User login
TICOSPA: Efficacy of treat-to-target strategy suggested in axial spondyloarthritis
A treat-to-target strategy for managing patients with axial spondyloarthritis failed to meet its primary efficacy endpoint but still showed several suggestive indications of benefit compared with usual care in a multicenter, randomized study with 160 patients.
The treat-to-target management strategy tested aimed to get patients to an Ankylosing Spondylitis Disease Activity Score (ASDAS) of less than 2.1, as recommended for patients with axial spondyloarthritis (axSpA) by an international task force. Also notable about the study was its primary endpoint, at least a 30% improvement in the Assessment of Spondyloarthritis International Society Health Index (ASAS HI), a measure of health-related quality-of-life that the study organizers selected in part because of its distinction from the treatment target.
“For the first time in rheumatology, we targeted inflammation to have an impact on another domain of the disease. Despite not reaching statistical significance, we see a difference between the groups,” Anna Moltó, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
After 12 months in the study, the 80 axSpA patients assigned to the treat-to-target regimen had a 47% rate of attainment of the primary endpoint, compared with 36% of the 80 patients assigned to usual care, an 11% absolute between-group difference with a P value that came close to but failed to achieve the conventional standard of statistical significance after adjustment for potential confounders (P = .09). Six secondary outcomes showed statistically significant improvements compared with the control patients, including the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the ASAS 20, and ASAS 40. Five additional metrics showed nominal between-group improvements with the treat-to-target strategy that were not statistically significant, including various forms of the ASDAS.
One additional notable finding came from a cost-efficacy analysis run by Dr. Moltó and associates, which showed that the treat-to-target strategy was “dominant” over usual care by producing both better outcomes as well as a lower total cost, compared with control patients, even though twice as many patients on the treat-to-target strategy received a biologic disease-modifying antirheumatic drug (bDMARD) compared with patients in the usual care group. The incremental cost utility ratio for treat-to-target was 19,430 euros (about $22,000) per quality-adjusted life-year gained, putting the strategy into the range of a “cost effective” approach, and the two treatment arms also had comparable safety, said Dr. Moltó, a rheumatologist at Cochin Hospital in Paris.
The 11% increase in treat-to-target patients achieving at least a 30% improvement in their ASAS HI score “is potentially clinically relevant” because the comparator arm in the study received “very active” usual care and was not by any measure a true placebo control group, noted Maxime Dougados, MD, a rheumatologist and professor or medicine at Cochin Hospital and senior investigator for the study. In general, in treatment studies of rheumatologic diseases a 10% or greater absolute increase in the incidence of a beneficial outcome is considered clinically meaningful when compared with an actively-treated control arm, he noted.
“Using the ASAS HI score was very ambitious for the study, and it’s a very relevant outcome,” said Sofia Ramiro, MD, a rheumatologist at Leiden (the Netherlands) University Medical Center who was not associated with the study and chaired the session where Dr. Moltó gave her report. “We have had treat-to-target trials that showed benefit when disease activity was the endpoint.” But when a study “targets treatment to [reducing] disease activity and then uses disease activity as the outcome measure you expect to see an effect, but it is circular reasoning and we are left with challenges in interpreting the results. Now we have a trial that is formally [neutral] but with a different, more ambitious endpoint. All the indications are for benefit from treat-to-target for both the primary endpoint and for all the other endpoints.”
“We were in a difficult situation when choosing the outcome. We didn’t know whether a 30% improvement in the ASAS HI was really relevant, but it seems to be,” said Désirée van der Heijde, MD, a rheumatologist and professor of medicine at Leiden University Medical Center and a collaborator on Dr. Moltó’s study. “I’d choose ASAS HI again as a primary endpoint” for a treat-to-target study in patients with axSpA, she said, but added that a 30% improvement in this score as the response threshold may warrant reconsideration. Both Dr. van der Heijde and Dr. Dougados agreed that at least one additional study with a somewhat similar design is needed to better document and confirm a role for a treat-to-target strategy in axSpA patients.
The Tight Control in Spondyloarthritis (TICOSPA) study ran at 10 French centers and 4 centers each in Belgium and the Netherlands. The study enrolled adults with rheumatologist-diagnosed axSpA with an ASDAS score greater than 2.1 who had not yet received a bDMARD, had not yet maxed out on their dosage of NSAIDs, and had certain baseline immunologic and imaging findings available. The researchers randomized 160 patients to either treat-to-target or usual care management by the center they attended to prevent cross contamination of management strategies. The treat-to-target regimen involved office examinations and consultations every 4 weeks rather than every 3 months with usual care, and also required a predefined management strategy with treatment prompts based on the strategy sent to the treating clinicians via the EMR. The average age of the patients was 38 years, they had been diagnosed with axSpA for an average of just under 4 years, and their mean ASDAS score at entry was 3. During the 12 months of management, 56% of the patients in the treat-to-target arm initiated treatment with a bDMARD, compared with 28% among the controls. Use of NSAIDs was similar between the two study subgroups.
TICOSPA was sponsored by UCB. Dr. Moltó has been a consultant to and received research funding from AbbVie, Bristol-Myers Squibb, Merck, Pfizer, and UCB. Dr. Dougados has had financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Novartis, Merck, Pfizer, and UCB. Dr. van der Heijde has had financial relationships with more than 20 companies including UCB. Dr. Ramiro had been a consultant to or received research funding from AbbVie, Eli Lilly, Merck Sharp & Dohme, Novartis, and Sanofi.
SOURCE: Moltó A et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:413.
A treat-to-target strategy for managing patients with axial spondyloarthritis failed to meet its primary efficacy endpoint but still showed several suggestive indications of benefit compared with usual care in a multicenter, randomized study with 160 patients.
The treat-to-target management strategy tested aimed to get patients to an Ankylosing Spondylitis Disease Activity Score (ASDAS) of less than 2.1, as recommended for patients with axial spondyloarthritis (axSpA) by an international task force. Also notable about the study was its primary endpoint, at least a 30% improvement in the Assessment of Spondyloarthritis International Society Health Index (ASAS HI), a measure of health-related quality-of-life that the study organizers selected in part because of its distinction from the treatment target.
“For the first time in rheumatology, we targeted inflammation to have an impact on another domain of the disease. Despite not reaching statistical significance, we see a difference between the groups,” Anna Moltó, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
After 12 months in the study, the 80 axSpA patients assigned to the treat-to-target regimen had a 47% rate of attainment of the primary endpoint, compared with 36% of the 80 patients assigned to usual care, an 11% absolute between-group difference with a P value that came close to but failed to achieve the conventional standard of statistical significance after adjustment for potential confounders (P = .09). Six secondary outcomes showed statistically significant improvements compared with the control patients, including the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the ASAS 20, and ASAS 40. Five additional metrics showed nominal between-group improvements with the treat-to-target strategy that were not statistically significant, including various forms of the ASDAS.
One additional notable finding came from a cost-efficacy analysis run by Dr. Moltó and associates, which showed that the treat-to-target strategy was “dominant” over usual care by producing both better outcomes as well as a lower total cost, compared with control patients, even though twice as many patients on the treat-to-target strategy received a biologic disease-modifying antirheumatic drug (bDMARD) compared with patients in the usual care group. The incremental cost utility ratio for treat-to-target was 19,430 euros (about $22,000) per quality-adjusted life-year gained, putting the strategy into the range of a “cost effective” approach, and the two treatment arms also had comparable safety, said Dr. Moltó, a rheumatologist at Cochin Hospital in Paris.
The 11% increase in treat-to-target patients achieving at least a 30% improvement in their ASAS HI score “is potentially clinically relevant” because the comparator arm in the study received “very active” usual care and was not by any measure a true placebo control group, noted Maxime Dougados, MD, a rheumatologist and professor or medicine at Cochin Hospital and senior investigator for the study. In general, in treatment studies of rheumatologic diseases a 10% or greater absolute increase in the incidence of a beneficial outcome is considered clinically meaningful when compared with an actively-treated control arm, he noted.
“Using the ASAS HI score was very ambitious for the study, and it’s a very relevant outcome,” said Sofia Ramiro, MD, a rheumatologist at Leiden (the Netherlands) University Medical Center who was not associated with the study and chaired the session where Dr. Moltó gave her report. “We have had treat-to-target trials that showed benefit when disease activity was the endpoint.” But when a study “targets treatment to [reducing] disease activity and then uses disease activity as the outcome measure you expect to see an effect, but it is circular reasoning and we are left with challenges in interpreting the results. Now we have a trial that is formally [neutral] but with a different, more ambitious endpoint. All the indications are for benefit from treat-to-target for both the primary endpoint and for all the other endpoints.”
“We were in a difficult situation when choosing the outcome. We didn’t know whether a 30% improvement in the ASAS HI was really relevant, but it seems to be,” said Désirée van der Heijde, MD, a rheumatologist and professor of medicine at Leiden University Medical Center and a collaborator on Dr. Moltó’s study. “I’d choose ASAS HI again as a primary endpoint” for a treat-to-target study in patients with axSpA, she said, but added that a 30% improvement in this score as the response threshold may warrant reconsideration. Both Dr. van der Heijde and Dr. Dougados agreed that at least one additional study with a somewhat similar design is needed to better document and confirm a role for a treat-to-target strategy in axSpA patients.
The Tight Control in Spondyloarthritis (TICOSPA) study ran at 10 French centers and 4 centers each in Belgium and the Netherlands. The study enrolled adults with rheumatologist-diagnosed axSpA with an ASDAS score greater than 2.1 who had not yet received a bDMARD, had not yet maxed out on their dosage of NSAIDs, and had certain baseline immunologic and imaging findings available. The researchers randomized 160 patients to either treat-to-target or usual care management by the center they attended to prevent cross contamination of management strategies. The treat-to-target regimen involved office examinations and consultations every 4 weeks rather than every 3 months with usual care, and also required a predefined management strategy with treatment prompts based on the strategy sent to the treating clinicians via the EMR. The average age of the patients was 38 years, they had been diagnosed with axSpA for an average of just under 4 years, and their mean ASDAS score at entry was 3. During the 12 months of management, 56% of the patients in the treat-to-target arm initiated treatment with a bDMARD, compared with 28% among the controls. Use of NSAIDs was similar between the two study subgroups.
TICOSPA was sponsored by UCB. Dr. Moltó has been a consultant to and received research funding from AbbVie, Bristol-Myers Squibb, Merck, Pfizer, and UCB. Dr. Dougados has had financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Novartis, Merck, Pfizer, and UCB. Dr. van der Heijde has had financial relationships with more than 20 companies including UCB. Dr. Ramiro had been a consultant to or received research funding from AbbVie, Eli Lilly, Merck Sharp & Dohme, Novartis, and Sanofi.
SOURCE: Moltó A et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:413.
A treat-to-target strategy for managing patients with axial spondyloarthritis failed to meet its primary efficacy endpoint but still showed several suggestive indications of benefit compared with usual care in a multicenter, randomized study with 160 patients.
The treat-to-target management strategy tested aimed to get patients to an Ankylosing Spondylitis Disease Activity Score (ASDAS) of less than 2.1, as recommended for patients with axial spondyloarthritis (axSpA) by an international task force. Also notable about the study was its primary endpoint, at least a 30% improvement in the Assessment of Spondyloarthritis International Society Health Index (ASAS HI), a measure of health-related quality-of-life that the study organizers selected in part because of its distinction from the treatment target.
“For the first time in rheumatology, we targeted inflammation to have an impact on another domain of the disease. Despite not reaching statistical significance, we see a difference between the groups,” Anna Moltó, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
After 12 months in the study, the 80 axSpA patients assigned to the treat-to-target regimen had a 47% rate of attainment of the primary endpoint, compared with 36% of the 80 patients assigned to usual care, an 11% absolute between-group difference with a P value that came close to but failed to achieve the conventional standard of statistical significance after adjustment for potential confounders (P = .09). Six secondary outcomes showed statistically significant improvements compared with the control patients, including the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the ASAS 20, and ASAS 40. Five additional metrics showed nominal between-group improvements with the treat-to-target strategy that were not statistically significant, including various forms of the ASDAS.
One additional notable finding came from a cost-efficacy analysis run by Dr. Moltó and associates, which showed that the treat-to-target strategy was “dominant” over usual care by producing both better outcomes as well as a lower total cost, compared with control patients, even though twice as many patients on the treat-to-target strategy received a biologic disease-modifying antirheumatic drug (bDMARD) compared with patients in the usual care group. The incremental cost utility ratio for treat-to-target was 19,430 euros (about $22,000) per quality-adjusted life-year gained, putting the strategy into the range of a “cost effective” approach, and the two treatment arms also had comparable safety, said Dr. Moltó, a rheumatologist at Cochin Hospital in Paris.
The 11% increase in treat-to-target patients achieving at least a 30% improvement in their ASAS HI score “is potentially clinically relevant” because the comparator arm in the study received “very active” usual care and was not by any measure a true placebo control group, noted Maxime Dougados, MD, a rheumatologist and professor or medicine at Cochin Hospital and senior investigator for the study. In general, in treatment studies of rheumatologic diseases a 10% or greater absolute increase in the incidence of a beneficial outcome is considered clinically meaningful when compared with an actively-treated control arm, he noted.
“Using the ASAS HI score was very ambitious for the study, and it’s a very relevant outcome,” said Sofia Ramiro, MD, a rheumatologist at Leiden (the Netherlands) University Medical Center who was not associated with the study and chaired the session where Dr. Moltó gave her report. “We have had treat-to-target trials that showed benefit when disease activity was the endpoint.” But when a study “targets treatment to [reducing] disease activity and then uses disease activity as the outcome measure you expect to see an effect, but it is circular reasoning and we are left with challenges in interpreting the results. Now we have a trial that is formally [neutral] but with a different, more ambitious endpoint. All the indications are for benefit from treat-to-target for both the primary endpoint and for all the other endpoints.”
“We were in a difficult situation when choosing the outcome. We didn’t know whether a 30% improvement in the ASAS HI was really relevant, but it seems to be,” said Désirée van der Heijde, MD, a rheumatologist and professor of medicine at Leiden University Medical Center and a collaborator on Dr. Moltó’s study. “I’d choose ASAS HI again as a primary endpoint” for a treat-to-target study in patients with axSpA, she said, but added that a 30% improvement in this score as the response threshold may warrant reconsideration. Both Dr. van der Heijde and Dr. Dougados agreed that at least one additional study with a somewhat similar design is needed to better document and confirm a role for a treat-to-target strategy in axSpA patients.
The Tight Control in Spondyloarthritis (TICOSPA) study ran at 10 French centers and 4 centers each in Belgium and the Netherlands. The study enrolled adults with rheumatologist-diagnosed axSpA with an ASDAS score greater than 2.1 who had not yet received a bDMARD, had not yet maxed out on their dosage of NSAIDs, and had certain baseline immunologic and imaging findings available. The researchers randomized 160 patients to either treat-to-target or usual care management by the center they attended to prevent cross contamination of management strategies. The treat-to-target regimen involved office examinations and consultations every 4 weeks rather than every 3 months with usual care, and also required a predefined management strategy with treatment prompts based on the strategy sent to the treating clinicians via the EMR. The average age of the patients was 38 years, they had been diagnosed with axSpA for an average of just under 4 years, and their mean ASDAS score at entry was 3. During the 12 months of management, 56% of the patients in the treat-to-target arm initiated treatment with a bDMARD, compared with 28% among the controls. Use of NSAIDs was similar between the two study subgroups.
TICOSPA was sponsored by UCB. Dr. Moltó has been a consultant to and received research funding from AbbVie, Bristol-Myers Squibb, Merck, Pfizer, and UCB. Dr. Dougados has had financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Novartis, Merck, Pfizer, and UCB. Dr. van der Heijde has had financial relationships with more than 20 companies including UCB. Dr. Ramiro had been a consultant to or received research funding from AbbVie, Eli Lilly, Merck Sharp & Dohme, Novartis, and Sanofi.
SOURCE: Moltó A et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:413.
REPORTING FROM THE EULAR 2020 E-CONGRESS
Opioid use up after TNF inhibitor for inflammatory arthritis
Opioid use does not decline after patients with inflammatory arthritis start TNF inhibitor therapy; in fact, average use appears to increase, results from a new study show.
“Starting a TNF inhibitor, you would think the pain would go down, and we were hoping the dose of opioids would go down with it,” said investigator Olafur Palsson, MD, from the University of Iceland in Reykjavik and Lund University in Sweden.
“But this research shows that the insertion of a TNF inhibitor has only a minor effect on that,” he told Medscape Medical News.
The findings are an “important reminder” to rheumatologists that they should broaden their consideration of other pain treatments and techniques for patients with inflammatory arthritis, Dr. Palsson said. “They should focus on trying other tactics to get patients’ pain and stiffness under control; there may be some underlying factors.”
The investigators compared opioid prescription rates in 940 patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and undifferentiated arthritis with a control group of 4,700 matched subjects. Dr. Palsson presented the findings at the virtual European League Against Rheumatism (EULAR) 2020 Congress.
The team assessed nationwide databases that capture all patients taking biologics for rheumatic diseases and more than 90% of all drug prescriptions. They found that patients with inflammatory arthritis in Iceland were more likely to have received at least one opioid prescription than control subjects (75% vs. 43%).
During the study period, average yearly opioid dose rose much more in the patient group than in the control group. And 2 years after the initiation of TNF inhibitors, the number of patients taking opioids was unchanged from baseline, at about 40%.
Overall, the patient group was prescribed nearly six times more opioids than the control group. The investigators used a bootstrapping analysis to obtain a reliable confidence interval.
“In a way, the data are extremely skewed,” Dr. Palsson explained. “Most patients were taking very low doses of opioids and a few were taking extremely high doses. It’s hard to do a statistical analysis.”
“With bootstrapping, you don’t detect small fluctuations in data,” he said, acknowledging this study limitation. Also, “prescription data don’t necessarily reflect consumption” of a drug. People prescribed high doses may not necessarily be consuming high doses.”
Additionally, the risk for addiction is low when opioids are used as intended, said John Isaacs, MBBS, PhD, from Newcastle University in Newcastle Upon Tyne, United Kingdom, who is chair of the EULAR scientific program committee.
To alleviate chronic pain, opioids “should, in any case, only be part of a comprehensive therapy program in which doctors, psychologists, and physiotherapists work together,” Dr. Isaacs said in a EULAR news release.
Dr. Palsson has disclosed no relevant financial relationships. Dr. Isaacs is a consultant or has received honoraria or grants from Pfizer, AbbVie, Amgen, Merck, Roche, and UCB.
This article first appeared on Medscape.com.
Opioid use does not decline after patients with inflammatory arthritis start TNF inhibitor therapy; in fact, average use appears to increase, results from a new study show.
“Starting a TNF inhibitor, you would think the pain would go down, and we were hoping the dose of opioids would go down with it,” said investigator Olafur Palsson, MD, from the University of Iceland in Reykjavik and Lund University in Sweden.
“But this research shows that the insertion of a TNF inhibitor has only a minor effect on that,” he told Medscape Medical News.
The findings are an “important reminder” to rheumatologists that they should broaden their consideration of other pain treatments and techniques for patients with inflammatory arthritis, Dr. Palsson said. “They should focus on trying other tactics to get patients’ pain and stiffness under control; there may be some underlying factors.”
The investigators compared opioid prescription rates in 940 patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and undifferentiated arthritis with a control group of 4,700 matched subjects. Dr. Palsson presented the findings at the virtual European League Against Rheumatism (EULAR) 2020 Congress.
The team assessed nationwide databases that capture all patients taking biologics for rheumatic diseases and more than 90% of all drug prescriptions. They found that patients with inflammatory arthritis in Iceland were more likely to have received at least one opioid prescription than control subjects (75% vs. 43%).
During the study period, average yearly opioid dose rose much more in the patient group than in the control group. And 2 years after the initiation of TNF inhibitors, the number of patients taking opioids was unchanged from baseline, at about 40%.
Overall, the patient group was prescribed nearly six times more opioids than the control group. The investigators used a bootstrapping analysis to obtain a reliable confidence interval.
“In a way, the data are extremely skewed,” Dr. Palsson explained. “Most patients were taking very low doses of opioids and a few were taking extremely high doses. It’s hard to do a statistical analysis.”
“With bootstrapping, you don’t detect small fluctuations in data,” he said, acknowledging this study limitation. Also, “prescription data don’t necessarily reflect consumption” of a drug. People prescribed high doses may not necessarily be consuming high doses.”
Additionally, the risk for addiction is low when opioids are used as intended, said John Isaacs, MBBS, PhD, from Newcastle University in Newcastle Upon Tyne, United Kingdom, who is chair of the EULAR scientific program committee.
To alleviate chronic pain, opioids “should, in any case, only be part of a comprehensive therapy program in which doctors, psychologists, and physiotherapists work together,” Dr. Isaacs said in a EULAR news release.
Dr. Palsson has disclosed no relevant financial relationships. Dr. Isaacs is a consultant or has received honoraria or grants from Pfizer, AbbVie, Amgen, Merck, Roche, and UCB.
This article first appeared on Medscape.com.
Opioid use does not decline after patients with inflammatory arthritis start TNF inhibitor therapy; in fact, average use appears to increase, results from a new study show.
“Starting a TNF inhibitor, you would think the pain would go down, and we were hoping the dose of opioids would go down with it,” said investigator Olafur Palsson, MD, from the University of Iceland in Reykjavik and Lund University in Sweden.
“But this research shows that the insertion of a TNF inhibitor has only a minor effect on that,” he told Medscape Medical News.
The findings are an “important reminder” to rheumatologists that they should broaden their consideration of other pain treatments and techniques for patients with inflammatory arthritis, Dr. Palsson said. “They should focus on trying other tactics to get patients’ pain and stiffness under control; there may be some underlying factors.”
The investigators compared opioid prescription rates in 940 patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and undifferentiated arthritis with a control group of 4,700 matched subjects. Dr. Palsson presented the findings at the virtual European League Against Rheumatism (EULAR) 2020 Congress.
The team assessed nationwide databases that capture all patients taking biologics for rheumatic diseases and more than 90% of all drug prescriptions. They found that patients with inflammatory arthritis in Iceland were more likely to have received at least one opioid prescription than control subjects (75% vs. 43%).
During the study period, average yearly opioid dose rose much more in the patient group than in the control group. And 2 years after the initiation of TNF inhibitors, the number of patients taking opioids was unchanged from baseline, at about 40%.
Overall, the patient group was prescribed nearly six times more opioids than the control group. The investigators used a bootstrapping analysis to obtain a reliable confidence interval.
“In a way, the data are extremely skewed,” Dr. Palsson explained. “Most patients were taking very low doses of opioids and a few were taking extremely high doses. It’s hard to do a statistical analysis.”
“With bootstrapping, you don’t detect small fluctuations in data,” he said, acknowledging this study limitation. Also, “prescription data don’t necessarily reflect consumption” of a drug. People prescribed high doses may not necessarily be consuming high doses.”
Additionally, the risk for addiction is low when opioids are used as intended, said John Isaacs, MBBS, PhD, from Newcastle University in Newcastle Upon Tyne, United Kingdom, who is chair of the EULAR scientific program committee.
To alleviate chronic pain, opioids “should, in any case, only be part of a comprehensive therapy program in which doctors, psychologists, and physiotherapists work together,” Dr. Isaacs said in a EULAR news release.
Dr. Palsson has disclosed no relevant financial relationships. Dr. Isaacs is a consultant or has received honoraria or grants from Pfizer, AbbVie, Amgen, Merck, Roche, and UCB.
This article first appeared on Medscape.com.
Age leads COVID-19 hospitalization risk factors in RMDs
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
FROM THE EULAR 2020 E-CONGRESS
Some biologics may be better than others for averting anterior uveitis
Among patients with ankylosing spondylitis or undifferentiated spondyloarthritis, risk for anterior uveitis may hinge on the choice of biologic disease-modifying antirheumatic drug (bDMARD), a large Swedish cohort study suggests.
Study results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“Randomized, controlled trials indicate that compared to tumor necrosis factor (TNF) inhibitors, secukinumab has similar efficacy regarding axial inflammation in spondyloarthritis and better efficacy regarding cutaneous psoriasis, but is inferior in inflammatory bowel disease,” noted lead investigator Ulf Lindström, MD, PhD, of the department of rheumatology and inflammation research in the Institute of Medicine at the University of Gothenburg (Sweden). “However, the efficacy of secukinumab, compared to TNF inhibitors, in anterior uveitis has not been extensively studied.”
The investigators used national registry data to study 3,568 patients with ankylosing spondylitis or undifferentiated spondyloarthritis who started bDMARDs in 2005-2018. They considered four agents: the anti–interleukin-17A antibody secukinumab (Cosentyx) and the TNF inhibitors etanercept (Enbrel), adalimumab (Humira), and infliximab (Remicade).
Analyses based on 4,523 treatment episodes showed that after excluding the 23% of patients who had previously experienced anterior uveitis, merely 0.9% of patients experienced new-onset anterior uveitis while on their bDMARD, Dr. Lindström reported.
There was confounding by indication, whereby patients with previous anterior uveitis were channeled toward adalimumab and infliximab, and away from secukinumab and etanercept. In addition, there was confounding by line of treatment, with secukinumab usually used in the third line.
After excluding patients who had experienced anterior uveitis in the past year to partly address confounding, the adjusted risk for first on-treatment anterior uveitis was about twice as high with secukinumab and with etanercept as compared with adalimumab. After additionally excluding all biologic treatment episodes beyond the third line, elevation of risk remained significant only for etanercept.
“There is probably a higher occurrence of anterior uveitis on treatment with secukinumab, compared to adalimumab, but there may still be residual confounding and bias that we need to consider,” Dr. Lindström concluded. “As seen previously, there is a higher occurrence of anterior uveitis on etanercept compared to adalimumab or infliximab.”
Findings in context
“These results are not surprising as we have known that secukinumab and etanercept are not good for controlling recurrent and chronic uveitis,” Nigil Haroon MD, PhD, DM, commented in an interview. However, “a single episode of uveitis or infrequent episodes are not usually considered a contraindication to starting these drugs.”
Study caveats included lack of adjustment for uveitis severity and potentially missed uveitis episodes in patients who treated it themselves with steroid eyedrops, he said. “Standard practice is to keep drops with them to start at the earliest possible time point.”
“It would be useful to know the number of patients who stopped medications as a result of uveitis,” added Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto. “Time-to-event analysis may also be interesting.”
“The study raises an important point regarding channeling bias, and this is important to consider when interpreting clinical trial data as well. Investigators are unlikely to include patients with history of uveitis (or strong family history of inflammatory bowel disease or personal history of gut symptoms) in studies with IL-17 inhibitors and etanercept. Hence, the results have to be interpreted with caution.”
Study details
Dr. Lindström and coinvestigators assessed incidences of any anterior uveitis (ascertained from outpatient ophthalmology visits having this diagnostic code) and of anterior uveitis flares (the subset occurring after a gap of at least 90 days without the diagnosis).
When they excluded patients who had experienced anterior uveitis in the year before starting therapy, secukinumab and etanercept carried the highest incidences of anterior uveitis (6.8 and 7.5 per 100 patient-years, respectively) and anterior uveitis flares (2.8 per 100 patient-years for each), he reported.
With adalimumab as the comparator, adjusted risk for first on-treatment anterior uveitis in this population was significantly higher with secukinumab (hazard ratio, 2.23) and etanercept (hazard ratio, 1.80).
When the investigators additionally excluded episodes of therapy beyond the third line, only etanercept carried notably higher incidences of anterior uveitis (7.0 per 100 patient-years) and anterior uveitis flares (2.6 per 100 patient-years). “This could imply that some of the higher incidence rate seen for secukinumab could be due to the fact that these patients are harder to treat and have received more biologics before,” Dr. Lindström proposed.
With adalimumab again as the comparator, the adjusted risk for first on-treatment anterior uveitis in this population was significantly higher only with etanercept (hazard ratio, 1.85).
A final analysis included all patients who started adalimumab in 2004-2018 and then switched to one of the other agents, dramatically reducing confounding by indication. In this population, the incidence rate ratio of anterior uveitis flares was 3.05 for secukinumab, 1.79 for etanercept, and 0.53 for infliximab, compared with adalimumab.
Dr. Lindström disclosed that he had no relevant conflicts of interest. The study did not receive any specific funding. Dr. Haroon disclosed consulting for Amgen, Abbvie, Eli Lilly, Janssen, Novartis, and UCB.
SOURCE: Lindström U et al. Ann Rheum Dis. 2020;79[suppl 1]:9, Abstract OP0014.
Among patients with ankylosing spondylitis or undifferentiated spondyloarthritis, risk for anterior uveitis may hinge on the choice of biologic disease-modifying antirheumatic drug (bDMARD), a large Swedish cohort study suggests.
Study results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“Randomized, controlled trials indicate that compared to tumor necrosis factor (TNF) inhibitors, secukinumab has similar efficacy regarding axial inflammation in spondyloarthritis and better efficacy regarding cutaneous psoriasis, but is inferior in inflammatory bowel disease,” noted lead investigator Ulf Lindström, MD, PhD, of the department of rheumatology and inflammation research in the Institute of Medicine at the University of Gothenburg (Sweden). “However, the efficacy of secukinumab, compared to TNF inhibitors, in anterior uveitis has not been extensively studied.”
The investigators used national registry data to study 3,568 patients with ankylosing spondylitis or undifferentiated spondyloarthritis who started bDMARDs in 2005-2018. They considered four agents: the anti–interleukin-17A antibody secukinumab (Cosentyx) and the TNF inhibitors etanercept (Enbrel), adalimumab (Humira), and infliximab (Remicade).
Analyses based on 4,523 treatment episodes showed that after excluding the 23% of patients who had previously experienced anterior uveitis, merely 0.9% of patients experienced new-onset anterior uveitis while on their bDMARD, Dr. Lindström reported.
There was confounding by indication, whereby patients with previous anterior uveitis were channeled toward adalimumab and infliximab, and away from secukinumab and etanercept. In addition, there was confounding by line of treatment, with secukinumab usually used in the third line.
After excluding patients who had experienced anterior uveitis in the past year to partly address confounding, the adjusted risk for first on-treatment anterior uveitis was about twice as high with secukinumab and with etanercept as compared with adalimumab. After additionally excluding all biologic treatment episodes beyond the third line, elevation of risk remained significant only for etanercept.
“There is probably a higher occurrence of anterior uveitis on treatment with secukinumab, compared to adalimumab, but there may still be residual confounding and bias that we need to consider,” Dr. Lindström concluded. “As seen previously, there is a higher occurrence of anterior uveitis on etanercept compared to adalimumab or infliximab.”
Findings in context
“These results are not surprising as we have known that secukinumab and etanercept are not good for controlling recurrent and chronic uveitis,” Nigil Haroon MD, PhD, DM, commented in an interview. However, “a single episode of uveitis or infrequent episodes are not usually considered a contraindication to starting these drugs.”
Study caveats included lack of adjustment for uveitis severity and potentially missed uveitis episodes in patients who treated it themselves with steroid eyedrops, he said. “Standard practice is to keep drops with them to start at the earliest possible time point.”
“It would be useful to know the number of patients who stopped medications as a result of uveitis,” added Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto. “Time-to-event analysis may also be interesting.”
“The study raises an important point regarding channeling bias, and this is important to consider when interpreting clinical trial data as well. Investigators are unlikely to include patients with history of uveitis (or strong family history of inflammatory bowel disease or personal history of gut symptoms) in studies with IL-17 inhibitors and etanercept. Hence, the results have to be interpreted with caution.”
Study details
Dr. Lindström and coinvestigators assessed incidences of any anterior uveitis (ascertained from outpatient ophthalmology visits having this diagnostic code) and of anterior uveitis flares (the subset occurring after a gap of at least 90 days without the diagnosis).
When they excluded patients who had experienced anterior uveitis in the year before starting therapy, secukinumab and etanercept carried the highest incidences of anterior uveitis (6.8 and 7.5 per 100 patient-years, respectively) and anterior uveitis flares (2.8 per 100 patient-years for each), he reported.
With adalimumab as the comparator, adjusted risk for first on-treatment anterior uveitis in this population was significantly higher with secukinumab (hazard ratio, 2.23) and etanercept (hazard ratio, 1.80).
When the investigators additionally excluded episodes of therapy beyond the third line, only etanercept carried notably higher incidences of anterior uveitis (7.0 per 100 patient-years) and anterior uveitis flares (2.6 per 100 patient-years). “This could imply that some of the higher incidence rate seen for secukinumab could be due to the fact that these patients are harder to treat and have received more biologics before,” Dr. Lindström proposed.
With adalimumab again as the comparator, the adjusted risk for first on-treatment anterior uveitis in this population was significantly higher only with etanercept (hazard ratio, 1.85).
A final analysis included all patients who started adalimumab in 2004-2018 and then switched to one of the other agents, dramatically reducing confounding by indication. In this population, the incidence rate ratio of anterior uveitis flares was 3.05 for secukinumab, 1.79 for etanercept, and 0.53 for infliximab, compared with adalimumab.
Dr. Lindström disclosed that he had no relevant conflicts of interest. The study did not receive any specific funding. Dr. Haroon disclosed consulting for Amgen, Abbvie, Eli Lilly, Janssen, Novartis, and UCB.
SOURCE: Lindström U et al. Ann Rheum Dis. 2020;79[suppl 1]:9, Abstract OP0014.
Among patients with ankylosing spondylitis or undifferentiated spondyloarthritis, risk for anterior uveitis may hinge on the choice of biologic disease-modifying antirheumatic drug (bDMARD), a large Swedish cohort study suggests.
Study results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“Randomized, controlled trials indicate that compared to tumor necrosis factor (TNF) inhibitors, secukinumab has similar efficacy regarding axial inflammation in spondyloarthritis and better efficacy regarding cutaneous psoriasis, but is inferior in inflammatory bowel disease,” noted lead investigator Ulf Lindström, MD, PhD, of the department of rheumatology and inflammation research in the Institute of Medicine at the University of Gothenburg (Sweden). “However, the efficacy of secukinumab, compared to TNF inhibitors, in anterior uveitis has not been extensively studied.”
The investigators used national registry data to study 3,568 patients with ankylosing spondylitis or undifferentiated spondyloarthritis who started bDMARDs in 2005-2018. They considered four agents: the anti–interleukin-17A antibody secukinumab (Cosentyx) and the TNF inhibitors etanercept (Enbrel), adalimumab (Humira), and infliximab (Remicade).
Analyses based on 4,523 treatment episodes showed that after excluding the 23% of patients who had previously experienced anterior uveitis, merely 0.9% of patients experienced new-onset anterior uveitis while on their bDMARD, Dr. Lindström reported.
There was confounding by indication, whereby patients with previous anterior uveitis were channeled toward adalimumab and infliximab, and away from secukinumab and etanercept. In addition, there was confounding by line of treatment, with secukinumab usually used in the third line.
After excluding patients who had experienced anterior uveitis in the past year to partly address confounding, the adjusted risk for first on-treatment anterior uveitis was about twice as high with secukinumab and with etanercept as compared with adalimumab. After additionally excluding all biologic treatment episodes beyond the third line, elevation of risk remained significant only for etanercept.
“There is probably a higher occurrence of anterior uveitis on treatment with secukinumab, compared to adalimumab, but there may still be residual confounding and bias that we need to consider,” Dr. Lindström concluded. “As seen previously, there is a higher occurrence of anterior uveitis on etanercept compared to adalimumab or infliximab.”
Findings in context
“These results are not surprising as we have known that secukinumab and etanercept are not good for controlling recurrent and chronic uveitis,” Nigil Haroon MD, PhD, DM, commented in an interview. However, “a single episode of uveitis or infrequent episodes are not usually considered a contraindication to starting these drugs.”
Study caveats included lack of adjustment for uveitis severity and potentially missed uveitis episodes in patients who treated it themselves with steroid eyedrops, he said. “Standard practice is to keep drops with them to start at the earliest possible time point.”
“It would be useful to know the number of patients who stopped medications as a result of uveitis,” added Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto. “Time-to-event analysis may also be interesting.”
“The study raises an important point regarding channeling bias, and this is important to consider when interpreting clinical trial data as well. Investigators are unlikely to include patients with history of uveitis (or strong family history of inflammatory bowel disease or personal history of gut symptoms) in studies with IL-17 inhibitors and etanercept. Hence, the results have to be interpreted with caution.”
Study details
Dr. Lindström and coinvestigators assessed incidences of any anterior uveitis (ascertained from outpatient ophthalmology visits having this diagnostic code) and of anterior uveitis flares (the subset occurring after a gap of at least 90 days without the diagnosis).
When they excluded patients who had experienced anterior uveitis in the year before starting therapy, secukinumab and etanercept carried the highest incidences of anterior uveitis (6.8 and 7.5 per 100 patient-years, respectively) and anterior uveitis flares (2.8 per 100 patient-years for each), he reported.
With adalimumab as the comparator, adjusted risk for first on-treatment anterior uveitis in this population was significantly higher with secukinumab (hazard ratio, 2.23) and etanercept (hazard ratio, 1.80).
When the investigators additionally excluded episodes of therapy beyond the third line, only etanercept carried notably higher incidences of anterior uveitis (7.0 per 100 patient-years) and anterior uveitis flares (2.6 per 100 patient-years). “This could imply that some of the higher incidence rate seen for secukinumab could be due to the fact that these patients are harder to treat and have received more biologics before,” Dr. Lindström proposed.
With adalimumab again as the comparator, the adjusted risk for first on-treatment anterior uveitis in this population was significantly higher only with etanercept (hazard ratio, 1.85).
A final analysis included all patients who started adalimumab in 2004-2018 and then switched to one of the other agents, dramatically reducing confounding by indication. In this population, the incidence rate ratio of anterior uveitis flares was 3.05 for secukinumab, 1.79 for etanercept, and 0.53 for infliximab, compared with adalimumab.
Dr. Lindström disclosed that he had no relevant conflicts of interest. The study did not receive any specific funding. Dr. Haroon disclosed consulting for Amgen, Abbvie, Eli Lilly, Janssen, Novartis, and UCB.
SOURCE: Lindström U et al. Ann Rheum Dis. 2020;79[suppl 1]:9, Abstract OP0014.
FROM EULAR 2020 E-CONGRESS
Most rheumatology drugs don’t increase COVID-19 hospitalization risk
The vast majority of patients with rheumatic and musculoskeletal diseases who contract COVID-19 recover from the virus, regardless of which medication they receive for their rheumatic condition, new international research suggests.
“These results provide, for the first time, information about the outcome of COVID-19 in patients with rheumatic and musculoskeletal diseases,” said study investigator Pedro Machado, MD, PhD, from University College London. “They should provide some reassurance to patients and healthcare providers.”
Machado and his colleagues looked at 600 COVID-19 patients from 40 countries, and found that those taking TNF inhibitors for their rheumatic disease were less likely to be hospitalized for COVID-19. However, treatment with more than 10 mg of prednisone daily — considered a moderate to high dose — was associated with a higher probability of hospitalization.
In addition, hospitalization was not associated with biologics; JAK inhibitors; conventional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; antimalarials, such as hydroxychloroquine; or nonsteroidal anti-inflammatory drugs (NSAIDs) — either alone or in combination with other biologics, such as TNF-alpha inhibitors.
The findings were presented at the virtual European League Against Rheumatism (EULAR) 2020 Congress and were published online in Annals of the Rheumatic Diseases.
“Initially, there was a huge concern that these drugs could affect the outcome of patients getting COVID-19, but what this is showing is that probably these drugs do not increase their risk of severe outcome,” Machado, who is chair of the EULAR standing committee on epidemiology and health services research, told Medscape Medical News.
As of June 1, 1061 patients from 28 participating countries had been entered into the EULAR COVID-19 database, which was launched as part of the international Global Rheumatology Alliance registry. Patient data are categorized by factors such as top rheumatology diagnosis, comorbidities, top-five COVID-19 symptoms, and DMARD therapy at the time of virus infection. Anonymized data will be shared with an international register based in the United States.
Machado’s team combined data from the EULAR and Global Rheumatology Alliance COVID-19 registries from March 24 to April 20. They looked at patient factors — such as age, sex, smoking status, rheumatic diagnosis, comorbidities, and rheumatic therapies — to examine the association of rheumatic therapies with hospitalization rates and COVID-19 disease course.
Of the 277 patients (46%) in the study cohort who required hospitalization, 55 (9%) died. But this finding shouldn’t be viewed as the true rate of hospitalization or death in patients with rheumatic disease and COVID-19, said Gerd Burmester, MD, from Charité–University Medicine Berlin.
“There’s tremendous bias in terms of more serious cases of COVID-19 being reported to the registries,” he explained, “because the mild cases won’t even show up at their rheumatologist’s office.”
“This can skew the idea that COVID-19 is much more dangerous to rheumatic patients than to the regular population,” Burmester told Medscape Medical News. “It scares the patients, obviously, but we believe this is not justified.”
It’s still unclear whether rituximab use raises the risk for severe COVID-19, he said. “It appears to be the only biologic for which the jury is still out,” he said.
“Anti-TNFs and anti-IL-6 drugs may even be beneficial, although we don’t have robust data,” he added.
The study can only highlight associations between rheumatic drugs and COVID-19 outcomes. “We cannot say there is a causal relationship between the findings,” Machado said.
Longer-term data, when available, should illuminate “more granular” aspects of COVID-19 outcomes in rheumatic patients, including their risks of requiring ventilation or developing a cytokine storm, he noted.
Burmester and Machado agree that research needs to continue as the pandemic rages on. But so far, “there are no data suggesting that, if you’re on a targeted, dedicated immunomodulator, your risk is higher to have a worse course of COVID-19 than the general population,” Burmester said.
“We simply didn’t know that when the pandemic started, and some patients even discontinued their drugs out of this fear,” he added. “It’s more reassuring than we originally thought.”
This article first appeared on Medscape.com.
The vast majority of patients with rheumatic and musculoskeletal diseases who contract COVID-19 recover from the virus, regardless of which medication they receive for their rheumatic condition, new international research suggests.
“These results provide, for the first time, information about the outcome of COVID-19 in patients with rheumatic and musculoskeletal diseases,” said study investigator Pedro Machado, MD, PhD, from University College London. “They should provide some reassurance to patients and healthcare providers.”
Machado and his colleagues looked at 600 COVID-19 patients from 40 countries, and found that those taking TNF inhibitors for their rheumatic disease were less likely to be hospitalized for COVID-19. However, treatment with more than 10 mg of prednisone daily — considered a moderate to high dose — was associated with a higher probability of hospitalization.
In addition, hospitalization was not associated with biologics; JAK inhibitors; conventional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; antimalarials, such as hydroxychloroquine; or nonsteroidal anti-inflammatory drugs (NSAIDs) — either alone or in combination with other biologics, such as TNF-alpha inhibitors.
The findings were presented at the virtual European League Against Rheumatism (EULAR) 2020 Congress and were published online in Annals of the Rheumatic Diseases.
“Initially, there was a huge concern that these drugs could affect the outcome of patients getting COVID-19, but what this is showing is that probably these drugs do not increase their risk of severe outcome,” Machado, who is chair of the EULAR standing committee on epidemiology and health services research, told Medscape Medical News.
As of June 1, 1061 patients from 28 participating countries had been entered into the EULAR COVID-19 database, which was launched as part of the international Global Rheumatology Alliance registry. Patient data are categorized by factors such as top rheumatology diagnosis, comorbidities, top-five COVID-19 symptoms, and DMARD therapy at the time of virus infection. Anonymized data will be shared with an international register based in the United States.
Machado’s team combined data from the EULAR and Global Rheumatology Alliance COVID-19 registries from March 24 to April 20. They looked at patient factors — such as age, sex, smoking status, rheumatic diagnosis, comorbidities, and rheumatic therapies — to examine the association of rheumatic therapies with hospitalization rates and COVID-19 disease course.
Of the 277 patients (46%) in the study cohort who required hospitalization, 55 (9%) died. But this finding shouldn’t be viewed as the true rate of hospitalization or death in patients with rheumatic disease and COVID-19, said Gerd Burmester, MD, from Charité–University Medicine Berlin.
“There’s tremendous bias in terms of more serious cases of COVID-19 being reported to the registries,” he explained, “because the mild cases won’t even show up at their rheumatologist’s office.”
“This can skew the idea that COVID-19 is much more dangerous to rheumatic patients than to the regular population,” Burmester told Medscape Medical News. “It scares the patients, obviously, but we believe this is not justified.”
It’s still unclear whether rituximab use raises the risk for severe COVID-19, he said. “It appears to be the only biologic for which the jury is still out,” he said.
“Anti-TNFs and anti-IL-6 drugs may even be beneficial, although we don’t have robust data,” he added.
The study can only highlight associations between rheumatic drugs and COVID-19 outcomes. “We cannot say there is a causal relationship between the findings,” Machado said.
Longer-term data, when available, should illuminate “more granular” aspects of COVID-19 outcomes in rheumatic patients, including their risks of requiring ventilation or developing a cytokine storm, he noted.
Burmester and Machado agree that research needs to continue as the pandemic rages on. But so far, “there are no data suggesting that, if you’re on a targeted, dedicated immunomodulator, your risk is higher to have a worse course of COVID-19 than the general population,” Burmester said.
“We simply didn’t know that when the pandemic started, and some patients even discontinued their drugs out of this fear,” he added. “It’s more reassuring than we originally thought.”
This article first appeared on Medscape.com.
The vast majority of patients with rheumatic and musculoskeletal diseases who contract COVID-19 recover from the virus, regardless of which medication they receive for their rheumatic condition, new international research suggests.
“These results provide, for the first time, information about the outcome of COVID-19 in patients with rheumatic and musculoskeletal diseases,” said study investigator Pedro Machado, MD, PhD, from University College London. “They should provide some reassurance to patients and healthcare providers.”
Machado and his colleagues looked at 600 COVID-19 patients from 40 countries, and found that those taking TNF inhibitors for their rheumatic disease were less likely to be hospitalized for COVID-19. However, treatment with more than 10 mg of prednisone daily — considered a moderate to high dose — was associated with a higher probability of hospitalization.
In addition, hospitalization was not associated with biologics; JAK inhibitors; conventional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; antimalarials, such as hydroxychloroquine; or nonsteroidal anti-inflammatory drugs (NSAIDs) — either alone or in combination with other biologics, such as TNF-alpha inhibitors.
The findings were presented at the virtual European League Against Rheumatism (EULAR) 2020 Congress and were published online in Annals of the Rheumatic Diseases.
“Initially, there was a huge concern that these drugs could affect the outcome of patients getting COVID-19, but what this is showing is that probably these drugs do not increase their risk of severe outcome,” Machado, who is chair of the EULAR standing committee on epidemiology and health services research, told Medscape Medical News.
As of June 1, 1061 patients from 28 participating countries had been entered into the EULAR COVID-19 database, which was launched as part of the international Global Rheumatology Alliance registry. Patient data are categorized by factors such as top rheumatology diagnosis, comorbidities, top-five COVID-19 symptoms, and DMARD therapy at the time of virus infection. Anonymized data will be shared with an international register based in the United States.
Machado’s team combined data from the EULAR and Global Rheumatology Alliance COVID-19 registries from March 24 to April 20. They looked at patient factors — such as age, sex, smoking status, rheumatic diagnosis, comorbidities, and rheumatic therapies — to examine the association of rheumatic therapies with hospitalization rates and COVID-19 disease course.
Of the 277 patients (46%) in the study cohort who required hospitalization, 55 (9%) died. But this finding shouldn’t be viewed as the true rate of hospitalization or death in patients with rheumatic disease and COVID-19, said Gerd Burmester, MD, from Charité–University Medicine Berlin.
“There’s tremendous bias in terms of more serious cases of COVID-19 being reported to the registries,” he explained, “because the mild cases won’t even show up at their rheumatologist’s office.”
“This can skew the idea that COVID-19 is much more dangerous to rheumatic patients than to the regular population,” Burmester told Medscape Medical News. “It scares the patients, obviously, but we believe this is not justified.”
It’s still unclear whether rituximab use raises the risk for severe COVID-19, he said. “It appears to be the only biologic for which the jury is still out,” he said.
“Anti-TNFs and anti-IL-6 drugs may even be beneficial, although we don’t have robust data,” he added.
The study can only highlight associations between rheumatic drugs and COVID-19 outcomes. “We cannot say there is a causal relationship between the findings,” Machado said.
Longer-term data, when available, should illuminate “more granular” aspects of COVID-19 outcomes in rheumatic patients, including their risks of requiring ventilation or developing a cytokine storm, he noted.
Burmester and Machado agree that research needs to continue as the pandemic rages on. But so far, “there are no data suggesting that, if you’re on a targeted, dedicated immunomodulator, your risk is higher to have a worse course of COVID-19 than the general population,” Burmester said.
“We simply didn’t know that when the pandemic started, and some patients even discontinued their drugs out of this fear,” he added. “It’s more reassuring than we originally thought.”
This article first appeared on Medscape.com.
Working group proposes MRI definitions of structural lesions indicative of axial spondyloarthritis
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that showed a high degree of specificity in identifying such lesions in the disease.
“There is a lack of consensus as to what defines a structural lesion on MRI of the sacroiliac joint [SIJ] typical of axial spondyloarthritis. Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies. The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Making a definitive diagnosis of axSpA can be difficult because MRI can show a variety of SIJ abnormalities in healthy people as well as those with axSpA, said Dr. Maksymowych, chief medical officer of CARE Arthritis and professor in rheumatology at the University of Alberta in Edmonton, said in an interview prior to his presentation at the e-congress. “People who evaluate MRI scans are looking for clues as to what types of lesions they can be confident are indicative of axSpA.”
That started a process by the ASAS MRI group to evaluate scans from the landmark ASAS Classification Cohort study (Ann Rheum Dis. 2019;78:1550-8). “But,” said Dr. Maksymowych, “the MRI scans from that study were never evaluated.” So that work was handed off to the working group, whose 25 members included 7 expert image readers who evaluated the MRI scans.
The group adopted a standardized approach for evaluating MRIs of the SIJ in 148 cases, dividing each SIJ into quadrants and then evaluating consecutive MRI slices. The readers first documented whether they observed a definite structural lesion on the scan, which they then used as an external reference standard. They then analyzed which lesion, and in how many SIJ quadrants or slices, best reflected this external standard.
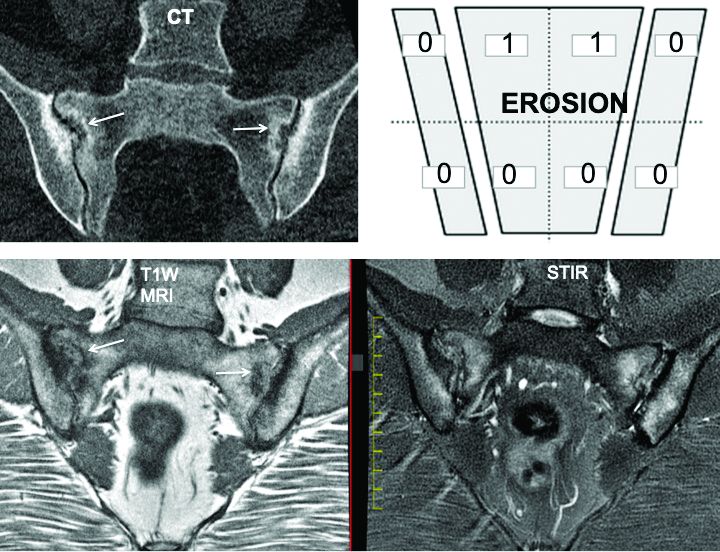
The investigators defined an erosion as “a defect in subchondral bone associated with full-thickness loss of a dark appearance of the subchondral cortex at its expected location, with loss of signal on a T1-weighted, non–fat-suppressed sequence, compared with the normal bright appearance of adjacent bone marrow.” They defined a fat lesion or fat metaplasia as a “bright signal seen on a T1-weighted, non–fat-suppressed sequence that is brighter than normal bone marrow which meets the following requirements: It is homogeneously bright, located in a typical anatomical area (specifically subchondral bone), and has a sharply defined border along its nonarticular border with normal bone marrow.”
An erosion in one quadrant isn’t sufficient to define a scan as positive for a definite structural lesion, said Dr. Maksymowych; but an erosion in three quadrants or in two or more consecutive slices meets the group’s designation of a definite structural lesion. “This showed over a 95% specificity for being associated with a definite structural lesion as defined by a majority of the seven experts,” he said.
The group also determined that a fat lesion typical of axSpA has a homogeneous white appearance on T1-weighted scans with a sharply defined border. The group also determined that such a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant is strongly indicative of axSpA.
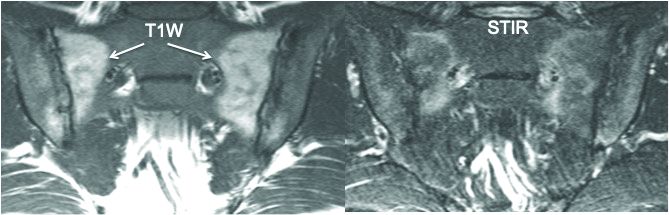
“So we now have definitions for two structural lesions, erosion and fat lesions, that reflect what a majority of experts consider to be a definite structural lesion according to at least 95% specificity,” he said. Sensitivity values were 90% for erosion in three quadrants and 83% for erosions in two or more consecutive slices. and 59% for a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant.
The second part of the analysis evaluated the predictive capacity of these lesion definitions for a rheumatologic diagnosis of axSpA at 4.4 years of follow-up. “These lesions predicted SpA with over 95% positive predictive value,” he said. “In other words, if you see them at baseline they’re going to predict SpA with high certainty at follow-up after 4.4 years.”
Three aspects of this study design are unique, Dr. Maksymowych noted. First is the high number of expert MRI readers who evaluated the scans. “There aren’t really too many studies I can think of that used more than two or three expert MRI readers,” he said.
Second is the way in which the study “very precisely and in a very standardized way” applied all the consensus-based ASAS definitions of structural SIJ lesions. “In the past, a variety of ways were used to define these lesions,” he said. “A good example would be the different ways in which erosions have been defined.”
The third novel aspect of the study is that the expert readers’ assessment of what constitutes a definite structural lesion was used as an external reference standard. For example, the study calculated sensitivity and specificity for numbers of SIJ quadrants and consecutive slices with erosion, sclerosis, and fat lesions where a majority of readers agreed on the presence of a structural lesion typical of axSpA with high confidence (3 or greater on a scale of 1-4). “The reason this was put in place is because we recognize sometimes lesions are very subtle and you can’t be certain that they’re reflecting SpA,” he said.
The investigators disclosed relationships with AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Merck, Novo Nordisk, Novartis, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB.
Maksymowych WP et al. Ann Rheum Dis, 2020;79[suppl 1]:53. Abstract OP0079.
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that showed a high degree of specificity in identifying such lesions in the disease.
“There is a lack of consensus as to what defines a structural lesion on MRI of the sacroiliac joint [SIJ] typical of axial spondyloarthritis. Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies. The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Making a definitive diagnosis of axSpA can be difficult because MRI can show a variety of SIJ abnormalities in healthy people as well as those with axSpA, said Dr. Maksymowych, chief medical officer of CARE Arthritis and professor in rheumatology at the University of Alberta in Edmonton, said in an interview prior to his presentation at the e-congress. “People who evaluate MRI scans are looking for clues as to what types of lesions they can be confident are indicative of axSpA.”
That started a process by the ASAS MRI group to evaluate scans from the landmark ASAS Classification Cohort study (Ann Rheum Dis. 2019;78:1550-8). “But,” said Dr. Maksymowych, “the MRI scans from that study were never evaluated.” So that work was handed off to the working group, whose 25 members included 7 expert image readers who evaluated the MRI scans.
The group adopted a standardized approach for evaluating MRIs of the SIJ in 148 cases, dividing each SIJ into quadrants and then evaluating consecutive MRI slices. The readers first documented whether they observed a definite structural lesion on the scan, which they then used as an external reference standard. They then analyzed which lesion, and in how many SIJ quadrants or slices, best reflected this external standard.

The investigators defined an erosion as “a defect in subchondral bone associated with full-thickness loss of a dark appearance of the subchondral cortex at its expected location, with loss of signal on a T1-weighted, non–fat-suppressed sequence, compared with the normal bright appearance of adjacent bone marrow.” They defined a fat lesion or fat metaplasia as a “bright signal seen on a T1-weighted, non–fat-suppressed sequence that is brighter than normal bone marrow which meets the following requirements: It is homogeneously bright, located in a typical anatomical area (specifically subchondral bone), and has a sharply defined border along its nonarticular border with normal bone marrow.”
An erosion in one quadrant isn’t sufficient to define a scan as positive for a definite structural lesion, said Dr. Maksymowych; but an erosion in three quadrants or in two or more consecutive slices meets the group’s designation of a definite structural lesion. “This showed over a 95% specificity for being associated with a definite structural lesion as defined by a majority of the seven experts,” he said.
The group also determined that a fat lesion typical of axSpA has a homogeneous white appearance on T1-weighted scans with a sharply defined border. The group also determined that such a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant is strongly indicative of axSpA.

“So we now have definitions for two structural lesions, erosion and fat lesions, that reflect what a majority of experts consider to be a definite structural lesion according to at least 95% specificity,” he said. Sensitivity values were 90% for erosion in three quadrants and 83% for erosions in two or more consecutive slices. and 59% for a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant.
The second part of the analysis evaluated the predictive capacity of these lesion definitions for a rheumatologic diagnosis of axSpA at 4.4 years of follow-up. “These lesions predicted SpA with over 95% positive predictive value,” he said. “In other words, if you see them at baseline they’re going to predict SpA with high certainty at follow-up after 4.4 years.”
Three aspects of this study design are unique, Dr. Maksymowych noted. First is the high number of expert MRI readers who evaluated the scans. “There aren’t really too many studies I can think of that used more than two or three expert MRI readers,” he said.
Second is the way in which the study “very precisely and in a very standardized way” applied all the consensus-based ASAS definitions of structural SIJ lesions. “In the past, a variety of ways were used to define these lesions,” he said. “A good example would be the different ways in which erosions have been defined.”
The third novel aspect of the study is that the expert readers’ assessment of what constitutes a definite structural lesion was used as an external reference standard. For example, the study calculated sensitivity and specificity for numbers of SIJ quadrants and consecutive slices with erosion, sclerosis, and fat lesions where a majority of readers agreed on the presence of a structural lesion typical of axSpA with high confidence (3 or greater on a scale of 1-4). “The reason this was put in place is because we recognize sometimes lesions are very subtle and you can’t be certain that they’re reflecting SpA,” he said.
The investigators disclosed relationships with AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Merck, Novo Nordisk, Novartis, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB.
Maksymowych WP et al. Ann Rheum Dis, 2020;79[suppl 1]:53. Abstract OP0079.
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that showed a high degree of specificity in identifying such lesions in the disease.
“There is a lack of consensus as to what defines a structural lesion on MRI of the sacroiliac joint [SIJ] typical of axial spondyloarthritis. Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies. The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Making a definitive diagnosis of axSpA can be difficult because MRI can show a variety of SIJ abnormalities in healthy people as well as those with axSpA, said Dr. Maksymowych, chief medical officer of CARE Arthritis and professor in rheumatology at the University of Alberta in Edmonton, said in an interview prior to his presentation at the e-congress. “People who evaluate MRI scans are looking for clues as to what types of lesions they can be confident are indicative of axSpA.”
That started a process by the ASAS MRI group to evaluate scans from the landmark ASAS Classification Cohort study (Ann Rheum Dis. 2019;78:1550-8). “But,” said Dr. Maksymowych, “the MRI scans from that study were never evaluated.” So that work was handed off to the working group, whose 25 members included 7 expert image readers who evaluated the MRI scans.
The group adopted a standardized approach for evaluating MRIs of the SIJ in 148 cases, dividing each SIJ into quadrants and then evaluating consecutive MRI slices. The readers first documented whether they observed a definite structural lesion on the scan, which they then used as an external reference standard. They then analyzed which lesion, and in how many SIJ quadrants or slices, best reflected this external standard.

The investigators defined an erosion as “a defect in subchondral bone associated with full-thickness loss of a dark appearance of the subchondral cortex at its expected location, with loss of signal on a T1-weighted, non–fat-suppressed sequence, compared with the normal bright appearance of adjacent bone marrow.” They defined a fat lesion or fat metaplasia as a “bright signal seen on a T1-weighted, non–fat-suppressed sequence that is brighter than normal bone marrow which meets the following requirements: It is homogeneously bright, located in a typical anatomical area (specifically subchondral bone), and has a sharply defined border along its nonarticular border with normal bone marrow.”
An erosion in one quadrant isn’t sufficient to define a scan as positive for a definite structural lesion, said Dr. Maksymowych; but an erosion in three quadrants or in two or more consecutive slices meets the group’s designation of a definite structural lesion. “This showed over a 95% specificity for being associated with a definite structural lesion as defined by a majority of the seven experts,” he said.
The group also determined that a fat lesion typical of axSpA has a homogeneous white appearance on T1-weighted scans with a sharply defined border. The group also determined that such a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant is strongly indicative of axSpA.

“So we now have definitions for two structural lesions, erosion and fat lesions, that reflect what a majority of experts consider to be a definite structural lesion according to at least 95% specificity,” he said. Sensitivity values were 90% for erosion in three quadrants and 83% for erosions in two or more consecutive slices. and 59% for a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant.
The second part of the analysis evaluated the predictive capacity of these lesion definitions for a rheumatologic diagnosis of axSpA at 4.4 years of follow-up. “These lesions predicted SpA with over 95% positive predictive value,” he said. “In other words, if you see them at baseline they’re going to predict SpA with high certainty at follow-up after 4.4 years.”
Three aspects of this study design are unique, Dr. Maksymowych noted. First is the high number of expert MRI readers who evaluated the scans. “There aren’t really too many studies I can think of that used more than two or three expert MRI readers,” he said.
Second is the way in which the study “very precisely and in a very standardized way” applied all the consensus-based ASAS definitions of structural SIJ lesions. “In the past, a variety of ways were used to define these lesions,” he said. “A good example would be the different ways in which erosions have been defined.”
The third novel aspect of the study is that the expert readers’ assessment of what constitutes a definite structural lesion was used as an external reference standard. For example, the study calculated sensitivity and specificity for numbers of SIJ quadrants and consecutive slices with erosion, sclerosis, and fat lesions where a majority of readers agreed on the presence of a structural lesion typical of axSpA with high confidence (3 or greater on a scale of 1-4). “The reason this was put in place is because we recognize sometimes lesions are very subtle and you can’t be certain that they’re reflecting SpA,” he said.
The investigators disclosed relationships with AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Merck, Novo Nordisk, Novartis, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB.
Maksymowych WP et al. Ann Rheum Dis, 2020;79[suppl 1]:53. Abstract OP0079.
FROM EULAR 2020 E-CONGRESS
FDA approves ixekizumab for nonradiographic axSpA
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
Nonpharmacologic ankylosing spondylitis recommendations not followed
Nonpharmacologic recommendations for ankylosing spondylitis aren’t often followed by rheumatologists in the Boston-based Partners Healthcare system, and probably elsewhere, according to a review presented at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The American College of Rheumatology, Spondylitis Association of America, and SPARTAN released joint guidelines for ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis in 2016. Nonpharmacologic recommendations for AS included regular disease activity monitoring using a validated measure and C-reactive protein or erythrocyte sedimentation rate; physical therapy (PT) or home back exercises; and screening for osteoporosis with dual x-ray absorptiometry (DXA) scanning.
However, “the extent to which these recommendations are followed in clinical practice is unknown,” said lead investigator Akash Patel, of the Brigham and Women’s Hospital Division of Rheumatology, Immunology, and Allergy, in Boston.
To find out, the team reviewed electronic records for 304 AS patients who had 564 rheumatology clinic visits with Brigham and Women’s and other Partners Healthcare physicians from July 1, 2016, to June 30, 2019.
Records documented DXA scans in less than 20% of visits. PT was documented in only 9% of visits, and home back exercise in just 7%. Inflammatory marker measurement was documented in about half of visits, and disease activity was measured in only 17%.
Comparing the first year of the study – right after the recommendations came out – to the third year, the team found just an 8% increase in disease activity documentation, and about a 3% increase in documentation of PT and back exercises.
In short, the recommendations “were performed at low frequencies in this study population,” Mr. Patel said at the meeting, which was held online this year because of the COVID-19 pandemic.
It’s unclear what’s going on. Perhaps some physicians disagree with the 2016 advice – the regular monitoring of disease activity, after all, was a conditional recommendation based on low-quality evidence. Other times, physicians might not have had enough time to talk about exercise or draw blood for AS biomarkers. Maybe they didn’t bring up PT when they knew their patients couldn’t afford the out-of-pocket cost.
Whatever the case, future iterations of the guidelines should include advice on how to implement them. “We believe that including some sort of strategy for rheumatologists may help increase compliance,” Mr. Patel said.
A member of the online viewing audience suggested that the problem may be widespread in rheumatology. "I think if we did this at my institution,” for example, “it would also look abysmal. I think we all just suck at this,” the attendee said.*
Mr. Patel and his team presented the results to Brigham and Women’s rheumatologists in February 2020, but it’s too early to tell if it made a difference.
It was a typical AS cohort. Almost three-quarters of the subjects were men; the average age was 50 years old; and the diagnosis was made by imaging. The majority of patients were HLA-B27 positive, and over one-third had a history of uveitis.
The study’s funding source and disclosures – if any – weren’t reported.
*Correction, 6/3/2020: A previous version of this story misattributed this quote.
SOURCE: Patel A et al. SPARTAN 2020 abstract session May 15.
Nonpharmacologic recommendations for ankylosing spondylitis aren’t often followed by rheumatologists in the Boston-based Partners Healthcare system, and probably elsewhere, according to a review presented at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The American College of Rheumatology, Spondylitis Association of America, and SPARTAN released joint guidelines for ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis in 2016. Nonpharmacologic recommendations for AS included regular disease activity monitoring using a validated measure and C-reactive protein or erythrocyte sedimentation rate; physical therapy (PT) or home back exercises; and screening for osteoporosis with dual x-ray absorptiometry (DXA) scanning.
However, “the extent to which these recommendations are followed in clinical practice is unknown,” said lead investigator Akash Patel, of the Brigham and Women’s Hospital Division of Rheumatology, Immunology, and Allergy, in Boston.
To find out, the team reviewed electronic records for 304 AS patients who had 564 rheumatology clinic visits with Brigham and Women’s and other Partners Healthcare physicians from July 1, 2016, to June 30, 2019.
Records documented DXA scans in less than 20% of visits. PT was documented in only 9% of visits, and home back exercise in just 7%. Inflammatory marker measurement was documented in about half of visits, and disease activity was measured in only 17%.
Comparing the first year of the study – right after the recommendations came out – to the third year, the team found just an 8% increase in disease activity documentation, and about a 3% increase in documentation of PT and back exercises.
In short, the recommendations “were performed at low frequencies in this study population,” Mr. Patel said at the meeting, which was held online this year because of the COVID-19 pandemic.
It’s unclear what’s going on. Perhaps some physicians disagree with the 2016 advice – the regular monitoring of disease activity, after all, was a conditional recommendation based on low-quality evidence. Other times, physicians might not have had enough time to talk about exercise or draw blood for AS biomarkers. Maybe they didn’t bring up PT when they knew their patients couldn’t afford the out-of-pocket cost.
Whatever the case, future iterations of the guidelines should include advice on how to implement them. “We believe that including some sort of strategy for rheumatologists may help increase compliance,” Mr. Patel said.
A member of the online viewing audience suggested that the problem may be widespread in rheumatology. "I think if we did this at my institution,” for example, “it would also look abysmal. I think we all just suck at this,” the attendee said.*
Mr. Patel and his team presented the results to Brigham and Women’s rheumatologists in February 2020, but it’s too early to tell if it made a difference.
It was a typical AS cohort. Almost three-quarters of the subjects were men; the average age was 50 years old; and the diagnosis was made by imaging. The majority of patients were HLA-B27 positive, and over one-third had a history of uveitis.
The study’s funding source and disclosures – if any – weren’t reported.
*Correction, 6/3/2020: A previous version of this story misattributed this quote.
SOURCE: Patel A et al. SPARTAN 2020 abstract session May 15.
Nonpharmacologic recommendations for ankylosing spondylitis aren’t often followed by rheumatologists in the Boston-based Partners Healthcare system, and probably elsewhere, according to a review presented at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The American College of Rheumatology, Spondylitis Association of America, and SPARTAN released joint guidelines for ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis in 2016. Nonpharmacologic recommendations for AS included regular disease activity monitoring using a validated measure and C-reactive protein or erythrocyte sedimentation rate; physical therapy (PT) or home back exercises; and screening for osteoporosis with dual x-ray absorptiometry (DXA) scanning.
However, “the extent to which these recommendations are followed in clinical practice is unknown,” said lead investigator Akash Patel, of the Brigham and Women’s Hospital Division of Rheumatology, Immunology, and Allergy, in Boston.
To find out, the team reviewed electronic records for 304 AS patients who had 564 rheumatology clinic visits with Brigham and Women’s and other Partners Healthcare physicians from July 1, 2016, to June 30, 2019.
Records documented DXA scans in less than 20% of visits. PT was documented in only 9% of visits, and home back exercise in just 7%. Inflammatory marker measurement was documented in about half of visits, and disease activity was measured in only 17%.
Comparing the first year of the study – right after the recommendations came out – to the third year, the team found just an 8% increase in disease activity documentation, and about a 3% increase in documentation of PT and back exercises.
In short, the recommendations “were performed at low frequencies in this study population,” Mr. Patel said at the meeting, which was held online this year because of the COVID-19 pandemic.
It’s unclear what’s going on. Perhaps some physicians disagree with the 2016 advice – the regular monitoring of disease activity, after all, was a conditional recommendation based on low-quality evidence. Other times, physicians might not have had enough time to talk about exercise or draw blood for AS biomarkers. Maybe they didn’t bring up PT when they knew their patients couldn’t afford the out-of-pocket cost.
Whatever the case, future iterations of the guidelines should include advice on how to implement them. “We believe that including some sort of strategy for rheumatologists may help increase compliance,” Mr. Patel said.
A member of the online viewing audience suggested that the problem may be widespread in rheumatology. "I think if we did this at my institution,” for example, “it would also look abysmal. I think we all just suck at this,” the attendee said.*
Mr. Patel and his team presented the results to Brigham and Women’s rheumatologists in February 2020, but it’s too early to tell if it made a difference.
It was a typical AS cohort. Almost three-quarters of the subjects were men; the average age was 50 years old; and the diagnosis was made by imaging. The majority of patients were HLA-B27 positive, and over one-third had a history of uveitis.
The study’s funding source and disclosures – if any – weren’t reported.
*Correction, 6/3/2020: A previous version of this story misattributed this quote.
SOURCE: Patel A et al. SPARTAN 2020 abstract session May 15.
FROM SPARTAN 2020
Inflammatory back pain likely underrecognized in primary care
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
REPORTING FROM SPARTAN 2020
TNF inhibitors may dampen COVID-19 severity
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
REPORTING FROM SOTA 2020




















