User login
Acetaminophen linked to diminished response to immunotherapy in cancer
The team found a strong association between the use of acetaminophen and a decreased response to immune checkpoint inhibitors in a study of three clinical cohorts involving more than 600 patients with advanced cancer.
Patients who took acetaminophen at the start of immunotherapy – with acetaminophen exposure confirmed by plasma testing – were found to have worse overall survival and progression-free survival than patients who did not take the analgesic. Multivariate analysis confirmed the association independent of other prognostic factors. “It is unlikely that our data are the result of bias or unmeasured confounding,” the authors comment.
The findings “present a compelling case for caution” in using acetaminophen in patients with cancer who are receiving immune checkpoint blockers, senior investigator Antoine Italiano, MD, PhD, a medical oncologist at the University of Bordeaux (France), and colleagues concluded.
The study was presented at the annual meeting of the American Society of Clinical Oncology and published simultaneously in Annals of Oncology.
“Patients with advanced cancer taking [acetaminophen] during immunotherapy experience worse clinical outcomes, which suggests that [acetaminophen] decreases T cell–mediated antitumor immunity,” the authors comment.
They also report bench research and blood studies in four healthy volunteers, which showed an up-regulation of immunosuppressive regulatory T cells (Tregs) with acetaminophen, and other findings that together suggest that acetaminophen undermines the antitumor immune processes by which checkpoint inhibitors work.
Reconsider acetaminophen pretreatment
After hearing Dr. Italiano present the results at the meeting, a Polish oncologist in the audience said he was concerned that his clinic premedicates with acetaminophen before immune checkpoint blockade and wanted to know if they should stop doing it.
“I don’t think inducing Tregs ... in cancer patients is a good approach. I do a lot of clinical trials,” and “I do not understand why in several cases sponsors required mandatory premedication with acetaminophen. I think ... we should reconsider this approach,” Dr. Italiano said.
There’s precedence for the findings. Acetaminophen – also known as paracetamol – has been shown in some studies to limit immune cell proliferation, T-cell–dependent antibody response, and viral clearance, among other things. After a randomized trial showing blunted responses to vaccines in individuals who were taking acetaminophen, the World Health Organization recommended in 2015 against concurrent use of acetaminophen with vaccines.
Steroids, antibiotics, and proton pump inhibitors have also recently been shown to worsen outcomes with pembrolizumab, noted invited discussant, Margaret Gatti-Mays, MD, a medical oncologist at Ohio State University, Columbus.
“We are starting to understand that ... commonly used medications may have a larger impact on the efficacy and toxicity of immune checkpoint blockade than historically seen with chemotherapy,” she said.
However, she expressed some uncertainty over the French findings, as she was concerned that even the multivariate analysis didn’t completely rule out that acetaminophen users had worse disease to begin with and so would be expected to have worse outcomes.
She was also unsure of how much acetaminophen is too much.
Acetaminophen has a half-life of around 3 hours or less, where the immune checkpoint inhibitors have a half-life of around 20 days or more.
Given that, Dr. Gatti-Mays wondered whether “a single dose of acetaminophen [is] enough to derail the benefit of checkpoint inhibition? Does exposure need to be continuous?”
She allowed that acetaminophen use may turn out to be one more of the many patient-level factors emerging lately – such as chronic stress, diet, body flora, and physiological age, among others – that might help explain why checkpoint inhibition works in only about 20% of eligible patients with cancer.
Study details
Dr. Italiano and his team analyzed plasma samples from 297 participants in the CheckMate 025 trial of nivolumab for renal cancer; 34 participants in the BIP study into actionable molecular alterations in cancer; and 297 participants in the PREMIS immune-related adverse events study. The patients in these last two studies had a variety of cancers and were taking various agents.
All 628 patients were on checkpoint inhibitors. The investigators divided them according to who had acetaminophen or its metabolite acetaminophen glucuronide in their plasma when they started checkpoint inhibition and those who did not.
In CheckMate 025, overall survival was significantly worse among participants who had detectable acetaminophen or its metabolite in plasma (hazard ratio, 0.67; P = .004).
None of the acetaminophen-positive participants in the BIP study responded to checkpoint blockade, compared with almost 30% of those who were negative. Acetaminophen-positive participants also trended toward worse progression-free survival (median, 1.87 vs. 4.72 months) and overall survival (median, 7.87 vs. 16.56 months).
In PREMIS, progression-free survival was a median of 2.63 months in the acetaminophen group versus 5.03 months in negative participants (P = .009); median overall survival was 8.43 months versus 14.93 months, respectively (P < .0001).
A multivariate analysis was performed in PREMIS. Acetaminophen exposure was associated with both progression-free survival (hazard ratio, 1.43; P =.015) and overall survival (HR, 1.78; P =.006) independently of performance status, liver metastases, bone metastases, number of metastases sites, tumor type, number of previous lines of treatment, steroid/antibiotic use, lactate dehydrogenase levels, and other factors.
There was no funding for the work. Dr. Italiano is a consultant for AstraZeneca, Bayer, Chugai, Deciphera, Merck, Parthenon, Roche, and Springworks, He also has grants from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, MSD, Novartis, Pharmamar, and Roche. Two authors work for Explicyte and one works for Amgen. Dr. Gatti-Mays is a consultant for Seattle Genetics.
A version of this article first appeared on Medscape.com.
The team found a strong association between the use of acetaminophen and a decreased response to immune checkpoint inhibitors in a study of three clinical cohorts involving more than 600 patients with advanced cancer.
Patients who took acetaminophen at the start of immunotherapy – with acetaminophen exposure confirmed by plasma testing – were found to have worse overall survival and progression-free survival than patients who did not take the analgesic. Multivariate analysis confirmed the association independent of other prognostic factors. “It is unlikely that our data are the result of bias or unmeasured confounding,” the authors comment.
The findings “present a compelling case for caution” in using acetaminophen in patients with cancer who are receiving immune checkpoint blockers, senior investigator Antoine Italiano, MD, PhD, a medical oncologist at the University of Bordeaux (France), and colleagues concluded.
The study was presented at the annual meeting of the American Society of Clinical Oncology and published simultaneously in Annals of Oncology.
“Patients with advanced cancer taking [acetaminophen] during immunotherapy experience worse clinical outcomes, which suggests that [acetaminophen] decreases T cell–mediated antitumor immunity,” the authors comment.
They also report bench research and blood studies in four healthy volunteers, which showed an up-regulation of immunosuppressive regulatory T cells (Tregs) with acetaminophen, and other findings that together suggest that acetaminophen undermines the antitumor immune processes by which checkpoint inhibitors work.
Reconsider acetaminophen pretreatment
After hearing Dr. Italiano present the results at the meeting, a Polish oncologist in the audience said he was concerned that his clinic premedicates with acetaminophen before immune checkpoint blockade and wanted to know if they should stop doing it.
“I don’t think inducing Tregs ... in cancer patients is a good approach. I do a lot of clinical trials,” and “I do not understand why in several cases sponsors required mandatory premedication with acetaminophen. I think ... we should reconsider this approach,” Dr. Italiano said.
There’s precedence for the findings. Acetaminophen – also known as paracetamol – has been shown in some studies to limit immune cell proliferation, T-cell–dependent antibody response, and viral clearance, among other things. After a randomized trial showing blunted responses to vaccines in individuals who were taking acetaminophen, the World Health Organization recommended in 2015 against concurrent use of acetaminophen with vaccines.
Steroids, antibiotics, and proton pump inhibitors have also recently been shown to worsen outcomes with pembrolizumab, noted invited discussant, Margaret Gatti-Mays, MD, a medical oncologist at Ohio State University, Columbus.
“We are starting to understand that ... commonly used medications may have a larger impact on the efficacy and toxicity of immune checkpoint blockade than historically seen with chemotherapy,” she said.
However, she expressed some uncertainty over the French findings, as she was concerned that even the multivariate analysis didn’t completely rule out that acetaminophen users had worse disease to begin with and so would be expected to have worse outcomes.
She was also unsure of how much acetaminophen is too much.
Acetaminophen has a half-life of around 3 hours or less, where the immune checkpoint inhibitors have a half-life of around 20 days or more.
Given that, Dr. Gatti-Mays wondered whether “a single dose of acetaminophen [is] enough to derail the benefit of checkpoint inhibition? Does exposure need to be continuous?”
She allowed that acetaminophen use may turn out to be one more of the many patient-level factors emerging lately – such as chronic stress, diet, body flora, and physiological age, among others – that might help explain why checkpoint inhibition works in only about 20% of eligible patients with cancer.
Study details
Dr. Italiano and his team analyzed plasma samples from 297 participants in the CheckMate 025 trial of nivolumab for renal cancer; 34 participants in the BIP study into actionable molecular alterations in cancer; and 297 participants in the PREMIS immune-related adverse events study. The patients in these last two studies had a variety of cancers and were taking various agents.
All 628 patients were on checkpoint inhibitors. The investigators divided them according to who had acetaminophen or its metabolite acetaminophen glucuronide in their plasma when they started checkpoint inhibition and those who did not.
In CheckMate 025, overall survival was significantly worse among participants who had detectable acetaminophen or its metabolite in plasma (hazard ratio, 0.67; P = .004).
None of the acetaminophen-positive participants in the BIP study responded to checkpoint blockade, compared with almost 30% of those who were negative. Acetaminophen-positive participants also trended toward worse progression-free survival (median, 1.87 vs. 4.72 months) and overall survival (median, 7.87 vs. 16.56 months).
In PREMIS, progression-free survival was a median of 2.63 months in the acetaminophen group versus 5.03 months in negative participants (P = .009); median overall survival was 8.43 months versus 14.93 months, respectively (P < .0001).
A multivariate analysis was performed in PREMIS. Acetaminophen exposure was associated with both progression-free survival (hazard ratio, 1.43; P =.015) and overall survival (HR, 1.78; P =.006) independently of performance status, liver metastases, bone metastases, number of metastases sites, tumor type, number of previous lines of treatment, steroid/antibiotic use, lactate dehydrogenase levels, and other factors.
There was no funding for the work. Dr. Italiano is a consultant for AstraZeneca, Bayer, Chugai, Deciphera, Merck, Parthenon, Roche, and Springworks, He also has grants from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, MSD, Novartis, Pharmamar, and Roche. Two authors work for Explicyte and one works for Amgen. Dr. Gatti-Mays is a consultant for Seattle Genetics.
A version of this article first appeared on Medscape.com.
The team found a strong association between the use of acetaminophen and a decreased response to immune checkpoint inhibitors in a study of three clinical cohorts involving more than 600 patients with advanced cancer.
Patients who took acetaminophen at the start of immunotherapy – with acetaminophen exposure confirmed by plasma testing – were found to have worse overall survival and progression-free survival than patients who did not take the analgesic. Multivariate analysis confirmed the association independent of other prognostic factors. “It is unlikely that our data are the result of bias or unmeasured confounding,” the authors comment.
The findings “present a compelling case for caution” in using acetaminophen in patients with cancer who are receiving immune checkpoint blockers, senior investigator Antoine Italiano, MD, PhD, a medical oncologist at the University of Bordeaux (France), and colleagues concluded.
The study was presented at the annual meeting of the American Society of Clinical Oncology and published simultaneously in Annals of Oncology.
“Patients with advanced cancer taking [acetaminophen] during immunotherapy experience worse clinical outcomes, which suggests that [acetaminophen] decreases T cell–mediated antitumor immunity,” the authors comment.
They also report bench research and blood studies in four healthy volunteers, which showed an up-regulation of immunosuppressive regulatory T cells (Tregs) with acetaminophen, and other findings that together suggest that acetaminophen undermines the antitumor immune processes by which checkpoint inhibitors work.
Reconsider acetaminophen pretreatment
After hearing Dr. Italiano present the results at the meeting, a Polish oncologist in the audience said he was concerned that his clinic premedicates with acetaminophen before immune checkpoint blockade and wanted to know if they should stop doing it.
“I don’t think inducing Tregs ... in cancer patients is a good approach. I do a lot of clinical trials,” and “I do not understand why in several cases sponsors required mandatory premedication with acetaminophen. I think ... we should reconsider this approach,” Dr. Italiano said.
There’s precedence for the findings. Acetaminophen – also known as paracetamol – has been shown in some studies to limit immune cell proliferation, T-cell–dependent antibody response, and viral clearance, among other things. After a randomized trial showing blunted responses to vaccines in individuals who were taking acetaminophen, the World Health Organization recommended in 2015 against concurrent use of acetaminophen with vaccines.
Steroids, antibiotics, and proton pump inhibitors have also recently been shown to worsen outcomes with pembrolizumab, noted invited discussant, Margaret Gatti-Mays, MD, a medical oncologist at Ohio State University, Columbus.
“We are starting to understand that ... commonly used medications may have a larger impact on the efficacy and toxicity of immune checkpoint blockade than historically seen with chemotherapy,” she said.
However, she expressed some uncertainty over the French findings, as she was concerned that even the multivariate analysis didn’t completely rule out that acetaminophen users had worse disease to begin with and so would be expected to have worse outcomes.
She was also unsure of how much acetaminophen is too much.
Acetaminophen has a half-life of around 3 hours or less, where the immune checkpoint inhibitors have a half-life of around 20 days or more.
Given that, Dr. Gatti-Mays wondered whether “a single dose of acetaminophen [is] enough to derail the benefit of checkpoint inhibition? Does exposure need to be continuous?”
She allowed that acetaminophen use may turn out to be one more of the many patient-level factors emerging lately – such as chronic stress, diet, body flora, and physiological age, among others – that might help explain why checkpoint inhibition works in only about 20% of eligible patients with cancer.
Study details
Dr. Italiano and his team analyzed plasma samples from 297 participants in the CheckMate 025 trial of nivolumab for renal cancer; 34 participants in the BIP study into actionable molecular alterations in cancer; and 297 participants in the PREMIS immune-related adverse events study. The patients in these last two studies had a variety of cancers and were taking various agents.
All 628 patients were on checkpoint inhibitors. The investigators divided them according to who had acetaminophen or its metabolite acetaminophen glucuronide in their plasma when they started checkpoint inhibition and those who did not.
In CheckMate 025, overall survival was significantly worse among participants who had detectable acetaminophen or its metabolite in plasma (hazard ratio, 0.67; P = .004).
None of the acetaminophen-positive participants in the BIP study responded to checkpoint blockade, compared with almost 30% of those who were negative. Acetaminophen-positive participants also trended toward worse progression-free survival (median, 1.87 vs. 4.72 months) and overall survival (median, 7.87 vs. 16.56 months).
In PREMIS, progression-free survival was a median of 2.63 months in the acetaminophen group versus 5.03 months in negative participants (P = .009); median overall survival was 8.43 months versus 14.93 months, respectively (P < .0001).
A multivariate analysis was performed in PREMIS. Acetaminophen exposure was associated with both progression-free survival (hazard ratio, 1.43; P =.015) and overall survival (HR, 1.78; P =.006) independently of performance status, liver metastases, bone metastases, number of metastases sites, tumor type, number of previous lines of treatment, steroid/antibiotic use, lactate dehydrogenase levels, and other factors.
There was no funding for the work. Dr. Italiano is a consultant for AstraZeneca, Bayer, Chugai, Deciphera, Merck, Parthenon, Roche, and Springworks, He also has grants from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, MSD, Novartis, Pharmamar, and Roche. Two authors work for Explicyte and one works for Amgen. Dr. Gatti-Mays is a consultant for Seattle Genetics.
A version of this article first appeared on Medscape.com.
FROM ASCO 2022
Women are not being warned that anesthetic may reduce birth pill efficacy
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
FROM EUROANAESTHESIA
Antipsychotic tied to dose-related weight gain, higher cholesterol
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.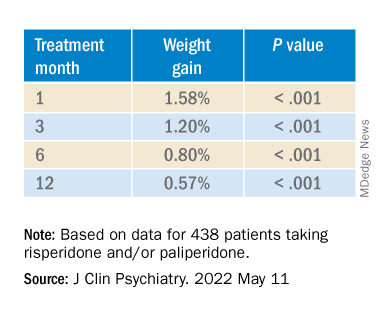
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.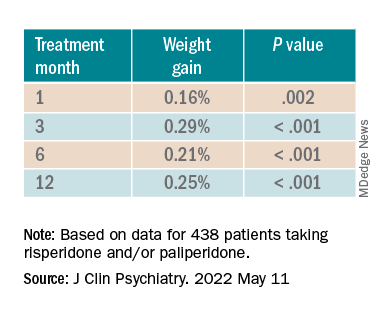
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.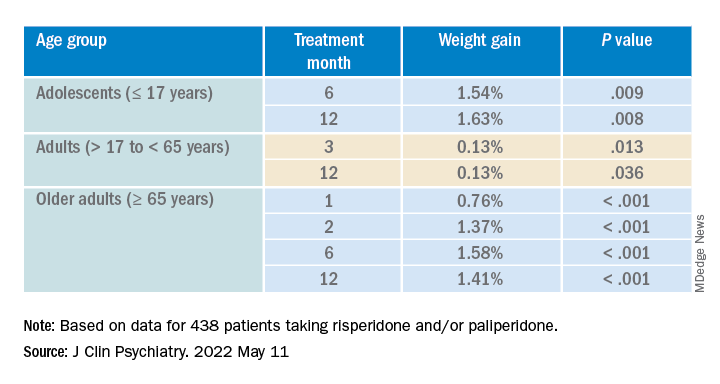
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
‘Great optimism’ greets immunotherapy responses in dMMR rectal cancer
Thus far, the study has involved only 12 patients, but all of them have had a clinical complete response to treatment. They continue to show no signs of cancer (during follow-up ranging from 6 to 25 months) and have not undergone surgery or had radiation and chemotherapy, which are the standard treatment approaches.
The results were presented (Abstract 16) at the American Society of Clinical Oncology annual meeting and simultaneously published in the New England Journal of Medicine.
“In our study, the elimination of tumors after 6 months of therapy with PD-1 blockade enabled us to omit both chemoradiotherapy and surgery and to proceed with observation alone,” said the authors, led by Andrea Cercek, MD, Memorial Sloan Kettering Cancer Center, New York.
About 5%-10% of patients with rectal cancer have tumors with dMMR.
“The implications for quality of life are substantial, especially among patients in whom standard treatment would affect child-bearing potential [and] given that the incidence of rectal cancer is rising among young adults of childbearing age, the use of PD-1 blockade to eliminate the need for chemoradiotherapy and surgery may confer a particular benefit in that age group,” the authors wrote.
The results of the current study are cause for “great optimism, but such an approach cannot yet supplant our current curative treatment approach,” Dr. Hanna K. Sanoff, MD, University of North Carolina at Chapel Hill, wrote in an accompanying editorial.
Single-agent dostarlimab
For the study, all patients were treated with single-agent dostarlimab every 3 weeks for 6 months.
Dostarlimab is already approved by the Food and Drug Administration for use in the treatment of recurrent or advanced endometrial cancer with dMMR. Rectal cancer is an off-label use.
All patients had mismatch repair-deficient stage 2 or 3 rectal adenocarcinoma. The authors noted that these tumors respond poorly to standard chemotherapy regimens, including neoadjuvant chemotherapy. The median age of enrolled patients was 54 years and 62% were women.
For the study, investigators planned that patients who had a clinical complete response after completion of dostarlimab were to proceed to observation without undergoing either chemoradiotherapy or surgery, while those who did not have a complete response were to have received these standard treatments.
As it turned out, all 12 patients achieved a complete response and have been followed by observation alone. The median follow-up from time of enrollment to data cutoff for the 12 patients was 12 months.
“Therapeutic responses were rapid,” the authors noted, “with resolution of symptoms within 8 weeks after initiation of dostarlimab in 81% of the patients.”
To date, four patients have had 1 year of sustained clinical complete response after completion of the anti-PD-1 course.
In addition to the 12 patients documented in the study, another four patients have received at least one dose of dostarlimab and continue to receive treatment.
Adverse events occurred in most patients but none were grade 3 or higher. The most common grade 1 or 2 adverse events were rash or dermatitis, pruritus, fatigue, nausea and, in one patient, thyroid-function abnormalities.
The authors speculated that in addition to the extremely high tumor mutational burden associated with mismatch-repair deficiency, a tumor cell–extrinsic factor such as the gut microbiome may be driving the exceptionally good response to PD-1 blockade seen in this patient population.
Editorial commentary
In the editorial, Dr. Sanoff emphasized that the approach remains experimental and should not replace current curative treatment. She noted that cancer recurrences have been seen in other studies using both chemotherapy and immunotherapy.
For example, with chemotherapy and radiation, those patients who achieve a clinical complete response have a better prognosis compared with those who do not, but she cautioned that “cancer regrowth occurs in 20% to 30% of such patients when the cancer is managed nonoperatively.”
Dr. Sanoff noted that recurrences were seen when this approach of PD-1 inhibition has been used for metastatic colorectal cancer with dMMR. In the KEYNOTE-177 trial with pembrolizumab (Keytruda), only 55% of patients were reported to be alive without cancer progression at 12 months, and of the patients who initially had a strong response, only 70% had an ongoing response 3 years later.
“These recurrence dynamics may (or may not) differ between immunotherapy and chemoradiotherapy and between early and late-stage disease,” Dr. Sanoff said.
“In fact, very little is known about the duration of time needed to find out whether a clinical complete response to dostarlimab equates to cure,” she added.
In addition, Dr. Sanoff warned that the decision not to pursue further treatment and to follow patients with observation alone requires very close monitoring.
The current study was conducted at a top U.S. cancer center, Memorial Sloan Kettering Cancer Center. The authors noted that the complete responses (after a minimum of 6 months of follow-up) were measured by the combination of rectal MRI, visual endoscopic inspection, and digital rectal examination.
The completeness of these responses was further supported by the absence of residual tumor on serial endoscopic biopsies and the resolution of 18F-fluorodeoxyglucose uptake on PET scans, the authors added.
In the editorial, Dr. Sanoff said that “safe nonoperative management [also] involves access to specialty care for direct intraluminal visualization and expertise in interpretation of rectal magnetic resonance imaging ... Such expertise is not available in all communities and without it, patients could miss the opportunity for curative resection if tumor regrowth occurred.”
The study was sponsored by the Simon and Eve Colin Foundation, GlaxoSmithKline, and Stand Up to Cancer, among others.
A version of this article first appeared on Medscape.com.
Thus far, the study has involved only 12 patients, but all of them have had a clinical complete response to treatment. They continue to show no signs of cancer (during follow-up ranging from 6 to 25 months) and have not undergone surgery or had radiation and chemotherapy, which are the standard treatment approaches.
The results were presented (Abstract 16) at the American Society of Clinical Oncology annual meeting and simultaneously published in the New England Journal of Medicine.
“In our study, the elimination of tumors after 6 months of therapy with PD-1 blockade enabled us to omit both chemoradiotherapy and surgery and to proceed with observation alone,” said the authors, led by Andrea Cercek, MD, Memorial Sloan Kettering Cancer Center, New York.
About 5%-10% of patients with rectal cancer have tumors with dMMR.
“The implications for quality of life are substantial, especially among patients in whom standard treatment would affect child-bearing potential [and] given that the incidence of rectal cancer is rising among young adults of childbearing age, the use of PD-1 blockade to eliminate the need for chemoradiotherapy and surgery may confer a particular benefit in that age group,” the authors wrote.
The results of the current study are cause for “great optimism, but such an approach cannot yet supplant our current curative treatment approach,” Dr. Hanna K. Sanoff, MD, University of North Carolina at Chapel Hill, wrote in an accompanying editorial.
Single-agent dostarlimab
For the study, all patients were treated with single-agent dostarlimab every 3 weeks for 6 months.
Dostarlimab is already approved by the Food and Drug Administration for use in the treatment of recurrent or advanced endometrial cancer with dMMR. Rectal cancer is an off-label use.
All patients had mismatch repair-deficient stage 2 or 3 rectal adenocarcinoma. The authors noted that these tumors respond poorly to standard chemotherapy regimens, including neoadjuvant chemotherapy. The median age of enrolled patients was 54 years and 62% were women.
For the study, investigators planned that patients who had a clinical complete response after completion of dostarlimab were to proceed to observation without undergoing either chemoradiotherapy or surgery, while those who did not have a complete response were to have received these standard treatments.
As it turned out, all 12 patients achieved a complete response and have been followed by observation alone. The median follow-up from time of enrollment to data cutoff for the 12 patients was 12 months.
“Therapeutic responses were rapid,” the authors noted, “with resolution of symptoms within 8 weeks after initiation of dostarlimab in 81% of the patients.”
To date, four patients have had 1 year of sustained clinical complete response after completion of the anti-PD-1 course.
In addition to the 12 patients documented in the study, another four patients have received at least one dose of dostarlimab and continue to receive treatment.
Adverse events occurred in most patients but none were grade 3 or higher. The most common grade 1 or 2 adverse events were rash or dermatitis, pruritus, fatigue, nausea and, in one patient, thyroid-function abnormalities.
The authors speculated that in addition to the extremely high tumor mutational burden associated with mismatch-repair deficiency, a tumor cell–extrinsic factor such as the gut microbiome may be driving the exceptionally good response to PD-1 blockade seen in this patient population.
Editorial commentary
In the editorial, Dr. Sanoff emphasized that the approach remains experimental and should not replace current curative treatment. She noted that cancer recurrences have been seen in other studies using both chemotherapy and immunotherapy.
For example, with chemotherapy and radiation, those patients who achieve a clinical complete response have a better prognosis compared with those who do not, but she cautioned that “cancer regrowth occurs in 20% to 30% of such patients when the cancer is managed nonoperatively.”
Dr. Sanoff noted that recurrences were seen when this approach of PD-1 inhibition has been used for metastatic colorectal cancer with dMMR. In the KEYNOTE-177 trial with pembrolizumab (Keytruda), only 55% of patients were reported to be alive without cancer progression at 12 months, and of the patients who initially had a strong response, only 70% had an ongoing response 3 years later.
“These recurrence dynamics may (or may not) differ between immunotherapy and chemoradiotherapy and between early and late-stage disease,” Dr. Sanoff said.
“In fact, very little is known about the duration of time needed to find out whether a clinical complete response to dostarlimab equates to cure,” she added.
In addition, Dr. Sanoff warned that the decision not to pursue further treatment and to follow patients with observation alone requires very close monitoring.
The current study was conducted at a top U.S. cancer center, Memorial Sloan Kettering Cancer Center. The authors noted that the complete responses (after a minimum of 6 months of follow-up) were measured by the combination of rectal MRI, visual endoscopic inspection, and digital rectal examination.
The completeness of these responses was further supported by the absence of residual tumor on serial endoscopic biopsies and the resolution of 18F-fluorodeoxyglucose uptake on PET scans, the authors added.
In the editorial, Dr. Sanoff said that “safe nonoperative management [also] involves access to specialty care for direct intraluminal visualization and expertise in interpretation of rectal magnetic resonance imaging ... Such expertise is not available in all communities and without it, patients could miss the opportunity for curative resection if tumor regrowth occurred.”
The study was sponsored by the Simon and Eve Colin Foundation, GlaxoSmithKline, and Stand Up to Cancer, among others.
A version of this article first appeared on Medscape.com.
Thus far, the study has involved only 12 patients, but all of them have had a clinical complete response to treatment. They continue to show no signs of cancer (during follow-up ranging from 6 to 25 months) and have not undergone surgery or had radiation and chemotherapy, which are the standard treatment approaches.
The results were presented (Abstract 16) at the American Society of Clinical Oncology annual meeting and simultaneously published in the New England Journal of Medicine.
“In our study, the elimination of tumors after 6 months of therapy with PD-1 blockade enabled us to omit both chemoradiotherapy and surgery and to proceed with observation alone,” said the authors, led by Andrea Cercek, MD, Memorial Sloan Kettering Cancer Center, New York.
About 5%-10% of patients with rectal cancer have tumors with dMMR.
“The implications for quality of life are substantial, especially among patients in whom standard treatment would affect child-bearing potential [and] given that the incidence of rectal cancer is rising among young adults of childbearing age, the use of PD-1 blockade to eliminate the need for chemoradiotherapy and surgery may confer a particular benefit in that age group,” the authors wrote.
The results of the current study are cause for “great optimism, but such an approach cannot yet supplant our current curative treatment approach,” Dr. Hanna K. Sanoff, MD, University of North Carolina at Chapel Hill, wrote in an accompanying editorial.
Single-agent dostarlimab
For the study, all patients were treated with single-agent dostarlimab every 3 weeks for 6 months.
Dostarlimab is already approved by the Food and Drug Administration for use in the treatment of recurrent or advanced endometrial cancer with dMMR. Rectal cancer is an off-label use.
All patients had mismatch repair-deficient stage 2 or 3 rectal adenocarcinoma. The authors noted that these tumors respond poorly to standard chemotherapy regimens, including neoadjuvant chemotherapy. The median age of enrolled patients was 54 years and 62% were women.
For the study, investigators planned that patients who had a clinical complete response after completion of dostarlimab were to proceed to observation without undergoing either chemoradiotherapy or surgery, while those who did not have a complete response were to have received these standard treatments.
As it turned out, all 12 patients achieved a complete response and have been followed by observation alone. The median follow-up from time of enrollment to data cutoff for the 12 patients was 12 months.
“Therapeutic responses were rapid,” the authors noted, “with resolution of symptoms within 8 weeks after initiation of dostarlimab in 81% of the patients.”
To date, four patients have had 1 year of sustained clinical complete response after completion of the anti-PD-1 course.
In addition to the 12 patients documented in the study, another four patients have received at least one dose of dostarlimab and continue to receive treatment.
Adverse events occurred in most patients but none were grade 3 or higher. The most common grade 1 or 2 adverse events were rash or dermatitis, pruritus, fatigue, nausea and, in one patient, thyroid-function abnormalities.
The authors speculated that in addition to the extremely high tumor mutational burden associated with mismatch-repair deficiency, a tumor cell–extrinsic factor such as the gut microbiome may be driving the exceptionally good response to PD-1 blockade seen in this patient population.
Editorial commentary
In the editorial, Dr. Sanoff emphasized that the approach remains experimental and should not replace current curative treatment. She noted that cancer recurrences have been seen in other studies using both chemotherapy and immunotherapy.
For example, with chemotherapy and radiation, those patients who achieve a clinical complete response have a better prognosis compared with those who do not, but she cautioned that “cancer regrowth occurs in 20% to 30% of such patients when the cancer is managed nonoperatively.”
Dr. Sanoff noted that recurrences were seen when this approach of PD-1 inhibition has been used for metastatic colorectal cancer with dMMR. In the KEYNOTE-177 trial with pembrolizumab (Keytruda), only 55% of patients were reported to be alive without cancer progression at 12 months, and of the patients who initially had a strong response, only 70% had an ongoing response 3 years later.
“These recurrence dynamics may (or may not) differ between immunotherapy and chemoradiotherapy and between early and late-stage disease,” Dr. Sanoff said.
“In fact, very little is known about the duration of time needed to find out whether a clinical complete response to dostarlimab equates to cure,” she added.
In addition, Dr. Sanoff warned that the decision not to pursue further treatment and to follow patients with observation alone requires very close monitoring.
The current study was conducted at a top U.S. cancer center, Memorial Sloan Kettering Cancer Center. The authors noted that the complete responses (after a minimum of 6 months of follow-up) were measured by the combination of rectal MRI, visual endoscopic inspection, and digital rectal examination.
The completeness of these responses was further supported by the absence of residual tumor on serial endoscopic biopsies and the resolution of 18F-fluorodeoxyglucose uptake on PET scans, the authors added.
In the editorial, Dr. Sanoff said that “safe nonoperative management [also] involves access to specialty care for direct intraluminal visualization and expertise in interpretation of rectal magnetic resonance imaging ... Such expertise is not available in all communities and without it, patients could miss the opportunity for curative resection if tumor regrowth occurred.”
The study was sponsored by the Simon and Eve Colin Foundation, GlaxoSmithKline, and Stand Up to Cancer, among others.
A version of this article first appeared on Medscape.com.
FROM ASCO 2022
Panitumumab beats bevacizumab in left-sided mCRC
A suspicion from retrospective data has now been confirmed by a prospective clinical trial: Adding panitumumab (Vectibix) to standard chemotherapy in left-sided RAS wild-type metastatic colorectal cancer (mCRC) is more effective than adding bevacizumab (Avastin).
Patients treated with panitumumab alongside chemotherapy saw a 16% improvement in overall survival versus those given bevacizumab after a median follow-up of over 5 years.
The overall survival benefit rose to 18% in those with left-sided tumors.
However, there was no difference in overall survival between the two treatment groups in the small subgroup of patients with right-sided primary tumors.
These findings come from the PARADIGM trial conducted in Japan.
The results were presented during a plenary session at the annual meeting of the American Society of Clinical Oncology.
“If gene testing shows that a tumor is RAS wild-type, the choice of initial treatment with panitumumab plus mFOLFOX6 chemotherapy is superior ... for those people with left-sided tumors,” said lead researcher Takayuki Yoshino, MD, PhD, department of gastrointestinal oncology, National Cancer Center Hospital East, Chiba, Japan, in an ASCO press release.
“It has long been believed that the sequence of metastatic colorectal cancer treatment does not matter as long as patients had access to the drugs at some point, which has now been disproven,” he noted.
Dr. Yoshino added in a press conference about the trial that the results establish “a standard first-line combination regimen for patients with RAS wild-type, left-sided mCRC.”
commented Cathy Eng, MD, ASCO Expert in gastrointestinal cancers.
The findings “emphasize the importance of taking into account sidedness, as well as including comprehensive biomarker testing,” she said.
Dr. Eng underlined that this is especially the case for RAS gene status testing, “which is critical for all colorectal cancer patients at the time of diagnosis of metastatic disease.”
These results are of particular relevance in the United States, where the choice between an anti-EGFR or anti-VEGF antibody for the treatment of mCRC has been an area of “controversy” because of the lack of supporting data.
Panitumumab is a human monoclonal antibody that targets EGFR. It was approved in 2006 for use in mCRC by the U.S. Food and Drug Administration and also approved in 2014 for use in combination with FOLFOX for the first-line treatment of patients with wild-type KRAS (exon 2 in codons 12 or 13) mCRC, having previously been shown to be equally effective as cetuximab (another EGFR inhibitor) in this population.
In contrast, bevacizumab is a monoclonal antibody that targets the VEGF receptor. It was approved by the FDA for use in mCRC in 2004 in combination with intravenous 5-fluorouracil–based chemotherapy.
Dr. Yoshino explained that around 36% of patients with CRC have metastatic tumors at diagnosis and that adding an anti-EGFR or anti-VEGF antibody to chemotherapy improves overall survival in these patients by up to 30 months.
There has been “accumulating” evidence from retrospective studies suggesting that patients with RAS wild-type mCRC whose primary tumor is on the left side, which accounts for approximately 35% of mCRC cases, have a longer survival benefit with an anti-EGFR antibody, he commented.
Despite this, both antibody types continue to be used in these patients, he added.
PARADIGM was the first prospective trial to compare the two antibody types. Patients were randomized to receive either panitumumab or bevacizumab plus the combination chemotherapy regimen modified FOLFOX6 (mFOLFOX6).
The trial involved 823 Japanese patients with previously untreated wild-type mCRC with unresectable disease. Most patients had left-sided primary tumors (312 of 400 patients in the panitumumab group, and 292 of 402 patients in the bevacizumab group).
After a median follow-up of 61 months, panitumumab was associated with a significant improvement in overall survival in the overall study population, at a hazard ratio of 0.84 (P = .030, with the boundary of significance set at P < .05).
In addition, panitumumab was associated with a significant improvement in overall survival in the large subgroup of patients with left-sided primary tumors, at 37.9 versus 34.3 months, or a hazard ratio of 0.82 (P = .031).
However, there was no significant difference in overall survival between the two treatment groups in the smaller subgroup of patients with right-sided tumors, at a hazard ratio of 1.09.
Median progression-free survival was no different between the panitumumab and bevacizumab groups, at 13.7 versus 13.2 months in patients with a left-sided tumor and 12.9 versus 12.0 months in the overall cohort.
There was, however, a difference in response rates in left-sided patients between those receiving the two antibodies, at 80.2% with panitumumab versus 68.6% with bevacizumab, and in curative resection rates, at 18.3% and 11.6%, respectively.
These results demonstrate the “superiority of first-line panitumumab versus bevacizumab in combination with mFOLFOX6 in the left-sided and overall populations,” Dr. Yoshino concluded.
He also highlighted that the team has undertaken a large-scale biomarker analysis of pre- and posttreatment plasma and tissue samples from patients in the PARADIGM study to identify potential biomarkers of treatment response.
At the plenary session, discussant for this abstract Chiara Cremolini, MD, PhD, professor of medical oncology, Pisa (Italy) University Hospital, commented that “location matters” when it comes to mCRC tumors.
Dr. Cremolini pointed out that the separation of the survival curves at 28 months suggests that the 40% of patients with left-sided tumors who survived only up until that time point receive an equal benefit from panitumumab and bevacizumab.
In contrast, the remainder who survived for longer showed better outcomes with panitumumab.
Overall, she said, in her opinion and based on the findings from other studies, the current results support the use of panitumumab plus mFOLFOX6 as first-line therapy in patients with microsatellite stable RAS wild-type and with BRAF wild-type left-sided mCRC.
Dr. Cremolini emphasized that patients should be warned that, if they opt for doublet chemotherapy plus bevacizumab, they could face a median 3.6-month loss in overall survival, as well as poorer treatment activity.
However, patients with high microsatellite instability should receive immunotherapy up front, she added, while those with BRAF mutations should be given FOLFOX upfront plus bevacizumab, followed by encorafenib plus cetuximab in the case of progression.
Dr. Cremolini ended by noting that there has, as yet, been no prospective comparison of doublet chemotherapy plus an anti-EGFR antibody with triplet chemotherapy plus bevacizumab in this population.
The study was funded by Takeda. Dr. Yoshino has reported relationships with Bayer Yakuhin, Chugai Pharmaceutical, Merck, and MSD. Dr. Eng has reported relationships with Bayer Health, Gilead/Forty Seven, GlaxoSmithKline, Hookipa Biotech, Mirati Therapeutics, Natera, Pfizer, Elevar, Fruquitinib, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
A suspicion from retrospective data has now been confirmed by a prospective clinical trial: Adding panitumumab (Vectibix) to standard chemotherapy in left-sided RAS wild-type metastatic colorectal cancer (mCRC) is more effective than adding bevacizumab (Avastin).
Patients treated with panitumumab alongside chemotherapy saw a 16% improvement in overall survival versus those given bevacizumab after a median follow-up of over 5 years.
The overall survival benefit rose to 18% in those with left-sided tumors.
However, there was no difference in overall survival between the two treatment groups in the small subgroup of patients with right-sided primary tumors.
These findings come from the PARADIGM trial conducted in Japan.
The results were presented during a plenary session at the annual meeting of the American Society of Clinical Oncology.
“If gene testing shows that a tumor is RAS wild-type, the choice of initial treatment with panitumumab plus mFOLFOX6 chemotherapy is superior ... for those people with left-sided tumors,” said lead researcher Takayuki Yoshino, MD, PhD, department of gastrointestinal oncology, National Cancer Center Hospital East, Chiba, Japan, in an ASCO press release.
“It has long been believed that the sequence of metastatic colorectal cancer treatment does not matter as long as patients had access to the drugs at some point, which has now been disproven,” he noted.
Dr. Yoshino added in a press conference about the trial that the results establish “a standard first-line combination regimen for patients with RAS wild-type, left-sided mCRC.”
commented Cathy Eng, MD, ASCO Expert in gastrointestinal cancers.
The findings “emphasize the importance of taking into account sidedness, as well as including comprehensive biomarker testing,” she said.
Dr. Eng underlined that this is especially the case for RAS gene status testing, “which is critical for all colorectal cancer patients at the time of diagnosis of metastatic disease.”
These results are of particular relevance in the United States, where the choice between an anti-EGFR or anti-VEGF antibody for the treatment of mCRC has been an area of “controversy” because of the lack of supporting data.
Panitumumab is a human monoclonal antibody that targets EGFR. It was approved in 2006 for use in mCRC by the U.S. Food and Drug Administration and also approved in 2014 for use in combination with FOLFOX for the first-line treatment of patients with wild-type KRAS (exon 2 in codons 12 or 13) mCRC, having previously been shown to be equally effective as cetuximab (another EGFR inhibitor) in this population.
In contrast, bevacizumab is a monoclonal antibody that targets the VEGF receptor. It was approved by the FDA for use in mCRC in 2004 in combination with intravenous 5-fluorouracil–based chemotherapy.
Dr. Yoshino explained that around 36% of patients with CRC have metastatic tumors at diagnosis and that adding an anti-EGFR or anti-VEGF antibody to chemotherapy improves overall survival in these patients by up to 30 months.
There has been “accumulating” evidence from retrospective studies suggesting that patients with RAS wild-type mCRC whose primary tumor is on the left side, which accounts for approximately 35% of mCRC cases, have a longer survival benefit with an anti-EGFR antibody, he commented.
Despite this, both antibody types continue to be used in these patients, he added.
PARADIGM was the first prospective trial to compare the two antibody types. Patients were randomized to receive either panitumumab or bevacizumab plus the combination chemotherapy regimen modified FOLFOX6 (mFOLFOX6).
The trial involved 823 Japanese patients with previously untreated wild-type mCRC with unresectable disease. Most patients had left-sided primary tumors (312 of 400 patients in the panitumumab group, and 292 of 402 patients in the bevacizumab group).
After a median follow-up of 61 months, panitumumab was associated with a significant improvement in overall survival in the overall study population, at a hazard ratio of 0.84 (P = .030, with the boundary of significance set at P < .05).
In addition, panitumumab was associated with a significant improvement in overall survival in the large subgroup of patients with left-sided primary tumors, at 37.9 versus 34.3 months, or a hazard ratio of 0.82 (P = .031).
However, there was no significant difference in overall survival between the two treatment groups in the smaller subgroup of patients with right-sided tumors, at a hazard ratio of 1.09.
Median progression-free survival was no different between the panitumumab and bevacizumab groups, at 13.7 versus 13.2 months in patients with a left-sided tumor and 12.9 versus 12.0 months in the overall cohort.
There was, however, a difference in response rates in left-sided patients between those receiving the two antibodies, at 80.2% with panitumumab versus 68.6% with bevacizumab, and in curative resection rates, at 18.3% and 11.6%, respectively.
These results demonstrate the “superiority of first-line panitumumab versus bevacizumab in combination with mFOLFOX6 in the left-sided and overall populations,” Dr. Yoshino concluded.
He also highlighted that the team has undertaken a large-scale biomarker analysis of pre- and posttreatment plasma and tissue samples from patients in the PARADIGM study to identify potential biomarkers of treatment response.
At the plenary session, discussant for this abstract Chiara Cremolini, MD, PhD, professor of medical oncology, Pisa (Italy) University Hospital, commented that “location matters” when it comes to mCRC tumors.
Dr. Cremolini pointed out that the separation of the survival curves at 28 months suggests that the 40% of patients with left-sided tumors who survived only up until that time point receive an equal benefit from panitumumab and bevacizumab.
In contrast, the remainder who survived for longer showed better outcomes with panitumumab.
Overall, she said, in her opinion and based on the findings from other studies, the current results support the use of panitumumab plus mFOLFOX6 as first-line therapy in patients with microsatellite stable RAS wild-type and with BRAF wild-type left-sided mCRC.
Dr. Cremolini emphasized that patients should be warned that, if they opt for doublet chemotherapy plus bevacizumab, they could face a median 3.6-month loss in overall survival, as well as poorer treatment activity.
However, patients with high microsatellite instability should receive immunotherapy up front, she added, while those with BRAF mutations should be given FOLFOX upfront plus bevacizumab, followed by encorafenib plus cetuximab in the case of progression.
Dr. Cremolini ended by noting that there has, as yet, been no prospective comparison of doublet chemotherapy plus an anti-EGFR antibody with triplet chemotherapy plus bevacizumab in this population.
The study was funded by Takeda. Dr. Yoshino has reported relationships with Bayer Yakuhin, Chugai Pharmaceutical, Merck, and MSD. Dr. Eng has reported relationships with Bayer Health, Gilead/Forty Seven, GlaxoSmithKline, Hookipa Biotech, Mirati Therapeutics, Natera, Pfizer, Elevar, Fruquitinib, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
A suspicion from retrospective data has now been confirmed by a prospective clinical trial: Adding panitumumab (Vectibix) to standard chemotherapy in left-sided RAS wild-type metastatic colorectal cancer (mCRC) is more effective than adding bevacizumab (Avastin).
Patients treated with panitumumab alongside chemotherapy saw a 16% improvement in overall survival versus those given bevacizumab after a median follow-up of over 5 years.
The overall survival benefit rose to 18% in those with left-sided tumors.
However, there was no difference in overall survival between the two treatment groups in the small subgroup of patients with right-sided primary tumors.
These findings come from the PARADIGM trial conducted in Japan.
The results were presented during a plenary session at the annual meeting of the American Society of Clinical Oncology.
“If gene testing shows that a tumor is RAS wild-type, the choice of initial treatment with panitumumab plus mFOLFOX6 chemotherapy is superior ... for those people with left-sided tumors,” said lead researcher Takayuki Yoshino, MD, PhD, department of gastrointestinal oncology, National Cancer Center Hospital East, Chiba, Japan, in an ASCO press release.
“It has long been believed that the sequence of metastatic colorectal cancer treatment does not matter as long as patients had access to the drugs at some point, which has now been disproven,” he noted.
Dr. Yoshino added in a press conference about the trial that the results establish “a standard first-line combination regimen for patients with RAS wild-type, left-sided mCRC.”
commented Cathy Eng, MD, ASCO Expert in gastrointestinal cancers.
The findings “emphasize the importance of taking into account sidedness, as well as including comprehensive biomarker testing,” she said.
Dr. Eng underlined that this is especially the case for RAS gene status testing, “which is critical for all colorectal cancer patients at the time of diagnosis of metastatic disease.”
These results are of particular relevance in the United States, where the choice between an anti-EGFR or anti-VEGF antibody for the treatment of mCRC has been an area of “controversy” because of the lack of supporting data.
Panitumumab is a human monoclonal antibody that targets EGFR. It was approved in 2006 for use in mCRC by the U.S. Food and Drug Administration and also approved in 2014 for use in combination with FOLFOX for the first-line treatment of patients with wild-type KRAS (exon 2 in codons 12 or 13) mCRC, having previously been shown to be equally effective as cetuximab (another EGFR inhibitor) in this population.
In contrast, bevacizumab is a monoclonal antibody that targets the VEGF receptor. It was approved by the FDA for use in mCRC in 2004 in combination with intravenous 5-fluorouracil–based chemotherapy.
Dr. Yoshino explained that around 36% of patients with CRC have metastatic tumors at diagnosis and that adding an anti-EGFR or anti-VEGF antibody to chemotherapy improves overall survival in these patients by up to 30 months.
There has been “accumulating” evidence from retrospective studies suggesting that patients with RAS wild-type mCRC whose primary tumor is on the left side, which accounts for approximately 35% of mCRC cases, have a longer survival benefit with an anti-EGFR antibody, he commented.
Despite this, both antibody types continue to be used in these patients, he added.
PARADIGM was the first prospective trial to compare the two antibody types. Patients were randomized to receive either panitumumab or bevacizumab plus the combination chemotherapy regimen modified FOLFOX6 (mFOLFOX6).
The trial involved 823 Japanese patients with previously untreated wild-type mCRC with unresectable disease. Most patients had left-sided primary tumors (312 of 400 patients in the panitumumab group, and 292 of 402 patients in the bevacizumab group).
After a median follow-up of 61 months, panitumumab was associated with a significant improvement in overall survival in the overall study population, at a hazard ratio of 0.84 (P = .030, with the boundary of significance set at P < .05).
In addition, panitumumab was associated with a significant improvement in overall survival in the large subgroup of patients with left-sided primary tumors, at 37.9 versus 34.3 months, or a hazard ratio of 0.82 (P = .031).
However, there was no significant difference in overall survival between the two treatment groups in the smaller subgroup of patients with right-sided tumors, at a hazard ratio of 1.09.
Median progression-free survival was no different between the panitumumab and bevacizumab groups, at 13.7 versus 13.2 months in patients with a left-sided tumor and 12.9 versus 12.0 months in the overall cohort.
There was, however, a difference in response rates in left-sided patients between those receiving the two antibodies, at 80.2% with panitumumab versus 68.6% with bevacizumab, and in curative resection rates, at 18.3% and 11.6%, respectively.
These results demonstrate the “superiority of first-line panitumumab versus bevacizumab in combination with mFOLFOX6 in the left-sided and overall populations,” Dr. Yoshino concluded.
He also highlighted that the team has undertaken a large-scale biomarker analysis of pre- and posttreatment plasma and tissue samples from patients in the PARADIGM study to identify potential biomarkers of treatment response.
At the plenary session, discussant for this abstract Chiara Cremolini, MD, PhD, professor of medical oncology, Pisa (Italy) University Hospital, commented that “location matters” when it comes to mCRC tumors.
Dr. Cremolini pointed out that the separation of the survival curves at 28 months suggests that the 40% of patients with left-sided tumors who survived only up until that time point receive an equal benefit from panitumumab and bevacizumab.
In contrast, the remainder who survived for longer showed better outcomes with panitumumab.
Overall, she said, in her opinion and based on the findings from other studies, the current results support the use of panitumumab plus mFOLFOX6 as first-line therapy in patients with microsatellite stable RAS wild-type and with BRAF wild-type left-sided mCRC.
Dr. Cremolini emphasized that patients should be warned that, if they opt for doublet chemotherapy plus bevacizumab, they could face a median 3.6-month loss in overall survival, as well as poorer treatment activity.
However, patients with high microsatellite instability should receive immunotherapy up front, she added, while those with BRAF mutations should be given FOLFOX upfront plus bevacizumab, followed by encorafenib plus cetuximab in the case of progression.
Dr. Cremolini ended by noting that there has, as yet, been no prospective comparison of doublet chemotherapy plus an anti-EGFR antibody with triplet chemotherapy plus bevacizumab in this population.
The study was funded by Takeda. Dr. Yoshino has reported relationships with Bayer Yakuhin, Chugai Pharmaceutical, Merck, and MSD. Dr. Eng has reported relationships with Bayer Health, Gilead/Forty Seven, GlaxoSmithKline, Hookipa Biotech, Mirati Therapeutics, Natera, Pfizer, Elevar, Fruquitinib, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
FROM ASCO 2022
Cannabis may relieve pain as effectively as opioids, but more research is needed
Several other systematic reviews have recently evaluated cannabinoids for treating chronic pain, but the new study’s methodology was “distinct” in “important ways,” leading to “conclusions that differ from other reviews,” according to the authors of the paper published in the Annals of Internal Medicine.
In the new systematic review, synthetic products with high THC:CBD ratios were associated with moderate improvements in pain, whereas plant-based products with comparable THC:CBD ratios offered less relief, said study author Marian S. McDonagh, PharmD, professor of medical informatics and clinical epidemiology, and codirector of the Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues.
Specifically, the investigators stratified cannabis-based interventions according to relative content of two key cannabinoids: THC and CBD. Products were sorted into five categories: high THC:CBD ratio (at least 2:1), comparable THC:CBD ratio (less than 2:1 but more than 1:2), low THC:CBD ratio (no more than 1:2), whole-plant cannabis products, and other cannabinoids.
“In preclinical studies, THC and related compounds have demonstrated analgesic properties, although its psychoactive effects and addiction potential may limit its suitability as an analgesic,” the investigators wrote. “CBD and other cannabinoids may also have some analgesic or anti-inflammatory properties and are not believed to be psychoactive or addictive. Given the variation in analgesic effect with THC and CBD, response may differ according to the ratio of THC to CBD in products used to treat pain.”
The final analysis included 18 randomized placebo-controlled trials involving 1,740 individuals and 7 cohort studies involving 13,095 individuals. Most of the studies were short-term, lasting 1-6 months.
Pain was scored on a ten-point scale, with improvements reported as the mean difference from baseline to post treatment. A mean difference in pain score of 0.5-1.0 was considered a “small effect,” an improvement of 1-2 points was considered a “moderate effect,” and an improvement greater than 2 points was considered a “large effect.”
Cannabis-based products with relatively high THC:CBD ratios showed efficacy
Synthetic products with high THC:CBD ratios offered moderate pain relief, based on a mean difference in pain score of –1.15 (95% confidence interval, –1.99 to –0.54), whereas products with comparable THC:CBD ratios were associated with a small effect on pain, with a mean difference of –0.52 (95% CI, –0.95 to –0.19).
According to Dr. McDonagh, treatment response rates were on par with response rates for more conventional treatments, “such as opioids or specific antidepressant drugs,” but data for the cannabis-based products are weaker.
“The amount of evidence available for cannabis-related products is very limited for [response rates], and therefore less certain,” Dr. McDonagh said in an interview. “The average reduction in pain severity is also similar to some other treatments, but we do not have studies directly comparing these treatments to draw conclusions.”
Although the cannabis-based products with relatively high and comparable THC:CBD ratios showed efficacy, they were also associated with “moderate to large increased risk for dizziness, sedation, and nausea,” the investigators wrote, noting that evidence was insufficient to characterize other “key adverse event outcomes” that may occur with long-term use, such as “psychosis, cannabis use disorder, and cognitive deficits.”
For products with low THC:CBD ratios, or without reported THC:CBD ratios, data were too scarce to reach any conclusions at all about safety or efficacy, highlighting the sizable knowledge gaps that remain in the area, the authors said.
“The current evidence on cannabis-related products for chronic pain is quite limited,” Dr. McDonagh said in an interview. “Patients with chronic pain should consult with their doctor to discuss which of the many options for treating chronic pain is best for them to start with.”
Patients may face resistance when asking about cannabis
According to Kevin F. Boehnke, PhD, and Daniel J. Clauw, MD, of the anesthesiology department and Chronic Pain and Fatigue Research Center at the University of Michigan, Ann Arbor, patients with chronic pain may face resistance, or even risk of being reported, when asking about cannabis-based products.
“Some physicians cite lack of data as rationale for not engaging with patients who wish to use or currently use cannabis,” Dr. Boehnke and Dr. Clauw wrote in an accompanying editorial. “Such practices may reflect consideration of cannabis solely as a drug of misuse (even in the 37 states where medical cannabis is legal) and requirements to refer patients who disclose or test positive for cannabis use to addiction services or decline to refill opioid prescriptions.”
Instead of shutting patients out, Dr. Boehnke and Dr. Clauw suggested clinicians engage in an “open information exchange” with their patients that focuses on “pragmatism, patient experience, known cannabinoid effects, and harm reduction.” In these conversations, the editorialists recommend noting that, “as with other analgesics, some persons will benefit, and others will not.”
They also offered some practical guidance: “Clinicians could suggest using tinctures (effect onset, 15-45 minutes) for breakthrough pain and edibles or capsules (which last about 6-8 hours) for extended relief. ... The scientific literature suggests that CBD doses could start at 5-10 mg twice daily and increase to 40-50 mg daily, whereas THC doses could start at 0.5-3 mg (initially at night) and increase to 30-40 mg/day.”
David Copenhaver, MD, MPH, clinical professor and chief of the division of pain medicine at UC Davis Health, Sacramento, shared a similar clinical mindset for patients choosing between opioids and cannabis-based products, specifically, CBD.
Compared with opioids, “the side-effect profile for CBD is less and the risk of mortality is less,” Dr. Copenhaver said in an interview, pointing out that nobody, to his knowledge, has ever died from an overdose of cannabis alone, and that CBD doses up to 1,000 mg/kg have been safely tolerated in people. “You present that, and most patients will say, ‘You know, I’d like to give this a try.’”
If so, Dr. Copenhaver makes sure patients know about a nonmedical risk: “The risk to the pocketbook.” Unlike opioids, which are covered under most insurance policies, most cannabis-based therapies are self-pay.
Buyers may get what they pay for, Dr. Copenhaver said, since products vary in quality, as do the dispensaries, from “very modest,” to highly sophisticated, with some even using chromatographic datasets to support the purity of their products.
Dr. Copenhaver steers his patients toward these more sophisticated retailers. Their expertise appears to be paying off, he said, not only in relief for patients, but also in market share. “Survival of the most fit will occur in the marketplace based on the results,” he said. “Unfortunately, some of that information doesn’t get percolated out into the literature.”
For investigators to fully uncover what cannabis-based products can do for chronic pain, Dr. Copenhaver said they need to get as “granular” as the leading dispensaries, which may first require recognition of the “very expansive opportunity” that less-studied cannabinoids may provide.
The study was supported by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services. The investigators, Dr. Boehnke, Dr. Clauw, and Dr. Copenhaver, disclosed no conflicts of interest.
Several other systematic reviews have recently evaluated cannabinoids for treating chronic pain, but the new study’s methodology was “distinct” in “important ways,” leading to “conclusions that differ from other reviews,” according to the authors of the paper published in the Annals of Internal Medicine.
In the new systematic review, synthetic products with high THC:CBD ratios were associated with moderate improvements in pain, whereas plant-based products with comparable THC:CBD ratios offered less relief, said study author Marian S. McDonagh, PharmD, professor of medical informatics and clinical epidemiology, and codirector of the Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues.
Specifically, the investigators stratified cannabis-based interventions according to relative content of two key cannabinoids: THC and CBD. Products were sorted into five categories: high THC:CBD ratio (at least 2:1), comparable THC:CBD ratio (less than 2:1 but more than 1:2), low THC:CBD ratio (no more than 1:2), whole-plant cannabis products, and other cannabinoids.
“In preclinical studies, THC and related compounds have demonstrated analgesic properties, although its psychoactive effects and addiction potential may limit its suitability as an analgesic,” the investigators wrote. “CBD and other cannabinoids may also have some analgesic or anti-inflammatory properties and are not believed to be psychoactive or addictive. Given the variation in analgesic effect with THC and CBD, response may differ according to the ratio of THC to CBD in products used to treat pain.”
The final analysis included 18 randomized placebo-controlled trials involving 1,740 individuals and 7 cohort studies involving 13,095 individuals. Most of the studies were short-term, lasting 1-6 months.
Pain was scored on a ten-point scale, with improvements reported as the mean difference from baseline to post treatment. A mean difference in pain score of 0.5-1.0 was considered a “small effect,” an improvement of 1-2 points was considered a “moderate effect,” and an improvement greater than 2 points was considered a “large effect.”
Cannabis-based products with relatively high THC:CBD ratios showed efficacy
Synthetic products with high THC:CBD ratios offered moderate pain relief, based on a mean difference in pain score of –1.15 (95% confidence interval, –1.99 to –0.54), whereas products with comparable THC:CBD ratios were associated with a small effect on pain, with a mean difference of –0.52 (95% CI, –0.95 to –0.19).
According to Dr. McDonagh, treatment response rates were on par with response rates for more conventional treatments, “such as opioids or specific antidepressant drugs,” but data for the cannabis-based products are weaker.
“The amount of evidence available for cannabis-related products is very limited for [response rates], and therefore less certain,” Dr. McDonagh said in an interview. “The average reduction in pain severity is also similar to some other treatments, but we do not have studies directly comparing these treatments to draw conclusions.”
Although the cannabis-based products with relatively high and comparable THC:CBD ratios showed efficacy, they were also associated with “moderate to large increased risk for dizziness, sedation, and nausea,” the investigators wrote, noting that evidence was insufficient to characterize other “key adverse event outcomes” that may occur with long-term use, such as “psychosis, cannabis use disorder, and cognitive deficits.”
For products with low THC:CBD ratios, or without reported THC:CBD ratios, data were too scarce to reach any conclusions at all about safety or efficacy, highlighting the sizable knowledge gaps that remain in the area, the authors said.
“The current evidence on cannabis-related products for chronic pain is quite limited,” Dr. McDonagh said in an interview. “Patients with chronic pain should consult with their doctor to discuss which of the many options for treating chronic pain is best for them to start with.”
Patients may face resistance when asking about cannabis
According to Kevin F. Boehnke, PhD, and Daniel J. Clauw, MD, of the anesthesiology department and Chronic Pain and Fatigue Research Center at the University of Michigan, Ann Arbor, patients with chronic pain may face resistance, or even risk of being reported, when asking about cannabis-based products.
“Some physicians cite lack of data as rationale for not engaging with patients who wish to use or currently use cannabis,” Dr. Boehnke and Dr. Clauw wrote in an accompanying editorial. “Such practices may reflect consideration of cannabis solely as a drug of misuse (even in the 37 states where medical cannabis is legal) and requirements to refer patients who disclose or test positive for cannabis use to addiction services or decline to refill opioid prescriptions.”
Instead of shutting patients out, Dr. Boehnke and Dr. Clauw suggested clinicians engage in an “open information exchange” with their patients that focuses on “pragmatism, patient experience, known cannabinoid effects, and harm reduction.” In these conversations, the editorialists recommend noting that, “as with other analgesics, some persons will benefit, and others will not.”
They also offered some practical guidance: “Clinicians could suggest using tinctures (effect onset, 15-45 minutes) for breakthrough pain and edibles or capsules (which last about 6-8 hours) for extended relief. ... The scientific literature suggests that CBD doses could start at 5-10 mg twice daily and increase to 40-50 mg daily, whereas THC doses could start at 0.5-3 mg (initially at night) and increase to 30-40 mg/day.”
David Copenhaver, MD, MPH, clinical professor and chief of the division of pain medicine at UC Davis Health, Sacramento, shared a similar clinical mindset for patients choosing between opioids and cannabis-based products, specifically, CBD.
Compared with opioids, “the side-effect profile for CBD is less and the risk of mortality is less,” Dr. Copenhaver said in an interview, pointing out that nobody, to his knowledge, has ever died from an overdose of cannabis alone, and that CBD doses up to 1,000 mg/kg have been safely tolerated in people. “You present that, and most patients will say, ‘You know, I’d like to give this a try.’”
If so, Dr. Copenhaver makes sure patients know about a nonmedical risk: “The risk to the pocketbook.” Unlike opioids, which are covered under most insurance policies, most cannabis-based therapies are self-pay.
Buyers may get what they pay for, Dr. Copenhaver said, since products vary in quality, as do the dispensaries, from “very modest,” to highly sophisticated, with some even using chromatographic datasets to support the purity of their products.
Dr. Copenhaver steers his patients toward these more sophisticated retailers. Their expertise appears to be paying off, he said, not only in relief for patients, but also in market share. “Survival of the most fit will occur in the marketplace based on the results,” he said. “Unfortunately, some of that information doesn’t get percolated out into the literature.”
For investigators to fully uncover what cannabis-based products can do for chronic pain, Dr. Copenhaver said they need to get as “granular” as the leading dispensaries, which may first require recognition of the “very expansive opportunity” that less-studied cannabinoids may provide.
The study was supported by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services. The investigators, Dr. Boehnke, Dr. Clauw, and Dr. Copenhaver, disclosed no conflicts of interest.
Several other systematic reviews have recently evaluated cannabinoids for treating chronic pain, but the new study’s methodology was “distinct” in “important ways,” leading to “conclusions that differ from other reviews,” according to the authors of the paper published in the Annals of Internal Medicine.
In the new systematic review, synthetic products with high THC:CBD ratios were associated with moderate improvements in pain, whereas plant-based products with comparable THC:CBD ratios offered less relief, said study author Marian S. McDonagh, PharmD, professor of medical informatics and clinical epidemiology, and codirector of the Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues.
Specifically, the investigators stratified cannabis-based interventions according to relative content of two key cannabinoids: THC and CBD. Products were sorted into five categories: high THC:CBD ratio (at least 2:1), comparable THC:CBD ratio (less than 2:1 but more than 1:2), low THC:CBD ratio (no more than 1:2), whole-plant cannabis products, and other cannabinoids.
“In preclinical studies, THC and related compounds have demonstrated analgesic properties, although its psychoactive effects and addiction potential may limit its suitability as an analgesic,” the investigators wrote. “CBD and other cannabinoids may also have some analgesic or anti-inflammatory properties and are not believed to be psychoactive or addictive. Given the variation in analgesic effect with THC and CBD, response may differ according to the ratio of THC to CBD in products used to treat pain.”
The final analysis included 18 randomized placebo-controlled trials involving 1,740 individuals and 7 cohort studies involving 13,095 individuals. Most of the studies were short-term, lasting 1-6 months.
Pain was scored on a ten-point scale, with improvements reported as the mean difference from baseline to post treatment. A mean difference in pain score of 0.5-1.0 was considered a “small effect,” an improvement of 1-2 points was considered a “moderate effect,” and an improvement greater than 2 points was considered a “large effect.”
Cannabis-based products with relatively high THC:CBD ratios showed efficacy
Synthetic products with high THC:CBD ratios offered moderate pain relief, based on a mean difference in pain score of –1.15 (95% confidence interval, –1.99 to –0.54), whereas products with comparable THC:CBD ratios were associated with a small effect on pain, with a mean difference of –0.52 (95% CI, –0.95 to –0.19).
According to Dr. McDonagh, treatment response rates were on par with response rates for more conventional treatments, “such as opioids or specific antidepressant drugs,” but data for the cannabis-based products are weaker.
“The amount of evidence available for cannabis-related products is very limited for [response rates], and therefore less certain,” Dr. McDonagh said in an interview. “The average reduction in pain severity is also similar to some other treatments, but we do not have studies directly comparing these treatments to draw conclusions.”
Although the cannabis-based products with relatively high and comparable THC:CBD ratios showed efficacy, they were also associated with “moderate to large increased risk for dizziness, sedation, and nausea,” the investigators wrote, noting that evidence was insufficient to characterize other “key adverse event outcomes” that may occur with long-term use, such as “psychosis, cannabis use disorder, and cognitive deficits.”
For products with low THC:CBD ratios, or without reported THC:CBD ratios, data were too scarce to reach any conclusions at all about safety or efficacy, highlighting the sizable knowledge gaps that remain in the area, the authors said.
“The current evidence on cannabis-related products for chronic pain is quite limited,” Dr. McDonagh said in an interview. “Patients with chronic pain should consult with their doctor to discuss which of the many options for treating chronic pain is best for them to start with.”
Patients may face resistance when asking about cannabis
According to Kevin F. Boehnke, PhD, and Daniel J. Clauw, MD, of the anesthesiology department and Chronic Pain and Fatigue Research Center at the University of Michigan, Ann Arbor, patients with chronic pain may face resistance, or even risk of being reported, when asking about cannabis-based products.
“Some physicians cite lack of data as rationale for not engaging with patients who wish to use or currently use cannabis,” Dr. Boehnke and Dr. Clauw wrote in an accompanying editorial. “Such practices may reflect consideration of cannabis solely as a drug of misuse (even in the 37 states where medical cannabis is legal) and requirements to refer patients who disclose or test positive for cannabis use to addiction services or decline to refill opioid prescriptions.”
Instead of shutting patients out, Dr. Boehnke and Dr. Clauw suggested clinicians engage in an “open information exchange” with their patients that focuses on “pragmatism, patient experience, known cannabinoid effects, and harm reduction.” In these conversations, the editorialists recommend noting that, “as with other analgesics, some persons will benefit, and others will not.”
They also offered some practical guidance: “Clinicians could suggest using tinctures (effect onset, 15-45 minutes) for breakthrough pain and edibles or capsules (which last about 6-8 hours) for extended relief. ... The scientific literature suggests that CBD doses could start at 5-10 mg twice daily and increase to 40-50 mg daily, whereas THC doses could start at 0.5-3 mg (initially at night) and increase to 30-40 mg/day.”
David Copenhaver, MD, MPH, clinical professor and chief of the division of pain medicine at UC Davis Health, Sacramento, shared a similar clinical mindset for patients choosing between opioids and cannabis-based products, specifically, CBD.
Compared with opioids, “the side-effect profile for CBD is less and the risk of mortality is less,” Dr. Copenhaver said in an interview, pointing out that nobody, to his knowledge, has ever died from an overdose of cannabis alone, and that CBD doses up to 1,000 mg/kg have been safely tolerated in people. “You present that, and most patients will say, ‘You know, I’d like to give this a try.’”
If so, Dr. Copenhaver makes sure patients know about a nonmedical risk: “The risk to the pocketbook.” Unlike opioids, which are covered under most insurance policies, most cannabis-based therapies are self-pay.
Buyers may get what they pay for, Dr. Copenhaver said, since products vary in quality, as do the dispensaries, from “very modest,” to highly sophisticated, with some even using chromatographic datasets to support the purity of their products.
Dr. Copenhaver steers his patients toward these more sophisticated retailers. Their expertise appears to be paying off, he said, not only in relief for patients, but also in market share. “Survival of the most fit will occur in the marketplace based on the results,” he said. “Unfortunately, some of that information doesn’t get percolated out into the literature.”
For investigators to fully uncover what cannabis-based products can do for chronic pain, Dr. Copenhaver said they need to get as “granular” as the leading dispensaries, which may first require recognition of the “very expansive opportunity” that less-studied cannabinoids may provide.
The study was supported by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services. The investigators, Dr. Boehnke, Dr. Clauw, and Dr. Copenhaver, disclosed no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Non-White subjects are sparse in DMT trials for MS
NATIONAL HARBOR, MD. -- Over 25 years of clinical research, phase 3 trials of approved disease-modifying therapies (DMTs) for multiple sclerosis (MS) were overwhelmingly made up of White subjects, a new analysis finds, and many studies failed to report percentages of non-White subjects at all. Researchers also found that the websites of multiple major drug manufacturers don’t include any trial data about how medications may affect people of different races and ethnicities.
It’s clear that “non-White participants are significantly underreported and unrepresented,” said study corresponding author and Dell Medical School/The University of Texas at Austin neurologist Leorah Freeman, MD, PhD, in an interview.
The study was presented at the annual meeting of the Consortium of Multiple Sclerosis Centers and published in Neurology.
“The lack of diversity in MS research is something that has been sporadically discussed in the past. By conducting this systematic review of MS phase 3 trials, we wanted to put numbers on this issue and review the evidence systematically,” Dr. Freeman said. “By doing so, we hoped to raise awareness about the problem of underreporting and underrepresentation of non-White participants in trials so that we, as a community involved in MS research, can start having the difficult conversations needed for change to occur.”
25 years of clinical research
The researchers reviewed 44 phase 3 studies from 1995-2020 that represented 45 trials. “We wanted to capture data from the very first global trials being conducted for the approval of MS DMTs, and the first was published in 1995,” Dr. Freeman said. “We were interested in understanding the impact of trial globalization over a long period of time on diversity of enrollment.”
The studies include trials of mainstays of MS treatment such as interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, diroximel fumarate, fingolimod, natalizumab, and others.
The researchers found that 17 (37.8%) of the trials did not report race or ethnicity, 14 (31.1%) reported race and ethnicity as proportion of White participants only, and 14 (31.1%) reported 2 or more races/ethnicities.
Of the 28 trials with racial breakdowns, the median percentage of White participants was 93.8% (range 78.5-99.6% across 28 studies), 1.9% for Black participants (range 0.1-8.1% across 14 studies), and 0.5% for Asian participants (range 0.1-14.5% across 11 studies).
The studies often failed to account for non-White subjects even though “Black people, in particular, have been shown to have a more severe disease course,” Dr. Freeman said.
A 2022 study of more than 2.6 million Southern California adults finds that prevalence of MS was similar among White and Black people at about 230 per 100,000. “Taken together with previous studies, these findings indicate that the burden of MS in the United States Black community has long been underrecognized,” the researchers wrote.
According to Dr. Freeman, it’s unclear why the studies were so dominated by White subjects. “Lack of awareness about the importance of this information likely explains why this information often goes unreported.”
She highlighted the TOWER (teriflunomide) and DEFINE and CONFIRM (dimethyl fumarate) studies as positive examples. “We noted the inclusion of trial sites in Asia and consequently a higher representation of Asian people with MS in those trials. We felt these studies were examples of how trial globalization can support better representation of underrepresented groups.”
And she noted that the ongoing CHIMES trial is examining the use of ocrelizumab in Black and Hispanic people with MS. “This study was designed in partnership with MS patients and advocacy groups to bridge gaps in clinical trial participation in these communities,” she said. “Innovative strategies were developed to increase participation of Black and Hispanic patients in this trial.”
What should happen next?
Going forward, Dr. Freeman said, “MS researchers, DMT manufacturers, sponsors, and publishers need to set better standards for racial and ethnic representation and reporting in trial publications.”
In an interview, epidemiologist Luisa N. Borrell, DDS, PhD, a professor who studies race and medicine at City University of New York, said the new study is valid and useful. She noted that it reflects the findings of a 2022 analysis of more than 20,500 clinical trials in the U.S. from 2000-2020: Only 43% reported racial/ethnic breakdowns, and the subjects were much more White than the population at large.
Possible reasons for the disparity include distrust among possible participants and lack of health literacy, she said, which are both “modifiable issues.”
Dr. Borrell added: “Clinical trials should aim to recruit populations affected by the outcome of interest. That would allow for the intervention to effectively treat those who need it the most. Moreover, the lack of diversity of trials brings issues of generalizability and transportability of the findings.”
No funding is reported. Dr. Freeman and some of the other study authors report various disclosures.
NATIONAL HARBOR, MD. -- Over 25 years of clinical research, phase 3 trials of approved disease-modifying therapies (DMTs) for multiple sclerosis (MS) were overwhelmingly made up of White subjects, a new analysis finds, and many studies failed to report percentages of non-White subjects at all. Researchers also found that the websites of multiple major drug manufacturers don’t include any trial data about how medications may affect people of different races and ethnicities.
It’s clear that “non-White participants are significantly underreported and unrepresented,” said study corresponding author and Dell Medical School/The University of Texas at Austin neurologist Leorah Freeman, MD, PhD, in an interview.
The study was presented at the annual meeting of the Consortium of Multiple Sclerosis Centers and published in Neurology.
“The lack of diversity in MS research is something that has been sporadically discussed in the past. By conducting this systematic review of MS phase 3 trials, we wanted to put numbers on this issue and review the evidence systematically,” Dr. Freeman said. “By doing so, we hoped to raise awareness about the problem of underreporting and underrepresentation of non-White participants in trials so that we, as a community involved in MS research, can start having the difficult conversations needed for change to occur.”
25 years of clinical research
The researchers reviewed 44 phase 3 studies from 1995-2020 that represented 45 trials. “We wanted to capture data from the very first global trials being conducted for the approval of MS DMTs, and the first was published in 1995,” Dr. Freeman said. “We were interested in understanding the impact of trial globalization over a long period of time on diversity of enrollment.”
The studies include trials of mainstays of MS treatment such as interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, diroximel fumarate, fingolimod, natalizumab, and others.
The researchers found that 17 (37.8%) of the trials did not report race or ethnicity, 14 (31.1%) reported race and ethnicity as proportion of White participants only, and 14 (31.1%) reported 2 or more races/ethnicities.
Of the 28 trials with racial breakdowns, the median percentage of White participants was 93.8% (range 78.5-99.6% across 28 studies), 1.9% for Black participants (range 0.1-8.1% across 14 studies), and 0.5% for Asian participants (range 0.1-14.5% across 11 studies).
The studies often failed to account for non-White subjects even though “Black people, in particular, have been shown to have a more severe disease course,” Dr. Freeman said.
A 2022 study of more than 2.6 million Southern California adults finds that prevalence of MS was similar among White and Black people at about 230 per 100,000. “Taken together with previous studies, these findings indicate that the burden of MS in the United States Black community has long been underrecognized,” the researchers wrote.
According to Dr. Freeman, it’s unclear why the studies were so dominated by White subjects. “Lack of awareness about the importance of this information likely explains why this information often goes unreported.”
She highlighted the TOWER (teriflunomide) and DEFINE and CONFIRM (dimethyl fumarate) studies as positive examples. “We noted the inclusion of trial sites in Asia and consequently a higher representation of Asian people with MS in those trials. We felt these studies were examples of how trial globalization can support better representation of underrepresented groups.”
And she noted that the ongoing CHIMES trial is examining the use of ocrelizumab in Black and Hispanic people with MS. “This study was designed in partnership with MS patients and advocacy groups to bridge gaps in clinical trial participation in these communities,” she said. “Innovative strategies were developed to increase participation of Black and Hispanic patients in this trial.”
What should happen next?
Going forward, Dr. Freeman said, “MS researchers, DMT manufacturers, sponsors, and publishers need to set better standards for racial and ethnic representation and reporting in trial publications.”
In an interview, epidemiologist Luisa N. Borrell, DDS, PhD, a professor who studies race and medicine at City University of New York, said the new study is valid and useful. She noted that it reflects the findings of a 2022 analysis of more than 20,500 clinical trials in the U.S. from 2000-2020: Only 43% reported racial/ethnic breakdowns, and the subjects were much more White than the population at large.
Possible reasons for the disparity include distrust among possible participants and lack of health literacy, she said, which are both “modifiable issues.”
Dr. Borrell added: “Clinical trials should aim to recruit populations affected by the outcome of interest. That would allow for the intervention to effectively treat those who need it the most. Moreover, the lack of diversity of trials brings issues of generalizability and transportability of the findings.”
No funding is reported. Dr. Freeman and some of the other study authors report various disclosures.
NATIONAL HARBOR, MD. -- Over 25 years of clinical research, phase 3 trials of approved disease-modifying therapies (DMTs) for multiple sclerosis (MS) were overwhelmingly made up of White subjects, a new analysis finds, and many studies failed to report percentages of non-White subjects at all. Researchers also found that the websites of multiple major drug manufacturers don’t include any trial data about how medications may affect people of different races and ethnicities.
It’s clear that “non-White participants are significantly underreported and unrepresented,” said study corresponding author and Dell Medical School/The University of Texas at Austin neurologist Leorah Freeman, MD, PhD, in an interview.
The study was presented at the annual meeting of the Consortium of Multiple Sclerosis Centers and published in Neurology.
“The lack of diversity in MS research is something that has been sporadically discussed in the past. By conducting this systematic review of MS phase 3 trials, we wanted to put numbers on this issue and review the evidence systematically,” Dr. Freeman said. “By doing so, we hoped to raise awareness about the problem of underreporting and underrepresentation of non-White participants in trials so that we, as a community involved in MS research, can start having the difficult conversations needed for change to occur.”
25 years of clinical research
The researchers reviewed 44 phase 3 studies from 1995-2020 that represented 45 trials. “We wanted to capture data from the very first global trials being conducted for the approval of MS DMTs, and the first was published in 1995,” Dr. Freeman said. “We were interested in understanding the impact of trial globalization over a long period of time on diversity of enrollment.”
The studies include trials of mainstays of MS treatment such as interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, diroximel fumarate, fingolimod, natalizumab, and others.
The researchers found that 17 (37.8%) of the trials did not report race or ethnicity, 14 (31.1%) reported race and ethnicity as proportion of White participants only, and 14 (31.1%) reported 2 or more races/ethnicities.
Of the 28 trials with racial breakdowns, the median percentage of White participants was 93.8% (range 78.5-99.6% across 28 studies), 1.9% for Black participants (range 0.1-8.1% across 14 studies), and 0.5% for Asian participants (range 0.1-14.5% across 11 studies).
The studies often failed to account for non-White subjects even though “Black people, in particular, have been shown to have a more severe disease course,” Dr. Freeman said.
A 2022 study of more than 2.6 million Southern California adults finds that prevalence of MS was similar among White and Black people at about 230 per 100,000. “Taken together with previous studies, these findings indicate that the burden of MS in the United States Black community has long been underrecognized,” the researchers wrote.
According to Dr. Freeman, it’s unclear why the studies were so dominated by White subjects. “Lack of awareness about the importance of this information likely explains why this information often goes unreported.”
She highlighted the TOWER (teriflunomide) and DEFINE and CONFIRM (dimethyl fumarate) studies as positive examples. “We noted the inclusion of trial sites in Asia and consequently a higher representation of Asian people with MS in those trials. We felt these studies were examples of how trial globalization can support better representation of underrepresented groups.”
And she noted that the ongoing CHIMES trial is examining the use of ocrelizumab in Black and Hispanic people with MS. “This study was designed in partnership with MS patients and advocacy groups to bridge gaps in clinical trial participation in these communities,” she said. “Innovative strategies were developed to increase participation of Black and Hispanic patients in this trial.”
What should happen next?
Going forward, Dr. Freeman said, “MS researchers, DMT manufacturers, sponsors, and publishers need to set better standards for racial and ethnic representation and reporting in trial publications.”
In an interview, epidemiologist Luisa N. Borrell, DDS, PhD, a professor who studies race and medicine at City University of New York, said the new study is valid and useful. She noted that it reflects the findings of a 2022 analysis of more than 20,500 clinical trials in the U.S. from 2000-2020: Only 43% reported racial/ethnic breakdowns, and the subjects were much more White than the population at large.
Possible reasons for the disparity include distrust among possible participants and lack of health literacy, she said, which are both “modifiable issues.”
Dr. Borrell added: “Clinical trials should aim to recruit populations affected by the outcome of interest. That would allow for the intervention to effectively treat those who need it the most. Moreover, the lack of diversity of trials brings issues of generalizability and transportability of the findings.”
No funding is reported. Dr. Freeman and some of the other study authors report various disclosures.
AT CMSC 2022
‘Extremely exciting’ study results guide MM treatment options
CHICAGO – New results from a trial in patients with newly diagnosed multiple myeloma (MM) offer some answers to questions about which treatment route to choose.
Patients who received the triplet of lenalidomide, bortezomib, and dexamethasone (RVD) plus ASCT had a median PFS of 67.5 months, compared with 46.2 months for those who received RVD but did not have a transplant soon after.
However, patients were just as likely to be alive more than 6 years after treatment regardless of whether or not they underwent an immediate stem cell transplant.
In addition, treatment-related adverse events of grade 3 or above were higher in the group that received the transplant immediately after the triplet therapy.
The results were presented during a plenary session at the American Society of Clinical Oncology annual meeting and simultaneously published in the New England Journal of Medicine.
“Our findings confirm the PFS benefit of transplantation as first-line treatment for patients with myeloma and confirms stem cell transplant as a standard of care with certain triplet therapy,” said lead author Paul G. Richardson, MD, professor of medicine, Harvard Medical School, and clinical program leader and director of clinical research at the Jerome Lipper Multiple Myeloma Center at Dana Farber Cancer Institute, Boston.
Another finding from the trial was that the use of maintenance lenalidomide in both groups continuously until progression conferred substantial clinical benefit.
“We can also say that the use of lenalidomide maintenance therapy is also a standard of care,” he added.
Study details
In this trial, Dr. Richardson and colleagues randomly assigned 873 patients newly diagnosed with multiple myeloma to the RVD-alone group (n = 357) or the transplantation group (n = 365). All patients had received one cycle of RVD prior to randomization and then received two additional RVD cycles plus stem-cell mobilization followed by either five additional RVD cycles (the RVD-alone group) or high-dose melphalan plus ASCT followed by two additional RVD cycles (the transplantation group). Lenalidomide was administered to all patients until disease progression, unacceptable side effects, or both.
At a median follow-up of 76.0 months, the risk of disease progression or death was 53% higher among patients who received RVD alone versus the transplantation group (hazard ratio [HR], 1.53; P < .001). The median duration of PFS among patients with a high-risk cytogenetic profile was 55.5 vs. 17.1 months, favoring the transplantation group.
The percentage of patients who were alive without progression at 5 years was 58.4% vs 41.6%, respectively (HR, 1.66) and median duration of response was 56.4 vs 38.9 months, also favoring transplantation (HR, 1.45).
The estimated 5-year overall survival was similar between groups: 80.7% for transplantation and 79.2% for RVD alone (HR for death, 1.10; P > .99). For patients with a high-risk cytogenetic profile, 5-year survival was 63.4% versus 54.3%, respectively.
“This tells us that for patients who had kept transplant in reserve, they had the same overall survival as those who had had a transplant right away, despite there being such impressive initial disease control for the patients in whom transplant was used early,” Dr. Richardson said in a press release from his institution.
Patients who did not undergo immediate transplant received treatment when their disease progressed with newer and active therapies, such as monoclonal antibodies and/or next-generation novel agents, he noted. Only 28% of patients used the reserve option of a transplant.
“It demonstrates the extent to which patients now have options and that we have new data to guide them in balancing the pluses and minuses of each approach,” he added.
When looking at safety, the authors noted that the most common treatment-related adverse events of grade 3 or higher occurred in 279 patients (78.2%) in the RVD-alone group and 344 patients (94.2%) in the transplantation group. Of those patients, 60.5% and 89.9%, respectively, reported hematologic events of grade 3 or higher (P < .001). The 5-year cumulative incidence of invasive second primary cancers was similar in both cohorts (RVD-alone group, 4.9%; transplantation group, 6.5%).
However, while the risk of secondary cancers was similar between groups, Dr. Richardson noted that there was a higher incidence of acute myeloid leukemia and myelodysplastic syndromes in the transplant cohort.
“There was also a significant drop in quality of life across transplant procedures, but the good news is that it was recoverable rapidly,” he said. “What is also really important is that we have prospective, multicenter, national comparative data on toxicity. That’s very important for providing patients with a choice as they move forward with their treatment plan.”
He noted that treatment continues to evolve. “This study was designed in 2009, begun in 2010, and now there is mature data in 2022,” Dr. Richardson said. “This is particularly relevant as we have now further improved the induction treatment for younger patients with newly diagnosed myeloma using quadruplet regimens incorporating monoclonal antibodies and novel next-generation therapies. The results from these studies are extremely exciting.
“Now more than ever, treatment for multiple myeloma can be adapted for each patient,” Dr. Richardson said. “Our study provides important information about the benefits of transplant in the era of highly effective novel therapies and continuous maintenance, as well as the potential risks, to help patients and their physicians decide what approach may be best for them. This is particularly relevant as we have now further improved the induction treatment for younger patients with newly diagnosed myeloma using quadruplet regimens incorporating monoclonal antibodies, such as RVD combined with daratumumab.”
Lack of difference in overall survival
These new results further support an already established role of autologous hematopoietic stem cell transplantation in the management of patients with multiple myeloma, said Samer Al-Homsi, MD, clinical professor of medicine and director of the blood and marrow transplant program at Perlmutter Cancer Center, NYU Langone, New York, who was approached for comment.
“The treatment regimen is applicable to patients who are determined by an expert in transplantation to be fit to receive autologous hematopoietic transplantation,” he added. “Although this study, like many others, establishes hematopoietic stem cell transplantation as part of the standard of care in multiple myeloma, only a fraction of patients are actually offered this important modality of treatment for a variety of reasons, including provider bias,” he noted. “In fact, although improvement in supportive care has enhanced the safety of the procedure, many patients are denied this therapy.”
Dr. Al-Homsi noted that the lack of difference in overall survival might be due to the fact that some patients (28%) in the RVD-alone group did end up undergoing transplantation at the time of progression. “Also, longer follow-up might reveal a difference in overall survival,” he said.
The toxicities are manageable, and the incidence of secondary malignancies was not significantly different between cohorts. “However,” he emphasized, “lenalidomide has been associated in other studies with increased incidence of secondary malignancies and it must be noted that this study used extended administration of lenalidomide until progression.”
Support for this study was provided by grants to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute, the National Cancer Institute, R. J. Corman Multiple Myeloma Foundation, Celgene/Bristol Myers Squibb, and Millennium/Takeda Pharmaceutical. Dr. Richardson has reported relationships with Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm Therapeutics, Oncopeptides, Sanofi, Secura Bio, Takeda, and Bristol Myers Squibb. Dr. Al-Homsi has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – New results from a trial in patients with newly diagnosed multiple myeloma (MM) offer some answers to questions about which treatment route to choose.
Patients who received the triplet of lenalidomide, bortezomib, and dexamethasone (RVD) plus ASCT had a median PFS of 67.5 months, compared with 46.2 months for those who received RVD but did not have a transplant soon after.
However, patients were just as likely to be alive more than 6 years after treatment regardless of whether or not they underwent an immediate stem cell transplant.
In addition, treatment-related adverse events of grade 3 or above were higher in the group that received the transplant immediately after the triplet therapy.
The results were presented during a plenary session at the American Society of Clinical Oncology annual meeting and simultaneously published in the New England Journal of Medicine.
“Our findings confirm the PFS benefit of transplantation as first-line treatment for patients with myeloma and confirms stem cell transplant as a standard of care with certain triplet therapy,” said lead author Paul G. Richardson, MD, professor of medicine, Harvard Medical School, and clinical program leader and director of clinical research at the Jerome Lipper Multiple Myeloma Center at Dana Farber Cancer Institute, Boston.
Another finding from the trial was that the use of maintenance lenalidomide in both groups continuously until progression conferred substantial clinical benefit.
“We can also say that the use of lenalidomide maintenance therapy is also a standard of care,” he added.
Study details
In this trial, Dr. Richardson and colleagues randomly assigned 873 patients newly diagnosed with multiple myeloma to the RVD-alone group (n = 357) or the transplantation group (n = 365). All patients had received one cycle of RVD prior to randomization and then received two additional RVD cycles plus stem-cell mobilization followed by either five additional RVD cycles (the RVD-alone group) or high-dose melphalan plus ASCT followed by two additional RVD cycles (the transplantation group). Lenalidomide was administered to all patients until disease progression, unacceptable side effects, or both.
At a median follow-up of 76.0 months, the risk of disease progression or death was 53% higher among patients who received RVD alone versus the transplantation group (hazard ratio [HR], 1.53; P < .001). The median duration of PFS among patients with a high-risk cytogenetic profile was 55.5 vs. 17.1 months, favoring the transplantation group.
The percentage of patients who were alive without progression at 5 years was 58.4% vs 41.6%, respectively (HR, 1.66) and median duration of response was 56.4 vs 38.9 months, also favoring transplantation (HR, 1.45).
The estimated 5-year overall survival was similar between groups: 80.7% for transplantation and 79.2% for RVD alone (HR for death, 1.10; P > .99). For patients with a high-risk cytogenetic profile, 5-year survival was 63.4% versus 54.3%, respectively.
“This tells us that for patients who had kept transplant in reserve, they had the same overall survival as those who had had a transplant right away, despite there being such impressive initial disease control for the patients in whom transplant was used early,” Dr. Richardson said in a press release from his institution.
Patients who did not undergo immediate transplant received treatment when their disease progressed with newer and active therapies, such as monoclonal antibodies and/or next-generation novel agents, he noted. Only 28% of patients used the reserve option of a transplant.
“It demonstrates the extent to which patients now have options and that we have new data to guide them in balancing the pluses and minuses of each approach,” he added.
When looking at safety, the authors noted that the most common treatment-related adverse events of grade 3 or higher occurred in 279 patients (78.2%) in the RVD-alone group and 344 patients (94.2%) in the transplantation group. Of those patients, 60.5% and 89.9%, respectively, reported hematologic events of grade 3 or higher (P < .001). The 5-year cumulative incidence of invasive second primary cancers was similar in both cohorts (RVD-alone group, 4.9%; transplantation group, 6.5%).
However, while the risk of secondary cancers was similar between groups, Dr. Richardson noted that there was a higher incidence of acute myeloid leukemia and myelodysplastic syndromes in the transplant cohort.
“There was also a significant drop in quality of life across transplant procedures, but the good news is that it was recoverable rapidly,” he said. “What is also really important is that we have prospective, multicenter, national comparative data on toxicity. That’s very important for providing patients with a choice as they move forward with their treatment plan.”
He noted that treatment continues to evolve. “This study was designed in 2009, begun in 2010, and now there is mature data in 2022,” Dr. Richardson said. “This is particularly relevant as we have now further improved the induction treatment for younger patients with newly diagnosed myeloma using quadruplet regimens incorporating monoclonal antibodies and novel next-generation therapies. The results from these studies are extremely exciting.
“Now more than ever, treatment for multiple myeloma can be adapted for each patient,” Dr. Richardson said. “Our study provides important information about the benefits of transplant in the era of highly effective novel therapies and continuous maintenance, as well as the potential risks, to help patients and their physicians decide what approach may be best for them. This is particularly relevant as we have now further improved the induction treatment for younger patients with newly diagnosed myeloma using quadruplet regimens incorporating monoclonal antibodies, such as RVD combined with daratumumab.”
Lack of difference in overall survival
These new results further support an already established role of autologous hematopoietic stem cell transplantation in the management of patients with multiple myeloma, said Samer Al-Homsi, MD, clinical professor of medicine and director of the blood and marrow transplant program at Perlmutter Cancer Center, NYU Langone, New York, who was approached for comment.
“The treatment regimen is applicable to patients who are determined by an expert in transplantation to be fit to receive autologous hematopoietic transplantation,” he added. “Although this study, like many others, establishes hematopoietic stem cell transplantation as part of the standard of care in multiple myeloma, only a fraction of patients are actually offered this important modality of treatment for a variety of reasons, including provider bias,” he noted. “In fact, although improvement in supportive care has enhanced the safety of the procedure, many patients are denied this therapy.”
Dr. Al-Homsi noted that the lack of difference in overall survival might be due to the fact that some patients (28%) in the RVD-alone group did end up undergoing transplantation at the time of progression. “Also, longer follow-up might reveal a difference in overall survival,” he said.
The toxicities are manageable, and the incidence of secondary malignancies was not significantly different between cohorts. “However,” he emphasized, “lenalidomide has been associated in other studies with increased incidence of secondary malignancies and it must be noted that this study used extended administration of lenalidomide until progression.”
Support for this study was provided by grants to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute, the National Cancer Institute, R. J. Corman Multiple Myeloma Foundation, Celgene/Bristol Myers Squibb, and Millennium/Takeda Pharmaceutical. Dr. Richardson has reported relationships with Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm Therapeutics, Oncopeptides, Sanofi, Secura Bio, Takeda, and Bristol Myers Squibb. Dr. Al-Homsi has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – New results from a trial in patients with newly diagnosed multiple myeloma (MM) offer some answers to questions about which treatment route to choose.
Patients who received the triplet of lenalidomide, bortezomib, and dexamethasone (RVD) plus ASCT had a median PFS of 67.5 months, compared with 46.2 months for those who received RVD but did not have a transplant soon after.
However, patients were just as likely to be alive more than 6 years after treatment regardless of whether or not they underwent an immediate stem cell transplant.
In addition, treatment-related adverse events of grade 3 or above were higher in the group that received the transplant immediately after the triplet therapy.
The results were presented during a plenary session at the American Society of Clinical Oncology annual meeting and simultaneously published in the New England Journal of Medicine.
“Our findings confirm the PFS benefit of transplantation as first-line treatment for patients with myeloma and confirms stem cell transplant as a standard of care with certain triplet therapy,” said lead author Paul G. Richardson, MD, professor of medicine, Harvard Medical School, and clinical program leader and director of clinical research at the Jerome Lipper Multiple Myeloma Center at Dana Farber Cancer Institute, Boston.
Another finding from the trial was that the use of maintenance lenalidomide in both groups continuously until progression conferred substantial clinical benefit.
“We can also say that the use of lenalidomide maintenance therapy is also a standard of care,” he added.
Study details
In this trial, Dr. Richardson and colleagues randomly assigned 873 patients newly diagnosed with multiple myeloma to the RVD-alone group (n = 357) or the transplantation group (n = 365). All patients had received one cycle of RVD prior to randomization and then received two additional RVD cycles plus stem-cell mobilization followed by either five additional RVD cycles (the RVD-alone group) or high-dose melphalan plus ASCT followed by two additional RVD cycles (the transplantation group). Lenalidomide was administered to all patients until disease progression, unacceptable side effects, or both.
At a median follow-up of 76.0 months, the risk of disease progression or death was 53% higher among patients who received RVD alone versus the transplantation group (hazard ratio [HR], 1.53; P < .001). The median duration of PFS among patients with a high-risk cytogenetic profile was 55.5 vs. 17.1 months, favoring the transplantation group.
The percentage of patients who were alive without progression at 5 years was 58.4% vs 41.6%, respectively (HR, 1.66) and median duration of response was 56.4 vs 38.9 months, also favoring transplantation (HR, 1.45).
The estimated 5-year overall survival was similar between groups: 80.7% for transplantation and 79.2% for RVD alone (HR for death, 1.10; P > .99). For patients with a high-risk cytogenetic profile, 5-year survival was 63.4% versus 54.3%, respectively.
“This tells us that for patients who had kept transplant in reserve, they had the same overall survival as those who had had a transplant right away, despite there being such impressive initial disease control for the patients in whom transplant was used early,” Dr. Richardson said in a press release from his institution.
Patients who did not undergo immediate transplant received treatment when their disease progressed with newer and active therapies, such as monoclonal antibodies and/or next-generation novel agents, he noted. Only 28% of patients used the reserve option of a transplant.
“It demonstrates the extent to which patients now have options and that we have new data to guide them in balancing the pluses and minuses of each approach,” he added.
When looking at safety, the authors noted that the most common treatment-related adverse events of grade 3 or higher occurred in 279 patients (78.2%) in the RVD-alone group and 344 patients (94.2%) in the transplantation group. Of those patients, 60.5% and 89.9%, respectively, reported hematologic events of grade 3 or higher (P < .001). The 5-year cumulative incidence of invasive second primary cancers was similar in both cohorts (RVD-alone group, 4.9%; transplantation group, 6.5%).
However, while the risk of secondary cancers was similar between groups, Dr. Richardson noted that there was a higher incidence of acute myeloid leukemia and myelodysplastic syndromes in the transplant cohort.
“There was also a significant drop in quality of life across transplant procedures, but the good news is that it was recoverable rapidly,” he said. “What is also really important is that we have prospective, multicenter, national comparative data on toxicity. That’s very important for providing patients with a choice as they move forward with their treatment plan.”
He noted that treatment continues to evolve. “This study was designed in 2009, begun in 2010, and now there is mature data in 2022,” Dr. Richardson said. “This is particularly relevant as we have now further improved the induction treatment for younger patients with newly diagnosed myeloma using quadruplet regimens incorporating monoclonal antibodies and novel next-generation therapies. The results from these studies are extremely exciting.
“Now more than ever, treatment for multiple myeloma can be adapted for each patient,” Dr. Richardson said. “Our study provides important information about the benefits of transplant in the era of highly effective novel therapies and continuous maintenance, as well as the potential risks, to help patients and their physicians decide what approach may be best for them. This is particularly relevant as we have now further improved the induction treatment for younger patients with newly diagnosed myeloma using quadruplet regimens incorporating monoclonal antibodies, such as RVD combined with daratumumab.”
Lack of difference in overall survival
These new results further support an already established role of autologous hematopoietic stem cell transplantation in the management of patients with multiple myeloma, said Samer Al-Homsi, MD, clinical professor of medicine and director of the blood and marrow transplant program at Perlmutter Cancer Center, NYU Langone, New York, who was approached for comment.
“The treatment regimen is applicable to patients who are determined by an expert in transplantation to be fit to receive autologous hematopoietic transplantation,” he added. “Although this study, like many others, establishes hematopoietic stem cell transplantation as part of the standard of care in multiple myeloma, only a fraction of patients are actually offered this important modality of treatment for a variety of reasons, including provider bias,” he noted. “In fact, although improvement in supportive care has enhanced the safety of the procedure, many patients are denied this therapy.”
Dr. Al-Homsi noted that the lack of difference in overall survival might be due to the fact that some patients (28%) in the RVD-alone group did end up undergoing transplantation at the time of progression. “Also, longer follow-up might reveal a difference in overall survival,” he said.
The toxicities are manageable, and the incidence of secondary malignancies was not significantly different between cohorts. “However,” he emphasized, “lenalidomide has been associated in other studies with increased incidence of secondary malignancies and it must be noted that this study used extended administration of lenalidomide until progression.”
Support for this study was provided by grants to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute, the National Cancer Institute, R. J. Corman Multiple Myeloma Foundation, Celgene/Bristol Myers Squibb, and Millennium/Takeda Pharmaceutical. Dr. Richardson has reported relationships with Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm Therapeutics, Oncopeptides, Sanofi, Secura Bio, Takeda, and Bristol Myers Squibb. Dr. Al-Homsi has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASCO 2022
‘Exciting’ new gene therapy yields promising results
In the first-in-human, phase 1 open-label study, known as ANTLER, 5 out of 5 patients with relapsed or refractory B cell non-Hodgkin lymphoma (r/r B-NHL) responded to a single dose of CB-010, an allogeneic CAR-T cell therapy designed to boost antitumor activity, according to the company.
The use of chimeric antigen receptor (CAR) T-cell therapy involves taking T cells out of the body, reprogramming them with CAR to better equip them to kill cancer cells, and putting them back into the body.
The study consists of two sections: an initial dose escalation following a 3 + 3 design, with prespecified, increasing doses, followed by an expanded trial in which all patients receive CB-010 at the dose determined in the first section.
The study population included 6 adults with r/r B-NHL who had relapsed after previous treatment with a median of 3 prior therapies. At baseline, all 6 patients underwent a lymphodepletion regimen consisting of cyclophosphamide at 60 mg/kg/day for 2 days, followed by 5 days of fludarabine at 25 mg/m2/day.
Then all patients received a single dose of 40x106 CAR-T cells. As of the Feb. 23, 2022, data cutoff date, 5 of the 6 patients had completed the 28-day dose-limiting toxicity (DLT) evaluation period. All 5 patients (100%) achieved a response; 4 achieved complete response and 1 achieved partial response. All 4 of the complete responders had ongoing complete response at 3 months, and the longest measured complete response was 6 months, according to the company.
“We are excited to see a 100% overall response rate with CB-010 at dose level 1 for these patients who have limited treatment options,” said Dr. Syed Rizvi, chief medical officer for Caribou Biosciences, in the press release. “We believe this initial level of activity is unparalleled for a single, starting dose of cell therapy. CB-010 was generally well-tolerated, with adverse events routinely observed in autologous or allogeneic anti-CD19 CAR-T cell therapies,” he said.
Based on the promising safety and efficacy results, the company is enrolling patients in a second cohort for treatment at dose level 2 (80x106 CAR-T cells), according to the news release.
Another allogeneic CAR-T cell therapy known as ALLO-501A is being studied in a similar trial conducted by the Moffitt Cancer Center.
Overall, CB-010 was well-tolerated, according to Caribou Biosciences. No cases of graft-versus-host disease were reported. A total of 3 patients developed grade 3 or 4 adverse events (AEs) within the first 28 days; the most common were neutropenia (50%), thrombocytopenia (33%), anemia (17%), and hypogammaglobulinemia (17%). One patient experienced both grade 1 cytokine release syndrome (CRS) and grade 3 Immune effector cell-Associated Neurotoxicity Syndrome (ICANS). This response was characterized as a dose-limiting toxicity. The patient was treated with tocilizumab and steroids, recovered within 39 hours, and went on to achieve a complete response, according to the company.
Although the safety profile in the current study was promising, prior research suggest that concerns associated with CRS and ICANS should not be ignored and may be barriers to treatment.
In an article published in Bone Marrow Transplant in 2021, Dr. Vipul Sheth and Dr. Jordan Gauthier of the Fred Hutchinson Cancer Center, Seattle, noted that adverse effects may remain a challenge to widespread use of CAR-T in patients with refractory or relapsed acute lymphoblastic leukemia, for which it has been approved by the U.S. Food and Drug Administration and several European agencies. However, “there is mounting evidence that earlier, and potentially more targeted, interventions can reduce these toxicities,” they wrote.
Study provides solid stepping stone
“CRS and ICANS are mild in most patients but can be severe and sometimes life-threatening in a subset of patients undergoing CD19 CAR T-cell therapy,” Dr. Gauthier said in an interview. “Different strategies are being investigated to mitigate or treat severe toxicities, such as the use of prophylactic corticosteroids, anakinra, lenzilumab, itacitinib. I am hopeful we will soon manage to prevent toxicities while maintaining potent anti-tumor effects,” he said.
“While autologous CD19 CAR-T cells have high efficacy in patients with refractory/relapsed large B-cell lymphoma, product manufacturing remains a complicated and lengthy process in the autologous setting,” Dr. Gauthier noted. “Commercial CAR T-cell manufacturing takes approximately 3-4 weeks, sometimes longer. Some patients won’t survive long enough to receive their infusion. In some patients, T-cell function is dramatically impaired, due to prior therapies or to the disease itself,” he said.
Dr. Gauthier said he was not surprised but that he was encouraged by the apparent early success of the ANTLER study. “The proof-of-concept that allogeneic CD19-targeted CAR T cells can induce high response rates in r/r LBCL has already been established,” he said. “Having said that, it is comforting to see prior findings confirmed by this new study, and those results are exciting for the field,” he added.
As for additional research, “we need longer follow-up after allogeneic CD19-targeted CAR T-cell therapy to ensure responses are durable,” Dr. Gauthier explained. “We also need to better understand the biology driving the antitumor effects and the side effects of CAR T-cells. This will help us build more efficacious and safer CAR T-cell therapies,” he said.
Response and side effects show promise for future research
The therapy is “the best CAR-T product” that clinicians can provide for patients knowing that autologous CAR-T works, said Dr. Ahmed Galal, of Duke University, Durham, N.C., in an interview. The current research supports the use of this treatment immediately for patients, he added.
Dr. Galal said he was somewhat surprised, but pleasantly so, by the 100% response rate. This rate is likely because of the small number of patients and may not hold up in further research, but “even 90% would be an amazing achievement,” he said. The tolerable safety profile is encouraging as well, he emphasized. Dr. Galal said that he did not foresee any real barriers to expanded use of the therapy and that technology should make it easier to deliver at authorized centers.
Limitations to the current study are those common to all phase 1 trials, such as the strict inclusion criteria, Dr. Galal said. As research progresses to phase 2, “I don’t think it will be an obstacle to find patients,” he said. However, patients should be aware of side effects, and clinicians should maintain a culture of education to help them understand the value of the therapy, he added.
The complete data from the preliminary findings are scheduled to be presented at the European Hematology Association (EHA) 2022 Hybrid Congress, Vienna, in June, as abstract P1455, titled “First-in-human trial of CB-010, a CRISPR-edited allogeneic anti-CD19 CAR-T cell therapy with a PD-1 knock out, in patients with relapsed or refractory B cell non-Hodgkin lymphoma (ANTLER study).” The findings are scheduled to be presented by Loretta J. Nastoupil, MD, of the University of Texas MD Anderson Cancer Center, according to Caribou Biosciences.
Dr. Gauthier had no financial conflicts to disclose. Dr. Galal had no financial conflicts to disclose.
In the first-in-human, phase 1 open-label study, known as ANTLER, 5 out of 5 patients with relapsed or refractory B cell non-Hodgkin lymphoma (r/r B-NHL) responded to a single dose of CB-010, an allogeneic CAR-T cell therapy designed to boost antitumor activity, according to the company.
The use of chimeric antigen receptor (CAR) T-cell therapy involves taking T cells out of the body, reprogramming them with CAR to better equip them to kill cancer cells, and putting them back into the body.
The study consists of two sections: an initial dose escalation following a 3 + 3 design, with prespecified, increasing doses, followed by an expanded trial in which all patients receive CB-010 at the dose determined in the first section.
The study population included 6 adults with r/r B-NHL who had relapsed after previous treatment with a median of 3 prior therapies. At baseline, all 6 patients underwent a lymphodepletion regimen consisting of cyclophosphamide at 60 mg/kg/day for 2 days, followed by 5 days of fludarabine at 25 mg/m2/day.
Then all patients received a single dose of 40x106 CAR-T cells. As of the Feb. 23, 2022, data cutoff date, 5 of the 6 patients had completed the 28-day dose-limiting toxicity (DLT) evaluation period. All 5 patients (100%) achieved a response; 4 achieved complete response and 1 achieved partial response. All 4 of the complete responders had ongoing complete response at 3 months, and the longest measured complete response was 6 months, according to the company.
“We are excited to see a 100% overall response rate with CB-010 at dose level 1 for these patients who have limited treatment options,” said Dr. Syed Rizvi, chief medical officer for Caribou Biosciences, in the press release. “We believe this initial level of activity is unparalleled for a single, starting dose of cell therapy. CB-010 was generally well-tolerated, with adverse events routinely observed in autologous or allogeneic anti-CD19 CAR-T cell therapies,” he said.
Based on the promising safety and efficacy results, the company is enrolling patients in a second cohort for treatment at dose level 2 (80x106 CAR-T cells), according to the news release.
Another allogeneic CAR-T cell therapy known as ALLO-501A is being studied in a similar trial conducted by the Moffitt Cancer Center.
Overall, CB-010 was well-tolerated, according to Caribou Biosciences. No cases of graft-versus-host disease were reported. A total of 3 patients developed grade 3 or 4 adverse events (AEs) within the first 28 days; the most common were neutropenia (50%), thrombocytopenia (33%), anemia (17%), and hypogammaglobulinemia (17%). One patient experienced both grade 1 cytokine release syndrome (CRS) and grade 3 Immune effector cell-Associated Neurotoxicity Syndrome (ICANS). This response was characterized as a dose-limiting toxicity. The patient was treated with tocilizumab and steroids, recovered within 39 hours, and went on to achieve a complete response, according to the company.
Although the safety profile in the current study was promising, prior research suggest that concerns associated with CRS and ICANS should not be ignored and may be barriers to treatment.
In an article published in Bone Marrow Transplant in 2021, Dr. Vipul Sheth and Dr. Jordan Gauthier of the Fred Hutchinson Cancer Center, Seattle, noted that adverse effects may remain a challenge to widespread use of CAR-T in patients with refractory or relapsed acute lymphoblastic leukemia, for which it has been approved by the U.S. Food and Drug Administration and several European agencies. However, “there is mounting evidence that earlier, and potentially more targeted, interventions can reduce these toxicities,” they wrote.
Study provides solid stepping stone
“CRS and ICANS are mild in most patients but can be severe and sometimes life-threatening in a subset of patients undergoing CD19 CAR T-cell therapy,” Dr. Gauthier said in an interview. “Different strategies are being investigated to mitigate or treat severe toxicities, such as the use of prophylactic corticosteroids, anakinra, lenzilumab, itacitinib. I am hopeful we will soon manage to prevent toxicities while maintaining potent anti-tumor effects,” he said.
“While autologous CD19 CAR-T cells have high efficacy in patients with refractory/relapsed large B-cell lymphoma, product manufacturing remains a complicated and lengthy process in the autologous setting,” Dr. Gauthier noted. “Commercial CAR T-cell manufacturing takes approximately 3-4 weeks, sometimes longer. Some patients won’t survive long enough to receive their infusion. In some patients, T-cell function is dramatically impaired, due to prior therapies or to the disease itself,” he said.
Dr. Gauthier said he was not surprised but that he was encouraged by the apparent early success of the ANTLER study. “The proof-of-concept that allogeneic CD19-targeted CAR T cells can induce high response rates in r/r LBCL has already been established,” he said. “Having said that, it is comforting to see prior findings confirmed by this new study, and those results are exciting for the field,” he added.
As for additional research, “we need longer follow-up after allogeneic CD19-targeted CAR T-cell therapy to ensure responses are durable,” Dr. Gauthier explained. “We also need to better understand the biology driving the antitumor effects and the side effects of CAR T-cells. This will help us build more efficacious and safer CAR T-cell therapies,” he said.
Response and side effects show promise for future research
The therapy is “the best CAR-T product” that clinicians can provide for patients knowing that autologous CAR-T works, said Dr. Ahmed Galal, of Duke University, Durham, N.C., in an interview. The current research supports the use of this treatment immediately for patients, he added.
Dr. Galal said he was somewhat surprised, but pleasantly so, by the 100% response rate. This rate is likely because of the small number of patients and may not hold up in further research, but “even 90% would be an amazing achievement,” he said. The tolerable safety profile is encouraging as well, he emphasized. Dr. Galal said that he did not foresee any real barriers to expanded use of the therapy and that technology should make it easier to deliver at authorized centers.
Limitations to the current study are those common to all phase 1 trials, such as the strict inclusion criteria, Dr. Galal said. As research progresses to phase 2, “I don’t think it will be an obstacle to find patients,” he said. However, patients should be aware of side effects, and clinicians should maintain a culture of education to help them understand the value of the therapy, he added.
The complete data from the preliminary findings are scheduled to be presented at the European Hematology Association (EHA) 2022 Hybrid Congress, Vienna, in June, as abstract P1455, titled “First-in-human trial of CB-010, a CRISPR-edited allogeneic anti-CD19 CAR-T cell therapy with a PD-1 knock out, in patients with relapsed or refractory B cell non-Hodgkin lymphoma (ANTLER study).” The findings are scheduled to be presented by Loretta J. Nastoupil, MD, of the University of Texas MD Anderson Cancer Center, according to Caribou Biosciences.
Dr. Gauthier had no financial conflicts to disclose. Dr. Galal had no financial conflicts to disclose.
In the first-in-human, phase 1 open-label study, known as ANTLER, 5 out of 5 patients with relapsed or refractory B cell non-Hodgkin lymphoma (r/r B-NHL) responded to a single dose of CB-010, an allogeneic CAR-T cell therapy designed to boost antitumor activity, according to the company.
The use of chimeric antigen receptor (CAR) T-cell therapy involves taking T cells out of the body, reprogramming them with CAR to better equip them to kill cancer cells, and putting them back into the body.
The study consists of two sections: an initial dose escalation following a 3 + 3 design, with prespecified, increasing doses, followed by an expanded trial in which all patients receive CB-010 at the dose determined in the first section.
The study population included 6 adults with r/r B-NHL who had relapsed after previous treatment with a median of 3 prior therapies. At baseline, all 6 patients underwent a lymphodepletion regimen consisting of cyclophosphamide at 60 mg/kg/day for 2 days, followed by 5 days of fludarabine at 25 mg/m2/day.
Then all patients received a single dose of 40x106 CAR-T cells. As of the Feb. 23, 2022, data cutoff date, 5 of the 6 patients had completed the 28-day dose-limiting toxicity (DLT) evaluation period. All 5 patients (100%) achieved a response; 4 achieved complete response and 1 achieved partial response. All 4 of the complete responders had ongoing complete response at 3 months, and the longest measured complete response was 6 months, according to the company.
“We are excited to see a 100% overall response rate with CB-010 at dose level 1 for these patients who have limited treatment options,” said Dr. Syed Rizvi, chief medical officer for Caribou Biosciences, in the press release. “We believe this initial level of activity is unparalleled for a single, starting dose of cell therapy. CB-010 was generally well-tolerated, with adverse events routinely observed in autologous or allogeneic anti-CD19 CAR-T cell therapies,” he said.
Based on the promising safety and efficacy results, the company is enrolling patients in a second cohort for treatment at dose level 2 (80x106 CAR-T cells), according to the news release.
Another allogeneic CAR-T cell therapy known as ALLO-501A is being studied in a similar trial conducted by the Moffitt Cancer Center.
Overall, CB-010 was well-tolerated, according to Caribou Biosciences. No cases of graft-versus-host disease were reported. A total of 3 patients developed grade 3 or 4 adverse events (AEs) within the first 28 days; the most common were neutropenia (50%), thrombocytopenia (33%), anemia (17%), and hypogammaglobulinemia (17%). One patient experienced both grade 1 cytokine release syndrome (CRS) and grade 3 Immune effector cell-Associated Neurotoxicity Syndrome (ICANS). This response was characterized as a dose-limiting toxicity. The patient was treated with tocilizumab and steroids, recovered within 39 hours, and went on to achieve a complete response, according to the company.
Although the safety profile in the current study was promising, prior research suggest that concerns associated with CRS and ICANS should not be ignored and may be barriers to treatment.
In an article published in Bone Marrow Transplant in 2021, Dr. Vipul Sheth and Dr. Jordan Gauthier of the Fred Hutchinson Cancer Center, Seattle, noted that adverse effects may remain a challenge to widespread use of CAR-T in patients with refractory or relapsed acute lymphoblastic leukemia, for which it has been approved by the U.S. Food and Drug Administration and several European agencies. However, “there is mounting evidence that earlier, and potentially more targeted, interventions can reduce these toxicities,” they wrote.
Study provides solid stepping stone
“CRS and ICANS are mild in most patients but can be severe and sometimes life-threatening in a subset of patients undergoing CD19 CAR T-cell therapy,” Dr. Gauthier said in an interview. “Different strategies are being investigated to mitigate or treat severe toxicities, such as the use of prophylactic corticosteroids, anakinra, lenzilumab, itacitinib. I am hopeful we will soon manage to prevent toxicities while maintaining potent anti-tumor effects,” he said.
“While autologous CD19 CAR-T cells have high efficacy in patients with refractory/relapsed large B-cell lymphoma, product manufacturing remains a complicated and lengthy process in the autologous setting,” Dr. Gauthier noted. “Commercial CAR T-cell manufacturing takes approximately 3-4 weeks, sometimes longer. Some patients won’t survive long enough to receive their infusion. In some patients, T-cell function is dramatically impaired, due to prior therapies or to the disease itself,” he said.
Dr. Gauthier said he was not surprised but that he was encouraged by the apparent early success of the ANTLER study. “The proof-of-concept that allogeneic CD19-targeted CAR T cells can induce high response rates in r/r LBCL has already been established,” he said. “Having said that, it is comforting to see prior findings confirmed by this new study, and those results are exciting for the field,” he added.
As for additional research, “we need longer follow-up after allogeneic CD19-targeted CAR T-cell therapy to ensure responses are durable,” Dr. Gauthier explained. “We also need to better understand the biology driving the antitumor effects and the side effects of CAR T-cells. This will help us build more efficacious and safer CAR T-cell therapies,” he said.
Response and side effects show promise for future research
The therapy is “the best CAR-T product” that clinicians can provide for patients knowing that autologous CAR-T works, said Dr. Ahmed Galal, of Duke University, Durham, N.C., in an interview. The current research supports the use of this treatment immediately for patients, he added.
Dr. Galal said he was somewhat surprised, but pleasantly so, by the 100% response rate. This rate is likely because of the small number of patients and may not hold up in further research, but “even 90% would be an amazing achievement,” he said. The tolerable safety profile is encouraging as well, he emphasized. Dr. Galal said that he did not foresee any real barriers to expanded use of the therapy and that technology should make it easier to deliver at authorized centers.
Limitations to the current study are those common to all phase 1 trials, such as the strict inclusion criteria, Dr. Galal said. As research progresses to phase 2, “I don’t think it will be an obstacle to find patients,” he said. However, patients should be aware of side effects, and clinicians should maintain a culture of education to help them understand the value of the therapy, he added.
The complete data from the preliminary findings are scheduled to be presented at the European Hematology Association (EHA) 2022 Hybrid Congress, Vienna, in June, as abstract P1455, titled “First-in-human trial of CB-010, a CRISPR-edited allogeneic anti-CD19 CAR-T cell therapy with a PD-1 knock out, in patients with relapsed or refractory B cell non-Hodgkin lymphoma (ANTLER study).” The findings are scheduled to be presented by Loretta J. Nastoupil, MD, of the University of Texas MD Anderson Cancer Center, according to Caribou Biosciences.
Dr. Gauthier had no financial conflicts to disclose. Dr. Galal had no financial conflicts to disclose.
Pembrolizumab before surgery improves survival in early triple negative breast cancer
for improving survival in patients with early triple negative breast cancer (TNBC).
The findings were presented in Chicago June 4 and 5 at the annual meeting of the American Society of Clinical Oncology by study author Lajos Pusztai, MD, D.Phil, director of Breast Cancer Translational Research at Yale University, New Haven, Conn.
KEYNOTE-522 is the first prospective, randomized, placebo-controlled phase 3 trial of pembrolizumab for early-stage TNBC in the neoadjuvant and adjuvant setting.
The study included 1,174 patients (median age 49 years) with previously untreated stage II or III triple-negative breast cancer. Patients were randomly assigned to receive neoadjuvant therapy with four cycles of pembrolizumab (200 mg) or placebo every 3 weeks plus paclitaxel and carboplatin, followed by four cycles of pembrolizumab or placebo plus doxorubicin-cyclophosphamide or epirubicin-cyclophosphamide. After surgery, patients received pembrolizumab or placebeo for 9 cycles or until recurrence or unacceptable toxicity. The primary end points were pathological complete response and event-free survival.
A total of 784 patients were treated with pembrolizumab and chemotherapy, and the second group of 390 patients received a placebo and chemotherapy. After surgery, patients received adjuvant pembrolizumab (pembrolizumab-chemotherapy group) or placebo and chemotherapy for every 3 weeks for up to nine cycles.
The estimated event-free survival at 36 months was 84.5% in the pembrolizumab-chemotherapy group, compared with 76.8% in the placebo-chemotherapy group (hazard ratio for event or death, 0.63; 95% confidence interval, 0.48 to 0.82; P <0.001). Adverse events occurred predominantly during the neoadjuvant phase and were consistent with the established safety profiles of pembrolizumab and chemotherapy.
At the first interim analysis, 64.8% achieved pathological complete response in the pembrolizumab group versus 51.2% in the placebo group. At the fourth interim analysis at 36 months, event-free survival was 76.8% in the placebo arm and 84.5% in the pembrolizumab arm. RCB-0 status was achieved by 63.4% and 56.2% of patients in the pembrolizumab and placebo arms, respectively.
Pembrolizumab did contribute immune-related adverse events, mostly grades 1-2, in about 17% of patients with thyroid function abnormalities most common with most occurring 20 weeks prior to surgical treatment.
Treatment with pembrolizumab added to chemotherapy, compared with chemotherapy alone, shifted residual cancer burden to lower categories across the entire spectrum of patients in the trial.
The hazard ratio for event-free survival with RCB-0, which Dr. Pusztai said is equivalent to a pathologic complete response (pCR), was 0.70 (0.38-1.31). For RCB-1 (minimal residual disease) it was 0.92 (0.39-2.20); for RCB-2 (moderate residual disease) it was 0.52 (0.32-0.82); and for RCB-3 (extensive residual disease) it was 1.24 (0.69-2.23).
“The most important finding is that patients in RCB-2, a group with a moderate amount of residual disease, experienced significant improvement with pembrolizumab. This clearly indicates not only that pembrolizumab leads to higher pCR rates but also that the pembrolizumCR/RCB-0 ... extends to patients who do not achieve pCR,” Dr. Pusztai said.
The benefit, he suggested, could be a result of the adjuvant pembrolizumab maintenance phase.
Patients in the RCB-3 category do poorly regardless of treatment (EFS of 34.6 % and 26.2% in the pembrolizumab and placebo arms, respectively).
“The RCB-3 population represents an unmet medical need, and they will need better drugs, and additional postoperative adjuvant therapy,” Dr. Pusztai said. The current standard of care is capecitabine for 6-8 cycles. Emerging new therapies, such as antibody drug conjugates, will be tested, he said.
In terms of limitations, adjuvant capecitabine was not allowed. “It remains uncertain how much better the RCB-2 and -3 patient outcomes would have been if capecitabine were administered,” he said.
The study was funded by Merck Sharp and Dohme, a subsidiary of Merck. Dr. Pusztai has received consulting fees and honoraria from Merck.
for improving survival in patients with early triple negative breast cancer (TNBC).
The findings were presented in Chicago June 4 and 5 at the annual meeting of the American Society of Clinical Oncology by study author Lajos Pusztai, MD, D.Phil, director of Breast Cancer Translational Research at Yale University, New Haven, Conn.
KEYNOTE-522 is the first prospective, randomized, placebo-controlled phase 3 trial of pembrolizumab for early-stage TNBC in the neoadjuvant and adjuvant setting.
The study included 1,174 patients (median age 49 years) with previously untreated stage II or III triple-negative breast cancer. Patients were randomly assigned to receive neoadjuvant therapy with four cycles of pembrolizumab (200 mg) or placebo every 3 weeks plus paclitaxel and carboplatin, followed by four cycles of pembrolizumab or placebo plus doxorubicin-cyclophosphamide or epirubicin-cyclophosphamide. After surgery, patients received pembrolizumab or placebeo for 9 cycles or until recurrence or unacceptable toxicity. The primary end points were pathological complete response and event-free survival.
A total of 784 patients were treated with pembrolizumab and chemotherapy, and the second group of 390 patients received a placebo and chemotherapy. After surgery, patients received adjuvant pembrolizumab (pembrolizumab-chemotherapy group) or placebo and chemotherapy for every 3 weeks for up to nine cycles.
The estimated event-free survival at 36 months was 84.5% in the pembrolizumab-chemotherapy group, compared with 76.8% in the placebo-chemotherapy group (hazard ratio for event or death, 0.63; 95% confidence interval, 0.48 to 0.82; P <0.001). Adverse events occurred predominantly during the neoadjuvant phase and were consistent with the established safety profiles of pembrolizumab and chemotherapy.
At the first interim analysis, 64.8% achieved pathological complete response in the pembrolizumab group versus 51.2% in the placebo group. At the fourth interim analysis at 36 months, event-free survival was 76.8% in the placebo arm and 84.5% in the pembrolizumab arm. RCB-0 status was achieved by 63.4% and 56.2% of patients in the pembrolizumab and placebo arms, respectively.
Pembrolizumab did contribute immune-related adverse events, mostly grades 1-2, in about 17% of patients with thyroid function abnormalities most common with most occurring 20 weeks prior to surgical treatment.
Treatment with pembrolizumab added to chemotherapy, compared with chemotherapy alone, shifted residual cancer burden to lower categories across the entire spectrum of patients in the trial.
The hazard ratio for event-free survival with RCB-0, which Dr. Pusztai said is equivalent to a pathologic complete response (pCR), was 0.70 (0.38-1.31). For RCB-1 (minimal residual disease) it was 0.92 (0.39-2.20); for RCB-2 (moderate residual disease) it was 0.52 (0.32-0.82); and for RCB-3 (extensive residual disease) it was 1.24 (0.69-2.23).
“The most important finding is that patients in RCB-2, a group with a moderate amount of residual disease, experienced significant improvement with pembrolizumab. This clearly indicates not only that pembrolizumab leads to higher pCR rates but also that the pembrolizumCR/RCB-0 ... extends to patients who do not achieve pCR,” Dr. Pusztai said.
The benefit, he suggested, could be a result of the adjuvant pembrolizumab maintenance phase.
Patients in the RCB-3 category do poorly regardless of treatment (EFS of 34.6 % and 26.2% in the pembrolizumab and placebo arms, respectively).
“The RCB-3 population represents an unmet medical need, and they will need better drugs, and additional postoperative adjuvant therapy,” Dr. Pusztai said. The current standard of care is capecitabine for 6-8 cycles. Emerging new therapies, such as antibody drug conjugates, will be tested, he said.
In terms of limitations, adjuvant capecitabine was not allowed. “It remains uncertain how much better the RCB-2 and -3 patient outcomes would have been if capecitabine were administered,” he said.
The study was funded by Merck Sharp and Dohme, a subsidiary of Merck. Dr. Pusztai has received consulting fees and honoraria from Merck.
for improving survival in patients with early triple negative breast cancer (TNBC).
The findings were presented in Chicago June 4 and 5 at the annual meeting of the American Society of Clinical Oncology by study author Lajos Pusztai, MD, D.Phil, director of Breast Cancer Translational Research at Yale University, New Haven, Conn.
KEYNOTE-522 is the first prospective, randomized, placebo-controlled phase 3 trial of pembrolizumab for early-stage TNBC in the neoadjuvant and adjuvant setting.
The study included 1,174 patients (median age 49 years) with previously untreated stage II or III triple-negative breast cancer. Patients were randomly assigned to receive neoadjuvant therapy with four cycles of pembrolizumab (200 mg) or placebo every 3 weeks plus paclitaxel and carboplatin, followed by four cycles of pembrolizumab or placebo plus doxorubicin-cyclophosphamide or epirubicin-cyclophosphamide. After surgery, patients received pembrolizumab or placebeo for 9 cycles or until recurrence or unacceptable toxicity. The primary end points were pathological complete response and event-free survival.
A total of 784 patients were treated with pembrolizumab and chemotherapy, and the second group of 390 patients received a placebo and chemotherapy. After surgery, patients received adjuvant pembrolizumab (pembrolizumab-chemotherapy group) or placebo and chemotherapy for every 3 weeks for up to nine cycles.
The estimated event-free survival at 36 months was 84.5% in the pembrolizumab-chemotherapy group, compared with 76.8% in the placebo-chemotherapy group (hazard ratio for event or death, 0.63; 95% confidence interval, 0.48 to 0.82; P <0.001). Adverse events occurred predominantly during the neoadjuvant phase and were consistent with the established safety profiles of pembrolizumab and chemotherapy.
At the first interim analysis, 64.8% achieved pathological complete response in the pembrolizumab group versus 51.2% in the placebo group. At the fourth interim analysis at 36 months, event-free survival was 76.8% in the placebo arm and 84.5% in the pembrolizumab arm. RCB-0 status was achieved by 63.4% and 56.2% of patients in the pembrolizumab and placebo arms, respectively.
Pembrolizumab did contribute immune-related adverse events, mostly grades 1-2, in about 17% of patients with thyroid function abnormalities most common with most occurring 20 weeks prior to surgical treatment.
Treatment with pembrolizumab added to chemotherapy, compared with chemotherapy alone, shifted residual cancer burden to lower categories across the entire spectrum of patients in the trial.
The hazard ratio for event-free survival with RCB-0, which Dr. Pusztai said is equivalent to a pathologic complete response (pCR), was 0.70 (0.38-1.31). For RCB-1 (minimal residual disease) it was 0.92 (0.39-2.20); for RCB-2 (moderate residual disease) it was 0.52 (0.32-0.82); and for RCB-3 (extensive residual disease) it was 1.24 (0.69-2.23).
“The most important finding is that patients in RCB-2, a group with a moderate amount of residual disease, experienced significant improvement with pembrolizumab. This clearly indicates not only that pembrolizumab leads to higher pCR rates but also that the pembrolizumCR/RCB-0 ... extends to patients who do not achieve pCR,” Dr. Pusztai said.
The benefit, he suggested, could be a result of the adjuvant pembrolizumab maintenance phase.
Patients in the RCB-3 category do poorly regardless of treatment (EFS of 34.6 % and 26.2% in the pembrolizumab and placebo arms, respectively).
“The RCB-3 population represents an unmet medical need, and they will need better drugs, and additional postoperative adjuvant therapy,” Dr. Pusztai said. The current standard of care is capecitabine for 6-8 cycles. Emerging new therapies, such as antibody drug conjugates, will be tested, he said.
In terms of limitations, adjuvant capecitabine was not allowed. “It remains uncertain how much better the RCB-2 and -3 patient outcomes would have been if capecitabine were administered,” he said.
The study was funded by Merck Sharp and Dohme, a subsidiary of Merck. Dr. Pusztai has received consulting fees and honoraria from Merck.
FROM ASCO 2022



