User login
Pandemic innovations that will outlast COVID
Editor’s note: Hospitalists told us about process changes that their teams have implemented during the COVID-19 pandemic.
Shyam Odeti, MD, SFHM
Ballad Health (Bristol, Tenn.)(Dr. Odeti was a hospitalist at Ballad Health during the period he describes below. He is currently chief of hospital medicine at Carilion Clinic, Roanoke, Va.)
Ballad Health is a 21-hospital health system serving 1.2 million population in 21 counties of rural Appalachia (northeast Tennessee, southwest Virginia, western North Carolina, and Kentucky). We saw a significant spike in COVID-19 numbers beginning in October 2020. We were at a 7.9% test positivity rate and 89 COVID-19 hospitalizations on Oct. 1, which rapidly increased to over 18% positivity rate and over 250 hospitalizations by mid-November. This alarming trend created concerns about handling the future inpatient volumes in an already strained health system.
There were some unique challenges to this region that were contributing to the increased hospitalizations. A significant part of the population we serve in this region has low health literacy, low socioeconomic status, and problems with transportation. Telehealth in an outpatient setting was rudimentary in parts of this region.
Ballad Health developed Safe At Home to identify lower-acuity COVID-19 patients and transition them to the home setting safely. This in turn would prevent their readmissions or return visits to the ED by implementing comprehensive oversight to their disease course. We achieved this through a collaborative approach of the existing teams, case management, telenurse team, primary care providers, and hospitalist-led transitional care. We leveraged the newly implemented EHR Epic and telehealth under the leadership of Ballad Health’s chief medical information officer, Dr. Mark Wilkinson.
Among the patients diagnosed with COVID-19 in ED and urgent care, low acuity cases were identified and enrolled into Safe At Home. Patients were provided with a pulse oximeter, thermometer, and incentive spirometer. They received phone calls the next 2 days from the telenurse team for a comprehensive interview, followed by daily phone calls during the first week. If no concerns were raised initially, then calls were spaced to every 3 days after that for up to 2 weeks. Any complaints or alarming symptoms would trigger a telehealth visit with primary care physicians, transitional care clinics, or a hospitalist.
The Safe At Home program was highly successful – in the past 5 months, over 1,500 patients were enrolled and hundreds of admissions were likely avoided. As we feared, the positivity rate in our region went close to 35% and inpatient COVID-19 census was over 350, with ICU utilization over 92%. If not for our innovative solution, this pandemic could have easily paralyzed health care in our region. Our patients also felt safe, as they were monitored daily and had help one call away, 24/7.
This innovation has brought solutions through technological advancements and process improvement. Safe At Home was also instrumental in breaking down silos and developing a culture of collaboration and cohesiveness among the inpatient, outpatient, and virtual teams of the health system. Lessons learned from this initiative can be easily replicated in the management of several chronic diseases to provide safe and affordable care to our patients in the comfort of their homes.
Vasundara Singh, MBBS
Mount Sinai West (New York)
At the onset of the pandemic in New York, our medium-sized midtown hospital used personal protective equipment briskly. One reason identified was the failure to cohort COVID-19 patients on a single floor. The other more important cause was that medicine teams in our hospital have patients scattered throughout the hospital in a nongeographic model across four different floors. Within 2 weeks, administration and hospital medicine leadership developed a geographic model. We started cohorting all COVID-19 positive patients on separate floors from negative patients. A geographic physician team model was also developed, which allowed physicians and nurses to don and doff at the entry and exit of each COVID-19 unit.
After the pandemic surge, hospital medicine and internal medicine residency program leadership made the collective decision to continue the geographic model for inpatient care. Care providers enjoyed working in a unit-based model, and noted increases in efficiency while rounding. Each of our four medicine floors has 36-40 beds, with variable occupancy. We restructured our resident teams and physician assistant teams by geography. Our outgoing chief residents led the change in May, designing a resident schedule to accommodate for a resident on each team to be available to admit and provide coverage until 8 p.m. each evening on their respective floors. The hospital medicine leadership put together a committee comprising representation of all stakeholders in this large transition of systems: attending hospitalists, physician assistants, chief residents, nurse managers, bed assignment, and administration. Since the transition and resumption of normal inpatient activity, we have encountered and addressed multiple concerns. Some notable hurdles in this transition included the high throughput on our telemetry team, movement of patients by bed board or nursing without involving the physicians in the decision, and variable nursing staffing that impacts teaching team caps because of geographic model.
This transition is very much still a work in progress, yet some benefits are already obvious. It has made bedside rounding more appealing and uncomplicated. Physicians in training learn very well at the bedside by role modeling. Greater acceptance of bedside rounding also affords the opportunity to teach physical exam skills, a dying art amongst newer generations of doctors. Another large gain is being able to involve nursing in bedside rounds, discussions, and decision-making. Finally, coordination with ancillary staff including social work and case management has become seamless as a result of having an entire floor to ourselves.
In summary, the silver lining of this pernicious pandemic at our hospital has been a transition to a geographic model for inpatient care. This is considered to be the gold standard for inpatient care across multiple health systems, and we hope to continue to refine this geographic model of care. Next steps would involve developing capabilities with flex acuity beds on each unit so that no matter what the patients need they can stay in one place.
Marina Farah, MD, MHA
Sound Physicians (Tacoma, Wash.)
With hospital programs in over 40 states, Sound Physicians has played an important role in the COVID-19 pandemic, treating approximately 6% of all COVID hospitalizations nationwide. To meet the needs of the crisis, Sound relied on innovation to expand coverage and improve outcomes at facilities across the country. Of one particular note, Sound Telemedicine partnered with the University of Maryland Medical System to open the state’s first COVID-only hospital. In March 2020, the UMMS needed to care for an emerging cohort of COVID-19 patients while maintaining high-quality care and minimizing exposure for non-COVID patients.
Sound collaborated with UMMS to rapidly reopen the University of Maryland Laurel Medical Center for COVID-only care, staffing the hospital with Sound’s telehospitalists. A model based on daily rounding delivered 100% by telemedicine providers and flexible staffing available 24/7 would let the program scale up or down to meet volume demands. Onsite physician support would be limited to one admitting doctor and a nocturnist. The COVID-only facility allowed a small group of doctors, nurses, and technicians to focus exclusively on an emerging disease, honing critical skills for treating COVID-19 patients.
Immediate benefits yielded big results. UMLMC’s capacity allowed UMMS to funnel COVID patients into fewer of their regional hospitals, limiting the risk of exposure. Rapid deployment got UMMS ahead of the surge, taking stress off other hospitals in the system and 24/7 telehospitalist coverage proved to be a successful long-term staffing strategy for UMLMC. Long-term benefits were recognized too. Sound’s staffing model and clinical processes significantly improved quality of care. Mortality rates dropped from 18% to 9% during the initial 60 days of the program. Vaccinations shifted COVID-19 needs, however, due to improvements in care and the flexibility offered, telemedicine remains an integral part of the UMMS’s long-term strategy
Emory Healthcare division of hospital medicine (Atlanta)
(Comments compiled by James Kim, MD, assistant professor in the division of hospital medicine) Ingrid Pinzon, MD, FACP Emory Johns Creek (Ga.) Hospital
When COVID-19 started, one of the things called to my attention was the disparity in education for the Hispanic population. Unfortunately, COVID showed how in our hospitals there is a lack of instructions and education in Spanish.
We started educating our Hispanic community with Facebook lives via the Latin American Association. I was also invited to the different Spanish news stations (Telemundo and Univision). I also educated this community through food drives, where I taught about the use of face masks, social distancing, and hand hygiene.
Reena Hemrajani, MD
Grady Memorial Hospital
At Grady, we transitioned our weekly educational conferences into virtual events, and this has increased our attendance, as more off-service people are likely to attend when they can log on remotely. This has also allowed us to record these sessions for later viewing by those were unable to make it in real time.
Yelena Burklin, MD, FHM
Emory University Hospital Midtown
In our Midtown group, we have started a few initiatives that we will continue post COVID. Hybrid didactic lectures have had great success with excellent attendance when our didactic sessions (lunch and learns, journal clubs, core lectures for step-down unit refresher series) have been conducted virtually.
During the pandemic’s height, when all resources were dedicated to COVID-19 patient care, there was a particular need to cognitively separate from “all things COVID” and provide additional topics to learn about, such as review of the management of different types of shock, chronic obstructive pulmonary disorder, sepsis, liver cirrhosis, etc. Attendance to these non–COVID-19 sessions was just as high.
We had a number of stressful and near-death experiences that tested our resilience, professional integrity, and overall wellness. These reflections prompted us to invite psychiatrists to one of the in-person–only sessions so that an informal conversation could be afforded in a safe space. Those hospitalists who felt the need to discuss their issues further received additional support and instructions from a subspecialist.
Ray Dantes, MD
Emory University Hospital Midtown
Post COVID, we will certainly utilize a hybrid approach to the didactic sessions when patient sensitive information is not being discussed. We will also preserve the continuity in addressing wellness and resilience, particularly, when our Midtown hospitalists had to work a lot of extra hours to cover the growing need at the time of pandemic, and need to emotionally decompress post pandemic. We are also taking infection control more seriously, and not coming to work with upper respiratory infections.
Rajasree Roy, MD
Emory Saint Joseph’s Hospital
At ESJH, we initiated a telemedicine pilot for our hospitalist team in order to sustain our service given census surge and physician illness.
Sara Millwee, DNP, APRN, FNP-BC
Emory chief of advanced practice providers
To help reduce exposure to COVID, our advanced practice providers (APPs) admitted patients from the ED (as they did pre-COVID) to the hospital medicine service, but the physicians administratively signed the note/orders. Emory Healthcare bylaws specify that patients are seen by a physician within 24 hours of admission. During the pandemic, at the time of admission, the APP discussed plan of care with the physician, but the patient was only seen by the APP upon initial evaluation/admission, as opposed to the physician and APP pre-COVID. This improved productivity, and facilitated communication and collaboration between APPs and physicians. This also fostered an environment where APPs were practicing at the top of their licenses and improved job satisfaction.
Additionally, across the hospital medicine division, several APPs were utilized from other divisions to assist with admissions and cross cover. As the volume was at incredibly high levels, this improved the workload and burden of the hospital medicine providers. The displaced APPs were utilized at several facilities and worked under the guidance and supervision of hospital medicine providers. Moving forward, this has prompted leadership to look at utilizing APPs from other divisions as “PRN” providers as well.
Editor’s note: Hospitalists told us about process changes that their teams have implemented during the COVID-19 pandemic.
Shyam Odeti, MD, SFHM
Ballad Health (Bristol, Tenn.)(Dr. Odeti was a hospitalist at Ballad Health during the period he describes below. He is currently chief of hospital medicine at Carilion Clinic, Roanoke, Va.)
Ballad Health is a 21-hospital health system serving 1.2 million population in 21 counties of rural Appalachia (northeast Tennessee, southwest Virginia, western North Carolina, and Kentucky). We saw a significant spike in COVID-19 numbers beginning in October 2020. We were at a 7.9% test positivity rate and 89 COVID-19 hospitalizations on Oct. 1, which rapidly increased to over 18% positivity rate and over 250 hospitalizations by mid-November. This alarming trend created concerns about handling the future inpatient volumes in an already strained health system.
There were some unique challenges to this region that were contributing to the increased hospitalizations. A significant part of the population we serve in this region has low health literacy, low socioeconomic status, and problems with transportation. Telehealth in an outpatient setting was rudimentary in parts of this region.
Ballad Health developed Safe At Home to identify lower-acuity COVID-19 patients and transition them to the home setting safely. This in turn would prevent their readmissions or return visits to the ED by implementing comprehensive oversight to their disease course. We achieved this through a collaborative approach of the existing teams, case management, telenurse team, primary care providers, and hospitalist-led transitional care. We leveraged the newly implemented EHR Epic and telehealth under the leadership of Ballad Health’s chief medical information officer, Dr. Mark Wilkinson.
Among the patients diagnosed with COVID-19 in ED and urgent care, low acuity cases were identified and enrolled into Safe At Home. Patients were provided with a pulse oximeter, thermometer, and incentive spirometer. They received phone calls the next 2 days from the telenurse team for a comprehensive interview, followed by daily phone calls during the first week. If no concerns were raised initially, then calls were spaced to every 3 days after that for up to 2 weeks. Any complaints or alarming symptoms would trigger a telehealth visit with primary care physicians, transitional care clinics, or a hospitalist.
The Safe At Home program was highly successful – in the past 5 months, over 1,500 patients were enrolled and hundreds of admissions were likely avoided. As we feared, the positivity rate in our region went close to 35% and inpatient COVID-19 census was over 350, with ICU utilization over 92%. If not for our innovative solution, this pandemic could have easily paralyzed health care in our region. Our patients also felt safe, as they were monitored daily and had help one call away, 24/7.
This innovation has brought solutions through technological advancements and process improvement. Safe At Home was also instrumental in breaking down silos and developing a culture of collaboration and cohesiveness among the inpatient, outpatient, and virtual teams of the health system. Lessons learned from this initiative can be easily replicated in the management of several chronic diseases to provide safe and affordable care to our patients in the comfort of their homes.
Vasundara Singh, MBBS
Mount Sinai West (New York)
At the onset of the pandemic in New York, our medium-sized midtown hospital used personal protective equipment briskly. One reason identified was the failure to cohort COVID-19 patients on a single floor. The other more important cause was that medicine teams in our hospital have patients scattered throughout the hospital in a nongeographic model across four different floors. Within 2 weeks, administration and hospital medicine leadership developed a geographic model. We started cohorting all COVID-19 positive patients on separate floors from negative patients. A geographic physician team model was also developed, which allowed physicians and nurses to don and doff at the entry and exit of each COVID-19 unit.
After the pandemic surge, hospital medicine and internal medicine residency program leadership made the collective decision to continue the geographic model for inpatient care. Care providers enjoyed working in a unit-based model, and noted increases in efficiency while rounding. Each of our four medicine floors has 36-40 beds, with variable occupancy. We restructured our resident teams and physician assistant teams by geography. Our outgoing chief residents led the change in May, designing a resident schedule to accommodate for a resident on each team to be available to admit and provide coverage until 8 p.m. each evening on their respective floors. The hospital medicine leadership put together a committee comprising representation of all stakeholders in this large transition of systems: attending hospitalists, physician assistants, chief residents, nurse managers, bed assignment, and administration. Since the transition and resumption of normal inpatient activity, we have encountered and addressed multiple concerns. Some notable hurdles in this transition included the high throughput on our telemetry team, movement of patients by bed board or nursing without involving the physicians in the decision, and variable nursing staffing that impacts teaching team caps because of geographic model.
This transition is very much still a work in progress, yet some benefits are already obvious. It has made bedside rounding more appealing and uncomplicated. Physicians in training learn very well at the bedside by role modeling. Greater acceptance of bedside rounding also affords the opportunity to teach physical exam skills, a dying art amongst newer generations of doctors. Another large gain is being able to involve nursing in bedside rounds, discussions, and decision-making. Finally, coordination with ancillary staff including social work and case management has become seamless as a result of having an entire floor to ourselves.
In summary, the silver lining of this pernicious pandemic at our hospital has been a transition to a geographic model for inpatient care. This is considered to be the gold standard for inpatient care across multiple health systems, and we hope to continue to refine this geographic model of care. Next steps would involve developing capabilities with flex acuity beds on each unit so that no matter what the patients need they can stay in one place.
Marina Farah, MD, MHA
Sound Physicians (Tacoma, Wash.)
With hospital programs in over 40 states, Sound Physicians has played an important role in the COVID-19 pandemic, treating approximately 6% of all COVID hospitalizations nationwide. To meet the needs of the crisis, Sound relied on innovation to expand coverage and improve outcomes at facilities across the country. Of one particular note, Sound Telemedicine partnered with the University of Maryland Medical System to open the state’s first COVID-only hospital. In March 2020, the UMMS needed to care for an emerging cohort of COVID-19 patients while maintaining high-quality care and minimizing exposure for non-COVID patients.
Sound collaborated with UMMS to rapidly reopen the University of Maryland Laurel Medical Center for COVID-only care, staffing the hospital with Sound’s telehospitalists. A model based on daily rounding delivered 100% by telemedicine providers and flexible staffing available 24/7 would let the program scale up or down to meet volume demands. Onsite physician support would be limited to one admitting doctor and a nocturnist. The COVID-only facility allowed a small group of doctors, nurses, and technicians to focus exclusively on an emerging disease, honing critical skills for treating COVID-19 patients.
Immediate benefits yielded big results. UMLMC’s capacity allowed UMMS to funnel COVID patients into fewer of their regional hospitals, limiting the risk of exposure. Rapid deployment got UMMS ahead of the surge, taking stress off other hospitals in the system and 24/7 telehospitalist coverage proved to be a successful long-term staffing strategy for UMLMC. Long-term benefits were recognized too. Sound’s staffing model and clinical processes significantly improved quality of care. Mortality rates dropped from 18% to 9% during the initial 60 days of the program. Vaccinations shifted COVID-19 needs, however, due to improvements in care and the flexibility offered, telemedicine remains an integral part of the UMMS’s long-term strategy
Emory Healthcare division of hospital medicine (Atlanta)
(Comments compiled by James Kim, MD, assistant professor in the division of hospital medicine) Ingrid Pinzon, MD, FACP Emory Johns Creek (Ga.) Hospital
When COVID-19 started, one of the things called to my attention was the disparity in education for the Hispanic population. Unfortunately, COVID showed how in our hospitals there is a lack of instructions and education in Spanish.
We started educating our Hispanic community with Facebook lives via the Latin American Association. I was also invited to the different Spanish news stations (Telemundo and Univision). I also educated this community through food drives, where I taught about the use of face masks, social distancing, and hand hygiene.
Reena Hemrajani, MD
Grady Memorial Hospital
At Grady, we transitioned our weekly educational conferences into virtual events, and this has increased our attendance, as more off-service people are likely to attend when they can log on remotely. This has also allowed us to record these sessions for later viewing by those were unable to make it in real time.
Yelena Burklin, MD, FHM
Emory University Hospital Midtown
In our Midtown group, we have started a few initiatives that we will continue post COVID. Hybrid didactic lectures have had great success with excellent attendance when our didactic sessions (lunch and learns, journal clubs, core lectures for step-down unit refresher series) have been conducted virtually.
During the pandemic’s height, when all resources were dedicated to COVID-19 patient care, there was a particular need to cognitively separate from “all things COVID” and provide additional topics to learn about, such as review of the management of different types of shock, chronic obstructive pulmonary disorder, sepsis, liver cirrhosis, etc. Attendance to these non–COVID-19 sessions was just as high.
We had a number of stressful and near-death experiences that tested our resilience, professional integrity, and overall wellness. These reflections prompted us to invite psychiatrists to one of the in-person–only sessions so that an informal conversation could be afforded in a safe space. Those hospitalists who felt the need to discuss their issues further received additional support and instructions from a subspecialist.
Ray Dantes, MD
Emory University Hospital Midtown
Post COVID, we will certainly utilize a hybrid approach to the didactic sessions when patient sensitive information is not being discussed. We will also preserve the continuity in addressing wellness and resilience, particularly, when our Midtown hospitalists had to work a lot of extra hours to cover the growing need at the time of pandemic, and need to emotionally decompress post pandemic. We are also taking infection control more seriously, and not coming to work with upper respiratory infections.
Rajasree Roy, MD
Emory Saint Joseph’s Hospital
At ESJH, we initiated a telemedicine pilot for our hospitalist team in order to sustain our service given census surge and physician illness.
Sara Millwee, DNP, APRN, FNP-BC
Emory chief of advanced practice providers
To help reduce exposure to COVID, our advanced practice providers (APPs) admitted patients from the ED (as they did pre-COVID) to the hospital medicine service, but the physicians administratively signed the note/orders. Emory Healthcare bylaws specify that patients are seen by a physician within 24 hours of admission. During the pandemic, at the time of admission, the APP discussed plan of care with the physician, but the patient was only seen by the APP upon initial evaluation/admission, as opposed to the physician and APP pre-COVID. This improved productivity, and facilitated communication and collaboration between APPs and physicians. This also fostered an environment where APPs were practicing at the top of their licenses and improved job satisfaction.
Additionally, across the hospital medicine division, several APPs were utilized from other divisions to assist with admissions and cross cover. As the volume was at incredibly high levels, this improved the workload and burden of the hospital medicine providers. The displaced APPs were utilized at several facilities and worked under the guidance and supervision of hospital medicine providers. Moving forward, this has prompted leadership to look at utilizing APPs from other divisions as “PRN” providers as well.
Editor’s note: Hospitalists told us about process changes that their teams have implemented during the COVID-19 pandemic.
Shyam Odeti, MD, SFHM
Ballad Health (Bristol, Tenn.)(Dr. Odeti was a hospitalist at Ballad Health during the period he describes below. He is currently chief of hospital medicine at Carilion Clinic, Roanoke, Va.)
Ballad Health is a 21-hospital health system serving 1.2 million population in 21 counties of rural Appalachia (northeast Tennessee, southwest Virginia, western North Carolina, and Kentucky). We saw a significant spike in COVID-19 numbers beginning in October 2020. We were at a 7.9% test positivity rate and 89 COVID-19 hospitalizations on Oct. 1, which rapidly increased to over 18% positivity rate and over 250 hospitalizations by mid-November. This alarming trend created concerns about handling the future inpatient volumes in an already strained health system.
There were some unique challenges to this region that were contributing to the increased hospitalizations. A significant part of the population we serve in this region has low health literacy, low socioeconomic status, and problems with transportation. Telehealth in an outpatient setting was rudimentary in parts of this region.
Ballad Health developed Safe At Home to identify lower-acuity COVID-19 patients and transition them to the home setting safely. This in turn would prevent their readmissions or return visits to the ED by implementing comprehensive oversight to their disease course. We achieved this through a collaborative approach of the existing teams, case management, telenurse team, primary care providers, and hospitalist-led transitional care. We leveraged the newly implemented EHR Epic and telehealth under the leadership of Ballad Health’s chief medical information officer, Dr. Mark Wilkinson.
Among the patients diagnosed with COVID-19 in ED and urgent care, low acuity cases were identified and enrolled into Safe At Home. Patients were provided with a pulse oximeter, thermometer, and incentive spirometer. They received phone calls the next 2 days from the telenurse team for a comprehensive interview, followed by daily phone calls during the first week. If no concerns were raised initially, then calls were spaced to every 3 days after that for up to 2 weeks. Any complaints or alarming symptoms would trigger a telehealth visit with primary care physicians, transitional care clinics, or a hospitalist.
The Safe At Home program was highly successful – in the past 5 months, over 1,500 patients were enrolled and hundreds of admissions were likely avoided. As we feared, the positivity rate in our region went close to 35% and inpatient COVID-19 census was over 350, with ICU utilization over 92%. If not for our innovative solution, this pandemic could have easily paralyzed health care in our region. Our patients also felt safe, as they were monitored daily and had help one call away, 24/7.
This innovation has brought solutions through technological advancements and process improvement. Safe At Home was also instrumental in breaking down silos and developing a culture of collaboration and cohesiveness among the inpatient, outpatient, and virtual teams of the health system. Lessons learned from this initiative can be easily replicated in the management of several chronic diseases to provide safe and affordable care to our patients in the comfort of their homes.
Vasundara Singh, MBBS
Mount Sinai West (New York)
At the onset of the pandemic in New York, our medium-sized midtown hospital used personal protective equipment briskly. One reason identified was the failure to cohort COVID-19 patients on a single floor. The other more important cause was that medicine teams in our hospital have patients scattered throughout the hospital in a nongeographic model across four different floors. Within 2 weeks, administration and hospital medicine leadership developed a geographic model. We started cohorting all COVID-19 positive patients on separate floors from negative patients. A geographic physician team model was also developed, which allowed physicians and nurses to don and doff at the entry and exit of each COVID-19 unit.
After the pandemic surge, hospital medicine and internal medicine residency program leadership made the collective decision to continue the geographic model for inpatient care. Care providers enjoyed working in a unit-based model, and noted increases in efficiency while rounding. Each of our four medicine floors has 36-40 beds, with variable occupancy. We restructured our resident teams and physician assistant teams by geography. Our outgoing chief residents led the change in May, designing a resident schedule to accommodate for a resident on each team to be available to admit and provide coverage until 8 p.m. each evening on their respective floors. The hospital medicine leadership put together a committee comprising representation of all stakeholders in this large transition of systems: attending hospitalists, physician assistants, chief residents, nurse managers, bed assignment, and administration. Since the transition and resumption of normal inpatient activity, we have encountered and addressed multiple concerns. Some notable hurdles in this transition included the high throughput on our telemetry team, movement of patients by bed board or nursing without involving the physicians in the decision, and variable nursing staffing that impacts teaching team caps because of geographic model.
This transition is very much still a work in progress, yet some benefits are already obvious. It has made bedside rounding more appealing and uncomplicated. Physicians in training learn very well at the bedside by role modeling. Greater acceptance of bedside rounding also affords the opportunity to teach physical exam skills, a dying art amongst newer generations of doctors. Another large gain is being able to involve nursing in bedside rounds, discussions, and decision-making. Finally, coordination with ancillary staff including social work and case management has become seamless as a result of having an entire floor to ourselves.
In summary, the silver lining of this pernicious pandemic at our hospital has been a transition to a geographic model for inpatient care. This is considered to be the gold standard for inpatient care across multiple health systems, and we hope to continue to refine this geographic model of care. Next steps would involve developing capabilities with flex acuity beds on each unit so that no matter what the patients need they can stay in one place.
Marina Farah, MD, MHA
Sound Physicians (Tacoma, Wash.)
With hospital programs in over 40 states, Sound Physicians has played an important role in the COVID-19 pandemic, treating approximately 6% of all COVID hospitalizations nationwide. To meet the needs of the crisis, Sound relied on innovation to expand coverage and improve outcomes at facilities across the country. Of one particular note, Sound Telemedicine partnered with the University of Maryland Medical System to open the state’s first COVID-only hospital. In March 2020, the UMMS needed to care for an emerging cohort of COVID-19 patients while maintaining high-quality care and minimizing exposure for non-COVID patients.
Sound collaborated with UMMS to rapidly reopen the University of Maryland Laurel Medical Center for COVID-only care, staffing the hospital with Sound’s telehospitalists. A model based on daily rounding delivered 100% by telemedicine providers and flexible staffing available 24/7 would let the program scale up or down to meet volume demands. Onsite physician support would be limited to one admitting doctor and a nocturnist. The COVID-only facility allowed a small group of doctors, nurses, and technicians to focus exclusively on an emerging disease, honing critical skills for treating COVID-19 patients.
Immediate benefits yielded big results. UMLMC’s capacity allowed UMMS to funnel COVID patients into fewer of their regional hospitals, limiting the risk of exposure. Rapid deployment got UMMS ahead of the surge, taking stress off other hospitals in the system and 24/7 telehospitalist coverage proved to be a successful long-term staffing strategy for UMLMC. Long-term benefits were recognized too. Sound’s staffing model and clinical processes significantly improved quality of care. Mortality rates dropped from 18% to 9% during the initial 60 days of the program. Vaccinations shifted COVID-19 needs, however, due to improvements in care and the flexibility offered, telemedicine remains an integral part of the UMMS’s long-term strategy
Emory Healthcare division of hospital medicine (Atlanta)
(Comments compiled by James Kim, MD, assistant professor in the division of hospital medicine) Ingrid Pinzon, MD, FACP Emory Johns Creek (Ga.) Hospital
When COVID-19 started, one of the things called to my attention was the disparity in education for the Hispanic population. Unfortunately, COVID showed how in our hospitals there is a lack of instructions and education in Spanish.
We started educating our Hispanic community with Facebook lives via the Latin American Association. I was also invited to the different Spanish news stations (Telemundo and Univision). I also educated this community through food drives, where I taught about the use of face masks, social distancing, and hand hygiene.
Reena Hemrajani, MD
Grady Memorial Hospital
At Grady, we transitioned our weekly educational conferences into virtual events, and this has increased our attendance, as more off-service people are likely to attend when they can log on remotely. This has also allowed us to record these sessions for later viewing by those were unable to make it in real time.
Yelena Burklin, MD, FHM
Emory University Hospital Midtown
In our Midtown group, we have started a few initiatives that we will continue post COVID. Hybrid didactic lectures have had great success with excellent attendance when our didactic sessions (lunch and learns, journal clubs, core lectures for step-down unit refresher series) have been conducted virtually.
During the pandemic’s height, when all resources were dedicated to COVID-19 patient care, there was a particular need to cognitively separate from “all things COVID” and provide additional topics to learn about, such as review of the management of different types of shock, chronic obstructive pulmonary disorder, sepsis, liver cirrhosis, etc. Attendance to these non–COVID-19 sessions was just as high.
We had a number of stressful and near-death experiences that tested our resilience, professional integrity, and overall wellness. These reflections prompted us to invite psychiatrists to one of the in-person–only sessions so that an informal conversation could be afforded in a safe space. Those hospitalists who felt the need to discuss their issues further received additional support and instructions from a subspecialist.
Ray Dantes, MD
Emory University Hospital Midtown
Post COVID, we will certainly utilize a hybrid approach to the didactic sessions when patient sensitive information is not being discussed. We will also preserve the continuity in addressing wellness and resilience, particularly, when our Midtown hospitalists had to work a lot of extra hours to cover the growing need at the time of pandemic, and need to emotionally decompress post pandemic. We are also taking infection control more seriously, and not coming to work with upper respiratory infections.
Rajasree Roy, MD
Emory Saint Joseph’s Hospital
At ESJH, we initiated a telemedicine pilot for our hospitalist team in order to sustain our service given census surge and physician illness.
Sara Millwee, DNP, APRN, FNP-BC
Emory chief of advanced practice providers
To help reduce exposure to COVID, our advanced practice providers (APPs) admitted patients from the ED (as they did pre-COVID) to the hospital medicine service, but the physicians administratively signed the note/orders. Emory Healthcare bylaws specify that patients are seen by a physician within 24 hours of admission. During the pandemic, at the time of admission, the APP discussed plan of care with the physician, but the patient was only seen by the APP upon initial evaluation/admission, as opposed to the physician and APP pre-COVID. This improved productivity, and facilitated communication and collaboration between APPs and physicians. This also fostered an environment where APPs were practicing at the top of their licenses and improved job satisfaction.
Additionally, across the hospital medicine division, several APPs were utilized from other divisions to assist with admissions and cross cover. As the volume was at incredibly high levels, this improved the workload and burden of the hospital medicine providers. The displaced APPs were utilized at several facilities and worked under the guidance and supervision of hospital medicine providers. Moving forward, this has prompted leadership to look at utilizing APPs from other divisions as “PRN” providers as well.
What does it mean to be a trustworthy male ally?
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
Hospitalist movers and shakers - November 2021
Vineet Chopra, MD, MSc, FHM, recently became chair of the Department of Medicine at the University of Colorado School of Medicine, Aurora. He had previously been the chief of the Division of Hospital Medicine at the University of Michigan Health system. He assumed his new role in October 2021.
Dr. Chopra, who specializes in research and mentorship in patient safety, helped create innovations in care delivery at the University of Michigan, including direct care hospitalist services at VA Ann Arbor Health Care and two other community hospitals.
In his safety-conscious research, Dr. Chopra focuses on preventing complications created within the hospital environment. He also is the first hospitalist to be named deputy editor of the Annals of Internal Medicine. He has written more than 250 peer-reviewed articles. Among the myriad awards he has received, Dr. Chopra recently earned the Kaiser Permanente Award for Clinical Teaching at the UM School of Medicine.
Steve Phillipson, MD, FHM, has been named regional director of hospital medicine at Aspirus Health (Wausau, Wisc.). Dr. Phillipson will oversee the hospitalist programs at 17 Aspirus hospitals in Wisconsin and Michigan.
Dr. Phillipson has worked with Aspirus since 2009, with stints in the emergency department and as a hospitalist. As Aspirus Wausau Hospital director of medicine, he chaired the facility’s COVID-19 treatment team.
Hackensack (N.J.) Meridian University Medical Center has hired Patricia (Patti) L. Fisher, MD, MHA, to be the institution’s chief medical officer. Dr. Fisher joined the medical center from Central Vermont Medical Center where she served as chief medical officer and chief safety officer, with direct oversight of hospital risk management, operations of all hospital-based services, IS services and quality including patient safety and regulatory compliance.
As a board-certified hospitalist, Dr. Fisher also served as clinical assistant professor in the Department of Family Medicine at the University of Vermont, Burlington. Dr. Fisher earned her medical degree from The University of Texas in Houston and completed residency through Forbes Family Practice Residency in Pittsburgh.
Martin Chaney, MD, has been chosen by the Maury Regional Health Board of Trustees to serve as interim chief executive officer. He was formerly the chief medical officer at MRH, which is based in Columbia, Tenn. Dr. Chaney began his new role in October, replacing Alan Watson, the CEO since 2012.
Dr. Chaney has spent 18 of his 25 years in medicine with MRH, where most recently he has focused on clinical quality, physician recruitment, and establishing and expanding the hospital medicine program.
Hyung (Harry) Cho, MD, SFHM, has been placed on Modern Healthcare’s Top 25 Innovators list for 2021, getting recognized for innovation and leadership in creating value and safety initiatives in New York City’s public health system. Dr. Cho became NYC Health + Hospitals’ first chief value officer in 2019, and his programs have created an estimated $11 million in savings per year by preventing unnecessary testing and treatment that can lead to patient harm.
A member of the Society of Hospital Medicine’s editorial advisory board, Dr. Cho is also SHM’s hospitalist liaison with the COVID-19 Real-Time Learning Network, which collaborates with the Centers for Disease Control and Prevention and the Infectious Diseases Society of America.
Raymond Kiser, MD, a hospitalist and nephrologist at Columbus (Ind.) Regional Health, has been named the Douglas J. Leonard Caregiver of the Year. The award is given by the Indiana Hospital Association to health care workers whose care is considered exemplary by both peers and patients.
Dr. Kiser has been with CRH for 7 years, including stints as associate chief medical officer and chief of staff.
Justin Buchholz, DO, has been elevated to medical director of the hospitalist teams at Regional Medical Center (Alamosa, Colo.) and Conejos County Hospital (La Jara, Colo.). Dr. Buchholz has been a full-time hospitalist and assistant medical director at Parkview Medical Center (Pueblo, Colo.) for the past 3 years. He also worked on a part-time basis seeing patients at the Regional Medical Center.
Dr. Buchholz completed his residency at Parkview Medical Center and was named Resident of the Year in his final year with the internal medicine program.
Kenneth Mishark, MD, SFHM, a hospitalist with the Mayo Clinic Hospital (Tucson, Ariz.), will serve on the board of directors for Anigent, a drug diversion-prevention company based in Chesterfield, Mo. He will be charged with helping Anigent better serve health systems with its drug-diversion software.
Dr. Mishark is vice-chair of diversion prevention across the whole Mayo Clinic. A one-time physician in the United States Air Force, Dr. Mishark previously has been the Mayo Clinic’s Healthcare Information Coordination Committee chair.
Core Clinical Partners (Tulsa, Okla.) has announced it will join with Hillcrest HealthCare System (Tulsa, Okla.) to provide hospitalist services to Hillcrest’s eight sites across Oklahoma. The partnership will begin at four locations in December 2021, and four others in March 2022.
In expanding its services, Core Clinical Partners will create 70 new physician positions, as well as a systemwide medical director. Core will manage hospitalist operations at Hillcrest Medical Center, Hillcrest Hospital South, Hillcrest Hospital Pryor, Hillcrest Hospital Claremore, Bailey Medical Center, Hillcrest Hospital Cushing, Hillcrest Hospital Henryetta, and Tulsa Spine and Specialty Hospital.
Vineet Chopra, MD, MSc, FHM, recently became chair of the Department of Medicine at the University of Colorado School of Medicine, Aurora. He had previously been the chief of the Division of Hospital Medicine at the University of Michigan Health system. He assumed his new role in October 2021.
Dr. Chopra, who specializes in research and mentorship in patient safety, helped create innovations in care delivery at the University of Michigan, including direct care hospitalist services at VA Ann Arbor Health Care and two other community hospitals.
In his safety-conscious research, Dr. Chopra focuses on preventing complications created within the hospital environment. He also is the first hospitalist to be named deputy editor of the Annals of Internal Medicine. He has written more than 250 peer-reviewed articles. Among the myriad awards he has received, Dr. Chopra recently earned the Kaiser Permanente Award for Clinical Teaching at the UM School of Medicine.
Steve Phillipson, MD, FHM, has been named regional director of hospital medicine at Aspirus Health (Wausau, Wisc.). Dr. Phillipson will oversee the hospitalist programs at 17 Aspirus hospitals in Wisconsin and Michigan.
Dr. Phillipson has worked with Aspirus since 2009, with stints in the emergency department and as a hospitalist. As Aspirus Wausau Hospital director of medicine, he chaired the facility’s COVID-19 treatment team.
Hackensack (N.J.) Meridian University Medical Center has hired Patricia (Patti) L. Fisher, MD, MHA, to be the institution’s chief medical officer. Dr. Fisher joined the medical center from Central Vermont Medical Center where she served as chief medical officer and chief safety officer, with direct oversight of hospital risk management, operations of all hospital-based services, IS services and quality including patient safety and regulatory compliance.
As a board-certified hospitalist, Dr. Fisher also served as clinical assistant professor in the Department of Family Medicine at the University of Vermont, Burlington. Dr. Fisher earned her medical degree from The University of Texas in Houston and completed residency through Forbes Family Practice Residency in Pittsburgh.
Martin Chaney, MD, has been chosen by the Maury Regional Health Board of Trustees to serve as interim chief executive officer. He was formerly the chief medical officer at MRH, which is based in Columbia, Tenn. Dr. Chaney began his new role in October, replacing Alan Watson, the CEO since 2012.
Dr. Chaney has spent 18 of his 25 years in medicine with MRH, where most recently he has focused on clinical quality, physician recruitment, and establishing and expanding the hospital medicine program.
Hyung (Harry) Cho, MD, SFHM, has been placed on Modern Healthcare’s Top 25 Innovators list for 2021, getting recognized for innovation and leadership in creating value and safety initiatives in New York City’s public health system. Dr. Cho became NYC Health + Hospitals’ first chief value officer in 2019, and his programs have created an estimated $11 million in savings per year by preventing unnecessary testing and treatment that can lead to patient harm.
A member of the Society of Hospital Medicine’s editorial advisory board, Dr. Cho is also SHM’s hospitalist liaison with the COVID-19 Real-Time Learning Network, which collaborates with the Centers for Disease Control and Prevention and the Infectious Diseases Society of America.
Raymond Kiser, MD, a hospitalist and nephrologist at Columbus (Ind.) Regional Health, has been named the Douglas J. Leonard Caregiver of the Year. The award is given by the Indiana Hospital Association to health care workers whose care is considered exemplary by both peers and patients.
Dr. Kiser has been with CRH for 7 years, including stints as associate chief medical officer and chief of staff.
Justin Buchholz, DO, has been elevated to medical director of the hospitalist teams at Regional Medical Center (Alamosa, Colo.) and Conejos County Hospital (La Jara, Colo.). Dr. Buchholz has been a full-time hospitalist and assistant medical director at Parkview Medical Center (Pueblo, Colo.) for the past 3 years. He also worked on a part-time basis seeing patients at the Regional Medical Center.
Dr. Buchholz completed his residency at Parkview Medical Center and was named Resident of the Year in his final year with the internal medicine program.
Kenneth Mishark, MD, SFHM, a hospitalist with the Mayo Clinic Hospital (Tucson, Ariz.), will serve on the board of directors for Anigent, a drug diversion-prevention company based in Chesterfield, Mo. He will be charged with helping Anigent better serve health systems with its drug-diversion software.
Dr. Mishark is vice-chair of diversion prevention across the whole Mayo Clinic. A one-time physician in the United States Air Force, Dr. Mishark previously has been the Mayo Clinic’s Healthcare Information Coordination Committee chair.
Core Clinical Partners (Tulsa, Okla.) has announced it will join with Hillcrest HealthCare System (Tulsa, Okla.) to provide hospitalist services to Hillcrest’s eight sites across Oklahoma. The partnership will begin at four locations in December 2021, and four others in March 2022.
In expanding its services, Core Clinical Partners will create 70 new physician positions, as well as a systemwide medical director. Core will manage hospitalist operations at Hillcrest Medical Center, Hillcrest Hospital South, Hillcrest Hospital Pryor, Hillcrest Hospital Claremore, Bailey Medical Center, Hillcrest Hospital Cushing, Hillcrest Hospital Henryetta, and Tulsa Spine and Specialty Hospital.
Vineet Chopra, MD, MSc, FHM, recently became chair of the Department of Medicine at the University of Colorado School of Medicine, Aurora. He had previously been the chief of the Division of Hospital Medicine at the University of Michigan Health system. He assumed his new role in October 2021.
Dr. Chopra, who specializes in research and mentorship in patient safety, helped create innovations in care delivery at the University of Michigan, including direct care hospitalist services at VA Ann Arbor Health Care and two other community hospitals.
In his safety-conscious research, Dr. Chopra focuses on preventing complications created within the hospital environment. He also is the first hospitalist to be named deputy editor of the Annals of Internal Medicine. He has written more than 250 peer-reviewed articles. Among the myriad awards he has received, Dr. Chopra recently earned the Kaiser Permanente Award for Clinical Teaching at the UM School of Medicine.
Steve Phillipson, MD, FHM, has been named regional director of hospital medicine at Aspirus Health (Wausau, Wisc.). Dr. Phillipson will oversee the hospitalist programs at 17 Aspirus hospitals in Wisconsin and Michigan.
Dr. Phillipson has worked with Aspirus since 2009, with stints in the emergency department and as a hospitalist. As Aspirus Wausau Hospital director of medicine, he chaired the facility’s COVID-19 treatment team.
Hackensack (N.J.) Meridian University Medical Center has hired Patricia (Patti) L. Fisher, MD, MHA, to be the institution’s chief medical officer. Dr. Fisher joined the medical center from Central Vermont Medical Center where she served as chief medical officer and chief safety officer, with direct oversight of hospital risk management, operations of all hospital-based services, IS services and quality including patient safety and regulatory compliance.
As a board-certified hospitalist, Dr. Fisher also served as clinical assistant professor in the Department of Family Medicine at the University of Vermont, Burlington. Dr. Fisher earned her medical degree from The University of Texas in Houston and completed residency through Forbes Family Practice Residency in Pittsburgh.
Martin Chaney, MD, has been chosen by the Maury Regional Health Board of Trustees to serve as interim chief executive officer. He was formerly the chief medical officer at MRH, which is based in Columbia, Tenn. Dr. Chaney began his new role in October, replacing Alan Watson, the CEO since 2012.
Dr. Chaney has spent 18 of his 25 years in medicine with MRH, where most recently he has focused on clinical quality, physician recruitment, and establishing and expanding the hospital medicine program.
Hyung (Harry) Cho, MD, SFHM, has been placed on Modern Healthcare’s Top 25 Innovators list for 2021, getting recognized for innovation and leadership in creating value and safety initiatives in New York City’s public health system. Dr. Cho became NYC Health + Hospitals’ first chief value officer in 2019, and his programs have created an estimated $11 million in savings per year by preventing unnecessary testing and treatment that can lead to patient harm.
A member of the Society of Hospital Medicine’s editorial advisory board, Dr. Cho is also SHM’s hospitalist liaison with the COVID-19 Real-Time Learning Network, which collaborates with the Centers for Disease Control and Prevention and the Infectious Diseases Society of America.
Raymond Kiser, MD, a hospitalist and nephrologist at Columbus (Ind.) Regional Health, has been named the Douglas J. Leonard Caregiver of the Year. The award is given by the Indiana Hospital Association to health care workers whose care is considered exemplary by both peers and patients.
Dr. Kiser has been with CRH for 7 years, including stints as associate chief medical officer and chief of staff.
Justin Buchholz, DO, has been elevated to medical director of the hospitalist teams at Regional Medical Center (Alamosa, Colo.) and Conejos County Hospital (La Jara, Colo.). Dr. Buchholz has been a full-time hospitalist and assistant medical director at Parkview Medical Center (Pueblo, Colo.) for the past 3 years. He also worked on a part-time basis seeing patients at the Regional Medical Center.
Dr. Buchholz completed his residency at Parkview Medical Center and was named Resident of the Year in his final year with the internal medicine program.
Kenneth Mishark, MD, SFHM, a hospitalist with the Mayo Clinic Hospital (Tucson, Ariz.), will serve on the board of directors for Anigent, a drug diversion-prevention company based in Chesterfield, Mo. He will be charged with helping Anigent better serve health systems with its drug-diversion software.
Dr. Mishark is vice-chair of diversion prevention across the whole Mayo Clinic. A one-time physician in the United States Air Force, Dr. Mishark previously has been the Mayo Clinic’s Healthcare Information Coordination Committee chair.
Core Clinical Partners (Tulsa, Okla.) has announced it will join with Hillcrest HealthCare System (Tulsa, Okla.) to provide hospitalist services to Hillcrest’s eight sites across Oklahoma. The partnership will begin at four locations in December 2021, and four others in March 2022.
In expanding its services, Core Clinical Partners will create 70 new physician positions, as well as a systemwide medical director. Core will manage hospitalist operations at Hillcrest Medical Center, Hillcrest Hospital South, Hillcrest Hospital Pryor, Hillcrest Hospital Claremore, Bailey Medical Center, Hillcrest Hospital Cushing, Hillcrest Hospital Henryetta, and Tulsa Spine and Specialty Hospital.
Rural hospitalists confront COVID-19
Unique demands of patient care in small hospitals
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
Unique demands of patient care in small hospitals
Unique demands of patient care in small hospitals
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
Opioid-induced adrenal insufficiency for the hospitalist
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4
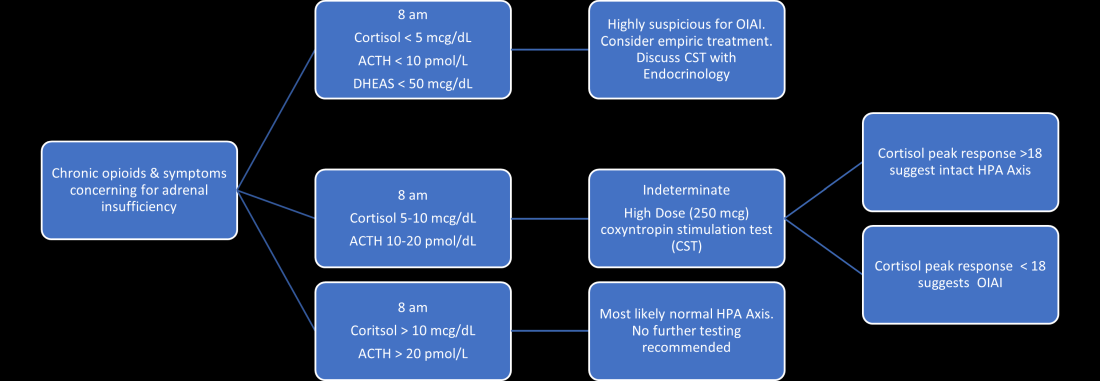
The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.
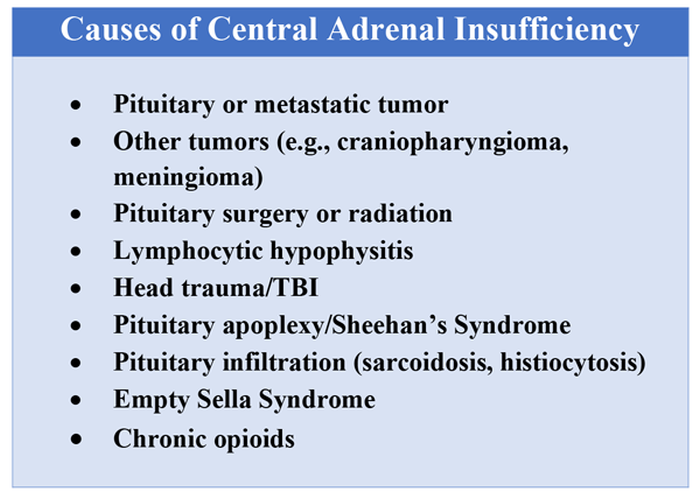
To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.
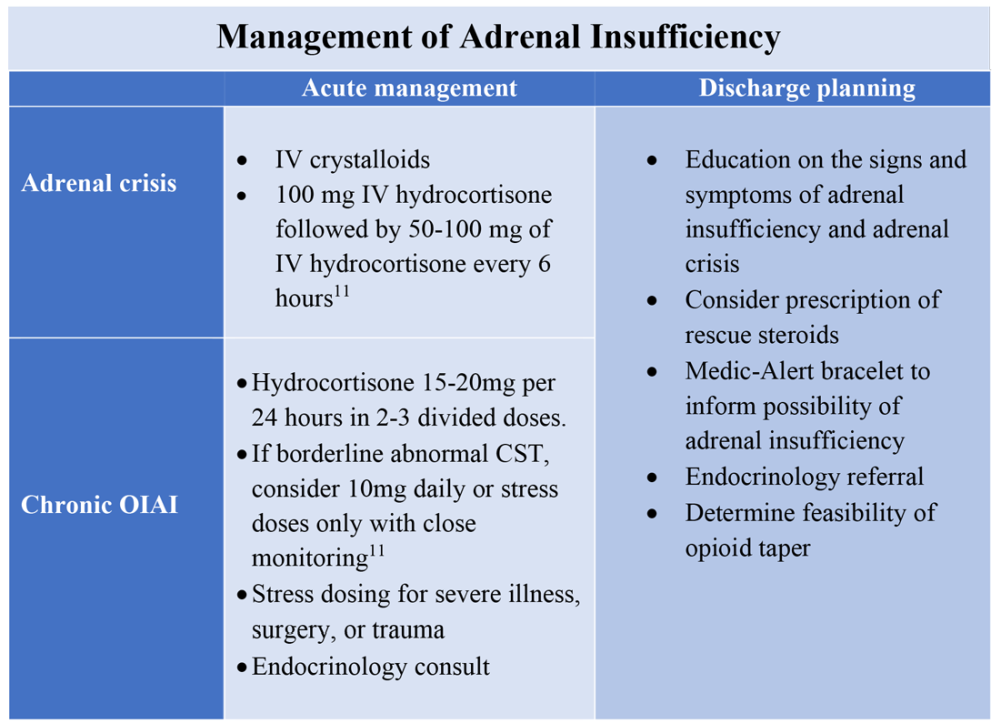
All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Consider OIAI, even among patients with common infections
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Benefit of combined ascorbic acid, corticosteroids, and thiamine in septic shock remains unproven
Background: Sepsis is a common reason for hospitalization, and studies of the combination of ascorbic acid, corticosteroids, and thiamine have had conflicting results.
Study design: Double-blind randomized controlled trial.
Setting: 14 hospitals in the United States.
Synopsis: A total of 205 patients were randomly assigned to receive parenteral ascorbic acid, hydrocortisone, and thiamine every 6 hours for 4 days or placebo in matching volumes and time points. The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score between enrollment and 72 hours. There was no statistically significant difference in SOFA scores (adjusted mean difference, –0.8; 95% CI, –1.7 to 0.2; P = .12), kidney failure (adjusted risk difference, 0.03; 95% CI, –0.1 to 0.2; P = .58), or 30-day mortality (HR, 1.3; 95% CI 0.8-2.2; P = .26) between the two groups. Adverse effects included hyperglycemia, hypernatremia, and new hospital-acquired infection.
Bottom line: The combination of ascorbic acid, corticosteroids, and thiamine in patients with septic shock does not improve SOFA score.
Citation: Moskowitz A et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA. 2020;324(7):642-50.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Background: Sepsis is a common reason for hospitalization, and studies of the combination of ascorbic acid, corticosteroids, and thiamine have had conflicting results.
Study design: Double-blind randomized controlled trial.
Setting: 14 hospitals in the United States.
Synopsis: A total of 205 patients were randomly assigned to receive parenteral ascorbic acid, hydrocortisone, and thiamine every 6 hours for 4 days or placebo in matching volumes and time points. The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score between enrollment and 72 hours. There was no statistically significant difference in SOFA scores (adjusted mean difference, –0.8; 95% CI, –1.7 to 0.2; P = .12), kidney failure (adjusted risk difference, 0.03; 95% CI, –0.1 to 0.2; P = .58), or 30-day mortality (HR, 1.3; 95% CI 0.8-2.2; P = .26) between the two groups. Adverse effects included hyperglycemia, hypernatremia, and new hospital-acquired infection.
Bottom line: The combination of ascorbic acid, corticosteroids, and thiamine in patients with septic shock does not improve SOFA score.
Citation: Moskowitz A et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA. 2020;324(7):642-50.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Background: Sepsis is a common reason for hospitalization, and studies of the combination of ascorbic acid, corticosteroids, and thiamine have had conflicting results.
Study design: Double-blind randomized controlled trial.
Setting: 14 hospitals in the United States.
Synopsis: A total of 205 patients were randomly assigned to receive parenteral ascorbic acid, hydrocortisone, and thiamine every 6 hours for 4 days or placebo in matching volumes and time points. The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score between enrollment and 72 hours. There was no statistically significant difference in SOFA scores (adjusted mean difference, –0.8; 95% CI, –1.7 to 0.2; P = .12), kidney failure (adjusted risk difference, 0.03; 95% CI, –0.1 to 0.2; P = .58), or 30-day mortality (HR, 1.3; 95% CI 0.8-2.2; P = .26) between the two groups. Adverse effects included hyperglycemia, hypernatremia, and new hospital-acquired infection.
Bottom line: The combination of ascorbic acid, corticosteroids, and thiamine in patients with septic shock does not improve SOFA score.
Citation: Moskowitz A et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA. 2020;324(7):642-50.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
New prescription for loop diuretic improves 30-day heart failure outcomes
Background: In patients with heart failure, loop diuretics are used to manage symptoms. However, the effect of loop diuretics on morbidity and mortality is not well studied.
Study design: Retrospective matched cohort study.
Setting: OPTIMIZE-HF registry.
Synopsis: Using the data from the OPTIMIZE-HF registry to develop a matched cohort of 2,191 pairs of patients, researchers showed that patients hospitalized for heart failure who were not previously taking any diuretic had significantly better 30-day clinical outcomes if they were discharged on a loop diuretic. Specifically, a loop diuretic on discharge resulted in a lower 30-day all-cause mortality (hazard ratio, 0.73; 95% CI, 0.57-0.94; P = .016) and a lower risk of 30-day heart failure readmission (HR, 0.79; 95% CI, 0.63-0.99; P = .037), compared with patients not discharged on a loop diuretic.
Neither of these associations was statistically significant during a 60-day follow-up, and the authors acknowledge that significant 30-day associations may be sensitive to an unmeasured confounder.
Bottom line: Starting an outpatient loop diuretic on discharge for patients hospitalized for heart failure improves 30-day all-cause mortality and lowers the risk of 30-day heart failure readmission.
Citation: Faselis C et al. Loop diuretic prescription and 30-day outcomes in older patients with heart failure. J Am Coll Cardiol. 2020;76:669-79.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Background: In patients with heart failure, loop diuretics are used to manage symptoms. However, the effect of loop diuretics on morbidity and mortality is not well studied.
Study design: Retrospective matched cohort study.
Setting: OPTIMIZE-HF registry.
Synopsis: Using the data from the OPTIMIZE-HF registry to develop a matched cohort of 2,191 pairs of patients, researchers showed that patients hospitalized for heart failure who were not previously taking any diuretic had significantly better 30-day clinical outcomes if they were discharged on a loop diuretic. Specifically, a loop diuretic on discharge resulted in a lower 30-day all-cause mortality (hazard ratio, 0.73; 95% CI, 0.57-0.94; P = .016) and a lower risk of 30-day heart failure readmission (HR, 0.79; 95% CI, 0.63-0.99; P = .037), compared with patients not discharged on a loop diuretic.
Neither of these associations was statistically significant during a 60-day follow-up, and the authors acknowledge that significant 30-day associations may be sensitive to an unmeasured confounder.
Bottom line: Starting an outpatient loop diuretic on discharge for patients hospitalized for heart failure improves 30-day all-cause mortality and lowers the risk of 30-day heart failure readmission.
Citation: Faselis C et al. Loop diuretic prescription and 30-day outcomes in older patients with heart failure. J Am Coll Cardiol. 2020;76:669-79.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Background: In patients with heart failure, loop diuretics are used to manage symptoms. However, the effect of loop diuretics on morbidity and mortality is not well studied.
Study design: Retrospective matched cohort study.
Setting: OPTIMIZE-HF registry.
Synopsis: Using the data from the OPTIMIZE-HF registry to develop a matched cohort of 2,191 pairs of patients, researchers showed that patients hospitalized for heart failure who were not previously taking any diuretic had significantly better 30-day clinical outcomes if they were discharged on a loop diuretic. Specifically, a loop diuretic on discharge resulted in a lower 30-day all-cause mortality (hazard ratio, 0.73; 95% CI, 0.57-0.94; P = .016) and a lower risk of 30-day heart failure readmission (HR, 0.79; 95% CI, 0.63-0.99; P = .037), compared with patients not discharged on a loop diuretic.
Neither of these associations was statistically significant during a 60-day follow-up, and the authors acknowledge that significant 30-day associations may be sensitive to an unmeasured confounder.
Bottom line: Starting an outpatient loop diuretic on discharge for patients hospitalized for heart failure improves 30-day all-cause mortality and lowers the risk of 30-day heart failure readmission.
Citation: Faselis C et al. Loop diuretic prescription and 30-day outcomes in older patients with heart failure. J Am Coll Cardiol. 2020;76:669-79.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Symptoms persist in patients after acute COVID-19
Background: A large proportion of Italian patients with COVID-19 presented with symptoms, most commonly cough, fever, dyspnea, myalgias, anosmia, and gastrointestinal symptoms. Information is lacking on persistent symptoms after recovery.
Study design: Retrospective observational study.
Setting: Hospital system in Rome.
Synopsis: A postacute outpatient service for individuals discharged after recovery from COVID-19 was established. All patients who met World Health Organization criteria for discontinuation of quarantine (no fever for 3 consecutive days, improved symptoms, and two negative SARS-CoV-2 tests 24 hours apart) were offered a comprehensive medical assessment. Patients were asked to retrospectively recount the presence or absence of symptoms during the acute phase of COVID-19 and whether each symptom persisted at the time of the visit.
From April 21 to May 29, 2020, 179 patients were potentially eligible; 143 ultimately were included. During hospitalization, 72.7% of participants had evidence of interstitial pneumonia. Patients were assessed a mean of 60.3 days after onset of the first COVID-19 symptom. Only 18 (12.6%) were completely free of any COVID-19–related symptoms, 32% had one or two symptoms, and 55% had three or more. Worsened quality of life was observed among 44.1% of patients.
Bottom line: 87.4% of patients who had recovered from COVID-19 reported persistence of at least one symptom, particularly fatigue and dyspnea.
Citation: Carfi A et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-5.
Dr. Walker is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: A large proportion of Italian patients with COVID-19 presented with symptoms, most commonly cough, fever, dyspnea, myalgias, anosmia, and gastrointestinal symptoms. Information is lacking on persistent symptoms after recovery.
Study design: Retrospective observational study.
Setting: Hospital system in Rome.
Synopsis: A postacute outpatient service for individuals discharged after recovery from COVID-19 was established. All patients who met World Health Organization criteria for discontinuation of quarantine (no fever for 3 consecutive days, improved symptoms, and two negative SARS-CoV-2 tests 24 hours apart) were offered a comprehensive medical assessment. Patients were asked to retrospectively recount the presence or absence of symptoms during the acute phase of COVID-19 and whether each symptom persisted at the time of the visit.
From April 21 to May 29, 2020, 179 patients were potentially eligible; 143 ultimately were included. During hospitalization, 72.7% of participants had evidence of interstitial pneumonia. Patients were assessed a mean of 60.3 days after onset of the first COVID-19 symptom. Only 18 (12.6%) were completely free of any COVID-19–related symptoms, 32% had one or two symptoms, and 55% had three or more. Worsened quality of life was observed among 44.1% of patients.
Bottom line: 87.4% of patients who had recovered from COVID-19 reported persistence of at least one symptom, particularly fatigue and dyspnea.
Citation: Carfi A et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-5.
Dr. Walker is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: A large proportion of Italian patients with COVID-19 presented with symptoms, most commonly cough, fever, dyspnea, myalgias, anosmia, and gastrointestinal symptoms. Information is lacking on persistent symptoms after recovery.
Study design: Retrospective observational study.
Setting: Hospital system in Rome.
Synopsis: A postacute outpatient service for individuals discharged after recovery from COVID-19 was established. All patients who met World Health Organization criteria for discontinuation of quarantine (no fever for 3 consecutive days, improved symptoms, and two negative SARS-CoV-2 tests 24 hours apart) were offered a comprehensive medical assessment. Patients were asked to retrospectively recount the presence or absence of symptoms during the acute phase of COVID-19 and whether each symptom persisted at the time of the visit.
From April 21 to May 29, 2020, 179 patients were potentially eligible; 143 ultimately were included. During hospitalization, 72.7% of participants had evidence of interstitial pneumonia. Patients were assessed a mean of 60.3 days after onset of the first COVID-19 symptom. Only 18 (12.6%) were completely free of any COVID-19–related symptoms, 32% had one or two symptoms, and 55% had three or more. Worsened quality of life was observed among 44.1% of patients.
Bottom line: 87.4% of patients who had recovered from COVID-19 reported persistence of at least one symptom, particularly fatigue and dyspnea.
Citation: Carfi A et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-5.
Dr. Walker is a hospitalist at the Lexington (Ky.) VA Health Care System.
Geographic cohorting increased direct care time and interruptions
Background: Geographic cohorting localizes hospitalist teams to a single unit. It has previously been shown to improve outcomes.
Design: Prospective time and motion study.
Setting: 11 geographically cohorted services and 4 noncohorted teams at Indiana University Health, a large academic medical center.
Synopsis: Geotracking was used to monitor time spent inside and outside of patient rooms for 17 hospitalists over at least 6 weeks. Eight hospitalists were also directly observed. Both groups spent roughly three times more time outside patient rooms than inside. Geographic cohorting was associated with longer patient visits (ranging from 69.6 to 101.7 minutes per day depending on team structure) and a higher percentage of time in patient rooms. Interruptions were more common with geographic cohorting. These hospitalists were interrupted every 14 minutes in the morning and every 8 minutes in the afternoon. Of these interruptions, 62% were face-to-face, 25% were electronic, and 13% were both simultaneously.
An important limitation of this study is that the investigators did not evaluate clinical outcomes or provider satisfaction. This may give some pause to the widespread push toward geographic cohorting.
Bottom line: More frequent interruptions may partially offset potential increases in patient-hospitalist interactions achieved through geographic cohorting.
Citation: Kara A et al. A time motion study evaluating the impact of geographic cohorting of hospitalists. J Hosp Med. 2020;15:338-44.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Geographic cohorting localizes hospitalist teams to a single unit. It has previously been shown to improve outcomes.
Design: Prospective time and motion study.
Setting: 11 geographically cohorted services and 4 noncohorted teams at Indiana University Health, a large academic medical center.
Synopsis: Geotracking was used to monitor time spent inside and outside of patient rooms for 17 hospitalists over at least 6 weeks. Eight hospitalists were also directly observed. Both groups spent roughly three times more time outside patient rooms than inside. Geographic cohorting was associated with longer patient visits (ranging from 69.6 to 101.7 minutes per day depending on team structure) and a higher percentage of time in patient rooms. Interruptions were more common with geographic cohorting. These hospitalists were interrupted every 14 minutes in the morning and every 8 minutes in the afternoon. Of these interruptions, 62% were face-to-face, 25% were electronic, and 13% were both simultaneously.
An important limitation of this study is that the investigators did not evaluate clinical outcomes or provider satisfaction. This may give some pause to the widespread push toward geographic cohorting.
Bottom line: More frequent interruptions may partially offset potential increases in patient-hospitalist interactions achieved through geographic cohorting.
Citation: Kara A et al. A time motion study evaluating the impact of geographic cohorting of hospitalists. J Hosp Med. 2020;15:338-44.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Geographic cohorting localizes hospitalist teams to a single unit. It has previously been shown to improve outcomes.
Design: Prospective time and motion study.
Setting: 11 geographically cohorted services and 4 noncohorted teams at Indiana University Health, a large academic medical center.
Synopsis: Geotracking was used to monitor time spent inside and outside of patient rooms for 17 hospitalists over at least 6 weeks. Eight hospitalists were also directly observed. Both groups spent roughly three times more time outside patient rooms than inside. Geographic cohorting was associated with longer patient visits (ranging from 69.6 to 101.7 minutes per day depending on team structure) and a higher percentage of time in patient rooms. Interruptions were more common with geographic cohorting. These hospitalists were interrupted every 14 minutes in the morning and every 8 minutes in the afternoon. Of these interruptions, 62% were face-to-face, 25% were electronic, and 13% were both simultaneously.
An important limitation of this study is that the investigators did not evaluate clinical outcomes or provider satisfaction. This may give some pause to the widespread push toward geographic cohorting.
Bottom line: More frequent interruptions may partially offset potential increases in patient-hospitalist interactions achieved through geographic cohorting.
Citation: Kara A et al. A time motion study evaluating the impact of geographic cohorting of hospitalists. J Hosp Med. 2020;15:338-44.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Open ICUs giveth and taketh away
Background: Some academic medical centers and many community centers use “open” ICU models in which primary services longitudinally follow patients into the ICU with intensivist comanagement.
Design: Semistructured interviews with 12 hospitalists and 8 intensivists.
Setting: Open 16-bed ICUs at the University of California, San Francisco. Teams round separately at the bedside and are informally encouraged to check in daily.
Synopsis: The authors iteratively developed the interview questions. Participants were selected using purposive sampling. The main themes were communication, education, and structure. Communication was challenging among teams as well as with patients and families. The open ICU was felt to affect handoffs and care continuity positively. Hospitalists focused more on longitudinal relationships, smoother transitions, and opportunities to observe disease evolution. Intensivists focused more on fragmentation during the ICU stay and noted cognitive disengagement among some team members with certain aspects of patient care. Intensivists did not identify any educational or structural benefits of the open ICU model.
This is the first qualitative study of hospitalist and intensivist perceptions of the open ICU model. The most significant limitation is the risk of bias from the single-center design and purposive sampling. These findings have implications for other models of medical comanagement.
Bottom line: Open ICU models offer a mix of communication, educational, and structural barriers as well as opportunities. Role clarity may help optimize the open ICU model.
Citation: Santhosh L and Sewell J. Hospital and intensivist experiences of the “open” intensive care unit environment: A qualitative exploration. J Gen Intern Med. 2020;35(8):2338-46.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Some academic medical centers and many community centers use “open” ICU models in which primary services longitudinally follow patients into the ICU with intensivist comanagement.
Design: Semistructured interviews with 12 hospitalists and 8 intensivists.
Setting: Open 16-bed ICUs at the University of California, San Francisco. Teams round separately at the bedside and are informally encouraged to check in daily.
Synopsis: The authors iteratively developed the interview questions. Participants were selected using purposive sampling. The main themes were communication, education, and structure. Communication was challenging among teams as well as with patients and families. The open ICU was felt to affect handoffs and care continuity positively. Hospitalists focused more on longitudinal relationships, smoother transitions, and opportunities to observe disease evolution. Intensivists focused more on fragmentation during the ICU stay and noted cognitive disengagement among some team members with certain aspects of patient care. Intensivists did not identify any educational or structural benefits of the open ICU model.
This is the first qualitative study of hospitalist and intensivist perceptions of the open ICU model. The most significant limitation is the risk of bias from the single-center design and purposive sampling. These findings have implications for other models of medical comanagement.
Bottom line: Open ICU models offer a mix of communication, educational, and structural barriers as well as opportunities. Role clarity may help optimize the open ICU model.
Citation: Santhosh L and Sewell J. Hospital and intensivist experiences of the “open” intensive care unit environment: A qualitative exploration. J Gen Intern Med. 2020;35(8):2338-46.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Some academic medical centers and many community centers use “open” ICU models in which primary services longitudinally follow patients into the ICU with intensivist comanagement.
Design: Semistructured interviews with 12 hospitalists and 8 intensivists.
Setting: Open 16-bed ICUs at the University of California, San Francisco. Teams round separately at the bedside and are informally encouraged to check in daily.
Synopsis: The authors iteratively developed the interview questions. Participants were selected using purposive sampling. The main themes were communication, education, and structure. Communication was challenging among teams as well as with patients and families. The open ICU was felt to affect handoffs and care continuity positively. Hospitalists focused more on longitudinal relationships, smoother transitions, and opportunities to observe disease evolution. Intensivists focused more on fragmentation during the ICU stay and noted cognitive disengagement among some team members with certain aspects of patient care. Intensivists did not identify any educational or structural benefits of the open ICU model.
This is the first qualitative study of hospitalist and intensivist perceptions of the open ICU model. The most significant limitation is the risk of bias from the single-center design and purposive sampling. These findings have implications for other models of medical comanagement.
Bottom line: Open ICU models offer a mix of communication, educational, and structural barriers as well as opportunities. Role clarity may help optimize the open ICU model.
Citation: Santhosh L and Sewell J. Hospital and intensivist experiences of the “open” intensive care unit environment: A qualitative exploration. J Gen Intern Med. 2020;35(8):2338-46.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.






























