User login
Debunking Psoriasis Myths: Is Psoriasis Infectious?
Myth: Psoriasis Is Infectious The precise cause of psoriasis is unknown, but researchers believe the immune system and genetics play major roles in its development, according to the National Psoriasis Foundation. The skin cells in patients with psoriasis grow at an abnormally fast rate, which causes the buildup of psoriasis lesions. Usually, something triggers psoriasis to flare.
A common misconception among patients is that psoriasis is caused by an infection. Psoriasis is not contagious and psoriasis lesions are not infectious.
However, psoriasis patients are more prone to infections than those without psoriasis. Risk factors for serious infections in psoriasis patients include immune dysregulation, systemic immunosuppressive medications, and comorbid health conditions such as diabetes mellitus or obesity. A 2016 study revealed an increased incidence of serious infections (eg, cellulitis, herpes simplex virus infection, any fungal infection, infectious arthritis, methicillin-resistant Staphylococcus aureus) in hospitalized patients with psoriasis. Higher rates were seen among nonwhite and non-privately insured patients.
In a 2011 study, the likelihood of infectious diseases in patients with psoriasis was twice as high as the reference population. The risk was highest in patients with more severe psoriasis but was not associated with recent systemic antipsoriatic drug dispensing. Respiratory tract, abdominal, and skin infections occurred most frequently in patients with psoriasis.
Poor access to adequate dermatologic care may contribute to higher rates of infections. Dermatologists must closely monitor patients with psoriasis for infection. More research is needed to develop interventions for prevention.
Expert Commentary Psoriasis patients have long faced discrimination because of an irrational fear that their disease was somehow contagious. In fact this is completely false. This highlights the need for education of the public, so that they understand the true causes and nature of the disease.
—Jeffrey M. Weinberg, MD (New York, New York)
About psoriasis. National Psoriasis Foundation website. http://www.psoriasis.org/about-psoriasis. Accessed September 9, 2016.
Hsu DY, Gordon K, Silverberg JI. Serious infections in hospitalized patients with psoriasis in the United States [published online June 17, 2016]. J Am Acad Dermatol. 2016;75:287-296.
Wakkee M, de Vries E, van den Haak P, et al. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65:1135-1144.
Myth: Psoriasis Is Infectious The precise cause of psoriasis is unknown, but researchers believe the immune system and genetics play major roles in its development, according to the National Psoriasis Foundation. The skin cells in patients with psoriasis grow at an abnormally fast rate, which causes the buildup of psoriasis lesions. Usually, something triggers psoriasis to flare.
A common misconception among patients is that psoriasis is caused by an infection. Psoriasis is not contagious and psoriasis lesions are not infectious.
However, psoriasis patients are more prone to infections than those without psoriasis. Risk factors for serious infections in psoriasis patients include immune dysregulation, systemic immunosuppressive medications, and comorbid health conditions such as diabetes mellitus or obesity. A 2016 study revealed an increased incidence of serious infections (eg, cellulitis, herpes simplex virus infection, any fungal infection, infectious arthritis, methicillin-resistant Staphylococcus aureus) in hospitalized patients with psoriasis. Higher rates were seen among nonwhite and non-privately insured patients.
In a 2011 study, the likelihood of infectious diseases in patients with psoriasis was twice as high as the reference population. The risk was highest in patients with more severe psoriasis but was not associated with recent systemic antipsoriatic drug dispensing. Respiratory tract, abdominal, and skin infections occurred most frequently in patients with psoriasis.
Poor access to adequate dermatologic care may contribute to higher rates of infections. Dermatologists must closely monitor patients with psoriasis for infection. More research is needed to develop interventions for prevention.
Expert Commentary Psoriasis patients have long faced discrimination because of an irrational fear that their disease was somehow contagious. In fact this is completely false. This highlights the need for education of the public, so that they understand the true causes and nature of the disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Myth: Psoriasis Is Infectious The precise cause of psoriasis is unknown, but researchers believe the immune system and genetics play major roles in its development, according to the National Psoriasis Foundation. The skin cells in patients with psoriasis grow at an abnormally fast rate, which causes the buildup of psoriasis lesions. Usually, something triggers psoriasis to flare.
A common misconception among patients is that psoriasis is caused by an infection. Psoriasis is not contagious and psoriasis lesions are not infectious.
However, psoriasis patients are more prone to infections than those without psoriasis. Risk factors for serious infections in psoriasis patients include immune dysregulation, systemic immunosuppressive medications, and comorbid health conditions such as diabetes mellitus or obesity. A 2016 study revealed an increased incidence of serious infections (eg, cellulitis, herpes simplex virus infection, any fungal infection, infectious arthritis, methicillin-resistant Staphylococcus aureus) in hospitalized patients with psoriasis. Higher rates were seen among nonwhite and non-privately insured patients.
In a 2011 study, the likelihood of infectious diseases in patients with psoriasis was twice as high as the reference population. The risk was highest in patients with more severe psoriasis but was not associated with recent systemic antipsoriatic drug dispensing. Respiratory tract, abdominal, and skin infections occurred most frequently in patients with psoriasis.
Poor access to adequate dermatologic care may contribute to higher rates of infections. Dermatologists must closely monitor patients with psoriasis for infection. More research is needed to develop interventions for prevention.
Expert Commentary Psoriasis patients have long faced discrimination because of an irrational fear that their disease was somehow contagious. In fact this is completely false. This highlights the need for education of the public, so that they understand the true causes and nature of the disease.
—Jeffrey M. Weinberg, MD (New York, New York)
About psoriasis. National Psoriasis Foundation website. http://www.psoriasis.org/about-psoriasis. Accessed September 9, 2016.
Hsu DY, Gordon K, Silverberg JI. Serious infections in hospitalized patients with psoriasis in the United States [published online June 17, 2016]. J Am Acad Dermatol. 2016;75:287-296.
Wakkee M, de Vries E, van den Haak P, et al. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65:1135-1144.
About psoriasis. National Psoriasis Foundation website. http://www.psoriasis.org/about-psoriasis. Accessed September 9, 2016.
Hsu DY, Gordon K, Silverberg JI. Serious infections in hospitalized patients with psoriasis in the United States [published online June 17, 2016]. J Am Acad Dermatol. 2016;75:287-296.
Wakkee M, de Vries E, van den Haak P, et al. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65:1135-1144.
Surgical Pearls in Total Knee Arthroplasty: A Lifetime of Lessons Learned
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
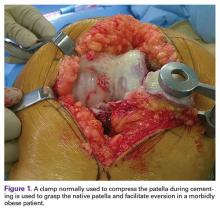
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
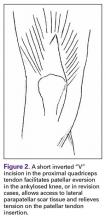
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
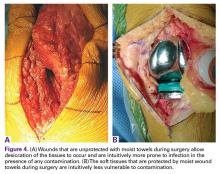
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
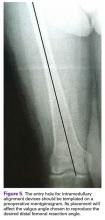
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
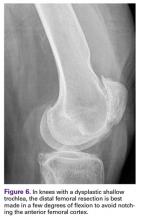
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
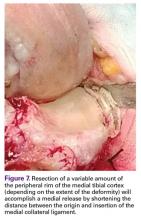
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
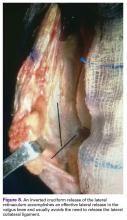
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
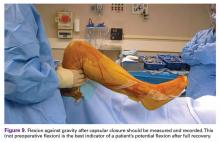
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
Engineered Bone Graft
Exactech
Optecure+ccc
(http://www.exac.com/products/biologics/optecure-optecure-ccc)
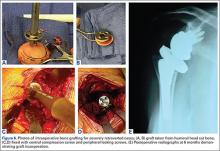
Autogenous bone graft remains the standard for augmenting the surgical care of severe fractures, promoting spinal fusion, filling bone voids, and treating nonunions. However, lingering problems with donor site morbidity, volume limitation, increased operative time, and increased case complexity have led to the growing use of bone graft substitutes.1 These alternatives include allograft bone, demineralized bone matrix, calcium sulfate and calcium phosphate, bioglass, growth factors (rhBMP-2, rhBMP-7, rhPDGF, and PRP [platelet-rich plasma]), collagen matrix, and new cellular-based compounds using mesenchymal stem cells. Since each individual class of bone substitute falls short of the optimal blend of osteoconduction, osteoinduction, and osteogenesis, novel composite grafts have been developed to combine the convenience, durability, and flexibility of synthetic grafts with the biologic activity of native bone.
Optecure+ccc (Exactech) is an engineered composite bone graft that contains demineralized bone mixed with gamma irradiated cortical cancellous chips in an absorbable synthetic hydrogel matrix (Figure). When mixed with saline, blood, autogenous bone, bone marrow aspirate, or PRP, it becomes a surprisingly robust and malleable 3-dimensional matrix that allows easy bone void filling with excellent osteoconductive and osteoinductive characteristics. Each individual lot is tested for sterility and endotoxin levels to confirm safety as well as in vivo testing in athymic mice to confirm osteoinductive potential. Optecure+ccc has been successfully used to augment healing when combined with bone marrow aspirate in minimally invasive spine fusion surgery.2
Surgical pearl: I treat a large number of bicycle injuries on Nantucket; many are quite serious. I have found Optecure+ccc to be particularly useful during locked volar plating of severe distal radius wrist fractures as a way to restore and support radial length when autogenous bone access is limited. In this application, Optecure’s ability to expand and mold into a functional bone scaffold is critical to create a stable, stress-resistant fracture construct.
After exposure of the comminuted fracture line of the distal radius, gentle axial traction is applied and a small osteotome or freer is used to carefully wedge open the cortex to allow metaphyseal window access. The Optecure+ccc is mixed with either blood or bone marrow aspirate to reach a “grape nuts cereal”-like consistency and then carefully packed into the metaphyseal window to backfill the void. Multiplanar fluoroscopy is used to monitor graft placement and gradual joint line restoration. Traction is then released after the void is filled sufficiently to support the provisional reduction. Additional grafting with standard Optecure without bone chips can be used to fill more difficult-to-access areas. Both forms of Optecure are resistant to diluent migration, giving them good intraoperative behavior. Excess graft can be easily wiped away from the fracture site prior to plate application.
After elevation and restoration of the joint line, the locking volar plate is then affixed, wrist alignment confirmed fluoroscopically, and the procedure completed. The result is a well-filled void and an improved fracture construct. While Optecure+ccc has proven its battle readiness in wrist fracture surgery, I have also found it very helpful in reconstructing complex proximal humerus and clavicle fractures. Its unique combination of intraoperative versatility and durability provides a welcome edge in challenging cases.
1. Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4(2):63-66.
2. Sasso RC, LeHuec JC, Shaffrey C; Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18 Suppl:S77-S81.
Exactech
Optecure+ccc
(http://www.exac.com/products/biologics/optecure-optecure-ccc)
Autogenous bone graft remains the standard for augmenting the surgical care of severe fractures, promoting spinal fusion, filling bone voids, and treating nonunions. However, lingering problems with donor site morbidity, volume limitation, increased operative time, and increased case complexity have led to the growing use of bone graft substitutes.1 These alternatives include allograft bone, demineralized bone matrix, calcium sulfate and calcium phosphate, bioglass, growth factors (rhBMP-2, rhBMP-7, rhPDGF, and PRP [platelet-rich plasma]), collagen matrix, and new cellular-based compounds using mesenchymal stem cells. Since each individual class of bone substitute falls short of the optimal blend of osteoconduction, osteoinduction, and osteogenesis, novel composite grafts have been developed to combine the convenience, durability, and flexibility of synthetic grafts with the biologic activity of native bone.
Optecure+ccc (Exactech) is an engineered composite bone graft that contains demineralized bone mixed with gamma irradiated cortical cancellous chips in an absorbable synthetic hydrogel matrix (Figure). When mixed with saline, blood, autogenous bone, bone marrow aspirate, or PRP, it becomes a surprisingly robust and malleable 3-dimensional matrix that allows easy bone void filling with excellent osteoconductive and osteoinductive characteristics. Each individual lot is tested for sterility and endotoxin levels to confirm safety as well as in vivo testing in athymic mice to confirm osteoinductive potential. Optecure+ccc has been successfully used to augment healing when combined with bone marrow aspirate in minimally invasive spine fusion surgery.2
Surgical pearl: I treat a large number of bicycle injuries on Nantucket; many are quite serious. I have found Optecure+ccc to be particularly useful during locked volar plating of severe distal radius wrist fractures as a way to restore and support radial length when autogenous bone access is limited. In this application, Optecure’s ability to expand and mold into a functional bone scaffold is critical to create a stable, stress-resistant fracture construct.
After exposure of the comminuted fracture line of the distal radius, gentle axial traction is applied and a small osteotome or freer is used to carefully wedge open the cortex to allow metaphyseal window access. The Optecure+ccc is mixed with either blood or bone marrow aspirate to reach a “grape nuts cereal”-like consistency and then carefully packed into the metaphyseal window to backfill the void. Multiplanar fluoroscopy is used to monitor graft placement and gradual joint line restoration. Traction is then released after the void is filled sufficiently to support the provisional reduction. Additional grafting with standard Optecure without bone chips can be used to fill more difficult-to-access areas. Both forms of Optecure are resistant to diluent migration, giving them good intraoperative behavior. Excess graft can be easily wiped away from the fracture site prior to plate application.
After elevation and restoration of the joint line, the locking volar plate is then affixed, wrist alignment confirmed fluoroscopically, and the procedure completed. The result is a well-filled void and an improved fracture construct. While Optecure+ccc has proven its battle readiness in wrist fracture surgery, I have also found it very helpful in reconstructing complex proximal humerus and clavicle fractures. Its unique combination of intraoperative versatility and durability provides a welcome edge in challenging cases.
Exactech
Optecure+ccc
(http://www.exac.com/products/biologics/optecure-optecure-ccc)
Autogenous bone graft remains the standard for augmenting the surgical care of severe fractures, promoting spinal fusion, filling bone voids, and treating nonunions. However, lingering problems with donor site morbidity, volume limitation, increased operative time, and increased case complexity have led to the growing use of bone graft substitutes.1 These alternatives include allograft bone, demineralized bone matrix, calcium sulfate and calcium phosphate, bioglass, growth factors (rhBMP-2, rhBMP-7, rhPDGF, and PRP [platelet-rich plasma]), collagen matrix, and new cellular-based compounds using mesenchymal stem cells. Since each individual class of bone substitute falls short of the optimal blend of osteoconduction, osteoinduction, and osteogenesis, novel composite grafts have been developed to combine the convenience, durability, and flexibility of synthetic grafts with the biologic activity of native bone.
Optecure+ccc (Exactech) is an engineered composite bone graft that contains demineralized bone mixed with gamma irradiated cortical cancellous chips in an absorbable synthetic hydrogel matrix (Figure). When mixed with saline, blood, autogenous bone, bone marrow aspirate, or PRP, it becomes a surprisingly robust and malleable 3-dimensional matrix that allows easy bone void filling with excellent osteoconductive and osteoinductive characteristics. Each individual lot is tested for sterility and endotoxin levels to confirm safety as well as in vivo testing in athymic mice to confirm osteoinductive potential. Optecure+ccc has been successfully used to augment healing when combined with bone marrow aspirate in minimally invasive spine fusion surgery.2
Surgical pearl: I treat a large number of bicycle injuries on Nantucket; many are quite serious. I have found Optecure+ccc to be particularly useful during locked volar plating of severe distal radius wrist fractures as a way to restore and support radial length when autogenous bone access is limited. In this application, Optecure’s ability to expand and mold into a functional bone scaffold is critical to create a stable, stress-resistant fracture construct.
After exposure of the comminuted fracture line of the distal radius, gentle axial traction is applied and a small osteotome or freer is used to carefully wedge open the cortex to allow metaphyseal window access. The Optecure+ccc is mixed with either blood or bone marrow aspirate to reach a “grape nuts cereal”-like consistency and then carefully packed into the metaphyseal window to backfill the void. Multiplanar fluoroscopy is used to monitor graft placement and gradual joint line restoration. Traction is then released after the void is filled sufficiently to support the provisional reduction. Additional grafting with standard Optecure without bone chips can be used to fill more difficult-to-access areas. Both forms of Optecure are resistant to diluent migration, giving them good intraoperative behavior. Excess graft can be easily wiped away from the fracture site prior to plate application.
After elevation and restoration of the joint line, the locking volar plate is then affixed, wrist alignment confirmed fluoroscopically, and the procedure completed. The result is a well-filled void and an improved fracture construct. While Optecure+ccc has proven its battle readiness in wrist fracture surgery, I have also found it very helpful in reconstructing complex proximal humerus and clavicle fractures. Its unique combination of intraoperative versatility and durability provides a welcome edge in challenging cases.
1. Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4(2):63-66.
2. Sasso RC, LeHuec JC, Shaffrey C; Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18 Suppl:S77-S81.
1. Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4(2):63-66.
2. Sasso RC, LeHuec JC, Shaffrey C; Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18 Suppl:S77-S81.
The Arthroscopic Superior Capsular Reconstruction
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
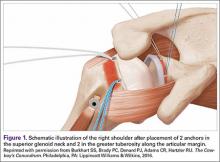
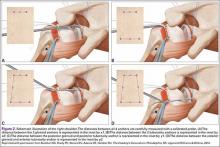
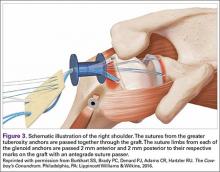
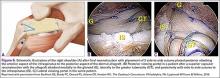
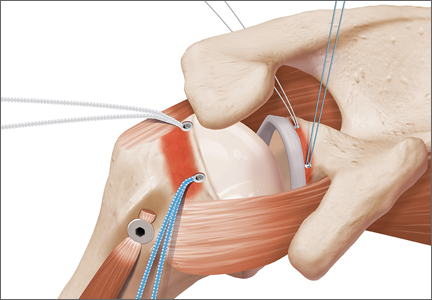
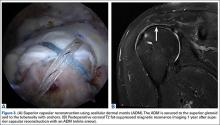
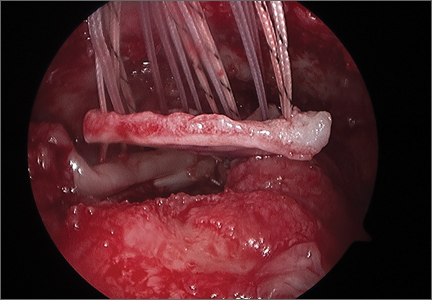
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
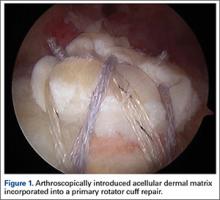
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
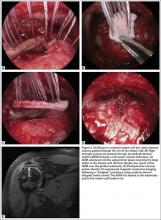
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
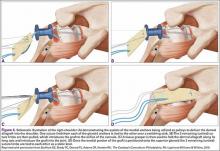
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
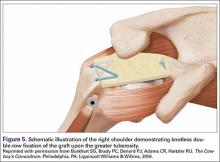
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
Platelet-Rich Plasma Can Be Used to Successfully Treat Elbow Ulnar Collateral Ligament Insufficiency in High-Level Throwers
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
Acellular Dermal Matrix in Rotator Cuff Surgery
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
Suture Anchor
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
A Guide to Ultrasound of the Shoulder, Part 2: The Diagnostic Evaluation
The musculoskeletal (MSK) ultrasound evaluation of the shoulder provides a cost- and time-efficient imaging modality with similar diagnostic power as magnetic resonance imaging (MRI).1,2 Its portable point-of-care applications can be used in the office, in the operating room, and in sideline athletic event coverage, as we discussed in Part 1 of this series.3
MSK ultrasound may seem difficult and daunting, and many articles have quoted steep learning curves.4,5 However, in our experience in teaching many ultrasound courses, this modality can be learned quite quickly with the proper instruction. Physicians are already familiar with anatomy and usually have had some exposure to MRI.4 Taking courses in MSK ultrasound or simply learning the basic concepts of ultrasound and then learning the machine controls is usually a good start.5-8 Practice scanning normal individuals, comparing the images from an MRI to learn how to reproduce the same planes and images. This will allow the user to become familiar with normal anatomy and how to see the images on the ultrasound screen.5-8 Vollman and colleagues9 showed that in trainees, combining MRI images with sonograms enhances the ability to correctly identify MSK ultrasound anatomy from 40.9% to 72.5%, when compared with learning from ultrasound images alone.
There are currently no certifications necessary to perform ultrasound scans or bill for them; however, some insurance carriers may require demonstrating relevant, documented training for reimbursement.3 Various organizations are trying to develop certifications and regulations for ultrasound to standardize the use of this modality. In the United States, the American Institute of Ultrasound in Medicine (AIUM) and the American Registry for Diagnostic Medical Sonography (ARDMS) provide guidelines and particular MSK ultrasound certifications.10,11
Basic Ultrasound Principles
The ultrasound machine creates electrical impulses that are turned into sound waves by piezoelectric crystals at the probe’s footprint. These sound waves bounce off tissues and return to the probe, where they are converted electronically to an image on the monitor. Depending on the echogenicity of the scanned tissue, the ultrasound beam will either reflect or be absorbed at different rates. This variance is transmitted on the monitor as a grayscale image. When ultrasound waves are highly reflective, like in bone or fat, they are characterized as hyperechoic. The opposite occurs when ultrasound waves are absorbed like in the fluid of a cystic cavity or joint effusion, and the image appears black. This is described as anechoic.12 Intermediate tissues such as tendons that are less reflective are seen as hypoechoic and appear gray. When a tissue has a similar echogenicity to its surrounding tissues, it is called isoechoic.12
The transducer is the scanning component of the ultrasound machine. Transducers come in 2 shapes: linear and curvilinear. The linear probe creates a straight image that is equal to the size of the transducer footprint. The curvilinear probe creates a wider, wedge-shaped panoramic image.
Linear probes are of higher frequency and generate higher resolution images of shallower structures, while curvilinear probes have greater depth penetration but generate lower resolution images. A high frequency of 10 to 15 MHz is preferred for anatomy between 2 cm to 4 cm depth.13 Midrange frequency of 5 to 10 MHz is preferred at 5 cm to 6 cm depth, and low-frequency 2 to 5 MHz probes are preferred for anatomical structures >6 cm depth.13
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. This anisotropic effect is dependent on the angle of the insonating beam. The maximum return echo occurs when the ultrasound beam is perpendicular to the tendon. Decreasing the insonating angle on a normal tendon will cause it to change from brightly hyperechoic (the actual echo from tightly bound tendon fibers) to darkly hypoechoic. If the angle is then increased, the tendon will again appear hyperechoic. If the artifact causes a normal tendon to appear hypoechoic, it may falsely lead to a diagnosis of tendinosis or tear.
Posterior acoustic shadowing is present when a hyperechoic structure reflects the ultrasound beam so much that it creates a dark shadow underneath it.12,14 This phenomenon is possible since the ultrasound beam cannot penetrate the hyperechoic structure and reflects off its inferior tissues. Reverberation is when the beam is repeated back and forth between 2 parallel highly reflective surfaces. The initial reflection will be displayed correctly, while the subsequent ultrasound waves will be delayed and appear at a farther distance from the transducer.12,14
The point where the beam is at its narrowest point generates the section of the image that is best visualized.15 This is called the focal zone, and it can be adjusted to highlight the desired area of evaluation. Gain controls adjust the amount of black, gray, and white on the monitor and can be adjusted to focus the desired image.13 Depth settings are fundamental in finding the desired targets. It is recommended to start with a higher depth setting to get an overview and progressively decrease the depth to key in on the desired anatomy.13 Color Doppler can be used to view movement within structures and to identify vessels, synovitis, and neovascularization in tendinopathy.13
Ultrasound of the Shoulder
Patients should be seated, if possible, on a rotating seat. The examiner’s shoulder should be higher than the patient’s shoulder.16 The user holds the ultrasound probe between the thumb and index fingers while resting the hypothenar eminence on the patient to serve as a fulcrum and steadying force. The examination should take 5 to 15 minutes, depending on the examiner’s expertise and the amount of anatomy being scanned.
Examining the body requires knowledge of anatomy. The examination and accuracy are determined by the technician using the probe. The probe can be angled any direction and be placed obliquely on the subject. The advantage here is that anatomy in the human body is not always planar. Muscles and tissues can run obliquely or even perpendicular to each other. When evaluating anatomy, the examiner should keep in mind what structure he or she is looking for; where it should be found; what landmarks can be used to easily locate it; what orientation it has; and what the normal anatomy should look like.
Muscle appears as a lattice with larger areas of hypoechoic muscle tissue and hyperechoic fascial perimysium layers traversing through it.17 The actual muscle tissue appears hypoechoic from the fluid or blood found within. Scarring, fibrosis, calcification, or chronic injury will change the tissue to appear denser or hyperechoic.17 Acute injury will appear hypoechoic from the inflammatory response and influx of blood. Tendon appears dense and hyperechoic with striations within the tissue, sometimes referred to as a horse’s tail.17 When torn, there will be a disassociation of the tissue with a hypoechoic region between the 2 ends. The attachment to the bone and muscle tissue should appear uniform. Hyperechoic areas within the tendon may be from calcification. Ligament appears similar to tendon but is more isoechoic and connects bone to bone. Evaluation of the entire length and the attachments to the bone are critical to evaluate for disease.
Bone appears bright hyperechoic, smooth, and flat, while hyaline cartilage is hypoechoic, smooth, and runs superiorly in a parallel pattern to its respective inferior cortical bone.17
Fibrocartilage is hyperechoic and typically triangularly shaped, such as in the glenohumeral labrum. Nerves appear fascicular and hypoechoic surrounded by hyperechoic epineurium.14
The epidermis and dermis are the most superficial structure on top of the screen, and are also hyperechoic.17
The Diagnostic Shoulder Examination
The proximal long head of the biceps tendon (LHBT) is the easiest structure in the shoulder to identify because of the anatomic structure, the bicipital groove. By keeping the arm relaxed, perpendicular to the ground, and in neutral rotation, the probe can be placed perpendicular to the arm over the proximal shoulder (Figure 1A).16-20 By finding the groove, the biceps tendon will usually be found resting within the groove (Figure 1B). This is the short axis view and is equivalent to an MRI in the axial plane.
The long axis view of the proximal biceps tendon is found by keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The user should be sure to visualize the entire tendon on the screen. If only part of the tendon is seen along only part of the screen, then the probe is oblique to the tendon. In this case, the probe area showing the tendon must be stabilized as the center or set point. The other part of the probe will then pivot until all of the tendon is seen on the screen. The MRI equivalent to the long axis of the proximal biceps tendon is the sagittal view.
Ultrasound is a dynamic evaluation. Moving the probe or moving the patient will change what and how something is imaged. The proximal biceps tendon is a good example of this concept. The bicipital groove is very deep proximally and flattens out as it travels distally to the mid-humerus. The examiner should continually adjust his or her hand/probe/patient position as well as depth/gain and other console functions to adapt to the dynamics of the scan. While keeping the bicep tendon in a short axis view, the tendon can be dynamically evaluated for subluxation by internally and externally rotating the arm.
To find the subscapularis, the arm remains in a neutral position with the hand supinated and the probe is held parallel with the ground. After finding the bicipital groove, the subscapularis tendon insertion is just medial to the groove (Figure 1B). By externally rotating the arm, the subscapularis tendon/muscle will come into a long axis view.16-20 The MRI equivalent to the long axis view of the subscapularis is the axial view. Dynamic testing can be done by internally and externally rotating the arm to evaluate for impingement of the subscapularis tendon as it slides underneath the coracoid process. To view the subscapularis tendon in short axis, the tendon is kept in the center of the screen/probe, and the probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The MRI equivalent is the sagittal view.
Some have recommended using the modified Crass or Middleton position to evaluate the supraspinatus, where the hand is in the “back pocket”.19 However, many patients with shoulder pain have trouble with this position. By resting the ipsilateral hand on the ipsilateral hip and then dropping the elbow, the supraspinatus insertion can still be brought out from under the acromion. This does bring the insertion anterior out of the scapular plane, so an adjustment is required in probe positioning to properly see the supraspinatus short and long axis. To find the long axis, the probe is placed parallel to a plane that spans the contralateral shoulder and ipsilateral hip (Figure 2A). The fibers of the supraspinatus should be inserting directly lateral to the humeral head without any intervening space (Figure 2B). If any space exists, a partial articular supraspinatus tendon avulsion (PASTA) lesion is present, and its thickness can be directly measured. Moving more posterior will show the flattening of the tuberosity and the fibers of the infraspinatus moving away from the humeral head—the bare spot. The MRI equivalent is the coronal view.
To view the supraspinatus tendon in short axis, maintain the arm in the same position, keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The probe should now be in a parallel plane between the ipsilateral shoulder and the contralateral hip. The biceps tendon in cross-section will be found anteriorly, and the articular cartilage will appear as a black layer over the bone. Dynamic testing includes placing the probe in a coronal plane between the acromion and greater tuberosity. When the patient abducts the arm while in internal rotation, the supraspinatus tendon will slide underneath the coracoacromial arch showing potential external impingement.15 The MRI equivalent is the sagittal plane.
The glenohumeral joint is best viewed posteriorly, limiting how much of the intra-articular portion of the joint can be imaged. The arm remains in a neutral position; palpate for the posterior acromion and place the probe just inferior to it, wedging up against it (Figure 3A). The glenohumeral joint will be seen by keeping the probe parallel to the ground (Figure 3B). The MRI equivalent is the axial plane. If a joint effusion exists, it can be seen in the posterior recess.15 A hyperechoic triangular region in between the humeral head and the glenoid will represent the glenoid labrum (Figure 3B). By internally and externally rotating the arm, the joint and labrum complex can be dynamically examined. From the labrum, scanning superior and medial can sometimes show the spinoglenoid notch where a paralabral cyst might be seen.15
Using the glenohumeral joint as a reference, the infraspinatus muscle is easily visualized. Maintaining the arm in neutral position with the probe over the glenohumeral joint, the infraspinatus will become apparent as it lays in long axis view superficially between the posterior deltoid and glenohumeral joint (Figure 3B).16-20 The teres minor lies just inferiorly. The MRI equivalent is the axial plane. To view the infraspinatus and teres minor in short axis, the probe is then rotated 90° on its center axis. The infraspinatus (superiorly) and teres minor (inferiorly) muscles will be visible in short axis within the infraspinatus fossa.15 The MRI equivalent is the sagittal view.
The acromioclavicular joint is superficial and easy to image. The arm remains in a neutral position, and we can palpate the joint for easy localization. The probe is placed anteriorly in a coronal plane over the acromion and clavicle. By scanning anteriorly and posteriorly, a joint effusion referred to as a Geyser sign might be seen. The MRI equivalent is the coronal view.
Available Certifications
The AIUM certification is a voluntary peer reviewed process that acknowledges that a practice is meeting national standards and aids in improving their respective MSK ultrasound protocols. They also provide guidelines on demonstrating training and competence on performing and/or interpreting diagnostic MSK examinations (Table).10 The ARDMS certification provides an actual individual certification referred to as “Registered” in MSK ultrasound.11 The physician must perform 150 diagnostic MSK ultrasound evaluations within 36 months of applying and pass a 200-question examination that is offered twice per year.11 None of these certifications are mandated by the American Medical Association (AMA) or American Osteopathic Association (AOA).
Maintenance and Continuing Medical Education (CME)
The AIUM recommends that a minimum of 50 diagnostic MSK ultrasound evaluations be performed per year for skill maintenance.10 Furthermore, 10 hours of AMA PRA Category 1 Credits™ or American Osteopathic Association Category 1-A Credits specific to MSK ultrasound must be completed by physicians performing and/or interpreting these examinations every 3 years.10 ARDMS recommends a minimum of 30 MSK ultrasound-specific CMEs in preparation for their “Registered” MSK evaluation.1
Conclusion
MSK ultrasound is a dynamic, real-time imaging modality that can improve cost efficiency and patient care. Its portability allows for its use anywhere. Learning the skill may seem daunting, but with the proper courses and education, the technology can be easily learned. By correlating a known modality like MRI, the user will easily begin to read ultrasound images. No current certification is needed to use or bill for ultrasound, but various institutions are developing criteria and testing. Two organizations, AIUM and ARDMS, provide guidelines and certifications to demonstrate competency, which may become necessary in the very near future.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Roy J-S, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis [published online ahead of print February 11, 2015]. Br J Sports Med. doi:10.1136/bjsports-2014-094148.
3. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
4. Hama M, Takase K, Ihata A, et al. Challenges to expanding the clinical application of musculoskeletal ultrasonography (MSUS) among rheumatologists: from a second survey in Japan. Mod Rheumatol. 2012;2:202-208.
5. Smith MJ, Rogers A, Amso N, Kennedy J, Hall A, Mullaney P. A training, assessment and feedback package for the trainee shoulder sonographer. Ultrasound. 2015;23(1):29-41.
6. Delzell PB, Boyle A, Schneider E. Dedicated training program for shoulder sonography: the results of a quality program reverberate with everyone. J Ultrasound Med. 2015;34(6):1037-1042.
7. Finnoff JT, Berkoff D, Brennan F, et al. American Medical Society for Sports Medicine (AMSSM) recommended sports ultrasound curriculum for sports medicine fellowships. PM R. 2015;7(2)e1-e11.
8. Adelman S, Fishman P. Use of portable ultrasound machine for outpatient orthopedic diagnosis: an implementation study. Perm J. 2013;17(3):18-22.
9. Vollman A, Hulen R, Dulchavsky S, et al. Educational benefits of fusing magnetic resonance imaging with sonograms. J Clin Ultrasound. 2014;42(5) 257-263.
10. Training guidelines for physicians and chiropractors who evaluate and interpret diagnostic musculoskeletal ultrasound examinations. Laurel, MD: American Institute of Ultrasound in Medicine; 2014. http://www.aium.org/resources/viewStatement.aspx?id=51. Accessed February 26, 2016.
11. Registered in musculoskeletal (RMSK) sonography. American Registry for Diagnostic Medical Sonography Web site. http://www.ardms.org/get-certified/RMSK/Pages/RMSK.aspx. Accessed February 26, 2016.
12. Silkowski C. Ultrasound nomenclature, image orientation, and basic instrumentation. In: Abraham D, Silkowski C, Odwin C, eds. Emergency Medicine Sonography Pocket Guide to Sonographic Anatomy and Pathology. Sudbury, MA: Jones and Bartlett; 2010:1-24.
13. Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4(3):55-62.
14. Taljanovic MS, Melville DM, Scalcione LR, Gimber LH, Lorenz EJ, Witte RS. Artifacts in musculoskeletal ultrasonography. Semin Musculoskelet Radiol. 2014;18(1):3-11.
15. Ng A, Swanevelder J. Resolution in ultrasound imaging. Continuing Educ Anaesth Crit Care Pain. 2011;11(5):186-192. http://ceaccp.oxfordjournals.org/content/11/5/186.full. Accessed March 3, 2016.
16. Nazarian L, Bohm-Velez M, Kan JH, et al. AIUM practice parameters for the performance of a musculoskeletal ultrasound examination. Laurel, MD: American Institute of Ultrasound in Medicine; 2012. http://www.aium.org/resources/guidelines/musculoskeletal.pdf. Accessed February 26, 2016.
17. Jacobson J. Fundamentals of Musculoskeletal Ultrasound. 2nd edition. Philadelphia, PA: Elsevier Saunders; 2013.
18. The Ultrasound Subcommittee of the European Society of Musculoskeletal Radiology. Musculoskeletal ultrasound: technique guidelines. Insights Imaging. 2010;1:99-141.
19. Corazza A, Orlandi D, Fabbro E, et al. Dynamic high-resolution ultrasound of the shoulder: how we do it. Eur J Radiol. 2015;84(2):266-277.
20. Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease processes. Eur J Radiol. 2008;68(1):137-146
The musculoskeletal (MSK) ultrasound evaluation of the shoulder provides a cost- and time-efficient imaging modality with similar diagnostic power as magnetic resonance imaging (MRI).1,2 Its portable point-of-care applications can be used in the office, in the operating room, and in sideline athletic event coverage, as we discussed in Part 1 of this series.3
MSK ultrasound may seem difficult and daunting, and many articles have quoted steep learning curves.4,5 However, in our experience in teaching many ultrasound courses, this modality can be learned quite quickly with the proper instruction. Physicians are already familiar with anatomy and usually have had some exposure to MRI.4 Taking courses in MSK ultrasound or simply learning the basic concepts of ultrasound and then learning the machine controls is usually a good start.5-8 Practice scanning normal individuals, comparing the images from an MRI to learn how to reproduce the same planes and images. This will allow the user to become familiar with normal anatomy and how to see the images on the ultrasound screen.5-8 Vollman and colleagues9 showed that in trainees, combining MRI images with sonograms enhances the ability to correctly identify MSK ultrasound anatomy from 40.9% to 72.5%, when compared with learning from ultrasound images alone.
There are currently no certifications necessary to perform ultrasound scans or bill for them; however, some insurance carriers may require demonstrating relevant, documented training for reimbursement.3 Various organizations are trying to develop certifications and regulations for ultrasound to standardize the use of this modality. In the United States, the American Institute of Ultrasound in Medicine (AIUM) and the American Registry for Diagnostic Medical Sonography (ARDMS) provide guidelines and particular MSK ultrasound certifications.10,11
Basic Ultrasound Principles
The ultrasound machine creates electrical impulses that are turned into sound waves by piezoelectric crystals at the probe’s footprint. These sound waves bounce off tissues and return to the probe, where they are converted electronically to an image on the monitor. Depending on the echogenicity of the scanned tissue, the ultrasound beam will either reflect or be absorbed at different rates. This variance is transmitted on the monitor as a grayscale image. When ultrasound waves are highly reflective, like in bone or fat, they are characterized as hyperechoic. The opposite occurs when ultrasound waves are absorbed like in the fluid of a cystic cavity or joint effusion, and the image appears black. This is described as anechoic.12 Intermediate tissues such as tendons that are less reflective are seen as hypoechoic and appear gray. When a tissue has a similar echogenicity to its surrounding tissues, it is called isoechoic.12
The transducer is the scanning component of the ultrasound machine. Transducers come in 2 shapes: linear and curvilinear. The linear probe creates a straight image that is equal to the size of the transducer footprint. The curvilinear probe creates a wider, wedge-shaped panoramic image.
Linear probes are of higher frequency and generate higher resolution images of shallower structures, while curvilinear probes have greater depth penetration but generate lower resolution images. A high frequency of 10 to 15 MHz is preferred for anatomy between 2 cm to 4 cm depth.13 Midrange frequency of 5 to 10 MHz is preferred at 5 cm to 6 cm depth, and low-frequency 2 to 5 MHz probes are preferred for anatomical structures >6 cm depth.13
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. This anisotropic effect is dependent on the angle of the insonating beam. The maximum return echo occurs when the ultrasound beam is perpendicular to the tendon. Decreasing the insonating angle on a normal tendon will cause it to change from brightly hyperechoic (the actual echo from tightly bound tendon fibers) to darkly hypoechoic. If the angle is then increased, the tendon will again appear hyperechoic. If the artifact causes a normal tendon to appear hypoechoic, it may falsely lead to a diagnosis of tendinosis or tear.
Posterior acoustic shadowing is present when a hyperechoic structure reflects the ultrasound beam so much that it creates a dark shadow underneath it.12,14 This phenomenon is possible since the ultrasound beam cannot penetrate the hyperechoic structure and reflects off its inferior tissues. Reverberation is when the beam is repeated back and forth between 2 parallel highly reflective surfaces. The initial reflection will be displayed correctly, while the subsequent ultrasound waves will be delayed and appear at a farther distance from the transducer.12,14
The point where the beam is at its narrowest point generates the section of the image that is best visualized.15 This is called the focal zone, and it can be adjusted to highlight the desired area of evaluation. Gain controls adjust the amount of black, gray, and white on the monitor and can be adjusted to focus the desired image.13 Depth settings are fundamental in finding the desired targets. It is recommended to start with a higher depth setting to get an overview and progressively decrease the depth to key in on the desired anatomy.13 Color Doppler can be used to view movement within structures and to identify vessels, synovitis, and neovascularization in tendinopathy.13
Ultrasound of the Shoulder
Patients should be seated, if possible, on a rotating seat. The examiner’s shoulder should be higher than the patient’s shoulder.16 The user holds the ultrasound probe between the thumb and index fingers while resting the hypothenar eminence on the patient to serve as a fulcrum and steadying force. The examination should take 5 to 15 minutes, depending on the examiner’s expertise and the amount of anatomy being scanned.
Examining the body requires knowledge of anatomy. The examination and accuracy are determined by the technician using the probe. The probe can be angled any direction and be placed obliquely on the subject. The advantage here is that anatomy in the human body is not always planar. Muscles and tissues can run obliquely or even perpendicular to each other. When evaluating anatomy, the examiner should keep in mind what structure he or she is looking for; where it should be found; what landmarks can be used to easily locate it; what orientation it has; and what the normal anatomy should look like.
Muscle appears as a lattice with larger areas of hypoechoic muscle tissue and hyperechoic fascial perimysium layers traversing through it.17 The actual muscle tissue appears hypoechoic from the fluid or blood found within. Scarring, fibrosis, calcification, or chronic injury will change the tissue to appear denser or hyperechoic.17 Acute injury will appear hypoechoic from the inflammatory response and influx of blood. Tendon appears dense and hyperechoic with striations within the tissue, sometimes referred to as a horse’s tail.17 When torn, there will be a disassociation of the tissue with a hypoechoic region between the 2 ends. The attachment to the bone and muscle tissue should appear uniform. Hyperechoic areas within the tendon may be from calcification. Ligament appears similar to tendon but is more isoechoic and connects bone to bone. Evaluation of the entire length and the attachments to the bone are critical to evaluate for disease.
Bone appears bright hyperechoic, smooth, and flat, while hyaline cartilage is hypoechoic, smooth, and runs superiorly in a parallel pattern to its respective inferior cortical bone.17
Fibrocartilage is hyperechoic and typically triangularly shaped, such as in the glenohumeral labrum. Nerves appear fascicular and hypoechoic surrounded by hyperechoic epineurium.14
The epidermis and dermis are the most superficial structure on top of the screen, and are also hyperechoic.17
The Diagnostic Shoulder Examination
The proximal long head of the biceps tendon (LHBT) is the easiest structure in the shoulder to identify because of the anatomic structure, the bicipital groove. By keeping the arm relaxed, perpendicular to the ground, and in neutral rotation, the probe can be placed perpendicular to the arm over the proximal shoulder (Figure 1A).16-20 By finding the groove, the biceps tendon will usually be found resting within the groove (Figure 1B). This is the short axis view and is equivalent to an MRI in the axial plane.
The long axis view of the proximal biceps tendon is found by keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The user should be sure to visualize the entire tendon on the screen. If only part of the tendon is seen along only part of the screen, then the probe is oblique to the tendon. In this case, the probe area showing the tendon must be stabilized as the center or set point. The other part of the probe will then pivot until all of the tendon is seen on the screen. The MRI equivalent to the long axis of the proximal biceps tendon is the sagittal view.
Ultrasound is a dynamic evaluation. Moving the probe or moving the patient will change what and how something is imaged. The proximal biceps tendon is a good example of this concept. The bicipital groove is very deep proximally and flattens out as it travels distally to the mid-humerus. The examiner should continually adjust his or her hand/probe/patient position as well as depth/gain and other console functions to adapt to the dynamics of the scan. While keeping the bicep tendon in a short axis view, the tendon can be dynamically evaluated for subluxation by internally and externally rotating the arm.
To find the subscapularis, the arm remains in a neutral position with the hand supinated and the probe is held parallel with the ground. After finding the bicipital groove, the subscapularis tendon insertion is just medial to the groove (Figure 1B). By externally rotating the arm, the subscapularis tendon/muscle will come into a long axis view.16-20 The MRI equivalent to the long axis view of the subscapularis is the axial view. Dynamic testing can be done by internally and externally rotating the arm to evaluate for impingement of the subscapularis tendon as it slides underneath the coracoid process. To view the subscapularis tendon in short axis, the tendon is kept in the center of the screen/probe, and the probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The MRI equivalent is the sagittal view.
Some have recommended using the modified Crass or Middleton position to evaluate the supraspinatus, where the hand is in the “back pocket”.19 However, many patients with shoulder pain have trouble with this position. By resting the ipsilateral hand on the ipsilateral hip and then dropping the elbow, the supraspinatus insertion can still be brought out from under the acromion. This does bring the insertion anterior out of the scapular plane, so an adjustment is required in probe positioning to properly see the supraspinatus short and long axis. To find the long axis, the probe is placed parallel to a plane that spans the contralateral shoulder and ipsilateral hip (Figure 2A). The fibers of the supraspinatus should be inserting directly lateral to the humeral head without any intervening space (Figure 2B). If any space exists, a partial articular supraspinatus tendon avulsion (PASTA) lesion is present, and its thickness can be directly measured. Moving more posterior will show the flattening of the tuberosity and the fibers of the infraspinatus moving away from the humeral head—the bare spot. The MRI equivalent is the coronal view.
To view the supraspinatus tendon in short axis, maintain the arm in the same position, keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The probe should now be in a parallel plane between the ipsilateral shoulder and the contralateral hip. The biceps tendon in cross-section will be found anteriorly, and the articular cartilage will appear as a black layer over the bone. Dynamic testing includes placing the probe in a coronal plane between the acromion and greater tuberosity. When the patient abducts the arm while in internal rotation, the supraspinatus tendon will slide underneath the coracoacromial arch showing potential external impingement.15 The MRI equivalent is the sagittal plane.
The glenohumeral joint is best viewed posteriorly, limiting how much of the intra-articular portion of the joint can be imaged. The arm remains in a neutral position; palpate for the posterior acromion and place the probe just inferior to it, wedging up against it (Figure 3A). The glenohumeral joint will be seen by keeping the probe parallel to the ground (Figure 3B). The MRI equivalent is the axial plane. If a joint effusion exists, it can be seen in the posterior recess.15 A hyperechoic triangular region in between the humeral head and the glenoid will represent the glenoid labrum (Figure 3B). By internally and externally rotating the arm, the joint and labrum complex can be dynamically examined. From the labrum, scanning superior and medial can sometimes show the spinoglenoid notch where a paralabral cyst might be seen.15
Using the glenohumeral joint as a reference, the infraspinatus muscle is easily visualized. Maintaining the arm in neutral position with the probe over the glenohumeral joint, the infraspinatus will become apparent as it lays in long axis view superficially between the posterior deltoid and glenohumeral joint (Figure 3B).16-20 The teres minor lies just inferiorly. The MRI equivalent is the axial plane. To view the infraspinatus and teres minor in short axis, the probe is then rotated 90° on its center axis. The infraspinatus (superiorly) and teres minor (inferiorly) muscles will be visible in short axis within the infraspinatus fossa.15 The MRI equivalent is the sagittal view.
The acromioclavicular joint is superficial and easy to image. The arm remains in a neutral position, and we can palpate the joint for easy localization. The probe is placed anteriorly in a coronal plane over the acromion and clavicle. By scanning anteriorly and posteriorly, a joint effusion referred to as a Geyser sign might be seen. The MRI equivalent is the coronal view.
Available Certifications
The AIUM certification is a voluntary peer reviewed process that acknowledges that a practice is meeting national standards and aids in improving their respective MSK ultrasound protocols. They also provide guidelines on demonstrating training and competence on performing and/or interpreting diagnostic MSK examinations (Table).10 The ARDMS certification provides an actual individual certification referred to as “Registered” in MSK ultrasound.11 The physician must perform 150 diagnostic MSK ultrasound evaluations within 36 months of applying and pass a 200-question examination that is offered twice per year.11 None of these certifications are mandated by the American Medical Association (AMA) or American Osteopathic Association (AOA).
Maintenance and Continuing Medical Education (CME)
The AIUM recommends that a minimum of 50 diagnostic MSK ultrasound evaluations be performed per year for skill maintenance.10 Furthermore, 10 hours of AMA PRA Category 1 Credits™ or American Osteopathic Association Category 1-A Credits specific to MSK ultrasound must be completed by physicians performing and/or interpreting these examinations every 3 years.10 ARDMS recommends a minimum of 30 MSK ultrasound-specific CMEs in preparation for their “Registered” MSK evaluation.1
Conclusion
MSK ultrasound is a dynamic, real-time imaging modality that can improve cost efficiency and patient care. Its portability allows for its use anywhere. Learning the skill may seem daunting, but with the proper courses and education, the technology can be easily learned. By correlating a known modality like MRI, the user will easily begin to read ultrasound images. No current certification is needed to use or bill for ultrasound, but various institutions are developing criteria and testing. Two organizations, AIUM and ARDMS, provide guidelines and certifications to demonstrate competency, which may become necessary in the very near future.
The musculoskeletal (MSK) ultrasound evaluation of the shoulder provides a cost- and time-efficient imaging modality with similar diagnostic power as magnetic resonance imaging (MRI).1,2 Its portable point-of-care applications can be used in the office, in the operating room, and in sideline athletic event coverage, as we discussed in Part 1 of this series.3
MSK ultrasound may seem difficult and daunting, and many articles have quoted steep learning curves.4,5 However, in our experience in teaching many ultrasound courses, this modality can be learned quite quickly with the proper instruction. Physicians are already familiar with anatomy and usually have had some exposure to MRI.4 Taking courses in MSK ultrasound or simply learning the basic concepts of ultrasound and then learning the machine controls is usually a good start.5-8 Practice scanning normal individuals, comparing the images from an MRI to learn how to reproduce the same planes and images. This will allow the user to become familiar with normal anatomy and how to see the images on the ultrasound screen.5-8 Vollman and colleagues9 showed that in trainees, combining MRI images with sonograms enhances the ability to correctly identify MSK ultrasound anatomy from 40.9% to 72.5%, when compared with learning from ultrasound images alone.
There are currently no certifications necessary to perform ultrasound scans or bill for them; however, some insurance carriers may require demonstrating relevant, documented training for reimbursement.3 Various organizations are trying to develop certifications and regulations for ultrasound to standardize the use of this modality. In the United States, the American Institute of Ultrasound in Medicine (AIUM) and the American Registry for Diagnostic Medical Sonography (ARDMS) provide guidelines and particular MSK ultrasound certifications.10,11
Basic Ultrasound Principles
The ultrasound machine creates electrical impulses that are turned into sound waves by piezoelectric crystals at the probe’s footprint. These sound waves bounce off tissues and return to the probe, where they are converted electronically to an image on the monitor. Depending on the echogenicity of the scanned tissue, the ultrasound beam will either reflect or be absorbed at different rates. This variance is transmitted on the monitor as a grayscale image. When ultrasound waves are highly reflective, like in bone or fat, they are characterized as hyperechoic. The opposite occurs when ultrasound waves are absorbed like in the fluid of a cystic cavity or joint effusion, and the image appears black. This is described as anechoic.12 Intermediate tissues such as tendons that are less reflective are seen as hypoechoic and appear gray. When a tissue has a similar echogenicity to its surrounding tissues, it is called isoechoic.12
The transducer is the scanning component of the ultrasound machine. Transducers come in 2 shapes: linear and curvilinear. The linear probe creates a straight image that is equal to the size of the transducer footprint. The curvilinear probe creates a wider, wedge-shaped panoramic image.
Linear probes are of higher frequency and generate higher resolution images of shallower structures, while curvilinear probes have greater depth penetration but generate lower resolution images. A high frequency of 10 to 15 MHz is preferred for anatomy between 2 cm to 4 cm depth.13 Midrange frequency of 5 to 10 MHz is preferred at 5 cm to 6 cm depth, and low-frequency 2 to 5 MHz probes are preferred for anatomical structures >6 cm depth.13
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. This anisotropic effect is dependent on the angle of the insonating beam. The maximum return echo occurs when the ultrasound beam is perpendicular to the tendon. Decreasing the insonating angle on a normal tendon will cause it to change from brightly hyperechoic (the actual echo from tightly bound tendon fibers) to darkly hypoechoic. If the angle is then increased, the tendon will again appear hyperechoic. If the artifact causes a normal tendon to appear hypoechoic, it may falsely lead to a diagnosis of tendinosis or tear.
Posterior acoustic shadowing is present when a hyperechoic structure reflects the ultrasound beam so much that it creates a dark shadow underneath it.12,14 This phenomenon is possible since the ultrasound beam cannot penetrate the hyperechoic structure and reflects off its inferior tissues. Reverberation is when the beam is repeated back and forth between 2 parallel highly reflective surfaces. The initial reflection will be displayed correctly, while the subsequent ultrasound waves will be delayed and appear at a farther distance from the transducer.12,14
The point where the beam is at its narrowest point generates the section of the image that is best visualized.15 This is called the focal zone, and it can be adjusted to highlight the desired area of evaluation. Gain controls adjust the amount of black, gray, and white on the monitor and can be adjusted to focus the desired image.13 Depth settings are fundamental in finding the desired targets. It is recommended to start with a higher depth setting to get an overview and progressively decrease the depth to key in on the desired anatomy.13 Color Doppler can be used to view movement within structures and to identify vessels, synovitis, and neovascularization in tendinopathy.13
Ultrasound of the Shoulder
Patients should be seated, if possible, on a rotating seat. The examiner’s shoulder should be higher than the patient’s shoulder.16 The user holds the ultrasound probe between the thumb and index fingers while resting the hypothenar eminence on the patient to serve as a fulcrum and steadying force. The examination should take 5 to 15 minutes, depending on the examiner’s expertise and the amount of anatomy being scanned.
Examining the body requires knowledge of anatomy. The examination and accuracy are determined by the technician using the probe. The probe can be angled any direction and be placed obliquely on the subject. The advantage here is that anatomy in the human body is not always planar. Muscles and tissues can run obliquely or even perpendicular to each other. When evaluating anatomy, the examiner should keep in mind what structure he or she is looking for; where it should be found; what landmarks can be used to easily locate it; what orientation it has; and what the normal anatomy should look like.
Muscle appears as a lattice with larger areas of hypoechoic muscle tissue and hyperechoic fascial perimysium layers traversing through it.17 The actual muscle tissue appears hypoechoic from the fluid or blood found within. Scarring, fibrosis, calcification, or chronic injury will change the tissue to appear denser or hyperechoic.17 Acute injury will appear hypoechoic from the inflammatory response and influx of blood. Tendon appears dense and hyperechoic with striations within the tissue, sometimes referred to as a horse’s tail.17 When torn, there will be a disassociation of the tissue with a hypoechoic region between the 2 ends. The attachment to the bone and muscle tissue should appear uniform. Hyperechoic areas within the tendon may be from calcification. Ligament appears similar to tendon but is more isoechoic and connects bone to bone. Evaluation of the entire length and the attachments to the bone are critical to evaluate for disease.
Bone appears bright hyperechoic, smooth, and flat, while hyaline cartilage is hypoechoic, smooth, and runs superiorly in a parallel pattern to its respective inferior cortical bone.17
Fibrocartilage is hyperechoic and typically triangularly shaped, such as in the glenohumeral labrum. Nerves appear fascicular and hypoechoic surrounded by hyperechoic epineurium.14
The epidermis and dermis are the most superficial structure on top of the screen, and are also hyperechoic.17
The Diagnostic Shoulder Examination
The proximal long head of the biceps tendon (LHBT) is the easiest structure in the shoulder to identify because of the anatomic structure, the bicipital groove. By keeping the arm relaxed, perpendicular to the ground, and in neutral rotation, the probe can be placed perpendicular to the arm over the proximal shoulder (Figure 1A).16-20 By finding the groove, the biceps tendon will usually be found resting within the groove (Figure 1B). This is the short axis view and is equivalent to an MRI in the axial plane.
The long axis view of the proximal biceps tendon is found by keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The user should be sure to visualize the entire tendon on the screen. If only part of the tendon is seen along only part of the screen, then the probe is oblique to the tendon. In this case, the probe area showing the tendon must be stabilized as the center or set point. The other part of the probe will then pivot until all of the tendon is seen on the screen. The MRI equivalent to the long axis of the proximal biceps tendon is the sagittal view.
Ultrasound is a dynamic evaluation. Moving the probe or moving the patient will change what and how something is imaged. The proximal biceps tendon is a good example of this concept. The bicipital groove is very deep proximally and flattens out as it travels distally to the mid-humerus. The examiner should continually adjust his or her hand/probe/patient position as well as depth/gain and other console functions to adapt to the dynamics of the scan. While keeping the bicep tendon in a short axis view, the tendon can be dynamically evaluated for subluxation by internally and externally rotating the arm.
To find the subscapularis, the arm remains in a neutral position with the hand supinated and the probe is held parallel with the ground. After finding the bicipital groove, the subscapularis tendon insertion is just medial to the groove (Figure 1B). By externally rotating the arm, the subscapularis tendon/muscle will come into a long axis view.16-20 The MRI equivalent to the long axis view of the subscapularis is the axial view. Dynamic testing can be done by internally and externally rotating the arm to evaluate for impingement of the subscapularis tendon as it slides underneath the coracoid process. To view the subscapularis tendon in short axis, the tendon is kept in the center of the screen/probe, and the probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The MRI equivalent is the sagittal view.
Some have recommended using the modified Crass or Middleton position to evaluate the supraspinatus, where the hand is in the “back pocket”.19 However, many patients with shoulder pain have trouble with this position. By resting the ipsilateral hand on the ipsilateral hip and then dropping the elbow, the supraspinatus insertion can still be brought out from under the acromion. This does bring the insertion anterior out of the scapular plane, so an adjustment is required in probe positioning to properly see the supraspinatus short and long axis. To find the long axis, the probe is placed parallel to a plane that spans the contralateral shoulder and ipsilateral hip (Figure 2A). The fibers of the supraspinatus should be inserting directly lateral to the humeral head without any intervening space (Figure 2B). If any space exists, a partial articular supraspinatus tendon avulsion (PASTA) lesion is present, and its thickness can be directly measured. Moving more posterior will show the flattening of the tuberosity and the fibers of the infraspinatus moving away from the humeral head—the bare spot. The MRI equivalent is the coronal view.
To view the supraspinatus tendon in short axis, maintain the arm in the same position, keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The probe should now be in a parallel plane between the ipsilateral shoulder and the contralateral hip. The biceps tendon in cross-section will be found anteriorly, and the articular cartilage will appear as a black layer over the bone. Dynamic testing includes placing the probe in a coronal plane between the acromion and greater tuberosity. When the patient abducts the arm while in internal rotation, the supraspinatus tendon will slide underneath the coracoacromial arch showing potential external impingement.15 The MRI equivalent is the sagittal plane.
The glenohumeral joint is best viewed posteriorly, limiting how much of the intra-articular portion of the joint can be imaged. The arm remains in a neutral position; palpate for the posterior acromion and place the probe just inferior to it, wedging up against it (Figure 3A). The glenohumeral joint will be seen by keeping the probe parallel to the ground (Figure 3B). The MRI equivalent is the axial plane. If a joint effusion exists, it can be seen in the posterior recess.15 A hyperechoic triangular region in between the humeral head and the glenoid will represent the glenoid labrum (Figure 3B). By internally and externally rotating the arm, the joint and labrum complex can be dynamically examined. From the labrum, scanning superior and medial can sometimes show the spinoglenoid notch where a paralabral cyst might be seen.15
Using the glenohumeral joint as a reference, the infraspinatus muscle is easily visualized. Maintaining the arm in neutral position with the probe over the glenohumeral joint, the infraspinatus will become apparent as it lays in long axis view superficially between the posterior deltoid and glenohumeral joint (Figure 3B).16-20 The teres minor lies just inferiorly. The MRI equivalent is the axial plane. To view the infraspinatus and teres minor in short axis, the probe is then rotated 90° on its center axis. The infraspinatus (superiorly) and teres minor (inferiorly) muscles will be visible in short axis within the infraspinatus fossa.15 The MRI equivalent is the sagittal view.
The acromioclavicular joint is superficial and easy to image. The arm remains in a neutral position, and we can palpate the joint for easy localization. The probe is placed anteriorly in a coronal plane over the acromion and clavicle. By scanning anteriorly and posteriorly, a joint effusion referred to as a Geyser sign might be seen. The MRI equivalent is the coronal view.
Available Certifications
The AIUM certification is a voluntary peer reviewed process that acknowledges that a practice is meeting national standards and aids in improving their respective MSK ultrasound protocols. They also provide guidelines on demonstrating training and competence on performing and/or interpreting diagnostic MSK examinations (Table).10 The ARDMS certification provides an actual individual certification referred to as “Registered” in MSK ultrasound.11 The physician must perform 150 diagnostic MSK ultrasound evaluations within 36 months of applying and pass a 200-question examination that is offered twice per year.11 None of these certifications are mandated by the American Medical Association (AMA) or American Osteopathic Association (AOA).
Maintenance and Continuing Medical Education (CME)
The AIUM recommends that a minimum of 50 diagnostic MSK ultrasound evaluations be performed per year for skill maintenance.10 Furthermore, 10 hours of AMA PRA Category 1 Credits™ or American Osteopathic Association Category 1-A Credits specific to MSK ultrasound must be completed by physicians performing and/or interpreting these examinations every 3 years.10 ARDMS recommends a minimum of 30 MSK ultrasound-specific CMEs in preparation for their “Registered” MSK evaluation.1
Conclusion
MSK ultrasound is a dynamic, real-time imaging modality that can improve cost efficiency and patient care. Its portability allows for its use anywhere. Learning the skill may seem daunting, but with the proper courses and education, the technology can be easily learned. By correlating a known modality like MRI, the user will easily begin to read ultrasound images. No current certification is needed to use or bill for ultrasound, but various institutions are developing criteria and testing. Two organizations, AIUM and ARDMS, provide guidelines and certifications to demonstrate competency, which may become necessary in the very near future.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Roy J-S, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis [published online ahead of print February 11, 2015]. Br J Sports Med. doi:10.1136/bjsports-2014-094148.
3. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
4. Hama M, Takase K, Ihata A, et al. Challenges to expanding the clinical application of musculoskeletal ultrasonography (MSUS) among rheumatologists: from a second survey in Japan. Mod Rheumatol. 2012;2:202-208.
5. Smith MJ, Rogers A, Amso N, Kennedy J, Hall A, Mullaney P. A training, assessment and feedback package for the trainee shoulder sonographer. Ultrasound. 2015;23(1):29-41.
6. Delzell PB, Boyle A, Schneider E. Dedicated training program for shoulder sonography: the results of a quality program reverberate with everyone. J Ultrasound Med. 2015;34(6):1037-1042.
7. Finnoff JT, Berkoff D, Brennan F, et al. American Medical Society for Sports Medicine (AMSSM) recommended sports ultrasound curriculum for sports medicine fellowships. PM R. 2015;7(2)e1-e11.
8. Adelman S, Fishman P. Use of portable ultrasound machine for outpatient orthopedic diagnosis: an implementation study. Perm J. 2013;17(3):18-22.
9. Vollman A, Hulen R, Dulchavsky S, et al. Educational benefits of fusing magnetic resonance imaging with sonograms. J Clin Ultrasound. 2014;42(5) 257-263.
10. Training guidelines for physicians and chiropractors who evaluate and interpret diagnostic musculoskeletal ultrasound examinations. Laurel, MD: American Institute of Ultrasound in Medicine; 2014. http://www.aium.org/resources/viewStatement.aspx?id=51. Accessed February 26, 2016.
11. Registered in musculoskeletal (RMSK) sonography. American Registry for Diagnostic Medical Sonography Web site. http://www.ardms.org/get-certified/RMSK/Pages/RMSK.aspx. Accessed February 26, 2016.
12. Silkowski C. Ultrasound nomenclature, image orientation, and basic instrumentation. In: Abraham D, Silkowski C, Odwin C, eds. Emergency Medicine Sonography Pocket Guide to Sonographic Anatomy and Pathology. Sudbury, MA: Jones and Bartlett; 2010:1-24.
13. Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4(3):55-62.
14. Taljanovic MS, Melville DM, Scalcione LR, Gimber LH, Lorenz EJ, Witte RS. Artifacts in musculoskeletal ultrasonography. Semin Musculoskelet Radiol. 2014;18(1):3-11.
15. Ng A, Swanevelder J. Resolution in ultrasound imaging. Continuing Educ Anaesth Crit Care Pain. 2011;11(5):186-192. http://ceaccp.oxfordjournals.org/content/11/5/186.full. Accessed March 3, 2016.
16. Nazarian L, Bohm-Velez M, Kan JH, et al. AIUM practice parameters for the performance of a musculoskeletal ultrasound examination. Laurel, MD: American Institute of Ultrasound in Medicine; 2012. http://www.aium.org/resources/guidelines/musculoskeletal.pdf. Accessed February 26, 2016.
17. Jacobson J. Fundamentals of Musculoskeletal Ultrasound. 2nd edition. Philadelphia, PA: Elsevier Saunders; 2013.
18. The Ultrasound Subcommittee of the European Society of Musculoskeletal Radiology. Musculoskeletal ultrasound: technique guidelines. Insights Imaging. 2010;1:99-141.
19. Corazza A, Orlandi D, Fabbro E, et al. Dynamic high-resolution ultrasound of the shoulder: how we do it. Eur J Radiol. 2015;84(2):266-277.
20. Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease processes. Eur J Radiol. 2008;68(1):137-146
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Roy J-S, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis [published online ahead of print February 11, 2015]. Br J Sports Med. doi:10.1136/bjsports-2014-094148.
3. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
4. Hama M, Takase K, Ihata A, et al. Challenges to expanding the clinical application of musculoskeletal ultrasonography (MSUS) among rheumatologists: from a second survey in Japan. Mod Rheumatol. 2012;2:202-208.
5. Smith MJ, Rogers A, Amso N, Kennedy J, Hall A, Mullaney P. A training, assessment and feedback package for the trainee shoulder sonographer. Ultrasound. 2015;23(1):29-41.
6. Delzell PB, Boyle A, Schneider E. Dedicated training program for shoulder sonography: the results of a quality program reverberate with everyone. J Ultrasound Med. 2015;34(6):1037-1042.
7. Finnoff JT, Berkoff D, Brennan F, et al. American Medical Society for Sports Medicine (AMSSM) recommended sports ultrasound curriculum for sports medicine fellowships. PM R. 2015;7(2)e1-e11.
8. Adelman S, Fishman P. Use of portable ultrasound machine for outpatient orthopedic diagnosis: an implementation study. Perm J. 2013;17(3):18-22.
9. Vollman A, Hulen R, Dulchavsky S, et al. Educational benefits of fusing magnetic resonance imaging with sonograms. J Clin Ultrasound. 2014;42(5) 257-263.
10. Training guidelines for physicians and chiropractors who evaluate and interpret diagnostic musculoskeletal ultrasound examinations. Laurel, MD: American Institute of Ultrasound in Medicine; 2014. http://www.aium.org/resources/viewStatement.aspx?id=51. Accessed February 26, 2016.
11. Registered in musculoskeletal (RMSK) sonography. American Registry for Diagnostic Medical Sonography Web site. http://www.ardms.org/get-certified/RMSK/Pages/RMSK.aspx. Accessed February 26, 2016.
12. Silkowski C. Ultrasound nomenclature, image orientation, and basic instrumentation. In: Abraham D, Silkowski C, Odwin C, eds. Emergency Medicine Sonography Pocket Guide to Sonographic Anatomy and Pathology. Sudbury, MA: Jones and Bartlett; 2010:1-24.
13. Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4(3):55-62.
14. Taljanovic MS, Melville DM, Scalcione LR, Gimber LH, Lorenz EJ, Witte RS. Artifacts in musculoskeletal ultrasonography. Semin Musculoskelet Radiol. 2014;18(1):3-11.
15. Ng A, Swanevelder J. Resolution in ultrasound imaging. Continuing Educ Anaesth Crit Care Pain. 2011;11(5):186-192. http://ceaccp.oxfordjournals.org/content/11/5/186.full. Accessed March 3, 2016.
16. Nazarian L, Bohm-Velez M, Kan JH, et al. AIUM practice parameters for the performance of a musculoskeletal ultrasound examination. Laurel, MD: American Institute of Ultrasound in Medicine; 2012. http://www.aium.org/resources/guidelines/musculoskeletal.pdf. Accessed February 26, 2016.
17. Jacobson J. Fundamentals of Musculoskeletal Ultrasound. 2nd edition. Philadelphia, PA: Elsevier Saunders; 2013.
18. The Ultrasound Subcommittee of the European Society of Musculoskeletal Radiology. Musculoskeletal ultrasound: technique guidelines. Insights Imaging. 2010;1:99-141.
19. Corazza A, Orlandi D, Fabbro E, et al. Dynamic high-resolution ultrasound of the shoulder: how we do it. Eur J Radiol. 2015;84(2):266-277.
20. Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease processes. Eur J Radiol. 2008;68(1):137-146
Management of the Biconcave (B2) Glenoid in Shoulder Arthroplasty: Technical Considerations
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.