User login
Debunking Melanoma Myths: Do Sunscreens Cause Cancer?
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Debunking Psoriasis Myths: Do Psoriasis Therapies Cause Depression?
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
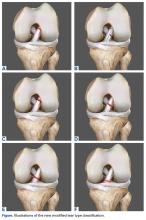
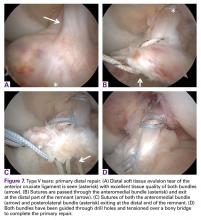
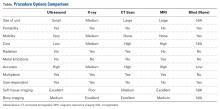
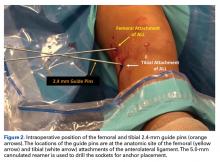
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
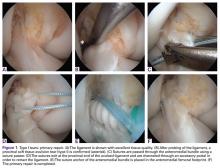
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
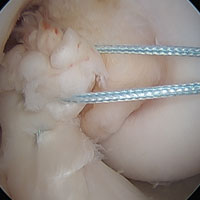
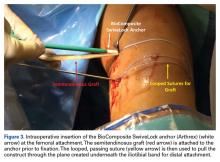
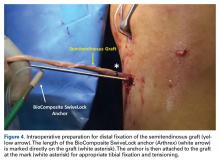
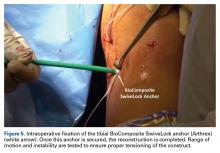
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
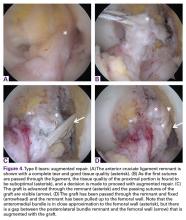
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
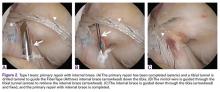
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
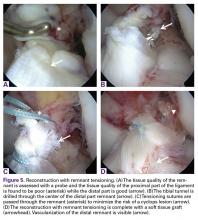
Type III Tears: Reconstruction With Remnant Tensioning
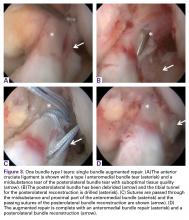
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
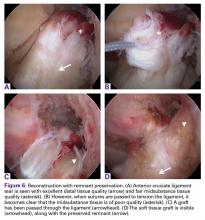
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
Preservation of the Anterior Cruciate Ligament: Surgical Techniques
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
A Guide to Ultrasound of the Shoulder, Part 3: Interventional and Procedural Uses
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
Ultrasound-Guided Percutaneous Reconstruction of the Anterolateral Ligament: Surgical Technique and Case Report
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
Periocular Fillers and Related Anatomy
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18
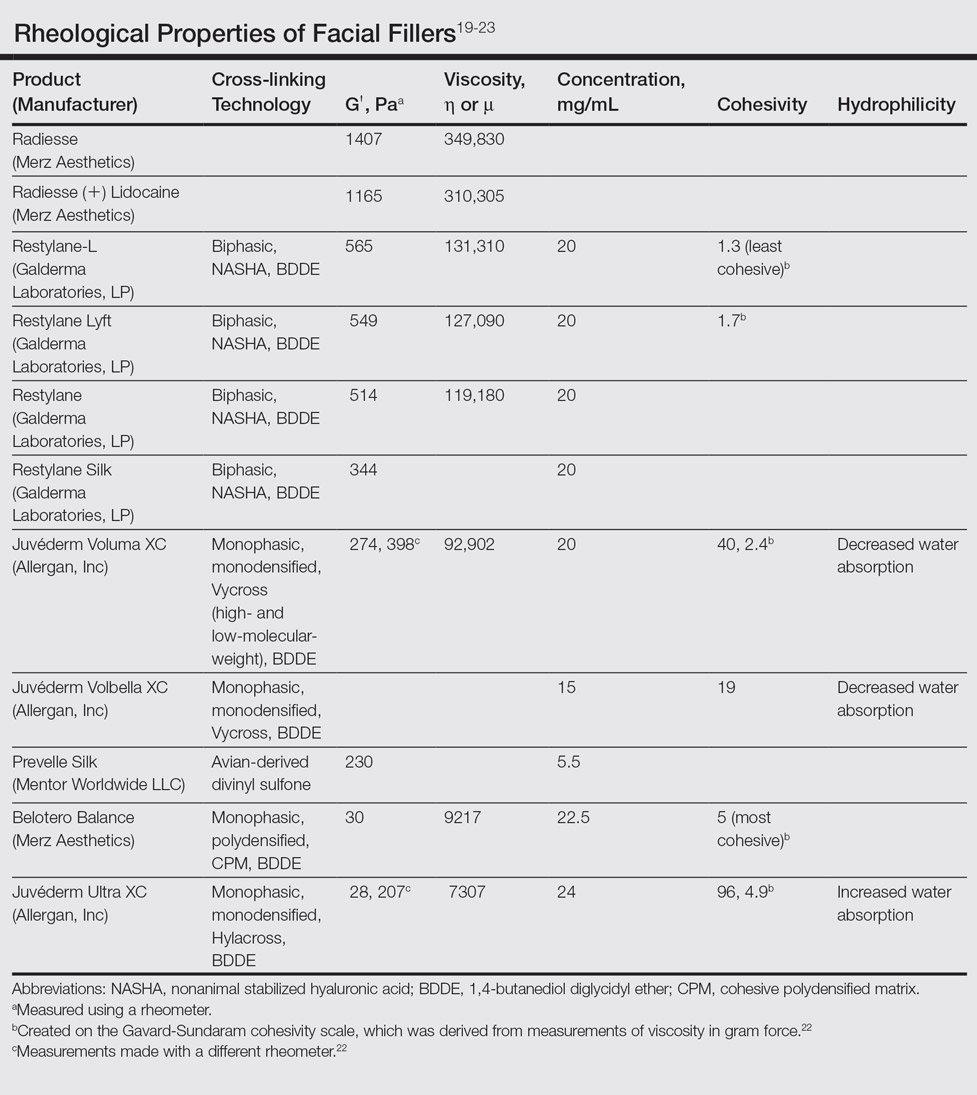
Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.
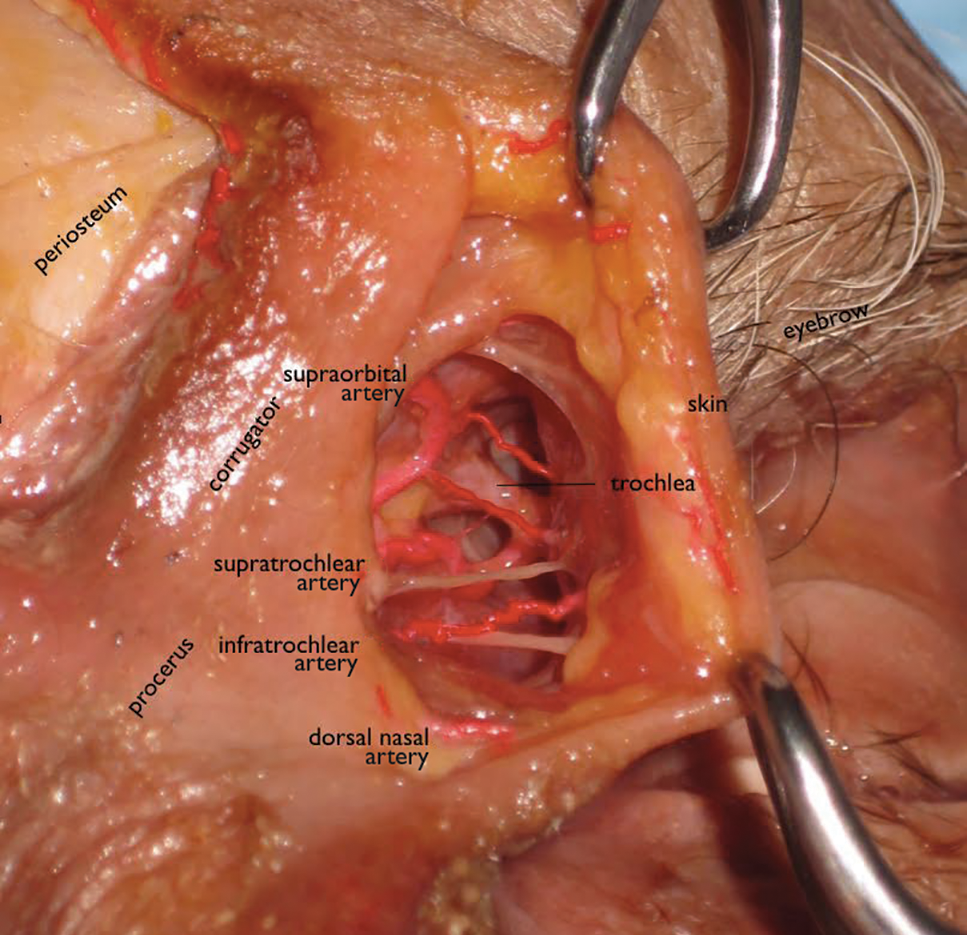
Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).
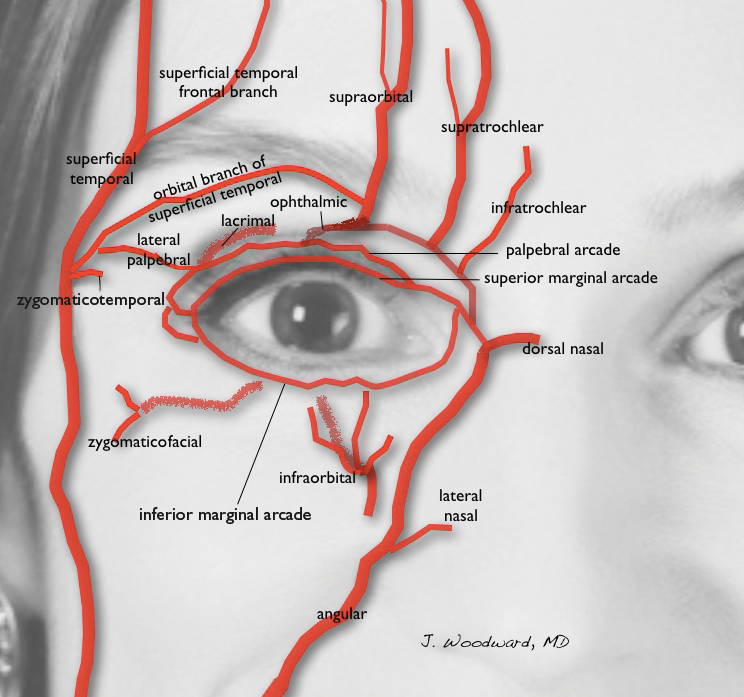
Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Practice Points
- When performing periocular dermal injections, physicians should understand the complicated anatomy surrounding the eyes and related changes with upper face aging.
- The different rheological properties of facial fillers impact product selection for various areas of the upper face.
- Physicians should be aware of the anatomical danger zones to avoid intravascular embolization.
Debunking Psoriasis Myths: Does UVB Phototherapy Cause Skin Cancer?
Myth: UVB phototherapy causes skin cancer
Phototherapy is a common treatment modality for psoriasis patients that can be used in the physician’s office or psoriasis clinic or at home. Options include UVB phototherapy (broadband and narrowband), which slows the growth of affected skin cells; psoralen plus UVA (PUVA), which slows excessive skin cell growth; and excimer laser therapy, which targets select areas of the skin affected by mild to moderate psoriasis and is particularly useful for scalp psoriasis. Each of these therapies may be combined with other topical and/or systemic psoriasis treatments. The effects of UV light on the skin and the connection to skin cancer is widely known. Therefore, patient education on the risk for skin cancer with phototherapy is essential.
Evidence suggests that UVB phototherapy remains a safe treatment modality. In a 2005 analysis of prospective and retrospective studies on skin cancer risk from UVB phototherapy, 11 studies (10 concerning psoriasis patients) were reviewed and the researchers concluded that all studies eventually showed no increased skin cancer risk with UVB phototherapy. One of the PUVA cohort studies examined genital skin cancers and found an increased rate of genital tumors associated with UVB phototherapy.
Another analysis to define the long-term carcinogenic risk for narrowband UVB treatment found that there was no association between narrowband UVB exposure alone (without PUVA) and any skin cancer. For patients treated with narrowband UVB and PUVA, there was a small increase in basal cell carcinomas.
Dermatologists should monitor psoriasis patients for self-administered treatment with tanning beds. Based on a questionnaire sent to approximately 14,000 subscribers of National Psoriasis Foundation emails, 62% of 617 tanners started tanning to treat psoriasis; they were more likely to have received medical phototherapy and had more severe psoriasis. Approximately 30% of these patients indicated that they used tanning as a self-treatment for psoriasis because of the inconvenience and cost of UV light treatment in a physician’s office as well as treatment failure of other therapies prescribed by the physician. “Our results imply that tanning bed usage among psoriasis sufferers is widespread and linked with tanning addiction,” reported Felton et al. “Practitioners should be particularly vigilant to the possibility of tanning bed usage in at-risk patients.” These patients may be at increased risk for skin cancer. Problematic tanning behaviors may be seen in younger female patients diagnosed with psoriasis at an early age as well as patients with severe psoriasis who were previously prescribed phototherapy treatment.
Expert Commentary on next page
Expert Commentary
UVB phototherapy is an effective therapy that does not increase the risk of nonmelanoma skin cancers (NMSCs), according to the 2 analyses mentioned above. When I discuss the risks and benefits of UVB phototherapy with psoriasis patients, I do say that there is a theoretical increased risk for NMSC but that the 2005 study mentioned above does not indicate an increased risk. However, UVB phototherapy and cyclosporine should not be combined, as this combination does increase the risk for NMSC.
Psoralen plus UVA definitely will increase the risk for NMSC, particularly squamous cell carcinoma. However, in this age of the biologics, PUVA use has fallen out of favor, partly due to the increased risk for NMSC, and many patients will not encounter dermatology practices that still use PUVA.
—Jashin J. Wu, MD (Los Angeles, California)
Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
Hearn RM, Kerr AC, Rahim KF, et al. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159:931-935.
Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44:355-360.
Phototherapy. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/treatments/phototherapy . Accessed October 4, 2016.
Myth: UVB phototherapy causes skin cancer
Phototherapy is a common treatment modality for psoriasis patients that can be used in the physician’s office or psoriasis clinic or at home. Options include UVB phototherapy (broadband and narrowband), which slows the growth of affected skin cells; psoralen plus UVA (PUVA), which slows excessive skin cell growth; and excimer laser therapy, which targets select areas of the skin affected by mild to moderate psoriasis and is particularly useful for scalp psoriasis. Each of these therapies may be combined with other topical and/or systemic psoriasis treatments. The effects of UV light on the skin and the connection to skin cancer is widely known. Therefore, patient education on the risk for skin cancer with phototherapy is essential.
Evidence suggests that UVB phototherapy remains a safe treatment modality. In a 2005 analysis of prospective and retrospective studies on skin cancer risk from UVB phototherapy, 11 studies (10 concerning psoriasis patients) were reviewed and the researchers concluded that all studies eventually showed no increased skin cancer risk with UVB phototherapy. One of the PUVA cohort studies examined genital skin cancers and found an increased rate of genital tumors associated with UVB phototherapy.
Another analysis to define the long-term carcinogenic risk for narrowband UVB treatment found that there was no association between narrowband UVB exposure alone (without PUVA) and any skin cancer. For patients treated with narrowband UVB and PUVA, there was a small increase in basal cell carcinomas.
Dermatologists should monitor psoriasis patients for self-administered treatment with tanning beds. Based on a questionnaire sent to approximately 14,000 subscribers of National Psoriasis Foundation emails, 62% of 617 tanners started tanning to treat psoriasis; they were more likely to have received medical phototherapy and had more severe psoriasis. Approximately 30% of these patients indicated that they used tanning as a self-treatment for psoriasis because of the inconvenience and cost of UV light treatment in a physician’s office as well as treatment failure of other therapies prescribed by the physician. “Our results imply that tanning bed usage among psoriasis sufferers is widespread and linked with tanning addiction,” reported Felton et al. “Practitioners should be particularly vigilant to the possibility of tanning bed usage in at-risk patients.” These patients may be at increased risk for skin cancer. Problematic tanning behaviors may be seen in younger female patients diagnosed with psoriasis at an early age as well as patients with severe psoriasis who were previously prescribed phototherapy treatment.
Expert Commentary on next page
Expert Commentary
UVB phototherapy is an effective therapy that does not increase the risk of nonmelanoma skin cancers (NMSCs), according to the 2 analyses mentioned above. When I discuss the risks and benefits of UVB phototherapy with psoriasis patients, I do say that there is a theoretical increased risk for NMSC but that the 2005 study mentioned above does not indicate an increased risk. However, UVB phototherapy and cyclosporine should not be combined, as this combination does increase the risk for NMSC.
Psoralen plus UVA definitely will increase the risk for NMSC, particularly squamous cell carcinoma. However, in this age of the biologics, PUVA use has fallen out of favor, partly due to the increased risk for NMSC, and many patients will not encounter dermatology practices that still use PUVA.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: UVB phototherapy causes skin cancer
Phototherapy is a common treatment modality for psoriasis patients that can be used in the physician’s office or psoriasis clinic or at home. Options include UVB phototherapy (broadband and narrowband), which slows the growth of affected skin cells; psoralen plus UVA (PUVA), which slows excessive skin cell growth; and excimer laser therapy, which targets select areas of the skin affected by mild to moderate psoriasis and is particularly useful for scalp psoriasis. Each of these therapies may be combined with other topical and/or systemic psoriasis treatments. The effects of UV light on the skin and the connection to skin cancer is widely known. Therefore, patient education on the risk for skin cancer with phototherapy is essential.
Evidence suggests that UVB phototherapy remains a safe treatment modality. In a 2005 analysis of prospective and retrospective studies on skin cancer risk from UVB phototherapy, 11 studies (10 concerning psoriasis patients) were reviewed and the researchers concluded that all studies eventually showed no increased skin cancer risk with UVB phototherapy. One of the PUVA cohort studies examined genital skin cancers and found an increased rate of genital tumors associated with UVB phototherapy.
Another analysis to define the long-term carcinogenic risk for narrowband UVB treatment found that there was no association between narrowband UVB exposure alone (without PUVA) and any skin cancer. For patients treated with narrowband UVB and PUVA, there was a small increase in basal cell carcinomas.
Dermatologists should monitor psoriasis patients for self-administered treatment with tanning beds. Based on a questionnaire sent to approximately 14,000 subscribers of National Psoriasis Foundation emails, 62% of 617 tanners started tanning to treat psoriasis; they were more likely to have received medical phototherapy and had more severe psoriasis. Approximately 30% of these patients indicated that they used tanning as a self-treatment for psoriasis because of the inconvenience and cost of UV light treatment in a physician’s office as well as treatment failure of other therapies prescribed by the physician. “Our results imply that tanning bed usage among psoriasis sufferers is widespread and linked with tanning addiction,” reported Felton et al. “Practitioners should be particularly vigilant to the possibility of tanning bed usage in at-risk patients.” These patients may be at increased risk for skin cancer. Problematic tanning behaviors may be seen in younger female patients diagnosed with psoriasis at an early age as well as patients with severe psoriasis who were previously prescribed phototherapy treatment.
Expert Commentary on next page
Expert Commentary
UVB phototherapy is an effective therapy that does not increase the risk of nonmelanoma skin cancers (NMSCs), according to the 2 analyses mentioned above. When I discuss the risks and benefits of UVB phototherapy with psoriasis patients, I do say that there is a theoretical increased risk for NMSC but that the 2005 study mentioned above does not indicate an increased risk. However, UVB phototherapy and cyclosporine should not be combined, as this combination does increase the risk for NMSC.
Psoralen plus UVA definitely will increase the risk for NMSC, particularly squamous cell carcinoma. However, in this age of the biologics, PUVA use has fallen out of favor, partly due to the increased risk for NMSC, and many patients will not encounter dermatology practices that still use PUVA.
—Jashin J. Wu, MD (Los Angeles, California)
Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
Hearn RM, Kerr AC, Rahim KF, et al. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159:931-935.
Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44:355-360.
Phototherapy. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/treatments/phototherapy . Accessed October 4, 2016.
Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
Hearn RM, Kerr AC, Rahim KF, et al. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159:931-935.
Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44:355-360.
Phototherapy. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/treatments/phototherapy . Accessed October 4, 2016.
Presenting Treatment Safety Data: Subjective Interpretations of Objective Information
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.
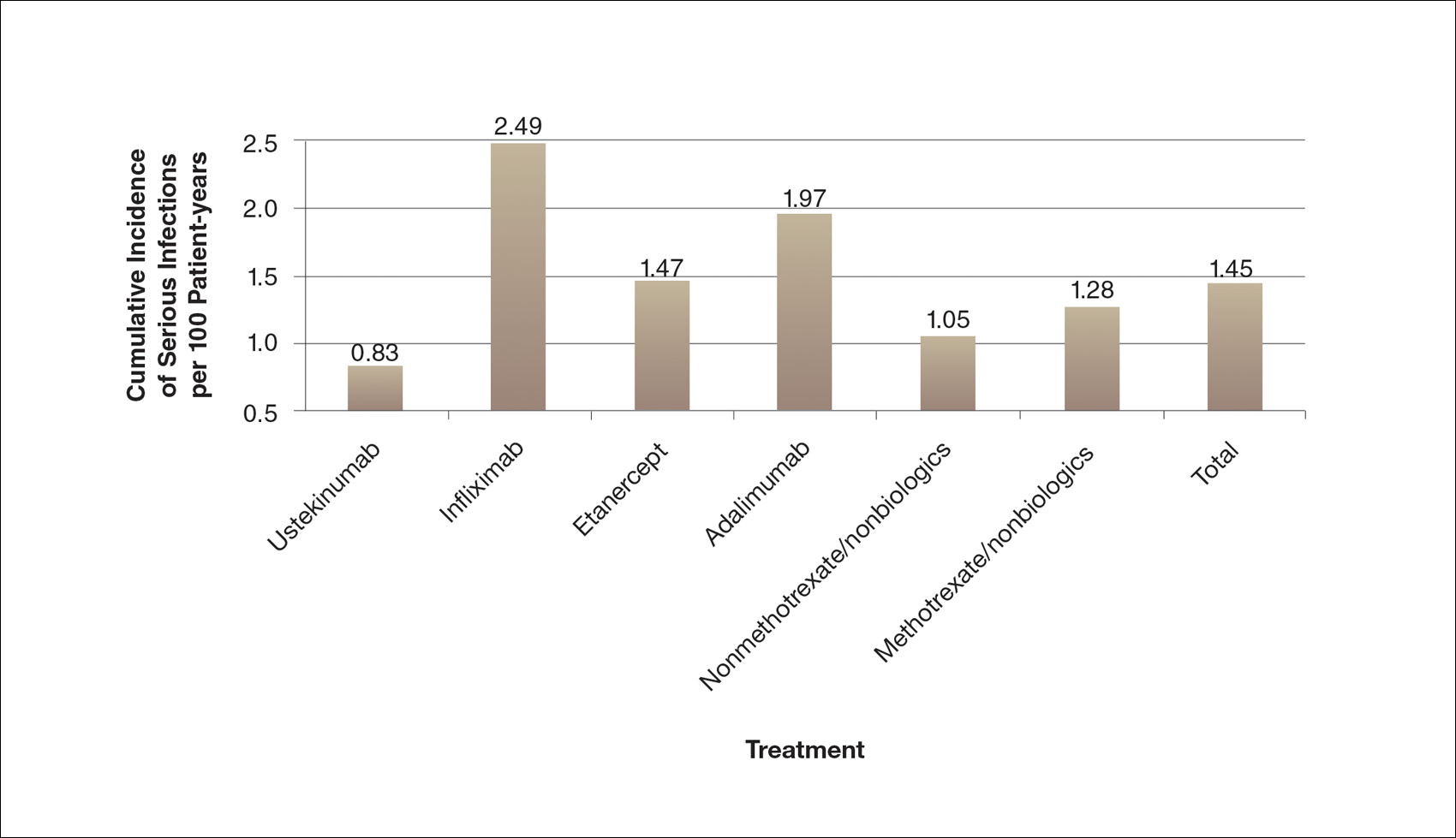
A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.
Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
Practice Points
- Physicians can guide patients’ perceptions of drug safety by the way safety data are presented.
- For patients who are concerned about rare treatment risks, presenting data on the patients who have not experienced adverse effects can be reassuring.
A Modified Levering Technique for Removing a Broken Solid Intramedullary Tibial Nail: A Technical Tip
In both elective and revision surgery, removal of retained hardware can be unpredictable. Broken hardware, whether identified before or during surgery, presents a significant challenge. Cases often require enlisting a large variety of equipment and techniques that often result in larger dissection and potential for wider soft-tissue or bony destruction. Broken intramedullary devices, located entirely within the cortices of bone, pose unique challenges.1,2 Various techniques have been used to remove broken cannulated nails.1-9 There is, however, a paucity of techniques for removing broken solid nails from within the tibia.1,2 Moreover, many of these techniques require significant metaphyseal and cortical bone destruction that may compromise the integrity of the long bone.1,3,9 In this article, we describe a modified technique for removal of a broken solid nail, with minimal cortical bone destruction, in the setting of a tibial nonunion.
Technique
A 23-year-old man presented with a symptomatic valgus nonunion of the tibia, which had been treated with a solid intramedullary 9-mm nail (Orthofix). The patient was taken to the operative theater for nonunion takedown and exchanged reamed intramedullary nailing. The proximal fragment of the anterograde intramedullary nail was removed in standard fashion using the Winquist Universal Extraction Set (Shukla Medical). When threading the extractor into the proximal aspect of the nail, we found it helpful to leave one of the cross-locks in place to prevent nail rotation.10 Inspection of the removed nail revealed a fracture of the device at the more proximal of 2 distal cross-locks (Figures 1A, 1B, 2).
To remove the distal fragment of the nail, we used a 5.0-mm smooth Steinmann pin. After cross-lock removal, the pin was placed unicortically through the distal medial cortex at the tip of the retained implant. The distal nail fragment was pushed proximally using the pin as a lever with the interposed cortical bone serving as a fulcrum (Figures 3A, 3B).
Discussion
Removal of broken solid intramedullary tibial nails presents orthopedic surgeons with a unique challenge. We have described a technique that modifies and incorporates previously described techniques while exploiting available surgical windows to facilitate hardware removal. This technique obviates the need for further bony and soft-tissue dissection, potentially mitigating surgical morbidity.
Other techniques for removing broken solid intramedullary devices have been reported. Krettek and colleagues7 described a technique in which the short distal fragment of a broken solid femoral intramedullary nail was removed with use of retrograde levering through a cortical window just proximal to the articular surface. The same window was then used for anterograde nail removal with a small Hohmann retractor serving as a guide. This technique is limited by the need for a large bony window, which potentially creates a stress riser within the distal segment. In addition, a short, distal nail fragment is required in order to facilitate manipulation through the metaphyseal bone. This technique is more readily used within the distal femur, given the large metaphyseal volume, in contrast with the distal tibial metaphysis. Giannoudis and colleagues1 described a method (for both tibia and femur) in which the intramedullary canal was proximally reamed to permit retrograde removal of an anterograde nail. The authors described reaming the canal to 4 mm larger than the nail to create access for a cleaning trephine and then a ratcheting extractor. This technique can be easily applied to the tibia or femur but requires special equipment that may not be readily available. Other retrograde techniques for the femur8 are not as suitable for the tibia, as they would cause significant chondral damage to the tibiotalar joint.
In developing our technique, which includes modifications of other methods, we used cortical windows, levering, and anterograde reaming to permit removal of a broken solid fragment through a nonunion site and with minimal additional destruction of bone. Although an existing cortical window was used, the newly created cortical window was significantly smaller than windows used in other techniques, and it avoids the articular surface. This technique can be performed with common, readily accessible equipment, which may be helpful in situations in which broken nails are encountered unexpectedly. In summary, this simple, safe, and effective technique uses standard equipment to preserve bone, decrease operative time, and alleviate surgeon frustration in complicated hardware removal surgeries.
Am J Orthop. 2016;45(6):E352-E354. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
2. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008;16(2):113-120.
3. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
4. Dawson GR Jr, Stader RO. Extractor for removing broken stuck intramedullary nail. Am J Orthop Surg. 1968;10(6):150-151.
5. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
6. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
7. Krettek C, Schandelmaier P, Tscherne H. Removal of a broken solid femoral nail: a simple push-out technique. A case report. J Bone Joint Surg Am. 1997;79(2):247-251.
8. Milia MJ, Vincent AB, Bosse MJ. Retrograde removal of an incarcerated solid titanium femoral nail after subtrochanteric fracture. J Orthop Trauma. 2003;17(7):521-524.
9. Whalley H, Thomas G, Hull P, Porter K. Surgeon versus metalwork—tips to remove a retained intramedullary nail fragment. Injury. 2009;40(7):783-789.
10. Smith G, Khan A, Marsh A. A novel way to remove a broken intramedullary nail. Ann R Coll Surg Engl. 2012;94(8):605.
In both elective and revision surgery, removal of retained hardware can be unpredictable. Broken hardware, whether identified before or during surgery, presents a significant challenge. Cases often require enlisting a large variety of equipment and techniques that often result in larger dissection and potential for wider soft-tissue or bony destruction. Broken intramedullary devices, located entirely within the cortices of bone, pose unique challenges.1,2 Various techniques have been used to remove broken cannulated nails.1-9 There is, however, a paucity of techniques for removing broken solid nails from within the tibia.1,2 Moreover, many of these techniques require significant metaphyseal and cortical bone destruction that may compromise the integrity of the long bone.1,3,9 In this article, we describe a modified technique for removal of a broken solid nail, with minimal cortical bone destruction, in the setting of a tibial nonunion.
Technique
A 23-year-old man presented with a symptomatic valgus nonunion of the tibia, which had been treated with a solid intramedullary 9-mm nail (Orthofix). The patient was taken to the operative theater for nonunion takedown and exchanged reamed intramedullary nailing. The proximal fragment of the anterograde intramedullary nail was removed in standard fashion using the Winquist Universal Extraction Set (Shukla Medical). When threading the extractor into the proximal aspect of the nail, we found it helpful to leave one of the cross-locks in place to prevent nail rotation.10 Inspection of the removed nail revealed a fracture of the device at the more proximal of 2 distal cross-locks (Figures 1A, 1B, 2).
To remove the distal fragment of the nail, we used a 5.0-mm smooth Steinmann pin. After cross-lock removal, the pin was placed unicortically through the distal medial cortex at the tip of the retained implant. The distal nail fragment was pushed proximally using the pin as a lever with the interposed cortical bone serving as a fulcrum (Figures 3A, 3B).
Discussion
Removal of broken solid intramedullary tibial nails presents orthopedic surgeons with a unique challenge. We have described a technique that modifies and incorporates previously described techniques while exploiting available surgical windows to facilitate hardware removal. This technique obviates the need for further bony and soft-tissue dissection, potentially mitigating surgical morbidity.
Other techniques for removing broken solid intramedullary devices have been reported. Krettek and colleagues7 described a technique in which the short distal fragment of a broken solid femoral intramedullary nail was removed with use of retrograde levering through a cortical window just proximal to the articular surface. The same window was then used for anterograde nail removal with a small Hohmann retractor serving as a guide. This technique is limited by the need for a large bony window, which potentially creates a stress riser within the distal segment. In addition, a short, distal nail fragment is required in order to facilitate manipulation through the metaphyseal bone. This technique is more readily used within the distal femur, given the large metaphyseal volume, in contrast with the distal tibial metaphysis. Giannoudis and colleagues1 described a method (for both tibia and femur) in which the intramedullary canal was proximally reamed to permit retrograde removal of an anterograde nail. The authors described reaming the canal to 4 mm larger than the nail to create access for a cleaning trephine and then a ratcheting extractor. This technique can be easily applied to the tibia or femur but requires special equipment that may not be readily available. Other retrograde techniques for the femur8 are not as suitable for the tibia, as they would cause significant chondral damage to the tibiotalar joint.
In developing our technique, which includes modifications of other methods, we used cortical windows, levering, and anterograde reaming to permit removal of a broken solid fragment through a nonunion site and with minimal additional destruction of bone. Although an existing cortical window was used, the newly created cortical window was significantly smaller than windows used in other techniques, and it avoids the articular surface. This technique can be performed with common, readily accessible equipment, which may be helpful in situations in which broken nails are encountered unexpectedly. In summary, this simple, safe, and effective technique uses standard equipment to preserve bone, decrease operative time, and alleviate surgeon frustration in complicated hardware removal surgeries.
Am J Orthop. 2016;45(6):E352-E354. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
In both elective and revision surgery, removal of retained hardware can be unpredictable. Broken hardware, whether identified before or during surgery, presents a significant challenge. Cases often require enlisting a large variety of equipment and techniques that often result in larger dissection and potential for wider soft-tissue or bony destruction. Broken intramedullary devices, located entirely within the cortices of bone, pose unique challenges.1,2 Various techniques have been used to remove broken cannulated nails.1-9 There is, however, a paucity of techniques for removing broken solid nails from within the tibia.1,2 Moreover, many of these techniques require significant metaphyseal and cortical bone destruction that may compromise the integrity of the long bone.1,3,9 In this article, we describe a modified technique for removal of a broken solid nail, with minimal cortical bone destruction, in the setting of a tibial nonunion.
Technique
A 23-year-old man presented with a symptomatic valgus nonunion of the tibia, which had been treated with a solid intramedullary 9-mm nail (Orthofix). The patient was taken to the operative theater for nonunion takedown and exchanged reamed intramedullary nailing. The proximal fragment of the anterograde intramedullary nail was removed in standard fashion using the Winquist Universal Extraction Set (Shukla Medical). When threading the extractor into the proximal aspect of the nail, we found it helpful to leave one of the cross-locks in place to prevent nail rotation.10 Inspection of the removed nail revealed a fracture of the device at the more proximal of 2 distal cross-locks (Figures 1A, 1B, 2).
To remove the distal fragment of the nail, we used a 5.0-mm smooth Steinmann pin. After cross-lock removal, the pin was placed unicortically through the distal medial cortex at the tip of the retained implant. The distal nail fragment was pushed proximally using the pin as a lever with the interposed cortical bone serving as a fulcrum (Figures 3A, 3B).
Discussion
Removal of broken solid intramedullary tibial nails presents orthopedic surgeons with a unique challenge. We have described a technique that modifies and incorporates previously described techniques while exploiting available surgical windows to facilitate hardware removal. This technique obviates the need for further bony and soft-tissue dissection, potentially mitigating surgical morbidity.
Other techniques for removing broken solid intramedullary devices have been reported. Krettek and colleagues7 described a technique in which the short distal fragment of a broken solid femoral intramedullary nail was removed with use of retrograde levering through a cortical window just proximal to the articular surface. The same window was then used for anterograde nail removal with a small Hohmann retractor serving as a guide. This technique is limited by the need for a large bony window, which potentially creates a stress riser within the distal segment. In addition, a short, distal nail fragment is required in order to facilitate manipulation through the metaphyseal bone. This technique is more readily used within the distal femur, given the large metaphyseal volume, in contrast with the distal tibial metaphysis. Giannoudis and colleagues1 described a method (for both tibia and femur) in which the intramedullary canal was proximally reamed to permit retrograde removal of an anterograde nail. The authors described reaming the canal to 4 mm larger than the nail to create access for a cleaning trephine and then a ratcheting extractor. This technique can be easily applied to the tibia or femur but requires special equipment that may not be readily available. Other retrograde techniques for the femur8 are not as suitable for the tibia, as they would cause significant chondral damage to the tibiotalar joint.
In developing our technique, which includes modifications of other methods, we used cortical windows, levering, and anterograde reaming to permit removal of a broken solid fragment through a nonunion site and with minimal additional destruction of bone. Although an existing cortical window was used, the newly created cortical window was significantly smaller than windows used in other techniques, and it avoids the articular surface. This technique can be performed with common, readily accessible equipment, which may be helpful in situations in which broken nails are encountered unexpectedly. In summary, this simple, safe, and effective technique uses standard equipment to preserve bone, decrease operative time, and alleviate surgeon frustration in complicated hardware removal surgeries.
Am J Orthop. 2016;45(6):E352-E354. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
2. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008;16(2):113-120.
3. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
4. Dawson GR Jr, Stader RO. Extractor for removing broken stuck intramedullary nail. Am J Orthop Surg. 1968;10(6):150-151.
5. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
6. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
7. Krettek C, Schandelmaier P, Tscherne H. Removal of a broken solid femoral nail: a simple push-out technique. A case report. J Bone Joint Surg Am. 1997;79(2):247-251.
8. Milia MJ, Vincent AB, Bosse MJ. Retrograde removal of an incarcerated solid titanium femoral nail after subtrochanteric fracture. J Orthop Trauma. 2003;17(7):521-524.
9. Whalley H, Thomas G, Hull P, Porter K. Surgeon versus metalwork—tips to remove a retained intramedullary nail fragment. Injury. 2009;40(7):783-789.
10. Smith G, Khan A, Marsh A. A novel way to remove a broken intramedullary nail. Ann R Coll Surg Engl. 2012;94(8):605.
1. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
2. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008;16(2):113-120.
3. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
4. Dawson GR Jr, Stader RO. Extractor for removing broken stuck intramedullary nail. Am J Orthop Surg. 1968;10(6):150-151.
5. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
6. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
7. Krettek C, Schandelmaier P, Tscherne H. Removal of a broken solid femoral nail: a simple push-out technique. A case report. J Bone Joint Surg Am. 1997;79(2):247-251.
8. Milia MJ, Vincent AB, Bosse MJ. Retrograde removal of an incarcerated solid titanium femoral nail after subtrochanteric fracture. J Orthop Trauma. 2003;17(7):521-524.
9. Whalley H, Thomas G, Hull P, Porter K. Surgeon versus metalwork—tips to remove a retained intramedullary nail fragment. Injury. 2009;40(7):783-789.
10. Smith G, Khan A, Marsh A. A novel way to remove a broken intramedullary nail. Ann R Coll Surg Engl. 2012;94(8):605.