User login
Stem-Based Repair of the Subscapularis in Total Shoulder Arthroplasty
Subscapularis integrity following total shoulder arthroplasty (TSA) is important to maintaining glenohumeral joint stability and functional outcome. In recent years increased emphasis has been placed on the management of the subscapularis during TSA. Options for management of the subscapularis during TSA include tenotomy, release of the tendon from the bone (peel technique), or a lesser tuberosity osteotomy (LTO). Several studies have demonstrated that subscapularis integrity is often impaired with a traditional tenotomy approach.1,2 Based on these studies, a subscapularis peel or LTO approach have gained popularity.3 This technical article describes a subscapularis peel repair technique that is integrated into a press-fit anatomical short-stem during TSA.
Technique
The repair technique demonstrated in this article features the Univers Apex (Arthrex) humeral stem, but it can be adapted to other stems with features that allow for the incorporation of sutures.
A standard deltopectoral approach is used to gain access to the shoulder. The biceps tendon is released or tenotomized to gain access to the bicipital groove. The rotator interval is then opened beginning at the superior subscapularis by following the course of the anterior side of the proximal biceps and then directing the release toward the base of the coracoid in order to protect the supraspinatus tendon. Next, the subscapularis is sharply released from the lesser tuberosity. The tendon and capsule are released as a unit and a 3-sided release of the subscapularis is performed.
The humeral canal is opened with a reamer and broached to accommodate an appropriately sized press-fit component. A polyethylene glenoid component is placed and then attention is returned to the humerus.
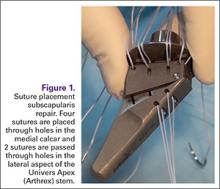
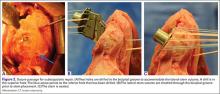
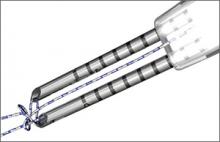
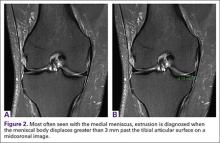
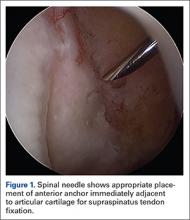
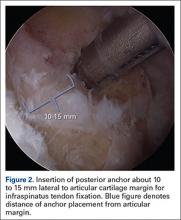
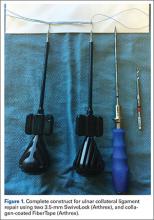
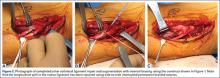
Prior to placement of the humeral stem, 6 No. 2 or No. 5 FiberWire (Arthrex) sutures are pre-placed through suture holes in the stem (Figure 1). Four sutures are passed by hand through the medial calcar component and 2 sutures are placed through holes in the lateral portion of the stem. A 2.0-mm or 2.5-mm drill is used to create 2 holes in the bicipital groove: 1 at the superior aspect of the lesser tuberosity, and 1 at the inferior aspect of the lesser tuberosity (Figure 2A). Prior to impacting the stem, the 4 lateral suture limbs (limbs A through D) are shuttled through the holes in the bicipital groove (Figure 2B). Then the stem is impacted and secured, the final humeral head is placed, the joint is reduced, and the subscapularis is repaired (Figure 2C).
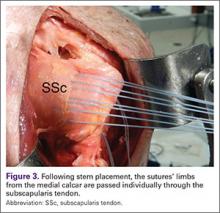
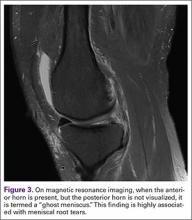
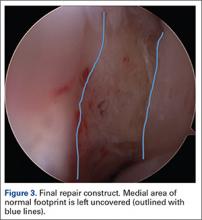
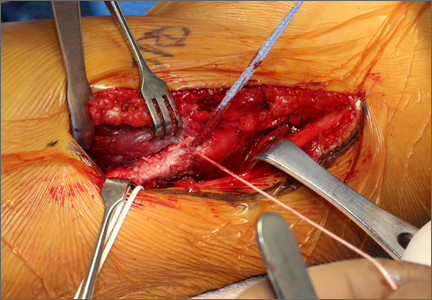
The 4 sutures passing through the medial calcar of the stem result in 8 suture limbs (limbs 1 through 8). Each limb is separately passed through the subscapularis tendon with a free needle, moving obliquely from inferior-medial to superior-lateral (Figure 3). Note: A variation is to pass 2 suture limbs at a time, but this technique has not been biomechanically investigated at the time of this writing.
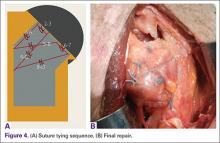
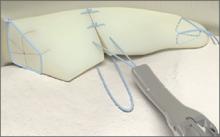
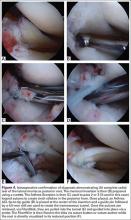
Prior to tying the sutures, it is helpful to place a stitch between the superolateral corner of the subscapularis and the anterior supraspinatus in order to facilitate reduction. The suture limbs are then tied with a specific sequence to create a suture-bridging construct with 2 additional medial mattress sutures as follows (Figures 4A, 4B):
1 to A
4 to C
5 to B
8 to D
2 to 3
6 to 7
In this technique, each suture limb is tied to a limb from another suture. When the last 2 pairs are tied (2 to 3 and 6 to 7), they are tensioned to remove any slack from the repair and equalize tension within all suture pairs. After the sutures are tied, the rotator interval may be closed with simple sutures if desired. The patient is immobilized in a sling for 4 to 6 weeks. Immediate passive forward flexion is allowed as well as external rotation to 30°. Strengthening is initiated at 8 weeks.
Discussion
The incidence of TSA has increased dramatically in the last decade and is projected to continue in the coming years.4 In the majority of cases, TSA leads to improvement in pain and function. However, failures continue to exist. In addition to glenoid loosening, prosthetic instability and rotator cuff insufficiency are the most common causes of failure.5 The latter 2 are intimately related since glenohumeral stability depends largely upon the rotator cuff. Therefore, optimization of outcome following TSA depends largely upon maintaining integrity of the rotator cuff. While the incidence of preoperative rotator cuff tears and fatty degeneration of the rotator are not modifiable, the management of the subscapularis is in the hands of the surgeon.
While subscapularis tenotomy has historically been used to access the glenohumeral joint during TSA, this approach is associated with an alarmingly high failure rate. Jackson and colleagues1 reported that 7 out of 15 (47%) of subscapularis tendons managed with tenotomy during TSA were completely torn on postoperative ultrasound. The patients with postoperative rupture had decreased internal rotation strength and DASH scores (4.6 intact vs. 25 ruptured; P = .04) compared to the patients with an intact tendon. Scalise and colleagues2 retrospectively compared a tenotomy approach to a LTO. They reported that 7 out of 15 subscapularis tenotomies were ruptured or attenuated postoperatively. By comparison, 18 out of 20 LTOs were healed. Regardless of approach, functional outcome was higher at 1 year postoperative when the subscapularis was intact.
The high failure rate with tendon-to-tendon healing following tenotomy has led to interest in a subscapularis peel to achieve tendon-to-bone healing or an LTO approach to achieve bone-to-bone healing. Lapner and colleagues3 compared a peel to an LTO in a randomized controlled trial of 87 patients. At 2 years postoperative, there was no difference in functional outcome between the 2 groups.
While both a peel and an LTO approach can be repaired with the technique described in this article, there are advantages to a peel approach. First, a peel approach may be considered more reproducible, particularly for surgeons who do a limited amount of shoulder arthroplasty. Whereas an LTO can vary in size, the subscapularis can nearly always be reproducibly peeled from the lesser tuberosity. Second, this technique uses a short stem, which relies upon proximal fixation. While this approach is bone-preserving, a large osteotomy has the potential to compromise fixation of the stem. Therefore, while one of us (PJD) uses a fleck LTO with a short stem, we advise a peel technique in most cases.
In summary, the subscapularis repair technique described here provides a reproducible and biomechanically sound approach to managing the subscapularis during TSA.
1. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
2. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
3. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
4. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
5. Australian Orthopaedic Association National Joint Replacement Registry. Shoulder Arthroplasty 2015 Annual Report. https://aoanjrr.sahmri.com/documents/10180/217645/Shoulder%20Arthroplasty. Accessed April 7, 2016.
Subscapularis integrity following total shoulder arthroplasty (TSA) is important to maintaining glenohumeral joint stability and functional outcome. In recent years increased emphasis has been placed on the management of the subscapularis during TSA. Options for management of the subscapularis during TSA include tenotomy, release of the tendon from the bone (peel technique), or a lesser tuberosity osteotomy (LTO). Several studies have demonstrated that subscapularis integrity is often impaired with a traditional tenotomy approach.1,2 Based on these studies, a subscapularis peel or LTO approach have gained popularity.3 This technical article describes a subscapularis peel repair technique that is integrated into a press-fit anatomical short-stem during TSA.
Technique
The repair technique demonstrated in this article features the Univers Apex (Arthrex) humeral stem, but it can be adapted to other stems with features that allow for the incorporation of sutures.
A standard deltopectoral approach is used to gain access to the shoulder. The biceps tendon is released or tenotomized to gain access to the bicipital groove. The rotator interval is then opened beginning at the superior subscapularis by following the course of the anterior side of the proximal biceps and then directing the release toward the base of the coracoid in order to protect the supraspinatus tendon. Next, the subscapularis is sharply released from the lesser tuberosity. The tendon and capsule are released as a unit and a 3-sided release of the subscapularis is performed.
The humeral canal is opened with a reamer and broached to accommodate an appropriately sized press-fit component. A polyethylene glenoid component is placed and then attention is returned to the humerus.
Prior to placement of the humeral stem, 6 No. 2 or No. 5 FiberWire (Arthrex) sutures are pre-placed through suture holes in the stem (Figure 1). Four sutures are passed by hand through the medial calcar component and 2 sutures are placed through holes in the lateral portion of the stem. A 2.0-mm or 2.5-mm drill is used to create 2 holes in the bicipital groove: 1 at the superior aspect of the lesser tuberosity, and 1 at the inferior aspect of the lesser tuberosity (Figure 2A). Prior to impacting the stem, the 4 lateral suture limbs (limbs A through D) are shuttled through the holes in the bicipital groove (Figure 2B). Then the stem is impacted and secured, the final humeral head is placed, the joint is reduced, and the subscapularis is repaired (Figure 2C).
The 4 sutures passing through the medial calcar of the stem result in 8 suture limbs (limbs 1 through 8). Each limb is separately passed through the subscapularis tendon with a free needle, moving obliquely from inferior-medial to superior-lateral (Figure 3). Note: A variation is to pass 2 suture limbs at a time, but this technique has not been biomechanically investigated at the time of this writing.
Prior to tying the sutures, it is helpful to place a stitch between the superolateral corner of the subscapularis and the anterior supraspinatus in order to facilitate reduction. The suture limbs are then tied with a specific sequence to create a suture-bridging construct with 2 additional medial mattress sutures as follows (Figures 4A, 4B):
1 to A
4 to C
5 to B
8 to D
2 to 3
6 to 7
In this technique, each suture limb is tied to a limb from another suture. When the last 2 pairs are tied (2 to 3 and 6 to 7), they are tensioned to remove any slack from the repair and equalize tension within all suture pairs. After the sutures are tied, the rotator interval may be closed with simple sutures if desired. The patient is immobilized in a sling for 4 to 6 weeks. Immediate passive forward flexion is allowed as well as external rotation to 30°. Strengthening is initiated at 8 weeks.
Discussion
The incidence of TSA has increased dramatically in the last decade and is projected to continue in the coming years.4 In the majority of cases, TSA leads to improvement in pain and function. However, failures continue to exist. In addition to glenoid loosening, prosthetic instability and rotator cuff insufficiency are the most common causes of failure.5 The latter 2 are intimately related since glenohumeral stability depends largely upon the rotator cuff. Therefore, optimization of outcome following TSA depends largely upon maintaining integrity of the rotator cuff. While the incidence of preoperative rotator cuff tears and fatty degeneration of the rotator are not modifiable, the management of the subscapularis is in the hands of the surgeon.
While subscapularis tenotomy has historically been used to access the glenohumeral joint during TSA, this approach is associated with an alarmingly high failure rate. Jackson and colleagues1 reported that 7 out of 15 (47%) of subscapularis tendons managed with tenotomy during TSA were completely torn on postoperative ultrasound. The patients with postoperative rupture had decreased internal rotation strength and DASH scores (4.6 intact vs. 25 ruptured; P = .04) compared to the patients with an intact tendon. Scalise and colleagues2 retrospectively compared a tenotomy approach to a LTO. They reported that 7 out of 15 subscapularis tenotomies were ruptured or attenuated postoperatively. By comparison, 18 out of 20 LTOs were healed. Regardless of approach, functional outcome was higher at 1 year postoperative when the subscapularis was intact.
The high failure rate with tendon-to-tendon healing following tenotomy has led to interest in a subscapularis peel to achieve tendon-to-bone healing or an LTO approach to achieve bone-to-bone healing. Lapner and colleagues3 compared a peel to an LTO in a randomized controlled trial of 87 patients. At 2 years postoperative, there was no difference in functional outcome between the 2 groups.
While both a peel and an LTO approach can be repaired with the technique described in this article, there are advantages to a peel approach. First, a peel approach may be considered more reproducible, particularly for surgeons who do a limited amount of shoulder arthroplasty. Whereas an LTO can vary in size, the subscapularis can nearly always be reproducibly peeled from the lesser tuberosity. Second, this technique uses a short stem, which relies upon proximal fixation. While this approach is bone-preserving, a large osteotomy has the potential to compromise fixation of the stem. Therefore, while one of us (PJD) uses a fleck LTO with a short stem, we advise a peel technique in most cases.
In summary, the subscapularis repair technique described here provides a reproducible and biomechanically sound approach to managing the subscapularis during TSA.
Subscapularis integrity following total shoulder arthroplasty (TSA) is important to maintaining glenohumeral joint stability and functional outcome. In recent years increased emphasis has been placed on the management of the subscapularis during TSA. Options for management of the subscapularis during TSA include tenotomy, release of the tendon from the bone (peel technique), or a lesser tuberosity osteotomy (LTO). Several studies have demonstrated that subscapularis integrity is often impaired with a traditional tenotomy approach.1,2 Based on these studies, a subscapularis peel or LTO approach have gained popularity.3 This technical article describes a subscapularis peel repair technique that is integrated into a press-fit anatomical short-stem during TSA.
Technique
The repair technique demonstrated in this article features the Univers Apex (Arthrex) humeral stem, but it can be adapted to other stems with features that allow for the incorporation of sutures.
A standard deltopectoral approach is used to gain access to the shoulder. The biceps tendon is released or tenotomized to gain access to the bicipital groove. The rotator interval is then opened beginning at the superior subscapularis by following the course of the anterior side of the proximal biceps and then directing the release toward the base of the coracoid in order to protect the supraspinatus tendon. Next, the subscapularis is sharply released from the lesser tuberosity. The tendon and capsule are released as a unit and a 3-sided release of the subscapularis is performed.
The humeral canal is opened with a reamer and broached to accommodate an appropriately sized press-fit component. A polyethylene glenoid component is placed and then attention is returned to the humerus.
Prior to placement of the humeral stem, 6 No. 2 or No. 5 FiberWire (Arthrex) sutures are pre-placed through suture holes in the stem (Figure 1). Four sutures are passed by hand through the medial calcar component and 2 sutures are placed through holes in the lateral portion of the stem. A 2.0-mm or 2.5-mm drill is used to create 2 holes in the bicipital groove: 1 at the superior aspect of the lesser tuberosity, and 1 at the inferior aspect of the lesser tuberosity (Figure 2A). Prior to impacting the stem, the 4 lateral suture limbs (limbs A through D) are shuttled through the holes in the bicipital groove (Figure 2B). Then the stem is impacted and secured, the final humeral head is placed, the joint is reduced, and the subscapularis is repaired (Figure 2C).
The 4 sutures passing through the medial calcar of the stem result in 8 suture limbs (limbs 1 through 8). Each limb is separately passed through the subscapularis tendon with a free needle, moving obliquely from inferior-medial to superior-lateral (Figure 3). Note: A variation is to pass 2 suture limbs at a time, but this technique has not been biomechanically investigated at the time of this writing.
Prior to tying the sutures, it is helpful to place a stitch between the superolateral corner of the subscapularis and the anterior supraspinatus in order to facilitate reduction. The suture limbs are then tied with a specific sequence to create a suture-bridging construct with 2 additional medial mattress sutures as follows (Figures 4A, 4B):
1 to A
4 to C
5 to B
8 to D
2 to 3
6 to 7
In this technique, each suture limb is tied to a limb from another suture. When the last 2 pairs are tied (2 to 3 and 6 to 7), they are tensioned to remove any slack from the repair and equalize tension within all suture pairs. After the sutures are tied, the rotator interval may be closed with simple sutures if desired. The patient is immobilized in a sling for 4 to 6 weeks. Immediate passive forward flexion is allowed as well as external rotation to 30°. Strengthening is initiated at 8 weeks.
Discussion
The incidence of TSA has increased dramatically in the last decade and is projected to continue in the coming years.4 In the majority of cases, TSA leads to improvement in pain and function. However, failures continue to exist. In addition to glenoid loosening, prosthetic instability and rotator cuff insufficiency are the most common causes of failure.5 The latter 2 are intimately related since glenohumeral stability depends largely upon the rotator cuff. Therefore, optimization of outcome following TSA depends largely upon maintaining integrity of the rotator cuff. While the incidence of preoperative rotator cuff tears and fatty degeneration of the rotator are not modifiable, the management of the subscapularis is in the hands of the surgeon.
While subscapularis tenotomy has historically been used to access the glenohumeral joint during TSA, this approach is associated with an alarmingly high failure rate. Jackson and colleagues1 reported that 7 out of 15 (47%) of subscapularis tendons managed with tenotomy during TSA were completely torn on postoperative ultrasound. The patients with postoperative rupture had decreased internal rotation strength and DASH scores (4.6 intact vs. 25 ruptured; P = .04) compared to the patients with an intact tendon. Scalise and colleagues2 retrospectively compared a tenotomy approach to a LTO. They reported that 7 out of 15 subscapularis tenotomies were ruptured or attenuated postoperatively. By comparison, 18 out of 20 LTOs were healed. Regardless of approach, functional outcome was higher at 1 year postoperative when the subscapularis was intact.
The high failure rate with tendon-to-tendon healing following tenotomy has led to interest in a subscapularis peel to achieve tendon-to-bone healing or an LTO approach to achieve bone-to-bone healing. Lapner and colleagues3 compared a peel to an LTO in a randomized controlled trial of 87 patients. At 2 years postoperative, there was no difference in functional outcome between the 2 groups.
While both a peel and an LTO approach can be repaired with the technique described in this article, there are advantages to a peel approach. First, a peel approach may be considered more reproducible, particularly for surgeons who do a limited amount of shoulder arthroplasty. Whereas an LTO can vary in size, the subscapularis can nearly always be reproducibly peeled from the lesser tuberosity. Second, this technique uses a short stem, which relies upon proximal fixation. While this approach is bone-preserving, a large osteotomy has the potential to compromise fixation of the stem. Therefore, while one of us (PJD) uses a fleck LTO with a short stem, we advise a peel technique in most cases.
In summary, the subscapularis repair technique described here provides a reproducible and biomechanically sound approach to managing the subscapularis during TSA.
1. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
2. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
3. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
4. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
5. Australian Orthopaedic Association National Joint Replacement Registry. Shoulder Arthroplasty 2015 Annual Report. https://aoanjrr.sahmri.com/documents/10180/217645/Shoulder%20Arthroplasty. Accessed April 7, 2016.
1. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
2. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
3. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
4. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
5. Australian Orthopaedic Association National Joint Replacement Registry. Shoulder Arthroplasty 2015 Annual Report. https://aoanjrr.sahmri.com/documents/10180/217645/Shoulder%20Arthroplasty. Accessed April 7, 2016.
Transitions (The Future of Orthopedics)
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
Technical Errors May Affect Accuracy of Torque Limiter in Locking Plate Osteosynthesis
Proper surgical technique must be used to ensure that surgical fracture management is long-lasting. Plate implantation and screw implantation are among the most common orthopedic procedures performed. Plate and screw osteosynthesis can be done with nonlocking or locking plate and screw constructs or with hybrid fixation that incorporates both methods.
Nonlocking plate and screw osteosynthesis uses friction-fit for fixation. In osteoporotic bone, less torque is generated because of poor bone quality, and thus less friction force between plate and bone.1,2 Locked plating has dramatically changed fracture management, especially in frail and comminuted osteoporotic bone, with significant advantages over conventional plating.3-7
Development of locked plating systems, including the Less Invasive Stabilization System (LISS; DePuy Synthes) with its soft-tissue and fracture-fragment preservation, has changed treatment of distal femur and proximal tibia fractures. Cole and colleagues8 reported stable fixation and union in 97% of their patients. The LISS system proved to be stable, but there were cases of implant removal difficulty with this titanium construct. In 1 of the 10 cases in which the LISS plate was removed, 4 of the 11 locking screws were welded to the plate.8
Cold welding, in which similar metals are chemically bonded together under extreme pressure, is a complication associated with use of titanium-only plates and screws.9 This process, which is more likely to happen if cross-threading occurs within the screw–plate interface, can make screw removal extremely difficult. Screw removal difficulty strips screw heads, and often the surgeon must use either metal cutting instruments or trephines to remove screw remnants, which often results in retained implant or debris and damage or necrosis to surrounding bone.9,10
Locking screws are often inserted under power with a torque-limiting device attached to the drill mechanism to reduce the risk of lock screw overtightening and to try to prevent difficult implant removal. Although standard practice is to insert the screw and stop just before screw head engagement, with final tightening with a torque limiter and hand power, final tightening is often inadvertently done under power.3 Most technique guides instruct surgeons how to insert screws under power while using a torque limiter, but the exact technique is not emphasized.
We conducted a study to determine if rotational speed of screw insertion affects maximum torque on screw with use of a torque limiter. We describe proper use of a torque limiter as well as possible pitfalls. We hypothesized that improper use would result in substantially higher than expected insertion torque.
Materials and Methods
Torque-Limiting Attachments, Torque Wrench, and Drill
The Small Fragment Locking Compression Plate System (Synthes) includes a 1.5-Nm torque-limiting attachment and quick-coupling wooden handles and Star Drive attachments. All devices in this study were in active use at 6 urban institutions (3 level I trauma centers, 2 level II trauma centers, 1 level III hospital). Permission to obtain and test each device was granted by each institution.
A 0.25-inch dial torque wrench (751LDIN; CDI Torque Products) was purchased through an established distributor. The manufacturer includes a traceable certificate of accuracy to verify correct calibration. The torque wrench has a torque range of 0 to 9 Nm with visual increment demarcations of 0.2 Nm and a memory needle to retain maximum torque measurement. The same torque wrench was used in each experiment in order to maintain consistent measurements between devices. It was reset to zero after each use.
This study used a 0.5-inch, 19.2-V lithium drill (Craftsman C3) with 2 speed options: 0 to 440 rpm high torque and 0 to 1600 rpm high speed. This device provides variable torque output with a maximum output of 38.4 Nm. For this study, all measurements were done with the device on its high torque setting.
Maximum Torque Determination for Different Scenarios
Each torque limiter was evaluated for variations in maximum torque under 4 different scenarios. In each scenario, the torque limiter was coupled to the Star Drive attachment and then to that scenario’s rotating force. The completed system was then inserted into the torque wrench, which was secured to a flat working surface and rotated in accordance with each scenario; maximum torque was measured and recorded (Figures 1, 2). A torque-limiting event was defined as a single audible click on the torque limiter.
In scenario 1, each torque-limiting attachment system was attached to a quick-coupling wooden handle. The completed system was then rotated at controlled low velocity under hand power until 1 torque-limiting event occurred. This scenario was also used as an internal control to verify that the torque limiters were calibrated correctly.
In Scenario 2, the device was again attached to a quick-coupling wooden handle. The completed system was rotated at high velocity under hand power until multiple torque-limiting events occurred in a row. High velocity was defined as the operator freely rotating the wooden handle in a single action with full power resulting in multiple torque-limiting events.
In Scenario 3, the device was attached to a power drill braced to the flat working surface and rotated at low velocity under power until 1 torque-limiting event occurred.
In Scenario 4, the device was again attached to a power drill braced to the flat working surface. The completed system was rotated at high velocity under power until multiple torque-limiting events occurred.
After each trial, we recorded maximum torque achieved before each device’s torque-limiting event. Either an orthopedic surgery resident or a qualified medical student tested each torque-limiting device in each standardized testing scenario.
Statistical Analysis
Experiments for each torque limiter were repeated for 3 trials of each of the 4 different scenarios. For comparative statistics between experiments, maximum torque measurements were expressed as means and SDs; 95% confidence interval (95% CI) was calculated and reported to determine extent of variation within a single group. One-way analysis of variance (ANOVA) and Tukey post hoc tests were performed between groups for comparison of the normally distributed data. Significance was set at P ≤ .05.
Results
During simulation, we successfully measured maximum torque achieved with each torque limiter under the 4 different scenarios. All testing was done by 2 operators. ANOVA demonstrated significant (P ≤ .001) differences in torque among the scenarios.
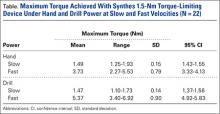
In scenario 1, mean (SD) maximum torque under hand power at low velocity was 1.49 (0.15) Nm (95% CI, 1.43-1.55), near the advertised maximum torque of 1.5 Nm, with relatively minimal variation between devices. This scenario confirmed proper calibration of properly used torque limiters. Mean maximum torque ranged from 1.25 to 1.93 Nm.
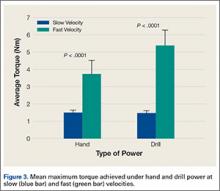
In scenario 2, mean (SD) maximum torque under hand power at high velocity was 3.73 (0.79) Nm (95% CI, 3.33-4.13), a 2.5-fold increase compared with scenario 1 (P < .0001) (Figure 3). There also was an increase in variation of maximum torque between trials of individual devices and between different devices. Mean maximum torque ranged from 2.27 to 5.53 Nm.
In scenario 3, mean (SD) maximum torque under drill power at controlled low velocity was 1.47 (0.14) Nm (95% CI, 1.37-1.56), again near the advertised maximum torque of 1.5 Nm, with relatively minimal variation. Mean maximum torque ranged from 1.10 to 1.73 Nm.
In scenario 4, mean (SD) maximum torque under drill power at full power/high velocity was 5.37 (0.90) Nm (95% CI, 4.92-5.83), a 3.65-fold increase compared with scenario 3 (P < .0001) (Figure 3). Mean maximum torque measured in 3 tests ranged from 3.40 to 6.92 Nm.
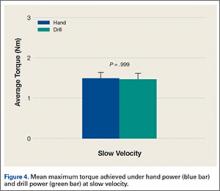
There was no significant difference in mean maximum torque between the scenarios of hand power at low velocity and drill power at low velocities (P = .999) (Figure 4). Highest maximum torque from any device was 9.0 Nm (drill at full power). Results are summarized in the Table. There was no statistical significance in the test between the 2 test operators.
Discussion
Maximum torque was measured using a torque-limiting attachment under 4 different simulated scenarios. Our goals were to determine if varying practice and rotational velocity would affect maximum insertional torque and to measure consistency among torque limiters. We designed the scenarios to mimic practice patterns, including hand insertion and power insertion of locking screws. Results demonstrated that misuse of a torque-limiting device may inadvertently produce insertional torque substantially higher than recommended. Highest maximum torque was 9.0 Nm, which is 6.0-fold higher than expected for a locking screw using a 1.5-Nm torque limiter.
Our study results showed that insertion under controlled hand power (and low-velocity drill power) until 1 torque-limiting event occurred produced the most consistent and predictable results. Insertion under drill power or high-velocity hand power produced multiple sequential torque-limiting events, yielding inaccurate insertion torque. Low-velocity insertion under hand power, or carefully controlled drill power, consistently produced torque similar to advertised values.
Manufacturers’ technique guides are available for proximal humerus locking compression plate (LCP) systems, small-fragment LCP systems, the Proximal Humeral Interlocking System (PHILOS; DePuy Synthes), and the LISS. These technique guides clearly state that insertion can be performed under power. Only the PHILOS and LISS guides state that insertion should be performed under power until a single click is heard or that final tightening should be completed under hand power. The proximal humerus LCP guide states that surgeons should insert the locking screw under power until the torque-limiting device clicks. The small-fragment LCP guide states that insertion under power should always be completed with the torque-limiting attachment; there is no mention of reducing power or a single click (this may give the surgeon a false sense of security).
Screw overtightening and head/thread stripping can make screw removal challenging.10 Removal rates for LISS plates range from 8% to 26%, and removal is often reported as taking longer than the index procedure, with complication rates as high as 47%.11-13 Bae and colleagues3 reported significant difficulty in removing 24 of 279 self-tapping locking screws (3.5 mm).
It is important to note that these complications, most notably cold welding, are mostly associated with titanium locking plate and screw constructs. Although stainless steel constructs have gained favor, titanium constructs are still widely used around the world.14,15
In 10% of cases in a laboratory setting, insertion of a 3.5-mm locking screw at 4 to 6 Nm damaged the screw.9 Removal of 3.5-mm locking screws had a stripping rate of 8.6%, and use of the torque limiter did not make removal easy all the time.3 Torque limiters are set specific to each screw diameter to reduce the risk of damage/stripping or even overtightening. Even when a surgeon intends to stop a drill before locking, final tightening often inadvertently occurs under power.3
Cold welding is often described as a cause of difficult implant removal.3,12 According to a newer definition, this process is independent of temperature and can occur when 2 metallic surfaces are in direct contact.16 High contact pressures between 2 similar metals can lead to this solid state welding.17 Theoretically, improper use of torque limiters can increase the risk of welding; however, it appears to be associated only with titanium locking plate and screw constructs.
Locked plating osteosynthesis is a valuable tool for fracture management, but improper use can have significant consequences, including morbid implant removal procedures, which are more difficult and time-consuming than the index surgery. We determined that proper use of torque limiters involves insertion under hand or power control at slow velocity until 1 torque-limiting event occurs. Many orthopedic surgeons may assume that torque limiters are accurate no matter how screws are inserted into locking plates. In addition, they may be unaware guidelines exist, as these are often deeply embedded within text. Therefore, we must emphasize that torque limiters can be inaccurate when used improperly.
One limitation of this study is that it tested only the Synthes 1.5-Nm torque-limiting attachment, though we can speculate that torque limiters designed for larger screws and limiters manufactured by different companies will behave similarly. Another limitation is that we did not obtain the hospitals’ service records for the tested equipment and assumed the equipment was properly checked for accuracy by the providing company. However, we hypothesized that, if maintenance were an issue, then our results would not be similar across all sites tested.
These tests involved a torque limiter linked to a torque-measuring device and may not perfectly represent actual torque measured at the locked screw–thread interface. However, we think our construct accurately determines the torque produced at the level of the driver tip. Also, we can speculate that the torque produced with improper use will lead to the complications mentioned and demonstrated in previous studies. Welding of the screw–plate interface may simply be a result of improper trajectory and cross-threading. However, if we assume that torque limiters prevent excessive torque no matter how they are used, high insertion speeds may compound the effect of welding. Additional biomechanical studies with full locked plate osteosynthesis constructs on bone specimens are planned to further characterize the potential complications of this issue.
1. Sommer C, Babst R, Müller M, Hanson B. Locking compression plate loosening and plate breakage: a report of four cases. J Orthop Trauma. 2004;18(8):571-577.
2. Schütz M, Südkamp NP. Revolution in plate osteosynthesis: new internal fixator systems. J Orthop Sci. 2003;8(2):252-258.
3. Bae JH, Oh JK, Oh CW, Hur CR. Technical difficulties of removal of locking screw after locking compression plating. Arch Orthop Trauma Surg. 2009;129(1):91-95.
4. Frigg R. Locking compression plate (LCP). An osteosynthesis plate based on the dynamic compression plate and the point contact fixator (PC-Fix). Injury. 2001;32(suppl 2):63-66.
5. Frigg R. Development of the locking compression plate. Injury. 2003;34(suppl 2):B6-B10.
6. Korner J, Lill H, Müller LP, Rommens PM, Schneider E, Linke B. The LCP-concept in the operative treatment of distal humerus fractures—biological, biomechanical and surgical aspects. Injury. 2003;34(suppl 2):B20-B30.
7. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
8. Cole PA, Zlowodzki M, Kregor PJ. Treatment of proximal tibia fractures using the Less Invasive Stabilization System: surgical experience and early clinical results in 77 fractures. J Orthop Trauma. 2004;18(8):528-535.
9. Ehlinger M, Adam P, Simon P, Bonnomet F. Technical difficulties in hardware removal in titanium compression plates with locking screws. Orthop Traumatol Surg Res. 2009;95(5):373-376.
10. Gopinathan NR, Dhillon MS, Kumar R. Surgical technique: simple technique for removing a locking recon plate with damaged screw heads. Clin Orthop Relat Res. 2013;471(5):1572-1575.
11. Pattison G, Reynolds J, Hardy J. Salvaging a stripped drive connection when removing screws. Injury. 1999;30(1):74-75.
12. Raja S, Imbuldeniya AM, Garg S, Groom G. Difficulties encountered removing locked plates. Ann R Coll Surg Engl. 2012;94(7):502-505.
13. Kumar G, Dunlop C. Case report: a technique to remove a jammed locking screw from a locking plate. Clin Orthop Relat Res. 2011;469(2):613-616.
14. Disegi JA. Titanium alloys for fracture fixation implants. Injury. 2000;31(suppl 4):14-17.
15. El-Zayat BF, Ruchholtz S, Efe T, Paletta J, Kreslo D, Zettl R. Results of titanium locking plate and stainless steel cerclage wire combination in femoral fractures. Indian J Orthop. 2013;47(5):454-458.
16. Van Nortwick SS, Yao J, Ladd AL. Titanium integration with bone, welding, and screw head destruction complicating hardware removal of the distal radius: report of 2 cases. J Hand Surg. 2012;37(7):1388-1392.
17. Ferguson GS, Chaudhury MK, Sigal GB, Whitesides GM. Contact adhesion of thin gold films on elastomeric supports: cold welding under ambient conditions. Science. 1991;253(5021):776-778.
Proper surgical technique must be used to ensure that surgical fracture management is long-lasting. Plate implantation and screw implantation are among the most common orthopedic procedures performed. Plate and screw osteosynthesis can be done with nonlocking or locking plate and screw constructs or with hybrid fixation that incorporates both methods.
Nonlocking plate and screw osteosynthesis uses friction-fit for fixation. In osteoporotic bone, less torque is generated because of poor bone quality, and thus less friction force between plate and bone.1,2 Locked plating has dramatically changed fracture management, especially in frail and comminuted osteoporotic bone, with significant advantages over conventional plating.3-7
Development of locked plating systems, including the Less Invasive Stabilization System (LISS; DePuy Synthes) with its soft-tissue and fracture-fragment preservation, has changed treatment of distal femur and proximal tibia fractures. Cole and colleagues8 reported stable fixation and union in 97% of their patients. The LISS system proved to be stable, but there were cases of implant removal difficulty with this titanium construct. In 1 of the 10 cases in which the LISS plate was removed, 4 of the 11 locking screws were welded to the plate.8
Cold welding, in which similar metals are chemically bonded together under extreme pressure, is a complication associated with use of titanium-only plates and screws.9 This process, which is more likely to happen if cross-threading occurs within the screw–plate interface, can make screw removal extremely difficult. Screw removal difficulty strips screw heads, and often the surgeon must use either metal cutting instruments or trephines to remove screw remnants, which often results in retained implant or debris and damage or necrosis to surrounding bone.9,10
Locking screws are often inserted under power with a torque-limiting device attached to the drill mechanism to reduce the risk of lock screw overtightening and to try to prevent difficult implant removal. Although standard practice is to insert the screw and stop just before screw head engagement, with final tightening with a torque limiter and hand power, final tightening is often inadvertently done under power.3 Most technique guides instruct surgeons how to insert screws under power while using a torque limiter, but the exact technique is not emphasized.
We conducted a study to determine if rotational speed of screw insertion affects maximum torque on screw with use of a torque limiter. We describe proper use of a torque limiter as well as possible pitfalls. We hypothesized that improper use would result in substantially higher than expected insertion torque.
Materials and Methods
Torque-Limiting Attachments, Torque Wrench, and Drill
The Small Fragment Locking Compression Plate System (Synthes) includes a 1.5-Nm torque-limiting attachment and quick-coupling wooden handles and Star Drive attachments. All devices in this study were in active use at 6 urban institutions (3 level I trauma centers, 2 level II trauma centers, 1 level III hospital). Permission to obtain and test each device was granted by each institution.
A 0.25-inch dial torque wrench (751LDIN; CDI Torque Products) was purchased through an established distributor. The manufacturer includes a traceable certificate of accuracy to verify correct calibration. The torque wrench has a torque range of 0 to 9 Nm with visual increment demarcations of 0.2 Nm and a memory needle to retain maximum torque measurement. The same torque wrench was used in each experiment in order to maintain consistent measurements between devices. It was reset to zero after each use.
This study used a 0.5-inch, 19.2-V lithium drill (Craftsman C3) with 2 speed options: 0 to 440 rpm high torque and 0 to 1600 rpm high speed. This device provides variable torque output with a maximum output of 38.4 Nm. For this study, all measurements were done with the device on its high torque setting.
Maximum Torque Determination for Different Scenarios
Each torque limiter was evaluated for variations in maximum torque under 4 different scenarios. In each scenario, the torque limiter was coupled to the Star Drive attachment and then to that scenario’s rotating force. The completed system was then inserted into the torque wrench, which was secured to a flat working surface and rotated in accordance with each scenario; maximum torque was measured and recorded (Figures 1, 2). A torque-limiting event was defined as a single audible click on the torque limiter.
In scenario 1, each torque-limiting attachment system was attached to a quick-coupling wooden handle. The completed system was then rotated at controlled low velocity under hand power until 1 torque-limiting event occurred. This scenario was also used as an internal control to verify that the torque limiters were calibrated correctly.
In Scenario 2, the device was again attached to a quick-coupling wooden handle. The completed system was rotated at high velocity under hand power until multiple torque-limiting events occurred in a row. High velocity was defined as the operator freely rotating the wooden handle in a single action with full power resulting in multiple torque-limiting events.
In Scenario 3, the device was attached to a power drill braced to the flat working surface and rotated at low velocity under power until 1 torque-limiting event occurred.
In Scenario 4, the device was again attached to a power drill braced to the flat working surface. The completed system was rotated at high velocity under power until multiple torque-limiting events occurred.
After each trial, we recorded maximum torque achieved before each device’s torque-limiting event. Either an orthopedic surgery resident or a qualified medical student tested each torque-limiting device in each standardized testing scenario.
Statistical Analysis
Experiments for each torque limiter were repeated for 3 trials of each of the 4 different scenarios. For comparative statistics between experiments, maximum torque measurements were expressed as means and SDs; 95% confidence interval (95% CI) was calculated and reported to determine extent of variation within a single group. One-way analysis of variance (ANOVA) and Tukey post hoc tests were performed between groups for comparison of the normally distributed data. Significance was set at P ≤ .05.
Results
During simulation, we successfully measured maximum torque achieved with each torque limiter under the 4 different scenarios. All testing was done by 2 operators. ANOVA demonstrated significant (P ≤ .001) differences in torque among the scenarios.
In scenario 1, mean (SD) maximum torque under hand power at low velocity was 1.49 (0.15) Nm (95% CI, 1.43-1.55), near the advertised maximum torque of 1.5 Nm, with relatively minimal variation between devices. This scenario confirmed proper calibration of properly used torque limiters. Mean maximum torque ranged from 1.25 to 1.93 Nm.
In scenario 2, mean (SD) maximum torque under hand power at high velocity was 3.73 (0.79) Nm (95% CI, 3.33-4.13), a 2.5-fold increase compared with scenario 1 (P < .0001) (Figure 3). There also was an increase in variation of maximum torque between trials of individual devices and between different devices. Mean maximum torque ranged from 2.27 to 5.53 Nm.
In scenario 3, mean (SD) maximum torque under drill power at controlled low velocity was 1.47 (0.14) Nm (95% CI, 1.37-1.56), again near the advertised maximum torque of 1.5 Nm, with relatively minimal variation. Mean maximum torque ranged from 1.10 to 1.73 Nm.
In scenario 4, mean (SD) maximum torque under drill power at full power/high velocity was 5.37 (0.90) Nm (95% CI, 4.92-5.83), a 3.65-fold increase compared with scenario 3 (P < .0001) (Figure 3). Mean maximum torque measured in 3 tests ranged from 3.40 to 6.92 Nm.
There was no significant difference in mean maximum torque between the scenarios of hand power at low velocity and drill power at low velocities (P = .999) (Figure 4). Highest maximum torque from any device was 9.0 Nm (drill at full power). Results are summarized in the Table. There was no statistical significance in the test between the 2 test operators.
Discussion
Maximum torque was measured using a torque-limiting attachment under 4 different simulated scenarios. Our goals were to determine if varying practice and rotational velocity would affect maximum insertional torque and to measure consistency among torque limiters. We designed the scenarios to mimic practice patterns, including hand insertion and power insertion of locking screws. Results demonstrated that misuse of a torque-limiting device may inadvertently produce insertional torque substantially higher than recommended. Highest maximum torque was 9.0 Nm, which is 6.0-fold higher than expected for a locking screw using a 1.5-Nm torque limiter.
Our study results showed that insertion under controlled hand power (and low-velocity drill power) until 1 torque-limiting event occurred produced the most consistent and predictable results. Insertion under drill power or high-velocity hand power produced multiple sequential torque-limiting events, yielding inaccurate insertion torque. Low-velocity insertion under hand power, or carefully controlled drill power, consistently produced torque similar to advertised values.
Manufacturers’ technique guides are available for proximal humerus locking compression plate (LCP) systems, small-fragment LCP systems, the Proximal Humeral Interlocking System (PHILOS; DePuy Synthes), and the LISS. These technique guides clearly state that insertion can be performed under power. Only the PHILOS and LISS guides state that insertion should be performed under power until a single click is heard or that final tightening should be completed under hand power. The proximal humerus LCP guide states that surgeons should insert the locking screw under power until the torque-limiting device clicks. The small-fragment LCP guide states that insertion under power should always be completed with the torque-limiting attachment; there is no mention of reducing power or a single click (this may give the surgeon a false sense of security).
Screw overtightening and head/thread stripping can make screw removal challenging.10 Removal rates for LISS plates range from 8% to 26%, and removal is often reported as taking longer than the index procedure, with complication rates as high as 47%.11-13 Bae and colleagues3 reported significant difficulty in removing 24 of 279 self-tapping locking screws (3.5 mm).
It is important to note that these complications, most notably cold welding, are mostly associated with titanium locking plate and screw constructs. Although stainless steel constructs have gained favor, titanium constructs are still widely used around the world.14,15
In 10% of cases in a laboratory setting, insertion of a 3.5-mm locking screw at 4 to 6 Nm damaged the screw.9 Removal of 3.5-mm locking screws had a stripping rate of 8.6%, and use of the torque limiter did not make removal easy all the time.3 Torque limiters are set specific to each screw diameter to reduce the risk of damage/stripping or even overtightening. Even when a surgeon intends to stop a drill before locking, final tightening often inadvertently occurs under power.3
Cold welding is often described as a cause of difficult implant removal.3,12 According to a newer definition, this process is independent of temperature and can occur when 2 metallic surfaces are in direct contact.16 High contact pressures between 2 similar metals can lead to this solid state welding.17 Theoretically, improper use of torque limiters can increase the risk of welding; however, it appears to be associated only with titanium locking plate and screw constructs.
Locked plating osteosynthesis is a valuable tool for fracture management, but improper use can have significant consequences, including morbid implant removal procedures, which are more difficult and time-consuming than the index surgery. We determined that proper use of torque limiters involves insertion under hand or power control at slow velocity until 1 torque-limiting event occurs. Many orthopedic surgeons may assume that torque limiters are accurate no matter how screws are inserted into locking plates. In addition, they may be unaware guidelines exist, as these are often deeply embedded within text. Therefore, we must emphasize that torque limiters can be inaccurate when used improperly.
One limitation of this study is that it tested only the Synthes 1.5-Nm torque-limiting attachment, though we can speculate that torque limiters designed for larger screws and limiters manufactured by different companies will behave similarly. Another limitation is that we did not obtain the hospitals’ service records for the tested equipment and assumed the equipment was properly checked for accuracy by the providing company. However, we hypothesized that, if maintenance were an issue, then our results would not be similar across all sites tested.
These tests involved a torque limiter linked to a torque-measuring device and may not perfectly represent actual torque measured at the locked screw–thread interface. However, we think our construct accurately determines the torque produced at the level of the driver tip. Also, we can speculate that the torque produced with improper use will lead to the complications mentioned and demonstrated in previous studies. Welding of the screw–plate interface may simply be a result of improper trajectory and cross-threading. However, if we assume that torque limiters prevent excessive torque no matter how they are used, high insertion speeds may compound the effect of welding. Additional biomechanical studies with full locked plate osteosynthesis constructs on bone specimens are planned to further characterize the potential complications of this issue.
Proper surgical technique must be used to ensure that surgical fracture management is long-lasting. Plate implantation and screw implantation are among the most common orthopedic procedures performed. Plate and screw osteosynthesis can be done with nonlocking or locking plate and screw constructs or with hybrid fixation that incorporates both methods.
Nonlocking plate and screw osteosynthesis uses friction-fit for fixation. In osteoporotic bone, less torque is generated because of poor bone quality, and thus less friction force between plate and bone.1,2 Locked plating has dramatically changed fracture management, especially in frail and comminuted osteoporotic bone, with significant advantages over conventional plating.3-7
Development of locked plating systems, including the Less Invasive Stabilization System (LISS; DePuy Synthes) with its soft-tissue and fracture-fragment preservation, has changed treatment of distal femur and proximal tibia fractures. Cole and colleagues8 reported stable fixation and union in 97% of their patients. The LISS system proved to be stable, but there were cases of implant removal difficulty with this titanium construct. In 1 of the 10 cases in which the LISS plate was removed, 4 of the 11 locking screws were welded to the plate.8
Cold welding, in which similar metals are chemically bonded together under extreme pressure, is a complication associated with use of titanium-only plates and screws.9 This process, which is more likely to happen if cross-threading occurs within the screw–plate interface, can make screw removal extremely difficult. Screw removal difficulty strips screw heads, and often the surgeon must use either metal cutting instruments or trephines to remove screw remnants, which often results in retained implant or debris and damage or necrosis to surrounding bone.9,10
Locking screws are often inserted under power with a torque-limiting device attached to the drill mechanism to reduce the risk of lock screw overtightening and to try to prevent difficult implant removal. Although standard practice is to insert the screw and stop just before screw head engagement, with final tightening with a torque limiter and hand power, final tightening is often inadvertently done under power.3 Most technique guides instruct surgeons how to insert screws under power while using a torque limiter, but the exact technique is not emphasized.
We conducted a study to determine if rotational speed of screw insertion affects maximum torque on screw with use of a torque limiter. We describe proper use of a torque limiter as well as possible pitfalls. We hypothesized that improper use would result in substantially higher than expected insertion torque.
Materials and Methods
Torque-Limiting Attachments, Torque Wrench, and Drill
The Small Fragment Locking Compression Plate System (Synthes) includes a 1.5-Nm torque-limiting attachment and quick-coupling wooden handles and Star Drive attachments. All devices in this study were in active use at 6 urban institutions (3 level I trauma centers, 2 level II trauma centers, 1 level III hospital). Permission to obtain and test each device was granted by each institution.
A 0.25-inch dial torque wrench (751LDIN; CDI Torque Products) was purchased through an established distributor. The manufacturer includes a traceable certificate of accuracy to verify correct calibration. The torque wrench has a torque range of 0 to 9 Nm with visual increment demarcations of 0.2 Nm and a memory needle to retain maximum torque measurement. The same torque wrench was used in each experiment in order to maintain consistent measurements between devices. It was reset to zero after each use.
This study used a 0.5-inch, 19.2-V lithium drill (Craftsman C3) with 2 speed options: 0 to 440 rpm high torque and 0 to 1600 rpm high speed. This device provides variable torque output with a maximum output of 38.4 Nm. For this study, all measurements were done with the device on its high torque setting.
Maximum Torque Determination for Different Scenarios
Each torque limiter was evaluated for variations in maximum torque under 4 different scenarios. In each scenario, the torque limiter was coupled to the Star Drive attachment and then to that scenario’s rotating force. The completed system was then inserted into the torque wrench, which was secured to a flat working surface and rotated in accordance with each scenario; maximum torque was measured and recorded (Figures 1, 2). A torque-limiting event was defined as a single audible click on the torque limiter.
In scenario 1, each torque-limiting attachment system was attached to a quick-coupling wooden handle. The completed system was then rotated at controlled low velocity under hand power until 1 torque-limiting event occurred. This scenario was also used as an internal control to verify that the torque limiters were calibrated correctly.
In Scenario 2, the device was again attached to a quick-coupling wooden handle. The completed system was rotated at high velocity under hand power until multiple torque-limiting events occurred in a row. High velocity was defined as the operator freely rotating the wooden handle in a single action with full power resulting in multiple torque-limiting events.
In Scenario 3, the device was attached to a power drill braced to the flat working surface and rotated at low velocity under power until 1 torque-limiting event occurred.
In Scenario 4, the device was again attached to a power drill braced to the flat working surface. The completed system was rotated at high velocity under power until multiple torque-limiting events occurred.
After each trial, we recorded maximum torque achieved before each device’s torque-limiting event. Either an orthopedic surgery resident or a qualified medical student tested each torque-limiting device in each standardized testing scenario.
Statistical Analysis
Experiments for each torque limiter were repeated for 3 trials of each of the 4 different scenarios. For comparative statistics between experiments, maximum torque measurements were expressed as means and SDs; 95% confidence interval (95% CI) was calculated and reported to determine extent of variation within a single group. One-way analysis of variance (ANOVA) and Tukey post hoc tests were performed between groups for comparison of the normally distributed data. Significance was set at P ≤ .05.
Results
During simulation, we successfully measured maximum torque achieved with each torque limiter under the 4 different scenarios. All testing was done by 2 operators. ANOVA demonstrated significant (P ≤ .001) differences in torque among the scenarios.
In scenario 1, mean (SD) maximum torque under hand power at low velocity was 1.49 (0.15) Nm (95% CI, 1.43-1.55), near the advertised maximum torque of 1.5 Nm, with relatively minimal variation between devices. This scenario confirmed proper calibration of properly used torque limiters. Mean maximum torque ranged from 1.25 to 1.93 Nm.
In scenario 2, mean (SD) maximum torque under hand power at high velocity was 3.73 (0.79) Nm (95% CI, 3.33-4.13), a 2.5-fold increase compared with scenario 1 (P < .0001) (Figure 3). There also was an increase in variation of maximum torque between trials of individual devices and between different devices. Mean maximum torque ranged from 2.27 to 5.53 Nm.
In scenario 3, mean (SD) maximum torque under drill power at controlled low velocity was 1.47 (0.14) Nm (95% CI, 1.37-1.56), again near the advertised maximum torque of 1.5 Nm, with relatively minimal variation. Mean maximum torque ranged from 1.10 to 1.73 Nm.
In scenario 4, mean (SD) maximum torque under drill power at full power/high velocity was 5.37 (0.90) Nm (95% CI, 4.92-5.83), a 3.65-fold increase compared with scenario 3 (P < .0001) (Figure 3). Mean maximum torque measured in 3 tests ranged from 3.40 to 6.92 Nm.
There was no significant difference in mean maximum torque between the scenarios of hand power at low velocity and drill power at low velocities (P = .999) (Figure 4). Highest maximum torque from any device was 9.0 Nm (drill at full power). Results are summarized in the Table. There was no statistical significance in the test between the 2 test operators.
Discussion
Maximum torque was measured using a torque-limiting attachment under 4 different simulated scenarios. Our goals were to determine if varying practice and rotational velocity would affect maximum insertional torque and to measure consistency among torque limiters. We designed the scenarios to mimic practice patterns, including hand insertion and power insertion of locking screws. Results demonstrated that misuse of a torque-limiting device may inadvertently produce insertional torque substantially higher than recommended. Highest maximum torque was 9.0 Nm, which is 6.0-fold higher than expected for a locking screw using a 1.5-Nm torque limiter.
Our study results showed that insertion under controlled hand power (and low-velocity drill power) until 1 torque-limiting event occurred produced the most consistent and predictable results. Insertion under drill power or high-velocity hand power produced multiple sequential torque-limiting events, yielding inaccurate insertion torque. Low-velocity insertion under hand power, or carefully controlled drill power, consistently produced torque similar to advertised values.
Manufacturers’ technique guides are available for proximal humerus locking compression plate (LCP) systems, small-fragment LCP systems, the Proximal Humeral Interlocking System (PHILOS; DePuy Synthes), and the LISS. These technique guides clearly state that insertion can be performed under power. Only the PHILOS and LISS guides state that insertion should be performed under power until a single click is heard or that final tightening should be completed under hand power. The proximal humerus LCP guide states that surgeons should insert the locking screw under power until the torque-limiting device clicks. The small-fragment LCP guide states that insertion under power should always be completed with the torque-limiting attachment; there is no mention of reducing power or a single click (this may give the surgeon a false sense of security).
Screw overtightening and head/thread stripping can make screw removal challenging.10 Removal rates for LISS plates range from 8% to 26%, and removal is often reported as taking longer than the index procedure, with complication rates as high as 47%.11-13 Bae and colleagues3 reported significant difficulty in removing 24 of 279 self-tapping locking screws (3.5 mm).
It is important to note that these complications, most notably cold welding, are mostly associated with titanium locking plate and screw constructs. Although stainless steel constructs have gained favor, titanium constructs are still widely used around the world.14,15
In 10% of cases in a laboratory setting, insertion of a 3.5-mm locking screw at 4 to 6 Nm damaged the screw.9 Removal of 3.5-mm locking screws had a stripping rate of 8.6%, and use of the torque limiter did not make removal easy all the time.3 Torque limiters are set specific to each screw diameter to reduce the risk of damage/stripping or even overtightening. Even when a surgeon intends to stop a drill before locking, final tightening often inadvertently occurs under power.3
Cold welding is often described as a cause of difficult implant removal.3,12 According to a newer definition, this process is independent of temperature and can occur when 2 metallic surfaces are in direct contact.16 High contact pressures between 2 similar metals can lead to this solid state welding.17 Theoretically, improper use of torque limiters can increase the risk of welding; however, it appears to be associated only with titanium locking plate and screw constructs.
Locked plating osteosynthesis is a valuable tool for fracture management, but improper use can have significant consequences, including morbid implant removal procedures, which are more difficult and time-consuming than the index surgery. We determined that proper use of torque limiters involves insertion under hand or power control at slow velocity until 1 torque-limiting event occurs. Many orthopedic surgeons may assume that torque limiters are accurate no matter how screws are inserted into locking plates. In addition, they may be unaware guidelines exist, as these are often deeply embedded within text. Therefore, we must emphasize that torque limiters can be inaccurate when used improperly.
One limitation of this study is that it tested only the Synthes 1.5-Nm torque-limiting attachment, though we can speculate that torque limiters designed for larger screws and limiters manufactured by different companies will behave similarly. Another limitation is that we did not obtain the hospitals’ service records for the tested equipment and assumed the equipment was properly checked for accuracy by the providing company. However, we hypothesized that, if maintenance were an issue, then our results would not be similar across all sites tested.
These tests involved a torque limiter linked to a torque-measuring device and may not perfectly represent actual torque measured at the locked screw–thread interface. However, we think our construct accurately determines the torque produced at the level of the driver tip. Also, we can speculate that the torque produced with improper use will lead to the complications mentioned and demonstrated in previous studies. Welding of the screw–plate interface may simply be a result of improper trajectory and cross-threading. However, if we assume that torque limiters prevent excessive torque no matter how they are used, high insertion speeds may compound the effect of welding. Additional biomechanical studies with full locked plate osteosynthesis constructs on bone specimens are planned to further characterize the potential complications of this issue.
1. Sommer C, Babst R, Müller M, Hanson B. Locking compression plate loosening and plate breakage: a report of four cases. J Orthop Trauma. 2004;18(8):571-577.
2. Schütz M, Südkamp NP. Revolution in plate osteosynthesis: new internal fixator systems. J Orthop Sci. 2003;8(2):252-258.
3. Bae JH, Oh JK, Oh CW, Hur CR. Technical difficulties of removal of locking screw after locking compression plating. Arch Orthop Trauma Surg. 2009;129(1):91-95.
4. Frigg R. Locking compression plate (LCP). An osteosynthesis plate based on the dynamic compression plate and the point contact fixator (PC-Fix). Injury. 2001;32(suppl 2):63-66.
5. Frigg R. Development of the locking compression plate. Injury. 2003;34(suppl 2):B6-B10.
6. Korner J, Lill H, Müller LP, Rommens PM, Schneider E, Linke B. The LCP-concept in the operative treatment of distal humerus fractures—biological, biomechanical and surgical aspects. Injury. 2003;34(suppl 2):B20-B30.
7. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
8. Cole PA, Zlowodzki M, Kregor PJ. Treatment of proximal tibia fractures using the Less Invasive Stabilization System: surgical experience and early clinical results in 77 fractures. J Orthop Trauma. 2004;18(8):528-535.
9. Ehlinger M, Adam P, Simon P, Bonnomet F. Technical difficulties in hardware removal in titanium compression plates with locking screws. Orthop Traumatol Surg Res. 2009;95(5):373-376.
10. Gopinathan NR, Dhillon MS, Kumar R. Surgical technique: simple technique for removing a locking recon plate with damaged screw heads. Clin Orthop Relat Res. 2013;471(5):1572-1575.
11. Pattison G, Reynolds J, Hardy J. Salvaging a stripped drive connection when removing screws. Injury. 1999;30(1):74-75.
12. Raja S, Imbuldeniya AM, Garg S, Groom G. Difficulties encountered removing locked plates. Ann R Coll Surg Engl. 2012;94(7):502-505.
13. Kumar G, Dunlop C. Case report: a technique to remove a jammed locking screw from a locking plate. Clin Orthop Relat Res. 2011;469(2):613-616.
14. Disegi JA. Titanium alloys for fracture fixation implants. Injury. 2000;31(suppl 4):14-17.
15. El-Zayat BF, Ruchholtz S, Efe T, Paletta J, Kreslo D, Zettl R. Results of titanium locking plate and stainless steel cerclage wire combination in femoral fractures. Indian J Orthop. 2013;47(5):454-458.
16. Van Nortwick SS, Yao J, Ladd AL. Titanium integration with bone, welding, and screw head destruction complicating hardware removal of the distal radius: report of 2 cases. J Hand Surg. 2012;37(7):1388-1392.
17. Ferguson GS, Chaudhury MK, Sigal GB, Whitesides GM. Contact adhesion of thin gold films on elastomeric supports: cold welding under ambient conditions. Science. 1991;253(5021):776-778.
1. Sommer C, Babst R, Müller M, Hanson B. Locking compression plate loosening and plate breakage: a report of four cases. J Orthop Trauma. 2004;18(8):571-577.
2. Schütz M, Südkamp NP. Revolution in plate osteosynthesis: new internal fixator systems. J Orthop Sci. 2003;8(2):252-258.
3. Bae JH, Oh JK, Oh CW, Hur CR. Technical difficulties of removal of locking screw after locking compression plating. Arch Orthop Trauma Surg. 2009;129(1):91-95.
4. Frigg R. Locking compression plate (LCP). An osteosynthesis plate based on the dynamic compression plate and the point contact fixator (PC-Fix). Injury. 2001;32(suppl 2):63-66.
5. Frigg R. Development of the locking compression plate. Injury. 2003;34(suppl 2):B6-B10.
6. Korner J, Lill H, Müller LP, Rommens PM, Schneider E, Linke B. The LCP-concept in the operative treatment of distal humerus fractures—biological, biomechanical and surgical aspects. Injury. 2003;34(suppl 2):B20-B30.
7. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
8. Cole PA, Zlowodzki M, Kregor PJ. Treatment of proximal tibia fractures using the Less Invasive Stabilization System: surgical experience and early clinical results in 77 fractures. J Orthop Trauma. 2004;18(8):528-535.
9. Ehlinger M, Adam P, Simon P, Bonnomet F. Technical difficulties in hardware removal in titanium compression plates with locking screws. Orthop Traumatol Surg Res. 2009;95(5):373-376.
10. Gopinathan NR, Dhillon MS, Kumar R. Surgical technique: simple technique for removing a locking recon plate with damaged screw heads. Clin Orthop Relat Res. 2013;471(5):1572-1575.
11. Pattison G, Reynolds J, Hardy J. Salvaging a stripped drive connection when removing screws. Injury. 1999;30(1):74-75.
12. Raja S, Imbuldeniya AM, Garg S, Groom G. Difficulties encountered removing locked plates. Ann R Coll Surg Engl. 2012;94(7):502-505.
13. Kumar G, Dunlop C. Case report: a technique to remove a jammed locking screw from a locking plate. Clin Orthop Relat Res. 2011;469(2):613-616.
14. Disegi JA. Titanium alloys for fracture fixation implants. Injury. 2000;31(suppl 4):14-17.
15. El-Zayat BF, Ruchholtz S, Efe T, Paletta J, Kreslo D, Zettl R. Results of titanium locking plate and stainless steel cerclage wire combination in femoral fractures. Indian J Orthop. 2013;47(5):454-458.
16. Van Nortwick SS, Yao J, Ladd AL. Titanium integration with bone, welding, and screw head destruction complicating hardware removal of the distal radius: report of 2 cases. J Hand Surg. 2012;37(7):1388-1392.
17. Ferguson GS, Chaudhury MK, Sigal GB, Whitesides GM. Contact adhesion of thin gold films on elastomeric supports: cold welding under ambient conditions. Science. 1991;253(5021):776-778.
All-Inside Meniscal Repair Devices
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Meniscal Root Tears: Identification and Repair
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
Arthroscopic Management of Full-Thickness Rotator Cuff Tears in Major League Baseball Pitchers: The Lateralized Footprint Repair Technique
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Ulnar Collateral Ligament Repair: An Old Idea With a New Wrinkle
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Novel Intraoperative Technique to Visualize the Lower Cervical Spine: A Case Series
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
Minimum 5-Year Results With Duracon Press-Fit Metal-Backed Patellae
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.