User login
Bullous Lesions in a Neonate
The Diagnosis: Incontinentia Pigmenti
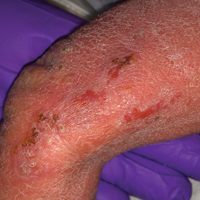
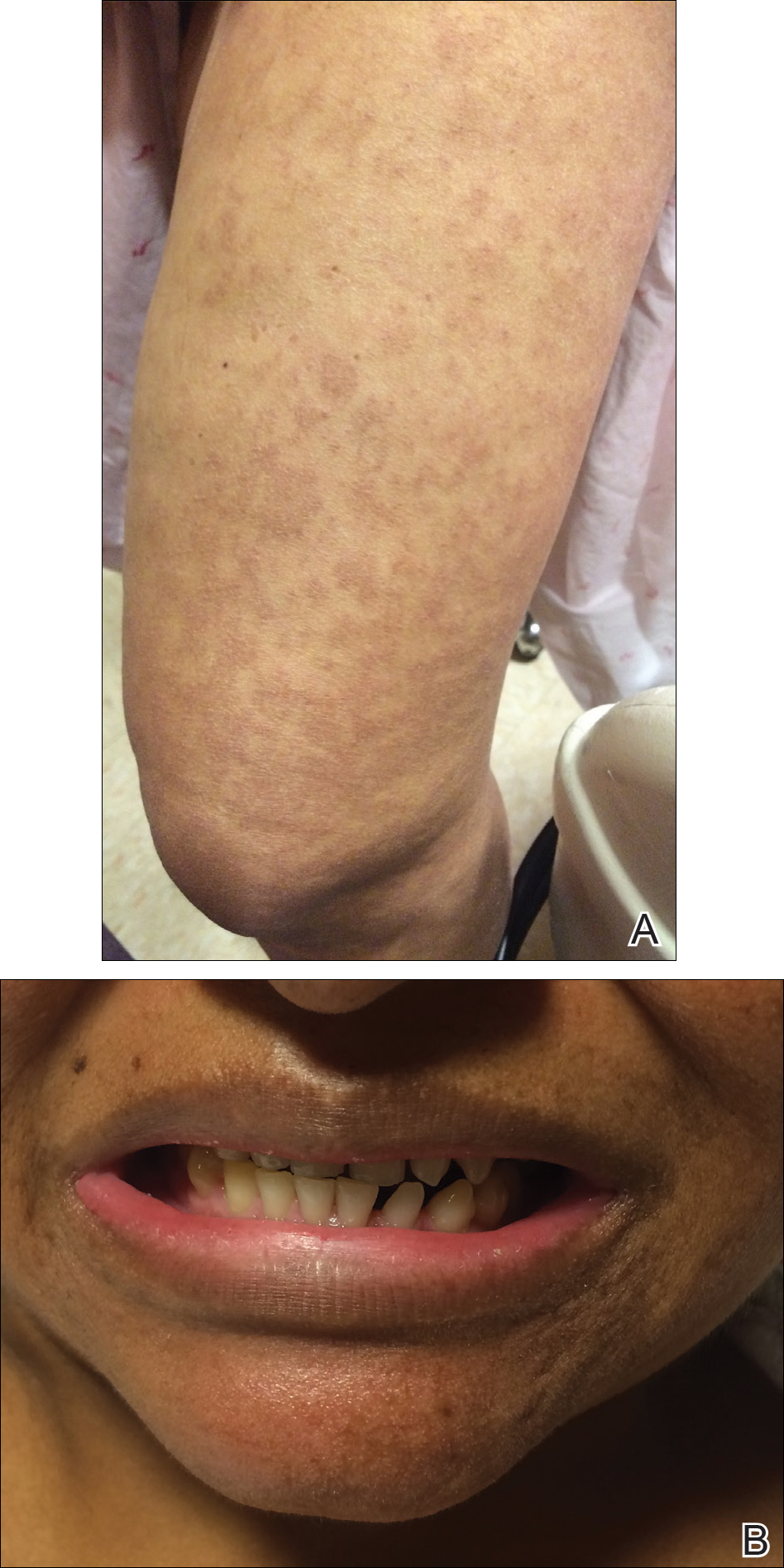
The infant's mother was noted to have diffuse hypopigmented patches over the trunk, arms, and legs (present since adolescence) with whorled cicatricial alopecia of the vertex scalp and peg-shaped teeth (Figure). Together, these findings suggested incontinentia pigmenti (IP), which the mother revealed she had been diagnosed with in childhood. The infant's characteristic lesions in the setting of her mother's diagnosed genodermatosis confirmed the diagnosis of IP.
Incontinentia pigmenti is an X-linked dominant disorder that presents with many classic dermatologic, dental, neurologic, and ophthalmologic findings. The causative mutation occurs in IKBKG/NEMO (inhibitor of κ polypeptide gene enhancer in B-cells, kinase γ/nuclear factor-κB essential modulator) gene on Xq28, disabling the resultant protein that normally protects cells from tumor necrosis factor family-induced apoptosis.1 Incontinentia pigmenti usually is lethal in males and causes an unbalanced X-inactivation in surviving female IP patients. Occurring at a rate of 1.2 per 100,000 births,2 IP typically presents in female infants with skin lesions patterned along Blaschko lines that evolve in 4 stages over a lifetime.3 Stage I, presenting in the neonatal period, manifests as vesiculobullous eruptions on the limbs and scalp. Stages II to IV vary in duration from months to years and are comprised of a verrucous stage, a hyperpigmented stage, and a hypopigmented stage, respectively.3 All stages of IP can overlap and coexist.
The vesiculobullous findings in infants with IP may be mistakenly attributed to other diseases with prominent vesicular or bullous components including herpes simplex virus, epidermolysis bullosa, and infantile acropustulosis. With neonatal herpes simplex virus infection, vesicular skin or mucocutaneous lesions occur 9 to 11 days after birth and can be confirmed by specimen culture or qualitative polymerase chain reaction, while stage I of IP appears within the first 6 to 8 weeks of life and can be present at birth.4 The hallmark of epidermolysis bullosa, caused by mutations in keratins 5 and 14, is blistering erosions of the skin in response to frictional stress,1 thus these lesions do not follow Blaschko lines. Infantile acropustulosis, a nonheritable vesiculopustular eruption of the hands and feet, rarely occurs in the immediate newborn period; it most often appears in the 3- to 6-month age range with recurrent eruptions at 3- to 4-week intervals.5 Focal dermal hypoplasia is another X-linked dominant disorder with blaschkolinear findings at birth that presents with pink or red, angular, atrophic macules, in contrast to the bullous lesions of IP.6
Incontinentia pigmenti may encompass a wide range of systemic symptoms in addition to the classic dermatologic findings. Notably, central nervous system defects are concurrent in up to 40% of IP cases, with seizures, mental retardation, and spastic paresis being the most common sequelae.7 Teeth defects, seen in 35% of patients, include delayed primary dentition and peg-shaped teeth. Many patients will experience ophthalmologic defects including vision problems (16%) and retinopathy (15%).7
The cutaneous eruptions of IP may be treated with topical corticosteroids or topical tacrolimus, and vesicles should be left intact and monitored for signs of infection.8,9 Seizures, if present, should be treated with anticonvulsants, and regular neuropsychiatric monitoring and physical rehabilitation may be warranted. Patients should be regularly monitored for retinopathy beginning at the time of diagnosis. Retinal fibrovascular proliferation is treated with xenon laser photocoagulation to reduce the high risk for retinal detachment in this population.10,11 Older and younger at-risk relatives must be evaluated by genetic testing or thorough physical examination to clarify their disease status and determine the need for additional genetic counseling.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012.
- Prevalence and incidence of rare diseases: bibliographic data. Orphanet Report Series, Rare Diseases collection. http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf. Published June 2017. Accessed July 13, 2017.
- Scheuerle AE, Ursini MV. Incontinentia pigmenti. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. Seattle, WA: University of Washington; 2015. http://www.ncbi.nlm.nih.gov/books/NBK1472/. Accessed July 25, 2017.
- James SH, Kimberlin DW. Neonatal herpes simplex virus infection. Infect Dis Clin North Am. 2015;29:391-400.
- Eichenfield LF, Frieden IJ, Mathes E, et al, eds. Neonatal and Infant Dermatology. Philadelphia, PA: Saunders; 2015.
- Temple IK, MacDowall P, Baraitser M, et al. Focal dermal hypoplasia (Goltz syndrome). J Med Genet. 1990;27:180-187.
- Fusco F, Paciolla M, Conte MI, et al. Incontinentia pigmenti: report on data from 2000 to 2013. Orphanet J Rare Dis. 2014;9:93.
- Jessup CJ, Morgan SC, Cohen LM, et al. Incontinentia pigmenti: treatment of IP with topical tacrolimus. J Drugs Dermatol. 2009;8:944-946.
- Kaya TI, Tursen U, Ikizoglu G. Therapeutic use of topical corticosteroids in the vesiculobullous lesions of incontinentia pigmenti [published online June 1, 2009]. Clin Exp Dermatol. 2009;34:E611-E613.
- Nguyen JK, Brady-Mccreery KM. Laser photocoagulation in preproliferative retinopathy of incontinentia pigmenti. J AAPOS. 2001;5:258-259.
- Chen CJ, Han IC, Tian J, et al. Extended follow-up of treated and untreated retinopathy in incontinentia pigmenti: analysis of peripheral vascular changes and incidence of retinal detachment. JAMA Ophthalmol. 2015;133:542-548.
The Diagnosis: Incontinentia Pigmenti
The infant's mother was noted to have diffuse hypopigmented patches over the trunk, arms, and legs (present since adolescence) with whorled cicatricial alopecia of the vertex scalp and peg-shaped teeth (Figure). Together, these findings suggested incontinentia pigmenti (IP), which the mother revealed she had been diagnosed with in childhood. The infant's characteristic lesions in the setting of her mother's diagnosed genodermatosis confirmed the diagnosis of IP.

Incontinentia pigmenti is an X-linked dominant disorder that presents with many classic dermatologic, dental, neurologic, and ophthalmologic findings. The causative mutation occurs in IKBKG/NEMO (inhibitor of κ polypeptide gene enhancer in B-cells, kinase γ/nuclear factor-κB essential modulator) gene on Xq28, disabling the resultant protein that normally protects cells from tumor necrosis factor family-induced apoptosis.1 Incontinentia pigmenti usually is lethal in males and causes an unbalanced X-inactivation in surviving female IP patients. Occurring at a rate of 1.2 per 100,000 births,2 IP typically presents in female infants with skin lesions patterned along Blaschko lines that evolve in 4 stages over a lifetime.3 Stage I, presenting in the neonatal period, manifests as vesiculobullous eruptions on the limbs and scalp. Stages II to IV vary in duration from months to years and are comprised of a verrucous stage, a hyperpigmented stage, and a hypopigmented stage, respectively.3 All stages of IP can overlap and coexist.
The vesiculobullous findings in infants with IP may be mistakenly attributed to other diseases with prominent vesicular or bullous components including herpes simplex virus, epidermolysis bullosa, and infantile acropustulosis. With neonatal herpes simplex virus infection, vesicular skin or mucocutaneous lesions occur 9 to 11 days after birth and can be confirmed by specimen culture or qualitative polymerase chain reaction, while stage I of IP appears within the first 6 to 8 weeks of life and can be present at birth.4 The hallmark of epidermolysis bullosa, caused by mutations in keratins 5 and 14, is blistering erosions of the skin in response to frictional stress,1 thus these lesions do not follow Blaschko lines. Infantile acropustulosis, a nonheritable vesiculopustular eruption of the hands and feet, rarely occurs in the immediate newborn period; it most often appears in the 3- to 6-month age range with recurrent eruptions at 3- to 4-week intervals.5 Focal dermal hypoplasia is another X-linked dominant disorder with blaschkolinear findings at birth that presents with pink or red, angular, atrophic macules, in contrast to the bullous lesions of IP.6
Incontinentia pigmenti may encompass a wide range of systemic symptoms in addition to the classic dermatologic findings. Notably, central nervous system defects are concurrent in up to 40% of IP cases, with seizures, mental retardation, and spastic paresis being the most common sequelae.7 Teeth defects, seen in 35% of patients, include delayed primary dentition and peg-shaped teeth. Many patients will experience ophthalmologic defects including vision problems (16%) and retinopathy (15%).7
The cutaneous eruptions of IP may be treated with topical corticosteroids or topical tacrolimus, and vesicles should be left intact and monitored for signs of infection.8,9 Seizures, if present, should be treated with anticonvulsants, and regular neuropsychiatric monitoring and physical rehabilitation may be warranted. Patients should be regularly monitored for retinopathy beginning at the time of diagnosis. Retinal fibrovascular proliferation is treated with xenon laser photocoagulation to reduce the high risk for retinal detachment in this population.10,11 Older and younger at-risk relatives must be evaluated by genetic testing or thorough physical examination to clarify their disease status and determine the need for additional genetic counseling.
The Diagnosis: Incontinentia Pigmenti
The infant's mother was noted to have diffuse hypopigmented patches over the trunk, arms, and legs (present since adolescence) with whorled cicatricial alopecia of the vertex scalp and peg-shaped teeth (Figure). Together, these findings suggested incontinentia pigmenti (IP), which the mother revealed she had been diagnosed with in childhood. The infant's characteristic lesions in the setting of her mother's diagnosed genodermatosis confirmed the diagnosis of IP.

Incontinentia pigmenti is an X-linked dominant disorder that presents with many classic dermatologic, dental, neurologic, and ophthalmologic findings. The causative mutation occurs in IKBKG/NEMO (inhibitor of κ polypeptide gene enhancer in B-cells, kinase γ/nuclear factor-κB essential modulator) gene on Xq28, disabling the resultant protein that normally protects cells from tumor necrosis factor family-induced apoptosis.1 Incontinentia pigmenti usually is lethal in males and causes an unbalanced X-inactivation in surviving female IP patients. Occurring at a rate of 1.2 per 100,000 births,2 IP typically presents in female infants with skin lesions patterned along Blaschko lines that evolve in 4 stages over a lifetime.3 Stage I, presenting in the neonatal period, manifests as vesiculobullous eruptions on the limbs and scalp. Stages II to IV vary in duration from months to years and are comprised of a verrucous stage, a hyperpigmented stage, and a hypopigmented stage, respectively.3 All stages of IP can overlap and coexist.
The vesiculobullous findings in infants with IP may be mistakenly attributed to other diseases with prominent vesicular or bullous components including herpes simplex virus, epidermolysis bullosa, and infantile acropustulosis. With neonatal herpes simplex virus infection, vesicular skin or mucocutaneous lesions occur 9 to 11 days after birth and can be confirmed by specimen culture or qualitative polymerase chain reaction, while stage I of IP appears within the first 6 to 8 weeks of life and can be present at birth.4 The hallmark of epidermolysis bullosa, caused by mutations in keratins 5 and 14, is blistering erosions of the skin in response to frictional stress,1 thus these lesions do not follow Blaschko lines. Infantile acropustulosis, a nonheritable vesiculopustular eruption of the hands and feet, rarely occurs in the immediate newborn period; it most often appears in the 3- to 6-month age range with recurrent eruptions at 3- to 4-week intervals.5 Focal dermal hypoplasia is another X-linked dominant disorder with blaschkolinear findings at birth that presents with pink or red, angular, atrophic macules, in contrast to the bullous lesions of IP.6
Incontinentia pigmenti may encompass a wide range of systemic symptoms in addition to the classic dermatologic findings. Notably, central nervous system defects are concurrent in up to 40% of IP cases, with seizures, mental retardation, and spastic paresis being the most common sequelae.7 Teeth defects, seen in 35% of patients, include delayed primary dentition and peg-shaped teeth. Many patients will experience ophthalmologic defects including vision problems (16%) and retinopathy (15%).7
The cutaneous eruptions of IP may be treated with topical corticosteroids or topical tacrolimus, and vesicles should be left intact and monitored for signs of infection.8,9 Seizures, if present, should be treated with anticonvulsants, and regular neuropsychiatric monitoring and physical rehabilitation may be warranted. Patients should be regularly monitored for retinopathy beginning at the time of diagnosis. Retinal fibrovascular proliferation is treated with xenon laser photocoagulation to reduce the high risk for retinal detachment in this population.10,11 Older and younger at-risk relatives must be evaluated by genetic testing or thorough physical examination to clarify their disease status and determine the need for additional genetic counseling.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012.
- Prevalence and incidence of rare diseases: bibliographic data. Orphanet Report Series, Rare Diseases collection. http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf. Published June 2017. Accessed July 13, 2017.
- Scheuerle AE, Ursini MV. Incontinentia pigmenti. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. Seattle, WA: University of Washington; 2015. http://www.ncbi.nlm.nih.gov/books/NBK1472/. Accessed July 25, 2017.
- James SH, Kimberlin DW. Neonatal herpes simplex virus infection. Infect Dis Clin North Am. 2015;29:391-400.
- Eichenfield LF, Frieden IJ, Mathes E, et al, eds. Neonatal and Infant Dermatology. Philadelphia, PA: Saunders; 2015.
- Temple IK, MacDowall P, Baraitser M, et al. Focal dermal hypoplasia (Goltz syndrome). J Med Genet. 1990;27:180-187.
- Fusco F, Paciolla M, Conte MI, et al. Incontinentia pigmenti: report on data from 2000 to 2013. Orphanet J Rare Dis. 2014;9:93.
- Jessup CJ, Morgan SC, Cohen LM, et al. Incontinentia pigmenti: treatment of IP with topical tacrolimus. J Drugs Dermatol. 2009;8:944-946.
- Kaya TI, Tursen U, Ikizoglu G. Therapeutic use of topical corticosteroids in the vesiculobullous lesions of incontinentia pigmenti [published online June 1, 2009]. Clin Exp Dermatol. 2009;34:E611-E613.
- Nguyen JK, Brady-Mccreery KM. Laser photocoagulation in preproliferative retinopathy of incontinentia pigmenti. J AAPOS. 2001;5:258-259.
- Chen CJ, Han IC, Tian J, et al. Extended follow-up of treated and untreated retinopathy in incontinentia pigmenti: analysis of peripheral vascular changes and incidence of retinal detachment. JAMA Ophthalmol. 2015;133:542-548.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012.
- Prevalence and incidence of rare diseases: bibliographic data. Orphanet Report Series, Rare Diseases collection. http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf. Published June 2017. Accessed July 13, 2017.
- Scheuerle AE, Ursini MV. Incontinentia pigmenti. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. Seattle, WA: University of Washington; 2015. http://www.ncbi.nlm.nih.gov/books/NBK1472/. Accessed July 25, 2017.
- James SH, Kimberlin DW. Neonatal herpes simplex virus infection. Infect Dis Clin North Am. 2015;29:391-400.
- Eichenfield LF, Frieden IJ, Mathes E, et al, eds. Neonatal and Infant Dermatology. Philadelphia, PA: Saunders; 2015.
- Temple IK, MacDowall P, Baraitser M, et al. Focal dermal hypoplasia (Goltz syndrome). J Med Genet. 1990;27:180-187.
- Fusco F, Paciolla M, Conte MI, et al. Incontinentia pigmenti: report on data from 2000 to 2013. Orphanet J Rare Dis. 2014;9:93.
- Jessup CJ, Morgan SC, Cohen LM, et al. Incontinentia pigmenti: treatment of IP with topical tacrolimus. J Drugs Dermatol. 2009;8:944-946.
- Kaya TI, Tursen U, Ikizoglu G. Therapeutic use of topical corticosteroids in the vesiculobullous lesions of incontinentia pigmenti [published online June 1, 2009]. Clin Exp Dermatol. 2009;34:E611-E613.
- Nguyen JK, Brady-Mccreery KM. Laser photocoagulation in preproliferative retinopathy of incontinentia pigmenti. J AAPOS. 2001;5:258-259.
- Chen CJ, Han IC, Tian J, et al. Extended follow-up of treated and untreated retinopathy in incontinentia pigmenti: analysis of peripheral vascular changes and incidence of retinal detachment. JAMA Ophthalmol. 2015;133:542-548.
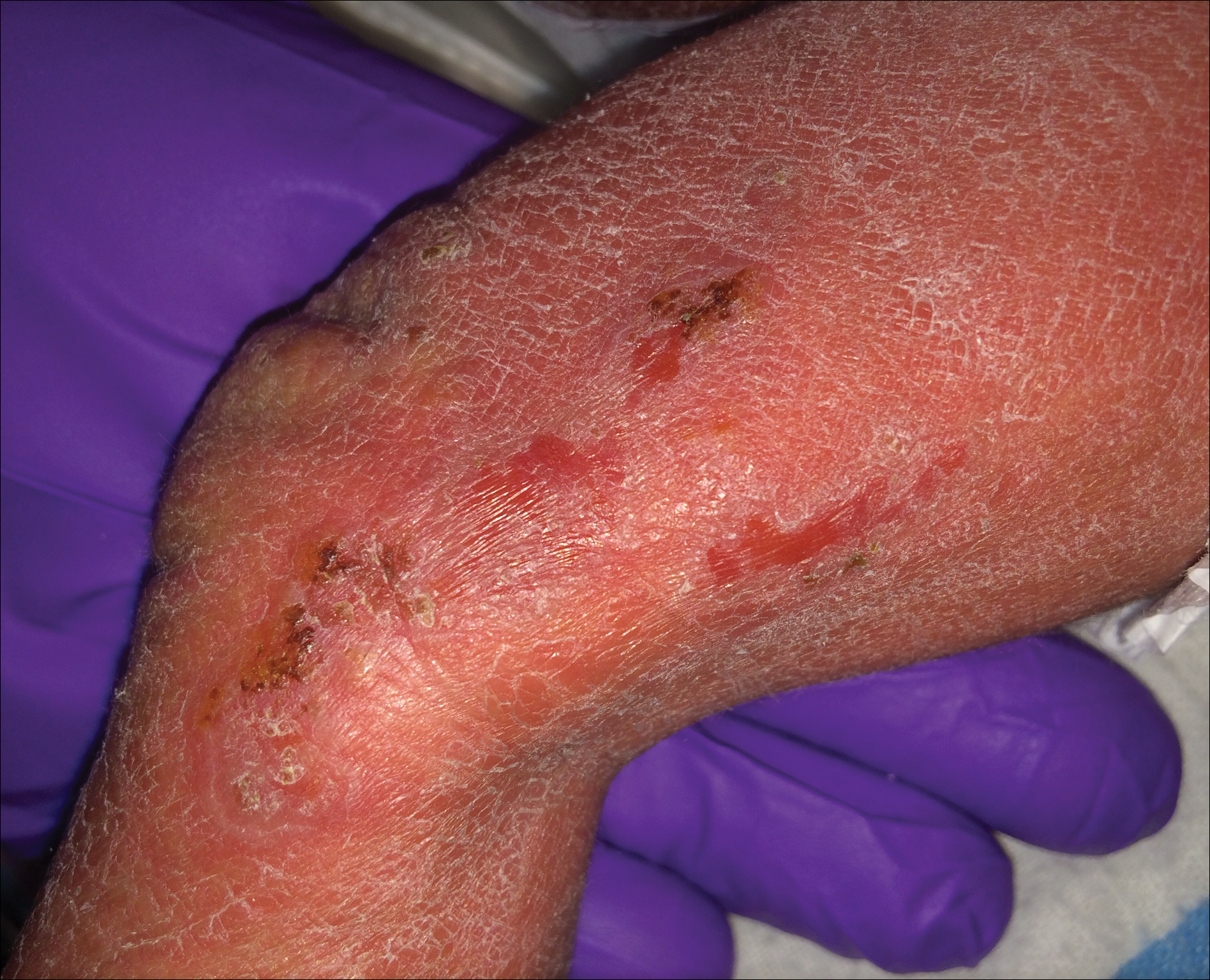
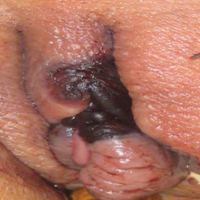
A 1-day-old Hispanic female infant was born via uncomplicated vaginal delivery at 41 weeks' gestation after a normal pregnancy. Linear plaques containing multiple ruptured vesicles and bullae following Blaschko lines were noted on the right medial thigh and anterior arm. The infant was afebrile and generally well-appearing.
Solitary Nodule With White Hairs
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.
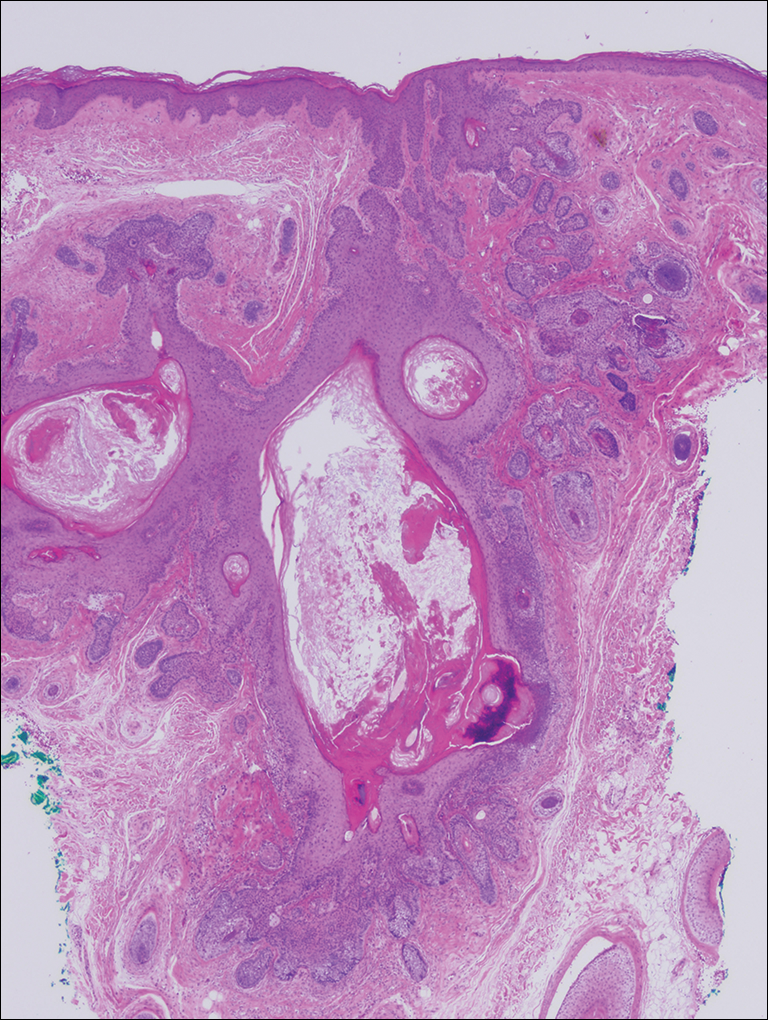
Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
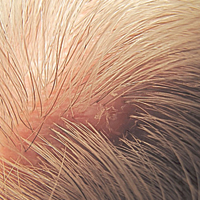
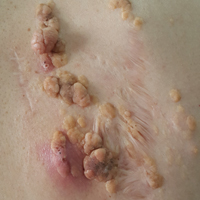
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.

Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.

Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
A 72-year-old man presented with a new asymptomatic 0.7-cm flesh-colored papule with a central tuft of white hairs on the posterior scalp. The remainder of the physical examination was unremarkable. Biopsy for histopathologic examination was performed to confirm diagnosis.
Necrotic Ulcer on the Thigh
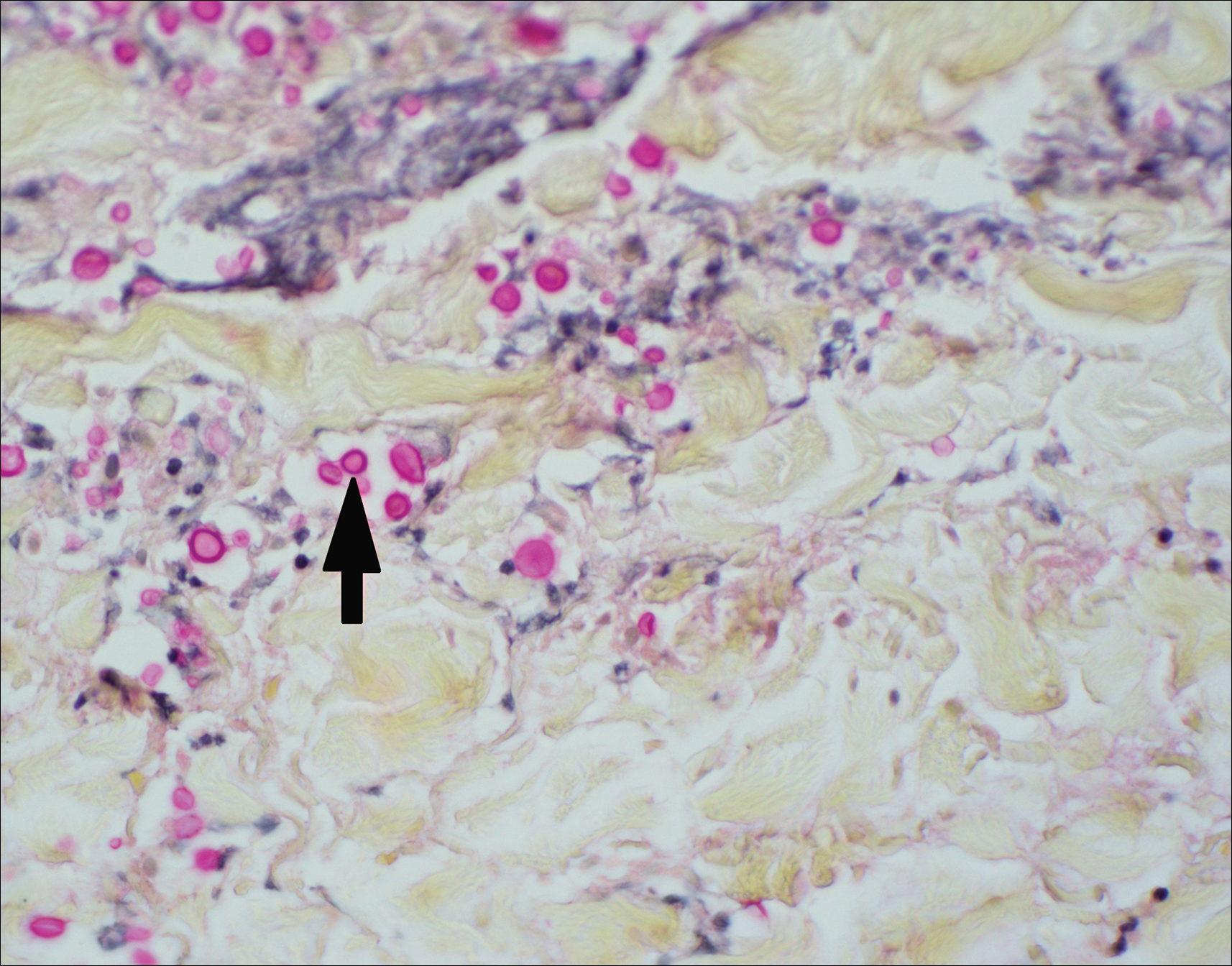
The Diagnosis: Disseminated Cryptococcosis
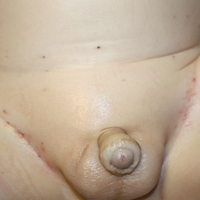
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.
Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
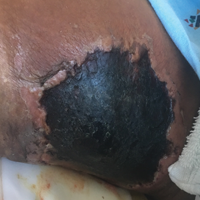
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.

Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.

Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
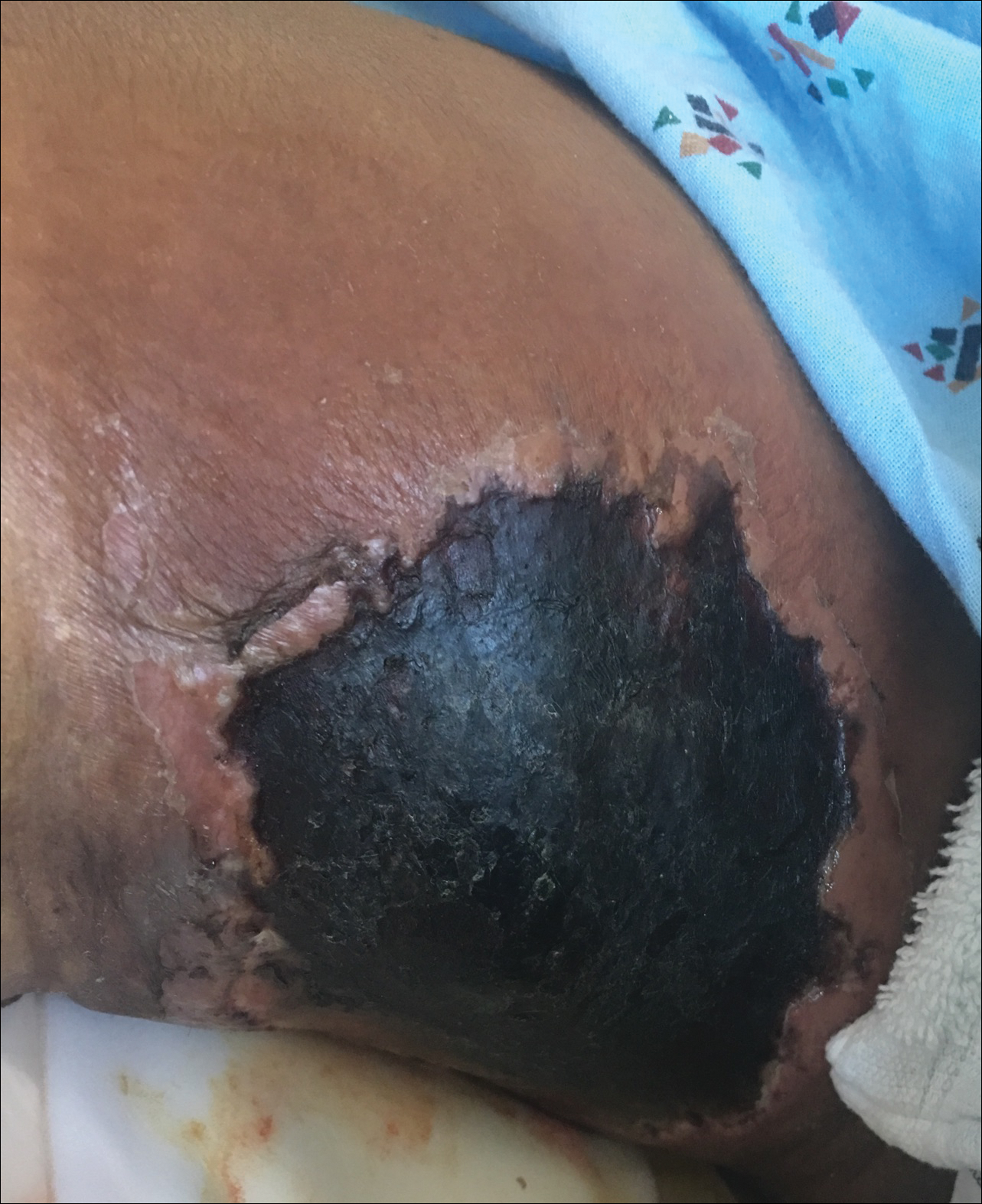
A 29-year-old man with a history of acute lymphoblastic leukemia was admitted for acute encephalopathy and a necrotic ulcer on the right thigh of 2 weeks' duration. He had received chemotherapy with pegaspargase and vincristine 6 weeks prior to admission. He reported headache with nausea and vomiting of 2 weeks' duration and had sustained a fall in the bathtub a week prior that initially resulted in a right thigh abrasion. He denied recent travel, unusual food consumption, animal exposure, exposure to sick persons, and alcohol or other drug use. On examination the patient was alert but was not oriented to person, place, or time. A 10.2 ×10-cm necrotic ulcer with surrounding mild erythema and tenderness was noted on the right inner thigh.
Chronic Diffuse Erythematous Papulonodules
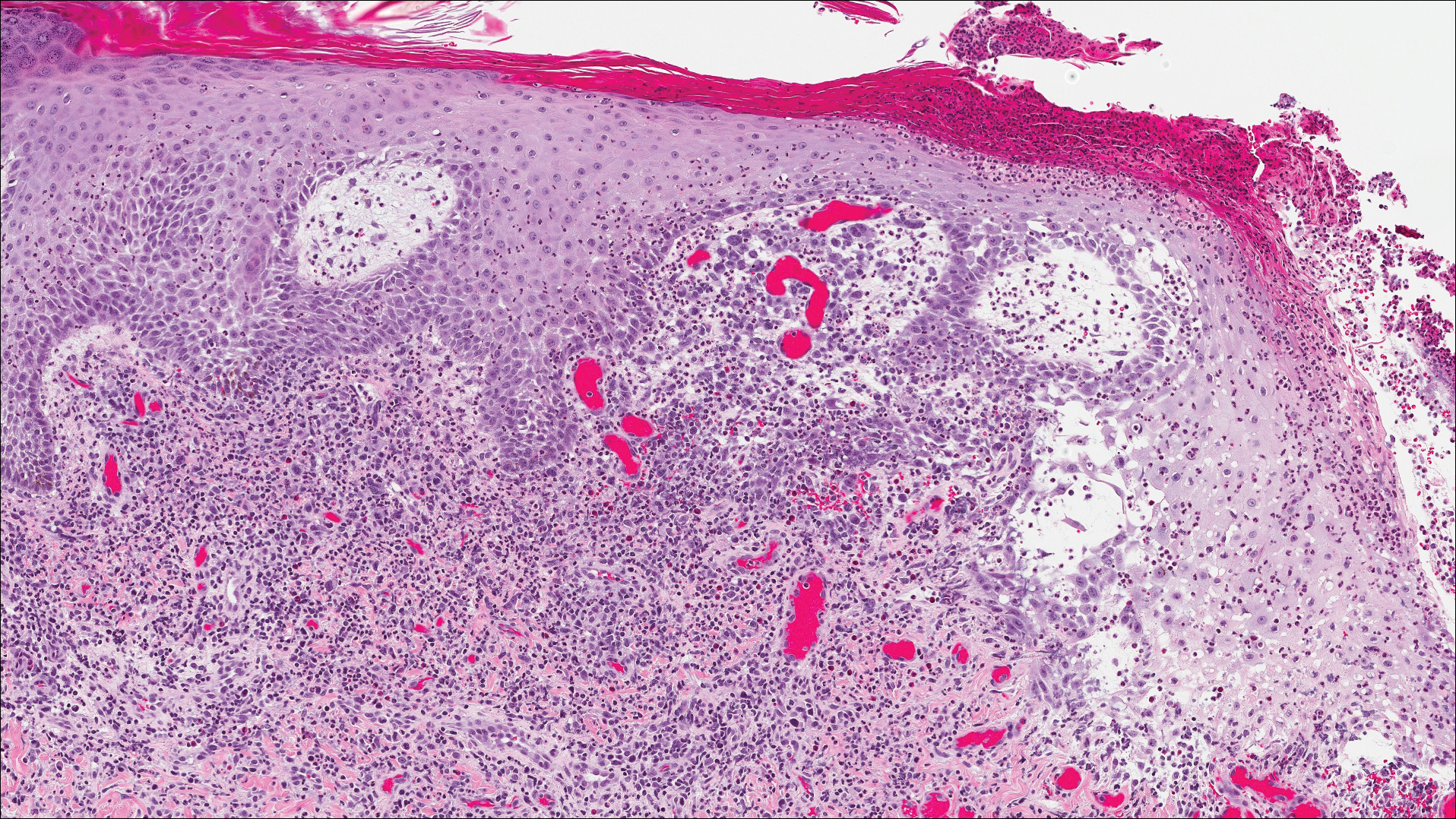
The Diagnosis: Lymphomatoid Papulosis
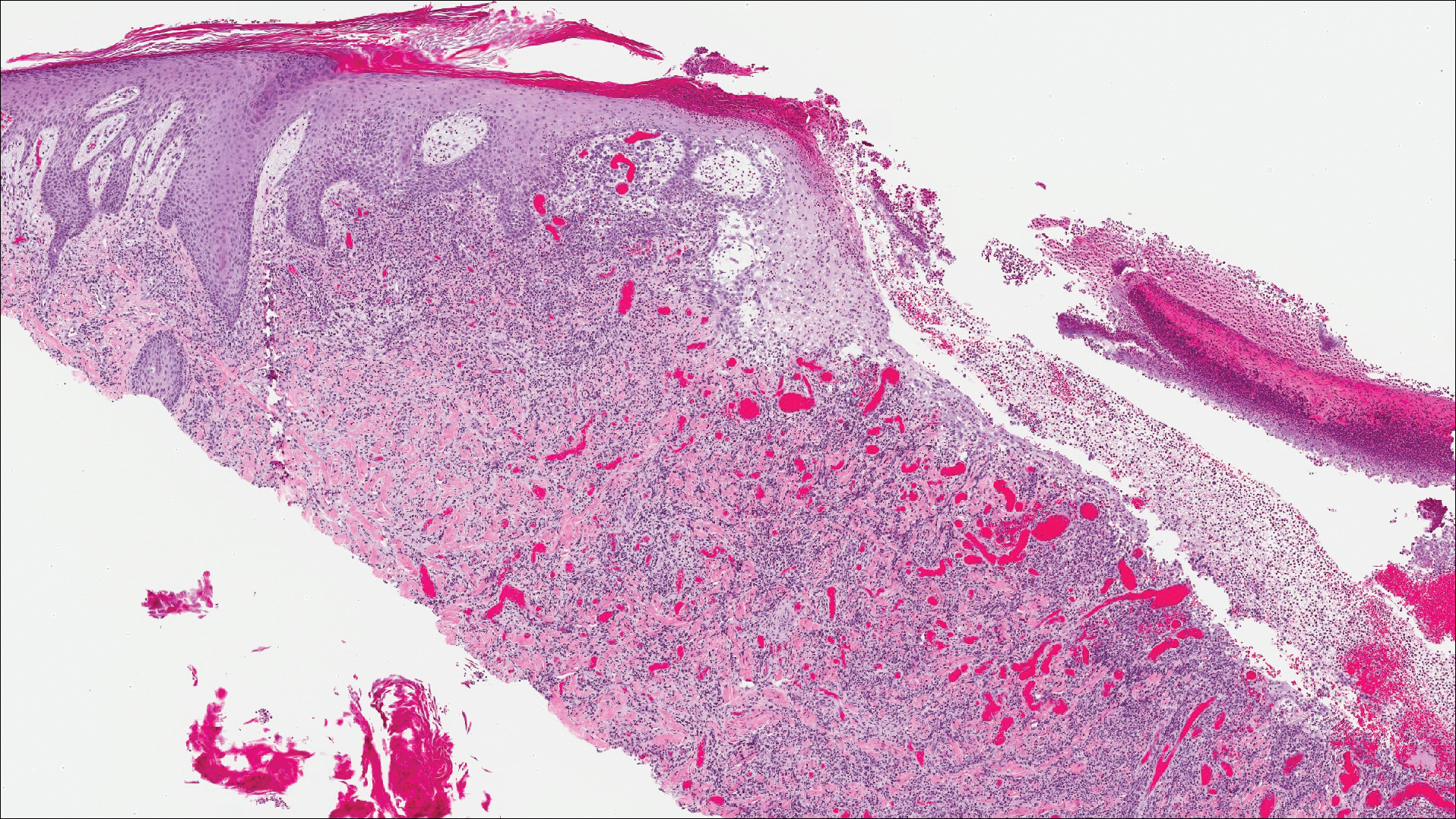
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).

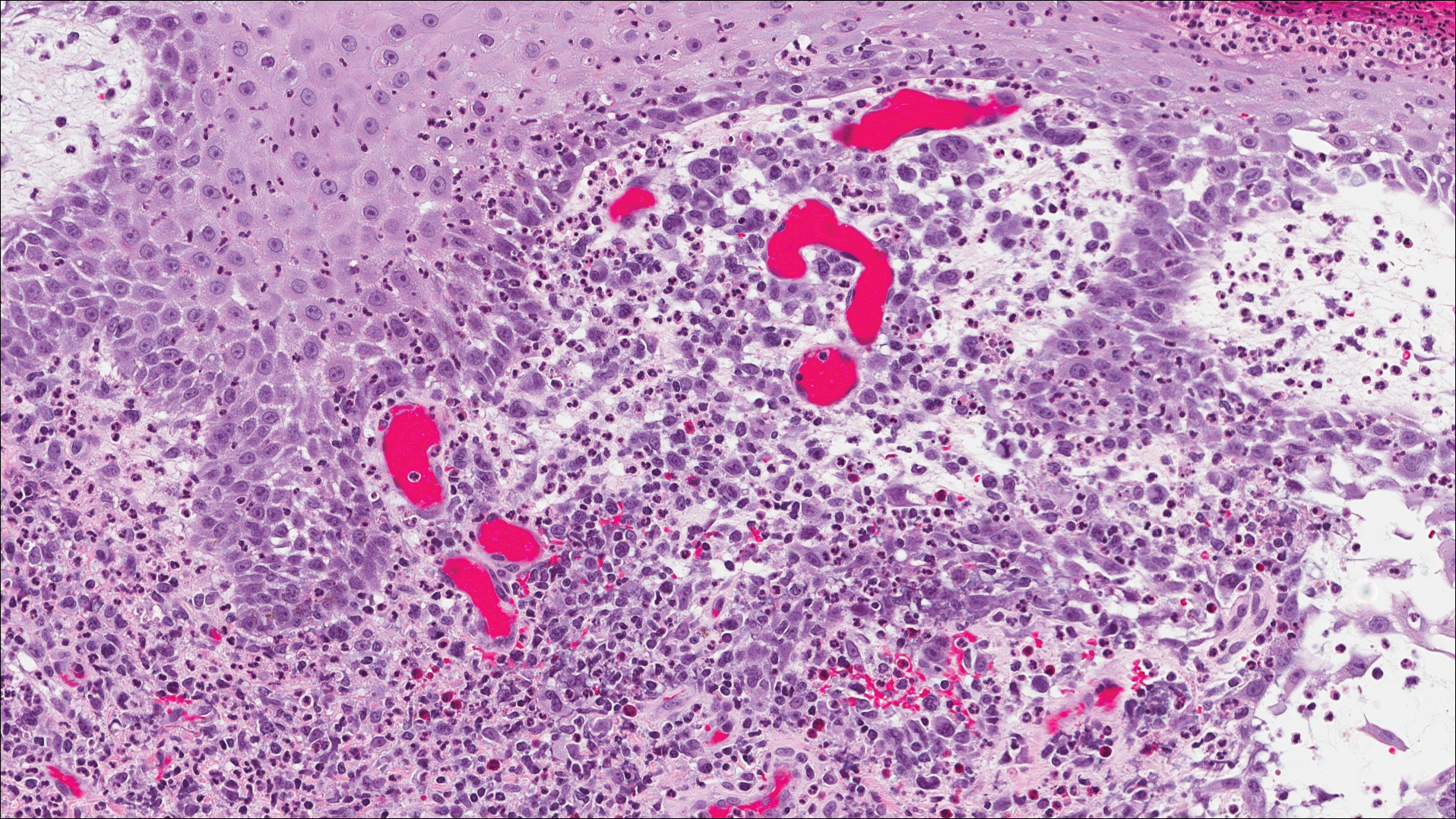
Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
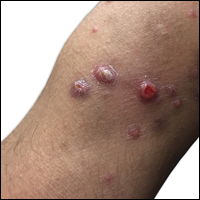
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
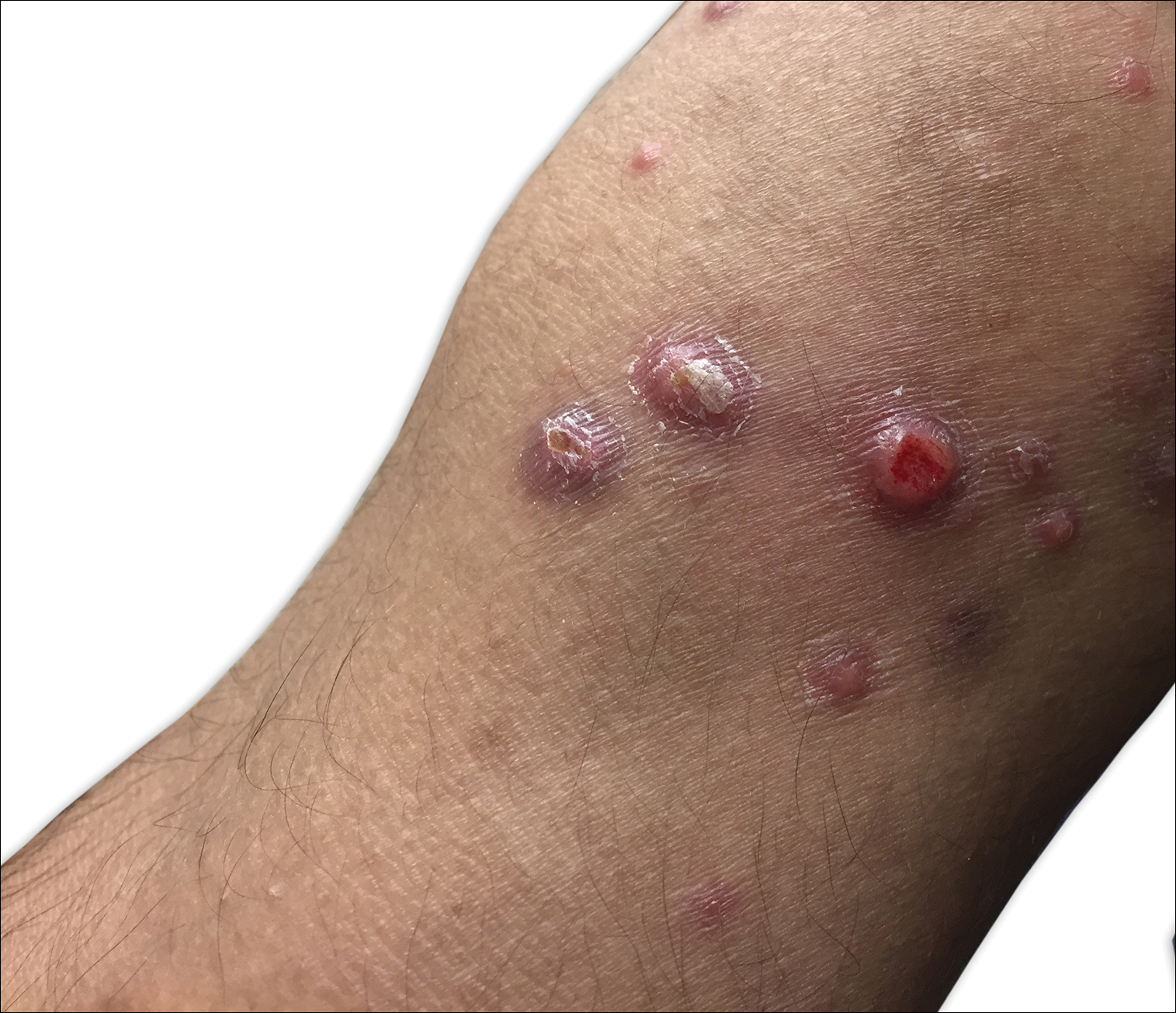
A 29-year-old man from Saudi Arabia presented with slightly tender skin lesions occurring in crops every few months over the last 7 years. The lesions typically would occur on the inguinal area, lower abdomen, buttocks, thighs, or arms, resolving within a few weeks despite no treatment. The patient denied having systemic symptoms such as fevers, chills, sweats, chest pain, shortness of breath, or unexpected weight loss. Physical examination revealed multiple erythematous papulonodules, some ulcerated with a superficial crust, grouped predominantly on the medial aspect of the right upper arm and left lower inguinal region. Isolated lesions also were present on the forearms, dorsal aspects of the hands, abdomen, and thighs. The grouped papulonodules were intermixed with faint hyperpigmented macules indicative of prior lesions. No oral lesions were noted, and there was no marked axillary or inguinal lymphadenopathy.
Eroded Plaque on the Lower Lip
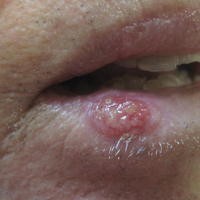
The Diagnosis: Squamous Cell Carcinoma
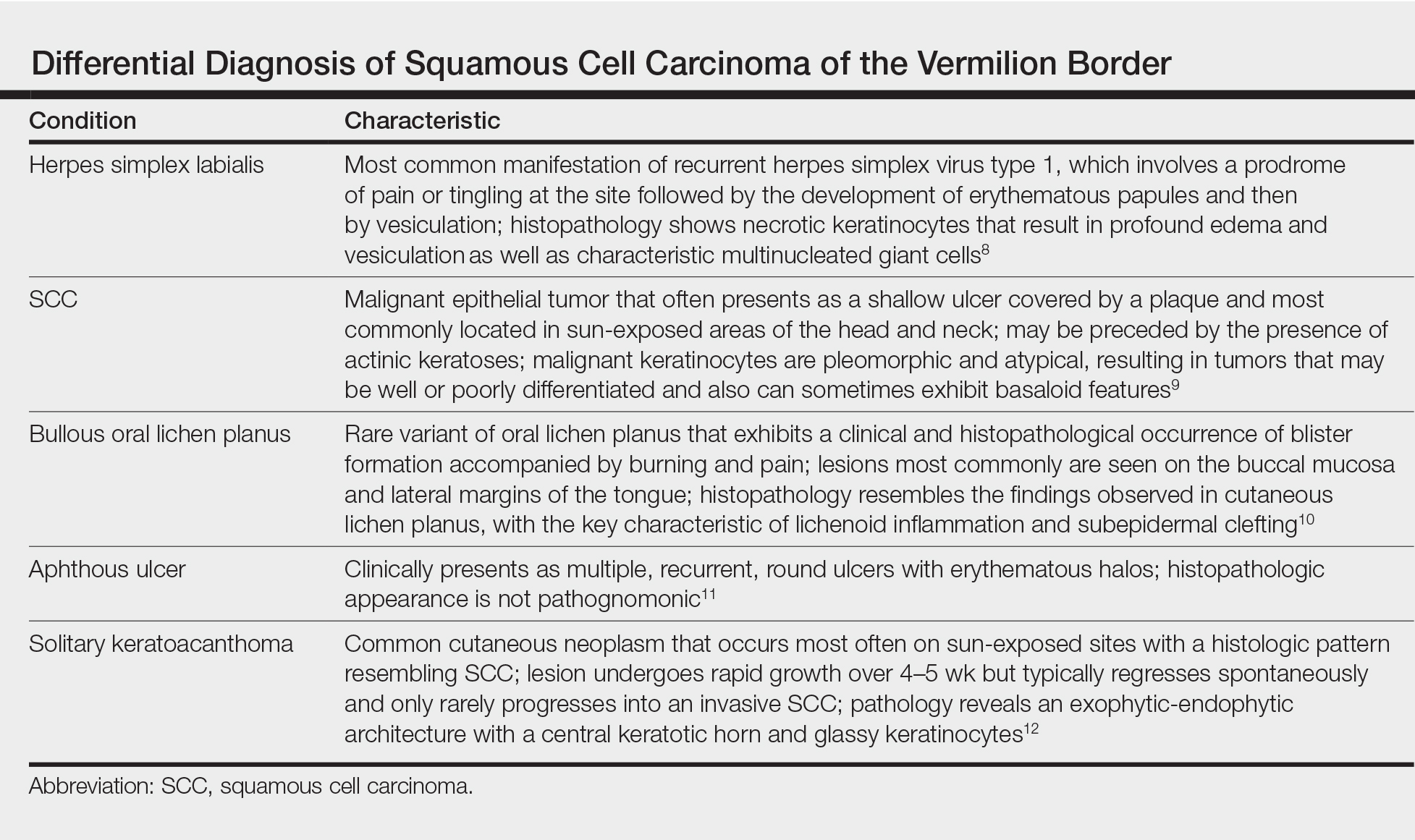
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).
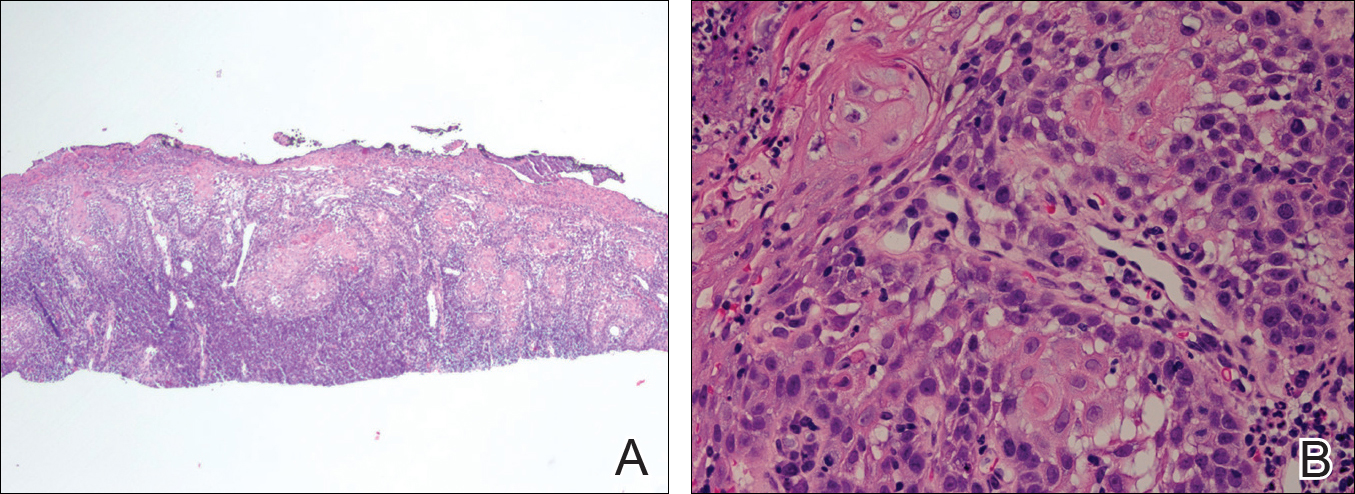
Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.
The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
The Diagnosis: Squamous Cell Carcinoma
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
The Diagnosis: Squamous Cell Carcinoma
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
An 83-year-old man presented with a new-onset 1.2-cm eroded plaque on the vermilion border of the right lower lip that reportedly developed 2 weeks prior and was increasing in size. The plaque was moist and was composed of confluent glistening papules. Medical history was notable for the presence of both basal cell and squamous cell carcinomas.
Black Adherence Nodules on the Scalp Hair Shaft
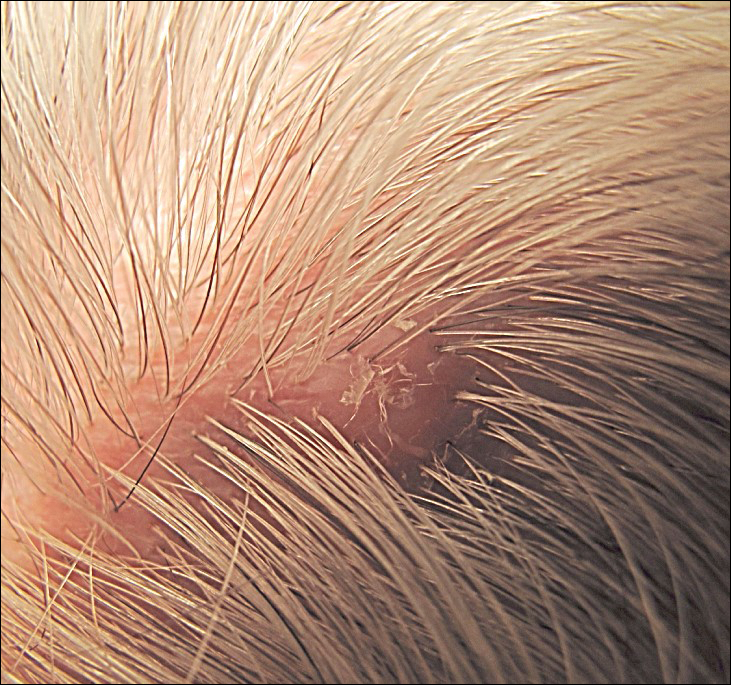
The Diagnosis: Piedra
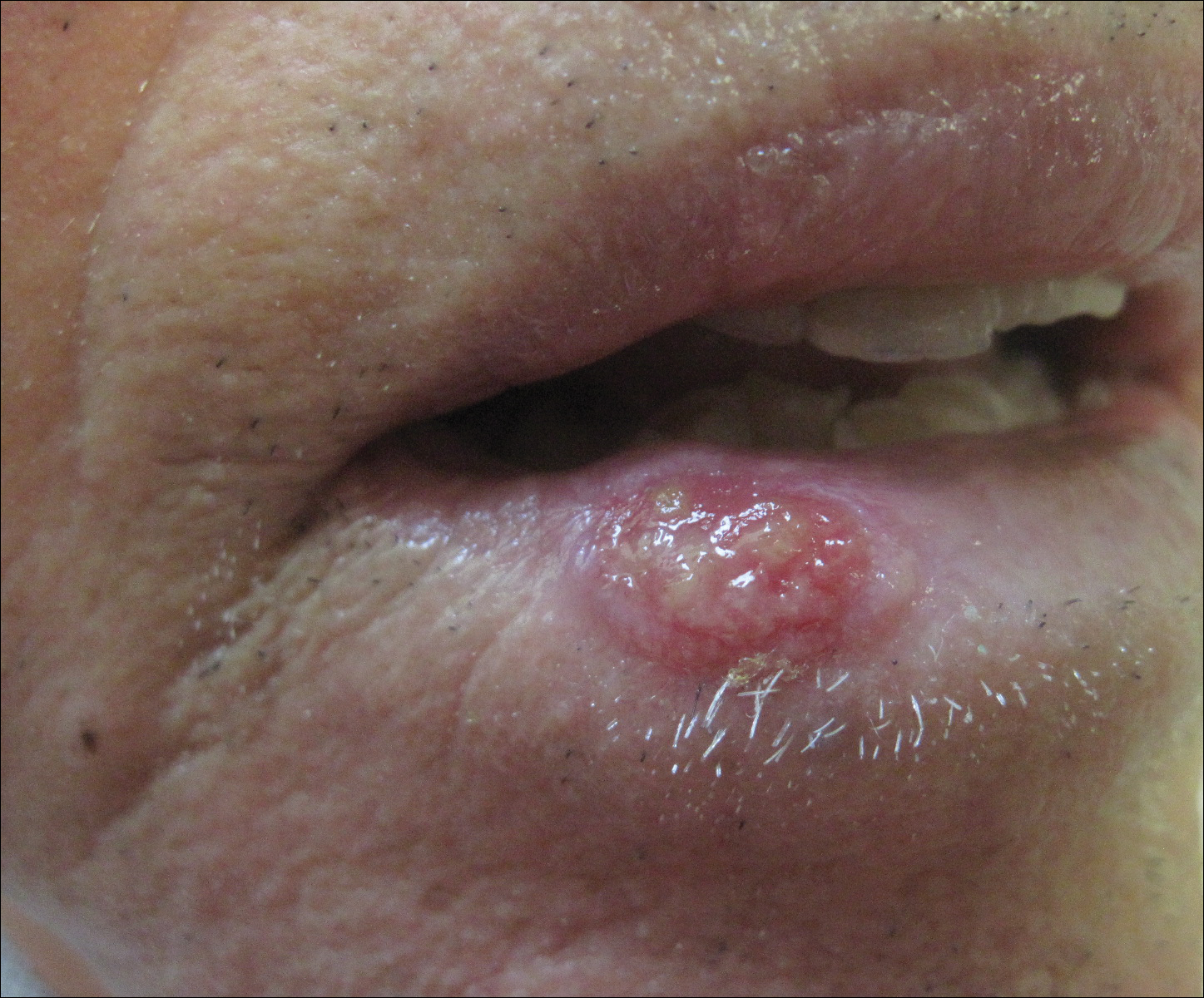
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).
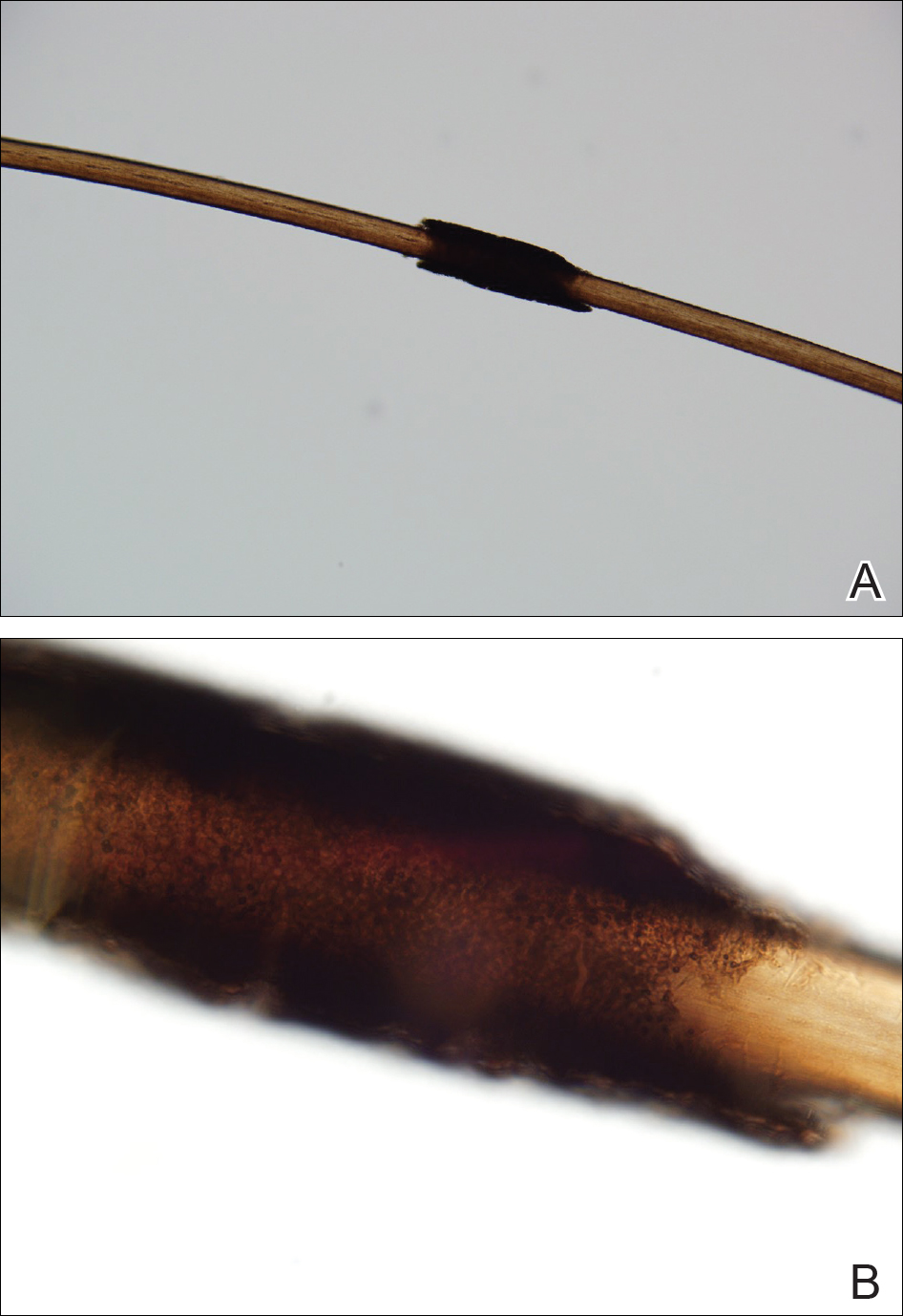
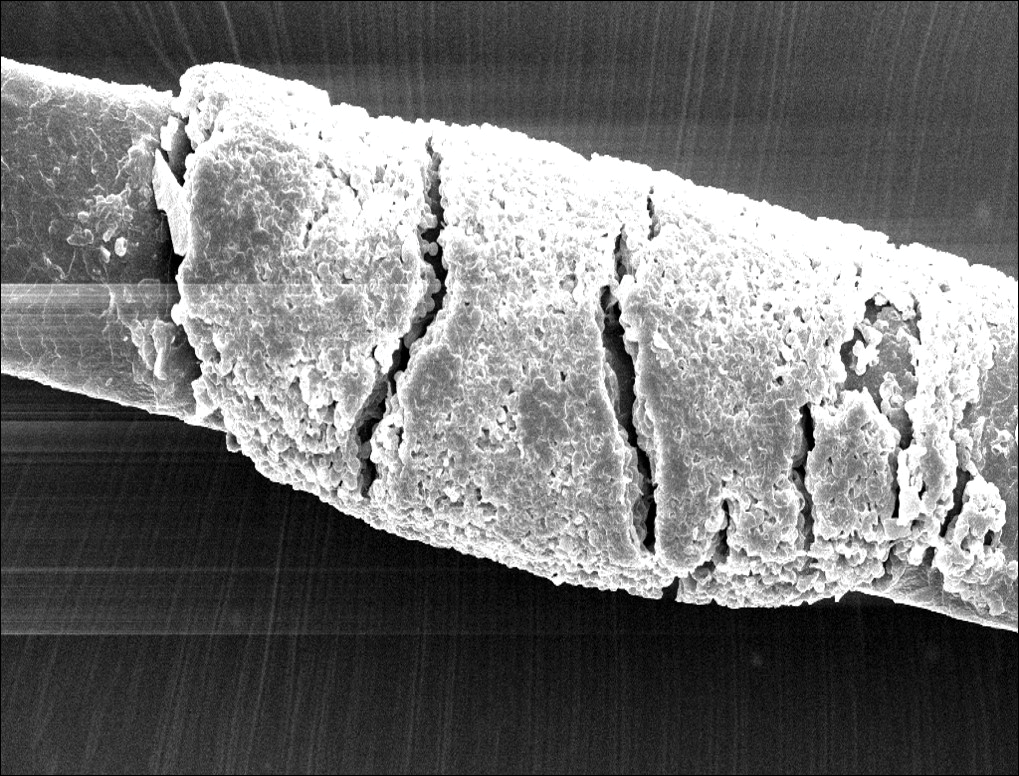
Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.
The Diagnosis: Piedra
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).


Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
The Diagnosis: Piedra
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).


Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.

A 21-year-old woman presented to the dermatology clinic with what she described as small black dots in her hair that she first noted 3 months prior to presentation. The black nodules were asymptomatic, but the patient noted that they seemed to be moving up the hair shaft. They were firmly attached and great effort was required to remove them. The patient's sister recently developed similar nodules. The patient and her sister work as missionaries and had spent time in India, Southeast Asia, and Central America within the last few years. Physical examination revealed firmly adherent black nodules involving the mid to distal portions of the hair shafts on the scalp. There were no nail or skin findings. Cultures were obtained, and microscopic examination was performed.
Painful Necrotic Ulcer on the Vulva
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.
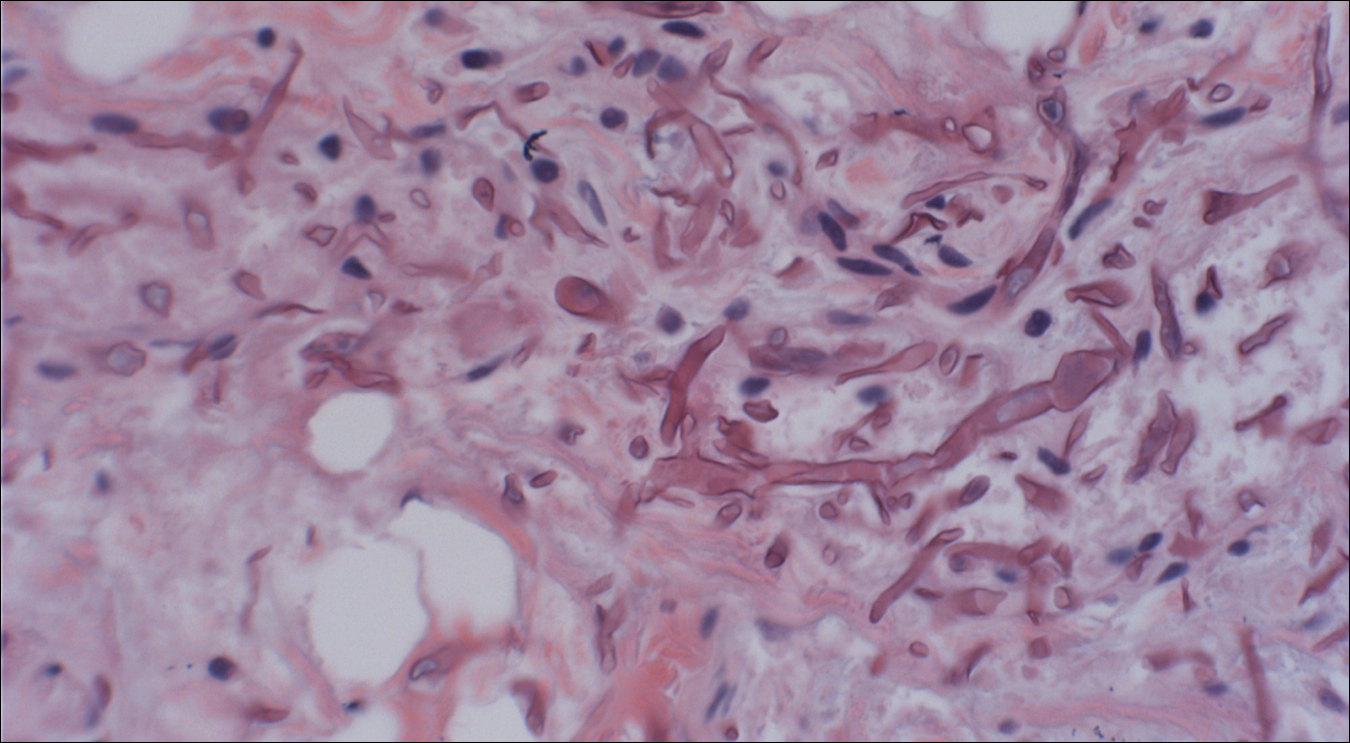
Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.



Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.



Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
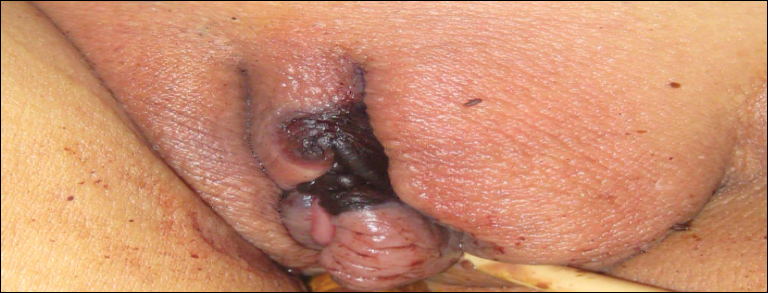
A 48-year-old woman with relapsed T-cell acute lymphoblastic leukemia was admitted to the oncology service for salvage chemotherapy and allogeneic stem cell transplant. Her admission was complicated by extended-spectrum β-lactamase-producing Escherichia coli sepsis and persistent pancytopenia, which required transfer to the intensive care unit. After 2 weeks and while still in the intensive care unit, she developed a painful necrotic vulvar ulcer over the right labia and clitoris that progressed and formed an overlying black eschar.
Recurring Yellowish Papules and Plaques on the Back
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.
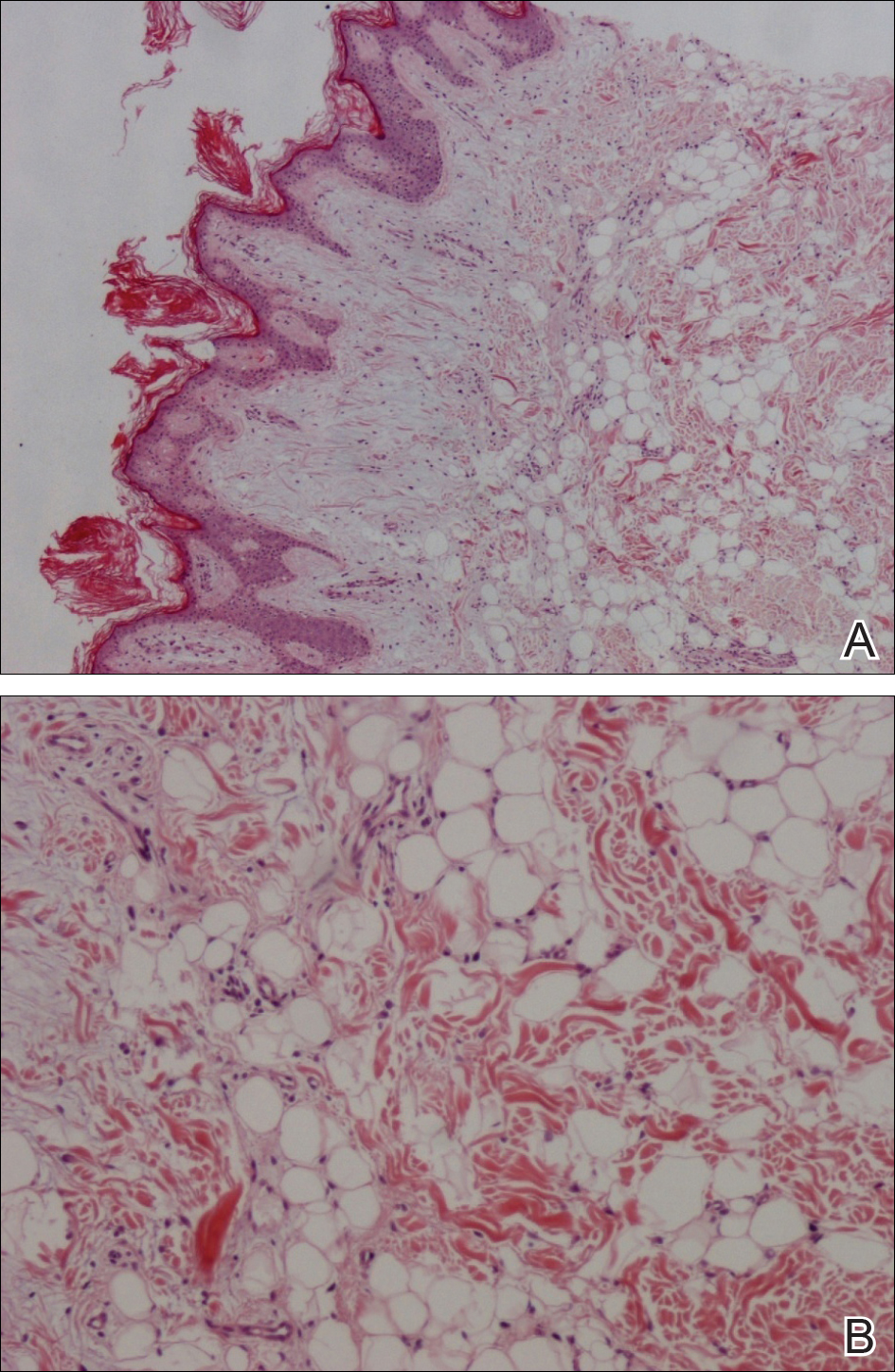
Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.

Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.

Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
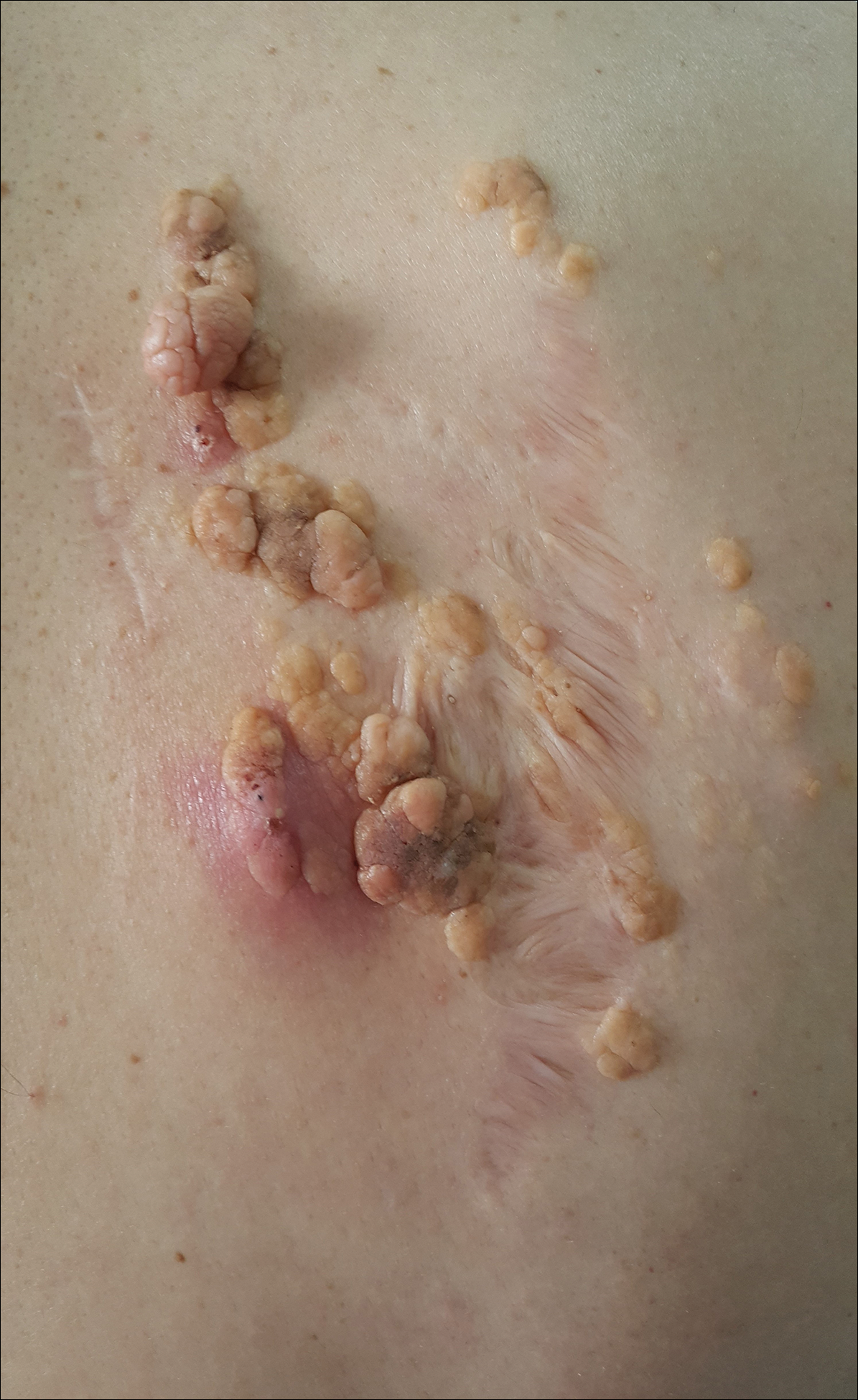
A 36-year-old man presented with a group of partially erythematous, yellowish papules and plaques ranging from 5 to 20 mm in diameter on the right side of the upper back of 20 years' duration. They were surgically excised 8 years prior but recurred and spread. The lesions occasionally were painful and tender with redness and discharge.
Purpuric Lesions of the Scalp, Axillae, and Groin of an Infant
The Diagnosis: Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a clonal proliferative disorder of Langerhans cells that can affect any organ, most commonly the skin and bones. It typically develops in children aged 1 to 3 years, with a male to female ratio of 2 to 1.1 Skin manifestations include purpuric papules, pustules, vesicles, erosions, and fissuring distributed predominantly on the scalp and flexural sites. Mucosal sites, particularly the oral mucosa, may be involved and usually present as erosions associated with underlying bone lesions.1 Langerhans cell histiocytosis should be considered in the differential diagnosis of recalcitrant diaper dermatitis in an infant, especially when there is purpura and erosions, as seen in our patient. Common conditions in infants such as cutaneous candidiasis (intense erythema with superficial erosions, peripheral scale and satellite pustules on flexural areas, potassium hydroxide microscopy revealing yeast forms and pseudohyphae) and seborrheic dermatitis (well-defined pink to red, moist, and often scaly patches favoring the folds) may be distinguished clinically from Hailey-Hailey disease (malodorous plaques with fissures and erosions favoring the folds), which is rare in infancy, and acrodermatitis enteropathica (erythema and erosions with scale-crust and desquamation on periorificial, acral, and intertriginous skin).
Histopathologic evaluation is instrumental in diagnosing the skin lesions of LCH. Further evaluation for systemic involvement is necessary once the diagnosis is made. Skin biopsy of the scalp and right inguinal fold revealed a wedge-shaped infiltrate of histiocytes with slightly folded nuclear contours in our patient (Figure 1). CD1a (Figure 2) and S-100 stains were markedly positive, which is characteristic of LCH. Complete blood cell count, renal function, liver function, urinalysis, and flow cytometry results were within reference range. A skeletal survey and echocardiogram were unremarkable; however, mild hepatosplenomegaly was noted on abdominal ultrasonography.
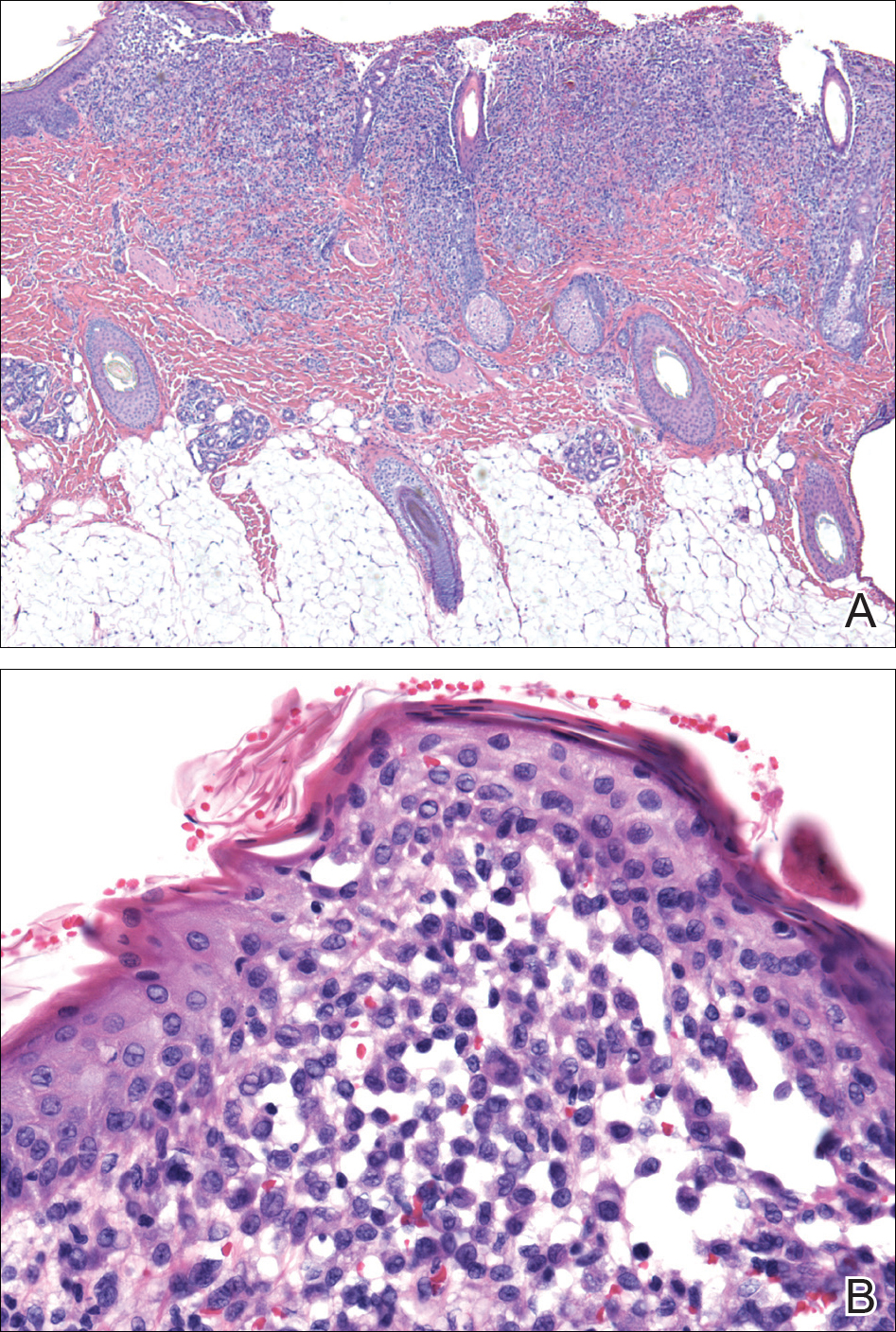
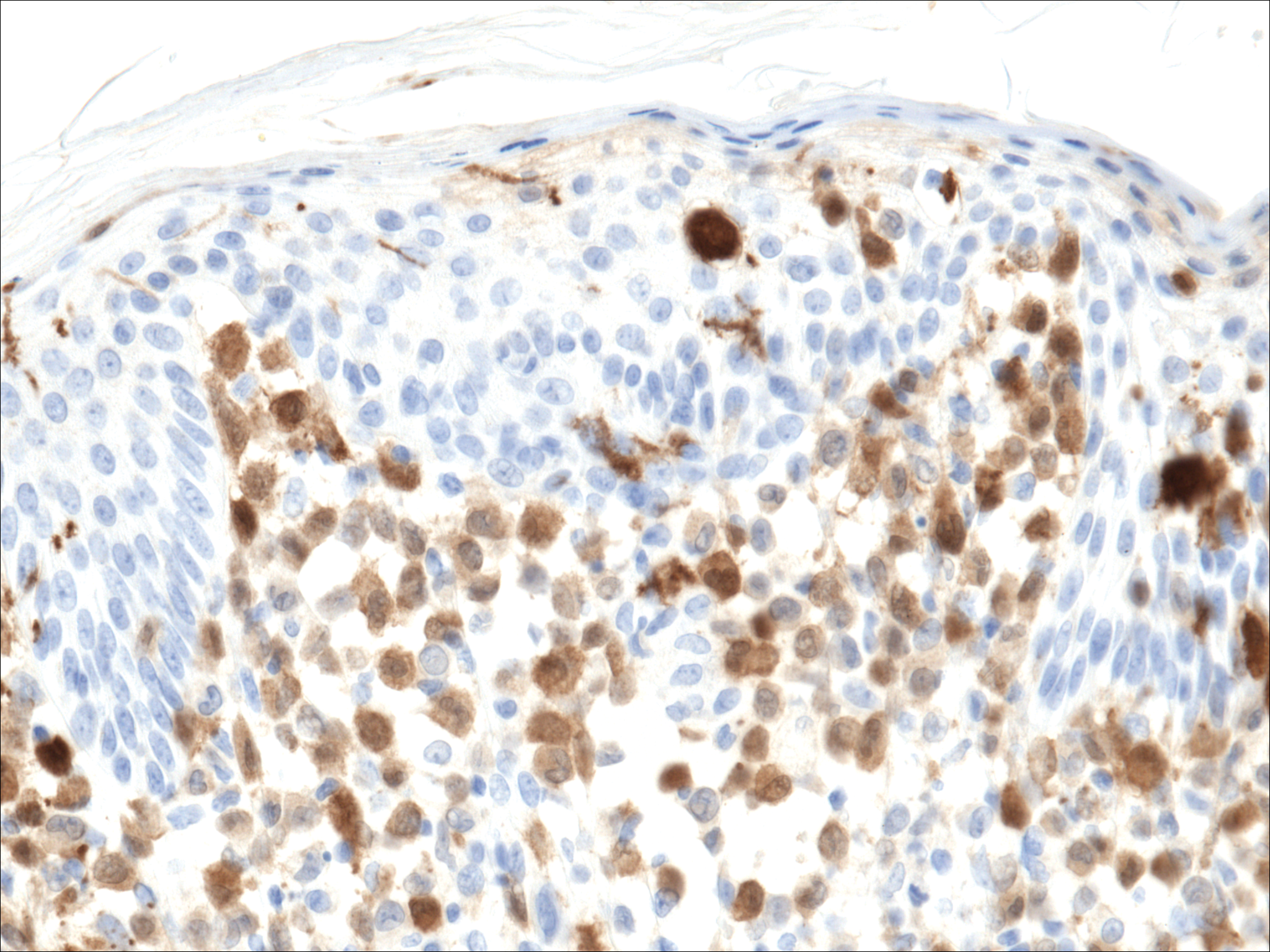
Treatment of LCH varies based on the extent of organ involvement. For isolated cutaneous disease, topical steroids, topical nitrogen mustard, phototherapy, and thalidomide may be employed.2 Multisystem disease requires chemotherapeutic agents including vinblastine and prednisone.2,3 Because more than half of patients with LCH have oncogenic BRAF V600E mutations,4 vemurafenib may have a therapeutic role in treatment. Rare case reports have documented disease response in patients with LCH and Erdheim-Chester disease.5,6
Prognosis varies based on age and extent of systemic involvement. Children younger than 2 years with multiorgan involvement have a poor prognosis (35%-55% mortality rate) compared to older children without hematopoietic, hepatosplenic, or lung involvement (100% survival rate). Additionally, response to treatment affects prognosis, as there is a 66% mortality rate in those who do not respond to treatment after 6 weeks.3 Long-term sequelae of LCH include endocrine dysfunction (ie, diabetes insipidus, growth hormone deficiencies), hearing impairment, orthopedic impairment, and neuropsychological disease; thus, multidisciplinary care often is neccessary.7
Given the multisystem involvement in our patient, he was treated with vinblastine, 6-mercaptopurine, and prednisolone with only partial and transient disease response. He was then treated with clofarabine with dramatic resolution of the mediastinal mass on follow-up positron emission tomography. The cutaneous lesions persisted and were managed with topical corticosteroids.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years [published online October 25, 2012]. Pediatr Blood Cancer. 2013;60:175-184.
- Gadner H, Grois N, Arico M, et al; Histiocyte Society. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728-734.
- Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-1500.
- Charles J, Beani JC, Fiandrino G, et al. Major response to vemurafenib in patient with severe cutaneous Langerhans cell histiocytosis harboring BRAF V600E mutation. J Am Acad Dermatol. 2014;71:E97-E99.
- Martin A, Macmillan S, Murphy D, et al. Langerhans cell histiocytosis: 23 years' paediatric experience highlights severe long-term sequelae. Scott Med J. 2014;59:149-157.
The Diagnosis: Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a clonal proliferative disorder of Langerhans cells that can affect any organ, most commonly the skin and bones. It typically develops in children aged 1 to 3 years, with a male to female ratio of 2 to 1.1 Skin manifestations include purpuric papules, pustules, vesicles, erosions, and fissuring distributed predominantly on the scalp and flexural sites. Mucosal sites, particularly the oral mucosa, may be involved and usually present as erosions associated with underlying bone lesions.1 Langerhans cell histiocytosis should be considered in the differential diagnosis of recalcitrant diaper dermatitis in an infant, especially when there is purpura and erosions, as seen in our patient. Common conditions in infants such as cutaneous candidiasis (intense erythema with superficial erosions, peripheral scale and satellite pustules on flexural areas, potassium hydroxide microscopy revealing yeast forms and pseudohyphae) and seborrheic dermatitis (well-defined pink to red, moist, and often scaly patches favoring the folds) may be distinguished clinically from Hailey-Hailey disease (malodorous plaques with fissures and erosions favoring the folds), which is rare in infancy, and acrodermatitis enteropathica (erythema and erosions with scale-crust and desquamation on periorificial, acral, and intertriginous skin).
Histopathologic evaluation is instrumental in diagnosing the skin lesions of LCH. Further evaluation for systemic involvement is necessary once the diagnosis is made. Skin biopsy of the scalp and right inguinal fold revealed a wedge-shaped infiltrate of histiocytes with slightly folded nuclear contours in our patient (Figure 1). CD1a (Figure 2) and S-100 stains were markedly positive, which is characteristic of LCH. Complete blood cell count, renal function, liver function, urinalysis, and flow cytometry results were within reference range. A skeletal survey and echocardiogram were unremarkable; however, mild hepatosplenomegaly was noted on abdominal ultrasonography.


Treatment of LCH varies based on the extent of organ involvement. For isolated cutaneous disease, topical steroids, topical nitrogen mustard, phototherapy, and thalidomide may be employed.2 Multisystem disease requires chemotherapeutic agents including vinblastine and prednisone.2,3 Because more than half of patients with LCH have oncogenic BRAF V600E mutations,4 vemurafenib may have a therapeutic role in treatment. Rare case reports have documented disease response in patients with LCH and Erdheim-Chester disease.5,6
Prognosis varies based on age and extent of systemic involvement. Children younger than 2 years with multiorgan involvement have a poor prognosis (35%-55% mortality rate) compared to older children without hematopoietic, hepatosplenic, or lung involvement (100% survival rate). Additionally, response to treatment affects prognosis, as there is a 66% mortality rate in those who do not respond to treatment after 6 weeks.3 Long-term sequelae of LCH include endocrine dysfunction (ie, diabetes insipidus, growth hormone deficiencies), hearing impairment, orthopedic impairment, and neuropsychological disease; thus, multidisciplinary care often is neccessary.7
Given the multisystem involvement in our patient, he was treated with vinblastine, 6-mercaptopurine, and prednisolone with only partial and transient disease response. He was then treated with clofarabine with dramatic resolution of the mediastinal mass on follow-up positron emission tomography. The cutaneous lesions persisted and were managed with topical corticosteroids.
The Diagnosis: Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a clonal proliferative disorder of Langerhans cells that can affect any organ, most commonly the skin and bones. It typically develops in children aged 1 to 3 years, with a male to female ratio of 2 to 1.1 Skin manifestations include purpuric papules, pustules, vesicles, erosions, and fissuring distributed predominantly on the scalp and flexural sites. Mucosal sites, particularly the oral mucosa, may be involved and usually present as erosions associated with underlying bone lesions.1 Langerhans cell histiocytosis should be considered in the differential diagnosis of recalcitrant diaper dermatitis in an infant, especially when there is purpura and erosions, as seen in our patient. Common conditions in infants such as cutaneous candidiasis (intense erythema with superficial erosions, peripheral scale and satellite pustules on flexural areas, potassium hydroxide microscopy revealing yeast forms and pseudohyphae) and seborrheic dermatitis (well-defined pink to red, moist, and often scaly patches favoring the folds) may be distinguished clinically from Hailey-Hailey disease (malodorous plaques with fissures and erosions favoring the folds), which is rare in infancy, and acrodermatitis enteropathica (erythema and erosions with scale-crust and desquamation on periorificial, acral, and intertriginous skin).
Histopathologic evaluation is instrumental in diagnosing the skin lesions of LCH. Further evaluation for systemic involvement is necessary once the diagnosis is made. Skin biopsy of the scalp and right inguinal fold revealed a wedge-shaped infiltrate of histiocytes with slightly folded nuclear contours in our patient (Figure 1). CD1a (Figure 2) and S-100 stains were markedly positive, which is characteristic of LCH. Complete blood cell count, renal function, liver function, urinalysis, and flow cytometry results were within reference range. A skeletal survey and echocardiogram were unremarkable; however, mild hepatosplenomegaly was noted on abdominal ultrasonography.


Treatment of LCH varies based on the extent of organ involvement. For isolated cutaneous disease, topical steroids, topical nitrogen mustard, phototherapy, and thalidomide may be employed.2 Multisystem disease requires chemotherapeutic agents including vinblastine and prednisone.2,3 Because more than half of patients with LCH have oncogenic BRAF V600E mutations,4 vemurafenib may have a therapeutic role in treatment. Rare case reports have documented disease response in patients with LCH and Erdheim-Chester disease.5,6
Prognosis varies based on age and extent of systemic involvement. Children younger than 2 years with multiorgan involvement have a poor prognosis (35%-55% mortality rate) compared to older children without hematopoietic, hepatosplenic, or lung involvement (100% survival rate). Additionally, response to treatment affects prognosis, as there is a 66% mortality rate in those who do not respond to treatment after 6 weeks.3 Long-term sequelae of LCH include endocrine dysfunction (ie, diabetes insipidus, growth hormone deficiencies), hearing impairment, orthopedic impairment, and neuropsychological disease; thus, multidisciplinary care often is neccessary.7
Given the multisystem involvement in our patient, he was treated with vinblastine, 6-mercaptopurine, and prednisolone with only partial and transient disease response. He was then treated with clofarabine with dramatic resolution of the mediastinal mass on follow-up positron emission tomography. The cutaneous lesions persisted and were managed with topical corticosteroids.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years [published online October 25, 2012]. Pediatr Blood Cancer. 2013;60:175-184.
- Gadner H, Grois N, Arico M, et al; Histiocyte Society. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728-734.
- Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-1500.
- Charles J, Beani JC, Fiandrino G, et al. Major response to vemurafenib in patient with severe cutaneous Langerhans cell histiocytosis harboring BRAF V600E mutation. J Am Acad Dermatol. 2014;71:E97-E99.
- Martin A, Macmillan S, Murphy D, et al. Langerhans cell histiocytosis: 23 years' paediatric experience highlights severe long-term sequelae. Scott Med J. 2014;59:149-157.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years [published online October 25, 2012]. Pediatr Blood Cancer. 2013;60:175-184.
- Gadner H, Grois N, Arico M, et al; Histiocyte Society. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728-734.
- Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-1500.
- Charles J, Beani JC, Fiandrino G, et al. Major response to vemurafenib in patient with severe cutaneous Langerhans cell histiocytosis harboring BRAF V600E mutation. J Am Acad Dermatol. 2014;71:E97-E99.
- Martin A, Macmillan S, Murphy D, et al. Langerhans cell histiocytosis: 23 years' paediatric experience highlights severe long-term sequelae. Scott Med J. 2014;59:149-157.
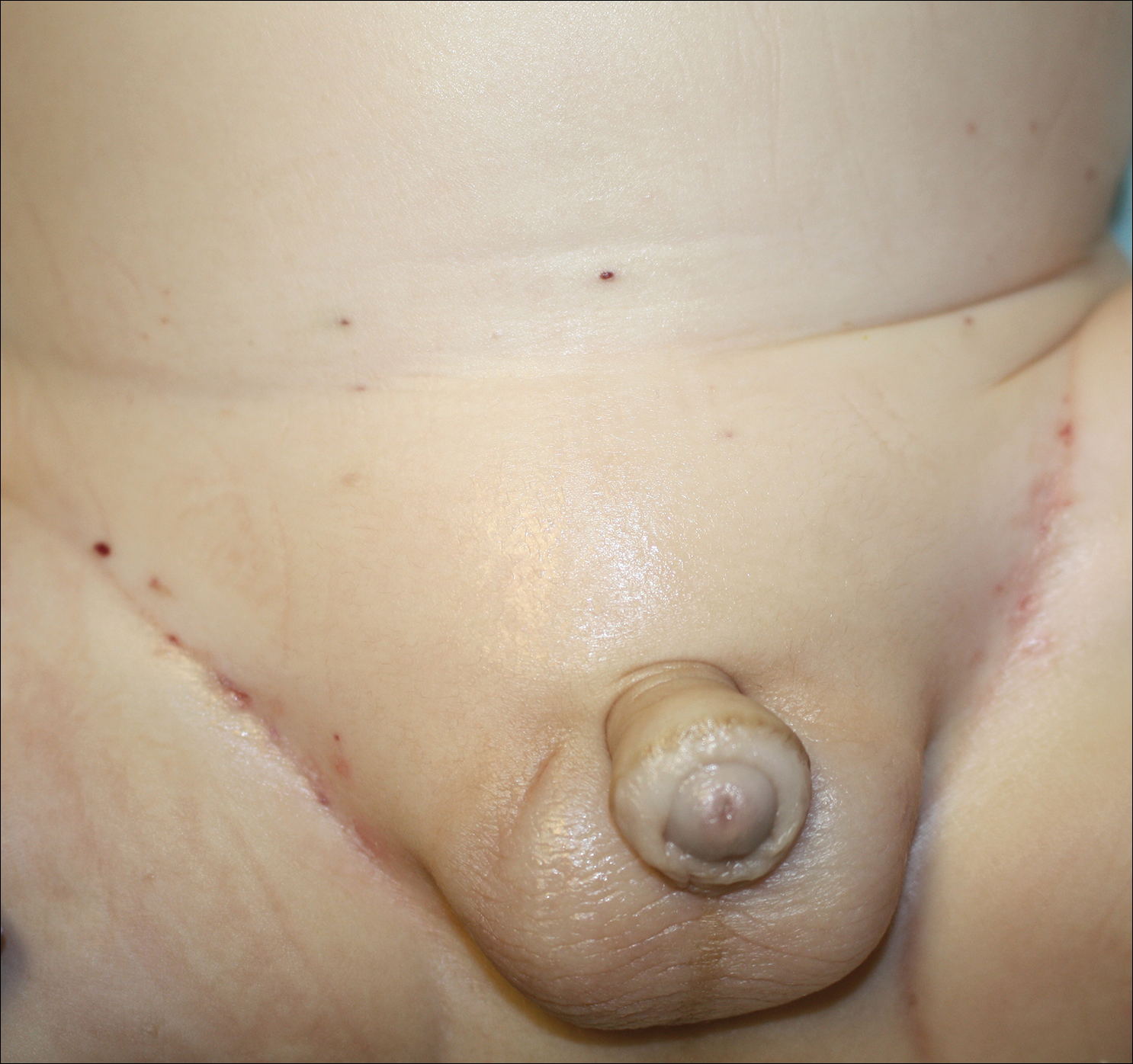
A 7-month-old boy admitted to the hospital with new-onset respiratory stridor was found to have a rash of the scalp, axillae, and groin of 1 month's duration that was unresponsive to treatment with mineral oil. Bronchoscopy revealed tracheal compression, and urgent magnetic resonance imaging of the chest demonstrated an anterior mediastinal mass. Prior to presentation, the patient was otherwise healthy with normal growth and development. On physical examination, scattered red-brown and purpuric papules with hemorrhagic crust were noted on the scalp. There were well-defined pink erosive patches and purpuric papules in the inguinal folds bilaterally and similar erosive patches in the axillae. Numerous punched out ulcerations were noted on the lower gingiva. There was no palpable lymphadenopathy. The hands, feet, penis, scrotum, and perianal area were spared. Biopsies of the skin and mediastinal mass were performed.
Perifollicular Papules on the Trunk
The Diagnosis: Disseminate and Recurrent Infundibulofolliculitis
A punch biopsy of a representative lesion on the trunk was performed. Histopathologic examination revealed a chronic lymphohistiocytic proliferation, focal spongiosis, and lymphocytic exocytosis primarily involving the isthmus of the hair follicle (Figure 1). At the follicular opening there was associated parakeratosis of the adjacent epidermis (Figure 2). Given these clinical and histopathological findings, a diagnosis of disseminate and recurrent infundibulofolliculitis (DRIF) was made.
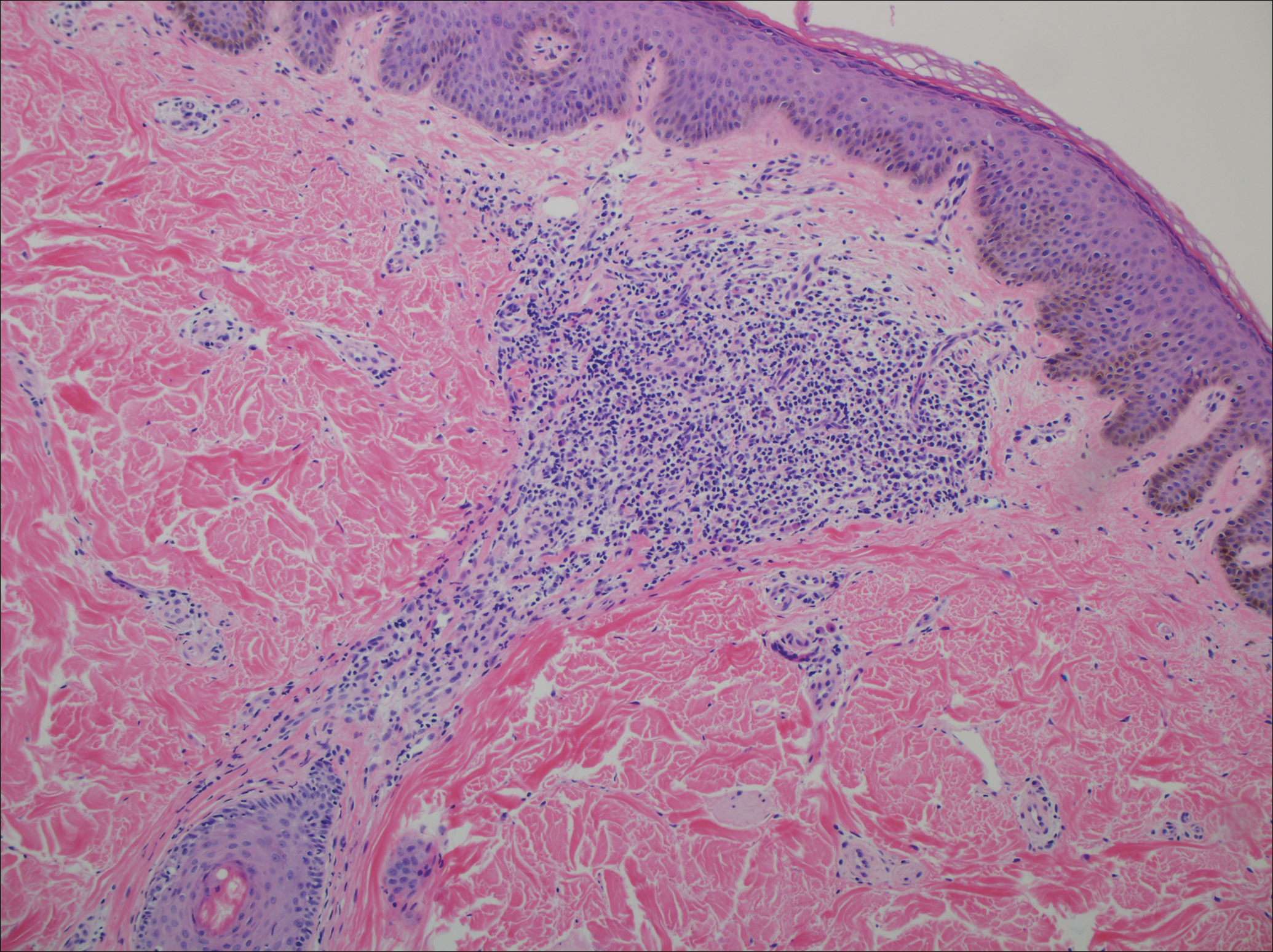
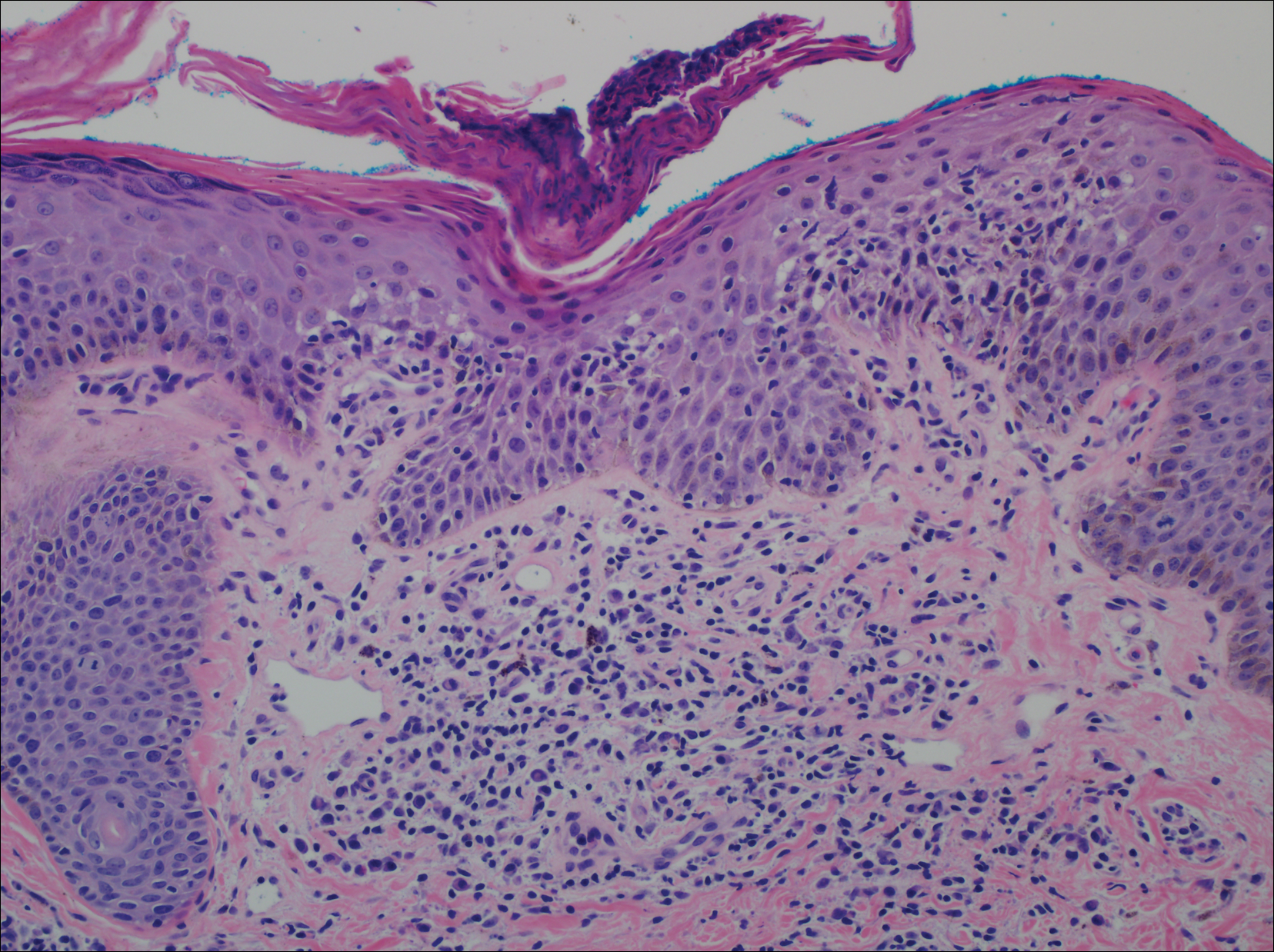
Disseminate and recurrent infundibulofolliculitis was first described by Hitch and Lund1 in 1968 in a healthy 27-year-old black man as a widespread recurrent follicular eruption. Disseminate and recurrent infundibulofolliculitis usually affects young adult males with darkly pigmented skin.2,3 It has less commonly been described in children, females, and white individuals.3,4 Associations with atopy, systemic diseases, or medications are unknown.3-6 The onset usually is sudden and the disease course may be characterized by intermittent recurrences. Pruritus usually is reported but may be mild.5
Histopathology is characterized by spongiosis centered on the infundibulum of the hair follicle and a primarily lymphocytic inflammatory infiltrate. Neutrophils also may be identified.3 Disseminate and recurrent infundibulofolliculitis can be differentiated histologically from clinically similar entities such as keratosis pilaris, which has a keratin plug filling the infundibulum; lichen nitidus, which is characterized by a clawlike downgrowth of the rete ridges surrounding a central foci of inflammation; or folliculitis, which is characterized by perifollicular suppurative inflammation.
Treatment of DRIF is anecdotal and limited to case reports. Vitamin A alone or in combination with vitamin E has been reported to lead to some improvement.5 Tetracycline-class antibiotics, keratolytics, antihistamines, and topical retinoids have not been successful, and mixed results have been seen with topical steroids.5-7 There is a reported case of improvement with a 3-week regimen of psoralen plus UVA followed by twice-weekly maintenance.8 Promising results in the treatment of DRIF have been shown with oral isotretinoin once daily.3-5 Finally, DRIF may resolve independently6; therefore, treatment of DRIF should be addressed on a case-by-case basis.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis: report of a case. Arch Dermatol. 1968;97:432-435.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis. Arch Dermatol. 1972;105:580-583.
- Calka O, Metin A, Ozen S. A case of disseminated and recurrent infundibulofolliculitis responsive to treatment with systemic isotretinoin. J Dermatol. 2002;29:431-434.
- Aroni K, Grapsa A, Agapitos E. Disseminate and recurrent infundibulofolliculitis: response to isotretinoin. J Drugs Dermatol. 2004;3:434-435.
- Aroni K, Aivaliotis M, Davaris P. Disseminated and recurrent infundibular folliculitis (D.R.I.F.): report of a case successfully treated with isotretinoin. J Dermatol. 1998;25:51-53.
- Owen WR, Wood C. Disseminate and recurrent infundibulofolliculitis. Arch Dermatol. 1979;115:174-175.
- Hinds GA, Heald PW. A case of disseminate and recurrent infundibulofolliculitis responsive to treatment with topical steroids. Dermatol Online J. 2008;14:11.
- Goihman-Yahr M. Disseminate and recurrent infundibulofolliculitis: response to psoralen plus UVA therapy. Int J Dermatol. 1999;38:75-78.
The Diagnosis: Disseminate and Recurrent Infundibulofolliculitis
A punch biopsy of a representative lesion on the trunk was performed. Histopathologic examination revealed a chronic lymphohistiocytic proliferation, focal spongiosis, and lymphocytic exocytosis primarily involving the isthmus of the hair follicle (Figure 1). At the follicular opening there was associated parakeratosis of the adjacent epidermis (Figure 2). Given these clinical and histopathological findings, a diagnosis of disseminate and recurrent infundibulofolliculitis (DRIF) was made.


Disseminate and recurrent infundibulofolliculitis was first described by Hitch and Lund1 in 1968 in a healthy 27-year-old black man as a widespread recurrent follicular eruption. Disseminate and recurrent infundibulofolliculitis usually affects young adult males with darkly pigmented skin.2,3 It has less commonly been described in children, females, and white individuals.3,4 Associations with atopy, systemic diseases, or medications are unknown.3-6 The onset usually is sudden and the disease course may be characterized by intermittent recurrences. Pruritus usually is reported but may be mild.5
Histopathology is characterized by spongiosis centered on the infundibulum of the hair follicle and a primarily lymphocytic inflammatory infiltrate. Neutrophils also may be identified.3 Disseminate and recurrent infundibulofolliculitis can be differentiated histologically from clinically similar entities such as keratosis pilaris, which has a keratin plug filling the infundibulum; lichen nitidus, which is characterized by a clawlike downgrowth of the rete ridges surrounding a central foci of inflammation; or folliculitis, which is characterized by perifollicular suppurative inflammation.
Treatment of DRIF is anecdotal and limited to case reports. Vitamin A alone or in combination with vitamin E has been reported to lead to some improvement.5 Tetracycline-class antibiotics, keratolytics, antihistamines, and topical retinoids have not been successful, and mixed results have been seen with topical steroids.5-7 There is a reported case of improvement with a 3-week regimen of psoralen plus UVA followed by twice-weekly maintenance.8 Promising results in the treatment of DRIF have been shown with oral isotretinoin once daily.3-5 Finally, DRIF may resolve independently6; therefore, treatment of DRIF should be addressed on a case-by-case basis.
The Diagnosis: Disseminate and Recurrent Infundibulofolliculitis
A punch biopsy of a representative lesion on the trunk was performed. Histopathologic examination revealed a chronic lymphohistiocytic proliferation, focal spongiosis, and lymphocytic exocytosis primarily involving the isthmus of the hair follicle (Figure 1). At the follicular opening there was associated parakeratosis of the adjacent epidermis (Figure 2). Given these clinical and histopathological findings, a diagnosis of disseminate and recurrent infundibulofolliculitis (DRIF) was made.


Disseminate and recurrent infundibulofolliculitis was first described by Hitch and Lund1 in 1968 in a healthy 27-year-old black man as a widespread recurrent follicular eruption. Disseminate and recurrent infundibulofolliculitis usually affects young adult males with darkly pigmented skin.2,3 It has less commonly been described in children, females, and white individuals.3,4 Associations with atopy, systemic diseases, or medications are unknown.3-6 The onset usually is sudden and the disease course may be characterized by intermittent recurrences. Pruritus usually is reported but may be mild.5
Histopathology is characterized by spongiosis centered on the infundibulum of the hair follicle and a primarily lymphocytic inflammatory infiltrate. Neutrophils also may be identified.3 Disseminate and recurrent infundibulofolliculitis can be differentiated histologically from clinically similar entities such as keratosis pilaris, which has a keratin plug filling the infundibulum; lichen nitidus, which is characterized by a clawlike downgrowth of the rete ridges surrounding a central foci of inflammation; or folliculitis, which is characterized by perifollicular suppurative inflammation.
Treatment of DRIF is anecdotal and limited to case reports. Vitamin A alone or in combination with vitamin E has been reported to lead to some improvement.5 Tetracycline-class antibiotics, keratolytics, antihistamines, and topical retinoids have not been successful, and mixed results have been seen with topical steroids.5-7 There is a reported case of improvement with a 3-week regimen of psoralen plus UVA followed by twice-weekly maintenance.8 Promising results in the treatment of DRIF have been shown with oral isotretinoin once daily.3-5 Finally, DRIF may resolve independently6; therefore, treatment of DRIF should be addressed on a case-by-case basis.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis: report of a case. Arch Dermatol. 1968;97:432-435.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis. Arch Dermatol. 1972;105:580-583.
- Calka O, Metin A, Ozen S. A case of disseminated and recurrent infundibulofolliculitis responsive to treatment with systemic isotretinoin. J Dermatol. 2002;29:431-434.
- Aroni K, Grapsa A, Agapitos E. Disseminate and recurrent infundibulofolliculitis: response to isotretinoin. J Drugs Dermatol. 2004;3:434-435.
- Aroni K, Aivaliotis M, Davaris P. Disseminated and recurrent infundibular folliculitis (D.R.I.F.): report of a case successfully treated with isotretinoin. J Dermatol. 1998;25:51-53.
- Owen WR, Wood C. Disseminate and recurrent infundibulofolliculitis. Arch Dermatol. 1979;115:174-175.
- Hinds GA, Heald PW. A case of disseminate and recurrent infundibulofolliculitis responsive to treatment with topical steroids. Dermatol Online J. 2008;14:11.
- Goihman-Yahr M. Disseminate and recurrent infundibulofolliculitis: response to psoralen plus UVA therapy. Int J Dermatol. 1999;38:75-78.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis: report of a case. Arch Dermatol. 1968;97:432-435.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis. Arch Dermatol. 1972;105:580-583.
- Calka O, Metin A, Ozen S. A case of disseminated and recurrent infundibulofolliculitis responsive to treatment with systemic isotretinoin. J Dermatol. 2002;29:431-434.
- Aroni K, Grapsa A, Agapitos E. Disseminate and recurrent infundibulofolliculitis: response to isotretinoin. J Drugs Dermatol. 2004;3:434-435.
- Aroni K, Aivaliotis M, Davaris P. Disseminated and recurrent infundibular folliculitis (D.R.I.F.): report of a case successfully treated with isotretinoin. J Dermatol. 1998;25:51-53.
- Owen WR, Wood C. Disseminate and recurrent infundibulofolliculitis. Arch Dermatol. 1979;115:174-175.
- Hinds GA, Heald PW. A case of disseminate and recurrent infundibulofolliculitis responsive to treatment with topical steroids. Dermatol Online J. 2008;14:11.
- Goihman-Yahr M. Disseminate and recurrent infundibulofolliculitis: response to psoralen plus UVA therapy. Int J Dermatol. 1999;38:75-78.
A 40-year-old black man presented with numerous perifollicular flesh-colored papules on the back, chest, abdomen, and proximal aspect of the arms of 6 years' duration. He described these lesions as persistent, nonpainful, and nonpruritic. He previously was treated with an unknown cream without any benefit. These lesions were cosmetically bothersome.