User login
WOW! You spend that much time on the EHR?
Unlike many of you, maybe even most of you, I can recall when my office records were handwritten, some would say scribbled, on pieces of paper. They were decipherable by a select few. Some veteran assistants never mastered the skill. Pages were sometimes lavishly illustrated with drawings of body parts, often because I couldn’t remember or spell the correct anatomic term. When I needed to send a referring letter to another provider I typed it myself because dictating never quite suited my personality.
When I joined a small primary care group, the computer-savvy lead physician and a programmer developed our own homegrown EHR. It relied on scanning documents, as so many of us still generated handwritten notes. Even the most vociferous Luddites among us loved the system from day 2.
However, for a variety of reasons, some defensible some just plain bad, our beloved system needed to be replaced after 7 years. We then invested in an off-the-shelf EHR system that promised more capabilities. We were told there would be a learning curve but the plateau would come quickly and we would enjoy our new electronic assistant.
You’ve lived the rest of the story. The learning curve was steep and long and the plateau was a time gobbler. I was probably the most efficient provider in the group, and after 6 months I was leaving the office an hour later than I had been and was seeing the same number of patients. Most of my coworkers were staying and/or working on the computer at home for an extra 2 hours. This change could be easily documented by speaking with our spouses and children. I understand from my colleagues who have stayed in the business that over the ensuing decade and a half since my first experience with the EHR, its insatiable appetite for a clinician’s time has not abated.
The authors of a recent article in Annals of Family Medicine offer up some advice on how this tragic situation might be brought under control. First, the investigators point out that the phenomenon of after-hours EHR work, sometimes referred to as WOW (work outside of work), has not gone unnoticed by health system administrators and vendors who develop and sell the EHRs. However, analyzing the voluminous data necessary is not any easy task and for the most part has resulted in metrics that cannot be easily applied over a variety of practice scenarios. Many health care organizations, even large ones, have simply given up and rely on the WOW data and recommendations provided by the vendors, obviously lending the situation a faint odor of conflict of interest.
The bottom line is that . It would seem to me just asking the spouses and significant others of the clinicians would be sufficient. But, authors of the paper have more specific recommendations. First, they suggest that time working on the computer outside of scheduled time with patients should be separated from any other calculation of EHR usage. They encourage vendors and time-management researchers to develop standardized and validated methods for measuring active EHR use. And, finally they recommend that all EHR work done outside of time scheduled with patients be attributed to WOW. They feel that clearly labeling it work outside of work offers health care organizations a better chance of developing policies that will address the scourge of burnout.
This, unfortunately, is another tragic example of how clinicians have lost control of our work environments. The fact that 20 years have passed and there is still no standardized method for determining how much time we spend on the computer is more evidence we need to raise our voices.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Unlike many of you, maybe even most of you, I can recall when my office records were handwritten, some would say scribbled, on pieces of paper. They were decipherable by a select few. Some veteran assistants never mastered the skill. Pages were sometimes lavishly illustrated with drawings of body parts, often because I couldn’t remember or spell the correct anatomic term. When I needed to send a referring letter to another provider I typed it myself because dictating never quite suited my personality.
When I joined a small primary care group, the computer-savvy lead physician and a programmer developed our own homegrown EHR. It relied on scanning documents, as so many of us still generated handwritten notes. Even the most vociferous Luddites among us loved the system from day 2.
However, for a variety of reasons, some defensible some just plain bad, our beloved system needed to be replaced after 7 years. We then invested in an off-the-shelf EHR system that promised more capabilities. We were told there would be a learning curve but the plateau would come quickly and we would enjoy our new electronic assistant.
You’ve lived the rest of the story. The learning curve was steep and long and the plateau was a time gobbler. I was probably the most efficient provider in the group, and after 6 months I was leaving the office an hour later than I had been and was seeing the same number of patients. Most of my coworkers were staying and/or working on the computer at home for an extra 2 hours. This change could be easily documented by speaking with our spouses and children. I understand from my colleagues who have stayed in the business that over the ensuing decade and a half since my first experience with the EHR, its insatiable appetite for a clinician’s time has not abated.
The authors of a recent article in Annals of Family Medicine offer up some advice on how this tragic situation might be brought under control. First, the investigators point out that the phenomenon of after-hours EHR work, sometimes referred to as WOW (work outside of work), has not gone unnoticed by health system administrators and vendors who develop and sell the EHRs. However, analyzing the voluminous data necessary is not any easy task and for the most part has resulted in metrics that cannot be easily applied over a variety of practice scenarios. Many health care organizations, even large ones, have simply given up and rely on the WOW data and recommendations provided by the vendors, obviously lending the situation a faint odor of conflict of interest.
The bottom line is that . It would seem to me just asking the spouses and significant others of the clinicians would be sufficient. But, authors of the paper have more specific recommendations. First, they suggest that time working on the computer outside of scheduled time with patients should be separated from any other calculation of EHR usage. They encourage vendors and time-management researchers to develop standardized and validated methods for measuring active EHR use. And, finally they recommend that all EHR work done outside of time scheduled with patients be attributed to WOW. They feel that clearly labeling it work outside of work offers health care organizations a better chance of developing policies that will address the scourge of burnout.
This, unfortunately, is another tragic example of how clinicians have lost control of our work environments. The fact that 20 years have passed and there is still no standardized method for determining how much time we spend on the computer is more evidence we need to raise our voices.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Unlike many of you, maybe even most of you, I can recall when my office records were handwritten, some would say scribbled, on pieces of paper. They were decipherable by a select few. Some veteran assistants never mastered the skill. Pages were sometimes lavishly illustrated with drawings of body parts, often because I couldn’t remember or spell the correct anatomic term. When I needed to send a referring letter to another provider I typed it myself because dictating never quite suited my personality.
When I joined a small primary care group, the computer-savvy lead physician and a programmer developed our own homegrown EHR. It relied on scanning documents, as so many of us still generated handwritten notes. Even the most vociferous Luddites among us loved the system from day 2.
However, for a variety of reasons, some defensible some just plain bad, our beloved system needed to be replaced after 7 years. We then invested in an off-the-shelf EHR system that promised more capabilities. We were told there would be a learning curve but the plateau would come quickly and we would enjoy our new electronic assistant.
You’ve lived the rest of the story. The learning curve was steep and long and the plateau was a time gobbler. I was probably the most efficient provider in the group, and after 6 months I was leaving the office an hour later than I had been and was seeing the same number of patients. Most of my coworkers were staying and/or working on the computer at home for an extra 2 hours. This change could be easily documented by speaking with our spouses and children. I understand from my colleagues who have stayed in the business that over the ensuing decade and a half since my first experience with the EHR, its insatiable appetite for a clinician’s time has not abated.
The authors of a recent article in Annals of Family Medicine offer up some advice on how this tragic situation might be brought under control. First, the investigators point out that the phenomenon of after-hours EHR work, sometimes referred to as WOW (work outside of work), has not gone unnoticed by health system administrators and vendors who develop and sell the EHRs. However, analyzing the voluminous data necessary is not any easy task and for the most part has resulted in metrics that cannot be easily applied over a variety of practice scenarios. Many health care organizations, even large ones, have simply given up and rely on the WOW data and recommendations provided by the vendors, obviously lending the situation a faint odor of conflict of interest.
The bottom line is that . It would seem to me just asking the spouses and significant others of the clinicians would be sufficient. But, authors of the paper have more specific recommendations. First, they suggest that time working on the computer outside of scheduled time with patients should be separated from any other calculation of EHR usage. They encourage vendors and time-management researchers to develop standardized and validated methods for measuring active EHR use. And, finally they recommend that all EHR work done outside of time scheduled with patients be attributed to WOW. They feel that clearly labeling it work outside of work offers health care organizations a better chance of developing policies that will address the scourge of burnout.
This, unfortunately, is another tragic example of how clinicians have lost control of our work environments. The fact that 20 years have passed and there is still no standardized method for determining how much time we spend on the computer is more evidence we need to raise our voices.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Daily multivitamins boost memory in older adults: A randomized trial
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. , known as COSMOS (Cocoa Supplement and Multivitamins Outcome Study). This is the second COSMOS trial to show a benefit of multivitamins on memory and cognition. This trial involved a collaboration between Brigham and Columbia University and was published in the American Journal of Clinical Nutrition. I’d like to acknowledge that I am a coauthor of this study, together with Dr. Howard Sesso, who co-leads the main COSMOS trial with me.
Preserving memory and cognitive function is of critical importance to older adults. Nutritional interventions play an important role because we know the brain requires several nutrients for optimal health, and deficiencies in one or more of these nutrients may accelerate cognitive decline. Some of the micronutrients that are known to be important for brain health include vitamin B12, thiamin, other B vitamins, lutein, magnesium, and zinc, among others.
The current trial included 3,500 participants aged 60 or older, looking at performance on a web-based memory test. The multivitamin group did significantly better than the placebo group on memory tests and word recall, a finding that was estimated as the equivalent of slowing age-related memory loss by about 3 years. The benefit was first seen at 1 year and was sustained across the 3 years of the trial.
Intriguingly, in both COSMOS and COSMOS-Web, and the earlier COSMOS-Mind study, which was done in collaboration with Wake Forest, the participants with a history of cardiovascular disease showed the greatest benefits from multivitamins, perhaps due to lower nutrient status. But the basis for this finding needs to be explored further.
A few important caveats need to be emphasized. First, multivitamins and other dietary supplements will never be a substitute for a healthy diet and healthy lifestyle and should not distract from those goals. But multivitamins may have a role as a complementary strategy. Another caveat is that the randomized trials tested recommended dietary allowances and not megadoses of these micronutrients. In fact, randomized trials of high doses of isolated micronutrients have not clearly shown cognitive benefits, and this suggests that more is not necessarily better and may be worse. High doses also may be associated with toxicity, or they may interfere with absorption or bioavailability of other nutrients.
In COSMOS, over the average 3.6 years of follow-up and in the earlier Physicians’ Health Study II, over 1 year of supplementation, multivitamins were found to be safe without any clear risks or safety concerns. A further caveat is that although Centrum Silver was tested in this trial, we would not expect that this is a brand-specific benefit, and other high-quality multivitamin brands would be expected to confer similar benefits. Of course, it’s important to check bottles for quality-control documentation such as the seals of the U.S. Pharmacopeia, National Science Foundation, ConsumerLab.com, and other auditors.
Overall, the finding that a daily multivitamin improved memory and slowed cognitive decline in two separate COSMOS randomized trials is exciting, suggesting that multivitamin supplementation holds promise as a safe, accessible, and affordable approach to protecting cognitive health in older adults. Further research will be needed to understand who is most likely to benefit and the biological mechanisms involved. Expert committees will have to look at the research and decide whether any changes in guidelines are indicated in the future.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School and director of the Division of Preventive Medicine, Brigham and Women’s Hospital, both in Boston. She reported receiving funding/donations from Mars Symbioscience.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. , known as COSMOS (Cocoa Supplement and Multivitamins Outcome Study). This is the second COSMOS trial to show a benefit of multivitamins on memory and cognition. This trial involved a collaboration between Brigham and Columbia University and was published in the American Journal of Clinical Nutrition. I’d like to acknowledge that I am a coauthor of this study, together with Dr. Howard Sesso, who co-leads the main COSMOS trial with me.
Preserving memory and cognitive function is of critical importance to older adults. Nutritional interventions play an important role because we know the brain requires several nutrients for optimal health, and deficiencies in one or more of these nutrients may accelerate cognitive decline. Some of the micronutrients that are known to be important for brain health include vitamin B12, thiamin, other B vitamins, lutein, magnesium, and zinc, among others.
The current trial included 3,500 participants aged 60 or older, looking at performance on a web-based memory test. The multivitamin group did significantly better than the placebo group on memory tests and word recall, a finding that was estimated as the equivalent of slowing age-related memory loss by about 3 years. The benefit was first seen at 1 year and was sustained across the 3 years of the trial.
Intriguingly, in both COSMOS and COSMOS-Web, and the earlier COSMOS-Mind study, which was done in collaboration with Wake Forest, the participants with a history of cardiovascular disease showed the greatest benefits from multivitamins, perhaps due to lower nutrient status. But the basis for this finding needs to be explored further.
A few important caveats need to be emphasized. First, multivitamins and other dietary supplements will never be a substitute for a healthy diet and healthy lifestyle and should not distract from those goals. But multivitamins may have a role as a complementary strategy. Another caveat is that the randomized trials tested recommended dietary allowances and not megadoses of these micronutrients. In fact, randomized trials of high doses of isolated micronutrients have not clearly shown cognitive benefits, and this suggests that more is not necessarily better and may be worse. High doses also may be associated with toxicity, or they may interfere with absorption or bioavailability of other nutrients.
In COSMOS, over the average 3.6 years of follow-up and in the earlier Physicians’ Health Study II, over 1 year of supplementation, multivitamins were found to be safe without any clear risks or safety concerns. A further caveat is that although Centrum Silver was tested in this trial, we would not expect that this is a brand-specific benefit, and other high-quality multivitamin brands would be expected to confer similar benefits. Of course, it’s important to check bottles for quality-control documentation such as the seals of the U.S. Pharmacopeia, National Science Foundation, ConsumerLab.com, and other auditors.
Overall, the finding that a daily multivitamin improved memory and slowed cognitive decline in two separate COSMOS randomized trials is exciting, suggesting that multivitamin supplementation holds promise as a safe, accessible, and affordable approach to protecting cognitive health in older adults. Further research will be needed to understand who is most likely to benefit and the biological mechanisms involved. Expert committees will have to look at the research and decide whether any changes in guidelines are indicated in the future.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School and director of the Division of Preventive Medicine, Brigham and Women’s Hospital, both in Boston. She reported receiving funding/donations from Mars Symbioscience.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. , known as COSMOS (Cocoa Supplement and Multivitamins Outcome Study). This is the second COSMOS trial to show a benefit of multivitamins on memory and cognition. This trial involved a collaboration between Brigham and Columbia University and was published in the American Journal of Clinical Nutrition. I’d like to acknowledge that I am a coauthor of this study, together with Dr. Howard Sesso, who co-leads the main COSMOS trial with me.
Preserving memory and cognitive function is of critical importance to older adults. Nutritional interventions play an important role because we know the brain requires several nutrients for optimal health, and deficiencies in one or more of these nutrients may accelerate cognitive decline. Some of the micronutrients that are known to be important for brain health include vitamin B12, thiamin, other B vitamins, lutein, magnesium, and zinc, among others.
The current trial included 3,500 participants aged 60 or older, looking at performance on a web-based memory test. The multivitamin group did significantly better than the placebo group on memory tests and word recall, a finding that was estimated as the equivalent of slowing age-related memory loss by about 3 years. The benefit was first seen at 1 year and was sustained across the 3 years of the trial.
Intriguingly, in both COSMOS and COSMOS-Web, and the earlier COSMOS-Mind study, which was done in collaboration with Wake Forest, the participants with a history of cardiovascular disease showed the greatest benefits from multivitamins, perhaps due to lower nutrient status. But the basis for this finding needs to be explored further.
A few important caveats need to be emphasized. First, multivitamins and other dietary supplements will never be a substitute for a healthy diet and healthy lifestyle and should not distract from those goals. But multivitamins may have a role as a complementary strategy. Another caveat is that the randomized trials tested recommended dietary allowances and not megadoses of these micronutrients. In fact, randomized trials of high doses of isolated micronutrients have not clearly shown cognitive benefits, and this suggests that more is not necessarily better and may be worse. High doses also may be associated with toxicity, or they may interfere with absorption or bioavailability of other nutrients.
In COSMOS, over the average 3.6 years of follow-up and in the earlier Physicians’ Health Study II, over 1 year of supplementation, multivitamins were found to be safe without any clear risks or safety concerns. A further caveat is that although Centrum Silver was tested in this trial, we would not expect that this is a brand-specific benefit, and other high-quality multivitamin brands would be expected to confer similar benefits. Of course, it’s important to check bottles for quality-control documentation such as the seals of the U.S. Pharmacopeia, National Science Foundation, ConsumerLab.com, and other auditors.
Overall, the finding that a daily multivitamin improved memory and slowed cognitive decline in two separate COSMOS randomized trials is exciting, suggesting that multivitamin supplementation holds promise as a safe, accessible, and affordable approach to protecting cognitive health in older adults. Further research will be needed to understand who is most likely to benefit and the biological mechanisms involved. Expert committees will have to look at the research and decide whether any changes in guidelines are indicated in the future.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School and director of the Division of Preventive Medicine, Brigham and Women’s Hospital, both in Boston. She reported receiving funding/donations from Mars Symbioscience.
A version of this article first appeared on Medscape.com.
The timekeeper
This little fellow greets you at my office. He’s been there for 25 years.
I don’t know where he came from originally. When I started out he was up front with the physician I subleased from and when he retired he passed him on to me (thanks, Fran!).
From the beginning he’s been the first thing I see when I arrive each morning. Because of my suprachiasmatic nucleus kicking me out of bed between 4 and 5 each morning, I’m always the first one in the office and so I update him. At this point he’s as much a part of my morning ritual as coffee and tea. I juggle the cubes to change the day (12 times a year I change the month) and once this is done I don’t think of him again until the next morning.
When I started setting him each morning I didn’t have kids. Now I have three, all grown. Patients, years, drug reps, and even a pandemic have all been marked by the clicking of his cubes when I change them each morning.
Now two-thirds of the way through my career, he’s taken on a different meaning. He’s counting down the days until I walk away and leave neurology in the hands of another generation. I don’t have a date for doing that, nor a plan to do so anytime soon, but sooner or later I’ll be changing his cubes for the last office day of my life as a neurologist.
What will happen to him then? Seems like a strange question to ask about an inanimate object, but after this much time I’ve gotten attached to the little guy. He’s come to symbolize more than just the date – he’s the passage of time. Maybe he’ll stay on a shelf at home, giving me something to do each morning of my retirement. Maybe one of my kids will want him.
Inevitably, he’ll probably end up at a charity store, awaiting a new owner. When that happens I hope he gives them something to pause, smile, and think about each day, like he did with me, as we travel around the sun together.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This little fellow greets you at my office. He’s been there for 25 years.
I don’t know where he came from originally. When I started out he was up front with the physician I subleased from and when he retired he passed him on to me (thanks, Fran!).
From the beginning he’s been the first thing I see when I arrive each morning. Because of my suprachiasmatic nucleus kicking me out of bed between 4 and 5 each morning, I’m always the first one in the office and so I update him. At this point he’s as much a part of my morning ritual as coffee and tea. I juggle the cubes to change the day (12 times a year I change the month) and once this is done I don’t think of him again until the next morning.
When I started setting him each morning I didn’t have kids. Now I have three, all grown. Patients, years, drug reps, and even a pandemic have all been marked by the clicking of his cubes when I change them each morning.
Now two-thirds of the way through my career, he’s taken on a different meaning. He’s counting down the days until I walk away and leave neurology in the hands of another generation. I don’t have a date for doing that, nor a plan to do so anytime soon, but sooner or later I’ll be changing his cubes for the last office day of my life as a neurologist.
What will happen to him then? Seems like a strange question to ask about an inanimate object, but after this much time I’ve gotten attached to the little guy. He’s come to symbolize more than just the date – he’s the passage of time. Maybe he’ll stay on a shelf at home, giving me something to do each morning of my retirement. Maybe one of my kids will want him.
Inevitably, he’ll probably end up at a charity store, awaiting a new owner. When that happens I hope he gives them something to pause, smile, and think about each day, like he did with me, as we travel around the sun together.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This little fellow greets you at my office. He’s been there for 25 years.
I don’t know where he came from originally. When I started out he was up front with the physician I subleased from and when he retired he passed him on to me (thanks, Fran!).
From the beginning he’s been the first thing I see when I arrive each morning. Because of my suprachiasmatic nucleus kicking me out of bed between 4 and 5 each morning, I’m always the first one in the office and so I update him. At this point he’s as much a part of my morning ritual as coffee and tea. I juggle the cubes to change the day (12 times a year I change the month) and once this is done I don’t think of him again until the next morning.
When I started setting him each morning I didn’t have kids. Now I have three, all grown. Patients, years, drug reps, and even a pandemic have all been marked by the clicking of his cubes when I change them each morning.
Now two-thirds of the way through my career, he’s taken on a different meaning. He’s counting down the days until I walk away and leave neurology in the hands of another generation. I don’t have a date for doing that, nor a plan to do so anytime soon, but sooner or later I’ll be changing his cubes for the last office day of my life as a neurologist.
What will happen to him then? Seems like a strange question to ask about an inanimate object, but after this much time I’ve gotten attached to the little guy. He’s come to symbolize more than just the date – he’s the passage of time. Maybe he’ll stay on a shelf at home, giving me something to do each morning of my retirement. Maybe one of my kids will want him.
Inevitably, he’ll probably end up at a charity store, awaiting a new owner. When that happens I hope he gives them something to pause, smile, and think about each day, like he did with me, as we travel around the sun together.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Don’t screen, just listen
A recent study published in the journal Academic Pediatrics suggests that during health maintenance visits clinicians are giving too little attention to their patients’ sleep problems. Using a questionnaire, researchers surveyed patients’ caregivers’ concerns and observations regarding a variety of sleep problems. The investigators then reviewed the clinicians’ documentation of what transpired at the visit and found that while over 90% of the caregivers reported their child had at least one sleep related problem, only 20% of the clinicians documented the problem. And, only 12% documented a management plan regarding the sleep concerns.
I am always bit skeptical about studies that rely on clinicians’ “documentation” because clinicians are busy people and don’t always remember to record things they’ve discussed. You and I know that the lawyers’ dictum “if it wasn’t documented it didn’t happen” is rubbish. However, I still find the basic finding of this study concerning. If we are failing to ask about or even listen to caregivers’ concerns about something as important as sleep, we are missing the boat ... a very large boat.
How could this be happening? First, sleep may have fallen victim to the bloated list of topics that well-intentioned single-issue preventive health advocates have tacked on to the health maintenance visit. It’s a burden that few of us can manage without cutting corners.
However, it is more troubling to me that so many clinicians have chosen sleep as one of those corners to cut. This oversight suggests to me that too many of us have failed to realize from our own observations that sleep is incredibly important to the health of our patients ... and to ourselves.
I will admit that I am extremely sensitive to the importance of sleep. Some might say my sensitivity borders on an obsession. But, the literature is clear and becoming more voluminous every year that sleep is important to the mental health of our patients and their caregivers to things like obesity, to symptoms that suggest an attention-deficit/hyperactivity disorder, to school success, and to migraine ... to name just a few.
It may be that most of us realize the importance of sleep but feel our society has allowed itself to become so sleep deprived that there is little chance we can turn the ship around by spending just a few minutes trying help a family undo their deeply ingrained sleep unfriendly habits.
I am tempted to join those of you who see sleep depravation as a “why bother” issue. But, I’m not ready to throw in the towel. Even simply sharing your observations about the importance of sleep in the whole wellness picture may have an effect.
One of the benefits of retiring in the same community in which I practiced for over 40 years is that at least every month or two I encounter a parent who thanks me for sharing my views on the importance of sleep. They may not recall the little tip or two I gave them, but it seems that urging them to put sleep near the top of their lifestyle priority list has made the difference for them.
If I have failed in getting you to join me in my crusade against sleep deprivation, at least take to heart the most basic message of this study. That is that the investigators found only 20% of clinicians were addressing a concern that 90% of the caregivers shared. It happened to be sleep, but it could have been anything.
The authors of the study suggest that we need to be more assiduous in our screening for sleep problems. On the contrary. You and I know we don’t need more screening. We just need to be better listeners.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A recent study published in the journal Academic Pediatrics suggests that during health maintenance visits clinicians are giving too little attention to their patients’ sleep problems. Using a questionnaire, researchers surveyed patients’ caregivers’ concerns and observations regarding a variety of sleep problems. The investigators then reviewed the clinicians’ documentation of what transpired at the visit and found that while over 90% of the caregivers reported their child had at least one sleep related problem, only 20% of the clinicians documented the problem. And, only 12% documented a management plan regarding the sleep concerns.
I am always bit skeptical about studies that rely on clinicians’ “documentation” because clinicians are busy people and don’t always remember to record things they’ve discussed. You and I know that the lawyers’ dictum “if it wasn’t documented it didn’t happen” is rubbish. However, I still find the basic finding of this study concerning. If we are failing to ask about or even listen to caregivers’ concerns about something as important as sleep, we are missing the boat ... a very large boat.
How could this be happening? First, sleep may have fallen victim to the bloated list of topics that well-intentioned single-issue preventive health advocates have tacked on to the health maintenance visit. It’s a burden that few of us can manage without cutting corners.
However, it is more troubling to me that so many clinicians have chosen sleep as one of those corners to cut. This oversight suggests to me that too many of us have failed to realize from our own observations that sleep is incredibly important to the health of our patients ... and to ourselves.
I will admit that I am extremely sensitive to the importance of sleep. Some might say my sensitivity borders on an obsession. But, the literature is clear and becoming more voluminous every year that sleep is important to the mental health of our patients and their caregivers to things like obesity, to symptoms that suggest an attention-deficit/hyperactivity disorder, to school success, and to migraine ... to name just a few.
It may be that most of us realize the importance of sleep but feel our society has allowed itself to become so sleep deprived that there is little chance we can turn the ship around by spending just a few minutes trying help a family undo their deeply ingrained sleep unfriendly habits.
I am tempted to join those of you who see sleep depravation as a “why bother” issue. But, I’m not ready to throw in the towel. Even simply sharing your observations about the importance of sleep in the whole wellness picture may have an effect.
One of the benefits of retiring in the same community in which I practiced for over 40 years is that at least every month or two I encounter a parent who thanks me for sharing my views on the importance of sleep. They may not recall the little tip or two I gave them, but it seems that urging them to put sleep near the top of their lifestyle priority list has made the difference for them.
If I have failed in getting you to join me in my crusade against sleep deprivation, at least take to heart the most basic message of this study. That is that the investigators found only 20% of clinicians were addressing a concern that 90% of the caregivers shared. It happened to be sleep, but it could have been anything.
The authors of the study suggest that we need to be more assiduous in our screening for sleep problems. On the contrary. You and I know we don’t need more screening. We just need to be better listeners.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A recent study published in the journal Academic Pediatrics suggests that during health maintenance visits clinicians are giving too little attention to their patients’ sleep problems. Using a questionnaire, researchers surveyed patients’ caregivers’ concerns and observations regarding a variety of sleep problems. The investigators then reviewed the clinicians’ documentation of what transpired at the visit and found that while over 90% of the caregivers reported their child had at least one sleep related problem, only 20% of the clinicians documented the problem. And, only 12% documented a management plan regarding the sleep concerns.
I am always bit skeptical about studies that rely on clinicians’ “documentation” because clinicians are busy people and don’t always remember to record things they’ve discussed. You and I know that the lawyers’ dictum “if it wasn’t documented it didn’t happen” is rubbish. However, I still find the basic finding of this study concerning. If we are failing to ask about or even listen to caregivers’ concerns about something as important as sleep, we are missing the boat ... a very large boat.
How could this be happening? First, sleep may have fallen victim to the bloated list of topics that well-intentioned single-issue preventive health advocates have tacked on to the health maintenance visit. It’s a burden that few of us can manage without cutting corners.
However, it is more troubling to me that so many clinicians have chosen sleep as one of those corners to cut. This oversight suggests to me that too many of us have failed to realize from our own observations that sleep is incredibly important to the health of our patients ... and to ourselves.
I will admit that I am extremely sensitive to the importance of sleep. Some might say my sensitivity borders on an obsession. But, the literature is clear and becoming more voluminous every year that sleep is important to the mental health of our patients and their caregivers to things like obesity, to symptoms that suggest an attention-deficit/hyperactivity disorder, to school success, and to migraine ... to name just a few.
It may be that most of us realize the importance of sleep but feel our society has allowed itself to become so sleep deprived that there is little chance we can turn the ship around by spending just a few minutes trying help a family undo their deeply ingrained sleep unfriendly habits.
I am tempted to join those of you who see sleep depravation as a “why bother” issue. But, I’m not ready to throw in the towel. Even simply sharing your observations about the importance of sleep in the whole wellness picture may have an effect.
One of the benefits of retiring in the same community in which I practiced for over 40 years is that at least every month or two I encounter a parent who thanks me for sharing my views on the importance of sleep. They may not recall the little tip or two I gave them, but it seems that urging them to put sleep near the top of their lifestyle priority list has made the difference for them.
If I have failed in getting you to join me in my crusade against sleep deprivation, at least take to heart the most basic message of this study. That is that the investigators found only 20% of clinicians were addressing a concern that 90% of the caregivers shared. It happened to be sleep, but it could have been anything.
The authors of the study suggest that we need to be more assiduous in our screening for sleep problems. On the contrary. You and I know we don’t need more screening. We just need to be better listeners.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Antibiotics for acute exacerbation of COPD: It’s still controversial
In late 2021, the Rome Proposal for diagnosing acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and grading their severity was published. The 2023 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) Report has adopted the Rome Proposal criteria. Given that an endorsement by GOLD is tantamount to acceptance by clinicians, researchers, and policymakers alike, I guess we’re all using them now.
Anyone who’s ever cared for patients with COPD knows that treatment and reduction of exacerbations is how we improve outcomes. AECOPD are associated with considerable morbidity, greater health care utilization and costs, and a long-term decline in lung function. While we hope our pharmacotherapies improve symptoms, we know they reduce AECOPD. If our pharmacotherapies have any impact on mortality, it’s probably via AECOPD prevention.
Since antibiotic indications are tied to severity, using the Rome Proposal criteria may affect management in unpredictable ways. As such, it’s worth reviewing the data on antibiotics for AECOPD.
What do the data reveal?
To start, it’s important to note that GOLD doesn’t equate having an AECOPD with needing an antibiotic. I myself have conflated the diagnosis with the indication and thereby overprescribed. The bar for diagnosis is quite low. In previous GOLD summaries, any “change in respiratory symptoms” would warrant the AECOPD label. Although the Rome Proposal definition is more specific, it leaves room for liberal interpretation. It’s likely to have a greater effect on research than on clinical practice. My guess is that AECOPD prevalence doesn’t change.
The antibiotic hurdle is slightly higher than that for diagnosis but is equally open to interpretation. In part, that’s related to the inherent subjectivity of judging symptoms, sputum production, and changes in color, but it’s also because the data are so poor. The meta-analyses that have been used to establish the indications include fewer than 1000 patients spread across 10 to 11 trials. Thus, the individual trials are small, and the sample size remains nominal even after adding them together. The addition of antibiotics – and it doesn’t seem to matter which class, type, or duration – will decrease mortality and hospital length of stay. One study says these effects are limited to inpatients while the other does not. After reading GOLD 2013, GOLD 2023, and both the meta-analyses they used to support their recommendations, I’m still not sure who benefits. Do you have to be hospitalized? Is some sort of ventilatory support required? Does C-reactive protein help or not?
In accordance with the classic Anthonisen criteria, GOLD relies on sputum volume and color as evidence of a bacterial infection. Soon after GOLD 2023 was published, a meta-analysis found that sputum color isn’t particularly accurate for detecting bacterial infection. Because it doesn’t seem to matter which antibiotic class is used, I always thought we were using antibiotics for their magical, pleiotropic anti-inflammatory effects anyway. I didn’t think the presence of an actual bacterial infection was important. If I saw an infiltrate on chest x-ray, I’d change my diagnosis from AECOPD to community-acquired pneumonia (CAP) and switch to CAP coverage. I’ve been doing this so long that I swear it’s in a guideline somewhere, though admittedly I couldn’t find said guideline while reading for this piece.
Key takeaways
In summary, I believe that the guidance reflects the data, which is muddy. The Rome Proposal should be seen as just that – a framework for moving forward with AECOPD classification and antibiotic indications that will need to be refined over time as better data become available. In fact, they allow for a more objective, point-of-care assessment of severity that can be validated and tied to antibiotic benefits. The Rome criteria aren’t evidence-based; they’re a necessary first step toward creating the evidence.
In the meantime, if your AECOPD patients are hospitalized, they probably warrant an antibiotic. If they’re not, sputum changes may be a reasonable surrogate for a bacterial infection. Considerable uncertainty remains.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
In late 2021, the Rome Proposal for diagnosing acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and grading their severity was published. The 2023 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) Report has adopted the Rome Proposal criteria. Given that an endorsement by GOLD is tantamount to acceptance by clinicians, researchers, and policymakers alike, I guess we’re all using them now.
Anyone who’s ever cared for patients with COPD knows that treatment and reduction of exacerbations is how we improve outcomes. AECOPD are associated with considerable morbidity, greater health care utilization and costs, and a long-term decline in lung function. While we hope our pharmacotherapies improve symptoms, we know they reduce AECOPD. If our pharmacotherapies have any impact on mortality, it’s probably via AECOPD prevention.
Since antibiotic indications are tied to severity, using the Rome Proposal criteria may affect management in unpredictable ways. As such, it’s worth reviewing the data on antibiotics for AECOPD.
What do the data reveal?
To start, it’s important to note that GOLD doesn’t equate having an AECOPD with needing an antibiotic. I myself have conflated the diagnosis with the indication and thereby overprescribed. The bar for diagnosis is quite low. In previous GOLD summaries, any “change in respiratory symptoms” would warrant the AECOPD label. Although the Rome Proposal definition is more specific, it leaves room for liberal interpretation. It’s likely to have a greater effect on research than on clinical practice. My guess is that AECOPD prevalence doesn’t change.
The antibiotic hurdle is slightly higher than that for diagnosis but is equally open to interpretation. In part, that’s related to the inherent subjectivity of judging symptoms, sputum production, and changes in color, but it’s also because the data are so poor. The meta-analyses that have been used to establish the indications include fewer than 1000 patients spread across 10 to 11 trials. Thus, the individual trials are small, and the sample size remains nominal even after adding them together. The addition of antibiotics – and it doesn’t seem to matter which class, type, or duration – will decrease mortality and hospital length of stay. One study says these effects are limited to inpatients while the other does not. After reading GOLD 2013, GOLD 2023, and both the meta-analyses they used to support their recommendations, I’m still not sure who benefits. Do you have to be hospitalized? Is some sort of ventilatory support required? Does C-reactive protein help or not?
In accordance with the classic Anthonisen criteria, GOLD relies on sputum volume and color as evidence of a bacterial infection. Soon after GOLD 2023 was published, a meta-analysis found that sputum color isn’t particularly accurate for detecting bacterial infection. Because it doesn’t seem to matter which antibiotic class is used, I always thought we were using antibiotics for their magical, pleiotropic anti-inflammatory effects anyway. I didn’t think the presence of an actual bacterial infection was important. If I saw an infiltrate on chest x-ray, I’d change my diagnosis from AECOPD to community-acquired pneumonia (CAP) and switch to CAP coverage. I’ve been doing this so long that I swear it’s in a guideline somewhere, though admittedly I couldn’t find said guideline while reading for this piece.
Key takeaways
In summary, I believe that the guidance reflects the data, which is muddy. The Rome Proposal should be seen as just that – a framework for moving forward with AECOPD classification and antibiotic indications that will need to be refined over time as better data become available. In fact, they allow for a more objective, point-of-care assessment of severity that can be validated and tied to antibiotic benefits. The Rome criteria aren’t evidence-based; they’re a necessary first step toward creating the evidence.
In the meantime, if your AECOPD patients are hospitalized, they probably warrant an antibiotic. If they’re not, sputum changes may be a reasonable surrogate for a bacterial infection. Considerable uncertainty remains.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
In late 2021, the Rome Proposal for diagnosing acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and grading their severity was published. The 2023 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) Report has adopted the Rome Proposal criteria. Given that an endorsement by GOLD is tantamount to acceptance by clinicians, researchers, and policymakers alike, I guess we’re all using them now.
Anyone who’s ever cared for patients with COPD knows that treatment and reduction of exacerbations is how we improve outcomes. AECOPD are associated with considerable morbidity, greater health care utilization and costs, and a long-term decline in lung function. While we hope our pharmacotherapies improve symptoms, we know they reduce AECOPD. If our pharmacotherapies have any impact on mortality, it’s probably via AECOPD prevention.
Since antibiotic indications are tied to severity, using the Rome Proposal criteria may affect management in unpredictable ways. As such, it’s worth reviewing the data on antibiotics for AECOPD.
What do the data reveal?
To start, it’s important to note that GOLD doesn’t equate having an AECOPD with needing an antibiotic. I myself have conflated the diagnosis with the indication and thereby overprescribed. The bar for diagnosis is quite low. In previous GOLD summaries, any “change in respiratory symptoms” would warrant the AECOPD label. Although the Rome Proposal definition is more specific, it leaves room for liberal interpretation. It’s likely to have a greater effect on research than on clinical practice. My guess is that AECOPD prevalence doesn’t change.
The antibiotic hurdle is slightly higher than that for diagnosis but is equally open to interpretation. In part, that’s related to the inherent subjectivity of judging symptoms, sputum production, and changes in color, but it’s also because the data are so poor. The meta-analyses that have been used to establish the indications include fewer than 1000 patients spread across 10 to 11 trials. Thus, the individual trials are small, and the sample size remains nominal even after adding them together. The addition of antibiotics – and it doesn’t seem to matter which class, type, or duration – will decrease mortality and hospital length of stay. One study says these effects are limited to inpatients while the other does not. After reading GOLD 2013, GOLD 2023, and both the meta-analyses they used to support their recommendations, I’m still not sure who benefits. Do you have to be hospitalized? Is some sort of ventilatory support required? Does C-reactive protein help or not?
In accordance with the classic Anthonisen criteria, GOLD relies on sputum volume and color as evidence of a bacterial infection. Soon after GOLD 2023 was published, a meta-analysis found that sputum color isn’t particularly accurate for detecting bacterial infection. Because it doesn’t seem to matter which antibiotic class is used, I always thought we were using antibiotics for their magical, pleiotropic anti-inflammatory effects anyway. I didn’t think the presence of an actual bacterial infection was important. If I saw an infiltrate on chest x-ray, I’d change my diagnosis from AECOPD to community-acquired pneumonia (CAP) and switch to CAP coverage. I’ve been doing this so long that I swear it’s in a guideline somewhere, though admittedly I couldn’t find said guideline while reading for this piece.
Key takeaways
In summary, I believe that the guidance reflects the data, which is muddy. The Rome Proposal should be seen as just that – a framework for moving forward with AECOPD classification and antibiotic indications that will need to be refined over time as better data become available. In fact, they allow for a more objective, point-of-care assessment of severity that can be validated and tied to antibiotic benefits. The Rome criteria aren’t evidence-based; they’re a necessary first step toward creating the evidence.
In the meantime, if your AECOPD patients are hospitalized, they probably warrant an antibiotic. If they’re not, sputum changes may be a reasonable surrogate for a bacterial infection. Considerable uncertainty remains.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Fibroid characteristics can help us anticipate postpartum hemorrhage
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
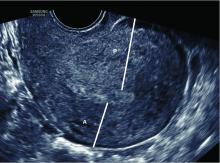
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Finding a home in psychiatry: A medical student’s story
Perched on a meditation cushion with the day’s first light creeping up the Himalayan foothills around me, I felt more at ease within myself than I could ever recall over my previous 19 years.
My immersion in daily conversations within the Tibetan monastic community on achieving a more harmonious relationship to our thoughts and feelings awoke a consideration of myself and my inner life in a way that I’d never truly contemplated before. These reflections gave me a vocabulary and a toolkit for navigating my own internal landscape that I have used ever since.
However, upon returning home, I was forced to acknowledge how fortunate I had been, and that these tools and the underlying spirit of inquiry are not commonplace in our society. Despite great strides in shifting views toward mental illness over the past few decades, our public discourse rarely captures the nuances of the mental health crisis that our culture has faced well before COVID-19 catalyzed even greater distress. We all pay the price of this cultural deficit to varying degrees, and I became captivated by the notion that things could be different.
I followed that thread of inquiry through the practices of Buddhist studies, massage therapy, yoga instruction, and refugee aid before coming to psychiatry as the unlikely yet ideal crucible for integrating my experiences in these spaces. Since arriving at medical school, however, my vision of myself as a psychiatrist has changed dramatically as my aspirations have collided with the realities of clinical experience and been tempered by the wisdom of mentors, colleagues, and patients, opening up a space for a deeper appreciation of what psychiatry might offer.
Clinical experience changes perspective
Short on clinical experience, I had previously imagined my future practice primarily as one of mindful listening and finding presence with each patient as a kind ear, supplemented by the ability to prescribe medication. Since then, working with patients has offered me insight into the ways in which my personality, perception, and potential access to a range of affective stances can serve as tools for skillfully developing the therapeutic encounter.
Moreover, “challenging” patients have taught me that my role is not always to offer unbounded empathetic support, but to potentially initiate compassionately tactful confrontation, shifting my sense of my role in the therapeutic relationship.
This is a lofty goal, which might entail modeling the successful navigation of potential ruptures and the subsequent repair of relationships so that they can live more adaptably in the world.
However, while I can support their envisioning of a realistic future for themselves and facilitate their acquisition of the tools needed to get there, my role is significant yet limited. This has been a hard truth to reckon with, but one that’s opened up pathways to greater empathy and a deeper understanding of each patient’s struggles. As a result, my view of pathology as a state has shifted to one of a dynamic process that emerges through the interaction of their genes, environment, life history, pharmacological supplements, psychodynamic tendencies, diet, and more.
Yet, while holding this reality of the complexities of mental illness, clinical decision-making often hinges on making binary choices regarding diagnoses, medications, and criteria for legal determinations. Developing this capacity to simultaneously practice different ways of knowing and sit with uncertainty excites me tremendously, not only equipping me to balance clinical practice with the demands of the modern health care system, but also nourishing the roots of a rich and ethical life.
Psychiatry calls to me for this expectation of sustaining an appropriate tension between uncertainty and decisiveness. It also inspires a deeper dive into the history of the field in order to learn the roots of its theories and perspectives so I can better understand how those inform contemporary practice in ways that are both helpful and harmful.
From individual to community
In tandem with this outer work of learning to appropriately position myself within individual patient relationships, the broader health care system, and the legacy of the field, I’ve also sought to develop a better understanding of how my own history, beliefs, and motivations shape my collaborative efforts.
Through my mindfulness practice and participation in exploratory psychoanalysis, I’ve caught glimpses of my own countertransference investments and opened up space for seeing how patients might experience me as a clinician. This has allowed for tuning in to my own response to them, identifying where in the typology of personality structures our reciprocal experiences might exist, and learning to manage those feelings to ultimately foster empathy through the interaction.
This has shifted my sense of the work from solely mindfully listening and thoughtfully responding to honing deliberate ways of both listening and responding in a way that is directly informed by the person sitting in front of me so I can best support them in creating change.
Given the responsibility inherent to this work, I have treated my medical education as an opportunity to build a foundation for stepping into this role. This has involved going beyond exploring these dynamics within individual clinician-patient relationships and carried over into my experiences with community-based research and program development. It has asked me to recognize the perceptual frames and prioritization of values that I bring to any given project.
This process has sharpened my aim of discovering each community’s understanding of their mental health needs so that I’m not implicitly imposing my own notions of psychological wholeness and “wellness” on others.
Working with San Diego’s Somali and Spanish-speaking populations has helped me to better understand each community’s own conceptualization of their strengths and needs, teaching me how to engage in reciprocal partnerships that honor each of our areas of expertise. Investing myself in medical school curricular reform represents the flip side of this coin, serving as an attempt to better understand my own medical community, how we think about health, and how we can best care for ourselves.
These experiences have offered opportunities to refine my skills in appreciative inquiry, coalition building, navigating institutional dynamics, and initiating and sustaining change within complex systems to carry the lessons of psychiatry beyond explicitly clinical spaces.
Toward integrative care
Ultimately, I view my community-based research and academic program development as outgrowths of my commitment to clinical psychiatry and my desire to learn how to provide people with the tools for changing their relationship to themselves, others, and their communities.
Equipped with formal medical training as the bedrock of this skill set, I have actively sought out opportunities to draw from practices that are outside the scope of the formal curriculum. These range from psychoanalysis and narrative medicine to cultural psychiatry and psychological anthropology, as well as my background in bodywork and mindfulness education. I’m eager to dive more fully into psychiatric practice as I work to integrate these disparate knowledge bases with the biomedical and psychodynamic views of the mind to develop a strengths-based practice that tends to patients’ bodies, minds, and spirits by bringing forth their own knowledge of themselves and their lives as they imagine what could be.
These realizations bring me back to that Himalayan sunrise more than a decade ago. They affirm that my heart lies with traversing disciplines to provide integrative psychiatric care in the community and developing infrastructure that supports these efforts. I’m filled with enthusiasm by the breadth of what psychiatry training offers as I continue expanding my capacity to support patients in this lifelong healing journey.
Alec Terrana is a rising fourth-year medical student at the University of California, San Diego, who intends to apply into psychiatry residency programs. He’s invested in exploring how we can more effectively conceptualize and measure mental health outcomes within San Diego’s Somali and Spanish-speaking communities, as well as advancing mindfulness and compassion training in undergraduate medical education. His professional interests also include implementation science, cultural psychiatry, psychodynamics, and strengthening public mental health infrastructure.
Perched on a meditation cushion with the day’s first light creeping up the Himalayan foothills around me, I felt more at ease within myself than I could ever recall over my previous 19 years.
My immersion in daily conversations within the Tibetan monastic community on achieving a more harmonious relationship to our thoughts and feelings awoke a consideration of myself and my inner life in a way that I’d never truly contemplated before. These reflections gave me a vocabulary and a toolkit for navigating my own internal landscape that I have used ever since.
However, upon returning home, I was forced to acknowledge how fortunate I had been, and that these tools and the underlying spirit of inquiry are not commonplace in our society. Despite great strides in shifting views toward mental illness over the past few decades, our public discourse rarely captures the nuances of the mental health crisis that our culture has faced well before COVID-19 catalyzed even greater distress. We all pay the price of this cultural deficit to varying degrees, and I became captivated by the notion that things could be different.
I followed that thread of inquiry through the practices of Buddhist studies, massage therapy, yoga instruction, and refugee aid before coming to psychiatry as the unlikely yet ideal crucible for integrating my experiences in these spaces. Since arriving at medical school, however, my vision of myself as a psychiatrist has changed dramatically as my aspirations have collided with the realities of clinical experience and been tempered by the wisdom of mentors, colleagues, and patients, opening up a space for a deeper appreciation of what psychiatry might offer.
Clinical experience changes perspective
Short on clinical experience, I had previously imagined my future practice primarily as one of mindful listening and finding presence with each patient as a kind ear, supplemented by the ability to prescribe medication. Since then, working with patients has offered me insight into the ways in which my personality, perception, and potential access to a range of affective stances can serve as tools for skillfully developing the therapeutic encounter.
Moreover, “challenging” patients have taught me that my role is not always to offer unbounded empathetic support, but to potentially initiate compassionately tactful confrontation, shifting my sense of my role in the therapeutic relationship.
This is a lofty goal, which might entail modeling the successful navigation of potential ruptures and the subsequent repair of relationships so that they can live more adaptably in the world.
However, while I can support their envisioning of a realistic future for themselves and facilitate their acquisition of the tools needed to get there, my role is significant yet limited. This has been a hard truth to reckon with, but one that’s opened up pathways to greater empathy and a deeper understanding of each patient’s struggles. As a result, my view of pathology as a state has shifted to one of a dynamic process that emerges through the interaction of their genes, environment, life history, pharmacological supplements, psychodynamic tendencies, diet, and more.
Yet, while holding this reality of the complexities of mental illness, clinical decision-making often hinges on making binary choices regarding diagnoses, medications, and criteria for legal determinations. Developing this capacity to simultaneously practice different ways of knowing and sit with uncertainty excites me tremendously, not only equipping me to balance clinical practice with the demands of the modern health care system, but also nourishing the roots of a rich and ethical life.
Psychiatry calls to me for this expectation of sustaining an appropriate tension between uncertainty and decisiveness. It also inspires a deeper dive into the history of the field in order to learn the roots of its theories and perspectives so I can better understand how those inform contemporary practice in ways that are both helpful and harmful.
From individual to community
In tandem with this outer work of learning to appropriately position myself within individual patient relationships, the broader health care system, and the legacy of the field, I’ve also sought to develop a better understanding of how my own history, beliefs, and motivations shape my collaborative efforts.
Through my mindfulness practice and participation in exploratory psychoanalysis, I’ve caught glimpses of my own countertransference investments and opened up space for seeing how patients might experience me as a clinician. This has allowed for tuning in to my own response to them, identifying where in the typology of personality structures our reciprocal experiences might exist, and learning to manage those feelings to ultimately foster empathy through the interaction.
This has shifted my sense of the work from solely mindfully listening and thoughtfully responding to honing deliberate ways of both listening and responding in a way that is directly informed by the person sitting in front of me so I can best support them in creating change.
Given the responsibility inherent to this work, I have treated my medical education as an opportunity to build a foundation for stepping into this role. This has involved going beyond exploring these dynamics within individual clinician-patient relationships and carried over into my experiences with community-based research and program development. It has asked me to recognize the perceptual frames and prioritization of values that I bring to any given project.
This process has sharpened my aim of discovering each community’s understanding of their mental health needs so that I’m not implicitly imposing my own notions of psychological wholeness and “wellness” on others.
Working with San Diego’s Somali and Spanish-speaking populations has helped me to better understand each community’s own conceptualization of their strengths and needs, teaching me how to engage in reciprocal partnerships that honor each of our areas of expertise. Investing myself in medical school curricular reform represents the flip side of this coin, serving as an attempt to better understand my own medical community, how we think about health, and how we can best care for ourselves.
These experiences have offered opportunities to refine my skills in appreciative inquiry, coalition building, navigating institutional dynamics, and initiating and sustaining change within complex systems to carry the lessons of psychiatry beyond explicitly clinical spaces.
Toward integrative care
Ultimately, I view my community-based research and academic program development as outgrowths of my commitment to clinical psychiatry and my desire to learn how to provide people with the tools for changing their relationship to themselves, others, and their communities.
Equipped with formal medical training as the bedrock of this skill set, I have actively sought out opportunities to draw from practices that are outside the scope of the formal curriculum. These range from psychoanalysis and narrative medicine to cultural psychiatry and psychological anthropology, as well as my background in bodywork and mindfulness education. I’m eager to dive more fully into psychiatric practice as I work to integrate these disparate knowledge bases with the biomedical and psychodynamic views of the mind to develop a strengths-based practice that tends to patients’ bodies, minds, and spirits by bringing forth their own knowledge of themselves and their lives as they imagine what could be.
These realizations bring me back to that Himalayan sunrise more than a decade ago. They affirm that my heart lies with traversing disciplines to provide integrative psychiatric care in the community and developing infrastructure that supports these efforts. I’m filled with enthusiasm by the breadth of what psychiatry training offers as I continue expanding my capacity to support patients in this lifelong healing journey.
Alec Terrana is a rising fourth-year medical student at the University of California, San Diego, who intends to apply into psychiatry residency programs. He’s invested in exploring how we can more effectively conceptualize and measure mental health outcomes within San Diego’s Somali and Spanish-speaking communities, as well as advancing mindfulness and compassion training in undergraduate medical education. His professional interests also include implementation science, cultural psychiatry, psychodynamics, and strengthening public mental health infrastructure.
Perched on a meditation cushion with the day’s first light creeping up the Himalayan foothills around me, I felt more at ease within myself than I could ever recall over my previous 19 years.
My immersion in daily conversations within the Tibetan monastic community on achieving a more harmonious relationship to our thoughts and feelings awoke a consideration of myself and my inner life in a way that I’d never truly contemplated before. These reflections gave me a vocabulary and a toolkit for navigating my own internal landscape that I have used ever since.
However, upon returning home, I was forced to acknowledge how fortunate I had been, and that these tools and the underlying spirit of inquiry are not commonplace in our society. Despite great strides in shifting views toward mental illness over the past few decades, our public discourse rarely captures the nuances of the mental health crisis that our culture has faced well before COVID-19 catalyzed even greater distress. We all pay the price of this cultural deficit to varying degrees, and I became captivated by the notion that things could be different.
I followed that thread of inquiry through the practices of Buddhist studies, massage therapy, yoga instruction, and refugee aid before coming to psychiatry as the unlikely yet ideal crucible for integrating my experiences in these spaces. Since arriving at medical school, however, my vision of myself as a psychiatrist has changed dramatically as my aspirations have collided with the realities of clinical experience and been tempered by the wisdom of mentors, colleagues, and patients, opening up a space for a deeper appreciation of what psychiatry might offer.
Clinical experience changes perspective
Short on clinical experience, I had previously imagined my future practice primarily as one of mindful listening and finding presence with each patient as a kind ear, supplemented by the ability to prescribe medication. Since then, working with patients has offered me insight into the ways in which my personality, perception, and potential access to a range of affective stances can serve as tools for skillfully developing the therapeutic encounter.
Moreover, “challenging” patients have taught me that my role is not always to offer unbounded empathetic support, but to potentially initiate compassionately tactful confrontation, shifting my sense of my role in the therapeutic relationship.
This is a lofty goal, which might entail modeling the successful navigation of potential ruptures and the subsequent repair of relationships so that they can live more adaptably in the world.
However, while I can support their envisioning of a realistic future for themselves and facilitate their acquisition of the tools needed to get there, my role is significant yet limited. This has been a hard truth to reckon with, but one that’s opened up pathways to greater empathy and a deeper understanding of each patient’s struggles. As a result, my view of pathology as a state has shifted to one of a dynamic process that emerges through the interaction of their genes, environment, life history, pharmacological supplements, psychodynamic tendencies, diet, and more.
Yet, while holding this reality of the complexities of mental illness, clinical decision-making often hinges on making binary choices regarding diagnoses, medications, and criteria for legal determinations. Developing this capacity to simultaneously practice different ways of knowing and sit with uncertainty excites me tremendously, not only equipping me to balance clinical practice with the demands of the modern health care system, but also nourishing the roots of a rich and ethical life.
Psychiatry calls to me for this expectation of sustaining an appropriate tension between uncertainty and decisiveness. It also inspires a deeper dive into the history of the field in order to learn the roots of its theories and perspectives so I can better understand how those inform contemporary practice in ways that are both helpful and harmful.
From individual to community
In tandem with this outer work of learning to appropriately position myself within individual patient relationships, the broader health care system, and the legacy of the field, I’ve also sought to develop a better understanding of how my own history, beliefs, and motivations shape my collaborative efforts.
Through my mindfulness practice and participation in exploratory psychoanalysis, I’ve caught glimpses of my own countertransference investments and opened up space for seeing how patients might experience me as a clinician. This has allowed for tuning in to my own response to them, identifying where in the typology of personality structures our reciprocal experiences might exist, and learning to manage those feelings to ultimately foster empathy through the interaction.
This has shifted my sense of the work from solely mindfully listening and thoughtfully responding to honing deliberate ways of both listening and responding in a way that is directly informed by the person sitting in front of me so I can best support them in creating change.
Given the responsibility inherent to this work, I have treated my medical education as an opportunity to build a foundation for stepping into this role. This has involved going beyond exploring these dynamics within individual clinician-patient relationships and carried over into my experiences with community-based research and program development. It has asked me to recognize the perceptual frames and prioritization of values that I bring to any given project.
This process has sharpened my aim of discovering each community’s understanding of their mental health needs so that I’m not implicitly imposing my own notions of psychological wholeness and “wellness” on others.
Working with San Diego’s Somali and Spanish-speaking populations has helped me to better understand each community’s own conceptualization of their strengths and needs, teaching me how to engage in reciprocal partnerships that honor each of our areas of expertise. Investing myself in medical school curricular reform represents the flip side of this coin, serving as an attempt to better understand my own medical community, how we think about health, and how we can best care for ourselves.
These experiences have offered opportunities to refine my skills in appreciative inquiry, coalition building, navigating institutional dynamics, and initiating and sustaining change within complex systems to carry the lessons of psychiatry beyond explicitly clinical spaces.
Toward integrative care
Ultimately, I view my community-based research and academic program development as outgrowths of my commitment to clinical psychiatry and my desire to learn how to provide people with the tools for changing their relationship to themselves, others, and their communities.
Equipped with formal medical training as the bedrock of this skill set, I have actively sought out opportunities to draw from practices that are outside the scope of the formal curriculum. These range from psychoanalysis and narrative medicine to cultural psychiatry and psychological anthropology, as well as my background in bodywork and mindfulness education. I’m eager to dive more fully into psychiatric practice as I work to integrate these disparate knowledge bases with the biomedical and psychodynamic views of the mind to develop a strengths-based practice that tends to patients’ bodies, minds, and spirits by bringing forth their own knowledge of themselves and their lives as they imagine what could be.
These realizations bring me back to that Himalayan sunrise more than a decade ago. They affirm that my heart lies with traversing disciplines to provide integrative psychiatric care in the community and developing infrastructure that supports these efforts. I’m filled with enthusiasm by the breadth of what psychiatry training offers as I continue expanding my capacity to support patients in this lifelong healing journey.
Alec Terrana is a rising fourth-year medical student at the University of California, San Diego, who intends to apply into psychiatry residency programs. He’s invested in exploring how we can more effectively conceptualize and measure mental health outcomes within San Diego’s Somali and Spanish-speaking communities, as well as advancing mindfulness and compassion training in undergraduate medical education. His professional interests also include implementation science, cultural psychiatry, psychodynamics, and strengthening public mental health infrastructure.
What’s in a drug name?
My use of drug names is a mixed bag of terms.
In medical school we learn drugs by their generic names, but it doesn’t take long before we realize that each has both a generic name and one (or more) brand names. I suppose there’s also the chemical names, but no one outside the lab uses those. They’re waaaaay too long.
There is, for better or worse, a lot of variability in this. The purists (almost always academics, or cardiologists, or academic cardiologists) insist on generic names only. In their notes, conversations, presentations, whatever. If you’re a medical student or resident under them, you learn fast not to use the brand name.
After 30 years of doing this ... I don’t care. My notes are a mishmash of both.
Let’s face it, brand names are generally shorter and easier to type, spell, and pronounce than the generic names. I still need to know both, but when I’m writing up a note Keppra is far easier than levetiracetam. And most patients find the brand names a lot easier to say and remember.
An even weirder point, which is my own, is that one of my teaching attendings insisted that we capitalize both generic and brand names while on his rotation. Why? He never explained that, but he was pretty insistent. Now, for whatever reason, the habit has stuck with me. I’m sure the cardiologist down the hall would love to send my notes back, heavily marked up with red ink.
There’s even a weird subdivisions in this: Aspirin is a brand name by Bayer. Shouldn’t it be capitalized in our notes? But it isn’t, and to make things more confusing that varies by country. Why? (if you’re curious, it’s a strange combination of 100-year-old patent claims, generic trademark rulings, and also what country you’re in, whether it was involved in World War I, and, if so, which side. Really).
So the medical lists in my notes are certainly understandable, though aren’t going to score me any points for academic correctness. Not that I care. As a medical Shakespeare might have written, Imitrex, Onzetra, Zembrace, Tosymra, Sumavel, Alsuma, Imigran, Migraitan, and Zecuity ... are still sumatriptan by any other name.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My use of drug names is a mixed bag of terms.
In medical school we learn drugs by their generic names, but it doesn’t take long before we realize that each has both a generic name and one (or more) brand names. I suppose there’s also the chemical names, but no one outside the lab uses those. They’re waaaaay too long.
There is, for better or worse, a lot of variability in this. The purists (almost always academics, or cardiologists, or academic cardiologists) insist on generic names only. In their notes, conversations, presentations, whatever. If you’re a medical student or resident under them, you learn fast not to use the brand name.
After 30 years of doing this ... I don’t care. My notes are a mishmash of both.
Let’s face it, brand names are generally shorter and easier to type, spell, and pronounce than the generic names. I still need to know both, but when I’m writing up a note Keppra is far easier than levetiracetam. And most patients find the brand names a lot easier to say and remember.
An even weirder point, which is my own, is that one of my teaching attendings insisted that we capitalize both generic and brand names while on his rotation. Why? He never explained that, but he was pretty insistent. Now, for whatever reason, the habit has stuck with me. I’m sure the cardiologist down the hall would love to send my notes back, heavily marked up with red ink.
There’s even a weird subdivisions in this: Aspirin is a brand name by Bayer. Shouldn’t it be capitalized in our notes? But it isn’t, and to make things more confusing that varies by country. Why? (if you’re curious, it’s a strange combination of 100-year-old patent claims, generic trademark rulings, and also what country you’re in, whether it was involved in World War I, and, if so, which side. Really).
So the medical lists in my notes are certainly understandable, though aren’t going to score me any points for academic correctness. Not that I care. As a medical Shakespeare might have written, Imitrex, Onzetra, Zembrace, Tosymra, Sumavel, Alsuma, Imigran, Migraitan, and Zecuity ... are still sumatriptan by any other name.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My use of drug names is a mixed bag of terms.
In medical school we learn drugs by their generic names, but it doesn’t take long before we realize that each has both a generic name and one (or more) brand names. I suppose there’s also the chemical names, but no one outside the lab uses those. They’re waaaaay too long.
There is, for better or worse, a lot of variability in this. The purists (almost always academics, or cardiologists, or academic cardiologists) insist on generic names only. In their notes, conversations, presentations, whatever. If you’re a medical student or resident under them, you learn fast not to use the brand name.
After 30 years of doing this ... I don’t care. My notes are a mishmash of both.
Let’s face it, brand names are generally shorter and easier to type, spell, and pronounce than the generic names. I still need to know both, but when I’m writing up a note Keppra is far easier than levetiracetam. And most patients find the brand names a lot easier to say and remember.
An even weirder point, which is my own, is that one of my teaching attendings insisted that we capitalize both generic and brand names while on his rotation. Why? He never explained that, but he was pretty insistent. Now, for whatever reason, the habit has stuck with me. I’m sure the cardiologist down the hall would love to send my notes back, heavily marked up with red ink.
There’s even a weird subdivisions in this: Aspirin is a brand name by Bayer. Shouldn’t it be capitalized in our notes? But it isn’t, and to make things more confusing that varies by country. Why? (if you’re curious, it’s a strange combination of 100-year-old patent claims, generic trademark rulings, and also what country you’re in, whether it was involved in World War I, and, if so, which side. Really).
So the medical lists in my notes are certainly understandable, though aren’t going to score me any points for academic correctness. Not that I care. As a medical Shakespeare might have written, Imitrex, Onzetra, Zembrace, Tosymra, Sumavel, Alsuma, Imigran, Migraitan, and Zecuity ... are still sumatriptan by any other name.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.
If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
Investigating the etiology of recurrent pregnancy loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.
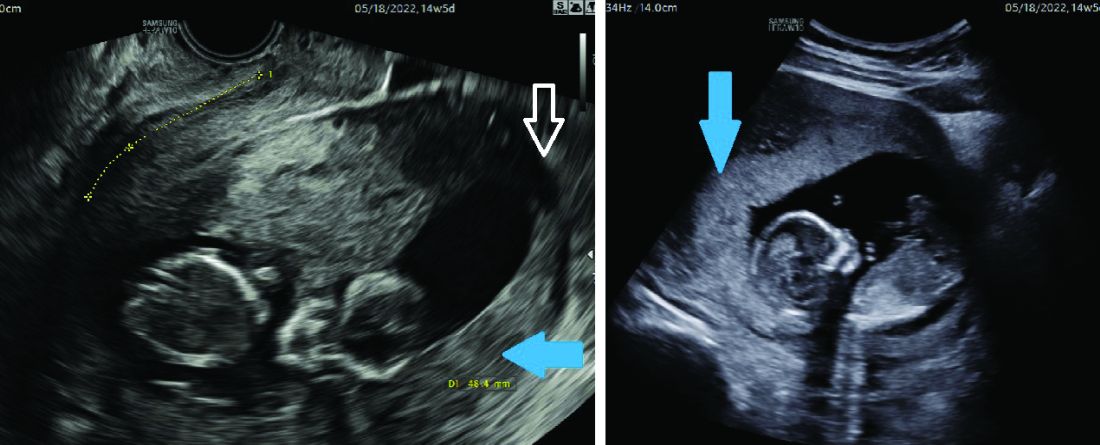
Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
With attention to the timing of loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.