User login
FDA okays first generic of ProAir HFA
Generic albuterol sulfate inhalation, from Perrigo Pharmaceutical, is indicated for the treatment or prevention of bronchospasm in people aged 4 years or older who have reversible obstructive airway disease, as well as for the prevention of exercise-induced bronchospasm.
“Approval of the first generic drug product for one of the most commonly used rescue inhalers in the US is part of our long-standing commitment to advance patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts, and to expand opportunities to bring generic copies of complex drugs to the market,” FDA Commissioner Stephen Hahn, MD, said in a news release.
Metered-dose inhalers are hard to duplicate because of the complexities of their formulation or mode of delivery. “As a result, too many complex drugs lack generic competition even after patents and exclusivities no longer block generic approval,” he explained.
“Supporting development and approval of generic copies of these complex medicines so that these products can get to patients has been a major focus of our efforts to improve competition and access and to lower drug prices. Getting more generic copies of complex drugs to the market is a key priority for how we’ll help bring new savings to consumers,” Hahn added.
In the United States, more than 26 million people suffer from asthma; about 7 million of these people are children.
Perrigo said it will immediately launch a limited quantity of generic albuterol sulfate and, in collaboration with its development and manufacturing partner, Catalent Pharma Solutions, is ramping up production to meet future demand.
The company “anticipates that we will be in a position to provide a steady supply of this product by the fourth quarter of 2020,” Perrigo Executive Vice President and Rx Pharmaceuticals President Sharon Kochan said in a statement.
This article originally appeared on Medscape.com.
Generic albuterol sulfate inhalation, from Perrigo Pharmaceutical, is indicated for the treatment or prevention of bronchospasm in people aged 4 years or older who have reversible obstructive airway disease, as well as for the prevention of exercise-induced bronchospasm.
“Approval of the first generic drug product for one of the most commonly used rescue inhalers in the US is part of our long-standing commitment to advance patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts, and to expand opportunities to bring generic copies of complex drugs to the market,” FDA Commissioner Stephen Hahn, MD, said in a news release.
Metered-dose inhalers are hard to duplicate because of the complexities of their formulation or mode of delivery. “As a result, too many complex drugs lack generic competition even after patents and exclusivities no longer block generic approval,” he explained.
“Supporting development and approval of generic copies of these complex medicines so that these products can get to patients has been a major focus of our efforts to improve competition and access and to lower drug prices. Getting more generic copies of complex drugs to the market is a key priority for how we’ll help bring new savings to consumers,” Hahn added.
In the United States, more than 26 million people suffer from asthma; about 7 million of these people are children.
Perrigo said it will immediately launch a limited quantity of generic albuterol sulfate and, in collaboration with its development and manufacturing partner, Catalent Pharma Solutions, is ramping up production to meet future demand.
The company “anticipates that we will be in a position to provide a steady supply of this product by the fourth quarter of 2020,” Perrigo Executive Vice President and Rx Pharmaceuticals President Sharon Kochan said in a statement.
This article originally appeared on Medscape.com.
Generic albuterol sulfate inhalation, from Perrigo Pharmaceutical, is indicated for the treatment or prevention of bronchospasm in people aged 4 years or older who have reversible obstructive airway disease, as well as for the prevention of exercise-induced bronchospasm.
“Approval of the first generic drug product for one of the most commonly used rescue inhalers in the US is part of our long-standing commitment to advance patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts, and to expand opportunities to bring generic copies of complex drugs to the market,” FDA Commissioner Stephen Hahn, MD, said in a news release.
Metered-dose inhalers are hard to duplicate because of the complexities of their formulation or mode of delivery. “As a result, too many complex drugs lack generic competition even after patents and exclusivities no longer block generic approval,” he explained.
“Supporting development and approval of generic copies of these complex medicines so that these products can get to patients has been a major focus of our efforts to improve competition and access and to lower drug prices. Getting more generic copies of complex drugs to the market is a key priority for how we’ll help bring new savings to consumers,” Hahn added.
In the United States, more than 26 million people suffer from asthma; about 7 million of these people are children.
Perrigo said it will immediately launch a limited quantity of generic albuterol sulfate and, in collaboration with its development and manufacturing partner, Catalent Pharma Solutions, is ramping up production to meet future demand.
The company “anticipates that we will be in a position to provide a steady supply of this product by the fourth quarter of 2020,” Perrigo Executive Vice President and Rx Pharmaceuticals President Sharon Kochan said in a statement.
This article originally appeared on Medscape.com.
FDA okays Palforzia, first drug for peanut allergy in children
The Food and Drug Administration has approved the first drug to combat peanut allergy in children, (Palforzia, Aimmune Therapeutics), although those who take it must continue to avoid peanuts in their diets.
The peanut (Arachis hypogaea) allergen powder is also the first drug ever approved to treat a food allergy. It is not a cure, but it mitigates allergic reactions, including anaphylaxis, that may occur with accidental exposure to peanuts, the FDA said in a news release.
Treatment with the oral powder, which is mixed into semisolid food – such as applesauce or yogurt – can be started in children aged 4 through 17 years who have a confirmed peanut allergy and then continued as a maintenance medication. Some 1 million American children have peanut allergy, and only a fifth will outgrow the allergy, the agency said.
“Because there is no cure, allergic individuals must strictly avoid exposure to prevent severe and potentially life-threatening reactions,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement.
An FDA advisory panel backed the medication in September 2019, but some committee members expressed concern about the large number of children in clinical trials who required epinephrine after receiving a dose of Palforzia.
The initial dose phase is given on a single day, while updosing consists of 11 increasing doses over several months. If the patient tolerates the first administration of an increased dose level, they may continue that dose daily at home. Daily maintenance begins after the completion of all updosing levels.
Palforzia will be available only through specially certified health care providers, health care settings, and pharmacies to patients enrolled in the REMS program, the agency said. Also, the initial dose escalation and first dose of each updosing level can be given only in a certified setting.
The agency said that patients or parents or caregivers must be counseled on the need for constant availability of injectable epinephrine, the need for continued dietary peanut avoidance, and on how to recognize the signs and symptoms of anaphylaxis.
‘Eagerly’ awaited
Palforzia’s effectiveness was based on a randomized, double-blind, placebo-controlled study involving about 500 peanut-allergic individuals that found that 67.2% of allergic patients tolerated an oral challenge with a single 600-mg dose of peanut protein with no more than mild allergic symptoms after 6 months of maintenance treatment, compared with 4% of placebo recipients, the FDA said.
In two double-blind, placebo-controlled studies looking at safety, the most commonly reported side effects among about 700 individuals involved in the research were abdominal pain, vomiting, nausea, tingling in the mouth, itching (including in the mouth and ears), cough, runny nose, throat irritation and tightness, hives, wheezing and shortness of breath, and anaphylaxis.
Palforzia should not be given to those with uncontrolled asthma and can’t be used for emergency treatment of allergic reactions, including anaphylaxis.
“The food allergy community has been eagerly awaiting an FDA-approved treatment that can help mitigate allergic reactions to peanut and, as allergists, we want nothing more than to have a treatment option to offer our patients that has demonstrated both the safety and efficacy to truly impact the lives of patients who live with peanut allergy,” said Christina Ciaccio, MD, chief of Allergy/Immunology and Pediatric Pulmonary Medicine at the University of Chicago Medical Center and Biological Sciences, in a company statement from Aimmune. “With today’s approval of Palforzia, we can – for the first time – offer children and teens with peanut allergy a proven medicine that employs an established therapeutic approach.”
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first drug to combat peanut allergy in children, (Palforzia, Aimmune Therapeutics), although those who take it must continue to avoid peanuts in their diets.
The peanut (Arachis hypogaea) allergen powder is also the first drug ever approved to treat a food allergy. It is not a cure, but it mitigates allergic reactions, including anaphylaxis, that may occur with accidental exposure to peanuts, the FDA said in a news release.
Treatment with the oral powder, which is mixed into semisolid food – such as applesauce or yogurt – can be started in children aged 4 through 17 years who have a confirmed peanut allergy and then continued as a maintenance medication. Some 1 million American children have peanut allergy, and only a fifth will outgrow the allergy, the agency said.
“Because there is no cure, allergic individuals must strictly avoid exposure to prevent severe and potentially life-threatening reactions,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement.
An FDA advisory panel backed the medication in September 2019, but some committee members expressed concern about the large number of children in clinical trials who required epinephrine after receiving a dose of Palforzia.
The initial dose phase is given on a single day, while updosing consists of 11 increasing doses over several months. If the patient tolerates the first administration of an increased dose level, they may continue that dose daily at home. Daily maintenance begins after the completion of all updosing levels.
Palforzia will be available only through specially certified health care providers, health care settings, and pharmacies to patients enrolled in the REMS program, the agency said. Also, the initial dose escalation and first dose of each updosing level can be given only in a certified setting.
The agency said that patients or parents or caregivers must be counseled on the need for constant availability of injectable epinephrine, the need for continued dietary peanut avoidance, and on how to recognize the signs and symptoms of anaphylaxis.
‘Eagerly’ awaited
Palforzia’s effectiveness was based on a randomized, double-blind, placebo-controlled study involving about 500 peanut-allergic individuals that found that 67.2% of allergic patients tolerated an oral challenge with a single 600-mg dose of peanut protein with no more than mild allergic symptoms after 6 months of maintenance treatment, compared with 4% of placebo recipients, the FDA said.
In two double-blind, placebo-controlled studies looking at safety, the most commonly reported side effects among about 700 individuals involved in the research were abdominal pain, vomiting, nausea, tingling in the mouth, itching (including in the mouth and ears), cough, runny nose, throat irritation and tightness, hives, wheezing and shortness of breath, and anaphylaxis.
Palforzia should not be given to those with uncontrolled asthma and can’t be used for emergency treatment of allergic reactions, including anaphylaxis.
“The food allergy community has been eagerly awaiting an FDA-approved treatment that can help mitigate allergic reactions to peanut and, as allergists, we want nothing more than to have a treatment option to offer our patients that has demonstrated both the safety and efficacy to truly impact the lives of patients who live with peanut allergy,” said Christina Ciaccio, MD, chief of Allergy/Immunology and Pediatric Pulmonary Medicine at the University of Chicago Medical Center and Biological Sciences, in a company statement from Aimmune. “With today’s approval of Palforzia, we can – for the first time – offer children and teens with peanut allergy a proven medicine that employs an established therapeutic approach.”
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first drug to combat peanut allergy in children, (Palforzia, Aimmune Therapeutics), although those who take it must continue to avoid peanuts in their diets.
The peanut (Arachis hypogaea) allergen powder is also the first drug ever approved to treat a food allergy. It is not a cure, but it mitigates allergic reactions, including anaphylaxis, that may occur with accidental exposure to peanuts, the FDA said in a news release.
Treatment with the oral powder, which is mixed into semisolid food – such as applesauce or yogurt – can be started in children aged 4 through 17 years who have a confirmed peanut allergy and then continued as a maintenance medication. Some 1 million American children have peanut allergy, and only a fifth will outgrow the allergy, the agency said.
“Because there is no cure, allergic individuals must strictly avoid exposure to prevent severe and potentially life-threatening reactions,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement.
An FDA advisory panel backed the medication in September 2019, but some committee members expressed concern about the large number of children in clinical trials who required epinephrine after receiving a dose of Palforzia.
The initial dose phase is given on a single day, while updosing consists of 11 increasing doses over several months. If the patient tolerates the first administration of an increased dose level, they may continue that dose daily at home. Daily maintenance begins after the completion of all updosing levels.
Palforzia will be available only through specially certified health care providers, health care settings, and pharmacies to patients enrolled in the REMS program, the agency said. Also, the initial dose escalation and first dose of each updosing level can be given only in a certified setting.
The agency said that patients or parents or caregivers must be counseled on the need for constant availability of injectable epinephrine, the need for continued dietary peanut avoidance, and on how to recognize the signs and symptoms of anaphylaxis.
‘Eagerly’ awaited
Palforzia’s effectiveness was based on a randomized, double-blind, placebo-controlled study involving about 500 peanut-allergic individuals that found that 67.2% of allergic patients tolerated an oral challenge with a single 600-mg dose of peanut protein with no more than mild allergic symptoms after 6 months of maintenance treatment, compared with 4% of placebo recipients, the FDA said.
In two double-blind, placebo-controlled studies looking at safety, the most commonly reported side effects among about 700 individuals involved in the research were abdominal pain, vomiting, nausea, tingling in the mouth, itching (including in the mouth and ears), cough, runny nose, throat irritation and tightness, hives, wheezing and shortness of breath, and anaphylaxis.
Palforzia should not be given to those with uncontrolled asthma and can’t be used for emergency treatment of allergic reactions, including anaphylaxis.
“The food allergy community has been eagerly awaiting an FDA-approved treatment that can help mitigate allergic reactions to peanut and, as allergists, we want nothing more than to have a treatment option to offer our patients that has demonstrated both the safety and efficacy to truly impact the lives of patients who live with peanut allergy,” said Christina Ciaccio, MD, chief of Allergy/Immunology and Pediatric Pulmonary Medicine at the University of Chicago Medical Center and Biological Sciences, in a company statement from Aimmune. “With today’s approval of Palforzia, we can – for the first time – offer children and teens with peanut allergy a proven medicine that employs an established therapeutic approach.”
This article first appeared on Medscape.com.
Despite PCV, pediatric asthma patients face pneumococcal risks
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
FROM PEDIATRICS
Fast, aggressive eczema treatment linked to fewer food allergies by age 2
Researchers in Japan report that
For their research published in the Journal of Allergy and Clinical Immunology: In Practice, Yumiko Miyaji, MD, PhD, of Japan’s National Center for Child Health and Development in Tokyo and colleagues looked at 3 years’ worth of records for 177 infants younger than 1 year of age seen at a hospital allergy center for eczema. Of these infants, 89 were treated with betamethasone valerate within 4 months of disease onset, and 88 were treated after 4 months of onset. Most (142) were followed-up at 22-24 months, when all were in complete remission or near remission from eczema.
At follow-up, clinicians collected information about anaphylactic reactions to food, administered specific food challenges, and tested serum immunoglobin E levels for food allergens. Dr. Miyaji and colleagues found a significant difference in the prevalence of allergies between the early-treated and late-treated groups to chicken egg, cow’s milk, wheat, peanuts, soy, or fish (25% vs. 46%, respectively; P equal to .013). For individual food allergies, only chicken egg was associated with a statistically significant difference in prevalence (15% vs 36%, P equal to .006).
“Our present study may be the first to demonstrate that early aggressive topical corticosteroid treatment to shorten the duration of eczema in infants was significantly associated with a decrease in later development of [food allergies],” Dr. Miyaji and colleagues wrote in their analysis.
The investigators acknowledged as limitations of their study some between-group differences at baseline, with characteristics such as Staphylococcus aureus infections and some inflammatory biomarkers higher in the early treatment group.
The Japan Agency for Medical Research and Development supported the study, and the investigators disclosed no conflicts of interest related to their findings.
SOURCE: Miyaji Y et al. J Allergy Clin Immunol Pract. 2019. doi: 10.1016/j.jaip.2019.11.036
Researchers in Japan report that
For their research published in the Journal of Allergy and Clinical Immunology: In Practice, Yumiko Miyaji, MD, PhD, of Japan’s National Center for Child Health and Development in Tokyo and colleagues looked at 3 years’ worth of records for 177 infants younger than 1 year of age seen at a hospital allergy center for eczema. Of these infants, 89 were treated with betamethasone valerate within 4 months of disease onset, and 88 were treated after 4 months of onset. Most (142) were followed-up at 22-24 months, when all were in complete remission or near remission from eczema.
At follow-up, clinicians collected information about anaphylactic reactions to food, administered specific food challenges, and tested serum immunoglobin E levels for food allergens. Dr. Miyaji and colleagues found a significant difference in the prevalence of allergies between the early-treated and late-treated groups to chicken egg, cow’s milk, wheat, peanuts, soy, or fish (25% vs. 46%, respectively; P equal to .013). For individual food allergies, only chicken egg was associated with a statistically significant difference in prevalence (15% vs 36%, P equal to .006).
“Our present study may be the first to demonstrate that early aggressive topical corticosteroid treatment to shorten the duration of eczema in infants was significantly associated with a decrease in later development of [food allergies],” Dr. Miyaji and colleagues wrote in their analysis.
The investigators acknowledged as limitations of their study some between-group differences at baseline, with characteristics such as Staphylococcus aureus infections and some inflammatory biomarkers higher in the early treatment group.
The Japan Agency for Medical Research and Development supported the study, and the investigators disclosed no conflicts of interest related to their findings.
SOURCE: Miyaji Y et al. J Allergy Clin Immunol Pract. 2019. doi: 10.1016/j.jaip.2019.11.036
Researchers in Japan report that
For their research published in the Journal of Allergy and Clinical Immunology: In Practice, Yumiko Miyaji, MD, PhD, of Japan’s National Center for Child Health and Development in Tokyo and colleagues looked at 3 years’ worth of records for 177 infants younger than 1 year of age seen at a hospital allergy center for eczema. Of these infants, 89 were treated with betamethasone valerate within 4 months of disease onset, and 88 were treated after 4 months of onset. Most (142) were followed-up at 22-24 months, when all were in complete remission or near remission from eczema.
At follow-up, clinicians collected information about anaphylactic reactions to food, administered specific food challenges, and tested serum immunoglobin E levels for food allergens. Dr. Miyaji and colleagues found a significant difference in the prevalence of allergies between the early-treated and late-treated groups to chicken egg, cow’s milk, wheat, peanuts, soy, or fish (25% vs. 46%, respectively; P equal to .013). For individual food allergies, only chicken egg was associated with a statistically significant difference in prevalence (15% vs 36%, P equal to .006).
“Our present study may be the first to demonstrate that early aggressive topical corticosteroid treatment to shorten the duration of eczema in infants was significantly associated with a decrease in later development of [food allergies],” Dr. Miyaji and colleagues wrote in their analysis.
The investigators acknowledged as limitations of their study some between-group differences at baseline, with characteristics such as Staphylococcus aureus infections and some inflammatory biomarkers higher in the early treatment group.
The Japan Agency for Medical Research and Development supported the study, and the investigators disclosed no conflicts of interest related to their findings.
SOURCE: Miyaji Y et al. J Allergy Clin Immunol Pract. 2019. doi: 10.1016/j.jaip.2019.11.036
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Atopic dermatitis in egg-, milk-allergic kids may up anaphylaxis risk
compared with allergic patients without atopic dermatitis, based on retrospective data from 347 individuals.
Atopic dermatitis has been associated with increased risk of food allergies, but the association and predictive factors of skin reactions to certain foods remain unclear, wrote Bryce C. Hoffman, MD, of National Jewish Health, Denver, and colleagues.
In a letter published in the Annals of Allergy, Asthma & Immunology, the researchers identified children aged 0-18 years with peanut, cow’s milk, and/or egg allergies with or without atopic dermatitis (AD) using an institutional research database and conducted a retrospective study of medical records.
Overall, children with egg and milk allergies plus AD had significantly higher rates of anaphylaxis than allergic children without AD (47% vs. 11% for egg, 50% vs. 19% for milk). Anaphylaxis rates were similar in children with peanut allergies with or without AD (27% vs. 23%).
“This finding may suggest that skin barrier dysfunction plays a role in the severity of [food allergy]. However, this is not universal to all food antigens, and other mechanisms are likely important in the association of anaphylaxis with a particular food,” the researchers noted.
Rates of tolerance for both baked egg and baked milk were similar between AD and non-AD patients (83% vs. 61% for milk; 82% vs. 67% for egg). In addition, levels of total IgE were increased in children with egg and milk allergies plus AD, compared with children without AD. However, children with peanut allergies plus AD had decreased total IgE, compared with children with peanut allergies but no AD. This “may support a link between Th2 polarization and [food allergy] severity, ” Dr. Hoffman and associates wrote.
The findings were limited by several factors, including the retrospective study design, exclusion of many patients, and lack of data on the amount of food that triggered anaphylactic reactions, the researchers noted.
Nonetheless, the results suggest that children with atopic dermatitis and allergies to eggs and milk are at increased risk and that clinicians should counsel these patients and families about the potential for more-severe reactions to oral food challenges, Dr. Hoffman and associates concluded.
The study was supported by National Jewish Health and the Edelstein Family Chair of Pediatric Allergy and Immunology. The researchers had no financial conflicts to disclose.
SOURCE: Hoffman BC et al. Ann Allergy Asthma Immunol. 2019 Sep 11. doi: 10.1016/j.anai.2019.09.008.
compared with allergic patients without atopic dermatitis, based on retrospective data from 347 individuals.
Atopic dermatitis has been associated with increased risk of food allergies, but the association and predictive factors of skin reactions to certain foods remain unclear, wrote Bryce C. Hoffman, MD, of National Jewish Health, Denver, and colleagues.
In a letter published in the Annals of Allergy, Asthma & Immunology, the researchers identified children aged 0-18 years with peanut, cow’s milk, and/or egg allergies with or without atopic dermatitis (AD) using an institutional research database and conducted a retrospective study of medical records.
Overall, children with egg and milk allergies plus AD had significantly higher rates of anaphylaxis than allergic children without AD (47% vs. 11% for egg, 50% vs. 19% for milk). Anaphylaxis rates were similar in children with peanut allergies with or without AD (27% vs. 23%).
“This finding may suggest that skin barrier dysfunction plays a role in the severity of [food allergy]. However, this is not universal to all food antigens, and other mechanisms are likely important in the association of anaphylaxis with a particular food,” the researchers noted.
Rates of tolerance for both baked egg and baked milk were similar between AD and non-AD patients (83% vs. 61% for milk; 82% vs. 67% for egg). In addition, levels of total IgE were increased in children with egg and milk allergies plus AD, compared with children without AD. However, children with peanut allergies plus AD had decreased total IgE, compared with children with peanut allergies but no AD. This “may support a link between Th2 polarization and [food allergy] severity, ” Dr. Hoffman and associates wrote.
The findings were limited by several factors, including the retrospective study design, exclusion of many patients, and lack of data on the amount of food that triggered anaphylactic reactions, the researchers noted.
Nonetheless, the results suggest that children with atopic dermatitis and allergies to eggs and milk are at increased risk and that clinicians should counsel these patients and families about the potential for more-severe reactions to oral food challenges, Dr. Hoffman and associates concluded.
The study was supported by National Jewish Health and the Edelstein Family Chair of Pediatric Allergy and Immunology. The researchers had no financial conflicts to disclose.
SOURCE: Hoffman BC et al. Ann Allergy Asthma Immunol. 2019 Sep 11. doi: 10.1016/j.anai.2019.09.008.
compared with allergic patients without atopic dermatitis, based on retrospective data from 347 individuals.
Atopic dermatitis has been associated with increased risk of food allergies, but the association and predictive factors of skin reactions to certain foods remain unclear, wrote Bryce C. Hoffman, MD, of National Jewish Health, Denver, and colleagues.
In a letter published in the Annals of Allergy, Asthma & Immunology, the researchers identified children aged 0-18 years with peanut, cow’s milk, and/or egg allergies with or without atopic dermatitis (AD) using an institutional research database and conducted a retrospective study of medical records.
Overall, children with egg and milk allergies plus AD had significantly higher rates of anaphylaxis than allergic children without AD (47% vs. 11% for egg, 50% vs. 19% for milk). Anaphylaxis rates were similar in children with peanut allergies with or without AD (27% vs. 23%).
“This finding may suggest that skin barrier dysfunction plays a role in the severity of [food allergy]. However, this is not universal to all food antigens, and other mechanisms are likely important in the association of anaphylaxis with a particular food,” the researchers noted.
Rates of tolerance for both baked egg and baked milk were similar between AD and non-AD patients (83% vs. 61% for milk; 82% vs. 67% for egg). In addition, levels of total IgE were increased in children with egg and milk allergies plus AD, compared with children without AD. However, children with peanut allergies plus AD had decreased total IgE, compared with children with peanut allergies but no AD. This “may support a link between Th2 polarization and [food allergy] severity, ” Dr. Hoffman and associates wrote.
The findings were limited by several factors, including the retrospective study design, exclusion of many patients, and lack of data on the amount of food that triggered anaphylactic reactions, the researchers noted.
Nonetheless, the results suggest that children with atopic dermatitis and allergies to eggs and milk are at increased risk and that clinicians should counsel these patients and families about the potential for more-severe reactions to oral food challenges, Dr. Hoffman and associates concluded.
The study was supported by National Jewish Health and the Edelstein Family Chair of Pediatric Allergy and Immunology. The researchers had no financial conflicts to disclose.
SOURCE: Hoffman BC et al. Ann Allergy Asthma Immunol. 2019 Sep 11. doi: 10.1016/j.anai.2019.09.008.
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Clinic goes to bat for bullied kids
NEW ORLEANS – After Massachusetts passed antibullying legislation in 2009, Peter C. Raffalli, MD, saw an opportunity to improve care for the increasing numbers of children presenting to his neurology practice at Boston Children’s Hospital who were victims of bullying – especially those with developmental disabilities.
“I had been thinking of a clinic to help kids with these issues, aside from just helping them deal with the fallout: the depression, anxiety, et cetera, that comes with being bullied,” Dr. Raffalli recalled at the annual meeting of the American Academy of Pediatrics. “I wanted to do something to help present to families the evidence-based strategies regarding bullying prevention, detection, and intervention that might help to stop the bullying.”
This led him to launch the Bullying and Cyberbullying Prevention and Advocacy Collaborative (BACPAC) at Boston Children’s Hospital, which began in 2009 as an educational resource for families, medical colleagues, and schools. Dr. Raffalli also formed an alliance with the Massachusetts Aggression Reduction Center at Bridgewater State University (Ann Neurol. 2016;79[2]:167-8).
Two years later in 2011, BACPAC became a formal clinic at Boston Children’s that serves as a subspecialty consult service for victims of bullying and their families. The clinic team consists of a child neurologist, a social worker, and an education resource specialist who meet with the bullying victim and his/her family in initial consultation for 90 minutes. The goal is to develop an evidence-based plan for bullying prevention, detection, and intervention that is individualized to the patient’s developmental and social needs.
“We tell families that bullying is recognized medically and legally as a form of abuse,” said Dr. Raffalli. “The medical and psychological consequences are similar to other forms of abuse. You’d be surprised how often patients do think the bullying is their fault.”
The extent of the problem
Researchers estimate that 25%-30% of children will experience some form of bullying between kindergarten and grade 12, and about 8% will engage in bullying themselves. When BACPAC began in 2009, Dr. Raffalli conducted an informal search of peer-reviewed literature on bullying in children with special needs; it yielded just four articles. “Since then, there’s been an exponential explosion of literature on various aspects of bullying,” he said. Now there is ample evidence in the peer-reviewed literature to show the increased risk for bullying/cyberbullying in children/teens, not just with neurodevelopmental disorders, but also for kids with other medical disorders such as obesity, asthma, and allergies.
“We’ve had a good number of kids over the years with peanut allergy who were literally threatened physically with peanut butter at school,” he said. “It’s incredible how callous some kids can be. Kids with oppositional defiant disorder, impulse control disorder, and callous/unemotional traits from a psychological standpoint are hardest to reach when it comes to getting them to stop bullying. You’d be surprised how frequently bullies use the phrase [to their victims], ‘You should kill yourself.’ They don’t realize the damage they’re doing to people. Bullying can lead to severe psychological but also long-term medical problems, including suicidal ideation.”
Published studies show that the highest incidences of bullying occur in children with neurodevelopmental conditions such as ADHD, autistic spectrum disorders, Tourette syndrome, and other learning disabilities (Eur J Spec Needs Ed. 2010;25[1]:77-91). This population of children is overrepresented in bullying “because the services they receive at school make their disabilities more visible,” explained Dr. Raffalli, who is also an assistant professor of neurology at Harvard Medical School, Boston. “They stand out, and they have social information–processing deficits or distortions that exacerbate bullying involvement. They also have difficulty interpreting social cues or attributing hostile characteristics to their peer’s behavior.”
The consequences of bullying
The psychological and educational consequences of bullying among children in general include being more likely to develop depression, loneliness, low self-esteem, alcohol and drug abuse, sleeping difficulties, self-harm, and suicidal ideation and attempts. “We’re social creatures, and when we don’t have those social connections, we get very depressed.”
Bullying victims also are more likely to develop school avoidance and absence, decreased school performance, poor concentration, high anxiety, and social withdrawal – all of which limit their opportunities to learn. “The No. 1 thing you can do to help these kids is to believe their story – to explain to them that it’s not their fault, and to explain that you are there for them and that you support them,” he said. “When a kid gets the feeling that someone is willing to listen to them and believe them, it does an enormous good for their emotional state.”
Dr. Raffalli added that a toxic stress response can occur when a child experiences strong, frequent, and/or prolonged adversity – such as physical or emotional abuse, chronic neglect, caregiver substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship – without adequate adult support. This kind of prolonged activation of the stress response systems can disrupt the development of brain architecture and other organ systems, and increase the risk for stress-related disease and cognitive impairment well into the adult years.
In the Harvard Review of Psychiatry, researchers set out to investigate what’s known about the long-term health effects of childhood bullying. They found that bullying can induce “aspects of the stress response, via epigenetic, inflammatory, and metabolic mediators [that] have the capacity to compromise mental and physical health, and to increase the risk of disease.” The researchers advised clinicians who care for children to assess the mental and physical health effects of bullying (Harv Rev Psychiatry. 2017;25[2]:89-95).
Additional vulnerabilities for bullying victims include parents and children whose primary language is not English, as well as parents with mental illness or substance abuse and families living in poverty. “We have to keep in mind how much additional stress they may be dealing with. This can make it harder for them to cope. Bullies also are shown to be at higher risk for psychological and legal trouble into adulthood, so we should be trying to help them too. We have to keep in mind that these are all developing kids.”
Cyberbullying
In Dr. Raffalli’s clinical experience, cyberbullying has become the bully’s weapon of choice. “I call it the stealth bomber of bullying,” he said. “Cyberbullying can start as early as the second or third grade. Most parents are not giving phones to second-graders. I’m worried that it’s going to get worse, though, with the excuse that ‘I feel safer if they have a cell phone so they can call me.’ I tell parents that they still make flip phones. You don’t have to get a smartphone for a second- or third-grader, or even for a sixth-grader.”
By the time kids reach fourth and fifth grade, he continued, they begin to form their opinion “about what they believe is cool and not cool, and they begin to get into cliques that have similar beliefs, and support each other, and may break off from old friends.” He added that, while adult predation “makes the news and is certainly something we should all be concerned about, the incidence of being harassed and bullied by someone in your own age group at school is actually much higher and still has serious outcomes, including the possibility of death.”
The Massachusetts antibullying law stipulates that all teachers and all school personnel have to participate in mandatory bullying training. Schools also are required to draft and follow a bullying investigative protocol.
“Apparently the schools have all done this, yet the number of times that schools use interventions that are not advisable, such as mediation, is incredible to me,” Dr. Raffalli said. “Bringing the bully and the victim together for a ‘cup of coffee and a handshake’ is not advisable. Mediation has been shown in a number of studies to be detrimental in bullying situations. Things can easily get worse.”
Often, family members who bring their child to the BACPAC “feel that their child’s school is not helping them,” he said. “We should try to figure out why those schools are having such a hard time and see if we can help them.”
Dr. Raffalli reported having no financial disclosures.
NEW ORLEANS – After Massachusetts passed antibullying legislation in 2009, Peter C. Raffalli, MD, saw an opportunity to improve care for the increasing numbers of children presenting to his neurology practice at Boston Children’s Hospital who were victims of bullying – especially those with developmental disabilities.
“I had been thinking of a clinic to help kids with these issues, aside from just helping them deal with the fallout: the depression, anxiety, et cetera, that comes with being bullied,” Dr. Raffalli recalled at the annual meeting of the American Academy of Pediatrics. “I wanted to do something to help present to families the evidence-based strategies regarding bullying prevention, detection, and intervention that might help to stop the bullying.”
This led him to launch the Bullying and Cyberbullying Prevention and Advocacy Collaborative (BACPAC) at Boston Children’s Hospital, which began in 2009 as an educational resource for families, medical colleagues, and schools. Dr. Raffalli also formed an alliance with the Massachusetts Aggression Reduction Center at Bridgewater State University (Ann Neurol. 2016;79[2]:167-8).
Two years later in 2011, BACPAC became a formal clinic at Boston Children’s that serves as a subspecialty consult service for victims of bullying and their families. The clinic team consists of a child neurologist, a social worker, and an education resource specialist who meet with the bullying victim and his/her family in initial consultation for 90 minutes. The goal is to develop an evidence-based plan for bullying prevention, detection, and intervention that is individualized to the patient’s developmental and social needs.
“We tell families that bullying is recognized medically and legally as a form of abuse,” said Dr. Raffalli. “The medical and psychological consequences are similar to other forms of abuse. You’d be surprised how often patients do think the bullying is their fault.”
The extent of the problem
Researchers estimate that 25%-30% of children will experience some form of bullying between kindergarten and grade 12, and about 8% will engage in bullying themselves. When BACPAC began in 2009, Dr. Raffalli conducted an informal search of peer-reviewed literature on bullying in children with special needs; it yielded just four articles. “Since then, there’s been an exponential explosion of literature on various aspects of bullying,” he said. Now there is ample evidence in the peer-reviewed literature to show the increased risk for bullying/cyberbullying in children/teens, not just with neurodevelopmental disorders, but also for kids with other medical disorders such as obesity, asthma, and allergies.
“We’ve had a good number of kids over the years with peanut allergy who were literally threatened physically with peanut butter at school,” he said. “It’s incredible how callous some kids can be. Kids with oppositional defiant disorder, impulse control disorder, and callous/unemotional traits from a psychological standpoint are hardest to reach when it comes to getting them to stop bullying. You’d be surprised how frequently bullies use the phrase [to their victims], ‘You should kill yourself.’ They don’t realize the damage they’re doing to people. Bullying can lead to severe psychological but also long-term medical problems, including suicidal ideation.”
Published studies show that the highest incidences of bullying occur in children with neurodevelopmental conditions such as ADHD, autistic spectrum disorders, Tourette syndrome, and other learning disabilities (Eur J Spec Needs Ed. 2010;25[1]:77-91). This population of children is overrepresented in bullying “because the services they receive at school make their disabilities more visible,” explained Dr. Raffalli, who is also an assistant professor of neurology at Harvard Medical School, Boston. “They stand out, and they have social information–processing deficits or distortions that exacerbate bullying involvement. They also have difficulty interpreting social cues or attributing hostile characteristics to their peer’s behavior.”
The consequences of bullying
The psychological and educational consequences of bullying among children in general include being more likely to develop depression, loneliness, low self-esteem, alcohol and drug abuse, sleeping difficulties, self-harm, and suicidal ideation and attempts. “We’re social creatures, and when we don’t have those social connections, we get very depressed.”
Bullying victims also are more likely to develop school avoidance and absence, decreased school performance, poor concentration, high anxiety, and social withdrawal – all of which limit their opportunities to learn. “The No. 1 thing you can do to help these kids is to believe their story – to explain to them that it’s not their fault, and to explain that you are there for them and that you support them,” he said. “When a kid gets the feeling that someone is willing to listen to them and believe them, it does an enormous good for their emotional state.”
Dr. Raffalli added that a toxic stress response can occur when a child experiences strong, frequent, and/or prolonged adversity – such as physical or emotional abuse, chronic neglect, caregiver substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship – without adequate adult support. This kind of prolonged activation of the stress response systems can disrupt the development of brain architecture and other organ systems, and increase the risk for stress-related disease and cognitive impairment well into the adult years.
In the Harvard Review of Psychiatry, researchers set out to investigate what’s known about the long-term health effects of childhood bullying. They found that bullying can induce “aspects of the stress response, via epigenetic, inflammatory, and metabolic mediators [that] have the capacity to compromise mental and physical health, and to increase the risk of disease.” The researchers advised clinicians who care for children to assess the mental and physical health effects of bullying (Harv Rev Psychiatry. 2017;25[2]:89-95).
Additional vulnerabilities for bullying victims include parents and children whose primary language is not English, as well as parents with mental illness or substance abuse and families living in poverty. “We have to keep in mind how much additional stress they may be dealing with. This can make it harder for them to cope. Bullies also are shown to be at higher risk for psychological and legal trouble into adulthood, so we should be trying to help them too. We have to keep in mind that these are all developing kids.”
Cyberbullying
In Dr. Raffalli’s clinical experience, cyberbullying has become the bully’s weapon of choice. “I call it the stealth bomber of bullying,” he said. “Cyberbullying can start as early as the second or third grade. Most parents are not giving phones to second-graders. I’m worried that it’s going to get worse, though, with the excuse that ‘I feel safer if they have a cell phone so they can call me.’ I tell parents that they still make flip phones. You don’t have to get a smartphone for a second- or third-grader, or even for a sixth-grader.”
By the time kids reach fourth and fifth grade, he continued, they begin to form their opinion “about what they believe is cool and not cool, and they begin to get into cliques that have similar beliefs, and support each other, and may break off from old friends.” He added that, while adult predation “makes the news and is certainly something we should all be concerned about, the incidence of being harassed and bullied by someone in your own age group at school is actually much higher and still has serious outcomes, including the possibility of death.”
The Massachusetts antibullying law stipulates that all teachers and all school personnel have to participate in mandatory bullying training. Schools also are required to draft and follow a bullying investigative protocol.
“Apparently the schools have all done this, yet the number of times that schools use interventions that are not advisable, such as mediation, is incredible to me,” Dr. Raffalli said. “Bringing the bully and the victim together for a ‘cup of coffee and a handshake’ is not advisable. Mediation has been shown in a number of studies to be detrimental in bullying situations. Things can easily get worse.”
Often, family members who bring their child to the BACPAC “feel that their child’s school is not helping them,” he said. “We should try to figure out why those schools are having such a hard time and see if we can help them.”
Dr. Raffalli reported having no financial disclosures.
NEW ORLEANS – After Massachusetts passed antibullying legislation in 2009, Peter C. Raffalli, MD, saw an opportunity to improve care for the increasing numbers of children presenting to his neurology practice at Boston Children’s Hospital who were victims of bullying – especially those with developmental disabilities.
“I had been thinking of a clinic to help kids with these issues, aside from just helping them deal with the fallout: the depression, anxiety, et cetera, that comes with being bullied,” Dr. Raffalli recalled at the annual meeting of the American Academy of Pediatrics. “I wanted to do something to help present to families the evidence-based strategies regarding bullying prevention, detection, and intervention that might help to stop the bullying.”
This led him to launch the Bullying and Cyberbullying Prevention and Advocacy Collaborative (BACPAC) at Boston Children’s Hospital, which began in 2009 as an educational resource for families, medical colleagues, and schools. Dr. Raffalli also formed an alliance with the Massachusetts Aggression Reduction Center at Bridgewater State University (Ann Neurol. 2016;79[2]:167-8).
Two years later in 2011, BACPAC became a formal clinic at Boston Children’s that serves as a subspecialty consult service for victims of bullying and their families. The clinic team consists of a child neurologist, a social worker, and an education resource specialist who meet with the bullying victim and his/her family in initial consultation for 90 minutes. The goal is to develop an evidence-based plan for bullying prevention, detection, and intervention that is individualized to the patient’s developmental and social needs.
“We tell families that bullying is recognized medically and legally as a form of abuse,” said Dr. Raffalli. “The medical and psychological consequences are similar to other forms of abuse. You’d be surprised how often patients do think the bullying is their fault.”
The extent of the problem
Researchers estimate that 25%-30% of children will experience some form of bullying between kindergarten and grade 12, and about 8% will engage in bullying themselves. When BACPAC began in 2009, Dr. Raffalli conducted an informal search of peer-reviewed literature on bullying in children with special needs; it yielded just four articles. “Since then, there’s been an exponential explosion of literature on various aspects of bullying,” he said. Now there is ample evidence in the peer-reviewed literature to show the increased risk for bullying/cyberbullying in children/teens, not just with neurodevelopmental disorders, but also for kids with other medical disorders such as obesity, asthma, and allergies.
“We’ve had a good number of kids over the years with peanut allergy who were literally threatened physically with peanut butter at school,” he said. “It’s incredible how callous some kids can be. Kids with oppositional defiant disorder, impulse control disorder, and callous/unemotional traits from a psychological standpoint are hardest to reach when it comes to getting them to stop bullying. You’d be surprised how frequently bullies use the phrase [to their victims], ‘You should kill yourself.’ They don’t realize the damage they’re doing to people. Bullying can lead to severe psychological but also long-term medical problems, including suicidal ideation.”
Published studies show that the highest incidences of bullying occur in children with neurodevelopmental conditions such as ADHD, autistic spectrum disorders, Tourette syndrome, and other learning disabilities (Eur J Spec Needs Ed. 2010;25[1]:77-91). This population of children is overrepresented in bullying “because the services they receive at school make their disabilities more visible,” explained Dr. Raffalli, who is also an assistant professor of neurology at Harvard Medical School, Boston. “They stand out, and they have social information–processing deficits or distortions that exacerbate bullying involvement. They also have difficulty interpreting social cues or attributing hostile characteristics to their peer’s behavior.”
The consequences of bullying
The psychological and educational consequences of bullying among children in general include being more likely to develop depression, loneliness, low self-esteem, alcohol and drug abuse, sleeping difficulties, self-harm, and suicidal ideation and attempts. “We’re social creatures, and when we don’t have those social connections, we get very depressed.”
Bullying victims also are more likely to develop school avoidance and absence, decreased school performance, poor concentration, high anxiety, and social withdrawal – all of which limit their opportunities to learn. “The No. 1 thing you can do to help these kids is to believe their story – to explain to them that it’s not their fault, and to explain that you are there for them and that you support them,” he said. “When a kid gets the feeling that someone is willing to listen to them and believe them, it does an enormous good for their emotional state.”
Dr. Raffalli added that a toxic stress response can occur when a child experiences strong, frequent, and/or prolonged adversity – such as physical or emotional abuse, chronic neglect, caregiver substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship – without adequate adult support. This kind of prolonged activation of the stress response systems can disrupt the development of brain architecture and other organ systems, and increase the risk for stress-related disease and cognitive impairment well into the adult years.
In the Harvard Review of Psychiatry, researchers set out to investigate what’s known about the long-term health effects of childhood bullying. They found that bullying can induce “aspects of the stress response, via epigenetic, inflammatory, and metabolic mediators [that] have the capacity to compromise mental and physical health, and to increase the risk of disease.” The researchers advised clinicians who care for children to assess the mental and physical health effects of bullying (Harv Rev Psychiatry. 2017;25[2]:89-95).
Additional vulnerabilities for bullying victims include parents and children whose primary language is not English, as well as parents with mental illness or substance abuse and families living in poverty. “We have to keep in mind how much additional stress they may be dealing with. This can make it harder for them to cope. Bullies also are shown to be at higher risk for psychological and legal trouble into adulthood, so we should be trying to help them too. We have to keep in mind that these are all developing kids.”
Cyberbullying
In Dr. Raffalli’s clinical experience, cyberbullying has become the bully’s weapon of choice. “I call it the stealth bomber of bullying,” he said. “Cyberbullying can start as early as the second or third grade. Most parents are not giving phones to second-graders. I’m worried that it’s going to get worse, though, with the excuse that ‘I feel safer if they have a cell phone so they can call me.’ I tell parents that they still make flip phones. You don’t have to get a smartphone for a second- or third-grader, or even for a sixth-grader.”
By the time kids reach fourth and fifth grade, he continued, they begin to form their opinion “about what they believe is cool and not cool, and they begin to get into cliques that have similar beliefs, and support each other, and may break off from old friends.” He added that, while adult predation “makes the news and is certainly something we should all be concerned about, the incidence of being harassed and bullied by someone in your own age group at school is actually much higher and still has serious outcomes, including the possibility of death.”
The Massachusetts antibullying law stipulates that all teachers and all school personnel have to participate in mandatory bullying training. Schools also are required to draft and follow a bullying investigative protocol.
“Apparently the schools have all done this, yet the number of times that schools use interventions that are not advisable, such as mediation, is incredible to me,” Dr. Raffalli said. “Bringing the bully and the victim together for a ‘cup of coffee and a handshake’ is not advisable. Mediation has been shown in a number of studies to be detrimental in bullying situations. Things can easily get worse.”
Often, family members who bring their child to the BACPAC “feel that their child’s school is not helping them,” he said. “We should try to figure out why those schools are having such a hard time and see if we can help them.”
Dr. Raffalli reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 2019
Asthma exacerbation in pregnancy impacts mothers, infants
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL
What to do when the evidence is not conclusive
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
When guideline treatment of asthma fails, consider a macrolide antibiotic
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
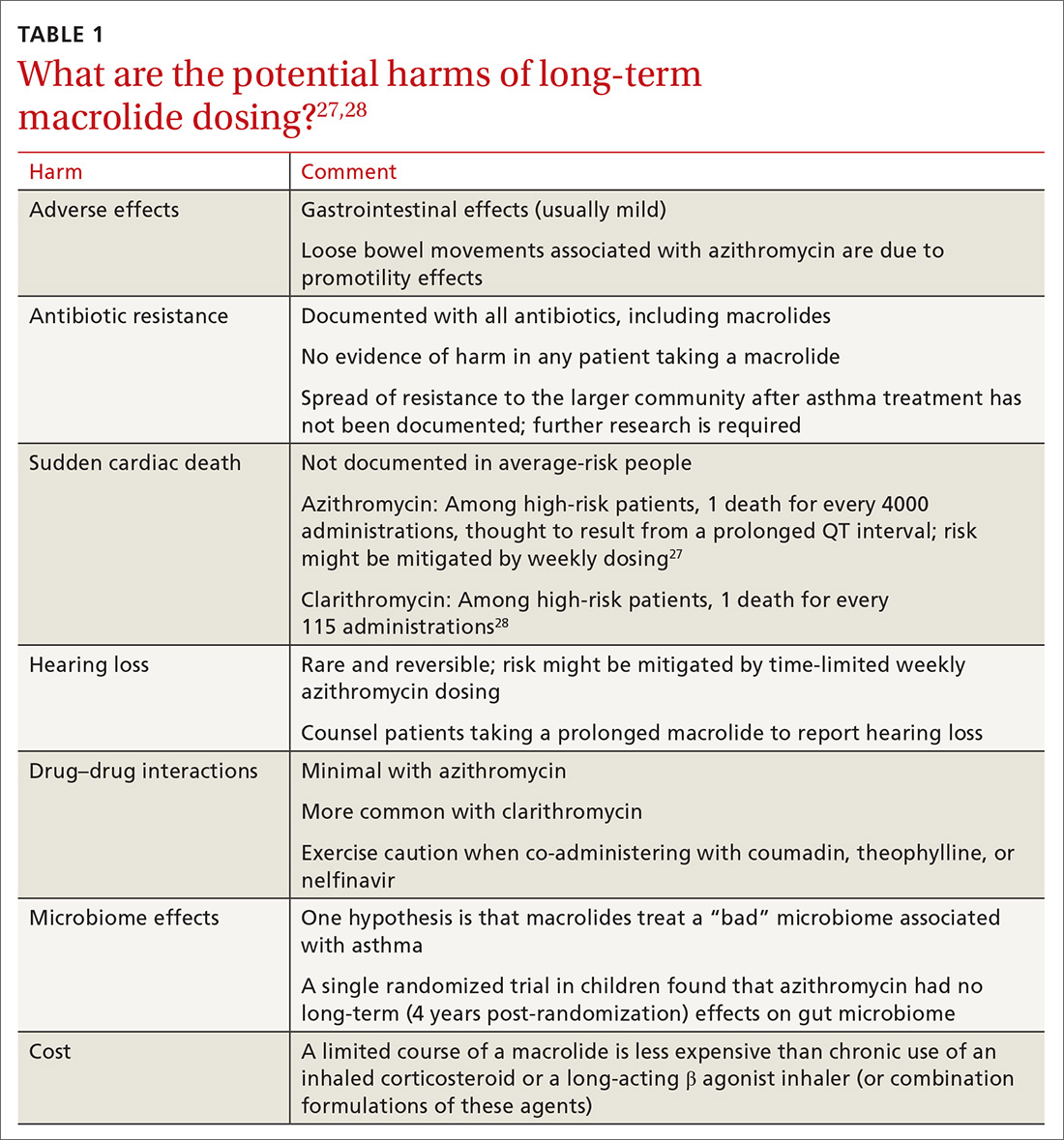
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
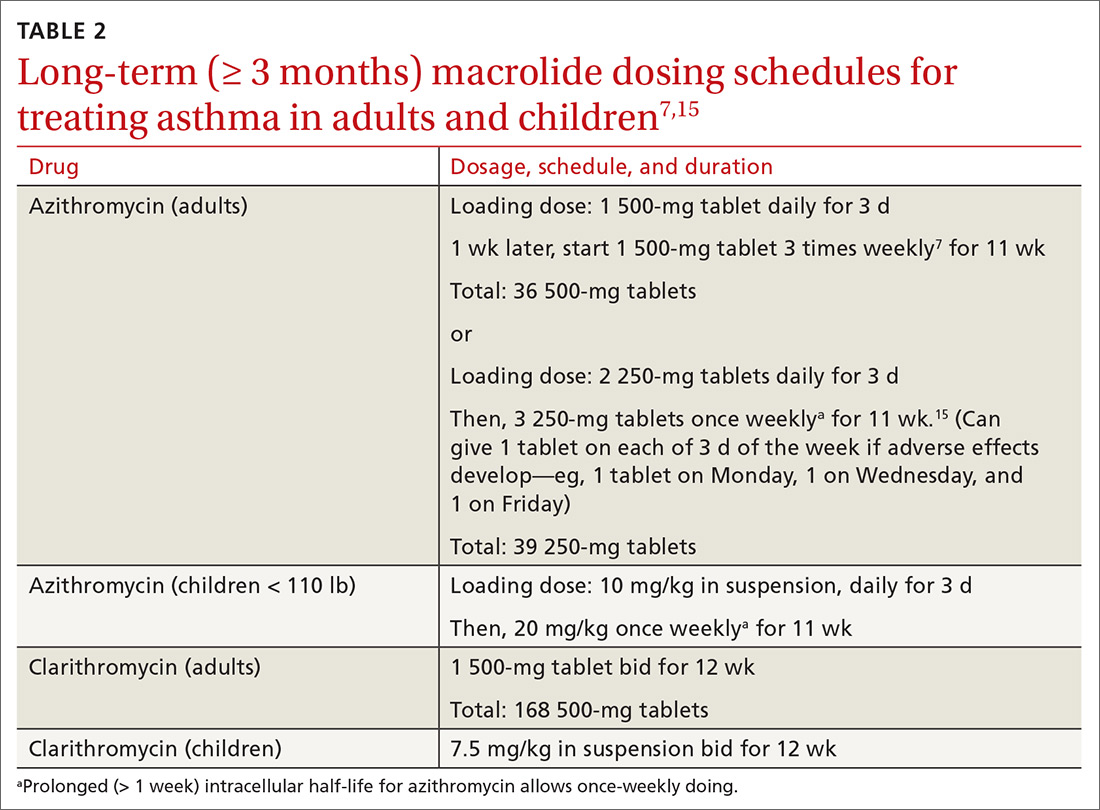
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
PRACTICE RECOMMENDATIONS
› Consider a trial of azithromycin for patients who have poorly controlled persistent asthma and are not responding to guideline treatment with the combination of an inhaled corticosteroid and either a long-acting bronchodilator or long-acting muscarinic antagonist. B
› Consider a trial of azithromycin in addition to first-line guideline therapy for patients who have new-onset asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dupilumab effective in early- and late-onset asthma
NEW ORLEANS – A new analysis suggests
Dupilumab may be more effective in reducing severe asthma exacerbations in patients with late-onset asthma, but the drug’s effect on lung function appeared the same regardless of asthma onset. Nicola Hanania, MD, of Baylor College of Medicine in Houston presented these results at the annual meeting of the American College of Chest Physicians.
Dr. Hanania and colleagues conducted a subanalysis of the LIBERTY ASTHMA QUEST study (NCT02414854). Previous data from this study showed that patients with uncontrolled, moderate to severe asthma who received dupilumab had fewer exacerbations and better lung function than did patients who received placebo (N Engl J Med. 2018;378:2486-96).
In their subanalysis, Dr. Hanania and his colleagues evaluated the efficacy of dupilumab, given at 200 mg or 300 mg every 2 weeks, in patients with early-onset asthma (at 40 years of age or younger) and late-onset asthma (at 41 years or older). The analysis included 919 patients with early-onset asthma who received dupilumab and 450 early-onset patients who received placebo. There were 345 patients with late-onset asthma who received dupilumab and 188 late-onset patients who received placebo.
Exacerbations
Dupilumab significantly reduced the adjusted annualized severe exacerbation rates during the 52-week treatment period. Significant reductions occurred in both early- and late-onset patients, though reductions were greater in the late-onset group.
In early-onset patients, dupilumab reduced severe exacerbations by 38% when given at 200 mg and by 37% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients, dupilumab reduced exacerbations by 64% and 69%, respectively (P less than .001 vs. placebo).
Dr. Hanania said it isn’t clear why late-onset patients appear to derive more benefit with regard to exacerbations. It may be because these patients have more comorbidities or because they aren’t using their inhalers correctly. The researchers are investigating these possibilities.
Dr. Hanania went on to note that reductions in exacerbation rates were greatest in patients with elevated blood eosinophils (150 cells/mcL or greater) or fractional exhaled nitric oxide (FeNO; 25 ppb or greater).
In patients with early-onset asthma and elevated eosinophils, dupilumab reduced severe exacerbations by 50% when given at 200 mg and by 55% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients with elevated eosinophils, dupilumab reduced exacerbations by 65% and 73%, respectively (P less than .001 vs. placebo).
In patients with early-onset asthma and elevated FeNO, dupilumab reduced severe exacerbations by 56% when given at 200 mg and by 52% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients with elevated FeNO, dupilumab reduced exacerbations by 79% and 71%, respectively (P less than .001 vs. placebo).
Lung function
Dupilumab also improved prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), compared with placebo, with similar results in early- and late-onset patients.
In early-onset patients, the P values were less than .001 for both doses of dupilumab at weeks 12 and 52. In late-onset patients, the P values were less than .001 for the 300-mg dose at week 12 and the 200-mg dose at week 52, less than .01 for the 200-mg dose at week 12, and less than .05 for the 300-mg dose at week 52.
The effects of dupilumab on pre-BD FEV1 were greatest in patients with elevated eosinophils or FeNO. At week 12, the P value was less than .001 for both doses of dupilumab in early-onset patients with elevated eosinophils or FeNO. The P value was less than .01 for both doses in late-onset patients with elevated eosinophils. And the P value was less than .001 for both doses in late-onset patients with elevated FeNO.
This research was sponsored by Sanofi and Regeneron. Dr. Hanania disclosed relationships with Genentech, Novartis, AstraZeneca, Boehringer Ingelheim, GSK, Regeneron, and Sanofi.
SOURCE: Hanania N et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.870.
NEW ORLEANS – A new analysis suggests
Dupilumab may be more effective in reducing severe asthma exacerbations in patients with late-onset asthma, but the drug’s effect on lung function appeared the same regardless of asthma onset. Nicola Hanania, MD, of Baylor College of Medicine in Houston presented these results at the annual meeting of the American College of Chest Physicians.
Dr. Hanania and colleagues conducted a subanalysis of the LIBERTY ASTHMA QUEST study (NCT02414854). Previous data from this study showed that patients with uncontrolled, moderate to severe asthma who received dupilumab had fewer exacerbations and better lung function than did patients who received placebo (N Engl J Med. 2018;378:2486-96).
In their subanalysis, Dr. Hanania and his colleagues evaluated the efficacy of dupilumab, given at 200 mg or 300 mg every 2 weeks, in patients with early-onset asthma (at 40 years of age or younger) and late-onset asthma (at 41 years or older). The analysis included 919 patients with early-onset asthma who received dupilumab and 450 early-onset patients who received placebo. There were 345 patients with late-onset asthma who received dupilumab and 188 late-onset patients who received placebo.
Exacerbations
Dupilumab significantly reduced the adjusted annualized severe exacerbation rates during the 52-week treatment period. Significant reductions occurred in both early- and late-onset patients, though reductions were greater in the late-onset group.
In early-onset patients, dupilumab reduced severe exacerbations by 38% when given at 200 mg and by 37% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients, dupilumab reduced exacerbations by 64% and 69%, respectively (P less than .001 vs. placebo).
Dr. Hanania said it isn’t clear why late-onset patients appear to derive more benefit with regard to exacerbations. It may be because these patients have more comorbidities or because they aren’t using their inhalers correctly. The researchers are investigating these possibilities.
Dr. Hanania went on to note that reductions in exacerbation rates were greatest in patients with elevated blood eosinophils (150 cells/mcL or greater) or fractional exhaled nitric oxide (FeNO; 25 ppb or greater).
In patients with early-onset asthma and elevated eosinophils, dupilumab reduced severe exacerbations by 50% when given at 200 mg and by 55% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients with elevated eosinophils, dupilumab reduced exacerbations by 65% and 73%, respectively (P less than .001 vs. placebo).
In patients with early-onset asthma and elevated FeNO, dupilumab reduced severe exacerbations by 56% when given at 200 mg and by 52% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients with elevated FeNO, dupilumab reduced exacerbations by 79% and 71%, respectively (P less than .001 vs. placebo).
Lung function
Dupilumab also improved prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), compared with placebo, with similar results in early- and late-onset patients.
In early-onset patients, the P values were less than .001 for both doses of dupilumab at weeks 12 and 52. In late-onset patients, the P values were less than .001 for the 300-mg dose at week 12 and the 200-mg dose at week 52, less than .01 for the 200-mg dose at week 12, and less than .05 for the 300-mg dose at week 52.
The effects of dupilumab on pre-BD FEV1 were greatest in patients with elevated eosinophils or FeNO. At week 12, the P value was less than .001 for both doses of dupilumab in early-onset patients with elevated eosinophils or FeNO. The P value was less than .01 for both doses in late-onset patients with elevated eosinophils. And the P value was less than .001 for both doses in late-onset patients with elevated FeNO.
This research was sponsored by Sanofi and Regeneron. Dr. Hanania disclosed relationships with Genentech, Novartis, AstraZeneca, Boehringer Ingelheim, GSK, Regeneron, and Sanofi.
SOURCE: Hanania N et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.870.
NEW ORLEANS – A new analysis suggests
Dupilumab may be more effective in reducing severe asthma exacerbations in patients with late-onset asthma, but the drug’s effect on lung function appeared the same regardless of asthma onset. Nicola Hanania, MD, of Baylor College of Medicine in Houston presented these results at the annual meeting of the American College of Chest Physicians.
Dr. Hanania and colleagues conducted a subanalysis of the LIBERTY ASTHMA QUEST study (NCT02414854). Previous data from this study showed that patients with uncontrolled, moderate to severe asthma who received dupilumab had fewer exacerbations and better lung function than did patients who received placebo (N Engl J Med. 2018;378:2486-96).
In their subanalysis, Dr. Hanania and his colleagues evaluated the efficacy of dupilumab, given at 200 mg or 300 mg every 2 weeks, in patients with early-onset asthma (at 40 years of age or younger) and late-onset asthma (at 41 years or older). The analysis included 919 patients with early-onset asthma who received dupilumab and 450 early-onset patients who received placebo. There were 345 patients with late-onset asthma who received dupilumab and 188 late-onset patients who received placebo.
Exacerbations
Dupilumab significantly reduced the adjusted annualized severe exacerbation rates during the 52-week treatment period. Significant reductions occurred in both early- and late-onset patients, though reductions were greater in the late-onset group.
In early-onset patients, dupilumab reduced severe exacerbations by 38% when given at 200 mg and by 37% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients, dupilumab reduced exacerbations by 64% and 69%, respectively (P less than .001 vs. placebo).
Dr. Hanania said it isn’t clear why late-onset patients appear to derive more benefit with regard to exacerbations. It may be because these patients have more comorbidities or because they aren’t using their inhalers correctly. The researchers are investigating these possibilities.
Dr. Hanania went on to note that reductions in exacerbation rates were greatest in patients with elevated blood eosinophils (150 cells/mcL or greater) or fractional exhaled nitric oxide (FeNO; 25 ppb or greater).
In patients with early-onset asthma and elevated eosinophils, dupilumab reduced severe exacerbations by 50% when given at 200 mg and by 55% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients with elevated eosinophils, dupilumab reduced exacerbations by 65% and 73%, respectively (P less than .001 vs. placebo).
In patients with early-onset asthma and elevated FeNO, dupilumab reduced severe exacerbations by 56% when given at 200 mg and by 52% when given at 300 mg (P less than .001 vs. placebo). In late-onset patients with elevated FeNO, dupilumab reduced exacerbations by 79% and 71%, respectively (P less than .001 vs. placebo).
Lung function
Dupilumab also improved prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), compared with placebo, with similar results in early- and late-onset patients.
In early-onset patients, the P values were less than .001 for both doses of dupilumab at weeks 12 and 52. In late-onset patients, the P values were less than .001 for the 300-mg dose at week 12 and the 200-mg dose at week 52, less than .01 for the 200-mg dose at week 12, and less than .05 for the 300-mg dose at week 52.
The effects of dupilumab on pre-BD FEV1 were greatest in patients with elevated eosinophils or FeNO. At week 12, the P value was less than .001 for both doses of dupilumab in early-onset patients with elevated eosinophils or FeNO. The P value was less than .01 for both doses in late-onset patients with elevated eosinophils. And the P value was less than .001 for both doses in late-onset patients with elevated FeNO.
This research was sponsored by Sanofi and Regeneron. Dr. Hanania disclosed relationships with Genentech, Novartis, AstraZeneca, Boehringer Ingelheim, GSK, Regeneron, and Sanofi.
SOURCE: Hanania N et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.870.
REPORTING FROM CHEST 2019