User login
What to know about COVID-19 vaccines and skin reactions
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
FROM AAD VMX 2021
Small increase seen in new COVID-19 cases among children
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
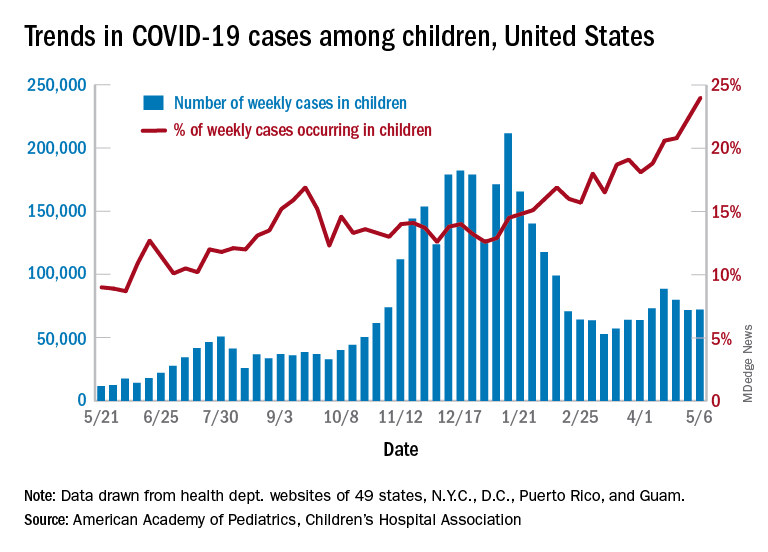
higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
Dr. Fauci: Feds may ease indoor mask mandates soon
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
NSAIDs don’t make COVID-19 worse in hospitalized patients
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
FROM THE LANCET RHEUMATOLOGY
In-hospital glucose management program gives dramatic savings
Initiatives targeting hypoglycemia and insulin pen wastage could lead to dramatic cost savings in small community hospitals, new data suggest.
The two projects are part of a dedicated inpatient glucose management service led by Mihail (“Misha”) Zilbermint, MD, one of the few full-time endocrine hospitalists in the United States and one of even fewer who work at a small community hospital.
In 2019, Dr. Zilbermint and colleagues reported that their inpatient glucose management program resulted in a 27% reduction in length of stay and a 10.7% lower 30-day readmission rate. The projected cost savings for the period January 2016 to May 2017 was $953,578.
Dr. Zilbermint’s team has written two new articles that document cost savings for specific elements of the program; namely, a set of hospital-wide hypoglycemia prevention measures, and an initiative that reduced duplicate inpatient insulin pen dispensing.
About 1 in 4 people in U.S. hospitals have diabetes or hyperglycemia. Large academic hospitals have endocrine divisions and training programs, but 85% of people receive care at small community hospitals.
“There are management guidelines, but they’re not always followed ... That’s why I’ve been advocating for endocrine hospitalists to be deployed nationally,” Dr. Zilbermint said. He is chief and director of endocrinology, diabetes, and metabolism at Johns Hopkins Community Physicians at Suburban Hospital, Bethesda, Maryland.
Asked to comment on behalf of the Society of Hospital Medicine (SHM), Greg Maynard, MD, program lead for SHM’s Electronic Quality Improvement Programs, said that Suburban’s overall program goals align with those of the SHM.
“Dedicated inpatient glycemic control teams are very important and desirable to improve the quality and safety of care for inpatients with hyperglycemia and diabetes,” he said.
Regarding specific initiatives, such as those aimed at reducing hypoglycemia and insulin pen wastage, Dr. Maynard said, “All of these are feasible in a wide variety of institutions. The main barrier is getting the institutional support for people to work on these interventions. This series of studies can help spread the word about the positive return on investment.”
Another barrier – the current lack of publicly reported measures or pay-for-performance programs for hypoglycemia prevention and glycemic control – may soon change, added Dr. Maynard, who is also chief quality officer at the University of California, Davis, Medical Center.
“The National Quality Forum has endorsed new measures, and the CDC’s National Healthcare Safety Network is working on ways to augment those measures and embed them into their infrastructure,” he said.
Although SHM doesn’t specifically endorse full-time glycemic control hospitalists over endocrinology-trained glycemic control experts, “certainly hospitalists who accrue added training are very well positioned to be an important part of these interdisciplinary teams,” Dr. Maynard said.
‘The nurses were so afraid of hypoglycemia’
Tackling hypoglycemia was Dr. Zilbermint’s first priority when he started the glycemic management program at Suburban in late 2015.
“One of the most common complaints from the nurses was that a lot of their patients had hypoglycemia, especially in the ICU, when patients were placed on insulin infusion protocols ... Every time, the nurse would have to call the attending and ask what to do,” he explains.
In addition, Dr. Zilbermint says, there was no standard for treating hypoglycemia. A nurse in one unit would give two cups of juice, another a 50% dextrose infusion, or another, milk. Even more concerning, “the nurses were so afraid of hypoglycemia they would reflexively discontinue all insulin, including basal.”
So one of the new initiatives, led by Carter Shelton, MSHCM, an administrative fellow at the Medical University of South Carolina, Charleston, was to implement a set of hospital-wide hypoglycemia prevention measures, as described in an article published online April 21 in the Journal of Diabetes Science and Technology.
Inpatient hypoglycemia rate was cut nearly in half
This began in 2016, when the multidisciplinary Suburban Hospital Glucose Steering Committee identified four main causes of insulin-induced hypoglycemia (defined as a blood glucose level of ≤70 mg/dL in a patient who had received at least one dose of insulin in the past 24 hours) and devised solutions for each:
1. Lack of a unified hypoglycemia protocol. A formal, evidence-based, nurse-driven treatment protocol with clinical decision support in the electronic medical record was developed. The Suburban team adapted much of the protocol from one that had been recently implemented at the flagship Johns Hopkins Hospital, in Baltimore, Maryland.
According to that protocol, if patients are able to swallow, they are given 15 g or 30 g of carbohydrates in order to achieve a blood glucose level of 50 to 70 mg/dL and <50 mg/dL, respectively. Levels are checked 15 minutes later. Intravenous D50 or glucagon is reserved for patients who can’t swallow.
2. For patients in critical care, the insulin infusion protocol that had been in use set blood glucose targets of 80 to 110 mg/dL, which resulted in hypoglycemia in nearly every patient who received an insulin infusion. This protocol was changed to the currently recommended 140 to 180 mg/dL.
3. Most patients were managed with sliding-scale insulin, an outdated yet still widely used regimen whereby insulin is given based only on current blood glucose without accounting for carbohydrates consumed with meals and not corrected until the subsequent meal. This was changed so that nurses give insulin after the patient has consumed at least 50% of their meal carbohydrates.
4. Lack of hypoglycemia reporting. A glucometrics dashboard – now used throughout the Johns Hopkins system – was adopted to produce daily hypoglycemia reports in the EMR system that could be reviewed by the inpatient glucose management service to track quality metrics and plan further interventions.
Between Jan. 1, 2016, and Sept. 30, 2019, out of a total 49,315 patient-days, there were 2,682 days on which any hypoglycemia occurred and 874 days on which moderate hypoglycemia occurred (≤54 mg/dL). Type 2 diabetes accounted for 84.4% of the total patient-days; type 1 accounted for 4.4%.
The overall frequency of any hypoglycemia patient-days per month decreased from 7.5% to 3.9% during the study period (P = .001). This was significant for the patients with type 2 diabetes (7.4% to 3.8%; P < .0001) but not for those with type 1 diabetes (18.5% to 18.0%; P = .08).
Rates of moderate hypoglycemia also decreased significantly among the patients with type 2 diabetes (1.9% to 1.0%; P = .03) but not for those with type 1 diabetes (7.4% to 6.0%; P = .14).
On the basis of these rates in reducing hypoglycemia, in which the inpatient hypoglycemia rate was cut nearly in half, the estimated savings in cost of care to the hospital was $98,635 during the period of January 2016 to September 2019.
Reducing insulin pen waste by minimizing duplicate prescriptions
Suburban Hospital had been using insulin vials and syringes when Dr. Zilbermint first arrived there. He lobbied the administration to allow use of pens, because they’re easier to use and they reduce the risk for needlestick injuries. Nurses were educated and retrained monthly in their use.
The switch to pens – aspart (Novolog Flexpen) for bolus insulin and glargine (Lantus SoloSTAR) – took place in 2018. The cost of the aspart pen was $16.19, and the cost of glargine was $25.08. Each holds 300 units of insulin.
After the first month, the team noticed a large increase in expenses. A quality improvement project was devised to address the issue.
“We were dispensing sometimes three or four pens per person. That’s a lot. Each pen holds 300 units, so one pen should last the entire hospital stay of an average 4- or 5-day stay,” Dr. Zilbermint explained. “We had to figure out where we were bleeding the money and where the pens were going.”
When pens disappeared, the pharmacy would have to dispense new ones. One problem was that when patients were transferred from one unit to another, the pen would be left behind and the room would be cleaned. Sometimes the pens weren’t stored properly or were misplaced. Often, they’d end up in a nurse’s pocket.
The second intervention was led by Urooj Najmi, MD, of the American International School of Medicine, Atlanta, Georgia. A program was instituted to reduce duplicate inpatient insulin pen dispensing, as detailed in an article published in the same issue of the Journal of Diabetes Science and Technology.
Solutions to reduce duplicate pen dispensing included having pharmacy track daily insulin pen reports and monitor duplicate orders, with “do not dispense” instructions conveyed via the EMR system. All multidose medications, including insulin pens, were to be placed in patients’ bins at the nursing station, and nurses were instructed to look for patients’ insulin pens prior to their being transferred to another unit, rather than ask for a replacement pen.
From July 2018 to July 2019, 3,121 patients received insulin, of whom 95% received aspart and 47% received glargine. Of the 9,516 pens dispensed, 68% were for aspart and 32% were for glargine. During the study period, the number of pens dispensed per patient dropped from 2.2 to 1.2 for aspart and from 2.1 to 1.3 for glargine; differences were highly significant (P = .0002 and P = .0005, respectively).
The total amount of unnecessary dispensing during the first 4 months after initiating the pen implementation program was 58%. The average monthly cost was $11,820.68; the projected cost per year was $141,848.
Six months after the waste reduction strategies were implemented, monthly waste had dropped to 42%, translating to an estimated potential cost savings of $66,261 over 12 months.
Because Suburban Hospital doesn’t have an outpatient dispensing license, there is still wastage when patients are discharged, because they can’t take their pens home with them. That remains a challenge, Dr. Zilbermint noted.
The team is working on implementing automatic A1c testing for patients admitted with hyperglycemia who either have a history of diabetes or whose blood glucose level is >140 mg/dL. Dr. Zilbermint said, “it’s in the guidelines, but it’s not always done.”
Dr. Zilbermint is a consultant for Guidepoint. Dr. Maynard, Mr. Shelton, and Dr. Najmi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initiatives targeting hypoglycemia and insulin pen wastage could lead to dramatic cost savings in small community hospitals, new data suggest.
The two projects are part of a dedicated inpatient glucose management service led by Mihail (“Misha”) Zilbermint, MD, one of the few full-time endocrine hospitalists in the United States and one of even fewer who work at a small community hospital.
In 2019, Dr. Zilbermint and colleagues reported that their inpatient glucose management program resulted in a 27% reduction in length of stay and a 10.7% lower 30-day readmission rate. The projected cost savings for the period January 2016 to May 2017 was $953,578.
Dr. Zilbermint’s team has written two new articles that document cost savings for specific elements of the program; namely, a set of hospital-wide hypoglycemia prevention measures, and an initiative that reduced duplicate inpatient insulin pen dispensing.
About 1 in 4 people in U.S. hospitals have diabetes or hyperglycemia. Large academic hospitals have endocrine divisions and training programs, but 85% of people receive care at small community hospitals.
“There are management guidelines, but they’re not always followed ... That’s why I’ve been advocating for endocrine hospitalists to be deployed nationally,” Dr. Zilbermint said. He is chief and director of endocrinology, diabetes, and metabolism at Johns Hopkins Community Physicians at Suburban Hospital, Bethesda, Maryland.
Asked to comment on behalf of the Society of Hospital Medicine (SHM), Greg Maynard, MD, program lead for SHM’s Electronic Quality Improvement Programs, said that Suburban’s overall program goals align with those of the SHM.
“Dedicated inpatient glycemic control teams are very important and desirable to improve the quality and safety of care for inpatients with hyperglycemia and diabetes,” he said.
Regarding specific initiatives, such as those aimed at reducing hypoglycemia and insulin pen wastage, Dr. Maynard said, “All of these are feasible in a wide variety of institutions. The main barrier is getting the institutional support for people to work on these interventions. This series of studies can help spread the word about the positive return on investment.”
Another barrier – the current lack of publicly reported measures or pay-for-performance programs for hypoglycemia prevention and glycemic control – may soon change, added Dr. Maynard, who is also chief quality officer at the University of California, Davis, Medical Center.
“The National Quality Forum has endorsed new measures, and the CDC’s National Healthcare Safety Network is working on ways to augment those measures and embed them into their infrastructure,” he said.
Although SHM doesn’t specifically endorse full-time glycemic control hospitalists over endocrinology-trained glycemic control experts, “certainly hospitalists who accrue added training are very well positioned to be an important part of these interdisciplinary teams,” Dr. Maynard said.
‘The nurses were so afraid of hypoglycemia’
Tackling hypoglycemia was Dr. Zilbermint’s first priority when he started the glycemic management program at Suburban in late 2015.
“One of the most common complaints from the nurses was that a lot of their patients had hypoglycemia, especially in the ICU, when patients were placed on insulin infusion protocols ... Every time, the nurse would have to call the attending and ask what to do,” he explains.
In addition, Dr. Zilbermint says, there was no standard for treating hypoglycemia. A nurse in one unit would give two cups of juice, another a 50% dextrose infusion, or another, milk. Even more concerning, “the nurses were so afraid of hypoglycemia they would reflexively discontinue all insulin, including basal.”
So one of the new initiatives, led by Carter Shelton, MSHCM, an administrative fellow at the Medical University of South Carolina, Charleston, was to implement a set of hospital-wide hypoglycemia prevention measures, as described in an article published online April 21 in the Journal of Diabetes Science and Technology.
Inpatient hypoglycemia rate was cut nearly in half
This began in 2016, when the multidisciplinary Suburban Hospital Glucose Steering Committee identified four main causes of insulin-induced hypoglycemia (defined as a blood glucose level of ≤70 mg/dL in a patient who had received at least one dose of insulin in the past 24 hours) and devised solutions for each:
1. Lack of a unified hypoglycemia protocol. A formal, evidence-based, nurse-driven treatment protocol with clinical decision support in the electronic medical record was developed. The Suburban team adapted much of the protocol from one that had been recently implemented at the flagship Johns Hopkins Hospital, in Baltimore, Maryland.
According to that protocol, if patients are able to swallow, they are given 15 g or 30 g of carbohydrates in order to achieve a blood glucose level of 50 to 70 mg/dL and <50 mg/dL, respectively. Levels are checked 15 minutes later. Intravenous D50 or glucagon is reserved for patients who can’t swallow.
2. For patients in critical care, the insulin infusion protocol that had been in use set blood glucose targets of 80 to 110 mg/dL, which resulted in hypoglycemia in nearly every patient who received an insulin infusion. This protocol was changed to the currently recommended 140 to 180 mg/dL.
3. Most patients were managed with sliding-scale insulin, an outdated yet still widely used regimen whereby insulin is given based only on current blood glucose without accounting for carbohydrates consumed with meals and not corrected until the subsequent meal. This was changed so that nurses give insulin after the patient has consumed at least 50% of their meal carbohydrates.
4. Lack of hypoglycemia reporting. A glucometrics dashboard – now used throughout the Johns Hopkins system – was adopted to produce daily hypoglycemia reports in the EMR system that could be reviewed by the inpatient glucose management service to track quality metrics and plan further interventions.
Between Jan. 1, 2016, and Sept. 30, 2019, out of a total 49,315 patient-days, there were 2,682 days on which any hypoglycemia occurred and 874 days on which moderate hypoglycemia occurred (≤54 mg/dL). Type 2 diabetes accounted for 84.4% of the total patient-days; type 1 accounted for 4.4%.
The overall frequency of any hypoglycemia patient-days per month decreased from 7.5% to 3.9% during the study period (P = .001). This was significant for the patients with type 2 diabetes (7.4% to 3.8%; P < .0001) but not for those with type 1 diabetes (18.5% to 18.0%; P = .08).
Rates of moderate hypoglycemia also decreased significantly among the patients with type 2 diabetes (1.9% to 1.0%; P = .03) but not for those with type 1 diabetes (7.4% to 6.0%; P = .14).
On the basis of these rates in reducing hypoglycemia, in which the inpatient hypoglycemia rate was cut nearly in half, the estimated savings in cost of care to the hospital was $98,635 during the period of January 2016 to September 2019.
Reducing insulin pen waste by minimizing duplicate prescriptions
Suburban Hospital had been using insulin vials and syringes when Dr. Zilbermint first arrived there. He lobbied the administration to allow use of pens, because they’re easier to use and they reduce the risk for needlestick injuries. Nurses were educated and retrained monthly in their use.
The switch to pens – aspart (Novolog Flexpen) for bolus insulin and glargine (Lantus SoloSTAR) – took place in 2018. The cost of the aspart pen was $16.19, and the cost of glargine was $25.08. Each holds 300 units of insulin.
After the first month, the team noticed a large increase in expenses. A quality improvement project was devised to address the issue.
“We were dispensing sometimes three or four pens per person. That’s a lot. Each pen holds 300 units, so one pen should last the entire hospital stay of an average 4- or 5-day stay,” Dr. Zilbermint explained. “We had to figure out where we were bleeding the money and where the pens were going.”
When pens disappeared, the pharmacy would have to dispense new ones. One problem was that when patients were transferred from one unit to another, the pen would be left behind and the room would be cleaned. Sometimes the pens weren’t stored properly or were misplaced. Often, they’d end up in a nurse’s pocket.
The second intervention was led by Urooj Najmi, MD, of the American International School of Medicine, Atlanta, Georgia. A program was instituted to reduce duplicate inpatient insulin pen dispensing, as detailed in an article published in the same issue of the Journal of Diabetes Science and Technology.
Solutions to reduce duplicate pen dispensing included having pharmacy track daily insulin pen reports and monitor duplicate orders, with “do not dispense” instructions conveyed via the EMR system. All multidose medications, including insulin pens, were to be placed in patients’ bins at the nursing station, and nurses were instructed to look for patients’ insulin pens prior to their being transferred to another unit, rather than ask for a replacement pen.
From July 2018 to July 2019, 3,121 patients received insulin, of whom 95% received aspart and 47% received glargine. Of the 9,516 pens dispensed, 68% were for aspart and 32% were for glargine. During the study period, the number of pens dispensed per patient dropped from 2.2 to 1.2 for aspart and from 2.1 to 1.3 for glargine; differences were highly significant (P = .0002 and P = .0005, respectively).
The total amount of unnecessary dispensing during the first 4 months after initiating the pen implementation program was 58%. The average monthly cost was $11,820.68; the projected cost per year was $141,848.
Six months after the waste reduction strategies were implemented, monthly waste had dropped to 42%, translating to an estimated potential cost savings of $66,261 over 12 months.
Because Suburban Hospital doesn’t have an outpatient dispensing license, there is still wastage when patients are discharged, because they can’t take their pens home with them. That remains a challenge, Dr. Zilbermint noted.
The team is working on implementing automatic A1c testing for patients admitted with hyperglycemia who either have a history of diabetes or whose blood glucose level is >140 mg/dL. Dr. Zilbermint said, “it’s in the guidelines, but it’s not always done.”
Dr. Zilbermint is a consultant for Guidepoint. Dr. Maynard, Mr. Shelton, and Dr. Najmi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initiatives targeting hypoglycemia and insulin pen wastage could lead to dramatic cost savings in small community hospitals, new data suggest.
The two projects are part of a dedicated inpatient glucose management service led by Mihail (“Misha”) Zilbermint, MD, one of the few full-time endocrine hospitalists in the United States and one of even fewer who work at a small community hospital.
In 2019, Dr. Zilbermint and colleagues reported that their inpatient glucose management program resulted in a 27% reduction in length of stay and a 10.7% lower 30-day readmission rate. The projected cost savings for the period January 2016 to May 2017 was $953,578.
Dr. Zilbermint’s team has written two new articles that document cost savings for specific elements of the program; namely, a set of hospital-wide hypoglycemia prevention measures, and an initiative that reduced duplicate inpatient insulin pen dispensing.
About 1 in 4 people in U.S. hospitals have diabetes or hyperglycemia. Large academic hospitals have endocrine divisions and training programs, but 85% of people receive care at small community hospitals.
“There are management guidelines, but they’re not always followed ... That’s why I’ve been advocating for endocrine hospitalists to be deployed nationally,” Dr. Zilbermint said. He is chief and director of endocrinology, diabetes, and metabolism at Johns Hopkins Community Physicians at Suburban Hospital, Bethesda, Maryland.
Asked to comment on behalf of the Society of Hospital Medicine (SHM), Greg Maynard, MD, program lead for SHM’s Electronic Quality Improvement Programs, said that Suburban’s overall program goals align with those of the SHM.
“Dedicated inpatient glycemic control teams are very important and desirable to improve the quality and safety of care for inpatients with hyperglycemia and diabetes,” he said.
Regarding specific initiatives, such as those aimed at reducing hypoglycemia and insulin pen wastage, Dr. Maynard said, “All of these are feasible in a wide variety of institutions. The main barrier is getting the institutional support for people to work on these interventions. This series of studies can help spread the word about the positive return on investment.”
Another barrier – the current lack of publicly reported measures or pay-for-performance programs for hypoglycemia prevention and glycemic control – may soon change, added Dr. Maynard, who is also chief quality officer at the University of California, Davis, Medical Center.
“The National Quality Forum has endorsed new measures, and the CDC’s National Healthcare Safety Network is working on ways to augment those measures and embed them into their infrastructure,” he said.
Although SHM doesn’t specifically endorse full-time glycemic control hospitalists over endocrinology-trained glycemic control experts, “certainly hospitalists who accrue added training are very well positioned to be an important part of these interdisciplinary teams,” Dr. Maynard said.
‘The nurses were so afraid of hypoglycemia’
Tackling hypoglycemia was Dr. Zilbermint’s first priority when he started the glycemic management program at Suburban in late 2015.
“One of the most common complaints from the nurses was that a lot of their patients had hypoglycemia, especially in the ICU, when patients were placed on insulin infusion protocols ... Every time, the nurse would have to call the attending and ask what to do,” he explains.
In addition, Dr. Zilbermint says, there was no standard for treating hypoglycemia. A nurse in one unit would give two cups of juice, another a 50% dextrose infusion, or another, milk. Even more concerning, “the nurses were so afraid of hypoglycemia they would reflexively discontinue all insulin, including basal.”
So one of the new initiatives, led by Carter Shelton, MSHCM, an administrative fellow at the Medical University of South Carolina, Charleston, was to implement a set of hospital-wide hypoglycemia prevention measures, as described in an article published online April 21 in the Journal of Diabetes Science and Technology.
Inpatient hypoglycemia rate was cut nearly in half
This began in 2016, when the multidisciplinary Suburban Hospital Glucose Steering Committee identified four main causes of insulin-induced hypoglycemia (defined as a blood glucose level of ≤70 mg/dL in a patient who had received at least one dose of insulin in the past 24 hours) and devised solutions for each:
1. Lack of a unified hypoglycemia protocol. A formal, evidence-based, nurse-driven treatment protocol with clinical decision support in the electronic medical record was developed. The Suburban team adapted much of the protocol from one that had been recently implemented at the flagship Johns Hopkins Hospital, in Baltimore, Maryland.
According to that protocol, if patients are able to swallow, they are given 15 g or 30 g of carbohydrates in order to achieve a blood glucose level of 50 to 70 mg/dL and <50 mg/dL, respectively. Levels are checked 15 minutes later. Intravenous D50 or glucagon is reserved for patients who can’t swallow.
2. For patients in critical care, the insulin infusion protocol that had been in use set blood glucose targets of 80 to 110 mg/dL, which resulted in hypoglycemia in nearly every patient who received an insulin infusion. This protocol was changed to the currently recommended 140 to 180 mg/dL.
3. Most patients were managed with sliding-scale insulin, an outdated yet still widely used regimen whereby insulin is given based only on current blood glucose without accounting for carbohydrates consumed with meals and not corrected until the subsequent meal. This was changed so that nurses give insulin after the patient has consumed at least 50% of their meal carbohydrates.
4. Lack of hypoglycemia reporting. A glucometrics dashboard – now used throughout the Johns Hopkins system – was adopted to produce daily hypoglycemia reports in the EMR system that could be reviewed by the inpatient glucose management service to track quality metrics and plan further interventions.
Between Jan. 1, 2016, and Sept. 30, 2019, out of a total 49,315 patient-days, there were 2,682 days on which any hypoglycemia occurred and 874 days on which moderate hypoglycemia occurred (≤54 mg/dL). Type 2 diabetes accounted for 84.4% of the total patient-days; type 1 accounted for 4.4%.
The overall frequency of any hypoglycemia patient-days per month decreased from 7.5% to 3.9% during the study period (P = .001). This was significant for the patients with type 2 diabetes (7.4% to 3.8%; P < .0001) but not for those with type 1 diabetes (18.5% to 18.0%; P = .08).
Rates of moderate hypoglycemia also decreased significantly among the patients with type 2 diabetes (1.9% to 1.0%; P = .03) but not for those with type 1 diabetes (7.4% to 6.0%; P = .14).
On the basis of these rates in reducing hypoglycemia, in which the inpatient hypoglycemia rate was cut nearly in half, the estimated savings in cost of care to the hospital was $98,635 during the period of January 2016 to September 2019.
Reducing insulin pen waste by minimizing duplicate prescriptions
Suburban Hospital had been using insulin vials and syringes when Dr. Zilbermint first arrived there. He lobbied the administration to allow use of pens, because they’re easier to use and they reduce the risk for needlestick injuries. Nurses were educated and retrained monthly in their use.
The switch to pens – aspart (Novolog Flexpen) for bolus insulin and glargine (Lantus SoloSTAR) – took place in 2018. The cost of the aspart pen was $16.19, and the cost of glargine was $25.08. Each holds 300 units of insulin.
After the first month, the team noticed a large increase in expenses. A quality improvement project was devised to address the issue.
“We were dispensing sometimes three or four pens per person. That’s a lot. Each pen holds 300 units, so one pen should last the entire hospital stay of an average 4- or 5-day stay,” Dr. Zilbermint explained. “We had to figure out where we were bleeding the money and where the pens were going.”
When pens disappeared, the pharmacy would have to dispense new ones. One problem was that when patients were transferred from one unit to another, the pen would be left behind and the room would be cleaned. Sometimes the pens weren’t stored properly or were misplaced. Often, they’d end up in a nurse’s pocket.
The second intervention was led by Urooj Najmi, MD, of the American International School of Medicine, Atlanta, Georgia. A program was instituted to reduce duplicate inpatient insulin pen dispensing, as detailed in an article published in the same issue of the Journal of Diabetes Science and Technology.
Solutions to reduce duplicate pen dispensing included having pharmacy track daily insulin pen reports and monitor duplicate orders, with “do not dispense” instructions conveyed via the EMR system. All multidose medications, including insulin pens, were to be placed in patients’ bins at the nursing station, and nurses were instructed to look for patients’ insulin pens prior to their being transferred to another unit, rather than ask for a replacement pen.
From July 2018 to July 2019, 3,121 patients received insulin, of whom 95% received aspart and 47% received glargine. Of the 9,516 pens dispensed, 68% were for aspart and 32% were for glargine. During the study period, the number of pens dispensed per patient dropped from 2.2 to 1.2 for aspart and from 2.1 to 1.3 for glargine; differences were highly significant (P = .0002 and P = .0005, respectively).
The total amount of unnecessary dispensing during the first 4 months after initiating the pen implementation program was 58%. The average monthly cost was $11,820.68; the projected cost per year was $141,848.
Six months after the waste reduction strategies were implemented, monthly waste had dropped to 42%, translating to an estimated potential cost savings of $66,261 over 12 months.
Because Suburban Hospital doesn’t have an outpatient dispensing license, there is still wastage when patients are discharged, because they can’t take their pens home with them. That remains a challenge, Dr. Zilbermint noted.
The team is working on implementing automatic A1c testing for patients admitted with hyperglycemia who either have a history of diabetes or whose blood glucose level is >140 mg/dL. Dr. Zilbermint said, “it’s in the guidelines, but it’s not always done.”
Dr. Zilbermint is a consultant for Guidepoint. Dr. Maynard, Mr. Shelton, and Dr. Najmi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dr. Topol talks: COVID-19 variants are innocent until proven guilty
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Infective endocarditis with stroke after TAVR has ‘dismal’ prognosis
Patients who suffer a stroke during hospitalization for infective endocarditis (IE) after transcatheter aortic valve replacement (TAVR) have a dismal prognosis, with more than half dying during the index hospitalization and two-thirds within the first year, a new study shows.
The study – the first to evaluate stroke as an IE-related complication following TAVR in a large multicenter cohort – is published in the May 11 issue of the Journal of the American College of Cardiology.
The authors, led by David del Val, MD, Quebec Heart & Lung Institute, Quebec City, explain that IE after TAVR is a rare but serious complication associated with a high mortality rate. Neurologic events, especially stroke, remain one of the most common and potentially disabling IE-related complications, but until now, no study has attempted to evaluate the predictors of stroke and outcomes in patients with IE following TAVR.
For the current study, the authors analyzed data from the Infectious Endocarditis after TAVR International Registry, including 569 patients who developed definite IE following TAVR from 59 centers in 11 countries.
Patients who experienced a stroke during IE admission were compared with patients who did not have a stroke.
Results showed that 57 patients (10%) had a stroke during IE hospitalization, with no differences in the causative microorganism between groups. Stroke patients had higher rates of acute renal failure, systemic embolization, and persistent bacteremia.
Factors associated with a higher risk for stroke during the index IE hospitalization included stroke before IE, moderate or higher residual aortic regurgitation after TAVR, balloon-expandable valves, IE within 30 days after TAVR, and vegetation size greater than 8 mm.
The stroke rate was 3.1% in patients with none of these risk factors; 6.1% with one risk factor; 13.1% with two risk factors; 28.9% with three risk factors, and 60% with four risk factors.
“The presence of such factors (particularly in combination) may be considered for determining an earlier and more aggressive (medical or surgical) treatment in these patients,” the researchers say.
IE patients with stroke had higher rates of in-hospital mortality (54.4% vs. 28.7%) and overall mortality at 1 year (66.3% vs. 45.6%).
Surgery rates were low (25%) even in the presence of stroke and failed to improve outcomes in this population.
Noting that consensus guidelines for managing patients with IE recommend surgery along with antibiotic treatment for patients developing systemic embolism, particularly stroke, the researchers say their findings suggest that such surgery recommendations may not be extrapolated to TAVR-IE patients, and specific guidelines are warranted for this particular population.
Furthermore, the possibility of early surgery in those patients with factors increasing the risk for stroke should be evaluated in future studies.
The authors note that TAVR has revolutionized the treatment of aortic stenosis and is currently moving toward less complex and younger patients with lower surgical risk. Despite the relatively low incidence of IE after TAVR, the number of procedures is expected to grow exponentially, increasing the number of patients at risk of developing this life-threatening complication. Therefore, detailed knowledge of this disease and its complications is essential to improve outcomes.
They point out that the 10% rate of stroke found in this study is substantially lower, compared with the largest surgical prosthetic-valve infective endocarditis registries, but they suggest that the unique clinical profile of TAVR patients may lead to an underdiagnosis of stroke, with a high proportion of elderly patients who more frequently present with nonspecific symptoms.
They conclude that “IE post-TAVR is associated with a poor prognosis with high in-hospital and late mortality rates. Our study reveals that patients with IE after TAVR complicated by stroke showed an even worse prognosis.”
“The progressive implementation of advanced imaging modalities for early IE diagnosis, especially nuclear imaging, may translate into a better prognosis in coming years. Close attention should be paid to early recognition of stroke-associated factors to improve clinical outcomes,” they add.
In an accompanying editorial, Vuyisile Nkomo, MD, Daniel DeSimone, MD, and William Miranda, MD, Mayo Clinic, Rochester, Minn., say the current study “highlights the devastating consequences of IE after TAVR and the even worse consequences when IE was associated with stroke.”
This points to the critical importance of efforts to prevent IE with appropriate antibiotic prophylaxis and addressing potential sources of infection (for example, dental screening) before invasive cardiac procedures.
“Patient education is critical in regard to recognizing early signs and symptoms of IE. In particular, patients must be informed to obtain blood cultures with any episode of fever, as identification of bacteremia is critical in the diagnosis of IE,” the editorialists comment.
Endocarditis should also be suspected in afebrile patients with increasing transcatheter heart valve gradients or new or worsening regurgitation, they state.
Multimodality imaging is important for the early diagnosis of IE to facilitate prompt antibiotic treatment and potentially decrease the risk for IE complications, especially systemic embolization, they add.
“Despite the unequivocal advances in the safety and periprocedural complications of TAVR, IE with and without stroke in this TAVR population remains a dreadful complication,” they conclude.
Dr. Del Val was supported by a research grant from the Fundación Alfonso Martin Escudero. The editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who suffer a stroke during hospitalization for infective endocarditis (IE) after transcatheter aortic valve replacement (TAVR) have a dismal prognosis, with more than half dying during the index hospitalization and two-thirds within the first year, a new study shows.
The study – the first to evaluate stroke as an IE-related complication following TAVR in a large multicenter cohort – is published in the May 11 issue of the Journal of the American College of Cardiology.
The authors, led by David del Val, MD, Quebec Heart & Lung Institute, Quebec City, explain that IE after TAVR is a rare but serious complication associated with a high mortality rate. Neurologic events, especially stroke, remain one of the most common and potentially disabling IE-related complications, but until now, no study has attempted to evaluate the predictors of stroke and outcomes in patients with IE following TAVR.
For the current study, the authors analyzed data from the Infectious Endocarditis after TAVR International Registry, including 569 patients who developed definite IE following TAVR from 59 centers in 11 countries.
Patients who experienced a stroke during IE admission were compared with patients who did not have a stroke.
Results showed that 57 patients (10%) had a stroke during IE hospitalization, with no differences in the causative microorganism between groups. Stroke patients had higher rates of acute renal failure, systemic embolization, and persistent bacteremia.
Factors associated with a higher risk for stroke during the index IE hospitalization included stroke before IE, moderate or higher residual aortic regurgitation after TAVR, balloon-expandable valves, IE within 30 days after TAVR, and vegetation size greater than 8 mm.
The stroke rate was 3.1% in patients with none of these risk factors; 6.1% with one risk factor; 13.1% with two risk factors; 28.9% with three risk factors, and 60% with four risk factors.
“The presence of such factors (particularly in combination) may be considered for determining an earlier and more aggressive (medical or surgical) treatment in these patients,” the researchers say.
IE patients with stroke had higher rates of in-hospital mortality (54.4% vs. 28.7%) and overall mortality at 1 year (66.3% vs. 45.6%).
Surgery rates were low (25%) even in the presence of stroke and failed to improve outcomes in this population.
Noting that consensus guidelines for managing patients with IE recommend surgery along with antibiotic treatment for patients developing systemic embolism, particularly stroke, the researchers say their findings suggest that such surgery recommendations may not be extrapolated to TAVR-IE patients, and specific guidelines are warranted for this particular population.
Furthermore, the possibility of early surgery in those patients with factors increasing the risk for stroke should be evaluated in future studies.
The authors note that TAVR has revolutionized the treatment of aortic stenosis and is currently moving toward less complex and younger patients with lower surgical risk. Despite the relatively low incidence of IE after TAVR, the number of procedures is expected to grow exponentially, increasing the number of patients at risk of developing this life-threatening complication. Therefore, detailed knowledge of this disease and its complications is essential to improve outcomes.
They point out that the 10% rate of stroke found in this study is substantially lower, compared with the largest surgical prosthetic-valve infective endocarditis registries, but they suggest that the unique clinical profile of TAVR patients may lead to an underdiagnosis of stroke, with a high proportion of elderly patients who more frequently present with nonspecific symptoms.
They conclude that “IE post-TAVR is associated with a poor prognosis with high in-hospital and late mortality rates. Our study reveals that patients with IE after TAVR complicated by stroke showed an even worse prognosis.”
“The progressive implementation of advanced imaging modalities for early IE diagnosis, especially nuclear imaging, may translate into a better prognosis in coming years. Close attention should be paid to early recognition of stroke-associated factors to improve clinical outcomes,” they add.
In an accompanying editorial, Vuyisile Nkomo, MD, Daniel DeSimone, MD, and William Miranda, MD, Mayo Clinic, Rochester, Minn., say the current study “highlights the devastating consequences of IE after TAVR and the even worse consequences when IE was associated with stroke.”
This points to the critical importance of efforts to prevent IE with appropriate antibiotic prophylaxis and addressing potential sources of infection (for example, dental screening) before invasive cardiac procedures.
“Patient education is critical in regard to recognizing early signs and symptoms of IE. In particular, patients must be informed to obtain blood cultures with any episode of fever, as identification of bacteremia is critical in the diagnosis of IE,” the editorialists comment.
Endocarditis should also be suspected in afebrile patients with increasing transcatheter heart valve gradients or new or worsening regurgitation, they state.
Multimodality imaging is important for the early diagnosis of IE to facilitate prompt antibiotic treatment and potentially decrease the risk for IE complications, especially systemic embolization, they add.
“Despite the unequivocal advances in the safety and periprocedural complications of TAVR, IE with and without stroke in this TAVR population remains a dreadful complication,” they conclude.
Dr. Del Val was supported by a research grant from the Fundación Alfonso Martin Escudero. The editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who suffer a stroke during hospitalization for infective endocarditis (IE) after transcatheter aortic valve replacement (TAVR) have a dismal prognosis, with more than half dying during the index hospitalization and two-thirds within the first year, a new study shows.
The study – the first to evaluate stroke as an IE-related complication following TAVR in a large multicenter cohort – is published in the May 11 issue of the Journal of the American College of Cardiology.
The authors, led by David del Val, MD, Quebec Heart & Lung Institute, Quebec City, explain that IE after TAVR is a rare but serious complication associated with a high mortality rate. Neurologic events, especially stroke, remain one of the most common and potentially disabling IE-related complications, but until now, no study has attempted to evaluate the predictors of stroke and outcomes in patients with IE following TAVR.
For the current study, the authors analyzed data from the Infectious Endocarditis after TAVR International Registry, including 569 patients who developed definite IE following TAVR from 59 centers in 11 countries.
Patients who experienced a stroke during IE admission were compared with patients who did not have a stroke.
Results showed that 57 patients (10%) had a stroke during IE hospitalization, with no differences in the causative microorganism between groups. Stroke patients had higher rates of acute renal failure, systemic embolization, and persistent bacteremia.
Factors associated with a higher risk for stroke during the index IE hospitalization included stroke before IE, moderate or higher residual aortic regurgitation after TAVR, balloon-expandable valves, IE within 30 days after TAVR, and vegetation size greater than 8 mm.
The stroke rate was 3.1% in patients with none of these risk factors; 6.1% with one risk factor; 13.1% with two risk factors; 28.9% with three risk factors, and 60% with four risk factors.
“The presence of such factors (particularly in combination) may be considered for determining an earlier and more aggressive (medical or surgical) treatment in these patients,” the researchers say.
IE patients with stroke had higher rates of in-hospital mortality (54.4% vs. 28.7%) and overall mortality at 1 year (66.3% vs. 45.6%).
Surgery rates were low (25%) even in the presence of stroke and failed to improve outcomes in this population.
Noting that consensus guidelines for managing patients with IE recommend surgery along with antibiotic treatment for patients developing systemic embolism, particularly stroke, the researchers say their findings suggest that such surgery recommendations may not be extrapolated to TAVR-IE patients, and specific guidelines are warranted for this particular population.
Furthermore, the possibility of early surgery in those patients with factors increasing the risk for stroke should be evaluated in future studies.
The authors note that TAVR has revolutionized the treatment of aortic stenosis and is currently moving toward less complex and younger patients with lower surgical risk. Despite the relatively low incidence of IE after TAVR, the number of procedures is expected to grow exponentially, increasing the number of patients at risk of developing this life-threatening complication. Therefore, detailed knowledge of this disease and its complications is essential to improve outcomes.
They point out that the 10% rate of stroke found in this study is substantially lower, compared with the largest surgical prosthetic-valve infective endocarditis registries, but they suggest that the unique clinical profile of TAVR patients may lead to an underdiagnosis of stroke, with a high proportion of elderly patients who more frequently present with nonspecific symptoms.
They conclude that “IE post-TAVR is associated with a poor prognosis with high in-hospital and late mortality rates. Our study reveals that patients with IE after TAVR complicated by stroke showed an even worse prognosis.”
“The progressive implementation of advanced imaging modalities for early IE diagnosis, especially nuclear imaging, may translate into a better prognosis in coming years. Close attention should be paid to early recognition of stroke-associated factors to improve clinical outcomes,” they add.
In an accompanying editorial, Vuyisile Nkomo, MD, Daniel DeSimone, MD, and William Miranda, MD, Mayo Clinic, Rochester, Minn., say the current study “highlights the devastating consequences of IE after TAVR and the even worse consequences when IE was associated with stroke.”
This points to the critical importance of efforts to prevent IE with appropriate antibiotic prophylaxis and addressing potential sources of infection (for example, dental screening) before invasive cardiac procedures.
“Patient education is critical in regard to recognizing early signs and symptoms of IE. In particular, patients must be informed to obtain blood cultures with any episode of fever, as identification of bacteremia is critical in the diagnosis of IE,” the editorialists comment.
Endocarditis should also be suspected in afebrile patients with increasing transcatheter heart valve gradients or new or worsening regurgitation, they state.
Multimodality imaging is important for the early diagnosis of IE to facilitate prompt antibiotic treatment and potentially decrease the risk for IE complications, especially systemic embolization, they add.
“Despite the unequivocal advances in the safety and periprocedural complications of TAVR, IE with and without stroke in this TAVR population remains a dreadful complication,” they conclude.
Dr. Del Val was supported by a research grant from the Fundación Alfonso Martin Escudero. The editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Trends in the management of pulmonary embolism
One of the newest trends in pulmonary embolism management is treatment of cancer associated venous thromboembolism (VTE) which encompasses deep vein thrombosis (DVT) and PE. Following the clinical management of cancer-associated venous thromboembolism in the hospital, direct oral anticoagulant therapy at discharge is your starting point, except in cases of intact luminal cancers, Scott Kaatz, DO, MSc, FACP, SFHM, said during SHM Converge, the annual conference of the Society of Hospital Medicine.
Dr. Kaatz, of the division of hospital medicine at Henry Ford Hospital, Detroit, based his remarks on emerging recommendations from leading medical societies on the topic, as well as a one-page algorithm from the Anticoagulation Forum that can be accessed at https://acforum-excellence.org/Resource-Center/resource_files/1638-2020-11-30-121425.pdf.
For the short-term treatment of VTE (3-6 months) for patients with active cancer, the American Society of Hematology guideline panel suggests direct oral anticoagulants, such as apixaban, edoxaban, or rivaroxaban, over low-molecular-weight heparin (LMWH) – a conditional recommendation based on low certainty in the evidence of effects.
Dr. Kaatz also discussed the latest recommendations regarding length of VTE treatment. After completion of primary treatment for patients with DVT and/or PE provoked by a chronic risk factor such as a surgery, pregnancy, or having a leg in a cast, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “On the other hand, patients with DVT and/or PE provoked by a transient factor typically do not require antithrombotic therapy after completion of primary treatment,” said Dr. Kaatz, who is also a clinical professor of medicine at Wayne State University, Detroit.
After completion of primary treatment for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “The recommendation does not apply to patients who have a high risk for bleeding complications,” he noted.
Transient or reversible risk factors should be also considered in length of VTE treatment. For example, according to guidelines from the European Society of Cardiology, the estimated risk for long-term VTE recurrence is high (defined as greater than 8% per year) for patients with active cancer, for patients with one or more previous episodes of VTE in the absence of a major transient or reversible factor, and for those with antiphospholipid antibody syndrome.
Dr. Kaatz also highlighted recommendations for the acute treatment of intermediate risk, or submassive PE. The ESC guidelines state that if anticoagulation is initiated parenterally, LMWH or fondaparinux is recommended over unfractionated heparin (UFH) for most patients. “The reason for that is, one drug-use evaluation study found that, after 24 hours using UFH, only about 24% of patients had reached their therapeutic goal,” Dr. Kaatz said. Guidelines for intermediate risk patients from ASH recommend anticoagulation as your starting point, while thrombolysis is reasonable to consider for submassive PE and low risk for bleeding in selected younger patients or for patients at high risk for decompensation because of concomitant cardiopulmonary disease. “The bleeding rates get much higher in patients over age 65,” he said.
Another resource Dr. Kaatz mentioned is the Pulmonary Embolism Response Team (PERT) Consortium, which was developed after initial efforts of a multidisciplinary team of physicians at Massachusetts General Hospital. The first PERT sought to coordinate and expedite the treatment of pulmonary embolus with a team of physicians from a variety of specialties. In 2019 the PERT Consortium published guidelines on the diagnosis, treatment, and follow-up of acute PE. “It includes detailed algorithms that are a little different from the ASH and ESC guidelines,” Dr. Kaatz said.
Dr. Kaatz disclosed that he is a consultant for Janssen, Pfizer, Portola/Alexion, Bristol-Myers Squibb, Novartis, and CSL Behring. He has also received research funding from Janssen, Bristol-Myers Squibb, and Osmosis. He also holds board positions with the AC Forum and the National Blood Clot Alliance Medical and Scientific Advisory Board.
One of the newest trends in pulmonary embolism management is treatment of cancer associated venous thromboembolism (VTE) which encompasses deep vein thrombosis (DVT) and PE. Following the clinical management of cancer-associated venous thromboembolism in the hospital, direct oral anticoagulant therapy at discharge is your starting point, except in cases of intact luminal cancers, Scott Kaatz, DO, MSc, FACP, SFHM, said during SHM Converge, the annual conference of the Society of Hospital Medicine.
Dr. Kaatz, of the division of hospital medicine at Henry Ford Hospital, Detroit, based his remarks on emerging recommendations from leading medical societies on the topic, as well as a one-page algorithm from the Anticoagulation Forum that can be accessed at https://acforum-excellence.org/Resource-Center/resource_files/1638-2020-11-30-121425.pdf.
For the short-term treatment of VTE (3-6 months) for patients with active cancer, the American Society of Hematology guideline panel suggests direct oral anticoagulants, such as apixaban, edoxaban, or rivaroxaban, over low-molecular-weight heparin (LMWH) – a conditional recommendation based on low certainty in the evidence of effects.
Dr. Kaatz also discussed the latest recommendations regarding length of VTE treatment. After completion of primary treatment for patients with DVT and/or PE provoked by a chronic risk factor such as a surgery, pregnancy, or having a leg in a cast, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “On the other hand, patients with DVT and/or PE provoked by a transient factor typically do not require antithrombotic therapy after completion of primary treatment,” said Dr. Kaatz, who is also a clinical professor of medicine at Wayne State University, Detroit.
After completion of primary treatment for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “The recommendation does not apply to patients who have a high risk for bleeding complications,” he noted.
Transient or reversible risk factors should be also considered in length of VTE treatment. For example, according to guidelines from the European Society of Cardiology, the estimated risk for long-term VTE recurrence is high (defined as greater than 8% per year) for patients with active cancer, for patients with one or more previous episodes of VTE in the absence of a major transient or reversible factor, and for those with antiphospholipid antibody syndrome.
Dr. Kaatz also highlighted recommendations for the acute treatment of intermediate risk, or submassive PE. The ESC guidelines state that if anticoagulation is initiated parenterally, LMWH or fondaparinux is recommended over unfractionated heparin (UFH) for most patients. “The reason for that is, one drug-use evaluation study found that, after 24 hours using UFH, only about 24% of patients had reached their therapeutic goal,” Dr. Kaatz said. Guidelines for intermediate risk patients from ASH recommend anticoagulation as your starting point, while thrombolysis is reasonable to consider for submassive PE and low risk for bleeding in selected younger patients or for patients at high risk for decompensation because of concomitant cardiopulmonary disease. “The bleeding rates get much higher in patients over age 65,” he said.
Another resource Dr. Kaatz mentioned is the Pulmonary Embolism Response Team (PERT) Consortium, which was developed after initial efforts of a multidisciplinary team of physicians at Massachusetts General Hospital. The first PERT sought to coordinate and expedite the treatment of pulmonary embolus with a team of physicians from a variety of specialties. In 2019 the PERT Consortium published guidelines on the diagnosis, treatment, and follow-up of acute PE. “It includes detailed algorithms that are a little different from the ASH and ESC guidelines,” Dr. Kaatz said.
Dr. Kaatz disclosed that he is a consultant for Janssen, Pfizer, Portola/Alexion, Bristol-Myers Squibb, Novartis, and CSL Behring. He has also received research funding from Janssen, Bristol-Myers Squibb, and Osmosis. He also holds board positions with the AC Forum and the National Blood Clot Alliance Medical and Scientific Advisory Board.
One of the newest trends in pulmonary embolism management is treatment of cancer associated venous thromboembolism (VTE) which encompasses deep vein thrombosis (DVT) and PE. Following the clinical management of cancer-associated venous thromboembolism in the hospital, direct oral anticoagulant therapy at discharge is your starting point, except in cases of intact luminal cancers, Scott Kaatz, DO, MSc, FACP, SFHM, said during SHM Converge, the annual conference of the Society of Hospital Medicine.
Dr. Kaatz, of the division of hospital medicine at Henry Ford Hospital, Detroit, based his remarks on emerging recommendations from leading medical societies on the topic, as well as a one-page algorithm from the Anticoagulation Forum that can be accessed at https://acforum-excellence.org/Resource-Center/resource_files/1638-2020-11-30-121425.pdf.
For the short-term treatment of VTE (3-6 months) for patients with active cancer, the American Society of Hematology guideline panel suggests direct oral anticoagulants, such as apixaban, edoxaban, or rivaroxaban, over low-molecular-weight heparin (LMWH) – a conditional recommendation based on low certainty in the evidence of effects.
Dr. Kaatz also discussed the latest recommendations regarding length of VTE treatment. After completion of primary treatment for patients with DVT and/or PE provoked by a chronic risk factor such as a surgery, pregnancy, or having a leg in a cast, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “On the other hand, patients with DVT and/or PE provoked by a transient factor typically do not require antithrombotic therapy after completion of primary treatment,” said Dr. Kaatz, who is also a clinical professor of medicine at Wayne State University, Detroit.
After completion of primary treatment for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “The recommendation does not apply to patients who have a high risk for bleeding complications,” he noted.
Transient or reversible risk factors should be also considered in length of VTE treatment. For example, according to guidelines from the European Society of Cardiology, the estimated risk for long-term VTE recurrence is high (defined as greater than 8% per year) for patients with active cancer, for patients with one or more previous episodes of VTE in the absence of a major transient or reversible factor, and for those with antiphospholipid antibody syndrome.
Dr. Kaatz also highlighted recommendations for the acute treatment of intermediate risk, or submassive PE. The ESC guidelines state that if anticoagulation is initiated parenterally, LMWH or fondaparinux is recommended over unfractionated heparin (UFH) for most patients. “The reason for that is, one drug-use evaluation study found that, after 24 hours using UFH, only about 24% of patients had reached their therapeutic goal,” Dr. Kaatz said. Guidelines for intermediate risk patients from ASH recommend anticoagulation as your starting point, while thrombolysis is reasonable to consider for submassive PE and low risk for bleeding in selected younger patients or for patients at high risk for decompensation because of concomitant cardiopulmonary disease. “The bleeding rates get much higher in patients over age 65,” he said.
Another resource Dr. Kaatz mentioned is the Pulmonary Embolism Response Team (PERT) Consortium, which was developed after initial efforts of a multidisciplinary team of physicians at Massachusetts General Hospital. The first PERT sought to coordinate and expedite the treatment of pulmonary embolus with a team of physicians from a variety of specialties. In 2019 the PERT Consortium published guidelines on the diagnosis, treatment, and follow-up of acute PE. “It includes detailed algorithms that are a little different from the ASH and ESC guidelines,” Dr. Kaatz said.
Dr. Kaatz disclosed that he is a consultant for Janssen, Pfizer, Portola/Alexion, Bristol-Myers Squibb, Novartis, and CSL Behring. He has also received research funding from Janssen, Bristol-Myers Squibb, and Osmosis. He also holds board positions with the AC Forum and the National Blood Clot Alliance Medical and Scientific Advisory Board.
FROM SHM CONVERGE 2021
How to utilize the updated PHM Core Competencies
Converge 2021 session
Making The Pediatric Hospital Medicine Core Competencies Work for You
Presenters
Erin Fisher, MD, MHM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sofia Teferi, MD, SFHM, FAAP
Session summary
The Pediatric Hospital Medicine (PHM) Core Competencies were originally published in the Journal of Hospital Medicine in 2010, and created a framework for graduate and continuing medical education, reflecting the roles and expectations for all pediatric hospitalists in the United States. Since that time, the field of PHM, scope of practice, and roles of hospitalists has evolved, making a substantial update to this dossier necessary.
The 2020 PHM Core Competencies consist of four sections, including common clinical diagnoses and conditions, specialized services, core skills, and the health care system. The four topics are covered in 66 chapters, which were updated or created for the present version.
The Core Competencies have many practical applications, including teaching or curriculum development, which may be used by trainees as well as PHM providers. The speakers gave real-world examples of the competencies’ application to evaluations, and the continuum of knowledge, skills, attitudes, and system implementation in the development of a trainee from student to practicing hospitalist. Trainees’ knowledge gaps can be identified using the competencies, and utilization of the provided compendium will help identify sources that can aid in teaching.
Professional development is an excellent way to utilize the Core Competencies. Division directors may identify a needed area for improvement and the competencies can serve as a road map for establishing goals, plan development, and analysis of results of the intervention. They are also a great resource for PHM board prep. Although the competencies were not developed specifically for the PHM boards, they do contain all 13 PHM content domains set forth by the American Board of Pediatrics for PHM.
The Core Competencies can also be used to justify service line needs and resources in discussions with administration. For instance, if one is a pediatric hospitalist at a community hospital and asked to take over the newborn nursery, the competencies can be used to get buy-in from the group, as a guide for additional training, to provide a framework for development of practice pathways, and to request resources needed.
The Pediatric Core Competencies are a great resource for pediatric hospitalists and group leaders with many uses, from board preparation to education and professional development. They provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Key takeaways
- Given a change in scope of practice of pediatric hospitalists over the past 10 years, the PHM Core Competencies were updated and published in the Journal of Hospital Medicine in 2020.
- The Core Competencies have many practical applications including education, curriculum development, professional development, and PHM board preparation.
- The Core Competencies provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Dr. Schwenk is a pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., where he serves as a medical director of inpatient services. He is an associate professor of pediatrics at the University of Louisville School of Medicine. He is a Senior Fellow of Hospital Medicine and has served on the executive council of the Pediatrics Special Interest Group and the Annual Meeting Committee for SHM Converge.
Converge 2021 session
Making The Pediatric Hospital Medicine Core Competencies Work for You
Presenters
Erin Fisher, MD, MHM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sofia Teferi, MD, SFHM, FAAP
Session summary
The Pediatric Hospital Medicine (PHM) Core Competencies were originally published in the Journal of Hospital Medicine in 2010, and created a framework for graduate and continuing medical education, reflecting the roles and expectations for all pediatric hospitalists in the United States. Since that time, the field of PHM, scope of practice, and roles of hospitalists has evolved, making a substantial update to this dossier necessary.
The 2020 PHM Core Competencies consist of four sections, including common clinical diagnoses and conditions, specialized services, core skills, and the health care system. The four topics are covered in 66 chapters, which were updated or created for the present version.
The Core Competencies have many practical applications, including teaching or curriculum development, which may be used by trainees as well as PHM providers. The speakers gave real-world examples of the competencies’ application to evaluations, and the continuum of knowledge, skills, attitudes, and system implementation in the development of a trainee from student to practicing hospitalist. Trainees’ knowledge gaps can be identified using the competencies, and utilization of the provided compendium will help identify sources that can aid in teaching.
Professional development is an excellent way to utilize the Core Competencies. Division directors may identify a needed area for improvement and the competencies can serve as a road map for establishing goals, plan development, and analysis of results of the intervention. They are also a great resource for PHM board prep. Although the competencies were not developed specifically for the PHM boards, they do contain all 13 PHM content domains set forth by the American Board of Pediatrics for PHM.
The Core Competencies can also be used to justify service line needs and resources in discussions with administration. For instance, if one is a pediatric hospitalist at a community hospital and asked to take over the newborn nursery, the competencies can be used to get buy-in from the group, as a guide for additional training, to provide a framework for development of practice pathways, and to request resources needed.
The Pediatric Core Competencies are a great resource for pediatric hospitalists and group leaders with many uses, from board preparation to education and professional development. They provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Key takeaways
- Given a change in scope of practice of pediatric hospitalists over the past 10 years, the PHM Core Competencies were updated and published in the Journal of Hospital Medicine in 2020.
- The Core Competencies have many practical applications including education, curriculum development, professional development, and PHM board preparation.
- The Core Competencies provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Dr. Schwenk is a pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., where he serves as a medical director of inpatient services. He is an associate professor of pediatrics at the University of Louisville School of Medicine. He is a Senior Fellow of Hospital Medicine and has served on the executive council of the Pediatrics Special Interest Group and the Annual Meeting Committee for SHM Converge.
Converge 2021 session
Making The Pediatric Hospital Medicine Core Competencies Work for You
Presenters
Erin Fisher, MD, MHM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sofia Teferi, MD, SFHM, FAAP
Session summary
The Pediatric Hospital Medicine (PHM) Core Competencies were originally published in the Journal of Hospital Medicine in 2010, and created a framework for graduate and continuing medical education, reflecting the roles and expectations for all pediatric hospitalists in the United States. Since that time, the field of PHM, scope of practice, and roles of hospitalists has evolved, making a substantial update to this dossier necessary.
The 2020 PHM Core Competencies consist of four sections, including common clinical diagnoses and conditions, specialized services, core skills, and the health care system. The four topics are covered in 66 chapters, which were updated or created for the present version.
The Core Competencies have many practical applications, including teaching or curriculum development, which may be used by trainees as well as PHM providers. The speakers gave real-world examples of the competencies’ application to evaluations, and the continuum of knowledge, skills, attitudes, and system implementation in the development of a trainee from student to practicing hospitalist. Trainees’ knowledge gaps can be identified using the competencies, and utilization of the provided compendium will help identify sources that can aid in teaching.
Professional development is an excellent way to utilize the Core Competencies. Division directors may identify a needed area for improvement and the competencies can serve as a road map for establishing goals, plan development, and analysis of results of the intervention. They are also a great resource for PHM board prep. Although the competencies were not developed specifically for the PHM boards, they do contain all 13 PHM content domains set forth by the American Board of Pediatrics for PHM.
The Core Competencies can also be used to justify service line needs and resources in discussions with administration. For instance, if one is a pediatric hospitalist at a community hospital and asked to take over the newborn nursery, the competencies can be used to get buy-in from the group, as a guide for additional training, to provide a framework for development of practice pathways, and to request resources needed.
The Pediatric Core Competencies are a great resource for pediatric hospitalists and group leaders with many uses, from board preparation to education and professional development. They provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Key takeaways
- Given a change in scope of practice of pediatric hospitalists over the past 10 years, the PHM Core Competencies were updated and published in the Journal of Hospital Medicine in 2020.
- The Core Competencies have many practical applications including education, curriculum development, professional development, and PHM board preparation.
- The Core Competencies provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Dr. Schwenk is a pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., where he serves as a medical director of inpatient services. He is an associate professor of pediatrics at the University of Louisville School of Medicine. He is a Senior Fellow of Hospital Medicine and has served on the executive council of the Pediatrics Special Interest Group and the Annual Meeting Committee for SHM Converge.
FROM SHM CONVERGE 2021







