User login
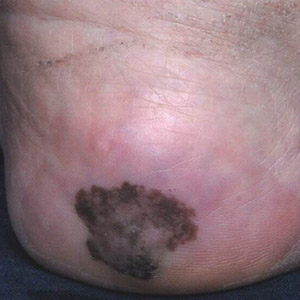
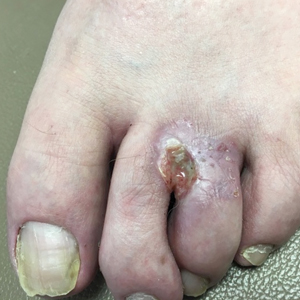
Irregularly Hyperpigmented Plaque on the Right Heel
The Diagnosis: Pigmented Bowen Disease
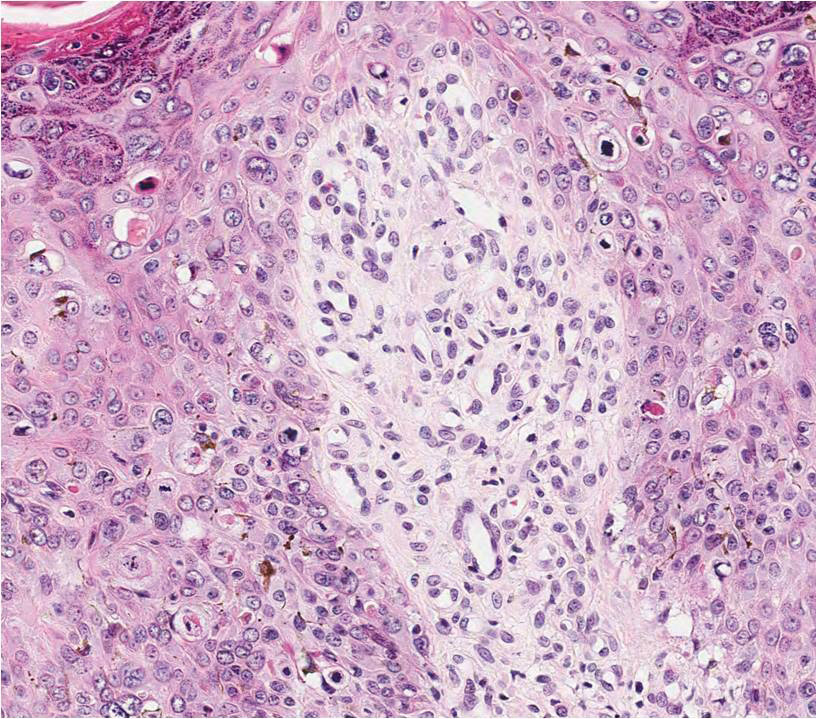
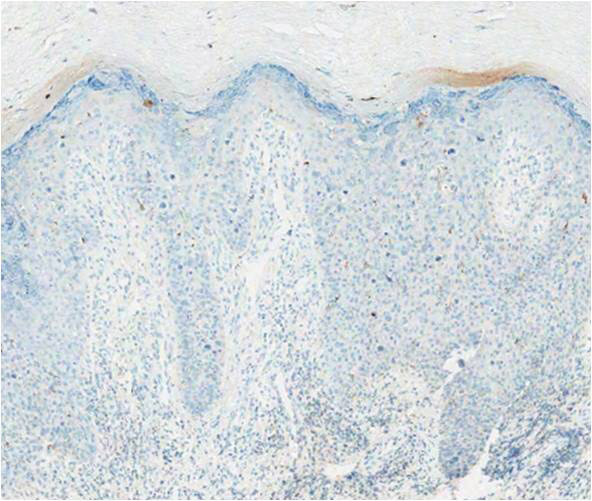
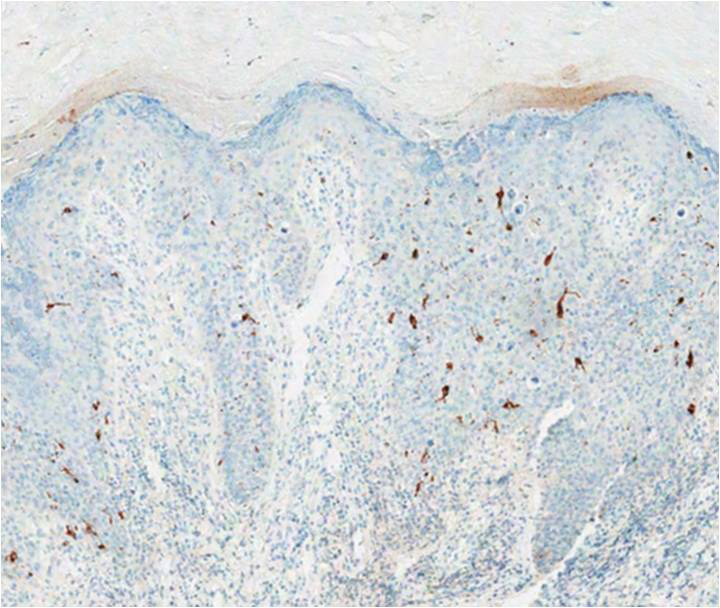
A biopsy of the lesion was performed for suspected acral malignant melanoma. Hematoxylin and eosin staining revealed acanthosis, elongation of rete ridges, and keratinocytes in complete disorder with atypical mitoses and pleomorphism affecting the full layer of the epidermis (Figure 1). The basement membrane was intact. Melanin pigmentation was increased in the lower epidermis and the upper dermis, and a lymphohistiocytic inflammatory infiltrate was present in the dermis. Staining for carcinoembryonic antigen (Figure 2) and melanoma
antigen (Figure 3) recognized by T cells (melan-A) both revealed negative results. Histopathologic findings led to the diagnosis of pigmented Bowen disease (BD).
Pigmented BD is a rare variant that accounts for 1.7% (N=420) to 5.5% (N=951) of all cases of BD.1,2 It is reported to affect men more than women and to be more prevalent in individuals with higher Fitzpatrick skin types.3 Furthermore, exposure to UV radiation, chemicals (eg, arsenic), or human papillomavirus, as well as immunosuppression, are known to be related to pigmented BD.2,4 Clinically, pigmented BD commonly involves nonexposed areas such as the anogenital area, trunk, and extremities, unlike typical BD that involves sun-exposed areas.5 In addition, it most frequently presents as a well-delineated, irregularly pigmented, asymptomatic
plaque and not as a scaly erythematous plaque. Therefore, the clinical diagnosis may be challenging. The differential diagnosis includes malignant melanoma, pigmented extramammary Paget disease, pigmented basal cell carcinoma, seborrheic keratosis, pigmented actinic keratosis, solar lentigo, and melanocytic nevi.
Histopathologically, a varying amount of melanin deposit is noted on hematoxylin and eosin staining, along with features of BD, including disarrayed atypical keratinocytes involving the full epidermis but not the basement membrane, with atypical individual cell keratinization.3,5,6 Pigmented extramammary Paget disease can mimic pigmented BD clinically and pathologically, but Paget cells stain positive for anticytokeratin (CAM 5.2), carcinoembryonic antigen, and mucicarmine, whereas cells in pigmented BD stain negative.7 Moreover, negative staining for human melanoma black, melan-A, and S-100 helps differentiate malignant melanoma from pigmented BD.8
The prognosis of pigmented BD is similar to classic BD and is independent of the presence of melanin pigment.6 Therefore, the treatment options do not differ from those for typical BD and include surgical excision, cryotherapy, laser ablation, topical imiquimod or 5-fluorouracil, curettage, electrosurgery, and photodynamic therapy (PDT).
In our case, the patient and her family did not want surgical removal; therefore, 1 course of fractional laser-assisted PDT and 2 courses of ablative laser-assisted PDT were performed. Unfortunately, the lesion persisted, possibly because it was too large and pigmented. Two months later, ingenol mebutate gel 0.05% was applied (4 courses) after using an ablative laser over 3 consecutive days with a 1-month interval between courses. The lesion resolved without any adverse events.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease [published online January 15, 2010]. J Am Acad Dermatol. 2010;62:597-604.
- Ragi G, Turner MS, Klein LE, et al. Pigmented Bowen’s disease and review of 420 Bowen’s disease lesions. J Dermatol Surg Oncol. 1988;14:765-769.
- Hernandez C, Ivkovic A, Fowler A. Growing plaque on foot. J Fam Pract. 2008;57:603-605.
- Hwang SW, Kim JW, Park SW, et al. Two cases of pigmented Bowen’s disease. Ann Dermatol 2002;14:127-129.
- Wilmer EM, Lee KC, Higgins W 2nd, et al. Hyperpigmented palmar plaque: an unexpected diagnosis of Bowen disease. Dermatol Online J. 2013;19:18573.
- Brinca A, Teixeira V, Gonçalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-884.
- Hilliard NJ, Huang C, Andea A. Pigmented extramammary Paget’s disease of the axilla mimicking melanoma: case report and review of the literature. J Cutan Pathol. 2009;36:995-1000.
- Öztürk Durmaz E, Dog˘ an Ekici I, Ozian F, et al. Pigmented Bowen’s disease of the genitalia masquerading as malignant melanoma. Acta Dermatovenerol Croat. 2015;23:130-133.
The Diagnosis: Pigmented Bowen Disease
A biopsy of the lesion was performed for suspected acral malignant melanoma. Hematoxylin and eosin staining revealed acanthosis, elongation of rete ridges, and keratinocytes in complete disorder with atypical mitoses and pleomorphism affecting the full layer of the epidermis (Figure 1). The basement membrane was intact. Melanin pigmentation was increased in the lower epidermis and the upper dermis, and a lymphohistiocytic inflammatory infiltrate was present in the dermis. Staining for carcinoembryonic antigen (Figure 2) and melanoma
antigen (Figure 3) recognized by T cells (melan-A) both revealed negative results. Histopathologic findings led to the diagnosis of pigmented Bowen disease (BD).



Pigmented BD is a rare variant that accounts for 1.7% (N=420) to 5.5% (N=951) of all cases of BD.1,2 It is reported to affect men more than women and to be more prevalent in individuals with higher Fitzpatrick skin types.3 Furthermore, exposure to UV radiation, chemicals (eg, arsenic), or human papillomavirus, as well as immunosuppression, are known to be related to pigmented BD.2,4 Clinically, pigmented BD commonly involves nonexposed areas such as the anogenital area, trunk, and extremities, unlike typical BD that involves sun-exposed areas.5 In addition, it most frequently presents as a well-delineated, irregularly pigmented, asymptomatic
plaque and not as a scaly erythematous plaque. Therefore, the clinical diagnosis may be challenging. The differential diagnosis includes malignant melanoma, pigmented extramammary Paget disease, pigmented basal cell carcinoma, seborrheic keratosis, pigmented actinic keratosis, solar lentigo, and melanocytic nevi.
Histopathologically, a varying amount of melanin deposit is noted on hematoxylin and eosin staining, along with features of BD, including disarrayed atypical keratinocytes involving the full epidermis but not the basement membrane, with atypical individual cell keratinization.3,5,6 Pigmented extramammary Paget disease can mimic pigmented BD clinically and pathologically, but Paget cells stain positive for anticytokeratin (CAM 5.2), carcinoembryonic antigen, and mucicarmine, whereas cells in pigmented BD stain negative.7 Moreover, negative staining for human melanoma black, melan-A, and S-100 helps differentiate malignant melanoma from pigmented BD.8
The prognosis of pigmented BD is similar to classic BD and is independent of the presence of melanin pigment.6 Therefore, the treatment options do not differ from those for typical BD and include surgical excision, cryotherapy, laser ablation, topical imiquimod or 5-fluorouracil, curettage, electrosurgery, and photodynamic therapy (PDT).
In our case, the patient and her family did not want surgical removal; therefore, 1 course of fractional laser-assisted PDT and 2 courses of ablative laser-assisted PDT were performed. Unfortunately, the lesion persisted, possibly because it was too large and pigmented. Two months later, ingenol mebutate gel 0.05% was applied (4 courses) after using an ablative laser over 3 consecutive days with a 1-month interval between courses. The lesion resolved without any adverse events.
The Diagnosis: Pigmented Bowen Disease
A biopsy of the lesion was performed for suspected acral malignant melanoma. Hematoxylin and eosin staining revealed acanthosis, elongation of rete ridges, and keratinocytes in complete disorder with atypical mitoses and pleomorphism affecting the full layer of the epidermis (Figure 1). The basement membrane was intact. Melanin pigmentation was increased in the lower epidermis and the upper dermis, and a lymphohistiocytic inflammatory infiltrate was present in the dermis. Staining for carcinoembryonic antigen (Figure 2) and melanoma
antigen (Figure 3) recognized by T cells (melan-A) both revealed negative results. Histopathologic findings led to the diagnosis of pigmented Bowen disease (BD).



Pigmented BD is a rare variant that accounts for 1.7% (N=420) to 5.5% (N=951) of all cases of BD.1,2 It is reported to affect men more than women and to be more prevalent in individuals with higher Fitzpatrick skin types.3 Furthermore, exposure to UV radiation, chemicals (eg, arsenic), or human papillomavirus, as well as immunosuppression, are known to be related to pigmented BD.2,4 Clinically, pigmented BD commonly involves nonexposed areas such as the anogenital area, trunk, and extremities, unlike typical BD that involves sun-exposed areas.5 In addition, it most frequently presents as a well-delineated, irregularly pigmented, asymptomatic
plaque and not as a scaly erythematous plaque. Therefore, the clinical diagnosis may be challenging. The differential diagnosis includes malignant melanoma, pigmented extramammary Paget disease, pigmented basal cell carcinoma, seborrheic keratosis, pigmented actinic keratosis, solar lentigo, and melanocytic nevi.
Histopathologically, a varying amount of melanin deposit is noted on hematoxylin and eosin staining, along with features of BD, including disarrayed atypical keratinocytes involving the full epidermis but not the basement membrane, with atypical individual cell keratinization.3,5,6 Pigmented extramammary Paget disease can mimic pigmented BD clinically and pathologically, but Paget cells stain positive for anticytokeratin (CAM 5.2), carcinoembryonic antigen, and mucicarmine, whereas cells in pigmented BD stain negative.7 Moreover, negative staining for human melanoma black, melan-A, and S-100 helps differentiate malignant melanoma from pigmented BD.8
The prognosis of pigmented BD is similar to classic BD and is independent of the presence of melanin pigment.6 Therefore, the treatment options do not differ from those for typical BD and include surgical excision, cryotherapy, laser ablation, topical imiquimod or 5-fluorouracil, curettage, electrosurgery, and photodynamic therapy (PDT).
In our case, the patient and her family did not want surgical removal; therefore, 1 course of fractional laser-assisted PDT and 2 courses of ablative laser-assisted PDT were performed. Unfortunately, the lesion persisted, possibly because it was too large and pigmented. Two months later, ingenol mebutate gel 0.05% was applied (4 courses) after using an ablative laser over 3 consecutive days with a 1-month interval between courses. The lesion resolved without any adverse events.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease [published online January 15, 2010]. J Am Acad Dermatol. 2010;62:597-604.
- Ragi G, Turner MS, Klein LE, et al. Pigmented Bowen’s disease and review of 420 Bowen’s disease lesions. J Dermatol Surg Oncol. 1988;14:765-769.
- Hernandez C, Ivkovic A, Fowler A. Growing plaque on foot. J Fam Pract. 2008;57:603-605.
- Hwang SW, Kim JW, Park SW, et al. Two cases of pigmented Bowen’s disease. Ann Dermatol 2002;14:127-129.
- Wilmer EM, Lee KC, Higgins W 2nd, et al. Hyperpigmented palmar plaque: an unexpected diagnosis of Bowen disease. Dermatol Online J. 2013;19:18573.
- Brinca A, Teixeira V, Gonçalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-884.
- Hilliard NJ, Huang C, Andea A. Pigmented extramammary Paget’s disease of the axilla mimicking melanoma: case report and review of the literature. J Cutan Pathol. 2009;36:995-1000.
- Öztürk Durmaz E, Dog˘ an Ekici I, Ozian F, et al. Pigmented Bowen’s disease of the genitalia masquerading as malignant melanoma. Acta Dermatovenerol Croat. 2015;23:130-133.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease [published online January 15, 2010]. J Am Acad Dermatol. 2010;62:597-604.
- Ragi G, Turner MS, Klein LE, et al. Pigmented Bowen’s disease and review of 420 Bowen’s disease lesions. J Dermatol Surg Oncol. 1988;14:765-769.
- Hernandez C, Ivkovic A, Fowler A. Growing plaque on foot. J Fam Pract. 2008;57:603-605.
- Hwang SW, Kim JW, Park SW, et al. Two cases of pigmented Bowen’s disease. Ann Dermatol 2002;14:127-129.
- Wilmer EM, Lee KC, Higgins W 2nd, et al. Hyperpigmented palmar plaque: an unexpected diagnosis of Bowen disease. Dermatol Online J. 2013;19:18573.
- Brinca A, Teixeira V, Gonçalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-884.
- Hilliard NJ, Huang C, Andea A. Pigmented extramammary Paget’s disease of the axilla mimicking melanoma: case report and review of the literature. J Cutan Pathol. 2009;36:995-1000.
- Öztürk Durmaz E, Dog˘ an Ekici I, Ozian F, et al. Pigmented Bowen’s disease of the genitalia masquerading as malignant melanoma. Acta Dermatovenerol Croat. 2015;23:130-133.
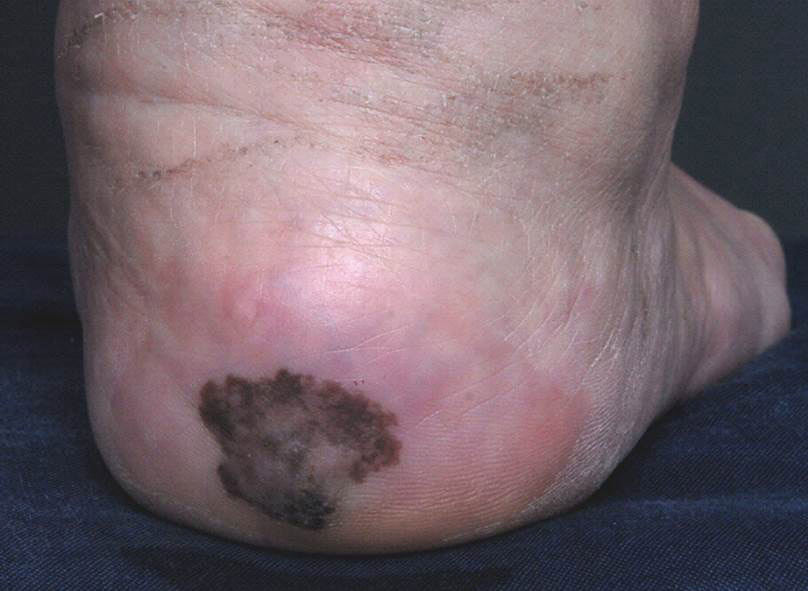
A 56-year-old woman presented with an asymptomatic plaque on the right heel that had grown
steadily over the last year. Pigmented lesions were not appreciated on other sites, and lymph nodes were not enlarged. Her medical history was otherwise normal, except for bilateral hearing loss due to encephalitis at the age of 5 years. None of her family members had similar symptoms. Physical examination revealed a well-defined, irregularly hyperpigmented plaque on the right heel.
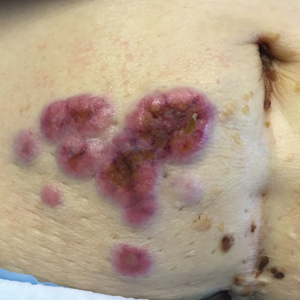
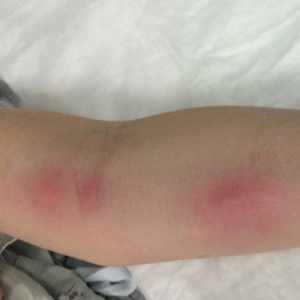
Large Hemorrhagic Plaque With Central Crusting
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
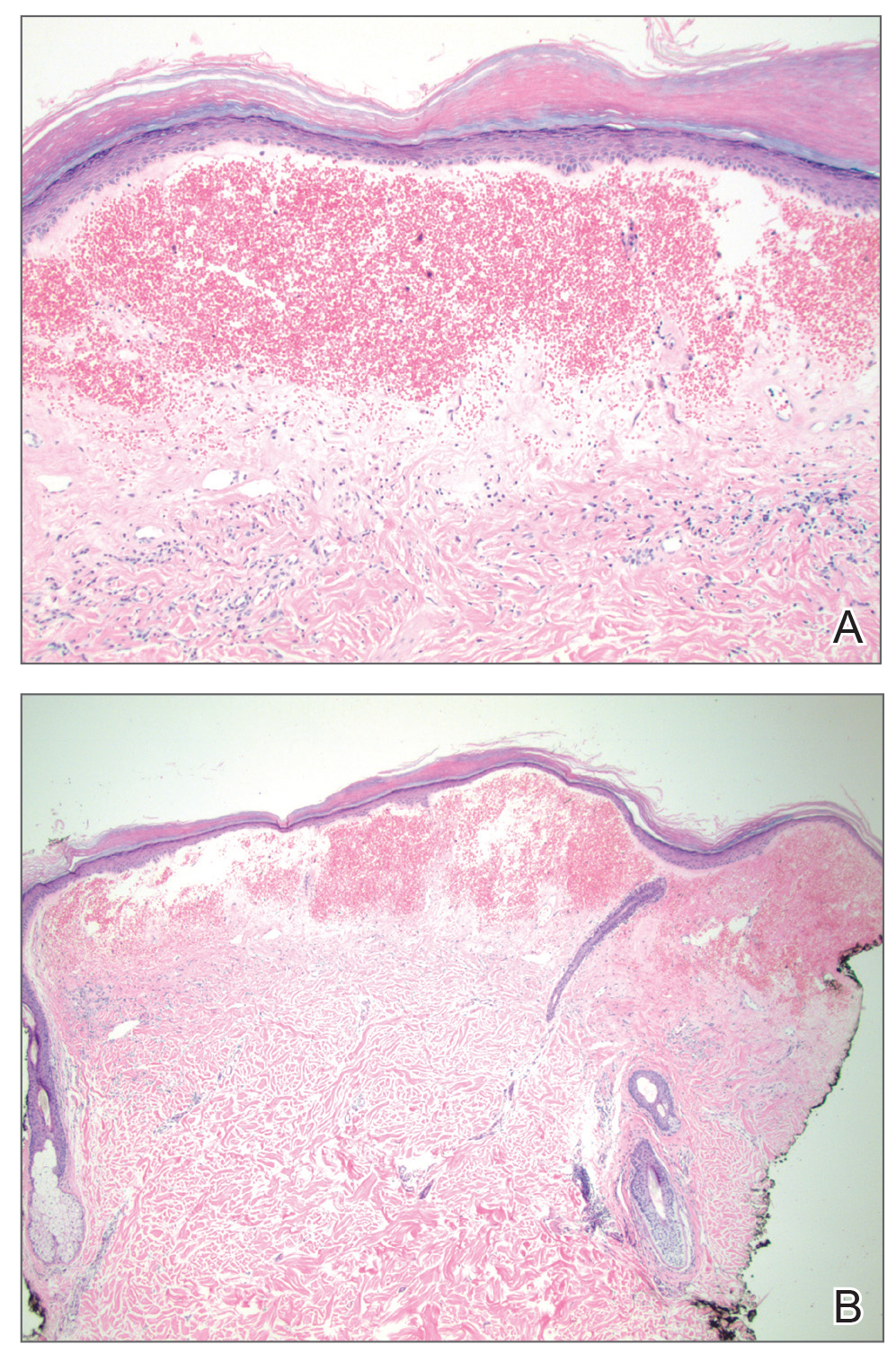
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.
Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.

Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.

Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.

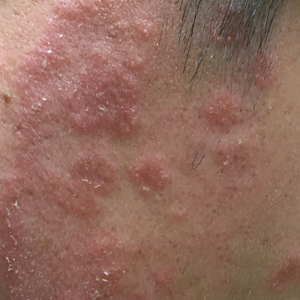
A 54-year-old woman with no notable medical history was referred to dermatology by her primary care provider for evaluation of a hematoma on the posterior neck that had developed gradually over 5 months. The lesion initially was asymptomatic but more recently had started to be painful and bleed intermittently. The patient denied any personal or family history of skin cancer. Physical examination revealed a large hemorrhagic plaque on the left side of the posterior neck with central brown-yellow crusting. There were few smaller, white, thin, sclerotic plaques with crinkling atrophy at the periphery of and inferolateral to the lesion. A punch biopsy specimen was obtained from the hemorrhagic plaque.
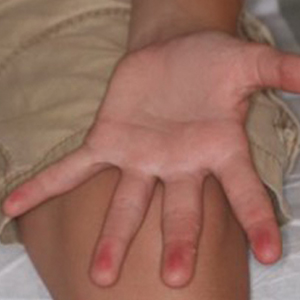
Erythematous Periumbilical Papules and Plaques
The Diagnosis: Metastatic Cancer
Further workup of patient 1 revealed an alkaline phosphatase level of 743 U/L (reference range, 30–120 U/L), total bilirubin level of 8.5 mg/dL (reference range, 0.3–1.2 mg/dL), and a white blood cell count of 14,000/μL (reference range, 4500–11,000/μL). Computed tomography of the abdomen and pelvis demonstrated cancer of unknown primary site that had metastasized to the colon, liver, and lungs. There was suspicion for potential colon cancer as the primary disease; however, based on the cutaneous findings, a skin biopsy was performed to confirm the diagnosis. Histology and immunohistochemistry revealed adenocarcinoma tumor cells positive for CDX2 (caudal type homeobox 2) and cytokeratin (CK) 7 with a subset positive for CK-20. The cells were negative for estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 (GATA binding protein 3). Immunohistochemistry was most consistent with pancreatic cancer. During palliative percutaneous transhepatic biliary drainage placement, a liver biopsy confirmed the skin biopsy results.
Further workup of patient 2 revealed a white blood cell count of 13,000/μL (reference range, 4500–11,000/μL). Computed tomography of the chest, abdomen, and pelvis revealed metastatic disease to the lungs with a suspicion for colon cancer as the primary site. Biopsy of the skin lesion revealed a mucin-producing adenocarcinoma, and immunohistochemistry was positive for keratin (AE1/AE3), CK-20, and CDX2, consistent with metastatic colon carcinoma. Immunohistochemistry of the biopsied skin lesion was nonreactive for CK-7. The patient had a colonoscopy that revealed a fungating, partially obstructing, circumferential large mass in the ascending colon.
Metastasis to the skin from visceral malignancies is not uncommon and may represent the first evidence of widespread disease, particularly in breast cancer or mucosal cancers of the head and neck.1 Cutaneous metastasis of colon cancer is uncommon and cutaneous metastasis of pancreatic cancer is rare. Furthermore, nonumbilical sites are much more common than umbilical sites for cutaneous metastatic disease.2 Pancreatic cancer is estimated to be the origin of a cutaneous umbilical metastasis, frequently termed Sister Mary Joseph nodule, in 7% to 9% of cases; colon cancer is estimated to account for 13% to 15% of cases.3 Sister Mary Joseph nodule or sign refers to a nodule often bulging into the umbilicus, signifying metastasis from a
malignant cancer.
In a study of cutaneous metastases, 10% (42/420) of patients with metastatic disease had cutaneous metastasis; 0.48% (2/420) were due to pancreatic cancer and 4.3% (18/420) were due to colon cancer.4 In another review, 63 cases of cutaneous metastasis of pancreatic cancer were found, 43 of which were nonumbilical.2
On immunohistochemistry, CK-7 positivity is highly specific for pancreatic cancer.2 Cytokeratin 7 often is used in conjunction with CK-20 to differentiate various types of glandular tumors. CDX2 is a highly sensitive and specific marker for adenocarcinomas of intestinal origin.5 The negative estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 stains are useful in excluding breast cancer (patient 1 had history of breast cancer).
When cutaneous involvement is present in pancreatic cancer, the disease usually is widespread. Multiple studies have reported involvement of other organs with cutaneous metastasis at rates of 88.9%,6 90.3%,7 and 93.5%.2 However, early recognition of metastatic cancerous lesions can lead to earlier diagnosis and earlier palliative treatment, perhaps prolonging median survival time in patients. In a review of 63 patients with cutaneous metastatic pancreatic cancer, the authors found a median survival time of 5 months, with surgery, chemotherapy, radiation therapy, or a combination helping to improve survival time from a median of 3.0 to 8.3 months.2
The location of lesions and duration of disease in both patients was atypical for arthropod assault. Acyclovir-resistant herpes zoster rarely is reported outside of human immunodeficiency patients; in addition, there was a lack of clear dermatomal distribution. Although cutaneous Crohn disease can manifest as pink papules, it is rare and unlikely as a presenting symptom. Cutaneous sarcoidosis can take many different skin manifestations, and patients can have cutaneous involvement without systemic manifestation. In both patients, medical history was more indicative of metastatic cancer than the other options in the differential diagnosis.
Cutaneous metastasis from colon cancer and pancreatic cancer is rare, and the prognosis is poor in these cases; however, in the appropriate clinical scenario, especially in a patient with a history of cancer, sinister etiologies should be considered for firm red papules of the umbilicus. Skin biopsy coupled with immunohistochemical staining can assist in identifying the primary malignancy.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-165.
- Zhou HY, Wang XB, Gao F, et al. Cutaneous metastasis from pancreatic cancer: a case report and systematic review of the literature [published online October 10, 2014]. Oncol Lett. 2014;8:2654-2660.
- Galvañ VG. Sister Mary Joseph's nodule. Ann Intern Med. 1998;128:410.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immnohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Takeuchi H, Kawano T, Toda T, et al. Cutaneous metastasis from pancreatic adenocarcinoma: a case report and a review of the literature. Hepatogastroenterology. 2003;50:275-277.
- Horino K, Hiraoka T, Kanemitsu K, et al. Subcutaneous metastases after curative resection for pancreatic carcinoma: a case report and review of the literature. Pancreas. 1999;19:406-408.
The Diagnosis: Metastatic Cancer
Further workup of patient 1 revealed an alkaline phosphatase level of 743 U/L (reference range, 30–120 U/L), total bilirubin level of 8.5 mg/dL (reference range, 0.3–1.2 mg/dL), and a white blood cell count of 14,000/μL (reference range, 4500–11,000/μL). Computed tomography of the abdomen and pelvis demonstrated cancer of unknown primary site that had metastasized to the colon, liver, and lungs. There was suspicion for potential colon cancer as the primary disease; however, based on the cutaneous findings, a skin biopsy was performed to confirm the diagnosis. Histology and immunohistochemistry revealed adenocarcinoma tumor cells positive for CDX2 (caudal type homeobox 2) and cytokeratin (CK) 7 with a subset positive for CK-20. The cells were negative for estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 (GATA binding protein 3). Immunohistochemistry was most consistent with pancreatic cancer. During palliative percutaneous transhepatic biliary drainage placement, a liver biopsy confirmed the skin biopsy results.
Further workup of patient 2 revealed a white blood cell count of 13,000/μL (reference range, 4500–11,000/μL). Computed tomography of the chest, abdomen, and pelvis revealed metastatic disease to the lungs with a suspicion for colon cancer as the primary site. Biopsy of the skin lesion revealed a mucin-producing adenocarcinoma, and immunohistochemistry was positive for keratin (AE1/AE3), CK-20, and CDX2, consistent with metastatic colon carcinoma. Immunohistochemistry of the biopsied skin lesion was nonreactive for CK-7. The patient had a colonoscopy that revealed a fungating, partially obstructing, circumferential large mass in the ascending colon.
Metastasis to the skin from visceral malignancies is not uncommon and may represent the first evidence of widespread disease, particularly in breast cancer or mucosal cancers of the head and neck.1 Cutaneous metastasis of colon cancer is uncommon and cutaneous metastasis of pancreatic cancer is rare. Furthermore, nonumbilical sites are much more common than umbilical sites for cutaneous metastatic disease.2 Pancreatic cancer is estimated to be the origin of a cutaneous umbilical metastasis, frequently termed Sister Mary Joseph nodule, in 7% to 9% of cases; colon cancer is estimated to account for 13% to 15% of cases.3 Sister Mary Joseph nodule or sign refers to a nodule often bulging into the umbilicus, signifying metastasis from a
malignant cancer.
In a study of cutaneous metastases, 10% (42/420) of patients with metastatic disease had cutaneous metastasis; 0.48% (2/420) were due to pancreatic cancer and 4.3% (18/420) were due to colon cancer.4 In another review, 63 cases of cutaneous metastasis of pancreatic cancer were found, 43 of which were nonumbilical.2
On immunohistochemistry, CK-7 positivity is highly specific for pancreatic cancer.2 Cytokeratin 7 often is used in conjunction with CK-20 to differentiate various types of glandular tumors. CDX2 is a highly sensitive and specific marker for adenocarcinomas of intestinal origin.5 The negative estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 stains are useful in excluding breast cancer (patient 1 had history of breast cancer).
When cutaneous involvement is present in pancreatic cancer, the disease usually is widespread. Multiple studies have reported involvement of other organs with cutaneous metastasis at rates of 88.9%,6 90.3%,7 and 93.5%.2 However, early recognition of metastatic cancerous lesions can lead to earlier diagnosis and earlier palliative treatment, perhaps prolonging median survival time in patients. In a review of 63 patients with cutaneous metastatic pancreatic cancer, the authors found a median survival time of 5 months, with surgery, chemotherapy, radiation therapy, or a combination helping to improve survival time from a median of 3.0 to 8.3 months.2
The location of lesions and duration of disease in both patients was atypical for arthropod assault. Acyclovir-resistant herpes zoster rarely is reported outside of human immunodeficiency patients; in addition, there was a lack of clear dermatomal distribution. Although cutaneous Crohn disease can manifest as pink papules, it is rare and unlikely as a presenting symptom. Cutaneous sarcoidosis can take many different skin manifestations, and patients can have cutaneous involvement without systemic manifestation. In both patients, medical history was more indicative of metastatic cancer than the other options in the differential diagnosis.
Cutaneous metastasis from colon cancer and pancreatic cancer is rare, and the prognosis is poor in these cases; however, in the appropriate clinical scenario, especially in a patient with a history of cancer, sinister etiologies should be considered for firm red papules of the umbilicus. Skin biopsy coupled with immunohistochemical staining can assist in identifying the primary malignancy.
The Diagnosis: Metastatic Cancer
Further workup of patient 1 revealed an alkaline phosphatase level of 743 U/L (reference range, 30–120 U/L), total bilirubin level of 8.5 mg/dL (reference range, 0.3–1.2 mg/dL), and a white blood cell count of 14,000/μL (reference range, 4500–11,000/μL). Computed tomography of the abdomen and pelvis demonstrated cancer of unknown primary site that had metastasized to the colon, liver, and lungs. There was suspicion for potential colon cancer as the primary disease; however, based on the cutaneous findings, a skin biopsy was performed to confirm the diagnosis. Histology and immunohistochemistry revealed adenocarcinoma tumor cells positive for CDX2 (caudal type homeobox 2) and cytokeratin (CK) 7 with a subset positive for CK-20. The cells were negative for estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 (GATA binding protein 3). Immunohistochemistry was most consistent with pancreatic cancer. During palliative percutaneous transhepatic biliary drainage placement, a liver biopsy confirmed the skin biopsy results.
Further workup of patient 2 revealed a white blood cell count of 13,000/μL (reference range, 4500–11,000/μL). Computed tomography of the chest, abdomen, and pelvis revealed metastatic disease to the lungs with a suspicion for colon cancer as the primary site. Biopsy of the skin lesion revealed a mucin-producing adenocarcinoma, and immunohistochemistry was positive for keratin (AE1/AE3), CK-20, and CDX2, consistent with metastatic colon carcinoma. Immunohistochemistry of the biopsied skin lesion was nonreactive for CK-7. The patient had a colonoscopy that revealed a fungating, partially obstructing, circumferential large mass in the ascending colon.
Metastasis to the skin from visceral malignancies is not uncommon and may represent the first evidence of widespread disease, particularly in breast cancer or mucosal cancers of the head and neck.1 Cutaneous metastasis of colon cancer is uncommon and cutaneous metastasis of pancreatic cancer is rare. Furthermore, nonumbilical sites are much more common than umbilical sites for cutaneous metastatic disease.2 Pancreatic cancer is estimated to be the origin of a cutaneous umbilical metastasis, frequently termed Sister Mary Joseph nodule, in 7% to 9% of cases; colon cancer is estimated to account for 13% to 15% of cases.3 Sister Mary Joseph nodule or sign refers to a nodule often bulging into the umbilicus, signifying metastasis from a
malignant cancer.
In a study of cutaneous metastases, 10% (42/420) of patients with metastatic disease had cutaneous metastasis; 0.48% (2/420) were due to pancreatic cancer and 4.3% (18/420) were due to colon cancer.4 In another review, 63 cases of cutaneous metastasis of pancreatic cancer were found, 43 of which were nonumbilical.2
On immunohistochemistry, CK-7 positivity is highly specific for pancreatic cancer.2 Cytokeratin 7 often is used in conjunction with CK-20 to differentiate various types of glandular tumors. CDX2 is a highly sensitive and specific marker for adenocarcinomas of intestinal origin.5 The negative estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 stains are useful in excluding breast cancer (patient 1 had history of breast cancer).
When cutaneous involvement is present in pancreatic cancer, the disease usually is widespread. Multiple studies have reported involvement of other organs with cutaneous metastasis at rates of 88.9%,6 90.3%,7 and 93.5%.2 However, early recognition of metastatic cancerous lesions can lead to earlier diagnosis and earlier palliative treatment, perhaps prolonging median survival time in patients. In a review of 63 patients with cutaneous metastatic pancreatic cancer, the authors found a median survival time of 5 months, with surgery, chemotherapy, radiation therapy, or a combination helping to improve survival time from a median of 3.0 to 8.3 months.2
The location of lesions and duration of disease in both patients was atypical for arthropod assault. Acyclovir-resistant herpes zoster rarely is reported outside of human immunodeficiency patients; in addition, there was a lack of clear dermatomal distribution. Although cutaneous Crohn disease can manifest as pink papules, it is rare and unlikely as a presenting symptom. Cutaneous sarcoidosis can take many different skin manifestations, and patients can have cutaneous involvement without systemic manifestation. In both patients, medical history was more indicative of metastatic cancer than the other options in the differential diagnosis.
Cutaneous metastasis from colon cancer and pancreatic cancer is rare, and the prognosis is poor in these cases; however, in the appropriate clinical scenario, especially in a patient with a history of cancer, sinister etiologies should be considered for firm red papules of the umbilicus. Skin biopsy coupled with immunohistochemical staining can assist in identifying the primary malignancy.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-165.
- Zhou HY, Wang XB, Gao F, et al. Cutaneous metastasis from pancreatic cancer: a case report and systematic review of the literature [published online October 10, 2014]. Oncol Lett. 2014;8:2654-2660.
- Galvañ VG. Sister Mary Joseph's nodule. Ann Intern Med. 1998;128:410.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immnohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Takeuchi H, Kawano T, Toda T, et al. Cutaneous metastasis from pancreatic adenocarcinoma: a case report and a review of the literature. Hepatogastroenterology. 2003;50:275-277.
- Horino K, Hiraoka T, Kanemitsu K, et al. Subcutaneous metastases after curative resection for pancreatic carcinoma: a case report and review of the literature. Pancreas. 1999;19:406-408.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-165.
- Zhou HY, Wang XB, Gao F, et al. Cutaneous metastasis from pancreatic cancer: a case report and systematic review of the literature [published online October 10, 2014]. Oncol Lett. 2014;8:2654-2660.
- Galvañ VG. Sister Mary Joseph's nodule. Ann Intern Med. 1998;128:410.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immnohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Takeuchi H, Kawano T, Toda T, et al. Cutaneous metastasis from pancreatic adenocarcinoma: a case report and a review of the literature. Hepatogastroenterology. 2003;50:275-277.
- Horino K, Hiraoka T, Kanemitsu K, et al. Subcutaneous metastases after curative resection for pancreatic carcinoma: a case report and review of the literature. Pancreas. 1999;19:406-408.

A 75-year-old woman (patient 1) with a history of localized invasive ductal breast cancer treated definitively with lumpectomy and radiation therapy more than a decade ago presented to the emergency department with jaundice, abdominal pain, weakness, and multiple periumbilical pink-red papules (top) of 2 weeks’ duration. Prior to presentation, the skin lesions did not improve with 10 days of acyclovir treatment prescribed by her primary care physician for presumed herpes zoster.
An 86-year-old man (patient 2) with chronic lymphocytic leukemia treated with ibrutinib presented to the emergency department with jaundice, abdominal pain, weakness, and multiple pink periumbilical papules (bottom) of 6 weeks’ duration. Prior to presentation, the skin lesions did not improve with 21 days of valacyclovir treatment prescribed by his oncologist for presumed herpes zoster.
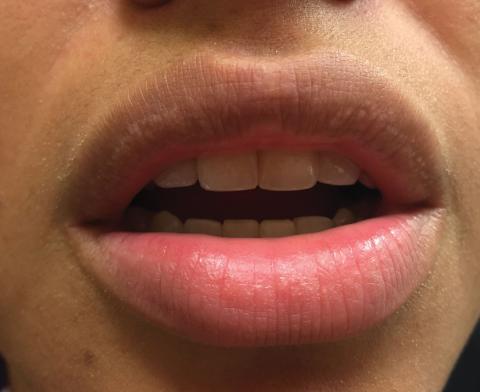
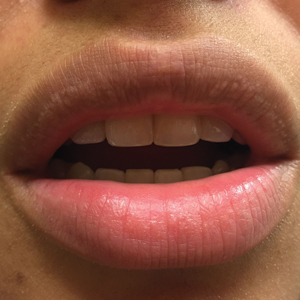
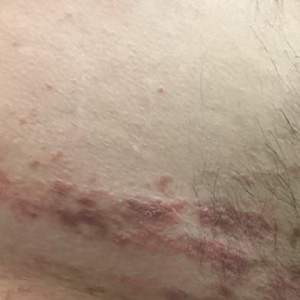
Small White Spots on the Lips
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
White Concretions on the Hair Shaft
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
A 35-year-old woman presented with possible nits on the hair of 1 year’s duration. She was previously evaluated by several outside medical providers and was unsuccessfully treated with topical and systemic medications for pediculosis. She reported sporadic scalp pruritus but denied hair loss, breakage, close contacts with similar symptoms, or recent travel outside the United States. She was otherwise healthy and was not taking any medications. Physical examination revealed small 1- to 2-mm, generalized, somewhat detachable, white concretions randomly distributed on the hair shafts. No broken hairs were observed. The eyebrows, eyelash hairs, and surrounding skin were normal. Potassium hydroxide mount was equivocal for nits.
Nonhealing Eroded Plaque on an Interdigital Web Space of the Foot
The Diagnosis: Basal Cell Nevus Syndrome
Given the patient’s history of numerous basal cell carcinomas (BCCs), odontogenic keratocysts, palmar pits, and a nonhealing ulcer, the clinical presentation was highly suggestive of interdigital BCC in the setting of basal cell nevus syndrome (BCNS). A shave biopsy was performed revealing islands of basaloid cells with peripheral palisading and a retraction artifact surrounded by fibromyxoid stroma, consistent with nodular and infiltrative BCC (Figure 1).
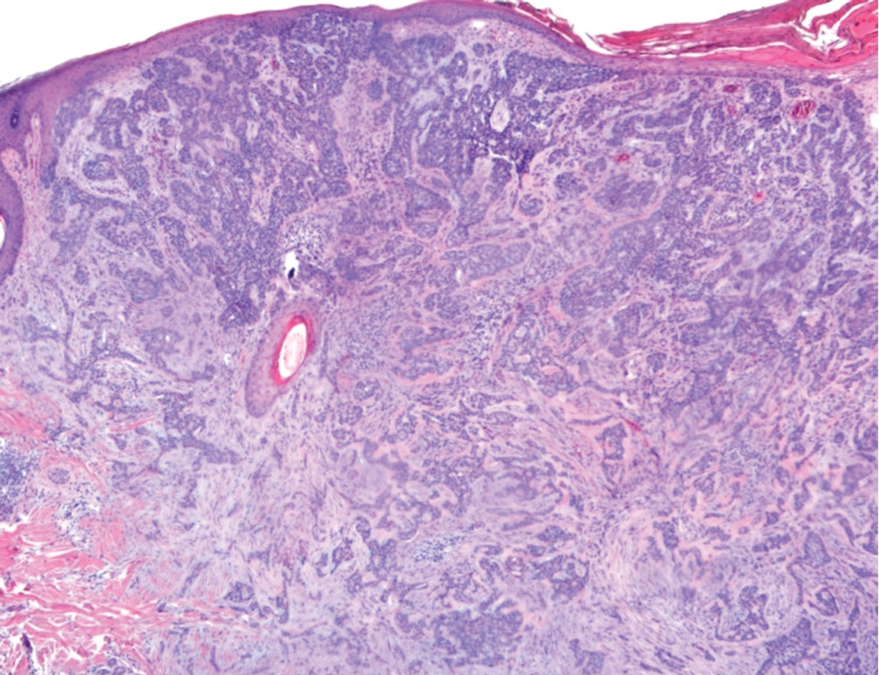
Basal cell nevus syndrome (also known as Gorlin syndrome) is a rare neurocutaneous syndrome that manifests with multiple BCCs; palmar and plantar pits (Figure 2); central nervous system tumors; and skeletal anomalies including jaw cysts, macrocephaly, frontal bossing, and bifid ribs.1 It is an autosomal-dominant condition caused by mutations in the PTCH1 gene, a tumor suppressor gene involved in the Hedgehog signaling pathway.2 Basal cell carcinoma is the most distinctive feature of BCNS, causing notable morbidity. Tumors typically present between puberty and 35 years of age, and patients can have anywhere from a few to thousands of tumors. They rarely become locally aggressive; however, with radiation therapy, proliferation and local invasion may occur within a few years. Therefore, radiotherapy should be avoided in these patients.1
Although the most common sites for BCCs in BCNS are the head, neck, and back, there is a higher rate of occurrence on sun-protected areas in BCNS compared to the general population.3 Our patient presented with interdigital BCC of the foot, which is an extremely rare occurrence. PubMed and Ovid searches using the terms basal cell carcinoma, BCC, foot, interdigital, and nonmelanoma skin cancer revealed only 3 cases of interdigital BCC of the foot. One case was associated with prior surgical trauma, the second presented as a junctional nevus, and the third did not appear to have any associated inciting factors.4-6 Dermatologists need to have a low threshold for biopsy for any unusual nonhealing lesions, especially in the setting of BCNS. Basal cell carcinomas in BCNS cannot be histologically differentiated from sporadic BCCs, and management largely depends on the size, location, recurrence, and number of lesions. Treatment methods range from topical agents to Mohs micrographic surgery.1
Nonhealing lesions of the foot may give an initial clinical impression of infection overlying peripheral vascular disease or diabetes mellitus with the possibility of associated osteomyelitis. Our patient had no clinical history to suggest peripheral vascular disease or diabetes mellitus, and he had palpable dorsalis pedis pulses as well as a normal neurologic examination. Clinicians also may consider fungal infection in the differential diagnosis. Erosio interdigitalis blastomycetica is a superficial yeast infection described as a well-defined, red, shiny plaque found in chronically wet areas, usually affecting the third or fourth interdigital spaces of the fingers.7 However, the lack of improvement with antibiotics and antifungals argued against bacterial or fungal infection in our patient. Although BCC also is a common feature of Bazex Dupré-Christol syndrome, it also is characterized by follicular atrophoderma, milia, hypohidrosis, and hypotrichosis,8 which were not evident in our patient. Pseudomonas hot foot syndrome is characterized by painful, plantar, erythematous nodules after exposure to ontaminated water that typically is self-limited but does respond to antibiotics for Pseudomonas.9
Our patient underwent Mohs micrographic surgery with a complex repair utilizing a full-thickness skin graft. There were no signs of recurrence at 3-month follow-up, and he was counseled on the importance of sun-protective behaviors along with regular dermatologic follow-up.
1. Gorlin RJ. Nevoid basal cell (Gorlin) syndrome. Genet Med. 2004; 6:530-539.
2. Bale A. The nevoid basal cell carcinoma syndrome: genetics and mechanism of carcinogenesis. Cancer Invest. 1997;15:180-186.
3. Goldstein AM, Bale SJ, Peck GL, et al. Sun exposure and basal cell carcinomas in nevoid basal cell carcinoma syndrome. J Am Acad Dermatol. 1993;29:34-41.
4. Silvers SH. Interdigital pedal basal cell carcinoma. Cutis. 1983;31:199-200.
5. Weitzner S. Basal cell carcinoma of toeweb presenting as a junctional nevus. Southwest Med. 1968;49:175.
6. Niwa A, Pimentel E. Basal cell carcinoma in unusual locations. An Bras Dermatol. 2006;81:281-284.
7. Mitchell JH. Erosio interdigitalis blastomycetica. Arch Derm Syphilol. 1922;6:675-679.
8. Kidd A, Carson L, Gregory DW, et al. A Scottish family with Bazex-Dupré-Christol syndrome: follicular atrophoderma, congenital hypotrichosis, and basal cell carcinoma. J Med Genet. 1996;33:493-497.
9. Yu Y, Cheng AS, Wang L, et al. Hot tub folliculitis or hot hand-foot syndrome caused by Pseudomonas aeruginosa. J Am Acad Dermatol. 2007;57:596-600.
The Diagnosis: Basal Cell Nevus Syndrome
Given the patient’s history of numerous basal cell carcinomas (BCCs), odontogenic keratocysts, palmar pits, and a nonhealing ulcer, the clinical presentation was highly suggestive of interdigital BCC in the setting of basal cell nevus syndrome (BCNS). A shave biopsy was performed revealing islands of basaloid cells with peripheral palisading and a retraction artifact surrounded by fibromyxoid stroma, consistent with nodular and infiltrative BCC (Figure 1).

Basal cell nevus syndrome (also known as Gorlin syndrome) is a rare neurocutaneous syndrome that manifests with multiple BCCs; palmar and plantar pits (Figure 2); central nervous system tumors; and skeletal anomalies including jaw cysts, macrocephaly, frontal bossing, and bifid ribs.1 It is an autosomal-dominant condition caused by mutations in the PTCH1 gene, a tumor suppressor gene involved in the Hedgehog signaling pathway.2 Basal cell carcinoma is the most distinctive feature of BCNS, causing notable morbidity. Tumors typically present between puberty and 35 years of age, and patients can have anywhere from a few to thousands of tumors. They rarely become locally aggressive; however, with radiation therapy, proliferation and local invasion may occur within a few years. Therefore, radiotherapy should be avoided in these patients.1

Although the most common sites for BCCs in BCNS are the head, neck, and back, there is a higher rate of occurrence on sun-protected areas in BCNS compared to the general population.3 Our patient presented with interdigital BCC of the foot, which is an extremely rare occurrence. PubMed and Ovid searches using the terms basal cell carcinoma, BCC, foot, interdigital, and nonmelanoma skin cancer revealed only 3 cases of interdigital BCC of the foot. One case was associated with prior surgical trauma, the second presented as a junctional nevus, and the third did not appear to have any associated inciting factors.4-6 Dermatologists need to have a low threshold for biopsy for any unusual nonhealing lesions, especially in the setting of BCNS. Basal cell carcinomas in BCNS cannot be histologically differentiated from sporadic BCCs, and management largely depends on the size, location, recurrence, and number of lesions. Treatment methods range from topical agents to Mohs micrographic surgery.1
Nonhealing lesions of the foot may give an initial clinical impression of infection overlying peripheral vascular disease or diabetes mellitus with the possibility of associated osteomyelitis. Our patient had no clinical history to suggest peripheral vascular disease or diabetes mellitus, and he had palpable dorsalis pedis pulses as well as a normal neurologic examination. Clinicians also may consider fungal infection in the differential diagnosis. Erosio interdigitalis blastomycetica is a superficial yeast infection described as a well-defined, red, shiny plaque found in chronically wet areas, usually affecting the third or fourth interdigital spaces of the fingers.7 However, the lack of improvement with antibiotics and antifungals argued against bacterial or fungal infection in our patient. Although BCC also is a common feature of Bazex Dupré-Christol syndrome, it also is characterized by follicular atrophoderma, milia, hypohidrosis, and hypotrichosis,8 which were not evident in our patient. Pseudomonas hot foot syndrome is characterized by painful, plantar, erythematous nodules after exposure to ontaminated water that typically is self-limited but does respond to antibiotics for Pseudomonas.9
Our patient underwent Mohs micrographic surgery with a complex repair utilizing a full-thickness skin graft. There were no signs of recurrence at 3-month follow-up, and he was counseled on the importance of sun-protective behaviors along with regular dermatologic follow-up.
The Diagnosis: Basal Cell Nevus Syndrome
Given the patient’s history of numerous basal cell carcinomas (BCCs), odontogenic keratocysts, palmar pits, and a nonhealing ulcer, the clinical presentation was highly suggestive of interdigital BCC in the setting of basal cell nevus syndrome (BCNS). A shave biopsy was performed revealing islands of basaloid cells with peripheral palisading and a retraction artifact surrounded by fibromyxoid stroma, consistent with nodular and infiltrative BCC (Figure 1).

Basal cell nevus syndrome (also known as Gorlin syndrome) is a rare neurocutaneous syndrome that manifests with multiple BCCs; palmar and plantar pits (Figure 2); central nervous system tumors; and skeletal anomalies including jaw cysts, macrocephaly, frontal bossing, and bifid ribs.1 It is an autosomal-dominant condition caused by mutations in the PTCH1 gene, a tumor suppressor gene involved in the Hedgehog signaling pathway.2 Basal cell carcinoma is the most distinctive feature of BCNS, causing notable morbidity. Tumors typically present between puberty and 35 years of age, and patients can have anywhere from a few to thousands of tumors. They rarely become locally aggressive; however, with radiation therapy, proliferation and local invasion may occur within a few years. Therefore, radiotherapy should be avoided in these patients.1

Although the most common sites for BCCs in BCNS are the head, neck, and back, there is a higher rate of occurrence on sun-protected areas in BCNS compared to the general population.3 Our patient presented with interdigital BCC of the foot, which is an extremely rare occurrence. PubMed and Ovid searches using the terms basal cell carcinoma, BCC, foot, interdigital, and nonmelanoma skin cancer revealed only 3 cases of interdigital BCC of the foot. One case was associated with prior surgical trauma, the second presented as a junctional nevus, and the third did not appear to have any associated inciting factors.4-6 Dermatologists need to have a low threshold for biopsy for any unusual nonhealing lesions, especially in the setting of BCNS. Basal cell carcinomas in BCNS cannot be histologically differentiated from sporadic BCCs, and management largely depends on the size, location, recurrence, and number of lesions. Treatment methods range from topical agents to Mohs micrographic surgery.1
Nonhealing lesions of the foot may give an initial clinical impression of infection overlying peripheral vascular disease or diabetes mellitus with the possibility of associated osteomyelitis. Our patient had no clinical history to suggest peripheral vascular disease or diabetes mellitus, and he had palpable dorsalis pedis pulses as well as a normal neurologic examination. Clinicians also may consider fungal infection in the differential diagnosis. Erosio interdigitalis blastomycetica is a superficial yeast infection described as a well-defined, red, shiny plaque found in chronically wet areas, usually affecting the third or fourth interdigital spaces of the fingers.7 However, the lack of improvement with antibiotics and antifungals argued against bacterial or fungal infection in our patient. Although BCC also is a common feature of Bazex Dupré-Christol syndrome, it also is characterized by follicular atrophoderma, milia, hypohidrosis, and hypotrichosis,8 which were not evident in our patient. Pseudomonas hot foot syndrome is characterized by painful, plantar, erythematous nodules after exposure to ontaminated water that typically is self-limited but does respond to antibiotics for Pseudomonas.9
Our patient underwent Mohs micrographic surgery with a complex repair utilizing a full-thickness skin graft. There were no signs of recurrence at 3-month follow-up, and he was counseled on the importance of sun-protective behaviors along with regular dermatologic follow-up.
1. Gorlin RJ. Nevoid basal cell (Gorlin) syndrome. Genet Med. 2004; 6:530-539.
2. Bale A. The nevoid basal cell carcinoma syndrome: genetics and mechanism of carcinogenesis. Cancer Invest. 1997;15:180-186.
3. Goldstein AM, Bale SJ, Peck GL, et al. Sun exposure and basal cell carcinomas in nevoid basal cell carcinoma syndrome. J Am Acad Dermatol. 1993;29:34-41.
4. Silvers SH. Interdigital pedal basal cell carcinoma. Cutis. 1983;31:199-200.
5. Weitzner S. Basal cell carcinoma of toeweb presenting as a junctional nevus. Southwest Med. 1968;49:175.
6. Niwa A, Pimentel E. Basal cell carcinoma in unusual locations. An Bras Dermatol. 2006;81:281-284.
7. Mitchell JH. Erosio interdigitalis blastomycetica. Arch Derm Syphilol. 1922;6:675-679.
8. Kidd A, Carson L, Gregory DW, et al. A Scottish family with Bazex-Dupré-Christol syndrome: follicular atrophoderma, congenital hypotrichosis, and basal cell carcinoma. J Med Genet. 1996;33:493-497.
9. Yu Y, Cheng AS, Wang L, et al. Hot tub folliculitis or hot hand-foot syndrome caused by Pseudomonas aeruginosa. J Am Acad Dermatol. 2007;57:596-600.
1. Gorlin RJ. Nevoid basal cell (Gorlin) syndrome. Genet Med. 2004; 6:530-539.
2. Bale A. The nevoid basal cell carcinoma syndrome: genetics and mechanism of carcinogenesis. Cancer Invest. 1997;15:180-186.
3. Goldstein AM, Bale SJ, Peck GL, et al. Sun exposure and basal cell carcinomas in nevoid basal cell carcinoma syndrome. J Am Acad Dermatol. 1993;29:34-41.
4. Silvers SH. Interdigital pedal basal cell carcinoma. Cutis. 1983;31:199-200.
5. Weitzner S. Basal cell carcinoma of toeweb presenting as a junctional nevus. Southwest Med. 1968;49:175.
6. Niwa A, Pimentel E. Basal cell carcinoma in unusual locations. An Bras Dermatol. 2006;81:281-284.
7. Mitchell JH. Erosio interdigitalis blastomycetica. Arch Derm Syphilol. 1922;6:675-679.
8. Kidd A, Carson L, Gregory DW, et al. A Scottish family with Bazex-Dupré-Christol syndrome: follicular atrophoderma, congenital hypotrichosis, and basal cell carcinoma. J Med Genet. 1996;33:493-497.
9. Yu Y, Cheng AS, Wang L, et al. Hot tub folliculitis or hot hand-foot syndrome caused by Pseudomonas aeruginosa. J Am Acad Dermatol. 2007;57:596-600.
A 53-year-old man with a history of numerous basal cell carcinomas and odontogenic keratocysts presented with a nonhealing erosion between the left second and third toes of several months’ duration. He was treated empirically with multiple courses of topical and systemic antibiotics as well as antifungals with minimal improvement. Physical examination revealed a 1.2×0.6-cm eroded plaque with rolled borders on the left second toe web; bilateral palmar pits; diffuse actinic damage; and several well-healed surgical scars on the head, neck, and back. Neurologic examination was normal, and dorsalis pedis pulses were equal and palpable bilaterally.
Ascending Erythematous Nodules on the Arm
The Diagnosis: Primary Cutaneous Nocardiosis
Comprehensive metabolic panel and complete blood cell count were unremarkable; human immunodeficiency virus screening was nonreactive. Punch biopsies were obtained for histopathology, as well as bacterial, fungal, and mycobacterial cultures. Histopathologic examination of a 4-mm punch biopsy of the forearm nodule showed a dermal abscess with neutrophilic infiltration in the dermis (Figure 1). No organisms were seen on Gram, methenamine-silver, periodic acid–Schiff, or acid-fast bacteria stains. Given the clinical suspicion for lymphocutaneous sporotrichosis, the patient was started on itraconazole. She reported modest improvement but subsequently developed a morbilliform eruption necessitating medication discontinuation.
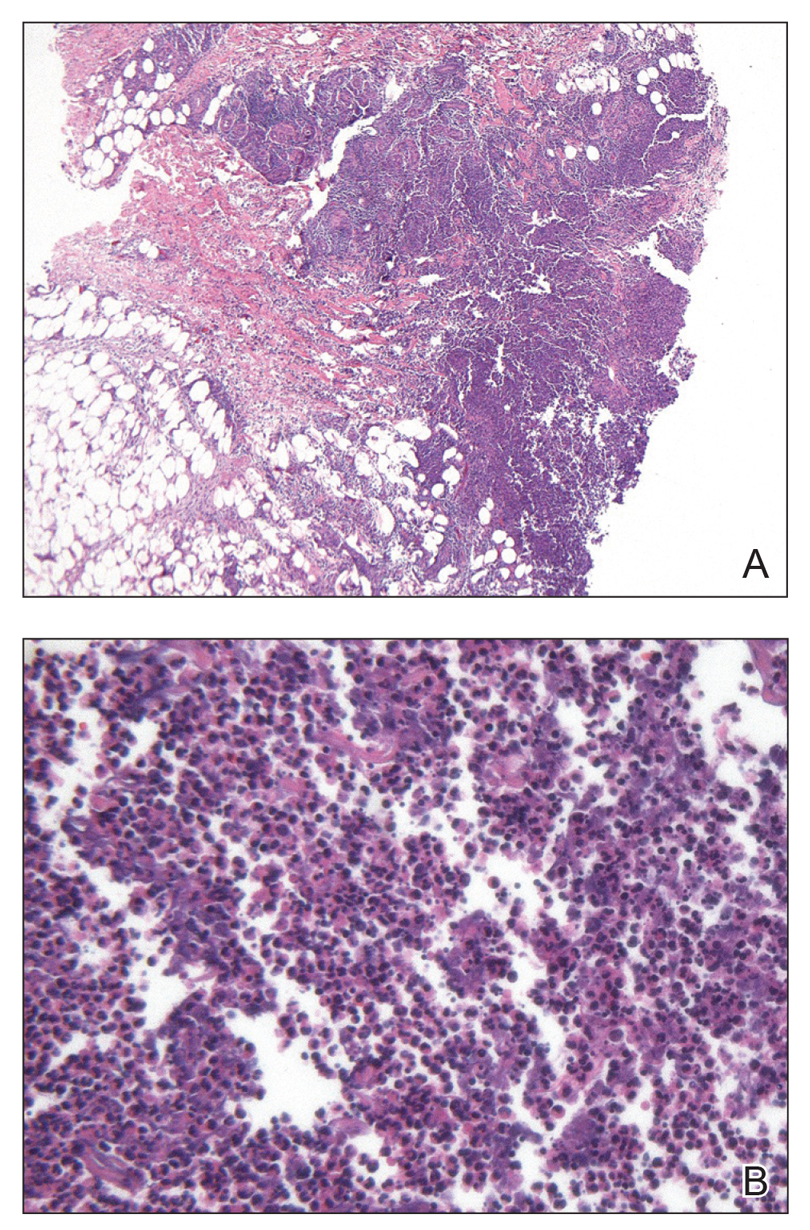
Eighteen days after obtaining the tissue culture, acid-fast organisms grew in culture. These organisms were subcultured on Middlebrook 7H11 agar (Sigma-Aldrich) with growth noted at 30°C and 37°C. Gram stain revealed filamentous gram-variable bacteria (Figure 2) that were identified as Nocardia brasiliensis by 16S ribosomal DNA analysis. Given the patient’s sulfonamide allergy, she started oral minocycline 100 mg twice daily. She responded to the therapy and subsequent testing confirmed susceptibility.
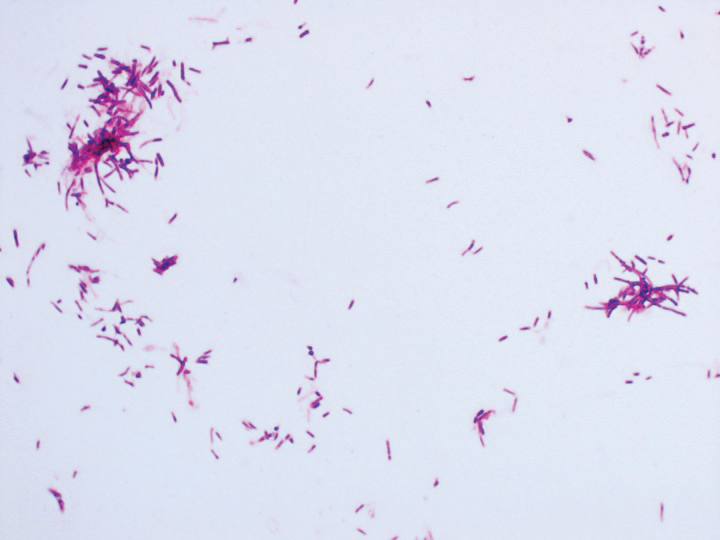
Nocardia brasiliensis, isolated from subculture on Gram stain (original
magnification ×1000).
The genus Nocardia consists of more than 50 species of gram-positive, weakly acid-fast, aerobic actinomycetes that can cause primary cutaneous infection via percutaneous inoculation. Nocardia brasiliensis is the leading cause (approximately 80% of cases) of primary cutaneous or subcutaneous nocardiosis and is found ubiquitously in soil and decaying vegetation.1 The clinical presentation varies, rendering definitive diagnosis a challenge without histopathologic and microbiologic testing.2 Patients presenting with nocardial cellulitis often are suspected to have Streptococcus pyogenes or Staphylococcus aureus infections. The differential diagnosis for patients presenting with nocardial nodular lymphangitis, also known as lymphocutaneous syndrome, includes atypical mycobacterial infections, leishmaniasis, and lymphocutaneous sporotrichosis.2
Histologic examination of nocardial nodules typically shows granulomatous or neutrophilic inflammation, and organisms may appear in small collections resembling sulfur granules.2 The organism itself is weakly positive on acid-fast stain, and useful stains include acid-fast bacteria, methenamine silver, and periodic acid–Schiff.2 Tissue culture often provides the definitive diagnosis, as the histology is nonspecific and organisms may not be visualized.
Oral trimethoprim-sulfamethoxazole 2.5 to 10 mg/kg and 12.5 to 50 mg/kg, respectively, twice daily is the treatment of choice for primary cutaneous nocardiosis. Minocycline 100 to 200 mg twice daily is an accepted alternative in case of sulfonamide allergy, as in our patient. Antibiotics should be tailored according to the susceptibility profile of the isolated organism.3
This case highlights the importance of forming a broad differential diagnosis for patients presenting with lymphocutaneous syndrome. The incidence and prevalence of N brasiliensis infection is difficult to determine due to its nonspecific clinical presentation and a lack of recent epidemiologic studies. Although primary cutaneous nocardiosis in the United States often is diagnosed in the South or Southwest, cases have been reported in other regions.4-6 Traumatic inoculation of contaminated soil, plants, and other organic matter, a well-known method of Sporothrix schenckii transmission, also is a method of N brasiliensis transmission. Because this organism may not be detected on histologic examination, empiric treatment should be considered if the diagnosis is suspected.
1. Brown-Eliot BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
2. Smego RA Jr, Castiglia M, Asperilla MO. Lymphocutaneous syndrome: a review of non-sporothrix causes. Medicine. 1999;78:38-63.
3. Lerner P. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
4. Smego RA Jr, Gallis HA. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev Infect Dis. 1984;6:164-180.
5. Fukuda H, Saotome A, Usami N, et al. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol. 2008;35:346-353.
6. Kil EH, Tsai CL, Kwark EH, et al. A case of nocardiosis with an uncharacteristically long incubation period. Cutis. 2005;76:33-36.
The Diagnosis: Primary Cutaneous Nocardiosis
Comprehensive metabolic panel and complete blood cell count were unremarkable; human immunodeficiency virus screening was nonreactive. Punch biopsies were obtained for histopathology, as well as bacterial, fungal, and mycobacterial cultures. Histopathologic examination of a 4-mm punch biopsy of the forearm nodule showed a dermal abscess with neutrophilic infiltration in the dermis (Figure 1). No organisms were seen on Gram, methenamine-silver, periodic acid–Schiff, or acid-fast bacteria stains. Given the clinical suspicion for lymphocutaneous sporotrichosis, the patient was started on itraconazole. She reported modest improvement but subsequently developed a morbilliform eruption necessitating medication discontinuation.

Eighteen days after obtaining the tissue culture, acid-fast organisms grew in culture. These organisms were subcultured on Middlebrook 7H11 agar (Sigma-Aldrich) with growth noted at 30°C and 37°C. Gram stain revealed filamentous gram-variable bacteria (Figure 2) that were identified as Nocardia brasiliensis by 16S ribosomal DNA analysis. Given the patient’s sulfonamide allergy, she started oral minocycline 100 mg twice daily. She responded to the therapy and subsequent testing confirmed susceptibility.

Nocardia brasiliensis, isolated from subculture on Gram stain (original
magnification ×1000).
The genus Nocardia consists of more than 50 species of gram-positive, weakly acid-fast, aerobic actinomycetes that can cause primary cutaneous infection via percutaneous inoculation. Nocardia brasiliensis is the leading cause (approximately 80% of cases) of primary cutaneous or subcutaneous nocardiosis and is found ubiquitously in soil and decaying vegetation.1 The clinical presentation varies, rendering definitive diagnosis a challenge without histopathologic and microbiologic testing.2 Patients presenting with nocardial cellulitis often are suspected to have Streptococcus pyogenes or Staphylococcus aureus infections. The differential diagnosis for patients presenting with nocardial nodular lymphangitis, also known as lymphocutaneous syndrome, includes atypical mycobacterial infections, leishmaniasis, and lymphocutaneous sporotrichosis.2
Histologic examination of nocardial nodules typically shows granulomatous or neutrophilic inflammation, and organisms may appear in small collections resembling sulfur granules.2 The organism itself is weakly positive on acid-fast stain, and useful stains include acid-fast bacteria, methenamine silver, and periodic acid–Schiff.2 Tissue culture often provides the definitive diagnosis, as the histology is nonspecific and organisms may not be visualized.
Oral trimethoprim-sulfamethoxazole 2.5 to 10 mg/kg and 12.5 to 50 mg/kg, respectively, twice daily is the treatment of choice for primary cutaneous nocardiosis. Minocycline 100 to 200 mg twice daily is an accepted alternative in case of sulfonamide allergy, as in our patient. Antibiotics should be tailored according to the susceptibility profile of the isolated organism.3
This case highlights the importance of forming a broad differential diagnosis for patients presenting with lymphocutaneous syndrome. The incidence and prevalence of N brasiliensis infection is difficult to determine due to its nonspecific clinical presentation and a lack of recent epidemiologic studies. Although primary cutaneous nocardiosis in the United States often is diagnosed in the South or Southwest, cases have been reported in other regions.4-6 Traumatic inoculation of contaminated soil, plants, and other organic matter, a well-known method of Sporothrix schenckii transmission, also is a method of N brasiliensis transmission. Because this organism may not be detected on histologic examination, empiric treatment should be considered if the diagnosis is suspected.
The Diagnosis: Primary Cutaneous Nocardiosis
Comprehensive metabolic panel and complete blood cell count were unremarkable; human immunodeficiency virus screening was nonreactive. Punch biopsies were obtained for histopathology, as well as bacterial, fungal, and mycobacterial cultures. Histopathologic examination of a 4-mm punch biopsy of the forearm nodule showed a dermal abscess with neutrophilic infiltration in the dermis (Figure 1). No organisms were seen on Gram, methenamine-silver, periodic acid–Schiff, or acid-fast bacteria stains. Given the clinical suspicion for lymphocutaneous sporotrichosis, the patient was started on itraconazole. She reported modest improvement but subsequently developed a morbilliform eruption necessitating medication discontinuation.

Eighteen days after obtaining the tissue culture, acid-fast organisms grew in culture. These organisms were subcultured on Middlebrook 7H11 agar (Sigma-Aldrich) with growth noted at 30°C and 37°C. Gram stain revealed filamentous gram-variable bacteria (Figure 2) that were identified as Nocardia brasiliensis by 16S ribosomal DNA analysis. Given the patient’s sulfonamide allergy, she started oral minocycline 100 mg twice daily. She responded to the therapy and subsequent testing confirmed susceptibility.

Nocardia brasiliensis, isolated from subculture on Gram stain (original
magnification ×1000).
The genus Nocardia consists of more than 50 species of gram-positive, weakly acid-fast, aerobic actinomycetes that can cause primary cutaneous infection via percutaneous inoculation. Nocardia brasiliensis is the leading cause (approximately 80% of cases) of primary cutaneous or subcutaneous nocardiosis and is found ubiquitously in soil and decaying vegetation.1 The clinical presentation varies, rendering definitive diagnosis a challenge without histopathologic and microbiologic testing.2 Patients presenting with nocardial cellulitis often are suspected to have Streptococcus pyogenes or Staphylococcus aureus infections. The differential diagnosis for patients presenting with nocardial nodular lymphangitis, also known as lymphocutaneous syndrome, includes atypical mycobacterial infections, leishmaniasis, and lymphocutaneous sporotrichosis.2
Histologic examination of nocardial nodules typically shows granulomatous or neutrophilic inflammation, and organisms may appear in small collections resembling sulfur granules.2 The organism itself is weakly positive on acid-fast stain, and useful stains include acid-fast bacteria, methenamine silver, and periodic acid–Schiff.2 Tissue culture often provides the definitive diagnosis, as the histology is nonspecific and organisms may not be visualized.
Oral trimethoprim-sulfamethoxazole 2.5 to 10 mg/kg and 12.5 to 50 mg/kg, respectively, twice daily is the treatment of choice for primary cutaneous nocardiosis. Minocycline 100 to 200 mg twice daily is an accepted alternative in case of sulfonamide allergy, as in our patient. Antibiotics should be tailored according to the susceptibility profile of the isolated organism.3
This case highlights the importance of forming a broad differential diagnosis for patients presenting with lymphocutaneous syndrome. The incidence and prevalence of N brasiliensis infection is difficult to determine due to its nonspecific clinical presentation and a lack of recent epidemiologic studies. Although primary cutaneous nocardiosis in the United States often is diagnosed in the South or Southwest, cases have been reported in other regions.4-6 Traumatic inoculation of contaminated soil, plants, and other organic matter, a well-known method of Sporothrix schenckii transmission, also is a method of N brasiliensis transmission. Because this organism may not be detected on histologic examination, empiric treatment should be considered if the diagnosis is suspected.
1. Brown-Eliot BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
2. Smego RA Jr, Castiglia M, Asperilla MO. Lymphocutaneous syndrome: a review of non-sporothrix causes. Medicine. 1999;78:38-63.
3. Lerner P. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
4. Smego RA Jr, Gallis HA. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev Infect Dis. 1984;6:164-180.
5. Fukuda H, Saotome A, Usami N, et al. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol. 2008;35:346-353.
6. Kil EH, Tsai CL, Kwark EH, et al. A case of nocardiosis with an uncharacteristically long incubation period. Cutis. 2005;76:33-36.
1. Brown-Eliot BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
2. Smego RA Jr, Castiglia M, Asperilla MO. Lymphocutaneous syndrome: a review of non-sporothrix causes. Medicine. 1999;78:38-63.
3. Lerner P. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
4. Smego RA Jr, Gallis HA. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev Infect Dis. 1984;6:164-180.
5. Fukuda H, Saotome A, Usami N, et al. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol. 2008;35:346-353.
6. Kil EH, Tsai CL, Kwark EH, et al. A case of nocardiosis with an uncharacteristically long incubation period. Cutis. 2005;76:33-36.
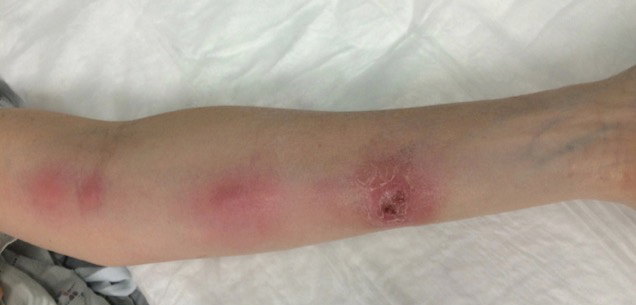
A 54-year-old woman called her primary care provider to report a painful pink nodule on the left wrist 1 week after sustaining thorn injuries while weeding in her garden. She started cephalexin and noted a pink streak with additional nodules extending up the arm over the next 2 days. She
was admitted to an outside hospital for incision and drainage of the wrist nodule and a 3-day course of intravenous vancomycin. Bacterial culture was negative, and she was discharged on oral clindamycin and doxycycline. Two days later, she presented to our emergency department with pain in the left axilla. Physical examination revealed 3 tender erythematous nodules in a linear distribution on the left arm with crusting at the incision and drainage site and painful left axillary lymphadenopathy. The patient was afebrile and otherwise asymptomatic.
Blanchable Erythematous Patches on the Fingers
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
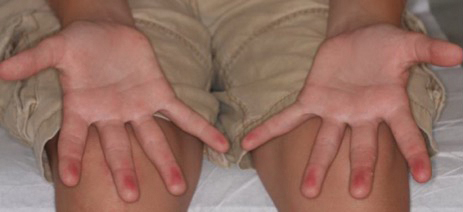
A 12-year-old boy presented with well-defined, blanchable, erythematous patches on the distal bilateral palmar aspects of the second to fifth fingers of 2 years’ duration. The patient stated that he skateboarded daily throughout the year and swam once weekly in the summer months. Furthermore, the patient cited frequent contact with the rough undersurface of the skateboard and concrete road surfaces while skateboarding. He stated that the lesions were always present and worsened in the summer months. The lesions had an occasional burning sensation when they were more prominently erythematous, and the patient denied any pattern of exacerbation, numbness, bleeding, or itching. There was no notable family history or evidence of systemic disease.
Verrucous Coalescing Dry Papules and Plaques on the Hip and Flank
The Diagnosis: Blaschkitis
A punch biopsy from the right lateral hip was performed. Histopathologic examination revealed orthokeratosis overlying mild psoriasiform epidermal hyperplasia associated with a lichenoid infiltrate composed almost entirely of lymphocytes (Figure). The infiltrate did not entirely obscure the dermoepidermal junction and spared the adnexal structures. The clinical presentation along with histopathologic analysis confirmed a diagnosis of blaschkitis. The lesions were treated with triamcinolone ointment twice daily as needed, and the patient reported symptomatic and clinical improvement with this intervention at 4-week follow-up.
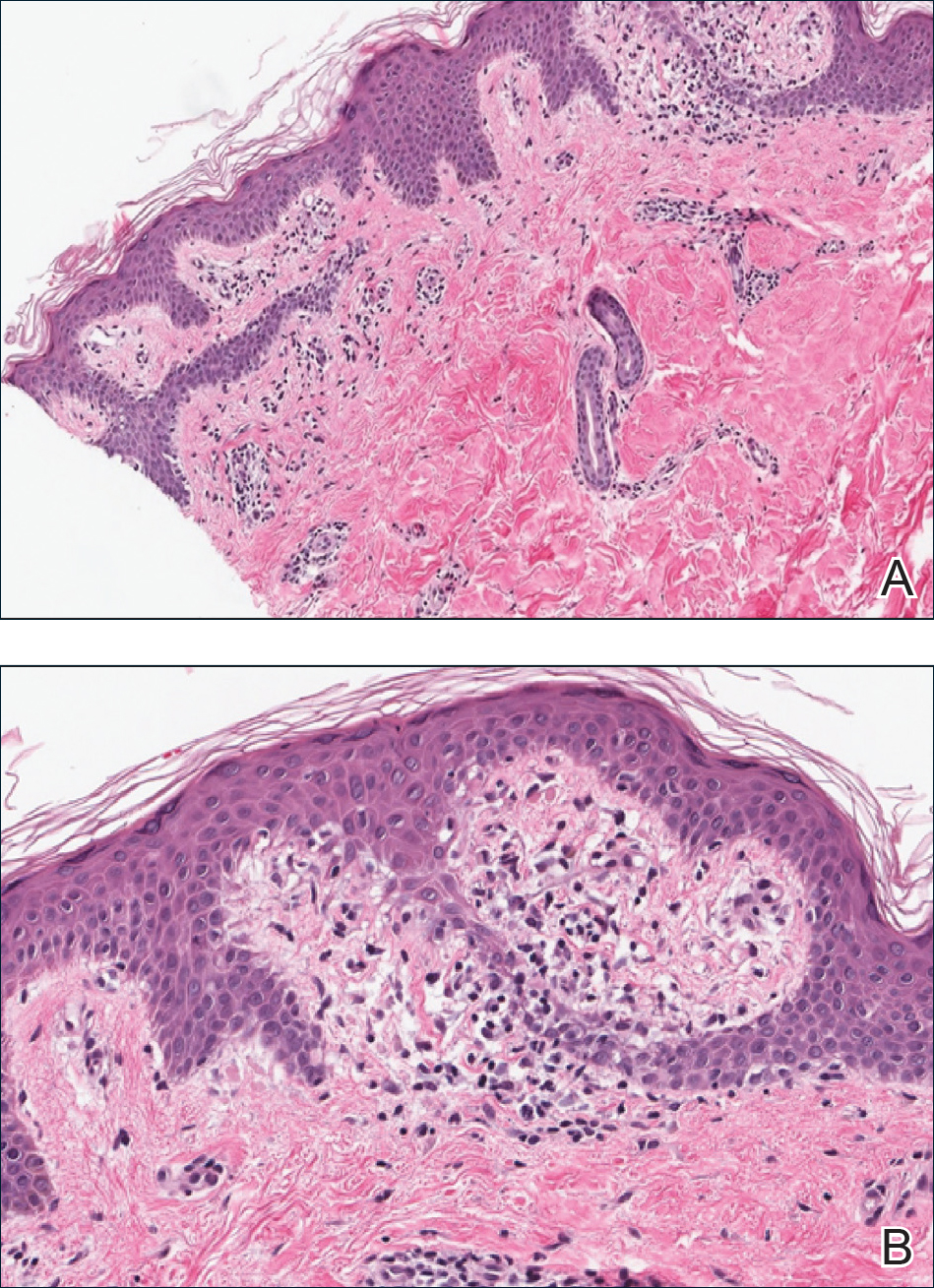
Described by Grosshans and Marot1 in 1990, blaschkitis is an acquired inflammatory dermatosis following the lines of Blaschko. It predominantly is seen on the trunk and typically presents with pruritic papules and vesicles. It frequently has a relapsing course and is more commonly found in adults. Blaschkitis is considered a form of cutaneous mosaicism representing somatic mutations affecting epidermal cell growth and migration during embryogenesis. It has been proposed that these aberrant cells are not clinically apparent at birth; however, viral infection and drug or other environmental triggers can induce antigen presentation of the clone cells activating a T cell–mediated inflammatory response.2-4
The differential diagnosis includes other acquired Blaschko-linear dermatoses such as lichen striatus, inflammatory linear verrucous epidermal nevus, unilateral lichen planus, linear lichen sclerosus, linear psoriasis, linear fixed drug reaction, lichen nitidus, and others.1,4 Given the overlap in clinical and histopathological presentations of the entities in the differential, it often is difficult to discern the etiology of the papular and vesicular eruption in question. Discrimination of one etiology from the others is further confounded by the fact that these lesions can all be pruritic and initially are treated with topical corticosteroids. A degree of clinical suspicion for blaschkitis coupled with prior understanding of lesional manifestations is helpful in this circumstance. Although classic lichen planus often affects the arms, legs, flexor surfaces, and occasionally the oral mucosa, blaschkitis generally is limited to the trunk. The lesions of lichen planus generally are violaceous, flattopped, polygonal papules that tend to coalesce. They often have a thin, transparent, and adherent scale overlying the papular lesions, and they occasionally demonstrate Wickham striae, which are faint white reticulated networks typically seen in oral mucosal lesions. In the case of lichen nitidus, lesions often follow a geometric line due to the Köbner response, whereas physical trauma from scratching or injury causes lesions to form along the line of insult. Assessing patients for any newly initiated medications can help eliminate lichenoid drug eruptions. Lichen striatus perhaps has the most overlap with blaschkitis, having been described as also following the lines of Blaschko but occurring in children rather than adults. Inflammatory linear verrucous epidermal nevi also can be distinguished from blaschkitis on this premise, as these lesions arise during the first 5 years of life and generally affect the lower extremities.4,5
Histopathology is somewhat variable but generally includes spongiotic dermatitis with concomitant interface
dermatitis characterized by T-cell infiltration. Spongiosis is a feature less commonly seen in lichen striatus. Lesions can progress over time from spongiotic dermatitis to spongiotic psoriasiform dermatitis and later to spongiotic psoriasiform lichenoid dermatitis.4 Treatment of blaschkitis should begin with reassurance of the benign nature of the dermatosis. Pruritic symptoms can be managed with a course of topical steroids.
Blaschkitis is a rare and self-limiting acquired dermatosis that should be incorporated into the differential diagnosis of Blaschko-linear dermatoses. Further investigation is needed to determine if blaschkitis and lichen striatus represent the ends of a disease spectrum or completely distinct entities.
- Grosshans E, Marot L. Blaschkitis in adults. Ann Dermatol Venereol. 1990;117:9-15.
- Müller CS, Schmaltz R, Vogt T, et al. Lichen striatus and blaschkitis: reappraisal of the concept of blaschkolinear dermatoses [published online November 29, 2010]. Br J Dermatol. 2011;164:257-262.
- Sun BK, Tsao H. X-chromosome inactivation and skin disease. J Invest Dermatol. 2008;128:2753-2759.
- Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:261-267.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
The Diagnosis: Blaschkitis
A punch biopsy from the right lateral hip was performed. Histopathologic examination revealed orthokeratosis overlying mild psoriasiform epidermal hyperplasia associated with a lichenoid infiltrate composed almost entirely of lymphocytes (Figure). The infiltrate did not entirely obscure the dermoepidermal junction and spared the adnexal structures. The clinical presentation along with histopathologic analysis confirmed a diagnosis of blaschkitis. The lesions were treated with triamcinolone ointment twice daily as needed, and the patient reported symptomatic and clinical improvement with this intervention at 4-week follow-up.

Described by Grosshans and Marot1 in 1990, blaschkitis is an acquired inflammatory dermatosis following the lines of Blaschko. It predominantly is seen on the trunk and typically presents with pruritic papules and vesicles. It frequently has a relapsing course and is more commonly found in adults. Blaschkitis is considered a form of cutaneous mosaicism representing somatic mutations affecting epidermal cell growth and migration during embryogenesis. It has been proposed that these aberrant cells are not clinically apparent at birth; however, viral infection and drug or other environmental triggers can induce antigen presentation of the clone cells activating a T cell–mediated inflammatory response.2-4
The differential diagnosis includes other acquired Blaschko-linear dermatoses such as lichen striatus, inflammatory linear verrucous epidermal nevus, unilateral lichen planus, linear lichen sclerosus, linear psoriasis, linear fixed drug reaction, lichen nitidus, and others.1,4 Given the overlap in clinical and histopathological presentations of the entities in the differential, it often is difficult to discern the etiology of the papular and vesicular eruption in question. Discrimination of one etiology from the others is further confounded by the fact that these lesions can all be pruritic and initially are treated with topical corticosteroids. A degree of clinical suspicion for blaschkitis coupled with prior understanding of lesional manifestations is helpful in this circumstance. Although classic lichen planus often affects the arms, legs, flexor surfaces, and occasionally the oral mucosa, blaschkitis generally is limited to the trunk. The lesions of lichen planus generally are violaceous, flattopped, polygonal papules that tend to coalesce. They often have a thin, transparent, and adherent scale overlying the papular lesions, and they occasionally demonstrate Wickham striae, which are faint white reticulated networks typically seen in oral mucosal lesions. In the case of lichen nitidus, lesions often follow a geometric line due to the Köbner response, whereas physical trauma from scratching or injury causes lesions to form along the line of insult. Assessing patients for any newly initiated medications can help eliminate lichenoid drug eruptions. Lichen striatus perhaps has the most overlap with blaschkitis, having been described as also following the lines of Blaschko but occurring in children rather than adults. Inflammatory linear verrucous epidermal nevi also can be distinguished from blaschkitis on this premise, as these lesions arise during the first 5 years of life and generally affect the lower extremities.4,5
Histopathology is somewhat variable but generally includes spongiotic dermatitis with concomitant interface
dermatitis characterized by T-cell infiltration. Spongiosis is a feature less commonly seen in lichen striatus. Lesions can progress over time from spongiotic dermatitis to spongiotic psoriasiform dermatitis and later to spongiotic psoriasiform lichenoid dermatitis.4 Treatment of blaschkitis should begin with reassurance of the benign nature of the dermatosis. Pruritic symptoms can be managed with a course of topical steroids.
Blaschkitis is a rare and self-limiting acquired dermatosis that should be incorporated into the differential diagnosis of Blaschko-linear dermatoses. Further investigation is needed to determine if blaschkitis and lichen striatus represent the ends of a disease spectrum or completely distinct entities.
The Diagnosis: Blaschkitis
A punch biopsy from the right lateral hip was performed. Histopathologic examination revealed orthokeratosis overlying mild psoriasiform epidermal hyperplasia associated with a lichenoid infiltrate composed almost entirely of lymphocytes (Figure). The infiltrate did not entirely obscure the dermoepidermal junction and spared the adnexal structures. The clinical presentation along with histopathologic analysis confirmed a diagnosis of blaschkitis. The lesions were treated with triamcinolone ointment twice daily as needed, and the patient reported symptomatic and clinical improvement with this intervention at 4-week follow-up.

Described by Grosshans and Marot1 in 1990, blaschkitis is an acquired inflammatory dermatosis following the lines of Blaschko. It predominantly is seen on the trunk and typically presents with pruritic papules and vesicles. It frequently has a relapsing course and is more commonly found in adults. Blaschkitis is considered a form of cutaneous mosaicism representing somatic mutations affecting epidermal cell growth and migration during embryogenesis. It has been proposed that these aberrant cells are not clinically apparent at birth; however, viral infection and drug or other environmental triggers can induce antigen presentation of the clone cells activating a T cell–mediated inflammatory response.2-4
The differential diagnosis includes other acquired Blaschko-linear dermatoses such as lichen striatus, inflammatory linear verrucous epidermal nevus, unilateral lichen planus, linear lichen sclerosus, linear psoriasis, linear fixed drug reaction, lichen nitidus, and others.1,4 Given the overlap in clinical and histopathological presentations of the entities in the differential, it often is difficult to discern the etiology of the papular and vesicular eruption in question. Discrimination of one etiology from the others is further confounded by the fact that these lesions can all be pruritic and initially are treated with topical corticosteroids. A degree of clinical suspicion for blaschkitis coupled with prior understanding of lesional manifestations is helpful in this circumstance. Although classic lichen planus often affects the arms, legs, flexor surfaces, and occasionally the oral mucosa, blaschkitis generally is limited to the trunk. The lesions of lichen planus generally are violaceous, flattopped, polygonal papules that tend to coalesce. They often have a thin, transparent, and adherent scale overlying the papular lesions, and they occasionally demonstrate Wickham striae, which are faint white reticulated networks typically seen in oral mucosal lesions. In the case of lichen nitidus, lesions often follow a geometric line due to the Köbner response, whereas physical trauma from scratching or injury causes lesions to form along the line of insult. Assessing patients for any newly initiated medications can help eliminate lichenoid drug eruptions. Lichen striatus perhaps has the most overlap with blaschkitis, having been described as also following the lines of Blaschko but occurring in children rather than adults. Inflammatory linear verrucous epidermal nevi also can be distinguished from blaschkitis on this premise, as these lesions arise during the first 5 years of life and generally affect the lower extremities.4,5
Histopathology is somewhat variable but generally includes spongiotic dermatitis with concomitant interface
dermatitis characterized by T-cell infiltration. Spongiosis is a feature less commonly seen in lichen striatus. Lesions can progress over time from spongiotic dermatitis to spongiotic psoriasiform dermatitis and later to spongiotic psoriasiform lichenoid dermatitis.4 Treatment of blaschkitis should begin with reassurance of the benign nature of the dermatosis. Pruritic symptoms can be managed with a course of topical steroids.
Blaschkitis is a rare and self-limiting acquired dermatosis that should be incorporated into the differential diagnosis of Blaschko-linear dermatoses. Further investigation is needed to determine if blaschkitis and lichen striatus represent the ends of a disease spectrum or completely distinct entities.
- Grosshans E, Marot L. Blaschkitis in adults. Ann Dermatol Venereol. 1990;117:9-15.
- Müller CS, Schmaltz R, Vogt T, et al. Lichen striatus and blaschkitis: reappraisal of the concept of blaschkolinear dermatoses [published online November 29, 2010]. Br J Dermatol. 2011;164:257-262.
- Sun BK, Tsao H. X-chromosome inactivation and skin disease. J Invest Dermatol. 2008;128:2753-2759.
- Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:261-267.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Grosshans E, Marot L. Blaschkitis in adults. Ann Dermatol Venereol. 1990;117:9-15.
- Müller CS, Schmaltz R, Vogt T, et al. Lichen striatus and blaschkitis: reappraisal of the concept of blaschkolinear dermatoses [published online November 29, 2010]. Br J Dermatol. 2011;164:257-262.
- Sun BK, Tsao H. X-chromosome inactivation and skin disease. J Invest Dermatol. 2008;128:2753-2759.
- Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:261-267.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
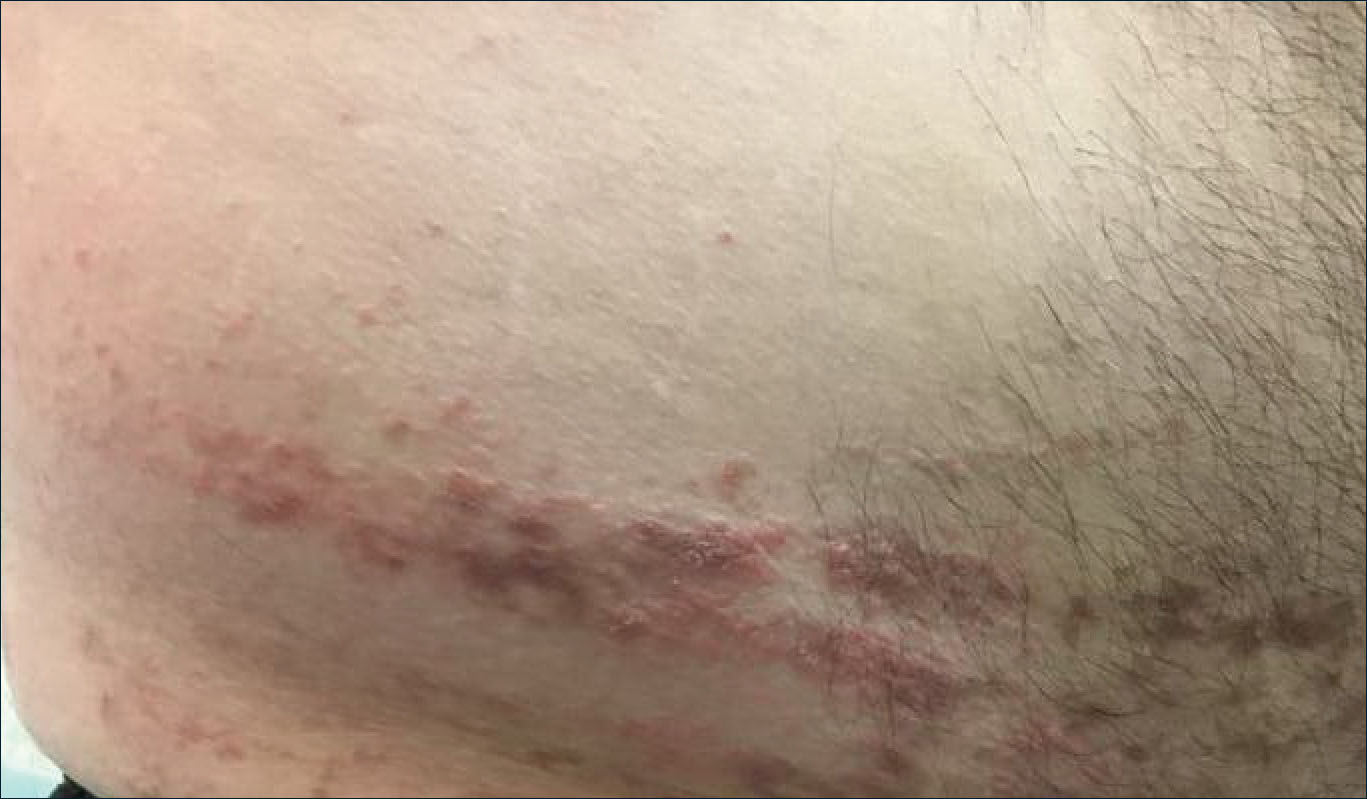
A 31-year-old man presented with a recurring pruritic rash on the right lateral flank and hip of 2 years’ duration. Physical examination revealed erythematous, verrucous, dry papules and plaques coalescing into larger plaques on the right flank and hip in dermatomal distributions involving the T10 and T11 dermatomes. A few papules were scattered in a linear eruption along the right flank and right upper thigh. Some postinflammatory changes were noted. No rash was noted over any other area of the body. Physical examination was otherwise unremarkable.
Erythematous Pruritic Plaque on the Cheek
The Diagnosis: Tinea Faciei
Given the morphology of the plaque, a potassium hydroxide preparation was performed and was positive for hyphal elements consistent with dermatophyte infection (Figure).
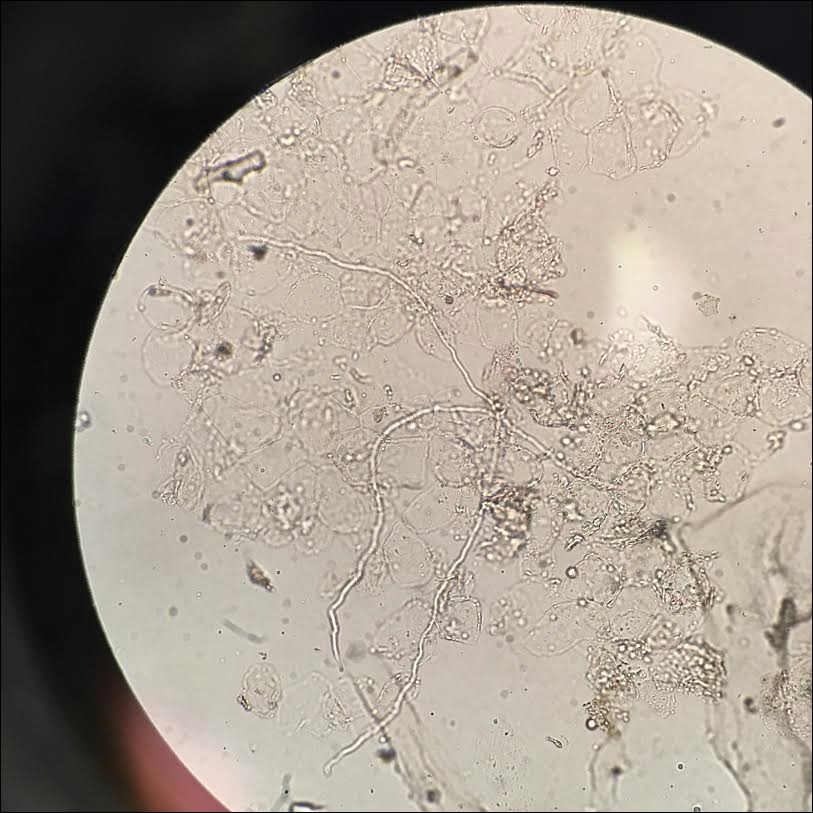
Tinea faciei is a fungal infection of the face caused by a dermatophyte that invades the stratum corneum.1 It is transmitted through direct contact with an infected individual or fomite.2 Infections typically are characterized by annular or serpiginous erythematous plaques with a scaly appearance and advancing edge. There may be associated vesicles, papules, or pustules with crusting around the advancing border.3 Tinea faciei can occur concomitantly with other dermatophytic infections and frequently presents atypically due to different characteristics of facial anatomy when compared to other tinea infections. As a result, it often is misdiagnosed.1
Tinea faciei represents roughly 19% of all superficial fungal infections and occurs more commonly in temperate humid regions.4 It can occur at any age but has bimodal peaks in incidence during childhood and early adulthood.5 The most common causative dermatophytes are Trichophyton tonsurans, Microsporum canis, Trichophyton mentagrophytes, and Trichophyton rubrum.1 Transmission is mainly through direct contact with infected individuals, animals, or soil, which likely occurred during the close quarters and exercises our patient experienced during basic training in the military.
Tinea faciei often is misdiagnosed and treated with topical corticosteroids. The steroids can give a false impression that the rash is resolving by initially decreasing the inflammatory component and reducing scale, which is referred to as tinea incognito. Once the steroid is stopped, however, the fungal infection often returns worse than the original presentation. The differential diagnosis includes subacute cutaneous lupus erythematosus, periorificial dermatitis, seborrheic dermatitis, psoriasis, rosacea, erythema annulare centrifugum, granuloma annulare, sarcoidosis, and contact dermatitis.1,3,6
Diagnosis of tinea faciei is best made with skin scraping of the active border of the lesion. The scraping is treated with potassium hydroxide 10%. Visualizing branching or curving hyphae confirms the diagnosis. Fungal speciation often is not performed due to the long time needed to culture. Wood lamp may fluoresce blue-green if tinea faciei is caused by Microsporum species; however, diagnosis in this manner is limited because other common species do not fluoresce.7
Options for treatment of tinea faciei include topical antifungals for 2 to 6 weeks for localized disease or oral antifungals for more extensive or unresponsive infections for 1 to 8 weeks depending on the agent that is used. If fungal folliculitis is present, oral medication should be given.1 Our patient was treated with oral terbinafine 250 mg once daily for 4 weeks with follow-up after that time to ensure resolution.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
- Raimer SS, Beightler EL, Hebert AA, et al. Tinea faciei in infants caused by Trichophyton tonsurans. Pediatr Dermatol. 1986;3:452-454.
- Shapiro L, Cohen HJ. Tinea faciei simulating other dermatoses. JAMA. 1971;215:2106-2107.
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(suppl 4):2-15.
- Jorquera E, Moreno JC, Camacho F. Tinea faciei: epidemiology. Ann Dermatol Venereol. 1991;119:101-104.
- Hsu S, Le EH, Khoshevis MR. Differential diagnosis of annular lesions. Am Fam Physician. 2001;64:289-296.
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
The Diagnosis: Tinea Faciei
Given the morphology of the plaque, a potassium hydroxide preparation was performed and was positive for hyphal elements consistent with dermatophyte infection (Figure).

Tinea faciei is a fungal infection of the face caused by a dermatophyte that invades the stratum corneum.1 It is transmitted through direct contact with an infected individual or fomite.2 Infections typically are characterized by annular or serpiginous erythematous plaques with a scaly appearance and advancing edge. There may be associated vesicles, papules, or pustules with crusting around the advancing border.3 Tinea faciei can occur concomitantly with other dermatophytic infections and frequently presents atypically due to different characteristics of facial anatomy when compared to other tinea infections. As a result, it often is misdiagnosed.1
Tinea faciei represents roughly 19% of all superficial fungal infections and occurs more commonly in temperate humid regions.4 It can occur at any age but has bimodal peaks in incidence during childhood and early adulthood.5 The most common causative dermatophytes are Trichophyton tonsurans, Microsporum canis, Trichophyton mentagrophytes, and Trichophyton rubrum.1 Transmission is mainly through direct contact with infected individuals, animals, or soil, which likely occurred during the close quarters and exercises our patient experienced during basic training in the military.
Tinea faciei often is misdiagnosed and treated with topical corticosteroids. The steroids can give a false impression that the rash is resolving by initially decreasing the inflammatory component and reducing scale, which is referred to as tinea incognito. Once the steroid is stopped, however, the fungal infection often returns worse than the original presentation. The differential diagnosis includes subacute cutaneous lupus erythematosus, periorificial dermatitis, seborrheic dermatitis, psoriasis, rosacea, erythema annulare centrifugum, granuloma annulare, sarcoidosis, and contact dermatitis.1,3,6
Diagnosis of tinea faciei is best made with skin scraping of the active border of the lesion. The scraping is treated with potassium hydroxide 10%. Visualizing branching or curving hyphae confirms the diagnosis. Fungal speciation often is not performed due to the long time needed to culture. Wood lamp may fluoresce blue-green if tinea faciei is caused by Microsporum species; however, diagnosis in this manner is limited because other common species do not fluoresce.7
Options for treatment of tinea faciei include topical antifungals for 2 to 6 weeks for localized disease or oral antifungals for more extensive or unresponsive infections for 1 to 8 weeks depending on the agent that is used. If fungal folliculitis is present, oral medication should be given.1 Our patient was treated with oral terbinafine 250 mg once daily for 4 weeks with follow-up after that time to ensure resolution.
The Diagnosis: Tinea Faciei
Given the morphology of the plaque, a potassium hydroxide preparation was performed and was positive for hyphal elements consistent with dermatophyte infection (Figure).

Tinea faciei is a fungal infection of the face caused by a dermatophyte that invades the stratum corneum.1 It is transmitted through direct contact with an infected individual or fomite.2 Infections typically are characterized by annular or serpiginous erythematous plaques with a scaly appearance and advancing edge. There may be associated vesicles, papules, or pustules with crusting around the advancing border.3 Tinea faciei can occur concomitantly with other dermatophytic infections and frequently presents atypically due to different characteristics of facial anatomy when compared to other tinea infections. As a result, it often is misdiagnosed.1
Tinea faciei represents roughly 19% of all superficial fungal infections and occurs more commonly in temperate humid regions.4 It can occur at any age but has bimodal peaks in incidence during childhood and early adulthood.5 The most common causative dermatophytes are Trichophyton tonsurans, Microsporum canis, Trichophyton mentagrophytes, and Trichophyton rubrum.1 Transmission is mainly through direct contact with infected individuals, animals, or soil, which likely occurred during the close quarters and exercises our patient experienced during basic training in the military.
Tinea faciei often is misdiagnosed and treated with topical corticosteroids. The steroids can give a false impression that the rash is resolving by initially decreasing the inflammatory component and reducing scale, which is referred to as tinea incognito. Once the steroid is stopped, however, the fungal infection often returns worse than the original presentation. The differential diagnosis includes subacute cutaneous lupus erythematosus, periorificial dermatitis, seborrheic dermatitis, psoriasis, rosacea, erythema annulare centrifugum, granuloma annulare, sarcoidosis, and contact dermatitis.1,3,6
Diagnosis of tinea faciei is best made with skin scraping of the active border of the lesion. The scraping is treated with potassium hydroxide 10%. Visualizing branching or curving hyphae confirms the diagnosis. Fungal speciation often is not performed due to the long time needed to culture. Wood lamp may fluoresce blue-green if tinea faciei is caused by Microsporum species; however, diagnosis in this manner is limited because other common species do not fluoresce.7
Options for treatment of tinea faciei include topical antifungals for 2 to 6 weeks for localized disease or oral antifungals for more extensive or unresponsive infections for 1 to 8 weeks depending on the agent that is used. If fungal folliculitis is present, oral medication should be given.1 Our patient was treated with oral terbinafine 250 mg once daily for 4 weeks with follow-up after that time to ensure resolution.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
- Raimer SS, Beightler EL, Hebert AA, et al. Tinea faciei in infants caused by Trichophyton tonsurans. Pediatr Dermatol. 1986;3:452-454.
- Shapiro L, Cohen HJ. Tinea faciei simulating other dermatoses. JAMA. 1971;215:2106-2107.
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(suppl 4):2-15.
- Jorquera E, Moreno JC, Camacho F. Tinea faciei: epidemiology. Ann Dermatol Venereol. 1991;119:101-104.
- Hsu S, Le EH, Khoshevis MR. Differential diagnosis of annular lesions. Am Fam Physician. 2001;64:289-296.
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
- Raimer SS, Beightler EL, Hebert AA, et al. Tinea faciei in infants caused by Trichophyton tonsurans. Pediatr Dermatol. 1986;3:452-454.
- Shapiro L, Cohen HJ. Tinea faciei simulating other dermatoses. JAMA. 1971;215:2106-2107.
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(suppl 4):2-15.
- Jorquera E, Moreno JC, Camacho F. Tinea faciei: epidemiology. Ann Dermatol Venereol. 1991;119:101-104.
- Hsu S, Le EH, Khoshevis MR. Differential diagnosis of annular lesions. Am Fam Physician. 2001;64:289-296.
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
A 19-year-old man with a medical history of keloids presented with a slowly enlarging, red, itchy plaque on the left cheek of 1 year's duration that first began to develop during basic training in the military. The patient denied other pain, pruritus, or separate dermatitis. He initially was treated with triamcinolone cream 0.1%, which he used for 8 days prior to referral to the dermatology department. The patient denied other acute concerns. On physical examination, multiple erythematous papules coalescing into a large, 10-cm, papulosquamous, arciform plaque were noted on the left preauricular cheek.