User login
Switching antipsychotics: A guide to dose equivalents
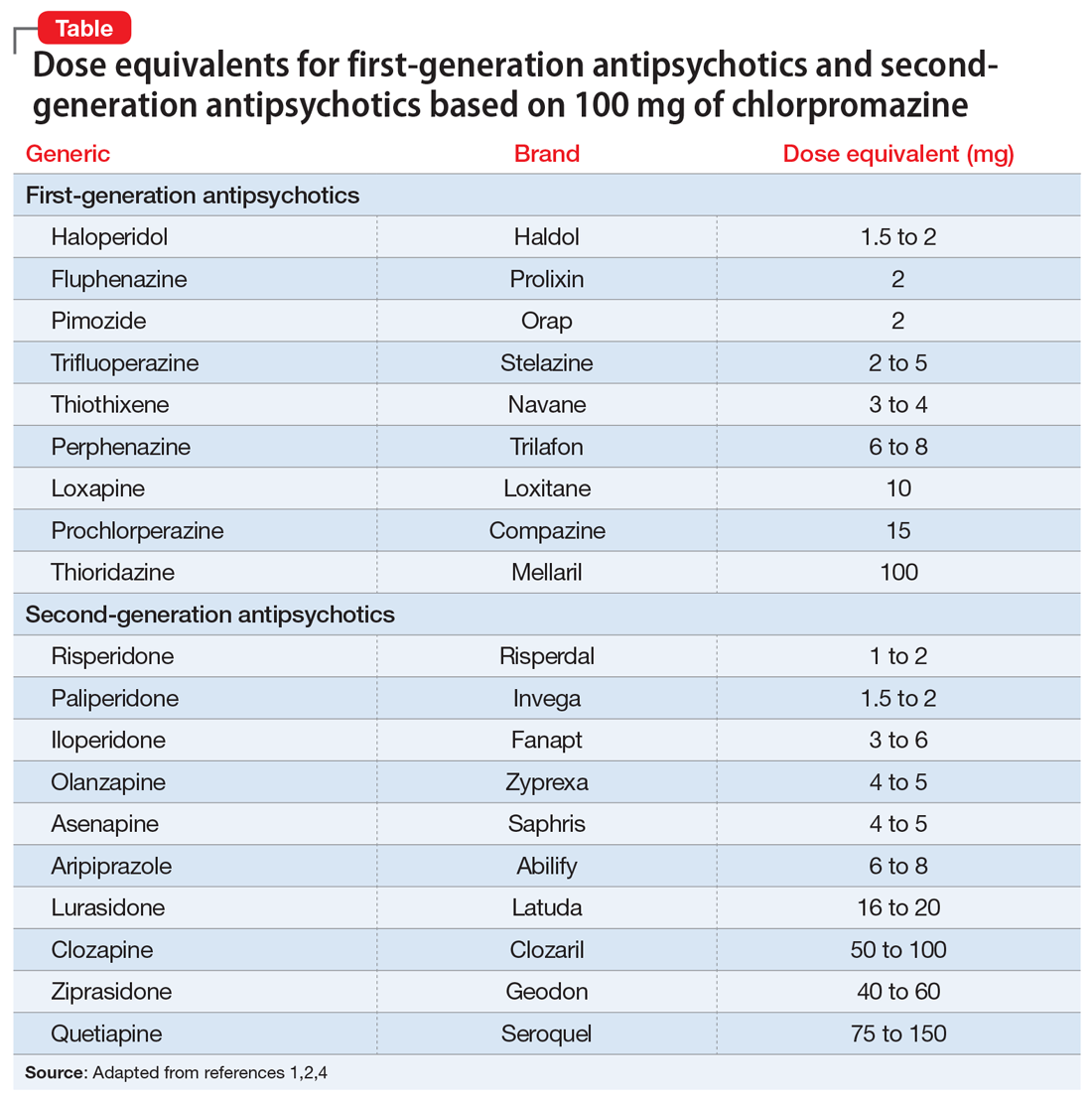
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”
While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”
While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”
While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how
Your patient refuses a suicide risk assessment. Now what?
On occasion, a patient may refuse to cooperate with a suicide risk assessment or is unable to participate due to the severity of a psychiatric or medical condition. In such situations, how can we conduct an assessment that meets our ethical, professional, and legal obligations?
First, skipping a suicide risk assessment is never an option. A patient’s refusal or inability to cooperate does not release us from our duty of care. We are obligated to gather information about suicide risk to anticipate the likelihood and severity of harm.1 Furthermore, collecting information helps us evaluate what types of precautions are necessary to reduce or eliminate suicide risk.
Some clinicians may believe that a suicide risk assessment is only possible when they can ask patients about ideation, intent, plans, and past suicidal behavior. While the patient’s self-report is valuable, it is only one data point, and in some cases, it may not be reliable or credible.2 So how should you handle such situations? Here I describe 3 steps to take to estimate a patient’s suicide risk without their participation.
1. Obtain information from other sources.
These can include:
- your recent contacts with the patient
- the patient’s responses to previous inquiries about suicidality
- collateral reports from staff
- the patient’s chart and past medical records
- past suicide attempts (including the precipitants, the patient’s reasons for the attempt, details of the actions taken and methods used, any medical outcome, and the patient’s reaction to surviving)3
- past nonsuicidal self-injury
- past episodes of suicidal thinking
- treatment progress to date
- mental status.
Documenting your sources of information will indicate that you made reasonable efforts to appreciate the risk despite imperfect circumstances. Furthermore, these sources of data can support your work to assess the severity of the patient’s current suicidality, to clinically formulate why the patient is susceptible to suicidal thoughts and behavior, and to anticipate circumstances that could constitute a high-risk period for your patient to attempt suicide.
2. Document the reasons you were unable to interview the patient. For patients who are competent to refuse services, document the efforts you made to gain the patient’s cooperation. If the patient’s psychiatric condition (eg, florid psychosis) was the main impediment, note this.
3. Explain the limitations of your assessment. This might include acknowledging that your estimation of the patient’s suicide risk is missing important information but is the best possible estimate at the time. Explain how you determined the level of risk with a statement such as, “Because the patient was unable to participate, I estimated risk based on….” If the patient’s lack of participation lowers your confidence in your risk estimate, this also should be documented. Reduced confidence may indicate the need for additional steps to assure the patient’s safety (eg, admission, delaying discharge, initiating continuous observation).
1. Obegi JH. Probable standards of care for suicide risk assessment. J Am Acad Psychiatry Law. 2017;45(4):452-459.
2. Hom MA, Stanley IH, Duffy ME, et al. Investigating the reliability of suicide attempt history reporting across five measures: a study of US military service members at risk of suicide. J Clin Psychol. 2019;75(7):1332-1349.
3. Rudd MD. Core competencies, warning signs, and a framework for suicide risk assessment in clinical practice. In: Nock MK, ed. The Oxford handbook of suicide and self-injury. Oxford University Press; 2014:323-336.
On occasion, a patient may refuse to cooperate with a suicide risk assessment or is unable to participate due to the severity of a psychiatric or medical condition. In such situations, how can we conduct an assessment that meets our ethical, professional, and legal obligations?
First, skipping a suicide risk assessment is never an option. A patient’s refusal or inability to cooperate does not release us from our duty of care. We are obligated to gather information about suicide risk to anticipate the likelihood and severity of harm.1 Furthermore, collecting information helps us evaluate what types of precautions are necessary to reduce or eliminate suicide risk.
Some clinicians may believe that a suicide risk assessment is only possible when they can ask patients about ideation, intent, plans, and past suicidal behavior. While the patient’s self-report is valuable, it is only one data point, and in some cases, it may not be reliable or credible.2 So how should you handle such situations? Here I describe 3 steps to take to estimate a patient’s suicide risk without their participation.
1. Obtain information from other sources.
These can include:
- your recent contacts with the patient
- the patient’s responses to previous inquiries about suicidality
- collateral reports from staff
- the patient’s chart and past medical records
- past suicide attempts (including the precipitants, the patient’s reasons for the attempt, details of the actions taken and methods used, any medical outcome, and the patient’s reaction to surviving)3
- past nonsuicidal self-injury
- past episodes of suicidal thinking
- treatment progress to date
- mental status.
Documenting your sources of information will indicate that you made reasonable efforts to appreciate the risk despite imperfect circumstances. Furthermore, these sources of data can support your work to assess the severity of the patient’s current suicidality, to clinically formulate why the patient is susceptible to suicidal thoughts and behavior, and to anticipate circumstances that could constitute a high-risk period for your patient to attempt suicide.
2. Document the reasons you were unable to interview the patient. For patients who are competent to refuse services, document the efforts you made to gain the patient’s cooperation. If the patient’s psychiatric condition (eg, florid psychosis) was the main impediment, note this.
3. Explain the limitations of your assessment. This might include acknowledging that your estimation of the patient’s suicide risk is missing important information but is the best possible estimate at the time. Explain how you determined the level of risk with a statement such as, “Because the patient was unable to participate, I estimated risk based on….” If the patient’s lack of participation lowers your confidence in your risk estimate, this also should be documented. Reduced confidence may indicate the need for additional steps to assure the patient’s safety (eg, admission, delaying discharge, initiating continuous observation).
On occasion, a patient may refuse to cooperate with a suicide risk assessment or is unable to participate due to the severity of a psychiatric or medical condition. In such situations, how can we conduct an assessment that meets our ethical, professional, and legal obligations?
First, skipping a suicide risk assessment is never an option. A patient’s refusal or inability to cooperate does not release us from our duty of care. We are obligated to gather information about suicide risk to anticipate the likelihood and severity of harm.1 Furthermore, collecting information helps us evaluate what types of precautions are necessary to reduce or eliminate suicide risk.
Some clinicians may believe that a suicide risk assessment is only possible when they can ask patients about ideation, intent, plans, and past suicidal behavior. While the patient’s self-report is valuable, it is only one data point, and in some cases, it may not be reliable or credible.2 So how should you handle such situations? Here I describe 3 steps to take to estimate a patient’s suicide risk without their participation.
1. Obtain information from other sources.
These can include:
- your recent contacts with the patient
- the patient’s responses to previous inquiries about suicidality
- collateral reports from staff
- the patient’s chart and past medical records
- past suicide attempts (including the precipitants, the patient’s reasons for the attempt, details of the actions taken and methods used, any medical outcome, and the patient’s reaction to surviving)3
- past nonsuicidal self-injury
- past episodes of suicidal thinking
- treatment progress to date
- mental status.
Documenting your sources of information will indicate that you made reasonable efforts to appreciate the risk despite imperfect circumstances. Furthermore, these sources of data can support your work to assess the severity of the patient’s current suicidality, to clinically formulate why the patient is susceptible to suicidal thoughts and behavior, and to anticipate circumstances that could constitute a high-risk period for your patient to attempt suicide.
2. Document the reasons you were unable to interview the patient. For patients who are competent to refuse services, document the efforts you made to gain the patient’s cooperation. If the patient’s psychiatric condition (eg, florid psychosis) was the main impediment, note this.
3. Explain the limitations of your assessment. This might include acknowledging that your estimation of the patient’s suicide risk is missing important information but is the best possible estimate at the time. Explain how you determined the level of risk with a statement such as, “Because the patient was unable to participate, I estimated risk based on….” If the patient’s lack of participation lowers your confidence in your risk estimate, this also should be documented. Reduced confidence may indicate the need for additional steps to assure the patient’s safety (eg, admission, delaying discharge, initiating continuous observation).
1. Obegi JH. Probable standards of care for suicide risk assessment. J Am Acad Psychiatry Law. 2017;45(4):452-459.
2. Hom MA, Stanley IH, Duffy ME, et al. Investigating the reliability of suicide attempt history reporting across five measures: a study of US military service members at risk of suicide. J Clin Psychol. 2019;75(7):1332-1349.
3. Rudd MD. Core competencies, warning signs, and a framework for suicide risk assessment in clinical practice. In: Nock MK, ed. The Oxford handbook of suicide and self-injury. Oxford University Press; 2014:323-336.
1. Obegi JH. Probable standards of care for suicide risk assessment. J Am Acad Psychiatry Law. 2017;45(4):452-459.
2. Hom MA, Stanley IH, Duffy ME, et al. Investigating the reliability of suicide attempt history reporting across five measures: a study of US military service members at risk of suicide. J Clin Psychol. 2019;75(7):1332-1349.
3. Rudd MD. Core competencies, warning signs, and a framework for suicide risk assessment in clinical practice. In: Nock MK, ed. The Oxford handbook of suicide and self-injury. Oxford University Press; 2014:323-336.
Procedural Competency Among Hospitalists: A Literature Review and Future Considerations
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).
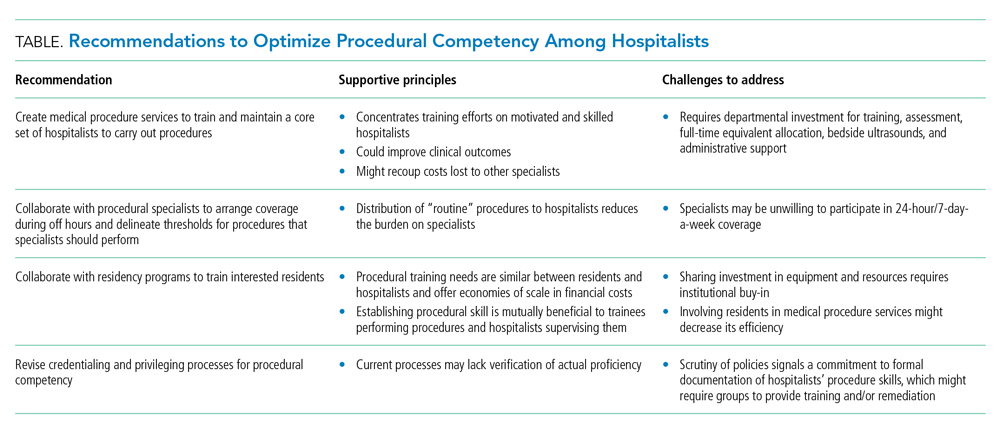
1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).

1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).

1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
© 2021 Society of Hospital Medicine
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
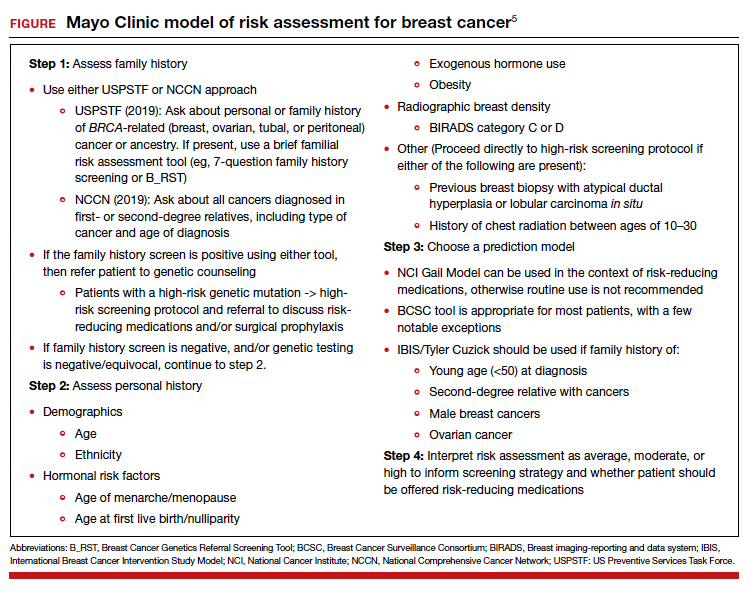
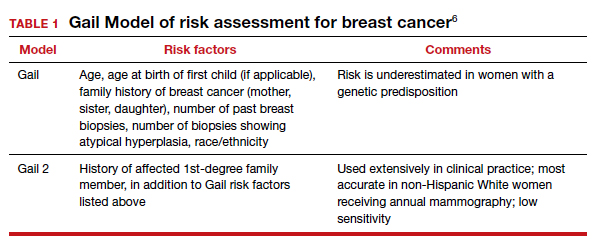
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
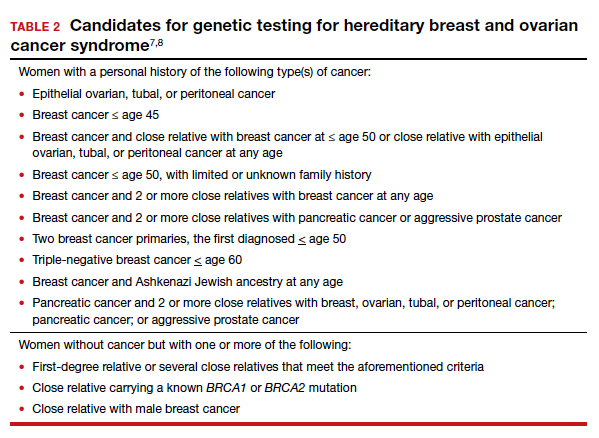
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
The lasting effects of childhood trauma
Childhood trauma, which is also called adverse childhood experiences (ACEs), can have lasting detrimental effects on individuals as they grow and mature into adulthood. ACEs may occur in children age ≤18 years if they experience abuse or neglect, violence, or other traumatic losses. More than 60% of people experience at least 1 ACE, and 1 in 6 individuals reported that they had experienced ≥4 ACEs.1 Subsequent additional ACEs have a cumulative deteriorating impact on the brain. This predisposes individuals to mental health disorders, substance use disorders, and other psychosocial problems. The efficacy of current therapeutic approaches provides only partial symptom resolution. For such individuals, the illness load and health care costs typically remain high across the lifespan.1,2
In this article, we discuss types of ACEs, protective factors and risk factors that influence the development of posttraumatic stress disorder (PTSD) in individuals who experience ACEs, how ACEs can negatively impact mental health in adulthood, and approaches to prevent or treat PTSD and other symptoms.
Types of trauma and correlation with PTSD
ACEs can be indexed as neglect or emotional, physical, or sexual abuse. Physical and sexual abuse strongly correlate with an increased risk of PTSD.3 Although neglect and emotional abuse do not directly predict the development of PTSD, these experiences foretell high rates of lifelong trauma exposure and are indirectly related to late PTSD symptoms.4,5 ACEs can impede an individual’s cognitive, social, and emotional development, diminish quality of life, and lead to an early death.6 The lifetime prevalence of PTSD is 6.1% to 9.2%.7 Compared with men, women are 4 times more likely to develop PTSD following a traumatic event.7
The development of PTSD is influenced by the nature, duration, and degree of trauma, and age at the time of exposure to trauma. Children who survive complex trauma (≥2 types of trauma) have a higher likelihood of developing PTSD.8 Prolonged trauma exposure has a more substantial negative impact than a one-time occurrence. However, it is an erroneous oversimplification to assume that each type of ACE has an equally traumatic effect.6
Factors that protect against PTSD
Factors that can protect against developing PTSD are listed in Table 1.7 Two of these are resilience and hope.
Resilience is defined as an individual’s strength to cope with difficulties in life.9 Resilience has internal psychological characteristics and external factors that aid in protecting against childhood adversities.10,11 The Brief Resilience Scale is a self-assessment that measures innate abilities to cope, including optimism, self-efficacy, patience, faith, and humor.12,13 External factors associated with resilience are family, friends, and community support.11,13
Hope can help in surmounting ACEs. The Adult Hope Scale has been used in many studies to assess this construct in individuals who have survived trauma.13 Some studies have found decreased hope in individuals who sustained early trauma and were diagnosed with PTSD in adulthood.14 A study examining children exposed to domestic violence found that children who showed high hope, endurance, and curiosity were better able to cope with adversities.15
Continue to: PTSD risk factors
PTSD risk factors
Many individual and societal risk factors can influence the likelihood of developing PTSD. Some of these factors are outlined in Table 1.7
Pathophysiology of PTSD
Multiple brain regions, pathways, and neurotransmitters are involved in the development of PTSD. Neuroimaging has identified volume and activity changes of the hippocampus, prefrontal cortex, and amygdala in patients with early trauma and PTSD. Some researchers have suggested a gross reduction in locus coeruleus neuronal volume in war veterans with a likely diagnosis of PTSD compared with controls.16,17 In other studies, chronic stress exposure has been found to cause neuronal cell death and affect neuronal plasticity in the limbic area of the brain.18
Diagnosing PTSD
More than 30% of individuals who experience ACEs develop PTSD.19 The DSM-5 diagnostic criteria for PTSD are outlined in Table 2.20 Several instruments are used to determine the diagnosis and assess the severity of PTSD. These include the Clinician-Administered PTSD Scale for DSM-5,21 which is a 30-item structured interview that can be administered in 45 to 60 minutes; the PTSD Symptom Scale Self-Report Version, which is a 17-item, Likert scale, self-report questionnaire; and the Structured Clinical Interview: PTSD Module, which is a semi-structured interview that can take up to several hours to administer.21
Other disorders. In addition to PTSD, individuals with ACEs are at high risk for other mental health issues throughout their lifetime. Individuals with ACE often experience depressive symptoms (approximately 40%); anxiety (approximately 30%); anger; guilt or shame; negative self-cognition; interpersonal difficulties; rumination; and thoughts of self-harm and suicide.22 Epidemiological studies suggest that patients who experience childhood sexual abuse are more likely to develop mood, anxiety, and substance use disorders in adulthood.23,24
Psychotherapeutic treatments for PTSD
Cognitive-behavioral therapy (CBT) addresses the relationship between an individual’s thoughts, emotions, and behaviors. CBT can be used to treat adults and children with PTSD. Before starting CBT, assess the patient’s current safety to ensure that they have the coping skills to manage distress related to their ACEs, and address any coexisting substance use.25
Continue to: According to the American Psychological Association...
According to the American Psychological Association, several CBT-based psychotherapies are recommended for treating PTSD26:
Trauma-focused–CBT includes psychoeducation, trauma narrative, processing, exposure, and relaxation skills training. It consists of approximately 12 to 16 sessions and incorporates elements of family therapy.
Cognitive processing therapy (CPT) focuses on helping patients develop adaptive cognitive domains about the self, the people around them, and the world. CPT therapists assist in information processing by accessing the traumatic memory and trying to eliminate emotions tied to it.25,27 CPT consists of 12 to 16 structured individual, group, or combined sessions.
Prolonged exposure (PE) targets fear-related emotions and works on the principles of habituation to extinguish trauma and fear response to the trigger. This increases self-reliance and competence and decreases the generalization of anxiety to innocuous triggers. PE typically consists of 9 to 12 sessions. PE alone or in combination with cognitive restructuring is successful in treating patients with PTSD, but cognitive restructuring has limited utility in young children.25,27
Cognitive exposure can be individual or group therapy delivered over 3 months, where negative self-evaluation and traumatic memories are challenged with the goal of interrupting maladaptive behaviors and thoughts.27
Continue to: Stress inoculation training
Stress inoculation training (SIT) provides psychoeducation, skills training, role-playing, deep muscle relaxation, paced breathing, and thought stopping. Emphasis is on coaching skills to alleviate anxiety, fear, and symptoms of depression associated with trauma. In SIT, exposures to traumatic memories are indirect (eg, role play), compared with PE, where the exposures are direct.25
The American Psychological Association conditionally recommended several other forms for psychotherapy for treating patients with PTSD26:
Brief eclectic psychotherapy uses CBT and psychodynamic approaches to target feelings of guilt and shame in 16 sessions.27
Narrative exposure therapy consists of 4 to 10 group sessions in which individuals provide detailed narration of the events; the focus is on self-respect and personal rights.27
Eye movement desensitization and reprocessing (EMDR) is a 6- to 12-session, 8-phase treatment that uses principles of accelerated information processing to target nonverbal expression of trauma and dissociative experiences. Patients with PTSD are suggested to have disrupted rapid eye movements. In EMDR, patients follow rhythmic movements of the therapist’s hands or flashed light. This is designed to decrease stress associated with accessing trauma memories, the emotional/physiologic response from the memories, and negative cognitive distortions about self, and to replace negative cognition distortions with positive thoughts about self.25,27
Continue to: Accelerated resolution therapy
Accelerated resolution therapy is a derivative of EMDR. It helps to reconsolidate the emotional and physical experiences associated with distressing memories by replacing them with positive ones or decreasing physiological arousal and anxiety related to the recall of traumatic memories.28
Pharmacologic treatments
Selective serotonin reuptake inhibitors (SSRIs). Multiple studies using different scales have found that paroxetine, sertraline, and fluoxetine can decrease PTSD symptoms. Approximately 60% of patients treated with SSRIs experience partial remission of symptoms, and 20% to 30% experience complete symptom resolution.29 Davidson et al30 found that 22% of patients with PTSD who received fluoxetine had a relapse of symptoms, compared with 50% of patients who received placebo.
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and other antidepressants. The SNRIs venlafaxine and duloxetine can help reduce hyperarousal symptoms and improve mood, anxiety, and sleep.26 Mirtazapine, an alpha 2A/2C adrenoceptor antagonist/5-HT 2A/2C/3 antagonist, can address PTSD symptoms from both serotonergic pathways and increase norepinephrine release by blocking autoreceptors and enhancing alpha-1 receptor activity. This alleviates hyperarousal symptoms and promotes sleep.29 In addition to having monoaminergic effects, antidepressant medications also regulate the hypothalamic–pituitary–adrenal (HPA) axis response to stress and promote neurogenesis in the hippocampal region.29
Adrenergic agents
Adrenergic receptor antagonists. Prazosin, an alpha-1 adrenoceptor antagonist, decreases hyperarousal symptoms, improves sleep, and decreases nightmares related to PTSD by decreasing noradrenergic hyperactivity.29
Beta-blockers such as propranolol can decrease physiological response to trauma but have mixed results in the prevention or improvement of PTSD symptoms.29,31
Continue to: Glucocorticoid receptor agonists
Glucocorticoid receptor agonists. In a very small study, low-dose cortisol decreased the severity of traumatic memory (consolidation phase).32 Glucocorticoid receptor agonists can also diminish memory retrieval (reconsolidation phase) through intrusive thoughts and flashbacks.29
Anticonvulsants, benzodiazepines, and antipsychotics
These medications have had a limited role in the treatment of PTSD.26,29
Future directions: Preventive treatments
Because PTSD has a profound impact on an individual’s quality of life and the development of other illnesses, there is strong interest in finding treatments that can prevent PTSD. Based on limited evidence primarily from animal studies, some researchers have suggested that certain agents may someday be helpful for PTSD prevention29:
Glucocorticoid antagonists such as corticotropin-releasing factor 1 (CRF1) antagonists or cholecystokinin 2 (CCK2) receptor antagonists might promote resilience to stress by inhibiting the HPA axis and influencing the amygdala by decreasing fear conditioning, as observed in animal models. Similarly, in animal models, CRF1 and CCK2 are predicted to decrease memory consolidation in response to exposure to stress.
Adrenoceptor antagonists and agonists also might have a role in preventive treatment, but the evidence is scarce. Prazosin, an alpha-1 adrenoceptor antagonist, was ineffective in animal models.29,31 Propranolol, a beta-adrenoceptor blocker, has had mixed results but can decrease trauma-induced physiological arousal when administered soon after exposure.29
Continue to: N-methyl-
N-methyl-
Bottom Line
Adverse childhood experiences (ACEs) are strong predictors for the development of posttraumatic stress disorder (PTSD) and other mental health or medical issues in late adolescence and adulthood. Experiencing a higher number of ACEs increases the risk of developing PTSD as an adult. Timely psychotherapeutic and pharmacologic interventions can help limit symptoms and reduce the severity of PTSD.
Related Resources
- Smith P, Dalglesih T, Meiser-Stedman R. Practitioner review: posttraumatic stress disorder and its treatment in children and adolescents. J Child Psychol Psychiatry. 2019;60(5):500-515.
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Duloxetine • Cymbalta
Fluoxetine • Prozac
Mirtazapine • Remeron
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal, Pronol
Sertraline • Zoloft
Venlafaxine • Effexor
1. Centers for Disease Control and Prevention. Preventing adverse childhood experiences. Published April 3, 2020. Accessed January 26, 2021. https://www.cdc.gov/violenceprevention/childabuseandneglect/aces/fastfact.html
2. Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378-385.
3. Norman RE, Byambaa M, De R, et al. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349
4. Spertus IL, Yehuda R, Wong CM, et al. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27(11):1247-1258.
5. Glück TM, Knefel M, Lueger-Schuster B. A network analysis of anger, shame, proposed ICD-11 post-traumatic stress disorder, and different types of childhood trauma in foster care settings in a sample of adult survivors. Eur J Psychotraumatol. 2017;8(suppl 3):1372543. doi: 10.1080/20008198.2017.1372543
6. Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453-1460.
7. Sareen J. Posttraumatic stress disorder in adults: epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. UpToDate. Updated December 3, 2020. Accessed January 26, 2021. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-manifestations-course-assessment-and-diagnosis
8. Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999:156;1223-1229.
9. Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57(3):316-331.
10. Ahern NR, Kiehl EM, Sole ML, et al. A review of instruments measuring resilience. Issues Compr Pediatr Nurs. 2006;29(2):103-125.
11. Zimmerman MA. Resiliency theory: a strengths-based approach to research and practice for adolescent health. Health Educ Behav. 2013;40(4):381-383.
12. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Munoz RT, Hanks H, Hellman CM. Hope and resilience as distinct contributors to psychological flourishing among childhood trauma survivors. Traumatology. 2020;26(2):177-184.
14. Baxter MA, Hemming EJ, McIntosh HC, et al. Exploring the relationship between adverse childhood experiences and hope. J Child Sex Abus. 2017;26(8):948-956.
15. Hellman CM, Gwinn C. Camp HOPE as an intervention for children exposed to domestic violence: a program evaluation of hope, and strength of character. Child Adolesc Soc Work J. 2017;34:269-276.
16. Bracha HS, Garcia-Rill E, Mrak RE, et al. Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J Neuropsychiatry Clin Neurosci. 2005;17(4):503-9.
17. de Lange GM. Understanding the cellular and molecular alterations in PTSD brains: the necessity of post-mortem brain tissue. Eur J Psychotraumatol. 2017;8(1):1341824. doi: 10.1080/20008198.2017.1341824
18. Zunszain PA, Anacker C, Cattaneo A, et al. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722-729.
19. Greeson JKP, Briggs EC, Kisiel CL, et al. Complex trauma and mental health in children and adolescents placed in foster care: findings from the national child traumatic stress network. Child Welfare. 2011;90(6):91-108.
20. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
21. American Psychological Association. PTSD assessment instruments. Updated September 26, 2018. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/assessment/
22. Bellis MA, Hughes K, Ford K, et al. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10):e517-e528. doi: 10.1016/S2468-2667(19)30145-8
23. Mullen PE, Martin JL, Anderson JC, et al. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721-732.
24. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women. An epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
25. Chard KM, Gilman R. Counseling trauma victims: 4 brief therapies meet the test. Current Psychiatry. 2005;4(8):50,55-58,61-62.
26. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. American Psychol. 2019;74(5):596-607.
27. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder. PTSD treatments. Updated June 2020. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/treatments/
28. Kip KE, Elk CA, Sullivan KL, et al. Brief treatment of symptoms of post-traumatic stress disorder (PTSD) by use of accelerated resolution therapy (ART(®)). Behav Sci (Basel). 2012;2(2):115-134.
29. Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62(2):617-627.
30. Davidson JR, Connor KM, Hertzberg MA, et al. Maintenance therapy with fluoxetine in posttraumatic stress disorder: a placebo-controlled discontinuation study. J Clin Psychopharmacol. 2005;25(2):166-169.
31. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
32. Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiat. 2004;161(8):1488-1490.
33. McGhee LL, Maani CV, Garza TH, et al. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64(2 suppl):S195-S198. doi: 10.1097/TA.0b013e318160ba1d
Childhood trauma, which is also called adverse childhood experiences (ACEs), can have lasting detrimental effects on individuals as they grow and mature into adulthood. ACEs may occur in children age ≤18 years if they experience abuse or neglect, violence, or other traumatic losses. More than 60% of people experience at least 1 ACE, and 1 in 6 individuals reported that they had experienced ≥4 ACEs.1 Subsequent additional ACEs have a cumulative deteriorating impact on the brain. This predisposes individuals to mental health disorders, substance use disorders, and other psychosocial problems. The efficacy of current therapeutic approaches provides only partial symptom resolution. For such individuals, the illness load and health care costs typically remain high across the lifespan.1,2
In this article, we discuss types of ACEs, protective factors and risk factors that influence the development of posttraumatic stress disorder (PTSD) in individuals who experience ACEs, how ACEs can negatively impact mental health in adulthood, and approaches to prevent or treat PTSD and other symptoms.
Types of trauma and correlation with PTSD
ACEs can be indexed as neglect or emotional, physical, or sexual abuse. Physical and sexual abuse strongly correlate with an increased risk of PTSD.3 Although neglect and emotional abuse do not directly predict the development of PTSD, these experiences foretell high rates of lifelong trauma exposure and are indirectly related to late PTSD symptoms.4,5 ACEs can impede an individual’s cognitive, social, and emotional development, diminish quality of life, and lead to an early death.6 The lifetime prevalence of PTSD is 6.1% to 9.2%.7 Compared with men, women are 4 times more likely to develop PTSD following a traumatic event.7
The development of PTSD is influenced by the nature, duration, and degree of trauma, and age at the time of exposure to trauma. Children who survive complex trauma (≥2 types of trauma) have a higher likelihood of developing PTSD.8 Prolonged trauma exposure has a more substantial negative impact than a one-time occurrence. However, it is an erroneous oversimplification to assume that each type of ACE has an equally traumatic effect.6
Factors that protect against PTSD
Factors that can protect against developing PTSD are listed in Table 1.7 Two of these are resilience and hope.
Resilience is defined as an individual’s strength to cope with difficulties in life.9 Resilience has internal psychological characteristics and external factors that aid in protecting against childhood adversities.10,11 The Brief Resilience Scale is a self-assessment that measures innate abilities to cope, including optimism, self-efficacy, patience, faith, and humor.12,13 External factors associated with resilience are family, friends, and community support.11,13
Hope can help in surmounting ACEs. The Adult Hope Scale has been used in many studies to assess this construct in individuals who have survived trauma.13 Some studies have found decreased hope in individuals who sustained early trauma and were diagnosed with PTSD in adulthood.14 A study examining children exposed to domestic violence found that children who showed high hope, endurance, and curiosity were better able to cope with adversities.15
Continue to: PTSD risk factors
PTSD risk factors
Many individual and societal risk factors can influence the likelihood of developing PTSD. Some of these factors are outlined in Table 1.7
Pathophysiology of PTSD
Multiple brain regions, pathways, and neurotransmitters are involved in the development of PTSD. Neuroimaging has identified volume and activity changes of the hippocampus, prefrontal cortex, and amygdala in patients with early trauma and PTSD. Some researchers have suggested a gross reduction in locus coeruleus neuronal volume in war veterans with a likely diagnosis of PTSD compared with controls.16,17 In other studies, chronic stress exposure has been found to cause neuronal cell death and affect neuronal plasticity in the limbic area of the brain.18
Diagnosing PTSD
More than 30% of individuals who experience ACEs develop PTSD.19 The DSM-5 diagnostic criteria for PTSD are outlined in Table 2.20 Several instruments are used to determine the diagnosis and assess the severity of PTSD. These include the Clinician-Administered PTSD Scale for DSM-5,21 which is a 30-item structured interview that can be administered in 45 to 60 minutes; the PTSD Symptom Scale Self-Report Version, which is a 17-item, Likert scale, self-report questionnaire; and the Structured Clinical Interview: PTSD Module, which is a semi-structured interview that can take up to several hours to administer.21
Other disorders. In addition to PTSD, individuals with ACEs are at high risk for other mental health issues throughout their lifetime. Individuals with ACE often experience depressive symptoms (approximately 40%); anxiety (approximately 30%); anger; guilt or shame; negative self-cognition; interpersonal difficulties; rumination; and thoughts of self-harm and suicide.22 Epidemiological studies suggest that patients who experience childhood sexual abuse are more likely to develop mood, anxiety, and substance use disorders in adulthood.23,24
Psychotherapeutic treatments for PTSD
Cognitive-behavioral therapy (CBT) addresses the relationship between an individual’s thoughts, emotions, and behaviors. CBT can be used to treat adults and children with PTSD. Before starting CBT, assess the patient’s current safety to ensure that they have the coping skills to manage distress related to their ACEs, and address any coexisting substance use.25
Continue to: According to the American Psychological Association...
According to the American Psychological Association, several CBT-based psychotherapies are recommended for treating PTSD26:
Trauma-focused–CBT includes psychoeducation, trauma narrative, processing, exposure, and relaxation skills training. It consists of approximately 12 to 16 sessions and incorporates elements of family therapy.
Cognitive processing therapy (CPT) focuses on helping patients develop adaptive cognitive domains about the self, the people around them, and the world. CPT therapists assist in information processing by accessing the traumatic memory and trying to eliminate emotions tied to it.25,27 CPT consists of 12 to 16 structured individual, group, or combined sessions.
Prolonged exposure (PE) targets fear-related emotions and works on the principles of habituation to extinguish trauma and fear response to the trigger. This increases self-reliance and competence and decreases the generalization of anxiety to innocuous triggers. PE typically consists of 9 to 12 sessions. PE alone or in combination with cognitive restructuring is successful in treating patients with PTSD, but cognitive restructuring has limited utility in young children.25,27
Cognitive exposure can be individual or group therapy delivered over 3 months, where negative self-evaluation and traumatic memories are challenged with the goal of interrupting maladaptive behaviors and thoughts.27
Continue to: Stress inoculation training
Stress inoculation training (SIT) provides psychoeducation, skills training, role-playing, deep muscle relaxation, paced breathing, and thought stopping. Emphasis is on coaching skills to alleviate anxiety, fear, and symptoms of depression associated with trauma. In SIT, exposures to traumatic memories are indirect (eg, role play), compared with PE, where the exposures are direct.25
The American Psychological Association conditionally recommended several other forms for psychotherapy for treating patients with PTSD26:
Brief eclectic psychotherapy uses CBT and psychodynamic approaches to target feelings of guilt and shame in 16 sessions.27
Narrative exposure therapy consists of 4 to 10 group sessions in which individuals provide detailed narration of the events; the focus is on self-respect and personal rights.27
Eye movement desensitization and reprocessing (EMDR) is a 6- to 12-session, 8-phase treatment that uses principles of accelerated information processing to target nonverbal expression of trauma and dissociative experiences. Patients with PTSD are suggested to have disrupted rapid eye movements. In EMDR, patients follow rhythmic movements of the therapist’s hands or flashed light. This is designed to decrease stress associated with accessing trauma memories, the emotional/physiologic response from the memories, and negative cognitive distortions about self, and to replace negative cognition distortions with positive thoughts about self.25,27
Continue to: Accelerated resolution therapy
Accelerated resolution therapy is a derivative of EMDR. It helps to reconsolidate the emotional and physical experiences associated with distressing memories by replacing them with positive ones or decreasing physiological arousal and anxiety related to the recall of traumatic memories.28
Pharmacologic treatments
Selective serotonin reuptake inhibitors (SSRIs). Multiple studies using different scales have found that paroxetine, sertraline, and fluoxetine can decrease PTSD symptoms. Approximately 60% of patients treated with SSRIs experience partial remission of symptoms, and 20% to 30% experience complete symptom resolution.29 Davidson et al30 found that 22% of patients with PTSD who received fluoxetine had a relapse of symptoms, compared with 50% of patients who received placebo.
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and other antidepressants. The SNRIs venlafaxine and duloxetine can help reduce hyperarousal symptoms and improve mood, anxiety, and sleep.26 Mirtazapine, an alpha 2A/2C adrenoceptor antagonist/5-HT 2A/2C/3 antagonist, can address PTSD symptoms from both serotonergic pathways and increase norepinephrine release by blocking autoreceptors and enhancing alpha-1 receptor activity. This alleviates hyperarousal symptoms and promotes sleep.29 In addition to having monoaminergic effects, antidepressant medications also regulate the hypothalamic–pituitary–adrenal (HPA) axis response to stress and promote neurogenesis in the hippocampal region.29
Adrenergic agents
Adrenergic receptor antagonists. Prazosin, an alpha-1 adrenoceptor antagonist, decreases hyperarousal symptoms, improves sleep, and decreases nightmares related to PTSD by decreasing noradrenergic hyperactivity.29
Beta-blockers such as propranolol can decrease physiological response to trauma but have mixed results in the prevention or improvement of PTSD symptoms.29,31
Continue to: Glucocorticoid receptor agonists
Glucocorticoid receptor agonists. In a very small study, low-dose cortisol decreased the severity of traumatic memory (consolidation phase).32 Glucocorticoid receptor agonists can also diminish memory retrieval (reconsolidation phase) through intrusive thoughts and flashbacks.29
Anticonvulsants, benzodiazepines, and antipsychotics
These medications have had a limited role in the treatment of PTSD.26,29
Future directions: Preventive treatments
Because PTSD has a profound impact on an individual’s quality of life and the development of other illnesses, there is strong interest in finding treatments that can prevent PTSD. Based on limited evidence primarily from animal studies, some researchers have suggested that certain agents may someday be helpful for PTSD prevention29:
Glucocorticoid antagonists such as corticotropin-releasing factor 1 (CRF1) antagonists or cholecystokinin 2 (CCK2) receptor antagonists might promote resilience to stress by inhibiting the HPA axis and influencing the amygdala by decreasing fear conditioning, as observed in animal models. Similarly, in animal models, CRF1 and CCK2 are predicted to decrease memory consolidation in response to exposure to stress.
Adrenoceptor antagonists and agonists also might have a role in preventive treatment, but the evidence is scarce. Prazosin, an alpha-1 adrenoceptor antagonist, was ineffective in animal models.29,31 Propranolol, a beta-adrenoceptor blocker, has had mixed results but can decrease trauma-induced physiological arousal when administered soon after exposure.29
Continue to: N-methyl-
N-methyl-
Bottom Line
Adverse childhood experiences (ACEs) are strong predictors for the development of posttraumatic stress disorder (PTSD) and other mental health or medical issues in late adolescence and adulthood. Experiencing a higher number of ACEs increases the risk of developing PTSD as an adult. Timely psychotherapeutic and pharmacologic interventions can help limit symptoms and reduce the severity of PTSD.
Related Resources
- Smith P, Dalglesih T, Meiser-Stedman R. Practitioner review: posttraumatic stress disorder and its treatment in children and adolescents. J Child Psychol Psychiatry. 2019;60(5):500-515.
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Duloxetine • Cymbalta
Fluoxetine • Prozac
Mirtazapine • Remeron
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal, Pronol
Sertraline • Zoloft
Venlafaxine • Effexor
Childhood trauma, which is also called adverse childhood experiences (ACEs), can have lasting detrimental effects on individuals as they grow and mature into adulthood. ACEs may occur in children age ≤18 years if they experience abuse or neglect, violence, or other traumatic losses. More than 60% of people experience at least 1 ACE, and 1 in 6 individuals reported that they had experienced ≥4 ACEs.1 Subsequent additional ACEs have a cumulative deteriorating impact on the brain. This predisposes individuals to mental health disorders, substance use disorders, and other psychosocial problems. The efficacy of current therapeutic approaches provides only partial symptom resolution. For such individuals, the illness load and health care costs typically remain high across the lifespan.1,2
In this article, we discuss types of ACEs, protective factors and risk factors that influence the development of posttraumatic stress disorder (PTSD) in individuals who experience ACEs, how ACEs can negatively impact mental health in adulthood, and approaches to prevent or treat PTSD and other symptoms.
Types of trauma and correlation with PTSD
ACEs can be indexed as neglect or emotional, physical, or sexual abuse. Physical and sexual abuse strongly correlate with an increased risk of PTSD.3 Although neglect and emotional abuse do not directly predict the development of PTSD, these experiences foretell high rates of lifelong trauma exposure and are indirectly related to late PTSD symptoms.4,5 ACEs can impede an individual’s cognitive, social, and emotional development, diminish quality of life, and lead to an early death.6 The lifetime prevalence of PTSD is 6.1% to 9.2%.7 Compared with men, women are 4 times more likely to develop PTSD following a traumatic event.7
The development of PTSD is influenced by the nature, duration, and degree of trauma, and age at the time of exposure to trauma. Children who survive complex trauma (≥2 types of trauma) have a higher likelihood of developing PTSD.8 Prolonged trauma exposure has a more substantial negative impact than a one-time occurrence. However, it is an erroneous oversimplification to assume that each type of ACE has an equally traumatic effect.6
Factors that protect against PTSD
Factors that can protect against developing PTSD are listed in Table 1.7 Two of these are resilience and hope.
Resilience is defined as an individual’s strength to cope with difficulties in life.9 Resilience has internal psychological characteristics and external factors that aid in protecting against childhood adversities.10,11 The Brief Resilience Scale is a self-assessment that measures innate abilities to cope, including optimism, self-efficacy, patience, faith, and humor.12,13 External factors associated with resilience are family, friends, and community support.11,13
Hope can help in surmounting ACEs. The Adult Hope Scale has been used in many studies to assess this construct in individuals who have survived trauma.13 Some studies have found decreased hope in individuals who sustained early trauma and were diagnosed with PTSD in adulthood.14 A study examining children exposed to domestic violence found that children who showed high hope, endurance, and curiosity were better able to cope with adversities.15
Continue to: PTSD risk factors
PTSD risk factors
Many individual and societal risk factors can influence the likelihood of developing PTSD. Some of these factors are outlined in Table 1.7
Pathophysiology of PTSD
Multiple brain regions, pathways, and neurotransmitters are involved in the development of PTSD. Neuroimaging has identified volume and activity changes of the hippocampus, prefrontal cortex, and amygdala in patients with early trauma and PTSD. Some researchers have suggested a gross reduction in locus coeruleus neuronal volume in war veterans with a likely diagnosis of PTSD compared with controls.16,17 In other studies, chronic stress exposure has been found to cause neuronal cell death and affect neuronal plasticity in the limbic area of the brain.18
Diagnosing PTSD
More than 30% of individuals who experience ACEs develop PTSD.19 The DSM-5 diagnostic criteria for PTSD are outlined in Table 2.20 Several instruments are used to determine the diagnosis and assess the severity of PTSD. These include the Clinician-Administered PTSD Scale for DSM-5,21 which is a 30-item structured interview that can be administered in 45 to 60 minutes; the PTSD Symptom Scale Self-Report Version, which is a 17-item, Likert scale, self-report questionnaire; and the Structured Clinical Interview: PTSD Module, which is a semi-structured interview that can take up to several hours to administer.21
Other disorders. In addition to PTSD, individuals with ACEs are at high risk for other mental health issues throughout their lifetime. Individuals with ACE often experience depressive symptoms (approximately 40%); anxiety (approximately 30%); anger; guilt or shame; negative self-cognition; interpersonal difficulties; rumination; and thoughts of self-harm and suicide.22 Epidemiological studies suggest that patients who experience childhood sexual abuse are more likely to develop mood, anxiety, and substance use disorders in adulthood.23,24
Psychotherapeutic treatments for PTSD
Cognitive-behavioral therapy (CBT) addresses the relationship between an individual’s thoughts, emotions, and behaviors. CBT can be used to treat adults and children with PTSD. Before starting CBT, assess the patient’s current safety to ensure that they have the coping skills to manage distress related to their ACEs, and address any coexisting substance use.25
Continue to: According to the American Psychological Association...
According to the American Psychological Association, several CBT-based psychotherapies are recommended for treating PTSD26:
Trauma-focused–CBT includes psychoeducation, trauma narrative, processing, exposure, and relaxation skills training. It consists of approximately 12 to 16 sessions and incorporates elements of family therapy.
Cognitive processing therapy (CPT) focuses on helping patients develop adaptive cognitive domains about the self, the people around them, and the world. CPT therapists assist in information processing by accessing the traumatic memory and trying to eliminate emotions tied to it.25,27 CPT consists of 12 to 16 structured individual, group, or combined sessions.
Prolonged exposure (PE) targets fear-related emotions and works on the principles of habituation to extinguish trauma and fear response to the trigger. This increases self-reliance and competence and decreases the generalization of anxiety to innocuous triggers. PE typically consists of 9 to 12 sessions. PE alone or in combination with cognitive restructuring is successful in treating patients with PTSD, but cognitive restructuring has limited utility in young children.25,27
Cognitive exposure can be individual or group therapy delivered over 3 months, where negative self-evaluation and traumatic memories are challenged with the goal of interrupting maladaptive behaviors and thoughts.27
Continue to: Stress inoculation training
Stress inoculation training (SIT) provides psychoeducation, skills training, role-playing, deep muscle relaxation, paced breathing, and thought stopping. Emphasis is on coaching skills to alleviate anxiety, fear, and symptoms of depression associated with trauma. In SIT, exposures to traumatic memories are indirect (eg, role play), compared with PE, where the exposures are direct.25
The American Psychological Association conditionally recommended several other forms for psychotherapy for treating patients with PTSD26:
Brief eclectic psychotherapy uses CBT and psychodynamic approaches to target feelings of guilt and shame in 16 sessions.27
Narrative exposure therapy consists of 4 to 10 group sessions in which individuals provide detailed narration of the events; the focus is on self-respect and personal rights.27
Eye movement desensitization and reprocessing (EMDR) is a 6- to 12-session, 8-phase treatment that uses principles of accelerated information processing to target nonverbal expression of trauma and dissociative experiences. Patients with PTSD are suggested to have disrupted rapid eye movements. In EMDR, patients follow rhythmic movements of the therapist’s hands or flashed light. This is designed to decrease stress associated with accessing trauma memories, the emotional/physiologic response from the memories, and negative cognitive distortions about self, and to replace negative cognition distortions with positive thoughts about self.25,27
Continue to: Accelerated resolution therapy
Accelerated resolution therapy is a derivative of EMDR. It helps to reconsolidate the emotional and physical experiences associated with distressing memories by replacing them with positive ones or decreasing physiological arousal and anxiety related to the recall of traumatic memories.28
Pharmacologic treatments
Selective serotonin reuptake inhibitors (SSRIs). Multiple studies using different scales have found that paroxetine, sertraline, and fluoxetine can decrease PTSD symptoms. Approximately 60% of patients treated with SSRIs experience partial remission of symptoms, and 20% to 30% experience complete symptom resolution.29 Davidson et al30 found that 22% of patients with PTSD who received fluoxetine had a relapse of symptoms, compared with 50% of patients who received placebo.
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and other antidepressants. The SNRIs venlafaxine and duloxetine can help reduce hyperarousal symptoms and improve mood, anxiety, and sleep.26 Mirtazapine, an alpha 2A/2C adrenoceptor antagonist/5-HT 2A/2C/3 antagonist, can address PTSD symptoms from both serotonergic pathways and increase norepinephrine release by blocking autoreceptors and enhancing alpha-1 receptor activity. This alleviates hyperarousal symptoms and promotes sleep.29 In addition to having monoaminergic effects, antidepressant medications also regulate the hypothalamic–pituitary–adrenal (HPA) axis response to stress and promote neurogenesis in the hippocampal region.29
Adrenergic agents
Adrenergic receptor antagonists. Prazosin, an alpha-1 adrenoceptor antagonist, decreases hyperarousal symptoms, improves sleep, and decreases nightmares related to PTSD by decreasing noradrenergic hyperactivity.29
Beta-blockers such as propranolol can decrease physiological response to trauma but have mixed results in the prevention or improvement of PTSD symptoms.29,31
Continue to: Glucocorticoid receptor agonists
Glucocorticoid receptor agonists. In a very small study, low-dose cortisol decreased the severity of traumatic memory (consolidation phase).32 Glucocorticoid receptor agonists can also diminish memory retrieval (reconsolidation phase) through intrusive thoughts and flashbacks.29
Anticonvulsants, benzodiazepines, and antipsychotics
These medications have had a limited role in the treatment of PTSD.26,29
Future directions: Preventive treatments
Because PTSD has a profound impact on an individual’s quality of life and the development of other illnesses, there is strong interest in finding treatments that can prevent PTSD. Based on limited evidence primarily from animal studies, some researchers have suggested that certain agents may someday be helpful for PTSD prevention29:
Glucocorticoid antagonists such as corticotropin-releasing factor 1 (CRF1) antagonists or cholecystokinin 2 (CCK2) receptor antagonists might promote resilience to stress by inhibiting the HPA axis and influencing the amygdala by decreasing fear conditioning, as observed in animal models. Similarly, in animal models, CRF1 and CCK2 are predicted to decrease memory consolidation in response to exposure to stress.
Adrenoceptor antagonists and agonists also might have a role in preventive treatment, but the evidence is scarce. Prazosin, an alpha-1 adrenoceptor antagonist, was ineffective in animal models.29,31 Propranolol, a beta-adrenoceptor blocker, has had mixed results but can decrease trauma-induced physiological arousal when administered soon after exposure.29
Continue to: N-methyl-
N-methyl-
Bottom Line
Adverse childhood experiences (ACEs) are strong predictors for the development of posttraumatic stress disorder (PTSD) and other mental health or medical issues in late adolescence and adulthood. Experiencing a higher number of ACEs increases the risk of developing PTSD as an adult. Timely psychotherapeutic and pharmacologic interventions can help limit symptoms and reduce the severity of PTSD.
Related Resources
- Smith P, Dalglesih T, Meiser-Stedman R. Practitioner review: posttraumatic stress disorder and its treatment in children and adolescents. J Child Psychol Psychiatry. 2019;60(5):500-515.
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Duloxetine • Cymbalta
Fluoxetine • Prozac
Mirtazapine • Remeron
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal, Pronol
Sertraline • Zoloft
Venlafaxine • Effexor
1. Centers for Disease Control and Prevention. Preventing adverse childhood experiences. Published April 3, 2020. Accessed January 26, 2021. https://www.cdc.gov/violenceprevention/childabuseandneglect/aces/fastfact.html
2. Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378-385.
3. Norman RE, Byambaa M, De R, et al. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349
4. Spertus IL, Yehuda R, Wong CM, et al. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27(11):1247-1258.
5. Glück TM, Knefel M, Lueger-Schuster B. A network analysis of anger, shame, proposed ICD-11 post-traumatic stress disorder, and different types of childhood trauma in foster care settings in a sample of adult survivors. Eur J Psychotraumatol. 2017;8(suppl 3):1372543. doi: 10.1080/20008198.2017.1372543
6. Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453-1460.
7. Sareen J. Posttraumatic stress disorder in adults: epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. UpToDate. Updated December 3, 2020. Accessed January 26, 2021. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-manifestations-course-assessment-and-diagnosis
8. Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999:156;1223-1229.
9. Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57(3):316-331.
10. Ahern NR, Kiehl EM, Sole ML, et al. A review of instruments measuring resilience. Issues Compr Pediatr Nurs. 2006;29(2):103-125.
11. Zimmerman MA. Resiliency theory: a strengths-based approach to research and practice for adolescent health. Health Educ Behav. 2013;40(4):381-383.
12. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Munoz RT, Hanks H, Hellman CM. Hope and resilience as distinct contributors to psychological flourishing among childhood trauma survivors. Traumatology. 2020;26(2):177-184.
14. Baxter MA, Hemming EJ, McIntosh HC, et al. Exploring the relationship between adverse childhood experiences and hope. J Child Sex Abus. 2017;26(8):948-956.
15. Hellman CM, Gwinn C. Camp HOPE as an intervention for children exposed to domestic violence: a program evaluation of hope, and strength of character. Child Adolesc Soc Work J. 2017;34:269-276.
16. Bracha HS, Garcia-Rill E, Mrak RE, et al. Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J Neuropsychiatry Clin Neurosci. 2005;17(4):503-9.
17. de Lange GM. Understanding the cellular and molecular alterations in PTSD brains: the necessity of post-mortem brain tissue. Eur J Psychotraumatol. 2017;8(1):1341824. doi: 10.1080/20008198.2017.1341824
18. Zunszain PA, Anacker C, Cattaneo A, et al. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722-729.
19. Greeson JKP, Briggs EC, Kisiel CL, et al. Complex trauma and mental health in children and adolescents placed in foster care: findings from the national child traumatic stress network. Child Welfare. 2011;90(6):91-108.
20. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
21. American Psychological Association. PTSD assessment instruments. Updated September 26, 2018. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/assessment/
22. Bellis MA, Hughes K, Ford K, et al. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10):e517-e528. doi: 10.1016/S2468-2667(19)30145-8
23. Mullen PE, Martin JL, Anderson JC, et al. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721-732.
24. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women. An epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
25. Chard KM, Gilman R. Counseling trauma victims: 4 brief therapies meet the test. Current Psychiatry. 2005;4(8):50,55-58,61-62.
26. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. American Psychol. 2019;74(5):596-607.
27. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder. PTSD treatments. Updated June 2020. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/treatments/
28. Kip KE, Elk CA, Sullivan KL, et al. Brief treatment of symptoms of post-traumatic stress disorder (PTSD) by use of accelerated resolution therapy (ART(®)). Behav Sci (Basel). 2012;2(2):115-134.
29. Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62(2):617-627.
30. Davidson JR, Connor KM, Hertzberg MA, et al. Maintenance therapy with fluoxetine in posttraumatic stress disorder: a placebo-controlled discontinuation study. J Clin Psychopharmacol. 2005;25(2):166-169.
31. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
32. Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiat. 2004;161(8):1488-1490.
33. McGhee LL, Maani CV, Garza TH, et al. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64(2 suppl):S195-S198. doi: 10.1097/TA.0b013e318160ba1d
1. Centers for Disease Control and Prevention. Preventing adverse childhood experiences. Published April 3, 2020. Accessed January 26, 2021. https://www.cdc.gov/violenceprevention/childabuseandneglect/aces/fastfact.html
2. Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378-385.
3. Norman RE, Byambaa M, De R, et al. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349
4. Spertus IL, Yehuda R, Wong CM, et al. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27(11):1247-1258.
5. Glück TM, Knefel M, Lueger-Schuster B. A network analysis of anger, shame, proposed ICD-11 post-traumatic stress disorder, and different types of childhood trauma in foster care settings in a sample of adult survivors. Eur J Psychotraumatol. 2017;8(suppl 3):1372543. doi: 10.1080/20008198.2017.1372543
6. Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453-1460.
7. Sareen J. Posttraumatic stress disorder in adults: epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. UpToDate. Updated December 3, 2020. Accessed January 26, 2021. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-manifestations-course-assessment-and-diagnosis
8. Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999:156;1223-1229.
9. Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57(3):316-331.
10. Ahern NR, Kiehl EM, Sole ML, et al. A review of instruments measuring resilience. Issues Compr Pediatr Nurs. 2006;29(2):103-125.
11. Zimmerman MA. Resiliency theory: a strengths-based approach to research and practice for adolescent health. Health Educ Behav. 2013;40(4):381-383.
12. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Munoz RT, Hanks H, Hellman CM. Hope and resilience as distinct contributors to psychological flourishing among childhood trauma survivors. Traumatology. 2020;26(2):177-184.
14. Baxter MA, Hemming EJ, McIntosh HC, et al. Exploring the relationship between adverse childhood experiences and hope. J Child Sex Abus. 2017;26(8):948-956.
15. Hellman CM, Gwinn C. Camp HOPE as an intervention for children exposed to domestic violence: a program evaluation of hope, and strength of character. Child Adolesc Soc Work J. 2017;34:269-276.
16. Bracha HS, Garcia-Rill E, Mrak RE, et al. Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J Neuropsychiatry Clin Neurosci. 2005;17(4):503-9.
17. de Lange GM. Understanding the cellular and molecular alterations in PTSD brains: the necessity of post-mortem brain tissue. Eur J Psychotraumatol. 2017;8(1):1341824. doi: 10.1080/20008198.2017.1341824
18. Zunszain PA, Anacker C, Cattaneo A, et al. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722-729.
19. Greeson JKP, Briggs EC, Kisiel CL, et al. Complex trauma and mental health in children and adolescents placed in foster care: findings from the national child traumatic stress network. Child Welfare. 2011;90(6):91-108.
20. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
21. American Psychological Association. PTSD assessment instruments. Updated September 26, 2018. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/assessment/
22. Bellis MA, Hughes K, Ford K, et al. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10):e517-e528. doi: 10.1016/S2468-2667(19)30145-8
23. Mullen PE, Martin JL, Anderson JC, et al. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721-732.
24. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women. An epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
25. Chard KM, Gilman R. Counseling trauma victims: 4 brief therapies meet the test. Current Psychiatry. 2005;4(8):50,55-58,61-62.
26. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. American Psychol. 2019;74(5):596-607.
27. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder. PTSD treatments. Updated June 2020. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/treatments/
28. Kip KE, Elk CA, Sullivan KL, et al. Brief treatment of symptoms of post-traumatic stress disorder (PTSD) by use of accelerated resolution therapy (ART(®)). Behav Sci (Basel). 2012;2(2):115-134.
29. Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62(2):617-627.
30. Davidson JR, Connor KM, Hertzberg MA, et al. Maintenance therapy with fluoxetine in posttraumatic stress disorder: a placebo-controlled discontinuation study. J Clin Psychopharmacol. 2005;25(2):166-169.
31. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
32. Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiat. 2004;161(8):1488-1490.
33. McGhee LL, Maani CV, Garza TH, et al. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64(2 suppl):S195-S198. doi: 10.1097/TA.0b013e318160ba1d
Sleep disorders in older adults
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24
Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24
Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24
Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
Metadata, malpractice claims, and making changes to the EHR
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
The ABCs of successful vaccinations: A role for psychiatry
While the implementation of mass vaccinations is a public health task, individual clinicians are critical for the success of any vaccination campaign. Psychiatrists may be well positioned to help increase vaccine uptake among psychiatric patients. They see their patients more frequently than primary care physicians do, which allows for patient engagement over time regarding vaccinations. Also, as physicians, psychiatrists are a trusted source of medical information, and they are well-versed in using the tools of nudging and motivational interviewing to manage ambivalence about receiving a vaccine (vaccine hesitancy).1
The “ABCs of successful vaccinations” (Figure) provide a framework that psychiatrists can use when speaking with their patients about vaccinations. The ABCs assess psychological factors that hinder acceptance of vaccination (A = Attitudes toward vaccination), practical challenges in vaccine access for patients who are willing to get vaccinated (B = Barriers to vaccination), and the actual outcome of “shot in the arm” (C = Completed vaccination series). The Figure provides examples of each area of focus.
How to talk to patients about vaccines
“Attitudes toward vaccination” is an area in which psychiatrists can potentially move patients from hesitancy to vaccine confidence and acceptance. First, express confidence in the vaccine (ie, make a clear statement: “You are an excellent candidate for this vaccine.”). Then, begin a discussion using presumptive language: “You must be ready to receive the vaccine.” In individuals who hesitate, elicit their concern: “What would make vaccination more acceptable?” In those who agree in principle about the benefits of vaccinations, ask about any impediments: “What would get in the way of getting vaccinated?” While some patients may require more information about the vaccine, others may need more time or mostly concrete help, such as assistance with scheduling a vaccine appointment. Do not to forget to follow up to see if a planned and complete vaccination series has taken place. The CDC offers an excellent online toolkit to help clinicians discuss vaccinations with their patients.2
Psychiatric patients, particularly those from disadvantaged and marginalized populations, have much to gain if psychiatrists are involved in preventive health care, including the coronavirus vaccination drive or the annual flu vaccination campaign.
1. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
2. Centers for Disease Control and Prevention. COVID-19 vaccination toolkits. Accessed February 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/toolkits.html
While the implementation of mass vaccinations is a public health task, individual clinicians are critical for the success of any vaccination campaign. Psychiatrists may be well positioned to help increase vaccine uptake among psychiatric patients. They see their patients more frequently than primary care physicians do, which allows for patient engagement over time regarding vaccinations. Also, as physicians, psychiatrists are a trusted source of medical information, and they are well-versed in using the tools of nudging and motivational interviewing to manage ambivalence about receiving a vaccine (vaccine hesitancy).1
The “ABCs of successful vaccinations” (Figure) provide a framework that psychiatrists can use when speaking with their patients about vaccinations. The ABCs assess psychological factors that hinder acceptance of vaccination (A = Attitudes toward vaccination), practical challenges in vaccine access for patients who are willing to get vaccinated (B = Barriers to vaccination), and the actual outcome of “shot in the arm” (C = Completed vaccination series). The Figure provides examples of each area of focus.
How to talk to patients about vaccines
“Attitudes toward vaccination” is an area in which psychiatrists can potentially move patients from hesitancy to vaccine confidence and acceptance. First, express confidence in the vaccine (ie, make a clear statement: “You are an excellent candidate for this vaccine.”). Then, begin a discussion using presumptive language: “You must be ready to receive the vaccine.” In individuals who hesitate, elicit their concern: “What would make vaccination more acceptable?” In those who agree in principle about the benefits of vaccinations, ask about any impediments: “What would get in the way of getting vaccinated?” While some patients may require more information about the vaccine, others may need more time or mostly concrete help, such as assistance with scheduling a vaccine appointment. Do not to forget to follow up to see if a planned and complete vaccination series has taken place. The CDC offers an excellent online toolkit to help clinicians discuss vaccinations with their patients.2
Psychiatric patients, particularly those from disadvantaged and marginalized populations, have much to gain if psychiatrists are involved in preventive health care, including the coronavirus vaccination drive or the annual flu vaccination campaign.
While the implementation of mass vaccinations is a public health task, individual clinicians are critical for the success of any vaccination campaign. Psychiatrists may be well positioned to help increase vaccine uptake among psychiatric patients. They see their patients more frequently than primary care physicians do, which allows for patient engagement over time regarding vaccinations. Also, as physicians, psychiatrists are a trusted source of medical information, and they are well-versed in using the tools of nudging and motivational interviewing to manage ambivalence about receiving a vaccine (vaccine hesitancy).1
The “ABCs of successful vaccinations” (Figure) provide a framework that psychiatrists can use when speaking with their patients about vaccinations. The ABCs assess psychological factors that hinder acceptance of vaccination (A = Attitudes toward vaccination), practical challenges in vaccine access for patients who are willing to get vaccinated (B = Barriers to vaccination), and the actual outcome of “shot in the arm” (C = Completed vaccination series). The Figure provides examples of each area of focus.
How to talk to patients about vaccines
“Attitudes toward vaccination” is an area in which psychiatrists can potentially move patients from hesitancy to vaccine confidence and acceptance. First, express confidence in the vaccine (ie, make a clear statement: “You are an excellent candidate for this vaccine.”). Then, begin a discussion using presumptive language: “You must be ready to receive the vaccine.” In individuals who hesitate, elicit their concern: “What would make vaccination more acceptable?” In those who agree in principle about the benefits of vaccinations, ask about any impediments: “What would get in the way of getting vaccinated?” While some patients may require more information about the vaccine, others may need more time or mostly concrete help, such as assistance with scheduling a vaccine appointment. Do not to forget to follow up to see if a planned and complete vaccination series has taken place. The CDC offers an excellent online toolkit to help clinicians discuss vaccinations with their patients.2
Psychiatric patients, particularly those from disadvantaged and marginalized populations, have much to gain if psychiatrists are involved in preventive health care, including the coronavirus vaccination drive or the annual flu vaccination campaign.
1. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
2. Centers for Disease Control and Prevention. COVID-19 vaccination toolkits. Accessed February 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/toolkits.html
1. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
2. Centers for Disease Control and Prevention. COVID-19 vaccination toolkits. Accessed February 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/toolkits.html
A case of BV during pregnancy: Best management approach
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.
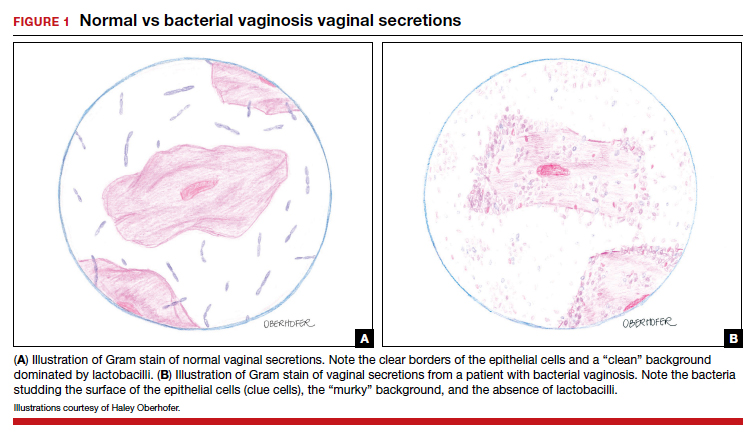
BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.
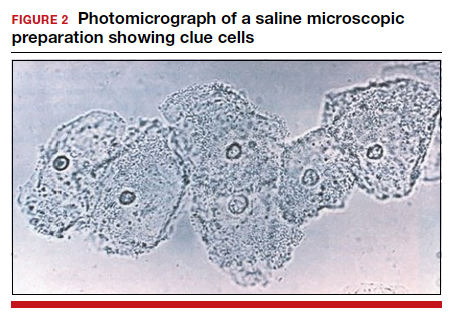
For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.
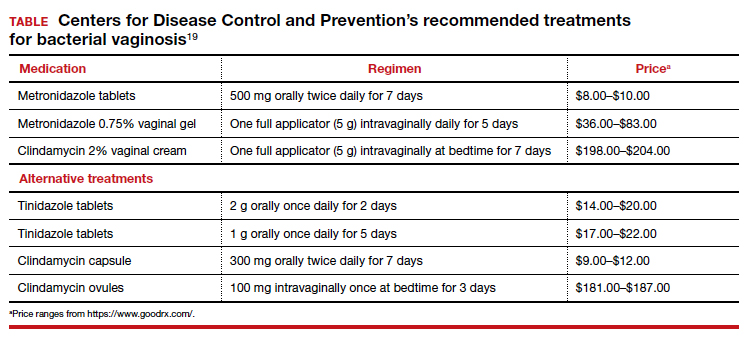
Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.