User login
Experts agree on optimal use of MRI in axSpA
BIRMINGHAM, ENGLAND – An evidence-based approach coupled with expert consensus has been used to determine the best way to use MRI for the diagnosis of axial spondyloarthritis (axSpA).
Working under the auspices of the British Society for Spondyloarthritis (BRITSpA), a task force of nine rheumatologists and nine musculoskeletal radiologists with an interest in axSpA developed a set of seven recommendations that provide guidance on how to best to acquire and then interpret MRI images of the spine and sacroiliac joints.
The recommendations, which were published online (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez173), cover how to perform MRI when axSpA is suspected, such as by imaging both the sacroiliac joints and the spine, and provide guidance on the sequences and order of MRI planes to be used, and what features may increase the diagnostic confidence of axSpA.
The recommendations are as follows:
• When requesting an MRI for suspected axSpA, imaging of both the sacroiliac joints and the spine is recommended.
• T1-weighted and fat-suppressed, fluid-sensitive sequences are recommended for suspected axSpA.
• The minimum protocol when requesting an MRI for suspected axSpA should include sagittal images of the spine with extended lateral coverage and images of the sacroiliac joints that are in an oblique coronal plane to the joint.
• In the sacroiliac joints, the presence of bone marrow edema, fatty infiltration, or erosion is suggestive of the diagnosis of axSpA. The presence of more than one of these features increases the diagnostic confidence of axSpA.
• In the spine, the presence of multiple corner inflammatory lesions and/or multiple corner fatty lesions increases the diagnostic confidence of axSpA.
• In the sacroiliac joints and/or spine, the presence of characteristic new bone formation increases the diagnostic confidence of axSpA.
• The full range and combination of active and structural lesions of the sacroiliac joints and spine should be taken into account when deciding if the MRI scan is suggestive of axSpA or not.
The recommendations “are intended to standardize practice around the use of MRI,” said Alexis Jones, MBBS, MS (Rheumatology), a senior clinical research fellow at University College London Hospitals NHS Foundation Trust. She presented the recommendations on behalf of the expert task force at the annual conference of the British Society for Rheumatology.
“MRI has become an essential tool in axial spondyloarthritis. It facilitates earlier diagnoses and therefore has allowed for earlier initiation of treatment. It can be used to monitor the burden of inflammation and may predict response to therapy,” Dr. Jones said. Despite this, “there is significant inconsistency in the use of MRI” in clinical practice.
For instance, a survey performed in the United Kingdom (J Rheumatol. 2017;44[6]:780-5) highlighted the need for better collaboration between rheumatology and radiology departments to identify axSpA MRI lesions and develop appropriate protocols.
That survey showed that a quarter of radiologists were not aware of the term axSpA, and just 31% and 25%, respectively, were aware of Assessment of Spondyloarthritis international Society (ASAS) criteria for a positive MRI of the sacroiliac joints and spine. Furthermore, 18% of radiologists did not recognize bone marrow edema as a diagnostic feature of axSpA.
The heterogeneity in the performance of MRI in clinical practice could lead to a delay in diagnosis and potentially misdiagnosis, the task force’s lead author and consultant rheumatologist, Pedro Machado, MD, said in an interview.
“I think everyone has been focusing on demonstrating the value of MRI in the condition but then they forgot to look at the standardization aspect,” said Dr. Machado, who works at University College Hospital and the National Hospital for Neurology and Neurosurgery in London.
With that in mind, the BRITSpA-endorsed task force was set up and met to determine the scope of the recommendations. They looked at the evidence for the use of MRI in the diagnosis of axSpA and used two overarching principles to draft the recommendations: 1) the diagnosis of axSpA is based on clinical, laboratory, and imaging features; 2) Some patients with axSpA have isolated inflammation of the sacroiliac joints or spine.
“All of the recommendations were met with a high level of agreement, indicating a strong consensus” among rheumatologists and radiologists, Dr. Jones noted.
“These recommendations can be immediately applied to clinical practice,” said Dr. Machado, who noted that they should standardize practice and decrease heterogeneity around the use of MRI. “This will help ensure a more informed and consistent approach to the diagnosis of axSpA.”
One of the potential impacts of the recommendations, if followed, is that they may actually help to reduce health care costs, Dr. Machado suggested, because an optimized protocol would be used, making MRI more cost effective by not including sequences that do not add value in the condition.
The next task is to share the recommendations more widely and make sure they are applied in clinical practice.
A systematic literature review on which the recommendations were based was published simultaneously with the conference presentation (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez172).
The work was supported by BRITSpA. The authors had no relevant disclosures.
SOURCE: Bray TJP et al. Rheumatology 2019;58(suppl 3): Abstract 033. doi: 10.1093/rheumatology/kez105.032.
BIRMINGHAM, ENGLAND – An evidence-based approach coupled with expert consensus has been used to determine the best way to use MRI for the diagnosis of axial spondyloarthritis (axSpA).
Working under the auspices of the British Society for Spondyloarthritis (BRITSpA), a task force of nine rheumatologists and nine musculoskeletal radiologists with an interest in axSpA developed a set of seven recommendations that provide guidance on how to best to acquire and then interpret MRI images of the spine and sacroiliac joints.
The recommendations, which were published online (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez173), cover how to perform MRI when axSpA is suspected, such as by imaging both the sacroiliac joints and the spine, and provide guidance on the sequences and order of MRI planes to be used, and what features may increase the diagnostic confidence of axSpA.
The recommendations are as follows:
• When requesting an MRI for suspected axSpA, imaging of both the sacroiliac joints and the spine is recommended.
• T1-weighted and fat-suppressed, fluid-sensitive sequences are recommended for suspected axSpA.
• The minimum protocol when requesting an MRI for suspected axSpA should include sagittal images of the spine with extended lateral coverage and images of the sacroiliac joints that are in an oblique coronal plane to the joint.
• In the sacroiliac joints, the presence of bone marrow edema, fatty infiltration, or erosion is suggestive of the diagnosis of axSpA. The presence of more than one of these features increases the diagnostic confidence of axSpA.
• In the spine, the presence of multiple corner inflammatory lesions and/or multiple corner fatty lesions increases the diagnostic confidence of axSpA.
• In the sacroiliac joints and/or spine, the presence of characteristic new bone formation increases the diagnostic confidence of axSpA.
• The full range and combination of active and structural lesions of the sacroiliac joints and spine should be taken into account when deciding if the MRI scan is suggestive of axSpA or not.
The recommendations “are intended to standardize practice around the use of MRI,” said Alexis Jones, MBBS, MS (Rheumatology), a senior clinical research fellow at University College London Hospitals NHS Foundation Trust. She presented the recommendations on behalf of the expert task force at the annual conference of the British Society for Rheumatology.
“MRI has become an essential tool in axial spondyloarthritis. It facilitates earlier diagnoses and therefore has allowed for earlier initiation of treatment. It can be used to monitor the burden of inflammation and may predict response to therapy,” Dr. Jones said. Despite this, “there is significant inconsistency in the use of MRI” in clinical practice.
For instance, a survey performed in the United Kingdom (J Rheumatol. 2017;44[6]:780-5) highlighted the need for better collaboration between rheumatology and radiology departments to identify axSpA MRI lesions and develop appropriate protocols.
That survey showed that a quarter of radiologists were not aware of the term axSpA, and just 31% and 25%, respectively, were aware of Assessment of Spondyloarthritis international Society (ASAS) criteria for a positive MRI of the sacroiliac joints and spine. Furthermore, 18% of radiologists did not recognize bone marrow edema as a diagnostic feature of axSpA.
The heterogeneity in the performance of MRI in clinical practice could lead to a delay in diagnosis and potentially misdiagnosis, the task force’s lead author and consultant rheumatologist, Pedro Machado, MD, said in an interview.
“I think everyone has been focusing on demonstrating the value of MRI in the condition but then they forgot to look at the standardization aspect,” said Dr. Machado, who works at University College Hospital and the National Hospital for Neurology and Neurosurgery in London.
With that in mind, the BRITSpA-endorsed task force was set up and met to determine the scope of the recommendations. They looked at the evidence for the use of MRI in the diagnosis of axSpA and used two overarching principles to draft the recommendations: 1) the diagnosis of axSpA is based on clinical, laboratory, and imaging features; 2) Some patients with axSpA have isolated inflammation of the sacroiliac joints or spine.
“All of the recommendations were met with a high level of agreement, indicating a strong consensus” among rheumatologists and radiologists, Dr. Jones noted.
“These recommendations can be immediately applied to clinical practice,” said Dr. Machado, who noted that they should standardize practice and decrease heterogeneity around the use of MRI. “This will help ensure a more informed and consistent approach to the diagnosis of axSpA.”
One of the potential impacts of the recommendations, if followed, is that they may actually help to reduce health care costs, Dr. Machado suggested, because an optimized protocol would be used, making MRI more cost effective by not including sequences that do not add value in the condition.
The next task is to share the recommendations more widely and make sure they are applied in clinical practice.
A systematic literature review on which the recommendations were based was published simultaneously with the conference presentation (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez172).
The work was supported by BRITSpA. The authors had no relevant disclosures.
SOURCE: Bray TJP et al. Rheumatology 2019;58(suppl 3): Abstract 033. doi: 10.1093/rheumatology/kez105.032.
BIRMINGHAM, ENGLAND – An evidence-based approach coupled with expert consensus has been used to determine the best way to use MRI for the diagnosis of axial spondyloarthritis (axSpA).
Working under the auspices of the British Society for Spondyloarthritis (BRITSpA), a task force of nine rheumatologists and nine musculoskeletal radiologists with an interest in axSpA developed a set of seven recommendations that provide guidance on how to best to acquire and then interpret MRI images of the spine and sacroiliac joints.
The recommendations, which were published online (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez173), cover how to perform MRI when axSpA is suspected, such as by imaging both the sacroiliac joints and the spine, and provide guidance on the sequences and order of MRI planes to be used, and what features may increase the diagnostic confidence of axSpA.
The recommendations are as follows:
• When requesting an MRI for suspected axSpA, imaging of both the sacroiliac joints and the spine is recommended.
• T1-weighted and fat-suppressed, fluid-sensitive sequences are recommended for suspected axSpA.
• The minimum protocol when requesting an MRI for suspected axSpA should include sagittal images of the spine with extended lateral coverage and images of the sacroiliac joints that are in an oblique coronal plane to the joint.
• In the sacroiliac joints, the presence of bone marrow edema, fatty infiltration, or erosion is suggestive of the diagnosis of axSpA. The presence of more than one of these features increases the diagnostic confidence of axSpA.
• In the spine, the presence of multiple corner inflammatory lesions and/or multiple corner fatty lesions increases the diagnostic confidence of axSpA.
• In the sacroiliac joints and/or spine, the presence of characteristic new bone formation increases the diagnostic confidence of axSpA.
• The full range and combination of active and structural lesions of the sacroiliac joints and spine should be taken into account when deciding if the MRI scan is suggestive of axSpA or not.
The recommendations “are intended to standardize practice around the use of MRI,” said Alexis Jones, MBBS, MS (Rheumatology), a senior clinical research fellow at University College London Hospitals NHS Foundation Trust. She presented the recommendations on behalf of the expert task force at the annual conference of the British Society for Rheumatology.
“MRI has become an essential tool in axial spondyloarthritis. It facilitates earlier diagnoses and therefore has allowed for earlier initiation of treatment. It can be used to monitor the burden of inflammation and may predict response to therapy,” Dr. Jones said. Despite this, “there is significant inconsistency in the use of MRI” in clinical practice.
For instance, a survey performed in the United Kingdom (J Rheumatol. 2017;44[6]:780-5) highlighted the need for better collaboration between rheumatology and radiology departments to identify axSpA MRI lesions and develop appropriate protocols.
That survey showed that a quarter of radiologists were not aware of the term axSpA, and just 31% and 25%, respectively, were aware of Assessment of Spondyloarthritis international Society (ASAS) criteria for a positive MRI of the sacroiliac joints and spine. Furthermore, 18% of radiologists did not recognize bone marrow edema as a diagnostic feature of axSpA.
The heterogeneity in the performance of MRI in clinical practice could lead to a delay in diagnosis and potentially misdiagnosis, the task force’s lead author and consultant rheumatologist, Pedro Machado, MD, said in an interview.
“I think everyone has been focusing on demonstrating the value of MRI in the condition but then they forgot to look at the standardization aspect,” said Dr. Machado, who works at University College Hospital and the National Hospital for Neurology and Neurosurgery in London.
With that in mind, the BRITSpA-endorsed task force was set up and met to determine the scope of the recommendations. They looked at the evidence for the use of MRI in the diagnosis of axSpA and used two overarching principles to draft the recommendations: 1) the diagnosis of axSpA is based on clinical, laboratory, and imaging features; 2) Some patients with axSpA have isolated inflammation of the sacroiliac joints or spine.
“All of the recommendations were met with a high level of agreement, indicating a strong consensus” among rheumatologists and radiologists, Dr. Jones noted.
“These recommendations can be immediately applied to clinical practice,” said Dr. Machado, who noted that they should standardize practice and decrease heterogeneity around the use of MRI. “This will help ensure a more informed and consistent approach to the diagnosis of axSpA.”
One of the potential impacts of the recommendations, if followed, is that they may actually help to reduce health care costs, Dr. Machado suggested, because an optimized protocol would be used, making MRI more cost effective by not including sequences that do not add value in the condition.
The next task is to share the recommendations more widely and make sure they are applied in clinical practice.
A systematic literature review on which the recommendations were based was published simultaneously with the conference presentation (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez172).
The work was supported by BRITSpA. The authors had no relevant disclosures.
SOURCE: Bray TJP et al. Rheumatology 2019;58(suppl 3): Abstract 033. doi: 10.1093/rheumatology/kez105.032.
REPORTING FROM BSR 2019
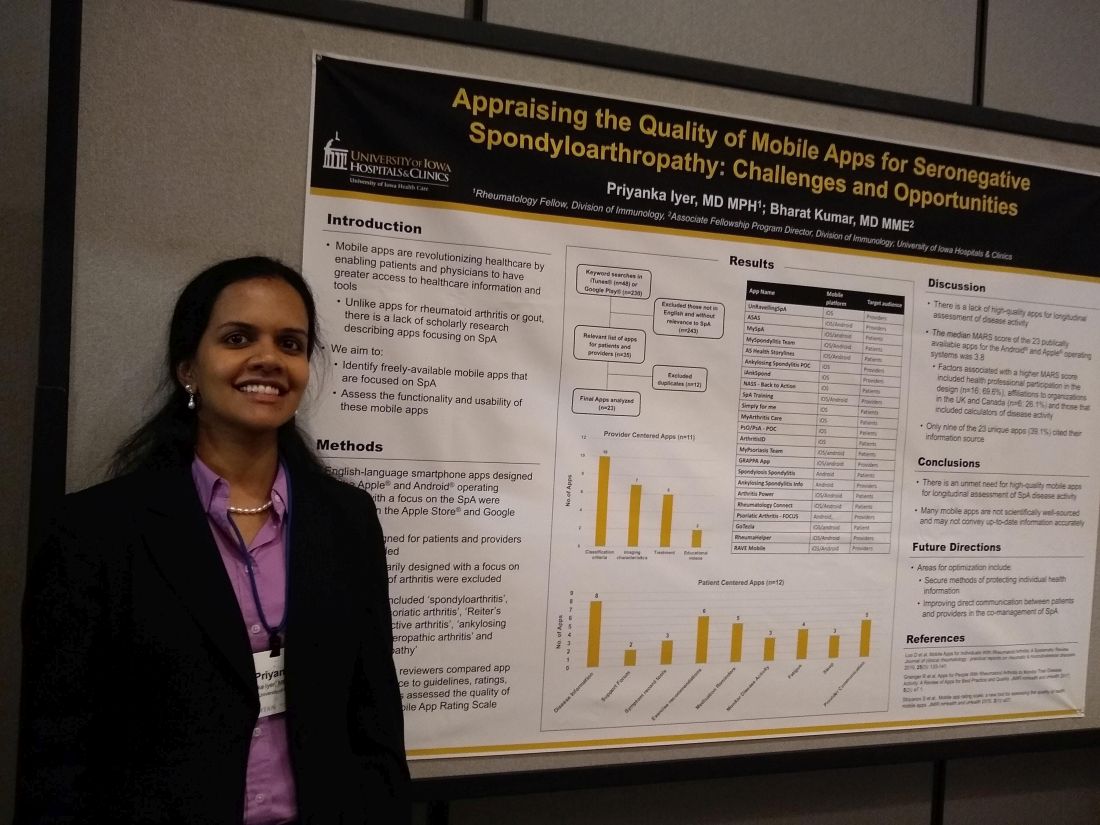
Mobile SpA apps abound, but there’s room for quality improvement
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
REPORTING FROM SPARTAN 2019
Smoking found not protective against uveitis attacks in axSpA patients
Smoking does not appear to have protective effects against anterior uveitis attacks in patients with axial spondyloarthritis, according to prospective registry study data.
Both current and ex-smokers had increased uveitis rates versus never-smokers in the study, suggesting that the supposed protective effect of smoking found in previous axial spondyloarthritis studies was not causal, Sizheng Steven Zhao, MD, and his colleagues reported in the Annals of the Rheumatic Diseases.
“Spurious relationships can emerge when studies restrict to a disease population,” the researchers wrote.
The present analysis by Dr. Zhao and colleagues included 2,420 patients with axial spondyloarthritis in the British Society for Rheumatology Biologics Registry for Ankylosing Spondylitis. Of that group, 632 (26%) had a diagnosis of acute anterior uveitis over a total of 1,457 patient-years of follow-up.
Researchers looked specifically at the number of uveitis episodes per 12-month period, which ranged from 0 to 15 in the overall study cohort.
Current smokers had a 33% higher incidence of acute anterior uveitis episodes versus never-smokers, while ex-smokers had a 19% higher incidence, although the findings did not reach statistical significance, according to the researchers.
Because some studies have suggested that smoking may influence response to biologic therapy, Dr. Zhao and coinvestigators stratified patients into biologic and nonbiologic cohorts. In the biologic cohort, they found a 76% higher incidence per year of uveitis attacks for current smokers versus never-smokers, and a 29% increased incidence for ex-smokers versus never-smokers.
These findings are “consistent with increased risk of uveitis observed among smokers in the general population,” the researchers said. “Although nicotine may have anti-inflammatory properties, cigarette smoking is overall pro-inflammatory.”
Those results provide “yet another line of evidence” that should compel spondyloarthritis patients to quit smoking, the researchers added. Previous studies have suggested that smoking may increase radiographic progression and may reduce response to treatment.
The authors declared no competing interests. The registry study is supported by the British Society for Rheumatology, which has received funding from Pfizer, AbbVie, and UCB for the study.
SOURCE: Zhao SS et al. Ann Rheum Dis. 2019 Apr 20. doi: 10.1136/annrheumdis-2019-215348
Smoking does not appear to have protective effects against anterior uveitis attacks in patients with axial spondyloarthritis, according to prospective registry study data.
Both current and ex-smokers had increased uveitis rates versus never-smokers in the study, suggesting that the supposed protective effect of smoking found in previous axial spondyloarthritis studies was not causal, Sizheng Steven Zhao, MD, and his colleagues reported in the Annals of the Rheumatic Diseases.
“Spurious relationships can emerge when studies restrict to a disease population,” the researchers wrote.
The present analysis by Dr. Zhao and colleagues included 2,420 patients with axial spondyloarthritis in the British Society for Rheumatology Biologics Registry for Ankylosing Spondylitis. Of that group, 632 (26%) had a diagnosis of acute anterior uveitis over a total of 1,457 patient-years of follow-up.
Researchers looked specifically at the number of uveitis episodes per 12-month period, which ranged from 0 to 15 in the overall study cohort.
Current smokers had a 33% higher incidence of acute anterior uveitis episodes versus never-smokers, while ex-smokers had a 19% higher incidence, although the findings did not reach statistical significance, according to the researchers.
Because some studies have suggested that smoking may influence response to biologic therapy, Dr. Zhao and coinvestigators stratified patients into biologic and nonbiologic cohorts. In the biologic cohort, they found a 76% higher incidence per year of uveitis attacks for current smokers versus never-smokers, and a 29% increased incidence for ex-smokers versus never-smokers.
These findings are “consistent with increased risk of uveitis observed among smokers in the general population,” the researchers said. “Although nicotine may have anti-inflammatory properties, cigarette smoking is overall pro-inflammatory.”
Those results provide “yet another line of evidence” that should compel spondyloarthritis patients to quit smoking, the researchers added. Previous studies have suggested that smoking may increase radiographic progression and may reduce response to treatment.
The authors declared no competing interests. The registry study is supported by the British Society for Rheumatology, which has received funding from Pfizer, AbbVie, and UCB for the study.
SOURCE: Zhao SS et al. Ann Rheum Dis. 2019 Apr 20. doi: 10.1136/annrheumdis-2019-215348
Smoking does not appear to have protective effects against anterior uveitis attacks in patients with axial spondyloarthritis, according to prospective registry study data.
Both current and ex-smokers had increased uveitis rates versus never-smokers in the study, suggesting that the supposed protective effect of smoking found in previous axial spondyloarthritis studies was not causal, Sizheng Steven Zhao, MD, and his colleagues reported in the Annals of the Rheumatic Diseases.
“Spurious relationships can emerge when studies restrict to a disease population,” the researchers wrote.
The present analysis by Dr. Zhao and colleagues included 2,420 patients with axial spondyloarthritis in the British Society for Rheumatology Biologics Registry for Ankylosing Spondylitis. Of that group, 632 (26%) had a diagnosis of acute anterior uveitis over a total of 1,457 patient-years of follow-up.
Researchers looked specifically at the number of uveitis episodes per 12-month period, which ranged from 0 to 15 in the overall study cohort.
Current smokers had a 33% higher incidence of acute anterior uveitis episodes versus never-smokers, while ex-smokers had a 19% higher incidence, although the findings did not reach statistical significance, according to the researchers.
Because some studies have suggested that smoking may influence response to biologic therapy, Dr. Zhao and coinvestigators stratified patients into biologic and nonbiologic cohorts. In the biologic cohort, they found a 76% higher incidence per year of uveitis attacks for current smokers versus never-smokers, and a 29% increased incidence for ex-smokers versus never-smokers.
These findings are “consistent with increased risk of uveitis observed among smokers in the general population,” the researchers said. “Although nicotine may have anti-inflammatory properties, cigarette smoking is overall pro-inflammatory.”
Those results provide “yet another line of evidence” that should compel spondyloarthritis patients to quit smoking, the researchers added. Previous studies have suggested that smoking may increase radiographic progression and may reduce response to treatment.
The authors declared no competing interests. The registry study is supported by the British Society for Rheumatology, which has received funding from Pfizer, AbbVie, and UCB for the study.
SOURCE: Zhao SS et al. Ann Rheum Dis. 2019 Apr 20. doi: 10.1136/annrheumdis-2019-215348
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Current smokers had a 33% higher incidence of acute anterior uveitis episodes versus never-smokers, while ex-smokers had a 19% higher incidence.
Study details: Analysis including 2,420 patients with axial spondyloarthritis in the British Society for Rheumatology Biologics Registry for Ankylosing Spondylitis.
Disclosures: The authors declared no competing interests. The study is supported by the British Society for Rheumatology, which has received funding from Pfizer, AbbVie, and UCB for the study.
Source: Zhao SS et al. Ann Rheum Dis. 2019 Apr 20. doi: 10.1136/annrheumdis-2019-215348.
FDA approves new etanercept biosimilar, Eticovo
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
Referral system aims to slash axial spondyloarthritis diagnostic delay
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.

But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.

But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.

But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Cimzia becomes first FDA-approved treatment for nonradiographic axial spondyloarthritis
, with objective evidence of inflammation, making it the first treatment approved by the agency for the condition.
The FDA approved the tumor necrosis factor inhibitor based on results from a randomized clinical trial in 317 adult patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had elevated C-reactive protein levels and/or sacroiliitis (inflammation of the sacroiliac joints) on MRI.
The trial entailed 52 weeks of double-blind therapy with certolizumab at a starting dose of 400 mg on weeks 0, 2, and 4 followed by 200 mg every 2 weeks, or placebo. The Ankylosing Spondylitis Disease Activity Score Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866).
The overall safety profile observed in the Cimzia treatment group was consistent with the known safety profile of certolizumab.
Cimzia was first approved in 2008 and has FDA-approved indications for adult patients with Crohn’s disease, moderate to severe rheumatoid arthritis, active ankylosing spondylitis and moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
, with objective evidence of inflammation, making it the first treatment approved by the agency for the condition.
The FDA approved the tumor necrosis factor inhibitor based on results from a randomized clinical trial in 317 adult patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had elevated C-reactive protein levels and/or sacroiliitis (inflammation of the sacroiliac joints) on MRI.
The trial entailed 52 weeks of double-blind therapy with certolizumab at a starting dose of 400 mg on weeks 0, 2, and 4 followed by 200 mg every 2 weeks, or placebo. The Ankylosing Spondylitis Disease Activity Score Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866).
The overall safety profile observed in the Cimzia treatment group was consistent with the known safety profile of certolizumab.
Cimzia was first approved in 2008 and has FDA-approved indications for adult patients with Crohn’s disease, moderate to severe rheumatoid arthritis, active ankylosing spondylitis and moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
, with objective evidence of inflammation, making it the first treatment approved by the agency for the condition.
The FDA approved the tumor necrosis factor inhibitor based on results from a randomized clinical trial in 317 adult patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had elevated C-reactive protein levels and/or sacroiliitis (inflammation of the sacroiliac joints) on MRI.
The trial entailed 52 weeks of double-blind therapy with certolizumab at a starting dose of 400 mg on weeks 0, 2, and 4 followed by 200 mg every 2 weeks, or placebo. The Ankylosing Spondylitis Disease Activity Score Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866).
The overall safety profile observed in the Cimzia treatment group was consistent with the known safety profile of certolizumab.
Cimzia was first approved in 2008 and has FDA-approved indications for adult patients with Crohn’s disease, moderate to severe rheumatoid arthritis, active ankylosing spondylitis and moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
Biologics boost work outcomes in axial spondyloarthritis
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Industry-funded rheumatology RCTs are higher quality
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
REPORTING FROM RWCS 2019
Recent trials advance axial spondyloarthritis therapy
MAUI, HAWAII – Arguably the most exciting therapeutic development in axial spondyloarthritis in the past year was the demonstrated efficacy and safety of the investigational oral selective Janus kinase 1 inhibitor filgotinib in the setting of active ankylosing spondylitis, speakers agreed at the 2019 Rheumatology Winter Clinical Symposium.
“This is big, big news,” commented symposium director Arthur Kavanaugh, MD. “This is going to be a big deal.”
Other recent clinical trials of note in axial spondyloarthritis (SpA) highlighted by Dr. Kavanaugh and Eric Ruderman, MD, professor of medicine at Northwestern University, Chicago, included a positive phase 3 study of certolizumab pegol (Cimzia) in nonradiographic SpA, two positive phase 3 trials of the interleukin-17A antagonist ixekizumab (Taltz) in radiographic SpA, a positive phase 2b trial of the dual IL-17A/F antagonist bimekizumab, and publication of three surprisingly negative phase 3 trials of the IL-12/23 inhibitor ustekinumab (Stelara).
Filgotinib
TORTUGA was a phase 2b, double-blind, multicenter trial of 116 European patients with active ankylosing spondylitis nonresponsive to NSAIDs who were randomized to oral filgotinib at 200 mg once daily or placebo for 12 weeks. Filgotinib reduced the Ankylosing Spondylitis Disease Activity Score (ASDAS) by a mean of 1.47 points from baseline, a significantly better result for the primary outcome than the 0.57-point decrease in controls (Lancet. 2018 Dec 1;392[10162]:2378-87).
Dr. Ruderman was also favorably impressed with the oral Janus kinase 1 (JAK1) inhibitor’s performance on the secondary outcome measures, including a mean 2.41-point reduction from baseline on the Bath Ankylosing Spondylitis Disease Activity Index, compared with a 1.44-point decrease in controls, with the difference being significant from week 8 onward. The filgotinib group also did significantly better on validated measures of physical function, spinal mobility, physical function, quality of life, peripheral arthritis, fatigue, and spinal and sacroiliac joint inflammation as assessed by MRI.
One patient in the filgotinib group, a smoker, developed pneumonia and another experienced deep venous thrombosis.
The study results are an exciting development because SpA treatments with new mechanisms of action are sorely needed. NSAIDs are considered first-line pharmacotherapy at present, with various tumor necrosis factor (TNF) inhibitors as well as the IL-17 inhibitor secukinumab (Cosentyx) the only approved biologic alternatives.
“This is the most impressive data I’ve seen that JAK inhibitors are effective in ankylosing spondyloarthritis,” commented Paul Emery, MD, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
TORTUGA was the first positive phase 2 trial of a selective JAK1 inhibitor in SpA. However, Dr. Kavanaugh noted that while a phase 2 trial of tofacitinib (Xeljanz) failed to meet its “very convoluted” primary endpoint, the JAK1/3 inhibitor was positive for key secondary endpoints, including favorable MRI changes. And a phase 3 trial of tofacitinib in SpA is underway.
A key remaining question pending the outcome of definitive phase 3 trials is whether specificity of JAK enzyme inhibition matters or if a class effect is at work, according to Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Certolizumab pegol
The TNF inhibitor is already approved for ankylosing spondylitis, among other indications, but it has now demonstrated efficacy and safety in nonradiographic SpA in a phase 3 trial structured with guidance from the Food and Drug Administration.
“It seems likely that UCB will get the indication for this,” according to Dr. Ruderman.
This 317-patient trial was remarkable in that it entailed a full 52 weeks of double-blind therapy with certolizumab at the standard dose of 200 mg every 2 weeks or placebo. The ASDAS Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% (ASAS 40) response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls in this trial, which was recently published (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866). All participants had to have baseline MRI evidence of sacroiliac joint inflammation and/or an elevated C-reactive protein.
By way of background, Dr. Ruderman explained that the FDA required 52 weeks of double-blind, placebo-controlled therapy because the agency’s advisory committee had formerly expressed reservations about considering an expanded indication for TNF inhibitors in nonradiographic as opposed to radiographic SpA.
“They were very concerned that approval could result in patients with mechanical back pain or fibromyalgia being treated with biologics. And they weren’t sure nonradiographic SpA was a discrete entity. They wondered if it remits on its own,” according to the rheumatologist.
Dr. Ruderman is curious to see how the FDA is going to handle this situation in light of the positive phase 3 certolizumab results. Will the agency require other companies that market TNF inhibitors to mount a similarly rigorous 52-week, double-blind, placebo-controlled trial in order to obtain an expanded indication? That would seem to pose ethical issues now. Or will the companies be able to gain an expanded indication by retrospective analysis of outcomes in patients with nonradiographic SpA in their existing trials databases? Stay tuned.
Ixekizumab
The COAST-V trial was a phase 3, randomized, double-blind, active- and placebo-controlled trial of 341 patients with radiographic SpA who hadn’t previously been treated with a biologic disease-modifying antirheumatic drug. They were assigned to 80 mg of ixekizumab every 2 weeks, 80 mg every 4 weeks, adalimumab (Humira) at 40 mg every 2 weeks, or placebo. The primary endpoint – an ASAS 40 response at week 16 – was achieved in 52% of patients on ixekizumab every 2 weeks, 48% of those who received ixekizumab every 4 weeks, 36% on adalimumab, and 18% on placebo (Lancet. 2018 Dec 8;392[10163]:2441-51).
In contrast, the phase 3 COAST-W trial included 316 patients with radiographic SpA who were inadequate responders or intolerant to one or more prior anti-TNF agents. The 16-week ASAS 40 response rate was 30.6% with ixekizumab every 2 weeks, similar at 25.4% with ixekizumab every 4 weeks, and 12.5% with placebo (Arthritis Rheumatol. 2018 Oct 20. doi: 10.1002/art.40753).
While the COAST-V trial convincingly showed both ixekizumab and adalimumab were more effective than placebo, the patient numbers were way too small to draw any conclusions about the relative efficacy of the two biologics, according to Dr. Ruderman.
“I don’t think these results are surprising,” Dr. Kavanaugh commented. “It would have been surprising if ixekizumab was ineffective, given that secukinumab works. But it’s nice to have the proof.”
Bimekizumab
This investigational dual IL-17A/F inhibitor demonstrated efficacy and safety for SpA in a 297-patient, phase 2b trial presented at the 2018 European Congress of Rheumatology.
“It’s effective, but it doesn’t look like it’s particularly more effective than either of the existing IL-17A inhibitors. We’ll see going forward if there truly is an advantage here to the additional inhibition of IL-17F in this population. I will say that the preclinical and laboratory data on the potential advantages of IL-17F inhibition are mostly in the psoriasis/psoriatic arthritis space. It’s not clear in ankylosing spondyloarthritis specifically whether we should expect to see a difference,” Dr. Ruderman said.
Ustekinumab
The IL-12/23 inhibitor proved no better than placebo in patients with SpA in three separate phase 3, randomized trials recently published as a single summary article (Arthritis Rheumatol. 2019 Feb;71[2]:258-70).
“Ustekinumab was effective in an earlier open study, so I think everybody was surprised by this,” Dr. Kavanaugh said.
He reported serving as a consultant to and/or receiving research funding from a dozen pharmaceutical companies. Dr. Ruderman reported financial relationships with eight companies.
MAUI, HAWAII – Arguably the most exciting therapeutic development in axial spondyloarthritis in the past year was the demonstrated efficacy and safety of the investigational oral selective Janus kinase 1 inhibitor filgotinib in the setting of active ankylosing spondylitis, speakers agreed at the 2019 Rheumatology Winter Clinical Symposium.
“This is big, big news,” commented symposium director Arthur Kavanaugh, MD. “This is going to be a big deal.”
Other recent clinical trials of note in axial spondyloarthritis (SpA) highlighted by Dr. Kavanaugh and Eric Ruderman, MD, professor of medicine at Northwestern University, Chicago, included a positive phase 3 study of certolizumab pegol (Cimzia) in nonradiographic SpA, two positive phase 3 trials of the interleukin-17A antagonist ixekizumab (Taltz) in radiographic SpA, a positive phase 2b trial of the dual IL-17A/F antagonist bimekizumab, and publication of three surprisingly negative phase 3 trials of the IL-12/23 inhibitor ustekinumab (Stelara).
Filgotinib
TORTUGA was a phase 2b, double-blind, multicenter trial of 116 European patients with active ankylosing spondylitis nonresponsive to NSAIDs who were randomized to oral filgotinib at 200 mg once daily or placebo for 12 weeks. Filgotinib reduced the Ankylosing Spondylitis Disease Activity Score (ASDAS) by a mean of 1.47 points from baseline, a significantly better result for the primary outcome than the 0.57-point decrease in controls (Lancet. 2018 Dec 1;392[10162]:2378-87).
Dr. Ruderman was also favorably impressed with the oral Janus kinase 1 (JAK1) inhibitor’s performance on the secondary outcome measures, including a mean 2.41-point reduction from baseline on the Bath Ankylosing Spondylitis Disease Activity Index, compared with a 1.44-point decrease in controls, with the difference being significant from week 8 onward. The filgotinib group also did significantly better on validated measures of physical function, spinal mobility, physical function, quality of life, peripheral arthritis, fatigue, and spinal and sacroiliac joint inflammation as assessed by MRI.
One patient in the filgotinib group, a smoker, developed pneumonia and another experienced deep venous thrombosis.
The study results are an exciting development because SpA treatments with new mechanisms of action are sorely needed. NSAIDs are considered first-line pharmacotherapy at present, with various tumor necrosis factor (TNF) inhibitors as well as the IL-17 inhibitor secukinumab (Cosentyx) the only approved biologic alternatives.
“This is the most impressive data I’ve seen that JAK inhibitors are effective in ankylosing spondyloarthritis,” commented Paul Emery, MD, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
TORTUGA was the first positive phase 2 trial of a selective JAK1 inhibitor in SpA. However, Dr. Kavanaugh noted that while a phase 2 trial of tofacitinib (Xeljanz) failed to meet its “very convoluted” primary endpoint, the JAK1/3 inhibitor was positive for key secondary endpoints, including favorable MRI changes. And a phase 3 trial of tofacitinib in SpA is underway.
A key remaining question pending the outcome of definitive phase 3 trials is whether specificity of JAK enzyme inhibition matters or if a class effect is at work, according to Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Certolizumab pegol
The TNF inhibitor is already approved for ankylosing spondylitis, among other indications, but it has now demonstrated efficacy and safety in nonradiographic SpA in a phase 3 trial structured with guidance from the Food and Drug Administration.
“It seems likely that UCB will get the indication for this,” according to Dr. Ruderman.
This 317-patient trial was remarkable in that it entailed a full 52 weeks of double-blind therapy with certolizumab at the standard dose of 200 mg every 2 weeks or placebo. The ASDAS Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% (ASAS 40) response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls in this trial, which was recently published (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866). All participants had to have baseline MRI evidence of sacroiliac joint inflammation and/or an elevated C-reactive protein.
By way of background, Dr. Ruderman explained that the FDA required 52 weeks of double-blind, placebo-controlled therapy because the agency’s advisory committee had formerly expressed reservations about considering an expanded indication for TNF inhibitors in nonradiographic as opposed to radiographic SpA.
“They were very concerned that approval could result in patients with mechanical back pain or fibromyalgia being treated with biologics. And they weren’t sure nonradiographic SpA was a discrete entity. They wondered if it remits on its own,” according to the rheumatologist.
Dr. Ruderman is curious to see how the FDA is going to handle this situation in light of the positive phase 3 certolizumab results. Will the agency require other companies that market TNF inhibitors to mount a similarly rigorous 52-week, double-blind, placebo-controlled trial in order to obtain an expanded indication? That would seem to pose ethical issues now. Or will the companies be able to gain an expanded indication by retrospective analysis of outcomes in patients with nonradiographic SpA in their existing trials databases? Stay tuned.
Ixekizumab
The COAST-V trial was a phase 3, randomized, double-blind, active- and placebo-controlled trial of 341 patients with radiographic SpA who hadn’t previously been treated with a biologic disease-modifying antirheumatic drug. They were assigned to 80 mg of ixekizumab every 2 weeks, 80 mg every 4 weeks, adalimumab (Humira) at 40 mg every 2 weeks, or placebo. The primary endpoint – an ASAS 40 response at week 16 – was achieved in 52% of patients on ixekizumab every 2 weeks, 48% of those who received ixekizumab every 4 weeks, 36% on adalimumab, and 18% on placebo (Lancet. 2018 Dec 8;392[10163]:2441-51).
In contrast, the phase 3 COAST-W trial included 316 patients with radiographic SpA who were inadequate responders or intolerant to one or more prior anti-TNF agents. The 16-week ASAS 40 response rate was 30.6% with ixekizumab every 2 weeks, similar at 25.4% with ixekizumab every 4 weeks, and 12.5% with placebo (Arthritis Rheumatol. 2018 Oct 20. doi: 10.1002/art.40753).
While the COAST-V trial convincingly showed both ixekizumab and adalimumab were more effective than placebo, the patient numbers were way too small to draw any conclusions about the relative efficacy of the two biologics, according to Dr. Ruderman.
“I don’t think these results are surprising,” Dr. Kavanaugh commented. “It would have been surprising if ixekizumab was ineffective, given that secukinumab works. But it’s nice to have the proof.”
Bimekizumab
This investigational dual IL-17A/F inhibitor demonstrated efficacy and safety for SpA in a 297-patient, phase 2b trial presented at the 2018 European Congress of Rheumatology.
“It’s effective, but it doesn’t look like it’s particularly more effective than either of the existing IL-17A inhibitors. We’ll see going forward if there truly is an advantage here to the additional inhibition of IL-17F in this population. I will say that the preclinical and laboratory data on the potential advantages of IL-17F inhibition are mostly in the psoriasis/psoriatic arthritis space. It’s not clear in ankylosing spondyloarthritis specifically whether we should expect to see a difference,” Dr. Ruderman said.
Ustekinumab
The IL-12/23 inhibitor proved no better than placebo in patients with SpA in three separate phase 3, randomized trials recently published as a single summary article (Arthritis Rheumatol. 2019 Feb;71[2]:258-70).
“Ustekinumab was effective in an earlier open study, so I think everybody was surprised by this,” Dr. Kavanaugh said.
He reported serving as a consultant to and/or receiving research funding from a dozen pharmaceutical companies. Dr. Ruderman reported financial relationships with eight companies.
MAUI, HAWAII – Arguably the most exciting therapeutic development in axial spondyloarthritis in the past year was the demonstrated efficacy and safety of the investigational oral selective Janus kinase 1 inhibitor filgotinib in the setting of active ankylosing spondylitis, speakers agreed at the 2019 Rheumatology Winter Clinical Symposium.
“This is big, big news,” commented symposium director Arthur Kavanaugh, MD. “This is going to be a big deal.”
Other recent clinical trials of note in axial spondyloarthritis (SpA) highlighted by Dr. Kavanaugh and Eric Ruderman, MD, professor of medicine at Northwestern University, Chicago, included a positive phase 3 study of certolizumab pegol (Cimzia) in nonradiographic SpA, two positive phase 3 trials of the interleukin-17A antagonist ixekizumab (Taltz) in radiographic SpA, a positive phase 2b trial of the dual IL-17A/F antagonist bimekizumab, and publication of three surprisingly negative phase 3 trials of the IL-12/23 inhibitor ustekinumab (Stelara).
Filgotinib
TORTUGA was a phase 2b, double-blind, multicenter trial of 116 European patients with active ankylosing spondylitis nonresponsive to NSAIDs who were randomized to oral filgotinib at 200 mg once daily or placebo for 12 weeks. Filgotinib reduced the Ankylosing Spondylitis Disease Activity Score (ASDAS) by a mean of 1.47 points from baseline, a significantly better result for the primary outcome than the 0.57-point decrease in controls (Lancet. 2018 Dec 1;392[10162]:2378-87).
Dr. Ruderman was also favorably impressed with the oral Janus kinase 1 (JAK1) inhibitor’s performance on the secondary outcome measures, including a mean 2.41-point reduction from baseline on the Bath Ankylosing Spondylitis Disease Activity Index, compared with a 1.44-point decrease in controls, with the difference being significant from week 8 onward. The filgotinib group also did significantly better on validated measures of physical function, spinal mobility, physical function, quality of life, peripheral arthritis, fatigue, and spinal and sacroiliac joint inflammation as assessed by MRI.
One patient in the filgotinib group, a smoker, developed pneumonia and another experienced deep venous thrombosis.
The study results are an exciting development because SpA treatments with new mechanisms of action are sorely needed. NSAIDs are considered first-line pharmacotherapy at present, with various tumor necrosis factor (TNF) inhibitors as well as the IL-17 inhibitor secukinumab (Cosentyx) the only approved biologic alternatives.
“This is the most impressive data I’ve seen that JAK inhibitors are effective in ankylosing spondyloarthritis,” commented Paul Emery, MD, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
TORTUGA was the first positive phase 2 trial of a selective JAK1 inhibitor in SpA. However, Dr. Kavanaugh noted that while a phase 2 trial of tofacitinib (Xeljanz) failed to meet its “very convoluted” primary endpoint, the JAK1/3 inhibitor was positive for key secondary endpoints, including favorable MRI changes. And a phase 3 trial of tofacitinib in SpA is underway.
A key remaining question pending the outcome of definitive phase 3 trials is whether specificity of JAK enzyme inhibition matters or if a class effect is at work, according to Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Certolizumab pegol
The TNF inhibitor is already approved for ankylosing spondylitis, among other indications, but it has now demonstrated efficacy and safety in nonradiographic SpA in a phase 3 trial structured with guidance from the Food and Drug Administration.
“It seems likely that UCB will get the indication for this,” according to Dr. Ruderman.
This 317-patient trial was remarkable in that it entailed a full 52 weeks of double-blind therapy with certolizumab at the standard dose of 200 mg every 2 weeks or placebo. The ASDAS Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% (ASAS 40) response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls in this trial, which was recently published (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866). All participants had to have baseline MRI evidence of sacroiliac joint inflammation and/or an elevated C-reactive protein.
By way of background, Dr. Ruderman explained that the FDA required 52 weeks of double-blind, placebo-controlled therapy because the agency’s advisory committee had formerly expressed reservations about considering an expanded indication for TNF inhibitors in nonradiographic as opposed to radiographic SpA.
“They were very concerned that approval could result in patients with mechanical back pain or fibromyalgia being treated with biologics. And they weren’t sure nonradiographic SpA was a discrete entity. They wondered if it remits on its own,” according to the rheumatologist.
Dr. Ruderman is curious to see how the FDA is going to handle this situation in light of the positive phase 3 certolizumab results. Will the agency require other companies that market TNF inhibitors to mount a similarly rigorous 52-week, double-blind, placebo-controlled trial in order to obtain an expanded indication? That would seem to pose ethical issues now. Or will the companies be able to gain an expanded indication by retrospective analysis of outcomes in patients with nonradiographic SpA in their existing trials databases? Stay tuned.
Ixekizumab
The COAST-V trial was a phase 3, randomized, double-blind, active- and placebo-controlled trial of 341 patients with radiographic SpA who hadn’t previously been treated with a biologic disease-modifying antirheumatic drug. They were assigned to 80 mg of ixekizumab every 2 weeks, 80 mg every 4 weeks, adalimumab (Humira) at 40 mg every 2 weeks, or placebo. The primary endpoint – an ASAS 40 response at week 16 – was achieved in 52% of patients on ixekizumab every 2 weeks, 48% of those who received ixekizumab every 4 weeks, 36% on adalimumab, and 18% on placebo (Lancet. 2018 Dec 8;392[10163]:2441-51).
In contrast, the phase 3 COAST-W trial included 316 patients with radiographic SpA who were inadequate responders or intolerant to one or more prior anti-TNF agents. The 16-week ASAS 40 response rate was 30.6% with ixekizumab every 2 weeks, similar at 25.4% with ixekizumab every 4 weeks, and 12.5% with placebo (Arthritis Rheumatol. 2018 Oct 20. doi: 10.1002/art.40753).
While the COAST-V trial convincingly showed both ixekizumab and adalimumab were more effective than placebo, the patient numbers were way too small to draw any conclusions about the relative efficacy of the two biologics, according to Dr. Ruderman.
“I don’t think these results are surprising,” Dr. Kavanaugh commented. “It would have been surprising if ixekizumab was ineffective, given that secukinumab works. But it’s nice to have the proof.”
Bimekizumab
This investigational dual IL-17A/F inhibitor demonstrated efficacy and safety for SpA in a 297-patient, phase 2b trial presented at the 2018 European Congress of Rheumatology.
“It’s effective, but it doesn’t look like it’s particularly more effective than either of the existing IL-17A inhibitors. We’ll see going forward if there truly is an advantage here to the additional inhibition of IL-17F in this population. I will say that the preclinical and laboratory data on the potential advantages of IL-17F inhibition are mostly in the psoriasis/psoriatic arthritis space. It’s not clear in ankylosing spondyloarthritis specifically whether we should expect to see a difference,” Dr. Ruderman said.
Ustekinumab
The IL-12/23 inhibitor proved no better than placebo in patients with SpA in three separate phase 3, randomized trials recently published as a single summary article (Arthritis Rheumatol. 2019 Feb;71[2]:258-70).
“Ustekinumab was effective in an earlier open study, so I think everybody was surprised by this,” Dr. Kavanaugh said.
He reported serving as a consultant to and/or receiving research funding from a dozen pharmaceutical companies. Dr. Ruderman reported financial relationships with eight companies.
REPORTING FROM RWCS 2019
Venous thromboembolism risk elevated in ankylosing spondylitis patients
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Newly diagnosed AS patients demonstrated increased risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, compared with controls.
Major finding: The relative risk for deep vein thrombosis was 63% higher for AS patients versus controls, but a 39% higher risk of pulmonary embolism did not reach statistical significance.
Study details: A population-based study including 7,190 incident AS cases and 71,900 matched controls from a health care database of residents of British Columbia.
Disclosures: The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
Source: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.