User login
A Microsponge Formulation of Hydroquinone 4% and Retinol 0.15% in the Treatment of Melasma and Postinflammatory Hyperpigmentation
Forest plots: Data summaries at a glance
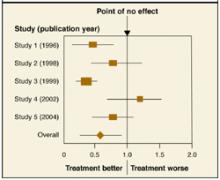
A forest plot, which most commonly appears in meta-analyses, summarizes results of individual studies and includes a combined or “pooled” estimate of the overall result.
The Figure shows a forest plot with the relative risk (RR) estimates from 5 studies of a new medication for the prevention of stroke. It includes the RR of the incidence of stroke with the new medication vs placebo. If the RR is <1.0, the treatment group has a lower incidence rate than placebo. An RR of 1.0 indicates no difference. The individual squares represent each study’s RR estimate. The lines extending from the squares represent the 95% confidence interval (CI) for the estimate. The size of the square corresponds to the size of the study and therefore the precision of the estimate.
In this example, Study 1 and Study 3 favor the new medication vs placebo. The larger square in Study 3 indicates a larger sample size and more precise result than Study 1. Study 2 also favors the new medication, but since its CI crosses the point of no effect, this result is not statistically significant. The pooled estimate is indicated with a diamond at bottom. Its CI does not cross 1.0, meaning, overall, the result of the meta-analysis is statistically significant and favors the new medication.
A forest plot is an effective way to represent data for a couple of reasons. Results of individual studies, usually with dates of publication and CIs, are summarized with a pooled result. One can also quickly see how much variation exists among studies (ie, whether the individual estimates are distributed tightly around one point or spread widely apart) and the degree of precision of each study.
Figure
Relative risk of the indicence of stroke
Correspondence
Julie Yeh, MD, UPMC St. Margaret Faculty Development Fellowship Program, 3937 Butler St, Pittsburgh, PA 15215. E-mail: [email protected].
REFERENCE
1. Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ 2001;322:1479-1480.
A forest plot, which most commonly appears in meta-analyses, summarizes results of individual studies and includes a combined or “pooled” estimate of the overall result.
The Figure shows a forest plot with the relative risk (RR) estimates from 5 studies of a new medication for the prevention of stroke. It includes the RR of the incidence of stroke with the new medication vs placebo. If the RR is <1.0, the treatment group has a lower incidence rate than placebo. An RR of 1.0 indicates no difference. The individual squares represent each study’s RR estimate. The lines extending from the squares represent the 95% confidence interval (CI) for the estimate. The size of the square corresponds to the size of the study and therefore the precision of the estimate.
In this example, Study 1 and Study 3 favor the new medication vs placebo. The larger square in Study 3 indicates a larger sample size and more precise result than Study 1. Study 2 also favors the new medication, but since its CI crosses the point of no effect, this result is not statistically significant. The pooled estimate is indicated with a diamond at bottom. Its CI does not cross 1.0, meaning, overall, the result of the meta-analysis is statistically significant and favors the new medication.
A forest plot is an effective way to represent data for a couple of reasons. Results of individual studies, usually with dates of publication and CIs, are summarized with a pooled result. One can also quickly see how much variation exists among studies (ie, whether the individual estimates are distributed tightly around one point or spread widely apart) and the degree of precision of each study.
Figure
Relative risk of the indicence of stroke
Correspondence
Julie Yeh, MD, UPMC St. Margaret Faculty Development Fellowship Program, 3937 Butler St, Pittsburgh, PA 15215. E-mail: [email protected].
A forest plot, which most commonly appears in meta-analyses, summarizes results of individual studies and includes a combined or “pooled” estimate of the overall result.
The Figure shows a forest plot with the relative risk (RR) estimates from 5 studies of a new medication for the prevention of stroke. It includes the RR of the incidence of stroke with the new medication vs placebo. If the RR is <1.0, the treatment group has a lower incidence rate than placebo. An RR of 1.0 indicates no difference. The individual squares represent each study’s RR estimate. The lines extending from the squares represent the 95% confidence interval (CI) for the estimate. The size of the square corresponds to the size of the study and therefore the precision of the estimate.
In this example, Study 1 and Study 3 favor the new medication vs placebo. The larger square in Study 3 indicates a larger sample size and more precise result than Study 1. Study 2 also favors the new medication, but since its CI crosses the point of no effect, this result is not statistically significant. The pooled estimate is indicated with a diamond at bottom. Its CI does not cross 1.0, meaning, overall, the result of the meta-analysis is statistically significant and favors the new medication.
A forest plot is an effective way to represent data for a couple of reasons. Results of individual studies, usually with dates of publication and CIs, are summarized with a pooled result. One can also quickly see how much variation exists among studies (ie, whether the individual estimates are distributed tightly around one point or spread widely apart) and the degree of precision of each study.
Figure
Relative risk of the indicence of stroke
Correspondence
Julie Yeh, MD, UPMC St. Margaret Faculty Development Fellowship Program, 3937 Butler St, Pittsburgh, PA 15215. E-mail: [email protected].
REFERENCE
1. Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ 2001;322:1479-1480.
REFERENCE
1. Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ 2001;322:1479-1480.
Mequinol 2%/Tretinoin 0.01% Solution: An Effective and Safe Alternative to Hydroquinone 3% in the Treatment of Solar Lentigines
Consider colonoscopy for young patients with hematochezia
- Nearly 12% of younger patients reporting rectal bleeding in this study had colon adenomas or cancer; thus, strong consideration should be given to colonoscopy in such individuals.
- Colonoscopy is a valuable diagnostic test and can help establish the source of rectal bleeding in nearly 80% of younger patients.
Background Hematochezia is a common complaint in adult patients aged <50 years. Most studies of lower endoscopy for rectal bleeding have concentrated on older patients or have failed to mention the location of lesions.
Objective To determine the findings of complete colonoscopy in adults younger than 50 years with rectal bleeding.
Methods Data were retrieved from medical records and included demographics, indications, endoscopic findings, and histology. Lesions were labeled according to location: proximal to the splenic flexure or distal to (and including) the splenic flexure. Excluded were those with a history of colitis, colorectal cancer, polyps, anemia, significant weight loss, severe bleeding, or strong family history of colorectal cancer.
Results The study included 223 patients with rectal bleeding aged <50 years who had undergone a colonoscopy. Normal findings were recorded for 48 (21.5%). Four (1.8%) were diagnosed with cancer in the distal colon, and 22 (9.9%) were found to have colon adenomas, 6 of whom had proximal adenomas only. Hemorrhoids were present in 135 patients (60.5%). Other findings included colitis, angiodysplasia, diverticulosis, anal fissures, and rectal ulcers.
Conclusions Colon neoplasms may be present even in younger adults with non-urgent rectal bleeding. Though most findings were benign and located in the distal colon, colonoscopy should be strongly considered for this patient group.
The role of colonoscopy is well established for patients aged more than 50 years with positive results on the fecal occult blood test. 1-3 For this population, colonoscopy has beenshown to reduce mortality from colorectal cancer, the second leading cause of cancer-related death in the United States. Colonoscopy has also been useful for diagnosing and treating lower gastrointestinal (GI) bleeding in older persons. 4-10
Some investigators have suggested the entire colon should be visualized in all patients with rectal bleeding. 4-11 Use of investigative colonoscopy has increased dramatically in recent years, particularly for younger patients, while use of sigmoidoscopy has declined. 12
Most of the literature on the investigation of rectal bleeding does not stratify patients by age. 4-8,13-23 Hence, there is no consensus on the proper evaluation of younger adults with rectal bleeding. The literature generally favors colonoscopy over sigmoidoscopy. But for adults aged younger than 50 years, data are sparse.
Rectal bleeding is common among younger patients
In a survey of patients aged 20 to 40 years, a history of rectal bleeding was reported in nearly 20%. 24 The concern with rectal bleeding is that it may indicate potentially serious disease, including colorectal cancer.
Deciding whether to subject a younger adult with non-urgent rectal bleeding to full colonoscopy can be difficult. A valid concern is that the incidence of colon neoplasms may be too low in younger adults to justify the widespread and costly use of colonoscopy. Colonoscopy has a small but finite risk of complications and imposes higher costs, greater discomfort, and more inconvenience for the patient than flexible sigmoidoscopy. On the other hand, the possibility of missing a neoplasm cannot be discounted.
The aim of this study was to review the diagnostic findings of colonoscopy in adults younger than 50 years who had non-urgent rectal bleeding (without alarm symptoms or signs).
Methods
Patients
We included all consecutive patients younger than 50 years who underwent colonoscopy for rectal bleeding at the University of Utah Medical Center or Salt Lake City Veterans Administration Medical Center between March 1997 and November 1999. Rectal bleeding was defined as the passage of bright blood on or within the stool, onto toilet paper, or into the toilet bowl. Patients were excluded if they had a history of colitis, colorectal cancer or polyps, severe bleeding requiring transfusion or hospitalization, unexplained weight loss greater than 5 pounds, iron-deficiency anemia, or a strong family history of colorectal cancer (at least 2 first-degree family members with colorectal cancer or 1 first-degree relative with colorectal cancer before the age of 50 years).
Data collection
Data were collected from medical records retrospectively. Patient demographics, indications for colonoscopy, endoscopic findings, and histology were retrieved.
Endoscopy
Gastroenterology faculty, or fellows under close supervision by the faculty, performed all endoscopic examinations. Informed written consent was obtained from each patient before every procedure. All endoscopic abnormalities were noted and biopsied if indicated, and all polyps were biopsied and removed. The distal colon was defined as that portion from the rectum through the splenic flexure.
Results
Two hundred twenty-three patients younger than 50 years with rectal bleeding underwent complete colonoscopy to the cecum or terminal ileum. Of the 223 patients, 170 (76%) were evaluated at the University of Utah Medical Center, and 53 (24%) were evaluated at the VA Medical Center. No major complications (hemorrhage, perforation, hypoxia) directly related to endoscopy were noted.
The Table summarizes colonoscopy findings. Of the 223 patients, 48 (21.5%) had a normal outcome. Abnormalities were found in 175 patients (78.5%). Hemorrhoids were the most common finding, present in 135 patients (60.5%). In 98 patients (73%), hemorrhoids were the only finding, excluding non-adenomatous polyps. In the other patients with hemorrhoids, coincident adenomas, colitis, and diverticulosis were also diagnosed. Other anorectal diseases, including rectal ulcers or anal fissures, were found in 14 patients (6.3%).
Twenty-six patients (11.6%) had colon neoplasms, either adenomas or adenocarcinomas. Four patients (1.8%) had adenomatous polyps 8 mm in the distal colon. Eighteen patients (8.1%) had adenomas <8 mm; 6 (2.7%) had polyps only in the proximal colon. Hyperplastic polyps were not included in this analysis. Four patients (1.8%) had adenocarcinomas. These cancers were located in the rectum or sigmoid colon. The ages of these patients ranged from 32 to 48 years. One cancer patient had a distant cousin who died of colon cancer at the age of 47; no others had a family history of colon cancer.
Biopsy-proven chronic colitis was found in 13 patients (5.8%). Among the 7 patients who had colitis in the proximal colon, colitis was present in the distal colon as well. Angiodysplasia was found in 2 of the patients (0.9%) and only affected the distal colon. Diverticulosis was found in 19 patients (8.5%).
TABLE
Colonoscopy findings in 223 patients with rectal bleeding
| Finding | Proximal | Distal | Total (%) |
|---|---|---|---|
| Carcinoma | 0 | 4 | 4 (1.8) |
| Colitis | 7 | 13 | 13 (5.8) |
| Tubular adenomas | |||
| ≥8 mm | 0 | 4 | 4 (1.8) |
| <8 mm | 6 | 14 | 18 (8.1) |
| Angiodysplasia | 0 | 2 | 2 (0.9) |
| Diverticulosis | 2 | 19 | 19 (8.5) |
| Hemorrhoids | 0 | 135 | 135 (60.5) |
| Fissure/Rectal ulcer | 0 | 14 | 14 (6.3) |
| Normal colonoscopy | 0 | 0 | 48 (21.5) |
Discussion
Rectal bleeding is a common problem in the US population. In a questionnaire sent by mail, 235 of 1643 respondents (15.5%) aged 20–64 years reported rectal bleeding. 24 The prevalence was higher in younger persons: 18.9% for those aged 20–40 years vs 11.3% for those older than 40 years (P<.001). Only 13.9% of all patients with rectal bleeding in this study had visited a physician for bowel problems in the past year.
A major challenge for the clinician is deciding if a diagnostic endoscopy is necessary and, if so,whether flexible sigmoidoscopy or colonoscopyshould be done. Certainly, the concern of missinga potentially early and curable colon neoplasm substantiates the argument favoring colonoscopy. However, the costs, risks, and inconvenience of doing colonoscopy on every patient with rectal bleeding may overshadow the benefit.
Normal/benign findings
Either normal findings or benign diseases are commonly documented in younger patients with rectal bleeding. Approximately 21% of patients in this study had normal findings on colonoscopy. Hemorrhoids are believed to be the most common cause of rectal bleeding in all age groups, accounting for 27%–72% of cases.8,19
In a random community sample of 202 people older than 30, with no history of cancer or inflammatory bowel disease, 16% reported rectal bleeding in the preceding 6 months. 25 About 43% of the respondents believed they had “hemorrhoids,” based on the presence of anal pain, bleeding, protrusion, or perianal itching. In our study, about 60% of patients had documented hemorrhoids and 6.3% had other anorectal pathology, including anal fissures and rectal ulcers.
Colitis
Colitis was found in nearly 6% of our patients, which is similar to the incidence reported in series on older patients. 26,26,26 Another study found that 6 of 102 patients under the age of 50 with rectal bleeding had colitis. 28 All the patients with colitis in our series were found to have involvement of the distal colon.
Colorectal cancer
Several studies have evaluated the prevalence of colorectal cancer among patients with rectal bleeding. An overall incidence of 4%–19% is reported in some series that included patients older than 50 years. 8,26
In a study of 280 patients younger than 40 by Acosta et al,11 the incidence of colon cancer was 0.03%. Lewis et al retrospectively evaluated 570 patients younger than 50 years with rectal bleeding and found only 1 patient with colorectal cancer.27 An additional 6.7% of patients had colorectal adenomas.
A limitation of this study, however, was that only 40% of patients had a colonoscopy; the other 60% had a flexible sigmoidoscopy. We found a colorectal cancer incidence of 1.8% among patients under 50 years old and all of these cancers were found in the distal colon.
Adenomas
Adenomas were found in 9.9% of our patients. A similar incidence was found in a series that studied the utility of anoscopy in addition to lower endoscopy.28 Only 1.8% of our patients had adenomas 8 mm, and all of these polyps were located in the distal colon. The incidence of adenomas <8 mm was 8.1%, and a third of the patients had polyps in the proximal colon. The relationship of these small adenomas to rectal bleeding is unclear as some of these patients also had hemorrhoids or diverticulosis. Polyps are common, bleed infrequently, and seem to be identified by chance during the investigation of GI bleeding.29-30
Choosing diagnostic tests for younger patients
Choosing between flexible sigmoidoscopy and colonoscopy for younger patients with rectal bleeding is a clinical dilemma. Most of the literature regarding the evaluation of rectal bleeding has either been directed towards older adults or has failed to stratify patients by age.4-8,13-22
One large study retrospectively studied the colonoscopic findings for rectal bleeding in 280 adults younger than 40 years.11 They found significant lesions, including cancers, polyps, colitis, angiodysplasia, diverticula, and rectal ulcers in 21% and concluded that full colonoscopy should be seriously considered even in this younger population. The study did not mention the location of the significant lesions within the colon, so the basis for recommending colonoscopy is unclear. Only 13.9% of patients with rectal bleeding had visited a physician for bowel problems in the past year Also, the study included a substantial number of hyperplastic polyps listed as significant pathology. To date, hyperplastic polyps do not appear to have malignant potential.
A prospective Canadian study found that, among 61 patients younger than 55 undergoing colonoscopy for rectal bleeding, most lesions, including colitis, polyps, cancers, diverticula, and hemorrhoids, were located within 60 cm of the anus.31 However, 1 cancer in a patient with massive bleeding and 1 small polyp were beyond 60 cm. A recent cost-effectiveness analysis by Lewis et al for the diagnosis of rectal bleeding in young persons demonstrated an incremental cost-effectiveness of colonoscopy as the age of the patient increased from 25 years to 45 years.32 At 35 years, the cost-effectiveness of evaluating the whole colon approximated the cost-effectiveness of repeat screening for colorectal cancer. At age 25 years, however, the cost-effectiveness of colonoscopy was more than $270,000 per year of life gained.
By comparison, several large studies have looked at colonoscopic findings in the screening population. Screening colonoscopy detected no colorectal cancers in 906 asymptomatic persons aged 40 to 49 years.33 Adenomatous polyps occurred in 8.7% of patients and advanced polyps (adenomas 10 mm, villous adenomas, adenomas with high-grade dysplasia) occurred in 3.5% patients; 55% of the lesions were located distally. In a Veterans Affairs study, advanced proximal neoplasias or invasive cancer were found in about 10% of patients older than 50 years undergoing screening colonoscopy.34 Of those with advanced proximal adenomas, only 48% had distal adenomas, supporting a role for colonoscopy over flexible sigmoidoscopy in the screening population.
Although none of the advanced adenomas or colon cancers were localized to the proximal colon, our study was not designed to determine the superiority of flexible sigmoidoscopy or colonoscopy. One important point is that flexible sigmoidoscopy at our institutions involves a full colon preparation and, in over 90% of cases, examines the distal 60 cm of colorectum (typically at or near the splenic flexure). Other studies reporting on flexible sigmoidoscopy use only enema preps and evaluate the distal colon less extensively.
The difficulty with more limited colon exams, such as anoscopy, rigid sigmoidoscopy, or flexible sigmoidoscopy, is whether or not a full colonoscopic exam should be performed when only benign anorectal pathology, namely hemorrhoids and anal fissures, are found. Hemorrhoids and anal fissures are the major cause of rectal bleeding and, because they are common, they can be coincident with more significant colon diseases, such as tumors and colitis. In our study, hemorrhoids were the only colonoscopy finding in 73% of the patients with hemorrhoids. The other 27% with hemorrhoids had coincident colorectal pathology, including adenomas and colitis, arguing that the discovery of hemorrhoids on a limited exam of the anorectum should not discourage practitioners from pursuing more detailed exams, such as colonoscopy. We can speculate that anoscopy alone would have missed a significant number of patients with cancers, adenomas, and chronic colitis.
Results and limitations of this study
The results of our study are significant in that approximately 12% of patients younger than 50 years with rectal bleeding had colon neoplasms, including 4 with colon cancers. Furthermore, an additional 13 patients had chronic colitis, another important finding with significant clinical implications for therapy and colorectal cancer surveillance.
Although a significant proportion of patients in this study was evaluated at a tertiary referral center, we believe that referral bias did not strongly influence the results of this study. Most endoscopic referrals originate from primary care providers within the University of Utah’s health care system. Furthermore, VA patients comprised approximately one fourth of the subjects, and VA patients typically receive all of their care within the VA Medical Center.
Because of the small numbers of patients in this study, it is difficult to conclude whether colonoscopy or flexible sigmoidoscopy is warranted in this patient population. However, based on current available evidence, we would strongly recommend consideration of colonoscopy in this patient population. Certainly, a large, prospective trial would be needed to answer the question of whether colonoscopy or flexible sigmoidoscopy is the appropriate test for patients younger than 50 years who present with rectal bleeding.
Corresponding author
Scott K. Kuwada, MD, Division of Gastroenterology, 4R-118 SOM, University of Utah Medical Center, 50 North Medical Drive, Salt Lake City, UT 84132. E-mail: [email protected].
1. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365-1371.
2. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471.
3. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477.
4. Helfand M, Marton KI, Zimmer-Gembeck MJ, Sox HC, Jr. History of visible rectal bleeding in a primary care population. Initial assessment and 10-year follow-up. JAMA. 1997;277:44-48.
5. Brenna E, Skreden K, Waldum HL, et al. The benefit of colonoscopy. Scand J Gastroenterol. 1990;25:81-88.
6. Editorial Investigation of rectal bleeding. Lancet. 1989;1:195-197.
7. Graham DJ, Pritchard TJ, Bloom AD. Colonoscopy for intermittent rectal bleeding: impact on patient management. J Surg Res. 1993;54:136-139.
8. Shinya H, Cwern M, Wolf G. Colonoscopy diagnosis and management of rectal bleeding. Surg Clin North Am. 1982;62:897-903.
9. Guillem JG, Forde KA, Treat MR, Neugut AI, Bodian CA. The impact of colonoscopy on early detection of colonic neoplasms in patients with rectal bleeding. Ann Surg. 1987;206:606-611.
10. Tedesco FJ, Waye JD, Raskin JB, Morris SJ, Greenwald RA. Colonoscopic evaluation of rectal bleeding: A study of 304 patients. Ann Intern Med. 1978;89:907-909.
11. Acosta JA, Fournier TK, Knutson TO, Ragland JJ. Colonoscopic evaluation of rectal bleeding in young adults. Am Surg. 1994;60:903-906.
12. Karasick S, Ehrlich SM, Levin DC, et al. Trends in use of barium enema examination, colonoscopy, and sigmoidoscopy: is use commensurate with risk of disease? Radiology. 1995;195:777-785.
13. Scrock TR. Colonoscopy diagnosis and treatment of lower GI bleeding. Surg Clin North Am. 1989;69:1309-1325.
14. Pines A, Shemesh E, Bat L. Pronged rectal bleeding associated with hemorrhoids: the diagnostic contribution of colonoscopy. South Med J. 1987;80:313-314.
15. Brand EJ, Sullivan BH, Jr, Sivak MV, Jr, Rankin GB. Colonoscopy in the diagnosis of unexpected rectal bleeding. Ann Surg. 1980;192:111-113.
16. Cheung PS, Wong SK, Boey J, Lai CK. Frank rectal bleeding: A prospective study of causes in patients over the age of 40. Postgrad Med J. 1988;64:364-368.
17. Dehn T, McGinn FP. Causes of ano-rectal bleeding. Postgrad Med J. 1982;58:92-93.
18. Gane EJ. In practice. Colonoscopy in unexplained lower GI bleeding. N Z Med J. 1992;105:31-33.
19. Goultson KJ, Cook I, Dent OF. How important is rectal bleeding in the diagnosis of bowel cancer and polyps? Lancet. 1986;2:261-264.
20. Kang JY. Investigation of rectal bleeding. Singapore Med J. 1991;32:327-328.
21. Neugut AI, Garbowski GC, Waye JD, et al. Diagnostic yield of colorectal neoplasia with colonoscopy for abdominal pain, change in bowel habits, and rectal bleeding. Am J Gastroenterol. 1993;88:1179-1183.
22. Teague RH, Manning AP, Thornton JR, Salmon PR. Colonoscopy for investigation of unexplained rectal bleeding. Lancet. 1978;1:1350-1352.
23. Swarback ET, Fevre DI, Hunt RH, Thomas BM, Williams CB. Colonoscopy for unexplained rectal bleeding. BMJ. 1978;2:1685-1687.
24. Talley NJ, Jones M. Self-reported rectal bleeding in a United States community: prevalence, risk factors, and health care seeking. Am J Gastroenterol. 1998;93:2179-2183.
25. Dent OF, Goulston KJ, Zubrzycki J, Chapuis PH. Bowel symptoms in an apparently well population. Dis Colon Rectum. 1986;29:243-247.
26. Segal WN, Greenberg PD, Rockey DC, Cello JP, McQuaid KR. The outpatient evaluation of hematochezia. Am J Gastroenterol. 1998;93:179-182.
27. Lewis JD, Shih CE, Blecker D. Endoscopy for hematochezia in patients under 50 years of age. Dig Dis Sci. 2001;46:2660-2665.
28. Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci. 1995;40:1520-1523.
29. Lang CA, Ransohoff DF. Fecal occult blood screening for colorectal cancer. Is mortality reduced by chance selection for screening colonoscopy? JAMA. 1994;271:1011-1013.
30. Ahlquist DA, Wieand HS, Moertel CG, et al. Accuracy of fecal occult blood screening for colorectal neoplasia. A prospective study using Hemoccult and HemoQuant tests. JAMA. 1993;269:1262-1267.
31. Van Rosendaal GM, Sutherland LR, Verhoef MJ, et al. Defining the role of fiberoptic sigmoidoscopy in the investigation of patients presenting with bright red rectal bleeding. Am J Gastroenterol. 2000;95:1184-1187.
32. Lewis JD, Brown AR, Localio R, Schwartz JS. Initial evaluation of rectal bleeding in young persons: a cost-effectiveness analysis. Ann Intern Med. 2002;136:99-110.
33. Imperiale TF, Wagner DR, Yin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346:1781-1785.
34. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-168.
- Nearly 12% of younger patients reporting rectal bleeding in this study had colon adenomas or cancer; thus, strong consideration should be given to colonoscopy in such individuals.
- Colonoscopy is a valuable diagnostic test and can help establish the source of rectal bleeding in nearly 80% of younger patients.
Background Hematochezia is a common complaint in adult patients aged <50 years. Most studies of lower endoscopy for rectal bleeding have concentrated on older patients or have failed to mention the location of lesions.
Objective To determine the findings of complete colonoscopy in adults younger than 50 years with rectal bleeding.
Methods Data were retrieved from medical records and included demographics, indications, endoscopic findings, and histology. Lesions were labeled according to location: proximal to the splenic flexure or distal to (and including) the splenic flexure. Excluded were those with a history of colitis, colorectal cancer, polyps, anemia, significant weight loss, severe bleeding, or strong family history of colorectal cancer.
Results The study included 223 patients with rectal bleeding aged <50 years who had undergone a colonoscopy. Normal findings were recorded for 48 (21.5%). Four (1.8%) were diagnosed with cancer in the distal colon, and 22 (9.9%) were found to have colon adenomas, 6 of whom had proximal adenomas only. Hemorrhoids were present in 135 patients (60.5%). Other findings included colitis, angiodysplasia, diverticulosis, anal fissures, and rectal ulcers.
Conclusions Colon neoplasms may be present even in younger adults with non-urgent rectal bleeding. Though most findings were benign and located in the distal colon, colonoscopy should be strongly considered for this patient group.
The role of colonoscopy is well established for patients aged more than 50 years with positive results on the fecal occult blood test. 1-3 For this population, colonoscopy has beenshown to reduce mortality from colorectal cancer, the second leading cause of cancer-related death in the United States. Colonoscopy has also been useful for diagnosing and treating lower gastrointestinal (GI) bleeding in older persons. 4-10
Some investigators have suggested the entire colon should be visualized in all patients with rectal bleeding. 4-11 Use of investigative colonoscopy has increased dramatically in recent years, particularly for younger patients, while use of sigmoidoscopy has declined. 12
Most of the literature on the investigation of rectal bleeding does not stratify patients by age. 4-8,13-23 Hence, there is no consensus on the proper evaluation of younger adults with rectal bleeding. The literature generally favors colonoscopy over sigmoidoscopy. But for adults aged younger than 50 years, data are sparse.
Rectal bleeding is common among younger patients
In a survey of patients aged 20 to 40 years, a history of rectal bleeding was reported in nearly 20%. 24 The concern with rectal bleeding is that it may indicate potentially serious disease, including colorectal cancer.
Deciding whether to subject a younger adult with non-urgent rectal bleeding to full colonoscopy can be difficult. A valid concern is that the incidence of colon neoplasms may be too low in younger adults to justify the widespread and costly use of colonoscopy. Colonoscopy has a small but finite risk of complications and imposes higher costs, greater discomfort, and more inconvenience for the patient than flexible sigmoidoscopy. On the other hand, the possibility of missing a neoplasm cannot be discounted.
The aim of this study was to review the diagnostic findings of colonoscopy in adults younger than 50 years who had non-urgent rectal bleeding (without alarm symptoms or signs).
Methods
Patients
We included all consecutive patients younger than 50 years who underwent colonoscopy for rectal bleeding at the University of Utah Medical Center or Salt Lake City Veterans Administration Medical Center between March 1997 and November 1999. Rectal bleeding was defined as the passage of bright blood on or within the stool, onto toilet paper, or into the toilet bowl. Patients were excluded if they had a history of colitis, colorectal cancer or polyps, severe bleeding requiring transfusion or hospitalization, unexplained weight loss greater than 5 pounds, iron-deficiency anemia, or a strong family history of colorectal cancer (at least 2 first-degree family members with colorectal cancer or 1 first-degree relative with colorectal cancer before the age of 50 years).
Data collection
Data were collected from medical records retrospectively. Patient demographics, indications for colonoscopy, endoscopic findings, and histology were retrieved.
Endoscopy
Gastroenterology faculty, or fellows under close supervision by the faculty, performed all endoscopic examinations. Informed written consent was obtained from each patient before every procedure. All endoscopic abnormalities were noted and biopsied if indicated, and all polyps were biopsied and removed. The distal colon was defined as that portion from the rectum through the splenic flexure.
Results
Two hundred twenty-three patients younger than 50 years with rectal bleeding underwent complete colonoscopy to the cecum or terminal ileum. Of the 223 patients, 170 (76%) were evaluated at the University of Utah Medical Center, and 53 (24%) were evaluated at the VA Medical Center. No major complications (hemorrhage, perforation, hypoxia) directly related to endoscopy were noted.
The Table summarizes colonoscopy findings. Of the 223 patients, 48 (21.5%) had a normal outcome. Abnormalities were found in 175 patients (78.5%). Hemorrhoids were the most common finding, present in 135 patients (60.5%). In 98 patients (73%), hemorrhoids were the only finding, excluding non-adenomatous polyps. In the other patients with hemorrhoids, coincident adenomas, colitis, and diverticulosis were also diagnosed. Other anorectal diseases, including rectal ulcers or anal fissures, were found in 14 patients (6.3%).
Twenty-six patients (11.6%) had colon neoplasms, either adenomas or adenocarcinomas. Four patients (1.8%) had adenomatous polyps 8 mm in the distal colon. Eighteen patients (8.1%) had adenomas <8 mm; 6 (2.7%) had polyps only in the proximal colon. Hyperplastic polyps were not included in this analysis. Four patients (1.8%) had adenocarcinomas. These cancers were located in the rectum or sigmoid colon. The ages of these patients ranged from 32 to 48 years. One cancer patient had a distant cousin who died of colon cancer at the age of 47; no others had a family history of colon cancer.
Biopsy-proven chronic colitis was found in 13 patients (5.8%). Among the 7 patients who had colitis in the proximal colon, colitis was present in the distal colon as well. Angiodysplasia was found in 2 of the patients (0.9%) and only affected the distal colon. Diverticulosis was found in 19 patients (8.5%).
TABLE
Colonoscopy findings in 223 patients with rectal bleeding
| Finding | Proximal | Distal | Total (%) |
|---|---|---|---|
| Carcinoma | 0 | 4 | 4 (1.8) |
| Colitis | 7 | 13 | 13 (5.8) |
| Tubular adenomas | |||
| ≥8 mm | 0 | 4 | 4 (1.8) |
| <8 mm | 6 | 14 | 18 (8.1) |
| Angiodysplasia | 0 | 2 | 2 (0.9) |
| Diverticulosis | 2 | 19 | 19 (8.5) |
| Hemorrhoids | 0 | 135 | 135 (60.5) |
| Fissure/Rectal ulcer | 0 | 14 | 14 (6.3) |
| Normal colonoscopy | 0 | 0 | 48 (21.5) |
Discussion
Rectal bleeding is a common problem in the US population. In a questionnaire sent by mail, 235 of 1643 respondents (15.5%) aged 20–64 years reported rectal bleeding. 24 The prevalence was higher in younger persons: 18.9% for those aged 20–40 years vs 11.3% for those older than 40 years (P<.001). Only 13.9% of all patients with rectal bleeding in this study had visited a physician for bowel problems in the past year.
A major challenge for the clinician is deciding if a diagnostic endoscopy is necessary and, if so,whether flexible sigmoidoscopy or colonoscopyshould be done. Certainly, the concern of missinga potentially early and curable colon neoplasm substantiates the argument favoring colonoscopy. However, the costs, risks, and inconvenience of doing colonoscopy on every patient with rectal bleeding may overshadow the benefit.
Normal/benign findings
Either normal findings or benign diseases are commonly documented in younger patients with rectal bleeding. Approximately 21% of patients in this study had normal findings on colonoscopy. Hemorrhoids are believed to be the most common cause of rectal bleeding in all age groups, accounting for 27%–72% of cases.8,19
In a random community sample of 202 people older than 30, with no history of cancer or inflammatory bowel disease, 16% reported rectal bleeding in the preceding 6 months. 25 About 43% of the respondents believed they had “hemorrhoids,” based on the presence of anal pain, bleeding, protrusion, or perianal itching. In our study, about 60% of patients had documented hemorrhoids and 6.3% had other anorectal pathology, including anal fissures and rectal ulcers.
Colitis
Colitis was found in nearly 6% of our patients, which is similar to the incidence reported in series on older patients. 26,26,26 Another study found that 6 of 102 patients under the age of 50 with rectal bleeding had colitis. 28 All the patients with colitis in our series were found to have involvement of the distal colon.
Colorectal cancer
Several studies have evaluated the prevalence of colorectal cancer among patients with rectal bleeding. An overall incidence of 4%–19% is reported in some series that included patients older than 50 years. 8,26
In a study of 280 patients younger than 40 by Acosta et al,11 the incidence of colon cancer was 0.03%. Lewis et al retrospectively evaluated 570 patients younger than 50 years with rectal bleeding and found only 1 patient with colorectal cancer.27 An additional 6.7% of patients had colorectal adenomas.
A limitation of this study, however, was that only 40% of patients had a colonoscopy; the other 60% had a flexible sigmoidoscopy. We found a colorectal cancer incidence of 1.8% among patients under 50 years old and all of these cancers were found in the distal colon.
Adenomas
Adenomas were found in 9.9% of our patients. A similar incidence was found in a series that studied the utility of anoscopy in addition to lower endoscopy.28 Only 1.8% of our patients had adenomas 8 mm, and all of these polyps were located in the distal colon. The incidence of adenomas <8 mm was 8.1%, and a third of the patients had polyps in the proximal colon. The relationship of these small adenomas to rectal bleeding is unclear as some of these patients also had hemorrhoids or diverticulosis. Polyps are common, bleed infrequently, and seem to be identified by chance during the investigation of GI bleeding.29-30
Choosing diagnostic tests for younger patients
Choosing between flexible sigmoidoscopy and colonoscopy for younger patients with rectal bleeding is a clinical dilemma. Most of the literature regarding the evaluation of rectal bleeding has either been directed towards older adults or has failed to stratify patients by age.4-8,13-22
One large study retrospectively studied the colonoscopic findings for rectal bleeding in 280 adults younger than 40 years.11 They found significant lesions, including cancers, polyps, colitis, angiodysplasia, diverticula, and rectal ulcers in 21% and concluded that full colonoscopy should be seriously considered even in this younger population. The study did not mention the location of the significant lesions within the colon, so the basis for recommending colonoscopy is unclear. Only 13.9% of patients with rectal bleeding had visited a physician for bowel problems in the past year Also, the study included a substantial number of hyperplastic polyps listed as significant pathology. To date, hyperplastic polyps do not appear to have malignant potential.
A prospective Canadian study found that, among 61 patients younger than 55 undergoing colonoscopy for rectal bleeding, most lesions, including colitis, polyps, cancers, diverticula, and hemorrhoids, were located within 60 cm of the anus.31 However, 1 cancer in a patient with massive bleeding and 1 small polyp were beyond 60 cm. A recent cost-effectiveness analysis by Lewis et al for the diagnosis of rectal bleeding in young persons demonstrated an incremental cost-effectiveness of colonoscopy as the age of the patient increased from 25 years to 45 years.32 At 35 years, the cost-effectiveness of evaluating the whole colon approximated the cost-effectiveness of repeat screening for colorectal cancer. At age 25 years, however, the cost-effectiveness of colonoscopy was more than $270,000 per year of life gained.
By comparison, several large studies have looked at colonoscopic findings in the screening population. Screening colonoscopy detected no colorectal cancers in 906 asymptomatic persons aged 40 to 49 years.33 Adenomatous polyps occurred in 8.7% of patients and advanced polyps (adenomas 10 mm, villous adenomas, adenomas with high-grade dysplasia) occurred in 3.5% patients; 55% of the lesions were located distally. In a Veterans Affairs study, advanced proximal neoplasias or invasive cancer were found in about 10% of patients older than 50 years undergoing screening colonoscopy.34 Of those with advanced proximal adenomas, only 48% had distal adenomas, supporting a role for colonoscopy over flexible sigmoidoscopy in the screening population.
Although none of the advanced adenomas or colon cancers were localized to the proximal colon, our study was not designed to determine the superiority of flexible sigmoidoscopy or colonoscopy. One important point is that flexible sigmoidoscopy at our institutions involves a full colon preparation and, in over 90% of cases, examines the distal 60 cm of colorectum (typically at or near the splenic flexure). Other studies reporting on flexible sigmoidoscopy use only enema preps and evaluate the distal colon less extensively.
The difficulty with more limited colon exams, such as anoscopy, rigid sigmoidoscopy, or flexible sigmoidoscopy, is whether or not a full colonoscopic exam should be performed when only benign anorectal pathology, namely hemorrhoids and anal fissures, are found. Hemorrhoids and anal fissures are the major cause of rectal bleeding and, because they are common, they can be coincident with more significant colon diseases, such as tumors and colitis. In our study, hemorrhoids were the only colonoscopy finding in 73% of the patients with hemorrhoids. The other 27% with hemorrhoids had coincident colorectal pathology, including adenomas and colitis, arguing that the discovery of hemorrhoids on a limited exam of the anorectum should not discourage practitioners from pursuing more detailed exams, such as colonoscopy. We can speculate that anoscopy alone would have missed a significant number of patients with cancers, adenomas, and chronic colitis.
Results and limitations of this study
The results of our study are significant in that approximately 12% of patients younger than 50 years with rectal bleeding had colon neoplasms, including 4 with colon cancers. Furthermore, an additional 13 patients had chronic colitis, another important finding with significant clinical implications for therapy and colorectal cancer surveillance.
Although a significant proportion of patients in this study was evaluated at a tertiary referral center, we believe that referral bias did not strongly influence the results of this study. Most endoscopic referrals originate from primary care providers within the University of Utah’s health care system. Furthermore, VA patients comprised approximately one fourth of the subjects, and VA patients typically receive all of their care within the VA Medical Center.
Because of the small numbers of patients in this study, it is difficult to conclude whether colonoscopy or flexible sigmoidoscopy is warranted in this patient population. However, based on current available evidence, we would strongly recommend consideration of colonoscopy in this patient population. Certainly, a large, prospective trial would be needed to answer the question of whether colonoscopy or flexible sigmoidoscopy is the appropriate test for patients younger than 50 years who present with rectal bleeding.
Corresponding author
Scott K. Kuwada, MD, Division of Gastroenterology, 4R-118 SOM, University of Utah Medical Center, 50 North Medical Drive, Salt Lake City, UT 84132. E-mail: [email protected].
- Nearly 12% of younger patients reporting rectal bleeding in this study had colon adenomas or cancer; thus, strong consideration should be given to colonoscopy in such individuals.
- Colonoscopy is a valuable diagnostic test and can help establish the source of rectal bleeding in nearly 80% of younger patients.
Background Hematochezia is a common complaint in adult patients aged <50 years. Most studies of lower endoscopy for rectal bleeding have concentrated on older patients or have failed to mention the location of lesions.
Objective To determine the findings of complete colonoscopy in adults younger than 50 years with rectal bleeding.
Methods Data were retrieved from medical records and included demographics, indications, endoscopic findings, and histology. Lesions were labeled according to location: proximal to the splenic flexure or distal to (and including) the splenic flexure. Excluded were those with a history of colitis, colorectal cancer, polyps, anemia, significant weight loss, severe bleeding, or strong family history of colorectal cancer.
Results The study included 223 patients with rectal bleeding aged <50 years who had undergone a colonoscopy. Normal findings were recorded for 48 (21.5%). Four (1.8%) were diagnosed with cancer in the distal colon, and 22 (9.9%) were found to have colon adenomas, 6 of whom had proximal adenomas only. Hemorrhoids were present in 135 patients (60.5%). Other findings included colitis, angiodysplasia, diverticulosis, anal fissures, and rectal ulcers.
Conclusions Colon neoplasms may be present even in younger adults with non-urgent rectal bleeding. Though most findings were benign and located in the distal colon, colonoscopy should be strongly considered for this patient group.
The role of colonoscopy is well established for patients aged more than 50 years with positive results on the fecal occult blood test. 1-3 For this population, colonoscopy has beenshown to reduce mortality from colorectal cancer, the second leading cause of cancer-related death in the United States. Colonoscopy has also been useful for diagnosing and treating lower gastrointestinal (GI) bleeding in older persons. 4-10
Some investigators have suggested the entire colon should be visualized in all patients with rectal bleeding. 4-11 Use of investigative colonoscopy has increased dramatically in recent years, particularly for younger patients, while use of sigmoidoscopy has declined. 12
Most of the literature on the investigation of rectal bleeding does not stratify patients by age. 4-8,13-23 Hence, there is no consensus on the proper evaluation of younger adults with rectal bleeding. The literature generally favors colonoscopy over sigmoidoscopy. But for adults aged younger than 50 years, data are sparse.
Rectal bleeding is common among younger patients
In a survey of patients aged 20 to 40 years, a history of rectal bleeding was reported in nearly 20%. 24 The concern with rectal bleeding is that it may indicate potentially serious disease, including colorectal cancer.
Deciding whether to subject a younger adult with non-urgent rectal bleeding to full colonoscopy can be difficult. A valid concern is that the incidence of colon neoplasms may be too low in younger adults to justify the widespread and costly use of colonoscopy. Colonoscopy has a small but finite risk of complications and imposes higher costs, greater discomfort, and more inconvenience for the patient than flexible sigmoidoscopy. On the other hand, the possibility of missing a neoplasm cannot be discounted.
The aim of this study was to review the diagnostic findings of colonoscopy in adults younger than 50 years who had non-urgent rectal bleeding (without alarm symptoms or signs).
Methods
Patients
We included all consecutive patients younger than 50 years who underwent colonoscopy for rectal bleeding at the University of Utah Medical Center or Salt Lake City Veterans Administration Medical Center between March 1997 and November 1999. Rectal bleeding was defined as the passage of bright blood on or within the stool, onto toilet paper, or into the toilet bowl. Patients were excluded if they had a history of colitis, colorectal cancer or polyps, severe bleeding requiring transfusion or hospitalization, unexplained weight loss greater than 5 pounds, iron-deficiency anemia, or a strong family history of colorectal cancer (at least 2 first-degree family members with colorectal cancer or 1 first-degree relative with colorectal cancer before the age of 50 years).
Data collection
Data were collected from medical records retrospectively. Patient demographics, indications for colonoscopy, endoscopic findings, and histology were retrieved.
Endoscopy
Gastroenterology faculty, or fellows under close supervision by the faculty, performed all endoscopic examinations. Informed written consent was obtained from each patient before every procedure. All endoscopic abnormalities were noted and biopsied if indicated, and all polyps were biopsied and removed. The distal colon was defined as that portion from the rectum through the splenic flexure.
Results
Two hundred twenty-three patients younger than 50 years with rectal bleeding underwent complete colonoscopy to the cecum or terminal ileum. Of the 223 patients, 170 (76%) were evaluated at the University of Utah Medical Center, and 53 (24%) were evaluated at the VA Medical Center. No major complications (hemorrhage, perforation, hypoxia) directly related to endoscopy were noted.
The Table summarizes colonoscopy findings. Of the 223 patients, 48 (21.5%) had a normal outcome. Abnormalities were found in 175 patients (78.5%). Hemorrhoids were the most common finding, present in 135 patients (60.5%). In 98 patients (73%), hemorrhoids were the only finding, excluding non-adenomatous polyps. In the other patients with hemorrhoids, coincident adenomas, colitis, and diverticulosis were also diagnosed. Other anorectal diseases, including rectal ulcers or anal fissures, were found in 14 patients (6.3%).
Twenty-six patients (11.6%) had colon neoplasms, either adenomas or adenocarcinomas. Four patients (1.8%) had adenomatous polyps 8 mm in the distal colon. Eighteen patients (8.1%) had adenomas <8 mm; 6 (2.7%) had polyps only in the proximal colon. Hyperplastic polyps were not included in this analysis. Four patients (1.8%) had adenocarcinomas. These cancers were located in the rectum or sigmoid colon. The ages of these patients ranged from 32 to 48 years. One cancer patient had a distant cousin who died of colon cancer at the age of 47; no others had a family history of colon cancer.
Biopsy-proven chronic colitis was found in 13 patients (5.8%). Among the 7 patients who had colitis in the proximal colon, colitis was present in the distal colon as well. Angiodysplasia was found in 2 of the patients (0.9%) and only affected the distal colon. Diverticulosis was found in 19 patients (8.5%).
TABLE
Colonoscopy findings in 223 patients with rectal bleeding
| Finding | Proximal | Distal | Total (%) |
|---|---|---|---|
| Carcinoma | 0 | 4 | 4 (1.8) |
| Colitis | 7 | 13 | 13 (5.8) |
| Tubular adenomas | |||
| ≥8 mm | 0 | 4 | 4 (1.8) |
| <8 mm | 6 | 14 | 18 (8.1) |
| Angiodysplasia | 0 | 2 | 2 (0.9) |
| Diverticulosis | 2 | 19 | 19 (8.5) |
| Hemorrhoids | 0 | 135 | 135 (60.5) |
| Fissure/Rectal ulcer | 0 | 14 | 14 (6.3) |
| Normal colonoscopy | 0 | 0 | 48 (21.5) |
Discussion
Rectal bleeding is a common problem in the US population. In a questionnaire sent by mail, 235 of 1643 respondents (15.5%) aged 20–64 years reported rectal bleeding. 24 The prevalence was higher in younger persons: 18.9% for those aged 20–40 years vs 11.3% for those older than 40 years (P<.001). Only 13.9% of all patients with rectal bleeding in this study had visited a physician for bowel problems in the past year.
A major challenge for the clinician is deciding if a diagnostic endoscopy is necessary and, if so,whether flexible sigmoidoscopy or colonoscopyshould be done. Certainly, the concern of missinga potentially early and curable colon neoplasm substantiates the argument favoring colonoscopy. However, the costs, risks, and inconvenience of doing colonoscopy on every patient with rectal bleeding may overshadow the benefit.
Normal/benign findings
Either normal findings or benign diseases are commonly documented in younger patients with rectal bleeding. Approximately 21% of patients in this study had normal findings on colonoscopy. Hemorrhoids are believed to be the most common cause of rectal bleeding in all age groups, accounting for 27%–72% of cases.8,19
In a random community sample of 202 people older than 30, with no history of cancer or inflammatory bowel disease, 16% reported rectal bleeding in the preceding 6 months. 25 About 43% of the respondents believed they had “hemorrhoids,” based on the presence of anal pain, bleeding, protrusion, or perianal itching. In our study, about 60% of patients had documented hemorrhoids and 6.3% had other anorectal pathology, including anal fissures and rectal ulcers.
Colitis
Colitis was found in nearly 6% of our patients, which is similar to the incidence reported in series on older patients. 26,26,26 Another study found that 6 of 102 patients under the age of 50 with rectal bleeding had colitis. 28 All the patients with colitis in our series were found to have involvement of the distal colon.
Colorectal cancer
Several studies have evaluated the prevalence of colorectal cancer among patients with rectal bleeding. An overall incidence of 4%–19% is reported in some series that included patients older than 50 years. 8,26
In a study of 280 patients younger than 40 by Acosta et al,11 the incidence of colon cancer was 0.03%. Lewis et al retrospectively evaluated 570 patients younger than 50 years with rectal bleeding and found only 1 patient with colorectal cancer.27 An additional 6.7% of patients had colorectal adenomas.
A limitation of this study, however, was that only 40% of patients had a colonoscopy; the other 60% had a flexible sigmoidoscopy. We found a colorectal cancer incidence of 1.8% among patients under 50 years old and all of these cancers were found in the distal colon.
Adenomas
Adenomas were found in 9.9% of our patients. A similar incidence was found in a series that studied the utility of anoscopy in addition to lower endoscopy.28 Only 1.8% of our patients had adenomas 8 mm, and all of these polyps were located in the distal colon. The incidence of adenomas <8 mm was 8.1%, and a third of the patients had polyps in the proximal colon. The relationship of these small adenomas to rectal bleeding is unclear as some of these patients also had hemorrhoids or diverticulosis. Polyps are common, bleed infrequently, and seem to be identified by chance during the investigation of GI bleeding.29-30
Choosing diagnostic tests for younger patients
Choosing between flexible sigmoidoscopy and colonoscopy for younger patients with rectal bleeding is a clinical dilemma. Most of the literature regarding the evaluation of rectal bleeding has either been directed towards older adults or has failed to stratify patients by age.4-8,13-22
One large study retrospectively studied the colonoscopic findings for rectal bleeding in 280 adults younger than 40 years.11 They found significant lesions, including cancers, polyps, colitis, angiodysplasia, diverticula, and rectal ulcers in 21% and concluded that full colonoscopy should be seriously considered even in this younger population. The study did not mention the location of the significant lesions within the colon, so the basis for recommending colonoscopy is unclear. Only 13.9% of patients with rectal bleeding had visited a physician for bowel problems in the past year Also, the study included a substantial number of hyperplastic polyps listed as significant pathology. To date, hyperplastic polyps do not appear to have malignant potential.
A prospective Canadian study found that, among 61 patients younger than 55 undergoing colonoscopy for rectal bleeding, most lesions, including colitis, polyps, cancers, diverticula, and hemorrhoids, were located within 60 cm of the anus.31 However, 1 cancer in a patient with massive bleeding and 1 small polyp were beyond 60 cm. A recent cost-effectiveness analysis by Lewis et al for the diagnosis of rectal bleeding in young persons demonstrated an incremental cost-effectiveness of colonoscopy as the age of the patient increased from 25 years to 45 years.32 At 35 years, the cost-effectiveness of evaluating the whole colon approximated the cost-effectiveness of repeat screening for colorectal cancer. At age 25 years, however, the cost-effectiveness of colonoscopy was more than $270,000 per year of life gained.
By comparison, several large studies have looked at colonoscopic findings in the screening population. Screening colonoscopy detected no colorectal cancers in 906 asymptomatic persons aged 40 to 49 years.33 Adenomatous polyps occurred in 8.7% of patients and advanced polyps (adenomas 10 mm, villous adenomas, adenomas with high-grade dysplasia) occurred in 3.5% patients; 55% of the lesions were located distally. In a Veterans Affairs study, advanced proximal neoplasias or invasive cancer were found in about 10% of patients older than 50 years undergoing screening colonoscopy.34 Of those with advanced proximal adenomas, only 48% had distal adenomas, supporting a role for colonoscopy over flexible sigmoidoscopy in the screening population.
Although none of the advanced adenomas or colon cancers were localized to the proximal colon, our study was not designed to determine the superiority of flexible sigmoidoscopy or colonoscopy. One important point is that flexible sigmoidoscopy at our institutions involves a full colon preparation and, in over 90% of cases, examines the distal 60 cm of colorectum (typically at or near the splenic flexure). Other studies reporting on flexible sigmoidoscopy use only enema preps and evaluate the distal colon less extensively.
The difficulty with more limited colon exams, such as anoscopy, rigid sigmoidoscopy, or flexible sigmoidoscopy, is whether or not a full colonoscopic exam should be performed when only benign anorectal pathology, namely hemorrhoids and anal fissures, are found. Hemorrhoids and anal fissures are the major cause of rectal bleeding and, because they are common, they can be coincident with more significant colon diseases, such as tumors and colitis. In our study, hemorrhoids were the only colonoscopy finding in 73% of the patients with hemorrhoids. The other 27% with hemorrhoids had coincident colorectal pathology, including adenomas and colitis, arguing that the discovery of hemorrhoids on a limited exam of the anorectum should not discourage practitioners from pursuing more detailed exams, such as colonoscopy. We can speculate that anoscopy alone would have missed a significant number of patients with cancers, adenomas, and chronic colitis.
Results and limitations of this study
The results of our study are significant in that approximately 12% of patients younger than 50 years with rectal bleeding had colon neoplasms, including 4 with colon cancers. Furthermore, an additional 13 patients had chronic colitis, another important finding with significant clinical implications for therapy and colorectal cancer surveillance.
Although a significant proportion of patients in this study was evaluated at a tertiary referral center, we believe that referral bias did not strongly influence the results of this study. Most endoscopic referrals originate from primary care providers within the University of Utah’s health care system. Furthermore, VA patients comprised approximately one fourth of the subjects, and VA patients typically receive all of their care within the VA Medical Center.
Because of the small numbers of patients in this study, it is difficult to conclude whether colonoscopy or flexible sigmoidoscopy is warranted in this patient population. However, based on current available evidence, we would strongly recommend consideration of colonoscopy in this patient population. Certainly, a large, prospective trial would be needed to answer the question of whether colonoscopy or flexible sigmoidoscopy is the appropriate test for patients younger than 50 years who present with rectal bleeding.
Corresponding author
Scott K. Kuwada, MD, Division of Gastroenterology, 4R-118 SOM, University of Utah Medical Center, 50 North Medical Drive, Salt Lake City, UT 84132. E-mail: [email protected].
1. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365-1371.
2. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471.
3. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477.
4. Helfand M, Marton KI, Zimmer-Gembeck MJ, Sox HC, Jr. History of visible rectal bleeding in a primary care population. Initial assessment and 10-year follow-up. JAMA. 1997;277:44-48.
5. Brenna E, Skreden K, Waldum HL, et al. The benefit of colonoscopy. Scand J Gastroenterol. 1990;25:81-88.
6. Editorial Investigation of rectal bleeding. Lancet. 1989;1:195-197.
7. Graham DJ, Pritchard TJ, Bloom AD. Colonoscopy for intermittent rectal bleeding: impact on patient management. J Surg Res. 1993;54:136-139.
8. Shinya H, Cwern M, Wolf G. Colonoscopy diagnosis and management of rectal bleeding. Surg Clin North Am. 1982;62:897-903.
9. Guillem JG, Forde KA, Treat MR, Neugut AI, Bodian CA. The impact of colonoscopy on early detection of colonic neoplasms in patients with rectal bleeding. Ann Surg. 1987;206:606-611.
10. Tedesco FJ, Waye JD, Raskin JB, Morris SJ, Greenwald RA. Colonoscopic evaluation of rectal bleeding: A study of 304 patients. Ann Intern Med. 1978;89:907-909.
11. Acosta JA, Fournier TK, Knutson TO, Ragland JJ. Colonoscopic evaluation of rectal bleeding in young adults. Am Surg. 1994;60:903-906.
12. Karasick S, Ehrlich SM, Levin DC, et al. Trends in use of barium enema examination, colonoscopy, and sigmoidoscopy: is use commensurate with risk of disease? Radiology. 1995;195:777-785.
13. Scrock TR. Colonoscopy diagnosis and treatment of lower GI bleeding. Surg Clin North Am. 1989;69:1309-1325.
14. Pines A, Shemesh E, Bat L. Pronged rectal bleeding associated with hemorrhoids: the diagnostic contribution of colonoscopy. South Med J. 1987;80:313-314.
15. Brand EJ, Sullivan BH, Jr, Sivak MV, Jr, Rankin GB. Colonoscopy in the diagnosis of unexpected rectal bleeding. Ann Surg. 1980;192:111-113.
16. Cheung PS, Wong SK, Boey J, Lai CK. Frank rectal bleeding: A prospective study of causes in patients over the age of 40. Postgrad Med J. 1988;64:364-368.
17. Dehn T, McGinn FP. Causes of ano-rectal bleeding. Postgrad Med J. 1982;58:92-93.
18. Gane EJ. In practice. Colonoscopy in unexplained lower GI bleeding. N Z Med J. 1992;105:31-33.
19. Goultson KJ, Cook I, Dent OF. How important is rectal bleeding in the diagnosis of bowel cancer and polyps? Lancet. 1986;2:261-264.
20. Kang JY. Investigation of rectal bleeding. Singapore Med J. 1991;32:327-328.
21. Neugut AI, Garbowski GC, Waye JD, et al. Diagnostic yield of colorectal neoplasia with colonoscopy for abdominal pain, change in bowel habits, and rectal bleeding. Am J Gastroenterol. 1993;88:1179-1183.
22. Teague RH, Manning AP, Thornton JR, Salmon PR. Colonoscopy for investigation of unexplained rectal bleeding. Lancet. 1978;1:1350-1352.
23. Swarback ET, Fevre DI, Hunt RH, Thomas BM, Williams CB. Colonoscopy for unexplained rectal bleeding. BMJ. 1978;2:1685-1687.
24. Talley NJ, Jones M. Self-reported rectal bleeding in a United States community: prevalence, risk factors, and health care seeking. Am J Gastroenterol. 1998;93:2179-2183.
25. Dent OF, Goulston KJ, Zubrzycki J, Chapuis PH. Bowel symptoms in an apparently well population. Dis Colon Rectum. 1986;29:243-247.
26. Segal WN, Greenberg PD, Rockey DC, Cello JP, McQuaid KR. The outpatient evaluation of hematochezia. Am J Gastroenterol. 1998;93:179-182.
27. Lewis JD, Shih CE, Blecker D. Endoscopy for hematochezia in patients under 50 years of age. Dig Dis Sci. 2001;46:2660-2665.
28. Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci. 1995;40:1520-1523.
29. Lang CA, Ransohoff DF. Fecal occult blood screening for colorectal cancer. Is mortality reduced by chance selection for screening colonoscopy? JAMA. 1994;271:1011-1013.
30. Ahlquist DA, Wieand HS, Moertel CG, et al. Accuracy of fecal occult blood screening for colorectal neoplasia. A prospective study using Hemoccult and HemoQuant tests. JAMA. 1993;269:1262-1267.
31. Van Rosendaal GM, Sutherland LR, Verhoef MJ, et al. Defining the role of fiberoptic sigmoidoscopy in the investigation of patients presenting with bright red rectal bleeding. Am J Gastroenterol. 2000;95:1184-1187.
32. Lewis JD, Brown AR, Localio R, Schwartz JS. Initial evaluation of rectal bleeding in young persons: a cost-effectiveness analysis. Ann Intern Med. 2002;136:99-110.
33. Imperiale TF, Wagner DR, Yin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346:1781-1785.
34. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-168.
1. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365-1371.
2. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471.
3. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477.
4. Helfand M, Marton KI, Zimmer-Gembeck MJ, Sox HC, Jr. History of visible rectal bleeding in a primary care population. Initial assessment and 10-year follow-up. JAMA. 1997;277:44-48.
5. Brenna E, Skreden K, Waldum HL, et al. The benefit of colonoscopy. Scand J Gastroenterol. 1990;25:81-88.
6. Editorial Investigation of rectal bleeding. Lancet. 1989;1:195-197.
7. Graham DJ, Pritchard TJ, Bloom AD. Colonoscopy for intermittent rectal bleeding: impact on patient management. J Surg Res. 1993;54:136-139.
8. Shinya H, Cwern M, Wolf G. Colonoscopy diagnosis and management of rectal bleeding. Surg Clin North Am. 1982;62:897-903.
9. Guillem JG, Forde KA, Treat MR, Neugut AI, Bodian CA. The impact of colonoscopy on early detection of colonic neoplasms in patients with rectal bleeding. Ann Surg. 1987;206:606-611.
10. Tedesco FJ, Waye JD, Raskin JB, Morris SJ, Greenwald RA. Colonoscopic evaluation of rectal bleeding: A study of 304 patients. Ann Intern Med. 1978;89:907-909.
11. Acosta JA, Fournier TK, Knutson TO, Ragland JJ. Colonoscopic evaluation of rectal bleeding in young adults. Am Surg. 1994;60:903-906.
12. Karasick S, Ehrlich SM, Levin DC, et al. Trends in use of barium enema examination, colonoscopy, and sigmoidoscopy: is use commensurate with risk of disease? Radiology. 1995;195:777-785.
13. Scrock TR. Colonoscopy diagnosis and treatment of lower GI bleeding. Surg Clin North Am. 1989;69:1309-1325.
14. Pines A, Shemesh E, Bat L. Pronged rectal bleeding associated with hemorrhoids: the diagnostic contribution of colonoscopy. South Med J. 1987;80:313-314.
15. Brand EJ, Sullivan BH, Jr, Sivak MV, Jr, Rankin GB. Colonoscopy in the diagnosis of unexpected rectal bleeding. Ann Surg. 1980;192:111-113.
16. Cheung PS, Wong SK, Boey J, Lai CK. Frank rectal bleeding: A prospective study of causes in patients over the age of 40. Postgrad Med J. 1988;64:364-368.
17. Dehn T, McGinn FP. Causes of ano-rectal bleeding. Postgrad Med J. 1982;58:92-93.
18. Gane EJ. In practice. Colonoscopy in unexplained lower GI bleeding. N Z Med J. 1992;105:31-33.
19. Goultson KJ, Cook I, Dent OF. How important is rectal bleeding in the diagnosis of bowel cancer and polyps? Lancet. 1986;2:261-264.
20. Kang JY. Investigation of rectal bleeding. Singapore Med J. 1991;32:327-328.
21. Neugut AI, Garbowski GC, Waye JD, et al. Diagnostic yield of colorectal neoplasia with colonoscopy for abdominal pain, change in bowel habits, and rectal bleeding. Am J Gastroenterol. 1993;88:1179-1183.
22. Teague RH, Manning AP, Thornton JR, Salmon PR. Colonoscopy for investigation of unexplained rectal bleeding. Lancet. 1978;1:1350-1352.
23. Swarback ET, Fevre DI, Hunt RH, Thomas BM, Williams CB. Colonoscopy for unexplained rectal bleeding. BMJ. 1978;2:1685-1687.
24. Talley NJ, Jones M. Self-reported rectal bleeding in a United States community: prevalence, risk factors, and health care seeking. Am J Gastroenterol. 1998;93:2179-2183.
25. Dent OF, Goulston KJ, Zubrzycki J, Chapuis PH. Bowel symptoms in an apparently well population. Dis Colon Rectum. 1986;29:243-247.
26. Segal WN, Greenberg PD, Rockey DC, Cello JP, McQuaid KR. The outpatient evaluation of hematochezia. Am J Gastroenterol. 1998;93:179-182.
27. Lewis JD, Shih CE, Blecker D. Endoscopy for hematochezia in patients under 50 years of age. Dig Dis Sci. 2001;46:2660-2665.
28. Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci. 1995;40:1520-1523.
29. Lang CA, Ransohoff DF. Fecal occult blood screening for colorectal cancer. Is mortality reduced by chance selection for screening colonoscopy? JAMA. 1994;271:1011-1013.
30. Ahlquist DA, Wieand HS, Moertel CG, et al. Accuracy of fecal occult blood screening for colorectal neoplasia. A prospective study using Hemoccult and HemoQuant tests. JAMA. 1993;269:1262-1267.
31. Van Rosendaal GM, Sutherland LR, Verhoef MJ, et al. Defining the role of fiberoptic sigmoidoscopy in the investigation of patients presenting with bright red rectal bleeding. Am J Gastroenterol. 2000;95:1184-1187.
32. Lewis JD, Brown AR, Localio R, Schwartz JS. Initial evaluation of rectal bleeding in young persons: a cost-effectiveness analysis. Ann Intern Med. 2002;136:99-110.
33. Imperiale TF, Wagner DR, Yin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346:1781-1785.
34. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-168.
Estimating mortality reduction by comparing survival curves
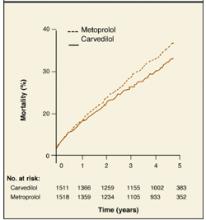
In the Carvedilol or Metoprolol European Trial (COMET),1 patients with heart failure were randomized to receive either carvedilol or metoprolol in addition to their current diuretic and angiotensin-converting enzyme inhibitor. A visual comparison of the survival curves shows a reduction in mortality in the carvedilol group compared with those in the metoprolol group (Figure 1).
Figure 1
Comparing survival curves
How to derive mortality reduction from survival curves
Different statistical methods are used to compare survival curves. Most commonly used is the hazard ratio, the increased speed with which one group is likely to experience an event at any given time in relation to another group. In the COMET trial, the hazard ratio for all-cause mortality was 0.83. This represents a 17% reduction in the risk of death with carvedilol compared with metoprolol.
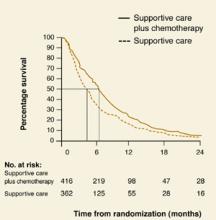
A rough estimate of the hazard ratio can be made by comparing median survival (the time point on each curve that corresponds to 50% survival) in both groups. In Figure 2, patients with non-small-cell lung cancer receiving supportive care (group A) had an approximate median survival of 4 months compared with 6 months in those who had also received chemotherapy (group B).
The hazard ratio is estimated by dividing the median survival time of group A by the median survival time of group B, or 4 months/6 months = 0.66. The reduction in risk of death for group B is therefore 37%. It is possible to estimate the hazard ratio this way only when the percent survival falls below 50% in each group.
Figure 2
Estimating hazard ratios
Correspondence
Mary K. Nordling, MD, Lawrence Family Practice Residency, 34 Haverhill Street, Lawrence, MA 01841. Email: [email protected].
1. Poole-Wilson PA, Swedberg K, Cleland JGF, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet 2003;362:7-13.
2. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899-909.
In the Carvedilol or Metoprolol European Trial (COMET),1 patients with heart failure were randomized to receive either carvedilol or metoprolol in addition to their current diuretic and angiotensin-converting enzyme inhibitor. A visual comparison of the survival curves shows a reduction in mortality in the carvedilol group compared with those in the metoprolol group (Figure 1).
Figure 1
Comparing survival curves
How to derive mortality reduction from survival curves
Different statistical methods are used to compare survival curves. Most commonly used is the hazard ratio, the increased speed with which one group is likely to experience an event at any given time in relation to another group. In the COMET trial, the hazard ratio for all-cause mortality was 0.83. This represents a 17% reduction in the risk of death with carvedilol compared with metoprolol.
A rough estimate of the hazard ratio can be made by comparing median survival (the time point on each curve that corresponds to 50% survival) in both groups. In Figure 2, patients with non-small-cell lung cancer receiving supportive care (group A) had an approximate median survival of 4 months compared with 6 months in those who had also received chemotherapy (group B).
The hazard ratio is estimated by dividing the median survival time of group A by the median survival time of group B, or 4 months/6 months = 0.66. The reduction in risk of death for group B is therefore 37%. It is possible to estimate the hazard ratio this way only when the percent survival falls below 50% in each group.
Figure 2
Estimating hazard ratios
Correspondence
Mary K. Nordling, MD, Lawrence Family Practice Residency, 34 Haverhill Street, Lawrence, MA 01841. Email: [email protected].
In the Carvedilol or Metoprolol European Trial (COMET),1 patients with heart failure were randomized to receive either carvedilol or metoprolol in addition to their current diuretic and angiotensin-converting enzyme inhibitor. A visual comparison of the survival curves shows a reduction in mortality in the carvedilol group compared with those in the metoprolol group (Figure 1).
Figure 1
Comparing survival curves
How to derive mortality reduction from survival curves
Different statistical methods are used to compare survival curves. Most commonly used is the hazard ratio, the increased speed with which one group is likely to experience an event at any given time in relation to another group. In the COMET trial, the hazard ratio for all-cause mortality was 0.83. This represents a 17% reduction in the risk of death with carvedilol compared with metoprolol.
A rough estimate of the hazard ratio can be made by comparing median survival (the time point on each curve that corresponds to 50% survival) in both groups. In Figure 2, patients with non-small-cell lung cancer receiving supportive care (group A) had an approximate median survival of 4 months compared with 6 months in those who had also received chemotherapy (group B).
The hazard ratio is estimated by dividing the median survival time of group A by the median survival time of group B, or 4 months/6 months = 0.66. The reduction in risk of death for group B is therefore 37%. It is possible to estimate the hazard ratio this way only when the percent survival falls below 50% in each group.
Figure 2
Estimating hazard ratios
Correspondence
Mary K. Nordling, MD, Lawrence Family Practice Residency, 34 Haverhill Street, Lawrence, MA 01841. Email: [email protected].
1. Poole-Wilson PA, Swedberg K, Cleland JGF, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet 2003;362:7-13.
2. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899-909.
1. Poole-Wilson PA, Swedberg K, Cleland JGF, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet 2003;362:7-13.
2. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899-909.
Safety and Efficacy of Combined Use of 4-Hydroxyanisole (mequinol) 2%/Tretinoin 0.01% Solution and Sunscreen in Solar Lentigines
Noxious Sensory Perceptions in Patients With Mild to Moderate Rosacea Treated With Azelaic Acid 15% Gel
Raloxifene reduces risk of vertebral fractures and breast cancer in postmenopausal women regardless of prior hormone therapy
- Consider prescribing raloxifene 60 mg/d for postmenopausal women, regardless of whether they have used hormone therapy, to reduce the incidence of vertebral fractures and breast cancer (SOR:B).
Objective: We examined whether past use of hormone therapy influences the effects of raloxifene on the risk of new vertebral fracture, cardiovascular events, or breast cancer.
Study Design: The Multiple Outcomes of Raloxifene Evaluation (MORE) trial examined vertebral fracture incidence as the primary endpoint, breast cancer incidence as a secondary endpoint. Cardiovascular events were collected as secondary safety endpoints.
Population: The MORE trial enrolled 7705 postmenopausal women. Of the 7682 women who reported their previous HT use status, 29% used HT before screening.
Outcomes Measured: Separate logistic regression models analyzed the relationships between prior HT use and the risk of vertebral fracture, cardiovascular events, or breast cancer. Interaction terms with P<.10 were considered to be statistically significant. Confidence intervals for relative risks (RR) were calculated using the Mantel-Haenszel method.
Results: Raloxifene 60 mg/d, the clinically approved dose for osteoporosis prevention and treatment, reduced the risk of vertebral fractures by 54% (RR=0.46) and 29% (RR=0.71) in women with and without prior HT use, respectively (interaction P=.05). A lower incidence of invasive breast cancer in women with prior HT use (RR=0.23) and in women without prior HT use [RR=0.31; interaction P=.60] was observed in women receiving raloxifene (pooled doses). Irrespective of prior HT use, women treated with raloxifene (pooled doses) had no change in incidence of cardiovascular events (interaction P=.56).
Conclusions: The risk of vertebral fractures was lower in women treated with raloxifene, regardless of prior HT use, but there was a suggestion that the effect was greater in women who had used HT. Women randomized to receive raloxifene exhibited a decreased incidence of invasive breast cancer, compared with women receiving placebo. No change occurred in the incidence of cardiovascular events, regardless of prior HT use.
Estrogen-containing hormone therapies (HT) have been used to alleviate menopausal symptoms and to prevent chronic diseases common to postmenopausal women, including osteoporosis and cardiovascular disease.1,2 In this analysis, we use the abbreviation “HT” to refer to postmenopausal hormone therapies, either estrogen alone or combined with progestin.
Based on the findings of the randomized, double-blind Women’s Health Initiative (WHI) study involving estrogen-progestin,3 the Food and Drug Administration (FDA) recommends using HT to treat moderate to severe symptoms of vulvar and vaginal atrophy and vasomotor symptoms associated with the menopause, and to prevent post-menopausal osteoporosis.4 When HT is prescribed only to prevent osteoporosis in women without menopausal symptoms, the FDA recommends that other approved, non-estrogen therapies be considered and that HT be used at the lowest dose for the shortest duration to achieve treatment goals.4 Many postmenopausal women have chosen to discontinue HT in light of these recommendations.5 However, discontinuing HT may increase bone resorption and accelerate bone loss,6,7 which, if untreated, places women at risk for osteoporotic fractures.
The serum estrogen receptor modulator (SERM) raloxifene is not an estrogen, a progestin, or a hormone,8 but it binds to the estrogen receptor to exert effects in the skeletal and cardiovascular systems and in breast tissue.9 In the 4-year Multiple Outcomes of Raloxifene Evaluation (MORE) osteoporosis treatment trial of postmenopausal women, raloxifene 60 mg/d, the approved dose for post-menopausal osteoporosis prevention and treatment, increased bone mineral density (BMD) and significantly reduced the risk for new vertebral fractures with sustained efficacy.10 With the declining use of long-term HT,5 it is clinically relevant and important to determine whether a history of HT use has any influence on the effects of other antiresorptive agents, such as raloxifene, which may be subsequently used for postmenopausal osteoporosis prevention and treatment. The objective of this analysis is to determine the effects of raloxifene on BMD, and the risks of vertebral fractures, cardiovascular events, and breast cancer in post-menopausal women who did or did not use HT prior to screening for the MORE osteoporosis study.
Materials and methods
Subjects and treatment
Details on subject recruitment and follow-up, and complete inclusion and exclusion criteria, were previously described for the MORE study.11 The trial examined the incidence of osteoporotic fractures as a primary endpoint and the incidence of breast cancer as a prespecified secondary endpoint, and it collected reports of cardiovascular events as a secondary safety endpoint.
Researchers enrolled 7705 women up to 80 years of age who were at least 2 years post-menopausal, and who had osteoporosis as defined by radiographically apparent vertebral fractures at baseline or BMD criteria. Women were randomly assigned to receive raloxifene 60 or 120 mg/d, or an identically appearing placebo.11 All women received daily supplements of calcium (500 mg) and vitamin D (400 to 600 IU). An ethical review board at each site approved the MORE study protocol. All women gave written informed consent to participate in the study in accordance with the ethical principles stated in the Declaration of Helsinki.
At study screening, women were asked if they had ever taken HT. Women were excluded from the study if they were experiencing clinically severe menopausal symptoms at the beginning of the study that required estrogen. Women were excluded if they had been treated with therapeutic doses of androgen, calcitonin, or estrogen (>1 cycle or 28 days) alone or with progestin (>1 cycle or 28 days) within 6 months of beginning the study. Women were permitted to have used systemic (oral or transdermal) estrogen and progestin for up to 1 cycle (28 days) during the 6 months before the study. No systemic estrogen and progestin use was allowed within 2 months before study entry. Occasional use of topical estrogens (3 times per week), and oral estriol (2 mg/d) for menopausal symptoms was permitted.
A 1-year double-blind extension phase was added to the 3-year treatment phase.10 All vertebral fracture, cardiovascular, and breast cancer endpoints that occurred over the 48-month study period were included in the present analyses.
Fracture and BMD assessment
New morphometric vertebral fractures, defined using semiquantitative assessment criteria,12 were identified by comparing spinal radiographs taken at 2, 3, and 4 years with baseline radiographs. A new vertebral fracture was defined as a vertebral fracture occurring in a vertebra that was not fractured at baseline. New clinical vertebral fractures were defined as those associated with signs or symptoms suggestive of vertebral fracture, such as back pain, reported either at an interim 6-month clinic visit or at any time between clinic visits,13 and which were subsequently corroborated with radiographs and adjudicated as previously described.10
Lumbar spine and femoral neck BMD were measured annually using dual-energy x-ray densitometry, as previously described.11
Cardiovascular event assessment
Cardiovascular events were collected as a secondary safety endpoint in the MORE trial, as previously described in detail.14 Women were asked at each clinic visit if they had had a myocardial infarction (MI), coronary bypass surgery, percutaneous coronary revascularization, or a stroke since the previous visit, and unsolicited reports of adverse cardiovascular events were recorded. All summaries of reported cardiovascular events were reviewed and adjudicated by 1 board-certified cardiologist, contracted by the sponsor, who was not associated with the trial and was blinded to treatment assignment.
Women with 4 or more risk points, assessed using the same criteria as for enrollment in the Raloxifene Use for The Heart (RUTH) trial,15 were considered to be at high risk for cardiovascular events. Coronary events included MI, unstable angina, or coronary ischemia.14 Cerebrovascular events included stroke and transient ischemic attack.14
Breast cancer assessment
Assessment of breast cancer was a prespecified secondary endpoint of the MORE trial, and was previously described in detail.16 All diagnoses of breast cancer were adjudicated by an independent oncology review board consisting of 5 physician specialists in breast cancer, and chaired by a pharmacologic scientist, none of whom were employed by the sponsor. Previous publications of the MORE breast cancer data have reported 61 cases of invasive breast cancer.16 A subsequent review of the MORE dataset found 1 fewer case of invasive breast cancer in each of the placebo and pooled raloxifene groups, so that 59 cases of invasive breast cancer were confirmed, and this number will be used in the present analysis. The change in the number of cases of invasive breast cancer was small, and had no impact on the overall interpretation of the breast cancer results from the MORE trial.
Results
Characteristics for all subjects at baseline and for women in the placebo group
Of the 7705 women enrolled, 7682 (99.7%) reported their status of previous HT use, with 2235 women (29.1%) having used HT before participating in MORE ( W1, available at www.jfponline.com). Baseline characteristics that were significantly different between women who reported prior HT use and those who reported no prior HT use included age, BMD, and the incidences of vertebral fractures, coronary angioplasty, hypertension, hyperlipidemia, and family histories of osteoporosis or breast cancer (Table W1, available at www.jfponline.com). In the subsets of women who did and who did not use HT previously, the baseline characteristics were not significantly different between the placebo and raloxifene groups, except for diabetes (placebo, 1.9%; raloxifene 60 mg/day, 3.3%; pooled raloxifene, 2.9%; P=.02).
In the placebo group, the incidence of new vertebral fractures, cardiovascular events, and breast cancer at 4 years were not significantly different in women with prior HT use compared with women without prior HT use.
Vertebral fracture events and bone mineral density
After 4 years of treatment with raloxifene 60 mg/d, women with and without prior HT use exhibited significant reductions in new vertebral fractures compared with those taking placebo ( Figure ). Vertebral fracture risk reductions between treatment groups were also statistically significant in subgroups of women with and without prevalent vertebral fractures ( Table1 ). Raloxifene 60 mg/d also reduced the risk of new clinically-apparent vertebral fractures, compared with placebo, in women with prior HT use (absolute risk reduction (ARR)=1.8%; RR=0.52 [95% CI, 0.28–0.96]), and in women without prior HT use (ARR=1.7%; RR=0.61 [95% CI, 0.43–0.87]; interaction P=.66]. The interaction P-values remained similar after adjusting for the baseline fracture risk factors (TableW1 , at www.jfponline.com) that were significantly different between women with and women without prior HT use. Women with and without prior HT use treated with raloxifene 60 mg/d had significant increases in BMD, at the lumbar spine (2.7% and 2.5%, respectively; interaction P=.54), and femoral neck (2.6% and 1.9% respectively; interaction P=.06), compared with placebo. Similar results were observed for fracture and BMD in women treated with the pooled raloxifene doses.
TABLE 1
Absolute and relative risks of new vertebral fractures with raloxifene 60 mg/d compared with placeboa
| Women who had used HT (n=1305) | Women who had notused HT (n=3232) | Overall study population d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=654) | Raloxifene 60 mg/d(n=651) | RR (95% CI)b [ARR] | Placebo (n=1634) | Raloxifene 60 md/d (n=1598) | RR (95% CI)[ARR] | Interaction P-valuec | RR (95% CI) | ||
| Women with prevalent fractures | 25.36% | 13.68% | 0.54(0.36–0.81)[11.68%] | 24.24% | 17.11% | 0.71(0.57–0.88)[7.13%] | .27 | 0.66(0.55–0.81) | |
| Women without prevalent vertebral | 6.29% | 1.82% | 0.29(0.13–0.63) [4.47%] | 5.54% | 3.46% | 0.62(0.41–0.95)[2.08%] | .09 | 0.51(0.35–0.73) | |
| a Results with raloxifene 60 mg/d are shown since this is the clinically approved dose for osteoporosis prevention and treatment. Results with pooled raloxifene doses were similar. Data from women with at least one post-baseline follow-up spinal radiograph were included in this table. | |||||||||
| b RR (95% CI) denotes relative risk (95% confidence interval) Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. Similar results were observed with raloxifene (pooled 60 mg/d and 120 mg/d doses) on vertebral fracture risk, in women who previously used HT [ARR=6.6%; RR=0.47 (95% CI, 0.35–0.63)], and in women without prior HT use [ARR=4.4%; RR=0.66 (95% CI, 0.55–0.78); interaction P=.06] | |||||||||
| c Results for the overall study population would be used unless the interaction effects between therapy group and prior HT use were statistically significant (P<.10), in which case, the results in the subgroups of women with and women without prior HT use should be used. | |||||||||
| d Delmas et al10 published the results for the overall study population, regardless of whether or not information on the participants’ HT use was available. | |||||||||
FIGURE
Percentage of women with and without prior HT use who experienced new vertebral fractures in the 4-year MORE study
This analysis included all women with at least 1 post-baseline follow-up vertebral radiograph, who reported their status of prior HT use. The relative risks (RR) and 95% confidence intervals (CI) are shown for women treated with either placebo or raloxifene 60 mg/d. The absolute risk reductions were 6.7% in women with prior HT use and 3.7% in women without prior HT use. The interaction P-value was .05.
Cardiovascular events
In women with and without prior HT use, treatment with raloxifene (pooled doses) did not result in statistically significant changes in the incidence of new cardiovascular, coronary, or cerebrovascu-lar events, compared with placebo ( Table2 ). In a subgroup of women who were at high risk of cardiovascular disease,15 prior HT use had no effect on the incidence of new cardiovascular events with raloxifene (pooled doses) treatment ( Table 2 ). The interaction P-values remained similar after adjusting for the baseline cardiovascular risk factors (Table W1, at www.jfponline.com) that were significantly different between women with and without prior HT use.
TABLE 2
Absolute and relative risks of cardiovascular events with raloxifene (pooled doses) compared with placeboa
| Women who had used HT (n=2235) | Women who had not used HT (n=5447) | Overall studypopulation | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo(n=738) | Pooled raloxifene(n=1497) | RR (95% CI)b [ARR] | Placebo (n=1833) | Pooled raloxifene (n=3614) | RR(95% CI)[ARR] | Interaction P-valuec | RR(95% CI) | |
| Cardiovascular events | 2.71% | 2.87% | 1.06(0.63–1.79)[–0.16%] | 4.15% | 3.68% | 0.89(0.67–1.17) [0.47%] | .56 | 0.92(0.72–1.18) |
| Coronary events | 1.49% | 1.74% | 1.17 (0.58–2.35)[–0.25%] | 2.40% | 2.08% | 0.87 (0.60–1.25) [0.32%] | .46 | 0.92(0.67–1.28) |
| Cerebrovascularevents | 1.22% | 1.14% | 0.93(0.42–2.08)[0.08%] | 1.75% | 1.63% | 0.94 (0.61–1.43)[.012%] | .99 | 0.93(0.64–1.36) |
| Cardiovascularevents in high-risk subgroupd | 12.66% | 5.91% | .047(0.21–1.06)[6.75%] | 13.08% | 8.54% | 0.65 (0.42–1.01)[4.54%] | .49 | 0.60(0.41–0.88) [4.54%] |
| a Pooled raloxifene doses were used in this analysis, since there were few events. There were no differences in the incidence of events between the raloxifene doses. | ||||||||
| b Relative risk (RR), 95% confidence interval (95% CI). Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. | ||||||||
| c The interaction effects between therapy group and prior HT use were not statistically significant (P>.10), so results from the overall study population would be used. Barrett-Connor et al14 reported the results for raloxifene 60 mg/d and raloxifene 120 mg/d in the overall study population, regardless of whether or not information on the participants’ HT use was available. | ||||||||
| d Of the 1029 women in the high-risk subgroup, 764 women had no prior history of HT use (placebo, n=237; raloxifene, n=527), and 265 women reported prior HT use (placebo, n=79; raloxifene, n=186). | ||||||||
Breast cancer
In women with and without prior HT use, similar reductions in the incidence of breast cancer (regardless of invasiveness), invasive breast cancer, and estrogen-receptor positive invasive breast cancer, were observed after raloxifene treatment (pooled doses) compared with placebo ( Table 3 ). The interaction P-values remained similar after adjusting for the baseline breast cancer risk factors (Table W1) that were significantly different between women with and without prior HT use.
TABLE 3
Absolute and relative risks of breast cancer with raloxifene (pooled doses) compared with placeboa
| Women who had used HT (n=2235) | Women who had notused HT (n=5447) | Overall study population | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo(n=738) | Pooledraloxifene(n=1497) | RR(95% CI)b [ARR] | Placebo(n=1833) | Pooled raloxifene(n=3614) | RR(95% CI)[ARR] | InteractionP-valuec | RR(95% CI) | |
| Breast cancer d | 2.30% | 0.73% | 0.32(0.15–0.68)[1.57%] | 1.47% | 0.64% | 0.43(0.25–0.75)[0.83%] | .52 | 0.38 (0.24–0.58) |
| Invasivebreast cancer | 2.03% | 0.47% | 0.23(0.09–0.56)[1.56%] | 1.25% | 0.39% | 0.31(0.16–0.60) [0.86%] | .60 | 0.28(0.17–0.46) |
| Invasiveestrogen-receptorpositive breastcancer | 1.76% | 0.27% | 0.15(0.05–0.46)[1.49%] | 0.87% | 0.17% | 0.19(0.08–0.49)[0.70%] | .75 | 0.16(0.09–0.30) |
| a Pooled raloxifene doses were used in this analysis, since there were few events. There were no differences in the incidence of events between the raloxifene doses. | ||||||||
| b Relative risk (RR), 95% confidence interval (95% CI). Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. | ||||||||
| c The interaction effects between therapy group and prior HT use were not statistically significant (P>.10), so results from the overall study population would be used. Cauley et al16 reported the results for in the overall study population, regardless of whether or not information on the participants’ HT use was available. | ||||||||
| d All breast cancer, regardless of invasiveness. | ||||||||
Discussion
This analysis examined the effects of raloxifene in women who reported their use of postmenopausal hormone therapies before enrolling in the MORE osteoporosis trial. Compared with placebo, women treated with raloxifene experienced significant decreases in the risks for new vertebral fractures and the incidence of breast cancer, without significant changes in the incidence of cardiovascular events, regardless of previous HT use. These analyses provide further information on the effects of raloxifene on the risks of vertebral fractures,10 cardiovascular events,14 and breast cancer16 seen in the overall MORE study population at 4 years.
This analysis found a differential reduction in vertebral fracture risk with raloxifene between women who did and did not have prior HT use, which may result from possible differences in women who chose to use HT before participating in MORE. In women with previous HT use, a greater proportion had a family history of osteo-porosis and a lower proportion had prevalent ver-tebral fractures at baseline, compared with women who had not used HT. Other unidentified confounding factors, such as the “healthy user” bias commonly associated with women who chose to use HT,17 may also contribute to the differential vertebral fracture risk reduction with raloxifene treatment. The Study of Osteoporotic Fractures showed that women with current estrogen use had significantly decreased fracture risks, but the risk reduction waned in women who discontinued estrogen.18 After HT discontinuation, BMD loss resumes at a rate similar to that seen in women shortly after menopause, suggesting that prior HT use may have limited residual effects on maintaining BMD.7,19 Such findings raise the urgency of evaluating the risk for osteoporosis in women who discontinue HT.
Women treated with raloxifene had no significant changes in the incidence of cardiovascular events, with no differential treatment effect based on prior HT use. In the HERS20 and WHI3 trials, which studied the outcomes of estrogen-progestin therapy in postmenopausal women, similar analyses did not show any significant differential effects of prior HT use on the incidence of cardiovascular events with estrogen-progestin during the respective trials.
In this analysis, women treated with raloxifene had a significantly lower incidence of breast cancer compared with those who received placebo, and this incidence was comparable between women with and without prior HT use. In contrast, women who had used HT before the WHI study had a significant increase in the risk of breast cancer with estrogen-progestin therapy during the study, compared with those who had not used HT.3
A limitation of our analysis that a history of HT use was based on participants’ self-report, which depended on their ability to recall medication they may have taken years earlier. Also, no information was obtained on therapy duration and the doses and formulations of HT. Since the MORE trial was conducted in 25 countries, the patterns and types of HT regimens are expected to be different. The strength of our analysis is that the MORE population was large enough to prospectively collect data on multiple clinical outcomes.
In summary, postmenopausal women treated with raloxifene experienced a significant risk reduction for vertebral fractures, regardless of prior HT use, but women who had used HT may exhibit greater reductions. Women who used raloxifene had no change in the incidence of cardiovascular events and a lower incidence of breast cancer, compared with placebo, regardless of their history of HT use. Since HT is becoming increasingly limited to short-term use for menopausal symptoms, women and their physicians may consider several other therapeutic options to address postmenopausal health concerns.
Acknowledgments
The authors acknowledge the contributions of Leo Plouffe Jr., MD, and Somnath Sarkar, PhD, for suggestions on manuscript content, and Sharon Xiaohan Zou, MS, for statistical programming. A complete list of all investigators in the MORE trial is found in J Clin Endocrinol Metab 2002; 87:3609–3617. Portions of this work were presented at the following meetings: Third European Symposium on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Barcelona, Spain, 2002; International Society for Clinical Densitometry (ISCD), Los Angeles, USA,2003; European Calcified Tissue Society (ECTS), Rome, Italy, 2003; International Bone and Mineral Society (IBMS), Osaka, Japan, 2003; European Menopause and Andropause Society (EMAS), Bucharest, Romania, 2003; Ninth Bath Conference on Osteoporosis, Bath, UK, 2003. Eli Lilly and Company sponsored the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial.
Corresponding author
Olof Johnell, MD, PhD, Department of Orthopedics, Universitetssjukhuset MAS, Malmo, SE-20502, Sweden. E-mail: [email protected].
1. Greendale GA, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-580.
2. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992;117:1016-1037.
3. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-333.
4. Bren L. The estrogen and progestin dilemma: New advice, labeling and guidelines. FDA Consumer 2003;37:10-11.
5. Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 2004;140:184-188.
6. Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 2002;87:4914-4923.
7. Tremollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant ver-tebral bone loss in postmenopausal women. Osteoporos Int 2001;12:385-390.
8. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators —mechanisms of action and application to clinical practice. N Engl J Med 2003;348:618-629.
9. Maricic M, Gluck O. Review of raloxifene and its clinical applications in osteoporosis. Expert Opin Pharmacother 2002;3:767-775.
10. Delmas PD, Ensrud KE, Adachi JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 2002;87:3609-3617.
11. Ettinger B, Black DM, Mitlak BH, et al. Reduction of verte-bral fracture risk in postmenopausal women with osteo-porosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999;282:637-645.
12. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-1148.
13. Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002;162:1140-1143.
14. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002;287:847-857.
15. Mosca L, Barrett-Connor E, Wenger NK, et al. Design and methods of the Raloxifene Use for The Heart (RUTH) study. Am J Cardiol 2001;88:392-395.
16. Cauley J, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year Results from the MORE Trial. Breast Cancer Res Treat 2001;65:125-134.
17. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med 2003;348:645-650.
18. Cauley J, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med 1995;122:9-16.
19. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-672.
20. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study Follow-up (HERS II). JAMA 2002;288:49-57.
21. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 1998;8:468-489.
- Consider prescribing raloxifene 60 mg/d for postmenopausal women, regardless of whether they have used hormone therapy, to reduce the incidence of vertebral fractures and breast cancer (SOR:B).
Objective: We examined whether past use of hormone therapy influences the effects of raloxifene on the risk of new vertebral fracture, cardiovascular events, or breast cancer.
Study Design: The Multiple Outcomes of Raloxifene Evaluation (MORE) trial examined vertebral fracture incidence as the primary endpoint, breast cancer incidence as a secondary endpoint. Cardiovascular events were collected as secondary safety endpoints.
Population: The MORE trial enrolled 7705 postmenopausal women. Of the 7682 women who reported their previous HT use status, 29% used HT before screening.
Outcomes Measured: Separate logistic regression models analyzed the relationships between prior HT use and the risk of vertebral fracture, cardiovascular events, or breast cancer. Interaction terms with P<.10 were considered to be statistically significant. Confidence intervals for relative risks (RR) were calculated using the Mantel-Haenszel method.
Results: Raloxifene 60 mg/d, the clinically approved dose for osteoporosis prevention and treatment, reduced the risk of vertebral fractures by 54% (RR=0.46) and 29% (RR=0.71) in women with and without prior HT use, respectively (interaction P=.05). A lower incidence of invasive breast cancer in women with prior HT use (RR=0.23) and in women without prior HT use [RR=0.31; interaction P=.60] was observed in women receiving raloxifene (pooled doses). Irrespective of prior HT use, women treated with raloxifene (pooled doses) had no change in incidence of cardiovascular events (interaction P=.56).
Conclusions: The risk of vertebral fractures was lower in women treated with raloxifene, regardless of prior HT use, but there was a suggestion that the effect was greater in women who had used HT. Women randomized to receive raloxifene exhibited a decreased incidence of invasive breast cancer, compared with women receiving placebo. No change occurred in the incidence of cardiovascular events, regardless of prior HT use.
Estrogen-containing hormone therapies (HT) have been used to alleviate menopausal symptoms and to prevent chronic diseases common to postmenopausal women, including osteoporosis and cardiovascular disease.1,2 In this analysis, we use the abbreviation “HT” to refer to postmenopausal hormone therapies, either estrogen alone or combined with progestin.
Based on the findings of the randomized, double-blind Women’s Health Initiative (WHI) study involving estrogen-progestin,3 the Food and Drug Administration (FDA) recommends using HT to treat moderate to severe symptoms of vulvar and vaginal atrophy and vasomotor symptoms associated with the menopause, and to prevent post-menopausal osteoporosis.4 When HT is prescribed only to prevent osteoporosis in women without menopausal symptoms, the FDA recommends that other approved, non-estrogen therapies be considered and that HT be used at the lowest dose for the shortest duration to achieve treatment goals.4 Many postmenopausal women have chosen to discontinue HT in light of these recommendations.5 However, discontinuing HT may increase bone resorption and accelerate bone loss,6,7 which, if untreated, places women at risk for osteoporotic fractures.
The serum estrogen receptor modulator (SERM) raloxifene is not an estrogen, a progestin, or a hormone,8 but it binds to the estrogen receptor to exert effects in the skeletal and cardiovascular systems and in breast tissue.9 In the 4-year Multiple Outcomes of Raloxifene Evaluation (MORE) osteoporosis treatment trial of postmenopausal women, raloxifene 60 mg/d, the approved dose for post-menopausal osteoporosis prevention and treatment, increased bone mineral density (BMD) and significantly reduced the risk for new vertebral fractures with sustained efficacy.10 With the declining use of long-term HT,5 it is clinically relevant and important to determine whether a history of HT use has any influence on the effects of other antiresorptive agents, such as raloxifene, which may be subsequently used for postmenopausal osteoporosis prevention and treatment. The objective of this analysis is to determine the effects of raloxifene on BMD, and the risks of vertebral fractures, cardiovascular events, and breast cancer in post-menopausal women who did or did not use HT prior to screening for the MORE osteoporosis study.
Materials and methods
Subjects and treatment
Details on subject recruitment and follow-up, and complete inclusion and exclusion criteria, were previously described for the MORE study.11 The trial examined the incidence of osteoporotic fractures as a primary endpoint and the incidence of breast cancer as a prespecified secondary endpoint, and it collected reports of cardiovascular events as a secondary safety endpoint.
Researchers enrolled 7705 women up to 80 years of age who were at least 2 years post-menopausal, and who had osteoporosis as defined by radiographically apparent vertebral fractures at baseline or BMD criteria. Women were randomly assigned to receive raloxifene 60 or 120 mg/d, or an identically appearing placebo.11 All women received daily supplements of calcium (500 mg) and vitamin D (400 to 600 IU). An ethical review board at each site approved the MORE study protocol. All women gave written informed consent to participate in the study in accordance with the ethical principles stated in the Declaration of Helsinki.
At study screening, women were asked if they had ever taken HT. Women were excluded from the study if they were experiencing clinically severe menopausal symptoms at the beginning of the study that required estrogen. Women were excluded if they had been treated with therapeutic doses of androgen, calcitonin, or estrogen (>1 cycle or 28 days) alone or with progestin (>1 cycle or 28 days) within 6 months of beginning the study. Women were permitted to have used systemic (oral or transdermal) estrogen and progestin for up to 1 cycle (28 days) during the 6 months before the study. No systemic estrogen and progestin use was allowed within 2 months before study entry. Occasional use of topical estrogens (3 times per week), and oral estriol (2 mg/d) for menopausal symptoms was permitted.
A 1-year double-blind extension phase was added to the 3-year treatment phase.10 All vertebral fracture, cardiovascular, and breast cancer endpoints that occurred over the 48-month study period were included in the present analyses.
Fracture and BMD assessment
New morphometric vertebral fractures, defined using semiquantitative assessment criteria,12 were identified by comparing spinal radiographs taken at 2, 3, and 4 years with baseline radiographs. A new vertebral fracture was defined as a vertebral fracture occurring in a vertebra that was not fractured at baseline. New clinical vertebral fractures were defined as those associated with signs or symptoms suggestive of vertebral fracture, such as back pain, reported either at an interim 6-month clinic visit or at any time between clinic visits,13 and which were subsequently corroborated with radiographs and adjudicated as previously described.10
Lumbar spine and femoral neck BMD were measured annually using dual-energy x-ray densitometry, as previously described.11
Cardiovascular event assessment
Cardiovascular events were collected as a secondary safety endpoint in the MORE trial, as previously described in detail.14 Women were asked at each clinic visit if they had had a myocardial infarction (MI), coronary bypass surgery, percutaneous coronary revascularization, or a stroke since the previous visit, and unsolicited reports of adverse cardiovascular events were recorded. All summaries of reported cardiovascular events were reviewed and adjudicated by 1 board-certified cardiologist, contracted by the sponsor, who was not associated with the trial and was blinded to treatment assignment.
Women with 4 or more risk points, assessed using the same criteria as for enrollment in the Raloxifene Use for The Heart (RUTH) trial,15 were considered to be at high risk for cardiovascular events. Coronary events included MI, unstable angina, or coronary ischemia.14 Cerebrovascular events included stroke and transient ischemic attack.14
Breast cancer assessment
Assessment of breast cancer was a prespecified secondary endpoint of the MORE trial, and was previously described in detail.16 All diagnoses of breast cancer were adjudicated by an independent oncology review board consisting of 5 physician specialists in breast cancer, and chaired by a pharmacologic scientist, none of whom were employed by the sponsor. Previous publications of the MORE breast cancer data have reported 61 cases of invasive breast cancer.16 A subsequent review of the MORE dataset found 1 fewer case of invasive breast cancer in each of the placebo and pooled raloxifene groups, so that 59 cases of invasive breast cancer were confirmed, and this number will be used in the present analysis. The change in the number of cases of invasive breast cancer was small, and had no impact on the overall interpretation of the breast cancer results from the MORE trial.
Results
Characteristics for all subjects at baseline and for women in the placebo group
Of the 7705 women enrolled, 7682 (99.7%) reported their status of previous HT use, with 2235 women (29.1%) having used HT before participating in MORE ( W1, available at www.jfponline.com). Baseline characteristics that were significantly different between women who reported prior HT use and those who reported no prior HT use included age, BMD, and the incidences of vertebral fractures, coronary angioplasty, hypertension, hyperlipidemia, and family histories of osteoporosis or breast cancer (Table W1, available at www.jfponline.com). In the subsets of women who did and who did not use HT previously, the baseline characteristics were not significantly different between the placebo and raloxifene groups, except for diabetes (placebo, 1.9%; raloxifene 60 mg/day, 3.3%; pooled raloxifene, 2.9%; P=.02).
In the placebo group, the incidence of new vertebral fractures, cardiovascular events, and breast cancer at 4 years were not significantly different in women with prior HT use compared with women without prior HT use.
Vertebral fracture events and bone mineral density
After 4 years of treatment with raloxifene 60 mg/d, women with and without prior HT use exhibited significant reductions in new vertebral fractures compared with those taking placebo ( Figure ). Vertebral fracture risk reductions between treatment groups were also statistically significant in subgroups of women with and without prevalent vertebral fractures ( Table1 ). Raloxifene 60 mg/d also reduced the risk of new clinically-apparent vertebral fractures, compared with placebo, in women with prior HT use (absolute risk reduction (ARR)=1.8%; RR=0.52 [95% CI, 0.28–0.96]), and in women without prior HT use (ARR=1.7%; RR=0.61 [95% CI, 0.43–0.87]; interaction P=.66]. The interaction P-values remained similar after adjusting for the baseline fracture risk factors (TableW1 , at www.jfponline.com) that were significantly different between women with and women without prior HT use. Women with and without prior HT use treated with raloxifene 60 mg/d had significant increases in BMD, at the lumbar spine (2.7% and 2.5%, respectively; interaction P=.54), and femoral neck (2.6% and 1.9% respectively; interaction P=.06), compared with placebo. Similar results were observed for fracture and BMD in women treated with the pooled raloxifene doses.
TABLE 1
Absolute and relative risks of new vertebral fractures with raloxifene 60 mg/d compared with placeboa
| Women who had used HT (n=1305) | Women who had notused HT (n=3232) | Overall study population d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=654) | Raloxifene 60 mg/d(n=651) | RR (95% CI)b [ARR] | Placebo (n=1634) | Raloxifene 60 md/d (n=1598) | RR (95% CI)[ARR] | Interaction P-valuec | RR (95% CI) | ||
| Women with prevalent fractures | 25.36% | 13.68% | 0.54(0.36–0.81)[11.68%] | 24.24% | 17.11% | 0.71(0.57–0.88)[7.13%] | .27 | 0.66(0.55–0.81) | |
| Women without prevalent vertebral | 6.29% | 1.82% | 0.29(0.13–0.63) [4.47%] | 5.54% | 3.46% | 0.62(0.41–0.95)[2.08%] | .09 | 0.51(0.35–0.73) | |
| a Results with raloxifene 60 mg/d are shown since this is the clinically approved dose for osteoporosis prevention and treatment. Results with pooled raloxifene doses were similar. Data from women with at least one post-baseline follow-up spinal radiograph were included in this table. | |||||||||
| b RR (95% CI) denotes relative risk (95% confidence interval) Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. Similar results were observed with raloxifene (pooled 60 mg/d and 120 mg/d doses) on vertebral fracture risk, in women who previously used HT [ARR=6.6%; RR=0.47 (95% CI, 0.35–0.63)], and in women without prior HT use [ARR=4.4%; RR=0.66 (95% CI, 0.55–0.78); interaction P=.06] | |||||||||
| c Results for the overall study population would be used unless the interaction effects between therapy group and prior HT use were statistically significant (P<.10), in which case, the results in the subgroups of women with and women without prior HT use should be used. | |||||||||
| d Delmas et al10 published the results for the overall study population, regardless of whether or not information on the participants’ HT use was available. | |||||||||
FIGURE
Percentage of women with and without prior HT use who experienced new vertebral fractures in the 4-year MORE study
This analysis included all women with at least 1 post-baseline follow-up vertebral radiograph, who reported their status of prior HT use. The relative risks (RR) and 95% confidence intervals (CI) are shown for women treated with either placebo or raloxifene 60 mg/d. The absolute risk reductions were 6.7% in women with prior HT use and 3.7% in women without prior HT use. The interaction P-value was .05.
Cardiovascular events
In women with and without prior HT use, treatment with raloxifene (pooled doses) did not result in statistically significant changes in the incidence of new cardiovascular, coronary, or cerebrovascu-lar events, compared with placebo ( Table2 ). In a subgroup of women who were at high risk of cardiovascular disease,15 prior HT use had no effect on the incidence of new cardiovascular events with raloxifene (pooled doses) treatment ( Table 2 ). The interaction P-values remained similar after adjusting for the baseline cardiovascular risk factors (Table W1, at www.jfponline.com) that were significantly different between women with and without prior HT use.
TABLE 2
Absolute and relative risks of cardiovascular events with raloxifene (pooled doses) compared with placeboa
| Women who had used HT (n=2235) | Women who had not used HT (n=5447) | Overall studypopulation | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo(n=738) | Pooled raloxifene(n=1497) | RR (95% CI)b [ARR] | Placebo (n=1833) | Pooled raloxifene (n=3614) | RR(95% CI)[ARR] | Interaction P-valuec | RR(95% CI) | |
| Cardiovascular events | 2.71% | 2.87% | 1.06(0.63–1.79)[–0.16%] | 4.15% | 3.68% | 0.89(0.67–1.17) [0.47%] | .56 | 0.92(0.72–1.18) |
| Coronary events | 1.49% | 1.74% | 1.17 (0.58–2.35)[–0.25%] | 2.40% | 2.08% | 0.87 (0.60–1.25) [0.32%] | .46 | 0.92(0.67–1.28) |
| Cerebrovascularevents | 1.22% | 1.14% | 0.93(0.42–2.08)[0.08%] | 1.75% | 1.63% | 0.94 (0.61–1.43)[.012%] | .99 | 0.93(0.64–1.36) |
| Cardiovascularevents in high-risk subgroupd | 12.66% | 5.91% | .047(0.21–1.06)[6.75%] | 13.08% | 8.54% | 0.65 (0.42–1.01)[4.54%] | .49 | 0.60(0.41–0.88) [4.54%] |
| a Pooled raloxifene doses were used in this analysis, since there were few events. There were no differences in the incidence of events between the raloxifene doses. | ||||||||
| b Relative risk (RR), 95% confidence interval (95% CI). Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. | ||||||||
| c The interaction effects between therapy group and prior HT use were not statistically significant (P>.10), so results from the overall study population would be used. Barrett-Connor et al14 reported the results for raloxifene 60 mg/d and raloxifene 120 mg/d in the overall study population, regardless of whether or not information on the participants’ HT use was available. | ||||||||
| d Of the 1029 women in the high-risk subgroup, 764 women had no prior history of HT use (placebo, n=237; raloxifene, n=527), and 265 women reported prior HT use (placebo, n=79; raloxifene, n=186). | ||||||||
Breast cancer
In women with and without prior HT use, similar reductions in the incidence of breast cancer (regardless of invasiveness), invasive breast cancer, and estrogen-receptor positive invasive breast cancer, were observed after raloxifene treatment (pooled doses) compared with placebo ( Table 3 ). The interaction P-values remained similar after adjusting for the baseline breast cancer risk factors (Table W1) that were significantly different between women with and without prior HT use.
TABLE 3
Absolute and relative risks of breast cancer with raloxifene (pooled doses) compared with placeboa
| Women who had used HT (n=2235) | Women who had notused HT (n=5447) | Overall study population | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo(n=738) | Pooledraloxifene(n=1497) | RR(95% CI)b [ARR] | Placebo(n=1833) | Pooled raloxifene(n=3614) | RR(95% CI)[ARR] | InteractionP-valuec | RR(95% CI) | |
| Breast cancer d | 2.30% | 0.73% | 0.32(0.15–0.68)[1.57%] | 1.47% | 0.64% | 0.43(0.25–0.75)[0.83%] | .52 | 0.38 (0.24–0.58) |
| Invasivebreast cancer | 2.03% | 0.47% | 0.23(0.09–0.56)[1.56%] | 1.25% | 0.39% | 0.31(0.16–0.60) [0.86%] | .60 | 0.28(0.17–0.46) |
| Invasiveestrogen-receptorpositive breastcancer | 1.76% | 0.27% | 0.15(0.05–0.46)[1.49%] | 0.87% | 0.17% | 0.19(0.08–0.49)[0.70%] | .75 | 0.16(0.09–0.30) |
| a Pooled raloxifene doses were used in this analysis, since there were few events. There were no differences in the incidence of events between the raloxifene doses. | ||||||||
| b Relative risk (RR), 95% confidence interval (95% CI). Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. | ||||||||
| c The interaction effects between therapy group and prior HT use were not statistically significant (P>.10), so results from the overall study population would be used. Cauley et al16 reported the results for in the overall study population, regardless of whether or not information on the participants’ HT use was available. | ||||||||
| d All breast cancer, regardless of invasiveness. | ||||||||
Discussion
This analysis examined the effects of raloxifene in women who reported their use of postmenopausal hormone therapies before enrolling in the MORE osteoporosis trial. Compared with placebo, women treated with raloxifene experienced significant decreases in the risks for new vertebral fractures and the incidence of breast cancer, without significant changes in the incidence of cardiovascular events, regardless of previous HT use. These analyses provide further information on the effects of raloxifene on the risks of vertebral fractures,10 cardiovascular events,14 and breast cancer16 seen in the overall MORE study population at 4 years.
This analysis found a differential reduction in vertebral fracture risk with raloxifene between women who did and did not have prior HT use, which may result from possible differences in women who chose to use HT before participating in MORE. In women with previous HT use, a greater proportion had a family history of osteo-porosis and a lower proportion had prevalent ver-tebral fractures at baseline, compared with women who had not used HT. Other unidentified confounding factors, such as the “healthy user” bias commonly associated with women who chose to use HT,17 may also contribute to the differential vertebral fracture risk reduction with raloxifene treatment. The Study of Osteoporotic Fractures showed that women with current estrogen use had significantly decreased fracture risks, but the risk reduction waned in women who discontinued estrogen.18 After HT discontinuation, BMD loss resumes at a rate similar to that seen in women shortly after menopause, suggesting that prior HT use may have limited residual effects on maintaining BMD.7,19 Such findings raise the urgency of evaluating the risk for osteoporosis in women who discontinue HT.
Women treated with raloxifene had no significant changes in the incidence of cardiovascular events, with no differential treatment effect based on prior HT use. In the HERS20 and WHI3 trials, which studied the outcomes of estrogen-progestin therapy in postmenopausal women, similar analyses did not show any significant differential effects of prior HT use on the incidence of cardiovascular events with estrogen-progestin during the respective trials.
In this analysis, women treated with raloxifene had a significantly lower incidence of breast cancer compared with those who received placebo, and this incidence was comparable between women with and without prior HT use. In contrast, women who had used HT before the WHI study had a significant increase in the risk of breast cancer with estrogen-progestin therapy during the study, compared with those who had not used HT.3
A limitation of our analysis that a history of HT use was based on participants’ self-report, which depended on their ability to recall medication they may have taken years earlier. Also, no information was obtained on therapy duration and the doses and formulations of HT. Since the MORE trial was conducted in 25 countries, the patterns and types of HT regimens are expected to be different. The strength of our analysis is that the MORE population was large enough to prospectively collect data on multiple clinical outcomes.
In summary, postmenopausal women treated with raloxifene experienced a significant risk reduction for vertebral fractures, regardless of prior HT use, but women who had used HT may exhibit greater reductions. Women who used raloxifene had no change in the incidence of cardiovascular events and a lower incidence of breast cancer, compared with placebo, regardless of their history of HT use. Since HT is becoming increasingly limited to short-term use for menopausal symptoms, women and their physicians may consider several other therapeutic options to address postmenopausal health concerns.
Acknowledgments
The authors acknowledge the contributions of Leo Plouffe Jr., MD, and Somnath Sarkar, PhD, for suggestions on manuscript content, and Sharon Xiaohan Zou, MS, for statistical programming. A complete list of all investigators in the MORE trial is found in J Clin Endocrinol Metab 2002; 87:3609–3617. Portions of this work were presented at the following meetings: Third European Symposium on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Barcelona, Spain, 2002; International Society for Clinical Densitometry (ISCD), Los Angeles, USA,2003; European Calcified Tissue Society (ECTS), Rome, Italy, 2003; International Bone and Mineral Society (IBMS), Osaka, Japan, 2003; European Menopause and Andropause Society (EMAS), Bucharest, Romania, 2003; Ninth Bath Conference on Osteoporosis, Bath, UK, 2003. Eli Lilly and Company sponsored the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial.
Corresponding author
Olof Johnell, MD, PhD, Department of Orthopedics, Universitetssjukhuset MAS, Malmo, SE-20502, Sweden. E-mail: [email protected].
- Consider prescribing raloxifene 60 mg/d for postmenopausal women, regardless of whether they have used hormone therapy, to reduce the incidence of vertebral fractures and breast cancer (SOR:B).
Objective: We examined whether past use of hormone therapy influences the effects of raloxifene on the risk of new vertebral fracture, cardiovascular events, or breast cancer.
Study Design: The Multiple Outcomes of Raloxifene Evaluation (MORE) trial examined vertebral fracture incidence as the primary endpoint, breast cancer incidence as a secondary endpoint. Cardiovascular events were collected as secondary safety endpoints.
Population: The MORE trial enrolled 7705 postmenopausal women. Of the 7682 women who reported their previous HT use status, 29% used HT before screening.
Outcomes Measured: Separate logistic regression models analyzed the relationships between prior HT use and the risk of vertebral fracture, cardiovascular events, or breast cancer. Interaction terms with P<.10 were considered to be statistically significant. Confidence intervals for relative risks (RR) were calculated using the Mantel-Haenszel method.
Results: Raloxifene 60 mg/d, the clinically approved dose for osteoporosis prevention and treatment, reduced the risk of vertebral fractures by 54% (RR=0.46) and 29% (RR=0.71) in women with and without prior HT use, respectively (interaction P=.05). A lower incidence of invasive breast cancer in women with prior HT use (RR=0.23) and in women without prior HT use [RR=0.31; interaction P=.60] was observed in women receiving raloxifene (pooled doses). Irrespective of prior HT use, women treated with raloxifene (pooled doses) had no change in incidence of cardiovascular events (interaction P=.56).
Conclusions: The risk of vertebral fractures was lower in women treated with raloxifene, regardless of prior HT use, but there was a suggestion that the effect was greater in women who had used HT. Women randomized to receive raloxifene exhibited a decreased incidence of invasive breast cancer, compared with women receiving placebo. No change occurred in the incidence of cardiovascular events, regardless of prior HT use.
Estrogen-containing hormone therapies (HT) have been used to alleviate menopausal symptoms and to prevent chronic diseases common to postmenopausal women, including osteoporosis and cardiovascular disease.1,2 In this analysis, we use the abbreviation “HT” to refer to postmenopausal hormone therapies, either estrogen alone or combined with progestin.
Based on the findings of the randomized, double-blind Women’s Health Initiative (WHI) study involving estrogen-progestin,3 the Food and Drug Administration (FDA) recommends using HT to treat moderate to severe symptoms of vulvar and vaginal atrophy and vasomotor symptoms associated with the menopause, and to prevent post-menopausal osteoporosis.4 When HT is prescribed only to prevent osteoporosis in women without menopausal symptoms, the FDA recommends that other approved, non-estrogen therapies be considered and that HT be used at the lowest dose for the shortest duration to achieve treatment goals.4 Many postmenopausal women have chosen to discontinue HT in light of these recommendations.5 However, discontinuing HT may increase bone resorption and accelerate bone loss,6,7 which, if untreated, places women at risk for osteoporotic fractures.
The serum estrogen receptor modulator (SERM) raloxifene is not an estrogen, a progestin, or a hormone,8 but it binds to the estrogen receptor to exert effects in the skeletal and cardiovascular systems and in breast tissue.9 In the 4-year Multiple Outcomes of Raloxifene Evaluation (MORE) osteoporosis treatment trial of postmenopausal women, raloxifene 60 mg/d, the approved dose for post-menopausal osteoporosis prevention and treatment, increased bone mineral density (BMD) and significantly reduced the risk for new vertebral fractures with sustained efficacy.10 With the declining use of long-term HT,5 it is clinically relevant and important to determine whether a history of HT use has any influence on the effects of other antiresorptive agents, such as raloxifene, which may be subsequently used for postmenopausal osteoporosis prevention and treatment. The objective of this analysis is to determine the effects of raloxifene on BMD, and the risks of vertebral fractures, cardiovascular events, and breast cancer in post-menopausal women who did or did not use HT prior to screening for the MORE osteoporosis study.
Materials and methods
Subjects and treatment
Details on subject recruitment and follow-up, and complete inclusion and exclusion criteria, were previously described for the MORE study.11 The trial examined the incidence of osteoporotic fractures as a primary endpoint and the incidence of breast cancer as a prespecified secondary endpoint, and it collected reports of cardiovascular events as a secondary safety endpoint.
Researchers enrolled 7705 women up to 80 years of age who were at least 2 years post-menopausal, and who had osteoporosis as defined by radiographically apparent vertebral fractures at baseline or BMD criteria. Women were randomly assigned to receive raloxifene 60 or 120 mg/d, or an identically appearing placebo.11 All women received daily supplements of calcium (500 mg) and vitamin D (400 to 600 IU). An ethical review board at each site approved the MORE study protocol. All women gave written informed consent to participate in the study in accordance with the ethical principles stated in the Declaration of Helsinki.
At study screening, women were asked if they had ever taken HT. Women were excluded from the study if they were experiencing clinically severe menopausal symptoms at the beginning of the study that required estrogen. Women were excluded if they had been treated with therapeutic doses of androgen, calcitonin, or estrogen (>1 cycle or 28 days) alone or with progestin (>1 cycle or 28 days) within 6 months of beginning the study. Women were permitted to have used systemic (oral or transdermal) estrogen and progestin for up to 1 cycle (28 days) during the 6 months before the study. No systemic estrogen and progestin use was allowed within 2 months before study entry. Occasional use of topical estrogens (3 times per week), and oral estriol (2 mg/d) for menopausal symptoms was permitted.
A 1-year double-blind extension phase was added to the 3-year treatment phase.10 All vertebral fracture, cardiovascular, and breast cancer endpoints that occurred over the 48-month study period were included in the present analyses.
Fracture and BMD assessment
New morphometric vertebral fractures, defined using semiquantitative assessment criteria,12 were identified by comparing spinal radiographs taken at 2, 3, and 4 years with baseline radiographs. A new vertebral fracture was defined as a vertebral fracture occurring in a vertebra that was not fractured at baseline. New clinical vertebral fractures were defined as those associated with signs or symptoms suggestive of vertebral fracture, such as back pain, reported either at an interim 6-month clinic visit or at any time between clinic visits,13 and which were subsequently corroborated with radiographs and adjudicated as previously described.10
Lumbar spine and femoral neck BMD were measured annually using dual-energy x-ray densitometry, as previously described.11
Cardiovascular event assessment
Cardiovascular events were collected as a secondary safety endpoint in the MORE trial, as previously described in detail.14 Women were asked at each clinic visit if they had had a myocardial infarction (MI), coronary bypass surgery, percutaneous coronary revascularization, or a stroke since the previous visit, and unsolicited reports of adverse cardiovascular events were recorded. All summaries of reported cardiovascular events were reviewed and adjudicated by 1 board-certified cardiologist, contracted by the sponsor, who was not associated with the trial and was blinded to treatment assignment.
Women with 4 or more risk points, assessed using the same criteria as for enrollment in the Raloxifene Use for The Heart (RUTH) trial,15 were considered to be at high risk for cardiovascular events. Coronary events included MI, unstable angina, or coronary ischemia.14 Cerebrovascular events included stroke and transient ischemic attack.14
Breast cancer assessment
Assessment of breast cancer was a prespecified secondary endpoint of the MORE trial, and was previously described in detail.16 All diagnoses of breast cancer were adjudicated by an independent oncology review board consisting of 5 physician specialists in breast cancer, and chaired by a pharmacologic scientist, none of whom were employed by the sponsor. Previous publications of the MORE breast cancer data have reported 61 cases of invasive breast cancer.16 A subsequent review of the MORE dataset found 1 fewer case of invasive breast cancer in each of the placebo and pooled raloxifene groups, so that 59 cases of invasive breast cancer were confirmed, and this number will be used in the present analysis. The change in the number of cases of invasive breast cancer was small, and had no impact on the overall interpretation of the breast cancer results from the MORE trial.
Results
Characteristics for all subjects at baseline and for women in the placebo group
Of the 7705 women enrolled, 7682 (99.7%) reported their status of previous HT use, with 2235 women (29.1%) having used HT before participating in MORE ( W1, available at www.jfponline.com). Baseline characteristics that were significantly different between women who reported prior HT use and those who reported no prior HT use included age, BMD, and the incidences of vertebral fractures, coronary angioplasty, hypertension, hyperlipidemia, and family histories of osteoporosis or breast cancer (Table W1, available at www.jfponline.com). In the subsets of women who did and who did not use HT previously, the baseline characteristics were not significantly different between the placebo and raloxifene groups, except for diabetes (placebo, 1.9%; raloxifene 60 mg/day, 3.3%; pooled raloxifene, 2.9%; P=.02).
In the placebo group, the incidence of new vertebral fractures, cardiovascular events, and breast cancer at 4 years were not significantly different in women with prior HT use compared with women without prior HT use.
Vertebral fracture events and bone mineral density
After 4 years of treatment with raloxifene 60 mg/d, women with and without prior HT use exhibited significant reductions in new vertebral fractures compared with those taking placebo ( Figure ). Vertebral fracture risk reductions between treatment groups were also statistically significant in subgroups of women with and without prevalent vertebral fractures ( Table1 ). Raloxifene 60 mg/d also reduced the risk of new clinically-apparent vertebral fractures, compared with placebo, in women with prior HT use (absolute risk reduction (ARR)=1.8%; RR=0.52 [95% CI, 0.28–0.96]), and in women without prior HT use (ARR=1.7%; RR=0.61 [95% CI, 0.43–0.87]; interaction P=.66]. The interaction P-values remained similar after adjusting for the baseline fracture risk factors (TableW1 , at www.jfponline.com) that were significantly different between women with and women without prior HT use. Women with and without prior HT use treated with raloxifene 60 mg/d had significant increases in BMD, at the lumbar spine (2.7% and 2.5%, respectively; interaction P=.54), and femoral neck (2.6% and 1.9% respectively; interaction P=.06), compared with placebo. Similar results were observed for fracture and BMD in women treated with the pooled raloxifene doses.
TABLE 1
Absolute and relative risks of new vertebral fractures with raloxifene 60 mg/d compared with placeboa
| Women who had used HT (n=1305) | Women who had notused HT (n=3232) | Overall study population d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=654) | Raloxifene 60 mg/d(n=651) | RR (95% CI)b [ARR] | Placebo (n=1634) | Raloxifene 60 md/d (n=1598) | RR (95% CI)[ARR] | Interaction P-valuec | RR (95% CI) | ||
| Women with prevalent fractures | 25.36% | 13.68% | 0.54(0.36–0.81)[11.68%] | 24.24% | 17.11% | 0.71(0.57–0.88)[7.13%] | .27 | 0.66(0.55–0.81) | |
| Women without prevalent vertebral | 6.29% | 1.82% | 0.29(0.13–0.63) [4.47%] | 5.54% | 3.46% | 0.62(0.41–0.95)[2.08%] | .09 | 0.51(0.35–0.73) | |
| a Results with raloxifene 60 mg/d are shown since this is the clinically approved dose for osteoporosis prevention and treatment. Results with pooled raloxifene doses were similar. Data from women with at least one post-baseline follow-up spinal radiograph were included in this table. | |||||||||
| b RR (95% CI) denotes relative risk (95% confidence interval) Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. Similar results were observed with raloxifene (pooled 60 mg/d and 120 mg/d doses) on vertebral fracture risk, in women who previously used HT [ARR=6.6%; RR=0.47 (95% CI, 0.35–0.63)], and in women without prior HT use [ARR=4.4%; RR=0.66 (95% CI, 0.55–0.78); interaction P=.06] | |||||||||
| c Results for the overall study population would be used unless the interaction effects between therapy group and prior HT use were statistically significant (P<.10), in which case, the results in the subgroups of women with and women without prior HT use should be used. | |||||||||
| d Delmas et al10 published the results for the overall study population, regardless of whether or not information on the participants’ HT use was available. | |||||||||
FIGURE
Percentage of women with and without prior HT use who experienced new vertebral fractures in the 4-year MORE study
This analysis included all women with at least 1 post-baseline follow-up vertebral radiograph, who reported their status of prior HT use. The relative risks (RR) and 95% confidence intervals (CI) are shown for women treated with either placebo or raloxifene 60 mg/d. The absolute risk reductions were 6.7% in women with prior HT use and 3.7% in women without prior HT use. The interaction P-value was .05.
Cardiovascular events
In women with and without prior HT use, treatment with raloxifene (pooled doses) did not result in statistically significant changes in the incidence of new cardiovascular, coronary, or cerebrovascu-lar events, compared with placebo ( Table2 ). In a subgroup of women who were at high risk of cardiovascular disease,15 prior HT use had no effect on the incidence of new cardiovascular events with raloxifene (pooled doses) treatment ( Table 2 ). The interaction P-values remained similar after adjusting for the baseline cardiovascular risk factors (Table W1, at www.jfponline.com) that were significantly different between women with and without prior HT use.
TABLE 2
Absolute and relative risks of cardiovascular events with raloxifene (pooled doses) compared with placeboa
| Women who had used HT (n=2235) | Women who had not used HT (n=5447) | Overall studypopulation | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo(n=738) | Pooled raloxifene(n=1497) | RR (95% CI)b [ARR] | Placebo (n=1833) | Pooled raloxifene (n=3614) | RR(95% CI)[ARR] | Interaction P-valuec | RR(95% CI) | |
| Cardiovascular events | 2.71% | 2.87% | 1.06(0.63–1.79)[–0.16%] | 4.15% | 3.68% | 0.89(0.67–1.17) [0.47%] | .56 | 0.92(0.72–1.18) |
| Coronary events | 1.49% | 1.74% | 1.17 (0.58–2.35)[–0.25%] | 2.40% | 2.08% | 0.87 (0.60–1.25) [0.32%] | .46 | 0.92(0.67–1.28) |
| Cerebrovascularevents | 1.22% | 1.14% | 0.93(0.42–2.08)[0.08%] | 1.75% | 1.63% | 0.94 (0.61–1.43)[.012%] | .99 | 0.93(0.64–1.36) |
| Cardiovascularevents in high-risk subgroupd | 12.66% | 5.91% | .047(0.21–1.06)[6.75%] | 13.08% | 8.54% | 0.65 (0.42–1.01)[4.54%] | .49 | 0.60(0.41–0.88) [4.54%] |
| a Pooled raloxifene doses were used in this analysis, since there were few events. There were no differences in the incidence of events between the raloxifene doses. | ||||||||
| b Relative risk (RR), 95% confidence interval (95% CI). Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. | ||||||||
| c The interaction effects between therapy group and prior HT use were not statistically significant (P>.10), so results from the overall study population would be used. Barrett-Connor et al14 reported the results for raloxifene 60 mg/d and raloxifene 120 mg/d in the overall study population, regardless of whether or not information on the participants’ HT use was available. | ||||||||
| d Of the 1029 women in the high-risk subgroup, 764 women had no prior history of HT use (placebo, n=237; raloxifene, n=527), and 265 women reported prior HT use (placebo, n=79; raloxifene, n=186). | ||||||||
Breast cancer
In women with and without prior HT use, similar reductions in the incidence of breast cancer (regardless of invasiveness), invasive breast cancer, and estrogen-receptor positive invasive breast cancer, were observed after raloxifene treatment (pooled doses) compared with placebo ( Table 3 ). The interaction P-values remained similar after adjusting for the baseline breast cancer risk factors (Table W1) that were significantly different between women with and without prior HT use.
TABLE 3
Absolute and relative risks of breast cancer with raloxifene (pooled doses) compared with placeboa
| Women who had used HT (n=2235) | Women who had notused HT (n=5447) | Overall study population | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo(n=738) | Pooledraloxifene(n=1497) | RR(95% CI)b [ARR] | Placebo(n=1833) | Pooled raloxifene(n=3614) | RR(95% CI)[ARR] | InteractionP-valuec | RR(95% CI) | |
| Breast cancer d | 2.30% | 0.73% | 0.32(0.15–0.68)[1.57%] | 1.47% | 0.64% | 0.43(0.25–0.75)[0.83%] | .52 | 0.38 (0.24–0.58) |
| Invasivebreast cancer | 2.03% | 0.47% | 0.23(0.09–0.56)[1.56%] | 1.25% | 0.39% | 0.31(0.16–0.60) [0.86%] | .60 | 0.28(0.17–0.46) |
| Invasiveestrogen-receptorpositive breastcancer | 1.76% | 0.27% | 0.15(0.05–0.46)[1.49%] | 0.87% | 0.17% | 0.19(0.08–0.49)[0.70%] | .75 | 0.16(0.09–0.30) |
| a Pooled raloxifene doses were used in this analysis, since there were few events. There were no differences in the incidence of events between the raloxifene doses. | ||||||||
| b Relative risk (RR), 95% confidence interval (95% CI). Absolute risk reductions, denoted [ARR], are the differences between the placebo and raloxifene groups. | ||||||||
| c The interaction effects between therapy group and prior HT use were not statistically significant (P>.10), so results from the overall study population would be used. Cauley et al16 reported the results for in the overall study population, regardless of whether or not information on the participants’ HT use was available. | ||||||||
| d All breast cancer, regardless of invasiveness. | ||||||||
Discussion
This analysis examined the effects of raloxifene in women who reported their use of postmenopausal hormone therapies before enrolling in the MORE osteoporosis trial. Compared with placebo, women treated with raloxifene experienced significant decreases in the risks for new vertebral fractures and the incidence of breast cancer, without significant changes in the incidence of cardiovascular events, regardless of previous HT use. These analyses provide further information on the effects of raloxifene on the risks of vertebral fractures,10 cardiovascular events,14 and breast cancer16 seen in the overall MORE study population at 4 years.
This analysis found a differential reduction in vertebral fracture risk with raloxifene between women who did and did not have prior HT use, which may result from possible differences in women who chose to use HT before participating in MORE. In women with previous HT use, a greater proportion had a family history of osteo-porosis and a lower proportion had prevalent ver-tebral fractures at baseline, compared with women who had not used HT. Other unidentified confounding factors, such as the “healthy user” bias commonly associated with women who chose to use HT,17 may also contribute to the differential vertebral fracture risk reduction with raloxifene treatment. The Study of Osteoporotic Fractures showed that women with current estrogen use had significantly decreased fracture risks, but the risk reduction waned in women who discontinued estrogen.18 After HT discontinuation, BMD loss resumes at a rate similar to that seen in women shortly after menopause, suggesting that prior HT use may have limited residual effects on maintaining BMD.7,19 Such findings raise the urgency of evaluating the risk for osteoporosis in women who discontinue HT.
Women treated with raloxifene had no significant changes in the incidence of cardiovascular events, with no differential treatment effect based on prior HT use. In the HERS20 and WHI3 trials, which studied the outcomes of estrogen-progestin therapy in postmenopausal women, similar analyses did not show any significant differential effects of prior HT use on the incidence of cardiovascular events with estrogen-progestin during the respective trials.
In this analysis, women treated with raloxifene had a significantly lower incidence of breast cancer compared with those who received placebo, and this incidence was comparable between women with and without prior HT use. In contrast, women who had used HT before the WHI study had a significant increase in the risk of breast cancer with estrogen-progestin therapy during the study, compared with those who had not used HT.3
A limitation of our analysis that a history of HT use was based on participants’ self-report, which depended on their ability to recall medication they may have taken years earlier. Also, no information was obtained on therapy duration and the doses and formulations of HT. Since the MORE trial was conducted in 25 countries, the patterns and types of HT regimens are expected to be different. The strength of our analysis is that the MORE population was large enough to prospectively collect data on multiple clinical outcomes.
In summary, postmenopausal women treated with raloxifene experienced a significant risk reduction for vertebral fractures, regardless of prior HT use, but women who had used HT may exhibit greater reductions. Women who used raloxifene had no change in the incidence of cardiovascular events and a lower incidence of breast cancer, compared with placebo, regardless of their history of HT use. Since HT is becoming increasingly limited to short-term use for menopausal symptoms, women and their physicians may consider several other therapeutic options to address postmenopausal health concerns.
Acknowledgments
The authors acknowledge the contributions of Leo Plouffe Jr., MD, and Somnath Sarkar, PhD, for suggestions on manuscript content, and Sharon Xiaohan Zou, MS, for statistical programming. A complete list of all investigators in the MORE trial is found in J Clin Endocrinol Metab 2002; 87:3609–3617. Portions of this work were presented at the following meetings: Third European Symposium on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, Barcelona, Spain, 2002; International Society for Clinical Densitometry (ISCD), Los Angeles, USA,2003; European Calcified Tissue Society (ECTS), Rome, Italy, 2003; International Bone and Mineral Society (IBMS), Osaka, Japan, 2003; European Menopause and Andropause Society (EMAS), Bucharest, Romania, 2003; Ninth Bath Conference on Osteoporosis, Bath, UK, 2003. Eli Lilly and Company sponsored the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial.
Corresponding author
Olof Johnell, MD, PhD, Department of Orthopedics, Universitetssjukhuset MAS, Malmo, SE-20502, Sweden. E-mail: [email protected].
1. Greendale GA, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-580.
2. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992;117:1016-1037.
3. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-333.
4. Bren L. The estrogen and progestin dilemma: New advice, labeling and guidelines. FDA Consumer 2003;37:10-11.
5. Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 2004;140:184-188.
6. Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 2002;87:4914-4923.
7. Tremollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant ver-tebral bone loss in postmenopausal women. Osteoporos Int 2001;12:385-390.
8. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators —mechanisms of action and application to clinical practice. N Engl J Med 2003;348:618-629.
9. Maricic M, Gluck O. Review of raloxifene and its clinical applications in osteoporosis. Expert Opin Pharmacother 2002;3:767-775.
10. Delmas PD, Ensrud KE, Adachi JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 2002;87:3609-3617.
11. Ettinger B, Black DM, Mitlak BH, et al. Reduction of verte-bral fracture risk in postmenopausal women with osteo-porosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999;282:637-645.
12. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-1148.
13. Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002;162:1140-1143.
14. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002;287:847-857.
15. Mosca L, Barrett-Connor E, Wenger NK, et al. Design and methods of the Raloxifene Use for The Heart (RUTH) study. Am J Cardiol 2001;88:392-395.
16. Cauley J, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year Results from the MORE Trial. Breast Cancer Res Treat 2001;65:125-134.
17. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med 2003;348:645-650.
18. Cauley J, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med 1995;122:9-16.
19. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-672.
20. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study Follow-up (HERS II). JAMA 2002;288:49-57.
21. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 1998;8:468-489.
1. Greendale GA, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-580.
2. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992;117:1016-1037.
3. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-333.
4. Bren L. The estrogen and progestin dilemma: New advice, labeling and guidelines. FDA Consumer 2003;37:10-11.
5. Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 2004;140:184-188.
6. Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 2002;87:4914-4923.
7. Tremollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant ver-tebral bone loss in postmenopausal women. Osteoporos Int 2001;12:385-390.
8. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators —mechanisms of action and application to clinical practice. N Engl J Med 2003;348:618-629.
9. Maricic M, Gluck O. Review of raloxifene and its clinical applications in osteoporosis. Expert Opin Pharmacother 2002;3:767-775.
10. Delmas PD, Ensrud KE, Adachi JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 2002;87:3609-3617.
11. Ettinger B, Black DM, Mitlak BH, et al. Reduction of verte-bral fracture risk in postmenopausal women with osteo-porosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999;282:637-645.
12. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-1148.
13. Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002;162:1140-1143.
14. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002;287:847-857.
15. Mosca L, Barrett-Connor E, Wenger NK, et al. Design and methods of the Raloxifene Use for The Heart (RUTH) study. Am J Cardiol 2001;88:392-395.
16. Cauley J, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year Results from the MORE Trial. Breast Cancer Res Treat 2001;65:125-134.
17. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med 2003;348:645-650.
18. Cauley J, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med 1995;122:9-16.
19. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-672.
20. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study Follow-up (HERS II). JAMA 2002;288:49-57.
21. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 1998;8:468-489.