User login
2016 Update on fertility
Patients seeking fertility care commonly ask the physician for advice regarding ways to optimize their conception attempts. While evidence from randomized controlled trials is not available, data from observational studies provide parameters that can inform patient decision making. Knowledge about the fertility window, the decline in fecundability with age, and lifestyle practices that promote conception may be helpful to clinicians and aid in their ability to guide patients.
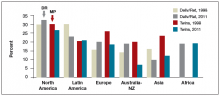
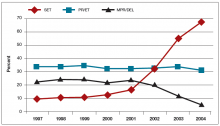
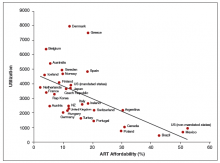
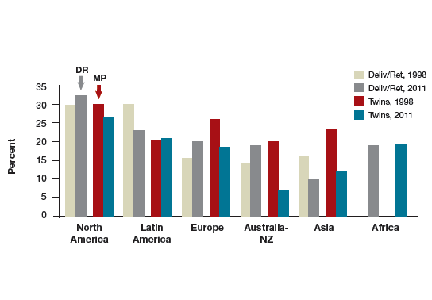
For those patients who will not achieve conception naturally, assisted reproductive technologies (ART) offer a promising alternative. ART options have improved greatly in effectiveness and safety since Louise Brown was born in 1978. More than 5 million babies have been born globally.1 However, even though the United States is wealthy, access to in vitro fertilization (IVF) is poor relative to many other countries, with not more than 1 in 3 people needing IVF actually receiving the treatment. Understanding the international experience enables physicians to take actions that help increase access for their patients who need IVF.
In this article we not only address ways in which your patients can optimize their natural fertility but also examine this country’s ability to offer ART options when they are needed. Without such examination, fundamental changes in societal attitudes toward infertility and payor attitudes toward reproductive care will not occur, and it is these changes, among others, that can move this country to more equitable ART access.
- Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the Assisted Reproductive Technologies (ART). Paper presented at International Federation of Fertility Societies/American Society for Reproductive Medicine Conjoint Meeting; October 12–17, 2013; Boston, Massachusetts.
- Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod. 1999;14(7):1835–1839.
- Keulers MJ, Hamilton CJ, Franx A, et al. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod. 2007;22(6):1652–1656.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–1521.
- Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83(6):1680–1686.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod. 2005;20(1):221–225.
- Check JH, Epstein R, Long R. Effect of time interval between ejaculations on semen parameters. Arch Androl. 1991;27(2):93–95.
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2013;100(3):631–637.
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundi G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20(5):1144–1447.
- Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. BMJ (Clin Res Ed). 1985;290(6483):1697–700.
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103(1):51–56.
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–1403.
- Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–1539.
- Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3223.
- Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. New Engl J Med. 1999;340(5):333–339.
- Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, London S. The effect of smoking on oogenesis, fertilization and implantation. Semin Reprod Med. 1989;7(4):291–304.
- Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol. 1982;9(2):121–130.
- Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334.
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456.
- Signorello LB, McLaughlin JK. Maternal caffeine consumption and spontaneous abortion: a review of the epidemiologic evidence. Epidemiology. 2004;15(2):229–239.
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol. 2002;37(1):87–92.
- Adamson GD; International Council of Medical Acupuncture and Related Techniques (ICMART). ICMART World Report 2011. Webcast presented at: Annual Meeting European Society of Human Reproduction and Embryology (ESHRE); June 16, 2015; Lisbon, Portugal.
- Chambers G, Phuong Hoang V, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101(1):191–198.
- Stovall DW, Allen BD, Sparks AE, Syrop CH, Saunders RG, VanVoorhis BJ. The cost of infertility evaluation and therapy: findings of a self-insured university healthcare plan. Fertil Steril. 1999;72(5):778–784.
- Chambers GM, Sullivan E, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–2294.
- Hamilton BH, McManus B. The effects of insurance mandates on choices and outcomes in infertility treatment markets. Health Econ. 2012;21(8):994–1016.
- Chambers GM, Adamson GD, Eijkemans MJC. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–327.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; ICMART, WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART); World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524.
Patients seeking fertility care commonly ask the physician for advice regarding ways to optimize their conception attempts. While evidence from randomized controlled trials is not available, data from observational studies provide parameters that can inform patient decision making. Knowledge about the fertility window, the decline in fecundability with age, and lifestyle practices that promote conception may be helpful to clinicians and aid in their ability to guide patients.
For those patients who will not achieve conception naturally, assisted reproductive technologies (ART) offer a promising alternative. ART options have improved greatly in effectiveness and safety since Louise Brown was born in 1978. More than 5 million babies have been born globally.1 However, even though the United States is wealthy, access to in vitro fertilization (IVF) is poor relative to many other countries, with not more than 1 in 3 people needing IVF actually receiving the treatment. Understanding the international experience enables physicians to take actions that help increase access for their patients who need IVF.
In this article we not only address ways in which your patients can optimize their natural fertility but also examine this country’s ability to offer ART options when they are needed. Without such examination, fundamental changes in societal attitudes toward infertility and payor attitudes toward reproductive care will not occur, and it is these changes, among others, that can move this country to more equitable ART access.
Patients seeking fertility care commonly ask the physician for advice regarding ways to optimize their conception attempts. While evidence from randomized controlled trials is not available, data from observational studies provide parameters that can inform patient decision making. Knowledge about the fertility window, the decline in fecundability with age, and lifestyle practices that promote conception may be helpful to clinicians and aid in their ability to guide patients.
For those patients who will not achieve conception naturally, assisted reproductive technologies (ART) offer a promising alternative. ART options have improved greatly in effectiveness and safety since Louise Brown was born in 1978. More than 5 million babies have been born globally.1 However, even though the United States is wealthy, access to in vitro fertilization (IVF) is poor relative to many other countries, with not more than 1 in 3 people needing IVF actually receiving the treatment. Understanding the international experience enables physicians to take actions that help increase access for their patients who need IVF.
In this article we not only address ways in which your patients can optimize their natural fertility but also examine this country’s ability to offer ART options when they are needed. Without such examination, fundamental changes in societal attitudes toward infertility and payor attitudes toward reproductive care will not occur, and it is these changes, among others, that can move this country to more equitable ART access.
- Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the Assisted Reproductive Technologies (ART). Paper presented at International Federation of Fertility Societies/American Society for Reproductive Medicine Conjoint Meeting; October 12–17, 2013; Boston, Massachusetts.
- Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod. 1999;14(7):1835–1839.
- Keulers MJ, Hamilton CJ, Franx A, et al. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod. 2007;22(6):1652–1656.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–1521.
- Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83(6):1680–1686.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod. 2005;20(1):221–225.
- Check JH, Epstein R, Long R. Effect of time interval between ejaculations on semen parameters. Arch Androl. 1991;27(2):93–95.
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2013;100(3):631–637.
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundi G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20(5):1144–1447.
- Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. BMJ (Clin Res Ed). 1985;290(6483):1697–700.
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103(1):51–56.
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–1403.
- Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–1539.
- Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3223.
- Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. New Engl J Med. 1999;340(5):333–339.
- Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, London S. The effect of smoking on oogenesis, fertilization and implantation. Semin Reprod Med. 1989;7(4):291–304.
- Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol. 1982;9(2):121–130.
- Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334.
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456.
- Signorello LB, McLaughlin JK. Maternal caffeine consumption and spontaneous abortion: a review of the epidemiologic evidence. Epidemiology. 2004;15(2):229–239.
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol. 2002;37(1):87–92.
- Adamson GD; International Council of Medical Acupuncture and Related Techniques (ICMART). ICMART World Report 2011. Webcast presented at: Annual Meeting European Society of Human Reproduction and Embryology (ESHRE); June 16, 2015; Lisbon, Portugal.
- Chambers G, Phuong Hoang V, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101(1):191–198.
- Stovall DW, Allen BD, Sparks AE, Syrop CH, Saunders RG, VanVoorhis BJ. The cost of infertility evaluation and therapy: findings of a self-insured university healthcare plan. Fertil Steril. 1999;72(5):778–784.
- Chambers GM, Sullivan E, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–2294.
- Hamilton BH, McManus B. The effects of insurance mandates on choices and outcomes in infertility treatment markets. Health Econ. 2012;21(8):994–1016.
- Chambers GM, Adamson GD, Eijkemans MJC. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–327.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; ICMART, WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART); World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524.
- Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the Assisted Reproductive Technologies (ART). Paper presented at International Federation of Fertility Societies/American Society for Reproductive Medicine Conjoint Meeting; October 12–17, 2013; Boston, Massachusetts.
- Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod. 1999;14(7):1835–1839.
- Keulers MJ, Hamilton CJ, Franx A, et al. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod. 2007;22(6):1652–1656.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–1521.
- Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83(6):1680–1686.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod. 2005;20(1):221–225.
- Check JH, Epstein R, Long R. Effect of time interval between ejaculations on semen parameters. Arch Androl. 1991;27(2):93–95.
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2013;100(3):631–637.
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundi G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20(5):1144–1447.
- Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. BMJ (Clin Res Ed). 1985;290(6483):1697–700.
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103(1):51–56.
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–1403.
- Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–1539.
- Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3223.
- Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. New Engl J Med. 1999;340(5):333–339.
- Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, London S. The effect of smoking on oogenesis, fertilization and implantation. Semin Reprod Med. 1989;7(4):291–304.
- Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol. 1982;9(2):121–130.
- Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334.
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456.
- Signorello LB, McLaughlin JK. Maternal caffeine consumption and spontaneous abortion: a review of the epidemiologic evidence. Epidemiology. 2004;15(2):229–239.
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol. 2002;37(1):87–92.
- Adamson GD; International Council of Medical Acupuncture and Related Techniques (ICMART). ICMART World Report 2011. Webcast presented at: Annual Meeting European Society of Human Reproduction and Embryology (ESHRE); June 16, 2015; Lisbon, Portugal.
- Chambers G, Phuong Hoang V, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101(1):191–198.
- Stovall DW, Allen BD, Sparks AE, Syrop CH, Saunders RG, VanVoorhis BJ. The cost of infertility evaluation and therapy: findings of a self-insured university healthcare plan. Fertil Steril. 1999;72(5):778–784.
- Chambers GM, Sullivan E, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–2294.
- Hamilton BH, McManus B. The effects of insurance mandates on choices and outcomes in infertility treatment markets. Health Econ. 2012;21(8):994–1016.
- Chambers GM, Adamson GD, Eijkemans MJC. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–327.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; ICMART, WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART); World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524.
In this Article
- Factors affecting the probability of conception
- Barriers to ART access
- Ways to increase ART funding
Vacuum extraction: Tips for achieving an optimal outcome
CASE: Is vacuum extraction right for this delivery?
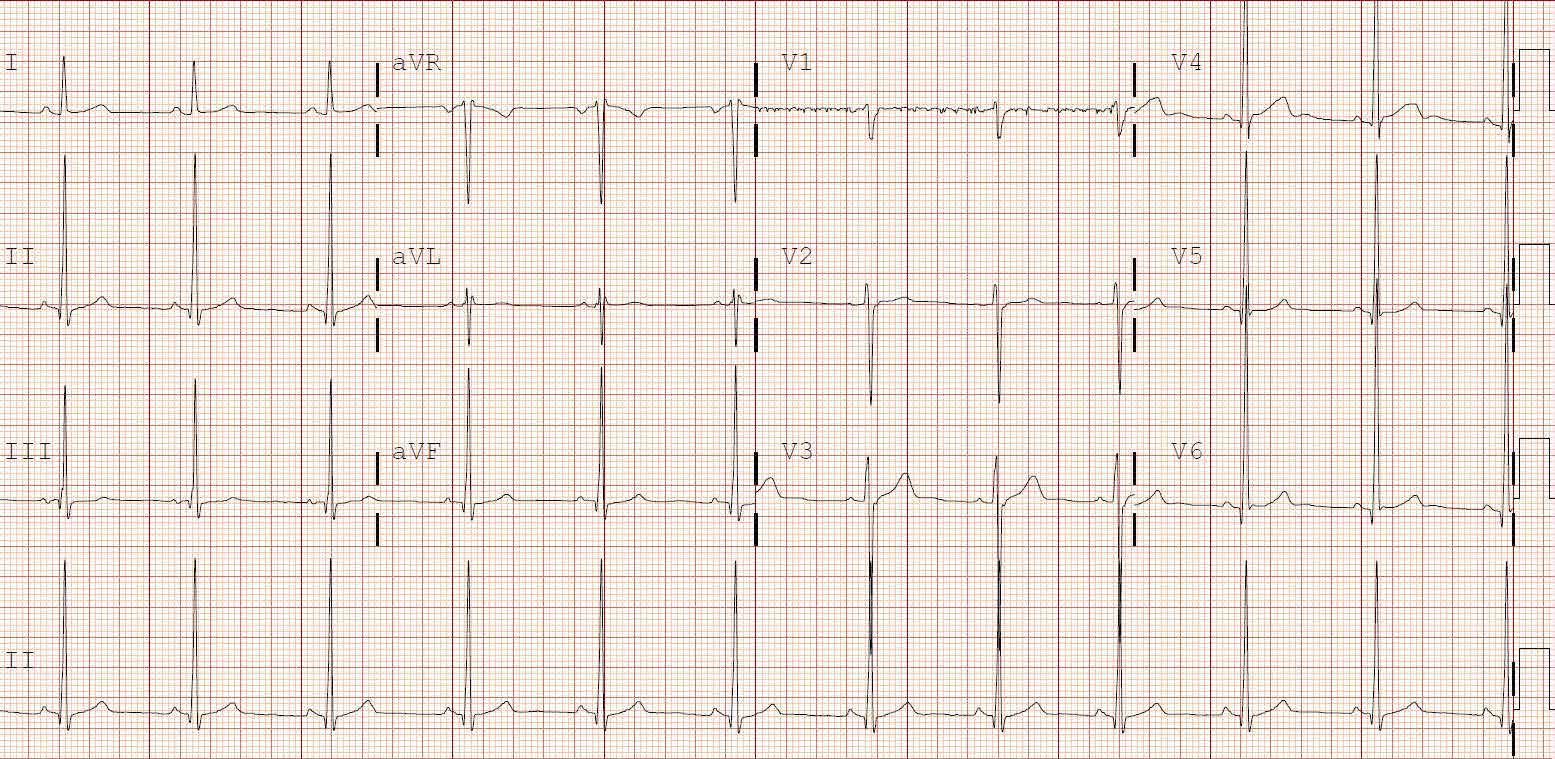
A 41-year-old woman (G2P2002) is at term in her third pregnancy, and the fetus exhibits prolonged deceleration that does not resolve while the mother pushes from a +3 station. The fetus, estimated to weigh 8 lb, is in the occiput anterior (OA) position. The mother is willing to consider vaginal extraction, and you must weigh the factors that may influence successful delivery.
Vacuum extraction (VE) is an effective method to facilitate delivery. From 2007 to 2013, VE was used to facilitate about 3% of vaginal deliveries in the United States.1 By contrast, cesarean delivery rates over the same period averaged about 30%.2
Controversy exists on the pros and cons of operative vaginal deliveries versus cesarean delivery, as well as on the instruments and operational approaches used. While opinion tends to be resolute and influential, evidence remains inconclusive.
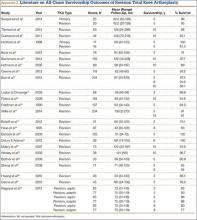
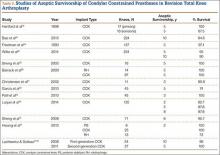
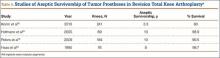
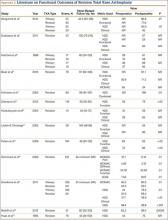
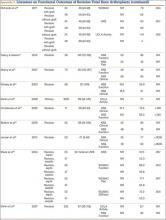
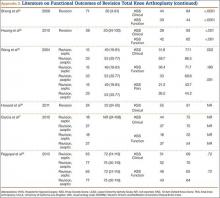
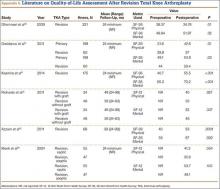
Multiple factors influence a decision on whether to choose VE. The clinician’s own bias regarding delivery routes and comfort level with performing VE are important. The patient, too, may have preconceived opinions about VE. Knowing the indications for VE and its benefits and risks (TABLE 1) can help the patient make an informed choice and the counseling on which will be needed in obtaining the patient’s informed consent. The expectations and desires of the patient in concert with the experience and skill of the clinician will serve to achieve the optimal decision.
Indications for VE
Maternal indications for the use of VE include prolongation or arrest of the second stage of labor. Another indication is the need to shorten the second stage due to a maternal cardiac or cardiovascular disorder or due to maternal exhaustion.
Fetal indications include nonreassuring fetal status or a need to correct for minor degrees of malposition (asynclitism, deflexion) that historically have been addressed with the use of obstetric forceps. VE delivery in these circumstances requires a very experienced and skilled operator.
Further selection criteria
Birthweight influences the consideration of VE. Low birthweight or prematurity are contraindications to the use of VE due to concerns about fetal/neonatal bleeding. Large fetuses will have issues with cephalopelvic disproportion, thus increasing the risk for 2 disorders: shoulder dystocia and fetal cranial bleeding.
Cranial bleeding, both intracranial and extracranial, can result in serious neonatal morbidity and mortality. Bleeding may occur spontaneously or with the use of VE. In using VE, force is transmitted to the fetal scalp. The scalp then has the tendency to pull on its contents and attachments—skull bones, brain, fluids, etc. The scalp attachments include vessels at right angles to the scalp, which may be traumatized or torn by the pulling force. This may lead to subgaleal hemorrhage, a collection of blood in the large potential space below the scalp and above the aponeurosis. Enough force may be generated to deform the intracranial contents and cause intracranial bleeding.
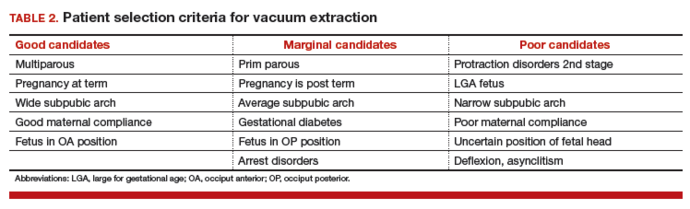
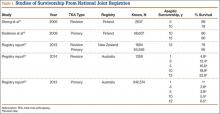
The likelihood of success with VE varies depending on maternal anatomy, the position of the fetal head, gestational age, and the presence or absence of gestational diabetes (TABLE 2).
Delivery by VE: Main considerations
After determining that a candidate is suitable for VE and obtaining informed consent, consider key operative factors:
- choice of extraction cup
- adequate anesthesia
- careful maternal positioning
- maternal bladder emptying
- review of fetal status.
Two major cup types are available: rigid and flexible.
Rigid plastic cup. This design is similar to the metal cup used by Malmström and attaches to the scalp via chignon formation. A variation of the rigid cup is the mityvac “M” that mimics the Malmström design but incorporates a semiflexible handle to facilitate proper cup placement and aid in the direction of pulling force.
Flexible cup. This type of cup flattens against the scalp with vacuum and may result in less minor scalp trauma than the rigid cup.
Greater force can be employed with rigid cup designs than with flexible cups, which can increase the chances of a successful delivery when the fetus is in the occiput posterior (OP) position. Flexible designs tend to cause less damage to the scalp than the rigid cup but are reported to have a higher failure rate.
Cardinal rule of any procedure. Prior to cup placement, remember this rule: abandon the procedure if it proves too difficult. Most deliveries will occur with 3 or 4 pulls.3 Difficulties include:
- failure to gain station with the initial pull
- repetitive cup pop-offs (3 or more)
- an excessive duration of the procedure (>10 minutes).
Less than optimal placement of the vacuum extractor will increase the risk of scalp trauma, particularly in nulliparous women.3
If the procedure is unsuccessful, the resulting options include cesarean delivery and expectant management.
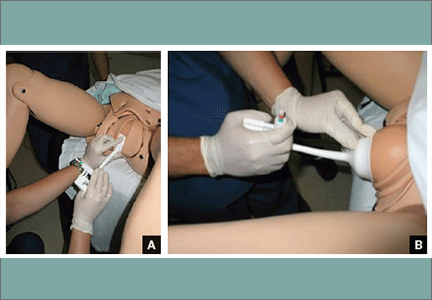
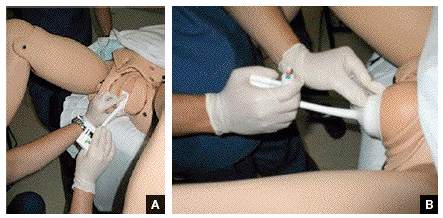
Tip. Use both hands during the pull to more reliably detect a problem with cup attachment, thereby minimizing the possibility of detachment and subsequent scalp trauma (FIGURE).
Key points of technique
Perform a careful and thorough pelvic examination to determine fetal station, position, attitude, and synclitism.
The optimal cup placement is 2- to 3-cm proximal to the posterior fontanel or, alternatively, 5- to 6-cm distal to the anterior fontanel, assuming the fetal head is properly flexed.4 The correct point of flexion will result in the smallest diameter of the fetal head presenting to the birth canal and should minimize the force necessary to achieve delivery.
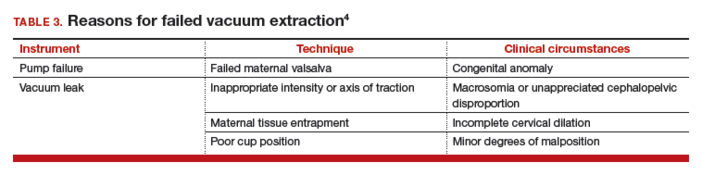
Use minimal vacuum to attach the cup to the fetal head. As the subsequent contraction develops, apply full vacuum with the hand device. Encourage maternal expulsive effort and use traction only in concert with pushing efforts. Three pushes facilitated with pulling may be achieved during a single contraction. Failure to bring about descent with the initial pull indicates potential failure with this approach and, in the absence of technical reasons for the failure, merits serious consideration of abandoning the procedure (TABLE 3).
In the event of failed delivery with VE, it is important to recognize that you should not make a second attempt with another instrument; the chance of success is low and the risk of injury is significantly increased.5
Carefully document the decision for VE and its implementation
The medical record is the most important witness to the event. Clearly record the following items, preferably as close in time to the decision/event as possible:
- the indication for the procedure
- the antecedent labor course
- maternal anesthesia
- personnel present
- instruments employed
- position and station of the fetal head
- force and duration of traction
- nature of the attempt
- immediate condition of the neonate, and any resuscitative efforts.
Closing reminders and advice
In preparing to discuss the patient’s preferences for delivery, understand clearly the risks and benefits of VE and develop a comfortable approach to sharing this information with your patient and her family. Also, if VE is selected, consider performing the procedure in the cesarean delivery room. This will serve to remind you to be mindful to abandon the procedure, if need be, at an appropriate point.
CASE: Resolved
You apply the vacuum extractor, and a small amount of vacuum demonstrates satisfactory attachment. On the second pull, the fetus easily delivers, and the Apgar scores are 8 and 8. The birthweight is 3,725 g. The vacuum-assisted delivery has resulted in the shortest delay in delivery and without adverse consequences for neonate or mother.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Committee on Practice Bulletins—Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 154 Summary: operative vaginal delivery. Obstet Gynecol. 2015;126(5):1118–1119.
- Baskett TF, Fanning CA, Young DC. A prospective observational study of 1000 vacuum assisted deliveries with the OmniCup device. J Obstet Gynaecol Can. 2008;30(7):573–580.
- O’Grady JP. Instrumental delivery. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello LA, Giordano K, eds. Operative Obstetrics. 2nd ed. New York, New York: Cambridge University Press; 2008:475.
- Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341(23):1709–1714.
CASE: Is vacuum extraction right for this delivery?
A 41-year-old woman (G2P2002) is at term in her third pregnancy, and the fetus exhibits prolonged deceleration that does not resolve while the mother pushes from a +3 station. The fetus, estimated to weigh 8 lb, is in the occiput anterior (OA) position. The mother is willing to consider vaginal extraction, and you must weigh the factors that may influence successful delivery.
Vacuum extraction (VE) is an effective method to facilitate delivery. From 2007 to 2013, VE was used to facilitate about 3% of vaginal deliveries in the United States.1 By contrast, cesarean delivery rates over the same period averaged about 30%.2
Controversy exists on the pros and cons of operative vaginal deliveries versus cesarean delivery, as well as on the instruments and operational approaches used. While opinion tends to be resolute and influential, evidence remains inconclusive.
Multiple factors influence a decision on whether to choose VE. The clinician’s own bias regarding delivery routes and comfort level with performing VE are important. The patient, too, may have preconceived opinions about VE. Knowing the indications for VE and its benefits and risks (TABLE 1) can help the patient make an informed choice and the counseling on which will be needed in obtaining the patient’s informed consent. The expectations and desires of the patient in concert with the experience and skill of the clinician will serve to achieve the optimal decision.
Indications for VE
Maternal indications for the use of VE include prolongation or arrest of the second stage of labor. Another indication is the need to shorten the second stage due to a maternal cardiac or cardiovascular disorder or due to maternal exhaustion.
Fetal indications include nonreassuring fetal status or a need to correct for minor degrees of malposition (asynclitism, deflexion) that historically have been addressed with the use of obstetric forceps. VE delivery in these circumstances requires a very experienced and skilled operator.
Further selection criteria
Birthweight influences the consideration of VE. Low birthweight or prematurity are contraindications to the use of VE due to concerns about fetal/neonatal bleeding. Large fetuses will have issues with cephalopelvic disproportion, thus increasing the risk for 2 disorders: shoulder dystocia and fetal cranial bleeding.
Cranial bleeding, both intracranial and extracranial, can result in serious neonatal morbidity and mortality. Bleeding may occur spontaneously or with the use of VE. In using VE, force is transmitted to the fetal scalp. The scalp then has the tendency to pull on its contents and attachments—skull bones, brain, fluids, etc. The scalp attachments include vessels at right angles to the scalp, which may be traumatized or torn by the pulling force. This may lead to subgaleal hemorrhage, a collection of blood in the large potential space below the scalp and above the aponeurosis. Enough force may be generated to deform the intracranial contents and cause intracranial bleeding.
The likelihood of success with VE varies depending on maternal anatomy, the position of the fetal head, gestational age, and the presence or absence of gestational diabetes (TABLE 2).
Delivery by VE: Main considerations
After determining that a candidate is suitable for VE and obtaining informed consent, consider key operative factors:
- choice of extraction cup
- adequate anesthesia
- careful maternal positioning
- maternal bladder emptying
- review of fetal status.
Two major cup types are available: rigid and flexible.
Rigid plastic cup. This design is similar to the metal cup used by Malmström and attaches to the scalp via chignon formation. A variation of the rigid cup is the mityvac “M” that mimics the Malmström design but incorporates a semiflexible handle to facilitate proper cup placement and aid in the direction of pulling force.
Flexible cup. This type of cup flattens against the scalp with vacuum and may result in less minor scalp trauma than the rigid cup.
Greater force can be employed with rigid cup designs than with flexible cups, which can increase the chances of a successful delivery when the fetus is in the occiput posterior (OP) position. Flexible designs tend to cause less damage to the scalp than the rigid cup but are reported to have a higher failure rate.
Cardinal rule of any procedure. Prior to cup placement, remember this rule: abandon the procedure if it proves too difficult. Most deliveries will occur with 3 or 4 pulls.3 Difficulties include:
- failure to gain station with the initial pull
- repetitive cup pop-offs (3 or more)
- an excessive duration of the procedure (>10 minutes).
Less than optimal placement of the vacuum extractor will increase the risk of scalp trauma, particularly in nulliparous women.3
If the procedure is unsuccessful, the resulting options include cesarean delivery and expectant management.
Tip. Use both hands during the pull to more reliably detect a problem with cup attachment, thereby minimizing the possibility of detachment and subsequent scalp trauma (FIGURE).

Key points of technique
Perform a careful and thorough pelvic examination to determine fetal station, position, attitude, and synclitism.
The optimal cup placement is 2- to 3-cm proximal to the posterior fontanel or, alternatively, 5- to 6-cm distal to the anterior fontanel, assuming the fetal head is properly flexed.4 The correct point of flexion will result in the smallest diameter of the fetal head presenting to the birth canal and should minimize the force necessary to achieve delivery.
Use minimal vacuum to attach the cup to the fetal head. As the subsequent contraction develops, apply full vacuum with the hand device. Encourage maternal expulsive effort and use traction only in concert with pushing efforts. Three pushes facilitated with pulling may be achieved during a single contraction. Failure to bring about descent with the initial pull indicates potential failure with this approach and, in the absence of technical reasons for the failure, merits serious consideration of abandoning the procedure (TABLE 3).
In the event of failed delivery with VE, it is important to recognize that you should not make a second attempt with another instrument; the chance of success is low and the risk of injury is significantly increased.5
Carefully document the decision for VE and its implementation
The medical record is the most important witness to the event. Clearly record the following items, preferably as close in time to the decision/event as possible:
- the indication for the procedure
- the antecedent labor course
- maternal anesthesia
- personnel present
- instruments employed
- position and station of the fetal head
- force and duration of traction
- nature of the attempt
- immediate condition of the neonate, and any resuscitative efforts.
Closing reminders and advice
In preparing to discuss the patient’s preferences for delivery, understand clearly the risks and benefits of VE and develop a comfortable approach to sharing this information with your patient and her family. Also, if VE is selected, consider performing the procedure in the cesarean delivery room. This will serve to remind you to be mindful to abandon the procedure, if need be, at an appropriate point.
CASE: Resolved
You apply the vacuum extractor, and a small amount of vacuum demonstrates satisfactory attachment. On the second pull, the fetus easily delivers, and the Apgar scores are 8 and 8. The birthweight is 3,725 g. The vacuum-assisted delivery has resulted in the shortest delay in delivery and without adverse consequences for neonate or mother.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Is vacuum extraction right for this delivery?
A 41-year-old woman (G2P2002) is at term in her third pregnancy, and the fetus exhibits prolonged deceleration that does not resolve while the mother pushes from a +3 station. The fetus, estimated to weigh 8 lb, is in the occiput anterior (OA) position. The mother is willing to consider vaginal extraction, and you must weigh the factors that may influence successful delivery.
Vacuum extraction (VE) is an effective method to facilitate delivery. From 2007 to 2013, VE was used to facilitate about 3% of vaginal deliveries in the United States.1 By contrast, cesarean delivery rates over the same period averaged about 30%.2
Controversy exists on the pros and cons of operative vaginal deliveries versus cesarean delivery, as well as on the instruments and operational approaches used. While opinion tends to be resolute and influential, evidence remains inconclusive.
Multiple factors influence a decision on whether to choose VE. The clinician’s own bias regarding delivery routes and comfort level with performing VE are important. The patient, too, may have preconceived opinions about VE. Knowing the indications for VE and its benefits and risks (TABLE 1) can help the patient make an informed choice and the counseling on which will be needed in obtaining the patient’s informed consent. The expectations and desires of the patient in concert with the experience and skill of the clinician will serve to achieve the optimal decision.
Indications for VE
Maternal indications for the use of VE include prolongation or arrest of the second stage of labor. Another indication is the need to shorten the second stage due to a maternal cardiac or cardiovascular disorder or due to maternal exhaustion.
Fetal indications include nonreassuring fetal status or a need to correct for minor degrees of malposition (asynclitism, deflexion) that historically have been addressed with the use of obstetric forceps. VE delivery in these circumstances requires a very experienced and skilled operator.
Further selection criteria
Birthweight influences the consideration of VE. Low birthweight or prematurity are contraindications to the use of VE due to concerns about fetal/neonatal bleeding. Large fetuses will have issues with cephalopelvic disproportion, thus increasing the risk for 2 disorders: shoulder dystocia and fetal cranial bleeding.
Cranial bleeding, both intracranial and extracranial, can result in serious neonatal morbidity and mortality. Bleeding may occur spontaneously or with the use of VE. In using VE, force is transmitted to the fetal scalp. The scalp then has the tendency to pull on its contents and attachments—skull bones, brain, fluids, etc. The scalp attachments include vessels at right angles to the scalp, which may be traumatized or torn by the pulling force. This may lead to subgaleal hemorrhage, a collection of blood in the large potential space below the scalp and above the aponeurosis. Enough force may be generated to deform the intracranial contents and cause intracranial bleeding.
The likelihood of success with VE varies depending on maternal anatomy, the position of the fetal head, gestational age, and the presence or absence of gestational diabetes (TABLE 2).
Delivery by VE: Main considerations
After determining that a candidate is suitable for VE and obtaining informed consent, consider key operative factors:
- choice of extraction cup
- adequate anesthesia
- careful maternal positioning
- maternal bladder emptying
- review of fetal status.
Two major cup types are available: rigid and flexible.
Rigid plastic cup. This design is similar to the metal cup used by Malmström and attaches to the scalp via chignon formation. A variation of the rigid cup is the mityvac “M” that mimics the Malmström design but incorporates a semiflexible handle to facilitate proper cup placement and aid in the direction of pulling force.
Flexible cup. This type of cup flattens against the scalp with vacuum and may result in less minor scalp trauma than the rigid cup.
Greater force can be employed with rigid cup designs than with flexible cups, which can increase the chances of a successful delivery when the fetus is in the occiput posterior (OP) position. Flexible designs tend to cause less damage to the scalp than the rigid cup but are reported to have a higher failure rate.
Cardinal rule of any procedure. Prior to cup placement, remember this rule: abandon the procedure if it proves too difficult. Most deliveries will occur with 3 or 4 pulls.3 Difficulties include:
- failure to gain station with the initial pull
- repetitive cup pop-offs (3 or more)
- an excessive duration of the procedure (>10 minutes).
Less than optimal placement of the vacuum extractor will increase the risk of scalp trauma, particularly in nulliparous women.3
If the procedure is unsuccessful, the resulting options include cesarean delivery and expectant management.
Tip. Use both hands during the pull to more reliably detect a problem with cup attachment, thereby minimizing the possibility of detachment and subsequent scalp trauma (FIGURE).

Key points of technique
Perform a careful and thorough pelvic examination to determine fetal station, position, attitude, and synclitism.
The optimal cup placement is 2- to 3-cm proximal to the posterior fontanel or, alternatively, 5- to 6-cm distal to the anterior fontanel, assuming the fetal head is properly flexed.4 The correct point of flexion will result in the smallest diameter of the fetal head presenting to the birth canal and should minimize the force necessary to achieve delivery.
Use minimal vacuum to attach the cup to the fetal head. As the subsequent contraction develops, apply full vacuum with the hand device. Encourage maternal expulsive effort and use traction only in concert with pushing efforts. Three pushes facilitated with pulling may be achieved during a single contraction. Failure to bring about descent with the initial pull indicates potential failure with this approach and, in the absence of technical reasons for the failure, merits serious consideration of abandoning the procedure (TABLE 3).
In the event of failed delivery with VE, it is important to recognize that you should not make a second attempt with another instrument; the chance of success is low and the risk of injury is significantly increased.5
Carefully document the decision for VE and its implementation
The medical record is the most important witness to the event. Clearly record the following items, preferably as close in time to the decision/event as possible:
- the indication for the procedure
- the antecedent labor course
- maternal anesthesia
- personnel present
- instruments employed
- position and station of the fetal head
- force and duration of traction
- nature of the attempt
- immediate condition of the neonate, and any resuscitative efforts.
Closing reminders and advice
In preparing to discuss the patient’s preferences for delivery, understand clearly the risks and benefits of VE and develop a comfortable approach to sharing this information with your patient and her family. Also, if VE is selected, consider performing the procedure in the cesarean delivery room. This will serve to remind you to be mindful to abandon the procedure, if need be, at an appropriate point.
CASE: Resolved
You apply the vacuum extractor, and a small amount of vacuum demonstrates satisfactory attachment. On the second pull, the fetus easily delivers, and the Apgar scores are 8 and 8. The birthweight is 3,725 g. The vacuum-assisted delivery has resulted in the shortest delay in delivery and without adverse consequences for neonate or mother.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Committee on Practice Bulletins—Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 154 Summary: operative vaginal delivery. Obstet Gynecol. 2015;126(5):1118–1119.
- Baskett TF, Fanning CA, Young DC. A prospective observational study of 1000 vacuum assisted deliveries with the OmniCup device. J Obstet Gynaecol Can. 2008;30(7):573–580.
- O’Grady JP. Instrumental delivery. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello LA, Giordano K, eds. Operative Obstetrics. 2nd ed. New York, New York: Cambridge University Press; 2008:475.
- Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341(23):1709–1714.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Committee on Practice Bulletins—Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 154 Summary: operative vaginal delivery. Obstet Gynecol. 2015;126(5):1118–1119.
- Baskett TF, Fanning CA, Young DC. A prospective observational study of 1000 vacuum assisted deliveries with the OmniCup device. J Obstet Gynaecol Can. 2008;30(7):573–580.
- O’Grady JP. Instrumental delivery. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello LA, Giordano K, eds. Operative Obstetrics. 2nd ed. New York, New York: Cambridge University Press; 2008:475.
- Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341(23):1709–1714.
In this Article
- Patient selection criteria
- Key technique points
- Important documentation
Troubleshooting the Left Ventricular Assist Device
Introduction
Between 2006 and 2013, over 9,000 continuous flow left ventricular assist devices (LVADs) were implanted in patients with end-stage heart failure; nearly 2,500 of these devices were placed in 2013 alone.1 As the number of patients with an LVAD continues to grow, so too does the likelihood of a patient with an LVAD presenting to the ED—even if the hospital is not a designated VAD center. An earlier article appearing in the February 2014 issue of Emergency Medicine addressed the management of the unique medical complications faced by patients with LVADs.2 This article focuses on troubleshooting the device when a patient with a LVAD presents to the ED.
Device Indications and Types
Left ventricular assist devices are mechanical implantable devices that provide circulatory support to patients with refractory advanced heart failure. A few of these devices are approved by the US Food and Drug Administration for use as either a bridge to heart transplant or as destination therapy if the patient is not a candidate for heart transplant.
Nearly all of the LVADs currently on the market are designed to provide continuous flow.3-6 The most commonly employed LVADs include the HeartMate II (Thoratec Corporation, Pleasanton, California), HeartWare Ventricular Assist System (HeartWare, Framingham, Massachusetts), and the Jarvik 2000 VAS (Jarvik Heart, New York, New York) (Table). These devices differ somewhat in configuration, but the initial management of LVAD patients with a device malfunction is essentially the same.
Even though the type of device is clearly marked on the patient’s controller, to ensure appropriate management and facilitate troubleshooting for malfunction, patients should always carry an information card identifying the type of device implanted, as well as the complete contact information for each of their health care providers, including their LVAD coordinator.3,7
Device Components
Left ventricular assist devices consist of several different components. All LVADs consist of a pump, controller, driveline, and batteries/battery pack that work together to augment function of the native heart.
Pump
The LVAD pump is surgically implanted into the patient’s abdominal or chest cavity, with an inflow cannula positioned in the left ventricle and an outflow cannula inserted into the ascending aorta. The pump is designed to have a single internal moving part called the impeller. The pump draws blood from the left ventricle and directs it to the aorta.
Controller and Driveline
The actions of the pump are directed by a controller, a mini-processor contained in a small box located outside the patient’s body. This processor is connected to the pump by a driveline inside the body. The driveline exits the body through the patient’s abdominal wall and connects to the controller. The controller regulates the pump speed and provides information about pump speed, flow, pulsatility index, and power of the LVAD.3,4
Speed Monitor. The LVAD speed on the controller is usually set at the time of discharge from the hospital; most devices cannot be changed manually.
Power Monitor. On the monitor, the "power" indictor reflects the voltage and current of the pump motor, whereas the "flow" indicator is a reflection of both power and speed (ie, the higher the power, the higher the flow and vice versa).
Batteries/Battery Pack. The LVAD is powered by two batteries attached to the controller or, in some models, to a power base unit that can be plugged into an electrical outlet. Each battery is connected to the controller by a separate connector wire. When battery power in the device becomes low, the batteries should be replaced with fully charged backup batteries, one battery at a time. If the controller is disconnected from both batteries at the same time, the LVAD will lose power and stop working. It is critical to patient health that the LVAD be powered at all times.3,5,7,8
Pulsatility Index. Changes in a patient’s blood pressure (BP) can affect flow, with higher BP causing a decrease in flow. The pulsatility index reflects the heart’s contractility and stretch, as well as the patient’s volume status. As the preload decreases (ie, due to a decrease in blood volume) the device will indicate a decreased pulsatility index. If the patient is volume overloaded, the pulsatility index will be increased. Pump speed and pulsatility index are inversely related. In some devices, if the controller detects a significant change in pulsatility index from the prior 15-second average, it will reduce the pump speed to low and then gradually accelerate to the set fixed speed.3,8 Significant changes in pulsatility index often indicate an event has occurred, such as an obstruction of the inflow cannula, a decrease in the patient’s volume status, an arrhythmia, or increased pulmonary artery pressure suggestive of right heart failure.3,4
Patient Responsibility
Following LVAD placement, patients are discharged from the hospital with extra batteries (usually four to six), a battery-charger station, a spare controller, and in certain models, a power base unit that can power the LVAD when they are at home and/or asleep. When patients are away from home, they should always have extra fully charged batteries, the spare controller, information about their device, and the complete names and contact information for their health care providers (ie, cardiologist, cardiothoracic surgeon, VAD coordinator) with them at all times.3,7,8
Patient Evaluation and Troubleshooting the Device
Perfusion and Mean Arterial Pressure
At presentation, the EP should evaluate the patient for signs of poor perfusion (eg, decreased mental status, pallor, cool skin) and, when indicated, provide a fluid bolus. Patients with an LVAD typically do not have palpable pulses due to the continuous flow of their devices.3-7 Therefore, a mean arterial pressure (MAP) using a Doppler and a manual BP cuff should be taken. The pressure at which the first sound is heard is used as the estimate of the MAP. The MAP for an LVAD patient generally should be between 70 and 90 mm Hg.3,4,7
Patients with an LVAD are afterload sensitive, and high BP must be addressed immediately to avoid morbidity. Elevated BP increases the work of the pump against increased peripheral resistance, which can lead to thrombus and stroke.7
Power and Connections
The EP should always check the controller to make sure the power light is on. Once this has been confirmed, she or he should auscultate over the patient’s chest and abdomen to detect the humming sound of the pump. If the pump is not powered or does not appear to be functioning, the controller should be replaced with the patient’s backup controller. Next, all connections to the power source and the connection between the driveline and the controller should be checked to confirm they are intact. After this has been completed, the connections should be disconnected and reconnected. Then the driveline should be evaluated for defects or damage.
Battery Assessment
While troubleshooting the LVAD for malfunction, the batteries on the device should be replaced with backup batteries or connected to the hospital’s power base unit (if one exists) or to the patient’s power base unit if it is present. If a battery replacement is required, before doing so, the patient should first be positioned flat on the stretcher or bed.7
Low-Flow Indicator
The EP should always check the controller to determine which alarms, if any, are flashing. Although the alarm buttons vary among the various LVAD devices, all types have a “low-flow” indicator. If the controller indicates low flow, the patient first should be given a fluid bolus. Patients with LVADs are preload dependent, and given their history of heart failure and fluid restriction, are often reluctant to maintain good fluid intake once an LVAD has been implanted.3,7
Another important etiology for a low-flow reading on the LVAD controller is pump thrombosis. Pump thrombosis should be considered when the MAP is low and the controller indicates a decreased pulsatility index and decreased flow. Often the RPMs (the speed) are increased as the controller attempts to adjust to the thrombosis with an increase in power. A bedside echocardiogram showing dilated right and left ventricles is consistent with a pump thrombosis. Treatment for pump thrombosis is anticoagulation with heparin or thrombolytics, and cardiothoracic surgery should be consulted.3
Suction Events
In addition to hypovolemia, another cause of hypotension is a “suction event” in which the left ventricle is not filling but the pump continues to attempt to pull blood from it and the walls of the ventricle suck in on themselves. Suction events can also be caused by cannula malposition, increased peripheral vascular resistance, and tamponade. A small left ventricle on bedside echocardiogram is consistent with a suction event. Often, the controller can sense this and will respond by slowing the pump speed and slowly bringing it back up to allow the ventricles to reaccumulate blood. Initial treatment consists of a fluid bolus and cardiothoracic surgery consultation.3,4
Low Battery
If an LVAD patient presents to the ED with a low battery, no backup batteries, and the hospital does not have a power base unit or other way to power the LVAD, the EP should call the patient’s VAD coordinator to assist. Often, the coordinator can identify a local LVAD patient who is willing to transport extra batteries to the ED.
To conserve power, the controller in some LVAD models is programmed to automatically reduce the pump speed when the battery power becomes low. This feature can be a cause of syncope or lightheadedness in in some patients. While some older LVAD models had the capability of being powered by a hand pump in the event of loss of function, the newer devices do not.4,7,8
Cardiac Assessment and Protocols
The presence of an LVAD should not have any effect on electrocardiography studies. When evaluating patients, standard advanced cardiac life support protocols should be followed, but extreme caution should be used before starting chest compressions as this can dislodge the cannula and lead to death. Prior to initiating chest compressions, the patient’s VAD coordinator should be contacted to ensure that any problems with the device itself have been considered, addressed, and ruled out. Defibrillation, cardioversion, and pacing are all acceptable in LVAD patients, but defibrillator or pacer pads should not be placed directly over the patient’s pump.7
Summary
As the number of patients with an implantable cardiac device continues to grow, EPs are likely to encounter an LVAD patient in the ED. An understanding of device function and knowledge of the basic trouble-shooting skills can prove life-saving in many instances. In addition, a familiarity with available LVAD resources, both online and within the health care community, is also essential to ensure the appropriate management and care of these patients.
- Troubleshooting the Left Ventricular Assist Device
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014; 33(6):555-564.
- Devine AS. Left ventricular assist devices: from mystery to mastery. Hardware for the heart: the increasing impact of pacemakers, ICDs, and LVADs. Emerg Med. 2014;46(2):72-75.
- Slaughter MS, Pagani FD, Rogers JG, et al; HeartMate II Clinical Investigators. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Klein T, Jacob MS. Management of implantable assisted circulation devices: emergency issues. Cardiol Clin. 2012;30(4):673-682.
- Felix SE, Martinia Jr, Kirkels JH, et al. Continuous-flow left ventricular assist device support in patients with advanced heart failure: points of interest for the daily management. Eur J Heart Fail. 2012;14(4):351-356
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251.
- Mechanical Circulatory Support Organization. EMS Guide, January 2015. http://mylvad.com/sites/mylvadrp/files/EMS%20Field%20Guides/MCSO%20EMS%20GUIDE%202015%20.pdf. Accessed February 2, 2016.
- Mylvad.com Web site. Accessed February 2, 2016.
Introduction
Between 2006 and 2013, over 9,000 continuous flow left ventricular assist devices (LVADs) were implanted in patients with end-stage heart failure; nearly 2,500 of these devices were placed in 2013 alone.1 As the number of patients with an LVAD continues to grow, so too does the likelihood of a patient with an LVAD presenting to the ED—even if the hospital is not a designated VAD center. An earlier article appearing in the February 2014 issue of Emergency Medicine addressed the management of the unique medical complications faced by patients with LVADs.2 This article focuses on troubleshooting the device when a patient with a LVAD presents to the ED.
Device Indications and Types
Left ventricular assist devices are mechanical implantable devices that provide circulatory support to patients with refractory advanced heart failure. A few of these devices are approved by the US Food and Drug Administration for use as either a bridge to heart transplant or as destination therapy if the patient is not a candidate for heart transplant.
Nearly all of the LVADs currently on the market are designed to provide continuous flow.3-6 The most commonly employed LVADs include the HeartMate II (Thoratec Corporation, Pleasanton, California), HeartWare Ventricular Assist System (HeartWare, Framingham, Massachusetts), and the Jarvik 2000 VAS (Jarvik Heart, New York, New York) (Table). These devices differ somewhat in configuration, but the initial management of LVAD patients with a device malfunction is essentially the same.
Even though the type of device is clearly marked on the patient’s controller, to ensure appropriate management and facilitate troubleshooting for malfunction, patients should always carry an information card identifying the type of device implanted, as well as the complete contact information for each of their health care providers, including their LVAD coordinator.3,7
Device Components
Left ventricular assist devices consist of several different components. All LVADs consist of a pump, controller, driveline, and batteries/battery pack that work together to augment function of the native heart.
Pump
The LVAD pump is surgically implanted into the patient’s abdominal or chest cavity, with an inflow cannula positioned in the left ventricle and an outflow cannula inserted into the ascending aorta. The pump is designed to have a single internal moving part called the impeller. The pump draws blood from the left ventricle and directs it to the aorta.
Controller and Driveline
The actions of the pump are directed by a controller, a mini-processor contained in a small box located outside the patient’s body. This processor is connected to the pump by a driveline inside the body. The driveline exits the body through the patient’s abdominal wall and connects to the controller. The controller regulates the pump speed and provides information about pump speed, flow, pulsatility index, and power of the LVAD.3,4
Speed Monitor. The LVAD speed on the controller is usually set at the time of discharge from the hospital; most devices cannot be changed manually.
Power Monitor. On the monitor, the "power" indictor reflects the voltage and current of the pump motor, whereas the "flow" indicator is a reflection of both power and speed (ie, the higher the power, the higher the flow and vice versa).
Batteries/Battery Pack. The LVAD is powered by two batteries attached to the controller or, in some models, to a power base unit that can be plugged into an electrical outlet. Each battery is connected to the controller by a separate connector wire. When battery power in the device becomes low, the batteries should be replaced with fully charged backup batteries, one battery at a time. If the controller is disconnected from both batteries at the same time, the LVAD will lose power and stop working. It is critical to patient health that the LVAD be powered at all times.3,5,7,8
Pulsatility Index. Changes in a patient’s blood pressure (BP) can affect flow, with higher BP causing a decrease in flow. The pulsatility index reflects the heart’s contractility and stretch, as well as the patient’s volume status. As the preload decreases (ie, due to a decrease in blood volume) the device will indicate a decreased pulsatility index. If the patient is volume overloaded, the pulsatility index will be increased. Pump speed and pulsatility index are inversely related. In some devices, if the controller detects a significant change in pulsatility index from the prior 15-second average, it will reduce the pump speed to low and then gradually accelerate to the set fixed speed.3,8 Significant changes in pulsatility index often indicate an event has occurred, such as an obstruction of the inflow cannula, a decrease in the patient’s volume status, an arrhythmia, or increased pulmonary artery pressure suggestive of right heart failure.3,4
Patient Responsibility
Following LVAD placement, patients are discharged from the hospital with extra batteries (usually four to six), a battery-charger station, a spare controller, and in certain models, a power base unit that can power the LVAD when they are at home and/or asleep. When patients are away from home, they should always have extra fully charged batteries, the spare controller, information about their device, and the complete names and contact information for their health care providers (ie, cardiologist, cardiothoracic surgeon, VAD coordinator) with them at all times.3,7,8
Patient Evaluation and Troubleshooting the Device
Perfusion and Mean Arterial Pressure
At presentation, the EP should evaluate the patient for signs of poor perfusion (eg, decreased mental status, pallor, cool skin) and, when indicated, provide a fluid bolus. Patients with an LVAD typically do not have palpable pulses due to the continuous flow of their devices.3-7 Therefore, a mean arterial pressure (MAP) using a Doppler and a manual BP cuff should be taken. The pressure at which the first sound is heard is used as the estimate of the MAP. The MAP for an LVAD patient generally should be between 70 and 90 mm Hg.3,4,7
Patients with an LVAD are afterload sensitive, and high BP must be addressed immediately to avoid morbidity. Elevated BP increases the work of the pump against increased peripheral resistance, which can lead to thrombus and stroke.7
Power and Connections
The EP should always check the controller to make sure the power light is on. Once this has been confirmed, she or he should auscultate over the patient’s chest and abdomen to detect the humming sound of the pump. If the pump is not powered or does not appear to be functioning, the controller should be replaced with the patient’s backup controller. Next, all connections to the power source and the connection between the driveline and the controller should be checked to confirm they are intact. After this has been completed, the connections should be disconnected and reconnected. Then the driveline should be evaluated for defects or damage.
Battery Assessment
While troubleshooting the LVAD for malfunction, the batteries on the device should be replaced with backup batteries or connected to the hospital’s power base unit (if one exists) or to the patient’s power base unit if it is present. If a battery replacement is required, before doing so, the patient should first be positioned flat on the stretcher or bed.7
Low-Flow Indicator
The EP should always check the controller to determine which alarms, if any, are flashing. Although the alarm buttons vary among the various LVAD devices, all types have a “low-flow” indicator. If the controller indicates low flow, the patient first should be given a fluid bolus. Patients with LVADs are preload dependent, and given their history of heart failure and fluid restriction, are often reluctant to maintain good fluid intake once an LVAD has been implanted.3,7
Another important etiology for a low-flow reading on the LVAD controller is pump thrombosis. Pump thrombosis should be considered when the MAP is low and the controller indicates a decreased pulsatility index and decreased flow. Often the RPMs (the speed) are increased as the controller attempts to adjust to the thrombosis with an increase in power. A bedside echocardiogram showing dilated right and left ventricles is consistent with a pump thrombosis. Treatment for pump thrombosis is anticoagulation with heparin or thrombolytics, and cardiothoracic surgery should be consulted.3
Suction Events
In addition to hypovolemia, another cause of hypotension is a “suction event” in which the left ventricle is not filling but the pump continues to attempt to pull blood from it and the walls of the ventricle suck in on themselves. Suction events can also be caused by cannula malposition, increased peripheral vascular resistance, and tamponade. A small left ventricle on bedside echocardiogram is consistent with a suction event. Often, the controller can sense this and will respond by slowing the pump speed and slowly bringing it back up to allow the ventricles to reaccumulate blood. Initial treatment consists of a fluid bolus and cardiothoracic surgery consultation.3,4
Low Battery
If an LVAD patient presents to the ED with a low battery, no backup batteries, and the hospital does not have a power base unit or other way to power the LVAD, the EP should call the patient’s VAD coordinator to assist. Often, the coordinator can identify a local LVAD patient who is willing to transport extra batteries to the ED.
To conserve power, the controller in some LVAD models is programmed to automatically reduce the pump speed when the battery power becomes low. This feature can be a cause of syncope or lightheadedness in in some patients. While some older LVAD models had the capability of being powered by a hand pump in the event of loss of function, the newer devices do not.4,7,8
Cardiac Assessment and Protocols
The presence of an LVAD should not have any effect on electrocardiography studies. When evaluating patients, standard advanced cardiac life support protocols should be followed, but extreme caution should be used before starting chest compressions as this can dislodge the cannula and lead to death. Prior to initiating chest compressions, the patient’s VAD coordinator should be contacted to ensure that any problems with the device itself have been considered, addressed, and ruled out. Defibrillation, cardioversion, and pacing are all acceptable in LVAD patients, but defibrillator or pacer pads should not be placed directly over the patient’s pump.7
Summary
As the number of patients with an implantable cardiac device continues to grow, EPs are likely to encounter an LVAD patient in the ED. An understanding of device function and knowledge of the basic trouble-shooting skills can prove life-saving in many instances. In addition, a familiarity with available LVAD resources, both online and within the health care community, is also essential to ensure the appropriate management and care of these patients.
Introduction
Between 2006 and 2013, over 9,000 continuous flow left ventricular assist devices (LVADs) were implanted in patients with end-stage heart failure; nearly 2,500 of these devices were placed in 2013 alone.1 As the number of patients with an LVAD continues to grow, so too does the likelihood of a patient with an LVAD presenting to the ED—even if the hospital is not a designated VAD center. An earlier article appearing in the February 2014 issue of Emergency Medicine addressed the management of the unique medical complications faced by patients with LVADs.2 This article focuses on troubleshooting the device when a patient with a LVAD presents to the ED.
Device Indications and Types
Left ventricular assist devices are mechanical implantable devices that provide circulatory support to patients with refractory advanced heart failure. A few of these devices are approved by the US Food and Drug Administration for use as either a bridge to heart transplant or as destination therapy if the patient is not a candidate for heart transplant.
Nearly all of the LVADs currently on the market are designed to provide continuous flow.3-6 The most commonly employed LVADs include the HeartMate II (Thoratec Corporation, Pleasanton, California), HeartWare Ventricular Assist System (HeartWare, Framingham, Massachusetts), and the Jarvik 2000 VAS (Jarvik Heart, New York, New York) (Table). These devices differ somewhat in configuration, but the initial management of LVAD patients with a device malfunction is essentially the same.
Even though the type of device is clearly marked on the patient’s controller, to ensure appropriate management and facilitate troubleshooting for malfunction, patients should always carry an information card identifying the type of device implanted, as well as the complete contact information for each of their health care providers, including their LVAD coordinator.3,7
Device Components
Left ventricular assist devices consist of several different components. All LVADs consist of a pump, controller, driveline, and batteries/battery pack that work together to augment function of the native heart.
Pump
The LVAD pump is surgically implanted into the patient’s abdominal or chest cavity, with an inflow cannula positioned in the left ventricle and an outflow cannula inserted into the ascending aorta. The pump is designed to have a single internal moving part called the impeller. The pump draws blood from the left ventricle and directs it to the aorta.
Controller and Driveline
The actions of the pump are directed by a controller, a mini-processor contained in a small box located outside the patient’s body. This processor is connected to the pump by a driveline inside the body. The driveline exits the body through the patient’s abdominal wall and connects to the controller. The controller regulates the pump speed and provides information about pump speed, flow, pulsatility index, and power of the LVAD.3,4
Speed Monitor. The LVAD speed on the controller is usually set at the time of discharge from the hospital; most devices cannot be changed manually.
Power Monitor. On the monitor, the "power" indictor reflects the voltage and current of the pump motor, whereas the "flow" indicator is a reflection of both power and speed (ie, the higher the power, the higher the flow and vice versa).
Batteries/Battery Pack. The LVAD is powered by two batteries attached to the controller or, in some models, to a power base unit that can be plugged into an electrical outlet. Each battery is connected to the controller by a separate connector wire. When battery power in the device becomes low, the batteries should be replaced with fully charged backup batteries, one battery at a time. If the controller is disconnected from both batteries at the same time, the LVAD will lose power and stop working. It is critical to patient health that the LVAD be powered at all times.3,5,7,8
Pulsatility Index. Changes in a patient’s blood pressure (BP) can affect flow, with higher BP causing a decrease in flow. The pulsatility index reflects the heart’s contractility and stretch, as well as the patient’s volume status. As the preload decreases (ie, due to a decrease in blood volume) the device will indicate a decreased pulsatility index. If the patient is volume overloaded, the pulsatility index will be increased. Pump speed and pulsatility index are inversely related. In some devices, if the controller detects a significant change in pulsatility index from the prior 15-second average, it will reduce the pump speed to low and then gradually accelerate to the set fixed speed.3,8 Significant changes in pulsatility index often indicate an event has occurred, such as an obstruction of the inflow cannula, a decrease in the patient’s volume status, an arrhythmia, or increased pulmonary artery pressure suggestive of right heart failure.3,4
Patient Responsibility
Following LVAD placement, patients are discharged from the hospital with extra batteries (usually four to six), a battery-charger station, a spare controller, and in certain models, a power base unit that can power the LVAD when they are at home and/or asleep. When patients are away from home, they should always have extra fully charged batteries, the spare controller, information about their device, and the complete names and contact information for their health care providers (ie, cardiologist, cardiothoracic surgeon, VAD coordinator) with them at all times.3,7,8
Patient Evaluation and Troubleshooting the Device
Perfusion and Mean Arterial Pressure
At presentation, the EP should evaluate the patient for signs of poor perfusion (eg, decreased mental status, pallor, cool skin) and, when indicated, provide a fluid bolus. Patients with an LVAD typically do not have palpable pulses due to the continuous flow of their devices.3-7 Therefore, a mean arterial pressure (MAP) using a Doppler and a manual BP cuff should be taken. The pressure at which the first sound is heard is used as the estimate of the MAP. The MAP for an LVAD patient generally should be between 70 and 90 mm Hg.3,4,7
Patients with an LVAD are afterload sensitive, and high BP must be addressed immediately to avoid morbidity. Elevated BP increases the work of the pump against increased peripheral resistance, which can lead to thrombus and stroke.7
Power and Connections
The EP should always check the controller to make sure the power light is on. Once this has been confirmed, she or he should auscultate over the patient’s chest and abdomen to detect the humming sound of the pump. If the pump is not powered or does not appear to be functioning, the controller should be replaced with the patient’s backup controller. Next, all connections to the power source and the connection between the driveline and the controller should be checked to confirm they are intact. After this has been completed, the connections should be disconnected and reconnected. Then the driveline should be evaluated for defects or damage.
Battery Assessment
While troubleshooting the LVAD for malfunction, the batteries on the device should be replaced with backup batteries or connected to the hospital’s power base unit (if one exists) or to the patient’s power base unit if it is present. If a battery replacement is required, before doing so, the patient should first be positioned flat on the stretcher or bed.7
Low-Flow Indicator
The EP should always check the controller to determine which alarms, if any, are flashing. Although the alarm buttons vary among the various LVAD devices, all types have a “low-flow” indicator. If the controller indicates low flow, the patient first should be given a fluid bolus. Patients with LVADs are preload dependent, and given their history of heart failure and fluid restriction, are often reluctant to maintain good fluid intake once an LVAD has been implanted.3,7
Another important etiology for a low-flow reading on the LVAD controller is pump thrombosis. Pump thrombosis should be considered when the MAP is low and the controller indicates a decreased pulsatility index and decreased flow. Often the RPMs (the speed) are increased as the controller attempts to adjust to the thrombosis with an increase in power. A bedside echocardiogram showing dilated right and left ventricles is consistent with a pump thrombosis. Treatment for pump thrombosis is anticoagulation with heparin or thrombolytics, and cardiothoracic surgery should be consulted.3
Suction Events
In addition to hypovolemia, another cause of hypotension is a “suction event” in which the left ventricle is not filling but the pump continues to attempt to pull blood from it and the walls of the ventricle suck in on themselves. Suction events can also be caused by cannula malposition, increased peripheral vascular resistance, and tamponade. A small left ventricle on bedside echocardiogram is consistent with a suction event. Often, the controller can sense this and will respond by slowing the pump speed and slowly bringing it back up to allow the ventricles to reaccumulate blood. Initial treatment consists of a fluid bolus and cardiothoracic surgery consultation.3,4
Low Battery
If an LVAD patient presents to the ED with a low battery, no backup batteries, and the hospital does not have a power base unit or other way to power the LVAD, the EP should call the patient’s VAD coordinator to assist. Often, the coordinator can identify a local LVAD patient who is willing to transport extra batteries to the ED.
To conserve power, the controller in some LVAD models is programmed to automatically reduce the pump speed when the battery power becomes low. This feature can be a cause of syncope or lightheadedness in in some patients. While some older LVAD models had the capability of being powered by a hand pump in the event of loss of function, the newer devices do not.4,7,8
Cardiac Assessment and Protocols
The presence of an LVAD should not have any effect on electrocardiography studies. When evaluating patients, standard advanced cardiac life support protocols should be followed, but extreme caution should be used before starting chest compressions as this can dislodge the cannula and lead to death. Prior to initiating chest compressions, the patient’s VAD coordinator should be contacted to ensure that any problems with the device itself have been considered, addressed, and ruled out. Defibrillation, cardioversion, and pacing are all acceptable in LVAD patients, but defibrillator or pacer pads should not be placed directly over the patient’s pump.7
Summary
As the number of patients with an implantable cardiac device continues to grow, EPs are likely to encounter an LVAD patient in the ED. An understanding of device function and knowledge of the basic trouble-shooting skills can prove life-saving in many instances. In addition, a familiarity with available LVAD resources, both online and within the health care community, is also essential to ensure the appropriate management and care of these patients.
- Troubleshooting the Left Ventricular Assist Device
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014; 33(6):555-564.
- Devine AS. Left ventricular assist devices: from mystery to mastery. Hardware for the heart: the increasing impact of pacemakers, ICDs, and LVADs. Emerg Med. 2014;46(2):72-75.
- Slaughter MS, Pagani FD, Rogers JG, et al; HeartMate II Clinical Investigators. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Klein T, Jacob MS. Management of implantable assisted circulation devices: emergency issues. Cardiol Clin. 2012;30(4):673-682.
- Felix SE, Martinia Jr, Kirkels JH, et al. Continuous-flow left ventricular assist device support in patients with advanced heart failure: points of interest for the daily management. Eur J Heart Fail. 2012;14(4):351-356
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251.
- Mechanical Circulatory Support Organization. EMS Guide, January 2015. http://mylvad.com/sites/mylvadrp/files/EMS%20Field%20Guides/MCSO%20EMS%20GUIDE%202015%20.pdf. Accessed February 2, 2016.
- Mylvad.com Web site. Accessed February 2, 2016.
- Troubleshooting the Left Ventricular Assist Device
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014; 33(6):555-564.
- Devine AS. Left ventricular assist devices: from mystery to mastery. Hardware for the heart: the increasing impact of pacemakers, ICDs, and LVADs. Emerg Med. 2014;46(2):72-75.
- Slaughter MS, Pagani FD, Rogers JG, et al; HeartMate II Clinical Investigators. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Klein T, Jacob MS. Management of implantable assisted circulation devices: emergency issues. Cardiol Clin. 2012;30(4):673-682.
- Felix SE, Martinia Jr, Kirkels JH, et al. Continuous-flow left ventricular assist device support in patients with advanced heart failure: points of interest for the daily management. Eur J Heart Fail. 2012;14(4):351-356
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251.
- Mechanical Circulatory Support Organization. EMS Guide, January 2015. http://mylvad.com/sites/mylvadrp/files/EMS%20Field%20Guides/MCSO%20EMS%20GUIDE%202015%20.pdf. Accessed February 2, 2016.
- Mylvad.com Web site. Accessed February 2, 2016.
Case Report: Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
Introduction
Coarctation of the aorta comprises approximately 5% to 8% of congenital heart defects and is often associated with valvular malformations.1 These defects are typically diagnosed early and are managed with surgical repair, balloon angioplasty, or endovascular stent placement. However, as the following case illustrates, complications can occur in this population despite early intervention.
Case
A 15-year-old male adolescent presented to the pediatric ED after repeated blood pressure (BP) checks by the school nurse revealed consistently elevated systolic and diastolic pressures. The patient’s hypertension was associated with symptoms of intermittent headache and light-headedness. His medical history was remarkable for a congenital aortic coarctation and a bicuspid aortic valve. The patient had undergone a subclavian flap repair prior to 1 month of age, followed by a balloon dilatation 1 year later for recurrent coarctation. The rest of the patient’s medical history was unremarkable, including normal renal function. He denied illicit drug or alcohol use, sexual activity, or trauma.
On evaluation, the patient’s cardiac examination revealed a regular rate and rhythm with normally split S2; there were no rubs, murmurs, or gallops on auscultation. He had normal and equal pulses of the upper and lower extremities bilaterally. The patient presented without cyanosis. He was alert and oriented with normal upper and lower extremity reflexes. The neurological examination, including cranial nerve, strength, and gait testing, was unremarkable. The gastrointestinal examination showed a soft, nondistended abdomen, with no pulsatile masses. There was no abnormal swelling of his extremities. Although the physical examination findings were unremarkable, the patient’s vital signs were concerning as BP in his right upper extremity was as high as 208/110 mm Hg, while BP in his right leg was 130/68 mm Hg.
The patient followed up with his cardiologist, who ordered cardiac magnetic resonance imaging (MRI). The MRI showed mild narrowing of the distal aortic arch with a minimal and clinically insignificant pressure gradient. Based on the MRI findings, the patient was referred to a pediatric nephrologist, who performed a 24-hour ambulatory BP evaluation. The results of this study showed the patient to have systolic hypertension at the 95th percentile for his age and height. Based on the patient’s athletic predilection, β-blockers were avoided, and he was instead started on the angiotensin-converting enzyme (ACE) inhibitor lisinopril, along with annual follow-up cardiac evaluation.
Discussion
The authors’ initial concern for this patient was the possibility of a recurrent coarctation causing a significant pressure gradient between the upper and lower extremities with associated symptoms. A review of the literature demonstrates such an occurrence is not uncommon in this patient population, especially in patients with a history of early intervention (ie, within the first year of life).2
Causes and Incidence
One of the factors believed to contribute to recurrent coarctation is insufficient growth versus retraction of the manipulated tissues over time. The rates of recurrence vary based on the initial technique used for repair. These recurrences have been found to be approximately 6% in patients who had subclavian flap repairs; 31% for those who had balloon angioplasty alone; and approximately 20% in patients who had aortic stenting.3-5 As seen in this case, balloon angioplasty is usually performed in patients requiring revascularization. However, up to 32% of these patients will require further intervention due to subsequent recurrence.6
Evaluation
Although emergency physicians (EPs) have numerous diagnostic modalities available to evaluate patients with suspected aortic coarctation, as long as the patient is in no acute distress, much of the work-up can be performed on an outpatient basis—in conjunction with the primary- and subspecialty-care team. Regarding appropriate imaging modalities, echocardiography with Doppler or 3D reconstruction of MR angiogram can be useful in detecting both anatomical abnormalities as well as the associated gradient dysfunction; computed tomography can be used for assessing the anatomy.7 All of these modalities can also be used to evaluate late-term complications of aortic coarctation pathology, including aortic aneurysms. To help ensure good outcome, the EP should always keep the possibility of recurrence in the differential when evaluating these patients, regardless of the number of previous interventions attempted.
Hypertension
As this case illustrates, patients with a history of coarctation repair often develop high BP. Unfortunately, up to 23% of these patients will go on to have BP above the 95th percentile.5 Moreover, a significant number of patients in this population will also suffer from exercise-induced hypertension, even when at-rest BP is controlled with antihypertensive medications.8
β-blockers, angiotensin-receptor blockers, and ACE inhibitors are considered the first-line medications for hypertension in adults and adult-sized patients with this condition.9
Since a high proportion of patients as young as age 7 years may develop high BP postrepair,10 the EP should discuss the initiation of an antihypertensive agent with the patient’s care team prior to discharge. It is also important to keep in mind that elevated BP is present to a significant degree even in patients without recurrent obstruction. The negative sequelae associated with uncontrolled hypertension is well known, and patients with congenital anatomical anomalies are at higher risk for such negative outcomes.
Conclusion
This case illustrates a common presentation of a teenaged patient with a chronic medical condition due to a corrected congenital cardiac defect. It also demonstrates the unique and early opportunity the EP has to evaluate and provide appropriate intervention for patients with potentially life-threatening diseases.
Patients with a history of corrective vascular surgery due to congenital heart malformations are an at-risk population. Therefore, during evaluation, the EP should always keep in mind that that these patients have a higher prevalence of related abnormalities at earlier ages than the general population. Steps initiated in the ED prior to discharge, in collaboration with the patient’s primary- and specialty-care team, can assist in expediting appropriate outpatient management of any sequelae. If a patient does not have a cardiologist, a referral to one should always be made prior to discharge.
Dr Smith is a postgraduate year 3 resident in the department of emergency medicine at Alpert Medical School of Brown University, Providence, Rhode Island. Dr Merritt is an assistant professor and pediatric emergency medicine attending in the department of emergency medicine, Brown Alpert Medical School, Providence, Rhode Island.
- Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
- Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg. 2015;6(2):257-265.
- Uchytil B, Ceryny J, Nicovsky J, et al. Surgery for coarctation of the aorta: long-term post-operative results. Scripta Medica. 2003;76(6):347-356.
- Jahangiri M, Shinebourne EA, Zurakowski D, Rigby ML, Redington AN, Lincoln C. Subclavian flap angioplasty: does the arch look after itself? J Thorac Cardiovasc Surg. 2000;120(2):224-229.
- Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120(6 Pt 1):1310-1304.
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010;76(4):553-563.
- Yetman AT, Nykanen D, McCrindle BW, et al. Balloon angioplasty of recurrent coarctation: a 12-year review. J Am Coll Cardiol. 1997;30(3):811-816.
- Bashore TM, Granger CB, Jackson KP, Patel MR. Heart disease. In: Current Medical Diagnosis and Treatment 2016. Papadakis MA, McPhee SJ. The McGraw-Hill Companies, Inc: New York; 2010:322,323
- Correia AS, Gonçalves A, Paiva M, et al. Long-term follow-up after aortic coarctation repair: the unsolved issue of exercise-induced hypertension. Rev Port Cardiol. 2013;32(11):879-883.
- Warnes CA, Williams RG, Bashore TM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. Circulation. 2008;111(23):e766,e767. Available at: http://circ.ahajournals.org/content/118/23/e714.full.pdf. Accessed January 12, 2016.
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88(2):163-166.
Introduction
Coarctation of the aorta comprises approximately 5% to 8% of congenital heart defects and is often associated with valvular malformations.1 These defects are typically diagnosed early and are managed with surgical repair, balloon angioplasty, or endovascular stent placement. However, as the following case illustrates, complications can occur in this population despite early intervention.
Case
A 15-year-old male adolescent presented to the pediatric ED after repeated blood pressure (BP) checks by the school nurse revealed consistently elevated systolic and diastolic pressures. The patient’s hypertension was associated with symptoms of intermittent headache and light-headedness. His medical history was remarkable for a congenital aortic coarctation and a bicuspid aortic valve. The patient had undergone a subclavian flap repair prior to 1 month of age, followed by a balloon dilatation 1 year later for recurrent coarctation. The rest of the patient’s medical history was unremarkable, including normal renal function. He denied illicit drug or alcohol use, sexual activity, or trauma.
On evaluation, the patient’s cardiac examination revealed a regular rate and rhythm with normally split S2; there were no rubs, murmurs, or gallops on auscultation. He had normal and equal pulses of the upper and lower extremities bilaterally. The patient presented without cyanosis. He was alert and oriented with normal upper and lower extremity reflexes. The neurological examination, including cranial nerve, strength, and gait testing, was unremarkable. The gastrointestinal examination showed a soft, nondistended abdomen, with no pulsatile masses. There was no abnormal swelling of his extremities. Although the physical examination findings were unremarkable, the patient’s vital signs were concerning as BP in his right upper extremity was as high as 208/110 mm Hg, while BP in his right leg was 130/68 mm Hg.
The patient followed up with his cardiologist, who ordered cardiac magnetic resonance imaging (MRI). The MRI showed mild narrowing of the distal aortic arch with a minimal and clinically insignificant pressure gradient. Based on the MRI findings, the patient was referred to a pediatric nephrologist, who performed a 24-hour ambulatory BP evaluation. The results of this study showed the patient to have systolic hypertension at the 95th percentile for his age and height. Based on the patient’s athletic predilection, β-blockers were avoided, and he was instead started on the angiotensin-converting enzyme (ACE) inhibitor lisinopril, along with annual follow-up cardiac evaluation.
Discussion
The authors’ initial concern for this patient was the possibility of a recurrent coarctation causing a significant pressure gradient between the upper and lower extremities with associated symptoms. A review of the literature demonstrates such an occurrence is not uncommon in this patient population, especially in patients with a history of early intervention (ie, within the first year of life).2
Causes and Incidence
One of the factors believed to contribute to recurrent coarctation is insufficient growth versus retraction of the manipulated tissues over time. The rates of recurrence vary based on the initial technique used for repair. These recurrences have been found to be approximately 6% in patients who had subclavian flap repairs; 31% for those who had balloon angioplasty alone; and approximately 20% in patients who had aortic stenting.3-5 As seen in this case, balloon angioplasty is usually performed in patients requiring revascularization. However, up to 32% of these patients will require further intervention due to subsequent recurrence.6
Evaluation
Although emergency physicians (EPs) have numerous diagnostic modalities available to evaluate patients with suspected aortic coarctation, as long as the patient is in no acute distress, much of the work-up can be performed on an outpatient basis—in conjunction with the primary- and subspecialty-care team. Regarding appropriate imaging modalities, echocardiography with Doppler or 3D reconstruction of MR angiogram can be useful in detecting both anatomical abnormalities as well as the associated gradient dysfunction; computed tomography can be used for assessing the anatomy.7 All of these modalities can also be used to evaluate late-term complications of aortic coarctation pathology, including aortic aneurysms. To help ensure good outcome, the EP should always keep the possibility of recurrence in the differential when evaluating these patients, regardless of the number of previous interventions attempted.
Hypertension
As this case illustrates, patients with a history of coarctation repair often develop high BP. Unfortunately, up to 23% of these patients will go on to have BP above the 95th percentile.5 Moreover, a significant number of patients in this population will also suffer from exercise-induced hypertension, even when at-rest BP is controlled with antihypertensive medications.8
β-blockers, angiotensin-receptor blockers, and ACE inhibitors are considered the first-line medications for hypertension in adults and adult-sized patients with this condition.9
Since a high proportion of patients as young as age 7 years may develop high BP postrepair,10 the EP should discuss the initiation of an antihypertensive agent with the patient’s care team prior to discharge. It is also important to keep in mind that elevated BP is present to a significant degree even in patients without recurrent obstruction. The negative sequelae associated with uncontrolled hypertension is well known, and patients with congenital anatomical anomalies are at higher risk for such negative outcomes.
Conclusion
This case illustrates a common presentation of a teenaged patient with a chronic medical condition due to a corrected congenital cardiac defect. It also demonstrates the unique and early opportunity the EP has to evaluate and provide appropriate intervention for patients with potentially life-threatening diseases.
Patients with a history of corrective vascular surgery due to congenital heart malformations are an at-risk population. Therefore, during evaluation, the EP should always keep in mind that that these patients have a higher prevalence of related abnormalities at earlier ages than the general population. Steps initiated in the ED prior to discharge, in collaboration with the patient’s primary- and specialty-care team, can assist in expediting appropriate outpatient management of any sequelae. If a patient does not have a cardiologist, a referral to one should always be made prior to discharge.
Dr Smith is a postgraduate year 3 resident in the department of emergency medicine at Alpert Medical School of Brown University, Providence, Rhode Island. Dr Merritt is an assistant professor and pediatric emergency medicine attending in the department of emergency medicine, Brown Alpert Medical School, Providence, Rhode Island.
Introduction
Coarctation of the aorta comprises approximately 5% to 8% of congenital heart defects and is often associated with valvular malformations.1 These defects are typically diagnosed early and are managed with surgical repair, balloon angioplasty, or endovascular stent placement. However, as the following case illustrates, complications can occur in this population despite early intervention.
Case
A 15-year-old male adolescent presented to the pediatric ED after repeated blood pressure (BP) checks by the school nurse revealed consistently elevated systolic and diastolic pressures. The patient’s hypertension was associated with symptoms of intermittent headache and light-headedness. His medical history was remarkable for a congenital aortic coarctation and a bicuspid aortic valve. The patient had undergone a subclavian flap repair prior to 1 month of age, followed by a balloon dilatation 1 year later for recurrent coarctation. The rest of the patient’s medical history was unremarkable, including normal renal function. He denied illicit drug or alcohol use, sexual activity, or trauma.
On evaluation, the patient’s cardiac examination revealed a regular rate and rhythm with normally split S2; there were no rubs, murmurs, or gallops on auscultation. He had normal and equal pulses of the upper and lower extremities bilaterally. The patient presented without cyanosis. He was alert and oriented with normal upper and lower extremity reflexes. The neurological examination, including cranial nerve, strength, and gait testing, was unremarkable. The gastrointestinal examination showed a soft, nondistended abdomen, with no pulsatile masses. There was no abnormal swelling of his extremities. Although the physical examination findings were unremarkable, the patient’s vital signs were concerning as BP in his right upper extremity was as high as 208/110 mm Hg, while BP in his right leg was 130/68 mm Hg.
The patient followed up with his cardiologist, who ordered cardiac magnetic resonance imaging (MRI). The MRI showed mild narrowing of the distal aortic arch with a minimal and clinically insignificant pressure gradient. Based on the MRI findings, the patient was referred to a pediatric nephrologist, who performed a 24-hour ambulatory BP evaluation. The results of this study showed the patient to have systolic hypertension at the 95th percentile for his age and height. Based on the patient’s athletic predilection, β-blockers were avoided, and he was instead started on the angiotensin-converting enzyme (ACE) inhibitor lisinopril, along with annual follow-up cardiac evaluation.
Discussion
The authors’ initial concern for this patient was the possibility of a recurrent coarctation causing a significant pressure gradient between the upper and lower extremities with associated symptoms. A review of the literature demonstrates such an occurrence is not uncommon in this patient population, especially in patients with a history of early intervention (ie, within the first year of life).2
Causes and Incidence
One of the factors believed to contribute to recurrent coarctation is insufficient growth versus retraction of the manipulated tissues over time. The rates of recurrence vary based on the initial technique used for repair. These recurrences have been found to be approximately 6% in patients who had subclavian flap repairs; 31% for those who had balloon angioplasty alone; and approximately 20% in patients who had aortic stenting.3-5 As seen in this case, balloon angioplasty is usually performed in patients requiring revascularization. However, up to 32% of these patients will require further intervention due to subsequent recurrence.6
Evaluation
Although emergency physicians (EPs) have numerous diagnostic modalities available to evaluate patients with suspected aortic coarctation, as long as the patient is in no acute distress, much of the work-up can be performed on an outpatient basis—in conjunction with the primary- and subspecialty-care team. Regarding appropriate imaging modalities, echocardiography with Doppler or 3D reconstruction of MR angiogram can be useful in detecting both anatomical abnormalities as well as the associated gradient dysfunction; computed tomography can be used for assessing the anatomy.7 All of these modalities can also be used to evaluate late-term complications of aortic coarctation pathology, including aortic aneurysms. To help ensure good outcome, the EP should always keep the possibility of recurrence in the differential when evaluating these patients, regardless of the number of previous interventions attempted.
Hypertension
As this case illustrates, patients with a history of coarctation repair often develop high BP. Unfortunately, up to 23% of these patients will go on to have BP above the 95th percentile.5 Moreover, a significant number of patients in this population will also suffer from exercise-induced hypertension, even when at-rest BP is controlled with antihypertensive medications.8
β-blockers, angiotensin-receptor blockers, and ACE inhibitors are considered the first-line medications for hypertension in adults and adult-sized patients with this condition.9
Since a high proportion of patients as young as age 7 years may develop high BP postrepair,10 the EP should discuss the initiation of an antihypertensive agent with the patient’s care team prior to discharge. It is also important to keep in mind that elevated BP is present to a significant degree even in patients without recurrent obstruction. The negative sequelae associated with uncontrolled hypertension is well known, and patients with congenital anatomical anomalies are at higher risk for such negative outcomes.
Conclusion
This case illustrates a common presentation of a teenaged patient with a chronic medical condition due to a corrected congenital cardiac defect. It also demonstrates the unique and early opportunity the EP has to evaluate and provide appropriate intervention for patients with potentially life-threatening diseases.
Patients with a history of corrective vascular surgery due to congenital heart malformations are an at-risk population. Therefore, during evaluation, the EP should always keep in mind that that these patients have a higher prevalence of related abnormalities at earlier ages than the general population. Steps initiated in the ED prior to discharge, in collaboration with the patient’s primary- and specialty-care team, can assist in expediting appropriate outpatient management of any sequelae. If a patient does not have a cardiologist, a referral to one should always be made prior to discharge.
Dr Smith is a postgraduate year 3 resident in the department of emergency medicine at Alpert Medical School of Brown University, Providence, Rhode Island. Dr Merritt is an assistant professor and pediatric emergency medicine attending in the department of emergency medicine, Brown Alpert Medical School, Providence, Rhode Island.
- Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
- Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg. 2015;6(2):257-265.
- Uchytil B, Ceryny J, Nicovsky J, et al. Surgery for coarctation of the aorta: long-term post-operative results. Scripta Medica. 2003;76(6):347-356.
- Jahangiri M, Shinebourne EA, Zurakowski D, Rigby ML, Redington AN, Lincoln C. Subclavian flap angioplasty: does the arch look after itself? J Thorac Cardiovasc Surg. 2000;120(2):224-229.
- Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120(6 Pt 1):1310-1304.
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010;76(4):553-563.
- Yetman AT, Nykanen D, McCrindle BW, et al. Balloon angioplasty of recurrent coarctation: a 12-year review. J Am Coll Cardiol. 1997;30(3):811-816.
- Bashore TM, Granger CB, Jackson KP, Patel MR. Heart disease. In: Current Medical Diagnosis and Treatment 2016. Papadakis MA, McPhee SJ. The McGraw-Hill Companies, Inc: New York; 2010:322,323
- Correia AS, Gonçalves A, Paiva M, et al. Long-term follow-up after aortic coarctation repair: the unsolved issue of exercise-induced hypertension. Rev Port Cardiol. 2013;32(11):879-883.
- Warnes CA, Williams RG, Bashore TM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. Circulation. 2008;111(23):e766,e767. Available at: http://circ.ahajournals.org/content/118/23/e714.full.pdf. Accessed January 12, 2016.
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88(2):163-166.
- Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
- Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg. 2015;6(2):257-265.
- Uchytil B, Ceryny J, Nicovsky J, et al. Surgery for coarctation of the aorta: long-term post-operative results. Scripta Medica. 2003;76(6):347-356.
- Jahangiri M, Shinebourne EA, Zurakowski D, Rigby ML, Redington AN, Lincoln C. Subclavian flap angioplasty: does the arch look after itself? J Thorac Cardiovasc Surg. 2000;120(2):224-229.
- Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120(6 Pt 1):1310-1304.
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010;76(4):553-563.
- Yetman AT, Nykanen D, McCrindle BW, et al. Balloon angioplasty of recurrent coarctation: a 12-year review. J Am Coll Cardiol. 1997;30(3):811-816.
- Bashore TM, Granger CB, Jackson KP, Patel MR. Heart disease. In: Current Medical Diagnosis and Treatment 2016. Papadakis MA, McPhee SJ. The McGraw-Hill Companies, Inc: New York; 2010:322,323
- Correia AS, Gonçalves A, Paiva M, et al. Long-term follow-up after aortic coarctation repair: the unsolved issue of exercise-induced hypertension. Rev Port Cardiol. 2013;32(11):879-883.
- Warnes CA, Williams RG, Bashore TM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. Circulation. 2008;111(23):e766,e767. Available at: http://circ.ahajournals.org/content/118/23/e714.full.pdf. Accessed January 12, 2016.
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88(2):163-166.
What Next When Metformin Isn't Enough For Type 2 Diabetes?
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5
What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1
Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1
In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral meds
For type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12
All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merck
manuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5
What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1
Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1
In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral meds
For type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12
All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5
What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1
Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1
In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral meds
For type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12
All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merck
manuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merck
manuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
Personality Disorders: A Measured Response
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
Smoking Cessation What Should You Recommend?
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
Outcomes and Aseptic Survivorship of Revision Total Knee Arthroplasty
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Implant Designs in Revision Total Knee Arthroplasty
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Nonoperative Treatment of Rotator Cuff Tears
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.