User login
Face to Face with Ebola — An Emergency Care Center in Sierra Leone
At 6 a.m., our medical team arrives at the Ebola case-management center in the Kailahun district of Sierra Leone to take blood samples. At our 80-bed center here near the borders of Liberia and Guinea, 8 new patients were admitted yesterday, 9 need to have a repeat test 72 hours after their symptoms began, and some we hope to discharge today: at least 18 blood samples to obtain. The center currently houses 64 patients in all, 4 of them children less than 5 years of age. We have already seen 2 patients die today.
I have been here for 7 weeks, working as a nurse and emergency coordinator for the Médecins sans Frontières (MSF) Ebola response. Today we're lucky: it's raining, so we won't be too hot in the personal protective equipment (PPE) we must wear. We control who goes into the isolation area, how often, and for how long. No one should wear the PPE for longer than 40 minutes; it's unbearable for any longer than that, but it's easy to lose track of time, so we have to monitor our colleagues. The process starts in the dressing room, where getting into the PPE takes about 5 minutes. We have a designated dresser, responsible solely for making sure that we are wearing our equipment properly and that not a square millimeter of skin is exposed. In case one layer is accidently perforated, we wear two pairs of gloves, two masks, and a heavy apron on top of the full-body overalls. When we exit the isolation area, we are sprayed down with chlorine solution and peel off the PPE layer by layer. Some of the equipment — goggles, apron, boots, thick gloves — can be sterilized and used again. Everything else — overalls, masks, headcover — is burned.
The center has two sections: the low-risk area, containing the pharmacy, dressing rooms, laundry, laboratory, water-chlorination points, and staff meeting area; and the high-risk, or isolation, area, where patients are admitted and staff must wear the full PPE. Our medical and water-sanitation teams go into the high-risk area with a clear plan: check vital signs, administer medication, serve meals, and clean the 10 tents. There are also teams that help new patients settle in, prepare patients for discharge, and disinfect and remove the dead.
Everyone working in the isolation area must follow the protocols and procedures to the letter. We use a buddy system — we're responsible for ourselves but must also put our lives in the hands of colleagues: one mistake could be deadly. The isolation area is divided into separate tents for patients with suspected, probable, and confirmed Ebola virus infection. Suspected cases are defined by fever and three or more other symptoms of the disease; probable cases, by symptoms plus known contact with someone who's had Ebola or with the body of someone who's died of the disease. There is a clear separation between the tents for these two types of patients, who are given instructions for minimizing the risk of cross-contamination — by washing their hands, for instance, and not touching other patients or their belongings. For the same reason, the staff follows a strict circuit, moving from the suspected-case tents to the probable-case tents and finally to the confirmed-case tents.
The isolation area also contains a waste area, laundry, latrines, showers, and the morgue. The staff works in three shifts a day, around the clock, but everything is organized to minimize the time we spend in the high-risk area. In reality, it's one of the safest places to be during this outbreak, because we know that the patients have Ebola, so every protective measure is in place.
In the suspected-case tents, most patients look quite well, but the probable-case area is a different story. Patients here have fever, pain, anorexia — but these symptoms could indicate malaria. In the on-site laboratory, a polymerase-chain-reaction test can determine whether a patient has Ebola, usually providing results on the same day or the next day. When the result comes in, the patient is either moved to the confirmed-case tents or discharged. Knowing what it means to be moved to these tents, patients are understandably frightened. We have a psychologist, a counselor, and health promoters to help and support patients, but there are just too many of them.
Standard treatment for Ebola is limited to supportive therapy: hydrating patients, maintaining their oxygen status and blood pressure, providing high-quality nutrition, and treating any complicating infections with antibiotics. Supportive treatment can help patients survive longer, and that extra time may be what their immune system needs to start fighting the virus.
There's also a tent for the most severely ill patients. I try to spend more time there than in the other tents, if only to hold patients' hands, give them painkillers, and sit on the edge of their beds so that they know they're not alone. But spending time is always difficult — there are so many patients waiting for help.
It's the children who distress me most. In the confirmed-case tents, I cared for a 6-year-old boy and his 3-year-old sister. Their parents and grandmother had died from Ebola. A midwife in their village then took care of the children, but they began to show symptoms of Ebola and were sent to us. Sadly, they came too late. When the boy died, we tried to console and calm his sister, but the PPE made it difficult to touch her, to hold her, even to speak with her. She died the next day. The midwife who had taken care of them also ended up at our center, and she, too, died. Another patient told me he doesn't remember how many members of his family have died — he thinks about 13. All he knows is that he is now alone.
For caregivers, there is a sad frustration in seeing patients arrive too late — and in knowing that many sick people are hiding, fearing the effects of a diagnosis of this stigmatized disease. Although community health care workers have received training, some of them still don't recognize Ebola.
But we are sometimes fortunate. Today we can discharge four patients who survived Ebola. The discharge criteria call for a negative blood test and 3 days without symptoms. People who have recovered from Ebola are immune from the strain of the virus that infected them. Discharging a patient is our happiest moment — we gather outside the center, clapping and dancing in a celebration that motivates us to keep going. And motivation is important, because today we also received eight new admissions.
The new patients sometimes arrive eight to an ambulance, those with suspected cases and those with probable cases all mixed together. We've given the ambulance drivers basic PPE to distribute to patients, but they're afraid to get close enough to hand it out.
One day, a surveillance officer from the Ministry of Health is admitted to the center. He was one of the few people who had come from Freetown, the capital city, to help in Kailahun, joining the surveillance team to assess new patients and deaths. He told me he'd come because the people here are his community, his friends, his colleagues. We laughed together, commiserated with one another, and then he was admitted to our center and, sadly, later died.
The Ebola outbreak has been out of control for months, but the global health community has taken a long time to react. All organizations have limits, and here in Kailahun, MSF's limit is in case management. The current international Ebola response remains dangerously inadequate. Last week, 250 contacts of infected persons were identified for contact tracing, but given the number of confirmed cases, there should have been more than 1500. The alert system — whereby an investigation team (and, if needed, an ambulance) is sent to a village when a suspected case or death is reported — is not functioning properly, and the Ministry of Health has only four ambulances in a district with about 470,000 people. Our health promotion teams are still visiting villages where no other health care provider has been. Every day sees deaths in the community that are surely caused by Ebola, but they are not counted by the Ministry of Health because the cause has not been confirmed by laboratory testing. The epidemiologic surveillance system is nonfunctional. We need to define the chains of Ebola transmission to interrupt them, but we lack key data.
My time in Kailahun has been frustrating and disappointing, because I know from previous outbreaks what is required to control this one. No single organization has the capacity to manage all that is needed to stop the outbreak. Other organizations must attack this outbreak in all its facets. But the response has been too slow. We need people who are hands-on and on the ground. We need to be one step ahead of this outbreak, but right now we are five steps behind.
Reprinted with permission from the New England Journal of Medicine (DOI: 10.1056/NEJMp1410179).
At 6 a.m., our medical team arrives at the Ebola case-management center in the Kailahun district of Sierra Leone to take blood samples. At our 80-bed center here near the borders of Liberia and Guinea, 8 new patients were admitted yesterday, 9 need to have a repeat test 72 hours after their symptoms began, and some we hope to discharge today: at least 18 blood samples to obtain. The center currently houses 64 patients in all, 4 of them children less than 5 years of age. We have already seen 2 patients die today.
I have been here for 7 weeks, working as a nurse and emergency coordinator for the Médecins sans Frontières (MSF) Ebola response. Today we're lucky: it's raining, so we won't be too hot in the personal protective equipment (PPE) we must wear. We control who goes into the isolation area, how often, and for how long. No one should wear the PPE for longer than 40 minutes; it's unbearable for any longer than that, but it's easy to lose track of time, so we have to monitor our colleagues. The process starts in the dressing room, where getting into the PPE takes about 5 minutes. We have a designated dresser, responsible solely for making sure that we are wearing our equipment properly and that not a square millimeter of skin is exposed. In case one layer is accidently perforated, we wear two pairs of gloves, two masks, and a heavy apron on top of the full-body overalls. When we exit the isolation area, we are sprayed down with chlorine solution and peel off the PPE layer by layer. Some of the equipment — goggles, apron, boots, thick gloves — can be sterilized and used again. Everything else — overalls, masks, headcover — is burned.
The center has two sections: the low-risk area, containing the pharmacy, dressing rooms, laundry, laboratory, water-chlorination points, and staff meeting area; and the high-risk, or isolation, area, where patients are admitted and staff must wear the full PPE. Our medical and water-sanitation teams go into the high-risk area with a clear plan: check vital signs, administer medication, serve meals, and clean the 10 tents. There are also teams that help new patients settle in, prepare patients for discharge, and disinfect and remove the dead.
Everyone working in the isolation area must follow the protocols and procedures to the letter. We use a buddy system — we're responsible for ourselves but must also put our lives in the hands of colleagues: one mistake could be deadly. The isolation area is divided into separate tents for patients with suspected, probable, and confirmed Ebola virus infection. Suspected cases are defined by fever and three or more other symptoms of the disease; probable cases, by symptoms plus known contact with someone who's had Ebola or with the body of someone who's died of the disease. There is a clear separation between the tents for these two types of patients, who are given instructions for minimizing the risk of cross-contamination — by washing their hands, for instance, and not touching other patients or their belongings. For the same reason, the staff follows a strict circuit, moving from the suspected-case tents to the probable-case tents and finally to the confirmed-case tents.
The isolation area also contains a waste area, laundry, latrines, showers, and the morgue. The staff works in three shifts a day, around the clock, but everything is organized to minimize the time we spend in the high-risk area. In reality, it's one of the safest places to be during this outbreak, because we know that the patients have Ebola, so every protective measure is in place.
In the suspected-case tents, most patients look quite well, but the probable-case area is a different story. Patients here have fever, pain, anorexia — but these symptoms could indicate malaria. In the on-site laboratory, a polymerase-chain-reaction test can determine whether a patient has Ebola, usually providing results on the same day or the next day. When the result comes in, the patient is either moved to the confirmed-case tents or discharged. Knowing what it means to be moved to these tents, patients are understandably frightened. We have a psychologist, a counselor, and health promoters to help and support patients, but there are just too many of them.
Standard treatment for Ebola is limited to supportive therapy: hydrating patients, maintaining their oxygen status and blood pressure, providing high-quality nutrition, and treating any complicating infections with antibiotics. Supportive treatment can help patients survive longer, and that extra time may be what their immune system needs to start fighting the virus.
There's also a tent for the most severely ill patients. I try to spend more time there than in the other tents, if only to hold patients' hands, give them painkillers, and sit on the edge of their beds so that they know they're not alone. But spending time is always difficult — there are so many patients waiting for help.
It's the children who distress me most. In the confirmed-case tents, I cared for a 6-year-old boy and his 3-year-old sister. Their parents and grandmother had died from Ebola. A midwife in their village then took care of the children, but they began to show symptoms of Ebola and were sent to us. Sadly, they came too late. When the boy died, we tried to console and calm his sister, but the PPE made it difficult to touch her, to hold her, even to speak with her. She died the next day. The midwife who had taken care of them also ended up at our center, and she, too, died. Another patient told me he doesn't remember how many members of his family have died — he thinks about 13. All he knows is that he is now alone.
For caregivers, there is a sad frustration in seeing patients arrive too late — and in knowing that many sick people are hiding, fearing the effects of a diagnosis of this stigmatized disease. Although community health care workers have received training, some of them still don't recognize Ebola.
But we are sometimes fortunate. Today we can discharge four patients who survived Ebola. The discharge criteria call for a negative blood test and 3 days without symptoms. People who have recovered from Ebola are immune from the strain of the virus that infected them. Discharging a patient is our happiest moment — we gather outside the center, clapping and dancing in a celebration that motivates us to keep going. And motivation is important, because today we also received eight new admissions.
The new patients sometimes arrive eight to an ambulance, those with suspected cases and those with probable cases all mixed together. We've given the ambulance drivers basic PPE to distribute to patients, but they're afraid to get close enough to hand it out.
One day, a surveillance officer from the Ministry of Health is admitted to the center. He was one of the few people who had come from Freetown, the capital city, to help in Kailahun, joining the surveillance team to assess new patients and deaths. He told me he'd come because the people here are his community, his friends, his colleagues. We laughed together, commiserated with one another, and then he was admitted to our center and, sadly, later died.
The Ebola outbreak has been out of control for months, but the global health community has taken a long time to react. All organizations have limits, and here in Kailahun, MSF's limit is in case management. The current international Ebola response remains dangerously inadequate. Last week, 250 contacts of infected persons were identified for contact tracing, but given the number of confirmed cases, there should have been more than 1500. The alert system — whereby an investigation team (and, if needed, an ambulance) is sent to a village when a suspected case or death is reported — is not functioning properly, and the Ministry of Health has only four ambulances in a district with about 470,000 people. Our health promotion teams are still visiting villages where no other health care provider has been. Every day sees deaths in the community that are surely caused by Ebola, but they are not counted by the Ministry of Health because the cause has not been confirmed by laboratory testing. The epidemiologic surveillance system is nonfunctional. We need to define the chains of Ebola transmission to interrupt them, but we lack key data.
My time in Kailahun has been frustrating and disappointing, because I know from previous outbreaks what is required to control this one. No single organization has the capacity to manage all that is needed to stop the outbreak. Other organizations must attack this outbreak in all its facets. But the response has been too slow. We need people who are hands-on and on the ground. We need to be one step ahead of this outbreak, but right now we are five steps behind.
Reprinted with permission from the New England Journal of Medicine (DOI: 10.1056/NEJMp1410179).
At 6 a.m., our medical team arrives at the Ebola case-management center in the Kailahun district of Sierra Leone to take blood samples. At our 80-bed center here near the borders of Liberia and Guinea, 8 new patients were admitted yesterday, 9 need to have a repeat test 72 hours after their symptoms began, and some we hope to discharge today: at least 18 blood samples to obtain. The center currently houses 64 patients in all, 4 of them children less than 5 years of age. We have already seen 2 patients die today.
I have been here for 7 weeks, working as a nurse and emergency coordinator for the Médecins sans Frontières (MSF) Ebola response. Today we're lucky: it's raining, so we won't be too hot in the personal protective equipment (PPE) we must wear. We control who goes into the isolation area, how often, and for how long. No one should wear the PPE for longer than 40 minutes; it's unbearable for any longer than that, but it's easy to lose track of time, so we have to monitor our colleagues. The process starts in the dressing room, where getting into the PPE takes about 5 minutes. We have a designated dresser, responsible solely for making sure that we are wearing our equipment properly and that not a square millimeter of skin is exposed. In case one layer is accidently perforated, we wear two pairs of gloves, two masks, and a heavy apron on top of the full-body overalls. When we exit the isolation area, we are sprayed down with chlorine solution and peel off the PPE layer by layer. Some of the equipment — goggles, apron, boots, thick gloves — can be sterilized and used again. Everything else — overalls, masks, headcover — is burned.
The center has two sections: the low-risk area, containing the pharmacy, dressing rooms, laundry, laboratory, water-chlorination points, and staff meeting area; and the high-risk, or isolation, area, where patients are admitted and staff must wear the full PPE. Our medical and water-sanitation teams go into the high-risk area with a clear plan: check vital signs, administer medication, serve meals, and clean the 10 tents. There are also teams that help new patients settle in, prepare patients for discharge, and disinfect and remove the dead.
Everyone working in the isolation area must follow the protocols and procedures to the letter. We use a buddy system — we're responsible for ourselves but must also put our lives in the hands of colleagues: one mistake could be deadly. The isolation area is divided into separate tents for patients with suspected, probable, and confirmed Ebola virus infection. Suspected cases are defined by fever and three or more other symptoms of the disease; probable cases, by symptoms plus known contact with someone who's had Ebola or with the body of someone who's died of the disease. There is a clear separation between the tents for these two types of patients, who are given instructions for minimizing the risk of cross-contamination — by washing their hands, for instance, and not touching other patients or their belongings. For the same reason, the staff follows a strict circuit, moving from the suspected-case tents to the probable-case tents and finally to the confirmed-case tents.
The isolation area also contains a waste area, laundry, latrines, showers, and the morgue. The staff works in three shifts a day, around the clock, but everything is organized to minimize the time we spend in the high-risk area. In reality, it's one of the safest places to be during this outbreak, because we know that the patients have Ebola, so every protective measure is in place.
In the suspected-case tents, most patients look quite well, but the probable-case area is a different story. Patients here have fever, pain, anorexia — but these symptoms could indicate malaria. In the on-site laboratory, a polymerase-chain-reaction test can determine whether a patient has Ebola, usually providing results on the same day or the next day. When the result comes in, the patient is either moved to the confirmed-case tents or discharged. Knowing what it means to be moved to these tents, patients are understandably frightened. We have a psychologist, a counselor, and health promoters to help and support patients, but there are just too many of them.
Standard treatment for Ebola is limited to supportive therapy: hydrating patients, maintaining their oxygen status and blood pressure, providing high-quality nutrition, and treating any complicating infections with antibiotics. Supportive treatment can help patients survive longer, and that extra time may be what their immune system needs to start fighting the virus.
There's also a tent for the most severely ill patients. I try to spend more time there than in the other tents, if only to hold patients' hands, give them painkillers, and sit on the edge of their beds so that they know they're not alone. But spending time is always difficult — there are so many patients waiting for help.
It's the children who distress me most. In the confirmed-case tents, I cared for a 6-year-old boy and his 3-year-old sister. Their parents and grandmother had died from Ebola. A midwife in their village then took care of the children, but they began to show symptoms of Ebola and were sent to us. Sadly, they came too late. When the boy died, we tried to console and calm his sister, but the PPE made it difficult to touch her, to hold her, even to speak with her. She died the next day. The midwife who had taken care of them also ended up at our center, and she, too, died. Another patient told me he doesn't remember how many members of his family have died — he thinks about 13. All he knows is that he is now alone.
For caregivers, there is a sad frustration in seeing patients arrive too late — and in knowing that many sick people are hiding, fearing the effects of a diagnosis of this stigmatized disease. Although community health care workers have received training, some of them still don't recognize Ebola.
But we are sometimes fortunate. Today we can discharge four patients who survived Ebola. The discharge criteria call for a negative blood test and 3 days without symptoms. People who have recovered from Ebola are immune from the strain of the virus that infected them. Discharging a patient is our happiest moment — we gather outside the center, clapping and dancing in a celebration that motivates us to keep going. And motivation is important, because today we also received eight new admissions.
The new patients sometimes arrive eight to an ambulance, those with suspected cases and those with probable cases all mixed together. We've given the ambulance drivers basic PPE to distribute to patients, but they're afraid to get close enough to hand it out.
One day, a surveillance officer from the Ministry of Health is admitted to the center. He was one of the few people who had come from Freetown, the capital city, to help in Kailahun, joining the surveillance team to assess new patients and deaths. He told me he'd come because the people here are his community, his friends, his colleagues. We laughed together, commiserated with one another, and then he was admitted to our center and, sadly, later died.
The Ebola outbreak has been out of control for months, but the global health community has taken a long time to react. All organizations have limits, and here in Kailahun, MSF's limit is in case management. The current international Ebola response remains dangerously inadequate. Last week, 250 contacts of infected persons were identified for contact tracing, but given the number of confirmed cases, there should have been more than 1500. The alert system — whereby an investigation team (and, if needed, an ambulance) is sent to a village when a suspected case or death is reported — is not functioning properly, and the Ministry of Health has only four ambulances in a district with about 470,000 people. Our health promotion teams are still visiting villages where no other health care provider has been. Every day sees deaths in the community that are surely caused by Ebola, but they are not counted by the Ministry of Health because the cause has not been confirmed by laboratory testing. The epidemiologic surveillance system is nonfunctional. We need to define the chains of Ebola transmission to interrupt them, but we lack key data.
My time in Kailahun has been frustrating and disappointing, because I know from previous outbreaks what is required to control this one. No single organization has the capacity to manage all that is needed to stop the outbreak. Other organizations must attack this outbreak in all its facets. But the response has been too slow. We need people who are hands-on and on the ground. We need to be one step ahead of this outbreak, but right now we are five steps behind.
Reprinted with permission from the New England Journal of Medicine (DOI: 10.1056/NEJMp1410179).
A Good Death — Ebola and Sacrifice
A friend of ours, Dr. Sam Brisbane, died recently. He was a Liberian doctor, and he died from Ebola, a horrible, nightmarish disease.
Information coming out of Liberia has been scarce. Since Dr. Brisbane's death, we've learned that other doctors and nurses with whom we've worked have also contracted Ebola and have died or are being treated in the types of rudimentary facilities we see on the news. As we live in dread of each phone call, questions about how we die and what we're willing to die for have been weighing on us.
The ancients had a concept of a “good death” — dying for one's country, for example, or gloriously on the battlefield. Solon, the sage of Athens, argued that one couldn't judge a person's happiness until one knew the manner of his death. The Greeks recognized that we're all destined to die and that the best we can hope for is a death that benefits our family or humanity.
For emergency-medicine clinicians like us, the concept of a good death can seem too abstract, intangible. Rarely are the deaths we see good or beneficial. We see young people who die in the throes of trauma; grandparents who die at the end of a long, debilitating illness; people who kill themselves; people who die from their excesses, whether of alcohol, food, or smoking.
Last year, as part of a new disaster-medicine fellowship program, we developed a partnership with John F. Kennedy Memorial Medical Center in Monrovia, the only academic referral hospital in Liberia. We collaborated with the hospital administration to develop disaster-planning and resilience programs and teamed up with the emergency department (ED) staff to enhance medical training and establish epidemiologic studies of trauma. It was there that we met Dr. Brisbane, the ED director. He immediately struck us as a genuine ED doc — at once caring and profane, light-hearted one minute, intense the next. A short, bald man with weathered skin and thick glasses, he spoke openly and easily; his laugh was best described as a giggle, and he swore frequently.
When we conducted an initial vulnerability analysis for the hospital, we discussed our concerns about severe supply and personnel shortages, regular power outages, and occasional electrical fires. Dr. Brisbane replied that what scared him the most was the potential for an epidemic of some viral hemorrhagic fever. He was right to be scared. We encountered rationing of gloves, a limited supply of hand soap, and an institutional hesitance to practice universal precautions, probably because of the limited resources. The hospital was not prepared for the kind of epidemic it's now facing — nor was the city of about 1.5 million people.
During our time at JFK, we became friends with Dr. Brisbane. We learned that he'd trained in Germany in the 1970s, had returned to Liberia to work, and had chosen to stay through the civil war and during Charles Taylor's despotic rule, continuing to see patients despite the bloodshed around him. He had welcomed the country's new democratic leadership and a new female administrator at the hospital — a first. He ran a successful coffee plantation and gave us bags of coffee every time we visited him. He was the father of eight biologic children and six adopted children, and he had numerous grandchildren around the world.
Within a few days after our return to Monrovia in June 2014, the city's first patients with Ebola presented at Redemption, the county hospital, and we soon got word that a doctor and some nurses there had died. Rumors were rampant, and staff quickly abandoned that hospital. At JFK, our colleagues grew nervous. There were tensions between the hospital administration and the public health ministry. There was no clear plan for what to do if a patient suspected of having Ebola showed up at the hospital. How would staff members protect themselves? How would they isolate the patient? How could they move the patient to one of the ministry's isolation centers? Dr. Brisbane was a wreck. He chattered nervously, his smile disappeared when he thought we weren't watching, and he openly wondered how he could protect himself. He told us bluntly, “Leave Monrovia.”
Then one morning, we arrived at the hospital at 7 o'clock and ran into Dr. Philip Zokonis Ireland, one of our young doctor friends. He was agitated, his fear evident in his face: there was a patient in the ED with suspected Ebola. The patient had lain in a bed in one of the small, crowded treatment areas for 6 hours, surrounded by nurses and other patients, until someone recognized his symptoms. We rushed to the room and met Dr. Brisbane and Dr. Abraham Borbor, the head of internal medicine. Others were sensing that something was wrong. Patients and their family members quickly disappeared, and nurses hung far back in the hallway.
The first priority was to get the patient out of the common room and into an isolation room, but the bed he was lying on was too wide for the doorway. So Dr. Brisbane, Dr. Borbor, and two custodians hastily donned gowns, gloves, and masks, then lifted the patient — mattress and all — and carried him into the isolation room, nearly dropping him in the process. The man had begun gasping for breath, and despite their efforts, within 5 minutes he was dead. Later that day, laboratory tests confirmed that he was indeed infected with Ebola virus. His body stayed in the now-otherwise-empty ED until it was retrieved hours later by the health ministry.
We remained in Monrovia for the next week and helped however we could. Dr. Brisbane brought his own thermometer and checked his temperature religiously, fearing the telltale sudden fever. He wore a fedora in the hospital as a protective talisman. And yet he still joked with us, displaying a sort of gallows humor.
A few days after we'd returned to the States, we got a call from a friend in Monrovia saying that Dr. Brisbane was in isolation and had tested positive for Ebola. The next call informed us of his death and hasty burial on his plantation. By late August, Dr. Ireland and one of the nurses we knew had contracted Ebola and were fighting for survival, and Dr. Borbor and a physician assistant who'd worked in the ED had died from the virus.
Dr. Brisbane didn't have to stay at JFK and continue to care for patients. He could easily have retired to his coffee plantation with his wife and children and grandchildren. He was terrified of Ebola, and yet we knew that every morning when we entered the ED, we'd find him there, seeing his patients.
Doctors and nurses have a duty of care toward their patients.1 We're expected, on the basis of our training and an unwritten social contract, to fulfill that duty even in less-than-ideal circumstances — in the face of depleted resources, for example, or undesirable patients. But we also have a duty to ourselves and our families, and when our work becomes life-threatening, we have to decide what benefit we will be to our patients and what cost it will exact from us. In such circumstances, we cannot be expected to uphold the same duty of care. But during the world's worst Ebola outbreak to date, clinicians like Dr. Brisbane are on the front lines — and are dying as a result. They care for patients despite the risks to themselves, despite the inadequate supplies and infrastructure, despite their insufficient training in infection control.
Dr. Sam Brisbane's death diminishes us as a people. But with apologies to his wife and family, who saw him die horribly and unjustly, and despite the deep loss we feel, we believe our friend died a good death — as did all the nurses and doctors who have sacrificed themselves caring for patients with this awful disease.
Note: This article was reprinted with permission of the New England Journal of Medicine (10.1056/NEJMp1410301).
References
1. Sokol, D.K. Virulent epidemics and scope of healthcare workers' duty of care. Emerg Infect Dis 2006;12:1238-1241
A friend of ours, Dr. Sam Brisbane, died recently. He was a Liberian doctor, and he died from Ebola, a horrible, nightmarish disease.
Information coming out of Liberia has been scarce. Since Dr. Brisbane's death, we've learned that other doctors and nurses with whom we've worked have also contracted Ebola and have died or are being treated in the types of rudimentary facilities we see on the news. As we live in dread of each phone call, questions about how we die and what we're willing to die for have been weighing on us.
The ancients had a concept of a “good death” — dying for one's country, for example, or gloriously on the battlefield. Solon, the sage of Athens, argued that one couldn't judge a person's happiness until one knew the manner of his death. The Greeks recognized that we're all destined to die and that the best we can hope for is a death that benefits our family or humanity.
For emergency-medicine clinicians like us, the concept of a good death can seem too abstract, intangible. Rarely are the deaths we see good or beneficial. We see young people who die in the throes of trauma; grandparents who die at the end of a long, debilitating illness; people who kill themselves; people who die from their excesses, whether of alcohol, food, or smoking.
Last year, as part of a new disaster-medicine fellowship program, we developed a partnership with John F. Kennedy Memorial Medical Center in Monrovia, the only academic referral hospital in Liberia. We collaborated with the hospital administration to develop disaster-planning and resilience programs and teamed up with the emergency department (ED) staff to enhance medical training and establish epidemiologic studies of trauma. It was there that we met Dr. Brisbane, the ED director. He immediately struck us as a genuine ED doc — at once caring and profane, light-hearted one minute, intense the next. A short, bald man with weathered skin and thick glasses, he spoke openly and easily; his laugh was best described as a giggle, and he swore frequently.
When we conducted an initial vulnerability analysis for the hospital, we discussed our concerns about severe supply and personnel shortages, regular power outages, and occasional electrical fires. Dr. Brisbane replied that what scared him the most was the potential for an epidemic of some viral hemorrhagic fever. He was right to be scared. We encountered rationing of gloves, a limited supply of hand soap, and an institutional hesitance to practice universal precautions, probably because of the limited resources. The hospital was not prepared for the kind of epidemic it's now facing — nor was the city of about 1.5 million people.
During our time at JFK, we became friends with Dr. Brisbane. We learned that he'd trained in Germany in the 1970s, had returned to Liberia to work, and had chosen to stay through the civil war and during Charles Taylor's despotic rule, continuing to see patients despite the bloodshed around him. He had welcomed the country's new democratic leadership and a new female administrator at the hospital — a first. He ran a successful coffee plantation and gave us bags of coffee every time we visited him. He was the father of eight biologic children and six adopted children, and he had numerous grandchildren around the world.
Within a few days after our return to Monrovia in June 2014, the city's first patients with Ebola presented at Redemption, the county hospital, and we soon got word that a doctor and some nurses there had died. Rumors were rampant, and staff quickly abandoned that hospital. At JFK, our colleagues grew nervous. There were tensions between the hospital administration and the public health ministry. There was no clear plan for what to do if a patient suspected of having Ebola showed up at the hospital. How would staff members protect themselves? How would they isolate the patient? How could they move the patient to one of the ministry's isolation centers? Dr. Brisbane was a wreck. He chattered nervously, his smile disappeared when he thought we weren't watching, and he openly wondered how he could protect himself. He told us bluntly, “Leave Monrovia.”
Then one morning, we arrived at the hospital at 7 o'clock and ran into Dr. Philip Zokonis Ireland, one of our young doctor friends. He was agitated, his fear evident in his face: there was a patient in the ED with suspected Ebola. The patient had lain in a bed in one of the small, crowded treatment areas for 6 hours, surrounded by nurses and other patients, until someone recognized his symptoms. We rushed to the room and met Dr. Brisbane and Dr. Abraham Borbor, the head of internal medicine. Others were sensing that something was wrong. Patients and their family members quickly disappeared, and nurses hung far back in the hallway.
The first priority was to get the patient out of the common room and into an isolation room, but the bed he was lying on was too wide for the doorway. So Dr. Brisbane, Dr. Borbor, and two custodians hastily donned gowns, gloves, and masks, then lifted the patient — mattress and all — and carried him into the isolation room, nearly dropping him in the process. The man had begun gasping for breath, and despite their efforts, within 5 minutes he was dead. Later that day, laboratory tests confirmed that he was indeed infected with Ebola virus. His body stayed in the now-otherwise-empty ED until it was retrieved hours later by the health ministry.
We remained in Monrovia for the next week and helped however we could. Dr. Brisbane brought his own thermometer and checked his temperature religiously, fearing the telltale sudden fever. He wore a fedora in the hospital as a protective talisman. And yet he still joked with us, displaying a sort of gallows humor.
A few days after we'd returned to the States, we got a call from a friend in Monrovia saying that Dr. Brisbane was in isolation and had tested positive for Ebola. The next call informed us of his death and hasty burial on his plantation. By late August, Dr. Ireland and one of the nurses we knew had contracted Ebola and were fighting for survival, and Dr. Borbor and a physician assistant who'd worked in the ED had died from the virus.
Dr. Brisbane didn't have to stay at JFK and continue to care for patients. He could easily have retired to his coffee plantation with his wife and children and grandchildren. He was terrified of Ebola, and yet we knew that every morning when we entered the ED, we'd find him there, seeing his patients.
Doctors and nurses have a duty of care toward their patients.1 We're expected, on the basis of our training and an unwritten social contract, to fulfill that duty even in less-than-ideal circumstances — in the face of depleted resources, for example, or undesirable patients. But we also have a duty to ourselves and our families, and when our work becomes life-threatening, we have to decide what benefit we will be to our patients and what cost it will exact from us. In such circumstances, we cannot be expected to uphold the same duty of care. But during the world's worst Ebola outbreak to date, clinicians like Dr. Brisbane are on the front lines — and are dying as a result. They care for patients despite the risks to themselves, despite the inadequate supplies and infrastructure, despite their insufficient training in infection control.
Dr. Sam Brisbane's death diminishes us as a people. But with apologies to his wife and family, who saw him die horribly and unjustly, and despite the deep loss we feel, we believe our friend died a good death — as did all the nurses and doctors who have sacrificed themselves caring for patients with this awful disease.
Note: This article was reprinted with permission of the New England Journal of Medicine (10.1056/NEJMp1410301).
References
1. Sokol, D.K. Virulent epidemics and scope of healthcare workers' duty of care. Emerg Infect Dis 2006;12:1238-1241
A friend of ours, Dr. Sam Brisbane, died recently. He was a Liberian doctor, and he died from Ebola, a horrible, nightmarish disease.
Information coming out of Liberia has been scarce. Since Dr. Brisbane's death, we've learned that other doctors and nurses with whom we've worked have also contracted Ebola and have died or are being treated in the types of rudimentary facilities we see on the news. As we live in dread of each phone call, questions about how we die and what we're willing to die for have been weighing on us.
The ancients had a concept of a “good death” — dying for one's country, for example, or gloriously on the battlefield. Solon, the sage of Athens, argued that one couldn't judge a person's happiness until one knew the manner of his death. The Greeks recognized that we're all destined to die and that the best we can hope for is a death that benefits our family or humanity.
For emergency-medicine clinicians like us, the concept of a good death can seem too abstract, intangible. Rarely are the deaths we see good or beneficial. We see young people who die in the throes of trauma; grandparents who die at the end of a long, debilitating illness; people who kill themselves; people who die from their excesses, whether of alcohol, food, or smoking.
Last year, as part of a new disaster-medicine fellowship program, we developed a partnership with John F. Kennedy Memorial Medical Center in Monrovia, the only academic referral hospital in Liberia. We collaborated with the hospital administration to develop disaster-planning and resilience programs and teamed up with the emergency department (ED) staff to enhance medical training and establish epidemiologic studies of trauma. It was there that we met Dr. Brisbane, the ED director. He immediately struck us as a genuine ED doc — at once caring and profane, light-hearted one minute, intense the next. A short, bald man with weathered skin and thick glasses, he spoke openly and easily; his laugh was best described as a giggle, and he swore frequently.
When we conducted an initial vulnerability analysis for the hospital, we discussed our concerns about severe supply and personnel shortages, regular power outages, and occasional electrical fires. Dr. Brisbane replied that what scared him the most was the potential for an epidemic of some viral hemorrhagic fever. He was right to be scared. We encountered rationing of gloves, a limited supply of hand soap, and an institutional hesitance to practice universal precautions, probably because of the limited resources. The hospital was not prepared for the kind of epidemic it's now facing — nor was the city of about 1.5 million people.
During our time at JFK, we became friends with Dr. Brisbane. We learned that he'd trained in Germany in the 1970s, had returned to Liberia to work, and had chosen to stay through the civil war and during Charles Taylor's despotic rule, continuing to see patients despite the bloodshed around him. He had welcomed the country's new democratic leadership and a new female administrator at the hospital — a first. He ran a successful coffee plantation and gave us bags of coffee every time we visited him. He was the father of eight biologic children and six adopted children, and he had numerous grandchildren around the world.
Within a few days after our return to Monrovia in June 2014, the city's first patients with Ebola presented at Redemption, the county hospital, and we soon got word that a doctor and some nurses there had died. Rumors were rampant, and staff quickly abandoned that hospital. At JFK, our colleagues grew nervous. There were tensions between the hospital administration and the public health ministry. There was no clear plan for what to do if a patient suspected of having Ebola showed up at the hospital. How would staff members protect themselves? How would they isolate the patient? How could they move the patient to one of the ministry's isolation centers? Dr. Brisbane was a wreck. He chattered nervously, his smile disappeared when he thought we weren't watching, and he openly wondered how he could protect himself. He told us bluntly, “Leave Monrovia.”
Then one morning, we arrived at the hospital at 7 o'clock and ran into Dr. Philip Zokonis Ireland, one of our young doctor friends. He was agitated, his fear evident in his face: there was a patient in the ED with suspected Ebola. The patient had lain in a bed in one of the small, crowded treatment areas for 6 hours, surrounded by nurses and other patients, until someone recognized his symptoms. We rushed to the room and met Dr. Brisbane and Dr. Abraham Borbor, the head of internal medicine. Others were sensing that something was wrong. Patients and their family members quickly disappeared, and nurses hung far back in the hallway.
The first priority was to get the patient out of the common room and into an isolation room, but the bed he was lying on was too wide for the doorway. So Dr. Brisbane, Dr. Borbor, and two custodians hastily donned gowns, gloves, and masks, then lifted the patient — mattress and all — and carried him into the isolation room, nearly dropping him in the process. The man had begun gasping for breath, and despite their efforts, within 5 minutes he was dead. Later that day, laboratory tests confirmed that he was indeed infected with Ebola virus. His body stayed in the now-otherwise-empty ED until it was retrieved hours later by the health ministry.
We remained in Monrovia for the next week and helped however we could. Dr. Brisbane brought his own thermometer and checked his temperature religiously, fearing the telltale sudden fever. He wore a fedora in the hospital as a protective talisman. And yet he still joked with us, displaying a sort of gallows humor.
A few days after we'd returned to the States, we got a call from a friend in Monrovia saying that Dr. Brisbane was in isolation and had tested positive for Ebola. The next call informed us of his death and hasty burial on his plantation. By late August, Dr. Ireland and one of the nurses we knew had contracted Ebola and were fighting for survival, and Dr. Borbor and a physician assistant who'd worked in the ED had died from the virus.
Dr. Brisbane didn't have to stay at JFK and continue to care for patients. He could easily have retired to his coffee plantation with his wife and children and grandchildren. He was terrified of Ebola, and yet we knew that every morning when we entered the ED, we'd find him there, seeing his patients.
Doctors and nurses have a duty of care toward their patients.1 We're expected, on the basis of our training and an unwritten social contract, to fulfill that duty even in less-than-ideal circumstances — in the face of depleted resources, for example, or undesirable patients. But we also have a duty to ourselves and our families, and when our work becomes life-threatening, we have to decide what benefit we will be to our patients and what cost it will exact from us. In such circumstances, we cannot be expected to uphold the same duty of care. But during the world's worst Ebola outbreak to date, clinicians like Dr. Brisbane are on the front lines — and are dying as a result. They care for patients despite the risks to themselves, despite the inadequate supplies and infrastructure, despite their insufficient training in infection control.
Dr. Sam Brisbane's death diminishes us as a people. But with apologies to his wife and family, who saw him die horribly and unjustly, and despite the deep loss we feel, we believe our friend died a good death — as did all the nurses and doctors who have sacrificed themselves caring for patients with this awful disease.
Note: This article was reprinted with permission of the New England Journal of Medicine (10.1056/NEJMp1410301).
References
1. Sokol, D.K. Virulent epidemics and scope of healthcare workers' duty of care. Emerg Infect Dis 2006;12:1238-1241
Quick tips for taming toxic feelings
Our team has been involved with a patient whose hospital stay is measured not in days, weeks, or months but in years. The scope of the problems surrounding not only the medical issues but also the family dynamic, out-of-hospital support system, and social situation can easily make the most seasoned providers feel paralyzed. It is fair to use words such as helpless, hopeless, overwhelmed, angry, confused, and conflicted for how the dozens of team members caring for this patient feel on a regular basis.
While the details of this case would eat up multiple columns, it might better for the reader to recall when a patient whose case you’ve been involved in has brought out strong, and often times, negative emotions from the faculty and staff that resulted in conflict. Finding a path to resolve conflict can be one of the most rewarding aspects of working within a system that relies on teamwork for desired, successful outcomes.
Toxic feelings build up easily in cases in which it seems like there is no end, no chance for our good intentions and hard work to salvage a patient’s spiraling course. Conflict with those we work with is almost inevitable. Hospitalists disagree with the surgeons, nursing staff and the pain team aren’t on the same page, hospital administration might seem to have the C-suite agenda. It is in times like this that conflict resolution smarts can preserve the peace. While maintaining a collegial attitude might not ultimately transform the patient’s outcome, the presence of on-going conflict amongst health care professionals is well studied and studded with unwanted outcomes.
There are conflict resolution books, courses, and even graduate degrees. For the hospitalist on the run, what principles can be applied immediately?
• Don’t react. Think it through. Emotions are strong and feelings differ. This is the crucible for doing something we might later regret. If you hear or read something that makes you upset, then first assume that your colleague has attempted to craft the absolute best plan for the patient. Before making statements or casting judgment, ask many questions. Never send a text or e-mail, make a phone call, or approach someone when you’re having trouble suppressing strong emotions.
• Practice active listening. This takes concentration, so rid yourself of distractions while engaging in listening. Use body language such as smiling or nodding to acknowledge the other person’s message. Pay attention to their gesticulations and nonverbal cues to their position. Avoid interruptions and defer judgment until they have finished. Summarize what you’ve heard from them to demonstrate that their message has been received. Even when you heartily disagree, show respect. We have the ability to find ways of being both candid and kind in our responses.
• Always go after the problem. It is too easy to attack a person rather than the underlying issue. Everyone involved in the care of a patient has the potential to contribute to the solution. By staying focused on the problem and not the people, you are building trust with your colleagues.
• Continue to show up. We all tire and become jaded with these marathon cases. Throwing your hands up in surrender and failing to remain a participating member of the team will send a message that you are not accepting responsibility. Showing up shouts that you’re committed.
• Be mindful of the words used in communication. Focus on "I" statements rather than "You" statements.As in "I need feedback on the plan of care I’ve proposed," rather than "You haven’t provided me with feedback on my plan." ... "I need more information before deciding on whether this procedure would be beneficial," rather than, "You haven’t told me why the procedure needs to be done now."
• Find common goals. Don’t spend time picking apart a nurse’s point of view or your consultant’s idea to work-up diagnoses that seem farfetched. Instead agree upon a goal or two and ask how we can get there together.
• Look toward tomorrow. Focusing on what has happened in the past leads only to war, litigation, or stagnation. Talking about how you can go forward from here can help reset the dynamic.
Like negotiation tools we’ve previously written about conflict resolution skills have broad applicability outside the work place and can make life better in a variety of situations.
As always if you’d like suggested readings or resources please contact us.
Dr. Bekanich and Dr. Leigh A. Fredholm are codirectors of Seton Palliative Care, part of the University of Texas Southwestern residency programs in Austin. They alternate contributions to the monthly Palliatively Speaking blog.
Our team has been involved with a patient whose hospital stay is measured not in days, weeks, or months but in years. The scope of the problems surrounding not only the medical issues but also the family dynamic, out-of-hospital support system, and social situation can easily make the most seasoned providers feel paralyzed. It is fair to use words such as helpless, hopeless, overwhelmed, angry, confused, and conflicted for how the dozens of team members caring for this patient feel on a regular basis.
While the details of this case would eat up multiple columns, it might better for the reader to recall when a patient whose case you’ve been involved in has brought out strong, and often times, negative emotions from the faculty and staff that resulted in conflict. Finding a path to resolve conflict can be one of the most rewarding aspects of working within a system that relies on teamwork for desired, successful outcomes.
Toxic feelings build up easily in cases in which it seems like there is no end, no chance for our good intentions and hard work to salvage a patient’s spiraling course. Conflict with those we work with is almost inevitable. Hospitalists disagree with the surgeons, nursing staff and the pain team aren’t on the same page, hospital administration might seem to have the C-suite agenda. It is in times like this that conflict resolution smarts can preserve the peace. While maintaining a collegial attitude might not ultimately transform the patient’s outcome, the presence of on-going conflict amongst health care professionals is well studied and studded with unwanted outcomes.
There are conflict resolution books, courses, and even graduate degrees. For the hospitalist on the run, what principles can be applied immediately?
• Don’t react. Think it through. Emotions are strong and feelings differ. This is the crucible for doing something we might later regret. If you hear or read something that makes you upset, then first assume that your colleague has attempted to craft the absolute best plan for the patient. Before making statements or casting judgment, ask many questions. Never send a text or e-mail, make a phone call, or approach someone when you’re having trouble suppressing strong emotions.
• Practice active listening. This takes concentration, so rid yourself of distractions while engaging in listening. Use body language such as smiling or nodding to acknowledge the other person’s message. Pay attention to their gesticulations and nonverbal cues to their position. Avoid interruptions and defer judgment until they have finished. Summarize what you’ve heard from them to demonstrate that their message has been received. Even when you heartily disagree, show respect. We have the ability to find ways of being both candid and kind in our responses.
• Always go after the problem. It is too easy to attack a person rather than the underlying issue. Everyone involved in the care of a patient has the potential to contribute to the solution. By staying focused on the problem and not the people, you are building trust with your colleagues.
• Continue to show up. We all tire and become jaded with these marathon cases. Throwing your hands up in surrender and failing to remain a participating member of the team will send a message that you are not accepting responsibility. Showing up shouts that you’re committed.
• Be mindful of the words used in communication. Focus on "I" statements rather than "You" statements.As in "I need feedback on the plan of care I’ve proposed," rather than "You haven’t provided me with feedback on my plan." ... "I need more information before deciding on whether this procedure would be beneficial," rather than, "You haven’t told me why the procedure needs to be done now."
• Find common goals. Don’t spend time picking apart a nurse’s point of view or your consultant’s idea to work-up diagnoses that seem farfetched. Instead agree upon a goal or two and ask how we can get there together.
• Look toward tomorrow. Focusing on what has happened in the past leads only to war, litigation, or stagnation. Talking about how you can go forward from here can help reset the dynamic.
Like negotiation tools we’ve previously written about conflict resolution skills have broad applicability outside the work place and can make life better in a variety of situations.
As always if you’d like suggested readings or resources please contact us.
Dr. Bekanich and Dr. Leigh A. Fredholm are codirectors of Seton Palliative Care, part of the University of Texas Southwestern residency programs in Austin. They alternate contributions to the monthly Palliatively Speaking blog.
Our team has been involved with a patient whose hospital stay is measured not in days, weeks, or months but in years. The scope of the problems surrounding not only the medical issues but also the family dynamic, out-of-hospital support system, and social situation can easily make the most seasoned providers feel paralyzed. It is fair to use words such as helpless, hopeless, overwhelmed, angry, confused, and conflicted for how the dozens of team members caring for this patient feel on a regular basis.
While the details of this case would eat up multiple columns, it might better for the reader to recall when a patient whose case you’ve been involved in has brought out strong, and often times, negative emotions from the faculty and staff that resulted in conflict. Finding a path to resolve conflict can be one of the most rewarding aspects of working within a system that relies on teamwork for desired, successful outcomes.
Toxic feelings build up easily in cases in which it seems like there is no end, no chance for our good intentions and hard work to salvage a patient’s spiraling course. Conflict with those we work with is almost inevitable. Hospitalists disagree with the surgeons, nursing staff and the pain team aren’t on the same page, hospital administration might seem to have the C-suite agenda. It is in times like this that conflict resolution smarts can preserve the peace. While maintaining a collegial attitude might not ultimately transform the patient’s outcome, the presence of on-going conflict amongst health care professionals is well studied and studded with unwanted outcomes.
There are conflict resolution books, courses, and even graduate degrees. For the hospitalist on the run, what principles can be applied immediately?
• Don’t react. Think it through. Emotions are strong and feelings differ. This is the crucible for doing something we might later regret. If you hear or read something that makes you upset, then first assume that your colleague has attempted to craft the absolute best plan for the patient. Before making statements or casting judgment, ask many questions. Never send a text or e-mail, make a phone call, or approach someone when you’re having trouble suppressing strong emotions.
• Practice active listening. This takes concentration, so rid yourself of distractions while engaging in listening. Use body language such as smiling or nodding to acknowledge the other person’s message. Pay attention to their gesticulations and nonverbal cues to their position. Avoid interruptions and defer judgment until they have finished. Summarize what you’ve heard from them to demonstrate that their message has been received. Even when you heartily disagree, show respect. We have the ability to find ways of being both candid and kind in our responses.
• Always go after the problem. It is too easy to attack a person rather than the underlying issue. Everyone involved in the care of a patient has the potential to contribute to the solution. By staying focused on the problem and not the people, you are building trust with your colleagues.
• Continue to show up. We all tire and become jaded with these marathon cases. Throwing your hands up in surrender and failing to remain a participating member of the team will send a message that you are not accepting responsibility. Showing up shouts that you’re committed.
• Be mindful of the words used in communication. Focus on "I" statements rather than "You" statements.As in "I need feedback on the plan of care I’ve proposed," rather than "You haven’t provided me with feedback on my plan." ... "I need more information before deciding on whether this procedure would be beneficial," rather than, "You haven’t told me why the procedure needs to be done now."
• Find common goals. Don’t spend time picking apart a nurse’s point of view or your consultant’s idea to work-up diagnoses that seem farfetched. Instead agree upon a goal or two and ask how we can get there together.
• Look toward tomorrow. Focusing on what has happened in the past leads only to war, litigation, or stagnation. Talking about how you can go forward from here can help reset the dynamic.
Like negotiation tools we’ve previously written about conflict resolution skills have broad applicability outside the work place and can make life better in a variety of situations.
As always if you’d like suggested readings or resources please contact us.
Dr. Bekanich and Dr. Leigh A. Fredholm are codirectors of Seton Palliative Care, part of the University of Texas Southwestern residency programs in Austin. They alternate contributions to the monthly Palliatively Speaking blog.
Five reasons physicians will use mobile health for patient care
Mobile health technologies will become a part of the health care landscape for all stakeholders at some point. Other sectors of society currently cannot function without mobile; for example, retail and financial services consider mobile a vital component of their business models.
There are many reasons for lag in adoption of mobile technologies by health care. Regulatory issues, the need for a digital cultural shift, lack of business models, and lack of proof of efficacy are certainly barriers.
But what is underappreciated by app developers and industry analysts is the fact that physicians will be key players in the future of mobile health. Physicians are the most trusted stakeholder by patients with regard to care planning. Issues that are important to consider from a clinician’s standpoint are reimbursement for coordinating digital care; the fresh, negative experience of poorly performing electronic health records (which should not be the face of other digital tech); the present lack of commitment to the philosophy of participatory medicine and that most health apps are consumer (not patient) oriented, with little proof of efficacy via clinical studies.
That said, there remain fundamental reasons that mobile health app prescribing will occur:
• Patients are mobile. According to the Pew Research Center’s Internet and American Life Project, 91% of adults in the United States own a cell phone. Few older adults use smart phones (18% in 2013), but effective mobile health can take the form of text messages, as has been proven with prenatal care and smoking cessation, as well as more sophisticated disease management apps such as WellDoc. Even though older patients might not be smart phone users now, a baby boomer turns 65 every 8 seconds. Many in the sandwich generation today and all in the future will be mobile health tech ready.
• There is a perfect storm of necessity and opportunity. The number of patients participating in health care has increased because of the Affordable Care Act. There is a well-recognized physician shortage, especially in primary care. Americans today do not want to live out their last years in an institutional setting as 70% of them do today. Digital technology will be required for this aging at home. Sensor technology, whether environmental or wearable, will be fundamental. Mobile technology not only will facilitate new care models, but will create them.
• Useful information and data will be at patients’ fingertips. New technologies – such as IBM’s Watson and Apple’s HealthKit – will hopefully serve as frameworks for many disease-specific apps. EHRs are repositories of huge amounts of data. The key to better health care lies in applying analytics to harness the power of this data and make it useful for better care on both population and individualized patient levels. Analytics will improve patient safety, proscribe therapy based on individual and population data, and increase efficiency.
• It is how patient content will be delivered. Physicians and health policy experts recognize the need for better patient education with regard to their diagnoses and medications. A research2guidance report on the disappointing diabetes app market illustrates the pharmaceutical industry’s heretofore slow uptake of mobile health. In general, the pharmaceutical and medical device industries (with 250 of the approximately 100,000 health and fitness apps) have so far concentrated on disease-specific content. The challenge remains to design apps that center on the clinician-patient interaction, not just the disease state. Interoperability with EHRs via more robust patient portals will help close this loop.
• It will create the engaged patient. "Patient engagement" is as overused as "innovation" when discussing technology in health care today. However, the concept is paramount to improving health and promoting wellness. I like a definition of patient engagement from the Center for Advanced Health: "Actions individuals must take to obtain the greatest benefit from the health care services available to them."
I believe that the basis of patient engagement is the combination of an informed patient (and caregiver) and shared decision making. It is not surprising that a significant percentage of patients leave the hospital or physician’s office not knowing their diagnosis or why a medication was prescribed. Mobile health is the potential holy grail of patient engagement. Behavioral change by both patients and providers in the broad sense (which includes payers, clinicians, and institutions) is imperative to affect patient engagement.
Health care must, for the first time, be approached as a rightful partnership between the patient and physician. I believe that mobile technology can utilize trending patient-derived data, transforming it into a useful actionable tool, and create a multidirectional (patient, provider, caregiver) platform of communication leading to better shared decision making.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
Mobile health technologies will become a part of the health care landscape for all stakeholders at some point. Other sectors of society currently cannot function without mobile; for example, retail and financial services consider mobile a vital component of their business models.
There are many reasons for lag in adoption of mobile technologies by health care. Regulatory issues, the need for a digital cultural shift, lack of business models, and lack of proof of efficacy are certainly barriers.
But what is underappreciated by app developers and industry analysts is the fact that physicians will be key players in the future of mobile health. Physicians are the most trusted stakeholder by patients with regard to care planning. Issues that are important to consider from a clinician’s standpoint are reimbursement for coordinating digital care; the fresh, negative experience of poorly performing electronic health records (which should not be the face of other digital tech); the present lack of commitment to the philosophy of participatory medicine and that most health apps are consumer (not patient) oriented, with little proof of efficacy via clinical studies.
That said, there remain fundamental reasons that mobile health app prescribing will occur:
• Patients are mobile. According to the Pew Research Center’s Internet and American Life Project, 91% of adults in the United States own a cell phone. Few older adults use smart phones (18% in 2013), but effective mobile health can take the form of text messages, as has been proven with prenatal care and smoking cessation, as well as more sophisticated disease management apps such as WellDoc. Even though older patients might not be smart phone users now, a baby boomer turns 65 every 8 seconds. Many in the sandwich generation today and all in the future will be mobile health tech ready.
• There is a perfect storm of necessity and opportunity. The number of patients participating in health care has increased because of the Affordable Care Act. There is a well-recognized physician shortage, especially in primary care. Americans today do not want to live out their last years in an institutional setting as 70% of them do today. Digital technology will be required for this aging at home. Sensor technology, whether environmental or wearable, will be fundamental. Mobile technology not only will facilitate new care models, but will create them.
• Useful information and data will be at patients’ fingertips. New technologies – such as IBM’s Watson and Apple’s HealthKit – will hopefully serve as frameworks for many disease-specific apps. EHRs are repositories of huge amounts of data. The key to better health care lies in applying analytics to harness the power of this data and make it useful for better care on both population and individualized patient levels. Analytics will improve patient safety, proscribe therapy based on individual and population data, and increase efficiency.
• It is how patient content will be delivered. Physicians and health policy experts recognize the need for better patient education with regard to their diagnoses and medications. A research2guidance report on the disappointing diabetes app market illustrates the pharmaceutical industry’s heretofore slow uptake of mobile health. In general, the pharmaceutical and medical device industries (with 250 of the approximately 100,000 health and fitness apps) have so far concentrated on disease-specific content. The challenge remains to design apps that center on the clinician-patient interaction, not just the disease state. Interoperability with EHRs via more robust patient portals will help close this loop.
• It will create the engaged patient. "Patient engagement" is as overused as "innovation" when discussing technology in health care today. However, the concept is paramount to improving health and promoting wellness. I like a definition of patient engagement from the Center for Advanced Health: "Actions individuals must take to obtain the greatest benefit from the health care services available to them."
I believe that the basis of patient engagement is the combination of an informed patient (and caregiver) and shared decision making. It is not surprising that a significant percentage of patients leave the hospital or physician’s office not knowing their diagnosis or why a medication was prescribed. Mobile health is the potential holy grail of patient engagement. Behavioral change by both patients and providers in the broad sense (which includes payers, clinicians, and institutions) is imperative to affect patient engagement.
Health care must, for the first time, be approached as a rightful partnership between the patient and physician. I believe that mobile technology can utilize trending patient-derived data, transforming it into a useful actionable tool, and create a multidirectional (patient, provider, caregiver) platform of communication leading to better shared decision making.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
Mobile health technologies will become a part of the health care landscape for all stakeholders at some point. Other sectors of society currently cannot function without mobile; for example, retail and financial services consider mobile a vital component of their business models.
There are many reasons for lag in adoption of mobile technologies by health care. Regulatory issues, the need for a digital cultural shift, lack of business models, and lack of proof of efficacy are certainly barriers.
But what is underappreciated by app developers and industry analysts is the fact that physicians will be key players in the future of mobile health. Physicians are the most trusted stakeholder by patients with regard to care planning. Issues that are important to consider from a clinician’s standpoint are reimbursement for coordinating digital care; the fresh, negative experience of poorly performing electronic health records (which should not be the face of other digital tech); the present lack of commitment to the philosophy of participatory medicine and that most health apps are consumer (not patient) oriented, with little proof of efficacy via clinical studies.
That said, there remain fundamental reasons that mobile health app prescribing will occur:
• Patients are mobile. According to the Pew Research Center’s Internet and American Life Project, 91% of adults in the United States own a cell phone. Few older adults use smart phones (18% in 2013), but effective mobile health can take the form of text messages, as has been proven with prenatal care and smoking cessation, as well as more sophisticated disease management apps such as WellDoc. Even though older patients might not be smart phone users now, a baby boomer turns 65 every 8 seconds. Many in the sandwich generation today and all in the future will be mobile health tech ready.
• There is a perfect storm of necessity and opportunity. The number of patients participating in health care has increased because of the Affordable Care Act. There is a well-recognized physician shortage, especially in primary care. Americans today do not want to live out their last years in an institutional setting as 70% of them do today. Digital technology will be required for this aging at home. Sensor technology, whether environmental or wearable, will be fundamental. Mobile technology not only will facilitate new care models, but will create them.
• Useful information and data will be at patients’ fingertips. New technologies – such as IBM’s Watson and Apple’s HealthKit – will hopefully serve as frameworks for many disease-specific apps. EHRs are repositories of huge amounts of data. The key to better health care lies in applying analytics to harness the power of this data and make it useful for better care on both population and individualized patient levels. Analytics will improve patient safety, proscribe therapy based on individual and population data, and increase efficiency.
• It is how patient content will be delivered. Physicians and health policy experts recognize the need for better patient education with regard to their diagnoses and medications. A research2guidance report on the disappointing diabetes app market illustrates the pharmaceutical industry’s heretofore slow uptake of mobile health. In general, the pharmaceutical and medical device industries (with 250 of the approximately 100,000 health and fitness apps) have so far concentrated on disease-specific content. The challenge remains to design apps that center on the clinician-patient interaction, not just the disease state. Interoperability with EHRs via more robust patient portals will help close this loop.
• It will create the engaged patient. "Patient engagement" is as overused as "innovation" when discussing technology in health care today. However, the concept is paramount to improving health and promoting wellness. I like a definition of patient engagement from the Center for Advanced Health: "Actions individuals must take to obtain the greatest benefit from the health care services available to them."
I believe that the basis of patient engagement is the combination of an informed patient (and caregiver) and shared decision making. It is not surprising that a significant percentage of patients leave the hospital or physician’s office not knowing their diagnosis or why a medication was prescribed. Mobile health is the potential holy grail of patient engagement. Behavioral change by both patients and providers in the broad sense (which includes payers, clinicians, and institutions) is imperative to affect patient engagement.
Health care must, for the first time, be approached as a rightful partnership between the patient and physician. I believe that mobile technology can utilize trending patient-derived data, transforming it into a useful actionable tool, and create a multidirectional (patient, provider, caregiver) platform of communication leading to better shared decision making.
Dr. Scher is an electrophysiologist with the Heart Group of Lancaster (Pa.) General Health. He is also director of DLS Healthcare Consulting, Harrisburg, Pa., and clinical associate professor of medicine at the Pennsylvania State University, Hershey.
Share supportive care while we wait for an Ebola remedy
While necessary vaccine research proceeds, resource-rich communities need to open their storage closets to send supportive care to where it is needed. Since the outbreak of Ebola in West Africa, and the subsequent introduction of an experimental treatment that may represent a cure for the disease, many have questioned how we expedite this research opportunity, while maximizing the integrity of the data and assisting those who do not have access to treatments.
Both of the Americans and a few others who received the controversial ZMapp treatment have survived. However, two individuals who received it still died. This inconsistency sparked a media campaign that focused on the ineffectiveness of the treatment. The campaign was launched despite the fact that there is currently no way to understand, completely, the reasons some have lived and some have died. That is the purpose of the research.
The accelerated research process sanctioned by the National Institutes of Health and the World Health Organization is an attempt to develop a much-needed vaccine to stop the spread. Unfortunately, research takes time and, if rushed, may result in inaccurate data that may erroneously find the vaccine effective. Exposing people to such a vaccine could cause further harm on many levels (side effects, death, loss of trust in medicine, etc.). Conversely, the hurried research could mistakenly find the vaccine ineffective and prevent entire populations from receiving a lifesaving treatment.
What to do right now
The harmful ramifications of getting this wrong for an already underserved, underprivileged, resource-poor, and extremely vulnerable population would be greater than anyone intends. The panel of experts convened by the World Health Organization determined that expediting the research can be ethically justified due to the emergent nature of the problem. Here we must agree that it should proceed, but insist on it proceeding properly. In the meantime, countries with resources need to determine what actions to take while people are suffering and dying.
The ethically correct action to take is to commit to our responsibilities as members of the global community and offer the supportive assistance that has already been shown to be helpful in managing the symptoms of the disease. Public health workers have enacted preventive strategies to contain the disease and decrease the spread. We in the United States should focus on providing the necessary interventions currently needed to support the masses effectively and less on gearing up to deal with the potential of having one or two people who happen to make it to our borders.
We have the resources in abundance, and it is our ethical duty to play an integral role in protecting others when we can. The media needs to put focus on countries and organizations that are sharing their resources and highlight ways for others to come together in support. We can play an integral part in addressing this crisis while time is taken to work on the research and set a precedent for dealing with such outbreaks in the future, come what may.
Nneka O. Mokwunye, Ph.D., is director of the Center for Ethics, Spiritual Care Department, at MedStar Washington Hospital Center and executive director of its Journal of Hospital Ethics.
While necessary vaccine research proceeds, resource-rich communities need to open their storage closets to send supportive care to where it is needed. Since the outbreak of Ebola in West Africa, and the subsequent introduction of an experimental treatment that may represent a cure for the disease, many have questioned how we expedite this research opportunity, while maximizing the integrity of the data and assisting those who do not have access to treatments.
Both of the Americans and a few others who received the controversial ZMapp treatment have survived. However, two individuals who received it still died. This inconsistency sparked a media campaign that focused on the ineffectiveness of the treatment. The campaign was launched despite the fact that there is currently no way to understand, completely, the reasons some have lived and some have died. That is the purpose of the research.
The accelerated research process sanctioned by the National Institutes of Health and the World Health Organization is an attempt to develop a much-needed vaccine to stop the spread. Unfortunately, research takes time and, if rushed, may result in inaccurate data that may erroneously find the vaccine effective. Exposing people to such a vaccine could cause further harm on many levels (side effects, death, loss of trust in medicine, etc.). Conversely, the hurried research could mistakenly find the vaccine ineffective and prevent entire populations from receiving a lifesaving treatment.
What to do right now
The harmful ramifications of getting this wrong for an already underserved, underprivileged, resource-poor, and extremely vulnerable population would be greater than anyone intends. The panel of experts convened by the World Health Organization determined that expediting the research can be ethically justified due to the emergent nature of the problem. Here we must agree that it should proceed, but insist on it proceeding properly. In the meantime, countries with resources need to determine what actions to take while people are suffering and dying.
The ethically correct action to take is to commit to our responsibilities as members of the global community and offer the supportive assistance that has already been shown to be helpful in managing the symptoms of the disease. Public health workers have enacted preventive strategies to contain the disease and decrease the spread. We in the United States should focus on providing the necessary interventions currently needed to support the masses effectively and less on gearing up to deal with the potential of having one or two people who happen to make it to our borders.
We have the resources in abundance, and it is our ethical duty to play an integral role in protecting others when we can. The media needs to put focus on countries and organizations that are sharing their resources and highlight ways for others to come together in support. We can play an integral part in addressing this crisis while time is taken to work on the research and set a precedent for dealing with such outbreaks in the future, come what may.
Nneka O. Mokwunye, Ph.D., is director of the Center for Ethics, Spiritual Care Department, at MedStar Washington Hospital Center and executive director of its Journal of Hospital Ethics.
While necessary vaccine research proceeds, resource-rich communities need to open their storage closets to send supportive care to where it is needed. Since the outbreak of Ebola in West Africa, and the subsequent introduction of an experimental treatment that may represent a cure for the disease, many have questioned how we expedite this research opportunity, while maximizing the integrity of the data and assisting those who do not have access to treatments.
Both of the Americans and a few others who received the controversial ZMapp treatment have survived. However, two individuals who received it still died. This inconsistency sparked a media campaign that focused on the ineffectiveness of the treatment. The campaign was launched despite the fact that there is currently no way to understand, completely, the reasons some have lived and some have died. That is the purpose of the research.
The accelerated research process sanctioned by the National Institutes of Health and the World Health Organization is an attempt to develop a much-needed vaccine to stop the spread. Unfortunately, research takes time and, if rushed, may result in inaccurate data that may erroneously find the vaccine effective. Exposing people to such a vaccine could cause further harm on many levels (side effects, death, loss of trust in medicine, etc.). Conversely, the hurried research could mistakenly find the vaccine ineffective and prevent entire populations from receiving a lifesaving treatment.
What to do right now
The harmful ramifications of getting this wrong for an already underserved, underprivileged, resource-poor, and extremely vulnerable population would be greater than anyone intends. The panel of experts convened by the World Health Organization determined that expediting the research can be ethically justified due to the emergent nature of the problem. Here we must agree that it should proceed, but insist on it proceeding properly. In the meantime, countries with resources need to determine what actions to take while people are suffering and dying.
The ethically correct action to take is to commit to our responsibilities as members of the global community and offer the supportive assistance that has already been shown to be helpful in managing the symptoms of the disease. Public health workers have enacted preventive strategies to contain the disease and decrease the spread. We in the United States should focus on providing the necessary interventions currently needed to support the masses effectively and less on gearing up to deal with the potential of having one or two people who happen to make it to our borders.
We have the resources in abundance, and it is our ethical duty to play an integral role in protecting others when we can. The media needs to put focus on countries and organizations that are sharing their resources and highlight ways for others to come together in support. We can play an integral part in addressing this crisis while time is taken to work on the research and set a precedent for dealing with such outbreaks in the future, come what may.
Nneka O. Mokwunye, Ph.D., is director of the Center for Ethics, Spiritual Care Department, at MedStar Washington Hospital Center and executive director of its Journal of Hospital Ethics.
Under Pressure
One of the most important elements of a psoriasis patient’s medical history is the medication list. We are aware of the possible association between induction or exacerbation of psoriasis and intake of drugs including beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, lithium, and nonsteroidal anti-inflammatory drugs. However, we are lacking comprehensive data on these putative relationships.
Wu et al1 evaluated the association of hypertension and antihypertensive medications with risk for psoriasis. The authors pointed out that psoriasis patients have an increased risk for hypertension, and medications for hypertension, especially beta-blockers, have been associated with the development of psoriasis. They noted, however, that there has been no prospective assessment of the association of existing hypertension and antihypertensive medications with risk for incident psoriasis.1
The authors performed a prospective cohort study (June 1996 to June 1998) of 77,728 women from the Nurses’ Health Study who provided biennially updated data on hypertension and antihypertensive medications.1 They documented 843 incidents of psoriasis during 1,066,339 person-years of follow-up. Women with hypertension tended to be older and had a higher body mass index. In addition, they had proportionately higher prevalence rates of cardiovascular disease, type 2 diabetes mellitus, and hypercholesterolemia. They also were less physically active than subjects without hypertension. Compared with those with normal blood pressure, women with hypertension lasting 6 years or more were at higher risk for development of psoriasis (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.03-1.57). In stratified analysis, the risk for psoriasis was higher among hypertensive women without medication use (HR, 1.49; 95% CI, 1.15-1.92) and among hypertensive women with current medication use (HR, 1.31; 95% CI, 1.10-1.55) when compared with those without hypertension and without medication use.1
Among the individual antihypertensive drugs, only beta-blockers were associated with an increased risk for psoriasis.1 Of interest, this association persisted in a duration-dependent manner, with a higher risk for psoriasis found among women who regularly used beta-blockers with a duration of use of 6 years or more (HR, 1.39; 95% CI, 1.11-1.73; P for trend=.009). No association was found between any other individual hypertension medication and the development of psoriasis.1
The authors concluded that their study provided evidence that a history of long-term hypertension was associated with an increased risk for psoriasis.1 Among the individual medications analyzed in the study, only beta-blockers were linked to an increased risk for psoriasis after long-term regular use (≥6 years). They noted that these findings provided insights into the relationship between hypertension, medications for the condition, and psoriasis. However, further work is necessary to confirm these findings and clarify the biological mechanisms that may explain these links.1
As we further evaluate the associations between psoriasis and systemic comorbidities, we are learning more about the complex interrelationship between these conditions. The findings reported by Wu et al1 serve as another reminder that clinicians should be proactive in having psoriasis patients actively monitor their blood pressure, either with the dermatologist or with the primary care physician. This type of novel prospective information serves as another piece of the puzzle in our comprehensive management of psoriasis.
- Wu S, Han J, Li WQ, et al. Hypertension, antihypertensive medication use, and risk of psoriasis [published online ahead of print July 2, 2014]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.9957.
One of the most important elements of a psoriasis patient’s medical history is the medication list. We are aware of the possible association between induction or exacerbation of psoriasis and intake of drugs including beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, lithium, and nonsteroidal anti-inflammatory drugs. However, we are lacking comprehensive data on these putative relationships.
Wu et al1 evaluated the association of hypertension and antihypertensive medications with risk for psoriasis. The authors pointed out that psoriasis patients have an increased risk for hypertension, and medications for hypertension, especially beta-blockers, have been associated with the development of psoriasis. They noted, however, that there has been no prospective assessment of the association of existing hypertension and antihypertensive medications with risk for incident psoriasis.1
The authors performed a prospective cohort study (June 1996 to June 1998) of 77,728 women from the Nurses’ Health Study who provided biennially updated data on hypertension and antihypertensive medications.1 They documented 843 incidents of psoriasis during 1,066,339 person-years of follow-up. Women with hypertension tended to be older and had a higher body mass index. In addition, they had proportionately higher prevalence rates of cardiovascular disease, type 2 diabetes mellitus, and hypercholesterolemia. They also were less physically active than subjects without hypertension. Compared with those with normal blood pressure, women with hypertension lasting 6 years or more were at higher risk for development of psoriasis (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.03-1.57). In stratified analysis, the risk for psoriasis was higher among hypertensive women without medication use (HR, 1.49; 95% CI, 1.15-1.92) and among hypertensive women with current medication use (HR, 1.31; 95% CI, 1.10-1.55) when compared with those without hypertension and without medication use.1
Among the individual antihypertensive drugs, only beta-blockers were associated with an increased risk for psoriasis.1 Of interest, this association persisted in a duration-dependent manner, with a higher risk for psoriasis found among women who regularly used beta-blockers with a duration of use of 6 years or more (HR, 1.39; 95% CI, 1.11-1.73; P for trend=.009). No association was found between any other individual hypertension medication and the development of psoriasis.1
The authors concluded that their study provided evidence that a history of long-term hypertension was associated with an increased risk for psoriasis.1 Among the individual medications analyzed in the study, only beta-blockers were linked to an increased risk for psoriasis after long-term regular use (≥6 years). They noted that these findings provided insights into the relationship between hypertension, medications for the condition, and psoriasis. However, further work is necessary to confirm these findings and clarify the biological mechanisms that may explain these links.1
As we further evaluate the associations between psoriasis and systemic comorbidities, we are learning more about the complex interrelationship between these conditions. The findings reported by Wu et al1 serve as another reminder that clinicians should be proactive in having psoriasis patients actively monitor their blood pressure, either with the dermatologist or with the primary care physician. This type of novel prospective information serves as another piece of the puzzle in our comprehensive management of psoriasis.
One of the most important elements of a psoriasis patient’s medical history is the medication list. We are aware of the possible association between induction or exacerbation of psoriasis and intake of drugs including beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, lithium, and nonsteroidal anti-inflammatory drugs. However, we are lacking comprehensive data on these putative relationships.
Wu et al1 evaluated the association of hypertension and antihypertensive medications with risk for psoriasis. The authors pointed out that psoriasis patients have an increased risk for hypertension, and medications for hypertension, especially beta-blockers, have been associated with the development of psoriasis. They noted, however, that there has been no prospective assessment of the association of existing hypertension and antihypertensive medications with risk for incident psoriasis.1
The authors performed a prospective cohort study (June 1996 to June 1998) of 77,728 women from the Nurses’ Health Study who provided biennially updated data on hypertension and antihypertensive medications.1 They documented 843 incidents of psoriasis during 1,066,339 person-years of follow-up. Women with hypertension tended to be older and had a higher body mass index. In addition, they had proportionately higher prevalence rates of cardiovascular disease, type 2 diabetes mellitus, and hypercholesterolemia. They also were less physically active than subjects without hypertension. Compared with those with normal blood pressure, women with hypertension lasting 6 years or more were at higher risk for development of psoriasis (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.03-1.57). In stratified analysis, the risk for psoriasis was higher among hypertensive women without medication use (HR, 1.49; 95% CI, 1.15-1.92) and among hypertensive women with current medication use (HR, 1.31; 95% CI, 1.10-1.55) when compared with those without hypertension and without medication use.1
Among the individual antihypertensive drugs, only beta-blockers were associated with an increased risk for psoriasis.1 Of interest, this association persisted in a duration-dependent manner, with a higher risk for psoriasis found among women who regularly used beta-blockers with a duration of use of 6 years or more (HR, 1.39; 95% CI, 1.11-1.73; P for trend=.009). No association was found between any other individual hypertension medication and the development of psoriasis.1
The authors concluded that their study provided evidence that a history of long-term hypertension was associated with an increased risk for psoriasis.1 Among the individual medications analyzed in the study, only beta-blockers were linked to an increased risk for psoriasis after long-term regular use (≥6 years). They noted that these findings provided insights into the relationship between hypertension, medications for the condition, and psoriasis. However, further work is necessary to confirm these findings and clarify the biological mechanisms that may explain these links.1
As we further evaluate the associations between psoriasis and systemic comorbidities, we are learning more about the complex interrelationship between these conditions. The findings reported by Wu et al1 serve as another reminder that clinicians should be proactive in having psoriasis patients actively monitor their blood pressure, either with the dermatologist or with the primary care physician. This type of novel prospective information serves as another piece of the puzzle in our comprehensive management of psoriasis.
- Wu S, Han J, Li WQ, et al. Hypertension, antihypertensive medication use, and risk of psoriasis [published online ahead of print July 2, 2014]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.9957.
- Wu S, Han J, Li WQ, et al. Hypertension, antihypertensive medication use, and risk of psoriasis [published online ahead of print July 2, 2014]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.9957.
Nostalgic for making diagnoses based on medical history alone? Me, neither
"It is clear that physicians take widely different attitudes towards investigations, some relying on them much more heavily than others."
– J.R. Hampton, et al., British Medical Journal, May 31, 1975
In 1975, a study was published in the British Medical Journal looking at new patients referred to a medical clinic. The study looked specifically at how a diagnosis was made for each new patient, and it concluded that in 66 out of 80 patients, a diagnosis was arrived at based on the history alone, that the physical exam was useful in an additional 7 patients, and that lab testing was useful in the last 7 patients (Br. Med. J. 1975;2:486-9). Since then, medical students have been taught that a good history will lead to the diagnosis about 80% of the time.
One of my professors in my internal medicine class taught me this aphorism. I thought he was brilliant. Surely, anyone who can come up with a diagnosis just by talking to a patient is a minor god? (He is a rheumatologist. He became my mentor, and is, in fact, one of the major influences in my choice of specialty.)
Medical school in the Philippines forces one to think that way anyhow. There is very little government-provided health care; everything else is paid for out of pocket. This means that every CBC I order, every electrolyte panel, every antinuclear antibody, every urinalysis, is charged to the patient. There are not enough hospital beds, ventilators, or MRI machines.
So patients waited a while before seeking medical attention, which means we took full advantage of a history that’s remarkably evolved, with classic, textbook physical exam findings. The general medicine wards were crammed with patients with jaundice, whether from hepatitis or from having the carcass of a dead ascaris worm lodged in their bile ducts. We saw fungating breast masses. Hyperthyroidism is not that hard to identify when the patient is in frank thyrotoxicosis. Patients with pulmonary tuberculosis had massive hemoptysis, buckets of blood, and acid-fast bacilli in the emergency department.
This is the environment in which I trained. Could I say then that I would be able to identify a problem just from taking a history alone? If you had months’, nay, years’ worth of history to work with, you’d be able to identify the problem, too. I’ll bet doctors who practiced in the 1960s and 1970s in the United States had similar experiences, having the benefit of witnessing full-blown cases of anything and everything.
Do I practice this way now? Not at all.
But that isn’t to say I don’t take a good enough history or physical exam, it’s just that patients seek medical attention earlier, and we have so many more resources at our disposal. We have the ability to detect illness before it wreaks havoc. (There are other, less charitable interpretations of this behavior, such as lack of time, patient expectations, etc. But that’s a topic for another time.)
I have a great deal of respect for my mentors who practice with very real limitations. I have no doubt they are better doctors than I am. And, of course, I feel nostalgic for the way we used to do things. It is easy to romanticize the sepia-toned snapshots of my third-world youth. But really, modernity is a blessing. We should celebrate our ability to find things early. Nostalgia is for meals and memories. Medicine is much more pedestrian than that.
Dr. Chan practices rheumatology in Pawtucket, R.I.
"It is clear that physicians take widely different attitudes towards investigations, some relying on them much more heavily than others."
– J.R. Hampton, et al., British Medical Journal, May 31, 1975
In 1975, a study was published in the British Medical Journal looking at new patients referred to a medical clinic. The study looked specifically at how a diagnosis was made for each new patient, and it concluded that in 66 out of 80 patients, a diagnosis was arrived at based on the history alone, that the physical exam was useful in an additional 7 patients, and that lab testing was useful in the last 7 patients (Br. Med. J. 1975;2:486-9). Since then, medical students have been taught that a good history will lead to the diagnosis about 80% of the time.
One of my professors in my internal medicine class taught me this aphorism. I thought he was brilliant. Surely, anyone who can come up with a diagnosis just by talking to a patient is a minor god? (He is a rheumatologist. He became my mentor, and is, in fact, one of the major influences in my choice of specialty.)
Medical school in the Philippines forces one to think that way anyhow. There is very little government-provided health care; everything else is paid for out of pocket. This means that every CBC I order, every electrolyte panel, every antinuclear antibody, every urinalysis, is charged to the patient. There are not enough hospital beds, ventilators, or MRI machines.
So patients waited a while before seeking medical attention, which means we took full advantage of a history that’s remarkably evolved, with classic, textbook physical exam findings. The general medicine wards were crammed with patients with jaundice, whether from hepatitis or from having the carcass of a dead ascaris worm lodged in their bile ducts. We saw fungating breast masses. Hyperthyroidism is not that hard to identify when the patient is in frank thyrotoxicosis. Patients with pulmonary tuberculosis had massive hemoptysis, buckets of blood, and acid-fast bacilli in the emergency department.
This is the environment in which I trained. Could I say then that I would be able to identify a problem just from taking a history alone? If you had months’, nay, years’ worth of history to work with, you’d be able to identify the problem, too. I’ll bet doctors who practiced in the 1960s and 1970s in the United States had similar experiences, having the benefit of witnessing full-blown cases of anything and everything.
Do I practice this way now? Not at all.
But that isn’t to say I don’t take a good enough history or physical exam, it’s just that patients seek medical attention earlier, and we have so many more resources at our disposal. We have the ability to detect illness before it wreaks havoc. (There are other, less charitable interpretations of this behavior, such as lack of time, patient expectations, etc. But that’s a topic for another time.)
I have a great deal of respect for my mentors who practice with very real limitations. I have no doubt they are better doctors than I am. And, of course, I feel nostalgic for the way we used to do things. It is easy to romanticize the sepia-toned snapshots of my third-world youth. But really, modernity is a blessing. We should celebrate our ability to find things early. Nostalgia is for meals and memories. Medicine is much more pedestrian than that.
Dr. Chan practices rheumatology in Pawtucket, R.I.
"It is clear that physicians take widely different attitudes towards investigations, some relying on them much more heavily than others."
– J.R. Hampton, et al., British Medical Journal, May 31, 1975
In 1975, a study was published in the British Medical Journal looking at new patients referred to a medical clinic. The study looked specifically at how a diagnosis was made for each new patient, and it concluded that in 66 out of 80 patients, a diagnosis was arrived at based on the history alone, that the physical exam was useful in an additional 7 patients, and that lab testing was useful in the last 7 patients (Br. Med. J. 1975;2:486-9). Since then, medical students have been taught that a good history will lead to the diagnosis about 80% of the time.
One of my professors in my internal medicine class taught me this aphorism. I thought he was brilliant. Surely, anyone who can come up with a diagnosis just by talking to a patient is a minor god? (He is a rheumatologist. He became my mentor, and is, in fact, one of the major influences in my choice of specialty.)
Medical school in the Philippines forces one to think that way anyhow. There is very little government-provided health care; everything else is paid for out of pocket. This means that every CBC I order, every electrolyte panel, every antinuclear antibody, every urinalysis, is charged to the patient. There are not enough hospital beds, ventilators, or MRI machines.
So patients waited a while before seeking medical attention, which means we took full advantage of a history that’s remarkably evolved, with classic, textbook physical exam findings. The general medicine wards were crammed with patients with jaundice, whether from hepatitis or from having the carcass of a dead ascaris worm lodged in their bile ducts. We saw fungating breast masses. Hyperthyroidism is not that hard to identify when the patient is in frank thyrotoxicosis. Patients with pulmonary tuberculosis had massive hemoptysis, buckets of blood, and acid-fast bacilli in the emergency department.
This is the environment in which I trained. Could I say then that I would be able to identify a problem just from taking a history alone? If you had months’, nay, years’ worth of history to work with, you’d be able to identify the problem, too. I’ll bet doctors who practiced in the 1960s and 1970s in the United States had similar experiences, having the benefit of witnessing full-blown cases of anything and everything.
Do I practice this way now? Not at all.
But that isn’t to say I don’t take a good enough history or physical exam, it’s just that patients seek medical attention earlier, and we have so many more resources at our disposal. We have the ability to detect illness before it wreaks havoc. (There are other, less charitable interpretations of this behavior, such as lack of time, patient expectations, etc. But that’s a topic for another time.)
I have a great deal of respect for my mentors who practice with very real limitations. I have no doubt they are better doctors than I am. And, of course, I feel nostalgic for the way we used to do things. It is easy to romanticize the sepia-toned snapshots of my third-world youth. But really, modernity is a blessing. We should celebrate our ability to find things early. Nostalgia is for meals and memories. Medicine is much more pedestrian than that.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Do not anger the ‘call gods’
"Do you feel lucky?"
It’s one of Clint Eastwood’s iconic lines (probably a notch below "go ahead, make my day") that wasn’t spoken to a home furnishing.
But it’s still a question many of us think of late on a Friday afternoon just before weekend call gets rolled over to us.
Weekend hospital call, like an approaching storm, brings foreboding and dread. You have no control over the circumstances that are about to whack you. If it’s busy, you’re out of luck. Whatever comes in, you have to deal with it.
I try to ameliorate the pain by using a quote from a residency attending: "It’s not busy, it’s profitable." I also often repeat Dory’s line from "Finding Nemo" – "just keep swimming, just keep swimming" – as I round endlessly.
But humans, by nature, are superstitious creatures. Our ancestors across the globe created pantheons of deities to explain the sun, storms, ocean, and other natural phenomenon they couldn’t control and prayed to them to try to do so.
Now we fear a nebulous group of beings named the "call gods."
It’s always plural, and it’s never been established how many there are or if they have individual names. But they’re feared by all who take hospital call. Amongst physicians, I find they’re universal. Doctors who are Christians, Jews, Hindus, Muslims, atheists ... all know and fear them.
The rules of this medical religion have never been put down and are passed on by verbal tradition. The main theme is that you never, ever, ever do anything to make them angry. This primarily involves not saying things like "it’s quiet so far" or "gee, my phone hasn’t rung all day," for doing so will most assuredly bring their wrath down upon you.
Likewise, even if things are quiet, you never say that until 7:01 on Monday morning, when it’s been rolled back over to your call partner. If someone asks, "how’s your call going?" – even if nothing has happened – you still say "steady" or "hopping" just to avoid challenging the unseen deities.
Of course, the call gods aren’t the only ones we fear. There are specialty-specific, and even procedure-specific, deities. Any neurologist attempting a bedside lumbar puncture on a large person will likely say a quick prayer to the LP gods.
Medicine has come a long way, over time, but even a field with a hefty base in science can’t overcome human nature and our inherent fear of forces beyond our control.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
"Do you feel lucky?"
It’s one of Clint Eastwood’s iconic lines (probably a notch below "go ahead, make my day") that wasn’t spoken to a home furnishing.
But it’s still a question many of us think of late on a Friday afternoon just before weekend call gets rolled over to us.
Weekend hospital call, like an approaching storm, brings foreboding and dread. You have no control over the circumstances that are about to whack you. If it’s busy, you’re out of luck. Whatever comes in, you have to deal with it.
I try to ameliorate the pain by using a quote from a residency attending: "It’s not busy, it’s profitable." I also often repeat Dory’s line from "Finding Nemo" – "just keep swimming, just keep swimming" – as I round endlessly.
But humans, by nature, are superstitious creatures. Our ancestors across the globe created pantheons of deities to explain the sun, storms, ocean, and other natural phenomenon they couldn’t control and prayed to them to try to do so.
Now we fear a nebulous group of beings named the "call gods."
It’s always plural, and it’s never been established how many there are or if they have individual names. But they’re feared by all who take hospital call. Amongst physicians, I find they’re universal. Doctors who are Christians, Jews, Hindus, Muslims, atheists ... all know and fear them.
The rules of this medical religion have never been put down and are passed on by verbal tradition. The main theme is that you never, ever, ever do anything to make them angry. This primarily involves not saying things like "it’s quiet so far" or "gee, my phone hasn’t rung all day," for doing so will most assuredly bring their wrath down upon you.
Likewise, even if things are quiet, you never say that until 7:01 on Monday morning, when it’s been rolled back over to your call partner. If someone asks, "how’s your call going?" – even if nothing has happened – you still say "steady" or "hopping" just to avoid challenging the unseen deities.
Of course, the call gods aren’t the only ones we fear. There are specialty-specific, and even procedure-specific, deities. Any neurologist attempting a bedside lumbar puncture on a large person will likely say a quick prayer to the LP gods.
Medicine has come a long way, over time, but even a field with a hefty base in science can’t overcome human nature and our inherent fear of forces beyond our control.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
"Do you feel lucky?"
It’s one of Clint Eastwood’s iconic lines (probably a notch below "go ahead, make my day") that wasn’t spoken to a home furnishing.
But it’s still a question many of us think of late on a Friday afternoon just before weekend call gets rolled over to us.
Weekend hospital call, like an approaching storm, brings foreboding and dread. You have no control over the circumstances that are about to whack you. If it’s busy, you’re out of luck. Whatever comes in, you have to deal with it.
I try to ameliorate the pain by using a quote from a residency attending: "It’s not busy, it’s profitable." I also often repeat Dory’s line from "Finding Nemo" – "just keep swimming, just keep swimming" – as I round endlessly.
But humans, by nature, are superstitious creatures. Our ancestors across the globe created pantheons of deities to explain the sun, storms, ocean, and other natural phenomenon they couldn’t control and prayed to them to try to do so.
Now we fear a nebulous group of beings named the "call gods."
It’s always plural, and it’s never been established how many there are or if they have individual names. But they’re feared by all who take hospital call. Amongst physicians, I find they’re universal. Doctors who are Christians, Jews, Hindus, Muslims, atheists ... all know and fear them.
The rules of this medical religion have never been put down and are passed on by verbal tradition. The main theme is that you never, ever, ever do anything to make them angry. This primarily involves not saying things like "it’s quiet so far" or "gee, my phone hasn’t rung all day," for doing so will most assuredly bring their wrath down upon you.
Likewise, even if things are quiet, you never say that until 7:01 on Monday morning, when it’s been rolled back over to your call partner. If someone asks, "how’s your call going?" – even if nothing has happened – you still say "steady" or "hopping" just to avoid challenging the unseen deities.
Of course, the call gods aren’t the only ones we fear. There are specialty-specific, and even procedure-specific, deities. Any neurologist attempting a bedside lumbar puncture on a large person will likely say a quick prayer to the LP gods.
Medicine has come a long way, over time, but even a field with a hefty base in science can’t overcome human nature and our inherent fear of forces beyond our control.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Should off-pump CABG be abandoned?
YES
I believe on-pump coronary artery bypass graft (CABG) surgery should be our primary operation, with off-pump CABG reserved only for certain situations.
In talking about these two surgical options, there are basically three main issues of interest: procedural outcomes, the quality of revascularization, and long-term effectiveness.
Procedural outcomes: In the 1990s, there was great excitement for the potential for off-pump CABG. We thought survival would be better, stroke less common, neurocognitive outcomes improved, etc., but after several randomized studies and a number of well-controlled observational studies, we found that, in terms of the important procedural outcomes – death, MI, stroke, and acute renal failure – there were actually no differences between off- and on-pump CABG. We saw this in ROOBY, with low-risk patients and less experienced surgeons; in the Canadian CORONARY trial, with experienced off-pump surgeons; and in the European GOPCABE trial, where the surgeons were very experienced and the patients were high risk.
There are some benefits with off-pump CABG in terms of what I call the "reversible" complications of surgery: fewer transfusions, less postoperative atrial fibrillation, fewer respiratory infections; but I believe these things have to be balanced against some of the negative effects of off-pump surgery in terms of its quality of revascularization and long-term effectiveness.
Probably one of the biggest disappointments we’ve had with off-pump CABG is that, in the trials, we didn’t see any major difference in neurocognitive dysfunction. What we learned is that cardiopulmonary bypass really is not that bad in terms of neurocognitive dysfunction after surgery.
One issue that tends to be forgotten is the risk associated with conversion. In a hemodynamically unstable patient, converting from off-pump to on-pump is associated with higher risks of death, bleeding, renal failure, stroke, respiratory failure, and GI complications. In CORONARY, with experienced surgeons, the conversion rate was almost 7.9%, and in GOPCABE, it was 9.7%. Even with experienced surgeons, conversions do occur, and they have negative consequences.
Quality of revascularization: We know that long-term survival is related to completeness of revascularization. The more ischemic myocardium that is left at risk after CABG, the more likely the patients is to have an MI or die, and this has been very clearly demonstrated in the surgical literature as well as the percutaneous coronary intervention literature. It has been shown multiple times that fewer grafts are done in off-pump patients, compared with on-pump patients. Indeed, in a recent Cochrane meta-analysis of more than 7,000 patients in 57 trials, there were significantly more grafts done in on-pump than off-pump cases.
Also in terms of graft patency, again, multiple studies have shown lower graft patency in off-pump patients, either directly or by means of showing a higher reintervention rate after off-pump as compared with on-pump surgery. In both CORONARY and GOPCABE, the repeat revascularization rate at 30 days was higher for off-pump CABG.
Long-term effectiveness: The long-term results from ROOBY are very sobering: significantly higher 1-year cardiac mortality in the off-pump arm and higher 1-year composite adverse events. Again, in the 2012 Cochrane meta-analysis, with more than 10,000 patients in 75 trials, mortality was significantly higher with off-pump patients, compared with on-pump, with a hazard ratio of 1.24 (95% CI, 1.01-1.53). When the authors of the Cochrane meta-analysis removed what they called the "studies with bias," this signal was even stronger. Also in observational studies, such as one published by Racz et al. in 2004 (J. Am. Coll. Cardiol. 2004; 43: 557-64), the best survival was found to be in patients who had on-pump CABG and the worst in those who had undergone off-pump CABG.
In summary, procedural outcomes with off-pump and on-pump CABG are similar, albeit with lower reversible complications in the off-pump patients, but a greater conversion risk, along with its associated complications. The quality of the revascularization is worse in off-pump patients, the completeness of revascularization is less and re-intervention rates are higher, and the bottom line, of course, it that there is higher long-term mortality after off-pump CABG.
"Should off-pump CABG be abandoned?" As our default procedure, yes. The vast majority of our patients are best served with on-pump revascularization.
Dr. Joseph Sabik is the chairman of the department of thoracic and cardiovascular surgery and the Sheik Hamdam Bin Rashid Al Maktoum Distinguished Chair at the Cleveland Clinic. He disclosed that he performs both off-pump and on-pump CABG.
References
Anesthesiology 2005;102:188-203
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Cochrane Database Syst. Rev. 2012; Mar 14. 3: CD007224 [doi:10.1002/14651858.CD007224.pub2])
J. Am. Coll. Cardiol. 2004;43:557-64
NO
Should off pump bypass be abandoned? Absolutely not, but let’s do it well.
The rationale for why off-pump CABG should be the preferred strategy is simple: cardiopulmonary bypass (CPB) entails extracorporeal circulation, aortic cannulation and clamping, global MI, hypothermia, and hemodilution, among other potentially deleterious phenomena. There are morbidities that can be attributed to these entities, and off-pump bypass avoids those effects by mechanically stabilizing each coronary artery target individually, while the rest of the heart beats and supports normal physiologic circulation. There is an important caveat to this, however, and that is if – and perhaps only if – a complete revascularization with precise anastomoses can be accomplished off pump, then the patient will in fact benefit.
At Emory University in Atlanta, we did a prospective, randomized trial on my own patients, which we called SMART (Surgical Management of Arterial Revascularization) trial. What we found was that in 200 unselected, consecutive patients undergoing either off- or on-pump CABG, we had lower myocardial enzyme release, fewer transfusions, more rapid extubation, and a shorter length of stay in hospital with off-pump CABG.
Completeness of revascularization is a very important issue. In SMART, the number of grafts per patient was exactly the same: 3.39 per patient with off-pump CABG and 3.4 with on-pump CABG. We coined the phrase "Index of Completeness of Revascularization," which we defined as the number of grafts we planned to do in examining the arteriogram prior to randomization and surgery divided by the number of grafts we actually did. We found no difference here, meaning we were able to do the operation we planned to do (1.00 vs. 1.01; P = not significant). Moreover, for the lateral wall, which is technically more difficult to reach in a beating heart, the number was similar in the off- and on-pump groups (0.97 vs. 0.98; P = not significant). We also used a similar percentage of arterial grafts in both groups.
CPB was an independent predictor of the need for transfusion by multivariate analysis with an odds ratio of 2.42 (P = .0073) and was associated with a longer length of stay by 1 day (5.1 days for off-pump and 6.1 for on-pump; P = .005 Wilcoxon).
Creatine phosphokinase of muscle band and troponin I release was about half as much in the off-pump group as in the on-pump group (P less than .001 Wilcoxon), and the rates of death, stroke, MI, angina, and reintervention were similar at both 30 days and 1 year, as was graft patency and quality of life. Off-pump CABG costs at 1 year were $1,955 less than on-pump, but this difference was not statistically significant (P = .08).
At 8 years, survival in SMART was still similar between groups (P = .33), as was graft patency in the small number of patients who had CT angiograms. PET scan results similarly showed no significant difference in ischemia between these two groups (P = .62). One patient in each group has had a percutaneous reintervention, and none have had a repeat coronary bypass in 10 years.
To see if these results could be replicated nationally, we turned to the STS database and looked at North American centers that performed more than 100 on-pump CABGs and more than 100 off-pump surgeries. This gave us 42,477 patients (16,245 off pump and 26,232 on pump) at 63 North American centers. We included the 2.2% of off-pump cases that were converted to on-pump cases in the off-pump group.
After risk adjustment for 32 variables, for the outcomes of death, stroke, MI, and major adverse cardiac events, off-pump bypass outperformed on-pump bypass in this huge cohort of patients from around the country. Looking at less-significant outcomes – renal failure, dialysis, sternal infection, reoperation, atrial fibrillation, prolonged ventilation, and length of stay greater than 14 days – all of them favored off-pump bypass.
We then looked at the Emory dataset (14,766 consecutive patients, 48% of whom had off-pump CABG and 52% on-pump) to see which patients benefitted more. For patients in the two lower quartiles of predicted risk, there was no difference in operative mortality. In the higher two risk quartiles, there was a mortality benefit with off-pump CABG, with a risk reduction for operative mortality of about 55% in the highest risk patients (P less than 0.001).
Logistic regression confirmed that there was an interaction between surgery type and predicted risk, and we now know that low-risk patients do not have the survival benefit of avoiding CPB. They do fine with on-pump CABG, but higher risk patients have a benefit from avoiding CPB and the higher the predicted risk, the greater the benefit to the patient.
We went back to the STS database and we looked at whether this applies only at some centers or all centers, some surgeons or all surgeons. We looked at almost a million cases, 210,469 of which were at sites that had a large off-pump CABG volume. With the usual adjustments, off-pump CABG was associated with significant reduction of risk of death, stroke, renal failure, any morbidity or mortality or prolonged length of stay, compared with on-pump bypass. This benefit was even more pronounced after adjustment for surgeon effect. Once again, the greater reduction was enjoyed by those patients who had the highest preoperative risk. In all predicted risk quartiles, off pump bypass reduced risk of death and stroke and that magnitude increased with increasing predicted risk of mortality. This was seen in large-volume centers and low-volume centers.
Similar results were seen in a recent multicenter, randomized, prospective trial by Lemma et al. that assigned 411 patients to either off- or on-pump coronary bypass (J. Thorac. Cardiovasc. Surg. 2012;143:625-31). There was reduced early mortality and morbidity among higher-risk patients. Interestingly, in this study, they used an experience-based randomization scheme, in which they had surgeons within each center who like to do off-pump CABG and those who like to do on-pump CABG, and each surgeon had hundreds of cases under his belt. For the primary endpoint, a composite of death, MI, stroke or TIA, renal failure, acute respiratory distress syndrome, or reoperation for bleeding, the rates were 5.8% of off-pump and 13.3% for on-pump patients (odds ratio, 2.5; P = .01).
I think the conclusions are clear, but not everyone has reached these same conclusions. In ROOBY, the results were different. Although the study was well conducted, it enrolled low-risk patients, in whom avoidance of CPB was unlikely to improve the expected excellent outcomes. And the operations were performed by residents, with supervising attendings who themselves only had to do 20 total career off-pump cases to be eligible. I think this lack of experience is well demonstrated by the 12.5% conversion rate from off-pump cases to on-pump in that trial. ROOBY enrolled the wrong patients and used the wrong surgeons.
In the CORONARY trial, conducted by Dr. Lamy in Canada but enrolling patients from 19 countries outside of Canada, there was no difference in the primary endpoint of death, stroke, MI, and renal failure at 30 days, but there was a decrease in transfusion, reoperation for bleeding, acute kidney injury, or respiratory complications. There wasn\'t a difference in stroke, interestingly, but the surgeons in this trial appropriately converted a hundred patients from on pump to off pump to avoid manipulating a calcified aorta. This was a good, well-conducted trial.
However, the primary outcome in CORONARY differed when assessed according to EuroSCORE. When the EuroSCORE was low, on pump outperformed off pump. When the EuroSCORE was high, off pump outperformed on pump.
These two trials offer important perspective: the ROOBY trial, enrolling low-risk patients, was actually in favor of on-pump CABG. The CORONARY trial, enrolling higher-risk patients, had a slight benefit in favor of off-pump CABG, and this was particularly evident in the Canadian cohort of 830 randomized patients, in which the primary outcome was, in fact, statistically significantly better in the off-pump group at 9.2% vs. 13.7%.
At the end of the day, I think it matters in whom you do off-pump CABG and how well you do it. It may not be for every patient or for every surgeon; off-pump CABG requires a focused and sustained effort to master a new set of physical and psychological skills to accomplish precise and complete revascularization. When we can do this, I think, we offer better outcomes for our patients.
Dr. John Puskas is the chairman of the department of cardiac surgery at Mount Sinai Beth Israel in New York. Dr. Puskas disclosed that he also does both on- and off-pump CABG. He received royalties from coronary surgical instruments marketed by Scanlan, as well.
References
JAMA 2004;291:1841-9
Ann. Thorac. Surg. 2009;88:1142-7
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2010;362:851
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Ann. Thorac. Surg. 2011;91:1836-42
J. Thorac. Cardiovasc. Surg. 2012;143:625-31
YES
I believe on-pump coronary artery bypass graft (CABG) surgery should be our primary operation, with off-pump CABG reserved only for certain situations.
In talking about these two surgical options, there are basically three main issues of interest: procedural outcomes, the quality of revascularization, and long-term effectiveness.
Procedural outcomes: In the 1990s, there was great excitement for the potential for off-pump CABG. We thought survival would be better, stroke less common, neurocognitive outcomes improved, etc., but after several randomized studies and a number of well-controlled observational studies, we found that, in terms of the important procedural outcomes – death, MI, stroke, and acute renal failure – there were actually no differences between off- and on-pump CABG. We saw this in ROOBY, with low-risk patients and less experienced surgeons; in the Canadian CORONARY trial, with experienced off-pump surgeons; and in the European GOPCABE trial, where the surgeons were very experienced and the patients were high risk.
There are some benefits with off-pump CABG in terms of what I call the "reversible" complications of surgery: fewer transfusions, less postoperative atrial fibrillation, fewer respiratory infections; but I believe these things have to be balanced against some of the negative effects of off-pump surgery in terms of its quality of revascularization and long-term effectiveness.
Probably one of the biggest disappointments we’ve had with off-pump CABG is that, in the trials, we didn’t see any major difference in neurocognitive dysfunction. What we learned is that cardiopulmonary bypass really is not that bad in terms of neurocognitive dysfunction after surgery.
One issue that tends to be forgotten is the risk associated with conversion. In a hemodynamically unstable patient, converting from off-pump to on-pump is associated with higher risks of death, bleeding, renal failure, stroke, respiratory failure, and GI complications. In CORONARY, with experienced surgeons, the conversion rate was almost 7.9%, and in GOPCABE, it was 9.7%. Even with experienced surgeons, conversions do occur, and they have negative consequences.
Quality of revascularization: We know that long-term survival is related to completeness of revascularization. The more ischemic myocardium that is left at risk after CABG, the more likely the patients is to have an MI or die, and this has been very clearly demonstrated in the surgical literature as well as the percutaneous coronary intervention literature. It has been shown multiple times that fewer grafts are done in off-pump patients, compared with on-pump patients. Indeed, in a recent Cochrane meta-analysis of more than 7,000 patients in 57 trials, there were significantly more grafts done in on-pump than off-pump cases.
Also in terms of graft patency, again, multiple studies have shown lower graft patency in off-pump patients, either directly or by means of showing a higher reintervention rate after off-pump as compared with on-pump surgery. In both CORONARY and GOPCABE, the repeat revascularization rate at 30 days was higher for off-pump CABG.
Long-term effectiveness: The long-term results from ROOBY are very sobering: significantly higher 1-year cardiac mortality in the off-pump arm and higher 1-year composite adverse events. Again, in the 2012 Cochrane meta-analysis, with more than 10,000 patients in 75 trials, mortality was significantly higher with off-pump patients, compared with on-pump, with a hazard ratio of 1.24 (95% CI, 1.01-1.53). When the authors of the Cochrane meta-analysis removed what they called the "studies with bias," this signal was even stronger. Also in observational studies, such as one published by Racz et al. in 2004 (J. Am. Coll. Cardiol. 2004; 43: 557-64), the best survival was found to be in patients who had on-pump CABG and the worst in those who had undergone off-pump CABG.
In summary, procedural outcomes with off-pump and on-pump CABG are similar, albeit with lower reversible complications in the off-pump patients, but a greater conversion risk, along with its associated complications. The quality of the revascularization is worse in off-pump patients, the completeness of revascularization is less and re-intervention rates are higher, and the bottom line, of course, it that there is higher long-term mortality after off-pump CABG.
"Should off-pump CABG be abandoned?" As our default procedure, yes. The vast majority of our patients are best served with on-pump revascularization.
Dr. Joseph Sabik is the chairman of the department of thoracic and cardiovascular surgery and the Sheik Hamdam Bin Rashid Al Maktoum Distinguished Chair at the Cleveland Clinic. He disclosed that he performs both off-pump and on-pump CABG.
References
Anesthesiology 2005;102:188-203
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Cochrane Database Syst. Rev. 2012; Mar 14. 3: CD007224 [doi:10.1002/14651858.CD007224.pub2])
J. Am. Coll. Cardiol. 2004;43:557-64
NO
Should off pump bypass be abandoned? Absolutely not, but let’s do it well.
The rationale for why off-pump CABG should be the preferred strategy is simple: cardiopulmonary bypass (CPB) entails extracorporeal circulation, aortic cannulation and clamping, global MI, hypothermia, and hemodilution, among other potentially deleterious phenomena. There are morbidities that can be attributed to these entities, and off-pump bypass avoids those effects by mechanically stabilizing each coronary artery target individually, while the rest of the heart beats and supports normal physiologic circulation. There is an important caveat to this, however, and that is if – and perhaps only if – a complete revascularization with precise anastomoses can be accomplished off pump, then the patient will in fact benefit.
At Emory University in Atlanta, we did a prospective, randomized trial on my own patients, which we called SMART (Surgical Management of Arterial Revascularization) trial. What we found was that in 200 unselected, consecutive patients undergoing either off- or on-pump CABG, we had lower myocardial enzyme release, fewer transfusions, more rapid extubation, and a shorter length of stay in hospital with off-pump CABG.
Completeness of revascularization is a very important issue. In SMART, the number of grafts per patient was exactly the same: 3.39 per patient with off-pump CABG and 3.4 with on-pump CABG. We coined the phrase "Index of Completeness of Revascularization," which we defined as the number of grafts we planned to do in examining the arteriogram prior to randomization and surgery divided by the number of grafts we actually did. We found no difference here, meaning we were able to do the operation we planned to do (1.00 vs. 1.01; P = not significant). Moreover, for the lateral wall, which is technically more difficult to reach in a beating heart, the number was similar in the off- and on-pump groups (0.97 vs. 0.98; P = not significant). We also used a similar percentage of arterial grafts in both groups.
CPB was an independent predictor of the need for transfusion by multivariate analysis with an odds ratio of 2.42 (P = .0073) and was associated with a longer length of stay by 1 day (5.1 days for off-pump and 6.1 for on-pump; P = .005 Wilcoxon).
Creatine phosphokinase of muscle band and troponin I release was about half as much in the off-pump group as in the on-pump group (P less than .001 Wilcoxon), and the rates of death, stroke, MI, angina, and reintervention were similar at both 30 days and 1 year, as was graft patency and quality of life. Off-pump CABG costs at 1 year were $1,955 less than on-pump, but this difference was not statistically significant (P = .08).
At 8 years, survival in SMART was still similar between groups (P = .33), as was graft patency in the small number of patients who had CT angiograms. PET scan results similarly showed no significant difference in ischemia between these two groups (P = .62). One patient in each group has had a percutaneous reintervention, and none have had a repeat coronary bypass in 10 years.
To see if these results could be replicated nationally, we turned to the STS database and looked at North American centers that performed more than 100 on-pump CABGs and more than 100 off-pump surgeries. This gave us 42,477 patients (16,245 off pump and 26,232 on pump) at 63 North American centers. We included the 2.2% of off-pump cases that were converted to on-pump cases in the off-pump group.
After risk adjustment for 32 variables, for the outcomes of death, stroke, MI, and major adverse cardiac events, off-pump bypass outperformed on-pump bypass in this huge cohort of patients from around the country. Looking at less-significant outcomes – renal failure, dialysis, sternal infection, reoperation, atrial fibrillation, prolonged ventilation, and length of stay greater than 14 days – all of them favored off-pump bypass.
We then looked at the Emory dataset (14,766 consecutive patients, 48% of whom had off-pump CABG and 52% on-pump) to see which patients benefitted more. For patients in the two lower quartiles of predicted risk, there was no difference in operative mortality. In the higher two risk quartiles, there was a mortality benefit with off-pump CABG, with a risk reduction for operative mortality of about 55% in the highest risk patients (P less than 0.001).
Logistic regression confirmed that there was an interaction between surgery type and predicted risk, and we now know that low-risk patients do not have the survival benefit of avoiding CPB. They do fine with on-pump CABG, but higher risk patients have a benefit from avoiding CPB and the higher the predicted risk, the greater the benefit to the patient.
We went back to the STS database and we looked at whether this applies only at some centers or all centers, some surgeons or all surgeons. We looked at almost a million cases, 210,469 of which were at sites that had a large off-pump CABG volume. With the usual adjustments, off-pump CABG was associated with significant reduction of risk of death, stroke, renal failure, any morbidity or mortality or prolonged length of stay, compared with on-pump bypass. This benefit was even more pronounced after adjustment for surgeon effect. Once again, the greater reduction was enjoyed by those patients who had the highest preoperative risk. In all predicted risk quartiles, off pump bypass reduced risk of death and stroke and that magnitude increased with increasing predicted risk of mortality. This was seen in large-volume centers and low-volume centers.
Similar results were seen in a recent multicenter, randomized, prospective trial by Lemma et al. that assigned 411 patients to either off- or on-pump coronary bypass (J. Thorac. Cardiovasc. Surg. 2012;143:625-31). There was reduced early mortality and morbidity among higher-risk patients. Interestingly, in this study, they used an experience-based randomization scheme, in which they had surgeons within each center who like to do off-pump CABG and those who like to do on-pump CABG, and each surgeon had hundreds of cases under his belt. For the primary endpoint, a composite of death, MI, stroke or TIA, renal failure, acute respiratory distress syndrome, or reoperation for bleeding, the rates were 5.8% of off-pump and 13.3% for on-pump patients (odds ratio, 2.5; P = .01).
I think the conclusions are clear, but not everyone has reached these same conclusions. In ROOBY, the results were different. Although the study was well conducted, it enrolled low-risk patients, in whom avoidance of CPB was unlikely to improve the expected excellent outcomes. And the operations were performed by residents, with supervising attendings who themselves only had to do 20 total career off-pump cases to be eligible. I think this lack of experience is well demonstrated by the 12.5% conversion rate from off-pump cases to on-pump in that trial. ROOBY enrolled the wrong patients and used the wrong surgeons.
In the CORONARY trial, conducted by Dr. Lamy in Canada but enrolling patients from 19 countries outside of Canada, there was no difference in the primary endpoint of death, stroke, MI, and renal failure at 30 days, but there was a decrease in transfusion, reoperation for bleeding, acute kidney injury, or respiratory complications. There wasn\'t a difference in stroke, interestingly, but the surgeons in this trial appropriately converted a hundred patients from on pump to off pump to avoid manipulating a calcified aorta. This was a good, well-conducted trial.
However, the primary outcome in CORONARY differed when assessed according to EuroSCORE. When the EuroSCORE was low, on pump outperformed off pump. When the EuroSCORE was high, off pump outperformed on pump.
These two trials offer important perspective: the ROOBY trial, enrolling low-risk patients, was actually in favor of on-pump CABG. The CORONARY trial, enrolling higher-risk patients, had a slight benefit in favor of off-pump CABG, and this was particularly evident in the Canadian cohort of 830 randomized patients, in which the primary outcome was, in fact, statistically significantly better in the off-pump group at 9.2% vs. 13.7%.
At the end of the day, I think it matters in whom you do off-pump CABG and how well you do it. It may not be for every patient or for every surgeon; off-pump CABG requires a focused and sustained effort to master a new set of physical and psychological skills to accomplish precise and complete revascularization. When we can do this, I think, we offer better outcomes for our patients.
Dr. John Puskas is the chairman of the department of cardiac surgery at Mount Sinai Beth Israel in New York. Dr. Puskas disclosed that he also does both on- and off-pump CABG. He received royalties from coronary surgical instruments marketed by Scanlan, as well.
References
JAMA 2004;291:1841-9
Ann. Thorac. Surg. 2009;88:1142-7
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2010;362:851
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Ann. Thorac. Surg. 2011;91:1836-42
J. Thorac. Cardiovasc. Surg. 2012;143:625-31
YES
I believe on-pump coronary artery bypass graft (CABG) surgery should be our primary operation, with off-pump CABG reserved only for certain situations.
In talking about these two surgical options, there are basically three main issues of interest: procedural outcomes, the quality of revascularization, and long-term effectiveness.
Procedural outcomes: In the 1990s, there was great excitement for the potential for off-pump CABG. We thought survival would be better, stroke less common, neurocognitive outcomes improved, etc., but after several randomized studies and a number of well-controlled observational studies, we found that, in terms of the important procedural outcomes – death, MI, stroke, and acute renal failure – there were actually no differences between off- and on-pump CABG. We saw this in ROOBY, with low-risk patients and less experienced surgeons; in the Canadian CORONARY trial, with experienced off-pump surgeons; and in the European GOPCABE trial, where the surgeons were very experienced and the patients were high risk.
There are some benefits with off-pump CABG in terms of what I call the "reversible" complications of surgery: fewer transfusions, less postoperative atrial fibrillation, fewer respiratory infections; but I believe these things have to be balanced against some of the negative effects of off-pump surgery in terms of its quality of revascularization and long-term effectiveness.
Probably one of the biggest disappointments we’ve had with off-pump CABG is that, in the trials, we didn’t see any major difference in neurocognitive dysfunction. What we learned is that cardiopulmonary bypass really is not that bad in terms of neurocognitive dysfunction after surgery.
One issue that tends to be forgotten is the risk associated with conversion. In a hemodynamically unstable patient, converting from off-pump to on-pump is associated with higher risks of death, bleeding, renal failure, stroke, respiratory failure, and GI complications. In CORONARY, with experienced surgeons, the conversion rate was almost 7.9%, and in GOPCABE, it was 9.7%. Even with experienced surgeons, conversions do occur, and they have negative consequences.
Quality of revascularization: We know that long-term survival is related to completeness of revascularization. The more ischemic myocardium that is left at risk after CABG, the more likely the patients is to have an MI or die, and this has been very clearly demonstrated in the surgical literature as well as the percutaneous coronary intervention literature. It has been shown multiple times that fewer grafts are done in off-pump patients, compared with on-pump patients. Indeed, in a recent Cochrane meta-analysis of more than 7,000 patients in 57 trials, there were significantly more grafts done in on-pump than off-pump cases.
Also in terms of graft patency, again, multiple studies have shown lower graft patency in off-pump patients, either directly or by means of showing a higher reintervention rate after off-pump as compared with on-pump surgery. In both CORONARY and GOPCABE, the repeat revascularization rate at 30 days was higher for off-pump CABG.
Long-term effectiveness: The long-term results from ROOBY are very sobering: significantly higher 1-year cardiac mortality in the off-pump arm and higher 1-year composite adverse events. Again, in the 2012 Cochrane meta-analysis, with more than 10,000 patients in 75 trials, mortality was significantly higher with off-pump patients, compared with on-pump, with a hazard ratio of 1.24 (95% CI, 1.01-1.53). When the authors of the Cochrane meta-analysis removed what they called the "studies with bias," this signal was even stronger. Also in observational studies, such as one published by Racz et al. in 2004 (J. Am. Coll. Cardiol. 2004; 43: 557-64), the best survival was found to be in patients who had on-pump CABG and the worst in those who had undergone off-pump CABG.
In summary, procedural outcomes with off-pump and on-pump CABG are similar, albeit with lower reversible complications in the off-pump patients, but a greater conversion risk, along with its associated complications. The quality of the revascularization is worse in off-pump patients, the completeness of revascularization is less and re-intervention rates are higher, and the bottom line, of course, it that there is higher long-term mortality after off-pump CABG.
"Should off-pump CABG be abandoned?" As our default procedure, yes. The vast majority of our patients are best served with on-pump revascularization.
Dr. Joseph Sabik is the chairman of the department of thoracic and cardiovascular surgery and the Sheik Hamdam Bin Rashid Al Maktoum Distinguished Chair at the Cleveland Clinic. He disclosed that he performs both off-pump and on-pump CABG.
References
Anesthesiology 2005;102:188-203
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Cochrane Database Syst. Rev. 2012; Mar 14. 3: CD007224 [doi:10.1002/14651858.CD007224.pub2])
J. Am. Coll. Cardiol. 2004;43:557-64
NO
Should off pump bypass be abandoned? Absolutely not, but let’s do it well.
The rationale for why off-pump CABG should be the preferred strategy is simple: cardiopulmonary bypass (CPB) entails extracorporeal circulation, aortic cannulation and clamping, global MI, hypothermia, and hemodilution, among other potentially deleterious phenomena. There are morbidities that can be attributed to these entities, and off-pump bypass avoids those effects by mechanically stabilizing each coronary artery target individually, while the rest of the heart beats and supports normal physiologic circulation. There is an important caveat to this, however, and that is if – and perhaps only if – a complete revascularization with precise anastomoses can be accomplished off pump, then the patient will in fact benefit.
At Emory University in Atlanta, we did a prospective, randomized trial on my own patients, which we called SMART (Surgical Management of Arterial Revascularization) trial. What we found was that in 200 unselected, consecutive patients undergoing either off- or on-pump CABG, we had lower myocardial enzyme release, fewer transfusions, more rapid extubation, and a shorter length of stay in hospital with off-pump CABG.
Completeness of revascularization is a very important issue. In SMART, the number of grafts per patient was exactly the same: 3.39 per patient with off-pump CABG and 3.4 with on-pump CABG. We coined the phrase "Index of Completeness of Revascularization," which we defined as the number of grafts we planned to do in examining the arteriogram prior to randomization and surgery divided by the number of grafts we actually did. We found no difference here, meaning we were able to do the operation we planned to do (1.00 vs. 1.01; P = not significant). Moreover, for the lateral wall, which is technically more difficult to reach in a beating heart, the number was similar in the off- and on-pump groups (0.97 vs. 0.98; P = not significant). We also used a similar percentage of arterial grafts in both groups.
CPB was an independent predictor of the need for transfusion by multivariate analysis with an odds ratio of 2.42 (P = .0073) and was associated with a longer length of stay by 1 day (5.1 days for off-pump and 6.1 for on-pump; P = .005 Wilcoxon).
Creatine phosphokinase of muscle band and troponin I release was about half as much in the off-pump group as in the on-pump group (P less than .001 Wilcoxon), and the rates of death, stroke, MI, angina, and reintervention were similar at both 30 days and 1 year, as was graft patency and quality of life. Off-pump CABG costs at 1 year were $1,955 less than on-pump, but this difference was not statistically significant (P = .08).
At 8 years, survival in SMART was still similar between groups (P = .33), as was graft patency in the small number of patients who had CT angiograms. PET scan results similarly showed no significant difference in ischemia between these two groups (P = .62). One patient in each group has had a percutaneous reintervention, and none have had a repeat coronary bypass in 10 years.
To see if these results could be replicated nationally, we turned to the STS database and looked at North American centers that performed more than 100 on-pump CABGs and more than 100 off-pump surgeries. This gave us 42,477 patients (16,245 off pump and 26,232 on pump) at 63 North American centers. We included the 2.2% of off-pump cases that were converted to on-pump cases in the off-pump group.
After risk adjustment for 32 variables, for the outcomes of death, stroke, MI, and major adverse cardiac events, off-pump bypass outperformed on-pump bypass in this huge cohort of patients from around the country. Looking at less-significant outcomes – renal failure, dialysis, sternal infection, reoperation, atrial fibrillation, prolonged ventilation, and length of stay greater than 14 days – all of them favored off-pump bypass.
We then looked at the Emory dataset (14,766 consecutive patients, 48% of whom had off-pump CABG and 52% on-pump) to see which patients benefitted more. For patients in the two lower quartiles of predicted risk, there was no difference in operative mortality. In the higher two risk quartiles, there was a mortality benefit with off-pump CABG, with a risk reduction for operative mortality of about 55% in the highest risk patients (P less than 0.001).
Logistic regression confirmed that there was an interaction between surgery type and predicted risk, and we now know that low-risk patients do not have the survival benefit of avoiding CPB. They do fine with on-pump CABG, but higher risk patients have a benefit from avoiding CPB and the higher the predicted risk, the greater the benefit to the patient.
We went back to the STS database and we looked at whether this applies only at some centers or all centers, some surgeons or all surgeons. We looked at almost a million cases, 210,469 of which were at sites that had a large off-pump CABG volume. With the usual adjustments, off-pump CABG was associated with significant reduction of risk of death, stroke, renal failure, any morbidity or mortality or prolonged length of stay, compared with on-pump bypass. This benefit was even more pronounced after adjustment for surgeon effect. Once again, the greater reduction was enjoyed by those patients who had the highest preoperative risk. In all predicted risk quartiles, off pump bypass reduced risk of death and stroke and that magnitude increased with increasing predicted risk of mortality. This was seen in large-volume centers and low-volume centers.
Similar results were seen in a recent multicenter, randomized, prospective trial by Lemma et al. that assigned 411 patients to either off- or on-pump coronary bypass (J. Thorac. Cardiovasc. Surg. 2012;143:625-31). There was reduced early mortality and morbidity among higher-risk patients. Interestingly, in this study, they used an experience-based randomization scheme, in which they had surgeons within each center who like to do off-pump CABG and those who like to do on-pump CABG, and each surgeon had hundreds of cases under his belt. For the primary endpoint, a composite of death, MI, stroke or TIA, renal failure, acute respiratory distress syndrome, or reoperation for bleeding, the rates were 5.8% of off-pump and 13.3% for on-pump patients (odds ratio, 2.5; P = .01).
I think the conclusions are clear, but not everyone has reached these same conclusions. In ROOBY, the results were different. Although the study was well conducted, it enrolled low-risk patients, in whom avoidance of CPB was unlikely to improve the expected excellent outcomes. And the operations were performed by residents, with supervising attendings who themselves only had to do 20 total career off-pump cases to be eligible. I think this lack of experience is well demonstrated by the 12.5% conversion rate from off-pump cases to on-pump in that trial. ROOBY enrolled the wrong patients and used the wrong surgeons.
In the CORONARY trial, conducted by Dr. Lamy in Canada but enrolling patients from 19 countries outside of Canada, there was no difference in the primary endpoint of death, stroke, MI, and renal failure at 30 days, but there was a decrease in transfusion, reoperation for bleeding, acute kidney injury, or respiratory complications. There wasn\'t a difference in stroke, interestingly, but the surgeons in this trial appropriately converted a hundred patients from on pump to off pump to avoid manipulating a calcified aorta. This was a good, well-conducted trial.
However, the primary outcome in CORONARY differed when assessed according to EuroSCORE. When the EuroSCORE was low, on pump outperformed off pump. When the EuroSCORE was high, off pump outperformed on pump.
These two trials offer important perspective: the ROOBY trial, enrolling low-risk patients, was actually in favor of on-pump CABG. The CORONARY trial, enrolling higher-risk patients, had a slight benefit in favor of off-pump CABG, and this was particularly evident in the Canadian cohort of 830 randomized patients, in which the primary outcome was, in fact, statistically significantly better in the off-pump group at 9.2% vs. 13.7%.
At the end of the day, I think it matters in whom you do off-pump CABG and how well you do it. It may not be for every patient or for every surgeon; off-pump CABG requires a focused and sustained effort to master a new set of physical and psychological skills to accomplish precise and complete revascularization. When we can do this, I think, we offer better outcomes for our patients.
Dr. John Puskas is the chairman of the department of cardiac surgery at Mount Sinai Beth Israel in New York. Dr. Puskas disclosed that he also does both on- and off-pump CABG. He received royalties from coronary surgical instruments marketed by Scanlan, as well.
References
JAMA 2004;291:1841-9
Ann. Thorac. Surg. 2009;88:1142-7
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2010;362:851
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Ann. Thorac. Surg. 2011;91:1836-42
J. Thorac. Cardiovasc. Surg. 2012;143:625-31
ID Consult: National immunization coverage and measles
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.
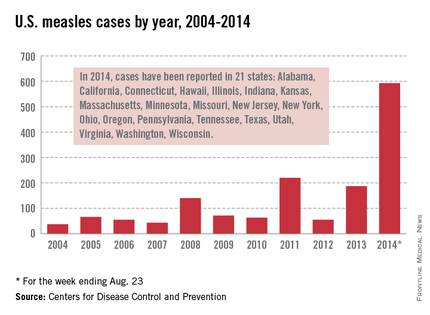
Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.
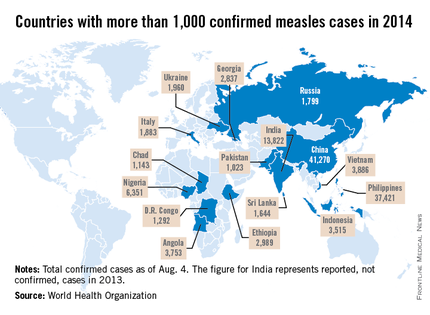
Measles
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.

Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.

Measles
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.

Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.

Measles
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].