User login
CPT and relative value changes that may affect reimbursement to your ObGyn practice
Another year brings changes to Current Procedural Terminology (CPT) codes (which are developed and copyrighted by the American Medical Association) in the form of additions and revisions, and payments related to resource-based relative value scale (RBRVS) revisions for selected services. As of January 1, 2018, 2 new Category I codes pertain to laparoscopic treatments for gynecologic cancer, and the 4 existing codes for colporrhaphy have been revised to include cystourethroscopy. New Category III codes include 4 for fetal magnetocardiography and 1 for transvaginal tactile imaging. Medicare also has reevaluated certain relative value units (RVUs) in outpatient and facility settings.
New and revised Category I codes
Laparoscopic treatments for gynecologic cancer. Technologic advances in performing laparoscopic procedures have allowed for more extensive laparoscopic surgery for various gynecologic cancers and, to this end, 2 new codes have been added.
First, a new code was added to capture comprehensive laparoscopic surgical staging for gynecologic cancer. This new code, 38573, Laparoscopy, surgical; with bilateral total pelvic lymphadenectomy and peri-aortic lymph node sampling, peritoneal washings, peritoneal biopsy(ies), omentectomy, and diaphragmatic washings, including diaphragmatic and other serosal biopsy(ies), when performed, may not be reported with any other code that includes lymphadenectomy, omentectomy, or hysterectomy. It is intended primarily for a stand-alone staging procedure after an initial biopsy shows a gynecologic malignancy such as ovarian cancer. This new code has been valued at 33.59 RVUs.
Second, a new code was added to capture laparoscopic debulking in conjunction with hysterectomy. The new code, 58575, Laparoscopy, surgical, total hysterectomy for resection of malignancy (tumor debulking), with omentectomy including salpingo-oophorectomy, unilateral or bilateral, when performed, has been valued at 53.62 RVUs. The open equivalent to this new code is 58953, Bilateral salpingo-oophorectomy with omentectomy, total abdominal hysterectomy and radical dissection for debulking.
Cystourethroscopy. The revisions involve no longer permitting separate reporting of 52000, Cystourethroscopy (separate procedure), with the colporrhaphy codes 57240−57265. The rationale behind this change was that surgeons were routinely performing cystoscopy at the time of these procedures and therefore it should become part of the surgical procedure. Currently the Medicare National Correct Coding Initiative (NCCI) bundles 52000 with these 4 codes, but only code 57250 allows for the use of a modifier -59 to bypass the edit if the purpose of the cystoscopy was evaluation of a distinct complaint or problem (such as evaluating patient-expressed urinary symptoms prior to the surgery that were investigated at the time of the prolapse surgery). When codes 57240, 57260, or 57265 are billed along with 52000, the cystoscopy will be denied and a modifier -59 cannot be reported to bypass this edit.
New Category III codes
The new Category III codes represent emerging technology, and it is important to report them, rather than an unlisted code, if the procedures described are performed so that data can be collected for later consideration to make these Category I CPT codes. Since these codes are not assigned relative values, the provider will need to let the payer know which existing CPT Category I code most closely represents the work involved.
Fetal magnetocardiography. The new Category III codes for fetal magnetocardiography describe essentially a fetal electrocardiogram (ECG) that would be performed to assess fetal arrhythmias by placing up to 3 leads on the mother’s abdomen. Possible comparison codes for physician work might include 59050, fetal monitoring by consultant during labor; 93000−93010, 12-lead ECG, or 93040−93042, rhythm strip up to 3 leads. However, because the equipment is very expensive, these codes would not capture practice expense and the physician would have to negotiate a reasonable reimbursement level with the payer, if the magnetocardiography was a covered service. The new codes are as follows:
- 0475T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording and storage, data scanning with signal extraction, technical analysis and result, as well as supervision, review, and interpretation of report by a physician or other qualified health care professional
- 0476T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording, data scanning, with raw electronic signal transfer of data and storage
- 0477T, Recording of fetal magnetic cardiac signal using at least 3 channels; signal extraction, technical analysis, and result.
Transvaginal tactile imaging. The new Category III code, 0487T, Biomechanical mapping, transvaginal, with report, describes the use of a pressure sensor probe inserted into the vaginal canal to measure and collect data on pelvic muscle strength, elasticity, tissue integrity, and tone. These data produce images in real time that are mapped to produce a report for physician review, interpretation, and report. The data allow quantification of pelvic floor dysfunction and may be useful in determining the most appropriate treatment (whether surgical or medical) for this gynecologic condition. The procedure uses a transvaginal probe like an ultrasound, so using 76830, transvaginal ultrasound, would not be unreasonable as a comparison code as a start.
Medicare relative value changes
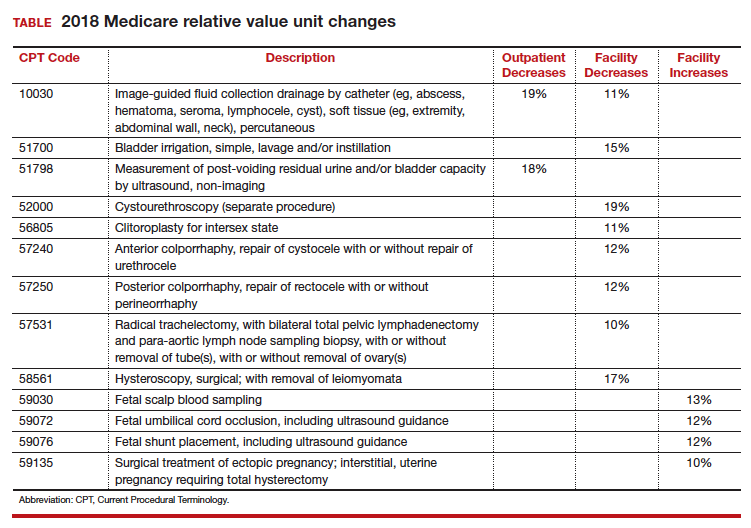
Every year, Medicare reevaluates potentially misvalued CPT codes and this year was no exception. The TABLE represents the winners and losers for codes in the outpatient and facility settings that have increased or decreased RVUs by more than 10%.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Another year brings changes to Current Procedural Terminology (CPT) codes (which are developed and copyrighted by the American Medical Association) in the form of additions and revisions, and payments related to resource-based relative value scale (RBRVS) revisions for selected services. As of January 1, 2018, 2 new Category I codes pertain to laparoscopic treatments for gynecologic cancer, and the 4 existing codes for colporrhaphy have been revised to include cystourethroscopy. New Category III codes include 4 for fetal magnetocardiography and 1 for transvaginal tactile imaging. Medicare also has reevaluated certain relative value units (RVUs) in outpatient and facility settings.
New and revised Category I codes
Laparoscopic treatments for gynecologic cancer. Technologic advances in performing laparoscopic procedures have allowed for more extensive laparoscopic surgery for various gynecologic cancers and, to this end, 2 new codes have been added.
First, a new code was added to capture comprehensive laparoscopic surgical staging for gynecologic cancer. This new code, 38573, Laparoscopy, surgical; with bilateral total pelvic lymphadenectomy and peri-aortic lymph node sampling, peritoneal washings, peritoneal biopsy(ies), omentectomy, and diaphragmatic washings, including diaphragmatic and other serosal biopsy(ies), when performed, may not be reported with any other code that includes lymphadenectomy, omentectomy, or hysterectomy. It is intended primarily for a stand-alone staging procedure after an initial biopsy shows a gynecologic malignancy such as ovarian cancer. This new code has been valued at 33.59 RVUs.
Second, a new code was added to capture laparoscopic debulking in conjunction with hysterectomy. The new code, 58575, Laparoscopy, surgical, total hysterectomy for resection of malignancy (tumor debulking), with omentectomy including salpingo-oophorectomy, unilateral or bilateral, when performed, has been valued at 53.62 RVUs. The open equivalent to this new code is 58953, Bilateral salpingo-oophorectomy with omentectomy, total abdominal hysterectomy and radical dissection for debulking.
Cystourethroscopy. The revisions involve no longer permitting separate reporting of 52000, Cystourethroscopy (separate procedure), with the colporrhaphy codes 57240−57265. The rationale behind this change was that surgeons were routinely performing cystoscopy at the time of these procedures and therefore it should become part of the surgical procedure. Currently the Medicare National Correct Coding Initiative (NCCI) bundles 52000 with these 4 codes, but only code 57250 allows for the use of a modifier -59 to bypass the edit if the purpose of the cystoscopy was evaluation of a distinct complaint or problem (such as evaluating patient-expressed urinary symptoms prior to the surgery that were investigated at the time of the prolapse surgery). When codes 57240, 57260, or 57265 are billed along with 52000, the cystoscopy will be denied and a modifier -59 cannot be reported to bypass this edit.
New Category III codes
The new Category III codes represent emerging technology, and it is important to report them, rather than an unlisted code, if the procedures described are performed so that data can be collected for later consideration to make these Category I CPT codes. Since these codes are not assigned relative values, the provider will need to let the payer know which existing CPT Category I code most closely represents the work involved.
Fetal magnetocardiography. The new Category III codes for fetal magnetocardiography describe essentially a fetal electrocardiogram (ECG) that would be performed to assess fetal arrhythmias by placing up to 3 leads on the mother’s abdomen. Possible comparison codes for physician work might include 59050, fetal monitoring by consultant during labor; 93000−93010, 12-lead ECG, or 93040−93042, rhythm strip up to 3 leads. However, because the equipment is very expensive, these codes would not capture practice expense and the physician would have to negotiate a reasonable reimbursement level with the payer, if the magnetocardiography was a covered service. The new codes are as follows:
- 0475T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording and storage, data scanning with signal extraction, technical analysis and result, as well as supervision, review, and interpretation of report by a physician or other qualified health care professional
- 0476T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording, data scanning, with raw electronic signal transfer of data and storage
- 0477T, Recording of fetal magnetic cardiac signal using at least 3 channels; signal extraction, technical analysis, and result.
Transvaginal tactile imaging. The new Category III code, 0487T, Biomechanical mapping, transvaginal, with report, describes the use of a pressure sensor probe inserted into the vaginal canal to measure and collect data on pelvic muscle strength, elasticity, tissue integrity, and tone. These data produce images in real time that are mapped to produce a report for physician review, interpretation, and report. The data allow quantification of pelvic floor dysfunction and may be useful in determining the most appropriate treatment (whether surgical or medical) for this gynecologic condition. The procedure uses a transvaginal probe like an ultrasound, so using 76830, transvaginal ultrasound, would not be unreasonable as a comparison code as a start.
Medicare relative value changes
Every year, Medicare reevaluates potentially misvalued CPT codes and this year was no exception. The TABLE represents the winners and losers for codes in the outpatient and facility settings that have increased or decreased RVUs by more than 10%.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Another year brings changes to Current Procedural Terminology (CPT) codes (which are developed and copyrighted by the American Medical Association) in the form of additions and revisions, and payments related to resource-based relative value scale (RBRVS) revisions for selected services. As of January 1, 2018, 2 new Category I codes pertain to laparoscopic treatments for gynecologic cancer, and the 4 existing codes for colporrhaphy have been revised to include cystourethroscopy. New Category III codes include 4 for fetal magnetocardiography and 1 for transvaginal tactile imaging. Medicare also has reevaluated certain relative value units (RVUs) in outpatient and facility settings.
New and revised Category I codes
Laparoscopic treatments for gynecologic cancer. Technologic advances in performing laparoscopic procedures have allowed for more extensive laparoscopic surgery for various gynecologic cancers and, to this end, 2 new codes have been added.
First, a new code was added to capture comprehensive laparoscopic surgical staging for gynecologic cancer. This new code, 38573, Laparoscopy, surgical; with bilateral total pelvic lymphadenectomy and peri-aortic lymph node sampling, peritoneal washings, peritoneal biopsy(ies), omentectomy, and diaphragmatic washings, including diaphragmatic and other serosal biopsy(ies), when performed, may not be reported with any other code that includes lymphadenectomy, omentectomy, or hysterectomy. It is intended primarily for a stand-alone staging procedure after an initial biopsy shows a gynecologic malignancy such as ovarian cancer. This new code has been valued at 33.59 RVUs.
Second, a new code was added to capture laparoscopic debulking in conjunction with hysterectomy. The new code, 58575, Laparoscopy, surgical, total hysterectomy for resection of malignancy (tumor debulking), with omentectomy including salpingo-oophorectomy, unilateral or bilateral, when performed, has been valued at 53.62 RVUs. The open equivalent to this new code is 58953, Bilateral salpingo-oophorectomy with omentectomy, total abdominal hysterectomy and radical dissection for debulking.
Cystourethroscopy. The revisions involve no longer permitting separate reporting of 52000, Cystourethroscopy (separate procedure), with the colporrhaphy codes 57240−57265. The rationale behind this change was that surgeons were routinely performing cystoscopy at the time of these procedures and therefore it should become part of the surgical procedure. Currently the Medicare National Correct Coding Initiative (NCCI) bundles 52000 with these 4 codes, but only code 57250 allows for the use of a modifier -59 to bypass the edit if the purpose of the cystoscopy was evaluation of a distinct complaint or problem (such as evaluating patient-expressed urinary symptoms prior to the surgery that were investigated at the time of the prolapse surgery). When codes 57240, 57260, or 57265 are billed along with 52000, the cystoscopy will be denied and a modifier -59 cannot be reported to bypass this edit.
New Category III codes
The new Category III codes represent emerging technology, and it is important to report them, rather than an unlisted code, if the procedures described are performed so that data can be collected for later consideration to make these Category I CPT codes. Since these codes are not assigned relative values, the provider will need to let the payer know which existing CPT Category I code most closely represents the work involved.
Fetal magnetocardiography. The new Category III codes for fetal magnetocardiography describe essentially a fetal electrocardiogram (ECG) that would be performed to assess fetal arrhythmias by placing up to 3 leads on the mother’s abdomen. Possible comparison codes for physician work might include 59050, fetal monitoring by consultant during labor; 93000−93010, 12-lead ECG, or 93040−93042, rhythm strip up to 3 leads. However, because the equipment is very expensive, these codes would not capture practice expense and the physician would have to negotiate a reasonable reimbursement level with the payer, if the magnetocardiography was a covered service. The new codes are as follows:
- 0475T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording and storage, data scanning with signal extraction, technical analysis and result, as well as supervision, review, and interpretation of report by a physician or other qualified health care professional
- 0476T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording, data scanning, with raw electronic signal transfer of data and storage
- 0477T, Recording of fetal magnetic cardiac signal using at least 3 channels; signal extraction, technical analysis, and result.
Transvaginal tactile imaging. The new Category III code, 0487T, Biomechanical mapping, transvaginal, with report, describes the use of a pressure sensor probe inserted into the vaginal canal to measure and collect data on pelvic muscle strength, elasticity, tissue integrity, and tone. These data produce images in real time that are mapped to produce a report for physician review, interpretation, and report. The data allow quantification of pelvic floor dysfunction and may be useful in determining the most appropriate treatment (whether surgical or medical) for this gynecologic condition. The procedure uses a transvaginal probe like an ultrasound, so using 76830, transvaginal ultrasound, would not be unreasonable as a comparison code as a start.
Medicare relative value changes
Every year, Medicare reevaluates potentially misvalued CPT codes and this year was no exception. The TABLE represents the winners and losers for codes in the outpatient and facility settings that have increased or decreased RVUs by more than 10%.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Beware the con
As I stepped from an exam room one recent busy morning, my office manager pulled me aside. “Someone from the county courthouse is on the phone and needs to talk to you,” she whispered.
“You know better than that,” I said. “While I’m seeing patients, I don’t take calls from anyone except colleagues and immediate family.”
I took the call.
“You failed to appear for jury duty,” the official-sounding voice said. “That’s a violation of state law, as you were warned when you received your summons. You’ll have to come down here and surrender yourself immediately, or else we’ll have to send deputies to your office. I don’t think you’ll want to be led through your waiting room in handcuffs.”
“Wait a minute,” I replied nervously. “I haven’t received a jury summons for 2 years, at least. There must be some mistake.”
“Perhaps we’ve confused you with a citizen with the same or a similar name,” he said. “Let me have your Social Security number and birth date.”
Alarm bells! “You should have that information already,” I replied. “Why don’t you read me what you have?”
A short silence, and then … click.
I immediately called the courthouse. “Citizens who fail to appear receive a warning letter and a new questionnaire, not a phone call,” said the jury manager. “And we use driver license numbers to keep track of jurors.”
The phone company traced the call, which dead-ended at a VoIP circuit, to no one’s surprise. The downside of VoIP (Voice over Internet Protocol) and similar technologies is that unscrupulous individuals can use them to appear to be calling you from a legitimate business when they are not.
Those of us of a certain age remember phony office calls offering great deals on supplies or waiting room magazine subscriptions. Those capers eventually disappeared; but scam artists are endlessly creative. This is especially true since the Internet took over, well, everything. There’s a real dark side to the information age.
The jury duty scheme, I learned, is an increasingly popular one. Others involve calls or e-mails from the “fraud department” of your bank, claiming to be investigating a breach of your account, or one of your credit or debit cards. Another purports to be a “Customs official” informing you that you owe a big duty payment on an overseas shipment. Victims of power outages due to natural disasters are hearing from crooks claiming to be from the local power company; the power won’t be restored, they say, without an advance payment.
In most cases, the common denominator – and the biggest red flag – is a request for a social security number, a birth date, a credit card number, or other private information that could be used to steal your identity or empty your accounts.
Here’s a summary of what my recent experience taught (or reminded) me:
- Never give out a bank account, social security, or credit card number online or over the telephone if you didn’t initiate the contact, no matter how legitimate the caller sounds. This is true of anyone claiming to be from a bank, a service company, or a government office, as well as anyone trying to sell you anything.
- No federal or state court will call to say you’ve missed jury duty – or that they are assembling jury pools and need to “prescreen” those who might be selected to serve on them. The jury manager I spoke with said she knew of no reason why anyone in my state would ever be called about jury service before mailing back a completed questionnaire, and even then, such a call would be extraordinary.
- Never send anyone a “commission” or “finder’s fee” as a condition of receiving funds. In legitimate transactions, such fees are merely deducted from the money being paid out.
- Examine your credit card and bank account statements each month. Immediately challenge any charges you don’t recognize.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As I stepped from an exam room one recent busy morning, my office manager pulled me aside. “Someone from the county courthouse is on the phone and needs to talk to you,” she whispered.
“You know better than that,” I said. “While I’m seeing patients, I don’t take calls from anyone except colleagues and immediate family.”
I took the call.
“You failed to appear for jury duty,” the official-sounding voice said. “That’s a violation of state law, as you were warned when you received your summons. You’ll have to come down here and surrender yourself immediately, or else we’ll have to send deputies to your office. I don’t think you’ll want to be led through your waiting room in handcuffs.”
“Wait a minute,” I replied nervously. “I haven’t received a jury summons for 2 years, at least. There must be some mistake.”
“Perhaps we’ve confused you with a citizen with the same or a similar name,” he said. “Let me have your Social Security number and birth date.”
Alarm bells! “You should have that information already,” I replied. “Why don’t you read me what you have?”
A short silence, and then … click.
I immediately called the courthouse. “Citizens who fail to appear receive a warning letter and a new questionnaire, not a phone call,” said the jury manager. “And we use driver license numbers to keep track of jurors.”
The phone company traced the call, which dead-ended at a VoIP circuit, to no one’s surprise. The downside of VoIP (Voice over Internet Protocol) and similar technologies is that unscrupulous individuals can use them to appear to be calling you from a legitimate business when they are not.
Those of us of a certain age remember phony office calls offering great deals on supplies or waiting room magazine subscriptions. Those capers eventually disappeared; but scam artists are endlessly creative. This is especially true since the Internet took over, well, everything. There’s a real dark side to the information age.
The jury duty scheme, I learned, is an increasingly popular one. Others involve calls or e-mails from the “fraud department” of your bank, claiming to be investigating a breach of your account, or one of your credit or debit cards. Another purports to be a “Customs official” informing you that you owe a big duty payment on an overseas shipment. Victims of power outages due to natural disasters are hearing from crooks claiming to be from the local power company; the power won’t be restored, they say, without an advance payment.
In most cases, the common denominator – and the biggest red flag – is a request for a social security number, a birth date, a credit card number, or other private information that could be used to steal your identity or empty your accounts.
Here’s a summary of what my recent experience taught (or reminded) me:
- Never give out a bank account, social security, or credit card number online or over the telephone if you didn’t initiate the contact, no matter how legitimate the caller sounds. This is true of anyone claiming to be from a bank, a service company, or a government office, as well as anyone trying to sell you anything.
- No federal or state court will call to say you’ve missed jury duty – or that they are assembling jury pools and need to “prescreen” those who might be selected to serve on them. The jury manager I spoke with said she knew of no reason why anyone in my state would ever be called about jury service before mailing back a completed questionnaire, and even then, such a call would be extraordinary.
- Never send anyone a “commission” or “finder’s fee” as a condition of receiving funds. In legitimate transactions, such fees are merely deducted from the money being paid out.
- Examine your credit card and bank account statements each month. Immediately challenge any charges you don’t recognize.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As I stepped from an exam room one recent busy morning, my office manager pulled me aside. “Someone from the county courthouse is on the phone and needs to talk to you,” she whispered.
“You know better than that,” I said. “While I’m seeing patients, I don’t take calls from anyone except colleagues and immediate family.”
I took the call.
“You failed to appear for jury duty,” the official-sounding voice said. “That’s a violation of state law, as you were warned when you received your summons. You’ll have to come down here and surrender yourself immediately, or else we’ll have to send deputies to your office. I don’t think you’ll want to be led through your waiting room in handcuffs.”
“Wait a minute,” I replied nervously. “I haven’t received a jury summons for 2 years, at least. There must be some mistake.”
“Perhaps we’ve confused you with a citizen with the same or a similar name,” he said. “Let me have your Social Security number and birth date.”
Alarm bells! “You should have that information already,” I replied. “Why don’t you read me what you have?”
A short silence, and then … click.
I immediately called the courthouse. “Citizens who fail to appear receive a warning letter and a new questionnaire, not a phone call,” said the jury manager. “And we use driver license numbers to keep track of jurors.”
The phone company traced the call, which dead-ended at a VoIP circuit, to no one’s surprise. The downside of VoIP (Voice over Internet Protocol) and similar technologies is that unscrupulous individuals can use them to appear to be calling you from a legitimate business when they are not.
Those of us of a certain age remember phony office calls offering great deals on supplies or waiting room magazine subscriptions. Those capers eventually disappeared; but scam artists are endlessly creative. This is especially true since the Internet took over, well, everything. There’s a real dark side to the information age.
The jury duty scheme, I learned, is an increasingly popular one. Others involve calls or e-mails from the “fraud department” of your bank, claiming to be investigating a breach of your account, or one of your credit or debit cards. Another purports to be a “Customs official” informing you that you owe a big duty payment on an overseas shipment. Victims of power outages due to natural disasters are hearing from crooks claiming to be from the local power company; the power won’t be restored, they say, without an advance payment.
In most cases, the common denominator – and the biggest red flag – is a request for a social security number, a birth date, a credit card number, or other private information that could be used to steal your identity or empty your accounts.
Here’s a summary of what my recent experience taught (or reminded) me:
- Never give out a bank account, social security, or credit card number online or over the telephone if you didn’t initiate the contact, no matter how legitimate the caller sounds. This is true of anyone claiming to be from a bank, a service company, or a government office, as well as anyone trying to sell you anything.
- No federal or state court will call to say you’ve missed jury duty – or that they are assembling jury pools and need to “prescreen” those who might be selected to serve on them. The jury manager I spoke with said she knew of no reason why anyone in my state would ever be called about jury service before mailing back a completed questionnaire, and even then, such a call would be extraordinary.
- Never send anyone a “commission” or “finder’s fee” as a condition of receiving funds. In legitimate transactions, such fees are merely deducted from the money being paid out.
- Examine your credit card and bank account statements each month. Immediately challenge any charges you don’t recognize.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Factors critical to reducing US maternal mortality and morbidity
More women die from pregnancy complications in the United States than in any other developed country. The United States is the only industrialized nation with a rising maternal mortality rate.
Those 2 sentences should stop us all in our tracks.
In fact, the United States ranks 47th globally with the worst maternal mortality rate. More than half these deaths are likely preventable, with suicide and drug overdose the leading causes of maternal death in many states. All this occurs despite our advanced medical system, premier medical colleges and universities, embrace of high-tech medical advances, and high percentage of gross domestic product spent on health care.
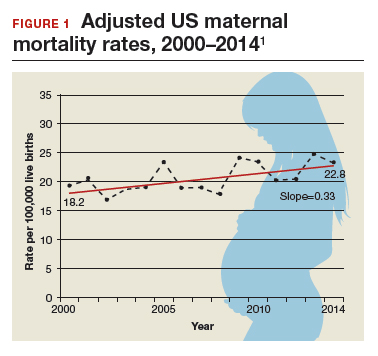
Need more numbers? According to a 2016 report in Obstetrics and Gynecology, the United States saw a 26% increase in the maternalmortality rate (unadjusted) in only 15 years: from 18.8 deaths per 100,000 live births in 2000 to 23.8 in 2014 (FIGURE 1).1
This problem received federal attention when, in 2000, the US Department of Health and Human Services launched Healthy People 2010. That health promotion and disease prevention agenda set a goal of reducing maternal mortality to 3.3 deaths per 100,000 live births by 2010, a goal clearly not met.
Considerable variations by race and by state
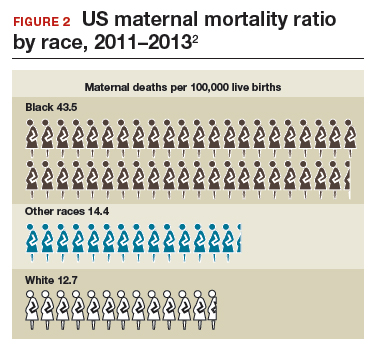
The racial disparities in maternal mortality are staggering and have not improved in more than 20 years: African American women are 3.4 times more likely to die than non-Hispanic white women of pregnancy-related complications. In 2011–2013, the maternal mortality ratio for non-Hispanic white women was 12.7 deaths per 100,000 live births compared with 43.5 deaths for non-Hispanic black women (FIGURE 2).2 American Indian or Alaska Native women, Asian women, and some Latina women also experience higher rates than non-Hispanic white women. The rate for American Indian or Alaska Native women is 16.9 deaths per 100,000 live births.3
Some states are doing better than others, showing that there is nothing inevitable about the maternal mortality crisis. Texas, for example, has seen the highest rate of maternal mortality increase. Its rate doubled from 2010 to 2012, while California reduced its maternal death rate by 30%, from 21.5 to 15.1, during roughly the same period.1
This is a challenge of epic proportions, and one that the American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Haywood Brown, MD, and Incoming President Lisa Hollier, MD, is determined to meet, ensuring that a high maternal death rate does not become our nation’s new normal.
Dr. Brown put it this way, “ACOG collaborative initiatives such as Levels of Maternal Care (LOMC) and implementation of OB safety bundles for hemorrhage, hypertension, and thromboembolism through the AIM [Alliance for Innovation on Maternal Health] Program target maternal morbidity and mortality at the community level. Bundles have also been developed to address the disparity in maternal mortality and for the opiate crisis.”
ACOG is making strides in putting in place nationwide meaningful, evidence-driven systems and care approaches that are proven to reduce maternal mortality and morbidity, saving mothers’ lives and keeping families whole.
Read about the AIM Program’s initiatives
ACOG’s AIM Program established to make an impact
The AIM Program (www.safehealthcare foreverywoman.org) is bringing together clinicians, public health officials, hospital administrators, patient safety organizations, and advocates to eliminate preventable maternal mortality throughout the United States. With funding and support from the US Health Resources and Services Administration, AIM is striving to:
- reduce maternal mortality by 1,000 deaths by 2018
- reduce severe maternal morbidity
- assist states and hospitals to improve outcomes
- create and encourage use of maternal safety bundles (evidence-based tool kits to guide the best care).
AIM offers participating physicians and hospitals online learning modules, checklists, work plans, and links to tool kits and published resources. Implementation data is shared with hospitals and states to further improve care. Physicians participating in AIM can receive Part IV maintenance of certification; continuing education units will soon be offered for nurses. In the future, AIM-participating hospitals may be able to receive reduced liability protection costs, too.
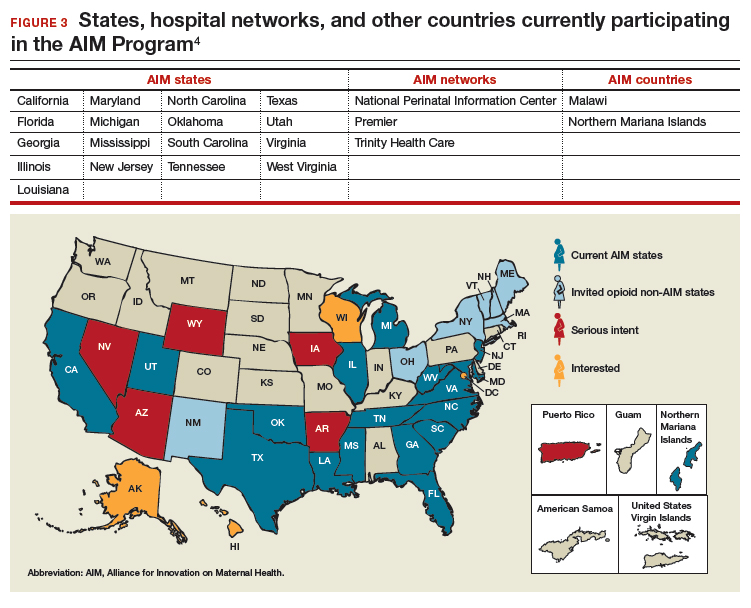
To date, 17 states are participating in the AIM initiative (FIGURE 3), with more states ready to enroll.4 States must demonstrate a commitment to lasting change to participate. Each AIM state must have an active maternal mortality review committee (MMRC); committed leadership from public health, hospital associations, and provider associations; and a commitment to report AIM data.
AIM thus far has released 9 obstetric patient safety bundles, including:
- reducing disparities in maternity care
- severe hypertension in pregnancy
- safe reduction of primary cesarean birth
- prevention of venous thromboembolism
- obstetric hemorrhage
- maternal mental health
- patient, family, and staff support following a severe maternal event
- postpartum care basics
- obstetric care of women with opioid use disorder (in use by Illinois, Massachusetts, Maryland, New Jersey, Maine, New Hampshire, Vermont, New York, Ohio, Oklahoma, Tennessee, Texas, and Virginia).
Read about how active MMRCS are critical to success
Review committees are critical to success
In use in many states, MMRCs are groups of local ObGyns, nurses, social workers, and other health care professionals who review specific cases of maternal deaths from their local area and recommend local solutions to prevent future deaths. MMRCs can be a critically important source of data to help us understand the underlying causes of maternal mortality.
Remember California’s success in reducing its maternal mortality rate, previously mentioned? That state was an early adopter of an active MMRC and has worked to bring best practices to maternity care throughout the state.
While every state should have an active MMRC, not every state does. ACOG is working with states, local leaders, and state and federal legislatures to help develop MMRCs in every state.
Dr. Brown pointed out that, “For several decades, Indiana had a legislatively authorized multidisciplinary maternal mortality review committee that I actively participated in and led in the late 1990s. The authorization for the program lapsed in the early 2000s, and the Indiana MMRC had to shut down. Bolstering the federal government’s capacity to help states like Indiana rebuild MMRCs, or start them from scratch, will help state public health officials, hospitals, and physicians take better care of moms and babies.”
Dr. Hollier explained, “In Texas, I chair our Maternal Mortality and Morbidity Task Force, which was legislatively authorized in 2013 in response to the rising rate of maternal death. The detailed state-based maternal mortality reviews provide critical information: verification of vital statistics data, assessment of the causes and contributing factors, and determination of pregnancy relatedness. These reviews identify opportunities for prevention and implementation of the most appropriate interventions to reduce maternal mortality on a local level. Support of essential review functions at the federal level would also enable data to be combined across jurisdictions for national learning that was previously not possible.”
Pending legislation will strengthen efforts
ACOG is working to enact into law the Preventing Maternal Deaths Act, HR 1318 and S1112. This is bipartisan legislation under which the Centers for Disease Control and Prevention would help states create or expand MMRCs and will require the Department of Health and Human Services to research ways to reduce disparities in maternal health outcomes.
Acknowledgement
The author thanks Jean Mahoney, ACOG’s Senior Director, AIM, for her generous assistance.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacDorman MF, Declerq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455.
- Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Updated November 9, 2017. Accessed February 16, 2018.
- Singh GK. Maternal mortality in the United States, 1935−2007: Substantial racial/ethnic, socioeconomic, and geographic disparities persist. A 75th Anniversary Publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, Maryland: US Department of Health and Human Services; 2010. https://www.hrsa.gov/sites/default/files/ourstories/mchb75th/mchb75maternalmortality.pdf. Accessed February 16, 2018.
- Council on Patient Safety in Women’s Health Care. Alliance for Innovation on Maternal Health Program: AIM states and systems. http://safehealthcareforeverywoman.org/aim-states-systems-2/#link_tab-1513011413196-9. Accessed February 20, 2018.
More women die from pregnancy complications in the United States than in any other developed country. The United States is the only industrialized nation with a rising maternal mortality rate.
Those 2 sentences should stop us all in our tracks.
In fact, the United States ranks 47th globally with the worst maternal mortality rate. More than half these deaths are likely preventable, with suicide and drug overdose the leading causes of maternal death in many states. All this occurs despite our advanced medical system, premier medical colleges and universities, embrace of high-tech medical advances, and high percentage of gross domestic product spent on health care.
Need more numbers? According to a 2016 report in Obstetrics and Gynecology, the United States saw a 26% increase in the maternalmortality rate (unadjusted) in only 15 years: from 18.8 deaths per 100,000 live births in 2000 to 23.8 in 2014 (FIGURE 1).1

This problem received federal attention when, in 2000, the US Department of Health and Human Services launched Healthy People 2010. That health promotion and disease prevention agenda set a goal of reducing maternal mortality to 3.3 deaths per 100,000 live births by 2010, a goal clearly not met.
Considerable variations by race and by state
The racial disparities in maternal mortality are staggering and have not improved in more than 20 years: African American women are 3.4 times more likely to die than non-Hispanic white women of pregnancy-related complications. In 2011–2013, the maternal mortality ratio for non-Hispanic white women was 12.7 deaths per 100,000 live births compared with 43.5 deaths for non-Hispanic black women (FIGURE 2).2 American Indian or Alaska Native women, Asian women, and some Latina women also experience higher rates than non-Hispanic white women. The rate for American Indian or Alaska Native women is 16.9 deaths per 100,000 live births.3

Some states are doing better than others, showing that there is nothing inevitable about the maternal mortality crisis. Texas, for example, has seen the highest rate of maternal mortality increase. Its rate doubled from 2010 to 2012, while California reduced its maternal death rate by 30%, from 21.5 to 15.1, during roughly the same period.1
This is a challenge of epic proportions, and one that the American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Haywood Brown, MD, and Incoming President Lisa Hollier, MD, is determined to meet, ensuring that a high maternal death rate does not become our nation’s new normal.
Dr. Brown put it this way, “ACOG collaborative initiatives such as Levels of Maternal Care (LOMC) and implementation of OB safety bundles for hemorrhage, hypertension, and thromboembolism through the AIM [Alliance for Innovation on Maternal Health] Program target maternal morbidity and mortality at the community level. Bundles have also been developed to address the disparity in maternal mortality and for the opiate crisis.”
ACOG is making strides in putting in place nationwide meaningful, evidence-driven systems and care approaches that are proven to reduce maternal mortality and morbidity, saving mothers’ lives and keeping families whole.
Read about the AIM Program’s initiatives
ACOG’s AIM Program established to make an impact
The AIM Program (www.safehealthcare foreverywoman.org) is bringing together clinicians, public health officials, hospital administrators, patient safety organizations, and advocates to eliminate preventable maternal mortality throughout the United States. With funding and support from the US Health Resources and Services Administration, AIM is striving to:
- reduce maternal mortality by 1,000 deaths by 2018
- reduce severe maternal morbidity
- assist states and hospitals to improve outcomes
- create and encourage use of maternal safety bundles (evidence-based tool kits to guide the best care).
AIM offers participating physicians and hospitals online learning modules, checklists, work plans, and links to tool kits and published resources. Implementation data is shared with hospitals and states to further improve care. Physicians participating in AIM can receive Part IV maintenance of certification; continuing education units will soon be offered for nurses. In the future, AIM-participating hospitals may be able to receive reduced liability protection costs, too.
To date, 17 states are participating in the AIM initiative (FIGURE 3), with more states ready to enroll.4 States must demonstrate a commitment to lasting change to participate. Each AIM state must have an active maternal mortality review committee (MMRC); committed leadership from public health, hospital associations, and provider associations; and a commitment to report AIM data.

AIM thus far has released 9 obstetric patient safety bundles, including:
- reducing disparities in maternity care
- severe hypertension in pregnancy
- safe reduction of primary cesarean birth
- prevention of venous thromboembolism
- obstetric hemorrhage
- maternal mental health
- patient, family, and staff support following a severe maternal event
- postpartum care basics
- obstetric care of women with opioid use disorder (in use by Illinois, Massachusetts, Maryland, New Jersey, Maine, New Hampshire, Vermont, New York, Ohio, Oklahoma, Tennessee, Texas, and Virginia).
Read about how active MMRCS are critical to success
Review committees are critical to success
In use in many states, MMRCs are groups of local ObGyns, nurses, social workers, and other health care professionals who review specific cases of maternal deaths from their local area and recommend local solutions to prevent future deaths. MMRCs can be a critically important source of data to help us understand the underlying causes of maternal mortality.
Remember California’s success in reducing its maternal mortality rate, previously mentioned? That state was an early adopter of an active MMRC and has worked to bring best practices to maternity care throughout the state.
While every state should have an active MMRC, not every state does. ACOG is working with states, local leaders, and state and federal legislatures to help develop MMRCs in every state.
Dr. Brown pointed out that, “For several decades, Indiana had a legislatively authorized multidisciplinary maternal mortality review committee that I actively participated in and led in the late 1990s. The authorization for the program lapsed in the early 2000s, and the Indiana MMRC had to shut down. Bolstering the federal government’s capacity to help states like Indiana rebuild MMRCs, or start them from scratch, will help state public health officials, hospitals, and physicians take better care of moms and babies.”
Dr. Hollier explained, “In Texas, I chair our Maternal Mortality and Morbidity Task Force, which was legislatively authorized in 2013 in response to the rising rate of maternal death. The detailed state-based maternal mortality reviews provide critical information: verification of vital statistics data, assessment of the causes and contributing factors, and determination of pregnancy relatedness. These reviews identify opportunities for prevention and implementation of the most appropriate interventions to reduce maternal mortality on a local level. Support of essential review functions at the federal level would also enable data to be combined across jurisdictions for national learning that was previously not possible.”
Pending legislation will strengthen efforts
ACOG is working to enact into law the Preventing Maternal Deaths Act, HR 1318 and S1112. This is bipartisan legislation under which the Centers for Disease Control and Prevention would help states create or expand MMRCs and will require the Department of Health and Human Services to research ways to reduce disparities in maternal health outcomes.
Acknowledgement
The author thanks Jean Mahoney, ACOG’s Senior Director, AIM, for her generous assistance.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
More women die from pregnancy complications in the United States than in any other developed country. The United States is the only industrialized nation with a rising maternal mortality rate.
Those 2 sentences should stop us all in our tracks.
In fact, the United States ranks 47th globally with the worst maternal mortality rate. More than half these deaths are likely preventable, with suicide and drug overdose the leading causes of maternal death in many states. All this occurs despite our advanced medical system, premier medical colleges and universities, embrace of high-tech medical advances, and high percentage of gross domestic product spent on health care.
Need more numbers? According to a 2016 report in Obstetrics and Gynecology, the United States saw a 26% increase in the maternalmortality rate (unadjusted) in only 15 years: from 18.8 deaths per 100,000 live births in 2000 to 23.8 in 2014 (FIGURE 1).1

This problem received federal attention when, in 2000, the US Department of Health and Human Services launched Healthy People 2010. That health promotion and disease prevention agenda set a goal of reducing maternal mortality to 3.3 deaths per 100,000 live births by 2010, a goal clearly not met.
Considerable variations by race and by state
The racial disparities in maternal mortality are staggering and have not improved in more than 20 years: African American women are 3.4 times more likely to die than non-Hispanic white women of pregnancy-related complications. In 2011–2013, the maternal mortality ratio for non-Hispanic white women was 12.7 deaths per 100,000 live births compared with 43.5 deaths for non-Hispanic black women (FIGURE 2).2 American Indian or Alaska Native women, Asian women, and some Latina women also experience higher rates than non-Hispanic white women. The rate for American Indian or Alaska Native women is 16.9 deaths per 100,000 live births.3

Some states are doing better than others, showing that there is nothing inevitable about the maternal mortality crisis. Texas, for example, has seen the highest rate of maternal mortality increase. Its rate doubled from 2010 to 2012, while California reduced its maternal death rate by 30%, from 21.5 to 15.1, during roughly the same period.1
This is a challenge of epic proportions, and one that the American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Haywood Brown, MD, and Incoming President Lisa Hollier, MD, is determined to meet, ensuring that a high maternal death rate does not become our nation’s new normal.
Dr. Brown put it this way, “ACOG collaborative initiatives such as Levels of Maternal Care (LOMC) and implementation of OB safety bundles for hemorrhage, hypertension, and thromboembolism through the AIM [Alliance for Innovation on Maternal Health] Program target maternal morbidity and mortality at the community level. Bundles have also been developed to address the disparity in maternal mortality and for the opiate crisis.”
ACOG is making strides in putting in place nationwide meaningful, evidence-driven systems and care approaches that are proven to reduce maternal mortality and morbidity, saving mothers’ lives and keeping families whole.
Read about the AIM Program’s initiatives
ACOG’s AIM Program established to make an impact
The AIM Program (www.safehealthcare foreverywoman.org) is bringing together clinicians, public health officials, hospital administrators, patient safety organizations, and advocates to eliminate preventable maternal mortality throughout the United States. With funding and support from the US Health Resources and Services Administration, AIM is striving to:
- reduce maternal mortality by 1,000 deaths by 2018
- reduce severe maternal morbidity
- assist states and hospitals to improve outcomes
- create and encourage use of maternal safety bundles (evidence-based tool kits to guide the best care).
AIM offers participating physicians and hospitals online learning modules, checklists, work plans, and links to tool kits and published resources. Implementation data is shared with hospitals and states to further improve care. Physicians participating in AIM can receive Part IV maintenance of certification; continuing education units will soon be offered for nurses. In the future, AIM-participating hospitals may be able to receive reduced liability protection costs, too.
To date, 17 states are participating in the AIM initiative (FIGURE 3), with more states ready to enroll.4 States must demonstrate a commitment to lasting change to participate. Each AIM state must have an active maternal mortality review committee (MMRC); committed leadership from public health, hospital associations, and provider associations; and a commitment to report AIM data.

AIM thus far has released 9 obstetric patient safety bundles, including:
- reducing disparities in maternity care
- severe hypertension in pregnancy
- safe reduction of primary cesarean birth
- prevention of venous thromboembolism
- obstetric hemorrhage
- maternal mental health
- patient, family, and staff support following a severe maternal event
- postpartum care basics
- obstetric care of women with opioid use disorder (in use by Illinois, Massachusetts, Maryland, New Jersey, Maine, New Hampshire, Vermont, New York, Ohio, Oklahoma, Tennessee, Texas, and Virginia).
Read about how active MMRCS are critical to success
Review committees are critical to success
In use in many states, MMRCs are groups of local ObGyns, nurses, social workers, and other health care professionals who review specific cases of maternal deaths from their local area and recommend local solutions to prevent future deaths. MMRCs can be a critically important source of data to help us understand the underlying causes of maternal mortality.
Remember California’s success in reducing its maternal mortality rate, previously mentioned? That state was an early adopter of an active MMRC and has worked to bring best practices to maternity care throughout the state.
While every state should have an active MMRC, not every state does. ACOG is working with states, local leaders, and state and federal legislatures to help develop MMRCs in every state.
Dr. Brown pointed out that, “For several decades, Indiana had a legislatively authorized multidisciplinary maternal mortality review committee that I actively participated in and led in the late 1990s. The authorization for the program lapsed in the early 2000s, and the Indiana MMRC had to shut down. Bolstering the federal government’s capacity to help states like Indiana rebuild MMRCs, or start them from scratch, will help state public health officials, hospitals, and physicians take better care of moms and babies.”
Dr. Hollier explained, “In Texas, I chair our Maternal Mortality and Morbidity Task Force, which was legislatively authorized in 2013 in response to the rising rate of maternal death. The detailed state-based maternal mortality reviews provide critical information: verification of vital statistics data, assessment of the causes and contributing factors, and determination of pregnancy relatedness. These reviews identify opportunities for prevention and implementation of the most appropriate interventions to reduce maternal mortality on a local level. Support of essential review functions at the federal level would also enable data to be combined across jurisdictions for national learning that was previously not possible.”
Pending legislation will strengthen efforts
ACOG is working to enact into law the Preventing Maternal Deaths Act, HR 1318 and S1112. This is bipartisan legislation under which the Centers for Disease Control and Prevention would help states create or expand MMRCs and will require the Department of Health and Human Services to research ways to reduce disparities in maternal health outcomes.
Acknowledgement
The author thanks Jean Mahoney, ACOG’s Senior Director, AIM, for her generous assistance.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacDorman MF, Declerq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455.
- Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Updated November 9, 2017. Accessed February 16, 2018.
- Singh GK. Maternal mortality in the United States, 1935−2007: Substantial racial/ethnic, socioeconomic, and geographic disparities persist. A 75th Anniversary Publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, Maryland: US Department of Health and Human Services; 2010. https://www.hrsa.gov/sites/default/files/ourstories/mchb75th/mchb75maternalmortality.pdf. Accessed February 16, 2018.
- Council on Patient Safety in Women’s Health Care. Alliance for Innovation on Maternal Health Program: AIM states and systems. http://safehealthcareforeverywoman.org/aim-states-systems-2/#link_tab-1513011413196-9. Accessed February 20, 2018.
- MacDorman MF, Declerq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455.
- Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Updated November 9, 2017. Accessed February 16, 2018.
- Singh GK. Maternal mortality in the United States, 1935−2007: Substantial racial/ethnic, socioeconomic, and geographic disparities persist. A 75th Anniversary Publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, Maryland: US Department of Health and Human Services; 2010. https://www.hrsa.gov/sites/default/files/ourstories/mchb75th/mchb75maternalmortality.pdf. Accessed February 16, 2018.
- Council on Patient Safety in Women’s Health Care. Alliance for Innovation on Maternal Health Program: AIM states and systems. http://safehealthcareforeverywoman.org/aim-states-systems-2/#link_tab-1513011413196-9. Accessed February 20, 2018.
The role of patient-reported outcomes in women’s health
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
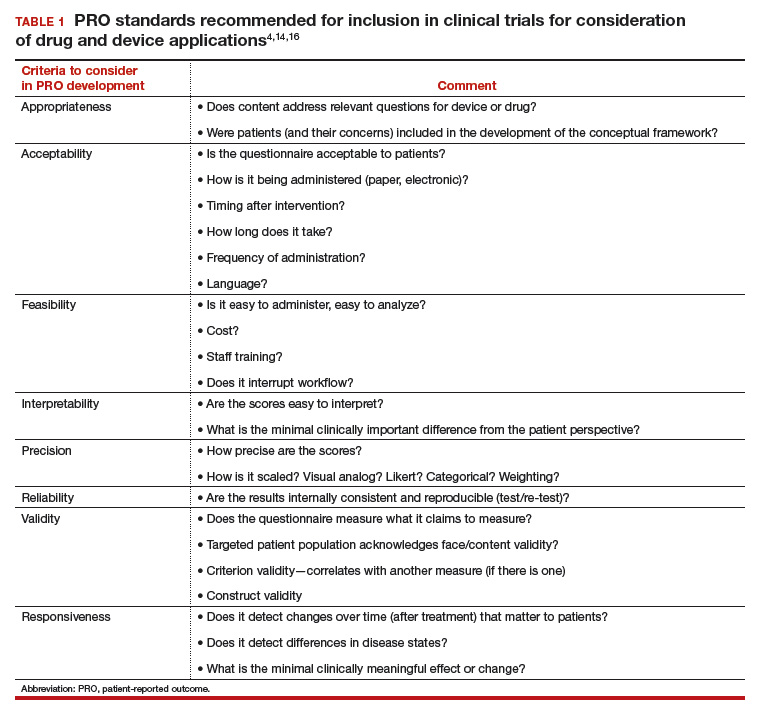
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16
Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
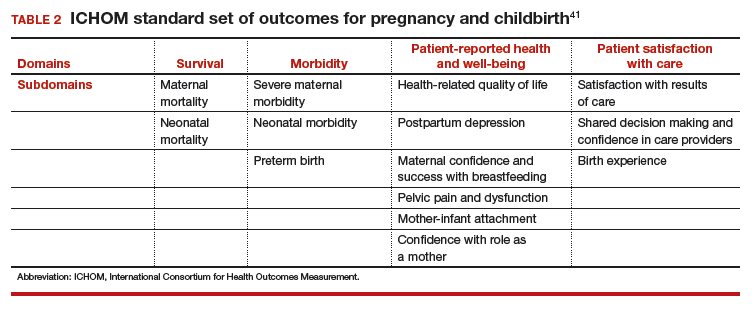
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.
Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16

Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.

Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16

Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.

Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost
Sharpening the saw
Few movies have universal appeal these days, but one that comes close is Bill Murray’s 1993 classic, “Groundhog Day,” in which Murray’s character is trapped in a time loop, living the same day over and over until he finally “gets it right.”
One reason that this film resonates with so many, I think, is that we are all, in essence, similarly trapped. Not in a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lesson, or a long weekend away with my wife. And we take longer vacations, without fail, each year.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations. It all averages out in the end.
Besides, this is much more important than money: This is breaking the routine, clearing the cobwebs, living your life. And trust me, your practice will still be there when you return.
More than once I’ve recounted the story of K. Alexander Müller, PhD, and J. Georg Bednorz, PhD, the Swiss Nobel laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Dr. Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Dr. Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you. Any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few movies have universal appeal these days, but one that comes close is Bill Murray’s 1993 classic, “Groundhog Day,” in which Murray’s character is trapped in a time loop, living the same day over and over until he finally “gets it right.”
One reason that this film resonates with so many, I think, is that we are all, in essence, similarly trapped. Not in a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lesson, or a long weekend away with my wife. And we take longer vacations, without fail, each year.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations. It all averages out in the end.
Besides, this is much more important than money: This is breaking the routine, clearing the cobwebs, living your life. And trust me, your practice will still be there when you return.
More than once I’ve recounted the story of K. Alexander Müller, PhD, and J. Georg Bednorz, PhD, the Swiss Nobel laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Dr. Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Dr. Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you. Any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few movies have universal appeal these days, but one that comes close is Bill Murray’s 1993 classic, “Groundhog Day,” in which Murray’s character is trapped in a time loop, living the same day over and over until he finally “gets it right.”
One reason that this film resonates with so many, I think, is that we are all, in essence, similarly trapped. Not in a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lesson, or a long weekend away with my wife. And we take longer vacations, without fail, each year.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations. It all averages out in the end.
Besides, this is much more important than money: This is breaking the routine, clearing the cobwebs, living your life. And trust me, your practice will still be there when you return.
More than once I’ve recounted the story of K. Alexander Müller, PhD, and J. Georg Bednorz, PhD, the Swiss Nobel laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Dr. Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Dr. Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you. Any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
What makes a quality “quality measure”?
The future of health care is value-based care. If Value equals Quality divided by Cost, then a defined, validated way to measure Quality is paramount to that equation. (Fortunately, Cost comes with convenient measurement units called dollars.) Payers now are asking health care providers to shift from a fee-for-service to a value-based reimbursement structure to encourage providers to deliver the best care at the lowest cost. Providers who can embrace this data-driven paradigm will succeed in this new environment.
So how do we define high-quality care? What makes a good quality measure? How do you actually measure what happens in a clinical encounter that impacts health outcomes?
To answer these questions, organizations have constructed standardized clinical quality measures. Clinical quality measures facilitate value-based care by providing a metric on which to measure a patient’s quality of care. They can be used 1) to decrease the overuse, underuse, and misuse of health care services and 2) to measure patient engagement and satisfaction with care.
What are quality measures?
The Academy of Medicine (formerly named the Institute of Medicine) defines health care quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1
Clearly defined components and terminology. From a quantitative standpoint, quality measures must have a clearly defined numerator and denominator and appropriate inclusions, exclusions, and exceptions. These components need to be expressed clearly in terms of publicly available terminologies, such as ICD (International Classification of Diseases) codes or SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms) terms. A measure that asks if “antihypertensive meds” have been given will not nearly be as specific as one that asks if “labetalol IV, or hydralazine IV, or nifedipine SL” has been administered. The decision to tie the data elements in a measure to administrative data, such as ICD codes, or to clinical data, such as SNOMED CT, also affects how these measures can be calculated.
Moving targets. The target of the measure also must carefully be considered. Quality measures can be used to evaluate care across the full range of health care settings—from individual providers, to care teams, to hospitals and hospital systems, to health plans. While some measures easily can be assigned to a specific provider, others are not as straightforward. For example, who gets assigned the cesarean delivery when a midwife turns the case over to an obstetrician?
Timeframe in outcomes measurement. The data infrastructure is currently set up to support measurement of immediate events, 30-day or 90-day episodes, and health insurance plan member years. Longer-term outcomes, such as over 5- and 10- year periods, are out of reach for most measures. To obtain an accurate view of the impact of medical interventions or disease conditions, however, it will be important to follow patients over time. For example, to know the failure rate of intrauterine systems, sterilization, or hormonal contraceptives, it is important to be able to track pregnancy occurrence during use of these methods for longer than 90 days. Failures can occur years after a method is initiated.
Another example is to create a performance measure focused on the overall improvement in quality of life and costs related to different treatments for abnormal uterine bleeding. How does the patient experience vary over time between treatment with hormonal contraception, endometrial ablation, or hysterectomy? Which option is most “valuable” over time when the patient experience and the cost are assessed for more than a 90-day episode? These important questions need to be answered as we maneuver into a value-based health system.
Risk adjustment. Quality measures also may need to be risk adjusted. The “My patients are sicker” refrain must be accounted for with full transparency and based on the best available data. Quality measures can be adjusted using an Observed/Expected factor, which helps to account for complicated cases.2
Clearly, social and behavioral determinants of health also play a role in these adjustments, but it can be more challenging to acquire the data elements needed for those types of adjustments. Including these data enables us to evaluate health disparities between populations, both demographically and socioeconomically.3 This is important for future development of minority inclusive quality measures. Some racial and ethnic minority populations have poorer health outcomes from preventable and treatable diseases. Evidence shows that these groups have differences in access to health care, quality of care, and health measures, including life expectancy and maternal mortality. Access to clinical data through quality measures allows for these health disparities to be brought into quantifiable perspective and assists in the development of future incentive programs to combat health inequalities and provide improved delivery of care.
Read about how to develop quality measures
Developing quality measures
Quality measures generally fall into 4 broad categories: structure, process, outcome, and patient experience (TABLE).4,5 Quality measure development begins with an assessment of the evidence, which is usually derived from clinical guidelines that link a particular process, structure, or outcome with improved patient health or experience of care. For example, the American College of Obstetricians and Gynecologists (ACOG) has developed a clinical practice guideline for screening, diagnosing, and managing gestational diabetes. The guideline addresses drug therapies, such as insulin, and alternative treatments, such as nutrition therapy. Much like the process for creating the guideline itself, translating the guideline into a quality measure requires a thoughtful, transparent, and well-defined process.
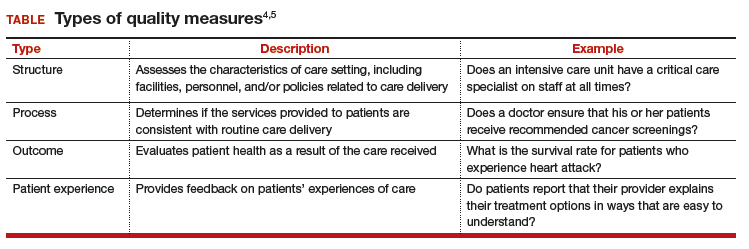
Role of the quality measure steward. Coordinating the process of translating evidence-based guidelines into quality measures requires a measure steward. Measure stewards usually are government agencies, nonprofit organizations, and/or for-profit companies. During the development process, the steward usually reaches out to additional stakeholders for feedback and consensus. Development process steps include:
- evaluation of the evidence, including the clinical practice guideline(s)
- consensus on the best measurement approach (consider the feasibility of the measurement and how it will be collected)
- development of detailed measure specifications (that is, what will be measured and how)
- feedback on the specifications from stakeholders, including professional societies and patient advocates
- testing of the measure logic and clinical validity against clinical data
- final approval by the measure steward.
Endorsement of quality measures. After a quality measure is developed, it is often endorsed by government agencies, professional societies, and/or consumer groups. Endorsement is a consensus-based process in which stakeholders evaluate a proposed measure based on established standards. Generally, stakeholders include health care professionals, consumers, payers, hospitals, health plans, and government agencies.
Evaluation of quality measures includes these important considerations:
- Are the necessary data fields available in a typical electronic health record (EHR) system?
- What is the data quality for those data fields?
- Can the measure be calculated reliably across different data sets or EHRs?
- Does the measure address one of the National Academy of Medicine quality properties? According to the academy, quality in the context of clinical care can be defined in terms of properties of effectiveness, equity, safety, efficiency, patient centeredness, and timeliness.1
Read about ACOG’s role in developing quality measures
ACOG’s role in developing quality measures
In October 2016, the Centers for Medicare and Medicaid Services released the final Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). Under this rule, the Merit-based Incentive Payment System (MIPS) was created, which was intended to drive “value” rather than “volume” in payment incentives. Measures are critical to defining value-based care. However, the law has limited or no impact on providers who do not care for Medicare patients.
Clinicians eligible to participate in MACRA must bill more than $90,000 a year in Medicare Part B allowed charges and provide care for more than 200 Medicare patients per year.6 This means that the MIPS largely overlooks ObGyns, as the bulk of our patients are insured either by private insurance or by Medicaid. However, maternity care spending is a significant part of both Medicaid and private insurers’ outlay, and both payers are actively considering using value-based financial models that will need to be fed by quality metrics. ACOG wants to be at the forefront of measure development for quality metrics that affect members and has committed resources to formation of a measure development team.
ACOG wants providers to be in control of how their practices are evaluated. For this reason, ACOG is focusing on measures that are based on clinical data entered by providers into an EHR at the point of care. At the same time, ACOG is cognizant of not increasing the documentation burden for providers. Understanding the quality of the data, as opposed to the quality of care, will be a fundamental task for the maternity care registry that ACOG is launching in 2018.
What can ObGyns do?
Quality measures are about more than just money. Public reporting of these measures on government and payer websites may influence public perception of a practice.7 The focus on patient-centered care means that patients have a voice in their care, financially as well as literally, so expect to see increased scrutiny of provider performance by patients as well as payers. One way to measure patient experience of treatments, symptoms, and quality of life is through patient-reported outcome measures (PROMs). Assessing PROMs in routine care ensures that information only the patient can provide is collected and analyzed, thus further enhancing the delivery of care and evaluating how that care is impacting the lives of your patients.
The transition from fee-for-service to a value-based system will not happen overnight, but it will happen. This transition—from being paid for the quantity of documentation to the quality of documentation—will require some change management, rethinking of workflows, and better documentation tools (such as apps instead of EHR customization).
Many in the medical profession are actively exploring these changes and new developments. These changes are too important to leave to administrators, coders, scribes, app developers, and policy makers. Someone in your practice, hospital, or health system is working on these issues today. Tomorrow, you need to be at the table. The voices of practicing ObGyns are critical as we work to address the current challenging environment in which we spend more per capita than any other nation with far inferior results. Measures that matter to us and to our patients will help us provide better and more cost-effective care that payers and patients value.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Academy of Sciences. Crossing the quality chasm: the IOM Health Care Quality Initiative. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Updated January 2, 2018. Accessed January 11, 2018.
- Agency for Healthcare Research and Quality. Selecting quality and resource use measures: a decision guide for community quality collaboratives. Part 2. Introduction to measures of quality (continued). https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/perfmeasguide/perfmeaspt2a.html. Reviewed 2014. Accessed December 12, 2017.
- Thomas SB, Fine MJ, Ibrahim SA. Health disparities: the importance of culture and health communication. Am J Public Health. 2004;94(12):2050.
- Agency for Healthcare Research and Quality. Types of quality measures. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/types.html. Reviewed 2011. Accessed December 12, 2017.
- Agency for Healthcare Research and Quality. Understanding quality measurement. https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/chtoolbx/understand/index.html. Reviewed November 2017. Accessed December 12, 2017.
- Centers for Medicare and Medicaid Services. Quality payment program. https://www.cms.gov/Medicare/Quality-Payment-Program/resource-library/QPP-Year-2-Final-Rule-Fact-Sheet.pdf. Published December 2017. Accessed December 12, 2017.
- Howell EA, Zeitlin J, Hebert PL, Balbierz, A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541.
- Tooker J. The importance of measuring quality and performance in healthcare. MedGenMed. 2005;7(2):49.
The future of health care is value-based care. If Value equals Quality divided by Cost, then a defined, validated way to measure Quality is paramount to that equation. (Fortunately, Cost comes with convenient measurement units called dollars.) Payers now are asking health care providers to shift from a fee-for-service to a value-based reimbursement structure to encourage providers to deliver the best care at the lowest cost. Providers who can embrace this data-driven paradigm will succeed in this new environment.
So how do we define high-quality care? What makes a good quality measure? How do you actually measure what happens in a clinical encounter that impacts health outcomes?
To answer these questions, organizations have constructed standardized clinical quality measures. Clinical quality measures facilitate value-based care by providing a metric on which to measure a patient’s quality of care. They can be used 1) to decrease the overuse, underuse, and misuse of health care services and 2) to measure patient engagement and satisfaction with care.
What are quality measures?
The Academy of Medicine (formerly named the Institute of Medicine) defines health care quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1
Clearly defined components and terminology. From a quantitative standpoint, quality measures must have a clearly defined numerator and denominator and appropriate inclusions, exclusions, and exceptions. These components need to be expressed clearly in terms of publicly available terminologies, such as ICD (International Classification of Diseases) codes or SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms) terms. A measure that asks if “antihypertensive meds” have been given will not nearly be as specific as one that asks if “labetalol IV, or hydralazine IV, or nifedipine SL” has been administered. The decision to tie the data elements in a measure to administrative data, such as ICD codes, or to clinical data, such as SNOMED CT, also affects how these measures can be calculated.
Moving targets. The target of the measure also must carefully be considered. Quality measures can be used to evaluate care across the full range of health care settings—from individual providers, to care teams, to hospitals and hospital systems, to health plans. While some measures easily can be assigned to a specific provider, others are not as straightforward. For example, who gets assigned the cesarean delivery when a midwife turns the case over to an obstetrician?
Timeframe in outcomes measurement. The data infrastructure is currently set up to support measurement of immediate events, 30-day or 90-day episodes, and health insurance plan member years. Longer-term outcomes, such as over 5- and 10- year periods, are out of reach for most measures. To obtain an accurate view of the impact of medical interventions or disease conditions, however, it will be important to follow patients over time. For example, to know the failure rate of intrauterine systems, sterilization, or hormonal contraceptives, it is important to be able to track pregnancy occurrence during use of these methods for longer than 90 days. Failures can occur years after a method is initiated.
Another example is to create a performance measure focused on the overall improvement in quality of life and costs related to different treatments for abnormal uterine bleeding. How does the patient experience vary over time between treatment with hormonal contraception, endometrial ablation, or hysterectomy? Which option is most “valuable” over time when the patient experience and the cost are assessed for more than a 90-day episode? These important questions need to be answered as we maneuver into a value-based health system.
Risk adjustment. Quality measures also may need to be risk adjusted. The “My patients are sicker” refrain must be accounted for with full transparency and based on the best available data. Quality measures can be adjusted using an Observed/Expected factor, which helps to account for complicated cases.2
Clearly, social and behavioral determinants of health also play a role in these adjustments, but it can be more challenging to acquire the data elements needed for those types of adjustments. Including these data enables us to evaluate health disparities between populations, both demographically and socioeconomically.3 This is important for future development of minority inclusive quality measures. Some racial and ethnic minority populations have poorer health outcomes from preventable and treatable diseases. Evidence shows that these groups have differences in access to health care, quality of care, and health measures, including life expectancy and maternal mortality. Access to clinical data through quality measures allows for these health disparities to be brought into quantifiable perspective and assists in the development of future incentive programs to combat health inequalities and provide improved delivery of care.
Read about how to develop quality measures
Developing quality measures
Quality measures generally fall into 4 broad categories: structure, process, outcome, and patient experience (TABLE).4,5 Quality measure development begins with an assessment of the evidence, which is usually derived from clinical guidelines that link a particular process, structure, or outcome with improved patient health or experience of care. For example, the American College of Obstetricians and Gynecologists (ACOG) has developed a clinical practice guideline for screening, diagnosing, and managing gestational diabetes. The guideline addresses drug therapies, such as insulin, and alternative treatments, such as nutrition therapy. Much like the process for creating the guideline itself, translating the guideline into a quality measure requires a thoughtful, transparent, and well-defined process.

Role of the quality measure steward. Coordinating the process of translating evidence-based guidelines into quality measures requires a measure steward. Measure stewards usually are government agencies, nonprofit organizations, and/or for-profit companies. During the development process, the steward usually reaches out to additional stakeholders for feedback and consensus. Development process steps include:
- evaluation of the evidence, including the clinical practice guideline(s)
- consensus on the best measurement approach (consider the feasibility of the measurement and how it will be collected)
- development of detailed measure specifications (that is, what will be measured and how)
- feedback on the specifications from stakeholders, including professional societies and patient advocates
- testing of the measure logic and clinical validity against clinical data
- final approval by the measure steward.
Endorsement of quality measures. After a quality measure is developed, it is often endorsed by government agencies, professional societies, and/or consumer groups. Endorsement is a consensus-based process in which stakeholders evaluate a proposed measure based on established standards. Generally, stakeholders include health care professionals, consumers, payers, hospitals, health plans, and government agencies.
Evaluation of quality measures includes these important considerations:
- Are the necessary data fields available in a typical electronic health record (EHR) system?
- What is the data quality for those data fields?
- Can the measure be calculated reliably across different data sets or EHRs?
- Does the measure address one of the National Academy of Medicine quality properties? According to the academy, quality in the context of clinical care can be defined in terms of properties of effectiveness, equity, safety, efficiency, patient centeredness, and timeliness.1
Read about ACOG’s role in developing quality measures
ACOG’s role in developing quality measures
In October 2016, the Centers for Medicare and Medicaid Services released the final Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). Under this rule, the Merit-based Incentive Payment System (MIPS) was created, which was intended to drive “value” rather than “volume” in payment incentives. Measures are critical to defining value-based care. However, the law has limited or no impact on providers who do not care for Medicare patients.
Clinicians eligible to participate in MACRA must bill more than $90,000 a year in Medicare Part B allowed charges and provide care for more than 200 Medicare patients per year.6 This means that the MIPS largely overlooks ObGyns, as the bulk of our patients are insured either by private insurance or by Medicaid. However, maternity care spending is a significant part of both Medicaid and private insurers’ outlay, and both payers are actively considering using value-based financial models that will need to be fed by quality metrics. ACOG wants to be at the forefront of measure development for quality metrics that affect members and has committed resources to formation of a measure development team.
ACOG wants providers to be in control of how their practices are evaluated. For this reason, ACOG is focusing on measures that are based on clinical data entered by providers into an EHR at the point of care. At the same time, ACOG is cognizant of not increasing the documentation burden for providers. Understanding the quality of the data, as opposed to the quality of care, will be a fundamental task for the maternity care registry that ACOG is launching in 2018.
What can ObGyns do?
Quality measures are about more than just money. Public reporting of these measures on government and payer websites may influence public perception of a practice.7 The focus on patient-centered care means that patients have a voice in their care, financially as well as literally, so expect to see increased scrutiny of provider performance by patients as well as payers. One way to measure patient experience of treatments, symptoms, and quality of life is through patient-reported outcome measures (PROMs). Assessing PROMs in routine care ensures that information only the patient can provide is collected and analyzed, thus further enhancing the delivery of care and evaluating how that care is impacting the lives of your patients.
The transition from fee-for-service to a value-based system will not happen overnight, but it will happen. This transition—from being paid for the quantity of documentation to the quality of documentation—will require some change management, rethinking of workflows, and better documentation tools (such as apps instead of EHR customization).
Many in the medical profession are actively exploring these changes and new developments. These changes are too important to leave to administrators, coders, scribes, app developers, and policy makers. Someone in your practice, hospital, or health system is working on these issues today. Tomorrow, you need to be at the table. The voices of practicing ObGyns are critical as we work to address the current challenging environment in which we spend more per capita than any other nation with far inferior results. Measures that matter to us and to our patients will help us provide better and more cost-effective care that payers and patients value.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The future of health care is value-based care. If Value equals Quality divided by Cost, then a defined, validated way to measure Quality is paramount to that equation. (Fortunately, Cost comes with convenient measurement units called dollars.) Payers now are asking health care providers to shift from a fee-for-service to a value-based reimbursement structure to encourage providers to deliver the best care at the lowest cost. Providers who can embrace this data-driven paradigm will succeed in this new environment.
So how do we define high-quality care? What makes a good quality measure? How do you actually measure what happens in a clinical encounter that impacts health outcomes?
To answer these questions, organizations have constructed standardized clinical quality measures. Clinical quality measures facilitate value-based care by providing a metric on which to measure a patient’s quality of care. They can be used 1) to decrease the overuse, underuse, and misuse of health care services and 2) to measure patient engagement and satisfaction with care.
What are quality measures?
The Academy of Medicine (formerly named the Institute of Medicine) defines health care quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1
Clearly defined components and terminology. From a quantitative standpoint, quality measures must have a clearly defined numerator and denominator and appropriate inclusions, exclusions, and exceptions. These components need to be expressed clearly in terms of publicly available terminologies, such as ICD (International Classification of Diseases) codes or SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms) terms. A measure that asks if “antihypertensive meds” have been given will not nearly be as specific as one that asks if “labetalol IV, or hydralazine IV, or nifedipine SL” has been administered. The decision to tie the data elements in a measure to administrative data, such as ICD codes, or to clinical data, such as SNOMED CT, also affects how these measures can be calculated.
Moving targets. The target of the measure also must carefully be considered. Quality measures can be used to evaluate care across the full range of health care settings—from individual providers, to care teams, to hospitals and hospital systems, to health plans. While some measures easily can be assigned to a specific provider, others are not as straightforward. For example, who gets assigned the cesarean delivery when a midwife turns the case over to an obstetrician?
Timeframe in outcomes measurement. The data infrastructure is currently set up to support measurement of immediate events, 30-day or 90-day episodes, and health insurance plan member years. Longer-term outcomes, such as over 5- and 10- year periods, are out of reach for most measures. To obtain an accurate view of the impact of medical interventions or disease conditions, however, it will be important to follow patients over time. For example, to know the failure rate of intrauterine systems, sterilization, or hormonal contraceptives, it is important to be able to track pregnancy occurrence during use of these methods for longer than 90 days. Failures can occur years after a method is initiated.
Another example is to create a performance measure focused on the overall improvement in quality of life and costs related to different treatments for abnormal uterine bleeding. How does the patient experience vary over time between treatment with hormonal contraception, endometrial ablation, or hysterectomy? Which option is most “valuable” over time when the patient experience and the cost are assessed for more than a 90-day episode? These important questions need to be answered as we maneuver into a value-based health system.
Risk adjustment. Quality measures also may need to be risk adjusted. The “My patients are sicker” refrain must be accounted for with full transparency and based on the best available data. Quality measures can be adjusted using an Observed/Expected factor, which helps to account for complicated cases.2
Clearly, social and behavioral determinants of health also play a role in these adjustments, but it can be more challenging to acquire the data elements needed for those types of adjustments. Including these data enables us to evaluate health disparities between populations, both demographically and socioeconomically.3 This is important for future development of minority inclusive quality measures. Some racial and ethnic minority populations have poorer health outcomes from preventable and treatable diseases. Evidence shows that these groups have differences in access to health care, quality of care, and health measures, including life expectancy and maternal mortality. Access to clinical data through quality measures allows for these health disparities to be brought into quantifiable perspective and assists in the development of future incentive programs to combat health inequalities and provide improved delivery of care.
Read about how to develop quality measures
Developing quality measures
Quality measures generally fall into 4 broad categories: structure, process, outcome, and patient experience (TABLE).4,5 Quality measure development begins with an assessment of the evidence, which is usually derived from clinical guidelines that link a particular process, structure, or outcome with improved patient health or experience of care. For example, the American College of Obstetricians and Gynecologists (ACOG) has developed a clinical practice guideline for screening, diagnosing, and managing gestational diabetes. The guideline addresses drug therapies, such as insulin, and alternative treatments, such as nutrition therapy. Much like the process for creating the guideline itself, translating the guideline into a quality measure requires a thoughtful, transparent, and well-defined process.

Role of the quality measure steward. Coordinating the process of translating evidence-based guidelines into quality measures requires a measure steward. Measure stewards usually are government agencies, nonprofit organizations, and/or for-profit companies. During the development process, the steward usually reaches out to additional stakeholders for feedback and consensus. Development process steps include:
- evaluation of the evidence, including the clinical practice guideline(s)
- consensus on the best measurement approach (consider the feasibility of the measurement and how it will be collected)
- development of detailed measure specifications (that is, what will be measured and how)
- feedback on the specifications from stakeholders, including professional societies and patient advocates
- testing of the measure logic and clinical validity against clinical data
- final approval by the measure steward.
Endorsement of quality measures. After a quality measure is developed, it is often endorsed by government agencies, professional societies, and/or consumer groups. Endorsement is a consensus-based process in which stakeholders evaluate a proposed measure based on established standards. Generally, stakeholders include health care professionals, consumers, payers, hospitals, health plans, and government agencies.
Evaluation of quality measures includes these important considerations:
- Are the necessary data fields available in a typical electronic health record (EHR) system?
- What is the data quality for those data fields?
- Can the measure be calculated reliably across different data sets or EHRs?
- Does the measure address one of the National Academy of Medicine quality properties? According to the academy, quality in the context of clinical care can be defined in terms of properties of effectiveness, equity, safety, efficiency, patient centeredness, and timeliness.1
Read about ACOG’s role in developing quality measures
ACOG’s role in developing quality measures
In October 2016, the Centers for Medicare and Medicaid Services released the final Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). Under this rule, the Merit-based Incentive Payment System (MIPS) was created, which was intended to drive “value” rather than “volume” in payment incentives. Measures are critical to defining value-based care. However, the law has limited or no impact on providers who do not care for Medicare patients.
Clinicians eligible to participate in MACRA must bill more than $90,000 a year in Medicare Part B allowed charges and provide care for more than 200 Medicare patients per year.6 This means that the MIPS largely overlooks ObGyns, as the bulk of our patients are insured either by private insurance or by Medicaid. However, maternity care spending is a significant part of both Medicaid and private insurers’ outlay, and both payers are actively considering using value-based financial models that will need to be fed by quality metrics. ACOG wants to be at the forefront of measure development for quality metrics that affect members and has committed resources to formation of a measure development team.
ACOG wants providers to be in control of how their practices are evaluated. For this reason, ACOG is focusing on measures that are based on clinical data entered by providers into an EHR at the point of care. At the same time, ACOG is cognizant of not increasing the documentation burden for providers. Understanding the quality of the data, as opposed to the quality of care, will be a fundamental task for the maternity care registry that ACOG is launching in 2018.
What can ObGyns do?
Quality measures are about more than just money. Public reporting of these measures on government and payer websites may influence public perception of a practice.7 The focus on patient-centered care means that patients have a voice in their care, financially as well as literally, so expect to see increased scrutiny of provider performance by patients as well as payers. One way to measure patient experience of treatments, symptoms, and quality of life is through patient-reported outcome measures (PROMs). Assessing PROMs in routine care ensures that information only the patient can provide is collected and analyzed, thus further enhancing the delivery of care and evaluating how that care is impacting the lives of your patients.
The transition from fee-for-service to a value-based system will not happen overnight, but it will happen. This transition—from being paid for the quantity of documentation to the quality of documentation—will require some change management, rethinking of workflows, and better documentation tools (such as apps instead of EHR customization).
Many in the medical profession are actively exploring these changes and new developments. These changes are too important to leave to administrators, coders, scribes, app developers, and policy makers. Someone in your practice, hospital, or health system is working on these issues today. Tomorrow, you need to be at the table. The voices of practicing ObGyns are critical as we work to address the current challenging environment in which we spend more per capita than any other nation with far inferior results. Measures that matter to us and to our patients will help us provide better and more cost-effective care that payers and patients value.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Academy of Sciences. Crossing the quality chasm: the IOM Health Care Quality Initiative. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Updated January 2, 2018. Accessed January 11, 2018.
- Agency for Healthcare Research and Quality. Selecting quality and resource use measures: a decision guide for community quality collaboratives. Part 2. Introduction to measures of quality (continued). https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/perfmeasguide/perfmeaspt2a.html. Reviewed 2014. Accessed December 12, 2017.
- Thomas SB, Fine MJ, Ibrahim SA. Health disparities: the importance of culture and health communication. Am J Public Health. 2004;94(12):2050.
- Agency for Healthcare Research and Quality. Types of quality measures. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/types.html. Reviewed 2011. Accessed December 12, 2017.
- Agency for Healthcare Research and Quality. Understanding quality measurement. https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/chtoolbx/understand/index.html. Reviewed November 2017. Accessed December 12, 2017.
- Centers for Medicare and Medicaid Services. Quality payment program. https://www.cms.gov/Medicare/Quality-Payment-Program/resource-library/QPP-Year-2-Final-Rule-Fact-Sheet.pdf. Published December 2017. Accessed December 12, 2017.
- Howell EA, Zeitlin J, Hebert PL, Balbierz, A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541.
- Tooker J. The importance of measuring quality and performance in healthcare. MedGenMed. 2005;7(2):49.
- National Academy of Sciences. Crossing the quality chasm: the IOM Health Care Quality Initiative. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Updated January 2, 2018. Accessed January 11, 2018.
- Agency for Healthcare Research and Quality. Selecting quality and resource use measures: a decision guide for community quality collaboratives. Part 2. Introduction to measures of quality (continued). https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/perfmeasguide/perfmeaspt2a.html. Reviewed 2014. Accessed December 12, 2017.
- Thomas SB, Fine MJ, Ibrahim SA. Health disparities: the importance of culture and health communication. Am J Public Health. 2004;94(12):2050.
- Agency for Healthcare Research and Quality. Types of quality measures. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/types.html. Reviewed 2011. Accessed December 12, 2017.
- Agency for Healthcare Research and Quality. Understanding quality measurement. https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/chtoolbx/understand/index.html. Reviewed November 2017. Accessed December 12, 2017.
- Centers for Medicare and Medicaid Services. Quality payment program. https://www.cms.gov/Medicare/Quality-Payment-Program/resource-library/QPP-Year-2-Final-Rule-Fact-Sheet.pdf. Published December 2017. Accessed December 12, 2017.
- Howell EA, Zeitlin J, Hebert PL, Balbierz, A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541.
- Tooker J. The importance of measuring quality and performance in healthcare. MedGenMed. 2005;7(2):49.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost
How to set up your own RSS feed
In my last column, I reviewed the reasons why RSS news feeds can be a useful tool for keeping abreast on frequently updated information, including blog entries, news headlines, audio, and video, without having to check multiple Web pages every day.
This can help increase readership on your website, publicize a podcast, or keep your patients up to date on the latest treatments and procedures in your practice. And if your name appears in news or gossip sites, you will be alerted immediately.
Alternatively, many organizations that publish their own articles and news stories use a content management system (CMS) to organize, store, and publish their material, including RSS feeds. Examples include Drupal and Plone, which are both free, open source programs. Stand-alone RSS creation programs also exist; one popular example is RSS Builder, also a free and open source.
Disadvantages of free systems include advertisements (which can sometimes be removed for a monthly fee) and little or no technical support – and you will probably be limited to a single feed. You’ll also have to add and update headlines, links, and descriptive text manually. Your free feed can become quite expensive if you or staffers are forced to spend an inordinate amount of time maintaining it. Paid RSS editors like FeedForAll and NewzAlert Composer allow easier and less time-consuming content creation and maintenance.
Once you have picked a service or application, you can create your first feed, a process that will be different from program to program. But all feeds will need some basic data: a name (which should be the same as your practice or website); the URL for your website, to help viewers link back to your home page; and a description – a sentence or two describing the general content on the feed.
The next step is to populate the feed with content. Enter the title of each article, blog post, podcast episode, etc.; the URL that links directly to that content; and the publishing date. Each entry should have its own short, interesting description, which is what potential readers will see before they choose to click your entry in their RSS readers, and a global unique identifier (GUID), which the RSS readers use to detect changes or updates.
When all of your content is entered, all that remains is to export your feed to an extensible markup language (XML) file, which will allow visitors to subscribe to it. Upload the XML file to your website, place it on your home page, and click the “publish feed” button.
Once your feed is live, you’ll want to list it on some of the many RSS feed directories to maximize its visibility on search engines. There are literally hundreds of such directories; look for medically oriented ones that do not charge fees, and do not require a reciprocal link back to their website. Add each directory’s URL to your XML file.
Addendum: In my December 2017 column (“Your Online Reputation”), I suggested encouraging your most devoted patients to post favorable reviews about you on the “rating” websites. Several readers (including a practice consultant) have suggested making a laptop or tablet available in your office for that purpose. While that sounds like a great idea, most rating portals track incoming IP addresses, and automatically reject multiple reviews originating from the same computer.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
In my last column, I reviewed the reasons why RSS news feeds can be a useful tool for keeping abreast on frequently updated information, including blog entries, news headlines, audio, and video, without having to check multiple Web pages every day.
This can help increase readership on your website, publicize a podcast, or keep your patients up to date on the latest treatments and procedures in your practice. And if your name appears in news or gossip sites, you will be alerted immediately.
Alternatively, many organizations that publish their own articles and news stories use a content management system (CMS) to organize, store, and publish their material, including RSS feeds. Examples include Drupal and Plone, which are both free, open source programs. Stand-alone RSS creation programs also exist; one popular example is RSS Builder, also a free and open source.
Disadvantages of free systems include advertisements (which can sometimes be removed for a monthly fee) and little or no technical support – and you will probably be limited to a single feed. You’ll also have to add and update headlines, links, and descriptive text manually. Your free feed can become quite expensive if you or staffers are forced to spend an inordinate amount of time maintaining it. Paid RSS editors like FeedForAll and NewzAlert Composer allow easier and less time-consuming content creation and maintenance.
Once you have picked a service or application, you can create your first feed, a process that will be different from program to program. But all feeds will need some basic data: a name (which should be the same as your practice or website); the URL for your website, to help viewers link back to your home page; and a description – a sentence or two describing the general content on the feed.
The next step is to populate the feed with content. Enter the title of each article, blog post, podcast episode, etc.; the URL that links directly to that content; and the publishing date. Each entry should have its own short, interesting description, which is what potential readers will see before they choose to click your entry in their RSS readers, and a global unique identifier (GUID), which the RSS readers use to detect changes or updates.
When all of your content is entered, all that remains is to export your feed to an extensible markup language (XML) file, which will allow visitors to subscribe to it. Upload the XML file to your website, place it on your home page, and click the “publish feed” button.
Once your feed is live, you’ll want to list it on some of the many RSS feed directories to maximize its visibility on search engines. There are literally hundreds of such directories; look for medically oriented ones that do not charge fees, and do not require a reciprocal link back to their website. Add each directory’s URL to your XML file.
Addendum: In my December 2017 column (“Your Online Reputation”), I suggested encouraging your most devoted patients to post favorable reviews about you on the “rating” websites. Several readers (including a practice consultant) have suggested making a laptop or tablet available in your office for that purpose. While that sounds like a great idea, most rating portals track incoming IP addresses, and automatically reject multiple reviews originating from the same computer.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
In my last column, I reviewed the reasons why RSS news feeds can be a useful tool for keeping abreast on frequently updated information, including blog entries, news headlines, audio, and video, without having to check multiple Web pages every day.
This can help increase readership on your website, publicize a podcast, or keep your patients up to date on the latest treatments and procedures in your practice. And if your name appears in news or gossip sites, you will be alerted immediately.
Alternatively, many organizations that publish their own articles and news stories use a content management system (CMS) to organize, store, and publish their material, including RSS feeds. Examples include Drupal and Plone, which are both free, open source programs. Stand-alone RSS creation programs also exist; one popular example is RSS Builder, also a free and open source.
Disadvantages of free systems include advertisements (which can sometimes be removed for a monthly fee) and little or no technical support – and you will probably be limited to a single feed. You’ll also have to add and update headlines, links, and descriptive text manually. Your free feed can become quite expensive if you or staffers are forced to spend an inordinate amount of time maintaining it. Paid RSS editors like FeedForAll and NewzAlert Composer allow easier and less time-consuming content creation and maintenance.
Once you have picked a service or application, you can create your first feed, a process that will be different from program to program. But all feeds will need some basic data: a name (which should be the same as your practice or website); the URL for your website, to help viewers link back to your home page; and a description – a sentence or two describing the general content on the feed.
The next step is to populate the feed with content. Enter the title of each article, blog post, podcast episode, etc.; the URL that links directly to that content; and the publishing date. Each entry should have its own short, interesting description, which is what potential readers will see before they choose to click your entry in their RSS readers, and a global unique identifier (GUID), which the RSS readers use to detect changes or updates.
When all of your content is entered, all that remains is to export your feed to an extensible markup language (XML) file, which will allow visitors to subscribe to it. Upload the XML file to your website, place it on your home page, and click the “publish feed” button.
Once your feed is live, you’ll want to list it on some of the many RSS feed directories to maximize its visibility on search engines. There are literally hundreds of such directories; look for medically oriented ones that do not charge fees, and do not require a reciprocal link back to their website. Add each directory’s URL to your XML file.
Addendum: In my December 2017 column (“Your Online Reputation”), I suggested encouraging your most devoted patients to post favorable reviews about you on the “rating” websites. Several readers (including a practice consultant) have suggested making a laptop or tablet available in your office for that purpose. While that sounds like a great idea, most rating portals track incoming IP addresses, and automatically reject multiple reviews originating from the same computer.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Value-based payment: What does it mean, and how can ObGyns get out ahead?
For ObGyns to be successful, understanding the basics of quality and cost measurement is essential, along with devoting more attention to what they are being evaluated on and held accountable for. But how will ObGyns be impacted by the push to incentivize them for delivering value in their work?
Although much of health care policy has become politically divisive lately, one area of agreement is that, in the United States, we have unsustainable health costs and the exorbitant amount our country pays for health care does not translate to improved outcomes. The United States spends more than most other developed nations on health care (roughly, $9,403 per capita in 2014) but has some of the lowest life expectancies, along with the highest maternal and infant mortality rates, compared with peer nations.1–4
One of the key culprits in our health system’s inefficiencies is the fee-for-service payment model. Fee-for-service incentivizes the delivery of a high volume of care without any way to determine whether that care is achieving the desired outcomes of improved health and quality of life. Not only does fee-for-service drive up the volume of care but it also rewards the delivery of high-cost services, regardless of whether those services provide what is best for the patient.
During the previous administration, Secretary of Health and Human Services Sylvia Mathews Burwell set goals for moving away from fee-for-service in Medicare and in the health system more broadly. Congress also passed legislation that provides incentives for Medicare providers to transition away from fee-for-service with the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). While fee-for-service remains the predominant form of payment for many physicians, value-based payment arrangements are gaining a toehold. In 2014, 86% of physicians reported working in a practice receiving fee-for-service. Those fees accounted for nearly 72% of revenue.5 This percentage likely will continue to decrease over the next few years as government and private payers seek to promote value-based payment systems.
Assessing quality
“Value” in the context of health care is often defined as quality or outcomes relative to costs.6 Before payers can reward value, there must be measurement of performance to determine the quality of care being delivered. Quality measures are tools to help quantify access to care, processes, outcomes, patient experience, and organizational structure within the health care system. ObGyns likely encounter process, outcome, and patient experience measures most frequently in their practice.
Although outcome measures are generally held as the gold standard for quality measurement, they are often hard to obtain—either because of issues of temporality and rarity of events or because the data are hard to capture through existing formats. In lieu of measuring outcomes, process measures are often used to determine whether certain services that are known to be tied to desired health outcomes were delivered. Patient experience measures are also rising in popularity and are seen as a critical tool to ensuring that care that purports to be patient-centered actually is so.
Measures are specified to different levels of accountability, ranging from the individual physician all the way to the population. Some measures also can be specified at multiple levels. One major concern is the problem of attribution—that is, the difficulty of assigning who is primarily responsible for a specific quality metric result. Because obstetrics and gynecology is an increasingly team-based specialty, the American College of Obstetricians and Gynecologists (ACOG) recommends that measures that are used to reward or penalize providers should reflect performance at the care team or practice level, not at the individual physician or health care provider level.7 As consolidation of providers continues, it is expected that team-based care will increase and that the use of advanced practice providers will increase.8
Data to determine performance can come from a variety of sources, including claims, electronic health records (EHRs), paper medical record abstraction, birth certificates, registries, surveys, and separate reporting mechanisms. There are pros and cons of these various sources. Because administrative claims data are so easily obtainable, many measures have been developed based on this data source, but there are significant limitations to assessments made with such data. These limitations include inherent problems with translating clinical diagnoses into specific codes and inadequate documentation to support particular diagnoses and procedure codes.9 Claims data are limited by what physicians and other health care providers code for in their claims, making proper coding an essential skill for ObGyns to master.
Although there has been an increase in measures that rely on clinical data found in EHRs and registries—which are more robust and capture a wider breadth of indicators—claims-based measures still form the basis for many reporting programs because of standardization and ease of access to data. Data quality will become increasingly more important in a value-based payment world because completeness, risk adjustment, and specificity will be determined by the data recorded. This need for data quality will require that improvements be made in the user interface of EHRs and that providers pay specific attention to making sure their documentation is complete. New designs for EHRs should assist in that task, and data extraction should become a by-product of documentation.10
Read about alternative payment models and how ObGyns can succeed.
Paying for value
In an attempt to move away from fee-for-service medicine, payers and employers are adopting alternative payment models (APMs) that are intended to reward physicians and other health care providers for delivering value. Although APMs can be a catchall term, the Health Care Payment Learning and Action Network (LAN), a multi-stakeholder collaborative convened by the US Centers for Medicare & Medicaid Services, has laid out a framework for the different types of APMs11 (FIGURE). This framework provides a common reference point for concepts related to value-based care.
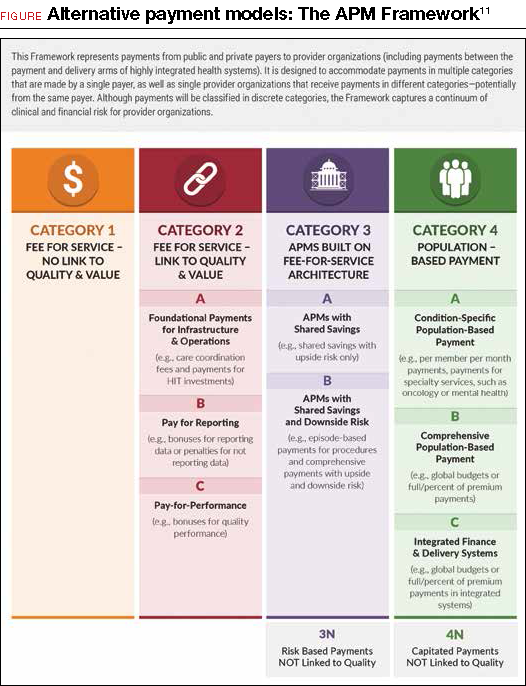
Although ACOG does not endorse all the concepts and principles included in the LAN white paper, it does support moving away from fee-for-service payments that lack any link to quality or outcomes. Originally, the LAN envisioned that all physicians, providers, and hospital systems would move in the direction of adopting Category 4 APMs, but in the recent “refresh” of the LAN’s white paper, the authors recognized that not all entities will be able to move toward population-based payments—nor will it be beneficial for all providers to do so. ACOG agrees that not all ObGyns will be able to thrive under population-based payments, so we must lead the way in developing models and measures that appropriately assess value in the care that ObGyns provide.
ACOG has undertaken its first foray into value-based payments by developing an “episode group” related to benign hysterectomy, with attendant quality measures. (An episode group is a collection of services associated with treating a condition or performing a procedure that are both clinically and temporally related.) The goal in creating episode groups is to create alignment across payers so that ObGyns are not faced with multitudinous payer-specific metrics and reporting requirements. As the benign hysterectomy episode group is refined and adopted by payers, ACOG plans to expand to other treatments and, eventually, develop condition-based episode groups that incentivize the most appropriate treatment options for patients.
Current forms of APMs are mostly Category 2 and 3 models. Rates of proper screening for cervical and breast cancer have been used as performance metrics for bonus payments. Major payers have pushed specific metrics as cutoffs for limiting narrow networks.12 For example, Covered California, the state health care exchange, has set a nulliparous term singleton vertex cesarean rate of 23.9% by 2018 as a necessary standard for inclusion of a hospital’s entire services (obstetric and nonobstetric) in their network. Episode group payments for total obstetric care included in the episode routine services, such as ultrasonography, have been previously utilized to discourage overutilization.
Such payment incentives can lead to underutilization of resources, however, which might lead to poorer outcomes and therefore result in overall greater cost. For example, poor screening for fetal anomalies or poorly managed medical conditions such as diabetes can lead to markedly increased costs in neonatal management. Therefore, some authorities have proposed tying incentives for obstetric care to performance outcome measures in neonatal care as a method of finding “sweet spots” for utilization of complex services and episode groups. Such models will depend on more robust clinical information sources and standardization.8
How can ObGyns succeed?
So what does success look like under these value-based payments for ObGyns? This is new territory, in a rapidly changing environment in which providers who flourished under the fee-for-service system will only survive under the new system if they become knowledgeable about the nuances of the new payment methods. Providers should understand that success is going to be defined as reaching the “Triple Aim”13 of improving the health of the population, containing costs, and improving the experience of health care.
Practice patient-centered care. One way to better position yourself is to focus on delivering patient-centered care and improving customer service in your practice. By implementing patient satisfaction surveys, you can identify where you are most vulnerable. One option is to utilize the Consumer Assessment of Healthcare Providers and Systems Clinician and Group Survey, developed by the US Department of Health and Human Services’ Agency for Healthcare Research & Quality. However, there are other assessment tools available, and you should investigate what works best for your practice.
Code properly. Another key to making sure you are in an optimal position is to properly document and code the services you deliver. Accurately capturing the clinical complexity of your patients will help down the road with risk adjustment and risk stratification for cost and quality measures. Many payment models, including episode groups, are built on the fee-for-service system, so coding for services is still important in the transition to alternative models. Modern EHRs are building new tools to assist clinician documentation, such as tools that aid coding. Carefully groomed and up-to-date problem lists can help providers keep track of appropriate testing and screening by enabling decision support tools that are imbedded in the systems. Although upgrading can be expensive, especially for small group practices, the development of “software as a service” or cloud-based EHRs will likely drive individual costs down.10
One example of point-of-care decision support that ACOG is spearheading to support our Fellows is the ACOG Prenatal Record (APR) by Dorsata.14 The APR is an application designed by ObGyns to work seamlessly with an existing EHR system to improve clinical workflow, save time, and help ObGyns support high-quality prenatal outcomes. The APR uses the same simplicity, flexibility, and familiarity of the original paper-based flowsheet, but in an electronic format to integrate ACOG guidance, which provides a more robust solution. The APR uses information such as gestational age, pregnancy history, the problem list, and other risk factors to provide patient and visit-specific care plans based on ACOG clinical practice guidelines. It was designed to help reduce physician burden by creating an easy-to-navigate electronic flowsheet that provides everything ObGyns need to know about each patient, succinctly captured in a single view.
ACOG also offers comprehensive coding workshops across the country and webinars on special coding topics to help Fellows learn to properly code their services. Availing yourself of these educational opportunities now so that you are better prepared to transition to value-based payment is a great way to ensure success in the future.
Chances are that some of your payers are already requiring you to report on metrics or tracking your performance using claims data. Pay attention to the performance measures that you are being held accountable for by payers when you review your payer contracts. Make sure you understand how your patients may fall into and out of the measure numerators and denominators. Ask yourself whether these metrics are ones that you can reasonably influence and that are within your control.
Of course, you can also reach out to ACOG for help. We are here to educate, inform, and guide you on these changes and provide assistance to ensure your success. Send inquiries to: [email protected].
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The World Bank. Health expenditure per capita (current US $). 2017. http://data.worldbank.org/indicator/SH.XPD.PCAP?year_high_desc=true. Accessed December 4, 2017.
- Gonzales S, Sawyer B. How does U.S. life expectancy compare to other countries? Peterson Center on Healthcare and the Kaiser Family Foundation. 2017. http://www.healthsystemtracker.org/chart-collection/u-s-life-expectancy-compare-countries/?_sf_s=life#item-start. Accessed December 4, 2017.
- World Health Organization. Trends in maternal mortality: 1990 to 2015: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. http://apps.who.int/iris/bitstream/10665/194254/1/9789241565141_eng.pdf?ua=1. Accessed December 4, 2017.
- MacDorman MF, Mathews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. Natl Vital Stat Rep. 2014;63(5):1-6.
- Kane, CK. American Medical Association Policy Research Perspectives. Payment and delivery in 2014: The prevalence of new models reported by physicians. 2015. https://www.ama-assn.org/sites/default/files/media-browser/member/health-policy/practicepay-prp2015_0.pdf. Accessed December 4, 2017.
- Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
- Task Force on Collaborative Practice. Collaboration in practice: Implementing team-based care. Washington, DC: American College of Obstetricians and Gynecologists. 2016. https://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Collaboration-in-Practice-Implementing-Team-Based-Care. Accessed December 4, 2017.
- Lagrew DC Jr, Jenkins TR. The future of obstetrics/gynecology in 2020: a clearer vision: finding true north and the forces of change. Am J Obstet Gynecol. 2014;211(6):617-622.
- Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 suppl 1):S51-S55.
- Lagrew DC Jr, Jenkins TR. The future of obstetrics/gynecology in 2020: a clearer vision. Transformational forces and thriving in the new system. Am J Obstet Gynecol. 2015;212(1):28-33.
- US Centers for Medicare & Medicaid Services. Health Care Payment Learning and Action Network. Alternative Payment Models (APM) Framework. 2017. https://innovation.cms.gov/initiatives/Health-Care-Payment-Learning-and-Action-Network/. Accessed December 4, 2017.
- Morse S. Covered California will exclude hospitals with high rates of C-sections. Healthcare Finance. 2016. http://www.healthcarefinancenews.com/news/covered-california-will-exclude-hospitals-high-rates-c-sections. Accessed December 4, 2017.
- Institute for Healthcare Improvement. The IHI Triple Aim. 2017. http://www.ihi.org/engage/initiatives/TripleAim/Pages/default.aspx. Accessed December 4, 2017.
- A pregnancy app for your EHR. 2017. https://www.dorsata.com/. Accessed December 4, 2017.
For ObGyns to be successful, understanding the basics of quality and cost measurement is essential, along with devoting more attention to what they are being evaluated on and held accountable for. But how will ObGyns be impacted by the push to incentivize them for delivering value in their work?
Although much of health care policy has become politically divisive lately, one area of agreement is that, in the United States, we have unsustainable health costs and the exorbitant amount our country pays for health care does not translate to improved outcomes. The United States spends more than most other developed nations on health care (roughly, $9,403 per capita in 2014) but has some of the lowest life expectancies, along with the highest maternal and infant mortality rates, compared with peer nations.1–4
One of the key culprits in our health system’s inefficiencies is the fee-for-service payment model. Fee-for-service incentivizes the delivery of a high volume of care without any way to determine whether that care is achieving the desired outcomes of improved health and quality of life. Not only does fee-for-service drive up the volume of care but it also rewards the delivery of high-cost services, regardless of whether those services provide what is best for the patient.
During the previous administration, Secretary of Health and Human Services Sylvia Mathews Burwell set goals for moving away from fee-for-service in Medicare and in the health system more broadly. Congress also passed legislation that provides incentives for Medicare providers to transition away from fee-for-service with the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). While fee-for-service remains the predominant form of payment for many physicians, value-based payment arrangements are gaining a toehold. In 2014, 86% of physicians reported working in a practice receiving fee-for-service. Those fees accounted for nearly 72% of revenue.5 This percentage likely will continue to decrease over the next few years as government and private payers seek to promote value-based payment systems.
Assessing quality
“Value” in the context of health care is often defined as quality or outcomes relative to costs.6 Before payers can reward value, there must be measurement of performance to determine the quality of care being delivered. Quality measures are tools to help quantify access to care, processes, outcomes, patient experience, and organizational structure within the health care system. ObGyns likely encounter process, outcome, and patient experience measures most frequently in their practice.
Although outcome measures are generally held as the gold standard for quality measurement, they are often hard to obtain—either because of issues of temporality and rarity of events or because the data are hard to capture through existing formats. In lieu of measuring outcomes, process measures are often used to determine whether certain services that are known to be tied to desired health outcomes were delivered. Patient experience measures are also rising in popularity and are seen as a critical tool to ensuring that care that purports to be patient-centered actually is so.
Measures are specified to different levels of accountability, ranging from the individual physician all the way to the population. Some measures also can be specified at multiple levels. One major concern is the problem of attribution—that is, the difficulty of assigning who is primarily responsible for a specific quality metric result. Because obstetrics and gynecology is an increasingly team-based specialty, the American College of Obstetricians and Gynecologists (ACOG) recommends that measures that are used to reward or penalize providers should reflect performance at the care team or practice level, not at the individual physician or health care provider level.7 As consolidation of providers continues, it is expected that team-based care will increase and that the use of advanced practice providers will increase.8
Data to determine performance can come from a variety of sources, including claims, electronic health records (EHRs), paper medical record abstraction, birth certificates, registries, surveys, and separate reporting mechanisms. There are pros and cons of these various sources. Because administrative claims data are so easily obtainable, many measures have been developed based on this data source, but there are significant limitations to assessments made with such data. These limitations include inherent problems with translating clinical diagnoses into specific codes and inadequate documentation to support particular diagnoses and procedure codes.9 Claims data are limited by what physicians and other health care providers code for in their claims, making proper coding an essential skill for ObGyns to master.
Although there has been an increase in measures that rely on clinical data found in EHRs and registries—which are more robust and capture a wider breadth of indicators—claims-based measures still form the basis for many reporting programs because of standardization and ease of access to data. Data quality will become increasingly more important in a value-based payment world because completeness, risk adjustment, and specificity will be determined by the data recorded. This need for data quality will require that improvements be made in the user interface of EHRs and that providers pay specific attention to making sure their documentation is complete. New designs for EHRs should assist in that task, and data extraction should become a by-product of documentation.10
Read about alternative payment models and how ObGyns can succeed.
Paying for value
In an attempt to move away from fee-for-service medicine, payers and employers are adopting alternative payment models (APMs) that are intended to reward physicians and other health care providers for delivering value. Although APMs can be a catchall term, the Health Care Payment Learning and Action Network (LAN), a multi-stakeholder collaborative convened by the US Centers for Medicare & Medicaid Services, has laid out a framework for the different types of APMs11 (FIGURE). This framework provides a common reference point for concepts related to value-based care.

Although ACOG does not endorse all the concepts and principles included in the LAN white paper, it does support moving away from fee-for-service payments that lack any link to quality or outcomes. Originally, the LAN envisioned that all physicians, providers, and hospital systems would move in the direction of adopting Category 4 APMs, but in the recent “refresh” of the LAN’s white paper, the authors recognized that not all entities will be able to move toward population-based payments—nor will it be beneficial for all providers to do so. ACOG agrees that not all ObGyns will be able to thrive under population-based payments, so we must lead the way in developing models and measures that appropriately assess value in the care that ObGyns provide.
ACOG has undertaken its first foray into value-based payments by developing an “episode group” related to benign hysterectomy, with attendant quality measures. (An episode group is a collection of services associated with treating a condition or performing a procedure that are both clinically and temporally related.) The goal in creating episode groups is to create alignment across payers so that ObGyns are not faced with multitudinous payer-specific metrics and reporting requirements. As the benign hysterectomy episode group is refined and adopted by payers, ACOG plans to expand to other treatments and, eventually, develop condition-based episode groups that incentivize the most appropriate treatment options for patients.
Current forms of APMs are mostly Category 2 and 3 models. Rates of proper screening for cervical and breast cancer have been used as performance metrics for bonus payments. Major payers have pushed specific metrics as cutoffs for limiting narrow networks.12 For example, Covered California, the state health care exchange, has set a nulliparous term singleton vertex cesarean rate of 23.9% by 2018 as a necessary standard for inclusion of a hospital’s entire services (obstetric and nonobstetric) in their network. Episode group payments for total obstetric care included in the episode routine services, such as ultrasonography, have been previously utilized to discourage overutilization.
Such payment incentives can lead to underutilization of resources, however, which might lead to poorer outcomes and therefore result in overall greater cost. For example, poor screening for fetal anomalies or poorly managed medical conditions such as diabetes can lead to markedly increased costs in neonatal management. Therefore, some authorities have proposed tying incentives for obstetric care to performance outcome measures in neonatal care as a method of finding “sweet spots” for utilization of complex services and episode groups. Such models will depend on more robust clinical information sources and standardization.8
How can ObGyns succeed?
So what does success look like under these value-based payments for ObGyns? This is new territory, in a rapidly changing environment in which providers who flourished under the fee-for-service system will only survive under the new system if they become knowledgeable about the nuances of the new payment methods. Providers should understand that success is going to be defined as reaching the “Triple Aim”13 of improving the health of the population, containing costs, and improving the experience of health care.
Practice patient-centered care. One way to better position yourself is to focus on delivering patient-centered care and improving customer service in your practice. By implementing patient satisfaction surveys, you can identify where you are most vulnerable. One option is to utilize the Consumer Assessment of Healthcare Providers and Systems Clinician and Group Survey, developed by the US Department of Health and Human Services’ Agency for Healthcare Research & Quality. However, there are other assessment tools available, and you should investigate what works best for your practice.
Code properly. Another key to making sure you are in an optimal position is to properly document and code the services you deliver. Accurately capturing the clinical complexity of your patients will help down the road with risk adjustment and risk stratification for cost and quality measures. Many payment models, including episode groups, are built on the fee-for-service system, so coding for services is still important in the transition to alternative models. Modern EHRs are building new tools to assist clinician documentation, such as tools that aid coding. Carefully groomed and up-to-date problem lists can help providers keep track of appropriate testing and screening by enabling decision support tools that are imbedded in the systems. Although upgrading can be expensive, especially for small group practices, the development of “software as a service” or cloud-based EHRs will likely drive individual costs down.10
One example of point-of-care decision support that ACOG is spearheading to support our Fellows is the ACOG Prenatal Record (APR) by Dorsata.14 The APR is an application designed by ObGyns to work seamlessly with an existing EHR system to improve clinical workflow, save time, and help ObGyns support high-quality prenatal outcomes. The APR uses the same simplicity, flexibility, and familiarity of the original paper-based flowsheet, but in an electronic format to integrate ACOG guidance, which provides a more robust solution. The APR uses information such as gestational age, pregnancy history, the problem list, and other risk factors to provide patient and visit-specific care plans based on ACOG clinical practice guidelines. It was designed to help reduce physician burden by creating an easy-to-navigate electronic flowsheet that provides everything ObGyns need to know about each patient, succinctly captured in a single view.
ACOG also offers comprehensive coding workshops across the country and webinars on special coding topics to help Fellows learn to properly code their services. Availing yourself of these educational opportunities now so that you are better prepared to transition to value-based payment is a great way to ensure success in the future.
Chances are that some of your payers are already requiring you to report on metrics or tracking your performance using claims data. Pay attention to the performance measures that you are being held accountable for by payers when you review your payer contracts. Make sure you understand how your patients may fall into and out of the measure numerators and denominators. Ask yourself whether these metrics are ones that you can reasonably influence and that are within your control.
Of course, you can also reach out to ACOG for help. We are here to educate, inform, and guide you on these changes and provide assistance to ensure your success. Send inquiries to: [email protected].
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
For ObGyns to be successful, understanding the basics of quality and cost measurement is essential, along with devoting more attention to what they are being evaluated on and held accountable for. But how will ObGyns be impacted by the push to incentivize them for delivering value in their work?
Although much of health care policy has become politically divisive lately, one area of agreement is that, in the United States, we have unsustainable health costs and the exorbitant amount our country pays for health care does not translate to improved outcomes. The United States spends more than most other developed nations on health care (roughly, $9,403 per capita in 2014) but has some of the lowest life expectancies, along with the highest maternal and infant mortality rates, compared with peer nations.1–4
One of the key culprits in our health system’s inefficiencies is the fee-for-service payment model. Fee-for-service incentivizes the delivery of a high volume of care without any way to determine whether that care is achieving the desired outcomes of improved health and quality of life. Not only does fee-for-service drive up the volume of care but it also rewards the delivery of high-cost services, regardless of whether those services provide what is best for the patient.
During the previous administration, Secretary of Health and Human Services Sylvia Mathews Burwell set goals for moving away from fee-for-service in Medicare and in the health system more broadly. Congress also passed legislation that provides incentives for Medicare providers to transition away from fee-for-service with the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). While fee-for-service remains the predominant form of payment for many physicians, value-based payment arrangements are gaining a toehold. In 2014, 86% of physicians reported working in a practice receiving fee-for-service. Those fees accounted for nearly 72% of revenue.5 This percentage likely will continue to decrease over the next few years as government and private payers seek to promote value-based payment systems.
Assessing quality
“Value” in the context of health care is often defined as quality or outcomes relative to costs.6 Before payers can reward value, there must be measurement of performance to determine the quality of care being delivered. Quality measures are tools to help quantify access to care, processes, outcomes, patient experience, and organizational structure within the health care system. ObGyns likely encounter process, outcome, and patient experience measures most frequently in their practice.
Although outcome measures are generally held as the gold standard for quality measurement, they are often hard to obtain—either because of issues of temporality and rarity of events or because the data are hard to capture through existing formats. In lieu of measuring outcomes, process measures are often used to determine whether certain services that are known to be tied to desired health outcomes were delivered. Patient experience measures are also rising in popularity and are seen as a critical tool to ensuring that care that purports to be patient-centered actually is so.
Measures are specified to different levels of accountability, ranging from the individual physician all the way to the population. Some measures also can be specified at multiple levels. One major concern is the problem of attribution—that is, the difficulty of assigning who is primarily responsible for a specific quality metric result. Because obstetrics and gynecology is an increasingly team-based specialty, the American College of Obstetricians and Gynecologists (ACOG) recommends that measures that are used to reward or penalize providers should reflect performance at the care team or practice level, not at the individual physician or health care provider level.7 As consolidation of providers continues, it is expected that team-based care will increase and that the use of advanced practice providers will increase.8
Data to determine performance can come from a variety of sources, including claims, electronic health records (EHRs), paper medical record abstraction, birth certificates, registries, surveys, and separate reporting mechanisms. There are pros and cons of these various sources. Because administrative claims data are so easily obtainable, many measures have been developed based on this data source, but there are significant limitations to assessments made with such data. These limitations include inherent problems with translating clinical diagnoses into specific codes and inadequate documentation to support particular diagnoses and procedure codes.9 Claims data are limited by what physicians and other health care providers code for in their claims, making proper coding an essential skill for ObGyns to master.
Although there has been an increase in measures that rely on clinical data found in EHRs and registries—which are more robust and capture a wider breadth of indicators—claims-based measures still form the basis for many reporting programs because of standardization and ease of access to data. Data quality will become increasingly more important in a value-based payment world because completeness, risk adjustment, and specificity will be determined by the data recorded. This need for data quality will require that improvements be made in the user interface of EHRs and that providers pay specific attention to making sure their documentation is complete. New designs for EHRs should assist in that task, and data extraction should become a by-product of documentation.10
Read about alternative payment models and how ObGyns can succeed.
Paying for value
In an attempt to move away from fee-for-service medicine, payers and employers are adopting alternative payment models (APMs) that are intended to reward physicians and other health care providers for delivering value. Although APMs can be a catchall term, the Health Care Payment Learning and Action Network (LAN), a multi-stakeholder collaborative convened by the US Centers for Medicare & Medicaid Services, has laid out a framework for the different types of APMs11 (FIGURE). This framework provides a common reference point for concepts related to value-based care.

Although ACOG does not endorse all the concepts and principles included in the LAN white paper, it does support moving away from fee-for-service payments that lack any link to quality or outcomes. Originally, the LAN envisioned that all physicians, providers, and hospital systems would move in the direction of adopting Category 4 APMs, but in the recent “refresh” of the LAN’s white paper, the authors recognized that not all entities will be able to move toward population-based payments—nor will it be beneficial for all providers to do so. ACOG agrees that not all ObGyns will be able to thrive under population-based payments, so we must lead the way in developing models and measures that appropriately assess value in the care that ObGyns provide.
ACOG has undertaken its first foray into value-based payments by developing an “episode group” related to benign hysterectomy, with attendant quality measures. (An episode group is a collection of services associated with treating a condition or performing a procedure that are both clinically and temporally related.) The goal in creating episode groups is to create alignment across payers so that ObGyns are not faced with multitudinous payer-specific metrics and reporting requirements. As the benign hysterectomy episode group is refined and adopted by payers, ACOG plans to expand to other treatments and, eventually, develop condition-based episode groups that incentivize the most appropriate treatment options for patients.
Current forms of APMs are mostly Category 2 and 3 models. Rates of proper screening for cervical and breast cancer have been used as performance metrics for bonus payments. Major payers have pushed specific metrics as cutoffs for limiting narrow networks.12 For example, Covered California, the state health care exchange, has set a nulliparous term singleton vertex cesarean rate of 23.9% by 2018 as a necessary standard for inclusion of a hospital’s entire services (obstetric and nonobstetric) in their network. Episode group payments for total obstetric care included in the episode routine services, such as ultrasonography, have been previously utilized to discourage overutilization.
Such payment incentives can lead to underutilization of resources, however, which might lead to poorer outcomes and therefore result in overall greater cost. For example, poor screening for fetal anomalies or poorly managed medical conditions such as diabetes can lead to markedly increased costs in neonatal management. Therefore, some authorities have proposed tying incentives for obstetric care to performance outcome measures in neonatal care as a method of finding “sweet spots” for utilization of complex services and episode groups. Such models will depend on more robust clinical information sources and standardization.8
How can ObGyns succeed?
So what does success look like under these value-based payments for ObGyns? This is new territory, in a rapidly changing environment in which providers who flourished under the fee-for-service system will only survive under the new system if they become knowledgeable about the nuances of the new payment methods. Providers should understand that success is going to be defined as reaching the “Triple Aim”13 of improving the health of the population, containing costs, and improving the experience of health care.
Practice patient-centered care. One way to better position yourself is to focus on delivering patient-centered care and improving customer service in your practice. By implementing patient satisfaction surveys, you can identify where you are most vulnerable. One option is to utilize the Consumer Assessment of Healthcare Providers and Systems Clinician and Group Survey, developed by the US Department of Health and Human Services’ Agency for Healthcare Research & Quality. However, there are other assessment tools available, and you should investigate what works best for your practice.
Code properly. Another key to making sure you are in an optimal position is to properly document and code the services you deliver. Accurately capturing the clinical complexity of your patients will help down the road with risk adjustment and risk stratification for cost and quality measures. Many payment models, including episode groups, are built on the fee-for-service system, so coding for services is still important in the transition to alternative models. Modern EHRs are building new tools to assist clinician documentation, such as tools that aid coding. Carefully groomed and up-to-date problem lists can help providers keep track of appropriate testing and screening by enabling decision support tools that are imbedded in the systems. Although upgrading can be expensive, especially for small group practices, the development of “software as a service” or cloud-based EHRs will likely drive individual costs down.10
One example of point-of-care decision support that ACOG is spearheading to support our Fellows is the ACOG Prenatal Record (APR) by Dorsata.14 The APR is an application designed by ObGyns to work seamlessly with an existing EHR system to improve clinical workflow, save time, and help ObGyns support high-quality prenatal outcomes. The APR uses the same simplicity, flexibility, and familiarity of the original paper-based flowsheet, but in an electronic format to integrate ACOG guidance, which provides a more robust solution. The APR uses information such as gestational age, pregnancy history, the problem list, and other risk factors to provide patient and visit-specific care plans based on ACOG clinical practice guidelines. It was designed to help reduce physician burden by creating an easy-to-navigate electronic flowsheet that provides everything ObGyns need to know about each patient, succinctly captured in a single view.
ACOG also offers comprehensive coding workshops across the country and webinars on special coding topics to help Fellows learn to properly code their services. Availing yourself of these educational opportunities now so that you are better prepared to transition to value-based payment is a great way to ensure success in the future.
Chances are that some of your payers are already requiring you to report on metrics or tracking your performance using claims data. Pay attention to the performance measures that you are being held accountable for by payers when you review your payer contracts. Make sure you understand how your patients may fall into and out of the measure numerators and denominators. Ask yourself whether these metrics are ones that you can reasonably influence and that are within your control.
Of course, you can also reach out to ACOG for help. We are here to educate, inform, and guide you on these changes and provide assistance to ensure your success. Send inquiries to: [email protected].
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The World Bank. Health expenditure per capita (current US $). 2017. http://data.worldbank.org/indicator/SH.XPD.PCAP?year_high_desc=true. Accessed December 4, 2017.
- Gonzales S, Sawyer B. How does U.S. life expectancy compare to other countries? Peterson Center on Healthcare and the Kaiser Family Foundation. 2017. http://www.healthsystemtracker.org/chart-collection/u-s-life-expectancy-compare-countries/?_sf_s=life#item-start. Accessed December 4, 2017.
- World Health Organization. Trends in maternal mortality: 1990 to 2015: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. http://apps.who.int/iris/bitstream/10665/194254/1/9789241565141_eng.pdf?ua=1. Accessed December 4, 2017.
- MacDorman MF, Mathews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. Natl Vital Stat Rep. 2014;63(5):1-6.
- Kane, CK. American Medical Association Policy Research Perspectives. Payment and delivery in 2014: The prevalence of new models reported by physicians. 2015. https://www.ama-assn.org/sites/default/files/media-browser/member/health-policy/practicepay-prp2015_0.pdf. Accessed December 4, 2017.
- Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
- Task Force on Collaborative Practice. Collaboration in practice: Implementing team-based care. Washington, DC: American College of Obstetricians and Gynecologists. 2016. https://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Collaboration-in-Practice-Implementing-Team-Based-Care. Accessed December 4, 2017.
- Lagrew DC Jr, Jenkins TR. The future of obstetrics/gynecology in 2020: a clearer vision: finding true north and the forces of change. Am J Obstet Gynecol. 2014;211(6):617-622.
- Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 suppl 1):S51-S55.
- Lagrew DC Jr, Jenkins TR. The future of obstetrics/gynecology in 2020: a clearer vision. Transformational forces and thriving in the new system. Am J Obstet Gynecol. 2015;212(1):28-33.
- US Centers for Medicare & Medicaid Services. Health Care Payment Learning and Action Network. Alternative Payment Models (APM) Framework. 2017. https://innovation.cms.gov/initiatives/Health-Care-Payment-Learning-and-Action-Network/. Accessed December 4, 2017.
- Morse S. Covered California will exclude hospitals with high rates of C-sections. Healthcare Finance. 2016. http://www.healthcarefinancenews.com/news/covered-california-will-exclude-hospitals-high-rates-c-sections. Accessed December 4, 2017.
- Institute for Healthcare Improvement. The IHI Triple Aim. 2017. http://www.ihi.org/engage/initiatives/TripleAim/Pages/default.aspx. Accessed December 4, 2017.
- A pregnancy app for your EHR. 2017. https://www.dorsata.com/. Accessed December 4, 2017.
- The World Bank. Health expenditure per capita (current US $). 2017. http://data.worldbank.org/indicator/SH.XPD.PCAP?year_high_desc=true. Accessed December 4, 2017.
- Gonzales S, Sawyer B. How does U.S. life expectancy compare to other countries? Peterson Center on Healthcare and the Kaiser Family Foundation. 2017. http://www.healthsystemtracker.org/chart-collection/u-s-life-expectancy-compare-countries/?_sf_s=life#item-start. Accessed December 4, 2017.
- World Health Organization. Trends in maternal mortality: 1990 to 2015: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. http://apps.who.int/iris/bitstream/10665/194254/1/9789241565141_eng.pdf?ua=1. Accessed December 4, 2017.
- MacDorman MF, Mathews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. Natl Vital Stat Rep. 2014;63(5):1-6.
- Kane, CK. American Medical Association Policy Research Perspectives. Payment and delivery in 2014: The prevalence of new models reported by physicians. 2015. https://www.ama-assn.org/sites/default/files/media-browser/member/health-policy/practicepay-prp2015_0.pdf. Accessed December 4, 2017.
- Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
- Task Force on Collaborative Practice. Collaboration in practice: Implementing team-based care. Washington, DC: American College of Obstetricians and Gynecologists. 2016. https://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Collaboration-in-Practice-Implementing-Team-Based-Care. Accessed December 4, 2017.
- Lagrew DC Jr, Jenkins TR. The future of obstetrics/gynecology in 2020: a clearer vision: finding true north and the forces of change. Am J Obstet Gynecol. 2014;211(6):617-622.
- Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 suppl 1):S51-S55.
- Lagrew DC Jr, Jenkins TR. The future of obstetrics/gynecology in 2020: a clearer vision. Transformational forces and thriving in the new system. Am J Obstet Gynecol. 2015;212(1):28-33.
- US Centers for Medicare & Medicaid Services. Health Care Payment Learning and Action Network. Alternative Payment Models (APM) Framework. 2017. https://innovation.cms.gov/initiatives/Health-Care-Payment-Learning-and-Action-Network/. Accessed December 4, 2017.
- Morse S. Covered California will exclude hospitals with high rates of C-sections. Healthcare Finance. 2016. http://www.healthcarefinancenews.com/news/covered-california-will-exclude-hospitals-high-rates-c-sections. Accessed December 4, 2017.
- Institute for Healthcare Improvement. The IHI Triple Aim. 2017. http://www.ihi.org/engage/initiatives/TripleAim/Pages/default.aspx. Accessed December 4, 2017.
- A pregnancy app for your EHR. 2017. https://www.dorsata.com/. Accessed December 4, 2017.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost