with APS will not open a case while a patient is in the ED or hospital, as it is deemed a safe environment and any investigation they undertake will only commence upon discharge. Because of this, contacting the local police department prior to discharge should be considered.
Mandatory elder abuse-reporting laws vary from state to state. Health care providers should therefore contact their respective state or city department of health to obtain local legislation.
Multidisciplinary Approach
Ideally, a multidisciplinary, ED-based intervention team modeled on child abuse teams18,19 would help to optimize treatment and ensure the safety and treatment of vulnerable older adults. These teams could conduct thorough medical, forensic, and social work assessments, allowing ED providers to attend to other patients. The team could also assist in arranging for appropriate and safe dispositions. An innovative Vulnerable Elder Protection Team was recently launched at New York-Presbyterian Weill Cornell Medical Center to provide these services, and its impact is currently being evaluated.
Case Conclusion
The EP who treated the patient realized that blows from a blunt object held by a right-handed person would tend to land on the left side of the victim’s face and upper torso, and that a right-handed victim who successfully blocked the blows intended for her face would instead sustain an isolated right ulna or radius midshaft fracture. These findings, together with the concomitant presence of both old and new fractures, led the EP to question the patient alone and, after obtaining a different history of the events that led to the injuries, admit her for further evaluation, treatment, and interventions to prevent continuing abuse.
Summary
Elder abuse has the potential to affect an increasing number of older adults in this growing population, and an ED visit may offer the only opportunity to identify victims and provide intervention, in turn reducing morbidity and mortality. The results of ongoing research will improve the ability of EPs and ED staff to accurately assess the presence or risk of elder abuse and respond more effectively. It is essential that EPs always consider elder abuse and neglect as a possible etiology when evaluating injuries in this population. Moreover, when identified, addressing elder mistreatment may dramatically improve quality of life or save the lives of these vulnerable patients.
1. Elder Mistreatment: Abuse, Neglect, and Exploitation in an Aging America. Bonnie RJ, Wallace RB, eds. Washington, DC: National Academies Press; 2003:1-552. https://www.nap.edu/read/10406/chapter/1. Accessed April 4, 2017.
2. Lifespan of Greater Rochester, Inc; Weill Cornell Medical Center of Cornell University; New York City Department for the Aging. Under the radar: New York state elder abuse prevalence study: self-reported prevalence and documented case surveys 2011.http://ocfs.ny.gov/main/reports/Under%20the%20Radar%2005%2012%2011%20final%20report.pdf. Published May 2011. Accessed April 4, 2017.
3. Connolly MT, Brandl B, Breckman R. The Elder Justice Roadmap: A Stakeholder Initiative to Respond to an Emerging Health, Justice, Financial, and Social Crisis. https://www.justice.gov/elderjustice/file/829266/download. National Center for Elder Abuse. Published January 2014. Accessed April 4, 2017.
4. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297. doi:10.2105/AJPH.2009.163089.
5. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272. doi:10.1016/S0140-6736(04)17144-4.
6. Lachs MS, Pillemer KA. Elder abuse. N Engl J Med. 2015;373(20):1947-1956. doi:10.1056/NEJMra1404688.
7. Ortmann C, Fechner G, Bajanowski T, Brinkmann B. Fatal neglect of the elderly. Int J Legal Med. 2001;114(3):191-193.
8. Schiamberg LB, Oehmke J, Zhang Z, et al. Physical abuse of older adults in nursing homes: a random sample survey of adults with an elderly family member in a nursing home. J Elder Abuse Negl. 2012;24(1):65-83. doi:10.1080/08946566.2011.608056.
9. Rosen T, Pillemer K, Lachs M. Resident-to-resident aggression in long-term care facilities: an understudied problem. Aggress Violent Behav. 2008;13(2):77-87. doi:10.1016/j.avb.2007.12.001.
10. Shinoda-Tagawa T, Leonard R, Pontikas J, McDonough JE, Allen D, Dreyer PI. Resident-to-resident violent incidents in nursing homes. JAMA. 2004;291(5):591-598. doi:10.1001/jama.291.5.591.
11. Dyer CB, Pavlik VN, Murphy KP, Hyman DJ. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
12. Lachs MS, Williams CS, O’Brien S, Pillemer KA, Charlson ME. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
13. Dong XQ, Simon MA, Beck TT, et al. Elder abuse and mortality: the role of psychological and social wellbeing. Gerontology. 2011;57(6):549-658. doi:10.1159/000321881.
14. Stevens TB, Richmond NL, Pereira GF, Shenvi CL, Platts-Mills TF. Prevalence of nonmedical problems among older adults presenting to the emergency department. Acad Emerg Med. 2014;21(6):651-658. doi:10.1111/acem.12395.
15. Rosen T, Hargarten S, Flomenbaum NE, Platts-Mills TF. Identifying elder abuse in the emergency department: toward a multidisciplinary team-based approach. Ann Emerg Med. 2016;68(3):378-382. doi:10.1016/j.annemergmed.2016.01.037.
16. Bond MC, Butler KH. Elder abuse and neglect: definitions, epidemiology, and approaches to emergency department screening. Clin Geriatr Med. 2013;29(1):257-273. doi:10.1016/j.cger.2012.09.004.
17. Heyborne RD. Elder abuse: keeping the unthinkable in the differential. Acad Emerg Med. 2007;14(6):566-567. doi:10.1197/j.aem.2007.01.015.
18. Kistin CJ, Tien I, Bauchner H, Parker V, Leventhal JM. Factors that influence the effectiveness of child protection teams. Pediatrics. 2010;126(1):94-100. doi:10.1542/peds.2009-3446.
19. Hochstadt NJ, Harwicke NJ. How effective is the multidisciplinary approach? A follow-up study. Child Abuse Negl. 1985;9(3):365-372.
20. Choo EK, Gottlieb AS, DeLuca M, Tape C, Colwell L, Zlotnick C. Systematic review of ED-based intimate partner violence intervention research. West J Emerg Med. 2015;16(7):1037-1042. doi:10.5811/westjem.2015.10.27586.
21. Rhodes KV, Rodgers M, Sommers M, et al. Brief motivational intervention for intimate partner violence and heavy drinking in the emergency department: a randomized clinical trial. JAMA. 2015;314(5):466-477. doi:10.1001/jama.2015.8369.
22. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 survey of state adult protective services: abuse of adults 60 years of age and older. http://www.napsa-now.org/wp-content/uploads/2012/09/2-14-06-FINAL-60+REPORT.pdf. Published February 2006. Accessed March 10, 2017.
23. Blakely BE, Dolon R. Another look at the helpfulness of occupational groups in the discovery of elder abuse and neglect. J Elder Abuse Negl. 2003;13:1-23.
24. Evans CS, Hunold KM, Rosen T, Platts-Mills TF. Diagnosis of elder abuse in U.S. emergency departments. J Am Geriatr Soc. 2017;65(1):91-97. doi:10.1111/jgs.14480.
25. Jones JS, Veenstra TR, Seamon JP, Krohmer J. Elder mistreatment: national survey of emergency physicians. Ann Emerg Med. 1997;30(4):473-479.
26. Amstadter AB, Zajac K, Strachan M, Hernandez MA, Kilpatrick DG, Acierno R. Prevalence and correlates of elder mistreatment in South Carolina: the South Carolina elder mistreatment study. J Interpers Violence. 2011;26(15):2947-2972. doi:10.1177/0886260510390959.
27. Friedman LS, Avila S, Tanouye K, Joseph K. A case-control study of severe physical abuse of older adults. J Am Geriatr Soc. 2011;59(3):417-422. doi:10.1111/j.1532-5415.2010.03313.x.
28. Pillemer K, Burnes D, Riffin C, Lachs MS. Elder Abuse: global situation, risk factors, and prevention strategies. Gerontologist. 2016;56 Suppl 2:S194-S205. doi:10.1093/geront/gnw004.
29. Laumann EO, Leitsch SA, Waite LJ. Elder mistreatment in the United States: prevalence estimates from a nationally representative study. J Gerontol B Psychol Sci Soc Sci. 2008;63(4):S248-S254.
30. Cooney C, Howard R, Lawlor B. Abuse of vulnerable people with dementia by their carers: can we identify those most at risk? Int J Geriatr Psychiatry. 2006;21(6):564-571. doi:10.1002/gps.1525.
31. Lachs MS, Williams C, O’Brien S, Hurst L, Horwitz R. Risk factors for reported elder abuse and neglect: a nine-year observational cohort study. Gerontologist. 1997;37(4):469-474.
32. Wiglesworth A, Mosqueda L, Mulnard R, Liao S, Gibbs L, Fitzgerald W. Screening for abuse and neglect of people with dementia. J Am Geriatr Soc. 2010;58(3):493-500. doi:10.1111/j.1532-5415.2010.02737.x.
33. Collins KA. Elder maltreatment: a review. Arch Pathol Lab Med. 2006;130(9):1290-1296. doi:10.1043/1543-2165(2006)130[1290:EMAR]2.0.CO;2.
34. Gibbs LM. Understanding the medical markers of elder abuse and neglect: physical examination findings. Clin Geriatr Med. 2014 Nov;30(4):687-712. doi:10.1016/j.cger.2014.08.002.
35. Palmer M, Brodell RT, Mostow EN. Elder abuse: dermatologic clues and critical solutions. J Am Acad Dermatol. 2013;68(2):e37-e42. doi:10.1016/j.jaad.2011.03.016.
36. Speck PM, Hartig MT, Likes W, et al. Case series of sexual assault in older persons. Clin Geriatr Med. 2014;30(4):779-806. doi:10.1016/j.cger.2014.08.007.
37. Chang AL, Wong JW, Endo JO, Norman RA. Geriatric dermatology: part II. Risk factors and cutaneous signs of elder mistreatment for the dermatologist. J Am Acad Dermatol. 2013;68(4):533.e1-.e10. doi:10.1016/j.jaad.2013.01.001.
38. Murphy K, Waa S, Jaffer H, Sauter A, Chan A. A literature review of findings in physical elder abuse. Can Assoc Radiol J. 2013;64(1):10-14. doi:10.1016/j.carj.2012.12.001.
39. Rosen T, Bloemen EM, LoFaso VM, Clark S, Flomenbaum NE, Lachs MS. Emergency department presentations for injuries in older adults independently known to be victims of elder abuse. J Emerg Med. 2016;50(3):518-526. doi:10.1016/j.jemermed.2015.10.037.
40. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196. doi:10.1111/j.1532-5415.2009.02330.x.
41. Rosen T, Bloemen EM, Harpe J, et al. Radiologists’ training, experience, and attitudes about elder abuse detection. AJR Am J Roentgenol. 2016;207:1210-1214.
42. Wong NZ, Rosen T, Sanchez AM, et al. Imaging findings in elder abuse: a role for radiologists in detection. Can Assoc Radiol J. 2017;68(1):16-20. doi:10.1016/j.carj.2016.06.001.
43. LoFaso VM, Rosen T. Medical and laboratory indicators of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):713-28. doi:10.1016/j.cger.2014.08.003.
44. Fulmer T, Guadagno L, Bitondo Dyer C, Connolly MT. Progress in elder abuse screening and assessment instruments. J Am Geriatr Soc. 2004;52(2):297-304.
45. Yaffe MJ, Wolfson C, Lithwick M, Weiss D. Development and validation of a tool to improve physician identification of elder abuse: the Elder Abuse Suspicion Index (EASI). J Elder Abuse Negl. 2008;20(3):276-300. doi:10.1080/08946560801973168.
46. National Initiative for the Care of the Elderly. EASI: Elder Abuse Suspicion Index. http://www.nicenet.ca/tools-easi-elder-abuse-suspicion-index. Accessed April 5, 2017.
1. Elder Mistreatment: Abuse, Neglect, and Exploitation in an Aging America. Bonnie RJ, Wallace RB, eds. Washington, DC: National Academies Press; 2003:1-552. https://www.nap.edu/read/10406/chapter/1. Accessed April 4, 2017.
2. Lifespan of Greater Rochester, Inc; Weill Cornell Medical Center of Cornell University; New York City Department for the Aging. Under the radar: New York state elder abuse prevalence study: self-reported prevalence and documented case surveys 2011.http://ocfs.ny.gov/main/reports/Under%20the%20Radar%2005%2012%2011%20final%20report.pdf. Published May 2011. Accessed April 4, 2017.
3. Connolly MT, Brandl B, Breckman R. The Elder Justice Roadmap: A Stakeholder Initiative to Respond to an Emerging Health, Justice, Financial, and Social Crisis. https://www.justice.gov/elderjustice/file/829266/download. National Center for Elder Abuse. Published January 2014. Accessed April 4, 2017.
4. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297. doi:10.2105/AJPH.2009.163089.
5. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272. doi:10.1016/S0140-6736(04)17144-4.
6. Lachs MS, Pillemer KA. Elder abuse. N Engl J Med. 2015;373(20):1947-1956. doi:10.1056/NEJMra1404688.
7. Ortmann C, Fechner G, Bajanowski T, Brinkmann B. Fatal neglect of the elderly. Int J Legal Med. 2001;114(3):191-193.
8. Schiamberg LB, Oehmke J, Zhang Z, et al. Physical abuse of older adults in nursing homes: a random sample survey of adults with an elderly family member in a nursing home. J Elder Abuse Negl. 2012;24(1):65-83. doi:10.1080/08946566.2011.608056.
9. Rosen T, Pillemer K, Lachs M. Resident-to-resident aggression in long-term care facilities: an understudied problem. Aggress Violent Behav. 2008;13(2):77-87. doi:10.1016/j.avb.2007.12.001.
10. Shinoda-Tagawa T, Leonard R, Pontikas J, McDonough JE, Allen D, Dreyer PI. Resident-to-resident violent incidents in nursing homes. JAMA. 2004;291(5):591-598. doi:10.1001/jama.291.5.591.
11. Dyer CB, Pavlik VN, Murphy KP, Hyman DJ. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
12. Lachs MS, Williams CS, O’Brien S, Pillemer KA, Charlson ME. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
13. Dong XQ, Simon MA, Beck TT, et al. Elder abuse and mortality: the role of psychological and social wellbeing. Gerontology. 2011;57(6):549-658. doi:10.1159/000321881.
14. Stevens TB, Richmond NL, Pereira GF, Shenvi CL, Platts-Mills TF. Prevalence of nonmedical problems among older adults presenting to the emergency department. Acad Emerg Med. 2014;21(6):651-658. doi:10.1111/acem.12395.
15. Rosen T, Hargarten S, Flomenbaum NE, Platts-Mills TF. Identifying elder abuse in the emergency department: toward a multidisciplinary team-based approach. Ann Emerg Med. 2016;68(3):378-382. doi:10.1016/j.annemergmed.2016.01.037.
16. Bond MC, Butler KH. Elder abuse and neglect: definitions, epidemiology, and approaches to emergency department screening. Clin Geriatr Med. 2013;29(1):257-273. doi:10.1016/j.cger.2012.09.004.
17. Heyborne RD. Elder abuse: keeping the unthinkable in the differential. Acad Emerg Med. 2007;14(6):566-567. doi:10.1197/j.aem.2007.01.015.
18. Kistin CJ, Tien I, Bauchner H, Parker V, Leventhal JM. Factors that influence the effectiveness of child protection teams. Pediatrics. 2010;126(1):94-100. doi:10.1542/peds.2009-3446.
19. Hochstadt NJ, Harwicke NJ. How effective is the multidisciplinary approach? A follow-up study. Child Abuse Negl. 1985;9(3):365-372.
20. Choo EK, Gottlieb AS, DeLuca M, Tape C, Colwell L, Zlotnick C. Systematic review of ED-based intimate partner violence intervention research. West J Emerg Med. 2015;16(7):1037-1042. doi:10.5811/westjem.2015.10.27586.
21. Rhodes KV, Rodgers M, Sommers M, et al. Brief motivational intervention for intimate partner violence and heavy drinking in the emergency department: a randomized clinical trial. JAMA. 2015;314(5):466-477. doi:10.1001/jama.2015.8369.
22. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 survey of state adult protective services: abuse of adults 60 years of age and older. http://www.napsa-now.org/wp-content/uploads/2012/09/2-14-06-FINAL-60+REPORT.pdf. Published February 2006. Accessed March 10, 2017.
23. Blakely BE, Dolon R. Another look at the helpfulness of occupational groups in the discovery of elder abuse and neglect. J Elder Abuse Negl. 2003;13:1-23.
24. Evans CS, Hunold KM, Rosen T, Platts-Mills TF. Diagnosis of elder abuse in U.S. emergency departments. J Am Geriatr Soc. 2017;65(1):91-97. doi:10.1111/jgs.14480.
25. Jones JS, Veenstra TR, Seamon JP, Krohmer J. Elder mistreatment: national survey of emergency physicians. Ann Emerg Med. 1997;30(4):473-479.
26. Amstadter AB, Zajac K, Strachan M, Hernandez MA, Kilpatrick DG, Acierno R. Prevalence and correlates of elder mistreatment in South Carolina: the South Carolina elder mistreatment study. J Interpers Violence. 2011;26(15):2947-2972. doi:10.1177/0886260510390959.
27. Friedman LS, Avila S, Tanouye K, Joseph K. A case-control study of severe physical abuse of older adults. J Am Geriatr Soc. 2011;59(3):417-422. doi:10.1111/j.1532-5415.2010.03313.x.
28. Pillemer K, Burnes D, Riffin C, Lachs MS. Elder Abuse: global situation, risk factors, and prevention strategies. Gerontologist. 2016;56 Suppl 2:S194-S205. doi:10.1093/geront/gnw004.
29. Laumann EO, Leitsch SA, Waite LJ. Elder mistreatment in the United States: prevalence estimates from a nationally representative study. J Gerontol B Psychol Sci Soc Sci. 2008;63(4):S248-S254.
30. Cooney C, Howard R, Lawlor B. Abuse of vulnerable people with dementia by their carers: can we identify those most at risk? Int J Geriatr Psychiatry. 2006;21(6):564-571. doi:10.1002/gps.1525.
31. Lachs MS, Williams C, O’Brien S, Hurst L, Horwitz R. Risk factors for reported elder abuse and neglect: a nine-year observational cohort study. Gerontologist. 1997;37(4):469-474.
32. Wiglesworth A, Mosqueda L, Mulnard R, Liao S, Gibbs L, Fitzgerald W. Screening for abuse and neglect of people with dementia. J Am Geriatr Soc. 2010;58(3):493-500. doi:10.1111/j.1532-5415.2010.02737.x.
33. Collins KA. Elder maltreatment: a review. Arch Pathol Lab Med. 2006;130(9):1290-1296. doi:10.1043/1543-2165(2006)130[1290:EMAR]2.0.CO;2.
34. Gibbs LM. Understanding the medical markers of elder abuse and neglect: physical examination findings. Clin Geriatr Med. 2014 Nov;30(4):687-712. doi:10.1016/j.cger.2014.08.002.
35. Palmer M, Brodell RT, Mostow EN. Elder abuse: dermatologic clues and critical solutions. J Am Acad Dermatol. 2013;68(2):e37-e42. doi:10.1016/j.jaad.2011.03.016.
36. Speck PM, Hartig MT, Likes W, et al. Case series of sexual assault in older persons. Clin Geriatr Med. 2014;30(4):779-806. doi:10.1016/j.cger.2014.08.007.
37. Chang AL, Wong JW, Endo JO, Norman RA. Geriatric dermatology: part II. Risk factors and cutaneous signs of elder mistreatment for the dermatologist. J Am Acad Dermatol. 2013;68(4):533.e1-.e10. doi:10.1016/j.jaad.2013.01.001.
38. Murphy K, Waa S, Jaffer H, Sauter A, Chan A. A literature review of findings in physical elder abuse. Can Assoc Radiol J. 2013;64(1):10-14. doi:10.1016/j.carj.2012.12.001.
39. Rosen T, Bloemen EM, LoFaso VM, Clark S, Flomenbaum NE, Lachs MS. Emergency department presentations for injuries in older adults independently known to be victims of elder abuse. J Emerg Med. 2016;50(3):518-526. doi:10.1016/j.jemermed.2015.10.037.
40. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196. doi:10.1111/j.1532-5415.2009.02330.x.
41. Rosen T, Bloemen EM, Harpe J, et al. Radiologists’ training, experience, and attitudes about elder abuse detection. AJR Am J Roentgenol. 2016;207:1210-1214.
42. Wong NZ, Rosen T, Sanchez AM, et al. Imaging findings in elder abuse: a role for radiologists in detection. Can Assoc Radiol J. 2017;68(1):16-20. doi:10.1016/j.carj.2016.06.001.
43. LoFaso VM, Rosen T. Medical and laboratory indicators of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):713-28. doi:10.1016/j.cger.2014.08.003.
44. Fulmer T, Guadagno L, Bitondo Dyer C, Connolly MT. Progress in elder abuse screening and assessment instruments. J Am Geriatr Soc. 2004;52(2):297-304.
45. Yaffe MJ, Wolfson C, Lithwick M, Weiss D. Development and validation of a tool to improve physician identification of elder abuse: the Elder Abuse Suspicion Index (EASI). J Elder Abuse Negl. 2008;20(3):276-300. doi:10.1080/08946560801973168.
46. National Initiative for the Care of the Elderly. EASI: Elder Abuse Suspicion Index. http://www.nicenet.ca/tools-easi-elder-abuse-suspicion-index. Accessed April 5, 2017.
Treatment Protocol for Acute Arterial Occlusion Secondary to Facial Revolumization Procedures
Artificial dermal fillers and autologous fat grafting have become increasingly popular in recent years, primarily because they augment existing soft tissue volumes, thus producing aesthetic improvements at a lower cost than traditional plastic surgery (ie, facelift), and with nearly no recovery time. According to the American Society for Aesthetic Plastic Surgery, more than 2 million hyaluronic acid (HA) dermal filler procedures were performed in 2016, an increase of 3% from 2015.1 In addition, 80,000 autologous fat grafting procedures were performed in 2016, an increase of 13% from 2015. In total, there were 2.6 million soft tissue filler procedures in 2016, an increase of 2% from 2015.1
With the increased demand and access to both artificial dermal fillers and autologous fat grafting, there has been a plethora of reported adverse events, ranging from expected erythema to acute blindness and stroke. Emergency physicians should have a thorough understanding of facial vascular anatomy, as well as the effects of available facial volumization products, including potential complications and treatment options. Through our review of two patient cases, we propose a simplified protocol for the treatment of patients with acute arterial occlusion secondary to facial volumization procedures.
Case 1
A 38-year-old white woman presented to the ED for evaluation of transient blurred vision and blanching of the left cheek and upper lip, which began approximately 40 minutes prior to presentation, immediately after her primary care physician (PCP) injected her left nasolabial fold with calcium hydroxyapatite (CaHA). The patient stated that her vision became blurry and her eyes began to tear within 1 minute of receiving the injection. She further noted that these visual changes were painless and lasted for approximately 30 seconds.
The patient’s PCP believed these symptoms were due to pain at the injection site. While the patient was at her PCP’s office, the reception clerk noticed the blanching of the patient’s left cheek and informed the PCP, who referred the patient to our ED for evaluation.
Workup
The patient’s vital signs at presentation were normal. Her medical history was unremarkable and negative for smoking, alcohol, or drug use. She was not taking any medications and had no known drug allergies. The patient’s history was negative for any prior cosmetic procedures, and she confirmed this was the first and only time had a facial revolumization.
Facial examination revealed a Fitzpatrick scale (FS; a numerical scoring system used to assess a patient’s reaction to ultraviolet radiation) score of type 3. She also had left-sided blanching that extended from the midpoint of the nose diagonally to the lateral midbuccal cheek to the level of the oral commissure, including the cutaneous upper lip, alar, and nasal side wall. There was minimal capillary refill with compression at the affected site, and sensation was diminished to fine touch and pinprick. The facial muscles were intact, and, with the exception of puncture marks along both nasolabial folds, the remainder of the facial examination was normal.
The ophthalmic examination revealed a reactive pupil at 2 mm, white sclera, pink conjunctiva, red reflex, and normal fundoscopic vessels. The patient’s bedside Snellen visual acuity and visual field assessments were normal. The neurological examination was likewise normal, and no other physical findings were noted.
Laboratory evaluation included complete blood count (CBC), Chem 7 panel (creatinine, blood urea nitrogen [BUN], carbon dioxide, chloride, glucose, sodium, and potassium), and international normalized ratio (INR), which were all within normal limits.
Diagnosis and Treatment
The patient was diagnosed with acute angular arterial occlusion and transient retinal artery embolism secondary to facial volumization with CaHA. She was treated with oral acetylsalicylic acid aspirin (ASA) 325 mg, prednisone 40 mg, and sildenafil 50 mg; and subcutaneous (SC) enoxaparin 60 mg (1 mg/kg). Topical nitroglycerin paste 2% was applied to the affected area.
Ophthalmology and plastic surgery services were contacted for consultation. Based on no acute findings on examination, the ophthalmologist provided no additional treatment recommendations. The patient was observed in the ED for 4 hours, during which time the facial blanching resolved and her capillary refill time returned to normal at 2 seconds.
After evaluating the patient, the plastic surgeon recommended discharge home with instructions to continue taking the oral ASA and sildenafil, as well as a methylprednisolone dose pack for 6 days. He also recommended the patient begin hyperbaric oxygen (HBO) therapy the day after discharge, since there was no HBO chamber available during her hospital stay.
The patient complied with all discharge instructions, including HBO therapy. At plastic surgery follow-up, the patient had no long-term adverse effects from the CaHA injection.
Case 2
A 54-year-old Asian woman presented to the ED for evaluation of a 24-hour history of progressive and persistent pain, swelling, and discoloration of the nasolabial and upper lip region. She stated her symptoms began within 1 hour of receiving a fat graft injection into the affected area by her cosmetic surgeon. After examining the patient, the cosmetic surgeon referred her to the ED for further evaluation. The patient stated that she had undergone six prior facial revolumization procedures, but noted the recent procedure was her first autologous fat graft.
Workup
The patient’s medical history was unremarkable. Her social history was positive for one glass of wine per day and negative for smoking. The patient was not taking any medications and had no known drug allergies.
The patient’s vital signs at presentation were normal. She was evaluated approximately 30 hours after the fat graft procedure. Facial examination revealed an FS of type 4 with right-sided ischemia along the cutaneous upper lip, alar, and cheek (Figure 1).
Capillary refill time with compression was 0 in the affected area. Sensation to fine touch and pinprick was 0. The facial muscles were intact and, with the exception of puncture marks along both nasolabial folds, the remainder of the facial examination was normal. The neurological examination was likewise normal, and no other physical findings were noted. Laboratory evaluation included CBC, Chem 7 panel, and INR, which were all within normal limits.
Diagnosis and Treatment
The patient was diagnosed with acute angular arterial occlusion with soft tissue ischemia secondary to facial revolumization with autologous fat grafting. She was given oral acyclovir 800 mg, ASA 325 mg, cephalexin 500 mg, prednisone 40 mg, and sildenafil 50 mg; and SC enoxaparin 60 mg (1 mg/kg). Topical nitroglycerin paste 2% was applied to the affected area.
Plastic surgery services were contacted for consultation. After evaluating the patient, the plastic surgeon recommended discharge home with instructions to continue taking the oral acyclovir, ASA, cephalexin, prednisone, and sildenafil for 6 days. He also recommended the patient start HBO therapy the day after discharge home.
The patient refused HBO therapy, but did visit a plastic surgeon for a follow-up examination 3 days after discharge from the ED. A photograph of the patient’s nasolabial and upper lip region taken during this visit is presented in Figure 2.
Five days after discharge from the ED, the patient presented to a plastic surgery clinic for evaluation; a photograph was also obtained at this visit (Figure 3). The plastic surgeon at this clinic referred the patient to a tertiary center for a second opinion regarding the need for HBO therapy. The plastic surgeon at the tertiary center affirmed the initial plastic surgeon’s diagnosis and recommendation for HBO therapy. Although the patient did not return for further evaluation, she underwent 10 HBO treatments at the tertiary center with an acceptable aesthetic result.
Noninvasive Injectable Cosmetic Facial Augmentation
Facial augmentation procedures include the use of autologous adipose bovine collagen, HA gels, CaHA, and plastic compounds to fill wrinkles, folds, or soft tissue defects due to normal aging or trauma. Plastic surgeons traditionally use adipose and manufactured products for scar revision, midfacial restoration of volume loss from aging or trauma, cheek and chin augmentation, tear-trough correction (the diagonal crease running from the inner eye canthus to the maxilla resulting in a groove that creates a tired appearance), nose reshaping, lip enhancement, and correction of facial asymmetry.
Today the use of manufactured soft tissue revolumization products (ie, fillers) is no longer solely in the purview of plastic surgeons, but rather has become ubiquitous with nonsurgeons and allied health care professionals. As the overall number of revolumization procedures increases, so too does the risk for local and distant vascular complications.
Dermal Fillers
Dermal fillers vary widely in their respective properties, solubility, injection-technique flow requirements, and inherent complication risks. Regardless of type, all dermal fillers have the potential to cause serious complications. Most adverse events are related to substance type, volume, and injection technique. Bruising and trauma-related edema following dermal filler procedures are considered normal.
Though complications from dermal filler injections are rarely lethal, serious adverse events can result in permanent functional and aesthetic deficits. With proper physician training, planning, and injection technique, most adverse events can be avoided.
Hyaluronic Acid. Hyaluronic acid (HA)-containing injectable gel fillers (eg, Belotero, Juvederm, Perlane, and Restylane) are one of the most commonly used volumization products—especially by nonplastic surgeons. These gel fillers, which vary in viscosity and elasticity, may be injected from the superficial dermis to the periosteum. Dilution, dispersion, and degradation may be achieved in vivo either by high arterial flow or hyaluronidase.
Calcium Hydroxylapatite. Calcium hydroxylapatite (Radiesse) microsphere fillers consist of a very viscous paste that is mixed with lidocaine prior to injection to increase its flowability. The CaHA solution is injected at the deep dermis to periosteum level. Since CaHA is not easily diluted, dispersed, or degraded by high arterial flow, it tends to retain its consistency. When this procedure is performed by a novice, it can result in complete occlusion at the injection site or through embolization via antegrade or retrograde flow.
Poly-L-lactic Acid. Poly-L-lactic acid (PLLA; Sculptra) is a low-viscosity fluid comprised of synthetic polymer beads. The PLLA microparticles are not dissolvable or degradable by high arterial flow, and are designed to induce an inflammatory response with neocollagenesis.
Polymethyl Methacrylate. Polymethyl methacrylate (PMMA; Bellafil) consists of a combination of microscopic synthetic polymer beads suspended in a variety of substances. For facial enhancement, PMMA is usually suspended in HA or bovine collagen. Off-label use of silicon oils and gels such as PMMA are gaining in popularity—often with disastrous consequences such as acute arterial occlusion, bone erosion, and skin ulcerations.2,3
Autologous Adipose Tissue. Plastic surgeons primarily use autologous adipose tissue to volumize the face, breasts, buttocks, and scars. Autologous fat grafts are typically placed in fat, superficial and deep muscles, and deep fat pads through a 2- to 2.5-mm facial fat grafting cannula using a multichannel technique that leaves minute amounts of fat in each channel. Fat embolization may occur when a nonfacial fat graft cannula or needle used to transplant the fat graft enters an artery either through direct sharp puncture or traumatic tear cannulation.4-9
Adverse Events
The first recorded manufactured adverse event from an injectable dermal filler was in 1991. At that time, the US Food and Drug Administration warned of adverse events secondary to collagen injections, including open sores, abscess formation associated with delayed healing of the skin, and partial blindness.10
Arterial Embolization and Cannulation. The most serious complications from dermal fillers are accidental injection and/or embolization of the filler into the arterial system. Since 1991, an increased number of cases of soft tissue necrosis, blindness, and stroke have been reported as a result of injection of fillers in the glabella, forehead creases, temple, crow’s feet, nose, cheeks, nasolabial folds, and lower lip.11-15
Accidental cannulation and inadvertent injection of fillers into the arterial vessels can have catastrophic complications. The potential of such inadvertent complications occurs despite skill level of the practitioner or surgeon. Therefore, recognition and treatment of a vascular occlusion must be immediate and aggressive to avoid devastating and potentially irreversible complications including blindness, stroke, and death.11-15
Acute Blindness and Stroke. The issues and concerns associated with local intra-arterial dermal filler injection and distal embolism are well described in the literature. However, the mechanism of retinal artery occlusion is much more complex given the need for deep placement of products and the force necessary to cause distention and elevation of the dermis. Hence, higher g-forces are applied via the plunger, forcing the intra-arterial filler proximally past the origin of the retinal artery. When the clinician stops the injection, the arterial systolic pressure immediately embolizes the filler into the distal branches of the ophthalmic artery. This causes acute pain and blindness (Figure 5). Depending on the g-force applied, filler can enter into the internal carotid artery and embolize resulting in cerebral ischemia. Signs of cerebral ischemia may be mild or overt.4-9
Intra-arterial placement results in pain out of proportion to the procedure and results in almost immediate skin blanching. Depending upon the duration of ischemia, there is progressive necrosis of the end target tissue (nasolabial dermis, mucosal and dermis lip, alar and nasal tip cartilage with dermis).14,16-19 Areas of tissue necrosis are also subject to secondary bacterial or viral infections, which is why the patient in Case 2 was given a course of acyclovir and cephalosporin.
Management
Patients with intra-arterial dermal filler injection constitute a medical emergency requiring immediate intervention. We recommend clinicians initiate the treatment protocols outlined in the Box and Table 1.
Hyaluronidase. Injection of hyaluronidase may assist in degrading the HA around the arterial puncture site, relieving compression, which may increase blood flow. There is a risk of distal embolization from a dislodged HA emboli.
Fat Graft Injections. There are no known degradation products for fat, CaHA, PLLA, or PMMA products. The use of normal saline or hyaluronidase has no proven efficacy and may increase the compression pressure in the artery and surrounding tissue, causing further ischemia.
Nitroglycerin. The use of topical nitroglycerin 2% will dilate the superficial vasculature with possible draw of blood from surrounding cross-feeding vessels. The nitroglycerin should be applied for 5-minute intervals every 1 to 2 hours. Adjusting the duration and frequency is necessary if the patient experiences headache or lightheadedness.
Corticosteroids. As corticosteroids help to decrease the inflammatory response in tissue ischemia, including edema, treatment should be initiated immediately. High doses (eg, 60 mg) of an oral corticosteroid for 4 days or a methylprednisolone dose pack for 6 days are both acceptable treatment options. There is no increased efficacy to giving corticosteroids via the intramuscular or intravenous (IV) route. Corticosteroid use in diabetic patients may increase blood glucose levels.
Subcutaneous enoxaparin. A low molecular weight heparin, enoxaparin should be given at a dose of 30 mg SC twice daily in patients in whom there is no known contraindication to heparins. Enoxaparin should not be given in combination with ASA therapy.
Acetylsalicylic Acid Aspirin. Patients should be given 325 mg ASA orally, which may assist fibrinolysis. Prophylactic treatment with an antacid is recommended to prevent gastritis/esophagitis associated with ASA therapy. Acetylsalicylic acid aspirin should not be given to patients in whom contraindications exist, or used in combination with enoxaparin.
Phosphodiesterase Type 5 Inhibitors. Phosphodiesterase type 5 inhibitors (eg, tadalafil, sildenafil, vardenafil) inhibit the degradation of cyclic guanosine monophosphate, allowing arterial muscle wall relaxation and increased vasodilation.
Antibiotic and Antiviral Therapy. Tissue ischemia requires treatment with prophylactic antibiotics and antivirals. Oral broad-spectrum coverage for gram-positive bacteria should be initiated (cephalosporin or penicillin). If the oral mucosa is compromised, the clinician should consider clindamycin.
Consultations. Ophthalmology consultation should be obtained if the patient has symptoms of retinal or ophthalmic artery compromise. Plastic surgery consultation should be obtained for possible HBO therapy and for possible surgical intervention. A neurology consultation should be obtained if the patient has symptoms of cerebral ischemia (Table 2).
Hyperbaric Oxygen Therapy. Patients in whom HBO therapy is recommended should receive treatment at 3 atmospheres of pressure for 45 minutes to drive oxygen into deep structures, raising the tissue oxygen tension 100-fold. Hyperbaric oxygen limits ischemic damage, cell death, and inflammation by decreasing lactate production and tissue acidosis. It also promotes collagen synthesis and angiogenesis.
Long-term Sequelae
Despite aggressive intervention, permanent complications of cerebral ischemia, blindness, and severe soft tissue necrosis may occur. In six cases in the literature, patients treated with ocular massage therapy,7,14 carbon dioxide rebreathing,14 HBO therapy,7,14 oral and IV corticosteroids,6-9 antiplatelet drugs,6 and fibrinolytic agents,7,16 or mechanical thrombolysis16 showed no clinical effects. Neither the treated nor the untreated patients in any of these case studies had any return of vision.4-7,9,20-27
Conclusion
Although artificial dermal fillers such as HA, CaHA, PLLA, and PMMA, and autologous adipose tissue grafts offer a minimally invasive alternative to plastic surgical procedures, they are not without complication or adverse effects, such as the acute arterial occlusion experienced by both of our patients. Patients presenting to the ED with adverse effects from such procedures must be managed promptly, employing the suggested management and treatment protocol, including consultation with ophthalmology, plastic surgery, and neurological services as indicated, to avoid permanent sequela and damage.
1. American Society of Plastic Surgeons. 2016 National Plastic Surgery Statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016-plastic-surgery-statistics-report.pdf. Accessed April 13, 2017.
2. Hilinski JM, Cohen SR. Soft tissue augmentation with ArteFill. Facial Plast Surg. 2009;25(2):114-119. doi:10.1055/s-0029-1220651.
3. Liu HL, Cheung WY. Complications of polyacrylamide hydrogel (PAAG) injection in facial augmentation. J Plast Reconstr Aesthet Surg. 2010;63(1):e9-e12. doi:10.1016/j.bjps.2009.05.013.
4. Danesh-Meyer HV, Savino PJ, Sergott RC. Case reports and small case series: ocular and cerebral ischemia following facial injection of autologous fat. Arch Ophthalmol. 2001;119(5):777-778.
5. Feinendegen DL, Baumgartner RW, Vuadens P, et al. Autologous fat injection for soft tissue augmentation in the face: a safe procedure? Aesthetic Plast Surg. 1998;22(3):163-167.
6. Egido JA, Arroyo R, Marcos A, Jiménez-Alfaro I. Middle cerebral artery embolism and unilateral visual loss after autologous fat injection into the glabellar area. Stroke. 1993;24(4):615-616.
7. Lee DH, Yang HN, Kim JC, Shyn KH. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80(11):1026-1027.
8. Thaunat O, Thaler F, Loirat P, Decroix JP, Boulin A. Cerebral fat embolism induced by facial fat injection. Plast Reconstr Surg. 2004;113(7):2235-2236.
9. Yoon SS, Chang DI, Chung KC. Acute fatal stroke immediately following autologous fat injection into the face. Neurology. 2003;61(8):1151-1152.
10. US Food and Drug Administration. Current and Useful Information on Collagen and Liquid Silicone Injections. FDA Backgrounder, August 1991. BG91-2.0.
11. Kwon SG, Hong JW, Roh TS, Kim YS, Rah DK, Kim SS. Ischemic oculomotor nerve palsy and skin necrosis caused by vascular embolization after hyaluronic Acid filler injection: a case report. Ann Plast Surg. 2013;71(4):333-334. doi:10.1097/SAP.0b013e31824f21da.
12. Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Exp Ophthalmol. 2006;34(4):363-364. doi:10.1111/j.1442-9071.2006.01224.x.
13. Kim YJ, Kim SS, Song WK, Lee SY, Yoon JS. Ocular ischemia with hypotony after injection of hyaluronic acid gel. Ophthal Plast Reconstr Surg. 2011;27(6):e152-e155. doi:10.1097/IOP.0b013e3182082f37.
14. Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. Dermatol Surg. 2009;35 Suppl 2:1635-1640. doi:10.1111/j.1524-4725.2009.01342.x.
15. Schanz S, Schippert W, Ulmer A, Rassner G, Fierlbeck G. Arterial embolization caused by injection of hyaluronic acid (Restylane). Br J Dermatol. 2002;146(5):928-929.
16. Georgescu D, Jones Y, McCann JD, Anderson RL. Skin necrosis after calcium hydroxylapatite injection into the glabellar and nasolabial folds. Ophthal Plast Reconstr Surg. 2009;25(6):498-499. doi:10.1097/IOP.0b013e3181b81082.
17. Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011;10(3):224-231. doi:10.1111/j.1473-2165.2011.00562.x.
18. Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006;32(2):276-281.
19. Burt B, Nakra T, Isaacs DK, Goldberg RA. Alar necrosis after facial injection of hyaluronic acid. Plast Reconstr Surg. 2010;125(5):199e-200e. doi:10.1097/PRS.0b013e3181d5152e.
20. Teimourian B. Blindness following fat injections. Plast Reconstr Surg. 1988;82(2):361.
21. Dreizen NG, Framm L. Sudden unilateral visual loss after autologous fat injection into the glabellar area. Am J Ophthalmol. 1989;107(1):85-87.
22. Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22(6):555-557. doi:10.1067/maj.2002.129625.
23. Allali J, Bernard A, Assaraf E, Bourges JL, Renard G. Multiple embolizations of the branches of the ophthalmic artery: an unknown serious complication of facial surgeries. [Article in French] J Fr Ophtalmol. 2006;29(1):51-57.
24. Mori K, Ohta K, Nagano S, Toshinori M, Yago T, Ichinose Y. A case of ophthalmic artery obstruction following autologous fat injection in the glabellar area. [Article in Japanese] Nippon Ganka Gakkai Zasshi. 2007;111(1):22-25.
25. Park SH, Sun HJ, Choi KS. Sudden unilateral visual loss after autologous fat injection into the nasolabial fold. Clin Ophthalmol. 2008;2(3):679-683.
26. Lee YJ, Kim HJ, Choi KD, Choi HY. MRI restricted diffusion in optic nerve infarction after autologous fat transplantation. J Neuroophthalmol. 2010;30(3):216-218. doi:10.1097/WNO.0b013e3181c5d147.
27. Park SJ, Woo SJ, Park KH, et al. Partial recovery after intraarterial pharmacomechanical thrombolysis in ophthalmic artery occlusion following nasal autologous fat injection. J Vasc Interv Radiol. 2011;22(2):251-254. doi:10.1016/j.jvir.2010.10.023.
Artificial dermal fillers and autologous fat grafting have become increasingly popular in recent years, primarily because they augment existing soft tissue volumes, thus producing aesthetic improvements at a lower cost than traditional plastic surgery (ie, facelift), and with nearly no recovery time. According to the American Society for Aesthetic Plastic Surgery, more than 2 million hyaluronic acid (HA) dermal filler procedures were performed in 2016, an increase of 3% from 2015.1 In addition, 80,000 autologous fat grafting procedures were performed in 2016, an increase of 13% from 2015. In total, there were 2.6 million soft tissue filler procedures in 2016, an increase of 2% from 2015.1
With the increased demand and access to both artificial dermal fillers and autologous fat grafting, there has been a plethora of reported adverse events, ranging from expected erythema to acute blindness and stroke. Emergency physicians should have a thorough understanding of facial vascular anatomy, as well as the effects of available facial volumization products, including potential complications and treatment options. Through our review of two patient cases, we propose a simplified protocol for the treatment of patients with acute arterial occlusion secondary to facial volumization procedures.
Case 1
A 38-year-old white woman presented to the ED for evaluation of transient blurred vision and blanching of the left cheek and upper lip, which began approximately 40 minutes prior to presentation, immediately after her primary care physician (PCP) injected her left nasolabial fold with calcium hydroxyapatite (CaHA). The patient stated that her vision became blurry and her eyes began to tear within 1 minute of receiving the injection. She further noted that these visual changes were painless and lasted for approximately 30 seconds.
The patient’s PCP believed these symptoms were due to pain at the injection site. While the patient was at her PCP’s office, the reception clerk noticed the blanching of the patient’s left cheek and informed the PCP, who referred the patient to our ED for evaluation.
Workup
The patient’s vital signs at presentation were normal. Her medical history was unremarkable and negative for smoking, alcohol, or drug use. She was not taking any medications and had no known drug allergies. The patient’s history was negative for any prior cosmetic procedures, and she confirmed this was the first and only time had a facial revolumization.
Facial examination revealed a Fitzpatrick scale (FS; a numerical scoring system used to assess a patient’s reaction to ultraviolet radiation) score of type 3. She also had left-sided blanching that extended from the midpoint of the nose diagonally to the lateral midbuccal cheek to the level of the oral commissure, including the cutaneous upper lip, alar, and nasal side wall. There was minimal capillary refill with compression at the affected site, and sensation was diminished to fine touch and pinprick. The facial muscles were intact, and, with the exception of puncture marks along both nasolabial folds, the remainder of the facial examination was normal.
The ophthalmic examination revealed a reactive pupil at 2 mm, white sclera, pink conjunctiva, red reflex, and normal fundoscopic vessels. The patient’s bedside Snellen visual acuity and visual field assessments were normal. The neurological examination was likewise normal, and no other physical findings were noted.
Laboratory evaluation included complete blood count (CBC), Chem 7 panel (creatinine, blood urea nitrogen [BUN], carbon dioxide, chloride, glucose, sodium, and potassium), and international normalized ratio (INR), which were all within normal limits.
Diagnosis and Treatment
The patient was diagnosed with acute angular arterial occlusion and transient retinal artery embolism secondary to facial volumization with CaHA. She was treated with oral acetylsalicylic acid aspirin (ASA) 325 mg, prednisone 40 mg, and sildenafil 50 mg; and subcutaneous (SC) enoxaparin 60 mg (1 mg/kg). Topical nitroglycerin paste 2% was applied to the affected area.
Ophthalmology and plastic surgery services were contacted for consultation. Based on no acute findings on examination, the ophthalmologist provided no additional treatment recommendations. The patient was observed in the ED for 4 hours, during which time the facial blanching resolved and her capillary refill time returned to normal at 2 seconds.
After evaluating the patient, the plastic surgeon recommended discharge home with instructions to continue taking the oral ASA and sildenafil, as well as a methylprednisolone dose pack for 6 days. He also recommended the patient begin hyperbaric oxygen (HBO) therapy the day after discharge, since there was no HBO chamber available during her hospital stay.
The patient complied with all discharge instructions, including HBO therapy. At plastic surgery follow-up, the patient had no long-term adverse effects from the CaHA injection.
Case 2
A 54-year-old Asian woman presented to the ED for evaluation of a 24-hour history of progressive and persistent pain, swelling, and discoloration of the nasolabial and upper lip region. She stated her symptoms began within 1 hour of receiving a fat graft injection into the affected area by her cosmetic surgeon. After examining the patient, the cosmetic surgeon referred her to the ED for further evaluation. The patient stated that she had undergone six prior facial revolumization procedures, but noted the recent procedure was her first autologous fat graft.
Workup
The patient’s medical history was unremarkable. Her social history was positive for one glass of wine per day and negative for smoking. The patient was not taking any medications and had no known drug allergies.
The patient’s vital signs at presentation were normal. She was evaluated approximately 30 hours after the fat graft procedure. Facial examination revealed an FS of type 4 with right-sided ischemia along the cutaneous upper lip, alar, and cheek (Figure 1).
Capillary refill time with compression was 0 in the affected area. Sensation to fine touch and pinprick was 0. The facial muscles were intact and, with the exception of puncture marks along both nasolabial folds, the remainder of the facial examination was normal. The neurological examination was likewise normal, and no other physical findings were noted. Laboratory evaluation included CBC, Chem 7 panel, and INR, which were all within normal limits.
Diagnosis and Treatment
The patient was diagnosed with acute angular arterial occlusion with soft tissue ischemia secondary to facial revolumization with autologous fat grafting. She was given oral acyclovir 800 mg, ASA 325 mg, cephalexin 500 mg, prednisone 40 mg, and sildenafil 50 mg; and SC enoxaparin 60 mg (1 mg/kg). Topical nitroglycerin paste 2% was applied to the affected area.
Plastic surgery services were contacted for consultation. After evaluating the patient, the plastic surgeon recommended discharge home with instructions to continue taking the oral acyclovir, ASA, cephalexin, prednisone, and sildenafil for 6 days. He also recommended the patient start HBO therapy the day after discharge home.
The patient refused HBO therapy, but did visit a plastic surgeon for a follow-up examination 3 days after discharge from the ED. A photograph of the patient’s nasolabial and upper lip region taken during this visit is presented in Figure 2.
Five days after discharge from the ED, the patient presented to a plastic surgery clinic for evaluation; a photograph was also obtained at this visit (Figure 3). The plastic surgeon at this clinic referred the patient to a tertiary center for a second opinion regarding the need for HBO therapy. The plastic surgeon at the tertiary center affirmed the initial plastic surgeon’s diagnosis and recommendation for HBO therapy. Although the patient did not return for further evaluation, she underwent 10 HBO treatments at the tertiary center with an acceptable aesthetic result.
Noninvasive Injectable Cosmetic Facial Augmentation
Facial augmentation procedures include the use of autologous adipose bovine collagen, HA gels, CaHA, and plastic compounds to fill wrinkles, folds, or soft tissue defects due to normal aging or trauma. Plastic surgeons traditionally use adipose and manufactured products for scar revision, midfacial restoration of volume loss from aging or trauma, cheek and chin augmentation, tear-trough correction (the diagonal crease running from the inner eye canthus to the maxilla resulting in a groove that creates a tired appearance), nose reshaping, lip enhancement, and correction of facial asymmetry.
Today the use of manufactured soft tissue revolumization products (ie, fillers) is no longer solely in the purview of plastic surgeons, but rather has become ubiquitous with nonsurgeons and allied health care professionals. As the overall number of revolumization procedures increases, so too does the risk for local and distant vascular complications.
Dermal Fillers
Dermal fillers vary widely in their respective properties, solubility, injection-technique flow requirements, and inherent complication risks. Regardless of type, all dermal fillers have the potential to cause serious complications. Most adverse events are related to substance type, volume, and injection technique. Bruising and trauma-related edema following dermal filler procedures are considered normal.
Though complications from dermal filler injections are rarely lethal, serious adverse events can result in permanent functional and aesthetic deficits. With proper physician training, planning, and injection technique, most adverse events can be avoided.
Hyaluronic Acid. Hyaluronic acid (HA)-containing injectable gel fillers (eg, Belotero, Juvederm, Perlane, and Restylane) are one of the most commonly used volumization products—especially by nonplastic surgeons. These gel fillers, which vary in viscosity and elasticity, may be injected from the superficial dermis to the periosteum. Dilution, dispersion, and degradation may be achieved in vivo either by high arterial flow or hyaluronidase.
Calcium Hydroxylapatite. Calcium hydroxylapatite (Radiesse) microsphere fillers consist of a very viscous paste that is mixed with lidocaine prior to injection to increase its flowability. The CaHA solution is injected at the deep dermis to periosteum level. Since CaHA is not easily diluted, dispersed, or degraded by high arterial flow, it tends to retain its consistency. When this procedure is performed by a novice, it can result in complete occlusion at the injection site or through embolization via antegrade or retrograde flow.
Poly-L-lactic Acid. Poly-L-lactic acid (PLLA; Sculptra) is a low-viscosity fluid comprised of synthetic polymer beads. The PLLA microparticles are not dissolvable or degradable by high arterial flow, and are designed to induce an inflammatory response with neocollagenesis.
Polymethyl Methacrylate. Polymethyl methacrylate (PMMA; Bellafil) consists of a combination of microscopic synthetic polymer beads suspended in a variety of substances. For facial enhancement, PMMA is usually suspended in HA or bovine collagen. Off-label use of silicon oils and gels such as PMMA are gaining in popularity—often with disastrous consequences such as acute arterial occlusion, bone erosion, and skin ulcerations.2,3
Autologous Adipose Tissue. Plastic surgeons primarily use autologous adipose tissue to volumize the face, breasts, buttocks, and scars. Autologous fat grafts are typically placed in fat, superficial and deep muscles, and deep fat pads through a 2- to 2.5-mm facial fat grafting cannula using a multichannel technique that leaves minute amounts of fat in each channel. Fat embolization may occur when a nonfacial fat graft cannula or needle used to transplant the fat graft enters an artery either through direct sharp puncture or traumatic tear cannulation.4-9
Adverse Events
The first recorded manufactured adverse event from an injectable dermal filler was in 1991. At that time, the US Food and Drug Administration warned of adverse events secondary to collagen injections, including open sores, abscess formation associated with delayed healing of the skin, and partial blindness.10
Arterial Embolization and Cannulation. The most serious complications from dermal fillers are accidental injection and/or embolization of the filler into the arterial system. Since 1991, an increased number of cases of soft tissue necrosis, blindness, and stroke have been reported as a result of injection of fillers in the glabella, forehead creases, temple, crow’s feet, nose, cheeks, nasolabial folds, and lower lip.11-15
Accidental cannulation and inadvertent injection of fillers into the arterial vessels can have catastrophic complications. The potential of such inadvertent complications occurs despite skill level of the practitioner or surgeon. Therefore, recognition and treatment of a vascular occlusion must be immediate and aggressive to avoid devastating and potentially irreversible complications including blindness, stroke, and death.11-15
Acute Blindness and Stroke. The issues and concerns associated with local intra-arterial dermal filler injection and distal embolism are well described in the literature. However, the mechanism of retinal artery occlusion is much more complex given the need for deep placement of products and the force necessary to cause distention and elevation of the dermis. Hence, higher g-forces are applied via the plunger, forcing the intra-arterial filler proximally past the origin of the retinal artery. When the clinician stops the injection, the arterial systolic pressure immediately embolizes the filler into the distal branches of the ophthalmic artery. This causes acute pain and blindness (Figure 5). Depending on the g-force applied, filler can enter into the internal carotid artery and embolize resulting in cerebral ischemia. Signs of cerebral ischemia may be mild or overt.4-9
Intra-arterial placement results in pain out of proportion to the procedure and results in almost immediate skin blanching. Depending upon the duration of ischemia, there is progressive necrosis of the end target tissue (nasolabial dermis, mucosal and dermis lip, alar and nasal tip cartilage with dermis).14,16-19 Areas of tissue necrosis are also subject to secondary bacterial or viral infections, which is why the patient in Case 2 was given a course of acyclovir and cephalosporin.
Management
Patients with intra-arterial dermal filler injection constitute a medical emergency requiring immediate intervention. We recommend clinicians initiate the treatment protocols outlined in the Box and Table 1.
Hyaluronidase. Injection of hyaluronidase may assist in degrading the HA around the arterial puncture site, relieving compression, which may increase blood flow. There is a risk of distal embolization from a dislodged HA emboli.
Fat Graft Injections. There are no known degradation products for fat, CaHA, PLLA, or PMMA products. The use of normal saline or hyaluronidase has no proven efficacy and may increase the compression pressure in the artery and surrounding tissue, causing further ischemia.

Nitroglycerin. The use of topical nitroglycerin 2% will dilate the superficial vasculature with possible draw of blood from surrounding cross-feeding vessels. The nitroglycerin should be applied for 5-minute intervals every 1 to 2 hours. Adjusting the duration and frequency is necessary if the patient experiences headache or lightheadedness.
Corticosteroids. As corticosteroids help to decrease the inflammatory response in tissue ischemia, including edema, treatment should be initiated immediately. High doses (eg, 60 mg) of an oral corticosteroid for 4 days or a methylprednisolone dose pack for 6 days are both acceptable treatment options. There is no increased efficacy to giving corticosteroids via the intramuscular or intravenous (IV) route. Corticosteroid use in diabetic patients may increase blood glucose levels.
Subcutaneous enoxaparin. A low molecular weight heparin, enoxaparin should be given at a dose of 30 mg SC twice daily in patients in whom there is no known contraindication to heparins. Enoxaparin should not be given in combination with ASA therapy.
Acetylsalicylic Acid Aspirin. Patients should be given 325 mg ASA orally, which may assist fibrinolysis. Prophylactic treatment with an antacid is recommended to prevent gastritis/esophagitis associated with ASA therapy. Acetylsalicylic acid aspirin should not be given to patients in whom contraindications exist, or used in combination with enoxaparin.
Phosphodiesterase Type 5 Inhibitors. Phosphodiesterase type 5 inhibitors (eg, tadalafil, sildenafil, vardenafil) inhibit the degradation of cyclic guanosine monophosphate, allowing arterial muscle wall relaxation and increased vasodilation.
Antibiotic and Antiviral Therapy. Tissue ischemia requires treatment with prophylactic antibiotics and antivirals. Oral broad-spectrum coverage for gram-positive bacteria should be initiated (cephalosporin or penicillin). If the oral mucosa is compromised, the clinician should consider clindamycin.
Consultations. Ophthalmology consultation should be obtained if the patient has symptoms of retinal or ophthalmic artery compromise. Plastic surgery consultation should be obtained for possible HBO therapy and for possible surgical intervention. A neurology consultation should be obtained if the patient has symptoms of cerebral ischemia (Table 2).

Hyperbaric Oxygen Therapy. Patients in whom HBO therapy is recommended should receive treatment at 3 atmospheres of pressure for 45 minutes to drive oxygen into deep structures, raising the tissue oxygen tension 100-fold. Hyperbaric oxygen limits ischemic damage, cell death, and inflammation by decreasing lactate production and tissue acidosis. It also promotes collagen synthesis and angiogenesis.
Long-term Sequelae
Despite aggressive intervention, permanent complications of cerebral ischemia, blindness, and severe soft tissue necrosis may occur. In six cases in the literature, patients treated with ocular massage therapy,7,14 carbon dioxide rebreathing,14 HBO therapy,7,14 oral and IV corticosteroids,6-9 antiplatelet drugs,6 and fibrinolytic agents,7,16 or mechanical thrombolysis16 showed no clinical effects. Neither the treated nor the untreated patients in any of these case studies had any return of vision.4-7,9,20-27
Conclusion
Although artificial dermal fillers such as HA, CaHA, PLLA, and PMMA, and autologous adipose tissue grafts offer a minimally invasive alternative to plastic surgical procedures, they are not without complication or adverse effects, such as the acute arterial occlusion experienced by both of our patients. Patients presenting to the ED with adverse effects from such procedures must be managed promptly, employing the suggested management and treatment protocol, including consultation with ophthalmology, plastic surgery, and neurological services as indicated, to avoid permanent sequela and damage.
Artificial dermal fillers and autologous fat grafting have become increasingly popular in recent years, primarily because they augment existing soft tissue volumes, thus producing aesthetic improvements at a lower cost than traditional plastic surgery (ie, facelift), and with nearly no recovery time. According to the American Society for Aesthetic Plastic Surgery, more than 2 million hyaluronic acid (HA) dermal filler procedures were performed in 2016, an increase of 3% from 2015.1 In addition, 80,000 autologous fat grafting procedures were performed in 2016, an increase of 13% from 2015. In total, there were 2.6 million soft tissue filler procedures in 2016, an increase of 2% from 2015.1
With the increased demand and access to both artificial dermal fillers and autologous fat grafting, there has been a plethora of reported adverse events, ranging from expected erythema to acute blindness and stroke. Emergency physicians should have a thorough understanding of facial vascular anatomy, as well as the effects of available facial volumization products, including potential complications and treatment options. Through our review of two patient cases, we propose a simplified protocol for the treatment of patients with acute arterial occlusion secondary to facial volumization procedures.
Case 1
A 38-year-old white woman presented to the ED for evaluation of transient blurred vision and blanching of the left cheek and upper lip, which began approximately 40 minutes prior to presentation, immediately after her primary care physician (PCP) injected her left nasolabial fold with calcium hydroxyapatite (CaHA). The patient stated that her vision became blurry and her eyes began to tear within 1 minute of receiving the injection. She further noted that these visual changes were painless and lasted for approximately 30 seconds.
The patient’s PCP believed these symptoms were due to pain at the injection site. While the patient was at her PCP’s office, the reception clerk noticed the blanching of the patient’s left cheek and informed the PCP, who referred the patient to our ED for evaluation.
Workup
The patient’s vital signs at presentation were normal. Her medical history was unremarkable and negative for smoking, alcohol, or drug use. She was not taking any medications and had no known drug allergies. The patient’s history was negative for any prior cosmetic procedures, and she confirmed this was the first and only time had a facial revolumization.
Facial examination revealed a Fitzpatrick scale (FS; a numerical scoring system used to assess a patient’s reaction to ultraviolet radiation) score of type 3. She also had left-sided blanching that extended from the midpoint of the nose diagonally to the lateral midbuccal cheek to the level of the oral commissure, including the cutaneous upper lip, alar, and nasal side wall. There was minimal capillary refill with compression at the affected site, and sensation was diminished to fine touch and pinprick. The facial muscles were intact, and, with the exception of puncture marks along both nasolabial folds, the remainder of the facial examination was normal.
The ophthalmic examination revealed a reactive pupil at 2 mm, white sclera, pink conjunctiva, red reflex, and normal fundoscopic vessels. The patient’s bedside Snellen visual acuity and visual field assessments were normal. The neurological examination was likewise normal, and no other physical findings were noted.
Laboratory evaluation included complete blood count (CBC), Chem 7 panel (creatinine, blood urea nitrogen [BUN], carbon dioxide, chloride, glucose, sodium, and potassium), and international normalized ratio (INR), which were all within normal limits.
Diagnosis and Treatment
The patient was diagnosed with acute angular arterial occlusion and transient retinal artery embolism secondary to facial volumization with CaHA. She was treated with oral acetylsalicylic acid aspirin (ASA) 325 mg, prednisone 40 mg, and sildenafil 50 mg; and subcutaneous (SC) enoxaparin 60 mg (1 mg/kg). Topical nitroglycerin paste 2% was applied to the affected area.
Ophthalmology and plastic surgery services were contacted for consultation. Based on no acute findings on examination, the ophthalmologist provided no additional treatment recommendations. The patient was observed in the ED for 4 hours, during which time the facial blanching resolved and her capillary refill time returned to normal at 2 seconds.
After evaluating the patient, the plastic surgeon recommended discharge home with instructions to continue taking the oral ASA and sildenafil, as well as a methylprednisolone dose pack for 6 days. He also recommended the patient begin hyperbaric oxygen (HBO) therapy the day after discharge, since there was no HBO chamber available during her hospital stay.
The patient complied with all discharge instructions, including HBO therapy. At plastic surgery follow-up, the patient had no long-term adverse effects from the CaHA injection.
Case 2
A 54-year-old Asian woman presented to the ED for evaluation of a 24-hour history of progressive and persistent pain, swelling, and discoloration of the nasolabial and upper lip region. She stated her symptoms began within 1 hour of receiving a fat graft injection into the affected area by her cosmetic surgeon. After examining the patient, the cosmetic surgeon referred her to the ED for further evaluation. The patient stated that she had undergone six prior facial revolumization procedures, but noted the recent procedure was her first autologous fat graft.
Workup
The patient’s medical history was unremarkable. Her social history was positive for one glass of wine per day and negative for smoking. The patient was not taking any medications and had no known drug allergies.
The patient’s vital signs at presentation were normal. She was evaluated approximately 30 hours after the fat graft procedure. Facial examination revealed an FS of type 4 with right-sided ischemia along the cutaneous upper lip, alar, and cheek (Figure 1).
Capillary refill time with compression was 0 in the affected area. Sensation to fine touch and pinprick was 0. The facial muscles were intact and, with the exception of puncture marks along both nasolabial folds, the remainder of the facial examination was normal. The neurological examination was likewise normal, and no other physical findings were noted. Laboratory evaluation included CBC, Chem 7 panel, and INR, which were all within normal limits.
Diagnosis and Treatment
The patient was diagnosed with acute angular arterial occlusion with soft tissue ischemia secondary to facial revolumization with autologous fat grafting. She was given oral acyclovir 800 mg, ASA 325 mg, cephalexin 500 mg, prednisone 40 mg, and sildenafil 50 mg; and SC enoxaparin 60 mg (1 mg/kg). Topical nitroglycerin paste 2% was applied to the affected area.
Plastic surgery services were contacted for consultation. After evaluating the patient, the plastic surgeon recommended discharge home with instructions to continue taking the oral acyclovir, ASA, cephalexin, prednisone, and sildenafil for 6 days. He also recommended the patient start HBO therapy the day after discharge home.
The patient refused HBO therapy, but did visit a plastic surgeon for a follow-up examination 3 days after discharge from the ED. A photograph of the patient’s nasolabial and upper lip region taken during this visit is presented in Figure 2.
Five days after discharge from the ED, the patient presented to a plastic surgery clinic for evaluation; a photograph was also obtained at this visit (Figure 3). The plastic surgeon at this clinic referred the patient to a tertiary center for a second opinion regarding the need for HBO therapy. The plastic surgeon at the tertiary center affirmed the initial plastic surgeon’s diagnosis and recommendation for HBO therapy. Although the patient did not return for further evaluation, she underwent 10 HBO treatments at the tertiary center with an acceptable aesthetic result.
Noninvasive Injectable Cosmetic Facial Augmentation
Facial augmentation procedures include the use of autologous adipose bovine collagen, HA gels, CaHA, and plastic compounds to fill wrinkles, folds, or soft tissue defects due to normal aging or trauma. Plastic surgeons traditionally use adipose and manufactured products for scar revision, midfacial restoration of volume loss from aging or trauma, cheek and chin augmentation, tear-trough correction (the diagonal crease running from the inner eye canthus to the maxilla resulting in a groove that creates a tired appearance), nose reshaping, lip enhancement, and correction of facial asymmetry.
Today the use of manufactured soft tissue revolumization products (ie, fillers) is no longer solely in the purview of plastic surgeons, but rather has become ubiquitous with nonsurgeons and allied health care professionals. As the overall number of revolumization procedures increases, so too does the risk for local and distant vascular complications.
Dermal Fillers
Dermal fillers vary widely in their respective properties, solubility, injection-technique flow requirements, and inherent complication risks. Regardless of type, all dermal fillers have the potential to cause serious complications. Most adverse events are related to substance type, volume, and injection technique. Bruising and trauma-related edema following dermal filler procedures are considered normal.
Though complications from dermal filler injections are rarely lethal, serious adverse events can result in permanent functional and aesthetic deficits. With proper physician training, planning, and injection technique, most adverse events can be avoided.
Hyaluronic Acid. Hyaluronic acid (HA)-containing injectable gel fillers (eg, Belotero, Juvederm, Perlane, and Restylane) are one of the most commonly used volumization products—especially by nonplastic surgeons. These gel fillers, which vary in viscosity and elasticity, may be injected from the superficial dermis to the periosteum. Dilution, dispersion, and degradation may be achieved in vivo either by high arterial flow or hyaluronidase.
Calcium Hydroxylapatite. Calcium hydroxylapatite (Radiesse) microsphere fillers consist of a very viscous paste that is mixed with lidocaine prior to injection to increase its flowability. The CaHA solution is injected at the deep dermis to periosteum level. Since CaHA is not easily diluted, dispersed, or degraded by high arterial flow, it tends to retain its consistency. When this procedure is performed by a novice, it can result in complete occlusion at the injection site or through embolization via antegrade or retrograde flow.
Poly-L-lactic Acid. Poly-L-lactic acid (PLLA; Sculptra) is a low-viscosity fluid comprised of synthetic polymer beads. The PLLA microparticles are not dissolvable or degradable by high arterial flow, and are designed to induce an inflammatory response with neocollagenesis.
Polymethyl Methacrylate. Polymethyl methacrylate (PMMA; Bellafil) consists of a combination of microscopic synthetic polymer beads suspended in a variety of substances. For facial enhancement, PMMA is usually suspended in HA or bovine collagen. Off-label use of silicon oils and gels such as PMMA are gaining in popularity—often with disastrous consequences such as acute arterial occlusion, bone erosion, and skin ulcerations.2,3
Autologous Adipose Tissue. Plastic surgeons primarily use autologous adipose tissue to volumize the face, breasts, buttocks, and scars. Autologous fat grafts are typically placed in fat, superficial and deep muscles, and deep fat pads through a 2- to 2.5-mm facial fat grafting cannula using a multichannel technique that leaves minute amounts of fat in each channel. Fat embolization may occur when a nonfacial fat graft cannula or needle used to transplant the fat graft enters an artery either through direct sharp puncture or traumatic tear cannulation.4-9
Adverse Events
The first recorded manufactured adverse event from an injectable dermal filler was in 1991. At that time, the US Food and Drug Administration warned of adverse events secondary to collagen injections, including open sores, abscess formation associated with delayed healing of the skin, and partial blindness.10
Arterial Embolization and Cannulation. The most serious complications from dermal fillers are accidental injection and/or embolization of the filler into the arterial system. Since 1991, an increased number of cases of soft tissue necrosis, blindness, and stroke have been reported as a result of injection of fillers in the glabella, forehead creases, temple, crow’s feet, nose, cheeks, nasolabial folds, and lower lip.11-15
Accidental cannulation and inadvertent injection of fillers into the arterial vessels can have catastrophic complications. The potential of such inadvertent complications occurs despite skill level of the practitioner or surgeon. Therefore, recognition and treatment of a vascular occlusion must be immediate and aggressive to avoid devastating and potentially irreversible complications including blindness, stroke, and death.11-15
Acute Blindness and Stroke. The issues and concerns associated with local intra-arterial dermal filler injection and distal embolism are well described in the literature. However, the mechanism of retinal artery occlusion is much more complex given the need for deep placement of products and the force necessary to cause distention and elevation of the dermis. Hence, higher g-forces are applied via the plunger, forcing the intra-arterial filler proximally past the origin of the retinal artery. When the clinician stops the injection, the arterial systolic pressure immediately embolizes the filler into the distal branches of the ophthalmic artery. This causes acute pain and blindness (Figure 5). Depending on the g-force applied, filler can enter into the internal carotid artery and embolize resulting in cerebral ischemia. Signs of cerebral ischemia may be mild or overt.4-9
Intra-arterial placement results in pain out of proportion to the procedure and results in almost immediate skin blanching. Depending upon the duration of ischemia, there is progressive necrosis of the end target tissue (nasolabial dermis, mucosal and dermis lip, alar and nasal tip cartilage with dermis).14,16-19 Areas of tissue necrosis are also subject to secondary bacterial or viral infections, which is why the patient in Case 2 was given a course of acyclovir and cephalosporin.
Management
Patients with intra-arterial dermal filler injection constitute a medical emergency requiring immediate intervention. We recommend clinicians initiate the treatment protocols outlined in the Box and Table 1.
Hyaluronidase. Injection of hyaluronidase may assist in degrading the HA around the arterial puncture site, relieving compression, which may increase blood flow. There is a risk of distal embolization from a dislodged HA emboli.
Fat Graft Injections. There are no known degradation products for fat, CaHA, PLLA, or PMMA products. The use of normal saline or hyaluronidase has no proven efficacy and may increase the compression pressure in the artery and surrounding tissue, causing further ischemia.

Nitroglycerin. The use of topical nitroglycerin 2% will dilate the superficial vasculature with possible draw of blood from surrounding cross-feeding vessels. The nitroglycerin should be applied for 5-minute intervals every 1 to 2 hours. Adjusting the duration and frequency is necessary if the patient experiences headache or lightheadedness.
Corticosteroids. As corticosteroids help to decrease the inflammatory response in tissue ischemia, including edema, treatment should be initiated immediately. High doses (eg, 60 mg) of an oral corticosteroid for 4 days or a methylprednisolone dose pack for 6 days are both acceptable treatment options. There is no increased efficacy to giving corticosteroids via the intramuscular or intravenous (IV) route. Corticosteroid use in diabetic patients may increase blood glucose levels.
Subcutaneous enoxaparin. A low molecular weight heparin, enoxaparin should be given at a dose of 30 mg SC twice daily in patients in whom there is no known contraindication to heparins. Enoxaparin should not be given in combination with ASA therapy.
Acetylsalicylic Acid Aspirin. Patients should be given 325 mg ASA orally, which may assist fibrinolysis. Prophylactic treatment with an antacid is recommended to prevent gastritis/esophagitis associated with ASA therapy. Acetylsalicylic acid aspirin should not be given to patients in whom contraindications exist, or used in combination with enoxaparin.
Phosphodiesterase Type 5 Inhibitors. Phosphodiesterase type 5 inhibitors (eg, tadalafil, sildenafil, vardenafil) inhibit the degradation of cyclic guanosine monophosphate, allowing arterial muscle wall relaxation and increased vasodilation.
Antibiotic and Antiviral Therapy. Tissue ischemia requires treatment with prophylactic antibiotics and antivirals. Oral broad-spectrum coverage for gram-positive bacteria should be initiated (cephalosporin or penicillin). If the oral mucosa is compromised, the clinician should consider clindamycin.
Consultations. Ophthalmology consultation should be obtained if the patient has symptoms of retinal or ophthalmic artery compromise. Plastic surgery consultation should be obtained for possible HBO therapy and for possible surgical intervention. A neurology consultation should be obtained if the patient has symptoms of cerebral ischemia (Table 2).

Hyperbaric Oxygen Therapy. Patients in whom HBO therapy is recommended should receive treatment at 3 atmospheres of pressure for 45 minutes to drive oxygen into deep structures, raising the tissue oxygen tension 100-fold. Hyperbaric oxygen limits ischemic damage, cell death, and inflammation by decreasing lactate production and tissue acidosis. It also promotes collagen synthesis and angiogenesis.
Long-term Sequelae
Despite aggressive intervention, permanent complications of cerebral ischemia, blindness, and severe soft tissue necrosis may occur. In six cases in the literature, patients treated with ocular massage therapy,7,14 carbon dioxide rebreathing,14 HBO therapy,7,14 oral and IV corticosteroids,6-9 antiplatelet drugs,6 and fibrinolytic agents,7,16 or mechanical thrombolysis16 showed no clinical effects. Neither the treated nor the untreated patients in any of these case studies had any return of vision.4-7,9,20-27
Conclusion
Although artificial dermal fillers such as HA, CaHA, PLLA, and PMMA, and autologous adipose tissue grafts offer a minimally invasive alternative to plastic surgical procedures, they are not without complication or adverse effects, such as the acute arterial occlusion experienced by both of our patients. Patients presenting to the ED with adverse effects from such procedures must be managed promptly, employing the suggested management and treatment protocol, including consultation with ophthalmology, plastic surgery, and neurological services as indicated, to avoid permanent sequela and damage.
1. American Society of Plastic Surgeons. 2016 National Plastic Surgery Statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016-plastic-surgery-statistics-report.pdf. Accessed April 13, 2017.
2. Hilinski JM, Cohen SR. Soft tissue augmentation with ArteFill. Facial Plast Surg. 2009;25(2):114-119. doi:10.1055/s-0029-1220651.
3. Liu HL, Cheung WY. Complications of polyacrylamide hydrogel (PAAG) injection in facial augmentation. J Plast Reconstr Aesthet Surg. 2010;63(1):e9-e12. doi:10.1016/j.bjps.2009.05.013.
4. Danesh-Meyer HV, Savino PJ, Sergott RC. Case reports and small case series: ocular and cerebral ischemia following facial injection of autologous fat. Arch Ophthalmol. 2001;119(5):777-778.
5. Feinendegen DL, Baumgartner RW, Vuadens P, et al. Autologous fat injection for soft tissue augmentation in the face: a safe procedure? Aesthetic Plast Surg. 1998;22(3):163-167.
6. Egido JA, Arroyo R, Marcos A, Jiménez-Alfaro I. Middle cerebral artery embolism and unilateral visual loss after autologous fat injection into the glabellar area. Stroke. 1993;24(4):615-616.
7. Lee DH, Yang HN, Kim JC, Shyn KH. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80(11):1026-1027.
8. Thaunat O, Thaler F, Loirat P, Decroix JP, Boulin A. Cerebral fat embolism induced by facial fat injection. Plast Reconstr Surg. 2004;113(7):2235-2236.
9. Yoon SS, Chang DI, Chung KC. Acute fatal stroke immediately following autologous fat injection into the face. Neurology. 2003;61(8):1151-1152.
10. US Food and Drug Administration. Current and Useful Information on Collagen and Liquid Silicone Injections. FDA Backgrounder, August 1991. BG91-2.0.
11. Kwon SG, Hong JW, Roh TS, Kim YS, Rah DK, Kim SS. Ischemic oculomotor nerve palsy and skin necrosis caused by vascular embolization after hyaluronic Acid filler injection: a case report. Ann Plast Surg. 2013;71(4):333-334. doi:10.1097/SAP.0b013e31824f21da.
12. Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Exp Ophthalmol. 2006;34(4):363-364. doi:10.1111/j.1442-9071.2006.01224.x.
13. Kim YJ, Kim SS, Song WK, Lee SY, Yoon JS. Ocular ischemia with hypotony after injection of hyaluronic acid gel. Ophthal Plast Reconstr Surg. 2011;27(6):e152-e155. doi:10.1097/IOP.0b013e3182082f37.
14. Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. Dermatol Surg. 2009;35 Suppl 2:1635-1640. doi:10.1111/j.1524-4725.2009.01342.x.
15. Schanz S, Schippert W, Ulmer A, Rassner G, Fierlbeck G. Arterial embolization caused by injection of hyaluronic acid (Restylane). Br J Dermatol. 2002;146(5):928-929.
16. Georgescu D, Jones Y, McCann JD, Anderson RL. Skin necrosis after calcium hydroxylapatite injection into the glabellar and nasolabial folds. Ophthal Plast Reconstr Surg. 2009;25(6):498-499. doi:10.1097/IOP.0b013e3181b81082.
17. Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011;10(3):224-231. doi:10.1111/j.1473-2165.2011.00562.x.
18. Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006;32(2):276-281.
19. Burt B, Nakra T, Isaacs DK, Goldberg RA. Alar necrosis after facial injection of hyaluronic acid. Plast Reconstr Surg. 2010;125(5):199e-200e. doi:10.1097/PRS.0b013e3181d5152e.
20. Teimourian B. Blindness following fat injections. Plast Reconstr Surg. 1988;82(2):361.
21. Dreizen NG, Framm L. Sudden unilateral visual loss after autologous fat injection into the glabellar area. Am J Ophthalmol. 1989;107(1):85-87.
22. Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22(6):555-557. doi:10.1067/maj.2002.129625.
23. Allali J, Bernard A, Assaraf E, Bourges JL, Renard G. Multiple embolizations of the branches of the ophthalmic artery: an unknown serious complication of facial surgeries. [Article in French] J Fr Ophtalmol. 2006;29(1):51-57.
24. Mori K, Ohta K, Nagano S, Toshinori M, Yago T, Ichinose Y. A case of ophthalmic artery obstruction following autologous fat injection in the glabellar area. [Article in Japanese] Nippon Ganka Gakkai Zasshi. 2007;111(1):22-25.
25. Park SH, Sun HJ, Choi KS. Sudden unilateral visual loss after autologous fat injection into the nasolabial fold. Clin Ophthalmol. 2008;2(3):679-683.
26. Lee YJ, Kim HJ, Choi KD, Choi HY. MRI restricted diffusion in optic nerve infarction after autologous fat transplantation. J Neuroophthalmol. 2010;30(3):216-218. doi:10.1097/WNO.0b013e3181c5d147.
27. Park SJ, Woo SJ, Park KH, et al. Partial recovery after intraarterial pharmacomechanical thrombolysis in ophthalmic artery occlusion following nasal autologous fat injection. J Vasc Interv Radiol. 2011;22(2):251-254. doi:10.1016/j.jvir.2010.10.023.
1. American Society of Plastic Surgeons. 2016 National Plastic Surgery Statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016-plastic-surgery-statistics-report.pdf. Accessed April 13, 2017.
2. Hilinski JM, Cohen SR. Soft tissue augmentation with ArteFill. Facial Plast Surg. 2009;25(2):114-119. doi:10.1055/s-0029-1220651.
3. Liu HL, Cheung WY. Complications of polyacrylamide hydrogel (PAAG) injection in facial augmentation. J Plast Reconstr Aesthet Surg. 2010;63(1):e9-e12. doi:10.1016/j.bjps.2009.05.013.
4. Danesh-Meyer HV, Savino PJ, Sergott RC. Case reports and small case series: ocular and cerebral ischemia following facial injection of autologous fat. Arch Ophthalmol. 2001;119(5):777-778.
5. Feinendegen DL, Baumgartner RW, Vuadens P, et al. Autologous fat injection for soft tissue augmentation in the face: a safe procedure? Aesthetic Plast Surg. 1998;22(3):163-167.
6. Egido JA, Arroyo R, Marcos A, Jiménez-Alfaro I. Middle cerebral artery embolism and unilateral visual loss after autologous fat injection into the glabellar area. Stroke. 1993;24(4):615-616.
7. Lee DH, Yang HN, Kim JC, Shyn KH. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80(11):1026-1027.
8. Thaunat O, Thaler F, Loirat P, Decroix JP, Boulin A. Cerebral fat embolism induced by facial fat injection. Plast Reconstr Surg. 2004;113(7):2235-2236.
9. Yoon SS, Chang DI, Chung KC. Acute fatal stroke immediately following autologous fat injection into the face. Neurology. 2003;61(8):1151-1152.
10. US Food and Drug Administration. Current and Useful Information on Collagen and Liquid Silicone Injections. FDA Backgrounder, August 1991. BG91-2.0.
11. Kwon SG, Hong JW, Roh TS, Kim YS, Rah DK, Kim SS. Ischemic oculomotor nerve palsy and skin necrosis caused by vascular embolization after hyaluronic Acid filler injection: a case report. Ann Plast Surg. 2013;71(4):333-334. doi:10.1097/SAP.0b013e31824f21da.
12. Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Exp Ophthalmol. 2006;34(4):363-364. doi:10.1111/j.1442-9071.2006.01224.x.
13. Kim YJ, Kim SS, Song WK, Lee SY, Yoon JS. Ocular ischemia with hypotony after injection of hyaluronic acid gel. Ophthal Plast Reconstr Surg. 2011;27(6):e152-e155. doi:10.1097/IOP.0b013e3182082f37.
14. Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. Dermatol Surg. 2009;35 Suppl 2:1635-1640. doi:10.1111/j.1524-4725.2009.01342.x.
15. Schanz S, Schippert W, Ulmer A, Rassner G, Fierlbeck G. Arterial embolization caused by injection of hyaluronic acid (Restylane). Br J Dermatol. 2002;146(5):928-929.
16. Georgescu D, Jones Y, McCann JD, Anderson RL. Skin necrosis after calcium hydroxylapatite injection into the glabellar and nasolabial folds. Ophthal Plast Reconstr Surg. 2009;25(6):498-499. doi:10.1097/IOP.0b013e3181b81082.
17. Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011;10(3):224-231. doi:10.1111/j.1473-2165.2011.00562.x.
18. Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006;32(2):276-281.
19. Burt B, Nakra T, Isaacs DK, Goldberg RA. Alar necrosis after facial injection of hyaluronic acid. Plast Reconstr Surg. 2010;125(5):199e-200e. doi:10.1097/PRS.0b013e3181d5152e.
20. Teimourian B. Blindness following fat injections. Plast Reconstr Surg. 1988;82(2):361.
21. Dreizen NG, Framm L. Sudden unilateral visual loss after autologous fat injection into the glabellar area. Am J Ophthalmol. 1989;107(1):85-87.
22. Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22(6):555-557. doi:10.1067/maj.2002.129625.
23. Allali J, Bernard A, Assaraf E, Bourges JL, Renard G. Multiple embolizations of the branches of the ophthalmic artery: an unknown serious complication of facial surgeries. [Article in French] J Fr Ophtalmol. 2006;29(1):51-57.
24. Mori K, Ohta K, Nagano S, Toshinori M, Yago T, Ichinose Y. A case of ophthalmic artery obstruction following autologous fat injection in the glabellar area. [Article in Japanese] Nippon Ganka Gakkai Zasshi. 2007;111(1):22-25.
25. Park SH, Sun HJ, Choi KS. Sudden unilateral visual loss after autologous fat injection into the nasolabial fold. Clin Ophthalmol. 2008;2(3):679-683.
26. Lee YJ, Kim HJ, Choi KD, Choi HY. MRI restricted diffusion in optic nerve infarction after autologous fat transplantation. J Neuroophthalmol. 2010;30(3):216-218. doi:10.1097/WNO.0b013e3181c5d147.
27. Park SJ, Woo SJ, Park KH, et al. Partial recovery after intraarterial pharmacomechanical thrombolysis in ophthalmic artery occlusion following nasal autologous fat injection. J Vasc Interv Radiol. 2011;22(2):251-254. doi:10.1016/j.jvir.2010.10.023.
Kratom: An Emerging Drug of Abuse
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Tavakoli HR, et al. Kratom: a new product in an expanding substance abuse market. Fed Prac. 2016;33[11]:132-136. http://www.fedprac.com).
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opioids, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most emergency physicians (EPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of this type of Web site and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite the purported raison d’être of these Web sites, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer et al4 of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patients’ digital habits, the researchers demonstrated that the majority of subjects used these Web sites and, as a result, either increased their drug use or learned about (and tried) new substances.
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opioid withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and has become increasingly popular in the United States and in the United Kingdom.2,5,6 In the United States, this poses a problem for EPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the US Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined Web sites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about $13 US currency).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a kratom product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least nine deaths.7
This article presents a case of kratom abuse. It describes a brief history of the substance, its pharmacological characteristics, the clinical presentation of kratom abuse, and the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the United States, a basic working knowledge of the drug is quickly becoming a must for EPs.
Case Presentation
At his employer’s request, a 33-year-old man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disk resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he became concerned that he was abusing his opioid medication, and it was discontinued. The patient was transferred to the local ED and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline, consistent with opioid withdrawal. Initial point-of-care (POC) admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s opioid withdrawal resolved, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia.2,3 The leaves of these trees contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-like effect, which at mega doses produces an intense euphoric state and has led to a steady growth in abuse worldwide. Although the government of Thailand banned the planting of Mitragyna speciosa as early as 1943, its continued proliferation in Southeast Asia and throughout the world has not ceased.2,3,6
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the two major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including mu-, delta-, and kappa-opioid receptors, leading to its opioid-like effects, including analgesia and euphoria. Also similar to common opioids, withdrawal symptomatology can present after only 5 days of daily use. There is limited evidence that mitragynine can activate postsynaptic alpha-2 adrenergic receptors, which may act synergistically with the mu-agonist with regard to its analgesic effect.2,5
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for EPs to be aware of the presentation of patients with kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from Web sites such as Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5-15 g of raw crushed leaves), it is believed that the mu-opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed with supportive and symptomatic care and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of a patient with an acute kratom overdose (typically requiring ingestion of >15 g of crushed leaves) begins with addressing airway support, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including POC glucose testing, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, as indicated).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong mu agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid withdrawal.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, alpha-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to EPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurological and mental status examinations.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and psychosis.3-7 Additionally, a study by Kittirattanapaiboon et al12 correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥14,000 individuals included in the study sample) with statistically significant higher suicide risk.
Detection
Because kratom is a relatively new compound in the United States, medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and two metabolites of mitragynine in urine.7 Le et al13 were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours. Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at the Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the case study patient’s urine because a urine test was not done until hospital day 5.
Case Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid-use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Conclusion
Kratom is an emerging drug of abuse in the Western world. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the United States and multiple other Western countries, kratom is easily accessible. Emergency physicians need to be aware of kratom, and during their evaluations, question appropriate patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Tavakoli HR, et al. Kratom: a new product in an expanding substance abuse market. Fed Prac. 2016;33[11]:132-136. http://www.fedprac.com).
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opioids, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most emergency physicians (EPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of this type of Web site and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite the purported raison d’être of these Web sites, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer et al4 of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patients’ digital habits, the researchers demonstrated that the majority of subjects used these Web sites and, as a result, either increased their drug use or learned about (and tried) new substances.
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opioid withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and has become increasingly popular in the United States and in the United Kingdom.2,5,6 In the United States, this poses a problem for EPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the US Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined Web sites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about $13 US currency).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a kratom product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least nine deaths.7
This article presents a case of kratom abuse. It describes a brief history of the substance, its pharmacological characteristics, the clinical presentation of kratom abuse, and the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the United States, a basic working knowledge of the drug is quickly becoming a must for EPs.
Case Presentation
At his employer’s request, a 33-year-old man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disk resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he became concerned that he was abusing his opioid medication, and it was discontinued. The patient was transferred to the local ED and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline, consistent with opioid withdrawal. Initial point-of-care (POC) admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s opioid withdrawal resolved, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia.2,3 The leaves of these trees contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-like effect, which at mega doses produces an intense euphoric state and has led to a steady growth in abuse worldwide. Although the government of Thailand banned the planting of Mitragyna speciosa as early as 1943, its continued proliferation in Southeast Asia and throughout the world has not ceased.2,3,6
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the two major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including mu-, delta-, and kappa-opioid receptors, leading to its opioid-like effects, including analgesia and euphoria. Also similar to common opioids, withdrawal symptomatology can present after only 5 days of daily use. There is limited evidence that mitragynine can activate postsynaptic alpha-2 adrenergic receptors, which may act synergistically with the mu-agonist with regard to its analgesic effect.2,5
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for EPs to be aware of the presentation of patients with kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from Web sites such as Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5-15 g of raw crushed leaves), it is believed that the mu-opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed with supportive and symptomatic care and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of a patient with an acute kratom overdose (typically requiring ingestion of >15 g of crushed leaves) begins with addressing airway support, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including POC glucose testing, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, as indicated).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong mu agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid withdrawal.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, alpha-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to EPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurological and mental status examinations.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and psychosis.3-7 Additionally, a study by Kittirattanapaiboon et al12 correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥14,000 individuals included in the study sample) with statistically significant higher suicide risk.
Detection
Because kratom is a relatively new compound in the United States, medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and two metabolites of mitragynine in urine.7 Le et al13 were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours. Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at the Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the case study patient’s urine because a urine test was not done until hospital day 5.
Case Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid-use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Conclusion
Kratom is an emerging drug of abuse in the Western world. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the United States and multiple other Western countries, kratom is easily accessible. Emergency physicians need to be aware of kratom, and during their evaluations, question appropriate patients about kratom and other legal highs.
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Tavakoli HR, et al. Kratom: a new product in an expanding substance abuse market. Fed Prac. 2016;33[11]:132-136. http://www.fedprac.com).
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opioids, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most emergency physicians (EPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of this type of Web site and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite the purported raison d’être of these Web sites, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer et al4 of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patients’ digital habits, the researchers demonstrated that the majority of subjects used these Web sites and, as a result, either increased their drug use or learned about (and tried) new substances.
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opioid withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and has become increasingly popular in the United States and in the United Kingdom.2,5,6 In the United States, this poses a problem for EPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the US Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined Web sites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about $13 US currency).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a kratom product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least nine deaths.7
This article presents a case of kratom abuse. It describes a brief history of the substance, its pharmacological characteristics, the clinical presentation of kratom abuse, and the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the United States, a basic working knowledge of the drug is quickly becoming a must for EPs.
Case Presentation
At his employer’s request, a 33-year-old man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disk resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he became concerned that he was abusing his opioid medication, and it was discontinued. The patient was transferred to the local ED and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline, consistent with opioid withdrawal. Initial point-of-care (POC) admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s opioid withdrawal resolved, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia.2,3 The leaves of these trees contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-like effect, which at mega doses produces an intense euphoric state and has led to a steady growth in abuse worldwide. Although the government of Thailand banned the planting of Mitragyna speciosa as early as 1943, its continued proliferation in Southeast Asia and throughout the world has not ceased.2,3,6
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the two major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including mu-, delta-, and kappa-opioid receptors, leading to its opioid-like effects, including analgesia and euphoria. Also similar to common opioids, withdrawal symptomatology can present after only 5 days of daily use. There is limited evidence that mitragynine can activate postsynaptic alpha-2 adrenergic receptors, which may act synergistically with the mu-agonist with regard to its analgesic effect.2,5
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for EPs to be aware of the presentation of patients with kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from Web sites such as Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5-15 g of raw crushed leaves), it is believed that the mu-opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed with supportive and symptomatic care and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of a patient with an acute kratom overdose (typically requiring ingestion of >15 g of crushed leaves) begins with addressing airway support, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including POC glucose testing, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, as indicated).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong mu agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid withdrawal.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, alpha-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to EPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurological and mental status examinations.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and psychosis.3-7 Additionally, a study by Kittirattanapaiboon et al12 correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥14,000 individuals included in the study sample) with statistically significant higher suicide risk.
Detection
Because kratom is a relatively new compound in the United States, medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and two metabolites of mitragynine in urine.7 Le et al13 were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours. Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at the Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the case study patient’s urine because a urine test was not done until hospital day 5.
Case Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid-use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Conclusion
Kratom is an emerging drug of abuse in the Western world. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the United States and multiple other Western countries, kratom is easily accessible. Emergency physicians need to be aware of kratom, and during their evaluations, question appropriate patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
First EDition: Medical “Merit Badges” for EPs, more
In a historic collaboration, 10 major emergency medicine organizations have joined forces to create The Coalition to Oppose Medical Merit Badges (COMMB). In its news release, the Coalition stated it “…believes that board-certified emergency physicians who actively maintain their board certification should not be required to complete short-course certification or acquire condition-specific continuing medical education credits in advanced
The COMMB consists of the American Academy of Emergency Medicine (AAEM), American Academy of Emergency Medicine/Resident and Student Association (AAEM/RSA), American Board of Emergency Medicine (ABEM), American College of Emergency Physicians (ACEP), Association of Academic Chairs of Emergency Medicine (AACEM), Council of Emergency Medicine Residency Directors (CORD), Emergency Medicine Residents’ Association (EMRA), Society for Academic Emergency Medicine (SAEM), American Osteopathic Board of Emergency Medicine (AOBEM), and American College of Osteopathic Emergency Physicians (ACOEP).
In a written statement signed by the president/chair of each member organization, COMMB further asserted:
Similarly, mandatory targeted continuing medical education (CME) requirements do not offer any meaningful value for the public or for the emergency physician who has achieved and maintained board certification. Such requirements are often promulgated by others who incompletely understand the foundation of knowledge and skills acquired by successfully completing an Accreditation Council for Graduate Medical Education-accredited or American Osteopathic Association-approved Emergency Medicine Residency Program. These “merit badges” add no additional value for board-certified emergency physicians. Instead, they devalue the board certification process, failing to recognize the rigor of the ABEM Maintenance of Certification (MOC) Program. In essence, medical merit badges set a lower bar than a diplomate’s education, training, and ongoing learning, as measured by initial board certification and maintenance of certification.
The Coalition finds no rational justification to require medical merit badges for board-certified emergency physicians who maintain their board certification. Our committed professional organizations provide the best opportunities for continuous professional development and medical merit badges dismiss the quality of those educational efforts.
Opposing the requirements for medical merit badges will be a long and challenging struggle. It will take time to help administrators and regulatory bodies to better understand the rigorous standards to which we adhere as board-certified emergency physicians. In the coming months, we will develop our long-term strategy to create success and a pathway to recognize clinical excellence. We welcome your thoughts and suggestions as to how we can best succeed. In the near future, we will ask for strong support and a loud and unified voice.
We will persist and we are up to the challenge—we are board-certified emergency physicians. Opposing medical merit badges is the right thing to do for our specialty. We will forever demonstrate a lifelong commitment to caring for anyone who is ill or injured, at any time, for any reason.
Study Nixed Magnesium for Infants With Acute Bronchiolitis
AMY KARON
FRONTLINE MEDICAL news
Intravenous (IV) magnesium does not benefit, and may harm, infants with moderate-to-severe acute bronchiolitis, investigators reported. Compared with placebo, adding a single IV dose of magnesium sulfate (100 mg/kg) to usual care did not reduce time to medical readiness for discharge, even when patients had eczema or a family history of asthma, and was tied to a more than 3-fold rise in the rate of short-term readmissions, Khalid Al Ansari, MD, of Hamad Medical Corp in Doha, Qatar, and his associates wrote in Chest. “To our knowledge, this is the first randomized study to investigate the effect of intravenous magnesium in a bronchiolitis population,” they added.
Bronchiolitis lacks new, inexpensive, readily available treatments, despite being a common reason for hospital admission, the researchers noted. For older children with moderate-to-severe exacerbations of asthma, a meta-analysis found that the addition of magnesium to usual care appeared to cut readmissions and shorten lengths of stay, compared with placebo. To explore magnesium therapy in younger children, the investigators enrolled 162 previously healthy infants up to 18 months old who had been admitted to the short-stay unit of a pediatric emergency center with a diagnosis of moderate-to-severe viral bronchiolitis. Patients received usual care with oral dexamethasone and nebulized 5% hypertonic saline in 1 mL of 1:1000 epinephrine, plus a 60-minute IV infusion with a blinded syringe of either 0.9% saline placebo or magnesium sulfate (100 mg/kg).
The primary endpoint, time to medical readiness for discharge, did not statistically differ between groups, averaging 24.1 (95% confidence interval [CI], 20.0-29.1) hours with magnesium and 25.3 (95% CI, 20.3-31.5) hours with placebo (P = .91). Among patients with a history of eczema or a family history of asthma, mean times to readiness for discharge resembled those for the entire cohort and did not statistically differ based on treatment. Average Wang bronchiolitis severity scores also were similar between groups, as were rates of outpatient clinic visits (33.8% with magnesium and 27.2% with placebo). Thus, the trial identified “no benefit in adding intravenous magnesium for infant bronchiolitis, even in patients characterized to be at a higher risk for asthma,” the researchers concluded.
Strikingly, 2-week readmission rates were 19.5% with magnesium (95% CI, 11.3-30.1) and 6.2% with placebo (95% CI, 0.02-13.8; P = .016). Among patients with eczema or a family history of asthma, 2-week readmission rates also were significantly higher with magnesium (26.3%; 95% CI, 13.4-43.1) than with placebo (7.5%; 95% CI, 1.6-20.4; P = .034) These might have been chance findings, or magnesium might have masked worse bronchiolitis, prolonged the disease course, or interacted with 5% hypertonic saline or systemic corticosteroids, the investigators said. Intravenous magnesium might contribute to secondary relapse, especially among patients with eczema or a family history of asthma, they added.
Patients in this study had a median age of 3.7 months (range, 22 days to 17.6 months), about half had eczema or a family history of asthma, and 86% had positive nasopharyngeal virus swabs. Cardiopulmonary monitoring revealed no acute events during treatment. Of 16 readmissions in the magnesium group, 11 entered the infirmary and four entered the hospital. The five placebo readmissions included four to the infirmary and one to the hospital.
“As with other ‘negative studies,’ we may have failed to identify a benefit from intravenous magnesium in a patient subgroup because of our limited sample size,” the investigators wrote. “But we think our findings are generalizable to a similarly heterogeneous group of patients presenting for bronchiolitis care in a busy urban emergency department.”
Alansari K, Sayyed R, Davidson BL, Al Jawala S, Ghadier M. Intravenous magnesium sulfate for bronchiolitis: A randomized trial. Chest. 2017;pii:S0012-3692(17):30361-30366. doi:10.1016/j.chest.2017.03.002. [Epub ahead of print]
CDC: Some Shigella Strains Show Reduced Ciprofloxacin Susceptibility
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
The Centers for Disease Control and Prevention (CDC) has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
- Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
- Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
- Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
- Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible—with special attention given to the MIC for fluoroquinolone antibiotics.
- Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
- Consult local or state health departments for guidance on when patients may return to childcare, school, or work.
- Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition, and all cases should be reported to one’s local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
Prenotification, Unequivocal Stroke Promote Ultrafast Door-to-Needle Time
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Ultrafast door-to-needle times (DNTs) of 10 minutes or less for IV acute ischemic stroke thrombolysis can be safely achieved in carefully selected cases, according to a review of cases at an Austrian teaching hospital.
Raffi Topakian, MD, and his colleagues at the Academic Teaching Hospital Wels-Grieskirchen in Wels, Austria, followed a multidisciplinary intervention to reinforce key components of the well-known Helsinki model of acute stroke care to improve the IV thrombolysis rate and the median DNT at the teaching hospital, and analyzed data from 361 patients who underwent intravenous thrombolysis (IVT) for stroke there between July 2014 and September 2016. The IVT rate increased from 19% to about 27% after intervention, and the DNT during the study period was 60 minutes or less in 316 patients (87.5%), 30 minutes or less in 181 patients (50.1%), and 10 minutes or less in 63 patients (17.5%).
“Over the study period, we reduced the DNT time from 49 minutes to 25 minutes. This was significant, and the door-to-needle times were astonishingly similar for the in-hours service and the out-of-hour service,” he said at the annual meeting of the American Academy of Neurology.
Further, the rate of prenotifications from emergency medical services (EMS) rose from about 30% to 63% during the study period.
Patients with ultrafast DNT vs those with slower DNT were older, had more chronic heart failure, had more severe stroke (National Institutes of Health Stroke Scale score of 10 vs 5), had more anterior circulation stroke and cardioembolic stroke, and had clear onset of stroke. Independent predictors of ultrafast DNT included prenotification by EMS, anterior circulation syndrome, chronic heart failure, and having a stroke neurologist on duty, Dr Topakian said.
“Ultrashort DNTs can be achieved safely. The key is that we are prenotified by the EMS, that we can get all the relevant history details during transport, that there is a dedicated multidisciplinary stroke team and EMS staff, and that we have a seemingly unequivocal clinical scenario,” he said. “Out-of-hours DNT matched in-hours DNT, but the caveat is we’re talking about highly selected candidates; safety must not be sacrificed for the sake of speed, in all of our patients.”
In a historic collaboration, 10 major emergency medicine organizations have joined forces to create The Coalition to Oppose Medical Merit Badges (COMMB). In its news release, the Coalition stated it “…believes that board-certified emergency physicians who actively maintain their board certification should not be required to complete short-course certification or acquire condition-specific continuing medical education credits in advanced
The COMMB consists of the American Academy of Emergency Medicine (AAEM), American Academy of Emergency Medicine/Resident and Student Association (AAEM/RSA), American Board of Emergency Medicine (ABEM), American College of Emergency Physicians (ACEP), Association of Academic Chairs of Emergency Medicine (AACEM), Council of Emergency Medicine Residency Directors (CORD), Emergency Medicine Residents’ Association (EMRA), Society for Academic Emergency Medicine (SAEM), American Osteopathic Board of Emergency Medicine (AOBEM), and American College of Osteopathic Emergency Physicians (ACOEP).
In a written statement signed by the president/chair of each member organization, COMMB further asserted:
Similarly, mandatory targeted continuing medical education (CME) requirements do not offer any meaningful value for the public or for the emergency physician who has achieved and maintained board certification. Such requirements are often promulgated by others who incompletely understand the foundation of knowledge and skills acquired by successfully completing an Accreditation Council for Graduate Medical Education-accredited or American Osteopathic Association-approved Emergency Medicine Residency Program. These “merit badges” add no additional value for board-certified emergency physicians. Instead, they devalue the board certification process, failing to recognize the rigor of the ABEM Maintenance of Certification (MOC) Program. In essence, medical merit badges set a lower bar than a diplomate’s education, training, and ongoing learning, as measured by initial board certification and maintenance of certification.
The Coalition finds no rational justification to require medical merit badges for board-certified emergency physicians who maintain their board certification. Our committed professional organizations provide the best opportunities for continuous professional development and medical merit badges dismiss the quality of those educational efforts.
Opposing the requirements for medical merit badges will be a long and challenging struggle. It will take time to help administrators and regulatory bodies to better understand the rigorous standards to which we adhere as board-certified emergency physicians. In the coming months, we will develop our long-term strategy to create success and a pathway to recognize clinical excellence. We welcome your thoughts and suggestions as to how we can best succeed. In the near future, we will ask for strong support and a loud and unified voice.
We will persist and we are up to the challenge—we are board-certified emergency physicians. Opposing medical merit badges is the right thing to do for our specialty. We will forever demonstrate a lifelong commitment to caring for anyone who is ill or injured, at any time, for any reason.
Study Nixed Magnesium for Infants With Acute Bronchiolitis
AMY KARON
FRONTLINE MEDICAL news
Intravenous (IV) magnesium does not benefit, and may harm, infants with moderate-to-severe acute bronchiolitis, investigators reported. Compared with placebo, adding a single IV dose of magnesium sulfate (100 mg/kg) to usual care did not reduce time to medical readiness for discharge, even when patients had eczema or a family history of asthma, and was tied to a more than 3-fold rise in the rate of short-term readmissions, Khalid Al Ansari, MD, of Hamad Medical Corp in Doha, Qatar, and his associates wrote in Chest. “To our knowledge, this is the first randomized study to investigate the effect of intravenous magnesium in a bronchiolitis population,” they added.
Bronchiolitis lacks new, inexpensive, readily available treatments, despite being a common reason for hospital admission, the researchers noted. For older children with moderate-to-severe exacerbations of asthma, a meta-analysis found that the addition of magnesium to usual care appeared to cut readmissions and shorten lengths of stay, compared with placebo. To explore magnesium therapy in younger children, the investigators enrolled 162 previously healthy infants up to 18 months old who had been admitted to the short-stay unit of a pediatric emergency center with a diagnosis of moderate-to-severe viral bronchiolitis. Patients received usual care with oral dexamethasone and nebulized 5% hypertonic saline in 1 mL of 1:1000 epinephrine, plus a 60-minute IV infusion with a blinded syringe of either 0.9% saline placebo or magnesium sulfate (100 mg/kg).
The primary endpoint, time to medical readiness for discharge, did not statistically differ between groups, averaging 24.1 (95% confidence interval [CI], 20.0-29.1) hours with magnesium and 25.3 (95% CI, 20.3-31.5) hours with placebo (P = .91). Among patients with a history of eczema or a family history of asthma, mean times to readiness for discharge resembled those for the entire cohort and did not statistically differ based on treatment. Average Wang bronchiolitis severity scores also were similar between groups, as were rates of outpatient clinic visits (33.8% with magnesium and 27.2% with placebo). Thus, the trial identified “no benefit in adding intravenous magnesium for infant bronchiolitis, even in patients characterized to be at a higher risk for asthma,” the researchers concluded.
Strikingly, 2-week readmission rates were 19.5% with magnesium (95% CI, 11.3-30.1) and 6.2% with placebo (95% CI, 0.02-13.8; P = .016). Among patients with eczema or a family history of asthma, 2-week readmission rates also were significantly higher with magnesium (26.3%; 95% CI, 13.4-43.1) than with placebo (7.5%; 95% CI, 1.6-20.4; P = .034) These might have been chance findings, or magnesium might have masked worse bronchiolitis, prolonged the disease course, or interacted with 5% hypertonic saline or systemic corticosteroids, the investigators said. Intravenous magnesium might contribute to secondary relapse, especially among patients with eczema or a family history of asthma, they added.
Patients in this study had a median age of 3.7 months (range, 22 days to 17.6 months), about half had eczema or a family history of asthma, and 86% had positive nasopharyngeal virus swabs. Cardiopulmonary monitoring revealed no acute events during treatment. Of 16 readmissions in the magnesium group, 11 entered the infirmary and four entered the hospital. The five placebo readmissions included four to the infirmary and one to the hospital.
“As with other ‘negative studies,’ we may have failed to identify a benefit from intravenous magnesium in a patient subgroup because of our limited sample size,” the investigators wrote. “But we think our findings are generalizable to a similarly heterogeneous group of patients presenting for bronchiolitis care in a busy urban emergency department.”
Alansari K, Sayyed R, Davidson BL, Al Jawala S, Ghadier M. Intravenous magnesium sulfate for bronchiolitis: A randomized trial. Chest. 2017;pii:S0012-3692(17):30361-30366. doi:10.1016/j.chest.2017.03.002. [Epub ahead of print]
CDC: Some Shigella Strains Show Reduced Ciprofloxacin Susceptibility
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
The Centers for Disease Control and Prevention (CDC) has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
- Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
- Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
- Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
- Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible—with special attention given to the MIC for fluoroquinolone antibiotics.
- Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
- Consult local or state health departments for guidance on when patients may return to childcare, school, or work.
- Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition, and all cases should be reported to one’s local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
Prenotification, Unequivocal Stroke Promote Ultrafast Door-to-Needle Time
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Ultrafast door-to-needle times (DNTs) of 10 minutes or less for IV acute ischemic stroke thrombolysis can be safely achieved in carefully selected cases, according to a review of cases at an Austrian teaching hospital.
Raffi Topakian, MD, and his colleagues at the Academic Teaching Hospital Wels-Grieskirchen in Wels, Austria, followed a multidisciplinary intervention to reinforce key components of the well-known Helsinki model of acute stroke care to improve the IV thrombolysis rate and the median DNT at the teaching hospital, and analyzed data from 361 patients who underwent intravenous thrombolysis (IVT) for stroke there between July 2014 and September 2016. The IVT rate increased from 19% to about 27% after intervention, and the DNT during the study period was 60 minutes or less in 316 patients (87.5%), 30 minutes or less in 181 patients (50.1%), and 10 minutes or less in 63 patients (17.5%).
“Over the study period, we reduced the DNT time from 49 minutes to 25 minutes. This was significant, and the door-to-needle times were astonishingly similar for the in-hours service and the out-of-hour service,” he said at the annual meeting of the American Academy of Neurology.
Further, the rate of prenotifications from emergency medical services (EMS) rose from about 30% to 63% during the study period.
Patients with ultrafast DNT vs those with slower DNT were older, had more chronic heart failure, had more severe stroke (National Institutes of Health Stroke Scale score of 10 vs 5), had more anterior circulation stroke and cardioembolic stroke, and had clear onset of stroke. Independent predictors of ultrafast DNT included prenotification by EMS, anterior circulation syndrome, chronic heart failure, and having a stroke neurologist on duty, Dr Topakian said.
“Ultrashort DNTs can be achieved safely. The key is that we are prenotified by the EMS, that we can get all the relevant history details during transport, that there is a dedicated multidisciplinary stroke team and EMS staff, and that we have a seemingly unequivocal clinical scenario,” he said. “Out-of-hours DNT matched in-hours DNT, but the caveat is we’re talking about highly selected candidates; safety must not be sacrificed for the sake of speed, in all of our patients.”
In a historic collaboration, 10 major emergency medicine organizations have joined forces to create The Coalition to Oppose Medical Merit Badges (COMMB). In its news release, the Coalition stated it “…believes that board-certified emergency physicians who actively maintain their board certification should not be required to complete short-course certification or acquire condition-specific continuing medical education credits in advanced
The COMMB consists of the American Academy of Emergency Medicine (AAEM), American Academy of Emergency Medicine/Resident and Student Association (AAEM/RSA), American Board of Emergency Medicine (ABEM), American College of Emergency Physicians (ACEP), Association of Academic Chairs of Emergency Medicine (AACEM), Council of Emergency Medicine Residency Directors (CORD), Emergency Medicine Residents’ Association (EMRA), Society for Academic Emergency Medicine (SAEM), American Osteopathic Board of Emergency Medicine (AOBEM), and American College of Osteopathic Emergency Physicians (ACOEP).
In a written statement signed by the president/chair of each member organization, COMMB further asserted:
Similarly, mandatory targeted continuing medical education (CME) requirements do not offer any meaningful value for the public or for the emergency physician who has achieved and maintained board certification. Such requirements are often promulgated by others who incompletely understand the foundation of knowledge and skills acquired by successfully completing an Accreditation Council for Graduate Medical Education-accredited or American Osteopathic Association-approved Emergency Medicine Residency Program. These “merit badges” add no additional value for board-certified emergency physicians. Instead, they devalue the board certification process, failing to recognize the rigor of the ABEM Maintenance of Certification (MOC) Program. In essence, medical merit badges set a lower bar than a diplomate’s education, training, and ongoing learning, as measured by initial board certification and maintenance of certification.
The Coalition finds no rational justification to require medical merit badges for board-certified emergency physicians who maintain their board certification. Our committed professional organizations provide the best opportunities for continuous professional development and medical merit badges dismiss the quality of those educational efforts.
Opposing the requirements for medical merit badges will be a long and challenging struggle. It will take time to help administrators and regulatory bodies to better understand the rigorous standards to which we adhere as board-certified emergency physicians. In the coming months, we will develop our long-term strategy to create success and a pathway to recognize clinical excellence. We welcome your thoughts and suggestions as to how we can best succeed. In the near future, we will ask for strong support and a loud and unified voice.
We will persist and we are up to the challenge—we are board-certified emergency physicians. Opposing medical merit badges is the right thing to do for our specialty. We will forever demonstrate a lifelong commitment to caring for anyone who is ill or injured, at any time, for any reason.
Study Nixed Magnesium for Infants With Acute Bronchiolitis
AMY KARON
FRONTLINE MEDICAL news
Intravenous (IV) magnesium does not benefit, and may harm, infants with moderate-to-severe acute bronchiolitis, investigators reported. Compared with placebo, adding a single IV dose of magnesium sulfate (100 mg/kg) to usual care did not reduce time to medical readiness for discharge, even when patients had eczema or a family history of asthma, and was tied to a more than 3-fold rise in the rate of short-term readmissions, Khalid Al Ansari, MD, of Hamad Medical Corp in Doha, Qatar, and his associates wrote in Chest. “To our knowledge, this is the first randomized study to investigate the effect of intravenous magnesium in a bronchiolitis population,” they added.
Bronchiolitis lacks new, inexpensive, readily available treatments, despite being a common reason for hospital admission, the researchers noted. For older children with moderate-to-severe exacerbations of asthma, a meta-analysis found that the addition of magnesium to usual care appeared to cut readmissions and shorten lengths of stay, compared with placebo. To explore magnesium therapy in younger children, the investigators enrolled 162 previously healthy infants up to 18 months old who had been admitted to the short-stay unit of a pediatric emergency center with a diagnosis of moderate-to-severe viral bronchiolitis. Patients received usual care with oral dexamethasone and nebulized 5% hypertonic saline in 1 mL of 1:1000 epinephrine, plus a 60-minute IV infusion with a blinded syringe of either 0.9% saline placebo or magnesium sulfate (100 mg/kg).
The primary endpoint, time to medical readiness for discharge, did not statistically differ between groups, averaging 24.1 (95% confidence interval [CI], 20.0-29.1) hours with magnesium and 25.3 (95% CI, 20.3-31.5) hours with placebo (P = .91). Among patients with a history of eczema or a family history of asthma, mean times to readiness for discharge resembled those for the entire cohort and did not statistically differ based on treatment. Average Wang bronchiolitis severity scores also were similar between groups, as were rates of outpatient clinic visits (33.8% with magnesium and 27.2% with placebo). Thus, the trial identified “no benefit in adding intravenous magnesium for infant bronchiolitis, even in patients characterized to be at a higher risk for asthma,” the researchers concluded.
Strikingly, 2-week readmission rates were 19.5% with magnesium (95% CI, 11.3-30.1) and 6.2% with placebo (95% CI, 0.02-13.8; P = .016). Among patients with eczema or a family history of asthma, 2-week readmission rates also were significantly higher with magnesium (26.3%; 95% CI, 13.4-43.1) than with placebo (7.5%; 95% CI, 1.6-20.4; P = .034) These might have been chance findings, or magnesium might have masked worse bronchiolitis, prolonged the disease course, or interacted with 5% hypertonic saline or systemic corticosteroids, the investigators said. Intravenous magnesium might contribute to secondary relapse, especially among patients with eczema or a family history of asthma, they added.
Patients in this study had a median age of 3.7 months (range, 22 days to 17.6 months), about half had eczema or a family history of asthma, and 86% had positive nasopharyngeal virus swabs. Cardiopulmonary monitoring revealed no acute events during treatment. Of 16 readmissions in the magnesium group, 11 entered the infirmary and four entered the hospital. The five placebo readmissions included four to the infirmary and one to the hospital.
“As with other ‘negative studies,’ we may have failed to identify a benefit from intravenous magnesium in a patient subgroup because of our limited sample size,” the investigators wrote. “But we think our findings are generalizable to a similarly heterogeneous group of patients presenting for bronchiolitis care in a busy urban emergency department.”
Alansari K, Sayyed R, Davidson BL, Al Jawala S, Ghadier M. Intravenous magnesium sulfate for bronchiolitis: A randomized trial. Chest. 2017;pii:S0012-3692(17):30361-30366. doi:10.1016/j.chest.2017.03.002. [Epub ahead of print]
CDC: Some Shigella Strains Show Reduced Ciprofloxacin Susceptibility
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
The Centers for Disease Control and Prevention (CDC) has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
- Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
- Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
- Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
- Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible—with special attention given to the MIC for fluoroquinolone antibiotics.
- Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
- Consult local or state health departments for guidance on when patients may return to childcare, school, or work.
- Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition, and all cases should be reported to one’s local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
Prenotification, Unequivocal Stroke Promote Ultrafast Door-to-Needle Time
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Ultrafast door-to-needle times (DNTs) of 10 minutes or less for IV acute ischemic stroke thrombolysis can be safely achieved in carefully selected cases, according to a review of cases at an Austrian teaching hospital.
Raffi Topakian, MD, and his colleagues at the Academic Teaching Hospital Wels-Grieskirchen in Wels, Austria, followed a multidisciplinary intervention to reinforce key components of the well-known Helsinki model of acute stroke care to improve the IV thrombolysis rate and the median DNT at the teaching hospital, and analyzed data from 361 patients who underwent intravenous thrombolysis (IVT) for stroke there between July 2014 and September 2016. The IVT rate increased from 19% to about 27% after intervention, and the DNT during the study period was 60 minutes or less in 316 patients (87.5%), 30 minutes or less in 181 patients (50.1%), and 10 minutes or less in 63 patients (17.5%).
“Over the study period, we reduced the DNT time from 49 minutes to 25 minutes. This was significant, and the door-to-needle times were astonishingly similar for the in-hours service and the out-of-hour service,” he said at the annual meeting of the American Academy of Neurology.
Further, the rate of prenotifications from emergency medical services (EMS) rose from about 30% to 63% during the study period.
Patients with ultrafast DNT vs those with slower DNT were older, had more chronic heart failure, had more severe stroke (National Institutes of Health Stroke Scale score of 10 vs 5), had more anterior circulation stroke and cardioembolic stroke, and had clear onset of stroke. Independent predictors of ultrafast DNT included prenotification by EMS, anterior circulation syndrome, chronic heart failure, and having a stroke neurologist on duty, Dr Topakian said.
“Ultrashort DNTs can be achieved safely. The key is that we are prenotified by the EMS, that we can get all the relevant history details during transport, that there is a dedicated multidisciplinary stroke team and EMS staff, and that we have a seemingly unequivocal clinical scenario,” he said. “Out-of-hours DNT matched in-hours DNT, but the caveat is we’re talking about highly selected candidates; safety must not be sacrificed for the sake of speed, in all of our patients.”
Head, Neck, and Shoulder Injuries in Ice Hockey: Current Concepts
Take-Home Points
- Hockey is a high-speed collision sport with one of the highest injury rates among all sports.
- Use of a helmet with visors or full-face shields significantly reduces the risk for eye injury.
- Broken portions of teeth should be found and placed in a protective medium such as saline, saliva, or milk for transport.
- A player with unresolved concussion symptoms should not be allowed to return to the ice.
- Shoulder dominance, which determines stick grip, is an important consideration in the treatment of shoulder instability in an ice hockey player.
On a surface of ice in Windsor, Nova Scotia in the middle of the 19th century, the modern game of ice hockey evolved.1 A blend of hurley, a Gaelic sport, and lacrosse, from the native Mi’kmaq culture, the sport of ice hockey gained rapidly in popularity throughout Canada and is now the country’s national sport. Hockey quickly spread to the United States and then Europe. It is presently played in 77 countries across the world.2
Hockey players can reach speeds of up to 48 km (~30 miles) per hour on razor-sharp skates on an ice surface surrounded by rigid plastic composite boards topped with plexiglass.3 They use sticks made of wood, aluminum, or a composite material to advance a 6-ounce vulcanized rubber puck on the opposing goal, and this puck sometimes reaches speeds over 160 km (~100 miles) per hour. Older, male players are allowed to make physical contact with their opposing counterparts to separate them from the puck (body-checking). Not surprisingly, the potential risk for injury in hockey is high. At the 2010 Winter Olympics, men’s ice hockey players had the highest rate of injury of any other competitors there—more than 30% were affected.4
Hockey is played and enjoyed by athletes ranging widely in age. Youth hockey leagues accept players as young as 5 years. Hockey can become a lifelong recreational activity. In North America, old timers’ leagues have many players up to age 70 years.6 According to International Ice Hockey Federation data for 2016, more than 543,000 and 639,500 people play hockey in the United States and Canada, respectively.2 Most of the rules, protective equipment, skates, ice surfaces, and goal sizes are the same in men’s and women’s hockey.7 The major difference is in body-checking—this practice is not allowed at any age in women’s ice hockey.
In this article, we review the evaluation and management of common head, neck, and shoulder hockey injuries for physicians who provide medical support and coverage for youth, amateur, and senior hockey teams.
Evaluation and Management of Common Hockey Injuries
Eye Injuries
Although eye injuries are less common than musculoskeletal injuries and concussions in hockey, they are a serious risk for recreational and competitive players alike. Furthermore, recovery may be difficult, and eye injuries can have serious lifelong consequences.8 In hockey, the most commonly reported eye injuries are periorbital contusions and lacerations, hyphema, corneal and conjunctival abrasions, orbital fractures, and ruptured globes (Table 2).9,10
As a contact sport, hockey often involves high-impact, blunt-force trauma. The trauma in hockey results from collisions with other players, the boards, hockey sticks, and pucks. It is therefore not surprising that the most common ocular injuries in this sport are periorbital contusions. Although most contusions cause only mild swelling and ecchymosis of the soft tissues around the eye, there is potential for serious consequences. In a Scandinavia study, Leivo and colleagues10 found that 9% of patients who sustained a periocular contusion also had a clinically significant secondary diagnosis, such as retinal tear or hemorrhage, eyelid laceration, vitreous hemorrhage, or retinal detachment. Although the study was hospital-based, and therefore biased toward more severe cases, its findings highlight the potential severity of eye injuries in hockey. Furthermore, the study found that the majority of players who sustained blunt trauma to the eye itself required lifelong follow-up because of increased risk for glaucoma. This is particularly true for hyphema, as this finding indicates significant damage to intraocular tissues.10Players can also sustain fractures of the orbital bones, including orbital blowout fractures. Typical signs and symptoms of blowout fractures include diplopia, proptosis or enophthalmos, infraorbital hypoesthesia, painful and decreased extraocular movement (particularly upgaze), and palpable crepitance caused by sinus air entering the lower eyelid.11 If orbital fracture is suspected, as it should be in any case in which the injured player experiences pain with eye movement or diplopia, the player should be referred to the ED for computed tomography (CT) and ophthalmologic evaluation.12 Continued participation seriously risks making the injury much worse, particularly should another impact occur. In addition, given the impact needed to cause orbital fractures, consideration must be given to the potential for a coexisting concussion injury.
Severe direct trauma to the eye—from a puck, a stick, or a fist—can result in a ruptured globe, a particularly serious injury that requires immediate surgical attention. Signs and symptoms of a ruptured globe are rarely subtle, but associated eyelid swelling or laceration may obscure the injury, delaying proper diagnosis and treatment. More obvious signs include severely reduced vision, hemorrhagic chemosis (swelling) of the conjunctiva, and an irregular or peaked pupil. If a rupture or any significant intraocular injury is suspected, it is crucial to avoid applying any pressure to the globe, as this can significantly worsen the damage to the intraocular tissues. Use of a helmet with protective shields and cages attached markedly reduces the risk for such injuries.13All eye injuries require prompt assessment, which allows for appropriate management and prevention of secondary damage.14 Initial evaluation of a patient with ocular trauma should begin with external examination for lacerations, swelling, or orbital rim step-off deformity. The physician should also check visual acuity in order to assess for significant vision impairment (counting fingers or reading a sign in the arena; confrontation visual fields). This should be done before attending to any periocular injuries, with the uninjured side serving as a control. Next, the physician should assess the extraocular eye movements as well as the size, shape, and reactivity of the pupils. Particular attention should be paid to detecting any deficit in extraocular movement or irregularity in pupil size, shape, or reactivity, as such findings are highly suggestive of serious injury to the globe.13 Hyphema (blood in anterior chamber of eye anterior to pupil) should be suspected if vision is reduced and the pupil cannot be clearly visualized. However, a bright red clot is not always apparent at time of injury or if the amount of blood is small. An irregular pupil, or a pupil that does not constrict well to light, is also a red flag for serious contusion injury to the eye, and requires ophthalmologic evaluation. It is important to keep in mind that blunt trauma severe enough to produce hyphema or an irregular and poorly reactive pupil is often associated with retinal damage as well, including retinal edema or detachment.
Minor injuries (eg, small foreign bodies, minor periocular contusions and lacerations) can often be managed rink-side. Foreign bodies not embedded in the cornea, but lodged under the upper eyelid, can sometimes be removed by everting the eyelid and sweeping with a moistened cotton swab or using diffuse, sterile saline irrigation.11 Corneal abrasions generally cause severe pain, photophobia, and tearing and are easily diagnosed with use of topical fluorescein and a blue light. A topical anesthetic can be extremely helpful in this setting, as it allows for proper pain-free evaluation, but should never be used in an ongoing manner for pain relief. Small lacerations of the brow can be sutured with 5-0 or 6-0 nylon or closed with 2-Octyl cyanoacrylate tissue adhesive (Dermabond). Eyelid lacerations, unless very small, are best managed by an ophthalmologist; care must be taken to rule out injury to the deeper orbital tissues and eye. If serious injury is suspected, or the eye cannot be appropriately evaluated, it should be stabilized and protected with a protective shield or plastic cup, and the player should be transferred to an ED for appropriate ophthalmologic evaluation.13Most eye injuries are accidental, caused by sticks or deflected pucks, but 18% are acquired in fights.8 Use of visors or full-face cages effectively minimizes the rate of eye injuries.8,13,15,16 In a cohort study of 282 elite amateur ice hockey players, the risk of eye injury was 4.7 times higher in players without face protection than in players who used half-face shields; there were no eye injuries in players who used full-face protection.13 For visors to prevent eye injury, they must be positioned to cover the eyes and the lower edge of the nose in all projections.10
Dental Injuries
The incidence and type of facial and dental injuries depend directly on the type of face protection used.11,17,18 In a study of face, head, and neck injuries in elite amateur ice hockey players, Stuart and colleagues13 found game-related injury rates of 158.9 per 1000 player-hours in players without face protection, 73.5 in players who used half-face shields, and 23.2 in players who used full-face shields. Players who wore full-face shields had facial, head, and neck injury rates of only 23.2 per 1000 player-game hours.13 Other studies clearly support the important role face shields play in lowering injury risk in hockey. Face and head injuries account for 20% to 40% of all hockey-related injuries,3,16,19 and dental injuries up to 11.5%.20 In a study from Finland, Lahti and colleagues19 found that over a 2-year period, 479 hockey players sustained injuries, including 650 separate dental injuries. The most commonly diagnosed dental injury was an uncomplicated crown fracture, and the most common cause was a hit with a hockey stick, which accounted for 52.7% and 40.3% of dental injuries in games and practices, respectively.19
In the management of dental fractures, the broken portions of teeth should be found and placed in a transportation-protective medium, such as saline, saliva, or milk,16 which can improve functional and esthetic replacement outcomes.21,22 Loose pieces of teeth should not be left in the player’s mouth. The residual tooth should be stabilized and exposure to air and occlusion limited. Dental fractures can affect the enamel, the enamel and dentin structures (uncomplicated fracture), or enamel, dentin, and pulp (complicated).23 Fractures involving only the enamel do not require urgent dental evaluation. Dentin or pulp involvement may cause temperature and air sensitivity.23 If a tooth is air-sensitive, the player should be referred to a specialist immediately.11
Direct trauma can cause instability without displacement (subluxation) or complete displacement of the tooth from its alveolar socket (avulsion).23 An avulsed tooth should be handled by the crown to avoid further damage to the root and periodontal ligament.16,24 The tooth should be rinsed gently with saline and reimplanted in its socket, ideally within 5 to 10 minutes,23with the athlete biting down gently on gauze to hold the tooth in place. A 1-mL supraperiosteal infiltration of 1% or 2% lidocaine hydrochloride (1:100,000 epinephrine) can be given into the apex of the tooth being anesthetized (Figure 1).
Concussions
A concussion is a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.”25 Concussion is largely a functional disturbance instead of a structural injury, owing to the rotational and/or shearing forces involved. Many studies have identified concussion as the most common type of injury in all of youth hockey.26 Concussions account for up to 19% of all injuries in men’s collegiate hockey.3
Concussion can be challenging to diagnose on the ice. The most important factor in concussion management is symptom reporting by the athlete.27 Despite significant efforts in education and awareness, student athletes, especially hockey players, withhold reporting a possible concussion.28 Reasons for underreporting include fear of letting down other players and coaches, thinking the injury is not severe enough to warrant evaluation, and fear of losing standing with the current team or future teams.28
As postinjury concussion assessments are ideal when comparisons can be made with preseason (baseline) scores, preseason testing is becoming standard in professional, college, junior, and high school hockey. This testing involves the Sport Concussion Assessment Tool, 3rd edition (SCAT3), and the King-Devick (K-D) test.30,31 Some youth leagues have baseline testing as well, though the frequency of baseline testing in their players is controversial,32 as the adolescent mind’s processing speed and memory increase exponentially.33 For these younger athletes, it may be necessary to perform baseline testing more frequently than annually.32 A physician can use baseline test results to help diagnose a concussion at the rink and then track the athlete’s recovery and help with return-to-play decisions.29 Vision involves almost half of the brain’s circuits,34 including areas vulnerable to head impact. A neuro-ophthalmologic test can assess for irregularities in accommodation, convergence, ocular muscle balance, pursuit, and saccades.29 The K-D test is a visual performance examination that allows easy and objective assessment of eye movements. Use of both the K-D test and the SCAT3 at the rink may increase the number of concussions detected.29,35 We recommend that physicians use both tests to assess for concussion at the hockey rink.
Initial treatment involves a period of physical rest and relative cognitive rest. Acute worsening of symptoms warrants urgent imaging to rule out a subdural or subarachnoid bleed. Once a player is symptom-free, a graded return-to-play protocol should be followed (Table 4).
On the prevention side, great efforts have been made to improve hockey helmets. (Some manufacturers claim to have made concussion-proof helmets, but there is no evidence supporting this claim.6) Numerous investigators have reported a lower overall injury rate in players who wear a helmet and a full-face shield.6,13 In addition, rule changes aimed at decreasing head contact have been implemented to decrease the incidence of sport-related concussions.36 Moreover, education on proper helmet use and wear should be emphasized. A study of the effects of hockey helmet fit on cervical motion found that 7 (39%) of 18 players wore a game or competition helmet so loosely that it could be removed without unbuttoning its chinstrap.37 Improperly worn helmets cannot prevent injury as well as properly worn helmets can.
Cervical Spine Injuries
Whereas American football is associated with a higher annual number of nonfatal catastrophic neck injuries, hockey has a 3 to 6 times higher incidence of cervical spine injuries and spinal cord damage.38,39 A Canadian Ice Hockey Spinal Injuries Registry review of the period 2006 to 2011 identified 44 cervical spine injuries, 7.3 per year on average.40 Severe injury, defined as complete motor and sensory loss, complete motor loss and incomplete sensory, or complete motor loss, occurred in 4 (9.1%) of the 44 injured players. In hockey, a major mechanism of cervical spine injury is an axial load to the slightly flexed spine.39 Of 355 hockey-related cervical spine injuries in a Canada study, 95 (35.5%) were caused by a check from behind.40,41 The Canadian neurosurgeons’ work led to rule changes prohibiting checks from behind, and this prohibition has reduced the incidence of cervical spine injuries in ice hockey.38,40
Team physicians should be comfortable managing serious neck and spine injuries on the ice. Initial evaluation should follow the standard ABCs (airway, breathing, circulation). The physician places a hand on each side of the head to stabilize the neck until the initial examination is complete. The goal is to minimize cervical spine motion until transportation to the hospital for advanced imaging and definitive treatment.37 The decision to remove or leave on the helmet is now controversial. Hockey helmets differ from football helmets in that their chinstraps do not afford significant cervical stabilization, and the helmets have less padding and cover less of the head; in addition, a shockingly high percentage of hockey players do not wear properly fitting helmets.37 In one study, 3-dimensional motion analysis of a hockey player during the logroll technique showed less transverse and sagittal cervical plane motion with the helmet removed than with the helmet (properly fitting or not) in place; the authors recommended removing the helmet to limit extraneous cervical spine motion during the technique.37 However, 2 other studies found that helmet removal can result in significantly increased cervical spine motion of the immobilized hockey player.42,43Recommendation 4 of the recently released interassociation consensus statement of the National Athletic Trainers’ Association reads, “Protective athletic equipment should be removed before transport to an emergency facility for an athlete-patient with suspected cervical spine instability.”44 This represents a shift from leaving the helmet and shoulder pads in place. For ice hockey players with suspected cervical spine injury, more research is needed on cervical motion during the entire sequence—partial logrolls, spine-boarding, placement of cervical collar before or after logroll, and different immobilization techniques for transport.37
The athlete must be carefully transferred to a spine board with either logroll or lift-and-slide. Although an extrication cervical collar can be placed before the spine board is placed, the effectiveness of this collar in executing the spine-board transfer is not proven.45 When the player is on the spine board, the head can be secured with pads and straps en route to the hospital.
Return-to-Play Criteria for Cervical Spine Injuries There is no clear consensus on return-to-play guidelines for cervical spine injuries in athletes.46
Shoulder Injuries
For hockey players, the upper extremity traditionally has been considered a well-protected area.48 However, shoulder pads are considerably more flexible in hockey than in football and other collision sports. In addition, hockey gloves allow a fair amount of motion for stick handling, and the wrist may be in maximal flexion or extension when a hit against the boards or the ice occurs. Open-ice checking, board collisions, and hockey stick use have been postulated as reasons for the high incidence of upper extremity injuries in hockey. Researchers in Finland found that upper extremity injuries accounted for up to 31% of all hockey injuries.49 More than 50% of these injuries resulted from checking or board collisions. Furthermore, study findings highlighted a low rate of injury in younger players and indicated the rate increases with age.49,50
In hockey players, the acromioclavicular (AC) joint is the most commonly injured shoulder structure.51 The mechanism of injury can be a board collision or an open-ice hit, but most often is a direct blow to the shoulder. The collision disrupts the AC joint and can sprain or tear the coracoclavicular ligaments. The Rockwood classification is used to categorize AC joint injuries (Figure 2).
Initial management involves icing the AC joint and placing a sling for comfort. Type I and type II injuries can be managed with progressive range-of-motion (ROM) exercises, strengthening, cryotherapy, and a period of rest. Treatment of type III injuries remains controversial,52 but in hockey players these injuries are almost always treated nonoperatively. Return to play requires full motion, normal strength, and minimal discomfort. Players return a few days to 2 weeks after a grade I injury; recovery from grade II injuries may take 2 to 3 weeks, and recovery from grade III injuries, 6 to 12 weeks. Surgical treatment is usually required in type IV and type V injuries, but we have had experience treating these injuries nonoperatively in high-level players. AC joint reinjury in hockey players is common, and surgical treatment should be approached cautiously, as delayed fracture after return to sport has been reported.53 Special precautions should be taken in collision athletes who undergo AC joint reconstruction. In the anatomical reconstruction described by Carofino and Mazzocca,54 2 holes are drilled in the clavicle; these holes are a potential source of fracture when the collision athlete returns to sport (Figure 3).
Clavicle fracture is another common hockey injury.55 Studies have shown clavicle fractures proportionally occur most often in people 15 to 19 years old.49 The injury presents with pain and deformity over the clavicle; in more severe fractures, skin tenting is identified. Initial management of suspected clavicle fracture includes cryotherapy, sling, and radiographs. Radiographs should include an AP view and then a 45° cephalad view, which eliminates overshadowing from the ribs. Most clavicle fractures are successfully managed nonoperatively, though there is evidence that significantly displaced or comminuted fractures have better union rates and shoulder function when treated with open reduction and internal fixation.56 After a clavicle fracture, return to skating and noncontact practice usually takes 8 weeks, with return to full contact occurring around 12 weeks.
Sternoclavicular injuries are relatively uncommon, but potentially serious. Special attention should also be given to adolescent athletes with sternoclavicular pain. Although sternoclavicular dislocations have been reported in hockey players, instead these likely are fractures involving the medial clavicle physis.57
The shoulder is the most commonly dislocated major joint, and the incidence of shoulder dislocation in elite hockey players is 8% to 21%.50,58 Anterior shoulder instability occurs from a fall with the shoulder in an abducted, externally rotated and extended position or from a direct anteriorly placed impact to the posterior shoulder. We recommend taking players off the ice for evaluation. Depending on physician comfort, the shoulder can be reduced in the training room, and the athlete sent for radiographs after reduction. If resources or support for closed reduction is not available at the rink, the athlete should be sent to the ED. Initial radiographic evaluation of a player with shoulder injury begins with plain radiographs, including a true AP (Grashey) view with the humerus in neutral, internal, and external rotation and an axillary view. The axillary radiograph is crucial in determining anterior or posterior dislocation. If the patient cannot tolerate the pain associated with having an axillary radiograph taken, a Velpeau radiograph can be used. This radiograph is taken with the patient’s arm in a sling and with the patient leaning back 30° while the x-ray beam is directed superior to inferior.
CT is performed for a suspected osseous injury. CT is more accurate than plain radiographs in showing glenoid and humeral fractures in the acute setting as well as the amount of bone loss in the case of chronic instability. Magnetic resonance arthrography is the imaging modality of choice for the diagnoses of capsulolabral injury.
After shoulder reduction, treatment with a sling, cryotherapy, and a nonsteroidal anti-inflammatory drug is initiated. In a Minnesota study of nonoperative management of shoulder instability, 9 of 10 hockey players were able to return to play the same season, and 6 of the 10 required surgery at the end of the season.59
Compared with noncontact athletes, hockey players and other collision athletes are at increased risk for recurrence.60-62 For collision athletes who want to continue playing their sport after recurrent instability, surgery is recommended. A shoulder instability study in Toronto found that more than 54% of 24 professional hockey players had associated Hill-Sachs lesions, but only 3 shoulders (12.5%) had glenoid defects.50 Arthroscopic and open techniques both demonstrate good results, and identification of bone loss can help determine which surgery to recommend.63 Hockey players can usually return to sport 6 months after shoulder stabilization.
Another important consideration in managing shoulder instability in hockey players is shoulder dominance, which determines stick grip. A left-handed player places the right hand on top of the stick for support, but most of the motion associated with shooting the puck—including abduction and external rotation—occurs with the left shoulder. Thus, a left-handed player with a history of previous left-side shoulder dislocation may dislocate with each shot, but a right-handed player with left shoulder instability may have considerably less trouble on the ice.58Shoulder and rotator cuff contusions (RCCs) occur in hockey and other collision sports.49,64 RCCs almost always result from a direct blow to the shoulder, and present with shoulder function loss, weakness, and pain.
Summary
Hockey is a high-speed collision sport with one of the highest injury rates among all sports. Physicians caring for youth, amateur, and senior hockey teams see a range of acute head, neck, and shoulder injuries. Although treatment of eye injuries, dental injuries, and concussions is not always considered orthopedic care, an orthopedic surgeon who is covering hockey needs to be comfortable managing these injuries acutely. Quality rink-side care minimizes the impact of the injury, maximizes the functional result, and expedites the safe return of the injured player back to the ice.
Am J Orthop. 2017;46(3):123-134. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Vaughan G. The Puck Starts Here: The Origin of Canada’s Great Winter Game, Ice Hockey. Fredericton, Canada: Goose Lane Editions; 1996.
2. IIHF member national associations. International Ice Hockey Federation website. http://www.iihf.com/iihf-home/the-iihf/members. Accessed April 6, 2017.
3. Flik K, Lyman S, Marx RG. American collegiate men’s ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
4. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
5. Deits J, Yard EE, Collins CL, Fields SK, Comstock RD. Patients with ice hockey injuries presenting to US emergency departments, 1990-2006. J Athl Train. 2010;45(5):467-474.
6. Brooks A, Loud KJ, Brenner JS, et al. Reducing injury risk from body checking in boys’ youth ice hockey. Pediatrics. 2014;133(6):1151-1157.
7. Agel J, Harvey EJ. A 7-year review of men’s and women’s ice hockey injuries in the NCAA. Can J Surg. 2010;53(5):319-323.
8. Micieli JA, Zurakowski D, Ahmed, II. Impact of visors on eye and orbital injuries in the National Hockey League. Can J Ophthalmol. 2014;49(3):243-248.
9. Pashby TJ. Ocular injuries in hockey. Int Ophthalmol Clin. 1988;28(3):228-231.
10. Leivo T, Haavisto AK, Sahraravand A. Sports-related eye injuries: the current picture. Acta Ophthalmol. 2015;93(3):224-231.
11. Cohn RM, Alaia MJ, Strauss EJ, Feldman AF. Rink-side management of ice hockey related injuries to the face, neck, and chest. Bull Hosp Jt Dis. 2013;71(4):253-256.
12. Reehal P. Facial injury in sport. Curr Sports Med Rep. 2010;9(1):27-34.
13. Stuart MJ, Smith AM, Malo-Ortiguera SA, Fischer TL, Larson DR. A comparison of facial protection and the incidence of head, neck, and facial injuries in Junior A hockey players. A function of individual playing time. Am J Sports Med. 2002;30(1):39-44.
14. MacEwen CJ, McLatchie GR. Eye injuries in sport. Scott Med J. 2010;55(2):22-24.
15. Stevens ST, Lassonde M, de Beaumont L, Keenan JP. The effect of visors on head and facial injury in National Hockey League players. J Sci Med Sport. 2006;9(3):238-242.
16. Moslener MD, Wadsworth LT. Ice hockey: a team physician’s perspective. Curr Sports Med Rep. 2010;9(3):134-138.
17. LaPrade RF, Burnett QM, Zarzour R, Moss R. The effect of the mandatory use of face masks on facial lacerations and head and neck injuries in ice hockey. A prospective study. Am J Sports Med. 1995;23(6):773-775.
18. Benson BW, Mohtadi NG, Rose MS, Meeuwisse WH. Head and neck injuries among ice hockey players wearing full face shields vs half face shields. JAMA. 1999;282(24):2328-2332.
19. Lahti H, Sane J, Ylipaavalniemi P. Dental injuries in ice hockey games and training. Med Sci Sports Exerc. 2002;34(3):400-402.
20. Sane J, Ylipaavalniemi P, Leppanen H. Maxillofacial and dental ice hockey injuries. Med Sci Sports Exerc. 1988;20(2):202-207.
21. Emerich K, Kaczmarek J. First aid for dental trauma caused by sports activities: state of knowledge, treatment and prevention. Sports Med. 2010;40(5):361-366.
22. Rosenberg H, Rosenberg H, Hickey M. Emergency management of a traumatic tooth avulsion. Ann Emerg Med. 2011;57(4):375-377.
23. Young EJ, Macias CR, Stephens L. Common dental injury management in athletes. Sports Health. 2015;7(3):250-255.
24. Andersson L, Andreasen JO, Day P, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol. 2012;28(2):88-96.
25. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19(3):185-200.
26. Schneider KJ, Meeuwisse WH, Kang J, Schneider GM, Emery CA. Preseason reports of neck pain, dizziness, and headache as risk factors for concussion in male youth ice hockey players. Clin J Sport Med. 2013;23(4):267-272.
27. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
28. Delaney JS, Lamfookon C, Bloom GA, Al-Kashmiri A, Correa JA. Why university athletes choose not to reveal their concussion symptoms during a practice or game. Clin J Sport Med. 2015;25(2):113-125.
29. Ventura RE, Balcer LJ, Galetta SL. The concussion toolbox: the role of vision in the assessment of concussion. Semin Neurol. 2015;35(5):599-606.
30. Vartiainen MV, Holm A, Peltonen K, Luoto TM, Iverson GL, Hokkanen L. King-Devick test normative reference values for professional male ice hockey players. Scand J Med Sci Sports. 2015;25(3):e327-e330.
31. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328(1-2):28-31.
32. Vernau BT, Grady MF, Goodman A, et al. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Dev Neuropsychol. 2015;40(1):7-11.
33. Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(1-3):1-34.
34. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1-47.
35. Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293.
36. Smith AM, Stuart MJ, Dodick DW, et al. Ice Hockey Summit II: zero tolerance for head hits and fighting. Curr Sports Med Rep. 2015;14(2):135-144.
37. Mihalik JP, Beard JR, Petschauer MA, Prentice WE, Guskiewicz KM. Effect of ice hockey helmet fit on cervical spine motion during an emergency log roll procedure. Clin J Sport Med. 2008;18(5):394-398.
38. Banerjee R, Palumbo MA, Fadale PD. Catastrophic cervical spine injuries in the collision sport athlete, part 1: epidemiology, functional anatomy, and diagnosis. Am J Sports Med. 2004;32(4):1077-1087.
39. Reynen PD, Clancy WG Jr. Cervical spine injury, hockey helmets, and face masks. Am J Sports Med. 1994;22(2):167-170.
40. Tator CH, Provvidenza C, Cassidy JD. Update and overview of spinal injuries in Canadian ice hockey, 1943 to 2011: the continuing need for injury prevention and education. Clin J Sport Med. 2016;26(3):232-238.
41. Tator CH, Edmonds VE, Lapczak L, Tator IB. Spinal injuries in ice hockey players, 1966-1987. Can J Surg. 1991;34(1):63-69.
42. Laprade RF, Schnetzler KA, Broxterman RJ, Wentorf F, Gilbert TJ. Cervical spine alignment in the immobilized ice hockey player. A computed tomographic analysis of the effects of helmet removal. Am J Sports Med. 2000;28(6):800-803.
43. Metz CM, Kuhn JE, Greenfield ML. Cervical spine alignment in immobilized hockey players: radiographic analysis with and without helmets and shoulder pads. Clin J Sport Med. 1998;8(2):92-95.
44. National Athletic Trainers’ Association. Appropriate prehospital management of the spine-injured athlete: updated from 1998 document. http://www.nata.org/sites/default/files/Executive-Summary-Spine-Injury-updated.pdf. Updated August 5, 2015. Accessed April 6, 2017.
45. Del Rossi G, Heffernan TP, Horodyski M, Rechtine GR. The effectiveness of extrication collars tested during the execution of spine-board transfer techniques. Spine J. 2004;4(6):619-623.
46. Morganti C, Sweeney CA, Albanese SA, Burak C, Hosea T, Connolly PJ. Return to play after cervical spine injury. Spine. 2001;26(10):1131-1136.
47. Huang P, Anissipour A, McGee W, Lemak L. Return-to-play recommendations after cervical, thoracic, and lumbar spine injuries: a comprehensive review. Sports Health. 2016;8(1):19-25.
48. Shindle MK, Marx RG, Kelly BT, Bisson L, Burke CJ 3rd. Hockey injuries: a pediatric sport update. Curr Opin Pediatr. 2010;22(1):54-60.
49. Molsa J, Kujala U, Myllynen P, Torstila I, Airaksinen O. Injuries to the upper extremity in ice hockey: analysis of a series of 760 injuries. Am J Sports Med. 2003;31(5):751-757.
50. Dwyer T, Petrera M, Bleakney R, Theodoropoulos JS. Shoulder instability in ice hockey players: incidence, mechanism, and MRI findings. Clin Sports Med. 2013;32(4):803-813.
51. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. Br J Sports Med. 2009;43(13):1000-1005.
52. Willimon SC, Gaskill TR, Millett PJ. Acromioclavicular joint injuries: anatomy, diagnosis, and treatment. Phys Sportsmed. 2011;39(1):116-122.
53. Martetschlager F, Horan MP, Warth RJ, Millett PJ. Complications after anatomic fixation and reconstruction of the coracoclavicular ligaments. Am J Sports Med. 2013;41(12):2896-2903.
54. Carofino BC, Mazzocca AD. The anatomic coracoclavicular ligament reconstruction: surgical technique and indications. J Shoulder Elbow Surg. 2010;19(2 suppl):37-46.
55. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. Br J Sports Med. 2014;48(1):4-10.
56. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
57. Lee JT, Nasreddine AY, Black EM, Bae DS, Kocher MS. Posterior sternoclavicular joint injuries in skeletally immature patients. J Pediatr Orthop. 2014;34(4):369-375.
58. Hovelius L. Shoulder dislocation in Swedish ice hockey players. Am J Sports Med. 1978;6(6):373-377.
59. Buss DD, Lynch GP, Meyer CP, Huber SM, Freehill MQ. Nonoperative management for in-season athletes with anterior shoulder instability. Am J Sports Med. 2004;32(6):1430-1433.
60. Mazzocca AD, Brown FM Jr, Carreira DS, Hayden J, Romeo AA. Arthroscopic anterior shoulder stabilization of collision and contact athletes. Am J Sports Med. 2005;33(1):52-60.
61. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
62. Cho NS, Hwang JC, Rhee YG. Arthroscopic stabilization in anterior shoulder instability: collision athletes versus noncollision athletes. Arthroscopy. 2006;22(9):947-953.
63. Griffin JW, Brockmeier SF. Shoulder instability with concomitant bone loss in the athlete. Orthop Clin North Am. 2015;46(1):89-103.
64. Cohen SB, Towers JD, Bradley JP. Rotator cuff contusions of the shoulder in professional football players: epidemiology and magnetic resonance imaging findings. Am J Sports Med. 2007;35(3):442-447.
65. Lorentzon R, Wedrèn H, Pietilä T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
66. Stuart MJ, Smith A. Injuries in Junior A ice hockey. A three-year prospective study. Am J Sports Med. 1995;23(4):458-461.
67. Voaklander DC, Saunders LD, Quinney HA, Macnab RB. Epidemiology of recreational and old-timer ice hockey injuries. Clin J Sport Med. 1996;6(1):15-21.
68. Mölsä J, Airaksinen O, Näsman O, Torstila I. Ice hockey injuries in Finland. A prospective epidemiologic study. Am J Sports Med. 1997;25(4):495-499.
69. Ferrara MS, Schurr KT. Intercollegiate ice hockey injuries: a casual analysis. Clin J Sport Med. 1999;9(1):30-33.
70. Pinto M, Kuhn JE, Greenfield ML, Hawkins RJ. Prospective analysis of ice hockey injuries at the Junior A level over the course of one season. Clin J Sport Med. 1999;9(2):70-74.
71. Emery CA, Meeuwisse WH. Injury rates, risk factors, and mechanisms of injury in minor hockey. Am J Sports Med. 2006;34(12):1960-1969.
72. Kuzuhara K, Shimamoto H, Mase Y. Ice hockey injuries in a Japanese elite team: a 3-year prospective study. J Athl Train. 2009;44(2):208-214.
73. Rishiraj N, Lloyd-Smith R, Lorenz T, Niven B, Michel M. University men’s ice hockey: rates and risk of injuries over 6-years. J Sports Med Phys Fitness. 2009;49(2):159-166.
74. Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men’s international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49(1):30-36.
75. Heckman JD, Bucholz RW. In: Rockwood CA, Green DP, Heckman JD, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults, Volume 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
Take-Home Points
- Hockey is a high-speed collision sport with one of the highest injury rates among all sports.
- Use of a helmet with visors or full-face shields significantly reduces the risk for eye injury.
- Broken portions of teeth should be found and placed in a protective medium such as saline, saliva, or milk for transport.
- A player with unresolved concussion symptoms should not be allowed to return to the ice.
- Shoulder dominance, which determines stick grip, is an important consideration in the treatment of shoulder instability in an ice hockey player.
On a surface of ice in Windsor, Nova Scotia in the middle of the 19th century, the modern game of ice hockey evolved.1 A blend of hurley, a Gaelic sport, and lacrosse, from the native Mi’kmaq culture, the sport of ice hockey gained rapidly in popularity throughout Canada and is now the country’s national sport. Hockey quickly spread to the United States and then Europe. It is presently played in 77 countries across the world.2
Hockey players can reach speeds of up to 48 km (~30 miles) per hour on razor-sharp skates on an ice surface surrounded by rigid plastic composite boards topped with plexiglass.3 They use sticks made of wood, aluminum, or a composite material to advance a 6-ounce vulcanized rubber puck on the opposing goal, and this puck sometimes reaches speeds over 160 km (~100 miles) per hour. Older, male players are allowed to make physical contact with their opposing counterparts to separate them from the puck (body-checking). Not surprisingly, the potential risk for injury in hockey is high. At the 2010 Winter Olympics, men’s ice hockey players had the highest rate of injury of any other competitors there—more than 30% were affected.4
Hockey is played and enjoyed by athletes ranging widely in age. Youth hockey leagues accept players as young as 5 years. Hockey can become a lifelong recreational activity. In North America, old timers’ leagues have many players up to age 70 years.6 According to International Ice Hockey Federation data for 2016, more than 543,000 and 639,500 people play hockey in the United States and Canada, respectively.2 Most of the rules, protective equipment, skates, ice surfaces, and goal sizes are the same in men’s and women’s hockey.7 The major difference is in body-checking—this practice is not allowed at any age in women’s ice hockey.
In this article, we review the evaluation and management of common head, neck, and shoulder hockey injuries for physicians who provide medical support and coverage for youth, amateur, and senior hockey teams.
Evaluation and Management of Common Hockey Injuries
Eye Injuries
Although eye injuries are less common than musculoskeletal injuries and concussions in hockey, they are a serious risk for recreational and competitive players alike. Furthermore, recovery may be difficult, and eye injuries can have serious lifelong consequences.8 In hockey, the most commonly reported eye injuries are periorbital contusions and lacerations, hyphema, corneal and conjunctival abrasions, orbital fractures, and ruptured globes (Table 2).9,10
As a contact sport, hockey often involves high-impact, blunt-force trauma. The trauma in hockey results from collisions with other players, the boards, hockey sticks, and pucks. It is therefore not surprising that the most common ocular injuries in this sport are periorbital contusions. Although most contusions cause only mild swelling and ecchymosis of the soft tissues around the eye, there is potential for serious consequences. In a Scandinavia study, Leivo and colleagues10 found that 9% of patients who sustained a periocular contusion also had a clinically significant secondary diagnosis, such as retinal tear or hemorrhage, eyelid laceration, vitreous hemorrhage, or retinal detachment. Although the study was hospital-based, and therefore biased toward more severe cases, its findings highlight the potential severity of eye injuries in hockey. Furthermore, the study found that the majority of players who sustained blunt trauma to the eye itself required lifelong follow-up because of increased risk for glaucoma. This is particularly true for hyphema, as this finding indicates significant damage to intraocular tissues.10Players can also sustain fractures of the orbital bones, including orbital blowout fractures. Typical signs and symptoms of blowout fractures include diplopia, proptosis or enophthalmos, infraorbital hypoesthesia, painful and decreased extraocular movement (particularly upgaze), and palpable crepitance caused by sinus air entering the lower eyelid.11 If orbital fracture is suspected, as it should be in any case in which the injured player experiences pain with eye movement or diplopia, the player should be referred to the ED for computed tomography (CT) and ophthalmologic evaluation.12 Continued participation seriously risks making the injury much worse, particularly should another impact occur. In addition, given the impact needed to cause orbital fractures, consideration must be given to the potential for a coexisting concussion injury.
Severe direct trauma to the eye—from a puck, a stick, or a fist—can result in a ruptured globe, a particularly serious injury that requires immediate surgical attention. Signs and symptoms of a ruptured globe are rarely subtle, but associated eyelid swelling or laceration may obscure the injury, delaying proper diagnosis and treatment. More obvious signs include severely reduced vision, hemorrhagic chemosis (swelling) of the conjunctiva, and an irregular or peaked pupil. If a rupture or any significant intraocular injury is suspected, it is crucial to avoid applying any pressure to the globe, as this can significantly worsen the damage to the intraocular tissues. Use of a helmet with protective shields and cages attached markedly reduces the risk for such injuries.13All eye injuries require prompt assessment, which allows for appropriate management and prevention of secondary damage.14 Initial evaluation of a patient with ocular trauma should begin with external examination for lacerations, swelling, or orbital rim step-off deformity. The physician should also check visual acuity in order to assess for significant vision impairment (counting fingers or reading a sign in the arena; confrontation visual fields). This should be done before attending to any periocular injuries, with the uninjured side serving as a control. Next, the physician should assess the extraocular eye movements as well as the size, shape, and reactivity of the pupils. Particular attention should be paid to detecting any deficit in extraocular movement or irregularity in pupil size, shape, or reactivity, as such findings are highly suggestive of serious injury to the globe.13 Hyphema (blood in anterior chamber of eye anterior to pupil) should be suspected if vision is reduced and the pupil cannot be clearly visualized. However, a bright red clot is not always apparent at time of injury or if the amount of blood is small. An irregular pupil, or a pupil that does not constrict well to light, is also a red flag for serious contusion injury to the eye, and requires ophthalmologic evaluation. It is important to keep in mind that blunt trauma severe enough to produce hyphema or an irregular and poorly reactive pupil is often associated with retinal damage as well, including retinal edema or detachment.
Minor injuries (eg, small foreign bodies, minor periocular contusions and lacerations) can often be managed rink-side. Foreign bodies not embedded in the cornea, but lodged under the upper eyelid, can sometimes be removed by everting the eyelid and sweeping with a moistened cotton swab or using diffuse, sterile saline irrigation.11 Corneal abrasions generally cause severe pain, photophobia, and tearing and are easily diagnosed with use of topical fluorescein and a blue light. A topical anesthetic can be extremely helpful in this setting, as it allows for proper pain-free evaluation, but should never be used in an ongoing manner for pain relief. Small lacerations of the brow can be sutured with 5-0 or 6-0 nylon or closed with 2-Octyl cyanoacrylate tissue adhesive (Dermabond). Eyelid lacerations, unless very small, are best managed by an ophthalmologist; care must be taken to rule out injury to the deeper orbital tissues and eye. If serious injury is suspected, or the eye cannot be appropriately evaluated, it should be stabilized and protected with a protective shield or plastic cup, and the player should be transferred to an ED for appropriate ophthalmologic evaluation.13Most eye injuries are accidental, caused by sticks or deflected pucks, but 18% are acquired in fights.8 Use of visors or full-face cages effectively minimizes the rate of eye injuries.8,13,15,16 In a cohort study of 282 elite amateur ice hockey players, the risk of eye injury was 4.7 times higher in players without face protection than in players who used half-face shields; there were no eye injuries in players who used full-face protection.13 For visors to prevent eye injury, they must be positioned to cover the eyes and the lower edge of the nose in all projections.10
Dental Injuries
The incidence and type of facial and dental injuries depend directly on the type of face protection used.11,17,18 In a study of face, head, and neck injuries in elite amateur ice hockey players, Stuart and colleagues13 found game-related injury rates of 158.9 per 1000 player-hours in players without face protection, 73.5 in players who used half-face shields, and 23.2 in players who used full-face shields. Players who wore full-face shields had facial, head, and neck injury rates of only 23.2 per 1000 player-game hours.13 Other studies clearly support the important role face shields play in lowering injury risk in hockey. Face and head injuries account for 20% to 40% of all hockey-related injuries,3,16,19 and dental injuries up to 11.5%.20 In a study from Finland, Lahti and colleagues19 found that over a 2-year period, 479 hockey players sustained injuries, including 650 separate dental injuries. The most commonly diagnosed dental injury was an uncomplicated crown fracture, and the most common cause was a hit with a hockey stick, which accounted for 52.7% and 40.3% of dental injuries in games and practices, respectively.19
In the management of dental fractures, the broken portions of teeth should be found and placed in a transportation-protective medium, such as saline, saliva, or milk,16 which can improve functional and esthetic replacement outcomes.21,22 Loose pieces of teeth should not be left in the player’s mouth. The residual tooth should be stabilized and exposure to air and occlusion limited. Dental fractures can affect the enamel, the enamel and dentin structures (uncomplicated fracture), or enamel, dentin, and pulp (complicated).23 Fractures involving only the enamel do not require urgent dental evaluation. Dentin or pulp involvement may cause temperature and air sensitivity.23 If a tooth is air-sensitive, the player should be referred to a specialist immediately.11
Direct trauma can cause instability without displacement (subluxation) or complete displacement of the tooth from its alveolar socket (avulsion).23 An avulsed tooth should be handled by the crown to avoid further damage to the root and periodontal ligament.16,24 The tooth should be rinsed gently with saline and reimplanted in its socket, ideally within 5 to 10 minutes,23with the athlete biting down gently on gauze to hold the tooth in place. A 1-mL supraperiosteal infiltration of 1% or 2% lidocaine hydrochloride (1:100,000 epinephrine) can be given into the apex of the tooth being anesthetized (Figure 1).
Concussions
A concussion is a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.”25 Concussion is largely a functional disturbance instead of a structural injury, owing to the rotational and/or shearing forces involved. Many studies have identified concussion as the most common type of injury in all of youth hockey.26 Concussions account for up to 19% of all injuries in men’s collegiate hockey.3
Concussion can be challenging to diagnose on the ice. The most important factor in concussion management is symptom reporting by the athlete.27 Despite significant efforts in education and awareness, student athletes, especially hockey players, withhold reporting a possible concussion.28 Reasons for underreporting include fear of letting down other players and coaches, thinking the injury is not severe enough to warrant evaluation, and fear of losing standing with the current team or future teams.28
As postinjury concussion assessments are ideal when comparisons can be made with preseason (baseline) scores, preseason testing is becoming standard in professional, college, junior, and high school hockey. This testing involves the Sport Concussion Assessment Tool, 3rd edition (SCAT3), and the King-Devick (K-D) test.30,31 Some youth leagues have baseline testing as well, though the frequency of baseline testing in their players is controversial,32 as the adolescent mind’s processing speed and memory increase exponentially.33 For these younger athletes, it may be necessary to perform baseline testing more frequently than annually.32 A physician can use baseline test results to help diagnose a concussion at the rink and then track the athlete’s recovery and help with return-to-play decisions.29 Vision involves almost half of the brain’s circuits,34 including areas vulnerable to head impact. A neuro-ophthalmologic test can assess for irregularities in accommodation, convergence, ocular muscle balance, pursuit, and saccades.29 The K-D test is a visual performance examination that allows easy and objective assessment of eye movements. Use of both the K-D test and the SCAT3 at the rink may increase the number of concussions detected.29,35 We recommend that physicians use both tests to assess for concussion at the hockey rink.
Initial treatment involves a period of physical rest and relative cognitive rest. Acute worsening of symptoms warrants urgent imaging to rule out a subdural or subarachnoid bleed. Once a player is symptom-free, a graded return-to-play protocol should be followed (Table 4).
On the prevention side, great efforts have been made to improve hockey helmets. (Some manufacturers claim to have made concussion-proof helmets, but there is no evidence supporting this claim.6) Numerous investigators have reported a lower overall injury rate in players who wear a helmet and a full-face shield.6,13 In addition, rule changes aimed at decreasing head contact have been implemented to decrease the incidence of sport-related concussions.36 Moreover, education on proper helmet use and wear should be emphasized. A study of the effects of hockey helmet fit on cervical motion found that 7 (39%) of 18 players wore a game or competition helmet so loosely that it could be removed without unbuttoning its chinstrap.37 Improperly worn helmets cannot prevent injury as well as properly worn helmets can.
Cervical Spine Injuries
Whereas American football is associated with a higher annual number of nonfatal catastrophic neck injuries, hockey has a 3 to 6 times higher incidence of cervical spine injuries and spinal cord damage.38,39 A Canadian Ice Hockey Spinal Injuries Registry review of the period 2006 to 2011 identified 44 cervical spine injuries, 7.3 per year on average.40 Severe injury, defined as complete motor and sensory loss, complete motor loss and incomplete sensory, or complete motor loss, occurred in 4 (9.1%) of the 44 injured players. In hockey, a major mechanism of cervical spine injury is an axial load to the slightly flexed spine.39 Of 355 hockey-related cervical spine injuries in a Canada study, 95 (35.5%) were caused by a check from behind.40,41 The Canadian neurosurgeons’ work led to rule changes prohibiting checks from behind, and this prohibition has reduced the incidence of cervical spine injuries in ice hockey.38,40
Team physicians should be comfortable managing serious neck and spine injuries on the ice. Initial evaluation should follow the standard ABCs (airway, breathing, circulation). The physician places a hand on each side of the head to stabilize the neck until the initial examination is complete. The goal is to minimize cervical spine motion until transportation to the hospital for advanced imaging and definitive treatment.37 The decision to remove or leave on the helmet is now controversial. Hockey helmets differ from football helmets in that their chinstraps do not afford significant cervical stabilization, and the helmets have less padding and cover less of the head; in addition, a shockingly high percentage of hockey players do not wear properly fitting helmets.37 In one study, 3-dimensional motion analysis of a hockey player during the logroll technique showed less transverse and sagittal cervical plane motion with the helmet removed than with the helmet (properly fitting or not) in place; the authors recommended removing the helmet to limit extraneous cervical spine motion during the technique.37 However, 2 other studies found that helmet removal can result in significantly increased cervical spine motion of the immobilized hockey player.42,43Recommendation 4 of the recently released interassociation consensus statement of the National Athletic Trainers’ Association reads, “Protective athletic equipment should be removed before transport to an emergency facility for an athlete-patient with suspected cervical spine instability.”44 This represents a shift from leaving the helmet and shoulder pads in place. For ice hockey players with suspected cervical spine injury, more research is needed on cervical motion during the entire sequence—partial logrolls, spine-boarding, placement of cervical collar before or after logroll, and different immobilization techniques for transport.37
The athlete must be carefully transferred to a spine board with either logroll or lift-and-slide. Although an extrication cervical collar can be placed before the spine board is placed, the effectiveness of this collar in executing the spine-board transfer is not proven.45 When the player is on the spine board, the head can be secured with pads and straps en route to the hospital.
Return-to-Play Criteria for Cervical Spine Injuries There is no clear consensus on return-to-play guidelines for cervical spine injuries in athletes.46
Shoulder Injuries
For hockey players, the upper extremity traditionally has been considered a well-protected area.48 However, shoulder pads are considerably more flexible in hockey than in football and other collision sports. In addition, hockey gloves allow a fair amount of motion for stick handling, and the wrist may be in maximal flexion or extension when a hit against the boards or the ice occurs. Open-ice checking, board collisions, and hockey stick use have been postulated as reasons for the high incidence of upper extremity injuries in hockey. Researchers in Finland found that upper extremity injuries accounted for up to 31% of all hockey injuries.49 More than 50% of these injuries resulted from checking or board collisions. Furthermore, study findings highlighted a low rate of injury in younger players and indicated the rate increases with age.49,50
In hockey players, the acromioclavicular (AC) joint is the most commonly injured shoulder structure.51 The mechanism of injury can be a board collision or an open-ice hit, but most often is a direct blow to the shoulder. The collision disrupts the AC joint and can sprain or tear the coracoclavicular ligaments. The Rockwood classification is used to categorize AC joint injuries (Figure 2).
Initial management involves icing the AC joint and placing a sling for comfort. Type I and type II injuries can be managed with progressive range-of-motion (ROM) exercises, strengthening, cryotherapy, and a period of rest. Treatment of type III injuries remains controversial,52 but in hockey players these injuries are almost always treated nonoperatively. Return to play requires full motion, normal strength, and minimal discomfort. Players return a few days to 2 weeks after a grade I injury; recovery from grade II injuries may take 2 to 3 weeks, and recovery from grade III injuries, 6 to 12 weeks. Surgical treatment is usually required in type IV and type V injuries, but we have had experience treating these injuries nonoperatively in high-level players. AC joint reinjury in hockey players is common, and surgical treatment should be approached cautiously, as delayed fracture after return to sport has been reported.53 Special precautions should be taken in collision athletes who undergo AC joint reconstruction. In the anatomical reconstruction described by Carofino and Mazzocca,54 2 holes are drilled in the clavicle; these holes are a potential source of fracture when the collision athlete returns to sport (Figure 3).
Clavicle fracture is another common hockey injury.55 Studies have shown clavicle fractures proportionally occur most often in people 15 to 19 years old.49 The injury presents with pain and deformity over the clavicle; in more severe fractures, skin tenting is identified. Initial management of suspected clavicle fracture includes cryotherapy, sling, and radiographs. Radiographs should include an AP view and then a 45° cephalad view, which eliminates overshadowing from the ribs. Most clavicle fractures are successfully managed nonoperatively, though there is evidence that significantly displaced or comminuted fractures have better union rates and shoulder function when treated with open reduction and internal fixation.56 After a clavicle fracture, return to skating and noncontact practice usually takes 8 weeks, with return to full contact occurring around 12 weeks.
Sternoclavicular injuries are relatively uncommon, but potentially serious. Special attention should also be given to adolescent athletes with sternoclavicular pain. Although sternoclavicular dislocations have been reported in hockey players, instead these likely are fractures involving the medial clavicle physis.57
The shoulder is the most commonly dislocated major joint, and the incidence of shoulder dislocation in elite hockey players is 8% to 21%.50,58 Anterior shoulder instability occurs from a fall with the shoulder in an abducted, externally rotated and extended position or from a direct anteriorly placed impact to the posterior shoulder. We recommend taking players off the ice for evaluation. Depending on physician comfort, the shoulder can be reduced in the training room, and the athlete sent for radiographs after reduction. If resources or support for closed reduction is not available at the rink, the athlete should be sent to the ED. Initial radiographic evaluation of a player with shoulder injury begins with plain radiographs, including a true AP (Grashey) view with the humerus in neutral, internal, and external rotation and an axillary view. The axillary radiograph is crucial in determining anterior or posterior dislocation. If the patient cannot tolerate the pain associated with having an axillary radiograph taken, a Velpeau radiograph can be used. This radiograph is taken with the patient’s arm in a sling and with the patient leaning back 30° while the x-ray beam is directed superior to inferior.
CT is performed for a suspected osseous injury. CT is more accurate than plain radiographs in showing glenoid and humeral fractures in the acute setting as well as the amount of bone loss in the case of chronic instability. Magnetic resonance arthrography is the imaging modality of choice for the diagnoses of capsulolabral injury.
After shoulder reduction, treatment with a sling, cryotherapy, and a nonsteroidal anti-inflammatory drug is initiated. In a Minnesota study of nonoperative management of shoulder instability, 9 of 10 hockey players were able to return to play the same season, and 6 of the 10 required surgery at the end of the season.59
Compared with noncontact athletes, hockey players and other collision athletes are at increased risk for recurrence.60-62 For collision athletes who want to continue playing their sport after recurrent instability, surgery is recommended. A shoulder instability study in Toronto found that more than 54% of 24 professional hockey players had associated Hill-Sachs lesions, but only 3 shoulders (12.5%) had glenoid defects.50 Arthroscopic and open techniques both demonstrate good results, and identification of bone loss can help determine which surgery to recommend.63 Hockey players can usually return to sport 6 months after shoulder stabilization.
Another important consideration in managing shoulder instability in hockey players is shoulder dominance, which determines stick grip. A left-handed player places the right hand on top of the stick for support, but most of the motion associated with shooting the puck—including abduction and external rotation—occurs with the left shoulder. Thus, a left-handed player with a history of previous left-side shoulder dislocation may dislocate with each shot, but a right-handed player with left shoulder instability may have considerably less trouble on the ice.58Shoulder and rotator cuff contusions (RCCs) occur in hockey and other collision sports.49,64 RCCs almost always result from a direct blow to the shoulder, and present with shoulder function loss, weakness, and pain.
Summary
Hockey is a high-speed collision sport with one of the highest injury rates among all sports. Physicians caring for youth, amateur, and senior hockey teams see a range of acute head, neck, and shoulder injuries. Although treatment of eye injuries, dental injuries, and concussions is not always considered orthopedic care, an orthopedic surgeon who is covering hockey needs to be comfortable managing these injuries acutely. Quality rink-side care minimizes the impact of the injury, maximizes the functional result, and expedites the safe return of the injured player back to the ice.
Am J Orthop. 2017;46(3):123-134. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Hockey is a high-speed collision sport with one of the highest injury rates among all sports.
- Use of a helmet with visors or full-face shields significantly reduces the risk for eye injury.
- Broken portions of teeth should be found and placed in a protective medium such as saline, saliva, or milk for transport.
- A player with unresolved concussion symptoms should not be allowed to return to the ice.
- Shoulder dominance, which determines stick grip, is an important consideration in the treatment of shoulder instability in an ice hockey player.
On a surface of ice in Windsor, Nova Scotia in the middle of the 19th century, the modern game of ice hockey evolved.1 A blend of hurley, a Gaelic sport, and lacrosse, from the native Mi’kmaq culture, the sport of ice hockey gained rapidly in popularity throughout Canada and is now the country’s national sport. Hockey quickly spread to the United States and then Europe. It is presently played in 77 countries across the world.2
Hockey players can reach speeds of up to 48 km (~30 miles) per hour on razor-sharp skates on an ice surface surrounded by rigid plastic composite boards topped with plexiglass.3 They use sticks made of wood, aluminum, or a composite material to advance a 6-ounce vulcanized rubber puck on the opposing goal, and this puck sometimes reaches speeds over 160 km (~100 miles) per hour. Older, male players are allowed to make physical contact with their opposing counterparts to separate them from the puck (body-checking). Not surprisingly, the potential risk for injury in hockey is high. At the 2010 Winter Olympics, men’s ice hockey players had the highest rate of injury of any other competitors there—more than 30% were affected.4
Hockey is played and enjoyed by athletes ranging widely in age. Youth hockey leagues accept players as young as 5 years. Hockey can become a lifelong recreational activity. In North America, old timers’ leagues have many players up to age 70 years.6 According to International Ice Hockey Federation data for 2016, more than 543,000 and 639,500 people play hockey in the United States and Canada, respectively.2 Most of the rules, protective equipment, skates, ice surfaces, and goal sizes are the same in men’s and women’s hockey.7 The major difference is in body-checking—this practice is not allowed at any age in women’s ice hockey.
In this article, we review the evaluation and management of common head, neck, and shoulder hockey injuries for physicians who provide medical support and coverage for youth, amateur, and senior hockey teams.
Evaluation and Management of Common Hockey Injuries
Eye Injuries
Although eye injuries are less common than musculoskeletal injuries and concussions in hockey, they are a serious risk for recreational and competitive players alike. Furthermore, recovery may be difficult, and eye injuries can have serious lifelong consequences.8 In hockey, the most commonly reported eye injuries are periorbital contusions and lacerations, hyphema, corneal and conjunctival abrasions, orbital fractures, and ruptured globes (Table 2).9,10
As a contact sport, hockey often involves high-impact, blunt-force trauma. The trauma in hockey results from collisions with other players, the boards, hockey sticks, and pucks. It is therefore not surprising that the most common ocular injuries in this sport are periorbital contusions. Although most contusions cause only mild swelling and ecchymosis of the soft tissues around the eye, there is potential for serious consequences. In a Scandinavia study, Leivo and colleagues10 found that 9% of patients who sustained a periocular contusion also had a clinically significant secondary diagnosis, such as retinal tear or hemorrhage, eyelid laceration, vitreous hemorrhage, or retinal detachment. Although the study was hospital-based, and therefore biased toward more severe cases, its findings highlight the potential severity of eye injuries in hockey. Furthermore, the study found that the majority of players who sustained blunt trauma to the eye itself required lifelong follow-up because of increased risk for glaucoma. This is particularly true for hyphema, as this finding indicates significant damage to intraocular tissues.10Players can also sustain fractures of the orbital bones, including orbital blowout fractures. Typical signs and symptoms of blowout fractures include diplopia, proptosis or enophthalmos, infraorbital hypoesthesia, painful and decreased extraocular movement (particularly upgaze), and palpable crepitance caused by sinus air entering the lower eyelid.11 If orbital fracture is suspected, as it should be in any case in which the injured player experiences pain with eye movement or diplopia, the player should be referred to the ED for computed tomography (CT) and ophthalmologic evaluation.12 Continued participation seriously risks making the injury much worse, particularly should another impact occur. In addition, given the impact needed to cause orbital fractures, consideration must be given to the potential for a coexisting concussion injury.
Severe direct trauma to the eye—from a puck, a stick, or a fist—can result in a ruptured globe, a particularly serious injury that requires immediate surgical attention. Signs and symptoms of a ruptured globe are rarely subtle, but associated eyelid swelling or laceration may obscure the injury, delaying proper diagnosis and treatment. More obvious signs include severely reduced vision, hemorrhagic chemosis (swelling) of the conjunctiva, and an irregular or peaked pupil. If a rupture or any significant intraocular injury is suspected, it is crucial to avoid applying any pressure to the globe, as this can significantly worsen the damage to the intraocular tissues. Use of a helmet with protective shields and cages attached markedly reduces the risk for such injuries.13All eye injuries require prompt assessment, which allows for appropriate management and prevention of secondary damage.14 Initial evaluation of a patient with ocular trauma should begin with external examination for lacerations, swelling, or orbital rim step-off deformity. The physician should also check visual acuity in order to assess for significant vision impairment (counting fingers or reading a sign in the arena; confrontation visual fields). This should be done before attending to any periocular injuries, with the uninjured side serving as a control. Next, the physician should assess the extraocular eye movements as well as the size, shape, and reactivity of the pupils. Particular attention should be paid to detecting any deficit in extraocular movement or irregularity in pupil size, shape, or reactivity, as such findings are highly suggestive of serious injury to the globe.13 Hyphema (blood in anterior chamber of eye anterior to pupil) should be suspected if vision is reduced and the pupil cannot be clearly visualized. However, a bright red clot is not always apparent at time of injury or if the amount of blood is small. An irregular pupil, or a pupil that does not constrict well to light, is also a red flag for serious contusion injury to the eye, and requires ophthalmologic evaluation. It is important to keep in mind that blunt trauma severe enough to produce hyphema or an irregular and poorly reactive pupil is often associated with retinal damage as well, including retinal edema or detachment.
Minor injuries (eg, small foreign bodies, minor periocular contusions and lacerations) can often be managed rink-side. Foreign bodies not embedded in the cornea, but lodged under the upper eyelid, can sometimes be removed by everting the eyelid and sweeping with a moistened cotton swab or using diffuse, sterile saline irrigation.11 Corneal abrasions generally cause severe pain, photophobia, and tearing and are easily diagnosed with use of topical fluorescein and a blue light. A topical anesthetic can be extremely helpful in this setting, as it allows for proper pain-free evaluation, but should never be used in an ongoing manner for pain relief. Small lacerations of the brow can be sutured with 5-0 or 6-0 nylon or closed with 2-Octyl cyanoacrylate tissue adhesive (Dermabond). Eyelid lacerations, unless very small, are best managed by an ophthalmologist; care must be taken to rule out injury to the deeper orbital tissues and eye. If serious injury is suspected, or the eye cannot be appropriately evaluated, it should be stabilized and protected with a protective shield or plastic cup, and the player should be transferred to an ED for appropriate ophthalmologic evaluation.13Most eye injuries are accidental, caused by sticks or deflected pucks, but 18% are acquired in fights.8 Use of visors or full-face cages effectively minimizes the rate of eye injuries.8,13,15,16 In a cohort study of 282 elite amateur ice hockey players, the risk of eye injury was 4.7 times higher in players without face protection than in players who used half-face shields; there were no eye injuries in players who used full-face protection.13 For visors to prevent eye injury, they must be positioned to cover the eyes and the lower edge of the nose in all projections.10
Dental Injuries
The incidence and type of facial and dental injuries depend directly on the type of face protection used.11,17,18 In a study of face, head, and neck injuries in elite amateur ice hockey players, Stuart and colleagues13 found game-related injury rates of 158.9 per 1000 player-hours in players without face protection, 73.5 in players who used half-face shields, and 23.2 in players who used full-face shields. Players who wore full-face shields had facial, head, and neck injury rates of only 23.2 per 1000 player-game hours.13 Other studies clearly support the important role face shields play in lowering injury risk in hockey. Face and head injuries account for 20% to 40% of all hockey-related injuries,3,16,19 and dental injuries up to 11.5%.20 In a study from Finland, Lahti and colleagues19 found that over a 2-year period, 479 hockey players sustained injuries, including 650 separate dental injuries. The most commonly diagnosed dental injury was an uncomplicated crown fracture, and the most common cause was a hit with a hockey stick, which accounted for 52.7% and 40.3% of dental injuries in games and practices, respectively.19
In the management of dental fractures, the broken portions of teeth should be found and placed in a transportation-protective medium, such as saline, saliva, or milk,16 which can improve functional and esthetic replacement outcomes.21,22 Loose pieces of teeth should not be left in the player’s mouth. The residual tooth should be stabilized and exposure to air and occlusion limited. Dental fractures can affect the enamel, the enamel and dentin structures (uncomplicated fracture), or enamel, dentin, and pulp (complicated).23 Fractures involving only the enamel do not require urgent dental evaluation. Dentin or pulp involvement may cause temperature and air sensitivity.23 If a tooth is air-sensitive, the player should be referred to a specialist immediately.11
Direct trauma can cause instability without displacement (subluxation) or complete displacement of the tooth from its alveolar socket (avulsion).23 An avulsed tooth should be handled by the crown to avoid further damage to the root and periodontal ligament.16,24 The tooth should be rinsed gently with saline and reimplanted in its socket, ideally within 5 to 10 minutes,23with the athlete biting down gently on gauze to hold the tooth in place. A 1-mL supraperiosteal infiltration of 1% or 2% lidocaine hydrochloride (1:100,000 epinephrine) can be given into the apex of the tooth being anesthetized (Figure 1).
Concussions
A concussion is a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.”25 Concussion is largely a functional disturbance instead of a structural injury, owing to the rotational and/or shearing forces involved. Many studies have identified concussion as the most common type of injury in all of youth hockey.26 Concussions account for up to 19% of all injuries in men’s collegiate hockey.3
Concussion can be challenging to diagnose on the ice. The most important factor in concussion management is symptom reporting by the athlete.27 Despite significant efforts in education and awareness, student athletes, especially hockey players, withhold reporting a possible concussion.28 Reasons for underreporting include fear of letting down other players and coaches, thinking the injury is not severe enough to warrant evaluation, and fear of losing standing with the current team or future teams.28
As postinjury concussion assessments are ideal when comparisons can be made with preseason (baseline) scores, preseason testing is becoming standard in professional, college, junior, and high school hockey. This testing involves the Sport Concussion Assessment Tool, 3rd edition (SCAT3), and the King-Devick (K-D) test.30,31 Some youth leagues have baseline testing as well, though the frequency of baseline testing in their players is controversial,32 as the adolescent mind’s processing speed and memory increase exponentially.33 For these younger athletes, it may be necessary to perform baseline testing more frequently than annually.32 A physician can use baseline test results to help diagnose a concussion at the rink and then track the athlete’s recovery and help with return-to-play decisions.29 Vision involves almost half of the brain’s circuits,34 including areas vulnerable to head impact. A neuro-ophthalmologic test can assess for irregularities in accommodation, convergence, ocular muscle balance, pursuit, and saccades.29 The K-D test is a visual performance examination that allows easy and objective assessment of eye movements. Use of both the K-D test and the SCAT3 at the rink may increase the number of concussions detected.29,35 We recommend that physicians use both tests to assess for concussion at the hockey rink.
Initial treatment involves a period of physical rest and relative cognitive rest. Acute worsening of symptoms warrants urgent imaging to rule out a subdural or subarachnoid bleed. Once a player is symptom-free, a graded return-to-play protocol should be followed (Table 4).
On the prevention side, great efforts have been made to improve hockey helmets. (Some manufacturers claim to have made concussion-proof helmets, but there is no evidence supporting this claim.6) Numerous investigators have reported a lower overall injury rate in players who wear a helmet and a full-face shield.6,13 In addition, rule changes aimed at decreasing head contact have been implemented to decrease the incidence of sport-related concussions.36 Moreover, education on proper helmet use and wear should be emphasized. A study of the effects of hockey helmet fit on cervical motion found that 7 (39%) of 18 players wore a game or competition helmet so loosely that it could be removed without unbuttoning its chinstrap.37 Improperly worn helmets cannot prevent injury as well as properly worn helmets can.
Cervical Spine Injuries
Whereas American football is associated with a higher annual number of nonfatal catastrophic neck injuries, hockey has a 3 to 6 times higher incidence of cervical spine injuries and spinal cord damage.38,39 A Canadian Ice Hockey Spinal Injuries Registry review of the period 2006 to 2011 identified 44 cervical spine injuries, 7.3 per year on average.40 Severe injury, defined as complete motor and sensory loss, complete motor loss and incomplete sensory, or complete motor loss, occurred in 4 (9.1%) of the 44 injured players. In hockey, a major mechanism of cervical spine injury is an axial load to the slightly flexed spine.39 Of 355 hockey-related cervical spine injuries in a Canada study, 95 (35.5%) were caused by a check from behind.40,41 The Canadian neurosurgeons’ work led to rule changes prohibiting checks from behind, and this prohibition has reduced the incidence of cervical spine injuries in ice hockey.38,40
Team physicians should be comfortable managing serious neck and spine injuries on the ice. Initial evaluation should follow the standard ABCs (airway, breathing, circulation). The physician places a hand on each side of the head to stabilize the neck until the initial examination is complete. The goal is to minimize cervical spine motion until transportation to the hospital for advanced imaging and definitive treatment.37 The decision to remove or leave on the helmet is now controversial. Hockey helmets differ from football helmets in that their chinstraps do not afford significant cervical stabilization, and the helmets have less padding and cover less of the head; in addition, a shockingly high percentage of hockey players do not wear properly fitting helmets.37 In one study, 3-dimensional motion analysis of a hockey player during the logroll technique showed less transverse and sagittal cervical plane motion with the helmet removed than with the helmet (properly fitting or not) in place; the authors recommended removing the helmet to limit extraneous cervical spine motion during the technique.37 However, 2 other studies found that helmet removal can result in significantly increased cervical spine motion of the immobilized hockey player.42,43Recommendation 4 of the recently released interassociation consensus statement of the National Athletic Trainers’ Association reads, “Protective athletic equipment should be removed before transport to an emergency facility for an athlete-patient with suspected cervical spine instability.”44 This represents a shift from leaving the helmet and shoulder pads in place. For ice hockey players with suspected cervical spine injury, more research is needed on cervical motion during the entire sequence—partial logrolls, spine-boarding, placement of cervical collar before or after logroll, and different immobilization techniques for transport.37
The athlete must be carefully transferred to a spine board with either logroll or lift-and-slide. Although an extrication cervical collar can be placed before the spine board is placed, the effectiveness of this collar in executing the spine-board transfer is not proven.45 When the player is on the spine board, the head can be secured with pads and straps en route to the hospital.
Return-to-Play Criteria for Cervical Spine Injuries There is no clear consensus on return-to-play guidelines for cervical spine injuries in athletes.46
Shoulder Injuries
For hockey players, the upper extremity traditionally has been considered a well-protected area.48 However, shoulder pads are considerably more flexible in hockey than in football and other collision sports. In addition, hockey gloves allow a fair amount of motion for stick handling, and the wrist may be in maximal flexion or extension when a hit against the boards or the ice occurs. Open-ice checking, board collisions, and hockey stick use have been postulated as reasons for the high incidence of upper extremity injuries in hockey. Researchers in Finland found that upper extremity injuries accounted for up to 31% of all hockey injuries.49 More than 50% of these injuries resulted from checking or board collisions. Furthermore, study findings highlighted a low rate of injury in younger players and indicated the rate increases with age.49,50
In hockey players, the acromioclavicular (AC) joint is the most commonly injured shoulder structure.51 The mechanism of injury can be a board collision or an open-ice hit, but most often is a direct blow to the shoulder. The collision disrupts the AC joint and can sprain or tear the coracoclavicular ligaments. The Rockwood classification is used to categorize AC joint injuries (Figure 2).
Initial management involves icing the AC joint and placing a sling for comfort. Type I and type II injuries can be managed with progressive range-of-motion (ROM) exercises, strengthening, cryotherapy, and a period of rest. Treatment of type III injuries remains controversial,52 but in hockey players these injuries are almost always treated nonoperatively. Return to play requires full motion, normal strength, and minimal discomfort. Players return a few days to 2 weeks after a grade I injury; recovery from grade II injuries may take 2 to 3 weeks, and recovery from grade III injuries, 6 to 12 weeks. Surgical treatment is usually required in type IV and type V injuries, but we have had experience treating these injuries nonoperatively in high-level players. AC joint reinjury in hockey players is common, and surgical treatment should be approached cautiously, as delayed fracture after return to sport has been reported.53 Special precautions should be taken in collision athletes who undergo AC joint reconstruction. In the anatomical reconstruction described by Carofino and Mazzocca,54 2 holes are drilled in the clavicle; these holes are a potential source of fracture when the collision athlete returns to sport (Figure 3).
Clavicle fracture is another common hockey injury.55 Studies have shown clavicle fractures proportionally occur most often in people 15 to 19 years old.49 The injury presents with pain and deformity over the clavicle; in more severe fractures, skin tenting is identified. Initial management of suspected clavicle fracture includes cryotherapy, sling, and radiographs. Radiographs should include an AP view and then a 45° cephalad view, which eliminates overshadowing from the ribs. Most clavicle fractures are successfully managed nonoperatively, though there is evidence that significantly displaced or comminuted fractures have better union rates and shoulder function when treated with open reduction and internal fixation.56 After a clavicle fracture, return to skating and noncontact practice usually takes 8 weeks, with return to full contact occurring around 12 weeks.
Sternoclavicular injuries are relatively uncommon, but potentially serious. Special attention should also be given to adolescent athletes with sternoclavicular pain. Although sternoclavicular dislocations have been reported in hockey players, instead these likely are fractures involving the medial clavicle physis.57
The shoulder is the most commonly dislocated major joint, and the incidence of shoulder dislocation in elite hockey players is 8% to 21%.50,58 Anterior shoulder instability occurs from a fall with the shoulder in an abducted, externally rotated and extended position or from a direct anteriorly placed impact to the posterior shoulder. We recommend taking players off the ice for evaluation. Depending on physician comfort, the shoulder can be reduced in the training room, and the athlete sent for radiographs after reduction. If resources or support for closed reduction is not available at the rink, the athlete should be sent to the ED. Initial radiographic evaluation of a player with shoulder injury begins with plain radiographs, including a true AP (Grashey) view with the humerus in neutral, internal, and external rotation and an axillary view. The axillary radiograph is crucial in determining anterior or posterior dislocation. If the patient cannot tolerate the pain associated with having an axillary radiograph taken, a Velpeau radiograph can be used. This radiograph is taken with the patient’s arm in a sling and with the patient leaning back 30° while the x-ray beam is directed superior to inferior.
CT is performed for a suspected osseous injury. CT is more accurate than plain radiographs in showing glenoid and humeral fractures in the acute setting as well as the amount of bone loss in the case of chronic instability. Magnetic resonance arthrography is the imaging modality of choice for the diagnoses of capsulolabral injury.
After shoulder reduction, treatment with a sling, cryotherapy, and a nonsteroidal anti-inflammatory drug is initiated. In a Minnesota study of nonoperative management of shoulder instability, 9 of 10 hockey players were able to return to play the same season, and 6 of the 10 required surgery at the end of the season.59
Compared with noncontact athletes, hockey players and other collision athletes are at increased risk for recurrence.60-62 For collision athletes who want to continue playing their sport after recurrent instability, surgery is recommended. A shoulder instability study in Toronto found that more than 54% of 24 professional hockey players had associated Hill-Sachs lesions, but only 3 shoulders (12.5%) had glenoid defects.50 Arthroscopic and open techniques both demonstrate good results, and identification of bone loss can help determine which surgery to recommend.63 Hockey players can usually return to sport 6 months after shoulder stabilization.
Another important consideration in managing shoulder instability in hockey players is shoulder dominance, which determines stick grip. A left-handed player places the right hand on top of the stick for support, but most of the motion associated with shooting the puck—including abduction and external rotation—occurs with the left shoulder. Thus, a left-handed player with a history of previous left-side shoulder dislocation may dislocate with each shot, but a right-handed player with left shoulder instability may have considerably less trouble on the ice.58Shoulder and rotator cuff contusions (RCCs) occur in hockey and other collision sports.49,64 RCCs almost always result from a direct blow to the shoulder, and present with shoulder function loss, weakness, and pain.
Summary
Hockey is a high-speed collision sport with one of the highest injury rates among all sports. Physicians caring for youth, amateur, and senior hockey teams see a range of acute head, neck, and shoulder injuries. Although treatment of eye injuries, dental injuries, and concussions is not always considered orthopedic care, an orthopedic surgeon who is covering hockey needs to be comfortable managing these injuries acutely. Quality rink-side care minimizes the impact of the injury, maximizes the functional result, and expedites the safe return of the injured player back to the ice.
Am J Orthop. 2017;46(3):123-134. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Vaughan G. The Puck Starts Here: The Origin of Canada’s Great Winter Game, Ice Hockey. Fredericton, Canada: Goose Lane Editions; 1996.
2. IIHF member national associations. International Ice Hockey Federation website. http://www.iihf.com/iihf-home/the-iihf/members. Accessed April 6, 2017.
3. Flik K, Lyman S, Marx RG. American collegiate men’s ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
4. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
5. Deits J, Yard EE, Collins CL, Fields SK, Comstock RD. Patients with ice hockey injuries presenting to US emergency departments, 1990-2006. J Athl Train. 2010;45(5):467-474.
6. Brooks A, Loud KJ, Brenner JS, et al. Reducing injury risk from body checking in boys’ youth ice hockey. Pediatrics. 2014;133(6):1151-1157.
7. Agel J, Harvey EJ. A 7-year review of men’s and women’s ice hockey injuries in the NCAA. Can J Surg. 2010;53(5):319-323.
8. Micieli JA, Zurakowski D, Ahmed, II. Impact of visors on eye and orbital injuries in the National Hockey League. Can J Ophthalmol. 2014;49(3):243-248.
9. Pashby TJ. Ocular injuries in hockey. Int Ophthalmol Clin. 1988;28(3):228-231.
10. Leivo T, Haavisto AK, Sahraravand A. Sports-related eye injuries: the current picture. Acta Ophthalmol. 2015;93(3):224-231.
11. Cohn RM, Alaia MJ, Strauss EJ, Feldman AF. Rink-side management of ice hockey related injuries to the face, neck, and chest. Bull Hosp Jt Dis. 2013;71(4):253-256.
12. Reehal P. Facial injury in sport. Curr Sports Med Rep. 2010;9(1):27-34.
13. Stuart MJ, Smith AM, Malo-Ortiguera SA, Fischer TL, Larson DR. A comparison of facial protection and the incidence of head, neck, and facial injuries in Junior A hockey players. A function of individual playing time. Am J Sports Med. 2002;30(1):39-44.
14. MacEwen CJ, McLatchie GR. Eye injuries in sport. Scott Med J. 2010;55(2):22-24.
15. Stevens ST, Lassonde M, de Beaumont L, Keenan JP. The effect of visors on head and facial injury in National Hockey League players. J Sci Med Sport. 2006;9(3):238-242.
16. Moslener MD, Wadsworth LT. Ice hockey: a team physician’s perspective. Curr Sports Med Rep. 2010;9(3):134-138.
17. LaPrade RF, Burnett QM, Zarzour R, Moss R. The effect of the mandatory use of face masks on facial lacerations and head and neck injuries in ice hockey. A prospective study. Am J Sports Med. 1995;23(6):773-775.
18. Benson BW, Mohtadi NG, Rose MS, Meeuwisse WH. Head and neck injuries among ice hockey players wearing full face shields vs half face shields. JAMA. 1999;282(24):2328-2332.
19. Lahti H, Sane J, Ylipaavalniemi P. Dental injuries in ice hockey games and training. Med Sci Sports Exerc. 2002;34(3):400-402.
20. Sane J, Ylipaavalniemi P, Leppanen H. Maxillofacial and dental ice hockey injuries. Med Sci Sports Exerc. 1988;20(2):202-207.
21. Emerich K, Kaczmarek J. First aid for dental trauma caused by sports activities: state of knowledge, treatment and prevention. Sports Med. 2010;40(5):361-366.
22. Rosenberg H, Rosenberg H, Hickey M. Emergency management of a traumatic tooth avulsion. Ann Emerg Med. 2011;57(4):375-377.
23. Young EJ, Macias CR, Stephens L. Common dental injury management in athletes. Sports Health. 2015;7(3):250-255.
24. Andersson L, Andreasen JO, Day P, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol. 2012;28(2):88-96.
25. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19(3):185-200.
26. Schneider KJ, Meeuwisse WH, Kang J, Schneider GM, Emery CA. Preseason reports of neck pain, dizziness, and headache as risk factors for concussion in male youth ice hockey players. Clin J Sport Med. 2013;23(4):267-272.
27. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
28. Delaney JS, Lamfookon C, Bloom GA, Al-Kashmiri A, Correa JA. Why university athletes choose not to reveal their concussion symptoms during a practice or game. Clin J Sport Med. 2015;25(2):113-125.
29. Ventura RE, Balcer LJ, Galetta SL. The concussion toolbox: the role of vision in the assessment of concussion. Semin Neurol. 2015;35(5):599-606.
30. Vartiainen MV, Holm A, Peltonen K, Luoto TM, Iverson GL, Hokkanen L. King-Devick test normative reference values for professional male ice hockey players. Scand J Med Sci Sports. 2015;25(3):e327-e330.
31. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328(1-2):28-31.
32. Vernau BT, Grady MF, Goodman A, et al. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Dev Neuropsychol. 2015;40(1):7-11.
33. Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(1-3):1-34.
34. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1-47.
35. Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293.
36. Smith AM, Stuart MJ, Dodick DW, et al. Ice Hockey Summit II: zero tolerance for head hits and fighting. Curr Sports Med Rep. 2015;14(2):135-144.
37. Mihalik JP, Beard JR, Petschauer MA, Prentice WE, Guskiewicz KM. Effect of ice hockey helmet fit on cervical spine motion during an emergency log roll procedure. Clin J Sport Med. 2008;18(5):394-398.
38. Banerjee R, Palumbo MA, Fadale PD. Catastrophic cervical spine injuries in the collision sport athlete, part 1: epidemiology, functional anatomy, and diagnosis. Am J Sports Med. 2004;32(4):1077-1087.
39. Reynen PD, Clancy WG Jr. Cervical spine injury, hockey helmets, and face masks. Am J Sports Med. 1994;22(2):167-170.
40. Tator CH, Provvidenza C, Cassidy JD. Update and overview of spinal injuries in Canadian ice hockey, 1943 to 2011: the continuing need for injury prevention and education. Clin J Sport Med. 2016;26(3):232-238.
41. Tator CH, Edmonds VE, Lapczak L, Tator IB. Spinal injuries in ice hockey players, 1966-1987. Can J Surg. 1991;34(1):63-69.
42. Laprade RF, Schnetzler KA, Broxterman RJ, Wentorf F, Gilbert TJ. Cervical spine alignment in the immobilized ice hockey player. A computed tomographic analysis of the effects of helmet removal. Am J Sports Med. 2000;28(6):800-803.
43. Metz CM, Kuhn JE, Greenfield ML. Cervical spine alignment in immobilized hockey players: radiographic analysis with and without helmets and shoulder pads. Clin J Sport Med. 1998;8(2):92-95.
44. National Athletic Trainers’ Association. Appropriate prehospital management of the spine-injured athlete: updated from 1998 document. http://www.nata.org/sites/default/files/Executive-Summary-Spine-Injury-updated.pdf. Updated August 5, 2015. Accessed April 6, 2017.
45. Del Rossi G, Heffernan TP, Horodyski M, Rechtine GR. The effectiveness of extrication collars tested during the execution of spine-board transfer techniques. Spine J. 2004;4(6):619-623.
46. Morganti C, Sweeney CA, Albanese SA, Burak C, Hosea T, Connolly PJ. Return to play after cervical spine injury. Spine. 2001;26(10):1131-1136.
47. Huang P, Anissipour A, McGee W, Lemak L. Return-to-play recommendations after cervical, thoracic, and lumbar spine injuries: a comprehensive review. Sports Health. 2016;8(1):19-25.
48. Shindle MK, Marx RG, Kelly BT, Bisson L, Burke CJ 3rd. Hockey injuries: a pediatric sport update. Curr Opin Pediatr. 2010;22(1):54-60.
49. Molsa J, Kujala U, Myllynen P, Torstila I, Airaksinen O. Injuries to the upper extremity in ice hockey: analysis of a series of 760 injuries. Am J Sports Med. 2003;31(5):751-757.
50. Dwyer T, Petrera M, Bleakney R, Theodoropoulos JS. Shoulder instability in ice hockey players: incidence, mechanism, and MRI findings. Clin Sports Med. 2013;32(4):803-813.
51. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. Br J Sports Med. 2009;43(13):1000-1005.
52. Willimon SC, Gaskill TR, Millett PJ. Acromioclavicular joint injuries: anatomy, diagnosis, and treatment. Phys Sportsmed. 2011;39(1):116-122.
53. Martetschlager F, Horan MP, Warth RJ, Millett PJ. Complications after anatomic fixation and reconstruction of the coracoclavicular ligaments. Am J Sports Med. 2013;41(12):2896-2903.
54. Carofino BC, Mazzocca AD. The anatomic coracoclavicular ligament reconstruction: surgical technique and indications. J Shoulder Elbow Surg. 2010;19(2 suppl):37-46.
55. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. Br J Sports Med. 2014;48(1):4-10.
56. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
57. Lee JT, Nasreddine AY, Black EM, Bae DS, Kocher MS. Posterior sternoclavicular joint injuries in skeletally immature patients. J Pediatr Orthop. 2014;34(4):369-375.
58. Hovelius L. Shoulder dislocation in Swedish ice hockey players. Am J Sports Med. 1978;6(6):373-377.
59. Buss DD, Lynch GP, Meyer CP, Huber SM, Freehill MQ. Nonoperative management for in-season athletes with anterior shoulder instability. Am J Sports Med. 2004;32(6):1430-1433.
60. Mazzocca AD, Brown FM Jr, Carreira DS, Hayden J, Romeo AA. Arthroscopic anterior shoulder stabilization of collision and contact athletes. Am J Sports Med. 2005;33(1):52-60.
61. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
62. Cho NS, Hwang JC, Rhee YG. Arthroscopic stabilization in anterior shoulder instability: collision athletes versus noncollision athletes. Arthroscopy. 2006;22(9):947-953.
63. Griffin JW, Brockmeier SF. Shoulder instability with concomitant bone loss in the athlete. Orthop Clin North Am. 2015;46(1):89-103.
64. Cohen SB, Towers JD, Bradley JP. Rotator cuff contusions of the shoulder in professional football players: epidemiology and magnetic resonance imaging findings. Am J Sports Med. 2007;35(3):442-447.
65. Lorentzon R, Wedrèn H, Pietilä T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
66. Stuart MJ, Smith A. Injuries in Junior A ice hockey. A three-year prospective study. Am J Sports Med. 1995;23(4):458-461.
67. Voaklander DC, Saunders LD, Quinney HA, Macnab RB. Epidemiology of recreational and old-timer ice hockey injuries. Clin J Sport Med. 1996;6(1):15-21.
68. Mölsä J, Airaksinen O, Näsman O, Torstila I. Ice hockey injuries in Finland. A prospective epidemiologic study. Am J Sports Med. 1997;25(4):495-499.
69. Ferrara MS, Schurr KT. Intercollegiate ice hockey injuries: a casual analysis. Clin J Sport Med. 1999;9(1):30-33.
70. Pinto M, Kuhn JE, Greenfield ML, Hawkins RJ. Prospective analysis of ice hockey injuries at the Junior A level over the course of one season. Clin J Sport Med. 1999;9(2):70-74.
71. Emery CA, Meeuwisse WH. Injury rates, risk factors, and mechanisms of injury in minor hockey. Am J Sports Med. 2006;34(12):1960-1969.
72. Kuzuhara K, Shimamoto H, Mase Y. Ice hockey injuries in a Japanese elite team: a 3-year prospective study. J Athl Train. 2009;44(2):208-214.
73. Rishiraj N, Lloyd-Smith R, Lorenz T, Niven B, Michel M. University men’s ice hockey: rates and risk of injuries over 6-years. J Sports Med Phys Fitness. 2009;49(2):159-166.
74. Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men’s international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49(1):30-36.
75. Heckman JD, Bucholz RW. In: Rockwood CA, Green DP, Heckman JD, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults, Volume 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
1. Vaughan G. The Puck Starts Here: The Origin of Canada’s Great Winter Game, Ice Hockey. Fredericton, Canada: Goose Lane Editions; 1996.
2. IIHF member national associations. International Ice Hockey Federation website. http://www.iihf.com/iihf-home/the-iihf/members. Accessed April 6, 2017.
3. Flik K, Lyman S, Marx RG. American collegiate men’s ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
4. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
5. Deits J, Yard EE, Collins CL, Fields SK, Comstock RD. Patients with ice hockey injuries presenting to US emergency departments, 1990-2006. J Athl Train. 2010;45(5):467-474.
6. Brooks A, Loud KJ, Brenner JS, et al. Reducing injury risk from body checking in boys’ youth ice hockey. Pediatrics. 2014;133(6):1151-1157.
7. Agel J, Harvey EJ. A 7-year review of men’s and women’s ice hockey injuries in the NCAA. Can J Surg. 2010;53(5):319-323.
8. Micieli JA, Zurakowski D, Ahmed, II. Impact of visors on eye and orbital injuries in the National Hockey League. Can J Ophthalmol. 2014;49(3):243-248.
9. Pashby TJ. Ocular injuries in hockey. Int Ophthalmol Clin. 1988;28(3):228-231.
10. Leivo T, Haavisto AK, Sahraravand A. Sports-related eye injuries: the current picture. Acta Ophthalmol. 2015;93(3):224-231.
11. Cohn RM, Alaia MJ, Strauss EJ, Feldman AF. Rink-side management of ice hockey related injuries to the face, neck, and chest. Bull Hosp Jt Dis. 2013;71(4):253-256.
12. Reehal P. Facial injury in sport. Curr Sports Med Rep. 2010;9(1):27-34.
13. Stuart MJ, Smith AM, Malo-Ortiguera SA, Fischer TL, Larson DR. A comparison of facial protection and the incidence of head, neck, and facial injuries in Junior A hockey players. A function of individual playing time. Am J Sports Med. 2002;30(1):39-44.
14. MacEwen CJ, McLatchie GR. Eye injuries in sport. Scott Med J. 2010;55(2):22-24.
15. Stevens ST, Lassonde M, de Beaumont L, Keenan JP. The effect of visors on head and facial injury in National Hockey League players. J Sci Med Sport. 2006;9(3):238-242.
16. Moslener MD, Wadsworth LT. Ice hockey: a team physician’s perspective. Curr Sports Med Rep. 2010;9(3):134-138.
17. LaPrade RF, Burnett QM, Zarzour R, Moss R. The effect of the mandatory use of face masks on facial lacerations and head and neck injuries in ice hockey. A prospective study. Am J Sports Med. 1995;23(6):773-775.
18. Benson BW, Mohtadi NG, Rose MS, Meeuwisse WH. Head and neck injuries among ice hockey players wearing full face shields vs half face shields. JAMA. 1999;282(24):2328-2332.
19. Lahti H, Sane J, Ylipaavalniemi P. Dental injuries in ice hockey games and training. Med Sci Sports Exerc. 2002;34(3):400-402.
20. Sane J, Ylipaavalniemi P, Leppanen H. Maxillofacial and dental ice hockey injuries. Med Sci Sports Exerc. 1988;20(2):202-207.
21. Emerich K, Kaczmarek J. First aid for dental trauma caused by sports activities: state of knowledge, treatment and prevention. Sports Med. 2010;40(5):361-366.
22. Rosenberg H, Rosenberg H, Hickey M. Emergency management of a traumatic tooth avulsion. Ann Emerg Med. 2011;57(4):375-377.
23. Young EJ, Macias CR, Stephens L. Common dental injury management in athletes. Sports Health. 2015;7(3):250-255.
24. Andersson L, Andreasen JO, Day P, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol. 2012;28(2):88-96.
25. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19(3):185-200.
26. Schneider KJ, Meeuwisse WH, Kang J, Schneider GM, Emery CA. Preseason reports of neck pain, dizziness, and headache as risk factors for concussion in male youth ice hockey players. Clin J Sport Med. 2013;23(4):267-272.
27. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
28. Delaney JS, Lamfookon C, Bloom GA, Al-Kashmiri A, Correa JA. Why university athletes choose not to reveal their concussion symptoms during a practice or game. Clin J Sport Med. 2015;25(2):113-125.
29. Ventura RE, Balcer LJ, Galetta SL. The concussion toolbox: the role of vision in the assessment of concussion. Semin Neurol. 2015;35(5):599-606.
30. Vartiainen MV, Holm A, Peltonen K, Luoto TM, Iverson GL, Hokkanen L. King-Devick test normative reference values for professional male ice hockey players. Scand J Med Sci Sports. 2015;25(3):e327-e330.
31. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328(1-2):28-31.
32. Vernau BT, Grady MF, Goodman A, et al. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Dev Neuropsychol. 2015;40(1):7-11.
33. Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(1-3):1-34.
34. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1-47.
35. Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293.
36. Smith AM, Stuart MJ, Dodick DW, et al. Ice Hockey Summit II: zero tolerance for head hits and fighting. Curr Sports Med Rep. 2015;14(2):135-144.
37. Mihalik JP, Beard JR, Petschauer MA, Prentice WE, Guskiewicz KM. Effect of ice hockey helmet fit on cervical spine motion during an emergency log roll procedure. Clin J Sport Med. 2008;18(5):394-398.
38. Banerjee R, Palumbo MA, Fadale PD. Catastrophic cervical spine injuries in the collision sport athlete, part 1: epidemiology, functional anatomy, and diagnosis. Am J Sports Med. 2004;32(4):1077-1087.
39. Reynen PD, Clancy WG Jr. Cervical spine injury, hockey helmets, and face masks. Am J Sports Med. 1994;22(2):167-170.
40. Tator CH, Provvidenza C, Cassidy JD. Update and overview of spinal injuries in Canadian ice hockey, 1943 to 2011: the continuing need for injury prevention and education. Clin J Sport Med. 2016;26(3):232-238.
41. Tator CH, Edmonds VE, Lapczak L, Tator IB. Spinal injuries in ice hockey players, 1966-1987. Can J Surg. 1991;34(1):63-69.
42. Laprade RF, Schnetzler KA, Broxterman RJ, Wentorf F, Gilbert TJ. Cervical spine alignment in the immobilized ice hockey player. A computed tomographic analysis of the effects of helmet removal. Am J Sports Med. 2000;28(6):800-803.
43. Metz CM, Kuhn JE, Greenfield ML. Cervical spine alignment in immobilized hockey players: radiographic analysis with and without helmets and shoulder pads. Clin J Sport Med. 1998;8(2):92-95.
44. National Athletic Trainers’ Association. Appropriate prehospital management of the spine-injured athlete: updated from 1998 document. http://www.nata.org/sites/default/files/Executive-Summary-Spine-Injury-updated.pdf. Updated August 5, 2015. Accessed April 6, 2017.
45. Del Rossi G, Heffernan TP, Horodyski M, Rechtine GR. The effectiveness of extrication collars tested during the execution of spine-board transfer techniques. Spine J. 2004;4(6):619-623.
46. Morganti C, Sweeney CA, Albanese SA, Burak C, Hosea T, Connolly PJ. Return to play after cervical spine injury. Spine. 2001;26(10):1131-1136.
47. Huang P, Anissipour A, McGee W, Lemak L. Return-to-play recommendations after cervical, thoracic, and lumbar spine injuries: a comprehensive review. Sports Health. 2016;8(1):19-25.
48. Shindle MK, Marx RG, Kelly BT, Bisson L, Burke CJ 3rd. Hockey injuries: a pediatric sport update. Curr Opin Pediatr. 2010;22(1):54-60.
49. Molsa J, Kujala U, Myllynen P, Torstila I, Airaksinen O. Injuries to the upper extremity in ice hockey: analysis of a series of 760 injuries. Am J Sports Med. 2003;31(5):751-757.
50. Dwyer T, Petrera M, Bleakney R, Theodoropoulos JS. Shoulder instability in ice hockey players: incidence, mechanism, and MRI findings. Clin Sports Med. 2013;32(4):803-813.
51. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. Br J Sports Med. 2009;43(13):1000-1005.
52. Willimon SC, Gaskill TR, Millett PJ. Acromioclavicular joint injuries: anatomy, diagnosis, and treatment. Phys Sportsmed. 2011;39(1):116-122.
53. Martetschlager F, Horan MP, Warth RJ, Millett PJ. Complications after anatomic fixation and reconstruction of the coracoclavicular ligaments. Am J Sports Med. 2013;41(12):2896-2903.
54. Carofino BC, Mazzocca AD. The anatomic coracoclavicular ligament reconstruction: surgical technique and indications. J Shoulder Elbow Surg. 2010;19(2 suppl):37-46.
55. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. Br J Sports Med. 2014;48(1):4-10.
56. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
57. Lee JT, Nasreddine AY, Black EM, Bae DS, Kocher MS. Posterior sternoclavicular joint injuries in skeletally immature patients. J Pediatr Orthop. 2014;34(4):369-375.
58. Hovelius L. Shoulder dislocation in Swedish ice hockey players. Am J Sports Med. 1978;6(6):373-377.
59. Buss DD, Lynch GP, Meyer CP, Huber SM, Freehill MQ. Nonoperative management for in-season athletes with anterior shoulder instability. Am J Sports Med. 2004;32(6):1430-1433.
60. Mazzocca AD, Brown FM Jr, Carreira DS, Hayden J, Romeo AA. Arthroscopic anterior shoulder stabilization of collision and contact athletes. Am J Sports Med. 2005;33(1):52-60.
61. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
62. Cho NS, Hwang JC, Rhee YG. Arthroscopic stabilization in anterior shoulder instability: collision athletes versus noncollision athletes. Arthroscopy. 2006;22(9):947-953.
63. Griffin JW, Brockmeier SF. Shoulder instability with concomitant bone loss in the athlete. Orthop Clin North Am. 2015;46(1):89-103.
64. Cohen SB, Towers JD, Bradley JP. Rotator cuff contusions of the shoulder in professional football players: epidemiology and magnetic resonance imaging findings. Am J Sports Med. 2007;35(3):442-447.
65. Lorentzon R, Wedrèn H, Pietilä T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
66. Stuart MJ, Smith A. Injuries in Junior A ice hockey. A three-year prospective study. Am J Sports Med. 1995;23(4):458-461.
67. Voaklander DC, Saunders LD, Quinney HA, Macnab RB. Epidemiology of recreational and old-timer ice hockey injuries. Clin J Sport Med. 1996;6(1):15-21.
68. Mölsä J, Airaksinen O, Näsman O, Torstila I. Ice hockey injuries in Finland. A prospective epidemiologic study. Am J Sports Med. 1997;25(4):495-499.
69. Ferrara MS, Schurr KT. Intercollegiate ice hockey injuries: a casual analysis. Clin J Sport Med. 1999;9(1):30-33.
70. Pinto M, Kuhn JE, Greenfield ML, Hawkins RJ. Prospective analysis of ice hockey injuries at the Junior A level over the course of one season. Clin J Sport Med. 1999;9(2):70-74.
71. Emery CA, Meeuwisse WH. Injury rates, risk factors, and mechanisms of injury in minor hockey. Am J Sports Med. 2006;34(12):1960-1969.
72. Kuzuhara K, Shimamoto H, Mase Y. Ice hockey injuries in a Japanese elite team: a 3-year prospective study. J Athl Train. 2009;44(2):208-214.
73. Rishiraj N, Lloyd-Smith R, Lorenz T, Niven B, Michel M. University men’s ice hockey: rates and risk of injuries over 6-years. J Sports Med Phys Fitness. 2009;49(2):159-166.
74. Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men’s international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49(1):30-36.
75. Heckman JD, Bucholz RW. In: Rockwood CA, Green DP, Heckman JD, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults, Volume 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
Joint-Preserving Osteotomies for Isolated Patellofemoral Osteoarthritis: Alternatives to Arthroplasty
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Measuring Malalignment on Imaging in the Treatment of Patellofemoral Instability
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.