User login
Coalescing Hyperkeratotic Plaques and Papules
The Diagnosis: X-Linked Ichthyosis
Immunohistochemical staining of a punch biopsy specimen from the left foot with cytokeratin markers AE1/3, 5/6, and 19 showed normal positive uptake. Further workup was recommended, and the patient was referred to genetics for an ichthyosis gene panel. DNA sequencing revealed a c.1121G>A transition in exon 10 of the steroid sulfatase gene, STS, consistent with X-linked ichthyosis (XLI).
X-linked ichthyosis, also known as steroid sulfatase deficiency and X-linked recessive ichthyosis, is a congenital skin disorder classified in 1965 by Wells and Kerr.1 Ichthyoses are a heterogenous group of acquired and congenital disorders of keratinization that manifest with xerosis, hyperkeratosis, and scaling.2 Of more than 20 ichthyoses, XLI is the second most common ichthyosis, with a prevalence of 1 in 6000 males.3 X-linked ichthyosis occurs almost exclusively in males, and although females can be carriers, they rarely exhibit skin manifestations.4
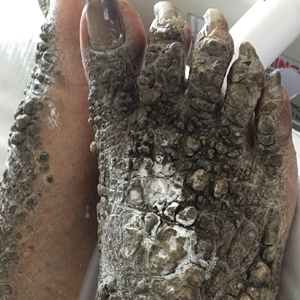
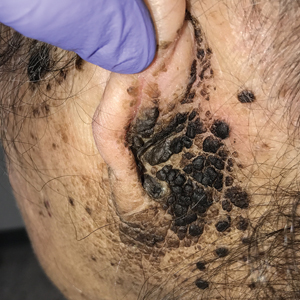
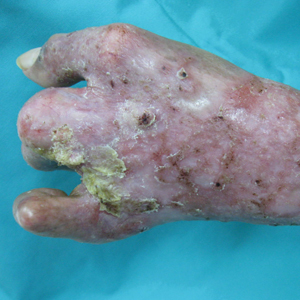
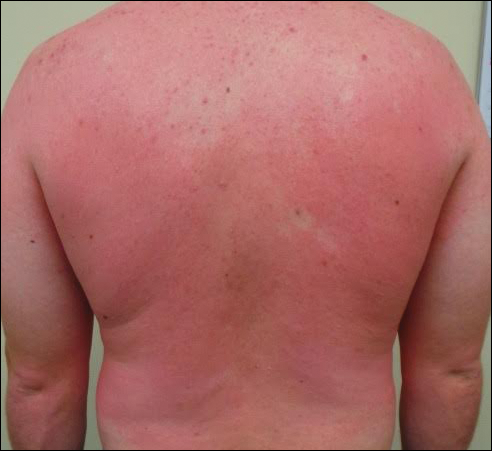
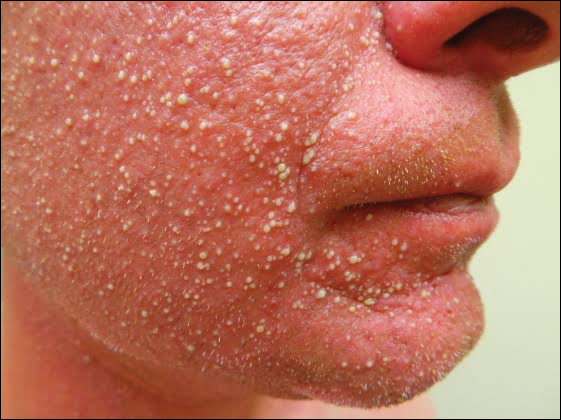
X-linked ichthyosis is caused by either a partial or full deletion or mutation in the STS gene on the X chromosome.2 The absence of STS activity results in the accumulation of cholesterol sulfate in the stratum corneum, leading to corneocyte cohesion, hyperkeratosis, and impaired skin permeability. The most common clinical phenotype is characterized by polygonal scales concentrated on the upper and lower extremities as well as the trunk (Figure), consistent with our patient's clinical presentation.5
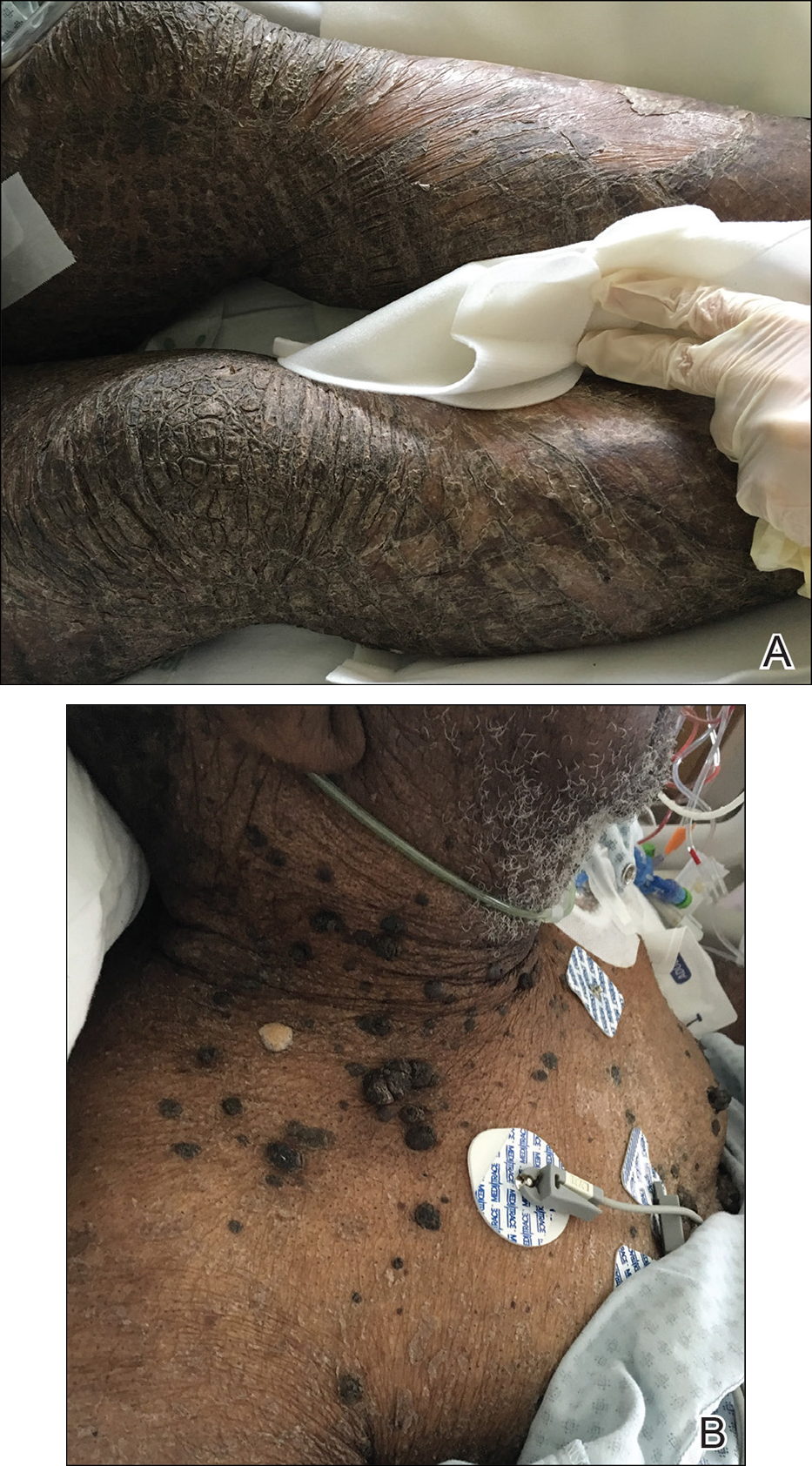
anterior knees (A) as well as large exophytic papules on the upper
chest and neck (B).
X-linked ichthyosis typically presents in the first 6 months of life as generalized desquamation and xerosis that progresses to fine scaling on the trunk and extremities, more commonly and heavily involving the legs; however, the extensor surfaces of the arms also may be affected.6 After the neonatal period, fine scaling persists on the trunk and extremities, but scales often become coarser and darker over time. Although scaling is generalized, it typically spares the antecubital and popliteal fossae, palms, soles, and midface. The lateral face, axillae, and neck always remain involved.4 The most common extracutaneous manifestations of XLI affect the ocular, genitourinary, and cognitive/behavioral systems. Patients can develop corneal comma-shaped opacities, hypogonadism, cryptorchidism, and an increased risk for testicular cancer. Female carriers may have prolonged delivery of affected neonates.2,5,7-9 Given the unrelated debilitating neurologic consequences of our patient's presenting subarachnoid hemorrhage, further workup was not pursued into these associations.
Although XLI is most commonly diagnosed in early childhood, it also must be considered in adult patients presenting with severe scaling of the trunk, arms, and legs who have not had prior dermatologic workup. Given the similarity of XLI presentation to other ichthyoses, particularly ichthyosis vulgaris, lamellar ichthyosis, and ichthyosis bullosa of Siemens, genetic analysis is the most accurate diagnostic tool and should be considered in patients with an atypical presentation. Rupioid psoriasis also may be considered and can be confirmed on biopsy. Diagnosis of XLI should prompt symptomatic treatment, genetic counseling, and workup for extracutaneous manifestations.
- Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol. 1965;92:1-6.
- Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62:480-485.
- Hernández-Martín A, González-Sarmiento R, De Unamuno P. X-linked ichthyosis: an update. Br J Dermatol. 1999;141:617-627.
- Elias PM, Williams ML, Choi EH, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis [published online November 27, 2013]. Biochim Biophys Acta. 2014;1841:353-361.
- Wu B, Paller AS. Ichthyosis, X-Linked. Treasure Island, FL: StatPearls Publishing LLC; 2019.
- Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5. doi:10.12688/f1000research.8584.1.
- Baek WS, Aypar U. Case report neurological manifestations of X-linked ichthyosis: case report and review of the literature [published online August 13, 2017]. 2017;2017:9086408.
- Brookes KJ, Hawi Z, Park J, et al. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153:1417-1424.
- Kent L, Emerton J, Bhadravathi V, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519-524.
The Diagnosis: X-Linked Ichthyosis
Immunohistochemical staining of a punch biopsy specimen from the left foot with cytokeratin markers AE1/3, 5/6, and 19 showed normal positive uptake. Further workup was recommended, and the patient was referred to genetics for an ichthyosis gene panel. DNA sequencing revealed a c.1121G>A transition in exon 10 of the steroid sulfatase gene, STS, consistent with X-linked ichthyosis (XLI).
X-linked ichthyosis, also known as steroid sulfatase deficiency and X-linked recessive ichthyosis, is a congenital skin disorder classified in 1965 by Wells and Kerr.1 Ichthyoses are a heterogenous group of acquired and congenital disorders of keratinization that manifest with xerosis, hyperkeratosis, and scaling.2 Of more than 20 ichthyoses, XLI is the second most common ichthyosis, with a prevalence of 1 in 6000 males.3 X-linked ichthyosis occurs almost exclusively in males, and although females can be carriers, they rarely exhibit skin manifestations.4
X-linked ichthyosis is caused by either a partial or full deletion or mutation in the STS gene on the X chromosome.2 The absence of STS activity results in the accumulation of cholesterol sulfate in the stratum corneum, leading to corneocyte cohesion, hyperkeratosis, and impaired skin permeability. The most common clinical phenotype is characterized by polygonal scales concentrated on the upper and lower extremities as well as the trunk (Figure), consistent with our patient's clinical presentation.5

anterior knees (A) as well as large exophytic papules on the upper
chest and neck (B).
X-linked ichthyosis typically presents in the first 6 months of life as generalized desquamation and xerosis that progresses to fine scaling on the trunk and extremities, more commonly and heavily involving the legs; however, the extensor surfaces of the arms also may be affected.6 After the neonatal period, fine scaling persists on the trunk and extremities, but scales often become coarser and darker over time. Although scaling is generalized, it typically spares the antecubital and popliteal fossae, palms, soles, and midface. The lateral face, axillae, and neck always remain involved.4 The most common extracutaneous manifestations of XLI affect the ocular, genitourinary, and cognitive/behavioral systems. Patients can develop corneal comma-shaped opacities, hypogonadism, cryptorchidism, and an increased risk for testicular cancer. Female carriers may have prolonged delivery of affected neonates.2,5,7-9 Given the unrelated debilitating neurologic consequences of our patient's presenting subarachnoid hemorrhage, further workup was not pursued into these associations.
Although XLI is most commonly diagnosed in early childhood, it also must be considered in adult patients presenting with severe scaling of the trunk, arms, and legs who have not had prior dermatologic workup. Given the similarity of XLI presentation to other ichthyoses, particularly ichthyosis vulgaris, lamellar ichthyosis, and ichthyosis bullosa of Siemens, genetic analysis is the most accurate diagnostic tool and should be considered in patients with an atypical presentation. Rupioid psoriasis also may be considered and can be confirmed on biopsy. Diagnosis of XLI should prompt symptomatic treatment, genetic counseling, and workup for extracutaneous manifestations.
The Diagnosis: X-Linked Ichthyosis
Immunohistochemical staining of a punch biopsy specimen from the left foot with cytokeratin markers AE1/3, 5/6, and 19 showed normal positive uptake. Further workup was recommended, and the patient was referred to genetics for an ichthyosis gene panel. DNA sequencing revealed a c.1121G>A transition in exon 10 of the steroid sulfatase gene, STS, consistent with X-linked ichthyosis (XLI).
X-linked ichthyosis, also known as steroid sulfatase deficiency and X-linked recessive ichthyosis, is a congenital skin disorder classified in 1965 by Wells and Kerr.1 Ichthyoses are a heterogenous group of acquired and congenital disorders of keratinization that manifest with xerosis, hyperkeratosis, and scaling.2 Of more than 20 ichthyoses, XLI is the second most common ichthyosis, with a prevalence of 1 in 6000 males.3 X-linked ichthyosis occurs almost exclusively in males, and although females can be carriers, they rarely exhibit skin manifestations.4
X-linked ichthyosis is caused by either a partial or full deletion or mutation in the STS gene on the X chromosome.2 The absence of STS activity results in the accumulation of cholesterol sulfate in the stratum corneum, leading to corneocyte cohesion, hyperkeratosis, and impaired skin permeability. The most common clinical phenotype is characterized by polygonal scales concentrated on the upper and lower extremities as well as the trunk (Figure), consistent with our patient's clinical presentation.5

anterior knees (A) as well as large exophytic papules on the upper
chest and neck (B).
X-linked ichthyosis typically presents in the first 6 months of life as generalized desquamation and xerosis that progresses to fine scaling on the trunk and extremities, more commonly and heavily involving the legs; however, the extensor surfaces of the arms also may be affected.6 After the neonatal period, fine scaling persists on the trunk and extremities, but scales often become coarser and darker over time. Although scaling is generalized, it typically spares the antecubital and popliteal fossae, palms, soles, and midface. The lateral face, axillae, and neck always remain involved.4 The most common extracutaneous manifestations of XLI affect the ocular, genitourinary, and cognitive/behavioral systems. Patients can develop corneal comma-shaped opacities, hypogonadism, cryptorchidism, and an increased risk for testicular cancer. Female carriers may have prolonged delivery of affected neonates.2,5,7-9 Given the unrelated debilitating neurologic consequences of our patient's presenting subarachnoid hemorrhage, further workup was not pursued into these associations.
Although XLI is most commonly diagnosed in early childhood, it also must be considered in adult patients presenting with severe scaling of the trunk, arms, and legs who have not had prior dermatologic workup. Given the similarity of XLI presentation to other ichthyoses, particularly ichthyosis vulgaris, lamellar ichthyosis, and ichthyosis bullosa of Siemens, genetic analysis is the most accurate diagnostic tool and should be considered in patients with an atypical presentation. Rupioid psoriasis also may be considered and can be confirmed on biopsy. Diagnosis of XLI should prompt symptomatic treatment, genetic counseling, and workup for extracutaneous manifestations.
- Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol. 1965;92:1-6.
- Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62:480-485.
- Hernández-Martín A, González-Sarmiento R, De Unamuno P. X-linked ichthyosis: an update. Br J Dermatol. 1999;141:617-627.
- Elias PM, Williams ML, Choi EH, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis [published online November 27, 2013]. Biochim Biophys Acta. 2014;1841:353-361.
- Wu B, Paller AS. Ichthyosis, X-Linked. Treasure Island, FL: StatPearls Publishing LLC; 2019.
- Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5. doi:10.12688/f1000research.8584.1.
- Baek WS, Aypar U. Case report neurological manifestations of X-linked ichthyosis: case report and review of the literature [published online August 13, 2017]. 2017;2017:9086408.
- Brookes KJ, Hawi Z, Park J, et al. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153:1417-1424.
- Kent L, Emerton J, Bhadravathi V, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519-524.
- Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol. 1965;92:1-6.
- Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62:480-485.
- Hernández-Martín A, González-Sarmiento R, De Unamuno P. X-linked ichthyosis: an update. Br J Dermatol. 1999;141:617-627.
- Elias PM, Williams ML, Choi EH, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis [published online November 27, 2013]. Biochim Biophys Acta. 2014;1841:353-361.
- Wu B, Paller AS. Ichthyosis, X-Linked. Treasure Island, FL: StatPearls Publishing LLC; 2019.
- Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5. doi:10.12688/f1000research.8584.1.
- Baek WS, Aypar U. Case report neurological manifestations of X-linked ichthyosis: case report and review of the literature [published online August 13, 2017]. 2017;2017:9086408.
- Brookes KJ, Hawi Z, Park J, et al. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153:1417-1424.
- Kent L, Emerton J, Bhadravathi V, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519-524.
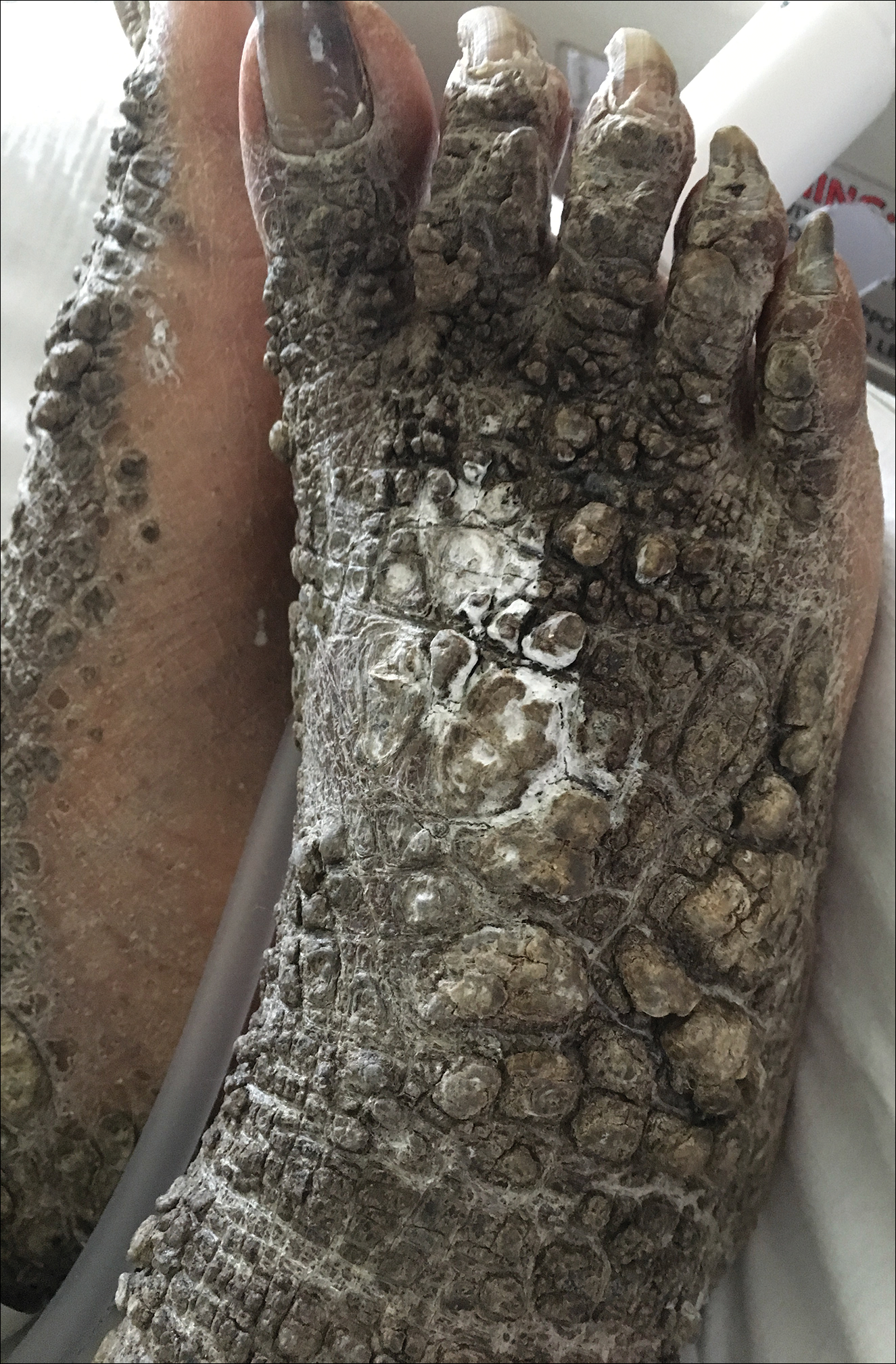
A 67-year-old man with a history of congestive heart failure, type 2 diabetes mellitus, hypertension, and schizophrenia was admitted to the hospital for subarachnoid hemorrhage and was noted to have heavy scaling on the bilateral legs. Given recent medical events, the patient was nonconversant at the time of consultation, but his daughter provided his medical history at bedside. The patient usually wore long-sleeved clothing and pants, thus no one had seen his skin in many years, and it was unclear how long the scaling had been present. His family history was notable for eczema in distant relatives but negative for comparable conditions. Physical examination revealed thick lichenified skin with many large, exophytic, brown papules (largest measured 1.5×1×1 cm) and platelike scaling on the anterior chest, abdomen, lateral arms, and forearms. Extensive coalescing hyperkeratotic plaques and papules (up to 1 cm in thickness) were present on the anterior legs and feet, and scattered verrucous brown papules were noted on the plantar aspects of the bilateral feet. A punch biopsy of the left foot revealed extensive, dense, compact, orthokeratotic hyperkeratosis with a preserved granular layer with no epidermolysis.
Lesions With a Distinct Black Pigment
The Diagnosis: Black-Spot Poison Ivy
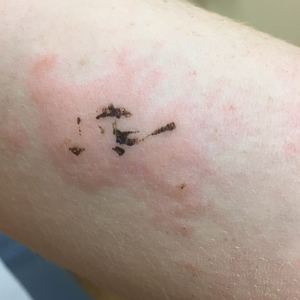
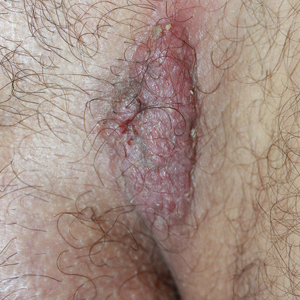
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.
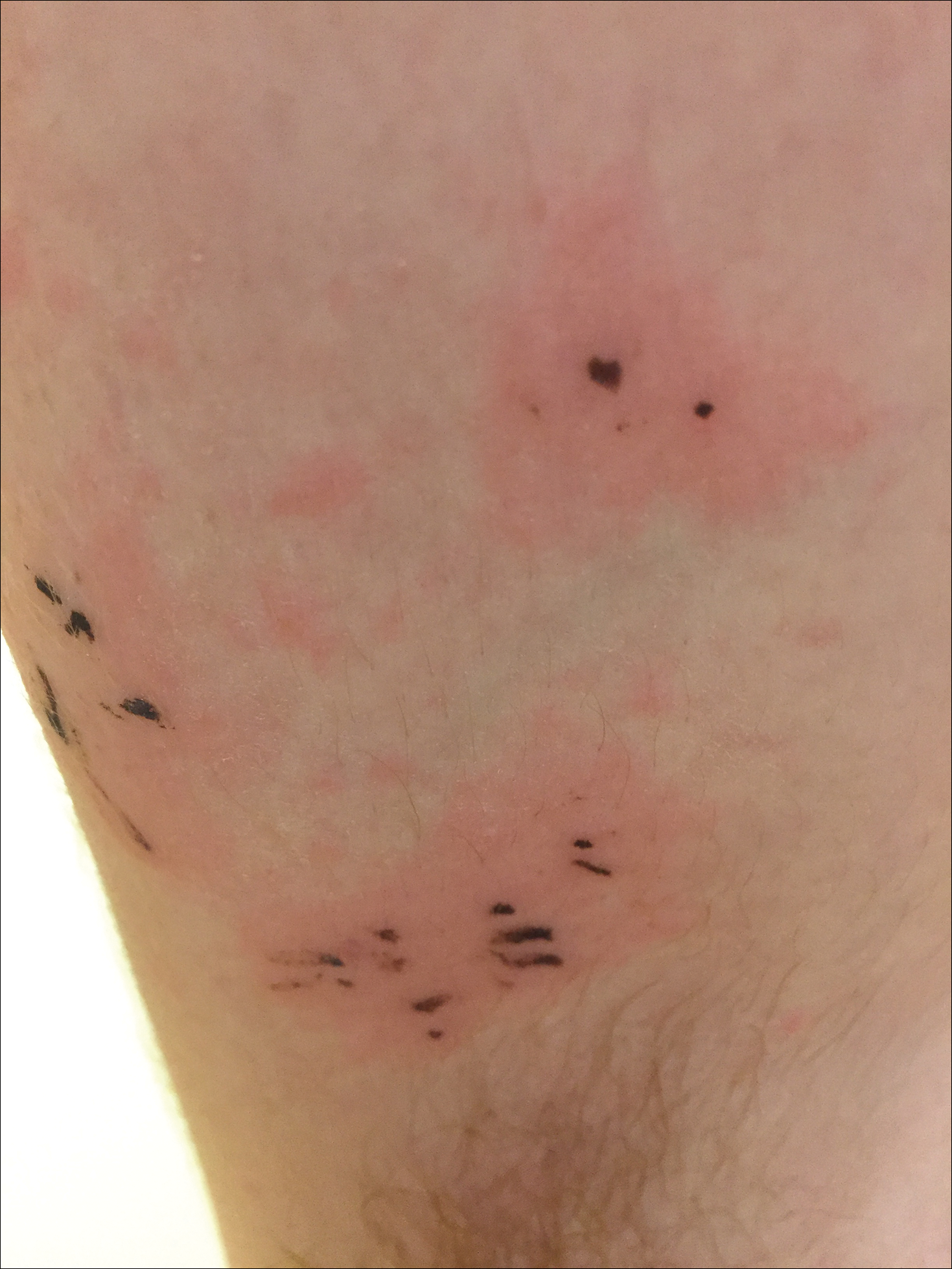
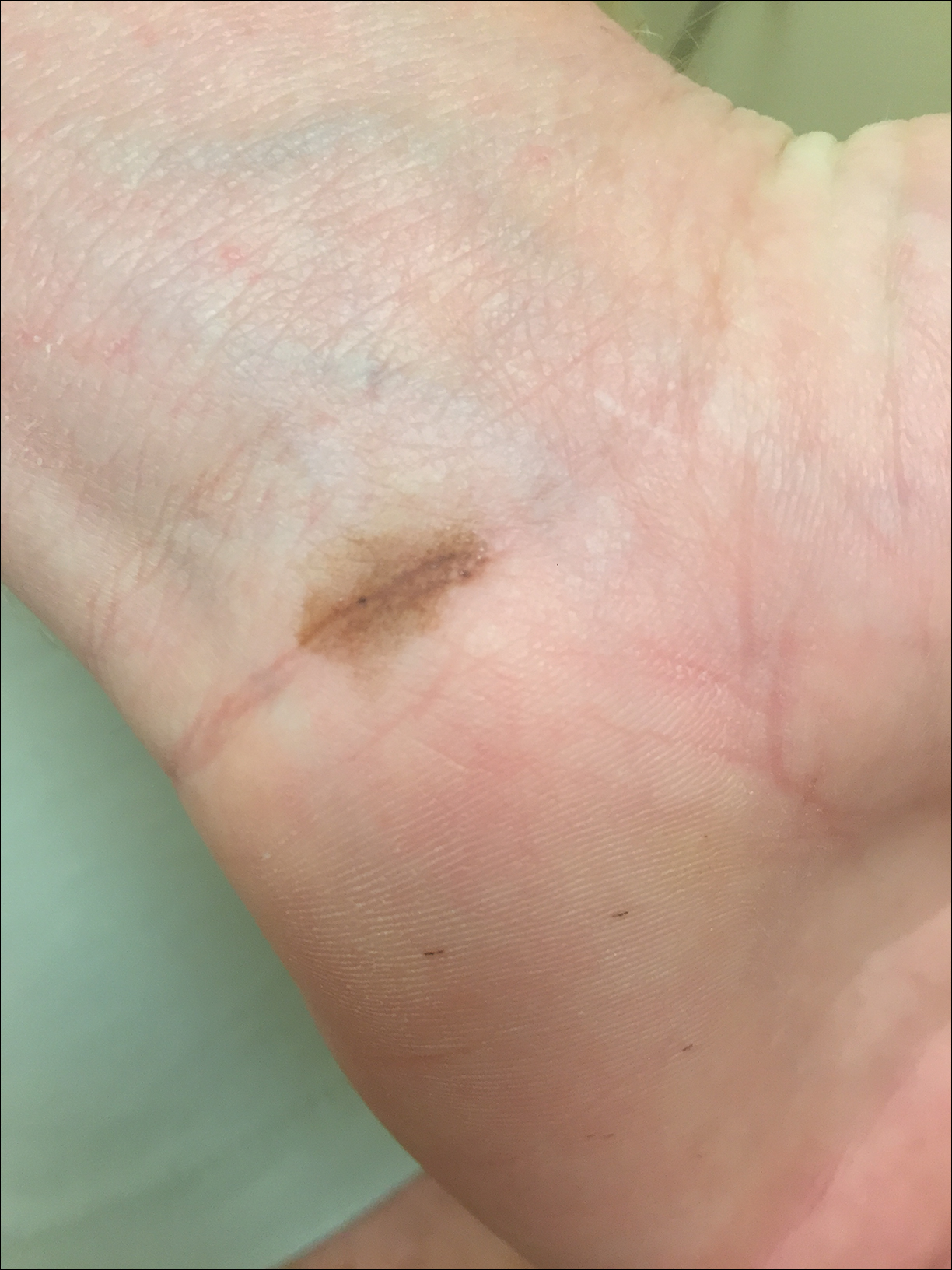
Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
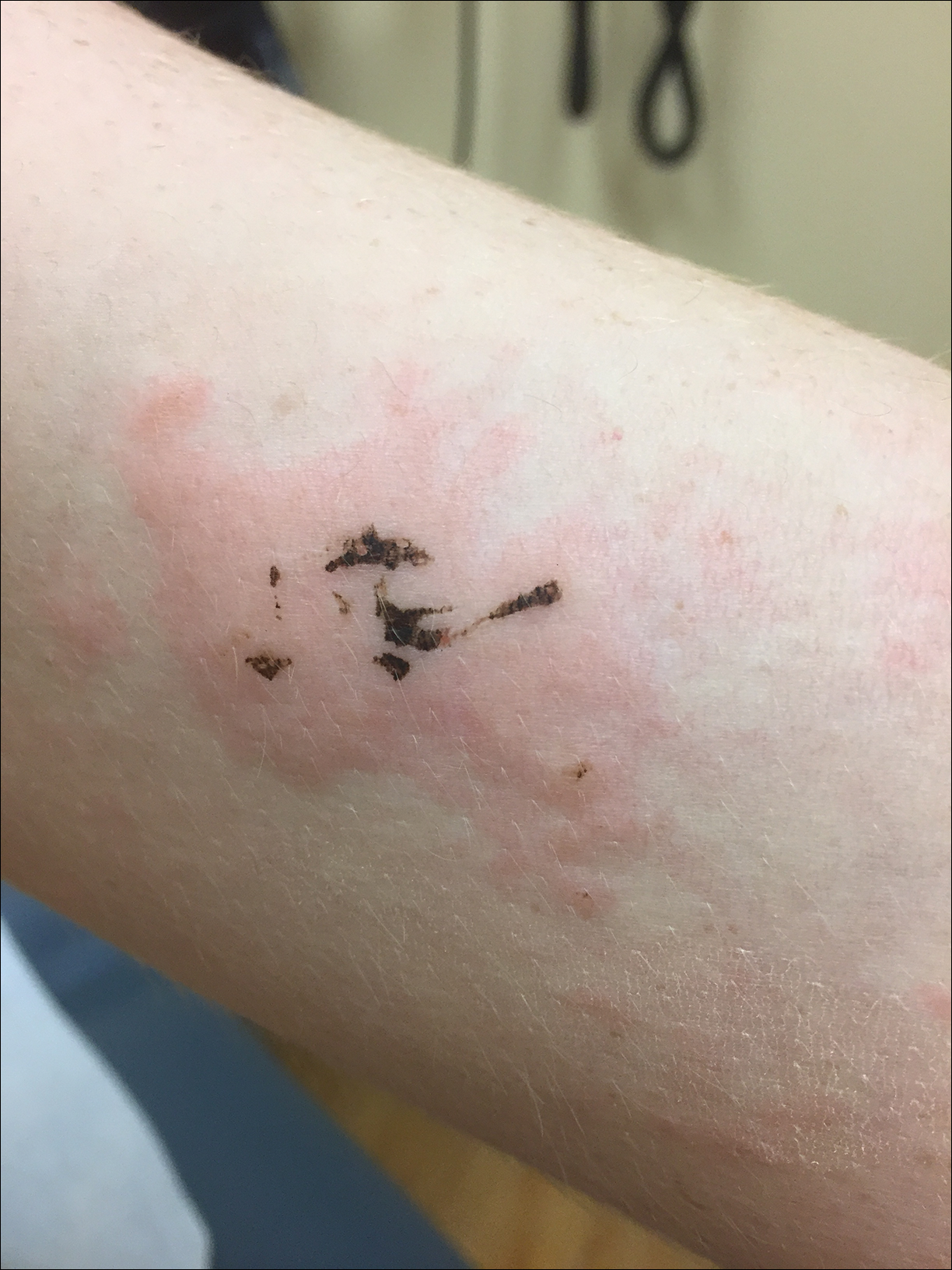
A 17-year-old adolescent boy presented to urgent care with a pruritic eruption on the bilateral arms of 1 day's duration. He was camping in the woods the night prior to presentation. On physical examination linear, erythematous, edematous plaques were observed bilaterally with overlying brown and black pigment on the arms. The pigment could not be removed with alcohol or vigorous scrubbing. The patient's condition improved with prednisone.
Diffuse Pustular Eruption Following Computed Tomography
The Diagnosis: Acute Generalized Exanthematous Pustulosis
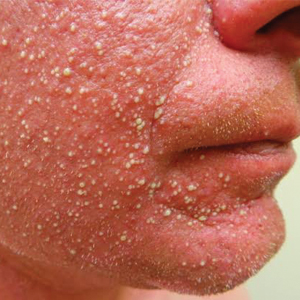
Histopathology demonstrated spongiosis with subcorneal pustules and an overlying basket-weave pattern stratum corneum. There was mild papillary dermal edema with scattered dermal neutrophils and rare eosinophils (Figure). The patient's clinical presentation and histopathology were consistent with acute generalized exanthematous pustulosis (AGEP). The inciting agent in this case was the contrast medium iopamidol. The patient was treated with a short course of prednisone, triamcinolone cream, diphenhydramine, and acetaminophen. Within 1 week the pustules and erythema had resolved.
Acute generalized exanthematous pustulosis is an uncommon T cell-mediated cutaneous reaction characterized by widespread progressive erythema with numerous nonfollicular pinpoint pustules. The patient usually is well appearing; however, he/she often will have concurrent fever and facial edema. Mucous membranes rarely are involved. Laboratory results typically are notable only for leukocytosis with neutrophilia.
The pustular eruption typically occurs within 1 to 2 days after exposure to an inciting agent1; however, this latent period can range from 1 hour to nearly 4 weeks in some studies.2 Systemic medications are the cause in approximately 90% of cases, with antibiotics being the most common category. Frequently implicated medications include β-lactams, macrolides, quinolones, sulfonamides, proton pump inhibitors, hydroxychloroquine, terbinafine, nonsteroidal anti-inflammatory drugs, diltiazem, ketoconazole, and fluconazole. Acute generalized exanthematous pustulosis also has been rarely reported following contact with mercury, viral and bacterial infections, and spider bites.3
Iodinated contrast agents have long been known to cause immediate and delayed adverse cutaneous reactions. However, one consensus study indicated that these reactions occur in only 0.05% to 0.10% of patients.4 Although rare, iodinated contrast media (eg, iopamidol, iohexol, ioversol, iodixanol, iomeprol, iobitridol, iopromide) have been reported as a cause of AGEP. A PubMed search of articles indexed for MEDLINE using the terms acute generalized exanthematous pustulosis, contrast, iodine, and iodinated revealed 10 adult cases reported in 6 articles in the English-language literature.1,5-9 The most recent articles focus on methods to identify the causative agent. If the etiology of the reaction is unclear, patch or intradermal testing can help to confirm the causative agent. These tests also can help determine similar agents to which the patient may cross-react.4,5
It can be difficult to differentiate AGEP from other cutaneous drug reactions and other nonfollicular pustular conditions. Drug-induced hypersensitivity syndrome typically presents with facial edema and a morbilliform rash. Although it can present with pustules, the latent period is longer (2-6 weeks), and there frequently are signs of multiorgan involvement including hepatic dysfunction, eosinophilia, atypical lymphocytosis, and lymphadenopathy. Patients with generalized pustular psoriasis often have a history of plaque psoriasis; the pustules are more concentrated in flexural sites; the eruption is gradual in onset; and histologically there tends to be features of psoriasis including parakeratosis, Munro microabscesses, and dilated blood vessels.10 Subcorneal pustular dermatosis also is more concentrated in flexural sites and frequently has an annular or serpiginous configuration. The onset also is gradual, and it follows a more chronic course than AGEP. Exfoliative erythroderma presents with widespread erythema and superficial desquamating scale. It often occurs in association with systemic symptoms and can be the result of a drug reaction or underlying inflammatory dermatosis such as psoriasis, mycosis fungoides, or pityriasis rubra pilaris.
Acute generalized exanthematous pustulosis usually resolves spontaneously within 2 weeks and is associated with a superficial desquamation as it clears. Appropriate treatment includes discontinuing the offending agent; monitoring for systemic involvement; and treating the patient's symptoms with antihistamines, analgesics, topical steroids, and emollients. In more severe or persistent cases, treatment with systemic steroids and tumor necrosis factor α inhibitors has been attempted, though their efficacy remains unclear. We report a case of iopamidol-induced AGEP that highlights the importance of eliciting a history of contrast exposure from a patient with suspected AGEP.
- Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis. Arch Dermatol. 2009;145:683-687.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;2015:1-8.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2016;73:843-848.
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26:144-155.
- Grandvuillemin A, Ripert C, Sgro C, et al. Iodinated contrast media-induced acute generalized exanthematous pustulosis confirmed by delayed skin tests. J Allergy Clin Immunol Pract. 2014;2:805-806.
- Bavbek S, Sözener ZÇ, Aydin Ö, et al. First case report of acute generalized exanthematous pustulosis due to intravenous iopromide. J Investig Allergol Clin Immunol. 2014;24:66-67.
- Kim SJ, Lee T, Lee YS, et al. Acute generalized exanthematous pustulosis caused by radiocontrast media. Ann Allergy Asthma Immunol. 2010;105:492-493.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. Am J Roentgenol. 2006;187:198-201.
- Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003;30:723-726.
- Halevy S, Kardaun S, Davidovici B, et al; EuroSCAR and RegiSCAR Study Group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010:163:1245-1252.
The Diagnosis: Acute Generalized Exanthematous Pustulosis
Histopathology demonstrated spongiosis with subcorneal pustules and an overlying basket-weave pattern stratum corneum. There was mild papillary dermal edema with scattered dermal neutrophils and rare eosinophils (Figure). The patient's clinical presentation and histopathology were consistent with acute generalized exanthematous pustulosis (AGEP). The inciting agent in this case was the contrast medium iopamidol. The patient was treated with a short course of prednisone, triamcinolone cream, diphenhydramine, and acetaminophen. Within 1 week the pustules and erythema had resolved.

Acute generalized exanthematous pustulosis is an uncommon T cell-mediated cutaneous reaction characterized by widespread progressive erythema with numerous nonfollicular pinpoint pustules. The patient usually is well appearing; however, he/she often will have concurrent fever and facial edema. Mucous membranes rarely are involved. Laboratory results typically are notable only for leukocytosis with neutrophilia.
The pustular eruption typically occurs within 1 to 2 days after exposure to an inciting agent1; however, this latent period can range from 1 hour to nearly 4 weeks in some studies.2 Systemic medications are the cause in approximately 90% of cases, with antibiotics being the most common category. Frequently implicated medications include β-lactams, macrolides, quinolones, sulfonamides, proton pump inhibitors, hydroxychloroquine, terbinafine, nonsteroidal anti-inflammatory drugs, diltiazem, ketoconazole, and fluconazole. Acute generalized exanthematous pustulosis also has been rarely reported following contact with mercury, viral and bacterial infections, and spider bites.3
Iodinated contrast agents have long been known to cause immediate and delayed adverse cutaneous reactions. However, one consensus study indicated that these reactions occur in only 0.05% to 0.10% of patients.4 Although rare, iodinated contrast media (eg, iopamidol, iohexol, ioversol, iodixanol, iomeprol, iobitridol, iopromide) have been reported as a cause of AGEP. A PubMed search of articles indexed for MEDLINE using the terms acute generalized exanthematous pustulosis, contrast, iodine, and iodinated revealed 10 adult cases reported in 6 articles in the English-language literature.1,5-9 The most recent articles focus on methods to identify the causative agent. If the etiology of the reaction is unclear, patch or intradermal testing can help to confirm the causative agent. These tests also can help determine similar agents to which the patient may cross-react.4,5
It can be difficult to differentiate AGEP from other cutaneous drug reactions and other nonfollicular pustular conditions. Drug-induced hypersensitivity syndrome typically presents with facial edema and a morbilliform rash. Although it can present with pustules, the latent period is longer (2-6 weeks), and there frequently are signs of multiorgan involvement including hepatic dysfunction, eosinophilia, atypical lymphocytosis, and lymphadenopathy. Patients with generalized pustular psoriasis often have a history of plaque psoriasis; the pustules are more concentrated in flexural sites; the eruption is gradual in onset; and histologically there tends to be features of psoriasis including parakeratosis, Munro microabscesses, and dilated blood vessels.10 Subcorneal pustular dermatosis also is more concentrated in flexural sites and frequently has an annular or serpiginous configuration. The onset also is gradual, and it follows a more chronic course than AGEP. Exfoliative erythroderma presents with widespread erythema and superficial desquamating scale. It often occurs in association with systemic symptoms and can be the result of a drug reaction or underlying inflammatory dermatosis such as psoriasis, mycosis fungoides, or pityriasis rubra pilaris.
Acute generalized exanthematous pustulosis usually resolves spontaneously within 2 weeks and is associated with a superficial desquamation as it clears. Appropriate treatment includes discontinuing the offending agent; monitoring for systemic involvement; and treating the patient's symptoms with antihistamines, analgesics, topical steroids, and emollients. In more severe or persistent cases, treatment with systemic steroids and tumor necrosis factor α inhibitors has been attempted, though their efficacy remains unclear. We report a case of iopamidol-induced AGEP that highlights the importance of eliciting a history of contrast exposure from a patient with suspected AGEP.
The Diagnosis: Acute Generalized Exanthematous Pustulosis
Histopathology demonstrated spongiosis with subcorneal pustules and an overlying basket-weave pattern stratum corneum. There was mild papillary dermal edema with scattered dermal neutrophils and rare eosinophils (Figure). The patient's clinical presentation and histopathology were consistent with acute generalized exanthematous pustulosis (AGEP). The inciting agent in this case was the contrast medium iopamidol. The patient was treated with a short course of prednisone, triamcinolone cream, diphenhydramine, and acetaminophen. Within 1 week the pustules and erythema had resolved.

Acute generalized exanthematous pustulosis is an uncommon T cell-mediated cutaneous reaction characterized by widespread progressive erythema with numerous nonfollicular pinpoint pustules. The patient usually is well appearing; however, he/she often will have concurrent fever and facial edema. Mucous membranes rarely are involved. Laboratory results typically are notable only for leukocytosis with neutrophilia.
The pustular eruption typically occurs within 1 to 2 days after exposure to an inciting agent1; however, this latent period can range from 1 hour to nearly 4 weeks in some studies.2 Systemic medications are the cause in approximately 90% of cases, with antibiotics being the most common category. Frequently implicated medications include β-lactams, macrolides, quinolones, sulfonamides, proton pump inhibitors, hydroxychloroquine, terbinafine, nonsteroidal anti-inflammatory drugs, diltiazem, ketoconazole, and fluconazole. Acute generalized exanthematous pustulosis also has been rarely reported following contact with mercury, viral and bacterial infections, and spider bites.3
Iodinated contrast agents have long been known to cause immediate and delayed adverse cutaneous reactions. However, one consensus study indicated that these reactions occur in only 0.05% to 0.10% of patients.4 Although rare, iodinated contrast media (eg, iopamidol, iohexol, ioversol, iodixanol, iomeprol, iobitridol, iopromide) have been reported as a cause of AGEP. A PubMed search of articles indexed for MEDLINE using the terms acute generalized exanthematous pustulosis, contrast, iodine, and iodinated revealed 10 adult cases reported in 6 articles in the English-language literature.1,5-9 The most recent articles focus on methods to identify the causative agent. If the etiology of the reaction is unclear, patch or intradermal testing can help to confirm the causative agent. These tests also can help determine similar agents to which the patient may cross-react.4,5
It can be difficult to differentiate AGEP from other cutaneous drug reactions and other nonfollicular pustular conditions. Drug-induced hypersensitivity syndrome typically presents with facial edema and a morbilliform rash. Although it can present with pustules, the latent period is longer (2-6 weeks), and there frequently are signs of multiorgan involvement including hepatic dysfunction, eosinophilia, atypical lymphocytosis, and lymphadenopathy. Patients with generalized pustular psoriasis often have a history of plaque psoriasis; the pustules are more concentrated in flexural sites; the eruption is gradual in onset; and histologically there tends to be features of psoriasis including parakeratosis, Munro microabscesses, and dilated blood vessels.10 Subcorneal pustular dermatosis also is more concentrated in flexural sites and frequently has an annular or serpiginous configuration. The onset also is gradual, and it follows a more chronic course than AGEP. Exfoliative erythroderma presents with widespread erythema and superficial desquamating scale. It often occurs in association with systemic symptoms and can be the result of a drug reaction or underlying inflammatory dermatosis such as psoriasis, mycosis fungoides, or pityriasis rubra pilaris.
Acute generalized exanthematous pustulosis usually resolves spontaneously within 2 weeks and is associated with a superficial desquamation as it clears. Appropriate treatment includes discontinuing the offending agent; monitoring for systemic involvement; and treating the patient's symptoms with antihistamines, analgesics, topical steroids, and emollients. In more severe or persistent cases, treatment with systemic steroids and tumor necrosis factor α inhibitors has been attempted, though their efficacy remains unclear. We report a case of iopamidol-induced AGEP that highlights the importance of eliciting a history of contrast exposure from a patient with suspected AGEP.
- Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis. Arch Dermatol. 2009;145:683-687.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;2015:1-8.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2016;73:843-848.
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26:144-155.
- Grandvuillemin A, Ripert C, Sgro C, et al. Iodinated contrast media-induced acute generalized exanthematous pustulosis confirmed by delayed skin tests. J Allergy Clin Immunol Pract. 2014;2:805-806.
- Bavbek S, Sözener ZÇ, Aydin Ö, et al. First case report of acute generalized exanthematous pustulosis due to intravenous iopromide. J Investig Allergol Clin Immunol. 2014;24:66-67.
- Kim SJ, Lee T, Lee YS, et al. Acute generalized exanthematous pustulosis caused by radiocontrast media. Ann Allergy Asthma Immunol. 2010;105:492-493.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. Am J Roentgenol. 2006;187:198-201.
- Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003;30:723-726.
- Halevy S, Kardaun S, Davidovici B, et al; EuroSCAR and RegiSCAR Study Group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010:163:1245-1252.
- Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis. Arch Dermatol. 2009;145:683-687.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;2015:1-8.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2016;73:843-848.
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26:144-155.
- Grandvuillemin A, Ripert C, Sgro C, et al. Iodinated contrast media-induced acute generalized exanthematous pustulosis confirmed by delayed skin tests. J Allergy Clin Immunol Pract. 2014;2:805-806.
- Bavbek S, Sözener ZÇ, Aydin Ö, et al. First case report of acute generalized exanthematous pustulosis due to intravenous iopromide. J Investig Allergol Clin Immunol. 2014;24:66-67.
- Kim SJ, Lee T, Lee YS, et al. Acute generalized exanthematous pustulosis caused by radiocontrast media. Ann Allergy Asthma Immunol. 2010;105:492-493.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. Am J Roentgenol. 2006;187:198-201.
- Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003;30:723-726.
- Halevy S, Kardaun S, Davidovici B, et al; EuroSCAR and RegiSCAR Study Group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010:163:1245-1252.
A 31-year-old man presented with a rapidly progressive, burning rash of 1 day's duration, along with malaise, nausea, and dizziness. At the time of presentation, he was hemodynamically stable and afebrile. Laboratory analysis revealed mild leukocytosis with neutrophilia. A complete metabolic panel was within normal limits. He had no chronic medical conditions and was taking no medications or supplements. One day prior to onset of the rash, he underwent contrast-enhanced (iopamidol) computed tomography of the abdomen. Physical examination revealed large edematous plaques on the face, neck, and trunk (top) that were studded with numerous pinpoint pustules (bottom). He also had subtle facial edema. There was relative sparing of the flexural sites and no involvement of the palms, soles, or mucous membranes. A shave biopsy was obtained from a pustular area on the neck.
Hemorrhagic Crusted Papule on the Arm
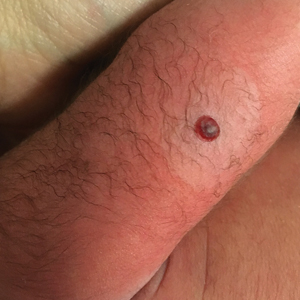
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
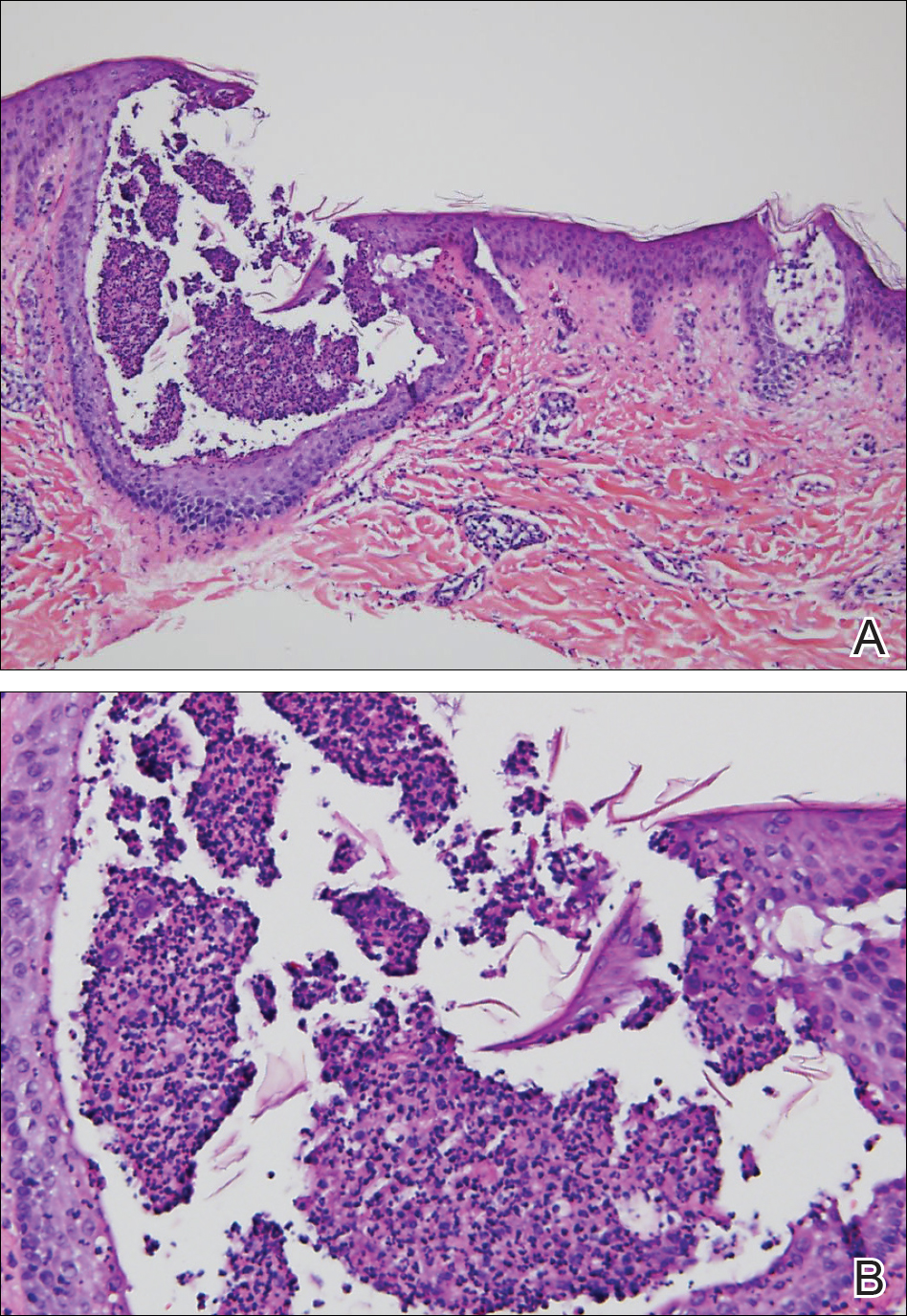
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).
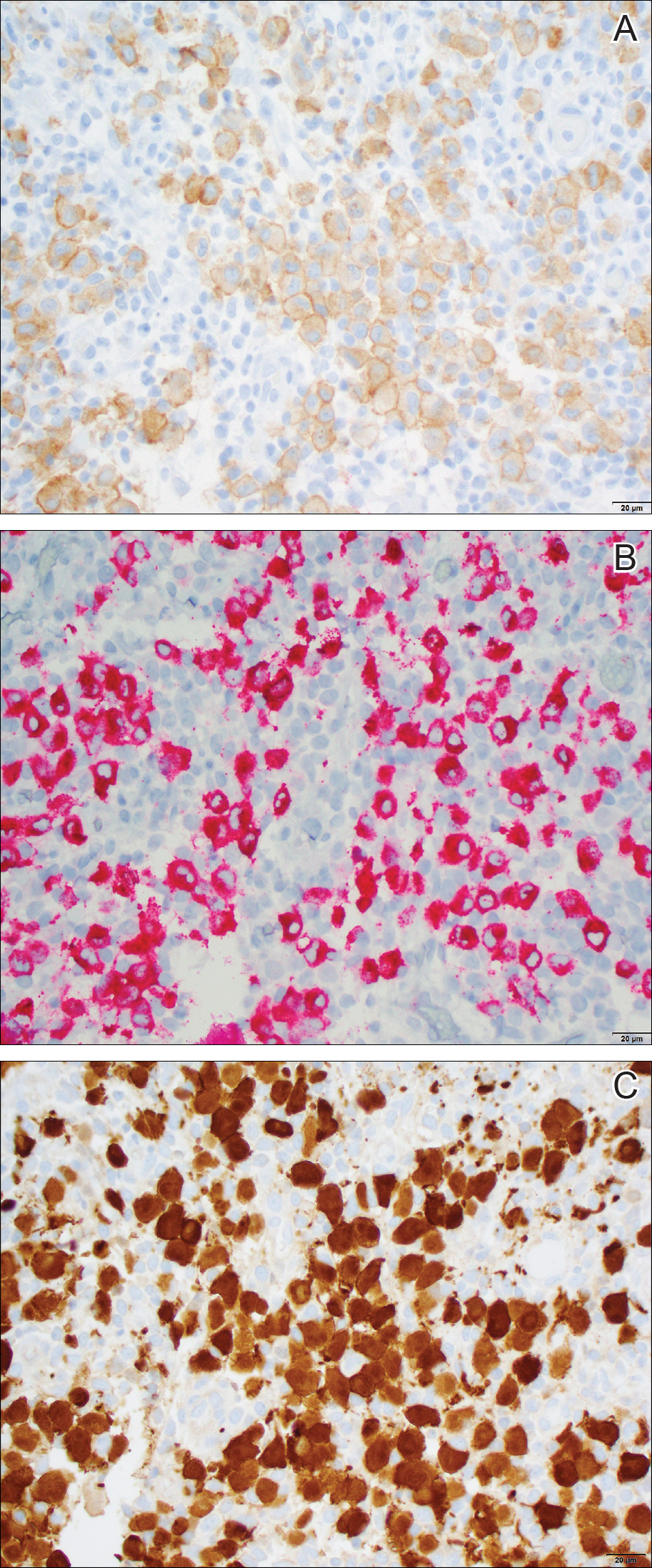
Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).

Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).

Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
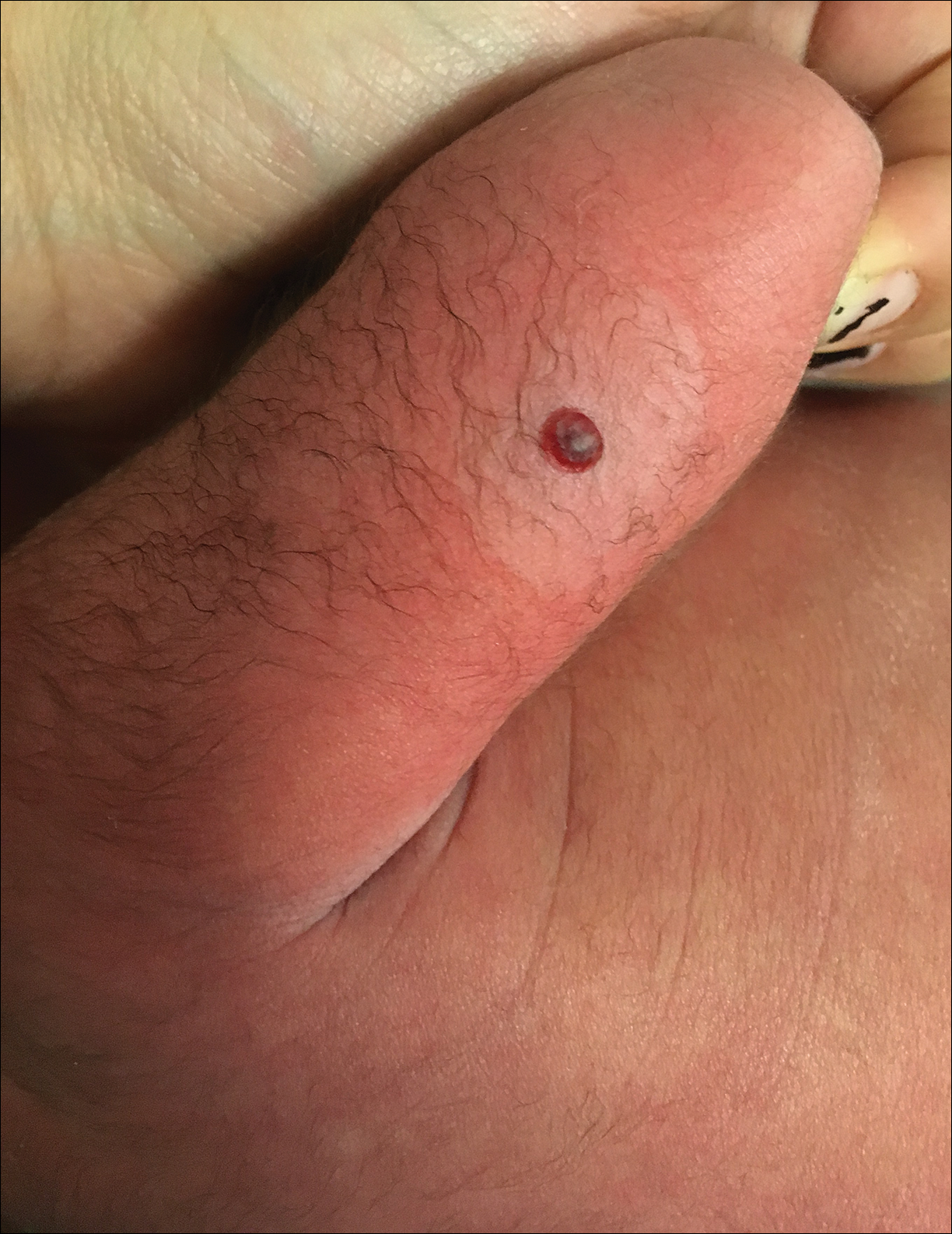
Dermatology consultation was called to the delivery room to evaluate a red, hemorrhagic, crusted, 5-mm papule on the right lateral upper arm of a preterm newborn. He appeared vigorous with an Apgar score of 7 at 1 minute and 8 at 5 minutes. Physical examination was otherwise normal. Of note, the mother presented late to prenatal care. Her herpes simplex and varicella-zoster virus status was unknown. A shave biopsy of the papule was performed at 3 days of age.
Pigmented Pruritic Macules in the Genital Area
The Diagnosis: Pediculosis Pubis
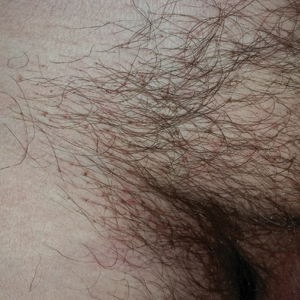
Dermoscopy of the pubic hair demonstrated a louse clutching multiple shafts of hairs (Figure) as well as scattered nits, confirming the presence of Phthirus pubis and diagnosis of pediculosis pubis. The key clinical diagnostic feature in this case was severe itching of the pubic area, with visible nits present on pubic hairs. Itching and infestation also can involve other hair-bearing areas such as the chest, legs, and axillae. The patient was treated with permethrin cream 5% applied to the pubic area and chest. Symptoms and infestation were resolved at 1-week follow-up.
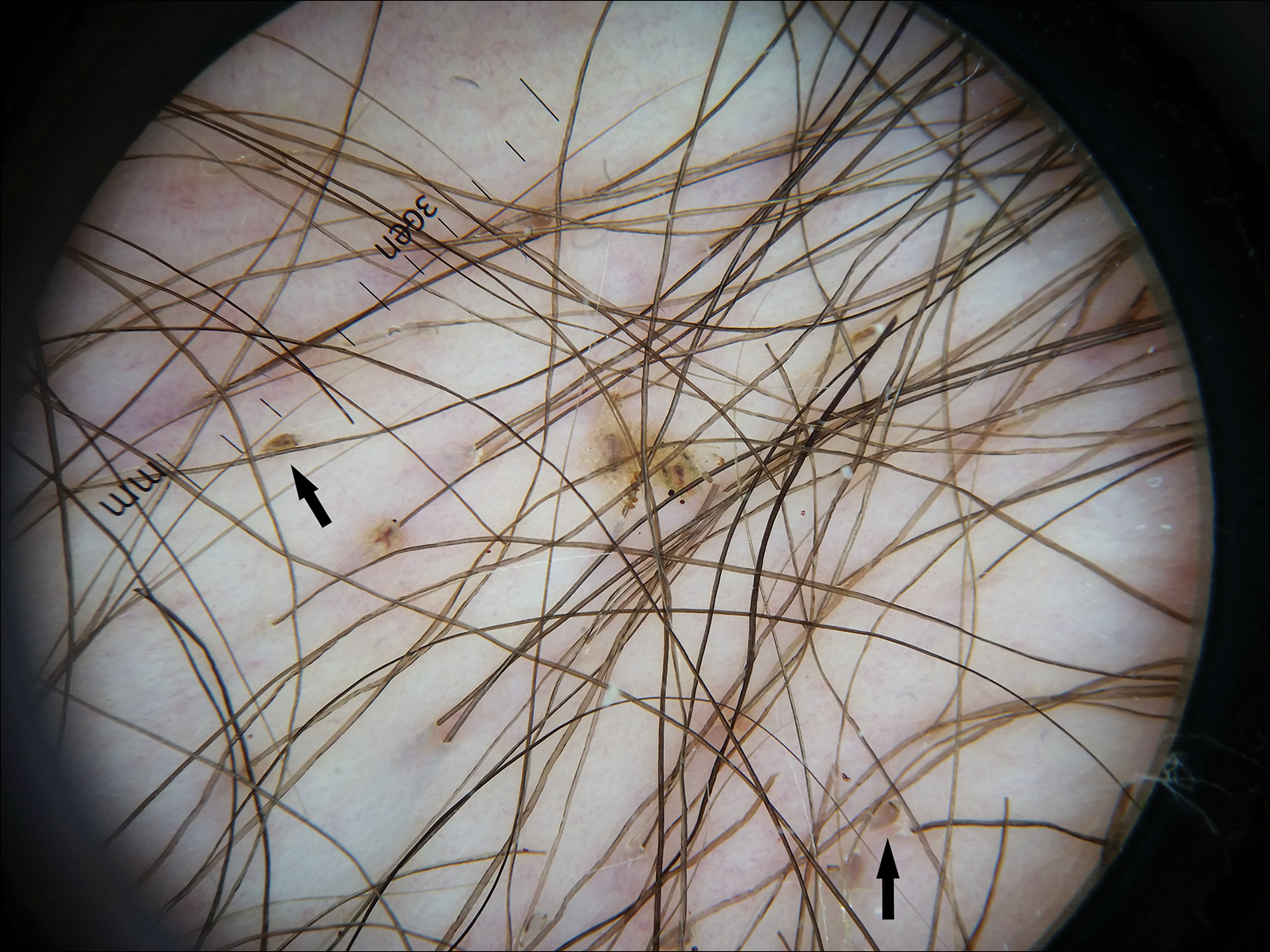
Pediculosis pubis is an infestation of pubic hairs by the pubic (crab) louse P pubis, which feeds on host blood. Other body areas covered with dense hair also may be involved; 60% of patients are infested in at least 2 different sites.1 Pediculosis pubis is most commonly sexually transmitted through direct contact.2 Worldwide prevalence has been estimated at approximately 2% of the adult population, and a survey of 817 US college students in 2009 indicated a lifetime prevalence of 1.3%.3 The prevalence is slightly higher in men, highest in men who have sex with men, and rare in individuals with shaved pubic hair.1 The most common symptom is pruritus of the genital area. Infested patients also may develop asymptomatic bluish gray macules (maculae ceruleae) secondary to hemosiderin deposition from louse bites.4
The diagnosis of pediculosis pubis is made by identification of P pubis, either by examination with the naked eye or confirmation with dermoscopy or microscopy. Although the 0.8- to 1.2-mm lice are visible to the naked eye, they can be difficult to see if not filled with blood, and nits on the hairs can be mistaken for white piedra (fungal infection of the hair shafts) or trichomycosis pubis (bacterial infection of the hair shafts).4 Scabies and tinea cruris do not present with attachments to the hairs. Scabies may present with papules and burrows and tinea cruris with scaly erythematous plaques. Small numbers of lice and nits may be missed by the naked eye or a traditional magnifying glass.5 The use of dermoscopy allows for fast and accurate identification of the characteristic lice and nits, even in these more challenging cases.5 Accurate diagnosis is important, as approximately 31% of infested patients have other concurrent sexually transmitted infections that warrant screening.6
The Centers for Disease Control and Prevention recommends first-line treatment with permethrin cream (1% or 5%) or pyrethrin with piperonyl butoxide applied to all affected areas and washed off after 10 minutes.7 Patients should be reevaluated after 1 week if symptoms persist and re-treated if lice are found on examination.7,8 Malathion lotion 0.5% (applied and washed off after 8-12 hours) or oral ivermectin (250 µg/kg, repeated after 2 weeks) may be used for alternative therapies or cases of permethrin or pyrethrin resistance.7 Ivermectin also is effective for involvement of eyelashes where topical insecticides should not be used.9 Sexual partners should be treated to prevent repeat transmission.7,8 Bedding and clothing can be decontaminated by machine wash on hot cycle or isolating from body contact for 72 hours.7 Patients also should be screened for other sexually transmitted infections, including human immunodeficiency virus.6
- Burkhart CN, Burkhart CG, Morrell DS. Infestations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier/Saunders; 2012:1429-1430.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Anderson AL, Chaney E. Pubic lice (Pthirus pubis): history, biology and treatment vs. knowledge and beliefs of US college students. Int J Environ Res Public Health. 2009;6:592-600.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12; quiz 13-14.
- Chuh A, Lee A, Wong W, et al. Diagnosis of pediculosis pubis: a novel application of digital epiluminescence dermatoscopy. J Eur Acad Dermatol Venereol. 2007;21:837-838.
- Chapel TA, Katta T, Kuszmar T, et al. Pediculosis pubis in a clinic for treatment of sexually transmitted diseases. Sex Transm Dis. 1979;6:257-260.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis. 2007;44(suppl 3):S153-S159.
- Burkhart CN, Burkhart CG. Oral ivermectin therapy for phthiriasis palpebrum. Arch Ophthalmol. 2000;118:134-135.
The Diagnosis: Pediculosis Pubis
Dermoscopy of the pubic hair demonstrated a louse clutching multiple shafts of hairs (Figure) as well as scattered nits, confirming the presence of Phthirus pubis and diagnosis of pediculosis pubis. The key clinical diagnostic feature in this case was severe itching of the pubic area, with visible nits present on pubic hairs. Itching and infestation also can involve other hair-bearing areas such as the chest, legs, and axillae. The patient was treated with permethrin cream 5% applied to the pubic area and chest. Symptoms and infestation were resolved at 1-week follow-up.

Pediculosis pubis is an infestation of pubic hairs by the pubic (crab) louse P pubis, which feeds on host blood. Other body areas covered with dense hair also may be involved; 60% of patients are infested in at least 2 different sites.1 Pediculosis pubis is most commonly sexually transmitted through direct contact.2 Worldwide prevalence has been estimated at approximately 2% of the adult population, and a survey of 817 US college students in 2009 indicated a lifetime prevalence of 1.3%.3 The prevalence is slightly higher in men, highest in men who have sex with men, and rare in individuals with shaved pubic hair.1 The most common symptom is pruritus of the genital area. Infested patients also may develop asymptomatic bluish gray macules (maculae ceruleae) secondary to hemosiderin deposition from louse bites.4
The diagnosis of pediculosis pubis is made by identification of P pubis, either by examination with the naked eye or confirmation with dermoscopy or microscopy. Although the 0.8- to 1.2-mm lice are visible to the naked eye, they can be difficult to see if not filled with blood, and nits on the hairs can be mistaken for white piedra (fungal infection of the hair shafts) or trichomycosis pubis (bacterial infection of the hair shafts).4 Scabies and tinea cruris do not present with attachments to the hairs. Scabies may present with papules and burrows and tinea cruris with scaly erythematous plaques. Small numbers of lice and nits may be missed by the naked eye or a traditional magnifying glass.5 The use of dermoscopy allows for fast and accurate identification of the characteristic lice and nits, even in these more challenging cases.5 Accurate diagnosis is important, as approximately 31% of infested patients have other concurrent sexually transmitted infections that warrant screening.6
The Centers for Disease Control and Prevention recommends first-line treatment with permethrin cream (1% or 5%) or pyrethrin with piperonyl butoxide applied to all affected areas and washed off after 10 minutes.7 Patients should be reevaluated after 1 week if symptoms persist and re-treated if lice are found on examination.7,8 Malathion lotion 0.5% (applied and washed off after 8-12 hours) or oral ivermectin (250 µg/kg, repeated after 2 weeks) may be used for alternative therapies or cases of permethrin or pyrethrin resistance.7 Ivermectin also is effective for involvement of eyelashes where topical insecticides should not be used.9 Sexual partners should be treated to prevent repeat transmission.7,8 Bedding and clothing can be decontaminated by machine wash on hot cycle or isolating from body contact for 72 hours.7 Patients also should be screened for other sexually transmitted infections, including human immunodeficiency virus.6
The Diagnosis: Pediculosis Pubis
Dermoscopy of the pubic hair demonstrated a louse clutching multiple shafts of hairs (Figure) as well as scattered nits, confirming the presence of Phthirus pubis and diagnosis of pediculosis pubis. The key clinical diagnostic feature in this case was severe itching of the pubic area, with visible nits present on pubic hairs. Itching and infestation also can involve other hair-bearing areas such as the chest, legs, and axillae. The patient was treated with permethrin cream 5% applied to the pubic area and chest. Symptoms and infestation were resolved at 1-week follow-up.

Pediculosis pubis is an infestation of pubic hairs by the pubic (crab) louse P pubis, which feeds on host blood. Other body areas covered with dense hair also may be involved; 60% of patients are infested in at least 2 different sites.1 Pediculosis pubis is most commonly sexually transmitted through direct contact.2 Worldwide prevalence has been estimated at approximately 2% of the adult population, and a survey of 817 US college students in 2009 indicated a lifetime prevalence of 1.3%.3 The prevalence is slightly higher in men, highest in men who have sex with men, and rare in individuals with shaved pubic hair.1 The most common symptom is pruritus of the genital area. Infested patients also may develop asymptomatic bluish gray macules (maculae ceruleae) secondary to hemosiderin deposition from louse bites.4
The diagnosis of pediculosis pubis is made by identification of P pubis, either by examination with the naked eye or confirmation with dermoscopy or microscopy. Although the 0.8- to 1.2-mm lice are visible to the naked eye, they can be difficult to see if not filled with blood, and nits on the hairs can be mistaken for white piedra (fungal infection of the hair shafts) or trichomycosis pubis (bacterial infection of the hair shafts).4 Scabies and tinea cruris do not present with attachments to the hairs. Scabies may present with papules and burrows and tinea cruris with scaly erythematous plaques. Small numbers of lice and nits may be missed by the naked eye or a traditional magnifying glass.5 The use of dermoscopy allows for fast and accurate identification of the characteristic lice and nits, even in these more challenging cases.5 Accurate diagnosis is important, as approximately 31% of infested patients have other concurrent sexually transmitted infections that warrant screening.6
The Centers for Disease Control and Prevention recommends first-line treatment with permethrin cream (1% or 5%) or pyrethrin with piperonyl butoxide applied to all affected areas and washed off after 10 minutes.7 Patients should be reevaluated after 1 week if symptoms persist and re-treated if lice are found on examination.7,8 Malathion lotion 0.5% (applied and washed off after 8-12 hours) or oral ivermectin (250 µg/kg, repeated after 2 weeks) may be used for alternative therapies or cases of permethrin or pyrethrin resistance.7 Ivermectin also is effective for involvement of eyelashes where topical insecticides should not be used.9 Sexual partners should be treated to prevent repeat transmission.7,8 Bedding and clothing can be decontaminated by machine wash on hot cycle or isolating from body contact for 72 hours.7 Patients also should be screened for other sexually transmitted infections, including human immunodeficiency virus.6
- Burkhart CN, Burkhart CG, Morrell DS. Infestations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier/Saunders; 2012:1429-1430.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Anderson AL, Chaney E. Pubic lice (Pthirus pubis): history, biology and treatment vs. knowledge and beliefs of US college students. Int J Environ Res Public Health. 2009;6:592-600.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12; quiz 13-14.
- Chuh A, Lee A, Wong W, et al. Diagnosis of pediculosis pubis: a novel application of digital epiluminescence dermatoscopy. J Eur Acad Dermatol Venereol. 2007;21:837-838.
- Chapel TA, Katta T, Kuszmar T, et al. Pediculosis pubis in a clinic for treatment of sexually transmitted diseases. Sex Transm Dis. 1979;6:257-260.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis. 2007;44(suppl 3):S153-S159.
- Burkhart CN, Burkhart CG. Oral ivermectin therapy for phthiriasis palpebrum. Arch Ophthalmol. 2000;118:134-135.
- Burkhart CN, Burkhart CG, Morrell DS. Infestations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier/Saunders; 2012:1429-1430.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Anderson AL, Chaney E. Pubic lice (Pthirus pubis): history, biology and treatment vs. knowledge and beliefs of US college students. Int J Environ Res Public Health. 2009;6:592-600.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12; quiz 13-14.
- Chuh A, Lee A, Wong W, et al. Diagnosis of pediculosis pubis: a novel application of digital epiluminescence dermatoscopy. J Eur Acad Dermatol Venereol. 2007;21:837-838.
- Chapel TA, Katta T, Kuszmar T, et al. Pediculosis pubis in a clinic for treatment of sexually transmitted diseases. Sex Transm Dis. 1979;6:257-260.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis. 2007;44(suppl 3):S153-S159.
- Burkhart CN, Burkhart CG. Oral ivermectin therapy for phthiriasis palpebrum. Arch Ophthalmol. 2000;118:134-135.
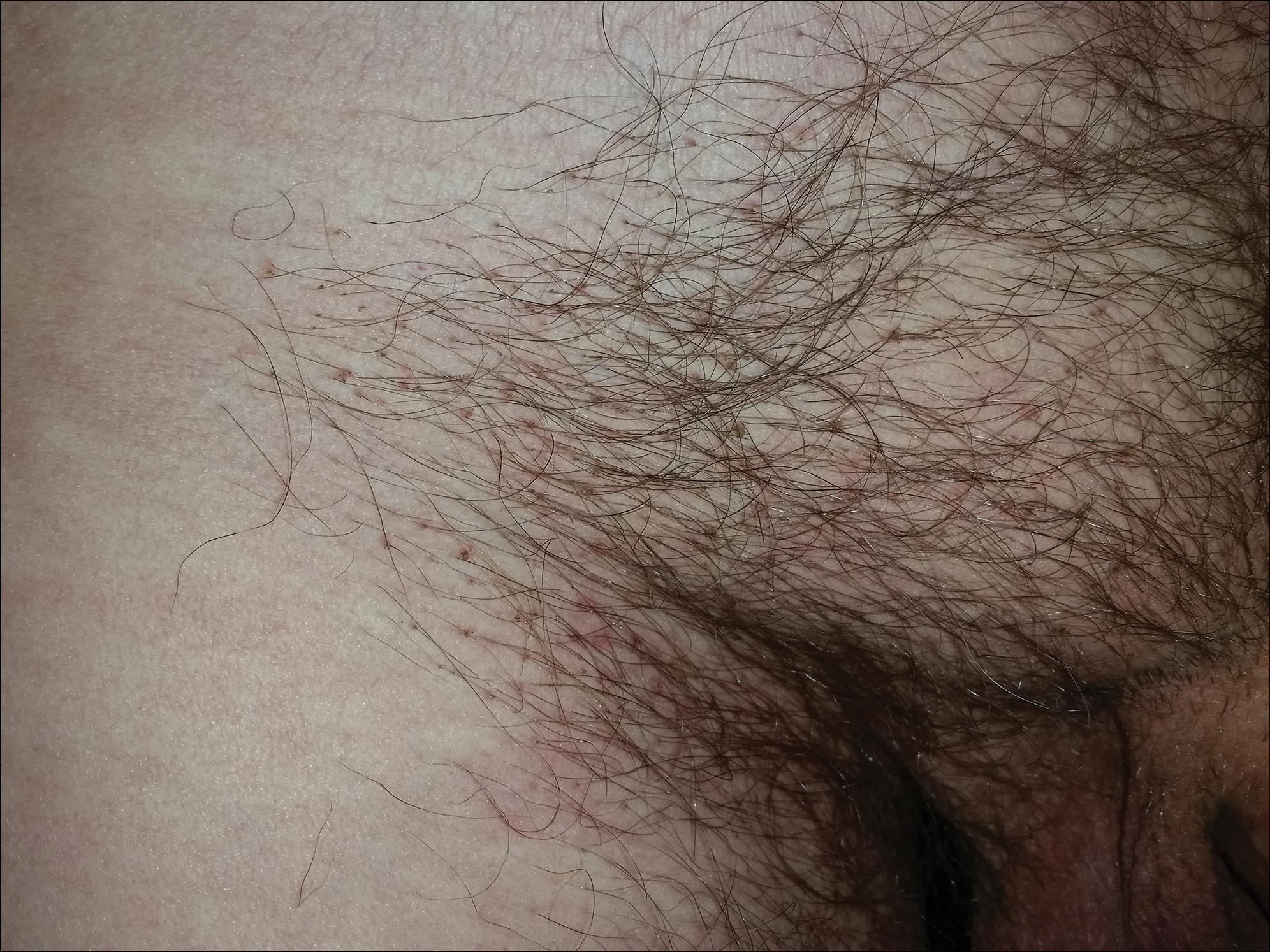
A 50-year-old man with a history of cerebrovascular accident presented with severe itching along the inguinal folds and over the chest of 2 months' duration. His last sexual encounter was 5 months prior. He had previously seen a primary care physician who told him he needed to clean the hair better. Examination of the genital area revealed pigmented macules and overlying particles among the pubic hair.
Solitary Exophytic Plaque on the Left Groin
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
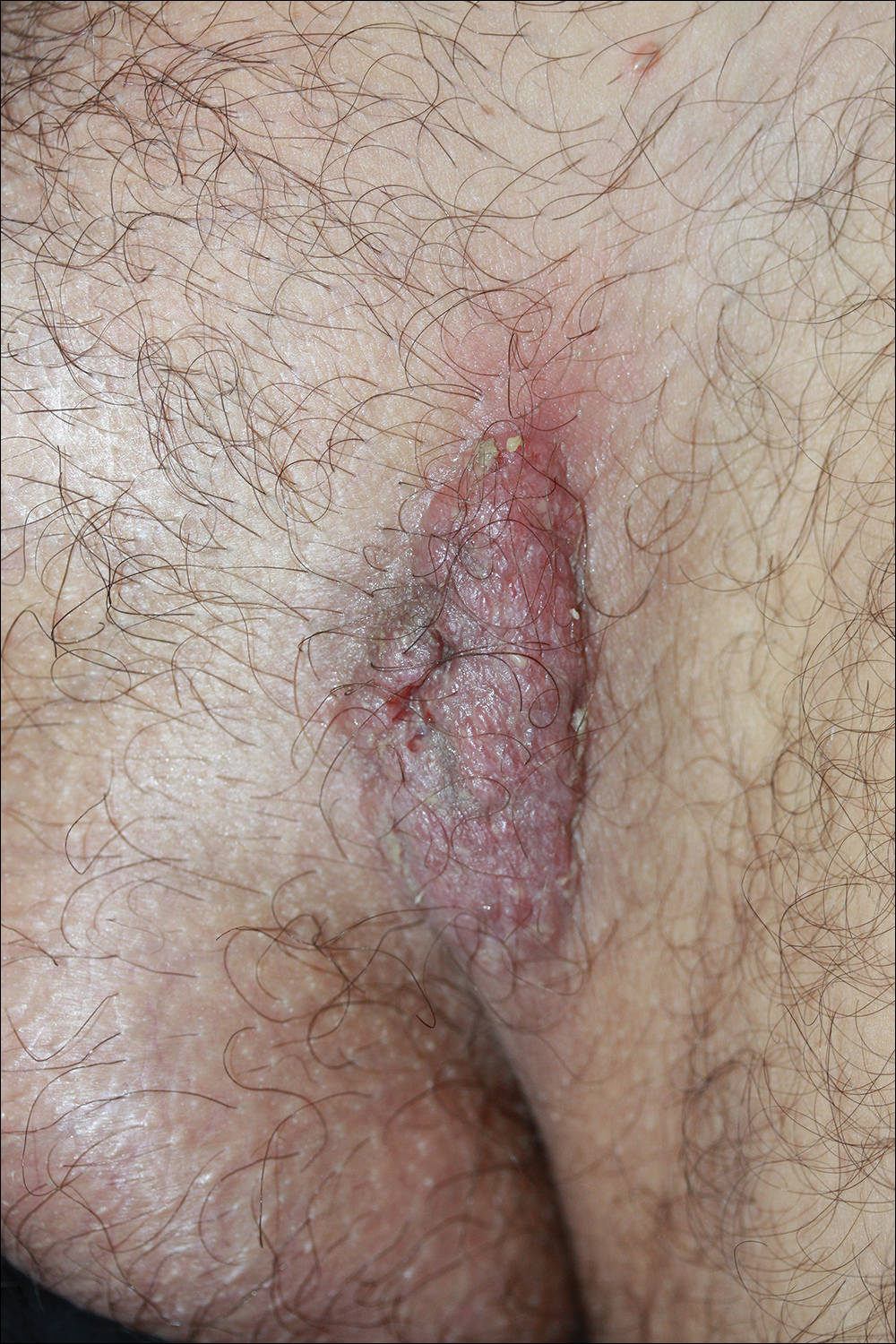
A 40-year-old otherwise healthy man presented with an exophytic plaque on the left groin of 1 month's duration. The lesion reportedly emerged as pustules that slowly expanded and coalesced. At an outside institution, cryotherapy was planned for a presumed diagnosis of condyloma acuminatum; however, the patient decided to get a second opinion. He denied recent intake of new drugs. Six months prior he had traveled to China and engaged in unprotected sexual intercourse. Physical examination revealed an approximately 4×2-cm exophytic plaque with a partially eroded and exudative surface on the left inguinal fold. Dermatologic examination, including the oral mucosa, was otherwise normal. Complete blood cell count and sexually transmitted disease panel were unremarkable.
Diffuse Nonscarring Alopecia
The Diagnosis: Trichotillomania
A scalp punch biopsy revealed pigmented hair casts, an increase in catagen and telogen follicles, and a lack of perifollicular inflammation (Figure). Based on the clinical and histopathological findings, a diagnosis of trichotillomania (TTM) was established.
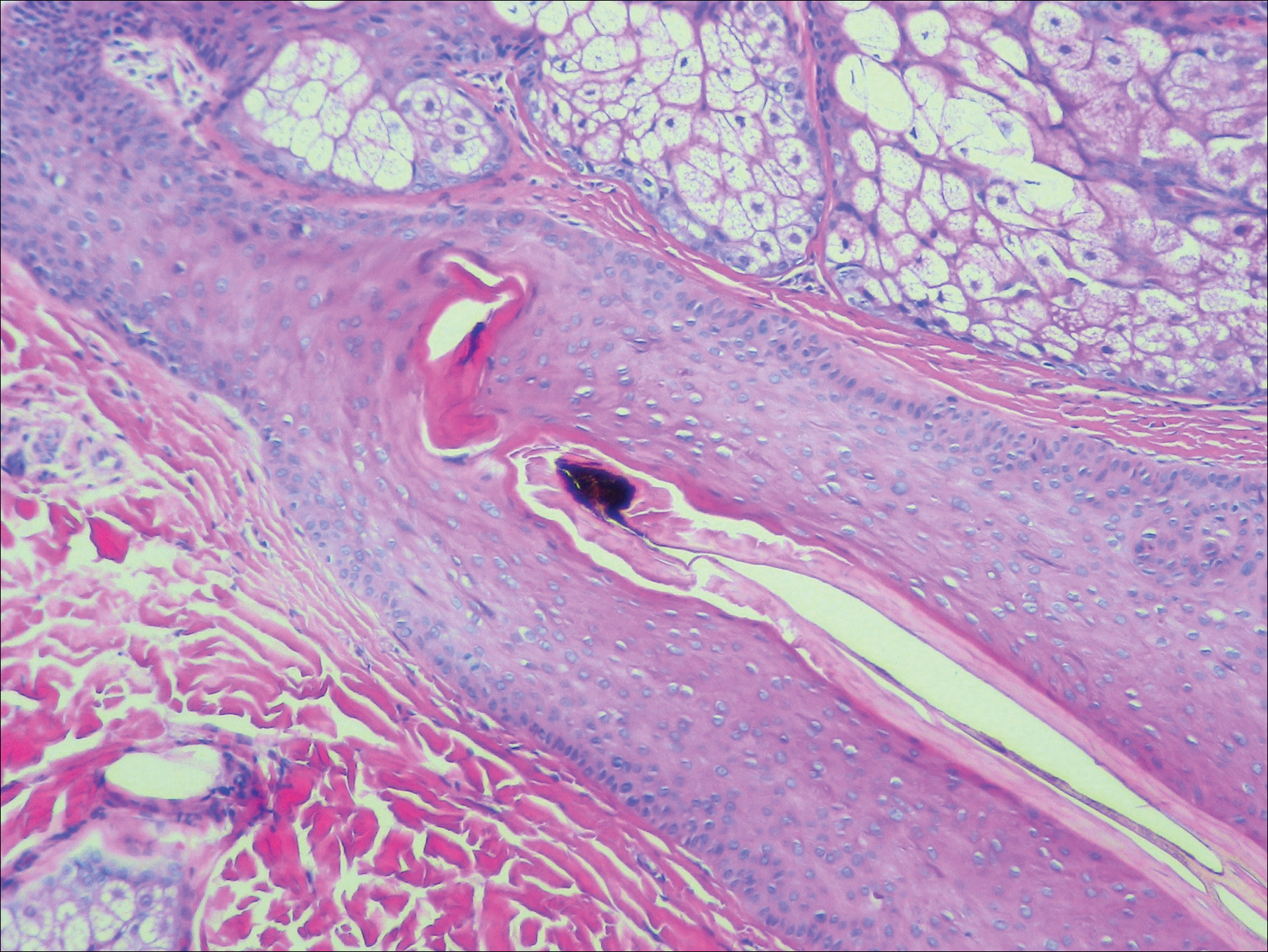
Trichotillomania is a hairpulling disorder with notable dermatologic and psychiatric overlap. Although previously considered an impulse control disorder, the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) reclassified it within obsessive-compulsive and related disorders, which also include body dysmorphic disorder and excoriation (skin-picking) disorder. Diagnostic criteria for TTM include the following: the patient must have recurrent pulling out of his/her hair resulting in hair loss despite repeated attempts to stop; underlying medical conditions and other psychiatric diagnoses must be excluded; and the patient must experience distress or impairment in social, occupational, or other areas of functioning from the hairpulling.1 Trichotillomania mainly occurs in children and young adults, with a lifetime prevalence of approximately 1% to 2%.2 The coexistence of a mood or anxiety disorder is common, as seen in our patient.
The diagnosis of TTM requires strong clinical suspicion because patients and their parents/guardians usually deny hairpulling. The main clinical differential diagnosis often is alopecia areata (AA) because both conditions can present as well-defined patches of nonscarring hair loss. Trichoscopy provides an invaluable noninvasive diagnostic tool that can be particularly useful in pediatric patients who may be reluctant to have a scalp biopsy. There are many overlapping trichoscopic findings of TTM and AA, including yellow dots, black dots, broken hairs, coiled hairs, and exclamation mark hairs.3 More specific trichoscopy findings for TTM include flame hairs (wavy proximal hair residue), V-sign (2 shafts within 1 follicle broken at the same length), and tulip hairs (dark, tulip-shaped ends of broken hairs).4 Hair breakage of varying lengths and trichoptilosis (split ends) can be better visualized using trichoscopy and support a diagnosis of TTM over AA.
Androgenetic alopecia (female pattern hair loss) presents with gradual thinning around the part line of the frontal and parietal scalp with trichoscopy showing miniaturization of hairs and decreased follicle density. The moth-eaten-like appearance of alopecia due to secondary syphilis may mimic alopecia areata clinically, but serologic testing can confirm the diagnosis of syphilis. Telogen effluvium does not have the trichoscopic features that are seen in TTM and is clinically distinguished by hair shedding and a positive hair pull test.
Biopsy can provide objective yet nonspecific support for the diagnosis, demonstrating trichomalacia, pigmented hair casts, empty follicles, and an increase in catagen hairs with a lack of inflammation. Normal and damaged hair follicles may be seen in close proximity, and hemorrhage may be seen secondary to trauma. Pigmented hair casts are not specific to TTM and are present in other traumatic hair disorders, such as traction alopecia; therefore, clinical correlation is essential for diagnosis.
Habit reversal training is the most effective treatment of TTM and involves 3 major components: awareness training with self-monitoring, stimulus control, and competing response procedures.5 Although numerous pharmacotherapies have been reported as effective treatments for TTM, a 2013 Cochrane review of 8 randomized controlled trials concluded that no medication has demonstrated reliable efficacy. Reported therapies included selective serotonin reuptake inhibitors, naltrexone, olanzapine, N-acetylcysteine, and clomipramine.6
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Schumer MC, Panza KE, Mulqueen JM, et al. Long-term outcome in pediatric trichotillomania. Depress Anxiety. 2015;32:737-743.
- Lencastre A, Tosti A. Role of trichoscopy on children's scalp and hair disorders. Pediatr Dermatol. 2013;30:674-682.
- Rakowska A, Slowinska M, Olszewska M, et al. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303-306.
- Morris S, Zickgraf H, Dingfelder H, et al. Habit reversal training in trichotillomania: guide for the clinician. Expert Rev Neurother. 2013;13:1069-1177.
- Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662.
The Diagnosis: Trichotillomania
A scalp punch biopsy revealed pigmented hair casts, an increase in catagen and telogen follicles, and a lack of perifollicular inflammation (Figure). Based on the clinical and histopathological findings, a diagnosis of trichotillomania (TTM) was established.

Trichotillomania is a hairpulling disorder with notable dermatologic and psychiatric overlap. Although previously considered an impulse control disorder, the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) reclassified it within obsessive-compulsive and related disorders, which also include body dysmorphic disorder and excoriation (skin-picking) disorder. Diagnostic criteria for TTM include the following: the patient must have recurrent pulling out of his/her hair resulting in hair loss despite repeated attempts to stop; underlying medical conditions and other psychiatric diagnoses must be excluded; and the patient must experience distress or impairment in social, occupational, or other areas of functioning from the hairpulling.1 Trichotillomania mainly occurs in children and young adults, with a lifetime prevalence of approximately 1% to 2%.2 The coexistence of a mood or anxiety disorder is common, as seen in our patient.
The diagnosis of TTM requires strong clinical suspicion because patients and their parents/guardians usually deny hairpulling. The main clinical differential diagnosis often is alopecia areata (AA) because both conditions can present as well-defined patches of nonscarring hair loss. Trichoscopy provides an invaluable noninvasive diagnostic tool that can be particularly useful in pediatric patients who may be reluctant to have a scalp biopsy. There are many overlapping trichoscopic findings of TTM and AA, including yellow dots, black dots, broken hairs, coiled hairs, and exclamation mark hairs.3 More specific trichoscopy findings for TTM include flame hairs (wavy proximal hair residue), V-sign (2 shafts within 1 follicle broken at the same length), and tulip hairs (dark, tulip-shaped ends of broken hairs).4 Hair breakage of varying lengths and trichoptilosis (split ends) can be better visualized using trichoscopy and support a diagnosis of TTM over AA.
Androgenetic alopecia (female pattern hair loss) presents with gradual thinning around the part line of the frontal and parietal scalp with trichoscopy showing miniaturization of hairs and decreased follicle density. The moth-eaten-like appearance of alopecia due to secondary syphilis may mimic alopecia areata clinically, but serologic testing can confirm the diagnosis of syphilis. Telogen effluvium does not have the trichoscopic features that are seen in TTM and is clinically distinguished by hair shedding and a positive hair pull test.
Biopsy can provide objective yet nonspecific support for the diagnosis, demonstrating trichomalacia, pigmented hair casts, empty follicles, and an increase in catagen hairs with a lack of inflammation. Normal and damaged hair follicles may be seen in close proximity, and hemorrhage may be seen secondary to trauma. Pigmented hair casts are not specific to TTM and are present in other traumatic hair disorders, such as traction alopecia; therefore, clinical correlation is essential for diagnosis.
Habit reversal training is the most effective treatment of TTM and involves 3 major components: awareness training with self-monitoring, stimulus control, and competing response procedures.5 Although numerous pharmacotherapies have been reported as effective treatments for TTM, a 2013 Cochrane review of 8 randomized controlled trials concluded that no medication has demonstrated reliable efficacy. Reported therapies included selective serotonin reuptake inhibitors, naltrexone, olanzapine, N-acetylcysteine, and clomipramine.6
The Diagnosis: Trichotillomania
A scalp punch biopsy revealed pigmented hair casts, an increase in catagen and telogen follicles, and a lack of perifollicular inflammation (Figure). Based on the clinical and histopathological findings, a diagnosis of trichotillomania (TTM) was established.

Trichotillomania is a hairpulling disorder with notable dermatologic and psychiatric overlap. Although previously considered an impulse control disorder, the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) reclassified it within obsessive-compulsive and related disorders, which also include body dysmorphic disorder and excoriation (skin-picking) disorder. Diagnostic criteria for TTM include the following: the patient must have recurrent pulling out of his/her hair resulting in hair loss despite repeated attempts to stop; underlying medical conditions and other psychiatric diagnoses must be excluded; and the patient must experience distress or impairment in social, occupational, or other areas of functioning from the hairpulling.1 Trichotillomania mainly occurs in children and young adults, with a lifetime prevalence of approximately 1% to 2%.2 The coexistence of a mood or anxiety disorder is common, as seen in our patient.
The diagnosis of TTM requires strong clinical suspicion because patients and their parents/guardians usually deny hairpulling. The main clinical differential diagnosis often is alopecia areata (AA) because both conditions can present as well-defined patches of nonscarring hair loss. Trichoscopy provides an invaluable noninvasive diagnostic tool that can be particularly useful in pediatric patients who may be reluctant to have a scalp biopsy. There are many overlapping trichoscopic findings of TTM and AA, including yellow dots, black dots, broken hairs, coiled hairs, and exclamation mark hairs.3 More specific trichoscopy findings for TTM include flame hairs (wavy proximal hair residue), V-sign (2 shafts within 1 follicle broken at the same length), and tulip hairs (dark, tulip-shaped ends of broken hairs).4 Hair breakage of varying lengths and trichoptilosis (split ends) can be better visualized using trichoscopy and support a diagnosis of TTM over AA.
Androgenetic alopecia (female pattern hair loss) presents with gradual thinning around the part line of the frontal and parietal scalp with trichoscopy showing miniaturization of hairs and decreased follicle density. The moth-eaten-like appearance of alopecia due to secondary syphilis may mimic alopecia areata clinically, but serologic testing can confirm the diagnosis of syphilis. Telogen effluvium does not have the trichoscopic features that are seen in TTM and is clinically distinguished by hair shedding and a positive hair pull test.
Biopsy can provide objective yet nonspecific support for the diagnosis, demonstrating trichomalacia, pigmented hair casts, empty follicles, and an increase in catagen hairs with a lack of inflammation. Normal and damaged hair follicles may be seen in close proximity, and hemorrhage may be seen secondary to trauma. Pigmented hair casts are not specific to TTM and are present in other traumatic hair disorders, such as traction alopecia; therefore, clinical correlation is essential for diagnosis.
Habit reversal training is the most effective treatment of TTM and involves 3 major components: awareness training with self-monitoring, stimulus control, and competing response procedures.5 Although numerous pharmacotherapies have been reported as effective treatments for TTM, a 2013 Cochrane review of 8 randomized controlled trials concluded that no medication has demonstrated reliable efficacy. Reported therapies included selective serotonin reuptake inhibitors, naltrexone, olanzapine, N-acetylcysteine, and clomipramine.6
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Schumer MC, Panza KE, Mulqueen JM, et al. Long-term outcome in pediatric trichotillomania. Depress Anxiety. 2015;32:737-743.
- Lencastre A, Tosti A. Role of trichoscopy on children's scalp and hair disorders. Pediatr Dermatol. 2013;30:674-682.
- Rakowska A, Slowinska M, Olszewska M, et al. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303-306.
- Morris S, Zickgraf H, Dingfelder H, et al. Habit reversal training in trichotillomania: guide for the clinician. Expert Rev Neurother. 2013;13:1069-1177.
- Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Schumer MC, Panza KE, Mulqueen JM, et al. Long-term outcome in pediatric trichotillomania. Depress Anxiety. 2015;32:737-743.
- Lencastre A, Tosti A. Role of trichoscopy on children's scalp and hair disorders. Pediatr Dermatol. 2013;30:674-682.
- Rakowska A, Slowinska M, Olszewska M, et al. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303-306.
- Morris S, Zickgraf H, Dingfelder H, et al. Habit reversal training in trichotillomania: guide for the clinician. Expert Rev Neurother. 2013;13:1069-1177.
- Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662.
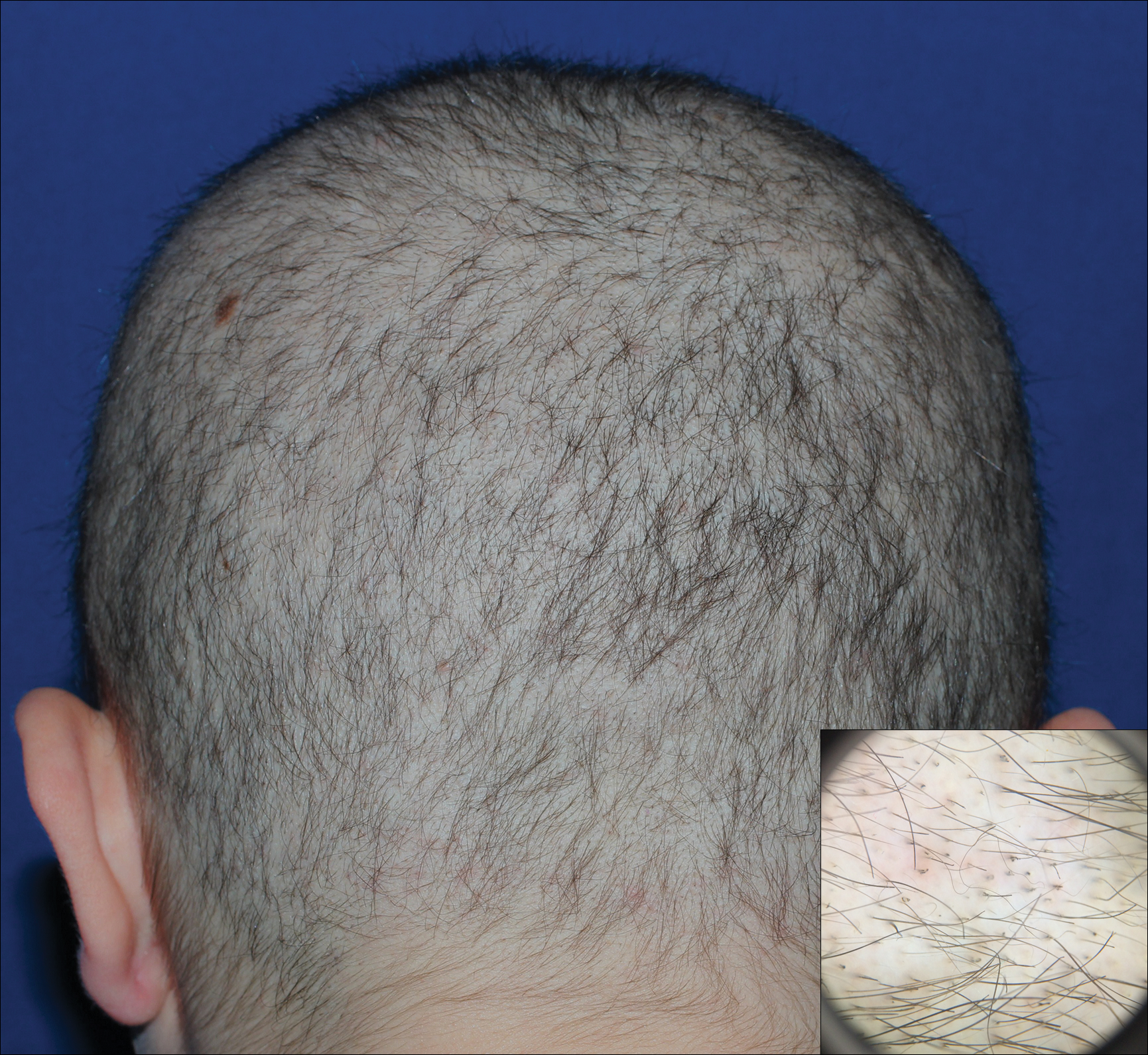
A 19-year-old woman with attention deficit hyperactivity disorder and an anxiety disorder presented with hair loss of 2 years' duration. She initially had small circular bald areas throughout the scalp that had progressed to diffuse hair loss of the entire scalp. She denied recent hairpulling but admitted to a remote prior history of eyelash and eyebrow pulling. She denied any voice changes, acne, or menstrual irregularities. Physical examination revealed short hairs of varying lengths throughout the scalp with no loss of follicles, erythema, scale, or exclamation point hairs. Eyebrows and eyelashes were normal. A hair-pull test was negative. Trichoscopy illuminated variation in hair shaft diameters, as well as short, irregularly broken hairs of different lengths (inset).
Bilateral Brown Plaques Behind the Ears
The Diagnosis: Terra Firma-Forme Dermatosis
Terra firma-forme dermatosis (TFFD), also known as Duncan dirty dermatosis, is an idiopathic benign cutaneous condition that is easily misdiagnosed or mismanaged. In 1987, Duncan et al1 first described the condition in children who had mothers that lamented over dirty skin spots that could not be washed off. The term terra firma translates in Latin to solid ground, which describes the characteristic dirtlike appearance of these lesions.
Terra firma-forme dermatosis most commonly affects children and young adults, though it can present in patients of any age without any known predisposing risk factors.1-4 The lesions have a predilection for the face, neck, shoulders, trunk, and ankles. Terra firma-forme dermatosis has no association with bathing and hygiene habits, and most patients describe unsuccessful removal of the lesions, even after vigorous scrubbing with soaps and detergents at home. The lesions are asymptomatic, and many patients present to dermatology for cosmetic concerns.1-8
The etiology of TFFD is not well understood and is considered a retention hyperkeratosis. Duncan et al1 postulated that TFFD is the result of partial or improper maturation of keratinocytes leading to keratinocyte and melanin retention. Hematoxylin and eosin stains demonstrate lamellar hyperkeratosis of the stratum corneum without parakeratosis as well as keratin pearls scattered throughout. Mild acanthosis and papillomatosis also have been reported.1,5-7 Fontana-Masson stain shows excess melanin in these lesions, extending from the basal layer to the stratum corneum. Fungal and bacterial stains as well as cultures often have no notable findings.1,7 Similarly, histopathologic examination of our patient's biopsy with hematoxylin and eosin stain revealed hyperorthokeratosis with scattered naked vellus hair shafts and incidental yeast forms (Figure 1).
The differential diagnosis for TFFD may include pityriasis versicolor, confluent and reticulated papillomatosis, acanthosis nigricans, ichthyosis, malignant melanoma, and seborrheic keratosis. All of these diagnoses can be ruled out by the easy removal of the lesions with isopropyl alcohol 70%, which was performed on our patient by scrubbing the lesions with soaked gauze (Figure 2). Indeed, removal with isopropyl alcohol 70% is both the therapeutic and diagnostic procedure for TFFD.1-8 Of note, dermatitis neglecta is histologically and clinically identical to TFFD, albeit with a history of uncleanly habits or exposure to dirty environments.
The diagnosis of TFFD often is discovered incidentally as physicians wipe the area with alcohol to prepare for biopsy.1 Occasionally, vigorous scrubbing is needed to completely remove the lesions, and without this effort the lesions may be easily mistaken for another cutaneous process.3 Failure to consider TFFD as a diagnosis has led to unnecessary endocrine workups and invasive biopsies.4 Therefore, physicians should have early clinical suspicion of TFFD and be aware of the bedside diagnostic procedure using isopropyl alcohol.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan's dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Moon J, Kim MW, Yoon HS, et al. A case of terra firma-forme dermatosis: differentiation from other dirty-appearing diseases. Ann Dermatol. 2016;28:413-415.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Chun SW, Lee SY, Kim JB, et al. A case of terra firma-forme dermatosis treated with salicylic acid alcohol peeling. Ann Dermatol. 2017;29:83-85.
- Aslan NC, Guler S, Demirci K, et al. Features of terra firma-forme dermatosis. Ann Fam Med. 2018;16:52-54.
The Diagnosis: Terra Firma-Forme Dermatosis
Terra firma-forme dermatosis (TFFD), also known as Duncan dirty dermatosis, is an idiopathic benign cutaneous condition that is easily misdiagnosed or mismanaged. In 1987, Duncan et al1 first described the condition in children who had mothers that lamented over dirty skin spots that could not be washed off. The term terra firma translates in Latin to solid ground, which describes the characteristic dirtlike appearance of these lesions.
Terra firma-forme dermatosis most commonly affects children and young adults, though it can present in patients of any age without any known predisposing risk factors.1-4 The lesions have a predilection for the face, neck, shoulders, trunk, and ankles. Terra firma-forme dermatosis has no association with bathing and hygiene habits, and most patients describe unsuccessful removal of the lesions, even after vigorous scrubbing with soaps and detergents at home. The lesions are asymptomatic, and many patients present to dermatology for cosmetic concerns.1-8
The etiology of TFFD is not well understood and is considered a retention hyperkeratosis. Duncan et al1 postulated that TFFD is the result of partial or improper maturation of keratinocytes leading to keratinocyte and melanin retention. Hematoxylin and eosin stains demonstrate lamellar hyperkeratosis of the stratum corneum without parakeratosis as well as keratin pearls scattered throughout. Mild acanthosis and papillomatosis also have been reported.1,5-7 Fontana-Masson stain shows excess melanin in these lesions, extending from the basal layer to the stratum corneum. Fungal and bacterial stains as well as cultures often have no notable findings.1,7 Similarly, histopathologic examination of our patient's biopsy with hematoxylin and eosin stain revealed hyperorthokeratosis with scattered naked vellus hair shafts and incidental yeast forms (Figure 1).

The differential diagnosis for TFFD may include pityriasis versicolor, confluent and reticulated papillomatosis, acanthosis nigricans, ichthyosis, malignant melanoma, and seborrheic keratosis. All of these diagnoses can be ruled out by the easy removal of the lesions with isopropyl alcohol 70%, which was performed on our patient by scrubbing the lesions with soaked gauze (Figure 2). Indeed, removal with isopropyl alcohol 70% is both the therapeutic and diagnostic procedure for TFFD.1-8 Of note, dermatitis neglecta is histologically and clinically identical to TFFD, albeit with a history of uncleanly habits or exposure to dirty environments.

The diagnosis of TFFD often is discovered incidentally as physicians wipe the area with alcohol to prepare for biopsy.1 Occasionally, vigorous scrubbing is needed to completely remove the lesions, and without this effort the lesions may be easily mistaken for another cutaneous process.3 Failure to consider TFFD as a diagnosis has led to unnecessary endocrine workups and invasive biopsies.4 Therefore, physicians should have early clinical suspicion of TFFD and be aware of the bedside diagnostic procedure using isopropyl alcohol.
The Diagnosis: Terra Firma-Forme Dermatosis
Terra firma-forme dermatosis (TFFD), also known as Duncan dirty dermatosis, is an idiopathic benign cutaneous condition that is easily misdiagnosed or mismanaged. In 1987, Duncan et al1 first described the condition in children who had mothers that lamented over dirty skin spots that could not be washed off. The term terra firma translates in Latin to solid ground, which describes the characteristic dirtlike appearance of these lesions.
Terra firma-forme dermatosis most commonly affects children and young adults, though it can present in patients of any age without any known predisposing risk factors.1-4 The lesions have a predilection for the face, neck, shoulders, trunk, and ankles. Terra firma-forme dermatosis has no association with bathing and hygiene habits, and most patients describe unsuccessful removal of the lesions, even after vigorous scrubbing with soaps and detergents at home. The lesions are asymptomatic, and many patients present to dermatology for cosmetic concerns.1-8
The etiology of TFFD is not well understood and is considered a retention hyperkeratosis. Duncan et al1 postulated that TFFD is the result of partial or improper maturation of keratinocytes leading to keratinocyte and melanin retention. Hematoxylin and eosin stains demonstrate lamellar hyperkeratosis of the stratum corneum without parakeratosis as well as keratin pearls scattered throughout. Mild acanthosis and papillomatosis also have been reported.1,5-7 Fontana-Masson stain shows excess melanin in these lesions, extending from the basal layer to the stratum corneum. Fungal and bacterial stains as well as cultures often have no notable findings.1,7 Similarly, histopathologic examination of our patient's biopsy with hematoxylin and eosin stain revealed hyperorthokeratosis with scattered naked vellus hair shafts and incidental yeast forms (Figure 1).

The differential diagnosis for TFFD may include pityriasis versicolor, confluent and reticulated papillomatosis, acanthosis nigricans, ichthyosis, malignant melanoma, and seborrheic keratosis. All of these diagnoses can be ruled out by the easy removal of the lesions with isopropyl alcohol 70%, which was performed on our patient by scrubbing the lesions with soaked gauze (Figure 2). Indeed, removal with isopropyl alcohol 70% is both the therapeutic and diagnostic procedure for TFFD.1-8 Of note, dermatitis neglecta is histologically and clinically identical to TFFD, albeit with a history of uncleanly habits or exposure to dirty environments.

The diagnosis of TFFD often is discovered incidentally as physicians wipe the area with alcohol to prepare for biopsy.1 Occasionally, vigorous scrubbing is needed to completely remove the lesions, and without this effort the lesions may be easily mistaken for another cutaneous process.3 Failure to consider TFFD as a diagnosis has led to unnecessary endocrine workups and invasive biopsies.4 Therefore, physicians should have early clinical suspicion of TFFD and be aware of the bedside diagnostic procedure using isopropyl alcohol.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan's dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Moon J, Kim MW, Yoon HS, et al. A case of terra firma-forme dermatosis: differentiation from other dirty-appearing diseases. Ann Dermatol. 2016;28:413-415.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Chun SW, Lee SY, Kim JB, et al. A case of terra firma-forme dermatosis treated with salicylic acid alcohol peeling. Ann Dermatol. 2017;29:83-85.
- Aslan NC, Guler S, Demirci K, et al. Features of terra firma-forme dermatosis. Ann Fam Med. 2018;16:52-54.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan's dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Moon J, Kim MW, Yoon HS, et al. A case of terra firma-forme dermatosis: differentiation from other dirty-appearing diseases. Ann Dermatol. 2016;28:413-415.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Chun SW, Lee SY, Kim JB, et al. A case of terra firma-forme dermatosis treated with salicylic acid alcohol peeling. Ann Dermatol. 2017;29:83-85.
- Aslan NC, Guler S, Demirci K, et al. Features of terra firma-forme dermatosis. Ann Fam Med. 2018;16:52-54.
A 94-year-old woman was referred to the dermatology department for biopsy of pigmented tumors behind the ears of unknown duration. The growths were asymptomatic. Her medical history included the early stages of Alzheimer disease. On physical examination dark brown, smooth, coalescing papules and plaques were noted extending from the posterior neck to the conchal bowls and ear folds bilaterally. The nodules were removed by scrubbing with isopropyl alcohol 70%. A nodule was submitted for histopathologic review.
Erythematous Verrucous Plaque on the Hand
The Diagnosis: Chromomycosis
Skin scrapings revealed brownish sclerotic bodies. A review of the skin biopsy performed 4 years prior showed florid pseudoepitheliomatous hyperplasia overlying dense mixed inflammatory infiltrates of predominantly granulomatous microabscesses in the dermis. Numerous sclerotic bodies were evident within multinucleated giant cells and scattered among epidermal and dermal microabscesses (Figure). Few atypical basal keratinocytes were noted, but frank pleomorphism and aberrant mitosis was absent.
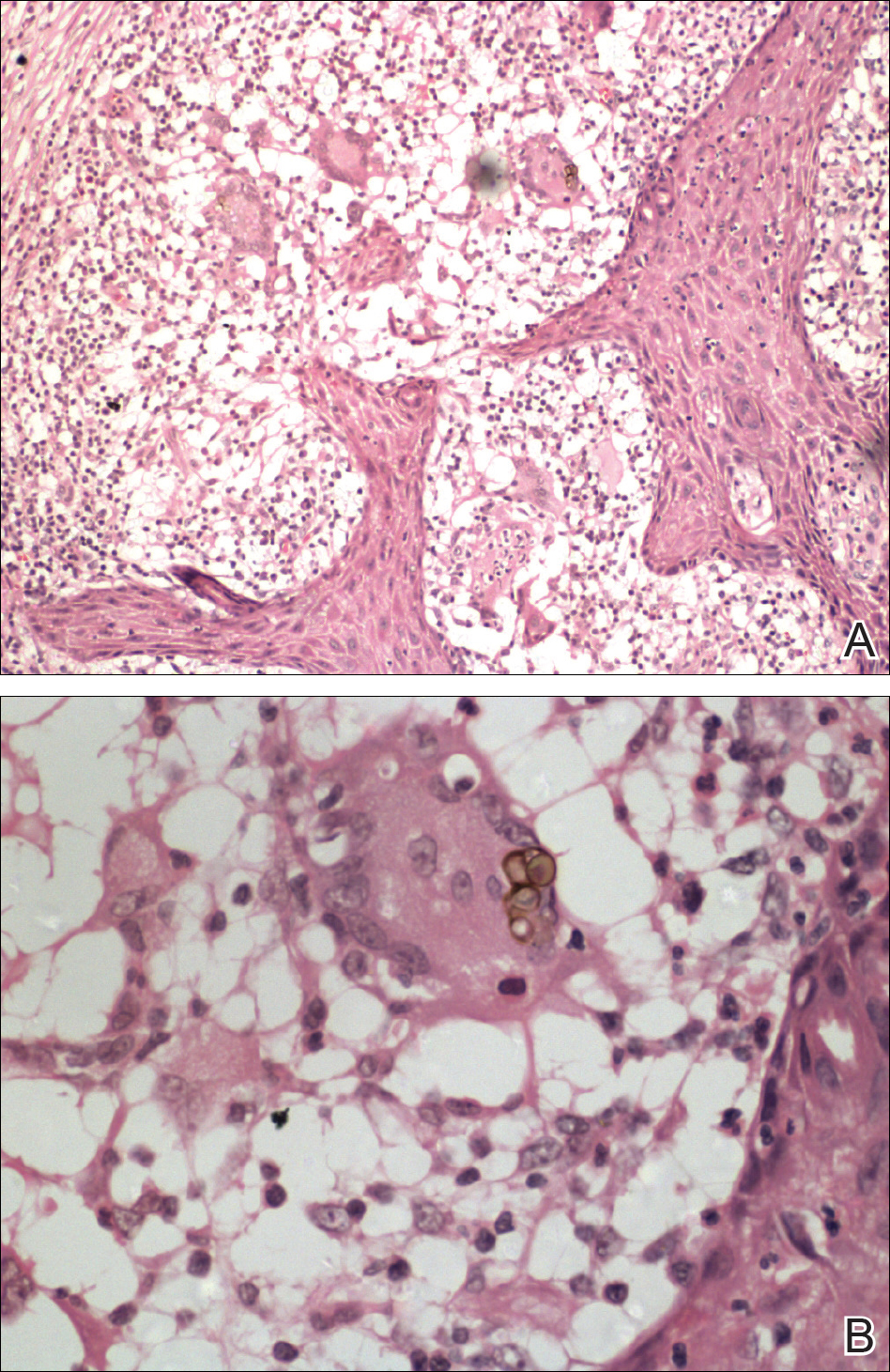
Chromomycosis is a chronic subcutaneous fungal infection caused by pigmented (dematiaceous) fungi growing in soil, decaying vegetables, and rotting wood. Infection usually occurs via traumatic inoculation from splinters and thorns. Some of the agents responsible include Fonsecaea pedrosoi, Cladophialophora carrionii, and Phialophora verrucosa.1
Diverse cutaneous manifestations have been observed with 5 different clinical forms: nodules, verrucous hyperkeratotic plaques, cicatricial lesions with central sparing, scaly plaques, and tumoral (cauliflowerlike) lesions.2 Of these clinical presentations, verrucous hyperkeratotic plaques are the most common, as seen in our patient. However, this presentation is not exclusive to chromomycosis because many conditions appear similarly, including sporotrichosis, nontuberculous mycobacterial infection, tuberculosis verrucosa cutis, and squamous cell carcinoma (SCC). The presence of small ulcerations may appear as the black dots seen on the plaques of chromomycosis, distinguishing chromomycosis from other conditions. Although this feature may be a fundamental clue for diagnosis, it should be emphasized that in many occasions, clinical differences between chromomycosis and its differentials are subtle. A study involving 9 patients with chromomycosis reported that only 1 was given the initial diagnosis of mycosis. Six patients initially were diagnosed with cutaneous malignancies, 1 patient with viral warts, and another patient with ganglion.3 Therefore, unless there is a high index of suspicion, these conditions may easily be mistaken for others by clinicians who are unfamiliar with their presentations, particularly in the setting of a busy clinic.
Chromomycosis routinely is diagnosed based on histologic examination and culture. Apart from sclerotic bodies, other histopathologic features include an inflammatory infiltrate characterized by neutrophilic microabscesses, multinucleated cells, fibrosis, acanthosis, papillomatosis, hyperkeratosis, and pseudoepitheliomatous hyperplasia (PEH).2 Pseudoepitheliomatous hyperplasia is an exaggerated proliferation of the epidermis, usually secondary to chronic inflammatory skin conditions.4 Because most verrucous lesions are thought to be neoplastic and carcinomas more commonly are seen and expected in dermatopathology, PEH can sometimes be mistaken for SCC. At times, the squamous epithelium of PEH can appear infiltrative, giving the illusion of well-differentiated SCC.5 However, absence of marked cellular atypia and abnormal mitotic activity should suggest otherwise. Thorough scrutiny for a concomitant infective process is necessary to avoid the overdiagnosis of SCC. Special stains for infectious agents such as periodic acid-Schiff and Grocott-Gomori methenamine-silver for fungal spores and Ziehl-Neelsen for acid-fast bacilli may reveal infectious organisms. Multilevel sections of deeper levels also may be essential to uncover sparse organisms.6
There is no standard treatment of chromomycosis. Some treatment options are available based on few open clinical studies and expert opinions. Systemic antifungals such as itraconazole or terbinafine most commonly are used with 15% to 80% cure rates.7 In invasive refractory cases, a combination of itraconazole and terbinafine has been employed as salvage therapy. Recently, the use of newer azoles such as posaconazole is favored due to its expanded-spectrum profile along with better pharmacodynamics and pharmacokinetic profile versus itraconazole. Physical methods such as cryotherapy, heat therapy, laser therapy, and photodynamic therapy frequently are practiced in conjunction with systemic antifungal therapy.8 Surgical procedures such as photocoagulation, Mohs micrographic surgery, and curettage sometimes are recommended for smaller well-defined lesions. Amputation, however, is rarely ever indicated, as there rarely is deep tissue involvement.2
Our case highlights the importance of clinicopathologic correlation in diagnosing squamous epithelial lesions. A high index of clinical suspicion and a wider list of differential diagnoses of verrucous plaques are necessary to minimize pitfalls in diagnosing lesions with squamous proliferation and therefore reduces the need for unnecessary interventions.
- Queiroz-Telles F, Esterre P, Perez-Blanco M, et al. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47:3-15.
- Krzyściak PM, Pindycka-Piaszczyńska M, Piaszczyński M. Chromoblastomycosis. Postepy Dermatol Allergol. 2014;31:310-321.
- Jayalakshmi P, Looi LM, Soo-Hoo TS. Chromoblastomycosis in Malaysia. Mycopathologica. 1990;109:27-31.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- El-Khoury J, Kibbi AG, Abbas O. Mucocutaneous pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2012;34:165-175.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- Queiroz-Telles F, Santos DW. Challenges in the therapy of chromoblastomycosis. Mycopathologia. 2013;175:477-488.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276.
The Diagnosis: Chromomycosis
Skin scrapings revealed brownish sclerotic bodies. A review of the skin biopsy performed 4 years prior showed florid pseudoepitheliomatous hyperplasia overlying dense mixed inflammatory infiltrates of predominantly granulomatous microabscesses in the dermis. Numerous sclerotic bodies were evident within multinucleated giant cells and scattered among epidermal and dermal microabscesses (Figure). Few atypical basal keratinocytes were noted, but frank pleomorphism and aberrant mitosis was absent.

Chromomycosis is a chronic subcutaneous fungal infection caused by pigmented (dematiaceous) fungi growing in soil, decaying vegetables, and rotting wood. Infection usually occurs via traumatic inoculation from splinters and thorns. Some of the agents responsible include Fonsecaea pedrosoi, Cladophialophora carrionii, and Phialophora verrucosa.1
Diverse cutaneous manifestations have been observed with 5 different clinical forms: nodules, verrucous hyperkeratotic plaques, cicatricial lesions with central sparing, scaly plaques, and tumoral (cauliflowerlike) lesions.2 Of these clinical presentations, verrucous hyperkeratotic plaques are the most common, as seen in our patient. However, this presentation is not exclusive to chromomycosis because many conditions appear similarly, including sporotrichosis, nontuberculous mycobacterial infection, tuberculosis verrucosa cutis, and squamous cell carcinoma (SCC). The presence of small ulcerations may appear as the black dots seen on the plaques of chromomycosis, distinguishing chromomycosis from other conditions. Although this feature may be a fundamental clue for diagnosis, it should be emphasized that in many occasions, clinical differences between chromomycosis and its differentials are subtle. A study involving 9 patients with chromomycosis reported that only 1 was given the initial diagnosis of mycosis. Six patients initially were diagnosed with cutaneous malignancies, 1 patient with viral warts, and another patient with ganglion.3 Therefore, unless there is a high index of suspicion, these conditions may easily be mistaken for others by clinicians who are unfamiliar with their presentations, particularly in the setting of a busy clinic.
Chromomycosis routinely is diagnosed based on histologic examination and culture. Apart from sclerotic bodies, other histopathologic features include an inflammatory infiltrate characterized by neutrophilic microabscesses, multinucleated cells, fibrosis, acanthosis, papillomatosis, hyperkeratosis, and pseudoepitheliomatous hyperplasia (PEH).2 Pseudoepitheliomatous hyperplasia is an exaggerated proliferation of the epidermis, usually secondary to chronic inflammatory skin conditions.4 Because most verrucous lesions are thought to be neoplastic and carcinomas more commonly are seen and expected in dermatopathology, PEH can sometimes be mistaken for SCC. At times, the squamous epithelium of PEH can appear infiltrative, giving the illusion of well-differentiated SCC.5 However, absence of marked cellular atypia and abnormal mitotic activity should suggest otherwise. Thorough scrutiny for a concomitant infective process is necessary to avoid the overdiagnosis of SCC. Special stains for infectious agents such as periodic acid-Schiff and Grocott-Gomori methenamine-silver for fungal spores and Ziehl-Neelsen for acid-fast bacilli may reveal infectious organisms. Multilevel sections of deeper levels also may be essential to uncover sparse organisms.6
There is no standard treatment of chromomycosis. Some treatment options are available based on few open clinical studies and expert opinions. Systemic antifungals such as itraconazole or terbinafine most commonly are used with 15% to 80% cure rates.7 In invasive refractory cases, a combination of itraconazole and terbinafine has been employed as salvage therapy. Recently, the use of newer azoles such as posaconazole is favored due to its expanded-spectrum profile along with better pharmacodynamics and pharmacokinetic profile versus itraconazole. Physical methods such as cryotherapy, heat therapy, laser therapy, and photodynamic therapy frequently are practiced in conjunction with systemic antifungal therapy.8 Surgical procedures such as photocoagulation, Mohs micrographic surgery, and curettage sometimes are recommended for smaller well-defined lesions. Amputation, however, is rarely ever indicated, as there rarely is deep tissue involvement.2
Our case highlights the importance of clinicopathologic correlation in diagnosing squamous epithelial lesions. A high index of clinical suspicion and a wider list of differential diagnoses of verrucous plaques are necessary to minimize pitfalls in diagnosing lesions with squamous proliferation and therefore reduces the need for unnecessary interventions.
The Diagnosis: Chromomycosis
Skin scrapings revealed brownish sclerotic bodies. A review of the skin biopsy performed 4 years prior showed florid pseudoepitheliomatous hyperplasia overlying dense mixed inflammatory infiltrates of predominantly granulomatous microabscesses in the dermis. Numerous sclerotic bodies were evident within multinucleated giant cells and scattered among epidermal and dermal microabscesses (Figure). Few atypical basal keratinocytes were noted, but frank pleomorphism and aberrant mitosis was absent.

Chromomycosis is a chronic subcutaneous fungal infection caused by pigmented (dematiaceous) fungi growing in soil, decaying vegetables, and rotting wood. Infection usually occurs via traumatic inoculation from splinters and thorns. Some of the agents responsible include Fonsecaea pedrosoi, Cladophialophora carrionii, and Phialophora verrucosa.1
Diverse cutaneous manifestations have been observed with 5 different clinical forms: nodules, verrucous hyperkeratotic plaques, cicatricial lesions with central sparing, scaly plaques, and tumoral (cauliflowerlike) lesions.2 Of these clinical presentations, verrucous hyperkeratotic plaques are the most common, as seen in our patient. However, this presentation is not exclusive to chromomycosis because many conditions appear similarly, including sporotrichosis, nontuberculous mycobacterial infection, tuberculosis verrucosa cutis, and squamous cell carcinoma (SCC). The presence of small ulcerations may appear as the black dots seen on the plaques of chromomycosis, distinguishing chromomycosis from other conditions. Although this feature may be a fundamental clue for diagnosis, it should be emphasized that in many occasions, clinical differences between chromomycosis and its differentials are subtle. A study involving 9 patients with chromomycosis reported that only 1 was given the initial diagnosis of mycosis. Six patients initially were diagnosed with cutaneous malignancies, 1 patient with viral warts, and another patient with ganglion.3 Therefore, unless there is a high index of suspicion, these conditions may easily be mistaken for others by clinicians who are unfamiliar with their presentations, particularly in the setting of a busy clinic.
Chromomycosis routinely is diagnosed based on histologic examination and culture. Apart from sclerotic bodies, other histopathologic features include an inflammatory infiltrate characterized by neutrophilic microabscesses, multinucleated cells, fibrosis, acanthosis, papillomatosis, hyperkeratosis, and pseudoepitheliomatous hyperplasia (PEH).2 Pseudoepitheliomatous hyperplasia is an exaggerated proliferation of the epidermis, usually secondary to chronic inflammatory skin conditions.4 Because most verrucous lesions are thought to be neoplastic and carcinomas more commonly are seen and expected in dermatopathology, PEH can sometimes be mistaken for SCC. At times, the squamous epithelium of PEH can appear infiltrative, giving the illusion of well-differentiated SCC.5 However, absence of marked cellular atypia and abnormal mitotic activity should suggest otherwise. Thorough scrutiny for a concomitant infective process is necessary to avoid the overdiagnosis of SCC. Special stains for infectious agents such as periodic acid-Schiff and Grocott-Gomori methenamine-silver for fungal spores and Ziehl-Neelsen for acid-fast bacilli may reveal infectious organisms. Multilevel sections of deeper levels also may be essential to uncover sparse organisms.6
There is no standard treatment of chromomycosis. Some treatment options are available based on few open clinical studies and expert opinions. Systemic antifungals such as itraconazole or terbinafine most commonly are used with 15% to 80% cure rates.7 In invasive refractory cases, a combination of itraconazole and terbinafine has been employed as salvage therapy. Recently, the use of newer azoles such as posaconazole is favored due to its expanded-spectrum profile along with better pharmacodynamics and pharmacokinetic profile versus itraconazole. Physical methods such as cryotherapy, heat therapy, laser therapy, and photodynamic therapy frequently are practiced in conjunction with systemic antifungal therapy.8 Surgical procedures such as photocoagulation, Mohs micrographic surgery, and curettage sometimes are recommended for smaller well-defined lesions. Amputation, however, is rarely ever indicated, as there rarely is deep tissue involvement.2
Our case highlights the importance of clinicopathologic correlation in diagnosing squamous epithelial lesions. A high index of clinical suspicion and a wider list of differential diagnoses of verrucous plaques are necessary to minimize pitfalls in diagnosing lesions with squamous proliferation and therefore reduces the need for unnecessary interventions.
- Queiroz-Telles F, Esterre P, Perez-Blanco M, et al. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47:3-15.
- Krzyściak PM, Pindycka-Piaszczyńska M, Piaszczyński M. Chromoblastomycosis. Postepy Dermatol Allergol. 2014;31:310-321.
- Jayalakshmi P, Looi LM, Soo-Hoo TS. Chromoblastomycosis in Malaysia. Mycopathologica. 1990;109:27-31.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- El-Khoury J, Kibbi AG, Abbas O. Mucocutaneous pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2012;34:165-175.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- Queiroz-Telles F, Santos DW. Challenges in the therapy of chromoblastomycosis. Mycopathologia. 2013;175:477-488.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276.
- Queiroz-Telles F, Esterre P, Perez-Blanco M, et al. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47:3-15.
- Krzyściak PM, Pindycka-Piaszczyńska M, Piaszczyński M. Chromoblastomycosis. Postepy Dermatol Allergol. 2014;31:310-321.
- Jayalakshmi P, Looi LM, Soo-Hoo TS. Chromoblastomycosis in Malaysia. Mycopathologica. 1990;109:27-31.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- El-Khoury J, Kibbi AG, Abbas O. Mucocutaneous pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2012;34:165-175.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- Queiroz-Telles F, Santos DW. Challenges in the therapy of chromoblastomycosis. Mycopathologia. 2013;175:477-488.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276.

A 75-year-old retired farmer presented with an erythematous verrucous plaque on the dorsal aspect of the left hand of 4 years' duration. Superficial biopsies from the lesion 4 years prior to presentation revealed pseudoepitheliomatous hyperplasia suggestive of squamous cell carcinoma, which led to the excision of the lesion along with 2 digits of the left hand. Despite surgery, the lesions promptly recurred and continued to progress. Physical examination revealed a verrucous plaque with crusting and small ulcerations (black dots) over the extensor aspect of the left hand and forearm.
Perianal Ulceration and Verrucous Papules
The Diagnosis: Herpes Simplex Virus Infection
Viral culture of the ulcer was positive for herpes simplex virus type 2 (HHV-2). Bacterial culture grew enteric flora. The patient was started on intravenous acyclovir 5 mg/kg every 8 hours for 7 days and then transitioned to oral acyclovir for chronic suppressive therapy. One month later, there was near-complete reepithelialization with 2 remaining 1-cm shallow ulcers. The verrucous lesions had dried up and were flaking off (Figure). At 6-month follow-up, the ulcers and verrucous lesions had completely resolved.
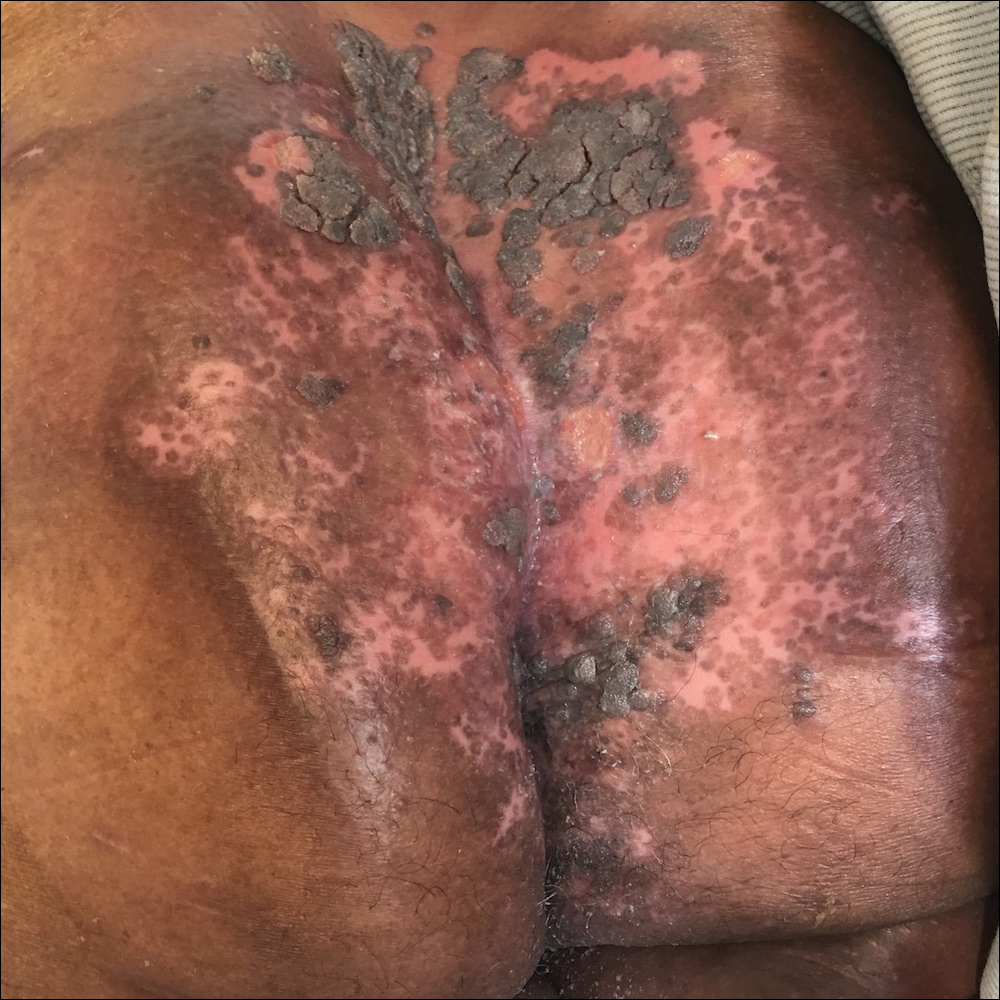
Herpes simplex virus type 2 is the most common cause of genital and perianal ulcers in immunocompromised individuals.1 Patients classically present with painful grouped vesicles followed by painful superficial ulcers that may rapidly progress to extensive confluent ulceration. A hypertrophic variant of genital herpes characterized by anogenital verrucous lesions, similar to condyloma acuminata, also can be seen in immunocompromised individuals.2 This form has almost exclusively been observed in patients with human immunodeficiency virus and may occur in isolation or together with the ulcerative form.1-5 A case of vegetative HHV infection of the genital area in a patient with common variable immunodeficiency has been reported.6 Verrucous lesions of the mouth secondary to HHV have been observed in Hodgkin lymphoma, acute myelogenous leukemia, and individuals on immunosuppressive medications.7-10
Perianal involvement of Crohn disease typically presents with fistulas, ulcers, abscesses, strictures, and skin tags in some cases. Invasive squamous cell carcinoma may arise within a chronic ulcer of the anogenital area or may itself manifest as an ulcer or anal fissure. Perianal ulcerative skin tuberculosis has been reported in the literature as a rare manifestation of extrapulmonary tuberculosis and should be considered in a patient with an appropriate clinical history. Pyoderma gangrenosum classically pre-sents as a large ulcer with irregular rolled borders, though a rare variant of vegetative pyoderma gangrenosum may manifest as a nodular or verrucous plaque.
Studies to diagnose HHV include viral cell culture, HHV polymerase chain reaction testing, HHV serology, and direct fluorescent antibody testing. Skin biopsy may be necessary to rule out underlying malignancy. Treatment of perianal HHV infection includes acyclovir, valacyclovir, or famciclovir.1,5,6 Hypertrophic lesions often are refractory to first-line antiviral therapy and may require surgical resection or treatment with alternative medications such as imiquimod, a topical immunomodulator.3,5,6,11
- Ranu H, Lee J, Chio M, et al. Tumour-like presentations of anogenital herpes simplex in HIV-positive patients. Int J STD AIDS. 2011;22:181-186.
- Tong P, Mutasim DF. Herpes simplex virus infection masquerading as condylomata accuminata in a patient with HIV disease. Br J Dermatol. 1996;134:797-800.
- Mosunjac M, Park J, Wang W, et al. Genital and perianal herpes simplex simulating neoplasia in patients with AIDS. AIDS Patient Care STDS. 2009;23:153-158.
- Gubinelli E, Cocuroccia B, Lazzarotto T, et al. Nodular perianal herpes simplex with prominent plasma cell infiltration. Sexually Transm Dis. 2003;30:157-159.
- Nadal SR, Calore EE, Manzione CR, et al. Hypertrophic herpes simplex simulating anal neoplasia in AIDS patients: report of five cases. Dis Colon Rectum. 2005;48:2289-2293.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Burke EM, Karp DL, Wu TC, et al. Atypical oral presentation of herpes simplex virus infection in a patient after orthotopic liver transplantation. Eur Arch Otorhinolaryngol. 1994;251:301-303.
- Tabaee A, Saltman B, Shutter J, et al. Recurrent oral herpes simplex virus infection presenting as a tongue mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:376-380.
- Leming PD, Martin SE, Zwelling LA. Atypical herpes simplex (HSV) infection in a patient with Hodgkin's disease. Cancer. 1984;54:3043-3047.
- Burgoyne M, Burke W. Atypical herpes simplex infection in patients with acute myelogenous leukemia recovering from chemotherapy. J Am Acad Dermatol. 1989;20:1125-1126.
- Deza G, Martin-Ezquerra G, Curto-Barredo L, et al. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42:1176-1178.
The Diagnosis: Herpes Simplex Virus Infection
Viral culture of the ulcer was positive for herpes simplex virus type 2 (HHV-2). Bacterial culture grew enteric flora. The patient was started on intravenous acyclovir 5 mg/kg every 8 hours for 7 days and then transitioned to oral acyclovir for chronic suppressive therapy. One month later, there was near-complete reepithelialization with 2 remaining 1-cm shallow ulcers. The verrucous lesions had dried up and were flaking off (Figure). At 6-month follow-up, the ulcers and verrucous lesions had completely resolved.

Herpes simplex virus type 2 is the most common cause of genital and perianal ulcers in immunocompromised individuals.1 Patients classically present with painful grouped vesicles followed by painful superficial ulcers that may rapidly progress to extensive confluent ulceration. A hypertrophic variant of genital herpes characterized by anogenital verrucous lesions, similar to condyloma acuminata, also can be seen in immunocompromised individuals.2 This form has almost exclusively been observed in patients with human immunodeficiency virus and may occur in isolation or together with the ulcerative form.1-5 A case of vegetative HHV infection of the genital area in a patient with common variable immunodeficiency has been reported.6 Verrucous lesions of the mouth secondary to HHV have been observed in Hodgkin lymphoma, acute myelogenous leukemia, and individuals on immunosuppressive medications.7-10
Perianal involvement of Crohn disease typically presents with fistulas, ulcers, abscesses, strictures, and skin tags in some cases. Invasive squamous cell carcinoma may arise within a chronic ulcer of the anogenital area or may itself manifest as an ulcer or anal fissure. Perianal ulcerative skin tuberculosis has been reported in the literature as a rare manifestation of extrapulmonary tuberculosis and should be considered in a patient with an appropriate clinical history. Pyoderma gangrenosum classically pre-sents as a large ulcer with irregular rolled borders, though a rare variant of vegetative pyoderma gangrenosum may manifest as a nodular or verrucous plaque.
Studies to diagnose HHV include viral cell culture, HHV polymerase chain reaction testing, HHV serology, and direct fluorescent antibody testing. Skin biopsy may be necessary to rule out underlying malignancy. Treatment of perianal HHV infection includes acyclovir, valacyclovir, or famciclovir.1,5,6 Hypertrophic lesions often are refractory to first-line antiviral therapy and may require surgical resection or treatment with alternative medications such as imiquimod, a topical immunomodulator.3,5,6,11
The Diagnosis: Herpes Simplex Virus Infection
Viral culture of the ulcer was positive for herpes simplex virus type 2 (HHV-2). Bacterial culture grew enteric flora. The patient was started on intravenous acyclovir 5 mg/kg every 8 hours for 7 days and then transitioned to oral acyclovir for chronic suppressive therapy. One month later, there was near-complete reepithelialization with 2 remaining 1-cm shallow ulcers. The verrucous lesions had dried up and were flaking off (Figure). At 6-month follow-up, the ulcers and verrucous lesions had completely resolved.

Herpes simplex virus type 2 is the most common cause of genital and perianal ulcers in immunocompromised individuals.1 Patients classically present with painful grouped vesicles followed by painful superficial ulcers that may rapidly progress to extensive confluent ulceration. A hypertrophic variant of genital herpes characterized by anogenital verrucous lesions, similar to condyloma acuminata, also can be seen in immunocompromised individuals.2 This form has almost exclusively been observed in patients with human immunodeficiency virus and may occur in isolation or together with the ulcerative form.1-5 A case of vegetative HHV infection of the genital area in a patient with common variable immunodeficiency has been reported.6 Verrucous lesions of the mouth secondary to HHV have been observed in Hodgkin lymphoma, acute myelogenous leukemia, and individuals on immunosuppressive medications.7-10
Perianal involvement of Crohn disease typically presents with fistulas, ulcers, abscesses, strictures, and skin tags in some cases. Invasive squamous cell carcinoma may arise within a chronic ulcer of the anogenital area or may itself manifest as an ulcer or anal fissure. Perianal ulcerative skin tuberculosis has been reported in the literature as a rare manifestation of extrapulmonary tuberculosis and should be considered in a patient with an appropriate clinical history. Pyoderma gangrenosum classically pre-sents as a large ulcer with irregular rolled borders, though a rare variant of vegetative pyoderma gangrenosum may manifest as a nodular or verrucous plaque.
Studies to diagnose HHV include viral cell culture, HHV polymerase chain reaction testing, HHV serology, and direct fluorescent antibody testing. Skin biopsy may be necessary to rule out underlying malignancy. Treatment of perianal HHV infection includes acyclovir, valacyclovir, or famciclovir.1,5,6 Hypertrophic lesions often are refractory to first-line antiviral therapy and may require surgical resection or treatment with alternative medications such as imiquimod, a topical immunomodulator.3,5,6,11
- Ranu H, Lee J, Chio M, et al. Tumour-like presentations of anogenital herpes simplex in HIV-positive patients. Int J STD AIDS. 2011;22:181-186.
- Tong P, Mutasim DF. Herpes simplex virus infection masquerading as condylomata accuminata in a patient with HIV disease. Br J Dermatol. 1996;134:797-800.
- Mosunjac M, Park J, Wang W, et al. Genital and perianal herpes simplex simulating neoplasia in patients with AIDS. AIDS Patient Care STDS. 2009;23:153-158.
- Gubinelli E, Cocuroccia B, Lazzarotto T, et al. Nodular perianal herpes simplex with prominent plasma cell infiltration. Sexually Transm Dis. 2003;30:157-159.
- Nadal SR, Calore EE, Manzione CR, et al. Hypertrophic herpes simplex simulating anal neoplasia in AIDS patients: report of five cases. Dis Colon Rectum. 2005;48:2289-2293.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Burke EM, Karp DL, Wu TC, et al. Atypical oral presentation of herpes simplex virus infection in a patient after orthotopic liver transplantation. Eur Arch Otorhinolaryngol. 1994;251:301-303.
- Tabaee A, Saltman B, Shutter J, et al. Recurrent oral herpes simplex virus infection presenting as a tongue mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:376-380.
- Leming PD, Martin SE, Zwelling LA. Atypical herpes simplex (HSV) infection in a patient with Hodgkin's disease. Cancer. 1984;54:3043-3047.
- Burgoyne M, Burke W. Atypical herpes simplex infection in patients with acute myelogenous leukemia recovering from chemotherapy. J Am Acad Dermatol. 1989;20:1125-1126.
- Deza G, Martin-Ezquerra G, Curto-Barredo L, et al. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42:1176-1178.
- Ranu H, Lee J, Chio M, et al. Tumour-like presentations of anogenital herpes simplex in HIV-positive patients. Int J STD AIDS. 2011;22:181-186.
- Tong P, Mutasim DF. Herpes simplex virus infection masquerading as condylomata accuminata in a patient with HIV disease. Br J Dermatol. 1996;134:797-800.
- Mosunjac M, Park J, Wang W, et al. Genital and perianal herpes simplex simulating neoplasia in patients with AIDS. AIDS Patient Care STDS. 2009;23:153-158.
- Gubinelli E, Cocuroccia B, Lazzarotto T, et al. Nodular perianal herpes simplex with prominent plasma cell infiltration. Sexually Transm Dis. 2003;30:157-159.
- Nadal SR, Calore EE, Manzione CR, et al. Hypertrophic herpes simplex simulating anal neoplasia in AIDS patients: report of five cases. Dis Colon Rectum. 2005;48:2289-2293.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Burke EM, Karp DL, Wu TC, et al. Atypical oral presentation of herpes simplex virus infection in a patient after orthotopic liver transplantation. Eur Arch Otorhinolaryngol. 1994;251:301-303.
- Tabaee A, Saltman B, Shutter J, et al. Recurrent oral herpes simplex virus infection presenting as a tongue mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:376-380.
- Leming PD, Martin SE, Zwelling LA. Atypical herpes simplex (HSV) infection in a patient with Hodgkin's disease. Cancer. 1984;54:3043-3047.
- Burgoyne M, Burke W. Atypical herpes simplex infection in patients with acute myelogenous leukemia recovering from chemotherapy. J Am Acad Dermatol. 1989;20:1125-1126.
- Deza G, Martin-Ezquerra G, Curto-Barredo L, et al. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42:1176-1178.
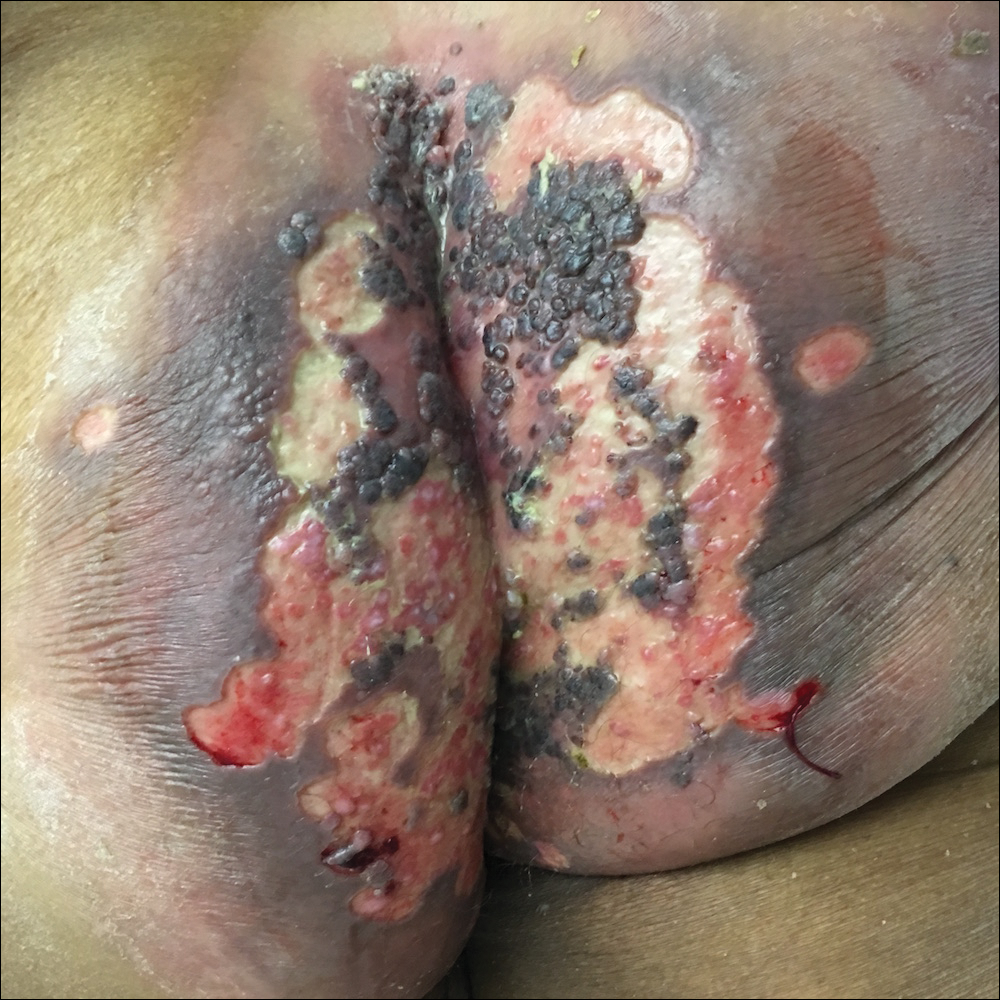
A 75-year-old woman with chronic lymphocytic leukemia undergoing ibrutinib targeted therapy presented to the emergency department with fever and perianal pain of 4 months' duration. The patient denied history of genital or perianal ulcers, warts, masses, bedsores, prolonged immobilization, anal surgeries, or recent travel. She had not been previously treated for the perianal pain. On physical examination there was an 18×15-cm shallow ulceration with rolled borders involving the intergluteal cleft and perianal area. There were numerous hyperpigmented verrucous papules clustered in the center of the ulceration. No vesicles or bullae were present. Laboratory results were pertinent for a white blood cell count of 3600/µL (reference range, 4500-11,000/µL) and absolute neutrophil count of 1300/µL (reference range, 1900-8000/µL). Human immunodeficiency virus testing was negative.