User login
Cultivating emotional awareness
A path to resilience and joy in the hospital
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
A path to resilience and joy in the hospital
A path to resilience and joy in the hospital
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Care of post–acute COVID-19 patients requires multidisciplinary collaboration
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
FROM SHM CONVERGE 2021
Some things pediatric hospitalists do for no reason
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
FROM SHM CONVERGE 2021
Procalcitonin-guided antibiotic stewardship for lower respiratory tract infection
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 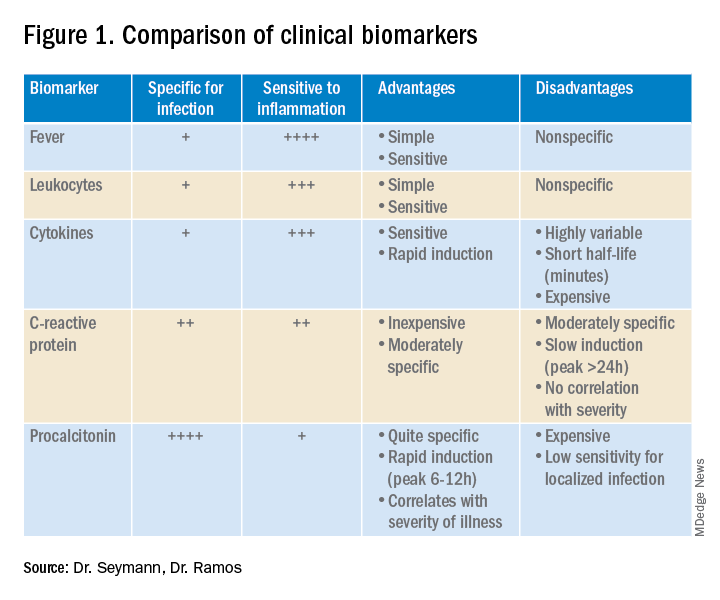
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)
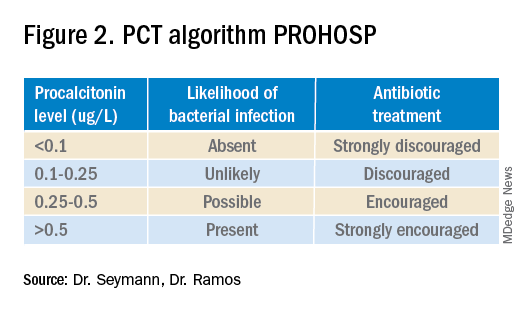
For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.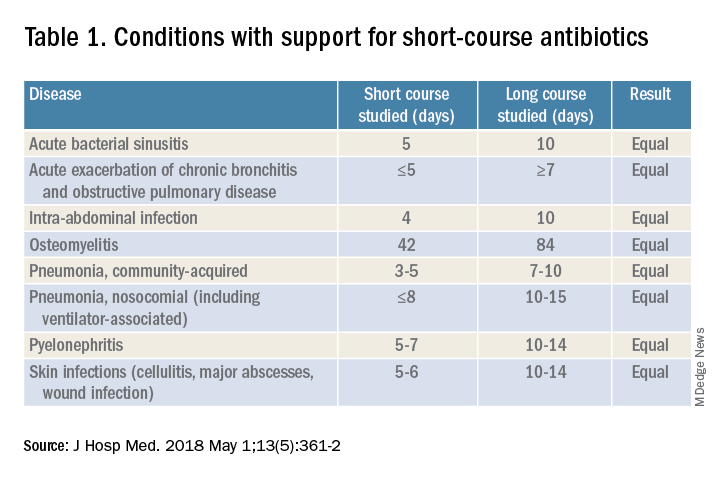
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.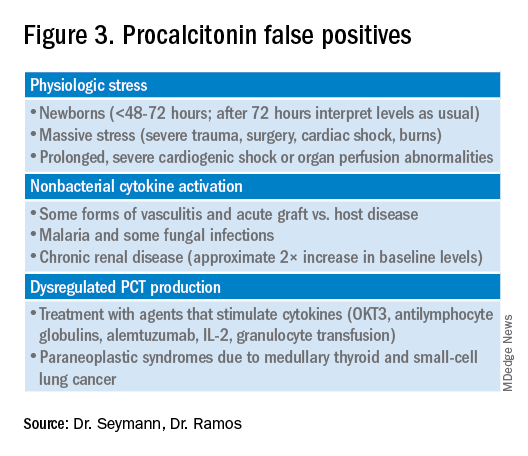
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Dynamics of the assay must be considered
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Planning for SHM Converge 2022 now underway
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
PHM groups issue Choosing Wisely® recommendations
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
SHM members involved from the start
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
Update in Hospital Medicine relays important findings
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
FROM SHM CONVERGE 2021
Trends in hospital medicine program operations during COVID-19
Staffing was a challenge for most groups
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
Staffing was a challenge for most groups
Staffing was a challenge for most groups
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
Smart prescribing strategies improve antibiotic stewardship
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
FROM SHM CONVERGE 2021
Mentor-mentee relationships in hospital medicine
Your mentor has been looking for someone to help lead a new project in your division, and tells you she’s been having a hard time finding someone – but that you would be great. The project isn’t something you are very interested in doing and you’re already swamped with other projects, but the mentor seems to need the help. What do you do?
Mentor-mentee relationships can be deeply beneficial, but the dynamics – in this situation and many others – can be complex. At SHM Converge, the annual conference of the Society of Hospital Medicine, panelists offered guidance on how best to navigate this terrain.
Vineet Arora, MD, MAPP, MHM, associate chief medical officer for clinical learning environment at the University of Chicago, suggested that, in the situation involving the mentor’s request to an uncertain mentee, the mentee should not give an immediate answer, but consider the pros and cons.
“It’s tough when it’s somebody who’s directly overseeing you,” she said. “If you’re really truly the best person, they’re going to want you in the job, and maybe they’ll make it work for you.” She said it would be important to find out why the mentor is having trouble finding someone, and suggested the mentee could find someone with whom to discuss it.
Calling mentoring a “team sport,” Dr. Arora described several types: the traditional mentor who helps many aspects of a mentee’s career, a “coach” who helps on a specific project or topic, a “sponsor” that can help elevate a mentee to a bigger opportunity, and a “connector” who can help a mentee begin new career relationships.
“Don’t invest in just one person,” she said. “Try to get that personal board of directors.”
She mentioned six things all mentors should do: Choose mentees carefully, establish a mentorship team, run a tight ship, head off rifts or resolve them, prepare for transitions when they take a new position and might have a new relationship with a mentee, and don’t commit “mentorship malpractice.”
Mentoring is a two-way street, with both people benefiting and learning, but mentoring can have its troubles, either through active, dysfunctional behavior that’s easy to spot, or passive behavior, such as the “bottleneck” problem when a mentor is too preoccupied with his or her own priorities to mentor well, the “country clubber” who mentors only for popularity and social capital but doesn’t do the work required, and the “world traveler” who is sought after but has little time for day-to-day mentoring.
Valerie Vaughan, MD, MSc, assistant professor of medicine at the University of Utah, described four “golden rules” of being a mentee. First, find a CAPE mentor (for capable, availability, projects of interest, and easy to get along with). Then, be respectful of a mentor’s time, communicate effectively, and be engaged and energizing.
“Mentors typically don’t get paid to mentor and so a lot of them are doing it because they find joy for doing it,” Dr. Vaughan said. “So as much as you can as a mentee, try to be the person who brings energy to the mentor-mentee relationship. It’s up to you to drive projects forward.”
Valerie Press, MD, MPH, SFHM, associate professor of medicine at the University of Chicago, offered tips for men who are mentoring women. She said that, while cross-gender mentorship is common and important, gender-based stereotypes and “unconscious assumptions” are alive and well. Women, she noted, have less access to mentorship and sponsorship, are paid less for the same work, and have high rates of attrition.
Male mentors have to meet the challenge of thinking outside of their own lived experience, combating stereotypes, and addressing these gender-based career disparities, she said.
She suggested that male mentors, for one thing, “rewrite gender scripts,” with comments such as, “This is a difficult situation, but I have confidence in you! What do you think your next move should be?” They should also “learn from each other on how to change the power dynamic,” and start and participate in conversations involving emotions, since they can be clues to what a mentee is experiencing.
When it comes to pushing for better policies, “be an upstander, not a bystander,” Dr. Press said.
“Use your organizational power and your social capital,” she said. “Use your voice to help make more equitable policies. Don’t just leave it to the women’s committee to come up with solutions to lack of lactation rooms, or paternity and maternity leave, or better daycare. These are family issues and everybody issues.”
Maylyn S. Martinez, MD, clinical associate professor of medicine at the University of Chicago, suggested that mentors for physicians from minority groups should resist the tendency to view their interests narrowly.
“Don’t assume that their interests are going to center on their gender or minority status – invite them to be on projects that have nothing to do with that,” she said. They should also not be encouraged to do projects that won’t help with career advancement any more than others would be encouraged to take on such projects.
“Be the solution,” she said. “Not the problem.”
Your mentor has been looking for someone to help lead a new project in your division, and tells you she’s been having a hard time finding someone – but that you would be great. The project isn’t something you are very interested in doing and you’re already swamped with other projects, but the mentor seems to need the help. What do you do?
Mentor-mentee relationships can be deeply beneficial, but the dynamics – in this situation and many others – can be complex. At SHM Converge, the annual conference of the Society of Hospital Medicine, panelists offered guidance on how best to navigate this terrain.
Vineet Arora, MD, MAPP, MHM, associate chief medical officer for clinical learning environment at the University of Chicago, suggested that, in the situation involving the mentor’s request to an uncertain mentee, the mentee should not give an immediate answer, but consider the pros and cons.
“It’s tough when it’s somebody who’s directly overseeing you,” she said. “If you’re really truly the best person, they’re going to want you in the job, and maybe they’ll make it work for you.” She said it would be important to find out why the mentor is having trouble finding someone, and suggested the mentee could find someone with whom to discuss it.
Calling mentoring a “team sport,” Dr. Arora described several types: the traditional mentor who helps many aspects of a mentee’s career, a “coach” who helps on a specific project or topic, a “sponsor” that can help elevate a mentee to a bigger opportunity, and a “connector” who can help a mentee begin new career relationships.
“Don’t invest in just one person,” she said. “Try to get that personal board of directors.”
She mentioned six things all mentors should do: Choose mentees carefully, establish a mentorship team, run a tight ship, head off rifts or resolve them, prepare for transitions when they take a new position and might have a new relationship with a mentee, and don’t commit “mentorship malpractice.”
Mentoring is a two-way street, with both people benefiting and learning, but mentoring can have its troubles, either through active, dysfunctional behavior that’s easy to spot, or passive behavior, such as the “bottleneck” problem when a mentor is too preoccupied with his or her own priorities to mentor well, the “country clubber” who mentors only for popularity and social capital but doesn’t do the work required, and the “world traveler” who is sought after but has little time for day-to-day mentoring.
Valerie Vaughan, MD, MSc, assistant professor of medicine at the University of Utah, described four “golden rules” of being a mentee. First, find a CAPE mentor (for capable, availability, projects of interest, and easy to get along with). Then, be respectful of a mentor’s time, communicate effectively, and be engaged and energizing.
“Mentors typically don’t get paid to mentor and so a lot of them are doing it because they find joy for doing it,” Dr. Vaughan said. “So as much as you can as a mentee, try to be the person who brings energy to the mentor-mentee relationship. It’s up to you to drive projects forward.”
Valerie Press, MD, MPH, SFHM, associate professor of medicine at the University of Chicago, offered tips for men who are mentoring women. She said that, while cross-gender mentorship is common and important, gender-based stereotypes and “unconscious assumptions” are alive and well. Women, she noted, have less access to mentorship and sponsorship, are paid less for the same work, and have high rates of attrition.
Male mentors have to meet the challenge of thinking outside of their own lived experience, combating stereotypes, and addressing these gender-based career disparities, she said.
She suggested that male mentors, for one thing, “rewrite gender scripts,” with comments such as, “This is a difficult situation, but I have confidence in you! What do you think your next move should be?” They should also “learn from each other on how to change the power dynamic,” and start and participate in conversations involving emotions, since they can be clues to what a mentee is experiencing.
When it comes to pushing for better policies, “be an upstander, not a bystander,” Dr. Press said.
“Use your organizational power and your social capital,” she said. “Use your voice to help make more equitable policies. Don’t just leave it to the women’s committee to come up with solutions to lack of lactation rooms, or paternity and maternity leave, or better daycare. These are family issues and everybody issues.”
Maylyn S. Martinez, MD, clinical associate professor of medicine at the University of Chicago, suggested that mentors for physicians from minority groups should resist the tendency to view their interests narrowly.
“Don’t assume that their interests are going to center on their gender or minority status – invite them to be on projects that have nothing to do with that,” she said. They should also not be encouraged to do projects that won’t help with career advancement any more than others would be encouraged to take on such projects.
“Be the solution,” she said. “Not the problem.”
Your mentor has been looking for someone to help lead a new project in your division, and tells you she’s been having a hard time finding someone – but that you would be great. The project isn’t something you are very interested in doing and you’re already swamped with other projects, but the mentor seems to need the help. What do you do?
Mentor-mentee relationships can be deeply beneficial, but the dynamics – in this situation and many others – can be complex. At SHM Converge, the annual conference of the Society of Hospital Medicine, panelists offered guidance on how best to navigate this terrain.
Vineet Arora, MD, MAPP, MHM, associate chief medical officer for clinical learning environment at the University of Chicago, suggested that, in the situation involving the mentor’s request to an uncertain mentee, the mentee should not give an immediate answer, but consider the pros and cons.
“It’s tough when it’s somebody who’s directly overseeing you,” she said. “If you’re really truly the best person, they’re going to want you in the job, and maybe they’ll make it work for you.” She said it would be important to find out why the mentor is having trouble finding someone, and suggested the mentee could find someone with whom to discuss it.
Calling mentoring a “team sport,” Dr. Arora described several types: the traditional mentor who helps many aspects of a mentee’s career, a “coach” who helps on a specific project or topic, a “sponsor” that can help elevate a mentee to a bigger opportunity, and a “connector” who can help a mentee begin new career relationships.
“Don’t invest in just one person,” she said. “Try to get that personal board of directors.”
She mentioned six things all mentors should do: Choose mentees carefully, establish a mentorship team, run a tight ship, head off rifts or resolve them, prepare for transitions when they take a new position and might have a new relationship with a mentee, and don’t commit “mentorship malpractice.”
Mentoring is a two-way street, with both people benefiting and learning, but mentoring can have its troubles, either through active, dysfunctional behavior that’s easy to spot, or passive behavior, such as the “bottleneck” problem when a mentor is too preoccupied with his or her own priorities to mentor well, the “country clubber” who mentors only for popularity and social capital but doesn’t do the work required, and the “world traveler” who is sought after but has little time for day-to-day mentoring.
Valerie Vaughan, MD, MSc, assistant professor of medicine at the University of Utah, described four “golden rules” of being a mentee. First, find a CAPE mentor (for capable, availability, projects of interest, and easy to get along with). Then, be respectful of a mentor’s time, communicate effectively, and be engaged and energizing.
“Mentors typically don’t get paid to mentor and so a lot of them are doing it because they find joy for doing it,” Dr. Vaughan said. “So as much as you can as a mentee, try to be the person who brings energy to the mentor-mentee relationship. It’s up to you to drive projects forward.”
Valerie Press, MD, MPH, SFHM, associate professor of medicine at the University of Chicago, offered tips for men who are mentoring women. She said that, while cross-gender mentorship is common and important, gender-based stereotypes and “unconscious assumptions” are alive and well. Women, she noted, have less access to mentorship and sponsorship, are paid less for the same work, and have high rates of attrition.
Male mentors have to meet the challenge of thinking outside of their own lived experience, combating stereotypes, and addressing these gender-based career disparities, she said.
She suggested that male mentors, for one thing, “rewrite gender scripts,” with comments such as, “This is a difficult situation, but I have confidence in you! What do you think your next move should be?” They should also “learn from each other on how to change the power dynamic,” and start and participate in conversations involving emotions, since they can be clues to what a mentee is experiencing.
When it comes to pushing for better policies, “be an upstander, not a bystander,” Dr. Press said.
“Use your organizational power and your social capital,” she said. “Use your voice to help make more equitable policies. Don’t just leave it to the women’s committee to come up with solutions to lack of lactation rooms, or paternity and maternity leave, or better daycare. These are family issues and everybody issues.”
Maylyn S. Martinez, MD, clinical associate professor of medicine at the University of Chicago, suggested that mentors for physicians from minority groups should resist the tendency to view their interests narrowly.
“Don’t assume that their interests are going to center on their gender or minority status – invite them to be on projects that have nothing to do with that,” she said. They should also not be encouraged to do projects that won’t help with career advancement any more than others would be encouraged to take on such projects.
“Be the solution,” she said. “Not the problem.”
FROM SHM CONVERGE 2021

























