User login
CDC encourages improved antibiotic stewardship
The Centers for Disease Control and Prevention is highlighting a series of new and ongoing initiatives to combat antibiotic resistance this week, officially designated “Get Smart About Antibiotics Week” by President Barack Obama.
The CDC estimates that 2 million Americans are infected with an antibiotic-resistant bacteria annually and that 23,000 of those patients die. The agency has made combating antibiotic resistance a top priority.
“Antibiotic resistance is one of the deadliest health threats facing the world,” CDC Director Tom Frieden said in a statement. “These pledges will help protect the antibiotics we have so we can use these miracle drugs to save lives for years to come.”
The CDC is tracking antibiotic use and the spread of antibiotic-resistant infections in the United States and exploring new ways to stop the rise of antibiotic resistance. The agency is using data to identify hotspots in need of attention, and the CDC-run isolate bank assists industry in developing new antibiotics and rapid diagnostic tests, contributing to the global effort to combat antibiotic resistance.
The CDC is highlighting a number of projects this week to promote antibiotic stewardship:
Ascension Health launches stewardship initiative: Ascension, the largest nonprofit health system in the United States, is creating stewardship programs throughout its care sites. A new center of excellence will focus on antimicrobial stewardship efforts system-wide.
Hospital Corporation of America: HCA joined with the CDC to track antibiotic prescriptions in HCA facilities by automatically collecting and reporting monthly antibiotic-use data using CDC’s National Healthcare Safety Network. Data can be analyzed and fed back to caregivers to guide patient-care decisions.
Premier Safety Institute: First launch of a collaborative of more than 50 hospitals working to implement CDC Core Elements of an Antibiotic Stewardship Programs as well as reducing the overuse of specific antimicrobials that were identified in research conducted by Premier and in collaboration with the CDC.
Walmart public service announcement (PSA) on appropriate antibiotic use: Walmart created educational videos for checkout lines across the country so that customers get clear information on antibiotic resistance and what they can do to improve antibiotic use.
Major airlines run in-flight PSA: State health departments are partnering to improve educational awareness about antibiotic stewardship. An in-flight PSA about antibiotic stewardship, produced by the Michigan Antibiotic Resistance Reduction (MARR) Coalition, is now featured on Jet Blue and other airlines.
Pew Charitable Trust briefing on Capitol Hill: A Pew coalition of “Supermoms against superbugs” will join the Pew Charitable Trust and CDC director Dr. Frieden at a Capitol Hill briefing on Nov. 18, 2015. Pew also is partnering with the CDC to establish national targets to improve the use of antibiotics in support of the goals outlined in the National Action Plan on Combating Antibiotic-Resistant Bacteria.
Consumer Reports: The CDC is partnering with Consumer Reports and the American Board of Internal Medicine Foundation in support of the Choosing Wisely campaign.
U.S. State Department toolkit: The State Department is piloting a toolkit for use by 10 U.S. embassies with a focus on improving antibiotic use.
Society for Hospital Medicine’s educational campaign for hospitalists: The society’s antibiotic stewardship campaign targets hospitalists – an important group for improving antibiotic use.
Global Twitter chat: Hosted by the European Union’s Antibiotic Awareness Day (Nov. 18, 2015), the 24-hour chat will use the hashtag #antibioticresistance and unite CDC experts and partners in a global conversation about antibiotic resistance. CDC experts will lead the conversation from 2 p.m. to 4 p.m. ET.
The latest U.S. antibiotic prescribing rate map: Although overuse of antibiotics is happening in every state across the country, community antibiotic prescribing rates in some states are two times greater than in other states, suggesting opportunities for improvement.
U.S. hospital stewardship programs map: The percentage of hospitals reported by states as having antibiotic stewardship programs that follow all seven of CDC Core Elements of Antibiotic Stewardship Programs varies from state to state from a low of 7% to a high of 58%. The national goal is for 100% of all U.S. hospitals to have antibiotic stewardship programs by 2020.
On Twitter @richpizzi
The Centers for Disease Control and Prevention is highlighting a series of new and ongoing initiatives to combat antibiotic resistance this week, officially designated “Get Smart About Antibiotics Week” by President Barack Obama.
The CDC estimates that 2 million Americans are infected with an antibiotic-resistant bacteria annually and that 23,000 of those patients die. The agency has made combating antibiotic resistance a top priority.
“Antibiotic resistance is one of the deadliest health threats facing the world,” CDC Director Tom Frieden said in a statement. “These pledges will help protect the antibiotics we have so we can use these miracle drugs to save lives for years to come.”
The CDC is tracking antibiotic use and the spread of antibiotic-resistant infections in the United States and exploring new ways to stop the rise of antibiotic resistance. The agency is using data to identify hotspots in need of attention, and the CDC-run isolate bank assists industry in developing new antibiotics and rapid diagnostic tests, contributing to the global effort to combat antibiotic resistance.
The CDC is highlighting a number of projects this week to promote antibiotic stewardship:
Ascension Health launches stewardship initiative: Ascension, the largest nonprofit health system in the United States, is creating stewardship programs throughout its care sites. A new center of excellence will focus on antimicrobial stewardship efforts system-wide.
Hospital Corporation of America: HCA joined with the CDC to track antibiotic prescriptions in HCA facilities by automatically collecting and reporting monthly antibiotic-use data using CDC’s National Healthcare Safety Network. Data can be analyzed and fed back to caregivers to guide patient-care decisions.
Premier Safety Institute: First launch of a collaborative of more than 50 hospitals working to implement CDC Core Elements of an Antibiotic Stewardship Programs as well as reducing the overuse of specific antimicrobials that were identified in research conducted by Premier and in collaboration with the CDC.
Walmart public service announcement (PSA) on appropriate antibiotic use: Walmart created educational videos for checkout lines across the country so that customers get clear information on antibiotic resistance and what they can do to improve antibiotic use.
Major airlines run in-flight PSA: State health departments are partnering to improve educational awareness about antibiotic stewardship. An in-flight PSA about antibiotic stewardship, produced by the Michigan Antibiotic Resistance Reduction (MARR) Coalition, is now featured on Jet Blue and other airlines.
Pew Charitable Trust briefing on Capitol Hill: A Pew coalition of “Supermoms against superbugs” will join the Pew Charitable Trust and CDC director Dr. Frieden at a Capitol Hill briefing on Nov. 18, 2015. Pew also is partnering with the CDC to establish national targets to improve the use of antibiotics in support of the goals outlined in the National Action Plan on Combating Antibiotic-Resistant Bacteria.
Consumer Reports: The CDC is partnering with Consumer Reports and the American Board of Internal Medicine Foundation in support of the Choosing Wisely campaign.
U.S. State Department toolkit: The State Department is piloting a toolkit for use by 10 U.S. embassies with a focus on improving antibiotic use.
Society for Hospital Medicine’s educational campaign for hospitalists: The society’s antibiotic stewardship campaign targets hospitalists – an important group for improving antibiotic use.
Global Twitter chat: Hosted by the European Union’s Antibiotic Awareness Day (Nov. 18, 2015), the 24-hour chat will use the hashtag #antibioticresistance and unite CDC experts and partners in a global conversation about antibiotic resistance. CDC experts will lead the conversation from 2 p.m. to 4 p.m. ET.
The latest U.S. antibiotic prescribing rate map: Although overuse of antibiotics is happening in every state across the country, community antibiotic prescribing rates in some states are two times greater than in other states, suggesting opportunities for improvement.
U.S. hospital stewardship programs map: The percentage of hospitals reported by states as having antibiotic stewardship programs that follow all seven of CDC Core Elements of Antibiotic Stewardship Programs varies from state to state from a low of 7% to a high of 58%. The national goal is for 100% of all U.S. hospitals to have antibiotic stewardship programs by 2020.
On Twitter @richpizzi
The Centers for Disease Control and Prevention is highlighting a series of new and ongoing initiatives to combat antibiotic resistance this week, officially designated “Get Smart About Antibiotics Week” by President Barack Obama.
The CDC estimates that 2 million Americans are infected with an antibiotic-resistant bacteria annually and that 23,000 of those patients die. The agency has made combating antibiotic resistance a top priority.
“Antibiotic resistance is one of the deadliest health threats facing the world,” CDC Director Tom Frieden said in a statement. “These pledges will help protect the antibiotics we have so we can use these miracle drugs to save lives for years to come.”
The CDC is tracking antibiotic use and the spread of antibiotic-resistant infections in the United States and exploring new ways to stop the rise of antibiotic resistance. The agency is using data to identify hotspots in need of attention, and the CDC-run isolate bank assists industry in developing new antibiotics and rapid diagnostic tests, contributing to the global effort to combat antibiotic resistance.
The CDC is highlighting a number of projects this week to promote antibiotic stewardship:
Ascension Health launches stewardship initiative: Ascension, the largest nonprofit health system in the United States, is creating stewardship programs throughout its care sites. A new center of excellence will focus on antimicrobial stewardship efforts system-wide.
Hospital Corporation of America: HCA joined with the CDC to track antibiotic prescriptions in HCA facilities by automatically collecting and reporting monthly antibiotic-use data using CDC’s National Healthcare Safety Network. Data can be analyzed and fed back to caregivers to guide patient-care decisions.
Premier Safety Institute: First launch of a collaborative of more than 50 hospitals working to implement CDC Core Elements of an Antibiotic Stewardship Programs as well as reducing the overuse of specific antimicrobials that were identified in research conducted by Premier and in collaboration with the CDC.
Walmart public service announcement (PSA) on appropriate antibiotic use: Walmart created educational videos for checkout lines across the country so that customers get clear information on antibiotic resistance and what they can do to improve antibiotic use.
Major airlines run in-flight PSA: State health departments are partnering to improve educational awareness about antibiotic stewardship. An in-flight PSA about antibiotic stewardship, produced by the Michigan Antibiotic Resistance Reduction (MARR) Coalition, is now featured on Jet Blue and other airlines.
Pew Charitable Trust briefing on Capitol Hill: A Pew coalition of “Supermoms against superbugs” will join the Pew Charitable Trust and CDC director Dr. Frieden at a Capitol Hill briefing on Nov. 18, 2015. Pew also is partnering with the CDC to establish national targets to improve the use of antibiotics in support of the goals outlined in the National Action Plan on Combating Antibiotic-Resistant Bacteria.
Consumer Reports: The CDC is partnering with Consumer Reports and the American Board of Internal Medicine Foundation in support of the Choosing Wisely campaign.
U.S. State Department toolkit: The State Department is piloting a toolkit for use by 10 U.S. embassies with a focus on improving antibiotic use.
Society for Hospital Medicine’s educational campaign for hospitalists: The society’s antibiotic stewardship campaign targets hospitalists – an important group for improving antibiotic use.
Global Twitter chat: Hosted by the European Union’s Antibiotic Awareness Day (Nov. 18, 2015), the 24-hour chat will use the hashtag #antibioticresistance and unite CDC experts and partners in a global conversation about antibiotic resistance. CDC experts will lead the conversation from 2 p.m. to 4 p.m. ET.
The latest U.S. antibiotic prescribing rate map: Although overuse of antibiotics is happening in every state across the country, community antibiotic prescribing rates in some states are two times greater than in other states, suggesting opportunities for improvement.
U.S. hospital stewardship programs map: The percentage of hospitals reported by states as having antibiotic stewardship programs that follow all seven of CDC Core Elements of Antibiotic Stewardship Programs varies from state to state from a low of 7% to a high of 58%. The national goal is for 100% of all U.S. hospitals to have antibiotic stewardship programs by 2020.
On Twitter @richpizzi
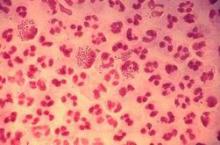
Resistant gonorrhea rates high in China, raise concerns in U.S.
SAN DIEGO – Experts in the field of Neisseria gonorrhoeae antimicrobial resistance are warily watching developments in China, where rates of nonsusceptibility to ceftriaxone are reportedly shooting through the roof.
In the United States at present, gonococcal resistance to ceftriaxone is exceedingly rare: well under 1% of isolates have elevated minimum inhibitory concentrations (MICs) to this cornerstone of empiric therapy. In Europe, the rate is about 3%. What’s really disturbing is that recent data from the World Health Organization Gonococcal Antimicrobial Surveillance Program indicate ceftriaxone nonsusceptibility rates in China are in the 20%-30%-plus range, Dr. Joseph Duncan said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is very concerning for the potential spread of this type of resistance across the world at some point. It’s just ready to explode,” observed Dr. Duncan of the University of North Carolina, Chapel Hill.
Anytime gonococcal resistance rates to a drug reach about 5%, it spells trouble.
“Gonorrhea treatment is often syndromic. An isolated swab is taken, identified by Gram stain, you give them empiric therapy, and the patients walk out of the clinic and never come back. So you don’t want to have a 5% failure rate for your initial therapy,” he explained.
Neisseria gonorrhoeae has acquired resistance to virtually every antibiotic ever used to treat the infection. Treatment options are running out, which is why the Centers for Disease Control and Prevention has designated gonorrhea as an immediate public health threat requiring urgent and aggressive action.
The 2015 CDC Sexually Transmitted Disease Treatment Guidelines recommend only one front-line treatment for gonorrhea: dual therapy with ceftriaxone at 250 mg IM in a single dose plus azithromycin 1 g orally, also in a single dose. Given N. gonorrhoeae’s adaptability, experts believe that’s not a long-term solution.
After all, 5%-10% of gonococcal isolates in Europe and China are azithromycin-resistant, and while CDC surveillance data showed less than 1% of strains in the United States were azithromycin-resistant through 2013, preliminary data for 2014 show a concerning jump to 2.5%, according to Dr. Duncan’s fellow panelist Dr. Kimberly Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 CDC STD guidelines.
Dr. Duncan described the state of the ongoing aggressive multipronged attempts to combat antimicrobial resistance in N. gonorrhoeae. This effort includes new antibiotics in the developmental pipeline, attempts to repurpose existing antibiotics, rapid point-of-care antibiotic susceptibility tests, and vaccine development.
He also highlighted the factors that have led to the bug’s capacity to acquire resistance to so many antibiotics having different mechanisms of action. One key factor is the organism’s sheer competence as expressed in genetic lability.
“This bacteria is continually sampling DNA from the environment. The organisms are constantly swapping genes and DNA segments with commensal Neisseria species. In fact, even human DNA sequences have been found inside the gonococcal genome,” Dr. Duncan said.
While high-level antibiotic resistance can be acquired in a single dramatic step, it can also come about through multiple smaller steps, each one yielding only low-level resistance. “This allows for the sort of creeping [minimum inhibitory concentration] that we’ve seen with the gonococcus, particularly with the extended-spectrum cephalosporin antibiotics,” Dr. Duncan continued.
Also, it’s apparent that even in the absence of antibiotics, other pressures can select for antibiotic-resistant strains. Dr. Duncan credited one of his mentors, William M. Shafer, Ph.D., of Emory University, with developing the hypothesis that fecal lipids might be an important driver of increased resistance rates. Supporting this hypothesis, he noted, is the finding in multiple studies that rectal gonococcal isolates consistently have a somewhat higher prevalence of resistance than those obtained from other sites.
Turning to the effort to curb antibiotic resistance, Dr. Duncan said the drug farthest along in the pipeline is solithromycin, a novel macrolide being developed by Cempra Pharmaceuticals in both oral and IV formulations. Solithromycin, a first-in-class fluoroketolide, is active against a broad range of Gram-positive organisms, among them Legionella, Chlamydia, Mycoplasma, and Ureaplasma, including macrolide-resistant strains. Its ability to bind to three ribosomal sites is thought to minimize development of resistance.
Two positive phase 3 clinical trials of solithromycin have been completed in patients with community-acquired bacterial pneumonia. A phase 3 trial in gonorrhea is ongoing after a positive phase 2 study.
Two bacterial topoisomerase inhibitors are in soon-to-be-completed phase 2 studies.
Delafloxacin, a new fluoroquinolone, was in a phase 3 clinical trial for gonorrhea that was terminated early due to ineffectiveness.
There is considerable interest in bringing back single-dose IM gentamicin (Garamycin) for the treatment of gonorrhea. A systematic review of five studies (Syst Rev. 2014 Sep 19;3:104. doi: 10.1186/2046-4053-3-104) reported cure rates that were mostly in the 90% range, but “not quite high enough to say you’d want to go to gentamicin as your front-line therapy,” in Dr. Duncan’s opinion.
Dr. Workowski noted that the National Institutes of Health is interested in trying to resurrect cefixime (Suprax) for gonorrhea after the CDC guidelines demoted it several years ago. An ongoing clinical trial is examining the impact of increasing the dose and duration of therapy.
Public health officials are also interested in spectinomycin, an antibiotic not currently available in the United States, which has achieved high cure rates as single-dose therapy in several foreign studies.
Dr. Duncan noted that a randomized trial of gentamicin plus azithromycin or gemifloxacin (Factive) plus azithromycin reported cure rates of 100% and 99.5%, respectively (Clin Infect Dis. 2014 Oct 15;59(8):1083-91). He found the study less than convincing, however: “These strains were almost all susceptibile to azithromycin, so you can’t actually say that gentamicin or gemifloxacin added a lot,” he said.
A number of researchers are working on rapid tests for susceptibility of a particular gonococcal strain so physicians could choose an effective antimicrobial for an individual patient right from the start.
“These are all in the developmental phase. It’s going to be difficult, because there are so many different mechanisms for resistance,” he said.
Similarly, vaccine development in the gonococcal field is a great challenge. The microorganism’s multiple mechanisms of immune evasion make it tough to identify correlates of protection, Dr. Duncan continued.
“Epidemiologic studies suggest there is not efficient development of natural immunity. Folks who get gonorrhea and then come back with reinfection often have the exact same strain,” he noted.
Dr. Duncan’s research is funded by the National Institutes of Health and the Burroughs Wellcome Fund. He reported having no financial conflicts of interest.
SAN DIEGO – Experts in the field of Neisseria gonorrhoeae antimicrobial resistance are warily watching developments in China, where rates of nonsusceptibility to ceftriaxone are reportedly shooting through the roof.
In the United States at present, gonococcal resistance to ceftriaxone is exceedingly rare: well under 1% of isolates have elevated minimum inhibitory concentrations (MICs) to this cornerstone of empiric therapy. In Europe, the rate is about 3%. What’s really disturbing is that recent data from the World Health Organization Gonococcal Antimicrobial Surveillance Program indicate ceftriaxone nonsusceptibility rates in China are in the 20%-30%-plus range, Dr. Joseph Duncan said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is very concerning for the potential spread of this type of resistance across the world at some point. It’s just ready to explode,” observed Dr. Duncan of the University of North Carolina, Chapel Hill.
Anytime gonococcal resistance rates to a drug reach about 5%, it spells trouble.
“Gonorrhea treatment is often syndromic. An isolated swab is taken, identified by Gram stain, you give them empiric therapy, and the patients walk out of the clinic and never come back. So you don’t want to have a 5% failure rate for your initial therapy,” he explained.
Neisseria gonorrhoeae has acquired resistance to virtually every antibiotic ever used to treat the infection. Treatment options are running out, which is why the Centers for Disease Control and Prevention has designated gonorrhea as an immediate public health threat requiring urgent and aggressive action.
The 2015 CDC Sexually Transmitted Disease Treatment Guidelines recommend only one front-line treatment for gonorrhea: dual therapy with ceftriaxone at 250 mg IM in a single dose plus azithromycin 1 g orally, also in a single dose. Given N. gonorrhoeae’s adaptability, experts believe that’s not a long-term solution.
After all, 5%-10% of gonococcal isolates in Europe and China are azithromycin-resistant, and while CDC surveillance data showed less than 1% of strains in the United States were azithromycin-resistant through 2013, preliminary data for 2014 show a concerning jump to 2.5%, according to Dr. Duncan’s fellow panelist Dr. Kimberly Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 CDC STD guidelines.
Dr. Duncan described the state of the ongoing aggressive multipronged attempts to combat antimicrobial resistance in N. gonorrhoeae. This effort includes new antibiotics in the developmental pipeline, attempts to repurpose existing antibiotics, rapid point-of-care antibiotic susceptibility tests, and vaccine development.
He also highlighted the factors that have led to the bug’s capacity to acquire resistance to so many antibiotics having different mechanisms of action. One key factor is the organism’s sheer competence as expressed in genetic lability.
“This bacteria is continually sampling DNA from the environment. The organisms are constantly swapping genes and DNA segments with commensal Neisseria species. In fact, even human DNA sequences have been found inside the gonococcal genome,” Dr. Duncan said.
While high-level antibiotic resistance can be acquired in a single dramatic step, it can also come about through multiple smaller steps, each one yielding only low-level resistance. “This allows for the sort of creeping [minimum inhibitory concentration] that we’ve seen with the gonococcus, particularly with the extended-spectrum cephalosporin antibiotics,” Dr. Duncan continued.
Also, it’s apparent that even in the absence of antibiotics, other pressures can select for antibiotic-resistant strains. Dr. Duncan credited one of his mentors, William M. Shafer, Ph.D., of Emory University, with developing the hypothesis that fecal lipids might be an important driver of increased resistance rates. Supporting this hypothesis, he noted, is the finding in multiple studies that rectal gonococcal isolates consistently have a somewhat higher prevalence of resistance than those obtained from other sites.
Turning to the effort to curb antibiotic resistance, Dr. Duncan said the drug farthest along in the pipeline is solithromycin, a novel macrolide being developed by Cempra Pharmaceuticals in both oral and IV formulations. Solithromycin, a first-in-class fluoroketolide, is active against a broad range of Gram-positive organisms, among them Legionella, Chlamydia, Mycoplasma, and Ureaplasma, including macrolide-resistant strains. Its ability to bind to three ribosomal sites is thought to minimize development of resistance.
Two positive phase 3 clinical trials of solithromycin have been completed in patients with community-acquired bacterial pneumonia. A phase 3 trial in gonorrhea is ongoing after a positive phase 2 study.
Two bacterial topoisomerase inhibitors are in soon-to-be-completed phase 2 studies.
Delafloxacin, a new fluoroquinolone, was in a phase 3 clinical trial for gonorrhea that was terminated early due to ineffectiveness.
There is considerable interest in bringing back single-dose IM gentamicin (Garamycin) for the treatment of gonorrhea. A systematic review of five studies (Syst Rev. 2014 Sep 19;3:104. doi: 10.1186/2046-4053-3-104) reported cure rates that were mostly in the 90% range, but “not quite high enough to say you’d want to go to gentamicin as your front-line therapy,” in Dr. Duncan’s opinion.
Dr. Workowski noted that the National Institutes of Health is interested in trying to resurrect cefixime (Suprax) for gonorrhea after the CDC guidelines demoted it several years ago. An ongoing clinical trial is examining the impact of increasing the dose and duration of therapy.
Public health officials are also interested in spectinomycin, an antibiotic not currently available in the United States, which has achieved high cure rates as single-dose therapy in several foreign studies.
Dr. Duncan noted that a randomized trial of gentamicin plus azithromycin or gemifloxacin (Factive) plus azithromycin reported cure rates of 100% and 99.5%, respectively (Clin Infect Dis. 2014 Oct 15;59(8):1083-91). He found the study less than convincing, however: “These strains were almost all susceptibile to azithromycin, so you can’t actually say that gentamicin or gemifloxacin added a lot,” he said.
A number of researchers are working on rapid tests for susceptibility of a particular gonococcal strain so physicians could choose an effective antimicrobial for an individual patient right from the start.
“These are all in the developmental phase. It’s going to be difficult, because there are so many different mechanisms for resistance,” he said.
Similarly, vaccine development in the gonococcal field is a great challenge. The microorganism’s multiple mechanisms of immune evasion make it tough to identify correlates of protection, Dr. Duncan continued.
“Epidemiologic studies suggest there is not efficient development of natural immunity. Folks who get gonorrhea and then come back with reinfection often have the exact same strain,” he noted.
Dr. Duncan’s research is funded by the National Institutes of Health and the Burroughs Wellcome Fund. He reported having no financial conflicts of interest.
SAN DIEGO – Experts in the field of Neisseria gonorrhoeae antimicrobial resistance are warily watching developments in China, where rates of nonsusceptibility to ceftriaxone are reportedly shooting through the roof.
In the United States at present, gonococcal resistance to ceftriaxone is exceedingly rare: well under 1% of isolates have elevated minimum inhibitory concentrations (MICs) to this cornerstone of empiric therapy. In Europe, the rate is about 3%. What’s really disturbing is that recent data from the World Health Organization Gonococcal Antimicrobial Surveillance Program indicate ceftriaxone nonsusceptibility rates in China are in the 20%-30%-plus range, Dr. Joseph Duncan said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is very concerning for the potential spread of this type of resistance across the world at some point. It’s just ready to explode,” observed Dr. Duncan of the University of North Carolina, Chapel Hill.
Anytime gonococcal resistance rates to a drug reach about 5%, it spells trouble.
“Gonorrhea treatment is often syndromic. An isolated swab is taken, identified by Gram stain, you give them empiric therapy, and the patients walk out of the clinic and never come back. So you don’t want to have a 5% failure rate for your initial therapy,” he explained.
Neisseria gonorrhoeae has acquired resistance to virtually every antibiotic ever used to treat the infection. Treatment options are running out, which is why the Centers for Disease Control and Prevention has designated gonorrhea as an immediate public health threat requiring urgent and aggressive action.
The 2015 CDC Sexually Transmitted Disease Treatment Guidelines recommend only one front-line treatment for gonorrhea: dual therapy with ceftriaxone at 250 mg IM in a single dose plus azithromycin 1 g orally, also in a single dose. Given N. gonorrhoeae’s adaptability, experts believe that’s not a long-term solution.
After all, 5%-10% of gonococcal isolates in Europe and China are azithromycin-resistant, and while CDC surveillance data showed less than 1% of strains in the United States were azithromycin-resistant through 2013, preliminary data for 2014 show a concerning jump to 2.5%, according to Dr. Duncan’s fellow panelist Dr. Kimberly Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 CDC STD guidelines.
Dr. Duncan described the state of the ongoing aggressive multipronged attempts to combat antimicrobial resistance in N. gonorrhoeae. This effort includes new antibiotics in the developmental pipeline, attempts to repurpose existing antibiotics, rapid point-of-care antibiotic susceptibility tests, and vaccine development.
He also highlighted the factors that have led to the bug’s capacity to acquire resistance to so many antibiotics having different mechanisms of action. One key factor is the organism’s sheer competence as expressed in genetic lability.
“This bacteria is continually sampling DNA from the environment. The organisms are constantly swapping genes and DNA segments with commensal Neisseria species. In fact, even human DNA sequences have been found inside the gonococcal genome,” Dr. Duncan said.
While high-level antibiotic resistance can be acquired in a single dramatic step, it can also come about through multiple smaller steps, each one yielding only low-level resistance. “This allows for the sort of creeping [minimum inhibitory concentration] that we’ve seen with the gonococcus, particularly with the extended-spectrum cephalosporin antibiotics,” Dr. Duncan continued.
Also, it’s apparent that even in the absence of antibiotics, other pressures can select for antibiotic-resistant strains. Dr. Duncan credited one of his mentors, William M. Shafer, Ph.D., of Emory University, with developing the hypothesis that fecal lipids might be an important driver of increased resistance rates. Supporting this hypothesis, he noted, is the finding in multiple studies that rectal gonococcal isolates consistently have a somewhat higher prevalence of resistance than those obtained from other sites.
Turning to the effort to curb antibiotic resistance, Dr. Duncan said the drug farthest along in the pipeline is solithromycin, a novel macrolide being developed by Cempra Pharmaceuticals in both oral and IV formulations. Solithromycin, a first-in-class fluoroketolide, is active against a broad range of Gram-positive organisms, among them Legionella, Chlamydia, Mycoplasma, and Ureaplasma, including macrolide-resistant strains. Its ability to bind to three ribosomal sites is thought to minimize development of resistance.
Two positive phase 3 clinical trials of solithromycin have been completed in patients with community-acquired bacterial pneumonia. A phase 3 trial in gonorrhea is ongoing after a positive phase 2 study.
Two bacterial topoisomerase inhibitors are in soon-to-be-completed phase 2 studies.
Delafloxacin, a new fluoroquinolone, was in a phase 3 clinical trial for gonorrhea that was terminated early due to ineffectiveness.
There is considerable interest in bringing back single-dose IM gentamicin (Garamycin) for the treatment of gonorrhea. A systematic review of five studies (Syst Rev. 2014 Sep 19;3:104. doi: 10.1186/2046-4053-3-104) reported cure rates that were mostly in the 90% range, but “not quite high enough to say you’d want to go to gentamicin as your front-line therapy,” in Dr. Duncan’s opinion.
Dr. Workowski noted that the National Institutes of Health is interested in trying to resurrect cefixime (Suprax) for gonorrhea after the CDC guidelines demoted it several years ago. An ongoing clinical trial is examining the impact of increasing the dose and duration of therapy.
Public health officials are also interested in spectinomycin, an antibiotic not currently available in the United States, which has achieved high cure rates as single-dose therapy in several foreign studies.
Dr. Duncan noted that a randomized trial of gentamicin plus azithromycin or gemifloxacin (Factive) plus azithromycin reported cure rates of 100% and 99.5%, respectively (Clin Infect Dis. 2014 Oct 15;59(8):1083-91). He found the study less than convincing, however: “These strains were almost all susceptibile to azithromycin, so you can’t actually say that gentamicin or gemifloxacin added a lot,” he said.
A number of researchers are working on rapid tests for susceptibility of a particular gonococcal strain so physicians could choose an effective antimicrobial for an individual patient right from the start.
“These are all in the developmental phase. It’s going to be difficult, because there are so many different mechanisms for resistance,” he said.
Similarly, vaccine development in the gonococcal field is a great challenge. The microorganism’s multiple mechanisms of immune evasion make it tough to identify correlates of protection, Dr. Duncan continued.
“Epidemiologic studies suggest there is not efficient development of natural immunity. Folks who get gonorrhea and then come back with reinfection often have the exact same strain,” he noted.
Dr. Duncan’s research is funded by the National Institutes of Health and the Burroughs Wellcome Fund. He reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM ICAAC 2015
Judicious antibiotic use key in ambulatory settings
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”
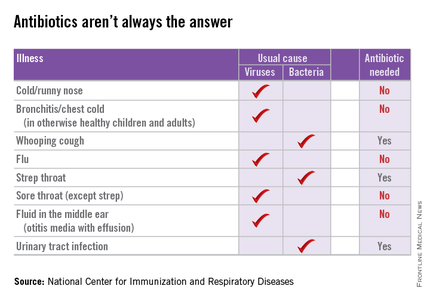
Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
Shared decision making reduces antibiotic use
Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use, according to a Cochrane review published Nov. 11.
“Shared decision making is … a set of communication and evidence-based practice skills that elicits patients’ expectations, clarifies any misperceptions, and discusses the best available evidence for benefits and harms of treatment,” wrote Peter Coxeter of the Centre for Research in Evidence-Based Practice at Bond University, Australia.
Dr. Coxeter and his coauthors analyzed 10 published reports from nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients, and found that shared decision making interventions were associated with a 39% overall reduction in antibiotic use (95% confidence interval, 0.55-0.68) within 6 weeks of the consultation, with a trend suggesting those reductions were maintained in the longer term.
The analysis also showed that this reduction did not lead to an increase in patient-initiated reconsultations or a decrease in patient satisfaction, although there were not enough data to determine the impact of these interventions on longer-term outcomes such as hospital admissions, pneumonia, or mortality (Cochrane Database Syst Rev. 2015 Nov 11. doi: 10.1002/14651858.CD010907.pub2).
“Further research should also aim to determine which aspects of these interventions provide the greatest benefit to adapt program implementation and uptake in diverse clinical settings,” the authors wrote.
The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use, according to a Cochrane review published Nov. 11.
“Shared decision making is … a set of communication and evidence-based practice skills that elicits patients’ expectations, clarifies any misperceptions, and discusses the best available evidence for benefits and harms of treatment,” wrote Peter Coxeter of the Centre for Research in Evidence-Based Practice at Bond University, Australia.
Dr. Coxeter and his coauthors analyzed 10 published reports from nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients, and found that shared decision making interventions were associated with a 39% overall reduction in antibiotic use (95% confidence interval, 0.55-0.68) within 6 weeks of the consultation, with a trend suggesting those reductions were maintained in the longer term.
The analysis also showed that this reduction did not lead to an increase in patient-initiated reconsultations or a decrease in patient satisfaction, although there were not enough data to determine the impact of these interventions on longer-term outcomes such as hospital admissions, pneumonia, or mortality (Cochrane Database Syst Rev. 2015 Nov 11. doi: 10.1002/14651858.CD010907.pub2).
“Further research should also aim to determine which aspects of these interventions provide the greatest benefit to adapt program implementation and uptake in diverse clinical settings,” the authors wrote.
The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use, according to a Cochrane review published Nov. 11.
“Shared decision making is … a set of communication and evidence-based practice skills that elicits patients’ expectations, clarifies any misperceptions, and discusses the best available evidence for benefits and harms of treatment,” wrote Peter Coxeter of the Centre for Research in Evidence-Based Practice at Bond University, Australia.
Dr. Coxeter and his coauthors analyzed 10 published reports from nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients, and found that shared decision making interventions were associated with a 39% overall reduction in antibiotic use (95% confidence interval, 0.55-0.68) within 6 weeks of the consultation, with a trend suggesting those reductions were maintained in the longer term.
The analysis also showed that this reduction did not lead to an increase in patient-initiated reconsultations or a decrease in patient satisfaction, although there were not enough data to determine the impact of these interventions on longer-term outcomes such as hospital admissions, pneumonia, or mortality (Cochrane Database Syst Rev. 2015 Nov 11. doi: 10.1002/14651858.CD010907.pub2).
“Further research should also aim to determine which aspects of these interventions provide the greatest benefit to adapt program implementation and uptake in diverse clinical settings,” the authors wrote.
The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point:Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use.
Major finding: Shared decision making interventions achieved an overall 39% reduction in antibiotic use in the 6 weeks after the consultation.
Data source: A review of nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients.
Disclosures: The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
New low-dose antibiotics, topicals offer options for acne
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Clinical Care Pathway for Cellulitis Can Help Reduce Antibiotic Use, Cost
Clinical question: How would an evidence-based clinical pathway for cellulitis affect process metrics, patient outcomes, and clinical cost?
Background: Cellulitis is a common hospital problem, but its evaluation and treatment vary widely. Specifically, broad-spectrum antibiotics and imaging studies are overutilized when compared to recommended guidelines. A standardized clinical pathway is proposed as a possible solution.
Study design: Retrospective, observational, pre-/post-intervention study.
Setting: University of Utah Health Sciences Center, Salt Lake City.
Synopsis: A multidisciplinary team created a guideline-based care pathway for cellulitis and enrolled 677 adult patients for retrospective analysis during a two-year period. The study showed an overall 59% decrease in the odds of ordering broad-spectrum antibiotics, 23% decrease in pharmacy cost, 44% decrease in laboratory cost, and 13% decrease in overall facility cost, pre-/post-intervention. It also demonstrated no adverse effect on length of stay or 30-day readmission rates.
Given the retrospective, single-center nature of this study, as well as some baseline characteristic differences between enrolled patients, careful conclusions regarding external validity on diverse patient populations must be considered; however, the history of clinical care pathways supports many of the study’s findings. The results make a compelling case for hospitalist groups to implement similar cellulitis pathways and research their effectiveness.
Bottom line: Clinical care pathways for cellulitis provide an opportunity to improve antibiotic stewardship and lower hospital costs without compromising quality of care.
Citation: Yarbrough PM, Kukhareva PV, Spivak ES, Hopkins C, Kawamoto K. Evidence-based care pathway for cellulitis improves process, clinical, and cost outcomes [published online ahead of print July 28, 2015]. J Hosp Med. doi: 10.1002/jhm.2433.
Clinical question: How would an evidence-based clinical pathway for cellulitis affect process metrics, patient outcomes, and clinical cost?
Background: Cellulitis is a common hospital problem, but its evaluation and treatment vary widely. Specifically, broad-spectrum antibiotics and imaging studies are overutilized when compared to recommended guidelines. A standardized clinical pathway is proposed as a possible solution.
Study design: Retrospective, observational, pre-/post-intervention study.
Setting: University of Utah Health Sciences Center, Salt Lake City.
Synopsis: A multidisciplinary team created a guideline-based care pathway for cellulitis and enrolled 677 adult patients for retrospective analysis during a two-year period. The study showed an overall 59% decrease in the odds of ordering broad-spectrum antibiotics, 23% decrease in pharmacy cost, 44% decrease in laboratory cost, and 13% decrease in overall facility cost, pre-/post-intervention. It also demonstrated no adverse effect on length of stay or 30-day readmission rates.
Given the retrospective, single-center nature of this study, as well as some baseline characteristic differences between enrolled patients, careful conclusions regarding external validity on diverse patient populations must be considered; however, the history of clinical care pathways supports many of the study’s findings. The results make a compelling case for hospitalist groups to implement similar cellulitis pathways and research their effectiveness.
Bottom line: Clinical care pathways for cellulitis provide an opportunity to improve antibiotic stewardship and lower hospital costs without compromising quality of care.
Citation: Yarbrough PM, Kukhareva PV, Spivak ES, Hopkins C, Kawamoto K. Evidence-based care pathway for cellulitis improves process, clinical, and cost outcomes [published online ahead of print July 28, 2015]. J Hosp Med. doi: 10.1002/jhm.2433.
Clinical question: How would an evidence-based clinical pathway for cellulitis affect process metrics, patient outcomes, and clinical cost?
Background: Cellulitis is a common hospital problem, but its evaluation and treatment vary widely. Specifically, broad-spectrum antibiotics and imaging studies are overutilized when compared to recommended guidelines. A standardized clinical pathway is proposed as a possible solution.
Study design: Retrospective, observational, pre-/post-intervention study.
Setting: University of Utah Health Sciences Center, Salt Lake City.
Synopsis: A multidisciplinary team created a guideline-based care pathway for cellulitis and enrolled 677 adult patients for retrospective analysis during a two-year period. The study showed an overall 59% decrease in the odds of ordering broad-spectrum antibiotics, 23% decrease in pharmacy cost, 44% decrease in laboratory cost, and 13% decrease in overall facility cost, pre-/post-intervention. It also demonstrated no adverse effect on length of stay or 30-day readmission rates.
Given the retrospective, single-center nature of this study, as well as some baseline characteristic differences between enrolled patients, careful conclusions regarding external validity on diverse patient populations must be considered; however, the history of clinical care pathways supports many of the study’s findings. The results make a compelling case for hospitalist groups to implement similar cellulitis pathways and research their effectiveness.
Bottom line: Clinical care pathways for cellulitis provide an opportunity to improve antibiotic stewardship and lower hospital costs without compromising quality of care.
Citation: Yarbrough PM, Kukhareva PV, Spivak ES, Hopkins C, Kawamoto K. Evidence-based care pathway for cellulitis improves process, clinical, and cost outcomes [published online ahead of print July 28, 2015]. J Hosp Med. doi: 10.1002/jhm.2433.
VIDEO: Gut microbiota may predict C. diff treatment response
HONOLULU – Could gut microbiota be a better predictor than clinical factors of a patient’s response to treatment for Clostridium difficile infection?
In a study of 88 patients with C. difficile, the overall treatment failure rate was 12.5% – but clinical factors such as age, sex, ongoing antibiotic exposure, and hospitalization status failed to predict which patients wouldn’t respond to treatment.
So, “we aimed to identify if there are any gut microbiota signatures to predict treatment response and treatment failure,” explained the study’s lead author, Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Khanna discussed the study results and why gut microbiota may be an effective predictor of treatment responders and nonresponders.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HONOLULU – Could gut microbiota be a better predictor than clinical factors of a patient’s response to treatment for Clostridium difficile infection?
In a study of 88 patients with C. difficile, the overall treatment failure rate was 12.5% – but clinical factors such as age, sex, ongoing antibiotic exposure, and hospitalization status failed to predict which patients wouldn’t respond to treatment.
So, “we aimed to identify if there are any gut microbiota signatures to predict treatment response and treatment failure,” explained the study’s lead author, Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Khanna discussed the study results and why gut microbiota may be an effective predictor of treatment responders and nonresponders.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HONOLULU – Could gut microbiota be a better predictor than clinical factors of a patient’s response to treatment for Clostridium difficile infection?
In a study of 88 patients with C. difficile, the overall treatment failure rate was 12.5% – but clinical factors such as age, sex, ongoing antibiotic exposure, and hospitalization status failed to predict which patients wouldn’t respond to treatment.
So, “we aimed to identify if there are any gut microbiota signatures to predict treatment response and treatment failure,” explained the study’s lead author, Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Khanna discussed the study results and why gut microbiota may be an effective predictor of treatment responders and nonresponders.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At ACG 2015
CHEST: Oral solithromycin shows pneumonia pivotal-trial efficacy
MONTREAL – A new, next-generation macrolide, solithromycin, showed safety and efficacy as a once-daily oral agent that was noninferior to the comparator oral antibiotic, the fluoroquinolone moxifloxacin, in a phase III trial.
Macrolide resistance among strains of Streptococcus pneumoniae that cause many U.S. cases of severe community-acquired pneumonia has become common, complicating treatment of this common infection with a macrolide, Dr. Carlos M. Barrera explained at the annual meeting of the American College of Chest Physicians.
The SOLITAIRE-ORAL (Efficacy and Safety Study of Oral Solithromycin [CEM-101] Compared to Oral Moxifloxacin in Treatment of Patients With Community-Acquired Bacterial Pneumonia) trial enrolled 860 patients with moderate to moderately severe community-acquired pneumonia.
About half of the patients enrolled in the trial underwent microbiologic assessment of their infecting pathogen, and about 40% of cases in each treatment arm had infections caused by S. pneumoniae. In this subgroup, the 5-day regimen of solithromycin tested in the study succeeded in clearing the infection in 89% of patients, comparable to the 83% success rate achieved with a 7-day course of moxifloxacin (Avelox), said Dr. Barrera, a pulmonologist who practices in Miami.
The study’s primary endpoint for Food and Drug Administration approval of solithromycin was early clinical response, defined as an improvement in at least two listed symptoms at 72 hours after onset of treatment. That endpoint occurred in 78% of patients enrolled in each of the two arms of the study.
The data make solithromycin look like a promising way to once again have a macrolide available for empiric oral treatment of more severe community-acquired pneumonia, pending full peer review of the data, commented Dr. Muthiah P. Muthiah, a pulmonologist at the University of Tennessee Health Science Center in Memphis.
“A couple of decades ago, you could comfortably treat a patient with severe community-acquired pneumonia with a macrolide, but you can’t do that anymore,” Dr. Muthiah said in an interview.
If the newly reported data on oral solithromycin hold up under further review, it would mean that solithromycin was as effective as a potent quinolone, which remains an effective monotherapy for community-acquired pneumonia in patients who do not require treatment in an intensive care unit, Dr. Muthiah noted.
A companion study, SOLITAIRE-IV, is a phase III pivotal trial assessing the safety and efficacy of solithromycin when begun intravenously for treating community-acquired pneumonia, followed by a switch to oral dosing, in comparison with intravenous followed by oral treatment with moxifloxacin.
Once those data are fully collected and analyzed, the company will submit the information from both trials to the FDA, said Dr. David Oldach, chief medical officer for Cempra.
Results from the intravenous trial, reported in a preliminary way by Cempra Oct. 16 in a press release, showed that the solithromycin treatment regimen tested in SOLITAIRE-IV met its noninferiority targets, compared with moxifloxacin. The safety results, however, showed that solithromycin produced a higher number of patients with a liver-enzyme elevation, compared with patients treated with moxifloxacin.
In SOLITAIRE-IV, Cempra reported that grade 3 increase in levels of alanine transaminase (ALT) occurred in 8% of patients on solithromycin and in 3% of patients on moxifloxacin. Grade 4 increases in ALT occurred in less than 1% of patients in both treatment arms.
In the current, orally administered trial, grade 3 ALT increases occurred in 5% of patients treated with solithromycin and in 2% of patients treated with moxifloxacin, Dr. Barrera reported. Grade 4 ALT increases occurred in 0.5% of patients treated with solithromycin and in 1.2% of those treated with moxifloxacin. No patients in either arm developed an elevation of both ALT and bilirubin, and the ALT increases seen were reversible and asymptomatic, Dr. Barrera said.
By other assessments, the safety profiles of solithromycin and moxifloxacin were similar: 7% of patients on solithromycin and 6% on moxifloxacin had a serious adverse event, and 4% of patients in each study arm discontinued treatment because of an adverse event.
SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
MONTREAL – A new, next-generation macrolide, solithromycin, showed safety and efficacy as a once-daily oral agent that was noninferior to the comparator oral antibiotic, the fluoroquinolone moxifloxacin, in a phase III trial.
Macrolide resistance among strains of Streptococcus pneumoniae that cause many U.S. cases of severe community-acquired pneumonia has become common, complicating treatment of this common infection with a macrolide, Dr. Carlos M. Barrera explained at the annual meeting of the American College of Chest Physicians.
The SOLITAIRE-ORAL (Efficacy and Safety Study of Oral Solithromycin [CEM-101] Compared to Oral Moxifloxacin in Treatment of Patients With Community-Acquired Bacterial Pneumonia) trial enrolled 860 patients with moderate to moderately severe community-acquired pneumonia.
About half of the patients enrolled in the trial underwent microbiologic assessment of their infecting pathogen, and about 40% of cases in each treatment arm had infections caused by S. pneumoniae. In this subgroup, the 5-day regimen of solithromycin tested in the study succeeded in clearing the infection in 89% of patients, comparable to the 83% success rate achieved with a 7-day course of moxifloxacin (Avelox), said Dr. Barrera, a pulmonologist who practices in Miami.
The study’s primary endpoint for Food and Drug Administration approval of solithromycin was early clinical response, defined as an improvement in at least two listed symptoms at 72 hours after onset of treatment. That endpoint occurred in 78% of patients enrolled in each of the two arms of the study.
The data make solithromycin look like a promising way to once again have a macrolide available for empiric oral treatment of more severe community-acquired pneumonia, pending full peer review of the data, commented Dr. Muthiah P. Muthiah, a pulmonologist at the University of Tennessee Health Science Center in Memphis.
“A couple of decades ago, you could comfortably treat a patient with severe community-acquired pneumonia with a macrolide, but you can’t do that anymore,” Dr. Muthiah said in an interview.
If the newly reported data on oral solithromycin hold up under further review, it would mean that solithromycin was as effective as a potent quinolone, which remains an effective monotherapy for community-acquired pneumonia in patients who do not require treatment in an intensive care unit, Dr. Muthiah noted.
A companion study, SOLITAIRE-IV, is a phase III pivotal trial assessing the safety and efficacy of solithromycin when begun intravenously for treating community-acquired pneumonia, followed by a switch to oral dosing, in comparison with intravenous followed by oral treatment with moxifloxacin.
Once those data are fully collected and analyzed, the company will submit the information from both trials to the FDA, said Dr. David Oldach, chief medical officer for Cempra.
Results from the intravenous trial, reported in a preliminary way by Cempra Oct. 16 in a press release, showed that the solithromycin treatment regimen tested in SOLITAIRE-IV met its noninferiority targets, compared with moxifloxacin. The safety results, however, showed that solithromycin produced a higher number of patients with a liver-enzyme elevation, compared with patients treated with moxifloxacin.
In SOLITAIRE-IV, Cempra reported that grade 3 increase in levels of alanine transaminase (ALT) occurred in 8% of patients on solithromycin and in 3% of patients on moxifloxacin. Grade 4 increases in ALT occurred in less than 1% of patients in both treatment arms.
In the current, orally administered trial, grade 3 ALT increases occurred in 5% of patients treated with solithromycin and in 2% of patients treated with moxifloxacin, Dr. Barrera reported. Grade 4 ALT increases occurred in 0.5% of patients treated with solithromycin and in 1.2% of those treated with moxifloxacin. No patients in either arm developed an elevation of both ALT and bilirubin, and the ALT increases seen were reversible and asymptomatic, Dr. Barrera said.
By other assessments, the safety profiles of solithromycin and moxifloxacin were similar: 7% of patients on solithromycin and 6% on moxifloxacin had a serious adverse event, and 4% of patients in each study arm discontinued treatment because of an adverse event.
SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
MONTREAL – A new, next-generation macrolide, solithromycin, showed safety and efficacy as a once-daily oral agent that was noninferior to the comparator oral antibiotic, the fluoroquinolone moxifloxacin, in a phase III trial.
Macrolide resistance among strains of Streptococcus pneumoniae that cause many U.S. cases of severe community-acquired pneumonia has become common, complicating treatment of this common infection with a macrolide, Dr. Carlos M. Barrera explained at the annual meeting of the American College of Chest Physicians.
The SOLITAIRE-ORAL (Efficacy and Safety Study of Oral Solithromycin [CEM-101] Compared to Oral Moxifloxacin in Treatment of Patients With Community-Acquired Bacterial Pneumonia) trial enrolled 860 patients with moderate to moderately severe community-acquired pneumonia.
About half of the patients enrolled in the trial underwent microbiologic assessment of their infecting pathogen, and about 40% of cases in each treatment arm had infections caused by S. pneumoniae. In this subgroup, the 5-day regimen of solithromycin tested in the study succeeded in clearing the infection in 89% of patients, comparable to the 83% success rate achieved with a 7-day course of moxifloxacin (Avelox), said Dr. Barrera, a pulmonologist who practices in Miami.
The study’s primary endpoint for Food and Drug Administration approval of solithromycin was early clinical response, defined as an improvement in at least two listed symptoms at 72 hours after onset of treatment. That endpoint occurred in 78% of patients enrolled in each of the two arms of the study.
The data make solithromycin look like a promising way to once again have a macrolide available for empiric oral treatment of more severe community-acquired pneumonia, pending full peer review of the data, commented Dr. Muthiah P. Muthiah, a pulmonologist at the University of Tennessee Health Science Center in Memphis.
“A couple of decades ago, you could comfortably treat a patient with severe community-acquired pneumonia with a macrolide, but you can’t do that anymore,” Dr. Muthiah said in an interview.
If the newly reported data on oral solithromycin hold up under further review, it would mean that solithromycin was as effective as a potent quinolone, which remains an effective monotherapy for community-acquired pneumonia in patients who do not require treatment in an intensive care unit, Dr. Muthiah noted.
A companion study, SOLITAIRE-IV, is a phase III pivotal trial assessing the safety and efficacy of solithromycin when begun intravenously for treating community-acquired pneumonia, followed by a switch to oral dosing, in comparison with intravenous followed by oral treatment with moxifloxacin.
Once those data are fully collected and analyzed, the company will submit the information from both trials to the FDA, said Dr. David Oldach, chief medical officer for Cempra.
Results from the intravenous trial, reported in a preliminary way by Cempra Oct. 16 in a press release, showed that the solithromycin treatment regimen tested in SOLITAIRE-IV met its noninferiority targets, compared with moxifloxacin. The safety results, however, showed that solithromycin produced a higher number of patients with a liver-enzyme elevation, compared with patients treated with moxifloxacin.
In SOLITAIRE-IV, Cempra reported that grade 3 increase in levels of alanine transaminase (ALT) occurred in 8% of patients on solithromycin and in 3% of patients on moxifloxacin. Grade 4 increases in ALT occurred in less than 1% of patients in both treatment arms.
In the current, orally administered trial, grade 3 ALT increases occurred in 5% of patients treated with solithromycin and in 2% of patients treated with moxifloxacin, Dr. Barrera reported. Grade 4 ALT increases occurred in 0.5% of patients treated with solithromycin and in 1.2% of those treated with moxifloxacin. No patients in either arm developed an elevation of both ALT and bilirubin, and the ALT increases seen were reversible and asymptomatic, Dr. Barrera said.
By other assessments, the safety profiles of solithromycin and moxifloxacin were similar: 7% of patients on solithromycin and 6% on moxifloxacin had a serious adverse event, and 4% of patients in each study arm discontinued treatment because of an adverse event.
SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT CHEST 2015
Key clinical point: A next-generation, orally administered macrolide, solithromycin, showed efficacy that was noninferior to moxifloxacin against moderate to moderately severe community-acquired pneumonia in a phase III pivotal trial.
Major finding: Both solithromycin and moxifloxacin produced a 78% early clinical response rate, the study’s primary endpoint for Food and Drug Administration approval.
Data source: SOLITAIRE-ORAL, a multicenter, international phase III trial involving 860 patients with community-acquired pneumonia.
Disclosures: SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
Experts debate fecal transplants as first-line therapy for CDI
SAN DIEGO – Fecal microbiota transplantation (FMT) is now first-line therapy for Clostridium difficile infection (CDI) in much of Scandinavia. At an annual conference on infectious diseases, two specialists debated whether that should be the case in the United States, too.
The epidemiology of CDI has changed greatly in the past decade, as other researchers have noted (Infect Drug Resist. 2014;7:63-72). Incidence, severity, and case-fatality rates have risen substantially, and individuals who lack the usual risk factors for CDI are now acquiring it in community settings. Moreover, CDI adds about 5-6 days to a patient’s average hospital stay, and almost one in three affected patients is rehospitalized (Am J Infect Control. 2015 Apr 1;43[4]:314-7.) – most often within a week of discharge, said Dr. Thomas Moore, who is at the University of Kansas, Wichita.
“Should FMT be used as first-line therapy? Not just yes, but hell, yes! It has superior efficacy,” Dr. Moore said.
He pointed to the recent landmark study (N Engl J Med. 2013;368:407-15) of CDI in which duodenal infusions of donor feces more than tripled the rates of relapse-free cure, compared with vancomycin monotherapy or vancomycin with bowel lavage (P less than .001 for both comparisons). Moreover, diarrhea resolved for 81% of patients after the first fecal infusion, and the observed superiority over the vancomycin regimens was so marked that investigators stopped the study after the interim analysis.
Evidence suggests FMT is safe as well as effective, said Dr. Moore. Centers in Norway, Sweden, Denmark, Finland, and Holland have treated at least 900 patients with no reported adverse effects and with cure rates of about 90%, he noted. Of more than 1,000 published FMT studies worldwide, there has been one only report of peritonitis after colonoscopy, one case of irritable bowel syndrome, three reports of mild enteritis, one case of upper gastrointestinal bleeding, one death from sepsis from a dislodged gastrostomy tube, and one case of new-onset obesity, which occurred after a patient received fecal microbiota donated by an obese relative, he added (Open Forum Infect Dis. 2015 Feb 1. doi: 10.1093/ofid/ofv004). Patients now receive fecal microbiota donations from normal-weight individuals, he noted (http://www.openbiome.org/stool-donation/).
“Fecal microbiota transplantation could save lives,” Dr. Moore concluded. Donor material is “cheap and unlimited, the procedure is cost-effective, easy to perform, can even be done at home, and patient satisfaction is very high.”
But Dr. Johan S. Bakken, an infectious disease specialist at St. Luke’s Hospital in Duluth, Minn., argued that FMT is not ready for first-line use for CDI in the United States. “There are no published FMT practice guidelines for initial therapy, even in Scandinavia, and no randomized controlled trials of FMT conducted anywhere,” said Dr. Bakken. He noted that because the Food and Drug Administration has not approved FMT for first-line use in CDI, utilization could require an approved Investigational New Drug application, leading to “unavoidable” treatment delays.
Clinicians also should not gloss over concerns about adverse effects with FMT, Dr. Bakken said. In addition to the case of new-onset obesity, there are risks of aspirating fecal material or perforating hollow viscera. Furthermore, the potential long-term adverse consequences of FMT are unknown, he said.
In contrast, the rate of resolution of initial CDI with per oral vancomycin or fidaxomicin is more than 80%, Dr. Bakken said. Moreover, liquid vancomycin at an appropriate dose and frequency for CDI costs about $4.25 per day in Duluth, he added. “No comparative outcomes data are available for FMT, but it is more costly than vancomycin, may not be locally available, and requires several days or weeks of planning,” he emphasized.
Cost-reimbursement issues with third-party payers are also likely with FMT, according to Dr. Bakken. “There also are potential or perceived medicolegal issues with FMT,” he said. “Keep in mind that about 80% of the world’s lawyers work and practice in the U.S.A.”
Dr. Moore had no disclosures. Dr. Bakken reported being an advisory board member of Rebiotix, which is developing a biologic drug to treat recurrent CDI.

|
| Dr. Christina Surawicz |
Fecal microbiota transplant is the best treatment for multiple recurrences of C. difficile infection that have not responded to standard therapy including a good pulse and taper course of vancomycin. There is such enthusiasm for FMT because of its simplicity and the ready availability of stool, which is a natural product. Why use an antibiotic to treat an illness that is usually a result of antibiotics? Is FMT therefore the best treatment for a primary infection of C. difficile infection? While treatment for recurrent C. difficile infection is supported by several randomized, controlled trials, including comparison with vancomycin, gut lavage, and sham colonoscopy, there are no randomized, controlled trials for FMT as a treatment of first episodes of CDI. Moreover, 80% of people with their first infection respond to standard antibiotic therapy. In addition, we are learning how important the microbiome is but we do not know the long-term consequences of FMT on an individual. I must agree with Dr. Bakken, it is not wise at this time to use FMT for first episodes of uncomplicated CDI.
Dr. Christina Surawicz is a professor of medicine at University of Washington, Seattle. She has no conflicts of interest.

|
| Dr. Christina Surawicz |
Fecal microbiota transplant is the best treatment for multiple recurrences of C. difficile infection that have not responded to standard therapy including a good pulse and taper course of vancomycin. There is such enthusiasm for FMT because of its simplicity and the ready availability of stool, which is a natural product. Why use an antibiotic to treat an illness that is usually a result of antibiotics? Is FMT therefore the best treatment for a primary infection of C. difficile infection? While treatment for recurrent C. difficile infection is supported by several randomized, controlled trials, including comparison with vancomycin, gut lavage, and sham colonoscopy, there are no randomized, controlled trials for FMT as a treatment of first episodes of CDI. Moreover, 80% of people with their first infection respond to standard antibiotic therapy. In addition, we are learning how important the microbiome is but we do not know the long-term consequences of FMT on an individual. I must agree with Dr. Bakken, it is not wise at this time to use FMT for first episodes of uncomplicated CDI.
Dr. Christina Surawicz is a professor of medicine at University of Washington, Seattle. She has no conflicts of interest.

|
| Dr. Christina Surawicz |
Fecal microbiota transplant is the best treatment for multiple recurrences of C. difficile infection that have not responded to standard therapy including a good pulse and taper course of vancomycin. There is such enthusiasm for FMT because of its simplicity and the ready availability of stool, which is a natural product. Why use an antibiotic to treat an illness that is usually a result of antibiotics? Is FMT therefore the best treatment for a primary infection of C. difficile infection? While treatment for recurrent C. difficile infection is supported by several randomized, controlled trials, including comparison with vancomycin, gut lavage, and sham colonoscopy, there are no randomized, controlled trials for FMT as a treatment of first episodes of CDI. Moreover, 80% of people with their first infection respond to standard antibiotic therapy. In addition, we are learning how important the microbiome is but we do not know the long-term consequences of FMT on an individual. I must agree with Dr. Bakken, it is not wise at this time to use FMT for first episodes of uncomplicated CDI.
Dr. Christina Surawicz is a professor of medicine at University of Washington, Seattle. She has no conflicts of interest.
SAN DIEGO – Fecal microbiota transplantation (FMT) is now first-line therapy for Clostridium difficile infection (CDI) in much of Scandinavia. At an annual conference on infectious diseases, two specialists debated whether that should be the case in the United States, too.
The epidemiology of CDI has changed greatly in the past decade, as other researchers have noted (Infect Drug Resist. 2014;7:63-72). Incidence, severity, and case-fatality rates have risen substantially, and individuals who lack the usual risk factors for CDI are now acquiring it in community settings. Moreover, CDI adds about 5-6 days to a patient’s average hospital stay, and almost one in three affected patients is rehospitalized (Am J Infect Control. 2015 Apr 1;43[4]:314-7.) – most often within a week of discharge, said Dr. Thomas Moore, who is at the University of Kansas, Wichita.
“Should FMT be used as first-line therapy? Not just yes, but hell, yes! It has superior efficacy,” Dr. Moore said.
He pointed to the recent landmark study (N Engl J Med. 2013;368:407-15) of CDI in which duodenal infusions of donor feces more than tripled the rates of relapse-free cure, compared with vancomycin monotherapy or vancomycin with bowel lavage (P less than .001 for both comparisons). Moreover, diarrhea resolved for 81% of patients after the first fecal infusion, and the observed superiority over the vancomycin regimens was so marked that investigators stopped the study after the interim analysis.
Evidence suggests FMT is safe as well as effective, said Dr. Moore. Centers in Norway, Sweden, Denmark, Finland, and Holland have treated at least 900 patients with no reported adverse effects and with cure rates of about 90%, he noted. Of more than 1,000 published FMT studies worldwide, there has been one only report of peritonitis after colonoscopy, one case of irritable bowel syndrome, three reports of mild enteritis, one case of upper gastrointestinal bleeding, one death from sepsis from a dislodged gastrostomy tube, and one case of new-onset obesity, which occurred after a patient received fecal microbiota donated by an obese relative, he added (Open Forum Infect Dis. 2015 Feb 1. doi: 10.1093/ofid/ofv004). Patients now receive fecal microbiota donations from normal-weight individuals, he noted (http://www.openbiome.org/stool-donation/).
“Fecal microbiota transplantation could save lives,” Dr. Moore concluded. Donor material is “cheap and unlimited, the procedure is cost-effective, easy to perform, can even be done at home, and patient satisfaction is very high.”
But Dr. Johan S. Bakken, an infectious disease specialist at St. Luke’s Hospital in Duluth, Minn., argued that FMT is not ready for first-line use for CDI in the United States. “There are no published FMT practice guidelines for initial therapy, even in Scandinavia, and no randomized controlled trials of FMT conducted anywhere,” said Dr. Bakken. He noted that because the Food and Drug Administration has not approved FMT for first-line use in CDI, utilization could require an approved Investigational New Drug application, leading to “unavoidable” treatment delays.
Clinicians also should not gloss over concerns about adverse effects with FMT, Dr. Bakken said. In addition to the case of new-onset obesity, there are risks of aspirating fecal material or perforating hollow viscera. Furthermore, the potential long-term adverse consequences of FMT are unknown, he said.
In contrast, the rate of resolution of initial CDI with per oral vancomycin or fidaxomicin is more than 80%, Dr. Bakken said. Moreover, liquid vancomycin at an appropriate dose and frequency for CDI costs about $4.25 per day in Duluth, he added. “No comparative outcomes data are available for FMT, but it is more costly than vancomycin, may not be locally available, and requires several days or weeks of planning,” he emphasized.
Cost-reimbursement issues with third-party payers are also likely with FMT, according to Dr. Bakken. “There also are potential or perceived medicolegal issues with FMT,” he said. “Keep in mind that about 80% of the world’s lawyers work and practice in the U.S.A.”
Dr. Moore had no disclosures. Dr. Bakken reported being an advisory board member of Rebiotix, which is developing a biologic drug to treat recurrent CDI.
SAN DIEGO – Fecal microbiota transplantation (FMT) is now first-line therapy for Clostridium difficile infection (CDI) in much of Scandinavia. At an annual conference on infectious diseases, two specialists debated whether that should be the case in the United States, too.
The epidemiology of CDI has changed greatly in the past decade, as other researchers have noted (Infect Drug Resist. 2014;7:63-72). Incidence, severity, and case-fatality rates have risen substantially, and individuals who lack the usual risk factors for CDI are now acquiring it in community settings. Moreover, CDI adds about 5-6 days to a patient’s average hospital stay, and almost one in three affected patients is rehospitalized (Am J Infect Control. 2015 Apr 1;43[4]:314-7.) – most often within a week of discharge, said Dr. Thomas Moore, who is at the University of Kansas, Wichita.
“Should FMT be used as first-line therapy? Not just yes, but hell, yes! It has superior efficacy,” Dr. Moore said.
He pointed to the recent landmark study (N Engl J Med. 2013;368:407-15) of CDI in which duodenal infusions of donor feces more than tripled the rates of relapse-free cure, compared with vancomycin monotherapy or vancomycin with bowel lavage (P less than .001 for both comparisons). Moreover, diarrhea resolved for 81% of patients after the first fecal infusion, and the observed superiority over the vancomycin regimens was so marked that investigators stopped the study after the interim analysis.
Evidence suggests FMT is safe as well as effective, said Dr. Moore. Centers in Norway, Sweden, Denmark, Finland, and Holland have treated at least 900 patients with no reported adverse effects and with cure rates of about 90%, he noted. Of more than 1,000 published FMT studies worldwide, there has been one only report of peritonitis after colonoscopy, one case of irritable bowel syndrome, three reports of mild enteritis, one case of upper gastrointestinal bleeding, one death from sepsis from a dislodged gastrostomy tube, and one case of new-onset obesity, which occurred after a patient received fecal microbiota donated by an obese relative, he added (Open Forum Infect Dis. 2015 Feb 1. doi: 10.1093/ofid/ofv004). Patients now receive fecal microbiota donations from normal-weight individuals, he noted (http://www.openbiome.org/stool-donation/).
“Fecal microbiota transplantation could save lives,” Dr. Moore concluded. Donor material is “cheap and unlimited, the procedure is cost-effective, easy to perform, can even be done at home, and patient satisfaction is very high.”
But Dr. Johan S. Bakken, an infectious disease specialist at St. Luke’s Hospital in Duluth, Minn., argued that FMT is not ready for first-line use for CDI in the United States. “There are no published FMT practice guidelines for initial therapy, even in Scandinavia, and no randomized controlled trials of FMT conducted anywhere,” said Dr. Bakken. He noted that because the Food and Drug Administration has not approved FMT for first-line use in CDI, utilization could require an approved Investigational New Drug application, leading to “unavoidable” treatment delays.
Clinicians also should not gloss over concerns about adverse effects with FMT, Dr. Bakken said. In addition to the case of new-onset obesity, there are risks of aspirating fecal material or perforating hollow viscera. Furthermore, the potential long-term adverse consequences of FMT are unknown, he said.
In contrast, the rate of resolution of initial CDI with per oral vancomycin or fidaxomicin is more than 80%, Dr. Bakken said. Moreover, liquid vancomycin at an appropriate dose and frequency for CDI costs about $4.25 per day in Duluth, he added. “No comparative outcomes data are available for FMT, but it is more costly than vancomycin, may not be locally available, and requires several days or weeks of planning,” he emphasized.
Cost-reimbursement issues with third-party payers are also likely with FMT, according to Dr. Bakken. “There also are potential or perceived medicolegal issues with FMT,” he said. “Keep in mind that about 80% of the world’s lawyers work and practice in the U.S.A.”
Dr. Moore had no disclosures. Dr. Bakken reported being an advisory board member of Rebiotix, which is developing a biologic drug to treat recurrent CDI.
EXPERT ANALYSIS AT IDWEEK 2015
IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
AT IDWEEK 2015
Key clinical point: A self-directed antibiotic time-out performed 72-96 hours into treatment was tied to increases in appropriate discontinuations of vancomycin.
Major finding: Discontinuation of vancomycin was higher than before the intervention (63% vs. 48%; P = .001).
Data source: Prospective single-center study.
Disclosures: CDC helped fund the study with a grant to the University of Utah. The researchers reported no conflicts of interest.