User login
Children and COVID: New cases up for third straight week
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.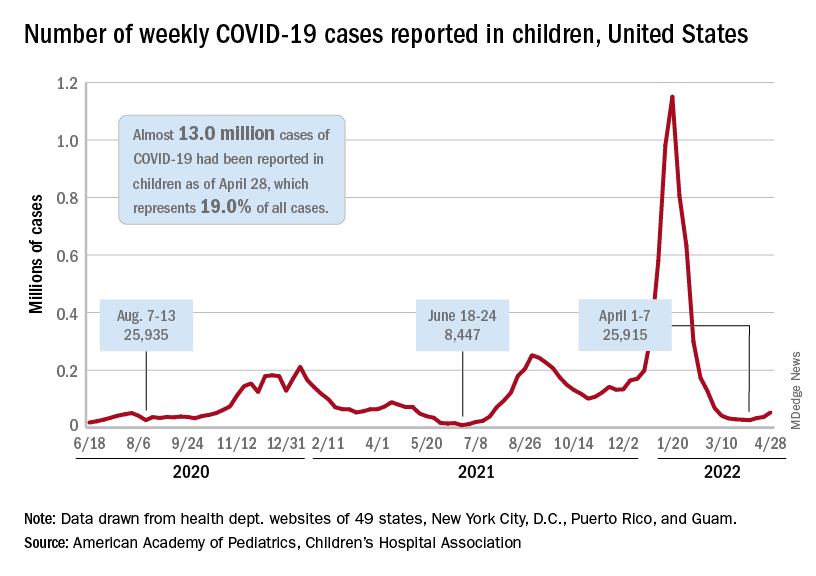
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”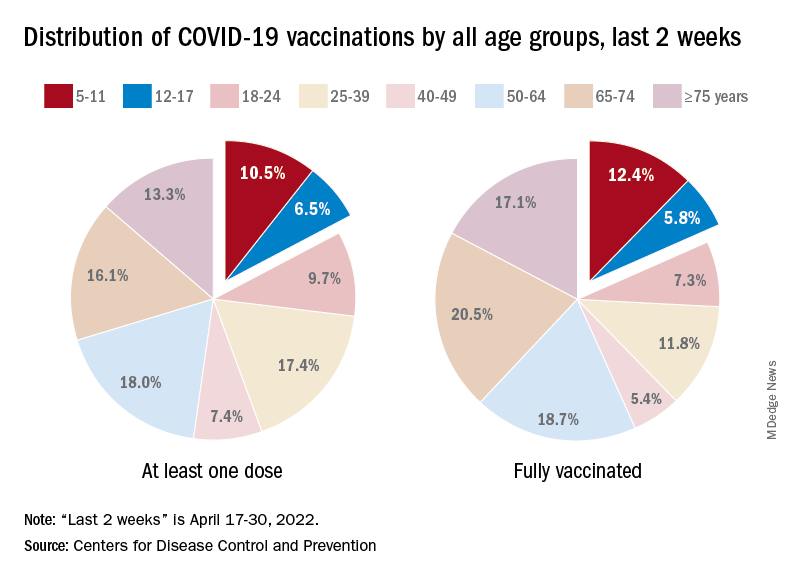
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
CDC reports first human case of H5 bird flu in the U.S.
A man who worked on a commercial poultry farm in Colorado has tested positive for avian influenza A(H5) virus, better known as H5 bird flu, the CDC announced on April 28.
This is the first case of H5 bird flu in humans in the United States and only the second case in the world, the CDC said in a news release. The first case was detected last December in a man who raised birds in the United Kingdom. That man had no symptoms.
The only symptom the man in Colorado reported was fatigue, the Colorado Department of Public Health and Environment (CDPHE) reported. He has recovered and is isolating and being treated with oseltamivir, an antiviral drug.
The CDC said the man was helping kill poultry that likely had the H5N1 bird flu.
He is a state prison inmate who was working on a commercial poultry farm in Montrose County in a prerelease employment program, the CDPHE said. The flock he was working with has been euthanized, and the response team and other inmates working on the farm were given protective equipment, the CDPHE said.
“Repeat testing on the person was negative for influenza,” the department said. “Because the person was in close contact with infected poultry, the virus may have been in the person’s nose without causing infection.”
This CDC said the case does not change the risk of bird flu for the general public, which is considered low. People who work with birds should continue to take safety precautions, such as wearing gloves when handling birds and avoiding birds that appear to be dead or ill, the CDC said.
“We want to reassure Coloradans that the risk to them is low,” said Rachel Herlihy, MD, state epidemiologist with the CDPHE. “I am grateful for the seamless collaboration between CDC, Department of Corrections, Department of Agriculture, and CDPHE, as we continue to monitor this virus and protect all Coloradans.”
The federal government says the H5N1 virus has been found in commercial and backyard birds in 29 states and in wild birds in 34 states since the first cases were detected in late 2021.
The CDC says it has tracked the health of 2,500 people exposed to birds infected with H5N1 and only found one case of human infection, in Colorado.
A version of this article first appeared on WebMD.com.
A man who worked on a commercial poultry farm in Colorado has tested positive for avian influenza A(H5) virus, better known as H5 bird flu, the CDC announced on April 28.
This is the first case of H5 bird flu in humans in the United States and only the second case in the world, the CDC said in a news release. The first case was detected last December in a man who raised birds in the United Kingdom. That man had no symptoms.
The only symptom the man in Colorado reported was fatigue, the Colorado Department of Public Health and Environment (CDPHE) reported. He has recovered and is isolating and being treated with oseltamivir, an antiviral drug.
The CDC said the man was helping kill poultry that likely had the H5N1 bird flu.
He is a state prison inmate who was working on a commercial poultry farm in Montrose County in a prerelease employment program, the CDPHE said. The flock he was working with has been euthanized, and the response team and other inmates working on the farm were given protective equipment, the CDPHE said.
“Repeat testing on the person was negative for influenza,” the department said. “Because the person was in close contact with infected poultry, the virus may have been in the person’s nose without causing infection.”
This CDC said the case does not change the risk of bird flu for the general public, which is considered low. People who work with birds should continue to take safety precautions, such as wearing gloves when handling birds and avoiding birds that appear to be dead or ill, the CDC said.
“We want to reassure Coloradans that the risk to them is low,” said Rachel Herlihy, MD, state epidemiologist with the CDPHE. “I am grateful for the seamless collaboration between CDC, Department of Corrections, Department of Agriculture, and CDPHE, as we continue to monitor this virus and protect all Coloradans.”
The federal government says the H5N1 virus has been found in commercial and backyard birds in 29 states and in wild birds in 34 states since the first cases were detected in late 2021.
The CDC says it has tracked the health of 2,500 people exposed to birds infected with H5N1 and only found one case of human infection, in Colorado.
A version of this article first appeared on WebMD.com.
A man who worked on a commercial poultry farm in Colorado has tested positive for avian influenza A(H5) virus, better known as H5 bird flu, the CDC announced on April 28.
This is the first case of H5 bird flu in humans in the United States and only the second case in the world, the CDC said in a news release. The first case was detected last December in a man who raised birds in the United Kingdom. That man had no symptoms.
The only symptom the man in Colorado reported was fatigue, the Colorado Department of Public Health and Environment (CDPHE) reported. He has recovered and is isolating and being treated with oseltamivir, an antiviral drug.
The CDC said the man was helping kill poultry that likely had the H5N1 bird flu.
He is a state prison inmate who was working on a commercial poultry farm in Montrose County in a prerelease employment program, the CDPHE said. The flock he was working with has been euthanized, and the response team and other inmates working on the farm were given protective equipment, the CDPHE said.
“Repeat testing on the person was negative for influenza,” the department said. “Because the person was in close contact with infected poultry, the virus may have been in the person’s nose without causing infection.”
This CDC said the case does not change the risk of bird flu for the general public, which is considered low. People who work with birds should continue to take safety precautions, such as wearing gloves when handling birds and avoiding birds that appear to be dead or ill, the CDC said.
“We want to reassure Coloradans that the risk to them is low,” said Rachel Herlihy, MD, state epidemiologist with the CDPHE. “I am grateful for the seamless collaboration between CDC, Department of Corrections, Department of Agriculture, and CDPHE, as we continue to monitor this virus and protect all Coloradans.”
The federal government says the H5N1 virus has been found in commercial and backyard birds in 29 states and in wild birds in 34 states since the first cases were detected in late 2021.
The CDC says it has tracked the health of 2,500 people exposed to birds infected with H5N1 and only found one case of human infection, in Colorado.
A version of this article first appeared on WebMD.com.
Upadacitinib earns FDA approval for ankylosing spondylitis
The Food and Drug Administration has approved upadacitinib (Rinvoq) as an oral treatment for active ankylosing spondylitis in adults, its manufacturer AbbVie announced April 29.
Upadacitinib, a selective and reversible Janus kinase inhibitor, is the second drug in its class to be FDA approved for ankylosing spondylitis, after tofacitinib (Xeljanz) in December.
Upadacitinib is now indicated for patients with active ankylosing spondylitis (AS) who have had an insufficient response or intolerance with one or more tumor necrosis factor (TNF) blockers. Upadacitinib is already approved by the FDA for adults with active psoriatic arthritis, moderately to severely active rheumatoid arthritis, and moderately to severely active ulcerative colitis who have had an insufficient response or intolerance with one or more TNF inhibitors. It also has been approved for adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis.
The European Medicines Agency gave marketing approval for upadacitinib in adults with active AS in January 2021.
Two main clinical studies form the basis for the FDA’s approval decision. The phase 3 SELECT-AXIS 2 clinical trial involved patients with an inadequate response or intolerance to one or two biologic disease-modifying antirheumatic drugs (bDMARDs). A total of 44.5% patients with AS who were randomly assigned to upadacitinib 15 mg once daily met the primary endpoint of at least 40% improvement in Assessment in Spondyloarthritis International Society response criteria (ASAS 40) at 14 weeks, compared against 18.2% with placebo.
The second study, the phase 2/3 SELECT-AXIS 1 clinical trial, tested upadacitinib in patients who had never taken bDMARDs and had an inadequate response or intolerance to at least two NSAIDs. In this study, significantly more patients randomly assigned to 15 mg upadacitinib achieved ASAS 40 at 14 weeks, compared with placebo (51% vs. 26%).
Patients randomly assigned to upadacitinib also showed significant improvements in signs and symptoms of AS, as well as improvements in physical function and disease activity, compared with placebo, after 14 weeks. The safety profile for patients with AS treated with upadacitinib was similar to that seen in studies of patients with rheumatoid arthritis or psoriatic arthritis. Potential severe side effects include increased risk for death in patients aged 50 years and older with at least one cardiovascular risk factor; increased risk of serious infections, such as tuberculosis; and increased risk of certain cancers, according to the company statement.
Read the complete prescribing information here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved upadacitinib (Rinvoq) as an oral treatment for active ankylosing spondylitis in adults, its manufacturer AbbVie announced April 29.
Upadacitinib, a selective and reversible Janus kinase inhibitor, is the second drug in its class to be FDA approved for ankylosing spondylitis, after tofacitinib (Xeljanz) in December.
Upadacitinib is now indicated for patients with active ankylosing spondylitis (AS) who have had an insufficient response or intolerance with one or more tumor necrosis factor (TNF) blockers. Upadacitinib is already approved by the FDA for adults with active psoriatic arthritis, moderately to severely active rheumatoid arthritis, and moderately to severely active ulcerative colitis who have had an insufficient response or intolerance with one or more TNF inhibitors. It also has been approved for adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis.
The European Medicines Agency gave marketing approval for upadacitinib in adults with active AS in January 2021.
Two main clinical studies form the basis for the FDA’s approval decision. The phase 3 SELECT-AXIS 2 clinical trial involved patients with an inadequate response or intolerance to one or two biologic disease-modifying antirheumatic drugs (bDMARDs). A total of 44.5% patients with AS who were randomly assigned to upadacitinib 15 mg once daily met the primary endpoint of at least 40% improvement in Assessment in Spondyloarthritis International Society response criteria (ASAS 40) at 14 weeks, compared against 18.2% with placebo.
The second study, the phase 2/3 SELECT-AXIS 1 clinical trial, tested upadacitinib in patients who had never taken bDMARDs and had an inadequate response or intolerance to at least two NSAIDs. In this study, significantly more patients randomly assigned to 15 mg upadacitinib achieved ASAS 40 at 14 weeks, compared with placebo (51% vs. 26%).
Patients randomly assigned to upadacitinib also showed significant improvements in signs and symptoms of AS, as well as improvements in physical function and disease activity, compared with placebo, after 14 weeks. The safety profile for patients with AS treated with upadacitinib was similar to that seen in studies of patients with rheumatoid arthritis or psoriatic arthritis. Potential severe side effects include increased risk for death in patients aged 50 years and older with at least one cardiovascular risk factor; increased risk of serious infections, such as tuberculosis; and increased risk of certain cancers, according to the company statement.
Read the complete prescribing information here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved upadacitinib (Rinvoq) as an oral treatment for active ankylosing spondylitis in adults, its manufacturer AbbVie announced April 29.
Upadacitinib, a selective and reversible Janus kinase inhibitor, is the second drug in its class to be FDA approved for ankylosing spondylitis, after tofacitinib (Xeljanz) in December.
Upadacitinib is now indicated for patients with active ankylosing spondylitis (AS) who have had an insufficient response or intolerance with one or more tumor necrosis factor (TNF) blockers. Upadacitinib is already approved by the FDA for adults with active psoriatic arthritis, moderately to severely active rheumatoid arthritis, and moderately to severely active ulcerative colitis who have had an insufficient response or intolerance with one or more TNF inhibitors. It also has been approved for adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis.
The European Medicines Agency gave marketing approval for upadacitinib in adults with active AS in January 2021.
Two main clinical studies form the basis for the FDA’s approval decision. The phase 3 SELECT-AXIS 2 clinical trial involved patients with an inadequate response or intolerance to one or two biologic disease-modifying antirheumatic drugs (bDMARDs). A total of 44.5% patients with AS who were randomly assigned to upadacitinib 15 mg once daily met the primary endpoint of at least 40% improvement in Assessment in Spondyloarthritis International Society response criteria (ASAS 40) at 14 weeks, compared against 18.2% with placebo.
The second study, the phase 2/3 SELECT-AXIS 1 clinical trial, tested upadacitinib in patients who had never taken bDMARDs and had an inadequate response or intolerance to at least two NSAIDs. In this study, significantly more patients randomly assigned to 15 mg upadacitinib achieved ASAS 40 at 14 weeks, compared with placebo (51% vs. 26%).
Patients randomly assigned to upadacitinib also showed significant improvements in signs and symptoms of AS, as well as improvements in physical function and disease activity, compared with placebo, after 14 weeks. The safety profile for patients with AS treated with upadacitinib was similar to that seen in studies of patients with rheumatoid arthritis or psoriatic arthritis. Potential severe side effects include increased risk for death in patients aged 50 years and older with at least one cardiovascular risk factor; increased risk of serious infections, such as tuberculosis; and increased risk of certain cancers, according to the company statement.
Read the complete prescribing information here.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases rise again, but more slowly
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.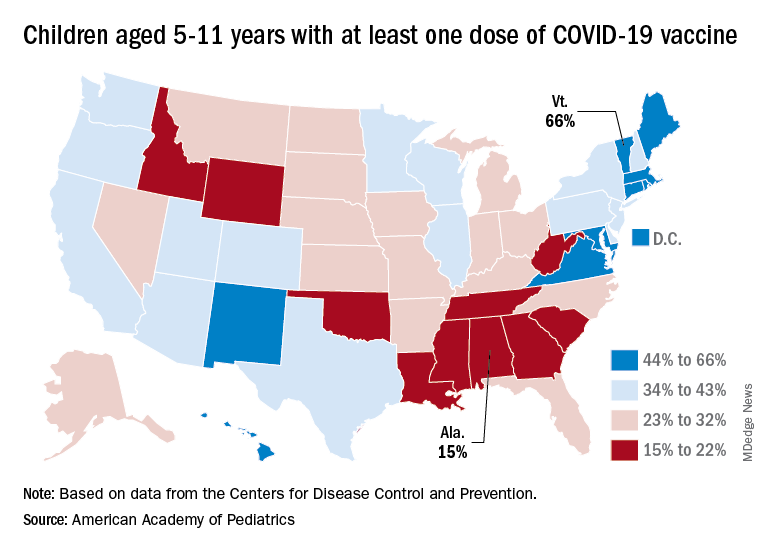
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
CDC panel lists reasons to get second COVID booster
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
Children and COVID: Decline in new cases comes to an end
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
FDA recommends switch to partially or fully disposable duodenoscope models
Health care facilities and providers should complete the transition to fully disposable duodenoscopes and those with disposable components, the U.S. Food and Drug Administration announced this week after an analysis of postmarket surveillance studies was completed.
The FDA’s directive updates its April 2020 recommendations on the subject. It cites concerns about cleaning fixed endcap duodenoscopes and the increasing availability of models that eliminate the need for reprocessing.
The announcement highlighted the potential for a dramatic difference in between-patient contamination risk, reducing it “by half or more as compared to reusable, or fixed endcaps.”
“Interim results from one duodenoscope model with a removable component show a contamination rate of just 0.5%, as compared to older duodenoscope models which had contamination rates as high as 6%,” the FDA writes.
Duodenoscopes are used in more than 500,000 procedures each year in the United States and are key in assessing and treating diseases and conditions of the pancreas and bile ducts.
Upgrade to new models to decrease infections
Manufacturers no longer market fixed endcap models in the United States, but some health care facilities continue to use them. The FDA recommends that all fixed endcap models be replaced.
The FDA says some manufacturers are offering replacement programs to upgrade to a model with a disposable component at no cost.
Two fully disposable models and five with disposable components have been cleared by the FDA. (One model is no longer marketed and thus not listed here.)
Fully Disposable:
Ambu Innovation GmbH, Duodenoscope model aScope Duodeno
Boston Scientific Corporation, EXALT Model D Single-Use Duodenoscope
Disposable Components:
Fujifilm Corporation, Duodenoscope model ED-580XT
Olympus Medical Systems, Evis Exera III Duodenovideoscope Olympus TJF-Q190V
Pentax Medical, Duodenoscope model ED34-i10T2
Pentax Medical, Duodenoscope model ED32-i10
Additionally, the failure to correctly reprocess a duodenoscope could result in tissue or fluid from one patient transferring to a subsequent patient.
“In rare cases, this can lead to patient-to-patient disease transmission,” the FDA says.
Postmarket surveillance studies
In 2015, the FDA ordered three manufacturers of reusable devices (Fujifilm, Olympus, and Pentax) to conduct postmarket surveillance studies to determine contamination rates after reprocessing.
In 2019, the FDA also ordered postmarket surveillance studies to the makers of duodenoscopes with disposable endcaps to verify that the new designs reduce the contamination rate.
The final results of the fixed endcap design indicate that contamination rates were as high as 6.6% with high-concern organisms after contamination. High-concern organisms are those more often associated with disease, such as E coli and Pseudomonas contamination.
“As a result, Pentax and Olympus are withdrawing their fixed endcap duodenoscopes from the market, and Fujifilm has completed withdrawal of its fixed endcap duodenoscope,” the FDA writes.
Studies are not yet complete for duodenoscopes with removable components. As of August 12, 2021, the Fujifilm ED-580XT duodenoscope with a removable cap had 57% of the samples required. Interim results indicate that no samples tested positive for enough low-concern organisms to indicate a reprocessing failure, and only 0.5% tested positive for high-concern organisms.
In addition to the contamination risk sampling, each manufacturer was ordered to do postmarket surveillance studies to evaluate whether staff could understand and follow the manufacturer’s reprocessing instructions in real-world health care settings.
According to the FDA, the results showed that users frequently had difficulty understanding and following the manufacturers’ instructions and were not able to successfully complete reprocessing with the older models.
However, the newer models had high user success rates for understanding instructions and correctly performing reprocessing tasks, the FDA says.
A version of this article first appeared on Medscape.com.
AGA supports FDA’s continued efforts to reduce the risk of disease transmission by duodenoscopes. Through the AGA Center for GI Innovation and Technology, AGA continues to support innovation in medical technology. To get up to date on past challenges with scope infections and future directions, check out AGA’s Innovation in Duodenoscope Design program, consisting of articles, webinars, and podcasts with leading experts in this space.
Health care facilities and providers should complete the transition to fully disposable duodenoscopes and those with disposable components, the U.S. Food and Drug Administration announced this week after an analysis of postmarket surveillance studies was completed.
The FDA’s directive updates its April 2020 recommendations on the subject. It cites concerns about cleaning fixed endcap duodenoscopes and the increasing availability of models that eliminate the need for reprocessing.
The announcement highlighted the potential for a dramatic difference in between-patient contamination risk, reducing it “by half or more as compared to reusable, or fixed endcaps.”
“Interim results from one duodenoscope model with a removable component show a contamination rate of just 0.5%, as compared to older duodenoscope models which had contamination rates as high as 6%,” the FDA writes.
Duodenoscopes are used in more than 500,000 procedures each year in the United States and are key in assessing and treating diseases and conditions of the pancreas and bile ducts.
Upgrade to new models to decrease infections
Manufacturers no longer market fixed endcap models in the United States, but some health care facilities continue to use them. The FDA recommends that all fixed endcap models be replaced.
The FDA says some manufacturers are offering replacement programs to upgrade to a model with a disposable component at no cost.
Two fully disposable models and five with disposable components have been cleared by the FDA. (One model is no longer marketed and thus not listed here.)
Fully Disposable:
Ambu Innovation GmbH, Duodenoscope model aScope Duodeno
Boston Scientific Corporation, EXALT Model D Single-Use Duodenoscope
Disposable Components:
Fujifilm Corporation, Duodenoscope model ED-580XT
Olympus Medical Systems, Evis Exera III Duodenovideoscope Olympus TJF-Q190V
Pentax Medical, Duodenoscope model ED34-i10T2
Pentax Medical, Duodenoscope model ED32-i10
Additionally, the failure to correctly reprocess a duodenoscope could result in tissue or fluid from one patient transferring to a subsequent patient.
“In rare cases, this can lead to patient-to-patient disease transmission,” the FDA says.
Postmarket surveillance studies
In 2015, the FDA ordered three manufacturers of reusable devices (Fujifilm, Olympus, and Pentax) to conduct postmarket surveillance studies to determine contamination rates after reprocessing.
In 2019, the FDA also ordered postmarket surveillance studies to the makers of duodenoscopes with disposable endcaps to verify that the new designs reduce the contamination rate.
The final results of the fixed endcap design indicate that contamination rates were as high as 6.6% with high-concern organisms after contamination. High-concern organisms are those more often associated with disease, such as E coli and Pseudomonas contamination.
“As a result, Pentax and Olympus are withdrawing their fixed endcap duodenoscopes from the market, and Fujifilm has completed withdrawal of its fixed endcap duodenoscope,” the FDA writes.
Studies are not yet complete for duodenoscopes with removable components. As of August 12, 2021, the Fujifilm ED-580XT duodenoscope with a removable cap had 57% of the samples required. Interim results indicate that no samples tested positive for enough low-concern organisms to indicate a reprocessing failure, and only 0.5% tested positive for high-concern organisms.
In addition to the contamination risk sampling, each manufacturer was ordered to do postmarket surveillance studies to evaluate whether staff could understand and follow the manufacturer’s reprocessing instructions in real-world health care settings.
According to the FDA, the results showed that users frequently had difficulty understanding and following the manufacturers’ instructions and were not able to successfully complete reprocessing with the older models.
However, the newer models had high user success rates for understanding instructions and correctly performing reprocessing tasks, the FDA says.
A version of this article first appeared on Medscape.com.
AGA supports FDA’s continued efforts to reduce the risk of disease transmission by duodenoscopes. Through the AGA Center for GI Innovation and Technology, AGA continues to support innovation in medical technology. To get up to date on past challenges with scope infections and future directions, check out AGA’s Innovation in Duodenoscope Design program, consisting of articles, webinars, and podcasts with leading experts in this space.
Health care facilities and providers should complete the transition to fully disposable duodenoscopes and those with disposable components, the U.S. Food and Drug Administration announced this week after an analysis of postmarket surveillance studies was completed.
The FDA’s directive updates its April 2020 recommendations on the subject. It cites concerns about cleaning fixed endcap duodenoscopes and the increasing availability of models that eliminate the need for reprocessing.
The announcement highlighted the potential for a dramatic difference in between-patient contamination risk, reducing it “by half or more as compared to reusable, or fixed endcaps.”
“Interim results from one duodenoscope model with a removable component show a contamination rate of just 0.5%, as compared to older duodenoscope models which had contamination rates as high as 6%,” the FDA writes.
Duodenoscopes are used in more than 500,000 procedures each year in the United States and are key in assessing and treating diseases and conditions of the pancreas and bile ducts.
Upgrade to new models to decrease infections
Manufacturers no longer market fixed endcap models in the United States, but some health care facilities continue to use them. The FDA recommends that all fixed endcap models be replaced.
The FDA says some manufacturers are offering replacement programs to upgrade to a model with a disposable component at no cost.
Two fully disposable models and five with disposable components have been cleared by the FDA. (One model is no longer marketed and thus not listed here.)
Fully Disposable:
Ambu Innovation GmbH, Duodenoscope model aScope Duodeno
Boston Scientific Corporation, EXALT Model D Single-Use Duodenoscope
Disposable Components:
Fujifilm Corporation, Duodenoscope model ED-580XT
Olympus Medical Systems, Evis Exera III Duodenovideoscope Olympus TJF-Q190V
Pentax Medical, Duodenoscope model ED34-i10T2
Pentax Medical, Duodenoscope model ED32-i10
Additionally, the failure to correctly reprocess a duodenoscope could result in tissue or fluid from one patient transferring to a subsequent patient.
“In rare cases, this can lead to patient-to-patient disease transmission,” the FDA says.
Postmarket surveillance studies
In 2015, the FDA ordered three manufacturers of reusable devices (Fujifilm, Olympus, and Pentax) to conduct postmarket surveillance studies to determine contamination rates after reprocessing.
In 2019, the FDA also ordered postmarket surveillance studies to the makers of duodenoscopes with disposable endcaps to verify that the new designs reduce the contamination rate.
The final results of the fixed endcap design indicate that contamination rates were as high as 6.6% with high-concern organisms after contamination. High-concern organisms are those more often associated with disease, such as E coli and Pseudomonas contamination.
“As a result, Pentax and Olympus are withdrawing their fixed endcap duodenoscopes from the market, and Fujifilm has completed withdrawal of its fixed endcap duodenoscope,” the FDA writes.
Studies are not yet complete for duodenoscopes with removable components. As of August 12, 2021, the Fujifilm ED-580XT duodenoscope with a removable cap had 57% of the samples required. Interim results indicate that no samples tested positive for enough low-concern organisms to indicate a reprocessing failure, and only 0.5% tested positive for high-concern organisms.
In addition to the contamination risk sampling, each manufacturer was ordered to do postmarket surveillance studies to evaluate whether staff could understand and follow the manufacturer’s reprocessing instructions in real-world health care settings.
According to the FDA, the results showed that users frequently had difficulty understanding and following the manufacturers’ instructions and were not able to successfully complete reprocessing with the older models.
However, the newer models had high user success rates for understanding instructions and correctly performing reprocessing tasks, the FDA says.
A version of this article first appeared on Medscape.com.
AGA supports FDA’s continued efforts to reduce the risk of disease transmission by duodenoscopes. Through the AGA Center for GI Innovation and Technology, AGA continues to support innovation in medical technology. To get up to date on past challenges with scope infections and future directions, check out AGA’s Innovation in Duodenoscope Design program, consisting of articles, webinars, and podcasts with leading experts in this space.
Children and COVID: Cases drop again, admission rate up slightly
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.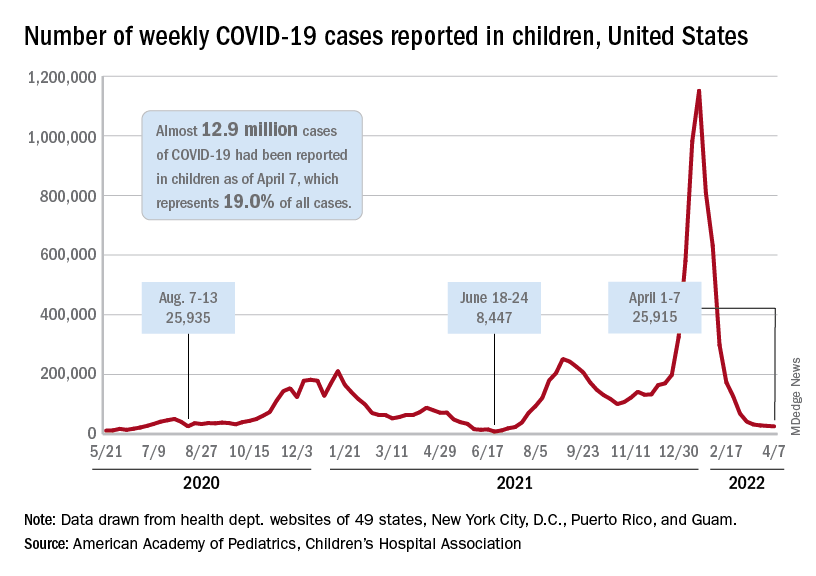
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
FDA okays first sublingual med for agitation in serious mental illness
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
FDA to decide by June on future of COVID vaccines
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.

