User login
How do you prescribe exercise in primary prevention?
To avoid cardiovascular disease, the American Heart Association (AHA) recommends performing at least 150 minutes of moderate-intensity aerobic activity every week, 75 minutes of intense aerobic activity every week, or a combination of both, preferably spread out throughout the week. But how knowledgeable are physicians when it comes to prescribing exercise, and how should patients be assessed so that appropriate physical activity can be recommended?
In a presentation titled, “Patient Evaluation and Exercise Prescription in Primary Prevention,”
“Exercise has cardioprotective, emotional, antiarrhythmic, and antithrombotic benefits, and it reduces stress,” she explained.
She also noted that the risk regarding cardiopulmonary and musculoskeletal components must be evaluated, because exercise can itself trigger coronary events, and the last thing intended when prescribing exercise is to cause complications. “We must recommend exercise progressively. We can’t suggest a high-intensity regimen to a patient if they haven’t had any preconditioning where collateral circulation could be developed and lung and cardiac capacity could be improved.”
Dr. Sánchez went on to say that, according to the AHA, patients should be classified as follows: those who exercise and those who don’t, those with a history of cardiovascular, metabolic, or renal disease, and those with symptomatic and asymptomatic diseases, in order to consider the parameters when recommending exercise.
“If the patient has symptoms and is doing light physical activity, like walking, they can keep doing this exercise and don’t need further assessments. But if they have a symptomatic disease and are not exercising, they need to be evaluated after exercise has been prescribed, and not just clinically, either. Some sort of diagnostic method should be considered. Also, for patients who are physically active and who desire to increase the intensity of their exercise, the recommendation is to perform a detailed clinical examination and, if necessary, perform additional imaging studies.”
Warning signs
- Dizziness.
- Orthopnea.
- Abnormal heart rate.
- Edema in the lower extremities.
- Chest pain, especially when occurring with exercise.
- Intermittent claudication.
- Heart murmurs.
- Dyspnea.
- Reduced output.
- Fatigue.
Calibrating exercise parameters
The parameters of frequency (number of sessions per week), intensity (perceived exertion measured by heart rate reached), time, and type (aerobic exercise vs. strength training) should be considered when forming an appropriate prescription for exercise, explained Dr. Sánchez.
“The big problem is that most physicians don’t know how to prescribe it properly. And beyond knowing how, the important thing is that, when we’re with the patient during the consultation, we ought to be doing more than just establishing a routine. We need to be motivators and we need to be identifying obstacles and the patient’s interest in exercise, because it’s clear that incorporating physical activity into our daily lives helps improve the quality and length of life,” the specialist added.
The recommendations are straightforward: for individuals aged 18-64 years, 150 minutes of moderate-intensity activity per week, whether aerobic, strength training, or mixed, should be prescribed. “We need to encourage moving more and sitting less, and recommend comprehensive programs that include coordination, balance, and muscle strengthening. If a sedentary lifestyle is a risk factor, we need to encourage patients to start performing physical activity for 1-2 minutes every hour, because any exercise must be gradual and progressive to avoid complications,” she noted.
Evaluate, then recommend
The specialist emphasized the importance of making personalized prescriptions, exercising caution, and performing adequate assessments to know which exercise routine to recommend. “The patient should also be involved in their self-care and must have an adequate diet and hydration, and we need to remind them that they shouldn’t be exercising if they have an infection, due to the risk of myocarditis and sudden death,” she added.
Rafaelina Concepción, MD, cardiologist from the Dominican Republic and vice president of the Inter-American Society of Cardiology for Central America and the Caribbean, agreed with the importance of assessing risk and risk factors for patients who request an exercise routine. “For example, in patients with prediabetes, it has been shown that exercising can slow the progression to diabetes. The essential thing is to use stratification and know what kind of exercise to recommend, whether aerobic, strength training, or a combination of the two, to improve functional capacity without reaching the threshold heart rate while reducing the risk of other comorbidities like hypertension, obesity, and high lipids, and achieving lifestyle changes.”
Carlos Franco, MD, a cardiologist in El Salvador, emphasized that there is no such thing as zero risk when evaluating a patient. “Of course, there’s a difference between an athlete and someone who isn’t physically active, but we need to profile all patients correctly, evaluate risk factors in detail, not overlook subclinical cardiovascular disease, and check whether they need stress testing or additional imaging to assess cardiac functional capacity. Also, exercise must be prescribed gradually, and the patient’s nutritional status must be assessed.”
Dr. Franco ended by explaining that physicians must understand how to prescribe the basics of exercise and make small interventions of reasonable intensity, provide practical advice, and, to the extent possible, rely on specialists such as physiatrists, sports specialists, and physical therapists.
This article was translated from the Medscape Spanish Edition. A version of this article appeared on Medscape.com.
To avoid cardiovascular disease, the American Heart Association (AHA) recommends performing at least 150 minutes of moderate-intensity aerobic activity every week, 75 minutes of intense aerobic activity every week, or a combination of both, preferably spread out throughout the week. But how knowledgeable are physicians when it comes to prescribing exercise, and how should patients be assessed so that appropriate physical activity can be recommended?
In a presentation titled, “Patient Evaluation and Exercise Prescription in Primary Prevention,”
“Exercise has cardioprotective, emotional, antiarrhythmic, and antithrombotic benefits, and it reduces stress,” she explained.
She also noted that the risk regarding cardiopulmonary and musculoskeletal components must be evaluated, because exercise can itself trigger coronary events, and the last thing intended when prescribing exercise is to cause complications. “We must recommend exercise progressively. We can’t suggest a high-intensity regimen to a patient if they haven’t had any preconditioning where collateral circulation could be developed and lung and cardiac capacity could be improved.”
Dr. Sánchez went on to say that, according to the AHA, patients should be classified as follows: those who exercise and those who don’t, those with a history of cardiovascular, metabolic, or renal disease, and those with symptomatic and asymptomatic diseases, in order to consider the parameters when recommending exercise.
“If the patient has symptoms and is doing light physical activity, like walking, they can keep doing this exercise and don’t need further assessments. But if they have a symptomatic disease and are not exercising, they need to be evaluated after exercise has been prescribed, and not just clinically, either. Some sort of diagnostic method should be considered. Also, for patients who are physically active and who desire to increase the intensity of their exercise, the recommendation is to perform a detailed clinical examination and, if necessary, perform additional imaging studies.”
Warning signs
- Dizziness.
- Orthopnea.
- Abnormal heart rate.
- Edema in the lower extremities.
- Chest pain, especially when occurring with exercise.
- Intermittent claudication.
- Heart murmurs.
- Dyspnea.
- Reduced output.
- Fatigue.
Calibrating exercise parameters
The parameters of frequency (number of sessions per week), intensity (perceived exertion measured by heart rate reached), time, and type (aerobic exercise vs. strength training) should be considered when forming an appropriate prescription for exercise, explained Dr. Sánchez.
“The big problem is that most physicians don’t know how to prescribe it properly. And beyond knowing how, the important thing is that, when we’re with the patient during the consultation, we ought to be doing more than just establishing a routine. We need to be motivators and we need to be identifying obstacles and the patient’s interest in exercise, because it’s clear that incorporating physical activity into our daily lives helps improve the quality and length of life,” the specialist added.
The recommendations are straightforward: for individuals aged 18-64 years, 150 minutes of moderate-intensity activity per week, whether aerobic, strength training, or mixed, should be prescribed. “We need to encourage moving more and sitting less, and recommend comprehensive programs that include coordination, balance, and muscle strengthening. If a sedentary lifestyle is a risk factor, we need to encourage patients to start performing physical activity for 1-2 minutes every hour, because any exercise must be gradual and progressive to avoid complications,” she noted.
Evaluate, then recommend
The specialist emphasized the importance of making personalized prescriptions, exercising caution, and performing adequate assessments to know which exercise routine to recommend. “The patient should also be involved in their self-care and must have an adequate diet and hydration, and we need to remind them that they shouldn’t be exercising if they have an infection, due to the risk of myocarditis and sudden death,” she added.
Rafaelina Concepción, MD, cardiologist from the Dominican Republic and vice president of the Inter-American Society of Cardiology for Central America and the Caribbean, agreed with the importance of assessing risk and risk factors for patients who request an exercise routine. “For example, in patients with prediabetes, it has been shown that exercising can slow the progression to diabetes. The essential thing is to use stratification and know what kind of exercise to recommend, whether aerobic, strength training, or a combination of the two, to improve functional capacity without reaching the threshold heart rate while reducing the risk of other comorbidities like hypertension, obesity, and high lipids, and achieving lifestyle changes.”
Carlos Franco, MD, a cardiologist in El Salvador, emphasized that there is no such thing as zero risk when evaluating a patient. “Of course, there’s a difference between an athlete and someone who isn’t physically active, but we need to profile all patients correctly, evaluate risk factors in detail, not overlook subclinical cardiovascular disease, and check whether they need stress testing or additional imaging to assess cardiac functional capacity. Also, exercise must be prescribed gradually, and the patient’s nutritional status must be assessed.”
Dr. Franco ended by explaining that physicians must understand how to prescribe the basics of exercise and make small interventions of reasonable intensity, provide practical advice, and, to the extent possible, rely on specialists such as physiatrists, sports specialists, and physical therapists.
This article was translated from the Medscape Spanish Edition. A version of this article appeared on Medscape.com.
To avoid cardiovascular disease, the American Heart Association (AHA) recommends performing at least 150 minutes of moderate-intensity aerobic activity every week, 75 minutes of intense aerobic activity every week, or a combination of both, preferably spread out throughout the week. But how knowledgeable are physicians when it comes to prescribing exercise, and how should patients be assessed so that appropriate physical activity can be recommended?
In a presentation titled, “Patient Evaluation and Exercise Prescription in Primary Prevention,”
“Exercise has cardioprotective, emotional, antiarrhythmic, and antithrombotic benefits, and it reduces stress,” she explained.
She also noted that the risk regarding cardiopulmonary and musculoskeletal components must be evaluated, because exercise can itself trigger coronary events, and the last thing intended when prescribing exercise is to cause complications. “We must recommend exercise progressively. We can’t suggest a high-intensity regimen to a patient if they haven’t had any preconditioning where collateral circulation could be developed and lung and cardiac capacity could be improved.”
Dr. Sánchez went on to say that, according to the AHA, patients should be classified as follows: those who exercise and those who don’t, those with a history of cardiovascular, metabolic, or renal disease, and those with symptomatic and asymptomatic diseases, in order to consider the parameters when recommending exercise.
“If the patient has symptoms and is doing light physical activity, like walking, they can keep doing this exercise and don’t need further assessments. But if they have a symptomatic disease and are not exercising, they need to be evaluated after exercise has been prescribed, and not just clinically, either. Some sort of diagnostic method should be considered. Also, for patients who are physically active and who desire to increase the intensity of their exercise, the recommendation is to perform a detailed clinical examination and, if necessary, perform additional imaging studies.”
Warning signs
- Dizziness.
- Orthopnea.
- Abnormal heart rate.
- Edema in the lower extremities.
- Chest pain, especially when occurring with exercise.
- Intermittent claudication.
- Heart murmurs.
- Dyspnea.
- Reduced output.
- Fatigue.
Calibrating exercise parameters
The parameters of frequency (number of sessions per week), intensity (perceived exertion measured by heart rate reached), time, and type (aerobic exercise vs. strength training) should be considered when forming an appropriate prescription for exercise, explained Dr. Sánchez.
“The big problem is that most physicians don’t know how to prescribe it properly. And beyond knowing how, the important thing is that, when we’re with the patient during the consultation, we ought to be doing more than just establishing a routine. We need to be motivators and we need to be identifying obstacles and the patient’s interest in exercise, because it’s clear that incorporating physical activity into our daily lives helps improve the quality and length of life,” the specialist added.
The recommendations are straightforward: for individuals aged 18-64 years, 150 minutes of moderate-intensity activity per week, whether aerobic, strength training, or mixed, should be prescribed. “We need to encourage moving more and sitting less, and recommend comprehensive programs that include coordination, balance, and muscle strengthening. If a sedentary lifestyle is a risk factor, we need to encourage patients to start performing physical activity for 1-2 minutes every hour, because any exercise must be gradual and progressive to avoid complications,” she noted.
Evaluate, then recommend
The specialist emphasized the importance of making personalized prescriptions, exercising caution, and performing adequate assessments to know which exercise routine to recommend. “The patient should also be involved in their self-care and must have an adequate diet and hydration, and we need to remind them that they shouldn’t be exercising if they have an infection, due to the risk of myocarditis and sudden death,” she added.
Rafaelina Concepción, MD, cardiologist from the Dominican Republic and vice president of the Inter-American Society of Cardiology for Central America and the Caribbean, agreed with the importance of assessing risk and risk factors for patients who request an exercise routine. “For example, in patients with prediabetes, it has been shown that exercising can slow the progression to diabetes. The essential thing is to use stratification and know what kind of exercise to recommend, whether aerobic, strength training, or a combination of the two, to improve functional capacity without reaching the threshold heart rate while reducing the risk of other comorbidities like hypertension, obesity, and high lipids, and achieving lifestyle changes.”
Carlos Franco, MD, a cardiologist in El Salvador, emphasized that there is no such thing as zero risk when evaluating a patient. “Of course, there’s a difference between an athlete and someone who isn’t physically active, but we need to profile all patients correctly, evaluate risk factors in detail, not overlook subclinical cardiovascular disease, and check whether they need stress testing or additional imaging to assess cardiac functional capacity. Also, exercise must be prescribed gradually, and the patient’s nutritional status must be assessed.”
Dr. Franco ended by explaining that physicians must understand how to prescribe the basics of exercise and make small interventions of reasonable intensity, provide practical advice, and, to the extent possible, rely on specialists such as physiatrists, sports specialists, and physical therapists.
This article was translated from the Medscape Spanish Edition. A version of this article appeared on Medscape.com.
Minimizing atrial pacing no benefit in sinus node disease: DANPACE II
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
The most important study from ESC: FRAIL-AF
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Advanced HF no obstacle to AFib ablation success: CASTLE-HTx
Catheter ablation had long taken atrial fibrillation (AF) rhythm control to the next level before clinical trials showed it could help keep AF patients with heart failure (HF) alive and out of the hospital.
But those trials didn’t include many patients with AF on top of advanced or even end-stage HF. Lacking much of an evidence base and often viewed as too sick to gain a lot from the procedure, patients with AF and advanced HF aren’t offered ablation very often.
Now a randomized trial suggests that, on the contrary, AF ablation may confer a similar benefit to patients with HF so advanced that they were referred for evaluation at a transplant center.
The study, modestly sized with fewer than 200 such patients and conducted at a single center, assigned half of them to receive ablation and the other half to continued medical management.
Risk for the composite primary endpoint plunged 76% over a median of 18 months for those who underwent ablation. The outcome comprised death from any cause, implantation of a left ventricular assist device (LVAD), or urgent heart transplantation.
The advantage for ablation emerged early enough that the trial, CASTLE-HTx, was halted for benefit only a year after reaching its planned enrollment, observed Christian Sohns, MD, when formally presenting the results in Amsterdam at the annual congress of the European Society of Cardiology.
The difference in the primary endpoint “in this severely sick cohort of advanced, end-stage heart failure patients,” he said, was driven mostly by fewer deaths, especially cardiovascular deaths, in the ablation group.
Ablation’s effect on outcomes was associated, perhaps causally, with significant gains in left ventricular (LV) function and more than triple the reduction in AF burden seen in the control group, noted Dr. Sohns, from the Heart and Diabetes Center North-Rhine Westphalia, Bad Oeynhausen, Germany.
states the CASTLE-HTx primary report, published in the New England Journal of Medicine, with Dr. Sohns as lead author, in tandem with his ESC presentation.
One of the study’s key messages “is that AF ablation is safe and effective in patients with end-stage heart failure” and “should be part of our armamentarium” for treating them, said Philipp Sommer, MD, also with Heart and Diabetes Center North-Rhine Westphalia, at a press conference preceding Dr. Sohns’ presentation of CASTLE-HTx.
The intervention could potentially help such patients survive longer on transplant wait lists and even delay need for the surgery, proposed Dr. Sommer, who is senior author on the trial’s publication.
CASTLE-HTx suggests that patients with advanced HF and even persistent AF, “if they have reasonably small atria, should be actually considered for ablation, as it may prevent the need for heart transplant or LVAD implant,” said invited discussant Finn Gustafsson, MD, PhD, DMSc, after Dr. Sohns’ presentation. “And that, of course, would be a huge achievement.”
The trial “should, if anything, help eradicate the current somewhat nihilistic approach to atrial fibrillation management in patients with advanced heart failure,” said Dr. Gustafsson, medical director of cardiac transplantation and mechanical circulatory support, Rigshopsitalet Copenhagen University Hospital.
Still, he disputed the characterization by the investigators and indeed the published report that the patients, or most of them, had “end-stage heart failure.”
For example, about a third of the trial’s patients started out in NYHA class 2, Dr. Gustafsson noted. Not that they weren’t “high-risk” or their HF wasn’t severe, he offered, but they don’t seem to have been “a truly advanced heart failure population.”
The trial population consisted of “patients referred to an advanced heart failure center, rather than patients with advanced heart failure,” agreed Mandeep R. Mehra, MD, director of the Center for Advanced Heart Disease at Brigham and Woman’s Hospital, Boston.
Also citing a large prevalence of patients in NYHA class-2, Dr. Mehra added that “we almost never see paroxysmal atrial fib in these patients. It’s usually an early-stage phenomenon.” In advanced HF, AF “is usually permanent,” he told this news organization. Yet it was paroxysmal in about 30% of cases.
To its credit, Dr. Mehra observed, the study does assert that advanced HF is no reason, necessarily, to avoid catheter ablation. Nor should an AF patient’s referral to an advanced-HF center “mean that you should rush to an LVAD or transplant” before considering ablation.
The study seems to be saying, “please exhaust all options before you biologically replace the heart or put in an LVAD,” Dr. Mehra said. “Certainly, this paper steers you in that direction.”
The trial entered 194 patients with symptomatic AF and HF of at least NYHA class 2, with impaired functional capacity by the 6-minute walk test, who had been referred to a major center in Germany for a heart-transplantation workup. With all on guideline-directed medical therapy, 97 were randomly assigned open-label to catheter ablation and 97 to continued standard care.
Catheter ablation was actually carried out in 81 patients (84%) who had been assigned to it and in 16 (16%) of those in the control group, the report states.
A total of 8 in the ablation group and 29 in the control arm died, received an LVAD, or went to urgent transplantation, for a hazard ratio of 0.24 (95% confidence interval, 0.11-0.52; P < .001) for the primary endpoint.
Death from any cause apparently played a big role in the risk reduction; its HR was 0.29 (95% CI, 0.12-0.72).
One peculiarity of the data, Dr. Mehra said, is that event curves for the primary endpoint and its individual components “diverge almost from day 1.” That would mean the ablation group right away started having fewer deaths, LVAD placements, or heart transplants than the control group.
“It is surprising to see such a large effect size on endpoints that are very much dependent on operators and diverge within the first day.” Probably, Dr. Mehra said, “it has to do with this being a single-center study that may not be generalizable to other practices.”
CASTLE HTx was supported by a grant from Else Kröner-Fresenius-Stiftung. Dr. Sommer discloses consulting for Abbott, Biosense Webster, Boston Scientific, and Medtronic. Dr. Sohns reported no relevant financial relationships. Dr. Gustafsson discloses receiving honoraria or fees for consulting from Abbott, Alnylam Amgen, Boehringer Ingelheim, Ionis, Novartis, and Pfizer; serving on a speakers bureau for Astra Zeneca and Orion; and receiving grants from Corvia Research. Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board for NuPulseCV, Leviticus, and FineHeart.
A version of this article first appeared on Medscape.com.
Catheter ablation had long taken atrial fibrillation (AF) rhythm control to the next level before clinical trials showed it could help keep AF patients with heart failure (HF) alive and out of the hospital.
But those trials didn’t include many patients with AF on top of advanced or even end-stage HF. Lacking much of an evidence base and often viewed as too sick to gain a lot from the procedure, patients with AF and advanced HF aren’t offered ablation very often.
Now a randomized trial suggests that, on the contrary, AF ablation may confer a similar benefit to patients with HF so advanced that they were referred for evaluation at a transplant center.
The study, modestly sized with fewer than 200 such patients and conducted at a single center, assigned half of them to receive ablation and the other half to continued medical management.
Risk for the composite primary endpoint plunged 76% over a median of 18 months for those who underwent ablation. The outcome comprised death from any cause, implantation of a left ventricular assist device (LVAD), or urgent heart transplantation.
The advantage for ablation emerged early enough that the trial, CASTLE-HTx, was halted for benefit only a year after reaching its planned enrollment, observed Christian Sohns, MD, when formally presenting the results in Amsterdam at the annual congress of the European Society of Cardiology.
The difference in the primary endpoint “in this severely sick cohort of advanced, end-stage heart failure patients,” he said, was driven mostly by fewer deaths, especially cardiovascular deaths, in the ablation group.
Ablation’s effect on outcomes was associated, perhaps causally, with significant gains in left ventricular (LV) function and more than triple the reduction in AF burden seen in the control group, noted Dr. Sohns, from the Heart and Diabetes Center North-Rhine Westphalia, Bad Oeynhausen, Germany.
states the CASTLE-HTx primary report, published in the New England Journal of Medicine, with Dr. Sohns as lead author, in tandem with his ESC presentation.
One of the study’s key messages “is that AF ablation is safe and effective in patients with end-stage heart failure” and “should be part of our armamentarium” for treating them, said Philipp Sommer, MD, also with Heart and Diabetes Center North-Rhine Westphalia, at a press conference preceding Dr. Sohns’ presentation of CASTLE-HTx.
The intervention could potentially help such patients survive longer on transplant wait lists and even delay need for the surgery, proposed Dr. Sommer, who is senior author on the trial’s publication.
CASTLE-HTx suggests that patients with advanced HF and even persistent AF, “if they have reasonably small atria, should be actually considered for ablation, as it may prevent the need for heart transplant or LVAD implant,” said invited discussant Finn Gustafsson, MD, PhD, DMSc, after Dr. Sohns’ presentation. “And that, of course, would be a huge achievement.”
The trial “should, if anything, help eradicate the current somewhat nihilistic approach to atrial fibrillation management in patients with advanced heart failure,” said Dr. Gustafsson, medical director of cardiac transplantation and mechanical circulatory support, Rigshopsitalet Copenhagen University Hospital.
Still, he disputed the characterization by the investigators and indeed the published report that the patients, or most of them, had “end-stage heart failure.”
For example, about a third of the trial’s patients started out in NYHA class 2, Dr. Gustafsson noted. Not that they weren’t “high-risk” or their HF wasn’t severe, he offered, but they don’t seem to have been “a truly advanced heart failure population.”
The trial population consisted of “patients referred to an advanced heart failure center, rather than patients with advanced heart failure,” agreed Mandeep R. Mehra, MD, director of the Center for Advanced Heart Disease at Brigham and Woman’s Hospital, Boston.
Also citing a large prevalence of patients in NYHA class-2, Dr. Mehra added that “we almost never see paroxysmal atrial fib in these patients. It’s usually an early-stage phenomenon.” In advanced HF, AF “is usually permanent,” he told this news organization. Yet it was paroxysmal in about 30% of cases.
To its credit, Dr. Mehra observed, the study does assert that advanced HF is no reason, necessarily, to avoid catheter ablation. Nor should an AF patient’s referral to an advanced-HF center “mean that you should rush to an LVAD or transplant” before considering ablation.
The study seems to be saying, “please exhaust all options before you biologically replace the heart or put in an LVAD,” Dr. Mehra said. “Certainly, this paper steers you in that direction.”
The trial entered 194 patients with symptomatic AF and HF of at least NYHA class 2, with impaired functional capacity by the 6-minute walk test, who had been referred to a major center in Germany for a heart-transplantation workup. With all on guideline-directed medical therapy, 97 were randomly assigned open-label to catheter ablation and 97 to continued standard care.
Catheter ablation was actually carried out in 81 patients (84%) who had been assigned to it and in 16 (16%) of those in the control group, the report states.
A total of 8 in the ablation group and 29 in the control arm died, received an LVAD, or went to urgent transplantation, for a hazard ratio of 0.24 (95% confidence interval, 0.11-0.52; P < .001) for the primary endpoint.
Death from any cause apparently played a big role in the risk reduction; its HR was 0.29 (95% CI, 0.12-0.72).
One peculiarity of the data, Dr. Mehra said, is that event curves for the primary endpoint and its individual components “diverge almost from day 1.” That would mean the ablation group right away started having fewer deaths, LVAD placements, or heart transplants than the control group.
“It is surprising to see such a large effect size on endpoints that are very much dependent on operators and diverge within the first day.” Probably, Dr. Mehra said, “it has to do with this being a single-center study that may not be generalizable to other practices.”
CASTLE HTx was supported by a grant from Else Kröner-Fresenius-Stiftung. Dr. Sommer discloses consulting for Abbott, Biosense Webster, Boston Scientific, and Medtronic. Dr. Sohns reported no relevant financial relationships. Dr. Gustafsson discloses receiving honoraria or fees for consulting from Abbott, Alnylam Amgen, Boehringer Ingelheim, Ionis, Novartis, and Pfizer; serving on a speakers bureau for Astra Zeneca and Orion; and receiving grants from Corvia Research. Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board for NuPulseCV, Leviticus, and FineHeart.
A version of this article first appeared on Medscape.com.
Catheter ablation had long taken atrial fibrillation (AF) rhythm control to the next level before clinical trials showed it could help keep AF patients with heart failure (HF) alive and out of the hospital.
But those trials didn’t include many patients with AF on top of advanced or even end-stage HF. Lacking much of an evidence base and often viewed as too sick to gain a lot from the procedure, patients with AF and advanced HF aren’t offered ablation very often.
Now a randomized trial suggests that, on the contrary, AF ablation may confer a similar benefit to patients with HF so advanced that they were referred for evaluation at a transplant center.
The study, modestly sized with fewer than 200 such patients and conducted at a single center, assigned half of them to receive ablation and the other half to continued medical management.
Risk for the composite primary endpoint plunged 76% over a median of 18 months for those who underwent ablation. The outcome comprised death from any cause, implantation of a left ventricular assist device (LVAD), or urgent heart transplantation.
The advantage for ablation emerged early enough that the trial, CASTLE-HTx, was halted for benefit only a year after reaching its planned enrollment, observed Christian Sohns, MD, when formally presenting the results in Amsterdam at the annual congress of the European Society of Cardiology.
The difference in the primary endpoint “in this severely sick cohort of advanced, end-stage heart failure patients,” he said, was driven mostly by fewer deaths, especially cardiovascular deaths, in the ablation group.
Ablation’s effect on outcomes was associated, perhaps causally, with significant gains in left ventricular (LV) function and more than triple the reduction in AF burden seen in the control group, noted Dr. Sohns, from the Heart and Diabetes Center North-Rhine Westphalia, Bad Oeynhausen, Germany.
states the CASTLE-HTx primary report, published in the New England Journal of Medicine, with Dr. Sohns as lead author, in tandem with his ESC presentation.
One of the study’s key messages “is that AF ablation is safe and effective in patients with end-stage heart failure” and “should be part of our armamentarium” for treating them, said Philipp Sommer, MD, also with Heart and Diabetes Center North-Rhine Westphalia, at a press conference preceding Dr. Sohns’ presentation of CASTLE-HTx.
The intervention could potentially help such patients survive longer on transplant wait lists and even delay need for the surgery, proposed Dr. Sommer, who is senior author on the trial’s publication.
CASTLE-HTx suggests that patients with advanced HF and even persistent AF, “if they have reasonably small atria, should be actually considered for ablation, as it may prevent the need for heart transplant or LVAD implant,” said invited discussant Finn Gustafsson, MD, PhD, DMSc, after Dr. Sohns’ presentation. “And that, of course, would be a huge achievement.”
The trial “should, if anything, help eradicate the current somewhat nihilistic approach to atrial fibrillation management in patients with advanced heart failure,” said Dr. Gustafsson, medical director of cardiac transplantation and mechanical circulatory support, Rigshopsitalet Copenhagen University Hospital.
Still, he disputed the characterization by the investigators and indeed the published report that the patients, or most of them, had “end-stage heart failure.”
For example, about a third of the trial’s patients started out in NYHA class 2, Dr. Gustafsson noted. Not that they weren’t “high-risk” or their HF wasn’t severe, he offered, but they don’t seem to have been “a truly advanced heart failure population.”
The trial population consisted of “patients referred to an advanced heart failure center, rather than patients with advanced heart failure,” agreed Mandeep R. Mehra, MD, director of the Center for Advanced Heart Disease at Brigham and Woman’s Hospital, Boston.
Also citing a large prevalence of patients in NYHA class-2, Dr. Mehra added that “we almost never see paroxysmal atrial fib in these patients. It’s usually an early-stage phenomenon.” In advanced HF, AF “is usually permanent,” he told this news organization. Yet it was paroxysmal in about 30% of cases.
To its credit, Dr. Mehra observed, the study does assert that advanced HF is no reason, necessarily, to avoid catheter ablation. Nor should an AF patient’s referral to an advanced-HF center “mean that you should rush to an LVAD or transplant” before considering ablation.
The study seems to be saying, “please exhaust all options before you biologically replace the heart or put in an LVAD,” Dr. Mehra said. “Certainly, this paper steers you in that direction.”
The trial entered 194 patients with symptomatic AF and HF of at least NYHA class 2, with impaired functional capacity by the 6-minute walk test, who had been referred to a major center in Germany for a heart-transplantation workup. With all on guideline-directed medical therapy, 97 were randomly assigned open-label to catheter ablation and 97 to continued standard care.
Catheter ablation was actually carried out in 81 patients (84%) who had been assigned to it and in 16 (16%) of those in the control group, the report states.
A total of 8 in the ablation group and 29 in the control arm died, received an LVAD, or went to urgent transplantation, for a hazard ratio of 0.24 (95% confidence interval, 0.11-0.52; P < .001) for the primary endpoint.
Death from any cause apparently played a big role in the risk reduction; its HR was 0.29 (95% CI, 0.12-0.72).
One peculiarity of the data, Dr. Mehra said, is that event curves for the primary endpoint and its individual components “diverge almost from day 1.” That would mean the ablation group right away started having fewer deaths, LVAD placements, or heart transplants than the control group.
“It is surprising to see such a large effect size on endpoints that are very much dependent on operators and diverge within the first day.” Probably, Dr. Mehra said, “it has to do with this being a single-center study that may not be generalizable to other practices.”
CASTLE HTx was supported by a grant from Else Kröner-Fresenius-Stiftung. Dr. Sommer discloses consulting for Abbott, Biosense Webster, Boston Scientific, and Medtronic. Dr. Sohns reported no relevant financial relationships. Dr. Gustafsson discloses receiving honoraria or fees for consulting from Abbott, Alnylam Amgen, Boehringer Ingelheim, Ionis, Novartis, and Pfizer; serving on a speakers bureau for Astra Zeneca and Orion; and receiving grants from Corvia Research. Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board for NuPulseCV, Leviticus, and FineHeart.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Anticoagulation no benefit in presumed AFib detected by cardiac devices
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
No reduction in AFib after noncardiac surgery with colchicine: COP-AF
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.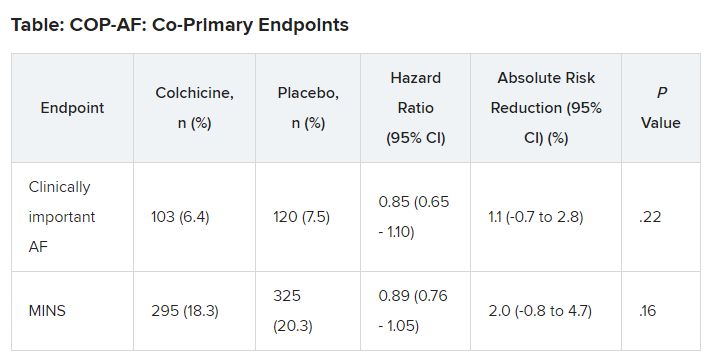
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023
Pulsed field ablation challenges conventional devices in AFib
in a head-to-head trial, an outcome that might favor PFA in the context of other considerations.
“The take-home message is that this is a new technology that has important safety benefits. Patients do not have to worry about the possibility – albeit rare – of esophageal fistulae and other problems. It is faster with at least the same efficacy,” reported Vivek Y. Reddy MD, director of cardiac arrhythmia services, Mount Sinai Hospital, New York.
As opposed to conventional catheter-based thermal ablation, which isolates pulmonary veins harboring AF triggers by heating or freezing the tissue, PFA uses microsecond high-voltage electrical fields to produce cellular necrosis. It is largely nonthermal, Dr. Reddy said.
New device might spare adjacent tissue
In experimental studies, PFA has demonstrated a high degree of ablative specificity, limiting effects on adjacent tissues, such as the esophagus and phrenic nerve, he explained.
Several previous clinical studies support the specificity of the PFA ablative effect, but the ADVENT trial, which Dr. Reddy presented Aug. 27 at the annual congress of the European Society of Cardiology, is the first trial in which patients have been randomly assigned to PFA or catheter-based ablation.
The study was published online in the New England Journal of Medicine simultaneously with the ESC presentation.
The primary efficacy endpoint was the absence of a composite of endpoints indicating incomplete ablation. These included an initial procedural failure, atrial tachyarrhythmias arising after a 3-month blanking period, subsequent use of antiarrhythmic drugs, cardioversion, or repeat ablation. The primary safety endpoint involved a composite of procedure-related adverse events.
The 607 patients enrolled in this trial had AF refractory to at least one antiarrhythmic drug class. They were randomly assigned in a 1:1 ratio to PFA with a catheter system (Farapulse–Boston Scientific) or to thermal ablation.
Of the thermal approaches, radiofrequency or cryoablation was permitted, but each center was required to use just one for the control arm. For the comparison to PFA, outcomes for the two thermal techniques, which were used in similar proportions of patients, were combined based on previous evidence that these approaches perform similarly.
At 1 year, 73.3% of patients in the PFA group and 71.3% of those in the control group met the primary outcome, meaning none of the events signaling ablation failure occurred. The numeric advantage of PFA confirmed noninferiority, although an evaluation of superiority for efficacy, which was triggered by the advantage of PFA, was not significant.
As predicted by previous studies, stratification of thermal ablation approaches showed that outcomes were similar, although the proportion of patients who remained free of events at 1 year was numerically higher in the cryoablation group relative to the radiofrequency group (73.6% vs. 69.2%).
An adverse safety event occurred in 2.1% of those who underwent PFA and in 1.5% of those who underwent thermal ablation. This 0.6–percentage point difference placed PFA well within the boundary of noninferiority for safety.
Of notable events, the only death in this study occurred in the PFA group, and the only stroke occurred in the control group. Phrenic nerve palsies occurred only in the control group (2 vs. 0) while pericarditis was seen only in the PFA group (2 vs. 0). One case of pulmonary edema occurred in each group.
“Catheter ablation is quite safe and effective,” said Dr. Reddy, explaining why this comparison was conducted on the basis of noninferiority.
Dr. Reddy emphasized that noninferiority for PFA was achieved by operators with little or no experience with this technology, whereas the catheter ablations were delivered by operators who typically had previously performed hundreds of interventions.
“With experience, one would expect even better rates of success. This is the floor,” Dr. Reddy said.
Procedure time faster with PFA
Despite working with a new technology, the mean procedure performance time with PFA was faster (105 vs. 123 minutes) even though mean fluoroscopy time was longer (21.1 vs 13.9 minutes). Dr. Reddy considers the difference in procedure time a meaningful demonstration of the efficiency of PFA.
“When you look at procedure performance, it is remarkable that the procedure times were statistically significantly shorter for a first-use technology in the hands of multiple operators,” Dr. Reddy said.
There was also a statistically significant advantage for PFA regarding change in the mean pulmonary vein cross-sectional area following the procedures (0.9% vs. 12%). Dr. Reddy acknowledged that small changes in pulmonary vein dimension are not clinically meaningful, but this result “gets at the question of whether we can achieve ablation without tissue proliferation that we see with conventional ablation.”
Overall, Dr. Reddy believes that the data from ADVENT provide several reasons “to get excited about PFA,” including the efficiency of this technique in the context of at least similar efficacy but a potential for fewer adverse events.
The ESC-invited discussant, Samuel Kiil Sørensen, MD, Gentofte University Hospital, Copenhagen, agreed that the ADVENT data support PFA as an alternative to thermal ablation. He suggested that the shorter procedure times are clinically meaningful given comparable safety and efficacy.
“Which property of PFA justifies noninferiority?” he asked. “Many of the complications of AF ablation are not specific to the energy modality. The devastating complications from damage to the esophagus, pulmonary veins, and phrenic nerve that the PFA technology may eliminate are rare, so they would not be expected to change the overall complication rate in a [randomized controlled trial] of realistic size.”
However, he suggested PFA might still prove to be an incremental advance for AF. He cited previous evidence that supports the specificity of its ablative activity and emphasized that ADVENT tested a first-generation device that might not capture the full advantages of the PFA technology.
The trial was supported by Farapulse–Boston Scientific. Dr. Reddy reports financial relationships with more than 30 pharmaceutical or device manufacturers, including Farapulse–Boston Scientific. Dr. Sørensen reports financial relationships with Medtronic and Biosense Webster.
A version of this article first appeared on Medscape.com.
in a head-to-head trial, an outcome that might favor PFA in the context of other considerations.
“The take-home message is that this is a new technology that has important safety benefits. Patients do not have to worry about the possibility – albeit rare – of esophageal fistulae and other problems. It is faster with at least the same efficacy,” reported Vivek Y. Reddy MD, director of cardiac arrhythmia services, Mount Sinai Hospital, New York.
As opposed to conventional catheter-based thermal ablation, which isolates pulmonary veins harboring AF triggers by heating or freezing the tissue, PFA uses microsecond high-voltage electrical fields to produce cellular necrosis. It is largely nonthermal, Dr. Reddy said.
New device might spare adjacent tissue
In experimental studies, PFA has demonstrated a high degree of ablative specificity, limiting effects on adjacent tissues, such as the esophagus and phrenic nerve, he explained.
Several previous clinical studies support the specificity of the PFA ablative effect, but the ADVENT trial, which Dr. Reddy presented Aug. 27 at the annual congress of the European Society of Cardiology, is the first trial in which patients have been randomly assigned to PFA or catheter-based ablation.
The study was published online in the New England Journal of Medicine simultaneously with the ESC presentation.
The primary efficacy endpoint was the absence of a composite of endpoints indicating incomplete ablation. These included an initial procedural failure, atrial tachyarrhythmias arising after a 3-month blanking period, subsequent use of antiarrhythmic drugs, cardioversion, or repeat ablation. The primary safety endpoint involved a composite of procedure-related adverse events.
The 607 patients enrolled in this trial had AF refractory to at least one antiarrhythmic drug class. They were randomly assigned in a 1:1 ratio to PFA with a catheter system (Farapulse–Boston Scientific) or to thermal ablation.
Of the thermal approaches, radiofrequency or cryoablation was permitted, but each center was required to use just one for the control arm. For the comparison to PFA, outcomes for the two thermal techniques, which were used in similar proportions of patients, were combined based on previous evidence that these approaches perform similarly.
At 1 year, 73.3% of patients in the PFA group and 71.3% of those in the control group met the primary outcome, meaning none of the events signaling ablation failure occurred. The numeric advantage of PFA confirmed noninferiority, although an evaluation of superiority for efficacy, which was triggered by the advantage of PFA, was not significant.
As predicted by previous studies, stratification of thermal ablation approaches showed that outcomes were similar, although the proportion of patients who remained free of events at 1 year was numerically higher in the cryoablation group relative to the radiofrequency group (73.6% vs. 69.2%).
An adverse safety event occurred in 2.1% of those who underwent PFA and in 1.5% of those who underwent thermal ablation. This 0.6–percentage point difference placed PFA well within the boundary of noninferiority for safety.
Of notable events, the only death in this study occurred in the PFA group, and the only stroke occurred in the control group. Phrenic nerve palsies occurred only in the control group (2 vs. 0) while pericarditis was seen only in the PFA group (2 vs. 0). One case of pulmonary edema occurred in each group.
“Catheter ablation is quite safe and effective,” said Dr. Reddy, explaining why this comparison was conducted on the basis of noninferiority.
Dr. Reddy emphasized that noninferiority for PFA was achieved by operators with little or no experience with this technology, whereas the catheter ablations were delivered by operators who typically had previously performed hundreds of interventions.
“With experience, one would expect even better rates of success. This is the floor,” Dr. Reddy said.
Procedure time faster with PFA
Despite working with a new technology, the mean procedure performance time with PFA was faster (105 vs. 123 minutes) even though mean fluoroscopy time was longer (21.1 vs 13.9 minutes). Dr. Reddy considers the difference in procedure time a meaningful demonstration of the efficiency of PFA.
“When you look at procedure performance, it is remarkable that the procedure times were statistically significantly shorter for a first-use technology in the hands of multiple operators,” Dr. Reddy said.
There was also a statistically significant advantage for PFA regarding change in the mean pulmonary vein cross-sectional area following the procedures (0.9% vs. 12%). Dr. Reddy acknowledged that small changes in pulmonary vein dimension are not clinically meaningful, but this result “gets at the question of whether we can achieve ablation without tissue proliferation that we see with conventional ablation.”
Overall, Dr. Reddy believes that the data from ADVENT provide several reasons “to get excited about PFA,” including the efficiency of this technique in the context of at least similar efficacy but a potential for fewer adverse events.
The ESC-invited discussant, Samuel Kiil Sørensen, MD, Gentofte University Hospital, Copenhagen, agreed that the ADVENT data support PFA as an alternative to thermal ablation. He suggested that the shorter procedure times are clinically meaningful given comparable safety and efficacy.
“Which property of PFA justifies noninferiority?” he asked. “Many of the complications of AF ablation are not specific to the energy modality. The devastating complications from damage to the esophagus, pulmonary veins, and phrenic nerve that the PFA technology may eliminate are rare, so they would not be expected to change the overall complication rate in a [randomized controlled trial] of realistic size.”
However, he suggested PFA might still prove to be an incremental advance for AF. He cited previous evidence that supports the specificity of its ablative activity and emphasized that ADVENT tested a first-generation device that might not capture the full advantages of the PFA technology.
The trial was supported by Farapulse–Boston Scientific. Dr. Reddy reports financial relationships with more than 30 pharmaceutical or device manufacturers, including Farapulse–Boston Scientific. Dr. Sørensen reports financial relationships with Medtronic and Biosense Webster.
A version of this article first appeared on Medscape.com.
in a head-to-head trial, an outcome that might favor PFA in the context of other considerations.
“The take-home message is that this is a new technology that has important safety benefits. Patients do not have to worry about the possibility – albeit rare – of esophageal fistulae and other problems. It is faster with at least the same efficacy,” reported Vivek Y. Reddy MD, director of cardiac arrhythmia services, Mount Sinai Hospital, New York.
As opposed to conventional catheter-based thermal ablation, which isolates pulmonary veins harboring AF triggers by heating or freezing the tissue, PFA uses microsecond high-voltage electrical fields to produce cellular necrosis. It is largely nonthermal, Dr. Reddy said.
New device might spare adjacent tissue
In experimental studies, PFA has demonstrated a high degree of ablative specificity, limiting effects on adjacent tissues, such as the esophagus and phrenic nerve, he explained.
Several previous clinical studies support the specificity of the PFA ablative effect, but the ADVENT trial, which Dr. Reddy presented Aug. 27 at the annual congress of the European Society of Cardiology, is the first trial in which patients have been randomly assigned to PFA or catheter-based ablation.
The study was published online in the New England Journal of Medicine simultaneously with the ESC presentation.
The primary efficacy endpoint was the absence of a composite of endpoints indicating incomplete ablation. These included an initial procedural failure, atrial tachyarrhythmias arising after a 3-month blanking period, subsequent use of antiarrhythmic drugs, cardioversion, or repeat ablation. The primary safety endpoint involved a composite of procedure-related adverse events.
The 607 patients enrolled in this trial had AF refractory to at least one antiarrhythmic drug class. They were randomly assigned in a 1:1 ratio to PFA with a catheter system (Farapulse–Boston Scientific) or to thermal ablation.
Of the thermal approaches, radiofrequency or cryoablation was permitted, but each center was required to use just one for the control arm. For the comparison to PFA, outcomes for the two thermal techniques, which were used in similar proportions of patients, were combined based on previous evidence that these approaches perform similarly.
At 1 year, 73.3% of patients in the PFA group and 71.3% of those in the control group met the primary outcome, meaning none of the events signaling ablation failure occurred. The numeric advantage of PFA confirmed noninferiority, although an evaluation of superiority for efficacy, which was triggered by the advantage of PFA, was not significant.
As predicted by previous studies, stratification of thermal ablation approaches showed that outcomes were similar, although the proportion of patients who remained free of events at 1 year was numerically higher in the cryoablation group relative to the radiofrequency group (73.6% vs. 69.2%).
An adverse safety event occurred in 2.1% of those who underwent PFA and in 1.5% of those who underwent thermal ablation. This 0.6–percentage point difference placed PFA well within the boundary of noninferiority for safety.
Of notable events, the only death in this study occurred in the PFA group, and the only stroke occurred in the control group. Phrenic nerve palsies occurred only in the control group (2 vs. 0) while pericarditis was seen only in the PFA group (2 vs. 0). One case of pulmonary edema occurred in each group.
“Catheter ablation is quite safe and effective,” said Dr. Reddy, explaining why this comparison was conducted on the basis of noninferiority.
Dr. Reddy emphasized that noninferiority for PFA was achieved by operators with little or no experience with this technology, whereas the catheter ablations were delivered by operators who typically had previously performed hundreds of interventions.
“With experience, one would expect even better rates of success. This is the floor,” Dr. Reddy said.
Procedure time faster with PFA
Despite working with a new technology, the mean procedure performance time with PFA was faster (105 vs. 123 minutes) even though mean fluoroscopy time was longer (21.1 vs 13.9 minutes). Dr. Reddy considers the difference in procedure time a meaningful demonstration of the efficiency of PFA.
“When you look at procedure performance, it is remarkable that the procedure times were statistically significantly shorter for a first-use technology in the hands of multiple operators,” Dr. Reddy said.
There was also a statistically significant advantage for PFA regarding change in the mean pulmonary vein cross-sectional area following the procedures (0.9% vs. 12%). Dr. Reddy acknowledged that small changes in pulmonary vein dimension are not clinically meaningful, but this result “gets at the question of whether we can achieve ablation without tissue proliferation that we see with conventional ablation.”
Overall, Dr. Reddy believes that the data from ADVENT provide several reasons “to get excited about PFA,” including the efficiency of this technique in the context of at least similar efficacy but a potential for fewer adverse events.
The ESC-invited discussant, Samuel Kiil Sørensen, MD, Gentofte University Hospital, Copenhagen, agreed that the ADVENT data support PFA as an alternative to thermal ablation. He suggested that the shorter procedure times are clinically meaningful given comparable safety and efficacy.
“Which property of PFA justifies noninferiority?” he asked. “Many of the complications of AF ablation are not specific to the energy modality. The devastating complications from damage to the esophagus, pulmonary veins, and phrenic nerve that the PFA technology may eliminate are rare, so they would not be expected to change the overall complication rate in a [randomized controlled trial] of realistic size.”
However, he suggested PFA might still prove to be an incremental advance for AF. He cited previous evidence that supports the specificity of its ablative activity and emphasized that ADVENT tested a first-generation device that might not capture the full advantages of the PFA technology.
The trial was supported by Farapulse–Boston Scientific. Dr. Reddy reports financial relationships with more than 30 pharmaceutical or device manufacturers, including Farapulse–Boston Scientific. Dr. Sørensen reports financial relationships with Medtronic and Biosense Webster.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
American Geriatrics Society 2023 updated Beers Criteria highlights
Every 4 years, an interprofessional panel of experts from the American Geriatrics Society provides updated guidelines on safe prescribing of medications in older adults, known as the Beers Criteria. A 2023 update was released in May 2023 after panel review of more 1,500 clinical trials and research studies published since the last update.
Anticoagulants
Notable changes to the 2023 guidelines include updated recommendations for anticoagulation. Warfarin should be avoided as initial therapy for venous thromboembolism or nonvalvular atrial fibrillation unless there are contraindications to direct oral anticoagulants (DOACs) or other substantial barriers to use.
Rivaroxaban should also be avoided, and dabigatran used with caution in favor of apixaban, which is felt to have a better safety profile in older adults. Rivaroxaban may be considered if once daily dosing is deemed to be more clinically appropriate. Financial barriers regarding drug coverage and formulary options were acknowledged as a significant barrier to equitable access to preferred direct oral anticoagulants in older adults.
Diabetes medication
Regarding diabetes management, short-acting sulfonylureas should be avoided in addition to long-acting sulfonylureas, because of the increased risk of hypoglycemia, and cardiovascular and all-cause mortality in older adults. Sodium-glucose cotransporter 2 inhibitors as an entire class are recommended to be used with caution, as older adults are at higher risk of euglycemic ketoacidosis and urogenital infections, particularly in women in the first month of initiating treatment.
Like DOACs, the panel acknowledged that financial considerations may lead to limited options for oral diabetic treatment. In circumstances where a sulfonylurea is used, short-acting forms are preferred over long acting to reduce the risk of prolonged hypoglycemia.
Aspirin for primary prevention
Alongside the U.S. Preventive Services Task Force guideline update in 2022 regarding aspirin for primary prevention of cardiovascular disease and stroke, the Beer’s Criteria recommend against initiation of aspirin for primary prevention in older adults. Ticagrelor and prasugrel should be used with caution because of the increased risk of major bleeding in older adults over the age of 75, compared with clopidogrel. If prasugrel is used, a lower dose of 5 mg is recommended, in line with guidelines by the American College of Cardiology and American Heart Association.
Pain medication
For pain management, the Beer’s Criteria updated recommendations to avoid NSAIDs, particularly when used in combination with steroids or anticoagulants. The panel highlights that even short-term use of NSAIDs is high risk when used in combination with steroids or anticoagulants. If no other alternatives are possible, patients should be placed on a proton pump inhibitor or misoprostol while taking NSAIDs.
Baclofen should be avoided in older adults with renal insufficiency (estimated glomerular filtration rate < 60 mL/min per 1.73 m2) because of the increased risk of encephalopathy, and when used, should be given at the lowest effective dose with close monitoring for mental status changes.
Androgen and estrogen replacement therapy
For androgen replacement therapy, the panel notes that testosterone supplementation should be avoided because of cardiovascular risks unless there is confirmed hypogonadism. The panel revised their recommendation on the basis of emerging data that a history of prostate cancer is not an absolute contraindication for exogenous testosterone. A risk versus benefit discussion about exogenous testosterone should be had with a medical oncologist or urologist in those with a history of prostate cancer.
Regarding estrogen, systemic formulations should not be initiated in women over the age of 60 because of increased risk of cardiovascular events, venous thromboembolism, and dementia. In women with a history of breast cancer, vaginal estrogens are generally felt to be safe to use at low doses, such as less than 25 mcg twice weekly.
Dr. Wang is a geriatrician and general internist at Harborview Medical Center, Seattle.
Every 4 years, an interprofessional panel of experts from the American Geriatrics Society provides updated guidelines on safe prescribing of medications in older adults, known as the Beers Criteria. A 2023 update was released in May 2023 after panel review of more 1,500 clinical trials and research studies published since the last update.
Anticoagulants
Notable changes to the 2023 guidelines include updated recommendations for anticoagulation. Warfarin should be avoided as initial therapy for venous thromboembolism or nonvalvular atrial fibrillation unless there are contraindications to direct oral anticoagulants (DOACs) or other substantial barriers to use.
Rivaroxaban should also be avoided, and dabigatran used with caution in favor of apixaban, which is felt to have a better safety profile in older adults. Rivaroxaban may be considered if once daily dosing is deemed to be more clinically appropriate. Financial barriers regarding drug coverage and formulary options were acknowledged as a significant barrier to equitable access to preferred direct oral anticoagulants in older adults.
Diabetes medication
Regarding diabetes management, short-acting sulfonylureas should be avoided in addition to long-acting sulfonylureas, because of the increased risk of hypoglycemia, and cardiovascular and all-cause mortality in older adults. Sodium-glucose cotransporter 2 inhibitors as an entire class are recommended to be used with caution, as older adults are at higher risk of euglycemic ketoacidosis and urogenital infections, particularly in women in the first month of initiating treatment.
Like DOACs, the panel acknowledged that financial considerations may lead to limited options for oral diabetic treatment. In circumstances where a sulfonylurea is used, short-acting forms are preferred over long acting to reduce the risk of prolonged hypoglycemia.
Aspirin for primary prevention
Alongside the U.S. Preventive Services Task Force guideline update in 2022 regarding aspirin for primary prevention of cardiovascular disease and stroke, the Beer’s Criteria recommend against initiation of aspirin for primary prevention in older adults. Ticagrelor and prasugrel should be used with caution because of the increased risk of major bleeding in older adults over the age of 75, compared with clopidogrel. If prasugrel is used, a lower dose of 5 mg is recommended, in line with guidelines by the American College of Cardiology and American Heart Association.
Pain medication
For pain management, the Beer’s Criteria updated recommendations to avoid NSAIDs, particularly when used in combination with steroids or anticoagulants. The panel highlights that even short-term use of NSAIDs is high risk when used in combination with steroids or anticoagulants. If no other alternatives are possible, patients should be placed on a proton pump inhibitor or misoprostol while taking NSAIDs.
Baclofen should be avoided in older adults with renal insufficiency (estimated glomerular filtration rate < 60 mL/min per 1.73 m2) because of the increased risk of encephalopathy, and when used, should be given at the lowest effective dose with close monitoring for mental status changes.
Androgen and estrogen replacement therapy
For androgen replacement therapy, the panel notes that testosterone supplementation should be avoided because of cardiovascular risks unless there is confirmed hypogonadism. The panel revised their recommendation on the basis of emerging data that a history of prostate cancer is not an absolute contraindication for exogenous testosterone. A risk versus benefit discussion about exogenous testosterone should be had with a medical oncologist or urologist in those with a history of prostate cancer.
Regarding estrogen, systemic formulations should not be initiated in women over the age of 60 because of increased risk of cardiovascular events, venous thromboembolism, and dementia. In women with a history of breast cancer, vaginal estrogens are generally felt to be safe to use at low doses, such as less than 25 mcg twice weekly.
Dr. Wang is a geriatrician and general internist at Harborview Medical Center, Seattle.
Every 4 years, an interprofessional panel of experts from the American Geriatrics Society provides updated guidelines on safe prescribing of medications in older adults, known as the Beers Criteria. A 2023 update was released in May 2023 after panel review of more 1,500 clinical trials and research studies published since the last update.
Anticoagulants
Notable changes to the 2023 guidelines include updated recommendations for anticoagulation. Warfarin should be avoided as initial therapy for venous thromboembolism or nonvalvular atrial fibrillation unless there are contraindications to direct oral anticoagulants (DOACs) or other substantial barriers to use.
Rivaroxaban should also be avoided, and dabigatran used with caution in favor of apixaban, which is felt to have a better safety profile in older adults. Rivaroxaban may be considered if once daily dosing is deemed to be more clinically appropriate. Financial barriers regarding drug coverage and formulary options were acknowledged as a significant barrier to equitable access to preferred direct oral anticoagulants in older adults.
Diabetes medication
Regarding diabetes management, short-acting sulfonylureas should be avoided in addition to long-acting sulfonylureas, because of the increased risk of hypoglycemia, and cardiovascular and all-cause mortality in older adults. Sodium-glucose cotransporter 2 inhibitors as an entire class are recommended to be used with caution, as older adults are at higher risk of euglycemic ketoacidosis and urogenital infections, particularly in women in the first month of initiating treatment.
Like DOACs, the panel acknowledged that financial considerations may lead to limited options for oral diabetic treatment. In circumstances where a sulfonylurea is used, short-acting forms are preferred over long acting to reduce the risk of prolonged hypoglycemia.
Aspirin for primary prevention
Alongside the U.S. Preventive Services Task Force guideline update in 2022 regarding aspirin for primary prevention of cardiovascular disease and stroke, the Beer’s Criteria recommend against initiation of aspirin for primary prevention in older adults. Ticagrelor and prasugrel should be used with caution because of the increased risk of major bleeding in older adults over the age of 75, compared with clopidogrel. If prasugrel is used, a lower dose of 5 mg is recommended, in line with guidelines by the American College of Cardiology and American Heart Association.
Pain medication
For pain management, the Beer’s Criteria updated recommendations to avoid NSAIDs, particularly when used in combination with steroids or anticoagulants. The panel highlights that even short-term use of NSAIDs is high risk when used in combination with steroids or anticoagulants. If no other alternatives are possible, patients should be placed on a proton pump inhibitor or misoprostol while taking NSAIDs.
Baclofen should be avoided in older adults with renal insufficiency (estimated glomerular filtration rate < 60 mL/min per 1.73 m2) because of the increased risk of encephalopathy, and when used, should be given at the lowest effective dose with close monitoring for mental status changes.
Androgen and estrogen replacement therapy
For androgen replacement therapy, the panel notes that testosterone supplementation should be avoided because of cardiovascular risks unless there is confirmed hypogonadism. The panel revised their recommendation on the basis of emerging data that a history of prostate cancer is not an absolute contraindication for exogenous testosterone. A risk versus benefit discussion about exogenous testosterone should be had with a medical oncologist or urologist in those with a history of prostate cancer.
Regarding estrogen, systemic formulations should not be initiated in women over the age of 60 because of increased risk of cardiovascular events, venous thromboembolism, and dementia. In women with a history of breast cancer, vaginal estrogens are generally felt to be safe to use at low doses, such as less than 25 mcg twice weekly.
Dr. Wang is a geriatrician and general internist at Harborview Medical Center, Seattle.
Crossed wires: Ischemia testing and monomorphic VT storm
Patients with a severe form of ventricular arrhythmia who may be referred for catheter ablation are often first tested for coronary artery disease (CAD) or ischemia.
The findings, they say, question such routine CAD/ischemia testing in patients like those studied, who had episodes of monomorphic ventricular tachycardia (VT) storm but not an acute coronary syndrome (ACS) and ultimately went to ablation.
Of 97 such patients, about 44% underwent CAD/ischemia testing by invasive angiography, myocardial functional imaging, or both. But the tests didn’t predict important ablation outcomes, including pre- or postablation VT inducibility. Nor did they significantly affect the likelihood or outcomes of preablation revascularization or 2-year survival.
The findings “argue against performing routine evaluations to rule out coronary [disease] or myocardial ischemia as culprits in monomorphic VT storm” in patients without evidence of ACS, write Feras Alkhalaileh, MD, Heart and Vascular Institute, Cleveland Clinic, and colleagues in their report published in JACC: Clinical Electrophysiology.
They suggest it’s “reasonable” not to perform tests for CAD or ischemia in such patients, senior author Ayman A. Hussein, MD, from the same center, said in an interview. Although such tests may be considered “case by case,” performed routinely they “aren’t going to add much to patient care, and as a matter of fact, may delay proper care and expose them to risks,” Dr. Hussein said.
It’s “reasonable” to test for CAD or ischemia in patients with polymorphic VT storm, which is likely ischemia-driven, he observed. In contrast, monomorphic VT storm is likely caused by myocardial scar, which revascularization cannot treat. “Because there’s scar substrate, we find that ischemic evaluations are technically without much yield.”
These issues are “not very controversial” among cardiac electrophysiologists, Dr. Hussein said, but it remains “common practice” for other specialists to order angiography or ischemia testing for patients with monomorphic VT storm, typically in the cardiac care unit (CCU), before considering ablation.
“Sometimes, as electrophysiologists, we don’t get to see them before an ischemic evaluation has already been done,” he added.
It’s “very hard to convince interventional cardiologists, CCU intensivists, or general cardiologists” that a VT may not be caused by ischemia, said electrophysiologist Roderick Tung, MD, University of Arizona College of Medicine, Phoenix, who was not involved in the current study.
In patients with monomorphic VT storm, “by the time we’re consulted, they’ve already had a cath. And it’s probably just not necessary,” Dr. Tung said. “That’s why this is such a great paper, because it has an immediate message [for nonelectrophysiologist clinicians and] the potential to change clinical practice.”
The study included 97 patients with monomorphic VT storm from a prospective VT-ablation registry covering about 7 years at a major referral center. Their mean age was 64 years, and 88% were men. Two-thirds were known to have ischemic cardiomyopathy and were in NYHA functional class II.
As reported, 10% of the cohort underwent coronary angiography after presentation with monomorphic VT storm, 26% had CT or PET myocardial functional imaging, and 9% had both tests.
Only four patients ultimately underwent coronary revascularization; no acute coronary occlusions were involved. Monomorphic VT recurred in all four cases, the report notes.
The 43 and 54 patients who did or did not get the CAD/ischemia tests, respectively, showed no significant procedural differences in extent of scar modification, prevalence of clinical or hemodynamically stable VT, or use of mechanical circulatory support; or in postablation, VT inducibility or overall mortality during follow-up averaging 24.3 months.
To address possible concerns about selection bias in the main cohort, all of whom underwent ablation, a secondary analysis was conducted with 91 patients with known asymptomatic coronary disease and monomorphic VT storm who were selected from the registry without regard to whether they underwent catheter ablation.
Of that cohort, 21 went to invasive angiography and 25 underwent stress testing; six of the latter went on to coronary angiography, the report states. Monomorphic VT later recurred in four of the five patients, who then underwent coronary revascularization.
Such patients with known coronary disease, Dr. Hussein said, are those “possibly more likely to get an ischemic evaluation.” And yet, “regardless of whether they had ablation, the yield of ischemic evaluations in these patients was low.”
By far most of the CAD/ischemia tests in the study’s primary cohort were performed using noninvasive imaging, notes an editorial accompanying the new report. “This raises the possibility of false negatives with very proximal and multivessel CAD, and with balanced ischemia,” write Saurabh Kumar, BSc (Med)/MBBS, PhD, and Ashwin Bhaskaran, MBBS, MSc, University of Sydney.
Ideally, the issues addressed by the study should be tested in large randomized, controlled trials, they state. “Achieving sufficient recruitment to address this clinical question may be difficult, leaving clinicians with the challenge of applying observational data to their patients.”
A version of this article appeared on Medscape.com.
Patients with a severe form of ventricular arrhythmia who may be referred for catheter ablation are often first tested for coronary artery disease (CAD) or ischemia.
The findings, they say, question such routine CAD/ischemia testing in patients like those studied, who had episodes of monomorphic ventricular tachycardia (VT) storm but not an acute coronary syndrome (ACS) and ultimately went to ablation.
Of 97 such patients, about 44% underwent CAD/ischemia testing by invasive angiography, myocardial functional imaging, or both. But the tests didn’t predict important ablation outcomes, including pre- or postablation VT inducibility. Nor did they significantly affect the likelihood or outcomes of preablation revascularization or 2-year survival.
The findings “argue against performing routine evaluations to rule out coronary [disease] or myocardial ischemia as culprits in monomorphic VT storm” in patients without evidence of ACS, write Feras Alkhalaileh, MD, Heart and Vascular Institute, Cleveland Clinic, and colleagues in their report published in JACC: Clinical Electrophysiology.
They suggest it’s “reasonable” not to perform tests for CAD or ischemia in such patients, senior author Ayman A. Hussein, MD, from the same center, said in an interview. Although such tests may be considered “case by case,” performed routinely they “aren’t going to add much to patient care, and as a matter of fact, may delay proper care and expose them to risks,” Dr. Hussein said.
It’s “reasonable” to test for CAD or ischemia in patients with polymorphic VT storm, which is likely ischemia-driven, he observed. In contrast, monomorphic VT storm is likely caused by myocardial scar, which revascularization cannot treat. “Because there’s scar substrate, we find that ischemic evaluations are technically without much yield.”
These issues are “not very controversial” among cardiac electrophysiologists, Dr. Hussein said, but it remains “common practice” for other specialists to order angiography or ischemia testing for patients with monomorphic VT storm, typically in the cardiac care unit (CCU), before considering ablation.
“Sometimes, as electrophysiologists, we don’t get to see them before an ischemic evaluation has already been done,” he added.
It’s “very hard to convince interventional cardiologists, CCU intensivists, or general cardiologists” that a VT may not be caused by ischemia, said electrophysiologist Roderick Tung, MD, University of Arizona College of Medicine, Phoenix, who was not involved in the current study.
In patients with monomorphic VT storm, “by the time we’re consulted, they’ve already had a cath. And it’s probably just not necessary,” Dr. Tung said. “That’s why this is such a great paper, because it has an immediate message [for nonelectrophysiologist clinicians and] the potential to change clinical practice.”
The study included 97 patients with monomorphic VT storm from a prospective VT-ablation registry covering about 7 years at a major referral center. Their mean age was 64 years, and 88% were men. Two-thirds were known to have ischemic cardiomyopathy and were in NYHA functional class II.
As reported, 10% of the cohort underwent coronary angiography after presentation with monomorphic VT storm, 26% had CT or PET myocardial functional imaging, and 9% had both tests.
Only four patients ultimately underwent coronary revascularization; no acute coronary occlusions were involved. Monomorphic VT recurred in all four cases, the report notes.
The 43 and 54 patients who did or did not get the CAD/ischemia tests, respectively, showed no significant procedural differences in extent of scar modification, prevalence of clinical or hemodynamically stable VT, or use of mechanical circulatory support; or in postablation, VT inducibility or overall mortality during follow-up averaging 24.3 months.
To address possible concerns about selection bias in the main cohort, all of whom underwent ablation, a secondary analysis was conducted with 91 patients with known asymptomatic coronary disease and monomorphic VT storm who were selected from the registry without regard to whether they underwent catheter ablation.
Of that cohort, 21 went to invasive angiography and 25 underwent stress testing; six of the latter went on to coronary angiography, the report states. Monomorphic VT later recurred in four of the five patients, who then underwent coronary revascularization.
Such patients with known coronary disease, Dr. Hussein said, are those “possibly more likely to get an ischemic evaluation.” And yet, “regardless of whether they had ablation, the yield of ischemic evaluations in these patients was low.”
By far most of the CAD/ischemia tests in the study’s primary cohort were performed using noninvasive imaging, notes an editorial accompanying the new report. “This raises the possibility of false negatives with very proximal and multivessel CAD, and with balanced ischemia,” write Saurabh Kumar, BSc (Med)/MBBS, PhD, and Ashwin Bhaskaran, MBBS, MSc, University of Sydney.
Ideally, the issues addressed by the study should be tested in large randomized, controlled trials, they state. “Achieving sufficient recruitment to address this clinical question may be difficult, leaving clinicians with the challenge of applying observational data to their patients.”
A version of this article appeared on Medscape.com.
Patients with a severe form of ventricular arrhythmia who may be referred for catheter ablation are often first tested for coronary artery disease (CAD) or ischemia.
The findings, they say, question such routine CAD/ischemia testing in patients like those studied, who had episodes of monomorphic ventricular tachycardia (VT) storm but not an acute coronary syndrome (ACS) and ultimately went to ablation.
Of 97 such patients, about 44% underwent CAD/ischemia testing by invasive angiography, myocardial functional imaging, or both. But the tests didn’t predict important ablation outcomes, including pre- or postablation VT inducibility. Nor did they significantly affect the likelihood or outcomes of preablation revascularization or 2-year survival.
The findings “argue against performing routine evaluations to rule out coronary [disease] or myocardial ischemia as culprits in monomorphic VT storm” in patients without evidence of ACS, write Feras Alkhalaileh, MD, Heart and Vascular Institute, Cleveland Clinic, and colleagues in their report published in JACC: Clinical Electrophysiology.
They suggest it’s “reasonable” not to perform tests for CAD or ischemia in such patients, senior author Ayman A. Hussein, MD, from the same center, said in an interview. Although such tests may be considered “case by case,” performed routinely they “aren’t going to add much to patient care, and as a matter of fact, may delay proper care and expose them to risks,” Dr. Hussein said.
It’s “reasonable” to test for CAD or ischemia in patients with polymorphic VT storm, which is likely ischemia-driven, he observed. In contrast, monomorphic VT storm is likely caused by myocardial scar, which revascularization cannot treat. “Because there’s scar substrate, we find that ischemic evaluations are technically without much yield.”
These issues are “not very controversial” among cardiac electrophysiologists, Dr. Hussein said, but it remains “common practice” for other specialists to order angiography or ischemia testing for patients with monomorphic VT storm, typically in the cardiac care unit (CCU), before considering ablation.
“Sometimes, as electrophysiologists, we don’t get to see them before an ischemic evaluation has already been done,” he added.
It’s “very hard to convince interventional cardiologists, CCU intensivists, or general cardiologists” that a VT may not be caused by ischemia, said electrophysiologist Roderick Tung, MD, University of Arizona College of Medicine, Phoenix, who was not involved in the current study.
In patients with monomorphic VT storm, “by the time we’re consulted, they’ve already had a cath. And it’s probably just not necessary,” Dr. Tung said. “That’s why this is such a great paper, because it has an immediate message [for nonelectrophysiologist clinicians and] the potential to change clinical practice.”
The study included 97 patients with monomorphic VT storm from a prospective VT-ablation registry covering about 7 years at a major referral center. Their mean age was 64 years, and 88% were men. Two-thirds were known to have ischemic cardiomyopathy and were in NYHA functional class II.
As reported, 10% of the cohort underwent coronary angiography after presentation with monomorphic VT storm, 26% had CT or PET myocardial functional imaging, and 9% had both tests.
Only four patients ultimately underwent coronary revascularization; no acute coronary occlusions were involved. Monomorphic VT recurred in all four cases, the report notes.
The 43 and 54 patients who did or did not get the CAD/ischemia tests, respectively, showed no significant procedural differences in extent of scar modification, prevalence of clinical or hemodynamically stable VT, or use of mechanical circulatory support; or in postablation, VT inducibility or overall mortality during follow-up averaging 24.3 months.
To address possible concerns about selection bias in the main cohort, all of whom underwent ablation, a secondary analysis was conducted with 91 patients with known asymptomatic coronary disease and monomorphic VT storm who were selected from the registry without regard to whether they underwent catheter ablation.
Of that cohort, 21 went to invasive angiography and 25 underwent stress testing; six of the latter went on to coronary angiography, the report states. Monomorphic VT later recurred in four of the five patients, who then underwent coronary revascularization.
Such patients with known coronary disease, Dr. Hussein said, are those “possibly more likely to get an ischemic evaluation.” And yet, “regardless of whether they had ablation, the yield of ischemic evaluations in these patients was low.”
By far most of the CAD/ischemia tests in the study’s primary cohort were performed using noninvasive imaging, notes an editorial accompanying the new report. “This raises the possibility of false negatives with very proximal and multivessel CAD, and with balanced ischemia,” write Saurabh Kumar, BSc (Med)/MBBS, PhD, and Ashwin Bhaskaran, MBBS, MSc, University of Sydney.
Ideally, the issues addressed by the study should be tested in large randomized, controlled trials, they state. “Achieving sufficient recruitment to address this clinical question may be difficult, leaving clinicians with the challenge of applying observational data to their patients.”
A version of this article appeared on Medscape.com.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Are fish oils on the hook for AFib risk?
Questions about omega-3 fatty acid supplements come up often in the atrial fibrillation (AFib) clinic.
The story begins with the simple observation that populations who eat lots of oily fish have fewer coronary events. This correlation provoked great interest in concentrating fish oils in pill form and studying their use to promote health.
OMENI secondary analysis
Peder Myhre, MD, and colleagues recently published a secondary analysis of the OMENI trial looking at both the risk and possible causes of AFib in the omega-3 group.
The OMENI trial randomly assigned slightly more than 1,000 older patients (mean age, 75 years) post–myocardial infarction to either 1.8 g/d of fish oil supplements versus placebo for 2 years. The supplements comprised 930 mg of eicosapentaenoic acid (EPA) and 660 mg of docosahexaenoic acid (DHA). The main trial reported no difference in a composite primary endpoint of MI, revascularization, stroke, death, or hospitalization for heart failure.
The secondary analysis explored the 75% of patients in the main trial who had no history of AFib. It looked at how many in each group developed either true clinical AFib or what the authors called micro-AFib, defined as short bursts of irregular atrial activity lasting seconds.
The sub-analysis had three main findings: Patients in the supplement arm had a 90% higher rate of AFib or micro-AFib, compared with patients on placebo, EPA had the strongest effect on the association, and there was a graded risk for AFib with increasing serum EPA levels.
The authors raised the possibility that more micro-AFib might be a possible mediator of AFib risk.
Trials of low-dose EPA and DHA
First, the low-dose trials. In the ASCEND trial from 2018, more than 15,000 patients with diabetes were randomly assigned to either 1 g of omega-3 fatty acids (460-mg EPA and 380-mg DHA) or mineral oil.
The trial was neutral. After 7.4 years, the primary endpoint of MI, stroke, transient ischemic attack, or cardiovascular death occurred in 8.9% of the supplement group versus 9.2% of the placebo arm.The incidence of AFib was higher in the omega-3 group but did not reach statistical significance (2.1% vs. 1.7% for placebo; hazard ratio, 1.23; 95% confidence interval, 0.98-1.54).
Another neutral CV trial, VITAL, specifically studied the effects of marine omega-3 pills (460-mg EPA and 380-mg DHA) in older adults without heart disease, cancer, or AFib. After slightly more than 5 years, AFib occurred at a similar rate in the active arm and placebo arms (3.7% vs. 3.4% for placebo; HR, 1.09; 95% CI, 0.96-1.24; P = .19)
Trials of very high-dose marine omega-3s
Next came trials of higher doses in higher-risk populations.
In 2020, JAMA published the STRENGTH trial, which compared 4 g/d of a carboxylic acid formulation of EPA and DHA with a corn oil placebo in more than 13,000 patients who either had established atherosclerotic CV disease (ASCVD) or were at high risk for ASCVD.
The trial was terminated early because of futility and a signal of increased AFib risk in the supplement arm.
Nearly the same number of patients in the supplement versus placebo arm experienced a primary composite endpoint of major adverse cardiac events: 12.0% versus 12.2%, respectively.
AFib was a tertiary endpoint in this trial. An increase in investigator-reported new-onset AFib was observed in the omega-3 group: 2.2% vs. 1.3% for corn oil (HR, 1.69; 95% CI, 1.29-2.21; nominal P < .001).
The REDUCE-IT trial randomly assigned more than 8,000 patients who had ASCVD or diabetes and high ASCVD risk and elevated triglyceride levels to either 4 g of icosapent ethyl daily, a concentrated form of EPA, or a mineral oil placebo.
After nearly 5 years, there was a 4.8% absolute risk reduction in the primary endpoint of CV death, MI, stroke, revascularization, or unstable angina with icosapent ethyl. An increase in atherogenic biomarkers in the mineral oil placebo complicated interpretation of this trial.
Hospitalization for AFib or flutter occurred in 3.1% of the active arm versus 2.1% of the mineral oil group (P = .004).
Meta-analysis of marine omega-3 supplement trials
In 2021, Baris Gencer and colleagues performed a meta-analysis of these five trials plus 2 more (GISSI-HF and RP) looking specifically at risk for AFib. Their final analysis included more than 81,000 patients followed for nearly 5 years.
Omega 3 fatty acid supplements associated with a 25% increase in the risk for AFib (HR, 1.25; 95% CI, 1.07-1.46P =.013). Exploring further, they noted a dose-dependent relationship. Most of the increased risk occurred in trials that tested greater than 1 g/d.
Summary
When faced with surprise findings, I like to think things through.
First about plausibility. Omega-3 fatty acids clearly exert electrophysiologic effects on cardiac cells, an increase in AFib risk is plausible. The exact underlying mechanism may be unknown, but exact mechanisms are less important than actual clinical effects (see sodium-glucose cotransporter 2 inhibitors).
What about causality? Factors supporting causality include plausibility, consistency of increased AFib risk in multiple studies, and a dose-response relationship.
I see multiple clinical implications of this observation.
The first is the power of the randomized trial to inform practice. If we relied only on observational evidence, we might have assumed that since high fish consumption in populations associated with lower rates of cardiac events, fish oil supplementation would also reduce cardiac events. Other than the outlier trial, REDUCE-IT, with its mineral oil placebo, the preponderance of the randomized controlled trial evidence does not support fish oils for the reduction of CV events.
Randomized controlled trials also exposed the AFib risk. This would have been difficult to sort out in nonrandom observational studies.
Another underappreciated lesson is the notion that drugs, including supplements, can have off-target effects.
Consider the case of statin drugs. It is widely assumed that statins reduce cardiac events by lowering low-density lipoprotein cholesterol (LDL-C). Yet, statins became a mainstay not because of LDL-C lowering but because multiple trials found that this class of drugs reduced cardiac events without increasing adverse effects.
Omega-3 fatty acids reduce triglyceride levels, but this is not enough to adopt the use of these pills. The lack of consistent reduction in CV events and the off-target signal of AFib risk argue against routine use of fish-oil pills.
I will close with uncertainty. Though there is plausibility and multiple reasons to infer causality of marine omega-3s in increasing AFib risk, the effect size remains unknown.
In an editorial accompanying the recent meta-analysis, epidemiologist Michelle Samuel, MPH, PhD, and electrophysiologist Stanley Nattel, MD, cautioned readers on a technical but important point. It concerns the matter of competing risks, such as death, in the analysis of AFib risk, meaning that patients who died may have developed AFib had they lived. They provide a detailed explanation in the open access article, but the take-home is that the exact effect size is difficult to quantify without patient-level original data.
No matter. I find the signal of increased AFib risk an important one to use at the bedside.
Intermittent AFib has an unpredictable natural history. It often resolves as mysteriously as it arises. When patients take fish-oil supplements, I cite these studies, note the lack of CV protection, then I recommend stopping the pills.
This allows for one of the most important interventions in AFib care: time.
Dr. Mandrola is a clinical electrophysiologist with Baptist Medical Associates, Louisville, Ky. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Questions about omega-3 fatty acid supplements come up often in the atrial fibrillation (AFib) clinic.
The story begins with the simple observation that populations who eat lots of oily fish have fewer coronary events. This correlation provoked great interest in concentrating fish oils in pill form and studying their use to promote health.
OMENI secondary analysis
Peder Myhre, MD, and colleagues recently published a secondary analysis of the OMENI trial looking at both the risk and possible causes of AFib in the omega-3 group.
The OMENI trial randomly assigned slightly more than 1,000 older patients (mean age, 75 years) post–myocardial infarction to either 1.8 g/d of fish oil supplements versus placebo for 2 years. The supplements comprised 930 mg of eicosapentaenoic acid (EPA) and 660 mg of docosahexaenoic acid (DHA). The main trial reported no difference in a composite primary endpoint of MI, revascularization, stroke, death, or hospitalization for heart failure.
The secondary analysis explored the 75% of patients in the main trial who had no history of AFib. It looked at how many in each group developed either true clinical AFib or what the authors called micro-AFib, defined as short bursts of irregular atrial activity lasting seconds.
The sub-analysis had three main findings: Patients in the supplement arm had a 90% higher rate of AFib or micro-AFib, compared with patients on placebo, EPA had the strongest effect on the association, and there was a graded risk for AFib with increasing serum EPA levels.
The authors raised the possibility that more micro-AFib might be a possible mediator of AFib risk.
Trials of low-dose EPA and DHA
First, the low-dose trials. In the ASCEND trial from 2018, more than 15,000 patients with diabetes were randomly assigned to either 1 g of omega-3 fatty acids (460-mg EPA and 380-mg DHA) or mineral oil.
The trial was neutral. After 7.4 years, the primary endpoint of MI, stroke, transient ischemic attack, or cardiovascular death occurred in 8.9% of the supplement group versus 9.2% of the placebo arm.The incidence of AFib was higher in the omega-3 group but did not reach statistical significance (2.1% vs. 1.7% for placebo; hazard ratio, 1.23; 95% confidence interval, 0.98-1.54).
Another neutral CV trial, VITAL, specifically studied the effects of marine omega-3 pills (460-mg EPA and 380-mg DHA) in older adults without heart disease, cancer, or AFib. After slightly more than 5 years, AFib occurred at a similar rate in the active arm and placebo arms (3.7% vs. 3.4% for placebo; HR, 1.09; 95% CI, 0.96-1.24; P = .19)
Trials of very high-dose marine omega-3s
Next came trials of higher doses in higher-risk populations.
In 2020, JAMA published the STRENGTH trial, which compared 4 g/d of a carboxylic acid formulation of EPA and DHA with a corn oil placebo in more than 13,000 patients who either had established atherosclerotic CV disease (ASCVD) or were at high risk for ASCVD.
The trial was terminated early because of futility and a signal of increased AFib risk in the supplement arm.
Nearly the same number of patients in the supplement versus placebo arm experienced a primary composite endpoint of major adverse cardiac events: 12.0% versus 12.2%, respectively.
AFib was a tertiary endpoint in this trial. An increase in investigator-reported new-onset AFib was observed in the omega-3 group: 2.2% vs. 1.3% for corn oil (HR, 1.69; 95% CI, 1.29-2.21; nominal P < .001).
The REDUCE-IT trial randomly assigned more than 8,000 patients who had ASCVD or diabetes and high ASCVD risk and elevated triglyceride levels to either 4 g of icosapent ethyl daily, a concentrated form of EPA, or a mineral oil placebo.
After nearly 5 years, there was a 4.8% absolute risk reduction in the primary endpoint of CV death, MI, stroke, revascularization, or unstable angina with icosapent ethyl. An increase in atherogenic biomarkers in the mineral oil placebo complicated interpretation of this trial.
Hospitalization for AFib or flutter occurred in 3.1% of the active arm versus 2.1% of the mineral oil group (P = .004).
Meta-analysis of marine omega-3 supplement trials
In 2021, Baris Gencer and colleagues performed a meta-analysis of these five trials plus 2 more (GISSI-HF and RP) looking specifically at risk for AFib. Their final analysis included more than 81,000 patients followed for nearly 5 years.
Omega 3 fatty acid supplements associated with a 25% increase in the risk for AFib (HR, 1.25; 95% CI, 1.07-1.46P =.013). Exploring further, they noted a dose-dependent relationship. Most of the increased risk occurred in trials that tested greater than 1 g/d.
Summary
When faced with surprise findings, I like to think things through.
First about plausibility. Omega-3 fatty acids clearly exert electrophysiologic effects on cardiac cells, an increase in AFib risk is plausible. The exact underlying mechanism may be unknown, but exact mechanisms are less important than actual clinical effects (see sodium-glucose cotransporter 2 inhibitors).
What about causality? Factors supporting causality include plausibility, consistency of increased AFib risk in multiple studies, and a dose-response relationship.
I see multiple clinical implications of this observation.
The first is the power of the randomized trial to inform practice. If we relied only on observational evidence, we might have assumed that since high fish consumption in populations associated with lower rates of cardiac events, fish oil supplementation would also reduce cardiac events. Other than the outlier trial, REDUCE-IT, with its mineral oil placebo, the preponderance of the randomized controlled trial evidence does not support fish oils for the reduction of CV events.
Randomized controlled trials also exposed the AFib risk. This would have been difficult to sort out in nonrandom observational studies.
Another underappreciated lesson is the notion that drugs, including supplements, can have off-target effects.
Consider the case of statin drugs. It is widely assumed that statins reduce cardiac events by lowering low-density lipoprotein cholesterol (LDL-C). Yet, statins became a mainstay not because of LDL-C lowering but because multiple trials found that this class of drugs reduced cardiac events without increasing adverse effects.
Omega-3 fatty acids reduce triglyceride levels, but this is not enough to adopt the use of these pills. The lack of consistent reduction in CV events and the off-target signal of AFib risk argue against routine use of fish-oil pills.
I will close with uncertainty. Though there is plausibility and multiple reasons to infer causality of marine omega-3s in increasing AFib risk, the effect size remains unknown.
In an editorial accompanying the recent meta-analysis, epidemiologist Michelle Samuel, MPH, PhD, and electrophysiologist Stanley Nattel, MD, cautioned readers on a technical but important point. It concerns the matter of competing risks, such as death, in the analysis of AFib risk, meaning that patients who died may have developed AFib had they lived. They provide a detailed explanation in the open access article, but the take-home is that the exact effect size is difficult to quantify without patient-level original data.
No matter. I find the signal of increased AFib risk an important one to use at the bedside.
Intermittent AFib has an unpredictable natural history. It often resolves as mysteriously as it arises. When patients take fish-oil supplements, I cite these studies, note the lack of CV protection, then I recommend stopping the pills.
This allows for one of the most important interventions in AFib care: time.
Dr. Mandrola is a clinical electrophysiologist with Baptist Medical Associates, Louisville, Ky. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Questions about omega-3 fatty acid supplements come up often in the atrial fibrillation (AFib) clinic.
The story begins with the simple observation that populations who eat lots of oily fish have fewer coronary events. This correlation provoked great interest in concentrating fish oils in pill form and studying their use to promote health.
OMENI secondary analysis
Peder Myhre, MD, and colleagues recently published a secondary analysis of the OMENI trial looking at both the risk and possible causes of AFib in the omega-3 group.
The OMENI trial randomly assigned slightly more than 1,000 older patients (mean age, 75 years) post–myocardial infarction to either 1.8 g/d of fish oil supplements versus placebo for 2 years. The supplements comprised 930 mg of eicosapentaenoic acid (EPA) and 660 mg of docosahexaenoic acid (DHA). The main trial reported no difference in a composite primary endpoint of MI, revascularization, stroke, death, or hospitalization for heart failure.
The secondary analysis explored the 75% of patients in the main trial who had no history of AFib. It looked at how many in each group developed either true clinical AFib or what the authors called micro-AFib, defined as short bursts of irregular atrial activity lasting seconds.
The sub-analysis had three main findings: Patients in the supplement arm had a 90% higher rate of AFib or micro-AFib, compared with patients on placebo, EPA had the strongest effect on the association, and there was a graded risk for AFib with increasing serum EPA levels.
The authors raised the possibility that more micro-AFib might be a possible mediator of AFib risk.
Trials of low-dose EPA and DHA
First, the low-dose trials. In the ASCEND trial from 2018, more than 15,000 patients with diabetes were randomly assigned to either 1 g of omega-3 fatty acids (460-mg EPA and 380-mg DHA) or mineral oil.
The trial was neutral. After 7.4 years, the primary endpoint of MI, stroke, transient ischemic attack, or cardiovascular death occurred in 8.9% of the supplement group versus 9.2% of the placebo arm.The incidence of AFib was higher in the omega-3 group but did not reach statistical significance (2.1% vs. 1.7% for placebo; hazard ratio, 1.23; 95% confidence interval, 0.98-1.54).
Another neutral CV trial, VITAL, specifically studied the effects of marine omega-3 pills (460-mg EPA and 380-mg DHA) in older adults without heart disease, cancer, or AFib. After slightly more than 5 years, AFib occurred at a similar rate in the active arm and placebo arms (3.7% vs. 3.4% for placebo; HR, 1.09; 95% CI, 0.96-1.24; P = .19)
Trials of very high-dose marine omega-3s
Next came trials of higher doses in higher-risk populations.
In 2020, JAMA published the STRENGTH trial, which compared 4 g/d of a carboxylic acid formulation of EPA and DHA with a corn oil placebo in more than 13,000 patients who either had established atherosclerotic CV disease (ASCVD) or were at high risk for ASCVD.
The trial was terminated early because of futility and a signal of increased AFib risk in the supplement arm.
Nearly the same number of patients in the supplement versus placebo arm experienced a primary composite endpoint of major adverse cardiac events: 12.0% versus 12.2%, respectively.
AFib was a tertiary endpoint in this trial. An increase in investigator-reported new-onset AFib was observed in the omega-3 group: 2.2% vs. 1.3% for corn oil (HR, 1.69; 95% CI, 1.29-2.21; nominal P < .001).
The REDUCE-IT trial randomly assigned more than 8,000 patients who had ASCVD or diabetes and high ASCVD risk and elevated triglyceride levels to either 4 g of icosapent ethyl daily, a concentrated form of EPA, or a mineral oil placebo.
After nearly 5 years, there was a 4.8% absolute risk reduction in the primary endpoint of CV death, MI, stroke, revascularization, or unstable angina with icosapent ethyl. An increase in atherogenic biomarkers in the mineral oil placebo complicated interpretation of this trial.
Hospitalization for AFib or flutter occurred in 3.1% of the active arm versus 2.1% of the mineral oil group (P = .004).
Meta-analysis of marine omega-3 supplement trials
In 2021, Baris Gencer and colleagues performed a meta-analysis of these five trials plus 2 more (GISSI-HF and RP) looking specifically at risk for AFib. Their final analysis included more than 81,000 patients followed for nearly 5 years.
Omega 3 fatty acid supplements associated with a 25% increase in the risk for AFib (HR, 1.25; 95% CI, 1.07-1.46P =.013). Exploring further, they noted a dose-dependent relationship. Most of the increased risk occurred in trials that tested greater than 1 g/d.
Summary
When faced with surprise findings, I like to think things through.
First about plausibility. Omega-3 fatty acids clearly exert electrophysiologic effects on cardiac cells, an increase in AFib risk is plausible. The exact underlying mechanism may be unknown, but exact mechanisms are less important than actual clinical effects (see sodium-glucose cotransporter 2 inhibitors).
What about causality? Factors supporting causality include plausibility, consistency of increased AFib risk in multiple studies, and a dose-response relationship.
I see multiple clinical implications of this observation.
The first is the power of the randomized trial to inform practice. If we relied only on observational evidence, we might have assumed that since high fish consumption in populations associated with lower rates of cardiac events, fish oil supplementation would also reduce cardiac events. Other than the outlier trial, REDUCE-IT, with its mineral oil placebo, the preponderance of the randomized controlled trial evidence does not support fish oils for the reduction of CV events.
Randomized controlled trials also exposed the AFib risk. This would have been difficult to sort out in nonrandom observational studies.
Another underappreciated lesson is the notion that drugs, including supplements, can have off-target effects.
Consider the case of statin drugs. It is widely assumed that statins reduce cardiac events by lowering low-density lipoprotein cholesterol (LDL-C). Yet, statins became a mainstay not because of LDL-C lowering but because multiple trials found that this class of drugs reduced cardiac events without increasing adverse effects.
Omega-3 fatty acids reduce triglyceride levels, but this is not enough to adopt the use of these pills. The lack of consistent reduction in CV events and the off-target signal of AFib risk argue against routine use of fish-oil pills.
I will close with uncertainty. Though there is plausibility and multiple reasons to infer causality of marine omega-3s in increasing AFib risk, the effect size remains unknown.
In an editorial accompanying the recent meta-analysis, epidemiologist Michelle Samuel, MPH, PhD, and electrophysiologist Stanley Nattel, MD, cautioned readers on a technical but important point. It concerns the matter of competing risks, such as death, in the analysis of AFib risk, meaning that patients who died may have developed AFib had they lived. They provide a detailed explanation in the open access article, but the take-home is that the exact effect size is difficult to quantify without patient-level original data.
No matter. I find the signal of increased AFib risk an important one to use at the bedside.
Intermittent AFib has an unpredictable natural history. It often resolves as mysteriously as it arises. When patients take fish-oil supplements, I cite these studies, note the lack of CV protection, then I recommend stopping the pills.
This allows for one of the most important interventions in AFib care: time.
Dr. Mandrola is a clinical electrophysiologist with Baptist Medical Associates, Louisville, Ky. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.



