User login
New assay hailed as a game changer for early Parkinson’s diagnosis
, and provides information on molecular subtypes, new research indicates.
“Identifying an effective biomarker for Parkinson’s disease pathology could have profound implications for the way we treat the condition, potentially making it possible to diagnose people earlier, identify the best treatments for different subsets of patients, and speed up clinical trials,” the study’s co-lead author Andrew Siderowf, MD, of the University of Pennsylvania, Philadelphia, said in a news release.
“Our findings suggest that the αSyn-SAA technique is highly accurate at detecting the biomarker for Parkinson’s disease regardless of the clinical features, making it possible to accurately diagnose the disease in patients at early stages,” added co-lead author Luis Concha-Marambio, PhD, director of research and development at Amprion, San Diego, Calif.
The study was published online in The Lancet Neurology.
‘New era’ in Parkinson’s disease
The researchers assessed the usefulness of αSyn-SAA in a cross-sectional analysis of 1,123 participants in the Parkinson’s Progression Markers Initiative (PPMI) cohort from 33 participating academic neurology outpatient practices in 12 countries.
The cohort included individuals with sporadic Parkinson’s disease from LRRK2 or GBA variants, healthy controls, individuals with clinical syndromes prodromal to Parkinson’s disease (rapid eye movement sleep behavior disorder [RBD] or hyposmia), and nonmanifesting carriers of LRRK2 and GBA variants. Cerebrospinal fluid (CSF) samples from each participant were analyzed using αSyn-SAA.
Overall, αSyn-SAA differentiated Parkinson’s disease from healthy controls with 87.7% sensitivity and 96.3% specificity.
Sensitivity of the assay varied across subgroups based on genetic and clinical features. Among genetic Parkinson’s disease subgroups, sensitivity was highest for GBA Parkinson’s disease (95.9%), followed by sporadic Parkinson’s disease (93.3%), and lowest for LRRK2 Parkinson’s disease (67.5%). Among clinical features, hyposmia was the most robust predictor of a positive assay result.
Among all Parkinson’s disease cases with hyposmia, the sensitivity of the assay was 97.2%, compared with 63.0% for Parkinson’s disease without olfactory dysfunction. Combining genetic and clinical features, the sensitivity of positive αSyn-SAA in sporadic Parkinson’s disease with olfactory deficit was 98.6%, compared with 78.3% in sporadic Parkinson’s disease without hyposmia. Most prodromal participants (86%) with RBD and hyposmia had positive αSyn-SAA results, indicating they had α-synuclein aggregates despite not yet being diagnosed with Parkinson’s disease.
Among those recruited based on their loss of smell, 89% (16 of 18 participants) had positive αSyn-SAA results. Similarly, in those with RBD, positive αSyn-SAA results were present in 85% of cases (28 of 33). No other clinical features were associated with a positive αSyn-SAA result.
In participants who carried LRRK2 or GBA variants but had no Parkinson’s disease diagnosis or prodromal symptoms (nonmanifesting carriers), 9% (14 of 159) and 7% (11 of 151), respectively, had positive αSyn-SAA results.
To date, this is the largest analysis of α-Syn-SAA for the biochemical diagnosis of Parkinson’s disease, the researchers said.
The results show that the assay classifies people with Parkinson’s disease with “high sensitivity and specificity, provides information about molecular heterogeneity, and detects prodromal individuals before diagnosis,” they wrote.
“These findings suggest a crucial role for the α-synuclein SAA in therapeutic development, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined at-risk cohorts,” they added.
Amprion has commercialized the assay (SYNTap test), which can be ordered online.
‘Seminal development’
The authors of an accompanying editorial noted the study “lays the foundation for a biological diagnosis” of Parkinson’s disease. “We have entered a new era of biomarker and treatment development for Parkinson’s disease. The possibility of detecting a misfolded α-synuclein, the pathological hallmark of Parkinson’s disease, by employing an SSA, is a seminal development,” wrote Daniela Berg, MD, PhD, and Christine Klein, MD, with University Hospital Schleswig-Holstein, Germany.
“However, to fully leverage the enormous potential of the α-synuclein seed amplification, the test would have to be performed in blood rather than the CSF, a less invasive approach that has proven to be viable,” they added.
“Although the blood-based method needs to be further elaborated for scalability, α-synuclein SAA is a game changer in Parkinson’s disease diagnostics, research, and treatment trials,” they concluded.
The study was funded by The Michael J. Fox Foundation for Parkinson’s Research and a consortium of more than 40 private and philanthropic partners. Dr. Siderowf has declared consulting for Merck and Parkinson Study Group, and receiving honoraria from Bial. A full list of author disclosures is available with the original article. Dr. Berg and Dr. Klein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and provides information on molecular subtypes, new research indicates.
“Identifying an effective biomarker for Parkinson’s disease pathology could have profound implications for the way we treat the condition, potentially making it possible to diagnose people earlier, identify the best treatments for different subsets of patients, and speed up clinical trials,” the study’s co-lead author Andrew Siderowf, MD, of the University of Pennsylvania, Philadelphia, said in a news release.
“Our findings suggest that the αSyn-SAA technique is highly accurate at detecting the biomarker for Parkinson’s disease regardless of the clinical features, making it possible to accurately diagnose the disease in patients at early stages,” added co-lead author Luis Concha-Marambio, PhD, director of research and development at Amprion, San Diego, Calif.
The study was published online in The Lancet Neurology.
‘New era’ in Parkinson’s disease
The researchers assessed the usefulness of αSyn-SAA in a cross-sectional analysis of 1,123 participants in the Parkinson’s Progression Markers Initiative (PPMI) cohort from 33 participating academic neurology outpatient practices in 12 countries.
The cohort included individuals with sporadic Parkinson’s disease from LRRK2 or GBA variants, healthy controls, individuals with clinical syndromes prodromal to Parkinson’s disease (rapid eye movement sleep behavior disorder [RBD] or hyposmia), and nonmanifesting carriers of LRRK2 and GBA variants. Cerebrospinal fluid (CSF) samples from each participant were analyzed using αSyn-SAA.
Overall, αSyn-SAA differentiated Parkinson’s disease from healthy controls with 87.7% sensitivity and 96.3% specificity.
Sensitivity of the assay varied across subgroups based on genetic and clinical features. Among genetic Parkinson’s disease subgroups, sensitivity was highest for GBA Parkinson’s disease (95.9%), followed by sporadic Parkinson’s disease (93.3%), and lowest for LRRK2 Parkinson’s disease (67.5%). Among clinical features, hyposmia was the most robust predictor of a positive assay result.
Among all Parkinson’s disease cases with hyposmia, the sensitivity of the assay was 97.2%, compared with 63.0% for Parkinson’s disease without olfactory dysfunction. Combining genetic and clinical features, the sensitivity of positive αSyn-SAA in sporadic Parkinson’s disease with olfactory deficit was 98.6%, compared with 78.3% in sporadic Parkinson’s disease without hyposmia. Most prodromal participants (86%) with RBD and hyposmia had positive αSyn-SAA results, indicating they had α-synuclein aggregates despite not yet being diagnosed with Parkinson’s disease.
Among those recruited based on their loss of smell, 89% (16 of 18 participants) had positive αSyn-SAA results. Similarly, in those with RBD, positive αSyn-SAA results were present in 85% of cases (28 of 33). No other clinical features were associated with a positive αSyn-SAA result.
In participants who carried LRRK2 or GBA variants but had no Parkinson’s disease diagnosis or prodromal symptoms (nonmanifesting carriers), 9% (14 of 159) and 7% (11 of 151), respectively, had positive αSyn-SAA results.
To date, this is the largest analysis of α-Syn-SAA for the biochemical diagnosis of Parkinson’s disease, the researchers said.
The results show that the assay classifies people with Parkinson’s disease with “high sensitivity and specificity, provides information about molecular heterogeneity, and detects prodromal individuals before diagnosis,” they wrote.
“These findings suggest a crucial role for the α-synuclein SAA in therapeutic development, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined at-risk cohorts,” they added.
Amprion has commercialized the assay (SYNTap test), which can be ordered online.
‘Seminal development’
The authors of an accompanying editorial noted the study “lays the foundation for a biological diagnosis” of Parkinson’s disease. “We have entered a new era of biomarker and treatment development for Parkinson’s disease. The possibility of detecting a misfolded α-synuclein, the pathological hallmark of Parkinson’s disease, by employing an SSA, is a seminal development,” wrote Daniela Berg, MD, PhD, and Christine Klein, MD, with University Hospital Schleswig-Holstein, Germany.
“However, to fully leverage the enormous potential of the α-synuclein seed amplification, the test would have to be performed in blood rather than the CSF, a less invasive approach that has proven to be viable,” they added.
“Although the blood-based method needs to be further elaborated for scalability, α-synuclein SAA is a game changer in Parkinson’s disease diagnostics, research, and treatment trials,” they concluded.
The study was funded by The Michael J. Fox Foundation for Parkinson’s Research and a consortium of more than 40 private and philanthropic partners. Dr. Siderowf has declared consulting for Merck and Parkinson Study Group, and receiving honoraria from Bial. A full list of author disclosures is available with the original article. Dr. Berg and Dr. Klein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and provides information on molecular subtypes, new research indicates.
“Identifying an effective biomarker for Parkinson’s disease pathology could have profound implications for the way we treat the condition, potentially making it possible to diagnose people earlier, identify the best treatments for different subsets of patients, and speed up clinical trials,” the study’s co-lead author Andrew Siderowf, MD, of the University of Pennsylvania, Philadelphia, said in a news release.
“Our findings suggest that the αSyn-SAA technique is highly accurate at detecting the biomarker for Parkinson’s disease regardless of the clinical features, making it possible to accurately diagnose the disease in patients at early stages,” added co-lead author Luis Concha-Marambio, PhD, director of research and development at Amprion, San Diego, Calif.
The study was published online in The Lancet Neurology.
‘New era’ in Parkinson’s disease
The researchers assessed the usefulness of αSyn-SAA in a cross-sectional analysis of 1,123 participants in the Parkinson’s Progression Markers Initiative (PPMI) cohort from 33 participating academic neurology outpatient practices in 12 countries.
The cohort included individuals with sporadic Parkinson’s disease from LRRK2 or GBA variants, healthy controls, individuals with clinical syndromes prodromal to Parkinson’s disease (rapid eye movement sleep behavior disorder [RBD] or hyposmia), and nonmanifesting carriers of LRRK2 and GBA variants. Cerebrospinal fluid (CSF) samples from each participant were analyzed using αSyn-SAA.
Overall, αSyn-SAA differentiated Parkinson’s disease from healthy controls with 87.7% sensitivity and 96.3% specificity.
Sensitivity of the assay varied across subgroups based on genetic and clinical features. Among genetic Parkinson’s disease subgroups, sensitivity was highest for GBA Parkinson’s disease (95.9%), followed by sporadic Parkinson’s disease (93.3%), and lowest for LRRK2 Parkinson’s disease (67.5%). Among clinical features, hyposmia was the most robust predictor of a positive assay result.
Among all Parkinson’s disease cases with hyposmia, the sensitivity of the assay was 97.2%, compared with 63.0% for Parkinson’s disease without olfactory dysfunction. Combining genetic and clinical features, the sensitivity of positive αSyn-SAA in sporadic Parkinson’s disease with olfactory deficit was 98.6%, compared with 78.3% in sporadic Parkinson’s disease without hyposmia. Most prodromal participants (86%) with RBD and hyposmia had positive αSyn-SAA results, indicating they had α-synuclein aggregates despite not yet being diagnosed with Parkinson’s disease.
Among those recruited based on their loss of smell, 89% (16 of 18 participants) had positive αSyn-SAA results. Similarly, in those with RBD, positive αSyn-SAA results were present in 85% of cases (28 of 33). No other clinical features were associated with a positive αSyn-SAA result.
In participants who carried LRRK2 or GBA variants but had no Parkinson’s disease diagnosis or prodromal symptoms (nonmanifesting carriers), 9% (14 of 159) and 7% (11 of 151), respectively, had positive αSyn-SAA results.
To date, this is the largest analysis of α-Syn-SAA for the biochemical diagnosis of Parkinson’s disease, the researchers said.
The results show that the assay classifies people with Parkinson’s disease with “high sensitivity and specificity, provides information about molecular heterogeneity, and detects prodromal individuals before diagnosis,” they wrote.
“These findings suggest a crucial role for the α-synuclein SAA in therapeutic development, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined at-risk cohorts,” they added.
Amprion has commercialized the assay (SYNTap test), which can be ordered online.
‘Seminal development’
The authors of an accompanying editorial noted the study “lays the foundation for a biological diagnosis” of Parkinson’s disease. “We have entered a new era of biomarker and treatment development for Parkinson’s disease. The possibility of detecting a misfolded α-synuclein, the pathological hallmark of Parkinson’s disease, by employing an SSA, is a seminal development,” wrote Daniela Berg, MD, PhD, and Christine Klein, MD, with University Hospital Schleswig-Holstein, Germany.
“However, to fully leverage the enormous potential of the α-synuclein seed amplification, the test would have to be performed in blood rather than the CSF, a less invasive approach that has proven to be viable,” they added.
“Although the blood-based method needs to be further elaborated for scalability, α-synuclein SAA is a game changer in Parkinson’s disease diagnostics, research, and treatment trials,” they concluded.
The study was funded by The Michael J. Fox Foundation for Parkinson’s Research and a consortium of more than 40 private and philanthropic partners. Dr. Siderowf has declared consulting for Merck and Parkinson Study Group, and receiving honoraria from Bial. A full list of author disclosures is available with the original article. Dr. Berg and Dr. Klein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET NEUROLOGY
Phototherapy a safe, effective, inexpensive new option for dementia?
It may be “one of the most promising interventions for improving core symptoms” of the disease.
A new meta-analysis shows that patients with dementia who received phototherapy experienced significant cognitive improvement, compared with those who received usual treatment. However, there were no differences between study groups in terms of improved depression, agitation, or sleep problems.
“Our meta-analysis indicates that phototherapy improved cognitive function in patients with dementia. ... This suggests that phototherapy may be one of the most promising non-pharmacological interventions for improving core symptoms of dementia,” wrote the investigators, led by Xinlian Lu, Peking University, Beijing.
The study was published online in Brain and Behavior.
A new treatment option?
“As drug treatment for dementia has limitations such as medical contraindications, limited efficacy, and adverse effects, nonpharmacological therapy has been increasingly regarded as a critical part of comprehensive dementia care,” the investigators noted.
Phototherapy, which utilizes full-spectrum bright light (usually > 600 lux) or wavelength-specific light (for example, blue-enriched or blue-green), is a “promising nonpharmacological therapy” that is noninvasive, inexpensive, and safe.
Most studies of phototherapy have focused on sleep. Findings have shown “high heterogeneity” among the interventions and the populations in the studies, and results have been “inconsistent.” In addition, the effect of phototherapy on cognitive function and behavioral and psychological symptoms of dementia (BPSD) “still need to be clarified.”
In the systematic review and meta-analysis, the investigators examined the effects of phototherapy on cognitive function, BPSD, and sleep in older adults with dementia.
They searched several databases for randomized controlled trials that investigated phototherapy interventions for elderly patients. The primary outcome was cognitive function, which was assessed via the Mini-Mental State Examination (MMSE).
Secondary outcomes included BPSD, including agitation, anxiety, irritability, depression, anxiety, and sleep disturbances, as assessed by the Cornell Scale for Depression in Dementia (CSDD), the Cohen-Mansfield Agitation Inventory (CMAI), the Neuropsychiatric Inventory (NPI), and measures of sleep, including total sleep time (TST), sleep efficiency (SE), and sleep disorders, as assessed by the Sleep Disorder Inventory (SDI).
To be included in the analysis, individual studies had to focus on elderly adults who had some form of dementia. In addition, a group receiving a phototherapy intervention had to be compared with a nonintervention group, and the study had to specify one of the above-defined outcomes.
The review included phototherapy interventions of all forms, frequencies, and durations, including use of bright light, LED light, and blue or blue-green light.
Regulating circadian rhythm
Twelve studies met the researchers’ criteria. They included a total of 766 patients with dementia – 426 in the intervention group and 340 in the control group. The mean ages ranged from 73.73 to 85.9 years, and there was a greater number of female than male participants.
Of the studies, seven employed routine daily light in the control group, while the others used either dim light (≤ 50 lux) or devices without light.
The researchers found “significant positive intervention effects” for global cognitive function. Improvements in postintervention MMSE scores differed significantly between the experimental groups and control groups (mean difference, 2.68; 95% confidence interval, 1.38-3.98; I2 = 0%).
No significant differences were found in the effects of intervention on depression symptoms, as evidenced in CSDD scores (MD, −0.70; 95% CI, −3.10 to 1.70; I2 = 81%).
Among patients with higher CMAI scores, which indicate more severe agitation behaviors, there was a “trend of decreasing CMAI scores” after phototherapy (MD, −3.12; 95% CI, −8.05 to 1.82; I2 = 0%). No significant difference in NPI scores was observed between the two groups.
Similarly, no significant difference was found between the two groups in TST, SE, or SDI scores.
Adverse effects were infrequent and were not severe. Two of the 426 patients in the intervention group experienced mild ocular irritation, and one experienced slight transient redness of the forehead.
Light “may compensate for the reduction in the visual sensory input of patients with dementia and stimulate specific neurons in the suprachiasmatic nucleus of the hypothalamus to regulate circadian rhythm,” the researchers suggested.
“As circadian rhythms are involved in optimal brain function, light supplementation may act on the synchronizing/phase-shifting effects of circadian rhythms to improve cognitive function,” they added.
They note that the light box is the “most commonly used device in phototherapy.” Light boxes provide full-spectrum bright light, usually greater than 2,500 lux. The duration is 30 minutes in the daytime, and treatment lasts 4-8 weeks.
The investigators cautioned that the light box should be placed 60 cm away from the patient or above the patient’s eye level. They said that a ceiling-mounted light is a “good choice” for providing whole-day phototherapy, since such lights do not interfere with the patient’s daily routine, reduce the demand on staff, and contribute to better adherence.
Phototherapy helmets and glasses are also available. These portable devices “allow for better control of light intensity and are ergonomic without interfering with patients’ normal activities.”
The researchers noted that “further well-designed studies are needed to explore the most effective clinical implementation conditions, including device type, duration, frequency, and time.”
Easy to use
Mariana Figueiro, PhD, professor and director of the Light and Health Research Center, department of population health medicine, Icahn School of Medicine at Mount Sinai, New York, said light is the “major stimulus for the circadian system, and a robust light-dark pattern daily (which can be given by light therapy during the day) improves sleep and behavior and reduces depression and agitation.”
Dr. Figueiro, who was not involved with the current study, noted that patients with dementia “have sleep issues, which can further affect their cognition; improvement in sleep leads to improvement in cognition,” and this may be an underlying mechanism associated with these results.
The clinical significance of the study “is that this is a nonpharmacological intervention and can be easily applied in the homes or controlled facilities, and it can be used with any other medication,” she pointed out.
“More importantly, sleep medications have negative side effects, so the use of nonpharmacological interventions improving sleep and cognition is great for clinical practice,” she added.
However, she took issue with the finding that phototherapy was not effective for depression and agitation, noting that there were “too few studies to say for sure that light therapy is ineffective at improving these outcomes.”
The research received no external funding. The authors and Dr. Figueiro disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It may be “one of the most promising interventions for improving core symptoms” of the disease.
A new meta-analysis shows that patients with dementia who received phototherapy experienced significant cognitive improvement, compared with those who received usual treatment. However, there were no differences between study groups in terms of improved depression, agitation, or sleep problems.
“Our meta-analysis indicates that phototherapy improved cognitive function in patients with dementia. ... This suggests that phototherapy may be one of the most promising non-pharmacological interventions for improving core symptoms of dementia,” wrote the investigators, led by Xinlian Lu, Peking University, Beijing.
The study was published online in Brain and Behavior.
A new treatment option?
“As drug treatment for dementia has limitations such as medical contraindications, limited efficacy, and adverse effects, nonpharmacological therapy has been increasingly regarded as a critical part of comprehensive dementia care,” the investigators noted.
Phototherapy, which utilizes full-spectrum bright light (usually > 600 lux) or wavelength-specific light (for example, blue-enriched or blue-green), is a “promising nonpharmacological therapy” that is noninvasive, inexpensive, and safe.
Most studies of phototherapy have focused on sleep. Findings have shown “high heterogeneity” among the interventions and the populations in the studies, and results have been “inconsistent.” In addition, the effect of phototherapy on cognitive function and behavioral and psychological symptoms of dementia (BPSD) “still need to be clarified.”
In the systematic review and meta-analysis, the investigators examined the effects of phototherapy on cognitive function, BPSD, and sleep in older adults with dementia.
They searched several databases for randomized controlled trials that investigated phototherapy interventions for elderly patients. The primary outcome was cognitive function, which was assessed via the Mini-Mental State Examination (MMSE).
Secondary outcomes included BPSD, including agitation, anxiety, irritability, depression, anxiety, and sleep disturbances, as assessed by the Cornell Scale for Depression in Dementia (CSDD), the Cohen-Mansfield Agitation Inventory (CMAI), the Neuropsychiatric Inventory (NPI), and measures of sleep, including total sleep time (TST), sleep efficiency (SE), and sleep disorders, as assessed by the Sleep Disorder Inventory (SDI).
To be included in the analysis, individual studies had to focus on elderly adults who had some form of dementia. In addition, a group receiving a phototherapy intervention had to be compared with a nonintervention group, and the study had to specify one of the above-defined outcomes.
The review included phototherapy interventions of all forms, frequencies, and durations, including use of bright light, LED light, and blue or blue-green light.
Regulating circadian rhythm
Twelve studies met the researchers’ criteria. They included a total of 766 patients with dementia – 426 in the intervention group and 340 in the control group. The mean ages ranged from 73.73 to 85.9 years, and there was a greater number of female than male participants.
Of the studies, seven employed routine daily light in the control group, while the others used either dim light (≤ 50 lux) or devices without light.
The researchers found “significant positive intervention effects” for global cognitive function. Improvements in postintervention MMSE scores differed significantly between the experimental groups and control groups (mean difference, 2.68; 95% confidence interval, 1.38-3.98; I2 = 0%).
No significant differences were found in the effects of intervention on depression symptoms, as evidenced in CSDD scores (MD, −0.70; 95% CI, −3.10 to 1.70; I2 = 81%).
Among patients with higher CMAI scores, which indicate more severe agitation behaviors, there was a “trend of decreasing CMAI scores” after phototherapy (MD, −3.12; 95% CI, −8.05 to 1.82; I2 = 0%). No significant difference in NPI scores was observed between the two groups.
Similarly, no significant difference was found between the two groups in TST, SE, or SDI scores.
Adverse effects were infrequent and were not severe. Two of the 426 patients in the intervention group experienced mild ocular irritation, and one experienced slight transient redness of the forehead.
Light “may compensate for the reduction in the visual sensory input of patients with dementia and stimulate specific neurons in the suprachiasmatic nucleus of the hypothalamus to regulate circadian rhythm,” the researchers suggested.
“As circadian rhythms are involved in optimal brain function, light supplementation may act on the synchronizing/phase-shifting effects of circadian rhythms to improve cognitive function,” they added.
They note that the light box is the “most commonly used device in phototherapy.” Light boxes provide full-spectrum bright light, usually greater than 2,500 lux. The duration is 30 minutes in the daytime, and treatment lasts 4-8 weeks.
The investigators cautioned that the light box should be placed 60 cm away from the patient or above the patient’s eye level. They said that a ceiling-mounted light is a “good choice” for providing whole-day phototherapy, since such lights do not interfere with the patient’s daily routine, reduce the demand on staff, and contribute to better adherence.
Phototherapy helmets and glasses are also available. These portable devices “allow for better control of light intensity and are ergonomic without interfering with patients’ normal activities.”
The researchers noted that “further well-designed studies are needed to explore the most effective clinical implementation conditions, including device type, duration, frequency, and time.”
Easy to use
Mariana Figueiro, PhD, professor and director of the Light and Health Research Center, department of population health medicine, Icahn School of Medicine at Mount Sinai, New York, said light is the “major stimulus for the circadian system, and a robust light-dark pattern daily (which can be given by light therapy during the day) improves sleep and behavior and reduces depression and agitation.”
Dr. Figueiro, who was not involved with the current study, noted that patients with dementia “have sleep issues, which can further affect their cognition; improvement in sleep leads to improvement in cognition,” and this may be an underlying mechanism associated with these results.
The clinical significance of the study “is that this is a nonpharmacological intervention and can be easily applied in the homes or controlled facilities, and it can be used with any other medication,” she pointed out.
“More importantly, sleep medications have negative side effects, so the use of nonpharmacological interventions improving sleep and cognition is great for clinical practice,” she added.
However, she took issue with the finding that phototherapy was not effective for depression and agitation, noting that there were “too few studies to say for sure that light therapy is ineffective at improving these outcomes.”
The research received no external funding. The authors and Dr. Figueiro disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It may be “one of the most promising interventions for improving core symptoms” of the disease.
A new meta-analysis shows that patients with dementia who received phototherapy experienced significant cognitive improvement, compared with those who received usual treatment. However, there were no differences between study groups in terms of improved depression, agitation, or sleep problems.
“Our meta-analysis indicates that phototherapy improved cognitive function in patients with dementia. ... This suggests that phototherapy may be one of the most promising non-pharmacological interventions for improving core symptoms of dementia,” wrote the investigators, led by Xinlian Lu, Peking University, Beijing.
The study was published online in Brain and Behavior.
A new treatment option?
“As drug treatment for dementia has limitations such as medical contraindications, limited efficacy, and adverse effects, nonpharmacological therapy has been increasingly regarded as a critical part of comprehensive dementia care,” the investigators noted.
Phototherapy, which utilizes full-spectrum bright light (usually > 600 lux) or wavelength-specific light (for example, blue-enriched or blue-green), is a “promising nonpharmacological therapy” that is noninvasive, inexpensive, and safe.
Most studies of phototherapy have focused on sleep. Findings have shown “high heterogeneity” among the interventions and the populations in the studies, and results have been “inconsistent.” In addition, the effect of phototherapy on cognitive function and behavioral and psychological symptoms of dementia (BPSD) “still need to be clarified.”
In the systematic review and meta-analysis, the investigators examined the effects of phototherapy on cognitive function, BPSD, and sleep in older adults with dementia.
They searched several databases for randomized controlled trials that investigated phototherapy interventions for elderly patients. The primary outcome was cognitive function, which was assessed via the Mini-Mental State Examination (MMSE).
Secondary outcomes included BPSD, including agitation, anxiety, irritability, depression, anxiety, and sleep disturbances, as assessed by the Cornell Scale for Depression in Dementia (CSDD), the Cohen-Mansfield Agitation Inventory (CMAI), the Neuropsychiatric Inventory (NPI), and measures of sleep, including total sleep time (TST), sleep efficiency (SE), and sleep disorders, as assessed by the Sleep Disorder Inventory (SDI).
To be included in the analysis, individual studies had to focus on elderly adults who had some form of dementia. In addition, a group receiving a phototherapy intervention had to be compared with a nonintervention group, and the study had to specify one of the above-defined outcomes.
The review included phototherapy interventions of all forms, frequencies, and durations, including use of bright light, LED light, and blue or blue-green light.
Regulating circadian rhythm
Twelve studies met the researchers’ criteria. They included a total of 766 patients with dementia – 426 in the intervention group and 340 in the control group. The mean ages ranged from 73.73 to 85.9 years, and there was a greater number of female than male participants.
Of the studies, seven employed routine daily light in the control group, while the others used either dim light (≤ 50 lux) or devices without light.
The researchers found “significant positive intervention effects” for global cognitive function. Improvements in postintervention MMSE scores differed significantly between the experimental groups and control groups (mean difference, 2.68; 95% confidence interval, 1.38-3.98; I2 = 0%).
No significant differences were found in the effects of intervention on depression symptoms, as evidenced in CSDD scores (MD, −0.70; 95% CI, −3.10 to 1.70; I2 = 81%).
Among patients with higher CMAI scores, which indicate more severe agitation behaviors, there was a “trend of decreasing CMAI scores” after phototherapy (MD, −3.12; 95% CI, −8.05 to 1.82; I2 = 0%). No significant difference in NPI scores was observed between the two groups.
Similarly, no significant difference was found between the two groups in TST, SE, or SDI scores.
Adverse effects were infrequent and were not severe. Two of the 426 patients in the intervention group experienced mild ocular irritation, and one experienced slight transient redness of the forehead.
Light “may compensate for the reduction in the visual sensory input of patients with dementia and stimulate specific neurons in the suprachiasmatic nucleus of the hypothalamus to regulate circadian rhythm,” the researchers suggested.
“As circadian rhythms are involved in optimal brain function, light supplementation may act on the synchronizing/phase-shifting effects of circadian rhythms to improve cognitive function,” they added.
They note that the light box is the “most commonly used device in phototherapy.” Light boxes provide full-spectrum bright light, usually greater than 2,500 lux. The duration is 30 minutes in the daytime, and treatment lasts 4-8 weeks.
The investigators cautioned that the light box should be placed 60 cm away from the patient or above the patient’s eye level. They said that a ceiling-mounted light is a “good choice” for providing whole-day phototherapy, since such lights do not interfere with the patient’s daily routine, reduce the demand on staff, and contribute to better adherence.
Phototherapy helmets and glasses are also available. These portable devices “allow for better control of light intensity and are ergonomic without interfering with patients’ normal activities.”
The researchers noted that “further well-designed studies are needed to explore the most effective clinical implementation conditions, including device type, duration, frequency, and time.”
Easy to use
Mariana Figueiro, PhD, professor and director of the Light and Health Research Center, department of population health medicine, Icahn School of Medicine at Mount Sinai, New York, said light is the “major stimulus for the circadian system, and a robust light-dark pattern daily (which can be given by light therapy during the day) improves sleep and behavior and reduces depression and agitation.”
Dr. Figueiro, who was not involved with the current study, noted that patients with dementia “have sleep issues, which can further affect their cognition; improvement in sleep leads to improvement in cognition,” and this may be an underlying mechanism associated with these results.
The clinical significance of the study “is that this is a nonpharmacological intervention and can be easily applied in the homes or controlled facilities, and it can be used with any other medication,” she pointed out.
“More importantly, sleep medications have negative side effects, so the use of nonpharmacological interventions improving sleep and cognition is great for clinical practice,” she added.
However, she took issue with the finding that phototherapy was not effective for depression and agitation, noting that there were “too few studies to say for sure that light therapy is ineffective at improving these outcomes.”
The research received no external funding. The authors and Dr. Figueiro disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BRAIN AND BEHAVIOR
Disordered sleep tied to a marked increase in stroke risk
Results of a large international study show stroke risk was more than three times higher in those who slept too little, more than twice as high in those who sleep too much, and two to three times higher in those with symptoms of severe obstructive sleep apnea.
The study also showed that the greater the number of sleep disorder symptoms, the greater the stroke risk. The 11% of study participants with five or more symptoms of disordered sleep had a fivefold increased risk for stroke.
Although the study data do not show a causal link between disordered sleep and stroke, the association between the two was strong.
“Given the association, sleep disturbance may represent a marker of somebody at increased risk of stroke, and further interventional studies are required to see if management can reduce this risk,” lead investigator Christine McCarthy, MD, PhD, a geriatric and stroke medicine physician and researcher with the University of Galway (Ireland), told this news organization. “In the interim, however, management of sleep disturbance may have a positive impact on a patient’s quality of life.”
The findings were published online in the journal Neurology.
More symptoms, more risk
Previous research shows severe OSA doubles the risk of stroke and increases the chance of recurrent stroke. A 2019 study showed that people with insomnia had a small increased risk of stroke.
“Both snoring and extremes of sleep duration have been previously associated with an increased risk of stroke in observational research, but less is known about other symptoms of sleep impairment, with less consistent findings,” Dr. McCarthy said.
Prior studies have also generally come from a single geographic region, which Dr. McCarthy noted could limit their generalizability.
For this effort, investigators used data from 4,496 participants in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. About half of the participants had a history of stroke.
Using information collected from a survey of sleep habits, researchers found an elevated stroke risk in those who received less than 5 hours of sleep per night (odds ratio, 3.15; 95% confidence interval, 2.09-4.76) or more than 9 hours of sleep per night (OR, 2.67; 95% CI, 1.89-3.78), compared with those who slept 7 hours a night.
Participants who took unplanned naps or naps lasting an hour or more (OR, 2.46; 95% CI, 1.69-3.57) and participants who reported poor quality sleep (OR,1.52; 95% CI, 1.32-1.75) were also at an increased risk for stroke.
Symptoms of OSA were also strongly associated with increased stroke risk, including snoring (OR, 1.91; 95% CI, 1.62-2.24), snorting (OR, 2.64; 95% CI, 2.17-3.20), and breathing cessation (OR, 2.87; 95% CI, 2.28-2.60).
Stroke risk increased as the number of sleep disturbance symptoms rose, with the greatest risk in the 11% of participants who had five or more symptoms (OR, 5.38; 95% CI, 4.03-7.18).
“This study finds an association between a broad range of sleep impairment symptoms and stroke, and a graded association with increasing symptoms, in an international setting,” Dr. McCarthy said.
Researchers aren’t sure what’s driving the higher stroke risk among people with sleep disturbances. Although the study did control for potential confounders, it wasn’t designed to get at what’s driving the association.
“Sleep disturbance may also have a bi-directional relationship with many stroke risk factors; for example, sleep disturbance may be a symptom of disease and exacerbate disease,” Dr. McCarthy said. “Future interventional studies are required to determine the true direction of the relationship.”
A marker of stroke risk
Daniel Lackland, DrPH, professor of neurology at the Medical University of South Carolina, Charleston, said the findings provide additional evidence of the link between sleep and stroke risk.
“The results confirm sleep disorders as a potential marker and part of the risk profile,” he said.
Collecting information about sleep using a validated assessment tool is an important piece of clinical care, Dr. Lackland said, especially among patients with other stroke risk factors.
One limitation of the study was that data on sleep was collected only at one point, and participants were not followed over time to see if changes in sleep affected stroke risk.
“This is an important point and should be a focus for future studies, as it is critical in the design of interventions,” Dr. Lackland said.
The INTERSTROKE study is funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Astra Zeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), MERCK, Sharp and Dohme, Swedish Heart and Lung Foundation, U.K. Chest, and U.K. Heart and Stroke. Dr. McCarthy and Lackland report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large international study show stroke risk was more than three times higher in those who slept too little, more than twice as high in those who sleep too much, and two to three times higher in those with symptoms of severe obstructive sleep apnea.
The study also showed that the greater the number of sleep disorder symptoms, the greater the stroke risk. The 11% of study participants with five or more symptoms of disordered sleep had a fivefold increased risk for stroke.
Although the study data do not show a causal link between disordered sleep and stroke, the association between the two was strong.
“Given the association, sleep disturbance may represent a marker of somebody at increased risk of stroke, and further interventional studies are required to see if management can reduce this risk,” lead investigator Christine McCarthy, MD, PhD, a geriatric and stroke medicine physician and researcher with the University of Galway (Ireland), told this news organization. “In the interim, however, management of sleep disturbance may have a positive impact on a patient’s quality of life.”
The findings were published online in the journal Neurology.
More symptoms, more risk
Previous research shows severe OSA doubles the risk of stroke and increases the chance of recurrent stroke. A 2019 study showed that people with insomnia had a small increased risk of stroke.
“Both snoring and extremes of sleep duration have been previously associated with an increased risk of stroke in observational research, but less is known about other symptoms of sleep impairment, with less consistent findings,” Dr. McCarthy said.
Prior studies have also generally come from a single geographic region, which Dr. McCarthy noted could limit their generalizability.
For this effort, investigators used data from 4,496 participants in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. About half of the participants had a history of stroke.
Using information collected from a survey of sleep habits, researchers found an elevated stroke risk in those who received less than 5 hours of sleep per night (odds ratio, 3.15; 95% confidence interval, 2.09-4.76) or more than 9 hours of sleep per night (OR, 2.67; 95% CI, 1.89-3.78), compared with those who slept 7 hours a night.
Participants who took unplanned naps or naps lasting an hour or more (OR, 2.46; 95% CI, 1.69-3.57) and participants who reported poor quality sleep (OR,1.52; 95% CI, 1.32-1.75) were also at an increased risk for stroke.
Symptoms of OSA were also strongly associated with increased stroke risk, including snoring (OR, 1.91; 95% CI, 1.62-2.24), snorting (OR, 2.64; 95% CI, 2.17-3.20), and breathing cessation (OR, 2.87; 95% CI, 2.28-2.60).
Stroke risk increased as the number of sleep disturbance symptoms rose, with the greatest risk in the 11% of participants who had five or more symptoms (OR, 5.38; 95% CI, 4.03-7.18).
“This study finds an association between a broad range of sleep impairment symptoms and stroke, and a graded association with increasing symptoms, in an international setting,” Dr. McCarthy said.
Researchers aren’t sure what’s driving the higher stroke risk among people with sleep disturbances. Although the study did control for potential confounders, it wasn’t designed to get at what’s driving the association.
“Sleep disturbance may also have a bi-directional relationship with many stroke risk factors; for example, sleep disturbance may be a symptom of disease and exacerbate disease,” Dr. McCarthy said. “Future interventional studies are required to determine the true direction of the relationship.”
A marker of stroke risk
Daniel Lackland, DrPH, professor of neurology at the Medical University of South Carolina, Charleston, said the findings provide additional evidence of the link between sleep and stroke risk.
“The results confirm sleep disorders as a potential marker and part of the risk profile,” he said.
Collecting information about sleep using a validated assessment tool is an important piece of clinical care, Dr. Lackland said, especially among patients with other stroke risk factors.
One limitation of the study was that data on sleep was collected only at one point, and participants were not followed over time to see if changes in sleep affected stroke risk.
“This is an important point and should be a focus for future studies, as it is critical in the design of interventions,” Dr. Lackland said.
The INTERSTROKE study is funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Astra Zeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), MERCK, Sharp and Dohme, Swedish Heart and Lung Foundation, U.K. Chest, and U.K. Heart and Stroke. Dr. McCarthy and Lackland report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large international study show stroke risk was more than three times higher in those who slept too little, more than twice as high in those who sleep too much, and two to three times higher in those with symptoms of severe obstructive sleep apnea.
The study also showed that the greater the number of sleep disorder symptoms, the greater the stroke risk. The 11% of study participants with five or more symptoms of disordered sleep had a fivefold increased risk for stroke.
Although the study data do not show a causal link between disordered sleep and stroke, the association between the two was strong.
“Given the association, sleep disturbance may represent a marker of somebody at increased risk of stroke, and further interventional studies are required to see if management can reduce this risk,” lead investigator Christine McCarthy, MD, PhD, a geriatric and stroke medicine physician and researcher with the University of Galway (Ireland), told this news organization. “In the interim, however, management of sleep disturbance may have a positive impact on a patient’s quality of life.”
The findings were published online in the journal Neurology.
More symptoms, more risk
Previous research shows severe OSA doubles the risk of stroke and increases the chance of recurrent stroke. A 2019 study showed that people with insomnia had a small increased risk of stroke.
“Both snoring and extremes of sleep duration have been previously associated with an increased risk of stroke in observational research, but less is known about other symptoms of sleep impairment, with less consistent findings,” Dr. McCarthy said.
Prior studies have also generally come from a single geographic region, which Dr. McCarthy noted could limit their generalizability.
For this effort, investigators used data from 4,496 participants in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. About half of the participants had a history of stroke.
Using information collected from a survey of sleep habits, researchers found an elevated stroke risk in those who received less than 5 hours of sleep per night (odds ratio, 3.15; 95% confidence interval, 2.09-4.76) or more than 9 hours of sleep per night (OR, 2.67; 95% CI, 1.89-3.78), compared with those who slept 7 hours a night.
Participants who took unplanned naps or naps lasting an hour or more (OR, 2.46; 95% CI, 1.69-3.57) and participants who reported poor quality sleep (OR,1.52; 95% CI, 1.32-1.75) were also at an increased risk for stroke.
Symptoms of OSA were also strongly associated with increased stroke risk, including snoring (OR, 1.91; 95% CI, 1.62-2.24), snorting (OR, 2.64; 95% CI, 2.17-3.20), and breathing cessation (OR, 2.87; 95% CI, 2.28-2.60).
Stroke risk increased as the number of sleep disturbance symptoms rose, with the greatest risk in the 11% of participants who had five or more symptoms (OR, 5.38; 95% CI, 4.03-7.18).
“This study finds an association between a broad range of sleep impairment symptoms and stroke, and a graded association with increasing symptoms, in an international setting,” Dr. McCarthy said.
Researchers aren’t sure what’s driving the higher stroke risk among people with sleep disturbances. Although the study did control for potential confounders, it wasn’t designed to get at what’s driving the association.
“Sleep disturbance may also have a bi-directional relationship with many stroke risk factors; for example, sleep disturbance may be a symptom of disease and exacerbate disease,” Dr. McCarthy said. “Future interventional studies are required to determine the true direction of the relationship.”
A marker of stroke risk
Daniel Lackland, DrPH, professor of neurology at the Medical University of South Carolina, Charleston, said the findings provide additional evidence of the link between sleep and stroke risk.
“The results confirm sleep disorders as a potential marker and part of the risk profile,” he said.
Collecting information about sleep using a validated assessment tool is an important piece of clinical care, Dr. Lackland said, especially among patients with other stroke risk factors.
One limitation of the study was that data on sleep was collected only at one point, and participants were not followed over time to see if changes in sleep affected stroke risk.
“This is an important point and should be a focus for future studies, as it is critical in the design of interventions,” Dr. Lackland said.
The INTERSTROKE study is funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Astra Zeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), MERCK, Sharp and Dohme, Swedish Heart and Lung Foundation, U.K. Chest, and U.K. Heart and Stroke. Dr. McCarthy and Lackland report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Antiamyloids linked to accelerated brain atrophy
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Magnesium-rich diet linked to lower dementia risk
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
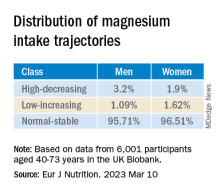
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.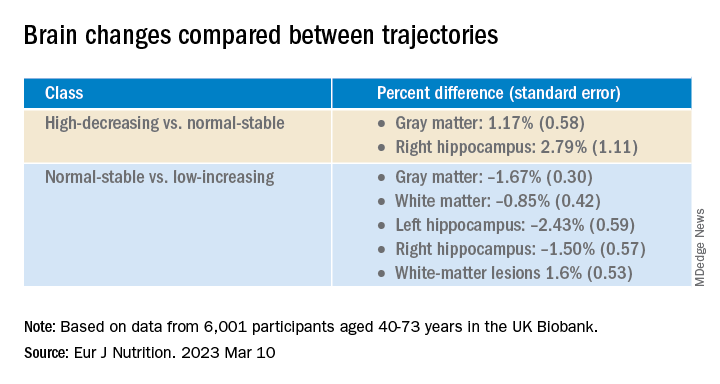
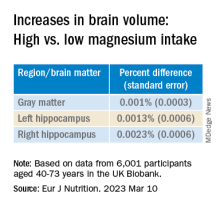
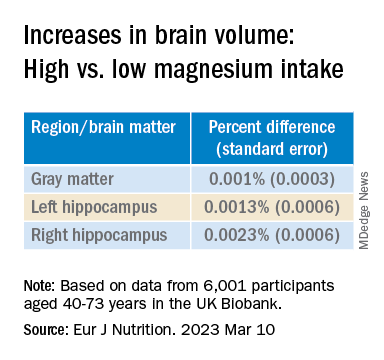
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below: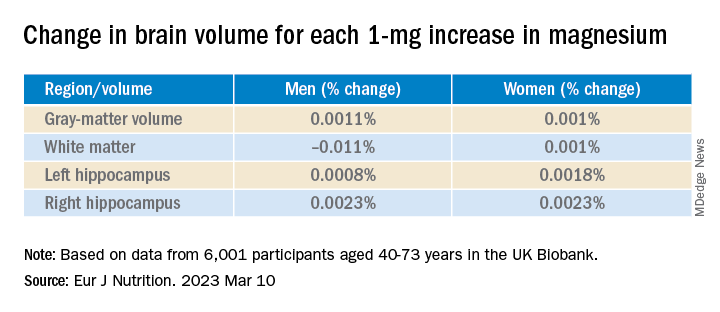
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF NUTRITION
Cancer risk elevated after stroke in younger people
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Cluster, migraine headache strongly linked to circadian rhythm
A meta-analysis of 16 studies showed a circadian pattern in 71% of cluster headache attacks (3,490 of 4,953), with a clear circadian peak between 9:00 p.m. and 3:00 a.m.
Migraine was also associated with a circadian pattern in 50% of cases (2,698 of 5,385) across eight studies, with a clear circadian trough between 11:00 p.m. and 7:00 a.m.
Seasonal peaks were also evident for cluster headache (spring and autumn) and migraine (April to October).
“In the short term, these findings help us explain the timing to patients – for example, it is possible that a headache at 8 a.m. is due to their internal body clock instead of their pillow, or breakfast food, or morning medications,” lead investigator Mark Burish, MD, PhD, associate professor, department of neurosurgery, at University of Texas Health Houston, told this news organization.
“In the long term, these findings do suggest that medications that target the circadian system could be effective in migraine and headache patients,” Dr. Burish added.
The study was published online in Neurology.
Treatment implications?
Across studies, chronotype was “highly variable” for both cluster headache and migraine, the investigators report.
Cluster headache was associated with lower melatonin and higher cortisol levels, compared with non–cluster headache controls.
On a genetic level, cluster headache was associated with two core circadian genes (CLOCK and REV-ERB–alpha), and five of the nine genes that increase the likelihood of having cluster headache are genes with a circadian pattern of expression.
Migraine headache was associated with lower urinary melatonin levels and with the core circadian genes, CK1-delta and ROR-alpha, and 110 of the 168 genes associated with migraine were clock-controlled genes.
“The data suggest that both of these headache disorders are highly circadian at multiple levels, especially cluster headache,” Dr. Burish said in a release.
“This reinforces the importance of the hypothalamus – the area of the brain that houses the primary biological clock – and its role in cluster headache and migraine. It also raises the question of the genetics of triggers such as sleep changes that are known triggers for migraine and are cues for the body’s circadian rhythm,” Dr. Burish said.
“We hope that future research will look into circadian medications as a new treatment option for migraine and cluster headache patients,” Dr. Burish told this news organization.
Importance of sleep regulation
The authors of an accompanying editorial note that even though the study doesn’t have immediate clinical implications, it offers a better understanding of the way chronobiologic factors may influence treatment.
“At a minimum, interventions known to regulate and improve sleep (e.g., melatonin, cognitive behavioral therapy), and which are safe and straightforward to introduce, may be useful in some individuals susceptible to circadian misalignment or sleep disorders,” write Heidi Sutherland, PhD, and Lyn Griffiths, PhD, with Queensland University of Technology, Brisbane, Australia.
“Treatment of comorbidities (e.g., insomnia) that result in sleep disturbances may also help headache management. Furthermore, chronobiological aspects of any pharmacological interventions should be considered, as some frequently used headache and migraine drugs can modulate circadian cycles and influence the expression of circadian genes (e.g., verapamil), or have sleep-related side effects,” they add.
A limitation of the study was the lack of information on factors that could influence the circadian cycle, such as medications; other disorders, such as bipolar disorder; or circadian rhythm issues, such as night-shift work.
The study was supported by grants from the Japan Society for the Promotion of Science, the National Institutes of Health, The Welch Foundation, and The Will Erwin Headache Research Foundation. Dr. Burish is an unpaid member of the medical advisory board of Clusterbusters, and a site investigator for a cluster headache clinical trial funded by Lundbeck. Dr. Sutherland has received grant funding from the U.S. Migraine Research Foundation, and received institute support from Queensland University of Technology for genetics research. Dr. Griffiths has received grant funding from the Australian NHMRC, U.S. Department of Defense, and the U.S. Migraine Research Foundation, and consultancy funding from TEVA.
A version of this article first appeared on Medscape.com.
A meta-analysis of 16 studies showed a circadian pattern in 71% of cluster headache attacks (3,490 of 4,953), with a clear circadian peak between 9:00 p.m. and 3:00 a.m.
Migraine was also associated with a circadian pattern in 50% of cases (2,698 of 5,385) across eight studies, with a clear circadian trough between 11:00 p.m. and 7:00 a.m.
Seasonal peaks were also evident for cluster headache (spring and autumn) and migraine (April to October).
“In the short term, these findings help us explain the timing to patients – for example, it is possible that a headache at 8 a.m. is due to their internal body clock instead of their pillow, or breakfast food, or morning medications,” lead investigator Mark Burish, MD, PhD, associate professor, department of neurosurgery, at University of Texas Health Houston, told this news organization.
“In the long term, these findings do suggest that medications that target the circadian system could be effective in migraine and headache patients,” Dr. Burish added.
The study was published online in Neurology.
Treatment implications?
Across studies, chronotype was “highly variable” for both cluster headache and migraine, the investigators report.
Cluster headache was associated with lower melatonin and higher cortisol levels, compared with non–cluster headache controls.
On a genetic level, cluster headache was associated with two core circadian genes (CLOCK and REV-ERB–alpha), and five of the nine genes that increase the likelihood of having cluster headache are genes with a circadian pattern of expression.
Migraine headache was associated with lower urinary melatonin levels and with the core circadian genes, CK1-delta and ROR-alpha, and 110 of the 168 genes associated with migraine were clock-controlled genes.
“The data suggest that both of these headache disorders are highly circadian at multiple levels, especially cluster headache,” Dr. Burish said in a release.
“This reinforces the importance of the hypothalamus – the area of the brain that houses the primary biological clock – and its role in cluster headache and migraine. It also raises the question of the genetics of triggers such as sleep changes that are known triggers for migraine and are cues for the body’s circadian rhythm,” Dr. Burish said.
“We hope that future research will look into circadian medications as a new treatment option for migraine and cluster headache patients,” Dr. Burish told this news organization.
Importance of sleep regulation
The authors of an accompanying editorial note that even though the study doesn’t have immediate clinical implications, it offers a better understanding of the way chronobiologic factors may influence treatment.
“At a minimum, interventions known to regulate and improve sleep (e.g., melatonin, cognitive behavioral therapy), and which are safe and straightforward to introduce, may be useful in some individuals susceptible to circadian misalignment or sleep disorders,” write Heidi Sutherland, PhD, and Lyn Griffiths, PhD, with Queensland University of Technology, Brisbane, Australia.
“Treatment of comorbidities (e.g., insomnia) that result in sleep disturbances may also help headache management. Furthermore, chronobiological aspects of any pharmacological interventions should be considered, as some frequently used headache and migraine drugs can modulate circadian cycles and influence the expression of circadian genes (e.g., verapamil), or have sleep-related side effects,” they add.
A limitation of the study was the lack of information on factors that could influence the circadian cycle, such as medications; other disorders, such as bipolar disorder; or circadian rhythm issues, such as night-shift work.
The study was supported by grants from the Japan Society for the Promotion of Science, the National Institutes of Health, The Welch Foundation, and The Will Erwin Headache Research Foundation. Dr. Burish is an unpaid member of the medical advisory board of Clusterbusters, and a site investigator for a cluster headache clinical trial funded by Lundbeck. Dr. Sutherland has received grant funding from the U.S. Migraine Research Foundation, and received institute support from Queensland University of Technology for genetics research. Dr. Griffiths has received grant funding from the Australian NHMRC, U.S. Department of Defense, and the U.S. Migraine Research Foundation, and consultancy funding from TEVA.
A version of this article first appeared on Medscape.com.
A meta-analysis of 16 studies showed a circadian pattern in 71% of cluster headache attacks (3,490 of 4,953), with a clear circadian peak between 9:00 p.m. and 3:00 a.m.
Migraine was also associated with a circadian pattern in 50% of cases (2,698 of 5,385) across eight studies, with a clear circadian trough between 11:00 p.m. and 7:00 a.m.
Seasonal peaks were also evident for cluster headache (spring and autumn) and migraine (April to October).
“In the short term, these findings help us explain the timing to patients – for example, it is possible that a headache at 8 a.m. is due to their internal body clock instead of their pillow, or breakfast food, or morning medications,” lead investigator Mark Burish, MD, PhD, associate professor, department of neurosurgery, at University of Texas Health Houston, told this news organization.
“In the long term, these findings do suggest that medications that target the circadian system could be effective in migraine and headache patients,” Dr. Burish added.
The study was published online in Neurology.
Treatment implications?
Across studies, chronotype was “highly variable” for both cluster headache and migraine, the investigators report.
Cluster headache was associated with lower melatonin and higher cortisol levels, compared with non–cluster headache controls.
On a genetic level, cluster headache was associated with two core circadian genes (CLOCK and REV-ERB–alpha), and five of the nine genes that increase the likelihood of having cluster headache are genes with a circadian pattern of expression.
Migraine headache was associated with lower urinary melatonin levels and with the core circadian genes, CK1-delta and ROR-alpha, and 110 of the 168 genes associated with migraine were clock-controlled genes.
“The data suggest that both of these headache disorders are highly circadian at multiple levels, especially cluster headache,” Dr. Burish said in a release.
“This reinforces the importance of the hypothalamus – the area of the brain that houses the primary biological clock – and its role in cluster headache and migraine. It also raises the question of the genetics of triggers such as sleep changes that are known triggers for migraine and are cues for the body’s circadian rhythm,” Dr. Burish said.
“We hope that future research will look into circadian medications as a new treatment option for migraine and cluster headache patients,” Dr. Burish told this news organization.
Importance of sleep regulation
The authors of an accompanying editorial note that even though the study doesn’t have immediate clinical implications, it offers a better understanding of the way chronobiologic factors may influence treatment.
“At a minimum, interventions known to regulate and improve sleep (e.g., melatonin, cognitive behavioral therapy), and which are safe and straightforward to introduce, may be useful in some individuals susceptible to circadian misalignment or sleep disorders,” write Heidi Sutherland, PhD, and Lyn Griffiths, PhD, with Queensland University of Technology, Brisbane, Australia.
“Treatment of comorbidities (e.g., insomnia) that result in sleep disturbances may also help headache management. Furthermore, chronobiological aspects of any pharmacological interventions should be considered, as some frequently used headache and migraine drugs can modulate circadian cycles and influence the expression of circadian genes (e.g., verapamil), or have sleep-related side effects,” they add.
A limitation of the study was the lack of information on factors that could influence the circadian cycle, such as medications; other disorders, such as bipolar disorder; or circadian rhythm issues, such as night-shift work.
The study was supported by grants from the Japan Society for the Promotion of Science, the National Institutes of Health, The Welch Foundation, and The Will Erwin Headache Research Foundation. Dr. Burish is an unpaid member of the medical advisory board of Clusterbusters, and a site investigator for a cluster headache clinical trial funded by Lundbeck. Dr. Sutherland has received grant funding from the U.S. Migraine Research Foundation, and received institute support from Queensland University of Technology for genetics research. Dr. Griffiths has received grant funding from the Australian NHMRC, U.S. Department of Defense, and the U.S. Migraine Research Foundation, and consultancy funding from TEVA.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Do B vitamins reduce Parkinson’s risk?
Though there was some evidence that vitamin B12 early in life was associated with decreased PD risk, the findings were inconsistent and were observed only in people whose daily intake was 10 times the recommended level.
“The results of this large prospective study do not support the hypothesis that increasing folate or vitamin B6 intakes above the current levels would reduce PD risk in this population of mostly White U.S. health professionals,” lead investigator Mario H. Flores-Torres, MD, PhD, a research scientist in the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
However, he added, the study “leaves open the possibility that in some individuals the intake of vitamin B12 contributes to PD risk – a finding that warrants further research.”
The findings were published online in Movement Disorders.
Mixed findings
Previous studies have suggested B vitamins – including folate, B6 and B12 – might affect PD risk, but results have been mixed.
The new study included 80,965 women from the Nurses’ Health Study (1984-2016) and 48,837 men from the Health Professionals Follow-up Study (1986-2016). The average age at baseline was 50 years in women and 54 years in men, and participants were followed for about 30 years.
Participants completed questionnaires about diet at the beginning of the study and again every 4 years.
To account for the possibility of reverse causation due to the long prodromal phase of PD, investigators conducted lagged analyses at 8, 12, 16, and 20 years.
During the follow-up period, 1,426 incident cases of PD were diagnosed (687 in women and 739 in men).
Researchers found no link between reduced PD risk and intake of vitamin B6 or folate.
Though the total cumulative average intake of vitamin B12 was not associated with PD risk, investigators noted a modest decrease in risk between those with highest baseline of B12 and participants with the lowest baseline levels (hazard ratio, 0.80; P = .01).
Individuals in the highest quintile of B12 intake at baseline had an average intake of 21-22 mcg/d, close to 10 times the recommended daily intake of 2.4 mcg/d.
“Although some of our results suggest that a higher intake of vitamin B12 may decrease the risk of PD in a population of U.S. health professionals, the associations we observed were modest and not entirely consistent,” Dr. Flores-Torres said.
“Additional studies need to confirm our findings to better understand whether people who take higher amounts of B12 younger in life may have a protective benefit against PD,” he added.
The whole picture?
Commenting on the findings for this article, Rebecca Gilbert, MD, PhD, chief scientific officer of the American Parkinson Disease Association, New York, noted that checking B vitamin levels is a fairly standard practice for most clinicians. In that regard, this study highlights why this is important.
“Neurologists will often test B12 levels and recommend a supplement if your level is below the normal range,” she said. “No one is questioning the value of B12 for nerves and recommend that B12 is in the normal to high normal range.”
But understanding how B vitamins may or may not affect PD risk might require a different kind of study.
“This analysis, much like many others, is trying so hard to figure out what is it in diets that affects Parkinson’s disease risk,” Dr. Gilbert said. “But we have yet to say these are the nutrients that prevent Parkinson’s or increase the risk.”
One reason for the conflicting results in studies such as this could be that the explanation for the link between diet and PD risk may not be in specific minerals consumed but rather in the diet as a whole.
“Focusing on specific elements of a diet may not give us the answer,” Dr. Gilbert said. “We should be analyzing diet as a complete holistic picture because it’s not just the elements but how everything in what we eat works together.”
The study was funded by the National Institutes of Health and the Parkinson’s Foundation. Dr. Flores-Torres and Dr. Gilbert report no relevant conflicts.
A version of this article originally appeared on Medscape.com.
Though there was some evidence that vitamin B12 early in life was associated with decreased PD risk, the findings were inconsistent and were observed only in people whose daily intake was 10 times the recommended level.
“The results of this large prospective study do not support the hypothesis that increasing folate or vitamin B6 intakes above the current levels would reduce PD risk in this population of mostly White U.S. health professionals,” lead investigator Mario H. Flores-Torres, MD, PhD, a research scientist in the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
However, he added, the study “leaves open the possibility that in some individuals the intake of vitamin B12 contributes to PD risk – a finding that warrants further research.”
The findings were published online in Movement Disorders.
Mixed findings
Previous studies have suggested B vitamins – including folate, B6 and B12 – might affect PD risk, but results have been mixed.
The new study included 80,965 women from the Nurses’ Health Study (1984-2016) and 48,837 men from the Health Professionals Follow-up Study (1986-2016). The average age at baseline was 50 years in women and 54 years in men, and participants were followed for about 30 years.
Participants completed questionnaires about diet at the beginning of the study and again every 4 years.
To account for the possibility of reverse causation due to the long prodromal phase of PD, investigators conducted lagged analyses at 8, 12, 16, and 20 years.
During the follow-up period, 1,426 incident cases of PD were diagnosed (687 in women and 739 in men).
Researchers found no link between reduced PD risk and intake of vitamin B6 or folate.
Though the total cumulative average intake of vitamin B12 was not associated with PD risk, investigators noted a modest decrease in risk between those with highest baseline of B12 and participants with the lowest baseline levels (hazard ratio, 0.80; P = .01).
Individuals in the highest quintile of B12 intake at baseline had an average intake of 21-22 mcg/d, close to 10 times the recommended daily intake of 2.4 mcg/d.
“Although some of our results suggest that a higher intake of vitamin B12 may decrease the risk of PD in a population of U.S. health professionals, the associations we observed were modest and not entirely consistent,” Dr. Flores-Torres said.
“Additional studies need to confirm our findings to better understand whether people who take higher amounts of B12 younger in life may have a protective benefit against PD,” he added.
The whole picture?
Commenting on the findings for this article, Rebecca Gilbert, MD, PhD, chief scientific officer of the American Parkinson Disease Association, New York, noted that checking B vitamin levels is a fairly standard practice for most clinicians. In that regard, this study highlights why this is important.
“Neurologists will often test B12 levels and recommend a supplement if your level is below the normal range,” she said. “No one is questioning the value of B12 for nerves and recommend that B12 is in the normal to high normal range.”
But understanding how B vitamins may or may not affect PD risk might require a different kind of study.
“This analysis, much like many others, is trying so hard to figure out what is it in diets that affects Parkinson’s disease risk,” Dr. Gilbert said. “But we have yet to say these are the nutrients that prevent Parkinson’s or increase the risk.”
One reason for the conflicting results in studies such as this could be that the explanation for the link between diet and PD risk may not be in specific minerals consumed but rather in the diet as a whole.
“Focusing on specific elements of a diet may not give us the answer,” Dr. Gilbert said. “We should be analyzing diet as a complete holistic picture because it’s not just the elements but how everything in what we eat works together.”
The study was funded by the National Institutes of Health and the Parkinson’s Foundation. Dr. Flores-Torres and Dr. Gilbert report no relevant conflicts.
A version of this article originally appeared on Medscape.com.
Though there was some evidence that vitamin B12 early in life was associated with decreased PD risk, the findings were inconsistent and were observed only in people whose daily intake was 10 times the recommended level.
“The results of this large prospective study do not support the hypothesis that increasing folate or vitamin B6 intakes above the current levels would reduce PD risk in this population of mostly White U.S. health professionals,” lead investigator Mario H. Flores-Torres, MD, PhD, a research scientist in the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
However, he added, the study “leaves open the possibility that in some individuals the intake of vitamin B12 contributes to PD risk – a finding that warrants further research.”
The findings were published online in Movement Disorders.
Mixed findings
Previous studies have suggested B vitamins – including folate, B6 and B12 – might affect PD risk, but results have been mixed.
The new study included 80,965 women from the Nurses’ Health Study (1984-2016) and 48,837 men from the Health Professionals Follow-up Study (1986-2016). The average age at baseline was 50 years in women and 54 years in men, and participants were followed for about 30 years.
Participants completed questionnaires about diet at the beginning of the study and again every 4 years.
To account for the possibility of reverse causation due to the long prodromal phase of PD, investigators conducted lagged analyses at 8, 12, 16, and 20 years.
During the follow-up period, 1,426 incident cases of PD were diagnosed (687 in women and 739 in men).
Researchers found no link between reduced PD risk and intake of vitamin B6 or folate.
Though the total cumulative average intake of vitamin B12 was not associated with PD risk, investigators noted a modest decrease in risk between those with highest baseline of B12 and participants with the lowest baseline levels (hazard ratio, 0.80; P = .01).
Individuals in the highest quintile of B12 intake at baseline had an average intake of 21-22 mcg/d, close to 10 times the recommended daily intake of 2.4 mcg/d.
“Although some of our results suggest that a higher intake of vitamin B12 may decrease the risk of PD in a population of U.S. health professionals, the associations we observed were modest and not entirely consistent,” Dr. Flores-Torres said.
“Additional studies need to confirm our findings to better understand whether people who take higher amounts of B12 younger in life may have a protective benefit against PD,” he added.
The whole picture?
Commenting on the findings for this article, Rebecca Gilbert, MD, PhD, chief scientific officer of the American Parkinson Disease Association, New York, noted that checking B vitamin levels is a fairly standard practice for most clinicians. In that regard, this study highlights why this is important.
“Neurologists will often test B12 levels and recommend a supplement if your level is below the normal range,” she said. “No one is questioning the value of B12 for nerves and recommend that B12 is in the normal to high normal range.”
But understanding how B vitamins may or may not affect PD risk might require a different kind of study.
“This analysis, much like many others, is trying so hard to figure out what is it in diets that affects Parkinson’s disease risk,” Dr. Gilbert said. “But we have yet to say these are the nutrients that prevent Parkinson’s or increase the risk.”
One reason for the conflicting results in studies such as this could be that the explanation for the link between diet and PD risk may not be in specific minerals consumed but rather in the diet as a whole.
“Focusing on specific elements of a diet may not give us the answer,” Dr. Gilbert said. “We should be analyzing diet as a complete holistic picture because it’s not just the elements but how everything in what we eat works together.”
The study was funded by the National Institutes of Health and the Parkinson’s Foundation. Dr. Flores-Torres and Dr. Gilbert report no relevant conflicts.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS
Exercise tied to reduced Parkinson’s motor symptoms and increased well-being
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Poor bone health is a ‘robust’ dementia risk factor
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY