User login
Brain differences suggest therapeutic targets in Takotsubo
A new study has identified differences in the brain present in patients with the cardiac disorder Takotsubo syndrome versus control scans, which may lead to new therapeutic targets.
Takotsubo syndrome is an acute heart failure cardiomyopathy mimicking an acute myocardial infarction in its presentation, but on investigation, no obstructive coronary disease is present. The syndrome, which mainly affects women, typically occurs in the aftermath of intense emotional or physical stress and has become known as “broken heart syndrome.”
The mechanism by which emotional processing in the context of stress leads to significant cardiac injury and acute left ventricular dysfunction is not understood. So, the current study examined both structural and functional effects in the brain in patients with Takotsubo syndrome to shed more light on the issue.
“The abnormalities in the thalamus-amygdala-insula and basal ganglia support the concept of involvement of higher-level function centers in Takotsubo syndrome, and interventions aimed at modulating these may be of benefit,” the authors conclude.
The study was published online in JACC: Heart Failure.
Lead author Hilal Khan, MB BCh, BAO, from the University of Aberdeen (Scotland), explained to this news organization that patients with Takotsubo syndrome have a substantial drop in heart function and show an apical ballooning of the heart.
It is a relatively newly defined condition and was first described in 1990 in Japan, and so named because the heart was thought to resemble the Takotsubo pot used by Japanese fishermen to trap octopus.
Although uncommon, the condition is not rare. Dr. Khan estimates that about 1 in 20 women with suspected MI turn out to have Takotsubo syndrome, with cases increasing in times of global stress such as in the recent pandemic.
While patients tend to recover in a few weeks and the pumping function of the heart usually returns to normal, there are some long-term cardiac complications including a reduction in global longitudinal strain, and patients have similar long-term outcomes as those with MI.
“It is believed that these cardiac changes may be triggered by changes in the brain caused by emotional stress, so we wanted to look at this more closely,” Dr. Khan said.
There have been a couple of studies published previously looking at brain changes in Takotsubo syndrome, but they haven’t reported patients in the acute stage of the condition and they haven’t compared the patients to controls, he noted.
For the current study, the researchers looked at brain scans for 25 acute Takotsubo patients and in 25 controls matched for age, gender, comorbidities, and medications. All the patients and controls were examined using the same MRI scanner in the same hospital.
“This is the largest structural and functional brain study of acute Takotsubo syndrome patients compared with matched control subjects,” Dr. Khan said.
The researchers looked at many different factors including brain volume in different regions, cortical thickness, small-vessel disease, and functional and structural connectivity to try and obtain a complete holistic view of the brain.
Key findings were that patients with Takotsubo syndrome had smaller brain volumes, compared with matched controls, driven by a reduction in brain surface area. In contrast, the insula and thalamus regions were larger.
“A reduction in brain volume could be caused by inflammation; this is often seen in depression,” Dr. Khan commented.
The researchers also found that certain areas of the brain had a reduction in functional connectivity, particularly the thalamus – the central autonomic area of the brain, which regulates the autonomic nervous system – and also the insula region, which is also involved in the autonomic regulation of the heart.
They suggest that there may be a loss of parasympathetic inhibition in Takotsubo syndrome, which would fit the theory that Takotsubo brings with it a surge of catecholamines, which could injure the heart.
Reduced functional connectivity was also seen in parts of the basal ganglia, abnormalities of which have been associated with an increased risk of both arrhythmias, and in the amygdala, similar to patients with a tendency to catastrophize events.
The other observation was that there appeared to be an increase in structural connectivity in certain areas of the brain.
“Structural pathways seem to be increased but functional connectivity was reduced, so while physical pathways are enhanced, they don’t seem to be doing anything,” Dr. Khan said. “We don’t know why this occurs, or if this has happened over time and made the brain and heart more vulnerable in some way.”
One possibility is that ,under a significant emotional stress, the brain may divert function from some areas to others to be able to cope, and that this results in reduced functioning in areas of the brain responsible for regulating the heart, Dr. Khan suggested.
“We believe this study confirms that the brain is involved in Takotsubo syndrome, and we have identified markers in the brain that may be contributing to the condition,” he said.
The researchers are planning to further study these markers and whether it might be possible to modulate these changes with various interventions such as exercise or mindfulness.
“We believe there is some interface between the brain changes and the impact on the heart. We don’t think it is just the release of catecholamines that causes damage to the heart. We think there is something else happening as well,” Dr. Khan commented.
It is also possible that the hearts of patients with Takotsubo syndrome are predisposed in some way and more vulnerable to this condition occurring.
“It will be important to obtain a greater understanding of the triggers and identify people who may be vulnerable,” Dr. Khan noted. “Around 10% of individuals who experience Takotsubo syndrome will have a recurrence, so we need to try and develop preventative strategies to reduce this.”
He suggested that possible preventive or therapeutic approaches may involve interventions such as exercise or mindfulness.
This work was supported by National Health Service Grampian Endowment. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study has identified differences in the brain present in patients with the cardiac disorder Takotsubo syndrome versus control scans, which may lead to new therapeutic targets.
Takotsubo syndrome is an acute heart failure cardiomyopathy mimicking an acute myocardial infarction in its presentation, but on investigation, no obstructive coronary disease is present. The syndrome, which mainly affects women, typically occurs in the aftermath of intense emotional or physical stress and has become known as “broken heart syndrome.”
The mechanism by which emotional processing in the context of stress leads to significant cardiac injury and acute left ventricular dysfunction is not understood. So, the current study examined both structural and functional effects in the brain in patients with Takotsubo syndrome to shed more light on the issue.
“The abnormalities in the thalamus-amygdala-insula and basal ganglia support the concept of involvement of higher-level function centers in Takotsubo syndrome, and interventions aimed at modulating these may be of benefit,” the authors conclude.
The study was published online in JACC: Heart Failure.
Lead author Hilal Khan, MB BCh, BAO, from the University of Aberdeen (Scotland), explained to this news organization that patients with Takotsubo syndrome have a substantial drop in heart function and show an apical ballooning of the heart.
It is a relatively newly defined condition and was first described in 1990 in Japan, and so named because the heart was thought to resemble the Takotsubo pot used by Japanese fishermen to trap octopus.
Although uncommon, the condition is not rare. Dr. Khan estimates that about 1 in 20 women with suspected MI turn out to have Takotsubo syndrome, with cases increasing in times of global stress such as in the recent pandemic.
While patients tend to recover in a few weeks and the pumping function of the heart usually returns to normal, there are some long-term cardiac complications including a reduction in global longitudinal strain, and patients have similar long-term outcomes as those with MI.
“It is believed that these cardiac changes may be triggered by changes in the brain caused by emotional stress, so we wanted to look at this more closely,” Dr. Khan said.
There have been a couple of studies published previously looking at brain changes in Takotsubo syndrome, but they haven’t reported patients in the acute stage of the condition and they haven’t compared the patients to controls, he noted.
For the current study, the researchers looked at brain scans for 25 acute Takotsubo patients and in 25 controls matched for age, gender, comorbidities, and medications. All the patients and controls were examined using the same MRI scanner in the same hospital.
“This is the largest structural and functional brain study of acute Takotsubo syndrome patients compared with matched control subjects,” Dr. Khan said.
The researchers looked at many different factors including brain volume in different regions, cortical thickness, small-vessel disease, and functional and structural connectivity to try and obtain a complete holistic view of the brain.
Key findings were that patients with Takotsubo syndrome had smaller brain volumes, compared with matched controls, driven by a reduction in brain surface area. In contrast, the insula and thalamus regions were larger.
“A reduction in brain volume could be caused by inflammation; this is often seen in depression,” Dr. Khan commented.
The researchers also found that certain areas of the brain had a reduction in functional connectivity, particularly the thalamus – the central autonomic area of the brain, which regulates the autonomic nervous system – and also the insula region, which is also involved in the autonomic regulation of the heart.
They suggest that there may be a loss of parasympathetic inhibition in Takotsubo syndrome, which would fit the theory that Takotsubo brings with it a surge of catecholamines, which could injure the heart.
Reduced functional connectivity was also seen in parts of the basal ganglia, abnormalities of which have been associated with an increased risk of both arrhythmias, and in the amygdala, similar to patients with a tendency to catastrophize events.
The other observation was that there appeared to be an increase in structural connectivity in certain areas of the brain.
“Structural pathways seem to be increased but functional connectivity was reduced, so while physical pathways are enhanced, they don’t seem to be doing anything,” Dr. Khan said. “We don’t know why this occurs, or if this has happened over time and made the brain and heart more vulnerable in some way.”
One possibility is that ,under a significant emotional stress, the brain may divert function from some areas to others to be able to cope, and that this results in reduced functioning in areas of the brain responsible for regulating the heart, Dr. Khan suggested.
“We believe this study confirms that the brain is involved in Takotsubo syndrome, and we have identified markers in the brain that may be contributing to the condition,” he said.
The researchers are planning to further study these markers and whether it might be possible to modulate these changes with various interventions such as exercise or mindfulness.
“We believe there is some interface between the brain changes and the impact on the heart. We don’t think it is just the release of catecholamines that causes damage to the heart. We think there is something else happening as well,” Dr. Khan commented.
It is also possible that the hearts of patients with Takotsubo syndrome are predisposed in some way and more vulnerable to this condition occurring.
“It will be important to obtain a greater understanding of the triggers and identify people who may be vulnerable,” Dr. Khan noted. “Around 10% of individuals who experience Takotsubo syndrome will have a recurrence, so we need to try and develop preventative strategies to reduce this.”
He suggested that possible preventive or therapeutic approaches may involve interventions such as exercise or mindfulness.
This work was supported by National Health Service Grampian Endowment. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study has identified differences in the brain present in patients with the cardiac disorder Takotsubo syndrome versus control scans, which may lead to new therapeutic targets.
Takotsubo syndrome is an acute heart failure cardiomyopathy mimicking an acute myocardial infarction in its presentation, but on investigation, no obstructive coronary disease is present. The syndrome, which mainly affects women, typically occurs in the aftermath of intense emotional or physical stress and has become known as “broken heart syndrome.”
The mechanism by which emotional processing in the context of stress leads to significant cardiac injury and acute left ventricular dysfunction is not understood. So, the current study examined both structural and functional effects in the brain in patients with Takotsubo syndrome to shed more light on the issue.
“The abnormalities in the thalamus-amygdala-insula and basal ganglia support the concept of involvement of higher-level function centers in Takotsubo syndrome, and interventions aimed at modulating these may be of benefit,” the authors conclude.
The study was published online in JACC: Heart Failure.
Lead author Hilal Khan, MB BCh, BAO, from the University of Aberdeen (Scotland), explained to this news organization that patients with Takotsubo syndrome have a substantial drop in heart function and show an apical ballooning of the heart.
It is a relatively newly defined condition and was first described in 1990 in Japan, and so named because the heart was thought to resemble the Takotsubo pot used by Japanese fishermen to trap octopus.
Although uncommon, the condition is not rare. Dr. Khan estimates that about 1 in 20 women with suspected MI turn out to have Takotsubo syndrome, with cases increasing in times of global stress such as in the recent pandemic.
While patients tend to recover in a few weeks and the pumping function of the heart usually returns to normal, there are some long-term cardiac complications including a reduction in global longitudinal strain, and patients have similar long-term outcomes as those with MI.
“It is believed that these cardiac changes may be triggered by changes in the brain caused by emotional stress, so we wanted to look at this more closely,” Dr. Khan said.
There have been a couple of studies published previously looking at brain changes in Takotsubo syndrome, but they haven’t reported patients in the acute stage of the condition and they haven’t compared the patients to controls, he noted.
For the current study, the researchers looked at brain scans for 25 acute Takotsubo patients and in 25 controls matched for age, gender, comorbidities, and medications. All the patients and controls were examined using the same MRI scanner in the same hospital.
“This is the largest structural and functional brain study of acute Takotsubo syndrome patients compared with matched control subjects,” Dr. Khan said.
The researchers looked at many different factors including brain volume in different regions, cortical thickness, small-vessel disease, and functional and structural connectivity to try and obtain a complete holistic view of the brain.
Key findings were that patients with Takotsubo syndrome had smaller brain volumes, compared with matched controls, driven by a reduction in brain surface area. In contrast, the insula and thalamus regions were larger.
“A reduction in brain volume could be caused by inflammation; this is often seen in depression,” Dr. Khan commented.
The researchers also found that certain areas of the brain had a reduction in functional connectivity, particularly the thalamus – the central autonomic area of the brain, which regulates the autonomic nervous system – and also the insula region, which is also involved in the autonomic regulation of the heart.
They suggest that there may be a loss of parasympathetic inhibition in Takotsubo syndrome, which would fit the theory that Takotsubo brings with it a surge of catecholamines, which could injure the heart.
Reduced functional connectivity was also seen in parts of the basal ganglia, abnormalities of which have been associated with an increased risk of both arrhythmias, and in the amygdala, similar to patients with a tendency to catastrophize events.
The other observation was that there appeared to be an increase in structural connectivity in certain areas of the brain.
“Structural pathways seem to be increased but functional connectivity was reduced, so while physical pathways are enhanced, they don’t seem to be doing anything,” Dr. Khan said. “We don’t know why this occurs, or if this has happened over time and made the brain and heart more vulnerable in some way.”
One possibility is that ,under a significant emotional stress, the brain may divert function from some areas to others to be able to cope, and that this results in reduced functioning in areas of the brain responsible for regulating the heart, Dr. Khan suggested.
“We believe this study confirms that the brain is involved in Takotsubo syndrome, and we have identified markers in the brain that may be contributing to the condition,” he said.
The researchers are planning to further study these markers and whether it might be possible to modulate these changes with various interventions such as exercise or mindfulness.
“We believe there is some interface between the brain changes and the impact on the heart. We don’t think it is just the release of catecholamines that causes damage to the heart. We think there is something else happening as well,” Dr. Khan commented.
It is also possible that the hearts of patients with Takotsubo syndrome are predisposed in some way and more vulnerable to this condition occurring.
“It will be important to obtain a greater understanding of the triggers and identify people who may be vulnerable,” Dr. Khan noted. “Around 10% of individuals who experience Takotsubo syndrome will have a recurrence, so we need to try and develop preventative strategies to reduce this.”
He suggested that possible preventive or therapeutic approaches may involve interventions such as exercise or mindfulness.
This work was supported by National Health Service Grampian Endowment. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Evolocumab’s LDL lowering surpassed inclisiran’s in ORION-3
Patients who received an injection of inclisiran (Leqvio), a small interfering RNA (siRNA) agent, every 6 months for as long as 4 years safely maintained about a 45% reduction from baseline in their level of low-density lipoprotein cholesterol (LDL-C) in an open-label extension study with 382 patients.
In addition to providing the longest reported treatment experience with inclisiran, which received Food and Drug Administration marketing approval a little over a year ago, the results also suggest with the most definitive evidence to date that inclisiran is less effective for lowering LDL-C, compared with a class of medications that reduce LDL-C by a related but distinct mechanism: antibodies that directly inhibit activity of the proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme, a drug class that includes alirocumab (Praluent) and evolocumab (Repatha). Inclisiran cuts PCSK9 activity by blocking this enzyme’s gene transcription in liver cells thereby interfering with PCSK9 production.
Results from this study, the ORION-3 trial, provide “the first prospective long-term evaluation of the durability and safety of an siRNA-based therapy to provide clinically meaningful reductions in LDL cholesterol with a convenient dosing schedule,” wrote Kausik K. Ray, MD, and coauthors in a report in The Lancet Diabetes & Endocrinology.
The findings “provide assurance that siRNA-based therapies are safe and have the potential to provide a convenient approach to managing” LDL-C, wrote Dr. Ray, a cardiologist and professor of public health at Imperial College London, and his associates.
Evolocumab surpasses inclisiran in crossover cohort
The new data from ORION-3 study included findings from 92 patients first treated with evolocumab injections every 2 weeks for a year, an intervention that lowered their LDL-C levels by an average of about 60%, compared with their pretreatment level. ORION-3’s study design then crossed these patients to treatment with injections of inclisiran twice a year during 3 further years of follow-up, during which their average LDL levels reset to a roughly 45% drop from baseline, a potentially clinically meaningful difference, commented Robert S. Rosenson, MD, a lipid management specialist who was not involved in the ORION-3 study.
“This is the first evidence that compared the two classes” within a single study, thereby avoiding a problematic cross-study comparison. “That’s why the data are important. They underscore that the monoclonal antibodies are more effective for lowering LDL-C,” compared with inclisiran, said Dr. Rosenson, professor and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai in New York.
The findings “confirm in a trial that the PCSK9 monoclonal antibodies are indeed more potent,” he said in an interview.
But Dr. Rosenson acknowledged that, while this analysis used data on patients treated with evolocumab and then switched to inclisiran collected prospectively in a single study, it has the limitation of involving a comparison that was not prespecified. The primary goal of the evolocumab-to-inclisiran switch included in ORION-3 was to assess the ease, safety, and efficacy of a switch to inclisiran from treatment with a PCSK9 antibody and was not intended to compare the two drug classes.
The roughly 15% absolute difference in LDL-C lowering between the two tested drug classes can have substantial clinical implications for patients who start treatment with highly elevated levels of LDL-C, more than 190 mg/dL, because they have heterozygous familial hypercholesterolemia, are unable to take a statin because of intolerance, or both. The difference in LDL-C reduction with an antibody or with inclisiran could mean the difference between whether or not a patient like this achieves their LDL-C goal level, Dr. Rosenson explained.
Inclisiran’s upside
On the other hand, inclisiran has a couple of important advantages. First, its mechanism of action means that effective treatment involves one injection every 6 months following a patient’s first two injections at onset and after 90 days, with all injections administered in a clinician’s office. In contrast, both of the monoclonal antibodies require injections every other week, a schedule that depends on patient self-injections using prefilled syringes obtained from a pharmacy.
Twice-a-year dosing by a clinician can be a major attraction because it helps ensure treatment compliance, aids patients with physical or psychological limitations to self-injection, reduces the pill burden for patients who require multiple medications, and facilitates frequent travelers who would otherwise need to carry syringes with them on trips, Dr. Rosenson noted.
The second big advantage of office-based administration of inclisiran for U.S. Medicare patients is that the treatment is billed under a patient’s part B coverage, usually resulting in easier coverage and a significantly lower patient co-pay, compared with Medicare’s coverage for a pharmacy-dispensed agent, which is covered under Medicare part D. “Part B coverage is financially more doable” for most Medicare patients, said Dr. Rosenson.
The administration schedule for inclisiran as well as its superior Medicare coverage makes the agent “transformative” for LDL-C lowering in patients for whom treatment delivery, frequency, and payment are issues, he said.
Inclisiran uptake modest after FDA approval
Despite these pluses, uptake of inclisiran has been modest since it received U.S. marketing approval in December 2021. In its most recent quarterly financial filing, in October 2022, Novartis reported total worldwide income from inclisiran (Leqvio) of $70 million during the first 9 months of 2022, although a Novartis spokesperson noted that the company has seen “positive trends in uptake” over the course of 2022. Inclisiran is labeled as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who require additional lowering” of LDL-C.
During 2022, inclisiran uptake lagged because of the usual problems that slow the introduction of new drugs and new drug classes, especially ones that require dosing by a clinician. Months were spent waiting for billing codes to roll out, for clinical staffs to incorporate inclisiran injections into their routines, and for commercial insurers to get up to speed on their coverage, Dr. Rosenson said.
Also, a key step for widespread uptake of a new medication for improving cardiovascular disease outcomes – results from phase 3 studies that document safety and efficacy for these outcomes – remains several years off. The ORION-4 trial and the VICTORION-2P trial, each assessing the impact of inclisiran on cardiovascular disease events in roughly 15,000 people, will need about another 3-4 years before their results become available.
Professional medical societies that issue cardiovascular-disease management guidelines “prefer agents with proven benefits in phase 3 trials,” Dr. Rosenson noted.
Hence, the most recent update to U.S. LDL-C–management guidelines, released in the second half of 2022 by the American College of Cardiology as an Expert Consensus Decision Pathway, said this about the current role for inclisiran: “At the present time, a PCSK9 monoclonal antibody is preferred as the initial PCSK9 inhibitor of choice in view of its demonstrated safety, efficacy, and benefits for cardiovascular outcomes in the FOURIER [for evolocumab] and ODYSSEY Outcomes [for alirocumab] trials. The ORION-4 and VICTORION-2P cardiovascular outcomes trials with inclisiran are currently underway, and their completion is anticipated in 2026 and 2027, respectively. In view of the twice-yearly dosing regimen, inclisiran may be considered in patients with demonstrated poor adherence to PCSK9 monoclonal antibodies. Patients with adverse effects from both PSCK9 monoclonal antibodies or those who may be unable to self-inject may also be considered for therapy with inclisiran.”
ORION-3 extended the ORION-1 trial
The ORION-1 study was a phase 2 placebo-controlled, dose-ranging safety and efficacy assessment of inclisiran that gave patients two injections of the drug, at day zero and 90 days, and followed them for an additional 120 days (210 days total follow-up duration), and in some cases for as long as 360 days total. Of the 370 patients who received inclisiran in ORION-1, 290 agreed to continue inclisiran in the open-label extension, ORION-3. ORION-1 also included 127 patients randomized to initial placebo treatment, and 92 of these patients agreed to continue in ORION-3 and became the patients initially treated with evolocumab injections every other week for 1 year followed by initiation of an inclisiran regimen.
The primary outcome of ORION-3 was the change in LDL-C from baseline (the ORION-1 baseline) after 210 days of receiving inclisiran in ORION-3 (or a total of roughly 570 days after the start of ORION-1). The primary endpoint showed that, at day 210 of ORION-3 the average reduction in LDL-C from the original baseline level was 47.5%.
But a “more important” outcome, said Dr. Ray when he first reported the ORION-3 results during the American Heart Association scientific sessions in Chicago in November 2022, was that, overall, during 4 years on inclisiran this cohort showed an average cut in LDL-C from baseline of about 45% that consistently remained at this level throughout the 4 years of treatment.
“This provides us with an idea of what happens with chronic inclisiran dosing,” Dr. Ray explained. “There was no loss of biological efficacy, and we achieved these clinically meaningful, time-averaged reductions with a good safety profile. The great thing is that when patients get their injections [every 6 months] you see a consistent LDL-C reduction. A twice-annual injection is an opportunity to redesign” the way patients receive preventive cardiology care and treatment to lower LDL-C, Dr Ray said.
ORION-1 was sponsored by The Medicines Company. ORION-3 was sponsored by Novartis (which acquired The Medicines Company). Dr. Ray has received consulting fees, personal fees, and research grants from Novartis, as well as consulting fees and research grants from Amgen, the company that markets evolocumab (Repatha), and research grants from Regeneron, the company that markets alirocumab (Praluent). He has also received consulting fee, personal fees, and research grants from numerous other companies. Dr. Rosenson has been a consultant to and has received research funding from Amgen, Novartis, and Regeneron, and he has received speaking fees from Amgen and Regeneron, and has ties to several other pharmaceutical companies.
This article was updated on 1/26/2023.
Patients who received an injection of inclisiran (Leqvio), a small interfering RNA (siRNA) agent, every 6 months for as long as 4 years safely maintained about a 45% reduction from baseline in their level of low-density lipoprotein cholesterol (LDL-C) in an open-label extension study with 382 patients.
In addition to providing the longest reported treatment experience with inclisiran, which received Food and Drug Administration marketing approval a little over a year ago, the results also suggest with the most definitive evidence to date that inclisiran is less effective for lowering LDL-C, compared with a class of medications that reduce LDL-C by a related but distinct mechanism: antibodies that directly inhibit activity of the proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme, a drug class that includes alirocumab (Praluent) and evolocumab (Repatha). Inclisiran cuts PCSK9 activity by blocking this enzyme’s gene transcription in liver cells thereby interfering with PCSK9 production.
Results from this study, the ORION-3 trial, provide “the first prospective long-term evaluation of the durability and safety of an siRNA-based therapy to provide clinically meaningful reductions in LDL cholesterol with a convenient dosing schedule,” wrote Kausik K. Ray, MD, and coauthors in a report in The Lancet Diabetes & Endocrinology.
The findings “provide assurance that siRNA-based therapies are safe and have the potential to provide a convenient approach to managing” LDL-C, wrote Dr. Ray, a cardiologist and professor of public health at Imperial College London, and his associates.
Evolocumab surpasses inclisiran in crossover cohort
The new data from ORION-3 study included findings from 92 patients first treated with evolocumab injections every 2 weeks for a year, an intervention that lowered their LDL-C levels by an average of about 60%, compared with their pretreatment level. ORION-3’s study design then crossed these patients to treatment with injections of inclisiran twice a year during 3 further years of follow-up, during which their average LDL levels reset to a roughly 45% drop from baseline, a potentially clinically meaningful difference, commented Robert S. Rosenson, MD, a lipid management specialist who was not involved in the ORION-3 study.
“This is the first evidence that compared the two classes” within a single study, thereby avoiding a problematic cross-study comparison. “That’s why the data are important. They underscore that the monoclonal antibodies are more effective for lowering LDL-C,” compared with inclisiran, said Dr. Rosenson, professor and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai in New York.
The findings “confirm in a trial that the PCSK9 monoclonal antibodies are indeed more potent,” he said in an interview.
But Dr. Rosenson acknowledged that, while this analysis used data on patients treated with evolocumab and then switched to inclisiran collected prospectively in a single study, it has the limitation of involving a comparison that was not prespecified. The primary goal of the evolocumab-to-inclisiran switch included in ORION-3 was to assess the ease, safety, and efficacy of a switch to inclisiran from treatment with a PCSK9 antibody and was not intended to compare the two drug classes.
The roughly 15% absolute difference in LDL-C lowering between the two tested drug classes can have substantial clinical implications for patients who start treatment with highly elevated levels of LDL-C, more than 190 mg/dL, because they have heterozygous familial hypercholesterolemia, are unable to take a statin because of intolerance, or both. The difference in LDL-C reduction with an antibody or with inclisiran could mean the difference between whether or not a patient like this achieves their LDL-C goal level, Dr. Rosenson explained.
Inclisiran’s upside
On the other hand, inclisiran has a couple of important advantages. First, its mechanism of action means that effective treatment involves one injection every 6 months following a patient’s first two injections at onset and after 90 days, with all injections administered in a clinician’s office. In contrast, both of the monoclonal antibodies require injections every other week, a schedule that depends on patient self-injections using prefilled syringes obtained from a pharmacy.
Twice-a-year dosing by a clinician can be a major attraction because it helps ensure treatment compliance, aids patients with physical or psychological limitations to self-injection, reduces the pill burden for patients who require multiple medications, and facilitates frequent travelers who would otherwise need to carry syringes with them on trips, Dr. Rosenson noted.
The second big advantage of office-based administration of inclisiran for U.S. Medicare patients is that the treatment is billed under a patient’s part B coverage, usually resulting in easier coverage and a significantly lower patient co-pay, compared with Medicare’s coverage for a pharmacy-dispensed agent, which is covered under Medicare part D. “Part B coverage is financially more doable” for most Medicare patients, said Dr. Rosenson.
The administration schedule for inclisiran as well as its superior Medicare coverage makes the agent “transformative” for LDL-C lowering in patients for whom treatment delivery, frequency, and payment are issues, he said.
Inclisiran uptake modest after FDA approval
Despite these pluses, uptake of inclisiran has been modest since it received U.S. marketing approval in December 2021. In its most recent quarterly financial filing, in October 2022, Novartis reported total worldwide income from inclisiran (Leqvio) of $70 million during the first 9 months of 2022, although a Novartis spokesperson noted that the company has seen “positive trends in uptake” over the course of 2022. Inclisiran is labeled as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who require additional lowering” of LDL-C.
During 2022, inclisiran uptake lagged because of the usual problems that slow the introduction of new drugs and new drug classes, especially ones that require dosing by a clinician. Months were spent waiting for billing codes to roll out, for clinical staffs to incorporate inclisiran injections into their routines, and for commercial insurers to get up to speed on their coverage, Dr. Rosenson said.
Also, a key step for widespread uptake of a new medication for improving cardiovascular disease outcomes – results from phase 3 studies that document safety and efficacy for these outcomes – remains several years off. The ORION-4 trial and the VICTORION-2P trial, each assessing the impact of inclisiran on cardiovascular disease events in roughly 15,000 people, will need about another 3-4 years before their results become available.
Professional medical societies that issue cardiovascular-disease management guidelines “prefer agents with proven benefits in phase 3 trials,” Dr. Rosenson noted.
Hence, the most recent update to U.S. LDL-C–management guidelines, released in the second half of 2022 by the American College of Cardiology as an Expert Consensus Decision Pathway, said this about the current role for inclisiran: “At the present time, a PCSK9 monoclonal antibody is preferred as the initial PCSK9 inhibitor of choice in view of its demonstrated safety, efficacy, and benefits for cardiovascular outcomes in the FOURIER [for evolocumab] and ODYSSEY Outcomes [for alirocumab] trials. The ORION-4 and VICTORION-2P cardiovascular outcomes trials with inclisiran are currently underway, and their completion is anticipated in 2026 and 2027, respectively. In view of the twice-yearly dosing regimen, inclisiran may be considered in patients with demonstrated poor adherence to PCSK9 monoclonal antibodies. Patients with adverse effects from both PSCK9 monoclonal antibodies or those who may be unable to self-inject may also be considered for therapy with inclisiran.”
ORION-3 extended the ORION-1 trial
The ORION-1 study was a phase 2 placebo-controlled, dose-ranging safety and efficacy assessment of inclisiran that gave patients two injections of the drug, at day zero and 90 days, and followed them for an additional 120 days (210 days total follow-up duration), and in some cases for as long as 360 days total. Of the 370 patients who received inclisiran in ORION-1, 290 agreed to continue inclisiran in the open-label extension, ORION-3. ORION-1 also included 127 patients randomized to initial placebo treatment, and 92 of these patients agreed to continue in ORION-3 and became the patients initially treated with evolocumab injections every other week for 1 year followed by initiation of an inclisiran regimen.
The primary outcome of ORION-3 was the change in LDL-C from baseline (the ORION-1 baseline) after 210 days of receiving inclisiran in ORION-3 (or a total of roughly 570 days after the start of ORION-1). The primary endpoint showed that, at day 210 of ORION-3 the average reduction in LDL-C from the original baseline level was 47.5%.
But a “more important” outcome, said Dr. Ray when he first reported the ORION-3 results during the American Heart Association scientific sessions in Chicago in November 2022, was that, overall, during 4 years on inclisiran this cohort showed an average cut in LDL-C from baseline of about 45% that consistently remained at this level throughout the 4 years of treatment.
“This provides us with an idea of what happens with chronic inclisiran dosing,” Dr. Ray explained. “There was no loss of biological efficacy, and we achieved these clinically meaningful, time-averaged reductions with a good safety profile. The great thing is that when patients get their injections [every 6 months] you see a consistent LDL-C reduction. A twice-annual injection is an opportunity to redesign” the way patients receive preventive cardiology care and treatment to lower LDL-C, Dr Ray said.
ORION-1 was sponsored by The Medicines Company. ORION-3 was sponsored by Novartis (which acquired The Medicines Company). Dr. Ray has received consulting fees, personal fees, and research grants from Novartis, as well as consulting fees and research grants from Amgen, the company that markets evolocumab (Repatha), and research grants from Regeneron, the company that markets alirocumab (Praluent). He has also received consulting fee, personal fees, and research grants from numerous other companies. Dr. Rosenson has been a consultant to and has received research funding from Amgen, Novartis, and Regeneron, and he has received speaking fees from Amgen and Regeneron, and has ties to several other pharmaceutical companies.
This article was updated on 1/26/2023.
Patients who received an injection of inclisiran (Leqvio), a small interfering RNA (siRNA) agent, every 6 months for as long as 4 years safely maintained about a 45% reduction from baseline in their level of low-density lipoprotein cholesterol (LDL-C) in an open-label extension study with 382 patients.
In addition to providing the longest reported treatment experience with inclisiran, which received Food and Drug Administration marketing approval a little over a year ago, the results also suggest with the most definitive evidence to date that inclisiran is less effective for lowering LDL-C, compared with a class of medications that reduce LDL-C by a related but distinct mechanism: antibodies that directly inhibit activity of the proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme, a drug class that includes alirocumab (Praluent) and evolocumab (Repatha). Inclisiran cuts PCSK9 activity by blocking this enzyme’s gene transcription in liver cells thereby interfering with PCSK9 production.
Results from this study, the ORION-3 trial, provide “the first prospective long-term evaluation of the durability and safety of an siRNA-based therapy to provide clinically meaningful reductions in LDL cholesterol with a convenient dosing schedule,” wrote Kausik K. Ray, MD, and coauthors in a report in The Lancet Diabetes & Endocrinology.
The findings “provide assurance that siRNA-based therapies are safe and have the potential to provide a convenient approach to managing” LDL-C, wrote Dr. Ray, a cardiologist and professor of public health at Imperial College London, and his associates.
Evolocumab surpasses inclisiran in crossover cohort
The new data from ORION-3 study included findings from 92 patients first treated with evolocumab injections every 2 weeks for a year, an intervention that lowered their LDL-C levels by an average of about 60%, compared with their pretreatment level. ORION-3’s study design then crossed these patients to treatment with injections of inclisiran twice a year during 3 further years of follow-up, during which their average LDL levels reset to a roughly 45% drop from baseline, a potentially clinically meaningful difference, commented Robert S. Rosenson, MD, a lipid management specialist who was not involved in the ORION-3 study.
“This is the first evidence that compared the two classes” within a single study, thereby avoiding a problematic cross-study comparison. “That’s why the data are important. They underscore that the monoclonal antibodies are more effective for lowering LDL-C,” compared with inclisiran, said Dr. Rosenson, professor and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai in New York.
The findings “confirm in a trial that the PCSK9 monoclonal antibodies are indeed more potent,” he said in an interview.
But Dr. Rosenson acknowledged that, while this analysis used data on patients treated with evolocumab and then switched to inclisiran collected prospectively in a single study, it has the limitation of involving a comparison that was not prespecified. The primary goal of the evolocumab-to-inclisiran switch included in ORION-3 was to assess the ease, safety, and efficacy of a switch to inclisiran from treatment with a PCSK9 antibody and was not intended to compare the two drug classes.
The roughly 15% absolute difference in LDL-C lowering between the two tested drug classes can have substantial clinical implications for patients who start treatment with highly elevated levels of LDL-C, more than 190 mg/dL, because they have heterozygous familial hypercholesterolemia, are unable to take a statin because of intolerance, or both. The difference in LDL-C reduction with an antibody or with inclisiran could mean the difference between whether or not a patient like this achieves their LDL-C goal level, Dr. Rosenson explained.
Inclisiran’s upside
On the other hand, inclisiran has a couple of important advantages. First, its mechanism of action means that effective treatment involves one injection every 6 months following a patient’s first two injections at onset and after 90 days, with all injections administered in a clinician’s office. In contrast, both of the monoclonal antibodies require injections every other week, a schedule that depends on patient self-injections using prefilled syringes obtained from a pharmacy.
Twice-a-year dosing by a clinician can be a major attraction because it helps ensure treatment compliance, aids patients with physical or psychological limitations to self-injection, reduces the pill burden for patients who require multiple medications, and facilitates frequent travelers who would otherwise need to carry syringes with them on trips, Dr. Rosenson noted.
The second big advantage of office-based administration of inclisiran for U.S. Medicare patients is that the treatment is billed under a patient’s part B coverage, usually resulting in easier coverage and a significantly lower patient co-pay, compared with Medicare’s coverage for a pharmacy-dispensed agent, which is covered under Medicare part D. “Part B coverage is financially more doable” for most Medicare patients, said Dr. Rosenson.
The administration schedule for inclisiran as well as its superior Medicare coverage makes the agent “transformative” for LDL-C lowering in patients for whom treatment delivery, frequency, and payment are issues, he said.
Inclisiran uptake modest after FDA approval
Despite these pluses, uptake of inclisiran has been modest since it received U.S. marketing approval in December 2021. In its most recent quarterly financial filing, in October 2022, Novartis reported total worldwide income from inclisiran (Leqvio) of $70 million during the first 9 months of 2022, although a Novartis spokesperson noted that the company has seen “positive trends in uptake” over the course of 2022. Inclisiran is labeled as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who require additional lowering” of LDL-C.
During 2022, inclisiran uptake lagged because of the usual problems that slow the introduction of new drugs and new drug classes, especially ones that require dosing by a clinician. Months were spent waiting for billing codes to roll out, for clinical staffs to incorporate inclisiran injections into their routines, and for commercial insurers to get up to speed on their coverage, Dr. Rosenson said.
Also, a key step for widespread uptake of a new medication for improving cardiovascular disease outcomes – results from phase 3 studies that document safety and efficacy for these outcomes – remains several years off. The ORION-4 trial and the VICTORION-2P trial, each assessing the impact of inclisiran on cardiovascular disease events in roughly 15,000 people, will need about another 3-4 years before their results become available.
Professional medical societies that issue cardiovascular-disease management guidelines “prefer agents with proven benefits in phase 3 trials,” Dr. Rosenson noted.
Hence, the most recent update to U.S. LDL-C–management guidelines, released in the second half of 2022 by the American College of Cardiology as an Expert Consensus Decision Pathway, said this about the current role for inclisiran: “At the present time, a PCSK9 monoclonal antibody is preferred as the initial PCSK9 inhibitor of choice in view of its demonstrated safety, efficacy, and benefits for cardiovascular outcomes in the FOURIER [for evolocumab] and ODYSSEY Outcomes [for alirocumab] trials. The ORION-4 and VICTORION-2P cardiovascular outcomes trials with inclisiran are currently underway, and their completion is anticipated in 2026 and 2027, respectively. In view of the twice-yearly dosing regimen, inclisiran may be considered in patients with demonstrated poor adherence to PCSK9 monoclonal antibodies. Patients with adverse effects from both PSCK9 monoclonal antibodies or those who may be unable to self-inject may also be considered for therapy with inclisiran.”
ORION-3 extended the ORION-1 trial
The ORION-1 study was a phase 2 placebo-controlled, dose-ranging safety and efficacy assessment of inclisiran that gave patients two injections of the drug, at day zero and 90 days, and followed them for an additional 120 days (210 days total follow-up duration), and in some cases for as long as 360 days total. Of the 370 patients who received inclisiran in ORION-1, 290 agreed to continue inclisiran in the open-label extension, ORION-3. ORION-1 also included 127 patients randomized to initial placebo treatment, and 92 of these patients agreed to continue in ORION-3 and became the patients initially treated with evolocumab injections every other week for 1 year followed by initiation of an inclisiran regimen.
The primary outcome of ORION-3 was the change in LDL-C from baseline (the ORION-1 baseline) after 210 days of receiving inclisiran in ORION-3 (or a total of roughly 570 days after the start of ORION-1). The primary endpoint showed that, at day 210 of ORION-3 the average reduction in LDL-C from the original baseline level was 47.5%.
But a “more important” outcome, said Dr. Ray when he first reported the ORION-3 results during the American Heart Association scientific sessions in Chicago in November 2022, was that, overall, during 4 years on inclisiran this cohort showed an average cut in LDL-C from baseline of about 45% that consistently remained at this level throughout the 4 years of treatment.
“This provides us with an idea of what happens with chronic inclisiran dosing,” Dr. Ray explained. “There was no loss of biological efficacy, and we achieved these clinically meaningful, time-averaged reductions with a good safety profile. The great thing is that when patients get their injections [every 6 months] you see a consistent LDL-C reduction. A twice-annual injection is an opportunity to redesign” the way patients receive preventive cardiology care and treatment to lower LDL-C, Dr Ray said.
ORION-1 was sponsored by The Medicines Company. ORION-3 was sponsored by Novartis (which acquired The Medicines Company). Dr. Ray has received consulting fees, personal fees, and research grants from Novartis, as well as consulting fees and research grants from Amgen, the company that markets evolocumab (Repatha), and research grants from Regeneron, the company that markets alirocumab (Praluent). He has also received consulting fee, personal fees, and research grants from numerous other companies. Dr. Rosenson has been a consultant to and has received research funding from Amgen, Novartis, and Regeneron, and he has received speaking fees from Amgen and Regeneron, and has ties to several other pharmaceutical companies.
This article was updated on 1/26/2023.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
After PCI, 1-month beats 12-month DAPT in high-risk patients
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
FROM JACC ASIA
PPI use in type 2 diabetes links with cardiovascular events
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
LDL cholesterol triglycerides ‘robust’ ASCVD risk marker
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Recount of FOURIER data finds higher mortality with evolocumab; trialists push back
Readjudication of mortality data from the FOURIER trial suggests a higher risk for cardiovascular death with evolocumab (Repatha) among patients with established atherosclerotic cardiovascular disease than originally reported for the first-in-class PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor.
The Restoring Invisible and Abandoned Trials (RIAT) investigators launched this review in 2018, citing “significant inconsistencies and misreporting” between information in death narratives in the trial’s clinical study report (CSR) and the 2017 New England Journal of Medicine publication of the primary trial results.
“After readjudication, deaths of cardiac origin were numerically higher in the evolocumab group than in the placebo group in the FOURIER trial, suggesting possible cardiac harm,” the researchers conclude in the new report published online in BMJ Open. “At the time the trial was terminated early, a non-significantly higher risk of cardiovascular mortality was observed with evolocumab, which was numerically greater in our adjudication.
“Our findings indicate that complete restoration of all clinical outcomes from the FOURIER trial is required,” they wrote. “Meanwhile, clinicians should be skeptical about benefits vs harms of prescribing evolocumab for patients with established atherosclerotic cardiovascular disease.”
Asked to comment on the reanalysis, FOURIER lead investigator Marc Sabatine, MD, MPH, a professor of medicine at Harvard Medical School and the Lewis Dexter distinguished chair in cardiovascular medicine at Brigham and Women’s Hospital, both in Boston, said: “It’s hard to call this science. I think it lacks all scientific rigor and is fundamentally flawed and, because their process was flawed, it has led them to erroneous conclusions.”
Reached for comment, Sanjay Kaul, MD, a cardiologist and professor of medicine at Cedars-Sinai Medical Center in Los Angeles, who was not involved with either study, said: “If I were to describe this in one sentence, I would say much ado about nothing. A tempest in a teapot.”
Evaluating hard outcomes
The Food and Drug Administration approved evolocumab in 2015 for lowering LDL cholesterol levels, but without results from any trial evaluating hard outcomes.
As previously reported in 2017, FOURIER showed that adding evolocumab to high-intensity statins slashed LDL cholesterol by 59% and was associated with a 15% reduction in the primary composite cardiovascular events endpoint, compared with placebo, but numerically more all-cause and CV mortality.
The NEJM data analysis reported the risk for cardiovascular mortality was 5% (hazard ratio, 1.05; 95% confidence interval, 0.88-1.25), whereas the new review found a still nonsignificant 20% relative risk (R95% CI, 0.95-1.51).
Cardiac deaths were also numerically higher in the evolocumab group (113 vs. 88), corresponding to a 28% higher relative risk (95% CI, 0.97-1.69). Vascular deaths were similar at 37 in both groups (RR, 1.00; 95% CI, 0.63-1.58).
For 360 of the 870 deaths, the cause of death adjudicated by the FOURIER clinical events committee differs from that identified by the local clinical investigators in the CSR death narrative, the authors said.
The RIAT investigators found 11 more deaths from myocardial infarction in the evolocumab group (36 vs. 25 in NEJM) and 3 fewer deaths in the placebo group (27 vs. 30). In addition, their review indicated that deaths as a result of cardiac failure in the evolocumab group were almost double those in the placebo group, at 31 versus 16, respectively.
An ‘obvious disconnect’
Thomas L. Perry, MD, a coauthor of the BMJ Open paper and a general internist in the department of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, said in an interview that the team repeatedly sought information from the FOURIER investigators but never received a response.
They petitioned and received the FOURIER CSR from the European Medicines Agency and Health Canada and made a similar request with the FDA but were told in October 2019 it would take up to 7 years to release the information. Case report forms were also requested but not received from all three agencies.
Dr. Perry noted that no autopsies were performed in the trial, a claim Dr. Sabatine rejected, and that their review of the death narratives in the CSR found 91 deaths classified by the local investigator as “undetermined” but subsequently adjudicated by the FOURIER clinical events committee as “sudden cardiac” deaths without any documented evidence to support the change.
At his request, Dr. Perry said they included two case examples (figures 1 and 2) in the BMJ Open paper of the “obvious disconnect” in death endpoints. Both of these were identified by the local investigator as a myocardial infarction but later “misreported” according to Dr. Perry, as a sudden cardiac death and noncardiovascular death (trauma), respectively.
“What’s so important about this is not only that it throws into doubt the reliability of what the people at Harvard and elsewhere reported in the New England Journal of Medicine in 2017, but also raises a question about any other large study like this where you rely on supposedly ethical local investigators to run the trial well and to report accurately what happens to people,” Dr. Perry said in an interview.
Although he never prescribed evolocumab after the initial results were published, Dr. Perry said he’s even less convinced of a benefit now. “Basically, I don’t believe that they are telling us the facts. I have no reason to say there’s an element of deliberately misleading us. I think it’s sloppiness, incompetence, laziness.”
Dr. Perry also favors readjudication of the mortality data in the ODYSSEY trial, which showed an all-cause mortality benefit with the PCSK9 inhibitor alirocumab (Praluent).
The ‘full picture’
Dr. Sabatine explained that when a patient had a cardiovascular event, including a death, it triggered the collection of a full dossier containing all available source documents, such as discharge summaries, laboratory and imaging data, and autopsy reports, that were independently reviewed by two board certified physicians blinded to treatment. To suggest, as the RIAT investigators have, that no autopsies were performed is “obviously ridiculous and wrong.”
In contrast, he said the new analysis was post hoc, involved unblinded individuals, and relied on serious adverse event narratives, which include a small text box that must be filled out with the site’s initial impression of the case and sent within 24 hours of the event.
Further, when the FOURIER investigators pulled the dossiers for the two more egregious examples cited in the paper, they found that the first patient died in his sleep at home. “The investigator then just said, ‘oh, I assume it’s an MI,’ but there’s no biochemical data, there’s no ECGs, there’s nothing to make the diagnosis of MI. So that’s why that is a sudden cardiac death per the FDA definition,” Dr. Sabatine said.
When the FOURIER investigators reviewed the full dossier for the second case example, they found the patient had slipped in his kitchen at home, sustained a serious head trauma, was brought into the emergency department, and died.
“That’s why we rely on the source documents. That gives the full picture,” he said. The FDA also reviewed the death narratives.
“They comment, ironically, that they were surprised at the inconsistencies between the investigator-reported causes of death and the central events committee-adjudicated ones, making it sound like something nefarious has happened. But that’s the whole point of adjudication, right? That you have a central events committee that reviews and then classifies based on all the data,” Dr. Sabatine said.
Dr. Sabatine said he sees no reason to reevaluate the ODYSSEY mortality data and that the RIAT analysis should not change the overall interpretation of FOURIER.
“I think this is in fact a disservice to the medical community because it’s not real science,” he said. “It’s just sensationalism and sends the wrong message. But I completely stand by the results that we published, as the FDA has.”
Dr. Kaul also thought the new analysis doesn’t materially change the overall benefit–risk balance. He observed that there isn’t a major difference between the reanalysis and the original evaluation. Total mortality was similar and, for cardiovascular deaths, the original NEJM paper lists 251 for evolocumab versus 240 for placebo and the reanalysis lists 150 versus 125, respectively.
Undetermined deaths were 144 for evolocumab and 164 for placebo in the reanalysis. “The conservative approach is to count them as presumed cardiovascular deaths,” Dr. Kaul said. “So, if you do the math and add those undetermined as cardiovascular deaths, we get a total of 294 (150 + 144) versus 289 (125 + 164). That’s five excess deaths with evolocumab.”
Open access
Although the RIAT group has called for the public release of the FOURIER data, commercial and legal issues will complicate that process, Steven Grover, MD, professor of medicine and director of the comprehensive health improvement program at McGill University, Montreal, said in an interview. Amgen is back in court over patent protection, filing an appeal with the Supreme Court after losing in the lower courts in a protracted battle, Reuters reported.
“One thing that’s for sure after they’ve raised questions about the results of this study [is that] somebody needs to take a good hard look at the adjudicated results,” said Dr. Grover, who coauthored several iterations of the Canadian Cardiovascular Society dyslipidemia guidelines, including the latest in 2021.
“I think the thing that got so many of us back in 2017 when the study was first published is the mortality data stuck out like a sore thumb,” he said in an interview. “It didn’t have to be statistically significant, but it did need to move in the same direction as the nonfatal coronary events. That’s what we’ve seen happen time and again and, in this case, it was going in the opposite direction.”
Dr. Sabatine said he doesn’t know whether the data will be released but that the FOURIER trialists plan to submit a rebuttal to BMJ Open to the RIAT analysis, which has caused a stir on CardioTwitter. “Now that people live with tweets of information, it necessitates then dispelling the misinformation that comes out. So yes, we will draft a rebuttal pointing out all the flaws in this analysis.”
Dr. Kaul commented that the FDA’s response not to provide the data was “rather curious” and that Dr. Sabatine and colleagues had the opportunity to address the RIAT group’s concerns, but the paper notes they did not even bother to respond. “You can’t be holier than thou in medicine. You have to treat every question with respect and humility and can’t be dismissive. ... He could have nipped the evil in the bud, so to speak.”
The study was funded by a grant from the University of Maryland, Baltimore. The authors, Dr. Kaul, and Dr. Grover reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Readjudication of mortality data from the FOURIER trial suggests a higher risk for cardiovascular death with evolocumab (Repatha) among patients with established atherosclerotic cardiovascular disease than originally reported for the first-in-class PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor.
The Restoring Invisible and Abandoned Trials (RIAT) investigators launched this review in 2018, citing “significant inconsistencies and misreporting” between information in death narratives in the trial’s clinical study report (CSR) and the 2017 New England Journal of Medicine publication of the primary trial results.
“After readjudication, deaths of cardiac origin were numerically higher in the evolocumab group than in the placebo group in the FOURIER trial, suggesting possible cardiac harm,” the researchers conclude in the new report published online in BMJ Open. “At the time the trial was terminated early, a non-significantly higher risk of cardiovascular mortality was observed with evolocumab, which was numerically greater in our adjudication.
“Our findings indicate that complete restoration of all clinical outcomes from the FOURIER trial is required,” they wrote. “Meanwhile, clinicians should be skeptical about benefits vs harms of prescribing evolocumab for patients with established atherosclerotic cardiovascular disease.”
Asked to comment on the reanalysis, FOURIER lead investigator Marc Sabatine, MD, MPH, a professor of medicine at Harvard Medical School and the Lewis Dexter distinguished chair in cardiovascular medicine at Brigham and Women’s Hospital, both in Boston, said: “It’s hard to call this science. I think it lacks all scientific rigor and is fundamentally flawed and, because their process was flawed, it has led them to erroneous conclusions.”
Reached for comment, Sanjay Kaul, MD, a cardiologist and professor of medicine at Cedars-Sinai Medical Center in Los Angeles, who was not involved with either study, said: “If I were to describe this in one sentence, I would say much ado about nothing. A tempest in a teapot.”
Evaluating hard outcomes
The Food and Drug Administration approved evolocumab in 2015 for lowering LDL cholesterol levels, but without results from any trial evaluating hard outcomes.
As previously reported in 2017, FOURIER showed that adding evolocumab to high-intensity statins slashed LDL cholesterol by 59% and was associated with a 15% reduction in the primary composite cardiovascular events endpoint, compared with placebo, but numerically more all-cause and CV mortality.
The NEJM data analysis reported the risk for cardiovascular mortality was 5% (hazard ratio, 1.05; 95% confidence interval, 0.88-1.25), whereas the new review found a still nonsignificant 20% relative risk (R95% CI, 0.95-1.51).
Cardiac deaths were also numerically higher in the evolocumab group (113 vs. 88), corresponding to a 28% higher relative risk (95% CI, 0.97-1.69). Vascular deaths were similar at 37 in both groups (RR, 1.00; 95% CI, 0.63-1.58).
For 360 of the 870 deaths, the cause of death adjudicated by the FOURIER clinical events committee differs from that identified by the local clinical investigators in the CSR death narrative, the authors said.
The RIAT investigators found 11 more deaths from myocardial infarction in the evolocumab group (36 vs. 25 in NEJM) and 3 fewer deaths in the placebo group (27 vs. 30). In addition, their review indicated that deaths as a result of cardiac failure in the evolocumab group were almost double those in the placebo group, at 31 versus 16, respectively.
An ‘obvious disconnect’
Thomas L. Perry, MD, a coauthor of the BMJ Open paper and a general internist in the department of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, said in an interview that the team repeatedly sought information from the FOURIER investigators but never received a response.
They petitioned and received the FOURIER CSR from the European Medicines Agency and Health Canada and made a similar request with the FDA but were told in October 2019 it would take up to 7 years to release the information. Case report forms were also requested but not received from all three agencies.
Dr. Perry noted that no autopsies were performed in the trial, a claim Dr. Sabatine rejected, and that their review of the death narratives in the CSR found 91 deaths classified by the local investigator as “undetermined” but subsequently adjudicated by the FOURIER clinical events committee as “sudden cardiac” deaths without any documented evidence to support the change.
At his request, Dr. Perry said they included two case examples (figures 1 and 2) in the BMJ Open paper of the “obvious disconnect” in death endpoints. Both of these were identified by the local investigator as a myocardial infarction but later “misreported” according to Dr. Perry, as a sudden cardiac death and noncardiovascular death (trauma), respectively.
“What’s so important about this is not only that it throws into doubt the reliability of what the people at Harvard and elsewhere reported in the New England Journal of Medicine in 2017, but also raises a question about any other large study like this where you rely on supposedly ethical local investigators to run the trial well and to report accurately what happens to people,” Dr. Perry said in an interview.
Although he never prescribed evolocumab after the initial results were published, Dr. Perry said he’s even less convinced of a benefit now. “Basically, I don’t believe that they are telling us the facts. I have no reason to say there’s an element of deliberately misleading us. I think it’s sloppiness, incompetence, laziness.”
Dr. Perry also favors readjudication of the mortality data in the ODYSSEY trial, which showed an all-cause mortality benefit with the PCSK9 inhibitor alirocumab (Praluent).
The ‘full picture’
Dr. Sabatine explained that when a patient had a cardiovascular event, including a death, it triggered the collection of a full dossier containing all available source documents, such as discharge summaries, laboratory and imaging data, and autopsy reports, that were independently reviewed by two board certified physicians blinded to treatment. To suggest, as the RIAT investigators have, that no autopsies were performed is “obviously ridiculous and wrong.”
In contrast, he said the new analysis was post hoc, involved unblinded individuals, and relied on serious adverse event narratives, which include a small text box that must be filled out with the site’s initial impression of the case and sent within 24 hours of the event.
Further, when the FOURIER investigators pulled the dossiers for the two more egregious examples cited in the paper, they found that the first patient died in his sleep at home. “The investigator then just said, ‘oh, I assume it’s an MI,’ but there’s no biochemical data, there’s no ECGs, there’s nothing to make the diagnosis of MI. So that’s why that is a sudden cardiac death per the FDA definition,” Dr. Sabatine said.
When the FOURIER investigators reviewed the full dossier for the second case example, they found the patient had slipped in his kitchen at home, sustained a serious head trauma, was brought into the emergency department, and died.
“That’s why we rely on the source documents. That gives the full picture,” he said. The FDA also reviewed the death narratives.
“They comment, ironically, that they were surprised at the inconsistencies between the investigator-reported causes of death and the central events committee-adjudicated ones, making it sound like something nefarious has happened. But that’s the whole point of adjudication, right? That you have a central events committee that reviews and then classifies based on all the data,” Dr. Sabatine said.
Dr. Sabatine said he sees no reason to reevaluate the ODYSSEY mortality data and that the RIAT analysis should not change the overall interpretation of FOURIER.
“I think this is in fact a disservice to the medical community because it’s not real science,” he said. “It’s just sensationalism and sends the wrong message. But I completely stand by the results that we published, as the FDA has.”
Dr. Kaul also thought the new analysis doesn’t materially change the overall benefit–risk balance. He observed that there isn’t a major difference between the reanalysis and the original evaluation. Total mortality was similar and, for cardiovascular deaths, the original NEJM paper lists 251 for evolocumab versus 240 for placebo and the reanalysis lists 150 versus 125, respectively.
Undetermined deaths were 144 for evolocumab and 164 for placebo in the reanalysis. “The conservative approach is to count them as presumed cardiovascular deaths,” Dr. Kaul said. “So, if you do the math and add those undetermined as cardiovascular deaths, we get a total of 294 (150 + 144) versus 289 (125 + 164). That’s five excess deaths with evolocumab.”
Open access
Although the RIAT group has called for the public release of the FOURIER data, commercial and legal issues will complicate that process, Steven Grover, MD, professor of medicine and director of the comprehensive health improvement program at McGill University, Montreal, said in an interview. Amgen is back in court over patent protection, filing an appeal with the Supreme Court after losing in the lower courts in a protracted battle, Reuters reported.
“One thing that’s for sure after they’ve raised questions about the results of this study [is that] somebody needs to take a good hard look at the adjudicated results,” said Dr. Grover, who coauthored several iterations of the Canadian Cardiovascular Society dyslipidemia guidelines, including the latest in 2021.
“I think the thing that got so many of us back in 2017 when the study was first published is the mortality data stuck out like a sore thumb,” he said in an interview. “It didn’t have to be statistically significant, but it did need to move in the same direction as the nonfatal coronary events. That’s what we’ve seen happen time and again and, in this case, it was going in the opposite direction.”
Dr. Sabatine said he doesn’t know whether the data will be released but that the FOURIER trialists plan to submit a rebuttal to BMJ Open to the RIAT analysis, which has caused a stir on CardioTwitter. “Now that people live with tweets of information, it necessitates then dispelling the misinformation that comes out. So yes, we will draft a rebuttal pointing out all the flaws in this analysis.”
Dr. Kaul commented that the FDA’s response not to provide the data was “rather curious” and that Dr. Sabatine and colleagues had the opportunity to address the RIAT group’s concerns, but the paper notes they did not even bother to respond. “You can’t be holier than thou in medicine. You have to treat every question with respect and humility and can’t be dismissive. ... He could have nipped the evil in the bud, so to speak.”
The study was funded by a grant from the University of Maryland, Baltimore. The authors, Dr. Kaul, and Dr. Grover reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Readjudication of mortality data from the FOURIER trial suggests a higher risk for cardiovascular death with evolocumab (Repatha) among patients with established atherosclerotic cardiovascular disease than originally reported for the first-in-class PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor.
The Restoring Invisible and Abandoned Trials (RIAT) investigators launched this review in 2018, citing “significant inconsistencies and misreporting” between information in death narratives in the trial’s clinical study report (CSR) and the 2017 New England Journal of Medicine publication of the primary trial results.
“After readjudication, deaths of cardiac origin were numerically higher in the evolocumab group than in the placebo group in the FOURIER trial, suggesting possible cardiac harm,” the researchers conclude in the new report published online in BMJ Open. “At the time the trial was terminated early, a non-significantly higher risk of cardiovascular mortality was observed with evolocumab, which was numerically greater in our adjudication.
“Our findings indicate that complete restoration of all clinical outcomes from the FOURIER trial is required,” they wrote. “Meanwhile, clinicians should be skeptical about benefits vs harms of prescribing evolocumab for patients with established atherosclerotic cardiovascular disease.”
Asked to comment on the reanalysis, FOURIER lead investigator Marc Sabatine, MD, MPH, a professor of medicine at Harvard Medical School and the Lewis Dexter distinguished chair in cardiovascular medicine at Brigham and Women’s Hospital, both in Boston, said: “It’s hard to call this science. I think it lacks all scientific rigor and is fundamentally flawed and, because their process was flawed, it has led them to erroneous conclusions.”
Reached for comment, Sanjay Kaul, MD, a cardiologist and professor of medicine at Cedars-Sinai Medical Center in Los Angeles, who was not involved with either study, said: “If I were to describe this in one sentence, I would say much ado about nothing. A tempest in a teapot.”
Evaluating hard outcomes
The Food and Drug Administration approved evolocumab in 2015 for lowering LDL cholesterol levels, but without results from any trial evaluating hard outcomes.
As previously reported in 2017, FOURIER showed that adding evolocumab to high-intensity statins slashed LDL cholesterol by 59% and was associated with a 15% reduction in the primary composite cardiovascular events endpoint, compared with placebo, but numerically more all-cause and CV mortality.
The NEJM data analysis reported the risk for cardiovascular mortality was 5% (hazard ratio, 1.05; 95% confidence interval, 0.88-1.25), whereas the new review found a still nonsignificant 20% relative risk (R95% CI, 0.95-1.51).
Cardiac deaths were also numerically higher in the evolocumab group (113 vs. 88), corresponding to a 28% higher relative risk (95% CI, 0.97-1.69). Vascular deaths were similar at 37 in both groups (RR, 1.00; 95% CI, 0.63-1.58).
For 360 of the 870 deaths, the cause of death adjudicated by the FOURIER clinical events committee differs from that identified by the local clinical investigators in the CSR death narrative, the authors said.
The RIAT investigators found 11 more deaths from myocardial infarction in the evolocumab group (36 vs. 25 in NEJM) and 3 fewer deaths in the placebo group (27 vs. 30). In addition, their review indicated that deaths as a result of cardiac failure in the evolocumab group were almost double those in the placebo group, at 31 versus 16, respectively.
An ‘obvious disconnect’
Thomas L. Perry, MD, a coauthor of the BMJ Open paper and a general internist in the department of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, said in an interview that the team repeatedly sought information from the FOURIER investigators but never received a response.
They petitioned and received the FOURIER CSR from the European Medicines Agency and Health Canada and made a similar request with the FDA but were told in October 2019 it would take up to 7 years to release the information. Case report forms were also requested but not received from all three agencies.
Dr. Perry noted that no autopsies were performed in the trial, a claim Dr. Sabatine rejected, and that their review of the death narratives in the CSR found 91 deaths classified by the local investigator as “undetermined” but subsequently adjudicated by the FOURIER clinical events committee as “sudden cardiac” deaths without any documented evidence to support the change.
At his request, Dr. Perry said they included two case examples (figures 1 and 2) in the BMJ Open paper of the “obvious disconnect” in death endpoints. Both of these were identified by the local investigator as a myocardial infarction but later “misreported” according to Dr. Perry, as a sudden cardiac death and noncardiovascular death (trauma), respectively.
“What’s so important about this is not only that it throws into doubt the reliability of what the people at Harvard and elsewhere reported in the New England Journal of Medicine in 2017, but also raises a question about any other large study like this where you rely on supposedly ethical local investigators to run the trial well and to report accurately what happens to people,” Dr. Perry said in an interview.
Although he never prescribed evolocumab after the initial results were published, Dr. Perry said he’s even less convinced of a benefit now. “Basically, I don’t believe that they are telling us the facts. I have no reason to say there’s an element of deliberately misleading us. I think it’s sloppiness, incompetence, laziness.”
Dr. Perry also favors readjudication of the mortality data in the ODYSSEY trial, which showed an all-cause mortality benefit with the PCSK9 inhibitor alirocumab (Praluent).
The ‘full picture’
Dr. Sabatine explained that when a patient had a cardiovascular event, including a death, it triggered the collection of a full dossier containing all available source documents, such as discharge summaries, laboratory and imaging data, and autopsy reports, that were independently reviewed by two board certified physicians blinded to treatment. To suggest, as the RIAT investigators have, that no autopsies were performed is “obviously ridiculous and wrong.”
In contrast, he said the new analysis was post hoc, involved unblinded individuals, and relied on serious adverse event narratives, which include a small text box that must be filled out with the site’s initial impression of the case and sent within 24 hours of the event.
Further, when the FOURIER investigators pulled the dossiers for the two more egregious examples cited in the paper, they found that the first patient died in his sleep at home. “The investigator then just said, ‘oh, I assume it’s an MI,’ but there’s no biochemical data, there’s no ECGs, there’s nothing to make the diagnosis of MI. So that’s why that is a sudden cardiac death per the FDA definition,” Dr. Sabatine said.
When the FOURIER investigators reviewed the full dossier for the second case example, they found the patient had slipped in his kitchen at home, sustained a serious head trauma, was brought into the emergency department, and died.
“That’s why we rely on the source documents. That gives the full picture,” he said. The FDA also reviewed the death narratives.
“They comment, ironically, that they were surprised at the inconsistencies between the investigator-reported causes of death and the central events committee-adjudicated ones, making it sound like something nefarious has happened. But that’s the whole point of adjudication, right? That you have a central events committee that reviews and then classifies based on all the data,” Dr. Sabatine said.
Dr. Sabatine said he sees no reason to reevaluate the ODYSSEY mortality data and that the RIAT analysis should not change the overall interpretation of FOURIER.
“I think this is in fact a disservice to the medical community because it’s not real science,” he said. “It’s just sensationalism and sends the wrong message. But I completely stand by the results that we published, as the FDA has.”
Dr. Kaul also thought the new analysis doesn’t materially change the overall benefit–risk balance. He observed that there isn’t a major difference between the reanalysis and the original evaluation. Total mortality was similar and, for cardiovascular deaths, the original NEJM paper lists 251 for evolocumab versus 240 for placebo and the reanalysis lists 150 versus 125, respectively.
Undetermined deaths were 144 for evolocumab and 164 for placebo in the reanalysis. “The conservative approach is to count them as presumed cardiovascular deaths,” Dr. Kaul said. “So, if you do the math and add those undetermined as cardiovascular deaths, we get a total of 294 (150 + 144) versus 289 (125 + 164). That’s five excess deaths with evolocumab.”
Open access
Although the RIAT group has called for the public release of the FOURIER data, commercial and legal issues will complicate that process, Steven Grover, MD, professor of medicine and director of the comprehensive health improvement program at McGill University, Montreal, said in an interview. Amgen is back in court over patent protection, filing an appeal with the Supreme Court after losing in the lower courts in a protracted battle, Reuters reported.
“One thing that’s for sure after they’ve raised questions about the results of this study [is that] somebody needs to take a good hard look at the adjudicated results,” said Dr. Grover, who coauthored several iterations of the Canadian Cardiovascular Society dyslipidemia guidelines, including the latest in 2021.
“I think the thing that got so many of us back in 2017 when the study was first published is the mortality data stuck out like a sore thumb,” he said in an interview. “It didn’t have to be statistically significant, but it did need to move in the same direction as the nonfatal coronary events. That’s what we’ve seen happen time and again and, in this case, it was going in the opposite direction.”
Dr. Sabatine said he doesn’t know whether the data will be released but that the FOURIER trialists plan to submit a rebuttal to BMJ Open to the RIAT analysis, which has caused a stir on CardioTwitter. “Now that people live with tweets of information, it necessitates then dispelling the misinformation that comes out. So yes, we will draft a rebuttal pointing out all the flaws in this analysis.”
Dr. Kaul commented that the FDA’s response not to provide the data was “rather curious” and that Dr. Sabatine and colleagues had the opportunity to address the RIAT group’s concerns, but the paper notes they did not even bother to respond. “You can’t be holier than thou in medicine. You have to treat every question with respect and humility and can’t be dismissive. ... He could have nipped the evil in the bud, so to speak.”
The study was funded by a grant from the University of Maryland, Baltimore. The authors, Dr. Kaul, and Dr. Grover reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Five thoughts on the Damar Hamlin collapse
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
STEMI times to treatment usually miss established goals
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).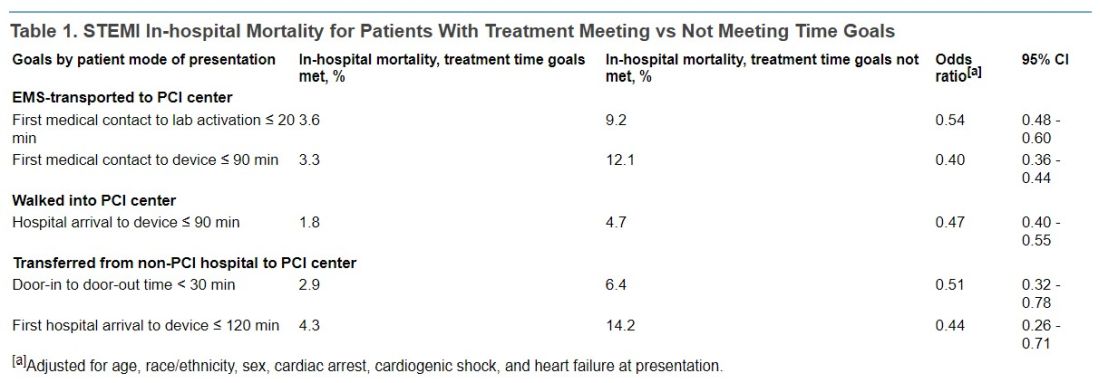
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Top cardiology societies call for revamp of clinical trials
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A doctor saves a drowning family in a dangerous river
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.







