User login
Shoulder Arthroplasty in Patients with Rheumatoid Arthritis: A Population-Based Study Examining Utilization, Adverse Events, Length of Stay, and Cost
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).
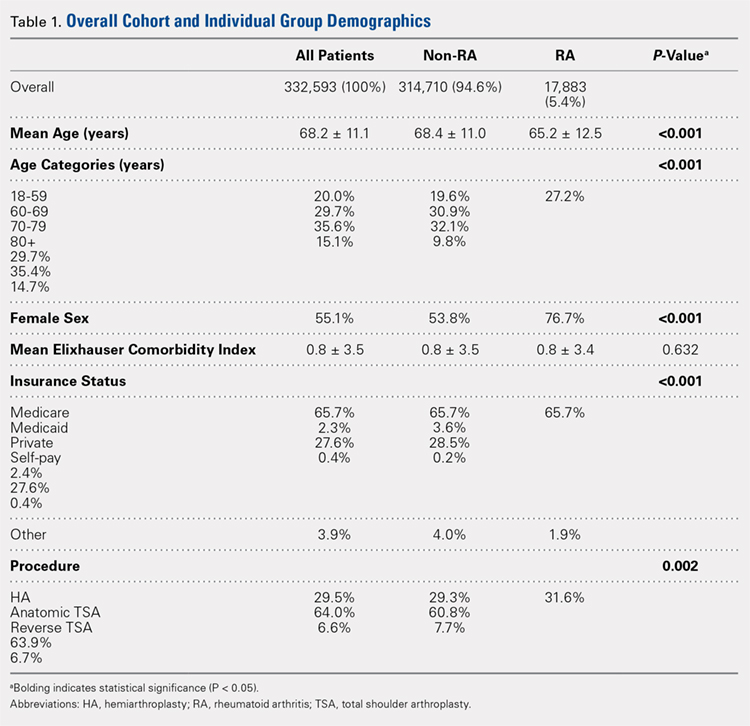
Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.
Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
TAKE-HOME POINTS
- There was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly TSA.
- There was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease.
- There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients.
- Non-RA patients had a significantly shorter length of stay.
- The utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continuous Cryotherapy vs Ice Following Total Shoulder Arthroplasty: A Randomized Control Trial
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
TAKE-HOME POINTS
- CC has been proposed as a means of improving postoperative pain control.
- CC represents a cost typically not covered by insurances.
- No difference was noted between the 2 groups in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval.
- While CC may offer convenience advantages, the increased cost associated with this type of unit may not be justified.
- The mechanism for CC for pain control is poorly understood.
When Would a Metal-Backed Component Become Cost-Effective Over an All-Polyethylene Tibia in Total Knee Arthroplasty?
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.
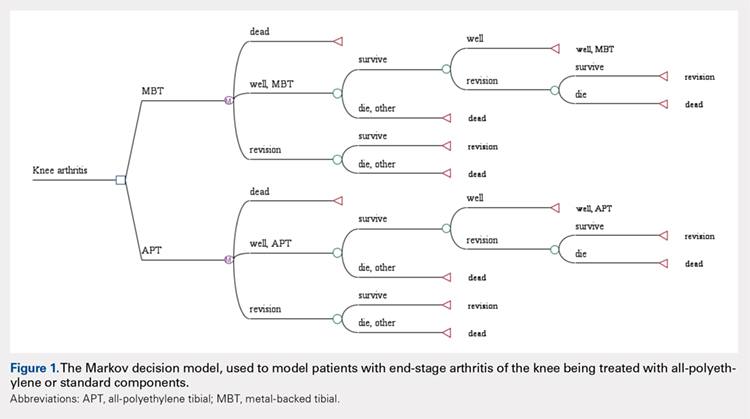
The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.
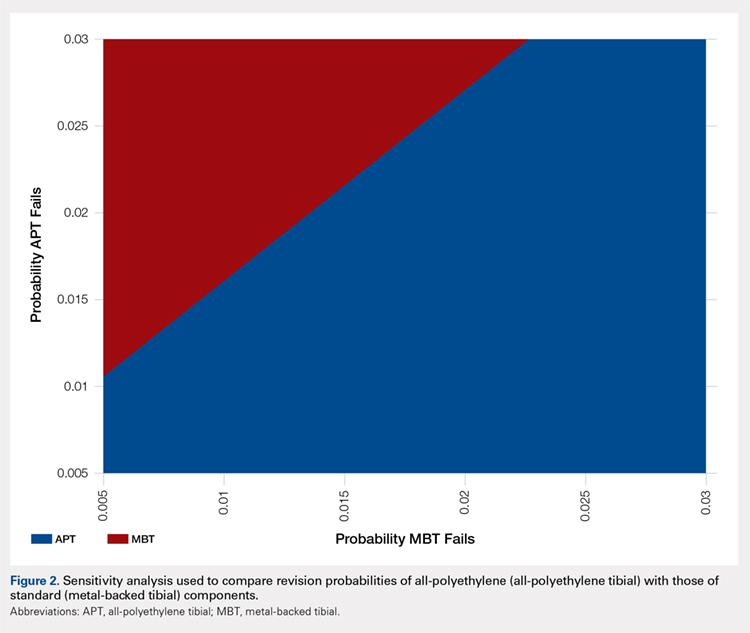
DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
TAKE-HOME POINTS
- APT components have been shown to be cost-effective when compared to MBT designs in TKA.
- Revision rates would have to be substantially lower in MBT to afford a cost advantage over APT components.
- Given that only a small percentage of surgeons routinely use APT components, factors other than cost-effectiveness must influence the choice of implant.
- Surgeons may find that APT components are more technically demanding to use and they do not allow for modular stems or augmentations.
- Institutional cost data is known to vary widely among institutions, and our conclusions regarding comparable revision rates would change with different cost inputs.
Radiographic Study of Humeral Stem in Shoulder Arthroplasty After Lesser Tuberosity Osteotomy or Subscapularis Tenotomy
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
TAKE-HOME POINTS
- LTO and ST remain viable options for takedown of the subscapularis.
- No difference exists in subsidence, lucent lines, and posterior subluxation on radiographic evaluation between LTO and ST.
- No clinically significant difference exists between outcome scores of patients with either technique.
- HAD was statistically significant but not clinically relevant between the 2 techniques.
- Results from the study do not apply to metaphyseal fitting stems, only diaphyseal fitting stems.
Blood Loss Reduction with Tranexamic Acid and a Bipolar Sealer in Direct Anterior Total Hip Arthroplasty
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
TAKE-HOME POINTS
- TXA reduces blood loss and transfusion requirements in THA.
- The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation.
- The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements.
- The addition of a bipolar sealer did not offer an advantage to transfusion requirements in anterior THA.
- TXA should be used routinely in THA.
Implant Survivorship and Complication Rates After Total Knee Arthroplasty With a Third-Generation Cemented System: 15-Year Follow-Up
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).
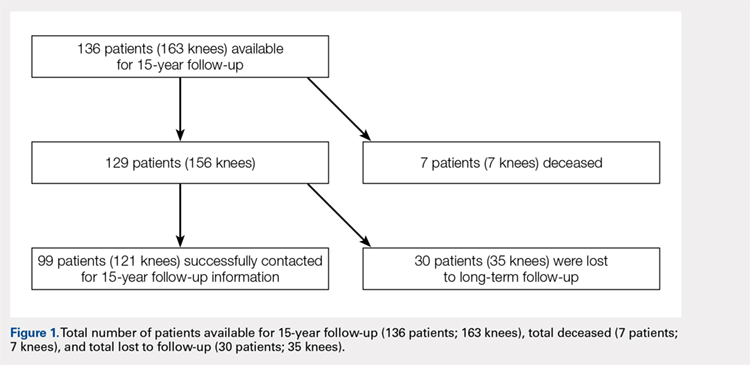
The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.
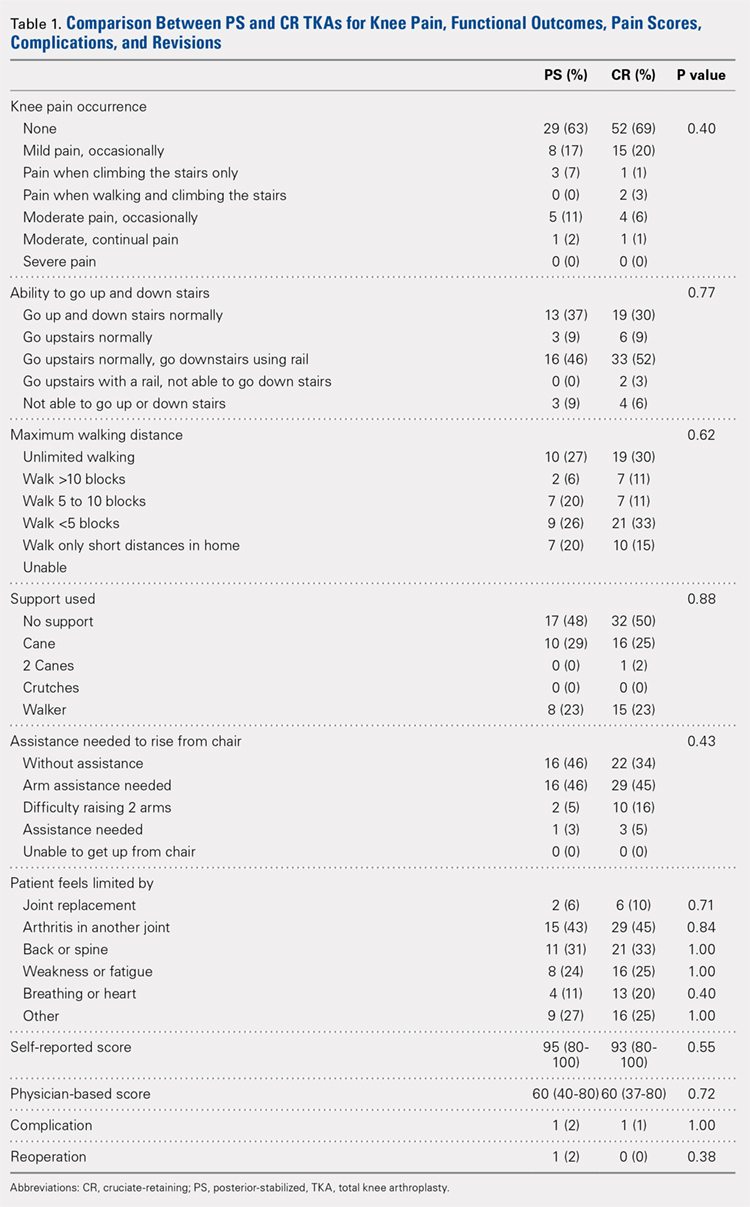
RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).
DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).

The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.

RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).

DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).

The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.

RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).

DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
TAKE-HOME POINTS
- TKA has a high success rate in pain relief and restoration of function in patients with severe osteoarthritis.
- NexGen (Zimmer Biomet) knee implants showed excellent functional outcomes at 15 years.
- There are no significant differences in functional outcomes between the PS and CR knee systems.
- NexGen knee implants showed excellent longevity and survivorship at 15-year follow-up with no evidence of aseptic loosening.
- There is an increased incidence of knee osteoarthritis in the younger population (<55 years of age).
The Prevention and Treatment of Femoral Trial Head Loss in Total Hip Arthroplasty
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
TAKE-HOME POINTS
- Femoral head trial loss is a complication that can occur during THA.
- This event can be a source of avoidable morbidity.
- Preventative measures can be taken to avoid this complication.
- If preventative measures fail, retrieval of the femoral trial head can be performed.
- A thorough understanding of preventative and retrieval methods is essential for surgeons that perform THA.
Managing Glenoid Bone Deficiency—The Augment Experience in Anatomic and Reverse Shoulder Arthroplasty
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.
Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.
Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.
Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
TAKE-HOME POINTS
- Glenoid defects are very common.
- Options for treating glenoid defects include eccentric reaming, bone grafting, and augmented glenoids.
- As computer-assisted surgery use becomes more widespread the use of augments in both TSA and RTSA will become very common.
- Subchondral bone is precious and cannot be replaced once reamed away. Eccentric glenoids introduce a mechanism to minimize reaming and preserve this precious bone.
- On short-term to midterm follow-up augments perform at least as well if not better than non-augmented glenoid components with complication rate and revisions likewise similar.
Patient-Specific Guides/Instrumentation in Shoulder Arthroplasty
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.
The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.

The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.

The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
TAKE-HOME POINTS
- Optimal outcomes following TSA and RSA are dependent on proper implant position.
- Patient-specific guides/instrumentation result in improved accuracy of implant positioning compared to traditional methods.
- Currently, there are no clinical studies demonstrating superiority of patient-specific guide/instrumentation use on patient outcomes.
- At this time there are 3 commercially available single use patient-specific instrumentation systems (DJO Global, Wright Medical Group, and Zimmer Biomet) and 1 commercially available reusable patient-specific instrumentation system (Arthrex).
- Limited research is available comparing the accuracy of different commercially available 3-D planning systems.
Humeral Bone Loss in Revision Shoulder Arthroplasty
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.
INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.
It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.
After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.
ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.
POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.

INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.

It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.

After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.

ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.

POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.

INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.

It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.

After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.

ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.

POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
TAKE-HOME POINTS
- Different preoperative diagnoses lead to distinct patterns of bone loss in revision shoulder arthroplasty.
- A variety of techniques should be utilized to address the specific pathologies encountered.
- Advanced proximal humeral bone loss results in limited substrate available for humeral component fixation.
- Monoblock humeral stems can be used without allografts in cases with mild humeral bone loss.
- The revision of loose humeral stems dictates the use of large diaphyseal allografts in the majority of cases.