User login
Advice on treating rheumatic diseases from a COVID-19 epicenter
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
ACR gives guidance on rheumatic disease management during pandemic
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
FROM ARTHRITIS & RHEUMATOLOGY
Case series suggests biologics, JAK inhibitors safe during pandemic
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
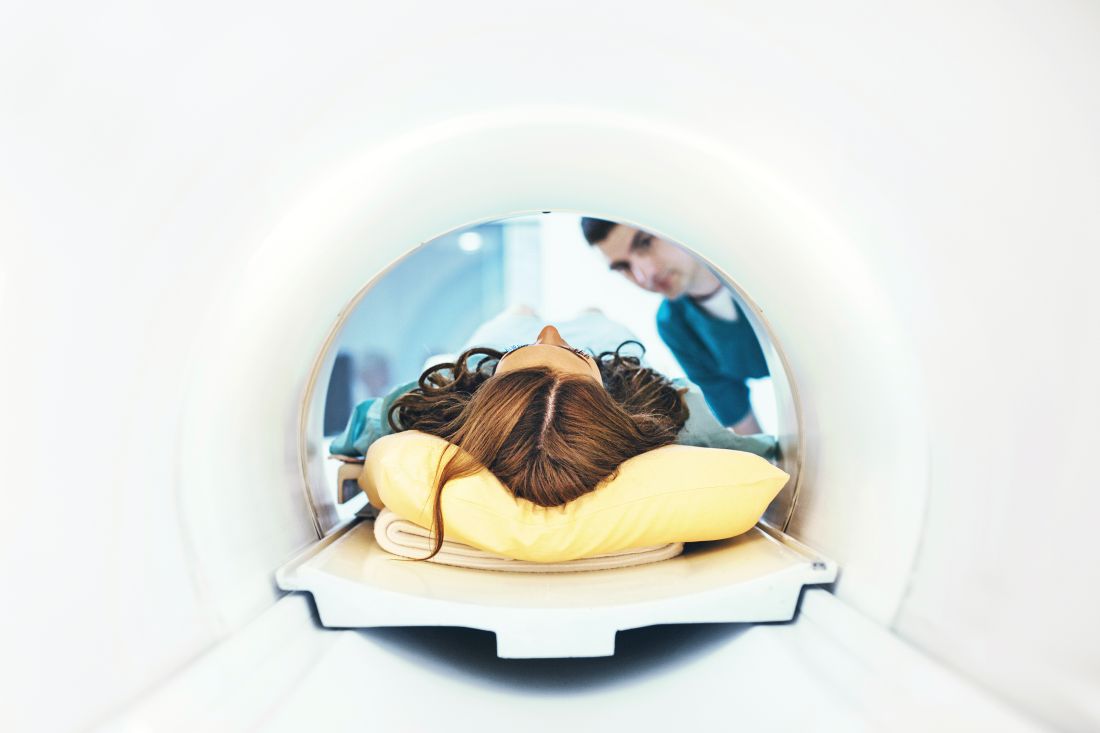
Sacroiliac bone marrow edema on MRI may be common postpartum
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
FROM ANNALS OF THE RHEUMATIC DISEASES
Differentiating hypersensitivity reactions to monoclonal antibodies
MAUI, HAWAII – Desensitization is a powerful and effective tool in patients with certain types of hypersensitivity reactions to therapeutic monoclonal antibodies, but it’s best considered a last resort reserved for individuals with no options left other than the offending biologic, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Why so selective? Desensitization is considered a high-risk intervention. It’s typically done as an inpatient procedure involving an overnight hospital stay followed by an elaborate 12-step protocol involving administration of small quantities of the culprit biologic in ascending concentrations over a 5- to 6-hour period.
Moreover, for an intravenous agent, such as infliximab (Remicade), desensitization has to be repeated prior to giving every dose of the biologic. So it makes sense to skip desensitization and simply switch to an alternative tumor necrosis factor inhibitor or a different class of biologic unless experience has shown that the culprit monoclonal antibody is the only one that works for that patient. It’s known, for example, that infliximab has no crossreactivity with adalimumab (Humira), explained Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
Defining type and severity of the hypersensitivity reaction
Dr. Postolova favors the hypersensitivity reaction classification system developed by Mariana Castells, MD, PhD, and coworkers at Brigham and Women’s Hospital, Boston.
They divide the field into immediate and delayed hypersensitivity reactions. Immediate hypersensitivity reactions arise rapidly, between minutes and a few hours. They can be categorized as infusion reactions, cytokine-release reactions, and IgE-mediated reactions. Phenotypically, infusion reactions and cytokine-release reactions are typically characterized by various combinations of chills, fever, flushing, hypertension, tachycardia, nausea, vomiting, syncope, and shortness of breath.
IgE-mediated reactions can also involve flushing and shortness of breath, and in addition itch, urticaria, and hypotension. These are anaphylactic reactions. Neither hypertension nor fever is part of the anaphylactic picture; those findings point instead to an infusion reaction or cytokine-release reaction.
Most allergists grade reaction severity on a 1-3 scale. Grade 1 reactions are considered mild and involve symptoms limited to the skin, such as flushing, or a single other organ system.
“That being said, if my patient is having a reaction with bronchospasm, I consider that a moderate, grade 2 reaction, and I stop the infusion. There’s only so much you can do for bronchospasm. It’s a very serious reaction,” Dr. Postolova observed.
Grade 2 reactions ordinarily involve two or more organ systems, but without hypotension or cyanosis. Grade 3 reactions are severe anaphylactic reactions with cardiovascular and/or neurologic compromise.
Delayed hypersensitivity reactions are of two types: serum sickness–like reactions and type IV cell-mediated mucocutaneous reactions.
Type IV reactions can range from a mild maculopapular rash to erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS (drug reaction with eosinophilia and systemic symptoms). Onset of type IV reactions can occur after 12 hours up to several weeks after exposure.
Serum sickness–like reactions typically begin 5-7 days after the infusion. These reactions are marked by evidence of immune overactivation: fever, arthralgia, arthritis, malaise, purpura, skin rash, and even renal failure.
Management of reactions
A patient with a grade 3 reaction who needs to continue using the culprit monoclonal antibody should be referred to an allergist for skin testing in an effort to identify an IgE-mediated reaction.
The timing of the referral for skin testing is important: The allergist wants to test roughly 4-6 weeks after the hypersensitivity reaction. Test too early and the results will be uninformative because the patient will still be anergic. On the other hand, after 7-8 weeks the patient will have lost the allergy. So there is a sweet spot.
If the patient is skin test positive – with the caveat that skin testing in this setting is not well validated – then the allergist will suggest desensitization, usually as an inpatient.
In contrast, infusion reactions can be handled in the rheumatologist’s infusion center. They are self-limited upon repeat exposure with premedication using antihistamines, NSAIDs, oral or injectable steroids, and perhaps montelukast (Singulair).
If a patient initially thought to have an infusion reaction continues to experience reactions even after the biologic is being delivered more slowly and under the protection of premedication, it’s time to consider the possibility that what’s really going on is a cytokine-release reaction or an IgE-mediated reaction. Diagnostic skin testing is in order.
For a skin test–negative patient with a suspected cytokine-release reaction, the allergist may propose a therapeutic challenge. This is reserved for patients who the allergist believe will not experience an immediate reaction, and unlike desensitization it’s not an intervention intended to induce drug tolerance. The challenge involves giving 10% of a full dose of the biologic, waiting in the allergist’s office for 30-60 minutes, then giving the other 90% of the medication, followed by an hour of in-office observation.
The solution to severe type IV delayed hypersensitivity reactions is strict medication avoidance, not desensitization, according to Dr. Postolova.
Top offending monoclonal antibodies
Infliximab and rituximab (Rituxan) are the most common culprits when it comes to immediate hypersensitivity reactions. About 10% of infliximab-treated patients develop these reactions. Although the reaction can occur with the first dose, the peak incidence is with the seventh infusion. Patients with anti-infliximab IgG antibodies are at 140%-300% increased risk; however, concomitant disease-modifying antirheumatic drug therapy lessens that risk.
Infusion reactions or cytokine-release reactions occur upon the first infusion of rituximab in about 25% of treated rheumatology patients and in a higher proportion of cancer patients. Most of these reactions are mild and don’t recur when the biologic is administered more slowly and with premedication. Severe recurrent reactions upon subsequent exposure are much more likely to be an IgE-mediated hypersensitivity reaction.
“Stop the medication, send the patient to your local allergist for skin testing, and they’ll use a desensitization protocol if rituximab is the best drug for your patient,” Dr. Postolova advised.
She reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – Desensitization is a powerful and effective tool in patients with certain types of hypersensitivity reactions to therapeutic monoclonal antibodies, but it’s best considered a last resort reserved for individuals with no options left other than the offending biologic, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Why so selective? Desensitization is considered a high-risk intervention. It’s typically done as an inpatient procedure involving an overnight hospital stay followed by an elaborate 12-step protocol involving administration of small quantities of the culprit biologic in ascending concentrations over a 5- to 6-hour period.
Moreover, for an intravenous agent, such as infliximab (Remicade), desensitization has to be repeated prior to giving every dose of the biologic. So it makes sense to skip desensitization and simply switch to an alternative tumor necrosis factor inhibitor or a different class of biologic unless experience has shown that the culprit monoclonal antibody is the only one that works for that patient. It’s known, for example, that infliximab has no crossreactivity with adalimumab (Humira), explained Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
Defining type and severity of the hypersensitivity reaction
Dr. Postolova favors the hypersensitivity reaction classification system developed by Mariana Castells, MD, PhD, and coworkers at Brigham and Women’s Hospital, Boston.
They divide the field into immediate and delayed hypersensitivity reactions. Immediate hypersensitivity reactions arise rapidly, between minutes and a few hours. They can be categorized as infusion reactions, cytokine-release reactions, and IgE-mediated reactions. Phenotypically, infusion reactions and cytokine-release reactions are typically characterized by various combinations of chills, fever, flushing, hypertension, tachycardia, nausea, vomiting, syncope, and shortness of breath.
IgE-mediated reactions can also involve flushing and shortness of breath, and in addition itch, urticaria, and hypotension. These are anaphylactic reactions. Neither hypertension nor fever is part of the anaphylactic picture; those findings point instead to an infusion reaction or cytokine-release reaction.
Most allergists grade reaction severity on a 1-3 scale. Grade 1 reactions are considered mild and involve symptoms limited to the skin, such as flushing, or a single other organ system.
“That being said, if my patient is having a reaction with bronchospasm, I consider that a moderate, grade 2 reaction, and I stop the infusion. There’s only so much you can do for bronchospasm. It’s a very serious reaction,” Dr. Postolova observed.
Grade 2 reactions ordinarily involve two or more organ systems, but without hypotension or cyanosis. Grade 3 reactions are severe anaphylactic reactions with cardiovascular and/or neurologic compromise.
Delayed hypersensitivity reactions are of two types: serum sickness–like reactions and type IV cell-mediated mucocutaneous reactions.
Type IV reactions can range from a mild maculopapular rash to erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS (drug reaction with eosinophilia and systemic symptoms). Onset of type IV reactions can occur after 12 hours up to several weeks after exposure.
Serum sickness–like reactions typically begin 5-7 days after the infusion. These reactions are marked by evidence of immune overactivation: fever, arthralgia, arthritis, malaise, purpura, skin rash, and even renal failure.
Management of reactions
A patient with a grade 3 reaction who needs to continue using the culprit monoclonal antibody should be referred to an allergist for skin testing in an effort to identify an IgE-mediated reaction.
The timing of the referral for skin testing is important: The allergist wants to test roughly 4-6 weeks after the hypersensitivity reaction. Test too early and the results will be uninformative because the patient will still be anergic. On the other hand, after 7-8 weeks the patient will have lost the allergy. So there is a sweet spot.
If the patient is skin test positive – with the caveat that skin testing in this setting is not well validated – then the allergist will suggest desensitization, usually as an inpatient.
In contrast, infusion reactions can be handled in the rheumatologist’s infusion center. They are self-limited upon repeat exposure with premedication using antihistamines, NSAIDs, oral or injectable steroids, and perhaps montelukast (Singulair).
If a patient initially thought to have an infusion reaction continues to experience reactions even after the biologic is being delivered more slowly and under the protection of premedication, it’s time to consider the possibility that what’s really going on is a cytokine-release reaction or an IgE-mediated reaction. Diagnostic skin testing is in order.
For a skin test–negative patient with a suspected cytokine-release reaction, the allergist may propose a therapeutic challenge. This is reserved for patients who the allergist believe will not experience an immediate reaction, and unlike desensitization it’s not an intervention intended to induce drug tolerance. The challenge involves giving 10% of a full dose of the biologic, waiting in the allergist’s office for 30-60 minutes, then giving the other 90% of the medication, followed by an hour of in-office observation.
The solution to severe type IV delayed hypersensitivity reactions is strict medication avoidance, not desensitization, according to Dr. Postolova.
Top offending monoclonal antibodies
Infliximab and rituximab (Rituxan) are the most common culprits when it comes to immediate hypersensitivity reactions. About 10% of infliximab-treated patients develop these reactions. Although the reaction can occur with the first dose, the peak incidence is with the seventh infusion. Patients with anti-infliximab IgG antibodies are at 140%-300% increased risk; however, concomitant disease-modifying antirheumatic drug therapy lessens that risk.
Infusion reactions or cytokine-release reactions occur upon the first infusion of rituximab in about 25% of treated rheumatology patients and in a higher proportion of cancer patients. Most of these reactions are mild and don’t recur when the biologic is administered more slowly and with premedication. Severe recurrent reactions upon subsequent exposure are much more likely to be an IgE-mediated hypersensitivity reaction.
“Stop the medication, send the patient to your local allergist for skin testing, and they’ll use a desensitization protocol if rituximab is the best drug for your patient,” Dr. Postolova advised.
She reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – Desensitization is a powerful and effective tool in patients with certain types of hypersensitivity reactions to therapeutic monoclonal antibodies, but it’s best considered a last resort reserved for individuals with no options left other than the offending biologic, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Why so selective? Desensitization is considered a high-risk intervention. It’s typically done as an inpatient procedure involving an overnight hospital stay followed by an elaborate 12-step protocol involving administration of small quantities of the culprit biologic in ascending concentrations over a 5- to 6-hour period.
Moreover, for an intravenous agent, such as infliximab (Remicade), desensitization has to be repeated prior to giving every dose of the biologic. So it makes sense to skip desensitization and simply switch to an alternative tumor necrosis factor inhibitor or a different class of biologic unless experience has shown that the culprit monoclonal antibody is the only one that works for that patient. It’s known, for example, that infliximab has no crossreactivity with adalimumab (Humira), explained Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
Defining type and severity of the hypersensitivity reaction
Dr. Postolova favors the hypersensitivity reaction classification system developed by Mariana Castells, MD, PhD, and coworkers at Brigham and Women’s Hospital, Boston.
They divide the field into immediate and delayed hypersensitivity reactions. Immediate hypersensitivity reactions arise rapidly, between minutes and a few hours. They can be categorized as infusion reactions, cytokine-release reactions, and IgE-mediated reactions. Phenotypically, infusion reactions and cytokine-release reactions are typically characterized by various combinations of chills, fever, flushing, hypertension, tachycardia, nausea, vomiting, syncope, and shortness of breath.
IgE-mediated reactions can also involve flushing and shortness of breath, and in addition itch, urticaria, and hypotension. These are anaphylactic reactions. Neither hypertension nor fever is part of the anaphylactic picture; those findings point instead to an infusion reaction or cytokine-release reaction.
Most allergists grade reaction severity on a 1-3 scale. Grade 1 reactions are considered mild and involve symptoms limited to the skin, such as flushing, or a single other organ system.
“That being said, if my patient is having a reaction with bronchospasm, I consider that a moderate, grade 2 reaction, and I stop the infusion. There’s only so much you can do for bronchospasm. It’s a very serious reaction,” Dr. Postolova observed.
Grade 2 reactions ordinarily involve two or more organ systems, but without hypotension or cyanosis. Grade 3 reactions are severe anaphylactic reactions with cardiovascular and/or neurologic compromise.
Delayed hypersensitivity reactions are of two types: serum sickness–like reactions and type IV cell-mediated mucocutaneous reactions.
Type IV reactions can range from a mild maculopapular rash to erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS (drug reaction with eosinophilia and systemic symptoms). Onset of type IV reactions can occur after 12 hours up to several weeks after exposure.
Serum sickness–like reactions typically begin 5-7 days after the infusion. These reactions are marked by evidence of immune overactivation: fever, arthralgia, arthritis, malaise, purpura, skin rash, and even renal failure.
Management of reactions
A patient with a grade 3 reaction who needs to continue using the culprit monoclonal antibody should be referred to an allergist for skin testing in an effort to identify an IgE-mediated reaction.
The timing of the referral for skin testing is important: The allergist wants to test roughly 4-6 weeks after the hypersensitivity reaction. Test too early and the results will be uninformative because the patient will still be anergic. On the other hand, after 7-8 weeks the patient will have lost the allergy. So there is a sweet spot.
If the patient is skin test positive – with the caveat that skin testing in this setting is not well validated – then the allergist will suggest desensitization, usually as an inpatient.
In contrast, infusion reactions can be handled in the rheumatologist’s infusion center. They are self-limited upon repeat exposure with premedication using antihistamines, NSAIDs, oral or injectable steroids, and perhaps montelukast (Singulair).
If a patient initially thought to have an infusion reaction continues to experience reactions even after the biologic is being delivered more slowly and under the protection of premedication, it’s time to consider the possibility that what’s really going on is a cytokine-release reaction or an IgE-mediated reaction. Diagnostic skin testing is in order.
For a skin test–negative patient with a suspected cytokine-release reaction, the allergist may propose a therapeutic challenge. This is reserved for patients who the allergist believe will not experience an immediate reaction, and unlike desensitization it’s not an intervention intended to induce drug tolerance. The challenge involves giving 10% of a full dose of the biologic, waiting in the allergist’s office for 30-60 minutes, then giving the other 90% of the medication, followed by an hour of in-office observation.
The solution to severe type IV delayed hypersensitivity reactions is strict medication avoidance, not desensitization, according to Dr. Postolova.
Top offending monoclonal antibodies
Infliximab and rituximab (Rituxan) are the most common culprits when it comes to immediate hypersensitivity reactions. About 10% of infliximab-treated patients develop these reactions. Although the reaction can occur with the first dose, the peak incidence is with the seventh infusion. Patients with anti-infliximab IgG antibodies are at 140%-300% increased risk; however, concomitant disease-modifying antirheumatic drug therapy lessens that risk.
Infusion reactions or cytokine-release reactions occur upon the first infusion of rituximab in about 25% of treated rheumatology patients and in a higher proportion of cancer patients. Most of these reactions are mild and don’t recur when the biologic is administered more slowly and with premedication. Severe recurrent reactions upon subsequent exposure are much more likely to be an IgE-mediated hypersensitivity reaction.
“Stop the medication, send the patient to your local allergist for skin testing, and they’ll use a desensitization protocol if rituximab is the best drug for your patient,” Dr. Postolova advised.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM RWCS 2020
Financial incentives affect the adoption of biosimilars
during the same time period in 2015-2019, according to an analysis published in Arthritis and Rheumatology.
The use of the biosimilars also was associated with cost savings at the VAMC, but not at the academic medical center, which illustrates that insufficient financial incentives can delay the adoption of biosimilars and the health care system’s realization of cost savings, according to the authors.
Medicare, which is not allowed to negotiate drug prices, is one of the largest payers for infused therapies. Medicare reimbursement for infused therapies is based on the latter’s average selling price (ASP) during the previous quarter. Institutions may negotiate purchase prices with drug manufacturers and receive Medicare reimbursement. Biosimilars generally have lower ASPs than their corresponding reference therapies, and biosimilar manufacturers may have less room to negotiate prices than reference therapy manufacturers. Consequently, a given institution might have a greater incentive to use reference products than to use biosimilars.
An examination of pharmacy data
The VA negotiates drug prices for all of its medical centers and has mandated that clinicians prefer biosimilars to their corresponding reference therapies, so Joshua F. Baker, MD, of the University of Pennsylvania and the Corporal Michael J. Crescenz VAMC, both in Philadelphia, and his colleagues hypothesized that the adoption of biosimilars had proceeded more quickly at a VAMC than at a nearby academic medical center.
The investigators examined pharmacy data from the University of Pennsylvania Health System (UPHS) electronic medical record and the Corporal Michael J. Crescenz VAMC to compare the frequency of prescribing biosimilars at these sites between Jan. 1, 2015, and May 31, 2019. Dr. Baker and his associates focused specifically on reference infliximab (Remicade) and the reference noninfusion therapies filgrastim (Neupogen) and pegfilgrastim (Neulasta) and on biosimilars of these therapies (infliximab-dyyb [Inflectra], infliximab-abda [Renflexis], filgrastim-sndz [Zarxio], and pegfilgrastim-jmdb [Fulphila]).
Because Medicare was the predominant payer, the researchers estimated reimbursement for reference and biosimilar infliximabs according to the Medicare Part B reimbursement policy. They defined an institution’s incentive to use a given therapy as the difference between the reimbursement and acquisition cost for that therapy. Dr. Baker and colleagues compared the incentives for UPHS with those for the VAMC.
VAMC saved 81% of reference product cost
The researchers identified 15,761 infusions of infliximab at UPHS and 446 at the VAMC during the study period. The proportion of infusions that used the reference product was 99% at UPHS and 62% at the VAMC. ASPs for biosimilar infliximab have been consistently lower than those for the reference product since July 2017. In December 2017, the VAMC switched to the biosimilar infliximab.
Institutional incentives based on Medicare Part B reimbursement and acquisitions costs for reference and biosimilar infliximab have been similar since 2018. In 2019, the institutional incentive favored the reference product by $49-$64 per 100-mg vial. But at the VAMC, the cost per 100-mg vial was $623.48 for the reference product and $115.58 for the biosimilar Renflexis. Purchasing the biosimilar thus yielded a savings of 81%. The current costs for the therapies are $546 and $116, respectively.
In addition, Dr. Baker and colleagues identified 46,683 orders for filgrastim or pegfilgrastim at UPHS. Approximately 90% of the orders were for either of the two reference products despite the ASP of biosimilar filgrastim being approximately 40% lower than that of its reference product. At the VAMC, about 88% of orders were for the reference products. Biosimilars became available in 2016. UPHS began using them at a modest rate, but their adoption was greater at the VAMC, which designated them as preferred products.
Tendering and a nationwide policy mandating use of biosimilars have resulted in financial savings for the VAMC, wrote Dr. Baker and colleagues. “These data suggest that, with current Medicare Part B reimbursement policy, the absence of financial incentives to encourage use of infliximab biosimilars has resulted in slower uptake of biosimilar use at institutions outside of the VA system. The implications of this are a slower reduction in costs to the health care system, since decreases in ASP over time are predicated on negotiations at the institutional level, which have been gradual and stepwise. ...
“Although some of our results may not be applicable to other geographical regions of the U.S., the comparison of two affiliated institutions in geographical proximity and with shared health care providers is a strength,” they continued. “Our findings should be replicated using national VAMC data or data from other health care systems.”
The researchers said that their findings may not apply to noninfused therapies, which are not covered under Medicare Part B, and they did not directly study the impact of pharmacy benefit managers. However, they noted that their data on filgrastim and pegfilgrastim support the hypothesis that pharmacy benefit managers receive “incentives that continue to promote the use of reference products that have higher manufacturer’s list prices, which likely will limit the uptake of both infused and injectable biosimilar therapies over time.” They said that “this finding has important implications for when noninfused biosimilars (e.g. etanercept and adalimumab) are eventually introduced to the U.S. market.”
European governments incentivize use of biosimilars
Government and institutional incentives have increased the adoption of biosimilars in Europe, wrote Guro Lovik Goll, MD, and Tore Kristian Kvien, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, in an accompanying editorial. Norway and Denmark have annual national tender systems in which biosimilars and reference products compete. The price of infliximab biosimilar was 39% lower than the reference product in 2014 and 69% lower in 2015. “Competition has caused dramatically lower prices both for biosimilars and also for the originator drugs competing with them,” wrote the authors.
In 2015, the government of Denmark mandated that patients on infliximab be switched to a biosimilar, and patients in Norway also have been switched to biosimilars. The use of etanercept in Norway increased by 40% from 2016 to 2019, and the use of infliximab has increased by more than threefold since 2015. “In Norway, the consequence of competition, national tenders, and availability of biosimilars have led to better access to therapy for more people in need of biologic drugs, while at the same time showing a total cost reduction of biologics for use in rheumatology, gastroenterology, and dermatology,” wrote the authors.
Health care costs $10,000 per capita in the United States, compared with $5,300 for other wealthy countries in the Organization for Economic Cooperation and Development. Low life expectancy and high infant mortality in the U.S. indicate that high costs are not associated with better outcomes. “As Americans seem to lose out on the cost-cutting potential of biosimilars, this missed opportunity is set to get even more expensive,” the authors concluded.
The U.S. Department of Veterans Affairs, the National Institutes of Health, and the American Diabetes Association contributed funding for the study. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb and Gilead, and another author reported receiving research support paid to his institution by Pfizer and UCB, as well as receiving consulting fees from nine pharmaceutical companies. Dr. Goll and Dr. Kvien both reported receiving fees for speaking and/or consulting from numerous pharmaceutical companies, including Pfizer.
SOURCES: Baker JF et al. Arthritis Rheumatol. 2020 Apr 6. doi: 10.1002/art.41277.
during the same time period in 2015-2019, according to an analysis published in Arthritis and Rheumatology.
The use of the biosimilars also was associated with cost savings at the VAMC, but not at the academic medical center, which illustrates that insufficient financial incentives can delay the adoption of biosimilars and the health care system’s realization of cost savings, according to the authors.
Medicare, which is not allowed to negotiate drug prices, is one of the largest payers for infused therapies. Medicare reimbursement for infused therapies is based on the latter’s average selling price (ASP) during the previous quarter. Institutions may negotiate purchase prices with drug manufacturers and receive Medicare reimbursement. Biosimilars generally have lower ASPs than their corresponding reference therapies, and biosimilar manufacturers may have less room to negotiate prices than reference therapy manufacturers. Consequently, a given institution might have a greater incentive to use reference products than to use biosimilars.
An examination of pharmacy data
The VA negotiates drug prices for all of its medical centers and has mandated that clinicians prefer biosimilars to their corresponding reference therapies, so Joshua F. Baker, MD, of the University of Pennsylvania and the Corporal Michael J. Crescenz VAMC, both in Philadelphia, and his colleagues hypothesized that the adoption of biosimilars had proceeded more quickly at a VAMC than at a nearby academic medical center.
The investigators examined pharmacy data from the University of Pennsylvania Health System (UPHS) electronic medical record and the Corporal Michael J. Crescenz VAMC to compare the frequency of prescribing biosimilars at these sites between Jan. 1, 2015, and May 31, 2019. Dr. Baker and his associates focused specifically on reference infliximab (Remicade) and the reference noninfusion therapies filgrastim (Neupogen) and pegfilgrastim (Neulasta) and on biosimilars of these therapies (infliximab-dyyb [Inflectra], infliximab-abda [Renflexis], filgrastim-sndz [Zarxio], and pegfilgrastim-jmdb [Fulphila]).
Because Medicare was the predominant payer, the researchers estimated reimbursement for reference and biosimilar infliximabs according to the Medicare Part B reimbursement policy. They defined an institution’s incentive to use a given therapy as the difference between the reimbursement and acquisition cost for that therapy. Dr. Baker and colleagues compared the incentives for UPHS with those for the VAMC.
VAMC saved 81% of reference product cost
The researchers identified 15,761 infusions of infliximab at UPHS and 446 at the VAMC during the study period. The proportion of infusions that used the reference product was 99% at UPHS and 62% at the VAMC. ASPs for biosimilar infliximab have been consistently lower than those for the reference product since July 2017. In December 2017, the VAMC switched to the biosimilar infliximab.
Institutional incentives based on Medicare Part B reimbursement and acquisitions costs for reference and biosimilar infliximab have been similar since 2018. In 2019, the institutional incentive favored the reference product by $49-$64 per 100-mg vial. But at the VAMC, the cost per 100-mg vial was $623.48 for the reference product and $115.58 for the biosimilar Renflexis. Purchasing the biosimilar thus yielded a savings of 81%. The current costs for the therapies are $546 and $116, respectively.
In addition, Dr. Baker and colleagues identified 46,683 orders for filgrastim or pegfilgrastim at UPHS. Approximately 90% of the orders were for either of the two reference products despite the ASP of biosimilar filgrastim being approximately 40% lower than that of its reference product. At the VAMC, about 88% of orders were for the reference products. Biosimilars became available in 2016. UPHS began using them at a modest rate, but their adoption was greater at the VAMC, which designated them as preferred products.
Tendering and a nationwide policy mandating use of biosimilars have resulted in financial savings for the VAMC, wrote Dr. Baker and colleagues. “These data suggest that, with current Medicare Part B reimbursement policy, the absence of financial incentives to encourage use of infliximab biosimilars has resulted in slower uptake of biosimilar use at institutions outside of the VA system. The implications of this are a slower reduction in costs to the health care system, since decreases in ASP over time are predicated on negotiations at the institutional level, which have been gradual and stepwise. ...
“Although some of our results may not be applicable to other geographical regions of the U.S., the comparison of two affiliated institutions in geographical proximity and with shared health care providers is a strength,” they continued. “Our findings should be replicated using national VAMC data or data from other health care systems.”
The researchers said that their findings may not apply to noninfused therapies, which are not covered under Medicare Part B, and they did not directly study the impact of pharmacy benefit managers. However, they noted that their data on filgrastim and pegfilgrastim support the hypothesis that pharmacy benefit managers receive “incentives that continue to promote the use of reference products that have higher manufacturer’s list prices, which likely will limit the uptake of both infused and injectable biosimilar therapies over time.” They said that “this finding has important implications for when noninfused biosimilars (e.g. etanercept and adalimumab) are eventually introduced to the U.S. market.”
European governments incentivize use of biosimilars
Government and institutional incentives have increased the adoption of biosimilars in Europe, wrote Guro Lovik Goll, MD, and Tore Kristian Kvien, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, in an accompanying editorial. Norway and Denmark have annual national tender systems in which biosimilars and reference products compete. The price of infliximab biosimilar was 39% lower than the reference product in 2014 and 69% lower in 2015. “Competition has caused dramatically lower prices both for biosimilars and also for the originator drugs competing with them,” wrote the authors.
In 2015, the government of Denmark mandated that patients on infliximab be switched to a biosimilar, and patients in Norway also have been switched to biosimilars. The use of etanercept in Norway increased by 40% from 2016 to 2019, and the use of infliximab has increased by more than threefold since 2015. “In Norway, the consequence of competition, national tenders, and availability of biosimilars have led to better access to therapy for more people in need of biologic drugs, while at the same time showing a total cost reduction of biologics for use in rheumatology, gastroenterology, and dermatology,” wrote the authors.
Health care costs $10,000 per capita in the United States, compared with $5,300 for other wealthy countries in the Organization for Economic Cooperation and Development. Low life expectancy and high infant mortality in the U.S. indicate that high costs are not associated with better outcomes. “As Americans seem to lose out on the cost-cutting potential of biosimilars, this missed opportunity is set to get even more expensive,” the authors concluded.
The U.S. Department of Veterans Affairs, the National Institutes of Health, and the American Diabetes Association contributed funding for the study. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb and Gilead, and another author reported receiving research support paid to his institution by Pfizer and UCB, as well as receiving consulting fees from nine pharmaceutical companies. Dr. Goll and Dr. Kvien both reported receiving fees for speaking and/or consulting from numerous pharmaceutical companies, including Pfizer.
SOURCES: Baker JF et al. Arthritis Rheumatol. 2020 Apr 6. doi: 10.1002/art.41277.
during the same time period in 2015-2019, according to an analysis published in Arthritis and Rheumatology.
The use of the biosimilars also was associated with cost savings at the VAMC, but not at the academic medical center, which illustrates that insufficient financial incentives can delay the adoption of biosimilars and the health care system’s realization of cost savings, according to the authors.
Medicare, which is not allowed to negotiate drug prices, is one of the largest payers for infused therapies. Medicare reimbursement for infused therapies is based on the latter’s average selling price (ASP) during the previous quarter. Institutions may negotiate purchase prices with drug manufacturers and receive Medicare reimbursement. Biosimilars generally have lower ASPs than their corresponding reference therapies, and biosimilar manufacturers may have less room to negotiate prices than reference therapy manufacturers. Consequently, a given institution might have a greater incentive to use reference products than to use biosimilars.
An examination of pharmacy data
The VA negotiates drug prices for all of its medical centers and has mandated that clinicians prefer biosimilars to their corresponding reference therapies, so Joshua F. Baker, MD, of the University of Pennsylvania and the Corporal Michael J. Crescenz VAMC, both in Philadelphia, and his colleagues hypothesized that the adoption of biosimilars had proceeded more quickly at a VAMC than at a nearby academic medical center.
The investigators examined pharmacy data from the University of Pennsylvania Health System (UPHS) electronic medical record and the Corporal Michael J. Crescenz VAMC to compare the frequency of prescribing biosimilars at these sites between Jan. 1, 2015, and May 31, 2019. Dr. Baker and his associates focused specifically on reference infliximab (Remicade) and the reference noninfusion therapies filgrastim (Neupogen) and pegfilgrastim (Neulasta) and on biosimilars of these therapies (infliximab-dyyb [Inflectra], infliximab-abda [Renflexis], filgrastim-sndz [Zarxio], and pegfilgrastim-jmdb [Fulphila]).
Because Medicare was the predominant payer, the researchers estimated reimbursement for reference and biosimilar infliximabs according to the Medicare Part B reimbursement policy. They defined an institution’s incentive to use a given therapy as the difference between the reimbursement and acquisition cost for that therapy. Dr. Baker and colleagues compared the incentives for UPHS with those for the VAMC.
VAMC saved 81% of reference product cost
The researchers identified 15,761 infusions of infliximab at UPHS and 446 at the VAMC during the study period. The proportion of infusions that used the reference product was 99% at UPHS and 62% at the VAMC. ASPs for biosimilar infliximab have been consistently lower than those for the reference product since July 2017. In December 2017, the VAMC switched to the biosimilar infliximab.
Institutional incentives based on Medicare Part B reimbursement and acquisitions costs for reference and biosimilar infliximab have been similar since 2018. In 2019, the institutional incentive favored the reference product by $49-$64 per 100-mg vial. But at the VAMC, the cost per 100-mg vial was $623.48 for the reference product and $115.58 for the biosimilar Renflexis. Purchasing the biosimilar thus yielded a savings of 81%. The current costs for the therapies are $546 and $116, respectively.
In addition, Dr. Baker and colleagues identified 46,683 orders for filgrastim or pegfilgrastim at UPHS. Approximately 90% of the orders were for either of the two reference products despite the ASP of biosimilar filgrastim being approximately 40% lower than that of its reference product. At the VAMC, about 88% of orders were for the reference products. Biosimilars became available in 2016. UPHS began using them at a modest rate, but their adoption was greater at the VAMC, which designated them as preferred products.
Tendering and a nationwide policy mandating use of biosimilars have resulted in financial savings for the VAMC, wrote Dr. Baker and colleagues. “These data suggest that, with current Medicare Part B reimbursement policy, the absence of financial incentives to encourage use of infliximab biosimilars has resulted in slower uptake of biosimilar use at institutions outside of the VA system. The implications of this are a slower reduction in costs to the health care system, since decreases in ASP over time are predicated on negotiations at the institutional level, which have been gradual and stepwise. ...
“Although some of our results may not be applicable to other geographical regions of the U.S., the comparison of two affiliated institutions in geographical proximity and with shared health care providers is a strength,” they continued. “Our findings should be replicated using national VAMC data or data from other health care systems.”
The researchers said that their findings may not apply to noninfused therapies, which are not covered under Medicare Part B, and they did not directly study the impact of pharmacy benefit managers. However, they noted that their data on filgrastim and pegfilgrastim support the hypothesis that pharmacy benefit managers receive “incentives that continue to promote the use of reference products that have higher manufacturer’s list prices, which likely will limit the uptake of both infused and injectable biosimilar therapies over time.” They said that “this finding has important implications for when noninfused biosimilars (e.g. etanercept and adalimumab) are eventually introduced to the U.S. market.”
European governments incentivize use of biosimilars
Government and institutional incentives have increased the adoption of biosimilars in Europe, wrote Guro Lovik Goll, MD, and Tore Kristian Kvien, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, in an accompanying editorial. Norway and Denmark have annual national tender systems in which biosimilars and reference products compete. The price of infliximab biosimilar was 39% lower than the reference product in 2014 and 69% lower in 2015. “Competition has caused dramatically lower prices both for biosimilars and also for the originator drugs competing with them,” wrote the authors.
In 2015, the government of Denmark mandated that patients on infliximab be switched to a biosimilar, and patients in Norway also have been switched to biosimilars. The use of etanercept in Norway increased by 40% from 2016 to 2019, and the use of infliximab has increased by more than threefold since 2015. “In Norway, the consequence of competition, national tenders, and availability of biosimilars have led to better access to therapy for more people in need of biologic drugs, while at the same time showing a total cost reduction of biologics for use in rheumatology, gastroenterology, and dermatology,” wrote the authors.
Health care costs $10,000 per capita in the United States, compared with $5,300 for other wealthy countries in the Organization for Economic Cooperation and Development. Low life expectancy and high infant mortality in the U.S. indicate that high costs are not associated with better outcomes. “As Americans seem to lose out on the cost-cutting potential of biosimilars, this missed opportunity is set to get even more expensive,” the authors concluded.
The U.S. Department of Veterans Affairs, the National Institutes of Health, and the American Diabetes Association contributed funding for the study. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb and Gilead, and another author reported receiving research support paid to his institution by Pfizer and UCB, as well as receiving consulting fees from nine pharmaceutical companies. Dr. Goll and Dr. Kvien both reported receiving fees for speaking and/or consulting from numerous pharmaceutical companies, including Pfizer.
SOURCES: Baker JF et al. Arthritis Rheumatol. 2020 Apr 6. doi: 10.1002/art.41277.
FROM ARTHRITIS & RHEUMATOLOGY
‘We’re in great distress here,’ infusion center CMO says
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
When the going gets tough, ophthalmologists call the rheumatologist
MAUI, HAWAII – When a rheumatologist gets a call from an ophthalmologist regarding a patient with an inflamed eye and elevated intraocular pressure unresponsive to the eye specialist’s customary array of topical, systemic, and intraocular implanted corticosteroids, that’s a patient who needs to be seen immediately, Alvin F. Wells, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
Elevated intraocular pressure due to uveitis or scleritis can result in blindness. Eye specialists call upon rheumatologists here because of their expertise in step-up therapy with methotrexate and other traditional oral disease-modifying antirheumatic drugs as well as biologic agents.
“Here’s my treatment approach to inflammatory eye disease: We’re pulling out all the guns,” declared Dr. Wells, a rheumatologist with a special interest in eye disease. He is director of the Rheumatology and Immunotherapy Center in Franklin, Wisc., with academic appointments to the Karolinska Institute in Stockholm, Duke University, and Marquette University.
Uveitis involves inflammation of the iris, choroid, and ciliary body. A straightforward case of noninfectious anterior uveitis will typically respond to 2 weeks of topical steroid drops, or sometimes even topical NSAID drops.
However, noninfectious posterior, intermediate, or panuveitis is another matter. In those circumstances, he gives the patient 125 mg of methylprednisolone by intramuscular injection and a 20-mg dose of oral methotrexate at that first clinic visit. The patient is sent home with a prescription for oral prednisone, tapering over 2-3 weeks, and another for methotrexate at 15-25 mg/week plus 1-2 mg/day of folic acid. Dr. Wells also gives consideration to add-on azathioprine or mycophenolate mofetil. He views multidrug therapy as having a sound rationale because multiple inflammatory pathways are involved in noninfectious uveitis.
“Ophthalmologists like to push for cyclophosphamide, but there’s no controlled data out there showing it’s effective in inflammatory eye disorders. It’s a pretty toxic regimen, and when you think about all the complications we see in using this drug to treat patients with lupus, I’d rather hold it in reserve for severe cases where we can go to it if we need to,” the rheumatologist explained.
He conducted a literature review to rank rheumatologic medications in terms of their evidence base for treatment of inflammatory ocular disorders. Among oral agents, at the top of the heap is methotrexate, whose efficacy for both noninfectious uveitis and scleritis is supported by multiple randomized, controlled studies. But mycophenolate mofetil is a reasonable alternative first-line corticosteroid-sparing agent, as demonstrated in the 265-patient multicenter FAST (First-line Antimetabolites as Steroid-sparing Treatment) trial sponsored by the National Eye Institute. That trial demonstrated no significant difference in treatment success at 6 months between methotrexate and mycophenolate mofetil.
Oral apremilast (Otezla) is approved for treatment of the oral ulcers of Behçet’s disease, but not for Behçet’s eye disease, where the experience is anecdotal.
Dr. Wells is quick to turn to adalimumab (Humira) when he deems a biologic to be warranted; indeed, it’s the only biologic approved for noninfectious uveitis. Of course, not everyone is a responder.
“Can we extrapolate that high-quality evidence of benefit for adalimumab to other drugs? Probably yes, and if you did that it would be for the IgG monoclonal antibodies that can cross the blood/aqueous barrier,” he said.
Infliximab (Remicade) is the biologic with the second-strongest supporting evidence in noninfectious uveitis. For the uveitis of Behçet’s disease, one of the most common rheumatic causes of inflammatory eye disease, Spanish investigators who conducted a nationwide nonrandomized study reported that both adalimumab and infliximab were effective, although adalimumab had superior outcomes at 1 year.
Uveitis is the most common extra-articular expression of axial spondyloarthritis (axSpA). In the open-label extension of the randomized RAPID-axSpA trial, patients randomized to certolizumab pegol (Cimzia) had a significantly lower incidence of uveitis flares than with placebo through 204 weeks of follow-up.
“The take-home message is we have some post hoc data here to say, ‘Hey, this could work in those patients who have inflammatory eye diseases in the setting of axSpA,’ ” Dr. Wells said.
The interleukin-6 receptor inhibitor tocilizumab (Actemra) “definitely works” for noninfectious uveitis, according to Dr. Wells, pointing to the positive results of the multicenter U.S. STOP-Uveitis study.
“The caveat here is tocilizumab has only been studied in the IV formulation. It’s too bad they didn’t use the [subcutaneous formulation]; you can’t get IV tocilizumab approved by payers in the U.S.,” according to the rheumatologist.
Based upon positive anecdotal case reports, Dr. Wells has a few patients on rituximab (Rituxan) for uveitis, with favorable results. The same for abatacept (Orencia).
It’s imperative that a patient on a biologic for uveitis undergo weekly ophthalmologic examinations. Only after the intraocular pressure is normal and inflammatory cells in the anterior chamber have waned is it appropriate to discontinue the biologic and slowly taper the methotrexate and any other oral disease-modifying antirheumatic drugs. Some experts argue for lifelong therapy in patients who’ve experienced uveitis. Dr. Wells disagrees, preferring to treat acute uveitis flares as they arise, although if underlying disease such as psoriatic arthritis or axSpA is present, some form of background therapy will probably be necessary.
Get to know teprotumumab
Rheumatologists who operate an infusion center are likely to increasingly be called upon by endocrinologists and ophthalmologists to administer intravenous teprotumumab-trbw (Tepezza), a human monoclonal antibody directed against the insulin-like growth factor 1 receptor that was approved earlier this year by the Food and Drug Administration as the first-ever drug for thyroid eye disease, a disfiguring and potentially blinding condition.
“This is really exciting,” Dr. Wells said. “The disease has an acute inflammatory stage, and that’s when you’ll be called on to give this drug. It makes a dramatic difference. Once a patient gets to the scarring phase there’s not a whole lot they can do other than surgery.”
In the pivotal phase 3 randomized trial, 83% of the teprotumumab group achieved the primary endpoint, a reduction in proptosis, or eye bulging, of at least 2 mm at week 24, compared with 10% of placebo-treated controls. The number needed to treat was 1.4. The chief side effects were muscle spasms, hair loss, fatigue, and nausea.
“You might say, ‘two millimeters, that’s nothing.’ But the primary drug used before teprotumumab was IV steroids, and there a 0.6-mm reduction in proptosis was considered improvement,” Dr. Wells observed.
Obtaining payer approval
“I’ve found over the last 10 years that when it comes to eye disease, insurance companies have a little more wiggle room,” he said. “They’re not going to let somebody go blind. You can get the references I’ve mentioned and show them the data. After all, we only have one biologic drug that’s been approved, and not everybody responds to it.
“Titrate your therapy based upon the intraocular pressure, the number of inflammatory cells in the anterior chamber, and any visual changes. You’ve got to be very aggressive with therapy, and don’t take no for an answer from the insurance companies,” he advised.
Dr. Wells reported serving as a member of an advisory board and/or speakers bureau for more than a dozen pharmaceutical companies.
MAUI, HAWAII – When a rheumatologist gets a call from an ophthalmologist regarding a patient with an inflamed eye and elevated intraocular pressure unresponsive to the eye specialist’s customary array of topical, systemic, and intraocular implanted corticosteroids, that’s a patient who needs to be seen immediately, Alvin F. Wells, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
Elevated intraocular pressure due to uveitis or scleritis can result in blindness. Eye specialists call upon rheumatologists here because of their expertise in step-up therapy with methotrexate and other traditional oral disease-modifying antirheumatic drugs as well as biologic agents.
“Here’s my treatment approach to inflammatory eye disease: We’re pulling out all the guns,” declared Dr. Wells, a rheumatologist with a special interest in eye disease. He is director of the Rheumatology and Immunotherapy Center in Franklin, Wisc., with academic appointments to the Karolinska Institute in Stockholm, Duke University, and Marquette University.
Uveitis involves inflammation of the iris, choroid, and ciliary body. A straightforward case of noninfectious anterior uveitis will typically respond to 2 weeks of topical steroid drops, or sometimes even topical NSAID drops.
However, noninfectious posterior, intermediate, or panuveitis is another matter. In those circumstances, he gives the patient 125 mg of methylprednisolone by intramuscular injection and a 20-mg dose of oral methotrexate at that first clinic visit. The patient is sent home with a prescription for oral prednisone, tapering over 2-3 weeks, and another for methotrexate at 15-25 mg/week plus 1-2 mg/day of folic acid. Dr. Wells also gives consideration to add-on azathioprine or mycophenolate mofetil. He views multidrug therapy as having a sound rationale because multiple inflammatory pathways are involved in noninfectious uveitis.
“Ophthalmologists like to push for cyclophosphamide, but there’s no controlled data out there showing it’s effective in inflammatory eye disorders. It’s a pretty toxic regimen, and when you think about all the complications we see in using this drug to treat patients with lupus, I’d rather hold it in reserve for severe cases where we can go to it if we need to,” the rheumatologist explained.
He conducted a literature review to rank rheumatologic medications in terms of their evidence base for treatment of inflammatory ocular disorders. Among oral agents, at the top of the heap is methotrexate, whose efficacy for both noninfectious uveitis and scleritis is supported by multiple randomized, controlled studies. But mycophenolate mofetil is a reasonable alternative first-line corticosteroid-sparing agent, as demonstrated in the 265-patient multicenter FAST (First-line Antimetabolites as Steroid-sparing Treatment) trial sponsored by the National Eye Institute. That trial demonstrated no significant difference in treatment success at 6 months between methotrexate and mycophenolate mofetil.
Oral apremilast (Otezla) is approved for treatment of the oral ulcers of Behçet’s disease, but not for Behçet’s eye disease, where the experience is anecdotal.
Dr. Wells is quick to turn to adalimumab (Humira) when he deems a biologic to be warranted; indeed, it’s the only biologic approved for noninfectious uveitis. Of course, not everyone is a responder.
“Can we extrapolate that high-quality evidence of benefit for adalimumab to other drugs? Probably yes, and if you did that it would be for the IgG monoclonal antibodies that can cross the blood/aqueous barrier,” he said.
Infliximab (Remicade) is the biologic with the second-strongest supporting evidence in noninfectious uveitis. For the uveitis of Behçet’s disease, one of the most common rheumatic causes of inflammatory eye disease, Spanish investigators who conducted a nationwide nonrandomized study reported that both adalimumab and infliximab were effective, although adalimumab had superior outcomes at 1 year.
Uveitis is the most common extra-articular expression of axial spondyloarthritis (axSpA). In the open-label extension of the randomized RAPID-axSpA trial, patients randomized to certolizumab pegol (Cimzia) had a significantly lower incidence of uveitis flares than with placebo through 204 weeks of follow-up.
“The take-home message is we have some post hoc data here to say, ‘Hey, this could work in those patients who have inflammatory eye diseases in the setting of axSpA,’ ” Dr. Wells said.
The interleukin-6 receptor inhibitor tocilizumab (Actemra) “definitely works” for noninfectious uveitis, according to Dr. Wells, pointing to the positive results of the multicenter U.S. STOP-Uveitis study.
“The caveat here is tocilizumab has only been studied in the IV formulation. It’s too bad they didn’t use the [subcutaneous formulation]; you can’t get IV tocilizumab approved by payers in the U.S.,” according to the rheumatologist.
Based upon positive anecdotal case reports, Dr. Wells has a few patients on rituximab (Rituxan) for uveitis, with favorable results. The same for abatacept (Orencia).
It’s imperative that a patient on a biologic for uveitis undergo weekly ophthalmologic examinations. Only after the intraocular pressure is normal and inflammatory cells in the anterior chamber have waned is it appropriate to discontinue the biologic and slowly taper the methotrexate and any other oral disease-modifying antirheumatic drugs. Some experts argue for lifelong therapy in patients who’ve experienced uveitis. Dr. Wells disagrees, preferring to treat acute uveitis flares as they arise, although if underlying disease such as psoriatic arthritis or axSpA is present, some form of background therapy will probably be necessary.
Get to know teprotumumab
Rheumatologists who operate an infusion center are likely to increasingly be called upon by endocrinologists and ophthalmologists to administer intravenous teprotumumab-trbw (Tepezza), a human monoclonal antibody directed against the insulin-like growth factor 1 receptor that was approved earlier this year by the Food and Drug Administration as the first-ever drug for thyroid eye disease, a disfiguring and potentially blinding condition.
“This is really exciting,” Dr. Wells said. “The disease has an acute inflammatory stage, and that’s when you’ll be called on to give this drug. It makes a dramatic difference. Once a patient gets to the scarring phase there’s not a whole lot they can do other than surgery.”
In the pivotal phase 3 randomized trial, 83% of the teprotumumab group achieved the primary endpoint, a reduction in proptosis, or eye bulging, of at least 2 mm at week 24, compared with 10% of placebo-treated controls. The number needed to treat was 1.4. The chief side effects were muscle spasms, hair loss, fatigue, and nausea.
“You might say, ‘two millimeters, that’s nothing.’ But the primary drug used before teprotumumab was IV steroids, and there a 0.6-mm reduction in proptosis was considered improvement,” Dr. Wells observed.
Obtaining payer approval
“I’ve found over the last 10 years that when it comes to eye disease, insurance companies have a little more wiggle room,” he said. “They’re not going to let somebody go blind. You can get the references I’ve mentioned and show them the data. After all, we only have one biologic drug that’s been approved, and not everybody responds to it.
“Titrate your therapy based upon the intraocular pressure, the number of inflammatory cells in the anterior chamber, and any visual changes. You’ve got to be very aggressive with therapy, and don’t take no for an answer from the insurance companies,” he advised.
Dr. Wells reported serving as a member of an advisory board and/or speakers bureau for more than a dozen pharmaceutical companies.
MAUI, HAWAII – When a rheumatologist gets a call from an ophthalmologist regarding a patient with an inflamed eye and elevated intraocular pressure unresponsive to the eye specialist’s customary array of topical, systemic, and intraocular implanted corticosteroids, that’s a patient who needs to be seen immediately, Alvin F. Wells, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
Elevated intraocular pressure due to uveitis or scleritis can result in blindness. Eye specialists call upon rheumatologists here because of their expertise in step-up therapy with methotrexate and other traditional oral disease-modifying antirheumatic drugs as well as biologic agents.
“Here’s my treatment approach to inflammatory eye disease: We’re pulling out all the guns,” declared Dr. Wells, a rheumatologist with a special interest in eye disease. He is director of the Rheumatology and Immunotherapy Center in Franklin, Wisc., with academic appointments to the Karolinska Institute in Stockholm, Duke University, and Marquette University.
Uveitis involves inflammation of the iris, choroid, and ciliary body. A straightforward case of noninfectious anterior uveitis will typically respond to 2 weeks of topical steroid drops, or sometimes even topical NSAID drops.
However, noninfectious posterior, intermediate, or panuveitis is another matter. In those circumstances, he gives the patient 125 mg of methylprednisolone by intramuscular injection and a 20-mg dose of oral methotrexate at that first clinic visit. The patient is sent home with a prescription for oral prednisone, tapering over 2-3 weeks, and another for methotrexate at 15-25 mg/week plus 1-2 mg/day of folic acid. Dr. Wells also gives consideration to add-on azathioprine or mycophenolate mofetil. He views multidrug therapy as having a sound rationale because multiple inflammatory pathways are involved in noninfectious uveitis.
“Ophthalmologists like to push for cyclophosphamide, but there’s no controlled data out there showing it’s effective in inflammatory eye disorders. It’s a pretty toxic regimen, and when you think about all the complications we see in using this drug to treat patients with lupus, I’d rather hold it in reserve for severe cases where we can go to it if we need to,” the rheumatologist explained.
He conducted a literature review to rank rheumatologic medications in terms of their evidence base for treatment of inflammatory ocular disorders. Among oral agents, at the top of the heap is methotrexate, whose efficacy for both noninfectious uveitis and scleritis is supported by multiple randomized, controlled studies. But mycophenolate mofetil is a reasonable alternative first-line corticosteroid-sparing agent, as demonstrated in the 265-patient multicenter FAST (First-line Antimetabolites as Steroid-sparing Treatment) trial sponsored by the National Eye Institute. That trial demonstrated no significant difference in treatment success at 6 months between methotrexate and mycophenolate mofetil.
Oral apremilast (Otezla) is approved for treatment of the oral ulcers of Behçet’s disease, but not for Behçet’s eye disease, where the experience is anecdotal.
Dr. Wells is quick to turn to adalimumab (Humira) when he deems a biologic to be warranted; indeed, it’s the only biologic approved for noninfectious uveitis. Of course, not everyone is a responder.
“Can we extrapolate that high-quality evidence of benefit for adalimumab to other drugs? Probably yes, and if you did that it would be for the IgG monoclonal antibodies that can cross the blood/aqueous barrier,” he said.
Infliximab (Remicade) is the biologic with the second-strongest supporting evidence in noninfectious uveitis. For the uveitis of Behçet’s disease, one of the most common rheumatic causes of inflammatory eye disease, Spanish investigators who conducted a nationwide nonrandomized study reported that both adalimumab and infliximab were effective, although adalimumab had superior outcomes at 1 year.
Uveitis is the most common extra-articular expression of axial spondyloarthritis (axSpA). In the open-label extension of the randomized RAPID-axSpA trial, patients randomized to certolizumab pegol (Cimzia) had a significantly lower incidence of uveitis flares than with placebo through 204 weeks of follow-up.
“The take-home message is we have some post hoc data here to say, ‘Hey, this could work in those patients who have inflammatory eye diseases in the setting of axSpA,’ ” Dr. Wells said.
The interleukin-6 receptor inhibitor tocilizumab (Actemra) “definitely works” for noninfectious uveitis, according to Dr. Wells, pointing to the positive results of the multicenter U.S. STOP-Uveitis study.
“The caveat here is tocilizumab has only been studied in the IV formulation. It’s too bad they didn’t use the [subcutaneous formulation]; you can’t get IV tocilizumab approved by payers in the U.S.,” according to the rheumatologist.
Based upon positive anecdotal case reports, Dr. Wells has a few patients on rituximab (Rituxan) for uveitis, with favorable results. The same for abatacept (Orencia).
It’s imperative that a patient on a biologic for uveitis undergo weekly ophthalmologic examinations. Only after the intraocular pressure is normal and inflammatory cells in the anterior chamber have waned is it appropriate to discontinue the biologic and slowly taper the methotrexate and any other oral disease-modifying antirheumatic drugs. Some experts argue for lifelong therapy in patients who’ve experienced uveitis. Dr. Wells disagrees, preferring to treat acute uveitis flares as they arise, although if underlying disease such as psoriatic arthritis or axSpA is present, some form of background therapy will probably be necessary.
Get to know teprotumumab
Rheumatologists who operate an infusion center are likely to increasingly be called upon by endocrinologists and ophthalmologists to administer intravenous teprotumumab-trbw (Tepezza), a human monoclonal antibody directed against the insulin-like growth factor 1 receptor that was approved earlier this year by the Food and Drug Administration as the first-ever drug for thyroid eye disease, a disfiguring and potentially blinding condition.
“This is really exciting,” Dr. Wells said. “The disease has an acute inflammatory stage, and that’s when you’ll be called on to give this drug. It makes a dramatic difference. Once a patient gets to the scarring phase there’s not a whole lot they can do other than surgery.”
In the pivotal phase 3 randomized trial, 83% of the teprotumumab group achieved the primary endpoint, a reduction in proptosis, or eye bulging, of at least 2 mm at week 24, compared with 10% of placebo-treated controls. The number needed to treat was 1.4. The chief side effects were muscle spasms, hair loss, fatigue, and nausea.
“You might say, ‘two millimeters, that’s nothing.’ But the primary drug used before teprotumumab was IV steroids, and there a 0.6-mm reduction in proptosis was considered improvement,” Dr. Wells observed.
Obtaining payer approval
“I’ve found over the last 10 years that when it comes to eye disease, insurance companies have a little more wiggle room,” he said. “They’re not going to let somebody go blind. You can get the references I’ve mentioned and show them the data. After all, we only have one biologic drug that’s been approved, and not everybody responds to it.
“Titrate your therapy based upon the intraocular pressure, the number of inflammatory cells in the anterior chamber, and any visual changes. You’ve got to be very aggressive with therapy, and don’t take no for an answer from the insurance companies,” he advised.
Dr. Wells reported serving as a member of an advisory board and/or speakers bureau for more than a dozen pharmaceutical companies.
REPORTING FROM RWCS 2020
Treatment for RA, SpA may not affect COVID-19 severity
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
FROM ANNALS OF THE RHEUMATIC DISEASES
JAK inhibitors may increase risk of herpes zoster
For patients with inflammatory bowel disease or other immune-mediated inflammatory diseases, Janus kinase (JAK) inhibitors appear generally safe, though they may increase the risk of herpes zoster infection, according to a large-scale systematic review and meta-analysis.
Data from more than 66,000 patients revealed no significant links between JAK inhibitors and risks of serious infections, malignancy, or major adverse cardiovascular events, reported lead author Pablo Olivera, MD, of Centro de Educación Médica e Investigación Clínica (CEMIC) in Buenos Aires and colleagues.
“To the best of our knowledge, this is the first systematic review evaluating the risk profile of JAK inhibitors in a wide spectrum of immune-mediated inflammatory diseases,” they wrote in Gastroenterology.
The investigators drew studies from the Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE from 1990 to 2019 and from conference databases from 2012 to 2018. Out of 973 studies identified, 82 were included in the final analysis, of which two-thirds were randomized clinical trials. In total, 101,925 subjects were included, of whom a majority had rheumatoid arthritis (n = 86,308), followed by psoriasis (n = 9,311), inflammatory bowel disease (n = 5,987), and ankylosing spondylitis (n = 319).
Meta-analysis of JAK inhibitor usage involved 66,159 patients. Four JAK inhibitors were included: tofacitinib, filgotinib, baricitinib, and upadacitinib. The primary outcomes were the incidence rates of adverse events and serious adverse events. The investigators also estimated incidence rates of herpes zoster infection, serious infections, mortality, malignancy, and major adverse cardiovascular events. These rates were compared with those of patients who received placebo or an active comparator in clinical trials.
Analysis showed that almost 9 out of 10 patients (87.16%) who were exposed to a JAK inhibitor received tofacitinib. The investigators described high variability in treatment duration and baseline characteristics of participants. Rates of adverse events and serious adverse events also fell across a broad spectrum, from 10% to 82% and from 0% to 29%, respectively.
“Most [adverse events] were mild, and included worsening of the underlying condition, probably showing lack of efficacy,” the investigators wrote.
Rates of mortality and most adverse events were not significantly associated with JAK inhibitor exposure. In contrast, relative risk of herpes zoster infection was 57% higher in patients who received a JAK inhibitor than in those who received a placebo or comparator (RR, 1.57; 95% confidence interval, 1.01-2.37).
“Regarding the risk of herpes zoster with JAK inhibitors, the largest evidence comes from the use of tofacitinib, but it appears to be a class effect, with a clear dose-dependent effect,” the investigators wrote.
Although risks of herpes zoster may be carried across the drug class, they may not be evenly distributed given that a subgroup analysis revealed that some JAK inhibitors may bring higher risks than others; specifically, tofacitinib and baricitinib were associated with higher relative risks of herpes zoster than were upadacitinib and filgotinib.
“Although this is merely a qualitative comparison, this difference could be related to the fact that both filgotinib and upadacitinib are selective JAK1 inhibitors, whereas tofacitinib is a JAK1/JAK3 inhibitor and baricitinib a JAK1/JAK2 inhibitor,” the investigators wrote. “Further studies are needed to determine if JAK isoform selectivity affects the risk of herpes zoster.”
The investigators emphasized this need for more research. While the present findings help illuminate the safety profile of JAK inhibitors, they are clouded by various other factors, such as disease-specific considerations, a lack of real-world data, and studies that are likely too short to accurately determine risk of malignancy, the investigators wrote.
“More studies with long follow-up and in the real world setting, in different conditions, will be needed to fully elucidate the safety profile of the different JAK inhibitors,” the investigators concluded.
The investigators disclosed relationships with AbbVie, Takeda, Pfizer, and others.
SOURCE: Olivera P et al. Gastroenterology. 2020 Jan 8. doi: 10.1053/j.gastro.2020.01.001.
The multiple different cytokines contributing to intestinal inflammation in IBD patients have been a major challenge in the design of therapies. Because the JAK signaling pathway (comprised of JAK1, JAK2, JAK3, and TYK2) is required for responses to a broad range of cytokines, therapies that inhibit JAK signaling have been an active area of interest. A simultaneous and important concern, however, has been the potential for adverse consequences when inhibiting the breadth of immune and hematopoietic molecules that depend on JAK family members for their functions. This meta-analysis by Olivera et al. examined adverse outcomes of four different JAK inhibitors in clinical trials across four immune-mediated diseases (rheumatoid arthritis, IBD, psoriasis, and ankylosing spondylitis), finding that herpes zoster infection was significantly increased (relative risk, 1.57). In contrast, patients treated with JAK inhibitors were not at a significantly increased risk for various other adverse events.
Reduced dosing of JAK inhibitors has been implemented as a means of improving safety profiles in select immune-mediated diseases. Another approach is more selective JAK inhibition, although it is unclear whether this will eliminate the risk of herpes zoster infection. In the current meta-analysis, about 87% of the studies had evaluated tofacitinib treatment, which inhibits both JAK1 and JAK3; more selective JAK inhibitors could not be evaluated in an equivalent manner. Of note, JAK1 is required for signaling by various cytokines that participate in the response to viruses, including type I IFNs and gamma c family members (such as IL-2 and IL-15); therefore, even the more selective JAK1 inhibitors do not leave this immune function fully intact. However, simply reducing the number of JAK family members inhibited simultaneously may be sufficient to reduce risk.
JAK inhibitors warrant further evaluation as additional infectious challenges arise, particularly with respect to viruses. In addition, more selective targeting of JAK inhibition of intestinal tissues may ultimately reduce systemic effects, including the risk of herpes zoster.
Clara Abraham, MD, professor of medicine, section of digestive diseases, Yale University, New Haven, Conn.
The multiple different cytokines contributing to intestinal inflammation in IBD patients have been a major challenge in the design of therapies. Because the JAK signaling pathway (comprised of JAK1, JAK2, JAK3, and TYK2) is required for responses to a broad range of cytokines, therapies that inhibit JAK signaling have been an active area of interest. A simultaneous and important concern, however, has been the potential for adverse consequences when inhibiting the breadth of immune and hematopoietic molecules that depend on JAK family members for their functions. This meta-analysis by Olivera et al. examined adverse outcomes of four different JAK inhibitors in clinical trials across four immune-mediated diseases (rheumatoid arthritis, IBD, psoriasis, and ankylosing spondylitis), finding that herpes zoster infection was significantly increased (relative risk, 1.57). In contrast, patients treated with JAK inhibitors were not at a significantly increased risk for various other adverse events.
Reduced dosing of JAK inhibitors has been implemented as a means of improving safety profiles in select immune-mediated diseases. Another approach is more selective JAK inhibition, although it is unclear whether this will eliminate the risk of herpes zoster infection. In the current meta-analysis, about 87% of the studies had evaluated tofacitinib treatment, which inhibits both JAK1 and JAK3; more selective JAK inhibitors could not be evaluated in an equivalent manner. Of note, JAK1 is required for signaling by various cytokines that participate in the response to viruses, including type I IFNs and gamma c family members (such as IL-2 and IL-15); therefore, even the more selective JAK1 inhibitors do not leave this immune function fully intact. However, simply reducing the number of JAK family members inhibited simultaneously may be sufficient to reduce risk.
JAK inhibitors warrant further evaluation as additional infectious challenges arise, particularly with respect to viruses. In addition, more selective targeting of JAK inhibition of intestinal tissues may ultimately reduce systemic effects, including the risk of herpes zoster.
Clara Abraham, MD, professor of medicine, section of digestive diseases, Yale University, New Haven, Conn.
The multiple different cytokines contributing to intestinal inflammation in IBD patients have been a major challenge in the design of therapies. Because the JAK signaling pathway (comprised of JAK1, JAK2, JAK3, and TYK2) is required for responses to a broad range of cytokines, therapies that inhibit JAK signaling have been an active area of interest. A simultaneous and important concern, however, has been the potential for adverse consequences when inhibiting the breadth of immune and hematopoietic molecules that depend on JAK family members for their functions. This meta-analysis by Olivera et al. examined adverse outcomes of four different JAK inhibitors in clinical trials across four immune-mediated diseases (rheumatoid arthritis, IBD, psoriasis, and ankylosing spondylitis), finding that herpes zoster infection was significantly increased (relative risk, 1.57). In contrast, patients treated with JAK inhibitors were not at a significantly increased risk for various other adverse events.
Reduced dosing of JAK inhibitors has been implemented as a means of improving safety profiles in select immune-mediated diseases. Another approach is more selective JAK inhibition, although it is unclear whether this will eliminate the risk of herpes zoster infection. In the current meta-analysis, about 87% of the studies had evaluated tofacitinib treatment, which inhibits both JAK1 and JAK3; more selective JAK inhibitors could not be evaluated in an equivalent manner. Of note, JAK1 is required for signaling by various cytokines that participate in the response to viruses, including type I IFNs and gamma c family members (such as IL-2 and IL-15); therefore, even the more selective JAK1 inhibitors do not leave this immune function fully intact. However, simply reducing the number of JAK family members inhibited simultaneously may be sufficient to reduce risk.
JAK inhibitors warrant further evaluation as additional infectious challenges arise, particularly with respect to viruses. In addition, more selective targeting of JAK inhibition of intestinal tissues may ultimately reduce systemic effects, including the risk of herpes zoster.
Clara Abraham, MD, professor of medicine, section of digestive diseases, Yale University, New Haven, Conn.
For patients with inflammatory bowel disease or other immune-mediated inflammatory diseases, Janus kinase (JAK) inhibitors appear generally safe, though they may increase the risk of herpes zoster infection, according to a large-scale systematic review and meta-analysis.
Data from more than 66,000 patients revealed no significant links between JAK inhibitors and risks of serious infections, malignancy, or major adverse cardiovascular events, reported lead author Pablo Olivera, MD, of Centro de Educación Médica e Investigación Clínica (CEMIC) in Buenos Aires and colleagues.
“To the best of our knowledge, this is the first systematic review evaluating the risk profile of JAK inhibitors in a wide spectrum of immune-mediated inflammatory diseases,” they wrote in Gastroenterology.
The investigators drew studies from the Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE from 1990 to 2019 and from conference databases from 2012 to 2018. Out of 973 studies identified, 82 were included in the final analysis, of which two-thirds were randomized clinical trials. In total, 101,925 subjects were included, of whom a majority had rheumatoid arthritis (n = 86,308), followed by psoriasis (n = 9,311), inflammatory bowel disease (n = 5,987), and ankylosing spondylitis (n = 319).
Meta-analysis of JAK inhibitor usage involved 66,159 patients. Four JAK inhibitors were included: tofacitinib, filgotinib, baricitinib, and upadacitinib. The primary outcomes were the incidence rates of adverse events and serious adverse events. The investigators also estimated incidence rates of herpes zoster infection, serious infections, mortality, malignancy, and major adverse cardiovascular events. These rates were compared with those of patients who received placebo or an active comparator in clinical trials.
Analysis showed that almost 9 out of 10 patients (87.16%) who were exposed to a JAK inhibitor received tofacitinib. The investigators described high variability in treatment duration and baseline characteristics of participants. Rates of adverse events and serious adverse events also fell across a broad spectrum, from 10% to 82% and from 0% to 29%, respectively.
“Most [adverse events] were mild, and included worsening of the underlying condition, probably showing lack of efficacy,” the investigators wrote.
Rates of mortality and most adverse events were not significantly associated with JAK inhibitor exposure. In contrast, relative risk of herpes zoster infection was 57% higher in patients who received a JAK inhibitor than in those who received a placebo or comparator (RR, 1.57; 95% confidence interval, 1.01-2.37).
“Regarding the risk of herpes zoster with JAK inhibitors, the largest evidence comes from the use of tofacitinib, but it appears to be a class effect, with a clear dose-dependent effect,” the investigators wrote.
Although risks of herpes zoster may be carried across the drug class, they may not be evenly distributed given that a subgroup analysis revealed that some JAK inhibitors may bring higher risks than others; specifically, tofacitinib and baricitinib were associated with higher relative risks of herpes zoster than were upadacitinib and filgotinib.
“Although this is merely a qualitative comparison, this difference could be related to the fact that both filgotinib and upadacitinib are selective JAK1 inhibitors, whereas tofacitinib is a JAK1/JAK3 inhibitor and baricitinib a JAK1/JAK2 inhibitor,” the investigators wrote. “Further studies are needed to determine if JAK isoform selectivity affects the risk of herpes zoster.”
The investigators emphasized this need for more research. While the present findings help illuminate the safety profile of JAK inhibitors, they are clouded by various other factors, such as disease-specific considerations, a lack of real-world data, and studies that are likely too short to accurately determine risk of malignancy, the investigators wrote.
“More studies with long follow-up and in the real world setting, in different conditions, will be needed to fully elucidate the safety profile of the different JAK inhibitors,” the investigators concluded.
The investigators disclosed relationships with AbbVie, Takeda, Pfizer, and others.
SOURCE: Olivera P et al. Gastroenterology. 2020 Jan 8. doi: 10.1053/j.gastro.2020.01.001.
For patients with inflammatory bowel disease or other immune-mediated inflammatory diseases, Janus kinase (JAK) inhibitors appear generally safe, though they may increase the risk of herpes zoster infection, according to a large-scale systematic review and meta-analysis.
Data from more than 66,000 patients revealed no significant links between JAK inhibitors and risks of serious infections, malignancy, or major adverse cardiovascular events, reported lead author Pablo Olivera, MD, of Centro de Educación Médica e Investigación Clínica (CEMIC) in Buenos Aires and colleagues.
“To the best of our knowledge, this is the first systematic review evaluating the risk profile of JAK inhibitors in a wide spectrum of immune-mediated inflammatory diseases,” they wrote in Gastroenterology.
The investigators drew studies from the Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE from 1990 to 2019 and from conference databases from 2012 to 2018. Out of 973 studies identified, 82 were included in the final analysis, of which two-thirds were randomized clinical trials. In total, 101,925 subjects were included, of whom a majority had rheumatoid arthritis (n = 86,308), followed by psoriasis (n = 9,311), inflammatory bowel disease (n = 5,987), and ankylosing spondylitis (n = 319).
Meta-analysis of JAK inhibitor usage involved 66,159 patients. Four JAK inhibitors were included: tofacitinib, filgotinib, baricitinib, and upadacitinib. The primary outcomes were the incidence rates of adverse events and serious adverse events. The investigators also estimated incidence rates of herpes zoster infection, serious infections, mortality, malignancy, and major adverse cardiovascular events. These rates were compared with those of patients who received placebo or an active comparator in clinical trials.
Analysis showed that almost 9 out of 10 patients (87.16%) who were exposed to a JAK inhibitor received tofacitinib. The investigators described high variability in treatment duration and baseline characteristics of participants. Rates of adverse events and serious adverse events also fell across a broad spectrum, from 10% to 82% and from 0% to 29%, respectively.
“Most [adverse events] were mild, and included worsening of the underlying condition, probably showing lack of efficacy,” the investigators wrote.
Rates of mortality and most adverse events were not significantly associated with JAK inhibitor exposure. In contrast, relative risk of herpes zoster infection was 57% higher in patients who received a JAK inhibitor than in those who received a placebo or comparator (RR, 1.57; 95% confidence interval, 1.01-2.37).
“Regarding the risk of herpes zoster with JAK inhibitors, the largest evidence comes from the use of tofacitinib, but it appears to be a class effect, with a clear dose-dependent effect,” the investigators wrote.
Although risks of herpes zoster may be carried across the drug class, they may not be evenly distributed given that a subgroup analysis revealed that some JAK inhibitors may bring higher risks than others; specifically, tofacitinib and baricitinib were associated with higher relative risks of herpes zoster than were upadacitinib and filgotinib.
“Although this is merely a qualitative comparison, this difference could be related to the fact that both filgotinib and upadacitinib are selective JAK1 inhibitors, whereas tofacitinib is a JAK1/JAK3 inhibitor and baricitinib a JAK1/JAK2 inhibitor,” the investigators wrote. “Further studies are needed to determine if JAK isoform selectivity affects the risk of herpes zoster.”
The investigators emphasized this need for more research. While the present findings help illuminate the safety profile of JAK inhibitors, they are clouded by various other factors, such as disease-specific considerations, a lack of real-world data, and studies that are likely too short to accurately determine risk of malignancy, the investigators wrote.
“More studies with long follow-up and in the real world setting, in different conditions, will be needed to fully elucidate the safety profile of the different JAK inhibitors,” the investigators concluded.
The investigators disclosed relationships with AbbVie, Takeda, Pfizer, and others.
SOURCE: Olivera P et al. Gastroenterology. 2020 Jan 8. doi: 10.1053/j.gastro.2020.01.001.
FROM GASTROENTEROLOGY