User login
Moderna warns of material drop in vaccine efficacy against Omicron
“There is no world, I think, where [the effectiveness] is the same level … we had with Delta,” Stephane Bancel told the Financial Times .
“I think it’s going to be a material drop,” he said. “I just don’t know how much, because we need to wait for the data. But all the scientists I’ve talked to … are like, ‘This is not going to be good.’”
Vaccine companies are now studying whether the new Omicron variant could evade the current shots. Some data is expected in about 2 weeks.
Mr. Bancel said that if a new vaccine is needed, it could take several months to produce at scale. He estimated that Moderna could make billions of vaccine doses in 2022.
“[Moderna] and Pfizer cannot get a billion doses next week. The math doesn’t work,” he said. “But could we get the billion doses out by the summer? Sure.”
The news caused some panic on Nov. 30, prompting financial markets to fall sharply, according to Reuters. But the markets recovered after European officials gave a more reassuring outlook.
“Even if the new variant becomes more widespread, the vaccines we have will continue to provide protection,” Emer Cooke, executive director of the European Medicines Agency, told the European Parliament.
Mr. Cooke said the agency could approve new vaccines that target the Omicron variant within 3 to 4 months, if needed. Moderna and Pfizer have announced they are beginning to tailor a shot to address the Omicron variant in case the data shows they are necessary.
Also on Nov. 30, the European Centre for Disease Prevention and Control announced that 42 Omicron cases had been identified in 10 European Union countries, according to Reuters.
The cases were mild or had no symptoms, although they were found in younger people who may have mild or no symptoms anyway.
“For the assessment of whether [Omicron] escapes immunity, we still have to wait until investigations in the laboratories with [blood samples] from people who have recovered have been carried out,” Andrea Ammon, MD, chair of the agency, said during an online conference.
The University of Oxford, which developed a COVID-19 vaccine with AstraZeneca, said Nov. 30 that there’s no evidence that vaccines won’t prevent severe disease from the Omicron variant, according to Reuters.
“Despite the appearance of new variants over the past year, vaccines have continued to provide very high levels of protection against severe disease and there is no evidence so far that Omicron is any different,” the university said in a statement. “However, we have the necessary tools and processes in place for rapid development of an updated COVID-19 vaccine if it should be necessary.”
A version of this article first appeared on WebMD.com.
“There is no world, I think, where [the effectiveness] is the same level … we had with Delta,” Stephane Bancel told the Financial Times .
“I think it’s going to be a material drop,” he said. “I just don’t know how much, because we need to wait for the data. But all the scientists I’ve talked to … are like, ‘This is not going to be good.’”
Vaccine companies are now studying whether the new Omicron variant could evade the current shots. Some data is expected in about 2 weeks.
Mr. Bancel said that if a new vaccine is needed, it could take several months to produce at scale. He estimated that Moderna could make billions of vaccine doses in 2022.
“[Moderna] and Pfizer cannot get a billion doses next week. The math doesn’t work,” he said. “But could we get the billion doses out by the summer? Sure.”
The news caused some panic on Nov. 30, prompting financial markets to fall sharply, according to Reuters. But the markets recovered after European officials gave a more reassuring outlook.
“Even if the new variant becomes more widespread, the vaccines we have will continue to provide protection,” Emer Cooke, executive director of the European Medicines Agency, told the European Parliament.
Mr. Cooke said the agency could approve new vaccines that target the Omicron variant within 3 to 4 months, if needed. Moderna and Pfizer have announced they are beginning to tailor a shot to address the Omicron variant in case the data shows they are necessary.
Also on Nov. 30, the European Centre for Disease Prevention and Control announced that 42 Omicron cases had been identified in 10 European Union countries, according to Reuters.
The cases were mild or had no symptoms, although they were found in younger people who may have mild or no symptoms anyway.
“For the assessment of whether [Omicron] escapes immunity, we still have to wait until investigations in the laboratories with [blood samples] from people who have recovered have been carried out,” Andrea Ammon, MD, chair of the agency, said during an online conference.
The University of Oxford, which developed a COVID-19 vaccine with AstraZeneca, said Nov. 30 that there’s no evidence that vaccines won’t prevent severe disease from the Omicron variant, according to Reuters.
“Despite the appearance of new variants over the past year, vaccines have continued to provide very high levels of protection against severe disease and there is no evidence so far that Omicron is any different,” the university said in a statement. “However, we have the necessary tools and processes in place for rapid development of an updated COVID-19 vaccine if it should be necessary.”
A version of this article first appeared on WebMD.com.
“There is no world, I think, where [the effectiveness] is the same level … we had with Delta,” Stephane Bancel told the Financial Times .
“I think it’s going to be a material drop,” he said. “I just don’t know how much, because we need to wait for the data. But all the scientists I’ve talked to … are like, ‘This is not going to be good.’”
Vaccine companies are now studying whether the new Omicron variant could evade the current shots. Some data is expected in about 2 weeks.
Mr. Bancel said that if a new vaccine is needed, it could take several months to produce at scale. He estimated that Moderna could make billions of vaccine doses in 2022.
“[Moderna] and Pfizer cannot get a billion doses next week. The math doesn’t work,” he said. “But could we get the billion doses out by the summer? Sure.”
The news caused some panic on Nov. 30, prompting financial markets to fall sharply, according to Reuters. But the markets recovered after European officials gave a more reassuring outlook.
“Even if the new variant becomes more widespread, the vaccines we have will continue to provide protection,” Emer Cooke, executive director of the European Medicines Agency, told the European Parliament.
Mr. Cooke said the agency could approve new vaccines that target the Omicron variant within 3 to 4 months, if needed. Moderna and Pfizer have announced they are beginning to tailor a shot to address the Omicron variant in case the data shows they are necessary.
Also on Nov. 30, the European Centre for Disease Prevention and Control announced that 42 Omicron cases had been identified in 10 European Union countries, according to Reuters.
The cases were mild or had no symptoms, although they were found in younger people who may have mild or no symptoms anyway.
“For the assessment of whether [Omicron] escapes immunity, we still have to wait until investigations in the laboratories with [blood samples] from people who have recovered have been carried out,” Andrea Ammon, MD, chair of the agency, said during an online conference.
The University of Oxford, which developed a COVID-19 vaccine with AstraZeneca, said Nov. 30 that there’s no evidence that vaccines won’t prevent severe disease from the Omicron variant, according to Reuters.
“Despite the appearance of new variants over the past year, vaccines have continued to provide very high levels of protection against severe disease and there is no evidence so far that Omicron is any different,” the university said in a statement. “However, we have the necessary tools and processes in place for rapid development of an updated COVID-19 vaccine if it should be necessary.”
A version of this article first appeared on WebMD.com.
Children and COVID: New cases, vaccinations both decline
States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
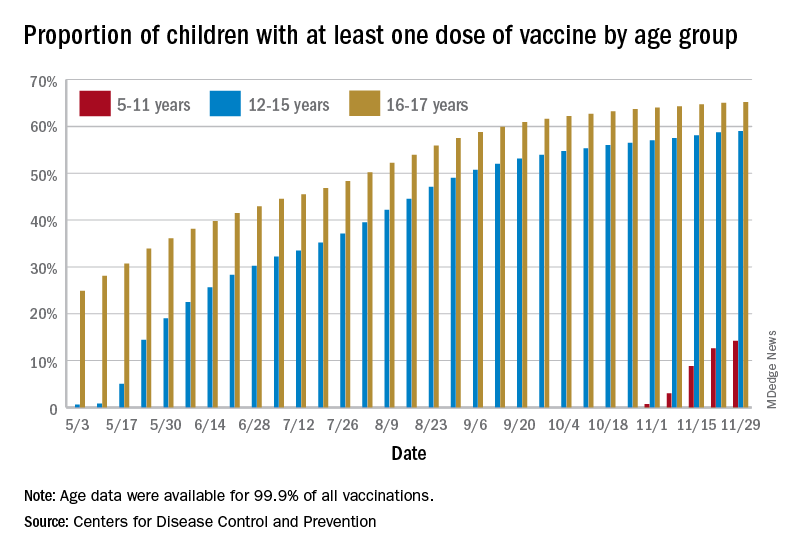
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.
Fauci: Omicron ‘very different from other variants’
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
FDA panel backs first pill for COVID-19 by a small margin
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
Optimizing perioperative cardiac risk assessment and management for noncardiac surgery
Background: There are extensive publications regarding preoperative risk assessment and optimization of risk management. This article is a review of current aggregate data from various meta-analyses and observational studies. It explores a systematic approach to preoperative risk assessment.
Study design: Literature review of meta-analyses and observational studies.
Setting: A review of the current literature available in the MEDLINE database and Cochrane Library from 1949 to January 2020, favoring meta-analyses and clinical practice guidelines.
Synopsis: A total of 92 publications were included in this review, which found history, physical exam, and functional capacity to be the best assessments of cardiac risk and should guide further preoperative management. Cardiovascular testing is rarely indicated except in those with clinical signs and symptoms of active cardiac conditions or with poor functional status undergoing high-risk surgery. Cardiac consultation should be considered for those with prior stents; high-risk conditions, including acute coronary syndrome, severe valvular disease, or active heart failure, among other conditions; or high-risk findings on cardiovascular testing. Preoperative medications should be individualized to patient-specific conditions. This study is limited by current available evidence and expert opinion, and the systematic approach suggested here has not been prospectively tested.
Bottom line: Preoperative risk assessment and management should be largely based on individualized history, physical exam, and functional status. Cardiovascular work-up should be pursued only if it would influence surgical decision-making and perioperative care.
Citation: Smilowitz NR, Berger JS. Perioperative cardiovascular risk assessment and management for noncardiac surgery: A review. JAMA. 2020 Jul 21;324:279-90. doi:
Dr. Young is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: There are extensive publications regarding preoperative risk assessment and optimization of risk management. This article is a review of current aggregate data from various meta-analyses and observational studies. It explores a systematic approach to preoperative risk assessment.
Study design: Literature review of meta-analyses and observational studies.
Setting: A review of the current literature available in the MEDLINE database and Cochrane Library from 1949 to January 2020, favoring meta-analyses and clinical practice guidelines.
Synopsis: A total of 92 publications were included in this review, which found history, physical exam, and functional capacity to be the best assessments of cardiac risk and should guide further preoperative management. Cardiovascular testing is rarely indicated except in those with clinical signs and symptoms of active cardiac conditions or with poor functional status undergoing high-risk surgery. Cardiac consultation should be considered for those with prior stents; high-risk conditions, including acute coronary syndrome, severe valvular disease, or active heart failure, among other conditions; or high-risk findings on cardiovascular testing. Preoperative medications should be individualized to patient-specific conditions. This study is limited by current available evidence and expert opinion, and the systematic approach suggested here has not been prospectively tested.
Bottom line: Preoperative risk assessment and management should be largely based on individualized history, physical exam, and functional status. Cardiovascular work-up should be pursued only if it would influence surgical decision-making and perioperative care.
Citation: Smilowitz NR, Berger JS. Perioperative cardiovascular risk assessment and management for noncardiac surgery: A review. JAMA. 2020 Jul 21;324:279-90. doi:
Dr. Young is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: There are extensive publications regarding preoperative risk assessment and optimization of risk management. This article is a review of current aggregate data from various meta-analyses and observational studies. It explores a systematic approach to preoperative risk assessment.
Study design: Literature review of meta-analyses and observational studies.
Setting: A review of the current literature available in the MEDLINE database and Cochrane Library from 1949 to January 2020, favoring meta-analyses and clinical practice guidelines.
Synopsis: A total of 92 publications were included in this review, which found history, physical exam, and functional capacity to be the best assessments of cardiac risk and should guide further preoperative management. Cardiovascular testing is rarely indicated except in those with clinical signs and symptoms of active cardiac conditions or with poor functional status undergoing high-risk surgery. Cardiac consultation should be considered for those with prior stents; high-risk conditions, including acute coronary syndrome, severe valvular disease, or active heart failure, among other conditions; or high-risk findings on cardiovascular testing. Preoperative medications should be individualized to patient-specific conditions. This study is limited by current available evidence and expert opinion, and the systematic approach suggested here has not been prospectively tested.
Bottom line: Preoperative risk assessment and management should be largely based on individualized history, physical exam, and functional status. Cardiovascular work-up should be pursued only if it would influence surgical decision-making and perioperative care.
Citation: Smilowitz NR, Berger JS. Perioperative cardiovascular risk assessment and management for noncardiac surgery: A review. JAMA. 2020 Jul 21;324:279-90. doi:
Dr. Young is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
We physicians must pull together as a knowledge community
The COVID-19 pandemic is a biosocial phenomenon. Patients and doctors alike find themselves assigned to groups designated as responsible and wise, or selfish and irrational, based strictly upon their personal assessments of medical risk. This trend in our culture is represented by threats of disciplinary action issued by medical regulators against physicians who are perceived to be undermining the public health message by spreading “misinformation.”
Our review of the literature reveals many references to “misinformation” but no definition narrow and precise enough to be interpreted consistently in a disciplinary environment. More pressing, this ambiguous word’s use is correlated with negative meaning and innuendo, often discrediting valuable information a priori without actual data points.
The most basic definition available is Merriam Webster’s: “incorrect or misleading information.” This definition includes no point of reference against which competing scientific claims can be measured.
Claudia E. Haupt, PhD, a political scientist and law professor, articulates a useful framework for understanding the relationship between medicine and state regulators. In the Yale Law Journal, Dr. Haupt wrote: “Knowledge communities have specialized expertise and are closest to those affected; they must have the freedom to work things out for themselves. The professions as knowledge communities have a fundamental interest in not having the state (or anyone else, for that matter) corrupt or distort what amounts to the state of the art in their respective fields.”
Injecting the artificial term “misinformation” into the science information ecosystem obfuscates and impedes the very ability of this vital knowledge community to perform its raison d’être. , rather than attending to healing or promoting progress.
Time has certainly shown us that science is anything but settled on all things COVID. If the scientific community accepts disrespect as the response of choice to difference of opinion and practice, we lose the trust in one another as colleagues; we need to keep scientific inquiry and exploration alive. Curiosity, equanimity, and tolerance are key components of the professional attitude as we deftly maneuver against the virus together.
In the face of deadly disease, it is especially imperative that intelligent, thoughtful, highly respected scientists, researchers, and physicians have room to safely share their knowledge and clinical experience. The Association of American Physicians and Surgeons has published a statement on scientific integrity that can be used as a measuring stick for claims about misinformation in medicine. We call on physicians to pull together as a knowledge community. Kindness and respect for patients starts with kindness and respect for one another as colleagues.
Dr. Kohanski is in private practice in Somerset, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She disclosed no relevant financial relationships. Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships.
The COVID-19 pandemic is a biosocial phenomenon. Patients and doctors alike find themselves assigned to groups designated as responsible and wise, or selfish and irrational, based strictly upon their personal assessments of medical risk. This trend in our culture is represented by threats of disciplinary action issued by medical regulators against physicians who are perceived to be undermining the public health message by spreading “misinformation.”
Our review of the literature reveals many references to “misinformation” but no definition narrow and precise enough to be interpreted consistently in a disciplinary environment. More pressing, this ambiguous word’s use is correlated with negative meaning and innuendo, often discrediting valuable information a priori without actual data points.
The most basic definition available is Merriam Webster’s: “incorrect or misleading information.” This definition includes no point of reference against which competing scientific claims can be measured.
Claudia E. Haupt, PhD, a political scientist and law professor, articulates a useful framework for understanding the relationship between medicine and state regulators. In the Yale Law Journal, Dr. Haupt wrote: “Knowledge communities have specialized expertise and are closest to those affected; they must have the freedom to work things out for themselves. The professions as knowledge communities have a fundamental interest in not having the state (or anyone else, for that matter) corrupt or distort what amounts to the state of the art in their respective fields.”
Injecting the artificial term “misinformation” into the science information ecosystem obfuscates and impedes the very ability of this vital knowledge community to perform its raison d’être. , rather than attending to healing or promoting progress.
Time has certainly shown us that science is anything but settled on all things COVID. If the scientific community accepts disrespect as the response of choice to difference of opinion and practice, we lose the trust in one another as colleagues; we need to keep scientific inquiry and exploration alive. Curiosity, equanimity, and tolerance are key components of the professional attitude as we deftly maneuver against the virus together.
In the face of deadly disease, it is especially imperative that intelligent, thoughtful, highly respected scientists, researchers, and physicians have room to safely share their knowledge and clinical experience. The Association of American Physicians and Surgeons has published a statement on scientific integrity that can be used as a measuring stick for claims about misinformation in medicine. We call on physicians to pull together as a knowledge community. Kindness and respect for patients starts with kindness and respect for one another as colleagues.
Dr. Kohanski is in private practice in Somerset, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She disclosed no relevant financial relationships. Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships.
The COVID-19 pandemic is a biosocial phenomenon. Patients and doctors alike find themselves assigned to groups designated as responsible and wise, or selfish and irrational, based strictly upon their personal assessments of medical risk. This trend in our culture is represented by threats of disciplinary action issued by medical regulators against physicians who are perceived to be undermining the public health message by spreading “misinformation.”
Our review of the literature reveals many references to “misinformation” but no definition narrow and precise enough to be interpreted consistently in a disciplinary environment. More pressing, this ambiguous word’s use is correlated with negative meaning and innuendo, often discrediting valuable information a priori without actual data points.
The most basic definition available is Merriam Webster’s: “incorrect or misleading information.” This definition includes no point of reference against which competing scientific claims can be measured.
Claudia E. Haupt, PhD, a political scientist and law professor, articulates a useful framework for understanding the relationship between medicine and state regulators. In the Yale Law Journal, Dr. Haupt wrote: “Knowledge communities have specialized expertise and are closest to those affected; they must have the freedom to work things out for themselves. The professions as knowledge communities have a fundamental interest in not having the state (or anyone else, for that matter) corrupt or distort what amounts to the state of the art in their respective fields.”
Injecting the artificial term “misinformation” into the science information ecosystem obfuscates and impedes the very ability of this vital knowledge community to perform its raison d’être. , rather than attending to healing or promoting progress.
Time has certainly shown us that science is anything but settled on all things COVID. If the scientific community accepts disrespect as the response of choice to difference of opinion and practice, we lose the trust in one another as colleagues; we need to keep scientific inquiry and exploration alive. Curiosity, equanimity, and tolerance are key components of the professional attitude as we deftly maneuver against the virus together.
In the face of deadly disease, it is especially imperative that intelligent, thoughtful, highly respected scientists, researchers, and physicians have room to safely share their knowledge and clinical experience. The Association of American Physicians and Surgeons has published a statement on scientific integrity that can be used as a measuring stick for claims about misinformation in medicine. We call on physicians to pull together as a knowledge community. Kindness and respect for patients starts with kindness and respect for one another as colleagues.
Dr. Kohanski is in private practice in Somerset, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She disclosed no relevant financial relationships. Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships.
Merck’s COVID-19 pill may be less effective than first hoped
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
Intranasal vs. intramuscular naloxone in reversing opioid overdose
Background: Naloxone is an opioid antagonist that works to treat opioid overdose. Few randomized trials have assessed the efficacy of intranasal administration, whereas more data have been published supporting use of intramuscular naloxone. This prospective trial examines the ability of the same dose (800 mcg per 1 mL solution) of intranasal naloxone vs. intramuscular naloxone at managing opioid overdose.
Study design: Double-blind double-dummy randomized clinical trial.
Setting: Single supervised injection center in Sydney.
Synopsis: In this study, 197 participants with opioid overdose were randomized to intramuscular or intranasal naloxone. If the patient did not respond to either (GSC score less than 13, RR less than 10, or oxygen saturation less than 95%), a rescue dose of intramuscular naloxone was given. Participants who received the intramuscular naloxone were less likely to need the rescue dose (8.6% vs. 23.1%; odds ratio, 0.35; P = .002). The time to achieve an RR greater than 10 (15 vs. 8 minutes) and GSC score greater than 13 (17 vs. 8 minutes) was longer in the intranasal than the intramuscular group. Limitations include the setting of a controlled environment. Also, this protocol called for an initial 5 minutes of ventilation prior to randomization, which selected for more severe overdose cases in the overall study population. More studies are needed to assess efficacy in the field, needlestick injuries, and larger intranasal doses.
Bottom line: Intranasal naloxone effectively reverses opioid overdose but not as effectively as intramuscular naloxone at the same dose.
Citation: Dietze P et al. Effect of intranasal vs intramuscular naloxone on opioid overdose: A randomized clinical trial. JAMA Netw Open. 2019;2:e1914977. doi: 1
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Naloxone is an opioid antagonist that works to treat opioid overdose. Few randomized trials have assessed the efficacy of intranasal administration, whereas more data have been published supporting use of intramuscular naloxone. This prospective trial examines the ability of the same dose (800 mcg per 1 mL solution) of intranasal naloxone vs. intramuscular naloxone at managing opioid overdose.
Study design: Double-blind double-dummy randomized clinical trial.
Setting: Single supervised injection center in Sydney.
Synopsis: In this study, 197 participants with opioid overdose were randomized to intramuscular or intranasal naloxone. If the patient did not respond to either (GSC score less than 13, RR less than 10, or oxygen saturation less than 95%), a rescue dose of intramuscular naloxone was given. Participants who received the intramuscular naloxone were less likely to need the rescue dose (8.6% vs. 23.1%; odds ratio, 0.35; P = .002). The time to achieve an RR greater than 10 (15 vs. 8 minutes) and GSC score greater than 13 (17 vs. 8 minutes) was longer in the intranasal than the intramuscular group. Limitations include the setting of a controlled environment. Also, this protocol called for an initial 5 minutes of ventilation prior to randomization, which selected for more severe overdose cases in the overall study population. More studies are needed to assess efficacy in the field, needlestick injuries, and larger intranasal doses.
Bottom line: Intranasal naloxone effectively reverses opioid overdose but not as effectively as intramuscular naloxone at the same dose.
Citation: Dietze P et al. Effect of intranasal vs intramuscular naloxone on opioid overdose: A randomized clinical trial. JAMA Netw Open. 2019;2:e1914977. doi: 1
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Naloxone is an opioid antagonist that works to treat opioid overdose. Few randomized trials have assessed the efficacy of intranasal administration, whereas more data have been published supporting use of intramuscular naloxone. This prospective trial examines the ability of the same dose (800 mcg per 1 mL solution) of intranasal naloxone vs. intramuscular naloxone at managing opioid overdose.
Study design: Double-blind double-dummy randomized clinical trial.
Setting: Single supervised injection center in Sydney.
Synopsis: In this study, 197 participants with opioid overdose were randomized to intramuscular or intranasal naloxone. If the patient did not respond to either (GSC score less than 13, RR less than 10, or oxygen saturation less than 95%), a rescue dose of intramuscular naloxone was given. Participants who received the intramuscular naloxone were less likely to need the rescue dose (8.6% vs. 23.1%; odds ratio, 0.35; P = .002). The time to achieve an RR greater than 10 (15 vs. 8 minutes) and GSC score greater than 13 (17 vs. 8 minutes) was longer in the intranasal than the intramuscular group. Limitations include the setting of a controlled environment. Also, this protocol called for an initial 5 minutes of ventilation prior to randomization, which selected for more severe overdose cases in the overall study population. More studies are needed to assess efficacy in the field, needlestick injuries, and larger intranasal doses.
Bottom line: Intranasal naloxone effectively reverses opioid overdose but not as effectively as intramuscular naloxone at the same dose.
Citation: Dietze P et al. Effect of intranasal vs intramuscular naloxone on opioid overdose: A randomized clinical trial. JAMA Netw Open. 2019;2:e1914977. doi: 1
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Comparing the efficacy and safety of common SIADH treatments
Background: Hyponatremia caused by SIADH is common in hospitalized patients, and most evidence for treatment comes from noncontrolled studies. This study aims to investigate the efficacy and safety of fluid restriction compared with furosemide, with or without NaCl supplementation, for treating SIADH.
Study design: Open-label randomized controlled trial.
Setting: Single center in Thailand.
Synopsis: There were 92 participants randomized to fluid restriction alone, fluid restriction and furosemide, or fluid restriction, furosemide, and NaCl supplementation. The authors assessed the primary outcome, change in sodium, at 4, 7, 14, and 28 days (baseline mean Na 125 mmol/L). By day 4, all groups had a significant increase in sodium (mean delta 5 mmol/L). The time to achieve a safe sodium level (Na less than 130 mmol/L) was not different among groups. Acute kidney injury was most common in patients who received furosemide and NaCl supplementation, compared with the fluid restriction and fluid restriction plus furosemide groups (32%, 10%, 17%, respectively; P = .07). Hypokalemia was also most common in the furosemide and NaCl group (42%, 13%, 23%, respectively; P = .01). Limitations include open-label study design, poor fluid restriction adherence (63% overall), and inflexible treatment regimens that excluded treatment with oral potassium.
Bottom line: In treatment of hyponatremia caused by SIADH, there was no benefit to adding furosemide with or without NaCl supplementation to fluid restriction. However, there was potential associated risk of acute kidney injury and hypokalemia.Citation: Krisanapan P et al. Efficacy of furosemide, oral sodium chloride, and fluid restriction for treatment of syndrome of inappropriate antidiuresis (SIADH): An open-label randomized controlled study (the EFFUSE-FLUID trial). Am J Kidney Dis. 2020 Aug;76(2):203-12. doi: 10.1053/j.ajkd.2019.11.012.
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Hyponatremia caused by SIADH is common in hospitalized patients, and most evidence for treatment comes from noncontrolled studies. This study aims to investigate the efficacy and safety of fluid restriction compared with furosemide, with or without NaCl supplementation, for treating SIADH.
Study design: Open-label randomized controlled trial.
Setting: Single center in Thailand.
Synopsis: There were 92 participants randomized to fluid restriction alone, fluid restriction and furosemide, or fluid restriction, furosemide, and NaCl supplementation. The authors assessed the primary outcome, change in sodium, at 4, 7, 14, and 28 days (baseline mean Na 125 mmol/L). By day 4, all groups had a significant increase in sodium (mean delta 5 mmol/L). The time to achieve a safe sodium level (Na less than 130 mmol/L) was not different among groups. Acute kidney injury was most common in patients who received furosemide and NaCl supplementation, compared with the fluid restriction and fluid restriction plus furosemide groups (32%, 10%, 17%, respectively; P = .07). Hypokalemia was also most common in the furosemide and NaCl group (42%, 13%, 23%, respectively; P = .01). Limitations include open-label study design, poor fluid restriction adherence (63% overall), and inflexible treatment regimens that excluded treatment with oral potassium.
Bottom line: In treatment of hyponatremia caused by SIADH, there was no benefit to adding furosemide with or without NaCl supplementation to fluid restriction. However, there was potential associated risk of acute kidney injury and hypokalemia.Citation: Krisanapan P et al. Efficacy of furosemide, oral sodium chloride, and fluid restriction for treatment of syndrome of inappropriate antidiuresis (SIADH): An open-label randomized controlled study (the EFFUSE-FLUID trial). Am J Kidney Dis. 2020 Aug;76(2):203-12. doi: 10.1053/j.ajkd.2019.11.012.
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Hyponatremia caused by SIADH is common in hospitalized patients, and most evidence for treatment comes from noncontrolled studies. This study aims to investigate the efficacy and safety of fluid restriction compared with furosemide, with or without NaCl supplementation, for treating SIADH.
Study design: Open-label randomized controlled trial.
Setting: Single center in Thailand.
Synopsis: There were 92 participants randomized to fluid restriction alone, fluid restriction and furosemide, or fluid restriction, furosemide, and NaCl supplementation. The authors assessed the primary outcome, change in sodium, at 4, 7, 14, and 28 days (baseline mean Na 125 mmol/L). By day 4, all groups had a significant increase in sodium (mean delta 5 mmol/L). The time to achieve a safe sodium level (Na less than 130 mmol/L) was not different among groups. Acute kidney injury was most common in patients who received furosemide and NaCl supplementation, compared with the fluid restriction and fluid restriction plus furosemide groups (32%, 10%, 17%, respectively; P = .07). Hypokalemia was also most common in the furosemide and NaCl group (42%, 13%, 23%, respectively; P = .01). Limitations include open-label study design, poor fluid restriction adherence (63% overall), and inflexible treatment regimens that excluded treatment with oral potassium.
Bottom line: In treatment of hyponatremia caused by SIADH, there was no benefit to adding furosemide with or without NaCl supplementation to fluid restriction. However, there was potential associated risk of acute kidney injury and hypokalemia.Citation: Krisanapan P et al. Efficacy of furosemide, oral sodium chloride, and fluid restriction for treatment of syndrome of inappropriate antidiuresis (SIADH): An open-label randomized controlled study (the EFFUSE-FLUID trial). Am J Kidney Dis. 2020 Aug;76(2):203-12. doi: 10.1053/j.ajkd.2019.11.012.
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Fueling an ‘already raging fire’: Fifth COVID surge approaches
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.






