User login
Serious arrhythmias playing video games ‘extremely rare’
provided their condition is properly treated, say researchers based on their large, single-center study.
Among more than 3,000 patients in the study with such a genetic vulnerability, just 6 – or less than 0.2% – experienced an electronic gaming–associated cardiac event.
A previous study had concluded that e-gaming, particularly with war games, might trigger potentially fatal arrhythmias in some vulnerable children. That study “sparked controversy in the field, with both clinicians and patients wondering whether electronic gaming is safe for patients with GHDs,” Michael J. Ackerman, MD, PhD, of Mayo Clinic in Rochester, Minn., said in an interview.
Dr. Ackerman and colleagues conducted the current study, published online in the Journal of the American College of Cardiology, to determine just how often e-gaming triggered cardiac events (CE) in these patients – and who was most at risk.
‘Extremely low’ risk
The investigators looked at records from all patients evaluated and treated at the Mayo Clinic’s genetic heart rhythm clinic from 2000 to 2022. They identified those with a history of playing electronic games at the time of their CE, defined here as such an event occurring before diagnosis, or breakthrough cardiac event (BCE), meaning an event occurring after diagnosis.
A total of 3,370 patients with a GHD (55% female) were included in the analysis. More than half (52%) were diagnosed with long-QT syndrome (LQTS). The remainder had various GHDs including, among others, catecholaminergic polymorphic ventricular tachycardia (CPVT) or hypertrophic cardiomyopathy (HCM).
The mean age at first evaluation was 27; 14% of the participants were age 6 or younger, 33% were age 7-20, and 53% were 21 or older. Most patients in each of the three age groups were diagnosed with either LQTS or CPVT.
Of the 3,370 GHD patients, 1,079 (32%) had a CE before diagnosis.
Six patients (0.5%) had a CE in the setting of e-gaming, including five for whom it was the sentinel CE. Five also had CEs in settings not involving e-gaming. Their average age at the time of the CE was 13.
Three of the six patients were diagnosed with CPVT (including two CPVT1 and one CPVT2). Of the others, one was diagnosed with LQT1, one with ventricular fibrillation triggered by premature ventricular contractions, and one with catecholamine-sensitive right ventricular outflow tract ventricular tachycardia (RVOT-VT).
After appropriate treatment, none of the six experienced a BCE during follow-ups ranging from 7 months to 4 years.
Among the full cohort of 3370 patients with GHD, 431 (13%) experienced one or more BCE during follow-up. Of those, one with catecholamine-sensitive RVOT-VT experienced an e-gaming–associated BCE.
“Although anecdotal e-gaming–associated cardiac events, including [sudden cardiac death], have been reported, the absolute risk is extremely low,” the authors wrote.
“Although there are no clear health benefits associated with e-gaming,” Dr. Ackerman said, “the risk of sudden death should not be used as an argument in an effort to curtail the amount of time patients spend e-gaming.”
Furthermore, he added, e-gaming is important to some patients’ quality of life. If patients are “properly diagnosed, risk stratified, and treated, it is okay to engage in e-gaming.”
However, “given that e-gaming may pose some risks, especially when compounded with additional factors such as dehydration, sleep deprivation, and use of performance-enhancing substances such as energy drinks, patients need to be counseled on the potential adverse health consequences,” Dr. Ackerman said.
“To this end,” he added, “we are proponents of incorporating e-gaming status into the clinical evaluation and electronic health record.”
“We would continue to urge common sense and individual risk assessment, with shared decision-making, for those where this may be an issue,” Claire M. Lawley, MBBS, PhD, Children’s Hospital at Westmead (Australia), said in an interview.
“Additionally, syncope during electronic gaming should prompt medical review,” said Dr. Lawley, lead author of the study that prompted Ackerman and colleagues to investigate the issue further.
Buddy system
Maully J. Shah, MBBS, led a study published in 2020 focusing on two case reports of syncope and potentially life-threatening ventricular arrhythmias provoked by emotional surges during play with violent video games.
Nevertheless, “we do not restrict patients from participating in e-games,” Dr. Shah, a pediatric cardiac electrophysiologist at the Cardiac Center at Children’s Hospital of Philadelphia, said in an interview. “We inform them about the available data regarding the very rare but possible occurrence of an event from e-gaming so that they can make an informed decision.”
Dr. Shah agreed that, “even in children not known to have a cardiac condition, syncope associated with emotional responses during violent video games should prompt cardiac evaluation, similar to exercise-induced syncope.”
If a patient wishes to play e-games, clinicians should ensure medication compliance and recommend a “buddy” system. “Don’t be alone while playing,” she said.
“The present study and previous reports make one pause to think whether these CEs and catecholaminergic drives can occur with sports only. If we now consider electronic gaming as a potential risk, what other activities need to be included?” wrote the authors of an accompanying editorial, led by Shankar Baskar, MD, Cincinnati Children’s Medical Center.
“A catecholaminergic drive can occur in many settings with activities of daily living or activities not considered to be competitive,” the editorialists wrote. “Ultimately these events [are] rare, but they can have life-threatening consequences, and at the same time they might not be altogether preventable and, as in electronic gaming, might be an activity that improves quality of life, especially in those who might be restricted from other sports.”
Dr. Ackerman disclosed consulting for Abbott, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Invitae, Medtronic, Tenaya Therapeutics, and UpToDate. Dr. Ackerman and the Mayo Clinic have license agreements with AliveCor, Anumana, ARMGO Pharma, Pfizer, and Thryv Therapeutics. The other coauthors reported no relevant relationships. Dr. Baskar and colleagues reported no relevant relationships. Dr. Shah disclosed she is a consultant to Medtronic.
A version of this article first appeared on Medscape.com.
provided their condition is properly treated, say researchers based on their large, single-center study.
Among more than 3,000 patients in the study with such a genetic vulnerability, just 6 – or less than 0.2% – experienced an electronic gaming–associated cardiac event.
A previous study had concluded that e-gaming, particularly with war games, might trigger potentially fatal arrhythmias in some vulnerable children. That study “sparked controversy in the field, with both clinicians and patients wondering whether electronic gaming is safe for patients with GHDs,” Michael J. Ackerman, MD, PhD, of Mayo Clinic in Rochester, Minn., said in an interview.
Dr. Ackerman and colleagues conducted the current study, published online in the Journal of the American College of Cardiology, to determine just how often e-gaming triggered cardiac events (CE) in these patients – and who was most at risk.
‘Extremely low’ risk
The investigators looked at records from all patients evaluated and treated at the Mayo Clinic’s genetic heart rhythm clinic from 2000 to 2022. They identified those with a history of playing electronic games at the time of their CE, defined here as such an event occurring before diagnosis, or breakthrough cardiac event (BCE), meaning an event occurring after diagnosis.
A total of 3,370 patients with a GHD (55% female) were included in the analysis. More than half (52%) were diagnosed with long-QT syndrome (LQTS). The remainder had various GHDs including, among others, catecholaminergic polymorphic ventricular tachycardia (CPVT) or hypertrophic cardiomyopathy (HCM).
The mean age at first evaluation was 27; 14% of the participants were age 6 or younger, 33% were age 7-20, and 53% were 21 or older. Most patients in each of the three age groups were diagnosed with either LQTS or CPVT.
Of the 3,370 GHD patients, 1,079 (32%) had a CE before diagnosis.
Six patients (0.5%) had a CE in the setting of e-gaming, including five for whom it was the sentinel CE. Five also had CEs in settings not involving e-gaming. Their average age at the time of the CE was 13.
Three of the six patients were diagnosed with CPVT (including two CPVT1 and one CPVT2). Of the others, one was diagnosed with LQT1, one with ventricular fibrillation triggered by premature ventricular contractions, and one with catecholamine-sensitive right ventricular outflow tract ventricular tachycardia (RVOT-VT).
After appropriate treatment, none of the six experienced a BCE during follow-ups ranging from 7 months to 4 years.
Among the full cohort of 3370 patients with GHD, 431 (13%) experienced one or more BCE during follow-up. Of those, one with catecholamine-sensitive RVOT-VT experienced an e-gaming–associated BCE.
“Although anecdotal e-gaming–associated cardiac events, including [sudden cardiac death], have been reported, the absolute risk is extremely low,” the authors wrote.
“Although there are no clear health benefits associated with e-gaming,” Dr. Ackerman said, “the risk of sudden death should not be used as an argument in an effort to curtail the amount of time patients spend e-gaming.”
Furthermore, he added, e-gaming is important to some patients’ quality of life. If patients are “properly diagnosed, risk stratified, and treated, it is okay to engage in e-gaming.”
However, “given that e-gaming may pose some risks, especially when compounded with additional factors such as dehydration, sleep deprivation, and use of performance-enhancing substances such as energy drinks, patients need to be counseled on the potential adverse health consequences,” Dr. Ackerman said.
“To this end,” he added, “we are proponents of incorporating e-gaming status into the clinical evaluation and electronic health record.”
“We would continue to urge common sense and individual risk assessment, with shared decision-making, for those where this may be an issue,” Claire M. Lawley, MBBS, PhD, Children’s Hospital at Westmead (Australia), said in an interview.
“Additionally, syncope during electronic gaming should prompt medical review,” said Dr. Lawley, lead author of the study that prompted Ackerman and colleagues to investigate the issue further.
Buddy system
Maully J. Shah, MBBS, led a study published in 2020 focusing on two case reports of syncope and potentially life-threatening ventricular arrhythmias provoked by emotional surges during play with violent video games.
Nevertheless, “we do not restrict patients from participating in e-games,” Dr. Shah, a pediatric cardiac electrophysiologist at the Cardiac Center at Children’s Hospital of Philadelphia, said in an interview. “We inform them about the available data regarding the very rare but possible occurrence of an event from e-gaming so that they can make an informed decision.”
Dr. Shah agreed that, “even in children not known to have a cardiac condition, syncope associated with emotional responses during violent video games should prompt cardiac evaluation, similar to exercise-induced syncope.”
If a patient wishes to play e-games, clinicians should ensure medication compliance and recommend a “buddy” system. “Don’t be alone while playing,” she said.
“The present study and previous reports make one pause to think whether these CEs and catecholaminergic drives can occur with sports only. If we now consider electronic gaming as a potential risk, what other activities need to be included?” wrote the authors of an accompanying editorial, led by Shankar Baskar, MD, Cincinnati Children’s Medical Center.
“A catecholaminergic drive can occur in many settings with activities of daily living or activities not considered to be competitive,” the editorialists wrote. “Ultimately these events [are] rare, but they can have life-threatening consequences, and at the same time they might not be altogether preventable and, as in electronic gaming, might be an activity that improves quality of life, especially in those who might be restricted from other sports.”
Dr. Ackerman disclosed consulting for Abbott, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Invitae, Medtronic, Tenaya Therapeutics, and UpToDate. Dr. Ackerman and the Mayo Clinic have license agreements with AliveCor, Anumana, ARMGO Pharma, Pfizer, and Thryv Therapeutics. The other coauthors reported no relevant relationships. Dr. Baskar and colleagues reported no relevant relationships. Dr. Shah disclosed she is a consultant to Medtronic.
A version of this article first appeared on Medscape.com.
provided their condition is properly treated, say researchers based on their large, single-center study.
Among more than 3,000 patients in the study with such a genetic vulnerability, just 6 – or less than 0.2% – experienced an electronic gaming–associated cardiac event.
A previous study had concluded that e-gaming, particularly with war games, might trigger potentially fatal arrhythmias in some vulnerable children. That study “sparked controversy in the field, with both clinicians and patients wondering whether electronic gaming is safe for patients with GHDs,” Michael J. Ackerman, MD, PhD, of Mayo Clinic in Rochester, Minn., said in an interview.
Dr. Ackerman and colleagues conducted the current study, published online in the Journal of the American College of Cardiology, to determine just how often e-gaming triggered cardiac events (CE) in these patients – and who was most at risk.
‘Extremely low’ risk
The investigators looked at records from all patients evaluated and treated at the Mayo Clinic’s genetic heart rhythm clinic from 2000 to 2022. They identified those with a history of playing electronic games at the time of their CE, defined here as such an event occurring before diagnosis, or breakthrough cardiac event (BCE), meaning an event occurring after diagnosis.
A total of 3,370 patients with a GHD (55% female) were included in the analysis. More than half (52%) were diagnosed with long-QT syndrome (LQTS). The remainder had various GHDs including, among others, catecholaminergic polymorphic ventricular tachycardia (CPVT) or hypertrophic cardiomyopathy (HCM).
The mean age at first evaluation was 27; 14% of the participants were age 6 or younger, 33% were age 7-20, and 53% were 21 or older. Most patients in each of the three age groups were diagnosed with either LQTS or CPVT.
Of the 3,370 GHD patients, 1,079 (32%) had a CE before diagnosis.
Six patients (0.5%) had a CE in the setting of e-gaming, including five for whom it was the sentinel CE. Five also had CEs in settings not involving e-gaming. Their average age at the time of the CE was 13.
Three of the six patients were diagnosed with CPVT (including two CPVT1 and one CPVT2). Of the others, one was diagnosed with LQT1, one with ventricular fibrillation triggered by premature ventricular contractions, and one with catecholamine-sensitive right ventricular outflow tract ventricular tachycardia (RVOT-VT).
After appropriate treatment, none of the six experienced a BCE during follow-ups ranging from 7 months to 4 years.
Among the full cohort of 3370 patients with GHD, 431 (13%) experienced one or more BCE during follow-up. Of those, one with catecholamine-sensitive RVOT-VT experienced an e-gaming–associated BCE.
“Although anecdotal e-gaming–associated cardiac events, including [sudden cardiac death], have been reported, the absolute risk is extremely low,” the authors wrote.
“Although there are no clear health benefits associated with e-gaming,” Dr. Ackerman said, “the risk of sudden death should not be used as an argument in an effort to curtail the amount of time patients spend e-gaming.”
Furthermore, he added, e-gaming is important to some patients’ quality of life. If patients are “properly diagnosed, risk stratified, and treated, it is okay to engage in e-gaming.”
However, “given that e-gaming may pose some risks, especially when compounded with additional factors such as dehydration, sleep deprivation, and use of performance-enhancing substances such as energy drinks, patients need to be counseled on the potential adverse health consequences,” Dr. Ackerman said.
“To this end,” he added, “we are proponents of incorporating e-gaming status into the clinical evaluation and electronic health record.”
“We would continue to urge common sense and individual risk assessment, with shared decision-making, for those where this may be an issue,” Claire M. Lawley, MBBS, PhD, Children’s Hospital at Westmead (Australia), said in an interview.
“Additionally, syncope during electronic gaming should prompt medical review,” said Dr. Lawley, lead author of the study that prompted Ackerman and colleagues to investigate the issue further.
Buddy system
Maully J. Shah, MBBS, led a study published in 2020 focusing on two case reports of syncope and potentially life-threatening ventricular arrhythmias provoked by emotional surges during play with violent video games.
Nevertheless, “we do not restrict patients from participating in e-games,” Dr. Shah, a pediatric cardiac electrophysiologist at the Cardiac Center at Children’s Hospital of Philadelphia, said in an interview. “We inform them about the available data regarding the very rare but possible occurrence of an event from e-gaming so that they can make an informed decision.”
Dr. Shah agreed that, “even in children not known to have a cardiac condition, syncope associated with emotional responses during violent video games should prompt cardiac evaluation, similar to exercise-induced syncope.”
If a patient wishes to play e-games, clinicians should ensure medication compliance and recommend a “buddy” system. “Don’t be alone while playing,” she said.
“The present study and previous reports make one pause to think whether these CEs and catecholaminergic drives can occur with sports only. If we now consider electronic gaming as a potential risk, what other activities need to be included?” wrote the authors of an accompanying editorial, led by Shankar Baskar, MD, Cincinnati Children’s Medical Center.
“A catecholaminergic drive can occur in many settings with activities of daily living or activities not considered to be competitive,” the editorialists wrote. “Ultimately these events [are] rare, but they can have life-threatening consequences, and at the same time they might not be altogether preventable and, as in electronic gaming, might be an activity that improves quality of life, especially in those who might be restricted from other sports.”
Dr. Ackerman disclosed consulting for Abbott, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Invitae, Medtronic, Tenaya Therapeutics, and UpToDate. Dr. Ackerman and the Mayo Clinic have license agreements with AliveCor, Anumana, ARMGO Pharma, Pfizer, and Thryv Therapeutics. The other coauthors reported no relevant relationships. Dr. Baskar and colleagues reported no relevant relationships. Dr. Shah disclosed she is a consultant to Medtronic.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
LAAO tied to fewer post-fall bleeds than DOACs in AF
Left atrial appendage occlusion (LAAO) is associated with fewer injuries and less bleeding from falls than anticoagulant medications in patients with atrial fibrillation (AF) and a previous stroke, a new cohort study suggests.
Investigators prospectively followed more than 1,250 patients with AF and a previous ischemic stroke. Approximately half underwent LAAO, while the other half were treated with direct oral anticoagulants (DOACs). Patients were followed for close to 2 years.
Slightly more than 20% of patients fell during that period in each group, and , who were not taking anticoagulants. The risk for a major bleed, including an intracranial bleed, was 70% lower in the LAAO group.
LAAO has previously been considered for people at risk of bleeding events – for example, those with gastrointestinal (GI) bleeds, bruising, or intracranial bleeding – but had not yet been studied in those at risk for falls, coauthor Moussa Mansour, MD, professor of medicine, Harvard Medical School, and director of the Atrial Fibrillation Program at Massachusetts General Hospital, Boston, said in an interview.
This is the first study to focus on LAAO specifically for those at risk for falling and demonstrated that the LAAO has utility in this population as well, which is important because the U.S. population is an aging population, and at advanced ages, “people’s balance becomes unsteady and they are at high risk of falling,” he said.
The findings were published online as a research letter in the Journal of the American College of Cardiology.
Multidisciplinary collaboration
“More than one in five of our neurology patients with AF fall – many with devastating consequences – making stroke prevention extremely challenging,” senior author MingMing Ning, MD, MMsc, associate professor of neurology, Harvard Medical School, and director of the Cardio-Neurology and the Clinical Proteomics Research Center at Massachusetts General Hospital, Boston, said in an interview.
“There is a dire need to tailor treatment to keep our patients safe while preventing future strokes,” she said.
Anticoagulants are effective in stroke prevention in these patients but are associated with a higher risk for major bleeding, especially after a fall.
The current prospective observational study recruited 1,266 stroke patients who were treated either with LAAO or DOACs (n = 570 and 696, respectively). Patients were followed for a median of 1.8 years (IQR: 0.9-3.0).
During the follow-up period, 22.6% of LAAO-treated patients and 22.7% of DOAC-treated patients sustained a fall (mean age 78.9 years, 57.4% male and 79.1 years, 52.5% male respectively).
Fall severity, evaluated via the Injury Severity Score, was less in the LAAO vs. the DOAC group, with ISS scores of 1 (IQR 1-4) vs. 4 (IQR 1.75-9).
LAAO was associated with significantly reduced severity of fall-related injuries (OR, –1.09, 95% confidence interval [CI], –1.52 to –0.66; P < .001) – a finding that remained statistically significant after adjustment for confounders such as age, sex, and comorbidities contributing to fall risk, such as hypertension, hyperlipidemia, and diabetes.
The incidence of major trauma (defined as ISS >15) was lower in the LAAO group, compared to the DOAC group (0.8% vs. 6.3%, respectively, P = .026), and LAAO-treated patients had a shorter length of hospital stay, with fewer LAAO patients compared with DOAC patients staying in the hospital for more than 3 days (17% vs. 29.1%, respectively, P = .018).
The risk for major post-fall bleeding was lower in the LAAO vs. the DOAC group (4.7% vs. 15.2%, AOR, 0.29; 95% CI, 0.11-0.73; P = .009) – a finding that included intracranial bleeding (3.1% vs. 9.5%; AOR, 0.29; 95% CI, 0.09-0.90; P = .033).
“Many people are living to advanced ages, where their balance becomes unsteady, and in addition, we have an increase in the prevalence of AF because people are living longer and it’s a disease of the elderly, because we have more hypertension, and we also have more tools to diagnose AF. It’s almost a ‘perfect storm’ situation, and we need effective interventions in this population,” said Dr. Mansour.
Before the study, people at risk for falling were not being considered for LAAO; but now, “we believe they should be considered,” he added. “And although people in the current study had all experienced an ischemic stroke, any patient at risk of a fall will potentially benefit.”
Beyond demonstrating the role of LAAO in reducing the risk of post-fall bleeding injuries, the study – which was conducted by specialists in neurology and cardiology among other fields – highlights the importance of multidisciplinary collaboration, which is “key” for effective stroke prevention, Dr. Ning said.
She emphasized that “we need to learn from our patients and tailor treatment to their needs. A patient’s risk of falling, lifestyle, and medication adherence are all important for individualizing care and improving quality of life.”
Better option
Commenting for this article, Andrea Natale, MD, executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the authors “should be commended for this prospective study on real-world patients that has yielded highly meaningful data from a clinical standpoint.”
Dr. Natale, who was not involved with the study, said it has “several strong points,” such as a fairly large sample size, exclusive population with a history of AF-related stroke, long follow-up duration, evaluation of fall incidents by blinded experts, and severity of fall assessed by a validated questionnaire.
“This is the first study that directly compared the outcomes of traumatic fall in patients receiving LAAO vs. DOAC,” he said. “Given that history of fall is an independent predictor of bleeding and death, the study findings by Deng et al. offer the hope for a safer life with the LAAO option in the aging, fall-prone AF population.”
The take-home message is that, in patients with history of stroke, LAAO “is a better option, in terms of significantly reduced injury severity and shortened hospital length of stay after traumatic falls,” Dr. Natale said.
This study was supported in part by research grants from Boston Scientific, the Leducq Foundation, and the National Institutes of Health. The authors reported no relevant financial relationships. Dr. Natale is a consultant for Abbott, Baylis, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
A version of this article appeared on Medscape.com.
Left atrial appendage occlusion (LAAO) is associated with fewer injuries and less bleeding from falls than anticoagulant medications in patients with atrial fibrillation (AF) and a previous stroke, a new cohort study suggests.
Investigators prospectively followed more than 1,250 patients with AF and a previous ischemic stroke. Approximately half underwent LAAO, while the other half were treated with direct oral anticoagulants (DOACs). Patients were followed for close to 2 years.
Slightly more than 20% of patients fell during that period in each group, and , who were not taking anticoagulants. The risk for a major bleed, including an intracranial bleed, was 70% lower in the LAAO group.
LAAO has previously been considered for people at risk of bleeding events – for example, those with gastrointestinal (GI) bleeds, bruising, or intracranial bleeding – but had not yet been studied in those at risk for falls, coauthor Moussa Mansour, MD, professor of medicine, Harvard Medical School, and director of the Atrial Fibrillation Program at Massachusetts General Hospital, Boston, said in an interview.
This is the first study to focus on LAAO specifically for those at risk for falling and demonstrated that the LAAO has utility in this population as well, which is important because the U.S. population is an aging population, and at advanced ages, “people’s balance becomes unsteady and they are at high risk of falling,” he said.
The findings were published online as a research letter in the Journal of the American College of Cardiology.
Multidisciplinary collaboration
“More than one in five of our neurology patients with AF fall – many with devastating consequences – making stroke prevention extremely challenging,” senior author MingMing Ning, MD, MMsc, associate professor of neurology, Harvard Medical School, and director of the Cardio-Neurology and the Clinical Proteomics Research Center at Massachusetts General Hospital, Boston, said in an interview.
“There is a dire need to tailor treatment to keep our patients safe while preventing future strokes,” she said.
Anticoagulants are effective in stroke prevention in these patients but are associated with a higher risk for major bleeding, especially after a fall.
The current prospective observational study recruited 1,266 stroke patients who were treated either with LAAO or DOACs (n = 570 and 696, respectively). Patients were followed for a median of 1.8 years (IQR: 0.9-3.0).
During the follow-up period, 22.6% of LAAO-treated patients and 22.7% of DOAC-treated patients sustained a fall (mean age 78.9 years, 57.4% male and 79.1 years, 52.5% male respectively).
Fall severity, evaluated via the Injury Severity Score, was less in the LAAO vs. the DOAC group, with ISS scores of 1 (IQR 1-4) vs. 4 (IQR 1.75-9).
LAAO was associated with significantly reduced severity of fall-related injuries (OR, –1.09, 95% confidence interval [CI], –1.52 to –0.66; P < .001) – a finding that remained statistically significant after adjustment for confounders such as age, sex, and comorbidities contributing to fall risk, such as hypertension, hyperlipidemia, and diabetes.
The incidence of major trauma (defined as ISS >15) was lower in the LAAO group, compared to the DOAC group (0.8% vs. 6.3%, respectively, P = .026), and LAAO-treated patients had a shorter length of hospital stay, with fewer LAAO patients compared with DOAC patients staying in the hospital for more than 3 days (17% vs. 29.1%, respectively, P = .018).
The risk for major post-fall bleeding was lower in the LAAO vs. the DOAC group (4.7% vs. 15.2%, AOR, 0.29; 95% CI, 0.11-0.73; P = .009) – a finding that included intracranial bleeding (3.1% vs. 9.5%; AOR, 0.29; 95% CI, 0.09-0.90; P = .033).
“Many people are living to advanced ages, where their balance becomes unsteady, and in addition, we have an increase in the prevalence of AF because people are living longer and it’s a disease of the elderly, because we have more hypertension, and we also have more tools to diagnose AF. It’s almost a ‘perfect storm’ situation, and we need effective interventions in this population,” said Dr. Mansour.
Before the study, people at risk for falling were not being considered for LAAO; but now, “we believe they should be considered,” he added. “And although people in the current study had all experienced an ischemic stroke, any patient at risk of a fall will potentially benefit.”
Beyond demonstrating the role of LAAO in reducing the risk of post-fall bleeding injuries, the study – which was conducted by specialists in neurology and cardiology among other fields – highlights the importance of multidisciplinary collaboration, which is “key” for effective stroke prevention, Dr. Ning said.
She emphasized that “we need to learn from our patients and tailor treatment to their needs. A patient’s risk of falling, lifestyle, and medication adherence are all important for individualizing care and improving quality of life.”
Better option
Commenting for this article, Andrea Natale, MD, executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the authors “should be commended for this prospective study on real-world patients that has yielded highly meaningful data from a clinical standpoint.”
Dr. Natale, who was not involved with the study, said it has “several strong points,” such as a fairly large sample size, exclusive population with a history of AF-related stroke, long follow-up duration, evaluation of fall incidents by blinded experts, and severity of fall assessed by a validated questionnaire.
“This is the first study that directly compared the outcomes of traumatic fall in patients receiving LAAO vs. DOAC,” he said. “Given that history of fall is an independent predictor of bleeding and death, the study findings by Deng et al. offer the hope for a safer life with the LAAO option in the aging, fall-prone AF population.”
The take-home message is that, in patients with history of stroke, LAAO “is a better option, in terms of significantly reduced injury severity and shortened hospital length of stay after traumatic falls,” Dr. Natale said.
This study was supported in part by research grants from Boston Scientific, the Leducq Foundation, and the National Institutes of Health. The authors reported no relevant financial relationships. Dr. Natale is a consultant for Abbott, Baylis, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
A version of this article appeared on Medscape.com.
Left atrial appendage occlusion (LAAO) is associated with fewer injuries and less bleeding from falls than anticoagulant medications in patients with atrial fibrillation (AF) and a previous stroke, a new cohort study suggests.
Investigators prospectively followed more than 1,250 patients with AF and a previous ischemic stroke. Approximately half underwent LAAO, while the other half were treated with direct oral anticoagulants (DOACs). Patients were followed for close to 2 years.
Slightly more than 20% of patients fell during that period in each group, and , who were not taking anticoagulants. The risk for a major bleed, including an intracranial bleed, was 70% lower in the LAAO group.
LAAO has previously been considered for people at risk of bleeding events – for example, those with gastrointestinal (GI) bleeds, bruising, or intracranial bleeding – but had not yet been studied in those at risk for falls, coauthor Moussa Mansour, MD, professor of medicine, Harvard Medical School, and director of the Atrial Fibrillation Program at Massachusetts General Hospital, Boston, said in an interview.
This is the first study to focus on LAAO specifically for those at risk for falling and demonstrated that the LAAO has utility in this population as well, which is important because the U.S. population is an aging population, and at advanced ages, “people’s balance becomes unsteady and they are at high risk of falling,” he said.
The findings were published online as a research letter in the Journal of the American College of Cardiology.
Multidisciplinary collaboration
“More than one in five of our neurology patients with AF fall – many with devastating consequences – making stroke prevention extremely challenging,” senior author MingMing Ning, MD, MMsc, associate professor of neurology, Harvard Medical School, and director of the Cardio-Neurology and the Clinical Proteomics Research Center at Massachusetts General Hospital, Boston, said in an interview.
“There is a dire need to tailor treatment to keep our patients safe while preventing future strokes,” she said.
Anticoagulants are effective in stroke prevention in these patients but are associated with a higher risk for major bleeding, especially after a fall.
The current prospective observational study recruited 1,266 stroke patients who were treated either with LAAO or DOACs (n = 570 and 696, respectively). Patients were followed for a median of 1.8 years (IQR: 0.9-3.0).
During the follow-up period, 22.6% of LAAO-treated patients and 22.7% of DOAC-treated patients sustained a fall (mean age 78.9 years, 57.4% male and 79.1 years, 52.5% male respectively).
Fall severity, evaluated via the Injury Severity Score, was less in the LAAO vs. the DOAC group, with ISS scores of 1 (IQR 1-4) vs. 4 (IQR 1.75-9).
LAAO was associated with significantly reduced severity of fall-related injuries (OR, –1.09, 95% confidence interval [CI], –1.52 to –0.66; P < .001) – a finding that remained statistically significant after adjustment for confounders such as age, sex, and comorbidities contributing to fall risk, such as hypertension, hyperlipidemia, and diabetes.
The incidence of major trauma (defined as ISS >15) was lower in the LAAO group, compared to the DOAC group (0.8% vs. 6.3%, respectively, P = .026), and LAAO-treated patients had a shorter length of hospital stay, with fewer LAAO patients compared with DOAC patients staying in the hospital for more than 3 days (17% vs. 29.1%, respectively, P = .018).
The risk for major post-fall bleeding was lower in the LAAO vs. the DOAC group (4.7% vs. 15.2%, AOR, 0.29; 95% CI, 0.11-0.73; P = .009) – a finding that included intracranial bleeding (3.1% vs. 9.5%; AOR, 0.29; 95% CI, 0.09-0.90; P = .033).
“Many people are living to advanced ages, where their balance becomes unsteady, and in addition, we have an increase in the prevalence of AF because people are living longer and it’s a disease of the elderly, because we have more hypertension, and we also have more tools to diagnose AF. It’s almost a ‘perfect storm’ situation, and we need effective interventions in this population,” said Dr. Mansour.
Before the study, people at risk for falling were not being considered for LAAO; but now, “we believe they should be considered,” he added. “And although people in the current study had all experienced an ischemic stroke, any patient at risk of a fall will potentially benefit.”
Beyond demonstrating the role of LAAO in reducing the risk of post-fall bleeding injuries, the study – which was conducted by specialists in neurology and cardiology among other fields – highlights the importance of multidisciplinary collaboration, which is “key” for effective stroke prevention, Dr. Ning said.
She emphasized that “we need to learn from our patients and tailor treatment to their needs. A patient’s risk of falling, lifestyle, and medication adherence are all important for individualizing care and improving quality of life.”
Better option
Commenting for this article, Andrea Natale, MD, executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the authors “should be commended for this prospective study on real-world patients that has yielded highly meaningful data from a clinical standpoint.”
Dr. Natale, who was not involved with the study, said it has “several strong points,” such as a fairly large sample size, exclusive population with a history of AF-related stroke, long follow-up duration, evaluation of fall incidents by blinded experts, and severity of fall assessed by a validated questionnaire.
“This is the first study that directly compared the outcomes of traumatic fall in patients receiving LAAO vs. DOAC,” he said. “Given that history of fall is an independent predictor of bleeding and death, the study findings by Deng et al. offer the hope for a safer life with the LAAO option in the aging, fall-prone AF population.”
The take-home message is that, in patients with history of stroke, LAAO “is a better option, in terms of significantly reduced injury severity and shortened hospital length of stay after traumatic falls,” Dr. Natale said.
This study was supported in part by research grants from Boston Scientific, the Leducq Foundation, and the National Institutes of Health. The authors reported no relevant financial relationships. Dr. Natale is a consultant for Abbott, Baylis, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Cognitive problems transient after AFib ablation
Investigators randomly assigned 100 patients with symptomatic AFib who had failed at least one anti-arrhythmic drug (AAD) to ongoing therapy or to AFib catheter ablation. Patients were followed for 1 year, and changes in cognitive performance were assessed at baseline and at 3, 6, and 12 months.
Although patients in the ablation arm initially showed more cognitive dysfunction than those in the medical arm, at 6 months, the gap was smaller, and at 12 months, no patients in the ablation arm showed signs of cognitive dysfunction. In fact, more than 1 in 10 showed signs of cognitive improvement, compared with no patients in the medical arm.
The study was published online in the July issue of JACC: Clinical Electrophysiology.
Important pillar
Previous research has shown that AFib is associated with cognitive dysfunction independently of stroke, “suggesting that AFib is an additional risk factor for cognitive impairment,” the authors write.
Catheter ablation is an “important pillar” in the management of patients with AFib that is refractory to medical therapy, but postoperative cognitive dysfunction (POCD) may occur in the immediate aftermath of the procedure, they note. Little is known about whether these cognitive changes persist long term, and no randomized studies have investigated this issue.
The researchers randomly assigned 100 patients with symptomatic paroxysmal or persistent AFib who had failed greater than or equal to 1 AAD to receive either medical management or catheter ablation. The mean age of the patients was 59 plus or minus 12 years, 32% were women, and 46% had persistent AFib.
Medical management consisted of optimization of AADs to maintain sinus rhythm. For those who underwent ablation, AADs were discontinued five half-lives prior to the procedure (with the exception of amiodarone).
Participants were followed for 12 months after enrollment. Clinical reviews and cognitive testing were performed at 3, 6, and 12 months during that time.
AADs and oral anticoagulation were weaned and were discontinued 3 months after the procedure, depending on each patient’s individual risk profile.
Cognitive testing included the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Auditory Verbal Learning Test and Semantic Fluency test; the Controlled Oral Word Association test; and the Trail Making Task (parts A and B).
Participants also completed the University of Toronto AFib Symptom Severity Scale at baseline and at all follow-up visits.
The primary endpoint was prevalence of new-onset cognitive dysfunction. Main secondary endpoints included improvement in cognitive function during follow-up; AFib recurrence and AFib function during follow-up; AAD use during follow-up; and changes to AFib symptom severity assessment scores during follow-up.
More research needed
Of the 100 participants, 96 completed the study protocol (52 in the ablation group and 48 in the medical management group). There were no significant differences between the groups regarding baseline demographics, clinical AFib risk factors, and echocardiographic parameters.
At 3 months, new-onset cognitive dysfunction was detected across a wide range of the neuropsychological tests in 14% of participants in the ablation arm, versus 2% of participants in the medical arm (P = .03)
But at 6 months, only 4% of patients in the ablation arm displayed cognitive dysfunction, compared again with 2% in the medical arm (P = .60). And by 12 months, there were no patients with detectable cognitive dysfunction in the ablation arm, compared with the same patient who showed cognitive impairment in the medical arm (P = .30).
Longer ablation time was an independent predictor of new-onset cognitive dysfunction (odds ratio, 1.30; 95% CI, 1.01-1.60; P = .003).
When patients with and those without new-onset cognitive dysfunction were compared, no differences were found in arrhythmia recurrence or AFib burden post ablation.
At 12 months, 14% of those in the ablation arm showed improvement in cognitive performance, compared with no participants in the medical arm (P = .007).
Compared with participants who had no change in cognitive performance, those who had a significant improvement had a trend toward lower AFib recurrence rates (29% vs. 48%; P = .30). However, both groups were found to have a low AFib burden over the 12 months. And the use of AADs at the 12-month mark was significantly lower among those with versus those without cognitive improvement (0% vs. 38%; P = .04).
As early as 3 months post procedure and then at 12 months, participants in the ablation group had significant improvement in AFib-related symptoms, compared with those in the medical arm (for both, P < .001).
“Among a contemporary cohort of symptomatic paroxysmal and persistent AFib patients, catheter ablation was associated with a transient decline in cognitive function in the short-term, followed by recovery at 12 months,” the authors conclude.
They note that further large studies “are required to determine with AFib ablation may prevent the longer-term neurocognitive decline and dementia development associated with AFib.”
‘Reassuring’ findings
In a comment, Andrea Natale, MD, executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said POCD is “very likely due to the vulnerable state of mind and the stress that the patients encounter while undergoing the cardiac procedure,” as well as postsurgical inflammation, which “can cause brief functional alterations in the brain, leading to temporary cognitive impairment.” Inadequate preprocedural anticoagulation may also play a role.
Dr. Natale, co-author of an accompanying editorial, said it’s “prudent” when evaluating cognitive function to use questionnaires that are “sensitive to mild cognitive impairment,” such as the Montreal Cognitive Assessment or the Mini-Mental State Examination.
Additionally, “post-ablation cognitive function should be assessed way after the blanking period to avoid any plausible impact of inflammation, medications, the feeling of being overwhelmed, and the stress of undergoing a cardiac procedure,” advised Dr. Natale, who was not involved with the study.
Also commenting, Matthew Hyman, MD, PhD, an electrophysiologist and assistant professor of medicine at the Hospital of the University of Pennsylvania, called it a “well-done and very reassuring study.”
Dr. Hyman, who was also not part of the research team, added that previous work has shown an association between AFib and dementia, “and it remains to be seen if patients with rhythm control over longer durations than the current studies have the best outcomes.”
The authors of the study are supported by the National Health and Medical Research Council research scholarship. One author of the study is supported by a practitioner fellowship from the National Health and Medical Research Council; has received research support from Biosense Webster, Boston Scientific, Abbott, and Medtronic; and has served on the advisory board of Boston Scientific and Biosense Webster. Dr. Natale is a consultant for Abbott, Baylis, Biosense Webster, Biotronik, Boston Scientific, and Medtronic. Dr. Hyman is a consultant/speaker for Abbott, Biosense Webster and Boston Scientific.
A version of this article first appeared on Medscape.com.
Investigators randomly assigned 100 patients with symptomatic AFib who had failed at least one anti-arrhythmic drug (AAD) to ongoing therapy or to AFib catheter ablation. Patients were followed for 1 year, and changes in cognitive performance were assessed at baseline and at 3, 6, and 12 months.
Although patients in the ablation arm initially showed more cognitive dysfunction than those in the medical arm, at 6 months, the gap was smaller, and at 12 months, no patients in the ablation arm showed signs of cognitive dysfunction. In fact, more than 1 in 10 showed signs of cognitive improvement, compared with no patients in the medical arm.
The study was published online in the July issue of JACC: Clinical Electrophysiology.
Important pillar
Previous research has shown that AFib is associated with cognitive dysfunction independently of stroke, “suggesting that AFib is an additional risk factor for cognitive impairment,” the authors write.
Catheter ablation is an “important pillar” in the management of patients with AFib that is refractory to medical therapy, but postoperative cognitive dysfunction (POCD) may occur in the immediate aftermath of the procedure, they note. Little is known about whether these cognitive changes persist long term, and no randomized studies have investigated this issue.
The researchers randomly assigned 100 patients with symptomatic paroxysmal or persistent AFib who had failed greater than or equal to 1 AAD to receive either medical management or catheter ablation. The mean age of the patients was 59 plus or minus 12 years, 32% were women, and 46% had persistent AFib.
Medical management consisted of optimization of AADs to maintain sinus rhythm. For those who underwent ablation, AADs were discontinued five half-lives prior to the procedure (with the exception of amiodarone).
Participants were followed for 12 months after enrollment. Clinical reviews and cognitive testing were performed at 3, 6, and 12 months during that time.
AADs and oral anticoagulation were weaned and were discontinued 3 months after the procedure, depending on each patient’s individual risk profile.
Cognitive testing included the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Auditory Verbal Learning Test and Semantic Fluency test; the Controlled Oral Word Association test; and the Trail Making Task (parts A and B).
Participants also completed the University of Toronto AFib Symptom Severity Scale at baseline and at all follow-up visits.
The primary endpoint was prevalence of new-onset cognitive dysfunction. Main secondary endpoints included improvement in cognitive function during follow-up; AFib recurrence and AFib function during follow-up; AAD use during follow-up; and changes to AFib symptom severity assessment scores during follow-up.
More research needed
Of the 100 participants, 96 completed the study protocol (52 in the ablation group and 48 in the medical management group). There were no significant differences between the groups regarding baseline demographics, clinical AFib risk factors, and echocardiographic parameters.
At 3 months, new-onset cognitive dysfunction was detected across a wide range of the neuropsychological tests in 14% of participants in the ablation arm, versus 2% of participants in the medical arm (P = .03)
But at 6 months, only 4% of patients in the ablation arm displayed cognitive dysfunction, compared again with 2% in the medical arm (P = .60). And by 12 months, there were no patients with detectable cognitive dysfunction in the ablation arm, compared with the same patient who showed cognitive impairment in the medical arm (P = .30).
Longer ablation time was an independent predictor of new-onset cognitive dysfunction (odds ratio, 1.30; 95% CI, 1.01-1.60; P = .003).
When patients with and those without new-onset cognitive dysfunction were compared, no differences were found in arrhythmia recurrence or AFib burden post ablation.
At 12 months, 14% of those in the ablation arm showed improvement in cognitive performance, compared with no participants in the medical arm (P = .007).
Compared with participants who had no change in cognitive performance, those who had a significant improvement had a trend toward lower AFib recurrence rates (29% vs. 48%; P = .30). However, both groups were found to have a low AFib burden over the 12 months. And the use of AADs at the 12-month mark was significantly lower among those with versus those without cognitive improvement (0% vs. 38%; P = .04).
As early as 3 months post procedure and then at 12 months, participants in the ablation group had significant improvement in AFib-related symptoms, compared with those in the medical arm (for both, P < .001).
“Among a contemporary cohort of symptomatic paroxysmal and persistent AFib patients, catheter ablation was associated with a transient decline in cognitive function in the short-term, followed by recovery at 12 months,” the authors conclude.
They note that further large studies “are required to determine with AFib ablation may prevent the longer-term neurocognitive decline and dementia development associated with AFib.”
‘Reassuring’ findings
In a comment, Andrea Natale, MD, executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said POCD is “very likely due to the vulnerable state of mind and the stress that the patients encounter while undergoing the cardiac procedure,” as well as postsurgical inflammation, which “can cause brief functional alterations in the brain, leading to temporary cognitive impairment.” Inadequate preprocedural anticoagulation may also play a role.
Dr. Natale, co-author of an accompanying editorial, said it’s “prudent” when evaluating cognitive function to use questionnaires that are “sensitive to mild cognitive impairment,” such as the Montreal Cognitive Assessment or the Mini-Mental State Examination.
Additionally, “post-ablation cognitive function should be assessed way after the blanking period to avoid any plausible impact of inflammation, medications, the feeling of being overwhelmed, and the stress of undergoing a cardiac procedure,” advised Dr. Natale, who was not involved with the study.
Also commenting, Matthew Hyman, MD, PhD, an electrophysiologist and assistant professor of medicine at the Hospital of the University of Pennsylvania, called it a “well-done and very reassuring study.”
Dr. Hyman, who was also not part of the research team, added that previous work has shown an association between AFib and dementia, “and it remains to be seen if patients with rhythm control over longer durations than the current studies have the best outcomes.”
The authors of the study are supported by the National Health and Medical Research Council research scholarship. One author of the study is supported by a practitioner fellowship from the National Health and Medical Research Council; has received research support from Biosense Webster, Boston Scientific, Abbott, and Medtronic; and has served on the advisory board of Boston Scientific and Biosense Webster. Dr. Natale is a consultant for Abbott, Baylis, Biosense Webster, Biotronik, Boston Scientific, and Medtronic. Dr. Hyman is a consultant/speaker for Abbott, Biosense Webster and Boston Scientific.
A version of this article first appeared on Medscape.com.
Investigators randomly assigned 100 patients with symptomatic AFib who had failed at least one anti-arrhythmic drug (AAD) to ongoing therapy or to AFib catheter ablation. Patients were followed for 1 year, and changes in cognitive performance were assessed at baseline and at 3, 6, and 12 months.
Although patients in the ablation arm initially showed more cognitive dysfunction than those in the medical arm, at 6 months, the gap was smaller, and at 12 months, no patients in the ablation arm showed signs of cognitive dysfunction. In fact, more than 1 in 10 showed signs of cognitive improvement, compared with no patients in the medical arm.
The study was published online in the July issue of JACC: Clinical Electrophysiology.
Important pillar
Previous research has shown that AFib is associated with cognitive dysfunction independently of stroke, “suggesting that AFib is an additional risk factor for cognitive impairment,” the authors write.
Catheter ablation is an “important pillar” in the management of patients with AFib that is refractory to medical therapy, but postoperative cognitive dysfunction (POCD) may occur in the immediate aftermath of the procedure, they note. Little is known about whether these cognitive changes persist long term, and no randomized studies have investigated this issue.
The researchers randomly assigned 100 patients with symptomatic paroxysmal or persistent AFib who had failed greater than or equal to 1 AAD to receive either medical management or catheter ablation. The mean age of the patients was 59 plus or minus 12 years, 32% were women, and 46% had persistent AFib.
Medical management consisted of optimization of AADs to maintain sinus rhythm. For those who underwent ablation, AADs were discontinued five half-lives prior to the procedure (with the exception of amiodarone).
Participants were followed for 12 months after enrollment. Clinical reviews and cognitive testing were performed at 3, 6, and 12 months during that time.
AADs and oral anticoagulation were weaned and were discontinued 3 months after the procedure, depending on each patient’s individual risk profile.
Cognitive testing included the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Auditory Verbal Learning Test and Semantic Fluency test; the Controlled Oral Word Association test; and the Trail Making Task (parts A and B).
Participants also completed the University of Toronto AFib Symptom Severity Scale at baseline and at all follow-up visits.
The primary endpoint was prevalence of new-onset cognitive dysfunction. Main secondary endpoints included improvement in cognitive function during follow-up; AFib recurrence and AFib function during follow-up; AAD use during follow-up; and changes to AFib symptom severity assessment scores during follow-up.
More research needed
Of the 100 participants, 96 completed the study protocol (52 in the ablation group and 48 in the medical management group). There were no significant differences between the groups regarding baseline demographics, clinical AFib risk factors, and echocardiographic parameters.
At 3 months, new-onset cognitive dysfunction was detected across a wide range of the neuropsychological tests in 14% of participants in the ablation arm, versus 2% of participants in the medical arm (P = .03)
But at 6 months, only 4% of patients in the ablation arm displayed cognitive dysfunction, compared again with 2% in the medical arm (P = .60). And by 12 months, there were no patients with detectable cognitive dysfunction in the ablation arm, compared with the same patient who showed cognitive impairment in the medical arm (P = .30).
Longer ablation time was an independent predictor of new-onset cognitive dysfunction (odds ratio, 1.30; 95% CI, 1.01-1.60; P = .003).
When patients with and those without new-onset cognitive dysfunction were compared, no differences were found in arrhythmia recurrence or AFib burden post ablation.
At 12 months, 14% of those in the ablation arm showed improvement in cognitive performance, compared with no participants in the medical arm (P = .007).
Compared with participants who had no change in cognitive performance, those who had a significant improvement had a trend toward lower AFib recurrence rates (29% vs. 48%; P = .30). However, both groups were found to have a low AFib burden over the 12 months. And the use of AADs at the 12-month mark was significantly lower among those with versus those without cognitive improvement (0% vs. 38%; P = .04).
As early as 3 months post procedure and then at 12 months, participants in the ablation group had significant improvement in AFib-related symptoms, compared with those in the medical arm (for both, P < .001).
“Among a contemporary cohort of symptomatic paroxysmal and persistent AFib patients, catheter ablation was associated with a transient decline in cognitive function in the short-term, followed by recovery at 12 months,” the authors conclude.
They note that further large studies “are required to determine with AFib ablation may prevent the longer-term neurocognitive decline and dementia development associated with AFib.”
‘Reassuring’ findings
In a comment, Andrea Natale, MD, executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said POCD is “very likely due to the vulnerable state of mind and the stress that the patients encounter while undergoing the cardiac procedure,” as well as postsurgical inflammation, which “can cause brief functional alterations in the brain, leading to temporary cognitive impairment.” Inadequate preprocedural anticoagulation may also play a role.
Dr. Natale, co-author of an accompanying editorial, said it’s “prudent” when evaluating cognitive function to use questionnaires that are “sensitive to mild cognitive impairment,” such as the Montreal Cognitive Assessment or the Mini-Mental State Examination.
Additionally, “post-ablation cognitive function should be assessed way after the blanking period to avoid any plausible impact of inflammation, medications, the feeling of being overwhelmed, and the stress of undergoing a cardiac procedure,” advised Dr. Natale, who was not involved with the study.
Also commenting, Matthew Hyman, MD, PhD, an electrophysiologist and assistant professor of medicine at the Hospital of the University of Pennsylvania, called it a “well-done and very reassuring study.”
Dr. Hyman, who was also not part of the research team, added that previous work has shown an association between AFib and dementia, “and it remains to be seen if patients with rhythm control over longer durations than the current studies have the best outcomes.”
The authors of the study are supported by the National Health and Medical Research Council research scholarship. One author of the study is supported by a practitioner fellowship from the National Health and Medical Research Council; has received research support from Biosense Webster, Boston Scientific, Abbott, and Medtronic; and has served on the advisory board of Boston Scientific and Biosense Webster. Dr. Natale is a consultant for Abbott, Baylis, Biosense Webster, Biotronik, Boston Scientific, and Medtronic. Dr. Hyman is a consultant/speaker for Abbott, Biosense Webster and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
S-ICD shows virtues, limits in ‘real-world’ postmarket study
In the latest chapter in the U.S. saga of the subcutaneous implantable cardioverter-defibrillator (S-ICD) system (Boston Scientific) – a large postmarket, multicenter registry study – the device performed at least as well as it did in earlier trials, researchers say.
The device met all prespecified safety and efficacy endpoints in a study that enrolled more than 1,600 patients and followed them for about 5 years, they noted in their report on the S-ICD Post-Approval Study (S-ICD-PAS) published online in the Journal of the American College of Cardiology.
the group reported.
The team was “pleasantly surprised” that the device’s safety and efficacy performance “was as good if not better than previous studies,” despite a sicker group of patients, lead author Michael R. Gold, MD, PhD, Medical University of South Carolina, Charleston, said in an interview.
No predictors of initial-shock failure were identified, suggesting the S-ICD should be effective in a broad range of ICD candidates without indications for pacing, Dr. Gold said.
The S-ICD was approved in Europe in 2008 and by the Food and Drug Administration in 2012. Clinical trials have suggested its performance and risk for inappropriate shocks are in line with transvenous-lead systems for most patients with ICD indications who don’t need pacing, while avoiding the sometimes serious risks posed by transvenous leads.
The S-ICD doesn’t have antitachycardia pacing (ATP), an alternative way to stop some arrhythmias and a universal feature of transvenous-lead systems. Its lack of ATP may be partly responsible for the device’s weak uptake in practice, some observers noted.
The S-ICD-PAS study “laudably included centers with variable prior experience with the S-ICD; however, data were not analyzed by center experience,” Jonathan S. Steinberg, MD, and Valentina Kutyifa, MD, PhD, University of Rochester (N.Y.) Medical Center, New York, wrote in an accompanying editorial regarding the report’s potential limitations.
Also of concern, they wrote, is the large proportion of patients who were lost to follow-up; almost 42% left the study before its prospectively defined end.
Still, wrote Dr. Steinberg and Dr. Kutyifa, S-ICD-PAS “provides robust, long-term evidence in favor of S-ICD use in a diverse cohort of younger patients receiving implants for primary or secondary prevention of sudden arrhythmic death.” Further analyses are needed, however, to clarify its performance “in centers with low vs high volume as well as in important clinical subgroups.”
It’s “reassuring to see the phase 4 postapproval study results sort of corroborate what the initial clinical study shows,” Miguel Leal, MD, Emory University, Atlanta, said in an interview.
The study’s significant attrition rate “does not negate the results because the performance curves of the device remained approximately stable over the 5 years,” he said, “suggesting that the patients who were lost [and whose clinical outcomes were not included] may not have made a significant impact when it comes to the final results.”
Although the S-ICD seems unlikely to cause complications related to endovascular occlusions or infection, “it can still cause complications related to the implant technique, particularly a device site erosion or device dislodgement,” said Dr. Leal, who chairs the American Heart Association Council on Clinical Cardiology–Electrocardiography & Arrhythmia Committee.
The S-ICD’s biggest contribution has been “the ability to promote efficacious therapy without a need for penetrating the endovascular space,” he observed. “We need to continue to push the envelope towards developing device-based technologies that spare the endovascular space.”
The study enrolled 1,643 patients at 86 U.S. centers; their mean age was 53 and 32% were women. Of the total, 1637 were implanted with the device, 665 completed the study, 288 died, and 686 left the study before completing follow-up. Of the latter, 102 (6.2% of the total) underwent S-ICD explantation, often because of infection.
In addition to the overall shock efficacy rate of 98.4%, induced-arrhythmia shock efficacy was 98.7%, first-shock efficacy for spontaneous arrhythmias was 92.2%, and the rate for either induced or spontaneous arrhythmia was 94.7%. A mean of 1.1 shocks was needed to terminate the arrhythmias; time to shock delivery averaged 17.5 seconds.
The rate of inappropriate shocks was 6.7% at 1 year and 15.8% at 5 years, notably with no significant differences between patients who had and had not undergone defibrillation threshold testing at implantation.
Of 516 inappropriate-shock episodes in 224 patients, almost 86% resulted from inappropriate sensing. Inappropriate shocks became less frequent with longer implantation times and during the course of the study.
The rate of freedom from type 1 complications, the primary safety endpoint, was 93.4%, besting the 85% performance goal. The rate of freedom from electrode-related complications was 99.3%, compared with the performance goal of 92.5%.
The S-ICD was replaced by a transvenous system because of the need for pacing in 1.6% of the cohort.
Sana M. Al-Khatib, MD, MHS, who chaired a 2017 multisociety guideline for managing ventricular arrhythmias and prevention of sudden death, acknowledged the 5-year safety and effectiveness of the S-ICD but also highlighted the “very high dropout rate.”
Moreover, given the cohort’s average age, “these results cannot be generalized to much older patients, in their 70s and 80s [for example]. More data on the S-ICD in older patients are needed, especially because some of these patients will need pacing, which is not provided by the S-ICD,” Dr. Al-Khatib, Duke University, Durham, N.C., said in an interview.
Longer follow-up of patients with the S-ICD is also needed, she added, and “having an S-ICD that is smaller with longer battery life would be great for my patients.”
The study was sponsored by Boston Scientific. Dr. Gold reported receiving consulting fees from Boston Scientific and Medtronic and participating in clinical trials with Boston Scientific, Medtronic, and Abbott. Dr. Steinberg, Dr. Kutyifa, Dr. Al-Khatib, and Dr. Leal reported no relevant relationships.
A version of this article first appeared on Medscape.com.
In the latest chapter in the U.S. saga of the subcutaneous implantable cardioverter-defibrillator (S-ICD) system (Boston Scientific) – a large postmarket, multicenter registry study – the device performed at least as well as it did in earlier trials, researchers say.
The device met all prespecified safety and efficacy endpoints in a study that enrolled more than 1,600 patients and followed them for about 5 years, they noted in their report on the S-ICD Post-Approval Study (S-ICD-PAS) published online in the Journal of the American College of Cardiology.
the group reported.
The team was “pleasantly surprised” that the device’s safety and efficacy performance “was as good if not better than previous studies,” despite a sicker group of patients, lead author Michael R. Gold, MD, PhD, Medical University of South Carolina, Charleston, said in an interview.
No predictors of initial-shock failure were identified, suggesting the S-ICD should be effective in a broad range of ICD candidates without indications for pacing, Dr. Gold said.
The S-ICD was approved in Europe in 2008 and by the Food and Drug Administration in 2012. Clinical trials have suggested its performance and risk for inappropriate shocks are in line with transvenous-lead systems for most patients with ICD indications who don’t need pacing, while avoiding the sometimes serious risks posed by transvenous leads.
The S-ICD doesn’t have antitachycardia pacing (ATP), an alternative way to stop some arrhythmias and a universal feature of transvenous-lead systems. Its lack of ATP may be partly responsible for the device’s weak uptake in practice, some observers noted.
The S-ICD-PAS study “laudably included centers with variable prior experience with the S-ICD; however, data were not analyzed by center experience,” Jonathan S. Steinberg, MD, and Valentina Kutyifa, MD, PhD, University of Rochester (N.Y.) Medical Center, New York, wrote in an accompanying editorial regarding the report’s potential limitations.
Also of concern, they wrote, is the large proportion of patients who were lost to follow-up; almost 42% left the study before its prospectively defined end.
Still, wrote Dr. Steinberg and Dr. Kutyifa, S-ICD-PAS “provides robust, long-term evidence in favor of S-ICD use in a diverse cohort of younger patients receiving implants for primary or secondary prevention of sudden arrhythmic death.” Further analyses are needed, however, to clarify its performance “in centers with low vs high volume as well as in important clinical subgroups.”
It’s “reassuring to see the phase 4 postapproval study results sort of corroborate what the initial clinical study shows,” Miguel Leal, MD, Emory University, Atlanta, said in an interview.
The study’s significant attrition rate “does not negate the results because the performance curves of the device remained approximately stable over the 5 years,” he said, “suggesting that the patients who were lost [and whose clinical outcomes were not included] may not have made a significant impact when it comes to the final results.”
Although the S-ICD seems unlikely to cause complications related to endovascular occlusions or infection, “it can still cause complications related to the implant technique, particularly a device site erosion or device dislodgement,” said Dr. Leal, who chairs the American Heart Association Council on Clinical Cardiology–Electrocardiography & Arrhythmia Committee.
The S-ICD’s biggest contribution has been “the ability to promote efficacious therapy without a need for penetrating the endovascular space,” he observed. “We need to continue to push the envelope towards developing device-based technologies that spare the endovascular space.”
The study enrolled 1,643 patients at 86 U.S. centers; their mean age was 53 and 32% were women. Of the total, 1637 were implanted with the device, 665 completed the study, 288 died, and 686 left the study before completing follow-up. Of the latter, 102 (6.2% of the total) underwent S-ICD explantation, often because of infection.
In addition to the overall shock efficacy rate of 98.4%, induced-arrhythmia shock efficacy was 98.7%, first-shock efficacy for spontaneous arrhythmias was 92.2%, and the rate for either induced or spontaneous arrhythmia was 94.7%. A mean of 1.1 shocks was needed to terminate the arrhythmias; time to shock delivery averaged 17.5 seconds.
The rate of inappropriate shocks was 6.7% at 1 year and 15.8% at 5 years, notably with no significant differences between patients who had and had not undergone defibrillation threshold testing at implantation.
Of 516 inappropriate-shock episodes in 224 patients, almost 86% resulted from inappropriate sensing. Inappropriate shocks became less frequent with longer implantation times and during the course of the study.
The rate of freedom from type 1 complications, the primary safety endpoint, was 93.4%, besting the 85% performance goal. The rate of freedom from electrode-related complications was 99.3%, compared with the performance goal of 92.5%.
The S-ICD was replaced by a transvenous system because of the need for pacing in 1.6% of the cohort.
Sana M. Al-Khatib, MD, MHS, who chaired a 2017 multisociety guideline for managing ventricular arrhythmias and prevention of sudden death, acknowledged the 5-year safety and effectiveness of the S-ICD but also highlighted the “very high dropout rate.”
Moreover, given the cohort’s average age, “these results cannot be generalized to much older patients, in their 70s and 80s [for example]. More data on the S-ICD in older patients are needed, especially because some of these patients will need pacing, which is not provided by the S-ICD,” Dr. Al-Khatib, Duke University, Durham, N.C., said in an interview.
Longer follow-up of patients with the S-ICD is also needed, she added, and “having an S-ICD that is smaller with longer battery life would be great for my patients.”
The study was sponsored by Boston Scientific. Dr. Gold reported receiving consulting fees from Boston Scientific and Medtronic and participating in clinical trials with Boston Scientific, Medtronic, and Abbott. Dr. Steinberg, Dr. Kutyifa, Dr. Al-Khatib, and Dr. Leal reported no relevant relationships.
A version of this article first appeared on Medscape.com.
In the latest chapter in the U.S. saga of the subcutaneous implantable cardioverter-defibrillator (S-ICD) system (Boston Scientific) – a large postmarket, multicenter registry study – the device performed at least as well as it did in earlier trials, researchers say.
The device met all prespecified safety and efficacy endpoints in a study that enrolled more than 1,600 patients and followed them for about 5 years, they noted in their report on the S-ICD Post-Approval Study (S-ICD-PAS) published online in the Journal of the American College of Cardiology.
the group reported.
The team was “pleasantly surprised” that the device’s safety and efficacy performance “was as good if not better than previous studies,” despite a sicker group of patients, lead author Michael R. Gold, MD, PhD, Medical University of South Carolina, Charleston, said in an interview.
No predictors of initial-shock failure were identified, suggesting the S-ICD should be effective in a broad range of ICD candidates without indications for pacing, Dr. Gold said.
The S-ICD was approved in Europe in 2008 and by the Food and Drug Administration in 2012. Clinical trials have suggested its performance and risk for inappropriate shocks are in line with transvenous-lead systems for most patients with ICD indications who don’t need pacing, while avoiding the sometimes serious risks posed by transvenous leads.
The S-ICD doesn’t have antitachycardia pacing (ATP), an alternative way to stop some arrhythmias and a universal feature of transvenous-lead systems. Its lack of ATP may be partly responsible for the device’s weak uptake in practice, some observers noted.
The S-ICD-PAS study “laudably included centers with variable prior experience with the S-ICD; however, data were not analyzed by center experience,” Jonathan S. Steinberg, MD, and Valentina Kutyifa, MD, PhD, University of Rochester (N.Y.) Medical Center, New York, wrote in an accompanying editorial regarding the report’s potential limitations.
Also of concern, they wrote, is the large proportion of patients who were lost to follow-up; almost 42% left the study before its prospectively defined end.
Still, wrote Dr. Steinberg and Dr. Kutyifa, S-ICD-PAS “provides robust, long-term evidence in favor of S-ICD use in a diverse cohort of younger patients receiving implants for primary or secondary prevention of sudden arrhythmic death.” Further analyses are needed, however, to clarify its performance “in centers with low vs high volume as well as in important clinical subgroups.”
It’s “reassuring to see the phase 4 postapproval study results sort of corroborate what the initial clinical study shows,” Miguel Leal, MD, Emory University, Atlanta, said in an interview.
The study’s significant attrition rate “does not negate the results because the performance curves of the device remained approximately stable over the 5 years,” he said, “suggesting that the patients who were lost [and whose clinical outcomes were not included] may not have made a significant impact when it comes to the final results.”
Although the S-ICD seems unlikely to cause complications related to endovascular occlusions or infection, “it can still cause complications related to the implant technique, particularly a device site erosion or device dislodgement,” said Dr. Leal, who chairs the American Heart Association Council on Clinical Cardiology–Electrocardiography & Arrhythmia Committee.
The S-ICD’s biggest contribution has been “the ability to promote efficacious therapy without a need for penetrating the endovascular space,” he observed. “We need to continue to push the envelope towards developing device-based technologies that spare the endovascular space.”
The study enrolled 1,643 patients at 86 U.S. centers; their mean age was 53 and 32% were women. Of the total, 1637 were implanted with the device, 665 completed the study, 288 died, and 686 left the study before completing follow-up. Of the latter, 102 (6.2% of the total) underwent S-ICD explantation, often because of infection.
In addition to the overall shock efficacy rate of 98.4%, induced-arrhythmia shock efficacy was 98.7%, first-shock efficacy for spontaneous arrhythmias was 92.2%, and the rate for either induced or spontaneous arrhythmia was 94.7%. A mean of 1.1 shocks was needed to terminate the arrhythmias; time to shock delivery averaged 17.5 seconds.
The rate of inappropriate shocks was 6.7% at 1 year and 15.8% at 5 years, notably with no significant differences between patients who had and had not undergone defibrillation threshold testing at implantation.
Of 516 inappropriate-shock episodes in 224 patients, almost 86% resulted from inappropriate sensing. Inappropriate shocks became less frequent with longer implantation times and during the course of the study.
The rate of freedom from type 1 complications, the primary safety endpoint, was 93.4%, besting the 85% performance goal. The rate of freedom from electrode-related complications was 99.3%, compared with the performance goal of 92.5%.
The S-ICD was replaced by a transvenous system because of the need for pacing in 1.6% of the cohort.
Sana M. Al-Khatib, MD, MHS, who chaired a 2017 multisociety guideline for managing ventricular arrhythmias and prevention of sudden death, acknowledged the 5-year safety and effectiveness of the S-ICD but also highlighted the “very high dropout rate.”
Moreover, given the cohort’s average age, “these results cannot be generalized to much older patients, in their 70s and 80s [for example]. More data on the S-ICD in older patients are needed, especially because some of these patients will need pacing, which is not provided by the S-ICD,” Dr. Al-Khatib, Duke University, Durham, N.C., said in an interview.
Longer follow-up of patients with the S-ICD is also needed, she added, and “having an S-ICD that is smaller with longer battery life would be great for my patients.”
The study was sponsored by Boston Scientific. Dr. Gold reported receiving consulting fees from Boston Scientific and Medtronic and participating in clinical trials with Boston Scientific, Medtronic, and Abbott. Dr. Steinberg, Dr. Kutyifa, Dr. Al-Khatib, and Dr. Leal reported no relevant relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Early or delayed AFib ablation after heart failure hospitalization?
TOPLINE:
Among patients with atrial fibrillation (AFib) hospitalized for worsening heart failure (HF), catheter (cath) ablation within 90 days of admission, compared with other times, is associated with reduced risk for all-cause mortality and HF-related mortality.
METHODOLOGY:
Cath ablation has become technically safer for patients with both AFib and HF, but the best timing for the ablation procedure after HF hospitalization has been unclear.
The study included 2,786 patients with HF who underwent cath ablation for AFib at 128 centers in the nationwide Japanese Registry of Acute Decompensated Heart Failure, were hospitalized with worsening HF, and survived at least 90 days after discharge.
The population included 103 individuals who underwent cath ablation within 90 days after admission; the remaining 2,683 participants served as the control group.
The researchers also looked at all-cause mortality 90 days after admission for HF in analysis of 83 early-ablation cases vs. 83 propensity-matched controls.
TAKEAWAY:
The early–cath ablation group was younger, predominantly male, had less history of prior HF hospitalizations, and greater incidence of paroxysmal AF, compared with the control group.
All-cause mortality was significantly lower in the early–cath ablation group than in the control group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.60; P < .001) over a median of 4.1 years.
Risk reductions were similarly significant for secondary endpoints, including cardiovascular (CV) mortality and HF mortality.
In the matched cohort analysis (83 in both groups) all-cause mortality was significantly reduced for those in the early–cath ablation group, compared with the matched controls (HR, 0.47; 95% CI, 0.25-0.88; P = .014), with similarly significant risk reductions for CV mortality and HF mortality.
IN PRACTICE:
the report states. Early catheter ablation, as early as during the hospitalization for HF, “might be a way to stabilize HF and solve the problems associated with long hospitalization periods and polypharmacy.”
SOURCE:
The study was conducted by Kazuo Sakamoto, MD, PhD, Kyushu University, Fukuoka, Japan, and colleagues. It was published online July 19, 2023 in JACC: Clinical Electrophysiology.
LIMITATIONS:
The early-ablation cohort was much smaller than the control group, and the analysis could not adjust for any variation in institutional characteristics, such as location and available equipment. Other unmeasured potential confounders include duration of AFib and patient lifestyle characteristics and success or failure of ablation.
DISCLOSURES:
The study was funded by Johnson & Johnson, the Japan Agency for Medical Research and Development, and Ministry of Health and Labor. Dr. Sakamoto reports no relevant conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with atrial fibrillation (AFib) hospitalized for worsening heart failure (HF), catheter (cath) ablation within 90 days of admission, compared with other times, is associated with reduced risk for all-cause mortality and HF-related mortality.
METHODOLOGY:
Cath ablation has become technically safer for patients with both AFib and HF, but the best timing for the ablation procedure after HF hospitalization has been unclear.
The study included 2,786 patients with HF who underwent cath ablation for AFib at 128 centers in the nationwide Japanese Registry of Acute Decompensated Heart Failure, were hospitalized with worsening HF, and survived at least 90 days after discharge.
The population included 103 individuals who underwent cath ablation within 90 days after admission; the remaining 2,683 participants served as the control group.
The researchers also looked at all-cause mortality 90 days after admission for HF in analysis of 83 early-ablation cases vs. 83 propensity-matched controls.
TAKEAWAY:
The early–cath ablation group was younger, predominantly male, had less history of prior HF hospitalizations, and greater incidence of paroxysmal AF, compared with the control group.
All-cause mortality was significantly lower in the early–cath ablation group than in the control group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.60; P < .001) over a median of 4.1 years.
Risk reductions were similarly significant for secondary endpoints, including cardiovascular (CV) mortality and HF mortality.
In the matched cohort analysis (83 in both groups) all-cause mortality was significantly reduced for those in the early–cath ablation group, compared with the matched controls (HR, 0.47; 95% CI, 0.25-0.88; P = .014), with similarly significant risk reductions for CV mortality and HF mortality.
IN PRACTICE:
the report states. Early catheter ablation, as early as during the hospitalization for HF, “might be a way to stabilize HF and solve the problems associated with long hospitalization periods and polypharmacy.”
SOURCE:
The study was conducted by Kazuo Sakamoto, MD, PhD, Kyushu University, Fukuoka, Japan, and colleagues. It was published online July 19, 2023 in JACC: Clinical Electrophysiology.
LIMITATIONS:
The early-ablation cohort was much smaller than the control group, and the analysis could not adjust for any variation in institutional characteristics, such as location and available equipment. Other unmeasured potential confounders include duration of AFib and patient lifestyle characteristics and success or failure of ablation.
DISCLOSURES:
The study was funded by Johnson & Johnson, the Japan Agency for Medical Research and Development, and Ministry of Health and Labor. Dr. Sakamoto reports no relevant conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with atrial fibrillation (AFib) hospitalized for worsening heart failure (HF), catheter (cath) ablation within 90 days of admission, compared with other times, is associated with reduced risk for all-cause mortality and HF-related mortality.
METHODOLOGY:
Cath ablation has become technically safer for patients with both AFib and HF, but the best timing for the ablation procedure after HF hospitalization has been unclear.
The study included 2,786 patients with HF who underwent cath ablation for AFib at 128 centers in the nationwide Japanese Registry of Acute Decompensated Heart Failure, were hospitalized with worsening HF, and survived at least 90 days after discharge.
The population included 103 individuals who underwent cath ablation within 90 days after admission; the remaining 2,683 participants served as the control group.
The researchers also looked at all-cause mortality 90 days after admission for HF in analysis of 83 early-ablation cases vs. 83 propensity-matched controls.
TAKEAWAY:
The early–cath ablation group was younger, predominantly male, had less history of prior HF hospitalizations, and greater incidence of paroxysmal AF, compared with the control group.
All-cause mortality was significantly lower in the early–cath ablation group than in the control group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.60; P < .001) over a median of 4.1 years.
Risk reductions were similarly significant for secondary endpoints, including cardiovascular (CV) mortality and HF mortality.
In the matched cohort analysis (83 in both groups) all-cause mortality was significantly reduced for those in the early–cath ablation group, compared with the matched controls (HR, 0.47; 95% CI, 0.25-0.88; P = .014), with similarly significant risk reductions for CV mortality and HF mortality.
IN PRACTICE:
the report states. Early catheter ablation, as early as during the hospitalization for HF, “might be a way to stabilize HF and solve the problems associated with long hospitalization periods and polypharmacy.”
SOURCE:
The study was conducted by Kazuo Sakamoto, MD, PhD, Kyushu University, Fukuoka, Japan, and colleagues. It was published online July 19, 2023 in JACC: Clinical Electrophysiology.
LIMITATIONS:
The early-ablation cohort was much smaller than the control group, and the analysis could not adjust for any variation in institutional characteristics, such as location and available equipment. Other unmeasured potential confounders include duration of AFib and patient lifestyle characteristics and success or failure of ablation.
DISCLOSURES:
The study was funded by Johnson & Johnson, the Japan Agency for Medical Research and Development, and Ministry of Health and Labor. Dr. Sakamoto reports no relevant conflicts.
A version of this article first appeared on Medscape.com.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Omega-3s and AFib: No added risk from eating fish but high-dose supplement questions persist
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
High-dose vitamin D may cut risk for new atrial fibrillation
TOPLINE:
a post hoc analysis from a randomized trial conducted in Finland suggests.
METHODOLOGY:
- Observational studies have suggested that vitamin D deficiency is associated with increased risk for AFib, but few randomized trials have looked at the effect of vitamin D supplementation on AFib incidence in healthy people.
- The study, a post hoc analysis from a trial that explored the effects of vitamin D3 supplementation on incidence of cardiovascular diseases and cancer, included 2,495 vitamin D–sufficient healthy older adults, mean age 68.2 years, of whom 43% were women.
- Participants had been randomized to one of three groups in which they received vitamin D3 at either 1,600 IU/day or 3,200 IU/day, or placebo.
- Serum 25(OH)D3 concentrations were measured and data on incident AFib were gathered from national health records.
TAKEAWAY:
- Atrial fibrillation was diagnosed in 190 participants.
- Over a follow-up averaging 4.1 years, risk for incident AFib was reduced by 27% for participants who received the 1,600 IU/day dose, compared with placebo; hazard ratio, 0.73 (95% confidence interval, 0.52-1.02; P = .07), and by 32% for those in the 3,200 IU/day arm; HR, 0.68 (95% CI, 0.48-0.96; P = .03).
- The incident-AFib risk was reduced by 30% in a comparison of the two vitamin D groups combined versus the placebo group; HR, 0.70 (95% CI, 0.53-0.94; P = .02).
- After exclusion of 122 participants who reported being on antiarrhythmic medications at baseline, the 1,600 IU/day group showed a significant 27% reduction in risk for AF (95% CI, 4%-58%; P = .03) and the 3,200 IU/day group a nonsignificant 30% (95% CI, 5%-53%; P = .08) reduction in risk.
IN PRACTICE:
High-dose vitamin D3 supplementation may reduce incidence of AFib in a generally healthy, largely vitamin D–sufficient elderly population, the authors proposed. Additional controlled trials are needed, especially in diverse populations.
STUDY DETAILS:
The study was conducted by Jyrki K. Virtanen, PhD, University of Eastern Finland, Institute of Public Health and Clinical Nutrition, Kuopio, and colleagues. It was published in the American Heart Journal.
LIMITATIONS:
Atrial fibrillation was not prespecified as a primary outcome, and the results differ from those of other randomized controlled trials. Information on type of AFib (whether paroxysmal or nonparoxysmal, for example) wasn’t available nor were participants’ history of AFib. All participants were White and from Finland, limiting generalizability of the results.
DISCLOSURES:
The study was supported by the Academy of Finland, University of Eastern Finland, the Juho Vainio Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and the Finnish Cultural Foundation. One coauthor disclosed receiving grants from the National Institutes of Health and Mars Edge. Another coauthor disclosed receipt of a grant from Orion. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
a post hoc analysis from a randomized trial conducted in Finland suggests.
METHODOLOGY:
- Observational studies have suggested that vitamin D deficiency is associated with increased risk for AFib, but few randomized trials have looked at the effect of vitamin D supplementation on AFib incidence in healthy people.
- The study, a post hoc analysis from a trial that explored the effects of vitamin D3 supplementation on incidence of cardiovascular diseases and cancer, included 2,495 vitamin D–sufficient healthy older adults, mean age 68.2 years, of whom 43% were women.
- Participants had been randomized to one of three groups in which they received vitamin D3 at either 1,600 IU/day or 3,200 IU/day, or placebo.
- Serum 25(OH)D3 concentrations were measured and data on incident AFib were gathered from national health records.
TAKEAWAY:
- Atrial fibrillation was diagnosed in 190 participants.
- Over a follow-up averaging 4.1 years, risk for incident AFib was reduced by 27% for participants who received the 1,600 IU/day dose, compared with placebo; hazard ratio, 0.73 (95% confidence interval, 0.52-1.02; P = .07), and by 32% for those in the 3,200 IU/day arm; HR, 0.68 (95% CI, 0.48-0.96; P = .03).
- The incident-AFib risk was reduced by 30% in a comparison of the two vitamin D groups combined versus the placebo group; HR, 0.70 (95% CI, 0.53-0.94; P = .02).
- After exclusion of 122 participants who reported being on antiarrhythmic medications at baseline, the 1,600 IU/day group showed a significant 27% reduction in risk for AF (95% CI, 4%-58%; P = .03) and the 3,200 IU/day group a nonsignificant 30% (95% CI, 5%-53%; P = .08) reduction in risk.
IN PRACTICE:
High-dose vitamin D3 supplementation may reduce incidence of AFib in a generally healthy, largely vitamin D–sufficient elderly population, the authors proposed. Additional controlled trials are needed, especially in diverse populations.
STUDY DETAILS:
The study was conducted by Jyrki K. Virtanen, PhD, University of Eastern Finland, Institute of Public Health and Clinical Nutrition, Kuopio, and colleagues. It was published in the American Heart Journal.
LIMITATIONS:
Atrial fibrillation was not prespecified as a primary outcome, and the results differ from those of other randomized controlled trials. Information on type of AFib (whether paroxysmal or nonparoxysmal, for example) wasn’t available nor were participants’ history of AFib. All participants were White and from Finland, limiting generalizability of the results.
DISCLOSURES:
The study was supported by the Academy of Finland, University of Eastern Finland, the Juho Vainio Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and the Finnish Cultural Foundation. One coauthor disclosed receiving grants from the National Institutes of Health and Mars Edge. Another coauthor disclosed receipt of a grant from Orion. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
a post hoc analysis from a randomized trial conducted in Finland suggests.
METHODOLOGY:
- Observational studies have suggested that vitamin D deficiency is associated with increased risk for AFib, but few randomized trials have looked at the effect of vitamin D supplementation on AFib incidence in healthy people.
- The study, a post hoc analysis from a trial that explored the effects of vitamin D3 supplementation on incidence of cardiovascular diseases and cancer, included 2,495 vitamin D–sufficient healthy older adults, mean age 68.2 years, of whom 43% were women.
- Participants had been randomized to one of three groups in which they received vitamin D3 at either 1,600 IU/day or 3,200 IU/day, or placebo.
- Serum 25(OH)D3 concentrations were measured and data on incident AFib were gathered from national health records.
TAKEAWAY:
- Atrial fibrillation was diagnosed in 190 participants.
- Over a follow-up averaging 4.1 years, risk for incident AFib was reduced by 27% for participants who received the 1,600 IU/day dose, compared with placebo; hazard ratio, 0.73 (95% confidence interval, 0.52-1.02; P = .07), and by 32% for those in the 3,200 IU/day arm; HR, 0.68 (95% CI, 0.48-0.96; P = .03).
- The incident-AFib risk was reduced by 30% in a comparison of the two vitamin D groups combined versus the placebo group; HR, 0.70 (95% CI, 0.53-0.94; P = .02).
- After exclusion of 122 participants who reported being on antiarrhythmic medications at baseline, the 1,600 IU/day group showed a significant 27% reduction in risk for AF (95% CI, 4%-58%; P = .03) and the 3,200 IU/day group a nonsignificant 30% (95% CI, 5%-53%; P = .08) reduction in risk.
IN PRACTICE:
High-dose vitamin D3 supplementation may reduce incidence of AFib in a generally healthy, largely vitamin D–sufficient elderly population, the authors proposed. Additional controlled trials are needed, especially in diverse populations.
STUDY DETAILS:
The study was conducted by Jyrki K. Virtanen, PhD, University of Eastern Finland, Institute of Public Health and Clinical Nutrition, Kuopio, and colleagues. It was published in the American Heart Journal.
LIMITATIONS:
Atrial fibrillation was not prespecified as a primary outcome, and the results differ from those of other randomized controlled trials. Information on type of AFib (whether paroxysmal or nonparoxysmal, for example) wasn’t available nor were participants’ history of AFib. All participants were White and from Finland, limiting generalizability of the results.
DISCLOSURES:
The study was supported by the Academy of Finland, University of Eastern Finland, the Juho Vainio Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and the Finnish Cultural Foundation. One coauthor disclosed receiving grants from the National Institutes of Health and Mars Edge. Another coauthor disclosed receipt of a grant from Orion. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves first leadless dual-chamber pacing system
, one based in part on an already-approved leadless single-chamber device, Abbott has announced.
The company’s AVEIR DR leadless pacing system consists of two percutaneously implanted devices, the single-chamber AVEIR VR leadless pacemaker, implanted within the right ventricle, and the novel AVEIR AR single-chamber pacemaker for implantation in the right atrium.
The AVEIR DR system relies on proprietary wireless technology to provide bidirectional, beat-to-beat communication between its two components to achieve dual-chamber synchronization, the company stated in a press release on the approval.
The system also provides real-time pacing analysis, Abbott said, allowing clinicians to assess proper device placement during the procedure and before implantation. The system is designed to be easily removed if the patient’s pacing needs evolve or its battery needs replacing.
Experienced operators achieved a 98% implantation success rate using the AVIER DR system in a 300-patient study conducted at 55 sites in Canada, Europe, and the United States. In that study, 63% of the patients had sinus-node dysfunction and 33% had AV block as their primary dual-chamber pacing indication.
The system exceeded its predefined safety and performance goals, providing AV-synchronous pacing in 97% of patients for at least 3 months, it was reported in May at the annual scientific sessions of the Heart Rhythm Society and in a simultaneous publication in The New England Journal of Medicine.
“Modern medicine has been filled with technological achievements that fundamentally changed how doctors approach patient care, and now we can officially add dual-chamber leadless pacing to that list of achievements,” coauthor Vivek Reddy, MD, director of cardiac arrhythmia services for Mount Sinai Hospital and the Mount Sinai Health System, New York, said in the press release.
A version of this article first appeared on Medscape.com.
, one based in part on an already-approved leadless single-chamber device, Abbott has announced.
The company’s AVEIR DR leadless pacing system consists of two percutaneously implanted devices, the single-chamber AVEIR VR leadless pacemaker, implanted within the right ventricle, and the novel AVEIR AR single-chamber pacemaker for implantation in the right atrium.
The AVEIR DR system relies on proprietary wireless technology to provide bidirectional, beat-to-beat communication between its two components to achieve dual-chamber synchronization, the company stated in a press release on the approval.
The system also provides real-time pacing analysis, Abbott said, allowing clinicians to assess proper device placement during the procedure and before implantation. The system is designed to be easily removed if the patient’s pacing needs evolve or its battery needs replacing.
Experienced operators achieved a 98% implantation success rate using the AVIER DR system in a 300-patient study conducted at 55 sites in Canada, Europe, and the United States. In that study, 63% of the patients had sinus-node dysfunction and 33% had AV block as their primary dual-chamber pacing indication.
The system exceeded its predefined safety and performance goals, providing AV-synchronous pacing in 97% of patients for at least 3 months, it was reported in May at the annual scientific sessions of the Heart Rhythm Society and in a simultaneous publication in The New England Journal of Medicine.
“Modern medicine has been filled with technological achievements that fundamentally changed how doctors approach patient care, and now we can officially add dual-chamber leadless pacing to that list of achievements,” coauthor Vivek Reddy, MD, director of cardiac arrhythmia services for Mount Sinai Hospital and the Mount Sinai Health System, New York, said in the press release.
A version of this article first appeared on Medscape.com.
, one based in part on an already-approved leadless single-chamber device, Abbott has announced.
The company’s AVEIR DR leadless pacing system consists of two percutaneously implanted devices, the single-chamber AVEIR VR leadless pacemaker, implanted within the right ventricle, and the novel AVEIR AR single-chamber pacemaker for implantation in the right atrium.
The AVEIR DR system relies on proprietary wireless technology to provide bidirectional, beat-to-beat communication between its two components to achieve dual-chamber synchronization, the company stated in a press release on the approval.
The system also provides real-time pacing analysis, Abbott said, allowing clinicians to assess proper device placement during the procedure and before implantation. The system is designed to be easily removed if the patient’s pacing needs evolve or its battery needs replacing.
Experienced operators achieved a 98% implantation success rate using the AVIER DR system in a 300-patient study conducted at 55 sites in Canada, Europe, and the United States. In that study, 63% of the patients had sinus-node dysfunction and 33% had AV block as their primary dual-chamber pacing indication.
The system exceeded its predefined safety and performance goals, providing AV-synchronous pacing in 97% of patients for at least 3 months, it was reported in May at the annual scientific sessions of the Heart Rhythm Society and in a simultaneous publication in The New England Journal of Medicine.
“Modern medicine has been filled with technological achievements that fundamentally changed how doctors approach patient care, and now we can officially add dual-chamber leadless pacing to that list of achievements,” coauthor Vivek Reddy, MD, director of cardiac arrhythmia services for Mount Sinai Hospital and the Mount Sinai Health System, New York, said in the press release.
A version of this article first appeared on Medscape.com.
Women with atrial fibrillation more likely to develop dementia
New data suggest a significantly stronger link in women compared with men between atrial fibrillation (AF) and mild cognitive impairment (MCI) and dementia.
“Our findings imply that women with AF may be at higher risk for MCI and dementia with potentially more rapid disease progression from normal cognition to MCI or dementia than women without AF or men with and without AF,” wrote authors of a new study led by Kathryn A. Wood, PhD, RN, Neil Hodgson Woodruff School of Nursing at Emory University in Atlanta.
The findings were published online in Alzheimer’s & Dementia.
Researchers used the National Alzheimer’s Coordinating Center data with 43,630 patients and analyzed sex differences between men and women with AF and their performance on neuropsychological tests and cognitive disease progression.
Higher odds of dementia, MCI in women
According to the paper, AF is associated with higher odds of dementia (odds ratio [OR], 3.00; 95% confidence interval [CI], 1.22-7.37) in women and MCI in women (OR, 3.43; 95% CI, 1.55-7.55) compared with men.
Women with AF and normal cognition at baseline had a higher risk of disease progression (hazard ratio [HR], 1.26; 95% CI, 1.06-1.50) from normal to MCI and from MCI to vascular dementia (HR, 3.27; 95% CI, 1.89-5.65) than that of men with AF or men and women without AF.
AF is a major public health problem linked with stroke and heart failure, and is an independent risk factor of increased mortality. It is associated with higher risk of cognitive impairment and dementia independent of stroke history.
Cognitive screening for AF patients
The authors wrote that cognitive screening, especially in women, should be part of yearly cardiology visits for patients with AF to help identify early those at highest risk for cognitive disease.
T. Jared Bunch, MD, professor of medicine in the division of cardiovascular medicine at University of Utah in Salt Lake City, said in an interview, “We have learned that how we treat atrial fibrillation can influence risk.”
First, he said, outcomes, including brain health, are better when rhythm control approaches are used within the first year of diagnosis.
“Restoring a normal heart rhythm improves brain perfusion and cognitive function. Next, aggressive rhythm control – such as catheter ablation – is associated with much lower long-term risks of dementia in the [patients]. Finally, early and effective use of anticoagulation in patients with atrial fibrillation lowers risk of stroke, dementia, and cognitive decline.”
Several factors unknown
Dr. Bunch said there are some unknowns in the study, such as how long patients were in atrial fibrillation.
He said one way to address the inequities is to refer women earlier as women are often referred later in disease to specialty care, which can have consequences.
He said it is not known how many people underwent early and effective rhythm control.
“Women also are less likely to receive catheter ablation, a cardioversion, or be placed on antiarrhythmic drugs,” said Dr. Bunch, who was not part of the study. “These also represent potential opportunities to improve outcomes by treating the rhythm in a similar and aggressive manner in both men and women.”
Also unknown is how many people were on effective oral anticoagulation, Dr. Bunch noted.
The study importantly highlights a significant problem surrounding the care of women with AF, he said, but there are strategies to improve outcomes.
In addition to earlier screening and referral for women, providers should recognize that men and women may present differently with different AF symptoms. He added that physicians should offer catheter ablation, the most effective treatment, equally to men and women who are candidates.
In all people, he said, it’s important “to start anticoagulation very early in the disease to lower the risk of micro- and macrothrombotic events that lead to poor brain health and function.”
The study authors and Dr. Bunch declared no relevant financial relationships.
New data suggest a significantly stronger link in women compared with men between atrial fibrillation (AF) and mild cognitive impairment (MCI) and dementia.
“Our findings imply that women with AF may be at higher risk for MCI and dementia with potentially more rapid disease progression from normal cognition to MCI or dementia than women without AF or men with and without AF,” wrote authors of a new study led by Kathryn A. Wood, PhD, RN, Neil Hodgson Woodruff School of Nursing at Emory University in Atlanta.
The findings were published online in Alzheimer’s & Dementia.
Researchers used the National Alzheimer’s Coordinating Center data with 43,630 patients and analyzed sex differences between men and women with AF and their performance on neuropsychological tests and cognitive disease progression.
Higher odds of dementia, MCI in women
According to the paper, AF is associated with higher odds of dementia (odds ratio [OR], 3.00; 95% confidence interval [CI], 1.22-7.37) in women and MCI in women (OR, 3.43; 95% CI, 1.55-7.55) compared with men.
Women with AF and normal cognition at baseline had a higher risk of disease progression (hazard ratio [HR], 1.26; 95% CI, 1.06-1.50) from normal to MCI and from MCI to vascular dementia (HR, 3.27; 95% CI, 1.89-5.65) than that of men with AF or men and women without AF.
AF is a major public health problem linked with stroke and heart failure, and is an independent risk factor of increased mortality. It is associated with higher risk of cognitive impairment and dementia independent of stroke history.
Cognitive screening for AF patients
The authors wrote that cognitive screening, especially in women, should be part of yearly cardiology visits for patients with AF to help identify early those at highest risk for cognitive disease.
T. Jared Bunch, MD, professor of medicine in the division of cardiovascular medicine at University of Utah in Salt Lake City, said in an interview, “We have learned that how we treat atrial fibrillation can influence risk.”
First, he said, outcomes, including brain health, are better when rhythm control approaches are used within the first year of diagnosis.
“Restoring a normal heart rhythm improves brain perfusion and cognitive function. Next, aggressive rhythm control – such as catheter ablation – is associated with much lower long-term risks of dementia in the [patients]. Finally, early and effective use of anticoagulation in patients with atrial fibrillation lowers risk of stroke, dementia, and cognitive decline.”
Several factors unknown
Dr. Bunch said there are some unknowns in the study, such as how long patients were in atrial fibrillation.
He said one way to address the inequities is to refer women earlier as women are often referred later in disease to specialty care, which can have consequences.
He said it is not known how many people underwent early and effective rhythm control.
“Women also are less likely to receive catheter ablation, a cardioversion, or be placed on antiarrhythmic drugs,” said Dr. Bunch, who was not part of the study. “These also represent potential opportunities to improve outcomes by treating the rhythm in a similar and aggressive manner in both men and women.”
Also unknown is how many people were on effective oral anticoagulation, Dr. Bunch noted.
The study importantly highlights a significant problem surrounding the care of women with AF, he said, but there are strategies to improve outcomes.
In addition to earlier screening and referral for women, providers should recognize that men and women may present differently with different AF symptoms. He added that physicians should offer catheter ablation, the most effective treatment, equally to men and women who are candidates.
In all people, he said, it’s important “to start anticoagulation very early in the disease to lower the risk of micro- and macrothrombotic events that lead to poor brain health and function.”
The study authors and Dr. Bunch declared no relevant financial relationships.
New data suggest a significantly stronger link in women compared with men between atrial fibrillation (AF) and mild cognitive impairment (MCI) and dementia.
“Our findings imply that women with AF may be at higher risk for MCI and dementia with potentially more rapid disease progression from normal cognition to MCI or dementia than women without AF or men with and without AF,” wrote authors of a new study led by Kathryn A. Wood, PhD, RN, Neil Hodgson Woodruff School of Nursing at Emory University in Atlanta.
The findings were published online in Alzheimer’s & Dementia.
Researchers used the National Alzheimer’s Coordinating Center data with 43,630 patients and analyzed sex differences between men and women with AF and their performance on neuropsychological tests and cognitive disease progression.
Higher odds of dementia, MCI in women
According to the paper, AF is associated with higher odds of dementia (odds ratio [OR], 3.00; 95% confidence interval [CI], 1.22-7.37) in women and MCI in women (OR, 3.43; 95% CI, 1.55-7.55) compared with men.
Women with AF and normal cognition at baseline had a higher risk of disease progression (hazard ratio [HR], 1.26; 95% CI, 1.06-1.50) from normal to MCI and from MCI to vascular dementia (HR, 3.27; 95% CI, 1.89-5.65) than that of men with AF or men and women without AF.
AF is a major public health problem linked with stroke and heart failure, and is an independent risk factor of increased mortality. It is associated with higher risk of cognitive impairment and dementia independent of stroke history.
Cognitive screening for AF patients
The authors wrote that cognitive screening, especially in women, should be part of yearly cardiology visits for patients with AF to help identify early those at highest risk for cognitive disease.
T. Jared Bunch, MD, professor of medicine in the division of cardiovascular medicine at University of Utah in Salt Lake City, said in an interview, “We have learned that how we treat atrial fibrillation can influence risk.”
First, he said, outcomes, including brain health, are better when rhythm control approaches are used within the first year of diagnosis.
“Restoring a normal heart rhythm improves brain perfusion and cognitive function. Next, aggressive rhythm control – such as catheter ablation – is associated with much lower long-term risks of dementia in the [patients]. Finally, early and effective use of anticoagulation in patients with atrial fibrillation lowers risk of stroke, dementia, and cognitive decline.”
Several factors unknown
Dr. Bunch said there are some unknowns in the study, such as how long patients were in atrial fibrillation.
He said one way to address the inequities is to refer women earlier as women are often referred later in disease to specialty care, which can have consequences.
He said it is not known how many people underwent early and effective rhythm control.
“Women also are less likely to receive catheter ablation, a cardioversion, or be placed on antiarrhythmic drugs,” said Dr. Bunch, who was not part of the study. “These also represent potential opportunities to improve outcomes by treating the rhythm in a similar and aggressive manner in both men and women.”
Also unknown is how many people were on effective oral anticoagulation, Dr. Bunch noted.
The study importantly highlights a significant problem surrounding the care of women with AF, he said, but there are strategies to improve outcomes.
In addition to earlier screening and referral for women, providers should recognize that men and women may present differently with different AF symptoms. He added that physicians should offer catheter ablation, the most effective treatment, equally to men and women who are candidates.
In all people, he said, it’s important “to start anticoagulation very early in the disease to lower the risk of micro- and macrothrombotic events that lead to poor brain health and function.”
The study authors and Dr. Bunch declared no relevant financial relationships.
FROM ALZHEIMER’S & DEMENTIA
ECG implant tightens AFib management, improves outcomes in MONITOR-AF
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.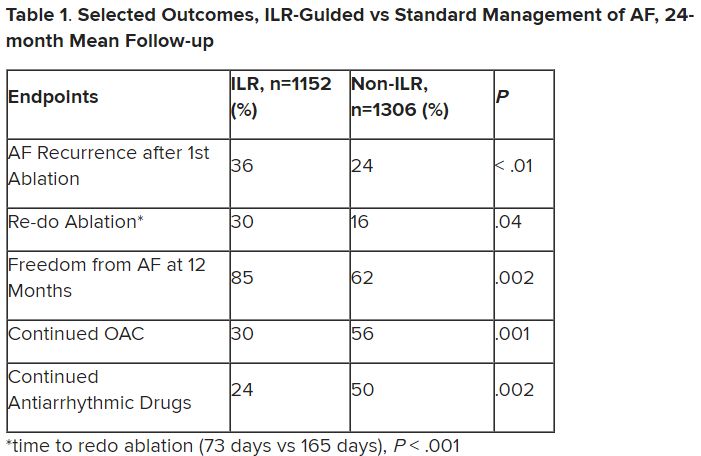
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.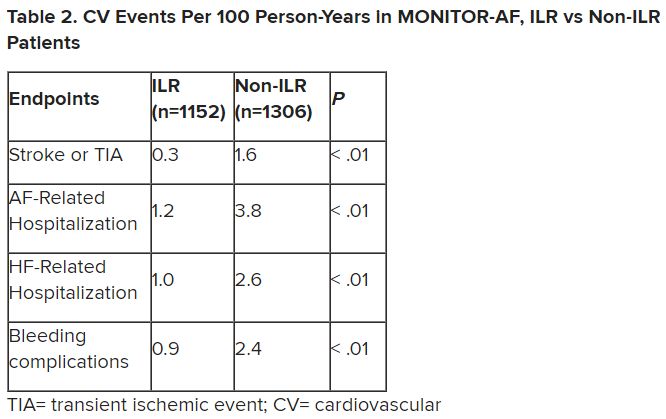
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023







