User login
How medical providers can observe LGBT Pride Month
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
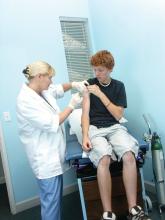
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
Is it measles? – Diagnosis and management for the pediatric provider
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
Evaluation, treatment of anxiety in children and adolescents with autism spectrum disorder
1 As ASD by definition involves deficits in communication and interaction, as well as restricted, repetitive patterns of behavior, interests, or activities, diagnosis and treatment of anxiety disorders in this population can present a significant challenge.
martinedoucet/E+/Getty Images
Clinical vignette
Sean is a 9-year-old boy in the fourth grade diagnosed with ASD. He is in a regular education classroom setting. Until this year, his grades have been above average. This year his mother has been getting calls from the teachers reporting that he is disruptive in class, and is having difficulty paying attention unless the subject relates to a specific interest of his. At home, his mother has been struggling to get him to do chores and homework, and even sitting at the dinner table is now a battle. He is significantly more irritable than usual. While he always preferred routines and familiar activities, deviations from them now trigger strong reactions and sometimes tantrums. He has started to insist on staying up late, and refuses to go to bed without his mother present. Notably his mother reports that she and Sean’s father recently separated, and that she believes he is very upset by this, although he refuses to talk about it.
Discussion
This case highlights the diagnostic complexity with which children with ASD may present. With the overlap between some of the core symptoms of ASD and anxiety, as well as the potential for other co-occurring disorders, a number of factors need to be explored before arriving at a treatment plan.
In evaluating behavior changes in children with ASD, I find it most helpful to start by looking for any medical or environmental factors. Medical problems such as illness or gastrointestinal difficulties may contribute to behavioral challenges and anxiety. Also, be sure to inquire if there are any precipitating events or change in the environment which might correlate with the change in behavior. In this case, we do have a situation – namely Sean’s parents separating – that may be contributing. While addressing Sean’s thoughts and feelings about this remains challenging, awareness of this factor certainly is important.
Understanding the educational setting and supports of a child with ASD is of significant importance. Academic challenges may result from learning or language difficulties, which can result in significant stress. While the vignette mentions that Sean’s grades had previously been above average, it is possible that increased complexity of material is contributing to his school struggles.
Next, it is worth looking at the question of whether Sean meets criteria for ADHD, which is estimated to occur in 30%-61% of people with ASD. In the case vignette, the mention of disruptions and attentional difficulties in the classroom warrant further investigation.
Finally, the question of whether insistence on routine, strong reactions to unfamiliar circumstances, disruptive behavior, and irritability meet criteria for an anxiety disorder is a complex one. Children with ASD may have difficulty communicating that they are anxious, making the behavioral observations of those around them especially important. An advantage pediatric primary care providers have in this circumstance is longitudinal experience with the child and family, which can help confirm whether the problem perceived as anxiety is a manifestation of core autism symptoms, or newer-onset phenomena. Assessing the severity and settings of the behavior also is necessary to guide treatment decisions. In the vignette, Sean’s irritability, acting out, and bedtime difficulties all are of relatively new onset, and occurring across multiple settings with significant functional consequences, making a diagnosis of an anxiety disorder the likely explanation.
As for treatment, cognitive behavioral therapy has been shown to be effective for anxiety in children with high functioning ASD.2 If a clinician with experience with this population is available, that certainly is preferred. If medication is being considered, there are no randomized controlled trials that have demonstrated efficacy of medication for anxiety specifically in children with co-occurring ASD. Treatment recommendations are taken from studies in typically developing children,3 where the SSRIs fluoxetine and sertraline have demonstrated efficacy in treatment of anxiety. When opting for pharmacotherapy in children with ASD, starting low, going slow, and carefully monitoring for side effects is recommended. Regardless of the method of treatment, a clear definition of the target symptoms ahead of time is critical for monitoring response and evaluating treatment effect.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Clin Child Fam Psychol Rev. 2011 Sep;14(3):302-17.
2. Child Psychiatry Hum Dev. 2015 Aug;46(4):533-47.
3. Pediatrics. 2016 Feb;137 Suppl 2:S115-23.
1 As ASD by definition involves deficits in communication and interaction, as well as restricted, repetitive patterns of behavior, interests, or activities, diagnosis and treatment of anxiety disorders in this population can present a significant challenge.
martinedoucet/E+/Getty Images
Clinical vignette
Sean is a 9-year-old boy in the fourth grade diagnosed with ASD. He is in a regular education classroom setting. Until this year, his grades have been above average. This year his mother has been getting calls from the teachers reporting that he is disruptive in class, and is having difficulty paying attention unless the subject relates to a specific interest of his. At home, his mother has been struggling to get him to do chores and homework, and even sitting at the dinner table is now a battle. He is significantly more irritable than usual. While he always preferred routines and familiar activities, deviations from them now trigger strong reactions and sometimes tantrums. He has started to insist on staying up late, and refuses to go to bed without his mother present. Notably his mother reports that she and Sean’s father recently separated, and that she believes he is very upset by this, although he refuses to talk about it.
Discussion
This case highlights the diagnostic complexity with which children with ASD may present. With the overlap between some of the core symptoms of ASD and anxiety, as well as the potential for other co-occurring disorders, a number of factors need to be explored before arriving at a treatment plan.
In evaluating behavior changes in children with ASD, I find it most helpful to start by looking for any medical or environmental factors. Medical problems such as illness or gastrointestinal difficulties may contribute to behavioral challenges and anxiety. Also, be sure to inquire if there are any precipitating events or change in the environment which might correlate with the change in behavior. In this case, we do have a situation – namely Sean’s parents separating – that may be contributing. While addressing Sean’s thoughts and feelings about this remains challenging, awareness of this factor certainly is important.
Understanding the educational setting and supports of a child with ASD is of significant importance. Academic challenges may result from learning or language difficulties, which can result in significant stress. While the vignette mentions that Sean’s grades had previously been above average, it is possible that increased complexity of material is contributing to his school struggles.
Next, it is worth looking at the question of whether Sean meets criteria for ADHD, which is estimated to occur in 30%-61% of people with ASD. In the case vignette, the mention of disruptions and attentional difficulties in the classroom warrant further investigation.
Finally, the question of whether insistence on routine, strong reactions to unfamiliar circumstances, disruptive behavior, and irritability meet criteria for an anxiety disorder is a complex one. Children with ASD may have difficulty communicating that they are anxious, making the behavioral observations of those around them especially important. An advantage pediatric primary care providers have in this circumstance is longitudinal experience with the child and family, which can help confirm whether the problem perceived as anxiety is a manifestation of core autism symptoms, or newer-onset phenomena. Assessing the severity and settings of the behavior also is necessary to guide treatment decisions. In the vignette, Sean’s irritability, acting out, and bedtime difficulties all are of relatively new onset, and occurring across multiple settings with significant functional consequences, making a diagnosis of an anxiety disorder the likely explanation.
As for treatment, cognitive behavioral therapy has been shown to be effective for anxiety in children with high functioning ASD.2 If a clinician with experience with this population is available, that certainly is preferred. If medication is being considered, there are no randomized controlled trials that have demonstrated efficacy of medication for anxiety specifically in children with co-occurring ASD. Treatment recommendations are taken from studies in typically developing children,3 where the SSRIs fluoxetine and sertraline have demonstrated efficacy in treatment of anxiety. When opting for pharmacotherapy in children with ASD, starting low, going slow, and carefully monitoring for side effects is recommended. Regardless of the method of treatment, a clear definition of the target symptoms ahead of time is critical for monitoring response and evaluating treatment effect.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Clin Child Fam Psychol Rev. 2011 Sep;14(3):302-17.
2. Child Psychiatry Hum Dev. 2015 Aug;46(4):533-47.
3. Pediatrics. 2016 Feb;137 Suppl 2:S115-23.
1 As ASD by definition involves deficits in communication and interaction, as well as restricted, repetitive patterns of behavior, interests, or activities, diagnosis and treatment of anxiety disorders in this population can present a significant challenge.
martinedoucet/E+/Getty Images
Clinical vignette
Sean is a 9-year-old boy in the fourth grade diagnosed with ASD. He is in a regular education classroom setting. Until this year, his grades have been above average. This year his mother has been getting calls from the teachers reporting that he is disruptive in class, and is having difficulty paying attention unless the subject relates to a specific interest of his. At home, his mother has been struggling to get him to do chores and homework, and even sitting at the dinner table is now a battle. He is significantly more irritable than usual. While he always preferred routines and familiar activities, deviations from them now trigger strong reactions and sometimes tantrums. He has started to insist on staying up late, and refuses to go to bed without his mother present. Notably his mother reports that she and Sean’s father recently separated, and that she believes he is very upset by this, although he refuses to talk about it.
Discussion
This case highlights the diagnostic complexity with which children with ASD may present. With the overlap between some of the core symptoms of ASD and anxiety, as well as the potential for other co-occurring disorders, a number of factors need to be explored before arriving at a treatment plan.
In evaluating behavior changes in children with ASD, I find it most helpful to start by looking for any medical or environmental factors. Medical problems such as illness or gastrointestinal difficulties may contribute to behavioral challenges and anxiety. Also, be sure to inquire if there are any precipitating events or change in the environment which might correlate with the change in behavior. In this case, we do have a situation – namely Sean’s parents separating – that may be contributing. While addressing Sean’s thoughts and feelings about this remains challenging, awareness of this factor certainly is important.
Understanding the educational setting and supports of a child with ASD is of significant importance. Academic challenges may result from learning or language difficulties, which can result in significant stress. While the vignette mentions that Sean’s grades had previously been above average, it is possible that increased complexity of material is contributing to his school struggles.
Next, it is worth looking at the question of whether Sean meets criteria for ADHD, which is estimated to occur in 30%-61% of people with ASD. In the case vignette, the mention of disruptions and attentional difficulties in the classroom warrant further investigation.
Finally, the question of whether insistence on routine, strong reactions to unfamiliar circumstances, disruptive behavior, and irritability meet criteria for an anxiety disorder is a complex one. Children with ASD may have difficulty communicating that they are anxious, making the behavioral observations of those around them especially important. An advantage pediatric primary care providers have in this circumstance is longitudinal experience with the child and family, which can help confirm whether the problem perceived as anxiety is a manifestation of core autism symptoms, or newer-onset phenomena. Assessing the severity and settings of the behavior also is necessary to guide treatment decisions. In the vignette, Sean’s irritability, acting out, and bedtime difficulties all are of relatively new onset, and occurring across multiple settings with significant functional consequences, making a diagnosis of an anxiety disorder the likely explanation.
As for treatment, cognitive behavioral therapy has been shown to be effective for anxiety in children with high functioning ASD.2 If a clinician with experience with this population is available, that certainly is preferred. If medication is being considered, there are no randomized controlled trials that have demonstrated efficacy of medication for anxiety specifically in children with co-occurring ASD. Treatment recommendations are taken from studies in typically developing children,3 where the SSRIs fluoxetine and sertraline have demonstrated efficacy in treatment of anxiety. When opting for pharmacotherapy in children with ASD, starting low, going slow, and carefully monitoring for side effects is recommended. Regardless of the method of treatment, a clear definition of the target symptoms ahead of time is critical for monitoring response and evaluating treatment effect.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Clin Child Fam Psychol Rev. 2011 Sep;14(3):302-17.
2. Child Psychiatry Hum Dev. 2015 Aug;46(4):533-47.
3. Pediatrics. 2016 Feb;137 Suppl 2:S115-23.
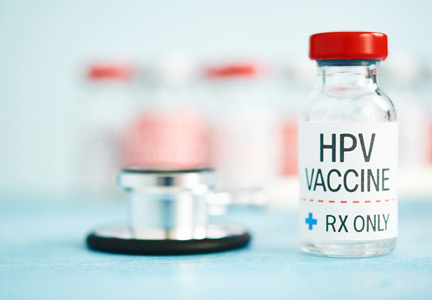
Flu vaccine visits reveal missed opportunities for HPV vaccination
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
REPORTING FROM PAS 2019
Pediatrician knowledge of tampon safety is low
BALTIMORE – and a remarkably high proportion of them lack adequate knowledge themselves about the topic, a new survey-based study found.
“Significant knowledge gaps [were] noted, for instance, [such as] the maximum time a tampon can safely remain in the body,” Miriam Singer of Cohen Children’s Medical Center of New York told attendees of the Pediatric Academic Societies annual meeting.
More than 80% of females aged 17-21 years have used tampons by themselves or with pads, Ms. Singer noted in her background information, yet many teens have low knowledge about their use and safety.
Past research has found that only 35% of high school junior and senior girls heard about tampon use from their mothers, yet many of these mothers showed low knowledge about proper tampon use as well. That same research found that less than 15% of girls aged 10-19 years reported getting information from a health professional about products for menstruation despite recommendations from the American Academy of Pediatrics to instruct girls on feminine hygiene product usage.
Other research has found minimal to no education about menstruation in schools “due to time constraints and stigma associated with menstruation,” Ms. Singer said.
She and her colleagues emailed 2,500 AAP members in November-December 2018 a 53-question online questionnaire about their self-rated and measured knowledge of proper tampon usage and safety and how frequently they discussed tampons with their female adolescent patients. The survey included questions asking pediatricians to self-rate their knowledge about tampon use and safety on a Likert scale of 1 (not at all knowledgeable) to 5 (extremely knowledgeable).
Two incentives provided for completing the survey were a Feminine Hygiene Fact Sheet offered in the first email and an ADHD Medication Guide offered in the third and final email.
Among the 518 pediatricians who responded (21% response rate), 462 met the inclusion criteria of being a primary care pediatrician currently practicing in the United States. Most were women (79%) and white (79%). Just over half of the pediatricians worked only in private practice (54%) and in a suburban area (52%). About a quarter (26%) were in an urban area and 20% in a rural area. Distribution of years in practice (from 1-5 years to over 25 years in 5-year increments) was fairly even across respondents.
Only 9% of respondents reported they very often or almost always talk to their female adolescent patients about how to insert a tampon. The most common tampon-related conversation pediatricians reported was how often to change tampons, which only 35% of respondents said they very often or almost always do.
Yet a similar proportion, 36%, rarely or almost never discuss how often to change tampons, and 62% said they rarely or almost never discuss how to insert a tampon or talk about using tampons while sleeping. Half of respondents (51%) almost never discuss using tampons while swimming (only 21% very often or almost always do), and 77% have not discussed how tampons might affect the hymen with their patients.
More pediatricians (36%) reported almost never discussing the risks of tampon use with female teens than those who sometimes (32%) or very often/almost always (31%) discussed risks.
Respondents also were generally much more willing to discuss tampons with older adolescents than younger ones. Only 18% of respondents said they were highly likely to discuss them with 12- and 13-year-olds, compared with almost twice as many (33%) who would discuss tampons with 16- and 17-year-olds (P less than .001).
Male pediatricians were significantly less likely to discuss any of these topics with their female adolescent patients than female pediatricians (P less than .001 for all questions except risks [P = .01] and hymen [P = .04]). They also rated their knowledge about tampons as significantly lower than self ratings by female pediatricians (P less than .001). Less than half of pediatricians (43%) rated their knowledge about tampons as high or very high, and one in five (20%) rated it as low.
Actual measured knowledge reflected the self-ratings, but still revealed substantial gaps in knowledge among male and female providers. Just over half of male pediatricians (52%) answered all questions about tampon use and safety correctly; however, female pediatricians were only slightly better, with 71% answering all questions correctly (P less than .001). Less than half of male and female pediatricians knew the maximum time a tampon could stay in before it should be removed to reduce risk of toxic shock syndrome (8 hours).
The only two questions that more than half of male pediatricians answered correctly were that girls can swim in the ocean while wearing a tampon and that it can, rarely but not typically, tear the hymen. Less than half knew girls could sleep while wearing a tampon and that a girl could start using a tampon with her first menstruation.
More than half of female pediatricians answered all these questions correctly, although only about two-thirds gave correct answers on how tampons can affect the hymen (the only question that more male pediatricians than female answered correctly), whether a girl can sleep in a tampon, and that patients should use the lowest effective absorbency tampon to minimize toxic shock syndrome risk.
Although the study is limited by a nonvalidated knowledge assessment instrument, self-reporting and potential selection bias means the study may not accurately represent U.S. primary care pediatricians nationwide; however, the findings still demonstrate notably low self-rated and measured knowledge about tampons.
“Given the AAP’s recommendation that pediatricians instruct girls on the use of feminine products, pediatricians must take steps to ensure they are educating patients about tampons,” Ms. Singer said. She also recommended the development of web-based resources targeting the improvement of pediatrician knowledge about tampon use and safety, and the need for the AAP to raise awareness about the importance of discussing tampons with female adolescent patients.
The study did not use external funding, and the authors reported no relevant financial disclosures.
BALTIMORE – and a remarkably high proportion of them lack adequate knowledge themselves about the topic, a new survey-based study found.
“Significant knowledge gaps [were] noted, for instance, [such as] the maximum time a tampon can safely remain in the body,” Miriam Singer of Cohen Children’s Medical Center of New York told attendees of the Pediatric Academic Societies annual meeting.
More than 80% of females aged 17-21 years have used tampons by themselves or with pads, Ms. Singer noted in her background information, yet many teens have low knowledge about their use and safety.
Past research has found that only 35% of high school junior and senior girls heard about tampon use from their mothers, yet many of these mothers showed low knowledge about proper tampon use as well. That same research found that less than 15% of girls aged 10-19 years reported getting information from a health professional about products for menstruation despite recommendations from the American Academy of Pediatrics to instruct girls on feminine hygiene product usage.
Other research has found minimal to no education about menstruation in schools “due to time constraints and stigma associated with menstruation,” Ms. Singer said.
She and her colleagues emailed 2,500 AAP members in November-December 2018 a 53-question online questionnaire about their self-rated and measured knowledge of proper tampon usage and safety and how frequently they discussed tampons with their female adolescent patients. The survey included questions asking pediatricians to self-rate their knowledge about tampon use and safety on a Likert scale of 1 (not at all knowledgeable) to 5 (extremely knowledgeable).
Two incentives provided for completing the survey were a Feminine Hygiene Fact Sheet offered in the first email and an ADHD Medication Guide offered in the third and final email.
Among the 518 pediatricians who responded (21% response rate), 462 met the inclusion criteria of being a primary care pediatrician currently practicing in the United States. Most were women (79%) and white (79%). Just over half of the pediatricians worked only in private practice (54%) and in a suburban area (52%). About a quarter (26%) were in an urban area and 20% in a rural area. Distribution of years in practice (from 1-5 years to over 25 years in 5-year increments) was fairly even across respondents.
Only 9% of respondents reported they very often or almost always talk to their female adolescent patients about how to insert a tampon. The most common tampon-related conversation pediatricians reported was how often to change tampons, which only 35% of respondents said they very often or almost always do.
Yet a similar proportion, 36%, rarely or almost never discuss how often to change tampons, and 62% said they rarely or almost never discuss how to insert a tampon or talk about using tampons while sleeping. Half of respondents (51%) almost never discuss using tampons while swimming (only 21% very often or almost always do), and 77% have not discussed how tampons might affect the hymen with their patients.
More pediatricians (36%) reported almost never discussing the risks of tampon use with female teens than those who sometimes (32%) or very often/almost always (31%) discussed risks.
Respondents also were generally much more willing to discuss tampons with older adolescents than younger ones. Only 18% of respondents said they were highly likely to discuss them with 12- and 13-year-olds, compared with almost twice as many (33%) who would discuss tampons with 16- and 17-year-olds (P less than .001).
Male pediatricians were significantly less likely to discuss any of these topics with their female adolescent patients than female pediatricians (P less than .001 for all questions except risks [P = .01] and hymen [P = .04]). They also rated their knowledge about tampons as significantly lower than self ratings by female pediatricians (P less than .001). Less than half of pediatricians (43%) rated their knowledge about tampons as high or very high, and one in five (20%) rated it as low.
Actual measured knowledge reflected the self-ratings, but still revealed substantial gaps in knowledge among male and female providers. Just over half of male pediatricians (52%) answered all questions about tampon use and safety correctly; however, female pediatricians were only slightly better, with 71% answering all questions correctly (P less than .001). Less than half of male and female pediatricians knew the maximum time a tampon could stay in before it should be removed to reduce risk of toxic shock syndrome (8 hours).
The only two questions that more than half of male pediatricians answered correctly were that girls can swim in the ocean while wearing a tampon and that it can, rarely but not typically, tear the hymen. Less than half knew girls could sleep while wearing a tampon and that a girl could start using a tampon with her first menstruation.
More than half of female pediatricians answered all these questions correctly, although only about two-thirds gave correct answers on how tampons can affect the hymen (the only question that more male pediatricians than female answered correctly), whether a girl can sleep in a tampon, and that patients should use the lowest effective absorbency tampon to minimize toxic shock syndrome risk.
Although the study is limited by a nonvalidated knowledge assessment instrument, self-reporting and potential selection bias means the study may not accurately represent U.S. primary care pediatricians nationwide; however, the findings still demonstrate notably low self-rated and measured knowledge about tampons.
“Given the AAP’s recommendation that pediatricians instruct girls on the use of feminine products, pediatricians must take steps to ensure they are educating patients about tampons,” Ms. Singer said. She also recommended the development of web-based resources targeting the improvement of pediatrician knowledge about tampon use and safety, and the need for the AAP to raise awareness about the importance of discussing tampons with female adolescent patients.
The study did not use external funding, and the authors reported no relevant financial disclosures.
BALTIMORE – and a remarkably high proportion of them lack adequate knowledge themselves about the topic, a new survey-based study found.
“Significant knowledge gaps [were] noted, for instance, [such as] the maximum time a tampon can safely remain in the body,” Miriam Singer of Cohen Children’s Medical Center of New York told attendees of the Pediatric Academic Societies annual meeting.
More than 80% of females aged 17-21 years have used tampons by themselves or with pads, Ms. Singer noted in her background information, yet many teens have low knowledge about their use and safety.
Past research has found that only 35% of high school junior and senior girls heard about tampon use from their mothers, yet many of these mothers showed low knowledge about proper tampon use as well. That same research found that less than 15% of girls aged 10-19 years reported getting information from a health professional about products for menstruation despite recommendations from the American Academy of Pediatrics to instruct girls on feminine hygiene product usage.
Other research has found minimal to no education about menstruation in schools “due to time constraints and stigma associated with menstruation,” Ms. Singer said.
She and her colleagues emailed 2,500 AAP members in November-December 2018 a 53-question online questionnaire about their self-rated and measured knowledge of proper tampon usage and safety and how frequently they discussed tampons with their female adolescent patients. The survey included questions asking pediatricians to self-rate their knowledge about tampon use and safety on a Likert scale of 1 (not at all knowledgeable) to 5 (extremely knowledgeable).
Two incentives provided for completing the survey were a Feminine Hygiene Fact Sheet offered in the first email and an ADHD Medication Guide offered in the third and final email.
Among the 518 pediatricians who responded (21% response rate), 462 met the inclusion criteria of being a primary care pediatrician currently practicing in the United States. Most were women (79%) and white (79%). Just over half of the pediatricians worked only in private practice (54%) and in a suburban area (52%). About a quarter (26%) were in an urban area and 20% in a rural area. Distribution of years in practice (from 1-5 years to over 25 years in 5-year increments) was fairly even across respondents.
Only 9% of respondents reported they very often or almost always talk to their female adolescent patients about how to insert a tampon. The most common tampon-related conversation pediatricians reported was how often to change tampons, which only 35% of respondents said they very often or almost always do.
Yet a similar proportion, 36%, rarely or almost never discuss how often to change tampons, and 62% said they rarely or almost never discuss how to insert a tampon or talk about using tampons while sleeping. Half of respondents (51%) almost never discuss using tampons while swimming (only 21% very often or almost always do), and 77% have not discussed how tampons might affect the hymen with their patients.
More pediatricians (36%) reported almost never discussing the risks of tampon use with female teens than those who sometimes (32%) or very often/almost always (31%) discussed risks.
Respondents also were generally much more willing to discuss tampons with older adolescents than younger ones. Only 18% of respondents said they were highly likely to discuss them with 12- and 13-year-olds, compared with almost twice as many (33%) who would discuss tampons with 16- and 17-year-olds (P less than .001).
Male pediatricians were significantly less likely to discuss any of these topics with their female adolescent patients than female pediatricians (P less than .001 for all questions except risks [P = .01] and hymen [P = .04]). They also rated their knowledge about tampons as significantly lower than self ratings by female pediatricians (P less than .001). Less than half of pediatricians (43%) rated their knowledge about tampons as high or very high, and one in five (20%) rated it as low.
Actual measured knowledge reflected the self-ratings, but still revealed substantial gaps in knowledge among male and female providers. Just over half of male pediatricians (52%) answered all questions about tampon use and safety correctly; however, female pediatricians were only slightly better, with 71% answering all questions correctly (P less than .001). Less than half of male and female pediatricians knew the maximum time a tampon could stay in before it should be removed to reduce risk of toxic shock syndrome (8 hours).
The only two questions that more than half of male pediatricians answered correctly were that girls can swim in the ocean while wearing a tampon and that it can, rarely but not typically, tear the hymen. Less than half knew girls could sleep while wearing a tampon and that a girl could start using a tampon with her first menstruation.
More than half of female pediatricians answered all these questions correctly, although only about two-thirds gave correct answers on how tampons can affect the hymen (the only question that more male pediatricians than female answered correctly), whether a girl can sleep in a tampon, and that patients should use the lowest effective absorbency tampon to minimize toxic shock syndrome risk.
Although the study is limited by a nonvalidated knowledge assessment instrument, self-reporting and potential selection bias means the study may not accurately represent U.S. primary care pediatricians nationwide; however, the findings still demonstrate notably low self-rated and measured knowledge about tampons.
“Given the AAP’s recommendation that pediatricians instruct girls on the use of feminine products, pediatricians must take steps to ensure they are educating patients about tampons,” Ms. Singer said. She also recommended the development of web-based resources targeting the improvement of pediatrician knowledge about tampon use and safety, and the need for the AAP to raise awareness about the importance of discussing tampons with female adolescent patients.
The study did not use external funding, and the authors reported no relevant financial disclosures.
REPORTING FROM PAS 2019
Key clinical point: U.S. pediatricians have low knowledge of and willingness to discuss proper tampon use and safety with adolescent patients.
Major finding: 35% of U.S. pediatricians reported they very often/almost always discuss how long to wear a tampon before removing it.
Study details: The findings are based on a survey of 462 U.S. pediatricians who responded to a 53-question online survey.
Disclosures: The study did not use external funding, and the authors reported no relevant financial disclosures.
No exudates or fever? Age over 11? Skip strep test
BALTIMORE – In children with pharyngitis, it’s safe to skip group A Streptococcus testing if there are no exudates, children are 11 years or older, and there is either no cervical adenopathy or adenopathy without fever, according to a Boston Children’s Hospital investigation.
The prevalence of group A Streptococcus among children who meet those criteria is 13%, less than the estimated asymptomatic carriage rate of about 15%. Among 67,127 children tested for strep and treated for sore throats in a network of retail health clinics across the United States, 35% fit the profile.
Investigators led by Daniel Shapiro, MD, a pediatrics fellow at Boston Children’s, concluded that “laboratory testing for GAS [group A Streptococcus] might be safely avoided in a large proportion of patients with sore throats. In doing so, we may avoid some of the downstream effects of unnecessary antibiotic use.” Incorporating the rules into EHRs “might help physicians identify patients who are at low risk of GAS pharyngitis.”
The study team tackled a long-standing and vexing problem in general pediatrics: how to distinguish viral from GAS pharyngitis. They often present the same way, so it’s difficult to tell them apart, but important to do so to prevent misuse of antibiotics. Health care providers generally rely on rapid strep tests and other assays to make the call, but they have to be used cautiously, because asymptomatic carriers also will test positive and be at risk for unnecessary treatment, Dr. Shapiro said at the Pediatric Academic Societies annual meeting.
To try to prevent that, the Infectious Disease Society of America (IDSA) recommends against strep testing in children who present with overt viral signs, including cough, rhinorrhea, oral ulcers, and hoarseness (Clin Infect Dis. 2012 Nov 15;55[10]:1279-82).
In a previous study at Boston Children’s ED, however, Dr. Shapiro and his colleagues found that 29% of children with overt viral features were positive for GAS, suggesting that the IDSA guidelines probably go too far (Pediatrics. 2017 May;139[5]. pii: e20163403).
“One might conclude that while it’s a good rule of thumb to avoid testing patients with viral features, some of the patients with viral features really do have GAS pharyngitis, so the recommendation to forgo testing in all these kids needs a little bit of refinement,” he said.
That was the goal of the new study; the team sought to identify viral features that signaled a low risk of GAS pharyngitis and, therefore, no need for testing. Low risk was defined as less than 15%, in keeping with the asymptomatic carriage rate.
The 67,127 patients were aged 3-21 years. Their signs and symptoms were collected at the retail clinics in a standardized form. The subjects had rapid strep tests, with negative results confirmed by DNA probe or culture.
Fifty-four percent had viral features, defined in the study as cough, runny nose, or hoarseness (oral ulcers weren’t collected on the form). The overall prevalence of GAS was 35%, similar to previous studies; 39% of children with no viral features tested positive for GAS versus 26% of children with all three. Exudates and age below 11 years were strongly associated with GAS among patients with viral features.
It turned out that just 23% of children without exudates were GAS positive; the number fell to 15% when limited to children 11 years or older, and to 13% when either no cervical adenopathy or adenopathy without fever were added to the mix.
There was no industry funding, and Dr. Shapiro didn’t have any disclosures.
BALTIMORE – In children with pharyngitis, it’s safe to skip group A Streptococcus testing if there are no exudates, children are 11 years or older, and there is either no cervical adenopathy or adenopathy without fever, according to a Boston Children’s Hospital investigation.
The prevalence of group A Streptococcus among children who meet those criteria is 13%, less than the estimated asymptomatic carriage rate of about 15%. Among 67,127 children tested for strep and treated for sore throats in a network of retail health clinics across the United States, 35% fit the profile.
Investigators led by Daniel Shapiro, MD, a pediatrics fellow at Boston Children’s, concluded that “laboratory testing for GAS [group A Streptococcus] might be safely avoided in a large proportion of patients with sore throats. In doing so, we may avoid some of the downstream effects of unnecessary antibiotic use.” Incorporating the rules into EHRs “might help physicians identify patients who are at low risk of GAS pharyngitis.”
The study team tackled a long-standing and vexing problem in general pediatrics: how to distinguish viral from GAS pharyngitis. They often present the same way, so it’s difficult to tell them apart, but important to do so to prevent misuse of antibiotics. Health care providers generally rely on rapid strep tests and other assays to make the call, but they have to be used cautiously, because asymptomatic carriers also will test positive and be at risk for unnecessary treatment, Dr. Shapiro said at the Pediatric Academic Societies annual meeting.
To try to prevent that, the Infectious Disease Society of America (IDSA) recommends against strep testing in children who present with overt viral signs, including cough, rhinorrhea, oral ulcers, and hoarseness (Clin Infect Dis. 2012 Nov 15;55[10]:1279-82).
In a previous study at Boston Children’s ED, however, Dr. Shapiro and his colleagues found that 29% of children with overt viral features were positive for GAS, suggesting that the IDSA guidelines probably go too far (Pediatrics. 2017 May;139[5]. pii: e20163403).
“One might conclude that while it’s a good rule of thumb to avoid testing patients with viral features, some of the patients with viral features really do have GAS pharyngitis, so the recommendation to forgo testing in all these kids needs a little bit of refinement,” he said.
That was the goal of the new study; the team sought to identify viral features that signaled a low risk of GAS pharyngitis and, therefore, no need for testing. Low risk was defined as less than 15%, in keeping with the asymptomatic carriage rate.
The 67,127 patients were aged 3-21 years. Their signs and symptoms were collected at the retail clinics in a standardized form. The subjects had rapid strep tests, with negative results confirmed by DNA probe or culture.
Fifty-four percent had viral features, defined in the study as cough, runny nose, or hoarseness (oral ulcers weren’t collected on the form). The overall prevalence of GAS was 35%, similar to previous studies; 39% of children with no viral features tested positive for GAS versus 26% of children with all three. Exudates and age below 11 years were strongly associated with GAS among patients with viral features.
It turned out that just 23% of children without exudates were GAS positive; the number fell to 15% when limited to children 11 years or older, and to 13% when either no cervical adenopathy or adenopathy without fever were added to the mix.
There was no industry funding, and Dr. Shapiro didn’t have any disclosures.
BALTIMORE – In children with pharyngitis, it’s safe to skip group A Streptococcus testing if there are no exudates, children are 11 years or older, and there is either no cervical adenopathy or adenopathy without fever, according to a Boston Children’s Hospital investigation.
The prevalence of group A Streptococcus among children who meet those criteria is 13%, less than the estimated asymptomatic carriage rate of about 15%. Among 67,127 children tested for strep and treated for sore throats in a network of retail health clinics across the United States, 35% fit the profile.
Investigators led by Daniel Shapiro, MD, a pediatrics fellow at Boston Children’s, concluded that “laboratory testing for GAS [group A Streptococcus] might be safely avoided in a large proportion of patients with sore throats. In doing so, we may avoid some of the downstream effects of unnecessary antibiotic use.” Incorporating the rules into EHRs “might help physicians identify patients who are at low risk of GAS pharyngitis.”
The study team tackled a long-standing and vexing problem in general pediatrics: how to distinguish viral from GAS pharyngitis. They often present the same way, so it’s difficult to tell them apart, but important to do so to prevent misuse of antibiotics. Health care providers generally rely on rapid strep tests and other assays to make the call, but they have to be used cautiously, because asymptomatic carriers also will test positive and be at risk for unnecessary treatment, Dr. Shapiro said at the Pediatric Academic Societies annual meeting.
To try to prevent that, the Infectious Disease Society of America (IDSA) recommends against strep testing in children who present with overt viral signs, including cough, rhinorrhea, oral ulcers, and hoarseness (Clin Infect Dis. 2012 Nov 15;55[10]:1279-82).
In a previous study at Boston Children’s ED, however, Dr. Shapiro and his colleagues found that 29% of children with overt viral features were positive for GAS, suggesting that the IDSA guidelines probably go too far (Pediatrics. 2017 May;139[5]. pii: e20163403).
“One might conclude that while it’s a good rule of thumb to avoid testing patients with viral features, some of the patients with viral features really do have GAS pharyngitis, so the recommendation to forgo testing in all these kids needs a little bit of refinement,” he said.
That was the goal of the new study; the team sought to identify viral features that signaled a low risk of GAS pharyngitis and, therefore, no need for testing. Low risk was defined as less than 15%, in keeping with the asymptomatic carriage rate.
The 67,127 patients were aged 3-21 years. Their signs and symptoms were collected at the retail clinics in a standardized form. The subjects had rapid strep tests, with negative results confirmed by DNA probe or culture.
Fifty-four percent had viral features, defined in the study as cough, runny nose, or hoarseness (oral ulcers weren’t collected on the form). The overall prevalence of GAS was 35%, similar to previous studies; 39% of children with no viral features tested positive for GAS versus 26% of children with all three. Exudates and age below 11 years were strongly associated with GAS among patients with viral features.
It turned out that just 23% of children without exudates were GAS positive; the number fell to 15% when limited to children 11 years or older, and to 13% when either no cervical adenopathy or adenopathy without fever were added to the mix.
There was no industry funding, and Dr. Shapiro didn’t have any disclosures.
REPORTING FROM PAS 2019
2019 Update: Contraceptives and unintended pregnancy rates
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
HHS finalizes controversial conscience regulation
Health care professionals may not be compelled to provide medical care, including abortion services or even referrals, if they object on religious or moral grounds under a federal regulation finalized May 2.
The regulation also requires health care entities that receive federal funding to alert their employees to their federal conscience rights.
This final rule “replaces a 2011 rule that has proven inadequate, and ensures that HHS implements the full set of tools appropriate for enforcing the conscience protections passed by Congress,” HHS officials said in a statement. “These federal laws protect providers, individuals, and other health care entities from having to provide, participate in, pay for, provide coverage of, or refer for, services such as abortion, sterilization, or assisted suicide. It also includes conscience protections with respect to advance directives.”
The regulation was first proposed in January 2018, shortly after the formation of the Conscience and Religious Freedom Division within the HHS Office of Civil Rights (OCR).
Application of the regulation extends beyond the clinic and hospital. The regulation notes that, on a case-by-case basis, those providing emergency services such as EMTs or even ambulance drivers could be protected should they choose to exercise their conscience and not provide services based on their religious beliefs.
“With this final rule, the Department seeks to educate protected entities and covered entities as to their legal rights and obligations; to encourage individuals and organizations with religious beliefs or moral convictions to enter, or remain in, the health care industry; and to prevent others from being dissuaded from filing complaints due to prior OCR complaint resolutions or sub-regulatory guidance that no reflect the views of the Department,” according to the regulation.
HHS officials denied accusations that the regulation puts the needs of providers over those of patients.
By “protecting a diversity of beliefs among health care providers, these protections ensure that options are available to patients who desire, and would feel most comfortable with, a provider whose religious beliefs or moral convictions match their own. Even where a patient and provider do not share the same religious beliefs or moral convictions, it is not necessarily the case that patients would want providers to be forced to violate their religious beliefs or moral convictions,” according to the regulation.
However, the American Civil Liberties Union and others see the new regulation as license to discriminate.
“Once again, this administration shows itself to be determined to use religious liberty to harm communities it deems less worthy of equal treatment under the law,” Louise Melling, ACLU deputy legal director, said in a statement. “This rule threatens to prevent people from accessing critical medical care and may endanger people’s lives. Religious liberty is a fundamental right, but it does not include the right to discriminate or harm others. Denying patients health care is not religious liberty. Discriminating against patients based on their gender or gender expression is not religious liberty. Medical standards, not religious beliefs, should guide medical care.”
The regulation does not yet have a scheduled publication date in the Federal Register, nor has it been posted as a preview document on the publication’s website. It will become effective 60 days after publication.
Health care professionals may not be compelled to provide medical care, including abortion services or even referrals, if they object on religious or moral grounds under a federal regulation finalized May 2.
The regulation also requires health care entities that receive federal funding to alert their employees to their federal conscience rights.
This final rule “replaces a 2011 rule that has proven inadequate, and ensures that HHS implements the full set of tools appropriate for enforcing the conscience protections passed by Congress,” HHS officials said in a statement. “These federal laws protect providers, individuals, and other health care entities from having to provide, participate in, pay for, provide coverage of, or refer for, services such as abortion, sterilization, or assisted suicide. It also includes conscience protections with respect to advance directives.”
The regulation was first proposed in January 2018, shortly after the formation of the Conscience and Religious Freedom Division within the HHS Office of Civil Rights (OCR).
Application of the regulation extends beyond the clinic and hospital. The regulation notes that, on a case-by-case basis, those providing emergency services such as EMTs or even ambulance drivers could be protected should they choose to exercise their conscience and not provide services based on their religious beliefs.
“With this final rule, the Department seeks to educate protected entities and covered entities as to their legal rights and obligations; to encourage individuals and organizations with religious beliefs or moral convictions to enter, or remain in, the health care industry; and to prevent others from being dissuaded from filing complaints due to prior OCR complaint resolutions or sub-regulatory guidance that no reflect the views of the Department,” according to the regulation.
HHS officials denied accusations that the regulation puts the needs of providers over those of patients.
By “protecting a diversity of beliefs among health care providers, these protections ensure that options are available to patients who desire, and would feel most comfortable with, a provider whose religious beliefs or moral convictions match their own. Even where a patient and provider do not share the same religious beliefs or moral convictions, it is not necessarily the case that patients would want providers to be forced to violate their religious beliefs or moral convictions,” according to the regulation.
However, the American Civil Liberties Union and others see the new regulation as license to discriminate.
“Once again, this administration shows itself to be determined to use religious liberty to harm communities it deems less worthy of equal treatment under the law,” Louise Melling, ACLU deputy legal director, said in a statement. “This rule threatens to prevent people from accessing critical medical care and may endanger people’s lives. Religious liberty is a fundamental right, but it does not include the right to discriminate or harm others. Denying patients health care is not religious liberty. Discriminating against patients based on their gender or gender expression is not religious liberty. Medical standards, not religious beliefs, should guide medical care.”
The regulation does not yet have a scheduled publication date in the Federal Register, nor has it been posted as a preview document on the publication’s website. It will become effective 60 days after publication.
Health care professionals may not be compelled to provide medical care, including abortion services or even referrals, if they object on religious or moral grounds under a federal regulation finalized May 2.
The regulation also requires health care entities that receive federal funding to alert their employees to their federal conscience rights.
This final rule “replaces a 2011 rule that has proven inadequate, and ensures that HHS implements the full set of tools appropriate for enforcing the conscience protections passed by Congress,” HHS officials said in a statement. “These federal laws protect providers, individuals, and other health care entities from having to provide, participate in, pay for, provide coverage of, or refer for, services such as abortion, sterilization, or assisted suicide. It also includes conscience protections with respect to advance directives.”
The regulation was first proposed in January 2018, shortly after the formation of the Conscience and Religious Freedom Division within the HHS Office of Civil Rights (OCR).
Application of the regulation extends beyond the clinic and hospital. The regulation notes that, on a case-by-case basis, those providing emergency services such as EMTs or even ambulance drivers could be protected should they choose to exercise their conscience and not provide services based on their religious beliefs.
“With this final rule, the Department seeks to educate protected entities and covered entities as to their legal rights and obligations; to encourage individuals and organizations with religious beliefs or moral convictions to enter, or remain in, the health care industry; and to prevent others from being dissuaded from filing complaints due to prior OCR complaint resolutions or sub-regulatory guidance that no reflect the views of the Department,” according to the regulation.
HHS officials denied accusations that the regulation puts the needs of providers over those of patients.
By “protecting a diversity of beliefs among health care providers, these protections ensure that options are available to patients who desire, and would feel most comfortable with, a provider whose religious beliefs or moral convictions match their own. Even where a patient and provider do not share the same religious beliefs or moral convictions, it is not necessarily the case that patients would want providers to be forced to violate their religious beliefs or moral convictions,” according to the regulation.
However, the American Civil Liberties Union and others see the new regulation as license to discriminate.
“Once again, this administration shows itself to be determined to use religious liberty to harm communities it deems less worthy of equal treatment under the law,” Louise Melling, ACLU deputy legal director, said in a statement. “This rule threatens to prevent people from accessing critical medical care and may endanger people’s lives. Religious liberty is a fundamental right, but it does not include the right to discriminate or harm others. Denying patients health care is not religious liberty. Discriminating against patients based on their gender or gender expression is not religious liberty. Medical standards, not religious beliefs, should guide medical care.”
The regulation does not yet have a scheduled publication date in the Federal Register, nor has it been posted as a preview document on the publication’s website. It will become effective 60 days after publication.
Human papillomavirus
To the Editor: I am an active primary care provider. After reading the update on human papillomavirus (HPV) in the March 2019 issue by Zhang and Batur,1 I was hoping for some clarification on a few points.
The statement is made that up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. I have pulled the reference2 but cannot find supporting data for this claim. Is this proven or optimistic thinking based on the decreased incidence of abnormal Papanicolaou (Pap) test results such as noted in the University of New Mexico HPV Pap registry database3? The authors do cite an additional reference4 documenting a decreased incidence of cervical cancer in the United States among 15- to 24-year-olds from 2003–2006 compared with 2011–2014. This study reported a 29% relative risk reduction in the group receiving the vaccine, with the absolute numbers 6 vs 8.4 cases per 1,000,000. Thus, can the authors provide further references to the statement that 70% of cervical cancers can be prevented by vaccination?
The authors also state that vaccine acceptance rates are highest when primary care providers announce that the vaccine is due rather than invite open-ended discussions. At first this shocked me, but then made me pause and wonder how often I do that—and when I do, why. I regularly do it with all the other vaccines recommended by the Advisory Committee on Immunization Practices. When the parent or patient asks for further information, I am armed to provide it. To date, I am struggling to provide data to educate the patient on the efficacy of the HPV vaccine, particularly the claim that it will prevent 70% of cervical cancers. Are there more data that I am missing?
Finally, let me state that I am a “vaccinator”—always have been, and always will be. I discuss the HPV vaccine with my patients and their parents and try to provide data to support my recommendation. However, I am concerned that this current practice regarding the HPV vaccine has been driven by scare tactics and has now turned to “just give it because I say so.” The University of New Mexico Center for HPV prevention reports up to a 50% reduction in cervical intraepithelial neoplasias (precancer lesions) in teens.3 This is exciting information and raises hope for the future successful battle against cervical cancer. I think it is also more accurate than stating to parents and patients that we have proof that we have prevented 70% of cervical cancers. When we explain it in this manner, the majority of parents and patients buy in and, I believe, enjoy and welcome this open-ended discussion.
- Zhang S, Batur P. Human papillomavirus in 2019: an update on cervical cancer prevention and screening guidelines. Cleve Clin J Med 2019; 86(3):173–178. doi:10.3949/ccjm.86a.18018
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3): 469-477.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017; 3(6):833–837. doi:10.1001/jamaoncol.2016.3609
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
To the Editor: I am an active primary care provider. After reading the update on human papillomavirus (HPV) in the March 2019 issue by Zhang and Batur,1 I was hoping for some clarification on a few points.
The statement is made that up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. I have pulled the reference2 but cannot find supporting data for this claim. Is this proven or optimistic thinking based on the decreased incidence of abnormal Papanicolaou (Pap) test results such as noted in the University of New Mexico HPV Pap registry database3? The authors do cite an additional reference4 documenting a decreased incidence of cervical cancer in the United States among 15- to 24-year-olds from 2003–2006 compared with 2011–2014. This study reported a 29% relative risk reduction in the group receiving the vaccine, with the absolute numbers 6 vs 8.4 cases per 1,000,000. Thus, can the authors provide further references to the statement that 70% of cervical cancers can be prevented by vaccination?
The authors also state that vaccine acceptance rates are highest when primary care providers announce that the vaccine is due rather than invite open-ended discussions. At first this shocked me, but then made me pause and wonder how often I do that—and when I do, why. I regularly do it with all the other vaccines recommended by the Advisory Committee on Immunization Practices. When the parent or patient asks for further information, I am armed to provide it. To date, I am struggling to provide data to educate the patient on the efficacy of the HPV vaccine, particularly the claim that it will prevent 70% of cervical cancers. Are there more data that I am missing?
Finally, let me state that I am a “vaccinator”—always have been, and always will be. I discuss the HPV vaccine with my patients and their parents and try to provide data to support my recommendation. However, I am concerned that this current practice regarding the HPV vaccine has been driven by scare tactics and has now turned to “just give it because I say so.” The University of New Mexico Center for HPV prevention reports up to a 50% reduction in cervical intraepithelial neoplasias (precancer lesions) in teens.3 This is exciting information and raises hope for the future successful battle against cervical cancer. I think it is also more accurate than stating to parents and patients that we have proof that we have prevented 70% of cervical cancers. When we explain it in this manner, the majority of parents and patients buy in and, I believe, enjoy and welcome this open-ended discussion.
To the Editor: I am an active primary care provider. After reading the update on human papillomavirus (HPV) in the March 2019 issue by Zhang and Batur,1 I was hoping for some clarification on a few points.
The statement is made that up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. I have pulled the reference2 but cannot find supporting data for this claim. Is this proven or optimistic thinking based on the decreased incidence of abnormal Papanicolaou (Pap) test results such as noted in the University of New Mexico HPV Pap registry database3? The authors do cite an additional reference4 documenting a decreased incidence of cervical cancer in the United States among 15- to 24-year-olds from 2003–2006 compared with 2011–2014. This study reported a 29% relative risk reduction in the group receiving the vaccine, with the absolute numbers 6 vs 8.4 cases per 1,000,000. Thus, can the authors provide further references to the statement that 70% of cervical cancers can be prevented by vaccination?
The authors also state that vaccine acceptance rates are highest when primary care providers announce that the vaccine is due rather than invite open-ended discussions. At first this shocked me, but then made me pause and wonder how often I do that—and when I do, why. I regularly do it with all the other vaccines recommended by the Advisory Committee on Immunization Practices. When the parent or patient asks for further information, I am armed to provide it. To date, I am struggling to provide data to educate the patient on the efficacy of the HPV vaccine, particularly the claim that it will prevent 70% of cervical cancers. Are there more data that I am missing?
Finally, let me state that I am a “vaccinator”—always have been, and always will be. I discuss the HPV vaccine with my patients and their parents and try to provide data to support my recommendation. However, I am concerned that this current practice regarding the HPV vaccine has been driven by scare tactics and has now turned to “just give it because I say so.” The University of New Mexico Center for HPV prevention reports up to a 50% reduction in cervical intraepithelial neoplasias (precancer lesions) in teens.3 This is exciting information and raises hope for the future successful battle against cervical cancer. I think it is also more accurate than stating to parents and patients that we have proof that we have prevented 70% of cervical cancers. When we explain it in this manner, the majority of parents and patients buy in and, I believe, enjoy and welcome this open-ended discussion.
- Zhang S, Batur P. Human papillomavirus in 2019: an update on cervical cancer prevention and screening guidelines. Cleve Clin J Med 2019; 86(3):173–178. doi:10.3949/ccjm.86a.18018
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3): 469-477.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017; 3(6):833–837. doi:10.1001/jamaoncol.2016.3609
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Zhang S, Batur P. Human papillomavirus in 2019: an update on cervical cancer prevention and screening guidelines. Cleve Clin J Med 2019; 86(3):173–178. doi:10.3949/ccjm.86a.18018
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3): 469-477.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017; 3(6):833–837. doi:10.1001/jamaoncol.2016.3609
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
In reply: Human papillomavirus
In Reply: We would like to thank Dr. Lichtenberg for giving us the opportunity to clarify and expand on questions regarding HPV vaccine efficacy.
Our statement “HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts” was based on a statement by Thaxton and Waxman, ie, that immunization against HPV types 16 and 18 has the potential to prevent 70% of cancers of the cervix plus a large percentage of other lower anogenital tract cancers.1 This was meant to describe the prevention potential of the quadrivalent vaccine. The currently available Gardasil 9 targets the HPV types that account for 90% of cervical cancers,2 with projected effectiveness likely to vary based on geographic variation in HPV subtypes, ranging from 86.5% in Australia to 92% in North America.3 It is difficult to precisely calculate the effectiveness of HPV vaccination alone, given that cervical cancer prevention is twofold, with primary vaccination and secondary screening (with several notable updates to US national screening guidelines during the same time frame as vaccine development).4
It is true that the 29% decrease in US cervical cancer incidence rates during the years 2011–2014 compared with 2003–2006 is less than the predicted 70%.5 However, not all eligible US females are vaccinated; according to reports from the US Centers for Disease Control and Prevention, 49% of adolescents were appropriately immunized against HPV in 2017, an increase over the rate of only 35% in 2014.6 Low vaccination rates undoubtedly negatively impact any benefits from herd immunity, though the exact benefits of this population immunity are difficult to quantify.7
In Australia, a national school-based HPV vaccination program was initiated in 2007, making the vaccine available for free. Over 70% of girls ages 12 and 13 were vaccinated, and follow-up within the same decade showed a greater than 90% reduction in genital warts, as well as a reduction in high-grade cervical lesions.8 In addition, the incidence of genital warts in unvaccinated heterosexual males during the prevaccination vs the vaccination period decreased by up to 81% (a marker of herd immunity).9
In the US, the HPV subtypes found in the quadrivalent vaccine decreased by 71% in those ages 14 to 19, within 8 years of vaccine introduction.10 An analysis of US state cancer registries between 2009 and 2012 showed that in Michigan, the rates of high-grade, precancerous lesions declined by 37% each year for women ages 15 to 19, thought to be due to changes in screening and vaccination guidelines.11 Similarly, an analysis of 9 million privately insured US females showed that the presence of high-grade precancerous lesions significantly decreased between the years 2007 and 2014 in those ages 15 to 24 (vaccinated individuals), but not in those ages 25 to 39 (unvaccinated individuals).12 Most recently, a study of 10,206 women showed a 21.9% decrease in cervical intraepithelial neoplasia grade 2 or worse lesions due to HPV subtypes 16 or 18 in those who have received at least 1 dose of the vaccine; reduced rates in unvaccinated women were also seen, representing first evidence of herd immunity in the United States.13 In contrast, the rates of high-grade lesions due to nonvaccine HPV subtypes remained constant. Given that progression to cervical cancer can take 10 to 15 years or longer after HPV infection, true vaccine benefits will emerge once increased vaccination rates are achieved and after at least a decade of follow-up.
We applaud Dr. Lichtenberg’s efforts to clarify vaccine efficacy for appropriate counseling, as this is key to ensuring patient trust. Immunization fears have fueled the re-emergence of vaccine-preventable illnesses across the world. Given the wave of vaccine misinformation on the Internet, we all face patients and family members skeptical of vaccine efficacy and safety. Those requesting more information deserve an honest, informed discussion with their provider. Interestingly, however, among 955 unvaccinated women, the belief of not being at risk for HPV was the most common reason for not receiving the vaccine.14 Effective education can be achieved by focusing on the personal risks of HPV to the patient, as well as the overall favorable risk vs benefits of vaccination. Quoting an exact rate of cancer reduction is likely a less effective counseling strategy, and these efficacy estimates will change as vaccination rates and HPV prevalence within the population change over time.
- Thaxton L, Waxman AG. Cervical cancer prevention: Immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JM, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Zhai L, Tumban E. Gardasil-9: A global survey of projected efficacy. Antiviral Res 2016 Jun;130:101–109. doi:10.1016/j.antiviral.2016.03.016
- Zhang S, Batur P. Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines. Cleve Clin J Med 2019; 86(3):173–178. doi:10.3949/ccjm.86a.18018
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young U.S. females after human papillomavirus vaccine Introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- US Centers for Disease Control and Prevention. Human papillomavirus (HPV) coverage data. https://www.cdc.gov/hpv/hcp/vacc-coverage/index.html. Accessed April 8, 2019.
- Nymark LS, Sharma T, Miller A, Enemark U, Griffiths UK. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine 2017; 35(49 Pt B):6828–6841. doi:10.1016/j.vaccine.2017.10.024
- Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Ther 2014; 36(1):17–23. doi:10.1016/j.clinthera.2013.12.005
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 2013; 346:f2032. doi:10.1136/bmj.f2032
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017; 216(5):594–603. doi:10.1093/infdis/jix244
- Watson M, Soman A, Flagg EW, et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries. Prev Med 2017; 103:60–65. doi:10.1016/j.ypmed.2017.07.027
- Flagg EW, Torrone EA, Weinstock H. Ecological association of human papillomavirus vaccination with cervical dysplasia prevalence in the United States, 2007–2014. Am J Public Health 2016; 106(12):2211–2218.
- McClung NM, Gargano JW, Bennett NM, et al; HPV-IMPACT Working Group. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008–2014. Cancer Epidemiol Biomarkers Prev 2019; 28(3):602–609. doi:10.1158/1055-9965.EPI-18-0885
- Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: findings from the 2006–2008 National Survey of Family Growth. Vaccine 2012; 30(16):2676–2682. doi:10.1016/j.vaccine.2012.02.007
In Reply: We would like to thank Dr. Lichtenberg for giving us the opportunity to clarify and expand on questions regarding HPV vaccine efficacy.
Our statement “HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts” was based on a statement by Thaxton and Waxman, ie, that immunization against HPV types 16 and 18 has the potential to prevent 70% of cancers of the cervix plus a large percentage of other lower anogenital tract cancers.1 This was meant to describe the prevention potential of the quadrivalent vaccine. The currently available Gardasil 9 targets the HPV types that account for 90% of cervical cancers,2 with projected effectiveness likely to vary based on geographic variation in HPV subtypes, ranging from 86.5% in Australia to 92% in North America.3 It is difficult to precisely calculate the effectiveness of HPV vaccination alone, given that cervical cancer prevention is twofold, with primary vaccination and secondary screening (with several notable updates to US national screening guidelines during the same time frame as vaccine development).4
It is true that the 29% decrease in US cervical cancer incidence rates during the years 2011–2014 compared with 2003–2006 is less than the predicted 70%.5 However, not all eligible US females are vaccinated; according to reports from the US Centers for Disease Control and Prevention, 49% of adolescents were appropriately immunized against HPV in 2017, an increase over the rate of only 35% in 2014.6 Low vaccination rates undoubtedly negatively impact any benefits from herd immunity, though the exact benefits of this population immunity are difficult to quantify.7
In Australia, a national school-based HPV vaccination program was initiated in 2007, making the vaccine available for free. Over 70% of girls ages 12 and 13 were vaccinated, and follow-up within the same decade showed a greater than 90% reduction in genital warts, as well as a reduction in high-grade cervical lesions.8 In addition, the incidence of genital warts in unvaccinated heterosexual males during the prevaccination vs the vaccination period decreased by up to 81% (a marker of herd immunity).9
In the US, the HPV subtypes found in the quadrivalent vaccine decreased by 71% in those ages 14 to 19, within 8 years of vaccine introduction.10 An analysis of US state cancer registries between 2009 and 2012 showed that in Michigan, the rates of high-grade, precancerous lesions declined by 37% each year for women ages 15 to 19, thought to be due to changes in screening and vaccination guidelines.11 Similarly, an analysis of 9 million privately insured US females showed that the presence of high-grade precancerous lesions significantly decreased between the years 2007 and 2014 in those ages 15 to 24 (vaccinated individuals), but not in those ages 25 to 39 (unvaccinated individuals).12 Most recently, a study of 10,206 women showed a 21.9% decrease in cervical intraepithelial neoplasia grade 2 or worse lesions due to HPV subtypes 16 or 18 in those who have received at least 1 dose of the vaccine; reduced rates in unvaccinated women were also seen, representing first evidence of herd immunity in the United States.13 In contrast, the rates of high-grade lesions due to nonvaccine HPV subtypes remained constant. Given that progression to cervical cancer can take 10 to 15 years or longer after HPV infection, true vaccine benefits will emerge once increased vaccination rates are achieved and after at least a decade of follow-up.
We applaud Dr. Lichtenberg’s efforts to clarify vaccine efficacy for appropriate counseling, as this is key to ensuring patient trust. Immunization fears have fueled the re-emergence of vaccine-preventable illnesses across the world. Given the wave of vaccine misinformation on the Internet, we all face patients and family members skeptical of vaccine efficacy and safety. Those requesting more information deserve an honest, informed discussion with their provider. Interestingly, however, among 955 unvaccinated women, the belief of not being at risk for HPV was the most common reason for not receiving the vaccine.14 Effective education can be achieved by focusing on the personal risks of HPV to the patient, as well as the overall favorable risk vs benefits of vaccination. Quoting an exact rate of cancer reduction is likely a less effective counseling strategy, and these efficacy estimates will change as vaccination rates and HPV prevalence within the population change over time.
In Reply: We would like to thank Dr. Lichtenberg for giving us the opportunity to clarify and expand on questions regarding HPV vaccine efficacy.
Our statement “HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts” was based on a statement by Thaxton and Waxman, ie, that immunization against HPV types 16 and 18 has the potential to prevent 70% of cancers of the cervix plus a large percentage of other lower anogenital tract cancers.1 This was meant to describe the prevention potential of the quadrivalent vaccine. The currently available Gardasil 9 targets the HPV types that account for 90% of cervical cancers,2 with projected effectiveness likely to vary based on geographic variation in HPV subtypes, ranging from 86.5% in Australia to 92% in North America.3 It is difficult to precisely calculate the effectiveness of HPV vaccination alone, given that cervical cancer prevention is twofold, with primary vaccination and secondary screening (with several notable updates to US national screening guidelines during the same time frame as vaccine development).4
It is true that the 29% decrease in US cervical cancer incidence rates during the years 2011–2014 compared with 2003–2006 is less than the predicted 70%.5 However, not all eligible US females are vaccinated; according to reports from the US Centers for Disease Control and Prevention, 49% of adolescents were appropriately immunized against HPV in 2017, an increase over the rate of only 35% in 2014.6 Low vaccination rates undoubtedly negatively impact any benefits from herd immunity, though the exact benefits of this population immunity are difficult to quantify.7
In Australia, a national school-based HPV vaccination program was initiated in 2007, making the vaccine available for free. Over 70% of girls ages 12 and 13 were vaccinated, and follow-up within the same decade showed a greater than 90% reduction in genital warts, as well as a reduction in high-grade cervical lesions.8 In addition, the incidence of genital warts in unvaccinated heterosexual males during the prevaccination vs the vaccination period decreased by up to 81% (a marker of herd immunity).9
In the US, the HPV subtypes found in the quadrivalent vaccine decreased by 71% in those ages 14 to 19, within 8 years of vaccine introduction.10 An analysis of US state cancer registries between 2009 and 2012 showed that in Michigan, the rates of high-grade, precancerous lesions declined by 37% each year for women ages 15 to 19, thought to be due to changes in screening and vaccination guidelines.11 Similarly, an analysis of 9 million privately insured US females showed that the presence of high-grade precancerous lesions significantly decreased between the years 2007 and 2014 in those ages 15 to 24 (vaccinated individuals), but not in those ages 25 to 39 (unvaccinated individuals).12 Most recently, a study of 10,206 women showed a 21.9% decrease in cervical intraepithelial neoplasia grade 2 or worse lesions due to HPV subtypes 16 or 18 in those who have received at least 1 dose of the vaccine; reduced rates in unvaccinated women were also seen, representing first evidence of herd immunity in the United States.13 In contrast, the rates of high-grade lesions due to nonvaccine HPV subtypes remained constant. Given that progression to cervical cancer can take 10 to 15 years or longer after HPV infection, true vaccine benefits will emerge once increased vaccination rates are achieved and after at least a decade of follow-up.
We applaud Dr. Lichtenberg’s efforts to clarify vaccine efficacy for appropriate counseling, as this is key to ensuring patient trust. Immunization fears have fueled the re-emergence of vaccine-preventable illnesses across the world. Given the wave of vaccine misinformation on the Internet, we all face patients and family members skeptical of vaccine efficacy and safety. Those requesting more information deserve an honest, informed discussion with their provider. Interestingly, however, among 955 unvaccinated women, the belief of not being at risk for HPV was the most common reason for not receiving the vaccine.14 Effective education can be achieved by focusing on the personal risks of HPV to the patient, as well as the overall favorable risk vs benefits of vaccination. Quoting an exact rate of cancer reduction is likely a less effective counseling strategy, and these efficacy estimates will change as vaccination rates and HPV prevalence within the population change over time.
- Thaxton L, Waxman AG. Cervical cancer prevention: Immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JM, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Zhai L, Tumban E. Gardasil-9: A global survey of projected efficacy. Antiviral Res 2016 Jun;130:101–109. doi:10.1016/j.antiviral.2016.03.016
- Zhang S, Batur P. Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines. Cleve Clin J Med 2019; 86(3):173–178. doi:10.3949/ccjm.86a.18018
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young U.S. females after human papillomavirus vaccine Introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- US Centers for Disease Control and Prevention. Human papillomavirus (HPV) coverage data. https://www.cdc.gov/hpv/hcp/vacc-coverage/index.html. Accessed April 8, 2019.
- Nymark LS, Sharma T, Miller A, Enemark U, Griffiths UK. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine 2017; 35(49 Pt B):6828–6841. doi:10.1016/j.vaccine.2017.10.024
- Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Ther 2014; 36(1):17–23. doi:10.1016/j.clinthera.2013.12.005
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 2013; 346:f2032. doi:10.1136/bmj.f2032
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017; 216(5):594–603. doi:10.1093/infdis/jix244
- Watson M, Soman A, Flagg EW, et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries. Prev Med 2017; 103:60–65. doi:10.1016/j.ypmed.2017.07.027
- Flagg EW, Torrone EA, Weinstock H. Ecological association of human papillomavirus vaccination with cervical dysplasia prevalence in the United States, 2007–2014. Am J Public Health 2016; 106(12):2211–2218.
- McClung NM, Gargano JW, Bennett NM, et al; HPV-IMPACT Working Group. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008–2014. Cancer Epidemiol Biomarkers Prev 2019; 28(3):602–609. doi:10.1158/1055-9965.EPI-18-0885
- Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: findings from the 2006–2008 National Survey of Family Growth. Vaccine 2012; 30(16):2676–2682. doi:10.1016/j.vaccine.2012.02.007
- Thaxton L, Waxman AG. Cervical cancer prevention: Immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JM, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Zhai L, Tumban E. Gardasil-9: A global survey of projected efficacy. Antiviral Res 2016 Jun;130:101–109. doi:10.1016/j.antiviral.2016.03.016
- Zhang S, Batur P. Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines. Cleve Clin J Med 2019; 86(3):173–178. doi:10.3949/ccjm.86a.18018
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young U.S. females after human papillomavirus vaccine Introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- US Centers for Disease Control and Prevention. Human papillomavirus (HPV) coverage data. https://www.cdc.gov/hpv/hcp/vacc-coverage/index.html. Accessed April 8, 2019.
- Nymark LS, Sharma T, Miller A, Enemark U, Griffiths UK. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine 2017; 35(49 Pt B):6828–6841. doi:10.1016/j.vaccine.2017.10.024
- Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Ther 2014; 36(1):17–23. doi:10.1016/j.clinthera.2013.12.005
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 2013; 346:f2032. doi:10.1136/bmj.f2032
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017; 216(5):594–603. doi:10.1093/infdis/jix244
- Watson M, Soman A, Flagg EW, et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries. Prev Med 2017; 103:60–65. doi:10.1016/j.ypmed.2017.07.027
- Flagg EW, Torrone EA, Weinstock H. Ecological association of human papillomavirus vaccination with cervical dysplasia prevalence in the United States, 2007–2014. Am J Public Health 2016; 106(12):2211–2218.
- McClung NM, Gargano JW, Bennett NM, et al; HPV-IMPACT Working Group. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008–2014. Cancer Epidemiol Biomarkers Prev 2019; 28(3):602–609. doi:10.1158/1055-9965.EPI-18-0885
- Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: findings from the 2006–2008 National Survey of Family Growth. Vaccine 2012; 30(16):2676–2682. doi:10.1016/j.vaccine.2012.02.007