User login
Certification of Point-of-Care Ultrasound Competency
Any conversation about point-of-care ultrasound (POCUS) inevitably brings up discussion about credentialing, privileging, and certification. While credentialing and privileging are institution-specific processes, competency certification can be extramural through a national board or intramural through an institutional process.
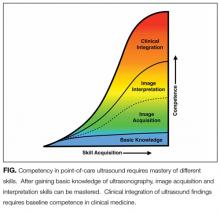
Some institutions have begun to develop intramural certification pathways for POCUS competency in order to grant privileges to hospitalists. In this edition of the Journal of Hospital Medicine, Mathews and Zwank2 describe a multidisciplinary collaboration to provide POCUS training, intramural certification, and quality assurance for hospitalists at one hospital in Minnesota. This model serves as a real-world example of how institutions are addressing the need to certify hospitalists in basic POCUS competency. After engaging stakeholders from radiology, critical care, emergency medicine, and cardiology, institutional standards were developed and hospitalists were assessed for basic POCUS competency. Certification included assessments of hospitalists’ knowledge, image acquisition, and image interpretation skills. The model described by Mathews did not assess competency in clinical integration but laid the groundwork for future evaluation of clinical outcomes in the cohort of certified hospitalists.
Although experts may not agree on all aspects of competency in POCUS, most will agree with the basic principles outlined by Mathews and Zwank. Initial certification should be based on training and an initial assessment of competency. Components of training should include ultrasound didactics, mentored hands-on practice, independent hands-on practice, and image interpretation practice. Ongoing certification should be based on quality assurance incorporated with an ongoing assessment of skills. Additionally, most experts will agree that competency can be recognized, and formative and summative assessments that combine a gestalt of provider skills with quantitative scoring systems using checklists are likely the best approach.
The real question is, what is the goal of certification of POCUS competency? Development of an institutional certification process demands substantive resources of the institution and time of the providers. Institutions would have to invest in equipment and staff to operate a full-time certification program, given the large number of providers that use POCUS and justify why substantive resources are being dedicated to certify POCUS skills and not others. Providers may be dissuaded from using POCUS if certification requirements are burdensome, which has potential negative consequences, such as reverting back to performing bedside procedures without ultrasound guidance or referring all patients to interventional radiology.
Conceptually, one may speculate that certification is required for providers to bill for POCUS exams, but certification is not required to bill, although institutions may require certification before granting privileges to use POCUS. However, based on the emergency medicine experience, a specialty that has been using POCUS for more than 20 years, billing may not be the main driver of POCUS use. A recent review of 2012 Medicare data revealed that <1% of emergency medicine providers received reimbursement for limited ultrasound exams.3 Despite the Accreditation Council for Graduate Medical Education (ACGME) requirement for POCUS competency of all graduating emergency medicine residents since 2001 and the increasing POCUS use reported by emergency medicine physicians,4,5 most emergency medicine physicians are not billing for POCUS exams. Maybe use of POCUS as a “quick look” or extension of the physical examination is more common than previously thought. Although billing for POCUS exams can generate some clinical revenue, the benefits for the healthcare system by expediting care,6,7 reducing ancillary testing,8,9 and reducing procedural complications10,11 likely outweigh the small gains from billing for limited ultrasound exams. As healthcare payment models evolve to reward healthcare systems that achieve good outcomes rather than services rendered, certification for the sole purpose of billing may become obsolete. Furthermore, concerns about billing increasing medical liability from using POCUS are likely overstated because few lawsuits have resulted from missed diagnoses by POCUS, and most lawsuits have been from failure to perform a POCUS exam in a timely manner.12,13
Many medical students graduating today have had some training in POCUS14 and, as this new generation of physicians enters the workforce, they will likely view POCUS as part of their routine bedside evaluation of patients. If POCUS training is integrated into medical school and residency curricula, and national board certification incorporates basic POCUS competency, then most institutions may no longer feel obligated to certify POCUS competency locally, and institutional certification programs, such as the one described by Mathews and Zwank, would become obsolete.
For now, until all providers enter the workforce with basic competency in POCUS and medical culture accepts that ultrasound is a diagnostic tool available to any trained provider, hospitalists may need to provide proof of their competence through intramural or extramural certification. The work of Mathews and Zwank provides an example of how local certification processes can be established. In a future edition of the Journal of Hospital Medicine, the Society of Hospital Medicine Point-of-Care Ultrasound Task Force will present a position statement with recommendations for certification of competency in bedside ultrasound-guided procedures.
Disclosure
Nilam Soni receives support from the U.S. Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1). Brian P. Lucas receives support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Bahner DP, Hughes D, Royall NA. I-AIM: a novel model for teaching and performing focused sonography. J Ultrasound Med. 2012;31:295-300. PubMed
2. Mathews BK, Zwank M. Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol. J Hosp Med. 2017;12(9):767-772. PubMed
3. Hall MK, Hall J, Gross CP, et al. Use of Point-of-Care Ultrasound in the Emergency Department: Insights From the 2012 Medicare National Payment Data Set. J Ultrasound Med. 2016;35:2467-2474. PubMed
4. Amini R, Wyman MT, Hernandez NC, Guisto JA, Adhikari S. Use of Emergency Ultrasound in Arizona Community Emergency Departments. J Ultrasound Med. 2017;36(5):913-921. PubMed
5. Herbst MK, Camargo CA, Jr., Perez A, Moore CL. Use of Point-of-Care Ultrasound in Connecticut Emergency Departments. J Emerg Med. 2015;48:191-196. PubMed
6. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538-542. PubMed
7. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124:766-774. PubMed
8. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146:1574-1577. PubMed
9. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH, Narasimhan M. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest. 2014;145:818-823. PubMed
10. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
11. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
12. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16:1-4. PubMed
13. Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338-341. PubMed
14. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686. PubMed
Any conversation about point-of-care ultrasound (POCUS) inevitably brings up discussion about credentialing, privileging, and certification. While credentialing and privileging are institution-specific processes, competency certification can be extramural through a national board or intramural through an institutional process.
Some institutions have begun to develop intramural certification pathways for POCUS competency in order to grant privileges to hospitalists. In this edition of the Journal of Hospital Medicine, Mathews and Zwank2 describe a multidisciplinary collaboration to provide POCUS training, intramural certification, and quality assurance for hospitalists at one hospital in Minnesota. This model serves as a real-world example of how institutions are addressing the need to certify hospitalists in basic POCUS competency. After engaging stakeholders from radiology, critical care, emergency medicine, and cardiology, institutional standards were developed and hospitalists were assessed for basic POCUS competency. Certification included assessments of hospitalists’ knowledge, image acquisition, and image interpretation skills. The model described by Mathews did not assess competency in clinical integration but laid the groundwork for future evaluation of clinical outcomes in the cohort of certified hospitalists.
Although experts may not agree on all aspects of competency in POCUS, most will agree with the basic principles outlined by Mathews and Zwank. Initial certification should be based on training and an initial assessment of competency. Components of training should include ultrasound didactics, mentored hands-on practice, independent hands-on practice, and image interpretation practice. Ongoing certification should be based on quality assurance incorporated with an ongoing assessment of skills. Additionally, most experts will agree that competency can be recognized, and formative and summative assessments that combine a gestalt of provider skills with quantitative scoring systems using checklists are likely the best approach.
The real question is, what is the goal of certification of POCUS competency? Development of an institutional certification process demands substantive resources of the institution and time of the providers. Institutions would have to invest in equipment and staff to operate a full-time certification program, given the large number of providers that use POCUS and justify why substantive resources are being dedicated to certify POCUS skills and not others. Providers may be dissuaded from using POCUS if certification requirements are burdensome, which has potential negative consequences, such as reverting back to performing bedside procedures without ultrasound guidance or referring all patients to interventional radiology.
Conceptually, one may speculate that certification is required for providers to bill for POCUS exams, but certification is not required to bill, although institutions may require certification before granting privileges to use POCUS. However, based on the emergency medicine experience, a specialty that has been using POCUS for more than 20 years, billing may not be the main driver of POCUS use. A recent review of 2012 Medicare data revealed that <1% of emergency medicine providers received reimbursement for limited ultrasound exams.3 Despite the Accreditation Council for Graduate Medical Education (ACGME) requirement for POCUS competency of all graduating emergency medicine residents since 2001 and the increasing POCUS use reported by emergency medicine physicians,4,5 most emergency medicine physicians are not billing for POCUS exams. Maybe use of POCUS as a “quick look” or extension of the physical examination is more common than previously thought. Although billing for POCUS exams can generate some clinical revenue, the benefits for the healthcare system by expediting care,6,7 reducing ancillary testing,8,9 and reducing procedural complications10,11 likely outweigh the small gains from billing for limited ultrasound exams. As healthcare payment models evolve to reward healthcare systems that achieve good outcomes rather than services rendered, certification for the sole purpose of billing may become obsolete. Furthermore, concerns about billing increasing medical liability from using POCUS are likely overstated because few lawsuits have resulted from missed diagnoses by POCUS, and most lawsuits have been from failure to perform a POCUS exam in a timely manner.12,13
Many medical students graduating today have had some training in POCUS14 and, as this new generation of physicians enters the workforce, they will likely view POCUS as part of their routine bedside evaluation of patients. If POCUS training is integrated into medical school and residency curricula, and national board certification incorporates basic POCUS competency, then most institutions may no longer feel obligated to certify POCUS competency locally, and institutional certification programs, such as the one described by Mathews and Zwank, would become obsolete.
For now, until all providers enter the workforce with basic competency in POCUS and medical culture accepts that ultrasound is a diagnostic tool available to any trained provider, hospitalists may need to provide proof of their competence through intramural or extramural certification. The work of Mathews and Zwank provides an example of how local certification processes can be established. In a future edition of the Journal of Hospital Medicine, the Society of Hospital Medicine Point-of-Care Ultrasound Task Force will present a position statement with recommendations for certification of competency in bedside ultrasound-guided procedures.
Disclosure
Nilam Soni receives support from the U.S. Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1). Brian P. Lucas receives support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Any conversation about point-of-care ultrasound (POCUS) inevitably brings up discussion about credentialing, privileging, and certification. While credentialing and privileging are institution-specific processes, competency certification can be extramural through a national board or intramural through an institutional process.
Some institutions have begun to develop intramural certification pathways for POCUS competency in order to grant privileges to hospitalists. In this edition of the Journal of Hospital Medicine, Mathews and Zwank2 describe a multidisciplinary collaboration to provide POCUS training, intramural certification, and quality assurance for hospitalists at one hospital in Minnesota. This model serves as a real-world example of how institutions are addressing the need to certify hospitalists in basic POCUS competency. After engaging stakeholders from radiology, critical care, emergency medicine, and cardiology, institutional standards were developed and hospitalists were assessed for basic POCUS competency. Certification included assessments of hospitalists’ knowledge, image acquisition, and image interpretation skills. The model described by Mathews did not assess competency in clinical integration but laid the groundwork for future evaluation of clinical outcomes in the cohort of certified hospitalists.
Although experts may not agree on all aspects of competency in POCUS, most will agree with the basic principles outlined by Mathews and Zwank. Initial certification should be based on training and an initial assessment of competency. Components of training should include ultrasound didactics, mentored hands-on practice, independent hands-on practice, and image interpretation practice. Ongoing certification should be based on quality assurance incorporated with an ongoing assessment of skills. Additionally, most experts will agree that competency can be recognized, and formative and summative assessments that combine a gestalt of provider skills with quantitative scoring systems using checklists are likely the best approach.
The real question is, what is the goal of certification of POCUS competency? Development of an institutional certification process demands substantive resources of the institution and time of the providers. Institutions would have to invest in equipment and staff to operate a full-time certification program, given the large number of providers that use POCUS and justify why substantive resources are being dedicated to certify POCUS skills and not others. Providers may be dissuaded from using POCUS if certification requirements are burdensome, which has potential negative consequences, such as reverting back to performing bedside procedures without ultrasound guidance or referring all patients to interventional radiology.
Conceptually, one may speculate that certification is required for providers to bill for POCUS exams, but certification is not required to bill, although institutions may require certification before granting privileges to use POCUS. However, based on the emergency medicine experience, a specialty that has been using POCUS for more than 20 years, billing may not be the main driver of POCUS use. A recent review of 2012 Medicare data revealed that <1% of emergency medicine providers received reimbursement for limited ultrasound exams.3 Despite the Accreditation Council for Graduate Medical Education (ACGME) requirement for POCUS competency of all graduating emergency medicine residents since 2001 and the increasing POCUS use reported by emergency medicine physicians,4,5 most emergency medicine physicians are not billing for POCUS exams. Maybe use of POCUS as a “quick look” or extension of the physical examination is more common than previously thought. Although billing for POCUS exams can generate some clinical revenue, the benefits for the healthcare system by expediting care,6,7 reducing ancillary testing,8,9 and reducing procedural complications10,11 likely outweigh the small gains from billing for limited ultrasound exams. As healthcare payment models evolve to reward healthcare systems that achieve good outcomes rather than services rendered, certification for the sole purpose of billing may become obsolete. Furthermore, concerns about billing increasing medical liability from using POCUS are likely overstated because few lawsuits have resulted from missed diagnoses by POCUS, and most lawsuits have been from failure to perform a POCUS exam in a timely manner.12,13
Many medical students graduating today have had some training in POCUS14 and, as this new generation of physicians enters the workforce, they will likely view POCUS as part of their routine bedside evaluation of patients. If POCUS training is integrated into medical school and residency curricula, and national board certification incorporates basic POCUS competency, then most institutions may no longer feel obligated to certify POCUS competency locally, and institutional certification programs, such as the one described by Mathews and Zwank, would become obsolete.
For now, until all providers enter the workforce with basic competency in POCUS and medical culture accepts that ultrasound is a diagnostic tool available to any trained provider, hospitalists may need to provide proof of their competence through intramural or extramural certification. The work of Mathews and Zwank provides an example of how local certification processes can be established. In a future edition of the Journal of Hospital Medicine, the Society of Hospital Medicine Point-of-Care Ultrasound Task Force will present a position statement with recommendations for certification of competency in bedside ultrasound-guided procedures.
Disclosure
Nilam Soni receives support from the U.S. Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1). Brian P. Lucas receives support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Bahner DP, Hughes D, Royall NA. I-AIM: a novel model for teaching and performing focused sonography. J Ultrasound Med. 2012;31:295-300. PubMed
2. Mathews BK, Zwank M. Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol. J Hosp Med. 2017;12(9):767-772. PubMed
3. Hall MK, Hall J, Gross CP, et al. Use of Point-of-Care Ultrasound in the Emergency Department: Insights From the 2012 Medicare National Payment Data Set. J Ultrasound Med. 2016;35:2467-2474. PubMed
4. Amini R, Wyman MT, Hernandez NC, Guisto JA, Adhikari S. Use of Emergency Ultrasound in Arizona Community Emergency Departments. J Ultrasound Med. 2017;36(5):913-921. PubMed
5. Herbst MK, Camargo CA, Jr., Perez A, Moore CL. Use of Point-of-Care Ultrasound in Connecticut Emergency Departments. J Emerg Med. 2015;48:191-196. PubMed
6. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538-542. PubMed
7. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124:766-774. PubMed
8. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146:1574-1577. PubMed
9. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH, Narasimhan M. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest. 2014;145:818-823. PubMed
10. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
11. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
12. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16:1-4. PubMed
13. Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338-341. PubMed
14. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686. PubMed
1. Bahner DP, Hughes D, Royall NA. I-AIM: a novel model for teaching and performing focused sonography. J Ultrasound Med. 2012;31:295-300. PubMed
2. Mathews BK, Zwank M. Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol. J Hosp Med. 2017;12(9):767-772. PubMed
3. Hall MK, Hall J, Gross CP, et al. Use of Point-of-Care Ultrasound in the Emergency Department: Insights From the 2012 Medicare National Payment Data Set. J Ultrasound Med. 2016;35:2467-2474. PubMed
4. Amini R, Wyman MT, Hernandez NC, Guisto JA, Adhikari S. Use of Emergency Ultrasound in Arizona Community Emergency Departments. J Ultrasound Med. 2017;36(5):913-921. PubMed
5. Herbst MK, Camargo CA, Jr., Perez A, Moore CL. Use of Point-of-Care Ultrasound in Connecticut Emergency Departments. J Emerg Med. 2015;48:191-196. PubMed
6. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538-542. PubMed
7. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124:766-774. PubMed
8. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146:1574-1577. PubMed
9. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH, Narasimhan M. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest. 2014;145:818-823. PubMed
10. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
11. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
12. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16:1-4. PubMed
13. Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338-341. PubMed
14. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686. PubMed
© 2017 Society of Hospital Medicine
A Video Is Worth a Thousand Words
There is no doubt about the importance of assessing, documenting, and honoring patient wishes regarding care. For hospitalized patients, code status is a critical treatment preference to document given that the need for cardiopulmonary resuscitation (CPR) arises suddenly, outcomes are often poor, and the default is for patients to receive the treatment unless they actively decline it. Hospitalists are expected to document code status for every hospitalized patient, but admission code status conversations are often brief—and that might be all right. A code status discussion for a 70-year-old man with no chronic medical problems and excellent functional status who has been admitted for pain after a motor vehicle accident may require only an introduction to the concept of advance care planning, the importance of having a surrogate, and confirmation of full code status. On the other hand, a 45-year-old woman with metastatic pancreatic cancer would likely benefit from a family meeting in which the hospitalist could review her disease course and prognosis, assess her values and priorities in the context of her advanced illness, make treatment recommendations—including code status—that are consistent with her values, and elicit questions.1,2 We need to free up hospitalists from spending time discussing code status with every patient so that they can spend more time in quality goals of care discussions with seriously ill patients. The paradigm of the one doctor—one patient admission code status conversation for every patient is no longer realistic.
As reported by Merino and colleagues in this issue of JHM, video decision aids about CPR for hospitalized patients can offer an innovative solution to determining code status for hospitalized patients.3 The authors conducted a prospective, randomized controlled trial, which enrolled older adults admitted to the hospital medicine service at the Veteran’s Administration (VA) Hospital in Minneapolis. Participants (N = 119) were randomized to usual care or to watch a 6-minute video that explained code status options, used a mannequin to illustrate a mock code, and provided information about potential complications and survival rates. Patients who watched the video were more likely to choose do not resuscitate/do not intubate status, with a large effect size (56% in the intervention group vs. 17% in the control group, P < 0.00001).
This study adds to a growing body of literature about this powerful modality to assist with advanced care planning. Over the past 10 years, studies—conducted primarily by Volandes, El-Jawahri, and colleagues—have demonstrated how video decision aids impact the care that patients want in the setting of cancer, heart failure, serious illness with short prognosis, and future dementia.4-9 This literature has also shown that video decision aids can increase patients’ knowledge about CPR and increase the stability of decisions over time. Further, video decision aids have been well accepted by patients, who report that they would recommended such videos to others. This body of evidence underscores the potential of video decision aids to improve concordance between patient preferences and care provided, which is key given the longstanding and widespread concern about patients receiving care that is inconsistent with their values at the end of life.10 In short, video decision aids work.
Merino and colleagues are the first to examine the use of a video decision aid about code status in a general population of older adults on a hospital medicine service and the second to integrate such a video into usual inpatient care, which are important advancements.2,3 There are several issues that warrant further consideration prior to widely disseminating such a video, however. As the authors note, the participants in this VA study were overwhelmingly white men and their average age was 75. Further, the authors found a nonsignificant trend towards patients in the intervention group having less trust that “my doctors and healthcare team want what is best for me” (76% in the intervention group vs. 93% in the control group; P = 0.083). Decision making about life-sustaining therapies and reactions to communication about serious illness are heavily influenced by cultural and socioeconomic factors, including health literacy.11 It will be important to seek feedback from a diverse group of patients and families to ensure that the video decision aid is interpreted accurately, renders decisions that are consistent with patients’ values, and does not negatively impact the clinician-patient relationship.12 Additionally, as the above cases illustrate, code status discussions should be tailored to patient factors, including illness severity and point in the disease course. Hospitalists will ultimately benefit from having access to multiple different videos about a range of advance care planning topics that can be used when appropriate.
In addition to selecting the right video for the right patient, the next challenge for hospitalists and health systems will be how to implement them within real-world clinical care and a broader approach to advance care planning. There are technical and logistical challenges to displaying videos in hospital rooms, and more significant challenges in ensuring timely follow-up discussions, communication of patients’ dynamic care preferences to their surrogates, changes to inpatient orders, documentation in the electronic medical record where it can be easily found in the future, and completion of advance directives and Physician Orders for Life Sustaining Treatment forms to communicate patients’ goals of care beyond the hospital and health system. Each of these steps is critical and is supported through videos and activities in the free, patient-facing, PREPARE web-based tool (https://www.prepareforyourcare.org/).2,13,14
The ubiquitous presence of videos in our lives speaks to their power to engage and affect us. Video decision aids provide detailed explanations and vivid images that convey more than words can alone. While there is more work to be done to ensure videos are appropriate for all hospitalized patients and support rather than detract from patient-doctor relationships, this study and others like it show that video decision aids are potent tools to promote better decision-making and higher value, more efficient care.
Disclosures
The authors have nothing to disclose.
1. Piscator E, Hedberg P, Göransson K, Djärv T. Survival after in-hospital cardiac arrest is highly associated with the Age-combined Charlson Co-morbidity Index in a cohort study from a two-site Swedish University hospital. Resuscitation. 2016;99:79-83. PubMed
2. Jain A, Corriveau S, Quinn K, Gardhouse A, Vegas DB, You JJ. Video decision aids to assist with advance care planning: a systematic review and meta-analysis. BMJ Open. 2015;5(6):e007491. PubMed
3. Merino AM, Greiner R, Hartwig K. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017:12(9):700-704. PubMed
4. Volandes AE, Levin TT, Slovin S, Carvajal RD, O’Reilly EM, et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention-postintervention study. Cancer. 2012;118(17):4331-4338. PubMed
5. El-Jawahri A, Paasche-Orlow MK, Matlock D, Stevenson LW, Lewis EF, Stewart G, et al. Randomized, ontrolled trial of an advance care planning video decision support tool for patients with advanced heart failure. Circulation. 2016;134(1):52-60. PubMed
6. El-Jawahri A, Mitchell SL, Paasche-Orlow MK, Temel JS, Jackson VA, Rutledge RR, et al. A randomized controlled trial of a CPR and intubation video decision support tool for hospitalized patients. J Gen Intern Med. 2015;30(8):1071-1080. PubMed
7. Volandes AE, Ferguson LA, Davis AD, Hull NC, Green MJ, Chang Y, et al. Assessing end-of-life preferences for advanced dementia in rural patients using an educational video: a randomized controlled trial. J Palliat Med. 2011;14(2):169-177. PubMed
8. Volandes AE, Paasche-Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009;338:b2159. PubMed
9. El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305-310. PubMed
10. IOM (Institute of Medicine). Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2015. PubMed
11. Castillo LS, Williams BA, Hooper SM, Sabatino CP, Weithorn LA, Sudore RL. Lost in translation: the unintended consequences of advance directive law on clinical care. Ann Intern Med. 2011;154(2):121-128. PubMed
12. Anderson WG, Cimino JW, Lo B. Seriously ill hospitalized patients’ perspectives on the benefits and harms of two models of hospital CPR discussions. Patient Educ Couns. 2013;93(3):633-640. PubMed
13. Sudore RL, Boscardin J, Feuz MA, McMahan RD, Katen MT, Barnes DE. Effect of the PREPARE website vs an easy-to-read advance directive on advance care planning documentation and engagement among veterans: a randomized clinical trial [published online ahead of print May 18, 2017]. JAMA Intern Med. 2017; May 18. doi: 10.1001/jamainternmed.20171607. PubMed
14. Improving Communication about Serious Illness: Implementation Toolkit. SHM Center for Quality Improvement. Society of Hospital Medicine. 2017. http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/EOL/Palliative_Care_Home_Society_of_Hospital_Medicine.aspx. Accessed June 13, 2017.
There is no doubt about the importance of assessing, documenting, and honoring patient wishes regarding care. For hospitalized patients, code status is a critical treatment preference to document given that the need for cardiopulmonary resuscitation (CPR) arises suddenly, outcomes are often poor, and the default is for patients to receive the treatment unless they actively decline it. Hospitalists are expected to document code status for every hospitalized patient, but admission code status conversations are often brief—and that might be all right. A code status discussion for a 70-year-old man with no chronic medical problems and excellent functional status who has been admitted for pain after a motor vehicle accident may require only an introduction to the concept of advance care planning, the importance of having a surrogate, and confirmation of full code status. On the other hand, a 45-year-old woman with metastatic pancreatic cancer would likely benefit from a family meeting in which the hospitalist could review her disease course and prognosis, assess her values and priorities in the context of her advanced illness, make treatment recommendations—including code status—that are consistent with her values, and elicit questions.1,2 We need to free up hospitalists from spending time discussing code status with every patient so that they can spend more time in quality goals of care discussions with seriously ill patients. The paradigm of the one doctor—one patient admission code status conversation for every patient is no longer realistic.
As reported by Merino and colleagues in this issue of JHM, video decision aids about CPR for hospitalized patients can offer an innovative solution to determining code status for hospitalized patients.3 The authors conducted a prospective, randomized controlled trial, which enrolled older adults admitted to the hospital medicine service at the Veteran’s Administration (VA) Hospital in Minneapolis. Participants (N = 119) were randomized to usual care or to watch a 6-minute video that explained code status options, used a mannequin to illustrate a mock code, and provided information about potential complications and survival rates. Patients who watched the video were more likely to choose do not resuscitate/do not intubate status, with a large effect size (56% in the intervention group vs. 17% in the control group, P < 0.00001).
This study adds to a growing body of literature about this powerful modality to assist with advanced care planning. Over the past 10 years, studies—conducted primarily by Volandes, El-Jawahri, and colleagues—have demonstrated how video decision aids impact the care that patients want in the setting of cancer, heart failure, serious illness with short prognosis, and future dementia.4-9 This literature has also shown that video decision aids can increase patients’ knowledge about CPR and increase the stability of decisions over time. Further, video decision aids have been well accepted by patients, who report that they would recommended such videos to others. This body of evidence underscores the potential of video decision aids to improve concordance between patient preferences and care provided, which is key given the longstanding and widespread concern about patients receiving care that is inconsistent with their values at the end of life.10 In short, video decision aids work.
Merino and colleagues are the first to examine the use of a video decision aid about code status in a general population of older adults on a hospital medicine service and the second to integrate such a video into usual inpatient care, which are important advancements.2,3 There are several issues that warrant further consideration prior to widely disseminating such a video, however. As the authors note, the participants in this VA study were overwhelmingly white men and their average age was 75. Further, the authors found a nonsignificant trend towards patients in the intervention group having less trust that “my doctors and healthcare team want what is best for me” (76% in the intervention group vs. 93% in the control group; P = 0.083). Decision making about life-sustaining therapies and reactions to communication about serious illness are heavily influenced by cultural and socioeconomic factors, including health literacy.11 It will be important to seek feedback from a diverse group of patients and families to ensure that the video decision aid is interpreted accurately, renders decisions that are consistent with patients’ values, and does not negatively impact the clinician-patient relationship.12 Additionally, as the above cases illustrate, code status discussions should be tailored to patient factors, including illness severity and point in the disease course. Hospitalists will ultimately benefit from having access to multiple different videos about a range of advance care planning topics that can be used when appropriate.
In addition to selecting the right video for the right patient, the next challenge for hospitalists and health systems will be how to implement them within real-world clinical care and a broader approach to advance care planning. There are technical and logistical challenges to displaying videos in hospital rooms, and more significant challenges in ensuring timely follow-up discussions, communication of patients’ dynamic care preferences to their surrogates, changes to inpatient orders, documentation in the electronic medical record where it can be easily found in the future, and completion of advance directives and Physician Orders for Life Sustaining Treatment forms to communicate patients’ goals of care beyond the hospital and health system. Each of these steps is critical and is supported through videos and activities in the free, patient-facing, PREPARE web-based tool (https://www.prepareforyourcare.org/).2,13,14
The ubiquitous presence of videos in our lives speaks to their power to engage and affect us. Video decision aids provide detailed explanations and vivid images that convey more than words can alone. While there is more work to be done to ensure videos are appropriate for all hospitalized patients and support rather than detract from patient-doctor relationships, this study and others like it show that video decision aids are potent tools to promote better decision-making and higher value, more efficient care.
Disclosures
The authors have nothing to disclose.
There is no doubt about the importance of assessing, documenting, and honoring patient wishes regarding care. For hospitalized patients, code status is a critical treatment preference to document given that the need for cardiopulmonary resuscitation (CPR) arises suddenly, outcomes are often poor, and the default is for patients to receive the treatment unless they actively decline it. Hospitalists are expected to document code status for every hospitalized patient, but admission code status conversations are often brief—and that might be all right. A code status discussion for a 70-year-old man with no chronic medical problems and excellent functional status who has been admitted for pain after a motor vehicle accident may require only an introduction to the concept of advance care planning, the importance of having a surrogate, and confirmation of full code status. On the other hand, a 45-year-old woman with metastatic pancreatic cancer would likely benefit from a family meeting in which the hospitalist could review her disease course and prognosis, assess her values and priorities in the context of her advanced illness, make treatment recommendations—including code status—that are consistent with her values, and elicit questions.1,2 We need to free up hospitalists from spending time discussing code status with every patient so that they can spend more time in quality goals of care discussions with seriously ill patients. The paradigm of the one doctor—one patient admission code status conversation for every patient is no longer realistic.
As reported by Merino and colleagues in this issue of JHM, video decision aids about CPR for hospitalized patients can offer an innovative solution to determining code status for hospitalized patients.3 The authors conducted a prospective, randomized controlled trial, which enrolled older adults admitted to the hospital medicine service at the Veteran’s Administration (VA) Hospital in Minneapolis. Participants (N = 119) were randomized to usual care or to watch a 6-minute video that explained code status options, used a mannequin to illustrate a mock code, and provided information about potential complications and survival rates. Patients who watched the video were more likely to choose do not resuscitate/do not intubate status, with a large effect size (56% in the intervention group vs. 17% in the control group, P < 0.00001).
This study adds to a growing body of literature about this powerful modality to assist with advanced care planning. Over the past 10 years, studies—conducted primarily by Volandes, El-Jawahri, and colleagues—have demonstrated how video decision aids impact the care that patients want in the setting of cancer, heart failure, serious illness with short prognosis, and future dementia.4-9 This literature has also shown that video decision aids can increase patients’ knowledge about CPR and increase the stability of decisions over time. Further, video decision aids have been well accepted by patients, who report that they would recommended such videos to others. This body of evidence underscores the potential of video decision aids to improve concordance between patient preferences and care provided, which is key given the longstanding and widespread concern about patients receiving care that is inconsistent with their values at the end of life.10 In short, video decision aids work.
Merino and colleagues are the first to examine the use of a video decision aid about code status in a general population of older adults on a hospital medicine service and the second to integrate such a video into usual inpatient care, which are important advancements.2,3 There are several issues that warrant further consideration prior to widely disseminating such a video, however. As the authors note, the participants in this VA study were overwhelmingly white men and their average age was 75. Further, the authors found a nonsignificant trend towards patients in the intervention group having less trust that “my doctors and healthcare team want what is best for me” (76% in the intervention group vs. 93% in the control group; P = 0.083). Decision making about life-sustaining therapies and reactions to communication about serious illness are heavily influenced by cultural and socioeconomic factors, including health literacy.11 It will be important to seek feedback from a diverse group of patients and families to ensure that the video decision aid is interpreted accurately, renders decisions that are consistent with patients’ values, and does not negatively impact the clinician-patient relationship.12 Additionally, as the above cases illustrate, code status discussions should be tailored to patient factors, including illness severity and point in the disease course. Hospitalists will ultimately benefit from having access to multiple different videos about a range of advance care planning topics that can be used when appropriate.
In addition to selecting the right video for the right patient, the next challenge for hospitalists and health systems will be how to implement them within real-world clinical care and a broader approach to advance care planning. There are technical and logistical challenges to displaying videos in hospital rooms, and more significant challenges in ensuring timely follow-up discussions, communication of patients’ dynamic care preferences to their surrogates, changes to inpatient orders, documentation in the electronic medical record where it can be easily found in the future, and completion of advance directives and Physician Orders for Life Sustaining Treatment forms to communicate patients’ goals of care beyond the hospital and health system. Each of these steps is critical and is supported through videos and activities in the free, patient-facing, PREPARE web-based tool (https://www.prepareforyourcare.org/).2,13,14
The ubiquitous presence of videos in our lives speaks to their power to engage and affect us. Video decision aids provide detailed explanations and vivid images that convey more than words can alone. While there is more work to be done to ensure videos are appropriate for all hospitalized patients and support rather than detract from patient-doctor relationships, this study and others like it show that video decision aids are potent tools to promote better decision-making and higher value, more efficient care.
Disclosures
The authors have nothing to disclose.
1. Piscator E, Hedberg P, Göransson K, Djärv T. Survival after in-hospital cardiac arrest is highly associated with the Age-combined Charlson Co-morbidity Index in a cohort study from a two-site Swedish University hospital. Resuscitation. 2016;99:79-83. PubMed
2. Jain A, Corriveau S, Quinn K, Gardhouse A, Vegas DB, You JJ. Video decision aids to assist with advance care planning: a systematic review and meta-analysis. BMJ Open. 2015;5(6):e007491. PubMed
3. Merino AM, Greiner R, Hartwig K. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017:12(9):700-704. PubMed
4. Volandes AE, Levin TT, Slovin S, Carvajal RD, O’Reilly EM, et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention-postintervention study. Cancer. 2012;118(17):4331-4338. PubMed
5. El-Jawahri A, Paasche-Orlow MK, Matlock D, Stevenson LW, Lewis EF, Stewart G, et al. Randomized, ontrolled trial of an advance care planning video decision support tool for patients with advanced heart failure. Circulation. 2016;134(1):52-60. PubMed
6. El-Jawahri A, Mitchell SL, Paasche-Orlow MK, Temel JS, Jackson VA, Rutledge RR, et al. A randomized controlled trial of a CPR and intubation video decision support tool for hospitalized patients. J Gen Intern Med. 2015;30(8):1071-1080. PubMed
7. Volandes AE, Ferguson LA, Davis AD, Hull NC, Green MJ, Chang Y, et al. Assessing end-of-life preferences for advanced dementia in rural patients using an educational video: a randomized controlled trial. J Palliat Med. 2011;14(2):169-177. PubMed
8. Volandes AE, Paasche-Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009;338:b2159. PubMed
9. El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305-310. PubMed
10. IOM (Institute of Medicine). Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2015. PubMed
11. Castillo LS, Williams BA, Hooper SM, Sabatino CP, Weithorn LA, Sudore RL. Lost in translation: the unintended consequences of advance directive law on clinical care. Ann Intern Med. 2011;154(2):121-128. PubMed
12. Anderson WG, Cimino JW, Lo B. Seriously ill hospitalized patients’ perspectives on the benefits and harms of two models of hospital CPR discussions. Patient Educ Couns. 2013;93(3):633-640. PubMed
13. Sudore RL, Boscardin J, Feuz MA, McMahan RD, Katen MT, Barnes DE. Effect of the PREPARE website vs an easy-to-read advance directive on advance care planning documentation and engagement among veterans: a randomized clinical trial [published online ahead of print May 18, 2017]. JAMA Intern Med. 2017; May 18. doi: 10.1001/jamainternmed.20171607. PubMed
14. Improving Communication about Serious Illness: Implementation Toolkit. SHM Center for Quality Improvement. Society of Hospital Medicine. 2017. http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/EOL/Palliative_Care_Home_Society_of_Hospital_Medicine.aspx. Accessed June 13, 2017.
1. Piscator E, Hedberg P, Göransson K, Djärv T. Survival after in-hospital cardiac arrest is highly associated with the Age-combined Charlson Co-morbidity Index in a cohort study from a two-site Swedish University hospital. Resuscitation. 2016;99:79-83. PubMed
2. Jain A, Corriveau S, Quinn K, Gardhouse A, Vegas DB, You JJ. Video decision aids to assist with advance care planning: a systematic review and meta-analysis. BMJ Open. 2015;5(6):e007491. PubMed
3. Merino AM, Greiner R, Hartwig K. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017:12(9):700-704. PubMed
4. Volandes AE, Levin TT, Slovin S, Carvajal RD, O’Reilly EM, et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention-postintervention study. Cancer. 2012;118(17):4331-4338. PubMed
5. El-Jawahri A, Paasche-Orlow MK, Matlock D, Stevenson LW, Lewis EF, Stewart G, et al. Randomized, ontrolled trial of an advance care planning video decision support tool for patients with advanced heart failure. Circulation. 2016;134(1):52-60. PubMed
6. El-Jawahri A, Mitchell SL, Paasche-Orlow MK, Temel JS, Jackson VA, Rutledge RR, et al. A randomized controlled trial of a CPR and intubation video decision support tool for hospitalized patients. J Gen Intern Med. 2015;30(8):1071-1080. PubMed
7. Volandes AE, Ferguson LA, Davis AD, Hull NC, Green MJ, Chang Y, et al. Assessing end-of-life preferences for advanced dementia in rural patients using an educational video: a randomized controlled trial. J Palliat Med. 2011;14(2):169-177. PubMed
8. Volandes AE, Paasche-Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009;338:b2159. PubMed
9. El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305-310. PubMed
10. IOM (Institute of Medicine). Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2015. PubMed
11. Castillo LS, Williams BA, Hooper SM, Sabatino CP, Weithorn LA, Sudore RL. Lost in translation: the unintended consequences of advance directive law on clinical care. Ann Intern Med. 2011;154(2):121-128. PubMed
12. Anderson WG, Cimino JW, Lo B. Seriously ill hospitalized patients’ perspectives on the benefits and harms of two models of hospital CPR discussions. Patient Educ Couns. 2013;93(3):633-640. PubMed
13. Sudore RL, Boscardin J, Feuz MA, McMahan RD, Katen MT, Barnes DE. Effect of the PREPARE website vs an easy-to-read advance directive on advance care planning documentation and engagement among veterans: a randomized clinical trial [published online ahead of print May 18, 2017]. JAMA Intern Med. 2017; May 18. doi: 10.1001/jamainternmed.20171607. PubMed
14. Improving Communication about Serious Illness: Implementation Toolkit. SHM Center for Quality Improvement. Society of Hospital Medicine. 2017. http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/EOL/Palliative_Care_Home_Society_of_Hospital_Medicine.aspx. Accessed June 13, 2017.
© 2017 Society of Hospital Medicine
Reducing Readmissions or Length of Stay—Which Is More Important?
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
© 2017 Society of Hospital Medicine
Continued Learning in Supporting Value-Based Decision Making
Physicians, researchers, and policymakers aspire to improve the value of healthcare, with reduced overall costs of care and improved outcomes. An important component of increasing healthcare costs in the United States is the rising cost of prescription medications, accounting for an estimated 17% of all spending in healthcare services.1 One potentially modifiable driver of low-value prescribing is poor awareness of medication cost.2 While displaying price to the ordering physician has reduced laboratory order volume and associated testing costs,3,4 applying cost transparency to medication ordering has produced variable results, perhaps reflecting conceptual differences in decision making regarding diagnosis and treatment.4-6
In this issue of the Journal of Hospital Medicine, Conway et al.7 performed a retrospective analysis applying interrupted times series models to measure the impact of passive cost display on the ordering frequency of 9 high-cost intravenous (IV) or inhaled medications that were identified as likely overused. For 7 of the IV medications, lower-cost oral alternatives were available; 2 study medications had no clear therapeutic alternatives. It was expected that lower-cost oral alternatives would have a concomitant increase in ordering rate as the order rate of the study medications decreased (eg, oral linezolid use would increase as IV linezolid use decreased). Order rate was the primary outcome, reported each week as treatment orders per 10,000 patient days, and was compared for both the pre- and postimplementation time periods. The particular methodology of segmented regressions allowed the research team to control for preintervention trends in medication ordering, as well as to analyze both immediate and delayed effects of the cost-display intervention. The research team framed the cost display as a passive approach. The intervention displayed average wholesale cost data and lower-cost oral alternatives on the ordering screen, which did not significantly reduce the ordering rate. Over the course of the study, outside influences led to 2 more active approaches to higher-cost medications, and Conway et al. wisely measured their effect as well. Specifically, the IV pantoprazole ordering rate decreased after restrictions secondary to a national medication shortage, and the oral voriconazole ordering rate decreased following an oncology order set change from oral voriconazole to oral posaconazole. These ordering-rate decreases were not temporally related to the implementation of the cost display intervention.
It is important to note several limitations of this study, some of which the authors discuss in the manuscript. Because 2 of the medications studied (eculizumab and calcitonin) do not have direct therapeutic alternatives, it is not surprising that price display alone would have no effect. The ordering providers who received this cost information had a more complex decision to make than they would in a scenario with a lower-cost alternative, essentially requiring them to ask “Does this patient need this class of medications at all?” rather than simply, “Is a lower-cost alternative appropriate?” Similarly, choosing medication alternatives that would require different routes of administration (ie, IV and oral) may have limited the effectiveness of a price intervention, given that factors such as illness severity also may influence the decision between IV and oral agents. Thus, the lack of an effect for the price display intervention for these specific medications may not be generalizable to all other medication decisions. Additionally, this manuscript offers limited data on the context in which the intervention was implemented and what adaptations, if any, were made based on early findings. The results may have varied greatly based on the visual design and how the cost display was presented within the electronic medical record. The wider organizational context may also have affected the intervention’s impact. A cost-display intervention appearing in isolation could understandably have a different impact, compared with an intervention within the context of a broader cost/value curriculum directed at house staff and faculty.
In summary, Conway et al. found that just displaying cost data did little to change prescribing patterns, but that more active approaches were quite efficacious. So where does this leave value-minded hospitalists looking to reduce overuse? Relatedly, what are the next steps for research and improvement science? We think there are 3 key strategic areas on which to focus. First, behavioral economics offers a critically important middle ground between the passive approaches studied here and more heavy-handed approaches that may limit provider autonomy, such as restricting drug use at the formulary.8 An improved choice architecture that presents the preferred higher-value option as the default selection may result in improved adoption of the high-value choice while also preserving provider autonomy and expertise required when clinical circumstances make the higher-cost drug the better choice.9,10 The second consideration is to minimize ethical tensions between cost displays that discourage use and a provider’s belief that a treatment is beneficial. Using available ethical frameworks for high-value care that engage both patient and societal concerns may help us choose and design interventions with more successful outcomes.11 Finally, research has shown that providers have poor knowledge of both cost and the relative benefits and harms of treatments and testing.12 Thus, the third opportunity for improvement is to provide appropriate clinical information (ie, relative therapeutic equivalency or adverse effects in alternative therapies) to support decision making at the point of order entry. Encouraging data already exists regarding how drug facts boxes can help patients understand benefits and side effects.13 A similar approach may aid physicians and may prove an easier task than improving patient understanding, given physicians’ substantial existing knowledge. These strategies may help guide providers to make a more informed value determination and obviate some ethical concerns related to clinical decisions based on cost alone. Despite their negative results, Conway et al.7 provided additional evidence that influencing complex decision making is not easy. However, we believe that continuing research into the factors that lead to successful value interventions has incredible potential for supporting high-value decision making in the future.
Disclosure
Nothing to report.
1. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858-871. PubMed
2. Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. PubMed
3. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
4. Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. PubMed
5. Guterman JJ, Chernof BA, Mares B, Gross-Schulman SG, Gan PG, Thomas D. Modifying provider behavior: a low-tech approach to pharmaceutical ordering. J Gen Intern Med. 2002;17(10):792-796. PubMed
6. Goetz C, Rotman SR, Hartoularos G, Bishop TF. The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835-842. PubMed
7. Conway SJ, Brotman DJ, Merola D, et al. Impact of displaying inpatient pharmaceutical costs at the time of order entry: lessons from a tertiary care center. J Hosp Med. 2017;12(8):639-645. PubMed
8. Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. New Haven: Yale University Press: 2008.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. PubMed
10. Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965-970. PubMed
11. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. PubMed
12. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. PubMed
13. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516-527. PubMed
Physicians, researchers, and policymakers aspire to improve the value of healthcare, with reduced overall costs of care and improved outcomes. An important component of increasing healthcare costs in the United States is the rising cost of prescription medications, accounting for an estimated 17% of all spending in healthcare services.1 One potentially modifiable driver of low-value prescribing is poor awareness of medication cost.2 While displaying price to the ordering physician has reduced laboratory order volume and associated testing costs,3,4 applying cost transparency to medication ordering has produced variable results, perhaps reflecting conceptual differences in decision making regarding diagnosis and treatment.4-6
In this issue of the Journal of Hospital Medicine, Conway et al.7 performed a retrospective analysis applying interrupted times series models to measure the impact of passive cost display on the ordering frequency of 9 high-cost intravenous (IV) or inhaled medications that were identified as likely overused. For 7 of the IV medications, lower-cost oral alternatives were available; 2 study medications had no clear therapeutic alternatives. It was expected that lower-cost oral alternatives would have a concomitant increase in ordering rate as the order rate of the study medications decreased (eg, oral linezolid use would increase as IV linezolid use decreased). Order rate was the primary outcome, reported each week as treatment orders per 10,000 patient days, and was compared for both the pre- and postimplementation time periods. The particular methodology of segmented regressions allowed the research team to control for preintervention trends in medication ordering, as well as to analyze both immediate and delayed effects of the cost-display intervention. The research team framed the cost display as a passive approach. The intervention displayed average wholesale cost data and lower-cost oral alternatives on the ordering screen, which did not significantly reduce the ordering rate. Over the course of the study, outside influences led to 2 more active approaches to higher-cost medications, and Conway et al. wisely measured their effect as well. Specifically, the IV pantoprazole ordering rate decreased after restrictions secondary to a national medication shortage, and the oral voriconazole ordering rate decreased following an oncology order set change from oral voriconazole to oral posaconazole. These ordering-rate decreases were not temporally related to the implementation of the cost display intervention.
It is important to note several limitations of this study, some of which the authors discuss in the manuscript. Because 2 of the medications studied (eculizumab and calcitonin) do not have direct therapeutic alternatives, it is not surprising that price display alone would have no effect. The ordering providers who received this cost information had a more complex decision to make than they would in a scenario with a lower-cost alternative, essentially requiring them to ask “Does this patient need this class of medications at all?” rather than simply, “Is a lower-cost alternative appropriate?” Similarly, choosing medication alternatives that would require different routes of administration (ie, IV and oral) may have limited the effectiveness of a price intervention, given that factors such as illness severity also may influence the decision between IV and oral agents. Thus, the lack of an effect for the price display intervention for these specific medications may not be generalizable to all other medication decisions. Additionally, this manuscript offers limited data on the context in which the intervention was implemented and what adaptations, if any, were made based on early findings. The results may have varied greatly based on the visual design and how the cost display was presented within the electronic medical record. The wider organizational context may also have affected the intervention’s impact. A cost-display intervention appearing in isolation could understandably have a different impact, compared with an intervention within the context of a broader cost/value curriculum directed at house staff and faculty.
In summary, Conway et al. found that just displaying cost data did little to change prescribing patterns, but that more active approaches were quite efficacious. So where does this leave value-minded hospitalists looking to reduce overuse? Relatedly, what are the next steps for research and improvement science? We think there are 3 key strategic areas on which to focus. First, behavioral economics offers a critically important middle ground between the passive approaches studied here and more heavy-handed approaches that may limit provider autonomy, such as restricting drug use at the formulary.8 An improved choice architecture that presents the preferred higher-value option as the default selection may result in improved adoption of the high-value choice while also preserving provider autonomy and expertise required when clinical circumstances make the higher-cost drug the better choice.9,10 The second consideration is to minimize ethical tensions between cost displays that discourage use and a provider’s belief that a treatment is beneficial. Using available ethical frameworks for high-value care that engage both patient and societal concerns may help us choose and design interventions with more successful outcomes.11 Finally, research has shown that providers have poor knowledge of both cost and the relative benefits and harms of treatments and testing.12 Thus, the third opportunity for improvement is to provide appropriate clinical information (ie, relative therapeutic equivalency or adverse effects in alternative therapies) to support decision making at the point of order entry. Encouraging data already exists regarding how drug facts boxes can help patients understand benefits and side effects.13 A similar approach may aid physicians and may prove an easier task than improving patient understanding, given physicians’ substantial existing knowledge. These strategies may help guide providers to make a more informed value determination and obviate some ethical concerns related to clinical decisions based on cost alone. Despite their negative results, Conway et al.7 provided additional evidence that influencing complex decision making is not easy. However, we believe that continuing research into the factors that lead to successful value interventions has incredible potential for supporting high-value decision making in the future.
Disclosure
Nothing to report.
Physicians, researchers, and policymakers aspire to improve the value of healthcare, with reduced overall costs of care and improved outcomes. An important component of increasing healthcare costs in the United States is the rising cost of prescription medications, accounting for an estimated 17% of all spending in healthcare services.1 One potentially modifiable driver of low-value prescribing is poor awareness of medication cost.2 While displaying price to the ordering physician has reduced laboratory order volume and associated testing costs,3,4 applying cost transparency to medication ordering has produced variable results, perhaps reflecting conceptual differences in decision making regarding diagnosis and treatment.4-6
In this issue of the Journal of Hospital Medicine, Conway et al.7 performed a retrospective analysis applying interrupted times series models to measure the impact of passive cost display on the ordering frequency of 9 high-cost intravenous (IV) or inhaled medications that were identified as likely overused. For 7 of the IV medications, lower-cost oral alternatives were available; 2 study medications had no clear therapeutic alternatives. It was expected that lower-cost oral alternatives would have a concomitant increase in ordering rate as the order rate of the study medications decreased (eg, oral linezolid use would increase as IV linezolid use decreased). Order rate was the primary outcome, reported each week as treatment orders per 10,000 patient days, and was compared for both the pre- and postimplementation time periods. The particular methodology of segmented regressions allowed the research team to control for preintervention trends in medication ordering, as well as to analyze both immediate and delayed effects of the cost-display intervention. The research team framed the cost display as a passive approach. The intervention displayed average wholesale cost data and lower-cost oral alternatives on the ordering screen, which did not significantly reduce the ordering rate. Over the course of the study, outside influences led to 2 more active approaches to higher-cost medications, and Conway et al. wisely measured their effect as well. Specifically, the IV pantoprazole ordering rate decreased after restrictions secondary to a national medication shortage, and the oral voriconazole ordering rate decreased following an oncology order set change from oral voriconazole to oral posaconazole. These ordering-rate decreases were not temporally related to the implementation of the cost display intervention.
It is important to note several limitations of this study, some of which the authors discuss in the manuscript. Because 2 of the medications studied (eculizumab and calcitonin) do not have direct therapeutic alternatives, it is not surprising that price display alone would have no effect. The ordering providers who received this cost information had a more complex decision to make than they would in a scenario with a lower-cost alternative, essentially requiring them to ask “Does this patient need this class of medications at all?” rather than simply, “Is a lower-cost alternative appropriate?” Similarly, choosing medication alternatives that would require different routes of administration (ie, IV and oral) may have limited the effectiveness of a price intervention, given that factors such as illness severity also may influence the decision between IV and oral agents. Thus, the lack of an effect for the price display intervention for these specific medications may not be generalizable to all other medication decisions. Additionally, this manuscript offers limited data on the context in which the intervention was implemented and what adaptations, if any, were made based on early findings. The results may have varied greatly based on the visual design and how the cost display was presented within the electronic medical record. The wider organizational context may also have affected the intervention’s impact. A cost-display intervention appearing in isolation could understandably have a different impact, compared with an intervention within the context of a broader cost/value curriculum directed at house staff and faculty.
In summary, Conway et al. found that just displaying cost data did little to change prescribing patterns, but that more active approaches were quite efficacious. So where does this leave value-minded hospitalists looking to reduce overuse? Relatedly, what are the next steps for research and improvement science? We think there are 3 key strategic areas on which to focus. First, behavioral economics offers a critically important middle ground between the passive approaches studied here and more heavy-handed approaches that may limit provider autonomy, such as restricting drug use at the formulary.8 An improved choice architecture that presents the preferred higher-value option as the default selection may result in improved adoption of the high-value choice while also preserving provider autonomy and expertise required when clinical circumstances make the higher-cost drug the better choice.9,10 The second consideration is to minimize ethical tensions between cost displays that discourage use and a provider’s belief that a treatment is beneficial. Using available ethical frameworks for high-value care that engage both patient and societal concerns may help us choose and design interventions with more successful outcomes.11 Finally, research has shown that providers have poor knowledge of both cost and the relative benefits and harms of treatments and testing.12 Thus, the third opportunity for improvement is to provide appropriate clinical information (ie, relative therapeutic equivalency or adverse effects in alternative therapies) to support decision making at the point of order entry. Encouraging data already exists regarding how drug facts boxes can help patients understand benefits and side effects.13 A similar approach may aid physicians and may prove an easier task than improving patient understanding, given physicians’ substantial existing knowledge. These strategies may help guide providers to make a more informed value determination and obviate some ethical concerns related to clinical decisions based on cost alone. Despite their negative results, Conway et al.7 provided additional evidence that influencing complex decision making is not easy. However, we believe that continuing research into the factors that lead to successful value interventions has incredible potential for supporting high-value decision making in the future.
Disclosure
Nothing to report.
1. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858-871. PubMed
2. Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. PubMed
3. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
4. Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. PubMed
5. Guterman JJ, Chernof BA, Mares B, Gross-Schulman SG, Gan PG, Thomas D. Modifying provider behavior: a low-tech approach to pharmaceutical ordering. J Gen Intern Med. 2002;17(10):792-796. PubMed
6. Goetz C, Rotman SR, Hartoularos G, Bishop TF. The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835-842. PubMed
7. Conway SJ, Brotman DJ, Merola D, et al. Impact of displaying inpatient pharmaceutical costs at the time of order entry: lessons from a tertiary care center. J Hosp Med. 2017;12(8):639-645. PubMed
8. Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. New Haven: Yale University Press: 2008.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. PubMed
10. Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965-970. PubMed
11. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. PubMed
12. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. PubMed
13. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516-527. PubMed
1. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858-871. PubMed
2. Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. PubMed
3. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
4. Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. PubMed
5. Guterman JJ, Chernof BA, Mares B, Gross-Schulman SG, Gan PG, Thomas D. Modifying provider behavior: a low-tech approach to pharmaceutical ordering. J Gen Intern Med. 2002;17(10):792-796. PubMed
6. Goetz C, Rotman SR, Hartoularos G, Bishop TF. The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835-842. PubMed
7. Conway SJ, Brotman DJ, Merola D, et al. Impact of displaying inpatient pharmaceutical costs at the time of order entry: lessons from a tertiary care center. J Hosp Med. 2017;12(8):639-645. PubMed
8. Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. New Haven: Yale University Press: 2008.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. PubMed
10. Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965-970. PubMed
11. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. PubMed
12. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. PubMed
13. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516-527. PubMed
© 2017 Society of Hospital Medicine
Syphilis 100 years later: Another lost opportunity?
According to a report from the US Centers for Disease Control and Prevention (CDC) on the incidence of sexually transmitted diseases (STDs), “Total combined cases of chlamydia, gonorrhea, and syphilis reported in 2015 reached the highest number ever”1 since the CDC was founded in July 1946.
Nearly 24,000 cases of primary and secondary syphilis were reported in 2015, a 19% increase from the previous year. And Dr. Jonathan Mermin, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, reported, “We have reached a decisive moment for the nation. STD rates are rising, and many of the country’s systems for preventing STDs have eroded. We must mobilize, rebuild, and expand services—or the human and economic burden will continue to grow.”1
Dr. Mermin stressed the need to rebuild services because, “In recent years more than half of state and local STD programs have experienced budget cuts, resulting in more than 20 health department STD clinic closures in one year alone. Fewer clinics mean reduced access to STD testing and treatment for those who need these services.”1
The CDC also reports that STD treatment costs the US healthcare system nearly $16 billion each year.
The CDC has identified several players whose engagement is necessary to stem the tide of this epidemic:
- Providers must make STD screening a standard part of medical care, especially in pregnant women, and integrate STD prevention and treatment into prenatal care and other routine visits.
- People need to talk openly about STDs, get tested regularly, and reduce risk by using condoms or practicing mutual monogamy if sexually active.
- Parents and providers need to offer young people safe, effective ways to get information and services.
- State and local health departments should continue to direct resources to people hardest hit by the STD epidemic and work with community partners to maximize their impact.1
STD CAMPAIGNS 100 YEARS AGO
This message sounds familiar. Let’s go back 100 years to World War I. The book No Magic Bullet by Allan M. Brandt2 provides fascinating details about this period in America’s battle against venereal diseases. While the book is well worth reading in its entirety, I will attempt here to summarize the pertinent facts.
In the late 1910s, antivenereal campaigns were in full swing, with publicly shown movies such as “Fit to Fight” to train soldiers about STD symptoms and prevention to keep them physically healthy for fighting in the war. Similar information was widely available stateside for both men and women in open, matter-of-fact formats to encourage STD prevention.
After the war ended, the national sentiment became split between sexual revolution and social moralism. “Social hygienists” blamed the widespread increase in promiscuity on the newly introduced sexually explicit philosophy of Sigmund Freud, the widespread availability of automobiles (ie, a mobile, private, backseat location for sex), popular “vulgar” dances, and social feminism, among many others. The sexual revolution clearly led to an increased risk of STDs. But the antivenereal campaigns that had been appropriate in wartime came to be considered amoral and unfit for public consumption, and a period of silence about venereal diseases ensued.
By the 1930s, the situation had worsened:
- Approximately 1 out of every 10 Americans suffered from syphilis.
- Each year, Americans contracted almost half a million new syphilis infections (twice as many cases as tuberculosis, and 100 times as many cases as polio).
- 18% of all deaths from organic heart disease could be attributed to syphilis.
- Up to 20% of all mental institution inmates suffered from tertiary syphilis.
- 60,000 children were born each year with congenital syphilis.2
Although penicillin was still a decade or more away from discovery, syphilis could be treated, though likely not cured, with arsenic compounds. A course of treatment from a private physician, however, could cost from $300 to $1,000. Many patients who could not pay these exorbitant prices turned to public clinics for help. However, funding for the Venereal Disease Division of the Public Health Service, originally $4 million in 1920, was cut to less than $60,000 by 1926.2 Some hospitals refused to admit patients with syphilis and other venereal diseases, deeming them “morally tainted and less deserving of care.”2
Things couldn’t get much worse.
Dr. Thomas Parran was the New York State health commissioner in 1930, at the start of the Great Depression. Realizing that arguments for moral responsibility to prevent and treat venereal diseases were not effective, Dr. Parran and other public health officials turned to financial arguments. Among the most persuasive arguments, “More than $15 million was spent annually for the ambulatory care of venereal patients…Experts argued that syphilis costs taxpayers between $40 and $50 million each year for the institutional care of the insane, paralyzed, and blind.”2 The American Medical Association calculated that “8 to 10 million workers lost 21 million working days each year at an average of $4 a day as a result of infection with these conditions.”2 The cost was estimated at more than $100,000,000 annually.2
But the general public was not a part of the larger conversation regarding treatment and prevention of syphilis, thanks to the social hygienists. In November 1934, Dr. Parran was scheduled to give a radio broadcast on future goals for public health in New York. Notified that he would not be able to mention syphilis or gonorrhea by name, he refused to give the speech. Dr. Parran went on to lead the charge to reduce the moral cloud that blocked the ability to address syphilis openly and scientifically. With his extensive experience in public health, he proposed plans that had been effective in controlling other infectious diseases as measures to control the spread of syphilis. He outlined the following:
- Identify cases of syphilis. Offer free diagnostic centers where individuals could obtain confidential blood tests.
- Offer prompt therapy for identified cases.
- Identify, locate, and test all contacts of infected patients, and treat them if they are infected too.
- Make blood testing mandatory before marriage and early in all pregnancies.
- Educate the public concerning syphilis.2
Do these approaches sound familiar?
Appointed US Surgeon General in 1936 by President Franklin Delano Roosevelt, Dr. Parran published “The next great plague to go,”3 an article focusing on the medical approach to treating syphilis and other venereal diseases, while refusing to address the moral and social issues.3 This was widely acclaimed by the public and the press. Two years after he was blocked from mentioning syphilis and gonorrhea on the radio, he was pictured on the cover of Time magazine for his groundbreaking work.
With the advent of penicillin, syphilis became not only treatable but curable. Over the next decades, the number of patients infected with syphilis and the morbidity it caused continually declined until the 1990s, when there were even whispers of eradication in the United States. This likely came in part due to the AIDS epidemic and the increased public discourse on safe sex.
However, the 1990s saw a new rise in cases of syphilis. This clearly could not be blamed on the social hygienists; rather, it was likely due to apathy and a decline in public health spending. We are now in a period of rapid rise in STDs.
We have the benefit of antibiotics. We have the benefit of hindsight. What we need is to heed the call to arms of Dr. Mermin, to be inspired by the wisdom of Dr. Parran, and to act. Identify the case of syphilis, offer treatment, educate the public. Drs. Coleman, Fiahlo, and Brateanu have accomplished all of these in their article in this issue of the Journal.4
- Centers for Disease Control and Prevention (CDC). 2015 STD surveillance report press release. Reported STDs at unprecedented high in the US. www.cdc.gov/nchhstp/newsroom/2016/std-surveillance-report-2015-press-release.html. Accessed June 5, 2017.
- Brandt AM. No Magic Bullet. A Social History of Venereal Disease in the United States Since 1880. Cambridge, MA: Oxford University Press; 1985.
- Parran T. The next plague to go. Survey Graphic 1936.
- Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med 2017; 84:510–511.
According to a report from the US Centers for Disease Control and Prevention (CDC) on the incidence of sexually transmitted diseases (STDs), “Total combined cases of chlamydia, gonorrhea, and syphilis reported in 2015 reached the highest number ever”1 since the CDC was founded in July 1946.
Nearly 24,000 cases of primary and secondary syphilis were reported in 2015, a 19% increase from the previous year. And Dr. Jonathan Mermin, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, reported, “We have reached a decisive moment for the nation. STD rates are rising, and many of the country’s systems for preventing STDs have eroded. We must mobilize, rebuild, and expand services—or the human and economic burden will continue to grow.”1
Dr. Mermin stressed the need to rebuild services because, “In recent years more than half of state and local STD programs have experienced budget cuts, resulting in more than 20 health department STD clinic closures in one year alone. Fewer clinics mean reduced access to STD testing and treatment for those who need these services.”1
The CDC also reports that STD treatment costs the US healthcare system nearly $16 billion each year.
The CDC has identified several players whose engagement is necessary to stem the tide of this epidemic:
- Providers must make STD screening a standard part of medical care, especially in pregnant women, and integrate STD prevention and treatment into prenatal care and other routine visits.
- People need to talk openly about STDs, get tested regularly, and reduce risk by using condoms or practicing mutual monogamy if sexually active.
- Parents and providers need to offer young people safe, effective ways to get information and services.
- State and local health departments should continue to direct resources to people hardest hit by the STD epidemic and work with community partners to maximize their impact.1
STD CAMPAIGNS 100 YEARS AGO
This message sounds familiar. Let’s go back 100 years to World War I. The book No Magic Bullet by Allan M. Brandt2 provides fascinating details about this period in America’s battle against venereal diseases. While the book is well worth reading in its entirety, I will attempt here to summarize the pertinent facts.
In the late 1910s, antivenereal campaigns were in full swing, with publicly shown movies such as “Fit to Fight” to train soldiers about STD symptoms and prevention to keep them physically healthy for fighting in the war. Similar information was widely available stateside for both men and women in open, matter-of-fact formats to encourage STD prevention.
After the war ended, the national sentiment became split between sexual revolution and social moralism. “Social hygienists” blamed the widespread increase in promiscuity on the newly introduced sexually explicit philosophy of Sigmund Freud, the widespread availability of automobiles (ie, a mobile, private, backseat location for sex), popular “vulgar” dances, and social feminism, among many others. The sexual revolution clearly led to an increased risk of STDs. But the antivenereal campaigns that had been appropriate in wartime came to be considered amoral and unfit for public consumption, and a period of silence about venereal diseases ensued.
By the 1930s, the situation had worsened:
- Approximately 1 out of every 10 Americans suffered from syphilis.
- Each year, Americans contracted almost half a million new syphilis infections (twice as many cases as tuberculosis, and 100 times as many cases as polio).
- 18% of all deaths from organic heart disease could be attributed to syphilis.
- Up to 20% of all mental institution inmates suffered from tertiary syphilis.
- 60,000 children were born each year with congenital syphilis.2
Although penicillin was still a decade or more away from discovery, syphilis could be treated, though likely not cured, with arsenic compounds. A course of treatment from a private physician, however, could cost from $300 to $1,000. Many patients who could not pay these exorbitant prices turned to public clinics for help. However, funding for the Venereal Disease Division of the Public Health Service, originally $4 million in 1920, was cut to less than $60,000 by 1926.2 Some hospitals refused to admit patients with syphilis and other venereal diseases, deeming them “morally tainted and less deserving of care.”2
Things couldn’t get much worse.
Dr. Thomas Parran was the New York State health commissioner in 1930, at the start of the Great Depression. Realizing that arguments for moral responsibility to prevent and treat venereal diseases were not effective, Dr. Parran and other public health officials turned to financial arguments. Among the most persuasive arguments, “More than $15 million was spent annually for the ambulatory care of venereal patients…Experts argued that syphilis costs taxpayers between $40 and $50 million each year for the institutional care of the insane, paralyzed, and blind.”2 The American Medical Association calculated that “8 to 10 million workers lost 21 million working days each year at an average of $4 a day as a result of infection with these conditions.”2 The cost was estimated at more than $100,000,000 annually.2
But the general public was not a part of the larger conversation regarding treatment and prevention of syphilis, thanks to the social hygienists. In November 1934, Dr. Parran was scheduled to give a radio broadcast on future goals for public health in New York. Notified that he would not be able to mention syphilis or gonorrhea by name, he refused to give the speech. Dr. Parran went on to lead the charge to reduce the moral cloud that blocked the ability to address syphilis openly and scientifically. With his extensive experience in public health, he proposed plans that had been effective in controlling other infectious diseases as measures to control the spread of syphilis. He outlined the following:
- Identify cases of syphilis. Offer free diagnostic centers where individuals could obtain confidential blood tests.
- Offer prompt therapy for identified cases.
- Identify, locate, and test all contacts of infected patients, and treat them if they are infected too.
- Make blood testing mandatory before marriage and early in all pregnancies.
- Educate the public concerning syphilis.2
Do these approaches sound familiar?
Appointed US Surgeon General in 1936 by President Franklin Delano Roosevelt, Dr. Parran published “The next great plague to go,”3 an article focusing on the medical approach to treating syphilis and other venereal diseases, while refusing to address the moral and social issues.3 This was widely acclaimed by the public and the press. Two years after he was blocked from mentioning syphilis and gonorrhea on the radio, he was pictured on the cover of Time magazine for his groundbreaking work.
With the advent of penicillin, syphilis became not only treatable but curable. Over the next decades, the number of patients infected with syphilis and the morbidity it caused continually declined until the 1990s, when there were even whispers of eradication in the United States. This likely came in part due to the AIDS epidemic and the increased public discourse on safe sex.
However, the 1990s saw a new rise in cases of syphilis. This clearly could not be blamed on the social hygienists; rather, it was likely due to apathy and a decline in public health spending. We are now in a period of rapid rise in STDs.
We have the benefit of antibiotics. We have the benefit of hindsight. What we need is to heed the call to arms of Dr. Mermin, to be inspired by the wisdom of Dr. Parran, and to act. Identify the case of syphilis, offer treatment, educate the public. Drs. Coleman, Fiahlo, and Brateanu have accomplished all of these in their article in this issue of the Journal.4
According to a report from the US Centers for Disease Control and Prevention (CDC) on the incidence of sexually transmitted diseases (STDs), “Total combined cases of chlamydia, gonorrhea, and syphilis reported in 2015 reached the highest number ever”1 since the CDC was founded in July 1946.
Nearly 24,000 cases of primary and secondary syphilis were reported in 2015, a 19% increase from the previous year. And Dr. Jonathan Mermin, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, reported, “We have reached a decisive moment for the nation. STD rates are rising, and many of the country’s systems for preventing STDs have eroded. We must mobilize, rebuild, and expand services—or the human and economic burden will continue to grow.”1
Dr. Mermin stressed the need to rebuild services because, “In recent years more than half of state and local STD programs have experienced budget cuts, resulting in more than 20 health department STD clinic closures in one year alone. Fewer clinics mean reduced access to STD testing and treatment for those who need these services.”1
The CDC also reports that STD treatment costs the US healthcare system nearly $16 billion each year.
The CDC has identified several players whose engagement is necessary to stem the tide of this epidemic:
- Providers must make STD screening a standard part of medical care, especially in pregnant women, and integrate STD prevention and treatment into prenatal care and other routine visits.
- People need to talk openly about STDs, get tested regularly, and reduce risk by using condoms or practicing mutual monogamy if sexually active.
- Parents and providers need to offer young people safe, effective ways to get information and services.
- State and local health departments should continue to direct resources to people hardest hit by the STD epidemic and work with community partners to maximize their impact.1
STD CAMPAIGNS 100 YEARS AGO
This message sounds familiar. Let’s go back 100 years to World War I. The book No Magic Bullet by Allan M. Brandt2 provides fascinating details about this period in America’s battle against venereal diseases. While the book is well worth reading in its entirety, I will attempt here to summarize the pertinent facts.
In the late 1910s, antivenereal campaigns were in full swing, with publicly shown movies such as “Fit to Fight” to train soldiers about STD symptoms and prevention to keep them physically healthy for fighting in the war. Similar information was widely available stateside for both men and women in open, matter-of-fact formats to encourage STD prevention.
After the war ended, the national sentiment became split between sexual revolution and social moralism. “Social hygienists” blamed the widespread increase in promiscuity on the newly introduced sexually explicit philosophy of Sigmund Freud, the widespread availability of automobiles (ie, a mobile, private, backseat location for sex), popular “vulgar” dances, and social feminism, among many others. The sexual revolution clearly led to an increased risk of STDs. But the antivenereal campaigns that had been appropriate in wartime came to be considered amoral and unfit for public consumption, and a period of silence about venereal diseases ensued.
By the 1930s, the situation had worsened:
- Approximately 1 out of every 10 Americans suffered from syphilis.
- Each year, Americans contracted almost half a million new syphilis infections (twice as many cases as tuberculosis, and 100 times as many cases as polio).
- 18% of all deaths from organic heart disease could be attributed to syphilis.
- Up to 20% of all mental institution inmates suffered from tertiary syphilis.
- 60,000 children were born each year with congenital syphilis.2
Although penicillin was still a decade or more away from discovery, syphilis could be treated, though likely not cured, with arsenic compounds. A course of treatment from a private physician, however, could cost from $300 to $1,000. Many patients who could not pay these exorbitant prices turned to public clinics for help. However, funding for the Venereal Disease Division of the Public Health Service, originally $4 million in 1920, was cut to less than $60,000 by 1926.2 Some hospitals refused to admit patients with syphilis and other venereal diseases, deeming them “morally tainted and less deserving of care.”2
Things couldn’t get much worse.
Dr. Thomas Parran was the New York State health commissioner in 1930, at the start of the Great Depression. Realizing that arguments for moral responsibility to prevent and treat venereal diseases were not effective, Dr. Parran and other public health officials turned to financial arguments. Among the most persuasive arguments, “More than $15 million was spent annually for the ambulatory care of venereal patients…Experts argued that syphilis costs taxpayers between $40 and $50 million each year for the institutional care of the insane, paralyzed, and blind.”2 The American Medical Association calculated that “8 to 10 million workers lost 21 million working days each year at an average of $4 a day as a result of infection with these conditions.”2 The cost was estimated at more than $100,000,000 annually.2
But the general public was not a part of the larger conversation regarding treatment and prevention of syphilis, thanks to the social hygienists. In November 1934, Dr. Parran was scheduled to give a radio broadcast on future goals for public health in New York. Notified that he would not be able to mention syphilis or gonorrhea by name, he refused to give the speech. Dr. Parran went on to lead the charge to reduce the moral cloud that blocked the ability to address syphilis openly and scientifically. With his extensive experience in public health, he proposed plans that had been effective in controlling other infectious diseases as measures to control the spread of syphilis. He outlined the following:
- Identify cases of syphilis. Offer free diagnostic centers where individuals could obtain confidential blood tests.
- Offer prompt therapy for identified cases.
- Identify, locate, and test all contacts of infected patients, and treat them if they are infected too.
- Make blood testing mandatory before marriage and early in all pregnancies.
- Educate the public concerning syphilis.2
Do these approaches sound familiar?
Appointed US Surgeon General in 1936 by President Franklin Delano Roosevelt, Dr. Parran published “The next great plague to go,”3 an article focusing on the medical approach to treating syphilis and other venereal diseases, while refusing to address the moral and social issues.3 This was widely acclaimed by the public and the press. Two years after he was blocked from mentioning syphilis and gonorrhea on the radio, he was pictured on the cover of Time magazine for his groundbreaking work.
With the advent of penicillin, syphilis became not only treatable but curable. Over the next decades, the number of patients infected with syphilis and the morbidity it caused continually declined until the 1990s, when there were even whispers of eradication in the United States. This likely came in part due to the AIDS epidemic and the increased public discourse on safe sex.
However, the 1990s saw a new rise in cases of syphilis. This clearly could not be blamed on the social hygienists; rather, it was likely due to apathy and a decline in public health spending. We are now in a period of rapid rise in STDs.
We have the benefit of antibiotics. We have the benefit of hindsight. What we need is to heed the call to arms of Dr. Mermin, to be inspired by the wisdom of Dr. Parran, and to act. Identify the case of syphilis, offer treatment, educate the public. Drs. Coleman, Fiahlo, and Brateanu have accomplished all of these in their article in this issue of the Journal.4
- Centers for Disease Control and Prevention (CDC). 2015 STD surveillance report press release. Reported STDs at unprecedented high in the US. www.cdc.gov/nchhstp/newsroom/2016/std-surveillance-report-2015-press-release.html. Accessed June 5, 2017.
- Brandt AM. No Magic Bullet. A Social History of Venereal Disease in the United States Since 1880. Cambridge, MA: Oxford University Press; 1985.
- Parran T. The next plague to go. Survey Graphic 1936.
- Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med 2017; 84:510–511.
- Centers for Disease Control and Prevention (CDC). 2015 STD surveillance report press release. Reported STDs at unprecedented high in the US. www.cdc.gov/nchhstp/newsroom/2016/std-surveillance-report-2015-press-release.html. Accessed June 5, 2017.
- Brandt AM. No Magic Bullet. A Social History of Venereal Disease in the United States Since 1880. Cambridge, MA: Oxford University Press; 1985.
- Parran T. The next plague to go. Survey Graphic 1936.
- Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med 2017; 84:510–511.
Labels matter: Challenging conversations or challenging people?
Anyone who has tried to appreciate the challenges we face in medicine has probably read the 1978 article by Groves, “Taking care of the hateful patient.”1 This and a later article by Strous et al2 label and group patients according to specific behaviors and, perhaps more importantly, how they make the clinician on the other end of the conversation feel.
How patients make us feel should not be underappreciated. Taking care of other human beings is a complex, intricate, intimate privilege. To characterize it as anything else—to simply consumerize it—is to not fully understand it.
Yet, now more than ever, the impact of challenges—not just with patients but in healthcare today—is staggering: 54% of US physicians report burnout,3 and significant numbers would not choose medicine again as a career. Too much time spent charting (up to 2 hours in a recent study4) and less time spent connecting as human beings are driving the meaning out of medicine. Calls are growing for more empathy in medicine and better services to meet the needs of patients and caregivers alike.
WORDS CAN STIGMATIZE, VALIDATE, DAMAGE, OR HEAL
As we read in the article by Schuermeyer et al in this issue of the Journal,5 there are steps forward and also continued opportunities. The article begins to shift us from labeling patients as “dependent clingers” and “entitled demanders” to a much needed and more meaningful discussion about difficult patient behaviors and how we might more effectively respond to them.
Even if we need to apply them in medicine at times, our labeling the type of person a patient is or how the patient behaves carries tremendous significance to our patients and should not be applied lightly. Depending on the words or labels we choose, our words can stigmatize, validate, damage, or heal. Have no doubt, however, that our words will be remembered.
PATIENTS LABEL US, TOO
As a chief experience officer, I review thousands of patient comments every month. And what patients say is that although their medical care may be spectacular, their emotional needs and expectations are not always met. Despite both valid and less-valid criticisms of patient satisfaction surveys, we have an obligation to listen and learn. We too are fallible.
We too could be—and most certainly are—labeled by patients. “Insensitive,” “uncaring,” and “rude” are words I too often hear from patients as they comment on the care they received from their physicians. These labels certainly do not embody the profound caring at the core of the healthcare profession, just as they do not embody our patients.
LABELING ENDS REFLECTION
An additional and unforeseen risk to labeling is the end of meaningful reflection. When we label, we stop asking who this person is. What trauma did the person suffer that makes trust so difficult? What is he or she most afraid of? What am I contributing to this ineffective dialogue, and how can I adapt my own language and behavior? We have a professional responsibility to respond to frustration or challenges with patients, not with labeling in return, but with humility, listening, and reflection.
BEYOND LABELS
To truly enhance communication and the experience of our patients, we must model empathic curiosity. People are not the label we give them. They are not the disease they have. The richness of their lives, experiences, and emotions cannot possibly be embodied in a single word that we have assigned. Our role as healers requires not judgment but the willingness to know more about who they are and the skill to more effectively express our intention and meaning. Only then will our patients feel truly “seen” and known by us.
To that end, there are a few models of effective communication. One of them, the Relationship: Establishment, Development, and Engagement (REDE) model, was developed at Cleveland Clinic,6 and a recent study found that when physicians were trained in it, patient satisfaction, physician empathy, and burnout improved.7 Another, the Four Habit model, has been effectively used by Kaiser Permanente for decades.8 These models provide a framework and detailed skills that can be used with any patient, loved one, or colleague, especially those we find “challenging.”
In addition, Groves and Schuermeyer et al highlight the impact these difficult conversations have on the clinician. Because most clinicians care deeply about the patients they serve, they are haunted by conversations that don’t go well. When patients are unhappy or angry with our care, we often feel that it is our fault or that we have failed in some way. Alternatively, we seek to distance ourselves from the patient we find challenging.
EMPATHY IS HARD WORK
The most difficult work actually goes on in the space between withdrawing from our patients in anger and continuing to enable inappropriate behavior at an emotional cost to ourselves and our colleagues. That in-between space is an opportunity for the clinician to set boundaries and be consistent, while also seeking to build relationships based on empathy and trust. Otherwise, both parties walk away labeling each other, which prevents us from building relationships with the patients whom we find difficult. Relationships still matter in healthcare and have therapeutic benefits for our patients and ourselves.
Empathy is hard work. When we connect with the patient in front of us, empathy may be easy. Yet the real need for empathy is when we don’t connect with the person in front of us—when we feel frustrated, tired, and angry. And I believe as healers—not just doctors—we are absolutely up for the challenge.
- Groves JE. Taking care of the hateful patient. N Engl J Med 1978; 298:883–887.
- Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med 2006; 17:387–393.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc 2015; 90:1600–1613.
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165:753–760.
- Schuermeyer IN, Sieke E, Dickstein L, Falcone T, Franco K. When patients challenge you: Strategies for communication. Cleve Clin J Med 2017; 84:535–542.
- Windover AK, Boissy A, Rice TW, Gilligan T, Velez VJ, Merlino J. The REDE model of healthcare communication: optimizing relationship as a therapeutic agent. J Patient Exper 2014; 1:8–13
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med 2016; 31:755–761.
- Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns 2005; 58:4–12.
Anyone who has tried to appreciate the challenges we face in medicine has probably read the 1978 article by Groves, “Taking care of the hateful patient.”1 This and a later article by Strous et al2 label and group patients according to specific behaviors and, perhaps more importantly, how they make the clinician on the other end of the conversation feel.
How patients make us feel should not be underappreciated. Taking care of other human beings is a complex, intricate, intimate privilege. To characterize it as anything else—to simply consumerize it—is to not fully understand it.
Yet, now more than ever, the impact of challenges—not just with patients but in healthcare today—is staggering: 54% of US physicians report burnout,3 and significant numbers would not choose medicine again as a career. Too much time spent charting (up to 2 hours in a recent study4) and less time spent connecting as human beings are driving the meaning out of medicine. Calls are growing for more empathy in medicine and better services to meet the needs of patients and caregivers alike.
WORDS CAN STIGMATIZE, VALIDATE, DAMAGE, OR HEAL
As we read in the article by Schuermeyer et al in this issue of the Journal,5 there are steps forward and also continued opportunities. The article begins to shift us from labeling patients as “dependent clingers” and “entitled demanders” to a much needed and more meaningful discussion about difficult patient behaviors and how we might more effectively respond to them.
Even if we need to apply them in medicine at times, our labeling the type of person a patient is or how the patient behaves carries tremendous significance to our patients and should not be applied lightly. Depending on the words or labels we choose, our words can stigmatize, validate, damage, or heal. Have no doubt, however, that our words will be remembered.
PATIENTS LABEL US, TOO
As a chief experience officer, I review thousands of patient comments every month. And what patients say is that although their medical care may be spectacular, their emotional needs and expectations are not always met. Despite both valid and less-valid criticisms of patient satisfaction surveys, we have an obligation to listen and learn. We too are fallible.
We too could be—and most certainly are—labeled by patients. “Insensitive,” “uncaring,” and “rude” are words I too often hear from patients as they comment on the care they received from their physicians. These labels certainly do not embody the profound caring at the core of the healthcare profession, just as they do not embody our patients.
LABELING ENDS REFLECTION
An additional and unforeseen risk to labeling is the end of meaningful reflection. When we label, we stop asking who this person is. What trauma did the person suffer that makes trust so difficult? What is he or she most afraid of? What am I contributing to this ineffective dialogue, and how can I adapt my own language and behavior? We have a professional responsibility to respond to frustration or challenges with patients, not with labeling in return, but with humility, listening, and reflection.
BEYOND LABELS
To truly enhance communication and the experience of our patients, we must model empathic curiosity. People are not the label we give them. They are not the disease they have. The richness of their lives, experiences, and emotions cannot possibly be embodied in a single word that we have assigned. Our role as healers requires not judgment but the willingness to know more about who they are and the skill to more effectively express our intention and meaning. Only then will our patients feel truly “seen” and known by us.
To that end, there are a few models of effective communication. One of them, the Relationship: Establishment, Development, and Engagement (REDE) model, was developed at Cleveland Clinic,6 and a recent study found that when physicians were trained in it, patient satisfaction, physician empathy, and burnout improved.7 Another, the Four Habit model, has been effectively used by Kaiser Permanente for decades.8 These models provide a framework and detailed skills that can be used with any patient, loved one, or colleague, especially those we find “challenging.”
In addition, Groves and Schuermeyer et al highlight the impact these difficult conversations have on the clinician. Because most clinicians care deeply about the patients they serve, they are haunted by conversations that don’t go well. When patients are unhappy or angry with our care, we often feel that it is our fault or that we have failed in some way. Alternatively, we seek to distance ourselves from the patient we find challenging.
EMPATHY IS HARD WORK
The most difficult work actually goes on in the space between withdrawing from our patients in anger and continuing to enable inappropriate behavior at an emotional cost to ourselves and our colleagues. That in-between space is an opportunity for the clinician to set boundaries and be consistent, while also seeking to build relationships based on empathy and trust. Otherwise, both parties walk away labeling each other, which prevents us from building relationships with the patients whom we find difficult. Relationships still matter in healthcare and have therapeutic benefits for our patients and ourselves.
Empathy is hard work. When we connect with the patient in front of us, empathy may be easy. Yet the real need for empathy is when we don’t connect with the person in front of us—when we feel frustrated, tired, and angry. And I believe as healers—not just doctors—we are absolutely up for the challenge.
Anyone who has tried to appreciate the challenges we face in medicine has probably read the 1978 article by Groves, “Taking care of the hateful patient.”1 This and a later article by Strous et al2 label and group patients according to specific behaviors and, perhaps more importantly, how they make the clinician on the other end of the conversation feel.
How patients make us feel should not be underappreciated. Taking care of other human beings is a complex, intricate, intimate privilege. To characterize it as anything else—to simply consumerize it—is to not fully understand it.
Yet, now more than ever, the impact of challenges—not just with patients but in healthcare today—is staggering: 54% of US physicians report burnout,3 and significant numbers would not choose medicine again as a career. Too much time spent charting (up to 2 hours in a recent study4) and less time spent connecting as human beings are driving the meaning out of medicine. Calls are growing for more empathy in medicine and better services to meet the needs of patients and caregivers alike.
WORDS CAN STIGMATIZE, VALIDATE, DAMAGE, OR HEAL
As we read in the article by Schuermeyer et al in this issue of the Journal,5 there are steps forward and also continued opportunities. The article begins to shift us from labeling patients as “dependent clingers” and “entitled demanders” to a much needed and more meaningful discussion about difficult patient behaviors and how we might more effectively respond to them.
Even if we need to apply them in medicine at times, our labeling the type of person a patient is or how the patient behaves carries tremendous significance to our patients and should not be applied lightly. Depending on the words or labels we choose, our words can stigmatize, validate, damage, or heal. Have no doubt, however, that our words will be remembered.
PATIENTS LABEL US, TOO
As a chief experience officer, I review thousands of patient comments every month. And what patients say is that although their medical care may be spectacular, their emotional needs and expectations are not always met. Despite both valid and less-valid criticisms of patient satisfaction surveys, we have an obligation to listen and learn. We too are fallible.
We too could be—and most certainly are—labeled by patients. “Insensitive,” “uncaring,” and “rude” are words I too often hear from patients as they comment on the care they received from their physicians. These labels certainly do not embody the profound caring at the core of the healthcare profession, just as they do not embody our patients.
LABELING ENDS REFLECTION
An additional and unforeseen risk to labeling is the end of meaningful reflection. When we label, we stop asking who this person is. What trauma did the person suffer that makes trust so difficult? What is he or she most afraid of? What am I contributing to this ineffective dialogue, and how can I adapt my own language and behavior? We have a professional responsibility to respond to frustration or challenges with patients, not with labeling in return, but with humility, listening, and reflection.
BEYOND LABELS
To truly enhance communication and the experience of our patients, we must model empathic curiosity. People are not the label we give them. They are not the disease they have. The richness of their lives, experiences, and emotions cannot possibly be embodied in a single word that we have assigned. Our role as healers requires not judgment but the willingness to know more about who they are and the skill to more effectively express our intention and meaning. Only then will our patients feel truly “seen” and known by us.
To that end, there are a few models of effective communication. One of them, the Relationship: Establishment, Development, and Engagement (REDE) model, was developed at Cleveland Clinic,6 and a recent study found that when physicians were trained in it, patient satisfaction, physician empathy, and burnout improved.7 Another, the Four Habit model, has been effectively used by Kaiser Permanente for decades.8 These models provide a framework and detailed skills that can be used with any patient, loved one, or colleague, especially those we find “challenging.”
In addition, Groves and Schuermeyer et al highlight the impact these difficult conversations have on the clinician. Because most clinicians care deeply about the patients they serve, they are haunted by conversations that don’t go well. When patients are unhappy or angry with our care, we often feel that it is our fault or that we have failed in some way. Alternatively, we seek to distance ourselves from the patient we find challenging.
EMPATHY IS HARD WORK
The most difficult work actually goes on in the space between withdrawing from our patients in anger and continuing to enable inappropriate behavior at an emotional cost to ourselves and our colleagues. That in-between space is an opportunity for the clinician to set boundaries and be consistent, while also seeking to build relationships based on empathy and trust. Otherwise, both parties walk away labeling each other, which prevents us from building relationships with the patients whom we find difficult. Relationships still matter in healthcare and have therapeutic benefits for our patients and ourselves.
Empathy is hard work. When we connect with the patient in front of us, empathy may be easy. Yet the real need for empathy is when we don’t connect with the person in front of us—when we feel frustrated, tired, and angry. And I believe as healers—not just doctors—we are absolutely up for the challenge.
- Groves JE. Taking care of the hateful patient. N Engl J Med 1978; 298:883–887.
- Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med 2006; 17:387–393.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc 2015; 90:1600–1613.
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165:753–760.
- Schuermeyer IN, Sieke E, Dickstein L, Falcone T, Franco K. When patients challenge you: Strategies for communication. Cleve Clin J Med 2017; 84:535–542.
- Windover AK, Boissy A, Rice TW, Gilligan T, Velez VJ, Merlino J. The REDE model of healthcare communication: optimizing relationship as a therapeutic agent. J Patient Exper 2014; 1:8–13
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med 2016; 31:755–761.
- Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns 2005; 58:4–12.
- Groves JE. Taking care of the hateful patient. N Engl J Med 1978; 298:883–887.
- Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med 2006; 17:387–393.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc 2015; 90:1600–1613.
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165:753–760.
- Schuermeyer IN, Sieke E, Dickstein L, Falcone T, Franco K. When patients challenge you: Strategies for communication. Cleve Clin J Med 2017; 84:535–542.
- Windover AK, Boissy A, Rice TW, Gilligan T, Velez VJ, Merlino J. The REDE model of healthcare communication: optimizing relationship as a therapeutic agent. J Patient Exper 2014; 1:8–13
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med 2016; 31:755–761.
- Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns 2005; 58:4–12.
Mobility assessment in the hospital: What are the “next steps”?
Mobility impairment (reduced ability to change body position or ambulate) is common among older adults during hospitalization1 and is correlated with higher rates of readmission,2 long-term care placement,3 and even death.4 Although some may perceive mobility impairment during hospitalization as a temporary inconvenience, recent research suggests disruptions of basic activities of daily life such as mobility may be “traumatic” 5 or “toxic”6 to older adults with long-term post-hospital effects.7 While these studies highlight the underestimated effects of low mobility during hospitalization, they are based on data collected for research purposes using mobility measurement tools not typically utilized in routine hospital care.
The absence of a standardized mobility measurement tool used as part of routine hospital care poses a barrier to estimating the effects of low hospital mobility and programs seeking to improve mobility levels in hospitalized patients. In this issue of the Journal of Hospital Medicine, Valiani et al.8 found a novel approach to measure mobility using a universally disseminated clinical scale (Braden). Using the activity subscale of the Braden scale, the authors found that mobility level changes during hospitalization can have a striking impact on post-discharge mortality. Their results indicate that older adults who develop mobility impairment during hospitalization had higher odds of death, specifically 1.23 times greater risk, within 6 months after discharge (23% decreased chance of survival). Most of the risk applies in the first 30 days and remains to a lesser extent for up to 5 years post-hospitalization. An equally interesting finding was that those who enter the hospital with low mobility but improve have a 46% higher survival rate. Again, most of the benefit is seen during hospitalization or immediately afterward, but the benefit persists for up to 5 years. A schematic of the results are presented in the Figure. Notably, Valiani et al.8 did not find regression to the mean Braden score of 3.
This novel use of the Braden activity subscale raises a question: Should we be using the Braden activity component to measure mobility in the hospital? Put another way, what scale should we be using in the hospital? Using the Braden activity subscale is convenient, since it capitalizes on data already being gathered. However, this subscale focuses solely on ambulation frequency; it doesn’t capture other mobility domains, such as ability to change body position. Ambulation is only half of the mobility story. It is interesting that although the Braden scale does have a mobility subscale that captures body position changes, the authors chose not to use it. This begs the question of whether an ideal mobility scale should encompass both components.
Previous studies of hospital mobility have deployed tools such as Katz Activities of Daily Living (ADLs)9 and the Short Physical Performance Battery (SPPB),10 and there is a recent trend toward using the Activity Measure for Post-Acute Care (AM-PAC).11 However, none of these tools, including the one discussed in this review, were designed to capture mobility levels in hospitalized patients. The Katz ADLs and the SPPB were designed for community living adults, and the AM-PAC was designed for a more mobile post-acute-care patient population. Although these tools do have limitations for use with hospitalized patients, they have shown promising results.10,12
What does all this mean for implementation? Do we have enough data on the existing scales to say we should be implementing them—or in the case of Braden, continuing to use them—to measure function and mobility in hospitalized patients? Implementing an ideal mobility assessment tool into the routinized care of the hospital patient may be necessary but insufficient. Complementing the use of these tools with more objective and precise mobility measures (eg, activity counts or steps from wearable sensors) would greatly increase the ability to accurately assess mobility and potentially enable providers to recommend specific mobility goals for patients in the form of steps or minutes of activity per day. In conclusion, the provocative results by Valiani et al.8 underscore the importance of mobility for hospitalized patients but also suggest many opportunities for future research and implementation to improve hospital care, especially for older adults.
Disclosure
Nothing to report.
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
2. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
3. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Amer Geriatr Soc. 2003;51(4):451-458. PubMed
4. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. PubMed
5. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. PubMed
6. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
7. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
8. Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden activity subscale for mobility status in hospitalized older adults. J Hosp Med. 2017;12(6):396-401. PubMed
9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
10. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Bio Sci Med Sci. 1994;49(2):M85-M94. PubMed
11. Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Arch Phys Med Rehabil. 2004;85(4):649-660. PubMed
12. Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108(4):64-71. PubMed
Mobility impairment (reduced ability to change body position or ambulate) is common among older adults during hospitalization1 and is correlated with higher rates of readmission,2 long-term care placement,3 and even death.4 Although some may perceive mobility impairment during hospitalization as a temporary inconvenience, recent research suggests disruptions of basic activities of daily life such as mobility may be “traumatic” 5 or “toxic”6 to older adults with long-term post-hospital effects.7 While these studies highlight the underestimated effects of low mobility during hospitalization, they are based on data collected for research purposes using mobility measurement tools not typically utilized in routine hospital care.
The absence of a standardized mobility measurement tool used as part of routine hospital care poses a barrier to estimating the effects of low hospital mobility and programs seeking to improve mobility levels in hospitalized patients. In this issue of the Journal of Hospital Medicine, Valiani et al.8 found a novel approach to measure mobility using a universally disseminated clinical scale (Braden). Using the activity subscale of the Braden scale, the authors found that mobility level changes during hospitalization can have a striking impact on post-discharge mortality. Their results indicate that older adults who develop mobility impairment during hospitalization had higher odds of death, specifically 1.23 times greater risk, within 6 months after discharge (23% decreased chance of survival). Most of the risk applies in the first 30 days and remains to a lesser extent for up to 5 years post-hospitalization. An equally interesting finding was that those who enter the hospital with low mobility but improve have a 46% higher survival rate. Again, most of the benefit is seen during hospitalization or immediately afterward, but the benefit persists for up to 5 years. A schematic of the results are presented in the Figure. Notably, Valiani et al.8 did not find regression to the mean Braden score of 3.
This novel use of the Braden activity subscale raises a question: Should we be using the Braden activity component to measure mobility in the hospital? Put another way, what scale should we be using in the hospital? Using the Braden activity subscale is convenient, since it capitalizes on data already being gathered. However, this subscale focuses solely on ambulation frequency; it doesn’t capture other mobility domains, such as ability to change body position. Ambulation is only half of the mobility story. It is interesting that although the Braden scale does have a mobility subscale that captures body position changes, the authors chose not to use it. This begs the question of whether an ideal mobility scale should encompass both components.
Previous studies of hospital mobility have deployed tools such as Katz Activities of Daily Living (ADLs)9 and the Short Physical Performance Battery (SPPB),10 and there is a recent trend toward using the Activity Measure for Post-Acute Care (AM-PAC).11 However, none of these tools, including the one discussed in this review, were designed to capture mobility levels in hospitalized patients. The Katz ADLs and the SPPB were designed for community living adults, and the AM-PAC was designed for a more mobile post-acute-care patient population. Although these tools do have limitations for use with hospitalized patients, they have shown promising results.10,12
What does all this mean for implementation? Do we have enough data on the existing scales to say we should be implementing them—or in the case of Braden, continuing to use them—to measure function and mobility in hospitalized patients? Implementing an ideal mobility assessment tool into the routinized care of the hospital patient may be necessary but insufficient. Complementing the use of these tools with more objective and precise mobility measures (eg, activity counts or steps from wearable sensors) would greatly increase the ability to accurately assess mobility and potentially enable providers to recommend specific mobility goals for patients in the form of steps or minutes of activity per day. In conclusion, the provocative results by Valiani et al.8 underscore the importance of mobility for hospitalized patients but also suggest many opportunities for future research and implementation to improve hospital care, especially for older adults.
Disclosure
Nothing to report.
Mobility impairment (reduced ability to change body position or ambulate) is common among older adults during hospitalization1 and is correlated with higher rates of readmission,2 long-term care placement,3 and even death.4 Although some may perceive mobility impairment during hospitalization as a temporary inconvenience, recent research suggests disruptions of basic activities of daily life such as mobility may be “traumatic” 5 or “toxic”6 to older adults with long-term post-hospital effects.7 While these studies highlight the underestimated effects of low mobility during hospitalization, they are based on data collected for research purposes using mobility measurement tools not typically utilized in routine hospital care.
The absence of a standardized mobility measurement tool used as part of routine hospital care poses a barrier to estimating the effects of low hospital mobility and programs seeking to improve mobility levels in hospitalized patients. In this issue of the Journal of Hospital Medicine, Valiani et al.8 found a novel approach to measure mobility using a universally disseminated clinical scale (Braden). Using the activity subscale of the Braden scale, the authors found that mobility level changes during hospitalization can have a striking impact on post-discharge mortality. Their results indicate that older adults who develop mobility impairment during hospitalization had higher odds of death, specifically 1.23 times greater risk, within 6 months after discharge (23% decreased chance of survival). Most of the risk applies in the first 30 days and remains to a lesser extent for up to 5 years post-hospitalization. An equally interesting finding was that those who enter the hospital with low mobility but improve have a 46% higher survival rate. Again, most of the benefit is seen during hospitalization or immediately afterward, but the benefit persists for up to 5 years. A schematic of the results are presented in the Figure. Notably, Valiani et al.8 did not find regression to the mean Braden score of 3.
This novel use of the Braden activity subscale raises a question: Should we be using the Braden activity component to measure mobility in the hospital? Put another way, what scale should we be using in the hospital? Using the Braden activity subscale is convenient, since it capitalizes on data already being gathered. However, this subscale focuses solely on ambulation frequency; it doesn’t capture other mobility domains, such as ability to change body position. Ambulation is only half of the mobility story. It is interesting that although the Braden scale does have a mobility subscale that captures body position changes, the authors chose not to use it. This begs the question of whether an ideal mobility scale should encompass both components.
Previous studies of hospital mobility have deployed tools such as Katz Activities of Daily Living (ADLs)9 and the Short Physical Performance Battery (SPPB),10 and there is a recent trend toward using the Activity Measure for Post-Acute Care (AM-PAC).11 However, none of these tools, including the one discussed in this review, were designed to capture mobility levels in hospitalized patients. The Katz ADLs and the SPPB were designed for community living adults, and the AM-PAC was designed for a more mobile post-acute-care patient population. Although these tools do have limitations for use with hospitalized patients, they have shown promising results.10,12
What does all this mean for implementation? Do we have enough data on the existing scales to say we should be implementing them—or in the case of Braden, continuing to use them—to measure function and mobility in hospitalized patients? Implementing an ideal mobility assessment tool into the routinized care of the hospital patient may be necessary but insufficient. Complementing the use of these tools with more objective and precise mobility measures (eg, activity counts or steps from wearable sensors) would greatly increase the ability to accurately assess mobility and potentially enable providers to recommend specific mobility goals for patients in the form of steps or minutes of activity per day. In conclusion, the provocative results by Valiani et al.8 underscore the importance of mobility for hospitalized patients but also suggest many opportunities for future research and implementation to improve hospital care, especially for older adults.
Disclosure
Nothing to report.
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
2. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
3. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Amer Geriatr Soc. 2003;51(4):451-458. PubMed
4. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. PubMed
5. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. PubMed
6. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
7. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
8. Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden activity subscale for mobility status in hospitalized older adults. J Hosp Med. 2017;12(6):396-401. PubMed
9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
10. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Bio Sci Med Sci. 1994;49(2):M85-M94. PubMed
11. Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Arch Phys Med Rehabil. 2004;85(4):649-660. PubMed
12. Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108(4):64-71. PubMed
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
2. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
3. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Amer Geriatr Soc. 2003;51(4):451-458. PubMed
4. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. PubMed
5. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. PubMed
6. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
7. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
8. Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden activity subscale for mobility status in hospitalized older adults. J Hosp Med. 2017;12(6):396-401. PubMed
9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
10. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Bio Sci Med Sci. 1994;49(2):M85-M94. PubMed
11. Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Arch Phys Med Rehabil. 2004;85(4):649-660. PubMed
12. Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108(4):64-71. PubMed
© 2017 Society of Hospital Medicine
It’s time for a strategic approach to observation care
After patients have experienced an illness requiring a hospital stay, they are increasingly finding that despite having received treatment in a hospital bed, they were never actually admitted—at least not from the perspective of their insurers. Instead, these patients were kept under observation, an outpatient designation that allows a hospital to bill for observation services without formally admitting a patient.
Recent studies have recorded significant increases in hospitals’ use of observation stays among the Medicare population,1-3 raising concerns about the financial ramifications for patients. Under observation, patients are potentially responsible for a greater share of the cost and bear the financial consequences of inappropriate observation stays. Currently, around 6% of Medicare patients hospitalized as outpatients spend more than 48 hours (or two midnights) in observation, sometimes much longer, exposing them to significant out-of-pocket costs.3 In addition, liberal use of observation can lead to increased hospital stays, for example among lower-severity emergency department (ED) patients who could have been safely discharged but were instead kept for a costly observation stay.4 At the same time, hospitals do not necessarily benefit from this cost shifting; in fact, hospital margin is worse for patients under Medicare observation care.5 Yet hospitals are obligated to be compliant with CMS observation regulations and may try to avoid the consequences (eg, audits, non-payment) for inpatient stays that are deemed inappropriate by CMS.
While the nuances of how CMS finances observation stays have made the practice controversial, the use of observation care in other payer groups that may not have the same reimbursement policies, and its impact on patients, have not been well studied. In this issue of the Journal of Hospital Medicine, Nuckols et al.6 begins to address this gap by carefully exploring trends in observation stays in a multipayer data set.
The authors use data for four states (Georgia, Nebraska, South Carolina, and Tennessee) from the Healthcare Cost and Utilization Project (Agency for Healthcare Quality and Research) and the American Community Survey (US Census Bureau) to calculate population based rates of ED visits, observation stays, and inpatient admissions. To date, this is the first study to examine and compare the use of observation stays in an all-payer data set. Similar to prior work that examined the Medicare population, the authors find increased rates of treat-and-release ED visits and observation stays over time with a corresponding decline in inpatient admissions. As this study clearly shows, observation stays are comprising a greater fraction of the total hospital care delivered to patients with acute illnesses.
In many ways, the findings of Nuckols et al.6 raise more questions than they answer. For example, does the rise in observation stays represent a fundamental shift in how hospitals deliver care, an alternative to costly inpatient admissions? Are changing payer incentives driving hospitals to be more prudent in their inpatient admission practices, or are similar services simply being delivered under a new billing designation? And, most important, does this shift have any repercussions for the quality and safety of patient care?
Ultimately, the answer to these questions is, “It depends.” As the authors mention, most US hospitals admit observation patients to general medical wards, where they receive care at the admitting provider’s discretion instead of utilizing specific care pathways or observation protocols.7 In some of these hospitals, there may be little to no difference in how the observation patient is treated compared with a similar patient who is hospitalized as an inpatient.
However, a minority of hospitals has been more strategic in their delivery of observation care and have developed observation units. While observation units vary in design, common features include a dedicated location in the hospital with dedicated staff, reliance on clear inclusion-exclusion criteria for admission to the unit, and the use of rapid diagnostic or treatment protocols for a limited number of conditions. About half of these observation units are ED-based, reducing transitions of care between services. Protocol-driven observation units have the potential to prevent unnecessary inpatient admissions, standardize evidence-based practice, and reduce practice variation and resource use, apparently without increasing adverse events.8 In addition, they may also lead to better experiences of care for many patients compared with inpatient admissions.
Medicare’s own policy on observation hospital care succinctly describes ED observation units: “Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment in order to make a decision concerning their admission or discharge…usually in less than 24 hours.” Due to regulatory changes and auditing pressure, observation care has expanded beyond this definition in length of stay, scope, and practice such that much of observation care now occurs on general hospital wards. Ideally, observation policy must be realigned with its original intent and investment made in ED observation units.
The shifting landscape of hospital-based care as described by Nuckols et al.6 highlights the need for a more strategic approach to the delivery of acute care. Unfortunately, to date, there has been a lack of attention among policymakers towards promoting a system of emergent and urgent care that is coordinated and efficient. Observation stays are one major area for which innovations in the acute care delivery system may result in meaningful improvement in patient outcomes and greater value for the healthcare system. Incentivizing a system of high-value observation care, such as promoting the use of observation units that employ evidence-based practices, should be a key priority when considering approaches to reducing the cost of hospital-based and other acute care.
One strategy is to better define and possibly expand the cohort of patients likely to benefit from care in an observation unit. Hospitals with significant experience using observation units treat not only common observation conditions like chest pain, asthma, or cellulitis, but also higher-risk inpatient conditions like syncope and diabetic ketoacidosis using rapid diagnostic and treatment protocols.
Identifying high-value observation care also will require developing patient outcome measures specific for observation stays. Observation-specific quality measures will allow a comparison of hospitals that use different care pathways for observation patients or treat certain populations of patients in observation units. This necessitates looking beyond resource use (costs and length of stay), which most studies on observation units have focused on, and examining a broader range of patient outcomes like time to symptomatic resolution, quality of life, or return to productivity after an acute illness.
Finally, observation care is also a good target for payment redesign. For example, incentive payments could be provided to hospitals that choose to develop observation units, employ observation units that utilize best known practices for observation care (such as protocols and clearly defined patient cohorts), or deliver particularly good acute care outcomes for patients with observation-amenable conditions. On the consumer side, value-based contracting could be used to shunt patients with acute conditions that require evaluation in an urgent care center or ED to hospitals that use observation units.
While the declines in inpatient admission and increases in treat-and-release ED patients have been well-documented over time, perhaps the biggest contribution of this study from Nuckols et al.6 lies in its identification of the changes in observation care, which have been increasing in all payer groups. Our opportunity now is to shape whether these shifts toward observation care deliver greater value for patients.
Acknowledgment
The authors thank Joanna Guo, BA, for her editorial and technical assistance.
Disclosure
Nothing to report.
1. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
2. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
3. Office of Inspector General. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. US Department of Health & Human Services. Published 2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed April 25, 2017.
4. Blecker S, Gavin NP, Park H, Ladapo JA, Katz SD. Observation units as substitutes for hospitalization or home discharge. Ann Emerg Med. 2016;67(6):706-713.e702. PubMed
5. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Published 2015. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf?sfvrsn=0). Accessed April 25, 2017.
6. Nuckols TN, Fingar KR, Barrett M, Steiner C, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):444-446. PubMed
7. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
8. Ross MA, Aurora T, Graff L, et al. State of the art: emergency department observation units. Crit Pathw Cardiol. 2012;11(3):128-138. PubMed
After patients have experienced an illness requiring a hospital stay, they are increasingly finding that despite having received treatment in a hospital bed, they were never actually admitted—at least not from the perspective of their insurers. Instead, these patients were kept under observation, an outpatient designation that allows a hospital to bill for observation services without formally admitting a patient.
Recent studies have recorded significant increases in hospitals’ use of observation stays among the Medicare population,1-3 raising concerns about the financial ramifications for patients. Under observation, patients are potentially responsible for a greater share of the cost and bear the financial consequences of inappropriate observation stays. Currently, around 6% of Medicare patients hospitalized as outpatients spend more than 48 hours (or two midnights) in observation, sometimes much longer, exposing them to significant out-of-pocket costs.3 In addition, liberal use of observation can lead to increased hospital stays, for example among lower-severity emergency department (ED) patients who could have been safely discharged but were instead kept for a costly observation stay.4 At the same time, hospitals do not necessarily benefit from this cost shifting; in fact, hospital margin is worse for patients under Medicare observation care.5 Yet hospitals are obligated to be compliant with CMS observation regulations and may try to avoid the consequences (eg, audits, non-payment) for inpatient stays that are deemed inappropriate by CMS.
While the nuances of how CMS finances observation stays have made the practice controversial, the use of observation care in other payer groups that may not have the same reimbursement policies, and its impact on patients, have not been well studied. In this issue of the Journal of Hospital Medicine, Nuckols et al.6 begins to address this gap by carefully exploring trends in observation stays in a multipayer data set.
The authors use data for four states (Georgia, Nebraska, South Carolina, and Tennessee) from the Healthcare Cost and Utilization Project (Agency for Healthcare Quality and Research) and the American Community Survey (US Census Bureau) to calculate population based rates of ED visits, observation stays, and inpatient admissions. To date, this is the first study to examine and compare the use of observation stays in an all-payer data set. Similar to prior work that examined the Medicare population, the authors find increased rates of treat-and-release ED visits and observation stays over time with a corresponding decline in inpatient admissions. As this study clearly shows, observation stays are comprising a greater fraction of the total hospital care delivered to patients with acute illnesses.
In many ways, the findings of Nuckols et al.6 raise more questions than they answer. For example, does the rise in observation stays represent a fundamental shift in how hospitals deliver care, an alternative to costly inpatient admissions? Are changing payer incentives driving hospitals to be more prudent in their inpatient admission practices, or are similar services simply being delivered under a new billing designation? And, most important, does this shift have any repercussions for the quality and safety of patient care?
Ultimately, the answer to these questions is, “It depends.” As the authors mention, most US hospitals admit observation patients to general medical wards, where they receive care at the admitting provider’s discretion instead of utilizing specific care pathways or observation protocols.7 In some of these hospitals, there may be little to no difference in how the observation patient is treated compared with a similar patient who is hospitalized as an inpatient.
However, a minority of hospitals has been more strategic in their delivery of observation care and have developed observation units. While observation units vary in design, common features include a dedicated location in the hospital with dedicated staff, reliance on clear inclusion-exclusion criteria for admission to the unit, and the use of rapid diagnostic or treatment protocols for a limited number of conditions. About half of these observation units are ED-based, reducing transitions of care between services. Protocol-driven observation units have the potential to prevent unnecessary inpatient admissions, standardize evidence-based practice, and reduce practice variation and resource use, apparently without increasing adverse events.8 In addition, they may also lead to better experiences of care for many patients compared with inpatient admissions.
Medicare’s own policy on observation hospital care succinctly describes ED observation units: “Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment in order to make a decision concerning their admission or discharge…usually in less than 24 hours.” Due to regulatory changes and auditing pressure, observation care has expanded beyond this definition in length of stay, scope, and practice such that much of observation care now occurs on general hospital wards. Ideally, observation policy must be realigned with its original intent and investment made in ED observation units.
The shifting landscape of hospital-based care as described by Nuckols et al.6 highlights the need for a more strategic approach to the delivery of acute care. Unfortunately, to date, there has been a lack of attention among policymakers towards promoting a system of emergent and urgent care that is coordinated and efficient. Observation stays are one major area for which innovations in the acute care delivery system may result in meaningful improvement in patient outcomes and greater value for the healthcare system. Incentivizing a system of high-value observation care, such as promoting the use of observation units that employ evidence-based practices, should be a key priority when considering approaches to reducing the cost of hospital-based and other acute care.
One strategy is to better define and possibly expand the cohort of patients likely to benefit from care in an observation unit. Hospitals with significant experience using observation units treat not only common observation conditions like chest pain, asthma, or cellulitis, but also higher-risk inpatient conditions like syncope and diabetic ketoacidosis using rapid diagnostic and treatment protocols.
Identifying high-value observation care also will require developing patient outcome measures specific for observation stays. Observation-specific quality measures will allow a comparison of hospitals that use different care pathways for observation patients or treat certain populations of patients in observation units. This necessitates looking beyond resource use (costs and length of stay), which most studies on observation units have focused on, and examining a broader range of patient outcomes like time to symptomatic resolution, quality of life, or return to productivity after an acute illness.
Finally, observation care is also a good target for payment redesign. For example, incentive payments could be provided to hospitals that choose to develop observation units, employ observation units that utilize best known practices for observation care (such as protocols and clearly defined patient cohorts), or deliver particularly good acute care outcomes for patients with observation-amenable conditions. On the consumer side, value-based contracting could be used to shunt patients with acute conditions that require evaluation in an urgent care center or ED to hospitals that use observation units.
While the declines in inpatient admission and increases in treat-and-release ED patients have been well-documented over time, perhaps the biggest contribution of this study from Nuckols et al.6 lies in its identification of the changes in observation care, which have been increasing in all payer groups. Our opportunity now is to shape whether these shifts toward observation care deliver greater value for patients.
Acknowledgment
The authors thank Joanna Guo, BA, for her editorial and technical assistance.
Disclosure
Nothing to report.
After patients have experienced an illness requiring a hospital stay, they are increasingly finding that despite having received treatment in a hospital bed, they were never actually admitted—at least not from the perspective of their insurers. Instead, these patients were kept under observation, an outpatient designation that allows a hospital to bill for observation services without formally admitting a patient.
Recent studies have recorded significant increases in hospitals’ use of observation stays among the Medicare population,1-3 raising concerns about the financial ramifications for patients. Under observation, patients are potentially responsible for a greater share of the cost and bear the financial consequences of inappropriate observation stays. Currently, around 6% of Medicare patients hospitalized as outpatients spend more than 48 hours (or two midnights) in observation, sometimes much longer, exposing them to significant out-of-pocket costs.3 In addition, liberal use of observation can lead to increased hospital stays, for example among lower-severity emergency department (ED) patients who could have been safely discharged but were instead kept for a costly observation stay.4 At the same time, hospitals do not necessarily benefit from this cost shifting; in fact, hospital margin is worse for patients under Medicare observation care.5 Yet hospitals are obligated to be compliant with CMS observation regulations and may try to avoid the consequences (eg, audits, non-payment) for inpatient stays that are deemed inappropriate by CMS.
While the nuances of how CMS finances observation stays have made the practice controversial, the use of observation care in other payer groups that may not have the same reimbursement policies, and its impact on patients, have not been well studied. In this issue of the Journal of Hospital Medicine, Nuckols et al.6 begins to address this gap by carefully exploring trends in observation stays in a multipayer data set.
The authors use data for four states (Georgia, Nebraska, South Carolina, and Tennessee) from the Healthcare Cost and Utilization Project (Agency for Healthcare Quality and Research) and the American Community Survey (US Census Bureau) to calculate population based rates of ED visits, observation stays, and inpatient admissions. To date, this is the first study to examine and compare the use of observation stays in an all-payer data set. Similar to prior work that examined the Medicare population, the authors find increased rates of treat-and-release ED visits and observation stays over time with a corresponding decline in inpatient admissions. As this study clearly shows, observation stays are comprising a greater fraction of the total hospital care delivered to patients with acute illnesses.
In many ways, the findings of Nuckols et al.6 raise more questions than they answer. For example, does the rise in observation stays represent a fundamental shift in how hospitals deliver care, an alternative to costly inpatient admissions? Are changing payer incentives driving hospitals to be more prudent in their inpatient admission practices, or are similar services simply being delivered under a new billing designation? And, most important, does this shift have any repercussions for the quality and safety of patient care?
Ultimately, the answer to these questions is, “It depends.” As the authors mention, most US hospitals admit observation patients to general medical wards, where they receive care at the admitting provider’s discretion instead of utilizing specific care pathways or observation protocols.7 In some of these hospitals, there may be little to no difference in how the observation patient is treated compared with a similar patient who is hospitalized as an inpatient.
However, a minority of hospitals has been more strategic in their delivery of observation care and have developed observation units. While observation units vary in design, common features include a dedicated location in the hospital with dedicated staff, reliance on clear inclusion-exclusion criteria for admission to the unit, and the use of rapid diagnostic or treatment protocols for a limited number of conditions. About half of these observation units are ED-based, reducing transitions of care between services. Protocol-driven observation units have the potential to prevent unnecessary inpatient admissions, standardize evidence-based practice, and reduce practice variation and resource use, apparently without increasing adverse events.8 In addition, they may also lead to better experiences of care for many patients compared with inpatient admissions.
Medicare’s own policy on observation hospital care succinctly describes ED observation units: “Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment in order to make a decision concerning their admission or discharge…usually in less than 24 hours.” Due to regulatory changes and auditing pressure, observation care has expanded beyond this definition in length of stay, scope, and practice such that much of observation care now occurs on general hospital wards. Ideally, observation policy must be realigned with its original intent and investment made in ED observation units.
The shifting landscape of hospital-based care as described by Nuckols et al.6 highlights the need for a more strategic approach to the delivery of acute care. Unfortunately, to date, there has been a lack of attention among policymakers towards promoting a system of emergent and urgent care that is coordinated and efficient. Observation stays are one major area for which innovations in the acute care delivery system may result in meaningful improvement in patient outcomes and greater value for the healthcare system. Incentivizing a system of high-value observation care, such as promoting the use of observation units that employ evidence-based practices, should be a key priority when considering approaches to reducing the cost of hospital-based and other acute care.
One strategy is to better define and possibly expand the cohort of patients likely to benefit from care in an observation unit. Hospitals with significant experience using observation units treat not only common observation conditions like chest pain, asthma, or cellulitis, but also higher-risk inpatient conditions like syncope and diabetic ketoacidosis using rapid diagnostic and treatment protocols.
Identifying high-value observation care also will require developing patient outcome measures specific for observation stays. Observation-specific quality measures will allow a comparison of hospitals that use different care pathways for observation patients or treat certain populations of patients in observation units. This necessitates looking beyond resource use (costs and length of stay), which most studies on observation units have focused on, and examining a broader range of patient outcomes like time to symptomatic resolution, quality of life, or return to productivity after an acute illness.
Finally, observation care is also a good target for payment redesign. For example, incentive payments could be provided to hospitals that choose to develop observation units, employ observation units that utilize best known practices for observation care (such as protocols and clearly defined patient cohorts), or deliver particularly good acute care outcomes for patients with observation-amenable conditions. On the consumer side, value-based contracting could be used to shunt patients with acute conditions that require evaluation in an urgent care center or ED to hospitals that use observation units.
While the declines in inpatient admission and increases in treat-and-release ED patients have been well-documented over time, perhaps the biggest contribution of this study from Nuckols et al.6 lies in its identification of the changes in observation care, which have been increasing in all payer groups. Our opportunity now is to shape whether these shifts toward observation care deliver greater value for patients.
Acknowledgment
The authors thank Joanna Guo, BA, for her editorial and technical assistance.
Disclosure
Nothing to report.
1. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
2. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
3. Office of Inspector General. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. US Department of Health & Human Services. Published 2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed April 25, 2017.
4. Blecker S, Gavin NP, Park H, Ladapo JA, Katz SD. Observation units as substitutes for hospitalization or home discharge. Ann Emerg Med. 2016;67(6):706-713.e702. PubMed
5. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Published 2015. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf?sfvrsn=0). Accessed April 25, 2017.
6. Nuckols TN, Fingar KR, Barrett M, Steiner C, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):444-446. PubMed
7. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
8. Ross MA, Aurora T, Graff L, et al. State of the art: emergency department observation units. Crit Pathw Cardiol. 2012;11(3):128-138. PubMed
1. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
2. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
3. Office of Inspector General. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. US Department of Health & Human Services. Published 2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed April 25, 2017.
4. Blecker S, Gavin NP, Park H, Ladapo JA, Katz SD. Observation units as substitutes for hospitalization or home discharge. Ann Emerg Med. 2016;67(6):706-713.e702. PubMed
5. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Published 2015. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf?sfvrsn=0). Accessed April 25, 2017.
6. Nuckols TN, Fingar KR, Barrett M, Steiner C, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):444-446. PubMed
7. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
8. Ross MA, Aurora T, Graff L, et al. State of the art: emergency department observation units. Crit Pathw Cardiol. 2012;11(3):128-138. PubMed
© 2017 Society of Hospital Medicine